PHARMACY DISTRIBUTION AND SERVICES AGREEMENT
Exhibit 10.1

PHARMACY DISTRIBUTION AND SERVICES AGREEMENT
THIS PHARMACY DISTRIBUTION AND SERVICES AGREEMENT (“Agreement”) is made effective as of the 1st day of July, 2017 (“Effective Date”) between:
CELGENE CORPORATION
00 Xxxxxx Xxxxxx
Xxxxxx, Xxx Xxxxxx 00000
(together with its subsidiaries and affiliates hereinafter collectively, “Celgene”),
and
DIPLOMAT PHARMACY, INC. d/b/a
DIPLOMAT SPECIALTY PHARMACY
0000 Xxxxx Xxxxxxx Xxxxxx
Xxxxx, XX 00000
(hereinafter, “Pharmacy”).
WHEREAS, Celgene is authorized to market and sell REVLIMID® (lenalidomide), POMALYST® (pomalidomide), and THALOMID® (thalidomide) in the United States of America and its territories; and
WHEREAS, Celgene and Pharmacy wish to enter into this Agreement under which Pharmacy, registered under REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS(1) (as defined below), will provide data reporting and other services to Celgene.
NOW THEREFORE, in consideration of the promises and mutual covenants herein contained in this Agreement, the parties hereby agree as follows:
1. DEFINITIONS
For purposes of this Agreement the following terms shall have the following meanings:
1.1 “Adverse Drug Experience” or “ADE” shall have the meaning set forth in 21 CFR 314.80 as well as any occurrence of an elevated Beta HCG or positive urine pregnancy tests, or a pregnancy or a possible exposure of a pregnant woman, whether involving a Customer or partner of a male Customer or a pregnant female who comes into contact with the Product while dispensing. Furthermore:
(a) the term “Adverse Drug Experience” or “ADE” shall also include cases of special situations, as required by Guidelines on good pharmacovigilance practices: Module VI - Management and reporting of adverse reactions to medicinal products, which requires companies with marketed products centrally authorized by the EMA to report situations such as, by way of example only, outcome of use of medicinal product during pregnancy, adverse reaction during breastfeeding, use of product in children, reports of lack of efficacy, suspected transmission of infectious agents, reports of overdose, abuse, misuse, medication error, and reports from compassionate/named-patient use; and,
(b) the term “suspected to be associated with the use of” shall mean the causal relationship between the medicinal product and the adverse drug experience is considered at least a reasonable possibility.
1.2 “Affiliates” shall mean, with respect to a given party, any corporation, firm, partnership or other entity that directly or indirectly controls or is controlled by or is under common control with such party. For purposes of this Section 1.2, “control” shall mean direct or indirect ownership of greater than fifty percent (50%) of the equity having the power to vote on or direct the affairs of the entity.
1.3 “Certified Counselor” shall mean a licensed healthcare professional certified by Celgene as a counselor for REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® program(s) and who is either a: Doctor of Osteopathy (“D.O.”); Licensed Practical Nurse (“LPN”); Pharmacist (“X.Xx.”); Pharmacy Intern; Physician (“M.D.”); Physician Assistant (“P.A.”); Nurse Practitioner (“N.P.”); or Registered Nurse (“RN”).
1.4 “Certified Pharmacist” shall mean a Pharmacist certified by Celgene as a Pharmacist for REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® program(s) to dispense REVLIMID®, POMALYST®, and THALOMID®, or counsel patients.
1.5 “Certified Prescriber” shall mean a licensed healthcare professional who is licensed to prescribe medication and certified in the REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® program(s).
1.6 “Customers” shall mean persons who are prescribed Product by a Certified Prescriber.
1.7 “Database” shall have the meaning set forth in Section 5.2.
1.8 “Dispensing” shall mean a prescription for Product was filled at the certified Dispensing Site, counseling for the patient to receive Product was completed and a confirmation number obtained, and the prescription for Product was either shipped (for greater clarity, to mean the prescribed Product has physically left the Dispensing Site or provided to the
patient or the patient’s authorized representative who has picked up the prescribed Product from the Dispensing Site.)
1.9 “Dispensing Site” shall mean Pharmacy’s facility(ies) that fill and ship prescriptions, listed on Schedule 1.8, attached hereto, as may be amended from time to time by upon mutual agreement of the parties, after being properly certified in REVLIMID REMS°, POMALYST REMS®, and THALOMID REMS®. In the case of physician networks who are dispensing, it shall be responsible for placing and receiving orders of Product, administration, and record keeping, as well as certifying itself and any of its other Dispensing Sites and implementing REVLIMID REMS (8), POMALYST REMS®, and THALOMID REMSR.
1.10 “EMA” shall mean the European Medicines Agency.
1.11 “FDA” shall mean the United States Food and Drug Administration.
1.12 “High Risk Deviation” shall mean any action taken by the Pharmacy that is inconsistent or non-compliant with any provision, or part thereof, of REVLIMID REMS®, POMALYST REMS ®, and THALOMID REMS® which or which may: (i) increases the risk of fetal exposure; or (ii) occurs on a consistent basis which evidences a negligent or willful disregard by the Pharmacy to the requirements of REVLIMID REMS ®, POMALYST REMS®, and THALOMID REMS®. Any High Risk Deviation by Pharmacy shall be considered a material breach of the terms of this Agreement.
1.13 “Pharmacist” shall mean an individual who is currently licensed by the state in which he/she is practicing to engage in the practice of preparing, preserving, compounding and dispensing medical drugs.
1.14 “Pharmacy” shall be the corporate entity(ies) at Pharmacy responsible for placing and receiving orders of Product, administration, and record keeping, as well as certifying-itself and all of its Dispensing Sites and implementing REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® program(s). Pharmacy may also fill and ship prescriptions.
1.15 “Pharmacy Intern” shall mean an individual who engages in the practice of pharmacy while under the personal supervision of a Pharmacist and is enrolled in a professional degree program of an accredited school or college of pharmacy and is satisfactorily progressing towards meeting requirements for licensure as a Pharmacist.
1.16 “REVLIMID REMS®, POMALYST REMS® and THALOMID REMS®” shall mean the controlled distribution program and component of the Risk Evaluation Mitigation Strategies (“REMS”) program specifically tailored to the sale and distribution of the Product(s) REVLIMID®, POMALYST®, and THALOMID® as described in Section 3 and in the Requirements document (“Requirements Document”), attached as Schedule 1.17, which may be updated from time to time by Celgene, at its sole discretion. In the event the Requirements Document is updated, Celgene shall inform Pharmacy within ten (10) business days, and allow Pharmacy thirty (30) days to meet requirements
of the updated Requirements Document, unless a shorter time frame is necessary to meet Regulatory Authority requirements.
1.17 “Product” shall mean Celgene’s REVLIMID (lenalidomide), or POMALYST® (pomalidomide), or THALOMIW (thalidomide), and “Products” shall mean any two or more of REVLIMID® (lenalidomide) and/or POMALYST® (pomalidomide) and/or THALOMID® (thalidomide).
1.18 “Regulatory Authority” shall mean any governmental authority, agency or other instrumentality having regulatory responsibility, control or oversight over the manufacture, use, labeling, packaging, shipping, distribution, dispensing or destruction of the Product.
1.19 “SOP” shall mean the written standard operating procedures, specifications and instructions, mutually agreed upon by the parties, which may be updated from time to time, upon the sole discretion of the party who the owns the SOP.
1.20 “Territory” shall mean the United States of America and its territories.
2. PRODUCT ORDERS, SHIPMENT, AND HANDLING
2.1 Orders. The parties hereto agree that, during the Term, as defined below, Pharmacy shall purchase REVLIMID®, POMALYST®, and/or THALOMID® directly and exclusively from Celgene at the commercial prices in effect at the time of order, and, subject to the right of Celgene to allocate supplies of Product under Section 2.6, Celgene shall supply Product to Pharmacy, for sale and distribution to Customers. Pharmacy shall order Product by phone, email, or EDI from Celgene in such quantities as are necessary to meet the demand for Product from Customers. All orders shall be firm, and Pharmacy may not change or cancel an order without the prior written approval from Celgene. All purchases of Product by Pharmacy shall be on the terms and conditions set forth in this Agreement. No purchase order, invoice or other form shall be deemed to vary the terms of this Agreement.
2.2 Shipment by Celgene. Celgene shall ship Product to Pharmacy by means of transportation (commercial truck or better) determined by Celgene and at Celgene’s cost. While Celgene shall use reasonable efforts to avoid any delay in delivering Product on the delivery dates agreed upon by the parties, Celgene shall not be liable to Pharmacy for late delivery.
2.3 Storage; Handling of Product; Inventory Accountability. Pharmacy shall unload each shipment of Product immediately upon receipt from Celgene in accordance with the applicable Pharmacy SOP. Pharmacy may transport Product to a Dispensing Site for storage and distribution; however, there shall be no transfer of Product between Dispensing Sites. Pharmacy shall use storage facilities and storage conditions for Product in compliance with applicable Celgene SOPs. Pharmacy shall at all times handle, store and distribute Product in accordance with applicable Celgene SOPs which shall incorporate by this reference the handling and storage provisions in the package labeling for Product. Pharmacy shall keep an inventory log of the Product, by strength,
reflecting its on-hand inventory, at all times. Pharmacy shall provide Celgene inventory reporting for all Products pharmacy is actively enrolled in a REMS program, to dispense REVLIMID”, POMALYST®, and/or THALOMID. Inventory reports will be sent to Celgene within five (5) days after the Date of Count (the “Date of Count” means the last business day of the quarter). Electronic counts will be provided at the end of Q1 and Q3, physical inventory counts will be provided at the end of Q2 and Q4. The inventory report template is detailed in Schedule 2.3. Pharmacy shall be responsible for all costs associated with storage, handling and shipment of Product from the Pharmacy/Dispensing Site to the Customer.
2.4 Inspection of Product; Remedies and Procedures for Defects. Pharmacy shall carefully examine Product upon delivery and shall notify Celgene within ten (10) business days of any non-delivery of a portion of a shipment or any defect in any Product that is reasonably discoverable upon visual inspection of the Product, without unloading individual shipping units. Along with notice of any defect, Pharmacy shall furnish to Celgene a detailed description of the nature of the defect. Upon receipt of notice of any defect or non-delivery, Celgene, at its option, shall (a) replace any defective Product, (b) issue Pharmacy a credit in the amount of the purchase price paid for any defective Product, or (c) replace, or issue Pharmacy a credit in the amount of purchase price paid for, any undelivered Product. Except as set forth in Section 14, the preceding sentence sets forth Celgene’s sole liability with respect to non-delivery of a portion of a shipment and Product defects reasonably discoverable upon visual inspection of the Product without unloading individual shipping units. Section 7.1 sets forth Celgene’s sole liability with respect to other Product returns, and except as set forth in this Section 2.4, and Sections 7.1 and 14, Celgene shall not be otherwise liable to Pharmacy for any Product delivery failures or Product defects. In the absence of written notice from Pharmacy to Celgene in accordance with the terms of this Section 2.4, a shipment of Product shall be deemed to have been delivered and accepted by Pharmacy as complete and in satisfactory condition. Pharmacy shall, at Celgene’s request and expense, follow Celgene’s instructions to return to Celgene or Celgene’s third party disposal company any Product delivered to Pharmacy, which are not in compliance with the documentation provided by Celgene. Pharmacy shall cooperate with Celgene in investigating the cause of any defect in Product.
2.5 Title and Risk of Loss. Title to Product and risk of loss of Product shall transfer to Pharmacy upon delivery of Product to the Pharmacy or Dispensing Site.
2.6 Shortages. Notwithstanding anything in this Agreement to the contrary, in the event of a shortage of the Product, Celgene reserves the right to allocate available supplies of the Product in its sole discretion, however, Celgene shall not allocate available supplies in a manner which intentionally disadvantages Pharmacy.
3. PHARMACY ORDERS AND DELIVERY
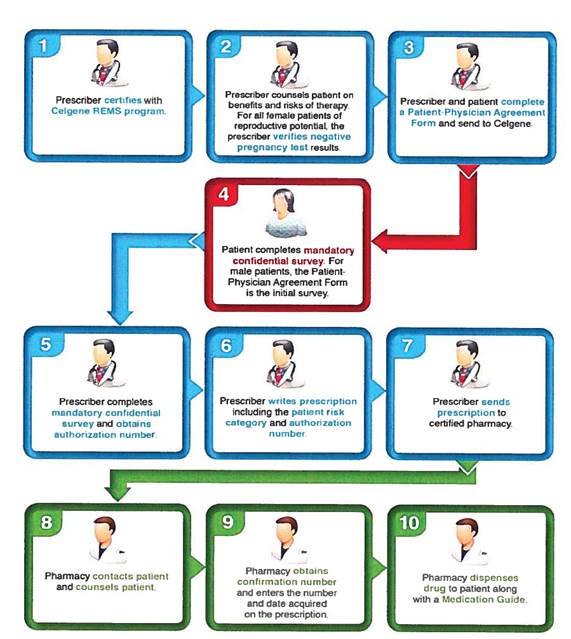
3.1 Acceptance of Prescriptions. Pharmacy shall only accept prescriptions with an authorization number generated by Celgene’s REMS system and patient risk category documented on the prescription. The authorization number on prescriptions for Females
of Reproductive Potential (FRP) are only valid for seven (7) days from the date of the last pregnancy test, the authorization number on prescriptions for all other Customers are valid for thirty (30) days, from the date the authorization number is obtained. Pharmacy shall not accept or otherwise fill any prescription for Product over the telephone or without a valid authorization number. Each unique authorization number on every prescription will require that a confirmation number must be obtained from Celgene Customer Care Center by way of: telephone; automated IVR system; or Celgene Web portal, prior to dispensing Product.
3.2 Counseling. Prior to obtaining a confirmation number related to each unique authorization number on each prescription, Pharmacy will contact Customers. Only Certified Counselors and/or Certified Pharmacists shall contact Customers and provide counseling, which contact shall be in accordance with the instructions set forth in this Section 3.1, and the Requirements Document. Pharmacy shall ensure there is a Certified Counselor available during normal business hours. Pharmacy shall educate Customers on the risks associated with the use of the Product, in accordance with the Requirements Document. Pharmacy must ensure that the Certified Counselors and/or Certified Pharmacists who will perform the counseling have been properly identified upon Pharmacy’s registration of the Pharmacy/Dispensing Site and upon staffing changes. Certified Counselors and/or Certified Pharmacists will be required to be recertified annually, or more frequently, as may be required by Celgene. Pharmacy shall inform Celgene of Certified Counselors and/or Certified Pharmacists who will no longer be counseling or dispensing for Celgene REMS products (example: employee no longer works there or has changed roles) when it occurs, and as directed on monthly or annual basis by Account Executive or Celgene Customer Care team. In the event that there are no Certified Counselors/Certified Pharmacists at a Pharmacy that have met their annual recertification obligation, the Pharmacy will be suspended from dispensing until such time as they have met their recertification obligations. Each pharmacy must have at least one Certified Counselor/Certified Pharmacist who is certified to dispense/counsel for REVLIMID®, POMALYST®, and/or THALOMID®. Pharmacy will not ship the Product to a Customer unless it has contacted Celgene to obtain a confirmation number and received a completed prescription from the Certified Prescriber (by facsimile or e-prescribed, or otherwise), which must indicate the authorization number and patient risk category, and has completed the additional Customer counseling, as outlined in the Requirements Document. Pharmacy shall fill valid prescriptions for Product in accordance with all applicable laws and regulations. For each subsequent Customer prescription, Pharmacy shall repeat counseling for such Customer upon receipt of additional Product prescriptions. Subsequent Customer prescriptions will only be allowed if fewer than seven (7) days of Product dosage remains on the previous prescription with respect to such Customer. In addition to those Customers that have not previously been prescribed Product, any Customer who has not received Product in the prior twelve (12) months will also be considered a “new Customer” and will have to re-enroll in POMALYST REMS®, REVLIMID REMS® and THALOMID REMS® program(s).
3.3 Filling Prescriptions. When filling prescriptions for Females of Reproductive Potential, Pharmacy/Dispensing Site shall ship Product to Customer on the same day of obtaining
the confirmation number by United Parcel Service or Federal Express, standard overnight delivery, or another similar carrier as agreed by the parties in writing, and in any case, such delivery shall require signature for delivery. For all other prescriptions Pharmacy/Dispensing Site shall ship Product to Customer within twenty four (24) hours of receipt of the confirmation number. Pharmacy/Dispensing Site shall package Products for shipment in accordance with its applicable SOP. Pharmacy/Dispensing Site shall use its best efforts to ship Product having the earliest expiration date first from available inventory. Each shipment of the Product shall be tracked and Pharmacy will maintain records of the disposition of all shipments, whether sent by Pharmacy or its Dispensing Site. Pharmacy/Dispensing Sites will require its shippers to receive written confirmation of delivery of Product, or to provide written notice of the non-delivery of a shipment of the Product within twenty four (24) hours of shipping. If the intended Customer does not receive a shipment of the Product, Pharmacy shall use reasonable efforts to track and retrieve the missing shipment, and shall inform Celgene of the missing shipment if it is not retrieved. In the event Customer will pick up the Product directly from Pharmacy/Dispensing Site, such pickup must occur within twenty four (24) hours of obtaining the confirmation number.
3.4 New Customers. In addition to Pharmacy meeting all of its obligations under this Agreement and POMALYST REMS®, REVLIMID REMS® and THALOMID REMS® program(s) for “new Customers” (for purposes of this Section 3.4 and for greater clarity, the term “new Customers” shall mean those customers that have not received Product in the prior twelve (12) months and/or those Customers that have not previously been prescribed Product), Pharmacy shall: (A) upon receipt of a prescription for Product immediately time-stamp such prescription, (B) fulfill obligations under the REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® Program requirements and dispense Product to such new Customers as quickly as possible and consistent with good clinical practice.
4. CORE SERVICES
4.1 Core Services. Pharmacy typically provides a wide array of services to patients and their caregivers for which Pharmacy does not receive any payment from manufacturers or vendors. Specifically, depending on the particular disease state, these core pharmacy activities include, but are not limited to, the following:
(a) Patient Intake: Initial patient processing, insurance eligibility and benefits verification, and scheduling of initial specialty medication order;
(b) Pharmacy Dispensing: Standard dispensing of specialty medications pursuant to a prescription in accordance with applicable law, the deposit of such specialty medications with a third party carrier (e.g., Federal Express) to facilitate the delivery of same as per the patient’s or prescribing physician’s instructions, and the provision of certain nominal ancillary supplies (e.g., syringes, needles, and alcohol swabs) and certain related items in connection with the specialty medication that may be necessary or useful to the patient in connection with the administration of the specialty medication; and
(c) Ongoing Clinical and Specialty Pharmacy Support: Standard patient education (no product marketing), patient assessment, and related clinical patient management activities and programs all of which are clinically objective; physician consultations; provision of information to prescribing physicians to facilitate patient coverage appeals; standard refill reminder follow-up calls; managing ongoing medication orders and shipment scheduling, and insurance follow-up and related ongoing delivery coordination.
4.2 No Payment for Core Services. Pharmacy represents and warrants that it intends to make the core pharmacy activities described in this Section 4 available to all Customers that are accepted by Pharmacy, and Pharmacy is not seeking any compensation from Celgene for these core pharmacy activities. Celgene shall not pay Pharmacy any fees for these core pharmacy activities.
5. ENHANCED SERVICES
5.1 Enhanced Services. Pharmacy agrees to provide, and Celgene agrees to purchase from Pharmacy, the enhanced pharmacy and data reporting services performed under the REVLIMID REMS®, POMALYST REMS®, and THALOMID REMSTM, as further detailed in Section 3 and the Requirements Document (Schedule 1.17); and the services described in Sections 5.2, 5.3, and 5.4 (collectively, the “Enhanced Services”).
5.2 Data. Pharmacy shall maintain a Celgene-specific data management system (the “Database”) from which reports can be generated and provided to Celgene and that contains information as required herein. In addition, and subject to Pharmacy obtaining appropriate Customer consent, Pharmacy shall maintain in the Database Customer-specific information as set forth herein. Pharmacy shall regularly update the Database with Product and Customer information set forth in Schedule 5.1, attached hereto. Celgene and Pharmacy may amend the information requested in Schedule 5.1, upon request by Celgene and mutual agreement of the parties. Celgene shall be the sole owner of the information compiled in the data reports, provided, however, that Pharmacy shall have full access to the Database in order to fulfill its obligations under this Agreement and to comply with all applicable laws and regulations. Celgene must authorize any release of the data to third parties, including but not limited to, sales data, or prescriptions filled.
5.3 Reports. Pharmacy shall generate and furnish data listed herein and in the format outlined in Schedule 5.1, for Vidaza®, Abraxane® and Istodax®. Pharmacy shall continue to provide this report on a monthly, basis, and periodic reporting as Celgene may reasonably request. Reports will include, but not be limited to the following information:
(a) Pharmacy site address
(b) Pharmacy site city
(c) Pharmacy site state
(d) Pharmacy site zip
(e) Date Dispensed
(f) Customer ID (specific to this customer)
(g) Prescriber First Name
(h) Prescriber Last Name
(i) Prescriber Address ID (if available)
(j) Prescriber Address
(k) Prescriber City
(l) (1) Prescriber State
(m) Prescriber Zip
(n) DEA (prescriber)
(o) NPI (prescriber)
(p) NDC
(q) Product
(r) QTY in vials
Report should be generated and received by Celgene no later than ten (10) business days following the close of each month. If no products were dispensed, report should reflect this. Report should be sent to xxxxxxxxxxxxxxx@xxxxxxx.xxx
Data Reports may be further defined or amended by Schedule 5.1. At Celgene’s request, Pharmacy will deliver the reports specified under this Section electronically through a secure connection in the format identified in Schedule 5.1.
5.4 Materials. Pharmacy shall maintain an inventory of current educational materials developed and provided by Celgene. Pharmacy shall include in shipments of Product any required material supplied and designated by Celgene for inclusion in Product shipments.
6. PAYMENT
6.1 Service Fee. Celgene shall pay Pharmacy a service fee for the Enhanced Services, based on the achievement of performance metrics set forth in Schedule 6.1 (“Service Fee”). The performance metrics are intended to encourage prompt compliance with the REMS and timely patient access to the Products. Celgene shall have the right to withhold or deny payment of the Service Fee in the event Pharmacy does not provide the Enhanced Services, or is in any way in breach of any of its obligations under this Agreement.
6.2 Service Fee Calculation. The Service Fee will be calculated and paid quarterly based on Celgene’s evaluation of Pharmacy’s achievement of performance metrics, as specified above.
6.3 Payment Due for Product Orders; Late Fee.
(a) All amounts due hereunder to Celgene for Product orders shall be payable by check or EFT to Celgene. Celgene shall invoice Pharmacy for all amounts due to Celgene hereunder, as adjusted to take into account credits due to Pharmacy, and Pharmacy shall pay all invoiced amounts when due. Any amounts remaining unpaid for more than thirty-five (35) days after date of invoice shall be subject to interest thereon equal to one and one half percent (1.5%) per month.
(b) Without limiting the generality of Section 6.3(a) and subject to Pharmacy being at all times in full compliance with all of the terms and conditions of this Agreement, Pharmacy shall be entitled to a two percent (2.0%) prompt payment cash discount off the invoice price if Celgene receives payment within thirty (30) days of the invoice date, net thirty one (31) (the “Prompt Pay Discount”).
(c) The parties agree that they have structured Prompt Pay Discount in a manner consistent with the statutory discount exception (42 U.S.C. § 1320a-7b(b)(3)(A)) and the discount safe harbor (42 C.F.R. § 1001.952(h)). The terms pursuant to which the Prompt Pay Discount is paid are fixed and set forth in this Agreement. This discount is not dependent on, and does not operate in conjunction with, (either explicitly or implicitly) any other arrangement or agreement between Celgene and Pharmacy. To the extent required under applicable law, Pharmacy will report the discounts to appropriate Federal health care programs, and will, upon the request of a governmental agency, (including the Secretary of Health and Human Services or a state healthcare agency), disclose information regarding the discounts to the requesting agency. Without limitation of the foregoing, all Prompt Pay Discounts and any other information that must be disclosed under applicable law, shall be disclosed to the Centers for Medicare and Medicaid Services (“CMS”) in accordance with: (1) CMS guidance (as it may be revised from time to time); (2) any contractual obligations with third parties; and (3) any other disclosure or reporting obligations or requirements imposed by federal or state laws, regulations, or guidance. Confidential treatment shall be requested for any disclosures made to CMS and Medicare Part D Plans to the extent permitted by law.
6.4 Costs and Expenses. Except as otherwise expressly set forth herein, Pharmacy shall be responsible for all costs and expenses associated with fulfilling its obligations under this Agreement.
6.5 Taxes. All prices are exclusive of federal, state and local excise, sales, use and other taxes levied or imposed on the sale, shipment, delivery, ownership, possession or resale of Product or any other activities contemplated under this Agreement. Except for taxes on Celgene’s income, Pharmacy shall be liable for and pay all taxes imposed in connection with the activities contemplated hereunder.
6.6 Financial Condition. At any time, when in Celgene’s reasonable opinion, the financial condition of Pharmacy or its parent company so warrants, or if Pharmacy consistently fails to make payments when due or otherwise defaults under this Agreement, Celgene may alter terms of payment (including but not limited to requiring full or partial payment in advance of delivery, eliminating the Prompt Pay Discount), suspend credit, delay or cancel shipping, request quarterly financial statements or other financial information on an ongoing basis, or pursue any remedies available at law or under this Agreement.
7. RETURN GOODS POLICY
7.1 Return Goods Policy. The current form of Celgene’s return goods policy is attached as Schedule 7.1 (the “Return Goods Policy”). Celgene may, if it deems necessary or desirable, update the Return Goods Policy from time to time. In the event Celgene updates the Return Goods Policy, Celgene shall provide Pharmacy with advance written notice of any changes to its Return Goods Policy.
7.2 Returns by Pharmacy. In the event Pharmacy returns or requests to return a Product, for any reason, Pharmacy shall promptly notify Celgene, and Celgene shall, upon receipt of Product, give Pharmacy a credit amount in accordance with the current Celgene Return Goods Policy, provided that the reason for the return of the Product does not arise from (i) the negligence or intentional misconduct of Pharmacy or any of its agents or employees, (ii) failure of Pharmacy to follow applicable SOPs or to otherwise comply with the terms of this Agreement or (iii) mis-delivery or loss of Product by a carrier used by Pharmacy. For any return of Product authorized by Celgene, Pharmacy shall send the Product, or shall instruct Customer to send the Product, to Celgene or Celgene’s designated disposal company as specified and in the manner described in the then current Return Goods Policy.
8. ADVERSE DRUG EXPERIENCE AND CUSTOMER COMPLAINTS
8.1 Adverse Drug Experience. Consistent with its internal SOPs, Pharmacy will promptly inform Celgene’s Drug Safety Group of any information regarding adverse drug experiences suspected to be associated with the use of Product and all cases of special situations it receives, with the appropriate information, as set forth in more detail on Schedule 8.1, to the address set forth therein.
8.2 Other Customer Complaints. Pharmacy shall give notice by email or phone to Celgene’s Customer Service Department at xxxxxxxxxxxxxxx@xxxxxxx.xxx or 000-000-0000 within ten (10) business days of receipt of any Customer complaint related to Product and/or services, other than Adverse Drug Events, and any labeling and package insert issues, specifying in detail the nature of the complaint or issue. Pharmacy shall provide Customer Complaints in a form substantially similar to the form attached as Schedule 8.2.
8.3 Cooperation. Pharmacy shall cooperate with Celgene in responding to or investigating any Customer complaints and Adverse Drug Events.
9. SUSPENSION OF DISTRIBUTION AND RECALLS
9.1 Suspension of Distribution. Pharmacy shall suspend distribution of Product if: (a) requested by Celgene as the result of a problem with Product quality or a directive from the FDA; (b) Celgene has determined, in Celgene’s sole and absolute discretion, Pharmacy to have deviated from, or otherwise be in non-compliance with, the requirements of REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® program(s); or (c) Pharmacy has engaged in High Risk Deviations. If multiple instances of deviations or noncompliance are uncovered, Celgene reserves the right to immediately and permanently suspend Pharmacy from dispensing any Product under a risk management program. Without otherwise limiting or waiving any other right or remedy available to Celgene under law or equity, certain specific consequences of Pharmacy deviations or non-compliance are as set forth in detail in the Requirements Documents. Any deviation from, or noncompliance with, REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® program(s), as determined by Celgene in Celgene’s sole and absolute discretion, shall be considered a breach of the terms and conditions herein. Any occurrence of a High Risk Deviation shall be considered a material breach of the terms of this Agreement.
9.2 Recalls. Celgene shall promptly notify Pharmacy of any recalls initiated by Celgene or requested by the FDA. Upon receipt of notice of a recall from Celgene, Pharmacy shall immediately notify the affected Customers. Celgene shall provide Pharmacy with the form of letter to be used in connection with notice of any recall which shall contain the appropriate instructions as to whether Customers should return or dispose of the affected Product. Celgene shall be responsible for the mailing, shipping and reasonable administrative expenses incurred by Pharmacy in connection with the recall as well as the cost of replacement Product for Customers, provided that the reason for the recall does not arise from (i) the negligence or intentional misconduct of Pharmacy or any of its agents or employees or (ii) failure of Pharmacy to follow applicable SOPs or to otherwise comply with the terms of this Agreement. Pharmacy shall cooperate in any recalls by providing relevant Product tracking information to Celgene.
9.3 Records. Pharmacy shall maintain during the Term and for three (3) years thereafter such information as may be reasonably required by Celgene to effect a Product recall, and shall make such information available to Celgene, at Celgene’s request, in the event of such a recall.
9.4 Cooperation. Pharmacy shall cooperate with Celgene in investigating any Product failure which resulted in the need for a recall.
10. REPRESENTATIONS, WARRANTIES AND COVENANTS OF PHARMACY
10.1 Compliance. In performing its obligations under this Agreement, Pharmacy represents and warrants that it shall comply with all applicable federal, state and local laws, regulations and ordinances, including without limitation (a) the Social Security Act; (b) the Health Insurance Portability and Accountability Act, (c) federal and state health care anti-fraud and abuse laws; (d) state drug product selection, dispensing, pharmacy
practice, biohazard disposal, privacy, and consumer protection laws; and (e) rules and regulations of the FDA, the Center for Medicare and Medicaid Services and any other Regulatory Authority by which Pharmacy may be governed. Pharmacy also shall comply with all applicable professional and industry standards and good business practices.
10.2 Services.
(a) Pharmacy represents and warrants: (1) Celgene has engaged Pharmacy to perform bona fide, legitimate, reasonable, and necessary Enhanced Services; (2) the Enhanced Services are not intended to serve, either directly or indirectly, as a means of marketing the Product; (3) the Enhanced Services are not intended to diminish the objectivity or professional judgment of Pharmacy or to interfere with the objectivity or professional discretion of any prescriber; (4) the Enhanced Services do not involve the counseling or promotion of any off-label use of the Products or a business arrangement or other activity that violates any state or federal law; (5) the Service Fees do not constitute a discount off the purchase price of the Product; (6) the Service Fees are not intended in any way as remuneration for referrals or for other business generated; (7) the Service Fees represent fair market value for the compensated services based on arms-length negotiations; and (8) the Service Fees are not intended in any way as a payment related to a drug formulary or drug formulary activities and have not been negotiated or discussed between the parties in connection with any such drug formulary or formulary activities.
(b) Pharmacy further represents and warrants: (1) no Enhanced Services shall be provided with respect to any Customer unless Pharmacy has received a prescription from the Customer’s prescribing physician; (2) Enhanced Services for which any Service Fees are to be paid hereunder are in addition to the services that Pharmacy typically performs for patients; (3) Pharmacy does not perform the Enhanced Services on behalf of any pharmaceutical manufacturer or other third party without being paid fair market value service fees for each such service; (4) the clinical judgment of the Customer’s treating physician (or other healthcare provider) shall not be undermined or otherwise usurped in the performance of the Enhanced Services; (5) Pharmacy shall not offer physicians or any other healthcare professionals any financial inducement to prescribe or switch patients to any Product; and (6) Pharmacy will not xxxx, and is not obligated by law or contract with, any third party for the performance of the Enhanced Services to be performed under this Agreement.
10.3 Federal Programs. Pharmacy represents and warrants that neither it nor any director, officer, employee, independent contractor, agent or other representative of Pharmacy (referred to collectively in this section as “Representative”) has been debarred pursuant to the Federal Food, Drug and Cosmetic Act or is currently excluded, debarred, suspended, or otherwise ineligible to participate in any Federal health care program (as defined in Section 1128B(f) of the Social Security Act (“Government Program”) or in Federal procurement or non-procurement programs. Pharmacy shall notify Celgene immediately if Pharmacy or any of its Representatives who are concerned with the performance of this
Agreement becomes debarred, excluded, suspended or ineligible, or is convicted of a criminal offense that falls within the ambit of the Federal statute providing for mandatory exclusion from participation in any Government Program, but has not yet been excluded, debarred, suspended, or otherwise declared ineligible to participate in those programs.
10.4 Quality of Employees and Monitoring. Pharmacy represents and warrants that it shall use a well-trained, knowledgeable team of employees to handle Product and to perform the services to be performed by Pharmacy under this Agreement. Pharmacy employees who will be providing the services will be required to be certified by Celgene in REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® program(s) and any other training required by Celgene. Pharmacy’s ability to maintain its certification, and its right to dispense Product is contingent upon completion of any such training required by Celgene, including compliance with recertification. Subject to applicable privacy laws, Celgene shall have the right, from time to time, to have a Celgene employee monitor Pharmacy’s responses during telephone calls transferred from Celgene’s customer support line, and Pharmacy shall cooperate with Celgene to enable such monitoring activities. Customer shall be notified at the beginning of a call to be monitored that monitoring for quality assurance purposes is to occur.
10.5 Medicaid Provider Status. Pharmacy represents and warrants that it is currently eligible to participate as a provider in the Medicaid program in each state in the Territory except those states listed on Schedule 10.3 (for avoidance of doubt, the list of those states to be provided by Pharmacy) attached hereto, and agrees to maintain such eligibility during the Term. Upon notice to Celgene, Pharmacy may amend Schedule 10.3 in its sole discretion to add additional states and shall provide Celgene with prompt notice of any such amendment, provided that Pharmacy shall not add any state to Schedule 10.3 unless the state has changed its laws to require an in-state pharmacy presence for eligibility in its Medicaid program. Pharmacy shall remove a state from Schedule 10.3 (and shall provide notice to Celgene of such removal) when the state no longer requires an in-state pharmacy presence for eligibility in the state’s Medicaid program.
10.6 Actions. Pharmacy shall not take any action which would materially adversely affect its standing or that of Celgene in the industry or with respect to Product customer base or which would undermine the image of Product.
10.7 Quality Reviews. Pharmacy shall periodically, but not less frequently than once per year, perform written quality reviews of Pharmacy’s performance in fulfilling its obligations under this Agreement, and shall provide Celgene with copies of such reviews. Pharmacy shall administer a validation checklist to each employee performing services related to Product upon completion of such employee’s initial training and annually thereafter, and shall provide Celgene with copies of such checklists. Pharmacy shall fully cooperate with Celgene in fulfilling all of its obligations under this Agreement, including, but not limited to, quality reviews.
10.8 Licenses. Pharmacy represents and warrants that it now has and shall maintain in full force during the Term all federal and state pharmacy, wholesaler and other licenses or approvals required by Pharmacy to fulfill its obligations under this Agreement.
Pharmacy shall provide Celgene with notice of any communications with pharmacy licensing boards or the FDA which relate to potential problems with facilities, operations or procedures used by Pharmacy in its distribution of Product that may affect Pharmacy’s performance of its obligations under this Agreement, including notices of inquiries, investigations or inspections and resulting findings.
10.9 Limitation on Promotion. Pharmacy shall not make any performance claims or engage in any promotional activities with respect to Product except for the distribution of required Product literature prepared by Celgene and any other activities directly related to the services to be provided by Pharmacy hereunder, in each case as expressly approved in writing by Celgene.
10.10 Use of Trademarks. Pharmacy shall not use the trademarks, trade names, trade dress or logos of Celgene except to the extent contained in Product literature provided by Celgene, on Product labels or as otherwise approved by Celgene in writing.
10.11 Authority. Pharmacy represents and warrants that (a) it has all requisite corporate power and authority to execute, deliver and perform this Agreement and any other agreements contemplated hereby and (b) it is not currently obligated nor will it assume any future obligation under any contract (including without limitation any commitment of any nature) or other agreement, instrument or arrangement that conflicts with its obligations under this Agreement.
10.12 Limitation of Liability. Except for its obligations of indemnification, or breach of its obligations of confidentiality, in no event shall Pharmacy be liable for any consequential, exemplary, punitive, incidental, indirect or special damages or costs, including without limitation, lost profits, of Celgene, whether or not Pharmacy has been advised of the possibility of such damages or costs.
10.13 Celgene Information. Pharmacy shall use information about Customers to whom Product has been dispensed, including any list of such Customers, and other Celgene Information (as defined below) solely to perform its obligations under this Agreement and to dispense Product to such Customers. Pharmacy shall not make such information (or any portion thereof) available to, or use such information for the benefit of, any third party other than an insurance provider and/or other third party payor (with respect to its covered persons). Notwithstanding the foregoing, Pharmacy may disclose information on or about Customers to whom Product has been dispensed, including any list of such Customers, as authorized under the Health Insurance Portability and Accountability Act and to Regulatory Authority(ies) and Pharmacy’s auditors, legal counsel and lenders, provided that each such party to whom such information is disclosed is obligated to preserve the confidentiality of such information.
11. REPRESENTATIONS, WARRANTIES AND COVENANTS OF CELGENE
11.1 Compliance with Law. Celgene shall be responsible for testing Product and ensuring that Product complies, when shipped to Pharmacy, with all applicable laws, regulations, directives and requirements of the FDA, including without limitation, packaging and
labeling requirements, product warning requirements, product design and safety requirements and advertising requirements.
11.2 Services. Celgene represents and warrants: (1) Celgene has engaged Pharmacy to perform bona fide, legitimate, reasonable, and necessary Enhanced Services; (2) the Enhanced Services are not intended to serve, either directly or indirectly, as a means of marketing the Product; (3) the Enhanced Services are not intended to diminish the objectivity or professional judgment of Pharmacy or to interfere with the objectivity or professional discretion of any prescriber; (4) the Enhanced Services do not involve the counseling or promotion of any off-label use of the Products or a business arrangement or other activity that violates any state or federal law; (5) the Service Fees do not constitute a discount off the purchase price of the Product; (6) the Service Fees are not intended in any way as remuneration for referrals or for other business generated; (7) the Service Fees represent fair market value for the Enhanced Services based on arms-length negotiations; and (8) the Service Fees are not intended in any way as a payment related to a drug formulary or drug formulary activities and have not been negotiated or discussed between the parties in connection with any such drug formulary or formulary activities.
11.3 Federal Programs. Celgene represents and warrants that neither it nor any director, officer, employee, independent contractor, agent or other representative of Celgene (referred to collectively in this section as “Representative”) has been debarred pursuant to the Federal Food, Drug and Cosmetic Act or is currently excluded, debarred, suspended, or otherwise ineligible to participate in any Government Program or in Federal procurement or non-procurement programs. Celgene shall notify Pharmacy immediately if Celgene or any of its Representatives who are concerned with the performance of this Agreement becomes debarred, excluded, suspended or ineligible, or is convicted of a criminal offense that falls within the ambit of the Federal statute providing for mandatory exclusion from participation in any Government Program, but has not yet been excluded, debarred, suspended, or otherwise declared ineligible to participate in those programs.
11.4 Use of Trademarks. Celgene shall not use the trademarks, trade names, trade dress or logos of Pharmacy except to the extent necessary for activities contemplated under this Agreement.
11.5 Warranty. Celgene represents and warrants that, upon the date of shipment to Pharmacy, Product will not be adulterated or misbranded within the meaning of the Federal Food, Drug and Cosmetic Act (the “Act”) and will not be articles which may not, under the provisions of the Act, be introduced into interstate commerce. THE EXPRESS WARRANTIES CONTAINED IN THIS SECTION 11 ARE IN LIEU OF ALL OTHER REPRESENTATIONS AND WARRANTIES WITH RESPECT TO PRODUCT. CELGENE DISCLAIMS ALL OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION ALL WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. Except as otherwise set forth in Section 14, Celgene’s sole liability and Pharmacy’s sole remedy for breach of warranty under this Agreement shall be for Celgene to replace the defective Product or to credit Pharmacy’s account in accordance with Section 2.4 and Section 7.1. Except for its obligations of indemnification, or breach of its obligations of
confidentiality, in no event shall Celgene be liable for any consequential, exemplary, punitive, incidental, indirect or special damages or costs, including without limitation, lost profits, of Pharmacy, whether or not Celgene has been advised of the possibility of such damages or costs.
11.6 License. Celgene represents and warrants to Pharmacy that Celgene has a valid and existing license from the FDA to market and sell Product in the Territory.
11.7 Authority. Celgene represents and warrants that (i) it has all requisite corporate power and authority to execute, deliver and perform this Agreement and any other agreements contemplated hereby and (ii) it is not currently obligated nor will it assume any future obligation under any contract (including without limitation any commitment of any nature) or other agreement, instrument or arrangement that conflicts with its obligations under this Agreement.
11.8 No Adverse Actions. Celgene shall not take any action which would adversely affect its standing or that of Pharmacy in the pharmaceutical and/or health care industry; provided that nothing in this Section 11.8 shall prevent Celgene from entering into agreements with other specialty distributors of Product within the Territory, terminating this Agreement pursuant to Section 12.2 or 12.3 or enforcing its rights under this Agreement.
12. TERM AND TERMINATION
12.1 Term. This Agreement shall become effective on the Effective Date and, unless earlier terminated in accordance with this Section 12, shall continue in effect for an initial term ending on June 30, 2019.
12.2 Voluntary Termination. Either party may terminate this Agreement for convenience at any time upon at least ninety (90) days’ prior written notice to the other party.
12.3 Termination for Breach. Either party may terminate this Agreement (i) for a material breach by the other party upon fifteen (15) days’ prior written notice unless the breaching party cures the breach within such fifteen (15) day period or (ii) in the event of any proceedings, voluntary or involuntary, in bankruptcy or insolvency, by or against the other party, or the appointment, with or without such other party’s consent, of a receiver for such other party.
12.4 Transition. Upon the expiration or earlier termination of this Agreement, as applicable, the parties shall begin to transition distribution of Product for Pharmacy’s Customers to a party to be designated by Celgene. Transition of distribution under this Section 12.4 shall mean the following:
(a) Celgene shall as soon as practicable begin referring Pharmacy Customers who contact Celgene’s Customer Service Department to a new designated distributor.
(b) At Celgene’s request, Pharmacy shall provide notice to all of Pharmacy Customers of the change in distributors.
(c) Pharmacy shall complete any Product shipments then underway, but otherwise shall refer Customers to the Celgene-designated distributor.
(d) Pharmacy shall transfer a copy of the Database and Customer information, including prescription files, to the Celgene-designated distributor, provided that if applicable patient confidentiality laws prohibit transfer of Customers’ names to such distributor, Pharmacy shall transfer the Database and Customer information using Customer numbers instead of names, and shall notify Customers of their respective numbers.
(e) Pharmacy’s obligation to order additional Product when its inventory falls to a one (1) week supply shall cease and Celgene shall repurchase any Product held in inventory by Pharmacy on the date of termination at the price paid for the Product by Pharmacy.
After (a) expiration of this Agreement or (b) upon the sending or receiving of any termination notice by Pharmacy in accordance with the terms hereof and (c) for a period of six (6) months after the event described in (a) or (b), as applicable, Pharmacy shall use commercially reasonable efforts to cooperate with Celgene in ensuring the smooth transition of the services provided by Pharmacy under this Agreement to any other distributor(s) designated by Celgene, provided that after the expiration or the effective date of any termination of this Agreement, as applicable, Celgene shall reimburse Pharmacy for its reasonable out-of-pocket, non-personnel-related expenses associated with such cooperation.
12.5 Survival. Sections 6 , 7, 8, 9, 10.3, 10.8, 10.9, 10.10, 10.12, 10.13, 11.1, 11.4, 11.5, 11.8, 12.4, 12.5, 13, 14, 15 and 17 shall survive termination or expiration of this Agreement for as long as necessary to permit their full discharge.
13. REGULATORY INSPECTIONS, INVESTIGATIONS, AUDITS
13.1 Regulatory Inspections. Pharmacy shall provide to the applicable Regulatory Authority or, at Celgene’s request, shall provide to Celgene all available documents and information requested by any Regulatory Authority or by Celgene in support of its regulatory filings. Copies of all documents to be provided to the applicable Regulatory Authority shall be provided to Celgene in advance, if practicable, or otherwise within two (2) business days of delivery to Regulatory Authority. Pharmacy shall notify Celgene immediately upon receipt of notice of any inspection by any Regulatory Authority directed specifically toward Product, and Celgene shall have the right to have an employee(s) and/or agent(s) present at any such inspection, if allowed by law. Pharmacy shall notify Celgene immediately of any notices, requests for information or other communications related to Product from the U.S. Department of Health and Human Services or any other Regulatory Authority or any state healthcare program or other state agency and, to the extent permitted under applicable law, shall promptly give Celgene copies of such communications.
13.2 Recalls, Returns or Investigations. Pharmacy shall provide Celgene, at Celgene’s request, any information reasonably required in connection with Celgene investigations relating to recalled or returned Product or any requests or investigations by or filings with any applicable Regulatory Authority, including without limitation the FDA, or in support of Celgene’s applications to the FDA. Pharmacy shall respond within two (2) business days to any reasonable request for information by Celgene.
13.3 Celgene Right to Audit. Throughout the Term and for a period of three (3) years following expiration or earlier termination of this Agreement, Pharmacy shall maintain complete and accurate records consistent with requirements of the REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS ® program(s) and sales of Product. First audits will occur within 3 months of a Pharmacy’s first dispense of REVLIMID®, POMALYST®, and THALOMID®, then an annual risk-based audit thereafter.
13.4 Inspection. Applicable records shall be made available for inspection at Pharmacy for three (3) years from the date of the initial Product receipt. Celgene and its representatives will not be required to sign additional non-disclosure or confidentiality agreements. Celgene and its representatives shall have the right to periodically visit Pharmacy and its Dispensing Sites during regular business hours, during the Term and for a period of three (3) years thereafter, upon reasonable prior notice to Pharmacy/Dispensing Site. During any such visit, Celgene and its representatives will have the right to (i) examine the books, ledgers and records, including inventory levels, of Pharmacy related to Celgene Product sales, (ii) audit quality control/assurance procedures, registrations, shipping records, supplements and regulatory correspondence to ensure that Pharmacy/Dispensing Site is in compliance with the SOPs and with applicable regulations, the Requirements Document or other procedures required by Celgene. The audit shall also include Celgene’s ability to interview Pharmacy’s Certified Counselors/Certified Pharmacists, monitor the Enhanced Services being provided, as well as access Pharmacy’s computer systems used to provide Enhanced Services for Celgene pursuant to this Agreement; provided that such access to Pharmacy’s computer systems used to provide Enhanced Service shall be limited in a manner to prevent the auditor from accessing Protected Health Information (as defined under the Health Insurance Portability and Accountability Act). Pharmacy shall take a course of action and resolution acceptable to Celgene in the event that Celgene finds any contractual or regulatory deficiencies during such audits. Each party shall bear its own costs of such examinations.
14. INDEMNIFICATION
14.1 Celgene Indemnification of Pharmacy. Celgene shall at all times during the Term and thereafter defend, indemnify and hold Pharmacy and its officers, directors, agents and employees harmless from and against any and all claims, suits, damages, liabilities, costs and expenses, including but not limited to court costs and reasonable attorneys’ fees (collectively, “Claims”), incurred in connection with any third-party claim arising out of the use of any Product by a Customer, except to the extent caused by (i) the negligence or intentional misconduct of Pharmacy or any of its officers, directors, agents or employees or (ii) breach by Pharmacy of any of the terms of this Agreement or (iii) acts of Pharmacy
or any of its officers, directors, agents or employees which are outside the scope of this Agreement.
14.2 Pharmacy Indemnification of Celgene. Pharmacy shall at all times during the Term and thereafter defend, indemnify and hold Celgene and its officers, directors, agents and employees harmless from and against any and all Claims incurred in connection with any third-party claim arising out of (i) the negligence or intentional misconduct of Pharmacy or any of its officers, directors, agents or employees, (ii) breach by Pharmacy of any of the terms of this Agreement, or (iii) acts of Pharmacy or any of its officers, directors, agents or employees which are outside the scope of this Agreement.
14.3 Procedures. A party that intends to seek indemnification under this Section 14 (the “indemnitee”) shall notify the other party (the “indemnitor”) promptly in writing of any Claim in respect of which the indemnitee believes it is entitled to claim indemnification, provided that the failure to give timely notice to the indemnitor shall not release the indemnitor from any liability to the indemnitee, except to the extent the indemnitor is prejudiced thereby. The indemnitor shall have the right, by notice to the indemnitee, to assume the defense of any such Claim within ten (10) days after the indemnitor’s receipt of notice of any Claim with counsel of the indemnitor’s choice and at the sole cost of the indemnitor. If the indemnitor so assumes such defense, the indemnitee may participate therein through counsel of its choice, but at the sole cost of the indemnitee; provided, however, that the indemnitor shall be obligated to pay fees and expenses of such indemnitee’s counsel if representation of the indemnitee by the counsel retained by the indemnitor would be inappropriate due to actual or potential conflicting interests between the indemnitee and any other party represented by such counsel in the investigation and defense of any such Claim. The party not assuming the defense of any such Claim shall render all reasonable assistance to the party assuming such defense, and all reasonable out-of-pocket costs of such assistance shall be for the account of the indemnitor. No such Claim shall be settled other than by the party defending the same, and then only with the consent of the other party which shall not be unreasonably withheld; provided that the indemnitee shall have no obligation to consent to any settlement of any such Claim which imposes on the indemnitee any liability or obligation which cannot be assumed and performed in full by the indemnitor, and the indemnitee shall have no right to withhold its consent to any settlement of any such Claim if the settlement involves only the payment of money by the indemnitor or its insurer.
15. CONFIDENTIALITY
15.1 Pharmacy Obligation. Pharmacy agrees to treat any confidential or proprietary information obtained from Celgene and any confidential or proprietary information generated by Pharmacy in performing its obligations under this Agreement, including Customer lists, information regarding Celgene’s pricing policies, information regarding reimbursement for the Product, information regarding the cost of providing services to Celgene and the information in the Database, and anything derived therefrom, (collectively, the “Celgene Information”) as the confidential and exclusive property of Celgene, and agrees not to disclose any of the Celgene Information to any third party without first obtaining the written consent of Celgene. Pharmacy agrees that it will use the Celgene Information
only for purposes of performing its obligations hereunder and for no other purpose without the prior written consent of Celgene. Pharmacy further agrees to take all practicable steps necessary to ensure that the Celgene Information (i) will be maintained in strict confidence, (ii) will not be disclosed to any third party except as expressly permitted herein, and (iii) will not be disclosed to or used by its directors, officers, employees or agents except pursuant to a written agreement with terms no less strict than those terms of confidentiality as aforesaid, set forth in this Section 15 and will be kept confidential by them.
The above provisions of confidentiality shall not apply to that part of the Celgene Information which Pharmacy is able to demonstrate by competent documentary evidence:
(a) was in Pharmacy’s possession prior to receipt from Celgene and prior to being generated under this Agreement;
(b) was in the public domain at the time of receipt from Celgene;
(c) became part of the public domain through no fault of Pharmacy, its directors, officers, employees or agents;
(d) was lawfully received by Pharmacy from a third party not disclosing the
information on behalf of Celgene and having a right of further disclosure; or
(e) is required to be disclosed by law or regulation applicable to Pharmacy, provided that Pharmacy gives prompt notice to Celgene that it is required to make such disclosure so that Celgene can take steps to limit the scope of the Celgene Information disclosed or otherwise to protect the Celgene Information, and provided further that Pharmacy limits such disclosure of the Celgene Information to the maximum extent practicable.
Specific aspects or details of the Celgene Information shall not be deemed to be within the public domain or in the possession of Pharmacy merely because the Celgene Information is embraced by general disclosures in the public domain or in the possession of Pharmacy. In addition, any combination of Celgene Information shall not be considered in the public domain or in the possession of Pharmacy merely because individual elements thereof are in the public domain or in the possession of Pharmacy unless the combination and its principles are in the public domain or in the possession of Pharmacy.
Pharmacy agrees that, at Celgene’s request, it shall return to Celgene all parts of the Celgene Information existing in documentary form, not including Pharmacy records, and will, at Celgene’s request, return or destroy any copies thereof made by Pharmacy, its directors, officers, employees or agents, except that Pharmacy shall retain a copy of the Database subject to the ongoing obligations of confidentiality set forth in this Section 15. Pharmacy shall not dispose of the information in the Database without first offering in writing, given at least five (5) business days prior to such disposal, to deliver such information to Celgene, at Celgene’s expense, without additional consideration.
15.2 Celgene Obligation. Celgene agrees to treat any confidential or proprietary information obtained from Pharmacy, (not including the Database and information about insurers’ reimbursement policies with respect to Product) and anything derived therefrom, (collectively, the “Pharmacy Information”) as the confidential and exclusive property of Pharmacy, and Celgene agrees not to disclose any of the Pharmacy Information to any third party without first obtaining the written consent of Pharmacy, provided that Celgene may disclose Pharmacy Information to any third party providing reimbursement-related services to Celgene as long as the third party is obligated to Pharmacy to keep such information confidential. Celgene agrees that it will use any Pharmacy Information only for purposes of activities contemplated hereunder and for no other purpose without the prior written consent of Pharmacy. Celgene further agrees to take all practicable steps necessary to ensure that the Pharmacy Information (i) will be maintained in strict confidence, (ii) will not be disclosed to any third party except as expressly permitted herein, and (iii) will not be disclosed to or used by its directors, officers, employees or agents except pursuant to a written agreement with terms no less strict than those set forth in this Section 15.
The above provisions of confidentiality shall not apply to that part of the Pharmacy Information which Celgene is able to demonstrate by competent documentary evidence:
(a) was in Celgene’s possession prior to receipt from Pharmacy;
(b) was in the public domain at the time of receipt from Pharmacy;
(c) became part of the public domain through no fault of Celgene, its directors, officers, employees or agents;
(d) was lawfully received by Celgene from a third party not disclosing the information on behalf of Pharmacy and having a right of further disclosure; or
(e) is required to be disclosed by law or regulation applicable to Celgene provided that Celgene gives prompt notice to Pharmacy that it is required to make such disclosure so that Pharmacy can take steps to limit the scope of the Pharmacy Information disclosed or otherwise to protect the Pharmacy Information, and provided further that Celgene limits such disclosure of the Pharmacy Information to the maximum extent practicable.
Specific aspects or details of the Pharmacy Information shall not be deemed to be within the public domain or in the possession of Celgene merely because the Pharmacy Information is embraced by general disclosures in the public domain or in the possession of Celgene. In addition, any combination of Pharmacy Information shall not be considered in the public domain or in the possession of Celgene merely because individual elements thereof are in the public domain or in the possession of Celgene unless the combination and its principles are in the public domain or in the possession of Celgene.
Celgene agrees that, at Pharmacy’s request, it shall return to Pharmacy all parts of the Pharmacy Information existing in documentary form and will, at Pharmacy’s request,
return or destroy any copies thereof made by Celgene, its directors, officers or employees.
15.3 No Implied Licenses. Nothing contained herein shall be deemed to grant to either party any rights or licenses under any patent applications or patents or to any know-how, technology, inventions or other intellectual property rights of the other party.
15.4 Publicity. Each party shall be permitted to make such public statements regarding its relationship with the other party as may be required by law or regulation or by obligations pursuant to any listing agreement with any securities exchange. Pharmacy shall not disclose the terms of this Agreement to any third party or, except as expressly set forth in this Section 15, make any public announcement of the existence of its relationship with Celgene without the prior written consent of Celgene except to its auditors and lawyers or as required by law.
15.5 Length of Obligation. The obligations of the parties under this Section 15 shall continue during the Term and for a period ending five (5) years after the expiration or earlier termination thereof.
16. INSURANCE
Pharmacy agrees (i) to obtain and maintain, while this Agreement is in effect, (a) commercial general liability insurance, including product liability insurance, and Pharmacists professional liability insurance, each with coverage limits of not less than $1,000,000 per occurrence and $3,000,000 in the aggregate, and (ii) not to cancel the insurance or reduce the coverage without giving at least thirty (30) days’ prior written notice to Celgene. Pharmacy shall cause Celgene to be provided notice on each insurance policy such that Celgene shall receive notice of any cancellation or change in the policy. At the request of Celgene, Pharmacy shall provide Celgene with a copy of a certificate of insurance to verify that insurance with the required coverage is in effect. All policies should be placed with an insurer with financial ratings of at least A- or better.
17. MISCELLANEOUS
17.1 Notices. Any notice required by this Agreement shall be given by prepaid, first class, certified mail, return receipt requested, or by air or other overnight courier, hand delivery or facsimile, or email provided that an original printed copy is also transmitted by any one of the methods prescribed in this sentence, to the parties at the addresses set forth below (or at such other address as either party may from time to time notify the other party in writing), provided, however, that updates and/or notice of updates to the Requirements Document or REMS may be communicated by Celgene to Pharmacy by email without requiring any subsequent provision of a printed notice. Notices:
if to Celgene: Celgene Corporation
00 Xxxxxx Xxxxxx
Xxxxxx, XX 00000
Attention: Xxxx Xxxxx
with a copy to: Celgene Corporation
00 Xxxxxx Xxxxxx
Xxxxxx, XX 00000
Attention: Legal Department
if to Pharmacy: DIPLOMAT PHARMACY, INC. d/b/a
DIPLOMAT SPECIALTY PHARMACY
0000 Xxxxx Xxxxxxx Xxxxxx
Xxxxx, XX 00000
Attention: General Counsel
With a copy to: DIPLOMAT PHARMACY, INC. d/b/a
DIPLOMAT SPECIALTY PHARMACY
0000 Xxxxx Xxxxxxx Xxxxxx
Xxxxx, XX 00000
Attention: Corporate Services, Contract Management
Any notice sent under this Section shall be deemed delivered within five (5) days if sent by mail and within twenty-four (24) hours if sent by fax, courier or hand delivery. In the event that any Section or subsection of this Agreement requires the notice contemplated therein to be sent to a different notice party and/or address, then that notice and/or address shall apply in lieu of the notice party and/or address in this Section, but the remainder of this Section shall apply.
17.2 Force Majeure. In the event a party (the “Affected Party”) shall be delayed or hindered in or prevented from the performance of any act required under this Agreement by reasons of fires, flood, earthquakes, accidents, explosions, sabotage, strikes, or other labor disturbances (regardless of the reasonableness of the demands of labor), civil commotions, riots, invasions, wars, acts, restraints, requisitions, regulations, or directions of governmental authorities, shortages of labor, fuel, power, or raw material, inability to obtain equipment or supplies, inability to obtain or delays in transportation, acts of God, or any other cause beyond its reasonable control (“Force Majeure”), then upon notice to the other party (the “Other Party”) performance of such act shall be excused for the period of such delay. Notice of the start and stop of any such Force Majeure and the nature and expected duration thereof shall be provided to the other party promptly following the occurrence thereof. The Affected Party shall use all commercially reasonable efforts to perform its obligations under this Agreement. In the event Force Majeure renders the Affected Party unable to perform its obligations under this Agreement for a period of ten (10) consecutive days or the aggregate of thirty (30) days in any twelve (12) month period, the Other Party may terminate this Agreement without penalty immediately upon written notice of termination to the Affected Party.
17.3 Binding Effect / No Amendments Unless in Writing. This Agreement shall be binding upon and shall inure to the benefit of the parties hereto and their permitted successors and permitted assigns. No agreements amending, altering or supplementing the terms hereof may be made except by means of a written document signed by the duly authorized representatives of both parties.
17.4 No Assignment/No Transfer Without Celgene’s Consent. This Agreement shall be personal to Pharmacy. Pharmacy shall not assign, sell, or otherwise transfer any of its rights and obligations (including, but not limited to, by operation-of-law, mergers, transfer of assets, change-of-ownership, or change-of-control) under this Agreement without the prior written consent of Celgene, which Celgene may grant or deny in its sole and absolute discretion. In the event Pharmacy attempts or otherwise enters into any agreement to assign, sell, or otherwise transfer this Agreement or any of its rights or obligations under this Agreement by way of any agreement or transaction which may involve any merger, transfer of assets, change-of-ownership, change-of-control or the like, Celgene shall have the right to immediately terminate this Agreement.
17.5 No Deemed Waiver. Failure of either party to enforce a right under this Agreement shall not act as a waiver of that right or the ability to later assert that right relative to the particular situation involved or to terminate this Agreement as a result of any subsequent default or breach.
17.6 No Publicity. Except as required by law, neither party shall use the name, logos, marks or trade names of the other party (or its affiliates) of the other party or of any employee of the other party in connection with any press release, public announcement or publicity without the prior written approval of the other party. The obligations in this section shall survive expiration or termination of the Agreement.
17.7 Severability. If any provision of this Agreement shall be found by a court to be void, invalid or unenforceable, the same shall either be reformed to comply with applicable law or stricken if not so conformable, so as not to affect the validity or enforceability of this Agreement, except if the principal intent of the Agreement is frustrated by such reformation or deletion in which case this Agreement shall terminate.
17.8 Independent Parties. The parties are, and shall be deemed to be, independent contractors and not agents or employees of the other party. Neither party shall have authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other party.
17.9 Counterparts. This Agreement may be executed in one or more counterparts, which taken together will constitute the one and the same Agreement, between the parties hereto.
17.10 Headings. Headings and section numbers included herein are for convenience only, and shall not be used to construe this Agreement.
17.11 Governing Law. This Agreement shall be construed and enforced in accordance with the laws of the state of New Jersey, without giving effect to its conflicts of law principles.
17.12 Schedules/Attachments. As of the Effective Date of this Agreement the following schedules, exhibits and/or attachments are attached to this Agreement:
· Schedule 1.8 — Pharmacy Dispensing Site List
· Schedule 1.17 — Requirements Document
· Schedule 2.3 — Sample Inventory
· Schedule 5.1 — Data Reports
· Schedule 6.1 — Performance Metrics
· Schedule 7.1 — Return Goods Policy
· Schedule 8.1 — Adverse Drug Experience Reporting Form
· Schedule 8.2 — Complaints Form
· Schedule 10.3 — Medicaid Program List
17.13 Entire Agreement. This Agreement, together with the attached schedules, exhibits and/or other attachments, constitutes the entire and only agreement between the parties relating to the subject matter hereof. Any and all other prior or contemporaneous negotiations, discussions, understandings, documents or agreements, are no longer of any force or effect, and are superseded by this Agreement. In the event of any conflict or inconsistency between the terms and conditions of this Agreement and any other document agreement concerning the subject matter hereof, the terms and conditions of this Agreement shall control.
[REMAINDER OF THIS PAGE LEFT BLANK, EXECUTION PAGE FOLLOWS]
[EXECUTION PAGE TO AGREEMENT]
THIS AGREEMENT WILL NOT BE CONSIDERED ACCEPTED, APPROVED, OR OTHERWISE EFFECTIVE UNTIL THE SIGNATURE OF EACH PARTY IS AFFIXED IN THE SPACE PROVIDED BELOW.
A FULLY EXECUTED AGREEMENT MUST BE RETURNED TO, AND RECEIVED BY, CELGENE BY NO LATER THAN MAY 31, 2017 OTHERWISE CELGENE RESERVES THE RIGHT TO RE-EVALUATE THE TERMS AND CONDITIONS OF THE AGREEMENT, INCLUDING BUT NOT LIMITED TO DECLARING THIS AGREEMENT NULL AND VOID.
IN WITNESS WHEREOF, the parties have executed and entered into this Agreement as of the Effective Date first above written.
|
|
DIPLOMAT PHARMACY, INC. d/b/a | ||||
|
CELGENE CORPORATION |
|
DIPLOMAT SPECIALTY PHARMACY | |||
|
|
|
|
| ||
|
By: |
/s/ Xxxxx Xxxxxxxxx |
|
By: |
/s/ Xxxx Xxxx | |
|
|
|
|
|
| |
|
Name: |
Xxxxx Xxxxxxxxx |
|
Name: |
Xxxx Xxxx | |
|
|
|
|
|
| |
|
Title: |
Exec. Dir. Pricing & Contracting |
|
Title: |
EVP, Operations | |
|
|
|
|
|
| |
|
Date: |
3/31/2017 |
|
Date: |
3/8/2017 | |
|
|
|
|
|
| |
|
Legal: |
xxxxxxxx@xxxxxxx.xxx |
|
|
| |
|
|
2017.03.10 13:45:02 -05’00’ |
|
|
| |

SCHEDULE 1.8
PHARMACY DISPENSING SITE LIST
Diplomat Specialty Pharmacy- Michigan
NPI: 0000000000 NCPDP: 2369797 DEA: XX0000000
0000 Xxxxx Xxxxxxx Xxxxxx, Xxxxx X
Xxxxx, XX 00000-0000
P: 000-000-0000 F: 000-000-0000
Diplomat Specialty Pharmacy- Michigan
NPI: 0000000000 NCPDP: 2321052 DEA: XX0000000
X-0000 Xxxxxxx Xxxx,
Xxxxx, XX 00000-0000
P: 000-000-0000 F: 000-000-0000
Diplomat Specialty Pharmacy- Florida
NPI: 0000000000 NCPDP: 1093929 DEA: XX0000000
000 XX 00xx Xxxxxx, Xxxxx 000
Xx. Xxxxxxxxxx, XX 00000-0000
P: 954-527-0440 F: 954-527-0940
Diplomat Specialty Pharmacy- Illinois
NPI: 0000000000 NCPDP: 1478189 DEA: XX0000000
0000 Xxxxx Xxxxxxx,
Xxxxxxx Xxxxx, XX 00000-0000
P: 000-000-0000 F: 000-000-0000
Diplomat Specialty Pharmacy- California
NPI: 0000000000 NCPDP: 0574764 DEA: XX0000000
0000 Xxxxxx Xxx. Xxxxx 000-000
Xxxxxxx, XX 00000-0000
P: 909-881-1728 F: 000-000-0000
SCHEDULE 1.17
REVLIMID®, POMALYST®, and THALOMID®
REQUIREMENTS DOCUMENT
1. BACKGROUND
1.1. REVLIMID®
REVLIMID® is approved by the United States Food and Drug Administration (FDA).
REVLIMID® is a thalidomide analogue, a known teratogen that causes severe birth defects or embryo-fetal death.
REVLIMID® is indicated for the treatment of patients with:
· Multiple myeloma (MM), in combination with dexamethasone.
· Transfusion-dependent anemia due to low- or intermediate-1-risk myelodysplastic syndromes (MDS) associated with a deletion 5q abnormality with or without additional cytogenetic abnormalities.
· Mantle cell lymphoma (MCL) whose disease has relapsed or progressed after two prior therapies, one of which included bortezomib.
Limitations of Use:
REVLIMID® is not indicated and is not recommended for the treatment of patients with chronic lymphocytic leukemia (CLL) outside of controlled clinical trials.
MM: The recommended starting dose of REVLIMID® is 25 mg orally once daily on Days 1-21 of repeated 28-day cycles in combination with dexamethasone.
MDS: The recommended starting dose is 10 mg daily.
MCL: The recommended starting dose of REVLIMID® is 25 mg/day orally on Days 1-21 of repeated 28-day cycles.
REVLIMID® is available in 2.5 mg (white and blue-green opaque), 5 mg (white opaque), 10 mg (blue/green and pale yellow opaque), 15 mg (powder blue and white opaque), 20 mg (powder blue and blue-green opaque), and 25 mg (white opaque) capsules.
2. BACKGROUND
2.1. POMALYST®
POMALYST® is approved by the United States Food and Drug Administration (FDA).
POMALYST® is a thalidomide analogue, a known teratogen that causes severe birth defects or embryo-fetal death.
POMALYST® is a thalidomide analogue indicated, in combination with dexamethasone, for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on or within 60 days of completion of the last therapy.
The recommended starting dose of POMALYST® is 4 mg per day taken orally on Days 1-21 of repeated 28-day cycles until disease progression. POMALYST® should be given in combination with dexamethasone.
POMALYST® is available in 1 mg (dark blue and yellow opaque), 2 mg (dark blue and orange opaque), 3 mg (dark blue and green opaque), and 4 mg (dark blue and blue opaque) capsules.
3. BACKGROUND
3.1. THALOMID®
THALOMID® is approved by the United States Food and Drug Administration (FDA).
THALOMID® is a known teratogen that causes severe birth defects or embryo-fetal death.
THALOMID® is indicated for the treatment of patients with:
· THALOMID® in combination with dexamethasone is indicated for the treatment of patients with newly diagnosed multiple myeloma (MM).
· THALOMID® is indicated for the acute treatment of the cutaneous manifestations of moderate to severe erythema nodosum leprosum (ENL).
· THALOMID® is not indicated as monotherapy for such ENL treatment in the presence of moderate to severe neuritis.
· THALOMID® is also indicated as maintenance therapy for prevention and suppression of the cutaneous manifestations of ENL recurrence.
MM: 200 mg orally once daily. The recommended dose of dexamethasone is 40 mg/day on days 1-4, 9-12, and 17-20 every 28 days.
ENL: 100 to 300 mg/day for an episode of cutaneous ENL. Up to 400 mg/day for severe cutaneous ENL.
THALOMID® is available in 50 mg (white opaque), 100 mg (tan), 150 mg capsules (tan and blue), and 200 mg (blue) capsules.
4. RISK EVALUATION AND MITIGATION STRATEGY
4.1. OVERVIEW
REVLIMID®, POMALYST®, AND THALOMID® are contraindicated in pregnancy. Thalidomide is a known teratogen that causes severe birth defects or embryo-fetal death. REVLIMID® and POMALYST® are analogues of thalidomide.
Celgene addresses the risks of REVLIMID®, POMALYST®, AND THALOMID® therapy not only through normal pharmacovigilance, but also through FDA-mandated Risk Evaluation and Mitigation Strategies, which include, among other things, the restricted distribution programs REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS®.
The REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® programs controls distribution of REVLIMID®, POMALYST®, AND THALOMID® through certified pharmacies. The REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs require education by telephone or in-person by trained, Certified Counselors who are licensed healthcare professionals, such as Nurses (“RNs”), Nurse Practitioners (“NPs”), Licensed Practical Nurses (“LPNs”), Pharmacists (“X.Xx,”), Pharmacy Interns, Physicians (“M.D.”), Doctors of Osteopathy (“D.O.”), and Physician Assistants (“P.A.”), before a prescription of REVLIMID®, POMALYST®, and/or THALOMID® can be dispensed by direct-shipment to a patient or the product is picked-up at the pharmacy. Education is the key component of the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs.
4.2. DEFINITION
A Risk Evaluation and Mitigation Strategy (“REMS”) is a strategy to manage a known or potential serious risk associated with a drug or biological product. A REMS program can include a Medication Guide, Patient Package Insert, a communication plan, elements to assure safe use, and an implementation system, and must include a timetable for assessment of the REMS.
4.3. CURRENT GOALS
Consistent with Title IX of Subtitle A of the Food and Drug Administration Amendments Act (“FDAAA”) Celgene Corporation has proposed the following REMS goals for REVLIMID®, POMALYST®, AND THALOMID® in each of the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs.
The goals of each of the REVLIMID®, POMALYST®, AND THALOMID® risk evaluation and mitigation strategies are as follows:
· To prevent the risk of embryo-fetal exposure to REVLIMID®, POMALYST®, and/or THALOMID®
· To inform prescribers, patients, and pharmacists on the serious risks and safe-use conditions for REVLIMID®, POMALYST®, and/or THALOMID®
4.4. REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® PROGRAMS PATIENT RISK CATEGORIES
The REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs categorize patients into six patient risk categories:
Female (4); and Male (2)
· Adult female of reproductive potential
· Adult female not of reproductive potential
· Female child of reproductive potential
· Female child not of reproductive potential
· Adult Male
· Male Child
What is a Female of Reproductive Potential (“FRP”)? An FRP is a female that:
· Is menstruating
· Is amenorrheic from previous medical treatments
· Is under 50 years of age
· Is perimenopausal
· Does not qualify for the adult female or female child not of reproductive potential category
4.5. REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® PROGRAMS PHARMACY BUSINESS REQUIREMENTS
4.5.1 Pharmacy Certification
Pharmacies must have executed a Pharmacy Distribution and Services Agreement (“Agreement”) with Celgene; completed a REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® program Pharmacy Inclusion Form; and successfully completed the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® program training and certification, in order to be certified with REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs.
All pharmacy distribution sites must be certified with the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs.
4.5.2 Training/Certification for Counselors and Staff
Pharmacy manager responsibilities
· Educate all staff regarding dispensing guidelines, including floater pharmacists, pharmacy technicians, or anyone else handling the product
· Make sure counselors are registered and certified in Compliance Wire and advise Celgene of inactive counselors
· Complete and return all documentation that pertains to non-compliance
Certified Counselors and Certified Pharmacists involved in dispensing REVLIMIW, POMALYST®, and THALOMID®
· For counselors to be certified they are required to be licensed healthcare professionals (See section 3.1). Counselors must complete Adverse Drug Reaction Reporting training modules, and REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® program training via onsite training (for newly certified sites), along with web based training modules, as may be required, to receive certification (Certified Counselor/Certified Pharmacist)). Additional personnel added to the program by a certified pharmacy must perform the web based training to receive certification prior to counseling patients. All Pharmacists involved in dispensing REVLIMID®, POMALYST®, and THALOMID® must complete the same training as a Certified Counselor.
· All Certified Counselors are required to take the Adverse Drug Reaction Reporting training modules and the REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® programs Certification Exam on an annual basis in order to maintain their certification status as counselors. Additionally, revised training or additional training, based upon enhanced REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® training requirements, or in response to identified regulatory issues, may be deployed using web based training and all Certified Counselors must complete the training by its scheduled due date in order to maintain their Certified Counselor status.
· A score of one hundred percent (100%) is required to pass the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs exam. If a Counselor fails the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs exam 4 times they will be locked out and be required to contact Celgene Customer Care and speak to a Risk & Compliance Specialist to request one additional attempt. The 5th attempt resulting in failure, will be reported to the Account Executive to provide additional training as needed.
The following table (Table 1: Certified Counselor Objectives and Tools) provides an overview of the objectives and tools for the Certified Counselor.
[REMAINDER OF PAGE BLANK, TABLE 1 FOLLOWS]
Table 1: Certified Counselor Objectives and Tools
|
Certified Counselor Objective |
|
Tools |
|
· Completion of Celgene-sponsored training on REVLIMID®, POMALYST®, and THALOMID® and REVLIMID REMS®, POMALYST REM®, and THALOMID REMS® programs to be able to counsel on the safe and effective use of REVLIMID®, POMALYST®, and THALOMID® annually
· Warn and educate Females of Reproductive Potential and males that REVLIMID®, POMALYST®, and/or THALOMID® can cause severe human birth defects and embryo-fetal death. Females of Reproductive Potential must use 2 forms of effective birth control at the same time or 4 weeks before, during, during dose interruptions and 4 weeks after discontinuation of therapy. Male patients must use a latex or synthetic condom as required in the REVLIMID REMS®, POMALYST REM®, and/or THALOMID REMS® programs
· Educate Females of Reproductive Potential of the requirements for pregnancy testing as scheduled during therapy
· Complete phone or in-person counseling with each patient, and complete the REVLIMID REMS®, POMALYST REM®, and/or THALOMID REMS® programs Education and Counseling Checklist prior to fulfillment of each prescription
· Understand and counsel on the risks of deep venous thrombosis (“DVT”) and pulmonary embolism (“PE”) with REVLIMID®, POMALYST®, and THALOMID® treatment and myocardial infarction (“MI”) and stroke risk for REVLIMID® and POMALYST®
· Understand and counsel on the risk of neutropenia and thrombocytopenia with REVLIMID® treatment |
|
· Specific training provided by Celgene to certified pharmacy
· Pharmacy Guides to REVLIMID REMS®, POMALYST REM®, and THALOMID REMS® programs
· REVLIMID®, POMALYST®, and THALOMID® Prescribing Information
· REVLIMID REMS®, POMALYST REM®, and THALOMID REMS® programs Education and Counseling Checklists for Pharmacies
· Adverse Drug Experience Reporting Procedure
· Celgene Medical Information Services |
The physician and/or nurse and Certified Counselor/Certified Pharmacist will work as a team to ensure that the patient is properly educated about the potential risks associated with REVLIMID®, POMALYST® and/or THALOMID® treatment.
Training for pharmacy staff involved in dispensing REVLIMID®, POMALYST®, and THALOMID®
· Other Pharmacy staff involved in dispensing REVLIMID®, POMALYST®, and THALOMID® under the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs that are not Counselors or Pharmacists must be educated on the dispensing guidelines and adverse event reporting, and may be required to complete web based training as may be deemed necessary by Celgene. These staff members must read the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs pharmacy training and Adverse Drug Reaction Reporting, and must sign and date the acknowledgment form (Please see: Figure 1) indicating that that they have read and understand the trainings. A Certified Counselor/Pharmacist or Diplomat training representative must also record and either sign or have an electronic system to verify and acknowledge that they reviewed the training staff member’s submitted training material to ensure the staff member’s understanding and completion. Additionally, revised training or additional training requirements, based on enhancements to training requirements or in response to identified regulatory issues, may be required to read and acknowledge.
4.5.3 Guidelines for Dispensing REVLIMID®, POMALYST®, AND THALOMID® (Please see Figure 2: Workflow for Dispensing REVLIMID®, POMALYST®, and THALOMID®))
(1) Fill valid prescriptions for REVLIMID®, POMALYST®, and THALOMID® in accordance with REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® programs and all applicable laws and regulations. Accept only prescriptions with an authorization number and patient risk category. The authorization number is a number issued by Celgene Customer Care to the prescriber after the completion of patient counseling by the prescriber and the prescriber survey.
(2) Prescriptions with corresponding authorization numbers are only valid for: (a) seven (7) days from the date of the last pregnancy test for FRP; and, (b) thirty (30) days from the date issued for all other patients. Prescriptions must be faxed, electronically transmitted (state law permitting), or written prescriptions presented in person. Faxed and written prescriptions must be signed and dated by the prescriber using ink. Stamped signatures are not permitted. Telephone prescriptions are not permitted. If a prescription does not have an authorization number and/or patient risk category, the pharmacy can contact the prescriber or Celgene Customer Care, obtain the authorization number and/or patient risk
category over the phone and write it on the prescription or include in electronic pharmacy records that can be linked to the original prescription, and is easily retrievable during a Celgene audit or as requested by Celgene. If the authorization number is available, but the patient risk category is missing, the Celgene web portal (if available) can provide the patient risk category. The pharmacist or designee will need to sign, date, and obtain name and title of person who provided the information, document on the hard copy of the prescription or in electronic pharmacy records that can be linked to the original prescription.
(3) Contact the patient through a Certified Counselor to provide the required counseling and to verify the shipping address. Before dispensing REVLIMID®, POMALYST®, or THALOMID®, a Certified Counselor will be required to complete the REVLIMID REMS®, POMALYST REMS®, or THALOMID REMS® programs Education and Counseling Checklist for Pharmacies (ECCP). (Please see: Figure 3) Certified Counselors/Certified Pharmacists are expected to use the most recently approved version of the ECCP when updates occur. Celgene will communicate these updates to Certified Pharmacies.
(4) Obtain confirmation number from Celgene by calling Celgene Customer Care Center at 0-000-000-0000 to use the IVR system 24 hours a day, 7 days a week, or speak to a live representative during business hours. Confirmation numbers can also be obtained through the Celgene web portal (if available.) Provide the following information to obtain a confirmation number:
(a) NCPDP# or DEA#
(b) Authorization number written on prescription
(c) Milligram strength and number of capsules being dispensed
(5) Celgene Customer Care Center will provide a confirmation number once the authorization number has been verified. Write the confirmation number on the prescription and date it, or include in pharmacy electronic records that can be linked to the original prescription. The confirmation number is valid for 24-hours once it is obtained from Celgene.
(6) Dispense no more than a 4-week (28-day) supply with no refills, along with the FDA approved MEDICATION GUIDE. A new prescription is required for subsequent dispenses.
(7) Dispense subsequent prescriptions only if 7 days or less of therapy remaining on the current prescription.
(8) In the event that the patient cannot be contacted or does not have access to a phone:
(a) Contact the Certified Prescriber; and
(b) Call and counsel patient in Certified Prescriber’s office after prescription has been faxed.
(9) Do not transfer REVLIMID®, POMALYST®, or THALOMID® inventory to another Pharmacy without prior permission from Celgene. Prescription transfers are allowed to be faxed to another pharmacy in the REVLIMID REMS®, POMALYST REMS®, AND THALOMID REMS® programs network (state law permitting) if the original pharmacy is not in the patient’s insurance network.
(10) Educate ALL STAFF involved in filling REVLIMID®, POMALYST®, and THALOMID® prescriptions about the dispensing procedures and document as per training requirements listed in Section 4.5.2 above.
(11) Keep a record of the prescription and REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® programs Education and Counseling Checklist with each REVLIMID®, POMALYST®, and THALOMID® prescription. Alternatively, the checklist and prescription can be linked to each other electronically in Pharmacy’s computer system. If Pharmacy uses an electronic Education and Counseling Checklist, it must be identical to the paper version (including all required elements from the paper form) provided by Celgene.
(12) The FDA approved MEDICATION GUIDE must be included with EACH filled prescription.
4.5.4 Shipping Requirements
· To avoid delays in the start of therapy, Product must be provided to patients as follows:
· Females of Reproductive Potential (High Risk) — Product must be shipped the same day the confirmation number is obtained or picked-up within twenty four (24) hours of obtaining the confirmation number.
· All other risk categories — Product must be shipped within twenty four (24) hours of obtaining the confirmation number or picked-up within twenty four (24) hours of obtaining the confirmation number.
· Use appropriate packing materials to protect the Product in transit
· Track each shipment to ensure on-time delivery
· Ship all packages for all patient risk categories Monday through Friday utilizing next calendar day delivery SIGNATURE REQUIRED.
· All shipments for FRP are required to ship for next calendar day delivery on the first attempt. For all FRP, all shipments on Fridays must be shipped for Saturday delivery with SIGNATURE REQUIRED.
· For all Male patients and all non-FRP female patients, all shipments on Fridays may be shipped for either Saturday delivery with SIGNATURE REQUIRED or Monday delivery with SIGNATURE REQUIRED.
IN ADDITION TO THE FOREGOING, PHARMACY MUST CANCEL THE CONFIRMATION NUMBER IF THE SHIPMENT IS “UNDELIVERABLE.” FOR PURPOSES OF THIS PARAGRAPH “UNDELIVERABLE” SHALL MEAN DELIVERY HAS BEEN ATTEMPTED BY THE CARRIER (UPS, FEDEX, ETC.) AND CARRIER WAS UNABLE TO DELIVER THE SHIPMENT AFTER 3 ATTEMPTS AT DELIVERY. THE SHIPMENT SHALL BE RETURNED TO THE PHARMACY AS “UNDELIVERABLE”. ONCE THE SHIPMENT IS RETURNED TO THE PHARMACY AS “UNDELIVERABLE”, PHARMACY MUST CONTACT CELGENE TO CANCEL THE CONFIRMATION NUMBER.
By way of example only: A shipment (for any patient risk category) is shipped to a patient on Friday, May 20, 2016 for Saturday delivery signature required. The patient is not home when the carrier arrives to deliver the package. The carrier’s next attempt to deliver is Monday, May 23, 2016. The patient is not home again to receive the package. The carrier reattempts the delivery on Tuesday, May 24, 2016 and the patient is not home once again. The carrier now returns the package to the Pharmacy as undeliverable. At this point, the Pharmacy must call Celgene the day they receive the undeliverable package to cancel the confirmation number. If the package is returned to the pharmacy on a Saturday or Sunday, the Pharmacy must call Celgene Customer Care first thing Monday morning to obtain a cancellation number.
· As a reminder, each prescription requires a new confirmation number when ready to ship again (after the shipment had been returned due to being undeliverable.). Record the confirmation number and the date that the confirmation number was received from Celgene Customer Care after you have counseled the patient, and prior to shipping product.
YOU MUST NOT DISPENSE OR SHIP REVLIMID®, POMALYST®, AND THALOMID® WITHOUT A VALID AND CURRENT CONFIRMATION NUMBER.
· The Pharmacy shall not ship or dispense REVLIMID®, POMALYST®, or THALOMID® to a patient until:
· The authorization numbers and patient risk category are recorded on the prescription, or REVLIMID®, POMALYST®, or THALOMID® Patient Prescription form (Please see: Figure 4), by the Prescriber or designee, or Pharmacy Certified Counselor or designee.
· The Certified Counselor has counseled the patient.
· The Pharmacy, through the Certified Counselor confirms by completing the Education and Counseling Checklist, that the patient understands the risks of REVLIMID®, POMALYST®, and/or THALOMID® therapy.
· NOTE: Any patient who has not received REVLIMID®, POMALYST®, or THALOMID® in the prior twelve (12) months will be considered a “new” patient and will have to be re-enroll into the REVLIMID REMS®, POMALYST REMS®, or THALOMID REMS® program.
· Celgene Customer Care provides the confirmation number to the Pharmacist or designee when the Pharmacist or designee calls to verify the authorization number. The Pharmacist or designee must write the confirmation number and date on the hard copy of the prescription or document in the Pharmacy’s electronic records linked to that prescription. The confirmation number obtained is valid for twenty four (24) hours. For prescriptions filled for Females of Reproductive Potential (FRP), the Pharmacy must ship the Product the same day that they receive the confirmation number or provide the Product directly to the patient within twenty four (24) hours of receipt of the confirmation number. For prescriptions for all other risk categories, Pharmacy shall ship within twenty-four (24) hours of obtaining the confirmation number.
· If the prescription is not shipped or provided to the patient in the proper timeframe, please call Celgene Customer Care to cancel the confirmation number. Document the cancellation number and date obtained on the prescription for auditing purposes.
DO NOT DISPENSE OR SHIP REVLIMID®, POMALYST®, OR THALOMID® IF:
(a) THE PATIENT IS PREGNANT;
(b) A FEMALE OF REPRODUCTIVE POTENTIAL STATES PREGNANCY TESTS WERE NOT CONDUCTED; OR
(c) THE PATIENT STATED THAT THEY DID NOT USE RECOMMENDED EFFECTIVE BIRTH CONTROL (UNLESS PRACTICING CONTINUOUS ABSTINENCE). See Education and Counseling Checklist for Pharmacies (see Figure 3) for acceptable birth control methods.
IF A, B, OR C OCCUR, CONTACT THE CERTIFIED PRESCRIBER AND CELGENE AT 0-000-000-0000, TO REPORT INDIVIDUAL INCIDENT(S).
4.5.5 Pharmacy Deviations:
The Pharmacy will be required to investigate and correct conditions that lead to deviations from the REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® programs. Celgene is committed to work with the Pharmacy to assist in implementing appropriate corrective actions, and establishing a timeframe for those actions.
These corrective action measures may include:
· Re-education of the pharmacy manager and staff by Celgene U.S. REMS and/or Account Executive
· Documentation of investigation of root cause and corrective action plan to prevent a future occurrence within the requested timeframe.
· Other risk mitigation measures or other actions if deemed suitable.
High Risk Deviations:
· In the event that Celgene deems any dispensing situation to be High Risk, Celgene shall have the right in its sole discretion to apply this provision and will contact the Pharmacy to advise that the subject dispense is deemed High Risk. See definition of “High Risk Deviation” on page 3 (1.12).
· Next steps may include correction action measures listed above, and will be considered for other risk mitigation options including deactivation, implementation of risk mitigation measures, or other actions as deemed suitable by Celgene.
· For any additional occurrence of a High Risk Deviation beyond 2 High Risk Deviations, the pharmacy may be deactivated and no longer permitted to dispense product.
· If the Pharmacy is deactivated, it will be informed of the decision and will not be permitted to order or dispense any Celgene Products.
Notwithstanding the foregoing, or anything to the contrary, if any corrective action measures or other actions deemed suitable by Celgene are not undertaken to Celgene’s satisfaction, Celgene has the right in its sole and absolute discretion to immediately deactivate the Pharmacy.
Request for reinstatement, following deactivation
· The Pharmacy must submit a letter of appeal directly to the CMO requesting reinstatement.
· The letter must document how circumstances have changed to ensure future compliance in the REMS Program(s).
· The CMO shall evaluate the appeal and determine whether the Pharmacy should be reactivated or remain deactivated.
· The pharmacy will be contacted by Celgene with the decision.
4.6. ADVERSE DRUG EXPERIENCE REPORTING
· All Adverse Drug Experiences suspected to be associated with the use of REVLIMID®, POMALYST®, or THALOMID® and all cases of special situations received by the Pharmacy from Prescribers or patients associated with the dispensing of REVLIMID®, POMALYST®, or THALOMID® must be reported within twenty four (24) hours to Celgene so that Celgene can meet all current health authority regulations and guidelines for reporting of Adverse Drug Experiences relating to REVLIMID®, POMALYST®, and THALOMID®.
· All reports of suspected pregnancy exposure must be reported immediately.
4.7. AUDIT
First audits will occur within 3 months of a Pharmacy’s first dispense of REVLIMID®, POMALYST®, and/or THALOMID®, then an annual risk-based audit thereafter.
FIGURE 1: REVLIMID REMS®, POMALYST REMS® AND THALOMID REMS®
NON-COUNSELOR STAFF TRAINING ACKNOWLEDGMENT

REVLIMID REMS® (formerly known as RevAssist®), POMALYST REMS® AND
THALOMID REMS® (formerly known as the S.T.E.P.S.® program)
NON COUNSELOR STAFF TRAINING ACKNOWLEDGMENT
REVLIMID REMS®, POMALYST REMS® AND THALOMID REMS® Training (REMS-GEN16777)
REMS ADVERSE DRUG REACTION REPORTING OVERVIEW PRESENTATION
(Version 7.0 January 2015 PPA)
REMS ADVERSE DRUG REACTION REPORTING FORM
(Version 6.0 March 2016)
REMS ADVERSE DRUG REACTION REPORTING FORM INSTRUCTIONS
(Instructions for ADR Report Form V4.1 February 2013_PPA)
REMS ADR REPORTING — IMPORTANT MEDICAL EVENTS LIST
(Version 7.1 March 01, 2018/ID/PPA)
I intend to assist in the dispensing of Celgene REMS products. I certify that I have read and understand the above listed REVLIMID REMS®, POMALYST REMS®, and THALOMID REMS® training and Adverse Event Reporting training, instructions, and reporting form. I understand that if there is revised training or additional training, based upon enhanced REVLIMID REMS®, POMALYST REMS®, or THALOMID REMS® training requirements or in response to identified regulatory issues, I will be required to complete additional training to ensure understanding, and I will sign another training form at that time.
|
|
|
PRINTED NAME OF PHARMACY STAFF MEMBER |
|
|
|
|
|
SIGNATURE OF PHARMACY STAFF MEMBER |
|
|
|
|
|
DATE |
|
|
|
I certify that I have assisted in training above pharmacy staff member on the CELGENE REMS requirements. |
|
|
|
|
|
PRINTED NAME OF CERTIFIED COUNSELOR/CERTIFIED PHARMACIST WITH CELGENE REMS/CELGENE STAFF |
|
|
|
|
|
SIGNATURE OF CERTIFIED COUNSELOR/CERTIFIED PHARMACIST WITH CELGENE REMS/CELGENE STAFF |
|
|
|
|
|
DATE |

FIGURE 3: REVLIMID REMS® EDUCATION AND COUNSELING CHECKLIST FOR PHARMACIES; POMALYST REMS® EDUCATION AND COUNSELING CHECKLIST FOR PHARMACIES; THALOMID REMS® EDUCATION AND COUNSELING CHECKLIST FOR PHARMACIES

FIGURE 4: REVLIMID® PATIENT PRESCRIPTION FORM;
POMALYST® PATIENT PRESCRIPTION FORM;
THALOMID® PATIENT PRESCRIPTION FORM
|
REVLIMID® (lenalidomide) Patient Prescription Form |
|
| ||||||||||
|
|
|
| ||||||||||
|
Today’s Date Date Rx Needed Patient Last Name Patient First Name Phone Number ( ) Shipping Address City State Zip Date of Birth Patient ID# Language Preference: ¨ English ¨ Spanish ¨ Other Best Time to Call Patient: ¨ AM ¨ PM Patient Diagnosis Patient Allergies
Other Current Medications |
|
Prescriber Name State License Number Prescriber Phone Number ( ) Ext Fax Number ( ) Prescriber Address
City State Zip
Patient Type From PPAF (Check one)
o Adult Female — NOT of Reproductive Potential ¨ Adult Female — Reproductive Potential ¨ Adult Male ¨ Female Child — Not of Reproductive Potential ¨ Female Child — Reproductive Potential ¨ Male Child | ||||||||||
|
|
|
| ||||||||||
|
PRESCRIPTION INSURANCE INFORMATION (Fill out entirely and fax a copy of patient’s insurance card, both sides)
Primary Insurance
Insured Policy # Group # Phone # Rx Drug Card #
Secondary Insurance
Insured |
|
TAPE PRESCRIPTION HERE PRIOR TO FAXING REFERRAL, OR COMPLETE THE FOLLOWING
Recommended Starting Dose: See below for dosage
Myelodysplastic Syndromes. The recommended starting dose of REVLIMID is 10 mg/day with water. Dosing is continued or modified based upon clinical and laboratory findings.
Multiple Myeloma and Mantle Cell Lymphoma: The recommended starting dose of REVLIMID is 25 mg/day orally for Days 1 – 21 of repeated 28-day cycles. Dosing is continued or modified based upon clinical and laboratory findings.
REVLIMID
| ||||||||||
|
Policy # |
|
Dose |
|
Quantity |
|
Directions |
| |||||
|
|
|
|
|
|
|
|
| |||||
|
Group # |
|
¨ 2.5 mg |
|
|
|
|
| |||||
|
Phone # |
|
¨ 5 mg |
|
|
|
|
| |||||
|
Rx Drug Card # |
|
¨ 10 mg |
|
|
|
|
| |||||
|
|
|
¨ 15 mg |
|
|
|
|
| |||||
|
|
|
¨ 20 mg |
|
|
|
|
| |||||
|
|
|
¨ 25 mg |
|
|
|
|
| |||||
|
|
|
¨ Dispense as Written |
|
¨ Substitution Permitted |
| |||||||
|
|
|
|
|
|
| |||||||
|
|
|
NO REFILLS ALLOWED (Maximum Quantity = 28 days) |
| |||||||||
|
|
|
|
|
|
|
| ||||||
|
|
|
Prescriber Signature |
|
|
Date |
|
| |||||
|
|
|
|
|
|
|
|
| |||||
|
|
|
Authorization # |
|
|
Date |
|
| |||||
|
|
|
(To be filled in by healthcare provider) |
| |||||||||
|
|
|
|
|
|
|
|
| |||||
|
|
|
Pharmacy Confirmation # |
|
|
Date |
|
| |||||
|
|
|
(To be filled in by pharmacy) |
|
|
|
| ||||||
For further information on REVLIMID, please refer to the full Prescribing Information
How to Fill a REVLIMID® (lenalidomide) Prescription
1. Healthcare Provider (HCP) instructs female patients to complete initial patient survey
2. HCP completes survey
3. HCP completes patient prescription form
4. HCP obtains REVLIMID REMS® (formerly known as the RevAssist® program) authorization number
5. HCP provides authorization number on patient prescription form
6. HCP faxes form, including prescription, to one of the Celgene Certified Pharmacy Network participants (see below)
7. HCP advises patient that a representative from the certified pharmacy will contact them
8. Certified pharmacy conducts patient education
9. Certified pharmacy obtains confirmation number
10. Certified pharmacy ships REVLIMID to patient with MEDICATION GUIDE
Please see xxx.Xxxxxxx.xxx/XxxxxxxxXxxxxxx for the list of pharmacy participants
Information about REVLIMID and the REVLIMID REMS® program can be obtained by calling the Celgene Customer Care Center toll-free at 0-000-000-0000, or at xxx.XxxxxxxXxxxXxxxxxxxxx.xxx.
|
|
|
|
|
POMALYST® (pomalidomide) Patient Prescription Form |
|
| ||||||||||
|
|
|
| ||||||||||
|
Today’s Date Date Rx Needed Patient Last Name Patient First Name Phone Number ( ) Shipping Address City State Zip Date of Birth Patient ID# Language Preference: ¨ English ¨ Spanish ¨ Other Best Time to Call Patient: ¨ AM ¨ PM Patient Diagnosis Patient Allergies
Other Current Medications |
|
Prescriber Name State License Number Prescriber Phone Number ( ) Ext Fax Number ( ) Prescriber Address
City State Zip
Patient Type From PPAF (Check one)
¨ Adult Female — NOT of Reproductive Potential ¨ Adult Female — Reproductive Potential ¨ Adult Male ¨ Female Child — Not of Reproductive Potential ¨ Female Child — Reproductive Potential ¨ Male Child | ||||||||||
|
|
|
| ||||||||||
|
PRESCRIPTION INSURANCE INFORMATION (Fill out entirely and fax a copy of patient’s insurance card, both sides)
Primary Insurance
Insured Policy # Group # Phone # Rx Drug Card #
|
|
TAPE PRESCRIPTION HERE PRIOR TO FAXING REFERRAL, OR COMPLETE THE FOLLOWING Recommended Starting Dose: See below for dosage
Multiple Myeloma: The recommended starting dose of POMALYST is 4 mg/day orally for Days 1 — 21 of repeated 28-day cycles. POMALYST should be given in combination with dexamethasone. Dosing is continued or modified based upon clinical and laboratory findings.
POMALYST | ||||||||||
|
Secondary Insurance |
|
Dose |
|
Quantity |
|
Directions |
| |||||
|
|
|
|
|
|
|
|
| |||||
|
Insured |
|
¨ 1 mg |
|
|
|
|
| |||||
|
Policy # |
|
¨ 2 mg |
|
|
|
|
| |||||
|
Group # |
|
¨ 3 mg |
|
|
|
|
| |||||
|
Phone # |
|
¨ 4 mg |
|
|
|
|
| |||||
|
Rx Drug Card # |
|
¨ Dispense as Written |
|
¨ Substitution Permitted |
| |||||||
|
|
|
|
|
|
| |||||||
|
|
|
NO REFILLS ALLOWED (Maximum Quantity = 28 days) |
| |||||||||
|
|
|
|
|
|
|
| ||||||
|
|
|
Prescriber Signature |
|
|
Date |
|
| |||||
|
|
|
|
|
|
|
|
| |||||
|
|
|
Authorization # |
|
|
Date |
|
| |||||
|
|
|
(To be filled in by healthcare provider) |
| |||||||||
|
|
|
|
|
|
|
|
| |||||
|
|
|
Pharmacy Confirmation # |
|
|
Date |
|
| |||||
|
|
|
(To be filled in by pharmacy) |
|
|
|
| ||||||
For further information on POMALYST, please refer to the full Prescribing Information
How to Fill a POMALYST® (pomalidomide) Prescription
1. Healthcare Provider (HCP) instructs female patients to complete initial patient survey
2. HCP completes survey
3. HCP completes patient prescription form
4. HCP obtains POMALYST REMS® authorization number
5. HCP provides authorization number on patient prescription form
6. HCP faxes form, including prescription, to one of the Celgene Certified Pharmacy Network participants (see below)
7. HCP advises patient that a representative from the certified pharmacy will contact them
8. Certified pharmacy conducts patient education
9. Certified pharmacy obtains confirmation number
10. Certified pharmacy ships POMALYST to patient with MEDICATION GUIDE
Please see xxx.Xxxxxxx.xxx/XxxxxxxxXxxxxxx for the list of pharmacy participants
Information about POMALYST and the POMALYST REMS® program can be obtained by calling the Celgene Customer Care Center toll-free at 0-000-000-0000, or at xxx.XxxxxxxXxxxXxxxxxxxxx.xxx.
|
|
|
|
|
THALOMID® (thalidomide) Patient Prescription Form |
|
| ||||||||||
|
Today’s Date Date Rx Needed Patient Last Name Patient First Name Phone Number ( ) Shipping Address City State Zip Date of Birth Patient ID# Language Preference: ¨ English ¨ Spanish ¨ Other Best Time to Call Patient: ¨ AM ¨ PM Patient Diagnosis Patient Allergies
Other Current Medications |
|
Prescriber Name State License Number Prescriber Phone Number ( ) Ext Fax Number ( ) Prescriber Address
City State Zip
Patient Type From PPAF (Check one)
¨ Adult Female — NOT of Reproductive Potential ¨ Adult Female — Reproductive Potential ¨ Adult Male ¨ Female Child — Not of Reproductive Potential ¨ Female Child — Reproductive Potential ¨ Male Child | ||||||||||
|
|
|
| ||||||||||
|
PRESCRIPTION INSURANCE INFORMATION (Fill out entirely and fax a copy of patient’s insurance card, both sides)
Primary Insurance
Insured Policy # Group # Phone # Rx Drug Card #
Secondary Insurance Insured Policy # Group # |
|
TAPE PRESCRIPTION HERE PRIOR TO FAXING REFERRAL, OR COMPLETE THE FOLLOWING
Recommended Starting Dose: See below for dosage
Multiple Myeloma: The recommended starting dose of THALOMID is 200 mg/day orally with water for a 28-day treatment cycle. Dosing is continued or modified based upon clinical and laboratory findings.
Erytherna Nodosum Leprosum: The recommended starting dose of THALOMID is 100 to 300 mg/day with water for an episode of cutaneous ENL. Up to 400 mg/day for severe cutaneous ENL. Dosing is continued or modified based upon clinical and laboratory findings.
THALOMID | ||||||||||
|
|
|
| ||||||||||
|
Phone # |
|
Dose |
|
Quantity |
|
Directions |
| |||||
|
|
|
|
|
|
|
|
| |||||
|
Rx Drug Card # |
|
¨ 50 mg |
|
|
|
|
| |||||
|
|
|
¨ 100 mg |
|
|
|
|
| |||||
|
|
|
¨ 150 mg |
|
|
|
|
| |||||
|
|
|
¨ 200 mg |
|
|
|
|
| |||||
|
|
|
¨ Dispense as Written |
|
¨ Substitution Permitted |
| |||||||
|
|
|
|
|
|
| |||||||
|
|
|
NO REFILLS ALLOWED (Maximum Quantity = 28 days) |
| |||||||||
|
|
|
|
|
|
|
| ||||||
|
|
|
Prescriber Signature |
|
|
Date |
|
| |||||
|
|
|
|
|
|
|
|
| |||||
|
|
|
Authorization # |
|
|
Date |
|
| |||||
|
|
|
(To be filled in by healthcare provider) |
| |||||||||
|
|
|
|
|
|
|
|
| |||||
|
|
|
Pharmacy Confirmation # |
|
|
Date |
|
| |||||
|
|
|
(To be filled in by pharmacy) |
|
|
|
| ||||||
For further information on THALOMID, please refer to the full Prescribing Information
How to Fill a THALOMID® (thalidomide) Prescription
1. Healthcare Provider (HCP) instructs female patients to complete initial patient survey
2. HCP completes survey
3. HCP completes patient prescription form
4. HCP obtains THALOMID REMS® (formerly known as the S.T.E.P.S.® program) authorization number
5. HCP provides authorization number on patient prescription form
6. HCP faxes form, including prescription, to one of the Celgene Certified Pharmacy Network participants (see below)
7. HCP advises patient that a representative from the certified pharmacy will contact them
8. Certified pharmacy conducts patient education
9. Certified pharmacy obtains confirmation number
10. Certified pharmacy ships THALOMID to patient with MEDICATION GUIDE
Please see xxx.Xxxxxxx.xxx/XxxxxxxxXxxxxxx for the list of pharmacy participants
Information about THALOMID and the THALOMID REMS® program can be obtained by calling the Celgene Customer Care Center toll-free at 0-000-000-0000, or at xxx.XxxxxxxXxxxXxxxxxxxxx.xxx.
|
|
|
|
|
|
|
|
FIGURE 1: REVLIMID REMS®, POMALYST REMS® AND THALOMID REMS® NON-COUNSELOR STAFF TRAINING ACKNOWLEDGMENT
SCHEDULE 2.3
SAMPLE INVENTORY REPORT
Quarterly Inventory Reporting Template
Required for all products pharmacy is actively enrolled in REMS to dispense (Revlimid, Pomalyst, or Thalomid)
Date of inventory count will be the last business day of the quarter (4x per year).
Electronic inventory count will be conducted at the end of Q1 and Q3, physical inventory count will be conducted at the end of Q2 and Q4.
All inventory reports must be submitted within 5 business days of count.
|
Pharmacy |
|
Pharmacy |
|
Pharmacy |
|
Pharmacy |
|
Pharmacy |
|
Pharmacy |
|
Pharmacy |
|
NDC#: |
|
Product |
|
Strength |
|
Unit of |
|
Units |
|
Capsules on |
|
Date of |
|
BD1234567 |
|
1112223 |
|
Main Street Pharmacy |
|
0 Xxxx Xx |
|
Xxx Xxxx |
|
XX |
|
00000 |
|
59572-405-00 |
|
Revlimid |
|
5MG |
|
Capsule |
|
100 |
|
106 |
|
3/31/2016 |
Submit Reports via e-mail to: Xxxxxxxxxxxxxxxx@xxxxxxx.xxx
SCHEDULE 5.1
DATA REPORTS
The table below outlines the fields, which are required for a report to be considered “complete”.
Celgene Sample File Format — Microsoft Excel.
|
Field Name |
|
Type |
|
Format |
|
|
Pharmacy site address |
|
VARCHAR |
|
|
|
|
Pharmacy site city |
|
ALPHA |
|
|
|
|
Pharmacy site state |
|
ALPHA |
|
|
|
|
Pharmacy site zip |
|
NUMERIC |
|
|
|
|
Date Dispensed |
|
DATE |
|
MM/DD/YYYY |
|
|
Customer ID (specific to this prescriber) |
|
VARCHAR |
|
|
|
|
Prescriber First Name |
|
ALPHA |
|
|
|
|
Prescriber First Name |
|
ALPHA |
|
|
|
|
Prescriber Address ID (if available) |
|
VARCHAR |
|
|
|
|
Prescriber Address |
|
VARCHAR |
|
|
|
|
Prescriber City |
|
ALPHA |
|
|
|
|
Prescriber State |
|
ALPHA |
|
|
|
|
Prescriber Zip |
|
NUMERIC |
|
|
|
|
DEA (prescriber) |
|
VARCHAR |
|
|
|
|
NPI (prescriber) |
|
NUMERIC |
|
|
|
|
NDC |
|
NUMERIC |
|
XXXXX-XXXX-XX |
|
|
Product |
|
ALPHA |
|
|
|
|
QTY in vials |
|
NUMERIC |
|
|
|
Submit Reports via e-mail to: xxxxxxxxxxxxxxx@xxxxxxx.xxx
SCHEDULE 6.1
PERFORMANCE METRICS
Pharmacy shall receive a Service Fee to pay for its performance of the Enhanced Services in connection with the first dispense of a Product to a Customer, based on the range in days between Pharmacy’s receipt of a prescription with a valid authorization from a Certified Prescriber and the dispensing of the Product, in an amount listed in the Time to First Dispense table for the Product. The Service Fee paid for Enhanced Services performed in connection with each subsequent dispense shall equal the applicable sum of the Time to Repeat Dispense and Subsequent Dispense Count amounts for the Product. All payments are subject to Section 6.2 of the Agreement.
![]()
|
Time to First Dispense | ||
|
|
|
|
|
Range |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
Time to Repeat Dispense | ||
|
|
|
|
|
Range |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
Subsequent Dispense Count | ||
|
|
|
|
|
Disp. |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
* Information redacted pursuant to a confidential treatment request by Diplomat Pharmacy, Inc. under 5 U.S.C. §522(b)(4) and Rule 24b-2 under the Securities Exchange Act of 1934 and submitted separately with the Securities and Exchange Commission.
Performance Metrics

Revlimid Specialty Pharmacy Scorecard
|
Time to First Dispense | ||
|
|
|
|
|
Range |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
Time to Repeat Dispense | ||
|
|
|
|
|
Range |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
Subsequent Dispense Count | ||
|
|
|
|
|
Disp. |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
* Information redacted pursuant to a confidential treatment request by Diplomat Pharmacy, Inc. under 5 U.S.C. §522(b)(4) and Rule 24b-2 under the Securities Exchange Act of 1934 and submitted separately with the Securities and Exchange Commission.

|
Time to First Dispense | ||
|
|
|
|
|
Range |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
Time to Repeat Dispense | ||
|
|
|
|
|
Range |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
Subsequent Dispense Count | ||
|
|
|
|
|
Disp. |
|
Payout in |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
|
[*] |
* Information redacted pursuant to a confidential treatment request by Diplomat Pharmacy, Inc. under 5 U.S.C. §522(b)(4) and Rule 24b-2 under the Securities Exchange Act of 1934 and submitted separately with the Securities and Exchange Commission.
SCHEDULE 7.1
RETURN GOODS POLICY

Return Goods Policy
Unless otherwise required by law, return of Celgene products will be handled in accordance with this Return Policy effective July 1, 2013.
Return Authorization Requests
1 ALL returns require prior authorization from Celgene Corporation by calling
· 0-000-000-0000 for All Celgene products
2 The customer must provide the following information for the products being returned in order to be considered for authorization
· Contact name
· NABP number or DEA number
· Lot number and quantity of each item to be returned
· Reason for return
· Advise if the product has been previously dispensed to a pattern (for Risk Managed Products)
3 Celgene Corporation reserves the right to deny credit for product returned without the accompanying return authorization documentation, or any product returned that falls outside of the guidelines stated in this policy
4. All products should be returned within five (5) business days of receiving authorization from Celgene Corporation
Products Eligible for Credit
· Products returned in ORIGINAL, UNOPENED, INTACT, FULL trade cartons, blister packs, bottles or vials maybe returned for credit unless Otib21,15, stated in the (-)Not Eligible for Credit(-) section below
· Products that are recalled or damaged during original shipment from Celgene are eligible for credit only if the customer reports any damages to the product within ten (10) business days of receipt of product shipment
· Products that are shipped in error by Celgene are eligible for credit only if the customer reports any errors or discrepancies in the order within ten (10) business days of receipt of product shipment
· All authorized products must be received within 3 months prior to expiration or up to 6 months after expiration date of the product to be eligible for credit
Celgene Corporation 00 Xxxxxx Xxxxxx Xxxxxx, XX 00000 phone (000) 000-0000 fax (000) 000-0000
· Handling fees apply
· 20% for products returned prior to 3 months expiration date
· 5% for products returned within 3 months prior expiration date or up to 6 months after expiration date
· Celgene (NDC # 59572), Pharmion (NDC # 67211) and Gloucester (NDC # 46026) Abraxis (NDC # 68817) trade dress product
Products NOT Eligible for Credit
· Product for professional sample, professional package, free goods, or with similar markings or special label
· Merchandise obtained other than through a Celgene authorized distributor of record or Celgene Corporation directly
· Product that has been used or dispensed to a patient
· Product obtained illegally or that has been diverted or resold by an account pursuant to a special price
· Product where the lot number or expiration date is missing, covered or unreadable
· When the intent of the customer is to temporarily reduce inventory
· Products involved in a bankruptcy sale or proceeding
· Product destroyed by the customer or a third party without prior written authorization from Celgene
· Product not manufactured by or on behalf of Celgene for distribution in the United States
· Expired product received by Celgene more than 6 (six) months past its expiration date
· Partial blister packs, bottles or vials
· Opened or unsealed commercial packages
Products NOT Eligible for Credit (continued)
· Products damaged by insurable catastrophes such as fire, smoke, acts of terrorism, flood, etc.
· Products sold, purchased or distributed contrary to federal, state or local law
· Product that is sold, purchased or distributed contrary to Celgene’s REMS programs
· BMS/Dupont trade dress product (NDC # 00056)
Terms of Return Policy
1. Credit for returned product will be determined by the return receipt date and upon review and inspection for compliance with all aspects of Celgene’s Product Return Policy.
2. A handling fee will be assessed on the original gross purchase price of the product as follows:
Celgene Corporation 00 Xxxxxx Xxxxxx Xxxxxx, XX 00000 phone (000) 000-0000 fax (000) 000-0000
a) 20% on in-date product returns, prior to 3 months before expiration unless otherwise agreed.
b) 5% on product returns up to 3 months before expiration and up to 6 months past expiration
*This fee does not apply to any product recalled, shipped damaged or shipped in error by Celgene or that which is ineligible for credit
3. Returns made in accordance with this policy will be credited, to the direct purchaser from Celgene, for the actual net price paid after any applicable handling fees, discounts, allowances or adjustments (including but not limited to, prompt payment discounts, government discounts, chargebacks or rebates)
4. Deductions from payables may not be taken until the credit memo is issued by Celgene Corporation. Unauthorized deductions from payables for returns may result in held orders
5. Credits will be processed through the account directly invoiced by Celgene Corporation. Customers who have been invoiced through a wholesaler or distributor should seek return credit from such wholesaler or distributor
6. No credit will be given for destroyed merchandise. A Returned Goods Authorization Form must accompany all returned product
7. Celgene Corporation reserves the right to destroy, without recourse, any returned product not eligible for credit
Shipping Information and Return Goods Addresses
1 All Authorized returns must be sent to:
Celgene Product Returns
0000 Xxxxxxxxxx Xxxx
Xxxxxxxxx, XX 00000
2. Shipping charges are the responsibility of the customer, unless the return is of products shipped in error by Celgene Corporation
3. Shipping labels will be provided when a pharmacy is returning product on behalf of a patient (returned product is ineligible for credit)
4. The return authorization number must be referenced on the shipping label to be considered as valid proof of delivery
Celgene reserves the right to destroy, without notification, credit, exchange or return to the customer, any merchandise that does not conform to the policy.
Celgene reserves the right to modify this policy in its discretion without advance notice.
Celgene Corporation 00 Xxxxxx Xxxxxx Xxxxxx, XX 00000 phone (000) 000-0000 fax (000) 000-0000
SCHEDULE 8.1
ADVERSE DRUG REACTION REPORTING
ADVERSE DRUG EXPERIENCES THAT ARE SUSPECTED TO BE ASSOCIATED WITH THE USE OF REVLIMID®, POMALYST®, OR THALOMID®, ANY SUSPECTED PREGNANCY OCCURRING DURING THE TREATMENT WITH REVLIMID®, POMALYST®, OR THALOMID®, AND ALL CASES OF SPECIAL SITUATIONS MUST BE REPORTED TO CELGENE WITHIN 24 HOURS. ANY REPORTS OF PREGNANCY EXPOSURE MUST BE REPORTED IMMEDIATELY.
Reporting to Celgene
Fill out Adverse Drug Reaction (ADR) Report Form as completely as possible (be sure to include reporter’s name on each page), then:
· Fax: 0-000-000-0000
· E-mail: xxxxxxxxxx@xxxxxxx.xxx
· Toll Free: 0-000-000-0000 (Global Drug Safety & Risk Management) 24 hours a day/7 days a week OR 0-000-000-0000 (Customer Care Center)
· Other electronic exchange as agreed upon by both parties.
If the report is of a possible exposure of a PREGNANT WOMAN, CALL Celgene Drug Safety IMMEDIATELY then follow-up with the ADR report form.
Reporting Procedures: Essential Information
· Reporter information
· Full Name/Title
· Address & Phone #
· Patient information (Sufficient information to enable Celgene to correspond with the treating physician)
· Initials
· Date of Birth, Age, Gender
· Adverse Drug Reaction (ADR) including causality assessment
· As complete a description as possible —Provide diagnosis and/or symptoms
· Key details —date started, date resolved and outcome
· Hospitalization or drug discontinued .... Ask why?
· Drug
· Did patient receive drug?
· If so, dose, frequency, dates of therapy, indication
· Lot # and expiration date
· Physician’s full name, address, phone number
Definitions: Cases of Special Situations
Market Authorization Holder (MAH) is responsible to report “Cases of Special Situations” which may include, but not be limited to:
· Outcomes of use of a medicinal product during pregnancy
· Adverse reactions during breastfeeding
· Use of product in pediatric or elderly population
· Reports of lack of therapeutic efficacy
· Reports in relation to overdose, abuse, off-label use, misuse; medication errors or occupational exposure
· Examples of Special Situations include but are not limited to:
· ADR in infant following exposure to product from breastfeeding
· Contamination of starting materials, or during product manufacturing
· Promotion of opportunist infections due to contamination
· Drug prescribed to a patient, accidentally taken by their child
· Patient took expired product or incorrect prescription given whether or not an adverse reaction occurred
SCHEDULE 8.2
COMPLAINT FORM
|
To: |
|
Celgene Customer Care Center |
|
Fax: |
|
0-000-000-0000 |
|
|
|
|
|
|
|
|
|
Specialty Pharmacy Name: |
|
|
|
Phone: |
|
|
|
|
|
|
|
|
|
|
|
From: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Title: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Date of Call: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Complainant Name: |
|
|
|
Address: |
|
|
|
|
|
|
|
|
|
|
|
Home Phone: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Work Phone: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product |
|
|
|
Gender: |
|
|
|
|
|
|
|
|
|
|
|
Lot Number: |
|
|
|
Language: |
|
|
|
|
|
|
|
|
|
|
|
Expiration Date: |
|
|
|
Address: |
|
|
|
|
|
|
|
|
|
|
|
Best Day to Call: |
|
|
|
Best Time to Call: |
|
|
|
|
|
|
|
|
|
|
|
Description of Problem: |
|
|
|
|
|
|