DEVELOPMENT SERVICES AND COMMERCIALIZATION AGREEMENT BY AND BETWEEN PAR PHARMACEUTICAL, INC. AND INTELGENX CORP. DATED AS OF DECEMBER 19, 2011
Exhibit 10.27
Confidential treatment has been requested for portions of this exhibit. The copy filed herewith omits the
information subject to the confidentiality request. Omissions are designated as [***]. A complete version of this
exhibit has been filed separately with the Securities and Exchange Commission.
information subject to the confidentiality request. Omissions are designated as [***]. A complete version of this
exhibit has been filed separately with the Securities and Exchange Commission.
DEVELOPMENT SERVICES AND
COMMERCIALIZATION AGREEMENT
BY AND BETWEEN
PAR PHARMACEUTICAL, INC.
AND INTELGENX CORP.
DATED AS OF DECEMBER 19, 2011
[EXECUTION COPY]
Table of Contents

TABLE OF CONTENTS
(Continued)

ii
THIS DEVELOPMENT SERVICES AND COMMERCIALIZATION AGREEMENT (this “Agreement”) is hereby entered into and effective as of December 19, 2011 (the “Effective Date”) by and between Par Pharmaceutical, Inc., a Delaware corporation with offices located at One Xxx Xxxxx Xxxx, Xxxxxx Xxxxxx, Xxx Xxxx 00000, X.X.X. (“Par”), and IntelGenx Corp., a Canadian corporation with offices located at 0000 xxx Xxxxxx, Xxxxx Xxxxxxx, Xxxxxx, Xxxxxx H4S-1X9 (“IntelGenx”).
WHEREAS, IntelGenx has undertaken certain development activities relating to the preparation of a generic pharmaceutical formulation of the Product (as defined below); and
WHEREAS, , Par desires to have IntelGenx exclusively develop, and IntelGenx desires to exclusively develop for Par generic versions of all strengths and presentations of Suboxone® Sublingual Film, as may be approved pursuant to the NDA (as defined below) for Suboxone® Sublingual Film, as further addressed below;
NOW, THEREFORE, in consideration of the mutual covenants and agreements of the Parties contained herein and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
ARTICLE 1. DEFINITIONS
Capitalized terms used in this Agreement shall have the following definitions:
1.1. “Acquisition Cost” means the fully allocated cost of acquiring the Product or AG Product by Par and/or its Affiliates, calculated in accordance with GAAP, including the following: (i) the transfer price payable by Par to the Manufacturer; (ii) all costs for inbound shipping, handling, intake testing, process validation and stability testing, and holding and storing the Product or AG Product; (iii) any amounts paid for the acquisition or supply of such AG Product; and (iv) any amounts payable to Third Parties on the sale or profits from such AG Product pursuant to an associated supply and/or license agreement or the like, less (in each case, to the extent applicable) any rebates or discounts afforded to and actually received by, or credited to, Par.
1.2. “Affiliate(s)” means as to a Party, any party which directly or indirectly controls, is controlled by, or is under common control with such Party. For purposes of the foregoing definition only, the term “control” (including with correlative meaning, the terms “controlling”, “controlled by”, and “under common control with”) as used with respect to the applicable Party, means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such Party, whether through ownership of equity, securities, or partnership interest or by contract, or otherwise. Ownership of more than fifty percent (50%) of the securities or other ownership interests representing the equity, the voting stock or general partnership interest in an entity, or greater than fifty percent (50%) interest in the income of such entity shall, without limitation, be deemed to be control for purposes of this definition.
1.3. “AG Agreement” has the meaning set forth in Section 6.7.
[EXECUTION COPY]
1.4. “AG Product” means a generically labeled version of the Brand Product that is approved for sale under the Regulatory Approval for such Brand Product.
1.5. “Agreement” has the meaning given to such term in the introductory paragraph of this Agreement.
1.6. “ANDA” means an Abbreviated New Drug Application pursuant to 21 U.S.C. § 355(j) et seq., and the regulations promulgated thereunder.
1.7. “API” means the active pharmaceutical ingredients in the Product.
1.8. “Applicable Laws” means all laws, rules, regulations and guidelines of any Governmental Authority with jurisdiction over the development, manufacturing, exportation, importation, promotion, marketing, sale or distribution of the Product and/or the performance of a Party’s obligations under this Agreement, to the extent applicable and relevant, and including specifically all cGMP or similar standards or guidelines of the FDA and compendial guidelines (e.g., United States Pharmacopeia or European Pharmacopeia), where applicable, as well as U.S. export control laws and the U.S. Foreign Corrupt Practices Act.
1.9. “Appointed Legal Counsel” has the meaning set forth in Section 6.9.4.
1.10. “Batch” means a specific quantity of Product, as mutually agreed upon by Par and IntelGenx, that (a) is intended to have a uniform character and quality within specified limits, and (b) is produced according to a single manufacturing order during the same cycle of manufacture.
1.11. “Bioequivalence Studies” means a study undertaken to satisfy the FDA’s requirements for bioequivalence in connection with establishing that a drug product subject to an ANDA is a Therapeutic Equivalent of the Brand Product referenced in such ANDA.
1.12. “Brand Product” means the Suboxone® (buprenorphine and naloxone) Sublingual Film Product that is the subject of NDA 022410, as may be amended or supplemented from time to time.
1.13. “Calendar Quarter” means a three (3) consecutive month period ending on March 31, June 30, September 30 or December 31.
1.14. “Clinical Expert” has the meaning set forth in Section 2.4.2.
1.15. “Commercial Launch” means the first commercial sale in the Territory of the Product by Par, its Affiliate or a permitted sublicensee, as the case may be, to a Third Party.
1.16. “Commercially Reasonable Efforts” means, with respect to each Party, efforts and commitment of resources in accordance with such Party’s reasonable business, legal, medical, and scientific judgment that are consistent with the efforts and resources that such Party uses for other products owned by it or to which it has exclusive rights, that are of similar market potential and at a similar stage in their life cycle, taking into account the competitiveness of the marketplace, the regulatory structure involved, the profitability of the applicable products and
2
[EXECUTION COPY]
other relevant factors, including technical, legal, scientific, medical, sales performance, and/or marketing factors, including the good faith performance of any associated commitments under this Agreement.
1.17. “Confidential Information” means, with respect to a Party disclosing such Information (the “Disclosing Party”), all non-public information of any kind whatsoever (including data, materials, compilations, formulae, models, patent disclosures, procedures, processes, projections, protocols, results of experimentation and testing, specifications, strategies, techniques and all non-public Intellectual Property as defined herein), and all tangible and intangible embodiments thereof of any kind whatsoever (including materials, samples, compositions, documents, drawings, patent applications, records and reports), that are disclosed by the Disclosing Party to the other Party (the “Receiving Party”), including any and all copies, replication or embodiments thereof.
Notwithstanding the foregoing, Confidential Information of a Disclosing Party shall not include information that the Receiving Party can establish by competent proof to have (a) been publicly known prior to disclosure of such information by the Disclosing Party to the Receiving Party, (b) become publicly known, without fault on the part of the Receiving Party, subsequent to disclosure of such information by the Disclosing Party to the Receiving Party, (c) been received by the Receiving Party from a source rightfully having possession of, and the right to disclose, such information free of an obligation of confidentiality, (d) been otherwise rightfully known by the Receiving Party prior to disclosure of such information by the Disclosing Party to the Receiving Party, or (e) been independently developed by employees or agents of the Receiving Party without the use of Confidential Information of the Disclosing Party.
1.18. “Control” means the legal or regulatory right (whether by ownership, license or otherwise) to grant access, right, title, a license or a sublicense to Intellectual Property without violating the terms of any Third Party agreement, court order, or other arrangement or legal obligation.
1.19. “Disclosing Party” has the meaning set forth in Section 1.17.
1.20. “Drug Product” means a drug product, as defined in 21 C.F.R. § 314.3, for administration to human subjects.
1.21. “Engineering Batch” means a Batch produced from an Engineering Run.
1.22. “Engineering Run” means a Run used for process developing or demonstrating and/or engineering of some or all of the Manufacturing Process steps.
1.23. “Effective Date” has the meaning given to such term in the introductory paragraph of this Agreement.
1.24. “FDA” means the United States Food and Drug Administration, and any successor agency thereto.
1.25. “First Applicant” means a first applicant, as defined in 21 U.S.C. § 355(j)(5)(B)(iv)(II)(bb), as amended.
3
[EXECUTION COPY]
1.26. “Force Majeure Event” has the meaning set forth in Section 13.10.
1.27. “GAAP” means generally accepted accounting principles in effect in the United
States from time to time, consistently applied.
1.28. “Governmental Authority” means any court, tribunal, arbitrator, agency, legislative body, commission, official or other instrumentality of (i) any government of any country, or (ii) a federal, state, province, county, city or other political subdivision thereof.
1.29. “Gross Amount” means the gross amount invoiced for the Product or AG Product, sold by Par, its Affiliate or a permitted sublicensee, as the case may be, in the Territory.
1.30. “Indemnitee” has the meaning set forth in Section 9.3.
1.31. “Indemnitor” has the meaning set forth in Section 9.3.
1.32. “IntelGenx” has the meaning given to such term in the introductory paragraph of this Agreement.
1.33. “IntelGenx Indemnitee” has the meaning set forth in Section 9.2.
1.34. “Intellectual Property” means all of the following: (i) patent applications, continuation applications, continuation-in-part applications, divisional applications, and United States patents corresponding to any of the foregoing that may grant or may have been granted on any of the foregoing, including reissues, re-examinations and extensions and any supplemental protection certificates, or the like; (ii) all Know-How, work product, trade secrets, inventions (whether patentable or otherwise), data, processes, techniques, procedures, compositions, devices, methods, formulas, protocols and information, whether patentable or not; (iii) copyrightable works, copyrights and applications, registrations and renewals; (iv) logos, trademarks, service marks, and all applications and registrations relating thereto; (v) other proprietary rights; (vi) ANDAs or other applications to market (including right of reference thereto); (vii) any regulatory exclusivities or the like; and (viii) copies and tangible embodiments of any one or more of the foregoing.
1.35. “Know-How” means all of the following: manufacturing protocols and methods, product specifications, analytical methods and assays, processes, product designs, plans, trade secrets, ideas, concepts, manufacturing information, engineering and other manuals and drawings, standard operating procedures, flow diagrams, chemical data, pharmacological data, pharmacokinetic data, toxicological data, pharmaceutical data, physical and analytical data, safety data, quality assurance data, quality control and clinical data, technical information, other data, and research records.
1.36. “Liabilities” has the meaning set forth in Section 9.1.
1.37. “Loss” has the meaning set forth in Section 5.5.2.
1.38. “Manufacturer” has the meaning set forth in Section 2.5.1.
4
[EXECUTION COPY]
1.39. “Manufacturing Process" means the production process for the manufacture of the Product, as such process may be changed from time to time in accordance with this Agreement.
1.40. “Marketing Cost Allowance” means an expense allowance used as an approximation (and not subject to adjustment) for any and all of Par’s costs and expenses in the marketing, promotion, distribution, sale, shipping and transport (from Par to its customers, including related insurance and freight expense) for the Product or AG Product, which shall be equal to [***] of Net Sales.
1.41. “NDA” means a New Drug Application, as defined in 21 U.S.C. § 355(b) et seq., and the regulations promulgated thereunder.
1.42. “Net Profits” means Net Sales, less Par’s Total Cost.
1.43. “Net Sales” means the Gross Amount, less all discounts and deductions that are customary in size and nature in the generic pharmaceutical products industry, including:
(a) sales credits for customer returns, returned goods allowances, billing and shipping errors, rejected goods; cash or term discounts; customer rebate programs; chargebacks and administration fees or similar credits or payments granted to customers pursuant to contract or other purchases; sales promotions, trade show discounts and stock allowances; price adjustments, including those on customer inventories following price changes; and Product or AG Product recalls;
(b) payments or rebates incurred pursuant to federal, state and local government assistance programs, whether now in existence or hereafter enacted;
(c) redistribution center (RDC) fees, information service agreement (ISA) fees, other fees that are customary in the industry and related to the sales of the Product or AG Product to customers, and ANDA filing fees;
(d) customs duties, and sales, use or excise taxes; and
(e) write-offs for unsold inventory or batches.
Par shall not sell the Product or AG Product as a loss leader, for any non-cash element or as part of a bundle, basket or group sale with any other product(s) not covered by this Agreement; provided, however, that the provision of a discount by Par to a customer based on the aggregate volume of such customer’s purchases of the Product or AG Product and other products shall not, for purposes of this Section 1.43, be considered a sale of such Product or AG Product as a loss leader or as part of a bundle, basket or group sale so long as such discount is (i) allocated on a proportionate basis to such Product or AG Product and such other products, and (ii) consistent with Par’s ordinary course of business for its products other than the Product or AG Product. For example, if the Product or AG Product and another product are sold under a volume discount arrangement and have a combined volume discount of $200,000 on a total undiscounted sales price of $1,000,000 and the units of such Product or AG Product included in such volume discount arrangement have an undiscounted sales price of $600,000 and the units of such other
5
[EXECUTION COPY]
product have an undiscounted sales price of $400,000, such discount shall not be considered a sale of such Product or AG Product as a loss leader or as part of a bundle, basket or group sale so long as no more than sixty percent (60%), or $120,000, of such discount is allocated to such Product or AG Product.
1.44. “Orange Book” means the FDA publication Approved Drug Products with Therapeutic Equivalence Evaluations, as may be amended from time to time.
1.45. “Paragraph IV Certification” means a certification pursuant to 21 U.S.C. § 355(j)(2)(A)(vii)(VI).
1.46. “Par” has the meaning given to such term in the introductory paragraph of this Agreement.
1.47. “Par Indemnitee” has the meaning set forth in Section 9.1.
1.48. “Par’s Total Cost” means the Acquisition Cost, plus the Marketing Cost Allowance.
1.49. “Party” means Par or IntelGenx, as applicable, and “Parties” means both Par and IntelGenx.
1.50. “Patent Litigation” has the meaning set forth in Section 6.9.
1.51. “Person” means an individual, corporation, partnership, limited liability company, firm, association, joint venture, estate, trust, governmental or administrative body or agency, or any other entity.
1.52. “Pilot Bioequivalence Study” means a Bioequivalence Study, the results of which are used to establish the bioequivalence benchmarks for the Pivotal Bioequivalence Study, including by validation of analytical methodology, assessment of variability, optimization of sample collection time intervals.
1.53. “Pivotal Bioequivalence Study” means a Bioequivalence Study that is submitted to the FDA for the purpose of seeking Regulatory Approval for the Product in the Territory.
1.54. “Proceedings” means governmental, judicial, administrative or adversarial proceedings (public or private), litigation, suits, patent oppositions, arbitration, disputes, claims, causes of action or investigations.
1.55. “Product” means a drug product that is formulated to be an A-rated Therapeutic Equivalent to the Brand Product, including all dosage strengths, and all packaging configurations thereof.
1.56. “Product ANDA” means an ANDA filed by Par for the Product pursuant to this Agreement to seek marketing approval by the FDA wherein the same may be supplemented and/or amended as required.
6
[EXECUTION COPY]
1.57. “Product Claim” has the meaning set forth in Section 9.4.
1.58. “Receiving Party” has the meaning set forth in Section 1.17.
1.59. “Regulatory Approval” means the applicable approval(s) necessary to market a Drug Product and/or active pharmaceutical ingredient, including all applicable product and/or establishment licenses, registrations, permits or other authorizations as may be necessary for the commercial manufacture, commercialization, use, storage, importation, transport, promotion, pricing, distribution or sale thereof.
1.60. “Regulatory Authority(ies)” means the Governmental Authority(ies) in the Territory with authority over the manufacture or distribution of a pharmaceutical product in the Territory (including the grant of Regulatory Approval by the FDA).
1.61. “Regulatory Litigation” has the meaning set forth in Section 6.9.
1.62. “Representatives” has the meaning set forth in Section 7.1.
1.63. “Run” means a single complete operation of all, or a discrete portion, of the Manufacturing Process at the Manufacturer.
1.64. “Specifications” means the specifications for the manufacture of the Product as set forth in the Product ANDA submitted for Regulatory Approval.
1.65. “Stable” means a Drug Product that meets FDA requirements for stability for purposes of an ANDA.
1.66. “Submission Batch” means a Batch that is manufactured in order to generate data, results and/or other information to be submitted or intended to be submitted to the FDA for the purpose of seeking the Regulatory Approval for the Product in the Territory.
1.67. “[***]”
1.68. “Tech Transfer Materials” has the meaning set forth in Section 2.6.
1.69. “Term” has the meaning set forth in Section 11.1.
1.70. “Territory” means the United States of America, and its territories, districts and possessions, including the Commonwealth of Puerto Rico; any installation, territory, location or jurisdiction under the purview of the FDA or control of the United States government; and any United States military bases and installations worldwide.
1.71. “Therapeutic Equivalent” has the meaning given to it by the FDA in the current edition of the Orange Book.
1.72. “Third Party” or “Third Parties” means any Person other than a Party or its Affiliates.
7
[EXECUTION COPY]
ARTICLE 2. DEVELOPMENT
2.1 IntelGenx Development Responsibilities. IntelGenx shall develop a final finished Stable dosage form of the Product corresponding to each strength and presentation of the Brand Product and conforming to the Specifications, and otherwise develop the Product to be Stable and an A-rated Therapeutic Equivalent to the corresponding Brand Product, as further provided herein. IntelGenx’s development responsibilities shall include completing the tasks set forth on Exhibit A hereto and making any changes that are necessary to support obtaining Regulatory Approval for the Product.
2.2 Cooperation. In carrying out its development responsibilities, IntelGenx shall cooperate and coordinate with Par, and Par shall have decision-making control with respect to all Specifications and development activities necessary to support the filing of the Product ANDA with the FDA.
2.3 API Supply. At the request of IntelGenx, accompanied by appropriate justification therefor, Par shall provide, at Par’s expense, (i) all reasonable quantities of API required to develop the formulation and Manufacturing Processes in respect of the Product, with the exception of API required for the Pilot Bioequivalence Study; (ii) samples of the Brand Product in reasonable quantities required to develop analytical methods and conduct stability and other testing; and (iii) any reference standards reasonably obtainable by Par from the supplier of the API for purposes of analysis, including in-process impurities and degradants, required to develop stability indicating methods.
2.4 Bioequivalence Studies.
2.4.1 IntelGenx shall be responsible, at its expense, for completion of the Pilot Bioequivalence Study. IntelGenx shall own any and all data, results, or other information developed and/or generated during the Pilot Bioequivalence Study.
2.4.2 In the event that the Pilot Bioequivalence Study is unsuccessful, as mutually agreed upon by the Parties, IntelGenx shall, at its expense, conduct at least one additional Pilot Bioequivalence Study. In the event that a dispute relating to the success criteria and/or successful completion of a Pilot Bioequivalence Study arises between the Parties, the Parties shall have the dispute settled by a mutually agreed upon independent Third Party consultant with relevant experience in the pharmaceutical industry (the “Clinical Expert”), and if the Clinical Expert determines that such Pilot Bioequivalence Study was unsuccessful, IntelGenx shall, at its expense, conduct at least one additional Pilot Bioequivalence Study.
2.4.3 In the event of successful completion of the Pilot Bioequivalence Study, Par shall be responsible, at its expense, for carrying out (or causing to be carried out by a Third Party selected by Par) the Pivotal Bioequivalence Study for the Product. Par may, at Par’s sole discretion, elect to conduct one or more additional Pivotal Bioequivalence Study for the Product. IntelGenx shall cooperate fully with Par in connection therewith, and shall promptly provide Par, as requested and at no additional charge, such technical and other assistance, including all available information and data in its control, reasonably necessary or useful for Par to conduct the Pivotal Bioequivalence Studies.
8
[EXECUTION COPY]
2.5 Manufacturer.
2.5.1 IntelGenx shall select one or more competent Third Party contract manufacturer(s), subject to Par’s consent, which consent shall not be unreasonably withheld, delayed or conditioned, to manufacture and supply the Product (the “Manufacturer”); and Par shall use Commercially Reasonable Efforts to negotiate a manufacture and supply agreement with the Manufacturer. Notwithstanding the foregoing, IntelGenx shall, at all times, retain all Intellectual Property rights related to the manufacture of the Product and invented or conceived by IntelGenx.
2.5.2 IntelGenx shall be responsible, at its expense, for the manufacture and supply of the Engineering Batch and all other Batches prior to the Submission Batches required by Par for and in the course of the Product development.
2.5.3 Par shall be responsible, at its expense, for causing the manufacture and supply of all Submission Batches.
2.6 Technology Transfer of the IntelGenx Formulation. Upon successful completion of the Pilot Bioequivalence Study, and on an ongoing basis thereafter, IntelGenx shall, at its own cost and expense, supply to the Manufacturer the materials and documentation reasonably necessary to enable the Manufacturer to develop and manufacture, on a commercial scale, a Stable, commercially saleable, final dosage form of the Product. Such materials and documentation shall include any and all information set forth on Exhibit B hereto (collectively, the “Tech Transfer Materials”) and all Know-How relating to the Product owned or controlled by IntelGenx, such as manufacturing formulae, information, methods and processes, analytical and processing techniques, product and API samples, stability data, or processing techniques, and any other knowledge, documentation and information that may be reasonably necessary or useful for the Manufacturer to complete commercial development of the Product.
2.7 Technology Transfer Assistance.
2.7.1 At Par’s request, IntelGenx shall make at least one (1) representative available at the Manufacturer’s facility during production of the exhibit and Submission Batches and during the validation of the analytical methods for the Product.
2.7.2 IntelGenx shall also provide all other reasonable assistance with respect to any development work that may be reasonably required in order for Par to submit the Product ANDA for Regulatory Approval and the commercial process validation for the Product, and for the Manufacturer to commercially manufacture the Product. IntelGenx shall reasonably make available IntelGenx personnel (or contractors) who are knowledgeable regarding the existing manufacturing processes in order to provide assistance to Par and/or the Manufacturer. IntelGenx’s obligation under this Section 2.7 shall continue until the Manufacturer successfully manufactures a Submission Batch. IntelGenx will bear all of its own costs and expenses required to perform its obligations under this Section 2.7.2.
2.8 Updates. IntelGenx shall keep Par informed of the progress of the development of the Product, as practical and reasonable, including responding in a prompt manner to Par’s inquiries, and participating in periodically scheduled telephone conferences regarding the status
9
[EXECUTION COPY]
of the development work. IntelGenx shall use its diligent efforts to complete timely requests from Par relating to the development and manufacture of the Product. IntelGenx shall provide updates to Par at Par’s request on the development of the Product, and shall promptly advise Par of any delays or problems encountered during development of the Product or the Manufacturing Process for the Product.
2.9 IntelGenx Facilities. All development work shall be conducted by IntelGenx at IntelGenx’s facilities; provided, however, that all work relating to process scale-up and Submission Batches shall be conducted, at Par’s direction based on IntelGenx’s formulation and manufacturing guidelines, at the Manufacturer’s facilities. Par shall, during the course of such development work, be permitted to inspect and audit such IntelGenx facilities once during each calendar year (and additionally in the event of a reasonable need or request by Par) during normal business hours upon reasonable advance notice of at least five (5) business days. Following the Effective Date, IntelGenx shall not subcontract any of its responsibilities under this Agreement without the prior written approval of Par, which shall not be unreasonably withheld, delayed or conditioned; provided, however, that IntelGenx may utilize another facility, subject to such facility passing an audit by Par, in Par’s sole discretion. IntelGenx shall notify Par in writing promptly, but in no event later than one (1) business day, after learning that any inspection, relating to the Product, by the FDA or other applicable Governmental Authority is being conducted or will be conducted. IntelGenx shall provide Par with copies of any Form FDA 483 or other correspondence from the FDA or other applicable Governmental Authority regarding the compliance with Applicable Laws, including cGMP and ICH Guidelines, within one (1) business day of receipt by IntelGenx of such correspondence.
ARTICLE 3. REGULATORY MATTERS
3.1 Ownership. Par shall exclusively own and control all Regulatory Approvals within the Territory (including all associated contents and correspondence) and applications therefor related to any Product, including the Product ANDA and any other marketing authorizations within the Territory.
3.1.1 In the event that Par intends to divest or sell the Product ANDA (other than in connection with a merger or acquisition or sale of all or substantially all of the assets of Par), Par shall provide written notice thereof to IntelGenx; and IntelGenx shall provide written notice to Par, within five (5) business days after delivery of such notice by Par, indicating whether it desires to have its rights under this Agreement included in such divestiture or sale.
(a) In the event that IntelGenx provides affirmative notice to Par in accordance with Section 3.1.1, Par shall use Commercially Reasonable Efforts to procure an offer to purchase all of the rights, title and interest in, to and under the Product ANDA; and if Par procures such an offer, Par shall provide written notice thereof, including the material economic terms with respect thereto. IntelGenx shall provide written notice to Par, within five (5) business days after delivery of such notice by Par, indicating whether, based on such terms, it desires to participate in such divestiture or sale.
10
[EXECUTION COPY]
(b) In the event that IntelGenx provides affirmative notice to Par in accordance with Section 3.1.1(a), Par shall use Commercially Reasonable Efforts to negotiate a definitive agreement based on such terms.
3.1.2 In the event that (i) IntelGenx does not provide affirmative notice described in Section 3.1.1 or 3.1.1(a) to Par, or (ii) IntelGenx provides such notice but, despite Par’s use of such Commercially Reasonable Efforts, Par is unable to negotiate a definitive agreement with respect to such terms, Par shall be entitled to sell the Product ANDA, subject to the rights set forth herein, including those set forth in Section 5.5.1.
3.2 Regulatory Approvals and Applications. Par shall author and assemble all aspects of the Product ANDA. IntelGenx shall fully support Par’s efforts to assemble the Product ANDA by providing such assistance as Par requests, including providing any necessary documents to Par in common technical document (CTD) format, as recognized by the FDA.
3.2.1 Par shall have the sole right and responsibility to communicate with the FDA and all other applicable Regulatory Authorities relating to the approval of any Product or submission for Regulatory Approval, and IntelGenx shall not submit material to the FDA or any Regulatory Authority related to the Product without Par’s prior written approval.
3.2.2 Notwithstanding anything else in this Agreement to the contrary, Par shall have sole control of and responsibility (including expenses) for preparing any patent certifications and related notice letters in connection with the Product ANDA and the prosecution and/or defense of any citizen’s petition associated with such ANDA, in each case as may be applicable in any jurisdiction in the Territory.
3.2.3 IntelGenx shall fully cooperate with Par in pursuing Regulatory Approval for the Product in the Territory, and shall promptly provide Par, as requested and at no additional charge, such technical and other assistance, including all available information and data in its control, reasonably necessary or useful for Par to apply for, obtain, and maintain Regulatory Approvals to manufacture, import, export, sell or otherwise commercialize a Product throughout the Territory.
3.2.4 IntelGenx shall, at Par’s direction, assist Par in (i) communications with or to applicable Regulatory Authorities, (ii) all activities relating to Regulatory Approvals for the Product, and (iii) responding to any Regulatory Authority request relating to the Product, API, or facilities used in, or proposed for use in, the development or manufacture of Product or API.
3.2.5 IntelGenx shall provide Par with written notice in the event IntelGenx intends to commercialize any product comprising the same active pharmaceutical ingredients, dosage form and strength(s) as the Product outside of the Territory. Upon receipt of such notice, Par shall, subject to the negotiation and execution of a written agreement by Par and IntelGenx in respect thereof, grant IntelGenx an exclusive, royalty-bearing license to use and have access to any information or Intellectual Property disclosed within the Product ANDA, including the results of the Pivotal Bioequivalence Studies, for the sole purpose of commercializing the Product outside the Territory.
11
[EXECUTION COPY]
ARTICLE 4. COMMERCIALIZATION AND MANUFACTURE
4.1 Product Commercialization. Par shall, in its sole discretion, determine the timing of the Commercial Launch taking into consideration the expected timing of the Regulatory Approval of the Product, availability of supply of the Product, and intellectual property and regulatory risks associated with such launch. Upon the Commercial Launch, Par will promote, market and sell the Product, from Par’s Spring Valley facility or such other Par or Third Party facility as Par may elect in its sole discretion, under Par’s label in a manner consistent with Par’s normal practices with respect to its other generic products.
4.2 Manufacture. The Manufacturer shall be responsible for the manufacture, labeling and packaging of all commercial supplies of the Product. Par shall test and release, or cause to be tested and released by a Third Party testing facility selected by Par, the Product manufactured pursuant to this Agreement for determining compliance in accordance with cGMP and all Applicable Laws.
4.3 API. Par shall be solely responsible, at its sole cost and expense, for procuring a commercially acceptable source of API supply for development (subject to the exception set forth in Section 2.3(i)) and commercialization performed under this Agreement (and IntelGenx shall confirm that such source is technically acceptable). IntelGenx shall cooperate with Par’s procurement of API under this Agreement.
ARTICLE 5. FINANCIAL PROVISIONS
5.1 Development Fee. Par shall pay to IntelGenx the following three (3) non- refundable development fees, if and as applicable:
5.1.1 [***] upon the execution of this Agreement by IntelGenx and Par;
5.1.2 Two Hundred Fifty Thousand Dollars ($250,000) upon successful completion of the Pivotal Bioequivalence Study; and
5.1.3 [***] upon acceptance for filing of the Product ANDA for the Product for all strengths and presentations of the Brand Product listed in the Orange Book as of the Effective Date.
5.2 Conditional Incentive Fee. If, and only if, Par is (a) the sole First Applicant with respect to the Product and (b) eligible at the time of final FDA approval of the Product ANDA for the 180-day marketing exclusivity under 21 U.S.C. § 355(J)(5)(B)(iv)(II)(aa), then Par shall pay to IntelGenx a one-time, conditional and non-refundable incentive fee of [***] upon obtaining final FDA approval of such ANDA or the first commercial sale in the Territory of an AG Product by Par, its Affiliate or a permitted sublicensee, as the case may be, to a Third Party.
5.3 Payment. Upon the occurrence of the applicable events under Sections 5.1 and 5.2, Par shall (i) promptly provide written notice thereof to IntelGenx and, (ii) within fourteen (14) days following the receipt of an invoice therefor provided by IntelGenx, remit the fee
12
[EXECUTION COPY]
payments payable to IntelGenx under Sections 5.1 and/or 5.2 (as applicable) by wire transfer of immediately available funds to a bank account designated in writing by IntelGenx.
5.4 Expenses. Each party shall bear all costs and expenses associated with its responsibilities under this Agreement, except as expressly set forth in this Agreement.
5.5 Royalties.
5.5.1 Royalty Rates. Par shall pay to IntelGenx a royalty equal to [***] of the Net Profits during the Term.
5.5.2 Payment of Royalties. Following Commercial Launch of the Product or commercial launch of the AG Product, within thirty (30) days of the end of each Calendar Quarter during the Term, Par shall, for Product or AG Product sold by Par during such Calendar Quarter, (i) compute in accordance with GAAP, the Net Sales and Net Profit and (ii) pay IntelGenx’s share of the Net Profit payable pursuant to Section 5.5.1. Each payment shall be accompanied by a written report (in the format attached as Exhibit C hereto) outlining the details surrounding the calculation of Net Profits.
5.5.3 Records and Audits. Par and its Affiliates shall keep and maintain or cause to be maintained books and records pertaining to the calculation of Net Profits during the Term and for three (3) years thereafter. Such books and records shall be maintained in accordance with GAAP and with all records and details necessary to enable IntelGenx to verify the foregoing. All factors included in the determination of the Net Profits shall be specific to the Product and/or AG Product, reasonably documented, and available for independent audit purposes. IntelGenx shall have the right once per calendar year, at its own expense, during the Term and for three (3) years thereafter, to have an independent public accountant, reasonably acceptable to Par, audit the relevant financial books and records of account of Par for up to the preceding three (3) years during normal business hours, upon reasonable advance notice, to determine or verify the applicable Net Profits. If errors are found, any deficiency shall be paid promptly following delivery of written d cumentation reasonably substantiating such deficiency, subject to Par having a reasonable period to verify the accuracy of such figures, and if errors are discovered as a result of such audit in IntelGenx’s favor exceeding the greater of five percent (5%) and Ten Thousand Dollars ($10,000) for the period audited (which shall be no less than one (1) year), Par shall reimburse IntelGenx for the reasonable expense of such audit.
5.5.4 Accounting. The Parties acknowledge that any expenses or costs deducted from Net Sales under this Agreement may be based upon accruals, which accruals will be compliant with GAAP; provided, however, that when the actual results become known relative to any accrued amount, any difference between the actual results and the accrual shall be accounted for in the subsequent payments due hereunder (subject to customary processing delays). To the extent that the difference between such accruals and the actual results has led to an underpayment, Par shall pay IntelGenx the amount of such underpayment on the next date payment is due to IntelGenx hereunder. To the extent that the difference between such accruals and the actual results has led to an overpayment to IntelGenx, Par may at its option set-off such overpayments against subsequent payments to be made to IntelGenx or issue an invoice for the overpayment, which shall be paid by IntelGenx within forty-five (45) days after IntelGenx’s
13
[EXECUTION COPY]
receipt thereof. By the date that is forty-five (45) days after the end of the sixth month following the expiration of the last lot of the Product and/or AG Product for which a sale was made pursuant to this Agreement, Par shall reconcile (and give to IntelGenx a report of such reconciliation) all accrued calculations and deductions used in the calculations of Net Sales with actual processed credits. If the report shows an underpayment to IntelGenx, Par shall pay IntelGenx the amount of the underpayment at the time it gives the report to IntelGenx. If the report shows an overpayment to IntelGenx, IntelGenx shall pay Par the amount of the overpayment within thirty (30) days of the receipt of such reconciliation.
ARTICLE 6. EXCLUSIVITY AND INTELLECTUAL PROPERTY
6.1 Exclusivity. During the Term, neither Party, by itself, its Affiliate or through any Third Party, shall develop, seek regulatory approval for, manufacture, import, market, sell, distribute, or otherwise commercialize in the Territory any Drug Product that is a Therapeutic Equivalent to the Brand Product or otherwise work on the development of, or supply of any Product, any AG Product, or any Drug Product that is a Therapeutic Equivalent to the Brand Product, except for the development and commercialization of the Product or commercialization of the AG Product pursuant to this Agreement.
6.2 Right of First Negotiation. In the event IntelGenx successfully completes a Pilot Bioequivalence Study for a Drug Product that is a Therapeutic Equivalent to the [***], as may be amended or supplemented from time to time (the “[***]”), IntelGenx shall promptly provide Par with written notice thereof. Par shall have the exclusive right, for a period of forty-five (45) days after receipt of such notice, to negotiate with IntelGenx to agree upon and execute a definitive agreement for Par to become the co-marketer, co-distributor or exclusive marketer and/or distributor in the Territory, as the case may be, for the Tablet Product. The Parties shall each negotiate in good faith with each other during such period. If, prior to the end of such forty-five (45) day period (or such longer period as may be mutually agreed upon by the Parties), a definitive agreement in respect thereof has not been executed by the Parties, IntelGenx shall thereafter owe no further obligation to Par with respect to the commercialization of the Tablet Product, and may negotiate and execute a definitive agreement with a Third Party in respect of the development and/or commercialization of the Tablet Product, but only if the terms and conditions of such agreement, taken as a whole, are not materially more favorable to such Third Party than the terms and conditions set forth in the last best written offer provided to Par by IntelGenx.
6.3 General Ownership. Except as expressly provided in this Agreement, each Party shall own its own Intellectual Property consistent with United States or other applicable international patent, trademark, and copyright law.
6.4 Product Intellectual Property.
6.4.1 IntelGenx shall have the exclusive right to enforce Intellectual Property that is Controlled by IntelGenx covering the Product against Third Parties that may (or may attempt to) make, have made, use, have used, sell, have sold, import or have imported, or otherwise market or commercialize any Drug Product containing the API and having the same dosage form as the Product, including the right to collect damages. Par shall, at IntelGenx’s cost
14
[EXECUTION COPY]
and expense, cooperate with IntelGenx in good faith in connection with the foregoing, as IntelGenx may reasonably request. In the event that IntelGenx elects not to enforce such Intellectual Property, Par shall have the right, but not the obligation, to enforce such Intellectual Property as set forth in this Section 6.4.1, and IntelGenx shall cooperate with Par in connection therewith.
6.4.2 Intellectual Property that is jointly invented or conceived during the Term under this Agreement shall be jointly owned by the Parties, unless otherwise agreed in writing. Employees of IntelGenx, whether serving as advisors or consultants to Par or serving Par in any other capacity, shall be considered employees of IntelGenx for the purpose of determining ownership of Intellectual Property.
6.4.3 For the avoidance of doubt, Intellectual Property covering inventions or improvements that are created or conceived in the course of developing the Product shall be owned solely by a Party if only its employees create or conceive such invention or improvement.
6.5 License Grant.
6.5.1 IntelGenx hereby grants to Par a limited, exclusive (even as to IntelGenx), irrevocable, perpetual, royalty-free license under the Intellectual Property that is Controlled by IntelGenx or its Affiliates to have manufactured, use, sell, have sold and import and/or otherwise for the sole purpose of the commercialization of the Product or AG Product in the Territory (including all components thereof).
6.5.2 The license granted to Par under Section 6.5.1 is sublicensable (and further sublicensable), in whole or in part, to Third Parties in arm’s-length transactions, subject to the following terms: (i) Par shall provide IntelGenx with written notice of any intended sublicense, including the name of the intended sublicensee and the material terms thereof; and (ii) IntelGenx shall, within ten (10 business days (or such shorter period as is reasonably specified by Par to address the exigencies of negotiation of an agreement with such sublicensee) after delivery of Par’s written notice to IntelGenx, provide written notice to Par indicating whether it approves the sublicense proposed by Par, such approval not to be unreasonably withheld, delayed or conditioned, it being acknowledged and agreed by IntelGenx that it shall consider in good faith the need to sublicense a substitute Third Party manufacturer in the event of any supply disruption involving the Manufacturer. The failure of IntelGenx to deliver such written notice to Par within such ten (10) business day period shall be deemed to be an approval of such proposed sublicense. Any sublicense approved or deemed approved under this Section
6.5.2 shall be consistent with the terms of this Agreement, including an obligation for such sublicensee to comply with obligations similar to those set forth in this Agreement.
6.6 Reserved Rights. Subject to Sections 6.1 and 6.5 hereof, Par acknowledges and agrees that IntelGenx may, now or in the future and without obligation to Par, develop, use or employ Intellectual Property that is Controlled by IntelGenx for other products, including formulation and process, various analytical methods, stability protocols and other methods, techniques or information similar to those used in connection with the Product hereunder (excluding Par’s Confidential Information) to pursue other business and product development activities that are part of IntelGenx’s business without obligation to Par.
15
[EXECUTION COPY]
6.7 Authorized Generic Product. Par shall be permitted, without requiring license or approval from IntelGenx, to enter into an agreement with the owner of the Brand Product under which Par may sell an AG Product (an “AG Agreement”), and Par may thereafter acquire, use, sell and otherwise market such AG Product pursuant to such AG Agreement in the Territory. Par shall be allowed to sell the AG Product in place of, or in addition to, the Product; provided, however, that in the event that Par enters into an AG Agreement, Par shall continue to be bound by its royalty obligations to IntelGenx under Section 5.5.1 during the Term, and will pay the applicable percentage of Net Profits as set forth in Section 5.5 on the sales of both AG Product and Product.
6.8 Notification. The Parties shall promptly notify each other of any allegation that any activity undertaken pursuant to this Agreement that infringes or may infringe the Intellectual Property rights of any Third Party. Each Party shall assist and cooperate with the other Party in the defense of any suit, action, Proceeding or claim relating to the Product (including consenting to being named as a nominal party thereto).
6.9 Patent and Regulatory Litigation.
6.9.1 Par’s legal counsel shall be responsible for managing any litigation brought by the Parties or by a Third Party seeking a judicial determination of whether the submission of Par’s ANDA or the importation, manufacture, use, sale or marketing of the Product infringes the patent rights of such Third Party (“Patent Litigation”). Par’s legal counsel shall also be responsible for managing the Parties’ participation in any Proceedings and litigation related to citizen’s petitions filed with the FDA regarding the Product or any claims based on or related to the Parties’ or a Third Party’s attempt to secure, challenge or appeal an FDA decision concerning the Product or competitive products (collectively, “Regulatory Litigation”). Par shall control and manage Patent Litigation and Regulatory Litigation and any other matters relating to Intellectual Property rights of a Third Party in its discretion, using counsel of its choice. In connection with such Patent Litigation, Regulatory Litigation or such other matters, each Party shall cooperate with each other at its own expense.
6.9.2 In connection with any Patent Litigation and/or Regulatory Litigation, Par’s legal counsel shall keep IntelGenx’s legal counsel (retained at IntelGenx’s option and expense) reasonably informed with respect to material events in the progress and settlement of such Proceedings and litigation. IntelGenx’s counsel may provide input relating to the management of Patent Litigation and Regulatory Litigation, and Par shall consider the suggestions of IntelGenx’s counsel in good faith and take such suggestions into account to the extent that, in the judgment of Par’s in-house counsel, such suggestions do not adversely affect Par’s position in any Intellectual Property and Regulatory Litigation.
6.9.3 IntelGenx’s legal counsel shall be permitted to monitor the progress of the Intellectual Property and Regulatory Litigation, and Par shall keep IntelGenx informed of any intended settlement. IntelGenx shall fully cooperate with Par in connection therewith.
6.9.4 In the event of any patent litigation brought by a Third Party solely against IntelGenx for inducement to infringe or contributory infringement as a result of the obligations set forth in this Agreement, IntelGenx shall have the right to defend such litigation using legal
16
[EXECUTION COPY]
counsel selected by Par, in its sole discretion (“Appointed Legal Counsel”), and at Par’s cost and expense.
(a) In the event of such litigation and selection by Par, each Party shall cooperate with each other in connection therewith, including entering into appropriate joint defense and/or joint privilege agreements. In the event that Par makes a determination to join as a party to such litigation, IntelGenx shall, at Par’s written request, move to implead Par as a party thereto.
(b) In connection therewith, IntelGenx shall ensure that the Appointed Legal Counsel shall keep Par informed with respect to the defense of such litigation (including access to all material documentation with regard thereto) and shall disclose to Par all material correspondence with the courts and adverse parties. If IntelGenx wishes to be represented with respect to such litigation by counsel of its own choosing (which counsel shall act in an advisory role only and shall not participate in the defense of such litigation), such representation shall be at IntelGenx’s sole cost and expense.
(c) Par shall, subject to Applicable Laws, make available its employees and relevant records in its possession or control, as applicable and to the extent reasonably necessary to assist in the defense of such litigation.
6.10 Settlement and Assertion of Rights. Par shall be entitled to settle or compromise any claim with respect to Patent Litigation or Regulatory Litigation, and to enter into any agreement in respect thereof, without the prior written consent of IntelGenx. IntelGenx shall not enter into any settlement agreement, other agreement, consent judgment or other voluntary final disposition of any Proceeding, threatened Proceeding, litigation or threatening litigation relating to the Product without the prior written consent of Par. Both Parties shall have the right to assert all Intellectual Property rights related to the Product against Third Parties, subject to mutual consultation. Notwithstanding the foregoing or any text to the contrary contained herein, with respect to matters relating to Intellectual Property rights of any Third Party other than Patent Litigation or Regulatory Litigation, neither Party shall, without the consent of the other Party, enter into any settlement or compromise or consent to any judgment in respect of any claim and/or proceeding related to rights licensed to Par under this Agreement, unless such settlement, compromise or consent includes an unconditional release of the other Party from all liability arising out of the claim, if any, and does not otherwise limit or impair the other Party’s rights.
ARTICLE 7. CONFIDENTIALITY AND PUBLIC DISCLOSURE
7.1 Treatment of Confidential Information. A Receiving Party shall retain in strict confidence, and not disclose, divulge or otherwise communicate to any other Person, any Confidential Information of the Disclosing Party, whether received prior to or after the Effective Date, and shall not use any such Confidential Information for any purpose, except pursuant to the terms of, and as required to carry out such Receiving Party’s obligations, under this Agreement, except that each Receiving Party may disclose Confidential Information of the Disclosing Party to the officers, directors, employees, agents, accountants, attorneys, consultants, subcontractors or other representatives of the Receiving Party or its Affiliates (the “Representatives”), who, in
17
[EXECUTION COPY]
each case, (a) need to know such Confidential Information for purposes of the implementation and performance by the Receiving Party of this Agreement, (b) will use the Confidential Information only for such limited purposes, and (c) are bound by confidentiality obligations no less protective than those set forth in this Agreement.
7.1.1 A Receiving Party hereby shall use at least the same standard of care in complying with its confidentiality obligations hereunder as it uses to protect its own Confidential Information of comparable sensitivity and to prevent and restrain the unauthorized disclosure of such Confidential Information by any of its Representatives, but no less than a reasonable standard of care. The Receiving Party shall be jointly and severally liable for any breach by any of its Representatives of the restrictions set forth in this Agreement.
7.1.2 Without limiting the generality of any of the foregoing, the Parties shall not make any disclosure of Confidential Information that would be reasonably likely to preclude the Disclosing Party from obtaining U.S. or foreign patents on any patentable invention or discovery described or otherwise embodied in such Party’s Confidential Information.
7.1.3 The Confidential Information of each Party includes information from Third Parties subject to confidentiality restrictions and disclosed by one Party to the other Party.
7.2 Release from Restrictions.
7.2.1 A Receiving Party may disclose Confidential Information to the extent that such Confidential Information disclosure is made in response to a valid order or subpoena of a court of competent jurisdiction or other Governmental Authority of a country or any political subdivision thereof of competent jurisdiction or otherwise required by law, in the opinion of counsel to the Receiving Party; provided, however, that, to the extent practicable, the Receiving Party shall first provide written notice to the Disclosing Party reasonably in advance under the circumstances in order to give the Disclosing Party a reasonable opportunity to quash such order or subpoena or to obtain a protective order requiring that the Confidential Information or documents that are the subject of such order be held in confidence by such court or Governmental Authority or, if disclosed, be used only for the purposes for which the order or subpoena was issued; and provided further that whether a disclosure order or subpoena is quashed or a protective order is obtained, the Confidential Information disclosed in response to such court or Governmental Authority order or subpoena shall be limited to that information that, in the opinion of counsel to the Receiving Party, is legally required to be disclosed in such response to such court or governmental order or subpoena. Par may also disclose Confidential Information to the extent that such disclosure is made to (i) a Governmental Authority as required in connection with any filing, application or request for Regulatory Approval with respect to the Product or under the reporting requirements of any securities exchange on which the securities of Par or its Affiliates are traded or (ii) a Third Party to which Par has a contractual obligation related to the Product, but only to the extent such information is required by such contractual obligation, provided that in each case (clauses (i) and (ii)) reasonable measures are taken to assure confidential treatment of such information.
7.2.2 A Receiving Party may disclose this Agreement to a Third Party in connection with or in conjunction with a proposed merger, consolidation, sale of assets that
18
[EXECUTION COPY]
includes those related to this Agreement, a permitted assignment of this Agreement or loan financing, raising of capital, or sale of securities, provided that the disclosing Party obtains an agreement for confidential treatment thereof on terms no less protective than those contained herein.
7.3 No Implied Rights. Except as otherwise expressly set forth in this Agreement, nothing herein shall be construed as granting any Receiving Party any right, title, interest in or ownership of the Confidential Information, proprietary information or Intellectual Property of the Disclosing Party. For the avoidance of doubt, specific information disclosed as part of Confidential Information shall not be deemed to be in the public domain or in the prior possession of the receiving Party merely because it is embraced by more general information in the public domain or by more general information in the prior possession of the receiving Party.
7.4 Survival of Confidentiality Obligations. The confidentiality obligations of the Parties contained in this Article 7 shall remain binding on both Parties during the Term and for a period of five (5) years after the expiration of the Term or the termination of this Agreement, regardless of the cause of such expiration or termination.
7.5 Use of Name and Disclosure of Term. No press release, public announcement, confirmation or other communication to the public or Third Parties regarding the existence or terms of this Agreement or related matters shall be made by either Party without the prior written consent of the other Party, including with respect to the form, content and timing of such press release, public announcement, confirmation or other communication to the public or Third Parties. Notwithstanding the foregoing or any text to the contrary contained herein, those communications required by applicable law, regulation or securities exchange rule (including, but not limited to, a public offering prospectus), disclosures of information for which consent has previously been obtained, and information of a similar nature to that which has been previously disclosed publicly with respect to this Agreement, will not require advance approval, but will be provided to the other Party as soon as practicable after the release or communication thereof.
7.6 Third Party Information.
7.6.1 IntelGenx shall not (i) violate or misappropriate the trade secrets, know- how, or confidential information, or knowingly violate or misappropriate any other proprietary rights, of any Third Party in developing the Product, and will not communicate any Third Party trade secrets to Par in connection with its rights and obligations under this Agreement without receiving permission from such Third Party and informing Par of communication of such trade secrets or (ii) provide or disclose any documents or information to Par unless IntelGenx is the owner thereof, or otherwise has the full and legal right to do so.
7.6.2 Par shall not (i) violate or misappropriate the trade secrets, know-how, or confidential information, or knowingly violate or misappropriate any other proprietary rights, of any Third Party in connection with its rights and obligations under this Agreement, and will not communicate any Third Party trade secrets to IntelGenx in connection with its rights and obligations under this Agreement without receiving permission from such Third Party and informing IntelGenx of communication of such trade secrets or (ii) provide or disclose any
19
[EXECUTION COPY]
documents or information to IntelGenx unless Par is the owner thereof, or otherwise has the full and legal right to do so.
7.7 Remedies. Each Party acknowledges and agrees that: (i) it will be too speculative to measure the damages that would be suffered by the other Party if such Party fails to comply with the obligations set forth in this Article 7 and that, in the event of any such failure, the other Party will be irreparably harmed and will not have an adequate remedy at law; (ii) the other Party shall, therefore, be entitled, in addition to any other rights and remedies, to obtain specific performance of such Party’s obligations and to obtain immediate injunctive relief without having to post a bond; and (iii) such Party shall not assert, as a defense to any proceeding for such specific performance or injunctive relief, that the other Party will not be irreparably harmed or that the other Party has an adequate remedy at law.
ARTICLE 8. REPRESENTATIONS AND WARRANTIES
8.1 By Par. Par hereby represents, warrants and covenants that:
(a) Par is a company duly organized, validly existing and in good standing under the laws of the jurisdiction of its formation;
(b) Par has the power and authority to enter into and be bound by the terms and conditions of this Agreement and to perform its obligations hereunder and to execute this Agreement;
(c) Par has taken all necessary action on its part to authorize the execution and delivery of this Agreement and this Agreement has been duly executed and delivered on behalf of Par and constitutes a legal, valid, binding obligation, enforceable against Par in accordance with its terms;
(d) Par is subject to no legal, contractual or other restrictions, limitations or conditions which conflict with its rights and obligations under this Agreement or which might affect adversely its ability to perform hereunder;
(e) Par will comply with all Applicable Laws applicable to its activities under this Agreement;
(f) Par has and will maintain appropriate skilled personnel and facilities to carry out its obligations under this Agreement; and
(g) No Par employees or other Persons performing services on behalf of Par under this Agreement have been debarred, or the subject of debarment Proceedings, under Section 306 of the FD&C Act; and if Par becomes aware that a Person performing on its behalf under this Agreement has been debarred, or has become the subject of debarment Proceedings, under Section 306 of the FD&C Act, Par shall promptly notify IntelGenx and shall prohibit such Person from performing on its behalf under this Agreement.
8.2 By IntelGenx. IntelGenx hereby represents and warrants that:
20
[EXECUTION COPY]
(a) IntelGenx is a company duly organized, validly existing and in good standing under the laws of the jurisdiction of its formation;
(b) IntelGenx has the power and authority to enter into and be bound by the terms and conditions of this Agreement and to perform its obligations hereunder;
(c) IntelGenx has taken all necessary action on its part to authorize the execution and delivery of this Agreement and this Agreement has been duly executed and delivered on behalf of IntelGenx and constitutes a legal, valid, binding obligation, enforceable against IntelGenx in accordance with its terms;
(d) IntelGenx is subject to no legal, contractual or other restrictions, limitations or conditions which conflict with its rights and obligations under this Agreement or which might affect adversely its ability to perform hereunder;
(e) IntelGenx has not misappropriated and will not misappropriate trade secrets of any Third Party in developing the Product, in the provision of services and the performance of its obligations under this Agreement or otherwise in connection with the Products;
(f) IntelGenx will comply with all Applicable Laws applicable to its activities under this Agreement;
(g) IntelGenx has and will maintain appropriate skilled personnel and facilities to carry out its obligations under this Agreement; and
(h) No IntelGenx employees or other Persons performing services on behalf of IntelGenx under this Agreement have been debarred, or the subject of debarment Proceedings, under Section 306 of the FD&C Act; and if IntelGenx becomes aware that a Person performing on its behalf under this Agreement has been debarred, or has become the subject of debarment Proceedings, under Section 306 of the FD&C Act, IntelGenx shall promptly notify Par and shall prohibit such Person from performing on its behalf under this Agreement.
ARTICLE 9. INDEMNIFICATION
9.1 Indemnification by IntelGenx. Subject to Section 9.3, IntelGenx shall defend, indemnify and hold harmless each of Par and its Affiliates, and each of their respective directors, officers and employees (each, a “Par Indemnitee”) from and against any and all liabilities, damages, settlements, penalties, fines, costs or expenses (including reasonable attorneys’ fees and other expenses of litigation) (collectively, “Liabilities”) arising, directly or indirectly, out of or in connection with Third Party claims, suits, actions, demands or judgments to the extent relating to or arising out of (i) any breach or alleged breach by IntelGenx of any representation, warranty, undertaking or covenant under this Agreement or (ii) any alleged negligence, gross negligence or willful misconduct by IntelGenx or its Affiliates, past or present employees or agents; except, in each case, for those Liabilities for which Par has an obligation to indemnify the IntelGenx Indemnitees pursuant to Section 9.2, as to which Liabilities each Party shall indemnify the other Party to the extent of its respective liability for such Liabilities.
21
[EXECUTION COPY]
9.2 Indemnification by Par. Subject to Section 9.3 and 11.4.4(b), Par shall defend, indemnify and hold harmless each of IntelGenx and its Affiliates, and each of their respective directors, officers and employees (each, an “IntelGenx Indemnitee”) from and against any and all Liabilities arising, directly or indirectly, out of or in connection with Third Party claims, suits, actions, demands or judgments to the extent relating to or arising out of (i) any breach or alleged breach by Par of any representation, warranty, undertaking or covenant under this Agreement, (ii) any alleged negligence, gross negligence or willful misconduct by Par or its Affiliates, past or present employees or agents, and (iii) Patent Litigation or Regulatory Litigation; except, in each case, for those Liabilities for which IntelGenx has an obligation to indemnify the Par Indemnitees pursuant to Section 9.1, as to which Liabilities each Party shall indemnify the other Party to the extent of its respective liability for such Liabilities.
9.3 Notice and Procedures. If an IntelGenx Indemnitee or a Par Indemnitee (the “Indemnitee”) intends to claim indemnification under this Article 9, it shall promptly notify the other Party (the “Indemnitor”) in writing of any such alleged Liabilities. In the event that the Indemnitor does not assume and pursue in a timely and diligent manner the defense of any Third Party claim (but in no event later than thirty (30) days, or such shorter period as required under Applicable Laws), then the Indemnitor shall be deemed to have ceded control of such claim and the Indemnitee shall be entitled to appoint counsel of its own choice for such defense, at the cost and expense of the Indemnitor. The Indemnitor shall have the right to control the defense thereof with counsel of its choice, provided that such counsel is reasonably acceptable to Indemnitee; and provided further that any Indemnitee shall have the right to retain its own counsel at its own expense, for any reason, including if representation of any Indemnitee by the counsel retained by the Indemnitor would be inappropriate due to actual or potential differing interests between such Indemnitee and any other Party reasonably represented by such counsel in such proceeding. The Indemnitee, its employees and agents, shall reasonably cooperate with the Indemnitor and its legal representatives in the investigation of any Liabilities covered by this Article 9. The obligations of this Section 9.3 shall not apply to amounts paid in settlement of any claim, demand, action or other proceeding if such settlement is effected without the consent of the Indemnitor (unless the Indemnitor is deemed to have ceded control of the applicable Third Party claim under this Section 9.3). The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any such action, if prejudicial to its ability to defend such action, shall relieve the Indemnitor of any obligation to the Indemnitee under this Section 9.3 to the extent that the Indemnitor is materially prejudiced by such delay. It is understood that only IntelGenx or Par may claim indemnity under this Article 9 (on its own behalf or on behalf of its Indemnitees), and other Persons may not directly claim indemnity hereunder.
9.4 Other Product Liability Claims. To the extent either Party incurs any Liabilities arising from or in connection with any product liability claim with respect to the Product to the extent arising from the actions not subject to the indemnity obligations set forth in Sections 9.1 or 9.2 (a “Product Claim”), each Party shall be liable for such portion of the Liabilities in accordance with such Party’s allocation of the Net Profits pursuant to Section 5.5.1; provided, however, that such Liabilities shall be shared initially by offsetting against the portion of Net Profits otherwise payable or retained pursuant to Section 5.5.1 and in the event of any shortfall thereafter, each Party’s share thereof shall be paid in accordance with such allocation. Par shall have sole control in addressing, defending, managing and conducting any negotiations,
22
[EXECUTION COPY]
litigation, threatened litigation or settlement regarding such Product Claim, using counsel of its choice. In the event that Par does not respond to any Product Claim against IntelGenx within (a) sixty (60) days following the notice of such claim or (b) ten (10) days before the time limit, if any, set forth in the appropriate laws and regulations for the filing of a response to such Product Claim, whichever comes first, IntelGenx shall have the right to control any such Product Claim, using counsel of its own choice. In the event of a Product Claim, IntelGenx shall cooperate fully with Par, including, if a party in such Product Claim, the furnishing of a power of attorney to defend IntelGenx in such litigation in IntelGenx name and/or being named as a party for the purposes of any cross claim or counterclaim, and Par shall keep IntelGenx and/or IntelGenx designated legal counsel reasonably informed as to the progress of such action. Neither Party shall enter into any settlement of a Product Claim, without the prior written consent of the other, such consent not to be unreasonably withheld, delayed or conditioned.
9.5 Exclusive Remedy. The rights of the Par Indemnitees and the IntelGenx Indemnitees under this Article 9 shall be the sole and exclusive remedy of the Par Indemnitees and the IntelGenx Indemnitees, as the case may be, with respect to matters covered hereunder.
ARTICLE 10. LIMITATION OF LIABILITY
NOTWITHSTANDING ANYTHING TO THE CONTRARY IN THIS AGREEMENT, EXCEPT WITH RESPECT TO A BREACH OF ARTICLE 7 HEREOF AND EXCEPT WITH RESPECT TO AMOUNTS PAYABLE ON LIABILITIES PURSUANT TO THE INDEMNIFICATION OBLIGATIONS SET FORTH IN ARTICLE 9, NO PARTY SHALL BE LIABLE TO THE OTHER FOR ANY CONSEQUENTIAL, INCIDENTAL OR INDIRECT DAMAGES, INCLUDING FOR LOST PROFITS, OR LOSS OF OPPORTUNITY OR USE OF ANY KIND SUFFERED BY THE A PARTY, WHETHER IN CONTRACT, TORT OR OTHERWISE.
ARTICLE 11. TERM AND TERMINATION
11.1 Term. Unless earlier terminated pursuant to this Article 11, the term of this Agreement shall continue in force from the Effective Date until the latter of (a) the end of the commercial life of the Product or AG Product or (b) the date that is ten (10) years following the earlier of Commercial Launch and the first commercial sale of an AG Product by Par, its Affiliate or a permitted sublicensee (the “Term”).
11.2 Termination for Breach. Either Party may terminate this Agreement, or suspend performance under this Agreement upon written notice to the other Party at any time during the Term of this Agreement, if the other Party is in material breach of this Agreement and such other Party has not cured such material breach within forty-five (45) days after notice requesting cure of the breach; provided, however, that if the pertinent breach is not capable of cure within forty- five (45) days, but is capable of cure, and the breaching Party has promptly commenced, and is and continues diligently pursuing in good faith the remedy of any such breach, then such cure period shall be extended for such period as may be reasonably required to effectuate such cure; providedfurther, however, that if such breach is not capable of cure, the non-breaching Party may terminate this Agreement, or suspend performance under this Agreement immediately by delivery of written notice thereof to such breaching Party.
23
[EXECUTION COPY]
11.3 Termination by Par.
11.3.1 Par may terminate this Agreement upon delivery of written notice to IntelGenx if:
(a) the Pilot Bioequivalence Study is deemed unsuccessful in accordance with Section 2.4.2, and IntelGenx conducts an additional Pilot Bioequivalence Study that is also unsuccessful (as determined in accordance with Section 2.4.2).
(b) the Pivotal Bioequivalence Study fails to demonstrate that the Product is bioequivalent to the Brand Product and (i) Par does not elect to conduct an additional Pivotal Bioequivalence Study pursuant to Section 2.4.3 within sixty (60) days after such failure or (ii) after such election, such additional Pivotal Bioequivalence Study fails again to demonstrate that the Product is bioequivalent to the Brand Product;
(c) Par is not the sole First Applicant with respect to the Product ANDA;
(d) at any time after the conclusion of Patent Litigation, the Product has become economically unviable; or
(e) following Commercial Launch, total Net Profits reach a level that is equal to or less than fifteen percent (15%) of Par’s (and its Affiliates’) Net Sales of the Products and such conditions persist for a period of two (2) or more consecutive Calendar Quarters;
and, in each case, Par is not, at the time, pursuing the commercial sale of an AG Product.
11.4 Effect of Expiration or Termination. Expiration of the Term or termination of this Agreement for any reason shall be without prejudice to:
11.4.1 IntelGenx’s right to receive all payments due and payable from Par as of the effective date of such termination, if any, pursuant to the terms of this Agreement;
11.4.2 Par’s right to sell, at its option, the Product remaining in its inventory at the time of termination (in which event, Net Profits on such sales shall continue to be shared as set forth above in Section 5.5); and
11.4.3Any other legal, equitable, or administrative remedies as to which either Party is or may become entitled.
11.4.4 In the event that Par wishes to terminate this Agreement pursuant to Section 11.3.1(e), Par’s written notice thereof shall be deemed an offer by Par to transfer its right, title, interest, ownership and/or control of the Product ANDA and all Intellectual Property to the extent solely and exclusively related to the Product to IntelGenx; and IntelGenx shall have the right, at its sole discretion, to accept such offer by delivering written notice thereof within twenty (20) business day following receipt of such Termination Notice. In the event of such acceptance, (i) IntelGenx shall, subject to Section 11.5 (as applicable), (x) assume and/or be
24
[EXECUTION COPY]
responsible for, at its own expense, all activities necessary to continue the commercialization the Product, as well as any Liabilities deriving therefrom, including the obligation to defend, indemnify and hold harmless each Par Indemnitee from any Liabilities asserted against Par for such commercialization by IntelGenx, and (y) pay Par a royalty equal to [***] of net amount received by IntelGenx from the sale of the Product; and (ii) Par shall have no further obligation to indemnify IntelGenx pursuant to Section 9.2 or 9.3. Each Party shall reasonably cooperate with each other in connection herewith, including negotiating in good faith appropriate documentation addressing the provisions in this Section 11.4.4.
11.5 Survival. In addition to specific indications throughout this Agreement that Articles and Sections of this Agreement shall survive expiration and termination of this Agreement, Articles 1, 7, 8, 9, 10, 12, 13, Sections 5.5.3, 5.5.4, 11.4, this Section 11.5, 11.6, and any other provisions necessary and proper to give effect to the intention of the Parties as to the effect of the Agreement after termination shall survive any expiration or termination of this Agreement. In addition, unless otherwise expressly set forth herein, no expiration or termination of this Agreement shall have any effect on any payment, obligation accruing or arising prior to such expiration or termination.
11.6 Accrued Rights and Surviving Obligations. The termination of this Agreement for any reason or expiration of the Term shall be without prejudice to any rights that shall have accrued to the benefit of either Party prior to such termination or expiration, including any damages arising from any breach hereunder. Such termination or expiration shall not relieve either Party from obligations which are expressly indicated to survive termination or expiration of this Agreement.
ARTICLE 12. INSURANCE
Each Party shall obtain and maintain at all times during the Term, prudent comprehensive general liability coverage appropriate to its activities with reputable and financially secure insurance carriers to cover its activities related to this Agreement. Additionally such insurance coverage shall include product liability coverage of an appropriate amount, not less than five million US dollars ($5,000,000) per occurrence, for so long as the Product is being sold pursuant to this Agreement.
ARTICLE 13. MISCELLANEOUS
13.1 Interpretation and Construction. Unless the context of this Agreement otherwise requires, (i) the terms “include,” “includes,” or “including” shall be deemed to be followed by the words “without limitation” unless otherwise indicated; (ii) words using the singular or plural number also include the other; (iii) the terms “hereof,” “herein,” “hereby,” and derivative or similar words refer to this entire Agreement; (iv) the terms “Article,” “Section” and “Exhibit” refer to the specified Article, Section and Exhibit of this Agreement, and (v) words of any gender include each other gender. Whenever this Agreement refers to a number of days, unless otherwise specified, such number shall refer to calendar days. The headings and paragraph captions in this Agreement are for reference and convenience purposes only and shall not affect the meaning or interpretation of this Agreement. This Agreement shall
25
[EXECUTION COPY]
not be interpreted or constructed in favor of or against either Party because of its effort in preparing it.
13.2 Independent Contractor Status. It is understood and agreed that nothing in this Agreement nor any agreements related hereto is intended to nor shall create a partnership between the Parties. The Parties are independent contractors and are engaged in the operation of their own respective businesses, and neither Party is to be considered the agent, partner, joint venturer or employee of the other Party for any purpose whatsoever and neither Party shall have any authority to enter into any contracts or assume any obligations for the other Party nor make any warranties or representations on behalf of that other Party.
13.3 Waiver. The waiver by either Party of a breach of any provision contained herein shall be in writing and shall in no way be construed as a waiver of any succeeding breach of such provision or the waiver of the provision itself.
13.4 Assignment. This Agreement shall be binding upon and inure to the benefit of each of the Parties and their respective successors and approved assigns; provided, however, that IntelGenx may not assign this Agreement without the prior written consent of Par, unless such assignment is in connection with a merger or acquisition or sale of all or substantially all of the assets of IntelGenx to which this Agreement relates. Par may assign this agreement at its sole discretion, subject to Section 3.1. Without in anyway limiting the preceding, each Party shall provide notice of any assignment of this Agreement to the other Party. Any assignment of this Agreement not in accordance with this provision shall be null and void.
13.5 Modification. This Agreement may not be changed, modified, amended or supplemented except by an express written instrument signed by both Parties.
13.6 Severability. If any provision of this Agreement shall be held illegal or unenforceable, such provision shall be limited or eliminated to the minimum extent necessary so that this Agreement shall otherwise remain in full force and effect and enforceable.
13.7 Further Assurances and Litigation Cooperation. Each Party hereto agrees to execute, acknowledge and deliver such further instruments and documents, and to do all such other acts, as may be reasonably necessary or appropriate in order to carry out the purposes and intent of this Agreement. Each Party shall invoice the other Party for all charges, costs and expenses which are the responsibility of the other Party, which shall be paid within thirty (30) days of receipt of such invoice. Each Party hereto agrees to provide all reasonable cooperation to the other Party, including providing documents and making its employees (and former employees) and contractors available for discussion and available for testimony, in connection with any litigation or regulatory proceedings (including citizens petitions) related to the Product or related Third Party products (such as competing products).
13.8 Notices. Any notice or other communication to be given under this Agreement by any Party to any other Party shall be in writing and shall be either (a) personally delivered, (b) mailed by registered or certified mail, postage prepaid with return receipt requested, (c) delivered by overnight express delivery service or same-day local courier service, or (d) delivered by telex or facsimile transmission (followed by a copy by the preceding (a), (b) or
26
[EXECUTION COPY]
(c)), to the address of the applicable Party as set forth below, or to such other address as may be designated by the Parties from time to time in accordance with this Section 13.8. Notices delivered personally, by overnight express delivery service or by local courier service shall be deemed given as of actual receipt. Mailed notices shall be deemed given three (3) business days after mailing. Notices delivered by telex or facsimile transmission shall be deemed given upon receipt by the sender of the answerback (in the case of a telex) or transmission confirmation (in the case of a facsimile transmission) if transmitted before 5:00 p.m. (recipient’s local time) on a business day, and otherwise on the following business day.
|
|
If to IntelGenx:
|
IntelGenx Corp.
|
|
|
0000 Xxxxxx
|
|
|
Xxxxx Xx-Xxxxxxx (Xxxxxx) X0X 0X0
|
|
|
Xxxxxx
|
|
|
Attention: President and CEO
|
|
|
Facsimile Number: (000) 000-0000
|
|
|
If to Par:
|
Par Pharmaceutical, Inc.
|
|
|
000 Xxxx Xxxxxxxxx
|
|
|
Xxxxxxxxx Xxxx, XX 00000
|
|
|
Attention: General Counsel
|
|
|
Facsimile Number: (000) 000-0000
|
13.9 Governing Law and Jurisdiction. This Agreement shall be governed by and construed in accordance with the laws of the State of New York without regard to the conflicts of law provisions thereof with the exception of Sections 5-1401 and 5-1402 of the New York General Obligations Law. The Parties irrevocably agree that the State and Federal Courts located in the State, City, and County of New York, shall have exclusive jurisdiction to deal with any disputes arising out of or in connection with this Agreement and that venue is proper in such Courts. Each Party hereby expressly consents and submits to the personal jurisdiction of Federal and State Courts in the State, City and County of New York. The UN Convention on Contracts for the International Sale of Goods shall not apply to this Agreement.
13.10 Force Majeure. A Party shall not be liable for nonperformance or delay in performance to the extent that such nonperformance or delay in performance is not due to its negligence and is caused by any event reasonably beyond the control of such Party, including wars, hostilities, revolutions, riots, civil commotion, national emergency, unavailability of supplies, epidemics, fire, flood, earthquake, force of nature, explosion, terrorist act, embargo, or any other Act of God, or any law, proclamation, regulation, ordinance, or other act or order of any court, Governmental Authority (each a “Force Majeure Event”). In the event that either Party is prevented from discharging its obligations under this Agreement on account of a Force Majeure Event, such Party shall notify the other forthwith, and shall nevertheless use Commercially Reasonable Efforts to discharge its said obligations, even if in a partial or compromised manner. If either Party is unable to perform its obligations hereunder as a result of a Force Majeure Event for a period of nine (9) months or greater, then the other Party shall have the right, upon its issuance of notice to the other Party, to terminate this Agreement.
27
[EXECUTION COPY]
13.11 Entire Agreement. This Agreement and any Exhibits attached hereto, constitute the entire agreement between Par and IntelGenx with respect to the Product and AG Product and supersede all prior representations, understandings and agreements with respect to such Product and AG Product. This Agreement and any Exhibits attached hereto shall prevail over those of any purchase order, agreement, or other document or understanding of any kind pertaining to such sale.
13.12 Counterparts. This Agreement may be executed in one or more counterparts, including by transmission of facsimile or PDF copies of signature pages, each of which shall for all purposes are deemed to be an original and all of which shall constitute on instrument.
13.13 Third Party Beneficiaries. Except as expressly provided herein, nothing in this Agreement, either express or implied, is intended to or shall confer upon any Third Party any legal or equitable right, benefit or remedy of any nature whatsoever under or by reason of this Agreement.
13.14 Cumulative Rights. The rights and remedies of each of the Parties under or pursuant to this Agreement are cumulative, may be exercised as often as such Party considers appropriate and are in addition to its rights and remedies under general law.
[Signature page follows]
28
[EXECUTION COPY]
IN WITNESS WHEREOF, the Parties hereto have executed this Development Services and Commercialization Agreement to be effective as of the Effective Date.
PAR PHARMACEUTICAL, INC.
By:____________________________________
Xxxx X. Xxxxxxxxxx, Chief Operating Officer
INTELGENX CORP.
By:_____________________________________
Xxxxx Xxxxx, Chief Executive Officer
Exhibit A
Listing of Activities Associated with the Development of an ANDA
1. Reference Listed Drug evaluation
|
|
a.
|
Drug product literature search
|
|
|
b.
|
Physico-chemical characterization of XX
|
|
|
x.
|
Perform 3 month elevated temperature stability tests if deemed necessary
|
|
|
d.
|
Evaluate innovator container/closure system
|
|
|
e.
|
Evaluate RLD impurity, stability profile evaluation (exposure to heat, light, oxygen, acid and base)
|
|
|
f.
|
Define packaging component specifications
|
2. Analytical Development
|
|
a.
|
Develop stability indicating assay methods for active ingredients, and other specific excipients, where possible
|
|
|
b.
|
Validate methods and provide associated methods validation reports
|
|
|
c.
|
Author all analytical test procedures for raw materials, packaging components and finished product
|
3. Container/Closure System Evaluation
|
|
a.
|
Review supplier specifications for all packaging (container/closure, filler, desiccant) components
|
|
|
b.
|
Establish packaging components (container/closure filler, desiccant) specifications
|
|
|
c.
|
Perform container/closure integrity studies and issue final report
|
|
|
d.
|
Perform light penetration studies, where applicable, and issue final report
|
4. Raw Materials and packaging materials
|
|
a.
|
Determine level of impurities/degradants allowed for active drug substance
|
|
|
b.
|
Establish incoming specifications for raw materials, packaging components, and labeling
|
5. Drug Development
|
|
a.
|
Develop the formulation composition and process, identifying critical processing parameters
|
|
|
b.
|
Establish master batch process (“Master Formula”), having all elements needed to assure compliance with cGMPs
|
|
|
c.
|
Develop processing narrative with key aspects for the production process
|
[EXECUTION COPY]
|
|
d.
|
Develop product stability criteria and provide justification for all stability criteria
|
|
|
e.
|
Establish developmental and commercial stability protocols
|
|
|
f.
|
Perform comparative impurity assessment between innovator and proposed product if required to justify stability of the product
|
|
|
g.
|
Perform a literature based product safety assessment (required when product impurity profiles exceed or differ from that of the innovator)
|
|
|
h.
|
Establish physicochemical equivalence between the product and RLD
|
|
|
i.
|
Provide a comparison of the qualitative/quantitative composition of proposed product and RLD formulation
|
|
|
j.
|
Write Product Development Report explaining the development approach justifying API grade, excipient, process, process parameters, and batch size.
|
|
|
k.
|
Provide assistance during the PAI, if needed.
|
|
|
l.
|
Provide technical support as needed during patent litigation.
|
6. Bioequivalence Pilot Study
|
|
a.
|
Evaluate and Recommend bioequivalence pilot study design to improve probability of success.
|
2
[EXECUTION COPY]
Exhibit B
Technology Transfer Materials
1. Information about raw materials including quantities and grades
2. Analytical method validated for finished product
3. Formulation for high and low dose
4. Ink and packaging identification (to be performed in collaboration with LTS)
5. Formulation development report
6. Process flow diagram
7. Informal stability data
3
[EXECUTION COPY]
Exhibit C
Net Profit Report
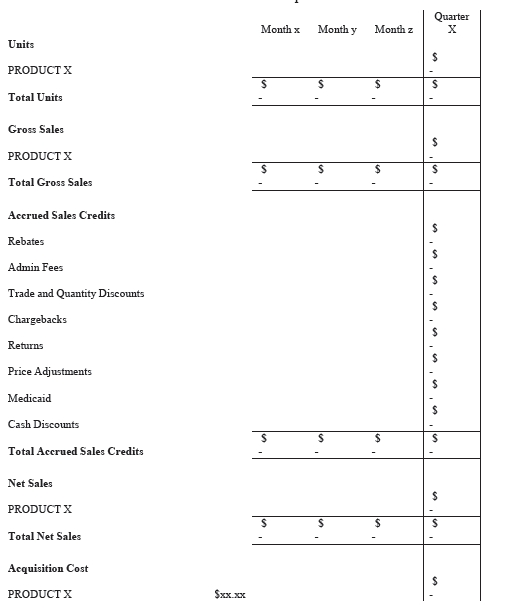
4
[EXECUTION COPY]
 5
5
