Contract

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. RESEARCH COLLABORATION AND LICENSE AGREEMENT by and between PFIZER INC. and CODEX DNA, INC. DECEMBER 20, 2021

Exhibit 10.21 i TABLE OF CONTENTS1 1. DEFINITIONS AND INTERPRETATION. ...................................................................... 1 1.1. Defined Terms ........................................................................................................ 1 1.2. Interpretation ......................................................................................................... 14 2. LICENSE GRANTS AND TECHNOLOGY TRANSFER. ............................................. 15 2.1. Reciprocal Non-Exclusive Research Program Licenses ....................................... 15 2.2. Non-Exclusive Commercial License under Codex Technology from Codex to Pfizer ..................................................................................................................... 15 2.3. Output Materials ................................................................................................... 15 2.4. Exclusive Option ................................................................................................... 16 2.5. Unblocking License .............................................................................................. 16 2.6. Right of Reference ................................................................................................ 16 2.7. Permitted Sublicensees ......................................................................................... 17 2.8. Direct Licenses to Affiliates ................................................................................. 17 2.9. No Implied Rights ................................................................................................. 17 2.10. Retained Rights ..................................................................................................... 18 3. PAYMENTS BY PFIZER TO CODEX ........................................................................... 18 3.1. Upfront Payment ................................................................................................... 18 3.2. Technical Milestone Payments ............................................................................. 18 3.3. Development Milestones ...................................................................................... 18 3.4. Sales Milestone Payments..................................................................................... 20 3.5. Products in Both Exclusive and Non-Exclusive Fields ........................................ 22 3.6. Milestone Payment Adjustment; Additional Payments for Previously Achieved Milestones ............................................................................................................. 22 3.7. Royalty Payments ................................................................................................. 22 3.8. Reports and Payments ........................................................................................... 24 3.9. Payment Terms ..................................................................................................... 25 4. RESEARCH PROGRAM. ................................................................................................ 28 4.1. Scope of Research ................................................................................................. 28 4.2. Research Plan ........................................................................................................ 28 4.3. Allocation of Responsibilities ............................................................................... 28

Exhibit 10.21 ii 4.4. Research Program Governance ............................................................................. 28 4.5. Research Term Extension ..................................................................................... 31 4.6. Research Program Expenses ................................................................................. 31 4.7. Scientific Records ................................................................................................. 31 4.8. Delegation and Subcontracting ............................................................................. 31 4.9. Transfer of Pfizer Materials .................................................................................. 32 5. PRODUCT DEVELOPMENT AND COMMERCIALIZATION. .................................. 34 5.1. General .................................................................................................................. 34 5.2. Regulatory Approvals ........................................................................................... 34 5.3. Commercialization Activities ............................................................................... 34 5.4. Manufacturing ....................................................................................................... 34 5.5. Reporting............................................................................................................... 34 5.6. Other Pfizer Matters .............................................................................................. 34 6. INTELLECTUAL PROPERTY ....................................................................................... 35 6.1. Ownership of Intellectual Property ....................................................................... 35 6.2. Patent Rights ......................................................................................................... 36 6.3. Enforcement and Defense of Patent Rights .......................................................... 38 6.4. Codex Third Party Agreements ............................................................................ 40 7. CONFIDENTIALITY....................................................................................................... 41 7.1. Confidentiality ...................................................................................................... 41 7.2. Authorized Disclosure .......................................................................................... 41 7.3. SEC Filings and Other Disclosures....................................................................... 42 7.4. Residual Knowledge Exception ............................................................................ 42 7.5. Public Announcements; Publications ................................................................... 43 7.6. Obligations in Connection with Change of Control ............................................. 43 8. REPRESENTATIONS, WARRANTIES AND COVENANTS. ..................................... 44 8.1. Mutual Representations and Warranties ............................................................... 44 8.2. Mutual Covenants ................................................................................................. 44 8.3. Representations and Warranties of Codex ............................................................ 44 8.4. Codex Covenants .................................................................................................. 47 8.5. Representation by Legal Counsel ......................................................................... 48 8.6. Disclaimer ............................................................................................................. 48

Exhibit 10.21 iii 9. TERM AND TERMINATION ......................................................................................... 49 9.1. Term ...................................................................................................................... 49 9.2. Termination by Codex .......................................................................................... 49 9.3. Termination by Pfizer ........................................................................................... 49 9.4. Effects of Termination .......................................................................................... 50 9.5. Provision for Insolvency ....................................................................................... 52 10. LIMITATION ON LIABILITY, INDEMNIFICATION AND INSURANCE. ............... 53 10.1. No Consequential Damages .................................................................................. 53 10.2. Indemnification by Pfizer ..................................................................................... 54 10.3. Indemnification by Codex..................................................................................... 54 10.4. Procedure .............................................................................................................. 55 10.5. Insurance ............................................................................................................... 56 11. ANTI-BRIBERY/ANTI-CORRUPTION. ........................................................................ 56 11.1. Foreign Corrupt Practices Act .............................................................................. 56 11.2. Representations and Warranties ............................................................................ 57 11.3. Use of Funds by Codex; Audit by Pfizer .............................................................. 57 12. MISCELLANEOUS. ........................................................................................................ 58 12.1. Assignment ........................................................................................................... 58 12.2. Change of Control of Codex ................................................................................. 58 12.3. Further Actions ..................................................................................................... 58 12.4. Force Majeure ....................................................................................................... 58 12.5. Notices .................................................................................................................. 58 12.6. Amendment ........................................................................................................... 59 12.7. Waiver ................................................................................................................... 59 12.8. Severability ........................................................................................................... 60 12.9. Descriptive Headings ............................................................................................ 60 12.10. Global Trade Control Laws .................................................................................. 60 12.11. Export Control ...................................................................................................... 61 12.12. Dispute Resolution ................................................................................................ 61 12.13. Governing Law ..................................................................................................... 62 12.14. Consent to Jurisdiction .......................................................................................... 62 12.15. Entire Agreement .................................................................................................. 62 12.16. Independent Contractors ....................................................................................... 62

Exhibit 10.21 iv 12.17. Counterparts .......................................................................................................... 62 12.18. No Third Party Rights or Obligations ................................................................... 62

Exhibit 10.21 v EXHIBITS Exhibit A Codex Patent Rights Existing as of the Effective Date Exhibit B Research Plan Exhibit C Pfizer Anti-Bribery and Anti-Corruption Principles SCHEDULES Schedule 3.7 Sample Royalty Calculation Schedule 7.5.1 Press Release Schedule 8.3 Codex’s Knowledge Parties

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 1 RESEARCH COLLABORATION AND LICENSE AGREEMENT This Research Collaboration and License Agreement (the “Agreement”) is entered into as of December 20, 2021 (the “Effective Date”), by and between PFIZER INC., a corporation organized and existing under the laws of Delaware and having a principal place of business at ▇▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇ (“Pfizer”) and CODEX DNA, Inc., a corporation organized and existing under the laws of Delaware and having a principal place of business at ▇▇▇▇ ▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ ▇▇, ▇▇▇ ▇▇▇▇▇, ▇▇ ▇▇▇▇▇ (“Codex”). Pfizer and Codex may each be referred to herein individually as a “Party” and collectively as the “Parties.” WHEREAS, Codex owns or otherwise controls certain patents, patent applications, technology, know-how, scientific and technical information and other proprietary rights and information relating to Instruments and Methods (each as defined below); WHEREAS, Pfizer has extensive experience and expertise in the development, manufacturing and commercialization of biopharmaceutical products, including mRNA vaccine products; WHEREAS, [***] (“Initial Instruments”), along with any instructions, methods, processes, workflows and other techniques or protocols for use of the Initial Instrument(s) that were provided to Pfizer to use such Initial Instruments (“Initial Methods”); WHEREAS, the Parties wish to collaborate to develop the Deliverables (as defined below) to synthesize DNA and RNA to meet the requirements described in the Research Plan (defined below); WHEREAS, subject to the terms of this Agreement, Codex wishes to grant to Pfizer, and Pfizer wishes to receive from Codex, a non-exclusive license in the Field (as defined below), with the exclusive option to an exclusive license in each Exclusive Field (as defined below) in the Territory (as defined below) under certain of Codex’s patents, patent applications, technology, know-how, scientific and technical information and other proprietary rights and information relating to the Deliverables to use, research, develop, manufacture, commercialize and otherwise exploit Products for the Field, including in each Exclusive Field, in each case in the Territory; NOW THEREFORE, in consideration of the mutual promises and covenants set forth below and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties hereby agree as follows: 1. DEFINITIONS AND INTERPRETATION. 1.1. Defined Terms. Capitalized terms used and not otherwise defined in this Agreement shall have the following meanings: 1.1.1. “Acquiring Entity” means (a) a Third Party that merges or consolidates with or acquires a Party, or to which a Party transfers all or substantially all of its assets to

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 2 which this Agreement pertains in a Change of Control transaction and (b) any Affiliate of such Third Party prior to the transaction described in clause (a) that is not also an Affiliate of the relevant Party prior to the transaction described in clause (a). 1.1.2. “Affiliate” means any entity directly or indirectly controlled by, controlling, or under common control with, a Person, but only for so long as such control will continue. For purposes of this definition, “control” (including, with correlative meanings, “controlled by”, “controlling” and “under common control with”) means (a) possession, direct or indirect, of the power to direct or cause direction of the management or policies of an entity (whether through ownership of securities or other ownership interests, by contract or otherwise), or (b) beneficial ownership of more than 50% (or the maximum ownership interest permitted by applicable Law) of the voting securities or other ownership or general partnership interest (whether directly or pursuant to any option, warrant or other similar arrangement) or other comparable equity interests of an entity; provided, however, that where an entity owns a majority of the voting power necessary to elect a majority of the board of directors or other governing board of another entity, but is restricted from electing such majority by contract or otherwise, such entity will not be considered to be in control of such other entity until such time as such restrictions are no longer in effect. 1.1.3. “Bankruptcy Code” means Title 11 of the United States Code, as amended. 1.1.4. “Binding Obligation” means, with respect to a Party (a) any oral or written agreement or arrangement that binds such Party, including any assignment, license agreement, loan agreement, guaranty, or financing agreement, (b) the provisions of such Party’s charter, bylaws or other organizational documents or (c) any order, writ, injunction, decree or judgment of any court or Governmental Authority entered against such Party or by which any of such Party’s operations or property are bound. 1.1.5. “Biosimilar Version” means, with respect to a Product that is being sold in a country or regulatory jurisdiction in the Territory (the “Reference Product”), a biopharmaceutical product sold by a Third Party (other than a Third Party acting on behalf of or in concert with Pfizer or any Affiliate or Sublicensee of Pfizer) in such country or regulatory jurisdiction in the Territory that through reference to the Regulatory Approval of the Reference Product, is eligible for and has achieved Regulatory Approval in such country or regulatory jurisdiction pursuant to an abbreviated follow-on biological approval pathway established by the Regulatory Authority in such country or regulatory jurisdiction pursuant to the applicable Law, or otherwise is approved for marketing and sale in such country or regulatory jurisdiction by an abridged procedure in reliance, in whole or in part, on the prior Regulatory Approval of the Reference Product or on the safety and efficacy data generated for the prior Regulatory Approval (in such country or regulatory jurisdiction) of the Reference Product, including any such biopharmaceutical product that (i) with respect to such biopharmaceutical product in the United States, has been approved

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 3 as a biosimilar or interchangeable product by the FDA pursuant to 42 U.S.C. § 262 of the Public Health Service Act, (ii) with respect to such biopharmaceutical product subject to the regulatory jurisdiction of the EMA, has been approved as a similar biological medicine product by EMA as described in CHMP/437/04, issued 30 October 2005, as may be amended, or any subsequent or superseding law, statute or regulation or (iii) with respect to such biopharmaceutical product outside the United States and in a country which is not subject to the regulatory jurisdiction of the EMA, has otherwise obtained Regulatory Approval from a Regulatory Authority pursuant to similar statutory or regulatory requirement as that described in the foregoing subsections (i) and (ii) in such other country or regulatory jurisdiction in the Territory. 1.1.6. “Business Day” means a day other than a Saturday, Sunday or bank or other public holiday in New York, New York. 1.1.7. “Calendar Quarter” means the respective periods of three consecutive calendar months ending on March 31, June 30, September 30 and December 31. 1.1.8. “Calendar Year” means any twelve (12) month period beginning on January 1 and ending on the next subsequent December 31. 1.1.9. “Change of Control” means, with respect to a Party (a) the acquisition of beneficial ownership, directly or indirectly, by any Person (other than such Party or an Affiliate of such Party, and other than by virtue of obtaining irrevocable proxies) of securities or other voting interest of such Party representing a majority or more of the combined voting power of such Party’s then outstanding securities or other voting interests, (b) any merger, reorganization, consolidation or business combination involving such Party with a Third Party that results in the holders of beneficial ownership (other than by virtue of obtaining irrevocable proxies) of the voting securities or other voting interests of such Party (or, if applicable, the ultimate parent of such Party) immediately prior to such merger, reorganization, consolidation or business combination ceasing to hold beneficial ownership of at least [***] of the combined voting power of the surviving entity immediately after such merger, reorganization, consolidation or business combination, (c) any sale, lease, exchange, contribution or other transfer (in one transaction or a series of related transactions) of all or substantially all of the assets of such Party to which this Agreement relates, other than a sale or disposition of such assets to an Affiliate of such Party or (d) the approval of any plan or proposal for the liquidation or dissolution of such Party (other than in circumstances where such Party is deemed a Debtor pursuant to Section 9.5). 1.1.10. “Clinical Trial” means a human clinical study conducted on sufficient numbers of human subjects that is designed to (a) establish that a pharmaceutical product is reasonably safe for continued testing, (b) investigate the safety and efficacy of the pharmaceutical product for its intended use, and to define warnings, precautions and adverse reactions that may be associated with the pharmaceutical product in the dosage

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 4 range to be prescribed or (c) support Regulatory Approval of such pharmaceutical product or label expansion of such pharmaceutical product. 1.1.11. “Codex Know-How” means any (a) Codex Sole Research Plan Know- How and Codex’s interest in any Joint Research Plan Know-How and (b) other Know-How that is (i) Controlled by Codex or any of its Affiliates as of the Effective Date or that comes into the Control of Codex or any of its Affiliates during the Term (other than through the grant of a license by Pfizer) and (ii) reasonably necessary for Pfizer to implement the Deliverables, reasonably necessary for Pfizer to practice the licenses or exercise other rights granted to Pfizer under this Agreement, or reasonably necessary for Pfizer to conduct activities under the Research Plan or (c) other Know-How that is otherwise provided by or on behalf of Codex to Pfizer hereunder. 1.1.12. “Codex Patent Right” means any (a) Codex Sole Research Plan Patent Right and Codex’s interest in any Joint Research Plan Patent Rights and (b) any other Patent Right that (i) is Controlled by Codex or any of its Affiliates as of the Effective Date (including the Codex Patent Rights listed in Exhibit A) or (ii) that comes into the Control of Codex or any of its Affiliates during the Term (other than through the grant of a license by Pfizer) and, in each case ((i) and (ii)), claims or discloses any invention included in any Codex Know-How. 1.1.13. “Codex Sole Research Plan Know-How” means any Research Plan Know- How that is invented solely by or on behalf of Codex or its Affiliates in the course of performing activities under the Research Plan; provided that Codex Sole Research Plan Know-How excludes the Output Materials Know-How and Pfizer Material Improvements. 1.1.14. “Codex Sole Research Plan Patent Right” means any Patent Right that claims or discloses any invention included in any Codex Sole Research Plan Know-How. 1.1.15. “Codex Technology” means any Codex Know-How and Codex Patent Rights. 1.1.16. “Codex Third Party Agreement” means any agreement between Codex (or any of its Affiliates) and any Third Party (such Third Party, a “Third Party Licensor”) pursuant to which Codex or any of its Affiliates obtains Control of any of the Codex Technology. 1.1.17. “Commercialize” or “Commercializing” means, with respect to a compound or product, to (a) market, promote, distribute, offer for sale, sell, have sold, import, have imported, export, have exported or otherwise commercialize such a compound or product and (b) conduct discovery, pre-clinical, research, or other Development activities with respect to a compound or product after such compound or product has received Regulatory Approval. When used as a noun, “Commercialization” means any and all activities involved in Commercializing.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 5 1.1.18. “Commercially Reasonable Efforts” means, with respect to the efforts to be expended by a Party with respect to any objective, those reasonable, good faith efforts to accomplish such objective as such Party would normally use to accomplish a similar objective under similar circumstances. 1.1.19. “Confidential Information” means, with respect to each Party, all Know- How or other information, including proprietary information and materials (whether or not patentable) regarding or embodying such Party’s or its Representatives’ technology, products, business information or objectives, that is communicated by or on behalf of the Disclosing Party to the Receiving Party or its permitted recipients, but only to the extent that such Know-How or other information in written form is marked in writing as “confidential” at the time of disclosure, and such Know-How or other information disclosed orally or in non-tangible form is (a) identified by the Disclosing Party as “confidential” at the time of disclosure and (b) within [***] days thereafter, the Disclosing Party provides a written summary of such Know-How or other information marked as “confidential”. Failure to ▇▇▇▇ or identify or summarize Confidential Information disclosed hereunder as “confidential” shall not cause the information to be considered non- confidential if such information should have been known by a reasonable person with expertise on the subject matter, based on the nature of the information and the circumstances of its disclosure, to be Confidential Information, provided that the Disclosing Party has otherwise made good faith efforts to clearly so ▇▇▇▇ or identify Confidential Information as such. Confidential Information does not include any Know- How or other information that (i) was already known by the Receiving Party (other than under an obligation of confidentiality to the Disclosing Party) at the time of disclosure by or on behalf of the Disclosing Party, (ii) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party, (iii) became generally available to the public or otherwise part of the public domain after its disclosure to the Receiving Party, other than through any act or omission of the Receiving Party in breach of its obligations under this Agreement, (iv) was disclosed to the Receiving Party, other than under an obligation of confidentiality, by a Third Party who had no direct or indirect obligation to the Disclosing Party not to disclose such information to the Receiving Party or (v) was independently discovered or developed by or on behalf of the Receiving Party without the use of or reference to any Confidential Information belonging to the Disclosing Party. The terms and conditions of this Agreement shall be considered Confidential Information of both Parties. 1.1.20. “Control” or “Controlled” means with respect to any intellectual property right or material (including any Patent Right, Know-How or other data, information or material), the ability (whether by sole, joint or other ownership interest, license or otherwise, other than pursuant to this Agreement) to, without violating the terms of any agreement with a Third Party, grant a license or sublicense or provide access or other right in, to or under such intellectual property right or material. If either Party undergoes a Change of Control during the Term, any Know-How or Patent Rights of the Acquiring Entity shall not be deemed to be Controlled by such Party unless: (i) prior to the

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 6 consummation of such Change of Control, such Party or any of its Affiliates also Controlled such Know-How or Patent Rights of the Acquiring Entity, (ii) any such Know- How or Patent Rights of the Acquiring Entity arises from participation by representatives of such Acquiring Entity in any activities under this Agreement after such Change of Control or (iii) such Know-How or Patent Rights of the Acquiring Entity were not used in the performance of activities under this Agreement prior to the consummation of such Change of Control, but after the consummation of such Change of Control, such Party or any of its Affiliates uses any such Know-How or Patent Rights in the performance of its obligations or exercise of its rights under this Agreement. 1.1.21. “Core Codex DNA Technology” means Codex’s proprietary systems, platforms and technologies, including its Instruments and proprietary Methods, together with related workflows and kits/reagents, in each case as proprietary to Codex and reasonably necessary to operate an Instrument or perform any Method, as applicable, and, in each case, (a) including any intellectual property rights (including Patent Rights) therein owned or Controlled by Codex, and (b) excluding any Research Plan Technology. 1.1.22. “Cover” means, with respect to the Instrument, a Product or a Deliverable and given Patent Right, that a Valid Claim of such Patent Right would, absent a license thereunder or ownership thereof, be infringed by the use or other Exploitation of such Instrument, Product or Deliverable. 1.1.23. [***] 1.1.24. “Current Licenses” means any agreement (a) that Codex or its Affiliates has entered into prior to the Effective Date and (b) pursuant to which Codex or its Affiliates are (i) granted rights to any Codex Technology as of the Effective Date or (ii) granted a license or otherwise transferred any right to practice under any Patent Rights or Know- How, in each case that are reasonably necessary to use the Deliverables or perform activities under this Agreement. 1.1.25. “Current Licensor” means any Third Party that is a party to a Current License. 1.1.26. “Deliverables” means any tangible Instrument, Method or other Know- How first made or developed by one or both Parties in the conduct of the Research Plan that are provided to Pfizer hereunder including in accordance with Section 2.11. 1.1.27. “Develop” or “Developing” means to discover, research or otherwise develop a process, compound or product, including conducting non-clinical and clinical research and development activities prior to Regulatory Approval. When used as a noun, “Development” means any and all activities involved in Developing. 1.1.28. “Development Milestone Payment” means any amounts payable by Pfizer upon achievement of any Development Milestones in accordance with Section 3.3.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 7 1.1.29. “DNA” means deoxyribonucleic acid. 1.1.30. “Exclusive Fields” means (a) the [***] or (b) [***], in each case (a) and (b), if Pfizer has exercised the Option for such under Section 2.4, in each case (a) and (b) unless and until such Field has converted to a Non-Exclusive Field pursuant to this Agreement (for example, under Sections 5.2 or 9.4.1). Each of the [***] and the [***] is an Exclusive Field once Pfizer has exercised the Option for such Field under Section 2.4. 1.1.31. “Exploit” means to Develop, Manufacture, Commercialize, use or otherwise exploit. Cognates of the word “Exploit” will have correlative meanings. 1.1.32. “FD&C Act” means the United States Federal Food, Drug, and Cosmetic Act, as amended, and the rules and regulations promulgated thereunder. 1.1.33. “FDA” means the United States Food and Drug Administration or any successor agency thereto. 1.1.34. “Field” means [***]. 1.1.35. “First Commercial Sale” means, with respect to any Product and with respect to any country in the Territory, the first sale of such Product (as applicable) by Pfizer or an Affiliate or Sublicensee of Pfizer to a Third Party in such country after such Product has been granted Regulatory Approval by the appropriate Regulatory Authority in such country. 1.1.36. [***] 1.1.37. “GAAP” means United States generally accepted accounting principles, consistently applied. 1.1.38. “Government Official”, to be broadly interpreted, means (a) any elected or appointed government official (e.g., a member of a ministry of health), (b) any employee or person acting for or on behalf of a government official, Governmental Authority, or other enterprise performing a governmental function, (c) any political party, candidate for public office, officer, employee, or person acting for or on behalf of a political party or candidate for public office, and (d) any employee or person acting for or on behalf of a public international organization (e.g., the United Nations). For clarity, healthcare providers employed by government-owned hospitals will be considered Government Officials. 1.1.39. “Governmental Authority” means any court, agency, department, authority or other instrumentality of any national, state, county, city or other political subdivision.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 8 1.1.40. “IND” means an Investigational New Drug Application submitted under the FD&C Act, or an analogous application or submission with any analogous agency or Regulatory Authority outside of the United States for the purposes of obtaining permission to conduct Clinical Trials. 1.1.41. “Indication” means the intended use of a Product for either therapeutic treatment or for the prevention of a distinct illness, sickness, interruption, cessation or disorder of a particular bodily function, system, tissue type or organ, or sign or symptom of any such items or conditions, regardless of the severity, frequency or route of any treatment, treatment regimen, dosage strength or patient class, for which Regulatory Approval is being or would be sought and which will be referenced on any Product labeling in any country. Label extensions shall not be deemed to be separate Indications. By way of example, each of the following would be considered a separate Indication: breast cancer, prostate cancer, colon cancer, gastric cancer, lung cancer, etc, but moving from one line of therapy to another would not be considered a new Indication. For clarity, a single Indication would include the primary disease and all variants or sub-divisions or sub- classifications within such primary disease, and regardless of prophylactic or therapeutic use, pediatric or adult use. For further clarity, any variant or sub-division or sub- classification within a primary disease shall constitute an Indication, but shall not be a distinct Indication from any other variant or sub-division or sub-classification within such primary disease. 1.1.42. “Instrument” means Codex’s proprietary fully automated, benchtop gene synthesis workstations existing during the Research Term that synthesize de novo gene constructs from digitally submitted sequences. 1.1.43. “Instrument/Methods Know-How” means any Research Plan Know-How that is invented, developed, or discovered, by either Party alone, or jointly with the other, whether or not patentable, predominantly directed to (a) any Instrument, (b) kit or reagent for operation of an Instrument, or (c) Methods, but not any Pfizer Material Improvement or Output Materials. 1.1.44. “Instrument/Methods Patent Rights” means any Research Plan Patent Right that claims or discloses any Instrument/Methods Know-How, but not any other Research Plan Know-How, Output Materials or Pfizer Material Improvement. 1.1.45. “Joint Research Committee” or “JRC” means the joint research committee described in Section 4.4.2(a). 1.1.46. “Joint Research Plan Know-How” means Research Plan Know-How other than Output Materials Know-How and Pfizer Material Improvements that is jointly invented, developed, discovered, or other Know-How, whether or not patentable, discovered, made or created jointly by (i) Codex or its Representatives and (ii) Pfizer or its Representatives under this Agreement.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 9 1.1.47. “Joint Research Plan Patent Right” means a Patent Right that claims or discloses any Joint Research Plan Know-How (and does not claim or disclose any invention included in any Pfizer Sole Research Plan Know-How or Codex Sole Research Plan Know- How). 1.1.48. “Joint Research Plan Technology” means the Joint Research Plan Know- How and the Joint Research Plan Patent Rights. 1.1.49. “Know-How” means any proprietary invention, discovery, development, data, information, process, method, technique, material (including any chemical or biological material), technology, result, cell line, compounds, probe, sequence or other know-how, whether or not patentable, and any physical embodiments of any of the foregoing. 1.1.50. “Law” means any law, statute, rule, regulation, order, judgment or ordinance of any Governmental Authority. 1.1.51. “Major EU Market Country” means any of [***]. 1.1.52. “Major Market Country” means any [***]. 1.1.53. “Manufacture” or “Manufacturing” means to make, produce, manufacture, process, fill, finish, package, label, perform quality assurance testing, release, ship or store, and for the purposes of further Manufacturing, distribute, import or export, a compound or product or any component thereof. When used as a noun, “Manufacture” or “Manufacturing” means any and all activities involved in Manufacturing a compound, protein, device or product or any component thereof. 1.1.54. “Methods” means any instructions, methods, processes, workflows and other techniques or protocols necessary or useful for use of the Instrument. 1.1.55. “Milestone Payments” shall mean the Technical Milestone Payments, the Development Milestone Payments and the Sales Milestone Payments. 1.1.56. “Milestones” means the Technical Milestones, the Development Milestones and the Sales Milestones. 1.1.57. “Net Sales” means: (a) with respect to a Product, the gross receipts from sales by Pfizer and its Affiliates and Sublicensees of such Product to Third Parties in the Territory, less in each case (i) bad debts and (ii) sales returns and allowances actually paid, granted or accrued, including trade, quantity and cash discounts and any other adjustments, including those granted on account of price adjustments, billing errors, rejected goods, damaged or defective goods, recalls, returns, rebates, chargeback rebates, reimbursements or similar payments granted or given to wholesalers or other distributors, buying groups, health care insurance carriers, chain pharmacies, mass merchandisers, staff model HMO’s,

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 10 pharmacy benefit managers or other institutions, adjustments arising from consumer discount programs or other similar programs, customs or excise duties, sales tax, consumption tax, value added tax, and other taxes (except income taxes and all to the extent paid by Pfizer and non-refundable in accordance with applicable Law) or duties relating to sales, compulsory or negotiated payments and cash rebates in respect of sales to the United States government, any state government or any foreign government, or to any other Governmental Authority, or with respect to any government-subsidized program or managed care organization, and freight (including storage, shipping and handling) and insurance (to the extent that Pfizer, its Affiliates or its Sublicensees bear the cost of freight (including storage, shipping and handling) and insurance for such Product). For clarity, the transfer of Product by or among Pfizer, its Affiliates or Sublicensees is not considered a sale, provided (1) such transfer is intended for further sale, transfer, lease, exchange, or other disposition and Pfizer, its Affiliates or Sublicensees are not the end users or consumers, and (2) any amount received by Pfizer, its Affiliates or Sublicensees in connection with the transfer from such entity to end users shall also be deemed part of Net Sales of such Product. Net Sales will be determined from books and records maintained in accordance with GAAP, as consistently applied by Pfizer with respect to sales of the Product, as applicable. 1.1.58. “Non-Exclusive Field” means, individually and collectively, the Field outside of the Exclusive Fields, on an Indication-by-Indication basis. 1.1.59. “Option Exercise Date” means, with respect to each Option, the date on which Pfizer has delivered written notice of Pfizer’s exercise of such Option to Codex pursuant to Section 2.4. 1.1.60. “Option Exercise Period” means the period of time beginning on the Effective Date and ending on the date that is [***]. 1.1.61. “Output Materials” means any chemical or biological materials, including any mRNA or DNA that are produced or generated through or from, or are the result or by- product of, the operation of an Instrument by a Party in the conduct of and in accordance with the Research Plan. 1.1.62. “Output Materials Know-How” means any Research Plan Know-How that is invented, developed, or discovered, by either Party alone, or jointly with the other, whether or not patentable, directed to or embodied in any Output Materials and/or the use thereof. 1.1.63. “Output Materials Patent Rights” means a Patent Right that claims or discloses Output Materials Know-How, but not any other Research Plan Know-How. 1.1.64. “Patent Rights” means any and all (a) issued patents, (b) pending patent applications, including all provisional applications, substitutions, continuations, continuations-in-part, divisions and renewals, and all patents granted thereon, (c) patents-

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 11 of-addition, reissues, reexaminations and extensions or restorations by existing or future extension or restoration mechanisms, including patent term adjustments, patent term extensions, supplementary protection certificates or the equivalent thereof, (d) inventor’s certificates, (e) other forms of government-issued rights substantially similar to any of the foregoing and (f) United States and foreign counterparts of any of the foregoing. 1.1.65. “Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, incorporated association, joint venture or similar entity or organization, including a government or political subdivision or department or agency of a government. 1.1.66. “Pfizer Know-How” means any Know-How that is (a) Controlled by Pfizer or any of its Affiliates on the Effective Date or that comes into the Control of Pfizer or any of its Affiliates during the Term (other than through the grant of a license by Codex) and is (b) either reasonably necessary for Codex to conduct activities under the Research Plan or otherwise provided to Codex hereunder. 1.1.67. “Pfizer Patent Right” means any Patent Right that (a) is Controlled by Pfizer or any of its Affiliates as of the Effective Date or (b) that comes into the Control of Pfizer or any of its Affiliates during the Term (other than through the grant of a license by Codex) and, in each case ((a) and (b)), claims or discloses any invention included in any Pfizer Know-How. 1.1.68. “Pfizer Quarter” means each of the four (4) thirteen (13) week periods (a) with respect to the United States, commencing on January 1 of any Pfizer Year and (b) with respect to any country in the Territory other than the United States, commencing on December 1 of any Pfizer Year. 1.1.69. “Pfizer Sole Research Plan Know-How” means any (a) Research Plan Know-How that is invented solely by or on behalf of Pfizer or its Affiliates in the course of performing activities under the Research Plan, (b) any Pfizer Material Improvement and (c) any Output Materials Know-How. 1.1.70. “Pfizer Sole Research Plan Patent Right” means any Patent Right that claims or discloses any invention included in any Pfizer Sole Research Plan Know-How. All Pfizer Material Improvement and Output Material Patent Rights will be Pfizer Sole Research Plan Patent Rights. 1.1.71. “Pfizer Technology” means the Pfizer Know-How and Pfizer Patent Rights. 1.1.72. “Pfizer Year” means the twelve-month fiscal periods observed by Pfizer (a) commencing on January 1 with respect to the United States and (b) commencing on December 1 with respect to any country in the Territory other than the United States.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 12 1.1.73. “Phase I Clinical Trial” means a Clinical Trial (whether a Phase Ia or a Phase Ib trial) that generally provides for the first introduction into humans of a pharmaceutical product with the primary purpose of determining safety, metabolism and pharmacokinetic properties and clinical pharmacology of such product, including in a manner that is generally consistent with 21 CFR § 312.21(a), as amended (or its successor regulation), or an equivalent clinical study required by a Regulatory Authority outside of the United States; provided, however, a Phase I Clinical Trial does not include any study generally characterized by the FDA as an “exploratory IND study” in CDER’s Guidance for Industry, Investigators, and Reviewers Exploratory IND Studies, January 2006, irrespective of whether or not such study is actually performed in the United States or under an IND. A so-called Phase I/II Clinical Trial shall be deemed to be a Phase I Clinical Trial. 1.1.74. “Price Approval” means, in any country where a Governmental Authority authorizes reimbursement for, or approves or determines pricing for, pharmaceutical products, receipt (or, if required to make such authorization, approval or determination effective, publication) of such reimbursement authorization or pricing approval or determination (as the case may be). 1.1.75. “Product” means a pharmaceutical or biopharmaceutical product in a formulation suitable for administration to humans, including mRNA encoding one or more polypeptides or fragments thereof, including naturally occurring or engineered variants thereof, for prophylaxis or treatment of a disease in humans, provided that such mRNA was (a) discovered, designed, encoded or created using the Deliverables by, or on behalf of, a Party or (b) was transcribed from DNA that was discovered, designed, encoded or created using the Deliverables by, or on behalf of, a Party. For the avoidance of doubt, Instruments, Deliverable(s), and Output Materials are not Products. For further clarity and by way of example, updates or changes to (y) the mRNA of a Product to account for changes to a Product’s formulation, or (z) dosage volume will not constitute a different Product, so long as such “updated” Product is for the same Indication. 1.1.76. “Public Health Service Act” means the United States Public Health Service Act (42 U.S.C. 201 et seq), as amended from time to time (including any rules and regulations promulgated thereunder) or any subsequent or superseding law, statute or regulation. 1.1.77. “Regulatory Approval” means all technical, medical and scientific licenses, registrations, authorizations and approvals (including approvals of NDAs, supplements and amendments, pre- and post- approvals and labeling approvals) of any Regulatory Authority, necessary or useful for the use, Development, Manufacture, and Commercialization of a pharmaceutical or biopharmaceutical product in a regulatory jurisdiction, including commercially reasonable Price Approvals and commercially reasonable Third Party reimbursement approvals. For clarity, an emergency use authorization pursuant to Section 564 of the Federal Food, Drug and Cosmetic Act, 21

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 13 U.S.C.A. 301, et. Seq. and the equivalent to such authorization outside the United States (an “Emergency Use Authorization”) shall be deemed a “Regulatory Approval”. 1.1.78. “Regulatory Authority” means, with respect to a country in the Territory, any national (e.g., the FDA), supra-national (e.g., the European Commission, the Council of the European Union, or the European Medicines Agency), regional, state or local regulatory agency, department, bureau, commission, council or other Governmental Authority with authority over the distribution, importation, exportation, manufacture, production, use, storage, transport, clinical testing or sale of a pharmaceutical product (including any Product) including, to the extent required in such country, Price Approval, for pharmaceutical products in such country. 1.1.79. “Representatives” means (a) with respect to Pfizer, its Affiliates, its Sublicensees and each of their respective officers, directors, employees, consultants, contractors and agents and (b) with respect to Codex, its Affiliates and each of their respective officers, directors, employees, consultants, contractors and agents. 1.1.80. “Research Plan” means the Research Plan attached hereto as Exhibit B, as may be amended from time to time pursuant to Section 4.4.2. 1.1.81. “Research Plan Know-How” means any and all Know-How, whether or not patentable, made solely by or on behalf of either Party or its Representatives in the conduct of activities under the Research Plan or made jointly by or on behalf of (i) Codex or its Representatives and (ii) Pfizer or its Representatives, in each case, in the conduct of activities under the Research Plan. 1.1.82. “Research Plan Patent Right” means any Patent Right that claims or discloses any invention included in any Research Plan Know-How. 1.1.83. “Research Plan Technology” means any and all Research Plan Know-How or Research Plan Patent Rights. 1.1.84. “Research Term” means the period of time beginning on the Effective Date and expiring on [***] thereof or such later date as may be established pursuant to Section 4.5, unless earlier terminated pursuant to the terms of this Agreement. 1.1.85. “Residual Knowledge” means knowledge, techniques, experience and Know-How that (a) are, or are based on any Confidential Information Controlled by the Disclosing Party and (b) are retained in the unaided memory of any authorized Representative of the Receiving Party after having access to such Confidential Information in accordance with this Agreement. An individual’s memory will be considered to be unaided if the individual has not intentionally memorized the Confidential Information for the purpose of retaining and subsequently using or disclosing it.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 14 1.1.86. “RNA” means any form of ribonucleic acid, including messenger RNA (mRNA), self-amplifying RNA (▇▇▇▇▇) or modified RNA (modRNA). 1.1.87. “Royalty Term” means, with respect to any particular Product or Exclusive Product (as the case may be) in any particular country in the Territory, the period that commences on the First Commercial Sale of such Product or Exclusive Product in such country in the Territory and ends on the earliest to occur of [***]. 1.1.88. “Sales Milestone Payment” means any amounts payable by Pfizer upon achievement of any Sales Milestones in accordance with Section 3. 1.1.89. “Sublicensee” means any Person to whom Pfizer grants or has granted, directly or indirectly, a license or sublicense with respect to a Product. 1.1.90. “Taxes” means all taxes, charges, fees, levies, or other assessments, including income, withholding, excise, value added, sales, payroll, transfer, and franchise taxes imposed by any Governmental Authority. Such term shall include any interest, penalties, or additions payable in connection with such taxes, charges, fees, levies, duties, or other assessments. 1.1.91. “Territory” means worldwide. 1.1.92. “Third Party” means any Person other than Pfizer, Codex or their respective Affiliates. 1.1.93. “Trademark” means any trademark, trade name, service ▇▇▇▇, service name, brand, domain name, trade dress, logo, slogan or other indicia of origin or ownership, including the goodwill and activities associated with each of the foregoing. 1.1.94. “Valid Claim” means, with respect to a particular country, a claim of a Codex Patent Right that is (a) issued and unexpired and has not been (i) held permanently revoked, unenforceable or invalid by a decision of a court or other Governmental Authority of competent jurisdiction, which decision is unappealable or unappealed within the time allowed for appeal, or (ii) cancelled, withdrawn, abandoned, disclaimed or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise, or (b) a bona fide claim in a pending patent application that has not been (i) cancelled, withdrawn or abandoned without being refiled in another application in the applicable jurisdiction or (ii) finally rejected by an administrative agency action, which action is unappealable or unappealed within the time allowed for appeal, [***] The following terms are defined in the section of this Agreement listed opposite each term: Defined Term Section in Agreement Agreement Preamble Codex Preamble
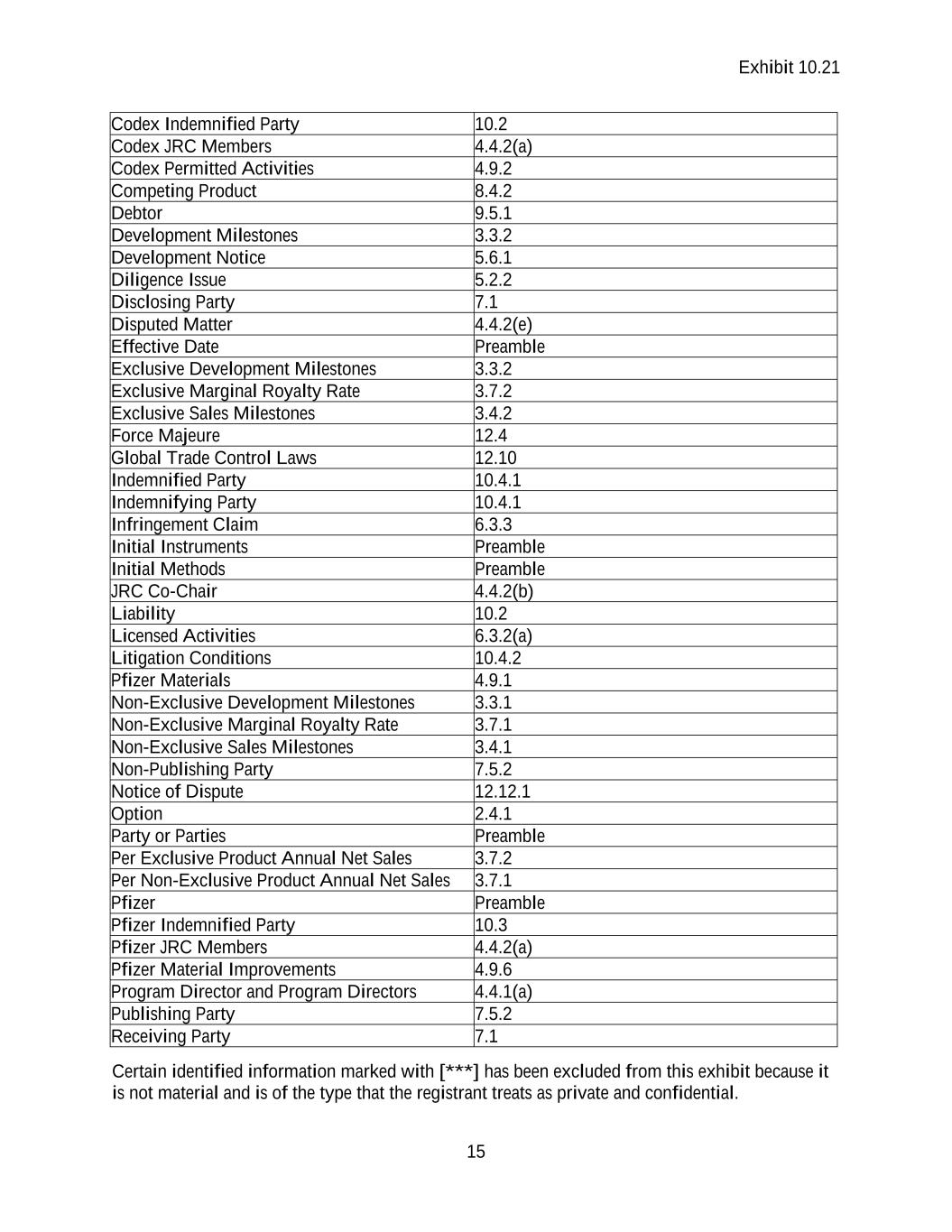
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 15 Codex Indemnified Party 10.2 Codex JRC Members 4.4.2(a) Codex Permitted Activities 4.9.2 Competing Product 8.4.2 Debtor 9.5.1 Development Milestones 3.3.2 Development Notice 5.6.1 Diligence Issue 5.2.2 Disclosing Party 7.1 Disputed Matter 4.4.2(e) Effective Date Preamble Exclusive Development Milestones 3.3.2 Exclusive Marginal Royalty Rate 3.7.2 Exclusive Sales Milestones 3.4.2 Force Majeure 12.4 Global Trade Control Laws 12.10 Indemnified Party 10.4.1 Indemnifying Party 10.4.1 Infringement Claim 6.3.3 Initial Instruments Preamble Initial Methods Preamble JRC Co-Chair 4.4.2(b) Liability 10.2 Licensed Activities 6.3.2(a) Litigation Conditions 10.4.2 Pfizer Materials 4.9.1 Non-Exclusive Development Milestones 3.3.1 Non-Exclusive Marginal Royalty Rate 3.7.1 Non-Exclusive Sales Milestones 3.4.1 Non-Publishing Party 7.5.2 Notice of Dispute 12.12.1 Option 2.4.1 Party or Parties Preamble Per Exclusive Product Annual Net Sales 3.7.2 Per Non-Exclusive Product Annual Net Sales 3.7.1 Pfizer Preamble Pfizer Indemnified Party 10.3 Pfizer JRC Members 4.4.2(a) Pfizer Material Improvements 4.9.6 Program Director and Program Directors 4.4.1(a) Publishing Party 7.5.2 Receiving Party 7.1
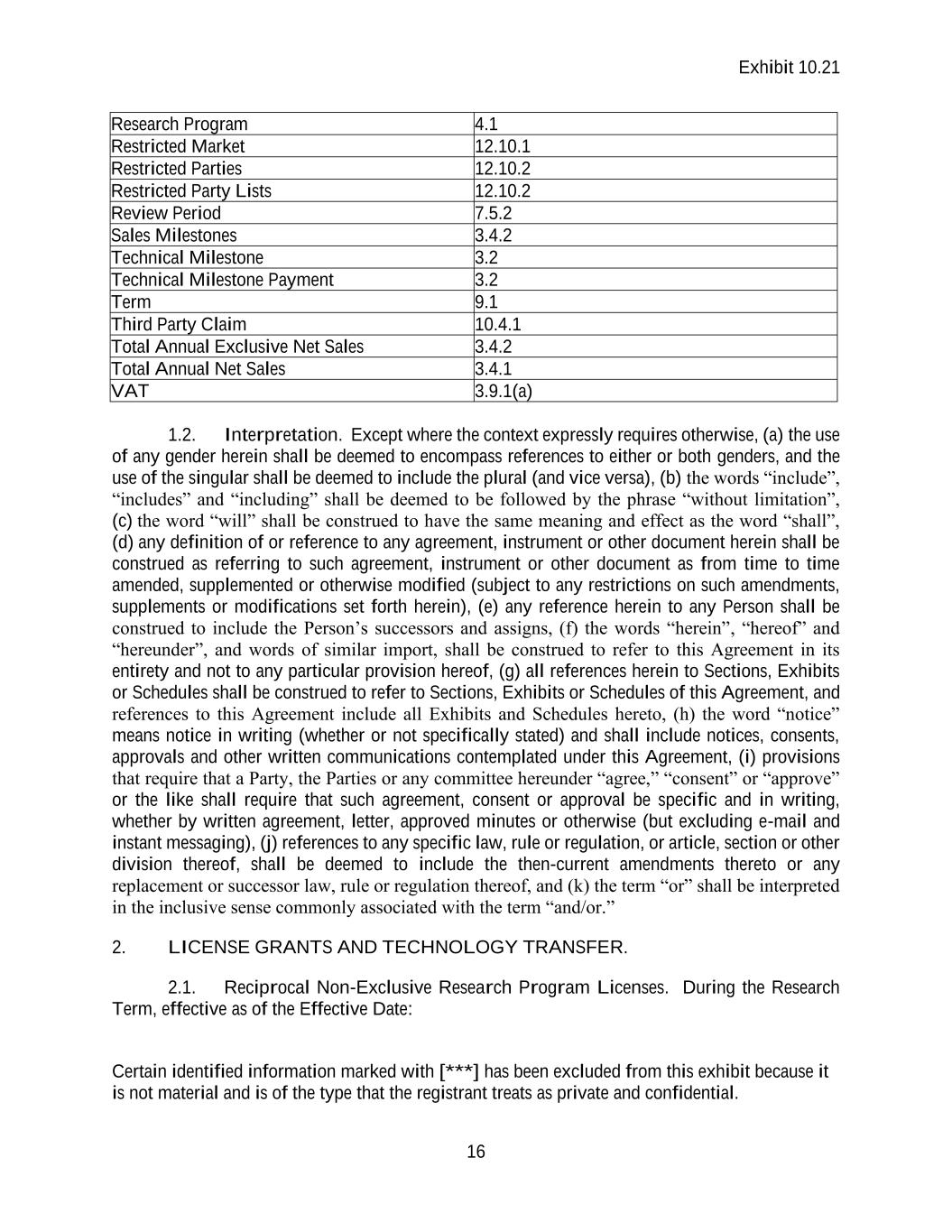
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 16 Research Program 4.1 Restricted Market 12.10.1 Restricted Parties 12.10.2 Restricted Party Lists 12.10.2 Review Period 7.5.2 Sales Milestones 3.4.2 Technical Milestone 3.2 Technical Milestone Payment 3.2 Term 9.1 Third Party Claim 10.4.1 Total Annual Exclusive Net Sales 3.4.2 Total Annual Net Sales 3.4.1 VAT 3.9.1(a) 1.2. Interpretation. Except where the context expressly requires otherwise, (a) the use of any gender herein shall be deemed to encompass references to either or both genders, and the use of the singular shall be deemed to include the plural (and vice versa), (b) the words “include”, “includes” and “including” shall be deemed to be followed by the phrase “without limitation”, (c) the word “will” shall be construed to have the same meaning and effect as the word “shall”, (d) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (e) any reference herein to any Person shall be construed to include the Person’s successors and assigns, (f) the words “herein”, “hereof” and “hereunder”, and words of similar import, shall be construed to refer to this Agreement in its entirety and not to any particular provision hereof, (g) all references herein to Sections, Exhibits or Schedules shall be construed to refer to Sections, Exhibits or Schedules of this Agreement, and references to this Agreement include all Exhibits and Schedules hereto, (h) the word “notice” means notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement, (i) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging), (j) references to any specific law, rule or regulation, or article, section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement or successor law, rule or regulation thereof, and (k) the term “or” shall be interpreted in the inclusive sense commonly associated with the term “and/or.” 2. LICENSE GRANTS AND TECHNOLOGY TRANSFER. 2.1. Reciprocal Non-Exclusive Research Program Licenses. During the Research Term, effective as of the Effective Date:

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 17 2.1.1. To Pfizer. Subject to the terms and conditions of this Agreement, including the Options in Section 2.4, Codex hereby grants to Pfizer a non-exclusive, royalty-free, fully paid-up license (and, to the extent any Codex Technology is Controlled by Codex pursuant to a Codex Third Party Agreement, a sublicense, as applicable), with no right to sublicense other than to Affiliates of Pfizer or Third Party subcontractors under the Codex Technology solely to the extent necessary to perform Pfizer’s activities under the Research Plan. 2.1.2. To Codex. Subject to the terms and conditions of this Agreement, Pfizer hereby grants to Codex a non-exclusive, royalty-free, fully paid-up license in the Territory, with no right to grant sublicenses other than to Affiliates of Codex or Third Party subcontractors pursuant to Section 4.8, under the Pfizer Technology solely to the extent necessary to perform Codex’s activities under the Research Plan. 2.2. Non-Exclusive Commercial License under Codex Technology from Codex to Pfizer. Effective as of the Effective Date, subject to the terms and conditions of this Agreement, Codex agrees to grant and hereby grants to Pfizer a non-exclusive, sublicensable (subject to Section 2.7) license (and, to the extent any Codex Technology is Controlled by Codex pursuant to a Codex Third Party Agreement, a sublicense, as applicable) under the Codex Technology to Develop and use the Deliverables solely to use, have used, Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized and otherwise Exploit Products in the Field. 2.3. Output Materials. Codex agrees that, as between the Parties, (a) Pfizer is the sole and exclusive owner of all right, title and interest in and to any Output Materials generated under the Research Plan by either Party and any Products containing, expressing or encoding such Output Materials and (b) Codex has no right, title or interest in or to any such Output Materials or Products. Codex further agrees that consistent with such ownership rights, Pfizer shall have sole authority over and control of the Development, Manufacture, Regulatory Approval and Commercialization of such Output Materials and Products in accordance with the terms of Section 5. 2.4. Exclusive Option. 2.4.1. Grant. Effective as of the Effective Date, subject to the terms and conditions of this Agreement (including Section 2.10 (Retained Rights)), Codex hereby grants to Pfizer exclusive options, on the terms set forth in this Section 2.4 (the “ Options” and each an “Option”), exercisable at Pfizer’s sole discretion during the Option Exercise Period pursuant to Section 2.4.2, on an Exclusive Field-by-Exclusive Field basis, to obtain an exclusive (even as to Codex), sublicensable (subject to Section 2.5) license (and, to the extent any Codex Technology is Controlled by Codex pursuant to a Codex Third Party Agreement, a sublicense, as applicable) under the Codex Technology (including any Deliverables therein), to use, have used, Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized and otherwise Exploit products in such Exclusive Field for which Pfizer has exercised the Option pursuant to Section 2.4.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 18 2.4.2. Exercise of the Option. At any time prior to the expiration of the Option Exercise Period, on an Option-by-Option basis, Pfizer may exercise its Option(s) in accordance with the procedure set forth in this Section 2.4.2. On or before the last day of the Option Exercise Period, Pfizer shall notify Codex in writing if Pfizer, in its sole discretion, elects to exercise the Option(s). For avoidance of doubt, the Exclusive Development Milestones, Exclusive Sales Milestones and Exclusive Royalties described in Section 3 shall solely be applicable to any Exclusive Products for use in an Exclusive Field for which Pfizer has exercised its Option in accordance with this Section 2. 2.4.3. Effects of Exercise of Option. Upon exercise of an Option in accordance with Section 2.4.2 in respect of an Exclusive Field, effective upon the Option Exercise Date with respect to such Exclusive Field and subject to the terms and conditions of this Agreement (including Section 2.10 (Retained Rights)), Codex hereby grants to Pfizer an exclusive (even as to Codex), sublicensable (subject to Section 2.7) license (and, to the extent any Codex Technology is Controlled by Codex pursuant to a Codex Third Party Agreement, a sublicense, as applicable) under the Codex Technology (including the Deliverables) to use, have used, Develop, have Developed, Manufacture, have Manufactured, Commercialize, have Commercialized and otherwise Exploit Products in such Exclusive Field. 2.5. Unblocking License. Subject to the terms and conditions of this Agreement and without limiting Section 2.10, Pfizer hereby grants to Codex a non-exclusive, royalty-free, perpetual, irrevocable, fully paid-up license in the Territory, with the right to grant sublicenses (subject to the remainder of this Section 2.5) to Third Parties without Pfizer’s prior written consent, under any Pfizer Sole Research Plan Patent Rights solely to the extent such Pfizer Sole Research Plan Patent Rights is directed to Instrument/Methods Know-How and not directed to Output Materials or Pfizer Material Improvements and is necessary for Codex to practice the Core Codex DNA Technology. Notwithstanding the forgoing, Codex would be permitted to sublicense the foregoing license to a third party licensor of Codex only if (i) Codex has a similar reciprocal arrangement with such Third Party licensee running to the benefit of Pfizer or (ii) Codex and Pfizer have agreed upon reasonable terms and conditions with respect to such right to sublicense to such Third Party, which the Parties agree to negotiate in good faith. 2.6. Right of Reference. Codex hereby grants to Pfizer, its Affiliates and its Sublicensees a “Right of Reference,” as that term is defined in 21 C.F.R. § 314.3(b) (or any analogous Law recognized outside of the United States), to all data (including any regulatory filings or Regulatory Approvals) Controlled by Codex or its Affiliates that relates generally to DNA or RNA Manufactured by an Instrument and Codex will provide a signed statement to this effect, if requested by Pfizer, in accordance with 21 C.F.R. § 314.50(g)(3) (or any analogous Law outside of the United States). For clarity, the foregoing obligation to provide any right or reference does not include any such obligation with respect to any specific DNA or RNA product or component thereof.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 19 2.7. Permitted Sublicensees. Pfizer shall have the right to freely grant sublicenses under any and all rights licensed to Pfizer under this Agreement to Third Party subcontractors, Affiliates or Third Parties; provided that (a) the rights licensed to Pfizer pursuant to Section 2.1.1 may not be sublicensed to any Sublicensee other than an Affiliate or Third Party subcontractors without Codex’s prior written consent, such consent not to be unreasonably withheld, delayed or conditioned and (b) the rights licensed to Pfizer pursuant to Sections 2.2 and 2.4.3 may be sublicensed to any Sublicensee without Codex’s prior written consent provided that such rights are licensed as part of an agreement between Pfizer or its Affiliate and a Third Party for such Third Party to Develop, Manufacture or Commercialize one or more Products (or further versions thereof). Upon Codex’s request, Pfizer shall furnish to Codex copies of such sublicense agreements, subject to redactions for financial, business and technical information (including confidential information of Third Parties) to the extent not required to ensure compliance with Section 2.7. Each sublicense granted by Pfizer shall be granted pursuant to a written agreement that is subject to and consistent with the terms and conditions of this Agreement. 2.8. Direct Licenses to Affiliates. Pfizer may, from time to time, request that Codex grant licenses directly to Affiliates of Pfizer by giving written notice, upon receipt of which Codex agrees to enter into and sign a separate direct license agreement with such designated Affiliate of Pfizer. All such direct license agreements shall be consistent with the terms and conditions of this Agreement, except for such modifications as may be required by applicable Laws in the country in which the direct license will be exercised. The Parties further agree to make any amendments to this Agreement that are necessary to conform the combined terms of such direct licenses and this Agreement to the terms of this Agreement as set forth on the Effective Date. All costs of making such direct license agreement(s), including Codex’s reasonable attorneys’ fees, under this Section 2.5 shall be borne by Pfizer. Pfizer shall remain responsible for the performance of its Affiliates under any such direct license agreement(s), and any breach of any such direct license agreement(s) by the Pfizer Affiliate that is a party thereto. 2.9. No Implied Rights. Except as expressly provided in this Agreement, neither Party shall be deemed to have granted the other Party (by implication, estoppel or otherwise) any right, title, license or other interest in or with respect to any intellectual property right, including any Patent Right, Know-How or information Controlled by such Party. For the avoidance of doubt, Pfizer shall not (a) sell, lease or otherwise transfer any Instrument, Method or Deliverable to a Third Party other than a Third Party subcontractor or Sublicensee or (b) use (or authorize, assist or enable an Affiliate or Third Party to use) any Instrument, Method or Deliverable to perform fee- for-service activities for or on behalf of a Third Party unless such activity is a part of an agreement with such Third Party for the Development, Manufacture or Commercialization of one or more Products. 2.10. Retained Rights. Notwithstanding the exclusive nature of the licenses granted pursuant to Section 2.4.3 following the exercise of an Option pursuant to Section 2.4.2, Codex expressly retains the rights to practice Codex Technology in the Exclusive Fields in the Territory solely in order to (a) perform its obligations under the Research Plan and (b) use, have used and otherwise Exploit the Instruments, Methods and Deliverables for research use (and authorize
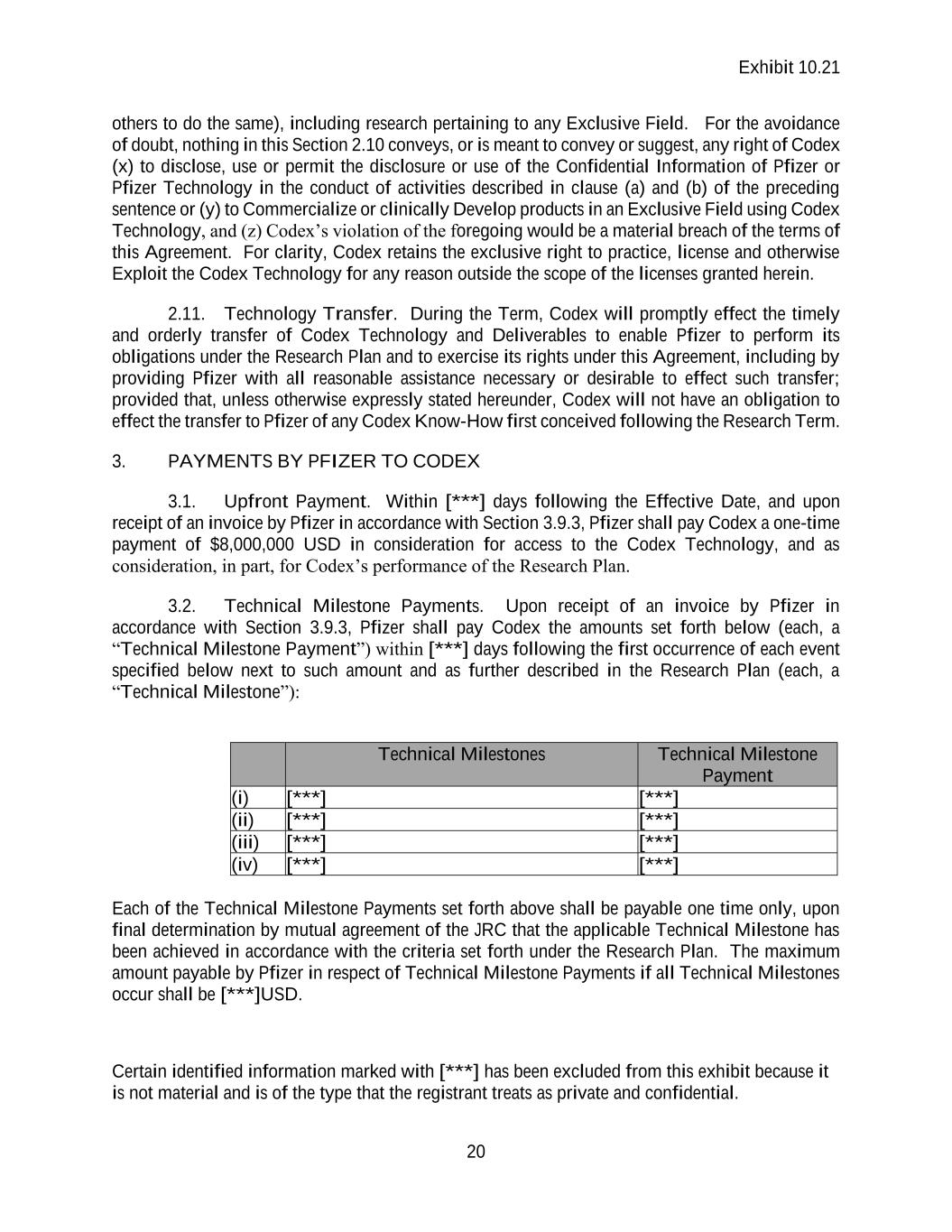
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 20 others to do the same), including research pertaining to any Exclusive Field. For the avoidance of doubt, nothing in this Section 2.10 conveys, or is meant to convey or suggest, any right of Codex (x) to disclose, use or permit the disclosure or use of the Confidential Information of Pfizer or Pfizer Technology in the conduct of activities described in clause (a) and (b) of the preceding sentence or (y) to Commercialize or clinically Develop products in an Exclusive Field using Codex Technology, and (z) Codex’s violation of the foregoing would be a material breach of the terms of this Agreement. For clarity, Codex retains the exclusive right to practice, license and otherwise Exploit the Codex Technology for any reason outside the scope of the licenses granted herein. 2.11. Technology Transfer. During the Term, Codex will promptly effect the timely and orderly transfer of Codex Technology and Deliverables to enable Pfizer to perform its obligations under the Research Plan and to exercise its rights under this Agreement, including by providing Pfizer with all reasonable assistance necessary or desirable to effect such transfer; provided that, unless otherwise expressly stated hereunder, Codex will not have an obligation to effect the transfer to Pfizer of any Codex Know-How first conceived following the Research Term. 3. PAYMENTS BY PFIZER TO CODEX 3.1. Upfront Payment. Within [***] days following the Effective Date, and upon receipt of an invoice by Pfizer in accordance with Section 3.9.3, Pfizer shall pay Codex a one-time payment of $8,000,000 USD in consideration for access to the Codex Technology, and as consideration, in part, for Codex’s performance of the Research Plan. 3.2. Technical Milestone Payments. Upon receipt of an invoice by Pfizer in accordance with Section 3.9.3, Pfizer shall pay Codex the amounts set forth below (each, a “Technical Milestone Payment”) within [***] days following the first occurrence of each event specified below next to such amount and as further described in the Research Plan (each, a “Technical Milestone”): Technical Milestones Technical Milestone Payment (i) [***] [***] (ii) [***] [***] (iii) [***] [***] (iv) [***] [***] Each of the Technical Milestone Payments set forth above shall be payable one time only, upon final determination by mutual agreement of the JRC that the applicable Technical Milestone has been achieved in accordance with the criteria set forth under the Research Plan. The maximum amount payable by Pfizer in respect of Technical Milestone Payments if all Technical Milestones occur shall be [***]USD.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 21 3.3. Development Milestones. 3.3.1. Products in the Non-Exclusive Field(s). Subject to Section 3.5, with respect to any Product upon receipt of an invoice by Pfizer in accordance with Section 3.9.3, Pfizer will pay Codex the amounts set forth below within [***] days following the first occurrence of each event described below (the “Non-Exclusive Development Milestones”) for the first Product in each Non-Exclusive Field (i.e., on an Indication-by- Indication basis) to achieve such Non-Exclusive Development Milestone. Pfizer shall provide Codex with notice of the occurrence of each Non-Exclusive Development Milestone within [***] of achievement. Non-Exclusive Development Milestone Development Milestone Payment for Products in Non-Exclusive Field [***] [***] [***] [***] [***] [***] [***] [***] [***] Each of the Development Milestone Payments payable pursuant to this Section 3.3.1 as set forth above will be payable one time only for each Non-Exclusive Field (regardless of the number of Products in such Non-Exclusive Field with respect to which the specified Non- Exclusive Development Milestone occurs). No Development Milestone Payments will be payable by Pfizer for any subsequent Product for each Non-Exclusive Field regardless of the number of Products Developed for each Non-Exclusive Field. Notwithstanding anything to the contrary in this Agreement, in the event [***] Product achieves the same Non-Exclusive Development Milestone for more than [***], the Development Milestone Payment shall be reduced by [***] for the [***] achievement of such Non-Exclusive Development Milestone by such Product and by [***] for the [***] achievement of such Non-Exclusive Development Milestone by such Product; provided that a Development Milestone Payment will not be paid for the achievement of a Non-Exclusive Development Milestone by the same Product after such Product has achieved such Non-Exclusive Development Milestone [***] (for clarity, [***]). For clarification, if one Product replaces another Product in Development for use in each Non-Exclusive Field, then such replacement Product will only be subject to Development Milestone Payments that have not previously been triggered by a Product in such Non-Exclusive Field. If the Non- Exclusive Development Milestones set forth in (ii) or (iii) of the table immediately above is achieved prior to the achievement of the Non-Exclusive Development Milestone set forth in (i), then Pfizer will pay the Development Milestone Payment for the Non-Exclusive Development Milestone in (i) of the table immediately above together with the payment for the most recently achieved Non-Exclusive Development Milestone. In the event the Non-Exclusive Development Milestone in (iii) of the table immediately above is achieved prior to the achievement of the Non-Exclusive Development Milestone in (ii) of the table immediately above, the Non-Exclusive Development Milestone in (ii) of the table immediately above will not be due or payable. The maximum amount payable by Pfizer
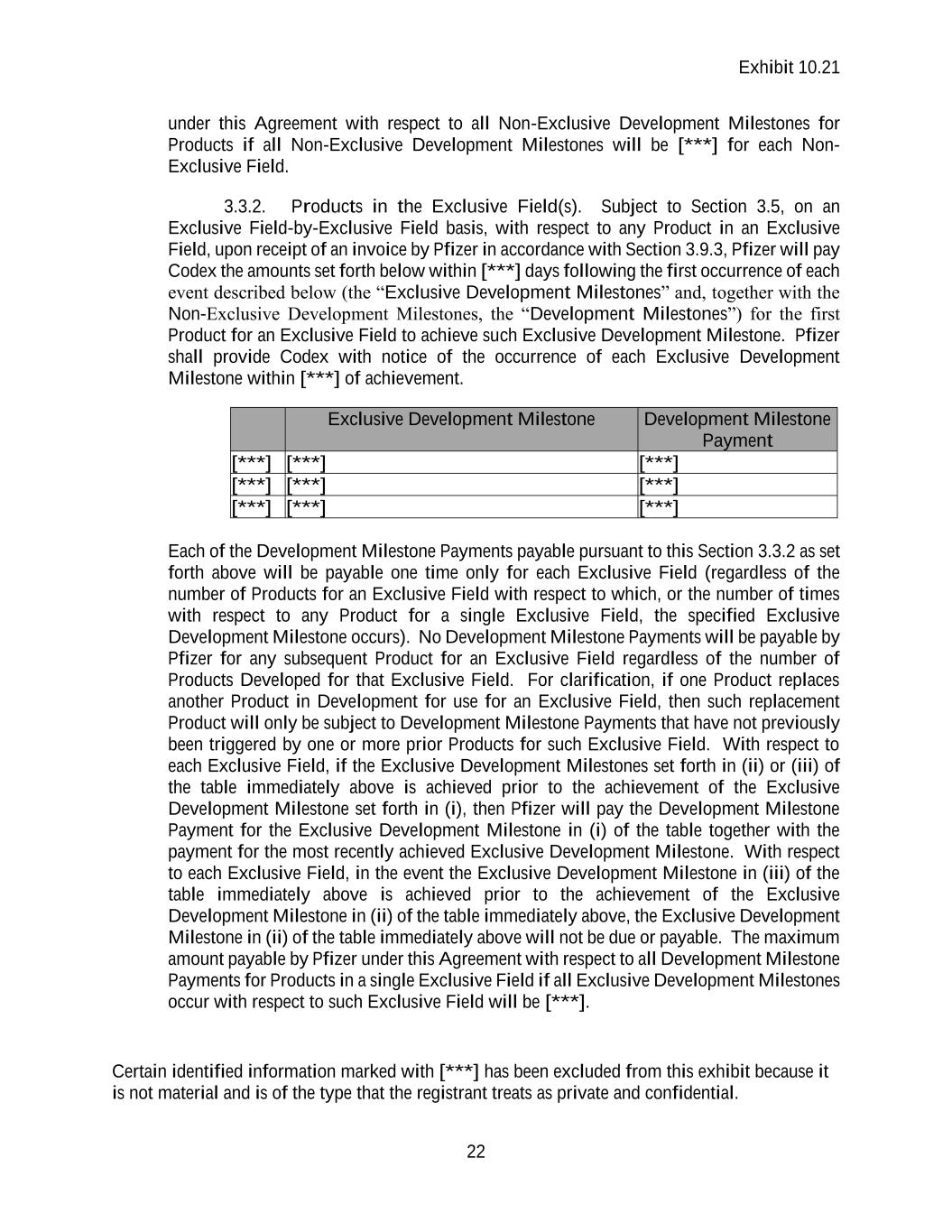
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 22 under this Agreement with respect to all Non-Exclusive Development Milestones for Products if all Non-Exclusive Development Milestones will be [***] for each Non- Exclusive Field. 3.3.2. Products in the Exclusive Field(s). Subject to Section 3.5, on an Exclusive Field-by-Exclusive Field basis, with respect to any Product in an Exclusive Field, upon receipt of an invoice by Pfizer in accordance with Section 3.9.3, Pfizer will pay Codex the amounts set forth below within [***] days following the first occurrence of each event described below (the “Exclusive Development Milestones” and, together with the Non-Exclusive Development Milestones, the “Development Milestones”) for the first Product for an Exclusive Field to achieve such Exclusive Development Milestone. Pfizer shall provide Codex with notice of the occurrence of each Exclusive Development Milestone within [***] of achievement. Exclusive Development Milestone Development Milestone Payment [***] [***] [***] [***] [***] [***] [***] [***] [***] Each of the Development Milestone Payments payable pursuant to this Section 3.3.2 as set forth above will be payable one time only for each Exclusive Field (regardless of the number of Products for an Exclusive Field with respect to which, or the number of times with respect to any Product for a single Exclusive Field, the specified Exclusive Development Milestone occurs). No Development Milestone Payments will be payable by Pfizer for any subsequent Product for an Exclusive Field regardless of the number of Products Developed for that Exclusive Field. For clarification, if one Product replaces another Product in Development for use for an Exclusive Field, then such replacement Product will only be subject to Development Milestone Payments that have not previously been triggered by one or more prior Products for such Exclusive Field. With respect to each Exclusive Field, if the Exclusive Development Milestones set forth in (ii) or (iii) of the table immediately above is achieved prior to the achievement of the Exclusive Development Milestone set forth in (i), then Pfizer will pay the Development Milestone Payment for the Exclusive Development Milestone in (i) of the table together with the payment for the most recently achieved Exclusive Development Milestone. With respect to each Exclusive Field, in the event the Exclusive Development Milestone in (iii) of the table immediately above is achieved prior to the achievement of the Exclusive Development Milestone in (ii) of the table immediately above, the Exclusive Development Milestone in (ii) of the table immediately above will not be due or payable. The maximum amount payable by Pfizer under this Agreement with respect to all Development Milestone Payments for Products in a single Exclusive Field if all Exclusive Development Milestones occur with respect to such Exclusive Field will be [***].
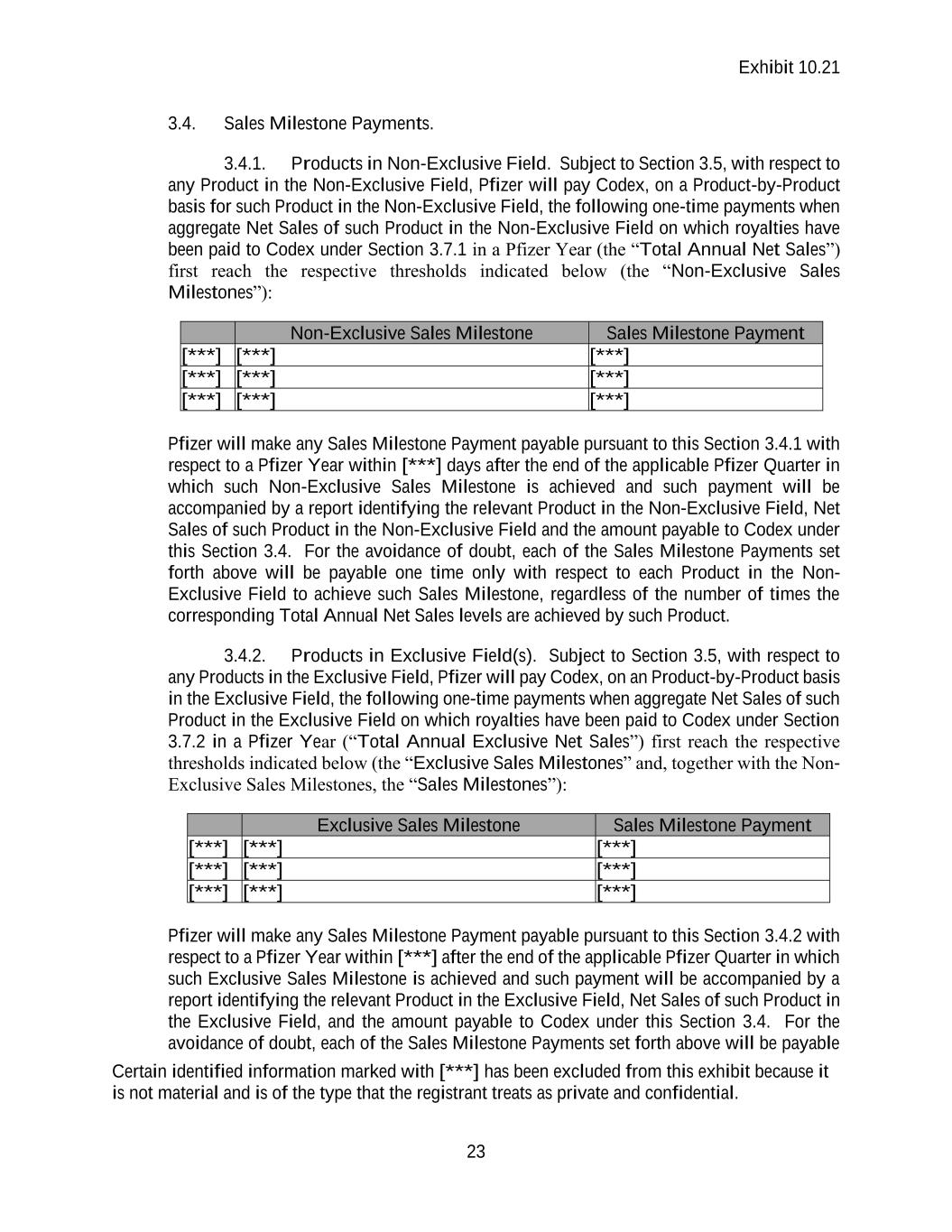
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 23 3.4. Sales Milestone Payments. 3.4.1. Products in Non-Exclusive Field. Subject to Section 3.5, with respect to any Product in the Non-Exclusive Field, Pfizer will pay Codex, on a Product-by-Product basis for such Product in the Non-Exclusive Field, the following one-time payments when aggregate Net Sales of such Product in the Non-Exclusive Field on which royalties have been paid to Codex under Section 3.7.1 in a Pfizer Year (the “Total Annual Net Sales”) first reach the respective thresholds indicated below (the “Non-Exclusive Sales Milestones”): Non-Exclusive Sales Milestone Sales Milestone Payment [***] [***] [***] [***] [***] [***] [***] [***] [***] Pfizer will make any Sales Milestone Payment payable pursuant to this Section 3.4.1 with respect to a Pfizer Year within [***] days after the end of the applicable Pfizer Quarter in which such Non-Exclusive Sales Milestone is achieved and such payment will be accompanied by a report identifying the relevant Product in the Non-Exclusive Field, Net Sales of such Product in the Non-Exclusive Field and the amount payable to Codex under this Section 3.4. For the avoidance of doubt, each of the Sales Milestone Payments set forth above will be payable one time only with respect to each Product in the Non- Exclusive Field to achieve such Sales Milestone, regardless of the number of times the corresponding Total Annual Net Sales levels are achieved by such Product. 3.4.2. Products in Exclusive Field(s). Subject to Section 3.5, with respect to any Products in the Exclusive Field, Pfizer will pay Codex, on an Product-by-Product basis in the Exclusive Field, the following one-time payments when aggregate Net Sales of such Product in the Exclusive Field on which royalties have been paid to Codex under Section 3.7.2 in a Pfizer Year (“Total Annual Exclusive Net Sales”) first reach the respective thresholds indicated below (the “Exclusive Sales Milestones” and, together with the Non- Exclusive Sales Milestones, the “Sales Milestones”): Exclusive Sales Milestone Sales Milestone Payment [***] [***] [***] [***] [***] [***] [***] [***] [***] Pfizer will make any Sales Milestone Payment payable pursuant to this Section 3.4.2 with respect to a Pfizer Year within [***] after the end of the applicable Pfizer Quarter in which such Exclusive Sales Milestone is achieved and such payment will be accompanied by a report identifying the relevant Product in the Exclusive Field, Net Sales of such Product in the Exclusive Field, and the amount payable to Codex under this Section 3.4. For the avoidance of doubt, each of the Sales Milestone Payments set forth above will be payable

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 24 one time only with respect to each Product in the Exclusive Field to achieve such Sales Milestone, regardless of the number of times the corresponding Total Annual Exclusive Net Sales levels are achieved by such Product. 3.5. Products in Both Exclusive and Non-Exclusive Field. If a Product contains mRNA encoding antigens in both a Non-Exclusive Field and Exclusive Field, such Product shall be deemed and treated as a Product in an Exclusive Field for purposes of this Agreement. To the extent that any payment to Codex under this Agreement is based on or for the same event or activity as a payment or reimbursement of costs under this Agreement or any other license agreement between Pfizer or any of its Affiliates and Codex or any of its Affiliates, such payment shall be payable to Codex only once, notwithstanding that the obligation to make such payment or reimbursement arises under both this Agreement and any such other agreement. 3.6. Milestone Payment Adjustment; Additional Payments for Previously Achieved Milestones. Each Development Milestone Payment for a Product in the Non-Exclusive Field or an Product in the Exclusive Field, as applicable, will be reduced by [***] if, at such time as the corresponding Development Milestone is achieved, neither the Manufacture or sale of the Product triggering such Milestone nor Pfizer’s use of the Deliverables used for the Development or Manufacture of such Product is Covered by a Valid Claim. Each Sales Milestone Payment for any Product, as applicable, will be reduced by [***] if, at such time as the corresponding Milestone is achieved, neither the Manufacture or sale of the Product(s) triggering such Milestone nor Pfizer’s use of the Deliverables used for the Development or Manufacture of such Product is Covered by a Valid Claim. In the event that more than one Sales Milestone is first achieved in a given Pfizer Year, Pfizer shall pay Codex the Sales Milestone Payment associated with each such Sales Milestone achieved during such Pfizer Year. Notwithstanding the above, in the event that a Development Milestone Payment is made with respect to a Product in the [***] or [***] (when such Field is part of the Non-Exclusive Field) and the [***] or [***], as applicable, becomes an Exclusive Field due to Pfizer’s exercise of the relevant Option for such Exclusive Field pursuant to this Agreement, Pfizer shall pay Codex the differential between the Development Milestone Payment in the Non-Exclusive Field and the Exclusive Field within [***] days of invoice. 3.7. Royalty Payments. 3.7.1. Royalties for Products in Non-Exclusive Field. Subject to the provisions of Section 3.7.4, Pfizer will pay Codex, on a Product-by-Product basis, royalties on a tiered marginal royalty rate basis as set forth below (the “Non-Exclusive Marginal Royalty Rates”) based on the annual aggregate Net Sales of such Product in the Non- Exclusive Field in the Territory during each Pfizer Year of the applicable Royalty Term for such Product in all Non-Exclusive Fields (each, the “Per Non-Exclusive Product Annual Net Sales”):
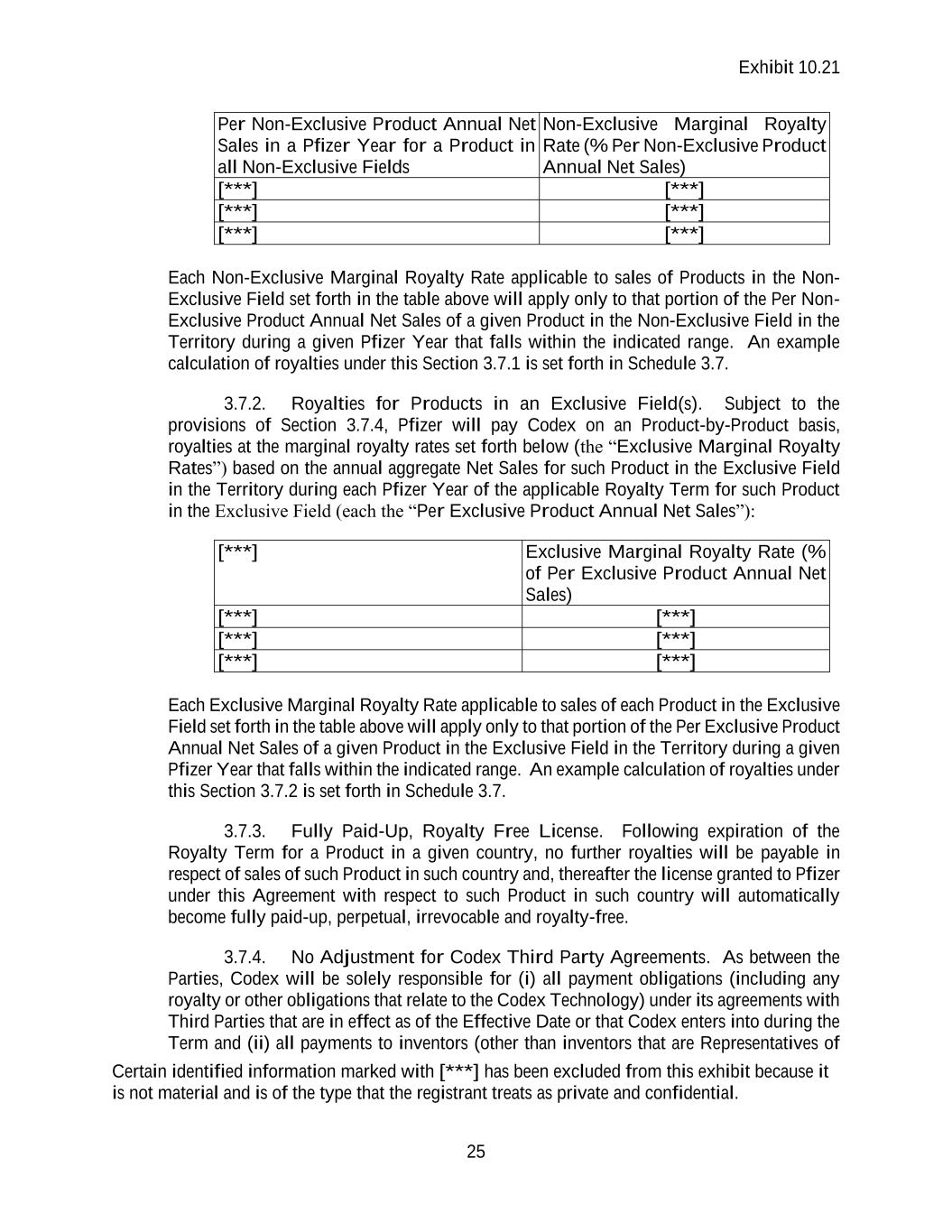
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 25 Per Non-Exclusive Product Annual Net Sales in a Pfizer Year for a Product in all Non-Exclusive Fields Non-Exclusive Marginal Royalty Rate (% Per Non-Exclusive Product Annual Net Sales) [***] [***] [***] [***] [***] [***] Each Non-Exclusive Marginal Royalty Rate applicable to sales of Products in the Non- Exclusive Field set forth in the table above will apply only to that portion of the Per Non- Exclusive Product Annual Net Sales of a given Product in the Non-Exclusive Field in the Territory during a given Pfizer Year that falls within the indicated range. An example calculation of royalties under this Section 3.7.1 is set forth in Schedule 3.7. 3.7.2. Royalties for Products in an Exclusive Field(s). Subject to the provisions of Section 3.7.4, Pfizer will pay Codex on an Product-by-Product basis, royalties at the marginal royalty rates set forth below (the “Exclusive Marginal Royalty Rates”) based on the annual aggregate Net Sales for such Product in the Exclusive Field in the Territory during each Pfizer Year of the applicable Royalty Term for such Product in the Exclusive Field (each the “Per Exclusive Product Annual Net Sales”): [***] Exclusive Marginal Royalty Rate (% of Per Exclusive Product Annual Net Sales) [***] [***] [***] [***] [***] [***] Each Exclusive Marginal Royalty Rate applicable to sales of each Product in the Exclusive Field set forth in the table above will apply only to that portion of the Per Exclusive Product Annual Net Sales of a given Product in the Exclusive Field in the Territory during a given Pfizer Year that falls within the indicated range. An example calculation of royalties under this Section 3.7.2 is set forth in Schedule 3.7. 3.7.3. Fully Paid-Up, Royalty Free License. Following expiration of the Royalty Term for a Product in a given country, no further royalties will be payable in respect of sales of such Product in such country and, thereafter the license granted to Pfizer under this Agreement with respect to such Product in such country will automatically become fully paid-up, perpetual, irrevocable and royalty-free. 3.7.4. No Adjustment for Codex Third Party Agreements. As between the Parties, Codex will be solely responsible for (i) all payment obligations (including any royalty or other obligations that relate to the Codex Technology) under its agreements with Third Parties that are in effect as of the Effective Date or that Codex enters into during the Term and (ii) all payments to inventors (other than inventors that are Representatives of

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 26 Pfizer) of Codex Technology who are Representatives of Codex, including payments under inventorship compensation Laws. 3.7.5. Biosimilar Entry. Notwithstanding the foregoing, for Net Sales based on sales of a Product in a country in the Territory, on a country-by-country basis, any payments owed with respect to such Product pursuant to this Section 3.7 will be reduced by [***] for the remainder of the applicable Royalty Term, if at any time (i) one or more Biosimilar Versions of such Product is available in such country and (ii) such one or more Biosimilar Versions in the aggregate have achieved in excess of [***] market penetration (based on unit volume). 3.8. Reports and Payments. 3.8.1. Cumulative Royalties. The obligation to pay royalties under this Agreement will be imposed only once with respect to any sale of any given unit of Product. 3.8.2. Royalty Statements and Payments. As soon as reasonably practicable (but in no event more than [***] after the end of each Calendar Quarter, Pfizer will deliver to Codex a report setting forth, for the most recent Pfizer Quarter ending during such Calendar Quarter, the following information, on a Product-by-Product in the Field, country-by-country and Territory-wide basis: [***] 3.9. Payment Terms. 3.9.1. Taxes and Withholding. (a) It is understood and agreed between the Parties that any payments made by Pfizer to Codex under this Agreement are exclusive of any value added or similar tax (“VAT”) imposed upon such payments. Where VAT is properly added to a payment made under this Agreement, the Party making the payment will pay the amount of VAT only on receipt of a valid tax invoice issued in accordance with the laws and regulations of the country in which the VAT is chargeable. In addition, in the event any payments made by Pfizer pursuant to this Agreement become subject to withholding taxes under the Laws or regulations of any jurisdiction or Governmental Authority, Pfizer will deduct and withhold the amount of such taxes for the account of Codex to the extent required by applicable Laws or regulations; such amounts payable to Codex will be reduced by the amount of taxes deducted and withheld; and Pfizer will pay the amounts of such taxes to the proper Governmental Authority in a timely manner and transmit to Codex an official tax certificate or other evidence of such tax obligations together with proof of payment from the relevant Governmental Authority of all amounts deducted and withheld sufficient to enable Codex to claim such payment of taxes. Any such withholding taxes required under applicable Laws or regulations to be paid or withheld will be an expense of, and borne solely by, Codex. Pfizer will provide Codex with reasonable assistance to enable Codex to recover such taxes as permitted by

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 27 applicable Laws or regulations. The Parties shall reasonably cooperate with each other in claiming exemptions from such deductions and withholdings under any agreement or treaty in effect at the relevant time. (b) Notwithstanding anything in this Agreement to the contrary, (i) if an action (including but not limited to any assignment, sublicense or exercise by any Affiliate of a Party’s rights or obligations under this Agreement, or payment by any Affiliate of any amount due under this Agreement, or any failure to comply with applicable Laws or filing or record retention requirements) by a Party leads to the imposition of withholding tax liability or VAT on the other Party that would not have been imposed in the absence of such action or in an increase in such liability above the liability that would have been imposed in the absence of such action, then the sum payable by that Party (in respect of which such deduction or withholding is required to be made) shall be increased to the extent necessary to ensure that the other Party receives a sum equal to the sum which it would have received had no such action occurred, (ii) otherwise, the sum payable by that Party (in respect of which such withholding is required to be made) shall be made to the other Party after deduction of the amount required to be so withheld, which withheld amount shall be remitted in accordance with applicable Law. 3.9.2. Currency. All amounts payable and calculations under this Agreement shall be in United States dollars. As applicable, Net Sales and any royalty deductions will be translated into United States dollars at the exchange rate used by Pfizer for public financial accounting purposes. If, due to restrictions or prohibitions imposed by national or international authority, a given payment cannot be made as provided in this Section 3, Pfizer shall continue to provide Net Sales reports for such royalty payments, such royalty payments shall continue to accrue in such country, and the Parties shall consult with a view to finding a prompt and acceptable solution. If the Parties are unable to identify a mutually acceptable solution regarding such payment, then Pfizer may elect, in its sole discretion, to deliver such payment in the relevant jurisdiction and in the local currency of the relevant jurisdiction. 3.9.3. Method of Payment. Except as permitted pursuant to Section 3.7.4, each payment hereunder will be made by electronic transfer in immediately available funds via either a bank wire transfer, an ACH (automated clearing house) mechanism, or any other means of electronic funds transfer, at Pfizer’s election, to such bank account as Codex will designate in writing to Pfizer at least [***] before the payment is due. All invoice or billing related questions should be referred to Pfizer’s Accounting Department at [***] or go to the Accounts Payable Invoice Portal at [***]. 3.9.4. Record Keeping. Pfizer and its Affiliates will keep and will contractually obligate its Sublicensees to keep books and accounts of record in connection with the sale of Products in the Field in sufficient detail to permit accurate determination of all figures necessary for verification of royalties and Sales Milestone Payments to be paid hereunder.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 28 Pfizer and its Affiliates will maintain and will contractually obligate its Sublicensees to maintain such records for a period of at least three years after the end of the Pfizer Quarter in which they were generated. 3.9.5. Audits. Upon [***] days prior notice from Codex, Pfizer will permit an independent certified public accounting firm of nationally recognized standing selected by Codex and reasonably acceptable to Pfizer, to examine, at Codex’s sole expense, the relevant books and records of Pfizer and its Affiliates as may be reasonably necessary to verify the amounts reported by Pfizer in accordance with Section 3.8.2 and the payment of royalties and Sales Milestone Payments hereunder. An examination by Codex under this Section 3.9.5 will occur not more than once in any Calendar Year and will be limited to the pertinent books and records for any Calendar Year ending not more than three years before the date of the request. The accounting firm will be provided access to such books and records at Pfizer’s or its Affiliates’ facility(ies) where such books and records are normally kept and such examination will be conducted during normal business hours. Pfizer may require the accounting firm to sign a reasonably acceptable non-disclosure agreement before providing the accounting firm with access to Pfizer’s or its Affiliates’ facilities or records. Upon completion of the audit, the accounting firm will provide both Pfizer and Codex a written report disclosing any discrepancies in the reports submitted by Pfizer or the royalties or Sales Milestone Payments paid by Pfizer, and, in each case, the specific details concerning any discrepancies. No other information will be provided to Codex. Pfizer shall use commercially reasonable efforts to obtain the right to inspect and audit such Sublicensee’s books and records for itself, and if such right is obtained, Pfizer shall disclose the results of any such audit to Codex in accordance with this Section 3.9.5 to the extent permitted by such Sublicensee. 3.9.6. Underpayments/Overpayments. If such accounting firm concludes that additional royalties or Sales Milestone Payments were due to Codex, then Pfizer will pay to Codex the additional royalties or Sales Milestone Payments within [***] of the date Pfizer receives such accountant’s written report. Further, if the amount of such underpayments exceeds more than [***] of the amount that was properly payable to Codex, then Pfizer will reimburse Codex for Codex’s out-of-pocket costs in connection with the audit. If such accounting firm concludes that Pfizer overpaid royalties or Sales Milestone Payments to Codex, then Codex will refund such overpayments to Pfizer, within [***] of the date Codex receives such accountant’s report. 3.9.7. Confidentiality. Notwithstanding any provision of this Agreement to the contrary, all reports and financial information of Pfizer, its Affiliates or its Sublicensees which are provided to or subject to review by Codex under this Section 3 will be deemed to be Pfizer’s Confidential Information and subject to the provisions of Section 7. 3.9.8. Acknowledgement. Pfizer and Codex acknowledge and agree that: (a) payments to Codex pursuant to Section 3.2 have been included in this Agreement on the basis that they are only payable or otherwise relevant if a Technical Milestone has been

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 29 achieved; (b) payments to Codex pursuant to Sections 3.3 and 3.4 have been included in this Agreement on the basis that they are only payable or otherwise relevant if the applicable Product in the applicable country (if any) is successfully Developed (in the case of Section 3.3) or Commercialized (in the case of Section 3.4); and (ii) are solely intended to allocate amounts that may be achieved upon such successful Development or Commercialization of such Product between Pfizer (who will receive all Product sales revenues) and Codex; (c) Milestone Payments are not intended to be used and will not be used as a measure of damages if this Agreement is terminated for any reason, including pursuant to Pfizer’s right to terminate at for convenience, before any such success is achieved and such amounts become due; and (d) Milestone Payments will only be triggered, and will only be relevant as provided in accordance with the terms and conditions of such provisions. Pfizer and Codex further acknowledge and agree that nothing in this Agreement, or in any document or presentation provided by Pfizer to Codex prior to the Effective Date will be construed as representing any estimate or projection of (i) the successful Development or Commercialization of any Product under this Agreement, (ii) the number of Products that will or may be successfully Developed or Commercialized under this Agreement, (iii) anticipated sales or the actual value of any Products that may be successfully Developed or Commercialized under this Agreement or (iv) the damages, if any, that may be payable if this Agreement is terminated for any reason. Pfizer makes no representation, warranty or covenant, either express or implied, that (A) it will successfully Develop, Manufacture, Commercialize or continue to Develop, Manufacture or Commercialize any Product in any country, (B) if Commercialized, that any Product will achieve any particular sales level, whether in any individual country or cumulatively throughout the Territory or (C) Pfizer will devote, or cause to be devoted, any level of diligence or resources to Developing or Commercializing any Product in any country, or in the Territory in general. 3.9.9. Disclosure of Fees. Consistent with any disclosure policy that may be implemented from time-to-time by Pfizer regarding payments made to members of the medical or scientific community, or in accordance with applicable laws or regulations, Pfizer shall have the right to disclose any terms related to this Agreement, including Codex’s name and the fees provided hereunder. Codex also agrees to disclose its relationship with Pfizer as needed to comply with any disclosure requirements of any healthcare institution, medical committee, or other medical or scientific organization with which Codex is affiliated. This duty to disclose will continue during the term of this Agreement and for [***] after its termination. 3.9.10. No Double Counting. To the extent that any payment to Codex or reimbursement of costs due to Codex under this Agreement is based on or for the same event or activity as a payment or reimbursement of costs under this Agreement or any other license agreement between Pfizer or any of its Affiliates and Codex or any of its Affiliates, such payment or reimbursement of costs shall be payable to Codex only once, notwithstanding that the obligation to make such payment or reimbursement arises under both this Agreement and any such other agreement.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 30 4. RESEARCH PROGRAM. 4.1. Scope of Research. Pfizer and Codex will collaborate during the Research Term to conduct the research under the Research Plan (the “Research Program”) in accordance with the terms set forth in this Section 4. 4.2. Research Plan. The Research Program will be performed by the Parties in accordance with the Research Plan and the terms and conditions set forth in this Section 4. 4.3. Allocation of Responsibilities. 4.3.1. General. Each Party shall use Commercially Reasonable Efforts to perform its obligations under the Research Plan in a professional and timely manner. Further, each Party shall perform its obligations under the Research Plan in compliance with all Laws applicable to its activities under the Research Plan. 4.4. Research Program Governance 4.4.1. Collaboration Management. (a) Program Directors. During the Research Term, the Research Program shall have a program director from each Party (each, a “Program Director” and together the “Program Directors”), initially [***] for Codex and [***] for Pfizer. Each Party may change its designated Program Director at any time upon written notice to the other Party. The Program Directors shall coordinate the research efforts of their respective Party in conducting the Research Program. Each Program Director shall: (i) use good faith efforts to attend (either in person or by telecommunications) all meetings of the JRC, but shall be a non-voting member at such meetings; and (ii) be the first point of referral for all matters of conflict resolution within the scope of the JRC’s decision-making authority, and bring any such disputes to the attention of the JRC in a timely manner. 4.4.2. Joint Research Committee. (a) Composition. The Parties shall establish a Joint Research Committee, comprised of two representatives of Codex and two representatives of Pfizer. As of the Effective Date, the JRC representatives shall be [***] for Pfizer (the ”Pfizer JRC Members”) and [***] for Codex (the “Codex JRC Members”). Each Party may replace its representatives to the JRC at any time upon notice to the other Party. Each Party may invite non-voting employees and, with the other Party’s JRC Chair’s prior written consent, Third Party consultants to attend

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 31 meetings of the JRC. All members of the JRC and any invitees of either Party described above shall agree in writing to be bound to obligations of confidentiality and assignment of inventions no less restrictive than those that bind the Parties under this Agreement. (b) Committee Chair. The JRC shall be co-chaired by a Pfizer JRC Member and a Codex JRC Member (each, a “JRC Co-Chair”) named by each Party. Each Party may replace its JRC Co-Chair at any time upon notice to the other Party. The responsibilities of the JRC Co-Chairs shall be: (i) to notify each Party at least [***] days in advance of each JRC meeting; (ii) to collect and organize agenda items for each JRC meeting; and (iii) to prepare the written minutes of each JRC meeting and circulate such minutes for review and approval by the Parties, and identify action items to be carried out by the Parties. (c) Meetings. During the Research Term, the JRC shall meet at least quarterly or as frequently as agreed upon by the Parties, either in-person or by audio or video teleconference. Either Party may call a special meeting of the JRC by videoconference or teleconference upon at least [***] Business Days prior written notice to the other Party if such Party reasonably believes that a significant matter must be addressed prior to the next scheduled meeting. Meetings of the JRC will only occur if at least one representative of each Party is present at the meeting or participating by teleconference or videoconference. Each Party shall be responsible for all of its own expenses of participating in such JRC meetings. The Parties shall endeavor to schedule meetings of the JRC in advance as mutually agreed. The JRC Co-Chairs shall use good faith efforts to (i) prepare and circulate to each JRC meeting agenda no later than [***] Business Days prior to the scheduled date for each JRC meeting and (ii) circulate for review and approval by the Parties written minutes of each JRC meeting within [***] days after such meeting. The Parties shall agree on the minutes of each meeting promptly, but in no event later than the next meeting of the JRC. (d) Responsibilities. The JRC shall oversee and supervise the overall performance of the Research Plan and within such scope shall: (i) review the efforts of the Parties under the Research Plan; (ii) review and approve any revised Research Plan; provided that any change to a Technical Milestone of the criteria for the achievement thereof shall require mutual agreement of the Parties;

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 32 (iii) as required under Section 3.2, determine by mutual agreement of the Parties, whether a Technical Milestone has been achieved in accordance with the criteria set forth under the Research Plan; (iv) address such other matters relating to the activities of the Parties under the Research Program as either Party may bring before the JRC, including any matters that are expressly for the JRC to decide as provided in this Agreement; and (v) attempt to resolve any disputes relating to the Research Program on an informal basis. (e) Decision-making. Notwithstanding the number of Pfizer JRC Members and Codex JRC Members, each Party will have one (1) vote, and the JRC will make decisions on a unanimous basis. The JRC will use good faith efforts to reach agreement on any and all matters properly brought before it. If, despite such good faith efforts, the JRC is unable to reach unanimous agreement on a particular matter within the scope of the JRC’s decision-making authority, within [***] days after the JRC first meets to consider such matter, or such later date as may be mutually acceptable to the Parties (each such matter, a “Disputed Matter”), then either Party may refer that Disputed Matter for resolution by the appropriate senior officer of each Party, and such senior officers will promptly initiate discussions in good faith to resolve such Disputed Matter. If the senior officers of each Party are unable to resolve the Disputed Matter within [***] of it being referred to them, then [***] will have final decision-making authority with respect to all Disputed Matters, subject only to specific issues identified in this Agreement as expressly requiring mutual consent of the Parties. For clarity, the decision-making of the JRC is limited to matters related to the Research Program and Research Plan (including the implementation thereof). (f) Limits on JRC Authority. Notwithstanding any provision of this Section 4.4 to the contrary, (i) each Party shall retain the rights, powers and discretion granted to it under this Agreement and no such rights, powers, or discretion shall be delegated to or vested in the JRC unless such delegation or vesting of rights is expressly provided for in this Agreement or the Parties expressly so agree in writing, (ii) the JRC shall not have the power to (A) impose any additional financial obligation on either Party or its Affiliates in a manner inconsistent with this Agreement, (B) resolve any dispute regarding the existence of amounts of any payment owed under this Agreement, (C) impose on either Party or its Affiliates a material obligation to allocate such Party’s or its Affiliate’s tangible or intangible resources or assets in a certain manner inconsistent with this Agreement or (D) amend this Agreement or otherwise modify or waive compliance with this Agreement in any manner and no decision of the JRC shall be in contravention of any term or condition of this Agreement and (iii) neither Party

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 33 shall require the other Party to (A) breach any obligation or agreement that such other Party may have with or to a Third Party or (B) perform any activities that are materially different or greater in scope or more costly than those provided for in the Research Plan then in effect. It is understood and agreed that issues to be formally decided by the JRC are limited to those specific issues that are expressly provided in Section 4.4.2(d) of this Agreement and disputes which relate to subjects other than those set forth in Section 4.4.2(d) will be handled according to Section 12.12. (g) Term. The JRC shall be dissolved immediately upon expiration of the Research Term. 4.5. Research Term Extension. If additional time is needed for the Parties to achieve the Technical Milestones described in the Research Plan, Pfizer may extend the Research Term at its option by up to one additional year by providing notice to Codex not later than [***] prior to the end of the first year of the Research Term. 4.6. Research Program Expenses. Each Party shall bear all costs and expenses it incurs in connection with its activities under the Research Program. 4.7. Scientific Records. Each Party shall maintain complete, current and accurate records of all activities conducted by it pursuant to the Research Program, and all data and other information resulting from such activities. Such records shall fully and properly reflect all work done and results achieved in the performance of such activities in good scientific manner appropriate for regulatory and patent purposes, and such records shall comply with applicable Laws, including applicable national and international guidelines such as the then-current Good Laboratory Practices promulgated or endorsed by the United States Food and Drug Administration, and any relevant guidelines under the International Congress on Harmonization. Each Party will provide the other Party with reasonable access to such records upon advance written notice as may be required to undertake the Research Program or to the extent necessary for regulatory purposes that are within the scope of such Party’s rights and responsibilities under this Agreement. 4.8. Delegation and Subcontracting. Codex shall not delegate or subcontract any of its obligations in connection with the Research Program to an Affiliate or Third Party, without Pfizer’s prior written consent. Any permitted Affiliate or Third Party subcontractors of either Party must have reasonably sufficient knowledge, experience and resources to perform such activities and, such Third Party, as applicable, must have entered into a binding subcontract with such Party under which such Third Party: 4.8.1. has agreed to assign and does assign to such Party all intellectual property rights generated during and in the course of its performance of the Research Program; 4.8.2. has agreed to terms and conditions with respect to confidentiality at least as restrictive as those described in Section 7; and

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 34 4.8.3. has agreed to comply with terms substantially similar to the provisions set out in Section 4.7 with respect to its performance of the Research Program. No delegation or subcontracting shall relieve either Party from its obligations under this Agreement, and the subcontracting Party shall remain fully responsible for the conduct of any Affiliate or Third Party subcontractor as though it were performing the activities itself. 4.9. Transfer of Pfizer Materials 4.9.1. Transfer. From time to time during the Research Term, Pfizer may, in its sole discretion, provide Codex with tangible chemical or biological materials, including Product(s) (the “Pfizer Materials”). Any Pfizer Materials are provided on an “as-is” basis without any representation or warranty of any type, express or implied, including any representation or warranty of merchantability, non-infringement, title or fitness for a particular purpose, each of which is hereby expressly disclaimed by Pfizer. 4.9.2. Permitted Use of Pfizer Materials. Codex shall use the Pfizer Materials solely in connection with conducting the activities specified in the Research Plan (the “Codex Permitted Activities”). Without limiting the generality of the foregoing, except in the performance of the Codex Permitted Activities, Codex shall not (a) make or attempt to make any analogues, progeny or derivatives of, or modifications to, the Pfizer Materials or attempt to reverse engineer, characterize or in any way try to ascertain the identity, chemical structure, sequence, mechanism of action or composition of the Pfizer Material or (b) use the Pfizer Materials for its own benefit or for the benefit of any of its Affiliates or any Third Party. Further, Codex shall not administer any Pfizer Material to any human. Codex shall comply with all Laws applicable to the handling and use of the Pfizer Materials. Codex shall retain possession over the Pfizer Materials and not provide any Pfizer Materials to any of its Affiliates or to any Third Party without Pfizer’s prior written consent, which consent may be withheld in Pfizer’s sole discretion. 4.9.3. Unauthorized Use of Pfizer Materials. If Codex uses any Pfizer Material in any manner other than in the performance of the Codex Permitted Activities, then any and all results of such unauthorized use, whether patentable or not, shall belong solely and exclusively to Pfizer. Codex, on behalf of itself and its Affiliates, hereby assigns and agrees to assign to Pfizer all of Codex’s and its Affiliates’ right, title and interest in and to all such discoveries and inventions. Codex further agrees to cooperate with Pfizer to execute and deliver any and all documents that Pfizer deems reasonably necessary to perfect and enforce Pfizer’s rights under this Section 4.9.3. Nothing in this Section 4.9.3 shall limit in any way any other remedy that Pfizer may have under this Agreement as a result of Codex’s unauthorized use of any Pfizer Materials. 4.9.4. Title to Pfizer Materials and Output Materials. All right, title and interest in and to the Pfizer Materials shall remain the sole and exclusive property of Pfizer notwithstanding the transfer to and use by Codex of the same. Pfizer shall also have any

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 35 and all right, title and interest in and to the Output Materials, which shall be the sole and exclusive property of Pfizer. Without limiting Section 6, Codex, on behalf of itself and its Affiliates, hereby assigns and agrees to assign to Pfizer all of Codex’s and its Affiliates’ right, title and interest in and to all Output Materials. 4.9.5. Return of Pfizer Materials. At the end of the Research Term (or such earlier time as Pfizer may request in writing), Codex shall either destroy or return to Pfizer, at Pfizer’s sole discretion, all unused Pfizer Materials and Output Materials, as applicable. 4.9.6. Ownership of Pfizer Material Improvements and Output Materials. “Pfizer Material Improvement” means any Research Plan Know-How that is invented, developed or discovered by either Party that is predominantly directed to any Pfizer Material or (b) constitutes an improvement or enhancement to, or a derivative or modification of, any Pfizer Material or any method of making or using, which is predominantly related to any Pfizer Material, including, without limitation, any Output Materials. Without limiting Section 6, Codex, on behalf of itself and its Affiliates, hereby assigns and agrees to assign to Pfizer all of Codex’s and its Affiliates’ right, title and interest in and to any and all Pfizer Material Improvements and Output Materials. Codex shall promptly notify Pfizer of any Pfizer Material Improvement made by Codex or its Affiliates and shall reasonably cooperate in obtaining patent and other proprietary protection for such Pfizer Material Improvement. Such protection shall be obtained in the name of Pfizer and at Pfizer’s cost and expense, and Codex shall, and shall cause its Affiliates to, execute and deliver all requested applications, assignments and other documents, and take such other actions as Pfizer may reasonably request, in order to perfect and enforce Pfizer’s rights in any Pfizer Material Improvement. 4.9.7. Activities. Notwithstanding anything to the contrary in this Agreement, nothing in this Agreement (including this Section 4.9) shall be deemed to prevent or restrict in any way the ability of either Party or its Affiliates to conduct any activities in the Territory, which activities would be allowed under any safe harbor, research exemption, government or executive declaration of urgent public health need, or similar right available in law or equity if conducted by a Third Party. 4.9.8. Confidentiality. Each Party’s obligations under this Section 4.9 are in addition to, and shall in no way limit, its obligations under Section 7.1 with respect to the other Party’s materials. 5. PRODUCT DEVELOPMENT AND COMMERCIALIZATION. 5.1. General. Pfizer shall have sole authority over and control of the Development, Manufacture, regulatory approval and Commercialization of its Products for all Fields in the Territory. 5.2. Diligence.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 36 5.2.1. General. Upon exercise of an Option with respect to an Exclusive Field in accordance with Section 2.4.2, Pfizer shall itself, or through its Affiliates or Sublicensees, use Commercially Reasonable Efforts to [***] Pfizer will have no other diligence obligations with respect to the Development, Regulatory Approval or Commercialization of Products under this Agreement. For clarity, if an Exclusive Field become a Non-Exclusive Field pursuant to this Agreement, Pfizer shall no longer have an obligation under this Section 5.2.1 with respect to such field. 5.2.2. Assertion of Pfizer Diligence Obligation Claims. If Codex is, becomes or reasonably should be aware of facts that might form a reasonable basis to allege that Pfizer has failed to meet its diligence obligation under Section 5.2.1, then Codex will promptly notify Pfizer in writing of such potential alleged performance failure (each such potential alleged performance failure, a “Diligence Issue”). Promptly upon Pfizer’s receipt of any notice of a Diligence Issue pursuant to this Section 5.2.2, the Parties shall use reasonable, good faith efforts to identify an appropriate corrective course of action if a Diligence Issue exists. If, no later than [***] days after Pfizer’s receipt of such a notice, (a) the Parties have not reached consensus regarding whether Pfizer has failed to satisfy its obligations pursuant to Section 5.2.1 or (b) the Parties have not agreed upon an appropriate corrective course of action for such Diligence Issue, then such Diligence Issue may be escalated by either Party and resolved pursuant to the dispute resolution provisions set forth in Section 12.12. If Codex fails to notify Pfizer of a Diligence Issue pursuant to this Section 5.2.2 within [***] days after the date that Codex first discovers or reasonably should have discovered such Diligence Issue, then Pfizer will be deemed to have satisfied its obligations under Section 5.2.1 with respect to such Diligence Issue. 5.2.3. Remedies for Breach of Pfizer Diligence Obligations. If it is determined under Section 5.2.2 that Pfizer failed to meet its diligence obligation with respect to an Exclusive Field and such failure is a material breach of Pfizer’s obligations under this Agreement, then Codex may elect to convert Pfizer’s exclusive license with respect to such Exclusive Field granted under Section 2.4.3 into a non-exclusive license and the restrictions on Codex under Section 8.4.2 with respect to such Exclusive Field shall be lifted. Codex acknowledges and agrees that the election set forth in this Section 5.2.3: (i) has been negotiated by the Parties to fully address any harm that Codex may incur as a result of Pfizer’s material breach of the Agreement in the form of a failure to meet its diligence obligation under Section 5.2.1 with respect to an Exclusive Field and (ii) if exercised, constitutes Codex’s sole and exclusive remedy with respect to any breach by Pfizer of its diligence obligation under Section 5.2.1 with respect to the applicable Exclusive Field. In the event that Codex elects to convert Pfizer’s exclusive license with respect to an Exclusive Field to a non-exclusive license, such Exclusive Field will become a Non-Exclusive Field and will no longer be an “Exclusive Field” under this Agreement, in each case immediately as of the time of conversion. 5.3. Regulatory Approvals. Pfizer or its designated Affiliate(s) shall have the sole authority to file applications for Regulatory Approval for its Products for all Fields in the Territory,

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 37 including communicating with any Regulatory Authority both prior to and following Regulatory Approval. To the extent Pfizer requests any information or support with respect to any such application for Regulatory Approval for a Product, Codex shall provide reasonably requested support or information with any reasonable out-of-pocket costs incurred to be at Pfizer’s expense. 5.4. Commercialization Activities. 5.4.1. General. Pfizer shall have sole and exclusive control over all matters relating to the Commercialization of its Products for the Field in the Territory, including sole and exclusive control over (a) pricing of such Products in the Field and (b) the negotiation of pricing with Regulatory Authorities and other Third Parties for such Products in the Field. 5.4.2. Branding. Pfizer or its designated Affiliates or Sublicensees shall select and own all Trademarks used in connection with the commercialization of any and all Output Materials and its Products for the Field in the Territory. Neither Codex nor its Affiliates shall use or seek to register, anywhere in the world, any Trademark not otherwise in use by Codex at the time of selection by Pfizer, its designated Affiliate or Sublicensee which is confusingly similar to any Trademark used by or on behalf of Pfizer, its Affiliates or Sublicensees in connection with any such Product for the Field in the Territory. 5.5. Manufacturing. Pfizer shall have the exclusive right to Manufacture, itself or through one or more Affiliates or Third Parties selected by Pfizer in its sole discretion, its Products for the Field in the Territory. For clarity, Pfizer shall have no diligence obligations with respect to the Manufacture of such Products. 5.6. Reporting. All information or written reports provided by Pfizer to Codex under this Section 5.6 shall be deemed Confidential Information of Pfizer and subject to Section 7. 5.6.1. Development Notices. Pfizer shall notify Codex in writing promptly following Pfizer’s decision to [***] with respect to a Product that has been (a) discovered, designed, encoded or created using the Deliverables or (b) transcribed from DNA that was discovered, designed, encoded or created using the Deliverables (such notice, a “Development Notice”). 5.6.2. Progress Reports. Until the First Commercial Sale of a Product, Pfizer will provide Codex with an annual written report summarizing, on a Product-by-Product basis, Pfizer’s activities to Develop Products in the Field. 5.7. Other Pfizer Matters. Codex understands and acknowledges that Pfizer may have present or future initiatives or opportunities, including initiatives or opportunities with its Affiliates or Third Parties, involving the manufacture or synthesis of DNA or RNA, including products, programs, technologies or processes that are similar to, and in some instances may compete with, a Product, Instrument, or other program, technology or processes covered by this Agreement. Codex acknowledges and agrees that nothing in this Agreement will be construed as a

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 38 representation, warranty, covenant or inference that Pfizer will not itself Develop, Manufacture or Commercialize or enter into business relationships with one or more of its Affiliates or Third Parties to develop, Manufacture or synthesize DNA or RNA, or Manufacture or Commercialize any products, instruments, programs, technologies or processes that are similar to or that may compete with any Instrument, Product or other program, technology or process covered by this Agreement. 6. INTELLECTUAL PROPERTY 6.1. Ownership of Intellectual Property. Except as otherwise explicitly set forth in this Agreement, the Parties hereby agree that ownership and inventorship of any Know-How made by any Party, solely or jointly, pursuant to this Agreement and any and all Patent Rights claiming or disclosing any invention included in such Know-How shall be determined in accordance with United States patent laws. 6.1.1. Ownership of Pfizer Technology. As between the Parties, subject to Section 6.1.3, Pfizer shall own all right, title and interest in and to the Pfizer Technology. Codex, on behalf of itself and its Affiliates, hereby assigns and agrees to assign to Pfizer all of Codex’s and its Affiliates’ right, title and interest in and to all Pfizer Sole Research Plan Know-how and Pfizer Sole Research Plan Patent Rights. Codex further agrees to cooperate with Pfizer (at Pfizer’s request and expense) to execute and deliver any and all documents that Pfizer deems reasonably necessary to perfect and enforce Pfizer’s rights in and to the Pfizer Technology under this Section 6.1.1. 6.1.2. Ownership of Codex Technology. As between the Parties, subject to Section 6.1.3, Codex shall own all right, title and interest in and to the Codex Technology. Pfizer further agrees to cooperate with Codex (at Codex’s request and expense) to execute and deliver any and all documents that Codex deems reasonably necessary to perfect and enforce Codex’s rights in and to the Codex Technology under this Section 6.1.2. 6.1.3. Ownership of Joint Research Plan Technology. The Parties will jointly own any Joint Research Plan Technology. Subject to the licenses or other rights granted to Pfizer under Sections 2.1, 2.2, 2.3, and 2.4 and the Parties’ other rights and obligations under this Agreement (including Codex’s obligations under Section 8.4), each Party shall be free to exploit, either itself or through the grant of licenses to Third Parties (which Third Party licenses may be further sublicensed), Joint Research Plan Technology throughout the world without restriction, without the need to obtain further consent from or provide notice to the other Party, and without any duty to account or otherwise make any payment of any compensation to the other Party. 6.1.4. Disclosure. Each Party will promptly disclose to the other Party all inventions within the Research Plan Know-How that it develops or invents, whether solely or jointly with others (in any event, prior to the filing of any patent application with respect to such inventions), including all invention disclosures or other similar documents

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 39 submitted to such Party by its or its Affiliates’ employees, agents, or independent contractors relating thereto. Each Party will also promptly respond to reasonable requests from the other Party for additional information relating thereto. 6.2. Patent Rights. 6.2.1. Filing, Prosecution and Maintenance of Patent Rights. (a) Codex Sole Research Plan Patent Rights and Instrument/Methods Patent Rights. Codex shall have the sole right, at its sole expense, to file, prosecute and maintain the Codex Sole Research Plan Patent Rights and Instrument/Methods Patent Rights in its sole discretion; provided, however that no Codex Sole Research Plan Patent Right shall claim or disclose any Pfizer Sole Research Plan Know-How, in each case without Pfizer’s prior written consent. (b) Pfizer Sole Research Plan Patent Rights. Pfizer shall have the sole right, at its sole expense, to file, prosecute and maintain the Pfizer Sole Research Plan Patent Rights, including Pfizer Material Improvements or Output Material Patent Rights, in its sole discretion; provided, however that no Pfizer Sole Research Plan Patent Right shall claim or disclose any Codex Sole Research Plan Know- How, in each case without Codex’s prior written consent. (c) Joint Research Plan Technology. Notwithstanding anything herein to the contrary, in the event the Parties make any Joint Research Plan Know- How that is not Instrument/Method Know-How, the Parties shall discuss the timing of the filing and content of Joint Research Plan Patent Rights with the mutual goal of preserving the value of the Joint Research Plan Patent Rights. Unless otherwise agreed in writing by the Parties, (1) Pfizer will have the sole right to file, prosecute and maintain any Joint Research Plan Patent Rights that Cover or are primarily directed to one or more Products and (2) Codex will have the first right to file, prosecute and maintain other Joint Research Plan Patent Rights, in each case at such Party’s own cost and expense. With respect to Joint Research Plan Patent Rights, each Party will, as applicable, (i) provide the other Party a reasonable opportunity and reasonable time to review and provide comment to such Party’s counsel regarding relevant substantive communications by such Party and drafts of any responses or other proposed substantive filings by such Party before any applicable filings are submitted to any relevant patent office (or Governmental Authority) and (ii) reflect any reasonable and timely comments offered by the other Party in any final filings submitted by such Party to any relevant patent office (or Governmental Authority). In addition, if the Party with the first right to file, prosecute and maintain elects to cease the prosecution or maintenance of any Joint Research Plan Patent Right in any country or jurisdiction, it shall notify the other Party in writing sufficiently in advance (but not less than [***] before any action is required) so that such other Party may, at its discretion, assume the responsibility for the filing,

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 40 prosecution or maintenance of such Joint Research Plan Patent Right in such designated country. The Parties agree that either Party may use internal patent counsel and agents, filing clerks, and paralegals employed by such Party, for coordinating worldwide filings of such Patent Rights, for prosecution before the European and Japanese Patent Offices, and for directly instructing US and ex-US outside counsel and patent agents, including by providing draft applications and responses, and that such Party may employ its preferred outside counsel and patent agents to conduct such activities as required for US and ex-US prosecution). (d) The Parties shall at all times reasonably cooperate with each other in order to reasonably implement the foregoing provisions of this Section 6.2.1. Such cooperation may include each Party’s execution of necessary legal documents, coordinating filing and/or prosecution of applications to avoid potential issues during prosecution (including novelty, enablement, estoppel and double patenting, execution of amendments and documents for reliance on the CREATE Act, if needed), and the assistance of each Party’s relevant personnel. Without limiting the foregoing, it is understood that even if a Party is permitted to reference the other Party’s technology created, discovered or utilized in the Research Program in a patent application filed pursuant to this Agreement, the filing Party shall not file any such patent application without first notifying the non-filing Party of the disclosure of the non-filing Party’s technology. If the non-filing Party determines that any such filing could adversely affect its filing strategy, the filing Party shall delay filing any such patent application and the Parties shall cooperate in accordance with this Section 6.2.1(d) to determine a strategy that would protect each Party’s interests. However, the filing Party will only be required to delay filing for a maximum of [***] days after notice to the non-filing Party unless the non-filing Party can show that a limited additional time period would allow it to protect its interests without prejudice to the filing Party’s interests. Pfizer shall not disclose and/or claim in any patent application, patent or publication any Codex Confidential Information without Codex’s prior written consent. Codex shall not disclose and/or claim in any patent application, patent or publication any Pfizer Confidential Information without Pfizer’s prior written consent. 6.2.2. Clarifications. For clarity, (i) prosecution under this Section 6.2 includes opposition, revocation, post-grant review or other patent office proceedings, unless such proceedings are concurrent with Third Party litigation under Section 6.3, in which case the provisions of Section 6.3 shall govern the Parties’ rights and obligations with respect to such proceedings, and (ii) Third Party declaratory judgment actions or other court actions relating to Patent Rights shall be governed by Sections 6.3.2 and 6.3.3, if applicable. 6.2.3. Liability. To the extent that a Party is obtaining, prosecuting or maintaining a Patent Right or otherwise exercising its rights under this Section 6.2, such Party, and its Affiliates, employees, agents or representatives, will not be liable to the other Party in respect of any act, omission, default or neglect on the part of any such Party, or its

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 41 Affiliates, employees, agents or representatives, in connection with such activities undertaken in good faith. 6.2.4. Recording. If Pfizer deems it necessary or desirable to register or record this Agreement or evidence of this Agreement with any patent office or other appropriate Governmental Authority(ies) in one or more jurisdictions in the Territory, Codex shall reasonably cooperate (at Pfizer’s request and expense) to execute and deliver to Pfizer any documents accurately reflecting or evidencing this Agreement that are necessary or desirable, in Pfizer’s reasonable judgment, to complete such registration or recordation. Pfizer shall reimburse Codex for all reasonable out-of-pocket expenses, including attorneys’ fees, incurred by Codex in complying with the provisions of this Section 6.2.4. 6.3. Enforcement and Defense of Patent Rights. 6.3.1. Enforcement. (a) Enforcement of Codex Patent Rights. Codex shall have the sole right, but no obligation, to take action to obtain a discontinuance of infringement or bring suit against a Third Party infringing or challenging the validity or enforceability of any Codex Patent Right other than a Joint Research Plan Patent Right. (b) Enforcement of Pfizer Patent Rights. Pfizer shall have the sole right, but no obligation, to take action to obtain a discontinuance of infringement or bring suit against a Third Party infringing or challenging the validity or enforceability of any Pfizer Patent Right other than a Joint Research Plan Patent Right. (c) Enforcement of Joint Research Plan Patent Rights. Notwithstanding anything herein to the contrary, each Party will promptly notify the other in the event of any actual, potential or suspected infringement of a patent under the Joint Research Plan Patent Rights by any Third Party. As between Pfizer and Codex, Pfizer will have the sole right, but not the obligation, to institute litigation or take other steps to remedy infringement in connection with the Joint Research Plan Patent Rights by the development, manufacture or commercialization of an Product in the Territory, and any such litigation or steps will be at Pfizer’s expense, subject to Codex’s obligation to indemnify Pfizer for such expenses pursuant to Section 10; provided that any infringement recoveries resulting from such litigation or steps relating to a claim of Third Party infringement, after deducting Pfizer’s out of pocket expenses (including counsel fees and expenses) in pursuing such claim, will be deemed Net Sales. Pfizer will not, without the prior written consent of Codex, enter into any compromise or settlement relating to such litigation that (i) admits the invalidity or unenforceability of any Codex Patent Right or Joint Research Plan Patent Right or (ii) requires Pfizer

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 42 to abandon any Joint Research Plan Patent Right. Codex, upon request of Pfizer, agrees to timely commence or to join in any such litigation, at Pfizer’s expense, and in any event to cooperate with Pfizer in such litigation or steps at Pfizer’s expense. Codex will have the right to consult with Pfizer about such litigation and to participate in and be represented by independent counsel in such litigation at Codex’s own expense. Neither Party will incur any liability to the other Party (other than that related to a Party’s indemnification obligation pursuant to Section 10) as a consequence of any litigation initiated or pursued pursuant to this Section 6.3 or any unfavorable decision resulting therefrom, including any decision holding any Codex Patent Right or Joint Research Plan Patent Right invalid or unenforceable. With respect to instituting litigation or taking other steps to remedy infringement in connection with the other Joint Research Plan Patent Rights, the Parties shall determine a mutually agreeable course of action. In no event shall a Party make an argument or settle a dispute which would render a claim in a Joint Research Plan Patent Right to be invalid or unenforceable without the other Party’s prior written consent. 6.3.2. Allegations of Infringement and Right to Seek Third Party Licenses. (a) Notice. If Codex receives written notice from a Third Party that the practice of any Codex Technology, or the exercise of any other right granted by Codex to Pfizer hereunder (collectively, the “Licensed Activities”) by Pfizer or any of its Affiliates or Sublicensees is alleged by such Third Party to infringe, misappropriate or otherwise violate such Third Party’s Patent Rights or other intellectual property rights, or Codex otherwise identifies any Third Party Patent Rights or other intellectual property rights that may be relevant to such activities, Codex shall, promptly upon becoming aware of such allegation or identification, notify Pfizer in writing. (b) Pfizer Option to Negotiate. If Pfizer determines, in its sole discretion, that, in order for Pfizer, its Affiliates or Sublicensees to engage in the Licensed Activities, it is necessary or desirable to obtain a license under one or more Patent Rights or other intellectual property rights Controlled by a Third Party, then Pfizer shall have the sole right, but not the obligation, to negotiate and enter into an exclusive license or other agreement with such Third Party in the Field under such Patent Rights or other intellectual property rights; provided that if and to the extent such Patent Rights are necessary to practice Core Codex DNA Technology, Pfizer shall only negotiate and enter into a non-exclusive license or other agreement with such Third Party with respect to the Non-Exclusive Field. 6.3.3. Third Party Infringement Suits. Each of the Parties shall promptly notify the other in the event that any Third Party files any suit or brings any other action alleging patent infringement by Pfizer or Codex or any of their respective Affiliates or Sublicensees with respect to the practice of any Codex Technology or Licensed Activities

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 43 (any such suit or other action referred to herein as an “Infringement Claim”). In the case of any Infringement Claim against Pfizer (including its Affiliates or Sublicensees) alone or against both Pfizer and Codex (including its Affiliates), Pfizer shall have the right, but not the obligation, to control the defense of such Infringement Claim, including control over any related litigation, settlement, appeal or other disposition arising in connection therewith. Codex, upon request of Pfizer, agrees to join in any litigation associated with any Infringement Claim at Pfizer’s expense and in any event to cooperate with Pfizer at Pfizer’s expense. Codex will have the right to consult with Pfizer concerning any Infringement Claim and to participate in and be represented by independent counsel in any associated litigation in which Codex is a party at Codex’s own expense. If Pfizer elects to control the defense of any Infringement Claim and Codex is obligated under Section 10.3 to indemnify Pfizer (including any Pfizer Indemnified Party) with respect to such Infringement Claim, then (a) Pfizer will bear [***] incurred in investigating, preparing or defending such Infringement Claim notwithstanding the provisions of Section 10.3 and (b) Codex will otherwise indemnify Pfizer and any applicable Pfizer Indemnified Parties to the full extent provided for under Section 10.3. In the case of any Infringement Claim against Codex alone, Pfizer shall have the right to consult with Codex concerning such Infringement Claim and Pfizer, upon request of Codex, will reasonably cooperate with Codex at Codex’s expense (but Pfizer shall have no obligation to join any Infringement Claim or associated litigation). 6.3.4. Enforcement and Defense of Know-How. (a) Misappropriation Actions Relating to Codex Know-How. Codex shall have the sole right, but no obligation, to take action to obtain a discontinuance of misappropriation or bring suit against a Third Party that is misappropriating, or that is suspected of misappropriating, any Codex Know-How other than a Joint Research Plan Know-How. (b) Misappropriation of Actions Relating to Pfizer Know-How. Pfizer shall have the sole right, but no obligation, to take action to obtain a discontinuance of misappropriation or bring suit against a Third Party that is misappropriating, or that is suspected of misappropriating, any Pfizer Know-How and Pfizer Sole Research Plan Know-How other than a Joint Research Plan Know-How. (c) Misappropriation of Joint Research Plan Know-How. Each Party will promptly notify the other in the event of any actual, potential or suspected misappropriation of Joint Research Plan Know-How by any Third Party, and the Parties shall determine a mutually agreeable course of action. In no event shall a Party, without the prior written consent of other Party, enter into any compromise or settlement relating to such misappropriation claim that (i) admits that all or any portion of the Joint Research Plan Know-How is not protectable under relevant trade secret Laws or (ii) requires the other Party to abandon trade secret protection for any Joint Research Plan Know-How.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 44 6.4. Codex Third Party Agreements. If on or after the Effective Date, Codex contemplates entering into a Codex Third Party Agreement under which Pfizer would be obligated to comply with any additional non-financial provisions as a sublicensee or transferee, then Codex shall (a) notify Pfizer of such agreement and (b) provide Pfizer [***] to review and provide comments to the terms of such agreement prior to signing with any reasonable comments provided by Pfizer to be considered and implemented in good faith by Codex; provided that for any such Codex Third Party Agreement, Codex shall notify Pfizer within [***] days of execution of such agreement, such notice to be accompanied by a copy of such Codex Third Party License Agreement, and Pfizer will not be granted a sublicense under such Codex Third Party Agreement unless it notifies Codex in writing of such sublicense acceptance within [***] days of Codex’s notice. 7. CONFIDENTIALITY 7.1. Confidentiality. Except to the extent expressly authorized by this Agreement, the Parties agree that, during the Term and for five years thereafter, each Party (the “Receiving Party”) receiving any Confidential Information of the other Party (the “Disclosing Party”) hereunder shall: (a) keep the Disclosing Party’s Confidential Information confidential; (b) not disclose, or permit the disclosure of, the Disclosing Party’s Confidential Information; and (c) not use, or permit to be used, the Disclosing Party’s Confidential Information for any purpose other than as expressly permitted under the terms of this Agreement. 7.2. Authorized Disclosure. 7.2.1. Disclosure to Party Representatives. Notwithstanding the foregoing provisions of Section 7.1, the Receiving Party may disclose Confidential Information belonging to the Disclosing Party to the Receiving Party’s Representatives who (a) have a need to know such Confidential Information in connection with the performance of the Receiving Party’s obligations or the exercise of the Receiving Party’s rights under this Agreement and (b) have agreed in writing to non-disclosure and non-use provisions with respect to such Confidential Information that are at least as restrictive as those set forth in this Section 7. 7.2.2. Disclosure to Third Parties. Notwithstanding the foregoing provisions of Section 7.1, each Party may disclose Confidential Information belonging to the other Party to the extent such disclosure is reasonably necessary: (a) to Governmental Authorities (i) to the extent desirable to obtain or maintain INDs or Regulatory Approvals for any Output Material or Product within the Territory, and (ii) in order to respond to inquiries, requests or investigations relating to Output Material, Products or this Agreement; (b) to outside consultants (including any professional advisor), potential acquisition partners (including any potential successors in interest), private investors or financing sources, contractors, advisory boards, managed care

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 45 organizations, and non-clinical and clinical investigators, in each case to the extent desirable to develop, register or market any Output Material, Product; provided that the Receiving Party shall obtain the same confidentiality obligations from such Third Parties as it obtains with respect to its own similar types of confidential information, and at least as restrictive as those set forth in this Section 7; (c) in connection with filing or prosecuting Patent Rights or Trademark rights as permitted by this Agreement, (d) in connection with prosecuting or defending litigation arising from this Agreement; (e) subject to the provisions of Section 7.5.2, in connection with or included in scientific presentations and publications relating to Output Material, Products, including abstracts, posters, journal articles and the like, and posting results of and other information about clinical trials to ▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ or PhRMA websites; (f) Pfizer may disclose Confidential Information belonging to Codex (including the terms of this Agreement) to any bona fide or potential Sublicensee or co-development or co-promotion partner who has agreed in writing to non- disclosure and non-use provisions with respect to such Confidential Information that are at least as restrictive as those set forth in this Section 7; and (g) to the extent necessary or desirable in order to enforce its rights under this Agreement. If a Party deems it reasonably necessary to disclose Confidential Information belonging to the other Party pursuant to this Section 7.2.2, then the Party disclosing pursuant to this Section 7.2.2 shall to the extent possible give reasonable advance written notice of such disclosure to the other Party and take such measures to ensure confidential treatment of such information as is reasonably required by the other Party, at the other Party’s expense. 7.3. SEC Filings and Other Disclosures. Either Party may disclose the terms of this Agreement and make any other public written disclosure regarding the existence of, or performance under, this Agreement, to the extent required, in the reasonable opinion of such Party’s legal counsel, to comply with applicable Law, including the rules and regulations promulgated by the United States Securities and Exchange Commission or any equivalent Governmental Authority, securities exchange or securities regulator in any country in the Territory. Before disclosing this Agreement or any of the terms hereof pursuant to this Section 7.3, the Parties will consult with one another on the terms of this Agreement to be redacted in making any such disclosure, with the Party disclosing pursuant to this Section 7.3 providing as much advanced notice as is feasible under the circumstances (but in no event less than [***] unless law requires otherwise), and giving good faith consideration to the comments of the other Party.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 46 Further, if a Party discloses this Agreement or any of the terms hereof in accordance with this Section 7.3, such Party shall, at its own expense, seek such confidential treatment of confidential portions of this Agreement and such other terms, as may be reasonably requested by the other Party and limit its disclosure of such Confidential Information to only that required to comply with applicable Law. 7.4. Residual Knowledge Exception. Notwithstanding any provision of this Agreement to the contrary, Residual Knowledge shall not be considered Confidential Information for purposes of this Section 7; provided that, for clarity, a Party’s rights to Residual Knowledge hereunder shall not include the right to practice any Patent Right owned or Controlled by the other Party that claims or discloses such Residual Knowledge unless otherwise expressly granted in another provision of this Agreement or in another agreement between the Parties. 7.5. Public Announcements; Publications. 7.5.1. Announcements. Except as may be expressly permitted under Section 7.3 or this Section 7.5, neither Party will make any public announcement regarding this Agreement without the prior written approval of the other Party. For the sake of clarity, nothing in this Agreement shall prevent Pfizer from making any scientific publications or public announcement with respect to any Product under this Agreement; provided, however, that, except as permitted under Section 7.2, Pfizer shall not disclose any of Codex’s Confidential Information in any such publication or announcement without obtaining Codex’s prior written consent to do so. The Parties agree that the Parties will issue a mutually agreed upon joint press release regarding the signing of this Agreement following the Effective Date, substantially similar to the draft set forth in Schedule 7.5.1. 7.5.2. Publications. During the Term, each Party (the “Publishing Party”) shall submit to the other Party (the “Non-Publishing Party”) for review and approval any proposed publication or public presentation which contains the Non-Publishing Party’s Confidential Information. In addition, except as otherwise permitted pursuant to Section 7.3 or Section 7.5.1, each Publishing Party shall submit to the Non-Publishing Party for review and approval any proposed publication or public presentation relating to the Research Program. In both instances, such review and approval will be conducted for the purposes of preserving the value of the Codex Technology and the Pfizer Technology, and the rights granted to Pfizer hereunder and determining whether any portion of the proposed publication or presentation containing the Non-Publishing Party’s Confidential Information should be modified or deleted. Written copies of such proposed publication or presentation required to be submitted hereunder shall be submitted to the Non- Publishing Party no later than [***] days before submission for publication or presentation (the “Review Period”). The Non-Publishing Party shall provide its comments with respect to such publications and presentations within [***] days of its receipt of such written copy. The Review Period may be extended for an additional [***] days in the event the Non- Publishing Party can, within [***] days of receipt of the written copy, demonstrate reasonable need for such extension including for the preparation and filing of patent

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 47 applications. Each Publishing Party will comply with standard academic practice regarding authorship of scientific publications and recognition of contribution of other parties in any publication governed by this Section 7.5.2, including International Committee of Medical Journal Editors standards regarding authorship and contributions. For the sake of clarity, Pfizer’s obligation to submit any publication to Codex for review and approval under this Section 7.5.2 shall not apply to any publication which does not contain Codex’s Confidential Information. In addition, Codex may use and disclose certain anonymized data obtained from performing the Research Program for the purpose of presenting the capabilities of the Codex Technology, Instrument or Methods to existing or prospective Codex partners, investors, acquirers or to members of the media; provided, that the use and disclosure of such anonymized data fall under one of the use rights as listed in Exhibit E, and Codex obtains Pfizer’s prior written consent from Pfizer prior to such disclosure. Such anonymized data to be used from the Research Program shall make no reference to the fact that the data was generated under this Agreement, using the Pfizer Materials provided by Pfizer or the name or mechanism of action of any Product. 7.6. Obligations in Connection with Change of Control. If Codex is subject to a Change of Control during the Research Term, Codex will, and it will cause its Representatives to, ensure that no Confidential Information of Pfizer is released to (a) any Affiliate of Codex that becomes an Affiliate as a result of the Change of Control or (b) any other Representatives of Codex (or of the relevant surviving entity of such Change of Control) who become Codex Representatives as a result of the Change of Control, unless such Affiliate or other Representatives, as applicable, have signed confidentiality agreements which include equivalent obligations to those set out in this Section 7. If any Change of Control of Codex occurs, Codex shall promptly notify Pfizer, share with Pfizer the policies and procedures it plans to implement in order to protect the confidentiality of Pfizer’s Confidential Information prior to such implementation and make any adjustments to such policies and procedures that are reasonably requested by Pfizer. 8. REPRESENTATIONS, WARRANTIES AND COVENANTS. 8.1. Mutual Representations and Warranties. Each of Codex and Pfizer hereby represents and warrants to the other Party that: 8.1.1. it is duly organized, validly existing and in good standing under the laws of the jurisdiction of its organization; 8.1.2. the execution, delivery and performance of this Agreement by such Party has been duly authorized by all requisite action under the provisions of its charter, bylaws and other organizational documents, and does not require any action or approval by any of its shareholders or other holders of its voting securities or voting interests; 8.1.3. it has the power and authority to execute and deliver this Agreement and to perform its obligations hereunder;

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 48 8.1.4. this Agreement has been duly executed and is a legal, valid and binding obligation on each Party, enforceable against such Party in accordance with its terms; and 8.1.5. the execution, delivery and performance by such Party of this Agreement and its compliance with the terms and provisions hereof does not and will not conflict with or result in a breach of or default under any Binding Obligation existing as of the Effective Date. 8.2. Mutual Covenants. Each of Codex and Pfizer hereby covenants to the other Party that, from the Effective Date until expiration or termination of this Agreement, it will perform its obligations under this Agreement in compliance with applicable Laws. 8.3. Representations and Warranties of Codex. As of the Effective Date, Codex hereby represents and warrants to Pfizer that: 8.3.1. the execution and delivery of this Agreement by Codex, and the consummation of the transactions contemplated hereby and thereby, do not require any material consent, waiver, authorization or approval of, or any notice to or other filing with, any Governmental Authority; 8.3.2. it is in compliance in all material respects with all Laws applicable to the ownership of the Initial Instrument and Initial Methods, including all manufacturing, development, marketing and regulatory filing and maintenance activities related to the Initial Instrument and Initial Methods to the extent necessary for Codex to perform those activities assigned to it under the Research Program; 8.3.3. [***]; 8.3.4. it has and will have the full right, power and authority to grant all of the right, title and interest in the licenses and other rights granted or to be granted to Pfizer, Pfizer’s Affiliates or Pfizer’s Sublicensees under this Agreement; 8.3.5. (a) Exhibit A sets forth a true and complete list of all Codex Patent Rights as of the Effective Date, (b) each such Codex Patent Right is in full force and effect and (c) Codex or its Affiliates have timely paid all filing and renewal fees payable with respect to such Codex Patent Rights; 8.3.6. (a) the issued and pending Codex Patent Rights as of the Effective Date, are, or upon issuance, will be, valid and enforceable patents and (b) [***]; 8.3.7. Codex, its Affiliates and Third Parties and Representatives acting on Codex’s behalf in connection with this Agreement have complied with all applicable Laws, including any disclosure requirements, in connection with the filing, prosecution and maintenance of the Codex Patent Rights;

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 49 8.3.8. Codex, its Affiliates, and to its knowledge, all Third Parties and Representatives acting on Codex’s behalf, have and will comply in all material respects with all applicable Law and accepted pharmaceutical industry business practices, including, to the extent applicable, the FD&C Act (21 U.S.C. § 301, et seq.), the Anti- Kickback Statute (42 U.S.C. § 1320a-7b), Civil Monetary Penalty Statute (42 U.S.C. § 1320a-7a), the False Claims Act (31 U.S.C. § 3729 et seq.), comparable state statutes, the regulations promulgated under all such statutes, and the regulations issued by the FDA, consistent with the ‘Compliance Program Guidance for Pharmaceutical Manufacturers’ published by the Office of Inspector General, U.S. Department of Health and Human Services; 8.3.9. [***]; 8.3.10. Codex, its Affiliates, and to its knowledge all Third Parties and Representatives acting on Codex’s behalf, have and will continue to comply with the laws and regulations of the countries where it operates, including anti-bribery and anti- corruption laws, including, to the extent applicable, the U.S. Foreign Corrupt Practices Act of 1977 and the U.K. ▇▇▇▇▇▇▇ ▇▇▇ ▇▇▇▇, accounting and record keeping laws, and laws relating to interactions with healthcare professionals or healthcare providers and Government Officials; 8.3.11. [***]; 8.3.12. [***]; 8.3.13. no Codex Technology existing as of the Effective Date is subject to any funding agreement with any government or Governmental Authority; 8.3.14. [***]; 8.3.15. there is no (a) claim, demand, suit, proceeding, arbitration, inquiry, investigation or other legal action of any nature, civil, criminal, regulatory or otherwise, pending or, to the best knowledge of Codex, threatened against Codex or any of its Affiliates or (b) judgment or settlement against or owed by Codex or any of its Affiliates, in each case with respect to the Codex Technology, the Current Licenses, or the Instrument, or the transactions contemplated by this Agreement; 8.3.16. [***]; 8.3.17. there are no Current Licenses and no Third Party has any right, title or interest in or to, or any license under, any Codex Technology; and 8.3.18. the (a) conduct of the Research Plan by or on behalf of Codex, including the use of the Initial Instrument and Initial Methods in the conduct of the Research Plan, (b) use by or on behalf of Pfizer of the Initial Instruments and Initial Methods in the conduct

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 50 of the Research Plan and (c) the use by Codex or the authorized use by Pfizer of the Deliverables, in each case ((a)-(c)), (x) does not and will not infringe any issued patent of any Third Party or (y) will not infringe the claims of any published Third Party patent application when and if such claims issue. For purposes of this Section 8.3, “knowledge” means the actual knowledge of the individuals listed in Schedule 8.3, after consulting with their direct reports. 8.4. Codex Covenants. In addition to the covenants made by Codex elsewhere in this Agreement, Codex hereby covenants to Pfizer that, from the Effective Date until expiration or termination of this Agreement: 8.4.1. it shall not, and shall cause its Affiliates not to (a) exclusively license, sell, assign or otherwise transfer (other than in a connection with a permitted assignment of this Agreement by Codex pursuant to Section 12.1) to any Person (other than Pfizer or its Affiliates or Sublicensees pursuant to the terms of this Agreement) any Codex Technology (or agree to do any of the foregoing) or (b) incur or permit to exist, with respect to any Codex Technology, any lien, encumbrance, charge, security interest, mortgage, liability, assignment, grant of license or other Binding Obligation that is or would be inconsistent with the licenses and other rights granted to Pfizer or its Affiliates under this Agreement; 8.4.2. [***]; 8.4.3. it will (a) not enter into any Codex Third Party Agreement that adversely affects (i) the rights granted to Pfizer, Pfizer’s Affiliates or Sublicensees hereunder or (ii) Codex’s ability to fully perform its obligations hereunder; (b) not amend or otherwise modify any Codex Third Party Agreement or consent or waive rights with respect thereto in any manner that (i) adversely affects the rights granted to Pfizer or Pfizer’s Affiliates or Sublicensees hereunder or (ii) Codex’s ability to fully perform its obligations hereunder; (c) promptly furnish Pfizer with true and complete copies of all Codex Third Party Agreements executed following the Effective Date and amendments thereto, in each case which may be redacted with respect to matters unrelated to the Agreement (including the rights and obligations hereunder); (d) remain, and cause its Affiliates to remain, in compliance in all material respects with all Codex Third Party Agreements; and (e) furnish Pfizer with copies of all notices received by Codex or its Affiliates of any alleged breach or default by Codex or its Affiliates under any Codex Third Party Agreement within five Business Days after receipt thereof; 8.4.4. it will not enter into or otherwise allow itself or its Affiliates to be subject to any agreement or arrangement which limits the ownership or licensed rights of Pfizer or its Affiliates with respect to, or limits the ability of Pfizer or its Affiliates to grant a license, sublicense or access, or provide or provide access or other rights in, to or under, any intellectual property right or material (including any Patent Right, Know-How or other data or information), in each case, that would, but for such agreement or arrangement, be

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 51 included in the rights licensed or assigned to Pfizer or its Affiliates pursuant to this Agreement; and 8.4.5. [***]. 8.4.6. [***] 8.5. Pfizer Covenants. In addition to the covenants made by Pfizer elsewhere in this Agreement, Pfizer hereby covenants to Codex that, from the Effective Date until expiration or termination of this Agreement: 8.5.1. [***]; and 8.5.2. [***]. 8.6. Representation by Legal Counsel. Each Party hereto represents that it has been represented by legal counsel in connection with this Agreement and acknowledges that it has participated in the drafting hereof. In interpreting and applying the terms and provisions of this Agreement, the Parties agree that no presumption shall exist or be implied against the Party which drafted such terms and provisions. 8.7. Disclaimer. THE FOREGOING REPRESENTATIONS AND WARRANTIES OF EACH PARTY ARE IN LIEU OF ANY OTHER REPRESENTATIONS AND WARRANTIES, EXPRESS OR IMPLIED, INCLUDING ANY IMPLIED WARRANTIES OF MERCHANTABILITY OR ANY IMPLIED WARRANTIES OF FITNESS FOR A PARTICULAR PURPOSE, ALL OF WHICH ARE HEREBY SPECIFICALLY EXCLUDED AND DISCLAIMED. 9. TERM AND TERMINATION 9.1. Term. The term of this Agreement (the “Term”) will commence on the Effective Date and expire upon the expiration of the last to expire Royalty Term, unless this Agreement is terminated earlier in accordance with this Section 9 or pursuant to Section 12.2. 9.2. Termination by Codex. Codex may terminate this Agreement for cause, at any time during the Term, by giving written notice to Pfizer in the event that Pfizer commits a material breach of its obligations under this Agreement and such material breach remains uncured for [***] days, measured from the date written notice of such material breach is given to Pfizer; provided, however, that if any breach is not reasonably curable within [***] days and if Pfizer is making a bona fide effort to cure such breach, such termination shall be delayed for a time period to be agreed by both Parties in order to permit Pfizer a reasonable period of time to cure such breach. If the alleged material breach relates to non-payment of any amount due under this Agreement, the cure period shall be tolled pending resolution of any bona fide dispute between the Parties as to whether such payment is due.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 52 9.3. Termination by Pfizer. 9.3.1. Termination for Convenience. Pfizer may (1) terminate this Agreement in its entirety without cause, for any or no reason, upon at least [***] days prior written notice to Codex or (2) it may terminate its exclusive license under Section 2.4 with respect to either Exclusive Field, without cause, for any or no reason, upon at least [***] days prior written notice to Codex. 9.3.2. Termination for Cause. Pfizer may terminate (1) this Agreement in its entirety for cause at any time during the Term or (2) its exclusive license under Section 2.4 with respect to either Exclusive Field, in each case ((1) and (2)), by giving written notice to Codex, in the event that Codex commits a material breach of its obligations under this Agreement and such material breach remains uncured for [***] days, measured from the date written notice of such material breach is given to Codex; provided, however, that if any breach is not reasonably curable within [***] days and if Codex is making a bona fide effort to cure such breach, such termination shall be delayed for a time period to be agreed by both Parties in order to permit Codex a reasonable period of time to cure such breach. 9.3.3. Termination for Global Trade Control Laws-related Breach. Notwithstanding anything to the contrary in this Agreement, Pfizer may terminate this Agreement in whole or relevant part, immediately and without regard to any cure period, if, in Pfizer’s reasonable opinion, a violation of Global Trade Control Laws has occurred. Any such termination will be deemed for cause under Section 9.2 or 9.3.2, under which Pfizer will not be responsible for any related payments due, even if activities have already occurred. Codex will be responsible for reimbursing Pfizer for any payments due to Pfizer under this Agreement that are specifically blocked due to violation of Global Trade Control Laws. 9.3.4. Termination for Compliance with the Law-related Breach. Pfizer may terminate this Agreement pursuant to Section 9.3.2 if (a) Codex breaches any of its representations or warranties set forth in Sections 8.3.7 through 8.3.10, or if Pfizer learns that improper payments are being or have been made to Government Officials by Codex with respect to services performed in connection with this Agreement. Any such termination will be deemed for cause under Section 9.3.2, under which Pfizer will not be responsible for any related payments due, even if activities have already occurred. 9.4. Effects of Termination 9.4.1. Effect of Termination. (a) Termination for Cause by Codex; Termination for Convenience by Pfizer. (i) Complete Termination. In the event that Codex terminates this Agreement in its entirety for cause pursuant to Section 9.2 or Pfizer

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 53 terminates this Agreement without cause pursuant to Section 9.3.1, except as otherwise expressly provided herein, all rights and obligations of each Party hereunder shall cease (including all rights and licenses and sublicenses granted by either Party to the other Party hereunder). (ii) Partial Termination. In the event that Pfizer, pursuant to Section 9.3.1(2), terminates its exclusive license under Section 2.4 with respect to an Exclusive Field, the exclusive license with respect to such Exclusive Field will terminate as of the effective date of such termination and, at Pfizer’s request, such Exclusive Field will become a Non- Exclusive Field subject to the non-exclusive license in Section 2.2 of this Agreement. (b) Termination for Cause by Pfizer. (i) Complete Termination. (A) In the event that Pfizer terminates this Agreement in its entirety pursuant to Section 9.3.2, except as otherwise expressly provided herein, all other rights and obligations of each Party with respect to all Products throughout the Territory shall cease. (B) [***]. (ii) Partial Termination. (A) In the event that Pfizer, pursuant to Section 9.3.2(2), terminates its exclusive license under Section 2.4 with respect to an Exclusive Field, the exclusive license with respect to such Exclusive Field will terminate as of the effective date of such termination and, at Pfizer’s request, such Exclusive Field will become a Non-Exclusive Field subject to the non-exclusive license in Section 2.2 of this Agreement. (B) [***]. 9.4.2. Accrued Rights. Expiration or termination of this Agreement for any reason shall be without prejudice to any right which shall have accrued to the benefit of either Party prior to such termination, including damages arising from any breach under this Agreement. Expiration or termination of this Agreement shall not relieve either Party from any obligation which is expressly indicated to survive such expiration or termination. 9.4.3. Survival. The following sections, together with any sections that expressly survive (including any perpetual licenses granted hereunder), shall survive

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 54 expiration or termination of this Agreement for any reason: Sections 1 (Definitions and Interpretation), 2.5 (Unblocking License), 2.9 (No Implied Rights), 2.10 (Retained Rights), 3.9.4 (Record Keeping), 3.9.5 (Audits), 3.9.6 (Underpayments/Overpayments), 4.9.2 (Permitted Use of Pfizer Materials), 4.9.3 (Unauthorized Use of Pfizer Materials), 4.9.4 (Title to Pfizer Materials and Output Materials), 4.9.5 (Return of Pfizer Materials), 4.9.6 (Ownership of Pfizer Material Improvements and Output Materials), 4.9.7 (Activities), 4.9.8 (Confidentiality), 6.1 (Ownership of Intellectual Property), 7 (Confidentiality) (for the period set forth therein), 9.4 (Effects of Termination), 9.5 (Provision for Insolvency), 10.1 (No Consequential Damages), 10.2 (Indemnification by Pfizer), 10.3 (Indemnification by Codex), 10.4 (Procedure), and 12 (Miscellaneous). 9.5. Provision for Insolvency. 9.5.1. Termination Right. Codex shall be deemed a “Debtor” under this Agreement if, at any time during the Term (a) a case is commenced by or against Codex under the Bankruptcy Code, (b) Codex files for or is subject to the institution of bankruptcy, reorganization, liquidation or receivership proceedings (other than a case under the Bankruptcy Code), (c) Codex assigns all or substantially all of its assets for the benefit of creditors, (d) a receiver or custodian is appointed for Codex’s business or (e) substantially all of Codex’s business is subject to attachment or similar process; provided, however, that in the case of any involuntary case under the Bankruptcy Code, Codex shall not be deemed a Debtor if the case is dismissed within [***] days after the commencement thereof. If Codex is deemed a Debtor, then Pfizer may terminate this Agreement by providing written notice to Codex. If Pfizer terminates this Agreement pursuant to this Section 9.5.1, then (i) all licenses granted to Pfizer under this Agreement shall become irrevocable and perpetual, (ii) Pfizer shall have no further obligations to Codex under this Agreement other than [***]. 9.5.2. Rights to Intellectual Property. All rights and licenses now or hereafter granted by Codex to Pfizer under or pursuant to any Section of this Agreement, including Sections 2.1.1, 2.2 and, following the exercise of an Option pursuant to Section 2.4.2, Section 2.4.3 hereof, are rights to “intellectual property” (as defined in the Bankruptcy Code). The Parties hereto acknowledge and agree that the payments provided for under Sections 3.1, 3.2, 3.3 and 3.4 and all other payments by Pfizer to Codex hereunder, other than royalty payments pursuant to Section 3.7, do not constitute royalties within the meaning of Section 365(n) of the Bankruptcy Code or relate to licenses of intellectual property hereunder. If (a) a case under the Bankruptcy Code is commenced by or against Codex, (b) this Agreement is rejected as provided in the Bankruptcy Code and (c) Pfizer elects to retain its rights hereunder as provided in Section 365(n) of the Bankruptcy Code, then Codex (in any capacity, including debtor-in-possession) and its successors and assigns (including any trustee) shall provide to Pfizer all intellectual property licensed hereunder, and agrees to grant and hereby grants to Pfizer and its Affiliates a right to access and to obtain possession of and to benefit from and, in the case of any chemical or biological material or other tangible item of which there is a fixed or limited quantity, to obtain a pro

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 55 rata portion of, each of the following to the extent related to any Output Material or Product and the rights and licenses granted to Pfizer under or pursuant to this Agreement: (i) copies of pre-clinical and clinical research data and results; (ii) all of the following (to the extent that any of the following are so related): cell lines, antibodies, assays, reagents and other biological materials; (iii) Product samples; (iv) Codex Technology, (v) laboratory notes and notebooks; (vi) Product data or filings, and (vii) Rights of Reference in respect of regulatory filings and approvals, all of which constitute “embodiments” of intellectual property pursuant to Section 365(n) of the Bankruptcy Code, and (viii) all other embodiments of such intellectual property, whether any of the foregoing are in Codex’s possession or control or in the possession and control of any Third Party but which Codex has the right to access or benefit from and to make available to Pfizer. Codex shall not interfere with the exercise by Pfizer or its Affiliates of rights and licenses to intellectual property licensed hereunder and embodiments thereof in accordance with this Agreement and agrees to use Commercially Reasonable Efforts to assist Pfizer and its Affiliates (at Pfizer’s request) to obtain such intellectual property and embodiments thereof in the possession or control of Third Parties as reasonably necessary or desirable for Pfizer or its Affiliates or Sublicensees to exercise such Person’s rights and licenses in accordance with this Agreement. 9.5.3. No Limitation of Rights. All rights, powers and remedies of Pfizer provided in this Section 9.5 are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at Law or in equity (including the Bankruptcy Code) in the event of the commencement of a case under the Bankruptcy Code involving Codex. 10. LIMITATION ON LIABILITY, INDEMNIFICATION AND INSURANCE. 10.1. No Consequential Damages. Except with respect to liability arising from a breach of Section 6 or 7, a breach by Codex of Section Error! Reference source not found., from any willful misconduct or intentionally wrongful act, or to the extent such Party may be required to indemnify the other Party under this Section 10, in no event will either Party or its Representatives be liable under this Agreement for any special (only as related to indirect, incidental or consequential damages), indirect, incidental, consequential or punitive damages, whether in contract, warranty, tort, negligence, strict liability or otherwise, including loss of profits or revenue suffered by the other Party or any of its Representatives. Without limiting the generality of the foregoing, “consequential damages” will be deemed to include, and neither Party will be liable to the other Party or any of such other Party’s Representatives or stockholders for any damages based on or measured by loss of projected or speculative future sales of the Products, any Milestone Payments due upon any unachieved Development Milestone under Section 3.3, any Sales Milestone Payment due upon any unachieved Sales Milestones under Section 3.4, any unearned royalties under Section 3.7 or any other unearned, speculative or otherwise contingent payments provided for in this Agreement. EXCEPT WITH RESPECT TO LIABILITY ARISING FROM A BREACH OF SECTION 6 OR 7, A BREACH BY CODEX OF SECTION Error! Reference source not found., FROM ANY WILLFUL MISCONDUCT OR INTENTIONALLY

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 56 WRONGFUL ACT OR OMISSION, OR TO THE EXTENT SUCH PARTY MAY BE REQUIRED TO INDEMNIFY THE OTHER PARTY UNDER THIS SECTION 10, A PARTY’S AGGREGATE LIABILITY UNDER OR IN CONNECTION WITH THIS AGREEMENT FOR ANY CLAIM (INCLUDING RELATED LOSSES, COSTS OR DAMAGES OF ANY NATURE) IN A GIVEN CALENDAR YEAR SHALL NOT EXCEED [***]. 10.2. Indemnification by Pfizer. Pfizer will indemnify, defend and hold harmless Codex, each of its Affiliates, and each of its and its Affiliates’ employees, officers, directors and agents (each, a “Codex Indemnified Party”) from and against any and all claims, causes, or allegations (whether threatened or pending), judgments, expenses, damages, liabilities, obligations, fees (including the reasonable fees of attorneys and other consulting or testifying professionals), costs and losses (collectively, “Liabilities”) that the Codex Indemnified Party is required to pay to one or more Third Parties resulting from or arising out of: 10.2.1. the Development, Manufacture, Commercialization or use of any Product by, on behalf of, or under the authority of Pfizer, its Affiliates or Sublicensees (other than by any Codex Indemnified Party) except to the extent that such Liabilities arise from a claim that the practice of the Codex Technology infringes or misappropriates any issued patent or other proprietary right owned or possessed by any Third Party or any breach by Codex of any representations, warranties or covenants pursuant to this Agreement; 10.2.2. the material breach by Pfizer of any of its representations, warranties or covenants set forth in Section 8.1 or 8.2; or 10.2.3. the gross negligence, recklessness or intentional acts of Pfizer or any Pfizer Indemnified Party; except, in each case, to the extent caused by the gross negligence, recklessness or intentionally wrongful acts of Codex or any Codex Indemnified Party. 10.3. Indemnification by Codex. Codex will indemnify, defend and hold harmless Pfizer, and each of its Affiliates, Sublicensees, permitted contractors, and distributors, and each of Pfizer’s and their respective employees, officers, directors and agents (each, a “Pfizer Indemnified Party”) from and against any and all Liabilities that the Pfizer Indemnified Party is required to pay to one or more Third Parties resulting from or arising out of: 10.3.1. the material breach by Codex of any of its representations, warranties or covenants set forth in Section 8.1, Section 8.2, Section 8.3 (except with respect to Section 8.3.18) or Section 8.4; 10.3.2. the breach by Codex of Section 8.3.18; or 10.3.3. the gross negligence, recklessness or intentional acts of Codex or any Codex Indemnified Party;

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 57 except, in each case, to the extent caused by the gross negligence, recklessness or intentionally wrongful acts of Pfizer or any Pfizer Indemnified Party. 10.4. Procedure. 10.4.1. Notice. Each Party will notify the other Party in writing in the event it becomes aware of a claim for which indemnification may be sought hereunder. In the event that any Third Party asserts a claim or other proceeding (including any governmental investigation) with respect to any matter for which a Party (the “Indemnified Party”) is entitled to indemnification hereunder (a “Third Party Claim”), then the Indemnified Party shall promptly notify the Party obligated to indemnify the Indemnified Party (the “Indemnifying Party”) thereof; provided, however, that no delay on the part of the Indemnified Party in notifying the Indemnifying Party shall relieve the Indemnifying Party from any obligation hereunder unless (and then only to the extent that) the Indemnifying Party is prejudiced thereby. 10.4.2. Control. Subject to Pfizer’s right to control any actions described in Section 6.3.3 (even where Codex is the Indemnifying Party), the Indemnifying Party shall have the right, exercisable by notice to the Indemnified Party within [***] after receipt of notice from the Indemnified Party of the commencement of or assertion of any Third Party Claim, to assume direction and control of the defense, litigation, settlement, appeal or other disposition of the Third Party Claim (including the right to settle the claim solely for monetary consideration) with counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnified Party; provided that (a) the Indemnifying Party has sufficient financial resources, in the reasonable judgment of the Indemnified Party, to satisfy the amount of any adverse monetary judgment that is sought, (b) the Third Party Claim seeks solely monetary damages and (c) the Indemnifying Party expressly agrees in writing that as between the Indemnifying Party and the Indemnified Party, the Indemnifying Party shall be solely obligated to satisfy and discharge the Third Party Claim in full (the conditions set forth in clauses (a), (b) and (c) above are collectively referred to as the “Litigation Conditions”). Within [***] after the Indemnifying Party has given notice to the Indemnified Party of its exercise of its right to defend a Third Party Claim, the Indemnified Party shall give notice to the Indemnifying Party of any objection thereto based upon the Litigation Conditions. If the Indemnified Party reasonably so objects, the Indemnified Party shall continue to defend the Third Party Claim, at the expense of the Indemnifying Party, until such time as such objection is withdrawn. If no such notice is given, or if any such objection is withdrawn, the Indemnifying Party shall be entitled, at its sole cost and expense, to assume direction and control of such defense, with counsel selected by the Indemnifying Party and reasonably acceptable to the Indemnified Party. During such time as the Indemnifying Party is controlling the defense of such Third Party Claim, the Indemnified Party shall cooperate, and shall cause its Affiliates and agents to cooperate upon request of the Indemnifying Party, in the defense or prosecution of the Third Party Claim, including by furnishing such records, information and testimony and attending such conferences, discovery proceedings, hearings, trials or appeals as may reasonably be

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 58 requested by the Indemnifying Party. In the event that the Indemnifying Party does not satisfy the Litigation Conditions or does not notify the Indemnified Party of the Indemnifying Party’s intent to defend any Third Party Claim within ten Business Days after notice thereof, the Indemnified Party may (without further notice to the Indemnifying Party) undertake the defense thereof with counsel of its choice and at the Indemnifying Party’s expense (including reasonable, out-of-pocket attorneys’ fees and costs and expenses of enforcement or defense). The Indemnifying Party or the Indemnified Party, as the case may be, shall have the right to join in (including the right to conduct discovery, interview and examine witnesses and participate in all settlement conferences), but not control, at its own expense, the defense of any Third Party Claim that the other Party is defending as provided in this Agreement. 10.4.3. Settlement. The Indemnifying Party shall not, without the prior written consent of the Indemnified Party, enter into any compromise or settlement that commits the Indemnified Party to take, or to forbear to take, any action. The Indemnified Party shall have the sole and exclusive right to settle any Third Party Claim, on such terms and conditions as it deems reasonably appropriate, to the extent such Third Party Claim involves equitable or other non-monetary relief to be provided solely by the Indemnified Party, but shall not have the right to settle such Third Party Claim to the extent such Third Party Claim involves monetary damages without the prior written consent of the Indemnifying Party. Each of the Indemnifying Party and the Indemnified Party shall not make any admission of liability in respect of any Third Party Claim without the prior written consent of the other Party, and the Indemnified Party shall use reasonable efforts to mitigate Liabilities arising from such Third Party Claim. 10.5. Insurance. Each Party further agrees to obtain and maintain, during the Term, commercial general liability insurance, including products liability insurance, with reputable and financially secure insurance carriers to cover its indemnification obligations under Section 10.2 or Section 10.3, as applicable, in each case with limits of not less than [***] per occurrence and in the aggregate. Insurance shall be procured with carriers having an A.M. Best Rating of A-VII or better. Notwithstanding any provision of this Section 10.5 to the contrary, either Party may meet its obligations under this Section 10.5 through self-insurance at such described limits above. Neither Party’s insurance will be construed to create a limit of liability with respect to its indemnification obligations under this Section 10. 11. ANTI-BRIBERY/ANTI-CORRUPTION. 11.1. Foreign Corrupt Practices Act. Codex will comply with the U.S. Foreign Corrupt Practices Act of 1977 (as may be amended or revised from time-to-time) and the principles relating thereto, as stated in Exhibit C to this Agreement, which is attached hereto and incorporated by reference herein.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 59 11.2. Representations and Warranties. Codex hereby represents and warrants that: 11.2.1. It is licensed, registered, or qualified under local law, regulations, policies, and administrative requirements to do business and, to the extent required by applicable Law, has obtained licenses or completed such registrations as may be necessary or required by Law to provide the goods or services encompassed within this Agreement (including, without limitation, Exhibit C); 11.2.2. It has not and will not directly or indirectly offer or pay, or authorize such offer or payment, of any money or anything of value to improperly or corruptly seek to influence any Government Official (as such term is defined under Exhibit C), and, if Codex is itself a Government Official, has not accepted, and will not accept in the future, such a payment; and 11.2.3. All information provided by Codex during Pfizer’s pre-contractual due diligence, including all information provided in the “Third Party Entity Due Diligence Questionnaire,” is complete, truthful and accurate. 11.2.4. It undertakes to update these representations or warranties if (during the Term) Codex becomes aware, or should have become aware, that Codex, or any of the employees or individuals who will be primarily responsible for performing under this Agreement, or a relative of such an employee or individual, becomes a Government Official or if a Governmental Authority or Government Official becomes an owner of Codex. 11.3. Audit by Pfizer. Codex shall permit, during the Term and for three years after final payment has been made under this Agreement, Pfizer’s internal and external auditors' access to any relevant books, documents, papers, and records of Codex involving transactions or activities related to this Agreement. 12. MISCELLANEOUS. 12.1. Assignment. Neither this Agreement nor any interest hereunder shall be assignable by a Party without the prior written consent of the other Party, except as follows: (a) subject to the provisions of Section 12.2, as applicable, a Party may assign its rights and obligations under this Agreement by way of sale of itself or the sale of the portion of its business to which this Agreement relates, through merger, sale of assets and/or sale of stock or ownership interest, provided that the assignee shall expressly agree to be bound by such Party’s obligations under this Agreement and that such sale is not primarily for the benefit of its creditors and (b) such Party may assign its rights and obligations under this Agreement to any of its Affiliates, provided that the assignee shall expressly agree to be bound by such Party’s obligations under this Agreement and that such Party shall remain liable for all of its rights and obligations under this Agreement. In addition, Pfizer may assign its rights and obligations under this Agreement to a Third Party where Pfizer or its Affiliate is required, or makes a good faith determination based on advice of counsel, to divest a Product in order to comply with Law or the order of any Governmental Authority as a result of a

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 60 merger or acquisition, provided that the assignee shall expressly agree to be bound by Pfizer’s obligations under this Agreement. Each Party shall promptly notify the other Party of any assignment or transfer under the provisions of this Section 12.1. This Agreement shall be binding upon the successors and permitted assigns of the Parties and the name of a Party appearing herein shall be deemed to include the names of such Party’s successors and permitted assigns to the extent necessary to carry out the intent of this Agreement. Any assignment not in accordance with this Section 12.1 shall be void. 12.2. Change of Control of Codex. Codex shall notify Pfizer in writing promptly (and in any event within [***]) following the entering into of a definitive agreement with respect to a Change of Control of Codex. 12.3. Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the express purposes and intent of this Agreement. 12.4. Force Majeure. Each Party shall be excused from the performance of its obligations under this Agreement to the extent that such performance is prevented by force majeure (defined below) and the nonperforming Party promptly provides notice of the prevention to the other Party. Such excuse shall be continued so long as the condition constituting force majeure continues and the nonperforming Party takes Commercially Reasonable Efforts to remove the condition. For purposes of this Agreement, “force majeure” shall include conditions beyond the control of the Parties, including an act of God, voluntary or involuntary compliance with any regulation, Law or order of any government, war, act of terror, civil commotion, labor strike or lock-out, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe. 12.5. Notices. Any notice or notification required or permitted to be provided pursuant to the terms and conditions of this Agreement (including any notice of force majeure, breach, termination, change of address, etc.) shall be in writing and shall be deemed given upon receipt if delivered personally or by facsimile transmission (receipt verified), five days after deposited in the mail if mailed by registered or certified mail (return receipt requested) postage prepaid, or on the next Business Day if sent by overnight delivery using a nationally recognized express courier service and specifying next Business Day delivery (receipt verified), to the Parties at the following addresses or facsimile numbers (or at such other address or facsimile number for a Party as shall be specified by like notice, provided, however, that notices of a change of address shall be effective only upon receipt thereof): All correspondence to Pfizer shall be addressed as follows: Pfizer Inc. Notices: R&D Business Development ▇▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 61 ▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇ Attn.: R&D BD Contract Notice with a copy to: Pfizer Inc. Notices: Pfizer Legal Division ▇▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇ Attn.: Chief Counsel, R&D Fax: [***] and electronic copies to: [***] All correspondence to Codex shall be addressed as follows: CODEX DNA, Inc. ▇▇▇▇ ▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ ▇▇ ▇▇▇ ▇▇▇▇▇, ▇▇ ▇▇▇▇▇ Attention: Legal Department 12.6. Amendment. No amendment, modification or supplement of any provision of this Agreement shall be valid or effective unless made in writing and signed by a duly authorized officer of each Party. 12.7. Waiver. No provision of this Agreement shall be waived by any act, omission or knowledge of a Party or its agents or employees except by an instrument in writing expressly waiving such provision and signed by a duly authorized officer of the waiving Party. The waiver by either of the Parties of any breach of any provision hereof by the other Party shall not be construed to be a waiver of any succeeding breach of such provision or a waiver of the provision itself. 12.8. Severability. If any clause or portion thereof in this Agreement is for any reason held to be invalid, illegal or unenforceable, the same shall not affect any other portion of this Agreement, as it is the intent of the Parties that this Agreement shall be construed in such fashion as to maintain its existence, validity and enforceability to the greatest extent possible. In any such event, this Agreement shall be construed as if such clause of portion thereof had never been contained in this Agreement, and there shall be deemed substituted therefor such mutually agreed provision as will most nearly carry out the intent of the Parties as expressed in this Agreement to the fullest extent permitted by applicable Law.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 62 12.9. Descriptive Headings. The descriptive headings of this Agreement are for convenience only and shall be of no force or effect in construing or interpreting any of the provisions of this Agreement. 12.10. Global Trade Control Laws. The Parties acknowledge that certain activities covered by or performed under this Agreement may be subject to laws, regulations or orders regarding economic sanctions, import controls or export controls (“Global Trade Control Laws”). Each of the Parties will perform all activities under this Agreement in compliance with all applicable Global Trade Control Laws. Furthermore, with respect to the activities performed under this Agreement, each of the Parties represents, warrants and covenants that: 12.10.1. Each Party will not, for activities under this Agreement, (i) engage in any such activities in a Restricted Market; (ii) involve individuals ordinarily resident in a Restricted Market; or (iii) include companies, organizations, Governmental Authorities or Government Officials from or located in a Restricted Market. “Restricted Market” for purposes of this Agreement means the Crimean Peninsula, Cuba, the Donbass Region, Iran, North Korea, Sudan, and Syria, or any other country or region sanctioned by the United States or European Union. 12.10.2. Each Party represents and warrants that it is not a Restricted Party and is not owned or controlled by a Restricted Party. With respect to activities performed under this Agreement, neither Party will engage or delegate to any Restricted Parties for any activities under this Agreement. Each Party will screen all relevant Third Parties involved by such Party in the activities under this Agreement under the relevant Restricted Party Lists. “Restricted Parties” for purposes of this Agreement means any individual or entity on any of the following “Restricted Party Lists”: the list of sanctioned entities maintained by the United Nations; the Specially Designated Nationals List and the Sectoral Sanctions Identifications List of the U.S. Treasury Department’s Office of Foreign Assets Control; the U.S. Denied Persons List, the U.S. Entity List, and the U.S. Unverified List of the U.S. Department of Commerce; entities subject to restrictive measures and the Consolidated List of Persons, Groups and Entities Subject to E.U. Financial Sanctions, as implemented by the E.U. Common Foreign & Security Policy; the List of Excluded Individuals / Entities published by the U.S. Health and Human Services’ Office of Inspector General; any lists of prohibited or debarred parties established under the U.S. Federal Food Drug and Cosmetic Act; the list of parties suspended or debarred from contracting with the U.S. government; and similar lists of restricted parties maintained by the Governmental Authorities of the countries that have jurisdiction over the activities conducted under this Agreement. 12.10.3. Neither Party will knowingly transfer to the other Party any goods, software, technology or services that are (i) controlled under the U.S. International Traffic in Arms Regulations or at a level other than EAR99 under the U.S. Export Administration Regulations; or (ii) specifically identified as an E.U. Dual Use Item or on an applicable export control list of another country.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 63 12.11. Export Control. This Agreement is made subject to any restrictions concerning the export of products or technical information from the United States of America or other countries which may be imposed upon or related to Codex or Pfizer from time to time. Each Party agrees that it will not export, directly or indirectly, any technical information acquired from the other Party under this Agreement or any products using such technical information to a location or in a manner that at the time of export requires an export license or other governmental approval, without first obtaining the written consent to do so from the appropriate agency or other governmental entity. 12.12. Dispute Resolution. If any dispute or disagreement arises between Pfizer and Codex in respect of this Agreement that is not within the scope of the JRC’s decision-making authority as provided in Section 4.4 (all such disputes that are not within the scope of the JRC’s decision-making authority would include disputes arising with respect to the interpretation, enforcement, termination or invalidity of this Agreement), they shall follow the following procedures in an attempt to resolve the dispute or disagreement: 12.12.1. The Party claiming that such a dispute exists shall give notice in writing (“Notice of Dispute”) to the other Party of the nature of the dispute. 12.12.2. Within [***] of receipt of a Notice of Dispute, the Pfizer Program Director and the Codex Program Director shall meet in person or by teleconference and exchange written summaries reflecting, in reasonable detail, the nature and extent of the dispute, and at this meeting they shall use their reasonable endeavors to resolve the dispute. 12.12.3. If the Program Directors are unable to resolve the dispute during the meeting described in Section 12.12.2 or if for any reason such meeting does not take place within the period specified in Section 12.12.2, then the Chief Scientific Officer of Viral Vaccines Research of Pfizer and the Chief Executive Officer of Codex shall meet at a mutually agreed-upon time and location for the purpose of resolving such dispute. 12.12.4. If, within a further period of [***], or if in any event within [***] of initial receipt of the Notice of Dispute, the dispute has not been resolved, or if, for any reason, the meeting described in Section 12.12.3 has not been held within [***] of initial receipt of the Notice of Dispute, then the Parties agree that either Party may initiate litigation to resolve the dispute. Notwithstanding any provision of this Agreement to the contrary, either Party may immediately initiate litigation in any court of competent jurisdiction seeking any remedy at law or in equity, including the issuance of a preliminary, temporary or permanent injunction, to preserve or enforce its rights under this Agreement. The provisions of this Section 12.12 will survive the termination or expiration of this Agreement. 12.13. Governing Law. This Agreement, and all claims arising under or in connection therewith, shall be governed by and interpreted in accordance with the substantive laws of the State of New York, without regard to conflict of law principles thereof.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 64 12.14. Consent to Jurisdiction. Each Party to this Agreement hereby (a) irrevocably submits to the exclusive jurisdiction of the state courts of the State of New York or the United States District Court for the Southern District of New York for the purpose of any and all actions, suits or proceedings arising in whole or in part out of, related to, based upon or in connection with this Agreement or the subject matter hereof, (b) waives to the extent not prohibited by applicable Law, and agrees not to assert, by way of motion, as a defense or otherwise, in any such action, any claim that it is not subject personally to the jurisdiction of the above-named courts, that its property is exempt or immune from attachment or execution, that any such action brought in one of the above-named courts should be dismissed on grounds of forum non conveniens, should be transferred to any court other than one of the above-named courts, or should be stayed by reason of the pendency of some other proceeding in any other court other than one of the above-named courts, or that this Agreement or the subject matter hereof may not be enforced in or by such court, and (c) agrees not to commence any such action other than before one of the above-named courts nor to make any motion or take any other action seeking or intending to cause the transfer or removal of any such action to any court other than one of the above-named courts whether on the grounds of inconvenient forum or otherwise. 12.15. Entire Agreement. This Agreement constitutes and contains the complete, final and exclusive understanding and agreement of the Parties and cancels and supersedes any and all prior negotiations, correspondence, understandings and agreements, whether oral or written, between the Parties respecting the subject matter hereof and thereof, including that certain Non- Disclosure Agreement between the Parties [***], which is hereby terminated effective as of the Effective Date, provided that all confidential or proprietary information exchanged between the Parties under such Non-Disclosure Agreement will be deemed to have been disclosed under this Agreement and shall be subject to the terms and conditions of this Agreement. 12.16. Independent Contractors. Both Parties are independent contractors under this Agreement. Nothing herein contained shall be deemed to create an employment, agency, joint venture or partnership relationship between the Parties hereto or any of their agents or employees, or any other legal arrangement that would impose liability upon one Party for the act or failure to act of the other Party. Neither Party shall have any express or implied power to enter into any contracts or commitments or to incur any liabilities in the name of, or on behalf of, the other Party, or to bind the other Party in any respect whatsoever. 12.17. Counterparts. This Agreement may be executed in two counterparts, each of which shall be an original and both of which shall constitute together the same document. Counterparts may be signed and delivered by facsimile or digital (e.g., PDF) file, each of which shall be binding when received by the applicable Party. 12.18. No Third Party Rights or Obligations. No provision of this Agreement shall be deemed or construed in any way to result in the creation of any rights or obligation in any Person not a Party to this Agreement. However, Pfizer may decide, in its sole discretion, to use one or more of its Affiliates to perform its obligations and duties hereunder, provided that Pfizer shall remain liable hereunder for the performance by any such Affiliates of any such obligations.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 65 (Signature page follows.)

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 66 IN WITNESS WHEREOF, an authorized representative of each Party duly executed this Agreement as of the Effective Date to be effective as of the Effective Date. PFIZER INC. CODEX DNA, Inc. By___[***]________________________ By_[***]____ Name: Title: Name: Title:
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 67 Exhibit A Codex Patent Rights Existing as of the Effective Date [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 68 [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 69 [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 70 [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 71 [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 72 [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 73 [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** [***] [***] [***] [***]
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 74 *] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 75 [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 76 [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 77 [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 78 [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***]
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 79 [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 80 [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***]
Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. 81 [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [** *] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. Exhibit B Research Plan • [***]. [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] [***] Exhibit C Pfizer Anti-Bribery and Anti-Corruption Principles Pfizer has a longstanding corporate policy that prohibits colleagues or anyone acting on our behalf from providing any payment or benefit to any person or entity in order to improperly influence a government official or to gain an unfair business advantage. Pfizer is committed to performing with integrity, and acting ethically and legally in accordance with all applicable laws and regulations, including, but not limited to, anti-bribery and anti-corruption laws. We expect the same commitment from the consultants, agents, representatives or other companies and individuals acting on our behalf (“Business Associates”), as well as those acting on behalf of Business Associates, in connection with work for Pfizer. Bribery of Government Officials Most countries have laws that forbid making, offering or promising any payment or anything of value (directly or indirectly) to a government official when the payment is intended to influence an official act or decision to award or retain business. Under Pfizer’s policies,

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. “government official” is broadly interpreted and includes:(i) any elected or appointed government official (e.g., a member of a ministry of health); (ii) any employee or person acting for or on behalf of a government official, agency, or enterprise performing a governmental function; (iii) any political party, candidate for public office, officer, employee, or person acting for or on behalf of a political party or candidate for public office; or (iv) an employee or person acting for or on behalf of a public international organization (e.g., the United Nations). “Government” is meant to include all levels and subdivisions of governments (i.e., local, regional, or national and administrative, legislative, or executive). Because this definition of “government official” is so broad, it is likely that Business Associates will interact with a government official in the ordinary course of their business on behalf of Pfizer. For example, doctors employed by government-owned hospitals would be considered “government officials” under Pfizer’s policies. The U.S. Foreign Corrupt Practices Act of 1977 (the “FCPA”) prohibits making, promising, or authorizing the making of a payment or providing anything of value to a non-U.S. government official to improperly or corruptly induce that official to make any governmental act or decision to assist a company in obtaining or retaining business, or to otherwise obtain an improper advantage. The FCPA also prohibits a company or person from using another company or individual to engage in any of the foregoing activities. As a U.S. company, Pfizer must comply with the FCPA and could be held liable as a result of acts committed anywhere in the world by a Business Associate. Anti-Bribery and Anti-Corruption Principles Governing Interactions with Governments and Government Officials Business Associates must communicate and abide by the following principles with regard to their interactions with governments and government officials: • Business Associates, and those acting on their behalf in connection with work for Pfizer, may not directly or indirectly make, promise, or authorize the making of a corrupt payment or provide anything of value to any government official to induce that government official to make any governmental act or decision to help Pfizer obtain or retain business. Business Associates, and those acting on their behalf in connection with work for Pfizer, may never make a payment to or offer a government official any item or benefit, regardless of value, as an improper inducement for such government official to approve, reimburse, prescribe, or purchase a Pfizer product, to influence the outcome of a clinical trial, or otherwise improperly to benefit Pfizer’s business activities. • Business Associates, and those acting on their behalf in connection with work for Pfizer, need to understand whether local laws, regulations, or operating procedures (including requirements imposed by government entities such as government- owned hospitals or research institutions) impose any limits, restrictions, or disclosure requirements on compensation, financial support, donations, or gifts that may be provided to government officials. Business Associates, and those acting on their behalf in connection with work for Pfizer, must take into account and comply with any applicable restrictions in conducting their Pfizer-related activities. If a Business Associate is uncertain as to the meaning or applicability of any identified

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. limits, restrictions, or disclosure requirements with respect to interactions with government officials, that Business Associate should consult with his or her primary Pfizer contact before undertaking their activities. • Business Associates, and those acting on their behalf in connection with work for Pfizer, are not permitted to offer facilitation payments. A “facilitation payment” is a nominal, unofficial payment to a government official for the purpose of securing or expediting the performance of a routine, non-discretionary governmental action. Examples of facilitation payments include payments to expedite the processing of licenses, permits or visas for which all paperwork is in order. In the event that a Business Associate, or someone acting on their behalf in connection with work for Pfizer, receives or becomes aware of a request or demand for facilitation payment or bribe in connection with work for Pfizer, the Business Associate shall report such request or demand promptly to his or her primary Pfizer contact before taking any further action. Commercial Bribery Bribery and corruption can also occur in non-government, business to business relationships. Most countries have laws which prohibit offering, promising, giving, requesting, receiving, accepting, or agreeing to accept money or anything of value in exchange for an improper business advantage. Examples of prohibited conduct could include, but are not limited to, the provision of inappropriate gifts or hospitality, kickbacks, or investment opportunities offered to improperly induce the purchase of goods or services. Pfizer colleagues are not permitted to offer, give, solicit or accept bribes, and we expect our Business Associates, and those acting on their behalf in connection with work for Pfizer, to abide by the same principles. Anti-Bribery and Anti-Corruption Principles Governing Interactions with Private Parties and Pfizer Colleagues Business Associates, and those acting on their behalf in connection with work for Pfizer, may not directly or indirectly make, promise, or authorize the making of a corrupt payment or provide anything of value to any person to induce that person to provide an unlawful business advantage for Pfizer. • Business Associates, and those acting on their behalf in connection with work for Pfizer, may not directly or indirectly make, promise, or authorize the making of a corrupt payment or provide anything of value to any person to induce that person to provide an unlawful business advantage for Pfizer. • Business Associates, and those acting on their behalf in connection with work for Pfizer, may not directly or indirectly, solicit, agree to accept or receive a payment or anything of value as an improper inducement in connection with their business activities performed for Pfizer. Pfizer colleagues are not permitted to receive gifts, services, perks, entertainment, or other items of more than token or nominal monetary value from Business Associates, and those acting

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. on their behalf in connection with work for Pfizer. Moreover, gifts of nominal value are only permitted if they are received on an infrequent basis and only at appropriate occasions. Reporting Suspected or Actual Violations Business Associates, and those acting on their behalf in connection with work for Pfizer, are expected to raise concerns related to potential violations of these International Anti-Bribery and Anti-Corruption Principles or the law. Such reports can be made to a Business Associate’s primary point of contact at Pfizer, or if a Business Associate prefers, to Pfizer’s Compliance Group by e-mail at ▇▇▇▇▇▇▇▇▇.▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇ or by phone at ▇-▇▇▇-▇▇▇-▇▇▇▇.

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. Schedule 3.7 Sample Royalty Calculation [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. Schedule 7.5.1 Press Release [***]

Exhibit 10.21 Certain identified information marked with [***] has been excluded from this exhibit because it is not material and is of the type that the registrant treats as private and confidential. Schedule 8.3 [***] [***]

