Confidential Materials omitted and separately with the Securities and Exchange Commission. Asterisks denote omissions. LICENSE AGREEMENT
Exhibit 10.28
Confidential Materials omitted and separately with the
Securities and Exchange Commission. Asterisks denote omissions.
Securities and Exchange Commission. Asterisks denote omissions.
This License Agreement (the “Agreement”) is entered into as of the 4th day of February
2009 (the “Execution Date”) by and between Idenix Pharmaceuticals, Inc., a company
organized under the laws of the State of Delaware with its principal place of business at 00
Xxxxxxxxx Xxxxxx, Xxxxxxxxx, Xxxxxxxxxxxxx 00000, XXX (“Idenix”), and SmithKline Xxxxxxx
Corporation, doing business as GlaxoSmithKline, a company organized under the laws of the
Commonwealth of Pennsylvania, with its principal place of business at Xxx Xxxxxxxx Xxxxx,
Xxxxxxxxxxxx, Xxxxxxxxxxxx 00000 XXX (“GSK”).
INTRODUCTION
1. Idenix and GSK are each in the business of discovering, developing and commercializing
pharmaceutical products.
2. Idenix has developed the Licensed Compounds (as defined below) and possesses certain
intellectual property relating to such compounds.
3. GSK desires to exclusively license from Idenix such intellectual property for the purpose of
developing and commercializing the Products (as defined below), and Idenix desires to grant such a
license to GSK in accordance with the terms and conditions of this Agreement.
In consideration of the mutual covenants contained herein, and other good and valuable
consideration, the receipt of which is hereby acknowledged, GSK and Idenix agree as follows:
1. DEFINITIONS
When used in this Agreement, each of the following terms, whether used in the singular or
plural, shall have the meanings set forth in this Section 1.
1.1 “Affiliate” means any Person who directly or indirectly controls or is controlled
by or is under common control with another Person, for as long as such control exists. For
purposes of this definition, “control” or “controlled” means ownership, directly or through one or
more Affiliates, of fifty percent (50%) or more of the shares of stock entitled to vote for the
election of directors, in the case of a corporation, or fifty percent (50%) or more of the equity
interest in the case of any other type of legal entity, or status as a general partner in any
partnership. The Parties acknowledge that, in the case of certain entities organized under the
laws of certain countries, the maximum percentage ownership permitted by law for a foreign investor
may be less than fifty percent (50%), and in such case such lower percentage shall be substituted
in the preceding sentence; provided, that such foreign investor has the power to direct the
management and policies of such entity. The Parties agree and acknowledge that, for purposes of
this Agreement, the only Affiliates of Idenix are those Persons controlled by Idenix and,
specifically, that for the purposes of this Agreement, Novartis Pharma AG is not an Affiliate of
Idenix.
1.2 “AIDS” means acquired immune deficiency syndrome.
1.3 “ANDA” means an Abbreviated New Drug Application filed with the FDA or similar
foreign application or submission for Regulatory Approval.
1.4 “Annual Net Sales” means the Net Sales of Products in the Territory during a
Calendar Year.
1.5 “Business Day” means a day other than Saturday or Sunday on which the banks in New
York, New York are open for business.
1.6 “Calendar Quarter” means a calendar quarter ending on the last day of March, June,
September or December.
1.7 “Calendar Year” means a period of time commencing on January 1 and ending on the
following December 31.
1.8 “CFR” means the United States Code of Federal Regulations.
1.9 “cGMP” means all applicable standards relating to manufacturing practices for fine
chemicals, intermediates, bulk products or finished pharmaceutical products. For purposes of this
Agreement, cGMPs means (i) the principles detailed in the U.S. Current Good Manufacturing
Practices, 21 CFR Parts 210 and 211, and The Rules Governing Medicinal Products in the European
Community, Volume IV Good Manufacturing Practice for Medicinal Products and (ii) all other
applicable principles promulgated by any governmental body having jurisdiction over the manufacture
of the Initial Materials, in the form of laws, regulations or guidance documents (including but not
limited to advisory opinions, compliance policy guides and guidelines), which guidance documents
are being implemented within the pharmaceutical manufacturing industry similar products; in each
case as in effect at the Effective Date.
1.10 “Co-Labeling Right” means the non-exclusive right to label any HBV or HCV product
Controlled by Novartis Pharma AG or Idenix during the Term not containing a Licensed Compound as an
active ingredient, stating that such product can be used or administered with a Product to treat
patients coinfected with HBV or HCV on the one hand and HIV on the other hand.
1.11 “Co-Labeling Patent Rights” means (a) the following Idenix Patent Rights listed
in Exhibit A: [**], and (b) any patents that issue from the foregoing and all continuations,
continuations-in-part, divisions, reexaminations, renewals, registrations reissues, supplementary
protection certificates and extensions of any of the foregoing Patent Rights, both foreign and
domestic, Controlled by Idenix during the Term.
1.12 “Combination Product” means a Product that consists of (a) one or more Licensed
Compounds and (b) another active compound(s) that is not a Licensed Compound. “Sole Compound
Product” means a Product containing no compounds other than Licensed Compounds. “Double
Combination Product” means a Combination Product containing exactly one other compound other
than Licensed Compounds. “Triple Combination Product” means a Combination Product
containing two or more other compounds other than Licensed Compounds.
2
1.13 “Commercially Reasonable Efforts” means with respect to the efforts to be
expended by a Party with respect to any objective, reasonable, diligent, good faith efforts to
accomplish such objective as such Party would normally use to accomplish a similar objective under
similar circumstances, it being understood and agreed that with respect to the Exploitation of any
Product, such efforts shall be substantially equivalent to those efforts and resources commonly
used by such a Party for a product owned by it or to which it has rights, which product is at a
similar stage in its development or product life and is of similar market potential taking into
account efficacy, safety, approved labeling, the competitiveness of alternative products in the
marketplace, the patent and other proprietary position of the product, the likelihood of regulatory
approval given the regulatory structure involved, the profitability of the product, alternative
products and other relevant factors. Commercially Reasonable Efforts shall be determined on a
country-by-country and Product-by-Product basis, and it is anticipated that the level of effort
will change over time, reflecting changes in the status of the Product and the country involved.
1.14 “Confidential Information” means, with respect to a Party or its Affiliates
disclosing Information (the “Disclosing Party”), Information, regardless of the form in
which that Information is constituted, which is treated by the Disclosing Party as confidential and
relates either directly or indirectly to the business of such Disclosing Party. The Idenix
Know-How is Confidential Information of Idenix. All “Evaluation Materials” (as defined in the June
2008 CDA) disclosed by or on behalf of Idenix under the June 2008 CDA shall be deemed “Confidential
Information” of Idenix hereunder and shall be subject to the terms and conditions of this
Agreement. All “Confidential Information” (as defined in the February 2008 CDA) disclosed by or on
behalf of a Party under the February 2008 CDA shall be deemed “Confidential Information” of the
relevant Party hereunder and shall be subject to the terms and conditions of this Agreement. All
proprietary information disclosed by or on behalf of a Party under the MTA shall be deemed
“Confidential Information” of the relevant Party hereunder and shall be subject to the terms and
conditions of this Agreement.
Confidential Information of the Disclosing Party excludes any information that the other Party
or its Affiliates (the “Receiving Party”) can establish by written records:
(a) was known by the Receiving Party prior to the receipt from the Disclosing Party;
(b) was disclosed to the Receiving Party by a Third Party having the right to do so;
(c) was, or subsequently became, publicly known through no fault of the Receiving Party, its
Affiliates or any of the officers, directors, employees or agents of the Receiving Party or its
Affiliates; or
(d) was concurrently or subsequently developed by personnel of the Receiving Party without
having had access to the Disclosing Party’s Confidential Information.
3
1.15 “Control” or “Controlled” means, with respect to any item of Know-How or
any Intellectual Property Right, the possession of the right (whether by ownership, license or
otherwise (other than pursuant to a license granted under this Agreement)), to assign, or
grant a license, sublicense or other right to or under, such Know-How or Intellectual Property
Right as provided for herein without violating the terms of any agreement or other arrangement with
any Third Party.
1.16 “Cover”, “Covered” or “Covering” means, (a) with respect to a
patent, that, in the absence of a license granted to a Person under a Valid Claim included in such
patent, the practice by such Person of an invention claimed in such patent would infringe such
Valid Claim, or (b) with respect to a patent application, that, in the absence of a license granted
to a Person under a Valid Claim included in such patent application, the practice by such Person of
an invention claimed in such patent application would infringe such Valid Claim if such patent
application were to issue as a patent.
1.17 “Develop”, “Developing”, or “Development” means research,
pre-clinical and clinical development.
1.18 “DOJ” means the United States Department of Justice.
1.19 “Effective Date” means two (2) Business Days immediately following the HSR
Clearance Date as provided in Section 14.1 below.
1.20 “EMEA” means the European Medicines Agency or any successor agency thereof.
1.21 “Exploit” and, with correlative meaning, “Exploitation” means to Develop,
use, make, have made, market, offer to sell, sell, distribute, import or otherwise exploit.
1.22 “FDA” means the United States Food and Drug Administration or any successor
agency thereto.
1.23 “Field” means treatment or prophylaxis of any human diseases or conditions.
1.24 “First Commercial Sale” means, with respect to a Product and a country in the
Territory, the first sale for use or consumption of such Product in such country by GSK, its
Affiliate or Sublicensee, after the granting of Regulatory Approval in the Field for such Product
by the relevant Regulatory Authorities.
1.25 “FTC” means the United States Federal Trade Commission.
1.26 “Fully Allocated Cost of Goods” means, with respect to a unit of a Product,
whether such unit is in vial, tablet or other form, the standard fully allocated cost of
manufacturing and supplying such unit of such Product, which will be calculated consistently with
other products and in accordance with international financial reporting standards, consistently
applied.
4
1.27 “Generic Competition” means, with respect to a Product in a country in the
Territory, that one or more Generic Products of such Product are available in such country and that
the unit sales of such Generic Products in such country exceed [**] percent ([**]%) of the
total unit sales of such Product and such Generic Products in such country in a Calendar
Quarter as determined by reference to applicable sales data obtained from IMS Health, Verispan or
from such other source for such sales data as may be agreed upon by the Parties (provided,
that such other source, if any, shall be generally recognized as a reliable source for
pharmaceutical sales data among major pharmaceutical companies).
1.28 “Generic Product” means, with respect to a Product in a country in the Territory,
a pharmaceutical product sold by a Third Party not authorized by or on behalf of GSK, its
Affiliates or Sublicensees that is a “pharmaceutical equivalent” with a therapeutic equivalence
code of “A” with respect to such Product (as such term is used in the Approved Drug Products with
Therapeutic Equivalence Evaluations (a.k.a. the Orange Book) published by the FDA Center for Drug
Evaluation and Research or any successor publication) and is approved through the ANDA process and
is substitutable for such Product without a physician’s consent or additional prescription, or
similar foreign equivalent.
1.29 “GSK Invention” means any Invention made solely by one or more employees,
officers, directors, consultants or contractors of GSK.
1.30 “HBV” means hepatitis B virus.
1.31 “HCV” means hepatitis C virus.
1.32 “HIV” means human immunodeficiency virus.
1.33 “HSR Act” means the Xxxx-Xxxxx-Xxxxxx Antitrust Improvements Act of 1976, as
amended (15 U.S.C. §18a), and the rules and regulations promulgated thereunder.
1.34 “HSR Clearance Date” means the earlier of (a) the date on which the FTC or DOJ
shall notify the Parties of early termination of the waiting period under the HSR Act, or (b) the
date on which the applicable waiting period under the HSR Act expires; provided,
however, that if the FTC or DOJ shall commence any investigation by means of a second
request or otherwise, HSR Clearance Date means the date on which any investigation opened by the
FTC or DOJ shall have been terminated, without action to prevent the Parties from implementing the
transactions contemplated by this Agreement with respect to the United States.
1.35 “Idenix Entities” shall have the meaning set forth in the Novartis Waiver.
1.36 “Idenix Indemnitees” means Idenix, its Affiliates, and the agents, directors,
officers and employees of Idenix and its Affiliates.
1.37 “Idenix IP” means, collectively, Idenix Know-How and Idenix Patent Rights.
1.38 “Idenix Invention” means any Invention made solely by one or more employees,
officers, directors, consultants or contractors of Idenix.
5
1.39 “Idenix Know-How” means all Know-How in the Territory that is Controlled by
Idenix as of the Execution Date or during the Term, to the extent that such Know-How relates to
a Licensed Compound or a Product in the Field; provided, however, that Idenix
Know-How specifically excludes Joint Know-How.
1.40 “Idenix Patent Rights” means any Patent Right in the Territory that is Controlled
by Idenix as of the Execution Date or during the Term and Covers a Licensed Compound or a Product,
or the Exploitation thereof, in the Field, including the Patent Rights set forth in Exhibit
A; provided, however, that Idenix Patents Rights specifically exclude Joint
Patent Rights.
1.41 “IDX899” means
(2-Carbamoyl-5-chloro-1H-indol-3-yl)-[3-((E)-2-cyano-vinyl)-5-methyl-phenyl]-phosphinic acid methyl
ester, stereoisomers or salts thereof, including the compound having the following structure:
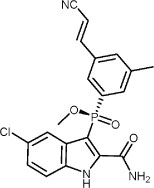 , and any prodrugs, active metabolites, polymorphs, salts, hydrates, solvates,
enantiomers and/or esters of the compound.
, and any prodrugs, active metabolites, polymorphs, salts, hydrates, solvates,
enantiomers and/or esters of the compound.
1.42 “IDX989” means (2-Carbamoyl-5-chloro-4-fluoro-1H-indol-3-yl)-[3-((E)-2-cyano-vinyl)-5-methyl-phenyl]-phosphinic
acid methyl ester, strereoisomers or salts thereof, including the compound having the following
structure:
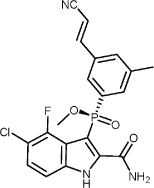 , and any prodrugs, active metabolites, polymorphs, salts, hydrates, solvates, enantiomers
and/or esters of the compound.
, and any prodrugs, active metabolites, polymorphs, salts, hydrates, solvates, enantiomers
and/or esters of the compound.
6
1.43 “IND” means an Investigational New Drug Application or similar foreign
application or submission for approval to conduct human clinical investigations.
1.44 “Information” means all tangible and intangible (a) techniques, technology,
practices, trade secrets, inventions (whether patentable or not), methods, knowledge, skill,
experience, test data and results (including pharmacological, toxicological and clinical test
data and results), analytical and quality control data, results or descriptions, software and
algorithms and (b) compositions of matter, cells, cell lines, assays, animal models and physical,
biological or chemical material; whether stored or transmitted in oral, documentary, electronic or
other form, including Regulatory Documentation.
1.45 “Intellectual Property Rights” means Patent Rights, Know-How, trade secret
rights, copyrights, trademarks and other forms of proprietary or industrial rights.
1.46 “Joint IP” means, collectively, Joint Know-How and Joint Patent Rights.
1.47 “Joint Invention” means any Invention made jointly by one or more employees,
officers, directors, consultants or contractors of Idenix and one or more employees, officers,
directors, consultants or contractors of GSK.
1.48 “Joint Know-How” means all Know-How in the Territory that is invented, developed,
conceived, reduced to practice or authored jointly by or on behalf of Idenix or its Affiliates, on
the one hand, and GSK or its Affiliates, on the other hand, during the Term that relates to the
Exploitation of Licensed Compounds and Products.
1.49 “Joint Patent Rights” means all Patent Rights throughout the world claiming the
Joint Inventions.
1.50 “Know-How” means any proprietary Information.
1.51 “Knowledge” or “Known” means, actual knowledge of the officers,
directors, and employees with day to day supervisory responsibility for the relevant subject matter
of the Party in question after reasonable inquiry of the management personnel of such Party who
are, in such Party’s judgment, best situated to know about the subject.
1.52 “Xx Xxxxx Agreement” means that certain agreement dated December 3, 2008, by and
between Idenix and Xxxxxxxxx Xx Xxxxx providing for certain payments by Idenix to Xxxxxxxxx Xx
Xxxxx based upon the sale of certain products containing certain NNRTI compounds.
1.53 “Law” means any law, statute, rule, regulation, ordinance or other pronouncement
having the effect of law, of any federal, national, multinational, state, provincial, county, city
or other political subdivision.
1.54 “Licensed Compound” means any NNRTI compound claimed in (a) the Idenix Patent
Rights listed on Exhibit A, including those referenced as [**] and/or (b) all
continuations, divisions, reexaminations, renewals, registrations, reissues and extensions of any
of the foregoing Patent Rights, both foreign and domestic, that are Controlled by Idenix during the
7
Term, including IDX899 and IDX989 and all back-up compounds, follow-on compounds, prodrugs and
metabolites of such compounds, as well as their isomers, salts, hydrates, solvates and polymorphs.
1.55 “Major Market” means any one of the following: the United States of America, the
United Kingdom, France, Italy, Spain, Germany or Japan.
1.56 “Measured COGS” means, with respect to a Calendar Quarter and a Product, on a
Product by Product basis, the Fully Allocated Cost of Goods for all units of such Product
(including all relevant Combination Products) sold during such Calendar Quarter (other than any
units of such Product sold in countries where a royalty rate reduction is applied pursuant to
Section 5.3(a)(iii)).
1.57 “Measured Net Sales” means, with respect to a Calendar Quarter and a Product, on
a Product by Product basis, the Net Sales for all units of such Product (including Net Sales for
all relevant Combination Products) sold during such Calendar Quarter (other than any units of such
Product sold in countries where a royalty rate reduction is applied pursuant to Section
5.3(a)(iii)).
1.58 “MHLW” means the Japanese Ministry of Health, Labor and Welfare, or a successor
agency thereto.
1.59 “NDA” means a New Drug Application filed with the FDA or similar foreign
application or submission for Regulatory Approval, including a Marketing Authorization Application
(“MAA”) filed with the EMEA or any other EU Regulatory Authority.
1.60 “Net Sales” means the gross invoice price of a unit of Product sold by GSK, its
Affiliates or Sublicensees (each, a “Seller”) to any Third Party other than a Sublicensee
less the following items, to the extent included in the gross invoiced sales price of such unit of
Product or otherwise directly paid or incurred by the Seller with respect to the sale of such unit
of Product:
(a) normal and customary trade and quantity discounts actually allowed and properly taken with
respect to sales of such Product;
(b) amounts repaid or credited by reason of rejections, recalls, returns, rebates and
allowances;
(c) chargebacks and other amounts paid on sale or dispensing of such Product;
(d) tariffs, duties, excise, sales value-added or other taxes (other than taxes based on
income);
(e) discounts pursuant to patient discount programs and coupon discounts; and
8
(f) the actual amount of any write offs for bad debt directly relating to sales of such
Product in the period; provided, that such amount shall not exceed [**] percent ([**]%) of
the aggregate amount invoiced with respect to such Product in the relevant Calendar Quarter.
There shall be no double-counting in determining the foregoing deductions from gross amounts
invoiced to calculate Net Sales. The Seller shall issue an invoice in respect of all Products that
are sold to Third Parties. The Seller shall make all sales or transfers of Products to Third
Parties on arm’s length terms.
In the case of any sale or other disposal of a Product between or among GSK, its Affiliates
and Sublicensees for resale, Net Sales shall be calculated on the value invoiced on the first
arm’s-length sale thereafter to a Third Party other than a Sublicensee.
Net Sales shall be determined in accordance with international financial reporting standards,
consistently applied.
In the event that non-monetary consideration is received for any Product, Net Sales shall be
calculated based on the average price charged per unit for such Product during the preceding
royalty period, or in the absence of such sales, the fair market value of the Product, as
determined by the Parties in good faith.
All such discounts, allowances, credits, rebates and other deductions shall be fairly and
equitably allocated to the Product and other products or services of GSK, and its Affiliates and
Sublicensees, such that the Product does not bear a disproportionate portion of such deductions.
1.61 “Novartis” means Novartis Pharma AG and its Affiliates.
1.62 “Novartis Protected Entities” shall have the meaning set forth in the Novartis
Waiver.
1.63 “Novartis Waiver” means the written agreement between Idenix and Novartis
attached as Exhibit B.
1.64 “NNRTI” means non-nucleoside reverse transcriptase inhibitor.
1.65 “Party” means Idenix or GSK, “Parties” means Idenix and GSK.
1.66 “Patent Rights” means (a) patent applications (including provisional
applications); (b) any patents issuing from such patent applications (including certificates of
invention); (c) all patents and patent applications based on, corresponding to or claiming the
priority date(s) of any of the foregoing; (d) any substitutions, extensions (including supplemental
protection certificates), registrations, confirmations, reissues, divisionals, continuations,
continuations-in-part, re-examinations, renewals and foreign counterparts thereof; and (e) all
patents claiming overlapping priority therefrom.
9
1.67 “Person” means any individual, corporation, limited or general partnership,
limited liability company, joint venture, trust, unincorporated association, governmental body,
authority, bureau or agency, or any other entity or body.
1.68 “Phase II Clinical Trial” means a human clinical trial in any country in the
Territory that would satisfy the requirements of 21 C.F.R. § 312.21(b) or its foreign counterpart.
1.69 “Phase III Clinical Trial” means a human clinical trial in any country in the
Territory that would satisfy the requirements of 21 C.F.R. § 312.21(c) or its foreign counterpart.
1.70 “Prior Confidentiality Agreements” means (a) the Confidential Disclosure
Agreement between the Parties, dated February 20, 2008, as amended on April 11, 2008 (the
“February 2008 CDA”), and (b) the Confidentiality Agreement between the Parties, dated
June 30, 2008 (the “June 2008 CDA”).
1.71 “Product” means any pharmaceutical product containing one or more Licensed
Compound as an active pharmaceutical ingredient; provided, however, that such
product may not contain a compound that is not a Licensed Compound, the Exploitation of which
compound is Covered by Patent Rights or uses Know-How owned or controlled by Idenix or its
Affiliates that has not been licensed to GSK hereunder. When the phrase “Product by Product” is
used herein Products (including Combination Products) that have the same: (i) indication, (ii)
route of administration and (iii) active ingredients will be considered to be the same Product.
1.72 “Xxxxxxxxx Xx Xxxxx” means Xxxxxxxxx Xxxxx Xx Xxxxx, Professor of Microbiology at
the University, an Italian citizen born on August 14, 1944 and currently residing at Xxxxxx Xxx
Xxxx, 0x Xxxxxx xx 00, Xxxxxxxxx (XX) — Italy.
1.73 “Regulatory Approval” means, with respect to a pharmaceutical product in a
country or regulatory jurisdiction, the act of a Regulatory Authority necessary for the marketing
and sale of such product in such country or regulatory jurisdiction, including the approval of an
NDA by the FDA.
1.74 “Regulatory Authority” means any applicable government regulatory authority
involved in granting approvals for the marketing and/or pricing of a pharmaceutical product in a
country or regulatory jurisdiction, including the FDA, the EMEA, the MHLW and foreign equivalents
thereof.
1.75 “Regulatory Documentation” means, with respect to a Product, all INDs, Regulatory
Approvals, pre-clinical and clinical data and information, regulatory materials, drug dossiers,
master files, and any other reports, records, regulatory correspondence and other materials
relating to the pre-clinical and clinical development and Regulatory Approval of such Product, or
required to manufacture, distribute and sell such Product, including any safety database.
1.76 “Royalty Term” means, with respect to a Product and country, the period of time
beginning with the First Commercial Sale of such Product in such country and continuing until the
later of: (a) the expiration of the last Valid Claim of the Idenix Patent Rights and Joint Patent
10
Rights which Cover the Exploitation of such Product in such country, or (b) ten (10) years after
First Commercial Sale of such Product in such country.
1.77 “Stock Purchase Agreement” means that Stock Purchase Agreement in the form of
Exhibit C hereto, dated as of the Execution Date, between Idenix and GSK.
1.78 “Sublicensee” means a Third Party to whom GSK, its Affiliates or another
Sublicensee grants a license or sublicense under the Idenix IP or Joint IP in accordance with the
terms of this Agreement.
1.79 “Successful Completion” of a particular milestone event has that meaning
described in Schedule 5.2(a).
1.80 “Territory” means each country in the world.
1.81 “Third Party” means any Person other than the Parties and their Affiliates.
1.82 “University” means the University of Cagliari or Universita’ Degli Studi Di
Cagliari, having a principal place of business at Cittadella Universitaria, SS 554 XX 0.0, 00000
Xxxxxxxxxx, Xxxxxxxx, Xxxxx.
1.83 “University Agreements” means certain agreements by and between Idenix and its
predecessors in interest and certain of their Affiliates and the University of Cagliari including:
(a) the Co-operative Antiviral Research Activity Agreement dated January 4, 1999, as amended April
10, 2002, May 8, 2003, June 30, 2004 and October 24, 2005 and (b) the License Agreement dated
December 14, 2000 as amended April 10, 2002, May 8, 2003, June 30, 2004 and October 24, 2005.
1.84 “University Amendment” means a written amendment agreement between Idenix and the
University of Cagliari amended for the purpose of permitting GSK, in lieu of Idenix, to exercise
certain rights under these arrangements in the form attached as Exhibit D
1.85 “U.S.C.” means the United States Code.
1.86 “Valid Claim” means a claim of (a) any issued, unexpired patent that has not been
revoked or held unenforceable or invalid by a decision of a court or governmental agency of
competent jurisdiction from which no appeal can be taken, or with respect to which an appeal is not
taken within the time (including any extensions) allowed for appeal, and that has not been
disclaimed or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise, or
(b) any patent application which has been pending for fewer than [**] years from the earliest
priority date for such application.
1.87 Other Defined Terms. Each of the following definitions is set forth in the
section of this Agreement indicated below:
11
| Definition | Section | |
1974 Convention |
14.7 | |
AAI |
5.3(a)(iii) | |
Agreement |
Preamble | |
Auditor |
5.9 | |
Bankruptcy Code |
14.4 | |
Breaching Party |
12.2(b) | |
Challenge |
12.2(d) | |
Combination Product Net Sales |
5.3(b)(i) | |
Committee |
4.5(a) | |
Development Plan |
4.3 | |
Disclosing Party |
1.14 | |
Execution Date |
Preamble | |
Executive Officer |
14.8 | |
February 2008 CDA |
1.70 | |
GSK |
Preamble | |
GSK Indemnitees |
8.2 | |
GSK IP |
12.3(a)(iii) | |
Xxxxx-Xxxxxx Act |
6.6 | |
Idenix |
Preamble | |
Initial Materials |
3.2(a) | |
Inventions |
6.1 | |
IP Committee |
4.5(a) | |
JSC |
4.5(a) | |
June 2008 CDA |
1.70 |
12
| Definition | Section | |
Losses |
8.1 | |
MAA |
1.59 | |
MTA |
3.2(a) | |
Non-Breaching Party |
12.2(b) | |
Paragraph IV Certification |
6.3(a) | |
Patent Term Extensions |
6.5(a) | |
Phase IIb Clinical Trial |
Schedule 5.2(a) | |
Product Trademarks |
2.2 | |
Receiving Party |
1.14 | |
Seller |
1.60 | |
Severed Clause |
14.10 | |
SPC |
6.5(a) | |
Specifications |
3.2 | |
Term |
12.1 |
1.88 Construction. In construing this Agreement, unless expressly specified
otherwise;
(a) references to Sections, Schedules and Exhibits are to sections of, and schedules and
exhibits to, this Agreement;
(b) use of either gender includes the other gender, and use of the singular includes the
plural and vice versa;
(c) headings and titles are for convenience only and do not affect the interpretation of this
Agreement;
(d) any list or examples following the word “including” shall be interpreted without
limitation to the generality of the preceding words; and
(e) the language used in this Agreement shall be deemed to be the language chosen by the
Parties to express their mutual intent, and no rule of strict construction shall be applied against
either Party.
13
2. LICENSES AND ASSIGNMENTS
2.1 License.
(a) Grants. Subject to the terms and conditions of this Agreement, Idenix hereby
grants to GSK an exclusive, royalty-bearing, sublicenseable (solely in accordance with Section
2.1(b)), non-transferable (except in accordance with Section 14.2) license, under the Idenix IP and
Idenix’s interests in the Joint IP, to make, have made, use, sell, offer for sale and import
Licensed Compounds and Products in the Field in the Territory.
(b) Sublicenses. GSK shall have the right to grant sublicenses under the license
granted in Section 2.1(a) to its Affiliates and Third Parties; provided, that, GSK may only
sublicense its rights under the license granted in Section 2.1(a) to any Third Party with Idenix’s
prior written consent, such consent not to be unreasonably withheld, [**]; provided, however, that
GSK may use subcontractors in the drug development, non-clinical and clinical testing,
formulation, manufacturing and distribution of the Licensed Compounds and Products without the
prior written approval of Idenix. Any such sublicense shall be in writing, shall be subject and
subordinate to, and consistent with, the terms and conditions of this Agreement, and shall provide
that such Affiliates and Sublicensees shall not further sublicense. Any sublicense shall not
absolve GSK of its obligations under this Agreement and GSK shall remain fully responsible for
performance of this Agreement. GSK shall notify Idenix in writing of any sublicense granted
pursuant to this Section 2.1(b) within [**] after the execution thereof. GSK shall ensure that all
its Affiliates and Sublicensees comply with the relevant provisions of this Agreement. GSK shall
be primarily liable for any failure of any of its Affiliates or Sublicensees to fail to comply with
this Agreement and any such failure shall be deemed a failure by GSK hereunder.
2.2 Trademark. GSK, its Affiliates and Sublicensees shall select and own the
trademarks under which they will market Products in the Field in the Territory (the “Product
Trademarks”); provided, that no such trademark shall contain the word “Idenix” or any
variation thereof and no name for the Product shall contain the words “Glaxo”, “Xxxxx”, “Xxxxx”,
“Xxxxxxx”, “GSK” or any combination or variation thereof.
2.3 Exclusivity. During the Term, neither Idenix nor any of its Affiliates, directly
or indirectly, alone or with a Third Party, shall Exploit NNRTI compounds or products for the
diagnosis and therapeutic or prophylactic treatment of HIV and/or AIDs or conditions caused by HIV
and/or AIDs.
2.4 Retained Rights. Except as expressly provided in Sections 2.1, 2.2, 2.3 and 6,
all rights in and to the Idenix IP and Idenix’s interests in the Joint IP, and any other
Intellectual Property Rights of Idenix and its Affiliates, are hereby retained by Idenix and its
Affiliates. Except as expressly provided in Sections 6 and 12.3, all rights in and to any
Intellectual Property Rights of GSK and its Affiliates are hereby retained by GSK and its
Affiliates. GSK agrees and acknowledges that (a) Novartis retains the non-exclusive right to
exercise the Co-Labeling Right under the Co-Labeling Patent Rights and (b) the licenses and other
rights granted to GSK with respect to the Co-Labeling Patent Rights are subject to Section 3(c) of
the Novartis Waiver and GSK shall, and shall cause its Affiliates and Sublicensees, successors and
assigns to, be bound by and comply with such provision.
14
3. TECHNOLOGY AND MATERIALS TRANSFER
3.1 Technology Transfer to GSK. Within [**] of the Effective Date, Idenix shall make
available to GSK, in a mutually-agreed upon format, all material and other mutually-agreed upon
information regarding the Idenix Know-How, and for a period of [**] after the Effective Date,
Idenix shall make its relevant scientific and technical personnel available to GSK to answer any
questions, consult or provide instruction as reasonably requested by GSK, and Idenix shall use
diligent efforts to obtain from its Third Party vendors such information as may be reasonably
requested by GSK, concerning the information delivered pursuant to this Section 3.1. Idenix shall
assign or transfer to GSK ownership of all Regulatory Documentation filed or Controlled by Idenix
prior to the Effective Date.
3.2 Materials Transfer to GSK.
(a) Within [**] after the Effective Date, Idenix shall deliver to GSK approximately [**] kg of
the IDX899 compound as described in Schedule 3.2(a), less the approximately [**] kg of the IDX899
compound delivered to GSK pursuant to the Material Transfer Agreement between Idenix and GSK dated
January 12, 2009, as amended (the “MTA”) (the “Initial Materials”). Prior to
delivery of the Initial Materials to GSK, Idenix shall have tested the Initial Materials in
accordance with the testing procedures described in the specifications attached in Exhibit
E (the “Specifications”), and shall provide GSK with the applicable master batch
record and a copy of the applicable deviation or other investigatory report, if any. In connection
with such delivery, Idenix shall provide GSK with a certificate of analysis and certificate of
compliance stating that the Initial Materials were produced in compliance with cGMPs and the
Specifications, and conform to such Specifications as of the last date of testing set forth on such
certificate of analysis. GSK retains the right (but not the obligation) to test the Products at
GSK’s discretion, but generally intends to accept or reject Products on the basis of the
certificates described in the preceding sentence. GSK shall be under no obligation to accept any
shipment of Product without the above-referenced certificates.
(b) GSK retains the right to test the Initial Materials within [**] of delivery, using the
same tests as Idenix uses to test such compound (which tests are set forth in the Specifications),
at GSK’s discretion and expense. If any Initial Materials are so found by GSK, in its reasonable
determination based on such test results, not to have been produced in compliance with cGMPs or the
Specifications, or not to conform to the Specifications, the Parties shall investigate such
non-conformity. If the Parties disagree as to whether Initial Materials meet the Specifications
or have been produced in compliance with cGMP, Idenix’s most senior quality assurance officer and
GSK’s designee for Quality and Regulatory Compliance, North America, or such other persons as they
may designate in writing, shall confer to review samples and/or batch records, as appropriate. If
the disagreement is not resolved, then samples, batch records and other data relating to the batch
in dispute shall promptly be submitted for testing and evaluation to an independent Third Party
(including a testing laboratory qualified to perform such testing using validated methods) approved
in writing by both Parties. The cost of the testing
15
and evaluation by the Third Party shall be
borne (x) by Idenix if the Initial Materials in question are ultimately found to fail to meet the
Specifications, or if Idenix is found not to have complied with the Specifications or cGMPs or (y)
by GSK if the Initial Materials in question are ultimately found to meet the Specifications and if
Idenix is not found to have failed to comply with the Specifications or cGMPs. Following the
receipt of the report from such independent Third Party, Idenix’s most senior quality assurance
officer and GSK’s Director of Contract Manufacturing, North America, or their respective designated
representatives, shall attempt to agree on the conformity or lack of conformity of the rejected or
held quantity. In the absence of any agreement, GSK shall have the right, with reasonable evidence
of a Nonconformity, to reject any affected quantities of Products. If any Initial Materials were
not produced in compliance with cGMPs or the Specifications, or do not conform to the
Specifications, GSK shall have the right to reject and return such Initial Materials to Idenix. For
clarity, it is understood and agreed by the Parties that in the event the Initial Materials do not
meet the Specification or are determined not to be in compliance with cGMPs or otherwise rejected
by GSK, under no circumstances shall such Initial Materials be used by GSK in human clinical
trials.
(c) IDENIX’S LIABILITY IN RESPECT OF ANY SUCH REJECTION OF INITIAL MATERIALS SHALL BE LIMITED
TO THE REMEDY PROVIDED IN THIS
SECTION 3.2. NOTHING IN THIS SECTION 3.2 IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION
RIGHTS OR OBLIGATIONS OF EITHER PARTY UNDER THIS AGREEMENT.
(d) All Initial Materials will be delivered EXW (INCOTERMS 2000) from Idenix to GSK. Title to
and risk of loss with respect to any Initial Materials shall pass from Idenix to GSK when the
Initial Materials are delivered to a common carrier selected by Idenix. If any Initial Materials
are rejected by GSK after delivery under this Agreement, and such Initial Materials are to be
returned to Idenix, then title to and risk of loss with respect to those rejected Initial Materials
shall pass from GSK to Idenix when such Initial Materials are placed in the possession of the
carrier for return to Idenix or for shipment on behalf of Idenix to a destination designated by
Idenix.
3.3 Other Assistance. Except as provided in Sections 3.1, 3.2, 4.5 or otherwise
expressly provided in this Agreement, Idenix and its Affiliates shall not have any obligation to
transfer technology or provide other assistance to GSK.
4. DEVELOPMENT AND COMMERCIALIZATION
4.1 Responsibility. GSK shall be solely responsible for Developing, manufacturing and
commercializing the Licensed Compounds and Products in the Field in the Territory during the Term,
in compliance with the terms and conditions of this Agreement.
4.2 Compliance with Laws. GSK shall, and shall ensure that its Affiliates and
Sublicensees shall, comply with all applicable Laws in exercising their rights and fulfilling their
obligations under this Agreement, including all Laws applicable to pharmacovigilance anywhere in
the Territory where a Product is Developed or commercialized in the Field.
16
4.3 Diligence.
(a) GSK shall, and shall ensure that its Affiliates and Sublicensees, use Commercially
Reasonable Efforts to Develop and commercialize at least [**] for HIV in each country in the
Territory, including obtaining Regulatory Approval of the Product, bringing the Product to market
and marketing the Product. In addition, GSK shall, and shall ensure that its Affiliates and
Sublicensees, use Commercially Reasonable Efforts to perform the Development activities described
in the development plan attached hereto as Exhibit F (the “Development Plan”).
Idenix may terminate this Agreement in its entirety if prior to the filing of an NDA for IDX899,
GSK halts Development of IDX899 for a period of [**] consecutive months for any reason, not
including any delays requested or required by Regulatory Authorities or any review periods by any
Regulatory Authorities.
(b) Idenix acknowledges and agrees that the drug development process is a process of
scientific discovery and as such is inherently unpredictable and delays to development of Products
may occur for reasons beyond GSK’s control as the Products are developed, and that the drug
development process is subject to a high level of governmental regulation and the requirements of
the regulatory process of seeking drug approval may result in delays beyond GSK’s control and that
such delays or other similar delays, without evidence of some other lack of Commercially Reasonable
Efforts, are not a breach of this Section 4.3; provided, however,
that this Section 4.3(b) is not intended to modify or qualify GSK’s obligations under
Section 4.3(a).
(c) GSK shall, and shall ensure that its Affiliates and Sublicensees shall, use Commercially
Reasonable Efforts to ensure that any Third Party manufacturing contracts relating to the Products
or any component (including any active pharmaceutical ingredient) thereof to which GSK or any of
its Affiliates or Sublicenseses is a party are assignable to Idenix; provided,
however at the time of negotiation and execution of such contracts neither GSK nor Idenix
will be required to pay any cost or expense associated with the acquisition of such assignment
rights.
4.4 Reporting. During the Term, GSK shall keep Idenix regularly informed in reasonable
detail regarding GSK’s worldwide Product Development and commercialization activities as follows:
(a) Prior to the First Commercial Sale of the first Product, such information sharing shall
occur primarily through JSC meetings. GSK shall provide [**] written reports to Idenix in [**] of
each Calendar Year summarizing the status of the Development and commercialization efforts of GSK
and its Affiliates and Sublicensees with respect to Licensed Compounds and Products, including
significant Development (including clinical trial progress and Regulatory Approval) and
commercialization plans, activities and results with respect to Products;
(b) From and after the First Commercial Sale of the first Product, GSK shall provide [**]
written reports to Idenix in [**] of each year summarizing the status of the Development and
commercialization efforts of GSK and its Affiliates and Sublicensees with
17
respect to Licensed
Compounds and Products, if any, including significant Development (including clinical trial
progress and Regulatory Approval) and commercialization plans, activities and results, including
sales trends, competitive landscape and potential and actual Generic Competition information with
respect to Products; and
(c) Throughout the Term, GSK shall notify Idenix promptly (either through one of the means
described in subparagraphs (a) through (b) above or by written notice) of the occurrence of the
following by each Product:
(i) initiation of any Phase II Clinical Trial;
(ii) initiation of any Phase IIb Clinical Trial;
(iii) initiation of any Phase III Clinical Trial;
(iv) NDA filing in any Major Market;
(v) NDA approval in any Major Market; and
(vi) First Commercial Sale in any Major Market or other European Union country.
4.5 Committees.
(a) Structure. The Parties shall establish a Joint Steering Committee (“JSC”)
and an Intellectual Property Committee (the “IP Committee”; each of the JSC and the IP
Committee, a “Committee”). Each Committee shall consist of [**] members, [**]
representatives from each Party, each of whom shall have the authority, experience and seniority
sufficient to enable him or her to make final decisions on behalf of the Party he or she
represents, and each JSC member will have the sufficient capacity and shall commit sufficient
effort to manage all aspects of the collaboration; provided, however, that the IP
Committee shall have less than [**] representatives from each Party if so agreed to by the Parties.
One member from among each Party’s JSC members who are members of such Party’s executive
management team will be selected as co-chair. Each Party may each change its Committee
representatives or designated co-chairs at any time by giving written notice to the other Party.
(b) JSC Responsibilities. The JSC’s overall responsibility shall be to encourage and
facilitate ongoing cooperation between the Parties with respect to the activities contemplated by
this Agreement and to perform the other obligations specifically delegated to the JSC by this
Agreement, subject to the limitations set forth in this Section 4.5(b). The JSC shall also serve
as the principal means by which GSK keeps Idenix informed regarding GSK’s development plans,
efforts and results with respect to Products as required under this Agreement.
(i) Decisions of the JSC shall be made by unanimous vote, with each Party’s representatives on
the JSC collectively having one (1) vote. No vote of the JSC may be taken unless at least [**] of
each Party’s representatives is present for the JSC vote.
18
Notwithstanding the foregoing, the
Parties agree to the following limitations with respect to the authority of the JSC:
(ii) The JSC’s role with respect to Products shall be solely informational and advisory, and
the JSC shall not have any decision-making authority, or any power to direct GSK’s Development
efforts or amend this Agreement. If, GSK determines in good faith that material changes to the
Development Plan are advisable, GSK shall promptly inform Idenix thereof and, at Idenix’s written
request, the Parties shall promptly convene a JSC meeting to discuss the nature of such changes and
the rationale therefor. In addition, if GSK determines, that a material delay in the performance
of the Development Plan is reasonably likely, GSK shall promptly inform Idenix thereof and, at
Idenix’s written request, the Parties shall promptly convene a JSC meeting to discuss the cause(s)
of such delay, the estimated duration of the delay and the possibility of avoiding or mitigating
such delay. Notwithstanding the foregoing GSK may not, without the prior written consent of
Idenix, not to be unreasonably withheld, make a change to the Development Plan that would remove or
significantly delay any Development milestone provided in Section 5.2(a) below, not including any
delays caused by Regulatory Authorities. However, in the event of a fundamental difference of
opinion between the Parties regarding the Development path for Products, Idenix or GSK shall have
the right to refer such matter to the Chief Executive Officer of Idenix and the SVP Medicines
Development Center, Infectious Diseases at GSK for further discussion for a period of [**] (unless
extended by mutual agreement of such officers). Such officers shall discuss such matter in good
faith and attempt to reach resolution. In the event the Parties still cannot agree on a matter
after such escalation to such officers, GSK shall have the ultimate decision-making authority with
respect
to any such Development matter subject to the limitations on such authority provided in
Section 14.8(a). For the avoidance of doubt, GSK shall have sole authority to manufacture the
Products.
(c) IP Committee Responsibilities. During its existence, the IP Committee shall be
responsible for coordinating communication between the Parties described in Section 6 and, to the
extent that a Party is obligated to communicate with the other Party pursuant to Section 6, such
Party shall ensure that such communication take place through the IP Committee. If there is a
reasonable need for urgency with respect to such communication (including notices of Paragraph IV
Certifications), either Party may convene a special meeting of the IP Committee within three (3)
Business Days. The Parties agree that, as set forth in Section 6, one or the other Party may have
the final decision-making authority with respect to certain decisions set forth therein, subject to
the limitations on such authority provided in Section 14.8(a).
(d) Committee Administration. Each Committee’s co-chairs shall be responsible for
calling meetings of such Committee and for leading the meetings. Each Committee shall meet at
least [**] during the Term, unless otherwise mutually agreed. Unless otherwise agreed by the
Parties, following each Committee meeting, the co-chairs shall jointly prepare draft meeting
minutes, and shall submit such draft minutes to the Committee members within [**] of such meeting
to allow adequate review and comment. Minutes shall provide a description of the topics discussed
at the meeting, including copies of any presentations and a list of any actions, decisions or
determinations approved by such Committee. Minutes of each Committee meeting shall be approved or
disapproved, and revised as necessary, at the next meeting and, following approval, shall be signed
by such Committee’s co-chairs. Final minutes of each meeting shall be distributed to the members
of such Committee by such Committee’s co-chairs.
19
(e) Committee Meetings. The location of meetings of each Committee shall alternate
between Idenix’s principal place of business and GSK’s principal place of business, or as otherwise
agreed by the Parties. Each Committee may also meet by means of a telephone conference call or
videoconference. Each Party shall use reasonable efforts to cause its representatives to attend
such meetings. If a representative of a Party is unable to attend a meeting, such Party may
designate an alternate to attend such meeting in place of the absent representative. In addition,
each Party may, at its discretion, invite non-voting employees, and, with the consent of the other
Party, consultants or scientific advisors, to attend the meetings of any Committee. Either Party
may convene a special meeting of any Committee for the purpose of resolving disputes.
(f) Termination of Committees. Notwithstanding anything to the contrary herein, no
Committee (or any other committee which the Parties may establish on which members of both Parties
participate) shall remain in existence after the tenth (10th) anniversary of the Effective Date,
unless otherwise agreed by the Parties. Notwithstanding the foregoing, Idenix may, at any time,
terminate the JSC.
5. PAYMENTS
5.1 Initial Payments. In partial consideration for the rights and licenses granted to
GSK hereunder, (a) GSK shall pay to Idenix, by wire transfer to an account designated by
Idenix, a non-refundable, non-creditable license fee in the amount of seventeen million U.S.
Dollars (US $17,000,000) within thirty (30) days of the Effective Date, (b) GSK and Idenix shall
enter into the Stock Purchase Agreement on the Execution Date.
5.2 Milestone Payments.
(a) Development Milestones. GSK shall pay to Idenix, by wire transfer to an account
designated by Idenix, the applicable non-refundable, non-creditable milestone payment listed below
within [**] after the first achievement of each milestone event in the Territory by a Product in
the Field:
| Milestone Event: | Milestone Payment: | |
(i) Successful Completion of development
of the first formulation of a Licensed
Compound
|
[**] U.S. Dollars (US $[**]) | |
(ii) Successful Completion of development
of the first fixed dose combination
formulation of a Licensed Compound
|
[**] U.S. Dollars (US $[**]) | |
(iii) Successful Completion of a segment
II reproductive toxicity study
|
[**] U.S. Dollars (US $[**]) |
20
| Milestone Event: | Milestone Payment: | |
(iv) Successful Completion of a six (6)
month rodent chronic toxicology study
|
[**] U.S. Dollars (US $[**]) | |
(v) Successful Completion of a chronic
toxicology non-rodent study of at least nine
(9) months
|
[**] U.S. Dollars (US $[**]) | |
(vi) Successful Completion of a
carcinogenicity program in at least one (1)
rodent species
|
[**] U.S. Dollars (US $[**]) | |
(vii) First patient dosed in first Phase II
Clinical Trial studying chronic dosing (24 —
48 weeks) in combination with other ARTs in
HIV-infected patients as defined in the
Development Plan
|
[**] U.S. Dollars (US $[**]) | |
(viii) First patient dosed in Phase III
Clinical Trial of a Product for HIV
|
[**] U.S. Dollars (US $[**]) | |
(ix) Acceptance for review by the FDA of an
NDA for first Product for HIV
|
[**] U.S. Dollars (US $[**]) | |
(x) Acceptance for review by EMEA of an NDA
for first Product for HIV
|
[**] U.S. Dollars (US $[**]) | |
(xi) Acceptance for review by MHLW of an NDA
for first Product for HIV
|
[**] U.S. Dollars (US $[**]) | |
(xii) Receipt of Regulatory Approval from
the FDA for first Product for HIV
|
[**] U.S. Dollars (US $[**]) | |
(xiii) First Commercial Sale of the first
Product for HIV in the first of one (1) of
the following countries: [**].
|
[**] U.S. Dollars (US $[**]) | |
(xiv) Receipt of Regulatory Approval from
the MHLW for first Product for HIV
|
[**] U.S. Dollars (US $[**]) |
(b) Sales Milestones. GSK shall pay to Idenix, by wire transfer to an account
designated by Idenix, the applicable non-refundable, non-creditable milestone payment listed below
within [**] after the first achievement of each milestone event for all Products, in the aggregate.
For purposes of clarity, the Parties understand and agree that for purposes of Section 5.3(b) the
Annual Net Sales shall include the aggregate Net Sales of any/all Product(s), including all
Combination Product Net Sales (as defined in Section 5.3(b)(i) below).
21
| Milestone Event: | Milestone Payment: | |
(i) Annual Net Sales greater than [**] U.S.
Dollars (US $[**])
|
[**] U.S. Dollars (US $[**]) | |
(ii) Annual Net Sales greater than [**]
U.S. Dollars (US $[**])
|
[**] U.S. Dollars (US $[**]) | |
(iii) Annual Net Sales greater than [**]
U.S. Dollars (US $[**])
|
[**] U.S. Dollars (US $[**]) |
(c) Overlapping Milestones. Each of the milestone payments set forth in Sections
5.2(a) and 5.2(b) shall be payable once. If a milestone event described in a clause in Section
5.2(b) occurs before or concurrently with a milestone event described in a preceding clause that
has yet to be achieved, GSK shall make the milestone payment corresponding the milestone event
described in such earlier clause in addition to the milestones payment corresponding to the
milestone event described in the later clause when the payment for the milestone event described
in such later clause is paid. If (A) the milestone event described in Section 5.2(a)(vii) occurs
prior to the milestone events described in 5.2(a)(iii), 5.2(a)(iv) or 5.2(a)(v), (B) the milestone
event described in Section 5.2(a)(viii) occurs prior to the milestone events described in Section
5.2(a)(i), Section 5.2(a)(iii), 5.2(a)(iv), 5.2(a)(v) or 5.2(a)(vii), (C) a milestone event
described in Section 5.2(a) clauses (ix) through (xi) occurs before or concurrently with a
milestone event described in Section 5.2(a) clauses (i) or Section 5.2(a) clauses (iii) through
(viii), (D) the milestone event described in Section 5.2(xii) occurs prior to the milestone event
described in Section 5.2(ix), (E) the milestone event described in Section
5.2(xiii) occurs prior to the milestone event described in Section 5.2(x), or (F) the
milestone event described in Section 5.2(xiv) occurs prior to the milestone event described in
Section 5.2(xi), GSK shall make the milestone payment corresponding to the milestone event
described in such earlier clause(s) in addition to the payment corresponding with the milestone
event described in the later clause when the payment corresponding with the milestone event
described in such later clause is paid; provided, however, the mechanism for
payment of overlapping milestones provided in this Section 5.2(c) will not apply to the milestone
event and corresponding payment described in Section 5.2(a)(ii) if the first Product to achieve the
milestone events described in Section 5.2(a) clauses (i) or Section 5.2(a) clauses (iii) through
(xiv) is a mono-therapy Product and the milestone event described in Section 5.2(a)(ii) is not
achieved.
5.3 Royalties.
(a) Base Rate.
(i) Subject to Sections 5.3(a)(ii), 5.3(a)(iii), 5.3(b), 5.3(c), 5.3(d) and 5.3(e), GSK shall
pay to Idenix the following tiered royalties on Annual Net Sales of Products in the Territory on a
Product by Product basis:
22
| Annual Net Sales Tiers: | Royalty Rate: | |
The portion of Annual Net Sales which is less than or equal
to [**] U.S. Dollars (US $[**])
|
[**]% | |
The portion of Annual Net Sales which is greater than [**]
U.S. Dollars (US $[**]), but less than or equal to [**] U.S.
Dollars (US $[**])
|
[**]% | |
The portion of Annual Net Sales which is greater than [**]
U.S. Dollars (US $[**]), but less than or equal to [**] U.S.
Dollars (US $[**])
|
[**]% | |
The portion of Annual Net Sales which is greater than [**]
U.S. Dollars (US $[**])
|
[**]% |
For example, if the Calendar Year Net Sales of a Product are [**] U.S. Dollars (US $[**]), then the
royalty payable to Idenix would be [**], which is calculated as follows: [**].
(ii) Notwithstanding the foregoing, if, with respect to a Calendar Quarter and a Product, on a
Product by Product basis, the Measured COGS of such Product, as a percentage of Measured Net Sales
of such Product, is greater than [**] percent ([**]%), then, solely with respect to such Calendar
Quarter and such Product, the royalty rate as provided in Section 5.3(a)(i) will be decreased by
[**] for every [**] by which such percentage exceeds [**] percent ([**]%). GSK will calculate the
Measured COGS, on a Product by Product basis, for all units of each Product sold during each
Calendar Quarter during the Term.
(iii) The royalty rates applied to Net Sales of the relevant Product sold by GSK, its
Affiliates or Sublicensees in UNAIDS Accelerating Access Initiative (“AAI”) countries
designated by the World Health Organization as set forth in Schedule 5.3(a) hereto, as may
be amended from time to time by the World Health Organization, for use in the management,
treatment, prevention or diagnosis of HIV will be [**] percent ([**]%) of the rates provided in
Section 5.3(a)(i) above; provided, however, that sales in AAI countries shall be
aggregated with all other sales of the relevant Product to determine the Annual Net Sales and,
thereby, to determine the applicable royalty rate(s).
(b) Calculation of Royalty Payments for Combination Products. If the Product is sold
as part of a Combination Product, the Net Sales of the Product, for the purposes of determining
royalty payments, shall be determined using the following formula:
(i) For any Product that is a Combination Product, the Net Sales shall be multiplied by [**]
(the product of which shall be deemed to be “Combination Product Net Sales”), and then the
Combination Product Net Sales will be multiplied by the applicable royalty rate(s) set forth in
Section 5.3(a)(i)-(iii) during the applicable royalty reporting period, subject to Sections 5.3(c),
(d) and (e).
For purposes of the immediately preceding sentence:
23
[**]
If sales of both the Sole Compound Product and the other active compounds did not occur during
the applicable royalty reporting period, then the most recent Calendar Quarter in which sales of
both occurred shall be used for determining A and B.
If such average sale price cannot be determined for both the Sole Compound Product and all
other active compounds included in the Combination Product, then Net Sales shall be multiplied by
[**], where:
[**].
In such event, GSK shall in good faith make a determination of the respective fair market
values of the Sole Compound Product and all other active compounds included in the Combination
Product, and shall notify Idenix of such determination and provide Idenix with GSK’s basis for such
determination. Idenix may review such determination. If Idenix does not agree with such
determination, Idenix shall give written notice of its disagreement within [**] after receiving the
relevant royalty report pursuant to Section 5.4, and the provisions of Section 14.8 shall apply.
(ii) Notwithstanding the foregoing provisions of Section 5.3(b)(i), royalty payments
calculated pursuant to Section 5.3(b) for any Combination Product shall equal the greater of (i)
the royalty payment as calculated pursuant to the foregoing provisions of Section 5.3(b) and (ii)
(A) with respect to a Double Combination Product, the royalty payment determined by multiplying
[**] percent ([**]%) of the Net Sales of such Combination Product by the applicable royalty rate(s)
set forth in Section 5.3(a) during the applicable royalty reporting period (i.e., without reference
to Section 5.3(b)) or (B) with respect to a Triple Combination
Product, the royalty payment determined by multiplying [**] percent ([**]%) of the Net Sales
of such Combination Product by the applicable royalty rate(s) set forth in Section 5.3(a) during
the applicable royalty reporting period (i.e., without reference to Section 5.3(b)).
(iii) [Intentionally Omitted]
(iv) On a Product by Product basis, GSK shall pay to Idenix royalties for Combination
Products as set forth in Section 5.3(b) of this Agreement; provided, however, that
sales of Combination Products shall be [**]. For example, if, with respect to a Calendar Year, the
Annual Net Sales for a particular Product, on a Product by Product basis, total $[**], with Net
Sales of the relevant Sole Compound Product of $[**] and Net Sales of the relevant Double
Combination Product of $[**]. Since [**] of the Annual Net Sales are attributable to the Sole
Compound Product and [**] of the Annual Net Sales are attributable to the Double Combination
Product, then, assuming that the [**] described in Schedule 5.3(b) applies to the Double
Combination Product, the royalties will = [**].
(v) For the sake of clarity, for any Product that is not a Combination Product, the Net Sales
shall be multiplied by the applicable royalty rate set forth in Section 5.3(a) during the
applicable royalty reporting period, subject to Sections 5.3(a)(ii), 5.3(a)(iii), 5.3(b), 5.3(c),
5.3 (d) and 5.3(e).
24
(c) Royalty Term. The royalty set forth in Section 5.3(a) or 5.3(b) shall be payable
during the Royalty Term for each Product in each country in the Territory; provided,
however, that, on a Product-by-Product and country-by-country basis, after the expiration
of the last Valid Claim of the Idenix Patent Rights and Joint Patent Rights which Covers the
Exploitation of such Product in such country, the royalty rate with respect to Net Sales of such
Product in such country shall be reduced to fifty percent (50%) of the rate set forth in Section
5.3(a)(i). Upon the expiration of the Royalty Term in a country, GSK shall have a fully paid-up,
non-exclusive, perpetual license to use the Idenix IP to Exploit such Product in the Field in such
country.
(d) Royalty Stacking. If GSK reasonably determines in good faith that, in order to
commercialize a Product in a country in the Territory, it is necessary to obtain a patent license
from a Third Party (other than a Sublicensee) in order to avoid infringing such Third Party’s
patents (including in connection with the settlement of a patent infringement claim), GSK shall be
entitled to deduct from the royalty payments it makes to Idenix with respect to sales of such
Product in such country during the applicable Calendar Quarter an amount up to [**] percent ([**]%)
of the amounts paid by GSK to such Third Party with respect to such license for use with such
Product in such country; provided, that in no event shall a deduction under this Section
5.3(d) reduce any royalty payment made by GSK pursuant to Section 5.3(a) or 5.3(b) (subject to
Section 5.3(c)) by more than [**] percent ([**]%). If, but for the proviso in the preceding
sentence, the deduction under this Section 5.3(d) would have reduced a royalty payment made by GSK
by more than [**] percent ([**]%), then the amount of such deduction that exceeds [**] percent
([**]%) will be carried over to subsequent royalty payments until the full amount that GSK would
have been entitled to deduct (absent the above limitation) is deducted. The provisions of this
Section 5.3(d) shall not apply in the instance where a patent
license from a Third Party is obtained by GSK for rights to the composition of matter or
method of treatment of an active or inactive ingredient in a Product that is not a Licensed
Compound.
(e) Generic Competition. If there is Generic Competition with respect to a Product in
a country in the Territory for a Calendar Quarter, then the royalty applicable to Net Sales of such
Product for such Calendar Quarter shall be reduced to [**] percent ([**]%) of the royalty otherwise
applicable to such Product in such country pursuant to Sections 5.3(a) or 5.3(b), subject to
Sections 5.3(c) and 5.3(d).
(f) No Discounting in Exchange for In-Kind Consideration. GSK and its Affiliates and
Sublicensees shall not sell or distribute Products at a discount (or without consideration) in
return for (i) concessions or consideration received in transactions involving products or services
other than Products or (ii) concessions from any government or governmental authority relating to
products or services other than Products.
(g) Existing Third Party Agreements. After the Effective Date, Idenix shall continue
to be solely responsible for the costs of any agreement existing on the Effective Date between
Idenix or its Affiliates, on the one hand, and a Third Party, on the other hand, pursuant to which
Idenix has in-licensed any Idenix IP, including the Xx Xxxxx Agreement and the University
Agreements.
25
5.4 Reports and Payments. GSK shall deliver to Idenix, within [**] after the end of
each Calendar Quarter, a report indicating estimated Net Sales on a country-by-country basis and
within [**] after the end of each Quarter, a royalty report together with the required payments.
The royalty report received [**] after the end of each Calendar Quarter shall indicate [**], the
calculation of royalties with respect thereto, each determined in accordance with international
financial reporting standards, consistently applied. All payments hereunder shall be payable in
U.S. dollars. When conversion of payments from any foreign currency is required, such conversion
shall be calculated using the average exchange rates as calculated and utilized by GSK’s group
reporting system and published accounts during the Calendar Quarter for which a payment is due.
All royalty payments shall be made in United States Dollars by wire transfer to an account
designated in advance by Idenix.
5.5 Tax Withholding. Idenix will pay any and all taxes levied on account of any
payments made to it under this Agreement. If any taxes are paid or required to be withheld by GSK
for the benefit of Idenix, or an entity that Idenix may assign this agreement to, on account of any
royalties or other payments payable to Idenix, or assignee of Idenix, under this Agreement, GSK
will (a) deduct such taxes from the amount of payment otherwise due to Idenix, or assignee of
Idenix, (b) timely pay the taxes to the proper taxing authority, and (c) provide a US Form 1042S
(Foreign Person’s U.S. Source Income Subject to Withholding) to Idenix, or assignee of Idenix, by
the end of March in the calendar year following the calendar year of any withholding tax
remittance. GSK agrees to use reasonable efforts to assist Idenix in claiming exemption from such
deductions or withholdings under any applicable double taxation or similar agreement or treaty.
26
5.6 Blocked Payments. Notwithstanding the provisions of Section 14.3, if at any time
legal restrictions in any country prevent prompt remittance of any royalties or other consideration
owed to Idenix by GSK, then GSK will pay Idenix directly from another source of funds in order to
remit the entire amount owed to Idenix.
5.7 Late Payments. Any payments that are not made on or before the due date shall
bear interest from the date due at a rate per annum equal to the annual prime rate of interest
quoted in the Money Rates section of The Wall Street Journal, Eastern Edition, for the date on
which payment was due until the date on which payment is received, calculated daily on the basis of
a 365-day year, or similar reputable data source mutually agreed upon by the Parties in good faith;
provided, however, that in no event shall such rate exceed the maximum legal annual
interest rate. Interest shall be payable for the period from the date on which such payment was due
through the date on which payment is actually made. The Parties agree this provision, unless
otherwise provided, will not apply to payments that are the result of a subsequent adjustment of an
estimated payment, including rebates, adjustments, returns or true-ups.
5.8 Financial Records. GSK shall maintain all of its and its Affiliates’ and
Sublicensees’ full, true and accurate books of accounts and other records relating to the
transactions and activities contemplated by this Agreement in sufficient detail to verify
compliance with the terms of this Agreement. GSK shall maintain such records for at least three
(3) years after the end of the Calendar Year to which such records relate.
5.9 Audit Right.
(a) [**] during each Calendar Year, Idenix may retain an independent certified public
accountant reasonably acceptable to GSK (the “Auditor”) to audit the records described in
Sections 5.4, and 5.8, upon reasonable notice to GSK, during regular business hours and under a
reasonable obligation of confidentiality to GSK. Idenix shall bear the costs of such audit, except
as provided below. The Auditor will execute a reasonable written confidentiality agreement with
GSK and will disclose to Idenix only such information as is reasonably necessary to provide Idenix
with information regarding any actual or potential discrepancies between amounts reported and
actually paid and amounts payable under this Agreement. The Auditor will send a copy of the report
to GSK at the same time it is sent to Idenix, but the report shall be considered GSK’s Confidential
Information. The report sent to both Parties will include the methodology and calculations used to
determine the results. If the audit demonstrates that the payments owed under this Agreement have
been understated, GSK shall pay the balance to Idenix, together with interest in accordance with
Section 5.7. Further, if the amount of the understatement is greater than five percent (5%) of the
amount owed to Idenix with respect to the audited period, then GSK shall reimburse Idenix for the
reasonable cost of the audit. All payments owed by GSK under this Section 5.9 shall be made within
[**] after the results of the audit are delivered to the Parties. If such audit discloses an
overpayment by GSK, then GSK will deduct the amount of such overpayment from amounts otherwise owed
to Idenix under this Agreement. If, however, no additional payments are due from GSK to Idenix
within the next [**] under the terms of this Agreement, then, upon request by GSK, Idenix will
refund such overpayment to GSK. Upon the expiration of [**] following the end of any Calendar
Year, the calculation of royalties payable with respect to such calendar year will be binding and
conclusive
27
upon Idenix except with respect to any audit then underway, and except for fraud or
misrepresentation, GSK and its Affiliates will be released from any liability or accountability
with respect to royalties for such Calendar Year, except that GSK shall remain liable for any
then-unpaid amounts described in the reports provided by GSK pursuant to Section 5.4 or determined
by the Auditor, pursuant to this Section 5.9, to have been due with respect to such Calendar Year.
(b) To the extent Idenix has rights to audit and inspect its contract manufacturers’ records
and those portions of each facility used in the manufacture, generation, storage, testing,
treatment, holding, transportation, distribution or other handling or receiving of the active
pharmaceutical ingredient, drug substance and drug product related to the Licensed Compound as of
the Effective Date, Idenix shall cooperate with GSK to enable GSK to perform such audit or
inspection.
5.10 Invoices. All fees to be paid by GSK hereunder shall be payable in accordance
with the timeframes expressly specified therefor following GSK’s receipt from Idenix of an original
invoice for the applicable fee generated in accordance with invoicing instructions reasonably
provided by GSK and including Idenix’s payee bank information and a contact name and number for
issue resolution. All fees and other charges set forth in this Agreement shall include all
applicable sales, use or other taxes, which, with respect to such taxes to be paid by GSK, shall
each be separately stated in the invoice. All invoices shall be sent to the address designated
below, unless otherwise specified in writing by GSK.
GlaxoSmithKline
Corporate Treasury
000 X. 00xx Xxxxxx, XX0000
Xxxxxxxxxxxx, XX 00000
Attn. U.S. Treasury
Corporate Treasury
000 X. 00xx Xxxxxx, XX0000
Xxxxxxxxxxxx, XX 00000
Attn. U.S. Treasury
5.11 Licensed Compounds or Products for Other Indications. In the event GSK, its
Affiliates or Sublicensees desires to Develop or commercialize a Licensed Compound or commercialize
a Product for an indication or therapeutic or prophylactic treatment in addition to HIV, then, GSK,
its Affiliates and Sublicensees shall not start such Development or commercialization until GSK and
Idenix mutually agree on a separate schedule of payments for the achievement of development and
sales milestones and royalties. In the event GSK and Idenix are not able to come to a mutual
agreement, any such dispute shall be resolved pursuant to Section 14.8(b).
5.12 Development Milestones, Sales Milestones and Royalties for Licensed Compounds Other
Than IDX899. If GSK develops a Licensed Compound other than IDX899, development milestones,
sales milestones and royalties for such Licensed Compound and the Product containing such Licensed
Compound would be negotiated by the Parties in good faith; provided, however,
Development milestones, sales milestones and royalties payable to Idenix for such Licensed
Compounds shall be determined relative to the amounts described above in Section 5.2 and 5.3 to
reflect the much earlier stage of development of the Licensed Compounds other than IDX899 and the
consequent cost and risk to be born solely by GSK during subsequent
28
development; and provided, further, development milestones for such Licensed
Compounds would begin with the milestone event described in Section 5.2(a)(viii) above (initiation
of Phase III Clinical Trials) and would be paid upon achievement of the events described in clauses
(viii) through (xiv).
6. INTELLECTUAL PROPERTY PROTECTION
6.1 Ownership of Inventions. Inventorship of inventions conceived of and reduced to
practice during the Term of this Agreement (“Inventions”) shall be determined in accordance
with United States patent Laws. Idenix shall solely own all Idenix Inventions, and GSK shall
solely own all GSK Inventions. All Joint Inventions shall be owned jointly by GSK and Idenix.
Subject to Section 6.2 below, Idenix shall have the sole right to seek protection for and enforce
the Idenix Inventions at Idenix’s sole cost and expense that are not Idenix Patent Rights. GSK
shall have the sole right to seek protection for and enforce GSK Inventions and Joint Inventions.
6.2 Prosecution and Maintenance of Idenix Patent Rights and Joint Patent Rights. GSK
shall have the sole right to file, prosecute and maintain the Idenix Patent Rights and Joint Patent
Rights in the Territory, as follows:
(a) Idenix shall, at GSK’s reasonable request, assist and cooperate in the filing, prosecution
and maintenance of the Idenix Patent Rights and Joint Patent Rights;
(b) GSK shall provide Idenix, sufficiently in advance for Idenix to comment, with copies of
all correspondence from patent authorities, summaries of correspondence from patent counsel and
draft copies of all patent applications and other material submissions and correspondence (or
summaries thereof) with any patent counsel or patent authority that pertain to the Idenix Patent
Rights and Joint Patent Rights;
(c) GSK shall give due consideration to Idenix’s comments, but GSK shall have the final say in
determining whether or not to incorporate such comments; provided, however, that
GSK shall not, without Idenix’s prior written consent, not to be unreasonably withheld, abandon or
narrow the scope of any claim in the Co-Labeling Patent Rights that Covers the Co-Labeling Right;
(d) GSK shall use Commercially Reasonable Efforts to maintain the claims set forth in the
Idenix Patent Rights in existence as of the Effective Date; and
(e) In the event GSK elects not to prosecute or maintain any Idenix Patent Rights or Joint
Patent Rights, Idenix shall have the right to do so at Idenix’s sole expense and Idenix shall also
then have the sole right to file, prosecute and maintain such Idenix Patent Rights and Joint Patent
Rights.
6.3 Enforcement of Idenix Patent Rights and Joint Patent Rights.
(a) Notice. Each Party shall, within [**], provide the other Party with written
notice reasonably detailing any known or alleged infringement by a Third Party of the Idenix Patent
Rights or Joint Patent Rights, including any “patent certification” filed in the United
29
States under 21 U.S.C. §355(b)(2) or 21 U.S.C. §355(j)(2) or similar provisions in other
jurisdictions (a “Paragraph IV Certification”), and of any declaratory judgment,
opposition, or similar action alleging the invalidity, unenforceability or non-infringement of the
Idenix Patent Rights or Joint Patent Rights.
(b) Infringement. With respect to any actual or suspected infringement of Idenix
Patent Rights or Joint Patent Rights by a Third Party in the Field:
(i) GSK shall have the initial right to initiate a legal action against such Third Party with
respect to such infringement, at GSK’s expense. Idenix shall join in such action as a party at
GSK’s request and expense in the event that an adverse party asserts, the court rules or other Laws
provide, or GSK determines in good faith, that a court would lack jurisdiction based on Idenix’s
absence as a party in such suit. Idenix may also at any time join in such action and may be
represented by counsel of its choice, at Idenix’s expense; but in any event control of such action
shall remain with GSK. At GSK’s reasonable request Idenix shall provide reasonable assistance to
GSK in connection with such action, provided that GSK will reimburse Idenix for any
reasonable out-of-pocket costs associated with such cooperation. GSK shall, through the IP
Committee and otherwise upon Idenix’s reasonable request, keep Idenix reasonably informed of the
progress and status of such action. Any recoveries resulting from such an action (by settlement or
otherwise) shall be applied as follows:
(A) First, to reimburse each Party for all out-of-pocket costs in connection
with such proceeding (on a pro rata basis, based on each Party’s respective
out-of-pocket costs, to the extent the recovery was less than all such costs); and
(B) Second, [**] percent ([**]%) of any remainder shall be paid to Idenix.
(ii) If GSK does not commence and vigorously pursue a legal action to enjoin such infringement
within [**] of being notified or otherwise becoming aware of such infringement (or such shorter
period of time as necessary to ensure that Idenix may reasonably protect the Idenix Patent Rights
or Joint Patent Rights), Idenix may, at its expense, commence the action. GSK shall join in such
action as a party at Idenix’s request and expense in the event that an adverse party asserts, the
court rules or other Laws provide, or Idenix determines in good faith, that a court would lack
jurisdiction based on GSK’s absence as a party in such suit, but control of such action shall
remain with Idenix. At Idenix’s reasonable request, GSK shall provide reasonable assistance to
Idenix in connection with such action provided that Idenix will reimburse GSK for
any reasonable out-of-pocket costs associated with such cooperation. Any recoveries resulting from
such an action shall be retained by Idenix.
(iii) Patent Rights Certifications and Infringement Suits. Notwithstanding any
provision herein to the contrary, the following provisions of this Section 6.3(b) will apply for
certifications claiming that any of the Idenix Patent Rights are invalid, unenforceable or that no
infringement will arise from the manufacture, use or sale of a Third Party’s product (i.e., a
Paragraph IV Certification under 21 U.S.C. Section 355 and 21 C.F.R.
30
Part 314). GSK will have the sole right, but not the obligation to bring suit against the
Third Party that filed the certification. If GSK decides to bring suit, GSK will have sole control
of all decisions regarding all aspects of such litigation including settlement; provided
that Idenix will have the right to participate in such litigation and to share in
recoveries from such litigation in the same manner as set forth in Section 6.3(b)(i)(B) with
respect to infringement litigations brought by GSK thereunder. Idenix will execute such legal
papers necessary for the prosecution of such suit and cooperate with GSK as may be reasonably
requested by GSK.
(c) No Admissions. Without the prior written consent of Idenix, not to be
unreasonably withheld, GSK shall not (i) enter into any settlement with respect to the Co-Labeling
Patent Rights (ii) enter into any settlement with respect to the invalidity or unenforceability of,
the Idenix Patent Rights or Joint Patent Rights or (iii) admit to the invalidity or
unenforceability of, the Idenix Patent Rights or Joint Patent Rights.
6.4 Claimed Infringement. If a Party becomes aware that the Exploitation of Products
in the Field in the Territory by GSK or its Affiliates infringes, or is likely or is alleged to
infringe, the Intellectual Property Rights of any Third Party, such Party shall promptly notify the
other Party, and GSK shall have the sole right and responsibility to take any action it deems
appropriate with respect thereto; provided, however, that, to the extent that any
action would involve the enforcement or defense of the Idenix IP, Section 6.3 shall apply with
respect to such enforcement or defense.
6.5 Patent Term Extensions.
(a) GSK shall use reasonable efforts to obtain all available patent term extensions,
adjustments or restorations, or supplementary protection certificates of Idenix Patent Rights and
Joint Patent Rights (an “SPC”, and together with patent term extensions, adjustments and
restorations, collectively “Patent Term Extensions”). Idenix shall cooperate with GSK in
obtaining Patent Term Extensions and GSK shall notify Idenix through the IP Committee, of the
Regulatory Approval of each Product for which Patent Term Extensions may be obtained within [**]
after receipt of such Regulatory Approval, and providing the date of issuance and patent number for
each such Idenix Patent Right and Joint Patent Right.
(b) GSK will select, which, if any, Idenix Patent Rights and Joint Patent Rights are to be
extended or restored with respect to each Product. In the event that more than one Idenix Patent
Right or Joint Patent Right is eligible for Patent Term Extension based upon the regulatory review
and subsequent approval of a particular Product, GSK will select, which, if any, Idenix Patent
Right or Joint Patent Right is to be extended or restored, such selection to be based solely on
GSK’s reasonable determination regarding which Patent Term Extension provides the best protection
for the Product, taking into account both duration and strength of patent coverage. GSK shall
promptly notify Idenix of any Idenix Patent Right or Joint Patent Right for which GSK seeks Patent
Term Extension and of any other Patent Controlled by GSK Covering such Product for which GSK seeks
extension or patent term restoration.
(c) GSK shall file for all such extensions at GSK’s expense, and Idenix shall execute such
authorizations and other documents and take such other actions as may be
31
reasonably requested to obtain such Patent Term Extensions, including designating GSK as its
agent for such purpose. Any such extension of any Idenix Patent Rights or Joint Patent Rights will
be included within the definition of Idenix Patent Rights or Joint Patent Rights, respectively.
6.6 Patent Rights Listings and Regulatory Data Protection. To the extent required or
permitted by applicable Law, GSK shall in its reasonable determination list with the applicable
Regulatory Authorities during the Term, applicable Idenix Patent Rights and Joint Patent Rights for
any Product that is the subject of a marketing application to secure statutory exclusivity rights,
including those available under the Xxxxx-Xxxxxx Act. For purposes hereof, “Xxxxx-Xxxxxx
Act” means the United States Drug Price Competition and Patent Term Restoration Act of 1984
(Pub. Law 98-417) (or any successor thereto), and any analogous laws in other countries, as in
effect from time to time during the Term. In connection with such listings, Idenix and GSK shall
meet to evaluate (which may include inspection of documentation in either Party’s possession
relating to potential listable Idenix Patent Rights and Joint Patent Rights) and identify all
potential applicable Idenix Patent Rights and Joint Patent Rights for any Product that GSK intends
or has begun to commercialize, and that has or will become the subject of a marketing application
submitted to FDA or equivalent foreign Regulatory Authority; provided, however,
that, notwithstanding any provision herein to the contrary, GSK will retain final decision-making
authority as to the listing (or delisting) of all applicable Idenix Patent Rights and Joint Patent
Rights.
7. CONFIDENTIAL INFORMATION
7.1 Non-Use and Non-Disclosure of Confidential Information. Each Receiving Party
agrees that all Confidential Information of the Disclosing Party (a) shall not be used by the
Receiving Party or its Affiliates except to perform its obligations or exercise its rights under
this Agreement, and (b) shall be maintained in confidence by the Receiving Party and its
Affiliates, and, except as permitted by Sections 7.2, 7.3 and 7.4, shall not be disclosed by the
Receiving Party or its Affiliates to any Person without the prior written consent of the Disclosing
Party.
7.2 Permitted Disclosures. The Receiving Party may provide the Disclosing Party’s
Confidential Information:
(a) to the Receiving Party’s and its Affiliates’ employees, consultants and advisors who have
a need to know such Confidential Information and are bound by an obligation to maintain the
confidentiality of the Disclosing Party’s Confidential Information to the same extent as if they
were parties hereto;
(b) to Regulatory Authorities in order to seek or obtain Regulatory Approval with respect to
the Product as contemplated by this Agreement; provided, that such disclosure may be made
only to the extent reasonably necessary to seek or obtain such approvals; and
(c) if such disclosure is required by Law (including by rules or regulations of any securities
exchange or NASDAQ) or to defend or prosecute litigation or arbitration; provided, that
prior to such disclosure, to the extent permitted by Law or such rules or regulations, the
Receiving Party promptly notifies the Disclosing Party of such requirement and
the Receiving Party furnishes only that portion of the Disclosing Party’s Confidential
Information that the Receiving Party is legally required to furnish.
32
7.3 Scientific Publications; Clinical Trial Registry.
(a) GSK, its Affiliates and Sublicensees shall have the right to make disclosures pertaining
to Products in the Field in scientific journals or other publications. GSK shall, and shall ensure
that its Affiliates and Sublicensees shall, with respect to any such publication to be published by
or on behalf of GSK, its Affiliates or Sublicensees, (i) provide Idenix with an advance copy of
such proposed publication, and Idenix shall then have sixty (60) days in which to recommend any
changes it reasonably believes are necessary to preserve its Patent Rights or Know-How which is
Confidential Information and (ii) provide that any such publication shall give appropriate
attribution for any work cited in such publication that was performed by an employee of Idenix. If
Idenix informs GSK that such publication, in Idenix’s reasonable judgment, could be expected to
have a material adverse effect on any patentable invention owned by or licensed, in whole or in
part, to Idenix (other than pursuant to a license granted under this Agreement), or on any Know-How
which is Confidential Information of Idenix, GSK shall, and shall ensure that its Affiliates and
Sublicensees shall, delay or prevent such publication as follows: (a) with respect to a patentable
invention, such publication shall be delayed sufficiently long (not to exceed sixty (60) days) to
permit the timely preparation and filing of a patent application; and (b) with respect to Know-How
which is Confidential Information of Idenix, such Know-How shall be deleted from the publication.
In the case of conference abstracts and other rapid communications such as press releases, the
Parties will complete the review process in ten (10) Business Days or less, provided that
Section 7.3(b)(iii) shall apply to such conference abstracts and other rapid communications.
(b) GSK shall have the right to publish the protocols for and summaries of results of, all
clinical trials conducted by either Party regarding the Licensed Compounds on the clinical trial
registries maintained by GSK or its Affiliates, provided, that, with respect to any such
publication to be published by or on behalf of GSK or its Affiliates, (i) any such publication
shall give appropriate attribution for any work cited in such publication that was performed by an
employee of Idenix or its Affiliates; (ii) GSK shall, and shall ensure that its Affiliates shall,
provide Idenix with an advance copy of such proposed publication, and Idenix shall then have ten
(10) Business Days in which to recommend any changes it reasonably believes are necessary,
including changes necessary to preserve its Patent Rights or Know-How which is Confidential
Information; and (iii) if Idenix informs GSK that such publication, in Idenix’s reasonable
judgment, could be expected to have a material adverse effect on any patentable invention owned by
or licensed, in whole or in part, to Idenix (other than pursuant to a license granted under this
Agreement), or on any Know-How which is Confidential Information of Idenix, GSK shall, and shall
ensure that its Affiliates shall, delay or prevent such publication as follows to the extent
permitted by applicable Law: (A) with respect to a patentable invention, such publication shall be
delayed sufficiently long (not to exceed sixty (60) days) to permit the timely preparation and
filing of a patent application; and (B) with respect to Know-How which is Confidential Information
of Idenix, such Know-How shall be deleted from the publication.
33
7.4 Publicity. No Party shall have the right to make any public announcements with
respect to this Agreement, nor publicly disclose the terms of this Agreement, without the prior
written consent of the other Party, except as follows:
(a) Within four (4) Business Days following the Execution Date, the Parties shall issue the
press release attached hereto as Exhibit G;
(b) Either Party may disclose the terms of this Agreement to its and its Affiliates’
employees, consultants and advisors who have a need to know such terms and are bound by an
obligation to maintain the confidentiality of such terms to the same extent as if they were parties
hereto;
(c) Each Party may disclose the terms of this Agreement to the extent such disclosure is
required by Law (including by rules or regulations of any securities exchange or NASDAQ) or to
defend or prosecute litigation or arbitration; provided, that, prior to such disclosure, to
the extent permitted by Law or such rules or regulations, the disclosing Party promptly notifies
the other Party of such requirement and the disclosing Party furnishes only those terms of this
Agreement that the disclosing Party is legally required to furnish;
(d) Each Party may publicly file this Agreement with the United States Securities and Exchange
Commission or any other relevant securities commission in any country and shall request, and use
Commercially Reasonable Efforts to obtain, confidential treatment with respect to the terms of this
Agreement; provided, that the redaction of such terms is permitted by the applicable rules
and regulations of the United States Securities and Exchange Commission or any such securities
commission;
(e) Pursuant to an agreement or professional responsibility to maintain confidentiality,
either Party may discuss the terms of this Agreement with, or provide a copy of this Agreement to,
its independent public accountants or its attorneys;
(f) Idenix may disclose this Agreement to (i) the University, (ii) Xxxxxxxxx Xx Xxxxx, (iii)
Idenix’s then-current and potential licensees of the Idenix IP and Joint IP outside the Field, and
(iv) Idenix’s then-current and potential lenders, investors and acquirers; provided, that
such Persons are bound to maintain the confidentiality of this Agreement to the same extent as if
they were parties hereto;
(g) The contents of any public announcement, press release or similar publicity which has been
reviewed and approved by the reviewing Party can be re-released by either Party in any form without
a requirement for re-approval.
8. INDEMNIFICATION
8.1 Indemnification by GSK. GSK agrees to defend the Idenix Indemnitees, at GSK’s
cost and expense, and will indemnify and hold harmless the Idenix Indemnitees from and against any
and all losses, costs, damages, fees or expenses (“Losses”) relating to or in connection
with a Third Party claim arising out of (a) the Exploitation of the Licensed Compounds or Products
by or on behalf of GSK, its Affiliates or Sublicensees, including any
34
claim of infringement, unauthorized use or misappropriation of any Third Party’s Intellectual
Property Right or any actual or alleged death, personal bodily injury, damage to real or tangible
personal property claimed to result, directly or indirectly, from the manufacture, possession, use
or consumption of, or treatment with, such Licensed Compounds or Products; (b) any breach by GSK of
its representations, warranties or covenants made under this Agreement; or (c) any negligent act or
omission or willful misconduct of GSK, its Affiliates or Sublicensees or any of their employees,
contractors or agents, in performing GSK’s obligations or exercising GSK’s rights under this
Agreement; provided, however, that the foregoing indemnity shall not apply to the
extent that any such Losses are attributable to (i) any breach by Idenix of its representations,
warranties or covenants made under this Agreement or (i) the negligence or willful misconduct of
the Idenix Indemnitees. In the event of any such claim against any Idenix Indemnitee, Idenix shall
promptly notify GSK in writing of the claim and GSK shall manage and control, at its sole expense,
the defense of the claim and its settlement. The relevant Idenix Indemnitees shall cooperate with
GSK and may, at such Idenix Indemnitees’ option and expense, be represented in any such action or
proceeding. GSK shall not be liable for any settlements, litigation costs or expenses incurred by
any Idenix Indemnitees without GSK’s written authorization. Without the prior written consent of
Idenix, GSK shall not enter into any settlement with respect to the Idenix Patent Rights.
8.2 Indemnification by Idenix. Idenix agrees to defend GSK, its Affiliates, and the
agents, directors, officers and employees of GSK and its Affiliates (collectively, the “GSK
Indemnitees”), at Idenix’s cost and expense, and will indemnify and hold harmless the GSK
Indemnitees from and against any and all Losses, relating to or in connection with a Third Party
claim arising out of (a) the Exploitation of the Licensed Compounds or Products by or on behalf of
Idenix, its Affiliates or Sublicensees (other than any Exploitation of the Licensed Compounds or
Products by or on behalf of GSK, its Affiliates or Sublicensees), including any claim of
infringement, unauthorized use or misappropriation of any Third Party’s Intellectual Property Right
or any actual or alleged death, personal bodily injury or damage to real or tangible personal
property claimed to result, directly or indirectly, from the manufacture, possession, use or
consumption of, or treatment with, such Initial Materials or its incorporation into clinical trial
materials, Licensed Compound or Product, (b) any breach by Idenix of its representations,
warranties or covenants made under this Agreement, or (c) any negligent act or omission or willful
misconduct of Idenix or its Affiliates, or any of their employees, contractors or agents, in
performing Idenix’s obligations or exercising Idenix’s rights under this Agreement;
provided, however, that the foregoing indemnity shall not apply to the extent that
any such Losses are attributable to any of the causes described in clauses (a), (b), or (c) of
Section 8.1. In the event of any such claim against any XXX Xxxxxxxxxx, XXX shall promptly notify
Idenix in writing of the claim and Idenix shall manage and control, at its sole expense, the
defense of the claim and its settlement. The relevant GSK Indemnitees shall cooperate with Idenix
and may, at such GSK Indemnitees’ option and expense, be represented in any such action or
proceeding. Idenix shall not be liable for any settlements, litigation costs or expenses incurred
by any GSK Indemnitees without Idenix’s written authorization.
8.3 Allocation. In the event a claim is based partially on an indemnified claim and
partially on a non-indemnified claim or based partially on a claim indemnified by one Party and
partially on a claim indemnified by the other Party, any payments in connection with such claims
are to be apportioned between the Parties in accordance with the degree of cause attributable
to each Party.
35
9. INSURANCE
GSK shall obtain and maintain an insurance policy that includes coverage for general liability and
products liability claims in an amount and coverage reasonably agreed upon by the Parties, but in
no even less than amounts and coverage typical for similarly situated global pharmaceutical
companies. Idenix shall have the right to request and to receive copies of the appropriate
certificates of insurance for the purpose of confirming the sufficiency and currency of such
coverage. GSK may satisfy its obligations under this Section 9 through self-insurance to the same
extent. The foregoing coverage shall continue during the Term and for a period of six (6) years
thereafter.
10. WARRANTIES AND COVENANTS
10.1 Mutual Warranties. As of the Effective Date, each Party warrants that:
(a) it is a corporation duly organized and in good standing under the Laws of the jurisdiction
of its incorporation, and it has full power and authority and the legal right to own and operate
its property and assets and to carry on its business as it is now being conducted and as it is
contemplated to be conducted by this Agreement;
(b) it has the full right, power and authority to enter into this Agreement and to grant the
rights and licenses granted by it under this Agreement (subject to Idenix providing the Novartis
Waiver and the University Amendment);
(c) there are no existing or, to its knowledge, threatened actions, suits or claims pending
with respect to the subject matter of this Agreement or its right to enter into and perform its
obligations under this Agreement;
(d) as of the Execution Date, it has taken all necessary action on its part to authorize the
execution and delivery of this Agreement and the performance of its obligations under this
Agreement (subject to obtaining all necessary governmental approvals with respect to the HSR Act);
(e) this Agreement has been duly executed and delivered on behalf of it, and constitutes a
legal, valid, binding obligation, enforceable against it in accordance with the terms hereof,
subject to the general principles of equity and to bankruptcy, insolvency, moratorium and other
similar Laws affecting the enforcement of creditors’ rights generally; and
(f) the execution and delivery of this Agreement and the performance of its obligations
hereunder do not conflict with, or constitute a default under, any of its contractual obligations.
36
10.2 Additional Idenix Warranties. Idenix hereby represents and warrants to GSK that
the statements contained in this Section 10.2 will be complete and accurate as of the Effective
Date.
(a) Exhibit A attached hereto contains a true and complete list of the existing Idenix
Patent Rights.
(b) The Patent Rights listed in Exhibit A include all of the Patent Rights owned by or
licensed to Idenix or its Affiliates that claim the Licensed Compounds or the manufacture, use,
sale, offer for sale or import thereof, and, except with respect to the rights of Novartis and
University, Idenix has the authority to direct the prosecution and maintenance of such Patent
Rights.
(c) To Idenix’s Knowledge, the issued claims included in the Idenix Patent Rights are valid
and enforceable.
(d) True, complete, and correct copies of the file wrappers and other documents and materials
that relate to the prosecution, defense, maintenance, validity and enforceability of the Idenix
Patent Rights filed in the United States and the European Union and all licenses and other
agreements regarding the ownership and licensing of Idenix Patent Rights have been made available
to GSK and to Idenix’s Knowledge the Idenix Patents have been diligently prosecuted in accordance
with all applicable Laws, maintained properly and correctly, and all applicable fees have been paid
to file and maintain the Idenix Patent Rights;
(e) Except as disclosed on Schedule 10.2, subsection (e), Idenix is the sole owner of all
right, title and interest in and to the Idenix IP, free and clear of all encumbrances, security
interests, options and licenses;
(f) (i) Except as disclosed on Schedule 10.2, subsection (f)(i), Idenix has not granted to any
Third Party or Affiliate any license or other right with respect to the Idenix IP. (ii) Except as
disclosed on Schedule 10.2, subsection (f)(ii), the Novartis Waiver completely and finally waives
any rights that Novartis may have to the Licensed Compounds or Products. (iii) During the Term,
Idenix shall not grant any rights inconsistent with the rights and licenses granted herein;
(g) Neither Idenix nor any of its Affiliates has received a written complaint from any Third
Party claiming that the development, manufacture, use, sale, offer for sale or import of any
Licensed Compound, or any other activity by Idenix or its Affiliates related to any Licensed
Compound, infringes or would infringe the patent or other Intellectual Property Rights of any Third
Party;
(h) Neither Idenix nor any of its Affiliates is a party to any legal action, suit or
proceeding relating to the Idenix IP, nor, to Idenix’s Knowledge, has Idenix or any of its
Affiliates received any written communication from any Third Party threatening such action, suit or
proceeding. During the Term, Idenix promptly shall notify GSK in writing upon learning of any such
threatened action, suit or proceeding;
37
(i) Except as disclosed on Schedule 10.2, subsection (i), to Idenix’s Knowledge, Idenix has
obtained the assignment of, or obligation to assign, all interests and all rights of any and all
Third Parties (including employees) and Affiliates involved in the creation of the existing Idenix
Know-How, and Idenix has taken reasonable measures to protect the confidentiality of the Idenix
Know-How;
(j) To Idenix’s Knowledge, there is no Third Party use, infringement or misappropriation of
the Idenix IP in derogation of the rights to be granted to GSK in this Agreement;
(k) To Idenix’s Knowledge, Idenix has disclosed to GSK prior to the Execution Date all Third
Party Patent Rights Known to Idenix that Idenix reasonably believes without having conducted any
special inquiry would be infringed by the Development, in accordance with the Development Plan and
in accordance in the formulation contemplated by Idenix as of the Execution Date, of a Product
containing IDX899 as the sole active ingredient;
(l) Except for consideration by the FDA or any equivalent Regulatory Authority outside the
United States of America of regulatory materials submitted by Idenix with respect to Licensed
Compounds, to Idenix’s Knowledge there are no investigations, inquiries, actions or other
proceedings pending before the FDA or any such Regulatory Authority with respect to any Licensed
Compounds, and, to Idenix’s Knowledge, Idenix has not received written notice threatening any such
investigation, inquiry, action or other proceeding. During the Term, Idenix promptly shall notify
GSK in writing upon learning of any such actual or threatened investigation, inquiry, action or
proceeding;
(m) The development, testing, manufacture, labeling, storage, and distribution of the Licensed
Compounds have been conducted by Idenix and its Affiliates and, its Third Party contractors, in
compliance in all material respects with all applicable Laws, including, to the extent applicable,
Laws with respect to investigational use, good clinical practices, good laboratory practices,
cGMPs, record keeping, security and filing of reports; and neither Idenix nor its Affiliates nor,
to Idenix’s Knowledge, its Third Party contractors have received any notice in writing which have
led Idenix to believe that any of the regulatory submissions relating to the Licensed Compounds are
not currently in good standing with the FDA;
(n) Idenix has disclosed in writing to GSK all licenses granted to Idenix by Third Parties
with respect to the Idenix IP and, to Idenix’s Knowledge, Idenix is not in breach or default under
any such agreement and has not received from any licensor any notice of breach or default; and
(o) Idenix has not intentionally withheld from GSK any material information in Idenix’s
possession requested by GSK in connection with GSK’s due diligence related to the pre-clinical and
clinical studies of the Licensed Compounds conducted by or on behalf of Idenix, and, to Idenix’s
Knowledge, all such information provided to GSK was up-to-date and accurate in all material
respects when provided to GSK.
38
10.3 Idenix Covenants. Idenix hereby covenants and agrees for the term of this
Agreement that: (i) it shall fully and faithfully perform its obligations and responsibilities
under the University Agreements and the Xx Xxxxx Agreement; (ii) it shall not knowingly take any
action or knowingly omit to take any action that could constitute a material breach of its
obligations under the University Agreements and the Xx Xxxxx Agreement; and (iii) it shall promptly
notify GSK if it receives notice that it is in breach of the University Agreements or the Xx Xxxxx
Agreement. Notwithstanding the foregoing, in the event that Idenix receives notice from the
University or Xxxxxxxxx Xx Xxxxx or any successor in interest to the University or Xxxxxxxxx Xx
Xxxxx that Idenix has committed a breach of its obligations under the University Agreements or the
Xx Xxxxx Agreement, or if Idenix anticipates such breach, such as may give rise to a right by the
University or Xxxxxxxxx Xx Xxxxx to terminate or otherwise diminish Idenix’s rights to the Idenix
Patent Rights or Idenix Know-How and/or otherwise diminish Idenix’s ability to perform its
obligations to GSK under this Agreement, Idenix shall immediately notify GSK of such situation, and
Idenix shall promptly cure such breach. However, if Idenix is unable to cure such breach, Idenix
shall permit GSK to cure such breach and to negotiate directly with the University or Xxxxxxxxx Xx
Xxxxx. Idenix covenants and agrees that it will not, without the prior written consent of GSK, not
to be unreasonably withheld, amend or alter the terms of the University Agreements, the University
Amendment, the Xx Xxxxx Agreement or the Novartis Waiver to the extent such amendment or alteration
would have a material adverse effect on GSK’s rights to the Licensed Compounds as granted to GSK by
Idenix. Idenix also hereby covenants and agrees that, during the Term, it will promptly notify GSK
regarding any information it becomes aware of from any source other than GSK with respect to side
effects, toxicity, adverse events or any instances of deleterious physical effects or reactions
alleged to result from the use of a Licensed Compound or Product.
10.4 Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS SECTION 10, NEITHER
PARTY MAKES ANY REPRESENTATIONS OR EXTENDS ANY WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED,
INCLUDING WARRANTIES OF MERCHANTABILITY, QUALITY, FITNESS FOR A PARTICULAR PURPOSE,
NONINFRINGEMENT, OR VALIDITY OF PATENT CLAIMS.
11. LIMITATION OF LIABILITY
UNLESS RESULTING FROM A PARTY’S WILLFUL MISCONDUCT OR FROM A PARTY’S BREACH OF SECTION 7, NEITHER
PARTY WILL BE LIABLE TO THE OTHER PARTY OR ITS AFFILIATES FOR SPECIAL, INCIDENTAL, CONSEQUENTIAL,
EXEMPLARY, PUNITIVE, MULTIPLE OR OTHER INDIRECT DAMAGES, OR FOR LOSS OF PROFITS, LOSS OF DATA, LOSS
OF REVENUE, OR LOSS OF USE DAMAGES, ARISING FROM OR RELATING TO THIS AGREEMENT, WHETHER BASED XXXX
XXXXXXXX, XXXXXXXX, XXXX, XXXXXXXXXX, XXXXXX LIABILITY OR OTHERWISE, REGARDLESS OF ANY NOTICE OF
SUCH DAMAGES. NOTHING IN THIS SECTION 11 IS INTENDED TO LIMIT OR RESTRICT THE INDEMNIFICATION
RIGHTS OR OBLIGATIONS OF EITHER PARTY UNDER THIS AGREEMENT.
39
12. TERMINATION
12.1 Term. This Agreement becomes effective as of the Effective Date and shall
continue in perpetuity until the earlier of (a) the termination of this Agreement in accordance
with Sections 12.2 or 14.3 or (b) the expiration of the last-to-expire of all Royalty Terms with
respect to all Products (the “Term”); provided, however, that Sections
12.3(e), 13 and 14.1 shall be effective as of the Execution Date.
12.2 Termination.
(a) Termination For Convenience. GSK may terminate this Agreement for convenience
upon ninety (90) days prior written notice to Idenix.
(b) Termination For Material Breach. If either Party (the “Non-Breaching
Party”) believes that the other Party (the “Breaching Party”) is in material breach of
this Agreement (including any material breach of a representation or warranty made in this
Agreement), then the Non-Breaching Party may deliver notice of such breach to the Breaching Party.
If the Breaching Party fails to cure such breach within the sixty (60) day period after the
Breaching Party’s receipt of such notice, the Non-Breaching Party may terminate this Agreement in
its entirety upon written notice to the Breaching Party.
(c) Termination for Bankruptcy. To the extent permitted under applicable Law, either
Party may terminate this Agreement effective immediately with written notice if the other Party
files for bankruptcy, is adjudicated bankrupt, files a petition under insolvency Laws, is dissolved
or has a receiver appointed for substantially all of its property.
(d) Termination for Patent Challenge. Except to the extent the following is
unenforceable under the applicable Laws of a particular jurisdiction where a patent application
within the Idenix Patent Rights is pending or a patent within the Idenix Patent Rights is issued,
Idenix may terminate this Agreement immediately upon written notice to GSK in the event that GSK or
any of its Affiliates or Sublicensees Challenges any Idenix Patent Rights or assists a Third Party
in initiating a Challenge of any Idenix Patent Right. “Challenge” means any challenge to
the validity or enforceability of any of the Idenix Patent Rights, including (except as provided
below) by (i) filing a declaratory judgment action in which any of the Idenix Patent Rights is
alleged to be invalid or unenforceable; (ii) citing prior art pursuant to 35 U.S.C. §301, filing a
request for re-examination of any of the Idenix Patent Rights pursuant to 35 U.S.C. §302 and/or
§311, or provoking or becoming a party to an interference with an application for any of the Idenix
Patent Rights pursuant to 35 U.S.C. §135; or (iii) filing or commencing any re-examination,
opposition, cancellation, nullity or similar proceedings against any of the Idenix Patent Rights in
any country; provided, that “Challenge” shall not include filing requests for
re-examination of Idenix Patent Rights or re-issue of Idenix Patent Rights to the extent that
Idenix agrees that such actions are in the best interest of the applicable Idenix Patent Rights.
12.3 Effects Of Termination.
(a) Upon termination of this Agreement:
40
(i) all licenses granted by Idenix to GSK hereunder shall terminate and all sublicenses
granted by GSK to its Affiliates and Sublicensees shall terminate;
(ii) GSK shall provide to Idenix a fair and accurate description of the status of the
Development and commercialization of the Products in the Territory through the effective date of
termination;
(iii) GSK hereby grants to Idenix, an exclusive, perpetual, irrevocable, royalty-free,
fully-paid license, with the right to grant sublicenses, under the Know-How and Patent Rights
Controlled by GSK or its Affiliates anywhere in the world, to the extent related to a Licensed
Compound or a Product in the Field (including GSK Inventions but excluding Joint Inventions and
Joint IP) (“GSK IP”) and GSK’s rights in any Joint Invention and Joint IP to Exploit the
Products in the Field in the Territory; provided, however, that Idenix covenants
not to exercise such license unless and until this Agreement is terminated pursuant to Section 12.2
or 14.3 rather than expires pursuant to Section 12.1;
(iv) GSK shall, within [**] after providing its notice of termination, transfer and assign to
Idenix (A) all Regulatory Documentation and other documented technical and other information or
materials necessary, and (B) all material Regulatory Documentation and other material documented
technical and other information or materials useful, in each case for the Exploitation of the
Products in the Territory, including reports, records and other materials in GSK’s or its
Affiliates’ or Sublicensees’ possession or control relating to process conditions, in-process
controls, analytical methodology and formulation relating to the manufacture of Products;
provided, that GSK may retain copies of such items for its records. Within [**] after
Idenix’s receipt of a detailed invoice therefor, Idenix shall reimburse GSK for GSK’s and its
Affiliates’ reasonable out-of-pocket expenses incurred solely in connection with implementing such
transfers and assignment. Within a reasonable period of time after the termination date, GSK and
Idenix shall agree to a transition plan, including costs and timelines, to (a) make available to
Idenix, in a mutually-agreed upon format, material information regarding the GSK Know-How, and (b)
make GSK and or its Affiliates relevant scientific and technical personnel available to Idenix to
answer any questions or provide instruction as reasonably requested by Idenix concerning the
information delivered pursuant to this Section 12.3(a)(iv);
(v) GSK shall, and shall ensure that its Affiliates and Sublicensees, promptly assign to
Idenix all Product Trademarks referred to in public with respect to the Product in the Territory,
including all names for the Product;
(vi) GSK shall keep Idenix reasonably apprised of the status of the filing, prosecution and
maintenance of the GSK IP and Joint IP throughout the Territory;
(vii) GSK shall execute any and all documents as reasonably requested by Idenix, including but
not limited to, assignments, declarations and powers of attorney, to permit Idenix the sole right
to file, prosecute and maintain the Idenix Patent Rights and Joint Patent Rights in the Territory;
41
(viii) the provisions of Sections 6.2, 6.3, 6.4, 6.5 and 6.6 shall apply to GSK, to Idenix and
to Joint IP, mutatis mutandis to the same extent as they apply to Idenix, to GSK and to Idenix
Patent Rights, respectively;
(ix) if requested by Idenix, at GSK’s discretion, GSK will either provide a sufficient
quantity of Product consistent with GSK’s anticipated twelve-month forecast, or manufacture and
supply Idenix’s requirements for the Product for the Territory in the Field from the date of such
termination until [**] after the effective date of termination. Idenix shall use diligent efforts
to secure a fully-functioning supply chain for the Product as promptly as reasonably practicable
following the date of termination. In addition, to the extent requested by Idenix, GSK shall
permit Idenix to purchase all or any part of GSK’s worldwide unsold inventory of raw materials for
Products, work-in-progress Products and finished Products. All Product supplied to Idenix by GSK
(EXW INCOTERMS 2000 GSK’s facilities) pursuant to this Section 12.3(a)(ix) shall be manufactured in
compliance with cGMP and shall be sold by GSK, and purchased by Idenix, at a price equal to [**];
(x) to the extent requested by Idenix and permitted by the terms of the relevant agreement,
GSK shall, and shall ensure that its Affiliates and Sublicensees, use Commercially Reasonable
Efforts to assign (to the extent assignable) to Idenix or its designated Affiliate any Third Party
manufacturing contracts relating to the Products or any component (including any active
pharmaceutical ingredient) thereof to which GSK or any of its Affiliates or Sublicensees is a party
(or the applicable provisions thereof, as the case may be). Idenix shall bear such amounts owed
to such Third Parties reasonably necessary to effect such assignment with respect to any agreement
for which GSK had complied with its obligations under Section 4.3(c). GSK and its Affiliates and
Sublicensees shall remain solely responsible for all costs and expenses owed to such Persons and
accruing prior to the effective date of such assignment, and all other obligations owed to such
Persons prior to the effective date of such assignment; and
(xi) If at the time of termination of this Agreement by GSK pursuant to Sections 12.2(b) or
12.2(c) there has been a First Commercial Sale of a Product in the Territory, then Idenix will pay
GSK a flat royalty equal to [**] percent ([**]%) of Net Sales of all Products in the Territory for
the remainder of the Royalty Term as provided herein, with the applicable definitions and the
provisions of Sections 5.3, 5.4, 5.5, 5.6, 5.7, 5.8, 5.9, 5.10 and 5.12 applying, mutatis mutandis,
to such royalty payments.
(b) Within [**] following the expiration or termination of this Agreement, each Party shall
(i) deliver to the other Party, or certify the destruction of, any and all tangible Confidential
Information of the other Party in its possession, (ii) to the extent practicable, remove
Confidential Information of the other Party from all databases and systems and in those instances
where removal is not practicable, segregate or otherwise indicate that such Confidential
Information is restricted, and (iii) treat all Confidential Information contained in lab notebooks
in accordance with the Party’s then current procedure for the status of the project and properly
note that such Confidential Information contained in such lab notebooks is restricted;
provided, however, that Idenix may retain GSK’s Confidential Information to the
extent necessary to exercise the rights and licenses granted to Idenix pursuant to Section 12.3(a)
expressly surviving expiration or termination of this Agreement.
42
(c) The following provisions shall survive the expiration or termination of this Agreement:
Sections 5.5, 5.7, 5.8, 5.9, 5.10, 7, 8, 9, 10.4, 11, 12.3 and 14 (except 14.1).
(d) Termination of this Agreement shall be in addition to, and shall not prejudice, the
Parties’ remedies at law or in equity, including the Parties’ ability to receive legal damages
and/or equitable relief with respect to any breach of this Agreement, regardless of whether or not
such breach was the reason for the termination.
(e) The MTA shall automatically terminate upon the termination of this Agreement or as
provided in the MTA; provided, however, that Sections 2.3, 3.1, 3.2, 3.3, 4.1, 4.2
and 6.2 of the MTA shall survive termination of the MTA.
13. HSR
13.1 HSR Filing. Promptly after the Execution Date, and in no event later than seven
(7) Business Days after the Execution Date, both Parties shall promptly file a copy of this
Agreement and their respective premerger notification and report forms with the FTC and the DOJ
pursuant to the HSR Act.
13.2 Cooperation.
(a) The Parties shall use Commercially Reasonable Efforts to promptly obtain clearance
required under the HSR Act for the consummation of this Agreement and the Stock Purchase Agreement
and the transactions contemplated hereby and thereby and shall keep each other apprised of the
status of any communications with, and any inquiries or requests for additional information from,
the FTC and the DOJ and shall comply promptly with any such inquiry or request; provided,
however, that neither Party shall be required to consent to the divestiture or other
disposition of any of its assets or assets of its Affiliates (or, with respect to Idenix, assets of
Novartis Pharma AG and its Affiliates) or to consent to any other structural or conduct remedy, and
each Party and its Affiliates (or, with respect to Idenix, assets of Novartis Pharma AG and its
Affiliates) shall have no obligation to contest, administratively or in court, any ruling, order or
other action of the FTC or DOJ or any Third Party respecting the transactions contemplated by this
Agreement.
(b) The Parties commit to instruct their respective counsel to cooperate with each other and
use Commercially Reasonable Efforts to facilitate and expedite the identification and resolution of
any such issues and, consequently, the expiration of the applicable HSR Act waiting period. Each
Party’s counsel will undertake (i) to keep each other appropriately informed of communications from
and to personnel of the reviewing antitrust authority, and (ii) to confer with each other regarding
appropriate contacts with and response to personnel of the FTC or DOJ. GSK shall be responsible
for the filing fee in connection with the HSR Act filing relating to the transactions contemplated
in this Agreement.
13.3 Termination Due to Passage of Time. Notwithstanding any other provisions of this
Agreement to the contrary, either Party may terminate this Agreement effective upon notice to the
other Party if the HSR Clearance Date shall not have occurred on or prior to the date that is
ninety (90) days after the Parties make their respective HSR Filings pursuant to Section 13.1. If
43
this Agreement is so terminated pursuant to this Section 13.3, then this Agreement shall
terminate in its entirety; provided, however, that, (i) the Prior Confidentiality
Agreements shall remain in full force and effect notwithstanding any termination of this Agreement
pursuant to this Section 13.3 and (ii) Section 12.3(e) shall apply.
14. MISCELLANEOUS
14.1 Effectiveness of the Agreement. This Agreement and the Stock Purchase Agreement
will become effective on the second Business Day immediately following the HSR Clearance Date.
14.2 Assignment. Neither this Agreement nor any of the rights or obligations
hereunder may be assigned by a Party without the prior written consent of the other Party, except
(a) each Party may assign this Agreement, in whole or in part, to an Affiliate of the assigning
Party; provided, that the assigning Party guarantees the performance of such Affiliate of
its obligations hereunder; and (b) each Party may assign this Agreement, in whole, to a Person that
acquires, by merger, sale of assets or otherwise, all or substantially all of the business of the
assigning Party to which the subject matter of this Agreement relates. Any assignment not in
accordance with the foregoing shall be void. This Agreement shall be binding upon, and shall inure
to the benefit of, all permitted successors and assigns. No provision of this Agreement is
intended to or will be deemed to confer upon Third Parties any right, benefit, remedy, claim,
liability, reimbursement, claim of action or other right of any nature whatsoever under or by
reason of this Agreement other than the Parties and, to the extent provided in Sections 8.1 and
8.2, the Idenix Indemnitees and GSK Indemnitees. Each Party agrees that, notwithstanding any
provisions of this Agreement to the contrary, in the event that this Agreement is assigned by
either Party in connection with the sale or transfer of all or substantially all of the business of
such Party or in connection with a merger, consolidation or similar transaction, the non-assigning
Party shall not be provided with rights or access to Know-How or intellectual property rights of
such assignee or the acquirer of such Party.
14.3 Force Majeure. Neither Party will be held liable or responsible to the other
Party nor be deemed to have breached this Agreement for failure or delay in fulfilling or
performing any provision of this Agreement when such failure or delay results from causes beyond
the reasonable control of the affected Party, which may include embargoes, acts of war (whether
declared or not), insurrections, riots, civil commotions, acts of terrorism, strikes, lockouts or
other labor disturbances, or acts of God. The affected Party will notify the other Party of such
force majeure circumstances as soon as reasonably practical and will make every reasonable effort
to mitigate the effects of such force majeure circumstances. If a Party is so delayed and such
failure or omission is not cured within ninety (90) days, the other Party may terminate this
Agreement.
14.4 Section 365(n) of the Bankruptcy Code. All rights and licenses granted under or
pursuant to any section of this Agreement are and shall otherwise be deemed to be, for purposes of
Section 365(n) of the United States Bankruptcy Code (Title 11, U.S.C.), as amended (the
“Bankruptcy Code”), licenses of rights to “intellectual property” as defined in Section
101(35A)
of the Bankruptcy Code. The Parties shall retain and may fully exercise all of their
respective rights and elections under the Bankruptcy Code.
44
14.5 Notices. Any notice required or provided for by the terms of this Agreement
shall be in writing, in the English language, and shall be (a) sent by certified or registered
mail, return receipt requested, postage prepaid, (b) sent via a reputable overnight international
courier service, (c) sent by facsimile transmission, or (d) delivered by hand. The effective date
of the notice shall be the actual date of receipt by the receiving Party.
Notices to GSK shall be addressed to:
GlaxoSmithKline
000 Xxxxxxxxx Xxxx
X.X. Xxx 0000
Xxxx xx Xxxxxxx, XX 00000-0000
Attention: Senior Vice President of Worldwide Business Development
Telephone: (610) 000- 0000
Facsimile: (000) 000-0000
000 Xxxxxxxxx Xxxx
X.X. Xxx 0000
Xxxx xx Xxxxxxx, XX 00000-0000
Attention: Senior Vice President of Worldwide Business Development
Telephone: (610) 000- 0000
Facsimile: (000) 000-0000
With copies to:
GlaxoSmithKline
0000 Xxxxxxxxxxx Xxxx.
Xxxx xx Xxxxxxx, XX 00000-0000
Attention: Vice President, R&D Legal Operations Business Development Transactions
Telephone: (000) 000-0000
Facsimile: (000) 000-0000
0000 Xxxxxxxxxxx Xxxx.
Xxxx xx Xxxxxxx, XX 00000-0000
Attention: Vice President, R&D Legal Operations Business Development Transactions
Telephone: (000) 000-0000
Facsimile: (000) 000-0000
Notices to Idenix shall be addressed to:
Idenix Pharmaceuticals Inc.
00 Xxxxxxxxx Xxxxxx
Xxxxxxxxx, XX 00000, XXX
Attention: Chief Executive Officer
Telephone: (000) 000-0000
Facsmilie: (000) 000-0000
00 Xxxxxxxxx Xxxxxx
Xxxxxxxxx, XX 00000, XXX
Attention: Chief Executive Officer
Telephone: (000) 000-0000
Facsmilie: (000) 000-0000
With a copy to:
Idenix Pharmaceuticals Inc.
00 Xxxxxxxxx Xxxxxx
Xxxxxxxxx, XX 00000, XXX
Attention: General Counsel
Telephone: (000) 000-0000
Facsmilie: (000) 000-0000
00 Xxxxxxxxx Xxxxxx
Xxxxxxxxx, XX 00000, XXX
Attention: General Counsel
Telephone: (000) 000-0000
Facsmilie: (000) 000-0000
45
Any Party may change its address by giving notice to the other Party in the manner provided in this
Section 14.5.
14.6 Relationship of the Parties. The Parties shall be deemed independent contractors
for all purposes hereunder. This Agreement does not constitute a partnership, joint venture or
agency between the Parties. Neither Party is an agent of the other Party and neither Party has any
authority to represent the other Party as to any matters.
14.7 Governing Law. This Agreement shall be governed by and construed in accordance
with the Laws of the State of Delaware, other than any principle of conflict or choice of laws that
would cause the application of the Laws of any other jurisdiction. The provisions of the United
Nations Convention on Contracts for the International Sale of Goods, the 1974 Convention on the
Limitation Period in the International Sale of Goods (the “1974 Convention”), and the
Protocol amending the 1974 Convention, done at Vienna April 11, 1980, shall not apply to this
Agreement or any subject matter hereof.
14.8 Dispute Resolution. Any dispute, controversy or claim arising out of or relating
to this Agreement, or the breach, termination or invalidity of this Agreement shall be resolved as
follows:
(a) To the extent that either Party is expressly given final decision-making authority under
this Agreement, such Party may exercise such right in accordance with the relevant provision and
such decision is not subject to the dispute resolution procedures described below;
provided, however, that, notwithstanding anything to the contrary herein, (i) no
decision so made shall obligate the other Party to spend money or devote resources outside those
expressly set forth in this Agreement, (ii) in no event may the deciding Party unilaterally amend
the terms of this Agreement; (iii) in no event may the deciding Party unilaterally determine that
it has fulfilled any obligations hereunder; and (iv) the deciding Party shall make its decision in
good faith.
(b) With respect to any dispute not finally determined by a Party in accordance with Section
14.8(a):
(i) each Party may request in writing a meeting of the Executive Officers of both Parties and
the Executive Officers of both Parties shall meet to attempt to resolve such dispute.
“Executive Officer” means, with respect to a Party, the Chief Executive Officer of such
Party (or the officer or employee of such Party then serving in a substantially equivalent
capacity) or his/her designee who reports directly to such Chief Executive Officer.
(ii) If the Executive Officers cannot resolve such disputes within [**] after either Party
requests such a meeting in writing, then upon written notice by either Party to the other Party,
such dispute, controversy or claim shall be finally resolved by binding arbitration conducted in
the English language in New York, New York under the Commercial Arbitration Rules of the American
Arbitration Association by three (3) arbitrators appointed in accordance with such rules. The
arbitrator(s) shall have the ability to grant injunctive or other equitable relief.
46
(c) Notwithstanding anything contained in this Section 14.8 to the contrary, each Party shall
have the right to institute judicial proceedings against the other Party or anyone acting by,
through or under such other Party, in order to enforce the instituting Party’s rights hereunder
through reformation of contract, specific performance, injunction or similar equitable relief.
14.9 Injunctive Relief. Each Party acknowledges and agrees that there can be no
adequate remedy at law for any breach of its obligations under Section 7 and that any such breach
may result in irreparable harm to the other Party, and therefore, that upon any such breach or any
threat thereof, such other Party shall be entitled to appropriate equitable relief in addition to
whatever remedies it might have at law, without the necessity of showing actual damages.
14.10 Severability. If, under applicable Law, any provision of this Agreement is
invalid or unenforceable, or otherwise directly or indirectly affects the validity of any other
material provision(s) of this Agreement (“Severed Clause”), the Parties mutually agree that
this Agreement shall endure except for the Severed Clause. The Parties shall consult and use their
best efforts to agree upon a valid and enforceable provision which shall be a reasonable substitute
for such Severed Clause in light of the intent of this Agreement.
14.11 Entire Agreement. This Agreement constitutes the entire agreement among the
Parties with respect to the subject matter hereof and supersedes all previous agreements (including
the Prior Confidentiality Agreements), whether written or oral, with respect to such subject
matter; provided, however, that (a) the MTA (as amended by Section 12.3(e)) shall
survive the execution and effectiveness of this Agreement and provided, further,
that, upon the Effective Date, Section 3.4 of the MTA shall terminate and any proprietary
information provided under the MTA shall be considered Confidential Information hereunder; (b)
Section 4(c) of the February 2008 CDA shall survive the execution and effectiveness of this
Agreement; and (c) Sections 2(d), 6 and 7 of the June 2008 CDA (provided that nothing in Section 7
of the June 2008 CDA shall limit GSK’s rights under the Stock Purchase Agreement) shall survive the
execution and effectiveness of this Agreement.
14.12 Amendment and Waiver. This Agreement may not be amended, nor any rights
hereunder waived, except in a written agreement that explicitly refers hereto that is signed by the
properly authorized representatives of each Party.
14.13 No Implied Waivers. The waiver by a Party of a breach of any provision of this
Agreement by the other Party shall not be construed as a waiver of any succeeding breach of the
same or any other provision, nor shall any delay or omission on the part of a Party to exercise or
avail itself of any right that it has or may have hereunder operate as a waiver of any right by
such Party.
14.14 Export Compliance. The Parties acknowledge that the exportation from the United
States of materials, products and related technical data (and the re-export from elsewhere of
United States origin items) may be subject to compliance with United States export Laws, including
Laws which restrict export, re-export and release of materials, products and their related
technical data, and the direct products of such technical data. The Parties agree to
47
comply with all export Laws and to commit no act that, directly or indirectly, would violate
any United States Law, or any other international treaty or agreement, relating to the export,
re-export, or release of any materials, products or their related technical data to which the
United States adheres or with which the United States complies.
14.15 Counterparts and Facsimile Signatures. This Agreement may be executed in
counterparts, each of which counterparts, when so executed and delivered, shall be deemed to be an
original, and all of which counterparts, taken together, shall constitute one and the same
instrument even if both Parties have not executed the same counterpart. Signatures provided by
facsimile transmission or in Adobe™ Portable Document Format (PDF) sent by electronic mail shall be
deemed to be original signatures.
14.16 Performance by Affiliates. Any obligation of a Party under or pursuant to this
Agreement may be satisfied, met or fulfilled either by such Party directly or by its Affiliate or
Sublicensees. With respect to any particular action, the clause “a Party shall”, “Idenix shall” or
“GSK shall” or similar clauses, also means the relevant Party “shall cause” the particular action
to be performed by its Affiliates or Sublicensees, as applicable. Each of the Parties guarantees
the performance of all actions, agreements and obligations to be performed by any of its Affiliates
or Sublicensees under the terms and conditions of this Agreement.
[Remainder of Page Intentionally Left Blank]
48
IN WITNESS WHEREOF, the Parties executed this Agreement as of the date first above written.
| Idenix PHARMACEUTICALS, Inc. |
||||
| By: | /s/ Xxxx-Xxxxxx Sommadossi, Ph.D. | |||
| Name: | Xxxx-Xxxxxx Sommadossi, Ph.D. | |||
| Title: | Chairman and CEO | |||
| SMITHKLINE XXXXXXX Corporation d/b/A Glaxosmithkline |
||||
| By: | /s/ Xxxxxxx X. Xxxxxx | |||
| Name: | Xxxxxxx X. Xxxxxx | |||
| Title: | Vice President and Secretary | |||
Signature Page
EXHIBIT A
IDENIX PATENT RIGHTS
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 4 pages were omitted pursuant to a request for confidential treatment. [**]
EXHIBIT B
HIV WAIVER AND CONSENT
This HIV Waiver and Consent to the Development License and Commercialization Agreement is made
and effective as of the 23 January 2009 (“Effective Date”) between Idenix Pharmaceuticals, Inc.,
with offices at 00 Xxxxxxxxx Xxxxxx, Xxxxxxxxx Xxxxxxxxxxxxx 00000, XXX (“Idenix US”),
Idenix (Cayman) Limited with offices at c/o Walkers SPV Limited, Xxxxxx House, Xxxx Street, Xxxxxx
Town, Grand Cayman, Cayman Islands (“Idenix Cayman” and together with Idenix US,
“Idenix”) and Novartis Pharma AG with offices at Forum 0, Xxxxxxxx Xxxxxx, 0000 Xxxxx,
Xxxxxxxxxxx (“Novartis”).
INTRODUCTION
A. Novartis and Idenix are parties to the Development, License and Commercialization Agreement
made as of 8 May 2003, as amended by Amendment No. 1 dated as of 30 April 2004, Amendment No. 2
dated as of 21 December 2004, Amendment No. 3 dated as of 27 February 2006 and Amendment No. 4
dated as of 28 September 2007 (as so amended, the “Novartis Licence Agreement”).
B. Under Section 3.3(a)(i)(A) of the Novartis Licence Agreement, Novartis has an ODC Option
over Other Drug Candidates, and under Section 3.3(a)(ii), Idenix has an obligation to provide to
Novartis a Completion Notice upon the occurrence of certain events (as more fully described in that
agreement).
C. In or around November 2007, March 2008 and September 2008, Idenix showed to Novartis
confidential data on IDX899 and IDX989 (as defined below), but at Novartis’ request has not
provided to Novartis a Completion Notice.
D. Idenix has advised Novartis of a possible Transaction (as defined below).
E. Solely in connection with the proposed Transaction and in accordance with the terms of this
HIV Waiver and Consent, Novartis has agreed to waive certain rights and grant certain consents
notwithstanding the terms of the Novartis Licence Agreement.
IN CONSIDERATION of the mutual covenants contained in this HIV Waiver and Consent,
Idenix and Novartis agree:
1. Definitions. Unless otherwise defined or amended by the terms of this HIV Waiver
and Consent, all initial capitalized defined terms used have the meanings as defined in the
Novartis Licence Agreement. In addition, for the purpose of this HIV Waiver and Consent, the
Parties agree to the following additional or amended definitions:
“[**] Patent Rights” means (a) the following Patent Rights listed in Schedule 1: [**],
and (b) any patents that issue from the above and all continuations, continuations in part,
divisions, reexaminations, renewals, registrations reissues, supplementary protection certificates
and extensions of any of the above Patent Rights, both foreign and domestic, that are owned or
controlled by Idenix during the Term.
“Affiliate” means, (a) with respect to Idenix, any Entity that is controlled by
Idenix, and (b) with respect to Novartis, any Entity that controls Novartis, or, except for Idenix
and Idenix Affiliates, is controlled by Novartis or is under common control with Novartis. For
purposes of this definition, “control” means (a) in the case of corporate entities, direct
or indirect ownership of at least fifty percent (50%) of the stock or shares having the right to
vote for the election of directors; (b) in the case of non-corporate entities, direct or indirect
ownership of at least fifty percent (50%) of the equity interest with the power to direct the
management and policies of such non-corporate entities; and (c) for Novartis, additionally to the
above, Affiliate includes The Novartis Research Foundation, Switzerland, The Genomics Institute of
the Novartis Research Foundation, USA, and The Xxxxxxxxx Xxxxxxxx Institute for Biomedical
Research, Switzerland. The Parties acknowledge that in the case of certain entities organized under
the laws of certain countries outside the United States, the maximum percentage ownership permitted
by law for a foreign investor may be less than fifty percent (50%), and that in such case such
lower percentage shall be substituted in the preceding sentence; provided that such foreign
investor has the power to direct the management and policies of such entity.
“Co-Labeling Right” means the labeling of any Novartis or Idenix HBV or HCV product
not containing a Waived Compound as an active ingredient(s), stating that the product can be used
or administered with a product containing a Waived Compound, to treat patients coinfected with HBV
or HCV, on the one hand, and HIV, on the other hand.
“Entity” means any corporation, company, partnership, joint venture or firm.
“Idenix Entities” means Idenix, its Affiliates, and their licensees (including any
Possible Licensee if a Transaction is entered into), successors and assigns.
“IDX899” means
(2-Carbamoyl-5-chloro-iH-indol-3-yl)-[3-((E)-2-cyano-vinyl)-5-methyl-phenyl]-phosphinic acid methyl
ester, a stereoisomer or a salt of the above, having the following structure:
 , and any prodrugs,
active metabolites, polymorphs, salts, hydrates, solvates, enantiomers
and/or esters having the same active moiety as the compound.
, and any prodrugs,
active metabolites, polymorphs, salts, hydrates, solvates, enantiomers
and/or esters having the same active moiety as the compound.
“IDX989” means
(2-Carbamoyl-5-chloro-4-fluoro-1H-indol-3-yl)-[3-((E)-2-cyano-vinyl)-5-methyl-phenyl]-phosphinic
acid methyl ester, a strereoisomer or a salt of the above, having the following structure:
3
 , and
any prodrugs, active metabolites, polymorphs, salts, hydrates, solvates, enantiomers
and/or esters having the same active moiety as the compound.
, and
any prodrugs, active metabolites, polymorphs, salts, hydrates, solvates, enantiomers
and/or esters having the same active moiety as the compound.
“Novartis Protected Entities” means Novartis, its Affiliates, their sublicensees and
distributors, and the direct or indirect customers of the above.
“Possible Licensees” means the Persons set out in Schedule 2.
“Terms Sheet” means, in respect of each Possible Licensee, the terms sheet attached as
Exhibit B to the General Waiver and Consent, dated the Effective Date, between Idenix US and
Novartis for that Possible Licensee (or terms and conditions which Novartis in its sole discretion
deems more favourable to Idenix than the terms and conditions set out in the terms sheet attached
for that Possible Licensee).
“Transaction” means a license agreement with one of the Possible Licensees, containing
substantially the terms and conditions set out in the Terms Sheet for that Possible Licensee, for
the research, development, manufacture and commercialization of Waived Compounds.
“Waived Compounds” means all non-nucleoside reverse transcriptase inhibitor compounds
claimed in (a) the Patent Rights on Schedule 1 referenced as [**] and/or (b) all continuations,
divisions, reexaminations, renewals, registrations reissues and extensions of any of the above
Patent Rights, both foreign and domestic, that are owned or controlled by Idenix or an Idenix
Entity, including, without limitation, IDX899 and IDX989 and all back-up compounds, follow-on
compounds, prodrugs and metabolites of the compounds, as well as their isomers, salts, hydrates,
solvants and polymorphs.
“Waived Compound Intellectual Property” means the know-how and Patent Rights owned or
otherwise licenseable by Idenix or its Affiliates that Cover a Waived Compound or a product
containing a Waived Compound, or the research, pre-clinical development, clinical development, use,
making, having made, marketing, offering to sell, selling, distributing, importing or otherwise
exploitation of a Waived Compound, including, without limitation, the Intellectual Property
relating to a Waived Compound developed after the date of this HIV Waiver and Consent, which to the
extent that that Intellectual Property comprises Patent Rights at the date of this HIV Waiver and
Consent, are set out in Schedule 1.
4
2. Waiver and Consent. Subject to Sections 3 and 4, and as set out in Section 2 (c)
and (d) below, provided that conditions (a) and (b) are satisfied, namely that:
(a) the Transaction includes a license only of Waived Compound Intellectual Property; and
(b) the Transaction is entered into by 31 March 2009;
then:
(c) except for Novartis’ non-exclusive right to exercise its Co-Labeling Right under the [**]
Patent Rights, Novartis waives all its rights to and under the Waived Compound Intellectual
Property (including, without limitation, any step-in patent prosecution and enforcement rights with
respect thereto pursuant to any agreement among Novartis, Idenix or its Affiliates and the
University of Cagliari or Universita’ Degli Studi Di Cagliari and all rights pursuant to Sections
10.2,10.3, 10.4(a) and 10.5 of the Novartis Licence Agreement); and
(d) Novartis agrees that the Waived Compounds are Rejected Compounds, and Idenix has no
obligation to provide a Completion Notice under Section 3.3(a) (ii) of the Novartis Licence
Agreement.
3. Representations. Warranties. Covenants and Indemnity.
(a) Idenix represents and warrants to Novartis that as of the Effective Date, except for the
Patent Rights in Schedule 1, referenced as [**], to the knowledge of Idenix, the Waived Compound
Intellectual Property in Schedule 1 does not Cover (i) the treatment of HBV or HCV nor (ii) the
manufacture, use, sale, offer for sale, or importation of LdT, LdC or Albuferon;
(b) Idenix represents and warrants to Novartis that the Transaction will provide that (i) the
Possible Licensee will not, without Idenix’s prior written consent, not to be unreasonably
withheld, abandon or narrow the scope of any claim in the [**] Patent Rights that Covers the
Co-Labeling Right; and (ii) the Possible Licensee will not, without the prior written consent of
Idenix, not to be unreasonably withheld, enter into any settlement with respect to, or admit to the
invalidity or unenforceability of, the [**] Patent Rights;
(c) Idenix, on behalf of the Idenix Entities, represents and warrants that the Idenix Entities
will not, directly or indirectly, assert against the Novartis Protected Entities, any claim that
the Novartis Protected Entities’ exercise of the Co-Labeling Right directly infringes, contributes
to the infringement of, or actively induces the infringement of any claims under the [**] Patent
Rights; and
Idenix indemnifies Novartis in respect of any breach of the representations, warranties and
covenants in this Section 3, under Section 11.5 of the Novartis Licence Agreement.
4. Novartis’ Retained Rights. Except as expressly provided in this document, this HIV
Waiver and Consent does not in any way limit or remove any other right Novartis has under Section
3.3 or any other part of the Novartis Licence Agreement.
5
5. Confidentiality. The Parties will keep confidential the terms of this HIV Waiver
and Consent except that Idenix may provide a copy or summary of this document (with Schedule 2
redacted appropriately to maintain the confidentiality of the identity of other Possible Licensees
and that licensee’s Terms Sheet from other Possible Licensees), for the purpose of negotiating the
Transaction, on the condition that the Possible Licensees keep confidential the contents of this
document or summary.
6. Governing Law. This HIV Waiver and Consent is governed by and interpreted under the
laws of the State of New York, excluding (a) its conflicts of laws principles; (b) the United
Nations Convention on Contracts for the International Sale of Goods; (c) the 1974 Convention; and
(d) the Protocol amending the 1974 Convention of 11 April 1980.
7. Entire Agreement. This HIV Waiver and Consent supersedes in its entirety that
Waiver and Consent signed by Novartis dated as of 15 December 2008.
[EXECUTION PAGE FOLLOWS]
6
EXECUTION Idenix and Novartis execute this HIV Waiver and Consent by their authorized
representatives, as of the date first written above.
| IDENIX PHARMACEUTICALS. INC. |
||||
| By: | /s/ Xxxx Xxxxxxxxxxx | |||
| Name: | Xxxx Xxxxxxxxxxx | |||
| Title: | Executive Vice Presidential and General Counsel | |||
| IDENIX (CAYMAN) LIMITED |
||||
| By: | /s/ Xxxx-Xxxxxx Sommadossi | |||
| Name: | Xxxx-Xxxxxx Sommadossi, Ph.D. | |||
| Title: | Director | |||
| NOVARTIS PHARMA AG |
||||
| By: | /s/ Xxxx Xxxxxxxx | |||
| Name: | Xxxx Xxxxxxxx | |||
| Title: | Head of Finance, Global Business Development & Licensing | |||
| By: | /s/ P.A. Ho | |||
| Name: | P.A. Ho | |||
| Title: | Senior Legal Counsel | |||
7
SCHEDULE 1
| Type | ||||||||
| Related/ | ||||||||
| Idenix Ref. No. | Country | Serial No. | Filed | Title | ||||
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 3 pages were omitted pursuant to a request for confidential treatment. [**]
8
SCHEDULE 2
Possible Licensees
| 1. | GlaxoSmithKline plc. |
[END OF SCHEDULE]
9
EXHIBIT C
STOCK PURCHASE AGREEMENT
[to be attached]
(Incorporated by reference to Exhibit 10.1 to the
Current Report on Form 8-K on February 6, 2009)
Current Report on Form 8-K on February 6, 2009)
EXHIBIT D
AMENDMENT AGREEMENT
This agreement (“Agreement”) is made by and between;
IDENIX PHARMACEUTICALS, INC., a corporation organized and existing under the laws of the State of
Delaware, having its principal offices located at 00 Xxxxxxxxx Xxxxxx, Xxxxxxxxx, XX 00000
represented by its legal representative Xx. Xxxx-Xxxxxx Xxxxxxxxxx (hereinafter referred to as
“Idenix”);
and
IDENIX SARL, a corporation organized and existing under the laws of France, having registered
offices located at Cap Gamma, 0000 xxx xx xx Xxxxxxxx. 00000 Montpellier Cedex 4 -France,
represented by its legal representative (hereinafter referred to as “Idenix SARL”);
and
SmithKline Xxxxxxx Corporation doing business as GLAXOSMITHKLINE, a corporation organized and
existing under the laws of Pennsylvania, having registered offices in Xxx Xxxxxxxx Xxxxx,
Xxxxxxxxxxxx, Xxxxxxxxxxxx 00000, represented by its legal representative Xx. Xxxxxxx X. Xxxxxx
(hereinafter referred to as “GSK”);
and
UN1VERSITA’ DEGLI STUDI DI CAGLIARI, having a principal place of business at Cittadella
Universitaria, SS 554 XX 0.0, 00000 Xxxxxxxxxx, Xxxxxxxx, Xxxxx, represented by its legal
representative (hereinafter referred to as “University”);
and
XXXX. XXXXX XX XXXXX, born in La Maddalena on August 14 1944, an Italian citizen residing in Italy
at Poggio Dei Pini, 5° Xxxxxx xx 00, Xxxxxxxxx (XX) - Xxxxx, full Professor of Microbiology at the
University of Cagliari (hereinafter referred to as “Xxxx. Xx Xxxxx”)
whereas
| A) | on 4 January 1999, the University and Idenix SARL, this last on behalf and for the benefit of Idenix, entered into an agreement entitled “Co-operative Antiviral Research Activity Agreement” (hereinafter referred to as the “Co-operative Agreement”) aimed at performing a joint research activity in the antiviral substances field; | |
| B) | in accordance with the Co-operative Agreement, on 14 December 2000, the University and Idenix entered into a license agreement (hereinafter the “License Agreement”) according to which the University grants to Idenix the exclusive license for the exploitation, whether direct or indirect, of the results obtained and that will be obtained from the performance of the joint research activity under the Co-operative Agreement; |
| C) | the above mentioned Co-operative Agreement and License Agreement (together, the “Original Agreements”) have been initially amended on 10 April 2002 (the “April 10, 2002 Amendment”); | |
| D) | the Original Agreements, as so amended, have been afterwards amended with the deed undersigned by the parties on 8 May 2003, also in consideration of the transactions at that time pending between Idenix and Novartis Pharma AG for the acquisition, by this last, of the majority of the shareholding of Idenix (the amendments indicated in this point have become effective on 8 May 2003, following to the occurred execution of a “Development, License and Commercialization Agreement” and of a “Manufacturing and Supply Agreement” by and among Idenix, Idenix (Cayman) Limited and Novartis) (together, the “Novartis Agreements”); the deed undersigned by the parties on 8 May 2003 provided, in favour of Novartis, the right to cure possible breaches of the Original Agreements by Idenix; | |
| E) | the parties further amended the Original Agreements, as previously amended, on 30 June 2004 (the “June 30, 2004 Letter Amendment”) and on 24 October 2005 (the “October 24, 2005 Amendment”). The Original Agreements, as amended through and including the April 10, 2002 Amendment, the June 30, 2004 Amendment, and the October 24, 2005 Amendment shall be collectively defined the “Cagliari Agreements”, provided that the “Cagliari Agreements” do not include the “Novartis Agreements”; | |
| F) | on 30 June 2004 the University and Xxxx. Xx Xxxxx executed an agreement (the “June 30, 2004 Assignment Agreement”) called “assignment of the intellectual property rights deriving from the execution of the Co-operative Antiviral Research Activity Agreement”; | |
| G) | on 22 December 2006 the parties have extended the validity of the Co-operative Agreement up to 22 January 2011 (the “December 22, 2006 Amendment”); | |
| H) | on 03 December, 2008 Idenix and Xxxx. Xxxxx Xx Xxxxx executed an agreement (hereinafter “[**] Agreement”, which is attached herewith under Enclosure B); | |
| I) | Idenix is currently carrying on negotiations with GSK in order to define an agreement relating to the granting of exclusive licenses to non-nucleoside reverse transcriptase inhibitor compounds owned and/or controlled by Idenix and namely: |
| (i) | an exclusive license, from Idenix to GSK, of the [**] patents covering inventions developed within the Co-operative Agreement and listed in Enclosure A to this agreement, including future patents related to the family of patents or compounds mentioned under such Enclosure A; | ||
| (ii) | an exclusive license, from Idenix to GSK, of the [**] patents listed in Enclosure B to this agreement and as the subject of the [**] Agreement, including future patents related to the family of patents or compounds mentioned under such Enclosure B; (collectively the “GSK Agreement”); |
12
| J) | art. 2.2 of the Licence Agreement allows Idenix (formerly Novirio) “to grant sublicenses under the license granted pursuant to Section 2.1 above to parties with whom Novirio or its Affiliates has agreed to jointly develop or commercialize Licensed Products”; | |
| K) | in the light of the negotiations currently pending between Idenix and GSK; |
| (i) | Idenix, Idenix SARL and the University hereby execute this amendment to the License Agreement and the Co-operative Agreement (to the extent that this latter refers to the licence granted by the University under art. 9.1 to Idenix) which shall be effective only if and when Idenix and GSK shall have executed the agreement mentioned under previous recital (I) and; | ||
| (ii) | Idenix and Xxxx. Xx Xxxxx hereby execute an amendment to the [**] Agreement which shall be effective only if and when Idenix and GSK shall have executed the agreement mentioned under previous recital (I), |
provided that, for purposes of this recital K), letters (i) and (ii), the effective date of such
agreement shall be defined as the “Effective Date”;
THEREFORE, in consideration of the premises, mutual covenants and definitions herein contained,
Idenix, Idenix SARL, GSK, the University of Cagliari and Xxxx. Xx Xxxxx agree as follows:
| 1) | The recitals and the Enclosures to this agreement shall represent an integral and substantial part of this agreement (hereinafter the “Amendment”). | |
| 2) | This Amendment shall represent an amendment to the License Agreement (as amended), the Co-operative Agreement (as amended) and to the [**] Agreement and shall become automatically effective among the parties hereof, if and when Idenix and GSK shall have executed the agreement mentioned under previous recital I, upon the Effective Date. | |
| 3a) | In relation to the provision mentioned under art. 2.2 of the License Agreement and article 9.1 of the Co-operative Agreement, the University hereby acknowledges and accepts that |
| (i) | Idenix is permitted to sublicense to GSK the patents, arising under the Co-operative Agreement and the License Agreement, listed in Enclosure A, including future patents related to the family of patents or compounds mentioned under such Enclosure A, for development or commercialization of the Licensed Products; | ||
| (ii) | such sublicense shall no longer be conditioned upon an agreement between Idenix (formerly Novirio) or its Affiliates and GSK to jointly develop or commercialize Licensed Products, and GSK shall be entitled to develop and commercialize Licensed Products solely and on its own behalf in accordance with the License Agreement; | ||
| (iii) | GSK is, in its turn, permitted to sublicense the patents mentioned under the Enclosure A, including future patents related to the family of patents or |
13
| compounds mentioned under such Enclosure A, to its affiliates or to third parties, solely in accordance with the agreement to be executed between Idenix and GSK, provided that, for purposes of this art. 3a) “affiliates” shall mean any corporation, company, partnership, joint venture and/or firm that controls, is controlled by, or is under common control with GSK. |
| 3b) | Idenix may disclose to GSK as required under the agreement between GSK and Idenix confidential information (as identified in art. 6 of the Co-operative Agreement) owned by the University of Cagliari it being understood that GSK may make further limited disclosures as permitted under the agreement between GSK and Idenix. | |
| 3c) | Idenix will license to GSK the [**] patents which represent the subject matter of the [**] Agreement (Enclosure B, including future patents related to the family of patents or compounds mentioned under such Enclosure B). | |
| 4a) | As of, and thereafter the Effective Date, should Idenix be in breach of one or more of the obligations mentioned under the License Agreement or the Co-operative Agreement and either Idenix or Novartis not cure such breach within the 3 months and 15 days term (“Idenix Cure Term”) granted pursuant to the amendment to the License Agreement or the Co-operative Agreement mentioned under recital D) to this Amendment, GSK shall have the right to cure such breach within 15 days (“Grace Term”) after expiration of the Idenix Cure Term provided that, if GSK cures the breach within the Grace Term, GSK shall then have the right, without the need for any further action by Idenix, to partially succeed to Idenix in the License Agreement with sole reference to the patents mentioned within the Enclosure A to this Amendment and to future patents related to the family of patents or compounds mentioned under such Enclosure A, or the Co-operative Agreement (to the sole extent it refers to the license granted by the University to Idenix pursuant to art. 9.1 of the same Co-operative Agreement), thereby assuming the rights and obligations of Idenix under such agreement, provided that |
| (i) | the License Agreement or the Co-operative agreement will not terminate in case Idenix or GSK demonstrate that the obligations have been contracted or proven the fault was a case of “force majeure” or the same is demonstrated by GSK within the Grace Term; | ||
| (ii) | the University of Cagliari shall provide written notice to GSK by registered mail (in addition to Idenix and Novartis) of any breach or default under the License Agreement or the Co-operative Agreement and with any related correspondence that is delivered to Idenix and at the same time that of delivery to Idenix. |
| 4b) | As of, and thereafter the Effective Date, should Idenix be in breach of one or more of the obligations mentioned under the [**] Agreement, Idenix shall have the right to cure such breach within 3 months after reception of a registered letter sent by Xxxx Xx Xxxxx to Idenix and GSK detailing the reasons for such complaint, provided that, should the breach be cured by Idenix, the [**] Agreement shall remain between Idenix and Xxxx. Xx Xxxxx. Should the breach not be cured within such 3 months term, the agreement shall remain in force for a further 30 days term starting from the expiration of |
14
| the 3 months period during which GSK shall still have the right to cure such breach in lieu of Idenix, provided that if GSK cures the breach within such 30 days term, GSK shall then have the right, without the need for any further action by Idenix to succeed to Idenix in the [**] Agreement, thereby assuming the rights and obligations of Idenix under such [**] Agreement. | ||
| 5) | It is understood that, should the agreement between Idenix and GSK state the right of Idenix to receive any up-front payment, milestone or other fixed amount (“Fixed Amount”) related to such agreement and pertaining to both the [**] patents and the [**] patents licensed by Idenix to GSK, the University and Xxxxxxxxx Xx Xxxxx shall, with specific reference to such Fixed Amount, receive from Idenix only the percentage provided under the Cagliari Agreements, provided that Xxxx. Xx Xxxxx shall not be entitled also to the percentages provided under the [**] Agreement in relation to the Fixed Amount. For purposes of clarity, it is understood by the parties that if Idenix and GSK enter into the agreement referenced herein and a payment is made by GSK to Idenix at the time of licensing both the [**] patents and the [**] patents, only one payment will be made to the University and Xxxxxxxxx XxXxxxx in relation to the [**] patents and the [**] patents. If future milestone or royalty payments are made based on a specific patent family, the respective agreement shall govern any such payment to be made to the University and Xxxxxxxxx Xx Xxxxx. | |
| 6) | The provisions of article 8.2 of the Co-operative Agreement and article 5.1 of the License Agreement are hereby expressly amended to permit GSK to file, prosecute and maintain patents licensed to GSK by Idenix and mentioned under Enclosure A (including future patents related to the family of patents or compounds mentioned under such Enclosure A) in lieu of Idenix’s first right to file, prosecute and maintain such patents, provided that the relevant expenses shall be borne by Idenix or by GSK as may be agreed between these last two entities and the University of Cagliari hereby permits and acknowledges such actions by GSK. It is understood that, with reference to the patents listed under Enclosure A, such filing, prosecution and maintenance shall be performed in the name and on behalf of Idenix and of University of Cagliari. | |
| 7) | Consistently with the amendment of article 5.1 of the License Agreement above, GSK or any of its affiliates or permitted sublicensees shall be permitted to exercise Idenix’ right to institute an action against a third party for infringement of the patents licensed to GSK by Idenix, including those mentioned under Enclosure A as Idenix’ designee and as may be agreed between Idenix and GSK. | |
| 8) | To this aim, with the signature of the present document, the parties acknowledge that as of the execution date, no breaches of the License Agreement and of the [**] Agreements have occurred. | |
| 9) | It is understood that, without limitation to the rights granted to GSK according to previous art. 4a) and 4b), the University of Cagliari and Xxxx. Xx Xxxxx expressly permit, respectively, the assignment of the License Agreement, the Co-operative Agreement and of the [**] Agreement by Idenix to GSK. |
15
IN WITNESS WHEREOF, Idenix, Idenix SARL, University of Cagliari, Xxxx. Xx Xxxxx and GSK have caused
this agreement to be duly executed by their authorized representatives.
| Idenix Pharmaceuticals, Inc. | ||||
By:
|
/s/ Xxxx X. Xxxxxxxxxxx
|
|||
| Title: Executive Vice President/General Counsel | ||||
21 January 2009
|
16
| Idenix SARI | ||||
By:
|
/s/ Xxxx-Xxxxxx Sommadossi
|
|||
| Title: Gerant | ||||
21 January 2009
|
17
| SmithKline Xxxxxxx Corporation Doing business as GLAXOSMITHKLINE | ||||
By:
|
/s/ Xxxxxxx X. Xxxxxx
|
|||
| Title: Vice President & Secretary | ||||
18
| Università degli Studi di Cagliari | ||||
By:
|
/s/ Xxxx. Xxxxxx Xxx
|
|||
| Title: | ||||
8 Gen. 2009
|
| Certifico io Ufficiale Rogante Sostituto dell’Università degli Studi di Cagliari che la firma, apposta in xxx xxxxxxxx dal Xxxx. Xxxxxx Xxx nuto a Ottana il 28.07.1943, Pro Rettore dell’Università di Cagliari e xxxxx xxx identità personale sono certo, è autentica L’Ufficiale Rogante Sostituto Xx. Xxxxxx Xxxxxxxxx Xxxxx |
||||
| /s/ Xx. Xxxxxx Xxxxxxxxx Xxxxx | SEAL | |||
19
| Xxxx. Xxxxx Xx Xxxxx | ||||
By:
|
/s/ Xxxxx Xx Xxxxx
|
|||
| Title: | ||||
8 Gen. 2009
|
| Certifico io Ufficiale Rogante
Sostituto dell’Università degli Studi di Cagliari che la firma, apposta in xxx xxxxxxxx dal Xxxx. Xxxxx Xx Xxxxx, nato a La Maddalena il 14.08.1944, Professore Orinario presso l’Università di Cagliari e xxxxx xxx identità personale sono certo, è autentica L’Ufficiale Rogante Sostituto Xx. Xxxxxx Xxxxxxxxx Xxxxx |
||||
| /s/ Xx. Xxxxxx Xxxxxxxxx Xxxxx | SEAL | |||
20
ENCLOSURE A
[**]
| Type | ||||||||
| Related/ | ||||||||
| Idenix Ref. No. | Country | Serial No. | Filed | Title | ||||
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 2 pages were omitted pursuant to a request for confidential treatment. [**]
21
ENCLOSURE B
[**]
| Type | ||||||||
| Related/ | ||||||||
| Idenix Ref. No. | Country | Serial No. | Filed | Title | ||||
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 2 pages were omitted pursuant to a request for confidential treatment. [**]
22
EXHIBIT E
SPECIFICATIONS
| IDX899 API | ||||
| Regulatory | ||||
| Specifications | ||||
ANALYSIS
|
METHOD(1) | Acceptance Criteria |
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 2 pages were omitted pursuant to a request for confidential treatment. [**]
23
IDX899 DP specification 50mg, 100mg 200mg capsules
| ANALYSIS | METHOD(1) | SPECIFICATIONS |
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 1 page was omitted pursuant to a request for confidential treatment. [**]
EXHIBIT F
DEVELOPMENT PLAN
The development plan for IDX-899 assumes API is received in February 09 after signing of contract.
The timing and activities described in this development plan could change over the course of
development based on data generated, feedback from regulatory agencies, or evolving clinical and/or
commercial insight.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 8 pages were omitted pursuant to a request for confidential treatment. [**]
25
EXHIBIT G
PRESS RELEASE
IDENIX PHARMACEUTICALS AND GLAXOSMITHKLINE SIGN WORLDWIDE LICENSE
AGREEMENT FOR IDX899, A NOVEL NNRTI FOR THE TREATMENT OF HIV
AGREEMENT FOR IDX899, A NOVEL NNRTI FOR THE TREATMENT OF HIV
Cambridge, MA and London, UK — February 6, 2009 — Idenix Pharmaceuticals, Inc. (NASDAQ: IDIX) and
GlaxoSmithKline (GSK) today announced the execution of a license agreement granting GSK exclusive
worldwide rights to IDX899. IDX899 is a novel non-nucleoside reverse transcriptase inhibitor
(NNRTI) in Phase II clinical development being developed by Idenix for the treatment of HIV/AIDS.
New NNRTIs are needed to address the increasing prevalence of viral resistance and side effects
associated with this drug class. To date, IDX899 has demonstrated high potency with low milligram
doses, a high barrier to drug resistance, favorable risk/benefit profile, and the convenience of
once-a-day administration.
Under the terms of the agreement, GSK will assume all development responsibility and associated
costs for IDX899, and Idenix will receive an upfront payment of $34 million and will be eligible to
receive up to $416 million in development, regulatory and sales milestones. Furthermore, if IDX899
is successfully developed and commercialized, Idenix will receive double-digit, tiered worldwide
royalties.
“GSK, with a well-established HIV franchise and substantial drug development experience, is the
ideal collaborator to help maximize the potential of IDX899, “ said Xxxx-Xxxxxx Sommadossi, Chief
Executive Officer of Idenix. “For Idenix, the significant value created through the license of
IDX899 enables us to focus all of our resources on advancing our core strategic HCV assets, which
include drug candidates from the three major classes of direct-acting HCV antivirals.”
“IDX899 may play a significant role in improving treatment options for people with HIV/AIDS,”
commented Zhi Hong, Senior Vice President of the Infectious Diseases Centre of Excellence for Drug
Discovery (ID CEDD) at GSK. “A once-daily, lower-dose NNRTI that offers an improved drug
resistance and pharmacokinetic profile would be valuable to HIV-treating physicians and patients.
The preliminary clinical evidence of IDX899 supports this and warrants continued clinical study as
part of GSK’s growing drug pipeline in HIV.”
The $34 million upfront payment by GSK to Idenix was split evenly between cash and the purchase of
Idenix common stock at $x.xx per share. The effectiveness of these transactions is subject to
approval under the Xxxx-Xxxxx-Xxxxxx Antitrust Improvements Act of 1976, as amended and other
customary closing conditions.
About XXX000
XXX000 is a potent non-nucleoside reverse transcriptase inhibitor (NNRTI) being developed for the
treatment of HIV-1. Idenix has advanced IDX899 through a Phase II proof-of-concept study in HIV-1
infected treatment-naïve patients that was completed in 2008. In the proof-of-concept study,
patients (n=32) receiving once-daily oral administration of IDX899 achieved mean viral load
reductions of 1.8 log10, after seven days of treatment as tested with the Roche
Amplicor® 1.5 assay. In this study, no treatment-related serious or non-serious adverse
events were reported and no patients discontinued. The most common adverse events observed were
dyspepsia, headache and nausea; the rate of these events was
similar between IDX899-treated patients and those receiving placebo. Additionally, no patterns in
laboratory abnormalities between treatment groups were observed during the treatment period.
26
About Idenix
Idenix Pharmaceuticals, Inc., headquartered in Cambridge, Massachusetts, is a biopharmaceutical
company engaged in the discovery and development of drugs for the treatment of human viral and
other infectious diseases. Idenix’s current focus is on the treatment of infections caused by
hepatitis C virus. For further information about Idenix, please refer to xxx.xxxxxx.xxx.
GlaxoSmithKline — one of the world’s leading research-based pharmaceutical and healthcare
companies — is committed to improving the quality of human life by enabling people to do more,
feel better and live longer. For further information please visit xxx.xxx.xxx
Idenix Pharmaceuticals Forward-looking Statements
This press release contains “forward-looking statements” within the meaning of The Private
Securities Litigation Reform Act of 1995. Such forward-looking statements can be identified by the
use of forward-looking terminology such as “anticipate,” “could,” “may,” “will,” or similar
expressions, or by express or implied statements with respect to the company’s clinical development
programs in HIV, or any potential pipeline candidates for the treatment of HIV, including any
expressed or implied statement regarding the efficacy and safety of IDX899 and any future clinical
trials involving IDX899. Such forward-looking statements involve known and unknown risks,
uncertainties and other factors that may cause actual results to be materially different from any
future results, performance or achievements expressed or implied by such statements. There can be
no guarantees that the company will advance any clinical product candidate or other component of
its potential pipeline to the clinic, to the regulatory process or to commercialization. In
particular, management’s expectations could be affected by unexpected regulatory actions or delays;
uncertainties relating to, or unsuccessful results of, pre-clinical studies and/or clinical trials,
including additional data relating to the ongoing pre-clinical studies and/or clinical trials
evaluating its product candidates, including IDX899; the company’s ability to obtain additional
funding required to conduct its research, development and commercialization activities; the
company’s dependence on its collaboration with Novartis Pharma AG; changes in the company’s
business plan or objectives; the ability of the company to attract and retain qualified personnel;
competition in general; and the company’s ability to obtain, maintain and enforce patent and other
intellectual property protection for its product candidates and its discoveries. These and other
risks which may impact management’s expectations are described in greater detail under the caption
“Risk Factors” in the company’s annual report on Form 10-K for the year ended December 31, 2007 and
the Quarterly Report on Form 10-Q for the quarter ended September 30, 2008, each as filed with the
Securities and Exchange Commission (SEC) and other filings that the company makes with the SEC.
All forward-looking statements reflect the company’s expectations only as of the date of this
release and should not be relied upon as reflecting the company’s views, expectations or beliefs at
any date subsequent to the date of this release. Idenix anticipates that subsequent events and
developments may cause these views, expectations and beliefs to change. However, while Idenix may
elect to update these forward-looking statements at some point in the future, it specifically
disclaims any obligation to do so.
Idenix Pharmaceuticals Contacts:
Xxx Xxxxxxxx 000-000-0000 (investors)
Xxxx Xxxxxxx 000-000-0000 (media)
Xxxx Xxxxxxx 000-000-0000 (media)
27
GlaxoSmithKline Contacts:
UK Media enquiries: |
Xxxxxx Xxxxxxx | (000) 0000 0000 | ||
| Xxxxx Xxxx | (000) 0000 0000 | |||
| Xxxxx Xxxxxxxxx | (000) 0000 0000 | |||
| Xxxxxxx Xxx | (000) 0000 0000 | |||
US Media enquiries: |
Xxxxx Xxxxxxx | (000) 000 0000 | ||
| Xxxx Xxxx Xxxxx | (000) 000 0000 | |||
| Xxxxx Xxxxxx | (000) 000 0000 | |||
| Xxxxx Xxxxxxx | (000) 000 0000 | |||
European
Analyst/Investor enquiries: |
Xxxxx Xxxxxxxx | (000) 0000 0000 | ||
| Xxxxx Xxxxxxxx | (000) 0000 0000 | |||
| Xxxx Xxxxxx | (000) 0000 0000 | |||
US Analyst/ Investor enquiries: |
Xxx Xxxxx | (000) 000 0000 | ||
| Xxx Xxxx | (000) 000 0000 |
###
28
Schedule 3.2(a)
Drug Substance Availability
The following drug substance supplies have been prepared and as of the Execution Date Idenix has
~[**] kg of GMP IDX-899 in stock.
Batch #
|
[**] | [**] | [**] | |||
Supplier
|
[**] | [**] | [**] | |||
DOM
|
[**] | [**] | [**] | |||
Size
|
[**] | [**] | [**] | |||
Use
|
[**] | [**] | [**] | |||
Form
|
[**] | [**] | [**] |
[**]
Schedule 5.2(a)
Successful Completion
| “Successful Completion” Definition for Corresponding | ||
| Development Milestone | Milestone Event | |
Successful Completion of development
of a commercially viable formulation
|
Achieved upon the first patient dosed in the first Phase IIb Clinical Trial using a once-daily solid oral dosage formulation developed and approved by GSK for its physical properties. “Phase IIb Clinical Trial” means a Phase II Clinical Trial to determine initial efficacy and dose range finding. | |
Successful Completion of a Segment
II reproductive toxicity study
|
Segment II reproductive toxicity study that demonstrates no direct teratogenic effects. | |
Successful Completion of 6-month
rodent chronic toxicology study.
|
Safety findings support dosing beyond 6 months at therapeutically and commercially relevant doses so that both FDA and applicable European Union (“EU”) health authorities authorize initiation of a Phase IIb Clinical Trial with IDX-899 and agree that data support the initiation of a Phase III Clinical Trial with IDX-899. | |
Successful Completion of at least
9-month non-rodent chronic
toxicology study.
|
Safety findings support dosing beyond 9 months at therapeutically and commercially relevant doses in NNRTI treatment-experienced patients so that both FDA and applicable EU health authorities authorize initiation of a Phase IIb Clinical Trial with IDX-899. | |
Successful Completion of 24-month
carcinogenicity studies in at least
one (1) rodent species.
|
Carcinogenicity program supports registration without additional studies per both FDA and EMEA agreement | |
Successful Completion of development
of a commercially viable fixed dose
combination formulation
|
Development of a once daily tablet [**] that is shown to be bioequivalent to individual products (80% — 125% per FDA definition of bioequivalence) as determined from a pivotal bioequivalence study as determined by the Development Plan in Exhibit F. |
30
Schedule 5.3(a)
UNAIDS Accelerating Access Initiative Countries
| Country | Country | |||||||
1
|
Angola | 32 | Malawi | |||||
2
|
Bangladesh | 33 | Maldives | |||||
3
|
Benin | 34 | Mali | |||||
4
|
Bhutan | 35 | Mauritania | |||||
5
|
Botswana | 36 | Mauritius | |||||
6
|
Burkina Faso | 37 | Mozambique | |||||
7
|
Burundi | 38 | Myanmar | |||||
8
|
Cambodia | 39 | Namibia | |||||
9
|
Cameroon | 40 | Nepal | |||||
00
|
Xxxx Xxxxx | 00 | Xxxxx | |||||
00
|
Xxxxxxx Xxxxxx Rep. | 42 | Nigeria | |||||
12
|
Chad | 43 | Rwanda | |||||
13
|
Comoros | 44 | Samoa | |||||
14
|
Congo | 45 | Sao Tome & Principe | |||||
15
|
Côte d’Ivoire | 46 | Senegal | |||||
16
|
Djibouti | 00 | Xxxxxxxxxx | |||||
00
|
XX Xxxxx (Xxxxx) | 48 | Sierra Leone | |||||
18
|
Equatorial Guinea | 49 | Solomon Islands | |||||
19
|
Eritrea | 50 | Somalia | |||||
00
|
Xxxxxxxx | 00 | Xxxxx Xxxxxx | |||||
21
|
Gabon | 52 | Sudan | |||||
22
|
Xxxxxx | 00 | Xxxxxxxxx | |||||
00
|
Xxxxx | 00 | Xxxxxxxx | |||||
00
|
Guinea | 55 | Togo | |||||
25
|
Guinea Bissau | 56 | Tuvalu | |||||
26
|
Haiti | 57 | Uganda | |||||
27
|
Kenya | 58 | Vanuatu | |||||
28
|
Kiribati | 59 | Yemen | |||||
29
|
Lao People’s DR | 60 | Zambia | |||||
30
|
Lesotho | 61 | Zimbabwe | |||||
31
|
Liberia |
Schedule 10.2
Disclosure Schedule
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
pursuant to a request for confidential treatment.
A total of 3 pages were omitted pursuant to a request for confidential treatment. [**]

