FACILITY SETUP AND CONTRACT MANUFACTURING AGREEMENT between Eckert & Ziegler Nuclitec GmbH Gieselweg 1 38110 Braunschweig Germany - hereinafter referred to as EZN - and Molecular Insight Pharmaceuticals, Inc Cambridge, MA 02142 USA - hereinafter...
Exhibit 10.6
FACILITY SETUP AND CONTRACT MANUFACTURING AGREEMENT
between
Xxxxxx & Xxxxxxx Nuclitec XxxX
Xxxxxxxxx 0
00000 Xxxxxxxxxxxx
Xxxxxxx
- hereinafter referred to as EZN -
and
Molecular Insight Pharmaceuticals, Inc
000 Xxxxxx Xxxxxx
Xxxxxxxxx, XX 00000
XXX
- hereinafter referred to as the MIPI -
TABLE OF CONTENTS
| 1 | DEFINITIONS | 3 | ||
| 2 | PURPOSE | 6 | ||
| 3 | TERM AND TERMINATION | 7 | ||
| 4 | REMEDIES UPON TERMINATION | 8 | ||
| 5 | EXCLUSIVITY AND NON-COMPETE | 9 | ||
| 6 | DEVELOPMENT AND KIT/SET SUPPLY PHASE | 10 | ||
| 7 | TECHNOLOGY TRANSFER | 11 | ||
| 8 | CONSTRUCTION OF FACILITY – FACILITY PROGRAM COST | 11 | ||
| 9 | ASSET OWNERSHIP AND ENVIRONMENTAL INDEMNITY OF FACILITY | 12 | ||
| 10 | MANUFACTURE AND SUPPLY OF FINAL PRODUCT | 13 | ||
| 11 | ORDERS AND SHIPMENTS | 14 | ||
| 12 | PAYMENTS | 15 | ||
| 13 | LICENSES, PATENTS AND TECHNOLOGY | 16 | ||
| 14 | REGULATORY MATTERS | 17 | ||
| 15 | DATA MANAGEMENT | 18 | ||
| 16 | DISCLOSURE OF TECHNOLOGY | 18 | ||
| 17 | RIGHT OF FIRST REFUSAL | 18 | ||
| 18 | RIGHT OF NEGOTIATION | 18 | ||
| 19 | CONFIDENTIALITY | 19 | ||
| 20 | NOTICES | 19 | ||
| 21 | FORCE MAJEURE | 20 | ||
1
| 22 | BACK-UP | 20 | ||
| 23 | INDEMNIFICATION AND INSURANCES | 21 | ||
| 24 | EXPORT CONTROL | 23 | ||
| 25 | GOVERNING LAW | 24 | ||
| 26 | GENERAL | 24 | ||
2
PREAMBLE
| A. | MIPI is the worldwide licensee of an yttrium-90 radiolabeled somatostatin peptide analog, formerly known as OctreoTher and now the brand name Onalta. Onalta is one of MIPI’s lead radiotherapeutic product candidates under development for the treatment of metastatic carcinoid and pancreatic neuroendocrine cancer in patients whose symptoms are not controlled by conventional somatostatin analog therapy. |
| B. | In May 2009, the European Medicines Agency (EMEA) has approved the Phase 3 protocol for Onalta and it is now intended to proceed with the final clinical trial that will position Onalta for marketing authorization in the EU expected for end of 2013/beginning of 2014. The proposed Phase 3 protocol will evaluate 194 patients in several European study sites. Additionally, Onalta shall be supplied to treatment centers participating in an approved compassionate use program. |
| C. | MIPI is interested in exclusively sourcing the centralized manufacturing of Onalta and its distribution in certain countries in Europe, the Middle East, North Africa, Russia and Turkey. The material shall be produced on a weekly basis by a batch process using the critical components [Y-90] Yttrium Chloride and a sterile pre-dispensed lyophilized preparation of DOTA-TOC in accordance with current Good Manufacturing Practices (cGMP). In order to avoid shipping and customs barriers to reliable and timely supply of the final drug product to clinical units, MIPI wishes to partner with an existing GMP compliant facility for the centralized production and distribution of radiolabeled [90Y]-Onalta. |
| D. | EZN operates a nuclear licensed site in Braunschweig, Germany, for the production and “cradle to grave” husbandry under ISO 9001 and ISO 13485 quality systems of a diverse range radioactive isotopes used in industrial and medical applications. The Braunschweig site includes an existing licensed radiopharmaceutical manufacturing facility where Yttriga (Y-90 Chloride), an EMEA approved “drug” is manufactured. |
| E. | MIPI desires that EZN plans, designs and constructs a commercial high throughput manufacturing facility suitable for the distribution of Onalta in the specified territories under compassionate use and following EMEA marketing authorization. Additionally, it is intended to provide a “provisional” cGMP compliant interim supply capability for the supply of Phase 3 included patients. |
NOW, THEREFORE, the parties agree as follows:
| 1. | DEFINITIONS |
| 1.1 | “Affiliated Company” shall mean either (a) a company which is at least majority owned or majority controlled by a Party hereto or which holds at least a majority interest or majority control in such Party; or (b) a parent company to one of the Parties hereto. |
| 1.2 | “Effective Date” shall mean the date of the last signature of this Agreement. |
3
| 1.3 | “Agreement” shall mean this Agreement together with all exhibits, schedules, and appendices attached to this Agreement, all as respectively amended, modified or supplemented by the Parties in accordance with the terms of this Agreement. |
| 1.4 | “Commercially Reasonable Effort” shall mean the efforts and resources customarily used in the industry by a company of similar size for a product with an equivalent sales and profit potential to the Final Product. |
| 1.5 | “Current Good Manufacturing Practices” or “cGMP” shall mean the Current Good Manufacturing Practices followed by the pharmaceutical industry and biotech firms to ensure that the products produced meet specific requirements for identity, strength, quality, and purity and as defined from time to time by the European Commission or other relevant Governmental Authority having jurisdiction over the development, manufacture or sale of the products in the Territory pursuant to its regulations, guidelines or otherwise. |
| 1.6 | “Final Product” shall mean Onalta, ready to use radiolabeled product. |
| 1.7 | “Y-90” shall mean Yttriga, a Yttrium-90 chloride solution with EMEA Marketing Authorization to be supplied cGMP compliant by EZN. |
| 1.8 | “Yttrium 90” shall mean Y90 chloride solution ordered by any Third Party other than MIPI and/or MIPI licensee and supplied by any Third Party other than EZN for the on-site labeling in treatment centers. |
| 1.9 | “Kit” shall mean the sterile pre-dispensed lyophilized preparation of DOTA-TOC to be supplied cGMP compliant by MIPI to EZN. |
| 1.10 | “Set” shall mean Y90 and Kit to be provided separately for the onsite labelling by radiopharmacists at treatment centers. |
| 1.11 | “Dose vial(s)” shall mean the Final Product with 120 mCi Y90 at calibration. |
| 1.12 | “Batch” shall mean a production batch of Final Product manufactured by EZN under this Agreement. |
| 1.13 | “EZN Technology” shall mean all EZN or its Affiliated Company(s) proprietary technology, including patents, copyrights, trademarks, know-how, techniques, methods, processes and trade secrets which is required for the purposes of performing the obligations of EZN under this Agreement and which is owned by EZN or its Affiliated Company(s), or which EZN is authorized to use, or which is licensed to EZN from third parties and which is in existence in the form of a written, description, prototype or can otherwise be demonstrated to be the property of EZN or its Affiliated Company(s), prior to the Effective Date. |
| 1.14 | “MIPI Technology” shall mean all MIPI proprietary technology, including patents, copyrights, trademarks, know-how, techniques, methods, processes and trade secrets which is required for the purposes of performing the obligations of MIPI under this Agreement and which is owned by MIPI, or which MIPI is authorized to use, or which is licensed to MIPI from third parties and which is in existence in the form of a written, description, prototype or can otherwise be demonstrated to be the property of MIPI, prior to the Effective Date. |
4
| 1.15 | “EMEA” shall mean the European Medicines Agency. |
| 1.16 | “Regulatory Authorities” shall mean the European Authority and/or the United States Authority and/or any other corresponding regulatory authority in any other geographic areas. |
| 1.17 | “AMG” shall mean the German Medicinal Products Act (Arzneimittelgesetz) as amended from time to time. |
| 1.18 | “Clinical Trials” shall mean Phase 3 human trials for clinical development of Onalta as approved by EMEA. |
| 1.19 | “Set Supply Phase” shall have the meaning set forth in Article 2.2. |
| 1.20 | “Interims Phase” shall mean the period in which EZN supplies Final Product for the clinical trials using a provisional cGMP compliant facility. |
| 1.21 | “Kit Supply Phase” shall have the meaning set forth in Article 2.2. |
| 1.22 | “Development Phase” shall mean the period commencing from the Effective Date until completion of the facility setup of the commercial high throughput production facility. |
| 1.22 | “Marketing Authorization” shall mean, with respect to a country in the Territory, the approval by the appropriate authority necessary for the commercialization of the Final Product in that country. Marketing Authorization shall not include the reimbursement approval. |
| 1.24 | “Facility” shall mean the Hot Cell(s) and Equipment to be constructed by EZN in its currently existing factory in Braunschweig, Germany, for the manufacture, dispensing, sterilization, testing, release, packaging and despatch of the Final Product. |
| 1.25 | “Provisional Facility” shall mean the assets already owned, to be purchased or manufactured by EZN for the supply of the Final Product for clinical trials during the lnterims Phase. |
| 1.26 | “Facility Program” shall mean Facility and Provisional Facility. |
| 1.27 | “Hot Cell(s)” shall mean the compatible isolator boxes to be purchased or manufactured by EZN for and on behalf of MIPI and installed in the currently existing factory in Braunschweig for the term of this Agreement unless EZN exercises the option under Article 9.3. |
| 1.28 | “Equipment” shall mean the moveable assets already owned, to be purchased or manufactured by EZN for the purpose of this Agreement, exclusive of the Hot Cell(s). |
| 1.29 | “Intellectual Property Rights” (IPR) shall mean all intellectual rights (including but not limited to) rights to inventions, patent rights, know-how, copyrights and design rights in any part of the world to the fullest extent and for the full period thereof (including without limitation any extensions, reversions and renewals) and all rights thereto and interests therein. |
5
| 1.30 | “Process” shall mean the process of formulation, preparation, labeling, dispensing into dose vials, sterilization, inspection and testing of Final Product initially transmitted by MIPI to EZN as set forth in Article 7. |
| 1.31 | “Scaled up Process” shall mean the process resulting from the development and improvement of the upon the Effective Date existing and initially transmitted Process advancing and optimizing the production capacity, manufacture and distribution of the Final Product. |
| 1.32 | “Specification(s)” shall mean those specifications for the Final Product set forth in Article 10.1 hereof. |
| 1.33 | “Packaging” means all primary containers, including bottles, cartons, shipping cases or any other like matter used in packaging or accompanying the Kit, Set and/or Final Product. |
| 1.34 | “Validation” shall mean the program by which documented evidence provides assurance that the Process/Scaled up Process will consistently produce the Final Product that meets Specifications and quality attributes, to the reasonable satisfaction of the appropriate Regulatory Authorities. |
| 1.35 | “Territory” shall mean those countries as of the Effective Date set forth in Exhibit A. |
| 1.36 | “Party” or “Parties” shall mean MIPI or EZN, or MIPI and EZN, whichever the context admits. |
| 1.37 | “Third Party” shall mean any Person or other entity other than MIPI, EZN or their respective Affiliates. |
| 2 | PURPOSE |
| 2.1 | The scope and object of the Agreement is to provide for the construction of a Facility at EZN’s manufacturing site in Braunschweig, Germany, for the exclusive manufacture and supply of Final Product during the term of this Agreement within the Territory. |
| 2.2 | Additionally, starting with the Effective Date of this Agreement, EZN will exclusively provide either for the supply of Sets for Y90 direct labeling by radiopharmacists at treatment centers in the Territory (Set Supply Phase) or single Kits for Yttrium 90 direct labeling by radiopharmacists at treatment centers in the Territory. (Kit Supply Phase). MIPI and MIPI licensee undertake to request Set supply and Kit supply only for the period until the Final Product can be delivered on a country-by country-basis for Clinical Trials, under compassionate use program and/or following Marketing Authorization. MIPI and MIPI licensee will apply for compassionate use and subsequent Marketing Authorisation in all countries of the EU and any other country within the Territory that duly requires such regulatory programs/approvals. It is further understood between the Parties that until Final Product is available, Set supply shall be the preferred method of supply. MIPI or MIPI licensee will communicate in writing in any materials provided to the treatment centers that Yttriga is the qualified active substance for the radioactive labeling with yttrium 90 chloride. MIPI and its licensee |
6
| agree that the Yttrium 90 to be used for the direct labeling by radiopharmacists at treatment centers will be delivered by duly qualified suppliers only, meaning that they meet the Specifications provided by MIPI which will have to be proven to MIPI or MIPI’s licensee. |
| 2.3 | It is anticipated that during the Term of this Agreement later duplication(s) of the Facility may be required by MIPI or its licensee at other sites in order to follow market demands. If MIPI has the intention to conduct a new site set-up within the Territory, EZN shall have a right of first refusal for recontracting with MIPI as facility set-up and contract manufacturing partner subject to the terms and conditions set forth herein. |
| 3 | TERM AND TERMINATION |
| 3.1 | The term of this Agreement shall commence upon the Effective Date and, unless terminated earlier pursuant to this Agreement, shall continue until the tenth anniversary of the Effective Date (“Term”). |
| 3.2 | Upon Forty-two (42) months following the Effective Date and for the following six (6) months, the Parties agree to meet in order to discuss, in good faith, the terms and conditions under which the Parties intend to continue the term of this Agreement beyond the fifth anniversary of the Effective Date (“Renegotiation Period”). If the Parties come to an agreement during the Renegotiation Period, then for the remaining five (5) years of this Agreement the renegotiated terms and conditions shall apply. In case the Parties will not reach an agreement, either Party has the right to terminate this Agreement giving to the other Party at least twelve (12) months written notice prior to the fifth anniversary of the Effective Date otherwise the Agreement will automatically continue for the remaining term on the terms and conditions set forth herein. |
| 3.3 | It is agreed between the Parties that in case of termination of the Agreement as set forth in Article 3.2, the manufacture and supply of Kit, Set and Final Product will continue on the terms and conditions as set forth in this Agreement for eighteen (18) months following the termination notice. |
| 3.4 | This Agreement may be terminated by either Party in the event of a material breach by the other Party of the terms and conditions hereof; provided, however, the other Party shall first give to the breaching Party written notice of the proposed termination of this Agreement (“Breach Notice”), specifying the grounds thereof. Upon receipt of such Breach Notice, the breaching Party shall have thirty (30) days to respond by curing such breach. If the breaching Party does not cure such breach within such period, the other Party may terminate the Agreement without prejudice to any other rights or remedies which may be available to the non-breaching Party. |
| 3.5 | Material breach of MIPI’s obligations under this Agreement shall mean and be limited to: (i) milestone payments for facility setup overdue for more than three (3) months, (ii) monthly payments overdue for more than three (3) months, (iii) payments for Final Product exceeding an accumulated amount of ***** overdue for more than three (3) month, (iv) funds held in the Escrow Account have not been made available to EZN for the purpose of decontamination and decommissioning of the Hot Cell(s) within three (3) month despite EZN’s notification |
*Confidential Treatment Requested*
7
| of commissioning of the high throughput facility, (v) breach of exclusivity clause, and (vi) violation of EZN’s right of first refusal and/or right of first negotiation. |
| 3.6 | Material breach of EZN’s obligations under this Agreement shall mean and be limited to: (i) EZN has failed, in any one contract year period, to fulfil more than four (4) orders of Kit, Set or Final Product consistent with the Specifications (a “Supply Breach”). It shall not be considered a Supply Breach in the event that (i) the failure to supply is attributable, in whole or in part, directly or indirectly, to MIPI or its licensee, (ii) EZN is able to supply an additional replacement of Kit, Set or Final Product meeting the Specifications in accordance with this Agreement within one (1) week of the delivery date of the originally scheduled order of Kit, Set or Final Product, or (iii) if the Kit, Set or Final Product failure is the result of conducting the Process under a deviation at the request of MIPI. Any failure by EZN to supply Kit, Set or Final Product due to maintenance, repairs and/or Force Majeure shall not be a material breach or Supply Breach under this Agreement. |
| 3.7 | Notwithstanding anything contained in this Agreement to the contrary, this Agreement may be terminated by either Party in the event the other Party files a petition in bankruptcy, is adjudicated a bankrupt, or files a petition or otherwise seeks relief under or pursuant to any bankruptcy, insolvency or reorganization statute or proceeding, or if a petition in bankruptcy is filed against it which is not dismissed within sixty (60) days or proceedings are taken to liquidate the assets of such Party which are not stayed within sixty (60) days. Any assets jointly owned by the two Parties including the Jointly Owned Arising IP shall become the property of the Party not seeking such relief. |
| 3.8 | Notice of Termination and Breach Notice are to be sent by registered letter. |
| 4 | REMEDIES UPON TERMINATION |
| 4.1 | If EZN terminates this Agreement, under Article 3, EZN, in addition to any claim for damages EZN may have, shall be entitled to: |
(i) retain all amounts paid by MIPI to EZN prior to such termination;
(ii) except for the Hot Cell(s), return to MIPI all the Equipment which is owned by MIPI and in EZN’s possession and for which MIPI has paid all amounts due to EZN pursuant to this Agreement, unless MIPI requests that EZN decommissions the Equipment by using the funds in the Escrow Account;
(iii) terminate all activities under this Agreement expeditiously so as to minimize costs incurred by MIPI therefore;
(iv) deliver all undelivered Kits or Dose Vials to MIPI, or destroy all undelivered Kits or Dose Vials, whichever MIPI may choose;
(v) immediately upon such termination, except as provided elsewhere in this agreement, terminate all licenses granted by EZN to MIPI under this Agreement which rights shall revert back to EZN; and
(vi) where applicable receive from MIPI written confirmation that the foregoing steps have been taken and that it has ceased using all patents data, information, technology, trade secrets and other intellectual property owned by EZN pursuant to this Agreement
8
MIPI shall further reimburse EZN for all reimbursable costs and work necessarily and properly incurred in relation to the orderly cessation of the work and sums owing but not invoiced prior to the effective date of any such termination by EZN under this Agreement. In addition, MIPI will if EZN so opts, either promptly transfer title of the Hot Cell(s) to EZN or allow the execution of the Decontamination and Decommissioning work by using the funds in the Escrow Account, whereupon MIPI shall have no further obligations under Article 9.4.
| 4.2 | If MIPI terminates this Agreement under Article 3, MIPI, in addition to any claim for damages MIPI may have, shall be entitled to: |
(i) within thirty (30) days of such termination at MIPI’s expense receive the Equipment and all related materials, in its then current condition (subject to decontamination);
(ii) exercise the option whether the Hot Cell(s) shall be returned by EZN to MIPI at its own expense or whether they shall be decontaminated and decommissioned by EZN by using the funds in the Escrow Account;
(iii) receive at MIPI’s expense all Kits and Dose Vials which have been ordered but not delivered and in possession of EZN;
(iv) immediately upon such termination, terminate all licenses granted by MIPI to EZN under this Agreement which rights shall revert back to MIPI; and
(v) receive from EZN written confirmation that the foregoing steps have been taken and that it has ceased using all patents data, information, technology, trade secrets and other intellectual property owned by MIPI pursuant to this Agreement.
If MIPI terminates this Agreement, MIPI shall reimburse EZN for all reimbursable costs and work necessarily and properly incurred in relation to the orderly cessation of the work and sums owing but not invoiced prior to the effective date of any such termination by MIPI under this Agreement. In addition, MIPI will if EZN so opts, either promptly transfer title of the Hot Cell(s) to EZN or allow the execution of the Decontamination and Decommissioning work by using the funds in the Escrow Account, whereupon MIPI shall have no further obligations under Article 9.4.
| 5 | EXCLUSIVITY AND NON-COMPETE |
| 5.1 | EZN shall not, and shall ensure that its Affiliates do not, directly or indirectly, manufacture Final Product for any person or entity for sale in the Territory other than MIPI or its licensee. MIPI shall not, and shall ensure that its Affiliates and licensees do not, directly or indirectly, manufacture Final Product destined for sale in the Territory. |
| 5.2 | MIPI shall exclusively order from EZN all of MIPI’s, its Affiliates’ and licensees’ Kit, Set and Final Product destined for the Clinical Trials, Compassionate Use and Commercial Supply in the Territory. |
| 5.3 | During the term of this Agreement and for a period of two (2) years following the termination or expiration of this Agreement, EZN shall not manufacture and/or sell Final Product or radio-labelled therapeutic somatostatin analogs with a somatostatin subtype receptor binding behaviour similar to Final Product. For the avoidance of doubt, diagnostic somatostatin analogs as well as radionuclides either in chemical or |
9
| pharm-grade form, and radiochemical and radiopharmaceutical synthesis, labelling and dispensing technology devices, are not subject of this Non-compete irrespective of their final use with the end user. |
| 5.4 | It is agreed between the Parties that in case of termination of this Agreement in accordance with Article 3.2 of this Agreement, the Non-compete term is considered to start on the fifth anniversary of the Effective Date irrespective any manufacturing and/or supply of Kit, Set or Final Product extending the fifth anniversary of the Effective Date according to Article 3.3. |
| 5.5 | In the event that MIPI or MIPI licensee decide to withdraw from the manufacture, marketing and distribution of Final Product for whatever reason, EZN’s obligation to non-compete shall cease. |
| 6 | DEVELOPMENT AND KIT/SET SUPPLY PHASE |
| 6.1 | The Development Phase shall (a) optimise the Process and establish the Scaled up Process for the cGMP manufacture of the Final Product, (b) provide for the planning, design, construction, qualification and validation of the Provisional Facility, (c) provide for the planning, design, construction, qualification and validation of the Facility and (d) provide for the planning, design and construction of IAEA compliant Type A packaging assembly for international transportation (Development Phase Projects). |
| 6.2 | Upon the Effective Date, EZN will prioritise the set up of a Provisional Facility and construction of transportation assembly needed for the initial Clinical Trial and Compassionate Use demand of Final Product which technical and regulatory completion is anticipated within 3 to 6 months upon Effective Date. |
| 6.3 | The completion of the Development Phase Projects may be reasonably delayed to the extent that such activities are premised on the work or provision of data, information or technology by the other Party which such other Party does not provide on a timely basis. Each Party will undertake commercially reasonable efforts in order to carry out their respective obligations and responsibilities with regards to the timeline of the Project Plan attached hereto as Exhibit B (“Project Plan”). |
| 6.4 | The Parties acknowledge and agree that Exhibit B may be amended during the course of the Development Phase to accommodate unforeseen events and results beyond the reasonable control of the Parties. All such changes to Exhibit B shall be made by written agreement of the Parties. |
| 6.5 | Progress of the Development Phase Projects will be reported by EZN via milestone reports and, upon request of MIPI, via regular conference calls at agreed preset times and quarterly review meetings. Both Parties will appoint project managers for the purpose of reviewing the status of the project and to assess the fulfilment of milestones. |
| 6.6 | Within thirty (30) days upon the Effective Date, MIPI will provide EZN free of charge with a reasonable minimum Kit inventory stock ensuring compatibility with expected purchase volumes of Kits and Sets to be delivered for Clinical Trials and Compassionate Use until the start of the Initial Phase. MIPI undertakes to inform EZN, within fifteen (15) days upon the Effective Date at the latest, of the storage conditions and |
10
| handling requirements to enable EZN to take all appropriate measurements and precautions for a duly transfer of Kits from MIPI to EZN. MIPI acknowledges that for the validation of cooling equipment for the Kit storage (refrigerators), EZN will need a lead time of at least eight (8) working days. |
| 6.7 | Prior to the first dispatch of a Kit and/or Set, MIPI and EZN will mutually determine and establish the process for packaging and distribution of the Kits and/ or Sets to the final destination. |
| 6.8 | For the ordering and shipment of the Kits and/or Sets the provisions of Article 11 shall apply accordingly. |
| 6.9 | In case the storage/shelf life of the Kits delivered by MIPI to EZN will pass before the Kits have been ordered by MIPI, MIPI will at its own expense be responsible for the Kits’ redemption and disposal and adequate replacement deliveries. |
| 6.10 | Additional development activities outside the scope of this Agreement will be agreed upon and billed separately by the Parties. |
| 7 | TECHNOLOGY TRANSFER |
| 7.1 | It is understood between the Parties that time is of essence in this project and that delays due to slow transfer of MIPI Technology and/or EZN Technology must be avoided. |
| 7.2 | The Parties undertake to use commercially best efforts to exchange all necessary Technology in a timely manner. MIPI and EZN will immediately upon the Effective Date schedule for a mutual meeting at the Braunschweig facility during which the Parties will disclose and exchange all information necessary for a successful and timely completion of the Development Phase and the Set-up of the Facility. If necessary, both Parties will, prior to the onsite meeting, exchange an agenda outlining the questions that should be addressed. It is agreed upon between the Parties that such a onsite meeting will take place within two (2) weeks following the Effective Date. |
| 7.3 | If and to the extent necessary, MIPI shall duly transmit additional documentation and provide technical training and consulting on the Process. MIPI will provide EZN within two (2) weeks following the Effective Date with the name and details of a contact person responsible for all questions and issues relating to technology transfer, documentation and training. |
| 8 | CONSTRUCTION OF FACILITY – FACILITY PROGRAM COST |
| 8.1 | Subject to successful completion of the relevant planning and design of the Facility Program to the satisfaction of MIPI, EZN shall conduct the Facility Program at its site in Braunschweig, Germany to carry out the manufacture of Final Product. EZN will use its commercially reasonable best efforts to complete the Facility Program in accordance with the Project Plan attached herewith as Exhibit B. Exhibit B may only be modified as agreed in writing by the Parties. |
| 8.2 | The actual capital cost of the Facility Program has been calculated on a time and materials basis. The budgeted capital cost for performance of the Facility Program by EZN is estimated at the Effective Date to be ******, |
*Confidential Treatment Requested*
11
| inclusive of contingency and EZN administration fees. Any cost in excess of the estimated budgeted capital cost shall be subject to the prior written authorization of MIPI. |
| 9 | ASSET OWNERSHIP AND ENVIRONMENTAL INDEMNITY OF FACILITY |
| 9.1 | EZN will purchase or manufacture, on behalf of MIPI, the Hot Cell(s) and Equipment, which will be installed in the Facility as described in the Project Plan (Exhibit B). Upon completion of the purchase or manufacture of the Hot Cell(s) and Equipment for the Facility, a warranty xxxx of sale in a form reasonably acceptable to MIPI, shall be executed and delivered to MIPI transferring full title to such Hot Cell(s) and Equipment dedicated to MIPI’s requirements free and clear of all liens, claims, or encumbrances. Subject to MIPI’s obligations to transfer ownership of the Hot Cell(s) and Equipment to EZN under circumstances as set forth in this Agreement, MIPI shall at all times hold all right, title and interest in the Hot Cell(s) and Equipment; provided, however, that during the term of this Agreement, use thereof shall exclusively be granted to EZN for the purposes of producing Final Product at the Braunschweig site. |
| 9.2 | EZN represents and warrants that during the term of this Agreement the Hot Cell(s) and Equipment shall be used for the purpose of producing Final Product only. |
| 9.3 | In partial consideration of the services to be performed hereunder by EZN and in consideration of the payment of ******, the sufficiency of which is hereby acknowledged, on the earlier of the expiration or termination of this Agreement by MIPI (for whatever reason other than the default of EZN), MIPI agrees, at EZN’s option, to transfer all of MIPI right, title and interest in and to the Hot Cell(s) and Equipment to EZN. |
| 9.4 | Upon expiration or termination of this Agreement, the Hot Cell(s) and Equipment have to be decontaminated and decommissioned in accordance with the applicable laws and regulations. Decontamination and decommissioning shall be the financial responsibility of MIPI unless agreed otherwise in accordance with this Agreement. At the time of commissioning of the Facility, MIPI agrees to furnish to EZN a lump sum deposit in the amount of ******. This amount shall be deposited by MIPI into a German Escrow Account in the name of EZN issued by a reputable bank or financial institution with a rating reasonably satisfactory to EZN (the “Escrow Account”). A copy of proof of deposit is to be provided to EZN within thirty (30) days at the latest following commissioning of the Facility. Failure of MIPI to deposit said amount or proof the deposit shall be a material breach and as such reason for termination of this Agreement in accordance with Article 3.4 of this Agreement. |
| 9.5 | If EZN does not exercise its option, or fails to be allowed to exercise the option due to EZN’s default under this Agreement, to own the Hot Cell(s) and the Equipment, then upon expiration or termination of this Agreement, the funds established by MIPI in the Escrow Account shall be made available to EZN for decontamination and decommissioning of the Hot Cell(s) and any other Equipment used for manufacture of Final Product, prior to their removal by MIPI from the Braunschweig site, in accordance with this Agreement. Decontamination, decommissioning and removal of the Hot Cell(s) and Equipment shall take place within six (6) months following expiration or termination of this Agreement. |
*Confidential Treatment Requested*
12
| 9.6 | If EZN exercises the option to own the Hot Cell(s) and the Equipment, then the funds held in the Escrow Account shall be made available to EZN for the purpose of decontamination and decommissioning of the Hot Cell(s) ad any other Equipment or forming a reserve for future decommissioning. If the Escrow Account has been made available to EZN, then MIPI shall have no further obligations under this Article. |
| 9.7 | Refund to Decommissioning Bond. If EZN’s revenues for Kits, Sets and Final Product (excluding shipment and packaging costs) aggregated until the fourth anniversary of completion of the high throughput facility exceed ****** then the MIPI funds held in the EZN Escrow Account shall be reduced by ******. If EZN’s revenues for Kits, Sets and Final Product (excluding shipment and packaging costs) aggregated until the fourth anniversary of completion of the high throughput facility exceed ****** then the MIPI funds held in the EZN Escrow Account shall completely convert back to MIPI in the form that MIPI cancels the Escrow Account. |
| 9.8 | Except as may be provided in accordance with this Agreement, EZN shall during the term of this Agreement not use or permit any Third Party to use the Hot Cell(s) and Equipment. |
| 9.9 | Until MIPI has transferred ownership of the Hot Cell(s) and Equipment as set out in this Agreement (the “Transfer Date”), EZN is granted a security interest (Sicherungsübereignung) in and to the Hot Cell(s) and Equipment. The security interest in the Hot Cell(s) and Equipment shall be perfected by possession of the Hot Cell(s) by EZN and shall be effective as of the date of commencement of installation of such Hot Cell(s) and Equipment and shall serve as collateral for the obligations and responsibilities of MIPI under this Agreement. MIPI shall execute all documents reasonably required to provide the above mentioned security interest in and to the Hot Cell(s) and Equipment to EZN. |
| 9.10 | It is understood by the Parties that ownership of the Provisional Facility used during the Interims Phase will be and remain with EZN including the responsibility for decontamination and decommission. |
| 10 | MANUFACTURE AND SUPPLY OF FINAL PRODUCT |
| 10.1 | EZN agrees to |
(a) manufacture all Y-90 for the Final Product,
(b) produce the Final Product,
(c) test and release the Final Product,
(d) store Final Product until delivery, and
(e) deliver Final Product to any person or entity as identified to EZN by MIPI,
in each case in accordance with
(i) the relevant Specifications attached hereto as Exhibit C (the “Specifications”),
*Confidential Treatment Requested*
13
(ii) the terms and conditions of the Quality Agreement attached hereto as Exhibit D (the “Quality Agreement”). The Parties agree to finalize and execute the Quality Agreement within 30 days of the Effective Date.
(iii) all applicable laws, including, without limitation, health, safety and environmental laws,
(iv) the terms and conditions of this Agreement.
| 10.2 | EZN shall promptly inform MIPI of decisions about, or changes to, the facilities used in the manufacturing of the Final Product. |
| 10.3 | EZN shall not make changes to Process and/or Scaled up Process without MIPI’s prior written consent and otherwise as provided in the Quality Agreement. |
| 10.4 | After the Facility is installed, EZN shall maintain such Facility, Hot Cell(s) and Equipment in satisfactory operating condition, as required to enable EZN to manufacture Final Product to Specification in accordance with the Scaled up Process and all other applicable laws, regulations, rules or orders. Routine repairs, preventive maintenance and service contracts for the Facility, Hot Cell(s) and Equipment shall be arranged by EZN. Routine repairs are repairs to a given asset entailing expenditures of the lesser of ******. |
| 10.5 | In the event of any conflict between or change of the applicable laws, regulations, rules or orders, EZN will notify MIPI of such conflict and the Parties shall act in good faith to resolve such conflict or change. Any repair, change, scale up or remodelling of the facility, Hot Cell(s) and/or equipment that are not due to normal tear and wear and/or necessary under routine repairs and preventive maintenance shall be the sole responsibility of MIPI. If requested, EZN will provide MIPI with a cost estimate on such activities. |
| 11 | ORDERS AND SHIPMENTS |
| 11.1 | During the term of this Agreement, MIPI or MIPI’s licensee will forward orders to EZN by facsimile (or other suitable means) at least ten (10) Business Days in advance of intended shipment. This lead time might be shorter in case of Kit orders only or might be longer in case of intended delivery of Set and/or Final Product outside the EU. Orders shall include at least the required quantities, identity of the recipient, requested delivery dates and destination. Delivery as directed by MIPI or MIPI’s licensee shall be ex-Works EZN’s facility in Braunschweig, Germany. Risk and title for the Set and/or Final Product shall pass to MIPI or MIPI’s licensee at point of delivery to the respective carrier. |
| 11.2 | Prior to the first shipment to any Third Party site, MIPI or its licensee shall obtain and forward to EZN from such Third Party its license evidencing proper legal authority for the receipt and possession of the Kit, Set and/or Final Product (Umgangsgenehmigung). If and to the extent necessary, MIPI or MIPI’s licensee shall further obtain all approvals, licenses and permits required to import Kit, Sets and/or Final Product into territories directed by MIPI or its licensee. |
*Confidential Treatment Requested*
14
| 11.3 | EZN shall deliver Kit, Set and/or Final Product in accordance with the quantities and requested delivery date(s) specified in the relevant order. EZN will pack the Kit, Set and/or Final Product for shipment and storage in accordance with the applicable Specifications given by MIPI. |
| 11.4 | If requested by MIPI or MIPI licensee, EZN shall make shipping arrangements with carriers from the ex-Works point to the delivery site. All transportation and packaging costs incurred to deliver Kit, Set and/or Final Product ordered by MIPI or MIPI licensee will be separately charged to and borne by MIPI. |
| 11.5 | If either Party or its designee discovers that a Batch and/or Dose Vial does not meet the Specifications, then the discovering Party shall promptly communicate in writing with the other Party to determine a mutually agreed course of action. With respect to any such Batch and/or Dose Vial which do not meet Specifications as a result of shortcomings in process or parameters under the direct control of EZN, then EZN will promptly replace such Batch and/or Dose Vial at no additional cost to MIPI. |
| 12 | PAYMENTS |
| 12.1 | The purchase prices for the Y-90, Final Product and Kit handling shall be as set forth in Exhibit E (“Product Price Table”). |
| 12.2 | Payment for the development and set-up of the Provisional Facility and Facility shall be as set forth in Exhibit F (“Milestone Schedule”). Upon completion of a milestone calling for payment, EZN shall invoice MIPI. |
| 12.3 | Transportation costs will be charged separately at rates to be calculated individually for each country in the Territory. Upon request of MIPI, EZN will forward a cost estimate of transportation costs to MIPI prior to delivery into the respective country. |
| 12.4 | Prices for the still to be designed and manufactured IAEA compliant Type A packaging devices for international transportation will be mutually agreed upon between the Parties following the first design and cost estimates provided by EZN to MIPI during the Development Phase. It is agreed that EZN will provide MIPI with a price for a separate shielded packaging container and MIPI will order and acquire, from EZN, prior to the start of the Interims phase the amount of shielded packaging containers MIPI considers reasonable to meet its demands. Any orders shall be forwarded to EZN with appropriate lead time. In case MIPI will place no orders with EZN, MIPI will reimburse EZN for the costs incurred regarding the planning, design and price calculations on man-hours basis. |
| 12.5 | Except as otherwise provided herein, all invoices shall be paid within forty-five (45) days upon date of invoice. All payments, costs and prices included in this Agreement are exclusive VAT and, unless otherwise specified, shall be in Euros. |
| 12.6 | For the term of this Agreement, EZN shall, additionally, be entitled to monthly payments of ******. All Monthly Payments shall be paid in advance, on the first day of each calendar month adding |
*Confidential Treatment Requested*
15
| the legally applicable VAT, currently nineteen percent (19%). In case this Agreement commences or expires later than the first day of a calendar month, then the monthly payments shall be calculated on a pro rata basis assuming that a month consists of thirty (30) days. |
| 12.7 | It is understood that the costs of operating the Facility can be suddenly increased as a result of direct or indirect Regulatory changes -such as via the costs of conditioning and disposal of radioactive arisings. Should such significant changes arise at any time after the Effective Date then EZN shall immediately inform MIPI and the Parties shall agree a price increase based upon EZN’s actual rise in costs. |
| 13 | LICENSES, PATENTS AND TECHNOLOGY |
| 13.1 | MIPI Technology shall be and remain the exclusive property of MIPI. MIPI hereby grants to EZN a non-exclusive, non-transferable, non-sublicensable, royalty free license during the term of this Agreement to use the MIPI Technology owned by MIPI for the sole purpose of assisting EZN in carrying out its obligations set out in this Agreement. To the extent the MIPI Technology includes third party technology exclusively controlled but not actually owned by MIPI (the “****** Technology”) exclusive of the Novartis patents which are sublicensed hereunder, then no sublicense rights as to the ****** Technology is granted to EZN but MIPI covenants that EZN shall have the right to use any Intellectual Property Rights in such ****** Technology in the performance of this Agreement, and MIPI will not assert any Intellectual Property Rights in such ****** Technology against EZN while EZN is an authorized manufacturer. MIPI shall hold EZN harmless of all damages and costs as a result of ****** asserting that the ****** Technology is infringed by the manufacture, marketing or distribution of Kit, Set or Final Product. |
| 13.2 | EZN Technology shall be and remain the exclusive property of EZN. EZN hereby grants to MIPI a non-exclusive, non-transferable, non-sublicensable royalty free license during the term of this Agreement to use EZN Technology for the sole purpose of assisting MIPI in carrying out its obligations set out in this Agreement. |
| 13.3 | All Intellectual Property Rights in any improvements conceived, written, created, developed or first reduced to practice in and related to the performance of this Agreement that relates to edotreotide and/or all Intellectual Property Rights in the Scaled up Process (the “Joint Improvements”) will be jointly owned by MIPI and EZN, irrespective of who conceives, writes, creates, develops or first reduces to practice any such Joint Improvements, and both MIPI and EZN shall, unless otherwise stated in this clause, have free use of such Joint Improvements during the Term of this Agreement or, if applicable, for as long as EZN is authorized to manufacture hereunder. Notwithstanding the above, neither Party shall during the term of this Agreement utilize such Joint Improvements in conjunction with any Third Party unless otherwise agreed upon between the Parties. Upon expiration or termination of this Agreement the Parties agree to the following restrictions on the use of Joint Improvements: MIPI shall only utilize any Joint Improvements in connection with edotreotide and MIPI’s other proprietary compounds publicly known as of the effective date hereof; EZN shall not utilize any Joint Improvements in connection with edotreotide other than with Novartis or any other Third Party being duly authorized by MIPI or Novartis to use edotreotide. |
*Confidential Treatment Requested*
16
| 13.4 | In case of termination of this Agreement by MIPI according to 3.2 or in case of termination of this Agreement by EZN due to a default of MIPI according to Article 3.5, MIPI shall pay to EZN a lump sum payment of ****** for the assignment of EZN’s Intellectual Property Rights in all Joint Improvements to MIPI. The payment shall be due at the latest within thirty (30) days following receipt of the respective termination notice. With due payment of the lump sum, EZN assigns and transfers all its Intellectual Property Rights in the Joint Improvements to MIPI who then shall be the sole owner of all Intellectual Property Rights in the Joint Improvements. For the avoidance of doubt and notwithstanding the full ownership of MIPI in all Intellectual Property Rights in the Joint Improvements under this paragraph, EZN shall have the right to freely use the Intellectual Property in the Joint Improvements for as long as EZN is authorized to manufacture. |
| 13.5 | EZN will use best efforts to preserve any Intellectual Property Rights arising out of the performance of the Agreement and to perfect rights in any Intellectual Property Rights to which MIPI can possibly be entitled, have a right to or interest in. It is acknowledged by the Parties that any such obligations will be subject to and be limited by the copyright and intellectual property rights of Germany, in particular the Employee’s Inventions Act (Arbeitnehmererfindungsgesetz) for claims to inventions of employees arising out of the performance of the Agreement. |
| 14 | REGULATORY MATTERS |
| 14.1 | It shall be the responsibility of MIPI or its licensee to file, obtain and maintain such licenses, registrations, listings, authorizations and approvals as the EMEA or any other competent Regulatory Authority may require to enable the application, marketing and distribution of the Kit, the Set and Final Product. |
| 14.2 | EZN shall be responsible for obtaining and maintaining all necessary facility licenses, registrations, authorizations and approvals for the manufacture, handling, storage, packaging and release of the Sets and Final Product in Germany. |
| 14.3 | Each Party shall promptly notify the other Party in case it learns of new regulatory requirements relevant to the manufacture, marketing and/or distribution of Set and/or Final Product. In such case, the Parties will use mutual commercially best efforts to comply with such requirements. |
| 14.4 | MIPI shall, as mutually agreed in the Quality Agreement (Exhibit D) be entitled to access to the Facility for the purpose of observing any Process or to audit the Facility for compliance with MIPI’s Specifications and other regulatory requirements. It is understood between the Parties that such right shall not extend to any licensee, designee or any other Third Party unless EZN and any such licensee, designee or other Third Party have entered into a separate Agreement providing for terms and conditions including but not limited to Disclosure, and Confidentiality, satisfactory to EZN. |
| 14.5 | In case of discrepancies between this Agreement and the Quality Agreement (Exhibit D), that relate to regulatory matters, the Quality Agreement shall prevail. |
*Confidential Treatment Requested*
17
| 15 | DATA MANAGEMENT |
Either Party shall retain all essential documents or data for as long as required by the applicable laws and guidelines. Essential documents shall be archived in a way that ensures that they are readily available, upon request, to the competent authorities. The Parties shall install security measures that prevent unauthorized access to archives.
| 16 | DISCLOSURE OF TECHNOLOGY |
Except as otherwise set out in this Agreement, it is agreed that disclosure of data, information or technology by MIPI or EZN to the other Party shall not, except to the extent granted herein, constitute any grant, option or license under any patent, technology or other rights held by MIPI or EZN.
| 17 | RIGHT OF FIRST REFUSAL |
| 17.1 | If MIPI has the intention to propose the initiation of a new manufacturing site in the Territory to a Third Party, EZN shall have a right of first refusal for a new contract with MIPI as facility set-up and contract manufacturing partner. |
| 17.2 | In the event MIPI desires to accept a bona fide Third Party offer for the initiation of a new manufacturing site in the Territory, MIPI shall promptly deliver to EZN written notice of the intended disposition (“Disposition Notice”) and the basic terms and conditions thereof. |
| 17.3 | EZN shall, for a period of sixty (60) days following receipt of the Disposition Notice, have the right to accept the facility set-up and manufacturing upon the same terms and conditions specified in the Disposition Notice. Such right shall be exercisable by written notice (the “Exercise Notice”) delivered to MIPI prior to the expiration of the sixty days exercise period. If such right is exercised, then MIPI and EZN shall enter into a new Facility Set-up and Manufacturing Agreement upon the terms set forth in the Disposition Notice not more than thirty (30) days after the delivery of the Exercise Notice. |
| 17.4 | In the event the Exercise Notice is not received by MIPI within sixty (60) days of the Disposition Notice, EZN shall be deemed to have waived its right of first refusal. |
| 18 | RIGHT OF NEGOTIATION |
| 18.1 | If MIPI has the intention to propose the initiation of a new manufacturing site in any other geographic area to a Third Party, then MIPI undertakes to inform EZN immediately of such intention and EZN shall have a right of negotiation with MIPI as facility set-up and contract manufacturing partner. |
| 18.2 | If MIPI or its licensee has the intention to offer radiochemical and radiopharmaceutical synthesis labelling and dispensing technology devices to any treatment sites purchasing Kit, Set or Final Product, MIPI or its licensee will recommend EZN or its respective affiliates as supplier for such devices. |
18
| 19 | CONFIDENTIALITY |
| 19.1 | During the term of this Agreement and for a period of five (5) years thereafter, each Party hereto shall maintain in confidence all technology including MIPI Technology, EZN Technology, Jointly Owned Arising IP and know-how, data, processes, methods, techniques, formulas, test data and other information disclosed to such Party by the other Party whether or not it is identified as “Confidential Information” by the disclosing Party (collectively “Confidential Information”). Each Party shall necessarily be free to disclose its own Technology under the terms of its own established process for the disclosure of its Confidential Information. If either Party needs to disclose Confidential Information pertaining to the manufacturing process covered by this Agreement to a Third Party then this shall be accommodated by the creation of a three way Confidential Information disclosure agreement. This obligation of confidentiality shall not apply to the extent that it can be established by the Party in receipt of such Confidential Information, that the information: |
| i) | was already known to the receiving Party at the time of disclosure; |
| ii) | was generally available to the public or otherwise part of the public domain at the time of its disclosure; |
| iii) | became generally available to the public or otherwise part of the public domain after its disclosure to the receiving Party through no act or omission of the receiving Party; |
| iv) | was disclosed to the receiving Party by a Third Party who had no obligation to restrict disclosure of such information; or |
| v) | was independently developed by the receiving Party without any use of Confidential Information of the disclosing Party. |
Notwithstanding the foregoing, MIPI and EZN may both disclose Confidential Information to an Affiliate or permitted assign provided that the Affiliate or permitted assign is bound by confidentiality to the same extent as MIPI and EZN hereunder. The Party disclosing Confidential Information to such Affiliate or permitted assign shall be liable for any unauthorized use or disclosure of the Confidential Information by the Affiliate or permitted assign.
| 20 | NOTICES |
All notices and other communications hereunder shall be sent with reference to the contract name and number:
| To MIPI: | ||
| Name: | Xxxx Xxxxxxx, General Counsel | |
| Address: | Molecular Insight Pharmaceuticals, Inc. | |
| 000 Xxxxxx Xxxxxx | ||
| Xxxxxxxxx, XX 00000 XXX | ||
| E-mail address: | xxxxxxxx@xxxxxxxxxxxxxxxx.xxx | |
| Phone: | 000-000-0000 | |
| Fax: | 000-000-0000 | |
19
| To EZN: | ||
| Name: | Xx. Xxxxx Xxxx | |
| Address: | Xxxxxx-Xxxxxxx-Str.10 | |
| E-mail address: | xxxxx.xxxx@xxxx.xx | |
| Phone: | x00 00 000000 000 | |
| Fax: | x00 00 000000 000 | |
| 21 | FORCE MAJEURE |
Neither party shall be liable to the other for failure to perform or delay in performing its obligations under this Agreement by virtue of the occurrence of an event of Force Majeure. In the event such Force Majeure affecting either party continues for more than ninety (90) days the party not subject of the Force Majeure may, upon thirty (30) days written notice terminate this Agreement. In the event such Force Majeure affecting either party continues for more than six (6) months either party, may upon thirty (30) days written notice terminate this Agreement. “Force Majeure” shall mean an occurrence arising from unforeseen circumstances beyond a party’s reasonable control which prevents, delays or interferes with the performance by such party of any of its obligations hereunder including without limitation an event that occurs by reason of any act of God, flood, power failure, fire, explosion, casualty or accident, failure of suppliers or usual suppliers to have available for supply sufficient raw materials, equipment or machinery, or war, revolution, civil commotion, acts of public enemies, act of terrorism, blockage or embargo, government acts or regulations, prohibitions or interventions, interruption of or delay in transportation, strike or labor disruption. In the event of Force Majeure, the party affected shall promptly notify the other and shall exert commercially reasonable efforts to eliminate, cure or overcome such event and to resume performance of its obligations.
| 22 | BACK-UP |
Upon completion of the high throughput facility at the latest, the Parties will enter into negotiations regarding the set-up of a back-up manufacturing facility for the unlikely case of unforeseen manufacturing stops or interruptions at EZN’s Braunschweig facility. MIPI and EZN will act in good faith to negotiate, complete and enter into a definite Back-up Facility Agreement.
EZN undertakes to enter into back-up cooperations to assure for a secondary supply of Yttrium 90 for the unlikely case of Y-90 shortages at the Braunschweig facility.
20
| 23 | INDEMNIFICATION AND INSURANCES |
| 23.1 | MIPI Insurance |
From the Effective Date of this Agreement and for as long as MIPI continues to make, sell, licence or otherwise commercialize the Kit, Set and/or Final Product, MIPI shall maintain in force and effect all insurance normally associated with the activities contemplated under this Agreement and in doing business generally, including but not limited to Third Party insurance, product liability insurance, general liability, business interruption, property and casualty insurance. Such insurances shall be issued by a reputable insurance company with a rating satisfactory to EZN. The product liability insurance shall (a) insure against damages resulting from or caused by (or claimed to be resulting from or caused by) the operation or use of any Kit, Set and/or Final Product manufactured, marketed or distributed by MIPI, and (b) shall have coverage limits of not less than ****** per occurrence and ****** in the annual aggregate. Upon request of EZN, MIPI will deliver to EZN copies of all policies effecting such insurance with a certificate (in English) of MIPIs insurance broker stating that all premiums then due have been paid.
The sponsor of the Clinical Trial shall establish separate insurance cover for the risks associated with the period of clinical trials and compassionate use as required by the pharmaceutical laws of the countries in the Territory. From the start of the commercial phase of this Agreement and for as long as MIPI or its licensee is marketing and distributing the Final Product under its name, MIPI or its licensee shall establish separate insurance cover for the risks associated with the administration of a medicinal product intended for human use as provided for in §§ 94, 88 AMG for the marketing and distribution in Germany or any comparable insurance required for the marketing and distribution in other countries in the Territory. MIPI will be liable towards EZN for any failure in establishing and duly maintaining such insurances.
| 23.2 | EZN INSURANCE |
From the Effective Date of this Agreement and for as long as MIPI continues to make, sell, licence or otherwise commercialize the Kit, Set and/or Final Product with the participation of EZN, EZN agrees to maintain in force and effect all insurance normally associated with the activities contemplated under this Agreement and in doing business generally, including but not limited to insurances covering loss or damage to:
| (i) | the Facility; |
| (ii) | any asset owned by MIPI in the possession of EZN under this Agreement; |
| (iii) | EZN’s facility located at Braunschweig, Germany. |
In addition, from the Effective Date of this Agreement and for as long as EZN continues to make and sell to MIPI the Y-90, Set and/or Final Product, EZN shall maintain in force and effect product liability insurance issued by a reputable insurance company with a rating reasonably satisfactory to MIPI. Such insurance shall (a) include coverage insuring against Damages resulting from or caused by (or claimed to be resulting from or caused by) the operation or use of the Y-90, Set and/or Final Product manufactured,
21
* Confidential Treatment Requested*
assembled and dispatched by EZN, and (b) shall have coverage limits of not less than ****** per occurrence and ****** in the annual aggregate. Upon request of MIPI, EZN will deliver to MIPI copies of all policies affecting such insurance with a certificate (in English) of EZN’s insurance broker stating that all premiums then due have been paid.
Each Party will notify the other without delay in the event that the cover is (or is to be) cancelled or made subject to a material change. The insurance coverages hereunder shall limit the liabilities of the Parties imposed by this Agreement.
| 23.3 | WARRANTY/LIABILITY EZN |
EZN shall not have any other obligations towards MIPI than those arising from the services described herein and shall therefore have no other liability towards MIPI than arising from default of such obligations.
In case of defects occurring during the performance of this Agreement, EZN is obligated to remedy such defects or to supply services or products free from defects as set forth in Article 3 of this Agreement. MIPI shall not have the option to terminate the Agreement unless the removal of defects fails or does not take place as set forth in Article 3 of this Agreement.
| 23.4 | WARRANTY/LIABILITY MIPI |
MIPI shall not have any other obligations towards EZN than those arising from the services described herein and shall therefore have no other liability towards EZN than arising from default of such obligations.
MIPI hereby warrants to EZN that all insurances necessary and appropriate for the performance of this Agreement have been issued and coverage has been paid prior to the first supply.
| 23.5 | INDEMNIFICATION BY MIPI |
MIPI agrees to indemnify, defend and hold EZN and its Affiliates and their respective directors, officers, employees and agents, harmless from and against any damages, claims, liabilities and expenses (including, but not limited to, reasonable attorney’s fees) resulting from any third party claims or suits (“General Claims Against EZN”) arising out of (a) MIPI’s, MIPI licensee’s or any other third party’s development, manufacture, use, handling, shipping, marketing, sale, distribution or other disposition of or treatment with Kit, Set or Final Product (b) MIPI’s breach of any of its material obligations, warranties or representations hereunder, (c) MIPI’s negligent acts or omissions or wilful misconduct, or (d) any failure of the Kit to meet applicable specification. Notwithstanding the foregoing, MIPI will not be required to indemnify, defend and hold EZN and its Affiliates and their respective directors, officers, employees and agents harmless from and against any General Claims Against EZN to the extent that such claims arise out of (i) EZN’s breach of any of its obligations, warranties or representations hereunder; (ii) EZN’s negligent acts or omissions or wilful misconduct; (iii) any failure of EZN to manufacture, handle, store, package and dispatch of Kit, Set or Final Product inconsistent with the Specifications, the Quality Agreement and the terms and conditions of this Agreement.
* Confidential Treatment Requested *
22
| 23.6 | INDEMNIFICATION BY EZN |
EZN agrees to indemnify, defend and hold MIPI and its Affiliates and their respective directors, officers, employees and agents, harmless from and against any damages, claims, liabilities and expenses (including, but not limited to, reasonable attorney’s fees) resulting from any third party claims or suits (“General Claims Against MIPI”) arising out of (a) EZN’s failure to manufacture, handle, store, package and dispatch of Kit, Set or Final Product inconsistent with the Specifications, the Quality Agreement and the terms and conditions of this Agreement (b) EZN’s breach of any of its material obligations, warranties or representations hereunder; or (c) EZN’s negligent acts or omissions or wilful misconduct; Notwithstanding the foregoing, EZN will not be required to indemnify, defend and hold MIPI and its Affiliates and their respective directors, officers, employees and agents harmless from and against any General Claims Against MIPI to the extent that such claims arise out of (i) MIPI’s breach of any of its obligations, warranties or representations hereunder; (ii) MIPI’s negligent acts or omissions or wilful misconduct; (iii) any defect or failure of Kits to meet applicable specifications; (iv) MIPI’s, MIPI licensee’s or any other third party’s development, manufacture, use, handling, shipping, marketing, sale, distribution or other disposition of or treatment with Kits, Sets, or Final Product or (v) MIPI’s or MIPI licensee’s incorrect information regarding Kit, Set and/or Final Product or failure of due disclosure to EZN of any risks regarding Kit, Set and/or Final Product.
| 22.7 | DISCLAIMER OF CONSEQUENTIAL DAMAGES |
In no event shall either Party be liable to the other for indirect, contingent, incidental, special or consequential damages, including, but not limited to, any claim for damages based on lost profits, cost of capital, loss of business opportunity or loss of time.
| 24 | EXPORT CONTROL |
| 24.1 | The Parties understand that materials and information resulting from the performance of this Agreement may be subject to export control laws and, except as otherwise provided in a separate Technical Agreement, MIPI and/or MIPI’s licensee will be responsible and liable for the compliance with such laws. |
| 24.2 | MIPI understands that the export of Kit, Set and/or Final Product will be conditional upon the issuance of a customs number for and the appointment of a Person Responsible for Export Control (Ausfuhrverantwortlicher) by MIPI or MIPI’s licensee as the party being responsible for the marketing and distribution of the Kit, Set and Final Product to the final destination. |
23
| 25 | GOVERNING LAW |
| 25.1 | This Agreement shall be construed in accordance with the laws of Germany. The Convention on the International Sale of Goods and the Law transforming the CISG into national law shall not apply. |
| 25.2 | All disputes arising out of or affecting this Agreement which cannot be resolved amicably shall be submitted to the exclusive jurisdiction of the courts of Basel Stadt, Switzerland. |
| 26 | GENERAL |
| 26.1 | This Agreement, including the Schedules hereto which are incorporated herein, constitute the entire agreement of the Parties with respect to the subject matter hereof and supersedes all proposals, oral or written, and all negotiations, conversations, or discussions. This Agreement may not be modified, amended, rescinded, cancelled or waived, in whole or in part, except by written amendment signed by both Parties hereto. |
| 26.2 | The Parties agree that, except as may otherwise be required by applicable laws, regulations, rules or orders, no information concerning this Agreement and the transactions contemplated herein shall be made public by either Party without the prior written consent of the other, which consent shall not be unreasonably withheld or delayed. In the event either Party decides to issue a press release announcing the execution of this Agreement, it shall not do so without the prior written approval of the other Party, which approval shall not be unreasonably withheld or delayed. A copy of any proposed press release shall be provided to the other Party at least three (3) business days prior to any proposed dissemination. The Parties agree that they will use reasonable efforts to coordinate the initial announcement or press release relating to the existence of this Agreement. |
| 26.3 | This Agreement shall endure to the benefit of and shall be binding upon the heirs, executors, administrators, successors and permitted assigns of the Parties. Neither MIPI nor EZN shall assign any portion of this Agreement without the written approval of the other Party, which approval shall not be unreasonably withheld or delayed. However, either Party has the right, without the consent of the other Party, to assign this agreement to an Affiliate, but in such case shall remain liable to the other Party for the performance of its Affiliate and shall indemnify the other Party and hold it harmless from and against all costs, claims, judgements and other expenses arising from the Affiliate’s performance or failure of performance. |
EZN shall be entitled without prior written consent of MIPI to subcontract to third parties parts of its obligations set out in this Agreement in order to carry out its obligations hereunder; provided, however, that such subcontractor shall agree to be bound by all of the relevant provisions hereof.
| 26.4 | This Agreement shall be carried out in compliance with all relevant laws, bylaws, rules, regulations and orders of government or manifestations thereof of Germany and the European Union. |
24
| 26.5 | Failure by either Party to enforce at any time any of the provisions of this Agreement shall not be construed as a waiver of its rights hereunder. Any waiver of a breach of any provision hereof shall not affect either Party’s rights in the event of any additional breach. |
| 26.6 | If any provision or term of this Agreement is found unenforceable under any of the laws or regulations applicable thereto, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect so long as the economic or legal substance of the Agreement or transactions contemplated herein are not affected in any manner materially adverse to any Party. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the Parties hereto shall negotiate in good faith to modify this Agreement to effect the original intent of the Parties as closely as possible in a mutually acceptable manner, in order that the transaction contemplated hereby be consummated as originally contemplated to the greatest extent possible. |
Berlin, this 20th day of October 2009
| /s/ Xxxxxxx Xxxxxxxx |
/s/ Xxxxx Xxxx & Xxxxxxx Xxxxxx | |||
| MIPI | EZN |
EXHIBIT A – LISTING OF COUNTRIES IN TERRITORY
EXHIBIT B – PROJECT PLAN
EXHIBIT C – SPECIFICATIONS
EXHIBIT D – QUALITY AGREEMENT
EXHIBIT E – PRODUCT PRICE TABLE
EXHIBIT F – MILESTONE SCHEDULE
25
Exhibit A—Listing of Countries in Territory
26
| European Union | 1 | Albania | European Free Trade |
00 | Xxxxxxxxxxxx | Xxxxxx Xxxx & Xxxxxx Xxxxxx |
62 | Mauritania | ||||||||||||
| 2 | Andorra | 36 | Norway | 63 | Morocco | |||||||||||||||
| 3 | Austria | Eastern Europe |
37 | Belarus | 00 | Xxxx | ||||||||||||||
| 0 | Xxxxxxx | X/X | Xxxxxxx* | 65 | Qatar | |||||||||||||||
| 5 | Boznia and Xxxxxxxxxxx | X/X | Xxxxxx* | 00 | Xxxxx Xxxxxx | |||||||||||||||
| 6 | Bulgaria | N/A | Lithuania* | |||||||||||||||||
| 7 | Croatia | 38 | Xxxxxxx | 00 | Somalia | |||||||||||||||
| 8 | Cyprus | 39 | Xxxxxxx | 00 | Xxxxx | |||||||||||||||
| 0 | Xxxxx Xxxxxxxx | Russia & CIS |
40 | Russia | 69 | Syria | ||||||||||||||
| 10 | Xxxxxxx | 00 | Xxxxxxx | 70 | Tunisia | |||||||||||||||
| 11 | Xxxxxxx | 00 | Xxxxxxxxxx | 00 | XXX | |||||||||||||||
| 00 | Xxxxxxx | N/A | Belarus* | 72 | Western Sahara | |||||||||||||||
| 13 | France | 43 | Georgia | |||||||||||||||||
| 14 | Germany | 44 | Xxxxxxxxxx | 00 | Xxxxx | |||||||||||||||
| 00 | Xxxxxx | 45 | Xxxxxxxxxx | Xxxxxx | 00 | Xxxxxx | ||||||||||||||
| 00 | Xxxxxxx | X/X | Xxxxxxx* | Xxxxx Xxxxxx |
X/X | Xxxxxxx* | ||||||||||||||
| 00 | Xxxxxxx | 46 | Tajikistan | N/A | Egypt* | |||||||||||||||
| 00 | Xxxxx | 00 | Xxxxxxxxxxxx | X/X | Xxxxx* | |||||||||||||||
| 00 | Xxxxxx | N/A | Ukraine* | N/A | Morocco* | |||||||||||||||
| 20 | Lithuania | 48 | Uzbekistan | N/A | Sudan* | |||||||||||||||
| 00 | Xxxxxxxxxx | Xxxxxx Xxxx & Xxxxxx |
49 | Algeria | N/A | Tunisia* | ||||||||||||||
| 22 | Xxxxx | 00 | Xxxxxxx | X/X | Xxxxxxx Xxxxxx* | |||||||||||||||
| 00 | Xxxxxxxxxxx | 51 | Chad | Europe: Non-Sovereign Locations |
75 | Åland | ||||||||||||||
| 24 | Poland | 52 | Comoros | 76 | Akrotiri and Dhekelia | |||||||||||||||
| 25 | Portugal | 53 | Dijibouti | 77 | Faroe Islands | |||||||||||||||
| 26 | Romania | 54 | Egypt | 78 | Gibraltar | |||||||||||||||
| 27 | Xxxxxxxx | 00 | Xxxxxxx | 00 | Xxxxxxxx | |||||||||||||||
| 00 | Xxxxxxxx | 56 | Iran | 80 | Isle of Man | |||||||||||||||
| 29 | Spain | 81 | Jersey | |||||||||||||||||
| 30 | Sweden | 57 | Iraq | Europe: Partially Recognized Locations |
00 | Xxxxxxxx | ||||||||||||||
| 00 | Xxxxxx Xxxxxxx | 58 | Jordan | 83 | Kosovo | |||||||||||||||
| 32 | Vatican City | 00 | Xxxxxx | 00 | Xxxxxxxx Xxxxxx | |||||||||||||||
| European Free Trade Assoc. |
33 | Switzerland | 60 | Lebanon | 85 | South Ossetia | ||||||||||||||
| 34 | Iceland | 61 | Libya | Europe: Unrecognized Locations | 86 | Nagorno-Karabakh | ||||||||||||||
| 87 | Transnistria |
| * | Countries are represented twice in two different territories, only counted once |
27
EXHIBIT B – PROJECT PLAN
28
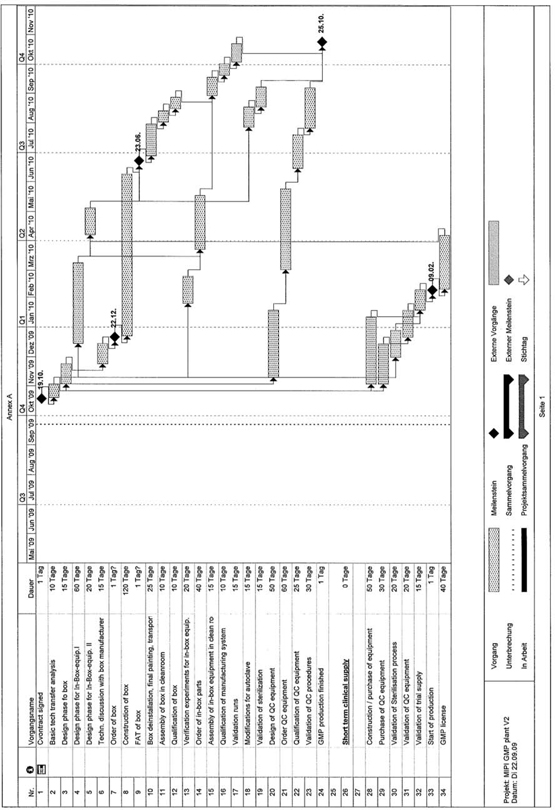
29
EXHIBIT C - SPECIFICATIONS
30
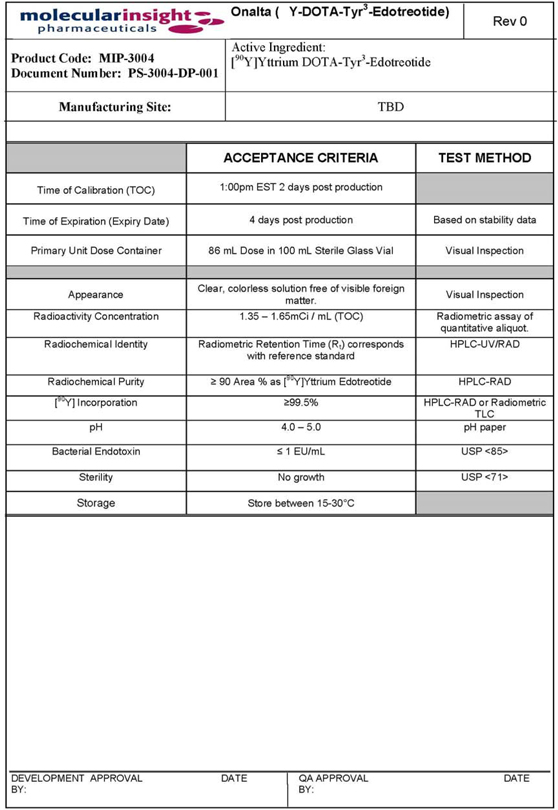
31
EXHIBIT D – QUALITY AGREEMENT
32
CHANGE CONTROL HISTORY
TO THE
QUALITY (GMP AND GDP) AGREEMENT
between
MIPI
and
EZN
[Not a part of the Agreement]
| Rev. |
Change |
Date | ||
| Date: |
|
|||||
| Number of pages: |
|
|||||
| Copies: |
|
|||||
| Name and number of Appendices: |
|
|||||
|
|
||||||
|
|
||||||
|
|
||||||
QUALITY (GMP and GDP) AGREEMENT
between
Xxxxxx & Xxxxxxx Nuclitec XxxX
Xxxxxxxxx 0
00000 Xxxxxxxxxxxx
Xxxxxxx
- hereinafter referred to as EZN -
and
Molecular Insight Pharmaceuticals, Inc
000 Xxxxxx Xxxxxx
Xxxxxxxxx, XX 00000
XXX
- hereinafter referred to as MIPI -
TABLE OF CONTENTS
| 1 | LEGAL REQUIREMENTS | 3 | ||
| 2 | ACTIVE PHARMACEUTICAL INGREDIENTS, EXCIPIENTS AND PACKAGING MATERIALS | 4 | ||
| 3 | MANUFACTURE | 4 | ||
| 4 | QUALITY CONTROLS DURING AND AFTER MANUFACTURE | 4 | ||
| 5 | REFERENCE AND RETENTION SAMPLES | 5 | ||
| 6 | DOCUMENTATION | 5 | ||
| 7 | CERTIFICATION/CONFIRMATION (RELEASE) | 5 | ||
| 8 | STORAGE, PACKAGING AND TRANSPORTATION | 5 | ||
| 9 | NOTIFICATION AND APPROVAL OF DEVIATIONS | 5 | ||
| 10 | CHANGE CONTROL | 6 | ||
| 11 | PRODUCT DEFECTS | 6 | ||
| 12 | CONTACT DEPARTMENTS AND INDIVIDUALS | 6 | ||
| 13 | CONFIDENTIALITY | 6 | ||
| 14 | FINAL CLAUSES | 7 | ||
| APPENDIX 1 | 9 | |||
| APPENDIX 2 | 10 | |||
| APPENDIX 3 | 14 | |||
| APPENDIX 4 | 15 | |||
| APPENDIX 5 | 16 | |||
| APPENDIX 6 | 17 | |||
Page 2 of 18
QUALITY AGREEMENT
THIS AGREEMENT (the “Quality Agreement”) is made on
between
Molecular Insight Pharmaceuticals, Inc whose registered office is at 000 Xxxxxx Xxxxxx, Xxxxxxxxx, XX 00000, XXX hereinafter referred to as “MIPI”,
and
Xxxxxx & Xxxxxxx Nuclitec whose registered office is at Xxxxxxxxx 0, 00000 Xxxxxxxxxxxx, Xxxxxxx hereinafter referred to as “EZN”,
each hereinafter referred to also as “a Party” and together as “the Parties”.
WITNESSETH
WHEREAS MIPI and EZN on the have entered into a Facility Setup and Contract Manufacturing Agreement (hereinafter the “Commercial Agreement”).
WHEREAS this Quality Agreement covers production and/or packaging and/or analysis and/or distribution of the product listed in the appendices; and
WHEREAS the main roles and responsibilities of the Parties are set out in Appendix 2 hereto;
Now THEREFORE, the Parties hereto have adopted and are bound by the following conditions of this Quality Agreement:
| 1 | LEGAL REQUIREMENTS |
| 1.1 | EZN affirms that it will be responsible for the application and issuance of the required Manufacturing Authorisation pursuant to the local legal requirements for the production and/or packaging and/or analysis and/or release of the product listed in Appendix 1 (herein referred to as “the Product”). |
| 1.2 | The Parties shall be obliged to immediately advise each other’s Contact Department and Individuals (cf. Article 12) of any changes in the Manufacturing Authorisation with regard to the Product. EZN shall be obliged to forward to MIPI a copy of the Manufacturing Authorisation (cf. Article 1.1). |
| 1.3 | EZN warrants that the Product from the first batch and onwards will be manufactured according to the documents agreed upon and as specified in Appendix 2. The manufacture will take place at EZN’s plant at Braunschweig, Germany. |
| 1.4 | EZN warrants that all activities related to the Product will be in accordance with (i) all current international rules and regulations according to ICH guidelines and (ii) with rules and regulations of the European Union, including but not limited to the EU-Guide to Good Manufacturing Practice for Medicinal Products, (iii) as well as the agreed directives of MIPI as specified in this Quality Agreement, and (iv) all rules and regulations as defined in the Commercial Agreement (collectively hereinafter called the “Provisions”). |
Page 3 of 18
| 1.5 | EZN will allow MIPI and the competent authorities in the Territories where Product is being marketed, after prior agreement and during normal working hours, to perform all the audits and inspections necessary in connection with the manufacture and supply of the Product covered by the Agreement. |
| 1.6 | MIPI will be responsible for compliance with the legal requirements and recommendations applicable to the Product and the manufacture hereof in the countries in which the Product is sold, and shall inform EZN of any changes hereto which could require changes at EZN. Implementation of any such changes must be agreed upon separately unless otherwise agreed upon in the Commercial Agreement. |
| 1.7 | EZN agrees not to assign or move the performance of any tasks or obligations covered by this Quality Agreement to any other site, any affiliate or any third party without having obtained the prior written consent of MIPI. |
| 2 | ACTIVE PHARMACEUTICAL INGREDIENTS, EXCIPIENTS AND PACKAGING MATERIALS |
| 2.1 | The active pharmaceutical ingredients, excipients and packaging materials listed in Appendix 3 shall be provided by EZN, checked for accordance with the specifications contained in the said Appendix, and tested and released for use in manufacturing the product by EZN. |
| 2.2 | The active pharmaceutical ingredients, excipients and packaging materials indicated in Appendix 4 shall be provided by MIPI, received, identity confirmed, approved and stored in accordance with the specifications in the said Appendix, and released for manufacturing of the product by EZN. |
| 3 | MANUFACTURE |
| 3.1 | The Product shall be manufactured in batches, ensuring adherence to the manufacturing instructions agreed upon between the Parties following the Scaled up Process as defined in the Commercial Agreement, as well as ensuring the quality in accordance with the Product specifications listed in Exhibit C of the Commercial Agreement. Manufacturing instructions shall be approved by MIPI in writing prior to routine manufacturing. Final definition of batches applying for this Quality Agreement will be subject to separate mutual definition of EZN and MIPI. |
| 4 | QUALITY CONTROLS DURING AND AFTER MANUFACTURE |
| 4.1 | EZN shall conduct in-process controls according to agreed upon manufacturing instructions. |
| 4.2 | EZN shall conduct final quality controls according to Exhibit C of the Commercial Agreement and any additional testing required for current European GMP guidelines or marketing authorization. |
Page 4 of 18
| 5 | REFERENCE AND RETENTION SAMPLES |
| 5.1 | EZN shall retain reference samples of all starting materials to the Product, including but not limited to active pharmaceutical ingredients, excipients (except for gases, solvents and water) and packaging materials (the “Starting Materials”) until expiry date of the last batch of Final Product it was used for or in compliance with current GMP guidelines. Retention samples of the manufactured Final Product must be taken in a quantity sufficient for two full analytic controls, and they must be kept for twenty-four (24) months past the expiry date. |
| 6 | DOCUMENTATION |
| 6.1 | EZN shall keep records on all manufacturing and control steps on the basis of GMP-guidelines and keep same for a minimum of five (5) years. All documentation will be in German. |
| 6.2 | EZN will issue an annual Product review on the manufacture, analytical testing and summarizing all changes implemented and deviations and submit such review to MIPI, in English and German. |
| 7 | BATCH RELEASE |
| 7.1 | MIPI shall receive the Confirmation by a Qualified Person (EU GMP Annex 16) of batch release as set forth in Appendix 5. |
| 8 | STORAGE, PACKAGING AND TRANSPORTATION |
| 8.1 | EZN shall be responsible for appropriate storage of the Starting Materials and the Product, as well as for packaging the Product for transportation in suitable and well-closed containers. |
| 8.2 | MIPI is responsible for the transportation of the Product in accordance with the Commercial agreement? International Hazardous material regulations? at all times governing European Guidelines for Good Distribution Practices and local legal requirements. |
| 9 | NOTIFICATION AND APPROVAL OF DEVIATIONS |
| 9.1 | EZN will investigate and document all deviations, out-of-specification results, non-compliance with Good Manufacturing Practices, production failures or product complaints or any other matter that may affect the quality, safety or efficacy of the Product. Documentation will be retained as part of the batch documentation for the batch affected. |
| 9.2 | EZN shall notify MIPI within forty-eight (48) hours of all significant deviations from agreed procedures or specifications, which might have an impact on Product quality and/or affect specifications and process compliance. |
Page 5 of 18
| 10 | CHANGE CONTROL |
| 10.1 | EZN will utilise a documented system of procedures for control of changes to raw materials, packaging materials, suppliers, equipment, facilities, utilities, manufacturing methods, product and material specifications and requirements, sampling, test methods and release requirements. |
| 10.2 | EZN shall inform the MIPI well in advance about any technical changes (e.g. in component sourcing or quality, utilities or environmental conditions), which could affect the regulatory documentation of the Product. |
| 10.3 | The Parties shall agree upon documentation necessary for the variation application and timeline of implementation, including necessary validation activities prior to such implementation. |
| 10.4 | MIPI will inform EZN in writing about the variation approval promptly after receipt of such approval from the competent authorities. Additionally, MIPI will inform EZN of any changes required in the process for receiving marketing authorization. |
| 11 | PRODUCT DEFECTS |
| 11.1 | EZN shall assist MIPI in investigating all Product complaints by reviewing Product and materials to determine the cause, if any. In addition, all appropriate documentation shall be reviewed by EZN. EZN shall diligently work to provide a written report of its determination within thirty (30) days from receipt of MIPI’s written request. |
| 11.2 | If a deviation or a product defect is discovered, e.g. during stability monitoring or out of customer complaints, which has an impact on the quality of the product after the product has been released; MIPI must be notified immediately. |
| 12 | CONTACT DEPARTMENTS AND INDIVIDUALS |
| 12.1 | EZN agrees to provide answers as soon as possible to all enquiries and complaints from MIPI. |
| 12.2 | Any communication between MIPI and EZN regarding the Quality Agreement shall be addressed to the Individuals mentioned in Appendix 6. |
| 13 | CONFIDENTIALITY |
| 13.1 | The Parties agree that information exchanged between them under the present Quality Agreement is of a proprietary and confidential nature Both Parties agree to keep confidential any and all information exchanged under the present Quality Agreement and any other information they acquire about each other during the term of the present Quality Agreement. Both Parties warrant to each other to do their utmost in order to ensure that their respective employees respect this confidentiality obligation. |
| 13.2 | This obligation does not extend to information for which the receiving Party can prove that: |
| 13.2.1 | it had the information prior to the time of disclosure; or |
Page 6 of 18
| 13.2.2 | the information has become generally available to the public domain after the date hereof through no fault of the receiving Party. |
| 13.2.3 | it has received the information from a third party whose direct or indirect source is not the disclosing Party. |
| 13.3 | The receiving Party shall further be entitled to disclose to the competent regulatory or legal authorities such part of the information received from the disclosing Party that is required by law to be disclosed to such authorities. In this event, the receiving Party shall ask the receiving regulatory or legal authority to maintain confidentiality. |
| 14 | FINAL CLAUSES |
| 14.1 | This Quality Agreement shall become effective upon signature by both Parties. |
| 14.2 | Supplements and amendments to this Quality Agreement shall be in writing and signed by the Parties. |
| 14.3 | This Quality Agreement shall remain in effect for the term of the Commercial Agreement. The termination or expiration of this Quality Agreement for any reason whatsoever shall be without prejudice to any obligations or rights on the part of either Party which have accrued prior to such termination, and shall not affect or prejudice any provision of this Agreement which is expressly (e.g. Retention of Samples in Article 5.1) or by implication provided to come into effect on, or continue in effect after such termination. |
| 14.4 | The Parties shall renegotiate the terms of this Quality Agreement if necessary due to major legal or regulatory changes. |
| 14.5 | Upon termination of this Quality Agreement and the corresponding Commercial Agreement, all documents provided by MIPI to EZN and all reference samples as well as the batch manufacturing and control documents archived according to Article 5 and 6 shall be returned to MIPI. |
| 14.6 | In the event that any provision in this Quality Agreement is held to be unlawful or invalid in any jurisdiction, the meaning of such provision will be construed to the greatest extent possible so as to render it enforceable. If no such construction can render such provision enforceable, it will be severed. The remainder of this Agreement will remain in full force and effect, and the Parties will negotiate in good faith a reasonable substitute provision that is valid and enforceable in such jurisdiction. |
| 14.7 | In case of discrepancy between any provision of the Commercial Agreement and the Quality Agreement, special terms shall prevail over general terms, and new terms prevail over old terms. |
| 14.8 | German law shall apply. |
| MIPI | EZN | |||
|
|
| |||
Page 7 of 18
| Name: | Name: | |
| Title: | Title: | |
| Date: | Date | |
Page 8 of 18
APPENDIX 1
TO THE
QUALITY (GMP AND GDP) AGREEMENT
between
MIPI
and
EZN
Product
Onalta™
Page 9 of 18
APPENDIX 2
TO THE
QUALITY (GMP AND GDP) AGREEMENT
between
MIPI
and
EZN
| MIPI | EZN | corresponding flies / documentation / remarks (e.g. title, No., enclosure, etc.) |
||||||
| Accordance with the registration documents | ¨ | X | ||||||
| sterile pre-dispensed Iyophilized preparation of DOTA-TOC “KIT”: |
||||||||
| Specification |
X | ¨ | ||||||
| Supplier qualification |
X | ¨ | ||||||
| Supplier review |
X | ¨ | ||||||
| Supply/ Procurement |
X | ¨ | ||||||
| Testing |
X | ¨ | ||||||
| Reference samples |
X | ¨ | ||||||
| Release |
X | ¨ | ||||||
| Storage Conditions |
X | ¨ | ||||||
| Shelf Life |
X | ¨ | ||||||
| Certain excipients: |
||||||||
| Specification |
X | ¨ | ||||||
| Supplier qualification |
¨ | X | ||||||
| Supplier review |
¨ | X | ||||||
| Supply / Procurement |
¨ | X | ||||||
| Testing |
¨ | X | ||||||
| Reference samples |
¨ | X | ||||||
| Release |
¨ | X | ||||||
| Other excipients: Not Applicable |
||||||||
| Specification |
¨ | ¨ | ||||||
| Supplier qualification |
¨ | ¨ | ||||||
| Supplier review |
¨ | ¨ | ||||||
| Supply/Procurement |
¨ | ¨ | ||||||
| Testing |
¨ | ¨ | ||||||
| Reference samples |
¨ | ¨ | ||||||
| Release |
¨ | ¨ | ||||||
Page 10 of 00
| XxXX | XXX | corresponding files / documentation / remarks (e.g. title, No., enclosure, etc.) | ||||||
| Primary packaging: |
||||||||
| Specification |
X | ¨ | ||||||
| Supplier qualification |
¨ | X | ||||||
| Supplier review |
¨ | X | ||||||
| Clearance for printing |
X | ¨ | ||||||
| Supply/Procurement |
¨ | X | ||||||
| Testing |
X | ¨ | ||||||
| Retention samples |
¨ | X | ||||||
| Release |
¨ | X | ||||||
| Printed secondary packaging: |
||||||||
| Label |
||||||||
| Specification |
X | ¨ | ||||||
| Supplier qualification |
¨ | X | ||||||
| Supplier review |
¨ | X | ||||||
| Clearance for printing |
¨ | X | ||||||
| Supply/Procurement |
¨ | X | ||||||
| Testing |
X | ¨ | ||||||
| Retention samples |
¨ | X | ||||||
| Release |
X | ¨ | ||||||
| Secondary packaging |
||||||||
| Specification |
¨ | X | ||||||
| Supplier qualification |
¨ | X | ||||||
| Supplier review |
¨ | X | ||||||
| Clearance for printing |
¨ | X | ||||||
| Supply/Procurement |
¨ | X | ||||||
| Testing |
X | ¨ | ||||||
| Retention samples |
¨ | X | ||||||
| Release |
X | ¨ | ||||||
Page 11 of 18
| MlPI | EZN | corresponding files / documentation / remarks (e.g. title, No., enclosure, etc.) | ||||||
| Package leaflet: |
||||||||
| Specification |
X | ¨ | ||||||
| Supplier qualification |
X | ¨ | ||||||
| Supplier review |
X | ¨ | ||||||
| Clearance for printing |
X | ¨ | ||||||
| Supply/Procurement |
X | ¨ | ||||||
| Testing/Readibility |
X | ¨ | ||||||
| Retention samples |
X | ¨ | ||||||
| Release |
X | ¨ | ||||||
| Finished product: |
||||||||
| Specification |
X | ¨ | ||||||
| Assignment of batch no. |
¨ | X | ||||||
| Determination of shelf life |
X | ¨ | ||||||
| Packaging procedures |
X | ¨ | ||||||
| In-process-control |
¨ | X | ||||||
| Packaging record |
¨ | X | ||||||
| Testing procedure |
X | ¨ | ||||||
| Testing record |
¨ | X | ||||||
| Certificate of analysis |
¨ | X | ||||||
| Release for shipment |
¨ | X | ||||||
| Final testing |
¨ | X | ||||||
| Retention samples |
¨ | X | ||||||
| Final release for market |
¨ | X | ||||||
| On-going stability |
¨ | X | ||||||
| On-going stability Procedure |
¨ | X | ||||||
EZN will perform the stability study based on MIPI protocol!
MIPI receives the following documentation:
| Every consignment | On request | |||
| Finished product: |
||||
| Packaging record, complete |
¨ | X | ||
| Packaging record, extract |
¨ | X | ||
| In-process control records |
¨ | X | ||
| Cert. of analysis for finished product |
¨ | X | ||
| Confirmation of proper manufacture/ testing |
¨ | X | ||
Page 12 of 18
Product Quality Review:
EZN submits documents / data for the activities performed by EZN.
The final evaluation of PQR is the responsibility of MIPI.
Other agreements / Special arrangements
(e.g. specific quality tests, shelf life tests, storage conditions, transport conditions)
On-going stability studies shall be limited to a maximum of analyses of 30 samples consisting at a maximum of tests conducted for a batch release.
| Done by (Signature / Date): | ||
| MIPI (Signature/ Date) Q.P.:
|
EZN (Signature/ Date) Q.P.:
| |
| Head of Production:
|
Head of Production:
| |
| Head of QC:
|
Head of QC:
| |
Page 13 of 18
APPENDIX 3
TO THE
QUALITY (GMP AND GDP) AGREEMENT
between
MIPI
and
EZN
[Edotreotide Y 90
And edotreotide In 111]
ACTIVE PHARMACEUTICAL INGREDIENTS, EXCIPIENTS AND PACKAGING MATERIALS TO BE PROVIDED BY EZN
Yttrium-90 as yttrium chloride
Indium-111 as indium chloride
Final primary container closure system (clean, sterile, pyrogen freel00 xX xxxxx vial with rubber serum cap and aluminium seal)
Qualify of Active Pharmaceutical Ingredients:
[Please include name and address of manufacturer of active pharmaceutical ingredient]
Quality of Excipients: Certificate of Analysis confirming the identity, purity and strength of the Y-90 and In-111 component isotopes.
Quality of Packaging Material:
Page 14 of 18
APPENDIX 4
TO THE
QUALITY (GMP AND GDP) AGREEMENT
between
MIPI
and
EZN
Edotreotide Y 90 and
Edotreotide In 111
ACTIVE PHARMACEUTICAL INGREDIENTS, EXCIPIENTS AND PACKAGING MATERIALS TO BE PROVIDED BY MIPI
Edotreotide reference standard
Kits for the production of edotreotide Y 90 and edotreotide In 111
Quality of Active Pharmaceutical Ingredients:
Bachem AG- GMP Edotreotide
Xxxxxxxxxx: GMP- freeze dried kits to product edotreotide
Quality of Excipients:
Excipients contained in GMP manufactured kits containing API
Quality of Packaging Material:
Page 15 of 18
APPENDIX 5
Form
Batch Release
| Product: |
|
|||
| Batch no.: |
|
|||
| Packaging size: |
|
|||
| Date of manufacture: |
|
|||
| Date of expiry: |
|
|||
| Quantity |
|
|||
| Name and address of manufacturing site: |
|
|||
Herewith we confirm that the above described batch has been produced incl. packaging and quality control (QC) within a.m. manufacturing site and in compliance with national pharmaceutical law and GMP requirements and in conformance with the manufacturing procedures and testing instructions released by CG.
The batch processing, packaging and analysis records were reviewed. That means, inter alia, that possible deviations (concerning the product covered by the Agreement and the starting materials) are listed and evaluated.
During the course of manufacturing, packaging and testing there were
[ ] no deviation / no OOS result
[ ] deviation / OOS result (deviation / OOS report attached)
[ ] additional relevant information regarding the quality of the product is attached
| Date: |
| |
| Sign: |
| |
| (Qualified Person CA | ||
Page 16 of 18
APPENDIX 6
CONTACT DEPARTMENT AND INDIVIDUALS
MIPI
| Name | Task | Address, Phone, Fax, E-mail | ||
EZN
| Name | Task | Address, Phone, Fax, E-mail | ||
Page 17 of 18
EXHIBIT E – PRODUCT PRICE TABLE
Final Product Price (in € per 120mCi dose)
| Item |
Year 1 (* |
Year 2 |
Year 3 |
Year 4 |
Year 5 to 10 | |||||
| Y90-DOTATOC | ***** |
***** |
***** |
***** |
***** |
| (* | Year 1 will commence upon completion of Facility and receipt of manufacturing authorization. For any dose of Final Product delivered during the Interims Phase, prices of Year 1 shall apply. |
All prices in addition to the Monthly Payments and Milestone Payments.
Y-90 Price (in € per 120mCi dose)
EZN will deliver Y-90 for the price of ***** per 120mCi dose.
All prices in addition to the Monthly Payments and Milestone Payments.
Kit Storage and Handling Fee
EZN will store and handle Kits for the price of ***** per patient dose/Kit (exclusive packaging and transportation costs).
All prices in addition to the Monthly Payments and Milestone Payments.
Preliminary non-binding volume forecast (Number of 120 mCi patient doses)
| Item |
Year 1 (* |
Year 2 |
Year 3 |
Year 4 |
Year 5 | |||||
| Y90-DOTATOC | ***** |
***** |
***** |
***** |
***** |
| (* | Year 1 will commence upon completion of Facility and receipt of manufacturing authorization. |
Projected Expected Volume Will Be Provided to EZN On An Agreed Upon Schedule
* Confidential Treatment Requested *
EXHIBIT F – MILESTONE SCHEDULE
Milestone schedule:
| 1. | Upon Effective date /signature of agreement ***** |
| 2. | Upon reaching the following milestones: ***** |
| • | technical readiness of Provisional Facility for Interim Supply, which shall mean three (3) consecutive production runs at the Provisional Facility that meet the product specifications to be proven to MIPI by EZN by means of signed respective batch release reports |
| • | design clearance for the Facility (high throughput supply capability), which shall mean submission of technical design specifications of the Facility (high throughput supply capability) by EZN to MIPI |
| 3. | Upon reaching the following milestones: ***** |
| • | regulatory clearance of Provisional Facility for Interim Supply, which shall mean the submission of a copy of a document by local authorities by EZN to MIPI |
| 4. | Upon reaching the following milestones: ***** |
| • | factory acceptance for the Hot Cell(s) for Facility (high throughput supply capability), which shall mean submission of the final report of FAT signed by EZN to MIPI |
| 5. | Upon reaching the following milestones: ***** |
| • | technical and regulatory clearance for the Facility (high throughput supply capability), which shall mean the submission of the final validation report for the Facility (high throughput supply capability) and a copy of a document by local authorities by EZN to MIPI |
A copy of the respective documents send by facsimile or via e-mail to the MIPI contact mentioned in Article 20 of the Facility Setup and Contract Manufacturing Agreement shall be sufficient for due submission.
* Confidential Treatment Requested *

