EXCLUSIVE COmmercialization LICENSE Agreement
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit 10.1
EXCLUSIVE COmmercialization LICENSE Agreement
This Exclusive Commercialization License Agreement (the “Agreement”) is entered into as of January 22nd, 2019 (the “Effective Date”), by and between Rigel Pharmaceuticals, Inc., a Delaware company having an address at 0000 Xxxxxxxx Xxxx., Xxxxx Xxx Xxxxxxxxx, XX 00000, XXX (“Rigel”) and Grifols Worldwide Operations Limited, an Irish company having an address at Grange Castle Business Park, Clondalkin, Xxxxxx 00, Xxxxxx, Xxxxxxx (“Grifols”). Rigel and Grifols may be referred to herein individually as a “Party” or collectively as the “Parties”.
Recitals
Whereas, Rigel, a biopharmaceutical company, owns or controls certain patents, know-how, and other intellectual property relating to its proprietary compound fostamatinib disodium hexahydrate, also known as TAVALISSE™ in the United States, which has been approved by the FDA (as defined below) for the treatment of chronic immune thrombocytopenia and is under development for the treatment of autoimmune hemolytic anemia, IgA nephropathy, and potentially other indications;
Whereas, Grifols, a healthcare company, possesses substantial resources and expertise in the development, commercialization, distribution and sale of pharmaceutical products;
Whereas, Grifols wishes to obtain, and Rigel wishes to grant Grifols, an exclusive license to develop, market, promote, distribute and sell the Product in the Field in the Grifols Territory (as such terms are defined below), subject to the terms and conditions included herein; and
Whereas, Rigel and Grifols are entering into a commercial supply agreement in relation to and for the purpose of this Agreement, pursuant to which Rigel shall manufacture and supply the Product to Grifols (the “Commercial Supply Agreement”) for use in accordance with the terms of this Agreement.
Agreement
Now, Therefore, in consideration of the foregoing premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Rigel and Grifols hereby agree as follows:
|
1.3 “Applicable Laws” means the applicable provisions of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidance, ordinances, judgments, decrees, directives, injunctions, orders, permits (including XXXx (as defined below)) of or from any court, Regulatory Authority or Governmental Authority (as such terms are defined below) having jurisdiction over or related to the subject item. |
1
|
1.4 “Auditor” has the meaning set forth in Section 9.4. |
|
1.5 “Calendar Quarter” means each respective period of three (3) consecutive months ending on March 31, June 30, September 30, and December 31. |
|
1.6 “Claim” has the meaning set forth in Section 12.3. |
|
1.7 “Commercialization” means the conduct of all activities undertaken (a)after Regulatory Approval (as defined below) relating to the promotion, sales, marketing, medical support, and distribution (including importing, exporting, transporting, customs clearance, warehousing, invoicing, handling, and delivering Products to customers) of Products in the Field in the Grifols Territory, including sales force efforts, detailing, advertising, market research, market access (including price and reimbursement activities), medical education and information services, publication, scientific and medical affairs, advisory and collaborative activities with opinion leaders and professional societies including symposia, marketing, sales force training, and sales (including receiving, accepting and filling Product orders) and distribution; and (b) any of the foregoing (but not marketing, promotion, sales, or distribution) that is conducted prior to Regulatory Approval in anticipation of and/or preparation for launch. “Commercialize” and “Commercializing” have correlative meanings. |
|
1.8 “Commercialization Plan” has the meaning set forth in Section 6.2. |
|
1.9 “Commercially Reasonable Efforts” means, with respect to a Party and its obligations under this Agreement, those commercially reasonable efforts and resources consistent with the usual practices of a similarly situated company for the development and commercialization of a pharmaceutical product originating from its own research and development department without a royalty obligation to others, which is at a similar stage of research, development, or commercialization, taking into account that product’s profile of efficacy and safety; proprietary position, including patent and regulatory exclusivity; regulatory status, including anticipated or approved labeling and anticipated or approved post-approval requirements; present and future market and commercial potential, including competitive market conditions (but not taking into account any payment owed to the other Party under this Agreement), and all other relevant factors, including technical, legal, scientific and/or medical factors. Commercially Reasonable Efforts requires that a Party: (a) at a minimum establishes a plan to achieve objectives and assigns specific responsibilities for the achievement of that plan and (b) makes and implements decisions and allocate resources designed to advance progress with respect to such objectives. |
|
1.10 “Competing Product” means any product or compound, other than the Compound (as defined below) and Product, that (a) [ * ], or (b) is [ * ], or (c) [ * ], or is any combination of (a), (b) or (c). For the avoidance of doubt, [ * ] Competing Products. |
|
1.11 “Compound” means fostamatinib disodium hexahydrate, having the chemical structure set forth in Exhibit A. |
|
1.12 “Confidentiality Agreement” means that certain Confidential Disclosure Agreement between Rigel and Grifols dated as of [ * ]. |
|
1.13 “Confidential Information” means all Know-How (as defined below) and other proprietary scientific, marketing, financial, or commercial information or data that is generated by or on behalf of a Party or its Affiliates or which one Party or any of its Affiliates has supplied or otherwise made available to the other Party or its Affiliates, whether made available orally, in writing, or in electronic form, including information comprising or relating to concepts, discoveries, inventions, data, designs, or formulae in relation to this Agreement; further all Rigel Technology (as defined below) will be deemed Rigel’s Confidential Information. |
|
1.14 “Control” or “Controlled” means, with respect to any Rigel Technology, the legal authority or right (whether by ownership, license, or otherwise, but without taking into account any rights granted by Rigel to Grifols pursuant to this Agreement) of Rigel to grant access, a license, or a sublicense of or under such Rigel |
2
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Technology to Grifols, or to otherwise disclose proprietary or trade secret information to Grifols, without breaching the terms of any agreement with a Third Party (as defined below), or misappropriating the proprietary or trade secret information of a Third Party. |
|
1.15 “Data” means any and all scientific, technical, test, marketing, or sales data pertaining to any Product that is generated by or on behalf of Rigel, Grifols, or their respective Affiliates and sublicensees, including research data, clinical pharmacology data, pre-clinical data, clinical data, clinical study reports, or submissions made in association with an IND or MAA with respect to any Product. |
|
1.17 “EMA” means the European Medicines Agency or its successor. |
|
1.18 “Europe” means Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Italy, Ireland, Latvia, Lithuania, Luxembourg, Malta, the Netherlands, Norway, Switzerland, Poland, Portugal, Romania, Spain, Slovakia, Slovenia, Sweden, and the United Kingdom. |
|
1.19 “Executive Officers” means [ * ] of Rigel and [ * ] of Grifols. |
|
1.20 “Export Control Laws” means all applicable U.S. laws and regulations relating to (a) sanctions and embargoes imposed by the Office of Foreign Assets Control of the U.S. Department of Treasury or (b) the export or re-export of commodities, technologies, or services, including the Export Administration Act of 1979, 24 U.S.C. §§ 2401-2420, the International Emergency Economic Powers Act, 50 U.S.C. §§ 1701-1706, the Trading with the Enemy Act, 50 U.S.C. §§ 1 et. seq., the Arms Export Control Act, 22 U.S.C. §§ 2778 and 2779, and the International Boycott Provisions of Section 999 of the U.S. Internal Revenue Code of 1986, in each case, as amended. |
|
1.21 “FCPA” means the U.S. Foreign Corrupt Practices Act (15 U.S.C. Section 78dd-1, et. seq.), as amended. |
|
1.22 “FDA” means the U.S. Food and Drug Administration or its successor. |
|
1.23 “Field” means the treatment, palliation, or prevention of human diseases, including chronic or persistent ITP, AIHA, and IgA nephropathy. |
|
1.24 “First Commercial Sale” means, on a Product-by-Product and country-by-country basis, the first sale of such Product in a country of the Grifols Territory by Grifols or its Affiliates or Sublicensees (as defined below) to a Third Party after Regulatory Approval for such Product has been obtained in such country. |
|
1.25 “Generic Product” means, with respect to a Product in a particular regulatory jurisdiction, any pharmaceutical product that (a) contains the same active pharmaceutical ingredient(s) as such Product; (b) is approved by the Regulatory Authority in such country as a substitutable generic for such Product on an expedited or abbreviated basis based on bioequivalence or interchangeability with the Product; and (c) is sold in such jurisdiction by a Third Party that is not a Sublicensee and did not purchase such product in a chain of distribution that included any of Rigel, Grifols, or their respective Affiliates, licensees, or sublicensees. |
3
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
1.26 “Governmental Authority” means any national, international, federal, state, provincial, or local government, or political subdivision thereof, or any multinational organization, or any authority, agency, or commission entitled to exercise any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power, or any court or tribunal (or any department, bureau or division thereof, or any governmental arbitrator or arbitral body). |
|
1.27 “Grifols Data” has the meaning set forth in Section 10.1(a). |
|
1.28 “Grifols Indemnitee” has the meaning set forth in Section 12.1. |
|
1.29 “Grifols Territory” means Europe and Turkey. |
|
1.30 “ICH” means the International Conference on Harmonization (of Technical Requirements for Registration of Pharmaceuticals for Human Use). |
|
1.31 “IFRS” means the International Financial Reporting Standards, existing as of the Effective Date and as may be amended from time to time during the Term (as defined below). |
|
1.32 “IND” means an investigational new drug application or equivalent application filed with the applicable Regulatory Authority, which application is required to commence human clinical trials in the applicable country. |
|
1.33 “Indemnitee” has the meaning set forth in Section 12.3. |
|
1.34 “Indemnitor” has the meaning set forth in Section 12.3. |
|
1.35 “Indication” means a separate and distinct disease, disorder, illness, or health condition and all of its associated signs, symptoms, stages, or progression (including precursor conditions), in each case for which a separate MAA may be filed. For clarity, subpopulations or patients with a primary disease or condition, however stratified (including stratification by stages or progression, particular combinations of symptoms associated with the primary disease or condition, prior treatment courses, response to prior treatment, family history, clinical history, phenotype, or other stratification) shall not be deemed to be separate “Indications” for the purposes of this Agreement. |
|
1.36 “Inventions” means all inventions, whether or not patentable, discovered, made, conceived, or reduced to practice in the course of activities contemplated by this Agreement. |
|
1.37 “ITP” means immune thrombocytopenia. |
|
1.38 “JSC” has the meaning set forth in Section 3.1. |
|
1.39 “Know-How” means all technical information, know-how, and data, including inventions, discoveries, trade secrets, specifications, instructions, processes, formulae, compositions of matter, cells, cell lines, assays, animal models, and other physical, biological, or chemical materials, expertise, and other technology applicable to development, registration, use, or marketing or to methods of assaying or testing them, and including all biological, chemical, pharmacological, biochemical, toxicological, pharmaceutical, physical, and analytical safety, nonclinical, and clinical data, regulatory documents, data and filings, instructions, processes, formulae, expertise, and information relevant to the research, development, use, importation, offering for sale, or sale of, or which may be useful in studying, testing, developing, Products. Know-How excludes Patents and manufacturing know-how for the Compound or Product. |
|
1.40 “Losses” has the meaning set forth in Section 12.1. |
4
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
1.41 “MAA” means a marketing authorization application or equivalent application, and all amendments and supplements thereto, filed with the applicable Regulatory Authority in any country or jurisdiction. For clarity, MAA does not include any application for Pricing and Reimbursement Approval (as such terms are defined below). |
|
1.42 “MAA Approval” means approval of an MAA by the applicable Regulatory Authority for marketing and sale of a Product in the applicable country or jurisdiction, but excluding any Pricing and Reimbursement Approval. |
|
1.44 “Medical Affairs” or “Medical Affairs Activities” means activities designed to ensure or improve appropriate medical use of, conduct medical education of, or further research regarding, the Product, including by way of example: (a) activities of medical scientific liaisons who, among their other functions, may: (i) conduct service based medical activities including providing input and assistance with consultancy meetings, proposing investigators for clinical trials sponsored or co-sponsored by a Party or Affiliate, and providing input in the design of such trials and other research related activities; and/or (ii) deliver non-promotional communications and conduct non-promotional activities; (b) grants to support continuing medical education, symposia, or Third Party research related to the Product; (c) development, publication, and dissemination of publications relating to the Product; (d) medical information services provided in response to inquiries communicated via sales representatives or received by letter, phone call, or email; (e) conducting advisory board meetings, international advisory board activities, or other consultant programs, including the engagement of key opinion leaders and health care professional in individual or group advisory and consulting arrangements; and (f) the evaluation of applications submitted to Grifols for support of investigator-initiated trials. |
|
1.45 “Middle East and North Africa” means Algeria, Bahrain, the Comoros Islands, Djibouti, Egypt, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Mauritiana, Oman, Palestine, Qatar, Saudi Arabia, Somalia, Sudan, Syria, Tunisia, the United Arab Emirates, and Yemen. |
|
(a) normal and customary trade, cash and quantity discounts actually allowed and properly taken directly with respect to sales of such Product; |
|
(b) credits or allowances given or made for rejection or return of previously sold Products or for retroactive price reductions and billing errors; |
|
(c) rebates and chargeback payments granted to managed health care organizations, pharmacy benefit managers (or equivalents thereof), national, state/provincial, local, and other governments, their agencies and purchasers and reimbursers, or to trade customers; |
|
(d) costs of freight, carrier insurance, and other transportation and/or delivery charges directly related to the distribution of such Product; |
|
(e) taxes, duties or other governmental charges (including any tax such as a value added or similar tax, other than any taxes based on income) directly levied on the sale, transportation, delivery or use of a Product which is paid by Grifols or measured by the billing amount for such Product, as adjusted for rebates and refunds; and |
5
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(f) any invoiced amounts (not to exceed [ * ]) not able to be reasonably collected by Grifols or its Affiliates or Sublicensees in each case written off by Grifols or its Affiliates or Sublicensees. |
In no event will any particular amount identified above be deducted more than once in calculating Net Sales. Sales of a Product between Grifols and its Affiliates or Sublicensees for resale shall be excluded from the computation of Net Sales, but the subsequent resale of such Product to a Third Party shall be included within the computation of Net Sales.
The supply of Product as samples, for use in non-clinical or clinical trials, or for use in any test or studies reasonably necessary to comply with any Applicable Laws, or as is otherwise normal and customary in the industry, shall not be included in the computation of Net Sales, so long as Grifols, its Affiliates, and Sublicensees do not receive payment for such Product in excess of the transfer price paid by Grifols to Rigel for such Product pursuant to the Commercial Supply Agreement.
|
1.47 “Option” has the meaning set forth in Section 2.4. |
|
1.48 “Option Fee” has the meaning set forth in Section 2.4(b). |
|
1.49 “Option Territory” means (a) the Middle East and North Africa, and (b) Russia/CIS (as defined below). |
|
1.50 “Patents” means (a) all patents, certificates of invention, applications for certificates of invention, priority patent filings, provisional patent applications and patent applications, and (b) any renewals, divisions, or continuations (in whole or in part) of any of such patents, certificates of invention and patent applications, and any all patents or certificates of invention issuing thereon, and any and all reissues, reexaminations, extensions, supplementary protection certificates, divisions, renewals, substitutions, confirmations, registrations, revalidations, revisions, and additions of or to any of the foregoing. |
|
1.51 “Pharmacovigilance Agreement” has the meaning set forth in Section 5.4(a). |
|
1.52 “Pricing and Reimbursement Approval” means, with respect to a Product, the approval, agreement, determination, or decision of any applicable Governmental Authority establishing the price or level of reimbursement for such Product, as required in a given country or jurisdiction prior to sale of such Product in such country or jurisdiction. |
|
1.53 “Product” means any pharmaceutical product in the final form as of the Effective Date, containing the Compound as the sole active ingredient in the form set forth in Exhibit A. |
|
1.54 “Product Infringement” has the meaning set forth in Section 10.3(a). |
|
1.55 “Public Official or Entity” means (a) any officer, employee (including physicians, hospital administrators, or other healthcare professionals), agent, representative, department, agency, de facto official, representative, corporate entity, instrumentality, or subdivision of any government, military, or international organization, including any ministry or department of health or any state-owned or affiliated company or hospital, or (b) any candidate for political office, any political party, or any official of a political party. |
|
1.56 “Recall” has the meaning set forth in Section 5.7. |
|
1.57 “Regulatory Approval” means, with respect to a country or jurisdiction, any and all approvals (including MAA Approval, and Pricing and Reimbursement Approval, if applicable), licenses, registrations, permits, notifications and authorizations (or waivers) of any Regulatory Authority that are necessary for the use, storage, import, transport, promotion, marketing, distribution, offer for sale, sale, or other commercialization of a Product in such country or jurisdiction. |
6
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
1.58 “Regulatory Authority” means any Governmental Authority that has responsibility in its applicable jurisdiction over the testing, development, manufacture, use, storage, import, transport, promotion, marketing, distribution, offer for sale, sale, or other commercialization of pharmaceutical products in a given jurisdiction, including the FDA and EMA. For countries where Pricing and Reimbursement Approval is required, Regulatory Authority shall also include any Governmental Authority whose grant of Pricing and Reimbursement Approval of the Product is required. |
|
1.60 “Rigel Data” has the meaning set forth in Section 10.1(a). |
|
1.61 “Rigel Indemnitee” has the meaning set forth in Section 12.2. |
|
1.63 “Rigel Patents” means all Patents in the Grifols Territory that Rigel Controls as of the Effective Date or during the Term that would be infringed, absent a license or other right to practice granted under such Patents, by the Development, use, importation, offer for sale or sale of any Compound or Product in the Field in the Grifols Territory (considering patent applications to be issued with the then-pending claims). The Rigel Patents existing as of the Effective Date are set forth in Exhibit B. |
|
1.64 “Rigel Technology” means the Rigel Know‑How and the Rigel Patents. |
|
1.65 “Rigel Territory” means the world outside the Grifols Territory. |
|
1.66 “Royalty Term” has the meaning set forth in Section 8.4(b). |
|
1.67 “Russia/CIS” means Russia, Armenia, Azerbaijan, Belarus, Kazakhstan, Kyrgyzstan, Moldova, Tajikistan, and Uzbekistan. |
|
1.68 “Safety Data” means Data related solely to any adverse drug experiences and serious adverse drug experience as such information is reportable to Regulatory Authorities. Safety Data also includes “adverse events”, “adverse drug reactions”, and “unexpected adverse drug reactions” as defined in the ICH Harmonised Tripartite Guideline for Clinical Safety Data Management: Definitions and Standards for Expedited Reporting. |
|
1.69 “SEC” means the U.S. Securities and Exchange Commission, or any successor entity or its foreign equivalent, as applicable. |
|
1.70 “Sublicensee” means a Third Party to whom Grifols grants a sublicense to use, import, promote, offer for sale, or sell any Product in the Field in the Grifols Territory, beyond the mere right to purchase Products from Grifols and its Affiliates, and excluding wholesalers and full-service distributors that do not promote the sale of the Product, and other similar physical distributors. In no event shall Rigel or any of its Affiliates be deemed a Sublicensee. |
|
1.71 “Sunshine Reporting Laws” has the meaning set forth in Section 6.10. |
|
1.72 “Term” has the meaning set forth in Section 14.1. |
7
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
1.73 “Third Party” means any entity that is not Rigel or Grifols or an Affiliate of Rigel or Grifols. |
|
1.75 “Year” means, initially, the period of time commencing on the date of the first shipment of Product from Rigel to Grifols under the Commercial Supply Agreement intended for the First Commercial Sale in any country of the Grifols Territory and ending December 31st of the same calendar year, and, thereafter, each consecutive 365-day period (or 366-day period in the event of a leap year) beginning on January 1st and ending on December 31st of each calendar year during the Term. |
|
2.1 License Grant to Grifols. Subject to the terms and conditions of this Agreement, Rigel hereby grants to Grifols, during the Term, (a) an exclusive (even as to Rigel and its Affiliates, except as expressly set forth herein), royalty-bearing license, with the right to grant sublicenses (through multiple tiers) solely as provided in Section 2.2, under the Rigel Technology to use, promote, sell, offer for sale, import, export, and otherwise Commercialize (but not to make or have made) the Product in the Field in the Grifols Territory and (b) a non-exclusive license, with the right to grant sublicenses (through multiple tiers) solely as provided in Section 2.2, under the Rigel Technology to Develop (but not to make or have made) the Product in the Grifols Territory and to use the Product for that purpose. |
|
2.2 Sublicenses. Grifols shall have the right to grant sublicenses under the licenses granted in Section 2.1: |
|
(a) to an Affiliate of Grifols without providing any written notice to Rigel; |
|
(b) to a Third Party in up to [ * ] Major Market Countries upon written notice to Rigel and to a Third Party in any of the remaining [ * ] Major Market Countries with Rigel’s express prior written consent; and |
|
(c) to a Third Party in any country in the Grifols Territory other than a Major Market Country upon written notice to Rigel. |
All sublicenses granted under the licenses granted to Grifols in Section 2.1 other than to an Affiliate of Grifols shall be in writing and shall be subject to, and consistent with, the terms and conditions of this Agreement and shall provide that any such Sublicensee shall not further sublicense except with the consent of Rigel. Grifols shall ensure that each agreement with a Sublicensee grants Rigel the same rights with respect to Regulatory Filings and Compound Inventions made or generated by such Sublicensee as if such Regulatory Filings and Compound Inventions were made or generated by Grifols pursuant to the terms and conditions set forth herein. Grifols shall be responsible for the compliance of its Affiliates, Sublicensees, and subcontractors with the terms and conditions of this Agreement.
|
(a) the right under the Rigel Technology to exercise its rights and perform its obligations under this Agreement, whether directly or through one or more licensees or subcontractors; and |
|
(b) all rights to practice, and to grant licenses under, the Rigel Technology outside of the scope of the licenses granted in Section 2.1, including the exclusive right to make and have made the Compound and Product anywhere in the world, the exclusive rights to practice the Rigel Patents and Rigel Know-How with respect to |
8
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
compounds and products other than Compound and Product anywhere in the world, and the non-exclusive right to develop the Compound and Product anywhere in the world. |
|
(a) subject to remainder of this Section 2.4, at any time during the Term by providing written notice thereof to Rigel; or |
|
(b) in the case Rigel notifies Grifols in writing of its intention to commercialize, directly or indirectly, the Product within the Option Territory, Grifols shall have a maximum term of [ * ] from receipt of Rigel’s notice to exercise the Option by providing Rigel with written notice of such exercise. |
Once Grifols exercises the Option, it shall pay to Rigel a one-time payment of [ * ] (the “Option Fee”) within [ * ] from the date of the corresponding invoice issued by Rigel and the Parties shall amend this Agreement to include the Option Territory as part of the Grifols Territory. For the avoidance of doubt, the Commercialization of the Products in the Field in the Option Territory will be subject to the same terms and conditions set forth in this Agreement and in the Commercial Supply Agreement and any and all references herein to the Grifols Territory will be understood as including the Option Territory. For the further avoidance of doubt, the Option Fee payment includes all countries of the Option Territory.
If Grifols fails to exercise the Option as set forth in this Section 2.4(b), the Option shall expire and Rigel shall have no further obligation to Grifols with respect to the Option Territory.
|
2.5 No Implied Licenses; Negative Covenant. Except as set forth in this Agreement, neither Party shall acquire any license or other intellectual property interest, by implication or otherwise, under or to any Patents, Know-How, or other intellectual property owned or controlled by the other Party. Neither Party shall, nor shall it permit any of its Affiliates or sublicensees to, practice any Patents or Know-How licensed to it by the other Party outside the scope of the licenses granted to it under this Agreement. |
|
2.6 Third Party Licenses. |
|
(b) If the Parties become aware of any Third Party Know-How or Patent that is useful, but not necessary, to exploit the license granted to Grifols in Section 2.1, the Parties will notify one another of such Know-How or Patent and evaluate, through the JSC, the benefit of obtaining a license under such Know-How or Patent. If the JSC and the Parties determine that obtaining a license to such Know-How or Patent is desirable, the Parties, through the JSC, will work together to determine the best strategy to obtaining such license. |
9
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(b) In the event that a Third Party becomes an assignee of this Agreement or an Affiliate of Grifols after the Effective Date through merger, acquisition, consolidation, or other similar transaction, and such Third Party, as of the closing date of such transaction, is engaged in the conduct of a Competing Program, then Rigel shall have the right to terminate this Agreement upon immediate written notice to Grifols if, within [ * ] after the closing of such transaction, the successor-in-interest of such Competing Program does not completely Divest such Competing Program. An additional [ * ] shall be provided as long as such assignee is using commercially reasonable efforts to Divest such Competing Program. “Divest” means (i) the sale or transfer of rights to the Competing Program to a Third Party (i.e., not an Affiliate of either Grifols or such successor-in-interest) without receiving a continuing share of profit, royalty payment, or other economic interest in the success of such Competing Program, or (ii) the termination of any direct or indirect commercialization activity for or in relation to such Competing Product. |
|
(a) review and discuss the strategy and progress of the Development and Commercialization of the Product in the Grifols Territory; |
|
(b) monitor and coordinate regulatory actions and pharmacovigilance and safety matters for the Product in the Grifols Territory; |
|
(c) review and discuss the Commercialization Plan for the Grifols Territory, including proposed amendments; |
|
(d) review the manufacturing and supply strategy and supply needs for the Grifols Territory; |
|
(e) establish joint subcommittees as it deems necessary or advisable to further the purpose of this Agreement; and |
|
(f) perform such other functions as appropriate to further the purposes of this Agreement, as expressly set forth in this Agreement or allocated to it by the Parties’ written agreement. |
|
3.2 JSC Membership and Meetings. |
|
(a) Committee Members. Each JSC representative shall have appropriate knowledge and expertise and sufficient seniority within the applicable Party to make decisions arising within the scope of the JSC’s responsibilities. Each Party may replace its representatives on the JSC on written notice to the other Party, but each Party shall strive to maintain continuity in the representation of its JSC members. The JSC chairperson shall [ * ]. The chairperson shall prepare and circulate agendas to JSC members at least [ * ] before each JSC meeting and shall direct the preparation of reasonably detailed minutes for each JSC meeting, which shall be approved by the chairperson and circulated to JSC members within [ * ] after such meeting. The Parties shall determine their respective initial members of the JSC promptly following the Effective Date. |
|
(b) Meetings. The JSC shall hold meetings at such times as it elects to do so, but in no event shall meetings of the JSC be held less frequently than once every [ * ] prior to [ * ] unless otherwise agreed to by the parties. The first JSC meeting shall be held within [ * ] after the Effective Date, at which meeting the dates for the first calendar year shall be set. JSC meetings may be held in person or by audio or video teleconference; provided that, unless otherwise agreed by both Parties, at least one (1) meeting per calendar year shall be held in person. In-person JSC meetings shall be held at locations alternately selected by the Parties. Each Party shall be responsible for |
10
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
all of its own expenses of participating in any JSC meeting. No action taken at any JSC meeting shall be effective unless at least [ * ] of each Party is participating. In addition, upon written notice to the other Party, either Party may request that a special ad hoc meeting of the JSC be convened for the purpose of resolving any disputes in connection with, or for the purpose of reviewing or making a decision pertaining to any material subject-matter within the scope of the JSC, the review or resolution of which cannot be reasonably postponed until the following scheduled JSC meeting. Such ad hoc meeting shall be convened at such time as may be mutually agreed by the Parties, but no later than [ * ] following the notification date of request that such meeting be held. |
|
(c) Non-Member Attendance. Each Party may from time to time invite a reasonable number of participants, in addition to its representatives, to attend JSC meetings in a non‑voting capacity; provided that if either Party intends to have any Third Party (including any consultant) attend such a meeting, such Party shall provide reasonable prior written notice to the other Party and obtain the other Party’s approval for such Third Party to attend such meeting, which approval shall not be unreasonably withheld or delayed. Such Party shall ensure that such Third Party is bound by written confidentiality and non-use obligations consistent with the terms of this Agreement. |
|
3.3 Decision-Making. |
|
(a) All decisions of the JSC shall be made by unanimous vote, with each Party’s representatives collectively having one (1) vote. If after reasonable discussion and good faith consideration of each Party’s view on a particular matter, the representatives of the Parties cannot reach an agreement as to such matter within [ * ] after such matter was brought to the JSC for resolution, then either Party at any time may refer such issue to the following Senior Managers of each Party: |
|
· |
For Rigel: [ * ] |
|
· For Grifols: [ * ] |
|
(b) If the Seniors Managers of the Parties cannot resolve such matter within [ * ] after such matter has been referred to them, then Article 15 of this Agreement applies. |
|
3.4 Limitations on Authority. The JSC shall have only such powers as are expressly assigned to it in this Agreement, and such powers shall be subject to the terms and conditions of this Agreement. Without limiting the generality of the foregoing, the JSC will not have the power to amend this Agreement, and no JSC decision may be in contravention of any terms and conditions of this Agreement. |
|
3.6 Alliance Managers. Promptly after the Effective Date, each Party shall appoint an employee of such Party having appropriate qualification and experience to act as the alliance manager for such Party (the “Alliance Manager”). Each Alliance Manager shall be responsible for coordinating and managing processes and interfacing between the Parties on a day-to-day basis throughout the Term and shall be permitted to attend meetings of the JSC as non-voting participants. The Alliance Managers shall be the primary contact for the Parties regarding the activities contemplated by this Agreement and shall facilitate all such activities hereunder. Each Party shall bear its own costs of its Alliance Manager. |
11
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
4.1 Development Activities. |
|
4.4 Diligence. Each Party shall use Commercially Reasonable Efforts to perform its obligations under this Article 4. |
|
4.5 Development Records. Each Party shall maintain complete, current, and accurate records of all Development activities conducted by it under this Agreement, and all data and other information resulting from such activities. Such records shall fully and properly reflect all work done and results achieved in the performance of the Development activities in good scientific manner appropriate for regulatory and patent purposes. Each Party shall document all non-clinical studies and clinical trials in formal written study reports according to Applicable Laws and national and international guidelines (e.g., ICH). |
12
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
4.7 Use of Subcontractors. Each Party may perform its Development activities under this Agreement through one or more subcontractors, provided that (a) such Party will remain responsible for the work allocated to, and payment to, such subcontractors to the same extent it would if it had done such work itself, (b) each subcontractor undertakes in writing obligations of confidentiality and non-use regarding Confidential Information that are substantially the same as those undertaken by the Parties pursuant to Article 13, and (c) each subcontractor agrees in writing to assign all intellectual property developed in the course of performing any such work to such Party (or, in the event such assignment is not feasible, a license to such intellectual property with the right to sublicense to such other Party). |
|
5.2 Rigel Transfer of Regulatory Filings and Right of Reference. |
|
(a) Rigel shall transfer to Grifols the XXXx submitted to any Regulatory Authority in the Grifols Territory for the Compound and Product that are in Rigel’s name and Controlled by Rigel promptly after the receipt of Regulatory Approval for the applicable MAA from such Regulatory Authority. |
|
5.3 Grifols Regulatory Information Sharing and Right of Reference. |
|
(a) In the case where Grifols submits any Regulatory Filings with respect to the Product, Grifols shall promptly provide Rigel with copies of such Regulatory Filings and documents of communication prepared (including any drafts), submitted, or received by Grifols in the Grifols Territory pertaining to the Product, including English translations, and Rigel shall have the right to review and comment on drafts of such Regulatory |
13
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(b) Grifols hereby grants to Rigel a right of reference to all Regulatory Filings pertaining to the Compound and Product submitted by or on behalf of Grifols. Rigel may use such right of reference to seek, obtain, and maintain Regulatory Approval of the Product in the Rigel Territory. |
|
(b) Rigel has established, and shall continue to hold (either by itself or through a vendor engaged by Rigel) the global safety database for the Product, and shall maintain such global safety database for so long as such Product is under Development or Commercialization by the Parties. Rigel shall [ * ] such database and preparing such reports. Rigel will ensure that each Party is able to access the data from the global safety database in order to meet legal and regulatory obligations. Grifols shall maintain its own safety database for the Product in the Grifols Territory and shall provide all Safety Data, including adverse event reports, in such database to Rigel in accordance with this Section 5.4 and the Pharmacovigilance Agreement. Grifols shall [ * ] such database for the Grifols Territory and preparing reports for the Grifols Territory. |
|
(c) Each Party shall be primarily responsible for reporting quality complaints, adverse events, and Safety Data related to the Product to any necessary Regulatory Authorities, and responding to safety issues and to all requests of Regulatory Authorities related to the Product under any MAA or Regulatory Approval for the Product held by such Party and filed with such Regulatory Authorities, [ * ]. Each Party agrees to comply with its respective obligations under the Pharmacovigilance Agreement and to cause its Affiliates, licensees, and sublicensees to comply with such obligations. |
|
5.5 No Harmful Actions. If a Party reasonably believes that the other Party is taking or intends to take any action with respect to a Product that could reasonably be expected to have a material adverse impact upon the regulatory status of such Product in the first Party’s territory, then such Party may bring the matter to the attention of the JSC and the Parties shall discuss in good faith to promptly resolve such concern. |
|
5.6 Notification of Threatened Action. Each Party shall notify the other Party within [ * ] of any information it receives regarding any threatened or pending action, inspection, or communication by any Regulatory Authority which may adversely affect the safety or efficacy claims of any Product or the continued Development or Commercialization of any Product. Upon receipt of such information, the Parties shall promptly consult with each other in an effort to arrive at a mutually acceptable procedure for taking appropriate action. |
14
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(a) General. During the Term, Grifols shall use Commercially Reasonable Efforts to Commercialize the Product for each and every Indication that has received or will receive Regulatory Approval in the Grifols Territory. |
|
(b) Product Launch. Grifols shall launch the Product for each Indication that has received Regulatory Approval in the Grifols Territory as soon as reasonably possible following receipt of such Regulatory Approval. As applicable, Grifols shall obtain all necessary Pricing and Reimbursement Approvals necessary to launch such Product as soon as reasonably possible following receipt of MAA Approval of such Product in any such country. Without limiting the generality of the foregoing, with respect to the Product for ITP, Grifols shall launch the Product in (i) [ * ] within [ * ] after receiving Regulatory Approval of the Product for ITP from the EMA, and (ii) [ * ] within [ * ] after receiving such Regulatory Approval. Thereafter, Grifols shall utilize Commercially Reasonable Efforts in the ongoing support for the Commercialization of the Product for ITP in the Grifols Territory. If Grifols fails to timely achieve the obligation in the foregoing subsections (i) (ii), the rights granted herein to Grifols for [ * ] in the case (i) |
15
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
6.7 Coordination of Commercialization Activities. |
|
(a) Generally. Grifols, through the JSC, shall update Rigel on Commercialization strategies for the Product (e.g., for branding and messaging, international congresses, advisory boards) in the Grifols Territory, and the Parties shall work together to identify and take advantage of any potential global strategies and messaging. The foregoing shall not be construed as requiring Grifols to seek Rigel’s consent in connection with Grifols establishing or implementing any sales, marketing, or medical affairs practices in the Grifols Territory. |
|
(c) Promotional Materials. Rigel shall provide Grifols with sufficient samples of Promotional Material (as defined below) produced and released for the US market so as to provide Grifols with ideas and information. Subsequently, Grifols shall, at its own expense, prepare, develop, produce, or otherwise obtain and utilize sales, promotional, advertising, marketing, website, educational, and training materials for the Products (the “Promotional Materials”) to support its Commercialization activities in the Grifols Territory, and shall ensure that such Promotional Materials, as well as all information contained therein, are accurate and comply with all Applicable Laws and are consistent with any Regulatory Approvals obtained for the Product in the applicable jurisdiction in the |
16
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Grifols Territory. At Rigel’s request, Grifols shall share samples of and updates to Promotional Materials with respect to the Commercialization of the Product with Rigel. |
|
6.8 Medical Affairs Activities. Grifols shall be responsible for Medical Affairs Activities in the Grifols Territory. Rigel shall collaborate, at its own cost, in knowledge transfer to Grifols and in support of Grifols’ execution of Medical Affairs Activities in the Grifols Territory in global support of the Product, based on the Commercialization Plan as discussed within the JSC from time to time. |
|
6.9 Diversion. Each Party hereby covenants and agrees that it and its Affiliates shall not, and it shall contractually obligate (and use Commercially Reasonable Efforts to enforce such contractual obligation) its sublicensees not to, directly or indirectly, actively promote, market, distribute, import, sell, or have sold any Product, including via the Internet or mail order, to any Third Party or to any address or Internet Protocol address or the like in the other Party’s territory. Neither Party shall engage, nor permit its Affiliates and sublicensees to engage, in any advertising or promotional activities relating to any Product for use directed primarily to customers or other buyers or users of such Product located in any country or jurisdiction in the other Party’s territory, or solicit orders from any prospective purchaser located in any country or jurisdiction in the other Party’s territory. If a Party or its Affiliates or sublicensees receives any order for a Product for use from a prospective purchaser located in a country or jurisdiction in the other Party’s territory, such Party shall immediately refer that order to such other Party and shall not accept any such orders. Neither Party shall, nor permit its Affiliates and sublicensees to, deliver or tender (or cause to be delivered or tendered) any Product for use in the other Party’s territory. |
|
7. |
Manufacture and Supply |
|
7.2 After the disbanding of the JSC, the Parties shall continue to exchange information regarding the manufacturing and supply activities with respect to the Compound and the Product by providing updates to each other on a periodic basis [ * ], through a manufacturing and supply review board. Decisions in such review board shall be made by unanimous vote with each Party's representative collectively having one (1) vote. |
|
8. |
Financial Provisions |
17
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
Milestone Event |
Milestone Payment |
|
1. MAA Approval by the EMA for the Product for the first Indication |
$17.5 million |
|
2. [ * ] |
$[ * ] |
|
3. [ * ] |
$[ * ] |
|
TOTAL |
$40 million |
|
(c) Notice and Payment. Each Party shall notify the other Party in writing within [ * ] after the achievement of any milestone set forth in this Section 8.2 by such Party or its Affiliates or Sublicensees. After the delivery or receipt of such notice, Grifols shall pay to Rigel the applicable milestone payment within [ * ] from the date of the corresponding invoice issued by Rigel. |
|
Aggregate Net Sales of all Products in the Grifols Territory in a Year |
Milestone Payment |
|
Equal or exceed$[ * ] |
$[ * ] |
|
Equal or exceed$[ * ] |
$[ * ] |
|
Equal or exceed $[ * ] |
$[ * ] |
|
Equal or exceed $[ * ] |
$[ * ] |
|
Equal or exceed $[ * ] |
$[ * ] |
|
TOTAL |
$255 million |
|
(b) Notice and Payment. As part of the report set forth in Section 9.1, Grifols shall provide written notice to Rigel if the aggregated Net Sales of all Products in the Grifols Territory in any Year first reach the |
18
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
values set forth in Section 8.3(a) above, and Grifols shall pay to Rigel the corresponding Net Sales milestone payment within [ * ] upon receipt of the corresponding invoice. |
|
Annual Net Sales of all Products in the Grifols Territory |
Royalty Rate |
|
Portion less than or equal to $[ * ] |
[ * ]% |
|
Portion greater than $[ * ] |
[ * ]% |
|
Portion greater than $[ * ] |
30% |
|
9. |
Payment; Records; Audits |
19
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Net Sales milestone under Section 8.3 has been achieved. Prior to the First Commercial Sale of the Product in the Grifols Territory, the Parties will agree on the form of royalty report through the JSC. Grifols shall submit a single report for all Net Sales during a Calendar Quarter, including all of Grifols’ and its Affiliates’ and Sublicensees’ Net Sales. |
|
9.2 Exchange Rate; Manner and Place of Payment. All references to dollars and “$” herein shall refer to U.S. dollars. All payments hereunder shall be payable in U.S. dollars. When conversion of Net Sales from any currency other than U.S. dollars is required, such conversion shall be at the exchange rate equal to the conversion rate for the U.S. dollar for the currency of the country in which the applicable Net Sales were made as published by [ * ]. All payments owed under this Agreement shall be made by wire transfer in immediately available funds to a bank and account designated in writing by Rigel, unless otherwise specified in writing by Rigel. |
|
(a) Taxes on Income. Each Party shall be solely responsible for the payment of all taxes imposed on its share of income arising directly or indirectly from the activities of the Parties under this Agreement. |
|
(b) Tax Cooperation. The Parties agree to cooperate with one another and use reasonable efforts to avoid or reduce tax withholding or similar obligations in respect of the milestone payments, royalty payments, and other payments made by Grifols to Rigel under this Agreement. To the extent that Grifols is required by Applicable Laws to deduct and withhold taxes on any payment to Rigel, Grifols shall pay the amounts of such taxes to the proper Governmental Authority in a timely manner and promptly transmit to Rigel an official tax certificate or other evidence of such payment sufficient to enable Rigel to claim such payment of taxes. Rigel shall provide Grifols any tax forms that may be reasonably necessary in order for Grifols to not withhold tax or to withhold tax at a reduced rate under an applicable bilateral income tax treaty, to the extent legally able to do so. Rigel shall use reasonable efforts to provide any such tax forms to Grifols in advance of the due date. Grifols shall provide Rigel with reasonable assistance to enable the recovery, as permitted by Applicable Laws, of withholding taxes or similar obligations resulting from payments made under this Agreement, such recovery to be for the benefit of Rigel. Grifols shall have the right to deduct any such tax, levy, or charge actually paid from payment due to Rigel. Each Party agrees to assist the other Party in claiming exemption from such deductions or withholdings under double taxation or similar agreement or treaty from time to time in force and in minimizing the amount required to be so withheld or deducted. |
|
(c) Taxes Resulting From Grifols’ Action. Grifols represents and warrants that, as of the Effective Date, Grifols is not required by Applicable Law to deduct or withhold taxes on the upfront payment, milestone payments, royalty payments, or other payments payable to Rigel under this Agreement. If a Party takes any action of its own discretion (not required by a Regulatory Authority) which results in a withholding or deduction obligation (“Withholding Tax Action”), then such Party shall pay the sum associated with such Withholding Tax Action. For clarity, if Grifols undertakes a Withholding Tax Action, then the sum payable by Grifols (in respect of which such deduction or withholding is required to be made) shall be increased to the extent necessary to ensure that Rigel receives a sum equal to the sum which it would have received had no such Withholding Tax Action occurred. If a change in Applicable Laws results in a withholding or deduction obligation absent either Party taking a Withholding Tax Action, then the amount of such withholding or deduction obligation shall be paid by Grifols to the applicable Governmental Authority on behalf of Rigel, provided that Grifols shall assist Rigel in minimizing or recovering such withholding or deduction obligation. The Parties shall use commercially reasonable efforts to invoke the application of any applicable bilateral income tax treaty that would reduce or eliminate otherwise applicable taxes with respect to payments payable pursuant to this Agreement. |
20
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
auditing Party the accuracy of the financial reports furnished by Grifols pursuant to this Agreement or of any payments made, or required to be made, by or to the audited Party pursuant to this Agreement. Before beginning its audit, the Auditor shall execute an undertaking acceptable to Grifols by which the Auditor agrees to keep confidential all information reviewed during the audit. Such audits may occur no more often than [ * ] each Year and not more frequently than [ * ] with respect to records covering any specific period of time. Rigel shall only be entitled to audit the books and records from the [ * ] prior to the Year in which the audit request is made. Other than the audit report, such Auditor shall not disclose the audited Party’s Confidential Information to the auditing Party, its Affiliates or Third Parties. In the event that the final result of the inspection reveals an undisputed underpayment or overpayment, the underpaid or overpaid amount shall be settled within [ * ] after the Auditor’s report. Rigel shall bear the full cost of such audit unless such audit reveals an underpayment by Grifols that resulted from a discrepancy in the financial report provided by Grifols for the audited period, which underpayment was more than [ * ] of the amount set forth in such report, in which case Grifols shall reimburse Rigel for the costs for such audit. In case of overpayment, Rigel shall, at Grifols’ discretion, reimburse Grifols the amounts overpaid by Grifols or settle them against any amount due or to be due by Grifols to Rigel pursuant to this Agreement. |
|
(b) Inventions. Inventorship of any Inventions will be determined in accordance with the standards of inventorship and conception under U.S. patent laws. The Parties will work together to resolve any issues regarding inventorship or ownership of Inventions. Ownership of Inventions will be allocated as follows: Rigel shall solely own all data, Inventions, and Patents claiming such Inventions that relate to the composition, manufacture, or use of the Compound, or any improvement of any such composition, manufacture, or use, including in combination with other agents or components (each, a “Compound Invention”). All Compound Inventions will be included in the Rigel Know-How, and Patents in the Grifols Territory claiming such Inventions will be included in the Rigel Patents, including any Compound Invention made by Grifols, its Affiliates, employees, agents, and Sublicensees. Grifols hereby assigns, or shall assign if under any Applicable Law assignment of future rights is not deemed effective, all of Grifols’ right, title, and interest in and to any and all Compound Inventions to Rigel and warrants that its Affiliates, employees, agents, and Sublicensees are or shall be contractually obliged to assign their right, title, and interest in and to any such Compound Inventions to Grifols to effectuate the foregoing assignment to Rigel. |
21
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(c) Cooperation. Notwithstanding Rigel's obligations under Section 10.2 (a), Grifols agrees to cooperate fully with Rigel, upon Rigel's request, in the preparation, filing, prosecution, maintenance, and defense, if any, of Patents under this Section 10.2 and in the obtaining and maintenance of any Patent term extensions and supplementary protection certificates and their equivalents. Such cooperation includes (i) executing all papers and instruments, or requiring its employees or contractors, to execute such papers and instruments, so as to enable Rigel to apply for and to maintain and prosecute patent applications in any country as permitted by this Section 10.2; and (ii) promptly informing Rigel of any matters coming to Grifols’ attention that may affect the preparation, filing, prosecution, or maintenance of any such patent application and the obtaining of any patent term extensions or supplementary protection certificates or their equivalents. |
|
(c) Collaboration. Each Party shall provide to the enforcing Party reasonable assistance in such enforcement, at such enforcing Party’s request and expense, including to be named in such action if required by Applicable Laws to pursue such action. The enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts, shall reasonably consider the other Party’s comments on any such efforts, including determination of litigation strategy and filing of material papers to the competent court. The non-enforcing Party shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall at all times cooperate fully with the enforcing Party. |
22
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
rights in a jurisdiction within the Grifols Territory, Grifols shall promptly notify Rigel and the Parties shall promptly meet to consider the claim or assertion and the appropriate course of action and may, if appropriate, agree on and enter into a “common interest agreement” wherein the Parties agree to their shared, mutual interest in the outcome of such potential dispute. Absent any agreement to the contrary, and subject to claims for indemnification under Article 12, each Party may defend itself from any such Third Party claim at its own cost and expense, provided, however, that the provisions of Section 10.3 shall govern the right of Grifols to assert a counterclaim of infringement of any Rigel Patents. |
|
10.5 Patents Licensed From Third Parties. Each Party’s rights under this Article 10 with respect to the prosecution and enforcement of any Rigel Patent shall be subject to the rights (a) retained by any upstream licensor to prosecute and enforce such Patent Right, if such Patent is subject to an upstream license agreement; and (b) granted to any Third Party prior to such Patent becoming subject to the license grant under this Agreement. |
|
10.6 Parties Cooperation. Further to the above, in the event of a Product Infringement or a Third Party intellectual property right infringement set forth in Section 10.3 and 10.4, the Parties shall meet to consider the impact of such infringements on Grifols’ rights pursuant to this Agreement. |
|
10.7 Trademarks. |
|
(a) Product Trademarks. Grifols shall utilize Rigel’s trade names, trade dresses, branding, and logos, to be used for the Product in the Field in the Grifols Territory, which are included in Exhibit C attached herein (the “Rigel Product Marks”); provided, however, that if any Product Xxxx is not approved for use in a country in the Grifols Territory, Grifols shall be responsible for identifying a trademark for the Product for use in such country (“Grifols Product Marks”). Rigel shall own all Rigel Product Marks and Grifols shall own all Grifols Product Marks. Each Party shall be responsible for the registration, maintenance, defense, and enforcement of its respective Product Marks at its own cost and using counsel of its own choice. Rigel shall keep Grifols informed of material progress with regard to the registration, prosecution, maintenance, and defense, if any, of any Rigel Product Marks in the Grifols Territory, including content, timing, and jurisdiction of the filing of such Rigel Product Marks in the Grifols Territory. |
|
(b) Trademark License. Grifols shall use the Rigel Product Marks and Grifols Product Marks, as applicable, to Commercialize the Product in the Field in the Grifols Territory. In addition, unless prohibited by Applicable Laws in any country of the Grifols Territory, Rigel’s corporate trademark shall be included in the on the packaging and product information of the Products sold in the Field in the Grifols Territory to indicate that the Product is licensed from Rigel. Rigel hereby grants to Grifols a limited (as to the Term of this Agreement), royalty-free license to use Rigel’s corporate trademark and Rigel Product Marks solely in connection with the Commercialization of the Product in the Field in the Grifols Territory under this Agreement. All use of the Rigel Product Marks and Rigel’s corporate trademark shall comply with Applicable Laws and shall be subject to Rigel’s review and approval. For clarity, Grifols may also include its (or its Affiliate’s or Sublicensee’s, as applicable) corporate trademarks and logo in the Product sold in the Grifols Territory. |
|
11. |
Representations and Warranties |
|
11.1 Mutual Representations and Warranties. Each Party represents and warrants to the other that, as of the Effective Date: (a) it is duly organized and validly existing under the laws of its jurisdiction of incorporation or formation, and has full corporate or other power and authority to enter into this Agreement and to carry out the provisions hereof, (b) it is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder, and the person or persons executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate or partnership action, (c) this Agreement is legally binding upon it, enforceable in accordance with its terms, and does not conflict with any agreement, instrument, or understanding, oral or written, to which it is a Party or by which it may be bound, nor violate any material law or regulation of any court, governmental body, or administrative or other agency having jurisdiction over it, and (d) it has the right to grant the licenses granted by it under this Agreement. |
23
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(a) Debarment. Each Party represents, warrants, and covenants to the other Party that it is not debarred or disqualified under the U.S. Federal Food, Drug and Cosmetic Act, as may be amended, or comparable laws in any country or jurisdiction other than the U.S., and it does not, and will not during the Term, employ or use the services of any person who is debarred or disqualified, in connection with activities relating to any Product. In the event that either Party becomes aware of the debarment or disqualification or threatened debarment or disqualification of any person providing services to such Party, including the Party itself or its Affiliates or sublicensees, that directly or indirectly relate to activities contemplated by this Agreement, such Party shall immediately notify the other Party in writing and such Party shall cease employing, contracting with, or retaining any such person to perform any such services. |
|
(i) In the performance of its obligations under this Agreement, each Party shall comply and shall cause its and its Affiliates’ employees and contractors to comply with all Applicable Laws. |
|
(i) Each Party and its and its Affiliates’ employees and contractors shall not, in connection with the performance of their respective obligations under this Agreement, directly or indirectly through Third Parties, pay, promise, or offer to pay, or authorize the payment of, any money or give any promise or offer to give, or authorize the giving of anything of value to a Public Official or Entity or other person for purpose of obtaining or retaining business for or with, or directing business to, any person, including, such Party (and such Party represents and warrants that as of the Effective Date, such Party, and to its knowledge, its and its Affiliates’ employees and contractors, have not directly or indirectly promised, offered, or provided any corrupt payment, gratuity, emolument, bribe, kickback, illicit gift, or hospitality or other illegal or unethical benefit to a Public Official or Entity or any other person in connection with the performance of such Party’s obligations under this Agreement, and such Party covenants that it and its Affiliates’ employees and contractors shall not, directly or indirectly, engage in any of the foregoing). |
|
(i) Each Party and its Affiliates, and their respective employees and contractors, in connection with the performance of their respective obligations under this Agreement, shall not violate or cause the violation of the FCPA, Export Control Laws, or any other Applicable Laws, or otherwise cause any reputational harm to the other Party. |
|
(i) Each Party shall immediately notify the other Party if such Party has any information or suspicion that there may be a violation of the FCPA, Export Control Laws, or any other Applicable Laws in connection with the performance of this Agreement or the Development, manufacture, or Commercialization of any Product. |
|
(i) In the event that a Party has violated or been suspected of violating any of the representations, warranties, or covenants in this Section 11.2(b), such Party will cause its or its |
24
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Affiliates’ personnel or others working under its direction or control to submit to periodic training that such Party will provide on anti-corruption law compliance. |
|
(i) Each Party shall have the right to suspend or terminate this Agreement in its entirety where there is a credible finding, after a reasonable investigation, that the other Party, its Affiliates, or its Sublicensees, in connection with performance of its obligations under this Agreement, has engaged in chronic or material violations of the FCPA. |
|
11.3 Additional Rigel Representations, Warranties, and Covenants. Rigel represents, warrants, and covenants, as applicable, to Grifols that, as of the Effective Date: |
|
(a) Exhibit B lists all Rigel Patents in the Grifols Territory and the Option Territory as of the Effective Date that claim the composition of matter or method of use or manufacture of the Compound and have been filed, prosecuted, and maintained in a manner consistent with Rigel’s standard practice, and in each applicable jurisdiction in which such Patent has been filed no official final deadlines with respect to prosecution thereof have been missed and all applicable fees have been paid on or before the due date for payment; |
|
(b) All inventors of Inventions claimed in the Rigel Patents listed on Exhibit B have assigned their entire right, title, and interest in and to such inventions to Rigel and the inventors listed are correct and there are no claims or assertions in writing received by Rigel regarding the inventorship of such Patent alleging that additional or alternative Inventors ought to be listed; |
|
(c) Rigel has the right to grant all rights and licenses it purports to grant to Grifols with respect to the Rigel Technology and the Rigel Product Marks under this Agreement; |
|
(d) Rigel has not granted any liens or security interests on the Rigel Technology; |
|
(e) to Rigel’s knowledge, Rigel has not received any written notice from a Third Party that the commercialization of the Product prior to the Effective Date has infringed any Patents of any Third Party within the Grifols Territory; |
|
(g) no claim or action has been brought or, to Rigel’s knowledge, threatened in writing, by any Third Party alleging that the Rigel Patents are invalid or unenforceable, and no Rigel Patent is the subject of any interference, opposition, cancellation, or other protest proceeding; |
|
(h) to Rigel’s knowledge, no Third Party is infringing or misappropriating or has materially infringed or misappropriated the Rigel Technology and Rigel Product Xxxx in the Grifols Territory; |
|
(i) to Rigel’s knowledge, it has disclosed to Grifols the clinical and non-clinical data in Rigel’s Control that is material to the evaluation of the safety, efficacy, and manufacturing process of the Product; |
|
(j) to Rigel’s knowledge, there are no issues or information, which to Rigel’s knowledge and reasonable opinion, are reasonably likely to have a material impact on the Product that have not been fully disclosed to Grifols in the course of Grifols’ due diligence; and |
25
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
11.4 Disclaimer. Except as expressly set forth in this Agreement, THE TECHNOLOGY AND INTELLECTUAL PROPERTY RIGHTS PROVIDED BY EACH PARTY HEREUNDER ARE PROVIDED “AS IS” AND EACH PARTY EXPRESSLY DISCLAIMS ANY AND ALL OTHER WARRANTIES OF ANY KIND, EXPRESS OR IMPLIED, INCLUDING THE WARRANTIES OF DESIGN, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NONINFRINGEMENT OF THE INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES, OR ARISING FROM A COURSE OF DEALING, USAGE OR TRADE PRACTICES, IN ALL CASES WITH RESPECT THERETO. Without limiting the foregoing, (a) neither Party represents or warrants that any Data obtained from conducting clinical trials in one country or jurisdiction will comply with the laws and regulations of any other country or jurisdiction, and (b) neither Party represents or warrants the success of any study or test conducted pursuant to this Agreement or the safety or usefulness for any purpose of the technology it provides hereunder. |
26
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
after the commencement of any action with respect to a Third Party Claim shall only relieve the Indemnitor of its indemnification obligations under this Article 12 if and to the extent the Indemnitor is actually prejudiced thereby. The Indemnitee shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action with respect to a Claim covered by this indemnification. |
27
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(a) filing, prosecuting, or maintaining Patents as permitted by this Agreement; |
|
(b) Regulatory Filings for Products that such Party has a license or right to Develop or Commercialize under this Agreement in a given country or jurisdiction; |
|
(d) complying with applicable court orders or governmental regulations, including regulations promulgated by securities exchanges; and |
|
(f) disclosure to actual and bona fide potential investors, acquirors, licensees, and other financial or commercial partners solely for the purpose of evaluating or carrying out an actual or potential investment, acquisition, or collaboration, in each case under written obligations of confidentiality and non-use at least as stringent as those herein, provided that the disclosing Party redacts the financial terms and other provisions of this Agreement that are not reasonably required to be disclosed in connection with such potential investment, acquisition, or collaboration, which redaction shall be prepared in consultation with the other Party. |
Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to Section 13.3(c) or 13.3(d), it will, except where impracticable, give reasonable advance notice to the other Party of such disclosure and use the same diligent efforts to secure confidential treatment of such Confidential Information as such Party would use to protect its own confidential information, but in no event less than reasonable efforts. In any event, the Parties agree to take all reasonable action to avoid disclosure of Confidential Information hereunder. Any information disclosed pursuant to Section 13.3(c) or 13.3(d) shall remain Confidential Information and subject to the restrictions set forth in this Agreement, including the foregoing provisions of this Article 13.
28
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
29
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
of the Product is so unfavorable that it would be incompatible with the welfare of patients to Develop or Commercialize or to continue to Develop or Commercialize such Product. Prior to any such termination, the terminating Party shall comply with such internal review and management approval processes as it would normally follow in connection with the termination of the development and commercialization of its own products for safety reasons. The terminating Party shall document the decisions of such committees or members of management and the basis therefor and shall make such minutes and documentation available to the other Party promptly upon written request. |
(i) within a period of [ * ] as of the Effective Date (or such longer term as the Parties may agree to in writing), Grifols has not been able to identify and negotiate a satisfactory agreement with a Third Party for the provision of pharmacovigilance services within the scope of this Agreement and the Commercial Supply Agreement; and/or
(ii) within a period of [ * ] as of the Effective Date (or such longer term as the Parties may agree to in writing), Grifols has not been able to identify and negotiate a satisfactory agreement with a Third Party for the provision of testing, labeling and packaging services within the scope of this Agreement and the Commercial Supply Agreement; and/or
(iii) within a period of [ * ] as of the Effective Date (or such longer term as the Parties may agree to in writing), Grifols or a Grifols' Affiliate, has not been able to obtain approval from the Spanish or other Regulatory Authorities to amend its license and/or permits in order to include the Products; and/or
(iv) within a period of [ * ] as of the Effective Date (or such longer term as the Parties may agree to in writing), if the Parties have not executed the Commercial Supply Agreement.
|
14.4 Termination due to Acquisition of Rigel by a Competing Company. In the case Rigel is acquired by a Competing Company and Grifols has not provided its consent to an assignment or transfer of this Agreement to such Competing Company during the Consent Process, this Agreement shall terminate upon closing of such an acquisition of Rigel by such a Competing Company and [ * ] written notice, and Rigel or the acquiring party shall pay Grifols a one-time payment of [ * ], within [ * ] from the closing date of such acquisition. The “Consent Process” shall be [ * ]. Upon [ * ], Grifols will have [ * ] to provide Rigel with Grifols’ written consent to assign or otherwise transfer this Agreement should such discussions result in an acquisition of Rigel. [ * ]. As used herein, a “Competing Company” means a company and/or its affiliated entities which [ * ]. |
|
(a) Licenses. The licenses granted by Rigel to Grifols will automatically terminate, including all sublicenses granted by Grifols to any Sublicensee. |
30
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(c) Development Wind-Down. Grifols shall either, as directed by Rigel, (i) wind-down any ongoing Development activities (including any Clinical Trials) of Grifols or its Affiliates and Sublicensees with respect to the Product in the Grifols Territory in an orderly fashion or (ii) promptly transfer such Development activities to Rigel or its designee, in each case in compliance with all Applicable Laws. |
|
(g) Trademarks. Grifols shall transfer and assign to Rigel, upon Rigel's request, all or part of the Grifols Product Marks, under the terms and conditions that the Parties may agree to at that time. |
31
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
or control containing Confidential Information of the other Party; provided that such Party may keep one copy of such materials for archival purposes only subject to continuing confidentiality obligations. All Grifols Data and Regulatory Filings assigned to Rigel upon termination of this Agreement will be deemed Rigel’s Confidential Information and no longer Grifols’ Confidential Information. |
|
14.7 Survival. Expiration or termination of this Agreement shall not relieve the Parties of any obligation or right accruing prior to such expiration or termination. Except as set forth below or elsewhere in this Agreement, the obligations and rights of the Parties under the following provisions of this Agreement shall survive expiration or termination of this Agreement: Sections 2.5, 10.1, 11.4, 13.1, 13.2, 13.3, 13.6, 14.4, 14.5, 14.6, 14.7, 14.8 and 14.9, and Articles 1, 8 (solely to the extent any payment obligation has accrued prior to the effective date of termination), 9 (solely to the extent any payment obligation has accrued prior to the effective date of termination or as part of the transition activities pursuant to the terms and conditions under this Article 14), 12, 15 and 16. |
32
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
|
(b) Notwithstanding the terms of and procedures set forth in Section 15.2 or 15.3(a), any applications, motions, or orders to show cause seeking temporary restraining orders, preliminary injunctions, or other similar preliminary or temporary legal or equitable relief (“Injunctive Relief”) concerning a Disputed Matter (including Disputed Matters arising out of a potential or actual breach of the confidentiality and non-use provisions in Article 13) may immediately be brought in the first instance and without invocation or exhaustion of the procedures set forth in subsections (a) and (b) for hearing and resolution in and by any court of competent jurisdiction. Alternatively, a party seeking Injunctive Relief may immediately institute arbitral proceedings without invocation or exhaustion of the procedures set forth in subsections (a) and (b), and any such Injunctive Relief proceedings will be administered by the AAA pursuant to its AAA emergency arbitration procedures then in effect and applying the substantive law specified in Section 16.1. In either event, once the Injunctive Relief proceedings have been conducted and a decision rendered thereon by the court or arbitral forum, the Parties shall, if the Disputed Matter is not finally resolved by the Injunctive Relief, proceed to resolve the Disputed Matter in accordance with the terms of Section 15.2 and 15.3(a). |
|
16.1 Governing Law. This Agreement, and all questions regarding the existence, validity, interpretation, breach, or performance of this Agreement, shall be governed by, and construed and enforced in accordance with, the laws of the State of New York, United States, without reference to its conflicts of law principles. |
|
16.2 Entire Agreement; Modification. This Agreement, including the exhibits, is both a final expression of the Parties’ agreement and a complete and exclusive statement with respect to all of its terms. This Agreement supersedes all prior and contemporaneous agreements and communications, whether oral, written, or |
33
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
otherwise, concerning any and all matters contained herein. This Agreement may only be modified or supplemented in a writing expressly stated for such purpose and signed by the Parties to this Agreement. |
|
16.3 Relationship Between the Parties. The Parties’ relationship, as established by this Agreement, is solely that of independent contractors. This Agreement does not create any partnership, joint venture, or similar business relationship between the Parties. Neither Party is a legal representative of the other Party, and neither Party can assume or create any obligation, representation, warranty, or guarantee, express or implied, on behalf of the other Party for any purpose whatsoever. |
|
16.4 Affiliates. Each Party may exercise its rights or fulfill its obligations under this Agreement through one or more of its Affiliates, provided that such Party shall remain primarily responsible to the other Party for the performance and action of such Affiliates. |
|
16.5 Non-Waiver. The failure of a Party to insist upon strict performance of any provision of this Agreement or to exercise any right arising out of this Agreement shall neither impair that provision or right nor constitute a waiver of that provision or right, in whole or in part, in that instance or in any other instance. Any waiver by a Party of a particular provision or right shall be in writing, shall be as to a particular matter and, if applicable, for a particular period of time and shall be signed by such Party. |
|
(b) to an Affiliate, provided that the assigning Party shall remain liable and responsible to the non-assigning Party hereto for the performance and observance of all such duties and obligations by such Affiliate, and provided further that if the entity to which this Agreement is assigned ceases to be an Affiliate of the assigning Party, the Agreement shall be automatically assigned back to the assigning Party or its successor. |
The rights and obligations of the Parties under this Agreement shall be binding upon and inure to the benefit of the successors and permitted assigns of the Parties specified above, and the name of a Party appearing herein will be deemed to include the name of such Party’s successors and permitted assigns to the extent necessary to carry out the intent of this Section 16.6. Any assignment not in accordance with this Section 16.6 shall be null and void.
|
16.7 Severability. If, for any reason, any part of this Agreement is adjudicated invalid, unenforceable, or illegal by a court of competent jurisdiction, such adjudication shall not, to the extent feasible, affect or impair, in whole or in part, the validity, enforceability, or legality of any remaining portions of this Agreement. All remaining portions shall remain in full force and effect as if the original Agreement had been executed without the invalidated, unenforceable, or illegal part. |
34
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
or (iv) if sent by facsimile, the date of confirmation of receipt if during the recipient’s normal business hours, otherwise the next business day. |
If to Grifols, notices must be addressed to:
Grifols
Grifols Worldwide Operations Limited
Grange Xxxxxx Xxxxxxxx Xxxx
Xxxxxxxxxx, Xxxxxx 00, Xxxxxxx
Attention: [ * ]
Facsimile: [ * ]
If to Rigel, notices must be addressed to:
Rigel Pharmaceuticals, Inc.
0000 Xxxxxxxx Xxxx.
Xxxxx Xxx Xxxxxxxxx, XX 00000
XXX
Attention: [ * ]
Facsimile: [ * ]
|
16.10 Interpretation. The headings of clauses contained in this Agreement preceding the text of the Sections, subsections, and paragraphs hereof are inserted solely for convenience and ease of reference only and shall not constitute any part of this Agreement, or have any effect on its interpretation or construction. All references in this Agreement to the singular shall include the plural where applicable. Unless otherwise specified, references in this Agreement to any Article shall include all Sections, subsections, and paragraphs in such Article, references to any Section shall include all subsections and paragraphs in such Section, and references in this Agreement to any subsection shall include all paragraphs in such subsection. The word “including” and similar words means including without limitation. The word “or” means “and/or” unless the context dictates otherwise because the subjects of the conjunction are, or are intended to be, mutually exclusive. The words “herein”, “hereof”, and “hereunder” and other words of similar import refer to this Agreement as a whole and not to any particular Section or other subdivision. All references to days in this Agreement mean calendar days, unless otherwise specified. Ambiguities and uncertainties in this Agreement, if any, shall not be interpreted against either Party, irrespective of which Party may be deemed to have caused the ambiguity or uncertainty to exist. This Agreement has been prepared in the English language and the English language shall control its interpretation. In addition, all notices required or permitted to be given hereunder, and all written, electronic, oral, or other communications between the Parties regarding this Agreement shall be in the English language. |
35
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
{Signature Page Follows}
36
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
In Witness Whereof, the Parties hereto have caused this Exclusive Commercialization License Agreement to be executed and entered into by their duly authorized representatives as of the Effective Date.
|
Rigel Pharmaceuticals, Inc. By: /s/ Xxxx Xxxxxxxxx Name: Xxxx Xxxxxxxxx Title: CEO & President |
Grifols Worldwide Operations Limited By: /s/ Xxxxxxx Xxxxxx Name: Xxxxxxx Xxxxxx Title: CFO |
37
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
List of Exhibits:
Exhibit A:The Compound and Product
Exhibit B:Rigel Patents
Exhibit C:Product Marks
Exhibit D:Press Release
38
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit A:
The Compound and Product
The chemical name for the Compound, fostamatinib disodium hexahydrate, is disodium [6-[[5-fluoro-2-(3,4,5-trimethoxyanilino) pyrimidin-4-yl]amino]-2,2-dimethyl-3-oxo-pyrido[3,2-b][1,4]oxazin-4-yl]methyl phosphate hexahydrate.
Fostamatinib disodium hexahydrate has the molecular formula C23H24FN6Na2O9P·6H2O, and the molecular weight is 732.52. The structural formula is:
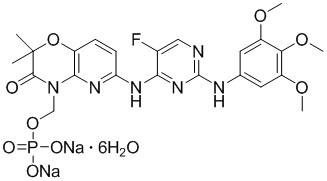
The Product is an oral tablet containing fostamatinib disodium hexahydrate in the amount equivalent to 100 mg and 150 mg of fostamatinib free acid. The inactive ingredients are: (Tablet Core) mannitol, sodium bicarbonate, sodium starch glycolate, povidone, magnesium stearate, (Coating) polyvinyl alcohol, titanium dioxide, polyethylene glycol 3350, talc, iron oxide yellow and iron oxide red.
39
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit B:
Rigel Patents
[ * ]
{Redacted content comprises approximately 16 pages.}
40
[ * ] = Certain confidential information contained in this document, marked by brackets, has been omitted because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
Exhibit C:
Trademark Protection Status
[ * ]
{Redacted content comprises approximately 2 pages.}
41
Exhibit D:
Press Release
Rigel Pharmaceuticals Enters Collaboration and License Agreement with Grifols, S.A. to Commercialize Fostamatinib in Europe
|
· |
Grifols gains exclusive rights to fostamatinib in all potential indications in Europe and Turkey |
|
· |
Rigel to receive stepped royalty payments reaching 30% of net sales of fostamatinib |
SOUTH SAN FRANCISCO, Calif., [ * ] /PRNewswire/ -- Rigel Pharmaceuticals, Inc. (Nasdaq: RIGL) today announced that it has entered into an exclusive license and supply agreement with Spain-based Grifols, S.A ( MCE: GRF, MCE: GRF.P, NASDAQ: GRFS) to commercialize fostamatinib disodium hexahydrate in all potential indications in Europe and Turkey. Grifols is a global healthcare company and a leading producer of plasma-derived medicines for the treatment of rare and chronic diseases, including intravenous immunoglobulin (IVIG) which is used in the treatment of ITP and AIHA. Fostamatinib is commercially available in the U.S. under the brand name TAVALISSE® (fostamatinib disodium hexahydrate) and is the first and only SYK inhibitor indicated for the treatment of thrombocytopenia in adult patients with chronic immune thrombocytopenia who have had an insufficient response to a previous treatment.
“Grifols has a broad presence in Europe and an established position in the hematology commercial landscape, which supports our goal of bringing fostamatinib to patients in these countries,” said Xxxx Xxxxxxxxx, president and CEO of Rigel. “Our marketing authorization application for fostamatinib in chronic ITP is currently under review by the European Medicines Agency, and we anticipate a decision by the end of 2019. This provides a potential opportunity for fostamatinib to begin generating revenue in the European market in 2020.”
Under terms of the agreement, Rigel will receive a $30 million upfront cash payment, with the potential for to $297.5 million in payments related to regulatory and commercial milestones, which includes a $20 million payment for EMA approval of fostamatinib for the treatment of chronic ITP. Rigel will receive significant stepped double-digit royalty payments based on tiered net sales which may reach 30% of net sales. In return, Grifols receives exclusive rights to fostamatinib in human diseases, including chronic ITP, autoimmune hemolytic anemia (AIHA), and IgA nephropathy (IgAN), in Europe and Turkey. Rigel retains the remaining global rights to fostamatinib outside the Grifols territories and those rights previously granted to Kissei Pharmaceuticals (in Japan, China, Taiwan and the Republic of Korea).
“Given our global leadership position as a manufacturer of plasma medicines and our in-depth knowledge and expertise in blood disorders, adding fostamatinib to our portfolio is a natural fit for Grifols,” said Xxxx Xxxxxxx, President, Bioscience Commercial Division of Grifols. “Its potential in multiple indications, including ITP, will provide significant benefit for patients and is a valuable addition to our portfolio.”
On October 4, 2018, the EMA validated the marketing authorization application for fostamatinib in adult chronic ITP, which was submitted by Rigel. The company anticipates a decision from the EMA’s Committee on Human Medicinal Products by the fourth quarter of 2019 and potential European approval by the end of 2019.
About ITP
In patients with ITP, the immune system attacks and destroys the body's own blood platelets, which play an active role in blood clotting and healing. Common symptoms of ITP are excessive bruising and bleeding. People suffering with chronic ITP may live with an increased risk of severe bleeding events that can result in serious medical
42
complications or even death. Current therapies for ITP include steroids, blood platelet production boosters (TPOs) and splenectomy. However, not all patients respond to existing therapies. As a result, there remains a significant medical need for additional treatment options for patients with ITP.
About TAVALISSE
Indication
TAVALISSE® (fostamatinib disodium hexahydrate) tablets is indicated in the US for the treatment of thrombocytopenia in adult patients with chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.
Important Safety Information
Warnings and Precautions
|
· |
Hypertension can occur with TAVALISSE treatment. Patients with pre-existing hypertension may be more susceptible to the hypertensive effects. Monitor blood pressure every 2 weeks until stable, then monthly, and adjust or initiate antihypertensive therapy for blood pressure control maintenance during therapy. If increased blood pressure persists, TAVALISSE interruption, reduction, or discontinuation may be required. |
|
· |
Elevated liver function tests (LFTs), mainly ALT and AST, can occur with TAVALISSE. Monitor LFTs monthly during treatment. If ALT or AST increase to >3 x upper limit of normal, manage hepatotoxicity using TAVALISSE interruption, reduction, or discontinuation. |
|
· |
Diarrhea occurred in 31% of patients and severe diarrhea occurred in 1% of patients treated with TAVALISSE. Monitor patients for the development of diarrhea and manage using supportive care measures early after the onset of symptoms. If diarrhea becomes severe (≥Grade 3), interrupt, reduce dose or discontinue TAVALISSE. |
|
· |
Neutropenia occurred in 6% of patients treated with TAVALISSE; febrile neutropenia occurred in 1% of patients. Monitor the ANC monthly and for infection during treatment. Manage toxicity with TAVALISSE interruption, reduction, or discontinuation. |
|
· |
TAVALISSE can cause fetal harm when administered to pregnant women. Advise pregnant women the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for at least 1 month after the last dose. Verify pregnancy status prior to initiating TAVALISSE. It is unknown if TAVALISSE or its metabolite is present in human milk. Because of the potential for serious adverse reactions in a breastfed child, advise a lactating woman not to breastfeed during TAVALISSE treatment and for at least 1 month after the last dose. |
Drug Interactions
|
· |
Concomitant use of TAVALISSE with strong CYP3A4 inhibitors increases exposure to the major active metabolite of TAVALISSE (R406), which may increase the risk of adverse reactions. Monitor for toxicities that may require a reduction in TAVALISSE dose. |
|
· |
It is not recommended to use TAVALISSE with strong CYP3A4 inducers, as concomitant use reduces exposure to R406. |
|
· |
Concomitant use of TAVALISSE may increase concentrations of some CYP3A4 substrate drugs and may require a dose reduction of the CYP3A4 substrate drug. |
|
· |
Concomitant use of TAVALISSE may increase concentrations of BCRP substrate drugs (eg, rosuvastatin) and P-Glycoprotein (P-gp) substrate drugs (eg, digoxin), which may require a dose reduction of the BCRP and P-gp substrate drug. |
Adverse Reactions
|
· |
Serious adverse drug reactions in the ITP double-blind studies were febrile neutropenia, diarrhea, pneumonia, and hypertensive crisis, which occurred in 1% of TAVALISSE patients. In addition, severe adverse reactions occurred including dyspnea and hypertension (both 2%), neutropenia, arthralgia, chest pain, diarrhea, dizziness, nephrolithiasis, pain in extremity, toothache, syncope, and hypoxia (all 1%). |
43
|
· |
Common adverse reactions (≥5% and more common than placebo) from the FIT-1 and FIT-2 phase 3 clinical trials included: diarrhea, hypertension, nausea, dizziness, ALT and AST increased, respiratory infection, rash, abdominal pain, fatigue, chest pain, and neutropenia. |
Please see xxx.XXXXXXXXX.xxx for full Prescribing Information.
To report side effects of prescription drugs to the FDA, visit xxx.xxx.xxx/xxxxxxxx or call 0-000-XXX-0000 (000-000-0000).
TAVALISSE is a trademark of Rigel Pharmaceuticals, Inc.
About Rigel (xxx.xxxxx.xxx)
Rigel Pharmaceuticals, Inc., is a biotechnology company dedicated to discovering, developing and providing novel small molecule drugs that significantly improve the lives of patients with immune and hematologic disorders, cancer and rare diseases. Rigel's pioneering research focuses on signaling pathways that are critical to disease mechanisms. The company's first FDA approved product is TAVALISSE™ (fostamatinib disodium hexahydrate), an oral spleen tyrosine kinase (SYK) inhibitor, for the treatment of adult patients with chronic immune thrombocytopenia who have had an insufficient response to a previous treatment. Rigel's current clinical programs include an upcoming Phase 3 study of fostamatinib in autoimmune hemolytic anemia and an ongoing Phase 1 study of R835, a proprietary molecule from its interleukin receptor associated kinase (IRAK) program. In addition, Rigel has product candidates in clinical development with partners BerGenBio AS, Daiichi Sankyo, and Aclaris Therapeutics.
About Grifols
Grifols is a global healthcare company with more than 75 years of legacy dedicated to improving the health and well-being of people around the world. Grifols produces essential plasma-derived medicines for patients, and provides hospitals and healthcare professionals with the tools, information and services they need to help them deliver expert medical care.
Grifols’ three main divisions – Bioscience, Diagnostic and Hospital – develop, produce and market innovative products and services that are available in more than 100 countries.
With a network of 250 plasma donation centers, Grifols is a leading producer of plasma-derived medicines used to treat rare, chronic and, at times, life-threatening conditions. As a recognized leader in transfusion medicine, Grifols offers a comprehensive portfolio of diagnostic products designed to support safety from donation through transfusion. The Hospital Division provides intravenous (IV) therapies, clinical nutrition products and hospital pharmacy systems, including systems that automate drug compounding and control drug inventory.
Grifols is headquartered in Barcelona, Spain, and has 20,000 employees in 30 countries. In 2017, sales exceeded 4,300 million euros. Grifols demonstrates its strong commitment to advancing healthcare by allocating a significant portion of its annual income to research, development and innovation.
The company’s class A shares are listed on the Spanish Stock Exchange, where they are part of the Ibex-35 (MCE:GRF). Grifols non-voting class B shares are listed on the Xxxxxxx Continuo (MCE:GRF.P) and on the US NASDAQ via ADRs (NASDAQ:GRFS).
For more information, visit xxx.xxxxxxx.xxx
Forward Looking Statements
This release contains forward-looking statements relating to, among other things, Rigel's partnership with Grifols; Rigel’s partnership with Kissei; Rigel's ability to achieve regulatory and commercial milestone payments under its agreement with Grifols; the potential opportunity for fostamatinib to begin generating revenue in the European market in 2020; Rigel's interactions with the EMA; and the timing of the EMA's MAA review process and when Rigel expects a decision. Any statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Words such as "planned," "will," "may," "expect," "anticipate," and
44
similar expressions are intended to identify these forward-looking statements. These forward-looking statements are based on Rigel's current expectations and inherently involve significant risks and uncertainties. Actual results and the timing of events could differ materially from those anticipated in such forward looking statements as a result of these risks and uncertainties, which include, without limitation, risks and uncertainties associated with the commercialization and marketing of TAVALISSE; risks that the FDA, EMA or other regulatory authorities may make adverse decisions regarding fostamatinib; risks that TAVALISSE clinical trials may not be predictive of real-world results or of results in subsequent clinical trials; risks that TAVALISSE may have unintended side effects, adverse reactions or incidents of misuses; the availability of resources to develop Rigel's product candidates; market competition; as well as other risks detailed from time to time in Rigel's reports filed with the Securities and Exchange Commission, including its Quarterly Report on Form 10-Q for the period ended September 30, 2018. Rigel does not undertake any obligation to update forward-looking statements and expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein.
Contact: Xxxxx Xxxxx
Phone: 000.000.0000
Email: xxxxxx@xxxxx.xxx
Media Contact: Xxxxxxx Xxxxxx
Phone: 000.000.0000
Email: xxxxxxx.xxxxxx@xxxxxxxxxxxx.xxx
45

