2- 91837512_5 deployment and administration, and that Customer will be solely responsible for the use, deployment and administration of Product to individuals in the Territory. The terms and conditions of this Agreement, including with respect to...

Exhibit 10.37 CERTAIN INFORMATION IDENTIFIED WITH [***] HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS BOTH (i) NOT MATERIAL AND (ii) WOULD BE COMPETITIVELY HARMFUL IF PUBLICLY DISCLOSED. -1- 91837512_5 ADVANCED PURCHASE AGREEMENT This ADVANCED PURCHASE AGREEMENT (this “Agreement”) is made as of 01/19/2021 (the “Effective Date”), by and between NOVAVAX, INC., incorporated and registered in the State of Delaware, with a principal place of business at ▇▇ ▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇ ▇.▇.▇. (“Novavax”), and Her Majesty the Queen in Right of Canada, as represented by the Minister of Public Works and Government Services, with offices at ▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇., ▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇, ▇▇, ▇▇▇▇▇▇, ▇▇▇▇▇▇ (collectively, “Customer”). Novavax and Customer may individually be referred to herein as a “Party” and, collectively, as the “Parties.” RECITALS WHEREAS, Novavax is currently developing a novel NVX-CoV2373 vaccine, consisting of a stable, prefusion protein made using its proprietary nanoparticle technology and coformulated with its proprietary Matrix-M™ adjuvant (the “Product”), which is intended to prevent SARS- CoV-2 (“COVID-19”) in humans; WHEREAS, if development of the vaccine is successful and Novavax receives Regulatory Approval (as defined below) to commercialize the Product in Canada, Novavax intends to commence commercial manufacture of the Product for supply to, and distribution by, Customer to individuals in Canada and to Canadian personnel who have been deployed offshore (e.g. members of the Canadian Armed Forces, Governmental foreign missions, Canadian consulates or embassies and their respective families) and locally engaged staff and their dependents (collectively, the “Territory”); a WHEREAS, in anticipation of Novavax receiving the government issued licenses, registrations, authorizations and approvals necessary to commercialize the Product in Canada including Expedited Authorization (as defined below) or any other conditional marketing approval (“Regulatory Approval”), Customer hereby orders an aggregate number of doses of Product from Novavax, to be supplied subject to the terms and conditions of this Agreement; WHEREAS, in reliance on such commitment by Customer, Novavax agrees to commence commercial manufacture of the Vaccine prior to Regulatory Approval for supply to, and distribution by, Customer to individuals in the Territory; NOW, THEREFORE, in consideration of the mutual covenants, terms and conditions set forth below, and for other good and valuable consideration the sufficiency and receipt of which is acknowledged by each Party, the Parties agree as follows: 1. Effects of COVID-19. Novavax and Customer hereby acknowledge and agree that to make Vaccine available [***] Novavax will commence manufacture of the Vaccine in advance of Regulatory Approval and the use, deployment and administration of the Product to individuals in the Territory will occur under epidemic conditions requiring such use,

-2- 91837512_5 deployment and administration, and that Customer will be solely responsible for the use, deployment and administration of Product to individuals in the Territory. The terms and conditions of this Agreement, including with respect to Product pricing, refund terms, indemnification and limitations of liability, reflect this understanding. 2. Sale of Product. 2.1. Generally. As of the Effective Date of this Agreement, Customer hereby commits to purchase from Novavax Fifty-Two Million (52,000,000) doses (the “Aggregate Amount”) of Product, which pending Regulatory Approval will be supplied in [***]. Such vials will be labelled in compliance with the applicable portions of the Food and Drugs Act, any applicable regulations or orders made under it and Health Canada interpretive guidance thereof. 2.2. Right to Additional Amounts. Prior to the end of the Term of the Agreement, subject to the terms of this Agreement, Customer has the irrevocable right, but not obligation, to purchase at multiple times, additional amount of Product from Novavax up to the additional amount of Product identified in Exhibit A. If Customer desires to exercise its right under this Section 2.2, Customer will notify Novavax in writing of the amount of Product Customer desires to procure, and the Parties will discuss, in good faith, the amount of Product Novavax can provide Customer based on its current supply. Likewise, if at any time during the Term of this Agreement, Novavax desires to sell any of the available Product identified in Exhibit A to a third party, Novavax shall notify Customer of the amount of Product in question (“Call Amount”). This notification will include relevant information for Customer to make a decision, including but not limited to, the delivery schedule. Within [***] of receipt of such notice, Customer will either place an order to purchase the portion of the Call Amount it desires to procure or notify Novavax it does not desire to purchase the Call Amount. If Customer fails to place an order or provide notice to Novavax within such [***], Customer’s additional amount of doses in the Call amount can be offered to other customer by Novavax and the amount of doses in the Call Amount will be available to Customer at a later date based on availabilities. Any amount of Product purchased by Customer under this Section 2.2. is the “Additional Amount”. The Parties acknowledge that if the Additional Amount is to be derived from a different manufacturing site than the one listed in the regulatory authorization, Novavax will need to obtain additional regulatory approval prior to shipment of the Additional Amount. 2.3. Customer will use the Product supplied hereunder solely (a) to vaccinate individuals in the Territory against COVID-19, subject to the immediately following sentences and (b) all in accordance with the terms and conditions of this Agreement. 2.3.1. Sale or Donations. Notwithstanding the territory restriction set forth in subclause 2.3 (a) hereof, subject to Customer’s obligation to indemnify Novavax as set forth in Section 9.3, Customer may donate or re-sell Product

-3- 91837512_5 supplied under this Agreement to one or more countries outside of the Territory, but only if (i) the intended purpose of such donation or resale is to vaccinate individual in such country(ies) against COVID-19, (ii) [***], (iii) [***] and (iv) [***]. 2.4. Delivery. Novavax intends to deliver monthly shipments of Product to Customer until the Aggregate Amount (and if applicable, Additional Amount) is supplied. Based upon Novavax’ projections and expectations as of the Effective Date, the anticipated quarterly delivery schedule of the Product is set forth in Exhibit B (“Delivery Schedule”). Customer acknowledges that the Delivery Schedule may change due to the impact of several variables including, but not limited to, speed of clinical trial enrollment and accrual of events, manufacturing delays and/or timing of Regulatory Approval. Novavax will use commercially reasonable efforts to deliver the Product to Customer in accordance with the Delivery Schedule and will, on at least on a [***] basis, communicate any anticipated changes to the Delivery Schedule to Customer. At least [***] in advance of the anticipated initial shipment under the Delivery Schedule, Novavax will inform Customer in writing of the date of the initial delivery of Product (“Delivery Start Date”) and provide Customer an updated Delivery Schedule detailing anticipated amounts and dates of each of monthly delivery of Product to meet the quarterly amounts identified in the Delivery Schedule. The Delivery Start Date is expected to be within [***] of the date the Product receives Regulatory Approval and there is sufficient Conforming Product in inventory for the complete first quarterly allotment in the Delivery Schedule, or a lower amount of Confirming Product than planned for the first quarterly allotment if it is deemed acceptable by Customer. 2.5. Expedited Authorization. If an Expedited Authorization is granted before [***], then Novavax will use commercially reasonable efforts to revise the Delivery Schedule to deliver Product to Customer earlier than indicated in the Delivery Schedule ("Adjusted Delivery Schedule"). “Expedited Authorization” means an expedited authorization for the Product granted by Health Canada that allows the Product to be placed on the market in Canada under the authority of the September 16, 2020 Interim Order Respecting the Importation, Sale and Advertising of Drugs in Relation to COVID-19 or other relevant interim order or regulation or authority. 2.5.1. Novavax Seeking Regulatory Approval. Novavax shall submit its application for Regulatory Approval in Canada no later than [***] of its first submission for Regulatory Approval in another priority market (such as the United States of America or the European Union). In the event that Novavax fails to submit as stated, then Customer’s sole and exclusive remedy shall be that it may terminate this Agreement [***] upon written notice to Novavax and [***] of the paid Advance Payment [***] will become due and refundable to Customer. 2.6. Variance. Customer hereby acknowledges and agrees that the Delivery Schedule is an estimate only and that notwithstanding anything herein to the contrary, (a) the quantity of Product actually delivered [***] may vary within [***] of the

-4- 91837512_5 Aggregate Amount and (b) the actual date of delivery may vary within [***] of the delivery date projected by Novavax pursuant to the updated Delivery Schedule provided to Customer pursuant to Section 2.44. Novavax will use commercially reasonable efforts to inform Customer of any variance in a quarterly allotment at least [***] prior to the expected first monthly delivery for doses from the impacted quarterly allotment. 2.7. Failure to Supply. If Novavax receives Regulatory Approval for the Product and thereafter fails to supply Customer with the quantity of Product units specified for a particular calendar quarter as set out in the Delivery Schedule, or, if applicable, the Adjusted Delivery Schedule, within the timeframe permitted by Section 2.6, then Novavax will [***] to Customer, in writing, the cause of the inability to supply and present Novavax’s good faith remedial plan, which should include [***] (“Remedial Plan”). Where such inability to supply results from Novavax’s inability to manufacture or source sufficient quantities of Product to supply all of its customers, Novavax shall allocate to Customer [***] for the period of short supply. 2.7.1. If the Remedial Plan does not resolve such inability or failure to supply to within [***] of the first missed monthly delivery giving rise to the plan, the Customer may [***], cancel delivery of any Product scheduled for delivery [***]. 2.7.2. If failure to supply is still ongoing after [***] of the initial missed monthly delivery giving rise to the plan, Customer may upon written notice to Novavax cancel future deliveries and terminate the Agreement. 2.7.3. [***]. 2.7.4. The remedies in this Section 2.7 shall be Customer’s sole recourse and Novavax’s entire liability with respect to any failure to supply. 2.8. Inconsistent Terms. All terms and conditions contained in any prior or subsequent oral or written communication or order, that are different from or in addition to this Agreement are hereby rejected by the Parties and will neither expand nor modify either Party’s obligations under this Agreement. 2.9. Regulatory. Notwithstanding Section 2.8 (Inconsistent Terms) in the event of any conflict between this Agreement and any applicable provision of the Food and Drugs Act (including any provision made under it), the Food and Drugs Act will prevail; provided, that in the event that a conflict between the terms of this Agreement and any applicable provision of the Food and Drugs Act exists or arises, (i) the Party determining that such conflict exists or arises will give [***] written notice (and in any event, within [***] of such determination) to the other Party, (ii) the Parties will cooperate in good faith to resolve any such conflict, including altering any resulting delivery schedules or remedies, and (iii) will negotiate a replacement provision that complies with the applicable Food and Drugs Act but preserves the intent of the Parties as provided hereunder (if necessary).

-5- 91837512_5 2.10. Support Obligations. During the Term of the Agreement, Novavax agrees to provide the Product Support obligations specified in Exhibit D. 3. Delivery and Inspection 3.1. Delivery, Title and Risk of Loss. Product will be delivered [***] to the delivery destinations in Canada set forth on Exhibit B hereto (the “Points of Entry”). Risk of loss and title to Product shall pass to Customer [***]. For clarity, Customer will be solely responsible for ensuring it has the necessary approvals for importing Product into the Territory and for distributing Product in the Territory. 3.2. Inspection. Customer (or its designee) shall, [***] (the “Inspection Period”) following Novavax’s delivery of Product to the Point of Entry, visually inspect such delivery to confirm that the Product has been supplied in the correct quantity and constitutes Conforming Product (as defined below). Notwithstanding the foregoing, Customer may request to extend the Inspection Period for an additional [***] and Novavax will reasonably and in good faith consider such extension request and provide written notice of approval to Customer if granted. If Customer determines that any shipment of Product contains missing or non-Conforming Product, then Customer must notify Novavax of the missing Product or reject the portion of the applicable delivery that constitutes non-Conforming Product, as applicable, by providing Novavax with written notice of such rejection during the Inspection Period. If a delivery of Product has not been rejected by Customer prior to expiry of the Inspection Period, Customer agrees that its remedies are limited to the warranty process set out in this paragraph if a Latent Defect (as defined below) is subsequently discovered. Notwithstanding the acceptance process established herein, in the event the failure of Product to constitute Conforming Product is not reasonably discoverable through customary visual inspection during the Inspection Period (each, a “Latent Defect”), Customer will have [***] following the discovery of the Latent Defect to make a warranty claim on the non-Conforming Products pursuant to Section 4.1 by providing Novavax with written notice of such Latent Defect; provided that Product may not be rejected for a Latent Defect beginning [***] prior to expiry of the shelf-life for the applicable Product. 4. Product Warranty. 4.1. Limited Product Warranty. Novavax warrants to Customer that, [***], such Product will (a) conform to the specifications for such Product as set out in the Regulatory Approval; (b) conform to the specifications set forth on Exhibit C hereto (the “Specifications”) and (c) have been manufactured in accordance with current good manufacturing practice (cGMP) as defined under applicable laws (Product satisfying clauses (a)-(c) hereof, “Conforming Product”). In case of a conflict between (a) and (b), (a) will take priority. Any claims by Customer that Product fails to meet this warranty set forth in this Section 4.1 must be made by Customer within [***] of its discovery of such deficiency, but in no event later than [***] prior to expiry of the shelf-life for the applicable Product.

-6- 91837512_5 4.2. Remedies for Non-Conforming Product. If Novavax accepts Customer’s rejection of Product as set forth in Section 3.2, accepts Customer’s warranty claim in Section 4.1 or if the Independent Expert determines that any Product is non-Conforming Product as set forth in Section 4.3, then Novavax shall, at Customer’s option and at no additional charge to Customer, either (a) replace the non-Conforming Product [***] or (b) credit or refund the pro-rated amount paid of the Total Price of such non-Conforming Product. If Novavax so requests, Customer shall, [***], return any non-Conforming Product to Novavax; otherwise, Customer shall dispose of Product in compliance with applicable laws and regulations. 4.3. Disputes. If Novavax disputes Customer’s rejection of Product as set forth in Section 3.2 or Section 4.1, then Novavax will provide Customer written notice of such dispute no later than [***] after the date of the notice from Customer that a Product is non-Conforming Product. Such dispute shall be resolved by having an independent, qualified third party expert (the “Independent Expert”), as selected by Novavax and acceptable to Customer (such acceptance not to be reasonably withheld or delayed) promptly test, analyze or otherwise evaluate the Product subject to the dispute to determine whether the Product in question meets the criteria specified in the warranty set forth in Section 4.1. The non-prevailing Party shall bear all out-of-pocket costs and expenses associated with the Independent Expert’s determination, including any reasonable out-of-pocket costs incurred by the prevailing Party in connection therewith. 4.4. Disclaimer. THE REMEDIES SET FORTH IN SECTION 4.2 SHALL BE CUSTOMER’S SOLE AND EXCLUSIVE REMEDY AND NOVAVAX’S ENTIRE LIABILITY FOR NON-ACCEPTANCE OF PRODUCT UNDER SECTION 3.2 OR ANY BREACH OF THE LIMITED WARRANTY SET FORTH IN SECTION 4.1. 5. Payment Terms. 5.1. Advance Payment. Customer shall pay to Novavax a total upfront payment of USD [***] (the “Advance Payment”). Customer acknowledges that in consideration of Novavax’s commitment to manufacture Product in advance of Regulatory Approval, [***] of the Advance Payment is non-refundable. The remaining [***] of the Advance Payment is refundable only as provided in Sections 2.7.3, 8.2.2, 8.2.3, and 8.3.1. 5.2. Price. Customer shall purchase the Product from Novavax at the Per-Unit Price for Product as set forth on Exhibit A (the “Price”). The Price shall be firm for the Term. The Price is exclusive of any and all governmental taxes, including without limitation, VAT, customs, charges or levies of every kind (“Taxes”) that Novavax may be required to collect or pay upon sale, transfer or shipment of Product to the Point of Entry under any applicable laws or regulations, but inclusive of [***]. Customer will be solely responsible for all such taxes, charges and levies, including any interest and penalties. If Customer is required under the law of any jurisdiction to deduct or withhold any sum of Taxes imposed on or in respect of any amount

-7- 91837512_5 due or payable to Novavax, the Taxes shall be paid and borne by Customer for Customer’s own account. “Total Price” means the Per-Unit Price multiplied by the Aggregate Amount and includes the Additional Amount if the Right for Additional Amount is exercised by Customer. 5.3. [***]. [***]. 5.4. Invoices. Novavax shall submit invoices to Customer for the Total Price as follows (a) the Advance Payment shall be invoiced [***] and (b) the Delivery Price shall be invoiced [***], which invoices shall be directed to the Contracting Authority and Technical Authority as defined in Section 6.1 and 6.2 of this Agreement (or to such other person or address if Customer notifies Novavax in writing pursuant to Section 14.2 that invoices should be sent to such other person or address). Further, the original and email copy of all invoices must also forwarded to the Public Health Agency of Canada for certification and payment at: Public Health Agency of Canada P2P Invoices ▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ Building 18th floor, RM ▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇ ▇▇▇ Email: [***] 5.5. Each invoice for the Delivery Price shall reflect the actual quantities of Product shipped to the Point of Entry, together with the per-unit Delivery Price [***] and the total Delivery Price [***] to be paid under such invoice. 5.5.1. All amounts set forth in each invoice for Product not rejected pursuant to Section 3.2 shall be payable by Customer within [***] of the date of Customer’s acceptance pursuant to Section 3.2, which will be made to Novavax, addressed as indicated on the applicable invoice, and made in United States Dollars. In the event Customer disputes all or any portion of an invoice submitted to it in accordance with this Section 5, then such dispute shall be resolved in accordance with Section 14.6. Customer will not be required to pay any amount disputed in good faith, unless and until such amount is finally determined to be owed to Novavax in accordance with the dispute resolution procedure set forth in Section 14.6. No interest shall be accrued on amounts disputed in good faith. 5.5.2. A payment is considered overdue on [***] following the date of Customer’s acceptance of Product pursuant to Section 3.2 and interest will be paid automatically in accordance with section 5.5.3. If the content of the invoice and its substantiating documentation are not materially in compliance of any the applicable invoicing requirements communicated to Novavax,

-8- 91837512_5 Customer will notify Novavax within [***] of receipt of the applicable invoice. The [***] payment period begins again upon acceptance of the revised invoice or the replacement or corrected Product if Product is rejected pursuant to Section 3.2. Failure by Customer to notify Novavax within [***] will only result in the date specified to apply for the sole purpose of calculating interest on overdue accounts. 5.5.3. For the purpose of this section: 5.5.3.1. "Average Rate" means the simple arithmetic mean of the Bank Rates in effect at 4:00 p.m. Eastern Time each day during the calendar month immediately before the calendar month in which payment is made; 5.5.3.2. "Bank Rate" means the rate of interest established from time to time by the Bank of Canada as the minimum rate at which the Bank of Canada makes short term advances to members of the Canadian Payments Association; 5.5.3.3. "date of payment" means the date of the negotiable instrument drawn by the Receiver General for Customer to pay any amount under the Agreement; 5.5.3.4. an amount becomes "overdue" when it is unpaid on [***] following the day on which it is due and payable according to the Agreement. 5.5.3.5. Customer will pay to Novavax simple interest at the Average Rate plus [***] per year on any amount that is overdue, from the date that amount becomes overdue until the day before the date of payment, inclusive. Novavax is not required to provide notice to Customer for interest to be payable. 5.5.4. Customer will pay interest in accordance with this section only if Customer is responsible for the delay in paying Novavax. Customer will not pay interest on overdue advance payments; however, Novavax shall be entitled to withhold shipments until overdue advance payments are received. 5.5.5. Novavax will maintain appropriate records and supporting documentation regarding sales of Product hereunder, that includes but is not limited to all invoices, receipts and vouchers. Novavax must retain records, including bills of lading and other evidence of transportation or delivery, for all deliveries made under the Agreement, to confirm that each invoice is limited to the actual quantities of Product shipped to the Point of Entry, which will be records and documentation will be maintained by Novavax for [***] following shipment of the relevant Product. Customer will have the right, upon reasonable advanced notice to Novavax, during normal business hours, to inspect any such records at the place such records are

-9- 91837512_5 normally kept. Novavax must provide all reasonably required facilities for any audit and inspection and must furnish information as the representatives of Customer may reasonably request from time to time require to perform a complete audit of the Agreement. In the event any such inspection reveals an overpayment by Customer, Novavax will [***] credit Customer the amount of such overpayment against future orders for Product, unless there are no such future orders, in which case, Novavax will [***] refund Customer for the amount of such overpayment. 6. Authorities and Representatives. 6.1. Contracting Authority (a) The Contracting Authority for this Agreement is: Name: [***] Telephone: [***] Email address: [***] (b) The Contracting Authority is responsible for the management of the Agreement on behalf of Customer and the Contracting Authority must authorize any changes to the Agreement in writing. Novavax must not perform, and shall not be obligated to perform, work in excess of or outside the scope of the Agreement based on verbal or written requests or instructions from anybody other than the Contracting Authority. 6.2. Technical Authority (a) The Technical Authority for this Agreement is: Name: [***] Telephone: [***] Email address: [***] (b) The Technical Authority is the representative of the department or agency for whom the Product is being carried out under the Agreement and is responsible for all matters concerning the technical content of the Product under the Agreement. Technical matters may be discussed with the Technical Authority, however the Technical Authority has no authority to authorize changes to the scope of the Product. Changes to the scope of the Product can only be made through a mutually agreed written contract amendment issued by the Contracting Authority and signed by both Parties.

-10- 91837512_5 6.3. Novavax Representatives (a) General enquiries: Name: [***] Telephone No.: [***] E-mail address: [***] (b) Delivery follow-up: Name: [***] Telephone No.: [***] E-mail address: [***] 7. Intellectual Property. As between Customer and Novavax, Customer hereby acknowledges and agrees that all rights, title and interests in, to and under any intellectual property that relate to the Product are and shall remain the sole and exclusive property of Novavax. This Agreement does not grant Customer and right, title or interest in, to or under any such intellectual property or any other intellectual property owned or controlled by Novavax. To the extent Customer, directly or indirectly, creates, discovers, reduces to practice or otherwise generates intellectual property relating to Product in connection with the activities contemplated by this Agreement, such intellectual property will be solely owned by Novavax. Customer shall assign, and hereby does assign, to Novavax all such intellectual property, and will take reasonable actions requested by Novavax, [***], to record and confirm Novavax’s ownership thereof, including executing formal documentation evidencing Novavax’s ownership thereof. 8. Term; Termination; Effects of Termination. 8.1. Term. This Agreement shall become effective upon the Effective Date and, unless sooner terminated as set forth in Section 8.2, shall continue in force and effect until Novavax has delivered to the Points of Entry and Customer has accepted an amount of Product equal to the Aggregate Amount (the “Term”). In the event that Customer exercises its right as per Section 2.2 (Right for Additional Amounts), the Term will automatically be extended to the end of the last delivery and acceptance under the revised Delivery Schedule provided by Novavax for the Additional Amount ordered. 8.2. Termination.

-11- 91837512_5 8.2.1. Material Breach. A Party may terminate this Agreement at any time prior to expiration of the Term upon written notice to the other Party if the other Party materially breaches this Agreement and such breach is not cured within [***] of written notice to the breaching Party describing such breach (excluding a failure by Customer to pay an undisputed amount when due, which must be cured within [***] of Novavax’s notice that Customer has failed to pay); provided, however, that if such breach (excluding a failure by Customer to pay an undisputed amount when due) is not reasonably curable within such [***] period and the breaching Party is using good faith efforts to cure such breach during such [***] period, then the breaching Party will have an additional [***] to cure such breach. Notwithstanding the foregoing, if the breaching Party has a bona fide dispute as to whether such breach has occurred or has been cured, it will so notify the non- breaching Party in writing and the cure period will be tolled until such dispute is resolved pursuant to Section 14.6. Upon a final determination of breach or failure to cure, the breaching Party will have the remainder of the cure period to cure such breach. 8.2.2. Regulatory Approval. On or before [***], if Novavax fails to receive Regulatory Approval of the Vaccine in the Territory or Novavax abandons the development of the Product or Novavax withdraws its application for Regulatory Approval in Canada, then Customer’s sole and exclusive remedy shall be that it may terminate this Agreement [***] upon written notice to Novavax and [***] of the paid Advance Payment will become due and refundable to Customer. Novavax will refund such amount within [***] of receipt of such termination notice. 8.2.3. Product Seizure. To the extent that Novavax fails to deliver Product Doses to Customer as a result of Product seizure by another country, Novavax shall, to the extent that replacement Product is not available in a timeframe acceptable to Customer, in Customer’s sole and absolute discretion and as Customer’s sole recourse and Novavax’s entire liability, return to Customer a minimum of [***] up to [***] of the paid Advance Payment for the Product that was not delivered to Customer. 8.3. Effects of Expiration or Termination. As of the effective date of expiration or any early termination of this Agreement, (a) neither Party shall be relieved of any obligation that accrued prior to such effective date of termination, (b) except as otherwise expressly provided herein, all rights and obligations of each Party hereunder will cease and (c) each Party shall return or destroy all Confidential Information of the other Party that is in its possession pursuant to the requirements of Section 13.6. 8.3.1. Breach by Novavax. In the event this Agreement is terminated pursuant to Section 8.2.1 after the first delivery due to a breach by Novavax, the Delivery Price for Conforming Product delivered to Customer in addition to the Advance Payment, shall become [***] due and payable to Novavax,

-12- 91837512_5 if not already paid by the Customer. Payment for quantities of Product not delivered to Customer as of the effective date of any such termination will not be paid by Customer. [***] of the paid Advance Payment for such Product units not delivered will be reimbursed at the discretion of Customer. 8.3.2. Breach by Customer. In the event this Agreement is terminated by Novavax pursuant to Section 8.2.1 due to a breachby Customer, [***], shall become [***] due and payable to Novavax. 9. Regulatory Matters. 9.1. Regulatory Assistance. Customer agrees to provide reasonable assistance to Novavax in obtaining Regulatory Approval for the Product in the Territory. The progress and outcome of the Regulatory Approval is wholly dependent on Novavax and the data it supplies to the Regulatory Approval authorities. Novavax acknowledges and agrees, and Customer represents to Novavax, that Customer will not influence decisions from the regulatory authorities to avoid conflict of interests regarding Novavax obtaining Regulatory Approval for the Product. 9.2. Product Recall or Withdrawal 9.2.1. This section is subject to section 21.3 of the Food and Drugs Act. In the event of a recall or a withdrawal of the Product, Novavax must notify Customer [***] from such recall or withdrawal, as the case may be, and must collect and destroy the delivered, recalled, or withdrawn Product [***], unless otherwise agreed to by the Parties. 9.2.2. Novavax must, upon the request of Customer, replace [***] any recalled or withdrawn Product [***], subject to Product availability as procured by Customer from Novavax. 9.2.3. If full replacement is not available in a timeframe acceptable to Customer, then Customer may, in addition to and without prejudice to any other remedy available, choose from one of the following options for the quantity of the Product affected: 9.2.3.1. full reimbursement of the Delivery Price, determined pro-rata for doses not replaced; or 9.2.3.2. partial replacement and partial reimbursement or partial credit under the Agreement of Delivery Price, determined pro-rata for those doses not replaced. Reimbursement and/or credit under this clause shall be made or applied, as applicable, by Novavax within [***] of Customer’s written notice of which option it selects. 9.3. Indemnification.

-13- 91837512_5 9.3.1. By Customer. Notwithstanding any contrary provision of this Agreement and to the fullest extent not prohibited by applicable laws, Customer will defend, indemnify and hold harmless Novavax and its affiliates and its or their respective officers, directors, employees, agents and contractors (each a “Novavax Indemnitee”) from and against any and all claims, demands, causes of action, damages, losses, liabilities, costs, expenses (including legal fees and litigation expenses), penalties, fines, settlements and judgments (collectively, “Losses”) resulting from a claim (each, a “Claim”) arising out of or in connection with any one or more of [***]. Notwithstanding any provision of this Agreement to the contrary, the provisions of this Section 9.3.1 shall apply and be binding on Customer regardless of whether any defect in the Product causing any Losses originates from the testing, development, manufacture, delivery, export, import, distribution, sale, offer for sale, administration, use or deployment of the Product. [***]. 9.3.2. Procedure. Any Novavax Indemnitee shall [***] notify Customer in writing of any Claim made against the Indemnified Party, specifying the basis given for such Claim; provided that [***]. Customer shall, upon giving written notice to a Novavax Indemnitee within [***] after its receipt of any notice of a Claim from the Novavax Indemnitee, undertake the defense of or, [***]. The election by Customer, pursuant to this Section to undertake the defense of a Claim shall not preclude the Novavax Indemnitee from participating or continuing to participate in such defense, so long as [***]. 10. Third Party Intellectual Property Infringement. IN THE EVENT THAT THE PRODUCTS INFRINGE ANY THIRD PARTY’S INTELLECTUAL PROPERTY RIGHTS, NOVAVAX’S SOLE OBLIGATION AND LIABILITY AND BUYER’S SOLE REMEDY IS EXPRESSLY LIMITED TO NOVAVAX REFUNDING TO CUSTOMER ANY AMOUNTS PAID FOR THE INFRINGING PRODUCT. IN THE EVENT OF SUCH INFRINGEMENT, CUSTOMER WILL, AT NOVAVAX’S REQUEST, RETURN THE INFRINGING PRODUCT TO NOVAVAX, AT NOVAVAX’S COST. 11. Representations and Covenants. 11.1. Mutual Representations. Each Party hereby represents and warrants to the other Party, as of the Effective Date, that: 11.1.1. it has all requisite power and authority, corporate or otherwise, to execute, deliver and perform this Agreement; 11.1.2. this Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms; 11.1.3. the execution, delivery and performance of this Agreement by such Party does not conflict with any agreement, instrument or understanding, oral or written, to which such Party is bound, nor violate any applicable law or any order, writ, judgment, injunction, decree, determination or award of any

-14- 91837512_5 court or governmental body or administrative or other agency presently in effect and applicable to such Party; and 11.1.4. in the performance of this Agreement, it shall comply with all applicable laws. 11.2. Novavax’s Covenant. Novavax hereby covenants to Customer that, at the time of delivery to the Points of Entry, Customer will have good title to the delivered Products, free and clear of all liens, encumbrances and security interests. 11.3. Disclaimer. EXCEPT FOR THOSE REPRESENTATIONS, WARRANTIES AND COVENANTS EXPRESSLY SET FORTH IN SECTION 4.1 OR THIS SECTION 11, TO THE FULLEST EXTENT NOT PROHIBITED BY APPLICABLE LAW, NOVAVAX EXPRESSLY DISCLAIMS ALL OTHER REPRESENTATIONS, WARRANTIES AND COVENANTS OF ANY KIND, WHETHER EXPRESS OR IMPLIED, WRITTEN OR ORAL, BY FACT OR LAW, INCLUDING ANY IMPLIED REPRESENTATIONS, WARRANTIES AND COVENANTS OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, SATISFACTORY QUALITY, NON-INFRINGEMENT AND ANY REPRESENTATIONS OR WARRANTIES OR CONDITIONS OR GUARANTEES ARISING FROM STATUTE, COURSE OF DEALING OR USAGE OF TRADE. EACH PARTY ACKNOWLEDGES THAT IT HAS NOT ENTERED INTO THIS AGREEMENT IN RELIANCE UPON ANY WARRANTY OR REPRESENTATION. FURTHER, THE PARTIES HEREBY ACKNOWLEDGE AND AGREE THAT NOTHING CONTAINED IN THIS AGREEMENT SHALL BE CONSTRUED AS A WARRANTY, EITHER EXPRESS OR IMPLIED, THAT NOVAVAX WILL OBTAIN A POSITIVE CLINICAL OUTCOME OR THAT THE PRODUCT WILL RECEIVE REGULATORY APPROVAL FOR COMMERCIAL USE 12. Limitation of Liability. 12.1. [***]. [***]. 12.2. Cap On Damages. EXCEPT WITH RESPECT TO CLAIMS FOR, UNDER OR ARISING OUT OF (A) A PARTY’S BREACH OF SECTION 1312, (B) A PARTY’S GROSS NEGLIGENCE, WILLFUL MISCONDUCT OR FRAUD OR (C) CLAIMS FOR WHICH A PARTY HAS AN INDEMNIFICATION OBLIGATION PURSUANT TO SECTION 9.3, IN NO EVENT WILL NOVAVAX’ LIABILITY TO CUSTOMER EXCEED [***]. 13. Confidential Information. 13.1. Definition. “Confidential Information” means any and all non-public or proprietary information provided by or on behalf of a Party to the other Party in connection with this Agreement, whether or not marked as “CONFIDENTIAL” or “PROPRIETARY,” and whether provided prior to, on or after the Effective Date, including all technical, scientific, business and other know-how, information, trade

-15- 91837512_5 secrets, methods, processes, practices, formulae, instructions, techniques, designs, drawings, data or results, but expressly excluding any information that (a) at the time of disclosure, is in the public domain, (b) after disclosure, becomes part of the public domain by publication or otherwise, through no fault of the receiving Party or its affiliates, (c) at the time of disclosure, is already in the receiving Party’s or its affiliates’ possession, except through prior disclosure by the disclosing Party, without any obligation of confidentiality or any restriction on its use, and such possession can be properly documented by the receiving Party or its affiliates in its written records, and was not made available to the receiving Party or its affiliates by any person or party owing an obligation of confidentiality to the disclosing Party, (d) is rightfully made available to the receiving Party or its affiliates from sources independent of the disclosing Party and (e) is independently discovered or developed by or on behalf of the receiving Party or its affiliates without the aid, use of, access to or application of any Confidential Information of the disclosing Party. For clarity, specific aspects or details of Confidential Information will not be deemed to be within the public domain or in the possession of the receiving Party merely because the Confidential Information is embraced by more general information in the public domain or in the possession of the receiving Party. 13.2. Obligation to Maintain in Confidence; Permitted Disclosure. Each Party agrees to (a) protect and maintain in confidence the disclosing Party’s Confidential Information using the same degree of care that it employs to protect the confidentiality of its own confidential information (but never less than a reasonable standard of care), (b) not disclose to any person or entity any the disclosing Party’s Confidential Information; provided that the receiving Party may disclose such Confidential Information to its affiliates and to its and their officers, directors, employees, contractors or agents who are bound by confidentiality obligations at least as restrictive as those set forth in this Section 13 and who reasonably need to know such Confidential Information in the performance of the receiving Party’s obligations under this Agreement, (c) ensure the full compliance of each of its affiliates and its and their officers, directors, employees, contractors or agents who have access to the disclosing Party’s Confidential Information with the confidentiality and non-use obligations in this Section 13 and (d) not use such Confidential Information for any purpose other than performing its obligations under this Agreement. Each Party acknowledges and agrees that its failure to comply with the provisions of this Section 13 may cause irreparable harm to the other Party that cannot be adequately compensated for in damages and, accordingly, that each Party will be entitled to claim, in addition to any other remedies available to it, interlocutory and permanent injunctive relief to restrain any anticipated, present or continuing breach of this Section 13 without the need to post bond or other security. The terms of this Agreement will be the Confidential Information of both Parties. 13.3. Notwithstanding Section 13.2, Customer will be permitted to disclose Confidential Information of Novavax for the purposes of government administration and operations, including for the purposes of vaccination program planning and vaccination program administration in collaboration with the Provinces and

-16- 91837512_5 Territories of Canada and its vaccine logistics provider, and in the exercise of Crown privileges. For greater clarity, this includes reporting to the Parliament of Canada, for public safety and national security purposes and for proactive disclosure required or permitted by applicable law. Any such disclosure of Confidential Information under this Section 13.3 will be limited to only that Confidential Information that is required to be disclosed under applicable law, and under and in compliance with the privacy, confidentiality and proactive disclosure policy regimes of the Government of Canada and in accordance with the Health Products and Food Branch of Health Canada practices. For the avoidance of doubt, the protections afforded to Novavax pursuant to Section 13.4 shall apply to disclosures of Confidential Information made by Customer pursuant to this Section in event Customer is compelled by law to disclose such Confidential Information. 13.4. Disclosures Required by Law. Each Party may disclose the Confidential Information of the other Party to the extent that such disclosure is required to be disclosed pursuant to applicable law (including the rules of any stock exchange) or a valid order of a court of competent jurisdiction or a supra-national, national, regional, state, provincial or local governmental body of competent jurisdiction. Prior to making any such disclosure, the receiving Party shall promptly advise the disclosing Party of the requirement to disclose as soon as the receiving Party becomes aware that such a requirement might become effective in order that, where possible, the disclosing Party may seek a protective order or such other remedy as the disclosing Party may consider appropriate in the circumstances. The receiving Party shall reasonably cooperate with the disclosing Party (at the disclosing Party’s cost) to the extent allowed by the applicable law in seeking a protective order or other relief. The receiving Party shall disclose only that portion of the disclosing Party’s Confidential Information that it is required to disclose. 13.5. Survival. The provisions of this Section 13 shall survive for a period of [***] from the date of any expiration or termination of this Agreement, but shall survive [***] with respect to any Confidential Information that is a trade secret for as long as such information remains a trade secret. 13.6. Return or Destruction. A Party may request that the other Party return or destroy any of its Confidential Information that is in the other Party’s possession at any time upon written notice to the other Party except for information submitted to Health Canada for regulatory purposes and subject to the Health Products and Food Branch of Health Canada practices. Upon expiration or termination of this Agreement, each Party shall return or destroy, at the other Party’s written election, all Confidential Information of the other Party that is in its possession as of the date of expiration or termination. Notwithstanding the foregoing, each Party may retain [***] of the other Party’s Confidential Information to ensure its compliance with this Agreement, and no Party will be required to destroy copies of the other Party’s Confidential Information that are included on disaster recover/backup tapes that are maintained by a Party pursuant to a bona fide disaster recovery plan. If requested by a Party, the returning or destroying Party will certify in writing to the other Party that such return or destruction has occurred.

-17- 91837512_5 14. Miscellaneous. 14.1. Force Majeure. Each Party’s obligations of performance under this Agreement will be temporarily suspended and excused for the period of interruption to the extent any failure of performance is due to (a) fire, earthquake, storm (including hurricanes, snow storms, blizzards or ice storms), hail, flood, act of war or terrorism, riot, civil commotion, pandemic (other than COVID-19, except to the extent that the effects of COVID-19 causes a material change on the resources such Party will use to perform its obligations under the Agreement materially adversely change following the Effective Date), epidemic (other than COVID-19, except to the extent that the effects of COVID-19 causes a material change on the resources such Party will use to perform its obligations under the Agreement materially adversely change following the Effective Date) or embargo, (b) changes to applicable law or (c) any other cause or event beyond the reasonable control of such Party (collectively, a “Force Majeure”). The affected Party will [***] notify the other Party of the anticipated period of interruption due to a Force Majeure and will take all reasonable measures to forthwith remedy the interruption. 14.2. Notices. Any notice given under this Agreement must be in writing and delivered either to the addresses set forth below in person or via overnight courier (or to such other addresses of which the Parties may from time to time be notified in writing), with a PDF copy sent by email: If to Novavax: Novavax, Inc. ▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ ▇.▇.▇. Attn: [***] Email: [***] If to Customer: As per Section 6.1 Contracting Authority of this Agreement. Such notice will be deemed to have been given as of the date delivered by hand, on the second (2nd) Business Day (at the place of delivery) after deposit with an internationally recognized overnight delivery service or upon written acknowledgement of the receiving Party. 14.3. Entire Agreement. This Agreement, including any schedules or exhibits hereto, contains the entire and exclusive agreement between the Parties in connection with the subject matter thereof and supersede all prior and collateral agreements, understandings, communications, representations and warranties between the Parties in relation thereto, including without limitation, the Memorandum of Understanding between the Parties dated August 27, 2020.

-18- 91837512_5 14.4. Amendment. No amendment or modification or supplement of this Agreement, including this provision, will be valid unless made in a writing signed by an authorized representative of each Party specifically referring to this Agreement. 14.5. Public Announcements. The Parties agree to determine jointly the contents of any public announcement informing the public about the existence of this Agreement between the Parties and, except as may be required by law or the rules of any national securities exchange, neither Party shall issue or cause the issuance of any such public announcement without the express prior approval of an executive officer of each Party. 14.6. Dispute Resolution; Equitable Relief. 14.6.1. With respect to any, dispute or controversy (“Dispute”) between the Parties and arising in whole or in part in connection with this Agreement, including whether a breach has occurred or been appropriate cured, the Parties shall first use good faith efforts to resolve such Dispute and, if such Dispute is not resolved within [***] from the date such Dispute arose, then Customer may submit the Dispute to binding arbitration in [***] or Novavax may submit the Dispute to binding arbitration in [***]. All matters so submitted to arbitration will be settled by three (3) arbitrators in accordance with the [***]. In the event of a conflict between [***] and this Agreement, this Agreement shall govern. Each Party will designate an arbitrator and the Parties will cause the designated arbitrators to mutually agree upon and to designate a third arbitrator who will serve as chairperson. The Parties shall arrange for a hearing to occur and be completed within [***] after the appointment of the third (3rd) arbitrator, which hearing shall last no longer than [***], unless the arbitral panel believes a longer period is required, in which case the hearing may last [***]. The Parties will cause the arbitrators to decide the matter to be arbitrated within [***] after the close of evidence unless the chairperson arbitrator determines, at the request of any Party or on his or her own initiative, that such time period should be extended, in which case such time period may not be extended beyond an additional [***] period. The final decision of the majority of the arbitrators shall be in writing, in all events follow governing law and will be furnished to all the Parties in such dispute. Judgment on such decision may be entered in any court having jurisdiction. 14.6.2. Notwithstanding any other terms of this Agreement, either Party may seek a preliminary injunction or other provisional equitable relief in any court of competent jurisdiction if, in its reasonable judgment, such action is necessary to avoid irreparable harm as permitted by applicable law. 14.7. Governing Law. This Agreement is made subject to the laws of [***], without regard to any conflict of laws principles. The United Nations Convention on Contracts for the International Sale of Goods and the United Nations Convention on the Limitation Period in the International Sale of Goods, if otherwise applicable,

-19- 91837512_5 each as the same may be amended or superseded, are hereby expressly excluded and will not be applicable to this Agreement. 14.8. Assignment. Neither Party will assign all or any portion of this Agreement or any right or obligation under this Agreement without the other Party’s prior written consent, which consent will not be unreasonably withheld or delayed. Any unauthorized assignment by a Party will be null and void of no force or effect. This Agreement will bind and inure to the benefit of the successors and permitted assigns of the respective Parties. 14.9. Survival. In order that the Parties may fully exercise their rights and perform their obligations in connection with this Agreement, any provisions of this Agreement that are required to ensure such exercise or performance (including any obligations accrued as of the termination date) or which are intended by their terms or by necessary implication to survive will survive the expiration or termination of this Agreement, including Sections 5 (with respect to accrued but unpaid amounts), 2.3, 7, 9.3, 9.3, 10, 13, 13, 14.2, 14.3, 14.6, 14.7, 14.9, 14.11, 14.12 and 14.13. 14.10. Waiver. Failure of either Party to exercise any right it has under this Agreement on one or more occasions will not operate or be construed as a waiver by such Party of its right to exercise the same right on another occasion. Any waiver must be in a writing signed by the waiving Party. 14.11. Severability. If any provision of this Agreement will be adjudicated to be invalid or unenforceable by a court of competent jurisdiction, it is the Parties’ intent that the remaining provisions of this Agreement will remain in full force and effect and the affected provision or portion thereof will be deemed modified so that it is enforceable to the maximum extent permissible to reflect as closely as possible the intentions of the Parties as evidenced from the provisions of this Agreement. 14.12. Independent Relationship of Parties; No Third-Party Beneficiary. The relationship of Novavax and Customer is that of independent contractors and under no circumstances will a Party, its agents or employees be partners, agents or representatives of another Party. Except as otherwise expressly provided in this Agreement, including any indemnification or limitation of liability provision, nothing in this Agreement will be construed as creating any direct or beneficial right in or on behalf of any third party. 14.13. Interpretation; Section Headings. For purposes of this Agreement, (a) the plural will include the singular and the singular the plural, (b) any gender will include any other gender, (c) the terms “included” or “including” or any variation are not words of exclusion and will be read to include “without limitation,” (d) the terms “hereof” or “herein” or any variation are intended to apply to this Agreement as a whole, (e) the word “or” is not exclusive and will be interpreted to have the meaning commonly associated with the phrase “and/or,” (f) references to any applicable law, rule or regulation, or article, section or other division thereof, will be deemed to include the then-current amendments thereto or any replacement or successor

-20- 91837512_5 applicable law, rule or regulation thereof and (g) the word the word “will” shall be construed to have the same meaning and effect as the word “shall.” The section headings used herein are intended for convenience of reference only and will not be considered in interpreting this Agreement. This Agreement shall be deemed to be the joint work product of the Parties and any rule of construction that a document shall be interpreted or construed against a drafter of such document shall not be applicable. 14.14. English Language. This Agreement shall be written and executed in, and all other communications under or in connection with this Agreement shall be in, the English language. Any translation into any other language shall not be an official version thereof, and in the event of any conflict in interpretation between the English version and such translation, the English version shall control. 14.15. Counterparts. This Agreement and all exhibits and schedules hereto may be executed and delivered by the Parties in one or more counterparts, each of which will be an original, and each of which may be delivered by facsimile, e-mail or other functionally equivalent electronic means of transmission and those counterparts will together constitute one and the same instrument. 15. Bio-Manufacturing. 15.1. Definitions: 15.2. Bio-Manufacturing consists of: 15.2.1. “Drug Substance Manufacturing” means the manufacture of Novavax adjuvant or nanoparticle bulk drug substance via [***]; and 15.2.2. “Final Formulation, Filling and Finishing” means the formulation of the drug substance with adjuvant and the subsequent filling, inspection, labelling and packaging of the final drug product. 15.3. Novavax acknowledges the potential benefits of Bio-Manufacturing in Canada and agrees to work in good faith with the Government and/or the private sector of Canada to establish a mutually beneficial contract manufacturing relationship(s) in Canada for one or more Novavax vaccines, including for Drug Substance Manufacturing and/or Final Formulation, Filling and Finishing. 16. Logistic Provider For Canada. 16.1. Customer has appointed [***] as the subcontractor with respect to the performance of importation into Canada, storage and transportation within Canada of the Products. [***] holds a Drug Establishment Licence authorizing it to import the drug. To ensure full compliance with Good Distribution Practice throughout the supply chain, Novavax and [***] will need to enter into a Quality Agreement prior to first delivery of Product.

-21- 91837512_5 [Remainder of page intentionally blank.]

[Signature Page to Advanced Purchase Agreement] 91837512_5 IN WITNESS WHEREOF, the parties hereto by their duly authorized officers have executed this Agreement as of the Effective Date. CUSTOMER By:/s/ ▇▇▇▇▇ ▇▇▇▇▇ Minister ▇▇▇▇▇ ▇▇▇▇▇ Printed Name Title: Minister of Public Works and Government Services NOVAVAX, INC. By:/s/ ▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇ III ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ III Printed Name EVP, CLO Title: 01/19/2021
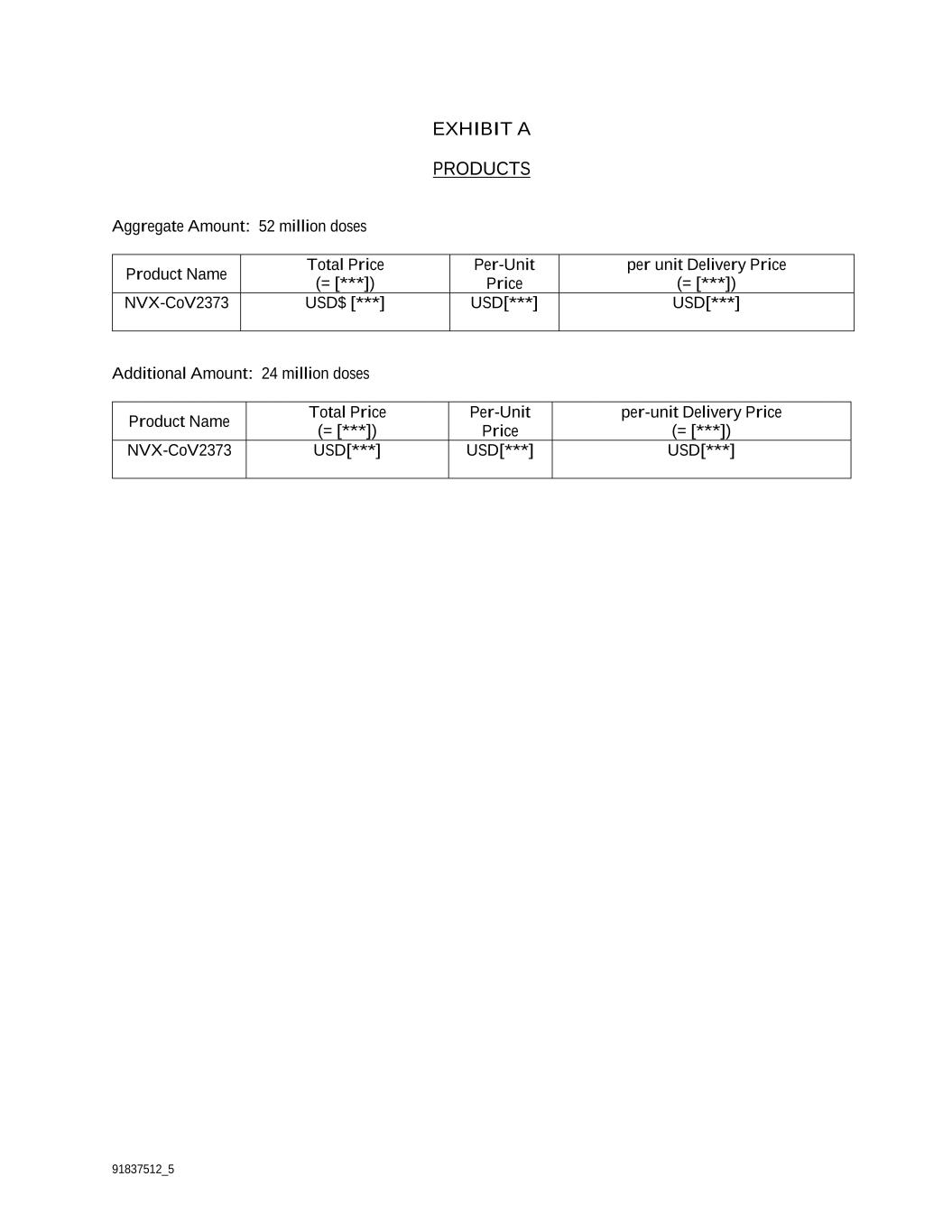
91837512_5 EXHIBIT A PRODUCTS Aggregate Amount: 52 million doses Product Name Total Price (= [***]) Per-Unit Price per unit Delivery Price (= [***]) NVX-CoV2373 USD$ [***] USD[***] USD[***] Additional Amount: 24 million doses Product Name Total Price (= [***]) Per-Unit Price per-unit Delivery Price (= [***]) NVX-CoV2373 USD[***] USD[***] USD[***]

91837512_5 EXHIBIT B DELIVERY SCHEDULE [Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the delivery schedule has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]

91837512_5 EXHIBIT C SPECIFICATIONS [Pursuant to Regulation S-K, Item 601(a)(5), this Exhibit setting forth the specifications has not been filed. The Registrant agrees to furnish supplementally a copy of any omitted exhibits to the Securities and Exchange Commission upon request; provided, however, that the Registrant may request confidential treatment of omitted items.]

91837512_5 EXHIBIT D SUPPORT OBLIGATIONS 1. Distribution in Canada Novavax must deliver the Product to, as a minimum, the destinations specified in Exhibit B in accordance with the directions of Customer. 2. Packaging Novavax must properly label and package the Product in compliance with the Food and Drugs Act (Canada) and any regulations, orders or instruments made thereunder, for the approved Product authorized for sale in Canada. To the extent a waiver of serialization is not available from Health Canada, Novavax will include bar codes on shipping package, secondary and primary package which includes variable data and which complies with GS1 standards that will enable tracking and tracing of Product from shipment to administration or other requirements specified by Health Canada. Unless otherwise agreed to in writing, smallest available package must not contain more than [***] doses 3. Maintenance of the Cold Chain During Transportation and the Use of Cold Chain Monitors The Product is a refrigerator-stable product, i.e. stored at 2-8 degrees Celsius, which permits leveraging standard vaccine distribution and delivery infrastructure. 4. Adverse Event Following Immunization (AEFI) Reporting Requirement Novavax must comply with all AEFI reporting requirements. 5. Customer Service (a) Novavax must identify a bilingual (English and French) Customer Support Contact, and maintain a bilingual customer support desk (reached via a toll free telephone number and an e-mail account) throughout a declared pandemic period to provide [***] customer support to Customer and to health care providers (“Requestors”). (b) The Customer Support Contact or Support Desk must, as a minimum:

91837512_5 (i) Be reachable Monday through Friday from [***] Newfoundland Standard Time to [***] Newfoundland Standard Time, with adjustments as necessary for daylight savings time (ii) Respond to general enquiries on product information concerning the use of the vaccine, its indications, contraindications, dosage and administration, drug interactions, storage and handling requirements; (iii) Provide scientific and technical advice and guidance in response to detailed technical and scientific questions; (i) Provide up-to-date information on product holds or suspensions and on product recalls and withdrawals; (ii) Provide technical advice concerning the continued ability to use vaccine which has experienced a cold chain excursion; (iii) Provide order status including real-time tracking of shipments; and (iv) Log reports received on adverse events following immunization. (c) For general enquiries, such as those regarding order tracking and status, product information, storage and administration, etc., a response must be communicated to the Requestor [***]. Enquiries of a scientific or technical nature that require more detailed review and investigation by Novavax may take longer; however, Novavax must inform the Requestor of the steps being taken to respond to the enquiry and of the expected timeline for a complete response. (d) Where requested, responses should be provided in writing. (e) Novavax must provide the Customer with an emergency contact and telephone number for any urgent enquiries that occur outside the regular business hours listed above, such as for urgent scientific or technical information or to report an adverse event. Novavax representative must call-back the Requestor within [***]. 6. Logistics Support Novavax must, as a minimum: (a) Provide to the Contracting Authority and the Technical Authority advance notice of production and delivery schedules (e.g. expected availability, timely updates on status of announced schedules, immediate notification of interruptions or delays in production and delivery schedules, etc.); and (b) Initiate and coordinate product holds, recalls or withdrawals, if necessary including coordinating trace back through the automated identification of vaccine products (AIVP);

91837512_5 provide clear and concise instructions on the activities necessary to implement the hold, recall or withdrawal; and provide regular updates on the status of same; 7. Support in the Management of Cold Chain Excursions As a minimum, Novavax must: (a) provide all published information on the stability of the product including continued stability if subject to temperature fluctuations (outside of recommended storage conditions); (b) clearly state all of the information required on a specific cold chain excursion to allow Novavax to properly assess the excursion; (c) provide clear and consistent written responses on the question of continued use of vaccine following an excursion; and (d) provide a written assessment on the viability of continued use of vaccine following an excursion within [***] of receipt of a request for assistance. 8. Technical Information and Data (a) During the Term, Novavax will upon written request, make available to the Technical Authority, who may in confidence make copies and take extracts therefrom, those reports, communications, etc. received from Health Canada which are exchanged for purposes of Regulatory Approval of the Vaccine. If requested, Novavax will make available to the Technical Authority on a confidential basis, where available, data and technical information concerning the Product being delivered under the Agreement, including such information as: (i) Clinical trial data – Canadian and International; (ii) Product safety data – Canadian and International; and (iii) Product quality and stability data. The Parties shall mutually agree on the scope of such data and information exchange in advance, taking into consideration the Technical Authority’s need to understand the clinical, safety, quality and stability profile of the Product and Novavax’ desire to provide such data and information in a manner that minimizes administrative burden and disruption to its operations. (b) Unless specifically prohibited by Novavax in writing, the information and data provided will be shared, on a confidential and need to know basis, within the Public Health Agency of Canada and Health Canada, as well as with Provincial and Territorial public health officials and experts in the field who provide public health advice to these officials, including but not limited to the National Advisory Committee on Immunization (NACI), to inform recommendations regarding the use of vaccine. Any information or data shared

91837512_5 in this manner will contain the notices provided by Novavax concerning the confidential or proprietary nature of the material submitted and the restrictions with respect to publication or further dissemination. If Customer wishes to present some aspect of the information or data in a scientific forum it will obtain the prior permission of Novavax in writing. 9. Timely Lot Release, Novavax’s Responsibility Novavax must submit all vaccine lots to Health Canada and such other information as required by Health Canada so as to ensure that the release of lots by Health Canada will occur early enough to allow Novavax to meet the delivery requirements of the Agreement. 10. Novavax Reporting on Production and Delivery Novavax must provide both the Contracting Authority and the Technical Authority with [***] written updates on the status of production and delivery. The Parties will cooperate to determine what details the Contracting Authority requires and what details Novavax has the capability on which to report. The information provided must be sufficiently detailed so as to allow Customer time to plan for vaccine availability. Without limiting the generality of the foregoing, Novavax will make commercially reasonable efforts to provide information on: (i) [***]; (ii) [***]; (iii) [***]; (iv) [***]; and (v) [***]. Where multiple lot numbers apply, all applicable lot numbers should be provided.

