EX-10.14 4 d381653dex1014.htm MASTER MANUFACTURING AND SUPPLY AGREEMENT (PFIZER AS MANUFACTURER) MASTER MANUFACTURING AND SUPPLY AGREEMENT BETWEEN PFIZER INC. AND ZOETIS INC. DATED AS OF OCTOBER 1, 2012 Exhibits: Exhibit A Form of Product Addendum...
Exhibit 10.14
MASTER MANUFACTURING AND SUPPLY AGREEMENT
BETWEEN
PFIZER INC.
AND
ZOETIS INC.
DATED AS OF OCTOBER 1, 2012
TABLE OF CONTENTS
| 1. | DEFINITIONS | 1 | ||||||
| 2. | SUPPLY OF PRODUCT | 7 | ||||||
| 2.1 | Agreement to Supply | 7 | ||||||
| 2.2 | Use of Facility, Equipment, Molds and Tooling | 8 | ||||||
| 2.3 | Capacity | 8 | ||||||
| 2.4 | Forecasts and Orders | 9 | ||||||
| 2.5 | Delivery; Risk of Loss | 11 | ||||||
| 2.6 | Procurement of Materials | 12 | ||||||
| 2.7 | Product Samples | 13 | ||||||
| 3. | PRICE; PAYMENT; TAXES | 13 | ||||||
| 3.1 | Purchase Price | 13 | ||||||
| 3.2 | Annual Price Adjustments to Reflect Future Budgeted Standard Costs | 14 | ||||||
| 3.3 | True-Up to Reflect Actual Standard Costs | 15 | ||||||
| 3.4 | Price Review and Audit Procedure | 16 | ||||||
| 3.5 | Invoices and Payment | 17 | ||||||
| 3.6 | Taxes | 17 | ||||||
| 4. | MANUFACTURING STANDARDS AND QUALITY ASSURANCE | 18 | ||||||
| 4.1 | Quality Agreement | 18 | ||||||
| 4.2 | Manufacturing Standards | 18 | ||||||
| 4.3 | Modifications in Specifications | 18 | ||||||
| 4.4 | Legal and Regulatory Filings and Requests | 18 | ||||||
| 4.5 | Quality Tests and Checks | 19 | ||||||
| 4.6 | Non-Complying Product | 19 | ||||||
| 4.7 | Responsibility for Rejected and Non-Complying Product | 19 | ||||||
| 4.8 | Rejection of Product; Disposal of Rejected Shipments | 19 | ||||||
| 4.9 | Disposal of Rejected and Non-Complying Product and Materials | 20 | ||||||
| 4.10 | Maintenance and Retention of Records | 21 | ||||||
| 4.11 | Government Inspections and Recalls | 21 | ||||||
| 4.12 | Segregation of Restricted Compounds | 22 | ||||||
| 4.13 | Packaging Material | 22 | ||||||
| 4.14 | Survival | 22 | ||||||
| 5. | 22 | |||||||
| 5.1 | Representations and Warranties and Covenants of Manufacturer | 22 | ||||||
| 5.2 | Representations and Warranties and Covenants of Customer | 24 | ||||||
| 5.3 | Warranty Disclaimer | 25 |
i
| 6. | INTELLECTUAL PROPERTY | 26 | ||||||
| 6.1 | Customer’s Intellectual Property | 26 | ||||||
| 6.2 | Improvements and Developments | 26 | ||||||
| 6.3 | Rights to Certain Improvements and Developments | 27 | ||||||
| 6.4 | Limited Right to Use | 28 | ||||||
| 6.5 | No Conflict with Separation Agreement or other Ancillary Agreements | 28 | ||||||
| 7. | INDEMNIFICATION; LIMITATION ON LIABILITY. | 28 | ||||||
| 7.1 | Indemnification of Customer | 28 | ||||||
| 7.2 | Indemnification of Manufacturer | 29 | ||||||
| 7.3 | Indemnification Obligations Net of Insurance Proceeds and Other Amounts | 30 | ||||||
| 7.4 | Procedures for Indemnification of Third Party Claims | 30 | ||||||
| 7.5 | Additional Matters | 32 | ||||||
| 7.6 | Limitation on Liability | 33 | ||||||
| 7.7 | Remedies Cumulative | 33 | ||||||
| 7.8 | Survival of Indemnities | 33 | ||||||
| 7.9 | Time Limit for Claims | 33 | ||||||
| 8. | INSURANCE | 34 | ||||||
| 9. | CUSTOMER-SUPPLIED MATERIALS. | 36 | ||||||
| 9.1 | Supply of Materials | 36 | ||||||
| 9.2 | Title and Risk of Loss | 36 | ||||||
| 9.3 | Reimbursement for Loss of Customer-Supplied Materials | 37 | ||||||
| 10. | CONFIDENTIAL INFORMATION | 37 | ||||||
| 10.1 | Non-Use and Xxx-Xxxxxxxxxx | 00 | ||||||
| 10.2 | Return of Confidential Information | 38 | ||||||
| 10.3 | Survival | 38 | ||||||
| 11. | TERM; TERMINATION | 39 | ||||||
| 11.1 | Term of Agreement | 39 | ||||||
| 11.2 | Termination for Cause | 39 | ||||||
| 11.3 | Termination in Event of Insolvency | 39 | ||||||
| 11.4 | Other Termination | 40 | ||||||
| 11.5 | Effect of Termination | 40 | ||||||
| 11.6 | Return of Customer-Supplied Materials, Tools and Equipment | 41 | ||||||
| 11.7 | Transitional Support | 42 |
ii
| 12. | SUPPLY CHAIN SECURITY | 44 | ||||||
| 12.1 | C-TPAT (US Manufacturers) | 44 | ||||||
| 12.2 | Ex-US Manufacturers | 45 | ||||||
| 13. | NO COUNTERFEIT | 45 | ||||||
| 14. | NOTICES | 45 | ||||||
| 15. | DISPUTE RESOLUTION | 46 | ||||||
| 15.1 | Disputes | 46 | ||||||
| 15.2 | Escalation; Mediation | 46 | ||||||
| 15.3 | Court Actions | 47 | ||||||
| 16. | MISCELLANEOUS | 47 | ||||||
| 16.1 | Publicity | 47 | ||||||
| 16.2 | Governing Law; Submission to Jurisdiction; Waiver of Jury Trial | 48 | ||||||
| 16.3 | No Agency | 48 | ||||||
| 16.4 | Assignment; Binding Effect | 48 | ||||||
| 16.5 | Force Majeure; Failure to Supply | 49 | ||||||
| 16.6 | Severability | 50 | ||||||
| 16.7 | Waiver of Default | 50 | ||||||
| 16.8 | Cumulative Effect | 50 | ||||||
| 16.9 | Further Documents | 50 | ||||||
| 16.10 | Forms | 51 | ||||||
| 16.11 | Headings | 51 | ||||||
| 16.12 | Counterparts; Entire Agreement; Corporate Power | 51 | ||||||
| 16.13 | Amendments | 51 |
Exhibits:
| Exhibit A | Form of Product Addendum | |
| Exhibit B | Form of Quality Agreement | |
| Exhibit C | Form of Monthly Inventory Report | |
| Exhibit D | Supply Chain Agreement Parameters | |
| Exhibit E | Pricing Exhibits |
iii
MASTER MANUFACTURING AND SUPPLY AGREEMENT
THIS MASTER MANUFACTURING AND SUPPLY AGREEMENT dated as of October 1, 2012 (the “Effective Date”) is made by and between Pfizer Inc., a corporation organized and existing under the laws of the State of Delaware, with offices at 000 Xxxx 00xx Xxxxxx, Xxx Xxxx, XX 00000 (hereinafter “Pfizer”) and Zoetis Inc., a corporation organized and existing under the laws of the State of Delaware, with offices at c/o Pfizer Inc. 000 Xxxx 00xx Xxxxxx, Xxx Xxxx, XX 00000 (hereinafter “Zoetis”). Pfizer and Zoetis may be referred to herein individually as a “Party” or collectively as the “Parties”.
| 1. | DEFINITIONS. |
Capitalized terms used herein and not otherwise defined herein shall have the meanings set forth for such terms in the Separation Agreement. The following terms used herein have the following meanings:
| 1.1 | “Action” means any demand, action, suit, countersuit, arbitration, inquiry, proceeding or investigation by or before any federal, state, local, foreign or international Governmental Authority or any arbitration or mediation tribunal. |
| 1.2 | “Additional Quantities” shall have the meaning set forth in Section 2.4(b). |
| 1.3 | “Affiliate” of any Person means a Person that controls, is controlled by, or is under common control with such Person. As used herein, “control” means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such entity, whether through ownership of voting securities or other interests, by contract or otherwise. It is expressly agreed that, from and after the Separation Date, solely for purposes of this Agreement (1) no member of the Company Group (as defined in the Separation Agreement) shall be deemed to be an Affiliate of any member of the Pfizer Group and (2) no member |
1
| of the Pfizer Group shall be deemed to be an Affiliate of any member of the Company Group. |
| 1.4 | “Agreement” means this Master Manufacturing and Supply Agreement, including all of the schedules and exhibits hereto. |
| 1.5 | “Business Day” means any day other than a Saturday, Sunday or a day on which banking institutions are authorized or obligated by Law to be closed in New York, New York. |
| 1.6 | “Confidential Information” means all confidential or proprietary information, in whatever form or manner presented, which: (a) relates to a disclosing party’s business or business plans, including suppliers, customers, prospective customers, contractors, clinical data, the content and format of various clinical and medical databases, utilization data, cost and pricing data, disease management data, software products, programming techniques, data warehouse and methodologies, know-how, trade secrets, technical and non-technical materials, products, specifications, processes, sales and marketing plans and strategies, designs, and any discussions and proceedings relating to any of the foregoing; (b) is disclosed pursuant to this Agreement or any Product Addendum (or is included in the Animal Health Assets or Excluded Assets (each as defined in the Separation Agreement), as applicable, and was lawfully in the recipient’s possession prior to the time of disclosure and is used in connection with performance of the recipient’s obligations or exercise of the recipient’s rights hereunder); and (c) is (i) disclosed in writing, electronically, or visually and is marked or stamped to indicate its confidential nature or is otherwise identified as confidential by the disclosing party at the time of such disclosure; (ii) disclosed orally and is identified as confidential by the disclosing party at the time of such disclosure and is confirmed in writing within fourteen (14) calendar days after such disclosure; or (iii) information, however disclosed, which should reasonably be considered to be confidential. Confidential Information of Customer for each Product pursuant to its applicable Product Addendum shall include Customer’s confidential information regarding such Product and Customer’s processes, customers, and suppliers, including Customer’s confidential manufacturing information regarding Customer’s processes and know-how, in whatever form or manner presented or disclosed (other than any Manufacturer-Owned Improvements and Developments, which shall be Confidential Information of Manufacturer). Notwithstanding anything to the contrary herein, any Confidential Information which is included in the Animal Health Assets (as defined in the Separation Agreement) shall be Confidential Information of Zoetis or its applicable Affiliate which is a Party hereto and any Confidential Information which is included in the Excluded Assets (as defined in the Separation Agreement) shall be Confidential Information of Pfizer or its applicable Affiliate which is a Party hereto. |
| 1.7 | “Current Good Manufacturing Practices” or “cGMP” mean the then-current requirements under all applicable laws and regulations for the manufacturing, preparation, processing, labeling, packaging, and distribution of products (and |
2
| components thereof), including as set forth in 21 U.S.C. Section 351, 21 C.F.R. parts 210 and 211, 21 U.S.C. Sections 151 – 159, 9 C.F.R. Parts 101 – 118, and as otherwise required by any Governmental Authority having jurisdiction over the Facility, as the same may be updated, supplemented or amended from time to time. |
| 1.8 | “Customer” shall mean, with respect to each Product Addendum, the Person set forth as the “Customer” in such Product Addendum. |
| 1.9 | “Customer-Exclusive Product” shall have the meaning set forth in Section 6.2(b). |
| 1.10 | “Customer Indemnitee” shall have the meaning set forth in Section 7.1. |
| 1.11 | “Customer-Owned Improvements and Developments” shall have the meaning set forth in Section 6.2(b). |
| 1.12 | “Customer Property” shall have the meaning set forth in Section 6.1. |
| 1.13 | “Customer-Supplied Materials” means the Product Materials supplied by Customer to Manufacturer under the terms of this Agreement as set forth in the applicable Product Addendum. |
| 1.14 | “Development” shall have the meaning set forth in Section 6.2(a). |
| 1.15 | “Effective Date” shall have the meaning set forth in the preamble above. |
| 1.16 | “Facilities” means Manufacturer’s manufacturing facility located at the address set forth in the applicable Product Addendum and such other facilities used by Manufacturer in the manufacture, packaging and storage of (a) Product or (b) materials utilized in the manufacture of Product hereunder. |
| 1.17 | “FDA” means the United States Food and Drug Administration or any successor agency. |
| 1.18 | “FD&C Act” means the United States Federal Food Drug and Cosmetic Act. |
| 1.19 | “Financial Year” means (i) with respect to each Product manufactured at a Facility within the United States, each consecutive twelve (12) month period starting on January 1 and ending on December 31, and (ii) with respect to each Product manufactured at a Facility outside the United States, each consecutive twelve (12) month period starting on December 1 and ending on November 30, in each case except to the extent Manufacturer changes its applicable fiscal year period and notifies Customer of such change. For clarity, the Term of each Product Addendum may commence and/or end during a Financial Year currently in progress. |
| 1.20 | “Forecast” means an MTO Forecast or VMR Forecast, as applicable. |
3
| 1.21 | “Governmental Authority” means any nation or government, any state, municipality or other political subdivision thereof, and any entity, body, agency, commission, department, board, bureau, court, tribunal or other instrumentality, whether federal, state, local, domestic, foreign or multinational, exercising executive, legislative, judicial, regulatory, administrative or other similar functions of, or pertaining to, government and any executive official thereof. |
| 1.22 | “Improvement” shall have the meaning set forth in Section 6.2(a). |
| 1.23 | “Indemnifying Party” shall have the meaning set forth in Section 7.3. |
| 1.24 | “Indemnitee” shall have the meaning set forth in Section 7.3. |
| 1.25 | “Indemnity Payment” shall have the meaning set forth in Section 7.3. |
| 1.26 | “Insurance Proceeds” means those monies: (i) received by an insured from a third party insurance carrier; (ii) paid by a third party insurance carrier on behalf of the insured; or (iii) received (including by way of setoff) from any third party in the nature of insurance, contribution or indemnification in respect of any Liability; in each such case net of any applicable premium adjustments (including reserves and retrospectively rated premium adjustments) and net of any costs or expenses incurred in the collection thereof and excluding, for the avoidance of doubt, proceeds from any self-insurance, captive insurance or similar program. |
| 1.27 | “Intellectual Property” means all intellectual property throughout the world, including all U.S. and foreign (i) patents, patent applications, invention disclosures, and all related continuations, continuations-in-part, divisionals, provisionals, renewals, reissues, re-examinations, additions, extensions (including all supplementary protection certificates) (“Patent Rights”), (ii) trademarks, service marks, names, corporate names, trade names, domain names, logos, slogans, trade dress, design rights, and other similar designations of source or origin, together with the goodwill symbolized by any of the foregoing, (iii) copyrights and copyrightable subject matter (“Copyrights”), (iv) rights in computer programs (whether in source code, object code, or other form), algorithms, databases, compilations and data, technology supporting the foregoing, and all documentation, including user manuals and training materials, related to any of the foregoing, (v) trade secrets and all other confidential information, ideas, know-how, inventions, proprietary processes, formulae, models, and methodologies, and (vi) all applications and registrations for the foregoing. |
| 1.28 | “Law” means any United States or non-United States federal, national, supranational, state, provincial, local or similar law (including common law), statute, ordinance, regulation, rule, code, order, treaty, license, permit, authorization, registration, approval, consent, decree, injunction, judgment, notice of liability, request for information, binding judicial or administrative interpretation or other requirement, in each case, enacted, promulgated, issued, entered or otherwise put into effect by a Governmental Authority. |
4
| 1.29 | “Liabilities” means any and all indebtedness, claims, debts, liabilities, demands, causes of actions, Actions and obligations, whether accrued, fixed or contingent, mature or inchoate, known or unknown, reflected on a balance sheet or otherwise, including those arising under any Law, Action or any judgment of any court of any kind or any award of any arbitrator of any kind, and those arising under any Contract, commitment or undertaking. |
| 1.30 | “Losses” means any and all damages, losses, deficiencies, Liabilities, Taxes, obligations, penalties, judgments, settlements, claims, payments, fines, charges, interest, costs and expenses, whether or not resulting from third party claims, including the costs and expenses of any and all Actions and demands, assessments, judgments, settlements and compromises relating thereto and the costs and expenses of attorneys’, accountants’, consultants’ and other professionals’ fees and expenses incurred in the investigation or defense thereof or the enforcement of rights hereunder. |
| 1.31 | “Manufacturer Indemnitee” shall have the meaning set forth in Section 7.2. |
| 1.32 | “Manufacturer-Owned Improvements and Developments” shall have the meaning set forth in Section 6.2(c). |
| 1.33 | “Made-to-Order Forecast” or “MTO Forecast” shall have the meaning set forth in Section 2.4(a). |
| 1.34 | “Made-to-Order Product” or “MTO Product” means each Product designated as an “MTO Product” in the applicable Product Addendum. |
| 1.35 | “Manufacturer” shall mean, with respect to each Product Addendum, the Person set forth as the “Manufacturer” in such Product Addendum. |
| 1.36 | “Non-Complying Product” shall have the meaning set forth in Section 4.6. |
| 1.37 | “Person” means an individual, a general or limited partnership, a corporation, a trust, a joint venture, an unincorporated organization, a limited liability entity, any other entity and any Governmental Authority. |
| 1.38 | “Price” means the price to be charged by Manufacturer for Product manufactured and supplied hereunder as delivered to Customer in accordance with Section 3.1, as may be adjusted in accordance with Section 3.2. |
| 1.39 | “Product” means, on a product-by-product basis, the product(s) identified in the applicable Product Addendum as more fully described in the Specifications for such product(s). |
| 1.40 | “Product Addendum” means a document executed by the Parties for Product to be manufactured pursuant to the terms of this Agreement, which shall be substantially in the form of addendum attached to this Agreement as Exhibit A and made a part hereof. |
5
| 1.41 | “Product Materials” means all raw materials (including active pharmaceutical ingredients and excipients), packaging materials and components needed for the manufacturing of the Product. |
| 1.42 | “Purchase Order” means a written or electronic order form submitted by Customer in accordance with the terms of this Agreement to Manufacturer authorizing the manufacture and supply of Product. |
| 1.43 | “Quality Agreement” means the supplemental quality assurance provisions set forth in the Quality Agreement for the applicable Product Addendum and made a part thereof, in the form attached to this Agreement as Exhibit B. |
| 1.44 | “Recall” shall mean “recall”, “seizure”, “correction” or “market withdrawal”. |
| 1.45 | “Sensitive Manufacturer Technology” shall have the meaning set forth in Section 6.2(b). |
| 1.46 | “Specifications” means the specifications for the Product set forth in the applicable Product Addendum, as such specifications may be amended or supplemented by mutual written agreement of the Parties. |
| 1.47 | “Standard Cost” shall have the meaning set forth in Section 3.1. |
| 1.48 | “Supply Chain Agreement” shall have the meaning set forth in Section 2.4(a). |
| 1.49 | “Term” with respect to this Agreement shall have the meaning set forth in Section 11.1 and with respect to a Product Addendum shall have the meaning set forth in the applicable Product Addendum. |
| 1.50 | “Territory” shall mean the countries in the world set forth in the applicable Product Addendum. |
| 1.51 | “Technical Support” shall have the meaning set forth in Section 11.7. |
| 1.52 | “Third Party Claim” shall have the meaning set forth in Section 7.4. |
| 1.53 | “USDA” means the United States Department of Agriculture. |
| 1.54 | “VAT” means, in relation to any jurisdiction within the European Union, the value added tax provided for in Council Directive 2006/112/EC and charged under the provisions of any national legislation implementing that directive or Council Directive 77/388/EEC together with legislation supplemental thereto and, in relation to any other jurisdiction, the equivalent tax (if any) in that jurisdiction. |
| 1.55 | “Vendor-Managed Replenishment Forecast” or “VMR Forecast” shall have the meaning set forth in Section 2.4(a). |
6
| 1.56 | “Vendor-Managed Replenishment Product” or “VMR Product” means each Product designated as a “VMR Product” in the applicable Product Addendum. |
Words in the singular shall be held to include the plural and vice versa and words of one gender shall be held to include the other genders as the context requires. The terms “hereof”, “herein” and “herewith” and words of similar import shall, unless otherwise stated, be construed to refer to this Agreement as a whole (including all of the schedules, exhibits and appendices hereto) and not to any particular provision of this Agreement. Article, Section, Exhibit, Schedule and Appendix references are to the Articles, Sections, Exhibits, Schedules and Appendices to this Agreement unless otherwise specified. The word “including” and words of similar import when used in this Agreement shall mean “including, without limitation”, unless the context otherwise requires or unless otherwise specified.
| 2. | SUPPLY OF PRODUCT. |
| 2.1 | Agreement to Supply. |
| (a) | Supply. During the Term of each Product Addendum, Manufacturer shall manufacture and supply Product to Customer for the Territory pursuant to the provisions of this Agreement and the applicable Product Addendum. |
| (b) | The terms of this Agreement shall be deemed to be incorporated into each Product Addendum that may be executed by the Parties and/or one or more of their respective Affiliates. Each Product Addendum constitutes a separate and distinct agreement from this Agreement and from any other Product Addendum. In addition, each Product Addendum may designate certain Products thereunder as “Tolling” Products (each a “Tolling Product”), and the provisions of Section 10 of such Product Addendum shall apply solely with respect to such Tolling Products. An Affiliate of Pfizer or Zoetis may execute a Product Addendum and, in such circumstances, all references in this Agreement (as incorporated in such Product Addendum) to Manufacturer shall be deemed to be to the applicable Person designated therein as Manufacturer and all references in this Agreement (as incorporated in such Product Addendum) to Customer shall be deemed to be to the applicable Person designated therein as Customer and all references in this Agreement (as incorporated in such Product Addendum) to a Party shall be deemed to be the applicable Manufacturer or Customer, as applicable, each of which shall be entitled to enforce the agreement constituted by such Product Addendum in its own name and shall be solely liable to the other Party for any obligations and liabilities undertaken thereunder, provided that, notwithstanding the foregoing, where an Affiliate of Zoetis is the Customer or Manufacturer under a Product Addendum, Zoetis hereby irrevocably and unconditionally guarantees and shall be directly liable to Pfizer and its Affiliate(s) that are party thereto for any and all obligations and liabilities of such Affiliate under such Product Addendum (including the terms of this Agreement as incorporated in such Product Addendum). For clarity, |
7
| any party to a Product Addendum that will be an Affiliate of Zoetis as of the Separation Date shall be deemed to be an Affiliate of Zoetis as of the Product Addendum Effective Date. In the event of a conflict between the terms of any Product Addendum and the terms of this Agreement, the terms of this Agreement shall prevail, except to the extent that the applicable Product Addendum expressly and specifically states an intent to supersede this Agreement on a specific matter. Any amendment to the terms of this Agreement contained in a Product Addendum shall be effective solely with respect to the terms of this Agreement as incorporated in such Product Addendum, and not to the terms of this Agreement or the terms of any other Product Addendum. Any amendment to the terms of this Agreement shall be effective for all Product Addenda, unless such amendment or Product Addendum specifies otherwise. |
| (c) | Non-Exclusivity; No Minimum Purchase Obligation. The Product under this Agreement shall be provided on a non-exclusive basis and Customer reserves the right to manufacture the Product for itself and to purchase the Product and similar products from any other party; provided that, for clarity, the foregoing shall be without limitation to any other agreement between the Parties or their respective Affiliates or the Intellectual Property rights of a Party or its Affiliates. Customer is not obligated to purchase any minimum or specific quantity or dollar amount of Product under this Agreement. |
| 2.2 | Use of Facility, Equipment, Molds and Tooling. |
For each Product, Manufacturer shall perform all manufacturing activities and all storage activities at the Facilities. Manufacturer may use other facilities for the manufacture and storage of Products under any Product Addendum, and such Product Addendum shall be deemed to be amended accordingly, provided that:
| (i) | such facilities have been approved for such manufacture by all applicable Governmental Authorities; and |
| (ii) | Manufacturer shall have obtained the prior written consent of Customer, such consent not to be unreasonably withheld. Manufacturer shall promptly notify Customer of its intent to use such alternate facilities, as far in advance as is reasonably practicable. |
| 2.3 | Capacity. |
Manufacturer shall use commercially reasonable efforts to devote adequate manufacturing capacity to be capable of manufacturing and supplying Product to Customer in accordance with the provisions of this Agreement; provided, however, that Manufacturer shall not be required to purchase any new equipment, install any equipment purchased or requested by Customer or add (or, for clarity, allocate or dedicate) any additional manufacturing or storage capacity for the
8
manufacturing and other activities to be carried out by Manufacturer hereunder. If Manufacturer agrees to provide additional capacity it shall be provided at the Customer’s expense including any costs associated with the write-off of abandoned assets related to the project.
| 2.4 | Forecasts and Orders. |
| (a) | Forecasts. |
| (i) | With respect to MTO Products: By the fifth (5th) Business Day of each calendar month during the Term, Customer (or Customer’s designee on behalf of Customer) shall submit a rolling forecast by Product of Customer’s anticipated demand of such Product for the next twenty-four (24) consecutive months, commencing with the month in which such forecast is sent (each, an “MTO Forecast”). The MTO Forecast shall show demand on a monthly basis, and for the first three months shall state the dates of delivery for such Product. Without limiting Section 2.4(b) each MTO Forecast, including the required delivery dates, is non-binding. In the event that Manufacturer has failed to receive an updated MTO Forecast by the fifth (5th) Business Day of any calendar month, Manufacturer shall notify Customer of such failure and, if Customer has not responded with an updated MTO Forecast by the tenth (10th) Business Day of such month, the most recent MTO Forecast shall be regarded as current. |
| (ii) | With respect to VMR Products: Customer shall provide Manufacturer access to Customer’s demand management system (e.g., Manugistics) for the purpose of managing supply to, and determining the forecasts for, Customer’s markets according to Planned Arrivals (as such term is used therein) calculated by the system in accordance with replenishment parameters agreed in writing between Customer and Manufacturer in the applicable Product Addendum and in the Supply Chain Agreement (including as may be listed in Exhibit D and as such parameters may be provided, updated and revised by mutual written agreement of the Parties from time to time) (the “Supply Chain Agreement”, and each such forecast, a “VMR Forecast”), and Manufacturer shall schedule production of such Product and delivery of such Product to Customer in accordance with such derived VMR Forecasts and the applicable Product lead times set forth in the applicable Product Addendum. Manufacturer shall confirm all such Product shipments via the replenishment planning system according to the Supply Chain Agreement parameters and the parameters in the applicable Product Addendum. |
9
| (iii) | With respect to any Product designated as a “Biological” in the applicable Product Addendum, the first six (6) month period of each Forecast with respect thereto shall be binding, and the corresponding portion of each subsequent Forecast shall be consistent with such period. |
| (iv) | Without limiting the foregoing, the Parties agree that certain Supply Chain Agreement parameters and replenishment parameters in the Product Addendum may not be available and included in Exhibit D or such Product Addendum as of the Effective Date and shall be included therein prior to the IPO Closing by written approval of each Party, such approval not to be unreasonably withheld. |
| (b) | Orders. Manufacturer shall provide Product to Customer pursuant to Purchase Orders issued by Customer to Manufacturer. For each Purchase Order: |
| (i) | With respect to MTO Products: Manufacturer shall provide to Customer such quantities of Products as may be ordered by Customer pursuant to such Purchase Orders, up to one hundred twenty-five percent (125%) (or such lower percentage as may be specified in the applicable Product Addendum) of the quantity set forth in the most recent MTO Forecast for the applicable time period. In the event that Customer orders quantities of Product above one hundred twenty-five percent (125%) (or such lower percentage as may be specified in the applicable Product Addendum) of the quantity set forth in the most recent MTO Forecast for the applicable time period (such quantities above 125% (or such lower percentage as may be specified in the applicable Product Addendum) referred to as “Additional Quantities”), Manufacturer shall use its commercially reasonably efforts to meet Customer’s requested quantities and delivery dates for such Additional Quantities as set forth in the applicable Purchase Order. For purposes of this Section 2.4(b), the most recent MTO Forecast for any given month shall mean the MTO Forecast submitted by Customer in the month prior to the month in which the applicable Purchase Order is issued. Customer will issue Purchase Orders for MTO Product no later than ninety (90) calendar days (or such longer time period as may be specified in the applicable Product Addendum) prior to the required shipping date. |
| (ii) | With respect to VMR Products: Manufacturer shall issue a purchase requisition using Customer’s demand management system, and promptly following receipt thereof, Customer shall issue binding Purchase Orders for each confirmed shipment of |
10
| VMR Product that conforms to the replenishment parameters set forth in the Supply Chain Agreement and the applicable Product Addendum. Manufacturer shall supply VMR Product to Customer pursuant to such Purchase Orders. Customer shall not be obligated to issue such Purchase Orders or to accept any deliveries for any shipments which do not conform to the replenishment parameters set forth in the Supply Chain Agreement and the applicable Product Addendum. For the purposes of clarity, a confirmed shipment shall be considered to be conforming to the replenishment parameters when it is based on the demand and inventory data available on the date that the net requirements are derived, taking into account the applicable lead time as set forth in the Supply Chain Agreement and the applicable Product Addendum, and Manufacturer shall not be responsible for changes in such data between such date and the date that the shipment is confirmed by Manufacturer. Manufacturer agrees that all Customer data provided to or obtained by Manufacturer pursuant to this paragraph shall be deemed Confidential Information of Customer, and Manufacturer shall only use such data in order to perform its obligations under this Agreement. |
| (c) | Order Quantities. Without limiting Section 2.1(c), minimum order quantities shall be identified in the applicable Product Addendum. Promptly following receipt of a Purchase Order, Manufacturer shall acknowledge receipt of said Purchase Order and confirm (in writing, including by email) its ability to manufacture, package and supply such Product to Customer in accordance with the terms of the Purchase Order, including delivering such Product by the date of delivery. Manufacturer shall deliver all Product so ordered on the requested delivery date (or such alternate delivery date as may be mutually agreed by the Parties as provided in this Section 2.4(c)). In the event Manufacturer will not be able to fulfill such Purchase Order in accordance with the terms herein, Manufacturer shall notify Customer in writing promptly upon becoming aware of such inability and in any event at least five (5) calendar days prior to the delivery date of such Purchase Order, and the Parties will discuss alternate delivery dates in good faith with a view to reaching agreement thereto, such agreement not to be unreasonably withheld by either Party. For clarity, changes to Forecast quantities shall not affect any Purchase Order quantity unless the Parties so agree in writing in each instance. |
| 2.5 | Delivery; Risk of Loss. |
| (a) | Manufacturer shall ship Product ordered by Customer and any Product samples requested by Customer to Customer throughout the Territory to the Customer location set forth in the applicable Purchase Order. Manufacturer shall deliver Product to Customer by the delivery date set |
11
| forth in the applicable Purchase Order, or such other date as may be agreed to in writing by the Parties from time to time. Manufacturer shall deliver Product to Customer at the applicable Facility (unless otherwise specified in the applicable Product Addendum) in accordance with the Incoterm set forth in the applicable Product Addendum. Manufacturer retains the option to transfer the responsibility and associated cost of outbound freight to Customer (with a corresponding adjustment to the applicable Standard Cost in accordance with Article 3). |
| (b) | Manufacturer shall include certificates of compliance and certificates of analysis with all shipments of Product. |
| (c) | Title to Product shall pass to Customer when the Product has been delivered to Customer pursuant to Section 2.5(a) unless otherwise specified by mutual written agreement of the Parties. |
| 2.6 | Procurement of Materials. |
| (a) | Manufacturer shall order sufficient quantities of all Product Materials (except Customer-Supplied Materials) to enable Manufacturer to manufacture and deliver Product in accordance with Customer’s Forecasts. The costs of all Product Materials shall be included in the Price for the Product as provided in Section 3.1. Without limiting the foregoing, Customer shall be solely responsible for all costs and expenses relating to designing and changing any artwork used in connection with the Product and its packaging, and any changes to such artwork shall be conducted in accordance with the processes used as of the Effective Date for changing such artwork. Customer shall be liable for excess or obsolete Product Materials (including Customer-Supplied Materials) purchased by Manufacturer to the extent the excess or obsolescence is caused by any: (i) change to, or cancellation of, a firm Purchase Order, (ii) Forecast variations (or, for clarity, any variation between a Forecast hereunder and a forecast applicable to the relevant Products prior to the Effective Date), (iii) without limiting Section 4.3, change to the Specifications or the specifications of such Product Materials after such Product Materials have been purchased by Manufacturer based upon a Customer firm Purchase Order or a Forecast quantity with respect to the binding portion of such Forecast, as applicable, or (iv) without limiting Section 2.4(a)(iii), with respect to any VMR Product, reduction in the quantity of such Product as specified in a VMR Forecast relative to the quantity of such Product that was specified in any previous VMR Forecast with respect to the same time period. In addition, Customer shall be liable for excess or obsolete Product Materials (including Customer-Supplied Materials) owned by Manufacturer prior to the applicable Product Addendum Effective Date. Customer shall reimburse Manufacturer for such excess or obsolescence described in this Section 2.6(a) on a quarterly basis within sixty (60) days following receipt of Manufacturer’s invoice therefor. |
12
| (b) | Without limiting the foregoing, at the end of the Term of each Product Addendum, Customer will purchase at cost any excess inventory of Product Materials, including any unique materials used to support production for Customer, and any works in process related to Products, in each case in accordance with Section 11.5(b), and any long lead time items (as specified in a Product Addendum) previously procured by Manufacturer (regardless of whether so procured in order to fulfill obligations under an existing Purchase Order). |
| (c) | The Parties agree that Manufacturer is responsible for, and shall administer, the procurement of such Product Materials (except Customer-Supplied Materials) in accordance with the terms herein, which responsibilities shall include: transporting, inspecting and storing the Product Materials; maintaining systems for material management; maintaining adequate supplies of Product Materials, to the extent required hereunder; and supplier and logistics management, in each case in accordance with the terms herein. If there is a failure to supply issue with a supplier as a result of a Force Majeure Event or a supplier selected by Customer fails to deliver the appropriate quantity and quality of Product Materials multiple times within the span of four consecutive orders, without limiting Section 16.5, Manufacturer shall so notify Customer in writing of such supply issue and if Customer concurs that such supplier is continuously failing to fulfill its obligations to Manufacturer, and unless Manufacturer requests otherwise, Customer shall, to the extent practicable, attempt to have a discussion with such supplier in an effort to resolve the issue. Customer shall have no obligations to continue discussions with such supplier following the initial discussion or after such offer of an initial discussion, if such supplier refused such discussion. |
| 2.7 | Product Samples. |
Manufacturer shall provide Customer (or any such other Person as Customer shall designate) with representative lot samples of each production batch of Product promptly upon request. Customer shall be entitled to review, upon reasonable prior written notice, all manufacturing records relating to such samples including all analytical procedures and cleaning validation relating to the equipment used in connection with the manufacture of the samples. Such Product samples shall be shipped to Customer (or such other Person as Customer shall designate) in accordance with the provisions set forth in Section 2.5 hereof, or as otherwise instructed by Customer. Customer shall pay for such samples (including all applicable shipping costs) when invoiced in accordance with Section 3.2 hereof.
| 3. | PRICE; PAYMENT; TAXES. |
| 3.1 | Purchase Price. |
Customer shall purchase the Product from Manufacturer at the Price therefor and in accordance with the terms of this Agreement. During the Term of each Product Addendum, the Price shall be equivalent to the product of: (x) the Standard Cost
13
of such Product during the then-current Financial Year, multiplied by (y) the then-current markup to such Standard Cost as set forth in Exhibit E-1 (which markup, for clarity, may change during a Financial Year currently in progress as set forth in Exhibit E-1). As used herein, “Standard Cost” means, with respect to each Product, all direct and indirect costs and expenses associated with the manufacturing and delivery of such Product, per quantity unit of such Product, calculated using the cost accounting methodology that is used at the applicable Facility during the applicable Financial Year (without limiting Section 3.2(c)), including all: (i) costs of Product Materials, labor, and other direct and indirect variable costs; (ii) costs of equipment pools; (iii) depreciation; and (iv) costs of plant operations and plant support services (including utilities, maintenance, engineering, safety, human resources, finance, plant management and other similar activities, as well as allocations to the Facility for center support and shipping costs to a finished goods warehouse of Customer or its Affiliates). The Standard Cost of each Product during the initial Financial Year during the Term of each Product Addendum is set forth in such Product Addendum, and Manufacturer shall notify Customer of, and such Product Addendum shall be deemed to be updated to include, the Standard Cost during the second Financial Year during such Term reasonably promptly following Manufacturer’s determination thereof.
| 3.2 | Annual Price Adjustments to Reflect Future Budgeted Standard Costs. |
| (a) | Annual Standard Cost Review. The Standard Cost of each Product shall be reviewed by the Manufacturer annually to determine the budgeted change thereof during the coming Financial Year. In order to facilitate Customer’s and Manufacturer’s relevant planning processes, multiple communications will occur between Customer and Manufacturer with respect to such Standard Cost reasonably promptly following Manufacturer’s determination thereof (and, to the extent reasonably practicable, according to the schedule set forth in Exhibit E-2). Without limiting Section 3.3 (or the Price markup as set forth in Exhibit E-1), the Price shall be automatically adjusted for the coming Financial Year to reflect such budgeted change in the Standard Cost thereof, subject to clause (b) below. |
| (b) | Audit Right. Only such an adjustment pursuant to clause (a) above that, on a Facility-by-Facility basis, would result in an increase of greater than five percent (5%) in the aggregate Standard Cost during the coming Financial Year relative to the then-current Financial Year of all Products manufactured at such Facility shall be subject to review under Section 3.4. |
| (c) | Changes to the Standard Cost Methodology. Manufacturer shall have the right to change the Standard Cost methodology once per Financial Year on a generally applicable basis with respect to each Facility. If Manufacturer elects to change the Standard Cost methodology, Manufacturer shall, on a pro-forma basis, (a) calculate both (x) the revised Standard Cost using the |
14
| methodology effective during the current Financial Year of the Term of the applicable Product Addendum and (y) the percentage change in Standard Cost, calculated on a Facility-by-Facility basis, caused by the change in methodology relative to such initial methodology, and (b) apply such percentage, on a Facility-by-Facility basis, to the Standard Cost calculated using the changed methodology so as to approximate the Standard Cost that would have applied to Customer had such change in the Standard Cost methodology not taken place. |
| 3.3 | True-Up to Reflect Actual Standard Costs. |
| (a) | No later than thirty (30) days following the end of each Financial Year during the Term of this Agreement, Manufacturer shall calculate, on a Facility-by-Facility basis, the Volume Variance and Purchase Price Variance with respect to such Financial Year for each such Facility. As used herein: |
| (i) | “Purchase Price Variance” means the result of calculating, on a Product Material-by-Product Material basis, (x) the difference between the Standard Costs budgeted to be incurred in connection with purchasing or otherwise obtaining such Product Material (as budgeted in accordance with Section 3.2(a)) and the actual costs incurred in connection with purchasing or otherwise obtaining such Product Material, multiplied by (y) the total quantity of such Product Material purchased or otherwise obtained with respect to each applicable Product, and aggregating the result over all Products manufactured at the applicable Facility during the applicable Financial Year. |
| (ii) | “Volume Variance” means the result of calculating, on a Product-by-Product basis, (x) the difference between the quantities of Product budgeted to be manufactured and the actual quantities of Product manufactured, multiplied by (y) the budgeted per-unit absorption rate of such Product (as budgeted in accordance with Section 3.2(a)), and aggregating the result over all Products manufactured at the applicable Facility during the applicable Financial Year, provided that (A) each calculated Volume Variance shall be reduced by a Facility-specific allowance for variable costs as determined by Manufacturer for each Financial Year and communicated to Customer, and (B) Customer shall not be responsible for Manufacturer’s failure to manufacture Product. As used herein, the “absorption rate” represents, with respect to the applicable Product, all budgeted Standard Cost elements less Product Material components thereof, as calculated in each case by Manufacturer. |
| (b) | With respect to each Facility, Manufacturer shall notify Customer of any calculated underpayment or overpayment, as applicable, and Customer |
15
| shall make payment to Manufacturer in the amount of any calculated underpayment, or Manufacturer shall make payment to Customer in the amount of any calculated overpayment, as applicable, within sixty (60) days of such notice, provided that the foregoing in each case shall apply only if and to the extent that either one or each of the Purchase Price Variance or Volume Variance with respect to such Facility individually exceeds a threshold of five percent (5%) of the applicable budgeted value relevant to the calculation of such Purchase Price Variance or Volume Variance, as applicable. Any such payment by Customer shall be subject to review under Section 3.4. |
| (c) | For purposes of clarity, examples of the calculations described in this Section 3.3 are provided in Exhibit E-4. |
| 3.4 | Price Review and Audit Procedure. |
Manufacturer shall maintain complete and accurate books and records that fairly reflect the Standard Costs of each Product and shall retain such books and records for a period of not less than two (2) years after the applicable Product was manufactured and delivered hereunder. With respect to any Standard Cost increase under Section 3.2 or required payment under Section 3.3 that in each case is subject to review under this Section 3.4, if Customer requests such a review in writing within thirty (30) days following notice to Customer of such increase or payment (as applicable): (i) the Parties shall reasonably discuss and attempt to resolve any disagreement with respect thereto, and (ii) if such disagreement is not resolved within thirty (30) days following commencement of such discussions, Customer shall have the right, on no less than thirty (30) days’ notice to Manufacturer, to appoint a reputable and internationally recognized independent accounting firm reasonably acceptable to Manufacturer to audit such relevant books and records, during normal business hours and on a confidential basis, to verify that the (i) with respect to Section 3.3, Volume Variance and/or Purchase Price Variance, as applicable, during the preceding Financial Year or (ii) with respect to Section 3.2, budgeted change in the Standard Cost for the coming Financial Year, as applicable, was accurately calculated by Manufacturer in accordance with this Article 3. Customer shall bear all costs and expenses of conducting such an audit, and such accounting firm shall work on an hourly or flat fee basis without a contingency fee or other performance or bonus fee. Such accounting firm shall provide in writing (i) a detailed report of such audit to Manufacturer and (ii) the applicable Standard Cost(s) as calculated by such accounting firm in accordance with this Article 3 to Manufacturer and Customer (provided that, for clarity, the Standard Cost methodology shall not be subject to audit). The Standard Cost(s) as calculated by such accounting firm absent manifest error shall be binding upon the Parties with respect to such increase or required payment, as applicable. Notwithstanding anything to the contrary in this Agreement, Customer shall have no right to dispute or request review of any Price or calculation thereof or change thereto, except as provided in this Section 3.4.
16
| 3.5 | Invoices and Payment. |
Manufacturer shall submit invoices to Customer upon shipment of Product to the address set forth in the applicable Purchase Order. Customer shall be obligated to pay only for actual quantities of Product delivered. Subject to the following sentence, Customer shall pay all amounts due in U.S. Dollars (unless another currency is specified in the applicable Product Addendum) within sixty (60) calendar days from the date of invoice. Payment by Customer shall not constitute a waiver of any of its rights under this Agreement.
| 3.6 | Taxes. |
| (a) | The Price includes all taxes except such sales and use taxes which Manufacturer is required by law to collect from Customer and except VAT. Such VAT and taxes, if any, will be payable in addition to the Price. Where Manufacturer is required by law to collect and/or account for such VAT and taxes from Customer, such VAT and taxes will be separately stated in Manufacturer’s invoice and will be paid by Customer to Manufacturer unless Customer provides an exemption to Manufacturer and, in the case of VAT, subject to Manufacturer providing a valid VAT invoice to Customer in the form and manner required by law to allow Customer to recover such VAT (to the extent Customer is allowed to do so by law). |
| (b) | Except where Customer is required by applicable Law to account for any VAT to the applicable Governmental Authority: |
| (i) | Manufacturer shall be solely responsible for the timely payment of all such VAT and taxes to the applicable Governmental Authority; and |
| (ii) | Manufacturer shall pay (without reimbursement by Customer), and shall hold Customer harmless against, any penalties, interest or additional VAT or taxes that may be levied or assessed as a result of the failure or delay of Manufacturer to pay any such VAT and taxes, except to the extent that such failure or delay of Manufacturer is caused by a failure or delay of Customer to pay such VAT and taxes to Manufacturer (in which case Customer shall reimburse Manufacturer for any related penalties, interest or additional VAT or taxes). |
| (c) | Notwithstanding the foregoing in this Section 3.6, Customer shall be responsible for the payment of all duties, tariffs, VAT, taxes and other charges payable on the exportation or importation of the Products. Without limiting any of Manufacturer’s obligations hereunder, Manufacturer shall cooperate with and assist Customer in all aspects of the shipment, exportation, importation and delivery process in order to ensure |
17
| the expeditious delivery of the Product to the designated delivery point, including assisting in obtaining any documents that may be required, provided that Customer shall reimburse Manufacturer for its out-of-pocket costs and expenses incurred in connection therewith as Manufacturer may invoice to Customer in writing, which invoices shall be payable within sixty (60) days following the date thereof. |
| 4. | MANUFACTURING STANDARDS AND QUALITY ASSURANCE. |
| 4.1 | Quality Agreement. |
The Parties agree to comply with the requirements and provisions set forth in the Quality Agreement. Unless expressly stated otherwise, in the event of a conflict between the terms of the Quality Agreement and the terms of this Agreement, the terms of the Quality Agreement shall prevail with respect to matters of Product quality and the terms of this Agreement shall prevail with respect to all other matters.
| 4.2 | Manufacturing Standards. |
Manufacturer shall manufacture and supply the Product (including disposing of all waste and other materials) in accordance with the Specifications, applicable Laws, the Quality Agreement and this Agreement.
| 4.3 | Modifications in Specifications. |
Neither Party shall make any revisions to the Specifications, manufacturing processes or Product Materials of a Product without the prior written consent of the other Party, such consent not to be unreasonably withheld. All costs for any and all revisions to the Specifications (including any additional Product or procurement costs) shall be borne by the Customer and shall be mutually agreed in writing by the Parties.
| 4.4 | Legal and Regulatory Filings and Requests. |
Except as otherwise agreed to in the Quality Agreements, Manufacturer shall use commercially reasonable efforts to cooperate with Customer in responding to all requests for information from, and in making all legally required filings with, Governmental Authorities having jurisdiction to make such requests or require such filings. Manufacturer shall (a) obtain and comply with all licenses, consents and permits, and (b) comply with all Laws to the extent applicable to its manufacturing and packaging processes, the Facility or for the performance of its obligations hereunder.
| 4.5 | Quality Tests and Checks. |
Manufacturer or permitted subcontractors shall perform all tests or checks required by the Specifications, the Quality Agreement, and the applicable Product Addendum (except in each case as may be specified in the applicable Product
18
Addendum). Customer acknowledges and agrees that the Price for each Product shall include all costs and expenses associated with such tests and checks in accordance with and subject to Article 3.
| 4.6 | Non-Complying Product. |
Manufacturer shall not release any Product for shipment that does not conform to the warranties set forth in Section 5.1(c) (“Non-Complying Product”), without the prior written approval of Customer. Manufacturer shall reimburse Customer for any Customer-Supplied Materials used to manufacture any such Non-Complying Product in accordance with Section 9.3.
| 4.7 | Responsibility for Rejected and Non-Complying Product. |
Manufacturer shall quarantine and properly tag all Non-Complying Product. Manufacturer shall promptly submit to Customer a report detailing the nature of such non-compliance, including the investigation and testing done and Manufacturer’s recommended disposition. Manufacturer shall provide any additional information regarding such Non-Complying Product as may reasonably be requested by Customer. Customer shall not be required to pay for any Non-Complying Product.
| 4.8 | Rejection of Product; Disposal of Rejected Shipments. |
| (a) | Customer may reject any Non-Complying Product by providing notice of rejection to Manufacturer within sixty (60) days following receipt by Customer of any shipment of such Product hereunder; provided, however, that Customer may provide notice of rejection of any shipments of Product having any latent defects or any defects not reasonably discoverable through visual inspection up until the date of expiration of such Product. Customer shall have no right to reject any Product hereunder other than Non-Complying Product. |
| (b) | Subject to Section 4.8(d), Customer shall return any shipments of Product (or portions thereof) rejected pursuant to this Section 4.8 to Manufacturer or Manufacturer’s designee at Manufacturer’s expense. In addition to any other rights or remedies of Customer hereunder, Manufacturer shall replace such rejected Product as soon as practicable at no additional charge to Customer (subject to Section 4.8(d)), and Manufacturer shall reimburse Customer for any Customer-Supplied Materials used to manufacture any such rejected Product in accordance with Section 9.3. |
| (c) | In the event Customer rejects any Product that Manufacturer reasonably believes to conform to the warranties set forth in Section 5.1(c), Manufacturer shall have the right to test such Product to determine whether or not it so conforms. Manufacturer shall hire an independent third party laboratory, subject to Customer’s prior written approval of such laboratory, to perform such tests in accordance with the Quality Agreement |
19
| and all applicable Laws and promptly provide the results thereof to Customer. The determination of such tests shall be binding upon the Parties for all purposes hereunder, provided that if such tests are unable to determine whether or not such rejected Product is Non-Complying Product, such Product shall be deemed to be Non-Complying Product. If such tests determine that the Product is, or such Product is so deemed to be, Non-Complying Product, Manufacturer shall bear the costs of such tests and the provisions of this Article 4 shall apply without limitation to such Non-Complying Product. However, if such tests determine that such Product is not Non-Complying Product, Customer shall bear the costs of such tests and shall remain obligated to pay Manufacturer the Price for such Product in accordance with Article 3, and, without limiting such obligation, if Customer reasonably so requests in writing, Manufacturer shall use commercially reasonable efforts to re-deliver such Product to Customer at Customer’s expense. |
| (d) | Notwithstanding anything to the contrary herein and without limiting Section 9.3(b), Customer shall be solely responsible for all costs and expenses incurred in connection with any Non-Complying Product to the extent any Customer-Supplied Materials resulted in such Product being Non-Complying Product, and shall reimburse Manufacturer for Manufacturer’s costs and expenses incurred in connection therewith. |
| (e) | The provisions of this Section 4.8 shall survive termination or expiration of this Agreement, provided that, subsequent to the termination or expiration of this Agreement, Manufacturer may, in lieu of replacing any rejected or missing quantities of Product, elect in its sole discretion to reimburse Customer for the costs (including any applicable freight charges) associated with the replacement of such rejected and/or missing quantities of Product by Customer or a third party selected by Customer. |
| 4.9 | Disposal of Rejected and Non-Complying Product and Materials. |
All Non-Complying Product and Product rejected pursuant to this Agreement and all non-conforming Product Materials not able to be used in the manufacture of Product, shall be removed (if applicable) and disposed of by Manufacturer in accordance with all applicable Laws, and as approved in advance by Customer (such disposal cost to be at the expense of the Party deemed to be responsible therefor pursuant to the terms of this Agreement). All Non-Complying Product shall be destroyed by Manufacturer prior to disposal, and Manufacturer shall deface and render unreadable all words or symbols that identify Customer including Customer’s trademarks and Customer’s logotypes that adorn any packaging containing such Product prior to disposal of such Product.
| 4.10 | Maintenance and Retention of Records. |
Manufacturer shall maintain detailed records with respect to Product Materials usage and finished Product production in accordance with the Quality Agreement.
20
| 4.11 | Government Inspections and Recalls. |
| (a) | Manufacturer shall notify Customer, in accordance with the Quality Agreement, of any inspection of Manufacturer’s facility proposed or scheduled with the FDA or any other Governmental Authority that relates to the Products or to general matters at the Facility that affects the Products. If the FDA or any other Governmental Authority conducts an inspection at Manufacturer’s Facility, seizes any Product and/or Product Materials, requests a Recall or Field Alert be issued for any Product, or otherwise notifies Manufacturer of any violation or potential violation of any applicable Law or of any intended inspection of the Facility, Manufacturer shall notify Customer, in accordance with the Quality Agreement, of such, and Manufacturer shall take such actions as may be required under the Specifications or Quality Agreement. For the purposes of this provision, “Field Alert” means a three-day NADA/ANADA field alert report described in 21 C.F.R. 514.80 (b)(1) that indicates the potential of a quality issue (e.g. bacterial contamination, significant chemical, physical degradation, adulterated or misbranded product). As applicable, Manufacturer shall promptly send any reports relating to such inspections, Recalls or violations or potential violations of Law to Customer, provided that Manufacturer may reasonably redact any such reports to protect its confidential information (including information regarding products not sold to or systems not used to manufacture Products for the other Party). In the event that any such Governmental Authority requests, but does not seize, a sample of the Product in connection with any such inspection, Manufacturer, as the case may be (i) shall promptly notify Customer of such request, (ii) if permitted by law, shall satisfy such request only after receiving Customer’s approval, such approval not to be unreasonably withheld or delayed, (iii) shall follow any reasonable procedures instructed by Customer in responding to such request and (iv) shall promptly send a sample of any Product requested by the Governmental Authority to Customer. Manufacturer shall not initiate any Recall of Product except as provided in the Quality Agreement without the prior written agreement by Customer. Manufacturer shall give and permit full and unrestricted access to all or any of its premises at any time to any authorized representative of any Governmental Authority in connection with its obligations hereunder and shall co-operate fully with any such representatives. |
| (b) | To the extent that such Recall results from any breach by Manufacturer of this Agreement, in addition to any other rights or remedies available under this Agreement, Manufacturer shall reimburse Customer for Customer’s costs and expenses associated with such Recall, including costs of Customer-Supplied Materials, shipping costs, administrative costs associated with arranging and coordinating the Recall, and all costs associated with the distribution of replacement Product; provided that Customer shall be solely responsible for all, and shall reimburse |
21
| Manufacturer for Manufacturer’s, costs and expenses associated with any Recall to the extent such Recall does not result from a breach by Manufacturer of this Agreement, in addition to any other rights or remedies available under this Agreement. |
| 4.12 | Segregation of Restricted Compounds. |
Manufacturer shall not use any equipment, dedicated change parts, molds or tooling that are used to manufacture Product to manufacture products containing any of the following compounds: (i) androgens, estrogens and progestin or (ii) herbicides, pesticides, rodentcides, agrochemicals and cytotoxins, in each case without such controls and segregation with respect to these compounds as provided in the Quality Agreement and required by applicable Laws. Manufacturer shall not manufacture penicillins, cephalosporins, beta lactams in the same building within the same Facility as the Product.
| 4.13 | Packaging Material. |
Customer shall determine and be responsible for the text (including any logos or other graphics) for all packaging material used in connection with Product. Manufacturer shall assure that all packaging materials are accurate and consistent with Customer’s specifications for such text or graphics including such matters as placement, size and colors.
| 4.14 | Survival. |
The obligations of Manufacturer under Sections 4.4, 4.8, 4.9, 4.11, and 4.12 of this Article shall survive the expiration or termination of this Agreement on a Product-by-Product basis until the expiration date of the applicable Product.
| 5. | REPRESENTATIONS AND WARRANTIES AND COVENANTS. |
| 5.1 | Representations and Warranties and Covenants of Manufacturer. |
Manufacturer represents and warrants to Customer that:
| (a) | The Facility and all equipment, tooling and molds utilized in the manufacture and supply of Product hereunder by Manufacturer shall, during the Term of this Agreement, be maintained in good operating condition and shall be maintained and operated in accordance with all applicable Laws, including cGMPs. |
| (b) | Manufacturer shall perform all of its obligations under this Agreement in full compliance with all applicable Laws in the Territory. Manufacturer shall hold during the Term of this Agreement all licenses, permits and similar authorizations required by any Governmental Authority in the Territory for Manufacturer to perform its obligations under this Agreement. |
22
| (c) | The Product furnished by Manufacturer to Customer under this Agreement: |
| (i) | shall conform to the Specifications; |
| (ii) | shall be manufactured, packaged, labeled, handled, stored and shipped in compliance with all applicable Laws including cGMPs, and in accordance with the Quality Agreement; |
| (iii) | shall not contain any Product Material that has not been used, handled or stored in accordance with the Specifications, all applicable Laws, and the Quality agreement, provided that the foregoing shall not apply to Customer-Supplied Materials as furnished to Manufacturer hereunder; |
| (iv) | shall not be adulterated or misbranded within the meaning of Sections 501 and 502, respectively, of the FD&C Act and any other applicable Laws and shall comply with the 1913 Virus-Serum-Toxin Act, 21 U.S.C. 151-159 and 21 C.F.R. Parts 101 to 118, as amended by the 1985 Food Security Act (as applicable with respect to veterinary biologics), in each case except to the extent resulting from (i) any Customer-Supplied Materials as furnished to Manufacturer hereunder; or (ii) Customer’s specifications for the text (including any logos or other graphics) for any packaging material used in connection with Product; |
| (v) | shall be manufactured with Product Materials (other than Customer-Supplied Materials as furnished to Manufacturer hereunder) that conform to the applicable specifications for such Product Materials; |
| (vi) | shall, at the time delivered, have a remaining minimum shelf-life as specified in the applicable Product Addendum (under the “Min. Effective Shelf Life” field). |
| (d) | Pfizer: |
| (i) | is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware; |
| (ii) | has power and authority to conduct its business as currently being conducted and as contemplated herein; and |
| (iii) | has power and authority to make, deliver and perform its obligations under this Agreement and has taken all necessary action to authorize the execution, delivery and performance of this Agreement. No consent of, authorization of, filing with or other act by or in respect of, any Governmental Authority or any other Person is or will be required in respect of Pfizer in connection with the execution, delivery, performance, validity or enforceability of this Agreement. This Agreement has been duly executed and delivered on behalf of Pfizer. This Agreement constitutes the legal, valid and binding obligations of Pfizer enforceable against Pfizer in accordance with its terms. |
23
| (e) | The execution, delivery and performance of this Agreement by Manufacturer will not violate any agreement or instrument to which Manufacturer is a party. |
| (f) | Manufacturer is not debarred by any applicable authority, including under subsections 306(a) or (b) of the FD&C Act and Manufacturer has not and shall not use in any capacity the services of any Person who has been debarred by any applicable authority with respect to Manufacturer’s performance of this Agreement. |
| 5.2 | Representations and Warranties and Covenants of Customer. |
Customer represents and warrants to Manufacturer that:
| (a) | Customer shall perform all of its obligations under this Agreement, and shall comply with all applicable Laws in the marketing, distribution and sale of each Product. Customer shall hold during the Term of this Agreement all licenses, permits and similar authorizations required by any Governmental Authority for Customer to perform its obligations under this Agreement, and to market, distribute and sell each Product in the applicable Territory. |
| (b) | The Customer-Supplied Materials, as furnished to Manufacturer under this Agreement, shall be used, handled or stored in accordance with the Specifications, all applicable Laws, and the Quality agreement and shall conform to the applicable specifications for such Product Materials. |
| (c) | Customer’s specifications for the text (including any trademarks, logos or other graphics) for all packaging material used in connection with Product, and any such packaging material for the Product provided by Customer or its designee, shall be true and accurate in all respects, comply with all applicable Laws and not infringe or otherwise violate the Intellectual Property of any Person. |
| (d) | None of the Customer-Supplied Materials as furnished to Manufacturer hereunder or Customer’s specifications for the packaging material used in connection with Product shall result in any Product being adulterated or misbranded within the meaning of Sections 501 and 502, respectively, of the FD&C Act and any other applicable Laws or non-compliant with the 1913 Virus-Serum-Toxin Act, 21 U.S.C. 151-159 and 21 C.F.R. Parts 101 to 118, as amended by the 1985 Food Security Act (as applicable with respect to veterinary biologics). |
24
| (e) | The Product as manufactured in accordance with the Specifications and cGMPs will comply with all applicable Laws and will not infringe or otherwise violate the Intellectual Property of any Person. |
| (f) | Zoetis: |
| (i) | is a corporation duly organized, validly existing and in good standing under the laws of the State of Delaware; |
| (ii) | has power and authority to conduct its business as currently being conducted and as contemplated herein; and |
| (iii) | has power and authority to make, deliver and perform its obligations under this Agreement and has taken all necessary action to authorize the execution, delivery and performance of this Agreement. No consent or authorization of, filing with or other act by or in respect of, any Governmental Authority or any other Person is or will be required in respect of Zoetis in connection with the execution, delivery, performance, validity or enforceability of this Agreement. This Agreement has been duly executed and delivered on behalf of Zoetis. This Agreement constitutes the legal, valid and binding obligations of Zoetis enforceable against Zoetis in accordance with its terms. |
| (g) | The execution, delivery and performance of this Agreement by Customer will not violate any agreement or instrument to which Customer is a party. |
| 5.3 | Warranty Disclaimer. |
EACH OF PFIZER (ON BEHALF OF ITSELF AND EACH MEMBER OF THE PFIZER GROUP) AND ZOETIS (ON BEHALF OF ITSELF AND EACH MEMBER OF THE COMPANY GROUP) UNDERSTANDS AND AGREES THAT, EXCEPT AS EXPRESSLY SET FORTH HEREIN OR IN THE SEPARATION AGREEMENT OR IN ANY OTHER ANCILLARY AGREEMENT, NEITHER PARTY MAKES ANY EXPRESS REPRESENTATIONS OR WARRANTIES, AND NO REPRESENTATION OR WARRANTY SHALL BE IMPLIED UNDER THIS AGREEMENT OR AT LAW, WITH RESPECT TO THIS AGREEMENT, THE PRODUCTS, PRODUCT MATERIALS, TECHNICAL SUPPORT OR OTHER SERVICES HEREUNDER OR OTHERWISE, INCLUDING WARRANTIES OF HABITABILITY, MERCHANTABILITY, FITNESS FOR ANY PARTICULAR PURPOSE, NON-INFRINGEMENT, VALIDITY AND ENFORCEABILITY, AND ALL OTHER WARRANTIES ARISING UNDER THE UNIFORM COMMERCIAL CODE (OR SIMILAR FOREIGN LAWS).
25
| 6. | INTELLECTUAL PROPERTY. |
| 6.1 | Customer’s Intellectual Property. |
Subject to Section 6.5, all Intellectual Property, together with all materials, data, writings and other property in any form whatsoever, which is provided to Manufacturer by or on behalf of Customer, and which was owned or controlled by Customer and/or its respective Affiliates (including pursuant to the Separation Agreement, this Agreement or any other Ancillary Agreement) prior to being provided to or used by Manufacturer hereunder, shall remain owned or controlled by Customer (the “Customer Property”). Without limiting Section 6.3(b), Customer hereby grants to Manufacturer a non-exclusive license to use any Customer Property solely in connection with Manufacturer performing its obligations hereunder. Manufacturer shall not acquire any other right, title or interest in or to the Customer Property as a result of its performance hereunder.
| 6.2 | Improvements and Developments. |
| (a) | Each Party acknowledges and agrees that improvements or modifications to Customer Property may be made by or on behalf of Manufacturer (“Improvements”), and creative ideas, proprietary information, developments, or inventions may be developed under or in connection with this Agreement by or on behalf of Manufacturer (“Developments”), in each case either alone or in concert with Customer or any third parties. |
| (b) | Manufacturer acknowledges and agrees that, as between the Parties, any Improvements or Developments that, as of the expiration or termination of such Product Addendum, are owned by Manufacturer and used by Manufacturer and its Affiliates exclusively for Products under such Product Addendum that relate exclusively to the AH Business (as defined in the Separation Agreement) (such Products, collectively, “Customer-Exclusive Products” and such Improvements and Developments, collectively, “Customer-Owned Improvements and Developments”) shall be the exclusive property of Customer, and Customer shall own all rights, title and interest in and to such Improvements and Developments. For clarity, the Customer-Owned Improvements and Developments shall not include, and the Manufacturer-Owned Improvements and Developments shall include, any Improvements or Developments that, as of the expiration or termination of the applicable Product Addendum, (i) are held for use or being researched, developed or commercialized for any purpose other than the AH Business, (ii) that Pfizer or any of its Affiliates intends to research, develop or commercialize for any purpose other than the AH Business, or (iii) relate to any “Sensitive Technology” as may be identified by written agreement of the Parties from time to time (collectively, “Sensitive Manufacturer Technology”). Manufacturer hereby irrevocably transfers, assigns and conveys, and shall cause its subcontractors and its and their employees to irrevocably transfer, assign and convey, all rights, title and interest in and to each of the Customer-Owned Improvements and |
26
| Developments to Customer free and clear of any encumbrances, and Manufacturer agrees to execute, and shall cause its subcontractors and its and their employees to execute, all documents necessary to do so. All such assignments shall include existing or prospective Copyrights and Patent Rights therein in any country. |
| (c) | Customer acknowledges and agrees that, as between the Parties, all Improvements and Developments other than Customer-Owned Improvements and Developments (such Improvements and Developments, collectively, “Manufacturer-Owned Improvements and Developments”) shall be the exclusive property of Manufacturer, and Manufacturer shall own all rights, title and interest in and to such Improvements and Developments. Customer hereby irrevocably transfers, assigns and conveys, and shall cause its subcontractors and its and their employees to irrevocably transfer, assign and convey, all rights, title and interest in and to each of the Manufacturer-Owned Improvements and Developments to Manufacturer free and clear of any encumbrances, and Customer agrees to execute, and shall cause its subcontractors and its and their employees to execute, all documents necessary to do so. All such assignments shall include existing or prospective Copyrights and Patent Rights therein in any country. |
| 6.3 | Rights to Certain Improvements and Developments. |
| (a) | Effective as of the expiration or termination of each Product Addendum, Manufacturer hereby grants to Customer a non-exclusive, royalty-free (except for any pass-through royalties or other payment owed to a third party therefor), sublicenseable license, solely with respect to the Product under such Product Addendum in the AH Field (as defined in the Separation Agreement), to use such Manufacturer-Owned Improvements and Developments that are used by Manufacturer in manufacturing such Product as of such expiration or termination, in each case solely to the extent necessary for Customer or an alternative source of supply designated by Customer to continue manufacturing such Product, or line extensions of such Products or combinations of such Products with other products of Customer in the AH Field, following such expiration or termination, provided that the foregoing shall not apply with respect to any Improvements or Developments that relate to any Sensitive Manufacturer Technology. |
| (b) | Effective as of the expiration or termination of each Product Addendum, Customer hereby grants to Manufacturer a non-exclusive, royalty-free (except for any pass-through royalties or other payment owed to a third party therefor), sublicenseable license to use any Customer-Owned Improvements and Developments for any and all purposes outside the AH Field. |
27
| (c) | With respect to any Product that, as of the expiration or termination of the applicable Product Addendum, requires any Sensitive Manufacturer Technology for the continued manufacturing thereof, at the request of a Party no earlier than two (2) years prior to such expiration or termination, the Parties shall discuss the continued supply needs of Customer with respect to such Product or the needs of Customer to identify and source third party technology as a replacement or alternative to Sensitive Manufacturer Technology, in each case without any obligation to enter into a long-term supply arrangement or license agreement with respect thereto or extend the Term of such Product Addendum unless and until mutually agreed by the Parties in writing. |
| 6.4 | Limited Right to Use. |
Subject to the provisions of Sections 6.1 to 6.3 and the terms of the Separation Agreement and any other Ancillary Agreement (as applicable), nothing set forth in this Agreement shall be construed to grant to either Party any title, right or interest in or to any Intellectual Property owned or controlled by the other Party or any of its Affiliates. Use by Manufacturer of any Customer Property, except to the extent expressly provided in an Ancillary Agreement, shall be limited exclusively to its performance of this Agreement. Use by Customer of any Manufacturer-Owned Improvements and Developments, except to the extent expressly provided in Section 6.3(a), shall be prohibited except with the prior written consent of Manufacturer in each instance.
| 6.5 | No Conflict with Separation Agreement or other Ancillary Agreements. |
Nothing in this Article 6 shall, or shall be deemed to, expand, limit or otherwise alter any rights of the Parties or their Affiliates pursuant to the Separation Agreement or any other Ancillary Agreement, and to the extent of any conflict between this Article 6 and the Separation Agreement or any other Ancillary Agreement, the Separation Agreement or such other Ancillary Agreement shall govern.
| 7. | INDEMNIFICATION; LIMITATION ON LIABILITY. |
| 7.1 | Indemnification of Customer. |
Except as provided in Section 7.3, Pfizer and Manufacturer shall, jointly and severally, indemnify, defend and hold harmless each member of the Customer’s Group and each of their Affiliates and each member of the Customer’s Group’s and their respective Affiliates’ respective directors, officers, employees and agents, and each of the heirs, executors, successors and assigns of any of the foregoing (collectively, the “Customer Indemnitees”), from and against any and all Losses of the Customer Indemnitees to the extent relating to, arising out of or resulting from any of the following items (without duplication and including any Losses arising by way of setoff, counterclaim or defense or enforcement of any Lien): (i) Manufacturer’s or its Affiliate’s breach of this Agreement or any Product Addendum or of any representation or warranty made by Manufacturer or
28
its Affiliate to Customer or its Affiliate under this Agreement or any Product Addendum; (ii) any injury to or death of any Person occurring on the premises of Manufacturer; (iii) Manufacturer’s supply of Non-Complying Product; (iv) the infringement of any third party Intellectual Property Rights relating to manufacturing processes or reagents caused solely by the use of any Manufacturer-Owned Improvements and Developments by or on behalf of Manufacturer to manufacture or supply the Product hereunder; or (v) any grossly negligent or reckless act or omission or misconduct on the part of Manufacturer, Affiliates of Manufacturer, subcontractors of Manufacturer, or its or their respective employees or agents; except in each case to the extent that Customer or its Affiliate is obligated to indemnify, defend and hold harmless any Manufacturer Indemnified Party pursuant to Section 7.2 below. Notwithstanding the foregoing, Manufacturer shall not be liable for Losses to the extent such Losses are caused by the gross negligence, recklessness or misconduct of Customer or its Affiliate or breach of any of the terms of this Agreement by Customer or its Affiliate or of any representation or warranty made by Customer or its Affiliate to Manufacturer or its Affiliate under this Agreement or any Product Addendum.
| 7.2 | Indemnification of Manufacturer. |
Except as provided in Section 7.3, Zoetis and Customer shall, jointly and severally, indemnify, defend and hold harmless each member of the Manufacturer’s Group and each of their Affiliates and each member of the Manufacturer’s Group’s and their respective Affiliates’ respective directors, officers, employees and agents, and each of the heirs, executors, successors and assigns of any of the foregoing (collectively, the “Manufacturer Indemnitees”), from and against any and all Losses of the Manufacturer Indemnitees to the extent relating to, arising out of or resulting from any of the following items (without duplication and including any Losses arising by way of setoff, counterclaim or defense or enforcement of any Lien): (i) Customer’s or its Affiliate’s breach of this Agreement or any Product Addendum or of any representation or warranty made by Customer or its Affiliate to Manufacturer or its Affiliate under this Agreement or any Product Addendum; (ii) any grossly negligent or reckless act or omission or misconduct on the part of Customer, Affiliates of Customer, or its or their respective employees or agents; (iii) the use of any Customer Property in accordance with this Agreement; or (iv) the manufacture, sale, marketing, use or distribution of any Products; except in each case to the extent that Manufacturer or its Affiliate is obligated to indemnify, defend and hold harmless any Customer Indemnified Party pursuant to Section 7.1 above. Notwithstanding the foregoing, Customer shall not be liable for Losses to the extent such Losses are caused by the gross negligence, recklessness, or misconduct of Manufacturer or its Affiliate or breach of any of the terms of this Agreement by Manufacturer or its Affiliate or of any representation or warranty made by Manufacturer or its Affiliate to Customer or its Affiliate under this Agreement or any Product Addendum.
29
| 7.3 | Indemnification Obligations Net of Insurance Proceeds and Other Amounts. |
| (a) | The Parties intend that any Loss subject to indemnification or reimbursement pursuant to this Article 7 will be net of Insurance Proceeds that actually reduce the amount of the Loss. Accordingly, the amount which any Party (an “Indemnifying Party”) is required to pay to any Person entitled to indemnification hereunder (an “Indemnitee”) will be reduced by any Insurance Proceeds theretofore actually recovered by or on behalf of the Indemnitee in respect of the related Loss. If an Indemnitee receives a payment (an “Indemnity Payment”) required by this Agreement from an Indemnifying Party in respect of any Loss and subsequently receives Insurance Proceeds, then the Indemnitee will pay to the Indemnifying Party an amount equal to the excess of the Indemnity Payment received over the amount of the Indemnity Payment that would have been due if the Insurance Proceeds had been received, realized or recovered before the Indemnity Payment was made. |
| (b) | An insurer who would otherwise be obligated to pay any claim shall not be relieved of the responsibility with respect thereto or, solely by virtue of the indemnification provisions hereof, have any subrogation rights with respect thereto, it being expressly understood and agreed that no insurer or any other third party shall be entitled to a “wind-fall” (i.e., a benefit such insurer or other third party would not be entitled to receive in the absence of the indemnification provisions) by virtue of the indemnification provisions hereof. Nothing contained in this Agreement or any Ancillary Agreement shall obligate any Person in any Group to seek to collect or recover any Insurance Proceeds. |
| (c) | If an indemnification claim is covered by the indemnification provisions of the Separation Agreement or any other Ancillary Agreement, the claim shall be made under the Separation Agreement or such Ancillary Agreement to the extent applicable and the provisions thereof shall govern such claim. In no event shall any party be entitled to double recovery from the indemnification provisions of this Agreement and the Separation Agreement or any other Ancillary Agreement. |
| 7.4 | Procedures for Indemnification of Third Party Claims. |
| (a) | If an Indemnitee shall receive notice or otherwise learn of the assertion by a Person (including any Governmental Authority) who is not a Person in the Pfizer Group or the Company Group of any claim or of the commencement by any such Person of any Action with respect to which an Indemnifying Party may be obligated to provide indemnification to such Indemnitee pursuant to Section 7.1 or Section 7.2 (collectively, a “Third Party Claim”), such Indemnitee shall give such Indemnifying Party written notice thereof as promptly as practicable (and in any event within thirty (30) days) after becoming aware of such Third Party Claim. Any such notice shall describe the Third Party Claim in reasonable detail. |
30
| Notwithstanding the foregoing, the failure of any Indemnitee or other Person to give notice as provided in this Section 7.4(a) shall not relieve the related Indemnifying Party of its obligations under this Article 7, except to the extent, and only to the extent, that such Indemnifying Party is materially prejudiced by such failure to give notice. |
| (b) | An Indemnifying Party may elect (but shall not be required) to defend, at such Indemnifying Party’s own expense and by such Indemnifying Party’s own counsel, any Third Party Claim, provided that the Indemnifying Party shall not be entitled to defend and shall pay the reasonable fees and expenses of one separate counsel for all Indemnities if the claim for indemnification relates to or arises in connection with any criminal action, indictment or allegation. Within thirty (30) days after the receipt of notice from an Indemnitee in accordance with Section 7.4(a) (or sooner, if the nature of such Third Party Claim so requires), the Indemnifying Party shall notify the Indemnitee of its election whether the Indemnifying Party will assume responsibility for defending such Third Party Claim, which election shall specify any reservations or exceptions to its defense. After notice from an Indemnifying Party to an Indemnitee of its election to assume the defense of a Third Party Claim, such Indemnitee shall have the right to employ separate counsel and to participate in (but not control) the defense, compromise, or settlement thereof, but the fees and expenses of such counsel shall be the expense of such Indemnitee; provided, however, in the event that (i) the Indemnifying Party has elected to assume the defense of the Third Party Claim but has specified, and continues to assert, any reservations or exceptions in such notice or (ii) the Third Party Claim involves injunctive or equitable relief, then, in any such case, the reasonable fees and expenses of one separate counsel for all Indemnitees shall be borne by the Indemnifying Party. |
| (c) | If an Indemnifying Party elects not to assume responsibility for defending a Third Party Claim, or fails to notify an Indemnitee of its election as provided in Section 7.4(b), such Indemnitee may defend such Third Party Claim at the cost and expense of the Indemnifying Party. |
| (d) | Unless the Indemnifying Party has failed to assume the defense of the Third Party Claim in accordance with the terms of this Agreement, no Indemnitee may settle or compromise any Third Party Claim without the consent of the Indemnifying Party. If an Indemnifying Party has failed to assume the defense of the Third Party Claim within the time period specified in clause (b) above, it shall not be a defense to any obligation to pay any amount in respect of such Third Party Claim that the Indemnifying Party was not consulted in the defense thereof, that such Indemnifying Party’s views or opinions as to the conduct of such defense were not accepted or adopted, that such Indemnifying Party does not approve of the quality or manner of the defense thereof or that such Third |
31
| Party Claim was incurred by reason of a settlement rather than by a judgment or other determination of liability. |
| (e) | In the case of a Third Party Claim, no Indemnifying Party shall consent to entry of any judgment or enter into any settlement of the Third Party Claim without the consent of the Indemnitee if the effect thereof is (i) to permit any injunction, declaratory judgment, other order or other non-monetary relief to be entered, directly or indirectly, against any Indemnitee or (ii) to ascribe any fault on any Indemnitee in connection with such defense. |
| 7.5 | Additional Matters. |
| (a) | Any claim on account of a Loss which does not result from a Third Party Claim shall be asserted by written notice given by the Indemnitee to the related Indemnifying Party. Such Indemnifying Party shall have a period of thirty (30) days after the receipt of such notice within which to respond thereto. If such Indemnifying Party does not respond within such 30-day period, such Indemnifying Party shall be deemed to have refused to accept responsibility to make payment. If such Indemnifying Party does not respond within such 30-day period or rejects such claim in whole or in part, such Indemnitee shall be free to pursue such remedies as may be available to such Indemnitee as contemplated by this Agreement. |
| (b) | In the event of payment by or on behalf of any Indemnifying Party to any Indemnitee in connection with any Third Party Claim, such Indemnifying Party shall be subrogated to and shall stand in the place of such Indemnitee as to any events or circumstances in respect of which such Indemnitee may have any right, defense or claim relating to such Third Party Claim against any claimant or plaintiff asserting such Third Party Claim or against any other Person. Such Indemnitee shall cooperate with such Indemnifying Party in a reasonable manner, and at the cost and expense of such Indemnifying Party, in prosecuting any subrogated right, defense or claim. |
| (c) | In the event of an Action in which the Indemnifying Party is not a named defendant, if either the Indemnitee or Indemnifying Party shall so request, the parties shall endeavor to substitute the Indemnifying Party for the named defendant or otherwise hold the Indemnifying Party as party thereto, if at all practicable. If such substitution or addition cannot be achieved for any reason or is not requested, the named defendant shall allow the Indemnifying Party to manage the Action as set forth in this Section, and the Indemnifying Party shall fully indemnify the named defendant against all costs of defending the Action (including court costs, sanctions imposed by a court, attorneys’ fees, experts fees and all other external expenses), the costs of any judgment or settlement, and the cost of any interest or penalties relating to any judgment or settlement with respect to such Third Party Claim. |
32
| 7.6 | Limitation on Liability. |
NOTWITHSTANDING ANY OTHER PROVISION OF THIS AGREEMENT TO THE CONTRARY, (I) IN NO EVENT WILL EITHER PARTY OR ANY OF ITS GROUP MEMBERS BE LIABLE FOR ANY SPECIAL, INCIDENTAL, INDIRECT, COLLATERAL, CONSEQUENTIAL OR PUNITIVE DAMAGES OR LOST PROFITS SUFFERED BY AN INDEMNIFIED PARTY, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY, IN CONNECTION WITH ANY DAMAGES ARISING HEREUNDER, AND (II) IN NO EVENT SHALL MANUFACTURER’S TOTAL LIABILITY TO CUSTOMER ARISING UNDER THIS AGREEMENT EXCEED, ON A PRODUCT-BY-PRODUCT BASIS, THE TOTAL PRICE PAID BY CUSTOMER FOR SUCH PRODUCT HEREUNDER; PROVIDED, HOWEVER, THAT (X) NOTHING IN THIS SECTION 7.6 SHALL LIMIT OR EXCLUDE ANY DAMAGES OR CLAIMS TO THE EXTENT ARISING OUT OF A BREACH OF ARTICLE 10 OF THIS AGREEMENT OR RESULTING FROM A PARTY’S GROSS NEGLIGENCE OR WILLFUL MISCONDUCT, AND (Y) TO THE EXTENT AN INDEMNIFIED PARTY IS REQUIRED TO PAY ANY DIRECT, SPECIAL, INCIDENTAL, INDIRECT, COLLATERAL, CONSEQUENTIAL OR PUNITIVE DAMAGES OR LOST PROFITS TO A PERSON WHO IS NOT A MEMBER OF EITHER GROUP IN CONNECTION WITH A THIRD PARTY CLAIM, SUCH DAMAGES WILL NOT BE SUBJECT TO ANY LIMITATIONS OR EXCLUSIONS SET FORTH IN THIS SECTION 7.6.
| 7.7 | Remedies Cumulative. The remedies provided in this Article 7 shall be cumulative and shall not preclude assertion by any Indemnitee of any other rights or the seeking of any and all other remedies against any Indemnifying Party. |
| 7.8 | Survival of Indemnities. The indemnity and contribution agreements contained in this Article 7 shall remain operative and in full force and effect, regardless of (i) any investigation made by or on behalf of any Indemnitee; and (ii) the knowledge by the Indemnitee of Liabilities for which it might be entitled to indemnification or contribution hereunder. The rights and obligations of each of Pfizer and Zoetis and their respective Indemnitees under this Article 7 shall survive the sale or other transfer by any party of any assets or businesses or the assignment by it of any Liabilities. |
| 7.9 | Time Limit for Claims. Any action for breach of this Agreement brought by a Party against the other Party must be commenced no later than twelve (12) months after expiration or termination of the Term (to the extent permitted by applicable Law), and any claim for indemnification hereunder must be asserted prior to expiration of the twelve (12) month period after the applicable breach, gross negligence or willful misconduct, or other applicable conduct or occurrence, respectively, for which the claim for indemnification is being brought hereunder (except to the extent such claim for indemnification is based upon a third party claim asserted after such twelve (12) month period by a Person who is not a member of either Group, and without limitation and subject to Section 7.4). |
33
| 8. | INSURANCE. |
| 8.1 | During the Term and for a period of three (3) years from the date of the last delivery of Product to Customer hereunder, Manufacturer shall self-insure (if Pfizer or its Affiliate is the Manufacturer) or shall provide and maintain such insurance coverage, in minimum types and amounts as described below in this Article. |
| (a) | Any and all deductibles for such insurance policies shall be assumed by, for the account of, and at Manufacturer’s sole risk. All deductibles and self-insured retention amounts must be acceptable to and approved, in writing (if required), by Customer in its reasonable discretion. |
| (b) | In respect of the Liabilities assumed by Manufacturer under this Agreement, such insurance policies shall be primary and non-contributing with respect to any other similar insurance policies available to Customer or its Affiliates. In respect of the Liabilities assumed by Manufacturer under this Agreement, except for Workers Compensation/Employers’ Liability all such policies shall include Customer and its Affiliates and any other such entities as Customer may reasonably request, as additional insureds. Where permitted by law, all such polices shall provide a waiver of subrogation in favor of Customer and its Affiliates. |
| (c) | Manufacturer shall furnish to Customer original certificates evidencing the specified insurance coverage (except to the extent self-insured, if Pfizer or its Affiliate is the Manufacturer), upon execution of this Agreement and at contract renewal or expiration of any one coverage, whichever occurs first. Such certificates shall provide that not less than thirty (30) days’ prior written notice of any policy cancellation, or material change shall be given to Customer. The Certificate(s) of Insurance shall be signed by a person authorized by the insurer(s) to bind coverage on its (their) behalf. |
| 8.2 | The insurance required under this Article shall be written for not less than any limits of liability specified herein or as required by law, whichever is greater. Manufacturer shall have the right to provide the total limits required by any combination of primary and Umbrella/Excess coverage; said insurance to include the following: |
| (a) | Insurance for liability under the Workers’ Compensation or occupational disease laws of any state or other jurisdiction in which services are performed (or be a qualified self-insurer in those states and jurisdictions) or otherwise applicable with respect to persons performing the services and Employer’s Liability insurance covering all claims by or in respect to the employees of Manufacturer and all subcontractors, providing: |
| (i) | Coverage for the statutory limits of all claims under the applicable State Workers’ Compensation Act or Acts. If the Scope of Work |
34
| will result in exposures under the U.S. Longshoreman’s Act and its amendments (work dockside or on water), the Xxxxx Act (involving xxxxxx, masters and crew of vessels) or the Federal Employer’s Liability Act (railroad exposure), coverage shall be extended to include insurance coverages mandated thereby; |
| (ii) | Employer’s Liability Insurance with a limit of not less than $1,000,000; |
| (iii) | Voluntary Compensation insurance covering all employees not subject to the applicable state Workers’ Compensation Act or Acts. |
| (b) | Commercial General Liability insurance with the following limits and forms/endorsements: |
| Each Occurrence | $ | 2,000,000 |
| (i) | Occurrence form including premises and operations coverage, broad form property damage, personal injury coverage, contractual liability, explosion, collapse, and underground (“XCU”) and watercraft liability coverage if services are performed on or near a body of water. |
| (ii) | Customer and its Affiliates shall be named as additional insureds via ISO form CG20101185 with respect to any legal liability of Customer or its Affiliates, arising out of Manufacturer’s performance. |
| (c) | Automobile and Truck Liability Insurance: $2,000,000 combined single limit for bodily injury and property damage arising out of all owned, non-owned and hired vehicles, including coverage for all automotive and truck equipment used in the performance of this Agreement and including the loading and unloading of same. |
| (d) | Umbrella (Excess) Liability Coverage in an amount not less than $3,000,000 per occurrence and in the aggregate. |
| (e) | If Manufacturer has care, custody or control of Customer property or inventory, Manufacturer shall be responsible for any loss or damage to it, and provide all risk Property Coverage at full replacement cost for property and at the costs-per-unit as specified in the Product Addendum for inventory. |
| (f) | Product Liability / Completed Operations insurance with the following limits: $5 million per occurrence and $5 million annual aggregate. Product Liability / Completed Operations coverage shall be maintained for a period of three (3) years following the date of the last delivery of Product to Customer hereunder. Products Liability/Completed Operations |
35
| coverage may be part of Commercial General Liability coverage and Umbrella/Excess coverage. |
| 8.3 | Acceptance of Insurance Certificate. Acceptance of any insurance certificate by Customer shall not constitute acceptance of the adequacy of coverage, compliance with the requirements of this Agreement, or serve as an amendment to this Agreement. |
| 9. | CUSTOMER-SUPPLIED MATERIALS. |
| 9.1 | Supply of Materials. |
| (a) | Customer shall at its own expense supply Manufacturer with the Customer-Supplied Materials identified in the applicable Product Addendum. At Customer’s option, the Customer-Supplied Materials may be delivered directly from Customer’s vendor to Manufacturer at the vendor’s or Customer’s expense. Customer or its vendor shall supply Manufacturer with a copy of the certificate of analysis for the Customer-Supplied Materials no later than delivery of the Customer-Supplied Materials to Manufacturer. |
| (b) | Customer and Manufacturer shall cooperate in good faith to determine when such Customer-Supplied Materials shall be supplied based upon Customer’s Forecasts for Products, and Manufacturer shall issue to Customer “pro forma” Purchase Orders for Customer-Supplied Materials according to parameters included in the applicable Product Addendum. Manufacturer shall be responsible to receive, sample, store and maintain the inventory of Customer-Supplied Materials at Manufacturer’s Facility. Manufacturer shall provide to Customer, on a monthly basis, an inventory report of Customer-Supplied Materials in Manufacturer’s possession in a format attached as Exhibit C to this Agreement. |
| 9.2 | Title and Risk of Loss. |
| (a) | Title to the Customer-Supplied Materials supplied by Customer to Manufacturer shall remain with Customer; provided, however, that risk of loss shall pass to Manufacturer at the time Customer-Supplied Materials arrive at Manufacturer’s Facility. Manufacturer shall not use Customer-Supplied Materials for any purposes other than those related to the manufacture of the Product pursuant to this Agreement. |
| (b) | The risk of loss or damage to Customer-Supplied Materials during the possession thereof by Manufacturer shall be solely with Manufacturer. |
| (c) | Manufacturer shall insure (or self-insure if Pfizer or its Affiliate is the Manufacturer) the Customer-Supplied Materials and the Product while such is in Manufacturer’s possession at the costs-per-unit as specified in the Product Addendum. |
36
| 9.3 | Reimbursement for Loss of Customer-Supplied Materials. |
| (a) | Manufacturer agrees to reimburse Customer for its costs-per-unit as specified in the Product Addendum per batch/lot pro-rated over the usable portion of the batch/lot, if applicable, for any loss of Customer-Supplied Materials, including any loss of Customer-Supplied Materials used to manufacture any Non-Complying Product, subject to Section 9.3(b). In addition, Manufacturer shall be responsible for all manufacturing costs incurred during the manufacture of such failed batch/lot, pro-rated over the usable portion of the batch/lot if applicable. Manufacturer shall also reimburse Customer for excess Customer-Supplied Materials used as a result of Manufacturer’s failure to achieve the minimum average yield or u-factor (as applicable) set forth in the applicable Product Addendum. During the month of January of each year Manufacturer will report to Customer the actual yield or u-factor (as applicable) achieved for all Customer-Supplied Materials used during the previous calendar year, as determined either by actual batch yield data or by u-factor calculations (as applicable). If the achieved average yield or u-factor (as applicable) is lower than the minimum acceptable yield or higher than the maximum acceptable u-factor (as applicable) specified in the applicable Product Addendum, calculated on a Facility-by-Facility basis, Manufacturer will reimburse to Customer the cost of the excess Customer-Supplied Materials used as set forth in the applicable Product Addendum. Rejected batches for which Manufacturer already compensated Customer for the loss of Customer-Supplied Materials will not be included in the annual yield or u-factor calculation (as applicable). |
| (b) | Notwithstanding anything to the contrary herein, Customer shall be solely responsible for all costs and expenses to the extent arising from the failure of any Customer-Supplied Materials to conform to the applicable specifications therefor, and shall reimburse Manufacturer for Manufacturer’s Standard Costs per batch/lot pro-rated over the usable portion of the batch/lot, if applicable, for any resulting loss of Product Materials, including any Product Materials used in the manufacture of any resulting Non-Complying Product or other rejected or discarded Product. |
| 10. | CONFIDENTIAL INFORMATION. |
| 10.1 | Non-Use and Non-Disclosure. |
Each Party to this Agreement or any Product Addendum shall maintain in strict confidence, and shall not disclose to any third party, all Confidential Information observed by or disclosed to it by or on behalf of the other Party pursuant to this Agreement or any Product Addendum. Each Party shall not use or disclose such Confidential Information except as permitted by this Agreement. Each Party shall safeguard the confidential and proprietary nature of the Confidential Information of the other Party with at least the same degree of care as it holds its own confidential or proprietary information of like kind, which shall be no less than a
37
reasonable degree of care. Notwithstanding the foregoing, the preceding restrictions shall not apply to information that the recipient can demonstrate (a) was lawfully in its possession prior to the time of disclosure (except where (i) Pfizer or one of its Affiliates is the recipient and such information is included in the Animal Health Assets or is otherwise owned by Zoetis or one of its Affiliates pursuant to the Separation Agreement, this Agreement or any other Ancillary Agreement, or (ii) Zoetis or one of its Affiliates is the recipient and such information is included in the Excluded Assets or is otherwise owned by Pfizer or one of its Affiliates pursuant to the Separation Agreement, this Agreement or any other Ancillary Agreement); (b) is or becomes public knowledge through no fault, omission, or other act of the recipient; (c) is obtained from a third party lawfully entitled to possession of such Confidential Information and under no obligation of confidentiality to the disclosing party; or (d) was independently developed by or for recipient without violating the terms of this Agreement. In addition, if recipient is requested to disclose the Confidential Information of the other Party in connection with a legal or administrative proceeding or otherwise to comply with a requirement under any Law, such recipient shall give the disclosing party prompt notice of such request so that the disclosing party may seek an appropriate protective order or other remedy, or waive compliance with the relevant provisions of this Agreement. If the disclosing party seeks a protective order or other remedy, recipient shall promptly cooperate with and reasonably assist the disclosing party in such efforts. If the disclosing party fails to obtain a protective order or waives compliance with the relevant provisions of this Agreement, the recipient shall disclose only that portion of Confidential Information which its legal counsel determines it is required to disclose.
| 10.2 | Return of Confidential Information. |
Upon the written request of the disclosing Party, the recipient shall promptly return or destroy, at such disclosing Party’s option, all Confidential Information of such disclosing Party (including all copies in whatever medium provided to, or made by, such recipient); provided, however, that, subject to the terms of this Agreement, recipient shall be entitled to retain one archival copy of such Confidential Information for purposes of determining its obligations under this Agreement. Notwithstanding recipient’s return or destruction of Confidential Information, recipient shall continue to be bound by its obligation of confidentiality and non-use under this Agreement.
| 10.3 | Survival. |
The provisions of this Article 10 shall survive the later of the termination or expiration of the applicable Product Addendum, or the last Purchase Order issued by Customer to Manufacturer hereunder, for a period of five (5) years.
38
| 11. | TERM; TERMINATION. |
| 11.1 | Term of Agreement. |
This Agreement shall commence on the Effective Date and shall continue for a period of five (5) years from the IPO Closing (the “Term” of this Agreement), unless extended or terminated pursuant to this Article or the mutual written agreement of the Parties. Each Product Addendum shall commence on the Product Addendum Effective Date thereof and, except as otherwise provided in such Product Addendum, shall continue until expiration or termination of this Agreement (the “Term” of such Product Addendum), in each case unless extended or terminated pursuant to this Article or the mutual written agreement of the Parties. Customer shall use commercially reasonable efforts to develop the capabilities and facilities to manufacture each Product so as to enable Customer to commence such manufacture on its own behalf, or to establish alternative sources of supply of such Product (including a long-term supply arrangement between the Parties, if mutually agreed to in writing by the Parties), and transition from such manufacture by Manufacturer hereunder, reasonably prior to the expiration of this Agreement, and Customer shall promptly respond to Manufacturer’s reasonable requests regarding Customer’s progress with respect to such matters.
| 11.2 | Termination for Cause. |
Either Party may terminate the applicable Product Addendum for cause immediately upon written notice to the other Party in the event that such other Party fails to perform any material obligation under such Product Addendum, through no fault of the Party initiating such termination, that remains uncured for ninety (90) calendar days following written notice to such Party of such breach.
| 11.3 | Termination in Event of Insolvency. |
In the event that either Party (the “Insolvent Party”): (i) becomes insolvent, or institutes or has instituted against it a petition for bankruptcy or is adjudicated bankrupt; or (ii) executes a xxxx of sale, deed of trust, or a general assignment for the benefit of creditors; or (iii) is dissolved or transfers a substantial portion of its assets to a third party; or (iv) a receiver is appointed for the benefit of its creditors, or a receiver is appointed on account of insolvency; then the Insolvent Party shall immediately notify the other Party of such event and such other Party shall be entitled to: (a) terminate this Agreement and/or any or all Product Addenda for cause immediately upon written notice to the Insolvent Party; or (b) request that the Insolvent Party or its successor provide adequate assurances of continued and future performance in form and substance acceptable to such other Party, which shall be provided by the Insolvent Party within ten (10) calendar days of such request, and the other Party may terminate this Agreement and/or any or all Product Addenda for cause immediately upon written notice to the Insolvent Party in the event that the Insolvent Party fails to provide such assurances acceptable to the other Party within such ten (10) day period.
39
| 11.4 | Other Termination. |
Any Product Addendum may be terminated as follows:
| (a) | By Customer or by Manufacturer pursuant to Section 16.5; |
| (b) | By Customer upon at least six (6) months’ prior written notice to Manufacturer (or such longer period as may be specified in the applicable Product Addendum) for any or no reason. |
| 11.5 | Effect of Termination. |
| (a) | The termination or expiration of this Agreement or any Product Addendum for any reason shall not release any Party hereto of any liability which at the time of termination or expiration had already accrued to the other Party in respect to any act or omission prior thereto. |
| (b) | Upon the effective date of expiration or termination of this Agreement or, with respect to applicable Product, any Product Addendum for any reason, without limiting Section 2.5(a), Manufacturer shall immediately deliver to Customer all Specifications (and copies thereof) and, at Customer’s direction, return or destroy (i) all remaining originals, masters and inventory of artwork, labels, bottles, premiums and packaging materials, and any other Product Materials bearing any trademarks or logos of Customer or its Affiliates or otherwise describing the Products, and (ii) all unused Customer-Supplied Materials. Additionally, within ninety (90) days after the effective date of the expiration or the termination for any reason of this Agreement or such Product Addendum, Customer shall purchase any finished Product and, at Manufacturer’s option, any work-in-process and/or Product Materials (other than Customer-Supplied Materials) that Manufacturer has purchased based upon a Customer firm Purchase Order or a Forecast quantity with respect to the binding portion of such Forecast, as applicable, for the production of Product under this Agreement or such Product Addendum, as applicable; provided that Manufacturer uses its commercially reasonable efforts to exhaust existing stocks of such Product Materials prior to the date of expiration or termination. Customer shall pay Manufacturer’s direct cost for work in process, and Manufacturer’s purchase price from its suppliers for such Product Materials. All delivery, removal and transportation costs incurred in connection with this Section 11.5(b) shall be borne by Customer except in the event Customer terminates this Agreement or such Product Addendum pursuant to Section 11.2 or 11.3, as applicable, in which case all such reasonable costs shall be borne by Manufacturer. |
| (c) | Without limiting the foregoing, upon the expiration or termination of each Product Addendum, Customer shall have the right to purchase from Manufacturer any item of equipment at the applicable Facility which is owned by Manufacturer or its Affiliates and used and held for use |
40
| exclusively for manufacturing the Product under such Product Addendum, provided that (i) Customer notifies Manufacturer that it is exercising such right no later than ninety (90) days prior to expiration or termination of such Product Addendum, and (ii) Customer pays Manufacturer the net book value for such item, as such net book value is determined by Manufacturer as of the date of such Customer notification (and Manufacturer shall provide such net book value to Customer within sixty (60) days following Customer’s written request therefor), within forty-five (45) days following such expiration or termination (each such item of purchased equipment, “Purchased Equipment”). Each such purchase shall be made pursuant to a written agreement to be entered into by Customer and Manufacturer, which agreement shall provide that such Purchased Equipment is sold “as is, where is, with all faults and defects.” For clarity, Customer shall have the right to exercise such right on an item-by-item basis with respect to such Purchased Equipment. Customer shall be solely responsible for all costs and expenses associated with removing such Purchased Equipment from the Facility (and repairing and restoring the Facility from any damage caused by such removal) in a manner that does not unreasonably interfere with Manufacturer’s operations at the Facility, and Customer shall reimburse Manufacturer within forty-five (45) days following Manufacturer’s invoice for such costs and expenses. Manufacturer and its Affiliates shall have no responsibility for marketing or insuring the Purchased Equipment and the Customer shall bear all risk of loss with respect thereto. |
| (d) | The termination or expiration of this Agreement or any Product Addendum shall not affect the survival and continuing validity of Article 5 (Representations and Warranties), Article 6 (Intellectual Property), Article 7 (Indemnification), Article 8 (Insurance), Article 14 (Notices), Article 15 (Dispute Resolution), Article 16 (Miscellaneous), or of any other provision which is expressly or by implication intended to continue in force after such termination or expiration. |
| 11.6 | Return of Customer-Supplied Materials, Tools and Equipment. |
| (a) | Upon the effective date of expiration or termination of this Agreement or, with respect to applicable Product, any Product Addendum for any reason whatsoever, Manufacturer shall immediately deliver to Customer all Specifications (and copies thereof), artwork, labels, bottles, all premiums and packaging materials purchased by Customer and all Product Materials and equipment, molds, tablet press tooling or proprietary materials provided by Customer or purchased by Manufacturer (and reimbursed by Customer), in each case that are used and held for use exclusively for the manufacture of Product for Customer and were purchased following consummation of the transactions under the Separation Agreement. Manufacturer will remove all such equipment, molds and tablet press tooling from the Facility and make such equipment, molds and tooling |
41
| available for pickup at the Facility by a carrier designated by Customer. All delivery, removal and transportation costs incurred in connection with this Section 11.6 shall be borne by Customer except in the event Customer terminates this Agreement pursuant to Section 11.2 or 11.3, in which case all such reasonable costs shall be borne by Manufacturer. |
| (b) | Any Product quarantined at the time of expiration or termination of this Agreement or, with respect to applicable Product, any Product Addendum shall be disposed of or destroyed (at the Customer’s expense, except to the extent provided otherwise in Article 4 or the Quality Agreement) in accordance with Customer’s instructions. |
| 11.7 | Transitional Support. |
| (a) | Upon or within a mutually agreed time period prior to the expiration or termination of the Term of each Product Addendum, with respect to each Customer-Exclusive Product, Manufacturer shall provide reasonable technical support to Customer, as set forth in this Section 11.7, to assist Customer in the technology transfer of production of each such Product to either one (1) facility of Customer or one (1) facility of an alternative source of supply as designated by Customer (“Technical Support”, and such facility, the “Receiving Site”). Such reasonable Technical Support shall consist of: |
| (i) | Supply of a technical package to facilitate the transfer of all relevant manufacturing information to the Receiving Site, including formulation descriptions, manufacturing instructions, specifications, methods and material supplier information, as applicable, except for any information that is (i) Sensitive Manufacturer Technology or (ii) subject to confidentiality obligations owing to a third party other than Customer or its Affiliates; |
| (ii) | Host one (1) site visit to the Manufacturer’s Facility by the Customer and/or such designated alternative source supplier to observe production of the applicable Product (such that, for clarity, no more than one (1) such site visit shall occur for each Product), in each case at a mutually agreed date and time during Manufacturer’s normal operating hours and subject to such confidentiality procedures or requirements as may be requested or implemented by Manufacturer, provided that (i) the request for each such visit shall be made so as to allow for sufficient advance preparation time and can be accommodated in the requested timeframe without interruption to Manufacturer’s routine production, and (ii) such visit shall not include access to Sensitive Manufacturer Technology; |
42
| (iii) | Performance of high-level consultation and answering queries for Customer through the transfer process; and |
| (iv) | Provision of Product samples required for transfer activities, in each case at the expense of Customer. |
| (b) | Customer and/or such designated alternative source supplier shall be responsible for providing leadership of any technology transfer from Manufacturer. For the avoidance of doubt, the Customer and/or such designated alternative source supplier shall be solely responsible for identifying any and all Technical Support that is required from Manufacturer to assure such technology transfer is successful. |
| (c) | The Parties shall reasonably cooperate and mutually agree to facilitate the provision of any additional reasonable Technical Support with respect to the applicable Product to Customer or such designated alternative source supplier, including assistance through the transfer process, Manufacturer personnel visits to the Receiving Site, and training and troubleshooting during the Receiving Site’s first production run of the Product, in each case as and to the extent agreed by Manufacturer in each instance (and subject to Section 11.7(f)). |
| (d) | Manufacturer shall have the right to prioritize its own projects and activities over any Technical Support hereunder with respect to staffing, production activities and other resources or obligations. In addition, Manufacturer shall have no obligation to hire or retain any individuals or make any capital expenditures in connection with the Technical Support, and Manufacturer’s obligation to provide Technical Support is contingent upon the continued employment by Manufacturer of those individuals capable of providing such Technical Support. Manufacturer’s obligation to provide any Technical Support under this Agreement shall terminate and be of no further force or effect if Customer or any its Affiliates hire any Manufacturer personnel involved in providing the Technical Support to Customer hereunder (without limiting any applicable non-solicitation obligations of Customer pursuant to the Separation Agreement or any other Ancillary Agreement). |
| (e) | Customer shall be solely responsible for any and all regulatory or other Governmental Authority requirements, activities and related costs and expenses that arise in conjunction with any Technical Support, technology transfer of production or production of each Product to or at the Receiving Site. These activities may also include, but are not limited to, creation of additional data or technical information, analytical method modifications or other work of a technical nature required to support regulatory queries or contemporary standards and guidelines driven by the manufacturing transfer (subject to Section 6.2). |
43
| (f) | Customer shall be responsible for, and shall promptly reimburse Manufacturer upon Manufacturer’s written request for, any and all out-of-pocket costs and expenses incurred by or on behalf of Manufacturer in connection with any Technical Support under this Agreement. |
| (g) | With respect to each Product, Manufacturer shall provide to Customer, in connection with the Technical Support or reasonably promptly following Customer’s written request therefor following termination of the applicable Product Addendum, such analytical materials and methods in Manufacturer’s possession or control that are required in connection with disclosures to any applicable Governmental Authority to qualify the applicable Product Materials or such Product itself for release testing to meet the then-current applicable Marketing Authorization, in each case in accordance with Section 10.1. |
| (h) | Notwithstanding anything to the contrary herein, except as expressly provided in Section 11.7(g), Manufacturer shall have no obligation to disclose, license or otherwise provide confidential or proprietary information of Manufacturer or any third party, including any Sensitive Manufacturer Technology, in connection with this Agreement or any Technical Support or technology transfer therein. |
| 12. | SUPPLY CHAIN SECURITY. |
| 12.1 | C-TPAT (US Manufacturers). |
| (a) | Manufacturer represents and warrants to Customer that it has reviewed its supply chain security procedures and that these procedures and their implementation are, and shall remain during the Term of this Agreement, in accordance with the importer security criteria set forth by the Customs-Trade Partnership Against Terrorism (“C-TPAT”) program of the U.S. Bureau of Customs and Border Protection. Manufacturer represents and warrants that it has developed and implemented, or shall develop and implement within sixty (60) calendar days of its execution of this Agreement, procedures for periodically reviewing and, if necessary, improving its supply chain security procedures to assure compliance with C-TPAT security criteria. |
| (b) | Customer is or has, or promptly following the Effective Date will have, applied to become a certified member of C-TPAT. Importers that have joined C-TPAT are expected to have substantially fewer of their imports inspected and, hence, fewer supply chain delays (the “C-TPAT Benefits”). As a C-TPAT member, Customer is (or will be) required to make periodic assessment of its international supply chain based upon C-TPAT security criteria. Manufacturer agrees to conduct an annual security audit at each of its facilities and to take all necessary corrective actions to ensure the continued participation of Customer in C-TPAT. Manufacturer agrees to share with Customer the results of such annual audits and agrees to |
44
| prepare and submit to Customer a report on the corrective actions taken in response thereto. Manufacturer agrees to notify Customer of any event that has resulted in or threatens the loss of its C-TPAT Benefits (if it is a member of the C-TPAT program) or alternatively jeopardizes Customer’s retention of its own C-TPAT Benefits. In an effort to secure each part of the supply chain, Manufacturer agrees to work in good faith to become a member of the C-TPAT program, if Manufacturer is organized or incorporated in the United States, Mexico or Canada, or the equivalent supply chain security program criteria administered by the customs administration in Manufacturer’s home country if Manufacturer is not organized or incorporated in the United States, Mexico or Canada. |
| 12.2 | Ex-US Manufacturers. |
| (a) | Manufacturer represents, warrants and covenants to Customer that it has reviewed its supply chain security procedures and that these procedures and their implementation are, and shall remain during the Term of this Agreement, in accordance with the conveyance security criteria set forth by Pfizer. Manufacturer represents and warrants that it has developed and implemented, or shall develop and implement within sixty (60) days of its execution of this Agreement, procedures for periodically reviewing and, if necessary, improving its supply chain security procedures to assure the integrity of the Product while in transit. |
| (b) | Manufacturer will adhere to all applicable Laws pertaining to security of the supply chain with respect to Product, as well as to standard industry practices. Manufacturer will make available to Pfizer any documentation outlining their internal supply chain security program with respect to Product upon request, and will work with the Customer to resolve gaps identified within that process. |
| 13. | NO COUNTERFEIT. |
In no event shall Manufacturer manufacture, supply or otherwise distribute for itself or a third party a counterfeit of the Product.
| 14. | NOTICES. |
All notices or other communications under this Agreement shall be in writing and shall be deemed to be duly given when (a) delivered in person or (b) deposited in the United States mail or private express mail, postage prepaid, addressed as follows:
| If to Pfizer or its applicable Affiliate that is party to a Product Addendum: | Pfizer Inc. 0000 Xxxxxxx Xxxx Xxxxxxxxx, XX 00000 Attn: President, Pfizer CentreSource Fax No. (000) 000-0000 |
45
| with copy to: | Pfizer Inc. 000 Xxxx 00xx Xxxxxx Xxx Xxxx, XX 00000 Attn: Executive Vice President and General Counsel Fax No.: (000) 000-0000 | |||
| If to Zoetis or its applicable Affiliate that is party to a Product Addendum: | Zoetis Inc. c/o Pfizer Inc. 000 Xxxx 00xx Xx. Xxx Xxxx, XX 00000 Attn: Xxxxxxx Xxxxxx | |||
| with copy to: | Zoetis Inc. 0 Xxxxxxx Xxxxx Xxxxxxx, XX 00000 Attn: Xxxxx Xxxx Fax. No.: (000) 000-0000 |
Any Party may, by notice to the other Party, change the address to which such notices are to be given.
| 15. | DISPUTE RESOLUTION. |
| 15.1 | Disputes. |
The procedures for discussion, negotiation and mediation set forth in this Article 15 shall apply to all disputes, controversies or claims (whether arising in contract, tort or otherwise) that may arise out of or relate to, or arise under or in connection with this Agreement, or the transactions contemplated hereby (including all actions taken in furtherance of the transactions contemplated hereby on or prior to the Separation Date), or the commercial or economic relationship of the Parties relating hereto or thereto, between or among any Person in the Pfizer Group and the Company Group.
| 15.2 | Escalation; Mediation. |
| (a) | It is the intent of the Parties to use their respective commercially reasonable efforts to resolve expeditiously any dispute, controversy or claim between or among them with respect to the matters covered hereby that may arise from time to time on a mutually acceptable negotiated basis. In furtherance of the foregoing, any Party involved in a dispute, controversy or claim with respect to such matters may deliver a notice (an “Escalation Notice”) demanding an in person meeting involving representatives of the Parties at a senior level of management of the parties (or if the Parties agree, of the appropriate strategic business unit or division within such entity). A copy of any such Escalation Notice shall be |
46
| given to the General Counsel, or like officer or official, of each party involved in the dispute, controversy or claim (which copy shall state that it is an Escalation Notice pursuant to this Agreement). Any agenda, location or procedures for such discussions or negotiations between the parties may be established by the Parties from time to time; provided, however, that the Parties shall use their commercially reasonable efforts to meet within thirty (30) days of the Escalation Notice. |
| (b) | If the Parties are not able to resolve the dispute, controversy or claim through the escalation process referred to above, then the matter shall be referred to mediation. The Parties shall retain a mediator to aid the Parties in their discussions and negotiations by informally providing advice to the parties. Any opinion expressed by the mediator shall be strictly advisory and shall not be binding on the Parties, nor shall any opinion expressed by the mediator be admissible in any other proceeding. The mediator may be chosen from a list of mediators previously selected by the Parties or by other agreement of the Parties. Costs of the mediation shall be borne equally by the Parties involved in the matter, except that each Party shall be responsible for its own expenses. Mediation shall be a prerequisite to the commencement of any Action by either Party. |
| 15.3 | Court Actions. |
| (a) | In the event that any Party, after complying with the provisions set forth in Section 15.2 above, desires to commence an Action, such Party, subject to Section 16.2, may submit the dispute, controversy or claim (or such series of related disputes, controversies or claims) to any court of competent jurisdiction. |
| (b) | Unless otherwise agreed in writing, the Parties will continue to provide service and honor all other commitments under this Agreement during the course of dispute resolution pursuant to the provisions of this Article 15, except to the extent such commitments are the subject of such dispute, controversy or claim. |
| 16. | MISCELLANEOUS. |
| 16.1 | Publicity. |
Neither Party shall issue any press release or other publicity materials, or make any presentation with respect to the existence of this Agreement or the terms and conditions hereof without the prior written consent of the other Party in each instance. Neither Party shall publicize or publicly use any name, trademarks or logos of the other Party in connection with this Agreement without the other Party’s prior written consent in each instance. This restriction shall not, however, apply to the extent that any such disclosures are required by applicable Laws, including as may be required in connection with any filings required to be made with the United States Securities and Exchange Commission or by the disclosure policies of a major stock exchange.
47
| 16.2 | Governing Law; Submission to Jurisdiction; Waiver of Jury Trial. |
| (a) | This Agreement shall be governed by and construed and interpreted in accordance with the Laws (other than Section 5–1401 and 5–1402 of the New York General Obligations Law) of the State of New York. THE PARTIES EXPRESSLY AGREE THAT THE APPLICATION OF THE UNITED NATIONS CONVENTION ON CONTRACTS FOR THE INTERNATIONAL SALE OF GOODS (1980) IS SPECIFICALLY EXCLUDED AND SHALL NOT APPLY TO THIS AGREEMENT. |
| (b) | With respect to any Action relating to or arising out of this Agreement, subject to the provisions of Article 15, each Party to this Agreement irrevocably (a) consents and submits to the exclusive jurisdiction of the courts of the State of New York and any court of the United States located in the Borough of Manhattan in New York City, (b) waives any objection which such Party may have at any time to the laying of venue of any Proceeding brought in any such court, waives any claim that such Proceeding has been brought in an inconvenient forum and further waives the right to object, with respect to such Proceeding, that such court does not have jurisdiction over such Party and (c) consents to the service of process at the address set forth for notices in Section 14; provided, however, that such manner of service of process shall not preclude the service of process in any other manner permitted under applicable Law. |
| (c) | THE PARTIES HERETO AGREE THAT THEY HEREBY IRREVOCABLY WAIVE AND AGREE TO CAUSE THEIR RESPECTIVE SUBSIDIARIES TO WAIVE THE RIGHT TO TRIAL BY JURY IN ANY ACTION TO ENFORCE OR INTERPRET THE PROVISIONS OF THIS AGREEMENT. |
| 16.3 | No Agency. |
Nothing contained herein shall be construed to place the Parties in the relationship of partners, joint venturers, principal and agent, or employer and employee. Neither Party shall have the power to assume, create, or incur liability or any obligation of any kind, express or implied, in the name of or on behalf of the other party by virtue of this Agreement.
| 16.4 | Assignment; Binding Effect. |
This Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and permitted assigns; provided, however, that no Party hereto may assign its respective rights or delegate its respective obligations under this Agreement without the express prior written consent of the other Party or Parties hereto; provided, further, that either Party may assign any of its rights and delegate or subcontract any of its duties and obligations under this Agreement to any of its Affiliates without the approval of the other Party (such assignment, delegation or subcontracting to an Affiliate shall not relieve such
48
Party of its responsibilities and liabilities hereunder and such Party shall remain liable to the other Party for the conduct and performance of its Affiliate), and, provided, further, that Customer may assign any of its rights and delegate or subcontract any of its duties and obligations hereunder with respect to any Product, without the approval of Manufacturer, to a third party that acquires all or substantially all of Customer’s rights to such Product (subject to Manufacturer’s reasonable approval or Customer’s guarantee of the creditworthiness of such third party), and, provided, further, that Pfizer may assign any of its rights and delegate or subcontract any of its duties and obligations hereunder in connection with the sale, transfer or other disposition of any Facility or related assets to a third party. Any subcontracting consented to by Customer shall not relieve Manufacturer of its responsibilities and liabilities hereunder and Manufacturer shall remain liable to Customer for the conduct and performance of each permitted subcontractor hereunder. Except for the indemnification rights under this Agreement of any Manufacturer Indemnitee or Customer Indemnitee in their respective capacities as such (a) the provisions of this Agreement are solely for the benefit of the Parties and are not intended to confer upon any Person (including employees of the Parties hereto) except the Parties any rights or remedies hereunder and (b) there are no third party beneficiaries of this Agreement and this Agreement shall not provide any third person (including employees of the Parties hereto) with any remedy, claim, liability, reimbursement, claim of action or other right in excess of those existing without reference to this Agreement.
| 16.5 | Force Majeure; Failure to Supply. |
| (a) | No Party shall be liable for any failure to perform or any delays in performance, and no Party shall be deemed to be in breach or default of its obligations set forth in this Agreement, if, to the extent and for so long as, such failure or delay is due to any causes that are beyond its reasonable control, including such causes as acts of God, natural disasters, fire, flood, severe storm, earthquake, civil disturbance, lockout, riot, order of any court or administrative body, embargo, acts of government, war (whether or not declared), acts of terrorism, failure of suppliers, strikes or other labor disputes or other similar causes, in each case to the extent beyond such Party’s reasonable control (“Force Majeure Event”). In the event of a Force Majeure Event, the Party prevented from or delayed in performing shall promptly give notice to the other Party and shall use commercially reasonable efforts to avoid or minimize the delay. In the event that the delay continues for a period of at least thirty (30) calendar days, the Party affected by the other Party’s delay may elect to: (i) suspend performance and extend the time for performance for the duration of the Force Majeure Event, or (ii) cancel all or any part of the unperformed part of the applicable Product Addendum and/or any Purchase Orders. |
| (b) | In the event that Manufacturer shall be unable or unwilling or shall fail to supply any Product in such quantities as Customer shall request and in compliance with the delivery periods set forth in Article 4 (whether due to |
49
| the occurrence of a Force Majeure Event, or otherwise) (hereinafter referred to as a “Failure to Supply”), then upon the occurrence of any such Failure to Supply and through and until such time as Manufacturer fully resumes its supply obligations hereunder: (a) Manufacturer shall provide advice and consultation in connection therewith; (b) Customer shall have no obligation to purchase Products from Manufacturer until any contractual obligations that Customer has assumed in connection with producing the same or obtaining such substitute source of supply shall have terminated; and (c) Customer shall use commercially reasonable efforts to terminate such alternate arrangements promptly following Manufacturer’s notice to Customer that Manufacturer is capable of fully resuming its supply obligations hereunder. |
| 16.6 | Severability. |
If any provision of this Agreement or the application thereof to any Person or circumstance is determined by a court of competent jurisdiction to be invalid, void or unenforceable, the remaining provisions hereof or the application of such provision to Persons or circumstances or in jurisdictions other than those as to which it has been held invalid or unenforceable, shall remain in full force and effect and shall in no way be affected, impaired or invalidated thereby, so long as the economic or legal substance of the transactions contemplated hereby is not affected in any manner adverse to any Party. Upon such determination, the Parties shall negotiate in good faith in an effort to agree upon such a suitable and equitable provision to effect the original intent of the Parties.
| 16.7 | Waiver of Default. |
Waiver by any Party of any default by the other Party of any provision of this Agreement shall not be deemed a waiver by the waiving Party of any subsequent or other default, nor shall it prejudice the rights of the other Party.
| 16.8 | Cumulative Effect. |
The rights and obligations of the Parties under this Agreement shall be cumulative to and not exclusive of the rights and obligations of the parties contained in the Separation Agreement; provided that the remedies provided in this Agreement shall not be cumulative with any duplicative remedies available pursuant to the Separation Agreement.
| 16.9 | Further Documents. |
Each Party hereto agrees to execute such further documents and take such further steps as may be reasonably necessary or desirable to effectuate the purposes of this Agreement.
50
| 16.10 | Forms. |
The Parties recognize that, during the term of this Agreement, a purchase order acknowledgment form or similar routine document (collectively, “Forms”) may be used to implement or administer provisions of this Agreement. The Parties agree that the terms of this Agreement shall prevail in the event of any conflict between terms of this Agreement and the terms of such Forms, and any additional or different terms contained in such Forms shall not apply to this Agreement.
| 16.11 | Headings. |
The article, section and paragraph headings contained in this Agreement are for reference purposes only and shall not affect in any way the meaning or interpretation of this Agreement.
| 16.12 | Counterparts; Entire Agreement; Corporate Power. |
| (a) | This Agreement may be executed in one or more counterparts, all of which shall be considered one and the same agreement, and shall become effective when one or more counterparts have been signed by each of the Parties and delivered to the other Party. Execution of this Agreement or any other documents pursuant to this Agreement by facsimile or other electronic copy of a signature shall be deemed to be, and shall have the same effect as, execution by an original signature. |
| (b) | This Agreement, the Separation Agreement, the other Ancillary Agreements, and the exhibits, the schedules and appendices hereto and thereto contain the entire agreement between the Parties with respect to the subject matter hereof and supersede all previous agreements, negotiations, discussions, writings, understandings, commitments and conversations with respect to such subject matter. There are no agreements or understandings between the Parties with respect to such subject matter other than those set forth or referred to herein or therein. |
| (c) | In the event of any inconsistency between this Agreement and the R&D Collaboration Agreement, to the extent such conflict relates to or is in connection with (i) Intellectual Property ownership, (ii) Intellectual Property use rights, or (iii) confidentiality, the R&D Collaboration Agreement shall control. |
| 16.13 | Amendments. |
No provisions of this Agreement shall be deemed waived, amended, supplemented or modified by any Party, unless such waiver, amendment, supplement or modification is in writing and signed by the authorized representative of the Party against whom it is sought to enforce such waiver, amendment, supplement or modification.
51
IN WITNESS WHEREOF, the Parties hereto have caused this Master Manufacturing and Supply Agreement to be duly executed by their duly authorized representatives.
| PFIZER INC. | ZOETIS INC. | |||||||
| By: | /s/ XXXX X. XXXXX | By: | /s/ XXXX XXXXX XXXXX | |||||
| Name: | Xxxx X. Xxxxx | Name: | Xxxx Xxxxx Xxxxx | |||||
| Title: | VP, Strategy & Transitioning Sites | Title: | Chief Executive Officer |
52
Exhibit A
Form of Product Addendum
This PRODUCT ADDENDUM (“Product Addendum”) to the Master Manufacturing and Supply Agreement dated [INSERT DATE OF MASTER AGREEMENT] between PFIZER INC. and ZOETIS INC. (the “Master Agreement”) is entered into as of [INSERT EFFECTIVE DATE OF PRODUCT ADDENDUM] (the “Product Addendum Effective Date”) by and between [INSERT FULL NAME OF PFIZER ENTITY EXECUTING ADDENDUM], a [INSERT JURISDICTION OF ORGANIZATION AND TYPE OF ENTITY] having an address of [INSERT PFIZER ENTITY’S ADDRESS] (“Manufacturer”) and [INSERT FULL NAME OF ZOETIS ENTITY EXECUTING ADDENDUM], a [INSERT JURISDICTION OF ORGANIZATION AND TYPE OF ENTITY] having an address of [INSERT OTHER ENTITY’S ADDRESS] (“Customer”).
The parties agree that the terms and conditions of the Master Agreement are incorporated herein as if fully set forth herein. Each of Manufacturer and Customer agrees to be bound by all of the terms and conditions of the Master Agreement applicable to (i) a Manufacturer or Customer, respectively, in the Master Agreement, including the representations and warranties set forth in the Master Agreement expressed to be made by Manufacturer or Customer, as the case may be, and (ii) the Party to the Master Agreement that is an Affiliate of Manufacturer or Customer, respectively, where an Affiliate of Manufacturer or Customer is the Party to the Master Agreement. All capitalized terms used in this Product Addendum and not otherwise defined herein shall have the meaning ascribed to such terms in the Master Agreement. For clarity, the terms “Manufacturer” and “Customer” shall, with respect to this Product Addendum, have the meanings set forth in the preamble above.
The Parties further agree as follows:
A-1
| 1. | Supply Parameters and Cost |
| Plant SKU | SKU Desc. | Market | Market Dest. | Dmd. Mgmt | UOM | Batch Size | MOQ | Incr. OQ | Min. Shelf Life | Abs. Shelf Life | Safety Stock Cov | Max Stock Cov | Reaction Time | Frozen Fence | ||||||||||||||
2012:
| Plant SKU | SKU Desc. | MFG Step | Supply Relationship | 2012 API Cost (€/unit) | 2012 API Markup (%) | 2012 API Markup (€/unit) | 2012 ext.matls & abs. (€/unit) | 2012 total site cost/mfg. fee (€/unit) | 2012 site Markup (%) | 2012 site Markup (€/unit) | 2012 Price (€/unit) | |||||||||||
A-2
2013: Pending updated prices.
| 2. | Product Specifications |
A. Product Specifications (including test methods & Xxxx of Materials) reside in the following systems:
[LIST SYSTEMS HERE]
B. Long Lead Time Items:
| Long Lead Time Item (Description) | Long Lead Time Item (Item #) | Lead time to procure item (days) | Associated SKU | Associated SKU Description | ||||
A-3
C. Certain Product Parameters:
[TO COME]
D. List of Biological Products:
[TO COME]
| 3. | Customer-Supplied Materials |
| CSM SKU / Item # | CSM Description | Cost (€/Unit) | UOM | Plant SKU (Addendum) | Associated SKU (Description) | [Min. Acceptable Yield][Max. Acceptable U-Factor] | ||||||
A-4
Supply Parameters & Cost
| Plant SKU | Item SKU code, as defined by plant budget | |
| SKU Description | Item SKU description, as defined by plant budget | |
| Market | Destination market based on plant budget | |
| Market Dest. | Destination market based on supply parameter data. This shall constitute the Territory with respect to the applicable Product. | |
| Dmd. Mgmt | Mechanism used to manage demand: VMR or MTO | |
| UOM | Unit of Measure of SKU | |
| Batch Size | Production batch size for SKU | |
| MOQ | For VMR: Minimum units the site will ship during one replenishment cycle. For MTO: Minimum units per firm order | |
| Incr. OQ | For VMR: Incremental units the site will ship above the MOQ. For MTO: Incremental units required per firm order, above the MOQ | |
| Min. Effective Shelf Life | Minimum remaining dating the Customer will accept delivery on (days) | |
| Abs. Shelf Life | Absolute shelf life from MFG to expiry (days) | |
| Safety Stock Cov | Time duration of future demand to be considered in the Safety Stock calculation (days) | |
| Max Stock Cov | Maximum days of supply the Customer will have available in stock | |
| Reaction Time | Applicable to VMR, duration required for manufacturing, testing and shipping to market destination (days) | |
| Frozen Fence | Applicable to MTO, time at which rolling forecast becomes firm (days) | |
| Supply relationship | Tolling: Customer procures and owns API/intermediate, consigns to Manufacturer. (For clarity, Section 10 of this Product Addendum applies with respect to “Tolling” Products.) Buy-Sell: Customer procures API/intermediate, sells API/intermediate to Manufacturer, then buys finished Product. Turnkey: All starting materials procured by Manufacturer, Customer buys finished Product. | |
| MFG Step | Manufacturing step, defined based on site budget | |
| 2012 API Cost (€/unit) | Cost of API (or intermediate) per unit of SKU | |
| 2012 API Markup (%) | % which API is marked up in supply from Customer to Manufacturer (applicable to Buy-Sell only) | |
| 2012 API Markup (€/unit) | Cost of API xxxx-up per unit of SKU |
A-5
| 2012 ext.matls & abs. (€/unit) | External materials cost and absoprtion cost incurred at site, per unit of SKU | |
| 2012 total site cost (€/unit) | For Turnkey and Buy-sell Products, total cost incurred by site per unit of SKU. For Tolling Products, manufacturing fee per unit of SKU. | |
| 2012 site Markup (%) | % which total site cost is marked up in supply from Manufacturer to Customer | |
| 2012 site Markup (€/unit) | Total site cost xxxx-up per SKU | |
| 2012 Price (€/unit) | Total price Customer pays per unit of SKU | |
| Long Lead Time Items | ||
| Long Lead time item | Description of items which must be procured more than 90 days prior to delivery date | |
| Lead time to procure | Days associated with procured item in previous column | |
| Associated SKU | SKU of product which requires the long lead time item for manufacturing | |
| Associated SKU Description | SKU description of product which requires the long lead time item for manufacturing | |
| Customer-Supplied Materials (CSM) | ||
| CSM SKU / Item# | Plant SKU (if source is Pfizer plant) or Item # (if other source) of API (or intermediate) supplied by the Customer | |
| CSM Description | Description of API (or intermediate) supplied by the Customer | |
| Cost / Unit | Cost per unit of CSM | |
| UOM | Unit of measure of CSM | |
| Associated SKU | Plant SKU generated by use of CSM | |
| [Min. Acceptable Yield][Max. Acceptable U-Factor] | [YIELD: Minimum acceptable yield of the CSM in the finished Product (as a percent of the actual CSM consumed during the manufacturing process)][U-FACTOR: Maximum acceptable Quantity of CSM consumed per unit of SKU produced.] |
A-6
| 4. | RESERVED. |
| 5. | Additional Provisions: |
| 5.1 | Facility Name and Address: |
[SPECIFY NAME AND ADDRESS OF THE FACILITY UNDER THIS PRODUCT ADDENDUM]
| 5.2 | Currency (if not U.S. Dollars): |
[SPECIFY LOCAL CURRENCY HERE]
| 5.3 | Incoterm: |
FCA (Incoterms 2010) at the Facility (freight included in Standard Cost in accordance with Section 3.1 of the Master Agreement)
| 5.4 | RESERVED. |
| 5.5 | RESERVED. |
| 5.6 | RESERVED. |
| 5.7 | RESERVED. |
| 5.8 | RESERVED. |
| 5.9 | RESERVED. |
| 5.10 | RESERVED. |
| 6. | Governing Law; Submission to Jurisdiction; Waiver of Jury Trial. |
| (a) | This Product Addendum shall be governed by and construed and interpreted in accordance with the Laws (other than Section 5–1401 and 5–1402 of the New York General Obligations Law) of the State of New York. THE PARTIES EXPRESSLY AGREE THAT THE APPLICATION OF THE UNITED NATIONS CONVENTION ON CONTRACTS FOR THE INTERNATIONAL SALE OF GOODS (1980) IS SPECIFICALLY EXCLUDED AND SHALL NOT APPLY TO THIS PRODUCT ADDENDUM. |
| (b) | With respect to any Action relating to or arising out of this Product Addendum, subject to the provisions of Article 15, each Party to this Product Addendum irrevocably (a) consents and submits to the exclusive jurisdiction of the courts of the State of New York and any court of the United States located in the Borough of Manhattan in New York City, (b) waives any objection which such Party may have at any time to the laying |
A-7
| of venue of any Proceeding brought in any such court, waives any claim that such Proceeding has been brought in an inconvenient forum and further waives the right to object, with respect to such Proceeding, that such court does not have jurisdiction over such Party and (c) consents to the service of process at the address set forth for notices in Section 14; provided, however, that such manner of service of process shall not preclude the service of process in any other manner permitted under applicable Law. |
| (c) | THE PARTIES HERETO AGREE THAT THEY HEREBY IRREVOCABLY WAIVE AND AGREE TO CAUSE THEIR RESPECTIVE SUBSIDIARIES TO WAIVE THE RIGHT TO TRIAL BY JURY IN ANY ACTION TO ENFORCE OR INTERPRET THE PROVISIONS OF THIS PRODUCT ADDENDUM. |
| 7. | In addition to the representations and warranties of Manufacturer set forth in Section 5.1 of the Master Agreement, Manufacturer represents and warrants to Customer that it: |
| (i) | is a corporation duly organized, validly existing and in good standing under the laws of [INSERT JURISDICTION OF ORGANIZATION]; |
| (ii) | has power and authority to conduct its business as currently being conducted and as contemplated herein; and |
| (iii) | has power and authority to make, deliver and perform its obligations under this Product Addendum and has taken all necessary action to authorize the execution, delivery and performance of this Product Addendum. No consent of authorization of, filing with or other act by or in respect of, any Governmental Authority or any other Person is or will be required in respect of Manufacturer in connection with the execution, delivery, performance, validity or enforceability of this Product Addendum. This Product Addendum has been duly executed and delivered on behalf of Manufacturer. This Product Addendum constitutes the legal, valid and binding obligations of Manufacturer enforceable against Manufacturer in accordance with its terms. |
| 8. | In addition to the representations and warranties of Customer set forth in Section 5.2 of the Master Agreement, Customer represents and warrants to Manufacturer that it: |
| (i) | is a corporation duly organized, validly existing and in good standing under the laws of [INSERT JURISDICTION OF ORGANIZATION]; |
| (ii) | has power and authority to conduct its business as currently being conducted and as contemplated herein; and |
| (iii) | has power and authority to make, deliver and perform its obligations under this Product Addendum and has taken all necessary action to authorize the execution, delivery and performance of this Product Addendum. No consent of authorization of, filing with or other act by or in respect of, any Governmental Authority or any |
A-8
| other Person is or will be required in respect of Customer in connection with the execution, delivery, performance, validity or enforceability of this Product Addendum. This Product Addendum has been duly executed and delivered on behalf of Customer. This Product Addendum constitutes the legal, valid and binding obligations of Customer enforceable against Customer in accordance with its terms. |
| 10. | Solely with respect to Tolling Products, the following terms of the Master Agreement are hereby revised with respect to the terms of the Master Agreement as incorporated into this Product Addendum as follows: |
| (a) | Article 1. |
| i. | Section 1.38 (definition of “Price”) of the Master Agreement is deleted in its entirety and replaced with the following new Section 1.38, and all references to “Price” in the Master Agreement are replaced by “Manufacturing Fee”: |
| “1.38 | “Manufacturing Fee” shall mean the manufacturing fee to be charged by Manufacturer for the Services to be provided to Customer hereunder in accordance with Section 3.1, as may be adjusted in accordance with Section 3.2.” |
| ii. | Section 1.42 (definition of “Purchase Order”) is revised as follows: the words “and supply” in the third line are deleted. |
| iii. | The following new Section 1.57 is added to the Master Agreement: |
| 1.57 | “Services” shall mean the operations and activities of Manufacturer related to the manufacture of the Product hereunder, including production, processing, testing, quality assurance, packaging and shipping as provided herein. |
| (b) | Article 2. |
| i. | The title of Article 2 is changed from “Supply of Product” to “Manufacturing Services”. |
| ii. | The title of Section 2.1 is changed from “Agreement to Supply” to “Agreement to Manufacture”. |
| iii. | Section 2.1(a) is deleted in its entirety and replaced with the following new Section 2.1(a): |
A-9
| “(a) | Manufacture of Product. During the Term of each Product Addendum, Manufacturer shall provide the Services to Customer for the manufacture of Product for the Territory pursuant to the provisions of this Agreement and the applicable Product Addendum.” |
| iv. | Section 2.1(c) is deleted in its entirety and replaced with the following new Section 2.1(c): |
| “(c) | Non-Exclusivity; No Minimum Purchase Obligation. The Services under this Agreement shall be provided on a non-exclusive basis and Customer reserves the right to manufacture the Product for itself and to purchase the Services and similar services from any other party. Customer is not obligated to purchase any minimum or specific quantity or dollar amount of Services under this Agreement. |
| v. | Section 2.3 (“Capacity”) is revised as follows: the words “manufacturing and supplying Product to Customer” therein are deleted and replaced by the words “providing Services to manufacture Product for Customer”. |
| vi. | Section 2.4(b) (“Orders”) is revised as follows: the words “quantities of Products” and similar terms in such Section are deleted and replaced with the words “Services for the manufacture of quantities of Product”, mutatis mutandis. |
| viii. | Section 2.5(a) is deleted in its entirety and replaced with the following new Section 2.5(a): |
| “(a) | Manufacturer shall ship Product manufactured pursuant to Purchase Orders issued by Customer and any Product samples requested by Customer to Customer throughout the Territory to the ship-to destination set forth in the applicable Purchase Order. Manufacturer shall ship Product manufactured pursuant to Purchase Orders issued by Customer to Customer by the delivery date set forth in the applicable Purchase Order, or such other date as may be agreed to in writing by the Parties from time to time. Manufacturer shall ship Product to Customer at the applicable Facility in accordance with the Incoterm set forth in the applicable Product Addendum (except to the extent that title and risk of loss passes to Customer pursuant to subsection (c) below). Manufacturer retains the option to transfer the responsibility and associated cost of outbound freight to Customer (with a corresponding adjustment to the applicable Standard Cost in accordance with Article 3).” |
A-10
| ix. | Section 2.5(c) is deleted in its entirety and replaced with the following new Section 2.5(c): |
| “(c) | Customer shall own title to all work in process related to Products and all finished Products manufactured by Manufacturer for Customer pursuant to this Agreement (without limiting Customer’s obligation to pay the Manufacturing Fee, for clarity).” |
| (c) | Article 3. |
| i. | The title of Article 3 is changed from “Price; Payment; Taxes” to “Manufacturing Fee; Payment; Taxes”. |
| ii. | The title of Section 3.1 is changed from “Purchase Price” to “Manufacturing Fee”. |
| iii. | Section 3.1 is revised by: |
(a) deleting the first sentence thereof and replacing it with the sentence below:
“Customer shall purchase Services from Manufacturer at the Manufacturing Fee therefor and in accordance with the terms of this Agreement.”; and
(b) replacing the word “delivery” in the definition of “Standard Cost” therein with the word “shipment”.
iv. The title of Section 3.2 is changed from “Annual Price Adjustments to Reflect Future Budgeted Standard Costs” to “Annual Manufacturing Fee Adjustments to Reflect Future Budgeted Standard Costs”.
iv. The title of Section 3.4 is changed from “Price Review and Audit Procedure” to “Manufacturing Fee Review and Audit Procedure”.
v. Section 3.5 (“Invoices and Payment”) is revised as follows: the second sentence in Section 3.5 is deleted in its entirety and replaced with the following new sentence:
“Customer shall be obligated to pay only for Services attributable to actual quantities of Product shipped.”
| (d) | Article 4. |
i. Section 4.2 (“Manufacturing Standards”) is revised as follows: the words “Manufacturer shall manufacture and supply the Product” therein are deleted and replaced with the words “Manufacturer shall perform all Services”.
A-11
| (e) | Article 5. |
i. Section 5.1(a) is revised as follows: the words “and supply” in the second line are deleted.
ii. Section 5.1(c) is revised as follows: the words “Product furnished by Manufacturer to Customer” in the first line are deleted and replaced by the words “Product manufactured by Manufacturer for Customer”.
| (f) | Article 6. |
i. Section 6.3(a) is revised as follows: the words “alternative source of supply” therein are deleted and replaced by “alternative source of manufacture”.
ii. Section 6.3(c) is revised as follows: the words “continued supply needs” therein are deleted and replaced by “continued manufacturing needs” and the words “long-term supply arrangement” are deleted and replaced by “long-term manufacturing arrangement”.
| (g) | Article 7. |
i. Section 7.1 is revised as follows: the words “Manufacturer’s supply of Non-Complying Product” in clause (iii) thereof are deleted and replaced by “Manufacturer’s shipment of Non-Complying Product” and the words “or supply” in clause (iv) thereof are deleted and replaced by “or ship”.
| (h) | Exhibit B. |
i. Section 5 is revised as follows: the words “supplies the Products” in the first paragraph thereof are deleted and replaced by “provides the Services” and the words “Products cease to be supplied” in such paragraph are deleted and replaced by “Services cease to be provided”.
| 11. | The Parties acknowledge and agree that the Quality Agreement between Customer and Manufacturer for the Facility shall apply with respect to the Product under this Product Addendum. |
(continued on next page)
A-12
| [INSERT PFIZER ENTITY NAME] | [INSERT ZOETIS ENTITY NAME] | |||||||
| By: |
| By: |
| |||||
| Name: |
| Name: |
| |||||
| Title: |
| Title: |
| |||||
| PFIZER INC. | ZOETIS INC. | |||||||
| By: |
| By: |
| |||||
| Name: |
| Name: |
| |||||
| Title: |
| Title: |
|
A-13
Exhibit B
FORM OF
QUALITY AGREEMENT1
Manufacturing Quality Agreement for [FACILITY]
(1 October 2012)
by and between
| Customer Name: | [INSERT CUSTOMER UNDER APPLICABLE PRODUCT ADDENDUM] | |
| Address: |
|
(“Customer”)
and
| Manufacturer Name: | [INSERT MANUFACTURER UNDER APPLICABLE PRODUCT ADDENDUM] | |
| Address: |
|
(“Manufacturer”)
Customer and Manufacturer may each be referred to herein individually as a “Party” and
collectively as the “Parties”.
for
the Product Addendum to the Primary Agreement (as defined below) entered into between
Customer and Manufacturer (“Product Addendum”)
with respect to each product set forth in the Product Addendum (“Product”)
| 1 | NTD: This form will be signed by the Quality Officers or other applicable representatives of the Customer and Manufacturer under each Product Addendum. |
B-1
The Parties wish to further define the individual responsibilities as to the quality aspects of manufacturing and acceptability of Products to ensure compliance with applicable Good Manufacturing Practices (GMPs), the marketing authorization of the Products, applicable regulatory requirements, and Customer’s requirements as specified by Customer (the “Customer Requirements”), in accordance with the terms and conditions of the Primary Agreement.
In order to achieve this purpose, this Quality Agreement includes a detailed listing of the activities associated with pharmaceutical production, analysis, evaluation and acceptability of Product. Unless otherwise indicated, responsibility for each activity is assigned to either Customer or Manufacturer, or to both Parties.
B-2
Agreement of the Parties to perform the activities detailed in this Quality Agreement is indicated by the responsible representatives approval below:
| [CUSTOMER] | [MANUFACTURER] | |||
|
|
| |||
| Signature | Signature | |||
|
|
| |||
| Name | Name | |||
|
|
| |||
| Title | Title | |||
|
|
| |||
| Date of Signature: 1 October 2012 | Date of Signature: 1 October 2012 |
B-3
Contents
Approval Page
| 1. | Effective Date |
| 2. | Scope |
| 3. | Other Agreements |
| 4. | Amendments to Quality Agreement |
| 5. | Termination of Quality Agreement |
| 6. | Resolution of quality Issues |
| 7. | Use of Third Parties |
| 8. | Quality Responsibilities Table |
| A. | Compliance Requirements | N. | Annual Product/Quality Reviews | |||||||
| B. | Right to Audit | O. | Annual Product Report (if applicable) | |||||||
| C. | Regulatory Inspections and Exchanges | P. | Production and In-Process Controls, Packaging and Labeling | |||||||
| D. | Regulatory Documentation | Q. | Process Equipment | |||||||
| E. | Animal Derived Materials | R. | Reprocess | |||||||
| F. | Buildings and Facilities | S. | Rework | |||||||
| G. | Personnel and Training | T. | Laboratory Controls | |||||||
| H. | Sub-Contracting and Testing | U. | Retest | |||||||
| I. | Change Control | V. | Stability (if applicable to service being provided) | |||||||
| J. | Validation/Qualification | W. | Storage and Distribution | |||||||
| K. | Preventive Maintenance and Calibration | X. | Control, Disposal and Destruction of Production Materials | |||||||
| L. | Manufacturing and Laboratory Investigations | Y. | Complaints | |||||||
| M. | Documentation and Records | Z. | Field Alerts, Biological Product Deviation Reports and Recalls | |||||||
| AA. | Adverse Experiences |
Appendices:
Appendix 1: Contacts and Responsibilities
Appendix 2: Significant Deviations Requiring Notification
Appendix 3: Change Notifications Not Requiring Review
B-4
| 1. | Effective Date |
This Quality Agreement shall become effective at the date of last signature (“Effective Date”).
| 2. | Scope |
This Quality Agreement outlines the responsibilities of Manufacturer and Customer with respect to the quality assurance of the Product manufactured and/or supplied by Manufacturer for Customer, as defined in the Product Addendum.
| 3. | Other Agreements |
This Quality Agreement shall complement and is without prejudice to the Master Manufacturing and Supply Agreement dated 1 October 2012 and made by and between Zoetis Inc. and Pfizer Inc. (“Primary Agreement”) and the Product Addendum thereunder between Customer and Manufacturer regarding the defined Products provided. If there are any direct conflicts between the terms of this Quality Agreement and the Primary Agreement or Product Addendum, the provisions in the Primary Agreement or Product Addendum (as applicable) shall govern, except where directly related to quality matters where this Agreement prevails.
| 4. | Amendments to Quality Agreement |
Amendments to this Quality Agreement shall be in writing and signed by the appropriate representatives of both Parties hereto.
The Parties agree to amend terms of this Quality Agreement that need to be amended in order to ensure the Product continues to meet regulatory requirements of applicable jurisdictions and current GMPs.
If an amendment to this Quality Agreement is proposed, the proposing Party will communicate the proposed amendment to the appropriate contact person at the other Party for review and approval. The appropriate contact person at Customer and Manufacturer is listed in Appendix 1 (Contacts and Responsibilities).
| 5. | Termination of Quality Agreement |
This Quality Agreement shall become effective as of the Effective Date and shall have the same duration and conditions for termination as the Product Addendum. In case no such terms are in force, this Quality Agreement shall continue in force while the Manufacturer supplies the Products to Customer. If the Products cease to be supplied by Manufacturer to Customer, this Quality Agreement can be terminated by either Party on providing written notice to the other Party.
Survival. All regulatory obligations required of Customer and Manufacturer by an applicable regulatory authority or effective regulations shall survive termination of this Quality Agreement as defined in Primary Agreement. Also, those provisions of the Primary Agreement between the Parties shall not be affected by a termination of this Quality Agreement, including any duties regarding confidentiality and non-disclosure.
| 6. | Resolution of Quality Issues |
B-5
Quality-related disagreements between Customer and Manufacturer that are not resolved in the normal course of business shall be brought to the attention of the appropriate contact person for notices at Customer and Manufacturer , in writing. The appropriate contact persons are listed in Appendix 1 (Contacts and Responsibilities). Both Parties shall use all reasonable efforts to agree to a resolution of the disagreement and agree to work jointly to develop a strategy for such resolution. Customer and Manufacturer further agree to record such resolution in writing.
In the event that resolution of a quality related disagreement cannot be reached, the dispute resolution procedures in the Primary Agreement shall be followed.
| 7. | Use of Third-Parties |
Manufacturer may not further subcontract any of its obligations under this Quality Agreement unless Customer provides prior written consent to Manufacturer for such subcontracting or unless this is permitted in the Primary Agreement. Before Customer grants any such written consent, Customer may require that Manufacturer enter into a written agreement with the third-party (“Third-Party Agreement”) to the satisfaction of Customer which requirement shall not apply in relation to the continuation by Manufacturer of the relationship between Manufacturer and the third-party in the period prior to the date of this Quality Agreement. This Third-Party Agreement shall define the respective quality responsibilities of Manufacturer and the third-party and shall provide for confidentiality and non-disclosure of all Customer confidential information requiring at least the same degree of protection for such confidential information as the obligations of confidentiality and non-disclosure that exist between Customer and Manufacturer .
Manufacturer shall remain responsible for the acts and omissions of any permitted Sub-Contractor, as if those acts and omissions has been carried out by the Manufacturer itself and in particular, but without limitation, will enter into an appropriate written contract with such Sub-Contractor and ensure the qualification and auditing of such Sub-Contractor.
In the event Customer policies and procedures require it or pursuant to Customer’s obligation to any competent authority, Manufacturer shall ensure that Customer will be permitted the right of access to Sub-Contractors in the presence of the Manufacturer, as if it were a Manufacturer site to carry out audits and other assessments and be accompanied by the Manufacturer representatives.
B-6
| 8. | Quality Responsibilities Table |
| § | Responsibilities | Customer | Manufacturer | |||
| A. | Compliance Requirements | |||||
| 1.01 | Follow applicable regulations and current Good Manufacturing Practices, as well as locally imposed requirements. | X | X | |||
| 1.02 | Manufacture, package, store and test the Product and materials in an environment meeting the applicable GMP regulations, which is designed, constructed and maintained in a manner that a) permits the operation therein to be performed under clean, sanitary and orderly conditions; b) permits the effective cleaning of all surfaces; and c) prevents the contamination of the Product and the addition of extraneous material to the Product. | X | ||||
| 1.03 | Manufacture the Product in adherence to the Manufacturing specifications and release specifications provided by Customer. | X | ||||
| 1.04 | Maintain a valid manufacturing license covering manufacture of the Product. | X | X | |||
| 1.05 | Refrain from activity that could adversely affect quality of the Product. | X | ||||
| 1.06 | Have management controls in place to track and trend investigations and commitments. | X | ||||
| 1.07 | Maintain a quality unit that is independent of production that fulfills both quality assurance and quality control responsibilities. | X | X | |||
| 1.08 | Disposition of Product by quality unit or qualified person (QP) after review of all relevant documents as listed in section 1.03 to ensure compliance with marketing authorization where applicable. | X | ||||
| 1.09 | Involve the quality unit in all Good Manufacturing Practices related matters. | X | X | |||
| 1.10 | Notify Customer of key organizational and/or key personnel changes. | X | ||||
| 1.11 | Maintain internal and external Good Manufacturing Practices audit program. | X | ||||
| 1.12 | Maintain effective quality systems that minimize the potential for product quality, regulatory and compliance issues. Notify Customer as soon as possible and not later than 3 business days of any failure of a quality system component (i.e. any product market action, major facility and/or equipment failure, critical/major regulatory authority observation, etc.) even though this failure may not result in an immediate, direct impact to Product or processes. | X | ||||
| B | Right to Audit | |||||
| 2.01 | Have the right to audit Manufacturer’s facilities and systems, as they relate to the manufacture of Product, upon reasonable prior notice of at least four (4) weeks. Customer retains the right to conduct “for cause” audits as necessary. | X | ||||
| 2.02 | Schedule visits and/or provide requests for Product specific documents for review to assure continued adherence to the agreed upon manufacturing process, applicable current Good Manufacturing Practices and other applicable requirements. | X | ||||
| 2.03 | Issue Manufacturer a confidential audit report summarizing audit observations. If required by Customer policies and procedures or by any competent authority, Manufacturer reserves the right to share the audit report with a third party undertaking QP release activities in relation to Product on behalf of Customer. | X |
B-7
| § | Responsibilities | Customer | Manufacturer | |||
| 2.04 | Issue responses to all observations in writing to Customer within thirty (30) days of receipt. Responses are to include timelines and plans for closure of all commitments. | X | ||||
| C | Regulatory Inspections and Exchanges | |||||
| 3.01 | Coordinate the activities necessary to maintain inspection readiness for all inspections. | X (for Customer specific inspections) | X | |||
| 3.02 | Notify Customer as soon as possible and not later than 3 business days of any pending or ongoing regulatory authority inspection or communication related to the Product or the facilities used to produce, test or warehouse the Product. In the event that the inspection is specific only to Product, Manufacturer shall permit a representative of Customer to be present during any such inspection. | X | ||||
| 3.03 | Provide copies to Customer within 3 business days of correspondence received from regulatory authorities (Boards of Health, Health Authority, etc.) related to Customer Product and related operations performed by the Manufacturer. | X | ||||
| 3.04 | Provide a copy of the regulatory inspection report, deficiency letter, or regulatory compliance observations, response and related correspondence to Customer, edited to exclude Manufacturer proprietary information within three (3) business days of receipt. Allow Customer to review and comment on the response, relevant to Product supplied to Customer prior to submission of the response to the regulatory authority. Customer feedback shall be provided within 5 business days at the latest. | X | ||||
| 3.05 | Determine establishment registration status and impact to Customer Products immediately upon receipt of regulatory observations, deficiencies and/or regulatory actions. Notify Customer within one (1) day if the site status has changed and disclose the new status (inspection classification code) | X | ||||
| 3.06 | Notify Manufacturer of any regulatory compliance observation received by Customer that pertains to operations performed by the Customer and requires Manufacturer information. | X | ||||
| 3.07 | Provide Customer with information requested to assure compliance with regulatory requirements within ten (10) days of notification, or as required to meet regulatory obligations. | X | ||||
| 3.08 | Provide Manufacturer with 4 weeks in advance written notification of supplemental regulatory submission/application that impact the operations performed by the Manufacturer. | X | ||||
| D. | Regulatory Documentation | |||||
| 4.01 | Maintain (including annual reports if required) Site Master File and all regulatory applications, as applicable, in accordance with the regulations of the applicable regulatory authority. | X | ||||
| 4.02 | Liaison with regulatory authorities for approval, maintenance and updating of product authorization where required. | X | ||||
| 4.03 | Notify Customer of Site master file, or regulatory application change | X |
B-8
| § | Responsibilities | Customer | Manufacturer | |||
| as applicable before submitting the change to authority and prior to implementation. | ||||||
| 4.04 | Provide sections of Product registration/regulatory submission relevant to manufacture and quality control of Product. | X | ||||
| E. | Animal Derived Materials | |||||
| 5.01 | Have an effective program in place (such as certificates of suitability from EDQM, supplier certificates, amongst others) that is aligned with Customer Requirements to evaluate and control the risk of transmissible spongiform encephalopathy for raw materials and components. | X | X | |||
| 5.02 | Maintain appropriate records for each lot of animal derived material to ensure traceability. Where required by local regulations of the country where product is manufactured, the location where animals lived or were slaughtered (if applicable) must be documented. | X | ||||
| F. | Buildings and Facilities | |||||
| 6.01 | Buildings and facilities used in the manufacture of the Product should be designed, constructed and maintained to facilitate cleaning, maintenance and operations and to assure orderly placement of equipment and materials to prevent mix-up and contamination as appropriate to the type and stage of manufacture. | X | ||||
| 6.02 | Ventilation systems will be designed and maintained to minimize the risk of contamination. | X | ||||
| 6.03 | Dispose of sewage, refuse and other waste in a safe and timely manner following applicable environmental health and safety regulations. | X | ||||
| 6.04 | Maintain a set of current drawings for critical utilities including water, electricity, compressed gasses and air handling as they pertain to operation for Customer. | X | ||||
| 6.05 | Maintain and document an adequate, effective pest control program ( such as a contract) as they pertain to operation for Customer. | X | ||||
| G. | Personnel and Training | |||||
| 7.01 | Provide sufficient training, including on applicable Good Manufacturing Requirements, to meet obligations of this Quality Agreement. | X | ||||
| 7.02 | Provide adequate number of personnel qualified by appropriate training and experience to perform and supervise the manufacture, testing, packaging and disposition of the Product as defined in the Primary Agreement. | X | ||||
| 7.03 | Assure training is regularly conducted, assessed and documented by qualified individuals. | X | ||||
| 7.04 | Have written job descriptions for positions responsible for performing Good Manufacturing Practices related activities. | X | ||||
| 7.05 | Assure that non-employees, including consultants, advising on the manufacture and control of the Product have sufficient education, training, and experience to advise on the subject for which they are retained. Non-employees will be supervised as required and trained in Good Manufacturing Practices. | X | X | |||
| H. | Sub-Contracting and Testing | |||||
| 8.01 | If Manufacturer sub-contracts any laboratory testing or | X |
B-9
| § | Responsibilities | Customer | Manufacturer | |||
| manufacturing operations to a third-party contract laboratory or third-party manufacturer (a “Sub-Contractor”), Manufacturer shall require that such Sub-Contractors shall operate in compliance with current Good Manufacturing Practices, compendia requirements and any other applicable regulations. | ||||||
| 8.02 | Not engage any new Sub-Contractors without the written consent of Customer or unless this is permitted in the Primary Agreement. | X | ||||
| 8.03 | Maintain Sub-Contractors as qualified following approved procedures according to a schedule. | X | ||||
| 8.04 | If Manufacturer engages a Sub-Contractors, Manufacturer shall require Sub-Contractors to grant access: (a) in the event Customer policies and procedures require it or pursuant to Customer’ obligation to any competent authority, to Customer (or Customer assignee) in the presence of a representative of the Sub-Contractor for the purpose of any Customer audit; and/or (b) to any applicable regulatory authority for purposes of any regulatory authority audits, on the same terms and conditions as such access is granted to Customer and/or any applicable regulatory authority by Manufacturer under the terms of this Quality Agreement and/or the terms and conditions of any other applicable agreement between Customer and Manufacturer. | X | ||||
| I. | Change Control | |||||
| 9.01 | Maintain approved written procedures for control of changes impacting the Product including, but not limited to, manufacturing components/raw materials or process, packaging materials, labeling, computer hardware/software, Product specifications, and test methods. Ensure any changes are reviewed/approved by quality unit. Include in written procedures the process and criteria for customer notification and approval, follow up and closure of changes. | X | ||||
| 9.02 | Notify Customer of all significant changes to facility, process, test methods, quality systems and specifications. Significant changes are those that impact Product identity, strength, safety, potency, purity, stability, regulatory status or validation/qualification. (See Appendix 3 for changes not requiring Customer review). Issue a change request for each change as soon as possible after the need for change is apparent, allowing sufficient time for Customer to comment and approve or reject changes prior to implementation. Customer feedback shall be provided within 5 business days at the latest. In the case of emergency changes, Customer and Manufacturer shall cooperate to expedite the review and approval process. | X | ||||
| 9.03 | Provide copies of change control documentation such as supporting data, validation/qualification reports and Customer approved change control forms for changes impacting Product as requested by Manufacturer. | X | ||||
| 9.04 | Implement compendia grade raw material monograph changes in order to meet compendia requirements. | X | ||||
| J. | Validation/Qualification | |||||
| 10.01 | Maintain a written master validation/qualification plan for the facilities, equipment/instruments, manufacturing process, cleaning procedures, analytical procedures, in process control tests and | X |
B-10
| § | Responsibilities | Customer | Manufacturer | |||
| computerized systems approved by the quality unit. | ||||||
| 10.02 | Prepare and maintain validation/qualification documentation approved by the quality unit, including protocols, reports and associated documentation. Provide such documents to Manufacturer upon request. | X | ||||
| Section 10.03 through 10.11 are only applicable to new or replaced equipment through the validity of the Product Addendum. | ||||||
| 10.03 | Validate/qualify as necessary all critical systems, utilities and equipment/instruments used for the manufacture and control of Product (Installation Qualification (IQ), Operational Qualification (OQ), and/or Performance Qualification (PQ)). | X | ||||
| 10.04 | Computer systems and associated software used in Good Manufacturing Practices related activities associated with the Product should be validated/qualified. Procedures must be in place to assure the integrity, archiving, retrieval and destruction of the electronic data that comply with applicable regulations. | X | ||||
| 10.05 | Validate/qualify methods and procedures for cleaning of equipment with acceptance criteria for residues defined and justified. | X | ||||
| 10.06 | Develop and execute a plan for process and method validation/qualification including definition of roles and responsibilities between Customer and Manufacturer for performing technology transfers, if applicable. | X | X | |||
| 10.07 | Where method validation is performed by Manufacturer: | |||||
| • Manufacturer shall write method validation protocol, | X | |||||
| • Customer and Manufacturer shall review and approve method validation protocol. Customer should make a good faith effort to provide feedback within 5 business days or notify Manufacturer. | X | X | ||||
| • Manufacturer shall execute method validation protocol, | X | |||||
| • Manufacturer shall write method validation report, and | X | |||||
| • Customer and Manufacturer shall review and approve method validation report. Customer should make a good faith effort to provide feedback within 5 business days or notify Manufacturer. | X | X | ||||
| 10.08 | For process validation: | |||||
| • Write process validation protocol, | X | |||||
| • Review and approve process validation protocol. Make a good faith effort to provide feedback within 5 business days or notify The author. | X | X | ||||
| • Execute process validation protocol, | X | |||||
| • Write process validation report, and | X | |||||
| • Review and approve process validation report. Make a good faith effort to provide feedback within 5 business days or notify the author. | X | X | ||||
| 10.09 | Validate/qualify all manufacturing processes, Product formulation, mixing operations and hold times for the formulation process. | X | ||||
| 10.10 | Qualify time limitations for each phase of production. | X | ||||
| 10.11 | Evaluate protocol deviations encountered during | X |
B-11
| § | Responsibilities | Customer | Manufacturer | |||
| validation/qualification to determine impact on validation/qualification studies, including need to conduct repeat studies. | ||||||
| 10.12 | Evaluate validated/qualified systems and processes periodically to verify they are still operating in a valid manner. | X | ||||
| 10.13 | Shall permit a representative of Customer to be present during process demonstration and validation. | X | ||||
| K | Preventive Maintenance and Calibration | |||||
| 11.01 | Maintain calibration and preventive maintenance procedures and schedules for equipment/instruments used in the manufacture, packaging, testing and validation/qualification of the Product. Include calibration tagging where appropriate. | X | ||||
| 11.02 | Document and review (including calibration performed by external companies) manufacturing and laboratory equipment/instrumentation calibration data. | X | ||||
| L | Manufacturing and Laboratory Investigations | |||||
| 12.01 | Maintain appropriate procedures for the identification, investigation, reporting, tracking, trending and closure of deviations as per applicable GMP requirements. Investigations must include but are not limited to known lab errors, atypical results and Out-of-Specification (OOS) results that occur during the manufacture and testing of the Product, including stability testing (as applicable). | X | ||||
| 12.02 | Document and notify Customer within 3 business days at the latest of any significant deviation. Significant deviations include, but are not limited to, those which affect the quality, identity, purity and/or strength of the Product; those which impact the GMP, validation or regulatory status; any OOS test result which cannot be promptly invalidated or any deviation resulting in the Product being outside of filed registration limits (see Appendix 2 for examples). Customer retains the right to request notification of additional deviations on a case by case basis. All deviations must be closed prior to release of Product. Conclusions related to significant deviations need to be endorsed by Manufacturer prior to Product release. | X | ||||
| 12.03 | Notify within 2 business days of first knowledge of all Out-of-Specification results generated during stability testing of the Product, unless the OOS can be promptly invalidated. | X | ||||
| 12.04 | Provide investigation documentation upon request. | X | ||||
| 12.05 | Assist in investigations when deemed appropriate. | X | ||||
| 12.06 | Complete investigations within thirty (30) calendar days of commencement. For investigations exceeding thirty (30) calendar days, an interim investigation report, including justification for extension of the completion date shall be issued. | X | ||||
| 12.07 | Complete corrective/preventive action (CAPA) commitments resulting from investigation closure within the planned timeframe. Provide evidence of closure of CAPA items for notified deviations. | X | ||||
| M | Documentation and Records | |||||
| 13.01 | Document all required process and testing steps at the time such process or testing step is executed. | X | X | |||
| 13.02 | Maintain a controlled system to initiate, review, revise, approve, | X |
B-12
| § | Responsibilities | Customer | Manufacturer | |||
| obsolete and archive all Good Manufacturing Practices documentation. | ||||||
| 13.03 | The Quality Unit must review and approve all Good Manufacturing Practices records. | X | ||||
| 13.04 | Records retention. | X | ||||
| 13.05 | Review and approve Master Batch Records. | X | ||||
| 13.06 | For laboratory control records, include complete data derived from all tests conducted to ensure compliance with specifications. These records will contain the date and the signature of a second qualified person showing review and verification of the records. | X | ||||
| 13.07 | Provide copies of documents or records needed to assure compliance with regulatory requirements of filings upon request. | X | ||||
| 13.08 | Maintain a document control system for specifications, including: raw materials, Product labeling, packaging materials and other materials that would likely affect Product quality. | X | ||||
| 13.09 | Maintain a document control system for Standard Operating Procedures. | X | ||||
| 13.10 | Maintain a document control system for specifications of reagents, solutions and laboratory standards, as appropriate. | X | ||||
| 13.11 | Provide a complete Certificate of Analysis for each shipment of the Product, containing at minimum the following information: • Manufacturer Product number, • Customer Product number, (if applicable) • Manufacturer’s lot number, • Name of Product, • Name of the test, • Test method, • Specification limit, • Expiration date (if applicable) • Actual test result (as a numerical value, unless designated Pass/Fail in the specification limit) and date tested, including retest results if required • Date tested (for each test) • Quality Assurance approval and date, • Manufacturing site (name and address), and • Manufacturing date. | X | X | |||
| 13.12 | Provide a document certifying product was manufactured in a current Good Manufacturing Practices compliant facility and was tested in accordance with and meets specifications. | X | ||||
| 13.13 | Keeps and makes accessible upon request all related equipment and training records for all activities related to the primary agreement. | X | ||||
| 13.14 | Retain, archive and destroy all Good Manufacturing Practices documents and data pertaining to Services performed for Manufacturer in accordance with Manufacturer and regulatory requirements as defined below: • keep all original analytical records including test results, records, charts, computer reports and graphs for a minimum period of five (5) years or if batch related one year in excess of expiration date, | X |
B-13
| § | Responsibilities | Customer | Manufacturer | |||
|
• keep all validation and technical transfer documents in connection with Service performed indefinitely, • keep original investigation and out-of-specification report documentation in connections with Services performed for a minimum of five (5) years or one year beyond expiration date of associated batch, • Notify prior to destruction of records, including a specific identification of the records. Manufacturer retains the right to maintain any such record at its’ discretion, • insure that original records are protected from fire and water damage in a limited access area and • Retain Batch Record documentation to meet applicable regulatory requirements and any special instruction. | ||||||
| 13.15 | Have all executed batch related records reviewed and approved by Quality unit prior to batch release. Assure records have a unique lot identification number. | X | ||||
| N | Annual Product/Quality Reviews | |||||
| 14.01 | Have procedures to conduct and document Annual Product/Quality Reviews on a scheduled basis. | X | X | |||
| O | Annual Product Report (if applicable) | |||||
| 15.01 | Provide data necessary for the submission of Annual Product Reports in accordance with an agreed upon schedule. | X | ||||
| 15.02 | Submit data in a mutually agreed to format. | X | ||||
| 15.03 | Where Manufacturer is registration holder, Manufacturer shall provide copy of Annual Product Report. | X | ||||
| P | Production and In Process Controls, Packaging and Labeling | |||||
| 16.01 | Have approved written procedures in place for qualification of Sub-Contractors that provide GMP-materials and services. | X | ||||
| 16.02 | Perform and maintain all Sub-Contractor qualifications in a current state as per applicable procedures. | X | ||||
| 16.03 | Make no changes in the sourcing of production materials (components/raw materials, packaging materials, processing aids) from the approved Sub-Contractor list without prior notification to Customer. | X | ||||
| 16.04 | Have a system for batch identification including assigning lot numbers. | X | ||||
| 16.05 | Implement and document specifications for raw materials, packaging materials, and Product labeling and processing aids that would likely affect Product quality pursuant to specification documentation provided by Customer. | X | ||||
| 16.06 | Have approved written procedures for all required in-process sampling and testing. | X | ||||
| 16.07 | Procure, test (as required), and disposition raw materials, components, packaging and labeling used in manufacture and packaging of Product. | X | ||||
| 16.08 | Document reconciliation for the Product, and evaluate actual yields versus theoretical in-process yield control limits for the filling process. | X |
B-14
| § | Responsibilities | Customer | Manufacturer | |||
| 16.09 | Provide Product expiration period for assigning Product expiry dates. | X | ||||
| 16.10 | Assign expiration dates | X | ||||
| 16.11 | Have appropriate inspection devices in place to assure the correct Product is packaged, the correct labeling is utilized, and any inline marking is completely and accurately located and legible. | X | ||||
| 16.12 | Include in shipper label provided by Customer: • name and address of the manufacturer, • unique identifying code, • batch number, • quantity of contents, • storage and special transport conditions, • manufacturing date, • expiry date, and • any special requirements (if applicable). | X | X | |||
| 16.13 | Where appropriate, develop all labeling and create master labels in accordance with applicable regulation (including for the country intended for distribution) and Marketing Authorization. | X | ||||
| 16.14 | Generate working Labels for approved Master | X | ||||
| 16.15 | Include a representative label in the batch record. | X | ||||
| 16.16 | Establish and maintain a program for environmental monitoring including tracking and trending processes. | X | ||||
| 16.17 | Retain reserve samples of raw materials, packaging materials and Product label, intermediates (if applicable) and final Product in accordance with written procedures. | X | X | |||
| Q | Process Equipment | |||||
| 17.01 | Process equipment must be uniquely identified, status tagged and managed with an equipment history log or equivalent system. Process lines will be appropriately identified. | X | ||||
| 17.02 | Use appropriate food grade machine lubricants and oils that contain no known animal derived materials. | X | ||||
| 17.03 | Maintain a current set of “as-built” drawings for equipment and facilities as far as it pertains to the Primary Agreement. | X | ||||
| R | Reprocessing | |||||
| 18.01 | Where reprocessing is required and permitted, review and approve all reprocessing steps. Document approval in specific reprocessing protocols or special batch record instructions. Reprocessing is defined as a repetition of a step (for example, redrying, remilling) using the same equipment and techniques as specified in the original procedure. In addition, an extension of an approved process step is also regarded as reprocessing. | X | ||||
| 18.02 | Perform reprocessing only where specified in protocol or specific batch record instructions approved by Manufacturer. | X | ||||
| S | Rework | |||||
| 19.01 | Where rework is required and permitted, have a protocol or procedure that has been approved by both Manufacturer and the Sub-Contractor for Product requiring rework describing the rationale and justification for the rework processes. Rework is a manufacturing step involving a technique or technology that is not part of the | X |
B-15
| § | Responsibilities | Customer | Manufacturer | |||
| approved process sequence. | ||||||
| 19.02 | Review and approve Product requiring rework. | X | X | |||
| T | Laboratory Controls | |||||
| 20.01 | Maintain written procedures for sample management, identification, testing, disposition and recording, approval, tracking, storage, retention and disposal of laboratory data. | X | ||||
| 20.02 | Hold samples and dispose of as required by specifications and procedures. | X | ||||
| 20.03 | Destroy samples and sample packaging in a secure and legal manner that prevents unauthorized use or diversion in accordance with approved procedures. Maintain destruction records. Destruction method must render any registrations, logos or trademarks unidentifiable. | X | ||||
| 20.04 | Maintain approved written procedures for management, preparation and use of reference standards, reference materials, reagents, and solutions including qualification/requalification of use period. | X | ||||
| 20.05 | Mutually agree on source, grade and characterization of reference standards/materials. | X | X | |||
| 20.06 | Have appropriate specifications and test procedures for the Product, which are consistent with the applicable, approved Marketing Authorization and/or compendia monograph. | X | ||||
| 20.07 | Test Product in accordance with qualified or validated methods as appropriate and with specifications using calibrated, qualified equipment. Shall not perform reduced testing regiment without meeting requirements of an established program or protocol reviewed and agreed to. | X | ||||
| 20.08 | In case of method changes verify compendia test methods (i.e. USP, EP, BP, JP). Supply a certificate of equivalency or verification report to Customer, if applicable. Follow approved change to test procedures for changing test methods. | X | ||||
| U. | Retest | |||||
| 21.01 | Perform retesting, when mutually agreed, in accordance with approved protocols or procedures. | X | ||||
| V. | Stability | |||||
| 22.01 | Maintain a documented, ongoing stability program to monitor the stability of the Product using stability indicating procedures. | X | ||||
| 22.02 | Store stability samples in market containers in accordance with approved protocols which include time stations and storage conditions. | X | ||||
| 22.03 | Write and review stability protocol and reports. | X | ||||
| 22.04 | Approve stability protocols prior to executing stability studies. | X | X | |||
| 22.05 | Provide approved stability protocols and stability reports upon request. | X | ||||
| 22.06 | Assign and approve appropriateness of storage conditions and retest or expiry date base on stability data. | X | X | |||
| 22.07 | Place the first three commercial production batches and at least one batch per year on stability or as required by applicable regulatory agencies. | X | ||||
| 22.08 | Perform stability testing of reworked/reprocessed batches or those | X |
B-16
| § | Responsibilities | Customer | Manufacturer | |||
| associated with investigations or revalidations/re-qualifications as required. | ||||||
| 22.09 | Any OOS (out of specification) results obtained during stability testing that cannot be invalidated within 24 hours (one day) must be documented and reported in 24 hours. Investigations are to be in accordance with section L of this Quality Agreement. | X | ||||
| W. | Storage and Distribution | |||||
| 23.01 | Validate/qualify and maintain storage facilities appropriate for conditions specified on the Product label. Maintain records of any critical parameters. | X | ||||
| 23.02 | Establish and maintain an environmental monitoring program including trending activities to assure adherence to specified Product, raw material, packaging material and component storage conditions (such as temperature). | X | ||||
| 23.03 | Maintain validated/qualified systems for controlling quarantined, rejected or recalled materials and segregate rejected or recalled materials. | X | ||||
| 23.04 | Storage of bulk material up to packaging and shipment | X | ||||
| 23.05 | Storage of finished Product | X | ||||
| 23.06 | Provide Product Material Safety Data Sheet or equivalent. | X | X | |||
| 23.07 | Ship Product in accordance with qualified transportation requirements in alignment with Customer instructions. | X | ||||
| 23.08 | In the event of an environmental excursion affecting Product, notify Customer within three (3) business days and make subsequent investigation available to Customer upon request. | X | ||||
| 23.09 | Have a system in place for assuring unreleased Product is not shipped unless authorized by Manufacturer Quality Assurance unit. | X | ||||
| X. | Control, Disposal and Destruction of Production Materials | |||||
| 24.01 | Maintain a procedure for the access, control, reconciliation, disposition, disposal, and destruction of obsolete or rejected production materials proprietary to Customer or bearing Customer proprietary information used in the Product manufacture, packaging and labeling that include but is not limited to: • Active Pharmaceutical Ingredient • Excepient Raw Materials • Tooling dies, printing rolls, plates and associated drawings used in the manufacturing of Product. • Printed components and materials used to print such component, including but not limited to: printed components, containers, closures, tools, dies, plates, drawings and artwork including all electronic files. | X | ||||
| 24.02 | Dispose and destroy obsolete or rejected production materials in a secure and compliant manner that prevents unauthorized use or diversion in compliance with environmental regulations. Maintain destruction records. Destruction method must render all Customer owned trademarks, logos, or registration marks as unidentifiable. | X | ||||
| Y. | Complaints | |||||
| 25.01 | Have written procedures in place which cover notification of complaints, documentation, issuing investigation reports, follow up | X |
B-17
| § | Responsibilities | Customer | Manufacturer | |||
| activities, responding to Manufacturer and management of all Product complaints. | ||||||
| 25.02 | Where Customer is the Marketing Authorization holder: | |||||
| • Notify within 24 hours of Product complaints received directly by the contractor (including those related to split lots) that impact compliance, quality, purity, safety or effectiveness of distributed Product, which includes but is not limited to those that may result in a Field Alert, Biological Product Deviation Report and/or recall. | X | |||||
| • Provide Preliminary Reports and associated documentation, where required, within two (2) business days from the date of notification. | X | |||||
| • Shall for complaint investigations provide the final complaint investigation report within thirty (30) calendar days from notification. | X | |||||
| • Communicate in those exceptional circumstances where an investigation report cannot be completed within thirty (30) calendar. An interim status report should be provided every thirty (30) calendar days until the complaint report | X | |||||
| • Retain complaint investigation records. | X | |||||
| • Evaluate trends and severity of complaints and Communicate where a trend is identified. | X | |||||
| • Track and implement corrective actions associated with complaints. | ||||||
| • Notify of any corrective actions where the due date is not met or where the corrective action is required to be rescheduled. | ||||||
| • Where available and applicable provide the complaint sample(s) | X | |||||
| • Perform a visual inspection of any returned complaint sample(s) within two (2) business days of receipt to confirm the assigned classification and priority. Any discrepancies shall be notified to Manufacturer within one (1) business day. | X | |||||
| • Reach agreement prior to performing analytical testing on returned complaint sample(s) or retain samples. | X | X | ||||
| • Forward complaints received by accident within 3 business day of Product complaints received. | X | |||||
| Z. | Field Alerts, Biological Product Deviation Reports and Recalls | |||||
| 26.1 | Manufacturer and Customer will comply with all requirements as listed in 21CFR regarding reporting requirements for Biological Product Defect Reports as well as Field Alert Reports. This includes making mutual notification so that any required reports can be completed according the regulatory requirements. Requirements for reporting specific events are noted within this agreements in addition to the requirements of 21CFR.
Manufacturer and Customer will mutually notify each other prior to taking any market action. | X | X | |||
| AA. | Adverse Experiences |
B-18
| § | Responsibilities | Customer | Manufacturer | |||
| 27.1 | Unless otherwise defined herein, the terms used surrounding adverse experiences shall have the meanings set forth in the International Conference on Harmonization (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. Customer shall be responsible for adverse experience reporting for the Product. In the event Manufacturer has or receives any information regarding any adverse experience which may be related to the use of the Product, Manufacturer shall within 24 hours of awareness, and immediately in the case of death or life-threatening adverse event, provide Customer with all pertinent information to facilitate investigation. When forwarding the information the date of first awareness of the event must be included. | X | X |
B-19
APPENDIX 1: Contacts and Responsibilities
Page 1 of Appendix 1
| Customer Quality Assurance | Manufacturer Quality Assurance | |||
| Name | ||||
| Title | ||||
| Phone/Fax | ||||
| Address (mail/delivery) | ||||
| Electronic | ||||
| Business Manager | Business Manager | |||
| Name | Xxxxxxx Xxxxxx | Xxxxxxx X. Xxxxx | ||
| Title | President, Zoetis Manufacturing and Supply | President, PCS | ||
| Phone/Fax | Ph: 000.000.0000 | Ph. 000-000-0000 | ||
| Address (mail/delivery) | Belgium Société Anonyme having an address of Xxx Xxxx Xxxxxxx, 0, 0000 Xxxxxxx-Xx-Xxxxx, Xxxxxxx | 0000 Xxxxxxx Xxxx Xxxxxxxxx, XX 00000 | ||
| Electronic | Xxxxxxx.Xxxxxx@xxxxxx.xxx | Xxxxxxx.X.Xxxxx@xxxxxx.xxx | ||
| And: | ||||
| Name | Xxxxx Xxxxx | Xxxxxxx X. Xxxxxxxx | ||
| Title | Vice President, Zoetis Manufacturing QA | VP Contract Operations Quality Assurance | ||
| Phone/Fax | Phone: 000- 000-0000 | Tel: 000-000 0000 | ||
| Address (mail/delivery) | 0000 Xxxxxxx Xxxx | 000 Xxxxx 000 Xxxxx Xxxxxxx, XX 00000 | ||
| Electronic | Xxxxx.x.xxxxx@xxxxxx.xxx | xxxxxxx.x.xxxxxxxx@xxxxxx.xxx |
B-20
APPENDIX 2: Contacts and Responsibilities
Page 2 of Appendix 1
Contact Person for Notices
(including Notices of Amendment, Assignment,
Termination, Resolution of Quality Issues)
| Customer | Manufacturer | |||
| Name | ||||
| Title | ||||
| Phone/Fax | ||||
| Address (mail/delivery) | ||||
| Electronic | ||||
| With a Copy to: | Zoetis Inc. | Pfizer Inc. | ||
| Name | Xxxxxxx Xxxxxx | Xxxxxxx X. Xxxxx | ||
| Title | President, Zoetis Manufacturing and Supply | President, PCS | ||
| Phone/Fax | Ph: 000.000.0000 | Ph. 000-000-0000 | ||
| Address (mail/delivery) | Belgium Société Anonyme having an address of Xxx Xxxx Xxxxxxx, 0, 0000 Xxxxxxx-Xx-Xxxxx, Xxxxxxx | 0000 Xxxxxxx Xxxx Xxxxxxxxx, XX 00000 | ||
| Electronic | Xxxxxxx.Xxxxxx@xxxxxx.xxx | Xxxxxxx.X.Xxxxx@xxxxxx.xxx | ||
| And: | ||||
| Name | Xxxxx Xxxxx | Xxxxxxx X. Xxxxxxxx | ||
| Title | Vice President, Zoetis Manufacturing QA | VP Contract Operations Quality Assurance | ||
| Phone/Fax | Phone: 000- 000-0000 | Tel: 000-000 0000 | ||
| Address (mail/delivery) | 0000 Xxxxxxx Xxxx | 000 Xxxxx 000 Xxxxx Xxxxxxx, XX 00000 | ||
| Electronic | Xxxxx.x.xxxxx@xxxxxx.xxx | xxxxxxx.x.xxxxxxxx@xxxxxx.xxx |
B-21
APPENDIX 2: Significant deviations requiring notification (Customer)
Any deviation that may potentially impact the quality, safety and efficacy of a Customer Product
| Significant deviations | List of specific examples (but not limited to) | |
| Deviations with direct and/or potential impact on marketed products | Stability OOS result Issues that could potentially result in Field Alert Report / BPDR, product defect report or recall Contamination found in components OOS result on complaint/retained samples Out of calibration results Labeling errors Issues related to expiration dating product mix-up
| |
| Deviations from specifications, regulatory filings and/or validated processes | Processing deviation from master batch record Critical process parameters OOS results in release testing Reject, rework, reprocessing of a batch or portion of a batch
| |
| Deviations showing a potential for physical and/ or microbiological contamination | Metal Cross-contamination Media fill positives Bioburden OOT or OOS Sterilization requalification failures EM results out of action limits Filter integrity check failures Sterility failures Endotoxin
| |
| Critical facility or utility failures | Water system HVAC Air / Gas systems |
B-22
APPENDIX 3: Significant deviations NOT requiring notification (Customer)
Typographical errors and formatting in batch documentation, specifications and analytical methods including spelling correction, punctuation or change in typeface (i.e., xxxxxxx or font size.)
Revision of master batch records concerning waste treatment and safety provisions.
Clarification of process details for operator instruction.
Waste Handling Procedures.
Computer hardware/software/programming (unless the change would impact the GXP).
Routine equipment repair, maintenance, calibration, and/or replacement (like for like) of auxiliary equipment (i.e. equipment that is not in direct contact with the Product) which does not impact the validated state. EXCEPTION: All changes in equipment for sterile products must be reviewed.
Maintenance, replacement (like for like), repair and/or calibration of lab equipment, unless the change would impact the equipment qualification, method validation, column (chromatography) and/or test method.
Routine maintenance and repair of utilities, services and support systems.
Archiving of obsolete documentation.
Non-GMP changes to plant areas such as area layout or establishing sample preparation rooms provided they do not change the environmental quality of utilities/services. EXCEPTION: Sterile Areas/Sterile Prep Areas (equipment/people).
Changes of Sub-Contractors and/or Manufacturer and/or material for tertiary packaging materials as long as it meets specifications.
Changes to raw materials for APIs, unless they are part of the regulatory filing or critical raw materials for the process.
Changes to GMP service providers (e.g. calibration providers, pest control, GMP documentation storage providers, preventive maintenances providers). EXCEPTION: Changes to sterilization services of finished products and/or devices.
Changes to Sub-Contractor part numbers provided they are not specifically referred to in batch records.
B-23
Change History Log for the Quality Agreement
| Version Number | Prepared By | Reason for Change | ||
B-24
Exhibit C
FORM OF
MONTHLY INVENTORY REPORT
| Manufacturer-owned API/BULK Activity Report | ||||||||||||||||||
| VENDOR: | PERIOD: | |||||||||||||||||
| PRODUCT NAME: | BULK/API NAME: | |||||||||||||||||
| PRODUCT CODE: | BULK/API CODE: | |||||||||||||||||
| CORP CODE
| ||||||||||||||||||
| OPENING | API/BULK RECEIPTS | DESCRIPTION | PRODUCTION DATA | API/BULK USED | CLOSING | |||||||||||||
|
INVENTORY | INVOICE | API | MANUFA
CTURER | QUANTITY | PRODUCT DESCRIPTION | FINISHED | SHIP | QUANTITY
BULK SHIPPED | INVENTORY | |||||||||
| NO | LOT | LOT | LOT # | DATE | ||||||||||||||
|
KG/GMS | DATE | NUMBER | NUMBER | KG/GMS | KG/GMS | KG/GMS | ||||||||||||
| - | - | |||||||||||||||||
| - | - | |||||||||||||||||
| - | - | |||||||||||||||||
| - | - | |||||||||||||||||
| - | - | |||||||||||||||||
| - | - | |||||||||||||||||
| - | - | |||||||||||||||||
| 0 | - | Sub Total | - | - | ||||||||||||||
| Inventory adjustments (Cycle Count) NMR or obsolete material destroyed | - | |||||||||||||||||
| MUV | ||||||||||||||||||
| - | ||||||||||||||||||
Note: Items in WIP should not be considered as SHIPPED
C-1
Exhibit D
SUPPLY CHAIN AGREEMENT PARAMETERS AND/OR APPLICABLE SYSTEMS
HOLDING SUCH PARAMETERS
See the applicable Product Addendum.
D-1
Exhibit E-1
PRICE MARKUP
The Price markup to the Standard Cost shall be as follows during the applicable period during the Term of the Product Addendum:
With respect to each Product manufactured in a Facility outside Brazil:
| Period | Markup | |
| From the Product Addendum Effective Date until the IPO Closing: | Fifteen percent (15%)* | |
| From the IPO Closing until the second (2nd) anniversary thereof: | Zero percent (0%) | |
| From the second (2nd) anniversary of the IPO Closing for the remainder of the Term of the applicable Product Addendum: | Fifteen percent (15%) |
| * | Except as specified otherwise in the applicable Product Addendum. |
With respect to each Product manufactured in a Facility within Brazil:
| Period | Markup | |
| During the Term of the Product Addendum: | Five percent (5%) |
E-1-1
Exhibit E-2
STANDARD COST REVIEW SCHEDULE
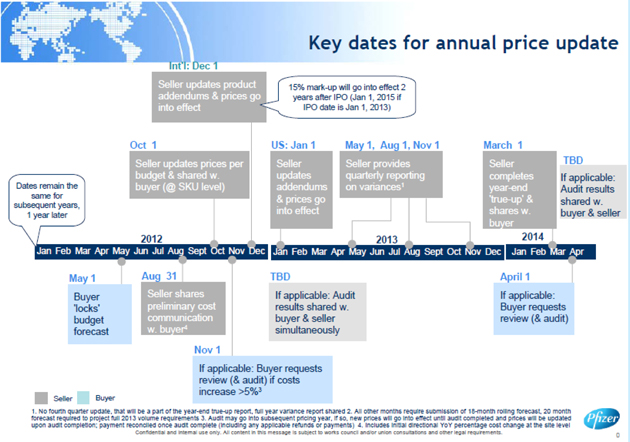
E-2-1
Exhibit E-3
CALCULATION EXAMPLES
Example 1:
Volume Variance Calculation
| Product | Budget units | Actual units | Unit Delta | Budget unit absorption rate | Total Budgeted $$ Absorption | Total Actual $$ Absorption | Absorption $$ Delta | |||||||||||||||||||||||||
| Product A | 10 | 10 | 0 | 5 | 50 | 50 | 0 | |||||||||||||||||||||||||
| Product B | 10 | 10 | 0 | 5 | 50 | 50 | 0 | |||||||||||||||||||||||||
| Product C | 100 | 200 | 100 | 6 | 600 | 1200 | (600 | ) | ||||||||||||||||||||||||
| Product D | 20 | 20 | 0 | 10 | 200 | 200 | 0 | |||||||||||||||||||||||||
| Product E | 20 | 20 | 0 | 8 | 160 | 160 | 0 | |||||||||||||||||||||||||
| Total | 1,060 | 1,660 | (600 | ) | ||||||||||||||||||||||||||||
| Volume Variance | (600 | ) | ||||||||||||||||||||||||||||||
| Less: 5% threshold | 53 | |||||||||||||||||||||||||||||||
|
|
| |||||||||||||||||||||||||||||||
| Base for credit | (547 | ) | ||||||||||||||||||||||||||||||
| Less: Variable portion | 164 | Assumed 30 | % | |||||||||||||||||||||||||||||
|
|
| |||||||||||||||||||||||||||||||
| Credit to buyer | (383 | ) | ||||||||||||||||||||||||||||||
|
|
|
E-3-1
Example 2:
Purchase Price Variance
| Qty Purchased | Std Unit cost | Actual Unit cost | Total value at Std | Total value at Actual | PPV | |||||||||||||||||||
| XX - 0 | 00 | 0 | 0 | 00 | 00 | 0 | ||||||||||||||||||
| XX - 0 | 20 | 4 | 5 | 80 | 100 | 20 | ||||||||||||||||||
| RM - 3 | 400 | 7 | 6 | 2,800 | 2,400 | (400 | ) | |||||||||||||||||
| RM - 4 | 40 | 3 | 3.5 | 120 | 140 | 20 | ||||||||||||||||||
| RM - 5 | 40 | 1 | 1 | 40 | 40 | 0 | ||||||||||||||||||
| Total | 3,060 | 2,700 | (360 | ) | ||||||||||||||||||||
| Purchase Price Variance | (360 | ) | ||||||||||||||||||||||
| Less: 5% threshold | 153 | |||||||||||||||||||||||
| Credit to Buyer | (207 | ) |
E-3-2
