DISTRIBUTOR of products that compete with the Products covered hereby and that DISTRIBUTOR is not precluded by any contractual obligation or any other reason from entering into or performing under this Agreement. DISTRIBUTOR agrees that during the...
|
|
EXHIBIT 10.43 INTERNATIONAL DISTRIBUTORSHIP AGREEMENT THIS INTERNATIONAL DISTRIBUTORSHIP AGREEMENT ("Agreement") is entered into effective as of the Effective Date contained in Schedule A, between ReShape Medical Inc., having its principal place of business at 0000 Xxxxx Xxxxxxxx, Xxx Xxxxxxxx, XXX ("RSM") and the company identified in Schedule A ("DISTRIBUTOR"). WITNESSETH WHEREAS, RSM is in the business of selling various medical devices primarily used to perform medical procedures; and WHEREAS, DISTRIBUTOR desires lo actively and diligently promote the sale, on its own behalf and for its own account, of certain of RSM's products; and WHEREAS, RSM and DISTRIBUTOR desire to enter into a non-exclusive distributorship agreement covering certain ReShape Medical product lines under the terms and conditions set out below. NOW, THEREFORE, in consideration of the premises and the mutual covenants contained herein, the parties agree as follows: l. DISTRIBUTION 1.1 Products. The products that are subject to this Agreement (the "Products") shall be those products identified on Schedule B hereto, together with such other products as may from time to time be included thereon by mutual written agreement of the parties. DISTRIBUTOR acknowledges that Schedule B will not necessarily include all products sold by RSM, and that Products are subject to modification or discontinuance by RSM upon notice to DISTRIBUTOR, and, upon DISTRIBUTOR's receipt of such notice, Schedule B will be deemed amended accordingly. 1.2 Appointment. l.2.1 Effective as of the Effective Date of this Agreement, RSM hereby appoints DISTRIBUTOR, and DISTRIBUTOR accepts such appointment, as a nonexclusive distributor of Products in the geographical area described on Schedule C hereto (the "Territory"), subject to the terms and conditions set forth in this Agreement. 1.2.2 DISTRIBUTOR shall not directly or indirectly deliver or promote the sale of the Products outside the Territory or locate or utilize an office, branch, or distribution depot for the sale or distribution of the Products outside the Territory. DISTRIBUTOR shall immediately notify RSM if it becomes aware that any DISTRIBUTOR customer exports or sells or plans to export or sell any of the Products outside the Territory. 1.3 Noncompetition. DISTRIBUTOR represents that as of the Effective Date there are no agreements in effect providing for the marketing, sale or distribution by «Distributorname» «Territory» 4782913v1 |
|
|
Schedule A Distributor and Term Distributor Information Corporate Name: SHIFLI GULF FOR TRADING IN DRUGS & EQUIPMENTs AND DEVICES COMPANY Address: STATE OF KUWAIT, HAWALLY, BLOCK 166, TOWW AR ZENAH, STREET BIN KHALDOUN, 3RD FLOOR P.O. ROX 543 |
|
|
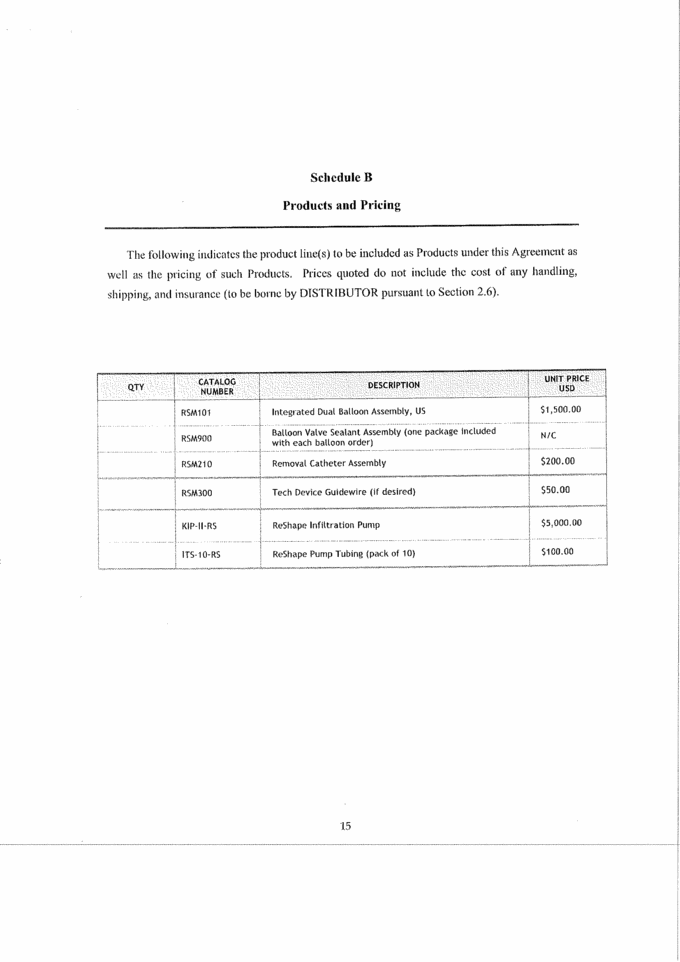
Schedule B Products and Pricing The following indicates the product line(s) to be included as Products under this Agreement as well as the pricing of such Products. Prices quoted do not include the cost of any handling, shipping, and insurance (to be borne by DISTRIBUTOR pursuant to Section 2.6). QTY CATALOG NUMBER DESCRIPTION UNIT PRICE USD RSM101 Integrated Dual Balloon Assembly, US $1,500.00 RSM900 Balloon Valve Sealant Assembly (one package included with each balloon order) N/C RSM210 Removal Catheter Assembly $200.00 RSM300 Tech Device Guidewire (if desired) $50.00 XXX-II-RS ReShape Infiltration Pump $5,000.00 ITS-10-RS ReShape Pump Tubing (pack of 10) $100.00 |
|
|
Schedule C Territory The customers and or geographical area subject to this Agreement shall be specifically and exclusively limited to the State of Kuwait. |
|
|
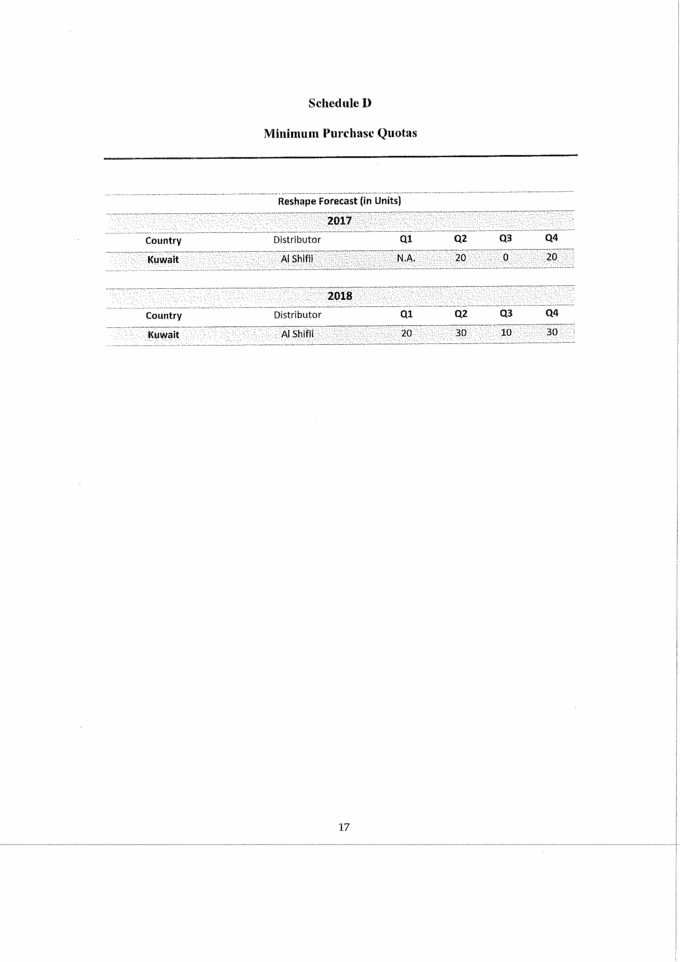
Schedule D Minimum Purchase Quotas Reshape Forecast (in Units) 2017 Country Distributor Ql Q2 Q3 Q4 Kuwait Al Shifli N.A. 20 0 20 2018 Country Distributor Ql Q2 Q3 Q4 Kuwait Al Shifli 20 30 10 30 |
|
|
Schedule E Payment Terms The first two orders for the ReShape Dual Balloon will be cash pay up front. After successfully executing these orders, future orders may be extended via the credit terms below. Payment for Products is due within 60 days after date of invoice on open account as long as DISTRIBUTOR’s credit remains good, payments to RSM are made on time, and the total amount owed by DISTRIBUTOR to RSM is within the credit limits determined by RSM from time to time. DISTRIBUTOR’s credit limit as of the effective date of this Agreement shall be U.S. currency although RSM reserves the right to require DISTRIBUTOR to provide adequate security for the amount of such credit limit as a condition to making sales on open account terms. RSM shall have the right to adjust DISTRIBUTOR’s payment terms and credit limits from time to time. OR DISTRIBUTOR shall pay RSM for Products in advance of delivery by cash or irrevocable letter of credit with order. Late Payments: DISTRIBUTOR will pay a late fee on all past due amounts at the rale of one percent per month or the highest rate permissible by law, whichever is lower, until paid in full. Taxes. All amounts payable lo RSM under this Agreement are exclusive of any income, sales, use, property, ad valorem, value added or other taxes, levies, imposts, duties, charges or withholdings of any nature (collectively, "Taxes"), arising out of any transaction contemplated by this Agreement and imposed against DISTRIBUTOR or the Products by any taxing authority in the Territory (excluding, however, any Taxes on, or measured solely by, the net income of RSM and Taxes imposed on RSM in the United States). DISTRIBUTOR shall pay all applicable Taxes or provide RSM with a certificate of exemption acceptable to the relevant taxing authority. In the event that any payments to RSM under this Agreement are subject to any withholding taxes, DISTRIBUTOR shall promptly provide all tax certificates, applications and related documents to RSM. If RSM is required to pay any Taxes in the Territory (excluding, however, any Taxes on, or measured solely by, the net income of RSM and Taxes imposed on RSM in the United States), DISTRIBUTOR shall promptly reimburse RSM upon written request therefore. |
|
|
ANNEX A- Quality Requirements A) Storage of Products: If applicable Distributor is required to store products in accordance with product labeling statements and within an environment that prevents any of their characteristics from being altered until delivered to the customer. The minimum storage requirements for RSM products include the following: If applicable, Distributor must establish a secure storage location and limit access to this location to only those personnel authorized by Distributor. Products must be stored within this location to prevent Products from being contaminated or tampered with in any way. Additionally, Products must be kept in a clean area, free of insects, rodents and any pests. Distributor shall have a process to prevent expired, rejected and/or quarantined Products from being sent to final customers. ReShape Medical RSM reserves the right to provide other instructions to Distributor regarding such Product, and Distributor agrees to comply with such instructions. B) Traceability: Distributor is required to maintain records to ensure the traceability of ReShape Medical products in accordance with applicable regulatory requirements, and to provide ReShape Medical or its authorized agents or representatives with reasonable access to such records. The minimum traceability requirements for ReShapc Medical Products include the following: Distributor is required to maintain a complete and current list of all customers who have purchased ReShapc Medical products from Distributor, the dates of such purchases, the quantity, and the lot numbers, UPNs, serial numbers and/or model numbers of the units purchased (as applicable) as identified on the product label. Distributor must ensure the traceability of all ReShape Medical products at UPN, lot level including the model and serial number where applicable. The final users for all products must be identified (units sold directly to hospitals, units sold directly to doctors, units sold directly to patients). If ReShape Medical products are consigned, the batches consumed at the account must be reconciled. The traceability of multi-pack boxes must be maintained and single units originally from multi-packs must never be re-boxed. C) Complaint Reporting and Handling: Distributor is required to promptly forward all complaints concerning the products, cooperate fully with ReShape Medical in dealing with customer complaints, and take such action to resolve such complaints as may be reasonably requested by ReShape Medical. The complaint reporting requirements for ReShape Medical products include the following: |
|
|
All customer complaints involving or contributing to serious adverse events (including patient death or serious injury) must be reported to ReShape Medical within 24 hours of Distributor's becoming aware. Complaints involving nonserious events including comments regarding product dissatisfaction, potential malfunctions, non-serious patient injury, or unanticipated medical or surgical intervention shall be reported to ReShape Medical within 48 hours. A Complaint Notification Form will be provided Lo Distributor by ReShape Medical and must be completed in full to document each complaint. Additionally, any ancillary documentation that may facilitate the complaint investigation process should also be attached, particularly if the product is not available for return. In cases where additional information is required from the customer, at least three (3) due diligent attempts must be performed by Distributor to try to collect this additional complaint information, if requested by ReShape Medical. Should requested information not be available, Distributor shall document the reason(s) it is not available and/or the 3 attempts. Products subject to complaints should be returned to ReShape Medical. Returned products must either, as directed by ReShape Medical, be accompanied by a disinfection certificate, even if the products have not been used; or be returned in biohazard controlled packaging and under safe handling controls. In cases where the customer has indicated that the complaint product is available to be returned but it has not been received, at least three (3) due diligent attempts must be made by Distributor to retrieve the product. |