LICENSE AGREEMENT BY AND BETWEEN CAMURUS AB AND BRAEBURN PHARMACEUTICALS BVBA SPRL NOVEMBER 14, 2014
EXHIBIT 10.10
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXECUTION COPY
BY AND BETWEEN
CAMURUS AB
AND
BRAEBURN PHARMACEUTICALS BVBA SPRL
NOVEMBER 14, 2014
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
TABLE OF CONTENTS
|
|
|
Page | |
|
1. |
DEFINITIONS |
1 | |
|
2. |
LICENSE GRANT TO BRAEBURN |
11 | |
|
|
2.1 |
License Grant |
11 |
|
|
2.2 |
Subcontracting |
11 |
|
|
2.3 |
Sublicenses |
11 |
|
|
2.4 |
Right of First Negotiation |
12 |
|
|
2.5 |
Option |
12 |
|
|
2.6 |
Co-Promotion |
13 |
|
|
2.7 |
Xxxxxxx Xxxxxxxxx |
00 |
|
|
2.8 |
Exclusivity |
13 |
|
|
2.9 |
Grant Back to Camurus |
14 |
|
3. |
DEVELOPMENT OF PRODUCT |
14 | |
|
|
3.1 |
Braeburn Development Responsibilities and Diligence |
14 |
|
|
3.2 |
Camurus Services |
14 |
|
|
3.3 |
Development of Products for Pain Indications |
15 |
|
|
3.4 |
Joint Steering Committee |
15 |
|
|
3.5 |
Composition of the JSC: Meetings of the JSC |
16 |
|
|
3.6 |
Coordination of Clinical Development |
17 |
|
|
3.7 |
Regulatory Filings and Approvals in the Licensed Territory |
17 |
|
|
3.8 |
Failure to file NDA Application |
18 |
|
|
3.9 |
Development Data |
18 |
|
|
3.10 |
Conduct of Development Activities |
18 |
|
|
3.11 |
Reporting Adverse Events |
19 |
|
4. |
COMMERCIALIZATION |
20 | |
|
|
4.1 |
Responsibility |
20 |
|
|
4.2 |
Launch Efforts |
20 |
|
|
4.3 |
Marketing Efforts |
20 |
|
|
4.4 |
Post Registration Studies; Publications |
20 |
|
|
4.5 |
Advertising and Promotion |
21 |
|
|
4.6 |
Commercialization Plan |
21 |
|
5. |
PAYMENT OBLIGATIONS |
21 | |
|
|
5.1 |
Signing Fee |
21 |
|
|
5.2 |
Reimbursement of Phase III Preparation Costs |
21 |
|
|
5.3 |
Development Milestone Payments |
21 |
|
|
5.4 |
Royalties |
22 |
|
|
5.5 |
Generic Product |
22 |
|
|
5.6 |
Sales Milestone Payments |
22 |
|
|
5.7 |
Royalty and Milestone Reports |
23 |
|
|
5.8 |
Payments |
23 |
|
|
5.9 |
Books and Records; Audit Rights |
23 |
|
6. |
MANUFACTURE |
24 | |
|
|
6.1 |
Identification of CMO |
24 |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
|
6.2 |
Technology Transfer |
24 |
|
|
6.3 |
Braeburn Supply Agreement |
25 |
|
|
6.4 |
Change in Product Specification and Manufacturing Process |
25 |
|
7. |
INTELLECTUAL PROPERTY |
25 | |
|
|
7.1 |
Trademarks |
25 |
|
|
7.2 |
Ownership of Collaboration Inventions |
26 |
|
|
7.3 |
Prosecution of Patents |
27 |
|
|
7.4 |
Camurus Platform IP and Camurus Product IP |
30 |
|
|
7.5 |
Assignment of IP |
30 |
|
|
7.6 |
Patent Term Extensions |
31 |
|
|
7.7 |
Third Party Intellectual Property |
31 |
|
|
7.8 |
Infringement |
31 |
|
|
7.9 |
Xxxxx-Xxxxxx Certifications |
33 |
|
8. |
CONFIDENTIALITY |
34 | |
|
9. |
REPRESENTATIONS, WARRANTIES AND COVENANTS |
36 | |
|
|
9.1 |
Mutual Representations and Warranties of Camurus and Braeburn |
36 |
|
|
9.2 |
Additional Representations and Warranties of Camurus |
37 |
|
|
9.3 |
Camurus Acknowledgement |
38 |
|
|
9.4 |
Additional Representations and Warranties of Braeburn |
38 |
|
|
9.5 |
Disclaimer of Warranties |
38 |
|
|
9.6 |
Mutual Covenants |
38 |
|
|
9.7 |
Camurus Covenant |
38 |
|
10. |
INDEMNIFICATION |
39 | |
|
|
10.1 |
Indemnification by Braeburn |
39 |
|
|
10.2 |
Indemnification by Camurus |
39 |
|
|
10.3 |
Notification of Liabilities/Losses |
39 |
|
|
10.4 |
Right to Participate in Defense |
40 |
|
|
10.5 |
Cooperation |
40 |
|
|
10.6 |
Exclusive Remedy |
40 |
|
|
10.7 |
Insurance |
40 |
|
11. |
TERM AND TERMINATION |
41 | |
|
|
11.1 |
Term of Agreement |
41 |
|
|
11.2 |
Braeburn Termination for Convenience |
41 |
|
|
11.3 |
Termination for Material Breach or Bankruptcy |
41 |
|
|
11.4 |
Effect of Termination |
42 |
|
|
11.5 |
Accrued Rights |
45 |
|
|
11.6 |
Surviving Provisions |
45 |
|
12. |
MISCELLANEOUS PROVISION’S |
45 | |
|
|
12.1 |
Consequential Damages |
45 |
|
|
12.2 |
Assignment |
45 |
|
|
12.3 |
Further Actions |
45 |
|
|
12.4 |
Compliance with Laws |
45 |
|
|
12.5 |
Force Majeure |
46 |
|
|
12.6 |
Notices |
46 |
|
|
12.7 |
Amendment |
47 |
|
|
12.8 |
Waiver |
47 |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
|
12.9 |
Counterparts |
47 |
|
|
12.10 |
Descriptive Headings |
47 |
|
|
12.11 |
Severability |
47 |
|
|
12.12 |
Entire Agreement |
47 |
|
|
12.13 |
Governing Law |
47 |
|
|
12.14 |
Dispute Resolution |
48 |
|
|
12.15 |
Independent Contractors |
48 |
EXHIBITS
|
Exhibit 1.16 |
|
Camurus Platform IP |
|
Exhibit 1.17 |
|
Camurus Product IP |
|
Exhibit 1.19 |
|
Camurus Trademarks |
|
Exhibit 2.6 |
|
Co-Promotion Terms |
|
Exhibit 3.1 |
|
CAM2038 Development Plan |
|
Exhibit 4.6 |
|
Commercialization Plan |
|
Exhibit 7.2(b) |
|
Expert’s Determination |
|
Exhibit 8.5 |
|
Press Release |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
This License Agreement is made as of the 14th day of November, 2014 (the “Effective Date”) between Camurus AB, a limited liability company organized and existing under the laws of Sweden and having its principal place of business at Ideon Science Park, Xxxxxxxxxx 00, XX-000 00 Xxxx, Xxxxxx (“Camurus”) and Braeburn Pharmaceuticals BVBA SPRL, a private limited company organized and existing under the laws of Belgium and having its principal place of business at Xxxxxxxxxx 000 X, 0000 Xxxxxxxx, Xxxxxxx (“Braeburn”) (each a “Party” and collectively, the “Parties”).
WITNESSETH
WHEREAS, Camurus is the owner of all right, title and interest in certain patents and know-how relating to its proprietary products for treatment of opioid dependence and pain referred to as CAM2038 and CAM2048, respectively, and Braeburn desires to further develop and commercialize CAM2038 and CAM2048 in the Licensed Territory (as defined below);
WHEREAS, Braeburn has capabilities in the development, manufacture, promotion, marketing, sales and life cycle management of pharmaceutical products in the field of treatment of opioid dependence and pain; and
WHEREAS, Camurus is willing to grant certain exclusive rights to Braeburn in respect of Products (as defined below) and related matters, and Braeburn is willing to accept exclusive rights in respect of Products and related matters, upon the terms and conditions hereinafter set forth.
NOW, THEREFORE, in consideration of the covenants and obligations expressed herein, and intending to be legally bound, the Parties agree as follows:
1. DEFINITIONS
1.1 “Adverse Events” shall have the meaning set out in Section 3.11.
1.2 “Affiliate” means, with respect to a Party, any entity or person that controls, is controlled by, or is under common control with that Party. For the purpose of this definition, “control” or “controlled” means, direct or indirect, ownership of 50% or more of the shares of stock entitled to vote for the election of directors in the case of a corporation or 50% or more of the equity interest in the case of any other type of legal entity; status as a general partner in any partnership; or any other arrangement whereby the entity or person controls or has the right to control the board of directors or equivalent governing body of a corporation or other entity or the ability to cause the direction of the management or policies of a corporation or other entity. The Parties acknowledge that in the case of entities organized under the laws of certain countries where the maximum percentage ownership permitted by law for a foreign investor is less than 50%, such lower percentage shall be substituted in the preceding sentence, provided that such foreign investor has the power to direct the management and policies of such entity. Notwithstanding the foregoing, the following entities shall not be considered Affiliates of Braeburn: Apple Tree Partners IV, L.P., Apple Tree Partners III, L.P. and their portfolio companies.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.3 “Braeburn” shall have the meaning set out in the Preamble.
1.4 “Braeburn Indemnified Party” shall have the meaning set out in Section 10.2.
1.5 “Braeburn IP” means the Braeburn Product IP; and Braeburn’s interest in any Joint IP.
1.6 “Braeburn Post-Registration Studies” shall have the meaning set out in Section 4.4.
1.7 “Braeburn Product IP” means (a) all Patent Rights; and (b) all other Intellectual Property Controlled by Braeburn or any of its Affiliates as of the Effective Date or during the Term hereof (whether as a result of activities under this Agreement or otherwise) that is necessary or useful to develop, make or have made, use, sell, offer for sale, import, market and promote the Products.
1.8 “Braeburn Supply Agreement” means the supply agreement between Braeburn and CMO governing the supply of the Products to Braeburn by CMO for noncommercial use and commercial sale in the Licensed Territory and, subject to Section 6.3, the Camurus Territory.
1.9 “Business Day” means a day on which banking institutions in New York, New York, United States and Malmo, Sweden are open for business.
1.10 “Calendar Year” means a period of 12 consecutive months beginning on January 1 and ending on December 31.
1.11 “CAM2038” means the products being developed by Camurus that comprise buprenorphine formulated with the FC Technology for opioid dependence indications. Current CAM2038 products are administered once-weekly (q4w) and once-monthly (q4w).
1.12 “CAM2048” means the products being developed by Camurus that comprise buprenorphine formulated with the FC Technology for pain indications. Current CAM2048 products are administered once-weekly (q4w).
1.13 “Camurus” shall have the meaning set out in the Preamble.
1.14 “Camurus Indemnified Party” shall have the meaning set out in Section 10.1.
1.15 “Camurus IP” means the Camurus Platform IP; the Camurus Product IP; and Camurus’ interest in any Joint IP.
1.16 “Camurus Platform IP” means (a) all Patent Rights listed in Exhibit 1.16, and (b) all other Intellectual Property, other than the Camurus Product IP, Controlled by Camurus or any of its Affiliates as of the Effective Date and during the Term hereof (whether as a result of activities under this Agreement or otherwise) that covers or claims the FC Technology and/or Camurus’ other proprietary formulations for injection all having an effective extended release duration of more than 24 hours and that is necessary or useful to develop, make or have made, use, sell, offer for sale, import, market and promote the Products.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.17 “Camurus Product IP” means (a) all Patent Rights listed in Exhibit 1.17, and (b) all other Intellectual Property Controlled by Camurus or any of its Affiliates as of the Effective Date and during the Term hereof (whether as a result of activities under this Agreement or otherwise), that relate solely to the Products and that is necessary or useful to develop, make or have made, use, sell, offer for sale, import, market and promote the Products.
1.18 “Camurus Territory” means all countries of the world excluding the countries of the Licensed Territory, including all their territories and possessions.
1.19 “Camurus Trademarks” means any Trademarks Controlled by Camurus, including FluidCrystal® and other Trademarks described in Exhibit 1.19, that relate to the FC Technology.
1.20 “Clinical Trials” means human clinical trials conducted on healthy volunteers or patients to provide data supporting Regulatory Approval of such drug or label expansion of such drug.
1.21 “CMO” means one or more Third Party contract manufacturing organization(s) that may be used to source ingredients, components, packaging materials and the like and to manufacture, package, label and quality release Braeburn’s requirements for Products for use and/or sale in the Territory, all pursuant to the Braeburn Supply Agreement.
1.22 “Collaboration Inventions” means all Intellectual Property conceived and reduced to practice by a Party or any of its Affiliates, or by a Third Party on behalf of such Party or any of its Affiliates, in the course of performing its obligations under this Agreement.
1.23 “Commercialization Plan” shall have the meaning set out in Section 4.6.
1.24 “Commercially Reasonable Efforts” means, [***].
1.25 “Competing Product” shall have the meaning set out in Section 2.8.
1.26 “Confidential Information” means the following, subject to the exceptions set forth in Section 8.1:
(a) the terms and conditions of this Agreement, for which each Party will be considered a Disclosing Party and a Recipient Party;
(b) Know-How within Camurus IP for which Camurus will be considered the Disclosing Party and Braeburn shall be the Recipient;
(c) Know-How within Braeburn IP for which Braeburn will be considered the Disclosing Party and Camurus shall be the Recipient; and
(d) any other non-public information, whether or not patentable, disclosed or provided by one Party to the other Party in connection with this Agreement, including, without limitation, information regarding such Party’s strategy, business plans, objectives, research, technology, products, intellectual property strategy, business affairs or finances, including information of the type that is customarily considered to be confidential information by parties
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
engaged in activities that are substantially similar to the activities being engaged in by the Parties under this Agreement, for which the Party making such disclosure will be considered the Disclosing Party and the receiver will be the Recipient.
1.27 “Control” or “Controlled” means possession by a Party of the right to grant to the other Party a license, sublicense or other right to use, of the scope provided for in this Agreement, to Intellectual Property and rights to access or cross-reference regulatory filings without violating the terms of any agreement or other arrangement with any Third Party.
1.28 “Development Data” means all chemistry, manufacturing and control, pre-clinical and clinical data, including, without limitation, pharmacological, pharmacokinetic, pharmaceutical development and toxicological data, relating to the Products, that is generated at any time during the Term of this Agreement by or for either Party or their Affiliates, licensees or sublicensees.
1.29 “Development Plan” and “Development Plans” shall have the meanings set forth in Section 3.1.
1.30 “Disclosing Part” means the Party which discloses Confidential Information to the other Party.
1.31 “Effective Date” shall have the meaning set out in the Preamble.
1.32 “EMA” means the European Medicines Agency for the Evaluation of Medicinal Products of European Union and/or the Committee for Human Medicinal Products, or any successor agency thereof or, to the extent the mutual recognition or decentralized procedure is used for a Product in the EU, any national governmental authority having the authority to regulate the sale of medicinal or pharmaceutical products in any country in the EU.
1.33 “EU” means the following member states of the European Union, including their territories and possessions: Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, and United Kingdom.
1.34 “FC Technology” means Camurus’ proprietary formulation technology that is referred to as FluidCrystal* injection depot technology, comprising a lipid based injectable liquid solution that, within minutes after injection, forms a controlled release liquid crystal gel matrix in situ on contact with body fluids at the site of injection.
1.35 “Financing Commitment” shall have the meaning set out in Section 9.4(a).
1.36 “First Commercial Sale” means the date on which a Product is first sold following Regulatory Approval in any country in the Licensed Territory by Braeburn or any of its Affiliates or Sublicensees to a Third Party (other than sales by Braeburn to its Affiliates) in a commercial arm’s length transaction.
1.37 “FTE” means [***].
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.38 “FTE Costs” means the cost of FTEs at the FTE Rate.
1.39 “FTE Rate” means the price of a single FTE per Calendar Year. The FTE Rate shall be [***] for senior scientists and [***] for other staff at the Effective Date. The FTE Rate reflects the fully burdened internal costs of an FTE including all employee-related compensation, including but not limited to, salaries, wages, bonuses, benefits, profit sharing, share option grants, and any other employment costs, including travel and associated subsistence costs (but excluding travel and subsistence costs incurred in any travel required hereunder) and professional dues and allocable overhead. On [***], the FTE Rate will be adjusted by the percentage change in inflation as measured by the Swedish Consumer Price Index published by the Statistiska Centralbyrån of Sweden on each 1 January of each Calendar Year. Camurus shall provide the JSC with the revised FTE Rate by [***] of each Calendar Year.
1.40 “GCP” means Good Clinical Practices, as set forth in the ICH Harmonized Guidance on Good Clinical Practice (CPMP/ICH/135/95) and the equivalent requirements and/or applicable guidance in any other jurisdiction in the Territory.
1.41 “Generic Product” means a product approved under an Abbreviated New Drug Application, or AND A, or any non-United States equivalent filing, with the Product as the reference product, that is “therapeutically equivalent” as evidenced by the assignment of any ‘A’ level therapeutic equivalence rating by the FDA, or any non-United States equivalent rating, such that the product that is therapeutically equivalent to the Product, or otherwise is generally substitutable by the pharmacist for the Product when filling a prescription written for the Product without having to seek authorization to do so from the physician writing such prescription.
1.42 “GMP” means Good Manufacturing Practices, as set forth in the Rules Governing Medicinal Products in the European Union volume 4 and the equivalent requirements and/or applicable guidance in any other jurisdiction in the Territory.
1.43 “IND” means an Investigational New Drug application (together with all subsequent submissions, supplements and amendments thereto, and any materials, documents or information referred to or relied upon thereby) filed with the FDA pursuant to Part 312 of Title 21 of the U.S. Code of Federal Regulations prior to beginning Clinical Trials in the United States or any comparable application filed with any Regulatory Authority outside the United States.
1.44 “Infringing Activity” shall have the meaning set out in Section 7.8(a).
1.45 “Intellectual Property” or “IP” means any Patent Rights, Trademarks, Know-How, Confidential Information, and any other intellectual property rights.
1.46 “Joint Invention” shall have the meaning set out in Section 7.2(b).
1.47 “Joint IP” means any Joint Invention and any Patent Rights claiming any Joint Invention.
1.48 “Joint Patents” shall have the meaning set out in Section 7.3(e).
1.49 “JSC” means the Joint Steering Committee referred to in Section 3.4.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.50 “Know-How” means technical and other information which is not in the public domain, including information comprising or relating to concepts, trade secrets, data, designs, discoveries, formulae, ideas, inventions, materials, methods, models, research plans, procedures, designs for experiments and tests and results of experimentation and testing (including results of research or development), processes (including manufacturing processes, specifications and techniques), laboratory records, chemical, pharmacological, toxicological, clinical, analytical and quality control data, clinical and non-clinical trial data, case report forms, data analyses, reports, manufacturing data or summaries and information contained in submissions to and information from ethical committees and regulatory authorities. Know-How includes documents containing Know-How, including any rights including trade secrets, copyright, database or design rights protecting such Know-How. The fact that an item is known to the public shall not be taken to preclude the possibility that a compilation including the item, and/or a development relating to the item, is not known to the public,
1.51 “Licensed Field” means any and all uses including, but not limited to, the treatment, prevention or diagnosis of any disease, disorder or condition.
1.52 “Licensed Territory” means the United States, Canada and Mexico, including all their territories and possessions.
1.53 “Losses” shall have the meaning set out in Section 10.1.
1.54 “Manufacturing Costs” means all of a Party’s costs and expenses, including with respect to Camurus FTE Costs, for the preparation, project management process development, scale-up, formulation, making and/or production of Products, including inspections, quality testing, stability studies, primary and secondary packaging and material, labeling, release and transportation of Products, including cost of procurement from a Third Party when a Party sources Products or services from a Third Party. A Party’s capital expenditures and costs not exclusively related to the Products shall not be deemed to be Manufacturing Costs, unless mutually agreed, but such expenditures and costs shall be deemed Manufacturing Costs when charged by a Third Party from which Products or services are sourced.
1.55 NDA” means a New Drug Application (together with all subsequent submissions, supplements and amendments thereto, and any materials, documents, documents or information referred to or relied upon thereby) filed with the FDA to obtain approval for commercial sale or use of the Product as a pharmaceutical or medicinal product in any formulation or dosage form (excluding any pricing and reimbursement approvals), or any comparable application filed with any Regulatory Authority outside the United States.
1.56 “NDA Approval” means approval of an NDA by the FDA or other applicable Regulatory Authority.
1.57 “Net Sales” means [***]
All such deductions shall be fairly and equitably allocated to the Products and other products or services of Braeburn, its Affiliates and Sublicensees, such that the Products do not bear a disproportionate portion of such deductions. The transfer of Products by Braeburn to an Affiliate
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
shall not be deemed a sale except where such Affiliate is an end user. Net Sales shall be calculated in accordance with generally accepted accounting principles in the United States (US GAAP), consistently applied.
1.58 “Non-Compete Period” shall have the meaning set out in Section 2.8.
1.59 “Notice of Exercise” means a written notice from Braeburn to Camurus that Braeburn intends to exercise its rights under Section 2.4, 2.5, 2.6 or 2.8, as further set forth in the Notice of Exercise.
1.60 “Option” shall have the meaning set out in Section 2.5.
1.61 “Option Term’’ means, on a Product-by-Product basis, the period beginning on the date of the NDA Approval for that Product in the United States and ending 3 months thereafter,
1.62 “Pain Trial” shall have the meaning set out in Section 3.3.
1.63 “Party” and “Parties” shall have the meanings set out in the Preamble.
1.64 “Patent Right” means (a) all national, regional and international patents and patent applications, including provisional patent applications; (b) all patent applications filed either from such patents, patent applications or provisional applications or from an application claiming priority from any of these, including utility applications, divisionals, continuations, continuations-in-part, provisionals, converted provisionals, and continued prosecution applications, and reissue applications; (c) any and all patents that have issued or in the future issue from the patent applications described in (a) and (b) above, including author certificates, inventor certificates, utility models, xxxxx patents and design patents and certificates of invention; (d) any and all extensions or restorations by existing or future extension or restoration mechanisms, including revalidations, reissues, reexaminations and extensions (including any supplementary protection certificates and the like) of the foregoing patents or patent applications described in (a), (b) and (c) above; and (e) any similar rights, including so-called pipeline protection (where the subject matter previously disclosed was not previously patentable in a particular jurisdiction but subsequently becomes patentable subject matter in such jurisdiction).
1.65 “Payment Report” shall have the meaning set out in Section 5.7.
1.66 “Phase I Clinical Trial” means a human clinical trial of a compound or product, the principal purpose of which is a determination of safety over a range of doses, as more fully defined in 21 C.F.R. §312.21(a), or its successor regulation, or the equivalent in any foreign country.
1.67 “Phase II Clinical Trial” means a human clinical trial of a compound or product for an indication, the principal purpose of which is a determination of safety and efficacy for such indication in a target patient population over a range of doses, as more fully defined in 21 C.F.R. §312.21(b), or its successor regulation, or the equivalent in any foreign country.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.68 “Phase III Clinical Trial” means a human clinical trial of a compound or product for an indication on a sufficient number of subjects that is designed to establish that the compound or product is safe and efficacious for its intended use, and to determine warnings, precautions, and adverse reactions that are associated with the compound or product in the dosage range to be prescribed, and to support Regulatory Approval of the compound or product for such indication, as more fully defined in 21 C.F.R. §312.21 (c), or its successor regulation, or the equivalent in any foreign country.
1.69 “Phase 111 Preparation Costs” means [***].
1.70 “Phase IV Clinical Trial” means a human clinical trial of a Product to acquire additional information about the Product’s risks, benefits and optimal use, commenced after receipt of Regulatory Approval for the Product in the indication for which the trial is being conducted.
1.71 “Product(s)” means a pharmaceutical product that is a sustained release injectable formulation of buprenorphine (in any form or formulation, including any pharmaceutically acceptable salts, esters, solvates, hydrates, polymorphs, crystal forms, prodrugs and tautomers) as sole active pharmaceutical ingredient claimed by or incorporating any Camurus IP, including but not limited to CAM2038 and CAM2048. Formulations for injection of buprenorphine Controlled by a Third Party acquirer (by way of merger, acquisition or otherwise) of Camurus prior to such acquisition are excluded from this definition.
1.72 “Product Trademarks” means any Trademarks Controlled by Braeburn or any of its Affiliates during the Term hereof used to market the Product in the Licensed Territory, excluding any Camurus Trademarks.
1.73 “Proposed Disclosure” shall have the meaning set out in Section 8.6.
1.74 “Prosecute” or “Prosecuting” means with regard to specified Patent Rights, preparing, filing, prosecuting, validating, maintaining and defending such Patent Rights, including with respect to any re-examination, reissue, revocation, interference or opposition proceedings including any appeal therefrom. For the avoidance of doubt, “Prosecuting” excludes any infringement suits or other legal proceedings to enforce the specified Patent Rights, regardless of whether or not such proceedings involve the defense of the Patent Rights in suit.
1.75 “Recipient” means the Party which receives Confidential Information from the other Party.
1.76 “Regulatory Approvals” means any NDA Approvals and other approvals, licenses, registrations, or authorizations granted or issued by any Regulatory Authority necessary for the manufacture, packaging, labeling, use, storage, transport, export, import, clinical testing, promotion or sale of the Product in a country, including pricing and reimbursement approvals to the extent the applicable Regulatory Authority in such country requires a pricing or reimbursement approval prior to commercialization of a Product in such country.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.77 “Regulatory Authority” means any national, supranational, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, including the United States Food and Drug Administration (“FDA”) or any foreign equivalent thereof, in any country involved in the granting or receipt as the case may be of INDs, NDAs or Regulatory Approvals.
1.78 “ROFN Countries” shall have the meaning set out in Section 2.4.
1.79 “ROFR Product” shall have the meaning set out in Section 2.8.
1.80 “Royalties” shall have the meaning set out in Section 5.4.
1.81 “Royalty Term” shall have the meaning set out in Section 5.4.
1.82 “Sublicensee” shall have the meaning set out in Section 2.3.
1.83 “Term” shall have the meaning set out in Section 11.1.
1.84 “Territory” means the Camurus Territory and the Licensed Territory.
1.85 “Third Party” means any entity other than Camurus or Braeburn or their respective Affiliates.
1.86 “Trademarks” means registered trademarks and applications therefor, unregistered trade or service marks and company names in each case with any and all associated goodwill and all rights or forms of protection of a similar or analogous nature including rights which protect goodwill whether arising or granted under the laws of any jurisdiction and, for purposes of this definition, trade dress.
1.87 “United States” or “US” means the United States of America and its territories and possessions.
1.88 “Valid Claim” means a claim of (a) an issued and unexpired patent within the Camurus IP, or (b) a patent application within the Camurus IP that has been pending approval for no more than [***], which claim (in each case, as applicable) has not been revoked or held unenforceable, unpatentable or invalid by a decision of a court or other governmental agency of competent jurisdiction, which is not appealable or has not been appealed within the time allowed for appeal, and which has not been cancelled, withdrawn from consideration, determined to be unallowable, abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination or disclaimer; provided, however, that if the holding of such court or agency is later reversed by a court or agency with overriding authority, the claim shall be reinstated as a Valid Claim with respect to Net Sales made after the date of such reversal; provided, further, however, on a country-by-country basis, a claim of a non-provisional patent application pending for more than [***], counted from the first to occur of (i) the date of national filing of such patent application or, if such patent application is a continuation, continuation-in-part or divisional patent application (each, a “Continuation”), the date of national filing of the parent patent application of a Continuation or (ii) if such patent application entered the national phase pursuant to a PCT application, the filing date of such PCT application, shall not be considered to be a Valid Claim unless and until a patent with
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
respect to such application issues with such claim or is accepted for grant with such a claim, in which case such claim will be reinstated and be deemed to be a Valid Claim, but only as of the date of issuance of such patent or the date of indication of acceptance of such a claim if earlier.
1.89 Interpretation:
(a) Whenever any provision of this Agreement uses the term “including” (or “includes”), such term shall be deemed to mean “including without limitation” and “including but not limited to” (or “includes without limitations” and “includes but is not limited to”) regardless of whether the words “without limitation” or “but not limited to” actually follow the term “including” (or “includes”);
(b) “Herein”, “hereby”, “hereunder”, “hereof and other equivalent words shall refer to this Agreement in its entirety and not solely to the particular portion of this Agreement in which any such word is used;
(c) All definitions set forth herein shall be deemed applicable whether the words defined are used herein in the singular or the plural;
(d) Wherever used herein, any pronoun or pronouns shall be deemed to include both the singular and plural and to cover all genders;
(e) The recitals set forth at the start of this Agreement, along with the Exhibits to this Agreement, and the terms and conditions incorporated in such recitals and Exhibits shall be deemed integral parts of this Agreement and all references in this Agreement to this Agreement shall encompass such recitals, Exhibits and the terms and conditions incorporated in such recitals and Exhibits; provided, that in the event of any conflict between the terms and conditions of this Agreement and any terms and conditions set forth in the Exhibits, the terms of this Agreement shall control;
(f) In the event of any conflict between the terms and conditions of this Agreement and any terms and conditions that may be set forth on any order, invoice, verbal agreement or otherwise, the terms and conditions of this Agreement shall govern;
(g) The Agreement shall be construed as if both Parties drafted it jointly, and shall not be construed against either Party as principal drafter;
(h) Unless otherwise provided, all references to Sections and Exhibits in this Agreement are to Sections and Exhibits of and to this Agreement;
(i) Unless otherwise provided, all references to days, months, quarters or years are references to calendar days, calendar months, calendar quarters or calendar years;
(j) Any reference to any federal, national, state, local or foreign statute or law shall be deemed to also refer to all rules and regulations promulgated thereunder, unless the context requires otherwise; and
(k) Wherever used, the word “shall” and the word “will” are each understood to be imperative or mandatory in nature and are interchangeable with one another.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
2. LICENSE GRANT TO BRAEBURN
2.1 License Grant. Camurus hereby grants to Braeburn, and Braeburn hereby accepts, the exclusive (even as to Camurus), royalty-bearing license, sublicenseable (subject to Section 2.3), under the Camurus IP to use, develop, make or have made, sell, offer for sale, import, market and promote Products in the Licensed Field in the Licensed Territory. Notwithstanding the foregoing, (a) each of Braeburn and its licensees (subject to Section 2.3) and Camurus and Camurus’ licensees may make and have made the Products anywhere in the world for development, use, sale or offering to sell the Products in their respective Territories, and (b) Braeburn may, with the prior consent of Camurus, such consent not to be unreasonably withheld, conditioned or delayed, conduct Clinical Trials for a Product at sites located in the Camurus Territory provided that such Clinical Trials are part of a Clinical Trial program for a Product initiated for the purpose of applying for Regulatory Approval in the Licensed Territory. Camurus acknowledges and agrees that due to the exclusive nature of the foregoing license. Camurus has no right under the Camurus IP to use, develop, make or have made, sell, offer for sale, import, market and promote in the Licensed Field in the Licensed Territory any pharmaceutical product that is a long acting (1-week or longer) injectable formulation of buprenorphine alone, or with one or more therapeutically active pharmaceutical ingredients.
2.2 Subcontracting. Notwithstanding Section 2.3 to the contrary, Braeburn shall have the right, without obtaining the written consent of Camurus, to subcontract its responsibilities under this Agreement (and grant any necessary sublicenses in connection therewith). The foregoing right shall include the right to engage contract sales organizations to supplement or complement Braeburn’s own sales force as well as to engage a Third Party to co-promote the Products in the Licensed Territory (provided that if the co-promotion partner books sales of the Products, then the co-promotion partner shall be deemed a Sublicensee covered by Section 2.3) and provided in each case that Braeburn retains control over strategic marketing and medical affairs decisions. Braeburn shall at all times be liable for all such activities as if such activities had been undertaken by Braeburn hereunder.
2.3 Sublicenses. Subject to Section 2.2, Braeburn may not grant sublicenses under the licenses granted under Section 2.1 without Camurus’ prior written consent, not to be unreasonably withheld, conditioned or delayed, except as follows.
(a) Braeburn may grant sublicenses to the Camurus IP as required to make and have made the Product;
(b) Braeburn may grant sublicenses or assign its rights to the Camurus IP to any of its Affiliates for so long as such entity remains an Affiliate of Braeburn; and
(c) On a country-by-country and Product-by-Product basis following the filing of an NDA for that Product in the United States, Braeburn may grant one sublicense to the Camurus IP to a Third Party (who may not grant further sublicenses) in each country in the Licensed Territory to use, develop, make and have made, sell, offer for sale, import, market and promote such Product in the Licensed Field without Camurus’ prior written approval; provided, that in each such case, (i) Braeburn shall be liable to Camurus as if Braeburn is exercising such sublicensed rights itself under this Agreement, (ii) the Sublicensee shall not be permitted to grant further sublicenses without Camurus’ prior written consent, such consent not to be
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
unreasonably withheld, conditioned or delayed, unless the Sublicensee is an Affiliate of Braeburn, in which case the Sublicensee may sublicense any portion of its rights to another Affiliate of Braeburn without Camurus’ consent for so long as such entity remains an Affiliate of Braeburn, and (iii) Braeburn shall ensure that each Sublicensee is subject to a written sublicense agreement requiring the Sublicensee to comply with confidentiality, indemnity, reporting, audit rights, access to data and information (including to obtain rights to access and copy Development Data and regulatory filings from Sublicensees) as well as diligence obligations at least equal to those set forth in this Agreement. Braeburn shall promptly provide notice to Camurus of any sublicense granted pursuant to this Section 2.3. Any person or entity that receives a sublicense or is otherwise granted the right to promote and sell the Product as permitted hereunder is a “Sublicensee.”
2.4 Right of First Negotiation. Camurus hereby grants to Braeburn a right of first negotiation to expand the license in Section 2.1 to include countries within the Camurus Territory that are outside the EU (the “ROFN Countries”) on the terms and conditions set forth in this Section 2.4.
If Camurus has received a bona fide offer or proposal from a Third Party encompassing key financial and commercial terms, or otherwise has agreed on such principal terms with a Third Party, in either case to commercialize a Product in one or more of the ROFN Countries, then prior to Camurus granting rights to such Third Party to commercialize a Product in one or more of the ROFN Countries, Camurus shall give Braeburn prompt written notice thereof. Braeburn shall have [***] after receipt of the notice to deliver a Notice of Exercise, covering all the ROFN Countries that are subject to the Third Party offer, to Camurus. Upon Camurus’ receipt of a Notice of Exercise, the Parties shall enter into good faith negotiations regarding an amendment to this Agreement on commercially reasonable terms to include in the Licensed Territory the ROFN Countries specified in the Notice of Exercise. If Braeburn has not delivered a Notice of Exercise within the [***] period, or the Parties are unable to reach agreement on an amendment to this Agreement within [***], then Braeburn shall have no further rights hereunder with respect to such ROFN Countries covered by the Third Party offer; provided, that if Camurus or one of its Affiliates do not execute a commercial agreement with a Third Party within [***] from the Notice of Exercise, then Braeburn’s rights with respect to the offered countries under this Section 2.4 shall reset.
2.5 Option. Camurus hereby grants Braeburn an exclusive option (the “Option”), which may be exercised during the Option Term, to include Japan, Taiwan, South Korea and China in the Licensed Territory. Braeburn shall have the right to exercise the Option on a country-by-country and Product-by-Product basis by delivering a Notice of Exercise to Camurus during the Option Term. The date when the Notice of Exercise is sent shall be the date of option exercise and the date when Braeburn’s rights in the countries set forth in the Notice of Exercise will take effect. The countries for which the Option was exercised shall become part of the Licensed Territory and no longer be considered part of the Camurus Territory and all applicable diligence, governance, milestone, royalty and other terms and conditions of this Agreement shall apply to those countries; provided, that Braeburn’s right to sublicense under Sections 2.3(a) and 2.3(c) shall, with respect to China, require the consent of Camurus, such consent not to be unreasonably withheld, conditioned or delayed. Within [***] after the Notice of Exercise, Braeburn shall submit to the JSC an updated Development Plan and Commercialization Plan including the countries for which the Option was exercised. With
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
respect to Japan it is anticipated that further Clinical Trials (in addition to the Phase III Clinical Trials covered by the Development Plan attached on the Effective Date) will be required to support a Japanese NDA application and, if so, Braeburn shall include in the applicable Development Plan such additional Clinical Trials required to support a Japanese NDA application.
2.6 Co-Promotion. Braeburn shall have the exclusive, nontransferable right for itself and its Affiliates, on a Product-by-Product basis, to co-promote the Product with Camurus, its Affiliates and licensees in the country of Belgium, pursuant to the terms of a separate agreement to be negotiated by the Parties, which agreement shall contain the terms set forth in Exhibit 2.6. Camurus shall provide Braeburn with written notice within [***] after Camurus or its Affiliate or licensee has filed the NDA for a Product in Belgium. Braeburn may exercise its right to co-promote by delivering a Notice of Exercise to Camurus no later than [***] after receipt of Camurus’ notice.
2.7 Camurus Territory. To the extent permitted under applicable law and except as otherwise permitted under this Agreement, Braeburn shall not directly or knowingly supply the Products to any Third Party located in any country in the Camurus Territory. The foregoing restrictions shall apply for the duration of the applicable Royalty Term.
2.8 Exclusivity. For a period beginning on the Effective Date and ending on the [***] of the First Commercial Sale of the first Product, on a country-by-country basis (the “Non-Compete Period”), Braeburn and Camurus and their respective Affiliates, as well as Braeburn’s Sublicensees (but with respect to Braeburn’s Sublicensees solely regarding the opioid dependence indication in the United States), shall not directly or indirectly promote, market, sell or have sold, including by means of a license to a Third Party, any other long acting (1-week or longer) injectable product containing buprenorphine as an active pharmaceutical ingredient for any indication (“Competing Product”), other than the Products, in the Licensed Territory. The foregoing restriction shall not prohibit Braeburn from researching, developing or commercializing a long-acting implantable (not injectable) buprenorphine product. Additionally, the foregoing restriction shall not apply to any Third Party that acquires an interest in a Party or the assets of a Party, in either case, otherwise sufficient for that Third Party to be deemed an “Affiliate” of such Party hereunder. In the event that Braeburn desires to promote, market, sell or have sold, including by means of license to a Third Party, a Competing Product in the Licensed Territory, it shall notify Camurus thereof in writing where after the Parties shall negotiate in good faith the terms and conditions of an agreement under which Braeburn may promote, market or sell such Competing Product, provided that neither Party shall have any obligation to enter into such an agreement.
If during the Non-Compete Period, Camurus has received a bona fide offer or proposal from a Third Party encompassing key financial and commercial terms, or otherwise has agreed on principal terms with a Third Party, in either case to develop or commercialize any pharmaceutical product for the indication of opioid addiction or pain that is a sustained release injectable formulation that does not contain buprenorphine as an active pharmaceutical ingredient and that is claimed by or incorporating any Camurus IP (the “ROFR Product”), then Camurus shall offer Braeburn a right of first refusal with respect to each such ROFR Product as follows: Prior to Camurus granting rights to such Third Party to develop and commercialize the ROFR Product in the Licensed Territory, Camurus shall give Braeburn prompt written notice
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
thereof. Braeburn shall have [***] after receipt of the notice to deliver a Notice of Exercise to Camurus. Upon Camurus’ receipt of a Notice of Exercise, the Parties shall enter into good faith negotiations for an exclusive license agreement on commercially reasonable terms. If Braeburn has not delivered a Notice of Exercise within the [***] period, or the Parties are unable to reach agreement on an exclusive license agreement within [***] from such Notice of Exercise, then Braeburn shall have no further rights hereunder with respect to such ROFR Product; provided, that if Camurus or one of its Affiliates do not execute a commercial agreement with a Third Party within [***] from the Notice of Exercise, then Braeburn’s rights with respect to the ROFR Product shall reset.
2.9 Grant Back to Camurus. Braeburn hereby grants to Camurus, and Camurus hereby accepts, an exclusive license, with the right to sublicense, to develop, make or have made, use, sell, offer for sale, market and promote under the Braeburn IP (but excluding Braeburn’s Development Data which is addressed in Section 3.9) Products in the Camurus Territory. During the Term and after the expiration of the Term, the foregoing licenses shall be fully paid-up, but if this Agreement is terminated and the foregoing licenses survive termination in accordance with Section 11.4(a), then the licenses shall be royalty-bearing in accordance with Section 11.4(a)(x).
3. DEVELOPMENT OF PRODUCT
3.1 Braeburn Development Responsibilities and Diligence. Braeburn shall have the responsibility to develop the Products within the scope of the rights granted to it hereunder at its own cost in accordance with the development plan and corresponding budget for each Product (each, a “Development Plan,” and collectively, the “Development Plans”). The initial Development Plan for CAM2038 is attached hereto as Exhibit 3.1. Braeburn shall exercise Commercially Reasonable Efforts to develop the Products in the Licensed Territory, including, without limitation, carrying out the development work within the timelines and as further provided in the Development Plans and in accordance with this Agreement. Notwithstanding anything to the contrary herein, the activities described as Key Elements (as defined in Section 3.5(d)) shall always be executed and shall accordingly not be subject to such Commercially Reasonable Efforts, provided that Braeburn may modify the timelines established in the Development Plans and applicable to the Key Elements as may be required due to guidance from a Regulatory Authority. However, Braeburn may assert that it is not required to perform one or more of the Key Elements to the extent that the activities are not permitted by applicable law, or require the approval of a Regulatory Authority that has not been granted or has been denied. Braeburn estimates that the budget for the development program for CAM2038 in the Development Plan is at least [***]. Braeburn shall keep the JSC regularly updated with respect to its efforts under the Development Plans. Braeburn shall provide to the JSC draft forms of protocols for Clinical Trials for the purpose of obtaining comments. Braeburn shall consider in good faith all comments provided by Camurus through the JSC within the [***] period following the JSC’s receipt of such protocols. Any Clinical Trials shall be conducted by Braeburn in accordance with GCP. Any proposed amendments to the Development Plans shall be addressed by the JSC in accordance with Sections 3.4 and 3.5.
3.2 Camurus Services. Camurus shall use Commercially Reasonable Efforts to perform the development activities specified in the initial Development Plan for CAM2038 and any other agreed development activities as shall be specified in an amendment to the
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Development Plan for CAM2038 or in Development Plans for other Products. Camurus shall at all times exercise Commercially Reasonable Efforts to perform its obligations under the Development Plans, including, without limitation, using Commercially Reasonable Efforts to carry out the development work within the timelines and as further provided in the Development Plans and in accordance with this Agreement. Braeburn shall reimburse Camurus for its costs and expenses, including FTE Costs, incurred in providing such services; provided, that Braeburn shall have no obligation to pay for any costs and expenses that exceed the budgeted costs and expenses in the Development Plan by more than [***] unless approved by Braeburn. Such costs and expenses incurred by Camurus may be invoiced to Braeburn on a monthly basis. Braeburn shall effect payment of all invoices to Camurus’ designated bank account within [***] after the date of Camurus’ invoice. Camurus shall together with such invoices provide reasonable available supporting documentation of such costs and expenses (including relevant Third Party invoices and a list of hours worked by Camurus and a summary of the work performed). Braeburn may withhold payment in respect of any Third Party costs that are in excess of [***] of the budgeted amount set forth in the Development Plan, if Braeburn disputes any amount set forth in an invoice and shall have the right to recoup or credit against future invoices any disputed amounts that, after final resolution, should not have been paid to Camurus. For clarity, Camurus shall not be obliged to carry out any activities that are not included within the Development Plan and the associated budget.
3.3 Development of Products for Pain Indications. Braeburn shall conduct a renew of the commercial potential of a Product for the treatment of pain in the Licensed Territory, such review to be completed and provided to the JSC within [***] after the Effective Date. As part of such review, Braeburn and Camurus shall develop a Development Plan to test the application of such Product for the treatment of pain through a Phase 1 Clinical Trial, a Phase II Clinical Trial or any other Clinical Trial that may be reasonably necessary in order to initiate a Phase III Clinical Trial (the “Pain Trial”). The budget for the Pain Trial shall in no event be less than [***], the IND for the Pain Trial shall be submitted no later than [***] after the Effective Date, and the first screening of a patient for participation in the Pain Trial shall occur no later than [***] after the later of the effectiveness of the IND for the Product for the treatment of pain and institutional review board/ethics committee approval. Braeburn shall be responsible for conducting, and shall bear the costs of, the Pain Trial. Any support provided by Camurus in connection with the Pain Trial shall be reimbursed as set forth in Section 3.2. Should the results of the Pain Trial warrant further clinical development work to explore the pain indication, then Braeburn shall propose to the JSC an updated Development Plan for such indication, which plan shall be deemed a Development Plan (as defined in Section 3.1).
3.4 Joint Steering Committee. Upon the Effective Date, the Parties shall appoint a Joint Steering Committee (the “JSC”) which shall be responsible for the ongoing oversight over the Parties’ activities under the Development Plans and Braeburn’s commercialization activities. The JSC shall be the primary forum for the exchange of information between the Parties and shall have the following responsibilities:
(a) monitoring the progress and results of Braeburn’s development and commercialization efforts and Camurus’ development efforts;
(b) recommending further development activities for the Products in the Licensed Territory, such as documentation of further indications;
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(c) reviewing, approving and amending the Development Plans and Commercialization Plan;
(d) agreeing on the strategy and procedure for filing of Joint Patents;
(e) discussing creation and coordination of a global branding strategy;
(f) discussing coordination of clinical development of Products in the Territory as provided in Section 3.6;
(g) monitoring the progress and results of Braeburn’s marketing, promotion and sales activities detailed in Section 4;
(h) monitoring the progress of Camurus’ technology transfer and the Parties’ manufacturing process development and scale-up activities in respect of the Product as detailed in Section 6; and
(i) discussing a publication strategy (such as abstract presentations at conferences, symposiums, etc.).
Notwithstanding the foregoing, the JSC shall not have the right to alter or amend this Agreement or either Party’s rights or obligations under this Agreement. For the avoidance of doubt, Braeburn shall have the sole right and responsibility to commercialize the Products in the Licensed Territory, subject to the terms of this Agreement.
3.5 Composition of the JSC: Meetings of the JSC.
(a) The JSC, which shall be chaired by Braeburn, shall consist of an equal number of representatives appointed by each of Camurus and Braeburn. One representative from each Party shall be a senior executive from such Party, and one representative shall be the project leader from such Party. Each Party shall have the right, at any time, to designate by written notice to the other Party, a sufficiently qualified replacement for any of such Party’s members on the JSC, including the chairperson. In addition, the JSC members may from time to time invite the participation of additional ad-hoc representatives from either Party on specific issues as the need arises.
(b) The JSC may establish project teams or subcommittees consisting of an equal number of representatives appointed by each of Camurus and Braeburn as the Parties may separately agree from time to time. The project teams or subcommittees may address any matters related to activities undertaken pursuant to this Agreement, including the exchange of information and Know-How in accordance with procedures instituted by the JSC. Such project teams or subcommittees shall report to the JSC.
(c) The JSC shall meet as necessary but in any event no less frequently than [***]. In lieu of in person meetings, meetings of the JSC may take place by telephonic or video conference. Minutes from the meetings shall be kept by the Chairman of the JSC and circulated to the members of the JSC within a reasonable time for comments and approval.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(d) Each Party shall bear its own costs, including travel and lodging for its personnel serving on the JSC or attending meetings of the JSC. All decisions by the JSC shall be made in agreement between all members being present. In order to form a quorum at least 1 member nominated by each Party shall be present at a meeting. If the members are unable to reach agreement on a matter within [***] from the date of the JSC meeting, then the disagreement shall be resolved as provided in Section 12.14(a); provided, that if the Parties’ CEOs are unable to reach agreement in respect of any matters relating to Braeburn’s Development Plan or the Commercialization Plan then, subject to Braeburn’s diligence obligations herein, and provided that the features of the Development Plan listed as “Key Elements” therein may not be amended without Camurus’ prior written approval, such approval not to be unreasonably withheld, conditioned or delayed, the opinion of the Chairman of the JSC shall prevail; provided, further, that in exercising such final say, the Chairman of the JSC may not amend the terms of this Agreement or materially increase the obligations of Camurus under this Agreement.
(e) Each Party shall submit to the members of the JSC [***] in advance of each JSC meeting reasonably detailed progress and other reports to keep the JSC informed of the current progress and status of the submitting Party’s activities under the Development Plans and to commercialize Products in their respective Territories.
3.6 Coordination of Clinical Development. The Parties undertake to use their Commercially Reasonable Efforts to coordinate through the JSC any clinical development efforts in their respective parts of the Territory in order to fully maximize the global position of the Product. Each Party shall use Commercially Reasonable Efforts to avoid activities in its Territory that could reasonably be expected to have a material adverse effect in the other Party’s Territory.
3.7 Regulatory Filings and Approvals in the Licensed Territory.
(a) Braeburn shall use Commercially Reasonable Efforts to apply for and obtain Regulatory Approvals for the Products in the Licensed Territory in accordance with the Development Plans, which applications and approvals shall be owned, held by and in the name of Braeburn. Braeburn shall use Commercially Reasonable Efforts to compile, submit, prosecute and maintain in a timely manner all necessary data, documents, INDs and NDAs (including labeling), in a format acceptable to the applicable Regulatory Authorities in the Licensed Territory.
(b) Camurus shall provide assistance as reasonably requested by Braeburn in (i) compiling an IND and NDA; (ii) providing support for meetings with Regulatory Authorities; and (iii) responding to questions from the Regulatory Authorities on technical (as opposed to pricing) questions about the Products. Camurus shall be reimbursed for such assistance at the FTE Rate and for reasonable, documented Third Party expenses. If and when Regulatory Approvals are secured, Braeburn shall use Commercially Reasonable Efforts to maintain and renew the Regulatory Approvals in the Licensed Territory and pay all user fees and other costs required to obtain and maintain such Regulatory Approvals. Promptly upon request by Braeburn, Camurus shall transfer to Braeburn, in each case to the extent Controlled by Camurus, copies of all existing Development Data, in the form it exists on the Effective Date, copies of all additional data and reports from ongoing work, such additional data and reports to
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
be provided if available in ECTD and otherwise in existing format, and copies of all material correspondence with Regulatory Authorities regarding the Products in the Territory.
3.8 Failure to file NDA Application. Without limiting the generality of the foregoing, on a Product-by-Product basis, if Braeburn (a) does not file an NDA for the Product in the United States within [***] after completion of the final report for the Phase III Clinical Trial for the Product, or (b) does not initiate within such [***] period additional clinical and/or non-clinical studies designed to generate data necessary to support an NDA Approval, then, without limiting any remedies available to Camurus hereunder, Braeburn shall notify Camurus thereof in writing within [***] from expiry of the [***] period and provide Camurus with a written explanation as to why timely filing of an NDA or the initiation of additional studies has not occurred.
3.9 Development Data.
(a) To the extent permitted by law, Braeburn grants Camurus, its Affiliates and licensees the right to use and cross-reference any Development Data and regulatory filings, including INDs, NDAs and Regulatory Approvals, Controlled by Braeburn, its Affiliates or Sublicensees as may be necessary or useful for Camurus, its Affiliates and licensees to make regulatory filings for (i) the Products, and (ii) any other products not being a Competing Product in the Territory, to the extent such Development Data and regulatory filings, including INDs, NDAs and Regulatory Approvals, relate to Cam urns Platform IP. Braeburn shall give Camurus, its Affiliates and licensees reasonable access (including the right to copy where reasonably required) to a copy of (i) the complete NDA for the Products filed by Braeburn, its Affiliates or Sublicensees with FDA or any other applicable Regulatory Authorities in the Licensed Territory, and (ii) Regulatory Approvals for the Products granted by FDA or any other applicable Regulatory Authorities in the Licensed Territory.
(b) To the extent permitted by law, Camurus grants Braeburn, its Affiliates and Sublicensees the right to use and cross-reference any Development Data and regulatory filings, including INDs, NDAs and Regulatory Approvals, Controlled by Camurus, its Affiliates or licensees as may be necessary or useful for Braeburn, its Affiliates and Sublicensees to make regulatory filings for the Products in the Licensed Territory. Camurus shall give Braeburn, its Affiliates and Sublicensees reasonable access (including the right to copy where reasonably required) to a copy of (i) the complete NDA for the Products filed by Camurus, its Affiliates or licensees with the EMA or any other applicable Regulatory Authorities in the Camurus Territory, and (ii) Regulatory Approvals for the Products granted to Camurus, its Affiliates or its licensees by the EMA or any other applicable Regulatory Authorities in the Camurus Territory. Notwithstanding the foregoing, Camurus shall not be required to provide Braeburn, its Affiliates and Sublicensees the rights described in this Section 3.9, if Camurus is restricted from doing so pursuant to an agreement with a Third Party in effect prior to the Effective Date.
3.10 Conduct of Development Activities.
(a) In performing its development activities under this Agreement, each Party shall use or retain personnel with sufficient skills and experience as are required to accomplish efficiently and expeditiously the activities of the Development Plans in a good scientific manner and in compliance in all respects with all applicable laws.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(b) In the course of performing development activities under the Development Plans, neither Party shall knowingly use any employee or consultant who is or has been debarred by the FDA or any other Regulatory Authority or, to the best of such Party’s knowledge, who is or has been the subject of debarment proceedings by the FDA or any such Regulatory Authority. Each Party shall promptly notify the other Party of and provide such other Party with a copy of any correspondence or other reports that such Party receives from any Third Party with respect to any use of a debarred employee or consultant in connection with such Party’s performance of its obligations under this Agreement.
(c) All clinical work conducted by or on behalf of Camurus and Braeburn will be completely and accurately recorded, in sufficient detail and in good scientific manner, in separate laboratory notebooks distinct from other work being conducted by Camurus and Braeburn, respectively. Each Party shall retain all development records required by law to be maintained.
(d) Each Party performing work under the Development Plans will permit the other Party’s representatives to examine or audit the work performed, and the facilities at which the work is conducted, by such Party or its designee, upon reasonable advance notice during regular business hours, to determine that the work is being conducted in accordance with the Development Plans and applicable law, and that the facilities are adequate. Such examination or audit shall always be subject to the restrictions imposed by the examined or audited Party in order to protect the confidential information of any Third Party. In addition, each Party shall promptly notify the other Party of any institutional review board/ethics committee inspections of Clinical Trial sites that may be reasonably expected to adversely affect the conduct of Clinical Trials for the Product or the resulting data.
3.11 Reporting Adverse Events. Promptly following the Effective Date but not later than 60 days thereafter, Braeburn and Camurus shall develop and agree upon safety data exchange procedures governing the coordination of collection, investigation, reporting, and exchange of information concerning adverse events (as defined in the then current edition of International Conference on Harmonization guidelines for good clinical practice, or ICH Guidelines, and any other relevant regulations or regulatory guidelines or any other safety problem of any significance, hereafter “Adverse Events”), product quality and product complaints involving Adverse Events, sufficient to permit each Party, its Affiliates, Sublicensees or licensees to comply with its legal obligations, including to the extent applicable, those obligations contained in ICH Guidelines. The safety data exchange procedures shall be promptly updated if required by changes in legal requirements or by agreement between the Parties. In any event, each Party shall inform the other Party of any Adverse Event of which it becomes aware in a timely manner commensurate with the seriousness of the Adverse Event. Braeburn or its Sublicensees shall be responsible for reporting all Adverse Events to the appropriate regulatory authorities in the countries in the Licensed Territory and Camurus or its licensees shall be responsible for reporting all Adverse Events to the appropriate regulatory authorities in the countries in the Camurus Territory. Each Party shall ensure that its Affiliates, Sublicensees and licensees, as applicable, comply with such reporting obligations. Each Party shall designate a safety liaison to be responsible for communicating with the other Party regarding the reporting of Adverse Events.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
4. COMMERCIALIZATION
4.1 Responsibility. Braeburn shall have responsibility at its own cost to commercialize the Products in the Licensed Territory; provided, that unless more strict requirements are set forth herein, Braeburn shall exercise Commercially Reasonable Efforts to commercialize the Products and shall comply with the terms and conditions of this Agreement, including the Commercialization Plan.
4.2 Launch Efforts. On a Product-by-Product and country-by-country basis, unless otherwise agreed by the Parties, Braeburn shall (a) within [***] after NDA Approval, apply for any other Regulatory Approvals necessary to launch the Product in that country, and (b) within [***] after the receipt of all required Regulatory Approvals in that country, launch the Product in that country.
4.3 Marketing Efforts. Braeburn shall have sole responsibility for and shall use Commercially Reasonable Efforts to commence marketing of, and to promote, market, sell and commercialize thereafter, the Products in the Licensed Territory within the scope of the rights granted to it hereunder. If Braeburn does not possess adequate internal resources to carry out such activities, it shall engage appropriate and competent Third Parties. Braeburn, its Affiliates or Sublicensees shall have available in the United States, not later than [***] after the first NDA Approval for a Product and continuing for the first [***] after launch of a Product, no less than a total of [***] full-time equivalent sales representatives promoting the Product in the first position and/or medical science liaisons providing medical education about the Product on a full-time equivalent basis. The detailed allocation of the sales force and/or medical science liaisons dedicated to the Products shall be specified in the Commercialization Plan, as may be amended from time to time except with respect to the foregoing requirements in respect of sales representatives and medical science liaisons. In performing all such marketing and promotion activities and disseminating Product information, Braeburn and its Affiliates and Sublicensees shall comply with all applicable laws, regulations and guidelines concerning such promotional activities in each country of the Licensed Territory in effect from time to time.
4.4 Post Registration Studies; Publications. Braeburn shall use Commercially Reasonable Efforts to prolong the lifecycle of the Products. To the extent that Braeburn performs post registration, Phase IV Clinical Trials or other Clinical Trials of the Product following receipt of Regulatory Approval for the Product (“Braeburn Post-Registration Studies”), Braeburn shall provide Camurus with draft forms of summary protocols for major studies before commencement of any such study. Each Party shall provide the other Party with draft publications relating to the Products [***] prior to submission of such drafts for publication. Camurus shall have the right to disclose such protocols, and each Party shall have the right to disclose such draft publications, in each case to its licensees for the Products for the purpose of obtaining comments and to facilitate coordination of clinical development and publication in the Territory. Braeburn shall consider in good faith all comments to draft protocols submitted by Camurus or its licensees and each Party shall consider in good faith all comments to draft publications submitted by the other Party, provided that in each case the comments are submitted within the [***] period following the reviewing Party’s receipt of such protocols and publications; provided, that Braeburn shall have the right to determine all final protocols and publications in the Licensed Territory so long as they conform to other requirements under this Agreement and Camurus shall have the right to determine all final publications in the Camurus
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Territory so long as they conform to other requirements under this Agreement. Braeburn shall bear the cost of all Braeburn Post-Registration Studies. For the avoidance of doubt, Braeburn Post-Registration Studies shall include any Clinical Trials required as a condition to, or for the maintenance of, Regulatory Approval of the Product in the Licensed Territory.
4.5 Advertising and Promotion. Each Party shall be given the opportunity to review and comment on the core promotional and training materials prepared by such Party for marketing the Products in such Party’s portion of the Territory.
4.6 Commercialization Plan. Beginning not later than [***] following filing of the first NDA for a Product in the Licensed Territory and on an annual basis thereafter, on a Product-by-Product basis Braeburn shall submit to the JSC Braeburn’s commercialization plan for the Product, including, without limitation, marketing plans for the Product for the following year in the Licensed Territory, plans related to the pricing and reimbursement strategy, pre-launch, launch, promotion and sale, including sales forecasts for the [***] following years, and the marketing, promotion and advertising campaigns proposed to be conducted, as well as associated budgets, including the number of detailing representatives, medical science liaisons, level of promotion, Braeburn Post-Registration Studies and its medical education activities (the “Commercialization Plan”). The initial Commercialization Plan for CAM2038 is attached hereto as Exhibit 4.6. The Commercialization Plan submitted by Braeburn shall be designed to fulfill Braeburn’s undertakings pursuant to this Section 4. Camurus, through the JSC, shall be given the opportunity to review and comment on the Commercialization Plan and subsequent amendments thereto and Braeburn shall consider in good faith any comments and reasonable requests for amendments.
5. PAYMENT OBLIGATIONS
5.1 Signing Fee. Within [***] after the Effective Date, provided that Braeburn has received an invoice from Camurus, Braeburn shall pay Camurus a non-refundable and non-creditable signing fee of US$20,000,000, payable by wire transfer of immediately available funds to an account designated in writing by Camurus.
5.2 Reimbursement of Phase III Preparation Costs. Within [***] after receipt of an invoice from Camurus, which invoice will be delivered by Camurus promptly after FDA authorization of the IND for the first Product for the treatment of opioid dependence, Braeburn shall reimburse Camurus for the Phase III Preparation Costs in the amount of US$1,250,000 payable by wire transfer of immediately available funds to an account designated in writing by Camurus.
5.3 Development Milestone Payments. Braeburn shall pay the following one-time, nonrefundable, non-creditable amounts upon the achievement of the following events, within [***] after each such event and upon receipt of an invoice from Camurus:
|
MILESTONE EVENT |
|
MILESTONE PAYMENT |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
MILESTONE EVENT |
|
MILESTONE PAYMENT |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
Each milestone event under this Section 5,3 shall be paid only once with respect to the first time such milestone is achieved by or with respect to a Product for the referenced indication, no matter how many times such milestone event is achieved by or with respect to a Product for the referenced indication. In no event shall development and approval milestone payments be payable in excess of US$56,000,000.
5.4 Royalties. On a Product-by-Product basis, Braeburn shall pay to Camurus royalties (“Royalties”) equal to [***] on the annual Net Sales of Products in the Licensed Territory. Royalties shall be payable on a Product-by-Product and country-by-country basis beginning on the First Commercial Sale of such Product in such country and ending on the later of (a) 12 years after the date of such First Commercial Sale of a Product in such country; and (b) the expiration of the last to expire Valid Claim of all Patent Rights included within the Camurus IP covering or claiming the Product in such country. Notwithstanding the foregoing, if after the expiration of the 12 year period set forth in (a) above the only Valid Claim of a Patent Right covering or claiming the Product in such country is a Patent Right included within the Joint IP, then the Royalties payable under this Section 5.4 shall be reduced to [***]. The period during which Royalties are payable in respect of a Product in any country is referred to as the “Royalty Term.”
5.5 Generic Product. If, on a country-by-country and Product-by-Product basis, a Generic Product is on the market in the Licensed Territory, then the Royalties due for such calendar quarter pursuant to Section 5.4 shall be reduced by (a) [***] if total dosage units of the Generic Product sold do not exceed [***] of the combined total aggregate dosage units sold of the Generic Products and the Product, (b) [***] if total dosage units of the Generic Product sold exceed [***] but are [***] or less of the combined total aggregate dosage units sold of the Generic Product and the Product, and (c) [***] if total dosage units of the Generic Product sold exceed [***] of the combined total aggregate dosage units sold of the Generic Product and the Product.
5.6 Sales Milestone Payments. Braeburn shall pay the following one-time, nonrefundable, non-creditable sales milestones upon the achievement of the following sales levels for the Products in the Licensed Territory. Payment for any such milestones shall be made within [***] of each event and upon receipt of an invoice from Camurus. Sales milestones will be paid on a Product-by-Product basis (except that different depot versions of the weekly and monthly CAM2038 and CAM2048, respectively, shall be aggregated) in accordance with the schedule below, with each milestone paid only once and on the first occurrence of the event, as set forth below. In the event more than one sales milestone is reached in the same year, then each such milestone shall be due that year.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
MILESTONE EVENT |
|
MILESTONE PAYMENT |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
In no event shall sales milestone payments be payable in excess of US$75,000,000.
5.7 Royalty and Milestone Reports. Royalty and sales milestone payments shall be paid within [***] of each year following the First Commercial Sale of a Product or the relevant milestone event, and shall include a written report with respect to the preceding quarter (the “Payment Report”) stating: (a) the gross sales and number of units sold of each presentation of the Product sold by Braeburn, its Affiliates and Sublicensees in each country in the Licensed Territory; (b) Net Sales of the Product sold by Braeburn, its Affiliates and Sublicensees, during such quarter on a country-by-country basis; (c) the date of any First Commercial Sale of the Product in each country during such quarter; (d) currency exchange rates used in determining the Royalties; and (e) a calculation of the amounts due to Camurus. Braeburn shall notify Camurus in writing promptly following the achievement of any milestone described in this Section 5.
5.8 Payments.
(a) All payments due under this Agreement shall be paid in immediately available funds in US Dollars to the bank account designated in writing by Camurus, as the case may be. To the extent Net Sales are accrued in currencies other than US Dollars, Net Sales shall be converted to US Dollars, as the case may be, at the average daily rate of exchange for the applicable calendar quarter as published by Financial Times (UK edition). The calculation of the average rate of exchange shall be stated in terms of US Dollars per foreign currency units.
(b) All payments hereunder are exclusive of any taxes, fees or charges imposed by any local or national authority. In the event that Braeburn reasonably determines that any tax, duty or other levy is required to be paid or withheld on account of Royalties or other payments payable to Camurus under this Agreement, such amounts shall be deducted from the amount of Royalties or other payments otherwise due. Braeburn shall secure and send to Camurus proof of any such taxes, duties or other levies withheld and paid by Braeburn for the benefit of Camurus and cooperate with any request to ensure that amounts withheld are reduced to the fullest extent permitted by the relevant jurisdiction.
(c) Any payments that are not paid within 30 days after the date such payments are due under this Agreement shall, after prior notice from Camurus and a 5 Business Day cure period, bear interest at an annual rate of interest equal to the London Interbank Offered Rate (LIBOR), plus [***] percentage points, calculated on the number of days such payment is delinquent.
5.9 Books and Records; Audit Rights. Beginning on the First Commercial Sale of a Product and continuing during the Term and for a period of [***] years thereafter, Braeburn shall keep full and true books of accounts and other records in sufficient detail so that the Royalties
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
payable hereunder can be properly ascertained. Braeburn shall, at the request of Camurus, permit a nationally recognized independent certified public accountant selected by Camurus to have access during ordinary business hours, to such books and records as may be necessary to determine the correctness of any Payment Report or payment made under this Agreement or to obtain information as to Royalties and milestones payable in case of failure to report or pay pursuant to the terms of this Agreement. The auditor shall execute a written confidentiality agreement with Braeburn and shall disclose to Camurus only the amount and accuracy of payments reported and actually paid or otherwise payable under this Agreement. The auditor shall send a copy of the report to Braeburn at the same time it is sent to Camurus. Such examination shall be conducted (a) after at least [***] prior written notice from Camurus, (b) at the facility(ies) where such books and records are maintained, and (c) no more frequently than once in any Calendar Year. Camurus shall be responsible for expenses for the independent certified public accountant, except that Braeburn shall reimburse Camurus in full thereof if the independent accountant determines the Royalties and milestones paid by Braeburn to Camurus are less than [***] of the amount actually owed for the period of the audit. As a condition to any sublicense granted by Braeburn hereunder, Braeburn shall ensure that Camurus has the same audit rights as those described in this Section 5.9 with respect to any such Braeburn Affiliate or Sublicensee.
6. MANUFACTURE
6.1 Identification of CMO. Braeburn shall use Commercially Reasonable Efforts to manufacture, package, label, quality release and to supply its requirements of Product for non-clinical, clinical and commercial use and sale in the Licensed Territory or identify and select a CMO to do so under the Braeburn Supply Agreement. Braeburn, or its CMO if applicable, shall be EU and US GMP approved and able to manufacture according to the Regulatory Approvals in the applicable countries in the Territory. Until the technology transfer process to Braeburn or CMO has been successfully completed as set forth in Section 6.2, Camurus shall use Commercially Reasonable Efforts to supply Braeburn’s noncommercial requirements of CAM2038 and CAM2048 for the performance of the Development Plans from Camurus’ existing manufacturer at Camurus’ Manufacturing Cost. The Parties shall enter into a separate supply agreement governing the terms and conditions of such non-commercial supply from Camurus to Braeburn and shall use good faith efforts to negotiate and enter into the foregoing within [***] from the Effective Date.
6.2 Technology Transfer. Within [***] after Braeburn’s request, Camurus shall use Commercially Reasonable Efforts to transfer to Braeburn tangible embodiments of its manufacturing technology as may be necessary or useful to enable Braeburn or CMO to manufacture the Products for non-clinical, clinical and commercial use. Upon reasonable request from Braeburn, Camurus shall use Commercially Reasonable Efforts to provide technical assistance to Braeburn to enable the use of such manufacturing technology to manufacture the Products. Unless otherwise separately agreed, the technology transfer process shall be deemed completed upon Braeburn’s or CMO’s successful process validation for manufacture of the Products. Prior to the technology transfer, the Parties will agree upon a technology transfer plan and corresponding budget. Braeburn shall reimburse Camurus for its direct, out-of-pocket costs and expenses as well as FTE Costs incurred in providing technology transfer assistance as requested by Braeburn. Camurus shall be reimbursed by Braeburn on a
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
monthly basis, within [***] of receipt of an invoice setting forth the costs and expenses together with reasonable supporting documentation.
6.3 Braeburn Supply Agreement. If Braeburn elects to use a CMO to manufacture the Products, Braeburn shall enter into one or more Braeburn Supply Agreements with the CMO covering the technology transfer, process development, scale-up and manufacture of the Products, Once Braeburn has commenced manufacture of the Products, either itself or through a CMO, Camurus shall be entitled to source its and its licensees’ non-clinical, clinical and commercial requirements of Products for the Camurus Territory from Braeburn at Braeburn’s Manufacturing Cost. The Parties shall enter into separate supply agreements governing the terms and conditions of such supply from Braeburn (or CMO if applicable) to Camurus. If Camurus cannot source its requirements of Products from CMO or Braeburn, as applicable, then Braeburn shall provide such manufacturing technology as may be reasonably necessary to enable Camurus’ designated CMO to manufacture the Product for Camurus’ non-clinical, clinical, and commercial use, and shall use Commercially Reasonable Efforts to provide technical assistance to effectively enable the use of such information and technology to manufacture the Products. Unless otherwise separately agreed, the technology transfer process shall be deemed completed upon Camurus’ CMO’s successful process validation for manufacture of the Products. Prior to such technology transfer, the Parties will agree upon a technology transfer plan and corresponding budget. Camurus shall reimburse Braeburn for its direct, out-of-pocket costs and expenses as well as FTE Costs incurred in providing such assistance as requested by Camurus. Braeburn shall be reimbursed by Camurus [***] of receipt of an invoice setting forth such costs and expenses.
6.4 Change in Product Specification and Manufacturing Process. Camurus and Braeburn shall ensure that all contemplated changes in the Product specification and manufacturing process as well as all documentation and information relating to a change that may require notification to any Regulatory Approval in respect of Products in the Territory shall be communicated to the other Party on a timely basis and such changes shall be discussed through the JSC. Subject to the foregoing sentence, all such activities shall be coordinated and finally controlled by Cam urns prior to successful process validation at Braeburn or its CMO and by Braeburn thereafter, in order to ensure conformity in respect of Product specification and manufacturing process between Product manufactured for the Licensed Territory and the Camurus Territory, respectively.
7. INTELLECTUAL PROPERTY
7.1 Trademarks.
(a) Braeburn shall have the right to select, after due consideration by the JSC, and shall register and maintain, at its expense, such Product Trademarks as shall be used for the promotion, marketing and sale of the Products in the Licensed Territory. Braeburn shall own such Product Trademarks and all goodwill associated therewith. Camurus is granted the option to obtain a non-exclusive, sublicensable, fully paid-up right and license to use the Product Trademarks in the distribution, marketing, promotion and sale of Products in the Camurus Territory. If Camurus elects to exercise the option, then Braeburn’s obligations under Section 7.1(c) with respect to the Camurus Trademarks shall apply to Camurus’ use of the Product Trademarks as if Camurus were Braeburn thereunder. Braeburn shall provide relevant
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
documents related to the Prosecution of the Product Trademarks in the Camurus Territory upon request from Camurus. In the event that Braeburn does not wish to file applications for Product Trademarks in any country in the Camurus Territory or Braeburn, after having filed, declines to further Prosecute any pending Product Trademarks in the Camurus Territory, Braeburn shall provide Camurus with written notice thereof. Such notice shall be given at least [***] prior to the expiration of any official substantive deadline relating to such activities. In any such circumstances Camurus shall have the right to decide that Camurus should continue to Prosecute such Product Trademarks at Camurus’ expense and in such case Camurus shall give written notice to Braeburn. Braeburn shall upon receipt of any such notice from Camurus transfer to Camurus all of its files relating to the relevant Product Trademarks and at Camurus’ reasonable cost and expense execute any documents to otherwise transfer control of such Prosecution to Camurus. All Product Trademarks shall, notwithstanding anything to the contrary herein, remain vested in and solely Controlled by Braeburn and be licensed to Camurus as provided above.
(b) Each Party shall use the Product Trademarks in accordance with sound trademark and trade name usage principles and in accordance with all applicable laws and regulations as reasonably necessary to maintain the validity and enforceability of the Product Trademarks.
(c) Camurus hereby grants Braeburn a non-exclusive, sublicensable, fully paid-up right and license to use the Camurus Trademarks for commercialization of the Products in the Licensed Territory. If Braeburn opts to use the Camurus Trademark, save to the extent Braeburn may be required to do so by a Regulatory Authority or pursuant to the requirements of a Regulatory Approval, Braeburn shall not conceal or otherwise obscure, remove or interfere with the Camurus Trademark. Braeburn shall not register or use any Trademark confusingly similar to any Camurus Trademark or any other Trademarks used by Camurus with the FC Technology. Braeburn shall ensure that each reference to and use of the Camurus Trademark in any marketing material related to the Products is accompanied by an acknowledgement that the Camurus Trademark is owned by Camurus and used by Braeburn under license. Braeburn shall adhere to any reasonable requests from Camurus relating to Braeburn’s use of the Camurus Trademark.
7.2 Ownership of Collaboration Inventions. Subject to the terms hereof, including the licenses and other rights granted hereunder, all Collaboration Inventions shall be owned as follows:
(a) All Collaboration Inventions, including Joint Inventions, conceived or created by either Party or a Third Party on behalf of such Party during the Term relating to (i) solely the FC Technology and/or other formulations for injection Controlled by Camurus and being part of the Camurus Platform IP all having an effective extended release duration of more than 24 hours, or (ii) the FC Technology and/or other formulations for injection Controlled by Camurus and being part of the Camurus Platform IP all having an effective extended release duration of more than 24 hours, in each case incorporating buprenorphine or any other active pharmaceutical ingredient, shall be exclusively owned by Camurus, subject to the license granted to Braeburn under Section 2.1.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(b) The Parties shall jointly own all Joint Inventions, other than those covered above in (a), and, subject to the rights granted each Party under this Agreement, each Party may make, use, sell, keep, license or assign its interest in Joint Inventions and otherwise undertake all activities a sole owner might undertake with respect to such Joint Inventions, without the consent of and without accounting to the other Party. “Joint Inventions” means Collaboration Inventions for which it is determined, in accordance with the patent law of England, that both: (i) one or more employees, consultants or agents of Camurus or its Affiliates or any other persons obligated to assign such Collaboration Invention to Camurus; and (ii) one or more employees, consultants or agents of Braeburn or its Affiliates or any other persons obligated to assign such Collaboration Invention to Braeburn, are joint inventors of the Collaboration Invention. For any Joint IP covered by this Section 7.2(b) that could be the subject of an application for a Patent Right, the JSC, in conjunction with each Party’s patent counsel, will make an initial determination of inventorship prior to filing the application therefor to confirm that it is Joint IP. Each Party will provide information and records relevant to such determination to the JSC and such patent counsel. If the JSC fails to agree whether there has been joint inventorship, the application for the Patent Right will continue to be filed as Joint IP under the procedures set out in this Section 7 and the dispute will be referred to a Third Party patent attorney acceptable to each of the Parties for Expert Determination as provided in Exhibit 7.2(b).
(c) Subject to appropriate confidentiality undertakings, each Party shall notify the other Party promptly after the completion of invention disclosure statements for each Collaboration Invention (or, if any provisional or other patent applications are filed claiming such invention, promptly after such filing), and, to the extent a Party is granted rights hereunder in such Collaboration Invention, shall provide a copy of the same to the other Party.
(d) For the avoidance of doubt, neither Party is granted any license rights to any Intellectual Property rights of the other Party which may be required for such Party to use a Collaboration Invention, unless otherwise expressly granted herein.
(e) Each of the Parties shall do all such acts and things, and execute all such deeds and documents, as may be necessary or desirable for them to perfect their rights of ownership as specified in Section 7.2 and otherwise implement the provisions of this Section 7. Each Party shall, and shall cause its applicable Affiliates, Third Party subcontractors, and their respective employees and agents, to perform at the requesting Party’s cost all reasonable acts reasonably requested, including the execution of confirmatory deeds and assignment documents of Patent Rights, as may be necessary or desirable for them to perfect their title therein in accordance with the forgoing provisions of this Section 7.2.
7.3 Prosecution of Patents.
(a) Patent Prosecution of Camurus Platform IP. Camurus shall control the Prosecution of the Camurus Platform IP at its own cost and expense using Commercially Reasonable Efforts. Camurus shall provide Braeburn with a copy of each new draft application and replies to substantive office actions within Patent Rights under the Camurus Platform IP (including, for clarity, divisional and continuation applications), shall keep Braeburn informed of all material developments in relation to the Camurus Platform IP, and shall provide Braeburn with copies of relevant documents related to the Prosecution of Camurus Platform IP in the
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Licensed Territory. Camurus shall provide relevant documents related to the Prosecution of the Camurus Platform IP in all other countries in the Territory upon request from Braeburn. In the event that, having filed, Camurus declines to further Prosecute any pending Camurus Platform IP in any country of the Licensed Territory, Camurus shall provide Braeburn with written notice thereof. Such notice shall be given at least [***] prior to the expiration of any official substantive deadline relating to such activities. In any such circumstances and provided that no earlier licensee of Camurus has assumed the right to Prosecute such Camurus Platform IP, Braeburn shall have the right to continue to Prosecute such Camurus Platform IP at Braeburn’s expense and in such case Braeburn shall give written notice to Camurus; provided, that if Camurus’ decision to decline to further Prosecute any pending Camurus Platform IP is as a result of the withdrawal of an unpublished patent application and the reason for such withdrawal was to enable the filing of another patent application relating to the same inventions, Braeburn shall not have the right to continue to Prosecute the withdrawn unpublished patent application. Camurus shall upon receipt of any such notice from Braeburn transfer to Braeburn copies of all of its files relating to the relevant Camurus Platform IP and at Braeburn’s reasonable cost and expense execute any documents to otherwise transfer control of such Prosecution to Braeburn. Camurus shall remain the owner of such Camurus Platform IP and Braeburn shall provide Camurus the same information and rights required under this Section 7.3 to be provided Braeburn concerning the Prosecution of such Patent Rights. The terms of this Section 7.3(a) shall be subject to the terms of any agreement with a Third Party under which Camurus acquired rights to any such Camurus Platform IP.
(b) Patent Prosecution of Camurus Product IP in the Licensed Territory. Braeburn shall control the Prosecution of the Camurus Product IP in the Licensed Territory at Braeburn’s expense using Commercially Reasonable Efforts. Braeburn shall provide Camurus the same information and rights required under Section 7.3(a) to be provided Braeburn concerning the Prosecution of such Patent Right and shall give Camurus an opportunity to comment upon draft patent applications and replies to substantive office actions before they are filed. Braeburn shall consider in good faith any reasonable comments made by Camurus in relation to the Prosecution of the above referred Camurus Product IP when making any submission to a patent office (including the scope of foreign filings) and in the conduct of any proceedings in relation to such Camurus Product IP, In the event that, having filed, Braeburn declines to further Prosecute any pending Camurus Product IP in any country of the Licensed Territory, Braeburn shall provide Camurus with written notice thereof. Such notice shall be given at least [***] prior to the expiration of any official substantive deadline relating to such activities. In any such circumstances Camurus shall have the right to decide that Camurus should continue to Prosecute such Camurus Product IP at Camurus’ expense and in such case Camurus shall give written notice to Braeburn. Braeburn shall upon receipt of any such notice from Camurus transfer to Camurus all of its files relating to the relevant Camurus Product IP and at Camurus’ reasonable cost and expense execute any documents to otherwise transfer control of such Prosecution to Camurus. The patent application and any patent issuing therefrom shall, notwithstanding anything to the contrary herein, remain vested in and solely Controlled by Camurus and shall be exclusively licensed to Braeburn as part of the licensed rights pursuant to Section 2. Camurus shall provide Braeburn the same information and rights required above to be provided Camurus concerning the Prosecution of such Patent Rights.
(c) Patent Prosecution of Camurus Product IP in the Camurus Territory. Camurus shall control the Prosecution of the Camurus Product IP in the Camurus Territory at
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Camurus’ expense using Commercially Reasonable Efforts. Camurus shall provide Braeburn the same information and rights required under Section 7.3(a) to be provided Braeburn concerning the Prosecution of such Patent Rights and shall give Braeburn an opportunity to comment upon draft patent applications and replies to substantive office actions before it is filed. Camurus shall consider in good faith any reasonable comments made by Braeburn in relation to the Prosecution of the above referred Camurus Product IP when making any submission to a patent office (including the scope of foreign filings) and in the conduct of any proceedings in relation to such Camurus Product IP. In the event that, having filed, Camurus declines to further Prosecute any pending Camurus Product IP in any country in the Camurus Territory, Camurus shall provide Braeburn with written notice thereof. Such notice shall be given at least [***] prior to the expiration of any official substantive deadline relating to such activities. In any such circumstances Braeburn shall have the right to decide that Braeburn should continue to Prosecute such Camurus Product IP at Braeburn’s expense and in such case Braeburn shall give written notice to Camurus, Camurus shall upon receipt of any such notice from Braeburn transfer to Braeburn all of its files relating to the relevant Camurus Product IP and at Braeburn’s reasonable cost and expense execute any documents to otherwise transfer control of such Prosecution to Braeburn. The patent application and any patent issuing therefrom shall, notwithstanding anything to the contrary herein, remain vested in and solely Controlled by Camurus and shall be exclusively licensed to Braeburn as part of the licensed rights pursuant to Section 2. Braeburn shall provide Camurus the same information and rights required above to be provided Braeburn concerning the Prosecution of such Patent Rights.
(d) Patent Prosecution of Braeburn Product IP in the Territory. Braeburn shall control the Prosecution of the Braeburn Product IP in the Territory at Braeburn’s expense using Commercially Reasonable Efforts. Braeburn shall provide Camurus the same information and rights required under Section 7.3(a) to be provided Braeburn concerning the Prosecution of such Braeburn Product IP and shall give Camurus an opportunity to comment upon draft patent applications and replies to substantive office actions before they are filed. Braeburn shall consider in good faith any reasonable comments made by Camurus in relation to the Prosecution of the above referred Braeburn Product IP when making any submission to a patent office (including the scope of foreign filings) and in the conduct of any proceedings in relation to such Braeburn Product IP. In the event that, having filed, Braeburn declines to further Prosecute any pending Braeburn Product IP in any country in the Territory, Braeburn shall provide Camurus with written notice thereof. Such notice shall be given at least 60 days prior to the expiration of any official substantive deadline relating to such activities. In any such circumstances Camurus shall have the right to decide that Camurus should continue to Prosecute such Braeburn Product IP at Camurus’ expense and in such case Camurus shall give written notice to Braeburn. Braeburn shall upon receipt of any such notice from Camurus transfer to Camurus all of its files relating to the relevant Braeburn Product IP and at Camurus(1) reasonable cost and expense execute any documents to otherwise transfer control of such Prosecution to Camurus. The patent application and any patent issuing therefrom shall, notwithstanding anything to the contrary herein, remain vested in and solely Controlled by Braeburn and shall be exclusively licensed to Camurus as part of the grant back license pursuant to Section 2. Camurus shall provide Braeburn the same information and rights required above to be provided Camurus concerning the Prosecution of such Patent Rights.
(e) Joint Patents. With respect to the Prosecution of patent applications claiming Joint Inventions covered by Section 7.2(b) (“Joint Patents”), Braeburn shall have the
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
right to take such actions as are necessary or appropriate to Prosecute Joint Patents in the Territory at its sole expense; provided, that all such patent applications and patents shall be owned jointly. Braeburn shall furnish Camurus with copies of such Joint Patents and other related correspondence relating to such Joint Inventions to and from patent offices throughout the Territory and permit Camurus to offer its comments thereon before Braeburn makes a submission to a patent office. Braeburn shall inform Camurus of the countries in which it intends to file patent applications. Camurus shall offer its comments promptly, including any request that the patents be filed in additional countries; provided, that Braeburn shall determine the appropriate action after considering in good faith any comments or requests from Camurus; provided, further, that in the event that delay would jeopardize any potential Patent Right, Braeburn shall have the right to proceed without awaiting Camurus’ comments. If Braeburn determines in its sole discretion not to Prosecute any patent application within the Joint Patents in any country in the Territory, and provided that no other patent applications or patents claiming the same or similar subject matter are then pending or issued in that same country, then Braeburn shall provide Camurus with [***] prior written notice (or such shorter time period that would permit Camurus a reasonable opportunity to respond in a timely manner) of such determination and Camurus shall have the right and opportunity to Prosecute such patent application on behalf of the Parties at Camurus’ sole cost and expense. Camurus shall provide Braeburn the same information and rights required above to be provided Camurus concerning the Prosecution of such Patent Rights.
(f) Each Party shall at the expense of the requesting Party execute such documents and take such other actions as may be reasonably requested by the other Party in conjunction with Prosecution of Patent Rights pursuant to this Section 7.3.
7.4 Camurus Platform IP and Camurus Product IP. If, after the Effective Date, any Collaboration Invention or Camurus Patent Right relates both to Camurus Platform IP or to FC Technology incorporating other active substances or products to the extent not constituting a Product, on the one hand, and Products, on the other hand, and patent claims can be made based on the same data, Patent Right or study result or separate applications can be filed or divided out with respect to existing Patent Rights, Camurus shall, in consultation with Braeburn, file separate patent applications on the same day, or shall file one or more divisional, continuation and/or continuation-in-part applications, as applicable to cover Camurus Platform IP or FC Technology incorporating no active substance or other active substances or products to the extent not constituting a Product, on the one hand, and Camurus Product IP, on the other hand, separately. Where Camurus Patent Rights existing and within its priority year at the Effective Date discloses aspects of the Products, Camurus shall, at the request of Braeburn, file two or more separate applications on the same day, each claiming priority from such existing Patent Rights, to cover Products on the one hand and other aspects on the other hand. Any such applications relating to Products shall become part of the Camurus Platform IP and/or Camurus Product IP hereunder, as applicable. Any such applications relating neither to Camurus Product IP nor Camurus Platform IP shall not be subject to this Agreement.
7.5 Assignment of IP. Each Party shall ensure that any employee, independent contractor, licensee or subcontractor of that Party involved in the performance of this Agreement shall be engaged on legally binding written terms which require the assignment of all Patent Rights and Know-How resulting from work carried out by that employee, independent contractor, licensee or subcontractor to the engaging Party. Each Party shall be responsible for
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
all payments to its employees or others in respect of obtaining rights to any such Patent Rights and Know-How.
7.6 Patent Term Extensions. For all patents within any Patent Rights relating to or claiming a Product for which NDA Approval has been obtained, the Parties shall use reasonable efforts, in each country where NDA Approval for a Product has been obtained and the law of such country permits application for a patent term extension (or any supplementary certificate), to apply for a patent term extension (or any supplementary certificate) for one or more selected patents within such Patent Rights chosen at (a) Braeburn’s reasonable discretion with respect to the Licensed Territory, and (b) Camurus reasonable discretion with respect to the Camurus Territory, provided, that the Parties shall, prior to filing any such extensions, work in good faith to establish and implement an agreed-upon approach for the Territory as a whole. Each Party agrees to cooperate with the other Party in the exercise of the authorizations granted under this Section 7.6, and to execute such documents and take such additional action as the other Party may reasonably request in connection therewith.
7.7 Third Party Intellectual Property. The Parties shall use reasonable efforts to avoid infringing or misappropriating any Third Party’s Patent Rights or other Intellectual Property rights in conducting any of its activities under this Agreement.
7.8 Infringement.
(a) In the case where either Party reasonably believes that an infringement by a Third Party of Camurus Product IP, Camurus Platform IP, Braeburn Product IP or any Joint IP by the development, manufacture or sale of any Product in the Licensed Field (an “Infringing Activity”) may be occurring, such Party shall disclose full details of the potential infringement to the other Party.
(b) Where an infringement of Camurus Product IP, Braeburn Product IP or any Joint IP by an Infringing Activity occurs in one or more countries of the Licensed Territory, Braeburn shall have the first right to, but shall not be obliged to, at its own cost and expense enforce the same in accordance with the below subparagraphs (i) through (iii).
(i) Braeburn shall have the sole right to conduct the claim and any proceedings, including any counterclaim for invalidity or unenforceability or any declaratory judgment action, and including the right to settle. If Braeburn decides to commence proceedings in relation to Camurus Product IP, Braeburn Product IP or any Joint IP, it shall be entitled to require Camurus to join Braeburn as co-plaintiff and Camurus shall have the right to join as co-plaintiff. Camurus shall, at Braeburn’s cost and expense, provide all necessary assistance to Braeburn in relation to any such proceeding. If Camurus elects to be separately represented (which shall be at Camurus’ discretion), then such separate representation shall be at Camurus’ cost and expense. Braeburn shall have the sole right to settle such proceedings (but excluding any counterclaim for invalidity or unenforceability, which shall require the written consent of Camurus not to be unreasonably withheld), provided that such settlement does not include a license under Camurus Product CP, Braeburn Product IP or Joint IP or causes Camurus to incur any losses, in which case Braeburn’s consent to the terms of such
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
license shall be required, such consent not to be unreasonably withheld, conditioned or delayed.
(ii) If Braeburn succeeds in any such infringement proceedings, whether at trial or by way of settlement, the proceeds of any award or damages or settlement in respect of such infringement proceedings shall first be applied (a) to reimburse Braeburn an amount equal to Braeburn’s costs of taking the proceedings, (b) to reimburse Camurus an amount equal to Camurus’ costs of assisting Braeburn with the proceedings, and (c) the remainder shall be retained by Braeburn less [***] thereof, which amount shall be paid to Camurus;
(iii) If Braeburn declines to initiate any such proceedings in respect of any Camurus Product IP, Braeburn Product IP or Joint IP in the Licensed Territory within 60 days of the date when Braeburn first became aware of the infringement, Camurus shall be entitled to do so at its own cost and expense in which case it shall have sole conduct of any claim or proceedings including any counterclaim for invalidity or unenforceability or any declaratory judgment action and shall be entitled to require Braeburn to join Camurus as co-plaintiff and Braeburn shall have the right to join as co-plaintiff, Braeburn shall, at Camurus’ cost and expense, provide all necessary assistance to Camurus in relation to such proceedings. If Braeburn elects to be separately represented (which shall be at Braeburn’s discretion), then such separate representation shall be at Braeburn’s cost and expense. Camurus shall have the sole right to settle such proceedings (but excluding any counterclaim for invalidity or unenforceability, which shall require the written consent of Braeburn not to be unreasonably withheld), provided that such settlement does not include a license under the Camurus Product IP, Braeburn Product IP or Joint IP or causes Braeburn to incur any losses in which case Braeburn’s consent to the terms of such license shall be required, such consent not to be unreasonably withheld, conditioned or delayed. If Camurus succeeds in any such proceedings, whether at trial or by way of settlement, the proceeds of any award or damages or settlement in respect of such proceedings shall first be applied (a) to reimburse Camurus an amount equal to Camurus’ costs of taking the proceedings, (b) to reimburse Braeburn an amount equal Braeburn’s costs of assisting Camurus with the proceedings, and (c) the remainder being retained by Camurus, less [***] thereof, which amount shall be paid to Braeburn.
(c) Where an infringement of Camurus Product IP, Braeburn Product IP or Joint IP by an Infringing Activity occurs in one or more countries in the Camurus Territory, then Camurus shall have the first right to, but shall not be obliged to, at its own cost and expense enforce the same in accordance with the above Sections 7.8(b)(i) through (iii).
(d) Where an infringement of the Camurus Platform IP by an Infringing Activity is occurring in one or more countries of the Territory, Camurus shall have the right to, but shall not be obliged to, at its own cost and expense to enforce the same. Braeburn shall, at Camurus’ cost and expense, provide all necessary assistance to Camurus in relation to any such proceeding. Camurus shall have the sole right to settle such proceedings, provided that such settlement does not include a license under the Camurus Platform IP or causes Braeburn to incur any losses in which case Braeburn’s consent to the terms of such license shall be required, such consent not to be unreasonably withheld, conditioned or delayed. If Camurus
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
succeeds in any such infringement proceedings whether at trial or by way of settlement and that relates to an Infringing Activity in the Licensed Territory, the proceeds of any award or damages or settlement in respect of such infringement proceedings shall first be applied (i) to reimburse Camurus an amount equal to Camurus’ costs of taking the proceedings, (ii) to reimburse Braeburn an amount equal to Braeburn’s costs of assisting Camurus with the proceedings, and (iii) the remainder shall be retained by Camurus less [***] thereof, which amount shall be paid to Braeburn. If Camurus elects not to enforce the Camurus Platform IP in the Licensed Territory, then Braeburn shall have the option to do so in accordance with the following, subject to the prior written consent of Camurus. If Camurus gives such consent, then the following procedures shall apply in these circumstances:
(i) Where Braeburn has requested and been granted approval by Camurus to commence proceedings in relation to Camurus Platform IP in the Licensed Territory, it shall be entitled to require Camurus to join Braeburn as co-plaintiff. In such case, Camurus shall have the right to join as co-plaintiff. Camurus shall, at Braeburn’s cost and expense, provide all necessary assistance to Braeburn in relation to any such proceeding. If Camurus elects to be separately represented (which shall be at Camurus’ discretion), then such separate representation shall be at Camurus’ cost and expense.
(ii) If Braeburn succeeds in any such infringement proceedings whether at trial or by way of settlement, the proceeds of any award or damages or settlement in respect of such infringement proceedings shall first be applied (a) to reimburse Braeburn an amount equal to Braeburn’s costs of taking the proceedings, (b) to reimburse Camurus an amount equal to Camurus’ costs of assisting Braeburn with the proceedings, and (c) the remainder shall be retained by Braeburn less [***] thereof, which amount shall be paid to Camurus;
(iii) Braeburn shall not enter into a settlement, consent judgment or other voluntary final disposition of an action or claim or counterclaim under this Section 7.8 without the prior written approval of Camurus.
7.9 Xxxxx-Xxxxxx Certifications. If either Party (a) reasonably believes that a Third Party may be filing or preparing or seeking to file a generic or abridged NDA that refers to or relies on regulatory documentation for a Product that was submitted by Braeburn to any Regulatory Authority, (b) receives any notice of certification regarding any Patent Rights included in Camurus IP or Braeburn IP pursuant to the Xxxxx-Xxxxxx Act claiming that any such Patent Rights are invalid or unenforceable or claiming that the any such Patent Rights will not be infringed by the manufacture, use, marketing or sale of a product for which an ANDA is filed, or (c) receives any equivalent or similar certification or notice in any other jurisdiction, it shall notify the other Party in writing, identifying the alleged applicant or potential applicant and furnishing the information upon which such determination is based, and provide the other Party a copy of any such notice of certification within [***] of receipt and the Parties’ rights and obligations with respect to any legal action as a result of such certification shall be as set forth above in Section 7.8.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
8. CONFIDENTIALITY
8.1 Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, each Recipient and its Affiliates, Sublicensees and licensees in possession of Confidential Information shall maintain such Confidential Information as confidential and use it only for the purposes of this Agreement in accordance with this Section 8. This obligation shall continue for a period equal to the longer of: (a) [***] after the date of expiration or termination of this Agreement; or (b) for so long as the exceptions set out below in the next subsequent paragraph do not apply to the relevant Confidential Information. Each Party shall guard such Confidential Information using the same degree of care as it normally uses to guard its own confidential, proprietary information of like importance, but in any event no less than reasonable care. Notwithstanding the foregoing, the Recipient of the categories of Confidential Information identified in Sections 1.26(b) through (d) inclusive shall be relieved of the confidentiality and limited use obligations of this Agreement to the extent that the Recipient establishes by written evidence that:
(a) the Confidential Information was previously known to the Recipient from sources other than the Disclosing Party at the time of disclosure and other than under an obligation of confidentiality and non-use;
(b) the Confidential Information was generally available to the public or otherwise part of the public domain at the time of its disclosure;
(c) the Confidential Information became generally available to the public or otherwise part of the public domain after its disclosure to the Recipient Party other than through any act or omission of the Recipient Party in breach of this Agreement;
(d) the Confidential Information is acquired in good faith in the future by the Recipient Party from a Third Party who has a lawful right to disclose such information and who is not under an obligation of confidence to the Disclosing Party with respect to such information; or
(e) the Confidential Information is subsequently developed by or on behalf of the Recipient Party without use of the Disclosing Party’s Confidential Information.
8.2 For clarity, specific aspects or details of Confidential Information shall not be deemed to be within the public domain or in the possession of the Recipient Party merely because the Confidential Information is embraced by more general information in the public domain or in the possession of the Recipient Party. Further, any combination of Confidential Information shall not be considered in the public domain or in the possession of the Recipient Party merely because individual elements of such Confidential Information are in the public domain or in the possession of the Recipient Party unless the combination is in the public domain or in the possession of the Recipient Party.
8.3 Notwithstanding the above obligations of confidentiality and non-use a Recipient Party may:
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(a) disclose Confidential Information to a Regulatory Authority as reasonably necessary to obtain Regulatory Approval in a particular jurisdiction to the extent consistent with the licenses granted under terms of this Agreement;
(b) disclose Confidential Information: (i) to the extent such disclosure is reasonably necessary to comply with the order of a court; or (ii) to the extent such disclosure is required to comply with a legal requirement, including to the extent such disclosure is required in publicly filed financial statements or other public statements under rules governing a stock exchange (e.g., the rules of the United States Securities and Exchange Commission, NASDAQ, NYSE, or any other stock exchange on which securities issued by either Party may be listed), subject to Section 8.6;
(c) disclose Confidential Information by filing or prosecuting Patent Rights, the filing or prosecution of which is contemplated by this Agreement, without violating the above secrecy provision; it being understood that publication of such filings occurs in some jurisdictions within 18 months of filing, and that such publication shall not violate the above secrecy provision;
(d) disclose Confidential Information to such Recipient’s employees, Affiliates, contractors (including clinical researchers and CMO), licensees, agents, consultants and potential business partners, as such Recipient reasonably determines is necessary to receive the benefit of the licenses and rights granted or available to it under this Agreement or to fulfill its obligations pursuant to this Agreement; provided, however, that any such persons must be obligated to substantially the same extent as set forth in Section 8.1 to hold in confidence and not make use of such Confidential Information for any purpose other than those permitted by this Agreement and breach by such persons of their confidentiality obligations shall be deemed a breach by the Recipient of its confidentiality obligations hereunder;
(e) disclose Confidential Information): (i) to its actual or potential investment bankers; (ii) to existing and potential investors in connection with an offering or placement of securities for purposes of obtaining financing for its business and to actual and prospective lenders for the purpose of obtaining financing for its business and to potential licensees to the FC Technology; and (iii) to a bona fide potential acquirer or merger partner for the purposes of evaluating entering into a merger or acquisition; provided, however, that any such persons must be obligated to substantially the same extent as set forth in Section 8.1 to hold in confidence and not make use of such Confidential Information for any purpose other than those permitted by this Agreement; and
(f) disclose Confidential Information to its legal advisers for the purpose of seeking advice.
8.4 Nothing in this Section 8 restricts either Party from using or disclosing any of its own Confidential Information for any purpose whatsoever; provided, that to the extent Know-How is exclusively licensed by one Party to the other, the licensor may not continue to use and disclose such Know-How in a manner not consistent with the exclusivity of the license granted.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
8.5 Other than the press release pertaining to this transaction that the Parties have agreed upon and attached as Exhibit 8.5 to this Agreement and save as permitted in Section 8.3:
(a) neither Party shall make any public announcement or statement to the public containing Confidential Information without the prior written consent of the other. No such public announcements or statements shall be made without the prior review and consent of the appropriate individual designated for the purpose by the other Party: and
(b) save as may otherwise be provided herein neither Party shall mention or otherwise use the name or Trademark of the other Party or its Affiliates in any publication, press release, promotional material or other form of publicity without the prior written consent of the appropriate individual designated for the purpose by the other Party.
8.6 With respect to public disclosure required to be made pursuant to regulatory requirements or stock exchange rules applicable to a Party, each such Party will submit to the other Party a draft of any public announcement (“Proposed Disclosure”) related to the Product for review and comment at least [***] prior to the date on which such Party plans to make such announcement, or if fewer days are reasonably practicable, then the maximum number of days available to the Party prior to the date that die disclosure is required, and will review and reasonably consider in good faith any comments provided in response by the other Party. Additionally, the Party seeking to make the Proposed Disclosure shall use reasonable efforts to limit the information disclosed by seeking “confidential treatment,” to the extent permitted pursuant under applicable laws.
8.7 Notwithstanding the foregoing, Camurus shall be entitled to include Braeburn within a list of collaborators.
9. REPRESENTATIONS, WARRANTIES AND COVENANTS
9.1 Mutual Representations and Warranties of Camurus and Braeburn. Each of Camurus and Braeburn hereby represents and warrants to the other Party as of the Effective Date as follows:
(a) It is duly organized, validly existing and in good standing under the laws of the jurisdiction of incorporation. It has the requisite legal and company power and authority to conduct its business as presently being conducted and as proposed to be conducted by it and is duly qualified to do business in those jurisdictions where its ownership of property or the conduct of its business requires.
(b) It has all requisite legal and corporate power and authority to enter into this Agreement and to grant the rights described herein. All corporate actions on its part, its boards of directors or managers, or similar governing body and its equity holders necessary for (i) the authorization, execution, delivery and performance by it of this Agreement, and (ii) the consummation of the transactions contemplated hereby, have been duly taken.
(c) This Agreement is a legally valid and binding obligation of it, enforceable against it in accordance with its terms (except in all cases as such enforceability may be limited
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
by applicable bankruptcy, insolvency, reorganization, moratorium, or similar laws affecting the enforcement of creditors’ rights generally and except that the availability of the equitable remedy of specific performance or injunctive relief is subject to the discretion of the court or other tribunal before which any proceeding may be brought).
(d) The execution, delivery and performance of this Agreement by it does not conflict with any material agreement, instrument or understanding, oral or written, to which it is a party or by which it is bound, nor violate any law or regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
(e) No government authorization, consent, approval, license, exemption of or filing or registration with any court or governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, under any applicable laws currently in effect, is necessary for the closing of transactions contemplated by this Agreement or any other agreement or instrument executed in connection herewith, or for the performance by it of its obligations under this Agreement.
(f) It has and shall continue to have written contracts with all Third Parties (including employees and subcontractors) performing services on its behalf under this Agreement where such services are intended to create inventions that may be Collaboration Inventions that assign to it all Collaboration Inventions and rights therein.
9.2 Additional Representations and Warranties of Camurus. Camurus hereby further represents and warrants to Braeburn as of the Effective Date that:
(a) Exhibits 1.16 and 1.17 set forth a complete and accurate list of the Patent Rights included within the Camurus Platform IP and the Camurus Product IP as of the Effective Date. Camurus has disclosed to Braeburn all material information received by Camurus concerning the institution of any interference, opposition, reexamination, reissue, revocation, nullification or any other similar official proceeding involving any such Patent Rights anywhere in the Licensed Territory and in South Korea, Japan, Taiwan and China.
(b) Camurus is not aware of any pending or threatened actions, suits or other similar proceedings against it that questions the validity of any issued Camurus Patent Rights forming part of Camurus IP.
(c) Camurus is not aware that any of the issued Camurus Patent Rights forming part of Camurus IP are invalid or that the manufacture, use or sale of CAM2038 and CAM2048 as now formulated would infringe issued Patent Rights of Third Parties.
(d) To the extent necessary to grant Braeburn the rights provided for in this Agreement, Camurus Controls sufficient rights in the Camurus Platform IP and the Camurus Product IP.
(e) Camurus has not granted any right or license to any Third Party relating to any of the Camurus IP that would conflict or interfere with any of the rights or licenses granted to Braeburn hereunder.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
9.3 Camurus Acknowledgement. Camurus acknowledges that, in entering into this Agreement, Braeburn has relied upon information made available by Camurus, on site and through an electronic data room. Camurus represents and warrants to Braeburn that, to Camurus’ knowledge, said information provided by Camurus in connection with this Agreement is accurate in all material respects. Camurus further warrants and represents to Braeburn that it has not, as of the Effective Date, intentionally omitted to furnish Braeburn with any material information known to Camurus concerning the Camurus IP, which would reasonably be considered to be material to Braeburn’s decision to enter into this Agreement and to undertake the commitments and obligations set forth herein.
9.4 Additional Representations and Warranties of Braeburn. Braeburn hereby further represents and warrants to Camurus as of the Effective Date that:
(a) Braeburn has previously delivered to Camurus evidence satisfactory to Camurus of up to an aggregate of [***] in funding commitments which are committed for the development and commercialization of CAM2038 and CAM2048 (the “Financing Commitment”). The Financing Commitment ensures that Braeburn will have sufficient cash and other cash equivalents to pay all amounts due and perform and consummate the obligations to be performed by Braeburn under this Agreement.
(b) Braeburn has utilized its own scientific, marketing and distribution expertise and experience to analyze and evaluate both the scientific and commercial value of Products in the Territory.
9.5 Disclaimer of Warranties. EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT OR MANDATED BY APPLICABLE LAW (WITHOUT THE RIGHT TO WAIVE OR DISCLAIM), NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY WITH RESPECT TO THE INTELLECTUAL PROPERTY LICENSED UNDER THIS AGREEMENT, THE PRODUCTS, ANY TECHNOLOGY, GOODS, SERVICES, RIGHTS, OR OTHER SUBJECT MATTER OF THIS AGREEMENT AND HEREBY DISCLAIMS ALL WARRANTIES, CONDITIONS OR REPRESENTATIONS OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING IMPLIED WARRANTIES OF PERFORMANCE, MERCHANTABILITY, SATISFACTORY QUALITY, FITNESS FOR A PARTICULAR PURPOSE OR NON-INFRINGEMENT OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS.
9.6 Mutual Covenants. Each Party hereby covenants to the other Party that:
(a) Such Party shall to the extent applicable, perform its activities pursuant to this Agreement in material compliance with applicable laws, including GMP and GCP.
(b) Each Party shall notify the other Party in writing promptly in the event that it has actual knowledge of the material breach of any covenant under this Section 9.6 or the material breach of any representation or warranty provided by either Party under Section 9.1, by Camurus under Sections 9.2 or 9.3 or by Braeburn under Section 9.4.
9.7 Camurus Covenant. Camurus hereby covenants and agrees that it will not, during the Term, grant any right or license to any Third Party relating to the Camurus IP, which would conflict with any of the rights or licenses granted to Braeburn hereunder.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
10. INDEMNIFICATION
10.1 Indemnification by Braeburn. Except to the extent required to be indemnified by Camurus under Section 10.2, Braeburn shall indemnify, defend and hold harmless Camurus, its Affiliates, and its and their respective, directors, officers, employees and agents (collectively, the “Camurus Indemnified Party”) against any and all claims, liabilities, losses, damages, costs or expenses, including reasonable attorneys’ fees, arising out of any claim or action brought by a Third Party (collectively, “Losses”) incurred or suffered by the Camurus Indemnified Party to the extent arising out of or caused by:
(a) the gross negligence or wrongful intentional acts or omissions of Braeburn and/or its Affiliates and/or its or their respective directors, officers, employees and agents, in connection with Braeburn’s performance of its obligations or exercise of its rights under this Agreement;
(b) the development, use, manufacture, distribution, marketing, promotion or sale of Products by or on behalf of Braeburn or its Affiliates or Sublicensees in the Licensed Territory (including any claims based upon product liability and any claims arising from Camurus’ or its Affiliates’ performance of the Development Plan); or
(c) the breach by Braeburn of one or more of its representations, warranties or other obligations under this Agreement.
10.2 Indemnification by Camurus. Except to the extent required to be indemnified by Braeburn under Section 10.1, Camurus shall indemnify, defend and hold harmless Braeburn, its Affiliates, and its and their respective, directors, officers, employees and agents (collectively, the “Braeburn Indemnified Party”) against any and all Losses (as defined above) incurred or suffered by the Braeburn Indemnified Party to the extent arising out of or caused by:
(a) the gross negligence or wrongful intentional acts or omissions of Camurus and/or its Affiliates and/or its or their respective directors, officers, employees and agents, in connection with Camurus’ performance of its obligations or exercise of its rights under this Agreement;
(b) the development, use, manufacture, distribution, marketing, promotion or sale of Products by or on behalf of Camurus or its Affiliates in the Camurus Territory (including any claims based upon product liability); or
(c) the breach by Camurus of one or more of its representations, warranties or other obligations under this Agreement.
10.3 Notification of Liabilities/Losses. In the event that either Party intends to seek
indemnification for any claim under any of Sections 10.3 or 10.2, it shall inform the other Party of the claim promptly after receiving notice of the claim.
(a) In the case of a claim for which Camurus seeks indemnification under Section 10.1, Camurus shall permit Braeburn to direct and control the defense of the claim and shall provide such reasonable assistance as is reasonably requested by Braeburn (at Braeburn’s cost) in the defense of the claim; provided, that nothing in this Section 10.3(a) shall
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
permit Braeburn to make any admission on behalf of Camurus or to settle any claim or litigation which would impose any financial obligations on Camurus without the prior written consent of Camurus, such consent not to be unreasonably withheld, conditioned or delayed.
(b) In the case of a claim for which Braeburn seeks indemnification under Section 10.2, Braeburn shall permit Camurus to direct and control the defense of the claim and shall provide such reasonable assistance as is reasonably requested by Camurus (at Camurus’ cost) in the defense of the claim; provided, that nothing in this Section 10.3(b) shall permit Camurus to make any admission on behalf of Braeburn, or to settle any claim or litigation which would impose any financial obligations on Braeburn without the prior written consent of Braeburn, such consent not to be unreasonably withheld, conditioned or delayed.
10.4 Right to Participate in Defense. Without limiting Section 10.3, any indemnitee will be entitled to participate in, but not control, the defense of a Third Party claim for which it has sought indemnification hereunder and to employ counsel of its choice for such purpose; provided, however, that such employment will be at the indemnitee’s own expense unless (a) the employment and reimbursement thereof has been specifically authorized by the indemnifying Party in writing, or (b) the indemnifying Party has failed to assume the defense of the claim and employ counsel in accordance with Section 10.3 (in which case the indemnified Party will control the defense),
10.5 Cooperation. If the indemnifying Party chooses to defend or prosecute any Third Party claim, the indemnified Party will, and will cause each other indemnitee to, cooperate in the defense or prosecution thereof and will furnish such records, information and testimony, provide such witnesses and attend such conferences, discovery proceedings, hearings, trials and appeals as may be reasonably requested in connection with such Third Party claim. Such cooperation will include access during normal business hours afforded to the indemnifying Party to, and reasonable retention by the indemnified Party of, records and information that are reasonably relevant to such Third Party claim, and making indemnities and other employees and agents available on a mutually convenient basis to provide additional information and explanation of any material! provided hereunder, and the indemnifying Party will reimburse the indemnified Party for all of its reasonable out-of-pocket expenses incurred in connection with such cooperation.
10.6 Exclusive Remedy. Each Party agrees that its sole and exclusive remedy with respect to Losses shall be pursuant to the indemnification provisions of this Section 10. NOTWITHSTANDING THE FOREGOING, NEITHER PARTY LIMITS OR EXCLUDES ITS LIABILITY FOR FRAUDULENT MISREPRESENTATION, DEATH OR PERSONAL INJURY ARISING FROM ITS GROSS NEGLIGENCE OR WILLFUL MISCONDUCT.
10.7 Insurance. Immediately (a) upon the first dosing of a Product to a human in a Clinical Trial in the Licensed Territory by Braeburn, its Affiliates or its permitted Sublicensees, and for a period of 5 years after the expiration of this Agreement or the earlier termination thereof, Braeburn shall maintain Clinical Trial Insurance in an amount not less than [***] per occurrence, and (b) upon the First Commercial Sale of a Product in the Licensed Territory by Braeburn, its Affiliates or its permitted Sublicensees, and for a period of [***] after the expiration of this Agreement or the earlier termination thereof, Commercial General Liability Insurance and Product Liability/Completed Operations Insurance, including contractual liability coverage, in an
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
amount that is customary in the industry for similarly situated parties but not less than [***] per occurrence and annual aggregate bodily injury/property damage combined. Immediately (i) upon the first Regulatory Approval of a Product in the Camurus Territory by Camurus, its Affiliates or its licensees, and for a period of [***] after the expiration of this Agreement or the earlier termination thereof, shall maintain Commercial General Liability Insurance and Product Liability coverage, in an amount not less than [***] per occurrence bodily injury/property damage combined and [***] aggregate annually. In addition, Camurus shall maintain insurance cover for Clinical Trials of Product for which Camurus is a sponsor in an amount required by local laws or customary for small and mid-sized Scandinavian pharmaceutical development company. Upon written request, the insuring Party shall provide the other Party with a certificate of insurance attesting to such coverage. It is understood and agreed that this insurance shall not be construed to limit either Party’s liability with respect to its indemnification obligations hereunder.
11. TERM AND TERMINATION
11.1 Term of Agreement. This Agreement shall become effective as of the Effective Date and, unless earlier terminated pursuant to this Section 11, shall continue in full force and effect until the expiration of all Royalty Terms (the “Term”). On a country-by-country and Product-by-Product basis, after expiration of the Royalty Term for the Product in each country in the Licensed Territory, Braeburn shall have a fully paid-up, irrevocable, perpetual license, sublicenseable without restriction, to develop, make or have made, use, sell, offer for sale, import, market and promote such Products in such country.
11.2 Braeburn Termination for Convenience. Braeburn may terminate this Agreement without cause on a Product-by-Product basis or in its entirety at any time by giving (a) if the Agreement is terminated in full prior to first NDA Approval of the first Product, 90 days’ prior written notice, (b) if the Agreement is terminated solely with respect to a Product prior to NDA Approval of such Product, 90 days’ prior written notice, or (c) 180 days’ prior written notice in all other cases.
11.3 Termination for Material Breach or Bankruptcy.
(a) Upon the material breach by one Party under this Agreement, the other Party may notify the breaching Party of such breach in writing and with specificity as to the alleged breach.
(b) In the event that a material breach by Braeburn is not cured within 60 days (or 30 days for any payment default) after written notice pursuant to Section 1 1.3(a), Camurus shall have the right to terminate this Agreement immediately upon written notice and all licenses granted by Camurus to Braeburn hereunder shall terminate, subject to the terms of Section 11.4.
(c) In the event that a material breach by Camurus is not cured within 60 days (or 30 days for any payment default) after written notice pursuant to Section 11.3(a), Braeburn shall have the right to immediately terminate this Agreement upon written notice and all licenses granted by Braeburn to Camurus hereunder shall terminate, subject to the terms of Section 11.4.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(d) If either Party reasonably disputes that the material breach described in the notice provided by the other Party exists, then the cure period described above shall be tolled until such time as it is finally determined under Section 12.14 whether the material breach exists; provided, that the negotiation period for the executive officers in Section 12.14(a) shall be limited to 10 days, the arbitration conducted under Sections 12.15(b) and (c), if any, shall be conducted and completed within 90 days of the appointment of arbitrators pursuant to Section 12.14(c), and the Parties shall adopt and comply with any additional rules or procedures instituted by the arbitrator in order to conduct and complete the arbitration within this expedited period.
(e) Either Party may, without limiting other available remedies, terminate this Agreement in whole by notice to the other Party in the event (i) the other Party shall have become bankrupt or shall have made an assignment for the benefit of its creditors; (ii) there shall have been appointed a trustee or receiver for the other Party or for all or a substantial part of its property; or (iii) any case or proceeding shall have been commenced or other action taken by or against the other Party in bankruptcy or seeking reorganization, liquidation, dissolution, winding-up, arrangement, composition or readjustment of its debts or any other relief under any bankruptcy, insolvency, reorganization or other similar act or law of any jurisdiction now or hereafter in effect, and any such event shall have continued for 60 days undisputed, undismissed, unbonded and/or undischarged.
(f) In the event that Braeburn or any of its Affiliates or Sublicensees commences or otherwise pursues, directly or indirectly (or voluntarily assists Third Parties to do so, other than as required by law or legal process), any proceeding seeking to have any of the Patent Rights included within the Camurus Platform IP or the Camurus Product IP revoked or declared invalid, unpatentable, or unenforceable, Camurus may declare a material breach hereunder with immediate effect; provided, that Braeburn may file requests for re-examination of or re-issue of Patent Rights included within the Camurus Platform IP or the Camurus Product IP to the extent that such actions are reasonably necessary or desirable to ensure adequate protection for Products.
11.4 Effect of Termination.
(a) Upon termination of this Agreement by Braeburn pursuant to Section 11.2 or by Camurus pursuant to Section 11.3(b), 11.3(e), 11.3(f) or 12.5:
(i) all licenses granted by Camurus to Braeburn shall terminate (including all rights to use any Camurus IP);
(ii) subject to subparagraph (v) below, all licenses and other rights granted Camurus to use any of the Braeburn Development Data, Regulatory Approvals and other Braeburn IP for use in the Camurus Territory shall continue, subject to all indemnity and other obligations of Camurus hereunder in respect thereof;
(iii) Braeburn shall assign and transfer all regulatory filings and Regulatory Approvals related to the Products to Camurus;
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(iv) If not already owned by Camurus, Braeburn shall license to Camurus on a royalty-free and exclusive basis all rights to all Product Trademarks for use with such Products in the Territory;
(v) Braeburn shall assign to Camurus Collaboration Inventions, Braeburn Development Data and Braeburn IP Controlled by Braeburn, if any, that relate to solely to the development, manufacture, use or sale of the Products; provided, that if such Collaboration Inventions, Development Data, and Braeburn IP Controlled by Braeburn are not solely related to, but are necessary for, the development, manufacture, use or sale of such Products, then Braeburn shall grant to Camurus the exclusive, worldwide, perpetual, paid-up license (with right to sublicense) under such Collaboration Inventions, Development Data and Braeburn IP to develop, manufacture, sell and use Products;
(vi) Braeburn shall complete at Camurus’ cost (or if reasonably practicable, allow Camurus or its designee to complete) any on-going Clinical Trials for which it has responsibility and in which patient dosing has commenced, subject to the payment by Braeburn for all costs associated with completion of such Clinical Trials through the effective date of termination;
(vii) If Braeburn has manufactured any Products, Braeburn at its option shall either transition the manufacturing process to Camurus or a Third Party contract manufacturer designated by Camurus or supply such Product to Camurus; provided, that if Braeburn chooses to transition the manufacturing process to Camurus or a mutually agreed Third Party contract manufacturer, Braeburn shall continue to supply such Product until the completion of the transition and associated regulatory approvals have been received, but in no event for a period exceeding 12 months from the date of termination;
(viii) Braeburn shall promptly transfer and assign to Camurus its and its Affiliates’ entire right, title and interest in and to any copyrights for promotional materials and training materials for the Products in the Territory and will execute all documents that may be necessary to transfer the title to such copyrights to Camurus;
(ix) Braeburn shall grant Camurus a world-wide, perpetual, non-exclusive license, with the right to sublicense, to develop, make or have made, use, sell, offer for sale, import, market and promote Products in the Territory under the Braeburn Product IP not covered by (v) above;
(x) If notice of termination is given after NDA Approval of a Product, then on a Product-by-Product basis for such Product, Camurus shall pay Royalties to Braeburn equal to [***] on the annual Net Sales of such Product in the Territory. Royalties shall be payable on a Product-by-Product and country-by-country basis beginning on the First Commercial Sale of such Product in such country and ending on the later of (a) 12 years after the date of such First Commercial Sale of a Product in such country; and (b) the expiration of the last to expire Valid Claim of all Patent Rights included within the Braeburn IP covering or claiming the Product in such country; and
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Section 5.5 shall apply mutatis mutandis to Camurus’ obligation to pay royalties with respect thereto;
(xi) Each of Braeburn’s Sublicensees will continue to have the rights and licenses set forth in their sublicense agreements, subject to the continued performance of their obligations thereunder and payment to Camurus by such Sublicensees of milestones and Royalties due hereunder in respect of such Sublicensees; provided, however, that each such Sublicensee agrees in writing that Camurus is entitled to enforce all relevant terms and conditions of such sublicense agreement directly against such Sublicensee; provided, further, that such Sublicensee is not then in breach of its sublicense agreement; and
(xii) Braeburn shall cooperate in any reasonable manner requested by Camurus to achieve a smooth transition of the development, manufacturing, marketing and sale of the Product to Camurus or its licensees in the Licensed Territory.
(b) Upon termination of this Agreement by Braeburn pursuant to Section 11.3(c), 11.3(e) or 12.5:
(i) all licenses granted by Braeburn to Camurus shall terminate (including all rights to use any Braeburn IP);
(ii) at Braeburn’s option, all licenses granted by Camurus to Braeburn hereunder shall continue in full force and effect, subject to the continuing obligation to pay Royalties as well as sales and development milestones; provided, that Braeburn’s obligation to pay Royalties and milestones shall apply only if Braeburn terminates this Agreement pursuant to Section 11.3(c) or 12.5, and not for a termination pursuant to Section 11.3(e); provided, further, that if Braeburn terminates this Agreement pursuant to Section 11.3(c), then the sales and development milestones payable under Sections 5.3 and 5.6 shall be reduced by an amount equivalent to the damages incurred by Braeburn resulting from Camurus’ breach of this Agreement;
(iii) Camurus’ obligations hereunder to provide Know-How and other materials and information to enable the use of such licenses shall continue;
(iv) Camurus’ right to use any Braeburn Development Data and Regulatory Approvals shall terminate;
(v) each of Camurus’ sublicensees will continue to have the rights and licenses set forth in their sublicense agreements, subject to the continued performance of their obligations thereunder; provided, however, that each such sublicensee agrees in writing that Braeburn is entitled to enforce all relevant terms and conditions of such sublicense agreement directly against such sublicensee; provided, further, that such sublicensee is not then in breach of its sublicense agreement; and
(vi) in addition to the surviving provisions set forth in Section 11.6 below, the following provisions shall survive termination of the Agreement if Braeburn elects the option to continue the licenses granted by Camurus under Section 11.4(b)(ii):
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Sections 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.8, 3.1, 3.3, 3.7, 3.8, 4.1, 4.2, 4.3, 4.6, 5, 7.1(c), 7.2, 7.3, 7.4, 7.5, 7.8, 7.9 and 9.7.
11.5 Accrued Rights. Termination of this Agreement for any reason will be without prejudice to any rights that will have accrued to the benefit of a Party prior to the effective date of such termination. Such termination will not relieve a Party from obligations that are expressly indicated to survive the termination of this Agreement.
11.6 Surviving Provisions. Except as otherwise provided in Section 1.4(b)(vi) above, the following Sections and Sections of this Agreement shall survive any expiration or termination of this Agreement for any reason: Sections 1 (to the extent applicable to the other surviving provisions), 8, 9.5, 10, 11.4, 11.5, 11.6 and 12.
12. MISCELLANEOUS PROVISION’S
12.1 Consequential Damages. IN NO EVENT SHALL EITHER PARTY OR THEIR AFFILIATES BE LIABLE FOR SPECIAL, PUNITIVE, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES AS WELL AS LOST PROFITS, WHETHER BASED ON CONTRACT, TORT OR ANY OTHER LEGAL THEORY AND IRRESPECTIVE OF WHETHER SUCH PARTY HAS BEEN ADVISED OF THE POSSIBILITY OF ANY SUCH LOSS OR DAMAGE; PROVIDED, THAT THIS LIMITATION SHALL NOT LIMIT THE INDEMNIFICATION OBLIGATION OF SUCH PARTY UNDER THE PROVISIONS OF SECTION 10 FOR SUCH DAMAGES CLAIMED BY A THIRD PARTY AND NOTHING IN THIS SECTION 12.1 IS INTENDED TO LIMIT BRAEBURN’S PAYMENT OBLIGATIONS UNDER SECTION 5.
12.2 Assignment. Neither Party shall have the right to assign this Agreement, nor any of its rights hereunder, nor delegate any of its obligations hereunder, without the prior written consent of the other Party, which shall not be unreasonably withheld or delayed. Notwithstanding the foregoing, (a) Camurus and Braeburn may assign this Agreement to any purchaser of all or substantially all of its assets or to any successor entity resulting from any merger or consolidation of Camurus or Braeburn with or into such entity, and (b) Camurus and Braeburn may assign this Agreement to any of its Affiliates but only for as long as such Affiliate remains an Affiliate of the assigning Party, provided that the assigning Party remains primarily liable for all of its obligations hereunder and such Affiliate agrees to be bound hereunder. Any attempt to assign this Agreement in breach of the foregoing shall be void. This Agreement shall be binding upon and inure to the benefit of the Parties hereto and each of their successors and permitted assigns.
12.3 Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
12.4 Compliance with Laws. Each Party shall review in good faith and cooperate in taking actions to ensure compliance of this Agreement and the Parties’ activities hereunder with all applicable laws, rules, ordinances, regulations and guidelines. Each Party shall provide the other Party such reasonable assistance as may be required for the Party requesting such assistance to comply with all such laws, rules, ordinances, regulations and guidelines of all governmental entities, bureaus, and agencies having jurisdiction pertaining to this Agreement,
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
including obtaining all import, export and other permits, certificates, licenses or the like required by such laws, rules, ordinances, regulations and guidelines necessary to permit the Parties to perform hereunder and to exercise their respective rights hereunder.
12.5 Force Majeure. Neither Party shall be responsible or liable in any way for failure or delay in carrying out the terms of this Agreement resulting from fire, flood, other natural disasters, war, labor difficulties, interruption of transit, accident, explosion, civil commotion, and acts of any governmental authority; provided, that the Party so affected shall give prompt notice thereof to the other. If any such cause prevents either Party from performing any of its material obligations hereunder for more than 180 days, the other Party may then terminate this Agreement upon 30 days prior notice. Except as provided in the preceding sentence, no such failure or delay shall terminate this Agreement, and each Party shall complete its obligations hereunder as promptly as reasonably practicable following cessation of the cause or circumstances of such failure or delay.
12.6 Notices. All notices and other communications hereunder shall be in writing and shall be deemed given when delivered personally or by facsimile transmission (receipt verified), 5 days after mailed by registered or certified air mail (return receipt requested), postage prepaid, or 2 days after sent by express courier service, to the Parties at the following addresses (or at such other address for a Party as shall be specified by like notice; provided, that notices of a change of address shall be effective only upon receipt thereof):
If to Camurus, addressed to:
Camurus AB
Xxxxx Xxxxxxx Xxxx, XX-000 00 Xxxx, Xxxxxx
Attn: Chief Executive Officer
Facsimile: x00 00 000 00 00
If to Braeburn, addressed to:
Braeburn Pharmaceuticals BVBA SPRL
c/o Braeburn Pharmaceuticals, Inc.
00 Xxxxxxx Xxxxxx
Xxxxxxxxx, Xxx Xxxxxx 00000
Xxxxxx Xxxxxx of America
Attn: Chief Executive Officer
Facsimile: 000-000-0000
With copies (which shall not constitute notice) to:
Braeburn Pharmaceuticals, Inc.
Attn: Xxxxxxxx Xxxxx, JD, PhD, General Counsel and VP Policy
Email: Xxxxxxxx@xxxxxxxxxxxxxx-xx.xxx
Xxxxx Lovells XX XXX
Xxxxxxxxxxxxx Xxxxx, Xxxxx 0000
Xxxxxxxxx, Xxxxxxxx 00000
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
United States of America
Attn: Xxxxx X. Xxxxx
Facsimile: 000-000 0000
Email: xxxxx.xxxxx@xxxxxxxxxxxx.xxx
12.7 Amendment. No amendment, modification or supplement of any provision of this Agreement shall be valid or effective unless made in a writing that explicitly refers to this Agreement and that is signed by a duly authorized officer of each Party.
12.8 Waiver. Except to the extent otherwise expressly set forth in this Agreement, the rights and remedies of the Parties set forth herein or otherwise available at law or equity are cumulative and not alternative. No provision of this Agreement shall be waived by any act, omission or knowledge of any Party or its agents or employees except by an instrument in writing expressly waiving such provision and signed by a duly authorized officer of the waiving Party.
12.9 Counterparts. This Agreement shall be executed in two or more counterparts, each of which shall contain the signature of the Parties and all such counterparts shall constitute one and the same agreement. Signatures transmitted via .pdf shall be treated as original signatures.
12.10 Descriptive Headings. The descriptive headings of this Agreement are for convenience only, and shall be of no force or effect in construing or interpreting any of the provisions of this Agreement.
12.11 Severability. Whenever possible, each provision of this Agreement shall be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be prohibited by or invalid under applicable law, such provision shall be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Agreement and the Parties shall in good faith seek to agree on an alternative provision reflecting the intent of the Parties that is enforceable.
12.12 Entire Agreement. This Agreement, together with the Exhibits hereto, shall constitute and contain the complete, final and exclusive understanding and agreement of the Parties and cancels and supersedes any and all prior negotiations, correspondence, understandings and agreements, whether oral or written, between the Parties with respect to the subject matter hereof. The Confidentiality Agreement previously entered into between the Parties shall terminate as of the Effective Date and the Parties rights and obligations in respect of the Confidential Information disclosed under the Confidentiality Agreement shall be governed by this Agreement.
12.13 Governing Law. This Agreement and all disputes arising out of it (including noncontractual disputes shall be governed by and interpreted in accordance with the substantive laws of England, without regard to the choice of law provisions thereof.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
12.14 Dispute Resolution.
(a) The Parties recognize that a bona fide dispute as to certain matters may from time to time arise during the Term of this Agreement that relate to any Party’s rights or obligations hereunder. In the event of the occurrence of any dispute arising out of or relating to this Agreement, including any question regarding its existence, validity or termination, either Party may, by written notice to the other, have such dispute referred to its respective officer designated below or their designee, for attempted resolution by good faith negotiations within 30 days after such notice is received. If either Party desires to pursue arbitration under Section 12.14(b) below to resolve any such dispute, a referral to such executives under this Section 12.14(a) shall be a mandatory condition precedent. Said designated officers are as follows.
For Camurus: Chief Executive Officer
For Braeburn: Chief Executive Officer
(b) In the event that the designated officers are unable to resolve the dispute within such 30 day period, then the dispute shall upon either Party’s written request be finally settled by binding arbitration as provided below.
(c) Any arbitration proceeding shall be administered by the Arbitration Institute of International Chamber of Commerce. The place of arbitration shall be London, England. The arbitration shall be conducted before [***] arbitrators, with one arbitrator appointed by each of Braeburn and Camurus and the third appointed by the Arbitration Institute and in English. The award of arbitration shall be final and binding upon both Parties. Each Party shall bear its own attorneys’ fees, costs and disbursements arising out of the arbitration, and shall pay an equal share of the fees and costs of the arbitrators; provided, that the arbitrator shall be authorized to determine whether a Party is the prevailing Party, and if so, to award to that prevailing Party reimbursement for its reasonable attorneys’ fees, costs and disbursements (including, for example, witness fees and expenses, photocopy charges and travel expenses). All proceedings and decisions of the arbitrator shall be deemed Confidential Information of each of the Parties, and shall be subject to Section 8.
(d) The procedures specified in this Section 12.14 shall be the sole and exclusive procedures for the resolution of disputes between the Parties arising out of or relating to this Agreement; provided, that a Party, without prejudice to the above procedures, may seek injunctive relief or other provisional judicial relief if in its sole judgment such action is necessary to avoid irreparable damage Despite such action the Parties shall continue to participate in good faith in the procedures specified in this Section 12.14.
(e) Each Party is required to continue to perform its obligations under this Agreement pending final resolution of any such dispute. From the date that a dispute is referred to arbitration until such time as the dispute has been finally settled, the time period during which a Party must cure an alleged breach of this Agreement that is the subject matter of the dispute shall be suspended.
12.15 Independent Contractors. Nothing herein shall be construed to create any relationship of employer and employee, agent and principal, partnership or joint venture between the Parties. Each Party is an independent contractor. Neither Party shall have
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
authority to make any statements, representations, or commitments of any kind, or to take any action which shall be binding on the other Party, except as may be explicitly provided for herein or otherwise authorized in writing.
[Remainder of Page Intentionally Left Blank - Signature Pages to Follow]
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
This Agreement has been executed in two original copies of which the Parties have taken one each. The Agreement shall come into force on the Effective Date.
|
For and on behalf of Camurus AB |
|
|
|
Date: |
|
|
|
Signed by: |
|
|
|
Full name: Xxxxxxx Xxxxxx |
|
|
|
Position: President and Chief Executive Officer |
|
|
|
|
|
For and on behalf of Braeburn Pharmaceuticals BVBA SPRL |
|
|
|
Date: |
|
|
|
Signed by: |
|
|
|
Full name: Xxxxxx Xxxxxxx |
|
|
|
Position: Manager |
|
|
|
|
|
Date: |
|
|
|
Signed by: |
|
|
|
Full name: Xxxxxxx Xxxxxxx |
|
|
|
Position: President and Chief Executive Officer |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 1.16
CAMURUS PLATFORM IP IN LICENSED TERRITORY, JAPAN, SOUTH KOREA,
TAIWAN AND CHINA
|
Case Ref. |
|
Country |
|
Application No. |
|
Internal Title |
|
Earliest Priority |
|
Application Date |
|
Patent No. |
|
Patent Date |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Case Ref. |
|
Country |
|
Application No. |
|
Internal Title |
|
Earliest Priority |
|
Application Date |
|
Patent No. |
|
Patent Date |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Case Ref. |
|
Country |
|
Application No. |
|
Internal Title |
|
Earliest Priority |
|
Application Date |
|
Patent No. |
|
Patent Date |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
|
|
[***] |
|
|
|
|
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Case Ref. |
|
Country |
|
Application No. |
|
Internal Title |
|
Earliest Priority |
|
Application Date |
|
Patent No. |
|
Patent Date |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 1.17
CAMURUS PRODUCT IP
|
Case Ref. |
|
Country |
|
Application No. |
|
Title |
|
Case Status |
|
Earliest Priority |
|
Application Xxxx |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 1.19
CAMURUS TRADEMARKS
|
Trademarks |
|
Country |
|
Classes |
|
Registration |
|
Application |
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
|
|
|
|
|
|
|
|
|
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
|
[***] |
|
[***] |
|
[***] |
|
|
|
[***] |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 2.6
CO-PROMOTION TERMS
|
Product Sales |
|
Camurus shall make and book all sales of the Products in Belgium, and shall be responsible for receiving and filling orders, controlling invoicing, collection of payments, returns, charge-backs and rebates on sales of the Products, and shall have sole control over pricing strategies and distribution of the Products, except for distribution of samples. |
|
|
|
|
|
Co-Promotion Payments |
|
Camurus shall pay to Braeburn a percentage of Net Sales to be determined based on the percentage of the sales force promoting the Product in Belgium provided by Braeburn: (i) X% during the first 12 months after launch, (ii) X% during the next 12 months and (iii) X% thereafter. |
|
|
|
|
|
Joint Commercialization Team |
|
Camurus and Braeburn shall establish a Joint Commercialization Team (“JCT”) to plan, implement and coordinate the promotion, marketing and sale of the Products. Beginning with the filing of the first application for NDA Approval in Belgium, the JCT shall meet as necessary, but in no event less than quarterly or as otherwise agreed by the Parries, at such locations as the Parties agree. Camurus will have final decision making authority on all commercialization matters. |
|
|
|
|
|
Establishment of Commercialization Plan |
|
Prior to launch of the Products and on an annual basis thereafter, the Parties shall develop a Commercialization Plan which shall include an annual plan and budget for the promotion and marketing of the Products in Belgium, which shall be approved by the JCT and shall be based upon a centrally developed, coordinated marketing approach. The Commercialization Plan shall contain the number of details to be carried out during the year, each Party’s allocation of details based upon either percentage or specialists, and the number of sales representatives required to effectively perform each Party’s level of detailing and other promotional activities. The allocation of Details shall be determined by Camurus in an equitable manner. |
|
|
|
|
|
Use and Distribution of Promotional Materials |
|
Pursuant to the Commercialization Plan, the Parties shall collaborate to develop advertising, promotional, educational and other related materials to distribute to Third Parties for the marketing, advertising and |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
|
|
promotion of the Product. Camurus shall have primary responsibility to produce all promotional materials developed in collaboration with Braeburn, and shall provide such materials to Braeburn in accordance with the Commercialization Plan. Each Party’s sales force shall use, and if applicable, distribute promotional materials to healthcare professionals to whom it details the Product. Promotional materials shall not be used unless approved by the JCT. All such promotional materials shall indicate that both Camurus and Braeburn participate in the development and commercialization of the Product, and shall display the name and trademarks/logos of the Parties with equal prominence. |
|
|
|
|
|
Samples |
|
The Parties shall use and distribute samples in accordance with the Commercialization Plan and all applicable laws. |
|
|
|
|
|
Sales Force Training |
|
The Parties shall each establish a sales force of sales representatives to co-promote the Product in Belgium. Braeburn shall develop training programs for the Product, and each Party shall utilize such training programs to ensure a consistent, focused promotional strategy. Braeburn shall be responsible for providing such training at no cost to Camurus and Braeburn shall be responsible for all costs for its sales force, including costs associated with recruitment, hiring, compensation and travel. Braeburn shall treat the Braeburn and Camurus sales representatives as a combined sales force and shall provide the Camurus representatives with the same support and assistance it provides its own representatives. |
|
|
|
|
|
Term |
|
Unless earlier terminated, the Co-Promotion Agreement shall remain in effect for so long as Camurus is selling the applicable Product in the Belgium. |
|
|
|
|
|
Trademarks/Logos |
|
Each Party shall grant the other Party such rights under its trademarks/logos as shall be necessary to perform the co-promotion activities. Each Party’s right to use the other Party’s trademarks shall be governed by the terms of the License Agreement. |
|
|
|
|
|
Compliance with Laws |
|
The Parties shall comply with all applicable laws and regulations, and regulatory authority policies and guidelines relating to the promotion of the Product. |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
|
Indemnification |
|
Camurus and Braeburn shall indemnify each other as provided in the License Agreement. |
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 3.1
CAM2038 DEVELOPMENT PLAN
See attached.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Exhibit 3.1
Development Plan for CAM2038 (q1w & q4w)
31 Nov 2014

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
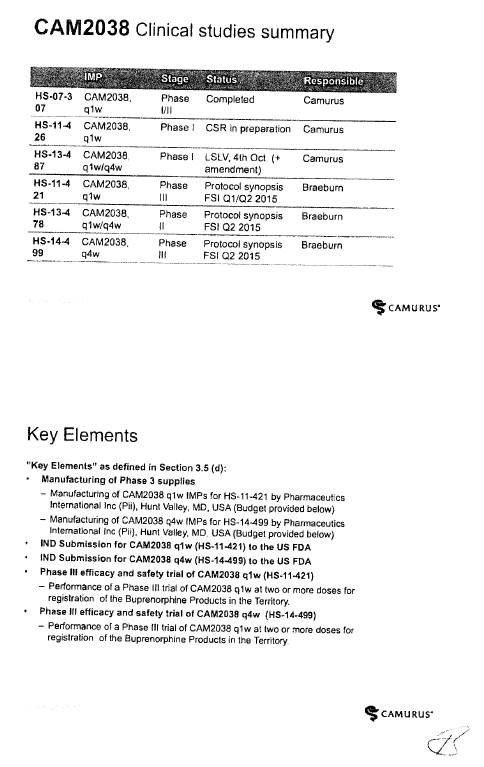
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
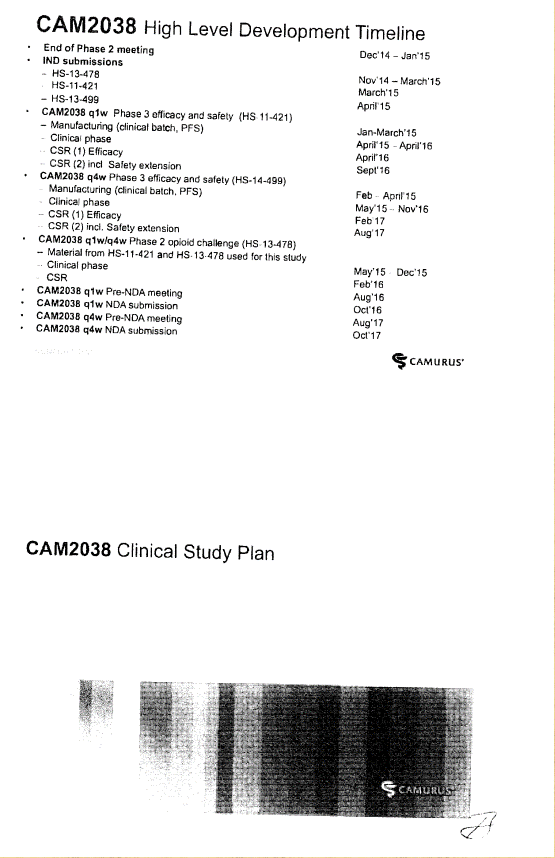
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
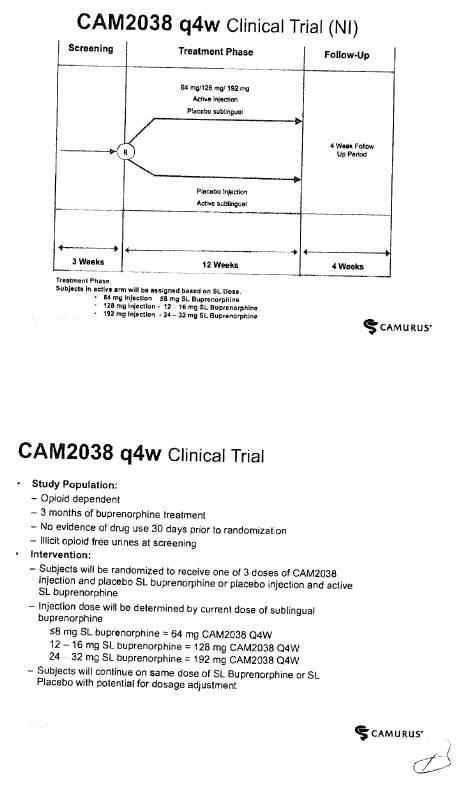
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
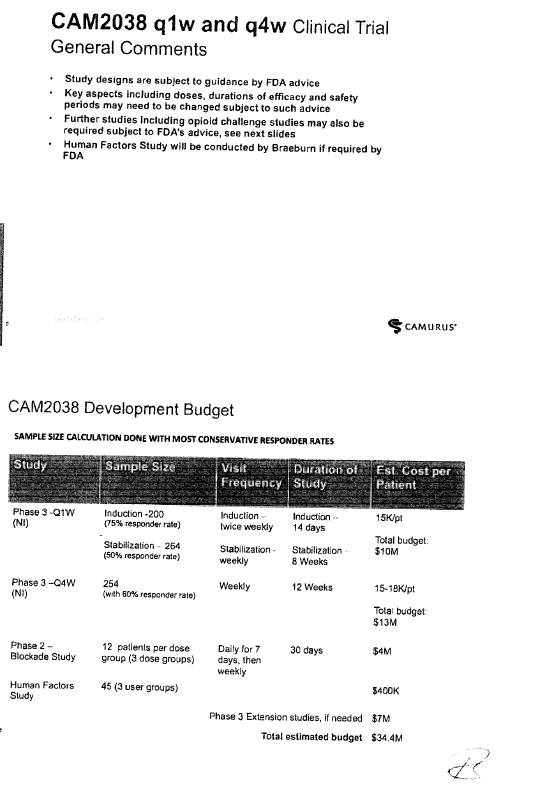
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
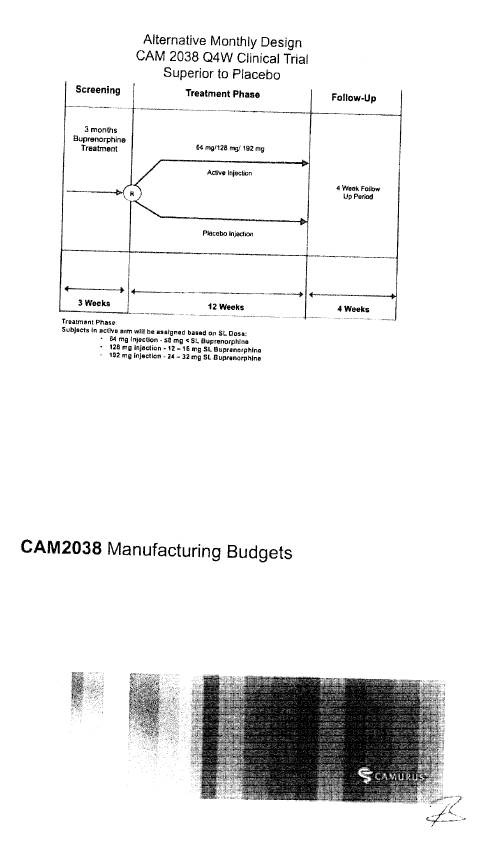
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
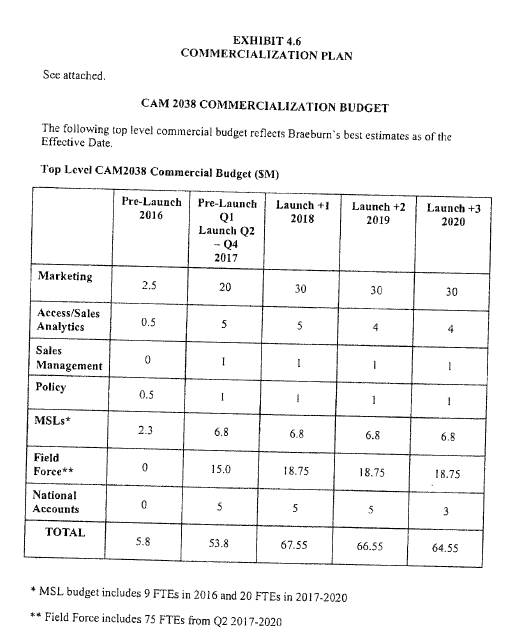
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
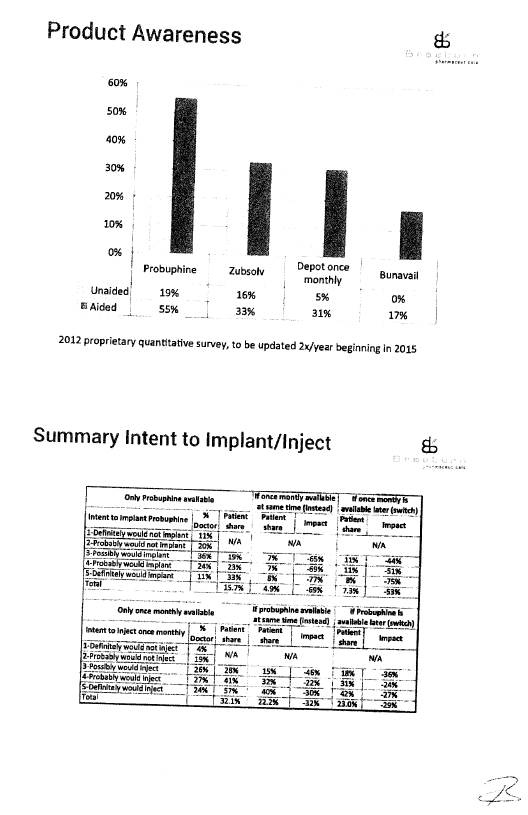
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
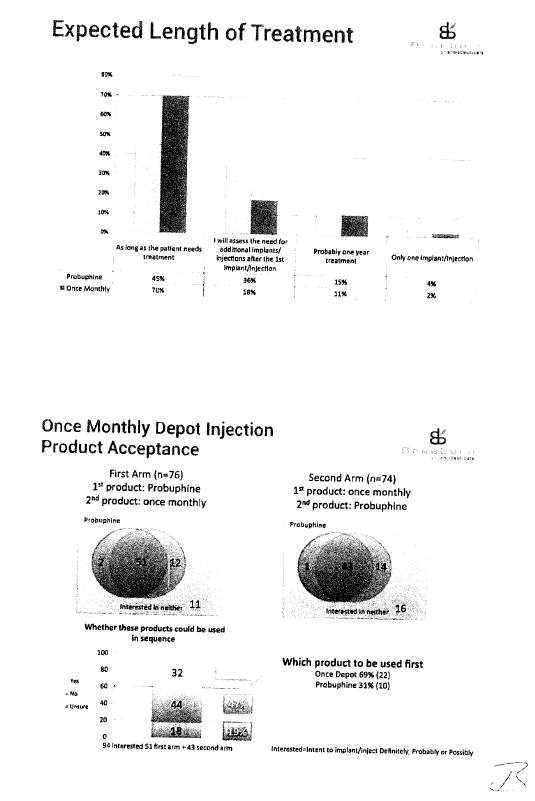
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
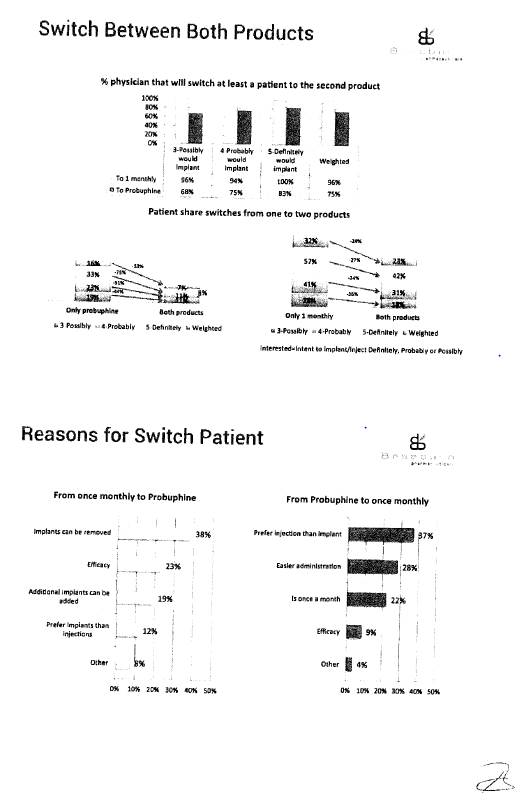
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
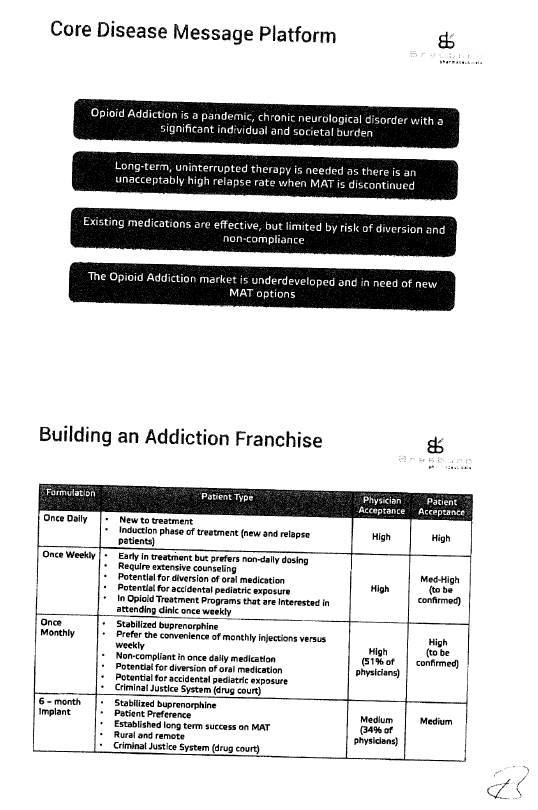
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
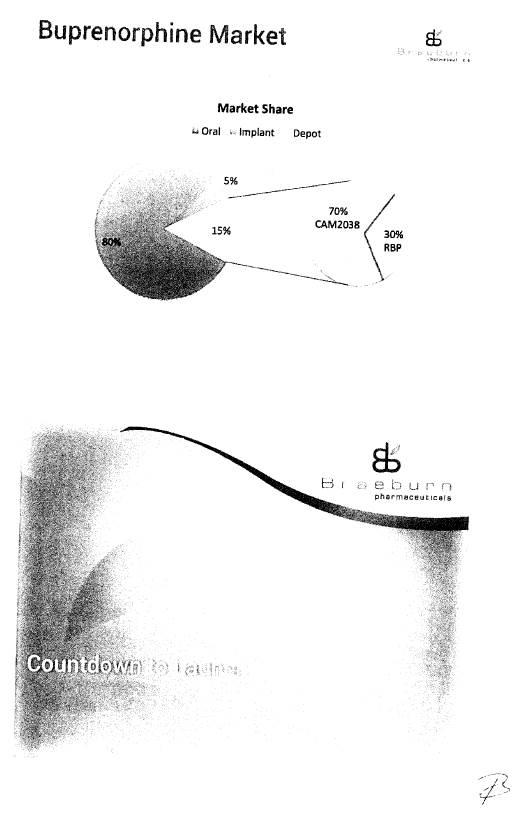
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
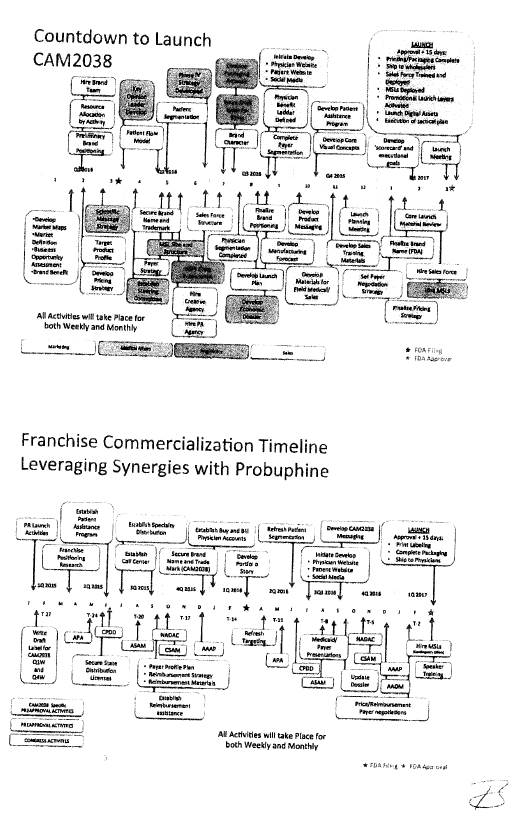
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
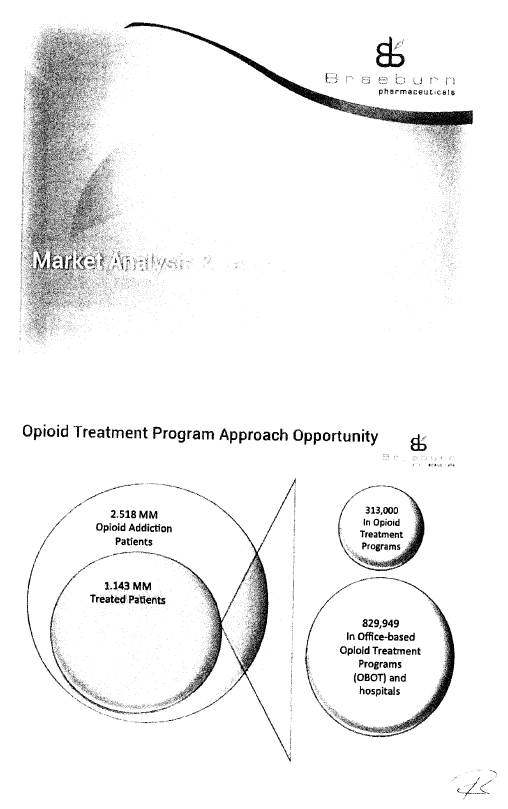
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
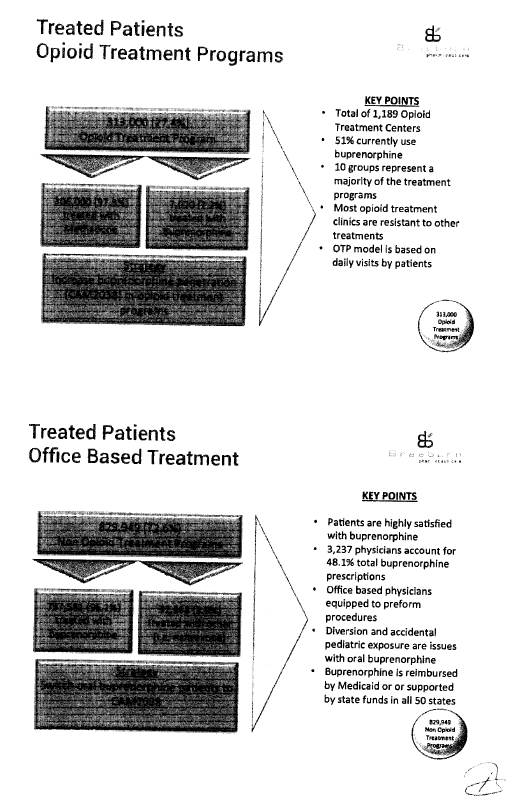
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
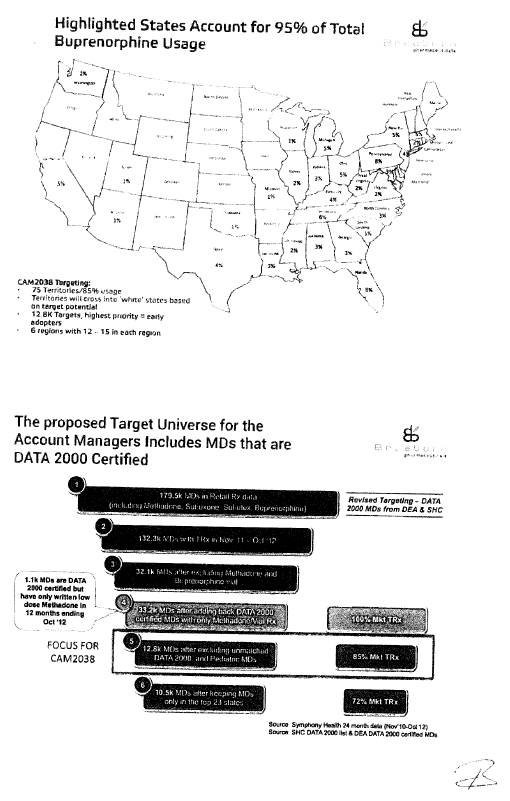
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
 f
f
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
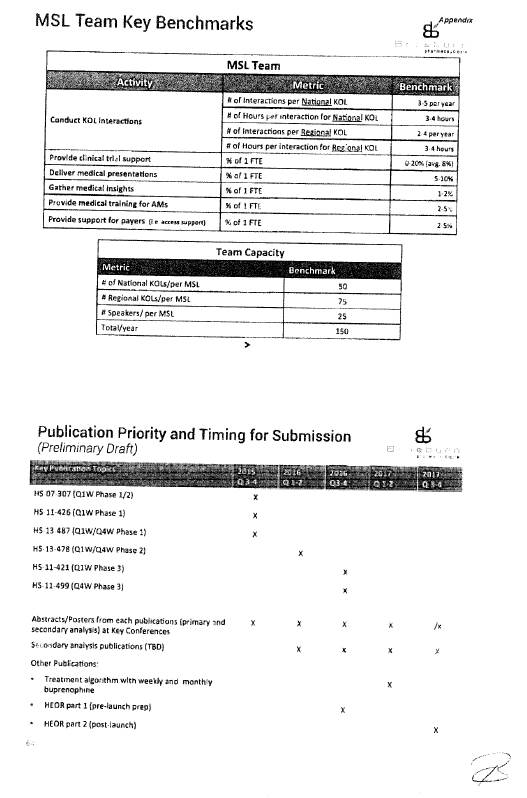
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
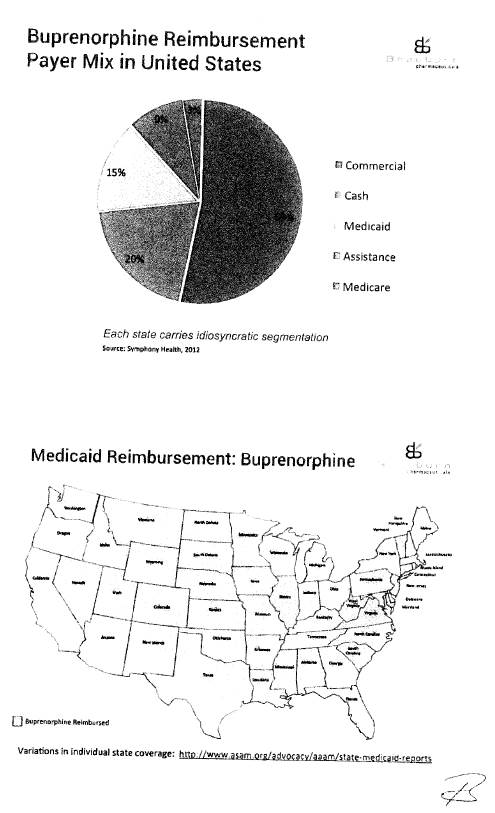
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
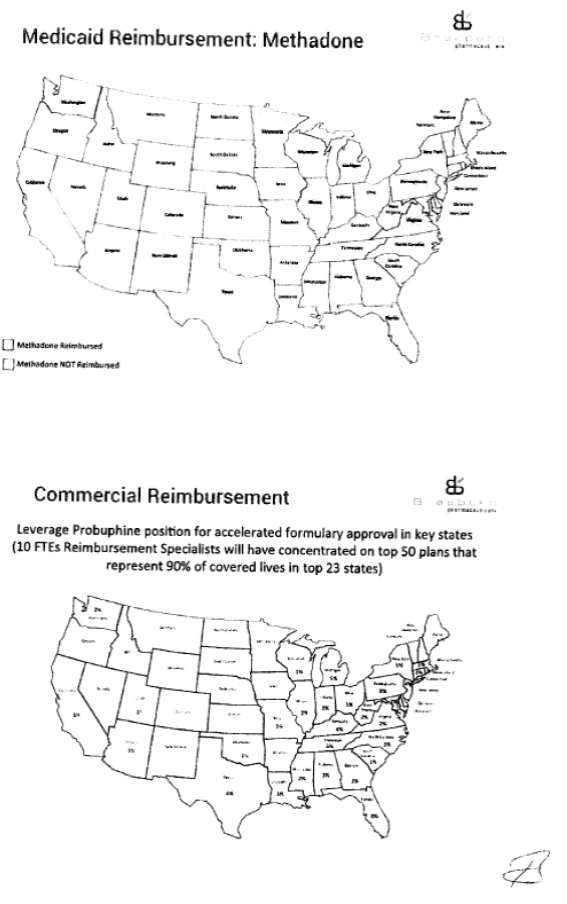
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
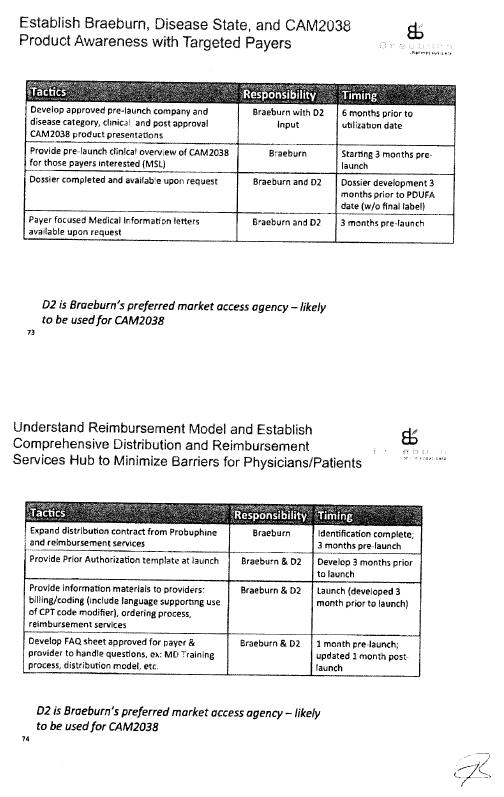
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
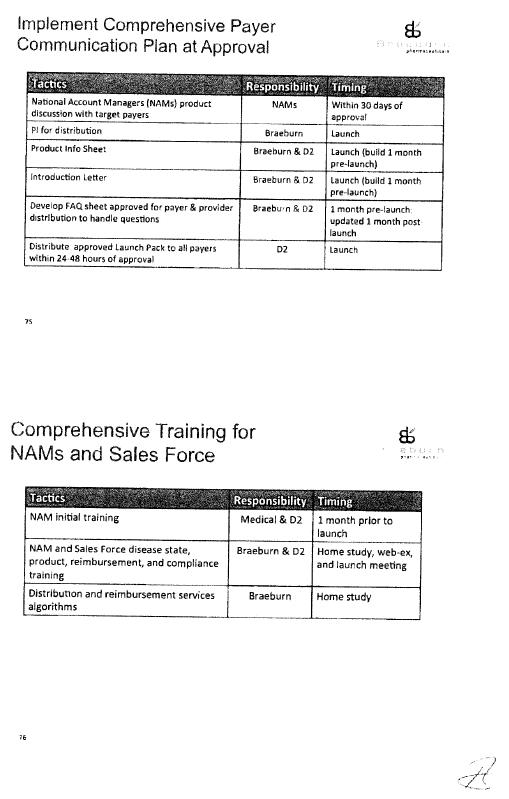
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
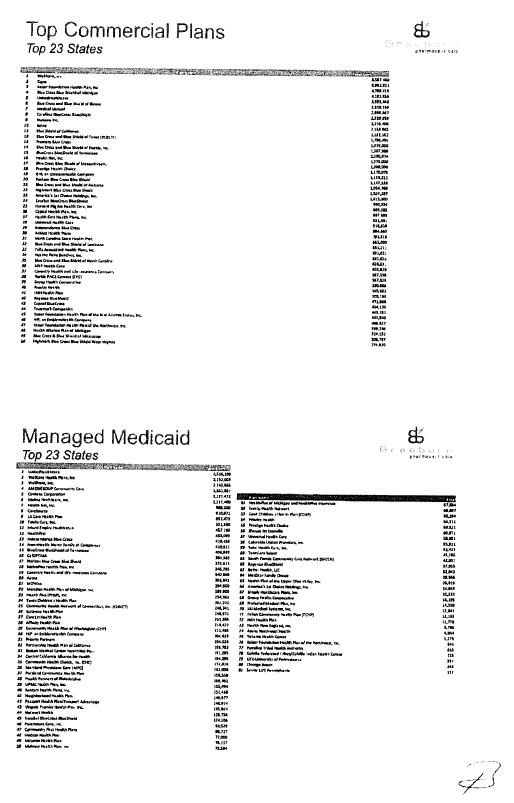
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
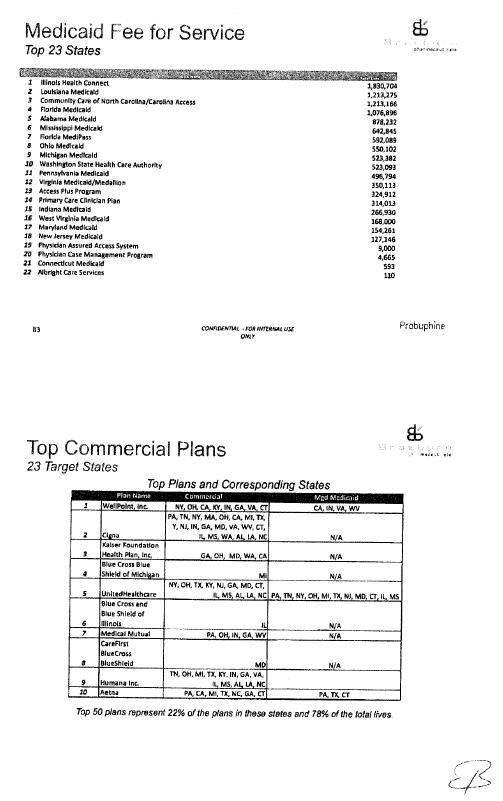
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
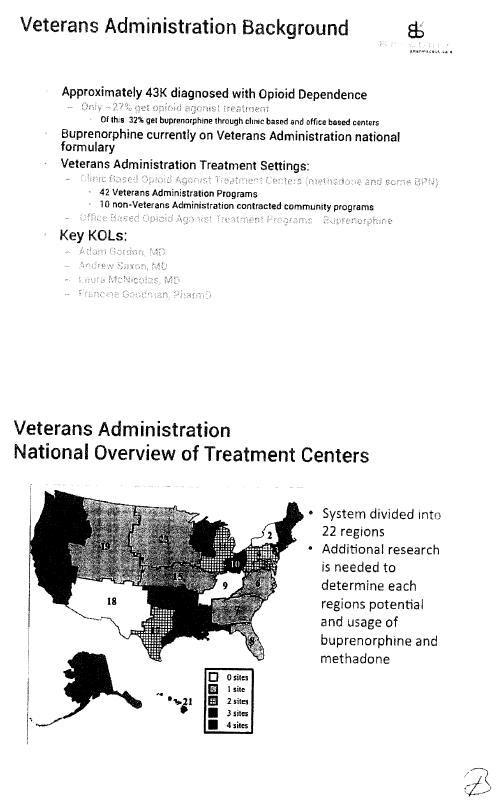
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
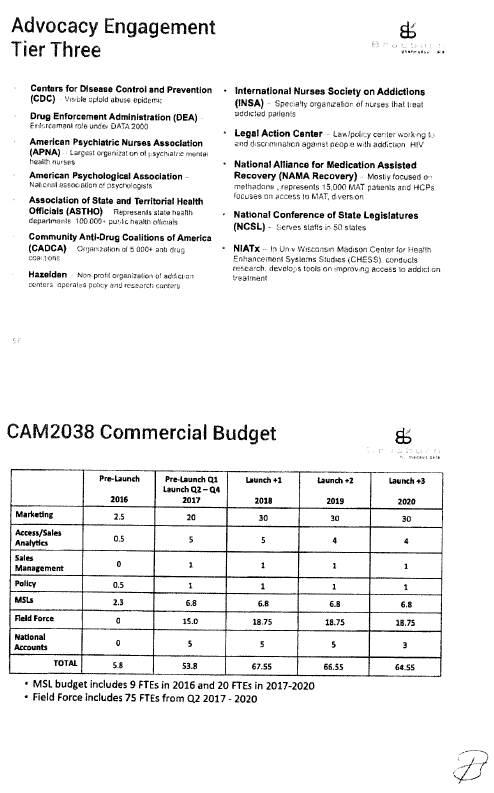
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
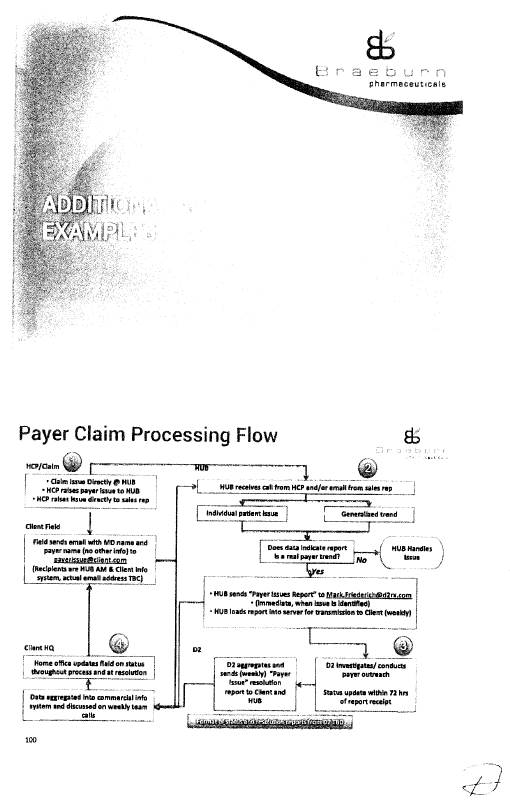
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
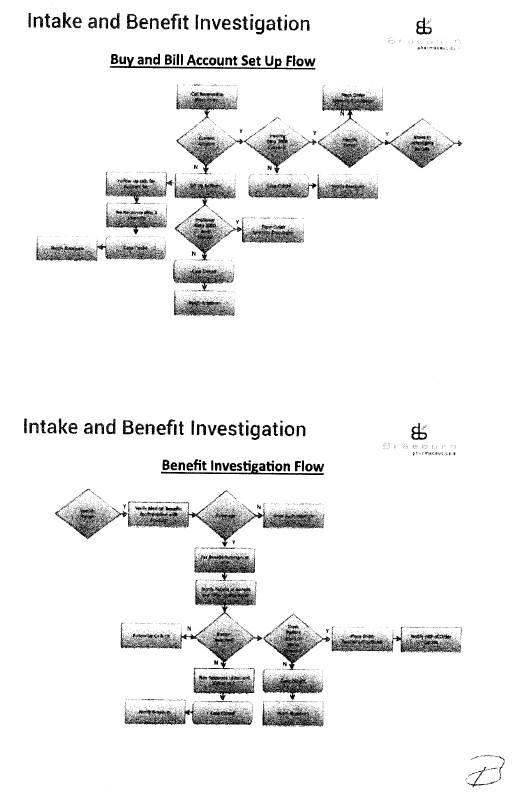
CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.

CERTAIN CONFIDENTIAL PORTIONS OF THIS EXHIBIT WERE OMITTED AND REPLACED WITH “[***]”. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECRETARY OF THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO AN APPLICATION REQUESTING CONFIDENTIAL TREATMENT PURSUANT TO RULE 24B-2 PROMULGATED UNDER THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.


