WHEREAS, pursuant to that certain Transition Services Agreement (such agreement, as may be amended or restated in accordance with the terms thereof and of the Credit Agreements, the “Transition Services Agreement”), dated as of the date hereof,...

Exhibit 10.2 PARTIAL RELEASE AND ACKNOWLEDGEMENT AGREEMENT This PARTIAL RELEASE AND ACKNOWLEDGEMENT AGREEMENT (this “Agreement”), dated as of July 22, 2020, is made by and among MIDCAP FINANCIAL TRUST, a Delaware statutory trust, as agent under Term Loan Credit Agreement referred to below (in such capacity and together its successors and assigns, the “Term Loan Agent”), MIDCAP FUNDING IV TRUST, a Delaware statutory trust, as agent under Revolving Credit Agreement referred to below (in such capacity and together its successors and assigns, the “Revolving Loan Agent” and, together with the Term Loan Agent, the “Agents”) RADIUS HEALTH, INC., a Delaware corporation (“Radius Health”), and RADIUS PHARMACEUTICALS, INC., a Delaware corporation (“Radius Pharma”, together with Radius Health, the “Grantors” and each, a “Grantor”). WHEREAS, pursuant to (i) that certain Credit and Security Agreement (Term Loan), dated as of January 10, 2020, among the Grantors, the Term Loan Agent and the Lenders and other parties thereto (as the same may have been amended, modified, restated, replaced or supplemented from time to time, the “Term Loan Credit Agreement”) and that certain Intellectual Property Security Agreement, dated as of January 10, 2020, among the Grantors and the Term Loan Agent (as the same may have been amended, modified, restated, replaced or supplemented from time to time, the “Term Loan IP Security Agreement”) and (ii) that certain Credit and Security Agreement (Revolving Loan), dated as of January 10, 2020, among the Grantors, the Revolving Loan Agent and the Lenders and other parties thereto (as the same may have been amended, modified, restated, replaced or supplemented from time to time, the “Revolving Credit Agreement”, together with the Term Loan Credit Agreement, the “Credit Agreements”) and that certain Intellectual Property Security Agreement, dated as of January 10, 2020, among the Grantors and the Revolving Loan Agent (as the same may have been amended, modified, restated, replaced or supplemented from time to time, the “Revolving IP Security Agreement”), the Grantors assigned and granted to each of (i) Revolving Loan Agent, for the benefit of itself and the Lenders under (and as defined in) the Revolving Credit Agreement and (ii) Term Loan Agent, for the benefit of itself and the Lenders under (and as defined in) the Term Loan Credit Agreement, a security interest in and to the personal property of the Grantors as specified therein, including the property set forth on Exhibit A hereto; WHEREAS, the Term Loan IP Security Agreement was duly filed and recorded with the United States Patent and Trademark Office on January 15, 2020 at reel/frame 51618/200 and on January 23, 2020 at reel/frame 6838/0823, and the Revolving IP Security Agreement was duly filed and recorded with the United States Patent and Trademark Office on January 15, 2020 at reel/frame 51618/758 and on January 23, 2020 at reel/frame 6838/0837; WHEREAS, pursuant to that certain License Agreement (such agreement, as may be amended or restated from time to time in accordance with the terms thereof and of the Credit Agreements, the “License Agreement”), dated as of the date hereof, between Radius Pharma and Berlin-Chemie AG - Menarini Group, organized under the laws of Germany having business offices at Xxxxxxxxxx Xxx 000, 00000 Xxxxxx, Xxxxxxx (including its permitted successors and assigns, “Licensee”), Radius Pharma has (a) granted to Licensee an exclusive, worldwide, royalty-bearing license under the Radius Elacestrant Patent Rights and the Radius Elacestrant Know-How to research, develop, have developed, make, have made, use, sell, offer for sale, commercialize, import and export one or more Licensed Compound(s) and Licensed Product(s) (each as defined in Exhibit A hereto) (such license grant, as contemplated under the License Agreement, the “License”); and (b) agreed to grant the right to use certain regulatory filings and other assets of the Grantors that are related solely to the Licensed Compounds and Licensed Products; WHEREAS, in connection with the License Agreement, the Grantors have requested that each Agent release, discharge, relinquish, terminate and dissolve its security interest in and continuing lien on, and security interest in and to, the Elacestrant Collateral (as defined in Exhibit A hereto); and

WHEREAS, pursuant to that certain Transition Services Agreement (such agreement, as may be amended or restated in accordance with the terms thereof and of the Credit Agreements, the “Transition Services Agreement”), dated as of the date hereof, between Radius Health and Licensee, Grantor has agree to perform certain clinical, regulatory and other activities relating to the Licensed Compound(s) and Licensed Products. NOW, THEREFORE, in consideration of the foregoing, the terms and conditions set forth in this Agreement, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, each Agent and the Grantors hereby agree as follows: 1. Recitals. This Agreement shall constitute a Financing Document under each Credit Agreement and the Recitals and each reference to the Credit Agreements, unless otherwise expressly noted, will be deemed to reference the Credit Agreements as supplemented hereby. Capitalized terms used but not otherwise defined herein shall have the meanings ascribed to them in the applicable Credit Agreement (including those capitalized terms used in the Recitals hereto). 2. Release. In reliance on the certifications set forth in Section 3(a) below, each Agent, without recourse, representation or warranty, automatically upon the execution and delivery of the License Agreement, and without any action required by any other Person, hereby (a) releases all of its right, title and interest in and to the Elacestrant Collateral, and reassigns and transfers all of its right, title and interest that such Agent may have in the Elacestrant Collateral, to the Grantors, and confirms that any Lien, security interest or other encumbrance of any kind in favor of such Agent on or in respect of the Elacestrant Collateral is hereby automatically discharged and released pursuant to Section 11.9 of each of the Credit Agreements, (b) authorizes Grantors or Grantors’ authorized representative (including the Licensee) to record this Agreement with the United States Patent and Trademark Office, and (c) agrees to file Uniform Commercial Code (UCC3) financing statement amendments in a form previously agreed by the Licensee with the Delaware Secretary of State promptly following the date hereof. Each Agent further agrees to execute and deliver to Grantors such further documents and instruments which any Grantor may reasonably request (at Grantors’ sole cost and expense) in order to release or terminate any security interest in favor of such Agent in the Elacestrant Collateral. Except as otherwise expressly set forth herein, this Agreement does not release any Lien granted by the Grantors in favor of either Agent, for the benefit of the applicable Lenders, pursuant to the Credit Agreements or any other Financing Documents. In the event the License Agreement is terminated or all or any portion of the Elacestrant Collateral is no longer subject to the License and the Grantors retain ownership of the Elacestrant Collateral (or such portion thereof), (i) a security interest in the Elacestrant Collateral (or such portion thereof) shall simultaneously and automatically be granted in favor of each Agent under Section 9 of the applicable Credit Agreement and the Elacestrant Collateral (or such portion thereof) shall be included in the Collateral and the Agents’ Liens shall reattach thereto and (ii) the Grantors shall promptly and duly take, execute, acknowledge and deliver all such further acts, documents and assurances as may be necessary or as either Agent may reasonably request in order to grant and evidence and perfect such security interests. 3. Acknowledgement by Grantors. a. The Grantors hereby certify that (i) the granting of the License pursuant to the License Agreement is an Asset Disposition permitted pursuant to clause (i) of the definition of Permitted Asset Dispositions in each of the Credit Agreements and (ii) no Event of Default has occurred and is continuing at the time such License is granted or would immediately result therefrom. Without limiting the foregoing, Grantors represent, warrant and covenant to each Agent that, at all times, the Elacestrant Collateral, including all assets set forth on Exhibit A, are Oncology Assets and that no other assets of any Credit Party (other than the Oncology Assets) are included in the definition of Elascestrant Collateral.

b. The Grantors hereby acknowledge that the License Agreement and any and all proceeds and products (other than any products that themselves constitute Elascestrant Collateral) thereof (including all Payment Rights (as defined below) thereunder) shall constitute Collateral and that all references to “Collateral” contained in the Credit Agreement or the other Financing Documents are hereby deemed for all purposes to include the License Agreement and the proceeds thereof (including all Payment Rights thereunder) as part of the Collateral. For the avoidance of doubt, in no event shall the License Agreement or the proceeds and products (other than any products that themselves constitute Elascestrant Collateral) thereof (including all Payment Rights thereunder) constitute Excluded Property. The term “Payment Rights” as used herein means, collectively: (i) all proceeds received by or on behalf of Radius Pharma under the License Agreement, (ii) all rights to payment of Radius Pharma under the License Agreement and (iii) all rights of Radius Pharma related, ancillary or incidental to the foregoing clauses (i) and (ii). For purposes of this clause (b), the term “proceeds” shall have the meaning set forth in the UCC. c. The Grantors agree, on behalf of themselves and the other Credit Parties, that they will not, without Agents’ prior consent, amend, supplement or otherwise modify the License Agreement, or any other material agreement related thereto, or enter into any agreement or other document related to the License Agreement or the Elascestrant Collateral, in each case, to the extent such amendment, supplement or modification or new agreement or document would be materially adverse to either Agent or the Lenders or their Collateral. 4. Acknowledgement by Each Agent. Each Agent (a) acknowledges and agrees on behalf of itself and (b) confirms that the Required Lenders under each of the Credit Agreements has acknowledged and agreed that, in each case, pursuant to Section 10.1(g) of the License Agreement, Radius Pharma has covenanted that it will not, nor will it permit any of its Affiliates to, directly or indirectly, create, assume or suffer to exist any Lien on or security interest in all or any portion of the Elacestrant Collateral for so long and during the period that the License Agreement remains in full force and effect or that the Elacestrant Collateral remains subject to the License (in accordance with its terms). 5. Miscellaneous. a. The agreements of each Agent hereunder are made without recourse to or warranty by such Agent. It is expressly agreed and understood that this is a partial release and that it shall in no manner release, affect or otherwise impair the liens and security interest in favor of either Agent, under the Credit Agreements or otherwise, against any Collateral other than the Elacestrant Collateral. Other than the Liens expressly released pursuant to Section 2 above, each Grantor confirms and agrees that all security interests and Liens granted to each Agent continue in full force and effect, and that all Collateral remains free and clear of any Liens, other than Permitted Liens. b. GOVERNING LAW. THIS AGREEMENT AND ALL DISPUTES AND OTHER MATTERS RELATING HERETO OR THERETO OR ARISING THEREFROM (WHETHER SOUNDING IN CONTRACT LAW, TORT LAW OR OTHERWISE), SHALL BE GOVERNED BY, AND SHALL BE CONSTRUED AND ENFORCED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF NEW YORK, WITHOUT REGARD TO CONFLICTS OF LAWS PRINCIPLES (OTHER THAN SECTION 5-1401 OF THE GENERAL OBLIGATIONS LAW). c. WAIVER OF JURY TRIAL. EACH PARTY HERETO HEREBY IRREVOCABLY WAIVES ANY AND ALL RIGHT TO TRIAL BY JURY IN ANY LEGAL ACTION OR PROCEEDING ARISING OUT OF OR RELATING TO THIS AGREEMENT OR THE TRANSACTIONS CONTEMPLATED HEREBY AND AGREES THAT ANY SUCH

ACTION OR PROCEEDING SHALL BE TRIED BEFORE A COURT AND NOT BEFORE A JURY. EACH PARTY HERETO ACKNOWLEDGES THAT THIS WAIVER IS A MATERIAL INDUCEMENT TO ENTER INTO A BUSINESS RELATIONSHIP, THAT EACH HAS RELIED ON THE WAIVER IN ENTERING INTO THIS AGREEMENT, AND THAT EACH WILL CONTINUE TO RELY ON THIS WAIVER IN THEIR RELATED FUTURE DEALINGS. EACH PARTY WARRANTS AND REPRESENTS THAT IT HAS HAD THE OPPORTUNITY OF REVIEWING THIS JURY WAIVER WITH LEGAL COUNSEL, AND THAT IT KNOWINGLY AND VOLUNTARILY WAIVES ITS JURY TRIAL RIGHTS. d. Counterparts. This Agreement may be signed in any number of counterparts, each of which shall be deemed an original and all of which when taken together shall constitute one and the same instrument. Delivery of an executed counterpart of this Agreement by facsimile or by electronic mail delivery of an electronic version (e.g., .pdf or .tif file) of an executed signature page shall be effective as delivery of an original executed counterpart hereof and shall bind the parties hereto. e. Successors and Assigns. This Agreement shall be binding upon the successors and assigns of each Agent, and shall be binding upon each Grantor and its successors and assigns. No Person other than the parties hereto shall have any rights hereunder or be entitled to rely on this Agreement and all third-party beneficiary rights are hereby expressly disclaimed; provided that, notwithstanding the foregoing, the Licensee shall be an express third-party beneficiary solely of clause (b) of Section 2 hereof, and such clause shall expressly inure to the benefit of the Licensee and the Licensee shall be entitled to rely on and enforce the provisions of such clause but not in respect of any other provision of this Agreement. [remainder of this page intentionally left blank]

IN WITNESS WHEREOF, intending to be legally bound, each of the parties have caused this Agreement to be executed as of the day and year first above mentioned. GRANTORS: RADIUS HEALTH, INC. By:/s/ Xxxx Xxxxxxx Name: Xxxx Xxxxxxx Title: Chief Financial Officer RADIUS PHARMACEUTICALS, INC. By: /s/ Xxxx Xxxxxxx Name: Xxxx Xxxxxxx Title: Chief Financial Officer 1

MIDCAP FINANCIAL TRUST, as Term Loan Agent By: Apollo Capital Management, L.P., its investment manager By: Apollo Capital Management GP, LLC, its general partner By: /s/ Xxxxxxx Xxxxxxxx Name: Xxxxxxx Xxxxxxxx Title: Authorized Signatory MIDCAP FUNDING IV TRUST, as Revolving Loan Agent By: Apollo Capital Management, L.P., its investment manager By: Apollo Capital Management GP, LLC, its general partner By: /s/ Xxxxxxx Xxxxxxxx Name: Xxxxxxx Xxxxxxxx Title: Authorized Signatory 2

Exhibit A “Elacestrant Collateral” means all term loan credit Collateral (as such term is defined in the Term Loan Credit Agreement) and all revolving credit Collateral (as such term is defined in the Revolving Credit Agreement), as applicable, that, in each case, is related solely to the Licensed Compounds or Licensed Products, specifically including (without limitation) the Radius Elacestrant Patent Rights, Radius Elacestrant Know-How, Elacestrant Contracts, Elacestrant Upstream Licenses and TSA Inventions; provided that Elacestrant Collateral shall not include any of the following assets or property: (i) cash or cash equivalents, (ii) deposit accounts or securities accounts, (iii) accounts or accounts receivable, (iv) the License Agreement (or any payments or rights to payment thereunder), (v) any other contract or agreement (or any payments or rights to payment thereunder) that provides for any payments to, or received by or on behalf of, any Grantor (or any of its Affiliates) by Licensee or any Affiliate thereof or any of their respective successors or assigns, (vi) any payments or rights to payment of any Grantor, or any Affiliate thereof, under any other contract or agreement related to the License Agreement or the Licensed Product or Licensed Compounds or (vii) any proceeds in respect of any of the foregoing. For purposes of the foregoing, the following terms shall have the following meanings: 1.1 “Affiliate” of a Person means any other Person which (directly or indirectly) is controlled by, controls or is under common control with such Person. For the purposes of this definition, the term “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”) as used with respect to any Person means (a) in the case of a corporate entity, direct or indirect ownership of voting securities entitled to cast at least fifty percent (50%) of the votes in the election of directors, (b) in the case of a non-corporate entity, direct or indirect ownership of at least fifty percent (50%), including ownership by trusts with substantially the same beneficial interest, of the equity interests with the power to direct the management and policies of such Person, provided that if local law restricts foreign ownership, control will be established by direct or indirect ownership of the maximum ownership percentage that may, under such local law, be owned by foreign interests, or (c) the power to direct the management or policies of a Person, whether through ownership of voting securities, by contract or otherwise. 1.2 “Combination” means a Combination Product or Combination Therapy. 1.3 “Combination Product” means a Licensed Product that includes at least one additional active ingredient other than Licensed Compound. Drug delivery vehicles, adjuvants, and excipients will not be deemed to be “active ingredients”, except in the case where such delivery vehicle, adjuvant, or excipient is recognized as an active ingredient in accordance with 21 C.F.R. § 210.3(b)(7) (as amended), or any foreign counterpart. 1.4 “Combination Therapy” means a therapy comprised of a Licensed Product and one or more other therapeutically or prophylactically active ingredients, whether priced and sold in a single package containing such multiple products, packaged separately but sold together for a single price, or sold under separate price points but labeled for use together, including all dosage forms, formulations, presentations, and package configurations. Drug delivery vehicles, adjuvants, and excipients will not be deemed to be “active ingredients”, except in the case where such delivery vehicle, adjuvant, or excipient is recognized as an active ingredient in accordance with 21 C.F.R. § 210.3(b)(7) (as amended), or any foreign counterpart. 1.5 “Confidential Information” means all Radius Elacestrant Know-How, marketing plans, strategies and customer lists, and other information or material that are disclosed or provided by Radius Health or its Affiliates to Licensee or its Affiliates pursuant to the terms of the Transition Services

Agreement, regardless of whether any of the foregoing are marked “confidential” or “proprietary” or communicated to the other by Radius Health or its Affiliates in oral, written, graphic, or electronic form. 1.6 “Duke License Agreement” means that certain Patent License Agreement dated as of December 8, 2017, by and between Radius Pharma and Duke University (“Duke”) as such agreement may be amended or restated from time to time in a manner that is not prohibited under the Financing Documents. 1.7 “Duke Patent Rights” means Duke’s legal rights under the patent laws of the United States or relevant foreign countries for all of the following: (a) the following United States and foreign patent(s) and/or patent application(s), including all divisionals, continuations, continuations-in-part arising from or claiming priority to any of the Duke Patent Rights and containing claims covering the chemical compound known as RAD1901 or elacestrant in any and all fields of use, and foreign counterparts of the same: US Patent 9,421,264 (US Application No.: 14/512,061), USSN 62/129,379, USSN 61/971,627, PCT/US2015/023216, USSN 15/214,187, USSN 15/129,197, EPO 15769394.6 (EP 3122426), CA 2,943,611. (b) United States and foreign patents issued from the applications listed in subparagraph (a) above, including any reviewed, reissued or re-examined patents, and supplementary protection certificates, and foreign counterparts of the same, based upon the same. (c) Any applications claiming priority from any of the listed applications. The Duke Patent Rights existing as of the Effective Date are set forth on Appendix 6 to this Exhibit A. 1.8 “Effective Date” means July 22, 2020. 1.9 “Eisai License Agreement” means that certain License Agreement, dated as of June 29, 2006, by and between Radius Pharma and Eisai Co., Ltd. (“Eisai”), as such agreement may be amended or restated from time to time in a manner that is not prohibited under the Financing Documents. 1.10 “Eisai Patents” means all patents and patent applications which are or become owned by Eisai and/or its Affiliates, or to which Eisai and/or its Affiliates, otherwise have, now or in the future, the right to grant licenses, and which generically or specifically claim Compound and/or Product, a use for Compound and/or Product, a process for manufacturing Compound and/or Product, or an intermediate use in such process. Included within the definition of Eisai Patents are all continuations, continuations-in-part, divisions, patents of addition, reissues, re-examinations, renewals or extensions thereof and all Supplementary Protection Certificates. Also included within the definition are any improvements on Compound and/or Product or intermediates or manufacturing process required or useful for production of Compound and/or Product which are developed by or for Eisai and/or its Affiliates, or to which Eisai and/or its Affiliates otherwise has the right to grant licenses, now or in the future, during the term of the License Agreement. Eisai

Patents also includes any patent application covering an invention solely owned by Eisai in accordance with Article 6.4 of the Eisai License Agreement. For purposes of this clause 1.10 (“Eisai Patents”), clause 1.17 (“Radius-Eisai Joint Invention”), clause 1.18 (“Radius-Eisai Joint Patents”), and clause 1.20 (“Radius Elacestrant Invented Patent”), (i) “Compound” means elacestrant or any derivative or analogue thereof, (ii) “Product” means any pharmaceutical drug in final packaged form containing the Compound or any derivative or analogue thereof, the development, manufacture, use or sale of which, absent the licenses granted to Licensee under the License Agreement, would infringe the Eisai Patents or which make use of any Radius-Eisai Joint Patents, and (iii) “Affiliates” means any corporation, firm, partnership or other entity which directly or indirectly owns, is owned by or is under common ownership with Eisai to the extent of more than fifty (50) percent of the equity having the power to vote on or direct the affairs of any such corporation, firm, partnership, or other entity The Eisai Patents existing as of the Effective Date as set forth on Appendix 6 to this Exhibit A. 1.11 “Elacestrant Contracts” means (a) the contracts listed on Appendix 4 to this Exhibit A, solely to the extent that such contracts are solely related to the Licensed Compounds or Licensed Products, and (b) any contracts that Radius Health or its Affiliates enter into after the Effective Date, solely for the purpose of carrying out the Services on behalf of Radius Health. 1.12 “Elacestrant Upstream Licenses” means the Duke License Agreement and Eisai License Agreement. 1.13 “Know-How” means all know-how, trade secrets, chemical and biological materials, formulations, information, documents, studies, results, data and regulatory approvals, data (including from clinical studies), filings and correspondence (including drug master files), including biological, chemical, pharmacological, toxicological, pre-clinical, clinical and assay data, manufacturing processes and data, specifications, sourcing information, assays, and quality control and testing procedures, whether or not patented or patentable. 1.14 “Licensed Compound” means the compound known as elacestrant (RAD1901), as further described on Appendix 1 to this Exhibit A, the backup compounds listed on Appendix 2 to this Exhibit A, and in each case, any modification, improvement, derivative or Structural Analog thereof, including any metabolite, salt, ester, free acid form, free base form, crystalline form, amorphous form, pro-drug form, racemate, chelate, polymorph, tautomer, solvate, or optical isomer thereof. 1.15 “Licensed Product” means any pharmaceutical product containing any Licensed Compound (alone or with other active ingredients), in all forms, presentations, formulations and dosage forms. For clarification, Licensed Product will include any Combination. 1.16 “Person” means an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, beneficiary or trustee of any trust, incorporated association, joint venture, or similar entity or organization, including a government or political subdivision or department or agency of a government. 1.17 “Radius-Eisai Joint Invention” means any invention developed by or on behalf of Radius Pharma and disclosed by Radius Pharma to Eisai pursuant to Section 6.3 of the Eisai License Agreement.

1.18 “Radius-Eisai Joint Patents” means any patent application claiming a Radius-Eisai Joint Invention, and any patent stemming therefrom. The Radius-Eisai Joint Patents existing as of the Effective Date are set forth in Appendix 6 to this Exhibit A. 1.19 “Radius Elacestrant Know-How” means (a) all Know-How that is related solely to the Licensed Compounds or Licensed Products and is controlled by Radius Pharma or any of its Affiliates as of the Effective Date, and (b) all Know-How within the TSA Inventions. 1.20 “Radius Elacestrant Invented Patents” means all patents and patent applications which are or become owned by Radius Pharma and/or its Affiliates, to which Radius Pharma and/or its Affiliates otherwise have, now or in the future, the right to grant licenses, and which generically or specifically claim Compound and/or Product, a use for Compound and/or Product, a process for manufacturing Compound and/or Product, or an intermediate used in such process. Included within the definition of Radius Elacestrant Invented Patents are all continuations, continuations-in-part, divisions, patents of addition, reissues, re-examinations, renewals or extensions thereof and all Supplementary Protection Certificates. Also included within the definition are all patents and patent applications any improvements on Compound and/or Product or intermediates or manufacturing process required or useful for production of Compound and/or Product which are developed by or for Radius Pharma and/or its Affiliates, or to which Radius Pharma and/or its Affiliates otherwise have the right to grant licenses, now or in the future, during the term of the License Agreement. Radius Elacestrant Invented Patents also includes any patent application covering an invention solely owned by Radius in accordance with Article 6.4 of the Eisai License Agreement. The Radius Elacestrant Invented Patents existing as of the Effective Date are set forth in in Appendix 5 to this Exhibit A. 1.21 “Radius Elacestrant Patent Rights” means (a) the patents and patent applications listed in Appendix 5 and Appendix 6 to this Exhibit A, plus any conversion, continuation, division or substitution thereon, any reissues, reexaminations or extensions thereof, any continuation-in-part application or patent that is entitled to the priority date of, and is directed specifically to subject matter specifically described in, at least one of the patents or patent applications described above, and any foreign counterparts of any of the foregoing, and (b) all patents and patent applications that specifically claim or cover the TSA Inventions, plus any conversion, continuation, division or substitution thereon, any reissues, reexaminations or extensions thereof, any continuation-in-part application or patent that is entitled to the priority date of, and is directed specifically to subject matter specifically described in, at least one of the patents or patent applications described above, and any foreign counterparts of any of the foregoing. For the sake of clarity, the Radius Elacestrant Patent Rights include the Duke Patent Rights, the Eisai Patents, the Radius-Eisai Joint Patents, and the Radius Elacestrant Invented Patents. 1.22 “Services” means the services to be performed by or on behalf of Radius Health in the interest of Licensee pursuant to the Transition Services Agreement. 1.23 “Structural Analog” means a compound covered by the chemical structure set forth on Appendix 3 to this Exhibit A. 1.24 “TSA Inventions” means any materials, data, processes, documents, deliverables, information (including Confidential Information), discoveries, inventions, know-how and the like developed or generated by or on behalf of Radius Health after the Effective Date during the course of performing the Services, whether or not patentable, and all related patent, copyright and other intellectual property rights in any of the foregoing.
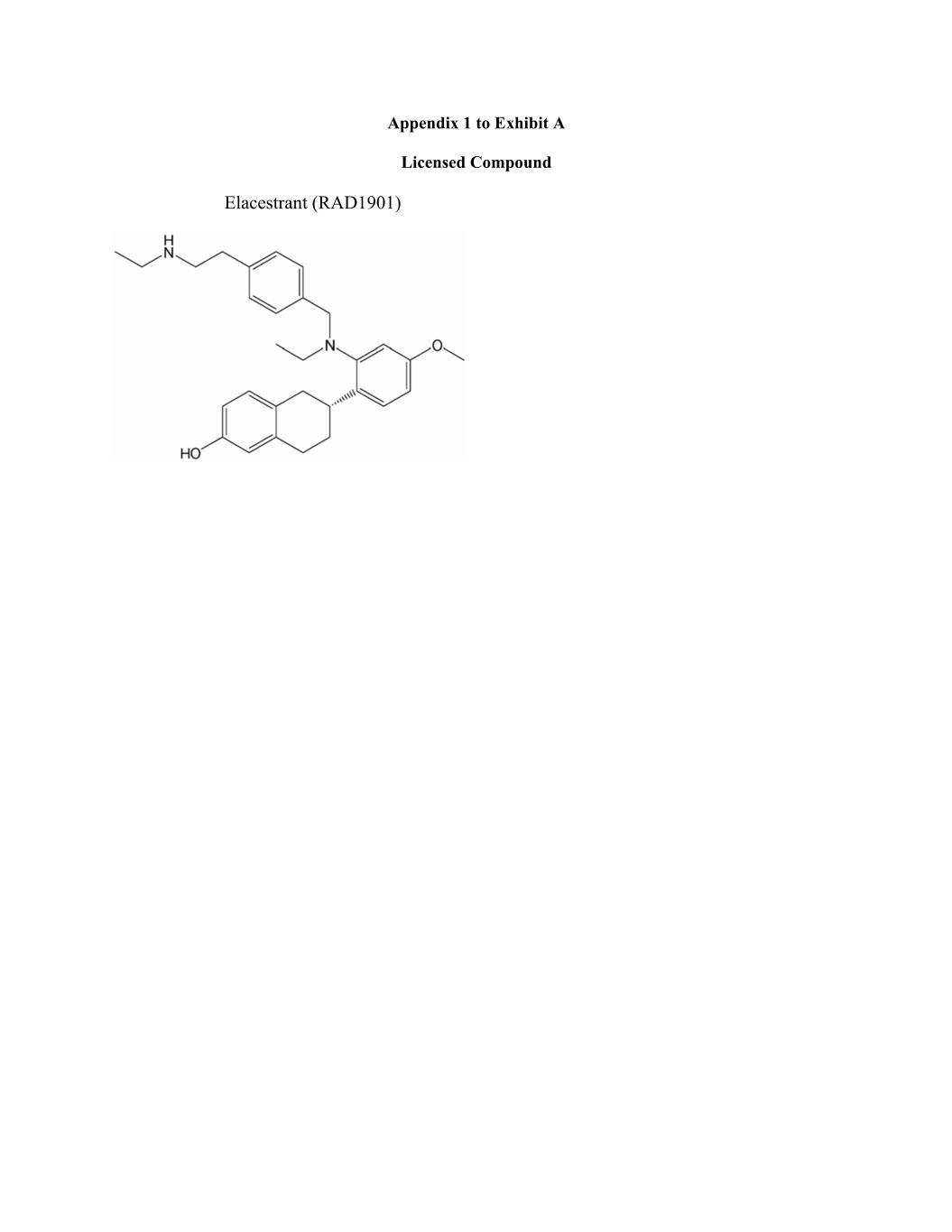
Appendix 1 to Exhibit A Licensed Compound Elacestrant (RAD1901)
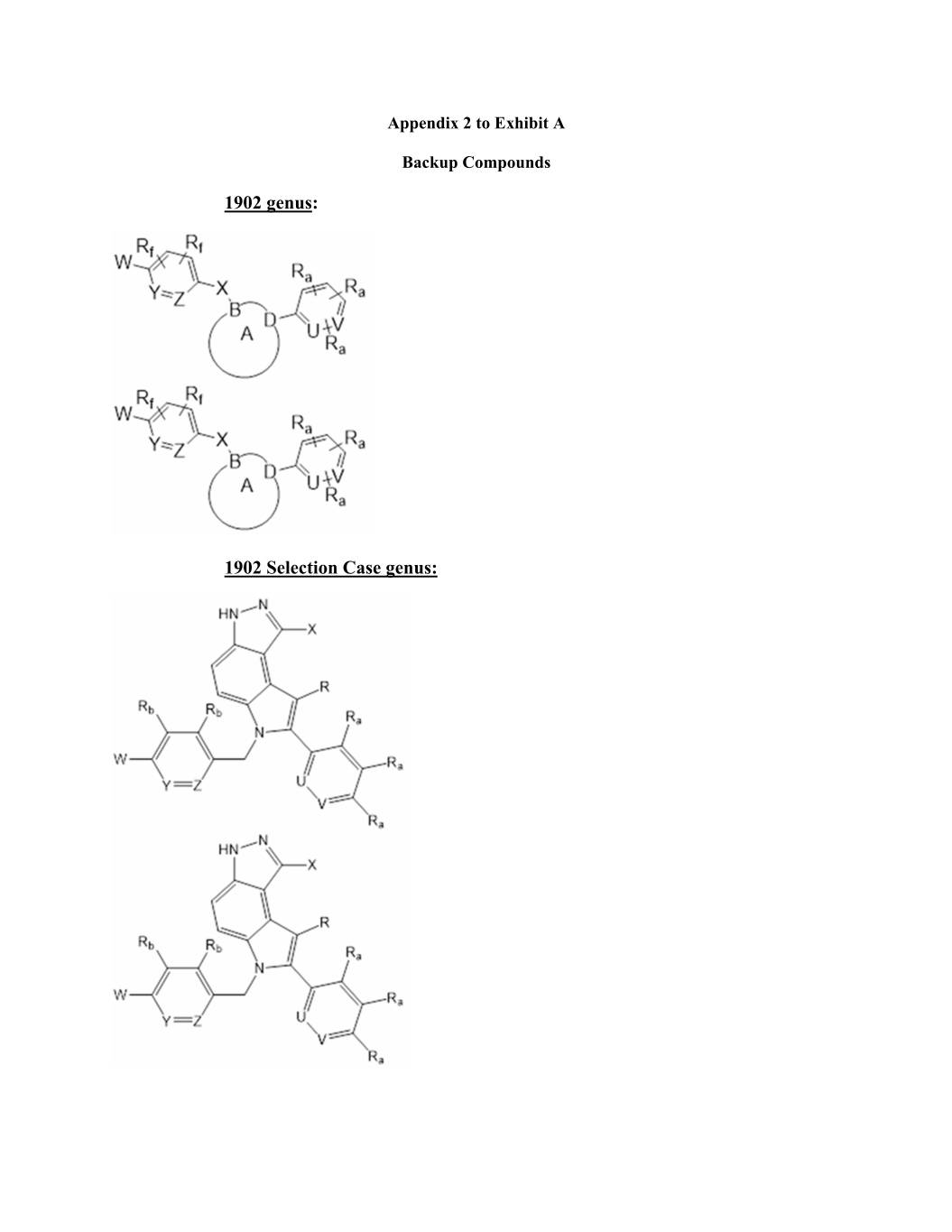
Appendix 2 to Exhibit A Backup Compounds 1902 genus: 1902 Selection Case genus:
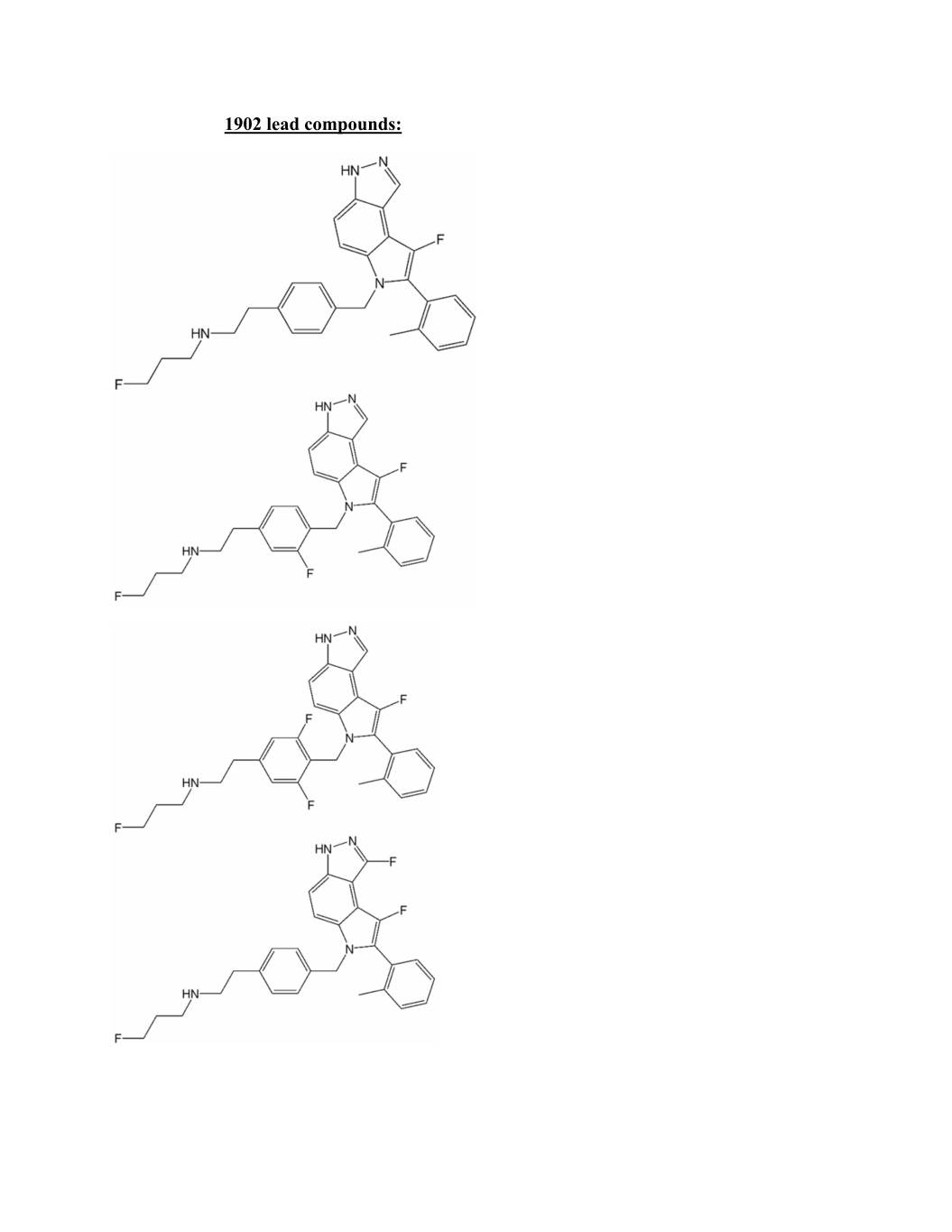
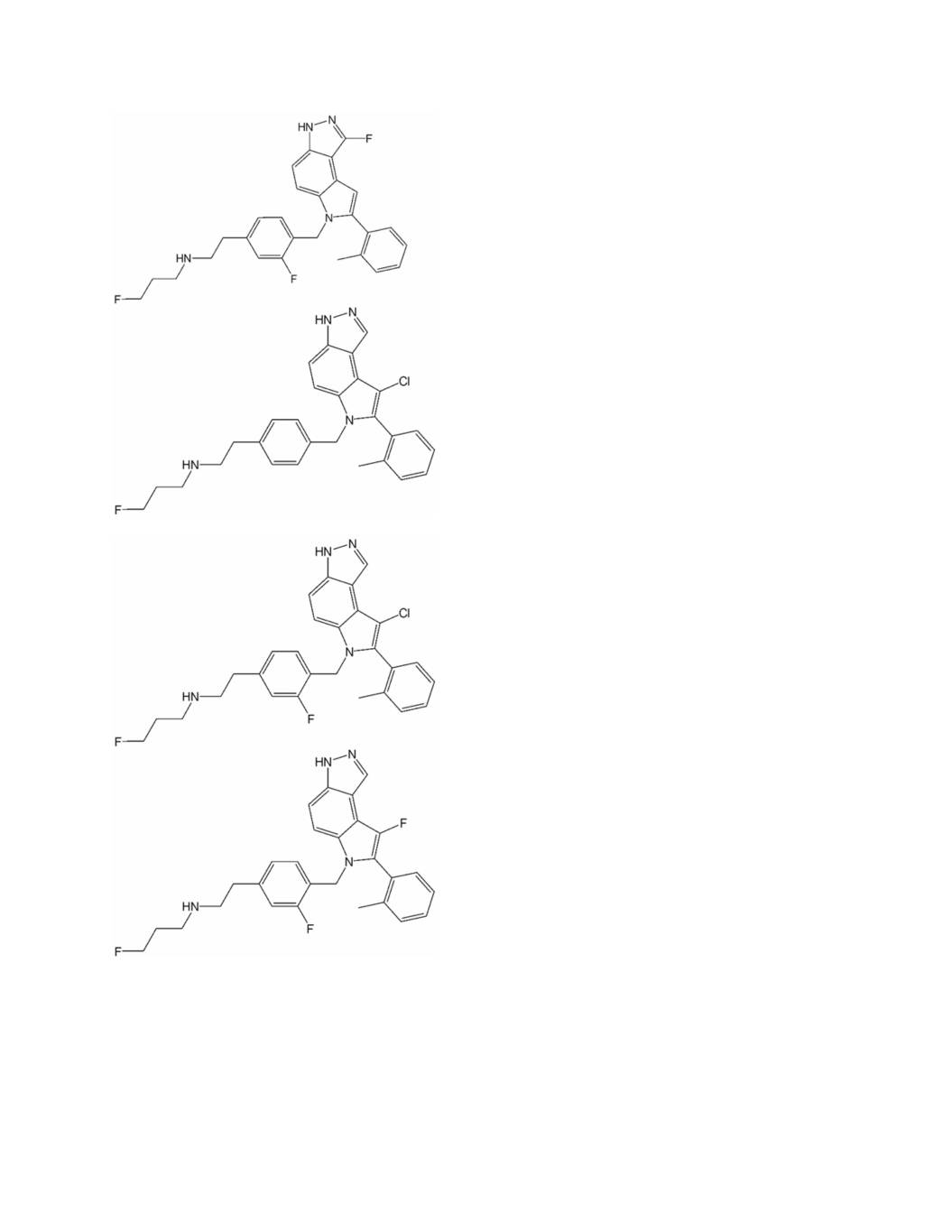
1902 lead compounds:
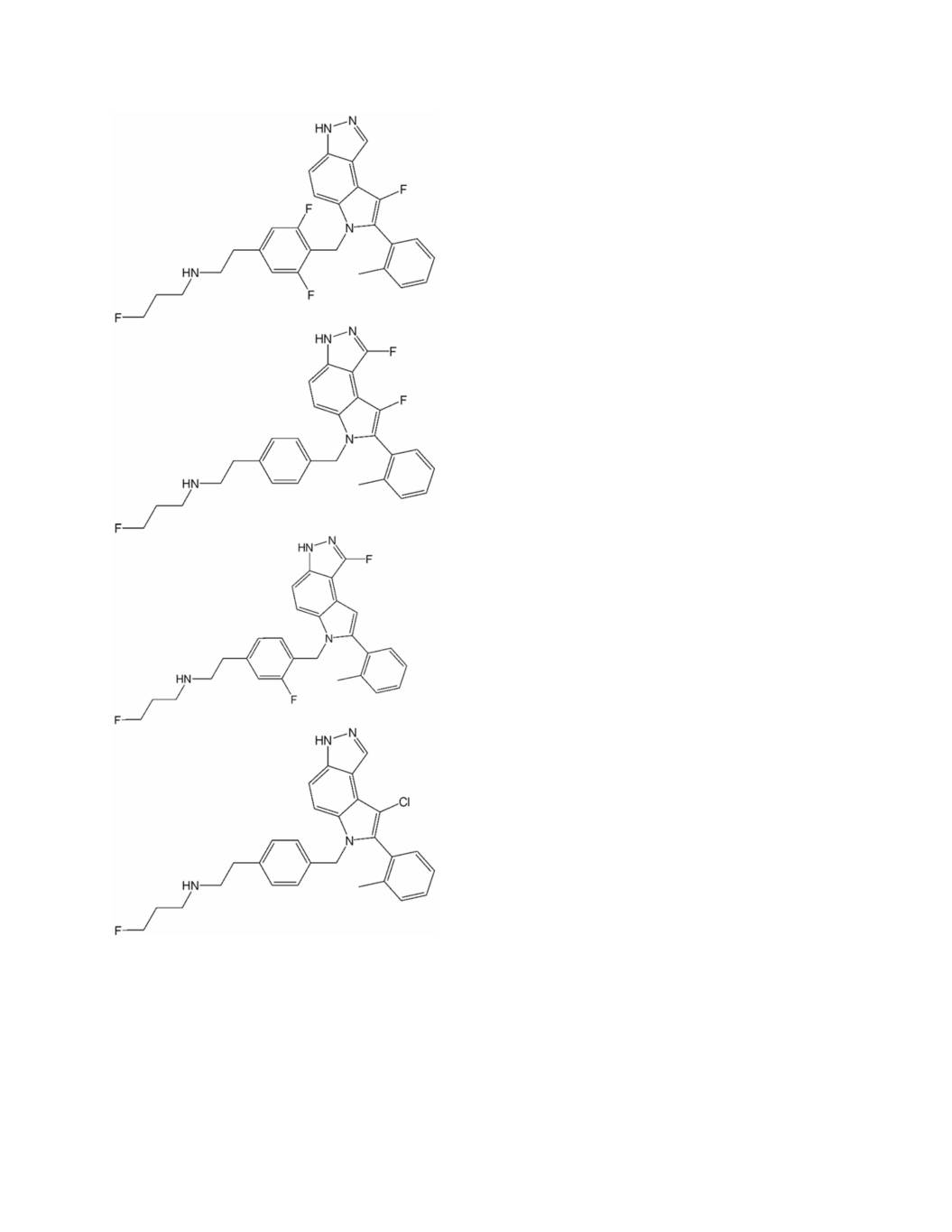
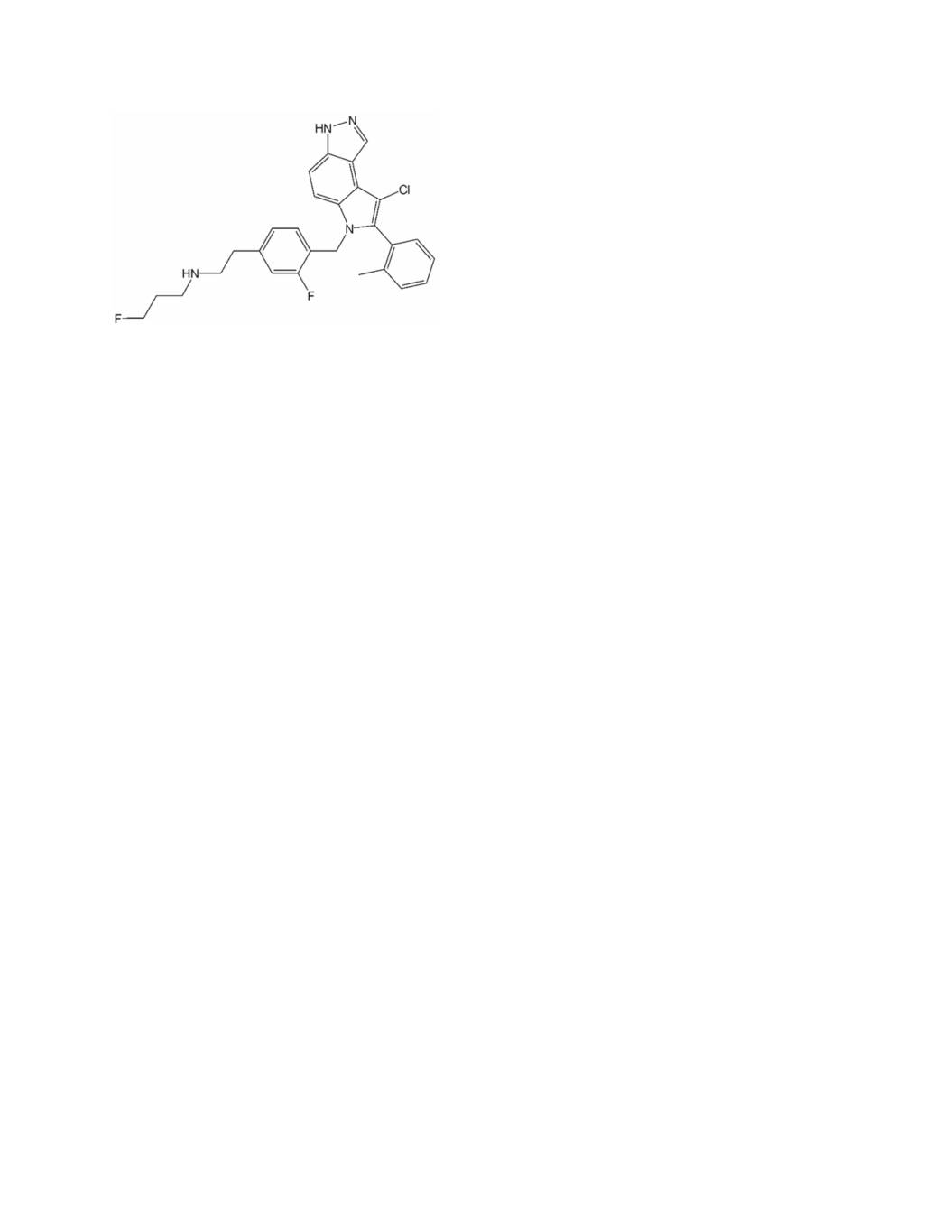
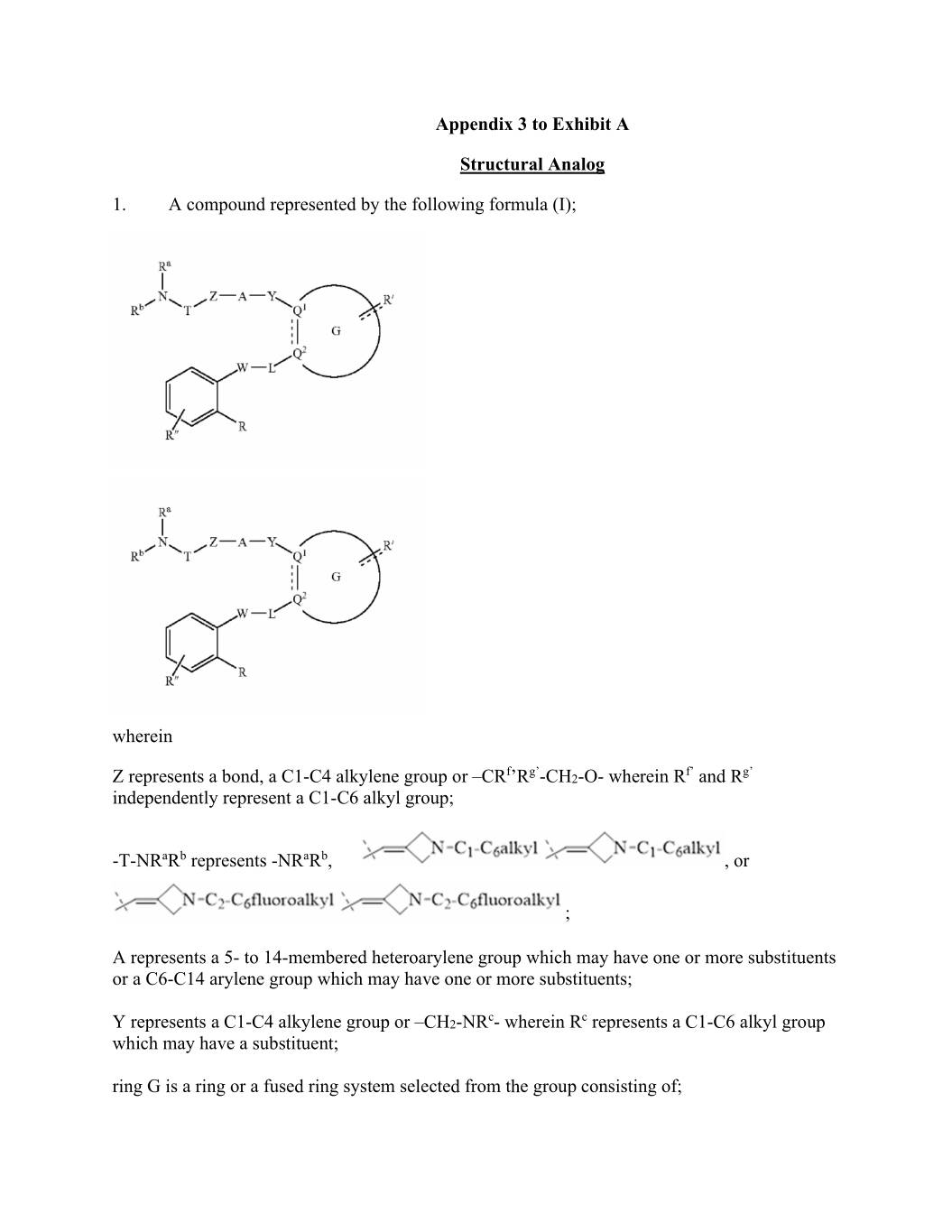
Appendix 3 to Exhibit A Structural Analog 1. A compound represented by the following formula (I); wherein f g’ f’ g’ Z represents a bond, a C1-C4 alkylene group or –CR ’R -CH2-O- wherein R and R independently represent a C1-C6 alkyl group; -T-NRaRb represents -NRaRb, , or ; A represents a 5- to 14-membered heteroarylene group which may have one or more substituents or a C6-C14 arylene group which may have one or more substituents; c c Y represents a C1-C4 alkylene group or –CH2-NR - wherein R represents a C1-C6 alkyl group which may have a substituent; ring G is a ring or a fused ring system selected from the group consisting of;
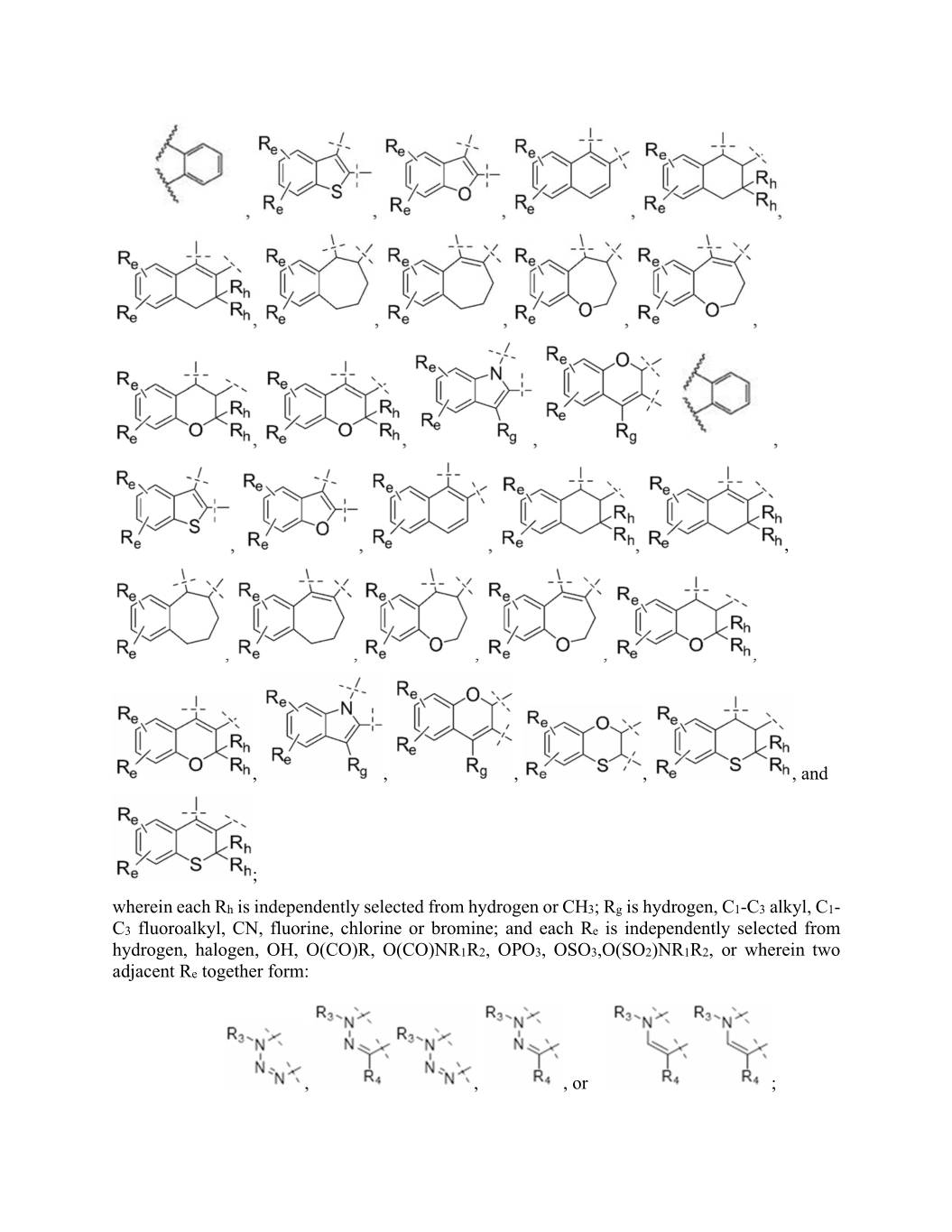
, , , , , , , , , , , , , , , , , , , , , , , , , , , , , and ; wherein each Rh is independently selected from hydrogen or CH3; Rg is hydrogen, C1-C3 alkyl, C1- C3 fluoroalkyl, CN, fluorine, chlorine or bromine; and each Re is independently selected from hydrogen, halogen, OH, O(CO)R, O(CO)NR1R2, XXX0, XXX0,X(XX0)XX0X0, or wherein two adjacent Re together form: , , , or ;
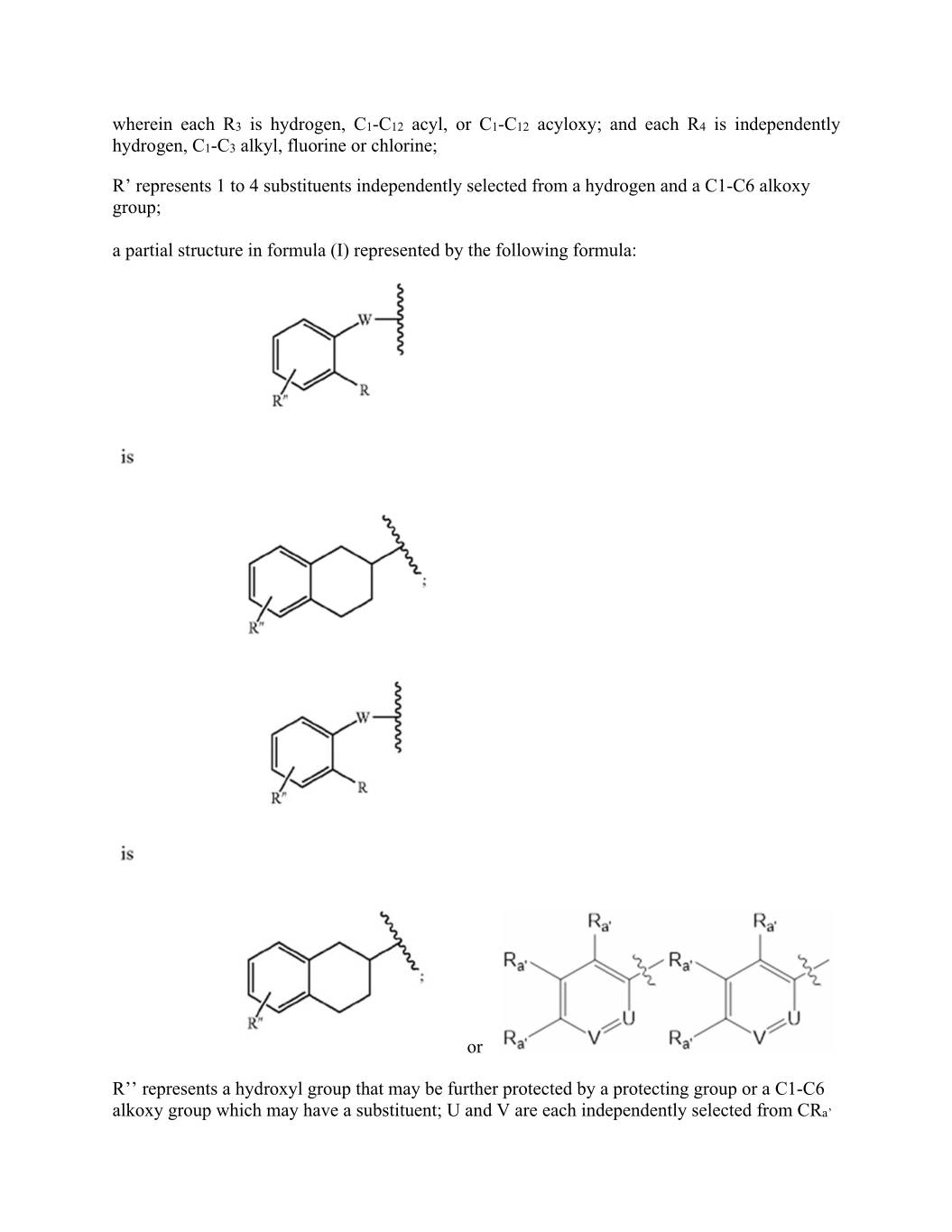
wherein each R3 is hydrogen, C1-C12 acyl, or C1-C12 acyloxy; and each R4 is independently hydrogen, C1-C3 alkyl, fluorine or chlorine; R’ represents 1 to 4 substituents independently selected from a hydrogen and a C1-C6 alkoxy group; a partial structure in formula (I) represented by the following formula: or R’’ represents a hydroxyl group that may be further protected by a protecting group or a C1-C6 alkoxy group which may have a substituent; U and V are each independently selected from CRa’

or N; each Ra’ is independently selected from: H, C1-C3 alkyl, C1-C3 fluoroalkyl, phenyl (optionally substituted with 1-3 groups selected from fluorine, chlorine, C1-C3 alkyl, CN, OC1-C3 alkyl, OH), OH, OC1-3alkyl, CN, fluorine, or chlorine; and Ra and Rb are the same as or different from each other and each represents a hydrogen atom, a C1-C6 alkyl group which may have one or more substituents, or a C3-C8 cycloalkyl group which may have one or more substituents, or when Ra and Rb are bonded together, they may form, together with the nitrogen atom that is adjacent to a Ra and Rb, a 4- to 10-membered single ring which may have one or more substituents; and L represents a single bond, or a salt thereof.

Appendix 4 to Exhibit A Elacestrant Contracts as of the Effective Date 1. Companion Diagnostics Master Collaboration Agreement, by and between Guardant Health, Inc. and Radius Pharmaceuticals, Inc., dated as of July 8, 2020 2. Master Services Agreement, by and between Radius Health, Inc. and Guardant Health, Inc., dated as of September 14, 2016 and amended October 22, 2018 3. Patient Referral Agreement, by and between Radius Health, Inc. and Guardant Health, Inc., dated as of February 7, 2020 4. Master Services Agreement, by and between Radius Health, Inc. and Mayne Pharma Inc., d/b/a Metrics Contract Services, dated as of October 30, 2015 and amended August 12, 2019 5. Master Services Agreement, by and between Radius Health, Inc. and Sambi Pharma PVT Ltd, dated as of January 7, 2020 6. Professional Services Agreement, by and between Radius Health, Inc. and Link2Trials B.V., dated as of May 1, 2019 7. Development and Manufacturing Services Agreement, by and between Radius Pharmaceuticals, Inc. and Asymchem, Inc., dated as of October 17, 2018 8. Master Services Agreement, by and between Radius Health, Inc. and Sai Life Sciences Limited, dated as of May 13, 2016 9. Master Services Agreement, by and between Radius Health, Inc. and ARC Trinova Limited trading as “Arcinova”, dated as of October 16, 2018 10. Master Services Agreement, by and between Radius Health, Inc. and CRF Health Management Limited, dated as of May 8, 2018 11. Master Agreement for Pharmaceutical Development and Technology Transfer Services, by and between Patheon, Inc. and Radius Pharmaceuticals, Inc., dated as of March 27, 2017 12. Master Clinical Contract Services Agreement, by and between Radius Health, Inc. and Parexel International (IRL) Limited, dated as of April 24, 2015 and amended April 24, 2018, but only with respect to Statement of Work RAD1901-308 dated as of December 14, 2017
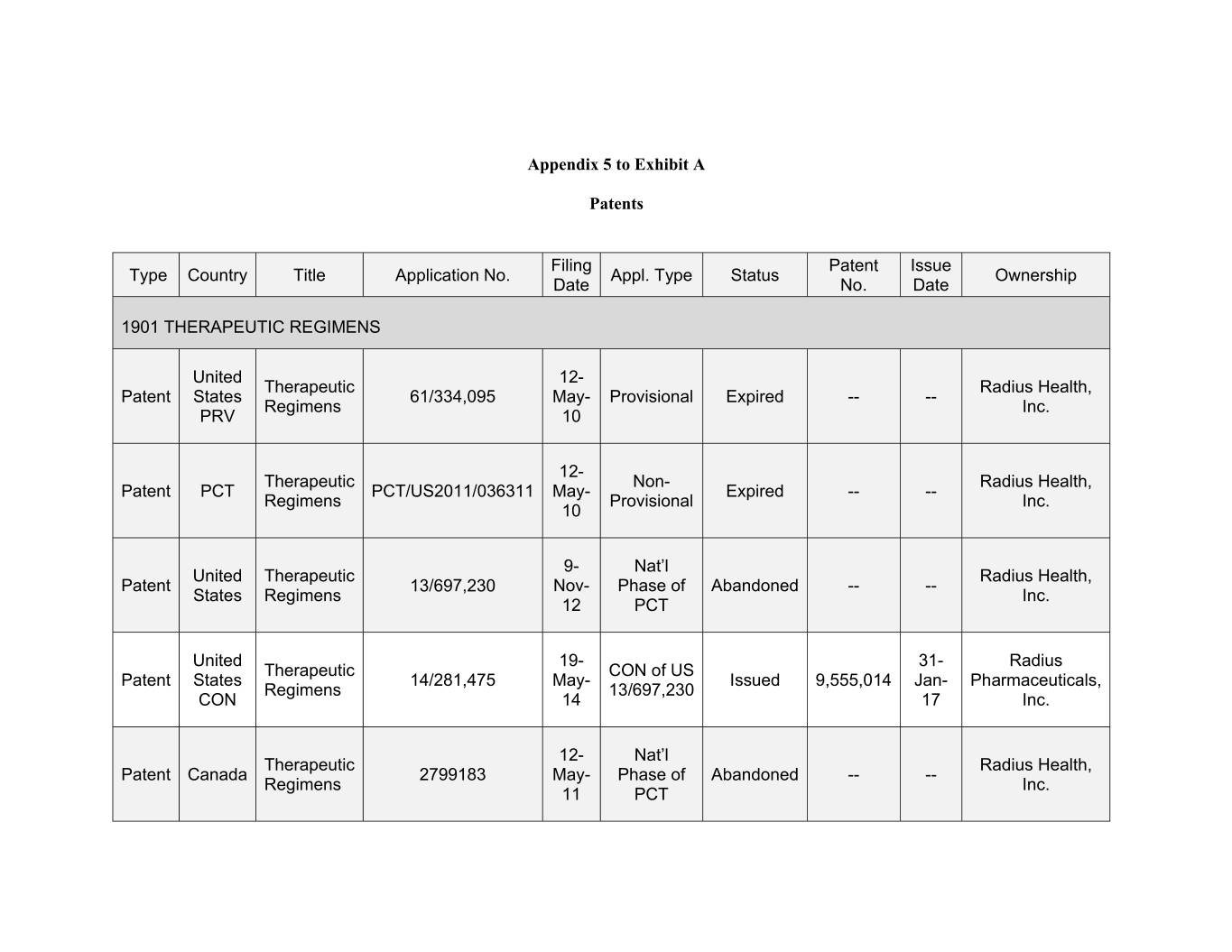
Appendix 5 to Exhibit A Patents Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 THERAPEUTIC REGIMENS United 12- Therapeutic Radius Health, Patent States 61/334,095 May- Provisional Expired -- -- Regimens Inc. PRV 10 12- Therapeutic Non- Radius Health, Patent PCT PCT/US2011/036311 May- Expired -- -- Regimens Provisional Inc. 10 9- Nat’l United Therapeutic Radius Health, Patent 13/697,230 Nov- Phase of Abandoned -- -- States Regimens Inc. 12 PCT United 19- 31- Radius Therapeutic CON of US Patent States 14/281,475 May- Issued 9,555,014 Jan- Pharmaceuticals, Regimens 13/697,230 CON 14 17 Inc. 12- Nat’l Therapeutic Radius Health, Patent Canada 2799183 May- Phase of Abandoned -- -- Regimens Inc. 11 PCT
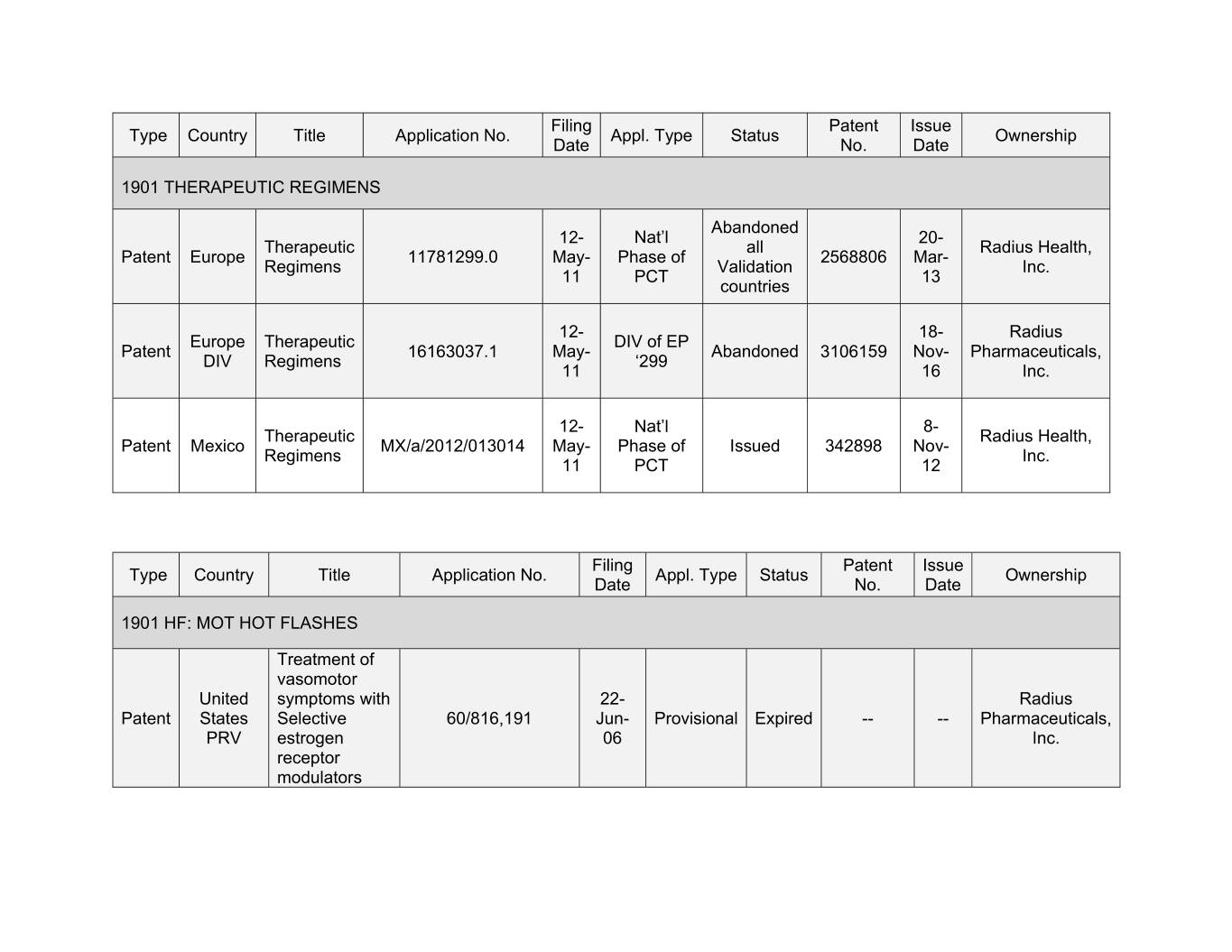
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 THERAPEUTIC REGIMENS Abandoned 12- Nat’l 20- Therapeutic all Radius Health, Patent Europe 11781299.0 May- Phase of 2568806 Mar- Regimens Validation Inc. 11 PCT 13 countries 12- 18- Radius Europe Therapeutic DIV of EP Patent 16163037.1 May- Abandoned 3106159 Nov- Pharmaceuticals, DIV Regimens ‘299 11 16 Inc. 12- Nat’l 8- Therapeutic Radius Health, Patent Mexico MX/a/2012/013014 May- Phase of Issued 342898 Nov- Regimens Inc. 11 PCT 12 Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 HF: MOT HOT FLASHES Treatment of vasomotor United symptoms with 22- Radius Patent States Selective 60/816,191 Jun- Provisional Expired -- -- Pharmaceuticals, PRV estrogen 06 Inc. receptor modulators
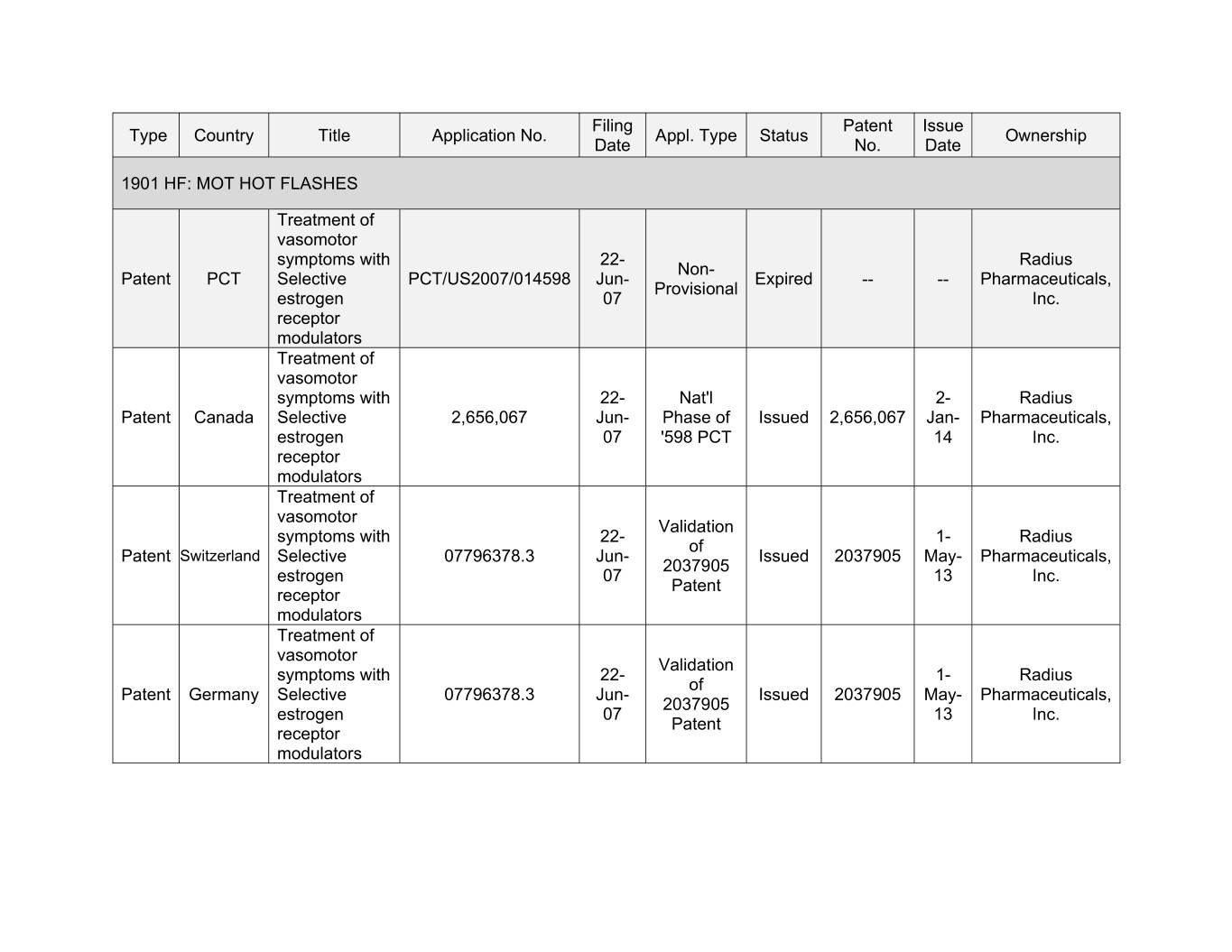
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 HF: MOT HOT FLASHES Treatment of vasomotor symptoms with 22- Radius Non- Patent PCT Selective PCT/US2007/014598 Jun- Expired -- -- Pharmaceuticals, Provisional estrogen 07 Inc. receptor modulators Treatment of vasomotor symptoms with 00- Xxx'x 0- Xxxxxx Xxxxxx Xxxxxx Selective 2,656,067 Jun- Phase of Issued 2,656,067 Jan- Pharmaceuticals, estrogen 07 '598 PCT 14 Inc. receptor modulators Treatment of vasomotor Validation symptoms with 22- 1- Radius of Patent Switzerland Selective 07796378.3 Jun- Issued 2037905 May- Pharmaceuticals, 2037905 estrogen 07 13 Inc. Patent receptor modulators Treatment of vasomotor Validation symptoms with 22- 1- Radius of Patent Germany Selective 07796378.3 Jun- Issued 2037905 May- Pharmaceuticals, 2037905 estrogen 07 13 Inc. Patent receptor modulators
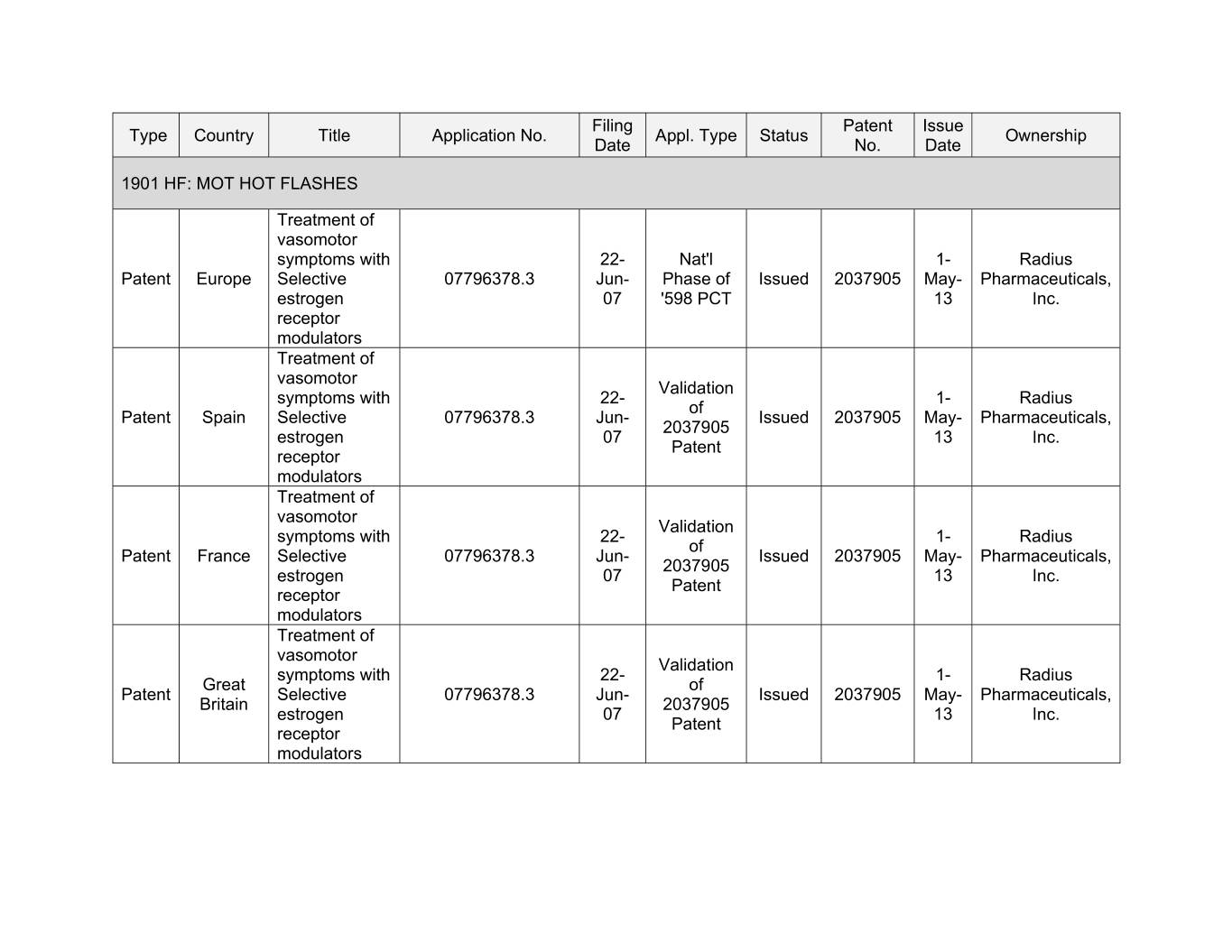
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 HF: MOT HOT FLASHES Treatment of vasomotor symptoms with 22- Nat'l 1- Radius Patent Europe Selective 07796378.3 Jun- Phase of Issued 2037905 May- Pharmaceuticals, estrogen 07 '598 PCT 13 Inc. receptor modulators Treatment of vasomotor Validation symptoms with 22- 1- Radius of Patent Spain Selective 07796378.3 Jun- Issued 2037905 May- Pharmaceuticals, 2037905 estrogen 07 13 Inc. Patent receptor modulators Treatment of vasomotor Validation symptoms with 22- 1- Radius of Patent France Selective 07796378.3 Jun- Issued 2037905 May- Pharmaceuticals, 2037905 estrogen 07 13 Inc. Patent receptor modulators Treatment of vasomotor Validation symptoms with 22- 1- Radius Great of Patent Selective 07796378.3 Jun- Issued 2037905 May- Pharmaceuticals, Britain 2037905 estrogen 07 13 Inc. Patent receptor modulators
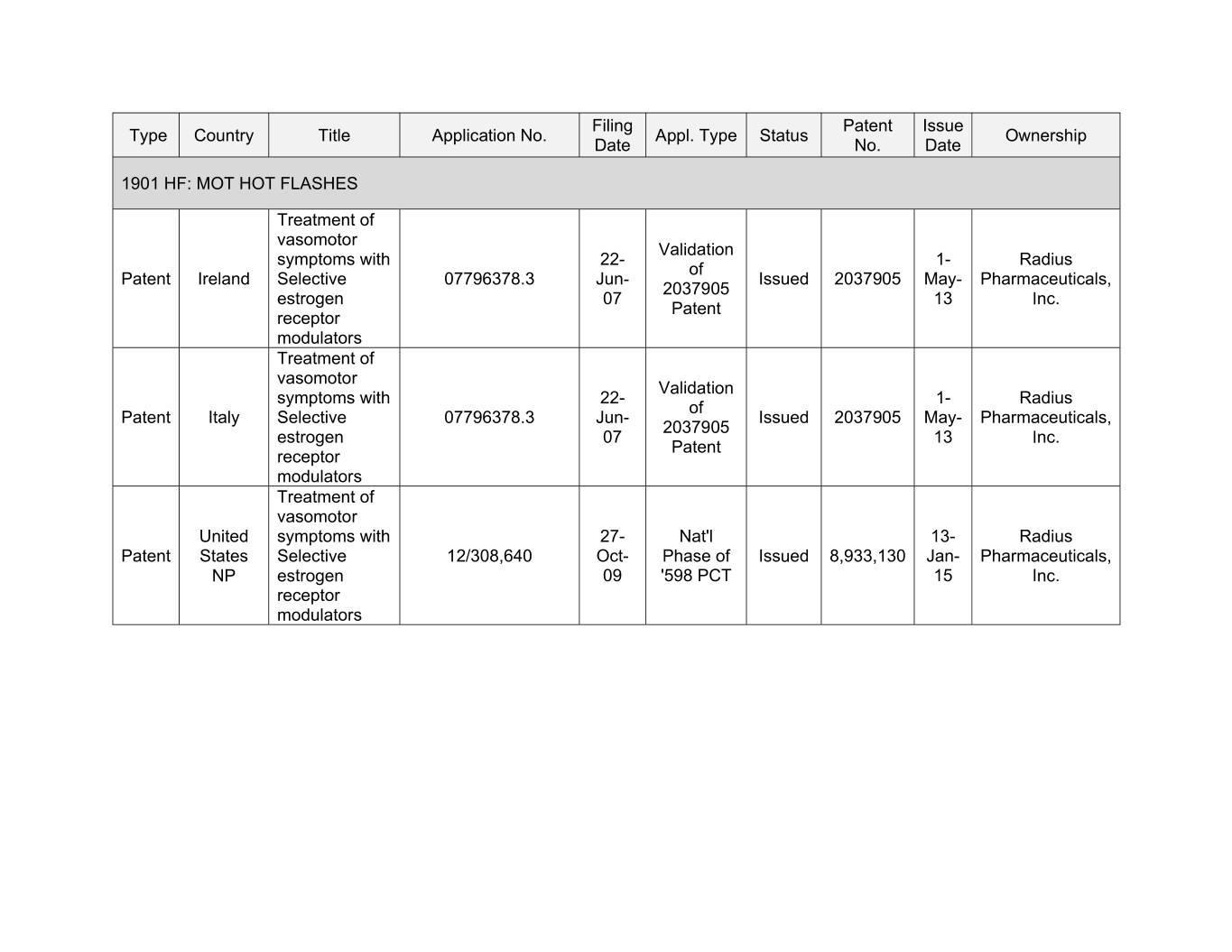
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 HF: MOT HOT FLASHES Treatment of vasomotor Validation symptoms with 22- 1- Radius of Patent Ireland Selective 07796378.3 Jun- Issued 2037905 May- Pharmaceuticals, 2037905 estrogen 07 13 Inc. Patent receptor modulators Treatment of vasomotor Validation symptoms with 22- 1- Radius of Patent Italy Selective 07796378.3 Jun- Issued 2037905 May- Pharmaceuticals, 2037905 estrogen 07 13 Inc. Patent receptor modulators Treatment of vasomotor United symptoms with 27- Nat'l 13- Radius Patent States Selective 12/308,640 Oct- Phase of Issued 8,933,130 Jan- Pharmaceuticals, NP estrogen 09 '598 PCT 15 Inc. receptor modulators
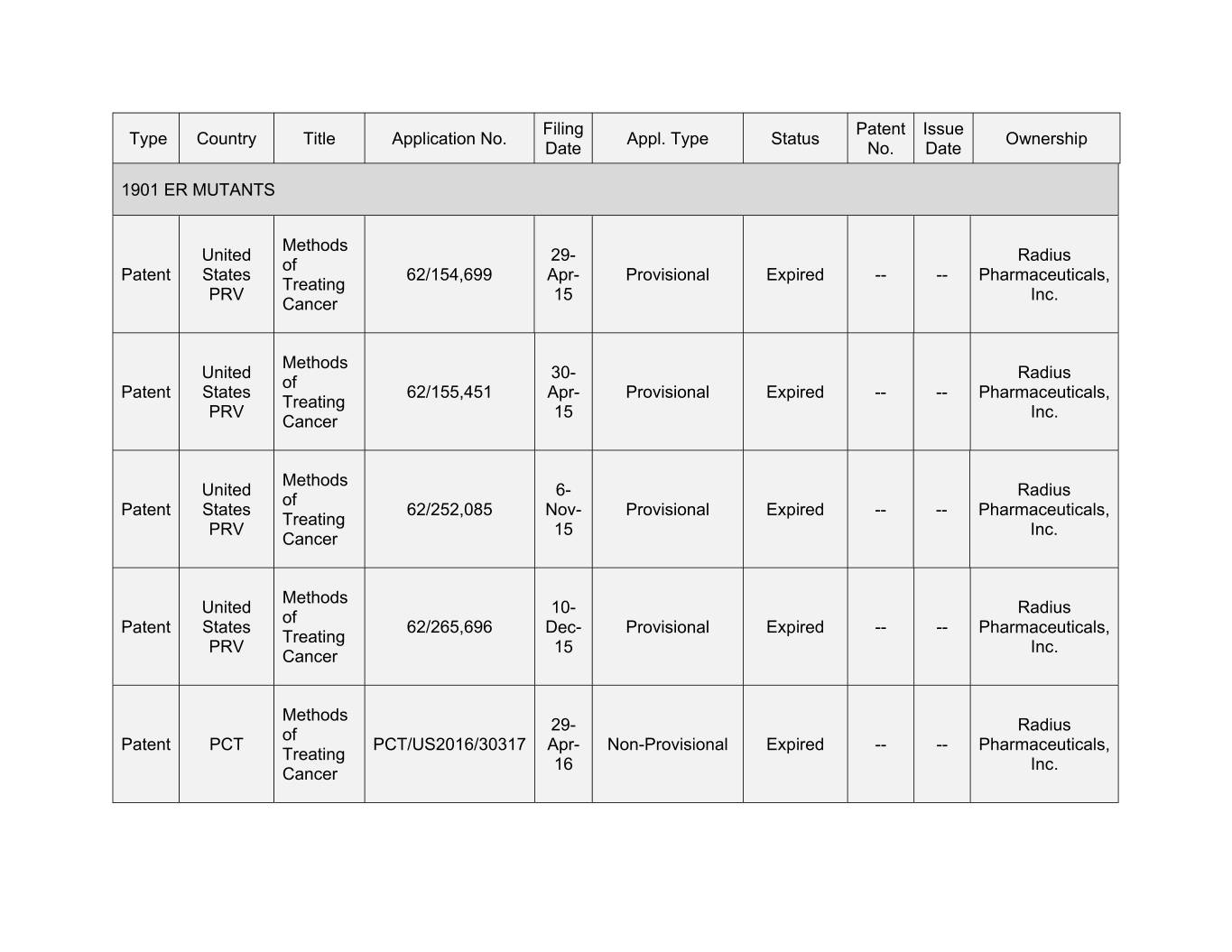
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 ER MUTANTS Methods United 29- Radius of Patent States 62/154,699 Apr- Provisional Expired -- -- Pharmaceuticals, Treating PRV 15 Inc. Cancer Methods United 30- Radius of Patent States 62/155,451 Apr- Provisional Expired -- -- Pharmaceuticals, Treating PRV 15 Inc. Cancer Methods United 6- Radius of Patent States 62/252,085 Nov- Provisional Expired -- -- Pharmaceuticals, Treating PRV 15 Inc. Cancer Methods United 10- Radius of Patent States 62/265,696 Dec- Provisional Expired -- -- Pharmaceuticals, Treating PRV 15 Inc. Cancer Methods 29- Radius of Patent PCT PCT/US2016/30317 Apr- Non-Provisional Expired -- -- Pharmaceuticals, Treating 16 Inc. Cancer
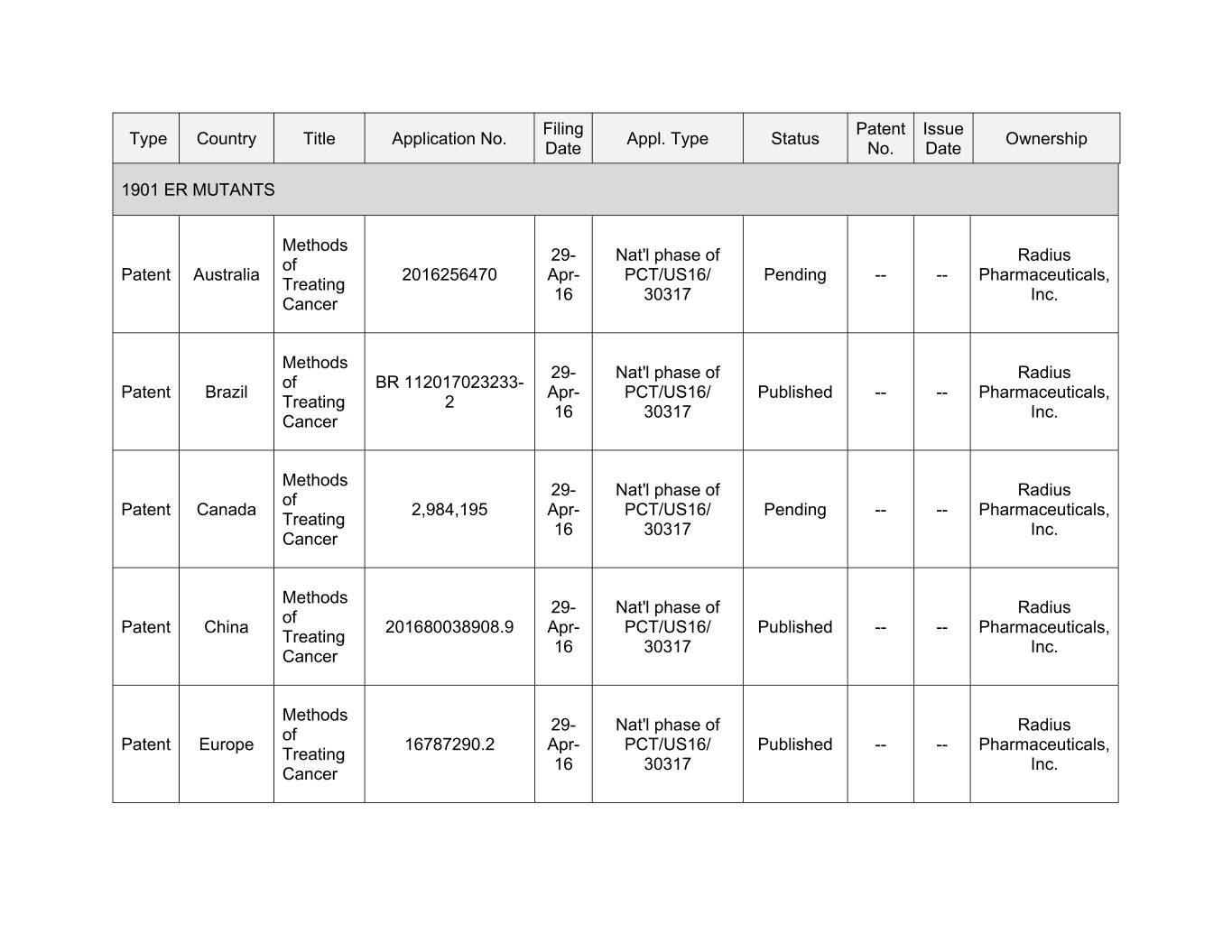
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 ER MUTANTS Methods 29- Nat'l phase of Radius of Patent Australia 2016256470 Apr- PCT/US16/ Pending -- -- Pharmaceuticals, Treating 16 30317 Inc. Cancer Methods 29- Nat'l phase of Radius of BR 112017023233- Patent Brazil Apr- PCT/US16/ Published -- -- Pharmaceuticals, Treating 2 16 30317 Inc. Cancer Methods 29- Nat'l phase of Radius of Patent Canada 2,984,195 Apr- PCT/US16/ Pending -- -- Pharmaceuticals, Treating 16 30317 Inc. Cancer Methods 29- Nat'l phase of Radius of Patent China 201680038908.9 Apr- PCT/US16/ Published -- -- Pharmaceuticals, Treating 16 30317 Inc. Cancer Methods 29- Nat'l phase of Radius of Patent Europe 16787290.2 Apr- PCT/US16/ Published -- -- Pharmaceuticals, Treating 16 30317 Inc. Cancer
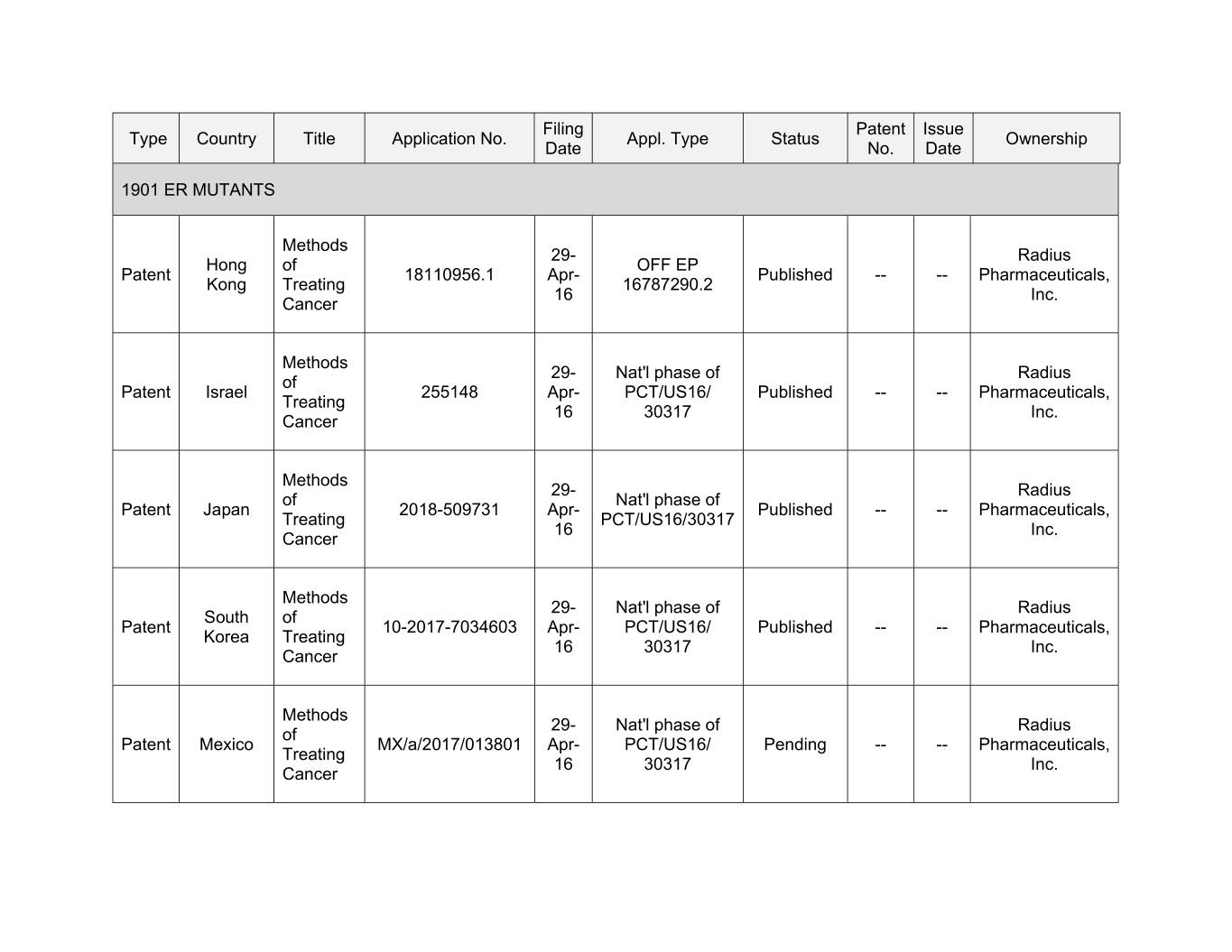
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 ER MUTANTS Methods 29- Radius Hong of OFF EP Patent 18110956.1 Apr- Published -- -- Pharmaceuticals, Kong Treating 16787290.2 16 Inc. Cancer Methods 29- Nat'l phase of Radius of Patent Israel 255148 Apr- PCT/US16/ Published -- -- Pharmaceuticals, Treating 16 30317 Inc. Cancer Methods 00- Xxxxxx xx Xxx'x xxxxx xx Xxxxxx Xxxxx 2018-509731 Apr- Published -- -- Pharmaceuticals, Treating PCT/US16/30317 16 Inc. Cancer Methods 29- Nat'l phase of Radius South of Patent 10-2017-7034603 Apr- PCT/US16/ Published -- -- Pharmaceuticals, Korea Treating 16 30317 Inc. Cancer Methods 29- Nat'l phase of Radius of Patent Mexico MX/a/2017/013801 Apr- PCT/US16/ Pending -- -- Pharmaceuticals, Treating 16 30317 Inc. Cancer
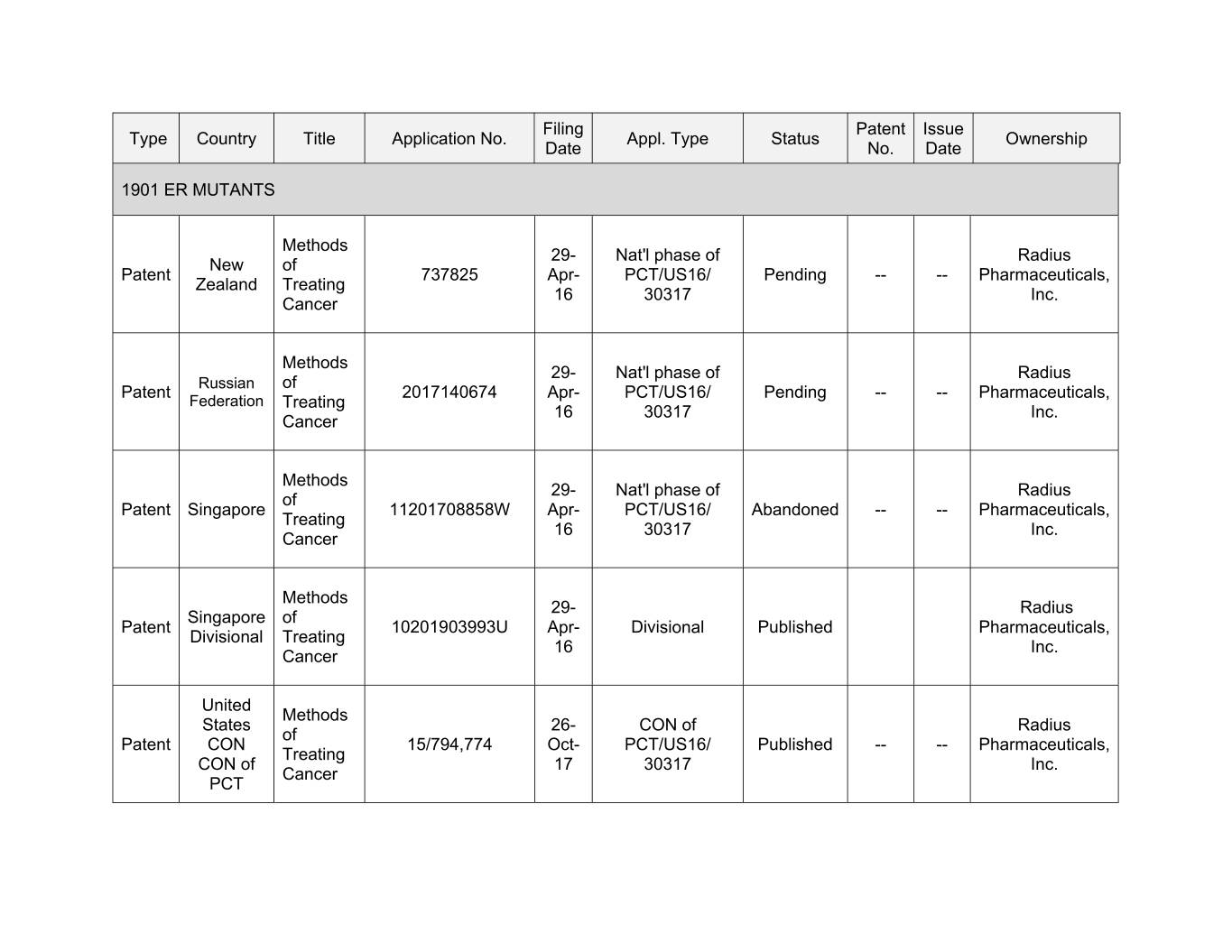
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 ER MUTANTS Methods 29- Nat'l phase of Radius New of Patent 737825 Apr- PCT/US16/ Pending -- -- Pharmaceuticals, Zealand Treating 16 30317 Inc. Cancer Methods 29- Nat'l phase of Radius Russian of Patent 2017140674 Apr- PCT/US16/ Pending -- -- Pharmaceuticals, Federation Treating 16 30317 Inc. Cancer Methods 29- Nat'l phase of Radius of Patent Singapore 11201708858W Apr- PCT/US16/ Abandoned -- -- Pharmaceuticals, Treating 16 30317 Inc. Cancer Methods 00- Xxxxxx Xxxxxxxxx of Patent 10201903993U Apr- Divisional Published Pharmaceuticals, Divisional Treating 16 Inc. Cancer United Methods States 26- CON of Radius of Patent CON 15/794,774 Oct- PCT/US16/ Published -- -- Pharmaceuticals, Treating CON of 17 30317 Inc. Cancer PCT
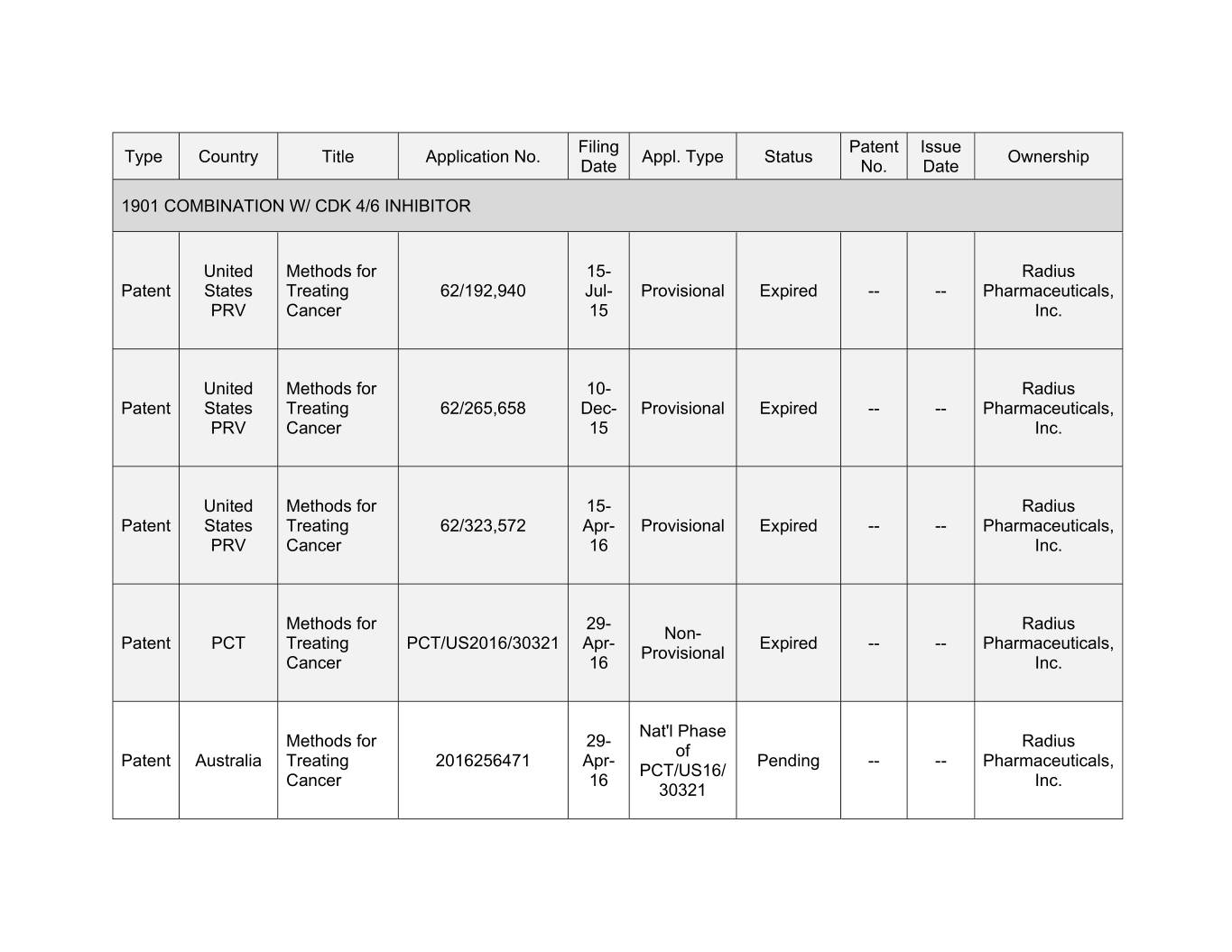
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ CDK 4/6 INHIBITOR United Methods for 15- Radius Patent States Treating 62/192,940 Jul- Provisional Expired -- -- Pharmaceuticals, PRV Cancer 15 Inc. United Methods for 10- Radius Patent States Treating 62/265,658 Dec- Provisional Expired -- -- Pharmaceuticals, PRV Cancer 15 Inc. United Methods for 15- Radius Patent States Treating 62/323,572 Apr- Provisional Expired -- -- Pharmaceuticals, PRV Cancer 16 Inc. Methods for 29- Radius Non- Patent PCT Treating PCT/US2016/30321 Apr- Expired -- -- Pharmaceuticals, Provisional Cancer 16 Inc. Nat'l Phase Methods for 29- Radius of Patent Australia Treating 2016256471 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321
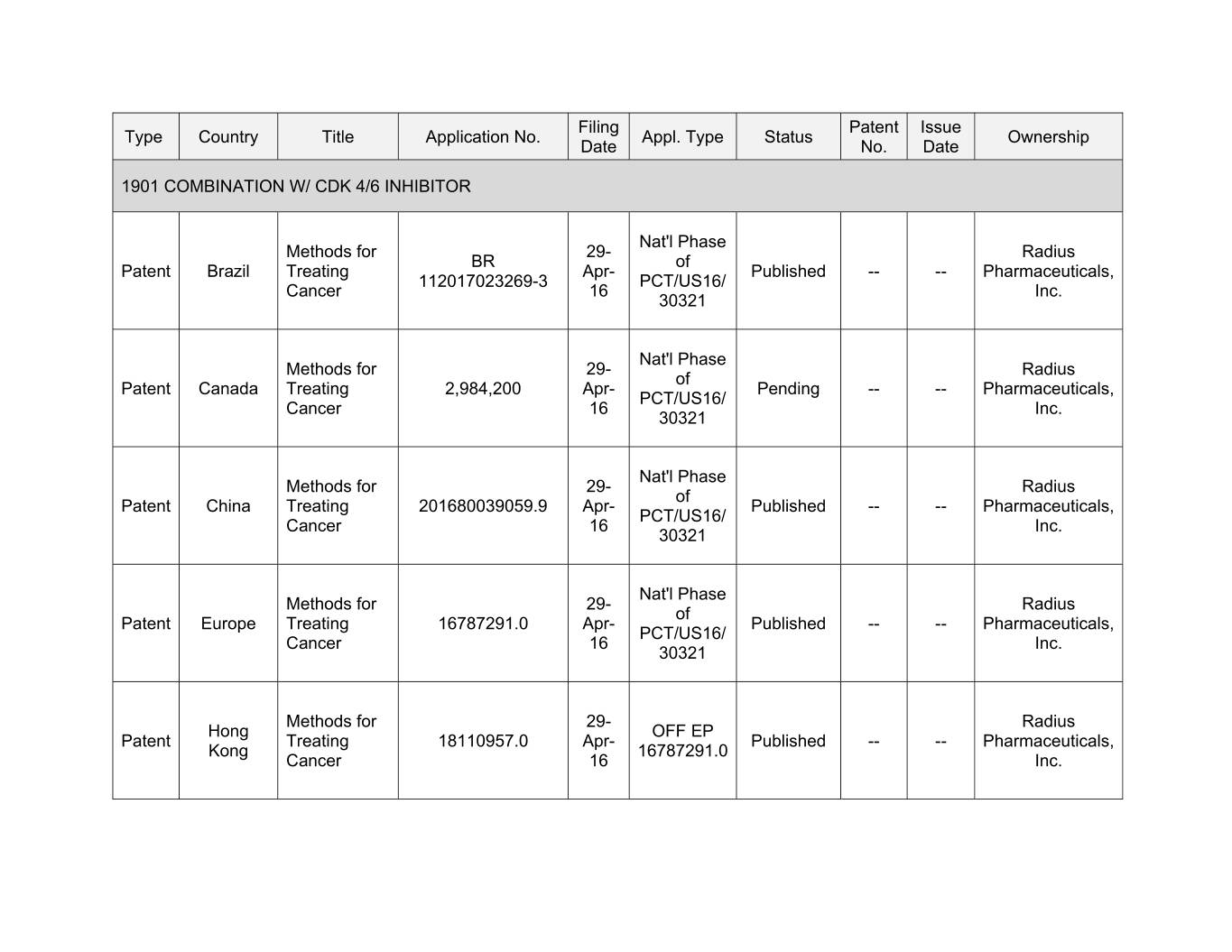
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ CDK 4/6 INHIBITOR Nat'l Phase Methods for 00- Xxxxxx XX xx Xxxxxx Xxxxxx Treating Apr- Published -- -- Pharmaceuticals, 112017023269-3 PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 00- Xxxxxx xx Xxxxxx Xxxxxx Treating 2,984,200 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 29- Radius of Patent China Treating 201680039059.9 Apr- Published -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 29- Radius of Patent Europe Treating 16787291.0 Apr- Published -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321 Methods for 29- Radius Hong OFF EP Patent Treating 18110957.0 Apr- Published -- -- Pharmaceuticals, Kong 16787291.0 Cancer 16 Inc.
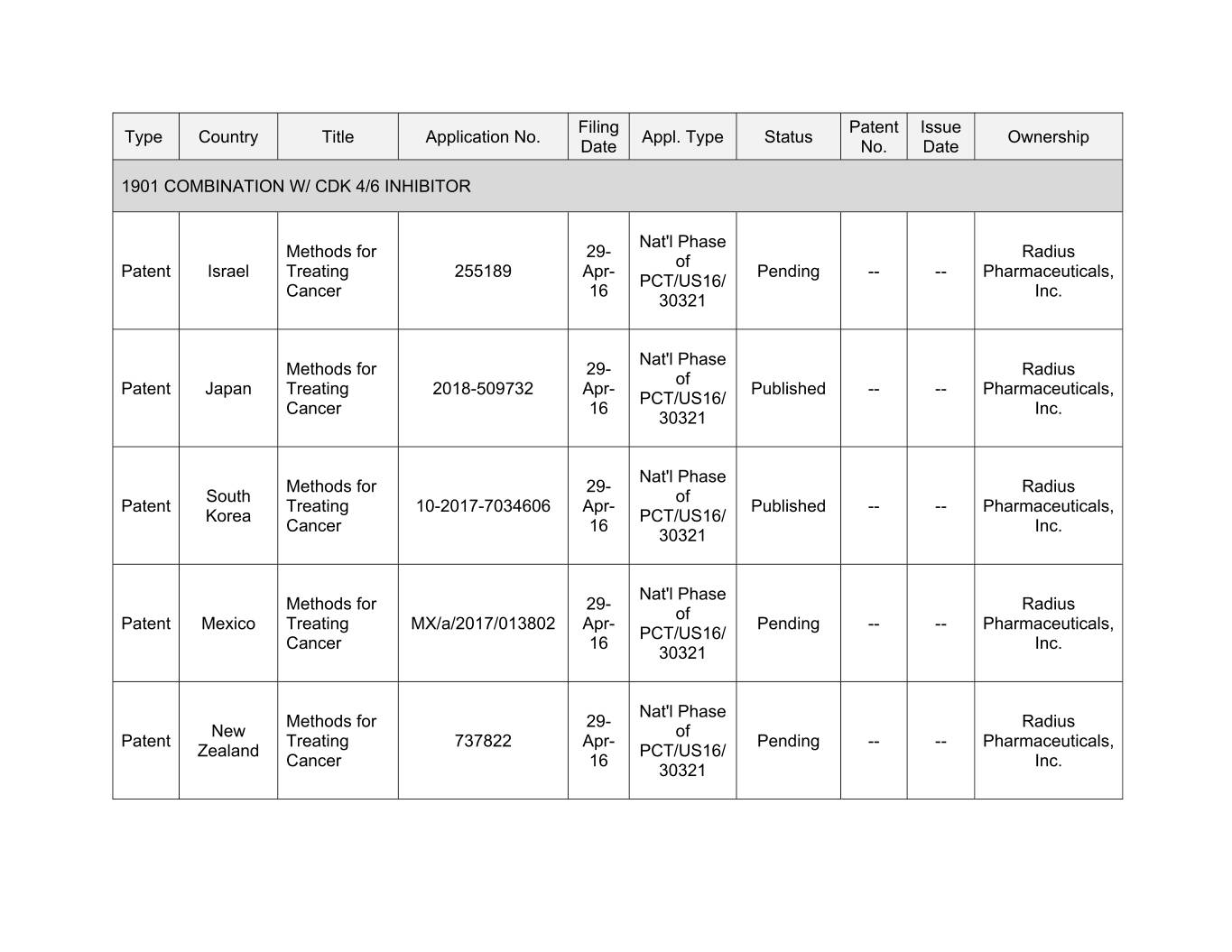
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ CDK 4/6 INHIBITOR Nat'l Phase Methods for 29- Radius of Patent Israel Treating 255189 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 29- Radius of Patent Japan Treating 2018-509732 Apr- Published -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 00- Xxxxxx Xxxxx xx Xxxxxx Treating 10-2017-7034606 Apr- Published -- -- Pharmaceuticals, Korea PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 29- Radius of Patent Mexico Treating MX/a/2017/013802 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 29- Radius New of Patent Treating 737822 Apr- Pending -- -- Pharmaceuticals, Zealand PCT/US16/ Cancer 16 Inc. 30321
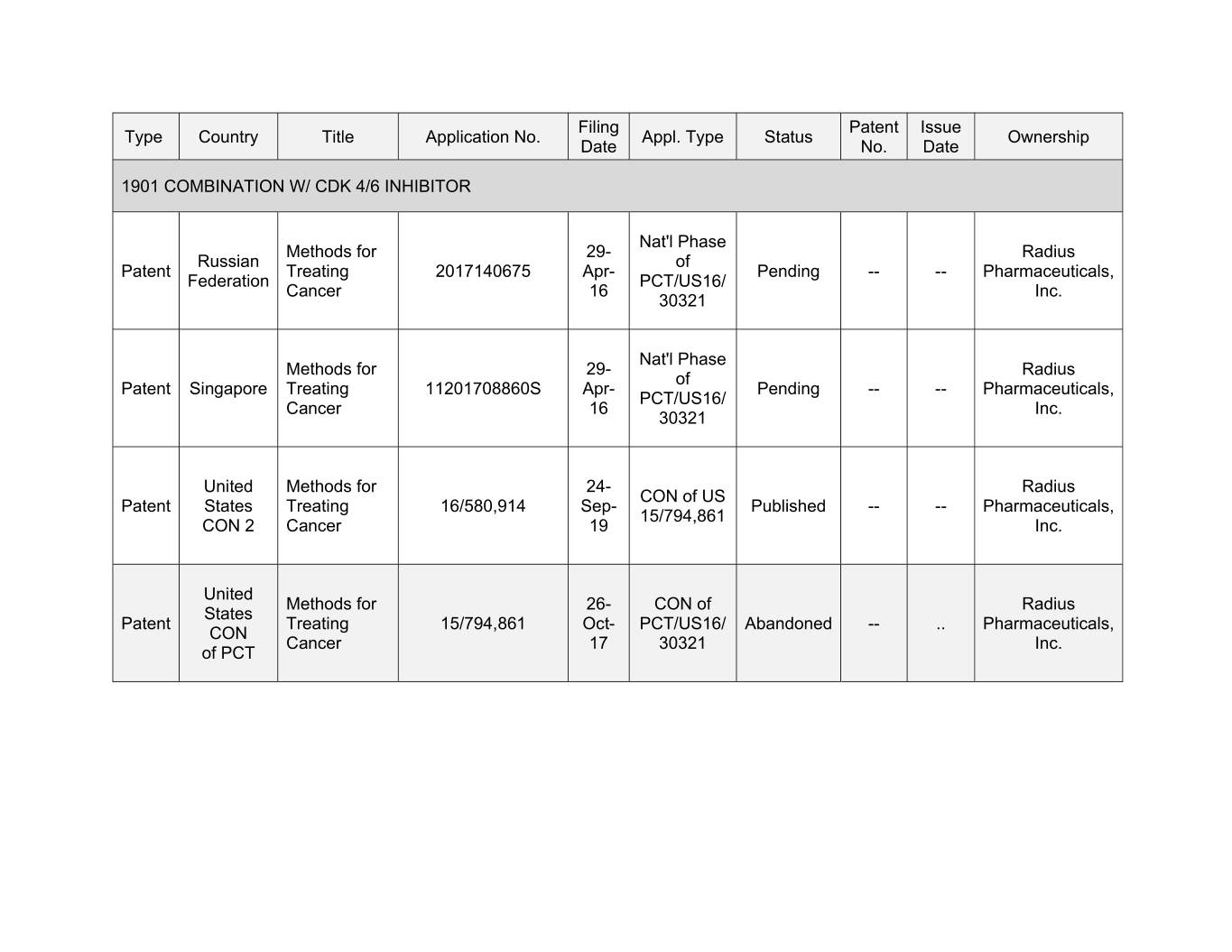
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ CDK 4/6 INHIBITOR Nat'l Phase Methods for 00- Xxxxxx Xxxxxxx of Patent Treating 2017140675 Apr- Pending -- -- Pharmaceuticals, Federation PCT/US16/ Cancer 16 Inc. 30321 Nat'l Phase Methods for 00- Xxxxxx xx Xxxxxx Xxxxxxxxx Treating 11201708860S Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30321 United Methods for 00- Xxxxxx XXX xx XX Xxxxxx Xxxxxx Treating 16/580,914 Sep- Published -- -- Pharmaceuticals, 15/794,861 CON 2 Cancer 19 Inc. United Methods for 00- XXX xx Xxxxxx Xxxxxx Patent Treating 15/794,861 Oct- PCT/US16/ Abandoned -- .. Pharmaceuticals, CON Cancer 17 30321 Inc. of PCT
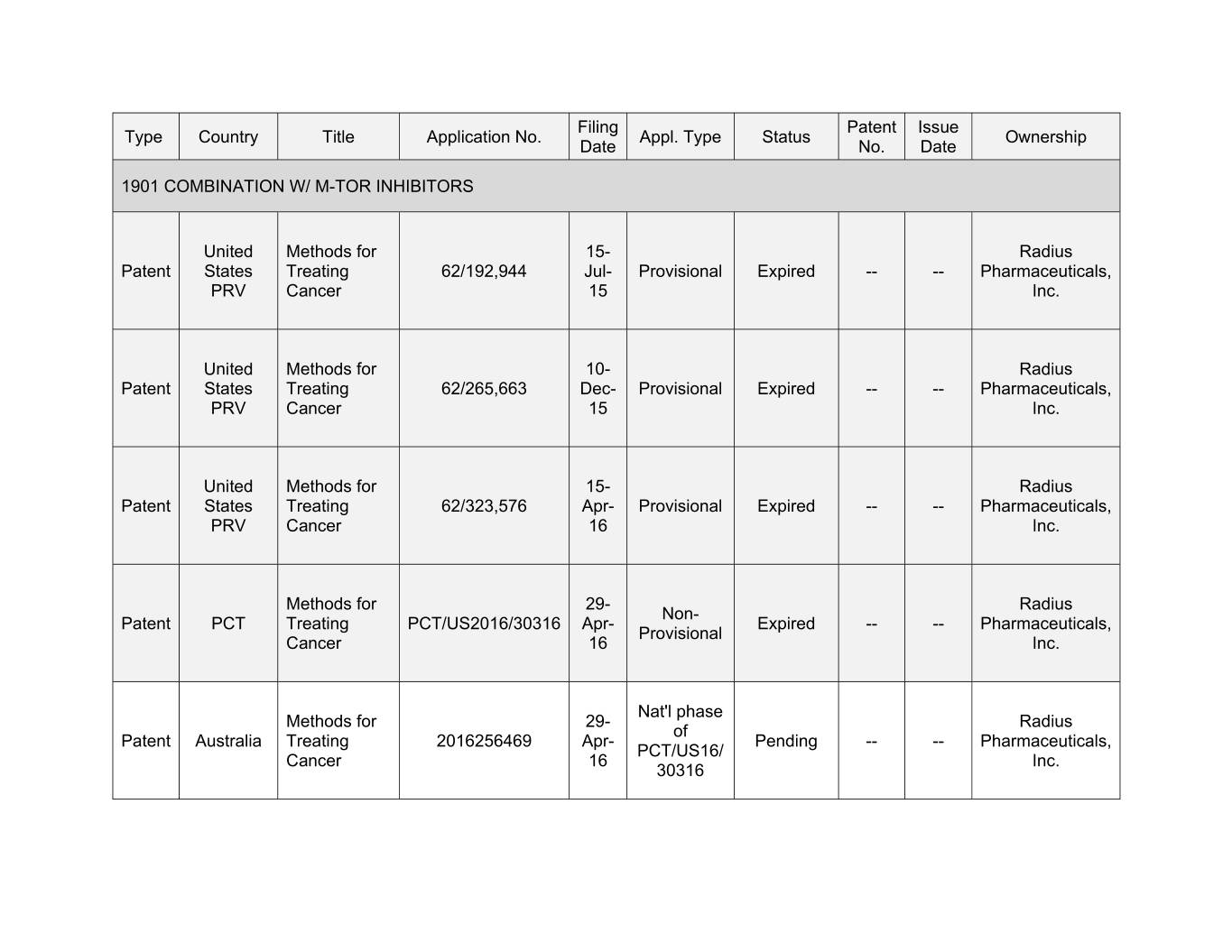
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ M-TOR INHIBITORS United Methods for 15- Radius Patent States Treating 62/192,944 Jul- Provisional Expired -- -- Pharmaceuticals, PRV Cancer 15 Inc. United Methods for 10- Radius Patent States Treating 62/265,663 Dec- Provisional Expired -- -- Pharmaceuticals, PRV Cancer 15 Inc. United Methods for 15- Radius Patent States Treating 62/323,576 Apr- Provisional Expired -- -- Pharmaceuticals, PRV Cancer 16 Inc. Methods for 29- Radius Non- Patent PCT Treating PCT/US2016/30316 Apr- Expired -- -- Pharmaceuticals, Provisional Cancer 16 Inc. Nat'l phase Methods for 29- Radius of Patent Australia Treating 2016256469 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316
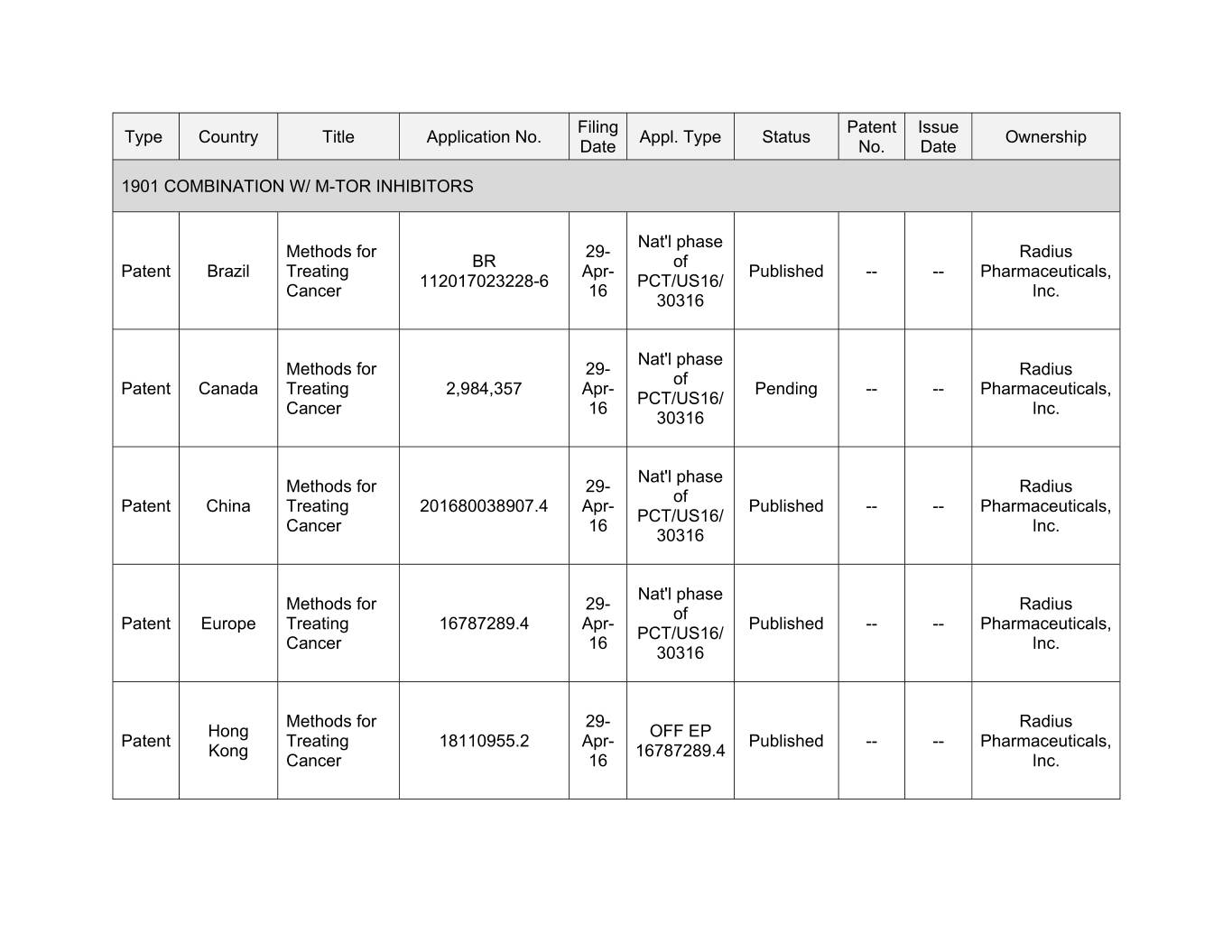
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ M-TOR INHIBITORS Nat'l phase Methods for 00- Xxxxxx XX xx Xxxxxx Xxxxxx Treating Apr- Published -- -- Pharmaceuticals, 112017023228-6 PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 00- Xxxxxx xx Xxxxxx Xxxxxx Treating 2,984,357 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 29- Radius of Patent China Treating 201680038907.4 Apr- Published -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 29- Radius of Patent Europe Treating 16787289.4 Apr- Published -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316 Methods for 29- Radius Hong OFF EP Patent Treating 18110955.2 Apr- Published -- -- Pharmaceuticals, Kong 16787289.4 Cancer 16 Inc.
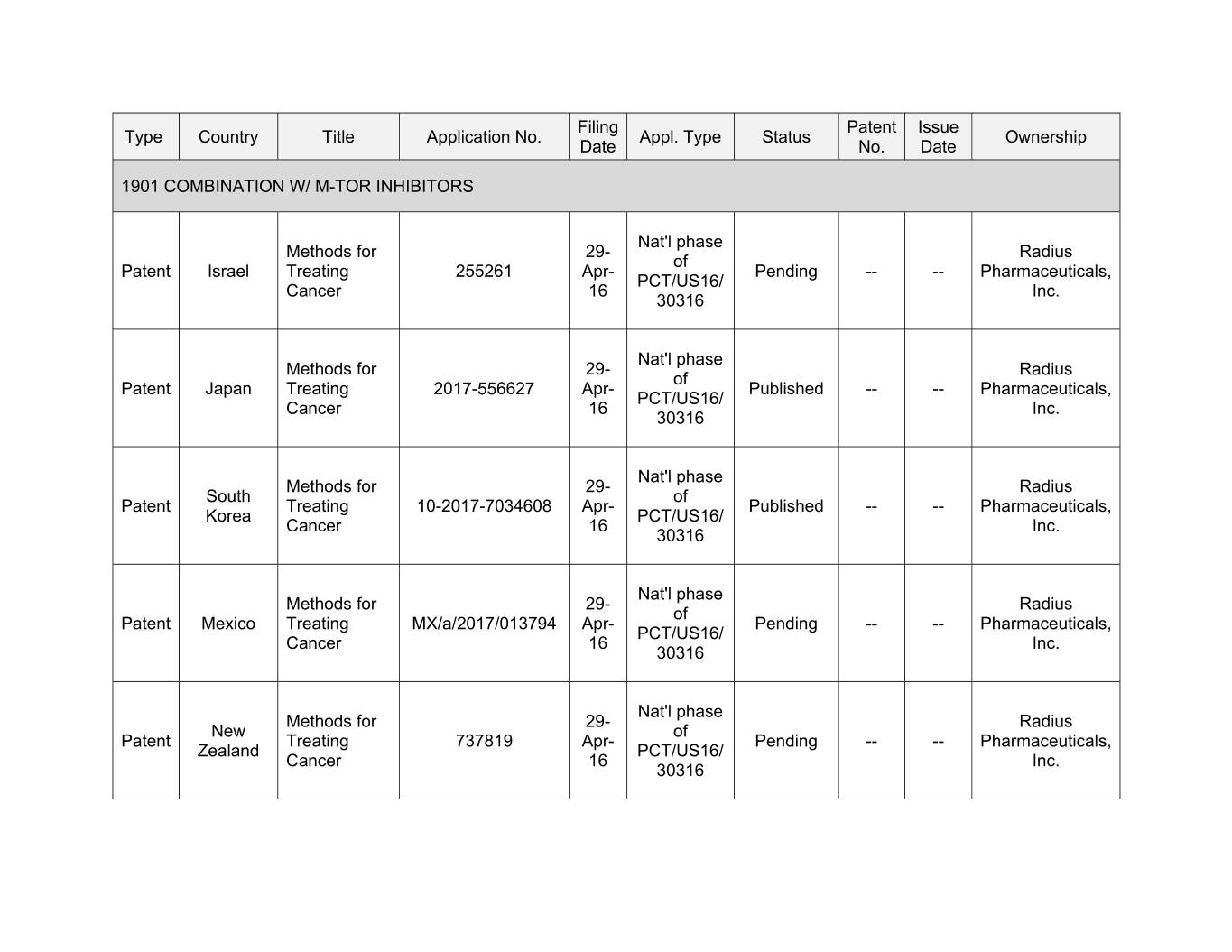
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ M-TOR INHIBITORS Nat'l phase Methods for 29- Radius of Patent Israel Treating 255261 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 29- Radius of Patent Japan Treating 2017-556627 Apr- Published -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 00- Xxxxxx Xxxxx xx Xxxxxx Treating 10-2017-7034608 Apr- Published -- -- Pharmaceuticals, Korea PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 29- Radius of Patent Mexico Treating MX/a/2017/013794 Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 29- Radius New of Patent Treating 737819 Apr- Pending -- -- Pharmaceuticals, Zealand PCT/US16/ Cancer 16 Inc. 30316
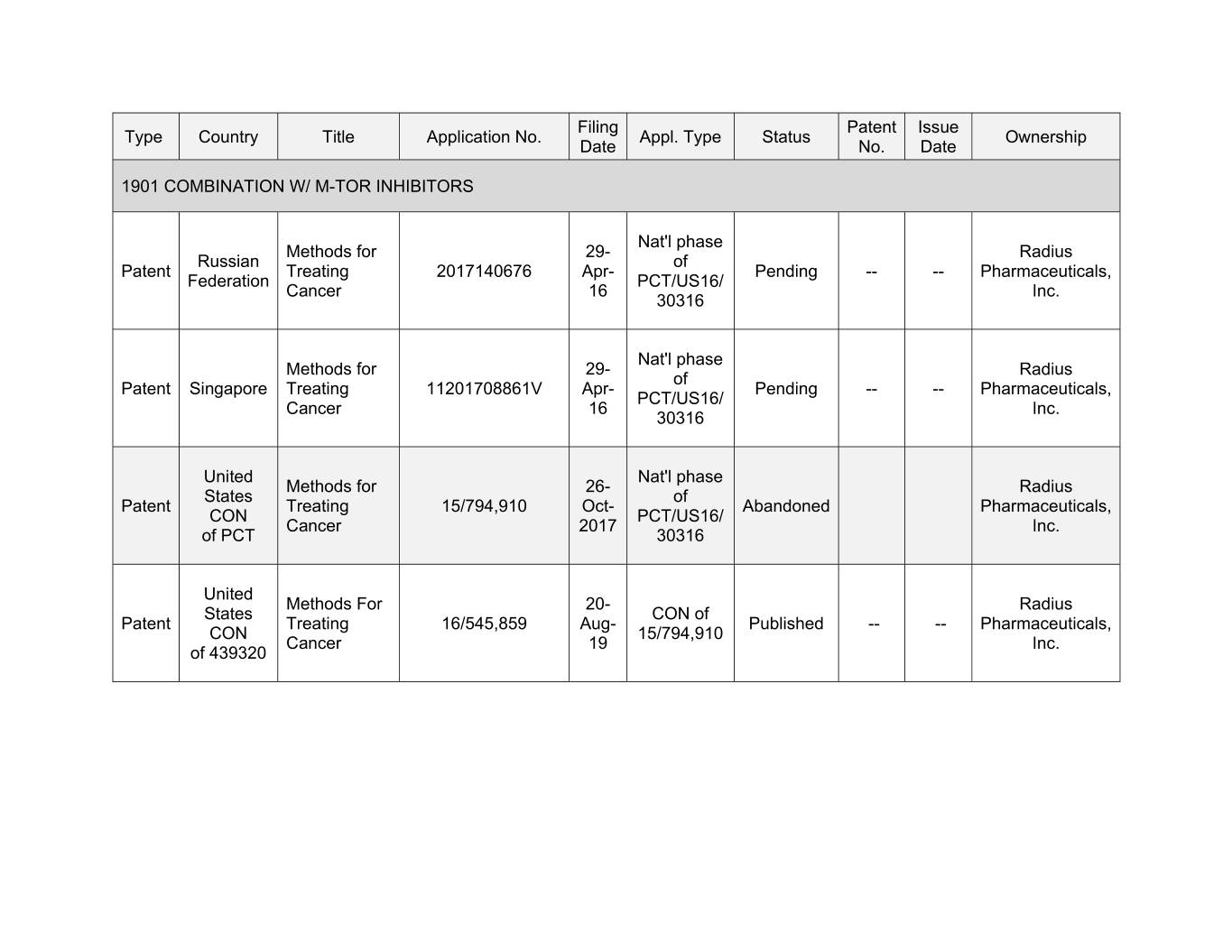
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 COMBINATION W/ M-TOR INHIBITORS Nat'l phase Methods for 29- Radius Russian of Patent Treating 0000000000 Apr- Pending -- -- Pharmaceuticals, Federation PCT/US16/ Cancer 16 Inc. 30316 Nat'l phase Methods for 00- Xxxxxx xx Xxxxxx Xxxxxxxxx Treating 11201708861V Apr- Pending -- -- Pharmaceuticals, PCT/US16/ Cancer 16 Inc. 30316 United Nat'l phase Methods for 26- Radius States of Patent Treating 15/794,910 Oct- Abandoned Pharmaceuticals, CON PCT/US16/ Cancer 2017 Inc. of PCT 30316 United Methods For 20- Radius States CON of Patent Treating 16/545,859 Aug- Published -- -- Pharmaceuticals, CON 15/794,910 Cancer 19 Inc. of 439320
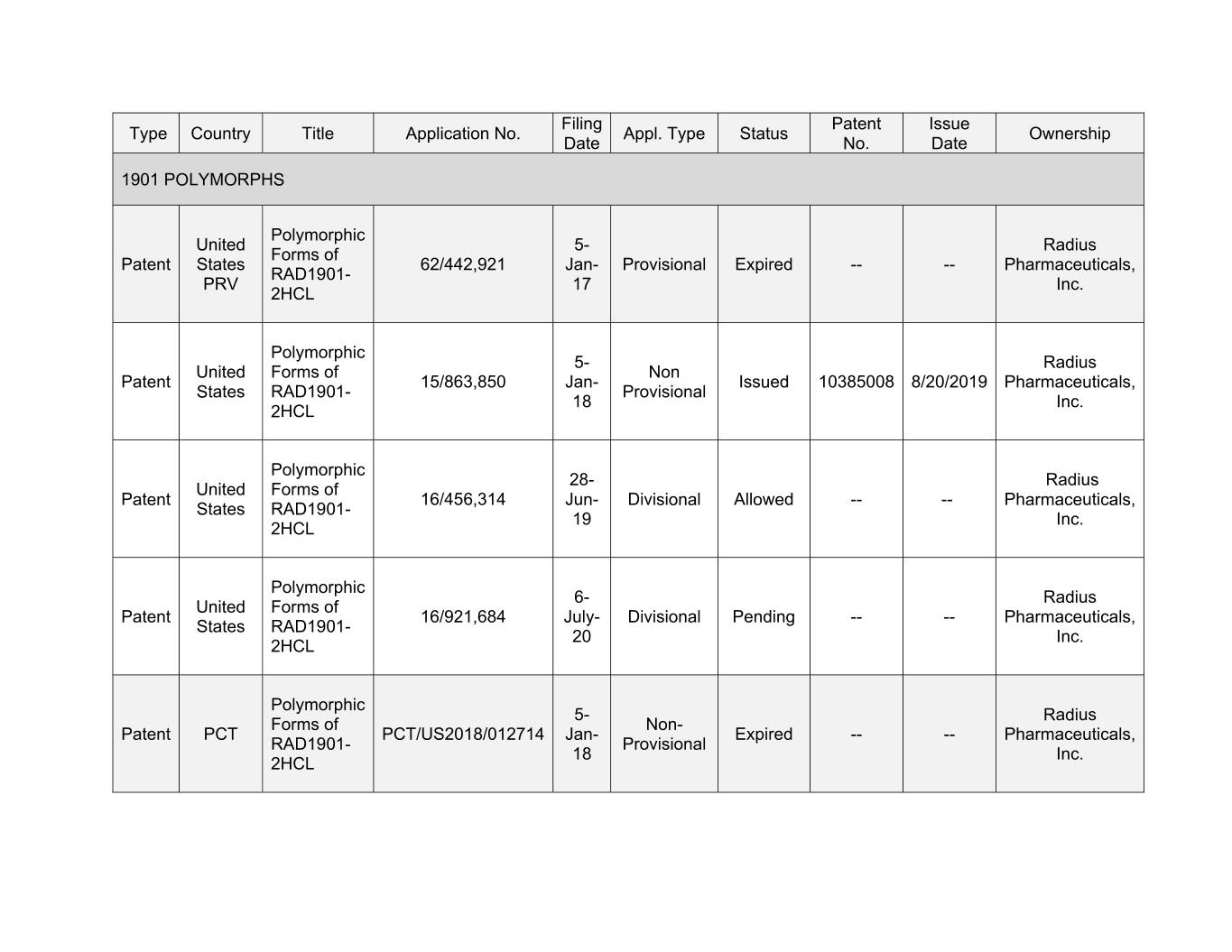
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 POLYMORPHS Polymorphic United 5- Radius Forms of Patent States 62/442,921 Jan- Provisional Expired -- -- Pharmaceuticals, RAD1901- PRV 17 Inc. 2HCL Polymorphic 5- Radius United Forms of Non Patent 15/863,850 Jan- Issued 10385008 8/20/2019 Pharmaceuticals, States RAD1901- Provisional 18 Inc. 2HCL Polymorphic 28- Radius United Forms of Patent 16/456,314 Jun- Divisional Allowed -- -- Pharmaceuticals, States RAD1901- 19 Inc. 2HCL Polymorphic 6- Radius United Forms of Patent 16/921,684 July- Divisional Pending -- -- Pharmaceuticals, States RAD1901- 20 Inc. 2HCL Polymorphic 5- Radius Forms of Non- Patent PCT PCT/US2018/012714 Jan- Expired -- -- Pharmaceuticals, RAD1901- Provisional 18 Inc. 2HCL
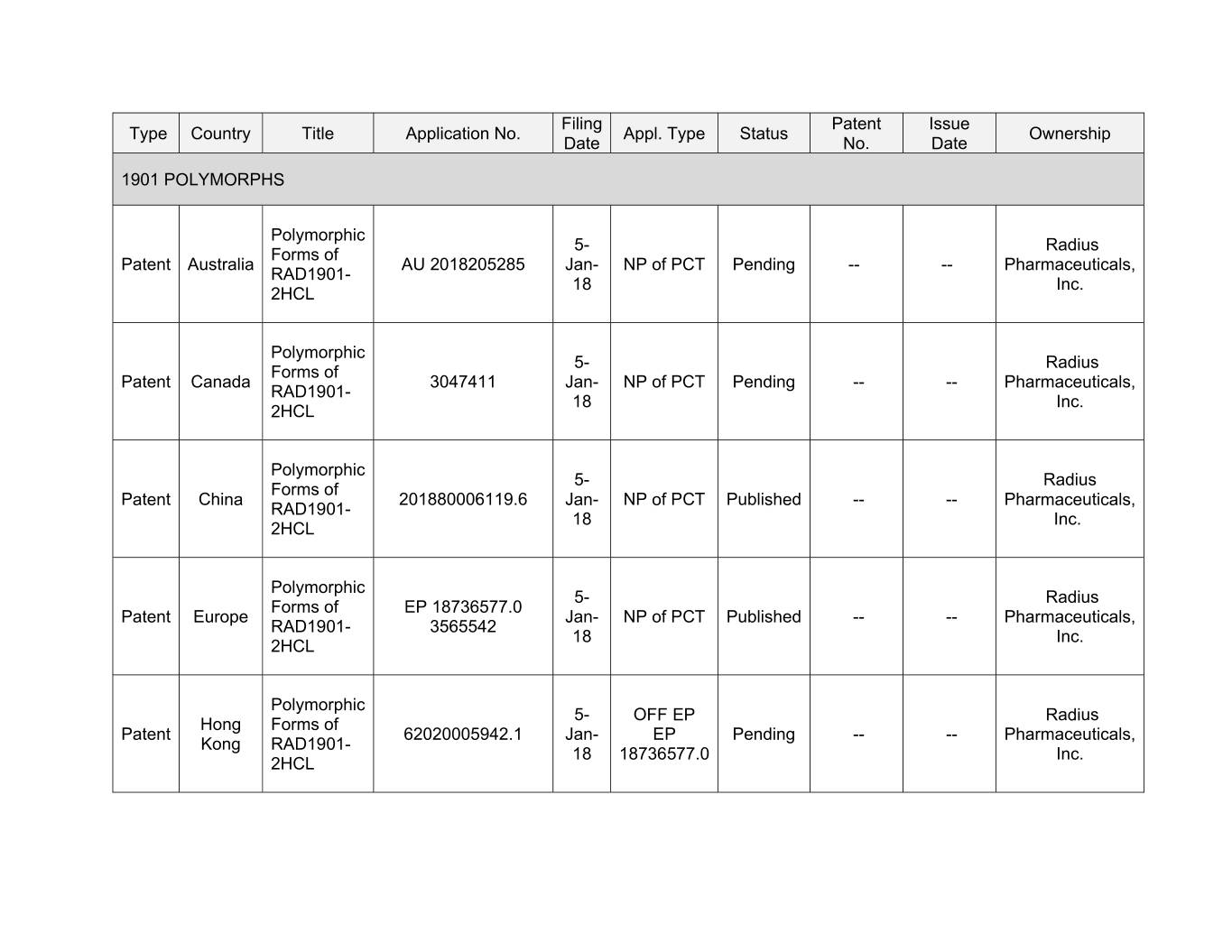
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 POLYMORPHS Polymorphic 5- Radius Forms of Patent Australia AU 2018205285 Jan- NP of PCT Pending -- -- Pharmaceuticals, RAD1901- 18 Inc. 2HCL Polymorphic 5- Radius Forms of Patent Canada 3047411 Jan- NP of PCT Pending -- -- Pharmaceuticals, RAD1901- 18 Inc. 2HCL Polymorphic 5- Radius Forms of Patent China 201880006119.6 Jan- NP of PCT Published -- -- Pharmaceuticals, RAD1901- 18 Inc. 2HCL Polymorphic 5- Radius Forms of EP 18736577.0 Patent Europe Jan- NP of PCT Published -- -- Pharmaceuticals, RAD1901- 3565542 18 Inc. 2HCL Polymorphic 5- OFF EP Radius Hong Forms of Patent 62020005942.1 Jan- EP Pending -- -- Pharmaceuticals, Kong RAD1901- 18 18736577.0 Inc. 2HCL
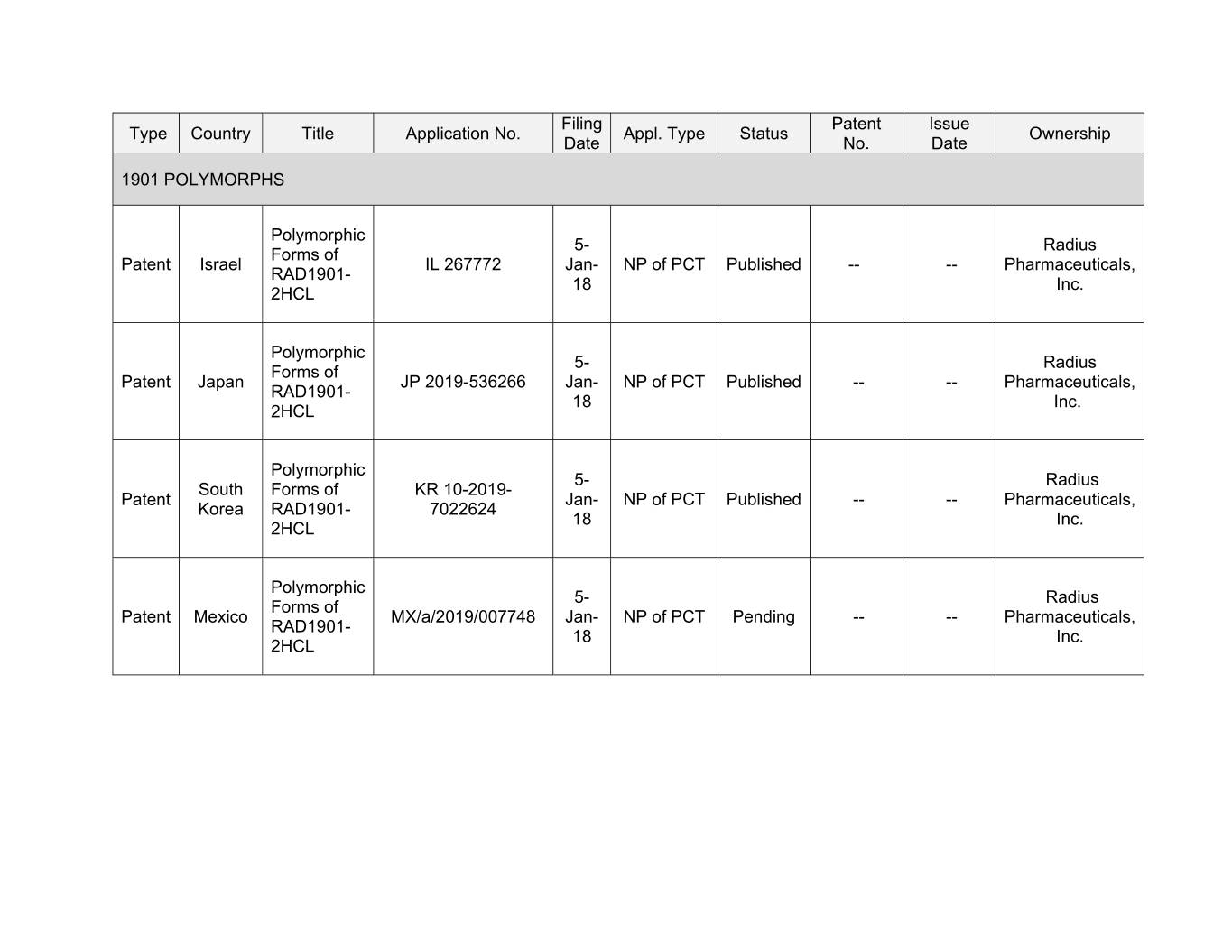
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 POLYMORPHS Polymorphic 5- Radius Forms of Patent Xxxxxx XX 000000 Jan- NP of PCT Published -- -- Pharmaceuticals, RAD1901- 18 Inc. 2HCL Polymorphic 5- Radius Forms of Patent Japan JP 2019-536266 Jan- NP of PCT Published -- -- Pharmaceuticals, RAD1901- 18 Inc. 2HCL Polymorphic 5- Radius South Forms of KR 10-2019- Patent Jan- NP of PCT Published -- -- Pharmaceuticals, Korea RAD1901- 7022624 18 Inc. 2HCL Polymorphic 5- Radius Forms of Patent Mexico MX/a/2019/007748 Jan- NP of PCT Pending -- -- Pharmaceuticals, RAD1901- 18 Inc. 2HCL
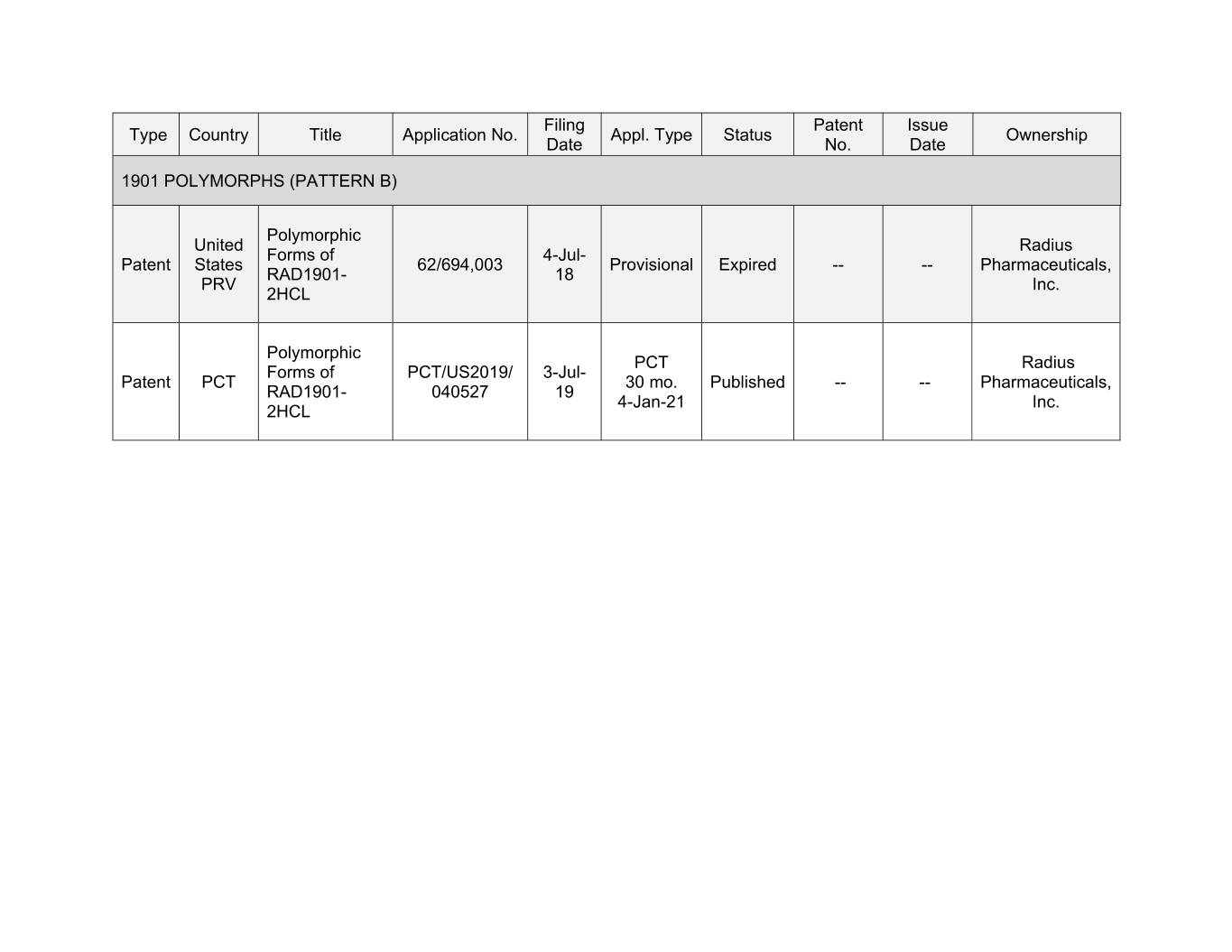
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 POLYMORPHS (PATTERN B) Polymorphic United Radius Forms of 4-Jul- Patent States 62/694,003 Provisional Expired -- -- Pharmaceuticals, RAD1901- 18 PRV Inc. 2HCL Polymorphic PCT Radius Forms of PCT/US2019/ 3-Jul- Patent PCT 30 mo. Published -- -- Pharmaceuticals, RAD1901- 040527 19 4-Jan-21 Inc. 2HCL
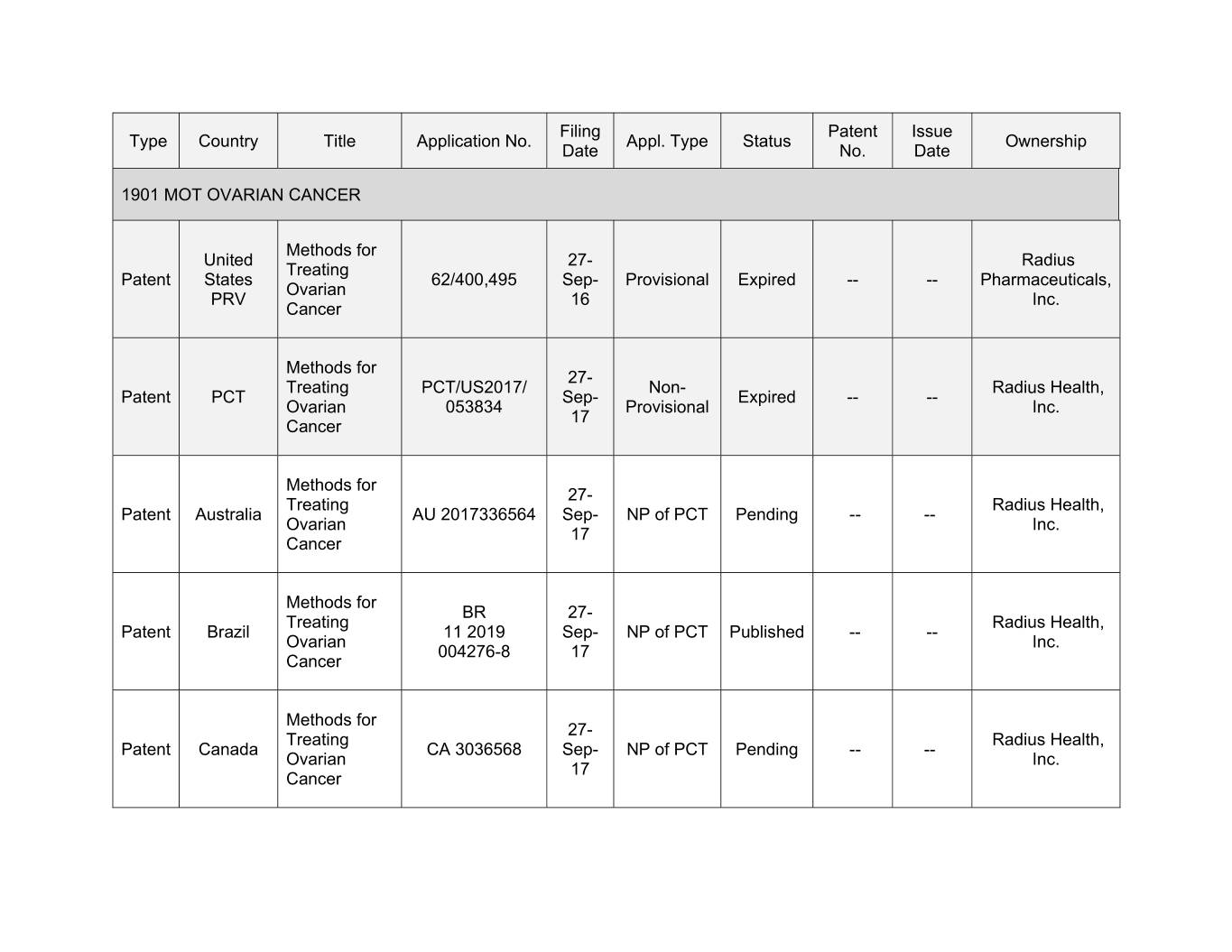
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 MOT OVARIAN CANCER Methods for United 27- Radius Treating Patent States 62/400,495 Sep- Provisional Expired -- -- Pharmaceuticals, Ovarian PRV 16 Inc. Cancer Methods for 27- Treating PCT/US2017/ Non- Radius Health, Patent PCT Sep- Expired -- -- Ovarian 053834 Provisional Inc. 17 Cancer Methods for 27- Treating Radius Health, Patent Australia AU 0000000000 Sep- NP of PCT Pending -- -- Ovarian Inc. 17 Cancer Methods for BR 27- Treating Radius Health, Patent Brazil 11 2019 Sep- NP of PCT Published -- -- Ovarian Inc. 004276-8 17 Cancer Methods for 27- Treating Radius Health, Patent Canada CA 3036568 Sep- NP of PCT Pending -- -- Ovarian Inc. 17 Cancer
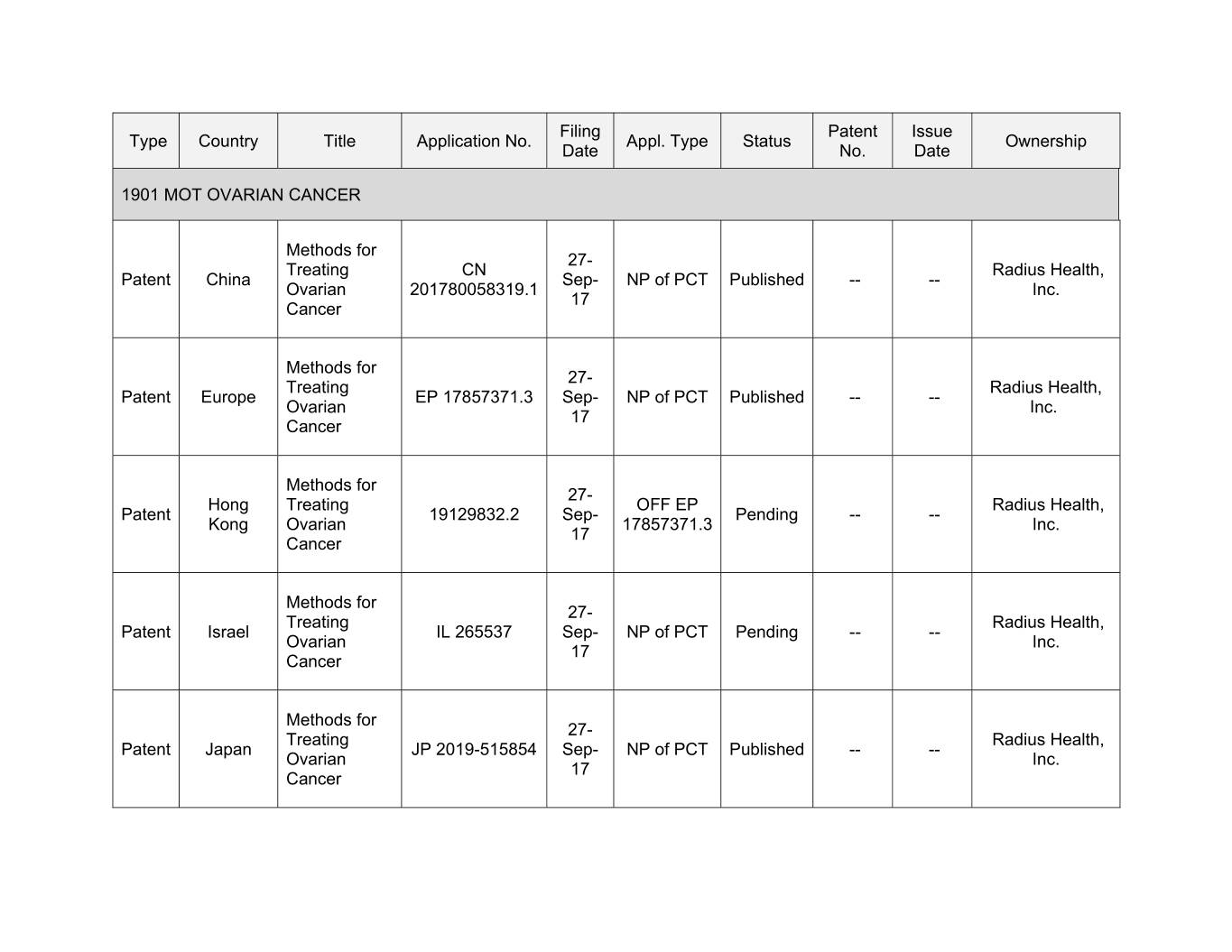
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 MOT OVARIAN CANCER Methods for 27- Treating CN Radius Health, Patent China Sep- NP of PCT Published -- -- Ovarian 201780058319.1 Inc. 17 Cancer Methods for 27- Treating Radius Health, Patent Europe EP 17857371.3 Sep- NP of PCT Published -- -- Ovarian Inc. 17 Cancer Methods for 27- Hong Treating OFF EP Radius Health, Patent 19129832.2 Sep- Pending -- -- Kong Ovarian 17857371.3 Inc. 17 Cancer Methods for 27- Treating Radius Health, Patent Xxxxxx XX 000000 Sep- NP of PCT Pending -- -- Ovarian Inc. 17 Cancer Methods for 27- Treating Radius Health, Patent Japan JP 2019-515854 Sep- NP of PCT Published -- -- Ovarian Inc. 17 Cancer
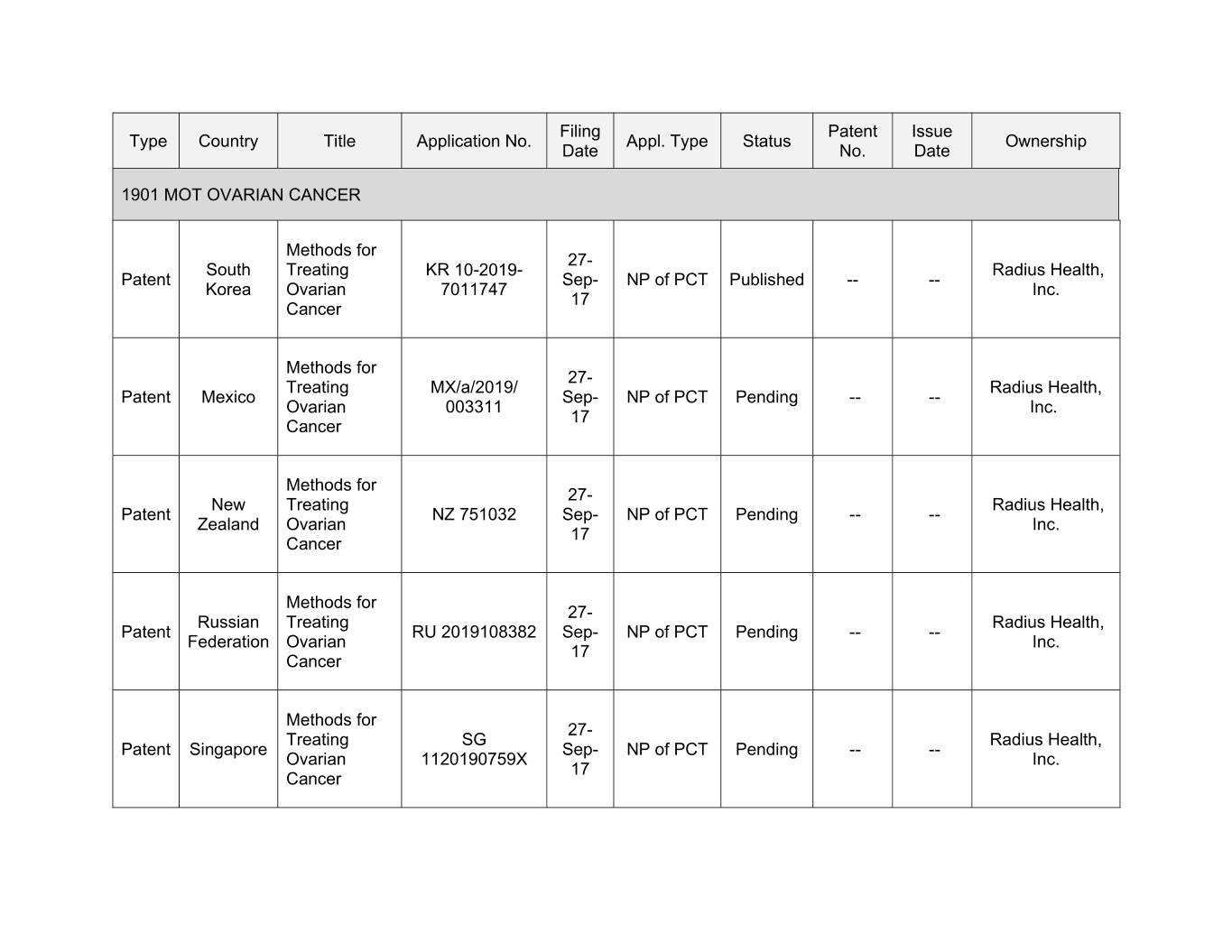
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 MOT OVARIAN CANCER Methods for 27- South Treating KR 10-2019- Radius Health, Patent Sep- NP of PCT Published -- -- Korea Ovarian 7011747 Inc. 17 Cancer Methods for 27- Treating MX/a/2019/ Radius Health, Patent Mexico Sep- NP of PCT Pending -- -- Ovarian 003311 Inc. 17 Cancer Methods for 27- New Treating Radius Health, Patent NZ 751032 Sep- NP of PCT Pending -- -- Zealand Ovarian Inc. 17 Cancer Methods for 27- Russian Treating Radius Health, Patent RU 0000000000 Sep- NP of PCT Pending -- -- Federation Ovarian Inc. 17 Cancer Methods for 27- Treating SG Radius Health, Patent Singapore Sep- NP of PCT Pending -- -- Ovarian 1120190759X Inc. 17 Cancer
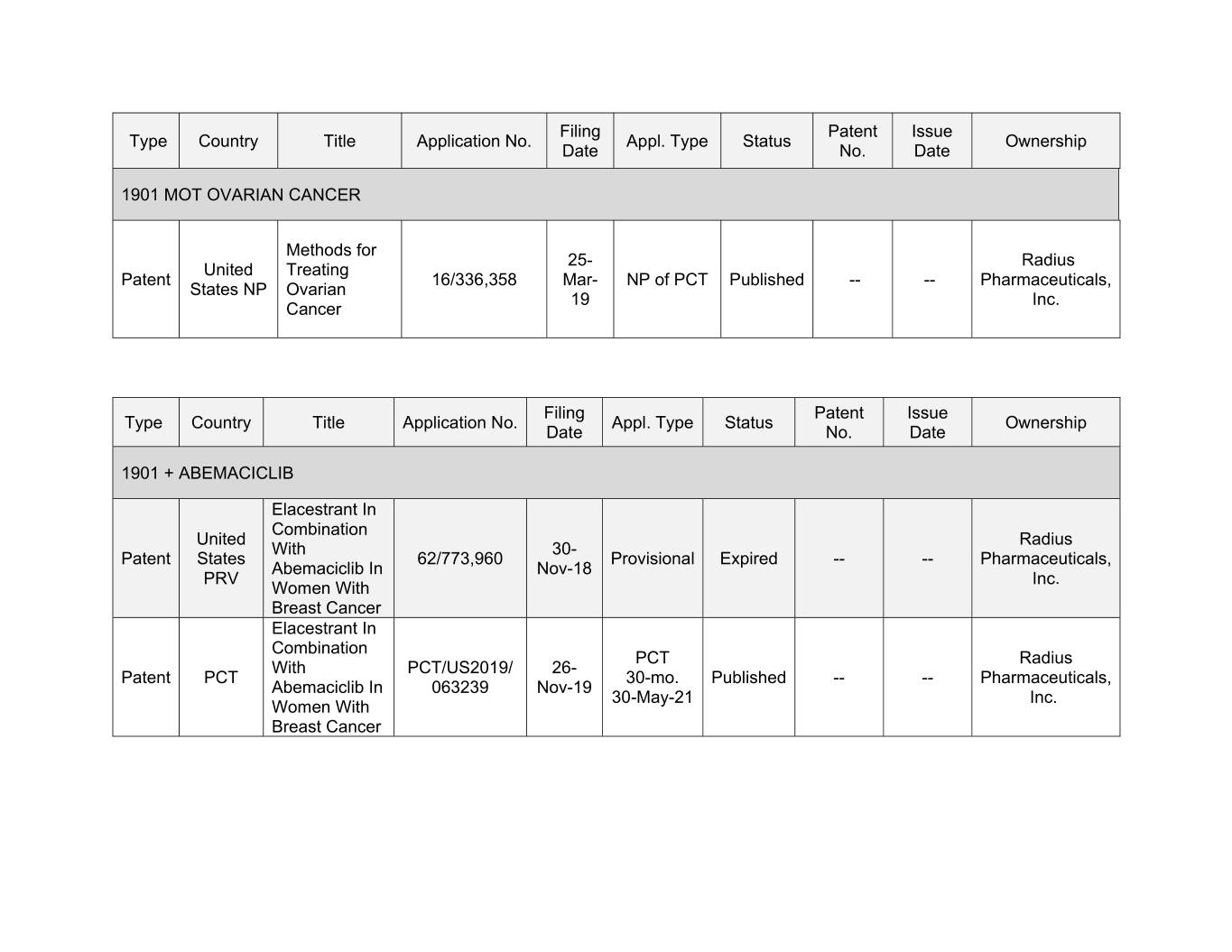
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 MOT OVARIAN CANCER Methods for 25- Radius United Treating Patent 16/336,358 Mar- NP of PCT Published -- -- Pharmaceuticals, States NP Ovarian 19 Inc. Cancer Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 + ABEMACICLIB Elacestrant In Combination United Radius With 30- Patent States 62/773,960 Provisional Expired -- -- Pharmaceuticals, Abemaciclib In Nov-18 PRV Inc. Women With Breast Cancer Elacestrant In Combination PCT Radius With PCT/US2019/ 26- Patent PCT 30-mo. Published -- -- Pharmaceuticals, Xxxxxxxxxxx Xx 000000 Nov-19 30-May-21 Inc. Women With Breast Cancer
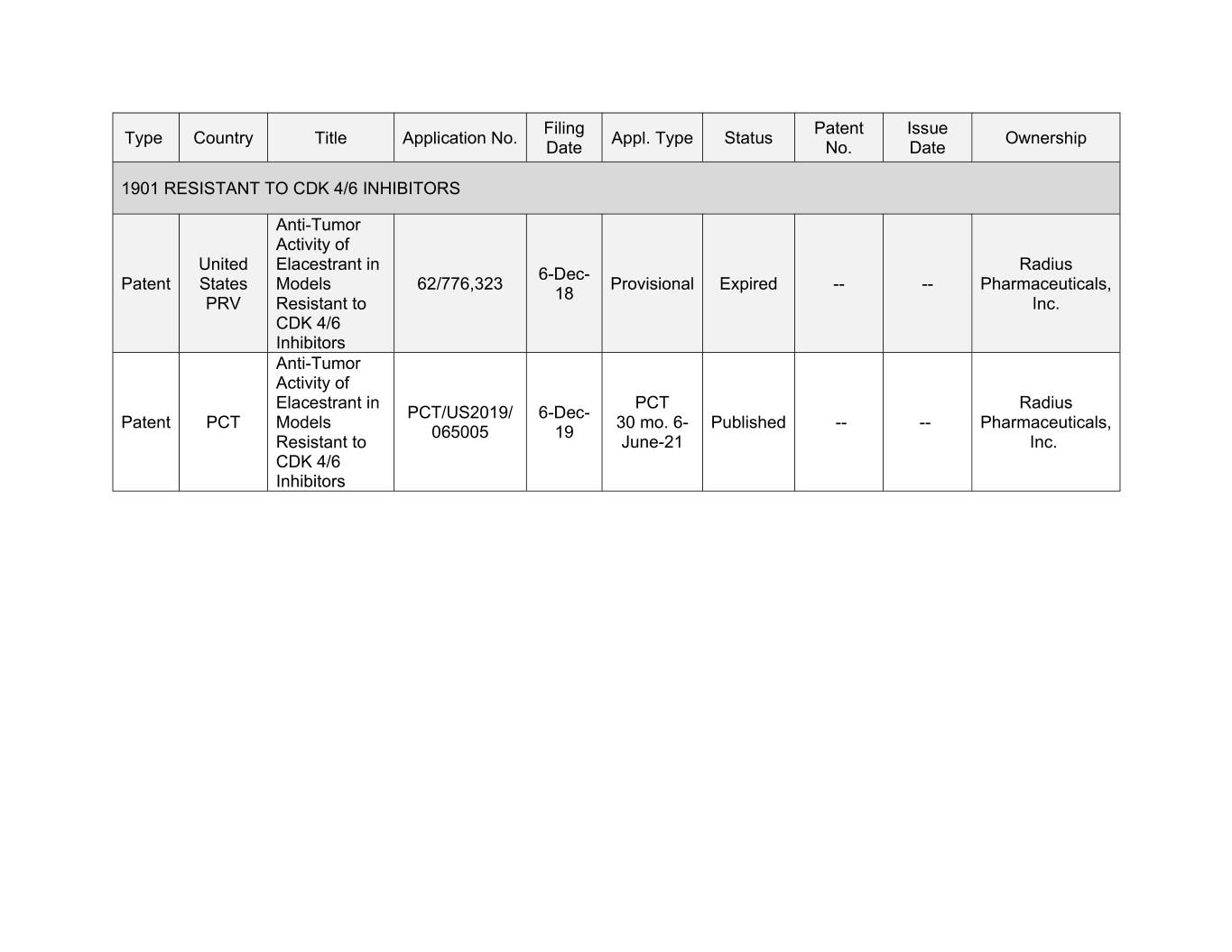
Filing Patent Issue Type Country Title Application No. Appl. Type Status Ownership Date No. Date 1901 RESISTANT TO CDK 4/6 INHIBITORS Anti-Tumor Activity of United Elacestrant in Radius 6-Dec- Patent States Models 62/776,323 Provisional Expired -- -- Pharmaceuticals, 18 PRV Resistant to Inc. CDK 4/6 Inhibitors Anti-Tumor Activity of Elacestrant in PCT Radius PCT/US2019/ 6-Dec- Patent PCT Models 30 mo. 6- Published -- -- Pharmaceuticals, 065005 19 Resistant to June-21 Inc. CDK 4/6 Inhibitors
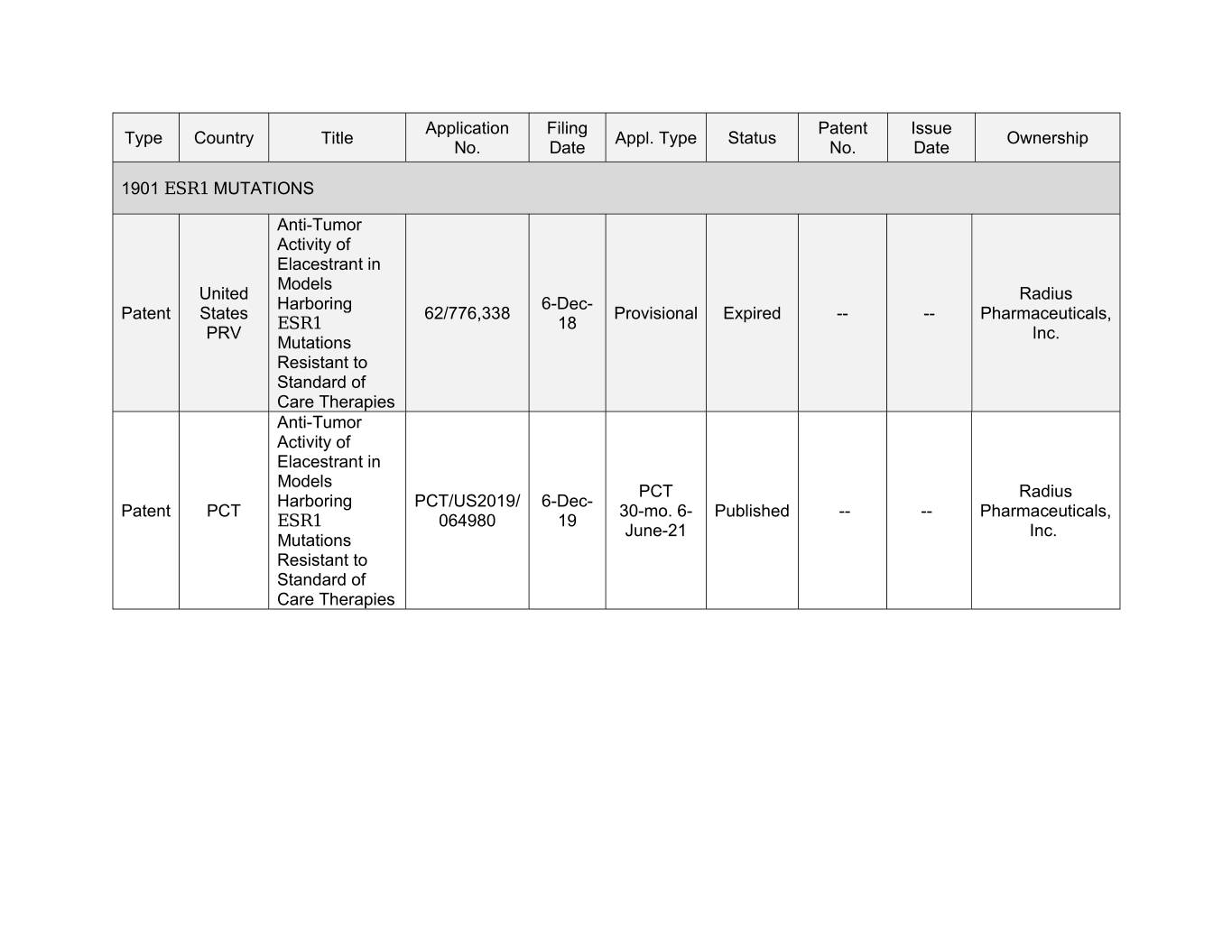
Application Filing Patent Issue Type Country Title Appl. Type Status Ownership No. Date No. Date 1901 ESR1 MUTATIONS Anti-Tumor Activity of Elacestrant in Models United Radius Harboring 6-Dec- Patent States 62/776,338 Provisional Expired -- -- Pharmaceuticals, ESR1 18 PRV Inc. Mutations Resistant to Standard of Care Therapies Anti-Tumor Activity of Elacestrant in Models PCT Radius Harboring PCT/US2019/ 6-Dec- Patent PCT 30-mo. 6- Published -- -- Pharmaceuticals, ESR1 064980 19 June-21 Inc. Mutations Resistant to Standard of Care Therapies
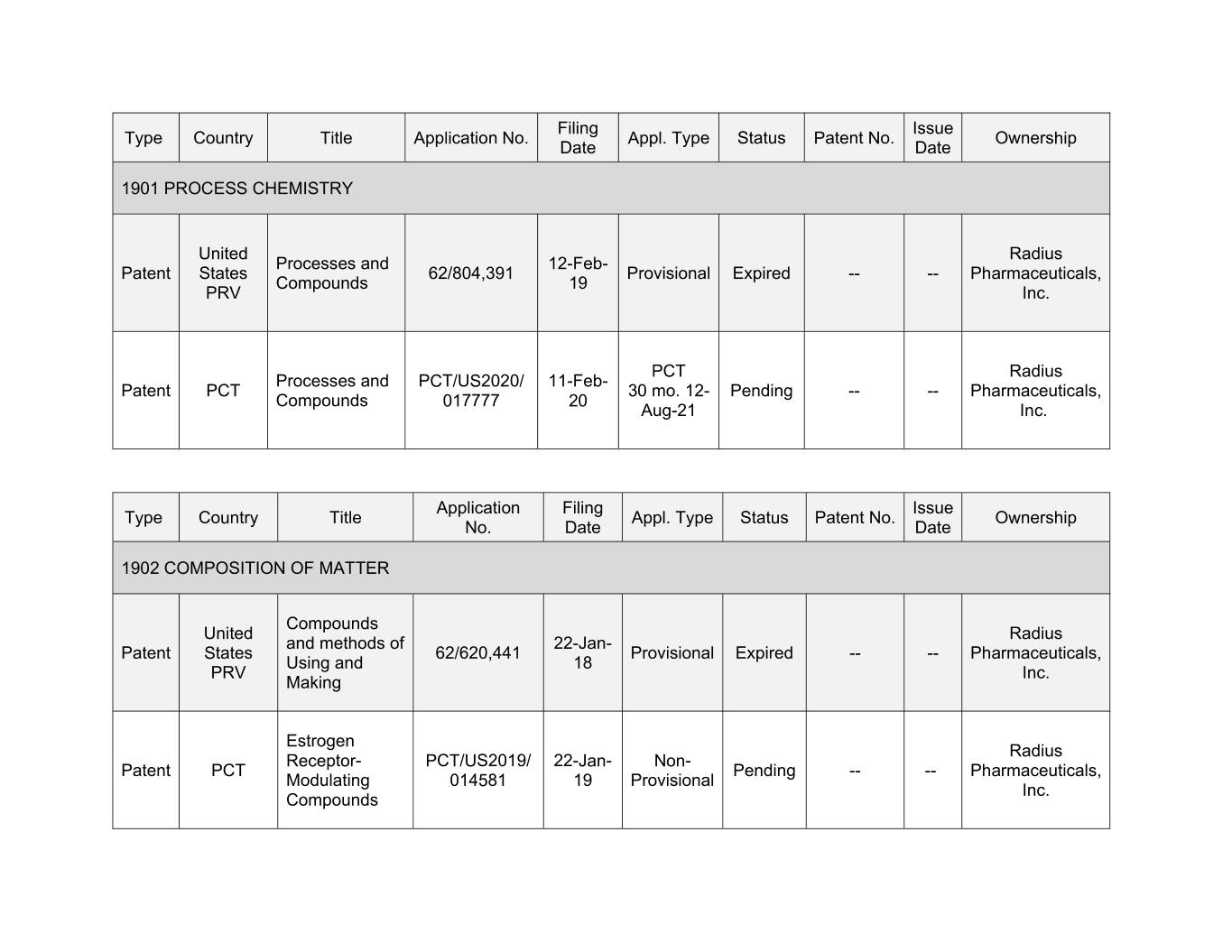
Filing Issue Type Country Title Application No. Appl. Type Status Patent No. Ownership Date Date 1901 PROCESS CHEMISTRY United Radius Processes and 12-Feb- Patent States 62/804,391 Provisional Expired -- -- Pharmaceuticals, Compounds 19 PRV Inc. PCT Radius Processes and PCT/US2020/ 11-Feb- Patent PCT 30 mo. 12- Pending -- -- Pharmaceuticals, Compounds 017777 20 Aug-21 Inc. Application Filing Issue Type Country Title Appl. Type Status Patent No. Ownership No. Date Date 1902 COMPOSITION OF MATTER Compounds United Radius and methods of 22-Jan- Patent States 62/620,441 Provisional Expired -- -- Pharmaceuticals, Using and 18 PRV Inc. Making Estrogen Radius Receptor- PCT/US2019/ 22-Jan- Non- Patent PCT Pending -- -- Pharmaceuticals, Modulating 014581 19 Provisional Inc. Compounds
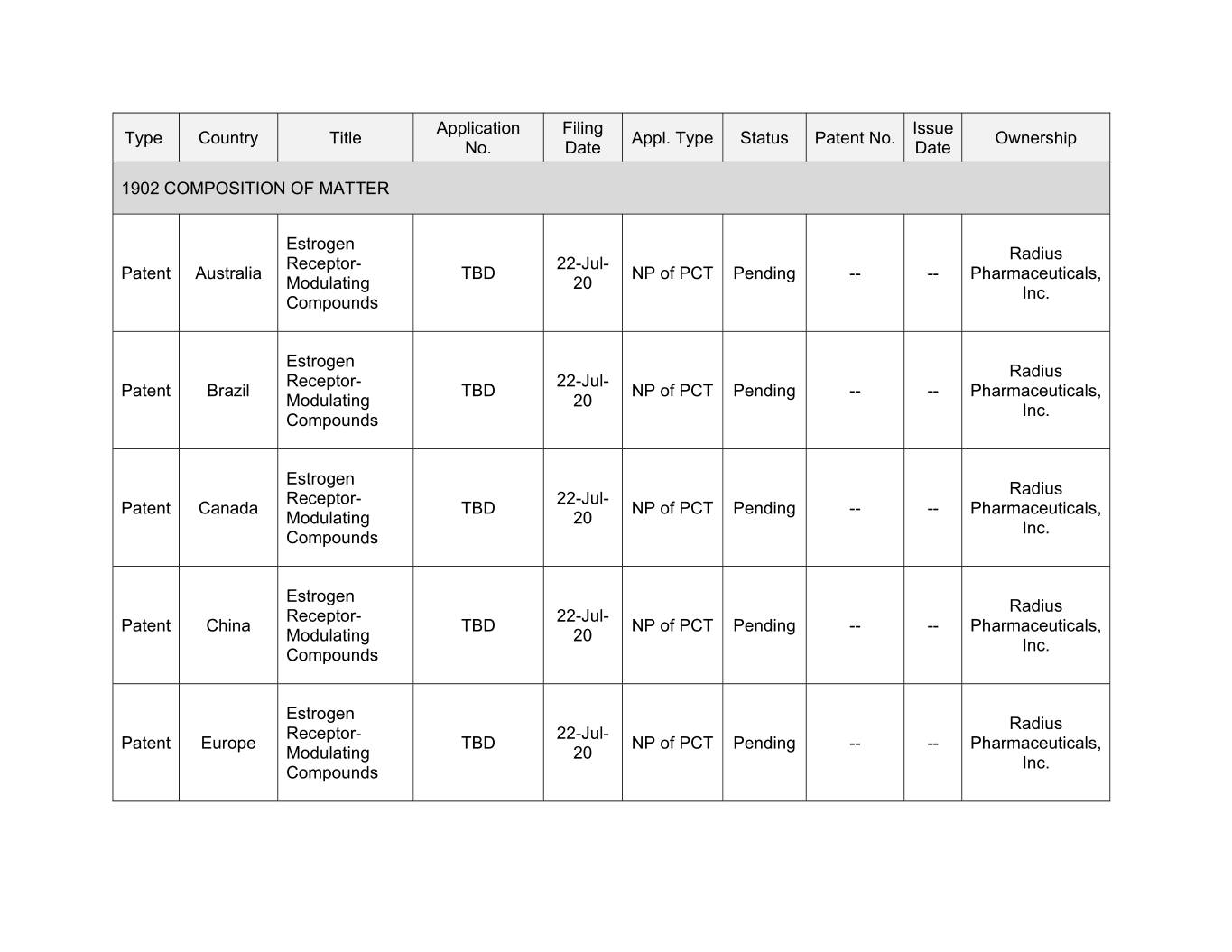
Application Filing Issue Type Country Title Appl. Type Status Patent No. Ownership No. Date Date 1902 COMPOSITION OF MATTER Estrogen Radius Receptor- 22-Jul- Patent Australia TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent Brazil TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent Canada TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent China TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent Europe TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds
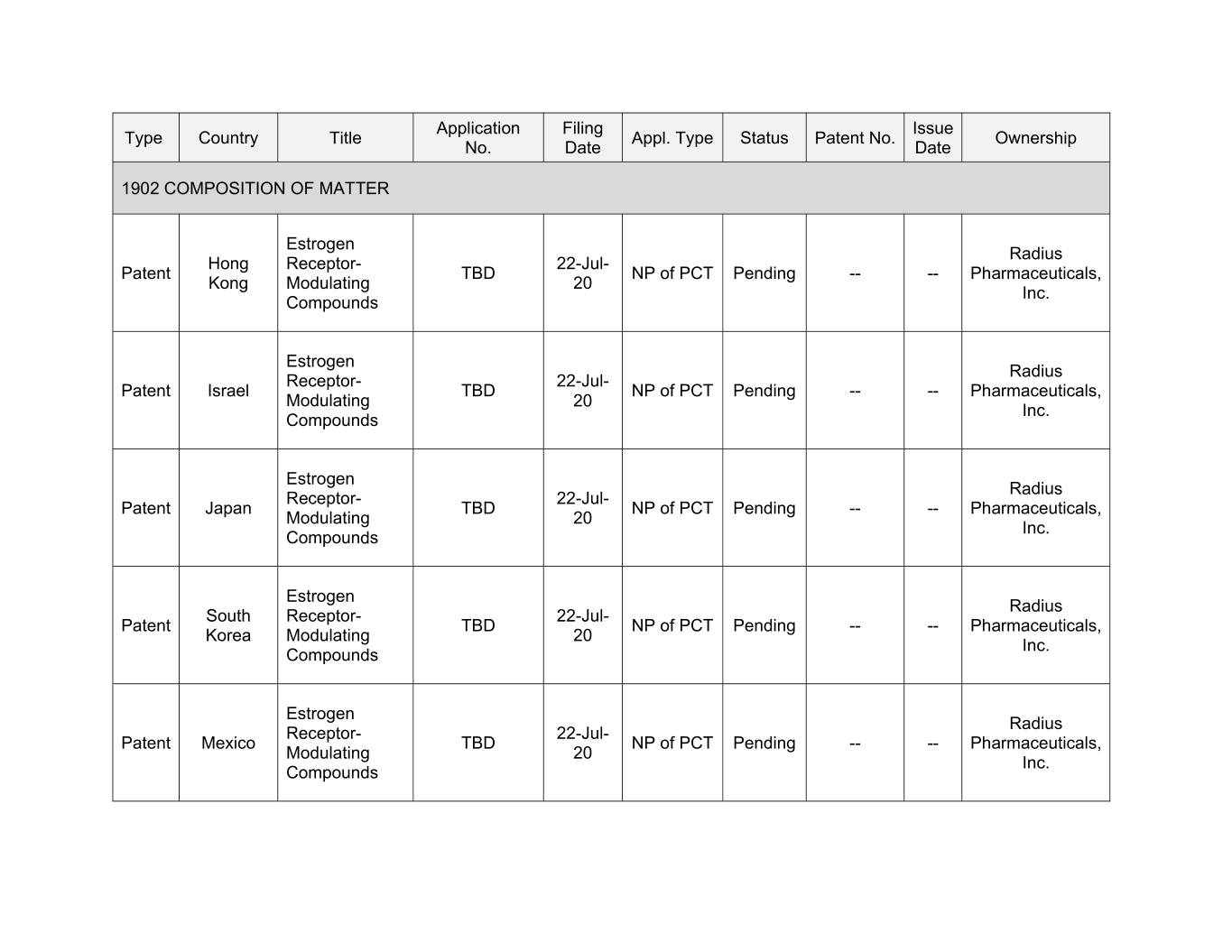
Application Filing Issue Type Country Title Appl. Type Status Patent No. Ownership No. Date Date 1902 COMPOSITION OF MATTER Estrogen Radius Hong Receptor- 22-Jul- Patent TBD NP of PCT Pending -- -- Pharmaceuticals, Kong Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent Israel TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent Japan TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds Estrogen Radius South Receptor- 22-Jul- Patent TBD NP of PCT Pending -- -- Pharmaceuticals, Korea Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent Mexico TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds
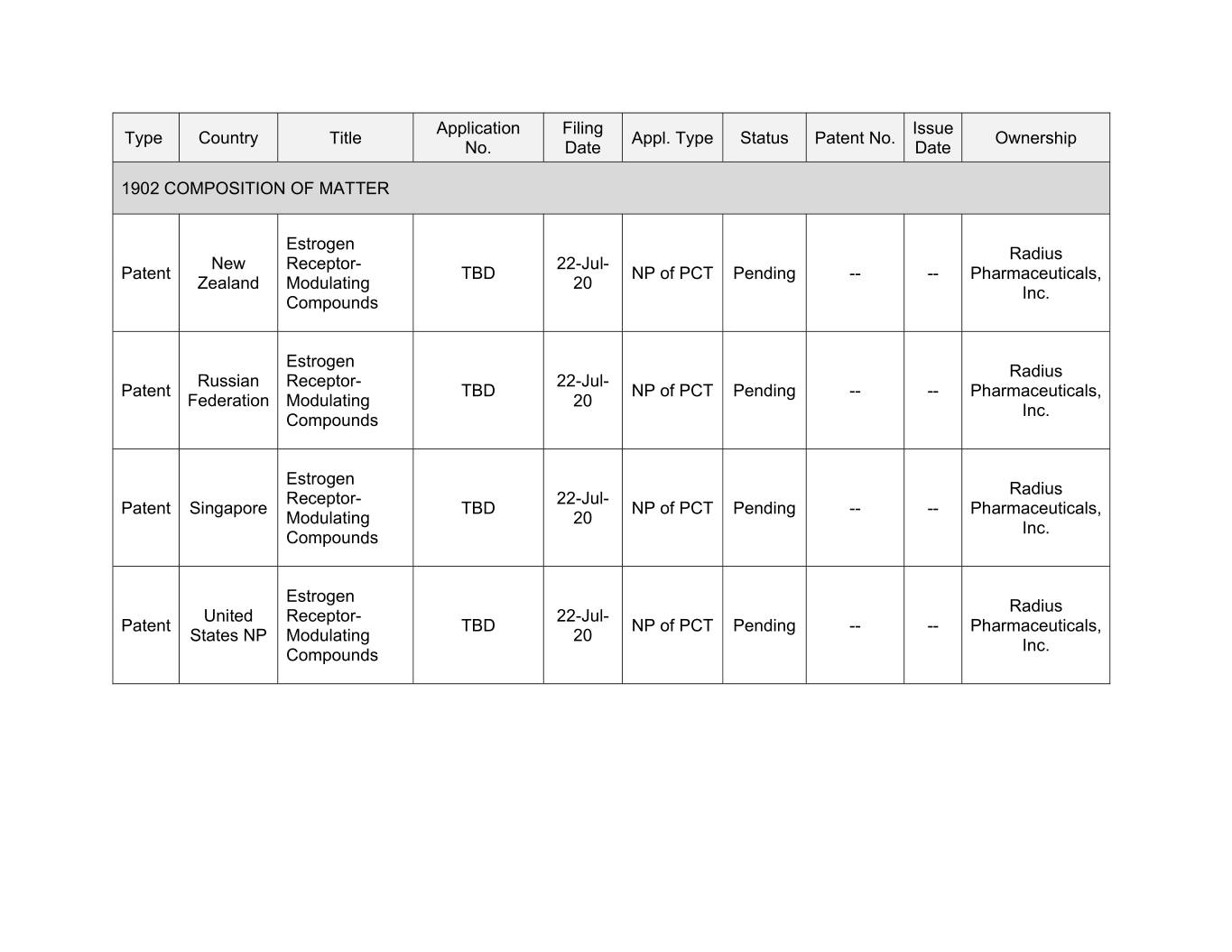
Application Filing Issue Type Country Title Appl. Type Status Patent No. Ownership No. Date Date 1902 COMPOSITION OF MATTER Estrogen Radius New Receptor- 22-Jul- Patent TBD NP of PCT Pending -- -- Pharmaceuticals, Zealand Modulating 20 Inc. Compounds Estrogen Radius Russian Receptor- 22-Jul- Patent TBD NP of PCT Pending -- -- Pharmaceuticals, Federation Modulating 20 Inc. Compounds Estrogen Radius Receptor- 22-Jul- Patent Singapore TBD NP of PCT Pending -- -- Pharmaceuticals, Modulating 20 Inc. Compounds Estrogen Radius United Receptor- 22-Jul- Patent TBD NP of PCT Pending -- -- Pharmaceuticals, States NP Modulating 20 Inc. Compounds
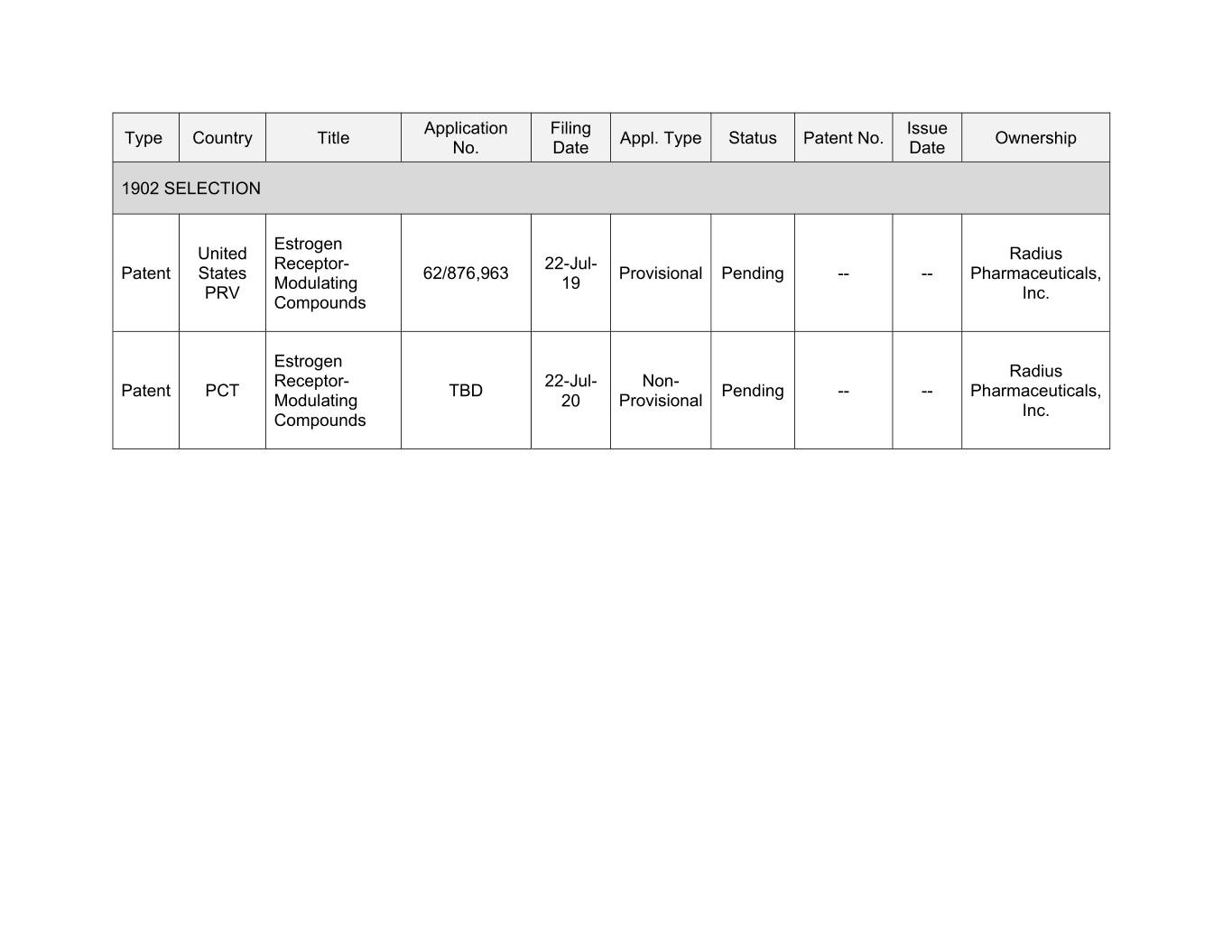
Application Filing Issue Type Country Title Appl. Type Status Patent No. Ownership No. Date Date 1902 SELECTION Estrogen United Radius Receptor- 22-Jul- Patent States 62/876,963 Provisional Pending -- -- Pharmaceuticals, Modulating 19 PRV Inc. Compounds Estrogen Radius Receptor- 22-Jul- Non- Patent PCT TBD Pending -- -- Pharmaceuticals, Modulating 20 Provisional Inc. Compounds
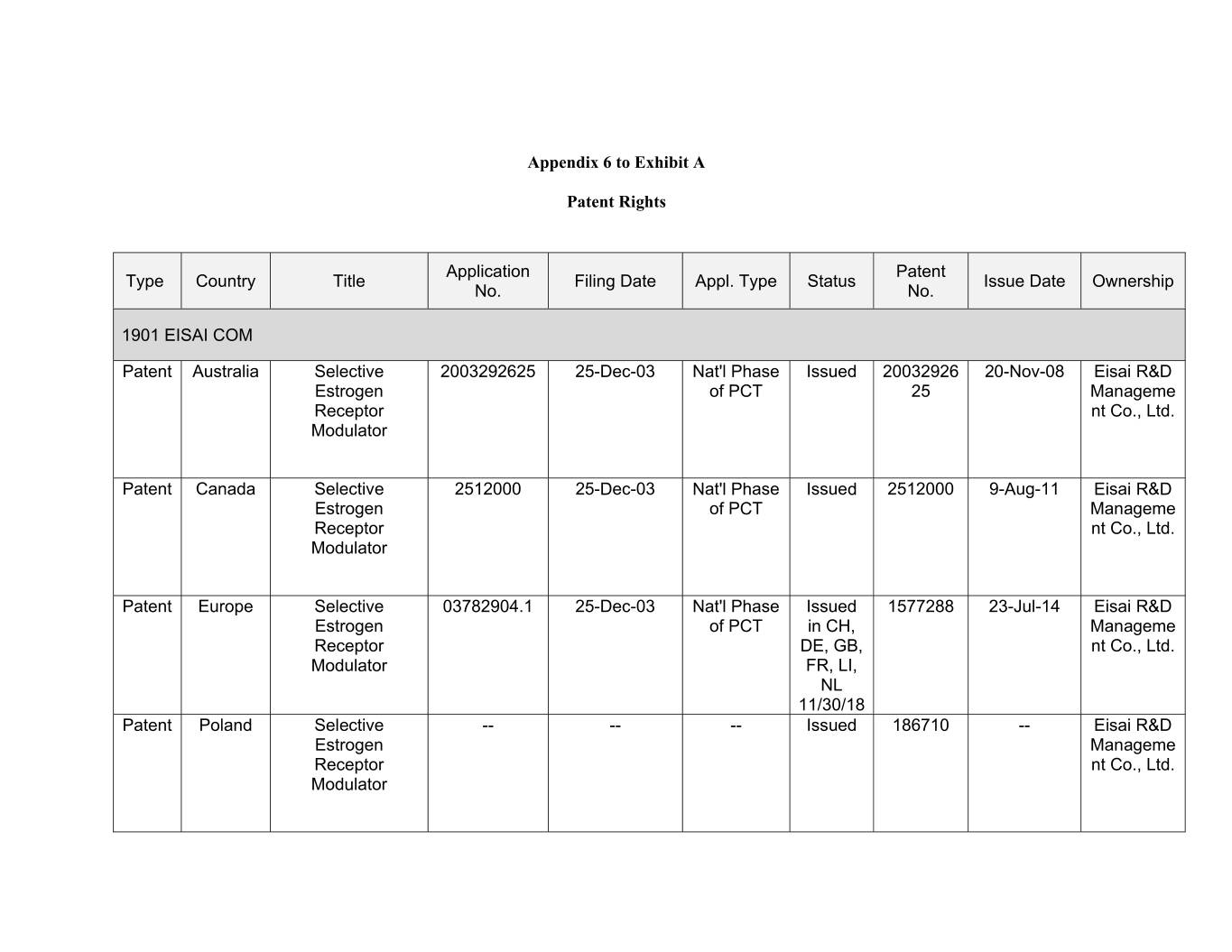
Appendix 6 to Exhibit A Patent Rights Application Patent Type Country Title Filing Date Appl. Type Status Issue Date Ownership No. No. 1901 EISAI COM Patent Australia Selective 2003292625 25-Dec-03 Nat'l Phase Issued 20032926 00-Xxx-00 Xxxxx R&D Estrogen of PCT 25 Manageme Receptor nt Co., Ltd. Modulator Patent Canada Selective 2512000 25-Dec-03 Nat'l Phase Issued 2512000 9-Aug-11 Eisai R&D Estrogen of PCT Manageme Receptor nt Co., Ltd. Modulator Patent Europe Selective 03782904.1 25-Dec-03 Nat'l Phase Issued 1577288 23-Jul-14 Eisai R&D Estrogen of PCT in CH, Manageme Receptor DE, GB, nt Co., Ltd. Modulator FR, LI, NL 11/30/18 Patent Poland Selective -- -- -- Issued 186710 -- Eisai R&D Estrogen Manageme Receptor nt Co., Ltd. Modulator
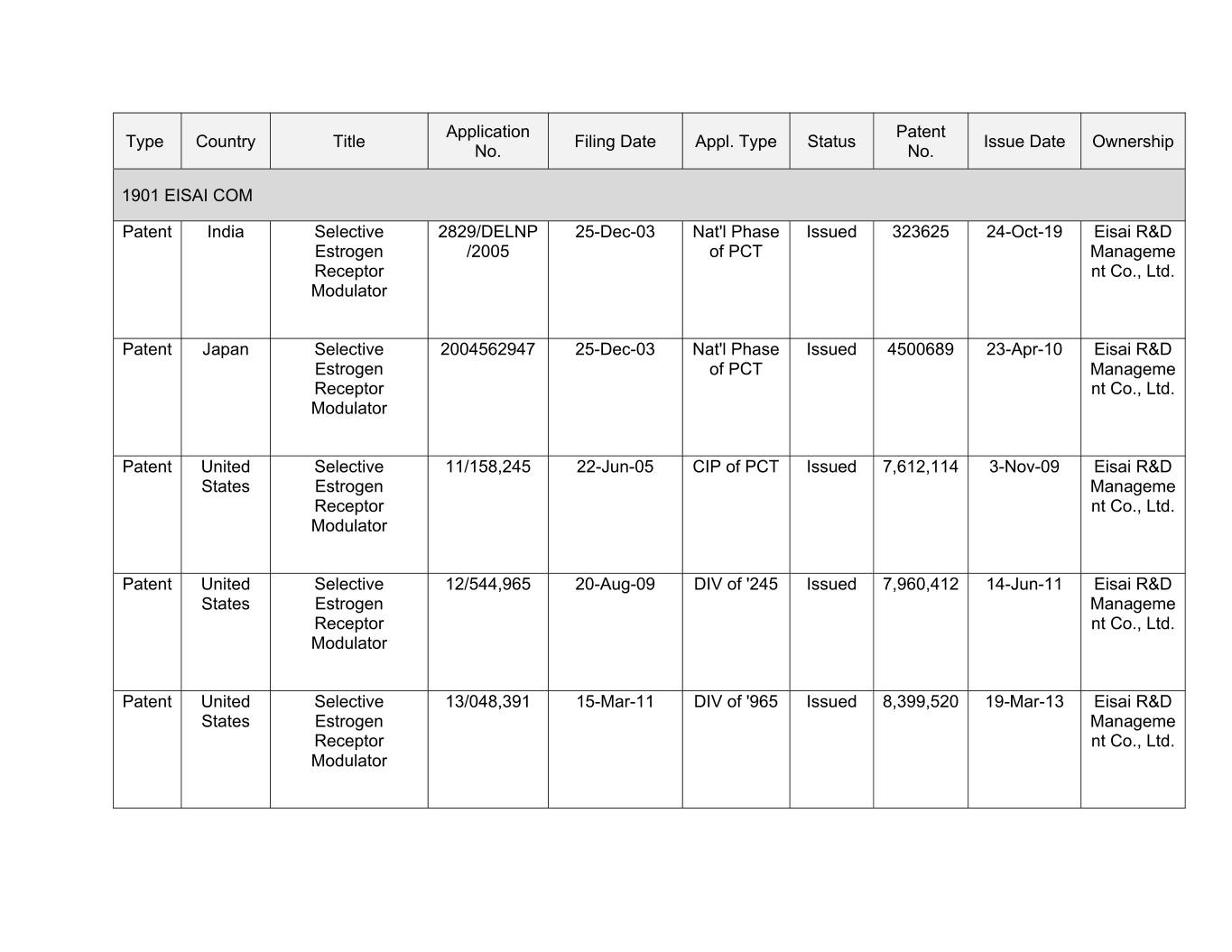
Application Patent Type Country Title Filing Date Appl. Type Status Issue Date Ownership No. No. 1901 EISAI COM Patent India Selective 2829/DELNP 25-Dec-03 Nat'l Phase Issued 323625 24-Oct-19 Eisai R&D Estrogen /2005 of PCT Manageme Receptor nt Co., Ltd. Modulator Patent Japan Selective 2004562947 25-Dec-03 Nat'l Phase Issued 4500689 23-Apr-10 Eisai R&D Estrogen of PCT Manageme Receptor nt Co., Ltd. Modulator Patent United Selective 11/158,245 22-Jun-05 CIP of PCT Issued 7,612,114 0-Xxx-00 Xxxxx R&D States Estrogen Manageme Receptor nt Co., Ltd. Modulator Patent United Selective 12/544,965 20-Aug-09 DIV of '245 Issued 7,960,412 14-Jun-11 Eisai R&D States Estrogen Manageme Receptor nt Co., Ltd. Modulator Patent United Selective 13/048,391 15-Mar-11 DIV of '965 Issued 8,399,520 19-Mar-13 Eisai R&D States Estrogen Manageme Receptor nt Co., Ltd. Modulator
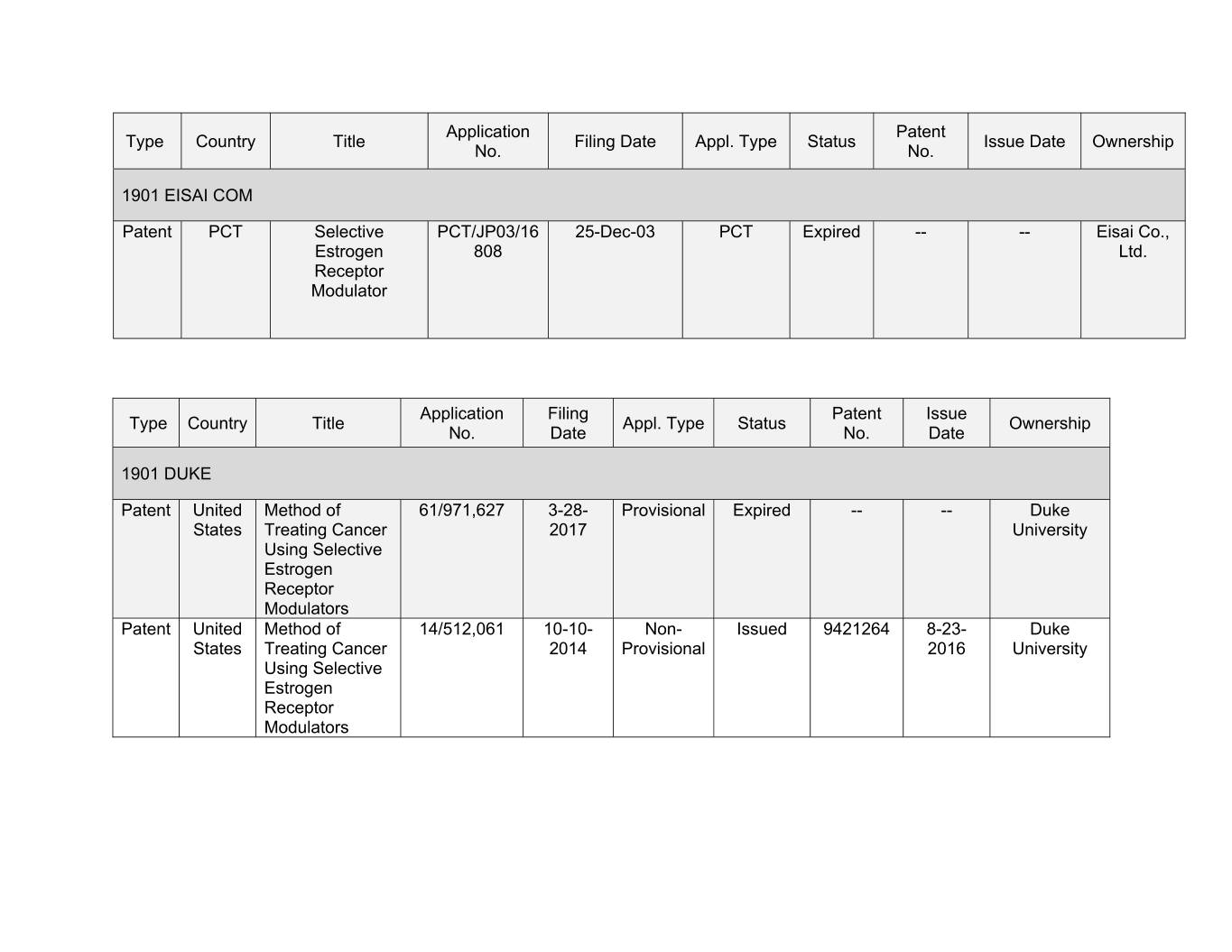
Application Patent Type Country Title Filing Date Appl. Type Status Issue Date Ownership No. No. 1901 EISAI COM Patent PCT Selective PCT/JP03/16 25-Dec-03 PCT Expired -- -- Eisai Co., Estrogen 808 Ltd. Receptor Modulator Application Filing Patent Issue Type Country Title Appl. Type Status Ownership No. Date No. Date 1901 DUKE Patent United Method of 61/971,627 3-28- Provisional Expired -- -- Duke States Treating Cancer 2017 University Using Selective Estrogen Receptor Modulators Patent United Method of 14/512,061 00-00- Xxx- Xxxxxx 0000000 0-00- Xxxx Xxxxxx Treating Cancer 2014 Provisional 2016 University Using Selective Estrogen Receptor Modulators
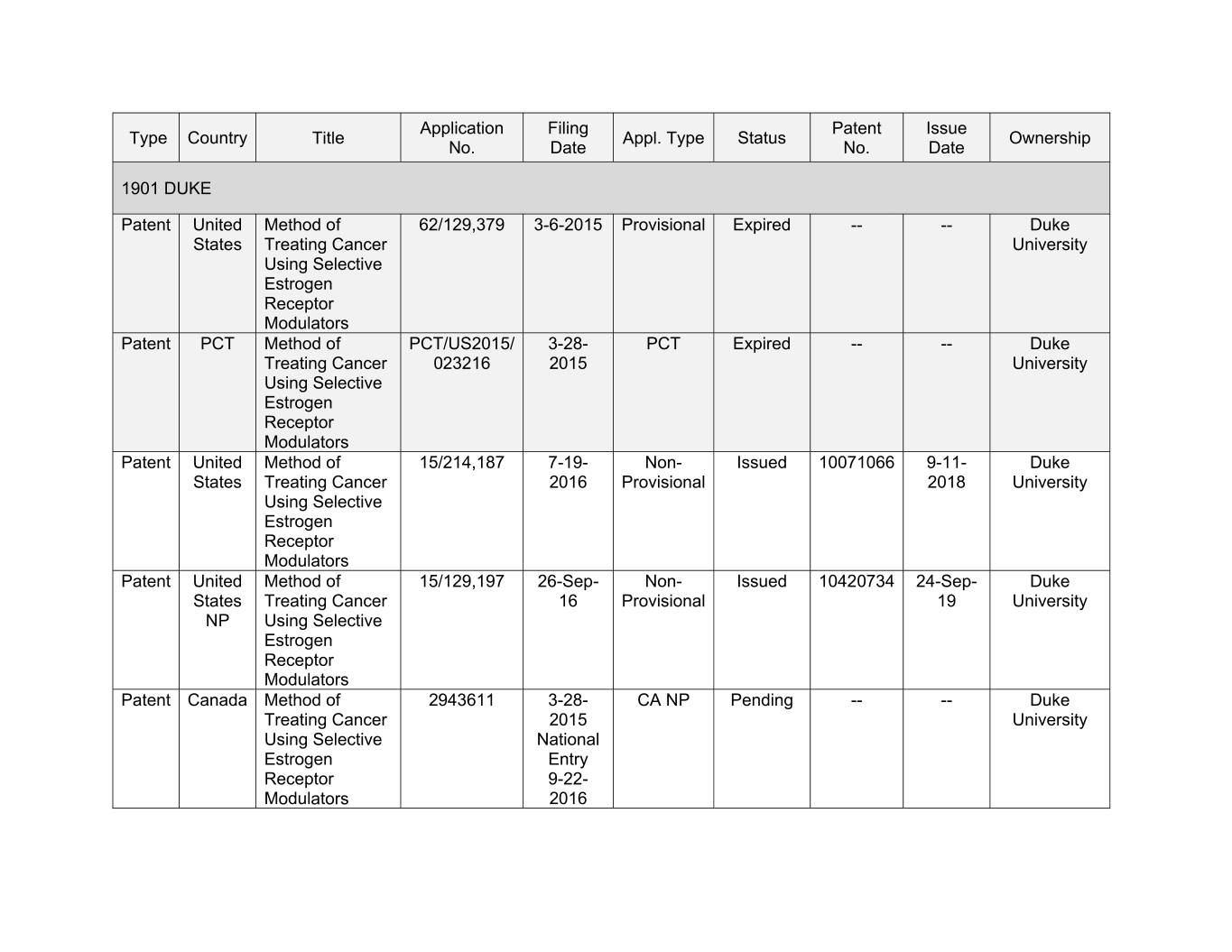
Application Filing Patent Issue Type Country Title Appl. Type Status Ownership No. Date No. Date 1901 DUKE Patent United Method of 62/129,379 3-6-2015 Provisional Expired -- -- Duke States Treating Cancer University Using Selective Estrogen Receptor Modulators Patent PCT Method of PCT/US2015/ 3-28- PCT Expired -- -- Duke Treating Cancer 023216 2015 University Using Selective Estrogen Receptor Modulators Patent United Method of 15/214,187 0-00- Xxx- Xxxxxx 00000000 0-00- Xxxx Xxxxxx Treating Cancer 2016 Provisional 2018 University Using Selective Estrogen Receptor Modulators Patent United Method of 15/129,197 26-Sep- Non- Issued 10420734 24-Sep- Duke States Treating Cancer 16 Provisional 19 University NP Using Selective Estrogen Receptor Modulators Patent Canada Method of 2943611 3-28- CA NP Pending -- -- Duke Treating Cancer 2015 University Using Selective National Estrogen Entry Receptor 9-22- Modulators 2016
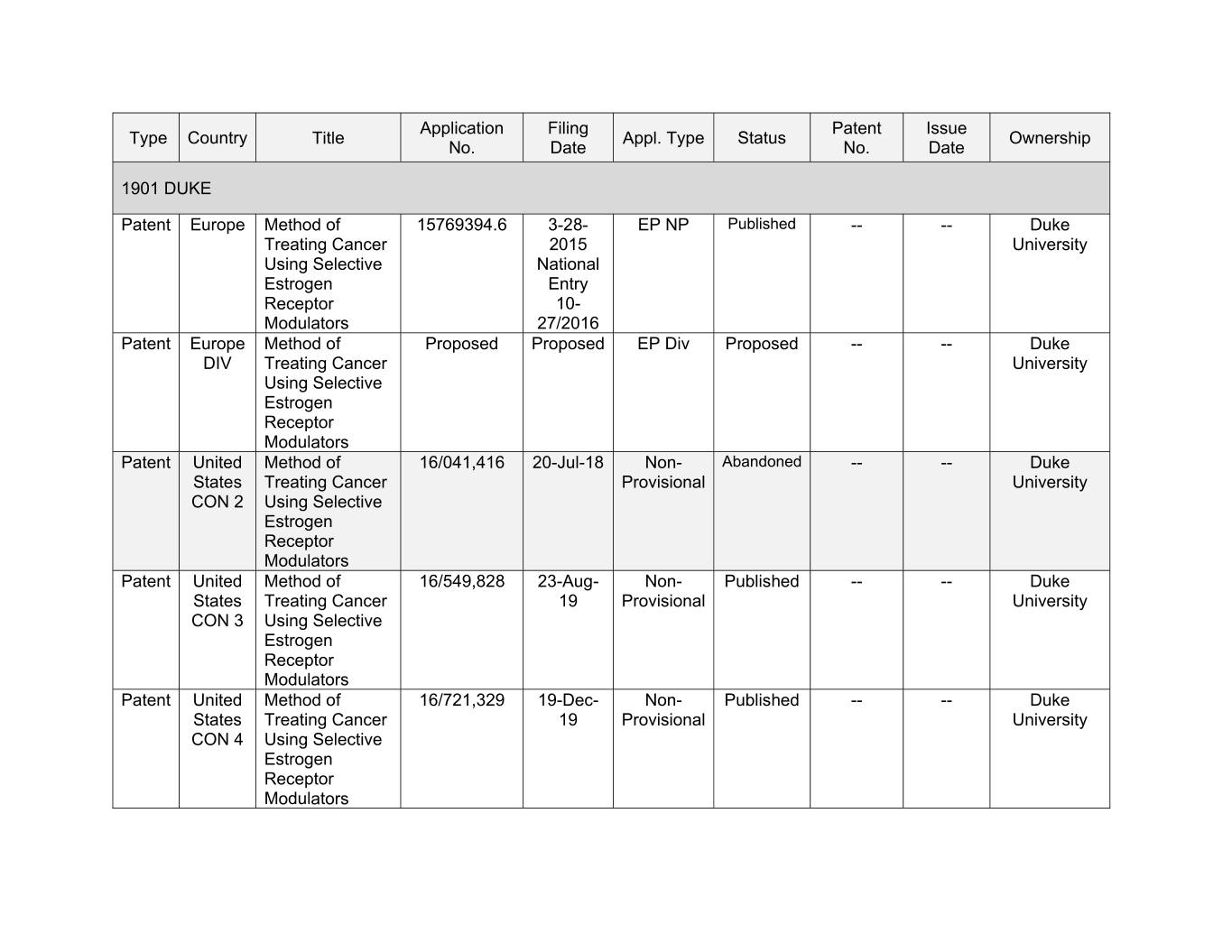
Application Filing Patent Issue Type Country Title Appl. Type Status Ownership No. Date No. Date 1901 DUKE Patent Europe Method of 15769394.6 3-28- EP NP Published -- -- Duke Treating Cancer 2015 University Using Selective National Estrogen Entry Receptor 10- Modulators 27/2016 Patent Europe Method of Proposed Proposed EP Div Proposed -- -- Duke DIV Treating Cancer University Using Selective Estrogen Receptor Modulators Patent United Method of 16/041,416 20-Jul-18 Non- Abandoned -- -- Duke States Treating Cancer Provisional University CON 2 Using Selective Estrogen Receptor Modulators Patent United Method of 16/549,828 23-Aug- Non- Published -- -- Duke States Treating Cancer 19 Provisional University CON 3 Using Selective Estrogen Receptor Modulators Patent United Method of 16/721,329 19-Dec- Non- Published -- -- Duke States Treating Cancer 19 Provisional University CON 4 Using Selective Estrogen Receptor Modulators

