DISTRIBUTOR of products that compete with the Products covered hereby and that DISTRIBUTOR is not precluded by any contractual obligation or any other reason from entering into or performing under this Agreement. DISTRIBUTOR agrees that during the...
|
|
EXHIBIT 10.44 INTERNATIONAL DISTRIBUTORSHIP AGREEMENT THIS INTERNATIONAL DISTRIBUTORSHIP AGREEMENT ("Agreement") is entered into effective as of the Effective Date contained in Schedule A, between ReShape Medical Inc., having its principal place of business at 0000 Xxxxx Xxxxxxxx, Xxx Xxxxxxxx, XXX ("RSM") and the company identified in Schedule A ("DISTRIBUTOR"). WITNESSETH WHEREAS, RSM is in the business of selling various medical devices primarily used to perform medical procedures; and WHEREAS, DISTRIBUTOR desires to actively and diligently promote the sale, on its own behalf and for its own account, of certain of RSM's products; and WHEREAS, RSM and DISTRIBUTOR desire to enter into an exclusive distributorship agreement covering certain ReShape Medical product lines under the terms and conditions set out below. NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein, the parties agree as follows: 1. DISTRIBUTION 1.1 Products. The products that are subject to this Agreement (the "Products") shall be those products identified on Schedule B hereto, together with such other products as may from time to time be included thereon by mutual written agreement of the parties. DISTRIBUTOR acknowledges that Schedule B will not necessarily include all products sold by RSM, and that Products are subject to modification or discontinuance by RSM upon notice to DISTRIBUTOR, and, upon DISTRIBUTOR's receipt of such notice, Schedule B will be deemed amended accordingly. 1.2 Appointment. 1.2.1 Effective as of the Effective Date of this Agreement, RSM hereby appoints DISTRIBUTOR, and DISTRIBUTOR accepts such appointment, as an exclusive distributor of Products in the geographical area described on Schedule C hereto (the "Territory"), subject to the terms and conditions set forth in this Agreement. 1.2.2 DISTRIBUTOR shall not directly or indirectly deliver or promote the sale of the Products outside the Territory or locate or utilize an office, branch, or distribution depot for the sale or distribution of the Products outside the Territory. DISTRIBUTOR shall immediately notify RSM if it becomes aware that any DISTRIBUTOR customer exports or sells or plans to export or sell any of the Products outside the Territory. 1.3 Noncompetition. DISTRIBUTOR represents that as of the Effective Date there are no agreements in effect providing for the marketing, sale or distribution by «Distributorname» «Territory» |
|
|
DISTRIBUTOR of products that compete with the Products covered hereby and that DISTRIBUTOR is not precluded by any contractual obligation or any other reason from entering into or performing under this Agreement. DISTRIBUTOR agrees that during the term of this Agreement DISTRIBUTOR will not, directly or indirectly, sell, promote or distribute any products that compete with the Products covered hereby. 1.4 Sub-Distributors. DISTRIBUTOR agrees that it will not establish any sub distributors without the prior written consent of RSM. It is understood that such appointment shall be made only in the name and for the account of DISTRIBUTOR and shall be for a term no greater than the term of this Agreement. DISTRIBUTOR shall not grant to any sub-distributor any rights greater than those which are granted by RSM to DISTRIBUTOR under this Agreement. DISTRIBUTOR shall also impose on any sub-distributor the same obligations as RSM has imposed on DISTRIBUTOR under this Agreement for the purpose of protecting the goodwill of RSM and the Products. DISTRIBUTOR shall insure that all its sub-distributors comply with any regulatory requirements with respect to the Products. DISTRIBUTOR shall defend, indemnify, and hold RSM harmless against any claim, loss, liability, or expense (including attorney's fees and court costs) arising out of or based upon any claim made by any of DISTRIBUTOR's sub-distributors, sales representatives, or employees against RSM. 1.5 Manufacturer's Representative. RSM or its affiliated companies shall have the right, at their option and expense, to maintain representatives in or supporting the Territory from time to time to participate in the marketing, sale, and after sale support of the Products. If RSM or its affiliated companies elect to maintain such representatives, DISTRIBUTOR shall share information and cooperate in good faith in connection with all material contacts and activities with customers and potential customers. DISTRIBUTOR agrees that during the term of this Agreement and for a period of one year thereafter it will not, without RSM's consent, induce or solicit any such ReShape Medical personnel to terminate their employment with ReShape Medical in order to become employed by, or otherwise affiliated with, DISTRIBUTOR. 1.6 Non-Agency. The parties acknowledge that DISTRIBUTOR is an independent contractor, and that neither the making of this Agreement nor the performance of any of the provisions hereof shall be construed to constitute DISTRIBUTOR or any of its agents acting hereunder an agent or legal representative of RSM for any purpose, nor shall this Agreement be deemed to establish a joint venture, partnership, franchise, agency, or employer-employee relationship. DISTRIBUTOR is not granted, and shall not exercise, the right or authority to assume or create any obligation or responsibility on behalf of or in the name of RSM or its affiliated companies. 1.7 Remuneration. Except as otherwise provided herein, DISTRIBUTOR shall not be entitled to any remuneration of any nature whatsoever other than the profit it makes on the delivery of Products to its customers in the Territory. 2. PURCHASE OF PRODUCTS AND TERMS OF SALE |
|
|
2.1 Quotas. 2.1.1 The parties agree to the quota levels set forth in Schedule D hereto (or as it may be amended from time to time by mutual agreement of the parties) as the minimum requirements for DISTRIBUTOR purchases of Products from RSM, and DISTRIBUTOR shall purchase Products in no less than such amounts in the applicable calendar quarters. 2.1.2 DISTRIBUTOR agrees that the minimum purchase requirements appearing on Schedule D hereto are reasonable in view of the market potential for Products in the Territory and acknowledges that all such requirements have been established as the result of a mutual examination of market potential and negotiations between the parties. 2.1.3 It is further agreed that in the event additional Products are added to Schedule B hereto, the minimum purchase requirements for such Products will be determined by RSM after consultation with DISTRIBUTOR, and such new minimum purchase requirements will be incorporated in and be made subject to the terms of this Agreement. 2.3 Orders. 2.3.1 DISTRIBUTOR shall purchase from RSM, and RSM shall sell to DISTRIBUTOR, such quantities of Products as DISTRIBUTOR may order from time to time pursuant to the terms of this Agreement. Orders shall be placed by written purchase order and submitted by e-mail or facsimile, or by other means agreed upon by the parties. No order shall be binding upon RSM until the same shall have been accepted in writing by RSM. In case of conflict between the standard printed terms of purchase/sale of DISTRIBUTOR and RSM, the terms of purchase/sale of RSM shall prevail, but in no event shall either party's standard terms override any provisions of this Agreement. 2.3.2 Notwithstanding any other provision hereof, it is agreed that the obligation of RSM to sell any Product to DISTRIBUTOR is subject to the availability of such Product. RSM shall make reasonable efforts to fill each order that is accepted, but RSM shall not be liable for damages caused by failure to ship or delay in shipment resulting from product shortage of any kind or conditions beyond the control of RSM, including, but not limited to, the unavailability of such Products because of the inability to obtain materials and supplies or to produce sufficient Products to meet sales demands. If RSM believes that it will not be able to satisfy DISTRIBUTOR's requirements for the Products, it shall promptly notify DISTRIBUTOR, specifying the reasons for the expected delay and its anticipated duration. 2.4. Prices. |
|
|
2.4.l Prices for the Products as of the Effective Date of this Agreement shall be as set forth on Schedule B hereto 2.5 Payments. All payments due to RSM pursuant to this Agreement shall be paid according to the payment terms set forth on Schedule E hereto. All payments to RSM pursuant to this Agreement shall be made in United States dollars, without set-off or counterclaim and without deduction for any other charges. RSM shall retain a security interest in the Products until full payment is made, and DISTRIBUTOR shall assist RSM in any local recording of such security interest. If DISTRIBUTOR fails to make any payment when due, RSM shall have the right to take whatever action it deems appropriate or necessary, including, but not limited to, requiring immediate return of unsold Products, refusal of further orders, requiring payment in full before shipment, or termination of this Agreement pursuant to Section 6.2 hereof. 2.6 Shipping. Except as set forth below, all fees for shipping to the DISTRIBUTOR will be paid by the DISTRIBUTOR. Products shall be shipped to DISTRIBUTOR at the address (es) specified by DISTRIBUTOR from time to time. Risk of loss and title to the Products will pass to DISTRIBUTOR at port of entry at the foreign airport destination in the Territory. DISTRIBUTOR shall bear the cost of any handling, shipping, and insurance, within the designated territory. DISTRIBUTOR shall be responsible for clearing the Products through customs unless RSM notifies DISTRIBUTOR otherwise. DISTRIBUTOR shall be responsible for paying any and all duties and taxes due in connection with the importation of the Products. DISTRIBUTOR will be responsible for inspecting Product upon receipt in the Territory. DISTRIBUTOR shall submit to RSM all claims for non-delivery, shortages in shipment or defects reasonably discoverable on careful inspection in writing within 10 days of receipt of such shipment by DISTRIBUTOR. If DISTRIBUTOR does not provide such written notice to RSM within the specified timeframe, RSM will be discharged from liability for any such non-delivery, short delivery or defect. RSM shall promptly file a notice of claim against the freight handler in the event that DISTRIBUTOR provides written notice to RSM that any of the Products arrive other than in external good order and condition. 3. OBLIGATIONS OF DISTRIBUTOR 3. l Distribution of Products. DISTRIBUTOR agrees to devote DISTRIBUTOR's best efforts to (i) develop and promote the use and sale of the Products in the Territory, and (ii) furnish such service of accounts as will enable DISTRIBUTOR adequately to develop and maintain the goodwill of customers and prospective customers and their acceptance of the Products. DISTRIBUTOR also agrees to abide by RSM's recommendations regarding the use of the Products, and plan orders adequately to meet customer delivery requirements. 3.2 Legal Requirements. Except as otherwise set forth in Section 3.4, DISTRIBUTOR will obtain and maintain, at its expense, all licenses, approvals, consents, and permits necessary for DISTRIBUTOR to perform its obligations under this Agreement. DISTRIBUTOR agrees to comply with all laws, statutes, |
|
|
regulations and other legal requirements and not to place RSM in jeopardy of not complying with any such requirements. DISTRIBUTOR understands that the ReShape Medical Code of Conduct requires that the Products be sold only on the basis of quality, service, price and other legitimate marketing attributes, and that the payment of bribes for any purpose has no place in DISTRIBUTOR'S performance under this Agreement and is absolutely prohibited. Furthermore, DISTRIBUTOR agrees to use good judgment, high ethical standards and honesty in DISTRIBUTOR's dealings with customers, end-users and employees, recognizing that even the appearance of unethical actions is not acceptable. DISTRIBUTOR acknowledges and expressly agrees that certain laws of the United States of America and other countries, including, without limitation, the United States Export Control Regulations, the United States Anti-Money Laundering laws, the United States Anti-Terrorism laws and the Foreign Corrupt Practices Act, may result in the imposition of sanctions on RSM or its affiliated companies in the event that, directly or indirectly, (i) Products are exported to various countries, including without limitation Cuba, Iran, North Korea, Syria, Sudan, or any country embargoed by Executive order or otherwise, or (ii) offers, promises, or payments are made to government officials or others for the purpose of influencing decisions favorable to RSM. DISTRIBUTOR expressly agrees, therefore, that in performing its obligations under this Agreement it shall comply at all times with such laws or regulations and refrain from making or promising to make payment or transfer of anything of value that would have the purpose or effect of public or commercial bribery, or acceptance of or acquiescence in extortion, kickbacks, or other unlawful or improper means of obtaining business. DISTRIBUTOR also agrees to furnish to RSM by affidavit or other reasonable means from time to time at RSM's request, and to RSM's reasonable satisfaction, assurances that the appointment of DISTRIBUTOR and DISTRIBUTOR's activities under this Agreement, and the payment to DISTRIBUTOR of any commissions, discounts, or any monies or consideration contemplated in this Agreement, are proper and lawful under said laws and regulations. DISTRIBUTOR further acknowledges that no person employed by it is an official of any government agency or a corporation owned by a governmental unit within the Territory and that no part of any monies or consideration paid pursuant to the terms and conditions of this Agreement or any proceeds from the sale of the Products in the Territory shall accrue for the benefit of any such official. Breach of this provision, or reasonable grounds for RSM to believe it has been breached (in RSM's sole discretion), will result in immediate termination of this Agreement. DISTRIBUTOR will not make any performance or safety claims with respect to Product not contained in the label or otherwise approved by RSM consistent with applicable laws. 3.3 Quality Requirements. RSM has, and requires of its distributors, a primary commitment to patient safety and product quality. To this end, DISTRIBUTOR agrees to comply with ReShape Medical's Quality requirements regarding the Products as specified in Annex A hereto or as they may be further communicated to DISTRIBUTOR from time to time. These include, without limitation, requirements regarding appropriate storage of the Products, maintaining traceability, prompt reporting and handling of complaints, and implementation of recalls and other field actions. These requirements shall survive the expiration or other termination of this Agreement. |
|
|
3.4 Product Registrations 3.4.1 Unless prohibited by applicable law, DISTRIBUTOR will assist RSM or its affiliates in securing all registrations and approvals pertaining to the Products and required for the sale or importation of the Products in the Territory, and all such approvals or registrations will be applied for and maintained in the name of RSM unless RSM agrees otherwise in writing. 3.4.2 RSM agrees to provide DISTRIBUTOR with all required documents in English upon request. DISTRIBUTOR will be responsible for all translation of necessary documents, and for answering any and all questions required by local government agencies ReShape Medical with the input and approval of RSM. RSM will assist in answering all questions. 3.4.4 In the event that any registrations are maintained in the name of DISTRIBUTOR, upon termination of this Agreement DISTRIBUTOR shall cooperate in any and all procedures (including, but not limited to, the completion of any documentation) required to transfer such registrations to RSM or its designee and shall not oppose any new registration for the Products by RSM or its designee. Copies of all regulatory permits shall be provided to RSM and copies of DISTRIBUTOR's files relating to such permits shall be provided to RSM on request. 3.4.5 DISTRIBUTOR shall not market or sell Product in the Territory prior to receipt of regulatory approval. 3.5 Reports and Other Information. DISTRIBUTOR agrees that during the term of this Agreement it will: 3.5.1 Respond in writing to any reasonable requests by RSM for market and inventory information, including information concerning competitive activity, pricing, distribution, and Territory surveys and forecasts and, as requested by RSM, meet with RSM representatives to review these matters. 3.5.2 Promptly forward to RSM any inquiry or other communication, including correspondence or notices from regulatory authorities in the Territory, received by DISTRIBUTOR concerning any of RSM 's products that appropriately should be responded to by RSM, and all inquiries related to the sale or distribution of Products outside the Territory. 3.5.3 If so requested by RSM, provide RSM reasonable financial information on a confidential basis or provide credit references to assure RSM of DIST RIBUTOR 's financial capability to conduct its ongoing business. |
|
|
3.6 Trademarks. RSM hereby grants to DISTRIBUTOR the right and license to use the trademarks, service marks, trade names, and trademark registrations of RSM and its affiliated companies for the Products in the Territory, but only in connection with sales in the Territory of the Products purchased from RSM during the term of this Agreement and solely in connection with trademark usage procedures provided by RSM. All right, title, and interest to the trademarks and other intellectual property rights of RSM and its affiliated companies shall remain with such companies, and no other license relating thereto is granted hereunder (except the right to use such trademarks as set forth herein). DISTRIBUTOR shall not market the Products under any tradename or trademark other than the trademarks and tradenames approved by RSM. 3.7 Expenses, Except as otherwise specifically provided herein, DISTRIBUTOR shall bear all costs and expenses associated with its performance of this Agreement, including (but not limited to) amounts due employees or agents of DISTRIBUTOR, advertising, bad debt expense, inventory losses, commissions, licensing fees, regulatory fees, and taxes. In no event shall RSM be liable for any expenses incurred by DISTRIBUTOR unless RSM has agreed in writing to pay such expense. 3.8 Proprietary Rights. DISTRIBUTOR shall report promptly to RSM: (i) any infringement of the patents, trademarks, or other intellectual property rights of RSM or its affiliated companies of which DISTRIBUTOR may learn, but DISTRIBUTOR shall not initiate any protective action with respect to such infringement without RSM's prior written authorization; and (ii) receipt of any notice or service of legal action against DISTRIBUTOR and/or RSM or its affiliated companies claiming any infringement, misappropriation or breach of any intellectual property right, including but not limited to patent, copyright, trademark, or trade name infringement, and RSM shall have full rights and responsibility to manage and control the defense of DISTRIBUTOR and RSM in any such action, including the right to settle on behalf of either or both, and DISTRIBUTOR agrees to cooperate to the fullest extent necessary to enable RSM to conduct such defense. 3.9 Business Review. DISTRIBUTOR hereby gives RSM the right, upon reasonable advance notice, to conduct business reviews involving an examination, either directly or through a designee, of DISTRIBUTOR'S inventory of Products, quality systems, copies of promotional materials, and business records, including financial and sales records relating to the business performed pursuant to this Agreement, in order to ensure DISTRIBUTOR's compliance with the terms of this Agreement. 3. 10 Product Materials. DISTRIBUTOR shall sell Product in the same containers and with the same labeling and packaging as provided by RSM or otherwise approved by RSM, and in all other respects in the same condition as when delivered to DISTRIBUTOR by RSM. DISTRIBUTOR will not use any promotional materials with respect to Product other than those provided by RSM or approved by RSM under Section 4.4. |
|
|
4. RSM's OBLIGATIONS 4.1 Quality Control. RSM agrees to maintain ongoing quality assurance and testing procedures sufficient to satisfy applicable regulatory requirements. 4.2 Assistance. RSM shall provide DISTRIBUTOR with reasonable access to its technical and marketing personnel at no charge to DISTRIBUTOR, except as otherwise agreed. 4.3 Training. RSM will provide DISTRIBUTOR with technical training seminars as RSM and DISTRIBUTOR agree is needed in the English language for DISTRIBUTOR's employees directly engaged in distributing the Products. Travel and living expenses for DISTRIBUTOR's employees connected with attendance at such training seminars shall be paid by DISTRIBUTOR. DISTRIBUTOR agrees that each of DISTRIBUTOR's employees directly engaged in distributing the Products who has not previously attended a RSM technical training seminar will attend such a seminar or will be trained by DISTRIBUTOR in a program approved by RSM within a reasonable period of time after the commencement of their involvement in the sale of the Products. 4.4 Advertising. RSM will furnish at no cost to DISTRIBUTOR reasonable quantities of promotional materials in the English language where available, such as sales literature, technical data, instruction manuals, and technical journal reprints. At the request of RSM, DISTRIBUTOR shall return all such literature or data in DISTRIBUTOR's custody or control at the time of such request. DISTRIBUTOR may print literature or brochures in other languages at its own cost; however, prior to the printing or distribution of any such translated Product literature, DISTRIBUTOR agrees to submit the translation for RSM's review and written approval. The copyright rights to any such translations shall be deemed assigned to RSM or its affiliated companies, and all translations shall have adequate copyright notices evidencing such rights. 5. WARRANTIES AND INDEMNIFICATION 5.1 Product Warranty. The Products are warranted to be free of defects in workmanship and material according to the written warranty contained in the literature that accompanies the Products, which warranty may be changed from time to time by RSM upon written notice to DISTRIBUTOR. RSM's obligation under this warranty shall be limited to the repair or replacement of any Products that RSM determines were defective when delivered to DISTRIBUTOR. The foregoing is the only express warranty made by RSM related to Product delivered under this Agreement. RSM EXPRESSLY DISCLAIMS ALL OTHER EXPRESS WARRANTIES AND ANY AND ALL IMPLIED WARRANTIES, INCLUDING IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. DISTRIBUTOR shall not add to or otherwise alter or modify any applicable warranty, nor make any false representation regarding RSM or the Products or any other representation that is not included in the Product labeling in promoting sales of the Products, including any misrepresentation regarding the permissible uses of the Products. DISTRIBUTOR will hold RSM, its officers, directors and |
|
|
employees harmless from and indemnify all of them against any liability that may arise out of or result from any such unauthorized warranty or representation. 5.2 Limitations. IN NO EVENT WILL RSM BE LIABLE FOR CONSEQUENTIAL, INCIDENTAL OR SPECIAL DAMAGES ARISING OUT OF THIS AGREEMENT OR OTHERWISE RELATED TO PRODUCT, INCLUDING ANY LOSS OF PROFITS, EVEN IF RSM HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. IN NO EVENT SHALL RSM'S LIABILITY TO DISTRIBUTOR EXCEED AN AMOUNT EQUAL TO THE AGGREGATE PRICES PAID BY DISTRIBUTOR TO RSM FOR PRODUCTS DURING THE LAST CALENDAR QUARTER PRECEDING THE DATE IN WHICH THE CLAIM IS MADE, except that this liability limitation shall not apply to RSM's indemnity obligation under Sections 5.2 and 5.3. 5.2 Intellectual Property Infringement. Subject to the following sentence, RSM will defend DISTRIBUTOR against any action, proceeding, or claim by any third party for RSM's infringement of any intellectual property rights (including patent, copyright, trademark, and trade name rights) of any third party. RSM shall at all times during the term of this Agreement and thereafter indemnify, defend, and hold DISTRIBUTOR harmless against any and all costs (including but not limited to reasonable attorneys' fees), expenses, loss, damages, or liability to any third party as a result of any such action, proceeding, or claim referred to in the preceding sentence, provided that DISTRIBUTOR promptly notifies RSM in writing of such action, proceeding, or claim, allows RSM to control defense of such claim, cooperates at RSM's request and expense in such defense and does not settle any such claim without RSM's prior written approval. 5.3 Indemnification. DISTRIBUTOR and RSM shall each defend, indemnify, and hold harmless the other party, its officers, directors, agents, insurers, employees, shareholders, and affiliated companies from and against any claim, loss, suit, liability, or expense (including but not limited to attorneys' fees and other costs associated with the handling of or defense of any such action or claim) arising out of or based upon a breach by the indemnifying party of any of the warranties in this Section 5 or other failure by the indemnifying party to comply with any material provision of this Agreement, provided that the indemnified party provides prompt written notice to the indemnifying party of such action, proceeding, or claim, allows the indemnifying party to control defense of such claim, cooperates at the indemnifying party's request and expense in such defense and does not settle any such claim without the indemnifying party's prior written approval. 6. TERM AND TERMINATION |
|
|
6.1 Term. This Agreement shall commence on the Effective Date, and, unless otherwise terminated earlier as provided below, shall remain in effect until the Expiration Date listed on Schedule A, at which time it shall expire automatically. Notwithstanding the number of renewals, this Agreement shall always be construed as a fixed-term contract. This Agreement is subject to DJSTRIBUTOR's cooperation with and satisfactory completion of a due diligence background check. If, within RSM's sole discretion, the results of the background check are unsatisfactory, RSM may suspend this Agreement upon immediate notice to DISTRIBUTOR. 6.2 Termination. Either party shall have the right to terminate this Agreement without liability therefore, after written notice to the other party, (i) effective immediately in the event of a breach by the other party of any of its obligations hereunder, which breach (if curable) is not cured within 10 days of written notice of such breach, or (ii) without cause upon 180 days' advance written notice to the other party. 6.3 Effect of Termination or Expiration. Upon termination or expiration of this Agreement, all amounts due by DISTRIBUTOR to RSM will become due and payable at the time of termination or expiration. The parties agree that neither party shall be liable to the other for damages or otherwise by reason of the nonrenewal of this Agreement or its termination as provided in this Section 6, provided that such nonrenewal or termination shall not operate to discharge or release either party of obligations assumed by it prior to such nonrenewal or termination and the foregoing is not intended to limit a party's liability for breach of this Agreement. Acceptance of orders from DISTRIBUTOR by RSM after termination will not constitute a renewal of this Agreement or a waiver of the right of RSM to treat this Agreement as terminated. The granting of any notice of nonrenewal or termination of this Agreement by RSM shall entitle RSM, before shipment of any pending or new orders, to require advance payment, or other security for payment, of all previously outstanding balances (whether or not otherwise due) plus the amount of the new order. Upon expiration or termination of this Agreement, DISTRIBUTOR shall return to RSM all technical and commercial materials, customer lists, price lists, and other materials that are RSM's property. 6.4 Inventory Repurchase. Upon termination of this Agreement for any reason, RSM may, at its sole option, elect to purchase back from DISTRIBUTOR, and by doing so require DISTRIBUTOR to sell to RSM, any unsold inventory of Products in DISTRIBUTOR's possession or control, provided that such Products are unopened and in saleable condition, the expiration date of sterility of such Products is at least 365 days beyond the effective date of repurchase, and such products are currently marketed by RSM and not obsolete. The price to be paid by RSM for the purchase of such inventory shall be the purchase price actually paid by DISTRIBUTOR for the Products, increased by transportation and customs duties, if any, paid by DISTRIBUTOR for the transportation of the Products into the Territory. Any Products designated for return by DISTRIBUTOR (and related storage and handling records) will be inspected by RSM or an authorized representative of RSM in order to determine if the Products to be purchased by RSM fulfill the conditions mentioned above. Upon |
|
|
issuance of a return authorization to DISTRIBUTOR by RSM, DISTRIBUTOR shall ship the Products to RSM or to any entity or individual designated by RSM, freight prepaid. 6.5 The Sections 3.4.4, 5, 6, and 7 and the last sentence of Sections 1.4 shall survive termination or expiration of this Agreement. 7. GENERAL TERMS 7.1 Confidential Information. DISTRIBUTOR agrees that it shall keep confidential and shall not publish or otherwise divulge or use for its own benefit or for the benefit of any third party any information of a proprietary nature furnished to it by or on behalf of RSM or its affiliated companies without the prior written approval of the communicating party, except (i) as required by court order, or (ii) as reasonably necessary to perform its sales-related obligations under this Agreement and in the case of any disclosure under clause (ii) subject to the obligations of confidentiality and restrictions on use at least as stringent as those set forth in this Agreement. Information of a proprietary nature shall include, but not be limited to, information concerning RSM's or its affiliated companies' products, proposed products, marketing plans, manufacturing processes, proprietary software, financial information, or any other marketing information or materials in whatever form not generally known to the public. 7.2 Force Majeure. In the event that a delay or failure of a party to comply with any obligation created by this Agreement is caused by a force majeure condition, that obligation shall be suspended during the continuance of the force majeure condition. For the purposes of this Agreement, the term "force majeure" shall mean any event beyond the control of the parties, including, without limitation, ·fire, flood, riots, strikes, epidemics, war (declared or undeclared and including the continuance, expansion or new outbreak of any war or conflict now in existence), embargoes, and governmental actions or decrees. 7.3 Assignment. This Agreement shall not be assignable by DISTRIBUTOR without the prior written consent of RSM, but this Agreement or any portion hereof is assignable by RSM without the consent of DISTRIBUTOR. 7.4 Non-Waiver. The waiver or failure of either party to exercise in any respect any right provided for herein shall not be deemed a waiver of any further right hereunder. 7.5 Notices. Any notice or request given under this Agreement shall be in writing and in the English language and may be delivered by hand or may be sent by telefax or certified or registered mail or commercial carrier (return receipt or confirmation of delivery requested) addressed to the other party at the address shown on the first page of this Agreement, or at such other address designated in writing to the other party. Any notices sent by telefax must be followed by a confirmation copy by airmail or other reliable means. |
|
|
7.6 Arbitration. Any and every dispute, controversy or claim between the parties and/or their valid and lawful assignees and successors, including, but not limited to (i) any and every dispute, controversy or claim arising out of or relating to this Agreement and/or its amendments, and (ii) any and every dispute, controversy or claim not arising out of or not relating to this Agreement and/or its amendments, shall be finally settled by arbitration in Orange County, U.S.A. in accordance with the International Arbitration Rules of the International Centre for Dispute Resolution ("ICDR"). Judgment on the award rendered by the arbitrator(s) may be entered in any court having jurisdiction thereof. A sole arbitrator shall be chosen at the mutual agreement of the parties from a list of ICDR proposed arbitrators under the ICDR's arbitration rules. If the parties fail to mutually agree on the choice of an arbitrator within 30 days of receipt of claimant's request for arbitration by the other party, the sole arbitrator shall be appointed by the ICDR in accordance with its International Arbitration Rules. The language of the arbitration proceedings shall be English and the law applied to the dispute shall be solely and exclusively the laws of the California. The award shall state with specificity the reasons upon which the Award is based, and shall contain the arbitrator's findings of fact. Except as required by law, neither party nor the arbitrator may disclose to a third party the existence, content, or results of any arbitration hereunder without the prior written consent of both parties. Notwithstanding the above, RSM shall, at its sole discretion, have the right to initiate in any court sitting in California, USA or in the Territory a non-jury collection lawsuit against DISTRIBUTOR in an effort to collect from DISTRIBUTOR any and all moneys charged by RSM to DISTRIBUTOR for the Products sold by RSM to DISTRIBUTOR or to obtain temporary injunctive relief. All other issues, without exception, must be arbitrated. 7.7 Entire Agreement. The terms and provisions contained in this Agreement and the attached Schedules constitute the entire Agreement between the parties and supersede all previous communications, representations, agreements, and understandings, whether oral or written, between the parties with respect to the subject matter hereof. Except as this Agreement specifically authorizes RSM to modify certain provisions of this Agreement or the attached Schedules upon written notice to DISTRIBUTOR, no agreement or understanding extending this Agreement or varying its terms (including any inconsistent terms in any purchase order, acknowledgment, or similar form) shall be binding upon either party unless it is in a writing specifically referring to this Agreement and signed by the duly authorized representatives of the respective parties. 7.8 Severability. Should any provision of this Agreement be determined to be unenforceable or prohibited by applicable law, such provision shall be ineffective only to the extent of such unenforceability or prohibition without invalidating the remainder of such provision or the remaining provisions of this Agreement. 7.9 Captions. The captions of provisions in this Agreement are for convenience only and shall not control or affect the meaning or construction of any of the provisions of this Agreement. |
|
|
7.10 Counterparts. This Agreement may be executed in any manner of counterparts, each of which shall be deemed to be an original as against any party whose signature appears thereon, and all of which shall together constitute one and the same instrument. This Agreement shall become binding when one or more counterparts hereof, individually or taken together, shall bear the signature of all of the parties reflected hereon as the signatories. 7.11 Governing Law, This Agreement shall be governed and construed in accordance with the laws of the California, United States of America, to the exclusion of both its rules or conflicts of laws and the provisions of the United Nations Convention on Contracts for the International Sale of Goods. 7.12 Release. In exchange for the agreement by RSM to enter into this Agreement with DISTRIBUTOR, DISTRIBUTOR hereby releases RSM and its affiliated companies from and waives any claims it may have had against RSM and its affiliated companies related to any previous distribution agreement or business dealings between DISTRIBUTOR and RSM or its affiliated companies. IN WITNESS WHEREOF, the parties have caused this Agreement to be executed as of the Effective Date. ReShape Medical Inc. By By Xxxxxxx X. Xxxxxxx Xxxx Xx Xxxxxx President 7/31/2017 Executive Director for and on behalf of Al Zahrawi Medical 18.7.2017 |
|
|
Schedule A Distributor and Term Distributor Information Corporate Name: Dar Al Zahrawi Medical LLC a Company incorporated under the laws of The Kingdom Of Saudi Arabia ("KSA"), having its registered office at "Al Rawdah - Riyadh", hereinafter referred to as the "Distributor". Office Address: DARALZAHRAWIMEDICALLLC Xx-Xxxxxx, Xxxxxxx Xxxx Xxxx 00, Xxxxxxxx Xx - Sadhan Market, Behind Al Jazira Bank P.0. Box: 26669 Xxxxxx 00000 Xxxxxxxx xx Xxxxx Xxxxxx Tel: x000 00 0000000 Warehouse Address: Dar Al Zahrawi Medical LLC Warehouse# 130 Al hair Road Dar Xx Xxxxx Area P.O. Box: 26669 Xxxxxx 00000 Xxxxxxxx xx Xxxxx Xxxxxx Tel: x000 0 00000000 00 4782913vl |
|
|
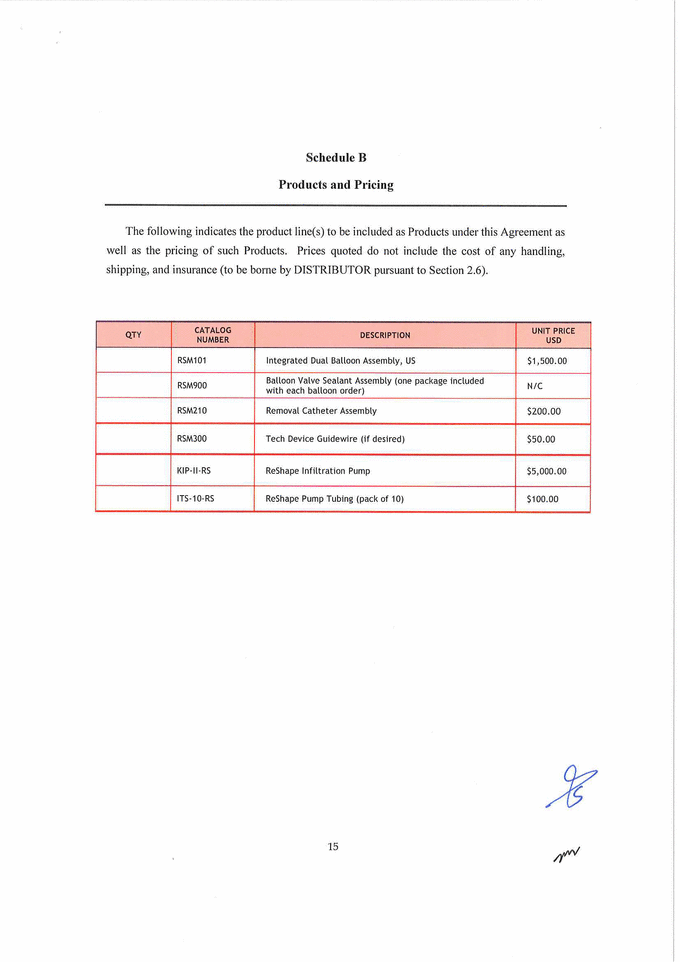
Schedule B Products and Pricing The following indicates the product line(s) to be included as Products under this Agreement as well as the pricing of such Products. Prices quoted do not include the cost of any handling, shipping, and insurance (to be borne by DISTRIBUTOR pursuant to Section 2.6). QTY CATALOG NUMBER DESCRIPTION UNIT PRICE USD RSM101 Integrated Dual Balloon Assembly, US $1,500.00 RSM900 Balloon Valve Sealant Assembly (one package included with each balloon order) N/ C RSM210 Removal Catheter Assembly $200.00 RSM300 Tech Device Guidewire (if desired) $50.00 XXX-II-RS ReShape Infiltration Pump $5,000. 00 ITS-10 -RS ReShape Pump Tubing (pack of 10) $100.00 |
|
|
Schedule C Territory The customers and or geographical area subject to this Agreement shall be specifically and exclusively limited to the Kingdom Of Saudi Arabia |
|
|
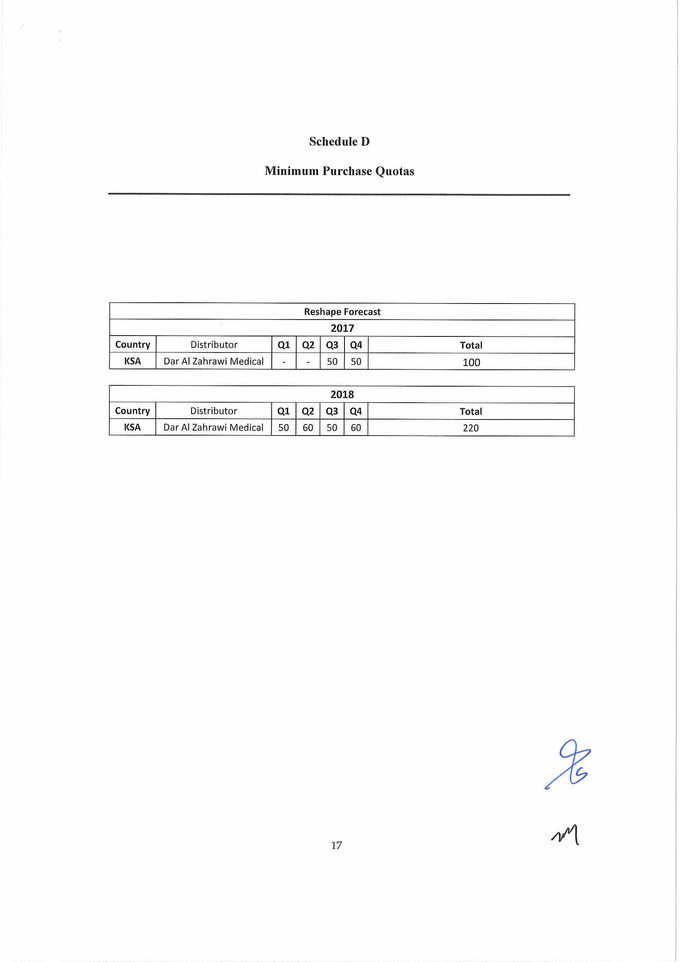
Schedule D Minimum Purchase Quotas Reshape Forecast 2017 Country J Distributor Ql Q2 Q3 Q4 Total KSA Dar Al Zahrawi Medical - - 50 50 100 2018 Country Distributor Q1 Q2 Q3 Q4 Total KSA Dar Al Zahrawi Medical 50 60 50 60 220 |
|
|
Schedule E Payment Terms The first two orders for the ReShape Dual Balloon will be cash pay up front. After successfully executing these orders, future orders may be extended via the credit terms below. Payment for Products is due within 60 days after date of invoice on open account as long as DISTRIBUTOR's credit remains good, payments to RSM are made on time, and the total amount owed by DISTRIBUTOR to RSM is within the credit limits determined by RSM from time to time. DISTRIBUTOR's credit limit as of the effective date of this Agreement shall be U.S. currency although RSM reserves the right to require DISTRIBUTOR to provide adequate security for the amount of such credit limit as a condition to making sales on open account terms. RSM shall have the right to adjust DISTRIBUTOR's payment terms and credit limits from time to time. OR DISTRIBUTOR shall pay RSM for Products in advance of delivery by cash or irrevocable letter of credit with order. Late Payments: DISTRIBUTOR will pay a late fee on all past due amounts at the rate of one percent per month or the highest rate permissible by law, whichever is lower, until paid in full. Taxes. All amounts payable to RSM under this Agreement are exclusive of any income, sales, use, property, ad valorem, value added or other taxes, levies, imposts, duties, charges or withholdings of any nature (collectively, "Taxes"), arising out of any transaction contemplated by this Agreement and imposed against DISTRIBUTOR or the Products by any taxing authority in the Territory (excluding, however, any Taxes on, or measured solely by, the net income of RSM and Taxes imposed on RSM in the United States). DISTRIBUTOR shall pay all applicable Taxes or provide RSM with a certificate of exemption acceptable to the relevant taxing authority. In the event that any payments to RSM under this Agreement are subject to any withholding taxes, DISTRIBUTOR shall promptly provide all tax certificates, applications and related documents to RSM. If RSM is required to pay any Taxes in the Territory (excluding, however, any Taxes on, or measured solely by, the net income of RSM and Taxes imposed on RSM in the United States), DISTRIBUTOR shall promptly reimburse RSM upon written request therefor. |
|
|
ANNEX A- Quality Requirements A) Storage of Products: If applicable Distributor is required to store products in accordance with product labeling statements and within an environment that prevents any of their characteristics from being altered until delivered to the customer. The minimum storage requirements for RSM products include the following: If applicable, Distributor must establish a secure storage location and limit access to this location to only those personnel authorized by Distributor. Products must be stored within this location to prevent Products from being contaminated or tampered with in any way. Additionally, Products must be kept in a clean area, free of insects, rodents and any pests. Distributor shall have a process to prevent expired, rejected and/or quarantined Products from being sent to final customers. ReShape Medical RSM reserves the right to provide other instructions to Distributor regarding such Product, and Distributor agrees to comply with such instructions. B) Traceability: Distributor is required to maintain records to ensure the traceability of ReShape Medical products in accordance with applicable regulatory requirements, and to provide ReShape Medical or its authorized agents or representatives with reasonable access to such records. The minimum traceability requirements for ReShape Medical Products include the following: Distributor is required to maintain a complete and current list of all customers who have purchased Reshape Medical products from Distributor, the dates of such purchases, the quantity, and the lot numbers, UPNs, serial numbers and/or model numbers of the units purchased (as applicable) as identified on the product label. Distributor must ensure the traceability of all ReShape Medical products at UPN, lot level including the model and serial number where applicable. The final users for all products must be identified (units sold directly to hospitals, units sold directly to doctors, units sold directly to patients). If ReShape Medical products are consigned, the batches consumed at the account must be reconciled. The traceability of multi-pack boxes must be maintained and single units originally from multi-packs must never be re-boxed. C) Complaint Reporting and Handling: Distributor is required to promptly forward all complaints concerning the products, cooperate fully with ReShape Medical in dealing with customer complaints, and take such action to resolve such complaints as may be reasonably requested by ReShape Medical. The complaint reporting requirements for ReShape Medical products include the following: |
|
|
All customer complaints involving or contributing to serious adverse events (including patient death or serious injury) must be reported to ReShape Medical within 24 hours of Distributor's becoming aware. Complaints involving nonserious events including comments regarding product dissatisfaction, potential malfunctions, non-serious patient injury, or unanticipated medical or surgical intervention shall be reported to ReShape Medical within 48 hours. A Complaint Notification Form will be provided to Distributor by ReShape Medical and must be completed in full to document each complaint. Additionally, any ancillary documentation that may facilitate the complaint investigation process should also be attached, particularly if the product is not available for return. In cases where additional information is required from the customer, at least three (3) due diligent attempts must be performed by Distributor to try to collect this additional complaint information, if requested by ReShape Medical. Should requested information not be available, Distributor shall document the reason(s) it is not available and/or the 3 attempts. • Products subject to complaints should be returned to ReShape Medical. Returned products must either, as directed by ReShape Medical, be accompanied by a disinfection certificate, even if the products have not been used; or be returned in biohazard controlled packaging and under safe handling controls. In cases where the customer has indicated that the complaint product is available to be returned but it has not been received, at least three (3) due diligent attempts must be made by Distributor to retrieve the product. |
|
|
D) Recalls and Other Field Actions: If ReShape Medical initiates a recall or other field action for any products, Distributor is required to implement such recall or other field action (including location and retrieval of the recalled product) with respect to Distributor's customers in accordance with the instructions provided by ReShape Medical. The minimum requirements for managing recalls and other field actions affecting ReShape Medical Products include the following: Recalls and other field actions must be acted upon immediately by Distributor after receiving the notification packet from ReShape Medical. An acknowledgement of the receipt of the field action notice must be promptly sent to ReShape Medical. Distributor must follow the instructions contained in the notification packet and ensure that actions are carried out in accordance with the timeframe specified. Where directed in the notice, Distributor must retrieve products from the following applicable locations: Distributor warehouse(s) inventories In-transit from ReShape Medical to Distributor Customer locations: whether sold, consigned or samples At least three (3) due diligent attempts must be performed and documented to try to retrieve products from customers. Once all product retrieval actions have been completed, the recalled stock must be reported to ReShape Medical using the Verification Form contained in the notification packet. (Note: the quantities documented on the verification forms must match the units physically returned to ReShape Medical.) The units must be returned to ReShape Medical following the instructions contained in the notification packet. ReShape Medical agrees at its option either to refund the purchase price, or to replace recalled products within a reasonable time at its expense unless the recall is attributable to Distributor's actions. |
|
|
E) Record Retention Time: All product related records (e.g. Traceability records, Complaints records, Recalls data, etc.) must be retained by the Distributor in accordance with the following requirements: Type of Product 1. Implantable Device 2. Equipment 3. All Other Products Record Retention Timeframe Indefinitely 2 years beyond dated removal from distribution or as otherwise indicated by ReShape Medical At least Product lifetime/expiry+ 2 years or as otherwise indicated by Reshape Medical. At termination of this agreement, Distributor shall deliver all records required under this section to ReShape Medical and shall direct future inquiries from customers to ReShape Medical. F) Demonstration Units: Demonstration Units (non-sterile, not for human use) are to be used by Distributor for demonstration purposes only and shall not be given to final customers. All provisions in this Annex are applicable to Demonstration Units. G) Literature and Label Control: All Product literature, labeling and the use of ReShape Medical logos, templates or trademarks is subject to ReShape Medical review and approval. Any "Internal Use" training materials provided by ReShape Medical cannot be given to the customers. |