SETTLEMENT AND LICENSE AGREEMENT among Biogen Swiss Manufacturing GmbH, Biogen International Holding Ltd., Forward Pharma A/S and Each of the Parties Listed on Appendix I Dated as of January 17, 2017
EXECUTION VERSION
SETTLEMENT AND LICENSE AGREEMENT
among
Biogen Swiss Manufacturing GmbH,
Biogen International Holding Ltd.,
and
Each of the Parties Listed on Appendix I
Dated as of January 17, 2017
TABLE OF CONTENTS
|
ARTICLE I | |
|
| |
|
Agreed Terms | |
|
|
|
|
SECTION 1.01. Definitions |
2 |
|
SECTION 1.02. Index of Defined Terms |
10 |
|
|
|
|
ARTICLE II | |
|
| |
|
Covenants | |
|
|
|
|
SECTION 2.01. Release of Claims, Dismissal of Claims and Covenant Not To ▇▇▇ |
14 |
|
SECTION 2.02. Intellectual Property Challenge or Contestation |
16 |
|
SECTION 2.03. Transfers of Intellectual Property; Liens |
16 |
|
SECTION 2.04. Access to Information; Confidentiality; Notification |
17 |
|
SECTION 2.05. Commercially Reasonable Efforts; Notification |
18 |
|
SECTION 2.06. Public Announcements |
21 |
|
SECTION 2.07. No Frustration |
21 |
|
SECTION 2.08. Existence |
21 |
|
SECTION 2.09. Solvency |
22 |
|
SECTION 2.10. TUPE Regulations; Indemnification |
23 |
|
SECTION 2.11. Specified Actions |
23 |
|
SECTION 2.12. Joinder |
23 |
|
SECTION 2.13. Ixchel |
24 |
|
SECTION 2.14. Shareholder Litigation |
24 |
|
|
|
|
ARTICLE III | |
|
| |
|
Licenses and Related Rights | |
|
|
|
|
SECTION 3.01. Co-Exclusive U.S. License |
24 |
|
SECTION 3.02. Exclusive U.S. License |
25 |
|
SECTION 3.03. Exclusive Worldwide (other than U.S.) License |
26 |
|
SECTION 3.04. Restrictions on Transfer |
26 |
|
SECTION 3.05. Use Through Affiliates |
26 |
|
SECTION 3.06. U.S. Purchase Option |
26 |
|
SECTION 3.07. Designated Countries Purchase Option |
28 |
|
|
|
|
ARTICLE IV | |
|
| |
|
Payment | |
|
|
|
|
SECTION 4.01. Upfront Fee |
30 |
|
SECTION 4.02. U.S. Royalty |
30 |
|
SECTION 4.03. Worldwide (other than U.S.) Royalties |
32 |
|
SECTION 4.04. Form of Payment |
34 |
|
SECTION 4.05. Late Penalties |
34 |
|
SECTION 4.06. Taxes |
34 |
|
SECTION 4.07. Audit Rights |
36 |
|
|
|
|
ARTICLE V | |
|
| |
|
Maintenance, Prosecution and Litigation | |
|
|
|
|
SECTION 5.01. Submitting Agreement to PTAB |
37 |
|
SECTION 5.02. IP Advisory Committee |
37 |
|
SECTION 5.03. Licensor Maintenance, Prosecution and Litigation |
38 |
|
SECTION 5.04. Licensee Maintenance, Prosecution and Litigation |
39 |
|
|
|
|
ARTICLE VI | |
|
| |
|
Conditions Precedent | |
|
|
|
|
SECTION 6.01. Conditions for the Benefit of Each Party |
42 |
|
SECTION 6.02. Frustration of Conditions to Effectiveness |
42 |
|
|
|
|
ARTICLE VII | |
|
| |
|
Representations and Warranties | |
|
| |
|
SECTION 7.01. Representations and Warranties Regarding Licensor |
42 |
|
SECTION 7.02. Representations and Warranties Regarding the Additional Parties |
48 |
|
SECTION 7.03. Representations and Warranties Regarding Licensee |
49 |
|
SECTION 7.04. No Additional Representations |
49 |
|
|
|
|
ARTICLE VIII | |
|
|
|
|
General Provisions | |
|
|
|
|
SECTION 8.01. Amendment; Extension; Waiver |
50 |
|
SECTION 8.02. Survival of Covenants, Agreements, Representations, Warranties, |
|
|
Obligations and Undertakings |
50 |
|
SECTION 8.03. Notices |
50 |
|
SECTION 8.04. Interpretation |
52 |
|
SECTION 8.05. Counterparts |
53 |
|
SECTION 8.06. Severability |
53 |
|
SECTION 8.07. Entire Agreement; Third-Party Beneficiaries; No Other Representations |
|
|
or Warranties |
53 |
|
SECTION 8.08. GOVERNING LAW |
54 |
|
SECTION 8.09. Assignment |
54 |
|
SECTION 8.10. Dispute Resolution |
55 |
|
SECTION 8.11. Authorized Agent |
57 |
|
SECTION 8.12. Specific Enforcement |
57 |
|
SECTION 8.13. Indirect Damages |
58 |
|
SECTION 8.14. WAIVER OF JURY TRIAL |
58 |
|
SECTION 8.15. Rights in Bankruptcy |
58 |
|
SECTION 8.16. Further Assurances |
60 |
|
SECTION 8.17. Costs |
60 |
|
SECTION 8.18. Independent Contractors |
60 |
|
SECTION 8.19. Representative of Additional Parties |
60 |
|
SECTION 8.20. Set Off |
60 |
|
|
|
|
APPENDICES |
|
|
|
|
|
Appendix A — Scheduled Patents and Patent Applications and Scheduled Trademark Rights |
|
|
Appendix B — Licensed Intellectual Property Contracts |
|
|
Appendix C — List of Non-Affiliates |
|
|
Appendix D — Specified Actions |
|
|
Appendix E — Draft Joint Submission of Agreement |
|
|
Appendix F — Aditech Addendum |
|
|
Appendix G — Schedule of Permitted Liens |
|
|
Appendix H — Scheduled Employment Matters |
|
|
Appendix I — Additional Parties |
|
SETTLEMENT AND LICENSE AGREEMENT (this “Agreement”) dated as of the Agreement Date (as defined below), among each of the following Parties:
Biogen Swiss Manufacturing GmbH, organized and existing under the Laws of Switzerland, having its principal place of business at Landys & ▇▇▇ ▇▇▇▇▇▇▇ ▇, ▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇ (“U.S. Licensee”);
Biogen International Holding Ltd., organized and existing under the Laws of Bermuda, having its registered office at ▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ (“Designated Countries Licensee” and together with the U.S. Licensee, “Licensee”);
Forward Pharma A/S, organized and existing under the Laws of Denmark, having its principal place of business at ▇▇▇▇▇▇▇▇▇ ▇▇▇, ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇ (“Licensor”); and
Each of the parties Listed on Appendix I (the “Additional Parties”) (each of the foregoing, a “Party” and collectively, the “Parties” hereunder).
RECITALS
WHEREAS Biogen Inc. (“Biogen”) and/or its Affiliates and Licensor are engaged in the following disputes, inter alia: (i) the Interference Proceeding (as defined below); (ii) the European Opposition Proceeding (as defined below); (iii) the opposition Licensor has filed against Biogen’s Affiliate Biogen MA Inc.’s European patent EP 2 137 537 (Application No. 8 725 256.5) at the European Patent Office; and (iv) the suits Licensor has filed in Germany under European patent EP 2 801 355 (Application No. 20140172398) and German Utility Model DE202005002112U1 against Biogen and/or its Affiliates;
WHEREAS Licensor and Licensee desire to reduce the expense and uncertainty of litigating their claims and have determined to resolve their disputes as set forth in this Agreement, including permitting the USPTO (as defined below) and the U.S. Court of Appeals for the Federal Circuit, as applicable, to make a determination of the Interference Proceeding to ensure that the applicable patentability and priority decisions are made according to U.S. Law and the European Patent Office and the Technical Board of Appeal and/or the Enlarged Board of Appeal, as applicable, to make a determination of the European Opposition Proceeding (as defined below) to ensure that the applicable patentability decisions are made according to European Union law;
WHEREAS Licensor and the Additional Parties wish to grant, and Licensee wishes to accept, the releases and the licenses to certain Intellectual Property (as defined below) granted herein;
WHEREAS, as of the Agreement Date (as defined below), the Boards of Directors of Licensee have approved this Agreement and the Transactions (as defined below), including the Licenses (as defined below), on the terms and subject to the conditions set forth in this Agreement;
WHEREAS, as of the Agreement Date, the respective Boards of Directors (or similar governing bodies) of the Additional Parties have approved this Agreement and the Transactions (as defined below), including the Licenses (as defined below), on the terms and subject to the conditions set forth in this Agreement;
WHEREAS, as of the Effective Date (as defined below) at a duly called and convened Licensor Shareholders’ Meeting (as defined below), Licensor obtained Licensor Shareholder Approval (as defined below); and
WHEREAS, as a condition and inducement to Licensee’s willingness to enter into this Agreement, certain shareholders of Licensor (the “Specified Shareholders”) entered into a shareholders commitment agreement (the “Shareholders Commitment Agreement”) in connection with the Transactions;
NOW, THEREFORE, in consideration of the mutual agreements herein contained, the sufficiency and receipt of which are hereby acknowledged, without admitting any liability on any claim or counterclaim asserted in any Litigation or any other wrongdoing whatsoever, the Parties, intending to be legally bound hereby, agree as follows:
ARTICLE I
Agreed Terms
SECTION 1.01. Definitions.
“Aditech Addendum” means the form of addendum between Licensor on the one hand and Aditech Pharma AG (“Aditech”) on the other hand, attached hereto as Appendix F, to which Licensee and each of its Affiliates shall be third party beneficiaries.
“Aditech Letter Agreement” means the letter agreement, dated as of the Agreement Date, between Licensor on the one hand and Aditech on the other hand.
“Affiliate” of any Person means another Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such first Person. For purposes of this definition, “control” (including the terms “controlled by” and “under common control with”), with respect to the relationship between or among two or more Persons, means the possession, directly or indirectly, of the power to direct or cause the direction of the affairs or management of a Person, whether through the ownership of shares of share capital or other equity or voting interests, by Contract or otherwise, including the ownership, directly or indirectly, of shares of share capital or other equity or voting interests having the power to elect a majority of the board of directors or comparable body governing the affairs of such Person. Such other Person shall be deemed to be an Affiliate only so long as such control exists. Notwithstanding the foregoing, none of (i) any entity not controlled by either ▇▇. ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ or Licensor and (ii) any entity (or such entity’s controlled Affiliates) listed on Appendix C, shall be an Affiliate of Licensor or of any Additional Party, or of any Licensor Releasing Party for purposes of this Agreement.
“Agreement Date” shall mean January 17, 2017.
“ANDA” means an abbreviated new drug application filed with the FDA, pursuant to its rules and regulations (or any equivalent or replacement mechanism).
“API” means an active pharmaceutical ingredient.
“Authorized Generic” means a generic product indicated for the treatment of a human for multiple sclerosis that is therapeutically equivalent to, and substitutable for an Infringing Product by orally administering dimethyl fumarate, wherein the therapeutically effective amount of dimethyl fumarate is 480 mg per day, that is (a) sold by or on behalf of Licensee or any of Licensee’s Affiliates, or its or their respective sublicensees, or (b) authorized by Licensee or any of Licensee’s Affiliates or its or their respective sublicensees by license, covenant not to ▇▇▇, settlement agreement, release or by any other arrangement or means (including with respect to ANDA filers) (i) for distribution in the U.S. under New Drug Application No. 204063 and/or any and all amendments or supplements thereto; or (ii) for distribution in a Designated Country.
“Business Day” means any day except a Saturday, a Sunday or other day on which the banks in the City of New York or Denmark are authorized or required by Law to be closed.
“Combination Products” means the Designated Country Combination Products and the U.S. Combination Products.
“Companies Act” means the Danish Act on Public and Private Limited Companies, as amended from time to time (Selskabsloven).
“Confidential Intellectual Property Information” means any Intellectual Property, information or data, in each case that is (i) confidential, (ii) related to or referencing the subject matter of the Licensed Intellectual Property, (iii) not protected by the attorney client privilege or work product immunity and (iv) not generally known, including any know-how, unpublished research, unpatented inventions, scientific or technical data, including all related ideas, concepts, methods, inventions, discoveries, data, formulae, processes, techniques, specifications, trade secrets and like technical information of a confidential nature in whatever form held, such as paper, electronically stored data, magnetic media film and microfilm and any material communications with or from any Third Parties.
“Contract” means any contract, agreement, deed, lease or similar instrument, and any legally binding obligation, commitment, arrangement or understanding, whether written or oral.
“Designated Countries” means every country in the world other than the United States.
“Designated Countries License Term” means the period beginning upon the date Licensor receives the Designated Countries Upfront Fee and continuing perpetually.
“Designated Countries Royalty Term” means the period beginning upon the date Licensor receives the Designated Countries Upfront Fee and ending on the expiration of the last to expire (or be invalidated in entirety by a final court ruling, from which no appeal can be taken or is timely taken) of the Patents (a) included in the Licensed Intellectual Property and (b) owned, registered or otherwise protected or enjoyable under the Laws of the Designated Countries.
“Designated Countries Upfront Fee” means an amount equal to USD$207,750,000.
“Designated Country Combination Product” means any fixed dose Designated Country Infringing Product that incorporates more than one API, at least one of which is not recited in a claim of a Patent included in the Designated Countries Licensed Intellectual Property, and has a duly authorized and valid marketing authorization.
“Designated Countries Royalty Consideration” has the meaning set forth in Section 4.03.
“Designated Country Generic Equivalent” means any product used, sold, offered for sale or imported in a Designated Country that is (a) both bioequivalent and therapeutically equivalent to, and substitutable for, an Infringing Product in such Designated Country, (b) not an Authorized Generic and (c) indicated for the treatment of multiple sclerosis.
“Designated Country Infringing Product” has the meaning set forth in Section 4.03.
“Effective Date” shall mean the date on which all Countersignatures (as defined in the Letter Agreement) have been released from escrow pursuant to and in accordance with the terms of the Letter Agreement.
“European Opposition Proceeding” means the Opposition against Licensor’s European patent EP 2801355 (Application No. 14172398.1) at the European Patent Office, including any appeals therefrom to the Technical Board of Appeal and/or the Enlarged Board of Appeal.
“Exclusive U.S. License Consideration” means one hundred thousand U.S. Dollars (USD$100,000).
“FDA” means the Food and Drug Administration of the United States, including any successor agency thereto.
“GAAP” means U.S. generally accepted accounting principles, consistently applied.
“Governmental Entity” means (i) any legislative, judicial or administrative authority, including any federal, state, local or foreign government (including, in each case, any self-regulatory organization), (ii) any court of competent jurisdiction, administrative agency or
commission or other governmental authority or instrumentality, domestic or foreign or (iii) any officials of any of the entities set forth in subclauses (i) or (ii).
“HSR Act” means the ▇▇▇▇-▇▇▇▇▇-▇▇▇▇▇▇ Antitrust Improvements Act of 1976.
“Infringed Claim” means (i) any extant claim of a Relevant Patent or (ii) any extant claim of a Patent included in the Licensed Intellectual Property, that, in each case, but for the rights granted pursuant to this Agreement, would be infringed by the making, using, sale, offering for sale, importation, exportation or supply of a product indicated for the treatment of multiple sclerosis having dimethyl fumarate as an API.
“Infringing Products” means the Designated Country Infringing Products and the U.S. Infringing Products.
“Intellectual Property” means (i) all Patents; (ii) any and all trademark rights in “FP-187”, including those trademark rights listed in Appendix A, together with all goodwill associated therewith (including all translations, adaptations, derivations and combinations of the foregoing); (iii) copyrights and copyrightable works; (iv) registrations, applications, renewals, reissues, continuations, continuations in part, divisions, revisions, extensions or reexaminations for any of the items set forth in clause (i), (ii) or (iii); (v) any Licensor proprietary computer software; and (vi) trade secrets, confidential information, know-how, regulatory, market and data clearance or exclusivity information (including with respect to regulatory filings relating to investigational or approved medicines), drug master files, clinical data, models, assays, testing data and the like, in each of the foregoing clauses (i) through (vi), in any jurisdiction in the world, relating to the treatment of any human disease or condition, and in each of the foregoing clauses (ii), (iii), (iv), (v), and (vi) , only to the extent used or planned by Licensor for use in connection with dimethyl fumarate; provided that Intellectual Property shall not include any voicemails, or any emails that have been or will be deleted in accordance with the owner’s retention policies in effect prior to the Agreement Date.
“Interference Proceeding” means the interference proceeding at the Patent Trial and Appeal Board of the USPTO (the “PTAB”) having the caption: Biogen MA Inc. v. Forward Pharma A/S, Interference 106,023 (PTAB Declared Apr. 13, 2015), including any appeals therefrom to the Federal Circuit (including any en banc review).
“Laws” means, collectively, any applicable statute, law, ordinance, decree, order, rule, regulation, treaty, principle of common law, directive, resolution, code, stock exchange rule, judgment, ruling, injunction or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Entity.
“Letter Agreement” means the letter agreement, dated as of the Agreement Date, between Licensor and Licensee.
“Licensed Intellectual Property” means all Intellectual Property anywhere in the Territory owned or controlled by Licensor, any of Licensor’s controlled Affiliates or, with respect to Relevant Patents and related Intellectual Property only, the Additional Parties or under which Licensor, any of Licensor’s controlled Affiliates or the Additional Parties has the
right to grant a license, as of the Agreement Date, and any Intellectual Property issuing from, based on, relating back to or claiming priority to any of the foregoing, including all such foregoing Intellectual Property (a) related to the treatment of any human disease or condition using dimethyl fumarate; (b) related to Licensor’s FP-187 product, including any and all Patents, Confidential Intellectual Property Information, regulatory exclusivity (including any period of data or marketing exclusivity) or regulatory clearance related thereto and all other Intellectual Property rights related to Licensor’s FP-187 product, (c) relating to the manufacture, formulation, method or means of delivery or administration of any therapeutic product for the treatment of any human disease or condition using dimethyl fumarate and (d) included in Appendix A hereto.
“Licensed Intellectual Property Rights” means any and all statutory and common law rights throughout the Territory in the Licensed Intellectual Property.
“Licensed Patents” means the Patents included in the Licensed Intellectual Property.
“Licensed Product” means any product made, used, sold, offered for sale or imported by or on behalf of Licensee, any of Licensee’s Affiliates or any of its or their respective sub-licensees and indicated for the treatment of multiple sclerosis that includes as an API a fumaric acid ester such as, by way of nonlimiting example, dimethyl fumarate or monomethyl fumarate. For clarity, and by way of nonlimiting example, Licensed Product includes Tecfidera and any Authorized Generic.
“License Term” means, collectively, the U.S. License Term and the Designated Countries License Term.
“Licenses” means the Co-Exclusive U.S. License, the Exclusive U.S. License and the Exclusive Designated Countries License.
“Licensor Articles” means the articles of association of Licensor as amended from time to time.
“Licensor Ordinary Shares” means the ordinary shares of Licensor, nominal value 0.10 DKK per share.
“Lien” means any lease, license, mortgage, deed of trust, pledge, lien, charge, hypothecation, option to purchase or otherwise acquire any interest, right of first refusal or offer or security interest, or any encumbrance created by any act of, or Contract entered into by, Licensor or any of its controlled Affiliates, of any kind or nature whatsoever, provided that none of (i) the Interference Proceeding, (ii) the European Opposition Proceeding or (iii) the Litigation involving Licensor’s European patent EP 2 801 355 (Application No. 20140172398)) shall be considered a “Lien”.
“Litigation” means any demand, suit, claim, counterclaim, action, cause of action, administrative action, arbitration, investigation, assessment or proceeding of any kind including any opposition proceeding.
“Net Sales” means the gross amount invoiced by Licensee, its Affiliates or sublicensees for the sale or other disposition of an Infringing Product in a country to Third Parties (including distributors, wholesalers and end-users), less the following deductions (such deductions, collectively, “Sales Returns and Allowances”):
(a) sales returns and allowances actually paid, granted or accrued on Infringing Products, including trade, quantity, prompt pay and cash discounts and any other adjustments, including those granted on account of price adjustments or billing errors;
(b) credits or allowances given or made for rejection, recall, return or wastage replacement of, and for uncollectible amounts on, Infringing Products or for rebates or retroactive price reductions (including Medicare, Medicaid, managed care and similar types of rebates and chargebacks);
(c) taxes, duties or other governmental charges levied on or measured by the billing amount for Infringing Products, as adjusted for rebates and refunds, including pharmaceutical excise taxes (such as those imposed on an Infringing Product by the United States Patient Protection and Affordable Care Act of 2010 and other comparable laws), but which shall not include any tax, duty, or other charge imposed on or measured by net income (however denominated) or any franchise taxes, branch profits taxes, or similar tax;
(d) charges for freight, customs and insurance directly related to the distribution of Infringing Products and wholesaler and distributor administration fees; and
(e) other future similar deductions, taken in the ordinary course of business and in accordance with applicable accounting standards and Licensee’s standard practices.
Net Sales shall not be imputed to transfers of Infringing Products without consideration or for nominal consideration for use in any clinical trial, or for any charitable, compassionate use or indigent patient program purpose where Infringing Products are sold or provided. For the avoidance of doubt, in the case of any transfer of any Infringing Product between or among Licensee and its Affiliates or sublicensees for resale, Net Sales shall be determined based only on the sale made by such Affiliate or sublicensee to a Third Party.
Notwithstanding the foregoing, in the event an Infringing Product is sold in a country as a component of a bona fide fixed dose Combination Product, Net Sales shall be calculated by multiplying the Net Sales of the Combination Product (calculated in the same manner as set forth above as if the Combination Product were an Infringing Product) in such country by the quotient m/n, where m equals the number of APIs in such Combination Product that would infringe a Patent included in the Licensed Intellectual Property and n equals the total number of APIs in such Combination Product.
“Patents” means all patents, patent applications, patent disclosures and inventions, including any reissues, reexaminations, replacements, continuations, continuations-in-part,
divisionals, adjustments or extensions thereof or any other periods of exclusivity that extend the patent term (statutory or otherwise), including pediatric exclusivities and supplementary protection certificates, in any jurisdiction in the world.
“Patent Transfer Agreement” means the Patent Transfer Agreement between Aditech Pharma AG and Forward Pharma A/S, dated as of May 4, 2010.
“Permitted Lien” means such Liens as are set forth in Appendix G.
“Person” means any individual, partnership, association, corporation, limited liability company, trust, governmental authority or other legal person or legal entity.
“Released Matters” means any and all claims, demands, damages, debts, liabilities, obligations, costs, expenses (including attorneys’ and accountants’ fees and expenses), actions and causes of action of any nature whatsoever, both at law and in equity, known or unknown, suspected or unsuspected, arising from the beginning of time to the Effective Date, that any Licensee Releasing Party or Licensor Releasing Party has as of the Effective Date, or at any time previously had, relating to either the treatment of multiple sclerosis or dimethyl fumarate, including Appeal T 1773/16-3.3.02 regarding the Opposition against Licensee’s European patent EP 2 137 537 (Application No. 8 725 256.5) at the European Patent Office, the Litigation involving Licensor’s European patent EP 2 801 355 (Application No. 20140172398), the challenge to the validity of Licensor’s German Utility Model DE202005002112U1 by Licensee and the Litigation involving Licensor’s German Utility Model DE202005002112U1; provided that Released Matters shall not include any of the foregoing to the extent relating to or arising out of (i) the Interference Proceeding, (ii) the European Opposition Proceeding (notwithstanding the inclusion in Released Matters of the Litigation involving Licensor’s European patent EP 2 801 355 (Application No. 20140172398)) or (iii) any right, benefit or obligation of Licensor, the Additional Parties or Licensee under this Agreement.
“Relevant Patent” means, on a country-by-country basis, any Patent rights in force in such country that include at least one extant claim covering treatment of a human for multiple sclerosis by orally administering dimethyl fumarate, wherein the therapeutically effective amount of dimethyl fumarate is 480 mg per day.
“Royalty Consideration” means the Designated Countries Royalty Consideration and the U.S. Royalty Consideration.
“Royalty Term” means the Designated Countries Royalty Term and the U.S. Royalty Term.
“SEC” means the U.S. Securities and Exchange Commission, or any successor Governmental Entity.
“Shareholder Meeting Materials” means, collectively, (a) the cover letter to the Notice of Meeting to the shareholders; (b) the Notice of Meeting and Licensor’s Board of Directors’ full proposal for adoption at the Licensor Shareholders’ Meeting, (c) information about the total number of shares and voting rights on the date of the notice; (d) the documents to
be presented at the general meeting, including this Agreement and the Aditech Addendum, (e) the form to be used for request for admission to the general meeting; and (f) the forms to be used for voting by proxy or voting by correspondence.
“Subsidiary” of any Person means any other Person, if any, (a) more than 50% of whose outstanding shares of capital stock or other equity or voting securities or interests representing the right to vote for the election of directors or other managing authority of such other Person are, at the time of such determination, owned or controlled, directly or indirectly, by such first Person, but such other Person shall be deemed to be a Subsidiary only so long as such ownership or control exists, or (b) which does not have outstanding shares of capital stock or other equity or voting securities or interests with such right to vote, as may be the case in a partnership, joint venture or unincorporated association, but more than 50% of whose ownership interest representing the right to make the decisions for such other Person is, at the time of such determination, owned or controlled, directly or indirectly, by such first Person, but such other Person shall be deemed to be a Subsidiary only so long as such ownership or control exists.
“Tecfidera” means the product sold as of the Agreement Date by Licensee under Licensee’s tradename and marketing authorization for its oral formulation of dimethyl fumarate indicated for the treatment of multiple sclerosis at the recommended dose of 480 mg/day associated with NDA No. 204063 and equivalent approvals in Designated Countries.
“Territory” means, collectively, the United States and the Designated Countries.
“Third Party” means any Person other than any Party or its Affiliates.
“Transactions” means the transactions contemplated by this Agreement, including the Licenses.
“TUPE Regulations” means the Danish Consolidation Act no. 710 of August 20, 2002, any other current or subsequent legislation based upon EU Directives 77/187 and 98/50 or any other applicable Law similar to any of the foregoing.
“United States” means all states and territories of the United States of America.
“Upfront Fee” means the Designated Countries Upfront Fee and the U.S. Upfront Fee.
“U.S. Combination Product” means any fixed dose U.S. Infringing Product that incorporates one or more APIs, at least one of which is not recited in a claim of a Patent included in the U.S. Licensed Intellectual Property and which is regulated by the FDA as either (i) a fixed-combination prescription drug as defined in 21 C.F.R. 300.5 (Fixed-combination prescription drugs for humans) (or any equivalent or replacement regulation) or (ii) a Combination Product as set forth in 21 CFR 3.2(e)(1) (or any equivalent or replacement definition).
“U.S. Generic Equivalent” means a product used, sold, offered for sale or imported in the United States (a) that is approved by the FDA pursuant to an ANDA filed under
21 U.S.C. §355(j) using New Drug Application No. 204063 as the reference product and (b) that is not an Authorized Generic.
“U.S. Infringing Product” has the meaning set forth in Section 4.02.
“U.S. License Term” means the period beginning upon the date Licensor receives the U.S. Upfront Fee and continuing perpetually.
“U.S. Patents” means all Patents owned, registered or otherwise protected or enjoyable under the laws of the United States.
“U.S. Royalty Consideration” has the meaning set forth in Section 4.02.
“U.S. Royalty Term” means the period beginning upon the date Licensor receives the U.S. Upfront Fee and ending on the expiration of the last to expire (or be invalidated in entirety by a final court ruling, from which no appeal can be taken or is timely taken) of the U.S. Patents included in the Licensed Intellectual Property.
“U.S. Upfront Fee” means an amount equal to USD$1,042,250,000.
“USPTO” means the United States Patent and Trademark Office.
SECTION 1.02. Index of Defined Terms.
|
Term |
|
Section |
|
Additional Parties |
|
Preamble |
|
Additional Parties Authorized Agent |
|
Section 8.11(b) |
|
Additional Party Representative |
|
Section 8.19(a) |
|
Aditech |
|
Section 1.01 |
|
Aditech Addendum |
|
Section 1.01 |
|
Aditech Letter Agreement |
|
Section 1.01 |
|
Affiliate |
|
Section 1.01 |
|
Agents |
|
Section 7.04 |
|
Agreement |
|
Preamble |
|
Agreement Date |
|
Section 1.01 |
|
ANDA |
|
Section 1.01 |
|
API |
|
Section 1.01 |
|
Applicable Payer |
|
Section 4.06(b) |
|
Auditor |
|
Section 4.07 |
|
Authorized Generic |
|
Section 1.01 |
|
Bankruptcy Code |
|
Section 8.15(a) |
|
Biogen |
|
Recitals |
|
Burdensome Condition |
|
Section 2.05(c) |
|
Business Day |
|
Section 1.01 |
|
Co-Exclusive |
|
Section 3.01 |
|
Co-Exclusive U.S. License |
|
Section 3.01 |
|
Combination Products |
|
Section 1.01 |
|
Companies Act |
|
Section 1.01 |
|
Confidential Information |
|
Section 2.04(b) |
|
Confidential Intellectual Property Information |
|
Section 1.01 |
|
Consent |
|
Section 2.05(a) |
|
Contract |
|
Section 1.01 |
|
Designated Countries |
|
Section 1.01 |
|
Designated Countries Acquisition Option |
|
Section 3.07(a) |
|
Designated Countries Acquisition Option Closing |
|
Section 3.07(c) |
|
Designated Countries Acquisition Option Closing Date |
|
Section 3.07(c) |
|
Designated Countries Acquisition Option Exercise Notice |
|
Section 3.07(b) |
|
Designated Countries License Term |
|
Section 1.01 |
|
Designated Countries Licensed Intellectual Property |
|
Section 3.03 |
|
Designated Countries Licensee |
|
Preamble |
|
Designated Countries Royalty Consideration |
|
Section 4.03 |
|
Designated Countries Royalty Term |
|
Section 1.01 |
|
Designated Countries Statement |
|
Section 4.03(g) |
|
Designated Countries Upfront Fee |
|
Section 1.01 |
|
Designated Country Combination Product |
|
Section 1.01 |
|
Designated Country Generic Entry Impact |
|
Section 4.03(h) |
|
Designated Country Generic Equivalent |
|
Section 1.01 |
|
Designated Country Infringing Product |
|
Section 4.03 |
|
Disclosing Parties |
|
Section 2.04(b) |
|
Disclosing Party |
|
Section 2.04(b) |
|
DOJ |
|
Section 2.05(b) |
|
Effective Date |
|
Section 1.01 |
|
European Opposition Proceeding |
|
Section 1.01 |
|
Exclusive Designated Countries License |
|
Section 3.03 |
|
Exclusive U.S. License |
|
Section 3.02(a) |
|
Exclusive U.S. License Consideration |
|
Section 1.01 |
|
Exclusive U.S. License Effective Date |
|
Section 3.02(a) |
|
Exclusive U.S. License Notice |
|
Section 3.02(b) |
|
FDA |
|
Section 1.01 |
|
Filing Party |
|
Section 8.15(a) |
|
FTC |
|
Section 2.05(b) |
|
GAAP |
|
Section 1.01 |
|
German Proceedings |
|
Section 2.01(b)(ii) |
|
Governmental Entity |
|
Section 1.01 |
|
HSR Act |
|
Section 1.01 |
|
Infringed Claim |
|
Section 1.01 |
|
Infringing Products |
|
Section 1.01 |
|
Intellectual Property |
|
Section 1.01 |
|
Interference Proceeding |
|
Section 1.01 |
|
IP Advisory Committee |
|
Section 5.02 |
|
JAMS |
|
Section 8.10(c) |
|
Laws |
|
Section 1.01 |
|
Legal Restraints |
|
Section 6.01(b) |
|
Letter Agreement |
|
Section 1.01 |
|
License Term |
|
Section 1.01 |
|
Licensed Intellectual Property |
|
Section 1.01 |
|
Licensed Intellectual Property Rights |
|
Section 1.01 |
|
Licensed Patents |
|
Section 1.01 |
|
Licensed Product |
|
Section 1.01 |
|
Licensee |
|
Preamble |
|
Licensee Released Parties |
|
Section 2.01(b)(i) |
|
Licensee Releasing Parties |
|
Section 2.01(c) |
|
Licenses |
|
Section 1.01 |
|
Licensor |
|
Preamble |
|
Licensor Articles |
|
Section 1.01 |
|
Licensor Authorized Agent |
|
Section 8.11(a) |
|
Licensor Ordinary Shares |
|
Section 1.01 |
|
Licensor Released Parties |
|
Section 2.01(c)(i) |
|
Licensor Releasing Parties |
|
Section 2.01(b) |
|
Licensor Shareholder Approval |
|
Section 7.01(b)(iii) |
|
Licensor Shareholders’ Meeting |
|
Section 7.01(b)(iii) |
|
Lien |
|
Section 1.01 |
|
Litigation |
|
Section 1.01 |
|
Net Sales |
|
Section 1.01 |
|
Non-Filing Party |
|
Section 8.15(a) |
|
Notice |
|
Section 8.03 |
|
Notice of Meeting |
|
Section 7.01(b)(ii) |
|
Order |
|
Section 7.01(b)(iv) |
|
Parties |
|
Preamble |
|
Party |
|
Preamble |
|
Patent Transfer Agreement |
|
Section 1.01 |
|
Patents |
|
Section 1.01 |
|
Permitted Lien |
|
Section 1.01 |
|
Person |
|
Section 1.01 |
|
PTAB |
|
Section 1.01 |
|
Receiving Parties |
|
Section 2.04(b) |
|
Receiving Party |
|
Section 2.04(b) |
|
Released Matters |
|
Section 1.01 |
|
Released Parties |
|
Section 2.01(d) |
|
Releasing Parties |
|
Section 2.01(f) |
|
Relevant Patent |
|
Section 1.01 |
|
Representatives |
|
Section 2.04(a) |
|
Royalty Consideration |
|
Section 1.01 |
|
Royalty Term |
|
Section 1.01 |
|
Sales Returns and Allowances |
|
Section 1.01 |
|
SEC |
|
Section 1.01 |
|
Section 2.05(b) Matters |
|
Section 2.05(b) |
|
Shareholder Meeting Materials |
|
Section 1.01 |
|
Shareholders Commitment Agreement |
|
Recitals |
|
Specified IP |
|
Section 8.15(b) |
|
Specified Shareholders |
|
Recitals |
|
Subsidiary |
|
Section 1.01 |
|
Tecfidera |
|
Section 1.01 |
|
Territory |
|
Section 1.01 |
|
the VAT Note |
|
Section 4.06(a)(ii) |
|
Third Party |
|
Section 1.01 |
|
Transactions |
|
Section 1.01 |
|
TUPE Regulations |
|
Section 1.01 |
|
TUPE Related Liabilities |
|
Section 2.10 |
|
U.S. Acquisition Option |
|
Section 3.06(a) |
|
U.S. Acquisition Option Closing |
|
Section 3.06(c) |
|
U.S. Acquisition Option Closing Date |
|
Section 3.06(c) |
|
U.S. Acquisition Option Exercise Notice |
|
Section 3.06(b) |
|
U.S. Combination Product |
|
Section 1.01 |
|
U.S. Generic Entry Impact |
|
Section 4.02(i) |
|
U.S. Generic Equivalent |
|
Section 1.01 |
|
U.S. Infringing Product |
|
Section 4.02 |
|
U.S. License Term |
|
Section 1.01 |
|
U.S. Licensed Intellectual Property |
|
Section 3.01 |
|
U.S. Licensee |
|
Preamble |
|
U.S. Outside Date |
|
Section 3.02(b) |
|
U.S. Patents |
|
Section 1.01 |
|
U.S. Royalty Consideration |
|
Section 4.02 |
|
U.S. Royalty Term |
|
Section 1.01 |
|
U.S. Statement |
|
Section 4.02(h) |
|
U.S. Upfront Fee |
|
Section 1.01 |
|
United States |
|
Section 1.01 |
|
Upfront Fee |
|
Section 1.01 |
|
USPTO |
|
Section 1.01 |
|
VAT |
|
Section 4.06(a) |
|
Verzichtsurteil |
|
Section 2.01(b)(ii) |
ARTICLE II
Covenants
SECTION 2.01. Release of Claims, Dismissal of Claims and Covenant Not To ▇▇▇. Effective from and after the Effective Date:
(a) Each Party hereby acknowledges and agrees that nothing herein shall be construed to be an admission of liability in connection with any Litigation. Each Party expressly denies any liability to any other Party related to any pending or ongoing Litigation between or among any of the Parties.
(b) Each of Licensor and the Additional Parties does for itself, its controlled Affiliates and its and its controlled Affiliates’ successors and assigns, as applicable, (the “Licensor Releasing Parties”),
(i) fully, finally, absolutely and forever, throughout the Territory, release, relinquish, acquit and discharge Licensee and each of its predecessors, successors, assigns, administrators, attorneys, agents, shareholders, representatives, officers, directors, employees, trustees, parents, Subsidiaries, customers, suppliers, distributors, importers, manufacturers and insurers and each of their respective Affiliates (collectively, the “Licensee Released Parties”) of, from and against any and all Released Matters, as applicable, anywhere in the Territory arising from the beginning of time to the Effective Date;
(ii) agree to undertake the necessary steps to withdraw with prejudice and no right to refile but with each party bearing its own costs and expenses in connection with the proceedings, promptly following the Effective Date, the (A) Litigation involving Licensor’s European patent EP 2 801 355 (Application No. 20140172398) and (B) Litigation involving Licensor’s German Utility Model DE202005002112U1 (the “German Proceedings”). The termination of such Litigation shall be effected by way of a waiver judgment (“Verzichtsurteil”) following Licensor’s waiver of all claims asserted and a subsequent “waiver judgment” of the court to be requested by Licensee. Licensor shall take, and shall cause each of its controlled Affiliates to take, any and all actions, including making any filings with any Governmental Entity, to effect such termination; and
(iii) covenant not to ▇▇▇, not to assign to any other Person a right to ▇▇▇ and not to authorize any other Person to ▇▇▇ any Licensee Released Party for, any and all Released Matters, as applicable, anywhere in the Territory.
(c) Licensee does for itself, its controlled Affiliates and its and its controlled Affiliates’ successors and assigns (the “Licensee Releasing Parties”),
(i) fully, finally, absolutely and forever, throughout the Territory, release, relinquish, acquit and discharge Licensor and each of its predecessors, successors and assigns and each of their respective administrators, attorneys, agents, shareholders,
representatives, officers, directors, employees, trustees, parents, Subsidiaries, customers, suppliers, distributors, importers, manufacturers and insurers (collectively, the “Licensor Released Parties”) of, from and against any and all Released Matters, as applicable, anywhere in the Territory arising from the beginning of time to the Effective Date;
(ii) agree to consent to the withdrawal from the German Proceedings described in Section 2.01(b)(ii) above; and
(iii) covenant not to ▇▇▇, not to assign to any other Person a right to ▇▇▇ and not to authorize any other Person to ▇▇▇ any Licensor Released Party for, any and all Released Matters, as applicable, anywhere in the Territory.
(d) It is the intention of the Parties in executing the releases contained in this Section 2.01 and in giving and receiving the consideration called for in this Agreement, that the release contained in this Section 2.01 shall be effective as a full and final accord and satisfaction and general release of and from all Released Matters and the final resolution by the Parties, the Licensee Released Parties and the Licensor Released Parties (collectively, the “Released Parties”) of all Released Matters. The invalidity or unenforceability of any part of this Section 2.01 shall not affect the validity or enforceability of the remainder of this Section 2.01 which shall remain in full force and effect. The releases granted in this Section 2.01 are ongoing, effective as of the Effective Date, and applicable to actions of any Released Party or occurring any time prior to or on the Effective Date.
(e) Each Party waives to the fullest extent permitted by Law the provisions and benefits of Section 1542 of the California Civil Code, which provides that: “A general release does not extend to claims which the creditor does not know or suspect to exist in his or her favor at the time of executing the release, which if known by him or her must have materially affected his or her settlement to the debtor,” and all similar provisions and benefits under the Laws of all countries, states, provinces, and other political subdivisions throughout the Territory.
(f) Each Party hereby represents, warrants and covenants to the other Parties that neither it nor any of its controlled Affiliates (collectively, the “Releasing Parties”) have, and Licensor hereby represents, warrants and covenants to the other Parties that none of the Additional Parties have, voluntarily or involuntarily assigned or transferred or purported to assign or transfer to any Person (other than a Subsidiary of such Person) any Released Matters and that no Person other than a Releasing Party has any interest in any Released Matter by Law or Contract or by virtue of any action or inaction by such Party or any of the Releasing Parties. Effective from and after the Effective Date, Licensor and the Additional Parties agree, severally but not jointly, to indemnify and hold harmless the Licensee Released Parties from and against all Litigation arising from any such alleged or actual assignment or transfer of such Released Matters by Licensor its controlled Affiliates or such Additional Party or its controlled Affiliates, respectively. Effective from and after the Effective Date, Licensee agrees to indemnify and hold harmless the Licensor Released Parties from and against all
Litigation arising from any such alleged or actual assignment or transfer of such Released Matters by a Licensee Releasing Party.
(g) Notwithstanding anything to the contrary in this Section 2.01, if Licensor has not received the Upfront Fee as required by Section 4.01, this Section 2.01 shall be null and void, nunc pro tunc.
SECTION 2.02. Intellectual Property Challenge or Contestation.
(a) Effective from and after the Agreement Date, the Parties hereby agree and covenant that, throughout the Territory, they shall not, and shall not agree or commit to, and shall cause their respective controlled Affiliates to not, and to not agree or commit to, (a) directly or indirectly challenge or contest in any Litigation, the validity or enforceability of any Intellectual Property owned or otherwise controlled by any of the Parties as of the Agreement Date relating to treating multiple sclerosis or of the Licensed Intellectual Property, or (b) assist any Third Party, directly or indirectly, to so challenge or contest in any such Litigation, except in each case (i) as the other Parties or such Affiliate may be compelled to respond to legal process in Litigation or proceedings initiated by a Third Party without any assistance or encouragement from any Party or any of their Affiliates or (ii) as related to the Interference Proceeding or the European Opposition Proceeding. In addition, Licensor and the Additional Parties shall not, and shall cause each of their respective controlled Affiliates not to, and shall not aid any Third Party to, oppose or object to any future amendments or supplements to NDA No. 204063.
(b) Notwithstanding anything to the contrary in this Section 2.02, if Licensor has not received the Upfront Fee as required by Section 4.01, this Section 2.02 shall be null and void, nunc pro tunc.
SECTION 2.03. Transfers of Intellectual Property; Liens. Effective from and after the Agreement Date, Licensor and the Additional Parties hereby agree and covenant that, throughout the Territory, they shall not, and shall cause each of their respective controlled Affiliates not to (i) sell, license, transfer, assign or otherwise dispose of, encumber or impair any Licensed Intellectual Property Rights except as permitted pursuant to and in accordance with Article III and/or Section 2.11, (ii) subject any Licensed Intellectual Property Rights to any Lien other than Permitted Liens (or authorize or allow any of the Licensed Intellectual Property Rights to become subject to any Lien other than Permitted Liens), (iii) enter into any Contract relating to the sale, licensing, transfer, disposition or assignment of any Licensed Intellectual Property Rights including any option related thereto; except, in the case of Licensor, to assign its Co-Exclusive rights pursuant to and in accordance with Section 3.01 or (iv) disclose to any Third Party, other than Representatives of Licensee under a confidentiality agreement or other legally binding duty of confidentiality, any Confidential Intellectual Property Information relating to the Licensed Intellectual Property in a way that results in a material loss of intellectual property protection for such Confidential Intellectual Property Information or any Licensed Intellectual Property. Licensor and the Additional Parties hereby agree and covenant that, throughout the Territory, they shall, and shall cause each of their respective controlled Affiliates to, comply in
all material respects with the terms of all third-party licenses and other obligations related to or included in the Licensed Intellectual Property.
SECTION 2.04. Access to Information; Confidentiality; Notification. Without limiting any other rights or obligations hereunder:
(a) Each of Licensor and the Additional Parties shall, and shall cause each of their respective controlled Affiliates to, give Licensee, and to Licensee’s Affiliates and its and their respective officers and employees and, solely when acting in their capacity as the following, attorneys and other advisors and representatives (collectively, “Representatives”), reasonable access during normal business hours and on reasonable advance notice, from the Effective Date until the earliest of the end of the Royalty Term and the termination of this Agreement in accordance with its terms, to all of their respective Confidential Intellectual Property Information, books and records and Contracts relating to the ownership of or Licensee’s rights in, to or under the Licensed Intellectual Property, and directors, officers, employees, contractors, consultants, attorneys, other advisors and representatives, in each case, to the extent related to the Licensed Intellectual Property; provided that in no event shall Licensor or the Additional Parties be required to provide any information that is subject to attorney-client privilege or work product immunity, to the extent (but only to the extent) that such privilege or immunity would reasonably be expected to be lost or reduced by disclosure to Licensee, or any Confidential Intellectual Property Information, in either case that is related to (i) the negotiation of this Agreement or any enforcement hereof or disputes hereunder (including establishing that a product is an Infringing Product), (ii) the Interference Proceeding or (iii) the European Opposition Proceeding, in each case prior to the conclusion of such matters; except that (A) in each case Licensor and the Additional Parties shall, and shall cause each of their respective controlled Affiliates to, use commercially reasonable efforts to provide the applicable information in a way, if any, that would not reasonably be expected to violate such privilege, as applicable, or materially adversely affect Licensor or the Additional Parties, as applicable, in the Interference Proceeding or the European Opposition Proceeding, as applicable; and (B) no investigation by Licensee or its Representatives shall affect or be deemed to modify or waive the representations and warranties of Licensor or the Additional Parties set forth in this Agreement. The Parties shall, and shall cause any of their respective applicable controlled Affiliates to, enter into a Joint Defense and Common Interest Agreement substantially in the form agreed upon by Licensor and Licensee and such other agreements as may be required to effectuate the purposes of this Agreement.
(b) Each Party shall keep confidential, and shall instruct its Representatives to keep confidential, information relating to the other Parties and their Affiliates provided by such other Parties or any of their Affiliates (each a “Disclosing Party” and, collectively with its respective Affiliates, the “Disclosing Parties”) to such receiving Party or any of its Affiliates (a “Receiving Party” and, collectively with its respective Affiliates, the “Receiving Parties”) and its Representatives pursuant to or in connection with this Agreement (the “Confidential Information”), except as may otherwise be requested or required by (i) applicable Law or stock exchange requirements
or (ii) judicial or legal process or by any Governmental Entity, in which case the Receiving Party will, to the extent permitted by applicable Law, provide the Disclosing Parties with prompt written notice of such requirement so that the Disclosing Parties may seek an appropriate protective order (at the Disclosing Parties’ sole expense). For purposes hereof, “Confidential Information” shall not include any information that (A) was or becomes generally available to the public other than as a result of a disclosure by the Receiving Party or any of its Representatives in violation of this Section 2.04(b), (B) was or becomes available to the Receiving Party or any of its Representatives from a source other than a Disclosing Party; provided that the provision of such information from such source is reasonably believed by the Receiving Party or its Representatives, as applicable, not to be subject to an obligation of confidentiality (whether by agreement or otherwise) to a Disclosing Party, (C) at the time of disclosure is already in the possession of the Receiving Party or any of its Representatives; provided that such information is reasonably believed by the Receiving Party or its Representatives, as applicable, not to be subject to an obligation of confidentiality (whether by agreement or otherwise) to a Disclosing Party or (D) was independently developed by the Receiving Party or any of its Representatives on the Receiving Party’s behalf without reference to, incorporation of, or other use of any Confidential Information. The Parties acknowledge that Licensor will file this Agreement and the Aditech Addendum with the SEC promptly after the Agreement Date, and that Licensor will also on or after the Agreement Date make this Agreement and the Aditech Addendum publically available on its website as part of the Shareholder Meeting Materials.
SECTION 2.05. Commercially Reasonable Efforts; Notification. Effective from and after the Effective Date:
(a) Upon the terms and subject to the conditions set forth in this Agreement, each Party shall, and shall cause its controlled Affiliates to, use its commercially reasonable efforts to take, or cause to be taken, all actions, and to do, or cause to be done, and to assist and cooperate with the other Parties in doing, all things necessary, proper or advisable to consummate and make effective, as promptly as practicable, the Transactions, including (i) the obtaining of all necessary or advisable actions or non-actions, waivers, approvals, licenses, permits, orders or other authorizations and consents (“Consent”) from, the making of all necessary registrations, declarations and filings with and the taking of all reasonable steps as may be necessary to avoid any Litigation by, any Governmental Entity or other Third Party with respect to this Agreement or the Transactions and (ii) the execution and delivery of any additional instruments necessary to consummate the Transactions and to fully carry out the purposes of this Agreement.
(b) Without limiting the generality of the Parties’ obligations under Section 2.05(a), and in furtherance thereof, each of the Parties shall, and shall cause their respective controlled Affiliates to, in consultation and cooperation with the other, file with the United States Federal Trade Commission (the “FTC”) and the United States Department of Justice (the “DOJ”), the notification and report form, if any, required under the HSR Act for any Transaction. Any such filings shall be in substantial
compliance with the requirements of the HSR Act. Each of the Parties shall, and shall cause each of their respective controlled Affiliates to, (i) furnish to the other Parties such necessary information and reasonable assistance as the other Parties may request in connection with its preparation of any filing or submission which is necessary under the HSR Act, (ii) give the other Parties reasonable prior notice of any such filings or submissions and, to the extent reasonably practicable, of any communication with, and any inquiries or requests for information from, the FTC, the DOJ or any other Governmental Entity regarding any of the Transactions, and permit the other Parties to review and discuss in advance, and consider in good faith the views of, and secure the participation of, the other Parties in connection with, any such filings, submissions, communications, inquiries or requests, (iii) unless prohibited by applicable Law or by the applicable Governmental Entity, and to the extent reasonably practicable, (A) not participate in or attend any meeting, or engage in any substantive conversation, with any Governmental Entity in respect of any of the Transactions without the other Parties, (B) give the other Parties reasonable prior notice of any such meeting or conversation, (C) in the event a Party is prohibited by applicable Law or by the applicable Governmental Entity from participating in or attending any such meeting or engaging in any such conversation, keep such Party apprised with respect thereto, (D) cooperate in the filing of any substantive memoranda, white papers, filings, correspondence or other written communications explaining or defending this Agreement or the Transactions, articulating any regulatory or competitive argument or responding to requests or objections made by any Governmental Entity and (E) furnish the other Parties with copies of all filings, submissions, correspondence and communications (and memoranda setting forth the substance thereof) between it and its controlled Affiliates and their respective Representatives, on the one hand, and any Governmental Entity or members of any Governmental Entity’s staff, on the other hand, with respect to this Agreement or the Transactions, including promptly furnishing the other Parties with copies of notices or other communications received or provided by such Party, or any of its controlled Affiliates, from or to any Third Party and/or Governmental Entity and (iv) comply with any inquiry or request from the FTC, the DOJ or any other Governmental Entity as promptly as reasonably practicable; provided, however, that Licensee and its Affiliates shall not be obligated to comply with any requests for substantial additional information and documentary material from the FTC and/or DOJ (or any analogous request by any non-U.S. Governmental Entity), which, for the avoidance of doubt, includes any “second request” from the FTC and/or the DOJ. In addition, no Party shall, and each shall cause its respective controlled Affiliates not to, take any action with the intention to, or that could reasonably be expected to, hinder or delay the expiration or termination of any waiting period under the HSR Act or the obtaining of any approval required by applicable Law from any Governmental Entity. Notwithstanding the foregoing, (I) the Parties agree that it is Licensee’s sole right to devise and implement the strategy for all filings, submissions, notifications and communications subject to this Section 2.05(b) (“Section 2.05(b) Matters”), including, in each case, the timing thereof, and to direct all Section 2.05(b) Matters with any Governmental Entity consistent with Licensee’s obligations hereunder, provided, however, that, in the event the PTAB’s determination in the Interference Proceeding (notwithstanding any appeal therefrom) results in the subsistence and ownership by Licensee (or an Affiliate of Licensee) of Patent US 8,399,514 B2 (and,
for the avoidance of doubt, does not result in Licensor obtaining a Relevant Patent), Licensee shall, subsequent to the issuance of such PTAB determination, consult with and consider in good faith the views of Licensor regarding the strategy for all Section 2.05(b) Matters, including, in each case, the timing thereof, and (II) each Party may, as each deems advisable and necessary, reasonably designate any competitively sensitive material provided to the other or its Affiliates under this Section 2.05(b) as “Counsel Only Material”, which such material and the information included therein shall be given only to the in house and outside counsel of the recipient and will not be disclosed by such counsel to employees, officers or directors of the recipient unless express permission is obtained in advance from the source of the materials or its legal counsel. Notwithstanding anything to the contrary contained in this Section 2.05, materials provided pursuant to this Section 2.05 may be redacted to remove references concerning any Confidential Information, as necessary to comply with contractual arrangements and as necessary to address reasonable confidentiality concerns.
(c) Notwithstanding anything to the contrary set forth in this Agreement, neither Licensee nor any of its Affiliates shall be required to, and Licensor and the Additional Parties may not, and shall cause their respective controlled Affiliates not to, without the prior written consent of Licensee, consent to, or offer or agree to, or otherwise take any action with respect to, any requirement, condition, limitation, understanding or agreement or order to (i) sell, license, grant an option or similar right under, assign, transfer, divest, hold separate or otherwise dispose of (A) in the case of Licensee or any of its Affiliates, any assets, business or portion of business of Licensee or any of its Affiliates and (B) in the case of Licensor, the Additional Parties or any of their respective Affiliates, any Licensed Intellectual Property, (ii) conduct, restrict, operate, invest or otherwise change in any manner (A) in the case of Licensee or any of its Affiliates, any assets, business or portion of business of Licensee or any of its Affiliates and (B) in the case of Licensor, the Additional Parties or any of their respective Affiliates, any Licensed Intellectual Property, (iii) impose any restriction, requirement or limitation on the operation of the business or portion of the business of Licensee or any of its Affiliates or (iv) modify any provision or term of this Agreement, the Aditech Addendum, the Patent Transfer Agreement or any other Contract with respect to the Licensed Intellectual Property (any of the foregoing, a “Burdensome Condition”). In addition, notwithstanding anything to the contrary set forth in this Agreement, (x) neither Licensee nor any of its Affiliates shall be required to defend or contest any Litigation, whether judicial or administrative, challenging this Agreement or the consummation of the Transactions, including seeking to have any stay or temporary restraining order entered by any court or other Governmental Entity vacated or reversed and (y) Licensor and the Additional Parties may not, and shall cause their respective controlled Affiliates not to, without the prior written consent of Licensee, defend or contest any Litigation whether judicial or administrative, challenging this Agreement or the consummation of the Transactions on antitrust or competition law grounds, including seeking to have any stay or temporary restraining order entered by any court or other Governmental Entity vacated or reversed. If requested by Licensee (x) Licensor and the Additional Parties shall, and shall cause their respective controlled Affiliates to become subject to, consent to, or offer or agree to, or otherwise take any action with respect to, any such
requirement, condition, limitation, understanding, agreement or order of any Governmental Entity so long as such requirement, condition, limitation, understanding, agreement or order is only binding on Licensor, the Additional Parties and their respective applicable controlled Affiliates if the Transactions are consummated and (y) prior to the U.S. Outside Date, Licensor and the Additional Parties shall, and shall cause each of their respective controlled Affiliates to, at each of their own respective cost and expense, defend or contest any Litigation referred to in clause (y) of the second sentence of this Section 2.05(c).
SECTION 2.06. Public Announcements. Effective from and after the Agreement Date, the Parties shall consult with each other before issuing, and provide each other the opportunity to review and comment upon, any press release or other public statements with respect to this Agreement, the Aditech Addendum or the Transactions or the transactions contemplated by the Aditech Addendum, and shall not issue any such press release or make any such public statement without the prior consent of the other (which consent shall not be unreasonably withheld, delayed or conditioned), except (a) as required by applicable Law, judicial or legal process or by obligations pursuant to any listing agreement with any securities exchange or the SEC; or (b) for press releases or other public statements which only include information relating to this Agreement or the Transactions that has been previously made public in accordance with the terms of this Agreement or (c) announcement of the Notice of Meeting on Licensor’s website or otherwise in accordance with Licensor Articles. The Parties agree that the initial press release to be issued with respect to the Transactions shall be in the form heretofore agreed to by the Parties in writing.
SECTION 2.07. No Frustration. Effective from and after the Agreement Date, Licensor and the Additional Parties shall not take, and shall cause each of their respective controlled Affiliates not to take, any action that would, or would reasonably be expected to, (i) result in any condition set forth in Article VI not being promptly satisfied or (ii) impair the ability of Licensor or any of the Additional Parties or any of their respective Affiliates to perform its obligations under this Agreement or prevent or impede, interfere with, hinder or delay (A) the consummation of any of the Transactions, (B) the performance of any of the Transactions following the Agreement Date or (C) Licensee, its Affiliates or sublicensees from realizing the benefits of the Licenses. For the avoidance of doubt, Licensor and the Additional Parties shall not take, and shall cause each of their respective controlled Affiliates not to take, any action permitted under the terms of the Licenses if such action would, or would reasonably be expected to, impede, interfere with, hinder or delay Licensee, its Affiliates or sublicensees from realizing the benefits of the Licenses.
SECTION 2.08. Existence. Effective from and after the Agreement Date, Licensor and, for so long as they retain any right in any Licensed Intellectual Property, the Additional Parties, if any, having any right in any of the Licensed Intellectual Property shall, and shall cause each of their respective controlled Affiliates having any right in any of the Licensed Intellectual Property to, do or cause to be done all things necessary to preserve, renew and keep in full force and effect its legal existence. In addition, effective from and after the Agreement Date, Licensor and, for so long as they retain any right in any Licensed Intellectual Property, the Additional Parties, if any, having any right in any of the Licensed Intellectual Property, shall not,
and shall cause each of their respective controlled Affiliates having any right in any of the Licensed Intellectual Property (for so long as such controlled Affiliates have any right in any of the Licensed Intellectual Property ) not to, commence or file any petition seeking (i) liquidation, reorganization or other relief in respect of any of Licensor, the Additional Parties, if any, having any right in any of the Licensed Intellectual Property or any of their respective Affiliates having any right in any of the Licensed Intellectual Property, or any of their respective debts, or of a substantial part of their respective assets, under any U.S. Federal, U.S. state, Danish or other bankruptcy, insolvency, receivership or similar Law now or hereafter in effect or (ii) the appointment of a receiver, trustee, custodian, sequestrator, conservator or similar official for any of Licensor, any of its controlled Affiliates having any right in any of the Licensed Intellectual Property or, for so long as they retain any right in any Licensed Intellectual Property, the Additional Parties, if any, having any right in any of the Licensed Intellectual Property, in each case for a substantial part of their respective assets.
SECTION 2.09. Solvency.
(a) During the period from the Agreement Date until the satisfaction of all of Licensor’s obligations under Section 2.10, Licensor shall not, and shall cause each of its controlled Affiliates not to, become insolvent or permit the cash and cash equivalents owned by Licensor and its Subsidiaries to be less than the amount required to satisfy its obligations as they come due, including its obligations under Section 2.10 plus any amounts required pursuant to Section 2.09(b) and any taxes thereon.
(b) During the period from the Agreement Date until the earlier of (i) the end of the Royalty Term and (ii) the later of (x) the Designated Countries Acquisition Option Closing Date and (y) the US Acquisition Option Closing Date, as applicable, Licensor shall not, and shall cause each of its controlled Affiliates not to, permit the assets, cash and cash equivalents owned by Licensor and its Subsidiaries to be less than the amount required to maintain Licensor as a going concern and a solvent entity, including the amount required to satisfy all tax liabilities of Licensor. In addition, and notwithstanding anything in the immediately preceding sentence to the contrary, from the Agreement Date until the satisfaction of all of Licensor’s obligations under Section 2.11 required to be performed before 18 months following the Agreement Date, Licensor shall not, and shall cause each of its controlled Affiliates not to, permit the assets, cash and cash equivalents owned by Licensor and its Subsidiaries to be less than the amount required to maintain Licensor as a going concern and a solvent entity, including, for the avoidance of doubt, the amount required to satisfy all tax and other liabilities of Licensor plus an additional $5,000,000.
(c) During the period from the Agreement Date until the end of the Royalty Term, as soon as reasonably practicable following the end of each Licensor fiscal year during such period, Licensor shall provide Licensee with a consolidated balance sheet of Licensor and its Subsidiaries as of the last day of the relevant fiscal year that has been prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board and audited by an internationally recognized independent accounting firm.
(d) During the period from the Agreement Date until the end of the Royalty Term, for so long as it has any right in any of the Licensed Intellectual Property, each of the Additional Parties, if any, having any right in any of the Licensed Intellectual Property shall not, and shall cause each of their respective controlled Affiliates having any right in any of the Licensed Intellectual Property not to, permit their respective assets, cash and cash equivalents, or those of their respective Subsidiaries, to be less than the amount required to maintain each such Person and each of their respective Subsidiaries as a going concern and a solvent entity.
SECTION 2.10. TUPE Regulations; Indemnification. Effective from and after the Agreement Date, Licensor shall indemnify Licensee and its Affiliates for any and all losses, damages, liabilities, costs and expenses arising in connection with the transactions contemplated by this Agreement as a result of the application of the TUPE Regulations to any directors, employees or other service providers of Licensor and any of its Affiliates (including those relating to claims for employment with or compensation from Licensee or any of its Affiliates or with respect to warrants or other equity or equity-based compensation issued by Licensor or any of its Affiliates) (the “TUPE Related Liabilities”). Without limiting the generality of the foregoing, Licensor and Licensee shall cooperate and use commercially reasonable efforts to take actions to mitigate any such TUPE Related Liabilities, which actions shall include (a) the vesting by Licensor of warrants and other equity or equity-based compensation which vest as a result of the Transactions in accordance with the terms and conditions of the applicable Licensor plans or programs and (b) in the case of any director, employee or service provider of Licensor or any of its Affiliates who successfully asserts a claim to become employed by Licensee or any of its Affiliates, (i) prompt written notification to Licensor of any such claim directly received by Licensee or any of its Affiliates and (ii) the termination of such director, employee or service provider by Licensee or such Affiliate, as applicable, as soon as practicable following a written request from Licensor to take such action if, and effective at the earliest time, such action is permissible under applicable Law.
SECTION 2.11. Specified Actions. Effective from and after the Effective Date, Licensor shall use its commercially reasonable efforts to, and to cause each of its controlled Affiliates to, (i) take the actions set forth on Appendix D as soon as reasonably practicable following the Effective Date, and (ii) consummate the transactions contemplated by, and in the manner and subject to the conditions described in, Appendix D within 270 days following the Agreement Date. If the transactions contemplated in Step 1 of Appendix D are not consummated substantially in accordance with the immediately foregoing sentence, the P/S Sub Restructuring Alternative (as a defined in Appendix D) will be consummated. The Parties shall, and shall cause each of their respective controlled Affiliates to, cooperate to make any amendments to this Agreement that are reasonably necessary to give effect to such transactions. Notwithstanding anything in this Agreement to the contrary, the Parties’ obligations set forth in this Section 2.11 shall terminate upon the later of (a) the Designated Countries Acquisition Option Closing Date and (b) the US Acquisition Option Closing Date.
SECTION 2.12. Joinder. With respect to any Person that is not a Party to this Agreement as of the Effective Date, but is contemplated by the terms of Appendix D to become a party to this Agreement after the Effective Date, the Parties (a) acknowledge the intent to join
such Person to this agreement in accordance with Appendix D, (b) shall execute and deliver or procure the execution and delivery of any instrument or agreement, and take such other action as may be necessary, to assure that the joinder to this Agreement of such Person in accordance with the terms of Appendix D occurs and is lawfully and validly carried out and (c) agree that upon the execution and delivery to the Parties of any such joinder agreement, each party thereto that was not a Party to this Agreement as of the Effective Date shall be deemed a Party to this Agreement from and after the date of such joinder agreement.
SECTION 2.13. Ixchel. Each of the Additional Parties and Licensor shall, and shall cause each of its respective controlled Affiliates to, terminate any and all existing, and not enter into any new, Contracts or obligations to Ixchel Pharma LLC, ▇▇. ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ and/or any other Person, to the extent related to the development by any of the Additional Parties, Licensor or any of their respective controlled Affiliates of any pharmaceutical product having dimethyl fumarate as an API for the treatment of a human for any indication, including Friedreich’s ataxia.
SECTION 2.14. Shareholder Litigation. Licensor shall give Licensee the opportunity to participate (at Licensee’s expense) in the defense or settlement of any shareholder litigation against Licensor or its officers and directors relating to this Agreement or the Transactions, and no such settlement involving any non-monetary damages shall be agreed to without Licensee’s prior written consent (such consent not to be unreasonably withheld, conditioned or delayed).
ARTICLE III
Licenses and Related Rights
SECTION 3.01. Co-Exclusive U.S. License. Effective upon U.S. Licensee’s payment of the U.S. Upfront Fee to Licensor pursuant to Section 4.01, each of Licensor and the Additional Parties, on behalf of itself and each of its respective controlled Affiliates, hereby grants to U.S. Licensee and its Affiliates, effective at all times during the U.S. License Term, a perpetual (until any grant of an Exclusive U.S. License (defined below) in accordance with Section 3.02), irrevocable, Co-Exclusive royalty-bearing (in accordance with Article IV) license, to make any and all use in the United States of the Licensed Intellectual Property owned, registered or otherwise protected or enjoyable under the Laws of the United States by Licensor, such Additional Party or such controlled Affiliate, as the case may be (it being understood that certain of such grantors (other than the Licensor) may not in fact own or hold or have any rights in or to any Licensed Intellectual Property) (such Licensed Intellectual Property, the “U.S. Licensed Intellectual Property”), with the right to sublicense, transfer or assign, (such Co-Exclusive license, the “Co-Exclusive U.S. License”). For the purpose of this Agreement, “Co-Exclusive” shall mean as to Licensor that Licensor has the limited license and right to itself (or through any of its wholly-owned Subsidiaries) make any and all use of the U.S. Licensed Intellectual Property in the United States, including by authorizing contractors to perform services for Licensor, including services to manufacture or import products and to perform wholesale and distribution services for Licensor and its wholly-owned Subsidiaries but shall not be permitted to otherwise directly or indirectly grant additional licenses under, sublicense, assign
or transfer the U.S. Licensed Intellectual Property to any Third Party or otherwise encumber the U.S. Licensed Intellectual Property in any way or use, deploy or operate the U.S. Licensed Intellectual Property for the benefit of any party other than Licensor and its wholly-owned Subsidiaries; provided, however, that if, after the U.S. Outside Date, as defined below, U.S. Licensee has not obtained the Exclusive U.S. License, as defined below, Licensor shall have the right on one occasion to assign its Co-Exclusive rights, in whole, but not in part, to a single Third Party, who shall have no additional right to assign or sublicense such Co-Exclusive rights (except to its wholly owned Subsidiaries) but shall have the right to authorize contractors to perform services (as contemplated above) for such assignee. For the avoidance of doubt, if Licensor assigns its Co-Exclusive rights to any Third Party in accordance with this Section 3.01, Licensor and the Additional Parties and their respective controlled Affiliates shall not be permitted to make any use of the U.S. Licensed Intellectual Property thereafter. The Co-Exclusive U.S. License granted under this Section 3.01 shall be binding on Licensor’s and each of the Additional Parties’ successors and assigns.
SECTION 3.02. Exclusive U.S. License.
(a) Subject to U.S. Licensee’s payment to Licensor of the U.S. Upfront Fee, (i) each of Licensor and the Additional Parties, on behalf of itself and each of its respective controlled Affiliates, hereby grants to U.S. Licensee and its Affiliates, a perpetual, irrevocable, exclusive (even as to Licensor, each of the Additional Parties and their respective Affiliates) royalty-bearing (in accordance with Article IV) license to the U.S. Licensed Intellectual Property, with the right to sublicense, transfer or assign, and to make any and all use thereof in the United States (such exclusive license, the “Exclusive U.S. License”), that shall be effective at all times during the period beginning on the date that is two Business Days following the later of (x) Licensee’s delivery of the Exclusive U.S. License Notice (as defined below) and (y) the satisfaction or waiver (by the Party or Parties entitled to the benefit thereof) of the conditions set forth in Article VI (the “Exclusive U.S. License Effective Date”) through the end of the U.S. License Term. U.S. Licensee shall pay, or cause to be paid, to Licensor, by wire transfer of immediately available funds to the account designated in writing by Licensor, the Exclusive U.S. License Consideration within five (5) Business Days after the Exclusive U.S. License Effective Date. The Exclusive U.S. License shall be binding on Licensor’s and each of the Additional Parties’ successors and assigns.
(b) U.S. Licensee shall deliver to Licensor and the Additional Parties a notice specifying its intention to take the Exclusive U.S. License (the “Exclusive U.S. License Notice”) on or prior to the date that is 75 days following the final decision in the Interference Proceeding, including any appeals therefrom to the Federal Circuit (including any en banc review); provided that U.S. Licensee may, at its option and upon written notice to Licensor and the Additional Parties, extend such date by an additional 140 days (such date, including such extension thereof, if exercised, the “U.S. Outside Date”); provided, further, that U.S. Licensee shall not be obligated to deliver an Exclusive U.S. License Notice if the conditions set forth in Section 6.01 have not been satisfied or waived (by the Party or Parties entitled to the benefit thereof) on or prior to the U.S. Outside Date. Notwithstanding the foregoing, U.S. Licensee may, at its option,
elect to deliver the Exclusive U.S. License Notice at any time prior to the U.S. Outside Date.
SECTION 3.03. Exclusive Worldwide (other than U.S.) License. Effective upon payment of the Designated Countries Upfront Fee to Licensor pursuant to Section 4.01, each of Licensor and the Additional Parties, on behalf of itself and each of its respective controlled Affiliates, hereby grants to Designated Countries Licensee and its Affiliates, effective at all times during the Designated Countries License Term, a perpetual, irrevocable, exclusive (even as to Licensor, each of the Additional Parties and their respective Affiliates), royalty-bearing (in accordance with Article IV) license to all Licensed Intellectual Property owned, registered or otherwise protected or enjoyable under the Laws of any country in the world other than the United States (the “Designated Countries Licensed Intellectual Property”) with the right to sublicense, transfer or assign, and to make any and all use thereof in the Designated Countries (the “Exclusive Designated Countries License”).
SECTION 3.04. Restrictions on Transfer. To the extent Licensor, any of the Additional Parties or any of their respective Affiliates assigns or otherwise transfers or grants rights under (by any means) to any Third Party any right, title or interest in, to or under any Licensed Intellectual Property from and after the Agreement Date, Licensor or such Additional Party, as applicable, shall and shall cause its applicable controlled Affiliate to make such assignment, transfer or other grant only as permitted hereunder and subject to the licenses and other rights granted under this Agreement, as applicable.
SECTION 3.05. Use Through Affiliates. For the avoidance of doubt, Licensee may exercise any or all of its rights under this Article III itself and with or through one or more of its Affiliates, and Licensee and its Affiliates may subcontract with their service providers under the license granted pursuant to this Article III to manufacture or import products licensed hereunder and to perform wholesale and distribution services for Licensee and its Affiliates regarding the same, without limitation.
SECTION 3.06. U.S. Purchase Option.
(a) If the Interference Proceeding (including any appeals therefrom to the Federal Circuit (including any en banc review)) results in the subsistence and ownership by Licensee (or an Affiliate of Licensee) of Patent US 8,399,514 B2 (and, for the avoidance of doubt, does not result in Licensor owning a Relevant Patent), then Licensee or its designated Affiliate shall have the option (but not the obligation) to acquire all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to and under the U.S. Licensed Intellectual Property (excluding any and all liabilities arising out of or in connection with the U.S. Licensed Intellectual Property) for the consideration set forth in Section 3.06(d) (the “U.S. Acquisition Option”).
(b) Licensee may exercise the U.S. Acquisition Option by delivering written notice of such exercise to Licensor and the Additional Parties (a “U.S. Acquisition Option Exercise Notice”) at any time permitted by Section 3.06(a), but in any event not later than 6 months following the date on which such option first becomes exercisable.
(c) Following the delivery of a U.S. Acquisition Option Exercise Notice, the U.S. Acquisition Option shall close on the later of (i) the date of delivery of the U.S. Acquisition Option Exercise Notice and (ii) the date on which the conditions to closing such U.S. Acquisition Option set forth in Section 3.06(g) have been satisfied or waived (by the Party or Parties entitled to the benefit thereof) (the “U.S. Acquisition Option Closing”). The date on which such U.S. Acquisition Option Closing shall occur is referred to herein as the “U.S. Acquisition Option Closing Date”.
(d) U.S. Licensee shall pay, or cause to be paid, to Licensor, by wire transfer of immediately available funds to the account designated in writing by Licensor, USD$50,000, on the U.S. Acquisition Option Closing Date.
(e) At all times from and after the U.S. Acquisition Option Closing Date, the acquisition of the U.S. Licensed Intellectual Property described in this Section 3.06 shall replace the licenses granted in Sections 3.01 and 3.02.
(f) Notwithstanding anything to the contrary in Article V, at all times from and after the U.S. Acquisition Option Closing Date, Licensee shall have sole control over the prosecution, maintenance, defense and assertion of the U.S. Licensed Intellectual Property and shall not be required to consult with Licensor regarding any of the foregoing.
(g) The U.S. Acquisition Option Closing shall be subject to the satisfaction or waiver of the following conditions:
(i) Payment of Upfront Fee. Timely payment in full by Licensee to Licensor of the Upfront Fee as contemplated by Section 4.01.
(ii) HSR Clearance. If Licensee reasonably determines in good faith that Licensee’s exercise of the U.S. Acquisition Option requires the filing of the notification and report form required by the HSR Act with the FTC and DOJ, any waiting period (and any extension thereof) applicable to the U.S. Acquisition Option shall have expired or been earlier terminated.
(iii) Legal Restraints. No Legal Restraints, whether temporary or permanent, restraining, enjoining, preventing, prohibiting or otherwise making illegal or ineffective Licensee’s acquisition of all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to or under the U.S. Licensed Intellectual Property pursuant to the U.S. Acquisition Option shall be in effect.
(h) At the U.S. Acquisition Option Closing, each of Licensor and the Additional Parties shall, and shall cause each of their respective controlled Affiliates to (i) execute all assignments, transfer forms, endorsements and such other customary instruments of sale, transfer or assumption required by applicable Law to vest in Licensee all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to or under the U.S. Licensed Intellectual Property, in form and substance reasonably satisfactory to Licensee, (ii) execute such other agreements,
documents and instruments as Licensee believes to be reasonably necessary to consummate its acquisition of all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to or under the U.S. Licensed Intellectual Property and (iii) take all actions that Licensee deems reasonably necessary or advisable in order to record, or assist Licensee or any of its Affiliates in recording, with any relevant Governmental Entity including the USPTO, the assignment of the U.S. Licensed Intellectual Property to Licensee, so as to perfect Licensee’s or an Affiliate of Licensee’s ownership thereof (including authorizing Licensee or an Affiliate of Licensee to record the documents, or forms of the documents, contemplated by the foregoing clauses (i) and (ii) with any relevant Governmental Entity including the USPTO). For the avoidance of doubt, notwithstanding any other provision of this Agreement or any other agreement, document or instrument executed pursuant to this Section 3.06, Licensee and its Affiliates shall not assume or be liable for any liabilities, obligations or commitments of Licensor, the Additional Parties or any of their respective Affiliates, of any kind, whether express or implied, liquidated, absolute, accrued, contingent or otherwise, or known or unknown, existing on or occurring prior to the U.S. Acquisition Option Closing.
SECTION 3.07. Designated Countries Purchase Option.
(a) If the European Opposition Proceeding (including any appeals therefrom to the Technical Board of Appeal and/or the Enlarged Board of Appeal) does not result in the subsistence and ownership by Licensor of European patent EP 2801355 (Application No. 14172398.1), then Licensee or its designated Affiliate shall have the option (but not the obligation) to acquire all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to and under the Designated Countries Licensed Intellectual Property (excluding any and all liabilities arising out of or in connection with the Designated Countries Licensed Intellectual Property) for the consideration set forth in Section 3.07(d) (the “Designated Countries Acquisition Option”).
(b) Licensee may exercise the Designated Countries Acquisition Option by delivering written notice of such exercise to Licensor and the Additional Parties (a “Designated Countries Acquisition Option Exercise Notice”) at any time permitted by Section 3.07(a), but in any event not later than 6 months following the date on which such option first becomes exercisable.
(c) Following the delivery of a Designated Countries Acquisition Option Exercise Notice, the Designated Countries Acquisition Option shall close on the later of (i) the date of delivery of the Designated Countries Acquisition Option Exercise Notice and (ii) the date on which the conditions to closing such Designated Countries Acquisition Option set forth in Section 3.07(g) have been satisfied or waived (by the Party or Parties entitled to the benefit thereof) (the “Designated Countries Acquisition Option Closing”). The date on which such Designated Countries Acquisition Option Closing shall occur is referred to herein as the “Designated Countries Acquisition Option Closing Date”.
(d) Designated Countries Licensee shall pay, or cause to be paid, to Licensor, by wire transfer of immediately available funds to the account designated in writing by Licensor, USD$50,000, on the Designated Countries Acquisition Option Closing Date.
(e) At all times from and after the Designated Countries Acquisition Option Closing Date, the acquisition of the Designated Countries Licensed Intellectual Property described in this Section 3.07 shall replace the license granted in Section 3.03.
(f) Notwithstanding anything to the contrary in Article V, at all times from and after the Designated Countries Acquisition Option Closing Date, Licensee shall have sole control over the prosecution, maintenance, defense and assertion of the Designated Countries Licensed Intellectual Property and shall not be required to consult with Licensor regarding any of the foregoing.
(g) The Designated Countries Acquisition Option Closing shall be subject to the satisfaction or waiver of the following condition:
(i) Payment of Upfront Fee. Timely payment in full by Licensee to Licensor of the Upfront Fee as contemplated by Section 4.01.
(ii) Legal Restraints. No Legal Restraints, whether temporary or permanent, restraining, enjoining, preventing, prohibiting or otherwise making illegal or ineffective Licensee’s acquisition of all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to or under the Designated Countries Licensed Intellectual Property pursuant to the Designated Countries Acquisition Option shall be in effect.
(h) At the Designated Countries Acquisition Option Closing, each of Licensor and the Additional Parties shall, and shall cause each of their respective controlled Affiliates to (i) execute all assignments, transfer forms, endorsements and such other customary instruments of sale, transfer or assumption required by applicable Law to vest in Licensee all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to or under the Designated Countries Licensed Intellectual Property, in form and substance reasonably satisfactory to Licensee, (ii) execute such other agreements, documents and instruments as Licensee believes to be reasonably necessary to consummate its acquisition of all of Licensor’s, the Additional Parties’ and their respective controlled Affiliates’ right, title and interest in, to or under the Designated Countries Licensed Intellectual Property and (iii) take all actions that Licensee reasonably deems necessary or advisable in order to record, or assist Licensee or any of its Affiliates in recording, with any relevant Governmental Entity, the assignment of the Designated Countries Licensed Intellectual Property to Licensee, so as to perfect Licensee’s or an Affiliate of Licensee’s ownership thereof (including authorizing Licensee or an Affiliate of Licensee to record the documents, or forms of the documents, contemplated by the foregoing clauses (i) and (ii) with any relevant Governmental Entity), in each case, in any country in the Territory. For the avoidance of doubt, notwithstanding any other provision of this Agreement or any other agreement,
document or instrument executed pursuant to this Section 3.07, Licensee and its Affiliates shall not assume or be liable for any liabilities, obligations or commitments of Licensor, the Additional Parties or any of their respective Affiliates, of any kind, whether express or implied, liquidated, absolute, accrued, contingent or otherwise, or known or unknown, existing on or occurring prior to the Designated Countries Acquisition Option Closing.
ARTICLE IV
Payment
SECTION 4.01. Upfront Fee. In consideration of the rights, licenses and releases granted by the Additional Parties and Licensor to Licensee pursuant to this Agreement, Designated Countries Licensee and U.S. Licensee, respectively, shall pay, or cause to be paid, to Licensor, by wire transfer of immediately available funds to the account designated in writing by Licensor, the Designated Countries Upfront Fee and the U.S. Upfront Fee, respectively, within five (5) Business Days after the Effective Date. The Parties acknowledge that the U.S. Upfront Fee and the Designated Countries Upfront Fee shall, once paid in full, be final and non-refundable.
SECTION 4.02. U.S. Royalty. If,
(a) the Interference Proceeding, which the Parties agree will be finally decided by exhausting, or failing to exhaust, any appeals to the Court of Appeals for the Federal Circuit without any petition for certiorari to the U.S. Supreme Court, results in Licensor obtaining a Relevant Patent in the United States, and
(b) the representations and warranties of Licensor set forth in Sections 7.01(a), 7.01(b) and 7.01(e)(i) and the representations of the Additional Parties set forth in Section 7.02 are true and correct in all material respects, and
(c) there is no Legal Restraint in effect, whether temporary or permanent, restraining, enjoining, preventing, prohibiting, revoking or otherwise making illegal or ineffective the grant of the Co-Exclusive U.S. License (during any time prior to the Exclusive U.S. License Effective Date) or the Exclusive U.S. License (during any time after the Exclusive U.S. License Effective Date), and
(d) the Aditech Addendum is in full force and effect.
(e) Each of Licensor and the Additional Parties have performed in all material respects all obligations required to be performed by it under Sections 2.01(b), 2.02, 2.03, 2.07, 2.08, 2.09 and 2.11;
then, U.S. Licensee shall pay Licensor royalties on Net Sales in the United States of (i) any product indicated for the treatment of multiple sclerosis that, but for the rights granted pursuant to this Agreement, would infringe a Relevant Patent arising out of the Interference Proceeding; and (ii) any product indicated for the treatment of multiple sclerosis having dimethyl fumarate as an API that, but for the rights granted pursuant to this Agreement, would infringe a Patent
included in the U.S. Licensed Intellectual Property (the foregoing (i) and (ii) collectively, a “U.S. Infringing Product”), according to the following terms (the royalty payments described in this Section 4.02, collectively, the “U.S. Royalty Consideration”):
(f) If U.S. Licensee is operating under the Exclusive U.S. License in accordance with Section 3.02, then (i) from January 1, 2021 to December 31, 2028, U.S. Licensee shall pay to Licensor a royalty of 10% on Net Sales in the United States of any U.S. Infringing Product; and (ii) from January 1, 2029 until the earlier of the expiration of the last to expire (or be invalidated by a final court ruling, from which no appeal can be taken or is timely taken) of the Infringed Claims included in the Patents included in the U.S. Licensed Intellectual Property, U.S. Licensee shall pay Licensor royalties of 20% on Net Sales in the United States of any U.S. Infringing Product; provided in the case of each of the foregoing clauses (i) and (ii) U.S. Licensee shall only be required to make any such payment if all conditions set forth in this Section 4.02 have been satisfied at all times throughout the calendar year prior to the time at which such payment is otherwise due and payable pursuant to the terms of this Section 4.02.
(g) If U.S. Licensee is operating under the Co-Exclusive U.S. License (and has not obtained the Exclusive U.S. License), then from January 1, 2023 until the earlier of the expiration of the last to expire (or be invalidated by a final court ruling, from which no appeal can be taken or is timely taken) of the Infringed Claims included in the Patents included in the U.S. Licensed Intellectual Property, U.S. Licensee shall pay to Licensor a 1% royalty on Net Sales in the United States of any U.S. Infringing Product; provided U.S. Licensee shall only be required to make any such payment if all conditions set forth in this Section 4.02 have been satisfied at all times throughout the calendar year prior to the time at which such payment is otherwise due and payable pursuant to the terms of this Section 4.02.
(h) Within 60 days after December 31 of each relevant year, Licensee shall submit a report to Licensor that sets forth, in reasonable detail, the calculation of Net Sales for such calendar year in the United States and the related U.S. Royalty Consideration owed by U.S. Licensee for such calendar year (such report, the “U.S. Statement”) and concurrently U.S. Licensee shall pay, or cause to be paid to, Licensor, in accordance with Section 4.04 of this Agreement, the amount of the U.S. Royalty Consideration owed to Licensor, pursuant to this Section 4.02 as set forth in such U.S. Statement.
(i) Notwithstanding anything to the contrary in this Section 4.02, (i) no U.S. Royalty Consideration shall be payable by U.S. Licensee with respect to Net Sales on any day on which any U.S. Generic Equivalent is offered for sale in the United States and (ii) in addition, if (A) there has been any U.S. Generic Equivalent offered for sale in the United States and (B) within two years following the last day of any such offer for sale of any U.S. Generic Equivalent, the average wholesale price of any branded U.S. Infringing Product, or product that is bioequivalent and therapeutically equivalent to and substitutable for a U.S. Infringing Product, sold, offered for sale or imported by Licensee or any other Person is 10% or more below the average wholesale price of Licensee’s U.S.
Infringing Products immediately prior to the offering for sale of such U.S. Generic Equivalent (the occurrence of the foregoing (A) and (B), a “U.S. Generic Entry Impact”), then U.S. Licensee will have no further obligation to pay any U.S. Royalty Consideration for the remainder of the U.S. Royalty Term. For the avoidance of doubt, for a year in which a U.S. Generic Entry Impact occurs, (x) U.S. Licensee will pay U.S. Royalty Consideration to Licensor with respect to the portion of such year prior to the date of such U.S. Generic Entry Impact and (y) no U.S. Royalty Consideration will be due with respect to any date thereafter.
SECTION 4.03. Worldwide (other than U.S.) Royalties. If,
(a) in the European Opposition Proceeding, Licensor obtains a Relevant Patent (in Swiss form or otherwise),
(b) the representations and warranties of Licensor set forth in Sections 7.01(a), 7.01(b) and 7.01(e)(i) and the representations of the Additional Parties set forth in Section 7.02 are true and correct in all material respects,
(c) there is no Legal Restraint in effect, whether temporary or permanent, restraining, enjoining, preventing, prohibiting, revoking or otherwise making illegal or ineffective the grant of the Exclusive Designated Countries License, on a country-by-country basis,
(d) The Aditech Addendum is in full force, and
(e) Each of Licensor and the Additional Parties have performed in all material respects all obligations required to be performed by it under Sections 2.01(b), 2.02, 2.03, 2.07, 2.08, 2.09 and 2.11;
then, Designated Countries Licensee shall pay Licensor royalties on Net Sales in each Designated Country of (i) any product indicated for the treatment of multiple sclerosis that, but for the rights granted pursuant to this Agreement, would infringe a Relevant Patent included in the Designated Countries Licensed Intellectual Property and (ii) any product indicated for the treatment of multiple sclerosis having dimethyl fumarate as an API that, but for the rights granted pursuant to this Agreement, would infringe a Patent included in the Designated Countries Licensed Intellectual Property (any products that fulfill the criteria in (i) or in (ii) or in both (i) and (ii) are referred to herein as a “Designated Country Infringing Product”), according to the following terms (the royalty payments described in this Section 4.03, collectively, the “Designated Countries Royalty Consideration”):
(f) (i) From January 1, 2021 to December 31, 2028, Designated Countries Licensee shall pay Licensor, on a country-by-country basis, royalties of 10% on Net Sales, due and payable in U.S. dollars, of any Designated Country Infringing Product in each Designated Country; and (ii) from January 1, 2029 until the expiration of the last to expire (or be invalidated by a final court ruling, from which no appeal can be taken or is timely taken) of the Infringed Claims included in the Patents included in the Designated Countries Licensed Intellectual Property, Designated Countries Licensee shall pay
Licensor, on a country-by-country basis, royalties of 20% on Net Sales, due and payable in U.S. dollars, of any Designated Country Infringing Product in each Designated Country; provided in the case of each of the foregoing clauses (i) and (ii) Designated Countries Licensee shall only be required to make any such payment if all conditions set forth in this Section 4.03(a)-(e) have been satisfied at all times throughout the calendar year prior to the time at which such payment is otherwise due and payable pursuant to the terms of this Section 4.03. For the avoidance of doubt, the condition set forth in Section 4.03(c) shall apply on a country by country basis and failure to satisfy such condition in an individual Designated Country shall not relieve Licensee of its obligation to pay royalties pursuant to this Section 4.03 in respect of other Designated Countries in which such condition has been satisfied.
(g) Within 60 days after December 31 of the relevant year, Licensee shall submit a report to Licensor that sets forth, in reasonable detail, the calculation of Net Sales on a country-by-country basis, converted to U.S. dollars calculated by converting the local currency to U.S. dollars using the average monthly foreign exchange rate for each applicable month used by Licensee for its external financial reporting, for such calendar year and the related Designated Countries Royalty Consideration owed by Designated Countries Licensee for such calendar year (such report, the “Designated Countries Statement”) and concurrently Designated Countries Licensee shall pay, or cause to be paid to, Licensor the amount of the Designated Countries Royalty Consideration owed to Licensor pursuant to this Section 4.03 in U.S. dollars as set forth in such Designated Countries Statement.
(h) Notwithstanding anything to the contrary in this Section 4.03, (i) no Designated Countries Royalty Consideration shall be payable by Designated Countries Licensee with respect to Net Sales of a Designated Country Infringing Product in a Designated Country on any day on which any Designated Country Generic Equivalent to such Designated Country Infringing Product is offered for sale in such Designated Country; and (ii) in addition, if (A) a Designated Country Generic Equivalent to a branded Designated Country Infringing Product is offered for sale in a Designated Country and (B) within two years following the last day of any such offer for sale of such Designated Country Generic Equivalent, the average wholesale price of such branded Designated Countries Infringing Product, or product that is bioequivalent and therapeutically equivalent to and substitutable for a Designated Countries Infringing Product, in such Designated Country, offered for sale by Licensee or any other Person, is 10% or more below the average wholesale price of such branded Designated Country Infringing Products in such Designated Country immediately prior to such last day (the occurrence of the foregoing (A) and (B), a “Designated Country Generic Entry Impact”), then Designated Countries Licensee will have no further obligation to pay any Designated Countries Royalty Consideration with respect to sales of such Designated Country Infringing Products in such Designated Country for the remainder of the Designated Countries Royalty Term. For the avoidance of doubt, for a year in which a Designated Country Generic Entry Impact occurs in respect of a Designated Country Infringing Product in a Designated Country, (x) Designated Countries Licensee will pay Designated Countries Royalty Consideration to Licensor with respect to the portion of
such year prior to the date of such Designated Country Generic Entry Impact, in such Designated Country and (y) no Designated Countries Royalty Consideration will be due with respect to any date thereafter in respect of such Designated Country Infringing Product in such Designated Country.
SECTION 4.04. Form of Payment. U.S. Licensee shall pay the U.S. Upfront Fee and the U.S. Royalty Consideration, and Designated Countries Licensee shall pay the Designated Countries Upfront Fee and the Designated Countries Royalty Consideration, in cash by wire transfer of immediately available funds (in U.S. dollars) to the account designated from time to time in writing by Licensor.
SECTION 4.05. Late Penalties. If U.S. Licensee or Designated Countries Licensee fails to pay Licensor within 15 Business Days of the relevant due dates set forth in this Article IV any amount otherwise due and payable to Licensor under this Agreement, U.S. Licensee or Designated Countries Licensee, as applicable, agrees to pay interest, calculated from the date such payment is due until such amount is paid in full, at the prime rate set by the Bank of America plus 1% per annum, or the maximum amount allowable by law, whichever is lower. U.S. Licensee or Designated Countries Licensee, as applicable, agrees to pay all reasonable, documented and out-of-pocket legal fees and costs incurred by Licensor in connection with Licensor’s collection efforts in the event U.S. Licensee or Designated Countries Licensee fails to make any payment under this Article IV that are due and payable and Licensor must take steps to collect the payments owed.
SECTION 4.06. Taxes.
(a) It is understood and agreed among the Parties hereto that any payments made by or for the benefit of Licensee or its Affiliates (or any assignee of Licensee) under this Agreement are exclusive of any value-added or similar tax (“VAT”) imposed upon such payments. Licensee represents that as of the date of payment of the Upfront Fee, Licensee will hold commercial licenses. Licensee shall promptly notify Licensor, if Licensee should cease to hold commercial licenses at any time prior to expiry of the Royalty Term. Licensor and Licensee agree that none of the payments under this Agreement are intended to be subject to VAT. Licensee and Licensor shall provide each other with any information and documentation reasonably requested to (i) mitigate the levying of any VAT on any payments made by or for the benefit of Licensee or its Affiliates (or any assignee of Licensee) under this Agreement and/or (ii) recover any VAT incurred on such payments.
(i) If any Party receives any claim or notice from a tax authority contending that a payment is or may be subject to VAT, then such Party shall promptly notify the other Party or Parties, as applicable. Licensor shall be entitled to control all audits or other proceedings in connection with such claim (provided that Licensee shall be entitled to participate at its own expense).
(ii) Licensor shall only be entitled to issue an invoice with the addition of VAT to Licensee when Licensor determines, based on a legal opinion received by Licensor and shared with Licensee, that Licensor is legally obliged to add VAT to its
invoice in order to ensure compliance with the requirements under the applicable VAT legislation, in which case Licensor may issue such invoice and Licensee shall pay, or cause to be paid to, Licensor the amount with the addition of VAT, which payment may, at Licensee’s discretion, be made in the form of a non-interest bearing promissory note (“the VAT Note”). Each Party shall take any commercially reasonable measures requested by another Party to recover any VAT incurred on payments under this Agreement in accordance with Section 45(1) of the Danish VAT Act. Licensor shall indemnify and hold Licensee harmless from (A) any VAT that cannot be recovered pursuant to the immediately preceding sentence (which indemnity, for the avoidance of doubt, may be settled in part or whole by offset against the corresponding VAT Note issued by Licensee) and (B) any reasonable expenses incurred by Licensee in recovering VAT; provided that no such obligation shall apply in respect of any VAT that cannot be recovered as a consequence of the Licensee not holding commercial licenses.
(b) In the event any payments made by or for the benefit of Licensee (or any assignee of Licensee) pursuant to this Agreement are or become subject to withholding taxes under applicable Law, the Person making such payment pursuant to this Agreement (the “Applicable Payer”) shall deduct and withhold the amount of such taxes to the extent required by applicable Law; amounts otherwise payable to Licensor pursuant to this Agreement shall be reduced by the amount of taxes deducted and withheld; the Applicable Payer shall pay the amounts of such taxes to the proper Governmental Entity in a timely manner and promptly transmit to Licensor an official tax certificate or other evidence of such tax obligations reasonably satisfactory to Licensor together with proof of payment reasonably satisfactory to Licensor of all amounts deducted and withheld sufficient to enable Licensor to claim such payment of taxes; and the amount of any such taxes so withheld or deducted shall be treated for all other purposes of this Agreement as if paid to Licensor. Any such withholding taxes required under applicable Law to be paid, deducted or withheld shall be an expense of, and borne solely by, Licensor. The Applicable Payer will provide Licensor with reasonable assistance, at Licensor’s expense to enable Licensor to recover such taxes as permitted by applicable Law. Should any payment required to be made to Licensor in accordance with the provisions of this Agreement be subject to withholding of any taxes by Licensee or its Affiliates or any assignee of Licensee, such Person shall (i) inform Licensor of such withholding requirement in advance of the first payment to be made by the Applicable Payer to Licensor hereunder, so as to allow Licensor to obtain and provide the Applicable Payer with an appropriate certificate of exemption, if available, and (ii) shall consult in good faith with Licensor prior to withholding any amounts and use commercially reasonable efforts to minimize any such withholding if a certificate of exemption is not available. No withholding shall be made to the extent an exemption from such withholding is timely obtained, and for as long as such exemption is valid. Notwithstanding anything to the contrary in this Agreement, provided (i) that Licensor provides the Applicable Payer with an executed Form W-8BEN-E claiming a complete exemption from U.S. withholding tax otherwise imposed on payments under this Agreement, including any VAT Note, under the U.S.-Denmark income tax treaty, the Applicable Payer shall not withhold U.S. tax from any payment under this Agreement or any VAT Note and (ii) no payment to Licensor under this Agreement shall be reduced by,
and Licensee, its Affiliates (and any assignee of Licensee) shall indemnify Licensor, its Affiliates (and any assignee of Licensor) and hold them harmless from, any taxes deducted or withheld which would not have been required to be deducted or withheld but for a sublicense or assignment by the U.S. Licensee or the Designated Countries Licensee of any or all of its rights under this Agreement or but for any other use of the Licensed Intellectual Property by a Person other than the Licensee. If any deduction or withholding described in clause (ii) of the prior sentence is required to be made under applicable Law, the payment otherwise due to Licensor under this agreement shall be increased as necessary so that after such deduction or withholding has been made (including such deductions and withholdings applicable to additional sums payable under this Section 4.06(b)) Licensor receives an amount equal to the sum it would have received had no such deduction or withholding been made. Upon the reasonable request of the Applicable Payer, Licensor shall update any Form W-8BEN-E or other form or certification previously delivered claiming an exemption from or reduction of withholding and, if such Form W-8BEN-E or any other such form or certification expires or becomes obsolete or inaccurate in any respect, or if any previously obtained exemption from withholding is otherwise no longer valid, Licensor shall promptly (and in any event within 10 days after such expiration, obsolescence, inaccuracy or invalidity) notify the Applicable Payor in writing of such expiration, obsolescence, inaccuracy or invalidity and, as applicable, update the form or certification if it is legally able to do so. Licensor shall indemnify and hold harmless any Applicable Payer from and against any taxes (together with any interest and penalties thereon) (I) that are U.S. withholding taxes due with respect to any such payments made pursuant to this Agreement or (II) that such Applicable Payer incurs as a result of the expiration, obsolescence or inaccuracy of any Form W-8BEN-E or other form, certification or documentation delivered by Licensor claiming an exemption from or reduction of withholding.
SECTION 4.07. Audit Rights. Licensee shall, and shall cause each of its controlled Affiliates to, from January 1, 2021 until the earlier of (a) the expiration of the last to expire of the Patents included in the Licensed Intellectual Property, or (b) a final court ruling, from which no appeal can be taken or from which no appeal is timely taken, that all otherwise extant claims of Patents included in the Licensed Intellectual Property are unenforceable or otherwise invalid in each jurisdiction in the Territory, keep and maintain books and records in accordance with its standard accounting procedures and in sufficient detail to verify the amount of any Royalty Consideration payable under this Agreement during such period. During the 45-day period following the later of (i) Licensee’s delivery of a U.S. Statement or Designated Countries Statement to Licensor pursuant to Section 4.02(h) or Section 4.03(g) and (ii) the mutual selection by Licensor and Licensee of an independent accounting firm as contemplated below, Licensor shall have the right on a reasonable period of notice to have an independent accounting firm that is mutually selected by Licensor and Licensee (the “Auditor”) examine such books and records of Licensee and its controlled Affiliates, in each case to the extent such books and records relate to the calculation of Net Sales and the related U.S. Royalty Consideration and Designated Countries Royalty Consideration for the preceding year set forth on such U.S. Statement or Designated Countries Statement, respectively, and solely for the purpose of verifying the related U.S. Royalty Consideration and Designated Countries Royalty Consideration set forth on such U.S. Statement or Designated Countries Statement and that the
Net Sales set forth on such U.S. Statement or Designated Countries Statement were calculated in accordance with the terms of this Agreement. For the avoidance of doubt, pursuant to this Section 4.07, the Auditor shall act as an expert and not an arbitrator and, in any event, shall not substitute its own accounting judgment for that of Licensee when auditing the accuracy of the calculation of Net Sales and related U.S. Royalty Consideration and Designated Countries Royalty Consideration set forth on a U.S. Statement or Designated Countries Statement. The Auditor may not be paid on a contingency or other basis related to the outcome of the audit, and shall execute a confidentiality agreement with Licensee and its Affiliates in a form reasonably satisfactory to Licensee that prohibits the Auditor from disclosing or using information obtained in connection with the audit (other than the disclosure to Licensor of the results and conclusions of such audit). Any such audit shall be conducted during normal business hours, in such a manner as not to materially interfere with the normal business activities, of Licensee and its Affiliates, and shall be at Licensor’s expense; provided, however, if such audit reveals an underpayment of more than 5% during the audited period, Licensee shall pay, or cause to be paid, all reasonable costs of the Auditor, but shall not be obligated to pay the fees, costs or expenses of any other Person in connection with such audit. Prompt adjustment and payment shall be made to correct for any underpayment or overpayment revealed by any such audit.
ARTICLE V
Maintenance, Prosecution and Litigation
SECTION 5.01. Submitting Agreement to PTAB. Immediately following the Effective Date, pursuant to 35 U.S.C. § 135(c) and 37 C.F.R. § 41.205, before termination of the Interference Proceeding, Licensor and Licensee agree to file, and/or to cause their relevant controlled Affiliates to file, a Joint Submission of Agreement in substantially the same form as that provided in Appendix E, or as otherwise directed by the PTAB, with the intention of providing a copy of this Agreement and all related agreements to be kept separate from the Interference file.
SECTION 5.02. IP Advisory Committee. Immediately following the Effective Date, Licensor and Licensee shall create an intellectual property advisory committee (the “IP Advisory Committee”) comprised of one or more individuals designated by Licensor and one or more individuals designated by Licensee, which IP Advisory Committee shall remain in effect from the Effective Date until (a) if the Exclusive U.S. License becomes effective, the later of the conclusion of the European Opposition Proceeding and the Exclusive U.S. License Effective Date or (b), if the Exclusive U.S. License does not become effective, the expiry of the last item to expire (or be invalidated in its entirety by a final court ruling, from which no appeal can be taken or is timely taken) of the Licensed Patents. The IP Advisory Committee shall cooperate and meet at regular intervals as agreed by Licensor and Licensee to discuss strategy and actions with respect to the filing, maintenance, prosecution and defense of the Licensed Intellectual Property and any Litigation related to the Licensed Intellectual Property (other than as set forth in this Section 5.02. Each of the Parties shall, and shall cause each of their respective controlled Affiliates to, take reasonable steps to make available to the members of the IP Advisory Committee documents reasonably related to, and keep the members of the IP Advisory Committee informed of, all maintenance, prosecution and defense activities related to the
Licensed Intellectual Property, any Litigation related to the Licensed Intellectual Property and any other correspondence involving such maintenance, prosecution, defense and Litigation; provided that in no event shall Licensor be required to provide any information that is subject to attorney-client privilege, or work product immunity, which privilege or immunity would reasonably be expected to be lost or reduced by disclosure to Licensee, or any Confidential Intellectual Property Information, in either case that is related to (i) the negotiation of this Agreement or any enforcement hereof or disputes hereunder (including, establishing that a product is an Infringing Product), (ii) the Interference Proceeding or (iii) the European Opposition Proceeding, in each case prior to the conclusion of such matters; except that in each case Licensor shall, and shall cause each of its controlled Affiliates to, use its commercially reasonable efforts to provide the applicable information in a way, if any, that would not reasonably be expected to violate such privilege, as applicable, or materially adversely affect Licensor in the Interference Proceeding or the European Opposition Proceeding, as applicable. As applicable, no Party shall take, or omit to take (and each Party shall cause each of its controlled Affiliates to not take or omit to take), any material action with respect to the filing, maintenance, prosecution or defense of the Licensed Intellectual Property (or any Litigation related to the Licensed Intellectual Property) without first consulting with and giving reasonable good faith consideration to the viewpoints of the IP Advisory Committee and its members. For the avoidance of doubt, (A) nothing contained in this Section 5.02 shall give Licensee or any of its Affiliates the right to direct or control the business operations of Licensor or any of the Additional Parties and (B) nothing contained in this Section 5.02 shall give any Party the right to information belonging to any other Party or its respective Affiliates related to the Interference Proceeding or the European Opposition Proceeding.
SECTION 5.03. Licensor Maintenance, Prosecution and Litigation. Except with respect to all Designated Countries Licensed Intellectual Property (other than Licensor’s European patent EP 2801355), effective from the Effective Date until the earlier of (a) the end of the Royalty Term or (b) (i) with respect to the U.S. Licensed Intellectual Property, the Exclusive U.S. License Effective Date and (ii) with respect to Licensor’s European patent EP 2801355 (Application No. 14172398.1), the date on which the European Opposition Proceeding has reached a final, unappealable conclusion:
(a) Costs and Expenses. Each of Licensor and the Additional Parties, if any, owning any Licensed Intellectual Property shall, and shall cause each of their respective controlled Affiliates to, at its or their sole cost and expense, take all reasonable measures to diligently file, prosecute and maintain the respective Licensed Patents. Each of Licensor and the Additional Parties, if any, owning any Licensed Intellectual Property shall, and shall cause each of their respective controlled Affiliates to, use commercially reasonable efforts not to decline to file, prosecute or maintain any Licensed Patents, elect to allow any Licensed Patents to lapse, or elect to terminate, abandon or otherwise impair any Licensed Patents, in each case without the prior written consent of Licensee, and Licensee shall have the right to assume the prosecution and/or maintenance of such Licensed Patents.
(b) Litigation. The Parties shall notify each other promptly in writing if any infringement or potential infringement of the Licensed Intellectual Property by a Third Party is observed or suspected by the Parties or any of their controlled Affiliates.
(i) Licensor shall have the initial right, but not the obligation, using counsel of its choice at its own cost, to enforce the Licensed Intellectual Property or defend any challenge with respect thereto. Licensor shall have sole control of any decisions or other aspects of any such Litigation. To the extent reasonably practicable, Licensor shall, and shall cause, where relevant, each of its controlled Affiliates to, keep Licensee informed of the status of, and shall consult with Licensee with respect to, any such Litigation (excepting the Interference Proceeding or the European Opposition Proceeding), including, for the avoidance of doubt, any defense, settlement, adjustment or compromise of any such Litigation. Upon request by Licensor, Licensee shall give to Licensor such reasonable assistance in the Litigation as Licensor may reasonably request, including by signing or executing any necessary documents and consenting to it being named as a party to the proceedings. Any and all recoveries from any such Litigation shall be solely and entirely for Licensor’s account.
(ii) If Licensor does not exercise its right to institute any such action, Licensor shall, and shall cause each of its controlled Affiliates to timely provide Licensee with Notice such that Licensee may, at its sole option and discretion, and at Licensee’s expense, enforce the Licensed Intellectual Property or defend against any challenge with respect thereto. In such case, (A) Licensee shall have sole control of any decisions or other aspects of any such Litigation, (B) to the extent reasonably practicable, Licensee shall keep Licensor informed of the status of, and shall consult with Licensor with respect to, any such Litigation, including, for the avoidance of doubt, any defense, settlement, adjustment or compromise of any such Litigation, (C) upon request by Licensee, Licensor shall, and shall cause each of its controlled Affiliates to, give to Licensee such reasonable assistance in the Litigation as Licensee may reasonably request, including by signing or executing any necessary documents and consenting to it being named as a party to the proceedings and (D) any and all recoveries from any such Litigation shall be solely and entirely for Licensee’s account.
(c) ANDA Litigation. Notwithstanding any provision of this Article V to the contrary, Licensee shall, at all times from and after the Effective Date, have sole control over, and shall have full authority to defend, litigate and control (at its own cost and expense, including any attorneys’ fees), and shall have no obligation to consult with the IP Advisory Committee with respect to, any ANDA-related challenges such as, for example, a challenge pursuant to 21 U.S.C. § 355(j)(2)(A)(vii)(IV) or any challenge instituted in or by the USPTO, or any similar Litigation or challenges anywhere in the Territory, to any (i) product of Licensee or any of its Affiliates (including Tecfidera) or (ii) Licensed Intellectual Property.
SECTION 5.04. Licensee Maintenance, Prosecution and Litigation. Effective with respect to (i) the Designated Countries Licensed Intellectual Property (other than Licensor’s European patent EP 2801355), as of the Effective Date, (ii) the U.S. Licensed Intellectual
Property, as of the Exclusive U.S. License Effective Date and (iii) Licensor’s European patent EP 2801355 (Application No. 14172398.1), as of the date on which the European Opposition Proceeding has reached a final, unappealable conclusion:
(a) Licensor Obligations. Each of Licensor and the Additional Parties shall provide, and shall cause each of their respective controlled Affiliates to provide, at Licensee’s cost and expense, any authorizations and powers of attorney as requested by Licensee in order for Licensee to take the actions contemplated by this Section 5.04(a), including to maintain the Licensed Patents, prosecute any applications included therein for registration and to opt-out from the exclusive competence of the European Unified Patent Court and to withdraw from any such opt-out.
(b) Licensee Prosecution Obligations. Licensee shall, at its sole cost and expense, take all reasonable measures to diligently file, prosecute and maintain the respective Licensed Intellectual Property and shall use commercially reasonable efforts not to decline to file, prosecute or maintain any Licensed Intellectual Property, elect to allow any Licensed Intellectual Property to lapse, or elect to terminate, abandon or otherwise impair any Licensed Intellectual Property, in each case without providing adequate advance notice to Licensor, and Licensor shall have the right to assume the prosecution and/or maintenance of such Licensed Intellectual Property.
(c) Litigation.
(i) Each of Licensor and the Additional Parties acknowledge and consent to Licensee’s sole right to institute Litigation under the applicable Licensed Intellectual Property, including the right to damages, equitable relief, and to settle without consent, royalty or consideration of any kind to Licensor, the Additional Parties or any of their respective Affiliates; provided that all costs and expenses associated with any of the foregoing activities will be paid by Licensee;
(ii) Each of Licensor and the Additional Parties shall, and shall cause each of their respective controlled Affiliates to, notify Licensee promptly in writing if any infringement or potential infringement of the Licensed Intellectual Property by a Third Party is observed or suspected by Licensor, the Additional Parties or any of their respective controlled Affiliates, whereupon (A) Licensee may, in its own sole discretion and at its own expense, institute Litigation against any infringer or alleged infringer and control and defend such Litigation and recover any damages, awards or settlements resulting therefrom; (B) Licensee shall have sole control over any such Litigation including any defense, settlement, adjustment or compromise of any such Litigation; and (C) any and all recoveries from any such Litigation shall be solely and entirely for Licensee’s account; and (D) if required by a Governmental Entity, applicable Law or Order to permit Licensee or any of its Affiliates to commence, pursue or defend any Litigation related to the Licensed Intellectual Property, each of Licensor and the Additional Parties shall, and shall cause each of their respective controlled Affiliates to, join as a party to any such Litigation if such joinder is reasonably necessary to advance Licensee’s position.
(iii) If Licensee does not exercise its right to institute any such action, Licensee shall timely provide Licensor and the Additional Parties with Notice such that Licensor and the Additional Parties may, at their sole option, discretion, and expense, enforce the Licensed Intellectual Property or defend against any challenge with respect thereto. Upon request by Licensor, Licensee shall give to Licensor such reasonable assistance in the Litigation as Licensor may reasonably request, including by signing or executing any necessary documents and consenting to being named as a party to the proceedings. In such case, (A) Licensor and the Additional Parties may, in their own sole discretion and at their own expense, institute Litigation against any infringer or alleged infringer and control and defend such Litigation and recover any damages, awards or settlements resulting therefrom; (B) Licensor and the Additional Parties shall have sole control over any such Litigation including any defense, settlement, adjustment or compromise of any such Litigation; and (C) any and all recoveries from any such Litigation shall be solely and entirely for Licensor’s and the Additional Parties’ account; and (D) if required by a Governmental Entity, applicable Law or Order to permit Licensor, the Additional Parties or any of their respective Subsidiaries to commence, pursue or defend any Litigation related to the Licensed Intellectual Property, Licensee shall, and shall cause each of its controlled Affiliates to, join as a party to any such Litigation if such joinder is reasonably necessary to advance Licensor’s and the Additional Parties’ position.
(d) Notwithstanding anything to the contrary in Section 5.03, if at any time prior to the Exclusive U.S. License Effective Date in respect of the U.S. Licensed Intellectual Property, or prior to the date on which the European Opposition Proceeding has reached a final, unappealable conclusion in respect of Licensor’s European patent EP 2801355 (Application No. 14172398.1), Licensor or any of the Additional Parties or any of their respective Affiliates has failed to, or notified Licensee that it does not intend to, diligently file, prosecute, and maintain the Licensed Intellectual Property and defend and pursue all Litigation against any infringer or alleged infringer of such Licensed Intellectual Property using its reasonable best efforts, Licensee shall have the right, but not the obligation, to file, prosecute, and maintain the Licensed Intellectual Property, and to defend and pursue Litigation, in which event, the foregoing Sections 5.04(a)-5.04(c) shall control.
(e) Notwithstanding Sections 5.04(a) and (b), if, at any time, Licensee has failed to, or has notified Licensor and the Additional Parties that it does not intend to, diligently file, prosecute, and maintain the Licensed Intellectual Property using its reasonable best efforts, Licensor and the Additional Parties shall have the right, but not the obligation, to, at Licensor’s and the Additional Parties’ sole cost and expense, file, prosecute and maintain the Licensed Intellectual Property upon reasonable notice to Licensee.
ARTICLE VI
Conditions Precedent
SECTION 6.01. Conditions for the Benefit of Each Party. The effectiveness of the Exclusive U.S. License is subject to the satisfaction or waiver (by each of the Parties) on or prior to the Exclusive U.S. License Effective Date of the following conditions:
(a) Governmental Approvals. Any waiting period under the HSR Act (including any extension thereof) applicable to the grant of the Exclusive U.S. License shall have expired or been earlier terminated and the authorizations, consents, orders or approvals of, or declarations or filings with, any Governmental Entity required by applicable Law, shall have occurred or been obtained (in each case, without the imposition of a Burdensome Condition).
(b) No Injunctions or Legal Restraints. No restraining order or injunction or other Order issued by any Governmental Entity of competent jurisdiction or Law or other legal restraint or prohibition (collectively, “Legal Restraints”), whether temporary or permanent, restraining, enjoining, preventing, prohibiting or otherwise making illegal or ineffective the grant of the Exclusive U.S. License shall be in effect.
SECTION 6.02. Frustration of Conditions to Effectiveness. None of Licensor, the Additional Parties or Licensee may rely on the failure of any condition set forth in this Article VI to be satisfied if such failure was caused by such Party’s failure to comply with the terms of this Agreement.
ARTICLE VII
Representations and Warranties
SECTION 7.01. Representations and Warranties Regarding Licensor. Licensor represents and warrants to Licensee as of the Effective Date:
(a) Organization, Standing and Corporate Power. Licensor is a Danish limited liability company duly organized and validly existing under the Laws of Denmark. Licensor has the requisite power and authority to execute, deliver and perform its obligations under this Agreement and to consummate the Transactions.
(b) Authority; Noncontravention; Voting Requirements. (i) Subject to the receipt of the Licensor Shareholder Approval, Licensor has all necessary corporate power and corporate authority to execute and deliver this Agreement and the Aditech Addendum and to perform its obligations hereunder and thereunder and to consummate the Transactions and the transactions contemplated by the Aditech Addendum. As of the Effective Date, this Agreement and the Aditech Addendum have been duly authorized, executed and delivered by Licensor and constitute legal, valid and binding agreement of Licensor, enforceable in accordance with their terms, except to the extent that enforcement hereof or thereof may be limited by bankruptcy, insolvency, fraudulent
conveyance, reorganization, moratorium or other Laws affecting enforcement of creditors’ rights or by general equitable principles. Except for obtaining the Licensor Shareholder Approval, no other corporate or shareholder action on the part of Licensor is necessary to authorize the execution, delivery and performance by Licensor (including any approval or action by the Board of Directors of Licensor and of this Agreement or the Aditech Addendum and the consummation by it of the Transactions or the transactions contemplated by the Aditech Addendum.
(ii) At a meeting of the Board of Directors of Licensor duly called and held (A) the disinterested members of the Board of Directors of Licensor, which do not form a quorum, declared in the best interests of Licensor and its shareholders, and therefore recommended the holders of Licensor Ordinary Shares approve the Transactions and the transactions contemplated by the Aditech Addendum and the execution, delivery and performance by Licensor of this Agreement and the Aditech Addendum and the consummation of the Transactions and the transactions contemplated by the Aditech Addendum, and (B) the Board of Directors of Licensor resolved to refer and submit the approval of this Agreement, the Aditech Addendum and the Transactions to a vote at a Licensor Shareholders’ Meeting in accordance with the terms of this Agreement. The Board of Directors of Licensor has taken all necessary actions in accordance with applicable Law and the Licensor Articles to duly call and give notice (such notice, a “Notice of Meeting”) of a meeting of holders of its Licensor Ordinary Shares for the purposes of obtaining Licensor Shareholder Approval.
(iii) As of the Effective Date, at a duly called and convened meeting of Licensor’s holders of its Ordinary Shares (the “Licensor Shareholders’ Meeting”), holders of at least two-thirds of the outstanding Licensor Ordinary Shares entitled to vote thereon, voting together as a single class, affirmatively voted (in person or by proxy) to approve the Transactions and the execution and delivery and performance by Licensor of this Agreement and the Aditech Addendum and the consummation of the Transactions and the transactions contemplated by the Aditech Addendum (the “Licensor Shareholder Approval”), and such Licensor Shareholder Approval, is the only vote of the holders of any class or series of capital stock of Licensor necessary to adopt this Agreement, the Aditech Addendum and approve and consummate the Transactions and the transactions contemplated by the Aditech Addendum.
(iv) The execution, delivery and performance by Licensor of this Agreement and the Aditech Addendum and the consummation of the Transactions and the transactions contemplated by the Aditech Addendum (in each case, alone or in combination with any other event) will not (A) conflict with or result in a breach or violation of any of the terms or provisions of, or constitute a default under any Contract to which Licensor or any of its Affiliates is a party or by which Licensor or any of its Affiliates is bound or to which any of the property or assets of Licensor or any of its Affiliates is subject, (B) impair or impose a Lien (other than the restrictions set forth in this Agreement or a Permitted Lien) on any of the Licensed Intellectual Property, (C) assuming the receipt of Licensor Shareholder Approval, violate any provision of the organizational documents of Licensor or any of its Affiliates, (D) violate any Law or
judgment, order, writ, injunction, legally binding agreement with a Governmental Entity, stipulation or decree, including any binding decree of any arbitrator (each, an “Order”) applicable to Licensor or any of its Affiliates or any of their respective properties, or (E) grant any rights of appraisal to any holder of Licensor Ordinary Shares, except, in the case of clauses (A) and (D), as would not reasonably be expected to impair in any material respect the ability of Licensor or any of its Affiliates to perform their obligations under this Agreement or the Aditech Addendum or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions and the transactions contemplated by the Aditech Addendum; and no filing with or Consent, approval, authorization, Order, registration or qualification of or with any Governmental Entity is required for the execution, delivery and performance by Licensor of its obligations under this Agreement or the Aditech Addendum, except, (i) in the case of this Agreement, for the filing of a notification and report by Licensor under the HSR Act and where the failure to obtain or make any such filing, Consent, approval, authorization, Order, registration or qualification would not reasonably be expected to impair in any material respect the ability of Licensor to perform its obligations under this Agreement or the Aditech Addendum or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions or the transactions contemplated by the Aditech Addendum.
(c) Shareholder Meeting Materials. As of the Effective Date and the time it or any amendment or supplement thereto was first published, sent or given to the shareholders of Licensor, or at the time of the Licensor Shareholders’ Meeting , the Shareholder Meeting Materials (including any amendment or supplement thereto) did not contain any untrue statement of a material fact or omit to state any material fact required to be stated therein or necessary in order to make the statements therein, in light of the circumstances under which they are made, not misleading. As of the same times, the Shareholder Meeting Materials complied as to form in all material respects with applicable Law. Notwithstanding the foregoing, Licensor makes no representation or warranty with respect to statements made or incorporated by reference therein based on information supplied by or on behalf of Licensee or any Affiliates thereof for inclusion or incorporation by reference in the Shareholder Meeting Materials.
(d) Legal Proceedings. Excepting the Litigation involving (i) the Interference; (ii) Licensor’s European patent EP 2 801 355 (Application No. 20140172398); (iii) Licensor’s German Utility Model DE202005002112U1; (iv) the European Opposition Proceeding; (v) Appeal T 1537/16-3.3.07 regarding the opposition to Licensor’s European patent EP 2 379 063 (Application No. 10 700 730.4) at the European Patent Office; and (vi) Appeal T 1490/15-3.3.07 regarding the opposition to Licensor’s European patent EP 2 316 430 (Application No. 10 182 198.1) at the European Patent Office, as of the Agreement Date there is no Litigation pending or, to the knowledge of Licensor, threatened in writing, before or by any Governmental Entity against Licensor or any of its Affiliates that involves or that would reasonably be expected to involve the Licensed Intellectual Property or that, individually or in the aggregate, would reasonably be expected to impair in any material respect the ability of Licensor or any of its controlled Affiliates to perform their obligations under this
Agreement or the Aditech Addendum or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions or any of the transactions contemplated by the Aditech Addendum, nor are there any Orders outstanding against Licensor or any of its Affiliates that involve or that would reasonably be expected to involve the Licensed Intellectual Property or that, individually or in the aggregate, would reasonably be expected to impair in any material respect the ability of Licensor or any of its controlled Affiliates to perform their obligations under this Agreement or the Aditech Addendum or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions or any of the transactions contemplated by the Aditech Addendum.
(e) Intellectual Property.
(i) As of the Agreement Date, Licensor or a wholly-owned Subsidiary of Licensor (1) owns or controls, or has the right to grant a license in, to or under the Licensed Intellectual Property, free and clear of all Liens (other than Permitted Liens) including licenses granted in, to or under the Licensed Intellectual Property and (2) possesses all rights necessary to grant the licenses contemplated by this Agreement under the Licensed Intellectual Property, except (w) for any adverse effect the Interference Proceeding, the European Opposition Proceeding, the Litigation involving European patent EP 2 801 355 (Application No. 20140172398) and German Utility Model DE202005002112U1, Appeal T 1537/16-3.3.07 regarding the opposition to Licensor’s European patent EP 2 379 063 (Application No. 10 700 730.4) at the European Patent Office, and Appeal T 1490/15-3.3.07 regarding the opposition to Licensor’s European patent EP 2 316 430 (Application No. 10 182 198.1) at the European Patent Office or the terms of this Agreement have on the ownership, control or ability to license the Licensed Intellectual Property and (x) with respect to the Licensed Intellectual Property other than the Patents included in the Licensed Intellectual Property, as would not be expected to have a material effect on Licensor, Licensee, any of their respective Affiliates or the Transactions; provided that Licensor does not represent or warrant that Licensed Intellectual Property which is licensed by a Third Party to Licensor or a wholly-owned Subsidiary of Licensor is free and clear of all Liens.
(ii) Other than the Additional Parties, none of Licensor’s Affiliates, has or has had at any time in the twelve (12) months prior to the Agreement Date, any right, title or interest in, to or under any Intellectual Property relating to the use of a fumaric acid ester to treat multiple sclerosis.
(iii) As of the Agreement Date, there is no Litigation (A) pending or threatened by Licensor, Aditech or any Affiliate of Licensor having (at any time) any right in any of the Licensed Intellectual Property to enforce the Licensed Intellectual Property or (B) pending, threatened in writing or, to the knowledge of Licensor, threatened orally or otherwise asserted, that challenges or contests the legality, validity, enforceability, registrability, alienability, use or ownership of any of the Licensed Intellectual Property, in each of (A) and (B), (x) except the Interference Proceeding, Appeal T 1773/16-3.3.02 regarding the Opposition against Licensee’s European patent
EP 2 137 537 (Application No. 8 725 256.5) at the European Patent Office, the Litigation involving Licensor’s European patent EP 2 801 355 (Application No. 20140172398) and the Litigation involving Licensor’s German Utility Model DE202005002112U1, Appeal T 1537/16-3.3.07 regarding the opposition to Licensor’s European patent EP 2 379 063 (Application No. 10 700 730.4) at the European Patent Office and Appeal T 1490/15-3.3.07 regarding the opposition to Licensor’s European patent EP 2 316 430 (Application No. 10 182 198.1) at the European Patent Office and (y) except, with respect to the Licensed Intellectual Property other than the Patents included in the Licensed Intellectual Property, the Licensor shall not be in breach of (A) and/or (B) above if the non compliance with (A) and/or (B) above does not have a material effect on Licensee, or any of its respective Affiliates or the Transactions.
(iv) (A) Licensor and each of its Affiliates that holds or has held any Licensed Intellectual Property have taken all commercially reasonable steps to obtain, maintain and protect the extant Patents of the Licensed Intellectual Property in the U.S. and Europe, except as would not have a material effect on Licensee and (B) excepting those agreements listed in Appendix B, Licensor has not, since December 31, 2015 transferred, sold, conveyed or assigned any Intellectual Property to any other Person.
(v) All Patents included in the Licensed Intellectual Property are lawfully held in Denmark. For avoidance of doubt, Patents are deemed to be lawfully held in Denmark where such Patents are owned by an entity organized under the laws of Denmark and having a principal place of business in Denmark.
(vi) Licensor does not own or hold any rights in, to or under any trademarks, service marks, trade dress, logos, trade names, corporate names or Internet domain names except (A) the trademark rights in “FP-187” licensed to Licensee hereunder, including those trademark rights listed in Appendix A and (B) any such rights including or containing the words “Forward Pharma”.
(vii) Licensor provides no warranty, express or implied, that any Patents of the Licensed Intellectual Property will issue after the Agreement Date or that any granted Patent which has issued or will issue from the Licensed Intellectual Property is or will be valid and enforceable, or that, after the date of this Agreement, the sale or distribution of any Licensed Product or Infringing Product will not infringe the patent or other proprietary rights of any Third Party.
(viii) The licenses and other rights granted in this Agreement do not (A) materially conflict with any Contract to which Licensor, or any of its Affiliates having any right in any of the Licensed Intellectual Property, is subject or (B) create or bring into effect any material rights involving any Third Party.
(ix) Licensor and each of its Affiliates having, on or at any time within the three years preceding the Agreement Date, any right in any of the Licensed Intellectual Property, are in material compliance with, and have at all times on and during the three years preceding the Agreement Date, been in material compliance with, the terms of all
third-party licenses and other obligations related to or included in the Licensed Intellectual Property.
(f) Shareholders’ Register. Licensor has delivered to Licensee an updated copy of its shareholders’ register which bears a notation indicating that the Specified Shareholders’ Licensor Ordinary Shares are subject to the provisions and restrictions of the Shareholders Commitment Agreement.
(g) Employment Matters. No current or former employee, director or other service provider of Licensor or any of its Affiliates other than those set forth on Appendix H, is or shall be entitled to employment with, or any compensation or benefit from, Licensee or any of its Affiliates as a result of the consummation of the Transactions (alone or in combination with any other event).
(h) Legal Matters. Licensor has delivered, or caused to be delivered to Licensee, the opinion of Danish counsel stating that Licensor, subject to the receipt of the Licensor Shareholder Approval, has the requisite power and authority to execute, deliver and perform its obligations under this Agreement.
(i) Finders or Brokers; Fees. No agent, broker, investment banker or other firm or Person is or will be entitled to any broker’s or finder’s fee or any other commission or similar fee in connection with any of the Transactions as a result of any action taken by Licensor or any Additional Party.
(j) Aditech Addendum. As of the Agreement Date, Licensor has made available to Licensee a true, complete and correct copy of the Patent Transfer Agreement and, as of the Effective Date, the Aditech Addendum between Licensor, on the one hand, and Aditech, on the other hand, and true, complete and correct copies of all other material Contracts, if any, between such parties and any of their respective Affiliates relating to the Licensed Intellectual Property.
(k) Documents Provided. As of the Agreement Date, Licensor has made available to Licensee true, complete and correct copies of (i) all material Contracts of Licensor or its Affiliates related to the Licensed Intellectual Property, including all licenses and other agreements, arrangements or understandings granting any rights or options in, to or under any items included in the Licensed Intellectual Property, (ii) all material nonpublic assignment documents; (iii) schedules demonstrating that any annuity fees, maintenance fees and the like that have become due prior to the Agreement Date have been timely paid for all Patents extant as of the Agreement Date and included in the Licensed Intellectual Property; (iv) references relating to all Patents included in the Licensed Intellectual Property; (v) any judgments relating to the Licensed Intellectual Property and any material documentation regarding any litigation, oppositions, interferences or any other adversarial proceedings related to the Licensed Intellectual Property or to third party intellectual property, as filed or otherwise asserted by or against Licensor, excluding the Interference Proceeding, the European Opposition Proceeding, Appeal T 1773/16-3.3.02 regarding the Opposition against Licensee’s European patent EP 2 137 537 (Application No. 8 725 256.5) at the European Patent Office, the Litigation
involving Licensor’s European patent EP 2 801 355 (Application No. 20140172398) and the Litigation involving Licensor’s German Utility Model DE202005002112U1, Appeal T 1537/16-3.3.07 regarding the opposition to Licensor’s European patent EP 2 379 063 (Application No. 10 700 730.4) at the European Patent Office and Appeal T 1490/15-3.3.07 regarding the opposition to Licensor’s European patent EP 2 316 430 (Application No. 10 182 198.1) at the European Patent Office and (vi) any assertions or claims of ownership, inventorship, or rights to practice any Patent included in the Licensed Intellectual Property by any party (other than Licensee and their Affiliates) not identified in the applicable Patent application as an owner or inventor.
(l) Maintenance Fees. Licensor has timely paid all maintenance fees and annuities related to the Patents of the Licensed Intellectual Property extant as of the Agreement Date.
SECTION 7.02. Representations and Warranties Regarding the Additional Parties. Each of the Additional Parties represents and warrants to Licensee as the Effective Date:
(a) Organization, Standing and Corporate Power. Such Additional Party is duly organized and validly existing. Such Additional Party has the requisite power and authority to execute, deliver and perform its obligations under this Agreement and to consummate the Transactions.
(b) Authority. As of the Effective Date, this Agreement has been duly authorized, executed and delivered by the applicable Additional Party and constitutes a legal, valid and binding agreement of the applicable Additional Party, enforceable in accordance with its terms, except to the extent that enforcement hereof may be limited by bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium or other Laws affecting enforcement of creditors’ rights or by general equitable principles.
(c) Noncontravention. The execution, delivery and performance by such Additional Parties of this Agreement and the consummation of the Transactions (alone or in combination with any other event) will not (i) conflict with or result in a breach or violation of any of the terms or provisions of, or constitute a default under any Contract to which such Additional Party or any of its Affiliates is a party or by which such Additional Party or any of its Affiliates is bound or to which any of the property or assets of such Additional Party or any of its Affiliates is subject, (ii) violate any provision of the organizational documents of such Additional Party or any of its Affiliates or (iii) violate any Law or Order applicable to such Additional Party or any of its Affiliates or their respective properties, except, in the case of clauses (i) and (iii), as would not reasonably be expected to impair in any material respect the ability of such Additional Party to perform its obligations under this Agreement or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions; and no filing with or Consent, approval, authorization, Order, registration or qualification of or with any Governmental Entity, is required for the execution, delivery and performance by such Additional Party of its obligations under this Agreement, except where the failure to obtain or make any such filing, Consent, approval, authorization, Order, registration or qualification would not reasonably be expected to impair in any material respect the
ability of such Additional Party to perform its obligations under this Agreement or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions.
SECTION 7.03. Representations and Warranties Regarding Licensee. Licensee represents and warrants to Licensor as of the Agreement Date and the Effective Date:
(a) Organization, Standing and Corporate Power. U.S. Licensee is a limited liability company duly organized, validly existing and in good standing under the Laws of Switzerland and Designated Countries Licensee is a limited company duly organized, validly existing and in good standing under the Laws of Bermuda. U.S. Licensee and Designated Countries Licensee have the requisite power and authority to execute, deliver and perform its obligations under this Agreement and the consummation of the Transactions.
(b) Authority. As of the Effective Date, this Agreement has been duly authorized, executed and delivered by Licensee and constitutes a legal, valid and binding agreement of Licensee, enforceable in accordance with its terms, except to the extent that enforcement hereof may be limited by bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium or other Laws affecting enforcement of creditors’ rights or by general equitable principles.
(c) Noncontravention. The execution, delivery and performance by Licensee of this Agreement and the consummation of the Transactions (alone or in combination with any other event) will not (i) conflict with or result in a breach or violation of any of the terms or provisions of, or constitute a default under any Contract to which Licensee or any of its Affiliates is a party or by which Licensee or any of its Affiliates is bound or to which any of the property or assets of Licensee or any of its Affiliates is subject, (ii) violate any provision of the organizational documents of Licensee or any of its Affiliates or (iii) violate any Law or Order applicable to Licensee or any of its Affiliates or their respective properties, except, in the case of clauses (i) and (iii), as would not reasonably be expected to impair in any material respect the ability of Licensee to perform its obligations under this Agreement or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions; and no filing with or Consent, approval, authorization, Order, registration or qualification of or with any Governmental Entity, is required for the execution, delivery and performance by Licensor of its obligations under this Agreement, except for the filing of a notification and report under the HSR Act and where the failure to obtain or make any such filing, Consent, approval, authorization, Order, registration or qualification would not reasonably be expected to impair in any material respect the ability of Licensee to perform its obligations under this Agreement or prevent or materially impede, interfere with, hinder or delay the consummation of any of the Transactions.
SECTION 7.04. No Additional Representations. Each Party represents and acknowledges that (i) none of the Parties or any of the respective Affiliates, shareholders, directors, officers, employees, counsel, advisors, representatives or agents (collectively, “Agents”) or any other Person has made any representation or warranty, express or implied, as to
any Licensed Intellectual Property, or the accuracy or completeness of any information regarding any Party or that any Party furnished or made available to any other Party or its Agents, except as expressly set forth in this Agreement, (ii) such Party has not relied on any representation or warranty from any other Party or any other Person in determining to enter into this Agreement, except as expressly set forth in this Agreement, and (iii) none of the Parties or any other Person shall have or be subject to any liability, whether in law or in equity and whether sounding in contract, tort or otherwise, to any Party or any other Person resulting from the provision of any such information to any other Party or its Agents, or use by any other Party or its Agents of any such information, including any information, documents or material made available in the data room or management presentations in expectation of the Transactions.
ARTICLE VIII
General Provisions
SECTION 8.01. Amendment; Extension; Waiver. This Agreement may not be amended except by an instrument in writing signed on behalf of each of the Parties; provided, however, there shall be no amendment or change to the provisions hereof which by applicable Law would require further approval by the shareholders of Licensor without such shareholder approval. Any agreement on the part of a Party to any extension or waiver with respect to this Agreement shall be valid only if set forth in an instrument in writing signed on behalf of such Party; provided, however, that there shall be no waiver of this Agreement which by applicable Law requires further approval by the shareholders of Licensor without such shareholder approval. The failure of any Party to this Agreement to assert any of its rights under this Agreement or otherwise shall not constitute a waiver of such rights.
SECTION 8.02. Survival of Covenants, Agreements, Representations, Warranties, Obligations and Undertakings. The representations, warranties, covenants, agreements, obligations and undertakings in this Agreement shall survive the Exclusive U.S. License Effective Date (unless otherwise indicated).
SECTION 8.03. Notices. All notices, requests, claims, demands and other communications under this Agreement shall be in writing and shall be given (and shall be deemed to have been duly given upon receipt) by delivery by hand, by registered or certified mail (postage prepaid, return receipt requested) or by email with a copy by mail (postage prepaid, return receipt requested) to the respective Parties at the following addresses (or at such other address for a Party as shall be specified by like notice) (each, a “Notice”):
if to Licensee, to:
Biogen Inc.
▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention: General Counsel
Email: ▇▇▇▇▇.▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
and
with a copy to (which copy does not constitute notice):
Cravath, Swaine & ▇▇▇▇▇ LLP
Worldwide Plaza
▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
Attention: ▇▇▇▇ ▇. ▇▇▇▇▇▇
▇▇▇▇▇ ▇. ▇▇▇▇▇▇
O. ▇▇▇▇▇ ▇▇▇▇▇▇, III
Email: ▇▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇
▇▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇
▇▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇
if to Licensor, to:
Forward Pharma FA ApS
Östergade ▇▇▇, ▇▇▇ ▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇,
▇▇▇▇▇▇▇
Attention: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇
Email: ▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
with a copy to (which copy does not constitute notice):
Sidley Austin LLP
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇
Email: ▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
▇▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
if to Licensor Authorized Agent, to:
Forward Pharma USA, LLC
▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇ ▇▇▇▇▇
▇▇▇▇▇▇ ▇▇▇▇▇▇
Attention: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇
Email: ▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
if to any or all of the Additional Parties, to:
The Representative
Forward Pharma A/S
▇▇▇▇▇▇▇▇▇ ▇▇▇, ▇
▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ K
Denmark
Attention: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇
Email: ▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
with a copy to (which copy does not constitute notice):
Sidley Austin LLP
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇
Email: ▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
▇▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
if to Additional Parties Authorized Agent, to:
Forward Pharma USA, LLC
▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇ ▇▇▇▇▇
▇▇▇▇▇▇ ▇▇▇▇▇▇
Attention: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇
Email: ▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇
with a copy to (which copy does not constitute notice):
Sidley Austin LLP
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇
Email: ▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
▇▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
SECTION 8.04. Interpretation. The headings contained in this Agreement and in the table of contents to this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement. The definitions of terms herein shall apply equally to the singular and plural forms of the terms defined. Whenever the context may require,
any pronoun shall include the corresponding masculine, feminine and neuter forms. The word “will” shall be construed to have the same meaning as the word “shall”. The words “include”, “includes” and “including” shall be deemed to be followed by the phrase “without limitation”. The word “extent” in the phrase “to the extent” shall mean the degree to which a subject or other thing extends, and such phrase shall not mean simply “if”. The word “or” shall not be exclusive. The phrase “date of this Agreement” shall be deemed to refer to the Agreement Date. All references to “dollars” or “$” shall refer to the lawful money of the United States, and all references to “krone” or “DKK” shall refer to the lawful money of Denmark. Unless the context requires otherwise (i) any definition of or reference to any Contract, instrument or other document or any Law herein shall be construed as referring to such Contract, instrument or other document or Law as from time to time amended, supplemented or otherwise modified, (ii) any reference herein to any Person shall be construed to include such Person’s successors and assigns, (iii) the words “herein”, “hereof” and “hereunder”, and words of similar import, shall be construed to refer to this Agreement in its entirety and not to any particular provision hereof and (iv) all references herein to articles, sections and appendices shall be construed to refer to articles and sections of, and appendices to, this Agreement. This Agreement shall be construed without regard to any presumption or rule requiring construction or interpretation against the party drafting or causing any instrument to be drafted.
SECTION 8.05. Counterparts. This Agreement may be executed in one or more counterparts, all of which shall be considered one and the same agreement and shall become effective when one or more counterparts have been signed by each of the Parties and delivered to the other Parties. Delivery of an executed counterpart of a signature page of this Agreement by facsimile or other electronic image scan transmission shall be effective as delivery of a manually executed counterpart of this Agreement.
SECTION 8.06. Severability. If any term or other provision of this Agreement is invalid, illegal or incapable of being enforced by any Law, or public policy, all other terms and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the Transactions contemplated hereby is not affected in any manner materially adverse to any Party or such Party waives its rights under this Section 8.06 with respect thereto. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the Parties hereto shall negotiate in good faith to modify this Agreement so as to effect the original intent of the Parties as closely as possible in an acceptable manner to the end that the Transactions are fulfilled to the extent possible. Notwithstanding the foregoing, the Parties intend that this Section 8.06 be construed as an integral provision of this Agreement and that the provisions of this Agreement shall not be severable in any manner that diminishes a Party’s rights hereunder or increases a Party’s liability or obligations hereunder.
SECTION 8.07. Entire Agreement; Third-Party Beneficiaries; No Other Representations or Warranties.
(a) This Agreement, the Aditech Addendum, the Aditech Letter Agreement and the Shareholders Commitment Agreement (i) constitute the entire agreement, and supersede all prior agreements and understandings, both written and oral, among the Parties and their Affiliates, or any of them, with respect to the subject matter
of this Agreement and the Shareholders Commitment Agreement and (ii) except as specified in Section 2.01, Section 2.10 and the Aditech Addendum, are not intended to confer upon any Person other than the Parties hereto or thereto and the Released Parties, as applicable, any rights or remedies.
(b) Except for the representations and warranties contained in Article VII, or in any certificate delivered to Licensee in connection with the Transactions, Licensee acknowledges that (i) none of Licensor, the Additional Parties or any Person on behalf of Licensor or the Additional Parties makes any other express or implied representation or warranty with respect to Licensor, the Additional Parties or any of their respective Affiliates or with respect to any other information made available to Licensee in connection with the Transactions, and (ii) Licensee has not relied on any representation, warranty or other statement made by Licensor, the Additional Parties or any Representative of Licensor or the Additional Parties other than those set forth in Section 7.01.
(c) Except for the representations and warranties contained in Article VII, each of Licensor and the Additional Parties acknowledges that (i) none of Licensee or any other Person on behalf of Licensee makes any other express or implied representation or warranty with respect to Licensee or with respect to any other information made available to Licensor in connection with the Transactions, and (ii) each of Licensor and the Additional Parties has not relied on any representation, warranty or other statement made by Licensee or any Representative of Licensee other than those set forth in Section 7.03.
SECTION 8.08. GOVERNING LAW. THIS AGREEMENT SHALL BE GOVERNED BY, AND CONSTRUED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW YORK, REGARDLESS OF THE LAWS THAT MIGHT OTHERWISE GOVERN UNDER APPLICABLE PRINCIPLES OF CONFLICTS OF LAWS THEREOF, EXCEPT (A) THAT ANY CONTROVERSY, CLAIM OR DISPUTE INVOLVING INFRINGEMENT OR VALIDITY OF INTELLECTUAL PROPERTY SHALL BE DETERMINED ACCORDING TO THE LAWS UNDER WHICH SUCH INTELLECTUAL PROPERTY IS REGISTERED OR OTHERWISE PROTECTED OR ENJOYABLE, ON A COUNTRY-BY-COUNTRY BASIS AND (B) TO THE EXTENT DANISH LAW IS MANDATORILY APPLICABLE TO THIS AGREEMENT OR THE TRANSACTIONS.
SECTION 8.09. Assignment. Licensee may assign, in whole or in part, in its sole discretion and without the consent of Licensor, this Agreement and any or all of its rights, interests and obligations hereunder to any other Person, but no such assignment shall relieve Licensee of its obligations under this Agreement if such assignee does not perform such obligations. Licensor and the Additional Parties may not assign this Agreement or any of their respective rights, interests, or obligations hereunder, in whole or in part, by operation of Law or otherwise to any other Person without the prior written consent of Licensee; provided, however, that Licensor may assign its rights to receive royalty payments pursuant to Sections 4.02 and 4.03 of this Agreement, and other ancillary rights as reasonably necessary to assign its rights to receive royalty payments, to another Person without the prior written consent of Licensee.
Subject to the first two sentences of this Section 8.09, this Agreement will be binding upon, inure to the benefit of and be enforceable by, the Parties and their respective successors and assigns.
SECTION 8.10. Dispute Resolution.
(a) Each of the Parties hereto irrevocably and unconditionally submits, for itself and its property, to the jurisdiction of the federal and state courts in the County of New York in the State of New York. Each of the Parties hereto further agrees that, to the fullest extent permitted by applicable Law, service of any process, summons, notice or document by U.S. registered mail to such Person’s respective address set forth in Section 8.03 shall be effective service of process for any such controversy, claim or dispute in New York with respect to any matters to which it has submitted to jurisdiction as set forth above in the immediately preceding sentence. Nothing in this Agreement will affect the right of any Party to this Agreement to serve process in any other manner permitted by applicable Law. Each of the Parties hereto irrevocably and unconditionally waives (and agrees not to plead or claim) any objection to the laying of venue of any such controversy, claim or dispute in the courts in the County of New York in the State of New York, or that any such controversy, claim or dispute brought in any court in the County of New York in the State of New York has been brought in an inconvenient forum.
(b) Any controversy, claim or dispute brought by Licensor or any of its Affiliates against Licensee or any of its Affiliates arising out of or relating to this Agreement or any breach hereof by Licensee shall be brought in the courts in the County of New York in the State of New York.
(c) Any controversy, claim or dispute brought by Licensee or any of its Affiliates against Licensor, the Additional Parties or any of their respective controlled Affiliates arising out of or relating to this Agreement or any breach hereof by Licensor or the Additional Parties may either be brought in the courts in the County of New York in the State of New York or resolved by confidential arbitration conducted and administered by JAMS or any successor entity thereto (“JAMS”), in accordance with its Comprehensive Rules and Procedures. The arbitration shall be conducted in the County of New York, in the State of New York.
(d) In the event of an arbitration, an organizational meeting shall be held within 90 days of service of the initial arbitration demand. The arbitration shall be conducted by a panel of three arbitrators. Where there is a conflict between the JAMS rules and this clause, the provisions of this clause shall govern. Each Party shall select one arbitrator and the two arbitrators shall select a third arbitrator, who will chair the panel. The persons considered for selection as arbitrators shall not be limited to persons identified by JAMS. The arbitrator(s) shall be neutral and independent of each Party. There shall be no ex parte communications with the Party arbitrator(s) after the first organizational meeting. The confidentiality of all proceedings related to any arbitration shall be strictly maintained, as shall the confidentiality of any documents, deposition testimony, or other information exchanged in relation to the arbitration proceedings
(except as information may be required in any judicial proceeding brought to enforce these arbitration provisions or any award rendered hereunder).
(i) The panel shall be requested to use reasonable efforts to render its decision and award within 30 days after the close of evidence and, in any event, within three months of the first organizational meeting. The panel shall allow reasonable discovery, relevant to the issues before it, subject to the goal of completing the proceedings within the specified time frame. Each Party shall be limited to a maximum total number of four depositions (each deposition not to exceed seven hours), except with respect to custodial depositions for purposes of authenticating documents and, in extraordinary cases, as approved by the panel.
(ii) The panel shall render findings of fact and conclusions of Law and a written opinion setting forth the basis and reasons for any decision reached. In rendering an award, the panel shall determine the rights and obligations of the Parties according to the substantive Laws of the State of New York and of the United States, except (A) that any controversy, claim or dispute involving infringement or validity of Intellectual Property shall be determined according to the Laws under which such Intellectual Property is registered or otherwise protected or enjoyable, on a country-by-country basis and (B) to the extent Danish law is mandatorily applicable. The decision of the panel shall be final and binding.
(iii) The panel shall have the authority to grant any equitable or legal relief that would be available in any judicial proceeding instituted to resolve the disputed matter, including interim relief, but the panel shall not have the authority to grant any remedies the Parties have waived in the Agreement or to award special, punitive or exemplary damages.
(iv) Any decision or award of the panel shall be subject to the appeal by any Party in accordance with the Optional Appeal Procedure of JAMS.
(v) Each of the Parties agrees that it will not bring any action relating to the interpretation, application or enforcement of the provisions of this Section 8.10(d) or seeking emergency or temporary relief prior to appointment of the panel, in any court other than (A) courts in the County of New York in the State of New York, or (B) the Danish Maritime and Commercial Court in Copenhagen, and the Laws of the State of New York shall apply to any such action, except (y) that any controversy, claim or dispute involving infringement or validity of Intellectual Property shall be determined according to the Laws under which such Intellectual Property is registered or otherwise protected or enjoyable, on a country-by-country basis and (z) to the extent Danish law is mandatorily applicable. With respect to any such action, each of the Parties hereby irrevocably consents to and submits itself to the personal jurisdiction of (1) the courts in the County of New York in the State of New York and (2) the Danish Maritime and Commercial Court in Copenhagen, and irrevocably waives any objection to the laying of venue of any such action in such court or that any such court is an inconvenient forum. Each of the Parties hereby waives any rights such Party may have to personal service of a summons, complaint or other process in connection with such an
action and agrees that service may be made by registered or certified mail addressed to such Party and sent in accordance with the provisions of this Agreement. The Parties acknowledge and agree that, upon appointment of the panel, it shall have the exclusive authority to grant relief.
(vi) The Parties hereby also consent to the personal jurisdiction of (A) the courts in the County of New York in the State of New York and (B) the Danish Maritime and Commercial Court in Copenhagen, for the purpose of confirming any award and entering judgment thereon and irrevocably waive any objection to the laying of venue of any such action in such court or that any such court is an inconvenient forum. Each of the Parties hereby waives any rights such Party may have to personal service of a summons, complaint or other process in connection with such an action and agrees that service may be made by registered or certified mail addressed to such Party and sent in accordance with the provisions of this Agreement.
(e) Each Party agrees that the losing party (as determined by the court or arbitral panel) in any Litigation or arbitration brought or held in accordance with this Section 8.10 shall promptly reimburse the other Parties for all their reasonable, documented, out-of-pocket attorneys’ fees and expenses incurred in connection with such Litigation or arbitration.
SECTION 8.11. Authorized Agent.
(a) Licensor hereby designates Forward Pharma USA, LLC as its authorized agent (the “Licensor Authorized Agent”), upon whom process may be served to enforce this Agreement in connection with any Litigation that may be instituted in any court described in this Section 8.11. Licensor hereby agrees to take any and all action, including the filing of any and all documents that may be necessary to establish and continue such appointment in full force and effect as aforesaid. Licensor hereby agrees that service of process upon the Licensor Authorized Agent shall be, in every respect, effective service of process upon Licensor.
(b) The Additional Parties hereby designate Forward Pharma USA, LLC as their authorized agent (the “Additional Parties Authorized Agent”), upon whom process may be served to enforce this Agreement in connection with any Litigation that may be instituted in any court described in this Section 8.11. The Additional Parties hereby agree to take any and all action, including the filing of any and all documents that may be necessary to establish and continue such appointment in full force and effect as aforesaid. The Additional Parties hereby agree that service of process upon the Additional Parties Authorized Agent shall be, in every respect, effective service of process upon the Additional Parties.
SECTION 8.12. Specific Enforcement. The Parties acknowledge and agree that irreparable damage would occur in the event that any of the provisions of this Agreement were not performed in accordance with its specific terms or were otherwise breached, and that monetary damages, even if available, would not be an adequate remedy therefor. It is accordingly agreed that the Parties shall be entitled to an injunction or injunctions, or any other
appropriate form of equitable relief, to prevent breaches of this Agreement and to enforce specifically the performance of the terms and provisions of this Agreement in any court or before any panel of arbitrators referred to in Section 8.10, without the necessity of proving the inadequacy of money damages as a remedy (and each Party hereby waives any requirement for the securing or posting of any bond in connection with such remedy), this being in addition to any other remedy to which they are entitled at law or in equity. Each of the Parties acknowledges and agrees that the right of specific enforcement is an integral part of the Transactions and without such right, none of the Parties would have entered into this Agreement.
SECTION 8.13. Indirect Damages. In no event shall either Party have any liability under any provision of this Agreement for any special, punitive, incidental, consequential, special or indirect damages, including (i) loss of future revenue or income, (ii) loss of business reputation or opportunity relating to the breach or alleged breach of this Agreement, or (iii) diminution of value or any damages based on any type of multiple, whether based on statute, contract, tort or otherwise, and whether or not arising from the other Party’s sole, joint, or concurrent negligence, strict liability, criminal liability or other fault.
SECTION 8.14. WAIVER OF JURY TRIAL. EACH PARTY HERETO HEREBY WAIVES, TO THE FULLEST EXTENT PERMITTED BY APPLICABLE LAW, ANY RIGHT IT MAY HAVE TO A TRIAL BY JURY IN RESPECT OF ANY LITIGATION ARISING OUT OF OR RELATED TO THIS AGREEMENT OR ANY TRANSACTION CONTEMPLATED HEREBY. EACH PARTY HERETO (A) CERTIFIES THAT NO REPRESENTATIVE, AGENT OR ATTORNEY OF ANY OTHER PARTY HAS REPRESENTED, EXPRESSLY OR OTHERWISE, THAT SUCH PARTY WOULD NOT, IN THE EVENT OF ANY LITIGATION, SEEK TO ENFORCE THE FOREGOING WAIVER AND (B) ACKNOWLEDGES THAT IT AND THE OTHER PARTIES HERETO HAVE BEEN INDUCED TO ENTER INTO THIS AGREEMENT BY, AMONG OTHER THINGS, THE MUTUAL WAIVER AND CERTIFICATIONS IN THIS SECTION 8.14.
SECTION 8.15. Rights in Bankruptcy.
(a) All rights and licenses granted to Licensee or any of its Affiliates under or pursuant to this Agreement are intended to be, and will be deemed to be, for purposes of Title 11 of the United States Code, as amended from time to time (the “Bankruptcy Code”), licenses of rights to “intellectual property” as defined under Section 101 of the Bankruptcy Code. The Parties agree that Licensee, any of its Affiliates or its or its Affiliates’ sublicensees will retain and may fully exercise all of their respective rights and elections as licensees of intellectual property in the event any case is commenced with respect to Licensor or any of its Affiliates under the Bankruptcy Code (whether a plenary case or an ancillary case under Chapter 15 of the Bankruptcy Code). The Parties further agree and acknowledge that enforcement by Licensee, any of its Affiliates or its or its Affiliates’ sublicensees of any of their respective rights under Section 365(n) of the Bankruptcy Code in connection with this Agreement shall not violate the automatic stay of Section 362 of the Bankruptcy Code and waive any right to object on such basis. If Licensor, the Additional Parties or any of their respective controlled Affiliates commence a case under the Bankruptcy Code after the Agreement Date or otherwise become the
subject of a case under the Bankruptcy Code commenced after the Agreement Date, voluntarily or involuntarily and whether a plenary case or an ancillary case under Chapter 15 of the Bankruptcy Code (such entity, a “Filing Party”), (i) Licensee, its Affiliates and its and its Affiliates’ sublicensees shall have all rights provided for under Section 365(n) of the Bankruptcy Code (and the Parties hereby agree and acknowledge that such rights are necessary to ensure that the interests of Licensee, and its Affiliates and its and its Affiliates’ sublicensees are “sufficiently protected” in the case of an ancillary case under Chapter 15 of the Bankruptcy Code) and (ii) in addition to and not in lieu of any other right or remedy Licensee or any of its Affiliates or their respective sublicensees (the “Non-Filing Party”) may have under this Agreement or Section 365(n) of the Bankruptcy Code, the Non-Filing Party shall have the right to obtain, and the Filing Party or any trustee for the Filing Party or its assets shall, at the Non-Filing Party’s written request to the Filing Party, deliver a copy of all embodiments held by the Filing Party of any Intellectual Property rights licensed to the Non-Filing Party under or pursuant to this Agreement, including such embodiments necessary for the Non-Filing Party to exercise its rights hereunder. In addition, the Filing Party shall take all steps reasonably requested by the Non-Filing Party to perfect, exercise and enforce its rights hereunder, including filings in the USPTO, U.S. Copyright Office or other similar Governmental Entity, and under the Uniform Commercial Code.
(b) To the extent any license of rights under or pursuant to this Agreement does not constitute a license to “intellectual property” as defined under Section 101 of the Bankruptcy Code (such licensed property, “Specified IP”), each of Licensor, the Additional Parties and their respective controlled Affiliates, in its position of licensor hereunder, hereby acknowledges and agrees that: (i) this Agreement is a material inducement to U.S. Licensee and Designated Countries Licensee paying Licensor their respective portions of the Upfront Fee and the Royalty Consideration pursuant to this Agreement and Licensee relying on this Agreement in connection with its business and investment planning; (ii) this Agreement is not an executory contract and does not contain any material, ongoing obligations on Licensee, any of its Affiliates or its or its Affiliates’ sublicensees relevant to the standard governing executory contracts; (iii) the Parties hereby acknowledge and agree that (A) any Specified IP is closely related to the other Licensed Intellectual Property that constitutes “intellectual property” as defined under Section 101 of the Bankruptcy Code and (B) in the event Licensee, any of its Affiliates or its or its Affiliates’ sublicensees were to lose their rights in and to any Specified IP included in the Licensed Intellectual Property, irreparable damage would occur to Licensee or such Affiliate or sublicensee for which monetary damages alone could not provide sufficient remedy to Licensee or such Affiliate or sublicensee; accordingly, Licensor and the Additional Parties (and any debtor-in-possession or trustee or foreign representative of the business of Licensor or the Additional Parties, as applicable) cannot and shall not attempt to reject this Agreement pursuant to Section 365 of the Bankruptcy Code or any foreign equivalent; and (iv) in the event Licensor or any of the Additional Parties (or any debtor-in-possession or trustee or foreign representative of the business of Licensor or such Additional Party, as applicable) does seek to reject this Agreement and in the event such relief is granted, (A) the rejection shall be treated merely as breach of the contract and not its avoidance, rescission, or termination,
(B) such rejection shall not terminate Licensee’s right to use such license and shall have no effect upon the contract’s continued existence, (C) Licensee, any of its Affiliates or its or its Affiliates’ sublicensees may elect rights under Section 365(n) of the Bankruptcy Code or any foreign equivalent, and (D) Licensee, any of its Affiliates or its or its Affiliates’ sublicensees shall be entitled to seek other equitable treatment relating to such rejection.
SECTION 8.16. Further Assurances. Each of the Parties agree to execute and deliver (and to cause their respective controlled Affiliates to execute and deliver), upon the written request of any Party hereto, any and all such further documents, certificates, papers, schedules and instruments as reasonably appropriate for the purpose of obtaining the full benefits of this Agreement.
SECTION 8.17. Costs. Each Party shall bear its own costs, fees and expenses incurred in connection with this Agreement and the Transactions.
SECTION 8.18. Independent Contractors. Nothing contained herein shall be deemed to create any relationship, whether in the nature of agency, joint venture, partnership or otherwise, between the Parties. No Party shall be authorized to bind or obligate the other Parties in any manner.
SECTION 8.19. Representative of Additional Parties.
(a) Each Additional Party hereby constitutes and appoints Forward Pharma A/S (the “Additional Party Representative”) as attorney-in-fact for such Additional Party with full power of substitution and authority, in its discretion, to enforce this Agreement against the parties hereto, and to execute any amendment or waiver of this Agreement and any other document or instrument necessary or advisable in order to carry out the provisions of this Agreement, to give and receive notices and communications relating to this Agreement and to agree to, negotiate, enter into settlements and compromises of, and to comply with Orders with respect to, any dispute relating to this Agreement and to take all actions necessary or appropriate in the judgment of the Additional Party Representative for the accomplishment of the foregoing.
(b) All decisions of and actions by the Additional Party Representative may be relied upon by Licensee, Licensor, their respective Affiliates and any Third Party, and shall be binding and conclusive upon each Additional Party.
(c) The Additional Parties may from time to time designate another Party hereto as the Additional Party Representative in substitution for the then Additional Party Representative, and upon written notice thereof to Licensee, such Party shall be the Additional Party Representative for all purposes hereof.
SECTION 8.20. Set Off. Notwithstanding anything in this Agreement to the contrary, the Parties hereby agree that each of the Parties shall have the right to set off any amounts owed by it to any other Party pursuant to this Agreement against any amounts otherwise concurrently due and payable to it pursuant to this Agreement.
IN WITNESS WHEREOF, each of the undersigned Parties has caused this Agreement to be signed by its signatories thereunto duly authorized as of the date first written above.
|
|
BIOGEN SWISS MANUFACTURING GMBH | |
|
|
| |
|
|
By: |
/s/ Fraderick ▇▇▇▇▇▇ |
|
|
|
Name: Fraderick ▇▇▇▇▇▇ |
|
|
|
Title: Managing Director |
|
|
|
|
|
|
|
|
|
|
By: |
/s/ ▇▇▇▇ ▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇ ▇▇▇▇▇ |
|
|
|
Title: Director |
|
|
| |
|
|
BIOGEN INTERNATIONAL HOLDING LTD. | |
|
|
|
|
|
|
By: |
/s/ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇ |
|
|
|
Title: Director |
[Signature Page to Settlement and License Agreement]
|
|
||
|
|
| |
|
|
By: |
/s/ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Title: Chairman of Board of Directors |
|
|
|
|
|
|
|
/s/ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ |
|
|
|
Title: Member of Board of Directors |
|
|
|
|
|
|
|
/s/ ▇▇▇▇▇ ▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇ ▇▇▇▇▇ |
|
|
|
Title: Member of Board of Directors |
|
|
|
|
|
|
|
/s/ Jan van ▇▇ ▇▇▇▇▇▇ |
|
|
|
Name: Jan van ▇▇ ▇▇▇▇▇▇ |
|
|
|
Title: Member of Board of Directors |
[Signature Page to Settlement and License Agreement]
|
|
ADITECH PHARMA AG | |
|
|
| |
|
|
|
/s/ ▇▇▇▇▇▇▇ ▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇▇▇ ▇▇▇▇▇ |
|
|
|
Title: Director |
|
|
|
|
|
|
NB FP INVESTMENT SLP APS | |
|
|
| |
|
|
|
/s/ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Title: CEO |
|
|
|
|
|
|
NB FP INVESTMENT GENERAL PARTNER APS | |
|
|
| |
|
|
|
/s/ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Title: CEO |
|
|
|
|
|
|
TECH GROWTH INVEST APS | |
|
|
| |
|
|
|
/s/ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Name: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Title: CEO |
[Signature Page to Settlement and License Agreement]
APPENDIX A
Scheduled Patents and Patent Applications
EP 2 879 672 and Family Members (Based on WO 2014/020156)
(Title: Combination Therapy for Treatment of Multiple Sclerosis)
|
No. |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
1 |
|
AU2013298517 (Al) |
|
3/5/2015 |
|
AU20130298517 |
|
8/2/2013 |
|
Australia |
|
2 |
|
CA2880742 (Al) |
|
2/6/2014 |
|
CA20132880742 |
|
8/2/2013 |
|
Canada |
|
3 |
|
CN104684553 (A) |
|
6/3/2015 |
|
CN2013852107 |
|
8/2/2013 |
|
China |
|
4 |
|
EA201590166 (Al); EA201590166 (A8) |
|
6/30/2015 |
|
EA20150090166 |
|
8/2/2013 |
|
Eurasia |
|
5 |
|
EP1940382 (A2) |
|
7/9/2008 |
|
EP20060791453 |
|
10/6/2006 |
|
European |
|
6 |
|
EP2692343 (Al) |
|
2/5/2014 |
|
EP20120179232 |
|
8/3/2012 |
|
European |
|
7 |
|
EP2692344 (Al) |
|
2/5/2014 |
|
EP20120187939 |
|
10/10/2012 |
|
European |
|
8 |
|
EP2879672 (Al) |
|
6/10/2015 |
|
EP20130745073 |
|
8/2/2013 |
|
European |
|
9 |
|
HK1211210 (Al) |
|
5/20/2016 |
|
HK20150112010 |
|
12/7/2015 |
|
Hong Kong |
|
10 |
|
872/CHENP/2015 A |
|
7/1/2016 |
|
872/CHENP/2015 |
|
2/12/2015 |
|
India |
|
11 |
|
JP2015523407 (A) |
|
8/13/2015 |
|
JP20150524803 |
|
8/2/2013 |
|
Japan |
|
12 |
|
KR20150040338 (A) |
|
4/14/2015 |
|
KR20157005612 |
|
8/2/2013 |
|
Korea |
|
13 |
|
17588/3A/16 |
|
8/18/2016 |
|
UA201501066 |
|
8/2/2013 |
|
Ukraine |
|
14 |
|
US20080300217 (Al) |
|
12/4/2008 |
|
12/089,004 |
|
4/2/2008 |
|
United States |
|
15 |
|
US20150164849 (Al) |
|
6/18/2015 |
|
14/419,031 |
|
▇/▇/▇▇▇▇ |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
16 |
|
WO2007042035 (A2); WO2007042035 (A3) |
|
4/19/2007 |
|
WO2006DK00563 |
|
10/6/2006 |
|
WIPO |
|
17 |
|
WO2014020156 (Al) |
|
2/6/2014 |
|
WO2013EP66285 |
|
8/2/2013 |
|
WIPO |
[Appendix A to Settlement and License Agreement]
EP 2 379 063 and Family Members (Based on WO 2010/079222)
(Title: Pharmaceutical Formulation Comprising One or More Fumaric Acid Esters in an Erosion Matrix)
|
No. |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
1 |
|
CN102369001 (A) |
|
3/7/2012 |
|
CN2010811800 |
|
1/8/2010 |
|
China |
|
2 |
|
DK2379063 (T3) |
|
4/22/2013 |
|
DK20100700730T |
|
1/8/2010 |
|
Denmark |
|
3 |
|
DK2564839 (T3) |
|
7/25/2016 |
|
DK20120193798T |
|
1/8/2010 |
|
Denmark |
|
4 |
|
EA201290596 (Al) |
|
1/30/2013 |
|
EA20120090596 |
|
1/8/2010 |
|
Eurasia |
|
5 |
|
▇▇▇▇▇▇▇▇▇ (Al); ▇▇▇▇▇▇▇▇▇ (B1) |
|
10/26/2011 |
|
EP20100700730 |
|
1/8/2010 |
|
European |
|
6 |
|
▇▇▇▇▇▇▇▇▇ (A2); ▇▇▇▇▇▇▇▇▇ (A3); ▇▇▇▇▇▇▇▇▇ |
|
3/6/2013 |
|
EP20120193798 |
|
1/8/2010 |
|
European |
|
7 |
|
EP3090733 (Al) |
|
11/9/2016 |
|
EP20160000993 |
|
1/8/2010 |
|
European |
|
8 |
|
ES2411972 (T3) |
|
7/9/2013 |
|
ES20100700730T |
|
1/8/2010 |
|
Spain |
|
9 |
|
ES2586761 (T3) |
|
10/18/2016 |
|
ES20120193798T |
|
1/8/2010 |
|
Spain |
|
10 |
|
HK1180943A0 |
|
11/01/2013 |
|
HK▇▇▇▇▇▇▇▇▇▇A |
|
7/11/2013 |
|
Hong Kong |
|
11 |
|
HRP20130480 (T1) |
|
6/30/2013 |
|
HR2013P000480T |
|
5/31/2013 |
|
Croatia |
|
12 |
|
HRP20160982 (T1) |
|
11/4/2016 |
|
HR2016P000982T |
|
8/1/2016 |
|
Croatia |
|
13 |
|
P6543 |
|
11/2/2016 |
|
GE201012819 |
|
8/1/2010 |
|
Georgia |
|
14 |
|
2814/KOLNP/2011 A |
|
1/20/2012 |
|
IN 2814/KOLNP/2011 |
|
7/5/2011 |
|
India |
|
15 |
|
JP2016006081 (A) |
|
1/14/2016 |
|
JP20150149886 |
|
7/29/2015 |
|
Japan |
|
16 |
|
▇▇▇▇▇▇▇▇▇ (B2); JP2012514624 (A) |
|
6/28/2012 |
|
JP20110544876 |
|
1/8/2010 |
|
Japan |
|
17 |
|
KR20110116027 (A) |
|
10/24/2011 |
|
KR20117018595 |
|
1/8/2010 |
|
Korea |
|
18 |
|
PL2564839T3 |
|
11/30/2016 |
|
PL2012193789T |
|
1/8/2010 |
|
Poland |
|
19 |
|
▇▇▇▇▇▇▇▇▇ (E) |
|
5/3/2013 |
|
PT20100700730T |
|
1/8/2010 |
|
Portugal |
|
20 |
|
RU2015113483 (A) |
|
11/27/2015 |
|
RU20150113483 |
|
1/8/2010 |
|
Russia |
|
21 |
|
RU2552951 (C2); RU2011128785 (A) |
|
2/20/2013 |
|
RU20110128785 |
|
1/8/2010 |
|
Russia |
[Appendix A to Settlement and License Agreement]
|
No. |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
22 |
|
SI2379063 (T1) |
|
4/30/2013 |
|
SI20100030161T |
|
1/8/2010 |
|
Slovenia |
|
23 |
|
SI2564839 (T1) |
|
9/30/2016 |
|
SI20100031248 |
|
1/8/2010 |
|
Slovenia |
|
24 |
|
SMT201300065 (B) |
|
9/6/2013 |
|
SM20130000065T |
|
6/17/2013 |
|
San Marino |
|
25 |
|
UA103844 (C2) |
|
11/25/2013 |
|
UA20120009635 |
|
1/8/2010 |
|
Ukraine |
|
26 |
|
1122975 |
|
11/25/2016 |
|
UA201307178/I |
|
1/8/2010 |
|
Ukraine |
|
27 |
|
Not known yet |
|
Not known yet |
|
UA201308182/I |
|
1/8/2010 |
|
Ukraine |
|
28 |
|
US20150272894 (A1) |
|
10/1/2015 |
|
14/561,010 |
|
▇▇/▇/▇▇▇▇ |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
29 |
|
US8906420 (B2); US20120034303 (A1) |
|
2/9/2012 |
|
13/143,498 |
|
▇/▇/▇▇▇▇ |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
30 |
|
WO2010079222 (A1) |
|
7/15/2010 |
|
WO2010EP50172 |
|
1/8/2010 |
|
WIPO |
[Appendix A to Settlement and License Agreement]
Chart EP 2 801 355 and Family Members (Based on WO 2006/037342)
|
No. |
|
Title |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
1 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
CN101056624 (A) |
|
10/17/2007 |
|
CN2005838572 |
|
10/7/2005 |
|
China |
|
2 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
CN101304732 (A) |
|
11/12/2008 |
|
CN2006841526 |
|
10/6/2006 |
|
China |
|
3 |
|
Gesteuerte Freisetzung von pharmazeutischen Zusammensetzungen mit Fumarinsäureester |
|
DE14172390 (T1) |
|
12/31/2014 |
|
DE14172390T |
|
10/7/2005 |
|
Germany |
|
4 |
|
Gesteuerte Freisetzung von pharmazeutischen Zusammensetzungen mit Fumarinsäureester |
|
DE14172396 (T1) |
|
1/8/2015 |
|
DE14172396T |
|
10/7/2005 |
|
Germany |
|
5 |
|
Gesteuerte Freisetzung von pharmazeutischen Zusammensetzungen mit Fumarinsäureester |
|
DE14172398 (T1) |
|
1/8/2015 |
|
DE14172398T |
|
10/7/2005 |
|
Germany |
|
6 |
|
Pharmazeutische Zusammensetzungen mit kontrollierter Freisetzung, umfassend einen Fumarinsäureester |
|
DE202005022112 (U1) |
|
4/24/2014 |
|
DE20052022112U |
|
10/7/2005 |
|
Germany |
|
7 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
DK1799196 (T3) |
|
8/15/2016 |
|
DK20050789026T |
|
10/7/2005 |
|
Denmark |
|
8 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
▇▇▇▇▇▇▇▇▇ (T3) |
|
7/23/2012 |
|
DK20100182198T |
|
10/7/2005 |
|
Denmark |
[Appendix A to Settlement and License Agreement]
|
No. |
|
Title |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
9 |
|
Pharmazeutische Zusammensetzungen mit kontrollierter Freisetzung, umfassend einen Fumarinsäureester |
|
DK2801355 (T3) |
|
7/6/2015 |
|
DK20140172398T |
|
10/7/2005 |
|
Denmark |
|
10 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
▇▇▇▇▇▇▇▇▇ (A2); |
|
6/27/2007 |
|
EP20050789026 |
|
10/7/2005 |
|
European |
|
11 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
EP1951206 (Al) |
|
8/6/2008 |
|
EP20060791451 |
|
10/6/2006 |
|
European |
|
12 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
EP2316430 (B1); |
|
5/4/2011 |
|
EP20100182198 |
|
10/7/2005 |
|
European |
|
13 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
▇▇▇▇▇▇▇▇▇ (A3); |
|
10/22/2014 |
|
EP20140172396 |
|
10/7/2005 |
|
European |
|
14 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
EP2801354 (Al) |
|
11/12/2014 |
|
EP20140172390 |
|
10/7/2005 |
|
European |
|
15 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
▇▇▇▇▇▇▇▇▇ (B1); |
|
11/12/2014 |
|
EP20140172398 |
|
10/7/2005 |
|
European |
|
16 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
EP2965751 (Al) |
|
1/13/2016 |
|
EP20150166243 |
|
10/7/2005 |
|
European |
|
17 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
EP3093012 (Al) |
|
11/16/16 |
|
EP20160001391 |
|
10/7/2005 |
|
European |
|
18 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
ES2387192 (T3) |
|
9/17/2012 |
|
ES20100182198T |
|
10/7/2005 |
|
Spain |
[Appendix A to Settlement and License Agreement]
|
No. |
|
Title |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
19 |
|
Pharmazeutische Zusammensetzungen mit kontrollierter Freisetzung, umfassend einen Fumarinsäureester |
|
ES2523796 (T1) |
|
12/1/2014 |
|
ES20140172396T |
|
10/7/2005 |
|
Spain |
|
20 |
|
Pharmazeutische Zusammensetzungen mit kontrollierter Freisetzung, umfassend einen Fumarinsäureester |
|
▇▇▇▇▇▇▇▇▇ (T1) |
|
12/23/2014 |
|
ES20140172390T |
|
10/7/2005 |
|
Spain |
|
21 |
|
Pharmazeutische Zusammensetzungen mit kontrollierter Freisetzung, umfassend einen Fumarinsäureester |
|
ES2525497 (T3); |
|
12/23/2014 |
|
ES20140172398T |
|
10/7/2005 |
|
Spain |
|
22 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
▇▇▇▇▇▇▇▇▇ (T3) |
|
9/16/2016 |
|
ES20050789026T |
|
10/7/2005 |
|
Spain |
|
23 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
HK1108836A |
|
5/23/2008 |
|
HK07114067.2 |
|
12/24/2007 |
|
Hong Kong |
|
24 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
IN200701583P2 |
|
7/27/2007 |
|
IN2007KN1583A |
|
5/3/2007 |
|
India |
|
25 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
JP2008515822 (A) |
|
5/15/2008 |
|
JP20070535023 |
|
▇▇/▇/▇▇▇▇ |
|
▇▇▇▇▇ |
|
26 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
JP2009510137 (A) |
|
3/12/2009 |
|
JP20080533870 |
|
▇▇/▇/▇▇▇▇ |
|
▇▇▇▇▇ |
|
27 |
|
Controlled release pharmaceutical composition comprising fumaric acid ester |
|
JP2013064007 (A) |
|
4/11/2013 |
|
JP20120267572 |
|
▇▇/▇/▇▇▇▇ |
|
▇▇▇▇▇ |
[Appendix A to Settlement and License Agreement]
|
No. |
|
Title |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
28 |
|
Controlled release pharmaceutical compositions comprising fumaric ester |
|
JP▇▇▇▇▇▇▇▇▇▇ (A) |
|
12/17/2015 |
|
JP20150139809 |
|
7/13/2015 |
|
Japan |
|
29 |
|
Pharmazeutische Zusammensetzungen mit kontrollierter Freisetzung, umfassend einen Fumarinsäureester |
|
LU92871 (I2) |
|
1/13/2016 |
|
LU20150092871C |
|
11/13/2015 |
|
Luxembourg |
|
30 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
PL2316430 (T3) |
|
11/30/2012 |
|
PL20100182198T |
|
10/7/2005 |
|
Poland |
|
31 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
PT2316430 (E) |
|
6/26/2012 |
|
PT20100182198T |
|
10/7/2005 |
|
Portugal |
|
32 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
▇▇▇▇▇▇▇▇▇ (E) |
|
9/18/2015 |
|
PT20050172398T |
|
10/7/2005 |
|
Portugal |
|
33 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
RS54187 (B1) |
|
12/31/2015 |
|
RS2015P000540 |
|
10/7/2005 |
|
Serbia |
|
34 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
SI1799196 (T1) |
|
10/28/2016 |
|
SI20050032086 |
|
10/7/2005 |
|
Slovenia |
|
35 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
SI2316430 (T1) |
|
9/28/2012 |
|
SI20050031565T |
|
10/7/2005 |
|
Slovenia |
|
36 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
SI2801355 (T1) |
|
9/30/2015 |
|
SI20050031993T |
|
10/7/2005 |
|
Slovenia |
|
37 |
|
Controlled Release Pharmaceutical Compositions Comprising a Fumaric Acid Ester |
|
US20080299196 (A1) |
|
12/04/2008 |
|
12/089,074 |
|
4/03/2008 |
|
United States |
[Appendix A to Settlement and License Agreement]
|
No. |
|
Title |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
38 |
|
Controlled release pharmaceutical compositions comprising fumaric acid ester |
|
US20090304790 (Al); |
|
12/10/2009 |
|
11/576,871 |
|
▇▇/▇/▇▇▇▇ |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
39 |
|
Controlled release pharmaceutical compositions comprising a |
|
US20130315993 (Al) |
|
11/28/2013 |
|
13/957,147 |
|
8/01/2013 |
|
United States |
|
40 |
|
CONTROLLED RELEASE PHARMACEUTICAL COMPOSITIONS COMPRISING A FUMARIC ACID ESTER (“Cmax and 480 mg per day dosing”) |
|
US20130316003 (Al) |
|
11/28/2013 |
|
13/957,220 |
|
8/01/2013 |
|
United States |
|
41 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester (“uptitration regimen including 480 mg per day dosing”) |
|
US20140037720 (Al) |
|
02/06/2014 |
|
13/957,117 |
|
8/01/2013 |
|
United States |
|
42 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140037740 (Al) |
|
02/06/2014 |
|
13/957,250 |
|
8/01/2013 |
|
United States |
|
43 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140199386 (Al) |
|
07/17/2014 |
|
14/209,712 |
|
3/13/2014 |
|
United States |
|
44 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140199387 (Al) |
|
07/17/2014 |
|
14/213,321 |
|
3/14/2014 |
|
United States |
|
45 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140199388 (Al) |
|
07/17/2014 |
|
14/213,673 |
|
3/14/2014 |
|
United States |
|
46 |
|
Methods for treating multiple sclerosis |
|
US20140199390 (Al) |
|
7/17/2014 |
|
14/212,503 |
|
3/14/2014 |
|
United States |
[Appendix A to Settlement and License Agreement]
|
No. |
|
Title |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
47 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140199392 (Al) |
|
7/17/2014 |
|
14/212,685 |
|
3/14/2014 |
|
United States |
|
48 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140199393 (Al) |
|
7/17/2014 |
|
14/209,756 |
|
3/13/2014 |
|
United States |
|
49 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140200272 (Al) |
|
7/17/2014 |
|
14/209,584 |
|
3/13/2014 |
|
United States |
|
50 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140200273 (Al) |
|
7/17/2014 |
|
14/209,651 |
|
3/13/2014 |
|
United States |
|
51 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20140205659 (Al) |
|
7/24/2014 |
|
14/213,399 |
|
3/14/2014 |
|
United States |
|
52 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20150024049 (Al) |
|
1/22/2015 |
|
14/209,823 |
|
3/13/2014 |
|
United States |
|
53 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
US20160271093 (A2); |
|
7/10/2014 |
|
14/209,480 |
|
3/13/2014 |
|
United States |
|
54 |
|
Controlled release pharmaceutical compositions fumaric acid ester |
|
WO2006037342 (A2); |
|
4/13/2006 |
|
WO2005DK00648 |
|
10/7/2005 |
|
WIPO |
|
55 |
|
Controlled release pharmaceutical compositions comprising a fumaric acid ester |
|
WO2007042034 (Al) |
|
4/19/2007 |
|
WO2006DK00561 |
|
10/6/2006 |
|
WIPO |
[Appendix A to Settlement and License Agreement]
|
No. |
|
Title |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
|
|
Forward Pharma has applied for Supplementary Protection Certificates based on EP 2 801 355 and the Marketing Authorisation for Tecfidera (EU/1/13/837) in the following countries: Albania, Austria, Belgium, Bosnia and Herzogovina, Cyprus, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Netherlands, Poland, Portugal, Serbia, Slovenia, Spain, Sweden, Switzerland, United Kingdom. SPCs have so far been granted in Cyprus, France, Greece, Luxembourg, Slovenia, Spain, and Sweden. The application in Serbia has been refused (lack of a market authorisation). | ||||||||||
[Appendix A to Settlement and License Agreement]
EP 3 038 606 and Family Members (Based on WO 2015/028472 or WO 2015/028473)
(Title: Pharmaceutical composition containing dimethyl fumarate for administration at a low daily dose)
|
No. |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
1 |
|
AU2014314230 (Al) |
|
4/7/2016 |
|
AU20140314230 |
|
8/26/2014 |
|
Australia |
|
2 |
|
AU2014314231 (Al) |
|
3/3/2016 |
|
AU20140314231 |
|
8/26/2014 |
|
Australia |
|
3 |
|
CA2918846 (Al) |
|
3/5/2015 |
|
CA20142918846 |
|
8/26/2014 |
|
Canada |
|
4 |
|
CA2918852 (Al) |
|
3/5/2015 |
|
CA20142918852 |
|
8/26/2014 |
|
Canada |
|
5 |
|
CN105658207 (A) |
|
6/8/2016 |
|
CN2014847436 |
|
8/26/2014 |
|
China |
|
6 |
|
CN105682648 (A) |
|
6/15/2016 |
|
CN2014847438 |
|
8/26/2014 |
|
China |
|
7 |
|
EA201690102 (Al) |
|
6/30/2016 |
|
EA20160090102 |
|
8/26/2014 |
|
Eurasia |
|
8 |
|
EA201690107 (Al) |
|
10/31/2016 |
|
EA20160090107 |
|
8/26/2014 |
|
Eurasia |
|
9 |
|
EP3038605 (Al) |
|
7/6/2016 |
|
EP20140755672 |
|
8/26/2014 |
|
European |
|
10 |
|
EP3038606 (Al) |
|
7/6/2016 |
|
EP20140755818 |
|
8/26/2014 |
|
European |
|
11 |
|
Not known yet |
|
Not known yet |
|
IN201637008837 |
|
8/26/2014 |
|
India |
|
12 |
|
Not published yet |
|
Not published yet |
|
IN201637008839 |
|
8/26/2014 |
|
India |
|
13 |
|
Not published yet |
|
Not published yet |
|
IL243660 |
|
8/26/2014 |
|
Israel |
|
14 |
|
Not known yet |
|
Not known yet |
|
IL243661 |
|
8/26/2014 |
|
Israel |
|
15 |
|
JP2016528302 (A) |
|
9/15/2016 |
|
JP20160537269 |
|
8/26/2014 |
|
Japan |
|
16 |
|
JP2016531912 (A) |
|
10/13/2016 |
|
JP20160537270 |
|
8/26/2014 |
|
Japan |
|
17 |
|
KR20160045728 (A) |
|
4/27/2016 |
|
KR20167004935 |
|
8/26/2014 |
|
Korea |
|
18 |
|
KR20160046813 (A) |
|
4/29/2016 |
|
KR20167004936 |
|
8/26/2014 |
|
Korea |
|
19 |
|
Not published yet |
|
Not published yet |
|
NZ716118 |
|
8/26/2014 |
|
New Zealand |
|
20 |
|
Not published yet |
|
Not published yet |
|
NZ716121 |
|
8/26/2014 |
|
New Zealand |
|
21 |
|
Not published yet |
|
Not published yet |
|
UA201600686 |
|
8/26/2014 |
|
Ukraine |
|
22 |
|
Not published yet |
|
Not published yet |
|
UA201600687 |
|
8/26/2014 |
|
Ukraine |
|
23 |
|
US20160206586 (Al) |
|
7/21/2016 |
|
14/914,025 |
|
8/26/2014 |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
▇▇ |
|
▇▇▇▇▇▇▇▇▇▇▇▇▇ (▇▇) |
|
7/21/2016 |
|
14/914,031 |
|
8/26/2014 |
|
United States |
|
25 |
|
WO2015028472 (Al) |
|
3/5/2015 |
|
WO2014EP68094 |
|
8/26/2014 |
|
WIPO |
|
26 |
|
WO2015028473 (Al) |
|
3/5/2015 |
|
WO2014EP68095 |
|
8/26/2014 |
|
WIPO |
[Appendix A to Settlement and License Agreement]
EP 2 379 062 and Family Members (Based on WO 2010/079221)
(Title: Pharmaceutical composition comprising one or more fumaric acid esters)
|
No. |
|
Publication No. |
|
Publication Date |
|
Application No. |
|
Application Date |
|
Country |
|
1 |
|
CN102369000 |
|
3/7/2012 |
|
CN2010811787 |
|
|
|
China |
|
2 |
|
EP2379062 |
|
10/26/2011 |
|
EP20100700231 |
|
1/8/2010 |
|
European |
|
3 |
|
JP2012514623 |
|
6/28/2012 |
|
JP2011544875 |
|
1/8/2010 |
|
Japanese |
|
4 |
|
US20120034274 |
|
2/9/2012 |
|
13/143,465 |
|
10/26/2011 |
|
United States |
|
5 |
|
US20130259906 |
|
10/3/2013 |
|
13/768,829 |
|
2/15/2013 |
|
United States |
|
6 |
|
WO2010079221 |
|
7/15/2010 |
|
WO2010EP50171 |
|
1/8/2010 |
|
WIPO |
(ii) Scheduled Trademark Rights
|
No. |
|
Trademark |
|
Serial Number |
|
Filing Date |
|
Registration Date |
|
Country |
|
Trademark Type |
|
Status |
|
1 |
|
FP187 |
|
86449799 |
|
▇▇/▇▇/▇▇▇▇ |
|
▇/▇ |
|
▇▇▇▇▇▇ ▇▇▇▇▇▇ |
|
Word |
|
On Appeal |
|
2 |
|
FP187 |
|
VR201500106 |
|
11/10/2014 |
|
1/14/2015 |
|
Denmark |
|
Word |
|
Registered |
[Appendix A to Settlement and License Agreement]
▇▇▇▇▇▇▇▇ ▇
Licensed Intellectual Property Contracts
1. Patent License Agreement Aditech-FP 01 Jul 2005
2. Patent License Agreement Assigment Aditech AB-Aditech AG 20 Aug 2009
3. Patent Transfer Agreement Aditech-FP 04 May 2010
4. Patent License Agreement FP AS-FP GmbH 23 May 2007
5. Termination Agreement (License Agreement AS - GmbH)
6. Consultancy Agreement (▇▇▇▇▇▇▇▇ ▇▇▇▇▇) 18.01.2016
7. Addendum to Consultancy Agreement (▇▇▇▇▇▇▇▇ ▇▇▇▇▇) 03062016
8. Addendum II to Consultancy Agreement (Prof. Dr. med. ▇▇▇▇▇▇▇▇ ▇▇▇▇▇)
9. Collaboration Agreement Ixchel Pharma
10. Consulting Agreement Ixchel Pharma
[Appendix B to Settlement and License Agreement]
APPENDIX C
List of Non-Affiliates
1) Genmab A/S
2) Jazz Pharmaceuticals plc
3) Thermo ▇▇▇▇▇▇ Scientific Inc.
[Appendix C to Settlement and License Agreement]
Step 1 – Drop-down* 1. Following the Effective Date, (A) Licensor shall (i) establish and form, as a wholly owned subsidiary of Licensor, a Danish limited liability company (“New SubCo”) and (ii) take all actions necessary to apply in a form and substance prepared by Licensor and approved by Licensee for written approval (in Danish: “tilladelse”) from SKAT (the “Danish Tax Authorities”) that Steps 1 and 2 can be carried out as a tax exempt drop down (in Danish: “skattefri tilførsel af aktiver”) and a tax exempt demerger (in Danish “skattefri spaltning”), respectively, under Danish tax Law, including in light of the fact that Steps 3 and 4 will also be completed and (B) Licensor in consultation with Licensee shall take all actions necessary to apply for approval from the Danish Business Authority (the “Danish Business Authority”) that the transactions contemplated by Steps 3 and 4 are permissible under applicable Danish Law. For the avoidance of doubt, Licensor shall not under any circumstances be required to carry out Step 1 and Step 2 unless the Danish Tax Authorities conclude that the transactions qualify for tax exemption according to the Danish legislation on tax exempt reorganizations; Shareholders 2. Following its receipt of the U.S. Upfront Fee and Designated Countries Upfront Fee pursuant to and in accordance with Section 4.01 of the License Agreement and approval of the Step 1 Contribution (as defined below) as tax exempt by the Danish Tax Authorities, Licensor shall contribute all of its assets (other than an amount of cash, if any, that Licensor determines to be in excess of the amount of cash necessary to satisfy the funding and operational requirements of New SubCo) and known liabilities, including the License Agreement, by way of a tax exempt business contribution to New SubCo (the “Step 1 Contribution”); and 3. Substantially concurrently with the consummation of the Step 1 Contribution, Licensor shall cause New SubCo to execute and deliver all instruments and agreements necessary to consummate the joinder of New SubCo to the License Agreement pursuant to and in accordance with Section 2.12 of the License Agreement (the “New SubCo Joinder”). For the avoidance of doubt, upon the consummation of the New SubCo Joinder, the parties to the License Agreement shall be Licensee, New SubCo, the Additional Parties and Licensor (unless Licensee consents to the removal of Licensor as a Party to the License Agreement); 4. Provided, however, that if the Step 1 Contribution is (i) not approved by the Danish Tax Authorities as tax exempt for any reason by May 1, 2017 (the “Step 1 Outside Date”), provided that Licensee may in its sole discretion elect to extend the Step 1 Outside Date by up to an additional 60 days, or (ii) approved by Danish Tax Authorities as tax exempt subject to the satisfaction of certain conditions, Licensor shall, at Licensee’s election in its sole discretion, (a) establish and form, as a wholly owned subsidiary of Licensor, a Danish partnership limited by shares (the “P/S Sub”) that is structured in a manner that would enable payments to the P/S Sub to qualify for benefits under the U.S.-Danish income tax treaty, (b) contribute all of the Licensed Intellectual Property by way of a tax exempt business contribution to the P/S Sub (the “P/S Sub Contribution”) and (c) substantially concurrently with the consummation of the P/S Sub Contribution, grant Licensee a pledge in all of the issued and outstanding shares of P/S Sub to secure Licensee’s rights under the License Agreement (the transactions contemplated by clauses (a)-(c) above, the “P/S Sub Restructuring Alternative”). *Capitalized terms used but not defined in this Appendix D shall have the meanings set forth in the Settlement and License Agreement (the “License Agreement”). [Appendix D to Settlement and License Agreement] 2 New SubCo Licensor
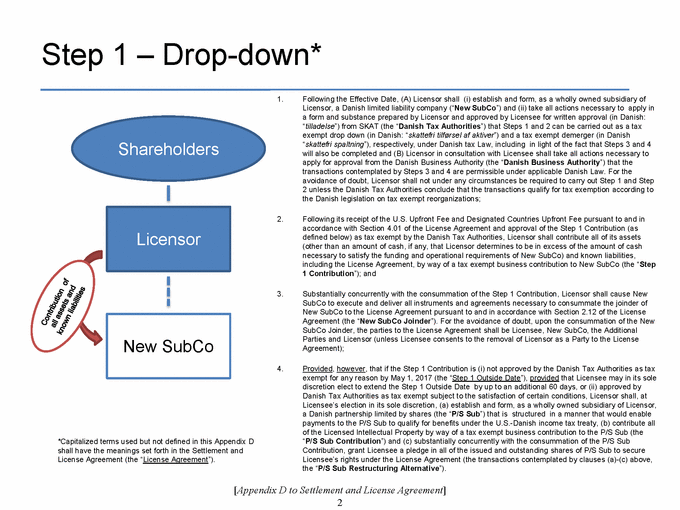
Step 2 – Demerger 1. In the event Licensor has received all necessary approvals of tax exemption from the Danish Tax Authorities, Licensor shall cause New SubCo to be demerged into two new Danish limited liability companies, SubCo 1 (“SubCo 1”) and (“SubCo 2”), which shall be wholly owned subsidiaries of Licensor (the “Demerger”), and whereby the assets and liabilities of New SubCo (which shall cease to exist upon consummation of the Demerger) will be allocated as follows: SubCo 1 shall hold all legal and beneficial right, title and interest to the Licensed Intellectual Property held by New SubCo prior to the Demerger and the payment rights set forth in Sections 3.06 and 3.07 of the License Agreement (but, for the avoidance of doubt, not including any other rights under the License Agreement other than those rights set forth in Shareholders o Sections 3.06 and 3.07 of the License Agreement) and shall hold and be responsible for (i) the liability to protect and maintain the Licensed Intellectual Property pursuant to the License Agreement held by New SubCo prior to the Demerger and (ii) the obligations under Sections 3.06 and 3.07 of the License Agreement held by New SubCo prior to the Demerger; SubCo 2 shall hold all of the assets held by New SubCo prior to the Demerger (other than such assets held by SubCo 1), including all payment rights under the License Agreement, and hold all liabilities held by New SubCo prior the Demerger (other than such liabilities held by SubCo 1). For the avoidance of doubt, SubCo 2 shall hold and be responsible for the obligation under Article V of the License Agreement to fund the protection and maintenance of the Licensed Intellectual Property that was held by NewCo prior to the Demerger. o 2. Licensor shall cause the corporate objects in the articles of association of SubCo 1 (in Danish: “formål”) to (i) require SubCo 1 to comply with its obligations under the License Agreement to maintain and protect the Licensed Intellectual Property (and to take any such steps reasonably requested by the Party to the License Agreement that, at the time of such request, has control of or a right to maintain or protect such Licensed Intellectual Property in connection with such maintenance and protection) and (ii) prohibit SubCo 1 from engaging in any other activities. Substantially concurrently with the consummation of the Demerger, SubCo 1 and SubCo 2 shall execute and deliver: all necessary instruments and agreements to consummate the joinder of SubCo 1 and SubCo 2, respectively, to the License Agreement pursuant to and in accordance with Section 2.12 of the License Agreement (the “SubCo Joinders”); for the avoidance of doubt, upon the consummation of the SubCo Joinders, the parties to the License Agreement shall be Licensee, SubCo 1, SubCo 2, the Additional Parties and Licensor (unless Licensee consents to the removal of Licensor as a Party to the License Agreement); and SubCo 2 SubCo 1 3. o o an agreement, reasonably satisfactory to Licensee, between SubCo 1 and SubCo 2 pursuant to which SubCo 2 agrees to provide SubCo 1 with the funds necessary for SubCo 1 to comply with its obligations under the License Agreement to protect and maintain the Licensed Intellectual Property to the extent such protection and maintenance is required by Article V of the License Agreement. [Appendix D to Settlement and License Agreement] 3 Licensor
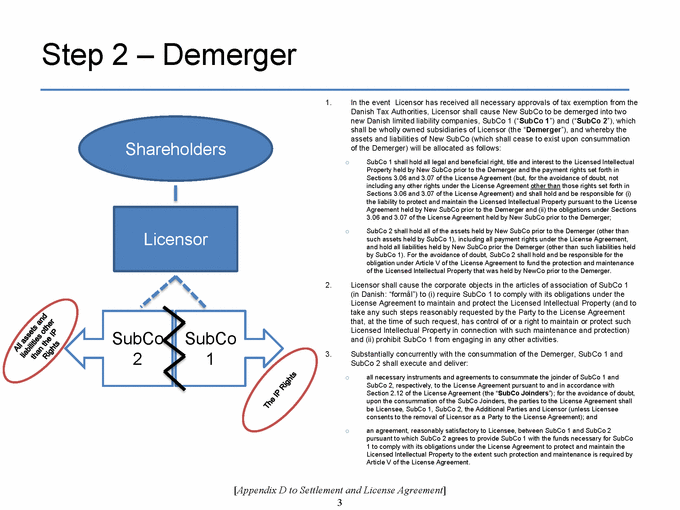
Step 3 – Establishment of a commercial foundation with a subsidiary 1. Following the consummation of the transactions contemplated by Step 2 and approval from the Danish Business Authority, a new Danish independent commercial foundation (in Danish: “erhvervsdrivende fond”) (the “Foundation”) shall be established with the Licensor as the founder by way of an irrevocable and unconditional contribution of capital from the Licensor equal to DKK 5 million in cash (the “Foundation Formation”) ”) and the Foundation shall be structured and operated in a manner that would enable payments to the Foundation to qualify for benefits under the U.S.-Danish income tax treaty; In connection with the Foundation Formation, Licensor shall cooperate in good faith with Licensee to prepare the articles of association of the Foundation, in form and substance approved by Licensee, which in any event shall: (i) be compliant in all respects with the Danish Act on Foundations Carrying on Business for Profit; (ii) allow the Foundation to own (directly or indirectly through a Subsidiary) the Licensed Intellectual Property; (iii) require the Foundation to cause its Subsidiaries to comply with their obligations under the License Agreement to the extent party thereto; (iv) provide detailed requirements on the qualifications to serve as an “independent” member of the board of directors of the Foundation (the “Foundation Board”); (v) provide that the Foundation Board consist of five members comprised of (a) three “independent” board members (the three initial “independent” board members shall be mutually selected by Licensee and Licensor at the Foundation Formation and any future vacancies shall be filled by the then remaining “independent” board members), (b) one non-independent board member appointed by Licensor and (c) one non-independent board member appointed by Licensee; (vi) require that all Foundation actions require unanimous approval of the Foundation Board; and (vii) provide that Licensor and Licensee shall each have the right following the Foundation Formation to provide additional capital contributions to the Foundation any time and from time to time as such Party deems necessary or desirable. All contact and communication with the Danish Business Authority regarding the establishment of the foundation shall be made jointly by the Licensee and Licensor. Promptly following the consummation of the Foundation Formation, the Foundation shall establish and form, as a wholly owned subsidiary of the Foundation, a new Danish private limited company (the “Holdco”), by cash subscription of DKK 50,000 (the “Holdco Formation”); and Promptly following the consummation of the Holdco Formation and prior to the consummation of the transactions set forth in Step 4, Licensor shall cause SubCo 1 and SubCo 2 (which, for the avoidance of doubt, are both wholly owned Subsidiaries of Licensor at such point) to enter into a trust agreement with Licensee and Licensor pursuant to which SubCo 1 agrees to hold, prosecute and maintain the Licensed Intellectual Property consistent with the requirements set forth in Step 2 in exchange for an annual fee of DKK 100,000 payable by SubCo 2; Provided, however, that if prior to the consummation of the transactions contemplated under this Step 3, Licensor and Licensee are informed (i) by the Danish Tax Authorities that Steps 1 and/or 2 (or modifications hereof proposed by the Licensee having no adverse effect to the Licensee or the Licensor compared to the structures described in Steps 1 and/or 2 in this Appendix D) will (a) not be approved as tax exempt or (b) will only be approved as tax exempt subject to the satisfaction of certain conditions or (ii) by the Danish Business Authority that the transactions contemplated by either Step 3 or Step 4 cannot be consummated, then Steps 3 and 4 shall automatically be abandoned (except in the case of clause (i)(b), where Steps 3 and 4 shall only be abandoned upon Licensee’s election in its sole discretion) and following any such abandonment of Steps 3 and 4 Licensor shall, at Licensee’s election in its sole discretion: grant Licensee a pledge in all of the issued and outstanding shares of SubCo 1 to secure the performance of its obligations under the License Agreement; allow the subscription by Licensee of 50% of the issued and outstanding shares of SubCo 1 at fair market value (as determined by a valuation report prepared by an auditor mutually selected by Licensor and Licensee); or cause SubCo 1 to consummate a P/S Sub Restructuring Alternative. 2. 3. 4. 5. o o o [Appendix D to Settlement and License Agreement] 4
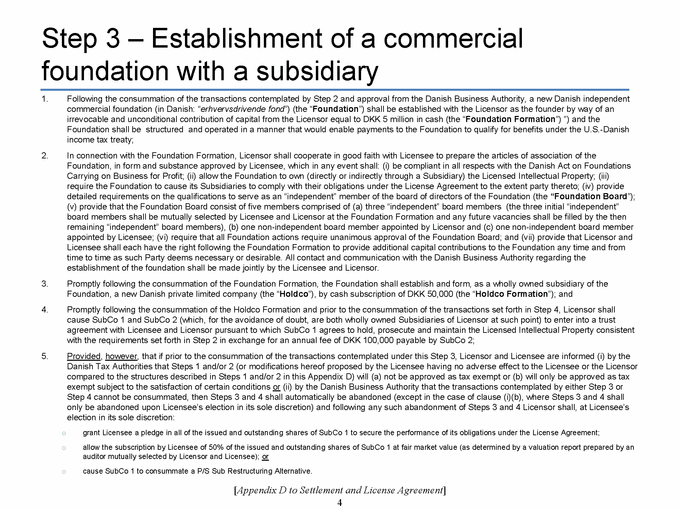
Step 4 – Transfer of shares 1. In the event Step 3 is consummated, Licensor shall then promptly sell all of the issued and outstanding shares of SubCo 1 to Holdco at fair market value (as determined by a valuation report prepared by an auditor mutually selected by Licensor and Licensee). Shareholders For the avoidance of doubt, following the consummation of this Step 4, SubCo 1 shall be a wholly owned Subsidiary of HoldCo and HoldCo shall remain a wholly owned Subsidiary of the Foundation. o Foundation Sale of shares [Appendix D to Settlement and License Agreement] 5 SubCo 1 SubCo 2 HoldCo Licensor
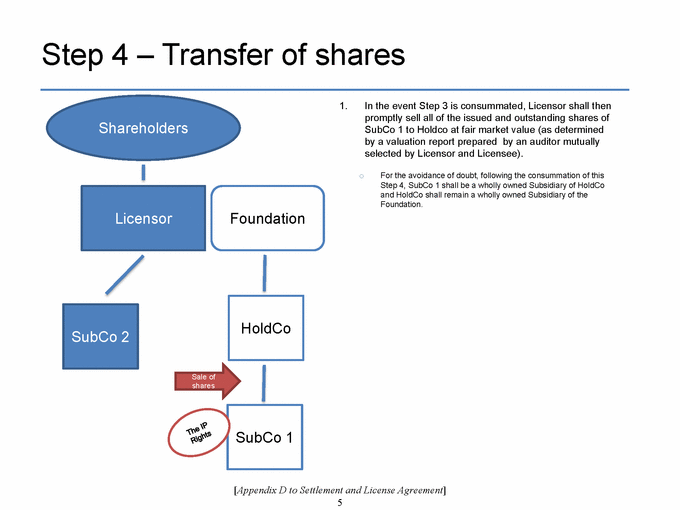
End structure following the consummation of the transactions contemplated by Step 4 1. Substantially concurrently with the consummation of Step 4, Holdco and Licensee shall enter into an agreement pursuant to which (i) Licensee shall receive the right to purchase all of the issued and outstanding shares of SubCo 1 from Holdco at a price corresponding to the intrinsic value (equity value) of SubCo 1 as per the most recent annual report for SubCo 1 at the time of exercise in the event SubCo 1 materially breaches its obligations under the License Agreement and Licensee shall be entitled to set off in the purchase price any claim it may have against HoldCo and/or SubCo 1 resulting from their breach of the License Agreement; and (ii) Holdco shall grant a pledge of all of the issued and outstanding shares of SubCo 1 in favor of Licensee as security for fulfilment of the purchase right described in clause (i) above. For the avoidance of doubt, exercise of this purchase right shall not affect Licensee’s obligations under Article IV of the License Agreement. Shareholders 2. The agreement described in Step 2 between SubCo 1 and SubCo 2 obligating SubCo 2 to fund SubCo 1’s obligations under the License Agreement to protect and maintain the Licensed Intellectual Property to the extent required by Article V of the License Agreement shall remain in full force and effect. Foundation 3. For the avoidance of doubt, if after the consummation of Step 4, Licensee exercises the U.S. Acquisition Option and/or the Designated Countries Acquisition Option, the Foundation shall cause SubCo 1 to (i) sell the applicable Licensed Intellectual Property to Licensee pursuant to and in accordance with Section 3.06 and/or Section 3.07 of the License Agreement, as applicable, and (ii) comply with all of its other obligations thereunder. 4. Where a Party to the License Agreement is given control of or a right to prosecute, maintain, defend and/or enforce any of the Licensed Intellectual Property, SubCo 1 (or P/S Sub, as the case may be) shall: • give such Party control of such actions; • give such Party an irrevocable right to take such actions in the name of SubCo 1 (or P/S Sub, as the case may be); • permit such Party to select attorneys to carry out such actions; • promptly provide such assistance and information to such Party as is reasonably necessary or desirable to enable such Party to carry out such actions (including joining as a party to any applicable Litigation if such joinder is reasonably necessary to advance such Party’s position) and such Party shall reimburse SubCo 1 (or P/S Sub, as the case may be)for any reasonable external costs reasonably incurred by it in providing such assistance and information; and • promptly copy to the Licensor and Licensee any correspondence or notifications received from a third party in relation to Licensed Intellectual Property, provided that SubCo 1 (or P/S Sub, as the case may be) shall not disclose to Licensee or its Affiliates such correspondence or notifications concerning Licensor’s (i) U.S. Patent Application 11/576,871, until the final, unappealable conclusion of the Interference Proceeding and (ii) European Patent Application EP 2801355, until the final, unappealable conclusion of the European Opposition Proceeding. License to IP Any sums or costs received by SubCo 1 (or P/S Sub, as the case may be) arising out of such action shall be paid to the Party taking or controlling such action. Any sums or costs awarded against SubCo 1 (or P/S Sub, as the case may be) arising out of such action shall be paid by the Party taking or controlling such action. [Appendix D to Settlement and License Agreement] 6 Licensee SubCo 1 HoldCo SubCo 2 Licensor
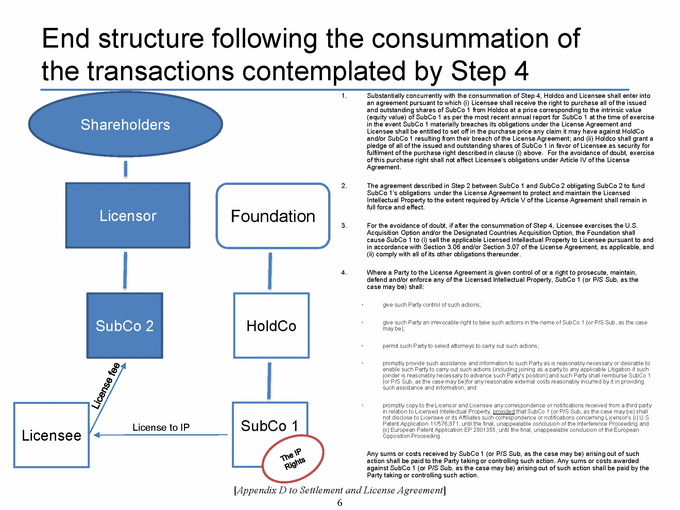
APPENDIX E
Draft Joint Submission of Agreement
|
Filed by: Junior Party BIOGEN MA INC. |
Paper No. |
By: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇
FINNEGAN, HENDERSON, FARABOW,
GARRETT & DUNNER, L.L.P.
▇▇▇ ▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇, ▇▇
▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
▇▇▇▇▇▇▇.▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇
▇▇▇▇▇▇▇.▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇
▇▇▇-▇▇▇-▇▇▇▇ tel
▇▇▇-▇▇▇-▇▇▇▇ fax
UNITED STATES PATENT AND TRADEMARK OFFICE
BEFORE THE PATENT TRIAL AND APPEAL BOARD
BIOGEN MA INC.
Junior Party
Patent 8,399,514 B2,
v.
FORWARD PHARMA A/S
Senior Party
Application 11/576,871.
Interference No. 106,023 (RES)
Technology Center 1600
JOINT SUBMISSION OF AGREEMENT AND JOINT REQUEST TO MAINTAIN AGREEMENT UNDER SEAL AND SEPARATE FROM THE INTERFERENCE FILE (pursuant to 35 U.S.C. §135(c) and 37 C.F.R. §41.205)
[Appendix E to Settlement and License Agreement]
Board of Patent Appeals and Interferences
United States Patent and Trademark ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇
▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Pursuant to 35 U.S.C. § 135(c), 37 C.F.R. § 41.205, and ¶ 205 of the Standing Order (Paper 2), Junior Party Biogen MA Inc. and Senior Party Forward Pharma A/S hereby give notice that the parties have made a written agreement relating to the Interference.
The agreement does not resolve any of the issues pending before the Board in this interference.
With the advance permission of the Board, the agreement and all related agreements are hereby submitted concurrently this day via hand delivery to the Board in a separate sealed envelope.
Pursuant to 35 U.S.C. § 135(c) and 37 C.F.R. §§ 41.205(c) and (d), the parties hereby request that the sealed agreements be kept separate from the Interference file and that the contents thereof be made available only to Government agencies on written request, or to any person only upon petition and on a showing of good cause. The parties further request that should such a petition be filed, each party be given the opportunity to comment on or oppose the request before a decision is made with respect to whether it should be granted or denied. Any such notification should be sent to the attention of:
For Junior Party Biogen MA Inc.:
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇
FINNEGAN, HENDERSON, FARABOW,
GARRETT & DUNNER, L.L.P.
▇▇▇ ▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇, ▇▇
▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
▇▇▇▇▇▇▇.▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇
▇▇▇▇▇▇▇.▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇
[Appendix E to Settlement and License Agreement]
▇▇▇-▇▇▇-▇▇▇▇ tel
▇▇▇-▇▇▇-▇▇▇▇ fax
For Senior Party Forward Pharma A/S
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
FITZPATRICK, CELLA, ▇▇▇▇▇▇ & ▇▇▇▇▇▇
▇▇▇▇ ▇▇▇▇▇▇ ▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇
▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
Tel: (▇▇▇) ▇▇▇-▇▇▇▇
Fax: (▇▇▇) ▇▇▇-▇▇▇▇
E-mail: ▇▇▇▇▇▇▇@▇▇▇▇.▇▇▇
The parties respectfully request acknowledgement of the filing of these agreements.
|
Dated: , 2017 |
Respectfully submitted, |
|
|
|
|
|
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ |
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇ | |
|
Reg. No. 27,276 |
Reg. No. 40,524 | |
|
Lead Attorney for Forward Pharma |
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇ | |
|
FITZPATRICK, CELLA, ▇▇▇▇▇▇ & ▇▇▇▇▇▇ |
Reg. No. 32,120 | |
|
1290 Avenue of the Americas |
FINNEGAN, HENDERSON, | |
|
New York, New York 10104-3800 |
FARABOW, GARRETT & DUNNER, | |
|
Telephone: (▇▇▇) ▇▇▇-▇▇▇▇ |
L.L.P. | |
|
Facsimile: (212) 218-2200 |
▇▇▇ ▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇, ▇▇ | |
|
|
▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇ | |
|
Of Counsel: |
▇▇▇▇▇▇▇.▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇ | |
|
▇▇▇▇▇ ▇. ▇▇▇▇▇▇ (admitted pro hac vice) |
▇▇▇▇▇▇▇.▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇.▇▇▇ | |
|
▇▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ & |
Telephone: ▇▇▇-▇▇▇-▇▇▇▇ | |
|
▇▇▇▇▇▇▇ LLP |
Facsimile: ▇▇▇-▇▇▇-▇▇▇▇ | |
|
▇▇▇▇ ▇▇▇▇▇▇ ▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇ |
| |
|
▇▇▇ ▇▇▇▇, ▇▇▇ ▇▇▇▇ |
Counsel for Biogen MA, Inc. | |
|
Telephone: (▇▇▇) ▇▇▇-▇▇▇▇ |
| |
|
|
| |
|
Counsel for Forward Pharma A/S |
| |
[Appendix E to Settlement and License Agreement]
APPENDIX F
Aditech Addendum
ADDENDUM TO PATENT TRANSFER AGREEMENT
|
between |
FORWARD PHARMA A/S |
|
|
|
|
and |
ADITECH PHARMA AG |
[Appendix F to Settlement and License Agreement]
This addendum, dated as of January 17, 2017 (the “Addendum”), to the Patent Transfer Agreement, including all schedules thereto, dated as of May 4, 2010 (the “Patent Transfer Agreement”)
between FORWARD PHARMA A/S
Company registration no. (CVR) 28865880
▇▇▇▇▇▇▇▇▇ ▇▇ ▇, ▇.
▇▇-▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇
(“Forward”)
and ADITECH PHARMA AG
Company registration no. CHE-114.631.207
c/o Domanda Verwaltungs GmbH
▇▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇
▇▇-▇▇▇▇ ▇▇▇
▇▇▇▇▇▇▇▇▇▇▇
(“Aditech”)
1. DEFINITIONS AND INTERPRETATIONS
1.1 Incorporation of definitions from Patent Transfer Agreement
Terms defined in the Patent Transfer Agreement shall have the same meaning when used in this Addendum, unless otherwise set out in this Addendum or unless the context otherwise requires; provided that Clause 1.2 of the Patent Transfer Agreement is hereby amended and replaced in its entirety with the definition of “Affiliate” set forth in Clause 1.2 of this Addendum.
1.2 Additional definitions
In addition to the definitions used in the Patent Transfer Agreement and the definitions set out in the header of this Addendum, the following definitions are used in this Addendum and shall have the following meanings in this Addendum (and, with respect to the definition of “Affiliate”, in the Patent Transfer Agreement):
“Addendum shall have the meaning set out in Clause 3.1;
Effective Date”
“Additional means each of the parties listed on Appendix I of the License Agreement (as
Parties” defined below);
[Appendix F to Settlement and License Agreement]
|
“Affiliate” |
|
means, with respect to any Person, any other Person that directly or indirectly, through one or more intermediaries, controls, is controlled by, or is under common control with, such first Person. For purposes of this definition, “control” (including the terms “controlled by” and “under common control with”), with respect to the relationship between or among two or more Persons, means the possession, directly or indirectly, of the power to direct or cause the direction of the affairs or management of a Person, whether through the ownership of shares of share capital or other equity or voting interests, by Contract or otherwise, including the ownership, directly or indirectly, of shares of share capital or other equity or voting interests having the power to elect a majority of the board of directors or comparable body governing the affairs of such Person. Such other Person shall be deemed to be an Affiliate only so long as such control exists; |
|
|
|
|
|
“Biogen |
|
means Biogen Swiss Manufacturing GmbH and Biogen International |
|
Parties” |
|
Holding Ltd. |
|
|
|
|
|
“Consent” |
|
means actions or non-actions, waivers, approvals, licenses, permits, orders or other authorizations and consents; |
|
|
|
|
|
“Contract” |
|
means any contract, agreement, deed, lease or similar instrument, and any legally binding obligation, commitment, arrangement or understanding, whether written or oral; |
|
|
|
|
|
“EU Relevant |
|
shall have the meaning set out in Clause 2.3; |
|
Patent” |
|
|
|
|
|
|
|
“European |
|
shall have the meaning set out in Clause 2.1; |
|
Opposition” |
|
|
|
|
|
|
|
“Final U.S. |
|
shall have the meaning set out in Clause 2.1; |
|
Interference |
|
|
|
Ruling” |
|
|
|
|
|
|
|
“Governmental |
|
means (i) any legislative, judicial or administrative authority, including any federal, state, local or foreign government (including, in each case, any self-regulatory organization), (ii) any court of competent jurisdiction, administrative agency or commission or other governmental authority or instrumentality, domestic or foreign or (iii) any officials of any of the entities set forth in sub-Clauses (i) or (ii); |
[Appendix F to Settlement and License Agreement]
|
“Intellectual |
|
means (i) all Patents; (ii) all trademarks, service marks, trade dress, logos, trade names, corporate names and Internet domain names, together with all goodwill associated therewith (including all translations, adaptations, derivations and combinations of the foregoing); (iii) copyrights and copyrightable works; (iv) registrations, applications, renewals, reissues, continuations, continuations in part, divisions, revisions, extensions or reexaminations for any of the items set forth in Clause (i), (ii) or (iii); (v) computer software; and (vi) trade secrets, confidential information, know-how, regulatory, market and data clearance or exclusivity information (including with respect to regulatory filings relating to investigational or approved medicines), drug master files, clinical data, models, assays, testing data and the like, in each of the foregoing Clauses (i) through (vi), in any jurisdiction in the world; |
|
|
|
|
|
“Laws” |
|
means, collectively, any applicable statute, law, ordinance, decree, order, rule, regulation, treaty, principle of common law, directive, resolution, code, stock exchange rule, judgment, ruling, injunction or requirement issued, enacted, adopted, promulgated, implemented or otherwise put into effect by or under the authority of any Governmental Entity; |
|
|
|
|
|
“License |
|
means the Settlement and License Agreement to be entered into by Biogen Swiss Manufacturing GmbH, Biogen International Holding LTD, Forward Pharma A/S and the Additional Parties; |
|
|
|
|
|
“Net Sales” |
|
shall have the meaning set out in the License Agreement; |
|
|
|
|
|
“Order” |
|
means any Law or judgment, order, writ, injunction, legally binding agreement with a Governmental Entity, stipulation or decree, including any binding decree of any arbitrator; |
|
|
|
|
|
“Patents” |
|
means all patents, patent applications, patent disclosures and inventions, including any reissues, reexaminations, replacements, continuations, continuations-in-part, divisionals, adjustments or extensions thereof or any other periods of exclusivity that extend the patent term (statutory or otherwise), including pediatric exclusivities and supplementary protection certificates, in any jurisdiction in the world; |
|
|
|
|
|
“Parties” |
|
means Forward and Aditech, collectively; |
|
|
|
|
|
“Party” |
|
means each of Forward and Aditech; |
|
|
|
|
|
“Person” |
|
means any individual, partnership, association, corporation, limited liability company, trust, governmental authority or other legal person or legal entity; and |
|
|
|
|
|
“US Relevant |
|
shall have the meaning set out in Clause 2.3. |
[Appendix F to Settlement and License Agreement]
|
1.3 |
|
Interpretation |
|
|
|
|
|
1.3.1 |
|
Unless the context otherwise requires, references to the singular number shall include references to the plural number and vice versa. References to Clauses are to Clauses, including sub-Clauses, of this Addendum. |
|
|
|
|
|
1.3.2 |
|
In this Addendum Clause headings are for ease of reference only and shall not affect in any way the meaning or interpretation of this Addendum. |
|
|
|
|
|
2. |
|
BACKGROUND |
|
|
|
|
|
2.1 |
|
Forward is involved in various Patent-related disputes with the Biogen Parties and/or their Affiliates both in Europe and in the United States concerning, among other things, the European opposition proceeding currently pending against Forward’s European Patent No. 2 801 355 (the “European Opposition”) and U.S. Patent and Trademark Office Interference No.106,023, or if such judgment is appealed, the judgment of the U.S. Court of Appeals for the Federal Circuit on such appeal (“Final U.S. Interference Ruling”). |
|
|
|
|
|
2.2 |
|
Forward and the Additional Parties are contemplating entering into the License Agreement with the Biogen Parties, which entitles Forward to receive certain payments from the Biogen Parties, as detailed in Clauses 2.3 and 2.4, as consideration for Forward and the Additional Parties granting the Biogen Parties and their Affiliates a license to certain of their Intellectual Property, including but not limited to the Patent Rights. |
|
|
|
|
|
2.3 |
|
The consideration to be received by Forward under the License Agreement is likely to consist of (i) a lump sum payment payable by the Biogen Parties to Forward regardless of the outcome of the Final U.S. Interference Ruling and the EU Opposition, so long as certain conditions are met, (ii) a royalty payment on certain products if the Final U.S. Interference Ruling results in Forward obtaining at least one issued U.S. Patent with at least one extant claim covering treatment of a human for multiple sclerosis by orally administering dimethyl fumarate, wherein the therapeutically effective amount of dimethyl fumarate is 480 mg per day (a “US Relevant Patent”), and (iii) a royalty payment on certain products if the final resolution of the European Opposition results in Forward obtaining at least one issued Patent with at least one extant claim covering treatment of a human for multiple sclerosis by orally administering dimethyl fumarate, wherein the therapeutically effective amount of dimethyl fumarate is 480 mg per day (a “EU Relevant Patent”). |
|
|
|
|
|
2.4 |
|
Under the License Agreement, subject to certain conditions, Forward and the Additional Parties will grant the Biogen Parties a co-exclusive license to the Patent Rights, the Fumaric Acid Products and/or the Fumaric Acid Processes in the United |
[Appendix F to Settlement and License Agreement]
|
|
|
States. Forward may also, subject to certain conditions, including any necessary antitrust clearance, grant an exclusive license to the Patent Rights to the Biogen Parties, and if Forward does not obtain a US Relevant Patent, Forward and the Additional Parties may at the Biogen Parties’ option transfer ownership of the Fumaric Acid Products and/or the Fumaric Acid Processes to the Biogen Parties. The size of the royalty payment referred to in Clause 2.3 (ii) if Forward obtains a US Relevant Patent will depend on whether the license granted under the License Agreement is exclusive or co-exclusive in the United States. The royalties payable for a co-exclusive license are lower than the royalties payable for an exclusive license. Under the License Agreement, subject to certain conditions, Forward and the Additional Parties will grant an exclusive license to the Patent Rights outside the United States. No royalties are payable under this exclusive license if Forward does not obtain an EU Relevant Patent from the EU Opposition. The royalty payments referred to Clause 2.3 may be suspended or terminated in the event of the entry into the market of a generic product that is therapeutically equivalent to and substitutable for certain products sold by or on behalf of either of the Biogen Parties. |
|
|
|
|
|
2.5 |
|
The purpose of this Addendum is (i) for the Parties to clarify, as set out in Clause 5, certain ambiguities with respect to the construction of the Patent Transfer Agreement in case Forward, the Additional Parties and the Biogen Parties enter into the License Agreement, and (ii) for Aditech to waive, as set out in Clause 6, certain rights under the Patent Transfer Agreement. |
|
|
|
|
|
3. |
|
EFFECTIVENESS OF THIS ADDENDUM |
|
|
|
|
|
3.1 |
|
The Parties hereby acknowledge and agree that this Addendum shall be effective as of January 17, 2017 (the “Addendum Effective Date”). |
|
|
|
|
|
4. |
|
REPRESENTATIONS AND WARRANTIES |
|
|
|
|
|
4.1 |
|
By signing this Addendum, each Party represents and warrants to the other Party as follows: |
|
|
|
|
|
4.1.1 |
|
Organization. Such Party is duly organized, validly existing and in good standing under the Laws of its jurisdiction of organization and has the requisite power and authority to execute, deliver and perform its obligations under this Addendum. |
|
|
|
|
|
4.2 |
|
Authority. This Addendum has been duly authorized, executed and delivered by such Party and constitutes a legal, valid, binding and enforceable agreement of such Party enforceable in accordance with its terms, except to the extent that enforcement hereof may be limited by bankruptcy, insolvency, fraudulent conveyance, reorganization, moratorium or other Laws affecting enforcement of creditors’ rights or by general equitable principles. |
[Appendix F to Settlement and License Agreement]
|
4.3 |
|
Noncontravention. The execution, delivery and performance by such Party of this Addendum will not (i) conflict with or result in a breach or violation of any of the terms or provisions of, or constitute a default under any Contract to which such Party or any of its Affiliates is a party or by which such Party or any of its Affiliates is bound or to which any of the property or assets of such Party or any of its Affiliates is subject, (ii) violate any provision of the organizational documents of such Party or any of its Affiliates or (iii) violate any Law or Order applicable to such Party or any of its Affiliates or their respective properties, except, in the case of sub-Clauses (i) and (iii), as would not reasonably be expected to impair in any material respect the ability of such Party to perform its obligations under this Addendum; and no filing with or Consent, registration or qualification of or with any Governmental Entity, is required for the execution, delivery and performance by such Party of its obligations under this Addendum, except for where the failure to obtain or make any such filing, Consent, approval, authorization, Order, registration or qualification would not reasonably be expected to impair in any material respect the ability of such Party to perform its obligations under this Addendum. |
|
|
|
|
|
5. |
|
CLARIFICATION OF THE CONSTRUCTION OF CERTAIN CLAUSES UNDER THE PATENT TRANSFER AGREEMENT |
|
|
|
|
|
5.1 |
|
The Parties hereby agree to the clarifications set out in this Clause 5 relating to the construction of the Patent Transfer Agreement in relation to the License Agreement. |
|
|
|
|
|
5.2 |
|
Notwithstanding any other possible construction of the Patent Transfer Agreement, the Parties hereby acknowledge and agree that any proceeds to be received by Forward from the Biogen Parties under the License Agreement shall only result in consideration being payable by Forward to Aditech under the Patent Transfer Agreement, as amended hereby, in accordance with paragraphs (a)-(d) below: |
|
|
|
|
|
|
|
a) Aditech shall be entitled to receive from Forward, and Forward shall pay to Aditech, promptly following its receipt thereof, a cash payment equal to 2% of any “lump sum” or “base consideration” consideration received by Forward from the Biogen Parties under the License Agreement, (prior to taking into account taxes, duties and VAT, if any) excluding, for the avoidance of doubt, any consideration referred to in paragraphs (b) and (c) of this Clause 5.2. By way of example, if Forward receives $1.25 billion under the License Agreement as an upfront fee, Forward shall pay $25 million to Aditech. |
|
|
|
|
|
|
|
b) In the event that Forward and the Additional Parties grant the Biogen Parties and their Affiliates an exclusive license to the Patent Rights (on a country-by-country basis) and Forward is entitled to receive royalty payments with respect to such Patent Rights in a country, Aditech shall be entitled to receive from Forward, and Forward shall pay to Aditech, promptly following its receipt |
[Appendix F to Settlement and License Agreement]
|
|
|
thereof, a cash payment equal to 2% of the Net Sales with respect to which Forward’s royalty percentage is calculated, accruing from the same period of time as any royalty payment payable by the Biogen Parties to Forward under the License Agreement (prior to taking into account taxes, duties and VAT, if any). Under no circumstances shall Forward be required to pay to Aditech an amount pursuant to this Clause 5.2(b) in excess of the royalty payment it receives from the Biogen Parties. By way of example, if the Biogen Parties pay Forward a royalty of 10% of the Biogen Parties’ Net Sales, then Forward shall pay to Aditech 20% of the amount received by Forward. |
|
|
|
|
|
|
|
c) In the event that Forward and the Additional Parties grant (i) the Biogen Parties and their Affiliates only a co-exclusive license to the Patent Rights in the United States and Forward is entitled to receive royalty payments with respect to such Patent Rights, and/or (ii) any other third party co-exclusive rights under the Patent Rights in the United States, Aditech shall be entitled to receive from Forward, and Forward shall pay to Aditech, promptly following its receipt thereof, a cash payment equal to 20% of (a) any royalty payment received by Forward from the Biogen Parties or (b) 20% of any royalty payment or any “lump sum” replacing a royalty payment, received by Forward from such other third party (prior to taking into account taxes, duties and VAT, if any). |
|
|
|
|
|
|
|
d) The Parties acknowledge and agree that any and all rights, title and interest in, to and under the Aditech Patent Rights have been lawfully, validly, and irrevocably transferred and assigned to Forward in accordance with Clause 2 of the Patent Transfer Agreement. If at any time from and after the date of this Addendum, any aspect of the immediately preceding sentence is not, for any reason, true and correct in all respects, Aditech agrees that it shall execute and deliver or procure the execution and delivery of any such instruments of transfer, conveyance, assignment and assumption, and take such other action as may be deemed necessary or desirable by Forward or the Biogen Parties, to confirm and assure that all rights, title and interest in, to and under the Aditech Patent Rights are lawfully, validly, and irrevocably transferred and assigned to Forward. |
|
|
|
|
|
|
|
e) For the avoidance of doubt, if Forward’s rights, including payment rights, with Aditech’s acceptance, are transferred to any other entity than Forward, including by means of merger, de-merger or similar, this Clause 5.2 shall be interpreted to include any payments made by the Biogen Parties to any such other entity. Hence, any such transfer shall in no event adversely affect Aditech’s rights pursuant to this Addendum and the Patent Transfer Agreement. Aditech irrevocably accepts and pre-approves the transfer of Forward’s rights, including payment rights, to a wholly-owned subsidiary of Forward as contemplated by Appendix D to the License Agreement. |
|
|
|
|
|
5.3 |
|
Aside from the consideration payable by Forward stipulated in Clause 5.2, the |
[Appendix F to Settlement and License Agreement]
|
|
|
Parties acknowledge and agree that Aditech shall not be entitled to receive any other consideration, royalty, proceeds or other form of payment or compensation from the Biogen Parties or Forward or any of their respective Affiliates under the Patent Transfer Agreement, as amended hereby, as a result of or in connection with (i) the License Agreement, and any licenses to be granted under the License Agreement and (ii) any rights to be granted to a third party (i.e., a permitted assignment of Forward’s co-exclusive rights). |
|
|
|
|
|
5.4 |
|
For the avoidance of doubt, each Party shall be responsible for and incur the full cost of paying any taxes, duties, VAT etc., which such Party itself may be subject to (including any withholding tax required to be deducted and withheld on a payment made to the other Party to such Party). |
|
|
|
|
|
6. |
|
WAIVER OF RIGHTS UNDER PATENT TRANSFER AGREEMENT |
|
|
|
|
|
6.1 |
|
Aditech hereby unconditionally and irrevocably waives all of its rights under the following Clauses of the Patent Transfer Agreement: 3; 4; 7 (with respect to any assignee or licensee of Forward); 8.1; 11.1; 11.3; 12.1; 12.3; 18.1; 18.2; and 18.7 (with respect to Clauses 7 and 12.3). |
|
|
|
|
|
6.2 |
|
For the avoidance of doubt, the Parties acknowledge and agree that the provisions of the Patent Transfer Agreement that are not amended, waived, terminated or clarified by this Addendum shall continue to be in full force and effect. |
|
|
|
|
|
6.3 |
|
For the avoidance of doubt, Aditech acknowledges and agrees that, from and after the date of this Addendum, it and its Affiliates shall have no rights in, to or under any of the Intellectual Property owned or controlled by Forward or any of its controlled Affiliates (including any Intellectual Property previously assigned by Aditech to Forward and any Intellectual Property under which Forward or any of its controlled Affiliates has the right to grant a license), worldwide, other than the right to the payments set forth in Clauses 5.2(a)-(c). |
|
|
|
|
|
6.4 |
|
The Parties unconditionally and irrevocably waive any breaches or other claims arising out of or under the Patent Transfer Agreement prior to the date of this Addendum. |
|
|
|
|
|
7. |
|
TERM AND TERMINATION |
|
|
|
|
|
7.1 |
|
Notwithstanding anything in the Patent Transfer Agreement to the contrary, the Parties hereby acknowledge and agree that the Patent Transfer Agreement and this Addendum shall remain in full force and effect indefinitely. The Parties further acknowledge and agree that no Party shall have the right to terminate or rescind, and shall not attempt to terminate or rescind, the Patent Transfer Agreement or this Addendum. |
[Appendix F to Settlement and License Agreement]
|
8. |
|
CHANGES TO THE LICENSE AGREEMENT |
|
|
|
|
|
|
|
Forward hereby agrees that it shall not amend the License Agreement if such amendment would have an adverse effect on Aditech’s rights under the Patent Transfer Agreement, as amended hereby, without Aditech’s prior written consent. |
|
|
|
|
|
9. |
|
OTHER PROVISIONS REMAIN EFFECTIVE |
|
|
|
|
|
|
|
Subject to the terms and conditions of this Addendum, the Patent Transfer Agreement shall remain in full force and effect and from and after the Addendum Effective Date, the Patent Transfer Agreement and this Addendum shall be read and construed as one document. |
|
|
|
|
|
10. |
|
CONFIDENTIALITY |
|
|
|
|
|
|
|
Clause 9 of the Patent Transfer Agreement shall apply mutatis mutandis to this Addendum and the Parties agree that the existence and contents of this Addendum and any information relating thereto and to the settlement negotiations with the Biogen Parties and their Affiliates shall be deemed Confidential Information for purposes of the Amended Patent Transfer Agreement. |
|
|
|
|
|
|
|
Notwithstanding Clause 9 of the Patent Transfer Agreement, the Parties agree that each Party shall be entitled to disclose the existence and contents of the Patent Transfer Agreement and this Addendum (i) as required by any applicable law or regulation, (ii) in connection with the extraordinary general meeting of Forward to be held in accordance with the License Agreement, including, for the avoidance of doubt, in connection with convening such general meeting, and (iii) as part of the negotiations of the License Agreement, under the Confidential Disclosure Agreement executed on October 14, 2016 between Biogen Inc. and Forward. |
|
|
|
|
|
11. |
|
LAW AND VENUE |
|
|
|
|
|
|
|
Clause 19 of the Patent Transfer Agreement shall apply mutatis mutandis to any dispute arising under this Addendum between the Parties or between any Party and any of the Biogen Parties. |
|
|
|
|
|
12. |
|
COUNTERPARTS |
|
|
|
|
|
|
|
This Addendum may be executed in one or more counterparts, all of which shall be considered one and the same agreement and shall become effective when one or more counterparts have been signed by each of the Parties and delivered to the other Parties. Delivery of an executed counterpart of a signature page of this Addendum by facsimile or other electronic image scan transmission shall be effective as delivery of a manually executed counterpart of this Addendum. |
[Appendix F to Settlement and License Agreement]
13. OTHER ACKNOWLEDGEMENTS
13.1 Third Party Beneficiaries
The Parties hereby acknowledge and agree that the Biogen Parties and each of their respective Affiliates, successors and assigns shall be an express third party beneficiary of this Addendum and shall have the right to directly enforce the terms and provisions of this Addendum. No Party may amend, agree to amend or waive any of its rights under this Addendum without the prior written consent of the Biogen Parties.
13.2 No Rights Under License Agreement
Aditech acknowledges and agrees that it does not and shall not have any rights in, to, under or with respect to the License Agreement, and that this Addendum does not grant it any such rights.
[Signatures to follow on the next page]
[Appendix F to Settlement and License Agreement]
|
FOR FORWARD PHARMA A/S |
|
FOR ADITECH PHARMA AG |
|
|
|
|
|
|
|
|
|
|
|
|
|
Signature |
|
Signature |
|
|
|
|
|
Name: ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ |
|
Name: ▇▇▇▇▇▇▇ ▇▇▇▇▇ |
|
|
|
|
|
Position: Chairman of Board of Directors |
|
Position: Director |
|
|
|
|
|
|
|
|
|
|
|
|
|
Signature |
|
|
|
|
|
|
|
Name: ▇▇▇▇▇ ▇▇▇▇▇▇▇▇ |
|
|
|
|
|
|
|
Position: Member of Board of Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Signature |
|
|
|
|
|
|
|
Name: ▇▇▇▇▇ ▇▇▇▇▇ |
|
|
|
|
|
|
|
Position: Member of Board of Directors |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Signature |
|
|
|
|
|
|
|
Name: Jan van ▇▇ ▇▇▇▇▇▇ |
|
|
|
|
|
|
|
Position: Member of Board of Directors |
|
|
[Appendix F to Settlement and License Agreement]
APPENDIX H
Scheduled Employment Matters
It is possible that employees listed below who are employed in jurisdictions in which the TUPE Regulations apply may assert claims under such regulations as a result of the transactions contemplated by the Agreement. The parties do not acknowledge hereby that any such claims will be accepted or are legally valid. Finally, Licensor has agreed to indemnify Licensee for any such claims pursuant to Section 2.10.
|
1. |
▇▇▇▇▇ Rundfeldt (employee) |
|
|
|
|
2. |
▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ (employee) |
|
|
|
|
3. |
▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ (CEO) |
|
|
|
|
4. |
▇▇▇▇ ▇▇▇▇▇▇ (employee) |
|
|
|
|
5. |
▇▇▇▇▇▇ ▇▇▇▇▇▇▇ (employee) |
|
|
|
|
6. |
▇▇▇▇▇▇ ▇▇▇▇▇▇ (employee) |
|
|
|
|
7. |
▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇ (employee) |
|
|
|
|
8. |
▇▇▇▇▇▇▇▇▇ ▇▇ ▇▇▇▇▇▇▇ (former employee) |
|
|
|
|
9. |
▇▇▇▇▇▇▇ ▇▇▇▇ (employee) |
|
|
|
|
10. |
Claus ▇▇ ▇▇▇▇▇▇▇▇ (employee) |
|
|
|
|
11. |
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇ (employee) |
|
|
|
|
12. |
▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇ (employee) |
|
|
|
|
13. |
Anders LivsØ (employee) |
|
|
|
|
14. |
▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇ (employee) |
|
|
|
|
15. |
Torben Tvermosegaard (employee) |
[Appendix H to Settlement and License Agreement]
APPENDIX I
Additional Parties
1. Aditech Pharma AG
2. NB FP Investment General Partner ApS
3. NB FP Investment SLP ApS
4. Tech Growth Invest ApS
[Appendix I to Settlement and License Agreement]

