EXCLUSIVE LICENSE AGREEMENT
Exhibit 10.11
Pages where confidential treatment has been requested are stamped ‘Confidential Treatment Requested and the
Redacted Material has been separately filed with the Commission,’ and the confidential section has been
marked as follows: [***].
This Exclusive License Agreement (Agreement), effective as of this day of November 1, 2001
(the “Effective Date”), is by and between Cambridge University Technical Services Ltd., an English
limited company (“CUTS”) and Ceres, Inc. (“Ceres”), a Delaware corporation, having a principal
place of business at 0000 Xxxxxx Xxxxxx Xxxx, Xxxxxx, Xxxxxxxxxx 00000.
W I T N E S S E T H:
WHEREAS, The Chancellor, Masters and Scholars of the University of Cambridge and Ceres have
entered into that certain Sponsored Research Agreement dated June 1, 2000 in support of research
and development work, including the screening of certain transgenic plants;
WHEREAS, CUTS is the owner of certain patent rights and other intellectual property developed
by Xx. Xxxxxxxx relating to (i) Arabidopsis transgenic plants and (ii) the HAP-1 and other
extensin-GFP constructs and Ceres desires to license such rights from CUTS;
WHEREAS, CUTS desires to provide to Ceres certain first rights of refusal to Other Project
Technology (defined below); and
WHEREAS, Ceres desires to grant to the University of Cambridge license rights to certain of
Ceres’ technologies for the University’s non-commercial research and teaching activities.
NOW, THEREFORE, in consideration of the foregoing premises and the following mutual covenants,
and other good and valuable consideration, the receipt of which is hereby acknowledged, and
intending to be legally bound hereby, the Parties agree as follows:
1. DEFINITIONS
As used in this Agreement, the following terms will have the meaning set forth below:
1.1 “Affiliate(s)” shall mean (a) any company owned or controlled to the extent of at least
fifty percent (50%) of its issued and voting capital by a Party to this Agreement and any other
company so owned or controlled (directly or indirectly) by any such company or the owner of any
such company, or (b) any partnership, joint venture or other entity directly or indirectly
controlled by, controlling, or under common control with, to the extent of at least fifty percent
(50%) of voting power (or otherwise having power to control its general activities), a Party to
this Agreement.
1.2 “Background Technology” shall mean the Technologies developed prior to the Effective Date
of the Sponsored Research Agreement, which CUTS or Ceres owns, or has license rights to, and which
are useful for the Purpose. The Party’s respective Background Technology shall be referred to as
Ceres Background Technology and CUTS Background Technology. CUTS Background Technology shall
include certain CUTS Technology Rights relating to Arabidopsis transgenic plants, as listed in
Exhibit A; except that CUTS Background Technology shall not include any HAP-1 Technology, nor any
Project Technology. In addition, Ceres Background Technology shall include Technologies relating to
recombinant transmembrane proteins as defined in Exhibit I to Amendment I to the Sponsored Research
Agreement, developed prior to the Effective Date of such Amendment I.
1.3 “Biological Material” means any plants, seeds, microorganisms, cells, parts of cells, DNA,
RNA, cDNA, proteins, peptides, enzymes, and any combination of the foregoing, and/or other organic
matter and/or biologically active compounds.
1.4 “Biological Product(s)” means any product comprising the Biological Materials.
1.5 “Confidential Information” means any information, disclosed by one Party to this Agreement
to the other Party, that has any commercial value to the disclosing Party’s business, research,
development or other activities. Confidential Information includes, without limitation,
inventions, biological materials, technical information, trade secrets, financial information,
product plans, customer lists, marketing plans and strategies, forecasts and other business
information, improvements, ideas, works of authorship, processes, computer programs, techniques,
schematics, data, gene sequences, gene expression data, protein sequences, protein structures,
regulatory sequences, and other data.
1.6 “HAP-1 Patent” shall mean any Patent Rights based on the patent application listed in
Exhibit B, which includes the HAP-1 Technology.
1.7 “HAP-1 Products” shall mean all products, processes, or services including Biological
Products that are to be commercialized, the manufacture, use or sale of which is covered by any
valid and subsisting claim of the HAP-1 Patent.
1.8 “HAP-1 Technology” means any and all Technology related to and including (i) the HAP-1
sequences or constructs and (ii) the extensin GFP gene constructs, as further described in Exhibit
B.
1.9 “Net Sales” shall mean the gross amount actually received on sales of HAP-1 Products to
third parties (except as set forth below) by Ceres, and its Affiliates, less the following: (i)
customary trade, quantity, or cash discounts and commissions to non-affiliated brokers or agents to
the extent actually allowed and taken; (ii) amounts repaid or credited by reason of rejection or
return; (iii) any sales, use, tariff, customs duties, V.A.T. and/or other taxes, duties and similar
governmental assessments (except taxes based on income), which are paid by or on behalf of Ceres;
and (iv) outbound transportation, shipping, packing, costs of insurance in transit, and other costs
paid or allowed by Ceres; subject in all cases (i) to (iii) being separately
- 2 -
charged on customer invoices or credit notes. In any transfers of Product between Ceres and
an Affiliate, Net Sales shall be calculated based only on the final sale of the Product to an
independent third party.
1.10 “Non-Commercial Research and Teaching” shall mean research and teaching activities whose
primary purpose is the advancement of science or academic learning and dissemination of knowledge
excluding (a) any research sponsored by a commercial entity other than Ceres or (b) any research
where the results will be provided to a commercial entity other than Ceres, either directly by CUTS
or the University or via collaboration or otherwise, and other than through publication in a
learned journal.
1.11 “Other Project Technology” shall mean any Technology, which the University develops,
which is based on or created by using Project Technology and/or which furthers the Purpose without
funding or information from Ceres during the Research Project.
1.12 “Party” means, CUTS or Ceres, collectively they are sometimes referred to as the
“Parties”.
1.13 “Patent Rights” shall mean all patents and patent applications throughout the world,
including any reissues, extensions, substitutions, continuations, divisions, and
continuations-in-part applications, reexaminations or extensions or other government actions which
extend the life of a patent, and all rights to apply for patent protection and all rights, if any,
to xxx or bring other actions for past, present or future infringement of such rights. The Party’s
respective Patent Rights shall be referred to as Ceres Patent Rights and CUTS Patent Rights.
1.14 “Products” shall mean all products, processes, or services including Biological Products
that are to be commercialized, the manufacture, use or sale of which is covered by any valid and
subsisting claim of the Patent Rights within CUTS Background Technology, and/or the Project
Technology, except that Products shall not include any HAP-1 Products.
1.15 “Project Technology” shall mean any and all Technology developed or obtained during and
resulting from the Research Project, but excluding any Background Technology, HAP-1 Technology and
Other Project Technology.
1.16 “Purpose” shall mean the generation and analysis and transfer to Ceres of data and large
numbers of transgenic Arabidopsis plants and/or seeds transformed with constructs containing the
GFP gene the expression of which is indirectly driven by a large amount of different plant
promoters, as further described in the Research Plan.
1.17 “Research Project” shall mean the collaborative research program under which the
University and Ceres have performed and shall perform certain research and development activities
in pursuit of the Purpose and in accordance with the Research Plan, as defined in the Sponsored
Research Agreement.
- 3 -
1.18 “Research Plan” shall mean the mutually agreed document attached as Exhibit A to the
Sponsored Research Agreement that describes the respective research experiments and the specific
responsibilities of Ceres and University in performing the Research Project.
1.19 “Sublicense Income” shall mean the gross amount actually received by either Ceres or its
Affiliates in consideration for sublicenses of any of the rights under the HAP-1 Patent granted
hereunder, including up-front fees, lump sum payments and any running royalties (on a
product-by-product and country-by-country basis), without deduction of any kind, but excluding the
following, in relation to which no payments shall be due to CUTS:
(a) Payments received by either Ceres or its Affiliates solely for performance of research and
development, including but not limited to milestone payments for achievement of objectives in
research and development, only to the extent that such payments (i) cover the actual cost of the
research and development work; (ii) cover the amounts of the milestone payments due under Paragraph
5.5 hereof; or, (iii) are directly related to development of products that would be covered by this
Agreement;
(b) Investments made by a sublicensee in either Ceres or its Affiliates;
(c) Payments made to either Ceres or its Affiliates solely to the extent that they cover the
actual costs of conducting testing and other activities in connection with obtaining regulatory
approval for a Product;
(d) Reimbursed expenses of either Ceres or its Affiliates.
1.20 “Sponsored Research Agreement” shall mean that certain sponsored research agreement dated
June 1, 2000, between the University and Ceres in respect of the Research Project.
1.21 “Technological Element” shall mean any individual Biological Material, data, methods,
protocols, procedures, processes arising out of the University’s performance of the Research
Project and which is employed or embodied in a Product and which can be separated from other such
Biological Material, data, methods, protocols, procedures, processes employed or embodied in the
Product and which, in the absence of a license, would infringe CUTS Patent Rights. There shall be
three (3) mutually exclusive types of Technological Elements, (i) those solely owned by CUTS, (ii)
those jointly owned by CUTS and Ceres, and (iii) all other Technological Elements. A non-inclusive
list of examples of Technological Elements can be found in Exhibit C hereto.
1.22 “Technology” shall mean any Biological Material, Biological Product, data, methods,
protocols, procedures, processes and the like, and the Patent Rights and Technology Rights relating
thereto.
1.23 “Technology Rights” shall mean existing and future proprietary rights, including but not
limited to know-how rights, trade secret rights, copyrights, design rights, and all other
- 4 -
intellectual property rights (including without limitation the right, if any, to xxx or bring
other actions for past, present or future infringement of such proprietary rights), but excluding
Patent Rights. The Party’s respective Technology Rights shall be referred to as Ceres Technology
Rights and CUTS Technology Rights.
1.24 “Term” shall mean the period beginning on the Effective Date, and ending on the earlier
of (i) the date of the expiration of the last to expire patent licensed hereunder, or if no patents
are licensed hereunder, ten (10) years from the Effective Date, or (ii) the termination hereof
pursuant to the terms of Section 6 of this Agreement.
1.25 “University” shall mean the University of Cambridge.
2. PROJECT TECHNOLOGY
CUTS acknowledges and agrees with Section 4.2 of the Sponsored Research Agreement on Ownership
of Project Technology.
3. LICENSE GRANTS
3.1 Subject to all the terms of this Agreement, CUTS hereby grants to Ceres, under CUTS Patent
Rights and CUTS Technology Rights, a fully paid-up, irrevocable, world-wide, non-exclusive license,
including the right to grant sublicenses, to make, have made, use, or have used, CUTS Background
Technology.
3.2 Subject to all the terms of this Agreement Ceres hereby grants to CUTS a limited license
to use the Project Technology solely for Non-Commercial Research and Teaching purposes. Ceres
acknowledges and agrees that such license will be implemented by CUTS solely at the University,
under the full responsibility of CUTS.
3.3 Subject to all the terms of this Agreement CUTS hereby grants to Ceres, under CUTS Patent
Rights and CUTS Technology Rights an irrevocable, world-wide, exclusive license, including the
right to grant sublicenses: (a) to possess, to make (e.g. propagate), have made, use, sell, have
sold, offer for sale, import and have imported the HAP-1 Technology, and (b) to make, have made,
use, sell, have sold, offer for sale, import and have imported HAP-1 Products; except that CUTS
shall retain the limited right to use such HAP-1 Technology for Non-Commercial Research and
Teaching purposes solely at the University under the full responsibility of CUTS.
3.4 Ceres will notify CUTS of each sublicense granted hereunder.
3.5 Subject to all the terms of this Agreement, and only in respect of Ceres Background
Technology that Ceres makes available to the University for conducting the Research Project, Ceres
hereby grants to CUTS, under Ceres Patent Rights and Ceres Technology Rights, a limited, fully
paid-up, non-exclusive license to use such Ceres Background Technology solely at the University and
only (i) to perform the Research Project and (ii) for
- 5 -
Non-Commercial Research and Teaching purposes.
4. OTHER PROJECT TECHNOLOGY
Prior to commercializing or granting any rights to any third party to commercialize any Other
Project Technology, CUTS shall offer to Ceres in writing terms for the commercialization of such
Other Project Technology (“Offer”), and Ceres will have a sixty (60) day period from the date of
delivery of the Offer in which to indicate its desire to accept such Offer, subject to negotiation
of definitive agreements. If the Offer is declined or is not accepted during such sixty (60) day
period, CUTS may either commercialize itself or grant a license to a third party on terms no more
favorable to the third party than the terms of the Offer. If Ceres indicates its desire to accept
the Offer in writing within said sixty (60) day period, the Parties agree to negotiate in good
faith the definitive agreements for such commercialization; provided, however, that if the Parties
are unable to agree upon the terms and conditions of any such license or acquisition agreement
within twelve months of delivery of the written notice of Ceres’ desire to accept the Offer, CUTS
may either commercialize itself or grant a license to a third party on terms no more favorable to
the third party than the terms offered by CUTS to Ceres. In determining whether the terms offered
to a third party are more favorable than those received or accepted by Ceres, all terms and
conditions of the respective offers shall be considered, including but not limited to, monetary
terms, scope of rights granted, warranties and indemnities.
5. CONSIDERATION
5.1 License Initiation Fee. Ceres agrees to pay to CUTS the amount of [***] United
States dollars (U.S. $[***]) within thirty (30) business days of the Effective Date. Such fee
includes the reimbursement of all costs incurred by CUTS in connection with the filing and
prosecution of the HAP-1 Patent.
5.2 Royalty on Net Sales. Ceres shall pay to CUTS an earned royalty on Net Sales on a
product-by-product and country-by-country basis. Earned royalties shall accrue in each country for
the duration of CUTS Patent Rights covering such HAP-1 Product in that country. Ceres shall pay
royalties to CUTS on Net Sales from the first date of commercial introduction of a HAP-1 Product,
which royalty shall be A. A is defined as follows: the lesser of [***] percent ([***]%) or
(W/X)(MRNS); where X shall be the total number of Technological Elements in the HAP-1 Product; W is
the sum of one half of the number of Technological Elements contributed to the HAP-1 Product which
are jointly owned by CUTS and Ceres plus the total number of Technological Elements contributed to
the HAP-1 Product solely by CUTS; and, MRNS shall be the maximum royalty on Net Sales, which is
[***] percent ([***]%). The Parties further agree to negotiate in good faith for another royalty
rate in the event of substantial market considerations.
5.3 Royalty on Sublicense Income. Ceres shall pay to CUTS an earned royalty on
Sublicense Income actually received by Ceres, which royalty shall be A. A is defined as follows:
the lesser of [***] percent ([***]%) or (W/X)(MRNS); where X shall be the total number of
Technological Elements in the sublicense; W is the sum of one half of the number of Technological
Elements contributed to the sublicense which are jointly owned by CUTS and
- 6 -
| Confidential Treatment Requested and the Redacted Material has been separately filed with the Commission. | ||
Ceres plus the total number of Technological Elements contributed to the sublicense solely by
CUTS; and, MRNS shall be the maximum royalty on Sublicense Income, which is [***] percent ([***]%).
The Parties further agree to negotiate in good faith for a lower royalty rate in the event of
substantial market considerations.
5.4 Milestone Payments. Ceres shall pay to CUTS the following Milestone payments:
(a) within thirty (30) days of Ceres’ successful validation of the technical evaluation
protocol for a dicotyledon plant, such as Arabidopsis, associated with the HAP-1 Technology and
described in Exhibit B of this Agreement, Ceres shall pay to CUTS [***] United States dollars (U.S.
$[***]). Ceres shall use all reasonable efforts to complete such validation within two (2) years
of the Effective Date;
(b) within thirty (30) days of Ceres’ successful validation of the technical evaluation
protocol for a monocotyledon plant, such as rice, associated with the HAP-1 Technology and
described in Exhibit B of this Agreement, Ceres shall pay to CUTS [***] United States dollars (U.S.
$[***]);
(c) within thirty (30) days of CUTS’ notice to Ceres that a United States patent under the
HAP-1 Patent, licensed to Ceres hereunder has been issued to CUTS, Ceres shall pay to CUTS [***]
United States dollars (U.S. $[***]);
(d) within thirty (30) days of CUTS’ notice to Ceres that a European patent under the HAP-1
Patent licensed to Ceres hereunder, has been issued to CUTS, Ceres shall pay to CUTS [***] United
States dollars (U.S. $[***]).
5.5 Royalty Reports; Payments; Records
(a) First Sale. Ceres shall report to CUTS the date of first commercial sale of any HAP-1
Product within thirty (30) business days of occurrence in each country.
(b) Reports and Payments. Within sixty (60) days after the conclusion of each calendar year
following First Sale, Ceres shall deliver to CUTS a report containing the following information:
- 7 -
| Confidential Treatment Requested and the Redacted Material has been separately filed with the Commission. | ||
(i) the number of HAP-1 Products sold to independent third parties in each
country;
(ii) the gross sales price for each HAP-1 Product sold by Ceres and its
Affiliates during the applicable year in each country;
(iii) the calculation of Net Sales and Sublicense Income for the applicable
year in each country, including a listing of applicable deductions and
credits applied;
(iv) the total royalty payable on Net Sales in U.S. dollars, together with
the exchange rates used for conversion; and,
(v) a statement indicating whether any Milestones have been attained
pursuant to Section 5.5.
All such reports shall be considered Ceres Confidential Information pursuant to Section 7, and
shall not be transferred to a third party. If no royalties are due to CUTS for any year, the
report shall so state. Concurrent with this report, Ceres shall remit to CUTS any payment due in
respect of Net Sales, Sublicense Income or Milestones for the applicable year. CUTS shall instruct
Ceres as to the method of payment.
(c) Records. Ceres shall maintain, and shall cause its Affiliates to maintain, reasonably
complete and accurate records of HAP-1 Product that is made, used, sold, or performed and
Sublicense Income received under this Agreement and any amounts payable to CUTS in relation to such
HAP-1 Product, which records shall contain sufficient information to permit CUTS to confirm the
accuracy of any reports delivered to CUTS under this Section 5.6. The relevant party shall retain
such records relating to a given royalty period for at least three (3) years after the conclusion
of that royalty period, during which time CUTS shall have the right, at its sole expense, to cause
an independent, certified public accountant to inspect such records once per calendar year, upon
thirty (30) days’ prior written notice, during normal business hours for the sole purpose of
verifying any reports and payments delivered under this Agreement. The parties shall reconcile any
underpayment or overpayment within thirty (30) days after the accountant delivers the results of
the audit. In the event that any audit performed under this Section reveals an aggregate
underpayment in excess of five percent (5%) during any calendar year, Ceres or the applicable
sublicensee or Affiliate shall bear the full cost of such audit.
6. TERM AND TERMINATION
6.1 Term. Unless otherwise terminated by operation of law or by acts of the Parties
in accordance with the terms of this Agreement, this Agreement will be in force for the Term.
6.2 Monies Due/Accrued Rights. Any termination of this Agreement shall not relieve
Ceres of its obligation to pay any monies due or owing at the time of such termination and will not
impair any accrued right of CUTS arising under this Agreement prior to such termination.
- 8 -
6.3 Termination Upon Breach. Upon material breach or default of any of the terms and
conditions of this Agreement, the defaulting Party shall be given notice of such default in writing
and a period of sixty (60) days after receipt of such notice to correct the breach or default. If
(a) the default or breach (i) is material to this Agreement taken as a whole, and (ii) is not
corrected within said sixty (60) day period and the defaulting Party has not taken reasonable steps
to cure the same, and (b) the Party not in default has fully complied with all of its obligations
under this Agreement and (c) the Party not in default has no adequate remedy from monetary changes,
the Party not in default shall have the right to terminate this Agreement. In the event that this
Agreement is terminated due to a breach by CUTS, Sections 3.1 and 3.3 of this Agreement shall
survive such termination.
6.4 Termination upon Bankruptcy. A Party shall have the right to terminate this
agreement upon the first to occur of the following events: (i) a petition of action is filed or
action taken by or against the other Party under any law dealing with insolvency or bankruptcy;
(ii) a receiver is appointed over the assets or undertaking of the other Party; (iii) the other
Party enters into a deed of arrangement or makes an assignment for the benefit of creditors; or
(iv) the other Party ceases to function as a going concern or an order is made or a resolution
passed to that effect.
6.5 Ceres Termination Rights. In addition to the above termination rights, Ceres
shall be entitled to terminate this Agreement at any time, with or without cause, upon providing
CUTS with ninety (90) days’ notice of termination in writing.
7. CONFIDENTIALITY
7.1 Mutual Non-Disclosure Obligations. Without prejudice to Sections 7.7 and 7.8 of
the Sponsored Research Agreement, each Party hereby agrees that it shall keep confidential and not
use for any purpose, except as provided herein, all Confidential Information supplied to it (the
“Recipient”) by the other Party (the “Disclosing Party”) during the term of this Agreement and for
five (5) years after termination or expiration hereof; provided, however, that the foregoing
obligations of confidentiality and non-use shall not apply to the extent that any Confidential
Information is demonstrated by written records to be (a) already known to the Recipient or one of
its Affiliates at the time of disclosure hereunder (provided the Recipient and/or its Affiliates
comply with any restrictions imposed by third parties); or (b) is hereafter developed by the
Recipient or one of its Affiliates in the course of work entirely independent of any disclosure
hereunder; or (c) publicly known prior to or after disclosure hereunder other than through acts or
omissions of the Recipient or one of its Affiliates; or (d) disclosed in good faith to the
Recipient or one of its Affiliates by a third party (provided the Recipient and/or its Affiliates
comply with any restrictions imposed by third parties). This does not prevent disclosure to third
parties by the Recipient under a secrecy or confidentiality agreement with essentially the same
confidentiality provisions provided herein in connection with the exercise of its rights under this
Agreement (but only to the extent permitted herein). In addition, disclosure may be made (i) to
Recipient’s employees, consultants, representatives, agents and advisors provided that such persons
are subject to confidentiality obligations consistent with the ones set
- 9 -
forth in this Section 7.1, and (ii) to governmental agencies to the extent required to secure
governmental approval for marketing of the Products; provided, however, that the Recipient shall
seek to limit disclosure and to obtain confidential treatment therefor.
7.2 Affiliates, Licensee and Sublicensees. Nothing herein shall be construed as
preventing Ceres from disclosing any information received from CUTS to an Affiliate, licensee or to
a sublicensee of Ceres, provided such Affiliate, licensee or sublicensee has undertaken a similar
obligation of confidentiality with respect to the Confidential Information.
7.3 Internet Communications. To the extent that the Parties use the Internet as a
means of communication, all e-mail and/or other Internet-based communications containing
Confidential Information shall be encrypted.
8. PRESS RELEASES AND USE OF NAMES AND TRADEMARKS
8.1 Press Releases. All press releases which one Party desires to make relating to
the Research Project or any of the matters contemplated hereunder shall be prepared by such Party
as a joint press release of the Parties and shall not be publicly released or released to the press
without the prior written consent of the other Party.
8.2 Use of Tradenames. Neither Party shall disclose or use the name of the other for
any purpose without the prior written consent of the named Party, except for the purposes of
referring to this Agreement in disclosures to be made in documents in connection with financings
and/or as required by law.
9. REPRESENTATIONS AND WARRANTIES
9.1 Ceres Representations and Warranties. Ceres represents to CUTS that:
(a) Ceres is a corporation duly organized, validly existing and in good standing under the
laws of the State of Delaware and has all requisite corporate power and authority to carry on its
business as now conducted;
(b) All corporate action on the part of Ceres and its officers and directors necessary for the
authorization, execution and delivery of this Agreement and the performance of all obligations of
Ceres hereunder has been taken, and this Agreement constitutes a valid and legally binding
obligation of Ceres, enforceable in accordance with its terms; and,
(c) Ceres warrants to CUTS that it has the lawful right to grant the licenses granted to CUTS
under this Agreement.
9.2 CUTS Representations and Warranties.
(a) CUTS is a company duly organized, validly existing and in good standing under the laws of
England and Wales has all requisite power and authority to carry on its
- 10 -
business as now conducted;
(b) All action on the part of CUTS and its officers and directors necessary for the
authorization, execution and delivery of this Agreement and the performance of all obligations of
CUTS hereunder has been taken, and this Agreement constitutes a valid and legally binding
obligation of CUTS, enforceable in accordance with its terms; and
(c) CUTS warrants to Ceres that it has the lawful right to grant the licenses granted to Ceres
under this Agreement. CUTS warrants that, to the best of its knowledge, CUTS owns and has full
rights, title and interest, through assignment by the University and/or inventors associated with
the University, in all Technology Rights, Patent Rights or other rights which are or shall or may
be licensed to Ceres pursuant to this Agreement; including without limitation, Technology Rights,
Patent Rights and other rights on CUTS Background Technology, on the HAP-1 Patent and HAP-1
Technology, and on Other Project Technology.
10. DISCLAIMERS
10.1 Project Technology and CUTS Background Technology. The Parties accept no
responsibility whatsoever for any use which may be made of any work carried out under or pursuant
to this Agreement, of the Project Technology, or of its Background Technology, and no liability
whatsoever either direct or indirect shall rest upon the a Party, its employees, students, agents
or appointees for the effects of any Product or process that may be developed, manufactured, used,
sold, imported or distributed by or on behalf of the other Party or any Affiliate or sublicensee of
the other Party, notwithstanding that such Product or process may be based upon the findings of the
Research Project, the results or upon any other advice or information furnished by a Party, its
employees, students, agents or appointees under this Agreement.
10.2 General. Except as expressly provided for in this Agreement the licenses granted
to the Parties under this Agreement and the associated Technology, Biological Materials, Patent
Rights, property rights, Products, and patent methods are provided WITHOUT WARRANTY OF
MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR ANY OTHER REPRESENTATION OR WARRANTY,
EXPRESS OR IMPLIED.
11. LIMITATION OF LIABILITY
11.1 EXCLUSIONS. IN NO EVENT WILL ANY PARTY BE LIABLE UNDER ANY CONTRACT, NEGLIGENCE,
STRICT LIABILITY OR OTHER THEORY FOR ANY INDIRECT, INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES
INCLUDING, BUT NOT LIMITED TO, LOSS OF REVENUES AND LOSS OF PROFITS IN CONNECTION WITH THIS
AGREEMENT OR IN CONNECTION WITH THE USE OF THE TECHNOLOGY, BIOLOGICAL MATERIALS, PATENT RIGHTS,
PROPERTY RIGHTS OR PRODUCTS.
11.2 Limitation. Under no circumstances whatsoever shall CUTS liability to Ceres
- 11 -
under or otherwise in connection with this Agreement exceed sums paid by Ceres to CUTS under
this Agreement together with the sums paid by Ceres to the University under the Sponsored Research
Agreement.
12. INDEMNIFICATION
12.1 Ceres Indemnity. Ceres agrees to indemnify, defend and hold harmless CUTS, its
employees, students, agents and appointees, including but not limited to, Xx. Xxxxxxxx, from and
against any and all liability, loss, damage, cost or expense (including reasonable legal fees,
court costs and other expenses of litigation) arising out of or in connection with third party
claims relating to:
(a) any alleged infringement of a third party’s intellectual property rights by reason of
Ceres’ activities in relation to the Research Project or this Agreement; or
(b) any HAP-1 Product or process developed, manufactured, used, sold, imported or distributed
by or on behalf of Ceres, its Affiliates or sublicensees arising out of the Research Project or in
any way out of this Agreement.
13. PATENT PROSECUTION AND MAINTENANCE
13.1 Responsibility.
(a) CUTS will diligently prosecute and maintain at its own expense, CUTS Patent Rights related
to CUTS Background Technology and Other Project Technology using counsel of its choice.
(b) Ceres shall have the sole right and discretion, at its own expense, to prepare, file,
prosecute and maintain patent applications and patents claiming Project Technology and the HAP-1
Patent, using patent counsel of its own choosing. CUTS will cause inventors to assign their
ownership rights in the Project Technology to Ceres and will cooperate with and assist Ceres in
preparation of such patents and patent applications. CUTS will cooperate with and assist Ceres, and
will cause the University to cooperate with and assist Ceres in assuming any ongoing patent
prosecution relating to the HAP-1 Patent or in the preparation and prosecution of any further such
patents and patent applications as may arise.
(c) The Parties will promptly provide each other with copies of all relevant documentation
associated with their respective Patent Rights to the extent that such Patent Rights relate to
Project Technology, the HAP-1 Patent, CUTS Background Technology or Other Project Technology and
all other reasonable assistance so that both Parties may be currently and promptly informed and
apprised of the continuing prosecution and may comment upon such documentation sufficiently in
advance of any initial deadline for filing a response; provided, however, that if the receiving
Party has not commented upon such documentation prior to ten (10) business days before the initial
deadline for filing a response with the relevant government patent office, then the Party providing
the documentation will be free to respond appropriately
- 12 -
without consideration of comments by the receiving Party, if any. Both Parties hereto will
keep this documentation in confidence in accordance with the provisions of Section 7 herein.
13.2 Choice to Not Prosecute. If subsequent to filing a patent application that
claims Project Technology or the HAP-1 Patent, Ceres elects not to prosecute or maintain such
patent application or ensuing patent or fund such prosecution, filing or maintenance, Ceres shall,
on a country-by-country basis, give CUTS notice thereof within a reasonable period prior to
allowing such patent application or patent to lapse or become abandoned or unenforceable and CUTS
may continue prosecution or maintenance of such patent application or patent at its sole expense
and for its exclusive benefit.
13.3 Claims. CUTS will use all reasonable efforts to amend any patent application to
include claims requested by Ceres and required to protect the products contemplated to be sold or
methods contemplated to be practiced under this Agreement.
13.4 Interferences/Oppositions. The costs of all interferences and oppositions
relating to such Patent Rights will be considered prosecution expenses and also will be borne by
the prosecuting Party.
14. PATENT INFRINGEMENT
14.1 Notice. In the event that a Party learns of any infringement of any Patent Right
licensed under this Agreement, that Party will call the attention of the other Party thereto in
writing and will provide the other Party with reasonable evidence of such infringement. The
Parties to this Agreement acknowledge and agree that during the period and in a jurisdiction where
Ceres has exclusive rights under this Agreement, CUTS will not notify a third party of the
infringement of any of Patent Rights without first obtaining consent of Ceres. The Parties will
use their diligent efforts in cooperation with each other to terminate such infringement without
litigation. Ceres shall have no obligation and CUTS shall have no right, to grant any rights to
such infringing third party in derogation of the exclusive licenses granted to Ceres under this
Agreement.
14.2 Legal Action. Ceres may request that CUTS take legal action against the
infringement of Patent Rights. Such request must be made in writing and must include reasonable
evidence of such infringement and damages to Ceres. If the infringing activity has not been abated
within ninety (90) days following the effective date of such request, CUTS will have the right to
elect to:
(a) commence suit on its own account; or
(b) refuse to participate in such suit;
and CUTS will give notice of its election in writing to Ceres by the end of the 100th day after
receiving notice of such request from Ceres. Ceres may thereafter bring suit for patent
infringement if and only if CUTS elects not to commence suit and if the infringement occurred
- 13 -
during the period and in a jurisdiction where Ceres had exclusive rights under this Agreement.
However, in the event Ceres elects to bring suit in accordance with this Section 14.2, CUTS may
thereafter join such suit at its own expense.
14.3 Expenses and Awards. Such legal action as is decided upon will be at the expense
of the Party on account of whom suit is brought and all recoveries recovered thereby will belong to
such Party; provided, however, that legal action brought jointly by CUTS and Ceres and participated
in by both will be at the joint expense of the Parties and all recoveries from such joint legal
action will be allocated in the following order:
(a) to each Party as reimbursement in equal amounts of the outside attorney’s costs, fees, and
other related expenses to the extent each Party paid for such costs, fees, and expenses until all
such costs, fees, and expenses are consumed for each Party, provided that if one Party paid more
for such costs, fees, and expenses, all of such Party’s costs, fees, and expenses shall be
reimbursed in full prior to the allocation of any remaining recovery to either Party under
Subsection 14.3(b); and
(b) any remaining amount to be divided by the Parties in the following manner: (i)
seventy-five percent (75%) to Ceres and twenty-five percent (25%) to CUTS of any recoveries based
on actual damages and lost profits; and (ii) fifty percent (50%) to Ceres and fifty percent (50%)
to CUTS of any recoveries based on punitive and statutory enhanced damages.
14.4 Cooperation. Each Party will cooperate with the other in litigation proceedings
instituted hereunder but at the expense of the Party on account of whom suit is brought. Such
litigation will be controlled by the Party bringing the suit, except that CUTS may be represented
by counsel of its choice, at its expense, in any suit brought by Ceres.
15. PATENT MARKING
Ceres will xxxx all Products made, used, or sold under the terms of this Agreement, or their
containers, in accordance with the applicable patent marking laws.
16. GOVERNMENT APPROVAL OR REGISTRATION
If this Agreement or any associated transaction is required by the law of any nation to be
either approved or registered with any governmental agency, Ceres will assume all legal obligations
to do so. Ceres will notify CUTS if it becomes aware that this Agreement is subject to a United
States or foreign government reporting or approval requirement. Ceres will make or cause to be
made all necessary filings and pay all costs including fees, penalties, and all other out-of-pocket
costs associated with such reporting or approval process. CUTS shall cooperate with Ceres, to the
extent it is able to do so within the law and established policy of CUTS, by providing
documentation and testimony to assist Ceres in obtaining such approval or registration. Any
expenses incurred by CUTS in cooperating with Ceres in obtaining approval or registration of this
Agreement in any country will be reimbursed within thirty (30) days after
- 14 -
receiving an itemized invoice for such expenses from CUTS.
17. MISCELLANEOUS
17.1 Reserved Rights. Ceres may during or after the Term of this Agreement
independently or with third parties perform any research, including, without limitation, research
related to the Purpose in any plant. Cambridge retains the right, after the Research Project is
complete, to perform research and development work related to the Purpose independent of Ceres.
17.2 Mediation and Governing Law.
(a) If any dispute arises out of or in connection with this Agreement the Parties will attempt
in good faith to settle it by negotiation.
(b) If the Parties are unable to settle any dispute by negotiation within twenty-eight (28)
days the Parties will attempt to settle it by mediation in accordance with the Centre for Dispute
Resolution (CEDR) Model Mediation Procedure.
(c) To initiate a mediation, a Party must give notice in writing to the other Parties
requesting a mediation in accordance with Section 17.7.
(d) This Agreement and all questions of construction, validity and performance under this
Agreement shall be governed by English law.
17.3 Independent Contractors. Nothing in this Agreement is intended or shall be
deemed to constitute a partnership, agency, employer-employee or joint venture relationship between
the Parties. All activities by the Parties hereunder shall be provided as independent contractors.
Neither Party shall incur any debts or make any commitments for the other except to the extent, if
at all, specifically provided herein.
17.4 Force Majeure. If the performance of any part of this Agreement by a Party is
prevented, restricted, interfered with or delayed by reason of any cause beyond the reasonable
control of the Party liable to perform, unless conclusive evidence to the contrary is provided, the
Party so affected shall use its diligent efforts to avoid or remove such causes of non-performance
and shall continue performance with the utmost dispatch whenever such causes are removed. When
such circumstances arise, the Parties shall discuss what, if any, modification of the terms of this
Agreement may be required in order to arrive at an equitable solution.
17.5 Construction. In the event any portion of this Agreement shall be held illegal,
void or ineffective, the remaining portions hereof shall remain in full force and effect, as long
as it does not materially alter the purpose and performance of this Agreement. If any of the terms
or provisions of this Agreement are in conflict with any applicable statute or rule of law, then
such terms or provisions shall be deemed inoperative to the extent that they may conflict therewith
and shall be deemed to be modified to conform with such statute or rule of law. In the
- 15 -
event that the terms and conditions of this Agreement are materially altered as a result of
this Section, the Parties will renegotiate the terms and conditions of this Agreement to resolve
any inequities.
17.6 Entire Agreement. Without prejudice to the Sponsored Research Agreement, this
Agreement constitutes the entire agreement between the Parties relating to the subject matter
hereof and supersedes all prior agreements, understandings, writings, and discussions between the
Parties relating to said subject matter. No terms or provisions of this Agreement shall be varied
or modified by any prior or subsequent statement, conduct or act of the Parties, except that the
Parties may amend this Agreement by written instruments specifically referring to and executed in
the same manner as this Agreement.
17.7 Notices. All notices pertaining to this Agreement, including but not limited to
notices concerning progress reports and royalty and other payments, shall be in writing and sent by
two-day delivery via an internationally recognized delivery service, to the Parties at the
following addresses or such other address as such Party shall have furnished in writing to the
other Parties in accordance with this Section 17.7:
For CUTS:
Cambridge University Technical Services, Ltd.
Research Services Division
00 Xxxx Xxxx
Xxxxxxxxx XX0 0XX
Great Britain
Attention: Contracts Officer
Research Services Division
00 Xxxx Xxxx
Xxxxxxxxx XX0 0XX
Great Britain
Attention: Contracts Officer
For Ceres:
A notice shall be deemed to have been received on the day after deposit with the delivery
service, if sent by overnight delivery.
17.8 No Third Party Beneficiaries and No Assignment. This Agreement shall be binding
upon and inure to the benefit of the successors in interest of the respective Parties. This
Agreement shall not be assigned by any Party without the written consent of the other Parties;
provided, however, Ceres may assign this Agreement to any Affiliates or to any corporation with
which it may merge or consolidate, or to which it may transfer all or substantially all of its
assets or business, without obtaining the consent of CUTS.
- 16 -
17.9 Further Assurances. Each Party hereto shall execute such further papers or
agreements as may be necessary to effect the purposes of this Agreement and carry out its
provisions.
17.10 Export Control Laws. The Parties will observe all applicable national laws with
respect to the transfer of materials and related technical data to foreign countries, including,
without limitation, the International Traffic in Arms Regulations (ITAR) and the Export
Administration Regulations.
17.11 No Waiver. The failure of either Party at any time or times to require
performance of any provision hereof shall in no manner affect its right at a later time to enforce
the same. No waiver by either Party of any condition or term in any one or more instances shall be
construed as a further or continuing waiver of such condition or term or of any other condition or
term.
17.12 Severability. If any term of this Agreement is held by a court of competent jurisdiction
to be unenforceable because it is invalid or in conflict with any law of any relevant jurisdiction,
the validity of the remaining provisions shall not be affected.
17.13
Headings. The headings of the several sections are inserted for convenience of
reference only and are not intended to be a part of or to affect the meaning or interpretation of
this Agreement.
17.14 Survival. Upon termination of this Agreement for any reason, Sections 3, 6, 7,
8, 10, 11, 12, 13, 14 and 17.2 and any accrued rights, obligations and causes of action shall
survive termination of this Agreement.
- 17 -
In Witness Whereof, both CUTS and Ceres have executed this Exclusive License Agreement, in
duplicate originals, by their respective officers hereunto duly authorized, as of the date and year
written on page one hereof.
| CAMBRIDGE UNIVERSITY TECHNICAL SERVICES LTD. | CERES, INC. | |||||
By
|
/s/ X.X. Xxxxxxxx | By | /s/ Xxxxxxx Xxxxxxx | |||
| Name: | Xx. X.X. Xxxxxxxx | Name: Xxxxxxx Xxxxxxx, CBE, FRS | ||||
| Title: | Director | Title: Chief Scientific Officer | ||||
| By | /s/ Xxxxx Xxxxxx | |||||
| Name: Xx. Xxxxx Xxxxxx | ||||||
| Title: Director of Product Development | ||||||
- 18 -
Exhibit A
CUTS Background Technology
CUTS Background Technology
HAP1 ENHANCER TRAP LINES
HAP1-VP16:HAP1-UAS-Extensin-emdGFP
HC10 — stomatal guard cells
HC03 — root base, cortex, endodermis
HC128.3 — petal vasculature
HC104.2 — leaf vasculature
HC03 — root base, cortex, endodermis
HC128.3 — petal vasculature
HC104.2 — leaf vasculature
HAP1-VP16:HAP1-UAS-histone-mCFP
With expression patterns in first screen:
HS69 — epidermis of roots, pili, abscission zones
HS135 — mature vasculature of silique and petals
HS151 — base of silique
HS164 — epidermis of roots, cotyledons, siliques, flowers
HS165 — style epidermis
HS176 — mature leaves
HS181 — silique vascular
HS135 — mature vasculature of silique and petals
HS151 — base of silique
HS164 — epidermis of roots, cotyledons, siliques, flowers
HS165 — style epidermis
HS176 — mature leaves
HS181 — silique vascular
HS222 — YOUNG ROOTS
HS230 — sepals and leaves
HS238 — vascular tissue roots, shoot meristem of embryo
HS241a — root tips
HS241b — hypocotyls and cotyledons, stem
HS251 — epidermis of stem/flower
HS357 — lateral root primordial
HS238 — vascular tissue roots, shoot meristem of embryo
HS241a — root tips
HS241b — hypocotyls and cotyledons, stem
HS251 — epidermis of stem/flower
HS357 — lateral root primordial
No expression in untreated seedlings, mature plants:
HS42
HS57
HS62
HS57
HS62
HS130
HS138
HS168
HS179
HS181
HS186
HS192
HS202
HS204
HS210
HS216
HS217
Hs220
HS221
HS225
HS228
HS231
HS232
HS243
HS247
HS250
HS253
HS359
HS630
HS361
HS364
HS366
HS368
HS369
HS371
HS138
HS168
HS179
HS181
HS186
HS192
HS202
HS204
HS210
HS216
HS217
Hs220
HS221
HS225
HS228
HS231
HS232
HS243
HS247
HS250
HS253
HS359
HS630
HS361
HS364
HS366
HS368
HS369
HS371
Exhibit B
HAP-1 Technology
HAP-1 Technology
Description of HAP-1 technology:
ET HAP1 EXT emdGFP [diagram of linear T-DNA vector transformation]
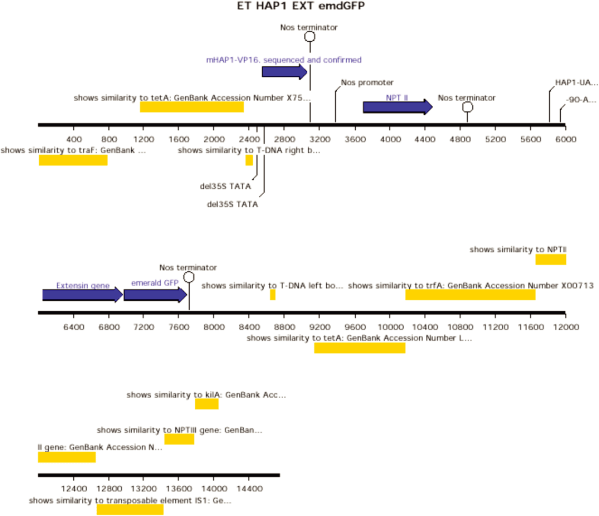




















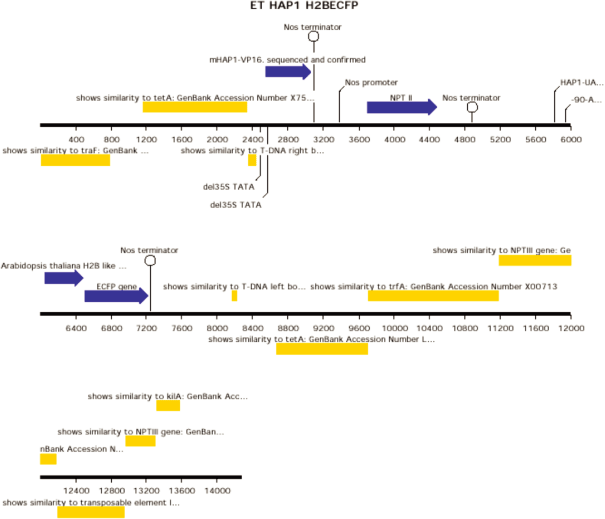
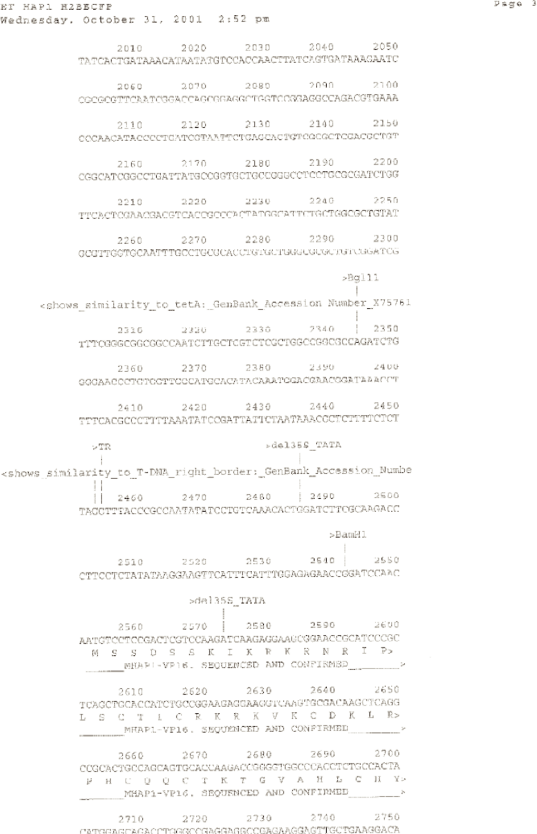
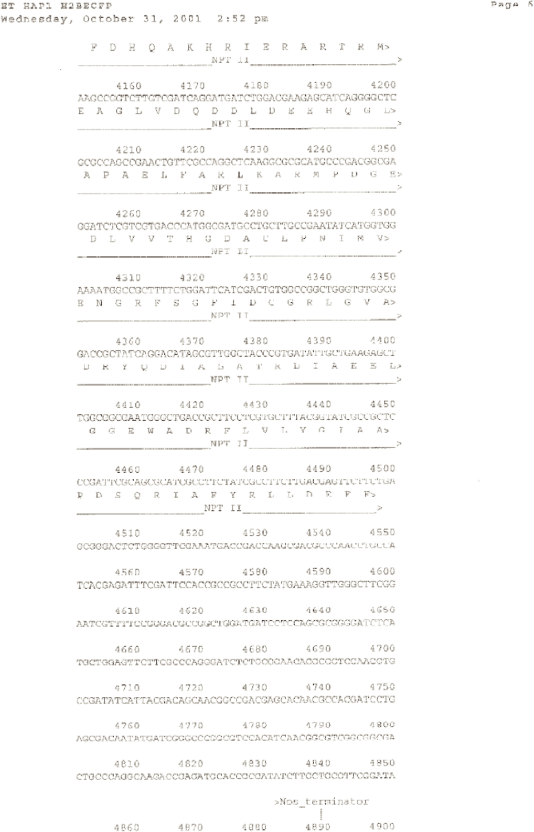
ET HAP1 H2BECFP [diagram of linear T-DNA vector transformation]

















HAP-1 Patent:
UK priority patent application number 0122828.7 filed on September 21, 2001, with title: Gene
expression construct.
Protocol associated with the validation of the use of HAP1 within the HAP-1 Technology:
Transgene activation by HAP1 in dicots: the following milestones should be achieved by
December 2002:
| § | Create Arabidopsis lines using a vector that contains a Ceres’ constitutive promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line A) | ||
| • | Create Arabidopsis lines using a vector that contains a “weak” cell/tissue specific promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line B) | ||
| • | Create Arabidopsis lines using a vector that contains UASHAP1 — driven aequorin or a spectrally distinct fluorescent protein (line C) | ||
| • | Cross line C into lines A and B (or retransform construct from C into A or B using a novel, non-PPT based selectable marker) | ||
| • | Evaluate RNA levels, and demonstrate presence of transactivated protein function in a range of at least 10 cell types in statistically relevant sets of independent Arabidopsis transformants. |
| Transgene activation by HAP1 in monocots: the following milestones should be achieved by December 2003: |
| • | Create rice lines using a vector that contains a Ceres’ constitutive promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line A) | ||
| • | Create rice lines using a vector that contains a “weak” cell/tissue specific promoter directing HAP1-VP16 expression as well as UASHAP1 — driven expression of a fluorescent protein (line B) | ||
| • | Create rice lines using a vector that contains UASHAP1 — driven aequorin or a spectrally distinct fluorescent protein (line C) | ||
| • | Cross line C into lines A and B (or retransform construct from C into A or B using a novel, non-PPT based selectable marker) | ||
| • | Evaluate RNA levels, and demonstrate presence of transactivated protein function in a range of at least 10 cell types in statistically relevant sets of independent rice transformants. |
Exhibit C
Exemplary Technological Elements
Exemplary Technological Elements
A polynucleotide sequence encoding a protein or polypeptide
A polynucleotide sequence regulating the expression of a coding sequence
A polynucleotide sequence regulating the transcription of a coding sequence
A polynucleotide regulating the stability of a transcript
A polynucleotide regulating the translation of a transcript
A polynucleotide sequence capable of suppressing the activity of another polynucleotide
A polynucleotide sequence capable of suppressing the activity of a polypeptide or protein
A process or method to transform a plant or a plant cell
A process or method to select a desirable transformant

