SECURITIES PURCHASE AGREEMENT
Exhibit 4.6
EXECUTION COPY
SECURITIES PURCHASE AGREEMENT (the “Agreement”), dated as of October 30, 2006 by and among Antigenics Inc., a Delaware corporation (the “Company”), and the investors listed on the Schedule of Buyers attached hereto (individually, a “Buyer” and collectively, the “Buyers”).
A. The Company and each Buyer is executing and delivering this Agreement in reliance upon the exemption from registration afforded by Section 4(2) of the Securities Act of 1933, as amended (the “1933 Act”), and Rule 506 of Regulation D (“Regulation D”) as promulgated by the United States Securities and Exchange Commission (the “SEC”) under the 1933 Act.
B. The Company has authorized the issuance and sale of (i) $25,000,000 in principal amount of senior secured convertible notes of the Company in the form attached hereto as Exhibit A (together with any senior secured convertible notes issued in replacement or exchange thereof in accordance with the terms thereof and any senior secured convertible notes issued to pay interest in the form attached hereto as Exhibit B (“PIK Notes”), the “Notes”), which Notes shall be, in accordance with the terms of the Notes, convertible into shares (as converted, the “Conversion Shares”) of the Company’s common stock, par value $.01 per share (“Company Common Stock”) or into Antigenics MA Shares (as defined below).
C. The Notes bear interest, which at the option of the Company, subject to certain conditions, may be paid in kind through the issuance of additional Notes.
D. Each Buyer wishes to purchase, severally but not jointly, and the Company wishes to sell, upon the terms and conditions stated in this Agreement, that aggregate principal amount of Notes set forth opposite such Buyer’s name in the Schedule of Buyers (which aggregate principal amount for all Buyers shall be $25,000,000).
E. The Company is agreeing to pledge all its shares (“Antigenics MA Shares”) in Antigenics Inc., a Massachusetts corporation (“Antigenics MA”), to secure its obligations to pay interest on and principal of the Notes and, as evidenced by the Pledge and Security Agreement, dated as of the date hereof, by and among the company and the Buyers, substantially in the form attached thereto as Exhibit C (the “Pledge and Security Agreement”) and in connection therewith the Company shall deliver the number of Antigenics MA Shares.
F. The Notes and the Conversion Shares collectively are referred to herein as the “Securities.”
1. PURCHASE AND SALE OF NOTES.
(a) Amount. Subject to the satisfaction (or waiver) of the conditions set forth in Sections 6 and 7 below, the Company shall issue and sell to each Buyer, and each Buyer severally, but not jointly, shall purchase from the Company on the Closing Date (as defined below), the principal amount of Notes set forth opposite such Buyer’s name in the Schedule of Buyers.
(b) Closing. The closing (the “Closing”) of the purchase of the Notes by the Buyers shall occur at the offices of Ropes & Xxxx, LLP, Xxx Xxxxxxxxxxxxx Xxxxx, Xxxxxx, Xxxxxxxxxxxxx 00000. The date and time of the Closing (the “Closing Date”) shall be 9:00 a.m., Boston City Time, on October 30, 2006, subject to notification of satisfaction (or waiver) of the conditions to the Closing set forth in Sections 6 and 7 below (or such later date as is mutually agreed to by the Company and each Buyer).
(c) Purchase Price. The purchase price for each Buyer (the “Purchase Price”) of the Notes at the Closing shall be equal to the principal amount set forth opposite such Buyer’s name on the Schedule of Buyers.
(d) Form of Payment. On the Closing Date, (i) each Buyer shall pay its aggregate applicable Purchase Price to the Company for the Notes to be issued and sold to such Buyer at the Closing, by wire transfer of immediately available funds in accordance with the Company’s written wire instructions, and (ii) the Company shall deliver to each Buyer the Notes which such Buyer is then purchasing, duly executed on behalf of the Company and registered in the name of such Buyer or its designee.
2. BUYER’S REPRESENTATIONS AND WARRANTIES.
Each Buyer represents and warrants with respect to only itself that:
(a) No Public Sale or Distribution. Such Buyer is acquiring the Notes, and, upon conversion of the Notes, will acquire the Conversion Shares, for its own account and not with a view towards, or for resale in connection with, the public sale or distribution thereof, except pursuant to sales registered or exempted under the 1933 Act; provided, however, that by making the representations herein, such Buyer does not agree to hold any of the Securities for any minimum or other specific term and reserves the right to dispose of the Securities at any time in accordance with or pursuant to a registration statement or an exemption under the 1933 Act. Such Buyer is acquiring the Securities hereunder in the ordinary course of its business. Such Buyer does not presently have any agreement or understanding, directly or indirectly, with any Person to distribute any of the Securities.
(b) Accredited Investor Status. Such Buyer is an “accredited investor” as that term is defined in Rule 501(a) of Regulation D.
(c) Reliance on Exemptions. Such Buyer understands that the Securities are being offered and sold to it in reliance on specific exemptions from the registration requirements of United States federal and state securities laws and that the Company is relying in part upon the truth and accuracy of, and such Buyer’s compliance with, the representations, warranties, agreements, acknowledgments and understandings of such Buyer set forth herein in order to determine the availability of such exemptions and the eligibility of such Buyer to acquire the Securities.
2
(d) Information. Such Buyer and its advisors, if any, have been furnished with all materials relating to the business, finances and operations of the Company and materials relating to the offer and sale of the Securities which have been requested by such Buyer. Such Buyer and its advisors, if any, have been afforded the opportunity to ask questions of the Company.
(e) Experience of Such Buyer. Such Buyer understands that its investment in the Securities involves a high degree of risk. Such Buyer, either alone or together with its representatives, has such knowledge, sophistication and experience in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the Securities, and has so evaluated the merits and risks of such investment. Such Buyer is able to bear the economic risk of an investment in the Securities and is able to afford a complete loss of such investment.
(f) No Governmental Review. Such Buyer understands that no United States federal or state agency or any other government or governmental agency has passed on or made any recommendation or endorsement of the Securities or the fairness or suitability of the investment in the Securities nor have such authorities passed upon or endorsed the merits of the offering of the Securities.
(g) Transfer or Resale. Such Buyer will not sell, offer to sell, assign, pledge, hypothecate or otherwise transfer any of the Securities unless (i) pursuant to an effective registration statement under the 1933 Act, (ii) such Buyer provides the Company with reasonable assurances and customary representations, that such Securities can be sold pursuant to Rule 144 under the 1933 Act. Notwithstanding anything to the contrary contained in the Agreement, such Buyer may transfer (without restriction) the Securities to its affiliates provided that such affiliate is an “accredited investor” under Regulation D and such affiliate agrees to be bound by the terms and conditions of the Agreement.
(h) Legends. Such Buyer understands that the certificates or other instruments representing the Notes and, until such time as the resale of the Conversion Shares have been registered under the 1933 Act, the stock certificates representing the Conversion Shares, except as set forth below, shall bear any legend as required by the “blue sky” laws of any state and a restrictive legend in substantially the following form (and a stop-transfer order may be placed against transfer of such certificates and instruments):
NEITHER THIS NOTE NOR THE SECURITIES INTO WHICH THIS NOTE IS CONVERTIBLE HAVE BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR APPLICABLE STATE SECURITIES LAWS. THE SECURITIES MAY NOT BE OFFERED FOR SALE, SOLD, TRANSFERRED OR ASSIGNED IN THE ABSENCE OF (A) AN EFFECTIVE REGISTRATION STATEMENT FOR THE SECURITIES UNDER THE SECURITIES ACT OF 1933, AS AMENDED, AND APPLICABLE STATE SECURITIES LAWS, OR (B) THE TRANSFEROR PROVIDES THE COMPANY WITH REASONABLE ASSURANCES AND CUSTOMARY REPRESENTATIONS THAT REGISTRATION IS NOT REQUIRED UNDER SAID ACT AND APPLICABLE STATE SECURITIES LAWS.
3
The legend set forth above shall be removed and the Company shall issue a certificate or instrument without such legend to the holder of the Securities upon which it is stamped, if, unless otherwise required by state securities laws, (i) such Securities are registered for resale under the 1933 Act; provided, that each Buyer has complied with or covenants and agrees that it will comply with the prospectus delivery requirements of the 1933 Act as applicable to it in connection with sales of such Securities pursuant to a registration statement or (ii) in connection with a sale, assignment or other transfer, such holder provides the Company with reasonable assurances and customary representations to the effect that such legend is not required under applicable requirements of the 1933 Act.
(i) Validity; Enforcement. This Agreement and Pledge and Security Agreement have been duly and validly authorized, executed and delivered on behalf of such Buyer and shall constitute the legal, valid and binding obligations of such Buyer, enforceable against such Buyer in accordance with their respective terms, except as such enforceability may be limited by general principles of equity or to applicable bankruptcy, insolvency, reorganization, moratorium, liquidation and other similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies.
(j) No Conflicts. The execution, delivery and performance by such Buyer of this Agreement and the Pledge and Security Agreement to which such Buyer is a party and the consummation by such Buyer of the transactions contemplated hereby and thereby will not (i) result in a violation of the organizational documents of such Buyer or (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which such Buyer is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws) applicable to such Buyer, except in the case of clauses (ii) and (iii), for such conflicts, defaults, rights or violations which would not, individually or in the aggregate, reasonably be expected to have a material adverse effect on the ability of such Buyer to perform its obligations hereunder.
(k) Residency. Such Buyer is a resident of that jurisdiction specified in its address on the Schedule of Buyers.
3. REPRESENTATIONS AND WARRANTIES OF THE COMPANY.
The Company represents and warrants to each of the Buyers that:
(a) Organization and Qualification. Each of the Company and each Subsidiary is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is organized and has all requisite corporate power and authority to carry on its business as now conducted. Each of the Company and each Subsidiary is duly qualified to transact business and is in good standing in each jurisdiction in which the failure so to qualify would have a Material Adverse Effect. As used in this Agreement, “Material Adverse Effect” means any material adverse effect on the business, properties, assets, operations, results of operations, or financial condition of the Company and its subsidiaries taken as a whole.
4
(b) Authorization; Enforcement; Validity. The Company has the requisite power and authority to enter into and perform its obligations under this Agreement, the Notes, the Pledge and Security Agreements and the Guaranty Agreement (collectively, the “Transaction Documents”) and to issue the Securities in accordance with the terms hereof and thereof. The execution and delivery of the Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby, including, without limitation, the issuance of the Notes, the reservation for issuance and the issuance of the Conversion Shares issuable upon conversion of the Notes, and the pledging of the Antigenics MA Shares have been duly authorized by the Company’s Board of Directors and no further corporate action is required by the Company. This Agreement and the other Transaction Documents have been duly executed and delivered by the Company, and constitute the legal, valid and binding obligations of the Company, enforceable against the Company in accordance with their respective terms, except as such enforceability may be limited by general principles of equity or applicable bankruptcy, insolvency, reorganization, moratorium, liquidation or similar laws relating to, or affecting generally, the enforcement of applicable creditors’ rights and remedies.
(c) Issuance of Securities. The issuance of the Notes and the pledging of the Antigenics MA Shares have been duly authorized by the Company. As of the Closing, a number of shares of Company Common Stock shall have been duly authorized and reserved for issuance which equals the sum of 100% of the maximum number of shares of Company Common Stock issuable upon conversion of the Notes on the Closing Date. Upon issuance or conversion in accordance with the Notes, the Conversion Shares will be validly issued, fully paid and nonassessable. Assuming the accuracy of the Buyers’ representations and warranties contained herein, the offer and issuance by the Company of the Notes will be exempt from registration under the 1933 Act.
(d) No Conflicts. The execution, delivery and performance of the Transaction Documents by the Company and the consummation by the Company of the transactions contemplated hereby and thereby (including, without limitation, the issuance of the Notes, the pledging of the Antigenics MA Shares and reservation for issuance and issuance of the Conversion Shares) will not (i) result in a violation of the Certificate of Incorporation (as defined in Section 3(o)) or Bylaws (as defined in Section 3(o)) or (ii) conflict with, or constitute a default (or an event which with notice or lapse of time or both would become a default) under, or give to others any rights of termination, amendment, acceleration or cancellation of, any agreement, indenture or instrument to which the Company is a party, or (iii) result in a violation of any law, rule, regulation, order, judgment or decree (including federal and state securities laws and regulations and the rules and regulations of the Nasdaq Stock Market (the “Principal Market”)) applicable to the Company or by which any property or asset of the Company is bound or affected, except in the case of clauses (ii) and (iii), for such conflicts, defaults, rights or violations which would have a Material Adverse Effect.
(e) Consents. Except as disclosed in Schedule 3(e) and except for those failure of which to obtain or make would not have a Material Adverse Effect, and any filings required pursuant to Section 4(b) hereof, the Company is not required to obtain any consent, authorization
5
or order of, or make any filing or registration with, any court, governmental agency or any regulatory or any other Person in order for it to execute, deliver or perform any of its obligations under or contemplated by the Transaction Documents, in each case in accordance with the terms hereof or thereof. All consents, authorizations, orders, filings and registrations which the Company is required to obtain pursuant to the preceding sentence have been obtained or effected on or prior to the Closing Date.
(f) Acknowledgment Regarding Buyer’s Purchase of Securities. The Company acknowledges that no Buyer is acting as a financial advisor or fiduciary of the Company (or in any similar capacity) with respect to the Transaction Documents and the transactions contemplated hereby and thereby, and any advice given by a Buyer or any of its representatives or agents in connection with the Transaction Documents and the transactions contemplated hereby and thereby is merely incidental to such Buyer’s purchase of the Securities. The Company further represents to each Buyer that the Company’s decision to enter into the Transaction Documents has been based solely on the independent evaluation by the Company and its representatives.
(g) No General Solicitation; Placement Agent’s Fees. Neither the Company, nor any Person acting on its behalf, has engaged in any form of general solicitation or general advertising (within the meaning of Regulation D) in connection with the offer or sale of the Notes. No broker has acted on behalf of the Company in connection with this Agreement, and there are no brokerage commissions, finders’ fees or similar fees or commissions payable in connection therewith based on any agreement, arrangement or understanding with the Company or any action taken by the Company.
(h) No Integrated Offering. Neither the Company, nor any Person acting on its behalf, has, directly or indirectly, made any offers or sales of any security or solicited any offers to buy any security, under circumstances that would require registration of the Notes to the Buyers under the 1933 Act or cause this offering of the Notes to be integrated with prior offerings by the Company for purposes of the 1933 Act.
(i) SEC Documents; Financial Statements. Since December 31, 2005, the Company has filed all reports, schedules, forms, statements and other documents required to be filed by it with the SEC pursuant to the reporting requirements of the Securities Exchange Act of 1934 (the “1934 Act”) (all of the foregoing and all exhibits included therein and financial statements, notes and schedules thereto and documents incorporated by reference therein being hereinafter referred to as the “SEC Documents”). As of their respective dates, the SEC Documents complied in all material respects with the requirements of the 1934 Act and the rules and regulations of the SEC promulgated thereunder and applicable thereto, and none of the SEC Documents, at the time they were filed with the SEC, contained any untrue statement of a material fact or omitted to state a material fact required to be stated therein or necessary in order to make the statements therein, in the light of the circumstances under which they were made, not misleading. As of their respective dates, the financial statements of the Company included in the SEC Documents complied as to form in all material respects with applicable accounting requirements and the published rules and regulations of the SEC with respect thereto. Such financial statements have been prepared in accordance with generally accepted accounting principles, consistently applied, during the periods involved (except (i) as may be otherwise indicated in such financial
6
statements or the notes thereto, or (ii) in the case of unaudited interim statements, to the extent they may exclude footnotes or may be condensed or summary statements) and fairly present in all material respects the financial position of the Company as of the dates thereof and the results of its operations and cash flows for the periods then ended (subject, in the case of unaudited statements, to normal year-end audit adjustments).
(j) Absence of Certain Changes. Except as disclosed in the SEC Documents since December 31, 2005, there has been no material adverse change and no material adverse development in the business, properties, assets, operations, results of operations, or financial condition of the Company or any Subsidiary.
(k) Regulatory Permits. Each of the Company and each Subsidiary possesses all certificates, authorizations and permits issued by the appropriate federal, state or foreign regulatory authorities necessary to conduct its business, except where the failure to possess such certificates, authorizations or permits would not have a Material Adverse Effect. Each of the Company and each Subsidiary is not in violation of any judgment, decree or order or any statute, ordinance, rule or regulation applicable to it, except for violations which would not have a Material Adverse Effect.
(l) Foreign Corrupt Practices. Neither the Company or Subsidiary, nor any director, officer, agent, employee or other Person acting on behalf of the Company or Subsidiary has, in the course of its actions for, or on behalf of, the Company or the Subsidiary (i) used any corporate funds for any unlawful contribution, gift, entertainment or other unlawful expenses relating to political activity; (ii) made any direct or indirect unlawful payment to any foreign or domestic government official or employee from corporate funds; (iii) violated or is in violation of any provision of the U.S. Foreign Corrupt Practices Act of 1977, as amended; or (iv) made any unlawful bribe, rebate, payoff, influence payment, kickback or other unlawful payment to any foreign or domestic government official or employee.
(m) Xxxxxxxx-Xxxxx Act. The Company is in compliance with any and all applicable requirements of the Xxxxxxxx-Xxxxx Act of 2002 that are effective as of the date hereof, and any and all applicable rules and regulations promulgated by the SEC thereunder that are effective as of the date hereof, except where such noncompliance would not have a Material Adverse Effect.
(n) Transactions With Affiliates. Except as disclosed in Schedule 3(n) and except as set forth in the SEC Documents, and other than the grant of stock options and restricted and non-restricted stock grants disclosed that are required to be publicly disclosed, none of the officers, directors or employees of the Company is presently a party to any transaction with the Company (other than for ordinary course services as employees, officers or directors) required to be disclosed pursuant to Regulation S-K Item 404, including any contract, agreement or other arrangement providing for the furnishing of services to or by, providing for rental of real or personal property to or from, or otherwise requiring payments to or from any such officer, director or employee or, to the knowledge of the Company, any corporation, partnership, trust or other entity in which any such officer, director, or employee has a substantial interest or is an officer, director, trustee or partner, which such transaction would be required to be disclosed.
7
(o) Equity Capitalization. The capitalization of the Company as of September 30, 2006 is as set forth on Schedule 3(o) hereto. All the outstanding shares of Company capital stock have been validly issued and are fully paid and nonassessable. Except as disclosed in Schedule 3(o): (i) none of the Company’s share capital is subject to preemptive rights that would be triggered upon issuance of the Securities; (ii) except as disclosed in the Form 10-K of the Company filed in March 2006 with the SEC or subsequently filed Forms 10-Q or Forms 8-K, there are no outstanding options, warrants, scrip, rights to subscribe for, calls or other rights convertible into, or exercisable or exchangeable for, any share capital of the Company by which the Company is bound to issue additional share capital of the Company, and (iii) there are no outstanding debt securities, notes, credit agreements, credit facilities or other agreements, documents or instruments evidencing Indebtedness (as defined in Section 3(p)) by which the Company is bound. The Company has made available to each Buyer true, correct and complete copies of the Company’s Certificate of Incorporation, as amended and as in effect on the date hereof (the “Certificate of Incorporation”), and the Company’s Bylaws, as amended and as in effect on the date hereof (the “Bylaws”).
(p) Indebtedness and Other Contracts. Except as disclosed in Schedule 3(p) or in the SEC Documents, each of the Company and each Subsidiary (i) has no outstanding Indebtedness (as defined below) other than Permitted Indebtedness (as defined in the Note), including Indebtedness secured by Permitted Liens, and (ii) is not in violation of any term of or in default under any contract, agreement or instrument relating to any Indebtedness, except where such violations and defaults would not result in a Material Adverse Effect. For purposes of this Agreement: (x) “Indebtedness” of any Person means, without duplication (A) all indebtedness for borrowed money, (B) all obligations issued, undertaken or assumed as the deferred purchase price of property or services (including, without limitation, “capital leases” in accordance with generally accepted accounting principals) (other than trade payables entered into in the ordinary course of business), (C) all reimbursement or payment obligations with respect to letters of credit, and surety bonds, (D) all obligations evidenced by notes, bonds, or debentures, (E) all indebtedness created or arising under any conditional sale or other title retention agreement, or incurred as financing, in either case with respect to any property or assets acquired with the proceeds of such indebtedness (even though the rights and remedies of the seller or bank under such agreement in the event of default are limited to repossession or sale of such property), (F) all monetary obligations under any leasing or similar arrangement which, in connection with generally accepted accounting principles, consistently applied for the periods covered thereby, is classified as a capital lease, (G) all indebtedness referred to in clauses (A) through (F) above secured by any mortgage, lien, pledge, charge, or security interest upon or in any property or assets (including accounts and contract rights) owned by any Person, even though the Person which owns such assets or property has not assumed or become liable for the payment of such indebtedness, and (H) all Contingent Obligations in respect of indebtedness or obligations of others of the kinds referred to in clauses (A) through (G) above; (y) “Contingent Obligation” means, as to any Person, any direct or indirect liability, contingent or otherwise, of that Person with respect to any indebtedness, lease, or other monetary obligation of another Person if the primary purpose or intent of the Person incurring such liability is to provide assurance to the obligee of such liability that such liability will be paid or discharged; and (z) “Person” means an individual, a limited liability company, a partnership, a joint venture, a corporation, a trust, an unincorporated organization and a government or any department or agency thereof.
8
(q) Litigation. Except as set forth in Schedule 3(q) or in the SEC Reports, there is no action, suit, proceeding, inquiry or investigation before any court, public board, government agency, self-regulatory organization or body pending other than those which would not have a Material Adverse Effect. Each of the Company and Antigenics MA, has, in all material respects, complied with all laws, regulations and orders applicable to its business, including Pharmaceutical Laws (as defined below), and has all material permits and licenses required thereby. For purposes of this Agreement, “Pharmaceutical Law” shall mean any federal, state, local or foreign law, statute, rule or regulation relating to the development, commercialization and sale of pharmaceutical and biotechnology products and devices, including all applicable regulations of the U.S. Food and Drug Administration and comparable applicable foreign regulatory authorities.
(r) Insurance. Each of the Company and each Subsidiary is insured by insurers of recognized financial responsibility against such losses and risks and in such amounts as are prudent and customary in the businesses in which either the Company or Subsidiary is engaged.
(s) Employee Relations. The Company is not a party to any collective bargaining agreement. The Company is in compliance with all federal, state, local and foreign laws and regulations respecting labor, employment and employment practices and benefits, terms and conditions of employment and wages and hours, except where failure to be in compliance would not reasonably be expected to result in a Material Adverse Effect.
(t) Intellectual Property Rights. Except as disclosed with the Securities and Exchange Commissions or as set forth on Schedule 3(t): (a) the Company and each of its subsidiaries owns or possesses adequate rights or licenses to use all trademarks, trade names, service marks, service xxxx registrations, service names, patents, patent rights, copyrights, trade secrets and other intellectual property rights (“Intellectual Property Rights”) necessary to conduct their respective businesses as now conducted; (b) the Company does not have any knowledge of any infringement by the Company of Intellectual Property Rights of others, nor does the Company have reason to believe that the Company has, or would infringe on the Intellectual Property Rights of others, the enforcement of which would result in a Materially Adverse Effect on financial conditions; (c) there is no claim, action or proceeding against the Company regarding its Intellectual Property Rights; (d) the Company has no knowledge of any infringement or improper use by any third party of any of the Company’s Intellectual Property Rights; (e) the Company has taken reasonable security measures to protect the secrecy, confidentiality and value of all of its Intellectual Property Rights; (f) the Company shall own, or shall cause Antigenics MA to at all times own all right, title and interest in all Intellectual Property Rights, which the Company or Antigenics MA, respectively own as of the Issuance Date and which may exist in the future, relating to QS-21 and AG-707. Such rights held by Antigenics MA shall include without limitation all rights, royalties or licensing revenues from the sale thereof relating to QS-21 and AG-707. Notwithstanding anything in this Section 3(t) to the contrary, the Company may consummate a Spin-off (as such term is defined the Pledge and Security Agreement), enter into partnership, license and collaboration agreements and other similar arrangements.
(u) Environmental Laws. Each of the Company and each Subsidiary (i) is in compliance with any and all Environmental Laws (as hereinafter defined), (ii) has received all
9
permits, licenses or other approvals required of them under applicable Environmental Laws to conduct their respective businesses and (iii) is in compliance with all terms and conditions of any such permit, license or approval where, in each of the foregoing clauses (i), (ii) and (iii), the failure to so comply could be reasonably expected to have, individually or in the aggregate, a Material Adverse Effect. The term “Environmental Laws” means all federal, state, local or foreign laws relating to pollution or protection of human health or the environment (including, without limitation, ambient air, surface water, groundwater, land surface or subsurface strata), including, without limitation, laws relating to emissions, discharges, releases or threatened releases of chemicals, pollutants, contaminants, or toxic or hazardous substances or wastes (collectively, “Hazardous Materials”) into the environment, or otherwise relating to the manufacture, processing, distribution, use, treatment, storage, disposal, transport or handling of Hazardous Materials, as well as all authorizations, codes, decrees, demands or demand letters, injunctions, judgments, licenses, notices or notice letters, orders, permits, plans or regulations issued, entered, promulgated or approved thereunder.
(v) Investment Company. The Company is not, and is not an affiliate of, an “investment company” within the meaning of the Investment Company Act of 1940, as amended.
(w) Tax Status. Except as would not have a Material Adverse Effect, the Company (i) has made or filed all foreign, federal and state income and all other tax returns, reports and declarations required by any jurisdiction to which it is subject, (ii) has paid all taxes and other governmental assessments and charges that are material in amount, shown or determined to be due on such returns, reports and declarations, except those being contested in good faith and (iii) has set aside on its books provision reasonably adequate for the payment of all taxes for periods subsequent to the periods to which such returns, reports or declarations apply.
(x) Internal Accounting and Disclosure Controls. The Company maintains a system of internal accounting controls sufficient to provide reasonable assurance that (i) transactions are executed in accordance with management’s general or specific authorizations, (ii) transactions are recorded as necessary to permit preparation of financial statements in conformity with generally accepted accounting principles and to maintain asset and liability accountability, (iii) access to assets or incurrence of liabilities is permitted only in accordance with management’s general or specific authorization and (iv) the recorded accountability for assets and liabilities is compared with the existing assets and liabilities at reasonable intervals and appropriate action is taken with respect to any difference. The Company maintains disclosure controls and procedures (as such term is defined in Rule 13a-14 under the 0000 Xxx) that are effective in ensuring that information required to be disclosed by the Company in the reports that it files or submits under the 1934 Act is recorded, processed, summarized and reported, within the time periods specified in the rules and forms of the SEC, including, without limitation, controls and procedures designed to ensure that information required to be disclosed by the Company in the reports that it files or submits under the 1934 Act is accumulated and communicated to the Company’s management, including its principal executive officer or officers and its principal financial officer or officers, as appropriate, to allow timely decisions regarding required disclosure.
(y) Off Balance Sheet Arrangements. There is no transaction, arrangement, or other relationship between the Company and an unconsolidated or other off balance sheet entity that is required to be disclosed by the Company in its Exchange Act filings and is not so disclosed or that otherwise would have a Material Adverse Effect.
10
(z) Ranking of Notes. Except as disclosed on Schedule 3(p) or the SEC Documents, no Indebtedness (other than Permitted Indebtedness (as defined in the Note), including Indebtedness secured by Permitted Liens) of the Company is senior to or ranks pari passu with the Notes in right of payment, whether with respect of payment of redemptions, interest, damages or upon liquidation or dissolution or otherwise.
(aa) Antigenics MA Shares.
i. The Company is the beneficial and record owner of all of the Antigenics MA Shares, free and clear of any Liens, and upon transfer in accordance with the applicable Transaction Documents, the Company will transfer to the Buyers good and marketable title to such Antigenics MA Shares, free and clear of any Liens imposed by or through the Company.
ii. The Company has no legal obligation, absolute or contingent, to any other Person to transfer or sell any of the Antigenics MA Shares. There is no action, claim, suit, investigation or proceeding pending or, to the knowledge of the Company, threatened by or against or affecting the Company or the Company’s ownership of the Antigenics MA Shares before any court or governmental or regulatory authority or body, that could materially impact the ability of the Company to pledge and transfer to the Buyers any Antigenics MA Shares. There are no writs, decrees, injunctions or orders of any court or governmental or regulatory agency, authority or body outstanding against the Company with respect to the Antigenics MA Shares.
(bb) Transfer Taxes. On the Closing Date, all stock transfer or other taxes (other than income or similar taxes) which are required to be paid in connection with the sale and transfer of the Notes to be sold to each Buyer hereunder will be, or will have been, fully paid or provided for by the Company, and all laws imposing such taxes will be or will have been complied with.
(cc) Manipulation of Price. The Company has not, and to its knowledge no one acting on its behalf has, (i) taken, directly or indirectly, any action designed to cause or to result in the stabilization or manipulation of the price of any security of the Company to facilitate the sale of the Notes or (ii) sold, bid for, purchased, or paid any compensation for soliciting purchases in the marketplace of, any of the Notes.
(dd) Seniority. As of the Closing Date, no Indebtedness of the Company will be senior to the Notes in right of payment, whether with respect to interest or upon liquidation or dissolution, except as disclosed on Schedule 3(p).
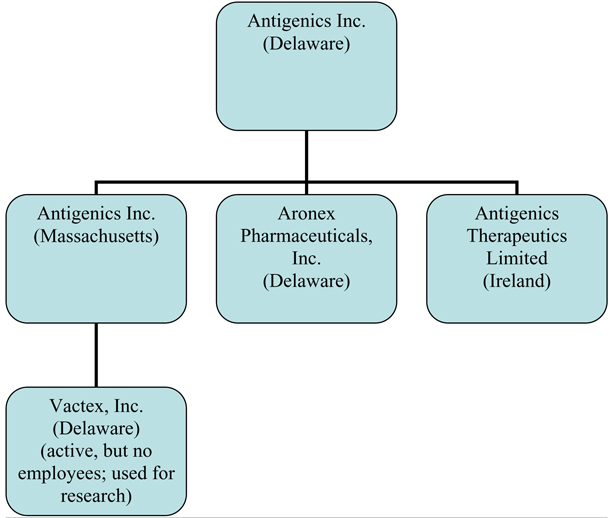
(ee) Subsidiaries. As of the Closing Date, each of the Company and Antigenics MA have no directly held Subsidiary other than those listed on Schedule 3 (ee). The Company is the beneficial owner (and the Company or the Company’s Subsidiary listed on Schedule 3(ee) is the record owner) of all of the equity interests in the Company’s Subsidiaries and holds such equity interests free and clear of all encumbrances except as are imposed by applicable securities laws.
11
4. COVENANTS.
(a) Efforts. Each party shall use its reasonable commercial efforts timely to satisfy each of the conditions to be satisfied by it as provided in Sections 6 and 7 of this Agreement.
(b) Form D and Blue Sky. The Company agrees to file a Form D with respect to the Notes and to provide a copy thereof to each Buyer promptly after such filing. The Company shall, on or before the Closing Date, take such action as the Company shall reasonably determine is necessary to be taken on or before the Closing Date in order to obtain an exemption for or to qualify the Notes for sale to the Buyers at the Closing pursuant to this Agreement under applicable securities or “Blue Sky” laws of the states of the United States (or to obtain an exemption from such qualification). The Company shall make all filings and reports relating to the offer and sale of the Notes required under applicable securities or “Blue Sky” laws of the states of the United States following the Closing Date.
(c) Use of Proceeds. The Company will use the proceeds from the sale of the Notes for working capital and other general corporate purposes.
5. REGISTER; TRANSFER AGENT INSTRUCTIONS.
(a) Register. The Company shall maintain at its principal executive offices (or such other office or agency of the Company as it may designate by notice to each holder of Notes), a register for the Notes, in which the Company shall record the name and address of the Person in whose name the Notes have been issued (including the name and address of each transferee) the principal amount of the Notes held by such Person. The Company shall keep the register open and available during business hours for inspection by any Buyer or its legal representatives upon prior written notice.
(b) Transfer Agent Instructions. The Company shall issue irrevocable instructions to its transfer agent, and any subsequent transfer agent, to issue certificates or credit shares to the applicable balance accounts at The Depository Trust Company, registered in the name of each Buyer or its respective nominee(s), for the Conversion Shares, if any, in such amounts as specified from time to time by each Buyer to the Company upon conversion of the Notes. The Company warrants that no instruction other than the Irrevocable Transfer Agent Instructions referred to in this Section 5(b), and stop transfer instructions to give effect to Section 2(g) hereof, will be given by the Company to its transfer agent with respect to the Securities, and that the Securities shall otherwise be freely transferable on the books and records of the Company, as applicable, and to the extent provided in this Agreement and the other Transaction Documents.
6. CONDITIONS TO THE COMPANY’S OBLIGATION TO SELL.
The obligation of the Company hereunder to issue and sell the Notes to each Buyer at the Closing is subject to the satisfaction, at or before the Closing Date, of each of the following conditions, provided that these conditions are for the Company’s sole benefit and may be waived by the Company at any time in its sole discretion by providing each Buyer with prior written notice thereof:
(a) All of the Buyers shall have executed each of the Transaction Documents to which it is a party and delivered the same to the Company.
12
(b) All of the Buyers shall have delivered to the Company the Purchase Price for the Notes being purchased by such Buyer at the Closing by wire transfer of immediately available funds pursuant to the wire instructions provided by the Company.
(c) The representations and warranties of each Buyer shall be true and correct as of the date when made and as of the Closing Date as though made at that time (except for representations and warranties that speak as of a specific date), and each Buyer shall have performed, satisfied and complied in all material respects with the covenants, agreements and conditions required by this Agreement to be performed, satisfied or complied with by such Buyer at or prior to the Closing Date.
7. CONDITIONS TO EACH BUYER’S OBLIGATION TO PURCHASE.
The obligation of each Buyer hereunder to purchase the Notes at the Closing is subject to the satisfaction, at or before the Closing Date, of each of the following conditions, provided that these conditions are for each Buyer’s sole benefit and may be waived by such Buyer at any time in its sole discretion by providing the Company with prior written notice thereof:
(a) The Company shall have executed and delivered to such Buyer (A) each of the Transaction Documents and (B) the Notes being purchased by such Buyer at the Closing pursuant to this Agreement.
(b) Such Buyer shall have received the opinion of internal or external counsel to the Company in form, scope and substance reasonably satisfactory to such Buyer.
(c) The Company shall have delivered to such Buyer a certificate evidencing the incorporation and good standing of the Company and each of its Subsidiaries.
(d) The Company shall have delivered to such Buyer a certificate evidencing the Company’s qualification as a foreign corporation issued by the Secretary of State of Massachusetts as of a date within five (5) days of the Closing Date.
(e) The Company shall have delivered to such Buyer a certified copy of the Certificate of Incorporation as certified by the Secretary of State of the State of Delaware within five (5) days of the Closing Date.
(f) The Company shall have delivered to such Buyer a certificate, executed by the Secretary of the Company and dated as of the Closing Date, as to (i) the resolutions adopted by the Company’s Board of Directors or a committee thereof in connection herewith, (ii) the Bylaws, and (iii) and the incumbency of the officers executing documents on behalf of the Company in connection herewith.
(g) The representations and warranties of the Company shall be true and correct in all material respects (except for those representations and warranties that are qualified by materiality or Material Adverse Effect, which shall be true and correct in all respects) as of the date of this
13
Agreement and as of the Closing Date as though made at that time (except for representations and warranties that speak as of a specific date) and the Company shall have performed, satisfied and complied in all material respects with the covenants, agreements and conditions required by the Transaction Documents to be performed, satisfied or complied with by the Company at or prior to the Closing Date. Such Buyer shall have received a certificate, executed by the Chief Executive Officer of the Company, dated as of the Closing Date, as to the foregoing.
8. TERMINATION.
In the event that the Closing shall not have occurred with respect to a Buyer on or before ten (10) Business Days from the date hereof due to the Company’s or such Buyer’s failure to satisfy the conditions set forth in Sections 6 and 7 above (and the nonbreaching party’s failure to waive such unsatisfied condition(s)), the nonbreaching party shall have the option to terminate this Agreement with respect to such breaching party at the close of business on such date without liability of any party to any other party.
9. COLLATERAL AGENT
(a) Collateral Agent’s Authority to Act, etc. Each of the Buyers appoints and authorizes Xxxxxxx & Xxxxxx LLC to act for Buyers as collateral agent (the “Collateral Agent”) in connection with the granting of a security interest in the Pledged Securities as contemplated by this Agreement and the Pledge and Security Agreement. All action in connection with the enforcement of, or the exercise of any remedies under this Agreement and the Pledge and Security Agreement shall be taken in the manner set forth therein.
(b) Collateral Agent’s Resignation. The Collateral Agent may resign at any time by giving at least 30 days’ prior written notice of its intention to do so to each of the Buyers and the Company and upon the appointment by the holders of at least 60% in principal amount of the outstanding Notes (the “Majority Noteholders”) of a successor Collateral Agent reasonably satisfactory to the Company. If no successor Collateral Agent shall have been so appointed and shall have accepted such appointment within 15 days after the retiring Collateral Agent’s giving of such notice of resignation, then the retiring Collateral Agent may appoint a successor Collateral Agent which shall be a bank or a trust company organized under the laws of the United States of America or any state thereof and having a combined capital, surplus and undivided profit of at least $500,000,000 (so long as no Event of Default exists) with the consent of the Company, which shall not be unreasonably withheld; provided, however, that any successor Collateral Agent appointed under this sentence may be removed upon the written request of the Majority Noteholders, which request shall also appoint a successor Collateral Agent (so long as no Event of Default exists) reasonably satisfactory to the Company. Upon the appointment of a new Collateral Agent hereunder, the term “Collateral Agent”, shall for all purposes of this Agreement and the Pledge & Security Agreement thereafter mean such successor. After any retiring Collateral Agent’s resignation hereunder as Collateral Agent, or the removal hereunder of any successor Collateral Agent, the provisions of this Agreement and the Pledge & Security Agreement shall continue to inure to the benefit of such retiring or removed Collateral Agent as to any actions taken or omitted to be taken by it while it was Collateral Agent under this Agreement and the Pledge & Security Agreement.
14
(c) Concerning the Collateral Agent
i. Action in Good Faith, etc. The Collateral Agent and its officers, directors, employees and agents shall have no liability to any of the Buyers or to any future holder of any Notes for any action or failure to act taken or suffered in good faith, and any action or failure to act in accordance with an opinion of its counsel shall conclusively be deemed to be in good faith. The Collateral Agent shall in all cases be entitled to rely, and shall be fully protected in relying, on instructions given to the Collateral Agent by the Majority Noteholders. The Collateral Agent may execute releases and other collateral termination documents with respect to assets disposed of by the Company as permitted by this Agreement.
ii. No Implied Duties, etc. The Collateral Agent shall have and may exercise such powers as are specifically delegated to the Collateral Agent under this Agreement or the Pledge & Security Agreement together with all other powers incidental thereto. The Collateral Agent shall have no implied duties to any Person or any obligation to take any action under this Agreement or the Pledge & Security Agreement except for action specifically provided for in this Agreement or the Pledge & Security Agreement to be taken by the Collateral Agent.
iii. Validity, etc. The Collateral Agent shall not be responsible to any Buyer or any future holder of any Notes (a) for the legality, validity, enforceability or effectiveness of this Agreement or the Pledge & Security Agreement, (b) for any recitals, reports, representations, warranties or statements contained in or made in connection with this Agreement or the Pledge & Security Agreement, (c) for the existence or value of any assets included in any security for the Credit Obligations (as defined in the Pledge & Security Agreement), (d) for the effectiveness of any lien purported to be included in the Pledged Securities, (e) for the specification or failure to specify any particular assets to be included in the Pledged Securities, or (f) unless the Collateral Agent shall have failed to comply with Section 9(c)(i), for the perfection of the security interests.
iv. Compliance. The Collateral Agent shall not be obligated to ascertain or inquire as to the performance or observance of any of the terms of this Agreement or the Pledge & Security Agreement.
v. Employment of Agents and Counsel. The Collateral Agent may execute any of its duties as Collateral Agent under this Agreement or the Pledge & Security Agreement by or through employees, agents and attorneys-in-fact and shall not be responsible to any of the Buyers, any future holder of any Notes, or the Company for the default or misconduct of any such agents or attorneys-in-fact selected by the Collateral Agent acting in good faith. The Collateral Agent shall be entitled to advice of counsel concerning all matters pertaining to the agency hereby created and its duties hereunder or under the Pledge & Security Agreement.
vi. Reliance on Documents and Counsel. The Collateral Agent shall be entitled to rely, and shall be fully protected in relying, upon any affidavit, certificate, cablegram, consent, instrument, letter, notice, order, document, statement, facsimile, telegram, telex or teletype message or writing reasonably believed in good faith by the Collateral Agent to be genuine and correct and to have been signed, sent or made by the Person in question, including any telephonic or oral statement made by such Person, and, with respect to legal matters, upon an opinion or the advice of counsel selected by the Collateral Agent.
15
vii. Collateral Agent’s Reimbursement. Each of the Buyers, and each future holder of Notes as successor to a Buyer severally agrees to reimburse the Collateral Agent, pro rata in accordance with such Buyer’s percentage interest (determined based on the ratio of the aggregate principal amount of the Notes held by such Buyer or holder of Notes to the aggregate amount of all outstanding Notes), for any reasonable expenses not reimbursed by the Company (without limiting the obligation of the Company to make such reimbursement): (a) for which the Collateral Agent is entitled to reimbursement by the Company under this Agreement or the Pledge & Security Agreement and (b) after the occurrence and during the continuance of an Event of Default, for any other reasonable expenses incurred by the Collateral Agent on the Buyers’ behalf in connection with the enforcement of the Buyers’ rights under this Agreement or the Pledge & Security Agreement; provided, however, that the Collateral Agent shall not be reimbursed for any such expenses arising as a result of its gross negligence or willful misconduct. Such reimbursement shall be provided within 30 days of a written notice by the Collateral Agent , providing proof of expenses and amounts due from each Buyer (calculated in the manner set forth in this Section 9(c)(vii))).
(d) Indemnification. The Buyers shall severally indemnify the Collateral Agent and its officers, directors, employees, agents, attorneys, accountants, consultants and controlling Persons (to the extent not reimbursed by the Buyers and without limiting the obligation of the Buyers to do so), pro rata in accordance with their respective percentage interests (as determined in accordance with Section 9(a)(vii)), from and against any and all liabilities, obligations, damages, penalties, actions, judgments, suits, losses (including accrued and unpaid Collateral Agent’s fees), costs, expenses or disbursements of any kind whatsoever which may at any time be imposed on, incurred by or asserted against a Collateral Agent or such Persons relating to or arising out of this Agreement, the Pledge & Security Agreement, the transactions contemplated hereby or thereby, or any action taken or omitted by the Collateral Agent in connection with any of the foregoing; provided, however, that the foregoing shall not extend to actions or omissions which are determined in a final, nonappealable judgment by a court of competent jurisdiction to have been taken by the Collateral Agent with gross negligence or willful misconduct. Such indemnification shall, in all cases, be provided within 30 days of a written notice by Collateral Agent, providing proof of expenses and amounts due from each Buyer (calculated in the manner set forth in Section 9(c)(vii)).
(e) Assumption of Agent’s Rights. Notwithstanding anything herein or in this Agreement or the Pledge & Security Agreement to the contrary, if at any time no Person constitutes a Collateral Agent hereunder or a Collateral Agent fails to act upon written directions from the Majority Noteholders, the Majority Noteholders shall be entitled to exercise any power, right or privilege granted to the Collateral Agent under this Agreement or the Pledge & Security Agreement and in so acting the Majority Noteholders shall have the same rights, privileges, indemnities and protections provided to the Collateral Agent under this Agreement or the Pledge & Security Agreement.
16
10. MISCELLANEOUS.
(a) Governing Law; Jurisdiction; Jury Trial. All questions concerning the construction, validity, enforcement and interpretation of this Agreement shall be governed by the internal laws of the State of New York, without giving effect to any choice of law or conflict of law provision or rule (whether of the State of New York or any other jurisdictions) that would cause the application of the laws of any jurisdictions other than the State of New York. Each party hereby irrevocably submits to the exclusive jurisdiction of the state and federal courts sitting in The City of New York, Borough of Manhattan, for the adjudication of any dispute hereunder or in connection herewith or with any transaction contemplated hereby or discussed herein, and hereby irrevocably waives, and agrees not to assert in any suit, action or proceeding, any claim that it is not personally subject to the jurisdiction of any such court, that such suit, action or proceeding is brought in an inconvenient forum or that the venue of such suit, action or proceeding is improper. Each party hereby irrevocably waives personal service of process and consents to process being served in any such suit, action or proceeding by mailing a copy thereof to such party at the address for such notices to it under this Agreement and agrees that such service shall constitute good and sufficient service of process and notice thereof. Nothing contained herein shall be deemed to limit in any way any right to serve process in any manner permitted by law.
(b) Counterparts. This Agreement may be executed in two or more identical counterparts, all of which shall be considered one and the same agreement and shall become effective when counterparts have been signed by each party and delivered to the other party; provided that a facsimile signature shall be considered due execution and shall be binding upon the signatory thereto with the same force and effect as if the signature were an original, not a facsimile signature.
(c) Headings. The headings of this Agreement are for convenience of reference and shall not form part of, or affect the interpretation of, this Agreement.
(d) Severability. If any provision of this Agreement shall be invalid or unenforceable in any jurisdiction, such invalidity or unenforceability shall not affect the validity or enforceability of the remainder of this Agreement in that jurisdiction or the validity or enforceability of any provision of this Agreement in any other jurisdiction.
(e) Entire Agreement; Amendments. This Agreement supersedes all other prior oral or written agreements between the Buyers, the Company, their affiliates and Persons acting on their behalf with respect to the matters discussed herein, and this Agreement and the instruments referenced herein contain the entire understanding of the parties with respect to the matters covered herein and therein and, except as specifically set forth herein or therein, neither the Company nor any Buyer makes any representation, warranty, covenant or undertaking with respect to such matters. No provision of this Agreement may be amended other than by an instrument in writing signed by the Company and the holders of Notes representing at least a majority of the aggregate principal amount of the Notes, or, if prior to the Closing Date, the Company and the Buyers listed on the Schedule of Buyers as being obligated to purchase at least a majority of the aggregate principal amount of the Notes, and any amendment to this Agreement made in conformity with the provisions of this Section 9(e) shall be binding on all Buyers and holders of Notes, as applicable. No provision hereof may be waived other than by an instrument in writing signed by the party against whom enforcement is sought. No such amendment shall
17
be effective to the extent that it applies to less than all of the holders of the Notes then outstanding. No consideration shall be offered or paid to any Person to amend or consent to a waiver or modification of any provision of any of the Transaction Documents unless the same consideration also is offered to all of the parties to the Transaction Documents. The Company has not, directly or indirectly, made any agreements with any Buyers relating to the terms or conditions of the transactions contemplated by the Transaction Documents except as set forth in the Transaction Documents.
(f) Notices. Any notices, consents, waivers or other communications required or permitted to be given under the terms of this Agreement must be in writing and will be deemed to have been delivered: (i) upon receipt, when delivered personally; (ii) upon receipt, when sent by facsimile (provided confirmation of transmission is mechanically or electronically generated and kept on file by the sending party); or (iii) one Business Day after deposit with an overnight courier service, in each case properly addressed to the party to receive the same. The addresses and facsimile numbers for such communications shall be:
If to the Company:
| Antigenics Inc. | ||
| 000 Xxxxx Xxxxxx | ||
| Xxxxx 0000 | ||
| Xxx Xxxx, XX 00000 | ||
| Telephone: | (000) 000-0000 | |
| Facsimile: | (000) 000-0000 | |
| Attention: | Xxxx X. Xxxxx | |
with a copy to:
| Ropes & Xxxx LLP | ||
| Xxx Xxxxxxxxxxxxx Xxxxx | ||
| Xxxxxx, Xxxxxxxxxxxxx 00000 | ||
| Telephone: | (000) 000-0000 | |
| Facsimile: | (000) 000-0000 | |
| Attention: | Xxxx X. Xxxxxxxx | |
If to a Buyer, to its address and facsimile number set forth on the Schedule of Buyers, with copies to such Buyer’s representatives as set forth on the Schedule of Buyers,
with a copy (for informational purposes only) to:
| Xxxxx & Xxxxxxx LLP | ||
| 000 Xxxx Xxxxxx | ||
| Xxx Xxxx, Xxx Xxxx 00000 | ||
| Telephone: | (000) 000-0000 | |
| Facsimile: | (000) 000-0000 | |
| Attention: | Xxxxxxxx X. Xxxxxxx | |
18
or to such other address and/or facsimile number and/or to the attention of such other Person as the recipient party has specified by written notice given to each other party five (5) days prior to the effectiveness of such change. Written confirmation of receipt (A) given by the recipient of such notice, consent, waiver or other communication, (B) mechanically or electronically generated by the sender’s facsimile machine containing the time, date, recipient facsimile number and an image of the first page of such transmission or (C) provided by an overnight courier service shall be rebuttable evidence of personal service, receipt by facsimile or receipt from an overnight courier service in accordance with clause (i), (ii) or (iii) above, respectively.
(g) Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the parties and their respective successors and assigns, including any purchasers of the Notes. The Company shall not assign this Agreement or any rights or obligations hereunder without the prior written consent of the holders of at least a majority of the Notes hereunder.
(h) No Third Party Beneficiaries. This Agreement is intended for the benefit of the parties hereto and their respective permitted successors and assigns, and is not for the benefit of, nor may any provision hereof be enforced by, any other Person.
(i) Replacement of Securities. If any certificate or instrument evidencing any Securities is mutilated, lost, stolen or destroyed, the Company shall issue or cause to be issued in exchange and substitution for and upon cancellation thereof, or in lieu of and substitution therefor, a new certificate or instrument, but only upon receipt of evidence reasonably satisfactory to the Company of such loss, theft or destruction and customary and reasonable indemnity, if requested. The applicants for a new certificate or instrument under such circumstances shall also pay any reasonable third-party costs associated with the issuance of such replacement Securities.
(j) Fees and expenses. Each party shall pay all costs and expenses that it incurs with respect to the negotiation, execution, delivery and performance of this Agreement; provided, however, that at the Closing, the Company shall reimburse the Buyers for their reasonable legal and other professional fees and out of pocket expenses up to $100,000 in the aggregate.
(k) Confidentiality. At no time will a party, without the prior written consent of any other party, make any press releases or public announcements concerning this Agreement or the other ancillary agreements; provided, however, that any party may make any public disclosure required by applicable law or any listing or trading agreement concerning its publicly traded securities (in which case the disclosing party will use its best efforts to advise the other parties prior to making the disclosure). Each party shall keep confidential any and all information obtained in connection with this Agreement and shall not disclose any such information to any other Person except: (a) with the prior written consent of the other party; (b) as required by law, regulation, or court order, but only after notice has been provided to such other party; or (c) for such information as shall have been publicly disclosed other than in violation of this Agreement or of any other obligation of confidentiality to such other party.
[Signature Page Follows]
19
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
| COMPANY: | ||
| ANTIGENICS INC. | ||
| By: | /s/ Xxxx X. Xxxxx | |
| Name: | Xxxx X. Xxxxx | |
| Title: | Chairman & Chief Executive Officer | |
Signature Page to Securities Purchase Agreement
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
| COLLATERAL AGENT: | ||
| XXXXXXX & XXXXXX LLC | ||
| By: |
/s/ Xxxxxx X. Xxxxxxx, Xx. | |
| Name: |
Xxxxxx X. Xxxxxxx, Xx. | |
| Title: |
Manager | |
Signature Page to Securities Purchase Agreement
IN WITNESS WHEREOF, each Buyer and the Company have caused their respective signature page to this Securities Purchase Agreement to be duly executed as of the date first written above.
| BUYERS: | ||
| Xxxxxxx & Xxxxxx Value Partners L.P. | ||
| By: | /s/ Xxxxxx X. Xxxxxxx, Xx. | |
| Name: | Xxxxxx X. Xxxxxxx, Xx. | |
| Title: | General Partner | |
| Penrith LTD | ||
| By: | /s/ Xxxxxx Xxxxxx | |
| Name: | ||
| Title: | President + C.E.O. | |
SCHEDULE OF BUYERS
| Buyer |
Address and Facsimile Number |
Aggregate Principal Amount of Notes | |||
| Xxxxxxx & Xxxxxx Value Partners L.P. |
c/o Ingalls & Xxxxxx LLC Attn: Xxx Xxxxxxx 00 Xxxxxxxx Xxx Xxxx, XX 00000
Facsimile: (000) 000-0000 |
$ | 20,000,000 | ||
| Penrith LTD |
c/o Ingalls & Xxxxxx LLC Attn: Xxx Xxxxxxx 00 Xxxxxxxx Xxx Xxxx, XX 00000
Facsimile: (000) 000-0000 |
$ | 5,000,000 | ||
EXHIBITS
| Exhibit A | Form of Notes | |
| Exhibit B | Form of PIK Notes | |
| Exhibit C | Form of Pledge and Security Agreement | |
| Exhibit D | Form of Irrevocable Transfer Agent Instructions |
SCHEDULES
| Schedule 3(e) | Consents | |
| Schedule 3(n) | Transaction with Affiliates | |
| Schedule 3(o) | Capitalization | |
| Schedule 3(p) | Indebtedness and Other Contracts/Seniority | |
| Schedule 3(q) | Litigation | |
| Schedule 3(t) | Intellectual Property Rights | |
| Schedule3(ee) | Subsidiaries |
Schedule 3(e)
Consents
None.
Schedule 3(n)
Transaction with Affiliates
None.
Schedule 3(o)
Capitalization
The following table sets forth our cash, cash equivalents and short-term investments and capitalization as of June 30, 2006:
| • | on an actual basis; and |
| • | on an adjusted basis to give effect to the sale of $25,000,000 aggregate principal amount of our 8.0% convertible senior notes due 2011, after deducting discounts and commissions and estimated offering expenses to be paid by us. |
| (in thousands, except per share data)
|
As of June 30, 2006 | |||||||
| Actual | As adjusted | |||||||
| (unaudited) | ||||||||
| Cash, cash equivalents and short-term investments |
$ | 31,867 | $ | 56,867 | ||||
| Current portion, long-term debt |
1,204 | 1,204 | ||||||
| Long-term debt, less current portion |
— | — | ||||||
| Convertible senior notes due 2025 |
50,000 | 50,000 | ||||||
| Convertible senior secured notes due 2011 |
— | 25,000 | ||||||
| Total |
51,204 | 76,204 | ||||||
| Stockholders’ equity: |
||||||||
| Preferred Stock, par value $0.01 per share; 25,000,000 shares authorized; Series A Convertible Preferred Stock, par value $0.01 per share; 31,620 shares designated, issued and outstanding, actual and as adjusted |
— | — | ||||||
| Common stock, $0.01 par value per share; 100,000,000 shares authorized; 45,810,870 shares issued and outstanding, actual and as adjusted |
458 | 458 | ||||||
| Additional paid in capital |
441,926 | 441,926 | ||||||
| Deferred compensation |
— | — | ||||||
| Accumulated other comprehensive (loss) income |
(52 | ) | (52 | ) | ||||
| Accumulated deficit |
(439,286 | ) | (439,286 | ) | ||||
| Total stockholders’ equity |
3,046 | 3,046 | ||||||
| Total capitalization |
$ | 54,250 | $ | 79,250 | ||||
The table above should be read in conjunction with our consolidated financial statements and related notes. The number of actual and as adjusted shares of our common stock outstanding excludes the following:
| • | 6,246,692 shares of our common stock issuable upon exercise of options outstanding as of June 30, 2006, at a weighted average exercise price of $8.31 per share, of which options to purchase 2,978,702 shares were exercisable as of that date; |
| • | 8,910 shares of our common stock issuable upon exercise of warrants outstanding as of June 30, 2006, at a weighted average exercise price of $54.71 per share, all of which were exercisable as of that date; |
| • | 79,203 shares outstanding under our Directors’ Deferred Compensation plan; |
| • | 2,432,910 shares of our common stock available for future grant under our equity incentive plans as of June 30, 2006; |
| • | 2,000,000 shares of our common stock issuable upon conversion of outstanding Series A Convertible Preferred Stock; |
| • | 4,645,115 shares of our common stock initially issuable upon conversion of $50,000,000 aggregate principal amount of our 5.25% convertible senior notes due 2025; and |
| • | 7,142,857 shares of our common stick initially issuable upon conversion of $25,000,000 aggregate principal amount of our 8.0% convertible senior secured notes due 2011. |
Schedule 3(p)
Indebtedness and Other Contracts/Seniority
1. The Company owes $1,065,800 as debt to General Electric Corporation.
2. The Company may owe a capital call of $375,000 to Applied Genomic Technology Capital Fund.
3. Letters of Credit in connection with security deposits in Lexington, Massachusetts, Framingham, Massachusetts and New York, New York.
4. Antigenics MA owes $146,061 in the form of subordinated debentures plus accrued interested of $51,490.
Schedule 3(q)
Litigation
None.
Schedule 3(t)
Intellectual Property Rights
| 1. | Antigenics MA has asserted a claim against Progenics Pharmaceuticals Inc. for possible infringement of Antigenics MA QS-21 patent rights and/or for misuse or misappropriation of QS-21 under its License and Supply Agreement and Material Transfer Agreement with the Antigenics MA. Progenics disputes the claim and the parties are discussing and negotiating a settlement. |
| 2. | The Company and its Subsidiaries from time to time in-licenses technology and patent rights that may related to the practice of the QS-21 and AG-707 Intellectual Property rights, and generally only holds non-exclusive license rights under such agreements. |
| 3. | The only agreements with third parties under which royalties are payable to third parties are the license agreements pertaining to AG-707 filed with the Securities and Exchange Commission. |
| 4. | Antigenics MA in the ordinary course of business enters into collaboration agreements, license agreements, and supply agreements with third parties seeking to develop and use QS-21 as a vaccine adjuvant under which Antigenics MA grants exclusive and non-exclusive license and supply rights under the Intellectual Property Rights to such third parties. |
| 5. | The Company and Antigenics MA have entered into a royalty-free cross license that provides Antigenics MA with exclusive license rights to all Intellectual Property Rights owned or controlled by the Company that are necessary to make, use, sell and import AG-707 and QS-21, and that provides the Company with exclusive license rights to all Intellectual Property Rights owned or controlled by Antigenics MA that are necessary to make, use, sell and import any products other than AG-707 and QS-21. |
| 6. | Antigenics MA and GlaxoSmithKline each hold an undivided joint ownership interest in 2 patent families covering the combined use of QS-21 and the combined use of saponins with an aminoalkyl glucosminide phosphase. Antigenics MA’s co-ownership interest was assigned to Antigenics by Corixa under an agreement between Corixa and Antigenics MA. Corixa’s joint ownership interest was subsequently transferred to GlaxoSmithKline in connection with its acquisition by GlaxoSmithKline. |
Schedule 3(ee)
Subsidiaries