LICENSE AGREEMENT
Exhibit 10.1
This LICENSE AGREEMENT (the “License Agreement”), dated June 14, 2016 (the “Effective Date”), is by and between NUVECTRA CORPORATION, a corporation and existing under the laws of the State of Delaware (hereinafter referred to as “Licensor”) and Aleva Neurotherapeutics SA, a Swiss share company registered under the federal identification number CHE-114.416.910, EPFL Xxxxxxxxxx Xxxx, Xxxxxxxx X, 0000 Xxxxxxxx, Xxxxxxxxxxx (hereinafter referred to as “Licensee”). The Licensor and the Licensee are sometimes referred to herein collectively as the “parties” and individually as a “party.”
WHEREAS, Licensor owns patents, patent applications and other intellectual property;
WHEREAS, Licensor desires to grant, and Licensee desires to obtain, a license with respect to the Licensed IP, including the Licensed Patent Applications (as defined below) and Licensed Patents (as defined below), which are listed on Exhibit A attached hereto, for applications in the Field of Use; and
NOW, THEREFORE, in consideration of the mutual covenants and agreements hereinafter set forth, the parties hereby agree as follows:
Article I.
DEFINITIONS
Section 1.01 Capitalized terms utilized in this License Agreement and not otherwise defined herein shall have the respective meanings assigned and ascribed to them under this Article I, as follows:
(a) “Affiliate” of a Person means any other Person that, whether now or in the future, controls, is controlled by or is under common control with, such Person. For the purposes of this definition, the terms “controls”, “controlled by” and “under common control with” means (i) to possess (directly or indirectly) the power to direct the management or affairs of a Person, whether through ownership of voting securities or other equity rights or by contract relating to voting rights or corporate governance or otherwise, or (ii) to own, directly or indirectly, more than fifty percent (50%) of the outstanding voting securities or other ownership interest of such Person.
(b) “Bankruptcy Proceeding” shall have the meaning assigned and ascribed to such term under Section 9.03(a) hereof.
(c) “Business Day” means a day other than a Saturday, Sunday or other day on which commercial banks in New York City are authorized or required by applicable law to be closed for business.
(d) “Change of Control” means (i) a consolidation or merger of a party or other change of control transaction in which the stockholders of a party immediately prior to such transaction do not continue to hold a greater than 50% interest in the successor or survivor entity immediately following such transaction, (ii) a transaction or series of transactions that results in the transfer of more than 50% of the voting power of a party to a person or entity, or (iii) the sale, lease, transfer or other disposition of all or substantially all of the assets of a party (which shall include any effective transfer of such assets regardless of the structure of any such transaction as a license or otherwise).
- 2 -
(e) “Confidential Information” of a party means any and all information of a confidential or proprietary nature disclosed by a party under this License Agreement, whether in oral, written, graphic or electronic format, which includes, but is not limited to, trade secrets, discoveries, ideas, concepts, know-how, techniques, designs, specifications, drawings, diagrams, data, business activities and operations, customer lists, reports, studies and other technical and business information.
(f) “Damages” shall have the meaning assigned and ascribed to such term in Section 12.01.
(g) “DBS System” means a neurostimulation medical device system for applications within the Field of Use that (i) is derived from, based upon or incorporates Technical Know-How or is within the scope of one or more claims of any Licensed Patent Application or Licensed Patent and (ii) consists primarily of components derived from or based upon the components of the Algovita Spinal Cord Stimulation System described on Exhibit B hereto.
(h) “Development Agreement” means that certain Development Agreement by and between Licensor and Licensee, dated as of January 29, 2016.
(i) “Effective Date” shall have the meaning assigned and ascribed to such term in the recitals to this License Agreement.
(j) “Exclusive Distribution License” shall have the meaning assigned and ascribed to such term in Section 2.01(a).
(k) “FDA Approval” means the receipt of all Regulatory Approvals from the FDA that would permit Licensor to sell the Licensed Products in the United States for applications within the Field of Use.
(l) “FDA” means the United States Food and Drug Administration or any successor entity.
(m) “Field of Use” means deep brain stimulation for movement disorders (i.e. Xxxxxxxxx’x disease and/or essential tremor).
(n) “Governmental Body” shall mean any (i) nation, state, county, city, town, village, district or other jurisdiction; (ii) federal, state, provincial, municipal, local, foreign or other government; or (iii) governmental or quasi-governmental authority (including any governmental agency branch, department, official or entity, and any court or other tribunal).
- 3 -
(o) “Greatbatch” means Greatbatch Ltd., a corporation incorporated under the laws of the state of New York.
(p) “Greatbatch Affiliate” means each Affiliate of Greatbatch.
(q) “Greatbatch License Agreement” means that certain Unrestricted License Agreement by and between Greatbatch and Licensor, dated as of March 14, 2016, as such agreement may be further amended from time to time.
(r) “Indemnified Parties” shall have the meaning assigned and ascribed to such term under Section 12.01.
(s) “Intellectual Property” means U.S. and foreign patents, patent applications, copyrights and copyright registrations and applications, mask works and registrations thereof, know-how and Inventions.
(t) “Invention” means any invention, discovery, know-how, copyright, trade secret, data, information, technology, process or concept, whether or not patented or patentable, and whether or not memorialized in writing.
(u) “License Agreement” shall have the meaning assigned and ascribed to such term in the recitals to this License Agreement.
(v) “License Conversion Date” means the date of the occurrence of a License Conversion Event.
(w) “License Conversion Event” means the first to occur of (i) receipt of a Conformité Européenne (CE) xxxx for the Licensed Products that permits sale of such Licensed Products within the European Economic Area for application within the Field of Use; (ii) receipt of FDA Approval or (iii) completion by the Licensee of a human clinical study demonstrating safety and efficacy required to obtain (A) a Conformité Européenne (CE) xxxx for the Licensed Products that permits sale of such Licensed Products within the European Economic Area for applications within the Field of Use, or (B) FDA Approval.
(x) “Licensed Patent Applications” shall mean those United States or foreign pending patent applications listed on Exhibit A.
(y) “Licensed Patents” shall mean those United States or foreign patents listed in Exhibit A; together with any patents resulting from (i) a Licensed Patent Application, (ii) any continuation, continuation-in-part, division, reissue, reexamination, and extension of such patents, and/or (iii) any invention disclosure report arising from an Invention that has been documented prior to or as of the Effective Date.
(z) “Licensed Products” shall mean the DBS System or any main component of the DBS System in such form and with such design and specifications as set forth in any regulatory submission, or any supplement related to such submission, used to obtain Regulatory Approval for the DBS System anywhere in the Territory; provided, however, that any product accessories, including, without limitation, torque wrenches, introducers, tunneling tools and adjustable belts, are not hereby included as Licensed Products. Furthermore, and for the avoidance of doubt, any products developed solely by Aleva and covered by Aleva’s Intellectual Property, but are not derived from or based upon, or do not incorporate, Licensed IP, including, without limitation, directional leads, xxxx hole covers, and other products solely developed by Aleva, are not hereby included as Licensed Products.
- 4 -
(aa) “Licensed IP” means the Licensed Patents, the Licensed Patent Applications, and the Technical Know-How.
(bb) “Licensee” shall have the meaning assigned and ascribed to such term in the recitals to this License Agreement.
(cc) “Licensor” shall have the meaning assigned and ascribed to such term in the recitals to this License Agreement.
(dd) “Licensor Affiliate” means each Affiliate of Licensor.
(ee) “Modified Product” means any Licensed Product that has been redesigned or modified as compared to the form, design or specifications for such Licensed Product set forth in any regulatory submission or any supplement related to such submission used to obtain Regulatory Approval for the DBS System or its components anywhere in the Territory, such that a separate regulatory submission and Regulatory Approval for such Licensed Product would be required prior to the sale of such Licensed Product in its modified form.
(ff) “Net Sales” means total gross amount of monies, cash or cash equivalent or other consideration paid by unaffiliated third parties to Licensee for sales of Licensed Products less the sum of the following: (a) discounts or rebates allowed in amounts customary in the trade; (b) sales, tariff duties and use taxes directly imposed and with reference to particular sales; (c) outbound transportation prepaid or allowed; and (d) amounts allowed or credited on returns. No deductions from Net Sales shall be made for commissions paid to individuals whether they are with independent sales agencies or regularly employed by Licensee and on its payroll, or for cost of collections. Licensed Products shall be considered “sold” when billed or invoiced.
For the purposes of calculating Net Sales, (a) all calculations of Net Sales shall be in accordance with United States generally accepted accounting principles consistently applied and based on, or valued as if based on, bona fide arms’ length transactions and not on any bundled, loss-leading or other blended or artificial selling or transfer price and (b) transfers of Licensed Products to an Affiliate for (i) end use (but not resale) by the Affiliate shall be treated as sales by Licensee at Licensee’s list price, or (ii) resale by an Affiliate shall be treated as the Affiliate’s sales at its list price.
Where Licensed Products are not sold, but are otherwise transferred or disposed of, the Net Sales of Licensed Product for the purposes of computing the Royalty Fee shall be the average Net Sales price at which products of similar kind and quality, sold in similar quantities and similar locations, are then currently being offered for sale by Licensee. Where such products are not then currently being offered for sale by Licensee, the Net Sales price of products otherwise disposed of, for the purpose of computing the Royalty Fee, shall be the average selling price at which products of similar kind and quality, sold in similar quantities and similar locations, are then currently being offered for sale by other manufacturers.
- 5 -
The expression “transferred or otherwise disposed of” means (y) not sold but delivered, directly or indirectly, by Licensee to others (including deliveries for export), regardless of any return or exchange consideration; or (z) exploited or otherwise used by Licensee for any purpose other than routine testing of such Licensed Products.
(gg) “Non-Exclusive Distribution License” shall have the meaning assigned and ascribed to such term in Section 2.01(a).
(hh) “Person” shall mean any individual, and any corporation, partnership, sole proprietorship, company, firm, association, trust, or governmental agency.
(ii) “Quarterly Period” means each three-month period commencing on January 1, April 1, July 1 and October 1 in each year.
(jj) “Regulatory Approvals” means all approvals necessary for the commercial sale of a Licensed Product for any indication in a given country or regulatory jurisdiction in the Territory, which shall include satisfaction of all applicable regulatory and notification requirements, but which shall exclude any pricing and reimbursement approvals.
(kk) “Royalty Fee” shall have the meaning assigned and ascribed to such term in Section 8.01.
(ll) “Supply Agreement” means that certain Supply Agreement by and between Greatbatch and Licensee, dated as of June 2, 2016.
(mm) “Technical Know-How” shall include all concepts, methods, devices and/or ideas directed or relating to the Inventions covered by the Licensed Patents or Licensed Patent Applications, some of which may be described or summarized in attached Exhibit A, as and to the extent presently configured, or as may be disclosed in a Licensed Patent Application.
(nn) “Term” shall have the meaning assigned and ascribed to such term under Section 9.01.
(oo) “Territory” shall mean the entire world.
(pp) “Third-Party Claim” shall have the meaning assigned and ascribed to such term under Section 12.02.
- 6 -
Article II.
LICENSE GRANT
Section 2.01 (a) Subject to the terms and conditions of this License Agreement, the Licensor hereby grants to the Licensee, and the Licensee hereby accepts, a non-exclusive, royalty-bearing right and license under the Licensed IP to use, make, have made, sell, offer to sell, distribute and import Licensed Products in the Territory during the Term for applications within the Field of Use; provided, however, that upon the occurrence of a License Conversion Event, the portion of the non-exclusive license under this Section 2.01(a) consisting of the non-exclusive, royalty-bearing license to sell, offer to sell, distribute and import Licensed Products in the Territory during the Term for applications within the Field of Use (the “Non-Exclusive Distribution License”) shall immediately, and without the need for any action on the part of either party, convert into an exclusive, royalty-bearing license to sell, offer to sell, distribute and import Licensed Products in the Territory during the Term for applications within the Field of Use (the “Exclusive Distribution License”); provided, however, that the exclusivity of such Exclusive Distribution License shall be subject to, and shall not impact, any license rights previously granted in favor of Greatbatch pursuant to the Greatbatch License Agreement; provided, further, however, that if the Licensee fails to obtain FDA Approval for the Licensed Products within six (6) years after the License Conversion Date, the Exclusive Distribution License shall become convertible by the Licensor, at its sole discretion, back into a Non-Exclusive Distribution License. For the avoidance of doubt, during the Term, the non-exclusive, royalty-bearing license to use, make and have made Licensed Products in the Territory during the Term for applications within the Field of Use shall continuously remain a non-exclusive license.
(b) The parties acknowledge and agree that the license granted to Licensee under Section 2.01(a) does not and shall not be construed to prohibit or restrict Licensor, any Licensor Affiliate, Greatbatch or any Greatbatch Affiliate from using or otherwise exploiting the Licensed IP (i) to make, have made, use, offer to sell, sell, distribute and import any Licensed Product for applications outside of the Field of Use or (ii) to make, have made, use, offer to sell, sell, distribute and import any Modified Product for applications within or outside of the Field of Use.
Section 2.02 Except for the rights and licenses granted by Licensor under this License Agreement, this License Agreement does not grant to Licensee or any other Person any right, title or interest by implication, estoppel, or otherwise. Without limitation of the foregoing, nothing in this License Agreement shall be construed as granting by implication, estoppel, or otherwise, any right, title or interest in, to or under any Licensor or Licensor Affiliate patent or patent application, other than the Licensed IP, regardless of whether such other patents are dominant or subordinate to any Licensed IP. All rights, titles and interests not specifically and expressly granted by Licensor hereunder are hereby reserved.
Section 2.03 For the avoidance of doubt, the parties acknowledge and confirm that Licensor retains the right to use, and to have its Affiliates use, the Licensed IP and any and all Inventions disclosed and/or claimed in the Licensed Patent Applications and the Licensed Patents for all applications outside of the Field of Use. In addition, Licensee shall not, and shall not permit any of its Affiliates to, use any Licensed IP outside of the specific scope of the license granted to it under this License Agreement.
- 7 -
Section 2.04 From time to time as reasonably necessary to reflect changes in the status of the Licensed IP, the Licensor will update Exhibit A and provide a copy thereof in writing to the Licensee. The Licensee may also make a written request that Exhibit A be updated, in which case the Licensor will promptly provide the Licensee with a revised Exhibit A that sets forth all of the then Licensed IP and the current status thereof.
Article III.
SUBLICENSES
The Licensee shall provide to Licensor all proposed, unexecuted written agreements pursuant to which Licensee would sublicense any Licensed IP for the Licensor’s prior review and written approval. The Licensee shall not enter into any sublicense agreement with respect to the Licensed IP without Licensor’s prior written consent, which consent may be withheld by Licensor in its absolute and sole discretion. Any permitted sublicensees shall be prohibited from granting any further sublicenses.
Article IV.
PATENT RIGHTS; INTELLECTUAL PROPERTY
Section 4.01 [Reserved.]
Section 4.02 Licensor agrees to use reasonable commercial efforts to protect the Licensed IP licensed hereunder to Licensee by obtaining and maintaining appropriate patent rights as recommended by reputable patent counsel; provided, however, that Licensee shall have the rights provided below in Section 4.03 of this License Agreement subject to the right of Licensor to determine, in good faith, not to file or prosecute an unpublished patent application and to maintain an Invention as a trade secret.
Section 4.03 In the event that Licensor decides to terminate prosecution of a Licensed Patent Application or discontinue maintaining any Licensed Patent in any country, then the Licensor shall provide the Licensee with prompt written notice of such decision but, in any event, such notice shall be provided to the Licensee at least sixty (60) days before any known bar date or non-extendable deadline. The Licensee, at its option, may then in writing request the Licensor to continue prosecution of any Licensed Patent Application and/or maintain any such Licensed Patent, as applicable, at the sole and exclusive expense of the Licensee. If the Licensor declines in writing to continue any such prosecution or maintenance obligation, the Licensee may provide the Licensor with written notice that the Licensee wishes to assume control of the prosecution of any such Licensed Patent Application and/or to maintain any such Licensed Patent, as applicable, at the sole and exclusive expense of the Licensee. If the Licensee assumes such control or maintenance obligation, the Licensor agrees to cooperate with the Licensee, its attorneys and agents in the prosecution of such Licensed Patent Application and to provide the Licensee with complete copies of any and all documents and other related materials that the Licensee deems necessary to undertake such control or obligation.
- 8 -
Article V.
ENFORCEMENT OF LICENSED PATENTS
Section 5.01 In the event of any third party infringement of any Licensed Patent, the party hereto having knowledge thereof shall promptly notify the other party of such infringement, whereupon the parties shall consult with a view to reaching an agreement as to the ways and means for eliminating such infringement. If both parties desire to litigate such infringement, they shall equally share any costs thereof and any recovery therein. The Licensor shall promptly advise the Licensee of all costs incurred with respect to any such litigation and provide supporting documentation with respect thereto. The Licensee shall within thirty (30) days of receipt of such documentation pay the Licensor its share of costs. In the event that either party desires to litigate such infringement and the other party refuses or fails to do so, the party desiring litigation may in its sole discretion, and at its sole expense, and by counsel of its own choice, bring suit to restrain such infringement, and shall be entitled to receive and retain, for its own use and benefit, any recovery awarded in such suit, including, without limitation, monetary damages. In the event that the Licensee desires to litigate such infringement and the Licensor refuses or fails to do so, the Licensor, agrees (a) to be joined as a party in such litigation, if so required by law, with all costs of Licensor’s participation in such litigation at the sole and exclusive expense of the Licensee and (b) that the Licensee shall have full control of such litigation.
Section 5.02 Unless the Licensor refuses or fails to initiate such litigation, the Licensor shall have primary control of the litigation, including the right to appoint counsel and the right to settle or compromise same with the written consent of the Licensee, which consent shall not be unreasonably withheld. In any event, the parties agree to use reasonable commercial efforts to cooperate with one another in any litigation relating to such third party infringement.
Article VI.
MARKING AND REGULATORY CLEARANCES
Section 6.01 Licensee shall comply with the patent marking provisions of 35 USC § 287(a) by marking an appropriate patent notice on each Licensed Product, or its packaging, labels, containers, displays or any associated printed materials, as appropriate, manufactured by or on behalf of the Licensee. Licensee shall include in all sublicense agreements, and require in any sublicense agreement granted by it or any sublicensee, a patent marking requirement substantially identical to this Section 6.01.
Section 6.02 The parties acknowledge and agree that the Licensee shall have the sole right to adopt trademarks of its own choosing with respect to all Licensed Products manufactured and/or sold under this License Agreement and that the Licensee shall own all right, title and interest to such trademarks. The Licensee shall not use any trademark, service xxxx, trade name, corporate name, or any other identifier of the Licensor, or any Affiliate of the Licensor, in connection with the Licensed Products in any manner without the express written consent of the Licensor.
- 9 -
Section 6.03 Licensee shall, at Licensee’s sole and exclusive expense, comply with all regulations and safety standards concerning Licensed Products developed and commercialized by or under the authority of Licensee, including, without limitation, the regulations and safety standards of the FDA, and obtain all necessary Regulatory Approvals for the development, production, distribution, sale and use of Licensed Products developed and commercialized by or under the authority of Licensee, including any safety or clinical studies. Licensee shall have responsibility for and provide suitable warning labels, packaging and instructions as to the use for such Licensed Products.
Article VII.
BOOKS AND RECORDS
Section 7.01 The Licensee agrees to maintain and keep full and accurate books and records regarding all Licensed Products and to retain such books and records for the Term and for a period of five (5) years thereafter in sufficient detail to identify all Licensed Products manufactured or provided by or on behalf of the Licensee and/or shipped, sold, or otherwise transferred or provided by the Licensee. Such books and records shall indicate: (a) invoice numbers, (b) invoice dates, (c) invoice prices, (d) customers, (e) quantity of Licensed Products shipped and sold, (f) shipping costs, regular trade and quantity discounts and taxes and (g) any other information reasonably necessary to audit the accuracy of Royalty Fee payments made hereunder. The Licensee agrees that such books and records shall be maintained in accordance with generally accepted and reasonable accounting principles in the United States.
Section 7.02 (a) The Licensee agrees that a certified public accountant selected by and compensated by the Licensor shall be permitted upon reasonable prior notice to inspect and copy, during Licensee’s normal business hours, said books and records of the Licensee. The Licensee further agrees that, at the Licensor’s expense, said certified public accountant shall have the right upon reasonable prior notice to audit the Licensee’s physical inventory (including component parts, work-in-progress, and finished goods) of Licensed Products.
(b) Licensor shall provide to Licensee a copy of the audit report within fourteen (14) days of Licensor’s receipt of the report. If the report shows that payment of Royalty Fees made by Licensee are deficient, Licensee shall pay Licensor the deficient amount plus interest on the deficient amount within thirty (30) days after Licensee’s receipt of the audit report. If payments made by Licensee are found to be deficient by more than five percent (5%), Licensee shall pay for the full cost of the audit.
- 10 -
Article VIII.
ROYALTIES
Section 8.01 In consideration of its license from the Licensor under this License Agreement, Licensee shall pay to Licensor a royalty fee (the “Royalty Fee”) in an amount equal to seven percent (7%) of the Net Sales of any Licensed Product (regardless of whether such sales are on-label or off-label) transferred or otherwise disposed of by or for Licensee in the Territory. The Royalty Fee shall be payable by the Licensee to the Licensor for the period beginning on the Effective Date and running until (and inclusive of) the year of the expiration of the Term.
Section 8.02 Royalties and other sums payable under this License Agreement are exclusive of taxes. Licensee shall be responsible for all sales, use, excise and value added taxes and any other similar taxes, duties and charges of any kind imposed by any federal, state or local Governmental Body on any amounts payable by Licensee hereunder and shall pay all such royalties and other sums payable hereunder free and clear of all deductions and withholdings whatsoever, unless the deduction or withholding is required by law. If any deduction or withholding is required by law, Licensee shall pay to Licensor such sum as will, after the deduction or withholding has been made, leave Licensor with the same amount as it would have been entitled to receive without any such requirement to make a deduction or withholding.
Section 8.03 (a) Licensee shall pay all Royalty Fees for each Quarterly Period within thirty (30) days after the end of such Quarterly Period. Licensee shall make all payments in United States dollars by wire transfer of immediately available funds to a bank account to be designated in writing by Licensor. For the purpose of converting the local currency in which any Royalty Fee arises into United States dollars, the rate of exchange to be applied shall be the rate of exchange in effect on the last Business Day of the Quarterly Period to which the payment relates as reported in the Wall Street Journal.
(b) On or before the due date for all payments paid to Licensor pursuant to Section 8.03, Licensee shall provide Licensor with a written statement including each of the following:
(i) the total number of Licensed Products (broken out by each Licensed Product, to the extent applicable) sold, transferred or otherwise disposed of by Licensee in the relevant Quarterly Period;
(ii) the total Net Sales of all Licensed Products (broken out by each Licensed Product, to the extent applicable) sold, transferred or otherwise disposed of by Licensee in the relevant Quarterly Period;
(iii) the calculation of the Royalty Fee for the relevant Quarterly Period; and
(iv) such other information and particulars as are reasonably necessary for an accurate accounting of the Royalty Fee paid pursuant to this License Agreement.
- 11 -
(c) If payments are not received by Licensor within thirty (30) days after becoming due, Licensee shall pay to Licensor interest on the overdue payment from the date such payment was due to the date of actual payment at a rate of one and a half percent (1.5%) per month, or if lower, the maximum amount permitted under applicable law.
Article IX.
TERM AND TERMINATION
Section 9.01 This License Agreement and the license hereunder shall be effective as of the Effective Date. This License Agreement shall, unless terminated in accordance with the provisions of this Article IX, be and remain in effect until the expiration of the last to expire Licensed Patent or the last Licensed Patent Application that becomes abandoned, whichever is later (herein referred to as the “Term”).
Section 9.02 In the event of any breach by the Licensee of this License Agreement, the Licensor shall deliver written notice thereof to the Licensee. The Licensee shall use all reasonable commercial efforts to cure such breach within forty-five (45) days of notice from the Licensor as aforesaid. If, within such period, the Licensee has not cured such breach, the Licensor shall have the right to terminate this License Agreement upon prior written notice to the Licensee.
Section 9.03 The Licensor, shall have the right to immediately terminate this License Agreement, upon prior written notice to the Licensee, if
(a) the Licensee: (i) becomes insolvent or generally fails to pay, or admits in writing its inability to pay, its debts as they become due; (ii) applies for or consents to the appointment of a trustee, receiver or other custodian, or makes a general assignment for the benefit of its creditors; (iii) commences any bankruptcy, reorganization, debt arrangement, or other case or proceeding under any bankruptcy or insolvency law, or any dissolution or liquidation proceedings (each, hereinafter referred to as a “Bankruptcy Proceeding’); or (iv) has a Bankruptcy Proceeding commenced against it and such Bankruptcy Proceeding is not dismissed within thirty (30) days of the date of commencement thereof;
(b) on or after a date that is two (2) years after the Effective Date, the Licensee or any of its Affiliates is not demonstrably and actively engaged in a research, development, manufacturing, marketing or sales activity for the Licensed Products, as appropriate, or has otherwise abandoned use of the Licensed IP;
(c) the Development Agreement is terminated for any reason, except as a result of a breach by the Licensor of its obligations under the Development Agreement;
(d) the Supply Agreement is terminated by Greatbatch or any Greatbatch Affiliate, as applicable, as a result of a breach by the Licensee of its obligations under the Supply Agreement;
- 12 -
(e) Licensee, directly or indirectly through one or more of its Affiliates, challenges the validity of, takes material and documented steps to support any proceeding with intended effect of invalidating, or otherwise attempts to limit the scope of any Licensed Patent, Licensed Patent Application or any other patent owned or licensed by Licensor or its Affiliates, in each case whether through post grant review, inter partes review, ex parte reexamination or other method;
(f) the Licensor is advised in writing by its outside legal counsel that it is not advisable for Licensee to continue with the commercialization of the Licensed Product as a result of an actual, threatened or perceived material safety, legal or economic risk regarding such Licensed Product as the result of any law, decree, resolution, liabilities resulting from a claim against any Person, or any decision of a Governmental Body or the FDA or change in the interpretation of any current law, decree, resolution or decision by a Governmental Body;
(g) there is a Change of Control of Licensee and, in connection with such Change of Control, Licensor does not believe in its reasonable judgment that Licensee’s successor in such Change of Control is able to perform Licensee’s obligations under this Agreement; or
(h) any of the milestones listed on Exhibit C hereto fails to be reached.
Section 9.04 Immediately upon termination pursuant to this Article IX, the Licensee shall cease and desist from making, having made, using, importing, distributing, selling and/or offering to sell the Licensed Products. After early termination of this License Agreement, the Licensor shall be free to grant to others exclusive rights under any or all of the Licensed IP. Termination of this License Agreement for any reason, or the expiration of this License Agreement, shall not relieve either party from performing obligations incurred prior to such termination or expiration.
Section 9.05 Neither party shall be in default hereunder by reason of any failure or delay in the performance of its obligations hereunder, except for Licensee’s payment obligations, where such failure or delay is due to any cause beyond its reasonable control, including strikes, labor disputes, civil disturbances, riot, rebellion, invasion, epidemic, hostilities, war, terrorist attack, embargo, natural disaster, acts of God, flood, fire, sabotage, loss and destruction of property or any other circumstances or causes beyond such party’s reasonable control.
Section 9.06 The provisions of Articles I and Articles VII through and including XIV shall survive termination or expiration of this License Agreement.
Article X.
REPRESENTATIONS AND WARRANTIES BY LICENSOR
Section 10.01 The Licensor, to its knowledge, represents, warrants and covenants that:
(a) The Licensor is a corporation duly incorporated, validly existing, and in good standing under the law of the State of Delaware, and has full corporate power to conduct the business in which it is presently engaged and to enter into and perform its obligations under this License Agreement.
- 13 -
(b) The Licensor has taken all necessary corporate action under the laws of the State of Delaware and its certificate of incorporation and by-laws to authorize the execution and consummation of this License Agreement and, when executed and delivered, this License Agreement shall constitute a valid and legally binding agreement of the Licensor enforceable against the Licensor in accordance with the terms hereof, except as may be limited by bankruptcy, insolvency or other laws affecting generally the enforceability of creditors’ rights and by limitations on the availability of equitable remedies.
(c) Neither the execution and delivery of this License Agreement nor the consummation of the transactions contemplated herein will violate any law, rule, regulation, writ, judgment, injunction, decree, determination, award or other order of any court, government or governmental agency or instrumentality, domestic or foreign, binding upon the Licensor, or conflict with or result in any breach of or event of termination under any of the terms of, or constitute a default under or result in the termination of or the creation or imposition of any mortgage, deed of trust, pledge, lien, security interest or other charge or encumbrance of any nature pursuant to, the terms of any contract or agreement to which the Licensor is a party or by which the Licensor or any of its assets and properties are bound.
Section 10.02 THE LICENSEE ACKNOWLEDGES THAT THE LICENSED PATENTS, THE LICENSED PATENT APPLICATIONS AND THE TECHNICAL KNOW-HOW ARE BEING LICENSED “AS-IS,” WITH NO WARRANTIES WHATSOEVER. WITHOUT LIMITING ANY PROVISION CONTAINED IN THIS LICENSE AGREEMENT, THE LICENSOR HEREBY DISCLAIMS ALL EXPRESS AND IMPLIED WARRANTIES, INCLUDING THOSE OF NON-INFRINGEMENT, VALIDITY, TITLE, FITNESS FOR A PARTICULAR PURPOSE AND MERCHANTABILITY.
Article XI.
REPRESENTATIONS AND WARRANTIES BY LICENSEE
Section 11.01 The Licensee, to its knowledge, represents, warrants and covenants that:
(a) The Licensee is a Swiss share company registered under the federal identification number CHE-114.416.910 and is duly organized, validly existing, and in good standing under the law of Switzerland, and has full right and authority to conduct the business in which it is presently engaged and to enter into and perform its obligations under this License Agreement.
(b) The Licensee has taken all necessary action under the laws of Switzerland and its articles of association to authorize the execution and consummation of this License Agreement and, when executed and delivered, this License Agreement shall constitute the valid and legally binding agreement of the Licensee enforceable against the Licensee in accordance with the terms hereof, except as may be limited by bankruptcy, insolvency or other laws affecting generally the enforceability of equitable remedies.
- 14 -
(c) Neither the execution and delivery of this License Agreement nor the consummation of the transactions contemplated herein will violate any law, rule, regulation, writ, judgment, injunction, decree, determination, award or other order of any court, government or governmental agency or instrumentality, domestic or foreign, binding upon the Licensee, or conflict with or result in any breach of or event of termination under any of the terms of, or constitute a default under or result in the termination of or the creation or imposition of any mortgage, deed of trust, pledge, lien, security interest or other charge or encumbrance of any nature pursuant to, the terms of any contract or agreement to which the Licensee is a party or by which the Licensee or any of its assets and properties are bound.
Article XII.
INDEMNIFICATION & INSURANCE
Section 12.01 The Licensee shall indemnify and hold harmless the Licensor and its Affiliates, and their respective directors, officers, members, managers, employees, agents, representatives, successors and assigns (herein referred to, collectively, as the “Indemnified Parties”), and each of them, from and against all damages, costs, expenses, interest (including prejudgment interest), losses, claims, demands, liabilities, deficiencies and/or obligations, including, without limitation, reasonable fees and disbursements of counsel (herein referred to, collectively, as “Damages”), which the Indemnified Parties, or any of them, may incur resulting, directly or indirectly, wholly or partly by reason of: (a) the breach of any representation, warranty, covenant, agreement or obligation to have been performed hereunder by the Licensee, or any of its Affiliates; or (b) the Licensee’s, any of its sublicensee’s, or any of its Affiliate’s use of the Licensed IP or sale of any Licensed Products after the Effective Date.
Section 12.02 If the facts that give rise to any indemnification hereunder shall involve any actual or threatened claim or demand (herein referred to as a “Third-Party Claim”) by any Person (including, without limitation any tax authority or other Governmental Body) other than a party hereto, its Affiliates, or their respective successors or assigns, notice thereof shall be given by the Indemnified Party to the Licensee no later than ten (10) days after the Indemnified Party shall have received written notice thereof from the third party making such Third Party Claim; provided, however, that if the Third-Party Claim is in the form of a pleading requiring an answer, such notice shall be given at least seven (7) Business Days prior to the due date of the answer or other response to the pleading; provided, further, however, that failure to give such notice shall not affect the indemnification provided hereunder. Thereafter, the Indemnified Parties shall deliver to the Licensee copies of all notices and documents (including court papers) received by the Indemnified Party relating to the Third-Party Claim.
Section 12.03 The Licensee shall have thirty (30) days from receipt of the notice provided by the Indemnified Party pursuant to Section 12.02 immediately preceding to provide the Indemnified Party with notice that it wishes to assume the defense of the Third-Party Claim, in which event the Indemnified Party shall have the right to participate in the defense at its own expense, including, without limitation, the right at its expense to employ counsel separate from counsel employed by the Licensee, except that such counsel shall be at the expense of the Licensee if (a) the Indemnified Parties are required to retain separate counsel due to a conflict of interest with the Licensee, or (b) the Licensee fails to act diligently in defending such action. In no event shall the Licensee be responsible for the fees and expenses of more than one firm of lead counsel and, to the extent required, one local counsel, representing all Indemnified Parties.
- 15 -
Section 12.04 If the Licensee chooses to defend a Third-Party Claim, the Indemnified Parties shall cooperate in the defense thereof. Such cooperation shall include the retention and the provision to the Licensee assuming the defense of records and information which are reasonably relevant to such Third-Party Claim and making relevant employees or agents reasonably available during normal business hours on a mutually convenient basis to provide additional information and explanation of any material provided hereunder. If the Licensee assumes the defense of a Third-Party Claim, each Indemnified Party shall agree to any settlement, compromise or discharge of such Third-Party Claim that the Licensee assuming the defense may recommend and that by its terms obligates the Licensee (a) to pay the full amount of the liability in connection with such Third-Party Claim; (b) releases the Indemnified Party completely in connection with such Third-Party Claim; and (c) would not otherwise adversely affect the Indemnified Party.
Section 12.05 In the event any Indemnified Party should have a claim against the Licensee under this Article XII that does not involve a Third-Party Claim, the Indemnified Party shall deliver notice of such claim with reasonable promptness to the Licensee, together with its request forthwith for payment subject to the provisions of this License Agreement; provided, however, that, the failure by any Indemnified Party so to notify the Licensee shall not relieve the Licensee from any liability that it may have to such Indemnified Party under this Article XII except to the extent that the Licensee demonstrates that it has been materially prejudiced by such failure.
Section 12.06 Licensee shall, at all times during the Term and for five (5) years thereafter, obtain and maintain at its own expense the following types of insurance, with limits of liability not less than those specified below:
(a) Commercial general liability insurance against claims for bodily injury and property damage which shall include contractual coverage and product liability coverage, with limits of not less than $10,000,000 per occurrence and $20,000,000 in the aggregate; provided, that Licensee’s obligation to obtain and maintain the insurance coverage described under this Section 12.06(a) shall not commence until sixty (60) days prior to the commencement of (i) a human clinical study for any Licensed Product, or (ii) the sale or transfer of any Licensed Product, whichever occurs first; provided, further, that Licensee shall deliver to Licensor certificates of insurance evidencing the insurance coverage described in this Section 12.06(a) at least sixty (60) days prior to the commencement of a human clinical study for any Licensed Product and the sale or transfer of any Licensed Product, and upon reasonable request thereafter. The Licensor shall be named as an additional insured; and
(b) Workers compensation and employers’ liability with limits to comply with the statutory requirements of the state(s) in which the License Agreement is to be performed. The policy shall include employers’ liability for not less than $5,000,000 per accident.
- 16 -
Licensee shall deliver certificates of insurance evidencing coverage to Licensor promptly after the execution of this License Agreement and upon reasonable request thereafter. All policies provided for herein shall expressly provide that such policies shall not be cancelled, terminated or altered without at least thirty (30) days prior written notice to the Licensee, and Licensee shall immediately notify the Licensor in the event that a policy provided for herein is cancelled, terminated or altered.
Article XIII.
CONFIDENTIALITY
Section 13.01 Confidential Information provided by the disclosing party and entitled to protection under this License Agreement shall be identified as such by an appropriate marking of “Confidential Information” on any document exchanged. If the disclosing party provides information other than in written form, such information shall be considered Confidential Information only if (a) the information by its nature would reasonably be considered of a confidential nature or if the receiving party, due to the context in which the information was disclosed, should have reasonably known it to be confidential, and (b) either the disclosing party gives written notice within thirty (30) days of disclosure that such information is to remain confidential or the disclosing party had previously confirmed in writing that such information was confidential.
Section 13.02 Each party acknowledges that the other party claims its trade secrets and other Confidential Information as special, valuable and unique assets. During the Restricted Period for itself and on behalf of its officers, directors, agents, and employees, each party agrees to the following:
(a) The receiving party will use the Confidential Information only for the purposes of exercising its rights or fulfilling its obligations under this License Agreement and will not otherwise use it for its own benefit. In no event shall the receiving party use less than the same degree of care to protect the Confidential Information as it would employ with respect to its own information of like importance which it does not desire to have published or disseminated.
(b) The receiving party will not disclose any Confidential Information to any third party or disclose to an employee unless:
(i) such disclosure is reasonably necessary (A) for the filing or prosecuting of Licensed Patents as contemplated by this License Agreement; (B) to comply with the requirement of a Governmental Body with respect to obtaining and maintaining Regulatory Approvals (or any pricing and reimbursement approvals) of any Licensed Product; or (C) for prosecuting or defending litigations as contemplated by this License Agreement;
(ii) such disclosure is reasonably necessary to its members, officers, directors, managers, employees, agents, consultants or contractors on a need-to-know basis for the sole purpose of performing its obligations or exercising its rights under this License Agreement; provided that in each case, the disclosees must be bound by written obligations of confidentiality and non-use consistent with those contained in this License Agreement;
- 17 -
(iii) such disclosure is reasonably necessary to any bona fide potential or actual investor, acquiror, merger partner, or other financial or commercial partner for the sole purpose of evaluating an actual or potential investment, acquisition or other business relationship; provided that in each case, the disclosees must be bound by written obligations of confidentiality and non-use consistent with those contained in this License Agreement; or
(iv) such disclosure is reasonably necessary to comply with applicable law, including regulations promulgated by applicable security exchanges, a valid order of a court of competent jurisdiction, administrative subpoena or order; provided, however, if the receiving party is subject to a valid order of a court of competent jurisdiction, administrative subpoena or order requiring disclosure of Confidential Information, then, prior to disclosing any such Confidential Information, the receiving party shall promptly notify the disclosing party in writing and, upon the disclosing party’s request, shall cooperate with the disclosing party in contesting such request or in obtaining a protective order or other similar injunctive relief.
(c) The parties acknowledge that either or both parties may be obligated to file a copy of this License Agreement with the United States Securities and Exchange Commission or similar stock exchange authorities or other governmental authorities. Each party shall be entitled to make such a required filing; provided, however, that it requests confidential treatment of the commercial terms and sensitive technical terms hereof and thereof to the extent such confidential treatment is reasonably available to such party. In the event of any such filing, each party shall provide the other party with a copy of this License Agreement marked to show provisions for which such party intends to seek confidential treatment and shall reasonably consider and incorporate the other party’s comments thereon to the extent consistent with the legal requirements, with respect to the filing party, governing disclosure of material agreements and material information that must be publicly filed.
(d) For purposes hereof, the term “Restricted Period” means (a) in the case of any Confidential Information that is designated as trade secrets of a disclosing party (which designation can be made at any reasonable time by the disclosing party), in perpetuity; and (b) in the case of other Confidential Information of a disclosing party, during the Term and for a period of ten (10) years thereafter.
Section 13.03 All information furnished under this License Agreement shall remain the property of the disclosing party and shall be returned to it or destroyed or purged promptly as requested by the disclosing party upon termination of this License Agreement. All documents, memoranda, notes and other tangible embodiments whatsoever prepared by the receiving party based on or which includes Confidential Information shall be destroyed to the extent necessary to remove all such Confidential Information upon the disclosing party’s request. An authorized officer of the receiving party shall, upon request, certify all destruction under this Section 13.03 in writing to the disclosing party.
- 18 -
Section 13.04 The confidentiality obligations in this Article XIII shall not apply to disclosed information which the receiving party can prove: (a) that the receiving party knew at the time of disclosure, free of any obligation to keep it confidential, as evidenced by written records; (b) that is or becomes generally publicly known through disclosure without breach of confidentiality obligations by the receiving party, (c) that the receiving party independently developed without the use of any Confidential Information as evidenced by written records; or (d) receiving party rightfully obtains from a third party who has the right to transfer or disclose it.
Section 13.05 Notwithstanding anything to the contrary contained in this License Agreement, neither party may initiate or make any public announcement or other disclosure concerning the terms and conditions or the subject matter of this License Agreement to any third party without the prior written approval of the other party except as may be required by law. In those circumstances where either party believes that any such disclosure is required by law, it shall (a) notify the other party on a timely basis in advance and (b) use its best efforts to seek confidential treatment of the material provisions of this License Agreement to the greatest extent permitted by applicable law.
Article XIV.
MISCELLANEOUS
Section 14.01 Licensee shall not assign or otherwise transfer any of its rights, or delegate or otherwise transfer any of its obligations or performance, under this License Agreement, in each case whether voluntarily, involuntarily, by operation of law or otherwise, without Licensor’s prior written consent, which consent Licensor may give or withhold in its sole discretion. No delegation or other transfer will relieve Licensee of any of its obligations or performance under this License Agreement. Any purported assignment, delegation or transfer in violation of Section 14.01 is void. Notwithstanding anything in this Section 14.01 to the contrary, Licensee shall assign this Agreement to Licensee’s successor in the Change of Control of Licensee; provided, that such successor, in the reasonable judgment of Licensor, is able to perform Licensee’s obligations under this Agreement. Licensor may freely assign or otherwise transfer all or any of its rights, or delegate or otherwise transfer all or any of its obligations or performance, under this License Agreement without Licensee's consent. This License Agreement is binding upon and inures to the benefit of the parties hereto and their respective permitted successors and assigns.
Section 14.02 Unless otherwise provided in this License Agreement, any notice to be given hereunder shall be in writing and (a) delivered personally (to be effective when so delivered), (b) mailed by registered or certified mail, return receipt requested (to be effective four days after the date it is mailed) or (c) sent by Federal Express or other overnight courier service (to be effective when received by the addressee), to the following addresses (or to such other addresses which any party shall designate in writing to the other parties):
If to Licensor:
Nuvectra Corporation
0000 Xxxxxxx Xxxxxxx
Xxxxx 000
Xxxxx, Xxxxx 00000
Attn: General Counsel
- 19 -
If to Licensee:
Aleva Neurotherapeutics SA
EPFL Xxxxxxxxxx Xxxx, Xxxxxxxx X
0000 Xxxxxxxx, Xxxxxxxxxxx
Attn: Chief Executive Officer
Section 14.03 This License Agreement and all exhibits attached hereto contain the entire agreement between the parties hereto with respect to the transactions contemplated hereby, and supersede all prior understandings, arrangements and agreements, written or oral, with respect to the subject matter hereof. No modification or amendments to this License Agreement shall be effective unless in writing and signed by the party against which it is sought to be enforced.
Section 14.04 Each of the parties hereto shall bear such party’s own expenses in connection with this License Agreement and the transactions contemplated hereby, except as may otherwise expressly be set forth herein. It is expressly understood that the parties are independent of one another and that neither has the authority to bind the other to any third person or otherwise to act in any way as the representative of the other, unless otherwise expressly agreed to in writing signed by both parties hereto.
Section 14.05 Each of the parties hereto shall use such party’s commercially reasonable efforts to take such actions as may be necessary or reasonably requested by the other party hereto to carry out and consummate the transactions contemplated by this License Agreement.
Section 14.06 This License Agreement shall be governed by and construed in accordance with the laws of the State of New York, without regard to the conflict of laws provisions thereof to the extent such principles or rules would require or permit the application of the laws of any jurisdiction other than those of the State of New York.
Section 14.07 The captions appearing herein are for the convenience of the parties only and shall not be construed to affect the meaning of the provisions of this License Agreement. All references in this License Agreement to Sections, Articles and Exhibits refer to the Sections, Articles and Exhibits of this License Agreement and exhibits attached hereto is hereby incorporated in and made a part of this License Agreement.
Section 14.08 Each party acknowledges and agrees that any controversy which may arise under this License Agreement is likely to involve complicated and difficult issues, and therefore each such party hereby irrevocably and unconditionally waives any right such Party may have to a trial by jury in respect of any litigation directly or indirectly arising out of or relating to this License Agreement, or the transactions contemplated by this License Agreement. Each Party certifies and acknowledges that (i) no representative, agent or attorney of any other Party has represented, expressly or otherwise, that such other Party would not, in the event of litigation, seek to enforce the foregoing waiver, (ii) each party makes this waiver voluntarily, and (iII) each Party has been induced to enter into this License Agreement by, among other things, the mutual waivers and certifications in this SECTION 14.08.
- 20 -
Section 14.09 The parties agree that irreparable damage would occur in the event that any of the provisions of this License Agreement were not performed in accordance with their specific terms or were otherwise breached. It is accordingly agreed that, except where this License Agreement is terminated in accordance with Article IX, the parties shall be entitled to an injunction or injunctions to prevent breaches or threatened breaches of this License Agreement and to specifically enforce the terms and provisions of this License Agreement and any other agreement or instrument executed in connection herewith. Each party waives any requirements for the securing or posting of any bond in connection with any such remedy. The parties further agree that (i) by seeking the remedies provided for in this Section 14.09, a party shall not in any respect waive its right to seek any other form of relief that may be available to a party and not otherwise specifically waived under this License Agreement, including monetary damages in the event that this License Agreement has been terminated or in the event that the remedies provided for in this Section 14.09 are not available or otherwise are not granted and (ii) nothing contained in this Section 14.09 shall require any party to institute any proceeding for (or limit any party’s right to institute any proceeding for) specific performance under this Section 14.09 before exercising any termination right under Article IX (and pursing damages after such termination) nor shall the commencement of any action pursuant to this Section 14.09 or anything contained in this Section 14.09 restrict or limit any party’s right to terminate this License Agreement in accordance with the terms of Article IX or pursue any other remedies under this License Agreement that may be available then or thereafter.
Section 14.10 Each of the parties hereto (i) irrevocably consents to the service of the summons and complaint and any other process in any action or proceeding contemplated by Section 14.09 or otherwise in any way relating to this License Agreement, on behalf of itself and/or officers, in accordance with the notice provision set forth in Section 14.02 or in such other manner as may be permitted by law, of copies of such process to such party, and nothing in this Section 14.10 shall affect the right of any party to serve legal process in any other manner permitted by law, (ii) irrevocably and unconditionally consents and submits itself and its property in any action or proceeding to the exclusive general jurisdiction of any state or federal court within the State of New York in the event any dispute arises out of this License Agreement or the transactions contemplated by this License Agreement, or for recognition and enforcement of any judgment in respect thereof, (iii) agrees that it will not attempt to deny or defeat such personal jurisdiction by motion or other similar relief from any such court, (iv) agrees that any actions or proceedings arising in connection with this License Agreement or the transactions contemplated by this License Agreement shall be brought, tried and determined only in a state or federal court within the State of New York, (v) waives any objection that it may now or hereafter have to the venue of any such action or proceeding in any such court or that such action or proceeding was brought in an inconvenient court and agrees not to plead or claim the same and (vi) agrees that it will not bring any action relating to this License Agreement or the license or other matters covered by this License Agreement in any court other than the aforesaid courts. Each of the parties agrees that a final judgment in any action or proceeding in such court as provided above shall be conclusive and may be enforced in other jurisdictions by suits on the judgment or in any other manner provided by law.
- 21 -
Section 14.11 This License Agreement and all of the provisions hereof shall be binding upon and inure to the benefit of the parties hereto and their respective successors and permitted assigns.
Section 14.12 Any term or provision of this License Agreement that is invalid or unenforceable in any jurisdiction shall, as to such jurisdiction, be ineffective to the extent of such invalidity or unenforceability without rendering invalid or unenforceable the remaining terms and provisions of this License Agreement, or any such terms in any other jurisdiction. If any provision of this License Agreement is so broad as to be unenforceable, such provision shall be interpreted to be only so broad as is enforceable.
Section 14.13 Nothing in this License Agreement, express or implied, is intended to confer on any Person other than the parties hereto, and their respective successors and permitted assigns any rights, remedies, obligations or liabilities under or by reason of this License Agreement; provided; however, the parties hereby designate Greatbatch as a third-party beneficiary of this License Agreement with the right to enforce this License Agreement.
Section 14.14 No waiver by any party of any of the provisions hereof shall be effective unless explicitly set forth in writing and signed by the party so waiving. No waiver by any party shall operate or be construed as a waiver in respect of any failure, breach or default not expressly identified by such written waiver, whether of a similar or different character, and whether occurring before or after that waiver. No failure to exercise, or delay in exercising, any right, remedy, power or privilege arising from this License Agreement shall operate or be construed as a waiver thereof; nor shall any single or partial exercise of any right, remedy, power or privilege hereunder preclude any other or further exercise thereof or the exercise of any other right, remedy, power or privilege.
Section 14.15 This License Agreement may be executed in counterparts, each of which shall be deemed an original, but all of which taken together shall constitute one and the same instrument. A signed copy of this License Agreement delivered by facsimile, e-mail or other means of electronic transmission (to which a PDF copy is attached) shall be deemed to have the same legal effect as delivery of an original signed copy of this License Agreement.
[SIGNATURE PAGE FOLLOWS]
- 22 -
IN WITNESS WHEREOF, the parties have caused this License Agreement to be properly executed and delivered as of the Effective Date.
|
|
NUVECTRA CORPORATION |
| |
|
|
|
|
|
|
|
|
|
|
|
|
By |
/s/ Xxxxxx X. Xxxxxx |
|
|
|
Name: |
Xxxxxx X. Xxxxxx |
|
|
|
Title: |
CFO |
|
|
|
ALEVA NEUROTHERAPEUTICS SA |
| |
|
|
|
|
|
|
|
|
|
|
|
|
By |
/s/ Xxxxx Xxxxxxxxxx |
|
|
|
Name: |
Xxxxx Xxxxxxxxxx |
|
|
|
Title: |
CTO |
|
EXHIBIT A
LIcensed IP
Patents and Patent Applications Licensed to Aleva (As of October 2015)
|
United States Issued Patents and Pending Applications | ||||
|
Patent No. |
Application No. |
Title |
Country | |
|
1 |
US 8, 954,148 |
13/170,558 |
KEY FOB CONTROLLER FOR AN IMPLANTABLE NEUROSTIMULATOR |
United States |
|
2 |
US 8,483,836 |
13/226,969 |
AUTOMATED SEARCH TO IDENTIFY A LOCATION FOR ELECTRICAL STIMULATION TO TREAT A PATIENT |
United States |
|
3 |
US 8,515,545 |
13/098,071 |
CURRENT STEERING NEUROSTIMULATOR DEVICE WITH UNIDIRECTIONAL CURRENT SOURCES |
United States |
|
4 |
US 8,571,667 |
13/175,283 |
ACTIVE CURRENT CONTROL USING THE ENCLOSURE OF AN IMPLANTED PULSE GENERATOR |
United States |
|
5 |
US 8,738,153 |
14/019,653 |
IMPLANTABLE LEAD WITH BRAIDED CONDUCTORS |
United States |
|
6 |
US 8,757,485 |
13/604,285 |
SYSTEM AND METHOD FOR USING CLINICIAN PROGRAMMER AND CLINICIAN PROGRAMMING DATA FOR INVENTORY AND MANUFACTURING PREDICTION AND CONTROL |
United States |
|
7 |
US 8,761,892 |
14/038,833 |
ACTIVE CURRENT CONTROL USING THE ENCLOSURE OF AN IMPLANTED PULSE GENERATOR |
United States |
|
8 |
US 8,761,897 |
14/015,032 |
METHOD AND SYSTEM OF GRAPHICAL REPRESENTATION OF LEAD CONNECTOR BLOCK AND IMPLANTABLE PULSE GENERATORS ON A CLINICIAN PROGRAMMER |
United States |
|
9 |
US 8,781,592 |
13/226,897 |
IDENTIFYING AN AREA FOR ELECTRICAL STIMULATION TO TREAT A PATIENT (see Claim 9) |
United States |
|
10 |
US 8,812,125 |
13/600,684 |
SYSTEMS AND METHODS FOR THE IDENTIFICATION AND ASSOCIATION OF MEDICAL DEVICES |
United States |
|
11 |
US 8,868,199 |
13/973,363 |
SYSTEM AND METHOD OF COMPRESSING MEDICAL MAPS FOR PULSE GENERATOR OR DATABASE STORAGE |
United States |
|
12 |
US 8,874,219 |
13/081,936 |
ARBITRARY WAVEFORM GENERATOR AND NEURAL STIMULATION APPLICATION |
United States |
|
13 |
US 8,903,496 |
13/600,875 |
CLINICIAN PROGRAMMING SYSTEM AND METHOD |
United States |
|
14 |
US 8,983,616 |
13/604,197 |
METHOD AND SYSTEM FOR ASSOCIATING PATIENT RECORDS WITH PULSE GENERATORS |
United States |
|
15 |
US 8,996,115 |
13/081,896 |
CHARGE BALANCING FOR ARBITRARY WAVEFORM GENERATOR AND NEURAL STIMULATION APPLICATION |
United States |
|
16 |
US 8,996,117 |
13/082,097 |
ARBITRARY WAVEFORM GENERATOR AND NEURAL STIMULATION APPLICATION WITH SCALABLE WAVEFORM FEATURE |
United States |
|
17 |
US 8,700,175 |
13/185,636 |
DEVICES AND METHODS FOR VISUALLY INDICATING THE ALIGNMENT OF A TRANSCUTANEOUS ENERGY TRANSFER DEVICE OVER AN IMPLANTED MEDICAL DEVICE |
United States |
|
18 |
US 9,002,466 |
13/442,283 |
DIVERSITY ANTENNAS FOR NEUROSTIMULATOR PROGRAMMING DEVICES |
United States |
|
19 |
US 9,031,664 |
13/943,869 |
CURRENT STEERING NEUROSTIMULATOR DEVICE WITH UNIDIRECTIONAL CURRENT SOURCES |
United States |
|
20 |
US 9,031,666 |
14/249,425 |
DEVICES AND METHODS FOR VISUALLY INDICATING THE ALIGNMENT OF A TRANSCUTANEOUS ENERGY TRANSFER DEVICE OVER AN IMPLANTED MEDICAL DEVICE |
United States |
|
21 |
US 9,067,074 |
13/937,463 |
AUTOMATED SEARCH TO IDENTIFY A LOCATION FOR ELECTRICAL STIMULATION TO TREAT A PATIENT |
United States |
|
22 |
US 9,072,903 |
13/118,775 |
SYSTEM AND METHOD OF ESTABLISHING A PROTOCOL FOR PROVIDING ELECTRICAL STIMULATION WITH A STIMULATION SYSTEM TO TREAT A PATIENT |
United States |
|
23 |
US 9,098,610 |
13/334,361 |
COMMUNICATION FOR IMPLANTABLE MEDICAL DEVICES |
United States |
|
24 |
US 9,101,767 |
13/110,466 |
MEASURING LOAD IMPEDANCE WITH ACTIVE STIMULATION PULSES IN AN IMPLANTED PULSE GENERATOR |
United States |
|
25 |
US 9,126,043 |
13/118,781 |
PATIENT HANDHELD DEVICE FOR USE WITH A SPINAL CORD STIMULATION SYSTEM |
United States |
|
26 |
US D663,035 |
29/403,072 |
TWO-PORT IMPLANTABLE MEDICAL DEVICE |
United States |
|
27 |
US D665,086 |
29/403,078 |
THREE-PORT IMPLANTABLE MEDICAL DEVICE |
United States |
|
28 |
US D665,087 |
29/403,085 |
MULTI-PORT IMPLANTABLE MEDICAL DEVICE |
United States |
|
29 |
US D671,900 |
29/408,300 |
POCKET CONTROLLER FOR IMPLANTABLE NEUROSTIMULATOR |
United States |
|
30 |
US D698,779 |
29/423,109 |
CLINICIAN PROGRAMMER FOR IMPLANTABLE NEUROSTIMULATOR |
United States |
|
31 |
NOA |
13/118,764 |
SYSTEM AND METHOD OF ESTABLISHING A PROTOCOL FOR PROVIDING ELECTRICAL STIMULATION WITH A STIMULATION SYSTEM TO TREAT A PATIENT |
United States |
|
32 |
NOA |
14/715,915 |
COMMUNICATION FOR IMPLANTABLE MEDICAL DEVICES |
United States |
|
33 |
NOA |
14/011,156 |
TOUCH SCREEN FINGER POSITION INDICATOR FOR A SPINAL CORD STIMULATION PROGRAMMING DEVICE |
United States |
|
34 |
NOA |
13/606,868 |
METHOD OF IMPROVING BATTERY RECHARGE EFFICIENCY BY STATISTICAL ANALYSIS |
United States |
|
35 |
NOA |
13/606,921 |
METHOD OF MINIMIZING INTERRUPTIONS TO IMPLANTABLE MEDICAL DEVICE RECHARGING |
United States |
|
36 |
Pending |
13/170,775 |
DUAL PATIENT CONTROLLERS |
United States |
|
37 |
Pending |
13/226,956 |
CONFIGURING ELECTRICAL STIMULATION TO TREAT A PATIENT |
United States |
|
38 |
Pending |
13/250,283 |
MEDICAL IMPLANT RANGE EXTENSION BRIDGE APPARATUS AND METHOD |
United States |
|
39 |
Pending |
13/359,739 |
HEAT DISPERSION FOR IMPLANTABLE MEDICAL DEVICES |
United States |
|
40 |
Pending |
13/366,705 |
METHOD OF PAIRING MULTIPLE PATIENT CONTROL DEVICES WITH A PATIENT MEDICAL DEVICE |
United States |
|
41 |
Pending |
13/600,943 |
COGNITION AND USABILITY APTITUDE EVALUATIONS FOR CLINICIAN PROGRAMMERS |
United States |
|
42 |
Pending |
13/601,449 |
VIRTUAL REALITY REPRESENTATION OF MEDICAL DEVICES |
United States |
|
43 |
Pending |
13/601,504 |
TOUCH SCREEN SAFETY CONTROLS FOR CLINICIAN PROGRAMMER |
United States |
|
44 |
Pending |
13/601,631 |
PROGRAMMING AND VIRTUAL REALITY REPRESENTATION OF STIMULATION PARAMETER GROUPS |
United States |
|
45 |
Pending |
13/607,037 |
IMPLANT CURRENT CONTROLLED BATTERY CHARGING BASED ON TEMPERATURE |
United States |
|
46 |
Pending |
13/800,729 |
DUAL PATIENT CONTROLLERS |
United States |
|
47 |
Pending |
13/828,102 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION WITH SCALABLE WAVEFORM FEATURE AND CHARGE BALANCING |
United States |
|
48 |
Pending |
13/973,292 |
METHOD AND SYSTEM OF BRACKETING STIUMULATION PARAMETERS ON CLINICIAN PROGRAMMERS |
United States |
|
49 |
Pending |
13/973,316 |
METHOD AND SYSTEM OF QUICK NEUROSTIMULATION ELECTRODE CONFIGURATION AND POSITIONING |
United States |
|
50 |
Pending |
14/010,872 |
METHOD AND SYSTEM OF MODEL SHADING AND REDUCTION OF VERTICES FOR 3D IMAGING ON A CLINICIAN PROGRAMMER |
United States |
|
51 |
Pending |
14/010,913 |
METHOD AND SYSTEM OF EMULATING A PATIENT PROGRAMMER |
United States |
|
52 |
Pending |
14/010,942 |
PREDEFINED INPUT FOR CLINICIAN PROGRAMMER DATA ENTRY |
United States |
|
53 |
Pending |
14/015,107 |
METHOD AND SYSTEM OF SIMULATING A PULSE GENERATOR ON A CLINICIAN PROGRAMMER |
United States |
|
54 |
Pending |
14/041,082 |
DIGITAL CONTROL FOR IMPLANTED PULSE GENERATORS |
United States |
|
55 |
Pending |
14/229,458 |
SYSTEM AND METHOD OF DEVELOPING A PROGRAM FOR PROVIDING THERAPEUTIC ELECTRICAL STIMULATION FOR TREATING A PATIENT |
United States |
|
56 |
Pending |
14/245,225 |
SYSTEMS, DEVICES, COMPONENTS AND METHODS FOR COMMUNICATING WITH AN IMD USING A PORTABLE ELECTRONIC DEVICE AND A MOBILE COMPUTING DEVICE |
United States |
|
57 |
Pending |
14/279,415 |
SYSTEM AND METHOD OF PROVIDING COMPUTER ASSISTED STIMULATION PROGRAMMING (CASP) |
United States |
|
58 |
Pending |
14/279,749 |
SYSTEM AND METHOD OF DISPLAYING STIMULATION MAP AND PAIN MAP OVERLAP COVERAGE REPRESENTATION |
United States |
|
59 |
Pending |
14/279,841 |
AUTOMATIC CURRENT BALANCING WITH LOCK CONTROL FOR A CLINICIAN PROGRAMMER |
United States |
|
60 |
Pending |
14/279,881 |
METHOD AND APPARATUS FOR VISUALIZING A MIGRATION HISTORY OF PAIN MAPS AND STIMULATION MAPS |
United States |
|
67 |
Pending |
14/279,925 |
METHOD AND APPARATUS FOR DISPLAYING A GRAPHICAL IMPEDANCE HISTORY FOR OUTPUT CHANNELS OF A LEAD |
United States |
|
68 |
Pending |
14/446,996 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION |
United States |
|
69 |
Pending |
14/552,880 |
CLINICIAN PROGRAMMING SYSTEM AND METHOD |
United States |
|
70 |
Pending |
14/628,318 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION WITH SCALABLE WAVEFORM FEATURE |
United States |
|
71 |
Pending |
14/670,539 |
DIVERSITY ANTENNAS FOR NEUROSTIMULATOR PROGRAMMING DEVICES |
United States |
|
72 |
Pending |
14/703,961 |
DEVICES AND METHODS FOR VISUALLY INDICATING THE ALIGNMENT OF A TRANSCUTANEOUS ENERGY TRANSFER DEVICE OVER AN IMPLANTED MEDICAL DEVICE |
United States |
|
73 |
Pending |
14/793,780 |
MEASURING LOAD IMPEDANCE WITH ACTIVE STIMULATION PULSES IN AN IMPLANTED PULSE GENERATOR |
United States |
|
74 |
Pending |
14/810,758 |
PATIENT HANDHELD DEVICE FOR USE WITH A SPINAL CORD STIMULATION SYSTEM |
United States |
|
Foreign issued patents and pending applications | ||||
|
Patent No. |
Application No. |
Title |
Country | |
|
75 |
DE602012008738 |
EP12163520.5 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION WITH SCALABLE WAVEFORM FEATURE |
Germany |
|
76 |
EP2508226 FR |
EP12163520.5 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION WITH SCALABLE WAVEFORM FEATURE |
France |
|
77 |
EP2508226 UK |
EP12163520.5 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION WITH SCALABLE WAVEFORM FEATURE |
United Kingdom |
|
78 |
Pending |
EP12163512.2 |
CHARGE BALANCING FOR ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION |
Europe |
|
79 |
Pending |
EP12163517.1 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION |
Europe |
|
80 |
Pending |
EP12166020.3 |
CURRENT STEERING NEUROSTIMULATOR DEVICE WITH UNIDIRECTIONAL CURRENT SOURCES |
Europe |
|
81 |
Pending |
EP12169536.5 |
PATIENT HANDHELD DEVICE FOR USE WITH A SPINAL CORD STIMULATION SYSTEM |
Europe |
|
82 |
Pending |
EP12169540.7 |
SYSTEM AND METHOD OF ESTABLISHING A PROTOCOL FOR PROVIDING ELECTRICAL STIMULATION WITH A STIMULATION SYSTEM TO TREAT A PATIENT |
Europe |
|
83 |
Pending |
EP12172998.2 |
KEY FOB CONTROLLER FOR AN IMPLANTABLE NEUROSTIMULATOR |
Europe |
|
84 |
Pending |
EP12173004.8 |
DUAL PATIENT PROGRAMMERS FOR AN IMPLANTABLE NEUROSTIMULATOR |
Europe |
|
85 |
Pending |
EP12173231.7 |
ACTIVE CURRENT CONTROL USING THE ENCLOSURE OF AN IMPLANTED PULSE GENERATOR |
Europe |
|
86 |
Pending |
EP12176586.1 |
DEVICES AND METHODS FOR VISUALLY INDICATING THE ALIGNMENT OF A TRANSCUTANEOUS ENERGY TRANSFER DEVICE OVER AN IMPLANTED MEDICAL DEVICE |
Europe |
|
87 |
Pending |
EP12182113.6 |
IDENTIFYING AN AREA FOR ELECTRICAL STIMULATION TO TREAT A PATIENT |
Europe |
|
88 |
Pending |
EP12182119.3 |
CONFIGURING ELECTRICAL STIMULATION TO TREAT A PATIENT |
Europe |
|
89 |
Pending |
EP12182121.9 |
AUTOMATED SEARCH TO IDENTIFY A LOCATION FOR ELECTRICAL STIMULATION TO TREAT A PATIENT |
Europe |
|
90 |
Pending |
EP13179897.7 |
CLINICIAN PROGRAMMING SYSTEM AND METHOD |
Europe |
|
91 |
Pending |
EP13182500.2 |
METHOD AND SYSTEM OF QUICK NEUROSTIMULATION ELECTRODE CONFIGURATION AND POSITIONING |
Europe |
|
92 |
Pending |
EP13182618.2 |
METHOD AND SYSTEM FOR ASSOCIATING PATIENT RECORDS WITH PULSE GENERATORS |
Europe |
|
93 |
Pending |
EP13183312.1 |
METHOD OF MINIMIZING INTERRUPTIONS TO IMPLANTABLE MEDICAL DEVICE RECHARGING |
Europe |
|
94 |
Pending |
EP13183319.6 |
IMPLANT CURRENT CONTROLLED BATTERY CHARGING BASED ON TEMPERATURE |
Europe |
|
95 |
Pending |
EP13186926.5 |
DIGITAL CONTROL FOR IMPLANTED PULSE GENERATORS |
Europe |
|
96 |
Pending DIV |
EP12163520.5 |
ARBITRARY WAVEFORM GENERATOR & NEURAL STIMULATION APPLICATION WITH SCALABLE WAVEFORM FEATURE |
Europe |
EXHIBIT B
COMPONENTS OF ALGOVITA NEUROSTIMULATION MEDICAL DEVICE
The Algovita Spinal Cord Stimulation System is a rechargeable, 24-electrode, spinal cord stimulation system for the treatment of chronic intractable pain of the trunk and/or limbs. The main components of the Algovita Spinal Cord Stimulation System include the following:
|
● |
Rechargeable, 24-channel implantable pulse generator (IPG or Stimulator) in two configurations |
|
o |
3 ports x 8 independent channels (3 leads, each with 8-electrodes) |
|
o |
2 ports x12 independent channels (2 leads, each with 12-electrodes) |
|
● |
Anatomically compliant implantable epidural leads in the following configurations and lengths: |
|
o |
percutaneous (delivered through a needle) in 45, 60, 75, 90 cm lengths |
|
o |
surgical paddle leads in 45 and 60 cm lengths |
|
● |
Extensions in 1x8 and 1x12 configurations with 20, 40, and 60 cm lengths |
|
● |
Clinician programmer (CP) |
|
● |
Programmer charger (PPC) |
|
● |
Pocket programmer (PoP) |
|
● |
Patient feedback tool (PFT) |
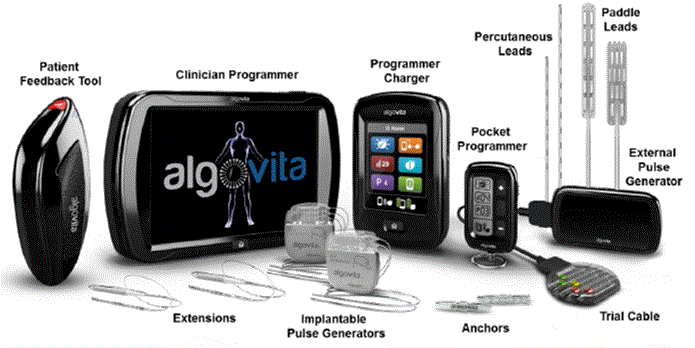
EXHIBIT C
MILESTONES
|
● |
Within two (2) years of the date of the substantial completion of the development of the Licensed Products under this Agreement, Licensee must receive a Conformité Européenne (CE) xxxx for such Licensed Products that permits the sale of such Licensed Products within the European Economic Area for application within the Field of Use (“CE Xxxx Approval”). |
|
● |
Within one (1) year of its receipt of the CE Xxxx Approval, Licensee must complete its first sale of the Licensed Products within the European Economic Area for application within the Field of Use. |
|
● |
Within two (2) years of its receipt of the CE Xxxx Approval, Licensee must receive the FDA’s approval of its Investigation Device Exemption application for the Licensed Products. |

