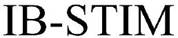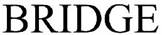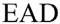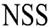LICENSE AND COLLABORATION AGREEMENT
Exhibit 10.1
Execution Version
LICENSE AND COLLABORATION AGREEMENT
This LICENSE AND COLLABORATION AGREEMENT is entered into as of April 9, 2020 (“Effective Date”), by and between Innovative Health Solutions, Inc., a corporation organized under the laws of the State of Indiana with principal offices at 000 X. Xxxxx Xxxxxx, Xxxxxxxxxx, XX 00000 (“Licensor”) and Masimo Corporation, a corporation organized under the laws of Delaware with principal offices at 00 Xxxxxxxxx, Xxxxxx, XX 00000 (“Licensee”) (this “Agreement”). Licensor and Licensee are each sometimes referred to individually herein as a “Party” and collectively as the “Parties”.
BACKGROUND
WHEREAS, concurrently with the execution of this Agreement, the Parties are entering into that certain Series A Preferred Stock Purchase Agreement (as may be amended or restated from time to time, the “Purchase Agreement”);
WHEREAS, as a material inducement to Licensee executing the Purchase Agreement, Licensee wishes to obtain certain exclusive rights and licenses from Licensor, and access to Licensor’s research and development capabilities, to enable the development, manufacture and commercialization of Products in the Field in the Territory (each as defined below) and Licensor is willing to grant such exclusive rights and licenses and access to Licensee; and
WHEREAS, the Parties also anticipate collaborating with each other to further develop and improve certain technologies and products (including Products); and
WHEREAS, the Parties now desire to enter into this Agreement to provide for and facilitate such exclusive rights and licenses, access, and mutual development activities and support.
NOW, THEREFORE, in consideration of the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereto, intending to be legally bound, hereby agree as follows:
ARTICLE 1
DEFINITIONS
Whenever used in this Agreement with an initial capital letter, the terms defined in this Article 1 shall have the meanings specified below:
“Affiliate” means, with respect to any specified Person, any other Person who, directly or indirectly, controls, is controlled by, or is under common control with such Person, including, without limitation, any general partner, managing member, officer, director or trustee of such Person, or any venture capital fund or registered investment company now or hereafter existing that is controlled by one or more general partners, managing members or investment advisers of, or shares the same management company or investment adviser with, such Person; provided that for purposes of this Agreement, Licensor and any entity controlled by Licensor will not be considered Affiliates of Licensee, and Licensee and its other Affiliates will not be considered Affiliates of Licensor or any entity controlled by Licensor.
“Applicable Law” means all federal, state, local, national and supra-national laws, statutes, rules and regulations, including any rules, regulations, or requirements of Regulatory Authorities, major national securities exchanges or major securities listing organizations, that may be in effect from time to time during the Term and applicable to a particular activity or exercise of rights hereunder.
| 1 |
“cGCP” means the then current Good Clinical Practice standards for the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials as set forth in the International Conference on Harmonization (ICH) guidelines entitled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” and equivalent regulations or standards in the Territory and any update thereto and any other policies or guidelines applicable to the design, conduct, performance, monitoring, auditing, recording, analysis, and reporting of clinical trials in the Territory, and/or any applicable foreign equivalents thereof, and any updates of any of the foregoing.
“Clinical Data” means all data with respect to a Product that is made, collected or otherwise generated anywhere in the world under or in connection with the Clinical Studies for a Product, as applicable (as opposed to Pre-Clinical Data or non-clinical data derived from laboratory studies, disease models and animal studies). Clinical Data includes, but is not limited to, validated clinical databases.
“Clinical Study(ies)” means any investigator initiated studies (including any Phase I Study, Phase II Study, Phase III Study, or Phase IV Study) conducted anywhere in the world, or such other tests or studies in humans conducted anywhere in the world, that are required by Applicable Law, or otherwise recommended by the Regulatory Authorities, to obtain or maintain Regulatory Approvals for a Product in the Territory.
“Commercialization” or “Commercialize” means any and all activities (whether before or after Regulatory Approval) directed to the manufacture, distribution, transportation, storage, use, sale, promotion, importation, exportation, use, and other commercialization of a Product, including commercial manufacturing, pre-launch and post-launch marketing, promoting, distributing, importing, offering to sell and selling a Product for the Territory. When used as a verb, “Commercializing” means to engage in Commercialization and “Commercialized” has a corresponding meaning.
“Confidential Information” means any and all confidential or proprietary information or material, whether oral, visual, in writing or in any other form, that has been or is provided, communicated or otherwise made known directly or indirectly by a Party or its Affiliates (the “Disclosing Party”) to the other Party or any of its Affiliates (the “Receiving Party”) pursuant to this Agreement or in connection with the transactions contemplated hereby or any discussions or negotiations with respect thereto, and that may be reasonably understood from notices or legends, the nature of such information itself or the circumstances of such information or materials’ disclosure to be confidential or proprietary to the Disclosing Party.
“Control” or “Controlled” means, with respect to any Intellectual Property or Regulatory Filing, the right of Licensor or an Affiliate thereof to license or sublicense such Intellectual Property or Regulatory Filing to Licensee pursuant to the terms and conditions of this Agreement without breaching any other agreement with respect to such Intellectual Property or Regulatory Filing.
“Development” or “Develop” means, with respect to a Product in the Territory, all research, all pre-clinical and clinical activities conducted relating to a Product (as applicable) in the Territory, including test method development and stability testing, toxicology, animal studies, formulation, process development, manufacturing scale-up, quality assurance and quality control development for Clinical Studies, statistical analysis and report writing, and Clinical Studies, including clinical trial design, operations, data collection and analysis and report writing, publication planning and support, risk assessment mitigation strategies, health economics outcomes research planning and support, clinical laboratory work, the preparation of Regulatory Filings, and obtaining and/or maintaining Regulatory Approvals for a Product in the Territory (including regulatory affairs activities and preparation of meetings with Regulatory Authorities in the Territory). When used as a verb, “Developing” means to engage in Development and “Developed” has a corresponding meaning.
| 2 |
“Disputes” means all disputes, differences, controversies or claims (whether based on contract, tort, statutory concepts, or any other legal doctrine) arising out of, in connection with, or relating to this Agreement (including without limitation the existence, validity, interpretation, performance, amendment, breach, default, or termination of this or the subject matter of this Agreement).
“Field” means and includes any treatment or management or other medical or therapeutic applications of, for or related to any of the following: pain or pain associated with medical conditions or procedures; substance abuse withdrawal symptoms; iatrogenic withdrawal syndrome; and post-operative nausea and vomiting. The Field excludes the following pediatric and adults conditions (including the associated symptoms and any pain caused thereby): chronic nausea, gastroparesis, functional gastrointestinal disorders, chemotherapy induced nausea/vomiting, concussions and post-concussion syndrome, headaches (migraine or benign, non-specified), symptoms resulting from traumatic brain injury, post-traumatic stress disorder, fatty liver disease, cyclic vomiting syndrome, movement disorder including Xxxxxxxxx’x disease, chronic sleep disorders, inflammatory bowel disease, pancreatitis, pulmonary inflammatory disorders, dysautonomia and postural orthostatic tachycardia syndrome, tic disorders, tinnitus, TMJ disorders, autoimmune disorders, seizure disorders, diabetes, and modulation of exercise physiology and recovery(the “Retained Fields”).
“Joint Invention” means any Invention, other than Improvements to Licensee IP, that is developed, conceived or reduced to practice jointly by one or more employees of Licensor or its Affiliates or a Third Party acting under authority of Licensor or its Affiliates, on the one hand, and one or more employees of Licensee or its Affiliates or a Third Party acting under authority of Licensee or its Affiliates, on the other hand.
“Intellectual Property” means any or all of the following and all rights, arising out of or associated therewith anywhere in the world: (i) all Patent Rights; (ii) all works of authorship (including software), user interfaces, URLs, web sites, copyrights, copyright registrations and applications therefor, and all other rights therein or corresponding thereto; (iii) all internet uniform resource locators, domain names, trade names, logos, brand names, slogans, product names, designs, common law trademarks and service marks, trademark and service mark registrations and applications therefor (collectively, “Trademarks”); (iv) all moral and economic rights of authors and inventors, however denominated; (v) all Know-How (whether or not embodied in any tangible form and including all tangible embodiments of the foregoing such as instruction manuals, laboratory notebooks, prototypes, samples, studies, and summaries); and (vi) any similar or equivalent rights in or to any of the foregoing.
“Invention” means any Intellectual Property that is developed, conceived or reduced to practice under and as a result of any work performed under or in connection with this Agreement, including the Development Activities.
“Know-How” means, collectively, trade secrets, proprietary information, inventions, ideas, discoveries, improvements, processes, methods, protocols, concepts, know-how, data (including any clinical or marketing studies data), databases, materials (including tangible chemical, biological or other physical materials), compositions of matter, formulae, APIs, schematics, specifications, designs, and all documentation relating to any of the foregoing.
“Licensed IP” means and includes any and all (i) Licensed Know-How and Technological Information, (ii) Licensed Patent Rights, and (iii) other Intellectual Property that is Controlled by Licensor or any of its Affiliates as of the Effective Date or at any time during the Term of this Agreement and that are, in each of the foregoing cases, or may be, used or held for use, necessary or otherwise relevant for or useful to the Development, Promotion, Commercialization or other exercise of Licensee’s rights under this Agreement or otherwise pertaining to the Field or any Products, including, without limitation the Intellectual Property set forth on Exhibit A hereto.
| 3 |
“Licensed Know-How and Technological Information” means and includes any and all Know- How that is Controlled by Licensor or any of its Affiliates as of the Effective Date or at any time during the Term of this Agreement and that are, in each of the foregoing cases, or may be, used or held for use, necessary or otherwise relevant for or useful to the Development, Promotion, Commercialization or other exercise of Licensee’s rights under this Agreement or otherwise pertaining to the Field or any Products. For clarity, Licensed Know-How and Technological Information includes any Pre-Clinical Data and Clinical Data with respect to any Products, Development Activities or otherwise generated in connection with this Agreement.
“Licensed Patent Rights” means and includes any and all Patent Rights that are Controlled by Licensor or any of its Affiliates as of the Effective Date or at any time during the Term of this Agreement that: (i) would be infringed, absent a license, by the Development, Promotion, Commercialization of any Product or other exercise of Licensee’s rights under this Agreement; or (ii) claim any (a) Licensed Know- How and Technological Information, or (b) Know-How made, conceived of or reduced to practice pursuant to or in connection with the activities contemplated under this Agreement.
“Licensed Trademarks” means any Trademarks included among the Licensed IP.
“Patent Prosecution” means activities directed to (i) preparing, filing and prosecuting applications (of all types) for any Patent Rights, (ii) managing any interference, opposition, re-issue, reexamination, supplemental examination, invalidation proceedings (including inter partes or post-grant review proceedings), revocation, nullification, or cancellation proceeding relating to the foregoing, (iii) deciding whether to abandon, extend or maintain Patents Rights, (iv) listing in regulatory publications (as applicable), and (v) settling any interference, opposition, reexamination, invalidation, revocation, nullification or cancellation proceeding, but excluding the defense of challenges to such Patent Rights as a counterclaim in an infringement proceeding with respect to the particular Patent Rights, and any appeals therefrom. For purposes of clarity, “Patent Prosecution” will not include any other enforcement actions taken with respect to a Patent Rights.
“Patent Rights” means the rights and interests in and to all patents and patent applications, including provisional applications, divisional applications, continuation applications, continuation-in-part applications, continued prosecution applications, certificate of inventions, utility models, xxxxx patents, extensions or restorations, including adjustments, revalidations, reissues, re-examinations, patent term extensions, supplementary protection certificates of or to any of the foregoing and any similar rights.
“Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, incorporated association, joint venture, or other entity or organization, in any case whether for-profit or not- for profit, and including, without limiting the generality of any of the foregoing, a government or political subdivision, department or agency of a government.
“Pre-Clinical Data” means data derived from a study to test a Product, including, but not limited to, laboratory studies, toxicology, safety pharmacology, disease models and animal models.
| 4 |
“Product” means any product, process, or service within the Field, including any and all products, processes, and services developed pursuant to the Development Activities. The term “Product” or “a Product” as used herein may be used to reference one or more than one Product(s).
“Promote” or “Promotion” means those activities normally undertaken by a medical device company’s sales force and marketing team to implement marketing plans and strategies aimed at encouraging the appropriate use of a particular medical device product, including detailing. When used as a verb, “Promote” means to engage in such activities.
“Regulatory Approval” means any and all approvals, licenses (including product and establishment licenses), permits, certifications, registrations, or authorizations of any Regulatory Authority necessary to Develop, Promote, or Commercialize a Product, as applicable, for use in the Territory.
“Regulatory Authority” means any national, supra-national, regional, federal, state, provincial or local regulatory agency, department, bureau, commission, council or other governmental entity having jurisdiction over the Development, Promotion, or Commercialization of a Product, as applicable, in the Territory.
“Regulatory Filings” means, with respect to a Product in the Territory, as applicable, all applications, registrations, submissions, dossiers, notifications, licenses, authorizations and approvals (including all Regulatory Approvals), all correspondence submitted to or received from the Regulatory Authorities (including minutes and official contract reports relating to any communications with any Regulatory Authority) and all supporting documents and all Pre-Clinical Data and Clinical Data, and all data contained in any of the foregoing.
“Sublicensee” means any Person (including a Licensee Affiliate) to whom Licensee sublicenses any rights as permitted hereunder.
“Territory” means worldwide.
ARTICLE 2
LICENSE GRANTS
2.1 Licensor Grants. Licensor, on behalf of itself and its Affiliates, hereby grants to Licensee, under the Licensed IP, an exclusive (even as to Licensor and its Affiliates), transferable, fully sublicensable (through multiple tiers), fully paid-up and royalty-free, perpetual, irrevocable, worldwide right and license to: (i) use and reference all Licensed Know-How and Technological Information to prepare for, support, obtain or maintain any Regulatory Filings or Regulatory Approvals made by or for Licensee or any of its Affiliates for a Product in the Field anywhere in the Territory; and (ii) Develop, Promote, and Commercialize any and all Products in the Field throughout the Territory.
2.2 Have Made and Right to Sublicense. Notwithstanding anything to the contrary contained in this Agreement, the licenses granted to Licensee under Section 2.1 shall include “have made” rights and the right of Licensee and its Affiliates to have any such licenses exercised by or on behalf of their behalf by any Third Party for their benefit and account. In addition to and not in lieu or limitation of the foregoing, Licensee and its Affiliates shall have the right to grant sublicenses to their respective Affiliates and any other Person (each of the foregoing, a “Sublicensee”).
| 5 |
2.3 No Conflicting Grant; Field Integrity. Licensor agrees that it shall not, and shall cause its Affiliates to not, at any time during the Term of this Agreement: (i) grant any licenses or other right, title or interest under or relating to any of the Licensed IP or proprietary rights Controlled by Licensor or any of its Affiliates, in each of the foregoing cases, within the Field that conflicts with Licensee’s rights under this Agreement, nor (ii) directly or indirectly develop, file for Regulatory Approval with respect to, make, have made, use, sell, offer for sale, import and otherwise commercialize any Product or any other product or service within the Field, except for or through Licensee and its designees, in accordance with the terms and conditions of this Agreement. For clarity, nothing in this paragraph shall restrict Licensor’s rights in the Retained Field or right to seek Regulatory Approval in the Retained Field, including the right to grant licenses in the Retained Field.
2.4 Licensed Trademarks. All use of the Licensed Trademarks by Licensee, in the Field and all goodwill associated with such use, shall inure to the benefit of Licensor. Licensee shall use commercially reasonable efforts intended to ensure that its respective use of Licensed Trademarks does not tarnish, blur, or dilute the quality associated with Licensed Trademarks or the associated goodwill and to cooperate with Licensor to address any reasonable concerns based on the quality associated with Licensed Trademarks caused by Licensee’s or its Sublicensee’s use thereof.
2.5 Subcontracting. Licensee may at its discretion, perform any activities in support of its Development, Promotion, and Commercialization of a Product in the Territory through contracting with a Third Party (“Subcontractor”) without prior consent of Licensor; provided that Licensee shall enter into an appropriate written agreement with any such Subcontractor such that the Subcontractor shall be bound by all applicable provisions of this Agreement to the same extent as Licensee. Upon any expiration or termination of this Agreement for any reason, all agreements with Subcontractors shall automatically terminate.
2.6 No Implied Licenses. No license or other right is or shall be created or granted hereunder by implication, estoppel or otherwise for any purpose. All such licenses and rights are or shall be granted only as expressly and specifically provided in this Agreement.
ARTICLE 3
UPFRONT FEE, TECHNOLOGY TRANSFER, RIGHTS OF REFERENCE & REGULATORY
3.1 Upfront Fee. As additional consideration for the licenses, rights and obligations granted and made by Licensor hereunder, Licensee shall pay to Licensor a one-time, upfront and non-refundable (except as provided below) fee of two hundred fifty thousand U.S. dollars ($250,000 USD) (the “Upfront Fee”) which shall be due upon execution of this Agreement. In the event of a material breach by Licensor of its obligations under this Section 3, where such breach remains uncured for thirty (30) days following Licensor’s receipt of written notice from Licensee specifying such breach, then Licensor shall promptly refund and pay to Licensee the Upfront Fee.
3.2 Technology Transfer. As soon as reasonably practicable after the Effective Date and from time to time during the Term of this Agreement, Licensor shall disclose and transfer to Licensee embodiments of the Licensed Patent Rights and any other Licensed IP Controlled by Licensor or any of its Affiliates, and all Licensed Know-How and Technological Information pertaining to the Field and any Products (collectively, the “Transferred Technology”). In addition, Licensor will, at the reasonable request of Licensee and at Licensee’s expense in accordance with Section 4.4 hereof: (i) support Licensee’s Commercialization of Products in the Field, and (ii) provide Licensee with reasonable assistance in connection with use the Transferred Technology to enable Licensee to use and exploit the Transferred Technology and otherwise exercise its rights and perform its obligations under this Agreement.
| 6 |
3.3 Regulatory Materials & Clinical Studies. During the Term, Licensor shall promptly provide to Licensee true and complete copies of all Regulatory Filings and Regulatory Approvals and other regulatory communications with respect to a Product in the Field received or submitted by or on behalf of Licensor, its Affiliates or Sublicensees. As reasonably requested during the Term, Licensor shall provide to Licensee true and complete copies of any Regulatory Filings and Regulatory Approvals and other material regulatory communications with respect to a Product outside the Field generated by or on behalf of, or received by, Licensor (or its Affiliates or respective Sublicensees or (sub)licensees). Without limiting the foregoing, Licensor shall provide Licensee (or its designees) with sufficient rights to reference and use any such documentation in connection with Licensee’s or its designees’ activities hereunder, including providing the appropriate authorizations to such Regulatory Authority(ies) allowing Licensee (or its designees) the right to reference and use any such documentation to support any Regulatory Filing for a Product in the Field consistent with the terms and conditions of this Agreement, including to support any necessary Regulatory Filing for the Products (or changes thereto) to permit manufacture by Licensee or its designee. Upon Licensee’s request, Licensor shall also introduce Licensee (or its designee) to all Third Parties who have handled or conducted any Clinical Studies with respect to any Product within the Field and provide reasonable assistance to Licensee to coordinate the transfer of such Clinical Studies to Licensee. Licensee acknowledges that, as of the Effective Date, Licensor has not conducted any Phase I Study, Phase II Study, Phase III Study, or Phase IV Study with respect to any Product in the Field.
3.4 Ownership. To the extent permitted by Applicable Law, Licensee shall own all Regulatory Filings (including all Regulatory Approvals) specific to Products in the Field and Licensor shall, upon Licensee’s request, assist Licensee and take such actions as Licensee may request to effect such transfer. Licensor agrees that neither it nor its Affiliates will do anything to adversely affect any such Regulatory Approvals or other Regulatory Filings. The Parties recognize all relevant investigator initiated studies are owned by the investigators that conducted the studies, not Licensor.
3.5 Cooperation. Licensor shall, at Licensee’s expense in accordance with Section 4.4 hereof, cooperate and consult with Licensee to assist Licensee in: (i) developing strategies for Regulatory Filings with respect to any Product in the Field; (ii) responding to any communications from Regulatory Authorities with respect to any Product in the Field; and (iii) Development, Promotion, and Commercialization of the Products in the Field, including procuring, shipping, selling and authorizing the manufacture of Product (including the NSS-2 Bridge product) to or for Licensee on terms equal to those obtained by Licensor.
ARTICLE 4
DEVELOPMENT ACTIVITIES; COMMERCIALIZATION
4.1 Joint Development Committee. Promptly following the execution of this Agreement, the Parties shall establish a joint development committee (the “Joint Development Committee”) to oversee the collaborative Product and technology development activities contemplated by this Agreement (such activities undertaken by either of the Parties, whether solely or jointly, hereunder, the “Development Activities”). The Joint Development Committee shall consist of two (2) representatives from each of Licensor and Licensee. Each Party may replace any or all of its representatives on the Joint Development Committee at any time upon written notice to the other Party. The Joint Development Committee will be held on a quarterly basis or as otherwise agreed upon by the Parties, as a forum to mutually discuss, exchange information and coordinate Development Activities.
| 7 |
4.2 Development Activities. Each Party shall perform its respective Development Activities and any related Clinical Studies activities in furtherance thereof in good scientific manner, in compliance with the current Good Clinical Practice standards set forth in the cGCP and Applicable Law, using personnel with sufficient skills and experience as are required to accomplish the objectives of the work. Each Party will maintain complete and accurate records of its Development Activities and regularly update the other Party through the Joint Development Committee regarding the results and progress of its Development Activities. All results of Licensee’s Development Activities shall be subject to the licenses granted to Licensee in Section 2.1. Unless otherwise agreed to by the Parties and set forth in writing or as otherwise provided herein, Licensor and Licensee hereby agree that each Party shall bear their own respective costs and expenses incurred by such Party in connection with any Development Activities.
4.3 Commercialization by Licensee. Licensee shall have the exclusive right, directly or through its Affiliates, Sublicensees and subcontractors, to develop and seek Regulatory Approvals for Products in the Field in the Territory and to commercialize Products in the Field in the Territory, including all Products developed in furtherance of the Development Activities. Licensee (itself or through its Affiliates or Sublicensees) shall have the sole and exclusive right and responsibility to determine, in its sole discretion, the trademarks, trade dress, style of packaging, labeling and domain names with respect to the packaging, marketing, distribution and sales of the Product in the Field in the Territory, and Licensor shall have no rights to such trademarks, trade dress, style of packaging, labeling or domain names.
4.4 Consulting Fees. All technology transfer, assistance, support, and development activities of Licensor in furtherance of Section 3 and Section 4 of this Agreement shall be provided to Licensee free of charge during the six (6) month period immediately following the Effective Date. Thereafter, to the extent Licensee requires Licensor to provide additional technology transfer, assistance, support or development activities under Section 3 or Section 4 then the Parties will negotiate in good faith a consulting agreement, which consulting agreement will provide, among other things, payment to Licensor of an hourly fee, at typical market rates, for such additional technology transfer, assistance, support, and development activities. The Parties agree that all fees payable to Licensor hereunder are subject to Licensee’s prior review and approval.
ARTICLE 5
REPRESENTATIONS AND WARRANTIES; LIABILITY
5.1 Mutual Representations, Warranties and Covenants. Licensor and Licensee each represents and warrants to the other, as of the Effective Date: (i) Such Party is duly organized, validly existing and in good standing under the laws of its jurisdiction of incorporation or formation, and has full corporate or other power and authority to enter into this Agreement and to perform its obligations hereunder; (ii) Such Party has taken all necessary corporate action required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder; (iii) this Agreement has been duly executed and delivered on behalf of such Party and constitutes a legal, valid and binding obligation of such Party and is enforceable against it in accordance with the terms hereof subject to the effects of bankruptcy, insolvency or other laws of general application affecting the enforcement of creditor rights and judicial principles affecting the availability of specific performance and general principles of equity, whether enforceability is considered a proceeding at law or equity; (iv) the execution and delivery of this Agreement and the performance of such Party’s obligations hereunder does not conflict with or violate any provision of the articles of incorporation, bylaws or any similar instrument of such Party, as applicable, in any material way and does not conflict with, violate or breach, or constitute a default or require any consent under, any contractual obligation or court or administrative order by which such Party is bound. In addition, each Party represents, warrants and covenants to the other: (a) that it shall, or shall cause its Affiliate to, obtain and maintain during the Term all authorizations, consents and approvals, governmental or otherwise, necessary for such Party to grant the rights and licenses granted by such Party under this Agreement and to perform its obligations under this Agreement; (b) it shall comply with Applicable Law relating to such Party’s rights, duties, responsibilities and obligations set forth in this Agreement; and (c) it will not enter into an agreement that is inconsistent with the rights, licenses and assignments granted to the other Party in this Agreement.
| 8 |
5.2 No Debarment; Anti-Bribery and Anti-Corruption. Each Party certifies as of the Effective Date that neither Party has been debarred by any Regulatory Authority, or, to such Party’s knowledge, is the subject of debarment proceedings by any Regulatory Authority. Each Party further certifies as of the Effective Date that it has not used prior to the Effective Date and shall not use during the Term, any employee, agent or independent contractor who has been debarred by any Regulatory Authority or, to such Party’s knowledge, is the subject of debarment proceedings by any Regulatory Authority. Each Party further represents, warrants and covenants that it has not been sanctioned, suspended, excluded or otherwise declared ineligible from any Regulatory Authority healthcare program, including, but not limited to any United States healthcare program, such as Medicare or Medicaid or comparable foreign healthcare program. In the event that during the Term, such Party (i) becomes debarred, suspended, excluded, sanctioned, or otherwise declared ineligible; or (ii) receives notice of an action or threat of an action with respect to any such debarment, suspension, exclusion, sanction or ineligibility, such Party shall immediately notify the other Party. In the event a Party becomes debarred by a Regulatory Authority during the Term, the other Party shall have a right to terminate this Agreement upon thirty (30) days written notice to the debarred Party.
5.3 Right to Grant Licenses; No Existing Claims. Licensor represents, warrants and covenants to Licensee that: (i) it Controls the Licensed IP and otherwise has the full power and authority to grant to Licensee the rights, licenses and assignments granted under this Agreement, free and clear of any and all encumbrances and without the need for any assignments, releases, consents, approvals, immunities or other rights not yet obtained; and (ii) no agreements or arrangements of any kind exist between Licensor and Third Parties that limit or restrict use of the Licensed IP for a Product in the Territory or that otherwise conflicts or is inconsistent with Licensee’s exercise of its rights under this Agreement. As of the Effective Date, Licensor represents and warrants that: (a) there are no claims, demands, Disputes or proceedings of any Person pertaining to or, to Licensor’s knowledge, any claim, demand, Dispute or proceeding (including interferences, reissues, reexaminations, cancellations, oppositions, nullity actions, invalidation actions or post-grant reviews) which are pending or threatened that, in either case, challenges Licensor’s right, title or interest in the Licensed IP in the Territory or makes any adverse claim of ownership thereof or which would affect Licensor’s ability to perform its obligations or grant the rights and licenses under this Agreement; (b) to the best of Licensor’s knowledge, after a reasonable investigation, the Licensed IP and Products and the exercise of Licensee’s rights in connection therewith and as otherwise contemplated by this Agreement and the performance of each Party’s obligations under this Agreement do not and will not violate, infringe or misappropriate the intellectual property or proprietary rights of any Third Party; and (c) none of the relevant Patent Rights and Trademarks in the Licensed IP are the subject of any judgment, injunction, order, decree, settlement or agreement restricting its use for or otherwise Licensee’s exercise of rights under this Agreement relating to a Product in the Field for the Territory. Licensor agrees that it shall notify Licensee immediately if Licensor becomes aware of any actual or potential Third Party Claims that could affect either Party’s ability to fully perform its obligations or to exercise its rights under this Agreement. Licensor represents, warrants and covenants that Licensor shall not assign or abandon, and shall maintain in full force and effect all Licensed IP.
5.4 No Additional Material Information. As of the Effective Date, Licensor has disclosed to Licensee all material scientific and technical information and all material information relating to safety and efficacy known to Licensor with respect to the Products. The documentation disclosed or made available to Licensee in connection with Licensee’s due diligence in entering into this Agreement is, in all material respects, true, complete and unredacted (except as expressly noted in such documentation). Licensor has not failed to disclose or make available any material information to Licensee.
| 9 |
5.5 Warranty Disclaimer. TO THE MAXIMUM EXTENT PERMITTED BY APPLICABLE LAW, EXCEPT AS OTHERWISE EXPRESSLY PROVIDED IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR WARRANTY WITH RESPECT TO THE SUBJECT MATTER OF THIS AGREEMENT AND EACH PARTY HEREBY EXPRESSLY AND SPECIFICALLY DISCLAIMS ALL REPRESENTATIONS AND WARRANTIES, WHETHER WRITTEN OR ORAL, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE, INCLUDING, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NON-INFRINGEMENT.
5.6 Limitation of Liability. NOTWITHSTANDING ANYTHING IN THIS AGREEMENT TO THE CONTRARY, TO THE MAXIMUM EXTENT PERMITTED BY APPLICABLE LAW, NEITHER LICENSEE NOR ANY OF ITS AFFILIATES SHALL BE LIABLE TO LICENSOR OR ANY OF ITS AFFILIATES FOR ANY SPECIAL, PUNITIVE, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES, INCLUDING LOST PROFITS OR LOST REVENUES, WHETHER UNDER ANY CONTRACT, WARRANTY, NEGLIGENCE, STRICT LIABILITY OR OTHER LEGAL OR EQUITABLE THEORY.
ARTICLE 6
INTELLECTUAL PROPERTY RIGHTS
6.1 Ownership of Inventions. Ownership of all Inventions shall be as set forth in this Article 6. Determination of inventorship of Inventions shall be made in accordance with US patent laws. Each Party will continue to own any Intellectual Property that it owned prior to the Effective Date or that it creates or obtains outside the scope of this Agreement that are not Inventions. Inventions that are made solely by or on behalf of Licensee shall be owned solely by Licensee. Any Inventions developed, conceived or reduced to practice by either Party (or jointly) that are improvements, modifications, derivative works or otherwise based upon any Intellectual Property owned by Licensee or its Affiliates shall be owned solely by Licensee, and Licensor, on behalf of itself and its Affiliates hereby irrevocably and unconditionally assigns to Licensee all of its (and their) respective right, title and interest in and to all such Inventions (collectively, “Improvements to Licensee IP”). Inventions, other than Improvements to Licensee IP that are made solely by or on behalf of Licensor shall be owned solely by Licensor but shall be subject to the exclusive licenses granted to Licensee pursuant to Section 2.1 (and, for the avoidance of doubt, exclude the Retained Field). Joint Inventions shall be jointly owned by Licensee and Licensor, without a duty of accounting, but shall be subject to the exclusive licenses granted to Licensee pursuant to Section 2.1 (and, for the avoidance of doubt, exclude the Retained Field).
6.2 Patent Prosecution and Maintenance of Licensed Patent Rights. Licensor, at Licensor’s expense, shall have the first right and responsibility to control the Patent Prosecution and maintenance of Licensed Patent Rights using patent counsel reasonably acceptable to Licensee. In addition to, and not in lieu of Licensor’s obligations under Section 6.4 hereof, Licensor shall keep Licensee reasonably informed with respect to the status of Patent Prosecution and maintenance of such Licensed Patent Rights and shall provide copies of all material submissions to any patent office related to Patent Prosecution of such Licensed Patent Rights. Licensor shall promptly (but not less than ninety (90) days, where possible, prior to the due date for the next applicable filing or payment) give notice to Licensee of any upcoming grant, lapse, revocation, surrender, invalidation or abandonment of any Licensed Patent Rights, and in the event of any upcoming lapse, revocation, surrender, invalidation or abandonment of any Licensed Patent Right in any country, Licensee may by notice to Xxxxxxxx assume control of the Patent Prosecution of such Licensed Patent Rights in such country at Licensee’s expense. Licensee shall have the sole right, but not the obligation, to control the Patent Prosecution and maintenance at Licensee’s expense of all Licensee- owned Intellectual Property, including all Improvements to Licensee IP. Each Party shall provide the other Party all reasonable assistance and cooperation in the Patent Prosecution efforts provided herein at the request and expense of the Party controlling such Patent Prosecution, including providing any necessary powers of attorney and assignments of employees of the Parties and their Affiliates and sublicensees and Third Party contractors and executing any other required documents or instruments for such prosecution.
| 10 |
6.3 Enforcement and Defense.
6.3.1 Notice. Each Party shall provide prompt notice to the other Party of any infringement of Licensed IP first occurring after the Effective Date within the Field of which such Party becomes aware. Licensor and Licensee shall thereafter consult and cooperate fully to determine a course of action, including the commencement of legal action by either or both Licensor and Licensee, to terminate any such infringement.
6.3.2 Licensed Patent Rights. In consultation with Licensor, Licensee shall have the first right to enforce the Licensed IP in the Field (including any product or service which Licensee reasonably believes to be competing with a Product in the Field (excluding, for clarity, all Retained Fields) (each, a “Competing Field Infringement”), and to defend any declaratory judgment action (or other challenge) with respect thereto, at Licensee’s expense and by counsel of its own choice and in the name of Licensee (at Licensee’s expense) and shall notify Licensor of such enforcement actions. If Licensee fails to bring or defend any such action against a Competing Field Infringement within (i) sixty (60) days following the notice of alleged Competing Field Infringement provided pursuant to the above or (ii) ten (10) days before the time limit, if any, set forth in Applicable Laws for the filing of such actions, whichever is earlier, Licensor shall have the right, but not the obligation, to bring and control any such action at its own expense and by counsel of its own choice, and Licensee shall have the right, at its own expense, to be represented in any such action by counsel of its own choice. In no event shall either Party admit the invalidity of, or after exercising its right to bring and control an action under this Section, fail to defend the validity of, any Licensed Patent Rights without the other Party’s prior written consent, which shall not be unreasonably withheld, conditioned or delayed.
6.3.3 Competing Field Infringement Action. In the event a Party brings a Competing Field Infringement action in accordance with this Section (the “Controlling Party”), such Controlling Party shall keep the other Party reasonably informed of the progress of any such action, and the other Party shall cooperate fully with the Controlling Party, at the Controlling Party’s request and expense, including by providing information, materials and fact witnesses and, if necessary or desirable to bring such action, be named as a party and join such action. Neither Party shall have the right to settle any Competing Field Infringement action under this Section without the prior written consent of the other Party, which shall not be unreasonably withheld, conditioned or delayed.
6.3.4 Recovery. Except as otherwise agreed by the Parties as part of a cost-sharing arrangement, any recovery obtained by either or both of Licensor and Licensee or as a result of any action with respect to a Competing Field Infringement contemplated by this Section, whether by settlement or otherwise, shall be shared in order as follows: (i) the Controlling Party shall recoup all of its costs and expenses incurred in connection with the action; (ii) the other Party shall then, to the extent possible, recover its costs and expenses incurred in connection with the action; and (c) the portion of any recovery remaining shall be shared by the Parties 75:25 in favor of the Controlling Party.
6.3.5 Defense of Infringement Claims. In the event that a claim is brought against either Party alleging the infringement, violation or misappropriation of any Third Party intellectual property right based on the manufacture, use, sale or importation of the Product, then, upon Licensee’s request, the Parties shall (i) promptly meet to discuss the defense of such claim and (ii) as appropriate, enter into a joint defense agreement with respect to the common interest privilege protecting communications regarding such claim in a form reasonably acceptable to the Parties. The Party against which such claim is brought shall have the right to control the defense of such claim and shall keep the other Party reasonably informed with respect thereto.
| 11 |
6.4 Information and Cooperation. During the Term, Licensor shall (i) provide Licensee with copies of all patent applications filed with respect to the Licensed Patent Rights in the Field and other material submissions and correspondence relating thereto, in sufficient time to allow for reasonable review and comment by Licensee, (ii) provide Licensee and its patent counsel with an opportunity to consult with Licensor and its patent counsel regarding the filing and contents of any such application, amendment, submission or response with respect to the Licensed Patent Rights in the Field and (iii) provide notice of filing of new Licensed Patent Rights in the Field to Licensee within ten (10) business days of such filing. Licensor hereby agrees that the advice and suggestions of Licensee and its patent counsel shall be taken into reasonable consideration by Xxxxxxxx and its patent counsel in connection with each filing; provided that Licensor and its patent counsel shall make the final determination in connection with each filing.
6.5 Right to Acquire Licensed Patent Rights. In the event Licensor wishes to assign any of the Licensed Patent Rights to a Third Party then Licensor will provide Licensee with written notice of the same and, at Licensee’s option, the Parties will negotiate in good faith a mutually acceptable agreement to assign such Licensed Patent Rights to Licensee. If the Parties fail to reach a mutually acceptable agreement then Licensor will be free to assign such Licensed Patent Rights to such Third Party (in all cases such assignment being subject to Licensee’s rights under this Agreement).
ARTICLE 7
TERM AND TERMINATION
7.1 Term. This Agreement is effective as of the Effective Date and continues in full force and effect in perpetuity unless terminated pursuant to Section 7.3, except that the licenses and rights granted hereunder for Intellectual Property shall expire upon the applicable expiration, if any, of each such form of Intellectual Property in accordance with an Applicable Law (the “Term”). Except as set forth herein, the licenses and rights granted hereunder shall not be terminable by the licensing Party.
7.2 Termination. Neither this Agreement nor the licenses or rights granted in Section 2.1 may be terminated in whole or in part by the Licensor for any reason. Licensor’s sole and exclusive remedy for any breach of or default under this Agreement by Licensee shall be to seek monetary damages and/or equitable remedies (other than rescission or termination of this Agreement) to prevent or stop any such breach or default. The rights granted to Licensor hereunder may be terminated by Licensee in the event of a material breach by Licensor of its obligations under this Agreement, where such breach remains uncured for thirty (30) days following Licensor’s receipt of written notice from Licensee specifying such breach. Licensee may terminate this Agreement at any time with or without cause by providing written notice of termination to Licensor, which termination shall be effective thirty (30) days following Licensor’s receipt of such written notice and no repayment or refund of the Upfront Payment shall be owed by Licensor to Licensee.
7.3 Effect of Termination; Surviving Provisions. The rights and obligations set forth in this Agreement shall extend beyond the Term or termination of this Agreement only to the extent expressly and specifically provided for in this Agreement. Without limiting the generality of the foregoing, it is agreed that the provisions of Articles 1, 8, and 9, Sections 5.5, 5.6, 6.1, and 7.3 and, to the extent applicable, all other Sections or Articles referenced in any such Section or Article, shall survive such expiration or termination.
| 12 |
ARTICLE 8
CONFIDENTIALITY AND NON-DISCLOSURE
8.1 Confidentiality.
8.1.1 Nondisclosure Obligations. The Receiving Party shall keep confidential and shall not publish or otherwise disclose or use for any purpose other than the purpose of the Parties to perform their respective obligations or in the exercise of their respective rights under this Agreement, any Confidential Information of the Disclosing Party. The Receiving Party shall treat Confidential Information as it would its own proprietary information which in no event shall be with less than a reasonable standard of care, and take reasonable precautions to prevent the disclosure of Confidential Information to a Third Party, except as explicitly set forth herein, without written consent of the Disclosing Party.
8.1.2 Exceptions to Confidentiality. The Receiving Party’s obligations set forth in this Agreement shall not extend to any Confidential Information of the Disclosing Party to the extent that such Confidential Information:
(a) is or hereafter becomes part of the public domain by public use, publication, general knowledge or the like or is made generally available by a Third Party, in each case, other than through a wrongful act, fault or negligence on the part of the Receiving Party or a breach of this Agreement;
(b) is received from a Third Party without restriction and with the right to disclose such Confidential Information;
(c) the Receiving Party can demonstrate by competent evidence was already in its possession without any limitation on use or disclosure prior to its receipt from the Disclosing Party;
(d) the Receiving Party can demonstrate by competent evidence was independently developed by or for the Receiving Party without reference to, use of or disclosure of the Disclosing Party’s Confidential Information;
(e) is released from the restrictions set forth in this Agreement by the express prior written consent of the Disclosing Party; or
(f) is reasonably necessary for the performance of the obligation or exercise of Receiving Party’s rights under this Agreement.
The restriction set forth in this Section 8.1 shall not apply to the extent that the Receiving Party is required to disclose any Confidential Information under law or by an order of a Governmental Authority; provided that the Receiving Party: (i) provides the Disclosing Party with prompt written notice of such disclosure requirement if legally permitted, (ii) affords the Disclosing Party an opportunity, and cooperates with the Disclosing Party’s efforts, to oppose or limit, or secure confidential treatment for such required disclosure (at the Disclosing Party’s expense), and (iii) if the Disclosing Party is unsuccessful in its efforts pursuant to subsection (ii), discloses only that portion of the Confidential Information that the Receiving Party is legally required to disclose as advised by the Receiving Party’s legal counsel.
| 13 |
ARTICLE 9
MISCELLANEOUS
9.1 Governing Law. This Agreement shall be governed by the laws of the State of Delaware, without regard to conflict of law principles. In relation to any legal action or proceedings (i) arising out of or in connection with this Agreement or its implementation or effect or (ii) relating to any non-contractual obligations arising out of or in connection with this Agreement, each of the parties irrevocably submits to the exclusive jurisdiction of the County of Orange, State of California, and waives any objection to proceedings in such courts on the grounds of venue or on the grounds that proceedings have been brought in an inappropriate forum., except that (a) questions affecting the construction and effect of any patent shall be determined by the law of the jurisdiction in which the patent has been granted, (b) matters related to Regulatory Filings and Regulatory Approval in the Territory, shall be governed by Applicable Law in the Territory and (c) any matters to be exclusively resolved pursuant to the Applicable Laws in the Territory as provided under this Agreement, shall be resolved by the Applicable Laws in the Territory. The Parties hereby exclude the United Nations Convention on Contracts for the International Sale of Goods from this Agreement.
9.2 Notice Requirements. All notices and other communications given or made pursuant to this Agreement shall be in writing and shall be deemed effectively given upon the earlier of actual receipt, or
(i) personal delivery to the party to be notified, (ii) when sent, if sent by electronic mail or facsimile during normal business hours of the recipient, and if not sent during normal business hours, then on the recipient’s next business day, (iii) five (5) days after having been sent by registered or certified mail, return receipt requested, postage prepaid, or (iv) one business day after deposit with a nationally recognized overnight courier, freight prepaid, specifying next business day delivery, with written verification of receipt. All communications shall be sent to the respective parties at their address as set forth below in Section 9.2.1, or to such e-mail address, facsimile number or address as subsequently modified by written notice given in accordance with this section.
9.2.1 Addresses for Notice
| For Licensee: | For Licensor: | |
Masimo Corporation 00 Xxxxxxxxx Xxxxxx, XX 00000 Attention: Xxxxx Xxxxx, CFO |
Innovative Health Solutions, Inc. 000 X. Xxxxx Xxxxxx Xxxxxxxxxx, XX 00000 Attention: Xxxxx Xxxxxxx, CEO |
9.3 Anti-Bribery and Anti-Corruption. Licensor and Licensee acknowledge and agree that there are anti-bribery and anti-corruption laws to which Licensor and Licensee are subject, that prohibit the payment, or offering, or receiving, as the case may be, of anything of value to, or from, a government employee, or official, or private individual, for the purpose of (i) inducing or influencing any governmental act, or decision affecting Licensor or Licensee, (ii) to help Licensor or Licensee obtain or retain any business, or (iii) to otherwise improperly benefit Licensor’s or Licensee’s business activities, and such laws prohibit Licensor and Licensee from being involved with clients, contractors, agents, advisors or other Third Parties involved in such activity. Each Party represents, warrants and covenants to refrain from any activity in connection with this Agreement that would constitute a contravention by that Party of such laws.
| 14 |
9.4 Section 365(n). The Parties agree that all rights and licenses granted under or pursuant to this Agreement to Licensee, including those rights and licenses granted in Section 2.1, are rights and licenses in “intellectual property” within the scope of the United States Bankruptcy Code or any foreign bankruptcy Law (each, a “Bankruptcy Law”). Licensee shall have the rights set forth in this Agreement with respect to the Licensed IP when and as developed or created. Licensee shall have the rights set forth in this Agreement with respect to the Licensed IP when and as developed or created. In addition, Licensee, as a licensee of the Licensed IP hereunder, shall have and may fully exercise all rights available to it under Bankruptcy Laws, including, without limitation, under Section 365(n) of Section 101(35A) (or its successors) of the United States Bankruptcy Code or its successors. In the event of a case under any Bankruptcy Law involving Licensor, in addition to and not in lieu of any other remedies available to Licensee, to the maximum extent available, Licensee shall have the right to obtain (and Licensor or any trustee for Licensor or its assets shall, at Licensee’s written request, deliver to Licensee) all Licensed IP granted to Licensee hereunder and a copy of all embodiments thereof, including, without limitation, embodiments necessary or desirable for Licensee to exercise its rights hereunder. In addition, Licensor shall take all steps reasonably requested by Licensee to perfect, exercise and enforce its rights hereunder, including, without limitation, filings in any Patent or Copyright office or with any other Regulatory Authorities with respect thereto.
9.5 Amendment; Waiver. Any term of this Agreement may be amended, modified or terminated and the observance of any term of this Agreement may be waived (either generally or in a particular instance, and either retroactively or prospectively) only with the written consent of Licensor and Licensee. No waivers of or exceptions to any term, condition, or provision of this Agreement, in any one or more instances, shall be deemed to be or construed as a further or continuing waiver of any such term, condition or provision.
9.6 Relationship of the Parties. Nothing in this Agreement shall be construed (i) to create or imply a partnership, association, joint venture or fiduciary duty between the Parties, (ii) to make either Party the agent of the other for any purpose, (iii) to alter, amend, supersede or vitiate any other arrangements between the Parties with respect to any subject matters not covered hereunder, or (iv) to give either Party the right to bind the other or to create any duties or obligations between the Parties, except as expressly set forth herein. All Persons employed by a Party shall be employees of such Party and not of the other Party and all costs/expenses and obligations incurred by reason of such employment shall be for the account and expense of such Party. The Parties agree that the rights and obligations under this Agreement are not intended to constitute a partnership or similar arrangement that will require separate reporting for tax purposes in the Territory.
9.7 Assignment and Successors. Subject to Section 6.5 hereof, Licensor and Licensee shall be free to assign or transfer this Agreement and its rights and obligations hereunder, in whole or in part, to any Person at any time, provided that Licensor must give Licensee (i) immediate written notice in the event Licensor receives an unsolicited offer to be acquired, or in the event Licensor receives an unsolicited offer to acquire any of Licensor’s business or assets related to this Agreement (including any of the Licensed IP), and (ii) at least thirty (30) days prior written notice of any intended assignment of this Agreement by Licensor or any potential merger, acquisition, change of control, or other sale of any business or assets of Licensor related to this Agreement.
9.8 Interpretation. Except where the context otherwise requires, wherever used, the singular shall include the plural, the plural the singular, the use of any gender shall be applicable to all genders. The term “including” as used herein shall mean including, without limiting the generality of any description preceding such term. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties. The Parties acknowledge and agree that: (i) the rule of construction to the effect that any ambiguities are resolved against the drafting Party shall not be employed in the interpretation of this Agreement; and (ii) the terms and provisions of this Agreement shall be construed fairly as to all Parties and not in favor of or against any Party, regardless of which Party was generally responsible for the preparation of this Agreement.
| 15 |
9.9 Severability. If any provision or provisions of this Agreement shall be held to be invalid, illegal or unenforceable for any reason whatsoever: (i) the validity, legality and enforceability of the remaining provisions of this Agreement (including without limitation, each portion of any Section of hereof containing any such provision held to be invalid, illegal or unenforceable, that is not itself invalid, illegal or unenforceable) shall not in any way be affected or impaired thereby and shall remain enforceable to the fullest extent permitted by law; (ii) such provision or provisions shall be deemed reformed to the extent necessary to conform to applicable law and to give the maximum effect to the intent of the parties hereto; and (iii) to the fullest extent possible, the provisions of this Agreement (including, without limitation, each portion of any Section of this Agreement containing any such provision held to be invalid, illegal or unenforceable, that is not itself invalid, illegal or unenforceable) shall be construed so as to give effect to the intent manifested thereby.
9.10 Entire Agreement. This Agreement, together with the Purchase Agreement, constitutes the full and entire understanding and agreement between the Parties with respect to the subject matter hereof, and any other written or oral agreement relating to the subject matter hereof existing between the Parties is expressly canceled.
9.11 Counterparts. This Agreement may be executed in two (2) or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. Counterparts may be delivered via facsimile, electronic mail (including pdf or any electronic signature complying with the U.S. federal ESIGN Act of 2000, e.g., xxx.xxxxxxxx.xxx) or other transmission method and any counterpart so delivered shall be deemed to have been duly and validly delivered and be valid and effective for all purposes.
9.12 Further Assurance. Licensor shall perform all further acts and things and execute and deliver such further documents as may be necessary or as Licensee may reasonably require to give effect to this Agreement.
[Remainder of page intentionally left blank.]
| 16 |
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their duly authorized representatives.
| MASIMO CORPORATION | INNOVATIVE HEALTH SOLUTIONS, INC. |
| By: | By: | /s/ Xxxxx Xxxxxxx |
| (Signature) | (Signature) |
| Xxxxx Xxxxx | Xxxxx Xxxxxxx | |
| Executive Vice President, Chief Financial Officer | Chief Executive Officer |
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their duly authorized representatives.
| MASIMO CORPORATION | INNOVATIVE HEALTH SOLUTIONS, INC. |
| By: | By: |
| (Signature) | (Signature) | |
| /s/ Xxxxx Xxxxx | ||
| Xxxxx Xxxxx | Xxxxx Xxxxxxx | |
| Executive Vice President, Chief Financial Officer | Chief Executive Officer |
[Signature Page to License and Collaboration Agreement]
Exhibit A
Licensed IP
PATENT RIGHTS
| Patent Number (Status) | Description | |
| U.S. Patent No. 9,662,269 (Issued May 30, 2017) | Directed to a device for generating and providing electrical stimulation pulses to a concentrated area of auricular neurovascular bundles via a needle array having needles for insertion into the skin surface of the concentrated area. | |
| U.S. Patent No. 9,839,577 (Issued December 12, 2017) | Directed at the same technical field as U.S. Patent No. 9,662,269 (above). | |
| U.S. Patent No. 10,010,479 (Issued July 3, 2018) | Directed at the same technical field as U.S. Patent No. 9,662,269 (above). | |
| U.S. Patent No. 10,322,062(Issued June 18, 2019) | Directed at auricular peripheral nerve field stimulator and method of operating same. | |
| U.S. Patent No. 10,413,719 (Issued September 17, 2019) | Directed at treating disease using auricular peripheral nerve field stimulation. |
TRADEMARKS
| Owner | Trademark | Reg. Date / (Filing Date) | Reg. No. / (Serial No.) | Live / Dead | ||||
| Innovative Health Solutions, Inc. |  |
(March 11, 2016) | (86937229) | Live | ||||
| Innovative Health Solutions, Inc. |
|
(March 11, 2016) | (86937225) | Live | ||||
| Innovative Health Solutions, Inc. |  |
(March 11, 2016) | (86937224) | Live |
| 1 |
| Owner | Trademark | Reg. Date / (Filing Date) | Reg. No. / (Serial No.) | Live / Dead | ||||
| Innovative Health Solutions, Inc. |
|
(March 11, 2016) | (86937221) | Live | ||||
| Innovative Health Solutions, Inc. |  |
(February 9, 2016) | (86902033) | Live | ||||
| Innovative Health Solutions, Inc. |
|
(February 9, 2016) | (86901992) | Live | ||||
| Innovative Health Solutions, Inc. |
|
November 8, 2016 | 5078513 | Live | ||||
| Innovative Health Solutions, Inc. |  |
November 8, 2016 | 5078512 | Live | ||||
| Innovative Health Solutions, Inc. |  |
April 19, 2016 | 4942293 | Live | ||||
| Innovative Health Solutions, Inc. |  |
April 19, 2016 | 4942292 | Live | ||||
| Innovative Health Solutions, Inc. |
|
November 10, 2015 | 4852008 | Live | ||||
| Innovative Health Solutions, Inc. |  |
December 20, 2016 | 5105258 | Live |
| 2 |
| Owner | Trademark | Reg. Date / (Filing Date) | Reg. No. / (Serial No.) | Live / Dead | ||||
| Innovative Health Solutions, Inc. |  |
December 20, 2016 | 5105257 | Live | ||||
| Innovative Health Solutions, Inc. |  |
February 23, 2016 | 4905470 | Live | ||||
| Innovative Health Solutions, Inc. | INNOVATIVE HEALTH SOLUTIONS | February 22, 2005 | 2927715 | Dead |
| 3 |