ASSET PURCHASE AGREEMENT BY AND BETWEEN ARADIGM CORPORATION. AND SJ2 THERAPEUTICS, INC. Dated as of August 25, 2006
Exhibit 10.9
CERTAIN MATERIAL (INDICATED BY AN ASTERISK) HAS BEEN OMITTED FROM THIS DOCUMENT PURSUANT TO A REQUEST FOR CONFIDENTIAL TREATMENT. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
BY AND BETWEEN
ARADIGM CORPORATION.
AND
SJ2 THERAPEUTICS, INC.
Dated as of August 25, 2006
TABLE OF CONTENTS
| Page | ||||
| ARTICLE I DEFINITIONS | 1 | |||
| Section 1.01 |
Certain Definitions |
1 | ||
| Section 1.02 |
Additional Definitions |
6 | ||
| ARTICLE II ASSIGNMENT TRANSFER AND LICENSE | 6 | |||
| Section 2.01 |
Assignment of Assigned Assets to Purchaser |
6 | ||
| Section 2.02 |
Asset Transfer |
6 | ||
| Section 2.03 |
Coordination Leads |
6 | ||
| Section 2.04 |
Transitional Services |
7 | ||
| Section 2.05 |
Assumption of Liabilities |
7 | ||
| Section 2.06 |
Consideration |
7 | ||
| Section 2.07 |
Closing, Closing Place, Time and Date |
9 | ||
| Section 2.08 |
Nontransferable Assets |
10 | ||
| Section 2.09 |
FTO Licenses |
11 | ||
| Section 2.10 |
Taking of Necessary Action; Further Action |
11 | ||
| ARTICLE III REPRESENTATIONS AND WARRANTIES OF ARADIGM | 12 | |||
| Section 3.01 |
Organization, Qualification, and Corporate Power |
12 | ||
| Section 3.02 |
Authorization |
12 | ||
| Section 3.03 |
Assets |
12 | ||
| Section 3.04 |
Transferred Books and Records |
12 | ||
| Section 3.05 |
Transferred Contracts |
12 | ||
| Section 3.06 |
Transferred Intellectual Property |
13 | ||
| ARTICLE IV REPRESENTATIONS AND WARRANTIES OF PURCHASER | 15 | |||
| Section 4.01 |
Organization, Qualification, and Corporate Power |
15 | ||
| Section 4.02 |
Authorization |
15 | ||
| ARTICLE V OTHER AGREEMENTS AND COVENANTS | 15 | |||
| Section 5.01 |
Additional Documents and Further Assurances |
15 | ||
| Section 5.02 |
Reasonable Cooperation of Purchaser |
15 | ||
| Section 5.03 |
Reasonable Efforts |
15 | ||
| Section 5.04 |
Indemnification |
15 | ||
| Section 5.05 |
Covenant Not to Compete |
17 | ||
| ARTICLE VI MISCELLANEOUS | 17 | |||
| Section 6.01 |
Press Releases and Public Announcements |
17 | ||
| Section 6.02 |
No Third-Party Beneficiaries |
17 | ||
| Section 6.03 |
Force Majeure |
17 | ||
| Section 6.04 |
Limitation of Liability |
17 | ||
| Section 6.05 |
Entire Agreement and Modification |
18 | ||
| Section 6.06 |
Amendment |
18 | ||
| Section 6.07 |
Waivers |
18 | ||
| Section 6.08 |
Successors and Assigns |
18 | ||
| Section 6.09 |
Counterparts |
18 | ||
| Section 6.10 |
Interpretation |
18 | ||
| Section 6.11 |
Notices |
19 | ||
| Section 6.12 |
Governing Law |
20 | ||
| Section 6.13 |
Severability |
20 | ||
| Section 6.14 |
Construction |
20 | ||
| Section 6.15 |
Attorneys’ Fees |
20 | ||
| Section 6.16 |
Further Assurances |
20 |
EXHIBITS
| EXHIBIT A | Transferred Assets (including Transferred Technology) | |
| EXHIBIT B | Transferred Books and Records | |
| EXHIBIT C | Transferred Contracts | |
| EXHIBIT D | Transferred Intellectual Property | |
| EXHIBIT E | General Assignment and Xxxx of Sale | |
| EXHIBIT F | Assumed Liabilities | |
| EXHIBIT G | Transfer Plan | |
| EXHIBIT H | Transitional Services Agreement | |
| EXHIBIT I | Intraject Delivery System | |
| EXHIBIT J | Nontransferable Assets | |
THIS ASSET PURCHASE AGREEMENT (this “Agreement”) is made and entered into as of August 25, 2006 by and between Aradigm Corporation, a California corporation (“Aradigm”), and SJ2 Therapeutics, Inc., a Delaware corporation (“Purchaser”). Aradigm and Purchaser are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
A. Aradigm desires to assign and transfer to Purchaser, and Purchaser desires to accept assignment and transfer from Aradigm, on the terms and subject to the conditions set forth herein, those certain assets of Aradigm related to the Intraject Delivery System.
B. Furthermore, Aradigm and Purchaser desire to make certain representations, warranties, covenants and other agreements in connection with the transactions contemplated hereby.
ARTICLE I
DEFINITIONS
Section 1.01 Certain Definitions. As used in this Agreement, the following terms have the following meanings (terms defined in the singular to have a correlative meaning when used in the plural and vice versa). Certain other terms are defined in the text of this Agreement.
(a) “Affiliate” means a corporation or any other entity that directly, or indirectly through one or more intermediaries, controls, is controlled by, or is under common control with, the designated Party, but only for so long as such control exists. As used in this definition only, “control” shall mean ownership of shares of stock having at least 50% of the voting power entitled to vote for the election of directors in the case of a corporation (or, in the case of an entity that is not a corporation, in the election of the corresponding managing authority), or otherwise having the power to directly or indirectly control the management of such entity.
(b) “Assigned Assets” shall mean any and all of Aradigm’s right, title and interest in and to the following:
(i) any and all tangible assets owned or otherwise transferable by Aradigm as of the Closing Date, in each case to the extent exclusively used or held for use in the Business, including those assets listed on Exhibit A (collectively, “Transferred Assets”);
(ii) the Books and Records listed on Exhibit B (collectively, “Transferred Books and Records”);
1
(iii) all agreements listed on Exhibit C (collectively, “Transferred Contracts”);
(iv) all Patents (including in each case all rights to Prosecute and Enforce the same) listed on Exhibit D (collectively, “Transferred Patents”);
(v) all Trademarks (including in each case all rights to Prosecute and Enforce the same) listed on Exhibit D (collectively, “Transferred Trademarks”);
(vi) any and all Technology owned or otherwise transferable by Aradigm as of the Closing Date, other than the Transferred Patents and Transferred Trademarks, in each case to the extent exclusively used or held for use in the Business, including that Technology listed on Exhibit A (collectively, “Transferred Technology”); and
(vii) any and all right to recover past, present and future damages for the breach, infringement or misappropriation, as the case may be, of any of the foregoing.
(c) “Books and Records” shall mean all papers and records (in any format including paper or electronic) kept or maintained including any and all laboratory notebooks, invention disclosures, purchasing and sales records, all data and communications relating to ongoing business development activities, preclinical and clinical data, all Regulatory Documents, vendor lists, accounting and financial records, product documentation, product specifications, marketing documents and the like, in each case pertaining to the Business or the Assigned Assets.
(d) “Business” shall mean the research, development, commercialization, manufacture, marketing, distribution, sale, support and other use and commercial exploitation of the Intraject Delivery System.
(e) “Business Intellectual Property” shall mean any and all Technology and any and all Intellectual Property Rights, including Registered Intellectual Property Rights, that is or are owned (in whole or in part) by or exclusively licensed to Aradigm, as of the Closing Date, in each case that are used in or necessary to the Business.
(f) “Dollars” shall refer to United States currency unless expressly specified otherwise.
(g) “Governmental Body” shall mean any: (i) nation, province, state, county, city, town, village, district, or other jurisdiction of any nature; (ii) federal, provincial, state, local, municipal, foreign, or other government; (iii) governmental or quasi-governmental authority of any nature (including any governmental agency, branch, department, official, or entity and any court or other tribunal); (iv) multi-national organization or body; or (v) body exercising, or entitled to exercise, any administrative, executive, judicial, legislative, police, regulatory, or taxing authority or power of any nature.
(h) “Intraject Delivery System” shall mean Aradigm’s Intraject® needle-free injection delivery system as more fully described in Exhibit I (the “Existing Delivery System”) or any modified, improved or derivative version thereof, in each case that includes one or more material elements of the Existing Delivery System.
2
(i) “Intellectual Property Rights” shall mean any or all of the following and all rights in, arising out of, or associated therewith: (i) all United States and foreign patents and utility models and applications therefor and all reissues, divisionals, re examinations, renewals, extensions, provisionals, supplementary protection certificates, continuations and continuations in-part thereof, and equivalent or similar registered rights anywhere in the world (“Patents”); (ii) all trade secrets and other rights in know-how and confidential or proprietary information, inventions and discoveries, including without limitation invention disclosures; (iii) all copyrights, works of authorship, copyright registrations and applications therefor and all other rights corresponding thereto throughout the world (“Copyrights”); (iv) all rights in Uniform Resource Locators, World Wide Web addresses and domain names and applications and registrations therefor (“Internet Property Rights”); (v) all trade names, logos, common law trademarks and service marks, trademark and service xxxx registrations and applications therefor and all goodwill associated therewith throughout the world (“Trademarks”); and (vi) any similar, corresponding or equivalent rights to any of the foregoing anywhere in the world, including, without limitation, moral rights.
(j) “Licensee” shall mean a Person other than an Affiliate to whom Purchaser or its Affiliate has granted the right, or to whom such a Person has sublicensed the right, to (i) make and sell any Product or (ii) sell any Product, provided that distributors, wholesalers and resellers as to which Purchaser does not receive compensation on resales of Products by such entity shall not be considered Licensees.
(k) “Lien” shall mean any mortgage, pledge, lien, charge, claim, security interest, adverse claims of ownership or use, restrictions on transfer, defect of title or other encumbrance of any sort, other than (i) mechanic’s, materialmen’s, and similar liens with respect to any amounts not yet due and payable, and (ii) liens for taxes not yet due and payable.
(l) “Net Sales” shall mean the amounts actually received by Purchaser, its Affiliates, or Licensees, in consideration of their sales of Product to Third Party customers, less: (i) normal and customary trade, cash and other discounts; (ii) credits or allowances for damaged goods, returns, rejections or recalls of Product; (iii) sales taxes, value added taxes, withholding, import/export taxes or other similar taxes (excluding taxes on the income of the selling entity) actually paid; (iv) normal and customary charge back payments or rebates; and (v) packaging, handling fees, prepaid freight, insurance and the like to the extent separately identified on the invoice. Sales between or among Purchaser, its Affiliates or Licensees for resale shall be excluded from the computation of Net Sales, but the subsequent re sale of such Products by Purchaser, its Affiliates or Licensees to an end user shall be included within the computation of Net Sales. Net Sales shall not include amounts in respect of Product sold or used for development applications (including for clinical trials) or commercial samples (i.e., items provided for free or at or below cost plus a nominal profit for promotional purposes).
(m) “Nontransferable Asset” shall have the meaning ascribed to the term in Section 9.
3
(n) “Non-Sumatriptan Product” shall mean any Product comprising the Intraject Delivery System combined with an applicable drug formulation, other than Sumatriptan.
(o) “Person” shall mean any individual, corporation (including any non-profit corporation), general or limited partnership, limited liability company, joint venture, estate, trust, association, organization, labor union, Governmental Body or other entity.
(p) “Product” shall mean any pharmaceutical product comprising the Intraject Delivery System combined with Sumatriptan or other applicable drug formulation.
(q) “Prosecution and Enforcement” shall mean (i) the preparation, filing for, prosecution, maintenance of registrations thereof and applications for any such registration (ii) the conduct of interferences, re examinations, reissues, oppositions or requests for term extensions with respect thereto and (iii) the conduct of any enforcement proceeding with respect thereto (whether infringement, misuse, misappropriation or otherwise) or any declaratory judgment proceeding with respect thereto; and “Prosecute and Enforce” shall have the correlative meaning.
(r) “Pulmonary Field” shall mean the delivery of one or more aerosolized active pharmaceutical ingredients directly into the bronchia or lungs.
(s) “Registered Intellectual Property Rights” shall mean all United States, international and foreign: (i) Patents, including applications therefor (each a “Registered Patent”); (ii) registered Trademarks, applications to register Trademarks, including intent-to use applications, or other registrations or applications related to Trademarks; (iii) Copyright registrations and applications to register Copyrights; and (iv) any other Technology or Intellectual Property Rights that is the subject of an application, certificate, filing, registration or other document issued by, filed with, or recorded by, any state, government or other public or private legal authority at any time.
(t) “Regulatory Documents” shall mean any and all regulatory submissions (whether completed or in process) to any Governmental Body anywhere in the world submitted by or on behalf of Aradigm relating to the Business (including any product developed in connection therewith), including all annual reports, adverse event reports, and other adverse event submission tracking information, and amendments and supplements to any of the foregoing. For purposes of clarity, “Regulatory Documents” shall not include any filing or other submission made to the United States Securities and Exchange Commission, the National Association of Securities Dealers, the Nasdaq Stock Exchange or any similar entity.
(u) “Representatives” shall mean, with respect to a Person, that Person’s officers, directors, employees, accountants, counsel, investment bankers, financial advisors, agents and other representatives.
4
(v) “Royalty Revenue” shall mean running royalties actually received by Purchaser from a Licensee for sales of Non-Sumatriptan Products by or under authority of such Licensee, plus any license fees or milestone or other payments receive by Purchaser from a Licensee to the extent not allocable to recovery of development or other costs incurred by Purchaser specific to the applicable Product. For clarity, Royalty Revenue shall exclude: (i) payments in consideration of goods (including Products) or services at Purchaser’s fully-burdened cost therefor (any amounts in excess of the fully-burdened cost shall be included in Royalty Revenue), (ii) payments in consideration for equity at the fair market value therefor (any amounts in excess of the fair market value shall be included in Royalty Revenue) and (iii) amounts received by Purchaser in consideration for a sale of all, or substantially all, of the business or assets of Purchaser (whether by way of merger, sale of stock, sale of assets or otherwise), if the successor to such business or assets has assumed the obligations under Section 2.06(a) of this Agreement.
(w) “Royalty Term” shall mean, for a given Product, the period commencing on the Closing Date and continuing until the later of (i) the ten-year anniversary of the first commercial sale of such Product in the United States, but no more than twenty years after the Closing Date and (ii) the later of expiration or abandonment of the last Valid Claim of the Transferred Patents covering the manufacture, use or sale of such Product.
(x) “Sumatriptan Product” shall mean any Product comprising the Intraject Delivery System combined with Sumatriptan.
(y) “Technology” shall mean any or all of the following: (i) works of authorship including, without limitation, computer programs, source code and executable code, whether embodied in software, firmware or otherwise, documentation, designs, files, net lists, records, data and mask works; (ii) inventions (whether or not patentable), improvements, and technology; (iii) proprietary and confidential information, including technical data and customer and supplier lists, trade secrets and know how; (iv) databases, data compilations and collections and technical data; (v) logos, trade names, trade dress, trademarks, service marks; (vi) World Wide Web addresses, domain names and sites; (vii) protocols, methods and processes; and (viii) all instantiations of the foregoing in any form and embodied in any media.
(z) “Territory” shall mean the entire world.
(aa) “Third Party” shall mean any Person other than Purchaser or Aradigm, or their respective Affiliates.
(bb) “Transfer Plan” shall mean the plan for the transfer of the Assigned Assets attached hereto as Exhibit G.
(cc) “Valid Claim” shall mean (i) a claim of an issued and unexpired patent, which has not been held unenforceable, unpatentable or invalid by a court or other governmental agency of competent jurisdiction, and which has not been admitted to be invalid or unenforceable through reissue, disclaimer or otherwise, or (ii) a claim in a pending patent application being prosecuted in good faith that has not been abandoned or finally rejected and that has been pending for fewer than five years after the earliest priority date to which it is entitled.
5
Section 1.02 Additional Definitions. Each of the following definitions shall have the meanings defined in the corresponding sections of this Agreement indicated below:
| Definition |
Section |
|||
| Agreement | Preamble | |||
| Aradigm Indemnities | Section 6.04(b) | |||
| Assumed Liabilities | Section 2.05(b) | |||
| Claim | Section 6.04(a) | |||
| Closing Date | Section 2.07 | |||
| Coordination Lead | Section 2.03 | |||
| Excluded Liabilities | Section 2.05(c) | |||
| Indemnitee | Section 6.04(c) | |||
| Indemnitor | Section 6.04(c) | |||
| Party | Preamble | |||
| PTO | Section 4.06(a) | |||
| Purchaser Indemnities | Section 6.04(a) |
ARTICLE II
ASSIGNMENT, TRANSFER AND LICENSE
Section 2.01 Assignment of Assigned Assets to Purchaser. Upon the terms and subject to the conditions set forth herein, Aradigm hereby assigns, conveys and transfers to Purchaser, at the Closing, all of Aradigm’s right, title and interest in and to the Assigned Assets, subject to the reservation on behalf of Aradigm of a perpetual, worldwide, royalty-free, non-exclusive license, under the Transferred Patents and Transferred Technology solely for purposes of the Pulmonary Field, which retained license shall include the right to grant sublicenses to Persons solely within the scope of such retained license in connection with the grant to such Persons of licenses under other Patents owned or controlled by Aradigm.
Section 2.02 Asset Transfer. Subject to the terms and conditions set forth in this Agreement, on the Closing Date, Aradigm shall transfer all Assigned Assets, in the shape, manner and form of their existence as of the date such Assigned Assets are transferred to Purchaser, in accordance with the Transfer Plan. Without limiting the specifics of the Transfer Plan, Aradigm shall promptly transfer those assets (to the extent not previously transferred to the Transferee hereunder) to Purchaser as required in the Transfer Plan and this Section 2.02. Unless otherwise specified in the Transfer Plan, the mode of such transfer shall be determined by the Coordination Leads with the goal of efficiency and cost-effectiveness. Without limiting the foregoing and in connection with such transfers of assets pursuant to this Section 2.02, Aradigm shall make available such personnel reasonably familiar with the Assigned Assets to consult with and assist Purchaser in implementing such assets at mutually agreeable times.
Section 2.03 Coordination Leads. In order to facilitate the transfer of assets pursuant to Section 2.02, each Party shall appoint, from time to time, by written notice to the other Party, one of its personnel as its coordination lead (each, a “Coordination Lead”). The Coordination Leads shall be responsible for oversight and coordination of the transfer of assets in accordance with Section 2.02 and the Transfer Plan. The Coordination Leads shall carry out their responsibilities by any reasonable means or practices as the Parties may mutually agree.
6
Section 2.04 Transitional Services. Aradigm shall provide all reasonable transitional services to Purchaser, including facilities, furnishings, access to systems, document control, quality systems, IT support, accounting, payroll, administration and other such services as the Parties may mutually agree, until December 31, 2006 or until such later date as mutually agreed to by the Parties, as more fully described in Exhibit H, and Purchaser shall pay the fees therefor set forth in Exhibit H in accordance with the schedule set forth therein.
Section 2.05 Assumption of Liabilities.
(a) Assumption. Upon the terms and subject to the conditions set forth herein, at the Closing, Purchaser shall assume from Aradigm, and Aradigm shall irrevocably convey, transfer and assign to Purchaser, all of the Assumed Liabilities (as defined in Section 2.05(b) hereof). Purchaser shall not assume any liabilities of Aradigm pursuant hereto, other than the Assumed Liabilities.
(b) Definition of Assumed Liabilities. For all purposes of and under this Agreement, the term “Assumed Liabilities” shall mean, refer to and include only those liabilities listed on Exhibit F.
(c) Definition of Excluded Liabilities. Except for the Assumed Liabilities, Purchaser does not assume and is not assuming any debt, liability, duty or other obligation (of any kind) of Aradigm, whether known or unknown, fixed or contingent, and regardless of when such liabilities or obligations may arise or may have arisen or when asserted, including any liabilities, or obligations related to the Assigned Assets which are outstanding or unpaid as of the Closing (the “Excluded Liabilities”), and Aradigm shall remain responsible for the Excluded Liabilities.
Section 2.06 Consideration. On the terms and subject to the conditions set forth in this Agreement, in addition to the payments contemplated by Section 2.07(a), the consideration for the Assigned Assets shall be the following:
(a) Royalties.
(i) In consideration for the assignment and transfer of the Assigned Assets, with respect to Net Sales Purchaser shall pay to Aradigm, during the Royalty Term:
(1) For each Non-Sumatriptan Product, [***] percent ([***]%) of Net Sales of such Non-Sumatriptan Product, provided that in the event and to the extent such Non-Sumatriptan Product is commercialized by a Licensee Purchaser may at its election pay to Aradigm either [***] percent ([***]%) of such Licensee’s Net Sales of such Non-Sumatriptan Product or [***] percent ([***]%) of Purchaser’s Royalty Revenues from such Licensee in respect of such Non-Sumatriptan Product. Purchaser shall make its election with respect to each such Non-Sumatriptan Product by written notice to Aradigm of its election on or before the date its first payment would be due under Section 2.06(a)(vi) in respect of such Non-Sumatriptan Product under either of the foregoing alternatives.
(2) For Sumatriptan Products, [***] percent ([***]%) of Net Sales of Sumatriptan Products.
| *** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
7
(ii) Combination Products. In the event that a Product is sold in the form of a combination product (a “Combination Product”) containing both (1) such Product and (2) another product or service for which no royalty would be due hereunder if sold separately, the Net Sales from such combination sales for purposes of calculating the amounts due under this Section 2.06(a) shall be calculated by multiplying Net Sales of the Combination Product by a fraction that reasonably reflects the fair value of the contribution of the Product in the Combination Product to the total market value of such Combination Product, which fraction shall be established by the Purchaser and Aradigm through good faith negotiations and mutual agreement, on a Combination Product-by-Combination Product basis.
(iii) Single Royalty. Only one royalty shall be paid with respect to each unit of Product that is subject to royalties under this Section 2.06(a), without regard to the number of transfers or otherwise. In no event shall more than one royalty be due under this Section 2.06(a) with respect to any Product unit.
(iv) Milestone Payment. Purchaser shall pay Aradigm $4,000,000 within 30 days of the first U.S. commercial sale of the Sumatriptan Product.
(v) Records. During the term of this Agreement and for a period of three years thereafter, Purchaser and its Affiliates shall keep, and shall cause its licensees and sublicensees to keep, complete and accurate records of their Net Sales in sufficient detail to enable the amounts payable under this Section 2.06(a) to be determined. Upon Aradigm’s written request, but not more frequently than once per calendar year, Purchaser shall permit representatives or agents of Aradigm, at Aradigm’s expense, to examine such records during Purchaser’s regular business hours for the purpose of and to the extent necessary to verify any report required under this Agreement with respect to Net Sales received not more than three years prior to the date of Aradigm’s request. In the event that the amounts due to Aradigm are determined to have been underpaid, Purchaser shall promptly pay to Aradigm any amount due and unpaid. In the event that it is determined, as a result of such examination, that the amount underpaid with respect to a given payment is in excess of 5% of the total amount of such payment, then Purchaser shall reimburse Aradigm for all costs incurred by Aradigm in conducting such examination.
(vi) Reports. Beginning with the first accrual of royalties or other payments due hereunder, Purchaser shall provide to Aradigm a quarterly royalty report as follows: Within 60 days after the end of each quarterly period, Purchaser shall deliver to Aradigm a true and accurate report, giving such particulars of the business conducted by Purchaser, its Affiliates and Licensees, during such quarterly period as are pertinent to account for payments due under this Section 2.06(a). Such report shall include, as applicable, at least (A) the total of Net Sales during such quarterly period; (B) the calculation of royalties; (C) the calculation of Royalty Revenue for each applicable Non-Sumatriptan Product and (D) the total royalties and other payments due Aradigm. Simultaneously with the delivery of each such report, Purchaser shall pay to Aradigm the total amount, if any, due to Aradigm for the period of such report. If no payment is due, Purchaser shall so report. Aradigm shall not provide to Third Parties any information contained in reports provided to Aradigm under this Section 2.06(a)(v), or learned by Aradigm under Section 2.06(a)(iii) above.
8
(vii) Payments. All amounts payable hereunder by Purchaser shall be payable in Dollars to Aradigm. If any currency conversion shall be required in connection with the payment of royalties hereunder, such conversion shall be made by using the exchange rates reported in the Wall Street Journal on the last business day of the quarter in respect of which such payment is made.
(viii) Taxes. Any withholding or other tax that is required by law to be withheld on behalf of Aradigm with respect to payments owed by Purchaser pursuant to this Agreement shall be deducted by Purchaser from such payment prior to remittance. Purchaser shall promptly furnish Aradigm evidence of any such taxes withheld.
(ix) Without limiting Section 2.06(a)(v) above, Purchaser shall take reasonable measures to keep Aradigm informed as to the progress of the development and commercialization of the Intraject Delivery System and Products arising therefrom until such time as Purchaser has fulfilled its royalty obligations to Aradigm pursuant to Section 2.06(a).
Section 2.07 Closing, Closing Place, Time and Date. The closing of the transactions contemplated by this Agreement (the “Closing”) shall be held at the offices of Cooley Godward llp, 0000 Xxxxxxx Xxxxxx, Xxxx Xxxx, Xxxxxxxxxx, at 10:00 a.m. on the date of the Agreement (the actual date on which the Closing shall occur being referred to herein as the “Closing Date”).
(a) Closing Deliveries.
(i) At the Closing, Purchaser shall deliver, or cause to be delivered, to Aradigm the following, dated as of the date of this Agreement and, where relevant, executed for and on behalf of Purchaser by a duly authorized officer thereof:
(1) any and all instruments, certificates and agreements as Aradigm may reasonably request in order to effectively make Purchaser responsible for all Assumed Liabilities pursuant hereto to the fullest extent permitted by applicable law;
(2) Purchaser shall have provided Aradigm with evidence demonstrating that Purchaser has obtained at least $15 million in equity financing;
(3) Purchaser shall have paid to Aradigm, by wire transfer, $4,000,000 in cash;
(4) Purchaser shall have reimbursed Aradigm for all documented expenses actually incurred by Aradigm from July 1, 2006 through the Closing Date, that were pre-approved in writing by Purchaser, up to $515,036;
(5) Each of Xxxxx Xxxx and Xxxx Xxxxxxx shall have provided Aradigm with a release of all claims over or rights to any severance payments relating to their cessation of services to Aradigm, in a form that is reasonably acceptable to Aradigm and including mutually agreed consideration for such releases; and
9
(6) the Transitional Services Agreement.
(ii) At the Closing, Aradigm shall deliver, or cause to be delivered, to Purchaser the following, dated as of the date of this Agreement and executed for and on behalf of Aradigm by a duly authorized officer thereof:
(1) a general assignment and xxxx of sale with respect to the Assigned Assets in the form attached hereto as Exhibit F;
(2) one or more instruments of assignment and assumption, in customary form and substance reasonably satisfactory to Purchaser and Aradigm and their respective counsel;
(3) an instrument of assignment of the Transferred Patents, the Transferred Trademarks, and any other Registered Intellectual Property Rights included in the Assigned Assets, in customary form and substance reasonably satisfactory to Purchaser and Aradigm and their respective counsel;
(4) any and all required third party consents including those consents necessary for the valid assignment and transfer of the Transferred Contracts;
(5) any and all other instruments, certificates and agreements as Purchaser may reasonably request in order to effectively transfer to Purchaser all of the Assigned Assets pursuant hereto and to the Transfer Plan to the fullest extent permitted by applicable law; and
(6) the Transitional Services Agreement.
(b) Closing. From and after the Closing, the Assigned Assets shall be held for the account and benefit, and at the risk, of Purchaser.
Section 2.08 Nontransferable Assets. To the extent that any Assigned Asset or Assumed Liability to be sold, conveyed, assigned, transferred, delivered or assumed to or by Purchaser pursuant hereto, or any claim, right or benefit arising thereunder or resulting therefrom, is not capable of being sold, conveyed, assigned, transferred or delivered without the approval, consent or waiver of the issuer thereof or the other Party thereto, or any third Person (including a Governmental Body), or if such sale, conveyance, assignment, transfer or delivery or attempted sale, conveyance, assignment, transfer or delivery would constitute a breach (or give rise to a termination right) thereof or a violation of any law, decree, order, regulation or other governmental edict (collectively, with respect to such Assigned Assets, as set forth on Exhibit J, the “Nontransferable Assets”), except as expressly otherwise provided herein, this Agreement shall not constitute a sale, conveyance, assignment, transfer or delivery thereof, or an attempted sale, conveyance, assignment, transfer or delivery thereof absent such approvals, consents or waivers. If any such approval, consent or waiver shall not be obtained, or if an attempted assignment of any such Assigned Asset or the assumption of any Assumed Liability by Purchaser would be ineffective so that Purchaser would not in fact receive all the Nontransferable Assets or assume all such Assumed Liabilities pursuant hereto, Aradigm and Purchaser shall cooperate in a mutually agreeable arrangement under which Purchaser would obtain the benefits and assume the obligations of such Assigned Assets and Assumed Liabilities, respectively, in accordance with this Agreement, including subcontracting, sub-licensing, or sub-leasing to Purchaser, or under which Aradigm, at Purchaser’s expense, would enforce for the benefit of Purchaser, with Purchaser assuming all of Aradigm’s obligations thereunder, any and all rights of Aradigm against a Third Party thereto.
10
Section 2.09 FTO Licenses.
(a) To Purchaser. Aradigm hereby grants to Purchaser a non-exclusive, fully-paid, world-wide, perpetual, irrevocable, transferable, sublicensable license to fully exercise any Intellectual Property Rights that are (i) owned, controlled or employed by Aradigm at any time prior to the Closing (or that arises thereafter to the extent covering Technology created, owned, controlled or employed by Aradigm prior to the Closing), (ii) necessary or useful for the operation of the Business and (iii) not included in the Assigned Assets that are actually assigned to Purchaser.
(b) To Aradigm. Purchaser hereby grants to Aradigm a non-exclusive, fully-paid, world-wide, perpetual, irrevocable, transferable, sublicensable license to fully exercise any Intellectual Property Rights that are (i) owned, controlled or employed by Purchaser as of the Closing (or that arises thereafter to the extent covering Technology created, owned, controlled or employed by Aradigm as of the Closing) and (ii) solely for use in the Pulmonary Field.
Section 2.10 Taking of Necessary Action; Further Action. From time to time after the Closing, at the request of either Party, the Parties hereto shall execute and deliver such other instruments of sale, transfer, conveyance, assignment and confirmation and take such action as the Parties may reasonably determine is necessary to transfer, convey and assign to Purchaser, and to confirm Purchaser’s title to or interest in the Assigned Assets, to put Purchaser in actual possession and operating control thereof and to assist Purchaser in exercising all rights with respect thereto. Aradigm hereby constitutes and appoints Purchaser and its successors and assigns as its true and lawful attorney in fact in connection with the transactions contemplated by this Agreement, with full power of substitution, in the name and stead of Aradigm but on behalf of and for the benefit of Purchaser and its successors and assigns, to demand and receive any and all of the Assigned Assets and to give receipt and releases for and in respect of the same and any part thereof, and from time to time to institute and prosecute, in the name of Aradigm or otherwise, for the benefit of Purchaser or its successors and assigns, proceedings at law, in equity, or otherwise, which Purchaser or its successors or assigns reasonably deem proper in order to collect or reduce to possession or endorse any of the Assigned Assets and to do all acts and things in relation to the Assigned Assets which Purchaser or its successors or assigns reasonably deem desirable.
11
ARTICLE III
REPRESENTATIONS AND WARRANTIES OF ARADIGM
Aradigm hereby represents and warrants to Purchaser as follows:
Section 3.01 Organization, Qualification, and Corporate Power. Aradigm (a) is a corporation duly organized, validly existing, and in good standing under the laws of the State of California, (b) has obtained all necessary corporate approvals to enter into and execute this Agreement and (c) has the full right, power and authority to enter into this Agreement.
Section 3.02 Authorization. Aradigm has full power and authority to execute and deliver this Agreement, and to consummate the transactions contemplated hereunder and to perform its obligations hereunder, and no other proceedings on the part of Aradigm are necessary to authorize the execution, delivery and performance of this Agreement. This Agreement constitutes the valid and legally binding obligations of Aradigm, enforceable against Aradigm in accordance with its terms and conditions, except as such enforceability may be limited by principles of public policy and subject to the laws of general application relating to bankruptcy, insolvency and the relief of debtors and rules of law governing specific performance, injunctive relief or other equitable remedies.
Section 3.03 Assets. The Assigned Assets include all assets of Aradigm and its Affiliates that are used or held for use by Aradigm and its Affiliates primarily in the operation or conduct of the Business.
(a) The Assigned Assets include all assets of Aradigm and its Affiliates that are used or held for use by Aradigm and its Affiliates primarily in the operation or conduct of the Business.
(b) Following the consummation of the transactions contemplated by this Agreement and the related agreements, and the execution of the instruments of transfer contemplated hereby and thereby, Purchaser will own, with good, valid and marketable title, or lease, under valid and subsisting leases, or otherwise acquire the interests of Aradigm in the Assigned Assets, free and clear of any Liens, and without incurring any penalty or similar transfer fee.
Section 3.04 Transferred Books and Records. The Transferred Books and Records listed on Exhibit B are all of the Books and Records maintained by Aradigm that pertain to the Business and the Assigned Assets.
Section 3.05 Transferred Contracts. The Transferred Contracts listed on Exhibit C are all of the contracts between Aradigm and any Third Party currently necessary for or primarily related to, the operation of the Business, and true and complete copies of all such Transferred Contracts have been delivered or made available to Purchaser or its representatives. Each Transferred Contract is in full force and effect and, to Aradigm’s knowledge, Aradigm is not subject to any default thereunder, nor, to Aradigm’s knowledge, is any party obligated to Aradigm pursuant to any such Transferred Contract subject to any default thereunder. Aradigm has neither breached, violated or defaulted under, nor received notice that Aradigm has breached, violated or defaulted under, any of the terms or conditions of any Transferred Contract. Aradigm has obtained, or will obtain prior to the Closing, all necessary consents, waivers and approvals of parties to any Transferred Contract as are required thereunder in connection with the Closing, or for any such Transferred Contract to be transferred to Purchaser, and to remain in full force and effect without limitation, modification or alteration after the Closing. Following the Closing, Purchaser will be permitted to exercise all of the rights Aradigm had under the Transferred Contracts without the payment of any additional amounts or consideration other than ongoing fees, royalties or payments which Aradigm would otherwise be required to pay pursuant to the terms of such Transferred Contracts had the transactions contemplated by this Agreement not occurred.
12
Section 3.06 Transferred Intellectual Property.
(a) The Exhibits listing the Transferred Patents and the Transferred Trademarks are, to Aradigm’s knowledge, complete and accurate. With respect to Transferred Patents, those Transferred Patents that are Registered Patents are currently in compliance with formal legal requirements (including payment of filing, examination and maintenance fees and proofs of use), and are not subject to any unpaid maintenance fees or taxes falling due within 90 days after the Closing Date. There are no proceedings or actions known to Aradigm before any court, tribunal (including the United States Patent and Trademark Office (the “PTO”) or equivalent authority anywhere in the world) related to any such Registered Patent.
(b) To Aradigm’s knowledge, each Registered Patent that is a Transferred Patent is properly filed and is currently pending or issued, and all necessary registration, maintenance and renewal fees in connection with such Registered Patent that is a Transferred Patent have been paid and all necessary documents and certificates in connection with such Registered Patent have been filed with the relevant patent authorities in the United States or foreign jurisdictions in which Aradigm has elected to pursue such Registered Patent, as the case may be, for the purposes of maintaining such Registered Patent. There are, to Aradigm’s knowledge, no actions that must be taken by Aradigm within 90 days after the Closing Date, including the payment of any registration, maintenance or renewal fees or the filing of any responses to PTO office actions, documents, applications or certificates for the purposes of obtaining, maintaining, perfecting or preserving or renewing any such Registered Patent. To the extent Aradigm has acquired from any Person any Technology or Intellectual Property Right, in each case that are included in the Assigned Assets, Aradigm has obtained a valid and enforceable assignment sufficient to irrevocably transfer all rights in such Technology and Intellectual Property Rights (including the right to seek past and future damages with respect thereto) to Aradigm. To the maximum extent provided for by, and in accordance with, applicable laws and regulations, Aradigm has recorded each such assignment of a Registered Intellectual Property Right assigned to Aradigm with the relevant Governmental Body, including the PTO, the U.S. Copyright Office, or their respective equivalents in any relevant foreign jurisdiction, as the case may be. Aradigm has not claimed a particular status, including “Small Entity Status,” in the application for any Registered Patent that is a Transferred Patent, which claim of status was not at the time made, or which has since become, inaccurate or false or that will no longer be true and accurate as of the Closing Date.
(c) Aradigm has no knowledge of any misrepresentation regarding, or failure to disclose, any fact or circumstances in any application for any Registered Patent that is a Transferred Patent that would materially and adversely affect the validity or enforceability of such Registered Patent that is a Transferred Patent.
13
(d) All Registered Intellectual Property Rights included in the Assigned Assets are free and clear of any Liens. Immediately prior to the Closing, Aradigm is the exclusive owner or exclusive licensee of all Business Intellectual Property.
(e) Schedule 3.06(e) sets forth a list of all Regulatory Documents.
(f) All Assigned Assets will be fully transferable, alienable or licensable by Purchaser without restriction and without payment of any kind to any Third Party, including royalty obligations, other than those restrictions and payments Aradigm would be subject to as of the Closing Date with respect to such Assigned Assets had the transactions contemplated by this Agreement not occurred.
(g) Each material item of Technology used in the conduct of the Business by Aradigm was (i) written and created by then-current employees of Aradigm acting within the scope of their employment or (ii) acquired or licensed by Aradigm from Third Parties who have validly and irrevocably assigned such item to Aradigm, or granted Aradigm a license to use such item of a sufficient scope to cover Aradigm’s use or prior use of thereof in the Business.
(h) To Aradigm’s knowledge, the conduct of the Business by Aradigm as it was previously conducted does not, infringe or misappropriate any Intellectual Property Right of any person, or constitute unfair competition or trade practices under the laws of any jurisdiction, and Aradigm has not received notice from any person claiming that such conduct by Aradigm infringes or misappropriates any Intellectual Property Right of any person or constitutes unfair competition or trade practices under the laws of any jurisdiction.
(i) Each employee and consultant of Aradigm that provides services to Aradigm in connection with the Business has entered into a valid and binding written agreement with Aradigm sufficient to vest title in Aradigm of all Technology and Intellectual Property Rights included in the Assigned Assets and created by such employee or consultant in the scope of his or her services or employment for Aradigm.
(j) Aradigm has not transferred ownership of, nor granted any exclusive license of or exclusive right to use, or authorized the retention of any exclusive rights to use or joint ownership of, any Technology or Intellectual Property Right that is or was used in connection with the Business, to any other person.
(k) To Aradigm’s knowledge, no person is infringing or misappropriating any Intellectual Property Right included in the Assigned Assets.
(l) No Business Intellectual Property is subject to any proceeding or outstanding decree, order, judgment or settlement agreement or stipulation against Aradigm or, to Aradigm’s knowledge, against any Third Parties from whom Aradigm acquired or licensed Business Intellectual Property that restricts in any material way the use, transfer or licensing of such Business Intellectual Property by Aradigm or is reasonably likely to affect the validity, use or enforceability of such Business Intellectual Property.
14
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF PURCHASER
Purchaser hereby represents and warrants to Aradigm as follows:
Section 4.01 Organization, Qualification, and Corporate Power. Purchaser (a) is a corporation duly organized, validly existing, and in good standing under the laws of the State of [Delaware], (b) has obtained all necessary corporate approvals to enter into and execute this Agreement and (c) has the full right, power and authority to enter into this Agreement.
Section 4.02 Authorization. Purchaser has full power and authority to execute and deliver this Agreement, and to consummate the transactions contemplated hereunder and to perform its obligations hereunder, and no other proceedings on the part of Purchaser are necessary to authorize the execution, delivery and performance of this Agreement. This Agreement constitutes the valid and legally binding obligations of Purchaser, enforceable against Purchaser in accordance with its terms and conditions, except as such enforceability may be limited by principles of public policy and subject to the laws of general application relating to bankruptcy, insolvency and the relief of debtors and rules of law governing specific performance, injunctive relief or other equitable remedies.
ARTICLE V
OTHER AGREEMENTS AND COVENANTS
Section 5.01 Additional Documents and Further Assurances. Each Party hereto, at the request of another Party hereto, shall execute and deliver such other instruments and do and perform such other acts and things as may be reasonably requested for effecting completely the consummation of the transactions contemplated hereby.
Section 5.02 Reasonable Cooperation of Purchaser. Purchaser shall cooperate, to the extent reasonable, with Aradigm’s efforts to obtain any Third Party consents; provided, however, that this Section 6.02 shall not obligate Purchaser to incur any additional expense or liability.
Section 5.03 Reasonable Efforts. Each of the Parties will use their reasonable efforts to take all action and to do all things necessary, proper, or advisable in order to consummate and make effective the transactions contemplated by this Agreement.
Section 5.04 Indemnification.
(a) Indemnification of Purchaser.
(i) Aradigm shall indemnify and hold harmless each of Purchaser and its Affiliates, and the directors, officers, and employees of Purchaser and of such Affiliates, and the successors and assigns of any of the foregoing (collectively, the “Purchaser Indemnitees”), from and against any and all liabilities, damages, settlements, claims, actions, suits, penalties, fines, costs and expenses (including, without limitation, reasonable attorneys’ fees and other expenses of settlement) (any of the foregoing, a “Claim”) incurred by any Purchaser Indemnitee, based upon a Claim of a Third Party, to the extent resulting from the breach of any of Aradigm’s express representations and warranties set forth in Article III of this Agreement. Aradigm’s obligations to the Purchaser Indemnitees pursuant to this Section 5.04(a)(i) shall be limited, in the aggregate, to amounts actually received by Aradigm by operation of Section 2.06(a)(i). Notwithstanding the foregoing, Aradigm shall not have any obligation to the Purchaser Indemnitees in respect of any breach of representations and warranties as to which Purchaser has actual knowledge (including for this purpose the actual knowledge of Xxxxx Xxxx, Xxxx Xxxxxxx or Xxxxxxxx Xxxxx) prior to the Closing.
15
(b) Aradigm shall indemnify and hold harmless the Purchaser Indemnitees from and against all Claims arising from the Excluded Liabilities.
(c) Indemnification of Aradigm. Purchaser shall indemnify and hold harmless each of Aradigm and its Affiliates, and the directors, officers, and employees of Aradigm and of such Affiliates, and the successors and assigns of any of the foregoing (collectively, the “Aradigm Indemnitees”), from and against any and all liabilities, damages, settlements, claims, actions, suits, penalties, fines, costs and expenses (including, without limitation, reasonable attorneys’ fees and other expenses of settlement) incurred by any Aradigm Indemnitee, based upon (i) a Claim of a Third Party, to the extent resulting from the breach of any of Purchaser’s express representations and warranties set forth in Article IV of this Agreement, (ii) a Claim relating to product liability concerning any of the Assigned Assets or (iii) a Claim relating to the Assumed Liabilities.
(d) Procedure. A Party that intends to claim indemnification under this Section 5.04 (the “Indemnitee”) shall promptly notify the other Party (the “Indemnitor”) in writing of any Claim in respect of which the Indemnitee intends to require such indemnification, and the Indemnitor shall have sole control of the defense and/or settlement thereof; provided that the Indemnitee shall have the right to participate, at its own expense, with counsel of its own choosing in the defense and/or settlement of such Claim. The indemnification obligations of the Parties in this Section 5.04 shall not apply to amounts paid in settlement of any Claim if such settlement is effected without the consent of the Indemnitor, which consent shall not be unreasonably withheld or delayed. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any such Claim, if prejudicial to Indemnitor’s ability to defend such action, shall relieve such Indemnitor of any liability to the Indemnitee under this Section 5.04, but the omission to so deliver written notice to the Indemnitor shall not relieve the Indemnitor of any liability to any Indemnitee otherwise than under this Section 5.04. The Indemnitee under this Section 5.04 and its directors, officers and employees shall cooperate fully with the Indemnitor and its legal representatives and provide full information in the investigation of any Claim covered by this indemnification.
(e) Sole Remedy. The indemnification rights provided for in this Article V shall constitute the sole and exclusive remedy and the sole basis and means of recourse among the Aradigm Indemnities and the Purchaser Indemnities with respect to Claims arising out of or in connection with any breach of or inaccuracy in any representation, warranty, covenant or agreement contained in this Agreement.
16
Section 5.05 Covenant Not to Compete. Aradigm and its Affiliates agree for a period of four (4) years after the Closing Date (the “Initial Period”) not to (i) conduct, participate in or sponsor, directly or indirectly, any activities directed toward the research, development of technologies or products for the delivery of one or more active pharmaceutical ingredients via needle free injection or the manufacture, marketing or distribution of such products (each, a “Competing Activity”) or (ii) appoint, license or otherwise authorize any Third Party, whether pursuant to such license, appointment, or authorization or otherwise to perform any Competing Activities; provided that during the Initial Period, Purchaser (itself or through one or more Third Parties) is diligently pursuing the development (including preclinical development) or commercialization of one or more Products. Thereafter during the Royalty Term, Aradigm and its Affiliates agree not to develop or commercialize any product for needle free injection of any active pharmaceutical ingredient for which Purchaser (itself or through one or more Third Parties) is then actively developing or commercializing a Product incorporating such active pharmaceutical ingredient (or any prodrug, metabolite, degradant, intermediate, salt form, hydrate, ester, isomer thereof).
ARTICLE VI
MISCELLANEOUS
Section 6.01 Press Releases and Public Announcements. No Party shall issue any press release or make any public announcement relating to the subject matter of this Agreement prior to the Closing without the prior written approval of the other Party; provided, however, that (a) either Party may make any public disclosure it believes in good faith is required by applicable law and (b) Aradigm may correspond with Third Parties in writings in form and substance reasonably satisfactory to Purchaser with respect to obtaining consents from such Third Parties. In furtherance of the foregoing sentence, the Parties agree and acknowledge that either party may issue a press release regarding this Agreement and the transactions contemplated herein at a time to be mutually agreed after the Closing Date, which press release shall not provide the financial terms of the Agreement. The Parties will provide to each other a copy of such press release at least five business days prior to its release and such press release shall be subject to written approval of the receiving Party, which approval shall not be unreasonably withheld or delayed.
Section 6.02 No Third-Party Beneficiaries. This Agreement shall not confer any rights or remedies upon any Person other than the Parties, and their respective successors and permitted assigns.
Section 6.03 Force Majeure. Except with respect to the payment of money, in the event either Party hereto is prevented from or delayed in the performance of any of its obligations hereunder by reason of acts of God, terrorism, war, invasion, strikes, riots, earthquakes, storms, fires, energy shortage, acts of government or governmental agencies, or any other cause whatsoever beyond the reasonable control of the Party, the Party so prevented or delayed shall be excused from the performance of any such obligation to the extent and during the period of such prevention or delay.
Section 6.04 Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY OR ANY THIRD PARTY FOR ANY SPECIAL, CONSEQUENTIAL, EXEMPLARY OR INCIDENTAL DAMAGES (INCLUDING LOST OR ANTICIPATED REVENUES OR PROFITS RELATING TO THE SAME), ARISING FROM ANY CLAIM RELATING TO THIS AGREEMENT, WHETHER SUCH CLAIM IS BASED ON CONTRACT, TORT (INCLUDING NEGLIGENCE) OR OTHERWISE, EVEN IF AN AUTHORIZED REPRESENTATIVE OF SUCH PARTY IS ADVISED OF THE POSSIBILITY OR LIKELIHOOD OF SAME.
17
Section 6.05 Entire Agreement and Modification. This Agreement (including the exhibits hereto) constitutes the entire agreement among the Parties with respect to the subject matter hereof and supersedes any prior understandings, agreements, or representations by or among the Parties, written or oral, to the extent they related in any way to the subject matter hereof. This Agreement may not be amended except by a written agreement executed by all Parties.
Section 6.06 Amendment. This Agreement may be amended by Purchaser and Aradigm or any successor thereto by execution by each Party (or their successors) of an instrument in writing.
Section 6.07 Waivers. The rights and remedies of the Parties to this Agreement are cumulative and not alternative. Neither the failure nor any delay by any Party in exercising any right, power or privilege under this Agreement or the documents referred to in this Agreement will operate as a waiver of such right, power or privilege, and no single or partial exercise of such right, power, or privilege will preclude any other or further exercise of such right, power, or privilege or the exercise of any other right, power, or privilege. To the maximum extent permitted by applicable law, (a) no claim or right arising out of this Agreement or the documents referred to in this Agreement can be discharged by one Party, in whole or in part, by a waiver or renunciation of the claim or right unless in writing signed by the other Party, (b) no waiver that may be given by a Party will be applicable except in the specific instance for which it is given and (c) no notice to or demand on one Party will be deemed to be a waiver of any obligation of such Party or of the right of the Party giving such notice or demand to take further action without notice or demand as provided in this Agreement or the documents referred to in this Agreement.
Section 6.08 Successors and Assigns. This Agreement shall be binding upon and inure to the benefit of the Parties named herein and their respective successors and permitted assigns. This Agreement shall not be assigned by either Party without the prior written consent of the other Party, except that either Party may assign this Agreement, in whole or in part, to an Affiliate of such Party or to the successor (including the surviving company in any consolidation, reorganization or merger) or assignee of all or substantially all of its business pertaining hereto. This Agreement will be binding upon any permitted assignee of either Party. No assignment shall have the effect of relieving any Party to this Agreement of any of its obligations hereunder.
Section 6.09 Counterparts. This Agreement may be executed in counterparts, each of which shall be deemed an original but all of which together will constitute one and the same instrument.
Section 6.10 Interpretation. The captions and headings to this Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions of this Agreement. Unless specified to the contrary, references to Articles, Sections or Exhibits mean the particular Articles, Sections and Exhibits to this Agreement and references to this Agreement include all such subparts. Unless context otherwise clearly requires, whenever used in this Agreement: (a) the words “include” or “including” shall be construed as incorporating, also, “but not limited to” or “without limitation”; (b) the word “day” or “year” means a calendar day or year unless otherwise specified; (c) the word “notice” means notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement; (d) the words “hereof,” “herein,” “hereby” and derivative or similar words refer to this Agreement (including any and all subparts); (e) the word “or” shall be construed as the inclusive meaning identified with the phrase “and/or”; (f) provisions that require that a Party, the Parties or any committee hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise; (g) words of any gender include the other gender; (h) words using the singular or plural number also include the plural or singular number, respectively; and (i) references to any specific Law or article, section or other division thereof shall be deemed to include the then-current amendments thereto or any replacement Law thereof.
18
Section 6.11 Notices. All notices and other communications required or permitted hereunder shall be in writing, shall be effective when given, and shall in any event be deemed to be given upon receipt or, if earlier, (a) five days after deposit with the U.S. Postal Service or other applicable postal service, if delivered by certified or registered first class mail, postage prepaid, return receipt requested, (b) upon delivery, if delivered by hand, (c) one (1) business day after the business day of deposit with Federal Express or similar overnight courier, freight prepaid or (d) one business day after the business day of facsimile transmission, if delivered by facsimile transmission with copy by certified or registered first class mail, postage prepaid, return receipt requested and shall be addressed to the intended recipient as set forth below:
| If to Purchaser: | ||
| Addressed to: | SJ2 Therapeutics, Inc. | |
| 0000 Xxxxx Xxxx Xxx | ||
| Xxxxxxx, Xxxxxxxxxx 00000 | ||
| Attention: President | ||
| Facsimile: (000) 000 0000 | ||
| With a copy to: | Wilson, Sonsini, Xxxxxxxx & Xxxxxx | |
| 000 Xxxx Xxxx Xx | ||
| Xxxx Xxxx, Xxxxxxxxxx 00000-0000 | ||
| Attn: J. Xxxxx XxXxxxx, Esq. | ||
| Facsimile: (000) 000-0000 | ||
| If to Aradigm: | ||
| Addressed to: | Aradigm Corporation. | |
| 0000 Xxxxx Xxxx Xxx | ||
| Xxxxxxx, Xxxxxxxxxx 00000 | ||
| Attention: Chief Financial Officer | ||
| Facsimile: (000) 000 0000 | ||
| With a copy to: | Xxxxxx Godward LLP | |
| 0000 Xxxxxxx Xxxxxx | ||
| Xxxx Xxxx, XX 00000-0000 | ||
| Attn: Xxxxx Xxxxx, Esq. | ||
| Facsimile: (000) 000-0000 | ||
19
Any Party may change the address to which notices, requests, demands, claims, and other communications hereunder are to be delivered by giving the other Party ten days’ advance written notice to the other Party pursuant to the provisions above.
Section 6.12 Governing Law. This Agreement shall be governed by and construed in accordance with the domestic laws of the State of California without giving effect to any choice or conflict of law provision or rule (whether of the State of California or any other jurisdiction) that would cause the application of the laws of any jurisdiction other than the State of California.
Section 6.13 Severability. Any term or provision of this Agreement that is invalid or unenforceable in any situation in any jurisdiction shall not affect the validity or enforceability of the remaining terms and provisions hereof or the validity or enforceability of the offending term or provision in any other situation or in any other jurisdiction.
Section 6.14 Construction. The Parties have participated jointly in the negotiation and drafting of this Agreement. In the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement. Any reference to any federal, state, local, or foreign statute or law shall be deemed also to refer to all rules and regulations promulgated thereunder, unless the context requires otherwise.
Section 6.15 Attorneys’ Fees. If any legal proceeding or other action relating to this Agreement is brought or otherwise initiated, the prevailing Party shall be entitled to recover reasonable attorney’s fees, costs and disbursements (in addition to any other relief to which the prevailing Party may be entitled).
Section 6.16 Further Assurances. The Parties agree (a) to furnish upon request to each other such further information, (b) to execute and deliver to each other such other documents and (c) to do such other acts and things, all as the other Party may reasonably request for the purpose of carrying out the intent of this Agreement and the documents referred to in this Agreement.
[The remainder of this page left intentionally blank; signature page follows]
20
| ARADIGM CORPORATION | ||
| By: | /s/ Xxx Xxxxxxxxxx | |
| Name: | Xxx Xxxxxxxxxx | |
| Title: | Senior Vice President and Chief Financial Officer | |
| SJ2 THERAPEUTICS, INC. | ||
| By: | /s/ Xxxxxxx X. Xxxx | |
| Name: | Xxxxxxx X. Xxxx | |
| Title: | President | |
Schedule 3.06(e)
Regulatory Documents
[***]
| *** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT A
Transferred Assets (including Transferred Technology)
[***]
*** Seven (7) pages have been omitted pursuant to a request for confidential treatment.
EXHIBIT B
Transferred Books and Records
[***]
*** Three hundred twenty four (324) pages have been omitted pursuant to a request for confidential treatment.
EXHIBIT C
Transferred Contracts
[***]
*** Two (2) pages have been omitted pursuant to a request for confidential treatment.
EXHIBIT D
Transferred Intellectual Property
[***]
*** Twenty-three (23) pages have been omitted pursuant to a request for confidential treatment.
EXHIBIT E
General Assignment and Xxxx of Sale
FORM OF XXXX OF SALE AND ASSIGNMENT AGREEMENT
This Xxxx of Sale and Assignment Agreement is made effective as of August 25, 2006, by and between SJ2 Therapeutics, Inc., a Delaware corporation (“Purchaser”), and Aradigm Corporation, a California corporation (“Aradigm”). All capitalized words and terms used in this Agreement and not defined herein shall have the respective meanings ascribed to them in the Asset Purchase Agreement dated August 25, 2006 between Aradigm and the Purchaser (the “Asset Purchase Agreement”).
WHEREAS, Aradigm and Purchaser have entered into the Asset Purchase Agreement, under which Aradigm has agreed to sell, convey, assign, transfer and deliver the Assigned Assets to Purchaser or its assigns.
| 1. | Sale. Aradigm does hereby sell, convey, assign, transfer and deliver to Purchaser, and Purchaser does hereby purchase, acquire and accept from Aradigm, all of Aradigm’s right, title and interest in and to the Assigned Assets, subject to the licensed reserved on behalf of Aradigm pursuant to Section 2.01 of the Asset Purchase Agreement. |
| 2. | Representations. All representations, warranties, agreements and indemnities of Aradigm with respect to the Assigned Assets set forth in the Asset Purchase Agreement will continue in effect as provided therein and will not be deemed to be amended, modified, terminated or superseded by or merged with this Xxxx of Sale and Assignment Agreement. |
| 3. | Miscellaneous Provisions. |
3.1. Amendments: Waiver. The terms, provisions and conditions of this Xxxx of Sale and Assignment Agreement may be amended only by agreement in writing of all parties. No waiver of any provision nor consent to any exception to the terms of this Xxxx of Sale and Assignment Agreement or any agreement contemplated hereby will be effective unless in writing and signed by the party to be bound and then only to the specific purpose, extent and instance so provided.
3.2. Further Assurances. Each party will execute and deliver, both before and after the Closing Date, such further certificates, agreements and other documents and take such other actions as the other party may reasonably request or as may be necessary or appropriate to consummate or implement the Transactions, including to more effectively transfer the Assigned Assets, or to evidence such events or matters.
3.3. Assignment. Neither this Xxxx of Sale and Assignment Agreement nor any rights or obligations under it are assignable by one party without the prior written consent of the other party.
3.4. Descriptive Headings. The descriptive headings of the sections and subsections of this Xxxx of Sale and Assignment Agreement are for convenience only and do not constitute a part of this Xxxx of Sale and Assignment Agreement.
3.5. Counterparts. This Xxxx of Sale and Assignment Agreement and any amendment hereto or any other agreement delivered pursuant hereto may be executed in one or more counterparts and by different parties in separate counterparts. All counterparts will constitute one and the same agreement and will become effective when one or more counterparts have been signed by each party and delivered to the other party. A facsimile signature page will be deemed an original.
3.6. Governing Laws. This Xxxx of Sale and Assignment Agreement and the legal relations between the parties will be governed by and construed in accordance with the laws of the State of California applicable to contracts made and performed in such State and without regard to conflicts of law doctrines unless certain matters are preempted by federal law.
3.7. Waiver. No failure on the part of any party to exercise or delay in exercising any right hereunder will be deemed a waiver thereof, nor will any single or partial exercise preclude any further or other exercise of such or any other right.
3.8. Representation By Counsel: Interpretation. The parties each acknowledge that each has been represented by counsel in connection with this Xxxx of Sale and Assignment Agreement and the transactions contemplated by the Asset Purchase Agreement. Accordingly, any rule of law or any legal decision that would require interpretation of any claimed ambiguities in this Xxxx of Sale and Assignment Agreement against the party that drafted it has no application and is expressly waived. The provisions of this Agreement will be interpreted in a reasonable manner to effect the intent of the parties hereto.
3.9. Severability. If any provision of this Xxxx of Sale and Assignment Agreement is held to be unenforceable for any reason, it will be adjusted rather than voided, if possible, to achieve the intent of the parties. All other provisions of this Xxxx of Sale and Assignment Agreement will be deemed valid and enforceable to the extent possible.
[signature page to follow]
2
IN WITNESS WHEREOF, Aradigm and Purchaser have caused this Xxxx of Sale and Assignment Agreement to be duly executed as of the day and year first above written.
| PURCHASER: | ARADIGM: | |||||||
| SJ2 THERAPEUTICS, INC. | ARADIGM CORPORATION | |||||||
| By: | /s/ |
By: | /s/ | |||||
| Name: | Xxxxxxx X. Xxxx |
Name: | X.X. Xxxxxxxxxx | |||||
| Title: | President |
Title: | SVP & CFO | |||||
EXHIBIT F
Assumed Liabilities
1. All obligations under Assumed Contracts, other than obligations due and owing as of the date of the Agreement to Third Parties that are parties to such Assumed Contracts.
2. Liabilities (other than Excluded Liabilities) incurred in the use of the Assigned Assets following the Closing Date.
3. See attached list for additional items.
[***]
*** Twenty-two (22) pages have been omitted pursuant to a request for confidential treatment.
EXHIBIT G
Transfer Plan
[***]
*** Six (6) pages have been omitted pursuant to a request for confidential treatment.
EXHIBIT H
Transitional Services Agreement
[ARADIGM LETTERHEAD]
August 25, 2006
SJ2 Therapeutics, Inc.
| Re: | Transition Services |
Ladies and Gentlemen:
SJ2 Therapeutics, Inc. (“SJ2”) and Aradigm Corporation (“Aradigm”) are entering into an Asset Purchase Agreement (the “APA”) dated as of the date of this letter (the “Effective Date”), which, among other things, provides for the sale to SJ2 of certain Aradigm assets related to the development, manufacture, and commercialization of Aradigm’s Intraject Delivery System.
1. Services. On the terms and subject to the conditions contained herein, Aradigm shall provide, or shall cause third parties designated or hired by it (such designated third parties, together with Aradigm, the “Service Providers”) to provide to SJ2 the following services (collectively, the “Services”) for the time period through December 31, 2006 (“Expiration Date”):
| (a) | General information technology services and support (e.g., e-mail access, computer equipment and software support, network access and support to SJ2’s server only, and other general computer technologies support) within Aradigm’s current systems and procedures until SJ2 vacates Aradigm’s facilities or the Expiration date, which ever is earlier, |
| (b) | Telephone and fax services and support, |
| (c) | Aradigm will provide SJ2 with document control support for the activities documented in Aradigm’s current document control processes, using Aradigm’s Document Control System (DCS) database. Aradigm has assumed that SJ2 will purchase the DCS on or shortly after the Effective Date. It is Aradigm’s intention to hire a temporary senior level Document Control Specialist, on or shortly after the Effective Date, who will be fully funded by SJ2, to allow Aradigm’s current document control personnel to provide document control support to SJ2 consistent with Aradigm’s current Document Control processes. If Aradigm is unable to hire a temporary senior level Document Control Specialist, or should the temporary employee hired leave Aradigm for any reason, Aradigm will not be able to provide the services described in this section 1(c). |
| (d) | Human resources services and support for Aradigm consultants transferring to SJ2, |
| (e) | Payment for individual Aradigm consultants transferring to SJ2, |
1
| (f) | Technical consulting as available and approved in writing by both parties, |
| (g) | Office facilities, furnishings, and services (e.g., utilities, maintenance, mail, etc.), and |
| (h) | Such other services as Aradigm and SJ2 may agree to as set forth in paragraph 4. |
2. Current Invoices. Exhibit A to this letter contains an invoice for transitional services provided by Aradigm to SJ2 through the months of July and August 2006. The parties acknowledge that a secondary invoice will be provided to SJ2 relating to transitional service provided at the time of closing Aradigm’s August accounting records. As Aradigm’s August accounting records have not been closed as of the Effective Date,
3. Term of Agreement. Except for the services performed prior to the Effective Date as referenced in paragraph 2, all services to be provided under this Agreement shall begin as of the Effective Date and shall terminate on the Expiration Date. Aradigm and SJ2 will negotiate in good faith if SJ2 needs to extend the term of this letter and/or any provision of any Service beyond the Expiration Date (and the parties hereby acknowledge that the negotiation of any such extension may involve a renegotiation of the charges with respect to any such Services). This letter may be extended upon the mutual agreement of the parties hereto in writing, either in whole or with respect to one or more of the Services; provided that, such extension shall only apply to the specific Services for which this letter was extended. Services shall be provided up to and including the applicable Expiration Date, subject to earlier termination as provided in this letter.
4. Additional Services. From time to time after the Effective Date, Aradigm and SJ2 may identify and mutually agree upon additional services to be provided to SJ2 in accordance with the terms of this letter (the “Additional Services”). At such times, the parties shall execute an addendum to this Agreement setting forth a description of any Additional Service, the time period during which such Additional Service will be provided, the charge for such Additional Service and any other terms applicable. Aradigm and SJ2 acknowledge that charges for Additional Services will include a profit margin consistent with industry standards for the provision of similar services. Additional Services may include, but shall not be limited to, regulatory consulting for the Intraject Sumatriptan NDA, clinical training of the CRO selected to conduct the Intraject bioequivalence study, submission of the Intraject Sumatriptan IND on behalf of SJ2 and assistance with R&D efforts.
5. Provision of Services. Aradigm will use commercially reasonable efforts to ensure that employees and Service Providers are available to perform its obligations hereunder. Aradigm shall provide the Services in accordance with the policies, procedures and practices in effect as of immediately prior to the Effective Date. Aradigm and SJ2 will use their commercially reasonable efforts to promote a smooth and efficient transition of operations.
2
6. No Warranties. ARADIGM WILL USE COMMERCIALLY REASONABLE EFFORTS TO CAUSE THE SERVICES TO BE PERFORMED IN A PROFESSIONAL AND COMPETENT MANNER; HOWEVER, ARADIGM DOES NOT MAKE ANY WARRANTIES, EXPRESS, IMPLIED OR STATUTORY, INCLUDING BUT NOT LIMITED TO THE IMPLIED WARRANTIES OF MERCHANTABILITY, BUSINESS CONTINUITY OR FITNESS FOR A PARTICULAR PURPOSE, WITH RESPECT TO THE SERVICES, MATERIALS OR OTHER DELIVERABLES PROVIDED, OR CAUSED TO BE PROVIDED, BY IT UNDER THIS LETTER.
7. Transition to SJ2 Systems and Personnel. During the term of this letter, Aradigm will use reasonable efforts to provide to SJ2, at SJ2’s expense, consultation, assistance and information as reasonably requested by SJ2, and will otherwise perform the Services, so as to effect a smooth transition from SJ2’s utilization of Aradigm’s systems and personnel to SJ2’s utilization of its own systems and personnel in connection with the development of the Intraject Delivery System prior to the termination or expiration of this letter.
8. Payments. SJ2 shall pay Aradigm on a monthly basis for documented actual charges for the performance of the Services. Aradigm will invoice SJ2 for its representatives’ activities using an hourly rate based on salary, benefits and overhead of the Aradigm representatives performing the Services. Aradigm will not apply a profit to its representatives’ hourly rates through the Expiration Date.
9. Discontinuation of Services. If SJ2 chooses to discontinue any Service prior to the Expiration Date, SJ2 shall give at least 30 days prior written notice, of its intent to terminate this letter as to that particular Service, which termination as to that particular service shall be effective on the last day of the month on which the 30 days prior written notice lapses. SJ2 will pay Aradigm hereto the fees and costs of any terminated Service up until the effective date of termination of such Service.
10. Termination. Notwithstanding anything to the contrary contained in this letter, this letter may be terminated, in whole or in part, at any time: (a) by the mutual consent of SJ2 and Aradigm; or (b) by either SJ2 or Aradigm in the event of any material breach or default by the other party of any of its obligations under this letter and the failure of such defaulting party to cure, or to take substantial steps towards the curing of, such breach or default within 14 days after receipt of written notice from the non-defaulting party requesting such breach or default to be cured.
11. No Implied Responsibilities or Obligations. NO PARTY HERETO ASSUMES ANY RESPONSIBILITY OR OBLIGATIONS WHATSOEVER, OTHER THAN THE RESPONSIBILITIES AND OBLIGATIONS EXPRESSLY SET FORTH IN THIS LETTER OR A SEPARATE WRITTEN AGREEMENT BETWEEN THE PARTIES. NOTWITHSTANDING ANYTHING CONTAINED IN THIS LETTER TO THE CONTRARY, IN NO EVENT SHALL ANY PARTY BE LIABLE TO ANY OTHER PARTY FOR ANY LOST PROFITS, LOSS OF DATA, LOSS OF USE, BUSINESS INTERRUPTION OR OTHER SPECIAL, INCIDENTAL, INDIRECT OR CONSEQUENTIAL DAMAGES.
3
12. Independent Contractors. The relationship between SJ2 and Aradigm established under this letter is that of independent contractors and no party shall be deemed an employee, agent, partner, or joint venturer of or with the other. Each Service Provider will be solely responsible for any employment-related taxes, insurance premiums or other employment benefits respecting its personnel’s performance of any Services. SJ2 agrees to grant to any applicable Service Provider’s personnel reasonable access to sites, systems and information as necessary for the Service Provider to perform its obligations under this letter. The personnel of SJ2 and Aradigm shall agree to obey any and all security regulations and other published policies of the other party relevant to the provision or receipt of any Services.
13. Confidentiality. Any information from time to time communicated or delivered by SJ2 or Aradigm to the other party, including without limitation trade secrets, business methods, and cost, supplier, manufacturing and customer information, shall be treated by SJ2 and Aradigm, respectively, as confidential information of the other party, and shall not be disclosed or revealed to any third party whatsoever or used in any manner except as expressly provided for in this letter; provided, however that such confidential information shall not be subject to the restrictions and prohibitions set forth in this paragraph 13 to the extent that such confidential information: (a) is available to the public in public literature or otherwise, or after disclosure by one party to the other becomes public knowledge through no default of the party receiving such confidential information; or (b) was known to the party (as demonstrated by the written records of such party) receiving such confidential information with no obligation to maintain confidentiality prior to the receipt of such confidential information by such party, whether received before or after the date of this letter; or (c) is obtained by the party receiving such confidential information from a third party not subject to a requirement of confidentiality with respect to such confidential information. For the avoidance of doubt, information will not be considered to be available to the public, in the public literature, or in the prior possession of the receiving party merely because individual elements thereof are available to the public, in the public literature, or in the prior possession of the receiving party, unless the combination of such elements is available to the public, in the public literature, or in the prior possession of the receiving party. SJ2 and Aradigm shall take all such precautions as it normally takes with its own confidential information to prevent any improper disclosure of such confidential information to any third party; provided that, such confidential information may be disclosed: (x) pursuant to any order of a court or government entity having jurisdiction and power to order such information to be released or made public; (y) within the limits required to obtain any authorization from any governmental or regulatory agency; or (z) with the prior written consent of the other party, which shall not be unreasonably withheld, as may otherwise be required in connection with the purposes of this letter.
14. Access to Aradigm Computer Systems. If SJ2 is given access to any computer equipment, computer, software, network, electronic files, or electronic data storage system owned or controlled by Aradigm (“Aradigm Computer Systems”), then
4
SJ2 shall limit access and use of such Aradigm Computer Systems solely to receive Services under this letter and shall not access, attempt to access or use any Aradigm Computer Systems, other than those specifically required to receive the Services. All user identification numbers and passwords disclosed to SJ2 and any of Aradigm’s confidential information obtained by SJ2 as a result of its access to and use of any such Aradigm Computer Systems shall be deemed to be, and shall be treated as, Aradigm’s confidential information under applicable provisions of this letter. SJ2 agrees to cooperate with Aradigm in the investigation of any apparent unauthorized access by SJ2 or its representatives to any Aradigm Computer Systems, or any apparent unauthorized release of Aradigm’s confidential information by the employees, contractors or advisers of SJ2.
15. Indemnification. SJ2 indemnifies Aradigm and its affiliates against, and agrees to hold each of them harmless from, any and all damage, loss, liability and expense (including reasonable expenses of investigation and reasonable attorneys’ fees and any incidental, indirect or consequential damages, losses, liabilities or expenses) (“Damages”) incurred or suffered by Aradigm or any of its affiliates (other than Damages incurred or suffered by Aradigm or any of its affiliates arising from any claims made by employees of Aradigm) that arise from any third-party claim for personal injury or damage to property based upon the performance of the Services by any of Aradigm’s employees, except to the extent such third-party claim arises out of such employee’s negligence, willful misconduct or breach of obligations under this letter. Aradigm indemnifies SJ2 and its affiliates against, and agrees to hold each of them harmless from, any and all Damages incurred or suffered by SJ2 or any of its affiliates (other than Damages incurred or suffered by SJ2 or any of its affiliates arising from any claims made by employees of SJ2) that arise from any third-party claim for personal injury or damage to property based upon actions by any of SJ2’s employees under the terms of this letter, except to the extent such third-party claim arises out of such employee’s negligence, willful misconduct or breach of obligations under this letter.
16. Existing Ownership Rights Unaffected. Neither SJ2 nor Aradigm will gain, by virtue of this letter, any rights or ownership of copyrights, patents, know-how, trade secrets, trademarks or any other intellectual property rights owned by the other party.
17. Dispute Resolution. All disputes arising out of this letter shall be settled as far as possible by negotiations between SJ2 and Aradigm. If SJ2 and Aradigm cannot agree on an amicable settlement within 30 days from written submission of the matter by one party to the other, the matter shall be shall be settled by binding arbitration in the County of Hayward in the State of California in accordance with the Commercial Arbitration Rules then in effect of JAMS/Endispute. Arbitration will be conducted by one arbitrator, mutually selected by SJ2 and Aradigm. If Aradigm and SJ2 fail to mutually select an arbitrator within 15 days following the submission of the matter to JAMS/Endispute, then arbitration will be conducted by three arbitrators: one selected by Aradigm; one selected by SJ2; and the third selected by the first two arbitrators. If SJ2 or Aradigm fails to select an arbitrator within ten days following the expiration of the initial 15 day period, then the other shall be entitled to select the second arbitrator. SJ2 and
5
Aradigm agree to use all reasonable efforts to cause the arbitration hearing to be conducted within 75 days after the appointment of the mutually selected arbitrator or the last of the three arbitrators, as the case may be, and to use all reasonable efforts to cause the decision of the arbitrators to be furnished within 95 days after the appointment of the mutually selected arbitrator or the last of the three arbitrators, as the case may be. SJ2 and Aradigm further agree that discovery shall be completed at least 10 days prior to the date of the arbitration hearing. The final decision of the arbitrators shall be furnished to SJ2 and Aradigm in writing and shall constitute a conclusive determination of the issues in question, binding upon SJ2 and Aradigm and shall not be contested by any of them. The non-prevailing party in any arbitration shall pay the reasonable expenses (including attorneys’ fees) of the prevailing party and the fees and expenses associated with the arbitration (including the arbitrators’ fees and expenses). For purposes of this paragraph 17, the non-prevailing party shall be determined solely by the arbitrators.
18. No Third Party Beneficiaries. This letter shall not confer any rights or remedies upon any person or entity other than SJ2 or Aradigm and their respective successors and permitted assigns.
19. Counterparts and Facsimile Signature. This letter may be executed in counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument. This letter may be executed by facsimile signature.
20. Notices. All notices and other communications under this letter will be in writing and deemed to have been duly given if given in accordance with Section 6.11 of the APA.
21. Successors and Assigns. The provisions of this letter shall be binding upon and inure to the benefit of SJ2 and Aradigm and their respective successors and assigns; provided, however, that except as expressly provided in this letter, no party may assign, delegate or otherwise transfer any of its rights or obligations under this letter without the consent of each other party.
[The remainder of this page is left intentionally blank; signature page follows]
6
If you are in agreement with the terms of this letter, please execute this letter where indicated below and return a copy of the signed letter to Aradigm.
| ARADIGM CORPORATION | ||
| By: | /s/ | |
| Name: | X.X. Xxxxxxxxxx | |
| Title: | SVP & CEO | |
| ACCEPTED AND AGREED TO: | ||
| SJ2 THERAPEUTICS, INC. | ||
| By: | /s/ | |
| Name: | Xxxxxxx X. Xxxx | |
| Title: | President | |
7
EXHIBIT I
Intraject Delivery System
EXHIBIT I
Technical Description of the Intraject System
Intraject is a pre-filled, disposable, sterile delivery device, which is designed to deliver up to 0.5mL into the subcutaneous tissue. Intraject demonstrably delivers the injectate into the subcutaneous tissue similar to needle injection. Intraject works by using a small canister of nitrogen gas, under pressure, to accelerate a metal rod towards a modified-PTFE piston. This piston is in contact with the liquid drug formulation, and the impact of the metal rod generates pressure in the drug formulation. The other end of the drug container has a small orifice, which is held in contact with the skin. The initial high pressure is sufficient to cause the liquid to be ejected through the orifice and to xxxxxx the skin. The bulk of the liquid is subsequently delivered at a lower pressure into the subcutaneous tissue where it is available for systemic absorption.
The Intraject System consists of the following components:
1. The Capsule sub-assembly, which stores the pre-filled sterile injection solution.
2. The Actuator sub-assembly, which is the mechanism that expels the injection solution when actuated.
3. The Setting Mechanism, which provides a convenient means for the user to set the actuator triggering mechanism
Appropriate materials that are compatible with the formulation, device capabilities and sterilization methods have been chosen as a result of the development program for the Intraject System.
Components of the Capsule sub-assembly
The primary packaging component of the Intraject® sumatriptan drug product is the Capsule sub-assembly. The formulation contact materials of the capsule sub-assembly are typical of those used in the manufacture of pharmaceutical products.
The Capsule sub-assembly has five components (Figure P.2-1).
The material, function and design rationale for use of each primary packaging component is described below:
| 1. | Glass capsule: The glass capsule material is a USP Type 1 borosilicate glass capsule, strengthened via an ion exchange surface treatment process, which stores the injection solution. The material was chosen for its known compatibility with drug formulations, for its clear appearance which allows observation of the formulation solution, and because it is resistant to chemical degradation, impermeable to solvent transport, and easy to sterilize. This material is widely used within the pharmaceutical industry as a formulation contact material. |
| 2. | Piston: Modified PTFE piston ([***]): The piston seals one end of the capsule and expels the injection solution when the device is actuated. The material was chosen to ensure appropriate amounts of friction between the piston and capsule during actuation, to provide sufficient transfer of energy between the ram and formulation solution during actuation, to ensure limited solvent transport out of the capsule, to ensure acceptable seal integrity across an appropriate temperature range, and to be easy to sterilize. The material was also chosen for its non-reactivity and compatibility with drug formulations. This material is used in medical implants and pharmaceutical processing. |
| 3. | Stopper: Chlorobutyl rubber stopper, which seals the other end of the capsule. The material was chosen because of its known compatibility with drug formulations, its elasticity which provides acceptable seal integrity across an appropriate temperature range, because it has low solvent transport properties, and is easy to sterilize. It is one of the most widely used elastomeric container-closure materials in the pharmaceutical industry. |
| 4. | Interface seal: Chlorobutyl rubber interface seal that allows sterile filling under vacuum. The material is the same as that used for the stopper, and was chosen for the same reasons. |
| 5. | Capsule sleeve: Polyurethane capsule sleeve, which protects the capsule and couples the capsule sub-assembly to the actuator. The material was chosen to be sterilizable, to provide visibility into the glass capsule, and to enable removal of the “snap-off’ end of the capsule sleeve by the user with an appropriate force. This material is medical-grade, and has very limited exposure to the formulation solution. |
Figure 2-1: Schematic of Capsule Sub-Assembly Components
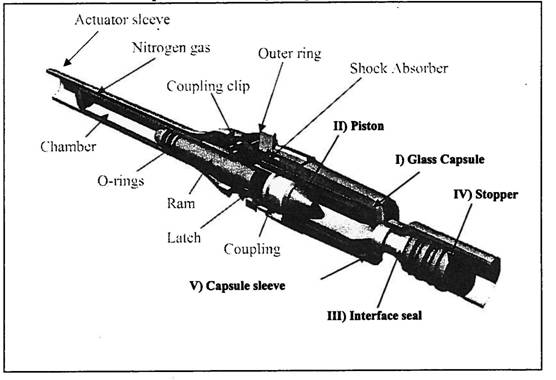
Components of Actuator Sub-Assembly
As shown in Figure P.2-2, the Actuator sub-assembly has twelve functional components. The actuator components have no drug formulation contact.
| I. | An actuator sleeve that restrains the latch until the device is actuated. | |||
| II. | A chamber that stores the pressurized gas for expelling the injection solution. | |||
| III. | Two-O-rings thatseal between the ram and the chamber to prevent gas loss. | |||
| IV. | A ram that drives the piston down the capsule when the device is actuated, thus expelling the injection solution through the drilled orifice. | |||
| V. | A latch, which restrains the ram from moving until the time of injection. | |||
| VI. | A coupling to join the actuator to the capsule sub-assembly. | |||
| VII. | An outer ring to strengthen the actuator sleeve where the latch pushes against it. | |||
| VIII. | A coupling clip to join the chamber to the coupling. | |||
| IX. | A shock absorber that controls the rise of pressure in the fluid and prevents shock waves. | |||
| 1 *** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
| 10. | [***]. |
| 11. | [***].2 |
| 12. | [***]. |
| 13. | [***]. |
| 14. | [***]. |
| 15. | [***]. |
Figure P.2-2: Schematic of Actuator Sub-Assembly Components
[***]
Table P.2-1 provides information on the materials used to manufacture the individual actuator components.
[***]
Components of Setting Mechanism
The presentation of the Intraject System consists of a Setting Mechanism, which encases the filled and assembled Actuator and Capsule sub-assemblies for the convenience of the user. The Setting Mechanism does not alter the drug delivery from the device and the components have no drug formulation contact.
The Setting Mechanism has been developed as a result of risk analysis and human factors evaluations and addresses issues identified during these assessments. The specific patient-device factors the Setting Mechanism addresses are:
| • | It provides a convenient means for the user to set the actuator triggering mechanism. |
| • | It improves the intuitiveness of use of the device by providing visual cues to the preparation steps and also forces the user to prepare the Intraject System for injection in the correct order. |
| • | It improves the ergonomics of the device by aiding removal of the capsule snap-off tip and provides the user with a grip to hold the device during snap-off, priming and injection. |
| 2 *** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
The Setting Mechanism consists of three functional parts as shown in Figure P.2-3:
Figure P.2-3: Setting Mechanism
| 1. | A snap-off cap, made up of two identical halves, which encloses the snap-off portion of the capsule sleeve. It provides an extended lever-arm to assist the user in breaking the snap-off while also releasing the setting lever (thereby forcing the correct sequence during use). |
| 2. | A setting lever that can only be operated when the snap-off cap is removed. When rotated and placed into the groove in the cover, the setting lever both sets the actuator triggering mechanism whilst also removing a block from between the actuator and capsule sub-assemblies (to facilitate subsequent actuation, only when pressed against the skin). |
| 3. | A grip, made up of the collar and cover components. The grip supports the actuator sub-assembly and the lever and is held by the patient during use. A pin on the collar component drives into and sets the actuator triggering mechanism as the user rotates the lever into the cover. At the same time, a block on the cover component is removed from between the two Intraject sub-assemblies to facilitate subsequent injection when pressed against the skin by the user. |
Table P.2-8 provides information on the materials used to manufacture the setting mechanism.
Table P.2-8: Components of the Setting Mechanism
[***].3
Table P.2-9 provides information on the actuator and setting mechanism materials, and rationale for their selection.
| 3 *** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
[***].4
Figure P.2-4 shows the three basic steps to using the Intraject® System with Setting Mechanism.
Figure P.2-4: Three Basic Steps for Using the Intraject System

| 4 *** | Certain information on this page has been omitted and filed separately with the Commission. Confidential treatment has been requested with respect to the omitted portions. |
EXHIBIT J
Nontransferable Assets
None.
