LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
Exhibit 10.3
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT
THIS LICENSE, DEVELOPMENT AND COMMERCIALIZATION AGREEMENT (“Agreement”) dated as of March 26, 2016 (“Execution Date”), is entered into between XenoPort, Inc., a Delaware corporation having its principal place of business at 0000 Xxxxxxx Xxxxxxxxxx, Xxxxx Xxxxx, XX 00000 (“XenoPort”) and Xx. Xxxxx’x Laboratories, S.A., a Swiss corporation having its principal place of business at Xxxxxxxxxxxxxxxxx 00, 0000, Xxxxx, Xxxxxxxxxxx (“DRL”).
BACKGROUND
A. XenoPort is developing a prodrug of monomethyl fumarate (as further defined below, “MMF”), known internally at XenoPort as XP23829, (as further defined below, the “Compound”), a formulation of which is currently targeted for the treatment of psoriasis and multiple sclerosis. XenoPort owns or controls certain patents, know-how and other intellectual property relating to such Compound, and products containing such Compound (as further defined below, “Products”);
B. DRL desires to develop, manufacture and commercialize Products in the United States, and XenoPort desires to have Products developed and commercialized in the United States by DRL, all in accordance with this Agreement.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
ARTICLE I
DEFINITIONS
1.1 “Affiliate” of a Party shall mean any person, corporation or other entity that, directly or indirectly through one or more intermediaries, controls, is controlled by or is under common control with such Party, as the case may be, for as long as such control exists. As used in this Section 1.1, “control” shall mean: (a) to possess, directly or indirectly, the power to direct the management and policies of such person, corporation or other entity, whether through ownership of voting securities or by contract relating to voting rights or corporate governance; or (b) direct or indirect beneficial ownership of at least fifty percent (50%) (or such lesser percentage that is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) of the voting share capital in such person, corporation or other entity. [ * ]
1.2 “ANDA” shall mean an Abbreviated New Drug Application (or its equivalent) as defined in Section 505(j) of the FDCA.
1.3 “Annual Net Sales” shall mean total Net Sales of either Equivalent Products or Non-Equivalent Products, as applicable, sold in the Territory in a particular fiscal year (i.e., April 1 to March 31). For such purposes, units of Product shall be considered sold when such Product is shipped to a customer or the revenue from such sale is recognized by the seller for financial reporting purposes, whichever occurs first.
1.4 “Applicable Laws” means the applicable provisions of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidances, ordinances, judgments, decrees, directives, injunctions, orders, permits (including Marketing Approvals) of or from any court, arbitrator, Regulatory Authority or governmental agency or authority having jurisdiction over or related to the subject item.
1.5 “Change of Control” shall mean either: (a) a sale of all or substantially all of the assets of a Party in one or a series of integrated transactions not in the ordinary course of business to a Third Party; or (b) the acquisition of control (as defined in Section 1.1 above) of a Party by a Third Party by means of any transaction or series of related transactions to which such Party is a party (including, any stock acquisition, merger or consolidation). For clarity, a Change of Control would not include any transaction or series of transactions in which the holders of voting securities of a Party outstanding immediately prior to such transaction continue to retain (either by such voting securities remaining outstanding or by such voting securities being converted into voting securities of the surviving entity), as a result of shares in the Party held by such holders prior to such transaction, fifty percent (50%) or more of the total voting power represented by the voting securities of the acquiring entity outstanding immediately after such transaction or series of transactions.
1.6 “Compound” shall mean that certain compound (N,N-Diethylcarbamoyl)methyl methyl (2E)but-2-ene-1,4-dioate or 1-[2-(diethylamino)-2-oxoethyl] 4-methyl (2E)-but-2-ene-1,4-dioate, referred to internally at XenoPort as XP23829, the structure of which is set forth on Exhibit 1.6, together with all isomers and/or mixtures of isomers thereof, and/or any salts of any of the foregoing.
1.7 “Control” (including any variations such as “Controlled” and “Controlling”) shall mean, in the context of intellectual property rights, Data or information, possession of the ability to grant an assignment, license or sublicense to such intellectual property, Data and/or information, and/or to disclose and deliver such Data and/or information, as the case may be, of or within the scope set forth in this Agreement, without violating the terms of any agreement or other arrangement with any Third Party.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.8 “Data” shall mean any and all research data, pharmacology data, preclinical data, clinical data and/or all regulatory documentation, information and submissions pertaining to, or made in association with an IND, NDA, Marketing Approval or the like for, the Compound or a Product, in each case that are Controlled by a Party as of the Effective Date or during the term of this Agreement after the Effective Date. Data also shall include any such data generated by DRL’s Affiliates or under authority of DRL or any of its Affiliates during the term of this Agreement.
1.9 “Diligent Efforts” shall mean, with respect to a Compound or Product, the carrying out of obligations under this Agreement in a sustained manner [ * ] the Compound or Product, as applicable, taking into account [ * ].
1.10 “DRL Know-How” shall mean all scientific, medical, technical, marketing, manufacturing, formulation, regulatory and other information relating to the Compound or a Product (including Data), that are owned or Controlled by DRL or its Affiliates during the term of this Agreement, [ * ] in order for XenoPort to exercise its rights or perform its obligations under this Agreement [ * ]. Notwithstanding the foregoing [ * ], DRL Know-How shall also include [ * ].
1.11 “Effective Date” shall mean the HSR Clearance Date.
1.12 [ * ] shall mean [ * ].
1.13 “Existing Inventory” shall mean [ * ] owned or controlled by XenoPort as of the Execution Date set forth on Exhibit 1.13, in the form described in such exhibit.
1.14 “FDA” shall mean the United States Food and Drug Administration, or any successor entity thereto performing similar functions.
1.15 “FDCA” shall mean the United States Federal Food, Drug, and Cosmetic Act, as amended.
1.16 “Field” shall mean the treatment, diagnosis and/or prevention of human and/or animal diseases and conditions.
1.17 “Filing” of an NDA shall [ * ].
1.18 [ * ] shall mean the [ * ].
1.19 “Generic Product” shall [ * ].
1.20 “IND” shall mean any Investigational New Drug Application (including any amendments thereto) filed with the FDA pursuant to 21 C.F.R. §321 before the commencement of clinical trials of a Product, or any comparable filings with any Regulatory Authority in any other jurisdiction.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.21 “Marketing Approval” shall mean all approvals, licenses, registrations or authorizations of the Regulatory Authority in a country, necessary for the manufacture, use, storage, import, marketing and sale of a Product in such country, excluding any governmental pricing and/or reimbursement approvals and/or authorizations.
1.22 “MMF” means [ * ] set forth on Exhibit 1.22.
1.23 “MS Indication” shall mean an indication for a Product involving multiple sclerosis.
1.24 “NDA” shall mean a new drug application (or its equivalent) submitted to the FDA, including new drug applications as defined by Sections 505(b)(1) and 505(b)(2) of the FDCA .
1.25 “Net Sales” shall mean the gross amount billed or invoiced by DRL and/or its Affiliates, as applicable, for all sales of Product to Third Party customers [ * ] To the extent applicable, components of Net Sales shall [ * ]. For the purposes of calculating Net Sales, the Parties understand and agree that: [ * ]; and [ * ]. If a Product is sold or transferred for consideration other than cash, the Net Sales from such sale or transfer shall be deemed the then fair market value of such Product.
In the event that [ * ], the Net Sales of [ * ], where [ * ], and [ * ], in each case in the Territory during the applicable reporting period or, if sales of [ * ], then the [ * ]. In the event that [ * ], then Net Sales for [ * ].
1.26 [ * ] shall mean: (a) [ * ] and including all [ * ]; or (b) any other [ * ].
1.27 “Orange Book” shall mean the Approved Drug Products with Therapeutic Equivalence Evaluation published as of the Execution Date by the FDA’s Center for Drug Evaluation and Research, as updated and modified from time to time, or a successor register thereto that has a similar function.
1.28 “Psoriasis Indication” shall mean an indication for a Product involving psoriasis, including psoriatic arthritis.
1.29 “Party” shall mean XenoPort or DRL individually, and “Parties” shall mean XenoPort and DRL collectively.
1.30 “Patent(s)” shall mean any patents and patent applications (whether provisional or nonprovisional), together with all additions, divisions, continuations, continuations-in-part, substitutions, reissues, re-examinations, extensions, registrations, patent term extensions, supplemental protection certificates and renewals of any of the foregoing.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
1.31 “Product” shall mean any pharmaceutical product containing the Compound, alone or in combination with one or more other active pharmaceutical ingredients, in any dosage form or formulation.
1.32 “Product Trademarks” shall mean (a) [ * ]; (b) all registrations and applications for registration for such trademarks [ * ]; (c) any corresponding trademarks, and registrations or applications for registration of such trademarks, [ * ]; (d) any renewals and extensions of any of the foregoing registrations and all other corresponding rights that are or may be secured under the laws; and (e) all good will associated with any of the foregoing.
1.33 “Regulatory Authority” shall mean the FDA, or a regulatory body with similar regulatory authority in any other jurisdiction.
1.34 “Regulatory Exclusivity” shall mean any exclusive marketing rights or data exclusivity rights conferred by an applicable Regulatory Authority or other governmental authority in the Territory, including any regulatory data protection exclusivity and any extensions to such exclusivity rights.
1.35 “ROW Territory” shall mean all countries in the world other than the Territory.
1.36 “Territory” shall mean the United States, and its territories and possessions.
1.37 “Third Party” shall mean any person, corporation, joint venture or other entity, other than XenoPort, DRL and their respective Affiliates.
1.38 “Valid Claim” means a claim (or claims) [ * ] that has (have) not been revoked or held unenforceable or invalid by a decision of a court or governmental agency of competent jurisdiction from which no appeal can be taken, or with respect to which an appeal is not taken within the time allowed for appeal, and that has not been disclaimed, denied or admitted to be invalid or unenforceable through reissue, interference proceedings, disclaimer or otherwise; [ * ].
1.39 “XenoPort IP” shall mean the XenoPort Know-How and XenoPort Patents.
1.40 “XenoPort Know-How” shall mean all scientific, medical, technical, manufacturing, formulation, regulatory and other information relating to the Compound and/or Products (including Data): (a) to the extent Controlled by XenoPort as of the Effective Date or, subject to [ * ], during the term of this Agreement after the Effective Date; and (b) that [ * ] the Compound or a Product prior to the Effective Date, or that [ * ] DRL to exercise its rights or perform its obligations under this Agreement.
1.41 “XenoPort Patents” shall mean all issued, unexpired patents and all reissues, renewals, re-examinations and extensions thereof, and patent applications therefor, and any divisions
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
or continuations, in whole or in part, thereof, including those patents and applications set forth in Exhibit 1.41 and, if applicable, XenoPort’s interest in patents and applications described in [ * ]: (a) to the extent Controlled by XenoPort as of the Effective Date or, subject to [ * ], during the term of this Agreement after the Effective Date; and (b) that [ * ].
1.42 Additional Definitions. Each of the following terms shall have the meaning described in the corresponding section of this Agreement indicated below:
| Term | Section Defined | |
| [ * ] | [ * ] | |
| [ * ] | [ * ] | |
| Agreement | Introduction | |
| CoAs | 8.1(a) | |
| Commercialization Plan | 5.1(a)(i) | |
| [ * ] | [ * ] | |
| Confidential Information | 9.1 | |
| [ * ] | [ * ] | |
| Delivery Date | 8.1(a) | |
| Development Plan | 4.2(a) | |
| DOJ | 16.2 | |
| DRL | Introduction | |
| DRL Indemnitees | 14.2 | |
| DRL IP | 10.1 | |
| Enforcement Action | 10.3(a) | |
| Execution Date | Introduction | |
| [ * ] |
[ * ] | |
| Existing Inventory Acceptance Notice | 8.1(a) | |
| FTC | 16.2 | |
| Xxxx-Xxxxx-Xxxxxx Act | 16.1 | |
| HSR Clearance Date | 16.1 | |
| HSR Conditions | 16.1 | |
| Improvements | 10.1 | |
| Improvement Patents | 10.1 | |
| Indemnitee | 14.3 | |
| Indemnitor | 14.3 | |
| Indirect Taxes | 7.2(b) | |
| Infringement Actions | 10.4 | |
| Infringing Product | 10.3(a) | |
| Joint Steering Committee / JSC | 3.1 | |
| Jointly-Owned IP | 10.1 | |
| Liabilities | 14.1 | |
| Orange Book | 1.37 | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| Paragraph IV Certification | 10.3(b) | |
| PK | 4.1(a)(i) |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
| Term | Section Defined | |
| Product Liability Claim | 14.1 | |
| Product Materials | 12.2(f) | |
| Prosecution and Maintenance / Prosecute and Maintain | 10.2(a) | |
| [ * ] | [ * ] | |
| Revenue | 2.5(b)(iv)(d) | |
| Royalty Term | 6.4(d) | |
| Senior Executives | 3.1(d) | |
| Stability Studies | 8.1(a) | |
| Term | 11.1 | |
| Third Party IP | 10.5 | |
| Third Party Claim | 14.1 | |
| [ * ] | [ * ] | |
| Withdrawal Notice | 3.2 | |
| XenoPort | Introduction | |
| XenoPort CMO | 8.1(a) | |
| XenoPort Indemnitees | 14.1 |
ARTICLE II
GRANT OF LICENSE
2.1 License. Subject to the terms and conditions of this Agreement, XenoPort hereby grants to DRL an exclusive license during the term of this Agreement under the XenoPort Patents and XenoPort Know-How to: (a) develop, use, make, have made, offer for sale, sell, import, market, distribute and promote Products; and (b) to use, make and have made the Compound for the purposes of developing, making and having made Products; in each case, solely in the Territory for use in the Field. In addition, DRL may: (i) manufacture or have manufactured the Compound and/or Product(s) outside the Territory solely for the purposes of (A) making Compound and/or Product for DRL and its Affiliates for sale and distribution of Products in the Territory for use within the Field or (B) providing Products for use in clinical trials for Products conducted by or under the authority of DRL outside the Territory in accordance with clause (ii) below, and (ii) perform clinical studies in countries of the ROW Territory as approved by the JSC and in accordance with the Development Plan, for the sole purpose of seeking and/or obtaining Marketing Approval in the Territory and for use within the Field. The rights and licenses granted in the first sentence of this Section 2.1 shall be exclusive even as to XenoPort, except that XenoPort shall continue to have the right to (and to authorize others to): (1) make and have made the Compound and Products in the Territory for use, distribution and/or sale outside the Territory or for use in clinical studies described in the following clause (2) and (2) to perform clinical studies in the Territory for the sole purpose of seeking and/or obtaining Marketing Approval in a jurisdiction(s) in the ROW Territory.
2.2 Affiliates; Sublicensees. DRL shall have the right, in accordance with this Section 2.2, to extend the licenses granted under Section 2.1 above to its Affiliates, solely for so long as such entity remains an Affiliate of DRL. DRL shall also have the right to grant sublicenses under
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
the license granted to DRL under Section 2.1 to the extent necessary to allow a Third Party contract manufacturer inside or outside the Territory to make the Compound and/or Products for the benefit of DRL and solely for the purposes of DRL and/or its Affiliates using such Compound and using and selling Products in the Territory for use in the Field or providing Products for use in clinical trials for Products conducted by or under the authority of DRL outside the Territory in accordance within this Agreement. [ * ] DRL shall ensure that each of its Affiliates and sublicensees is bound by a written agreement containing provisions at least as protective of the Compound, Products and XenoPort as this Agreement [ * ] and XenoPort shall be an intended third party beneficiary of each such agreement between DRL and its applicable Affiliate or sublicensee, as the case may be; and each such agreement between DRL and its applicable Affiliate or sublicensee, as the case may be, shall include a provision acknowledging and expressly agreeing that XenoPort is a third party beneficiary of such arrangement. In any event, DRL shall remain responsible to XenoPort for all activities of its Affiliates and sublicensees to the same extent as if such activities had been undertaken by DRL itself, and DRL shall be responsible for the payment to XenoPort of all milestone payments and royalties payable with respect to the activities and Net Sales of any Affiliate or sublicensee of DRL.
2.3 Activities Outside the Territory.
(a) DRL agrees that it shall not, and DRL shall cause its Affiliates not to, sell or provide the Compound or Products to any Third Party if DRL or its relevant Affiliate knows, or has reason to believe, that the Compound and/or Products, as the case may be, sold or provided to such Third Party would be sold or transferred, directly or indirectly, for use in the ROW Territory, except to the extent any such Products are for use in clinical trials conducted by or under the authority of DRL in the ROW Territory in accordance with this Agreement or such Compounds are being transferred for the purposes of manufacturing Product for use and/or sale in the Territory or for use in the foregoing clinical trials.
(b) XenoPort agrees that it will not sell or provide the Compound or Products to any Third Party if XenoPort knows, or has reason to believe, that the Compound and/or Products, as the case may be, sold or provided to such Third Party would be sold or transferred, directly or indirectly, for use in the Territory, except to the extent any such Products are for use in clinical trials conducted by or under the authority of XenoPort or its licensees in the Territory in accordance with this Agreement.
2.4 No Other Rights. Except for the rights and licenses expressly granted in this Agreement, XenoPort retains all rights under its intellectual property, and no additional rights shall be deemed granted to DRL by implication, estoppel or otherwise. For clarity, the licenses and rights granted in this Agreement shall not be construed to convey any licenses or rights under the XenoPort Patents with respect to any active pharmaceutical ingredient other than the Compound.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
2.5 Certain Covenants.
(a) DRL Exclusivity. During the Term, except for Products as expressly permitted in accordance with this Agreement [ * ] DRL agrees that it shall not, and DRL shall cause its Affiliates not to, develop, promote, market, distribute, manufacture, offer for sale, sell or provide [ * ] to any Third Party in any country; and DRL agrees that it shall not, and DRL shall cause its Affiliates not to, directly or indirectly, authorize or assist any Third Party to do any of the foregoing.
(i) For purposes of this Section 2.5(a), a [ * ] that: (1) is substantially [ * ]; and (2) is subject to, or has obtained, [ * ]; or [ * ] that: (1) is substantially [ * ]; provided that [ * ].
(ii) For the avoidance of doubt: (A) in no event shall anything in this Section 2.5 grant, or be construed as granting, to DRL or any of its Affiliates, by implication, estoppel or otherwise, any rights under any patents [ * ]; and (B) the only licenses and rights granted to or obtained by DRL (or, subject to Section 2.2, any of its Affiliates) under this Agreement are the express licenses granted by XenoPort to DRL in Section 2.1 above under the XenoPort Know-How and XenoPort Patents with respect to the Compound and Products and, in no event shall, DRL or any of its Affiliates have, or be construed to have, by implication, estoppel or otherwise, any license or other rights (including any rights to enforce) under any patents [ * ] or any other patent rights or other intellectual property owned or controlled by XenoPort, or with respect to [ * ] or other subject matter covered or included in any of the XenoPort Know-How and/or XenoPort Patents that is not a Compound or Product (or a method of manufacture or use of the foregoing).
(b) XenoPort Covenants.
(i) During the Term, and subject to this Section 2.5(b) below [ * ], XenoPort agrees that neither it, nor any of its Affiliates, will promote, market, offer for sale or sell, [ * ] in the Territory and [ * ] or, if DRL [ * ]; in each case, if the [ * ].
(ii) Nothing in this Section 2.5(b) or otherwise shall prevent or otherwise restrict or limit XenoPort, or any of its Affiliates, from granting a license or other rights to, or otherwise authorizing, any third party under any of the XenoPort Patents, [ * ] and/or any other patent rights or other intellectual property owned or controlled by XenoPort ([ * ]) to develop, promote, market, manufacture, distribute, offer for sale, sell or provide [ * ] within or outside the Territory and for any indication or use.
(iii) If, during the Term, XenoPort [ * ] or XenoPort [ * ] or, if DRL [ * ], an [ * ], then the [ * ] in the Territory.
(iv) Certain Definitions:
a) [ * ] shall mean those Patents (A) listed on Exhibit 2.5(b), together with any patents issuing thereon and all reissues, renewals, re-examinations and extensions, divisions or continuations of any such Patents; in each case, to the extent each such Patent: [ * ].
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
b) [ * ] shall mean [ * ].
c) [ * ] shall mean any [ * ] pursuant to which XenoPort: (1) [ * ]; or (2) [ * ].
d) [ * ] shall mean [ * ] that: (1) constitute [ * ] Psoriasis Indications and/or MS Indications in the Territory; or (2) represent [ * ] MS Indication; [ * ] will not include: (i) [ * ].
To the extent that any [ * ] Psoriasis Indications and/or MS Indications in the Territory and other intellectual property, undertakings or subject matter [ * ] a Psoriasis Indication or an MS Indication), then, for the purposes [ * ] for Psoriasis Indications and/or MS Indications in the Territory and such other intellectual property, undertakings or subject matter, [ * ].
ARTICLE III
GOVERNANCE
3.1 Joint Steering Committee. [ * ] after the Effective Date, the Parties shall establish a committee (the “Joint Steering Committee” or “JSC”) to oversee the development, Marketing Approval, manufacturing, and commercialization of the Compound and Products for the Territory.
(a) Composition. The JSC will consist of [ * ] from each Party, and at least [ * ] from each Party [ * ]. In case a representative of a Party is unavailable for a scheduled JSC meeting, upon reasonable notice to the other Party, such Party may substitute in place of such representative for such meeting, a competent person who is authorized by such Party to act on matters that will be presented to the JSC at such meeting. Either Party may also replace its respective JSC representatives at any time with prior notice to the other Party; provided that the criteria for composition of the JSC set forth in the first sentence of this Section 3.1(a) continues to be satisfied following any such replacement.
(b) Meetings. The JSC shall meet at least once each calendar half year during the term of this Agreement, unless otherwise agreed by the Parties or such other frequency as Parties reasonably mutually agree. Such meeting(s) shall be in person, alternating between each Party’s (or its Affiliate’s) facilities in the Territory, unless otherwise mutually agreed to in advance and in writing by the Parties, for example to conduct such meeting via teleconference, video conference or by other mutually agreeable means. At the discretion of each Party, other representatives of XenoPort or DRL may attend JSC meetings as non-voting observers. Each Party shall bear its own personnel and travel costs and expenses relating to such Party’s participation in JSC meetings.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(c) Responsibility. The JSC shall: (a) review and discuss material matters in connection with the development, manufacturing, and regulatory activities of the Compound and Products by or under the authority of each Party, provided that, in the case of XenoPort, XenoPort shall provide to the JSC and DRL information relating to development, manufacturing, regulatory or other activities conducted with respect to the Compound and/or Products for the ROW Territory only to the extent that it has the right to do so; (b) review and discuss DRL’s Development Plan, including any updates, modifications or additions thereto submitted to XenoPort and the JSC pursuant to Section 4.2(b) below; (c) oversee the implementation of the Development Plan; (d) review and discuss protocols for any clinical trials involving a Product being conducted by or under the authority of DRL in the Territory or in the ROW Territory for the purpose of seeking or obtaining Marketing Approval for such Product in the Territory; (e) review and discuss the summaries of any regulatory filings for Product in the Territory (including the precise wording of any Product label) and any regulatory filings pertaining to clinical trials involving a Product conducted by or under the authority of DRL in the ROW Territory; (f) facilitate the exchange of Data and other information and/or materials between the Parties as provided in this Agreement; (g) review and discuss DRL’s and/or its Affiliates’ manufacturing plans for the Compound and Product; (h) review and discuss the Commercialization Plan, including any updates, modifications or additions thereto submitted to XenoPort and the JSC pursuant to Section 5.1(a) below, and review and discuss the progress of DRL and/or its Affiliates thereunder; and (i) undertake and/or approve such other matters as are allocated to the authority of the JSC under this Agreement, or otherwise agreed by the Parties.
(d) Decisions. It is understood that the purpose of the JSC is to provide a forum for discussion and exchange of information between the Parties, as provided in this Agreement, and the JSC [ * ]. If the Parties are unable to mutually agree on a resolution with respect to any matter referred to the Senior Executives under this Section 3.1(d) above, DRL shall not proceed (or authorize any of its Affiliates or any other person or entity to proceed) with a disputed course of action unless and until such dispute is resolved.
(e) Scope of Governance. Notwithstanding the creation of the JSC, each Party shall retain the rights, powers and discretion granted to it hereunder, and the JSC shall not be delegated or vested with such rights, powers or discretion unless such delegation or vesting is expressly provided herein or the Parties expressly so agree in writing. The JSC shall not have the power to amend or modify this Agreement, and its decisions shall not be in contravention of any terms and conditions of this Agreement. It is understood and agreed that issues to be formally decided by the JSC are only those specific issues that are expressly provided in this Agreement to be decided by the JSC.
3.2 Withdrawal. Notwithstanding Section 3.1 above, it is understood that XenoPort’s participation in the JSC is not an obligation of, or a deliverable to be provided by, XenoPort under this Agreement and that such participation is a right of XenoPort that XenoPort may exercise or
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
waive, in its discretion. At any time and for any reason, XenoPort shall have the right to withdraw from participation in the JSC upon notice to DRL referencing this Section 3.2, which notice shall be effective immediately upon receipt (“Withdrawal Notice”). Following the issuance of a Withdrawal Notice, the JSC shall dissolve and neither Party shall be obligated to provide any further updates to the other party with respect to the matters set forth in Section 3.1(c) above.
3.3 General Communications. DRL shall keep XenoPort reasonably informed as to its (and its Affiliates’ and sublicensees’) progress and activities relating to the manufacture, development and commercialization of Products for the Territory (including with respect to regulatory matters and meetings with, or inspections by, Regulatory Authorities and the results thereof or any reports or notices issued in connection therewith), by way of updates to the JSC, at its meetings and as otherwise specified in this Agreement, or as reasonably requested by XenoPort. In connection therewith, DRL shall provide XenoPort with such information regarding such progress and activities under the Development Plan or otherwise relating to the Compound and Products, as XenoPort may reasonably request from time to time. [ * ].
ARTICLE IV
DEVELOPMENT
4.1 Development.
(a) In the Territory. Subject to Section 3.1(d) above, DRL shall be responsible for: (a) conducting, and shall use Diligent Efforts to conduct, such applicable preclinical studies and clinical trials and to obtain such regulatory approvals, including Marketing Approvals and pricing and/or reimbursement approvals, as may be necessary to commercialize Product [ * ] in the Territory; and (b) shall use Diligent Efforts to manufacture the Compound and Product as appropriate to support such clinical trials and regulatory approvals for Products for the Territory. Without limiting the foregoing, DRL agrees to use Diligent Efforts to conduct clinical trials to support, and to obtain, as soon as practical, Marketing Approval for a Product [ * ] in the Territory and to achieve the milestones set forth in Section 6.3. DRL agrees to timely report adverse events/experiences as defined in the SDEA which may occur in the conduct of these activities. It is understood and agreed that all such development and manufacturing efforts shall be [ * ], and shall be conducted in accordance with the Development Plan described in Section 4.2 of this Agreement. Notwithstanding the foregoing, the Parties agree that XenoPort shall [ * ], and upon [ * ] after the Execution Date in the performance of such study [ * ]. The ongoing preclinical carcinogenicity studies described in Exhibit 4.1(a) shall not be revised after the Execution Date by XenoPort without DRL’s consent.
(i) For [ * ], DRL shall promptly consult with the FDA regarding obtaining Marketing Approval [ * ]. In the event (A) the FDA [ * ] or (B) DRL [ * ]. Unless DRL provides notice as set forth in the preceding sentence of this Section 4.1(i), DRL shall use Diligent Efforts to obtain such regulatory approvals, including Marketing Approvals and pricing and/or reimbursement approvals, as may be necessary to commercialize [ * ] Product [ * ] in the Territory.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(b) Outside the Territory. XenoPort or its licensee(s) shall be solely responsible for all development, manufacturing and regulatory activities and expenses with respect to the Compound and Product for the ROW Territory.
4.2 DRL Development Plan.
(a) [ * ], DRL shall provide the JSC with a plan for its development of Product for the Territory for use in the Field for the JSC’s review and discussion (such plan, the “Development Plan”). Such Development Plan shall, at a minimum, outline all activities reasonably necessary for the development of the Compound and Product for the Territory in order to obtain Marketing Approval for Product [ * ] in the Territory, including any necessary preclinical and clinical trials.
(b) DRL agrees to provide to the JSC for its review updated written versions of the Development Plan [ * ], and also any modification or addition to the Development Plan within a reasonable period of time prior to adoption and implementation thereof.
4.3 Change in Formulation. Before modifying or creating a new formulation or dosage form of a Product, or developing the Compound in combination with another active ingredient, DRL shall discuss the proposed modifications or development with XenoPort at the JSC.
4.4 Conduct of Activities. DRL shall conduct, and, if applicable, shall cause its Affiliates and sublicensees to conduct, all activities under the Development Plan in compliance in all material respects with all Applicable Laws and in accordance with good scientific and clinical practices, applicable under the Applicable Laws of the country in which such activities are conducted. DRL shall provide XenoPort with prompt notice of any alleged violations of Applicable Laws, and any material issues relating to the development or manufacture of the Compound and Products.
4.5 Exchange of Data and Know-How.
(a) By XenoPort. After the Effective Date, XenoPort shall provide DRL with [ * ], including, [ * ]. Notwithstanding the foregoing, in no event shall XenoPort be obligated to provide [ * ]. If XenoPort, at its discretion, [ * ].
(b) By Each Party. During the term of this Agreement, each Party shall provide to the other Party, in a timely fashion and as promptly as possible, [ * ].
(c) Use; Disclosure. DRL may use and disclose XenoPort Know-How to its Affiliates or Third Parties only as required to obtain Marketing Approval for Products in the
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Territory and/or as necessary in performing its obligations and exercising its rights under and in accordance with this Agreement, [ * ]. DRL may not use any Data or XenoPort Know-How (and shall cause its any Third Party to whom DRL discloses any XenoPort Know-How and its Affiliates not to use Data or XenoPort Know-How) outside the Territory, except as required to manufacture the Compound or Products or to conduct clinical trials involving a Product in accordance with this Agreement, nor for any compound or products other than the Compound and Products. XenoPort may only use, and disclose to Affiliates and/or Third Parties, DRL Know-How provided by DRL as is reasonably necessary to (i) manufacture the Compound or Products solely for commercialization for the ROW Territory or for use in clinical trials or manufacturing activities conducted in the Territory in accordance with Section 2.1 above; (ii) develop and/or commercialize the Compound and Product for the ROW Territory; (iii) develop and/or commercialize the Compound and Product for the Territory after termination of this Agreement in accordance with Section 12.2; and/or (iv) with respect to the Compound and Product, as necessary under Applicable Laws, including for cross referencing drug master files or other regulatory filings by XenoPort and/or Third Parties for use in the ROW Territory.
(d) Provision of Data to JSC. Upon request by the JSC, DRL shall promptly provide the JSC with summaries in reasonable detail of all Data generated or obtained in the course of activities conducted under DRL’s Development Plan.
4.6 Regulatory Matters.
(a) Assignment of Regulatory Filings. [ * ] the Effective Date, XenoPort shall provide DRL full copies of [ * ] XenoPort shall also assign or cause to be assigned to DRL such [ * ]; provided, however, that, prior to [ * ] in connection with its activities under this Agreement.
(b) Responsibility for Regulatory Filings. Except as provided in Section 4.6(a) above, DRL shall be responsible [ * ] for filing, obtaining and maintaining approvals for the development and commercialization of the Compound and each Product in the Territory, including any such IND, NDA or Marketing Approval, as well as pricing or reimbursement approvals in the Territory and for any regulatory filings required in connection with clinical trials for Products conducted by or under the authority of DRL in the ROW Territory in accordance within this Agreement.
4.7 Regulatory Cooperation. DRL shall [ * ]. Without limiting the foregoing, DRL shall also provide to XenoPort a copy of any material documents, information and correspondence submitted to, or received from, the FDA or any other Regulatory Authority relating to activities conducted by or under the authority of DRL or any of its Affiliates involving the Compound or a Product as soon as reasonably practicable, together with summaries thereof, to the extent such summaries exist.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
4.8 Sharing of Regulatory Filings. Without limiting Section 4.5 above, each Party shall permit the other to access, and shall provide the other Party with sufficient rights to reference and use in association with exercising its rights and performing its obligations under this Agreement (including the right of XenoPort to commercialize the Product in the ROW Territory), all of such Party’s, and, in the case of DRL, its Affiliates’ and, to the extent a Party has the right to do so and subject to Section 10.5 if applicable, its licensees’ Data, regulatory filings and regulatory communications associated with any submissions of NDAs or other regulatory approvals for the Compound or a Product in such Party’s respective territory (i.e., in the Territory, in the case of DRL and in the ROW Territory, in the case of XenoPort).
ARTICLE V
COMMERCIALIZATION AND PROMOTION
5.1 Commercialization
(a) In the Territory. Subject to Section 3.1(d) above, DRL and/or its Affiliate(s), shall have the sole right to launch, manufacture and commercialize Products in the Territory, [ * ] and DRL shall use Diligent Efforts to launch a Product as soon as practicable in the Territory [ * ].
(i) Notwithstanding the foregoing, [ * ], DRL shall provide to the JSC, for its review and discuss, an initial commercialization plan setting forth the strategy for the commercialization of the Products in the Territory during the term of this (such plan, as presented to the JSC and as updated as provided in this Section 5.1(a)(i) below, the “Commercialization Plan”). The Commercialization Plan shall set forth at least estimated launch dates, and sales forecasts, including at least one year and three year sales forecasts and a high-level marketing plan for Products for all approved indications. DRL agrees to provide to the JSC, for its review and discussion, updated versions of the Commercialization Plan [ * ] in writing, including any material modification or addition to the Commercialization Plan [ * ] prior to adoption and implementation thereof.
(b) Outside the Territory. XenoPort and/or its licensee(s) shall have the sole right to launch, manufacture and commercialize Products outside the Territory in its sole discretion.
5.2 Reporting; Adverse Drug Reactions.
(a) [ * ] following the Effective Date, XenoPort will provide a draft Safety Data Exchange Agreement (SDEA) which shall be negotiated by the Parties in good faith and the Parties will enter into a Safety Data Exchange Agreement that more specifically sets forth the obligations of each Party with respect to the exchange of safety information and will require the Parties to comply with a standard operating procedure set forth therein governing the collection, investigation, reporting and exchange of safety information with respect to the Compound and Products, including
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
but not limited to adverse events, product quality, and product complaints, sufficient to permit each Party (and in the case of XenoPort, any of its licensees of the Compound and Products outside the Territory) to comply with their respective legal obligations, all in accordance with Applicable Law. The SDEA must be completed and agreed to prior to DRL’s commencement of any clinical trials involving a Product. The SDEA will be promptly updated if required by changes in Applicable Law. Each Party shall keep the other Party informed about any adverse events of which such Party (or in the case of DRL, any of its Affiliates) becomes aware or is informed regarding the use of Product in or outside the Territory. As between the Parties, DRL shall be responsible for reporting all adverse events/experiences with respect to activities involving the Compound and/or Product conducted by or under the authority of DRL to the appropriate Regulatory Authorities, and XenoPort shall be responsible for reporting all adverse events/experiences with respect to activities involving the Compound and/or Product conducted by or under its authority (other than by or under the authority of DRL) to the appropriate Regulatory Authorities, in accordance with the appropriate laws and regulations of the relevant countries and authorities. DRL shall ensure that its Affiliates comply with such safety reporting obligations. Each Party will designate a pharmacovigilance liaison to be responsible for communicating with the other Party regarding the reporting of adverse events/experiences.
(b) Without limiting Section 5.2(a), [ * ], each Party shall establish and thereafter maintain a safety database with respect to the Compound and Products in such Party’s territory (i.e., in the case of DRL, the Territory, and in the case of XenoPort, the ROW Territory), and shall provide the other Party with a copy of the data included in such safety database. The SDEA shall include provisions to facilitate and ensure that each Party has sufficient information to maintain such a database.
ARTICLE VI
PAYMENTS
6.1 Initial License Fee. In partial consideration of the costs incurred by XenoPort in connection with the research and development of Product and in exchange for the rights granted herein, DRL shall pay to XenoPort an initial license fee in the amount of Forty Seven Million Five Hundred Thousand Dollars ($47,500,000) on the Effective Date. XenoPort will issue an invoice to DRL for the Initial license fee amount on the Effective Date. The initial license fee set forth in this Section 6.1 shall not be refundable or creditable against any future milestone payments, royalties or other payments by DRL to XenoPort under this Agreement.
6.2 Inventory Payment. DRL shall pay to XenoPort an inventory payment in the amount of Two Million Five Hundred Thousand Dollars ($2,500,000) [ * ] after the Delivery Date (as defined in Section 8.1(a)). The inventory payment set forth in this Section 6.2 shall not be refundable or creditable against any future milestone payments, royalties or other payments by DRL to XenoPort under this Agreement, except to the extent expressly provided in Section 8.1 below.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
6.3 Milestone Payments.
(a) Milestone Payments. In addition, DRL shall pay to XenoPort the milestone payments set out below following the first achievement by DRL, or any of its Affiliates, of the corresponding milestone set out below, in accordance with this Section 6.3 and the payment provisions in Article 7:
| Milestone Event | Milestone Payment | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] |
(b) Reports and Payments. DRL shall notify XenoPort in writing promptly [ * ] with regard to Milestone Events [ * ] and [ * ] with regard to Milestone Events [ * ], after the achievement of each milestone set out in Section 6.3(a) by DRL or any of its Affiliates, and shall pay the appropriate milestone payment [ * ] as provided in Section 7.1. Any milestone payable by DRL pursuant to this Section 6.3 shall be made no more than once with respect to the achievement of each milestone set out in Section 6.3(a) by DRL, or any of its Affiliates, and in no event shall the aggregate amount to be paid by DRL under this Section 6.3 exceed Four Hundred Forty Million Dollars ($440,000,000). For the avoidance of doubt, the milestone payments set forth in this Section 6.3 shall not be refundable and shall not be creditable against future milestone payments, royalties or other payments to XenoPort under this Agreement. For the avoidance of doubt, [ * ].
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
6.4 Royalty Payments.
(a) Royalty Rate – [ * ]. Subject to the terms and conditions of this Agreement, in further consideration of the rights granted to DRL under this Agreement, DRL shall pay to XenoPort royalties at the rate set out below on the Net Sales of [ * ] Product(s) in the Territory:
| Annual Net Sales of [ * ] Product(s) in the United States | Royalty Rate | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] |
(b) Royalty Rate – [ * ]. Subject to the terms and conditions of this Agreement, in further consideration of the rights granted to DRL under this Agreement, DRL shall pay to XenoPort royalties at the rate set out below on Net Sales of [ * ] Product(s) in the Territory:
| Annual Net Sales of [ * ] Product(s) in the United States | Royalty Rate | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] | |
| [ * ] |
[ * ] |
(c) Discounting. DRL and its Affiliates shall set prices and discounts for Product in the Territory in the best interest of the commercial success of the Products in the Territory and not for the interest of their other products and services. Without limiting the foregoing, if DRL and/or any of its Affiliates sells any Product to a Third Party who also purchases other products or services from such entities, DRL and its Affiliates agree not to discount or price Products in a manner that would disadvantage Products in order to benefit sales or prices of such other products or services offered to such Third Party. However, the foregoing provisions in this Section 6.4(c) shall not be construed to dictate to DRL and/or its Affiliates any resale prices for Product in the Territory.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(d) Royalty Term.
(i) DRL’s obligations to pay royalties under Section 6.4 (“Royalty Term”) shall commence [ * ] on the [ * ] Product in the Territory and shall continue until the later to occur of: (A) [ * ] Product; (B) the expiration of the last to expire Valid Claim [ * ] in the Territory [ * ]; and (C) expiration of all Regulatory Exclusivity with respect to [ * ] in the Territory.
(ii) [ * ], if (A) a Generic Product [ * ] Product in the Territory, and (B) for [ * ], and (C) the [ * ]; then the royalty rate payable pursuant to Section 6.4(a) or (b), as applicable, on Net Sales of such Product in Territory shall [ * ] such Net Sales during such then-current calendar quarter.
(iii) The Parties acknowledge and agree that the royalty payments (including the royalty rates and term for such royalty payments) set forth in Section 6.4 are to be made in consideration for the licenses and rights granted by XenoPort to DRL with respect to [ * ], and have been agreed to by the Parties for the purpose of reflecting and advancing their mutual convenience, including the ease of calculation of such royalties and the payment of such royalties by DRL to XenoPort.
(e) Reports and Royalty Payment. [ * ] each calendar quarter, DRL shall deliver to XenoPort a report setting out in reasonable detail the information necessary to calculate the royalty payments due under this Section 6.4 with respect to Net Sales made in that calendar quarter, including the following, in each case, [ * ]:
(i) units of [ * ] Product(s) and [ * ] Product(s) sold in the Territory during the relevant calendar quarter;
(ii) gross sales of Products in the Territory in the relevant calendar quarter;
(iii) Net Sales in the relevant calendar quarter in the Territory; and
(iv) all relevant deductions or credits due to DRL in accordance with the terms of this Agreement.
Any amounts due under Section 6.4(a) and (b) for such calendar quarter shall accompany such statement.
ARTICLE VII
PAYMENTS; BOOKS AND RECORDS
7.1 Payment Method. For all payments covered under this Agreement, XenoPort will issue an invoice to DRL for each of such payments. All payments under this Agreement shall be
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
made by bank wire transfer in immediately available funds to an account designated by the Party to which such payments are due. Any payments or portions thereof due under this Agreement that are not paid by the date such payments are due under this Agreement shall bear interest at a rate equal to: [ * ]. This Section 7.1 shall in no way limit any other remedies available to the Parties. All amounts owed by DRL to XenoPort hereunder shall be paid by an entity resident in Switzerland from a bank account located in Switzerland.
7.2 Taxes.
(a) Withholding Taxes. If laws or regulations require withholding by DRL of any taxes imposed upon XenoPort on account of any initial license fees, milestone and/or royalties and/or other payments paid under this Agreement, such taxes shall be timely paid by DRL to the proper taxing authorities. Official receipts of payment of any withholding tax shall be secured and sent to XenoPort as evidence of such payment. The Parties will exercise their reasonable efforts to ensure that any withholding taxes imposed are reduced or eliminated as far as possible under the provisions of any applicable tax treaty, and shall cooperate in filing any forms required for such reduction. If any withholding taxes are imposed with respect to any initial license fees, milestone and/or royalties and/or other payments payable by DRL to XenoPort under this Agreement, DRL shall pay to XenoPort such additional amount as is necessary to ensure that the amount actually received by XenoPort with respect to such payment, free and clear of any withholding taxes (including any such withholding taxes imposed on such additional amount), shall equal the amount of the payment that would have been received if no such withholding taxes applied.
(b) In the event DRL is required to pay any withholding tax under Section 7.2(a) of this Agreement, both Parties will work together in good faith to minimize the amount of such withholding taxes to be paid by DRL. XenoPort shall make reasonable efforts to take credit or refund of withholding taxes paid by DRL by filing tax returns, as may be reasonably required, to claim the tax refund or credit. If XenoPort is successful in getting a refund or credit of such tax amount, then, after deducting any expenses thereof, shall provide refund or credit to DRL promptly following receipt of such credit or refunds.
(c) Indirect Taxes. All payment amounts specified in this Agreement are exclusive of value added taxes, sales taxes, consumption taxes and other similar taxes (the “Indirect Taxes”). If any Indirect Taxes are chargeable in respect of any payments, the paying Party shall pay such Indirect Taxes at the applicable rate in respect of such payments following receipt, where applicable, of an Indirect Taxes invoice in the appropriate form issued by the receiving Party in respect of those payments. The Parties shall issue invoices for all amounts payable under this Agreement consistent with Indirect Tax requirements and irrespective of whether the sums may be netted for settlement purposes. If the Indirect Taxes originally paid or otherwise borne by the paying Party are in whole or in part subsequently determined not to have been chargeable, reasonable steps will be taken by the receiving Party to apply for a refund of these overpaid Indirect Taxes from the applicable governmental authority or other fiscal authority and any amount of undue Indirect Taxes
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
repaid by such authority to the receiving Party will be transferred to the paying Party [ * ]. In the event that a government authority retroactively determines that a payment made by the paying Party to the receiving Party pursuant to this Agreement should have been subject to Indirect Taxes, and the receiving Party is required to remit such Indirect Taxes to the government authority, the receiving Party will have the right (i) to invoice the paying Party for such amount (which shall be payable by the paying Party [ * ]) or (ii) to pursue reimbursement by any other available remedy.
7.3 U.S. Dollars. All dollar amounts specified in, and all payments made under this Agreement, shall be in U.S. dollars.
7.4 Records; Inspection. DRL shall keep, and cause its Affiliates to keep, complete, true and accurate books of accounts and records for the purpose of determining the amounts payable to XenoPort pursuant to this Agreement. Such books and records shall be kept for [ * ]. Such records will be open for inspection during [ * ], by an independent auditor chosen by [ * ] for the purpose of verifying the amounts payable by DRL hereunder. Such inspections may be made [ * ], at reasonable times and [ * ] written notice. Such records [ * ] shall be subject to [ * ]. The independent auditor from the audit firm of international repute [ * ], shall be obligated to execute a reasonable confidentiality agreement prior to commencing any such inspection. Inspections conducted under this Section 7.4 shall be at the expense of [ * ], unless a variation or error producing [ * ] for a period covered by the inspection is established, in which case all reasonable costs relating to the inspection for such period and any [ * ] amounts that are discovered shall be paid by [ * ], together with interest on such [ * ] amounts at the rate set forth in Section 7.1 above. If the audit establishes that [ * ] period covered by the audit, then such [ * ] amount shall be credited against [ * ] under this Agreement after the completion of the audit. The Parties will endeavor in such inspection to minimize disruption of DRL’s normal business activities to the extent reasonably practicable.
ARTICLE VIII
MANUFACTURING AND SUPPLY
8.1 Transfer of Existing Inventory and Stability Studies.
(a) [ * ] XenoPort shall provide to DRL certificates of analysis (“CoAs”) for the quantities of Compound and Product included in the Existing Inventory, together with any additional information pertaining to the Existing Inventory in XenoPort’s possession that has been reasonably requested by DRL prior to the Execution Date. DRL shall [ * ] the CoAs for the Existing Inventory from XenoPort to notify XenoPort of DRL’s acceptance of the Existing Inventory; provided that if DRL fails to so notify XenoPort [ * ], DRL shall be deemed to have accepted the Existing Inventory (such notice or deemed notice, the “Existing Inventory Acceptance Notice”). [ * ] XenoPort’s [ * ] of the Existing Inventory Acceptance Notice from DRL or the Effective Date, [ * ] the “Delivery Date”), all XenoPort’s right, title and interest in and to the Existing Inventory, shall be, and is hereby, conveyed and assigned by XenoPort to DRL, and all such Existing Inventory shall be
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
deemed to have been delivered to DRL under this Agreement as of such date. DRL [ * ] to DRL from and after the Delivery Date and shall thereafter remain with DRL, notwithstanding that certain Third Parties may continue to have possession of the Existing Inventory pursuant to agreements between such Third Party and XenoPort. In addition, on and from the Delivery Date, DRL shall [ * ] conduct of the stability studies of the Compound and Product ongoing as of the Execution Date and being performed by each of the Third Parties identified on Exhibit 1.13 (each such Third Party, a “XenoPort CMO” and such studies, the “Stability Studies”).
(b) [ * ] the Delivery Date, XenoPort agrees to provide each XenoPort CMO with written notice that, as between the relevant XenoPort CMO, DRL and XenoPort: (i) if such XenoPort CMO has possession of any Existing Inventory, title to the Existing Inventory within such XenoPort CMO’s possession has passed to DRL [ * ] XenoPort of the Existing Inventory in such XenoPort CMO’s possession [ * ] DRL [ * ] the Delivery Date, and (ii) DRL [ * ] conduct of those of the Stability Studies [ * ] by such XenoPort CMO. XenoPort shall provide to DRL a copy of each such notice [ * ] to each XenoPort CMO.
(c) If DRL provides XenoPort with an Existing Inventory Acceptance Notice, DRL shall: (i) [ * ] the Existing Inventory held by each XenoPort CMO, [establish a new] storage [ * ] relevant XenoPort CMO with respect to the storage of such Existing Inventory, and (ii) [ * ] the XenoPort CMOs [ * ] DRL [ * ] the Stability Studies, including [ * ], in each case, [ * ] the Delivery Date. [ * ] XenoPort shall cooperate with DRL and [ * ] DRL to [ * ] the applicable quantities of Existing Inventory and [ * ] the Stability Studies [ * ] XenoPort’s [ * ] relevant XenoPort CMO; provided that DRL agrees [ * ] XenoPort to each XenoPort CMO in connection with (i) the storage of such Existing Inventory [ * ] and/or the performance of any services relating to the same (including, without limitation, services relating to the handling, delivery or disposal of such Existing Inventory), and/or (ii) the performance of the Stability Studies [ * ]. Any such amounts shall be paid by DRL to XenoPort [ * ] receipt of an invoice from XenoPort therefor.
(d) XenoPort represents and warrants to DRL [ * ] that, to its knowledge: (i) the storage of such Existing Inventory [ * ] and/or the performance of any handling, delivery or disposal services by each XenoPort CMO relating to the same have been conducted in compliance in all material respects with all Applicable Laws; (ii) the performance of the Stability Studies [ * ] by each such XenoPort CMO has been conducted in compliance in all material respects with all Applicable Laws; and (iii) the quantities of Compound, MMF, and Product specified in Exhibit 1.13 reflect the quantities of such items held at the relevant XenoPort CMO, as reported by such XenoPort CMO to XenoPort.
(e) DRL acknowledges and agrees that, unless otherwise agreed to by XenoPort in writing: (i) all of XenoPort’s obligations with respect to the storage of the Existing Inventory at each XenoPort CMO, and/or the performance of any activities relating thereto, and the continuation of the Stability Studies shall terminate [ * ] the Delivery Date; (ii) XenoPort shall have no further obligations to DRL with respect to the storage of the Existing Inventory, and/or the performance of
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
any activities relating thereto and/or with respect to the Stability Studies, [ * ]; and (iii) in any event, XenoPort’s obligations with respect to all such activities [ * ] shall be limited as described in this Agreement (including this Section 8).
(f) If, [ * ] DRL establishes that (i) any quantities of the Existing Inventory specified on Exhibit 1.13 were less than the actual quantities of Compound, MMF or Product included in the Existing Inventory being held at the relevant XenoPort CMO [ * ] or (ii)(A) any quantities of Compound, MMF or Product included in the Existing Inventory do not conform to the applicable specification therefor set out in Exhibit 8.1(f), (B) such failure to conform to the applicable specifications has not been not caused by any negligence, or failure to comply with Applicable Law or such specifications (including retesting the Existing Inventory as part of the Stability Studies) on the part of DRL or any of its Affiliates or any person storing or otherwise handling the applicable items on DRL’s or its Affiliate’s behalf, (C) the failure to conform is identified [ * ] and (D) DRL or one of its Affiliates has not determined to (or actually used) use such portion of the Existing Inventory in human clinical trials; then, in the case of each of clauses (i) and (ii) above, DRL may notify XenoPort (and shall include in such notice [ * ] description of the issue). [ * ] such notice to XenoPort, DRL shall have the right to deduct from future milestone payments due to XenoPort under Section 6.3, an amount equal to XenoPort’s manufacturing cost of the quantity of Existing Inventory that satisfies the requirements of clause (i) or (ii) above. The foregoing shall be DRL’s sole remedy with respect to any quantities of the Existing Inventory that are inaccurate [ * ] or that do not conform to the applicable specifications and in no event shall DRL be entitled under this Section 8.1(f) to deduct [ * ] from milestone payments otherwise due to XenoPort under Section 6.3 above. For the avoidance of doubt, DRL shall not have the right to assert that XenoPort is in material breach of this Agreement, nor to seek to terminate this Agreement under Section 11.2 below, if any quantities of the Existing Inventory actually held by a XenoPort CMO [ * ] are different to the applicable quantities specified in Exhibit 1.13 or fail to conform the applicable specifications therefor [ * ].
(g) EXCEPT AS EXPRESSLY SET FORTH IN THIS SECTION 8.1 ABOVE, XENOPORT MAKES NO REPRESENTATIONS NOR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, WITH RESPECT TO [ * ], INCLUDING ANY WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE OR AS TO THE CONDITION, VALUE OR QUALITY OF [ * ].
8.2 Transfer of Manufacturing and Supply Responsibilities. [ * ] DRL shall assume responsibility [ * ] for manufacturing and supply of the Compound and Products for use and/or sale by or under the authority of DRL in accordance with this Agreement. XenoPort agrees to [ * ] the Third Party suppliers that manufactured Compound and/or Product for XenoPort’s Phase II Trial, and shall [ * ] and DRL. The Parties acknowledge and agree that XenoPort shall not be obligated to assign or transfer to DRL any contracts with any such Third Party supplier or any other XenoPort CMO.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
8.3 Manufacturing and Supply for the Territory. [ * ], as between the Parties:
(a) Subject to the terms and conditions of this Agreement, during the Term, DRL shall have the exclusive right to manufacture the Compound and the Products for the purposes of commercial sale of Products in the Territory and to satisfy the development activities in the Development Plan that are conducted in accordance with this Agreement; except that XenoPort shall have the non-exclusive right to manufacture the Compound and Products at locations within the Territory solely for use and/or sale in the ROW Territory or for use in development activities in the Territory being conducted by or under the authority of XenoPort to support or obtain Marketing Approval for Products in a jurisdiction(s) within the ROW Territory. It is understood that XenoPort [ * ] DRL with respect to any of the foregoing activities.
(b) During the Term, each Party agrees that it will not, and in the case of DRL, it will cause its Affiliates not to, enter into any manufacturing agreements with Third Parties which expressly prohibits the other Party (or, in the case of XenoPort, XenoPort’s licensees) from purchasing the Compound or Product from the same Third Party supplier.
ARTICLE IX
CONFIDENTIALITY
9.1 Confidential Information. Except as expressly provided in this Agreement, the Parties agree that the receiving Party shall not publish or otherwise disclose and shall not use for any purpose any information furnished to it by the other Party hereto pursuant to this Agreement (collectively, “Confidential Information”). Notwithstanding the foregoing, Confidential Information shall not include information that, in each case as demonstrated by written documentation:
(a) was already known to the receiving Party, other than under an obligation of confidentiality, at the time of disclosure or, as shown by written documentation, was developed by the receiving Party prior to its disclosure by the disclosing Party;
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement;
(d) was subsequently lawfully disclosed to the receiving Party by a person other than the disclosing Party, and who did not directly or indirectly receive such information from disclosing Party; or
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(e) is developed by the receiving Party without use of or reference to any Confidential Information disclosed by the disclosing Party.
9.2 Permitted Disclosures. Notwithstanding the provisions of Section 9.1 above and subject to Sections 9.3 and 9.4 below, each Party hereto may use and disclose the other Party’s Confidential Information to its Affiliates, licensees, contractors and any other Third Parties to the extent such use and/or disclosure is reasonably necessary to exercise the rights granted to it, or reserved by it, under this Agreement (including in the case of XenoPort, in connection with the development, manufacture and/or commercialization of the Compound and/or Products for outside the Territory), prosecuting or defending litigation, complying with Applicable Laws, submitting information to tax or other governmental authorities or conducting clinical trials hereunder with respect to any Product. If a Party is required by law or regulations to make any such disclosure of the other Party’s Confidential Information, to the extent it may legally do so, it will give reasonable advance notice to such other Party of such disclosure and, save to the extent inappropriate in the case of patent applications or otherwise, will use its good faith efforts to secure confidential treatment of such Confidential Information prior to its disclosure (whether through protective orders or otherwise). For any other disclosures of the other Party’s Confidential Information, including to Affiliates, licensees, contractors and/or other Third Parties, a Party shall ensure that the recipient thereof is bound by a written confidentiality agreement as materially protective of such Confidential Information as this Article 9.
9.3 Confidential Terms. Each Party agrees not to disclose to any Third Party [ * ], except each Party may disclose [ * ] Notwithstanding the foregoing, the Parties [ * ].
9.4 Publication of Product Information. [ * ] Products [ * ] the Product [ * ] relating to the Compound and/or Products [ * ] the Parties. The contribution of each Party shall be noted in all publications or presentations by acknowledgment or co-authorship, whichever is appropriate.
9.5 Publicity Review. The Parties acknowledge the importance of supporting each other’s efforts to publicly disclose results and significant developments regarding the Compound and Products for the Territory and other activities pursuant to this Agreement that may reflect the terms of this Agreement or information that [ * ], beyond what is required by Applicable Law, and each Party may make such disclosures from time to time [ * ]. Such disclosures may include, without limitation, achievement of milestones, significant events in the development and regulatory process, commercialization activities and the like. When a Party [ * ] elects to make any such public disclosure under this Section 9.5, it will [ * ]. The principles to be observed in such disclosures shall be accuracy, compliance with Applicable Law, reasonable sensitivity to potential negative reactions of the FDA (and its foreign counterparts) and the need to keep investors informed regarding the [ * ] Party’s business. Accordingly, the [ * ] Party shall [ * ] disclosure that complies with such principles.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
9.6 Prior Non-Disclosure Agreements. Upon execution of this Agreement, the terms of this Article 9 shall supersede any prior non-disclosure, secrecy or confidentiality agreement between the Parties with respect to information relating to the Compound, Products and/or the business or operations of a Party. Any such information disclosed under such prior agreements shall be deemed disclosed under this Agreement.
ARTICLE X
INTELLECTUAL PROPERTY
10.1 Ownership of Inventions. As between the Parties, all rights, title and interest in and to all inventions and other intellectual property made (a) solely by personnel of DRL or any of its Affiliates in connection with this Agreement (“DRL IP”) shall be solely owned by DRL; (b) solely by personnel of XenoPort or any of its Affiliates in connection with this Agreement shall be solely owned by XenoPort; and (c) jointly by personnel of XenoPort and DRL (or their respective Affiliates) in connection with this Agreement (“Jointly-Owned IP”) shall be jointly owned by XenoPort and DRL. DRL hereby grants to XenoPort a non-exclusive, worldwide, irrevocable, fully paid-up license, with the right to sublicense, under any Improvement Patents to make, have made, use, sell, offer for sale, import, practice and otherwise exploit such Improvements, subject to the exclusive rights granted to DRL under this Agreement with respect to the Compound and Products in the Territory. As used herein, “Improvement Patents” means any Patent and any patent rights in inventions made by or under authority of DRL in connection with the research, development and/or commercialization of any Compound and/or Product that pertains to the Compound and/or Product and/or compositions, use, formulations, manufacture, packaging and/or testing of the Compound and/or Product, including Patents and patent rights within the DRL IP and DRL’s interest in any Jointly-Owned IP (“Improvements”).
10.2 Prosecution and Maintenance of XenoPort Patents.
(a) XenoPort Patent Rights. XenoPort shall, in its sole discretion and expense, control all Prosecution and Maintenance of Patents included in the XenoPort Patents [ * ], XenoPort shall [ * ]. For the purposes of this Section 10.2, “Prosecution and Maintenance” (including variations such as “Prosecute and Maintain”) shall mean, with respect to a Patent, the preparing, filing, prosecuting and maintenance of such Patent, as well as re-examinations, reissues and requests for Patent term extensions and the like with respect to such Patent, together with the conduct of interferences, the defense of oppositions and other similar proceedings with respect to a Patent.
(b) DRL Patents and Jointly-Owned Patents. DRL shall, in its sole discretion and expense, control all Prosecution and Maintenance of all Patents included within DRL IP, including Improvement Patents. Prosecution and Maintenance of any Patent included within Jointly-Owned IP, and [ * ].
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(c) Cooperation. Each Party shall cooperate with the other Party in connection with its Prosecution and Maintenance activities undertaken as set forth this Section 10.2, including: (i) making available in a timely manner any documents or information as the other Party reasonably requests to facilitate Prosecution and Maintenance of the XenoPort Patents, Patents included within DRL IP, and Patents included within Jointly-Owned IP; and (ii) if and as appropriate, signing (or causing to have signed), at the reasonable request of the other Party, all documents relating to the Prosecution and Maintenance of the XenoPort Patents and, during the Term, Patents included within DRL IP, and Patents included within Jointly-Owned IP. Each Party shall [ * ] provide to the other Party all information reasonably requested with regard to its Prosecution and Maintenance activities pursuant to this Section 10.2. Each Party shall hold all information disclosed to it under this Section as Confidential Information.
10.3 Enforcement.
(a) Notice. Subject to the provisions of this Section 10.3, in the event that DRL [ * ] that any XenoPort Patents in the Territory are infringed by a Third Party, or any of the XenoPort Patents are subject to a declaratory judgment action in the Territory arising from such infringement, in each case with respect to the manufacture, sale or use of a product containing a Compound (an “Infringing Product”), DRL shall [ * ] notify XenoPort. XenoPort shall have the initial right (but not the obligation), [ * ] to enforce the XenoPort Patents with respect to such infringement or defend any declaratory judgment action with respect thereto in the Territory (for purposes of this Section 10.3, an “Enforcement Action”). DRL shall, [ * ] have the right to join in as a party plaintiff [ * ] and, in any event, at all times shall give reasonable assistance to XenoPort with respect to such Enforcement Action. [ * ] XenoPort shall keep DRL reasonably informed of the progress of any such Enforcement Action. [ * ]XenoPort agrees not to settle any Enforcement Action, or make any admissions or assert any position in such Enforcement Action, [ * ] with respect to the Compound and Products in the Territory, [ * ].
(b) Initiating Enforcement Actions. In the event that, [ * ] XenoPort fails to initiate an Enforcement Action to enforce the XenoPort Patents against [ * ] infringement by a Third Party in the Territory, which infringement consists of the manufacture, sale or use of an Infringing Product in the Field in the Territory, [ * ], DRL may initiate an Enforcement Action against such infringement [ * ]; provided however that if the Infringing Product is the subject of a paragraph IV certification under the Hatch Waxman statute, 21 U.S.C. §355(j)(2)(A)(vii)(IV) or any successor provision thereto (“Paragraph IV Certification”), XenoPort shall [ * ] initiate such Enforcement Action; provided further that if any applicable XenoPort Patent contains claims directed to subject matter other than the Compound or Product, or a method of use or manufacture of the Compound or a Product, DRL shall [ * ]. [ * ] XenoPort shall cooperate in such Enforcement Action [ * ]. DRL shall keep XenoPort [ * ] informed of the progress of any such Enforcement Action. XenoPort shall, [ * ] have the right to join in as a party plaintiff and to give [ * ] assistance to such Enforcement Action. DRL agrees not to settle any Enforcement Action, or make any admissions or assert any
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
position in such Enforcement Action, in a manner that would materially adversely affect the validity, enforceability or scope of any XenoPort Patents or any Patents controlled by XenoPort relating to the Compound or Product (or any method of use or manufacture of any of the foregoing) outside the Territory [ * ].
(c) Recovery. DRL and XenoPort shall [ * ] associated with any litigation against infringers undertaken pursuant to Section 10.3(b) above or settlement thereof [ * ].
(d) Cooperation. The Parties shall keep one another informed of the status of their respective activities regarding any litigation or settlement thereof concerning any Enforcement Actions described in this Section 10.3 controlled by such Party and shall assist one another and cooperate in any such litigation at the other’s reasonable request (including joining as a party plaintiff to the extent necessary and requested by the other Party).
10.4 Third Party Infringement Claims. If the manufacture or use of the Compound or the manufacture, use or sale of a Product in the Territory pursuant to this Agreement results in a claim, suit or proceeding alleging patent infringement against XenoPort or DRL (or their respective Affiliates or licensees) (collectively, “Infringement Actions”), such Party shall [ * ] notify the other Party hereto in writing. The Party subject to such Infringement Action shall have the right to direct and control the defense thereof; provided, however, that the other Party may participate in the defense and/or settlement thereof [ * ]. In any event, the Party that is subject to the Infringement Action agrees to keep the other Party hereto [ * ] informed of all material developments in connection with any such Infringement Action. The Party who is subject to the Infringement Action agrees not to settle such Infringement Action, or make any admissions or assert any position in such Infringement Action, in a manner that would adversely affect the other Party’s rights with respect to the Compound or a Product in such other Party’s territory (i.e., in the case of DRL, the Territory, and in the case of XenoPort, the ROW Territory) or the manufacture or use of the Compound or manufacture, use or sale of any Product in such other Party’s respective territory, [ * ]. To the extent an Infringement Action is initiated during the Term, [ * ].
10.5 XenoPort IP Acquired after the Execution Date. If, after the Execution Date, XenoPort acquires from a Third Party subject matter within the XenoPort Patents, and/or XenoPort Know-How, as applicable, (“Third Party IP”) that is subject to royalty or other payment obligations to such Third Party, then the following shall apply. The licenses granted under Section 2.1 above with respect to such Third Party IP shall be subject [ * ] the XenoPort Patents, and/or XenoPort Know-How, as applicable, hereunder.
10.6 Patent Marking. DRL agrees to xxxx appropriately, and ensure that its Affiliates appropriately xxxx, all patented Product that it sells or distributes pursuant to this Agreement in accordance with the applicable patent statutes or regulations in the Territory to enable recovery of all damages or remedies available with respect to infringement of XenoPort Patents or any other Patents controlled by DRL relating to the Compound or Product.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
10.7 Regulatory Data Protection.
(a) To the extent required or permitted by Applicable Law, the Parties will use Diligent Efforts to promptly, accurately and completely list, with the applicable Regulatory Authorities during the Term all applicable XenoPort Patents, and/or any Patents that are Controlled by DRL or its Affiliates, for any Product and that the Parties intend, or have begun, to commercialize in the Territory and that have become the subject of an NDA submitted to FDA, such listings to include all Orange Book listings required under the Xxxxx-Xxxxxx Act (regardless of which Party is the sponsor of record of the NDA at such time).
(b) In connection with such listings, the Parties will meet to evaluate and identify all applicable Patents that are Controlled by each Party. DRL [ * ] the listing of all applicable Patents for any Product Controlled by DRL, and XenoPort [ * ] listing of any XenoPort Patent.
10.8 Product Trademarks. DRL shall not use, nor authorize the use of, the Product Trademarks except in the Territory, and agrees not to file or register the Product Trademarks, or any trademarks confusingly similar to the Product Trademarks, in any jurisdiction within the ROW Territory. DRL shall own, and shall be entitled to register, all Product Trademarks in the Territory in connection with packaging, marketing, promoting, distributing and selling Product(s), including all goodwill accruing from the use of such Product Trademarks in the Territory. XenoPort shall not knowingly take any action that would materially adversely affect the value of the Product Trademarks in the Territory.
ARTICLE XI
TERM, TERMINATION AND CONDITIONS TO OBLIGATIONS
11.1 Term. This Agreement shall commence on the Execution Date, and unless terminated earlier as provided in this Article 11, shall continue in full force and effect on a Product-by-Product basis until DRL has no remaining payment obligations to XenoPort with respect to such Product (the “Term”). Upon expiration (but not an earlier termination) of this Agreement, DRL shall [ * ] such Product in the Territory.
11.2 Breach. Either Party to this Agreement may terminate this Agreement in the event the other Party shall have materially breached or defaulted in the performance of any of its obligations hereunder, and such default shall have continued for [ * ] after written notice thereof was provided to the breaching Party by the non-breaching Party; provided, however, that if such breach is capable of being cured but cannot be cured within such [ * ] period and the breaching Party initiates actions to cure such breach within such [ * ] period and thereafter diligently pursues such actions, then upon written notice by the non-breaching Party to the non-breaching Party describing actions being taken to cure the applicable breach, the breaching Party shall have an additional period, but in no event longer than [ * ], to cure such breach. Any such termination shall become effective at the end of the applicable period unless the breaching Party has cured any such breach or default prior to the expiration of the applicable period.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
11.3 Termination For Convenience. DRL may terminate this Agreement in its entirety for any reason: (a) upon sixty (60) days’ prior written notice to XenoPort prior to the First Commercial Sale; and (b) upon one hundred eighty (180) days’ prior written notice to XenoPort following the First Commercial Sale.
11.4 Termination for Patent Challenge. XenoPort shall have the right to terminate this Agreement upon notice to DRL in the event that DRL, or any Affiliate or sublicensee of DRL, or any Third Party assigned or designated by DRL or any of its Affiliates, [ * ] in connection with a challenge to the validity, enforceability, scope, inventorship or ownership of any of the XenoPort Patents, or any other Patents controlled by XenoPort in the ROW Territory relating to the Compound or a Product, in any court or tribunal or before the United States Patent and Trademark Office or, any other patent office or in any arbitration proceeding, including in connection with an opposition proceeding, re-examination, or other post-grant proceeding, unless such challenge is withdrawn [ * ].
11.5 Rights in Bankruptcy. All rights and licenses granted under or pursuant to this Agreement by either Party are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction, licenses of right to “intellectual property” as defined under Section 101 of the U.S. Bankruptcy Code. The Parties agree that the Parties, as licensees of such rights under this Agreement, shall retain and may fully exercise all of their rights and elections under the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction. The Parties further agree that, in the event of the commencement of a bankruptcy proceeding by or against either Party under the U.S. Bankruptcy Code or any analogous provisions in any other country or jurisdiction, the Party that is not a Party to such proceeding shall be entitled to a complete duplicate of (or complete access to, as appropriate) any such intellectual property and all embodiments of such intellectual property, which, if not already in the non-subject Party’s possession, shall be promptly made available to it (a) upon any such commencement of a bankruptcy proceeding upon the non-subject Party’s written request therefor, unless the Party subject to such proceeding elects to continue to perform all of its obligations under this Agreement, or (b) if not made available under clause (a) above when required, following the rejection of this Agreement by or on behalf of the Party subject to such proceeding upon written request therefor by the non-subject Party.
11.6 Conditions to Obligations of DRL and XenoPort. As provided in Section 16.1 below, the rights and obligations of each of DRL and XenoPort pursuant to Articles 2 through 8 (excluding Section 8.1(a), 8.1(d) and 8.1(e)) and Article 10 shall not take effect until the HSR Clearance Date
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
and shall further be subject to the fulfillment by each Party, or waiver by the other Party, on or prior to the Effective Date, of the following conditions:
(a) The representations and warranties of each Party contained in Article 13 of this Agreement shall be true and correct in all respects (in the case of any representation or warranty qualified by materiality) or in all material respects (in the case of any representation or warranty not qualified by materiality) as of the Effective Date with the same effect as though made at and as of such date (except those representations and warranties that address matters only as of a specified date, which shall be true and correct in all respects as of that specified date).
(b) Each Party will have received from the other Party a certificate, signed by a duly authorized officer of such other Party, certifying that the condition set forth in Section 11.6(a) has been satisfied.
ARTICLE XII
EFFECT OF TERMINATION
12.1 Accrued Obligations. The expiration or termination of this Agreement for any reason shall not release either Party from any liability that, at the time of such expiration or termination, has already accrued to the other Party or that is attributable to a period prior to such expiration or termination, nor will any termination of this Agreement preclude either Party from pursuing all rights and remedies it may have under this Agreement, or at law or in equity, with respect to breach of this Agreement.
12.2 Rights on Termination. This Section 12.2 shall apply upon any termination of DRL’s rights under this Agreement in its entirety, excluding only termination of this Agreement pursuant to Section 11.2 for XenoPort’s breach.
(a) Wind-down Period.
(i) Development. In the event there are any on-going clinical trials of Product for the Territory and/or any on-going formulation studies (e.g. stability studies) of Product for the Territory, to the extent so requested by XenoPort, DRL agrees to either promptly transition such clinical trials and/or formulation studies to XenoPort (or its designee), or continue to conduct such clinical trials and/or formulation studies, at DRL’s own expense, and otherwise in accordance with the terms and conditions of this Agreement in effect prior to its termination.
(ii) Commercialization. To avoid a disruption in the supply of Product to patients in the Territory if this Agreement expires or is terminated after the First Commercial Sale of Product in the Territory, DRL and its Affiliates shall continue to distribute all Products for which Marketing Approval has been obtained in the Territory in accordance with the terms and conditions of this Agreement, [ * ]. Notwithstanding any other provision of this Agreement, [ * ] XenoPort shall have the right to engage one or more other distributor(s) and/or licensee(s) of the Compound and/or Products in all or part of the Territory. Any Product sold or disposed by DRL and its
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Affiliates in the Territory [ * ] shall be subject to the applicable payment obligations under Articles 6 and 7 above. [ * ] expiration or termination of this Agreement (or if applicable and at XenoPort’s election, [ * ]), DRL shall notify XenoPort of any quantity of the Compound and/or Product remaining in DRL’s inventory and, [ * ] DRL shall transfer to XenoPort (or its designee) all right, title and interest in and to any such quantities of the Compound and/or Product [ * ].
(b) Assignment of Regulatory Filings and Marketing Approvals. At XenoPort’s [ * ], DRL shall, at its expense, assign or cause to be assigned to XenoPort or its designee [ * ] all regulatory filings and registrations (including INDs, NDAs and Marketing Approvals) for the Compound and/or any Product in the Territory, including any such regulatory filings and registrations made or owned by its Affiliates. In each case, unless otherwise required by any Applicable Law or requested by XenoPort, the foregoing assignment (or availability) shall [ * ] such expiration or termination of this Agreement. In addition, DRL shall, [ * ] provide to XenoPort (in electronic form, to the extent the same exists in electronic form) a copy of all Data and DRL Know-How pertaining to the Compound and/or Product (including all development plans, life cycle management plans, medical education materials and/or commercialization plans), to the extent not previously provided to XenoPort, and XenoPort shall have the right to use (and authorize the use of), and to disclose, all such Data and other DRL Know-How following expiration or termination of this Agreement.
(c) Supply. DRL shall [ * ] transition to XenoPort, upon XenoPort’s request, any arrangements with any contractor from which DRL had arranged to obtain a supply of the Compound or Product for use or sale in the Territory. In any event, DRL shall continue to provide to (or procure for) XenoPort Compound and/or Products [ * ]. The [ * ] continued supply of Compound and/or Products to XenoPort pursuant to the transition supply terms of this Section 12.2(c) shall [ * ]. Without limiting the foregoing provisions of this Section 12.2(c), DRL shall, [ * ] provide to XenoPort [ * ] all DRL Know-How pertaining to the manufacture of the Compound and Products to the extent not previously provided to XenoPort during the Term or pursuant to Section 12.2(b), and XenoPort shall have the right to use (and authorize the use of) and to disclose all such DRL Know-How in connection with the manufacture of the Compound and Products following expiration or termination of this Agreement. Further, [ * ] DRL shall [ * ] to XenoPort (and/or its designated contract manufacturer(s)) DRL’s (or its relevant Third Party contract manufacturer’s) personnel with expertise in manufacturing any Compound and/or Products [ * ] to allow XenoPort (and/or its designated contract manufacturer(s)) to implement the manufacture of such Compound and/or Products at their facilities [ * ].
(d) Transition. DRL shall [ * ] XenoPort and/or its designee to effect a smooth and orderly transition in the development, sale and on-going marketing, promotion and commercialization of the Compound and Product in the Territory. Without limiting the foregoing, DRL shall conduct [ * ] activities to be conducted under Section 12.2. In addition, DRL shall refer all inquiries received by DRL [ * ] regarding the Compound or Products to XenoPort or its designee. Further, DRL shall [ * ] its Affiliates shall transition all Compound and Products back to XenoPort in the manner set forth in this Section 12.2 as if such Affiliate were named herein.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(e) Licenses; Assignment. DRL hereby grants to XenoPort an exclusive, worldwide, royalty-free license, with the right to grant and authorize sublicenses, under any DRL Know-How, and/or any Patents owned or Controlled by DRL or its Affiliates [ * ] in connection with, the development, manufacture and/or commercialization of the Compound and/or Product for the purposes of making, having made, using, developing, importing, selling, distributing, marketing, promoting and otherwise commercializing the Compound, product containing Compound and/or Product in or outside the Territory. Upon the effective date of a termination, DRL agrees to assign and does hereby assign to XenoPort, DRL’s right, title, and interest in and to the Product Trademarks in the Territory, and all goodwill accruing from the use of such Product Trademarks, and DRL shall cease all use of the Product Trademarks.
(f) Return of Materials. Except for any Product in DRL’s inventory that is transferred to XenoPort as set forth in Section 12.2(a)(ii), [ * ] DRL shall, [ * ] destroy all tangible items comprising, bearing or containing any Data provided by XenoPort and/or any Product Trademarks, including any samples, literature, sales and promotional aids relating to the Compound and/or Products (“Product Materials”) and Confidential Information of XenoPort, that is in DRL’s possession, and provide written certification of such destruction, or prepare such tangible items of Product Materials and Confidential Information for shipment to XenoPort, as XenoPort may direct [ * ].
12.3 Termination by DRL under Section 11.2. If this Agreement is terminated by DRL in accordance with Section 11.2 by reason of XenoPort’s material uncured breach, then the only provisions of this Agreement that will survive such termination are [ * ]
(a) [ * ].
12.4 Survival. Upon the expiration or termination of this Agreement, all rights and obligations of the Parties under this Agreement shall terminate except as expressly provided in Sections 12.1, 12.2 and 12.3 above and those rights and obligations of the Parties under the following Articles and Sections: [ * ].
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
ARTICLE XIII
REPRESENTATIONS, WARRANTIES AND COVENANTS
13.1 General Representations. Each Party hereby represents and warrants to the other Party as of the Execution Date as follows:
(a) Duly Organized. Such Party is a corporation duly organized, validly existing and is in good standing under the laws of the jurisdiction of its incorporation, is qualified to do business and is in good standing as a foreign corporation in each jurisdiction in which the conduct of its business or the ownership of its properties requires such qualification and failure to have such would prevent such Party from performing its obligations under this Agreement.
(b) Due Execution; Binding Agreement. This Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms. The execution, delivery and performance of this Agreement by such Party have been duly authorized by all necessary corporate action and do not and will not: (i) require any consent or approval of its stockholders; (ii) to such Party’s knowledge, violate any law, rule, regulation, order, writ, judgment, decree, determination or award of any court, governmental body or administrative or other agency having jurisdiction over such Party; nor (iii) conflict with, or constitute a default under, any agreement, instrument or understanding, oral or written, to which such Party is a party or by which it is bound.
(c) Debarment. Such Party has not been debarred and is not subject to debarment and neither it nor any of its Affiliates have knowingly used, nor will knowingly use in any material capacity, in connection with the activities directed to the development, manufacturing or commercialization of the Compound or Product for the Territory, any person or entity who has been debarred pursuant to Section 306 of the FDCA, or who is subject of a conviction described in such Section 306. Further, during the term of this Agreement, such Party agrees to inform the other Party in writing promptly if it or any person or entity who is performing activities directed to the development, manufacturing or commercialization of the Compound or Product for the Territory hereunder is debarred or is the subject of a conviction described in such Section 306, or if any action, suit, claim, investigation or legal administrative proceeding is pending or, to such Party’s knowledge, is threatened, relating to the debarment of such Party, its Affiliates or any person or entity used in any material capacity by such Party or its Affiliates in connection with the activities directed to the development, manufacturing or commercialization of the Compound or Product for the Territory.
(d) False Statements. Neither such Party, nor any officer, employee or agent of such Party, has made, or, during the term of this Agreement, will make an untrue statement of a material fact to the FDA with respect to the Compound or Product (whether in any submission to such Regulatory Authority or otherwise), or has knowingly failed or will knowingly fail to disclose a material fact required to be disclosed to the FDA with respect to the Compound or Product.
(e) Anti-Bribery; Anti-Corruption. To the extent applicable, each Party has complied, and will comply, in all material respects with all Applicable Laws pertaining to bribery, corruption and prohibited business practices of and has not, and will not, engage in any actions in relation to transactions that are in violation of such Applicable Laws, including, to the extent applicable, Prevention of Corruption Act, 1988, Foreign Corrupt Practices Act, 1977 and UK Xxxxxxx Xxx 0000.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
13.2 Representations, Warranties and Covenants of XenoPort. XenoPort represents, warrants and covenants to DRL that, as of the Execution Date:
(a) it Controls the XenoPort IP, and it has the full right and authority to grant the rights and licenses as provided herein;
(b) it has not previously granted, nor will it grant during the Term, any right, license or interest in or to the XenoPort IP, or any portion thereof, that is in conflict with the rights or licenses granted to DRL under this Agreement;
(c) to its knowledge, there are no Patents Controlled by XenoPort in the Territory not included in the XenoPort Patents that claim composition of matter (including, product by process), method of manufacture or use in the Field of the Compound or Product;
(d) to its knowledge, the research, development, manufacture, sale, offer for sale, import or export of the Compound or the Non-Equivalent Product described in clause (a) of Section 1.26, in each case, as they exist as of the Execution Date, does not infringe a claim of a Patent nor misappropriate any other intellectual property rights of any Third Party;
(e) to its knowledge, none of the XenoPort Patents are invalid or unenforceable, all fees required to be paid to applicable governmental patent offices in the Territory as of the Execution Date in order to prosecute or maintain such XenoPort Patents have been paid on or before the due date for payment, and all such XenoPort Patents have been filed and maintained in a manner consistent with XenoPort’s standard practice in the Territory;
(f) there are no actual, pending, or, to its knowledge, alleged or threatened actions, suits, claims, interference or governmental investigations in the Territory involving the Compound, the Product, or the XenoPort IP, by or against XenoPort, or any of its Affiliates. In particular, to its best knowledge, there is no pending or threatened product liability action nor intellectual property right litigation in the Territory in relation to the Compound;
(g) except for any filings that may be made by XenoPort with the FTC and DOJ pursuant to the Xxxx-Xxxxx-Xxxxxx Act and subject to satisfaction of the HSR Conditions as described in Article 16, all necessary consents, approvals and authorizations of all Regulatory Authorities, other governmental authorities and other persons or entities required to be obtained by XenoPort in order to enter into this Agreement have been obtained; and
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
(h) to its knowledge, there is no actual, pending, alleged or threatened infringement or misappropriation by a Third Party in the Territory of any of the XenoPort Patents or the XenoPort Know-How.
13.3 Representations and Warranties of DRL. DRL represents and warrants to XenoPort that, as of the Execution Date:
(a) it has the full right and authority to grant the rights granted herein;
(b) all necessary consents, approvals and authorizations of all Regulatory Authorities, other governmental authorities and other persons or entities required to be obtained by DRL in order to enter into this Agreement have been obtained; and
(c) DRL does not have any knowledge that any of XenoPort’s representations and warranties set forth in Sections 13.1 and 13.2 above are inaccurate.
13.4 Notice Obligations Between Execution Date and Effective Date. Each Party shall [ * ] notify the other Party if any representation or warranty made by it in this Article 13 becoming untrue or inaccurate in any material respect during the period commencing on the Execution Date and ending on the Effective Date; provided, however, that the delivery of any notice pursuant to this Section 13.4 shall not in and of itself be deemed to limit or modify such receiving Party’s payment or other obligations under this Agreement. For the avoidance of doubt, if the Effective Date occurs, the delivery of a notice by XenoPort to DRL pursuant to this Section 13.4 shall not, and shall not be deemed to, modify DRL’s obligations to pay the entire amounts specified in Section 6.1 and Section 6.2 above (with respect to Section 6.2, provided that DRL issues an Existing Inventory Acceptance Notice pursuant to Section 8.1(a) above).
13.5 DISCLAIMER. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTIES OF ANY KIND EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NONINFRINGEMENT OR VALIDITY OF ANY PATENTS ISSUED OR PENDING.
ARTICLE XIV
INDEMNIFICATION
14.1 Indemnification of XenoPort. DRL shall indemnify and hold harmless each of XenoPort, its Affiliates and licensees and their respective directors, officers and employees and the successors and assigns of any of the foregoing (the “XenoPort Indemnitees”), from and against any and all liabilities, damages, penalties, fines, costs, expenses (including reasonable attorneys’ fees and other expenses of litigation) (“Liabilities”) from any claims, actions, suits or proceedings brought by
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
a Third Party (a “Third Party Claim”) incurred by any XenoPort Indemnitee, arising from, or occurring as a result of: (a) the developing, making, having made, use, marketing, distribution, import, export and/or sale of the Compound and/or Products by or under the authority of DRL on or after the Effective Date, including, without limitation, any Third Party Claim alleging product liability, manufacturing, packaging or labeling defect, failure to warn, or any similar action relating to the formulation (to the extent such formulation of a Product was developed after the Effective Date), manufacture, use or safety of those Products sold or provided by or under authority of DRL (a “Product Liability Claim”) (b) the storage of the Existing Inventory by any XenoPort CMO on or after the Delivery Date and/or any activities or services performed on or after the Delivery Date by a XenoPort CMO relating to such Existing Inventory, or (c) any material breach of any representations, warranties or covenants by DRL in Section 13 above; except to the extent such Third Party Claims fall within the scope of XenoPort’s indemnification obligations set forth in Section 14.2 below.
14.2 Indemnification of DRL. XenoPort shall indemnify and hold harmless each of DRL, and its Affiliates and their respective directors, officers and employees and the successors and assigns of any of the foregoing (the “DRL Indemnitees”), from and against any and all Liabilities from any Third Party Claims incurred by any DRL Indemnitee, arising from, or occurring as a result of: (a) the developing, making, having made, use, marketing, distribution, import, export and/or sale of any Compound and/or Product by XenoPort or its Product licensee(s) (other than DRL or any Person under DRL’s authority), including, without limitation, any Third Party Claim alleging product liability, manufacturing, packaging or labeling defect, failure to warn, or any similar action relating to the formulation (to the extent such formulation was developed before the Effective Date), manufacture, use or safety of those Products sold or provided by or under authority of XenoPort or its Product licensee(s), (b) the storage of the Existing Inventory by any XenoPort CMO on or before the Delivery Date and/or any activities or services performed on or before the Delivery Date by a XenoPort CMO relating to such Existing Inventory, or (c) any material breach of any representations, warranties or covenants by XenoPort in Article 13 above.
14.3 Procedure. Except with respect to Third Party infringement claims subject to Section 10.3 above, a Party that intends to claim indemnification under Article 14 (the “Indemnitee”) shall promptly notify the other Party (the “Indemnitor”) in writing of any Third Party Claim, in respect of which the Indemnitee intends to claim such indemnification, and the Indemnitor shall have sole control of the defense and/or settlement thereof. The indemnity arrangement in Article 14 shall not apply to amounts paid in settlement of any action with respect to a Third Party Claim, if such settlement is effected without the consent of the Indemnitor, which consent shall not be withheld or delayed unreasonably. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim, if prejudicial to its ability to defend such action, shall relieve such Indemnitor of any liability to the Indemnitee under Article 14, but the omission to so deliver written notice to the Indemnitor shall not relieve the Indemnitor of any liability that it may have to any Indemnitee otherwise than under Article 14. The Indemnitee under Article 14 shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action with respect to a Third Party Claim covered by this indemnification.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
14.4 Insurance. Each Party shall secure and maintain in effect during the term of this agreement, and [ * ] insurance policy(ies) underwritten by a reputable insurance company and having [ * ] for exposures related to pharmaceutical products (including the Compound and Products) and their development, manufacture and/or commercialization. Such policies shall include coverage for [ * ]. Upon request by a Party, certificates of insurance evidencing the coverage required above shall be provided by the other Party to the requesting Party.
14.5 LIMITATION OF LIABILITY. EXCEPT WITH RESPECT TO (A) EACH PARTY’S INDEMNIFICATION OBLIGATIONS UNDER SECTION 14.1 OR SECTION 14.2, (B) EITHER PARTY’S BREACH OF ITS OBLIGATIONS UNDER SECTION 2.3, OR (C) EITHER PARTY’S BREACH OF ITS OBLIGATIONS UNDER SECTION 2.5, AS APPLICABLE, IN NO EVENT SHALL EITHER PARTY OR ANY OF ITS AFFILIATES BE LIABLE TO THE OTHER PARTY OR ANY OF ITS AFFILIATES FOR SPECIAL, INDIRECT, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES, INCLUDING LOSS OF PROFITS THAT ARE CONSEQUENTIAL DAMAGES, WHETHER IN CONTRACT, WARRANTY, TORT, NEGLIGENCE, WILLFUL MISCONDUCT, STRICT LIABILITY OR OTHERWISE, ARISING OUT OF OR RELATING TO THIS AGREEMENT, THE TRANSACTIONS CONTEMPLATED HEREIN OR ANY BREACH HEREOF.
ARTICLE XV
DISPUTE RESOLUTION
15.1 Senior Executives. In the event of any dispute between the Parties arising out of or in connection with this Agreement, either Party may, by written notice to the other, have such dispute referred to the Senior Executives of the Parties for attempted resolution by good faith negotiations [ * ] and, in such event, each Party shall cause its representative to meet and be available to attempt to resolve such issue. Notwithstanding the foregoing, neither Party shall be obligated to negotiate [ * ]. If the Parties should resolve such dispute or claim, a memorandum setting forth their agreement will be prepared and signed by both Parties if requested by either Party.
15.2 Jurisdiction. Any dispute, controversy or claim with respect to the breach, interpretation, performance or enforcement of this Agreement not resolved pursuant to Section 15.1 shall be subject to the exclusive jurisdiction of the state and federal courts in New York City, New York. Each Party hereby (a) submits, and irrevocably consents, to the exclusive jurisdiction and venue of such courts for the resolution of such disputes and (b) agrees that process shall be served upon such Party in the manner set forth in Section 17.7, and that service in such manner shall constitute valid and sufficient service of process. Each Party hereby waives the defense of any inconvenient forum for the maintenance of any action or proceeding in such jurisdiction for such
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
purpose and covenants not to assert or plead any objection that such Party might otherwise have to such jurisdiction, venue, and process. Each Party hereby agrees not to commence any legal proceedings relating to or arising out of this Agreement or the transactions contemplated hereby in any jurisdiction or courts other than as provided in this Section 15.2. Notwithstanding the foregoing, a Party will be entitled to seek enforcement of a judgment entered pursuant to this Section 15.2 in any court having competent jurisdiction thereof where enforcement is deemed necessary.
15.3 Interim Relief. Notwithstanding anything in this Article 15 to the contrary, DRL and XenoPort shall each have the right to apply to any court of competent jurisdiction for a temporary restraining order, preliminary injunction or other similar interim or conservatory relief, as necessary to protect the right or property of such Party.
ARTICLE XVI
XXXX-XXXXX-XXXXXX
16.1 Xxxx-Xxxxx-Xxxxxx Act Compliance. Notwithstanding anything to the contrary in this Agreement, this Agreement shall be binding upon the Parties as of the Execution Date; however, the provisions of Articles 2 through 8 (excluding Section 8.1(a), 8.1(d) and 8.1(e)) and Article 10 shall not take effect until the HSR Clearance Date. As used herein, the “HSR Clearance Date” shall mean such time as: (a) the Parties shall have complied with all applicable requirements of the Xxxx-Xxxxx-Xxxxxx Antitrust Improvements Act of 1976, as amended the (“Xxxx-Xxxxx-Xxxxxx Act”); (b) the waiting period under the Xxxx-Xxxxx-Xxxxxx Act shall have expired or been early terminated; (c) no judicial or administrative proceeding opposing consummation of all or any part of this Agreement shall be pending; (d) no injunction (whether temporary, preliminary or permanent) prohibiting consummation of the transactions contemplated by this Agreement or any material portion hereof shall be in effect; and (e) no requirements or conditions shall have been formally requested or imposed by the DOJ or FTC in connection therewith that are not reasonably and mutually satisfactory to the Parties (collectively, the “HSR Conditions”). In the event that the HSR Conditions are not met [ * ], then either Party may terminate this Agreement upon notice, in which case, notwithstanding any provisions that are stated to survive under Section 13.3 above, all provisions of this Agreement shall terminate and be of no force or effect whatsoever, except only that any liability of either Party for failing to comply this Article 16 shall survive.
16.2 HSR Filing. Both Parties shall promptly file following execution of this Agreement their respective pre-merger notification and report forms with the Federal Trade Commission (“FTC”) and the Department of Justice (“DOJ”) pursuant to the Xxxx-Xxxxx-Xxxxxx Act, which forms shall specifically request early termination of the initial Xxxx-Xxxxx-Xxxxxx Act waiting period. DRL shall pay the filing fee.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
16.3 Cooperation.
(a) The Parties shall [ * ] obtain [ * ] clearance required under the Xxxx-Xxxxx-Xxxxxx Act for the consummation of this Agreement and the transactions contemplated herein and shall keep each other apprised of the status of any communications with, and any inquiries or requests for additional information from, the FTC and the DOJ and shall comply [ * ] with any such inquiry or request; provided, however, that neither Party shall be required to consent to the divestiture or other disposition of any of its or its Affiliates’ assets or to consent to any other structural or conduct remedy, and each Party and its Affiliates shall have no obligation to contest, administratively or in court, any ruling, order or other action of the FTC or DOJ or any third party respecting the transactions contemplated by this Agreement.
(b) The Parties hereto commit to instruct their respective counsel to cooperate with each other and [ * ] facilitate and expedite the identification and resolution of any such issues and, consequently, the expiration of the applicable Xxxx-Xxxxx-Xxxxxx Act waiting period. In the context of this Section 16.3, [ * ] counsel’s undertaking: (a) to keep each other appropriately informed of communications received from and submitted to personnel of the reviewing antitrust authority; and (b) to confer with each other regarding appropriate contacts with and responses to personnel of the FTC or DOJ.
ARTICLE XVII
GENERAL PROVISIONS
17.1 Force Majeure. If the performance of any part of this Agreement (except for any payment obligation under this Agreement) by either Party is prevented, restricted, interfered with or delayed by reason of force majeure (including, fire, flood, embargo, power shortage or failure, acts of war, insurrection, riot, terrorism, strike, lockout or other labor disturbance or acts of God), the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such prevention, restriction, interference or delay; provided that the affected Party shall use its reasonable efforts to avoid or remove such causes of non-performance and shall continue performance with the utmost dispatch whenever such causes are removed.
17.2 Governing Law. This Agreement and all questions regarding its validity or interpretation, or the breach or performance of this Agreement, shall be governed by, and construed and enforced in accordance with, the laws of the State of New York, without reference to conflict of law principles. The U.N. Convention on the Sale of Goods shall not apply to this Agreement.
17.3 Waiver of Breach. Except as otherwise expressly provided in this Agreement, any term of this Agreement may be waived only by a written instrument executed by a duly authorized representative of the Party waiving compliance. The delay or failure of either Party at any time to require performance of any provision of this Agreement shall in no manner affect such Party’s rights at a later time to enforce the same. No waiver by either Party of any condition or term in any one or more instances shall be construed as a further or continuing waiver of such condition or term or of another condition or term.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
17.4 Modification. No amendment or modification of any provision of this Agreement shall be effective unless in writing signed by a duly authorized representative of each Party. No provision of this Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance or any other matter not set forth in an agreement in writing and signed by a duly authorized representative of each Party.
17.5 Severability. In the event any payment or other provision of this Agreement should be held invalid, illegal or unenforceable in any jurisdiction, the Parties shall negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and all other provisions of this Agreement shall remain in full force and effect in such jurisdiction. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such payment or other provision in any other jurisdiction. In the event a Party seeks to avoid a payment or other provision of this Agreement by asserting that such provision is invalid, illegal or otherwise unenforceable, the other Party shall have the right to terminate this Agreement upon [ * ] written notice to the asserting Party, unless such assertion is eliminated and the effect of such assertion cured within such [ * ] period. Any termination in accordance with the foregoing shall be deemed a termination pursuant to Section 11.3 if the Party who made the assertion was DRL, and shall be deemed a termination under Section 11.2 by reason of a breach by XenoPort, if XenoPort is the Party who made such assertion.
17.6 Entire Agreement. This Agreement (including the Exhibits attached hereto) constitutes the entire agreement between the Parties relating to its subject matter and supersedes all prior or contemporaneous agreements, understandings or representations, either written or oral, between XenoPort and DRL with respect to such subject matter.
17.7 Notices. Unless otherwise agreed by the Parties or specified in this Agreement, all notices and other communications between the Parties relating to, and all written documentation to be prepared and provided under, this Agreement shall be in the English language: (a) delivered personally; (b) sent by registered or certified mail (return receipt requested and postage prepaid); (c) sent by express courier service providing evidence of receipt, postage pre-paid where applicable; or (d) sent by facsimile (receipt verified and a copy promptly sent by another permissible method of providing notice described in paragraphs (b), (c) or (d) above), to the following addresses of the Parties or such other address for a Party as may be specified by like notice:
| To XenoPort:
XenoPort, Inc. 0000 Xxxxxxx Xxxxxxxxxx Xxxxx Xxxxx, XX 00000 Telephone: (000) 000-0000 Facsimile: (000) 000-0000 Attention: Secretary |
To DRL:
Xx. Xxxxx’x Laboratories SA Xxxxxxxxxxxxxxxxx 00 XX - 0000 Xxxxx Xxxxxxxxxxx T : x00 (0) 00 000 0000 F : x00 (0) 00 000 0000 Attention: [ * ] |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
| With a copy to:
Wilson, Sonsini, Xxxxxxxx & Xxxxxx 000 Xxxx Xxxx Xxxx Xxxx Xxxx, XX 00000 Telephone: (000) 000-0000 Facsimile: (000) 000-0000 Attention: [ * ] |
With a copy to:
Xx. Xxxxx’x Laboratories SA Xxxxxxxxxxxxxxxxx 00 XX - 0000 Xxxxx Xxxxxxxxxxx T : x00 (0) 00 000 0000 F : x00 (0) 00 000 0000 Attention: RGC – Europe |
Any notice required or permitted to be given concerning this Agreement shall be effective [ * ] or [ * ] whichever is earlier.
17.8 Assignment. This Agreement shall not be assignable by either Party to any Third Party hereto without the written consent of the other Party hereto; except either Party may assign this Agreement without the other Party’s consent to an entity that acquires substantially all of the business or assets of the assigning Party, whether by merger, acquisition or otherwise, provided that the Party to whom this Agreement is assigned assumes this Agreement in writing or by operation of law. In addition, either Party shall have the right to assign this Agreement to an Affiliate upon written notice to the non-assigning Party; provided that the assigning Party guarantees the performance of this Agreement by such Affiliate; and further provided that if the non-assigning Party reasonably believes such assignment could result in material adverse tax consequences to the non-assigning Party, such assignment shall not be made without the non-assigning Party’s consent. Subject to the foregoing, this Agreement shall inure to the benefit of each Party, its successors and permitted assigns. A Party assigning this Agreement shall provide written notice of any assignment to the other Party [ * ]. Any assignment of this Agreement in contravention of this Section 17.8 shall be null and void.
17.9 Effects of XenoPort Change of Control. In the event of a XenoPort Change of Control, the following provisions of this Section 17.9 shall apply:
(a) XenoPort Intellectual Property. All XenoPort Patents and XenoPort Know-How Controlled by XenoPort immediately prior to such XenoPort Change of Control shall continue to be XenoPort Patents and XenoPort Know-How for purposes of this Agreement.
(b) Existing Acquirer Intellectual Property. Patents, know-how and other intellectual property and subject matter that were Controlled by the entity acquiring XenoPort or such entity’s affiliates that were not Affiliates of XenoPort prior to such XenoPort Change of
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
Control (such entity together with such affiliates, collectively, the “Acquirer Entities”) shall not be included within the XenoPort Patents, XenoPort Know-How, the Compound or a Product and such Change of Control shall not provide DRL with a license, rights or access to any such pre-existing Patents, know-how and other intellectual property and subject matter.
(c) Independent Intellectual Property. Patent rights, know-how and other intellectual property or subject matter that, following such XenoPort Change of Control, are developed, made or otherwise acquired or Controlled by the Acquirer Entities without use of DRL’s Confidential Information, or any of the then-existing XenoPort Patents or confidential XenoPort Know-How in the Territory, shall not be included within the XenoPort Patents, XenoPort Know-How, the Compound or a Product and such Change of Control shall not provide DRL with a license, rights or access to any such independently developed or acquired Patents, know-how and other intellectual property and subject matter.
(d) Limitation on Exclusivity Obligations. The Acquirer Entity to which this Agreement, [ * ].
17.10 No Partnership or Joint Venture. Nothing in this Agreement is intended, or shall be deemed, to establish a joint venture or partnership between XenoPort and DRL. Neither Party to this Agreement shall have any express or implied right or authority to assume or create any obligations on behalf of, or in the name of, the other Party, or to bind the other Party to any contract, agreement or undertaking with any Third Party.
17.11 Interpretation. The captions to the several Articles and Sections of this Agreement are not a part of this Agreement, but are included for convenience of reference and shall not affect its meaning or interpretation. In this Agreement: (a) the word “including” shall be deemed to be followed by the phrase “without limitation” or like expression; (b) the singular shall include the plural and vice versa; (c) masculine, feminine and neuter pronouns and expressions shall be interchangeable; (d) the word “will” shall be construed as having the same meaning and effect as the word “shall”; (e) provision of information, documents or material to the JSC shall mean provision of such information, documents or material to each Parties’ representatives to the JSC and, unless timing is otherwise specified, shall occur reasonably in advance of the next scheduled JSC meeting; and (f) each reference in this Agreement to the conduct of activities or exercise of rights “by or under the authority of DRL” shall be deemed to include and apply to any Affiliates of DRL who may use or practice any of the intellectual property rights included in the XenoPort Patents and/or XenoPort Know-How and/or that may otherwise be involved in the activities relating to the Compound or any Product. Each accounting term used herein that is not specifically defined herein shall have the meaning given to it under U.S. Generally Accepted Accounting Principles, or other generally accepted cost accounting principles in the United States, but only to the extent consistent with its usage and the other definitions in this Agreement.
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
17.12 Export Laws. Notwithstanding anything to the contrary contained herein, all obligations of XenoPort and DRL are subject to prior compliance with the export regulations of the United States or any other relevant country and such other Applicable Laws in effect in the United States or any other relevant country as may be applicable, and to obtaining all necessary approvals required by the applicable agencies of the governments of the United States and any other relevant countries. XenoPort and DRL shall cooperate with each other and shall provide assistance to the other as reasonably necessary to obtain any required approvals.
17.13 Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original, and all of which together shall constitute one and the same instrument. Signatures to this Agreement delivered by facsimile or other form of electronic transmission (e.g., portable document format (PDF)) will be deemed binding as originals.
[Remainder of page intentionally left blank; signature page follows]
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the date first set forth above.
| XENOPORT, INC. | ||
| BY: | /s/ Xxxxxxx X. Xxxxxx | |
| NAME: | Xxxxxxx X. Xxxxxx | |
| TITLE: | Senior Vice President of Finance and Chief Financial Officer | |
| XX. XXXXX’X LABORATORIES, S.A. | ||
| BY: | /s/ Sameer Natu | |
| NAME: | Sameer Natu | |
| TITLE: | Senior Director & Head Finance (Europe) | |
| XX. XXXXX’X LABORATORIES, S.A. | ||
| BY: | /s/ Xxxxxxx Xxxxxxxx | |
| NAME: | Xxxxxxx Xxxxxxxx | |
| TITLE: | Director & Head | |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 1.6
Compound
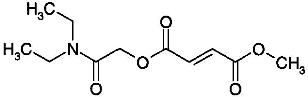
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 1.13
Existing Inventory
Drug Product
| Material |
Lot/Batch Number | Amount (kg) | Location | DOM | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| API | ||||||||
| API | ||||||||
| Description |
Lot# | Amount (kg) | Location | DOM | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||
XenoPort CMOs
[ * ]
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 1.22
MMF

[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 1.41
XenoPort Patents
[ * ]
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | ||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | ||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | ||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | ||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | |||||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
||||||||||||||
| Country |
Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 2.5(b)
[ * ] Patents
| Country | Appl. # | Filing Date | Publ. # | Publ. Date | Patent # | Issue Date | Status | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | ||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] | |||||||||
| [ * ] |
[ * ] | [ * ] | [ * ] | [ * ] | [ * ] |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 4.1(a)
Ongoing Carcinogenicity Studies
| 1. | [ * ] |
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 8.1(f)
Existing Inventory Specifications
Provided by [ * ]
[ * ] = CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
EXHIBIT 9.3
Press Release
(See attached.)

|

|
For Immediate Release
Xx. Xxxxx’x Laboratories and XenoPort Enter Into a
U.S. Licensing Agreement for XP23829
Xx. Xxxxx’x Laboratories Plans to Develop XP23829 in
Psoriasis and Multiple Sclerosis
Hyderabad, India, and Santa Clara, CA — March 28, 2016 — Xx. Xxxxx’x Laboratories (BSE: 500124, NSE: DRREDDY, NYSE: RDY) and XenoPort, Inc. (NASDAQ: XNPT) announced today that they have entered into a license agreement pursuant to which Xx. Xxxxx’x Laboratories will be granted exclusive U.S. rights for the development and commercialization of XenoPort’s clinical-stage oral new chemical entity, XP23829. Xx. Xxxxx’x Laboratories plans to develop XP23829 as a potential treatment for moderate-to-severe chronic plaque psoriasis and relapsing forms of multiple sclerosis (MS).
Under the terms of the agreement, Xx. Xxxxx’x Laboratories will receive exclusive U.S. rights to develop and commercialize XP23829 for all indications. In exchange for these rights, XenoPort will receive a $47.5 million up-front payment and an additional $2.5 million for transfer of certain clinical trial materials to Xx. Xxxxx’x Laboratories. XenoPort will also be eligible to receive up to $190 million upon the achievement by Xx. Xxxxx’x Laboratories of certain regulatory milestones, which could be achieved over a period of several years. In addition, XenoPort will be eligible to receive up to $250 million upon the achievement of commercial milestones, and up to mid-teens royalty payments based on potential net sales of XP23829 in the United States.
Dr. Xxxx Xxxxxxx, M.D., clinical professor of medicine, Dermatology, University of Louisville, stated, “Based on today’s available treatments, physicians need additional oral medications that are both safe and effective for patients with psoriasis. Fumaric acid esters possess a unique anti-inflammatory mechanism of action and have been used to treat psoriasis in Germany for over 20 years. XP23829, a novel fumaric acid ester, has the potential to be a meaningful treatment option for patients with moderate-to-severe psoriasis.”
“We see this compound complementing our internal development and focus to bring novel and relevant solutions to our providers,” said Xxxxxx Xxxxx, executive vice president, Proprietary Products Group, Xx. Xxxxx’x Laboratories. Further, XP23829 could enable Promius Pharma, a wholly owned marketing arm of Xx. Xxxxx’x that is focused on dermatology and neurology, to address the needs of a substantial portion of the 1.5 million moderate-to-severe psoriasis patients in the United States.”
“We are very pleased to announce this agreement with Xx. Xxxxx’x Laboratories,” said Xxxxxxx X. Xxxxxxx, chief executive officer, XenoPort, Inc. “As one of our key objectives for 2016, we were interested in finding a strong partner that would recognize the opportunity of this innovative therapy that we believe will make a significant difference in the lives of psoriasis and MS patients. We are now fully focused on our HORIZANT® (gabapentin enacarbil) Extended-Release Tablets commercialization effort.”
The agreement is subject to review by the U.S. Government under the Xxxx-Xxxxx-Xxxxxx (HSR) Antitrust Improvements Act, as amended, and will become effective only after clearing HSR review.
About XP23829
XP23829 is an investigational drug discovered by XenoPort. It is a novel, oral fumaric acid ester compound that is a prodrug of monomethyl fumarate (MMF). Fumaric acid ester compounds have shown immuno-modulatory and neuroprotective effects in cell-based systems and preclinical models of disease. TECFIDERA, which is approved for relapsing forms of MS in the United States and relapsing-remitting MS in the European Union and FUMADERM, which is approved in Germany for psoriasis, are based on another MMF prodrug known as dimethyl fumarate (DMF). XP23829 is protected by a U.S. composition-of-matter patent that currently has an expiration date of 2029.
In September 2015, XenoPort announced results of a Phase 2 clinical trial of XP23829 as a potential treatment for moderate-to-severe chronic plaque-type psoriasis.
About Psoriasis
Psoriasis is a chronic, systemic, inflammatory disease that manifests in the skin and/or joints. It typically manifests as thick scaling red plaques, with variable morphology and distribution, resulting from an unusually high rate of skin cell growth. There is no cure for psoriasis, and treatment often requires complex medical intervention. The main cause of psoriasis is uncertain, but it is thought to be caused by autoimmunity, genetic predisposition and environmental factors.
Psoriasis is the most prevalent autoimmune disease in the United States with as many as 7.5 million Americans suffering from the condition. It is estimated that approximately 1.5 million adults in the United States are considered to have moderate-to-severe psoriasis and between 150,000 and 260,000 new cases of psoriasis are diagnosed each year.
About MS
MS is a chronic and progressive neurodegenerative disease in which the body’s immune system attacks the myelin protein that wraps around nerve fibers. The disease typically strikes between the ages of 20 to 40 years, and because it is progressive in nature, disability accumulates over time and can lead to permanent impairment of mobility, cognition and the ability for self-care.
Although the exact prevalence is not known, it is estimated that approximately 250,000 to 350,000 people in the United States have been diagnosed with MS and that approximately one million people worldwide suffer from MS.
About Xx. Xxxxx’x
Xx. Xxxxx’x Laboratories Ltd. (BSE: 500124, NSE: DRREDDY, NYSE: RDY) is an integrated pharmaceutical company, committed to providing affordable and innovative medicines for healthier lives. Through its three businesses - Pharmaceutical Services & Active Ingredients, Global Generics and Proprietary Products – Xx. Xxxxx’x offers a portfolio of products and services including APIs, custom pharmaceutical services, generics, biosimilars and differentiated formulations. Its major therapeutic areas of focus are dermatology, gastro-intestinal, cardiovascular, diabetology, neurology, oncology, pain management and anti-infectives. Xx. Xxxxx’x operates in markets across the globe. Our major markets include – USA, Russia & CIS, Venezuela and India. For more information, log on to: xxx.xxxxxxxx.xxx.
About XenoPort
XenoPort, Inc. is a biopharmaceutical company focused on commercializing HORIZANT in the United States. XenoPort has entered into a clinical trial agreement with the National Institute on Alcohol Abuse and Alcoholism (NIAAA) under which the NIAAA has initiated a clinical trial evaluating HORIZANT as a potential treatment for patients with alcohol use disorder. REGNITE® (gabapentin enacarbil) Extended-Release Tablets is being marketed in Japan by Astellas Pharma Inc. XenoPort has granted exclusive world-wide rights for the development and commercialization of its clinical-stage oral product candidate, arbaclofen placarbil, to Indivior PLC for all indications. It has granted exclusive U.S. rights for the development and commercialization of its clinical-stage oral product candidate, XP23829, to Xx. Xxxxx’x Laboratories. XenoPort’s other clinical-stage product candidate, XP21279, is a prodrug of levodopa that is a potential treatment for patients with idiopathic Xxxxxxxxx’x disease.
To learn more about XenoPort, please visit the website at xxx.XxxxXxxx.xxx.
Xx. Xxxxx’x Disclaimer
This press release may include statements of future expectations and other forward-looking statements that are based on the management’s current views and assumptions and involve known or unknown risks and uncertainties that could cause actual results, performance or events to differ materially from those expressed or implied in such statements. In addition to statements which are forward-looking by reason of context, the words “believe,” “could,” “intend,” “plans,” “potential,” “will” and similar expressions identify forward-looking statements. Actual results, performance or events may differ materially from those in such statements due to without limitation, (i) general economic conditions such as performance of financial markets, credit defaults , currency exchange rates , interest rates , persistency levels and frequency / severity of insured loss events (ii) mortality and morbidity levels and trends, (iii) changing levels of competition and general competitive factors, (iv) changes in laws and regulations and in the policies of central banks and/or governments, (v) the impact of acquisitions or reorganization, including related integration issues.
XenoPort Forward-Looking Statements
This press release contains “forward-looking” statements, including, without limitation, all statements related to the anticipated effectiveness of XenoPort’s license agreement with Xx. Xxxxx’x Laboratories; Xx. Xxxxx’x Laboratories’ future clinical development program for XP23829; the therapeutic and commercial potential of XP23829; and XenoPort’s receipt of potential future regulatory and commercial milestone payments, as well as potential royalty payments, and the timing thereof. Any statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. Words such as “believe,” “could,” “intend,” “plans,” “potential,” “will” and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon XenoPort’s current expectations. Forward-looking statements involve risks and uncertainties. XenoPort’s actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation:[risks related to the ability of the parties to obtain clearance under the Xxxx-Xxxxx-Xxxxxx Antitrust Improvements Act, as amended; the difficulty and uncertainty of pharmaceutical product development and the uncertain results and timing of clinical trials and other studies, including the risk that success in preclinical testing and early clinical trials does not ensure that later clinical trials will be successful; the uncertainty of the FDA approval process and other regulatory requirements; the uncertain therapeutic and commercial value of XP23829; XenoPort’s dependence on collaborative partners, including the risks that if Xx. Xxxxx’x Laboratories were to breach or terminate the license agreement or otherwise fail to successfully develop and commercialize XP23829 thereunder and in a timely manner, XenoPort would not obtain the anticipated financial and other benefits of the license agreement and the clinical development or commercialization of XP23829 could be delayed or terminated; as well as risks related to future opportunities and plans, including the uncertainty of future financial and operating results. These and other risk factors are discussed under the heading “Risk Factors” in XenoPort’s Securities and Exchange Commission filings and reports, including in its Quarterly Report on Form 10-K for the year ended December 31, 2015, filed with the Securities and Exchange Commission on February 26, 2016. XenoPort expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in the company’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based.
| Xx. Xxxxx’x Laboratories | ||
| IR Contact | Media Contact | |
| Kedar Xxxxxxx | Xxxxxx Printer | |
| Phone: +91-40-6683 4297 | Phone: +91.40.4900 2121 | |
| Email: xxxxxx@xxxxxxx.xxx | Email: xxxxxxxxxxxxx@xxxxxxxx.xxx | |
| XenoPort IR and Media Contact | ||
| Xxxxxx Xxxxxxx | ||
| Phone: 000-000-0000 | ||
| Email: xx@XxxxXxxx.xxx | ||

