Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
Exhibit 10.4
DATED 10th April 2018
(1) FREELINE THERAPEUTICS LIMITED
-and-
(2) CELL THERAPY CATAPULT LIMITED
|
|

Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
THIS COLLABORATION AGREEMENT (the “Agreement) is dated 10 April 2018 (the “Effective Date”)
BETWEEN
| (1) | Freeline Therapeutics Limited a company incorporated in England with company number 09500073 and whose registered office is at Stevenage Bioscience Catalyst, Xxxxxxx Xxxx Xxxx, Xxxxxxxxx, Xxxxx XX0 0XX (“COLLABORATOR”); and |
| (2) | Cell Therapy Catapult Limited, trading as Cell and Gene Therapy Catapult, a company incorporated and registered in England & Wales with company number 07964711 whose registered office is at 12th Floor Tower Wing, Guys Hospital, Great Xxxx Xxxx, Xxxxxx, XX0 0XX, Xxxxxx Xxxxxxx (“Catapult”). |
BACKGROUND
| (A) | Catapult’s purpose in commissioning the cell and gene therapy manufacturing centre is to further its broader aims within the UK to develop novel technologies, processes, supply chains, facilities, skills, and working practices for simultaneous and cost effective large scale manufacture and distribution of multiple ATMP products. |
| (B) | COLLABORATOR is developing certain ATMP products. As part of this activity COLLABORATOR wishes to use the Centre in order to further develop and scale up manufacturing processes and capability for cell and gene therapy products. |
| (C) | COLLABORATOR and Catapult would each like to collaborate with the other as further set forth in this Agreement (“Project” or “Collaboration”, as further described in the work streams set out at Schedule 1). Other parties who collaborate with Catapult, and occupy space in the Centre will be referred to as “Collaborators”. |
| (D) | This document aims to record the contributions of each party with respect to this Agreement, and the terms under which COLLABORATOR and Catapult will work together within the Centre. |
OPERATIVE PROVISIONS
| 1. | DEFINITIONS AND INTERPRETATION |
In this Agreement, the following words shall have the following meanings:
| “Accompanied Access Areas” | the areas of the Centre marked yellow on the Plan which are accessible by any Collaborator, but on condition such access is in the company of Catapult personnel. | |
| “Activity Related Inputs” | the inputs provided by Catapult as set out in Clause 9.2 and more specifically set out in Schedule 3. | |
| “Activity Related Input Contributions” | the non-refundable financial contribution made by COLLABORATOR with respect to the provision of the Activity Related Inputs, as specifically set out in Schedule 3. | |
| “Actual Occupation Date” | means the date by which Catapult has completed its obligations contained in this Agreement enabling COLLABORATOR to occupy the Module. | |
| “Additional Inputs” | means Inputs COLLABORATOR requires Catapult to contribute to the Project, other than Activity Related Inputs and Integral Inputs, which will be arranged through the completion of an Additional Input Agreement in the form set out at Schedule 16 (“Additional Input Agreement”). | |
1
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| “Additional Input Contributions” |
the financial contribution made by COLLABORATOR with respect to the provision of any Additional Inputs requested from Catapult in the form set out at Schedule 16 (“Additional Input Agreement”). | |
| “Affiliate” |
In relation to a Party, means any person that Controls, is Controlled by, or is under common Control with that Party. | |
| “Applicable Law” |
any:
(a) statute, statutory instrument, by-law, order, regulation, directive, treaty, decree, decision of the European Council or law;
(b) legally binding rule, policy, guidance or recommendation issued by any governmental, statutory or regulatory body with jurisdiction over this Agreement or the activities conducted hereunder; which relates to the performance of this Agreement and/or the inputs by the relevant party and/or the activities which are comprised in the Project; and
(c) legally binding industry code of conduct or guideline. | |
| “Background Intellectual Property” |
(a) In relation to COLLABORATOR, means the Intellectual Property that is either (i) owned by or licensed to COLLABORATOR prior to the Effective Date, or (ii) that is developed or licensed by COLLABORATOR after the Effective Date and outside of the conduct of activities for the Project; and in the case of either (i) or (ii), that COLLABORATOR uses in the performance of the Project, other than Foreground Intellectual Property; and
(b) In relation to Catapult, means the Intellectual Property owned by or licensed to Catapult at the Effective Date, together with Intellectual Property that is developed by or licensed to Catapult after the Effective Date and outside of the conduct of activities for the Project; and in either case that Catapult uses in the performance of the Agreement, and that is not Foreground Intellectual Property. Catapult represents and warrants that to the best of Catapult’s knowledge and belief, as of the Effective Date, Catapult Background Intellectual Property, consists of the heads of Intellectual Property as set forth In the attached Schedule 4, which Catapult will update from time to time as additional Catapult Background Intellectual Property is brought into the Project. | |
| “Business Rates” |
meens the portion of the business rates chargeable against the Centre paid for by COLLABORATOR in accordance with Clause 8.4.2 and the amount set out at Schedule 3. | |
| “Catapult Board” |
means the directors of Cell Therapy Catapult Limited as registered at Companies House from time to time. | |
2
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| “Centre” |
the Cell and Gene Therapy Catapult Manufacturing Centre located at Cell and Gene Therapy Catapult Manufacturing Centre, Xxxxxxx Xxxx Xxxx, Xxxxxxxxx, Xxxxx, XX0 0XX, and edged blue on the Plans. | |
| “CNC corridor” |
means the controlled non-classified corridor forming part of the Common Access Areas and referred to in Schedule 12. | |
| “Code of Conduct” |
the code of conduct set out at Schedule 5. | |
| “Commissioning” |
has the meaning given in Clause 6.1. | |
| “Collaborator Forums” |
means the Quality Forum, the Health and Safety Forum, and the Operational Forum, each as more particularly referenced, and described in Clause 9.6 and Schedule 15. | |
| “COLLABORATOR Personnel” |
the employees, consultants or contractors of COLLABORATOR located at the Module or visiting the Module from time to time. | |
| “COLLABORATOR Process” |
means the process described in the Product Overview Document (or prior to this being in place, the Pre-Screen Questionnaire, as set out in Schedule 6) to be operated by COLLABORATOR under the Agreement in order to enable the production of COLLABORATOR Product on a large scale. It may be amended from time to time in accordance with Clause 7.2 and the QTA. | |
| “COLLABORATOR Product” |
the COLLABORATOR product, or products as defined in the QTA product list (or prior to this being in place, the Pre- Screen Questionnaire) to be produced through the use of the COLLABORATOR Process. | |
| “COLLABORATOR Responsibilities” |
the obligations on COLLABORATOR set out in Clause 10 (each one severally being a “COLLABORATOR Responsibility”). | |
| “Common Access Areas” |
any part of the Centre shown edged green on the Plan which does not form part of the Module, the Restricted Access Areas, or the Accompanied Access Areas, or that is designated by Catapult from time to time for common use by Catapult, COLLABORATOR, and other Collaborators in the Centre from time to time. | |
| “Compensations” |
has the meaning given to it in Clause 18.1. | |
| “Conducting Media” |
any media for the transmission of Supplies. | |
| “Confidential Information” |
means any Information in any form or medium which is either disclosed by one Party or such Party’s Affiliates, or their legal counsel, advisors, contractors or consultants (“the Disclosing Party”) to the other Party or Its Affiliates, or their legal counsel, advisors, contractors or consultants (“the Receiving Party”), or to which either Party gains access as a result of:
(a) COLLABORATOR’s occupancy of the Module and Centre;
(b) Catapult or Third Party’s (to include any other collaborator) use of or access to the Centre at any time; or | |
3
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| (c) as a result of either Party’s participation in any of the Collaborator Forums
concerning the business affairs, finances, technology, plans, strategy, products, manufacturing services, Know-how or services of (i) the Disclosing Party (ii) any of its Affiliates (iii) any other entity with which the Disclosing Party or any of its Affiliates is in business negotiations or has contracted or to which it owes a duty of confidence, or (iv) any other Collaborator ((iv) being an “Alternative Disclosing Party”), and all copies of the same. | ||
| “Control” |
means (a) the direct or indirect ownership of fifty percent (50%) or more of the total voting power of securities or other evidences of ownership interest in a party or (b) the power to direct or cause the direction of the management and policies of such party, directly or indirectly, whether through ownership of voting securities, by contract or otherwise; and the terms “controlling” and “controlled” have meanings correlative to the foregoing, as the case may be. | |
| “Disclosing Party” |
has the meaning given in the definition of Confidential Information. | |
| “Effective Date” |
means the date as defined in the preamble of this Agreement. | |
| “Establishment Inputs” |
the inputs provided by Catapult as provided for in Clause 9.3. | |
| “Establishment Input Contributions” |
the non-refundable financial contributions payable in accordance with Clause 8.4.5, and set out in the Establishment Input Contributions Statement in Schedule 6 to be made by COLLABORATOR with respect to the provision of the Establishment Inputs by Catapult. | |
| “Expected Occupation Date” |
1 July 2018, the contemplated date by which COLLABORATOR will occupy the Module, or such other earlier date as mutually agreed by the parties in writing. | |
| “Facility Contribution” |
the non-refundable financial contribution to be made by COLLABORATOR with respectvm to the provision of the Module and other capital aspects, as more particularly described at Clause 8.4.1, and at Schedule 3. | |
| “Financial Contributions” |
means the Activity Related Input Contributions, Integral Input Contributions, Establishment Input Contributions, the Facility Contributions, the Additional Input Contributions and/or any other contributions as agreed in writing between the Parties and provided by COLLABORATOR from time to time. | |
| “Foreground Intellectual Property” |
means the results, technical information, knowledge, inventions, improvements, experience, materials and data developed and arising directly from and as a direct result of the Project, together with any Intellectual Property in such items. | |
| “GMP” |
good manufacturing practice, being the standard required under Applicable Law. | |
| “GMP Requirements” | the guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use laid down in the Commission 2003/94/EC, or as replaced by Directive 2017/1572 and/or Regulation 2017/1569 as appropriate and set out in Volume 4 of Eudralex (the rules governing medicinal products in the European Union), and the MHRA Rules and Guidance for Pharmaceutical Manufacturers and Distributors (The Orange Guide). | |
4
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| “Health and Safety Forum” | means the forum in which COLLABORATOR, other Collaborators, and Catapult will convene to discuss health and safety matters as more particularly described in Schedule 15. | |
| “HVAC” | means heating, ventilation and air-conditioning. | |
| “Improvement” | Means, with respect to any Intellectual Property or material: (a) all Improvements, modifications and/or adaptions of such Intellectual Property or materials and (b) all other Intellectual Property or in, derived from, relating to, or Interest which would Impair or restrict the use of, application, or rights comprised in, such Intellectual Property or material. | |
| “Inputs” | the Activity Related Inputs, Integral inputs, Establishment Inputs and/or any other Inputs (“Additional Inputs”) as agreed in writing between the Parties and provided by Catapult to COLLABORATOR from time to time. | |
| “Integral Input Contributions” | the non-refundable financial contribution made by COLLABORATOR with respect to the provision of the Integral Inputs, as more particularly described in Clause 8.4.4, and at Schedule 3. | |
| “lntegral Inputs” | the inputs provided by Catapult set out at Clause 9.1. | |
| “Insured Risks” | the risks covered by the policies of insurance under Clause 19.1 and 19.2, In each case to the extent that cover is generally available on normal commercial terms in the UK insurance market at the time the insurance is taken out and any other risks against which Catapult reasonably insures from time to time, subject in all cases to any excesses, limitations and exclusions imposed by the insurers. | |
| “Intellectual Property” | any and all issued patents and patent applications, inventions, utility models, registered and unregistered trademarks and service marks, registered designs, unregistered design rights, domain names, trade or business names, copyright, database rights, rights in respect of confidential information, rights under data exclusivity laws, rights under licences, rights under orphan drug laws, property rights in biological or chemical materials, topography rights, Know-how, extension of the terms of any such rights (Including supplementary protection certificates), applications for and the right to apply any of the foregoing registered property and rights, and similar or analogous rights anywhere in the world. | |
| “lT Infrastructure” | the information technology facilities in. the Centre for use by COLLABORATOR and, where applicable, by other Collaborators as more particularly described in Schedule 10. | |
| “Know-how” | unpatented technical information (including without limitation information relating to inventions, discoveries, concepts, methodologies, models, research, development, and testing procedures; the results of experiments, tests, and trials; manufacturing processes, techniques, and specifications; and quality control data, analyses, reports, and submissions) that is not in the public domain. | |
5
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| “Lease” |
a lease dated 1 October 2015 made between the (1) Stevenage Bioscience Catalyst and (2) Cell Therapy Catapult Limited. | |
| “Liability” |
liability arising out of this Agreement, whether in contract, tort, misrepresentation, restitution, under statute or otherwise, including any liability under an indemnity contained in this Agreement, | |
| “Licence Period” |
means the Term. | |
| “MAL” |
means material airlock. | |
| “Manufacturing Office” |
the manufacturing office space forming part of the Module, allocated for COLLABORATOR’s use in accordance with Clause 3, and more particularly described in Schedule 12. | |
| “Manufacturing Space” |
the manufacturing space forming part of the Module, allocated for COLLABORATOR’s use in accordance with Clause 3, more particularly described in Schedule 12. | |
| “Module” |
the specific Manufacturing Space, Manufacturing Office, and Non-Manufacturing Office each allocated by Catapult under this Agreement for COLLABORATOR’s occupation and use at the Centre for carrying out the Project shown edged in red on the Plan, and which shall include all fixtures and fittings and plant and machinery set out in the Schedule of Condition and Inventory of Module Fixtures and Fittings at Schedule 7. | |
| “Necessary Consents” |
all planning permissions and all other consents, licences, permissions, certificates, authorisations and approvals whether of a public or private nature which shall be required by any regulatory authority for performance of Project. | |
| “Non-Manufacturing Office” |
means the office space allocated for COLLABORATOR’s use in Clause 3, forming part of the Module, the specifications for which are set out in Schedule 12. | |
| “On-boarding” |
part of the Establishment inputs and a process completed by Catapult together with COLLABORATOR involving the risk assessment and regulatory oversight required for the On-boarding Project as referred to in Clause 9.3.1(b), and more particularly set out at Schedule 6. | |
| “On-boarding Project” |
the establishment of COLLABORATOR’s Process and Product at the Centre in accordance with the process described in Schedule 6. | |
| “Operational Forum” |
means the forum in which COLLABORATOR, other Collaborators, and Catapult will convene to discuss operations matters connected with the Centre as more particularly described in Schedule 15. | |
| “PAL” |
means personnel airlock. | |
| “Parties” |
COLLABORATOR and Catapult; “Party” shall mean either of them, and “Parties” shall mean both COLLABORATOR and Catapult. | |
| “Permitted Use” |
activity strictly in connection with the performance of the Project. | |
| “PrAL” |
means product airlock. | |
6
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| “Product Overview Document” or “POD” |
means a quality document completed as part of the On-boarding Project defining the COLLABORATOR Process and Product. | |
| “Plans” |
the plans of the Module allocated to COLLABORATOR under this Agreement, and of the Centre generally, attached to this Agreement at Schedule 2, Part 4. | |
| “Process Transfer” |
means an Establishment Input, and the practical transfer of COLLABORATOR’S equipment and processes into the Centre under the control and responsibility of COLLABORATOR as referred to in Clause 9.3.1 and more particularly set out at Schedule 6. | |
| “Project” |
means the workstreams set out In Schedule 1. | |
| “Quality Forum” |
means the forum in which COLLABORATOR, other Collaborators, and Catapult will convene to discuss quality matters connected with the Centre as more particularly described in Schedule 15. | |
| “Quality Management System” |
a collection of business processes and governance structures focused on consistently meeting Regulatory Authority and GMP requirements. The Quality Management System is expressed as an organisational structure, policies, procedures, processes and resources needed to maintain compliance to Eudralex Vol 4, Chapter 1 that are set out In the Quality Technical Agreement. | |
| “Quality Technical Agreement” or “QTA” |
the agreement governing the quality aspects of the Centre that are comprised in the Quality Management System. | |
| “Quarter” |
a period of three months commencing on 1 January, 1 April, 1 July, or 1 October; and “Quarterly” shall be construed accordingly. | |
| “Receiving Party” |
has the meaning given in the definition of Confidential Information. | |
| “Registered Rights” |
patents, registrable design rights, trademarks, and all other registered Intellectual Property. | |
| “Regulatory Authority” |
the competent authority for each country or for any relevant grouping of countries legally responsible for authorising the manufacture, clinical trials or the sale or supply of human pharmaceutical products in that country or group of countries. | |
| “Restricted Access Area(s)” |
the parts of the Centre accessible only by Catapult personnel marked Pink on the Plan. | |
| “Service Level Commitments” |
the service delivery principles set out at Schedule 15. | |
| “Shared Restricted Access Area” |
means the areas shared between the Manufacturing Space and an adjacent manufacturing space belonging to another Collaborator marked in turquoise on the Plans. | |
| “Steering Committee” |
the representatives of the COLLABORATOR and Catapult appointed as set out in Clause 9.5. | |
| “Supplies” |
water, gas, air, foul and surface water, drainage, electricity, oil, telephone, heating, telecommunications, internet, data communications and similar supplies or utilities. | |
7
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| “Technology Transfer” | the transfer of COLLABORATOR’s existing production and/or manufacturing processes into the Module by COLLABORATOR. | |
| “Term” | the period specified in Clause 17.1 | |
| “Termination Date” | the date on which this Agreement expires or terminates for any reason. | |
| “Third Party” | any person other than a Party or its Affiliates. | |
| “UPS” | uninterrupted power supply. | |
| “Validation” | the action of proving, in accordance with the principles of Good Manufacturing Practice (Eudralex Volume 4, Annex 15), that any GMP process functions in accordance with predefined requirements, is robust and reproducible. | |
| “Warehouse and Procurement Management Provisions” | the standards and obligations relating to the management of the warehouse set out at Schedule 8. | |
| “Warehouse Space” | the warehouse space allocated for COLLABORATOR’s use in accordance with Clause 3 and Schedule 8, and more particularly described in Schedule 12. | |
| “Year” | means the financial year ending 31 March. | |
| 1.1 | In this Agreement, unless otherwise specified: |
| 1.1.1 | references to Clauses and Schedules are to the clauses of, and schedules to, this Agreement; |
| 1.1.2 | headings are for convenience only and do not affect the interpretation of this Agreement; |
| 1.1.3 | references to a person includes a body corporate or unincorporated body, and references to a company includes any company, corporation or other body corporate, wherever and however incorporated or established; |
| 1.1.4 | unless the context otherwise requires, words in the singular shall include the plural and vice versa; |
| 1.1.5 | references to approvals or notices being “in writing” or “written” shall include email; |
| 1.1.6 | any reference to a statute or statutory provision is a reference to it as amended, extended, re-enacted and/or replaced from time to time; and |
| 1.1.7 | ‘Including’ means ‘Including but not limited to’ and ‘include’ and ‘includes’ shall be construed accordingly. |
| 2. | CONDUCT OF THE PROJECT |
The Parties will undertake the Project in accordance with the provisions of this Agreement.
| 3. | OCCUPATION OF THE MODULE AND THE WAREHOUSE SPACE |
| 3.1 | Catapult permits COLLABORATOR to occupy the Module on the terms set out in Schedule 2. |
| 3.2 | Catapult permits COLLABORATOR to access and use the Warehouse Space in accordance with the terms in Schedule 8. |
8
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 4. | MODULE SPECIFICATION |
| 4.1 | Catapult will ensure the Manufacturing Space will be in accordance with the specifications at Schedule 12 Part A and will at all times comply with Applicable Laws (including GMP Requirements). |
| 4.2 | Catapult will ensure the Manufacturing Office and Non-Manufacturing Office will be in accordance with the specifications at Schedule 12 Part B. |
| 4.3 | Catapult will also ensure that use of the Shared Restricted Access Areas and Warehouse Space will at all times comply with Applicable Laws including EU-GMP Requirements, in relation to any collaborator other than COLLABORATOR. |
| 5. | CENTRE SPECIFICATIONS |
| The Centre will be a UK-licensed EU-GMP-compliant facility developed In close relationship with the Medicines and Healthcare Products Regulatory Agency comprising the facilities and services set out at Schedule 12, Part C. Catapult will also ensure that it has In place all consents and licenses required for operation of the Facility. |
| 6. | COMMISSIONING AND QUALIFICATION OF THE CENTRE |
| 6.1 | In advance of COLLABORATOR being granted access to the Manufacturing Space and subject to Clause 7, Catapult will test equipment, facilities and/or plant which is Catapult owned, or rented by Catapult and installed, (and following grant of access, test all Catapult owned or rented equipment, facilities and/or plant which is subsequently installed or up-graded) In order to verify it functions according to its design objectives or specifications (“Commissioning”). |
| 6.2 | Commissioning will not cover the formal qualification of manufacturing systems or manufacturing process equipment but will include the static and dynamic commissioning of the following by Catapult; |
| 6.2.1 | the Building Management System (BMS); |
| 6.2.2 | the electrical supply (single and three phase); |
| 6.2.3 | the boilers; |
| 6.2.4 | the chiller; |
| 6.2.5 | HVAC; |
| 6.2.6 | Quality Control area HVAC; |
| 6.2.7 | lighting – including emergency lighting; |
| 6.2.8 | back-up generator; |
| 6.2.9 | UPS systems; |
| 6.2.10 | door interlocks; |
| 6.2.11 | pharmaceutical grade gas supplies (air, oxygen, carbon dioxide and nitrogen) |
| 6.2.12 | CCTV |
| 6.2.13 | fire alarm |
| 6.2.14 | LN2 / Low level, temperature and oxygen monitors; |
| 6.2.15 | drainage; and |
| 6.2.16 | appropriate IT Infrastructure (including cable network, switches, and server rooms). |
9
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 6.3 | Where appropriate, Catapult will qualify the building, systems and equipment that form part of the Centre, and this will extend to installation qualification, operational qualification, and performance qualification of all GMP direct impacting systems and integral equipment. Any software related to GMP direct impacting systems and integral equipment at the Centre will be Validated by Catapult and will comply with EU GMP Annex 11. |
| 6.4 | Formal qualification will be undertaken (which includes installation qualification (IQ) and operational qualification (OQ)) concurrent with leveraging the output of Centre commissioning, performance qualification (PQ), which will only occur subsequent to the completion of commissioning, IQ and OQ, PQ will only be applied to those services, systems and items of equipment that have been identified as having direct impact on product quality according to a formal system level impact assessment. These include: |
| 6.4.1 | Manufacturing Space and all additional air locks HVAC; |
| 6.4.2 | Warehouse HVAC; |
| 6.4.3 | Grade C corridor and technical area HVAC; |
| 6.4.4 | Carbon dioxide system; |
| 6.4.5 | Nitrogen gas system; |
| 6.4.6 | Liquid nitrogen system, storage tanks (PQ will be carried out on conjunction with a collaborator) and shared Controlled Rate Freezing equipment (COLLABORATOR will be responsible for their own cycle development and PQ); |
| 6.4.7 | Oxygen system; |
| 6.4.8 | Cold room, fridges, freezers (PQ will be carried out in conjunction with COLLABORATOR); |
| 6.4.9 | the Environmental Monitoring System (EMS) (e.g. viable air sampler, non-viable particulate monitors); and |
| 6.4.10 | Environmental monitoring equipment, PQ will be carried out in conjunction with COLLABORATOR. |
| 7. | PROCESS AND PRODUCT |
| 7.1 | VALIDATION |
| Process validation and transfer of the COLLABORATOR Process into Module is entirely the responsibility of COLLABORATOR. |
| 7.2 | PROCESS AND PRODUCT AMENDMENT |
| 7.2.1 | Catapult confirms that it approved the information contained in the initial POD provided as part of the On-boarding Project as describing the COLLABORATOR Process and COLLABORATOR Product that can and may be developed, implemented, and manufactured in the Module by COLLABORATOR. The initial COLLABORATOR Product provided as part of the On-boarding Project has been included in the QTA Product List, and, therefore, constitutes a COLLABORATOR Product. It further confirms that such approval of COLLABORATOR Product will remain valid during the Term on the condition that no amendments ere made at a later stage. |
| 7.2.2 | The COLLABORATOR Product and Process will be again vetted and approved in advance of occupation as part of the On-boarding Project. In the event the On-boarding Project reveals any variations and/or additions to the information contained in the POD (“Product or Process Modifications”) these will be managed as changes in accordance with Clause 7.2.4. |
10
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 7.2.3 | Product or Process Modifications will be considered in accordance with the following procedure: |
| (a) | COLLABORATOR must notify Catapult in writing of its application for the intended changes (the “Notification”); and |
| (b) | In response, Catapult will apply the criteria set out in Clause 7.2.4. If the requested Product or Process Modifications meet the requirements of these steps, and are therefore capable of Introduction, Catapult will always endeavour to approve the earliest date feasible for introduction, allowing for logistical constraints, and the competing interests of other Collaborators in existence at the time of request (the “Introduction Date”), |
| (c) | Catapult will confirm the outcome of the application of the criteria in Clause 7.2.4 and, if applicable, of the Introduction Date in writing to COLLABORATOR as soon as it is able to from the date of notification under Clause 7.2.3(a) but in any event within 30 calendar days of receipt of Notification by Catapult. Where Catapult indicates that the Modifications will not be permitted, it will identify the reasons why such Notification has been refused. |
| 7.2.4 | Catapult will permit a new COLLABORATOR Product(s), and/or COLLABORATOR Process(es) or COLLABORATOR modification to such COLLABORATOR Product or COLLABORATOR Process if: |
| (a) | the new or modified COLLABORATOR Product(s), and/or COLLABORATOR Process(es) meet the requirements of the QTA; |
| (b) | the proposed product is not a restricted product listed at Clause 7.3; |
| (c) | it does not impact on Catapult’s Inputs or the operation of the Centre and as a result materially affect Catapult’s ability to comply with GMP or GMP Requirements; |
| (d) | it does not inherently compromise the safety of the Centre, or that of any other collaborator; |
| (e) | it does not place an additional, unreasonable demand on the resources of Catapult personnel and their ability to operate the Centre; |
| (f) | it does not interfere with the Catapult’s, or any other Collaborator’s compliance with their respective legal duties; and/or |
| (g) | it can be accommodated in the Centre, taking into account the overall capacity of the Centre. |
| 7.2.5 | In the event that COLLABORATOR does not agree with the outcome of Catapult’s application of the principles under Clause 7.2.4, the matter will be referred to the Steering Committee for resolution, and If no agreement is reached within a reasonable period, then the Parties will comply with the Expert Determination Procedure set out in Schedule 13. |
| 7.3 | RESTRICTED PRODUCTS |
| COLLABORATOR will not be permitted, and Catapult undertakes that it will not allow any other Collaborator to produce or utilise in their process the following products in the Centre (unless prior agreement is sought from all collaborators by Catapult): |
| 7.3.1 | B Lactam Antibiotics; |
| 7.3.2 | Other highly sensitising antibiotics; |
| 7.3.3 | Pathogenic Organisms (Containment Level 3 or 4); |
| 7.3.4 | GMO 3 and above; |
11
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 7.3.5 | Radiopharmaceuticals; |
| 7.3.6 | Ectoparasiticides; or |
| 7.3.7 | Sources of ionising radiation (but excluding low energy laboratory scale X-irradiators which have been assessed and approved by Catapult). |
| 8. | FINANCIAL CONTRIBUTIONS |
| 8.1 | A risk and capital contribution will be charged throughout the Term calculated at the rate of [**] of each Financial Contribution, except for the Facility Contribution and Business Rates. The risk and capital contribution will remain fixed at the rate of [**] throughout the Term. |
| 8.2 | VAT, if applicable, will be added to all Financial Contributions. |
| 8.3 | Any changes to the Financial Contributions (other than the Facility Contributions which are fixed for the Term) will be made once per year based on the new annual budget which will be discussed at the Operational Forum. |
| 8.4 | COLLABORATOR will make the following Financial Contributions to the costs of the Collaboration: |
| 8.4.1 | subject to Clauses 8.2 and 8.3, from the Actual Occupation Date, for each Module occupied by COLLABORATOR, the Facility Contribution, payable quarterly in advance; |
| 8.4.2 | subject to Clauses 8.1 to 8.3, from the Actual Occupation Date, for each Module occupied by COLLABORATOR, one-fifth of the total costs chargeable against Centre in the form of Business Rates, payable quarterly in advance; |
| 8.4.3 | the Activity Related Input Contributions as they are incurred, due Individually from COLLABORATOR and within 30 days of receipt of invoice; |
| 8.4.4 | subject to Clauses 8.1 to 8.3, from the Actual Occupation Date, the Integral input Contributions payable quarterly in advance and calculated in accordance with the following provisions of Clauses 8.4.4(a) and (b): |
| (a) | Catapult will estimate the aggregate Integral Input Contributions Incurred for 5 Modules in concurrent occupation for any 1 year (the “Estimated Aggregate Integral Input Contributions”), COLLABORATOR will be responsible for a fixed amount, as set out at Schedule 3, such amount to be based on a [**] share of this Estimated Aggregate Integral Input Contributions. When 5 Modules are in concurrent occupation (“Full Occupation”), the contributions model in Clause 8.4.4(b) will apply. |
| (b) | From the date Full Occupation is achieved, COLLABORATOR will continue to pay a [**] share of the Estimated Aggregate Integral Input Contributions Incurred, However, from and including the first anniversary date (the “First Anniversary Date”) that Full Occupation is achieved, a reconciliation will take place at the end of each Year and a refund will be made to or further contribution will be received from COLLABORATOR with respect to its share of the Integral Input Contributions based on the difference between the Estimated Aggregate Integral Input Contributions, and the pro rata actual aggregate Integral Input Contributions incurred for that Year. Reconciliation will be based on audited accounts, |
| 8.4.5 | the Establishment Input Contributions will be payable directly to Catapult as they are incurred on COLLABORATOR’S behalf and following receipt of invoice; and |
| 8.4.6 | Save as otherwise provided all contributions payable by COLLABORATOR to Catapult pursuant to this Agreement will be payable within 30 days of receipt of an accurate, complete and valid VAT invoice by COLLABORATOR for such costs. |
| 8.5 | Catapult will use reasonable endeavours to ensure the Actual Occupation Date is not later than the Expected Occupation Date, In the event the Actual Occupation Date is not achieved by the Expected Occupation Date, then Facility Contributions, Business Rates and Integral Input Contributions will only accrue on a pro rata basis from the Actual Occupation Date. In the event COLLABORATOR wishes to |
12
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| occupy the Module before the Expected Occupation Date It will notify Catapult of its requested Expected Occupation Date not less than 90 days’ In advance of the date It wishes to occupy the Module, Entry on such revised Expected Occupation Date will be subject to Catapult consent (not to be unreasonably withheld; however, for clarity, it will be reasonable to withhold consent if Catapult will not be able to complete Its obligations In order to enable occupation of the Module, in accordance with the definition of the “Actual Occupation Date” by the date requested by COLLABORATOR). However in the event COLLABORATOR wishes to delay its occupation to after the Expected Occupation Date, it will remain liable to pay all Contributions from the Expected Occupation Date. |
| 8.6 | By being part of the Centre, COLLABORATOR has access to the wider Catapult supporting infrastructure which includes, but is not limited to, reimbursement support, clinical trial support, process development capability, and regulatory and market access consultancy expertise, The cost of such Additional Inputs are to be agreed through separate negotiation and contractual agreement. |
| 8.7 | Catapult undertakes to keep full and proper books of account and records relating to the Integral Input Contributions and the Establishment Input Contributions. In addition COLLABORATOR will be provided with the opportunity to comment on such planned expenditure and consensus sought through participation in the Collaborator Forums (although for clarity, Catapult reserves its discretion in exercising its professional Judgment In relation to making any final decisions with respect to the Integral Input Contributions, Activity Related Input Contributions and Establishment Input Contributions incurred, while being consistent with the objectives set out in the terms of reference for the Collaborator Forums, particularly with respect to maintaining a suitable level of services required for robust operation of a licensed facility suitable for late stage clinical and commercial manufacture of ATMPs in the most economical way). |
| 8.8 | At the beginning of each Year during the Term Catapult will provide to all Collaborators a budget setting out all anticipated contributions for the Year with respect to Integral and Activity Related Inputs to be provided in that Year. In addition to this, from the date Full Occupation is achieved, a quarterly statement will be provided to all Collaborators in the Centre comparing actuals to the budgeted amounts. |
| 8.9 | Catapult will procure an audit for each Year during the Term to be carried out by an Independent auditor acceptable to all Collaborators. The Audit report will be made available to all Collaborators in the Centre. |
| 8.10 | In the event that an MHRA MIA (IMP) license or any other consent required for operation of the Centre by Catapult In accordance with GMP or Applicable Laws is not granted to Catapult on or before the Expected Occupation Date, then the Facility Contribution and Integral Input Contribution will be reduced [**] until the data that an MHRA MIA (IMP) license is granted. Catapult will also waive any additional [**] as a result of any such delay in establishing GMP compliance. For the avoidance of doubt, COLLABORATOR will remain liable for [**] |
| 8.11 | In recognition of the collaboration required to achieve an MHRA license, In the event COLLABORATOR elects to occupy the Module after the Actual Occupation Date, the revised deadline for an MHRA MIA (IMP) licence or Necessary Consent to be granted, will be delayed by a period of time equal to the number of days between the Actual Occupation Date and the subsequent later date that COLLABORATOR actually occupies the Module. |
| 8.12 | Catapult will use best efforts, in line with commercial practice, to minimise facility downtime and to plan any such downtime so as to minimise business impact for COLLABORATOR. Catapult will use best efforts to ensure [**] |
| [**] |
| [**] |
13
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| i. | [**] |
| [**] |
| [**] |
| [**] |
| [**] |
| In any event of conflict between this Clause 8.12 of this Agreement and the QTA, the QTA shall prevail. |
| 9. | CATAPULT INPUTS, FACILITIES AND SUPPORT |
| The operation of the Centre is dependent on a range of critical inputs split between: |
| (a) | Integral Inputs which are Inputs Catapult, using its reasonable judgment considers as fundamental to the operation of the Centre and that all collaborators will draw on In equal measure. Such Integral Inputs are listed in Clause 9.1, but may be varied from time to time by Catapult, in good faith, to cater for the common, but not necessarily universal, requirements of the collaborators in the Centre, while at all times maintaining robust compliance with health and safety and GMP guidelines; inputs included at Clause 9.1 may not be removed or varied without prior notification of COLLABORATOR, and |
| (b) | Activity Related Inputs which are inputs that are dependent on COLLABORATOR’s manufacturing processes and activity, and are listed at Clause 9.2 but may be varied from time to time by Catapult, in good faith, to meet COLLABORATOR’S requirements and with prior notification of COLLABORATOR, but may not include Items from Clause 9.1 without prior agreement of COLLABORATOR. |
| (c) | Establishment inputs which are activities to be performed by Catapult, in conjunction with COLLABORATOR, to support the On-boarding Project. |
| It is a condition of occupation that the Integral Inputs, Establishment Inputs, and Activity Related Inputs will be procured through Catapult. |
| (d) | Additional Inputs are inputs COLLABORATOR requires Catapult to contribute to the Project, that are not Integral Inputs, Establishment Inputs or Activity Related Inputs. These will be arranged through the completion and execution of an Additional Input Agreement, on reasonable terms to be agreed, in the form set out at Schedule 16 (“Additional Input Agreement”). Once signed by both Parties, the Additional Input Agreement will amend this agreement and an Additional Input will be deemed appended to the list of Additional Inputs at Clause 9.4 and any associated contributions from COLLABORATOR in consideration of the Additional Inputs will be included in the Additional Input Agreement, and inserted at Schedule 3. For the avoidance of doubt, such an Input which does not impact on the Centre, the Centre’s GMP compliance or other Collaborators may, alternatively where COLLABORATOR determines, be sourced by COLLABORATOR from a Third Party provider, Which will be reasonably facilitated by Catapult, where requested by COLLABORATOR. |
14
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 9.1 | Catapult shall provide the following Integral Inputs: |
| 9.1.1 | the Quality Management System and supporting quality assurance function assuring all GMP inputs contributed by Catapult are maintained in compliance with GMP Requirements; |
| 9.1.2 | management and governance of the Quality Management System GMP compliance process; |
| 9.1.3 | regulatory compliance of the Centre from start up, including handling of associated MHRA compliant activities such as routine audits; for the avoidance of doubt all audit of COLLABORATOR Process will be COLLABORATOR’S responsibility; |
| 9.1.4 | Catapult and Centre insurance as described in Clause 19; |
| 9.1.5 | safety systems and equipment such as emergency light testing, fire extinguishers, health and safety equipment outside the Manufacturing Space, and associated safety audits; |
| 9.1.6 | a managed reception during business hours, and the provision of a mechanism for COLLABORATOR to access the Module at any time (24 hours a day, 7 days a week, 365 days per year), except in situations of Centre shutdown / an emergency; |
| 9.1.7 | all utilities necessary for the operations of the Centre (but not the Manufacturing Space) as set out in Schedule 14; |
| 9.1.8 | support for IT Infrastructure. For the avoidance of doubt, this does not include applications support for COLLABORATOR; |
| 9.1.9 | hosting, maintenance and administration of all IT systems (BMS, EMS, eQMS, LIMS and WMS) plus all required access rights for IT systems required primarily for Centre functions and required even when no collaborators are in occupation (BMS, EMS); |
| 9.1.10 | receipt of incoming materials into the Centre within business hours; |
| 9.1.11 | a system for booking in and managing short term storage of COLLABORATOR’S inventory for raw materials, consumables, product contact materials and excipients within the warehouse; |
| 9.1.12 | short term storage of COLLABORATOR Products (including starting materials, intermediates, active substances, and final product thereof) subject to terms to be agreed; |
| 9.1.13 | out of hours call out system for all facilities alarms (bar the Manufacturing Space alarms); |
| 9.1.14 | scheduled cleaning and disinfection of all areas outside of the Manufacturing Space and outside of any Quality Control laboratory space occupied by any collaborator; |
| 9.1.15 | (save with respect to the Manufacturing Space which is provided for in Clause 9.2.1 and for any Quality Control laboratory space occupied by any collaborator) perform environmental monitoring in the form of viable and non-viable particulate monitoring In the Centre required to demonstrate maintenance of the appropriate environmental classifications; |
| 9.1.16 | access to a controlled rate freezer and allocated storage capacity of released starting materials and quarantined drug substance or drug product at the following temperatures; controlled room temperature, 2-8ºC, -20°C, -80°C and gas phase of liquid nitrogen; |
| 9.1.17 | COLLABORATOR relationship management, including via the Steering Committee; |
| 9.1.18 | a kitchen area and vending machines for snacks, hot and cold drinks within the Centre; |
| 9.1.19 | a dedicated secure Manufacturing Office and Non-Manufacturing Office per Module; |
| 9.1.20 | routine calibration, maintenance, requalification of facilities and equipment listed in Clause 6.3, as appropriate for their function; |
15
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 9.1.21 | access to a fair share of communal general refuse disposal facilities, for use by Catapult, COLLABORATOR and other Collaborators; |
| 9.1.22 | on request by COLLABORATOR, from time to time, Catapult shall provide general information on the Centre, equipment at the Centre, and general consumables, or as otherwise reasonably required in relation to the Centre or Module, for regulatory purposes or in relation to any investigation or inspection by any Regulatory Authority. The Catapult reserves the right to provide an Additional Input Statement for any such requests that will incur input from the Catapult above and beyond that expected during routine operation and; |
| 9.1.23 | If COLLABORATOR is the subject of any inspection or investigation by any Regulatory Authority, Catapult shall provide COLLABORATOR with all reasonable assistance, and, at COLLABORATOR’s request and expense, or as required by any Regulatory Authority, be present for any inspection of the Module by any Regulatory Authority. |
| 9.2 | Catapult shall provide the following Activity Related Inputs: |
| 9.2.1 | perform environmental monitoring in the form of viable monitoring and non-viable particulate monitoring required to demonstrate maintenance of the appropriate environmental classifications, including undertaking remote non-viable sampling in the Manufacturing Space. The COLLABORATOR is responsible for performing viable sampling in the Manufacturing Space which will then be processed by Catapult; |
| 9.2.2 | supply of measured electrical power, and all other necessary utilities, to the Manufacturing Space; |
| 9.2.3 | routine maintenance for the air handling system, including ULPA and HEPA filter changes |
| 9.2.4 | gowning for Catapult staff providing services to the collaborators, (COLLABORATOR gowning is ordered from Catapult warehouse stock); |
| 9.2.5 | provision of QA inputs to support COLLABORATOR activity within the module, in terms of handling non-process related deviations, Quality Events and planned changes, cleanroom environmental excursions, governance of Catapult generated GMP data provided to COLLABORATOR, providing GMP documentation to support QP certification of Drug Product; |
| 9.2.6 | Leased telephone line with a data package from the supplier if required; |
| 9.2.7 | Manufacturing Space decontamination on request from COLLABORATOR; |
| 9.2.8 | a measured supply of pharma grade oxygen, nitrogen, carbon dioxide, and compressed air; |
| 9.2.9 | transfer of decontaminated clinical, biological and hazardous chemical liquid waste from the liquid waste storage area and arrange its removal from the Centre by appropriately licensed contractors. The procedure for decontamination and disposal of are volume viral-contaminated waste will be defined between Catapult and COLLABORATOR; |
| 9.2.10 | packing and dispatch as described in Schedule 8; |
| 9.2.11 | access rights to IT systems required for COLLABORATOR activity within the module: eQMS, LIMS, WMS; |
| 9.2.12 | additional IT support if agreed in writing by the Parties (subject to request, and availability at the time of request); |
| 9.2.13 | a stand-by facility to receive incoming materials into the Centre outside of business hours; |
| 9.2.14 | Quality Assurance support for COLLABORATOR operation within the Module; and |
| 9.2.15 | Engineering and maintenance support for COLLABORATOR operation within the Module. |
| 9.3 | Catapult shall provide the following Establishment inputs: |
16
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 9.3.1 | Catapult will work in cooperation with COLLABORATOR to define and implement an agreed strategy for Technology Transfer, made up of; |
| (a) | Process Transfer; defining, implementing and/or supporting conduct of Process Transfer, but such process under the control and responsibility of COLLABORATOR, and |
| (b) | On-Boarding; defining, and implementing conduct of On-Boarding in collaboration with COLLABORATOR, but such process under the control and responsibility of the Catapult and described in Schedule 6. |
| 9.3.2 | Catapult will clean and decontaminate the Manufacturing Space and ensure the Manufacturing Space is operating at the specified cleanliness grade prior to COLLABORATOR occupation. |
| 9.3.3 | With respect to Collaborators, other then COLLABORATOR, Catapult will select and authorise all such Collaborators and ensure their processes and procedures meet the minimum standards required by Catapult. |
| 9.4 | Catapult shall provide the following Additional Inputs; |
| Additional Inputs are Catapult activities requested by COLLABORATOR (acting In Its sole discretion) because of a project identified need that are incorporated into this Agreement through the execution by both Parties of an Additional Input Agreement in accordance with Clause 9(d) above. |
| 9.5 | Steering Committee |
| The Parties will each nominate two representatives who will form the steering committee for the Project (“Steering Committee”). Where possible the representatives from both Parties will be the same as those serving on the steering committee for the On-boarding Project. The Steering Committee will meet (by telephone or in person) as required to discuss matters relating specifically to the COLLABORATOR and/or the Project (such as changes to Contributions and inputs, staffing updates, collaboration performance and resolution of issues). The Steering Committee will also participate in dispute resolution as set out In Clause 33 as required. |
| 9.6 | Collaborator Forums |
| Catapult undertakes to COLLABORATOR that it will ensure that the Collaborator Forums take place in accordance with the frequencies, the parameters, and all other terms set out in Schedule 16. |
| 9.7 | Catapult Staff |
| Catapult will ensure that all Catapult staff and third parties who are engaged by Catapult to provide the inputs set out in Clause 9 are appropriately skilled and trained. |
| 10. | COLLABORATOR RESPONSIBILITIES |
| 10.1 | COLLABORATOR shall, and shall ensure that COLLABORATOR Personnel shaft, comply with the following COLLABORATOR Responsibilities; |
| 10.1.1 | abide by the Code of Conduct and all other reasonable guidelines and protocols in force from time to time at the Centre; |
| 10.1.2 | handle all large volume liquid waste (such as culture media and buffers) within the Manufacturing Space and securely and safely transfer it to the handling area in the Centre (as designated by Catapult from time to time); |
| 10.1.3 | collect all small volume liquid waste in a sealable container within the Manufacturing Space and decontaminate it in-situ before removing it from the cleanroom via the MAL out to a waste staging and disposal area; |
| 10.1.4 | remove all solid waste from the cleanroom via the MAL out to a staging area for removal); |
| 10.1.5 | maintain and implement in accordance with Catapult’s standard operating procedures cleaning regimes for the Manufacturing Space; |
17
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 10.1.6 | unless otherwise agreed with Catapult, define and implement Process Transfer; |
| 10.1.7 | perform as required by Catapult all appropriate environmental monitoring within the Manufacturing Space and make the plates available to Catapult for analysis; |
| 10.1.8 | obtain, and maintain insurance for COLLABORATOR in accordance with Clause 19; and |
| 10.1.9 | comply with its obligations under the QTA. |
| 10.2 | If Catapult’s performance of its obligations under this Agreement is prevented or delayed by any act or omission of COLLABORATOR, its agents, contractors, sub-contractors or employees, Catapult shall not be liable for any costs, charges or loss sustained or incurred by COLLABORATOR arising directly from such prevention or delay. |
| 10.3 | if COLLABORATOR delays or does not perform its obligations set out in Clauses 10.1.1,10.1.2, 10.1.3, 10.1.4, 10.1.5, 10.1.7 and 10.1.9 above, Catapult may perform such obligations in its place. COLLABORATOR will reimburse Catapult for any reasonable out-of-pocket costs (as evidenced by appropriate invoices) that it incurs in discharging such obligations on COLLABORATOR’S behalf (for clarity, “obligations” as used in this clause Includes other terms commonly used synonymously with the term “obligation”, An example is “responsibility” as used in this Clause 10). |
| 11. | BACKGROUND INTELLECTUAL PROPERTY |
| 11.1 | Subject to the provisions of this Agreement, COLLABORATOR hereby grants to Catapult a non-exclusive, fully paid-up, royalty-free, licence, under COLLABORATOR’S Background Intellectual Property to undertake the Project with COLLABORATOR during the Term. |
| 11.2 | Subject to the provisions of this Agreement, Catapult hereby grants to COLLABORATOR a non-exclusive, fully paid-up, sub-licensabla, royalty-free, licence, under Catapult’s Background intellectual Property to undertake the Project and exploit COLLABORATOR’S Foreground Intellectual Property during the Term, |
| 11.3 | From the Termination Date, such license in Clause 11.2 will extend to permit COLLABORATOR to replicate the Module, and to such extent as required to enable COLLABORATOR to otherwise replicate, utilise and develop the COLLABORATOR Process, and/or to produce and exploit the COLLABORATOR Product and COLLABORATOR Foreground Intellectual Property, provided it is acknowledged that COLLABORATOR, at its own cost, will need to procure the consents required to use any Third Party’s Intellectual Property Including but not necessarily limited to any such Third Party Intellectual Property forming any part of the following Items that constitute the overall Catapult Background Intellectual Property when Catapult Background Intellectual Property is used by COLLABORATOR outside the Centre or from the Termination Date: the Electronic Quality Management System, the Laboratory Information Management System, Warehouse Management System and Environmental Monitoring System. It is acknowledged that, following termination or expiry of the Agreement, Catapult cannot procure the grant of such rights and that if COLLABORATOR does not procure such rights that Catapult accepts no liability whatsoever for claims resulting from breaches of any Third Party’s Intellectual Property resulting from COLLABORATOR’S use of the relevant Catapult Background Intellectual Property without a licence to the necessary Third Party’s Intellectual Property. |
| 11.4 | This Agreement does not affect the ownership of any Intellectual Property in any Background Intellectual Property or materials of a Party. Each Party will retain the sole and exclusive ownership rights in and to Its Background Intellectual Property and except for the license granted to Catapult in Clause 11.1 and to COLLABORATOR in Clause 11.2 and Clause 11.3, nothing in this Clause 11 will be construed as giving to either Party any rights to use any Background Intellectual Property of the other Party other than as expressly granted by this Agreement. Each Party will treat the other Party’s Background Intellectual Property as Confidential Information belonging to that other Party. |
| 12. | FOREGROUND INTELLECTUAL PROPERTY |
| 12.1 | All Foreground Intellectual Property excluding Catapult Foreground Intellectual Property (as defined in Clause 12.2 below), whether it is capable of being a Registered Right or not, shall be deemed to be the sole property of COLLABORATOR, regardless of which Party created such Foreground Intellectual Property (“COLLABORATOR Foreground Intellectual Property”). COLLABORATOR Foreground Intellectual Property shall constitute Confidential information belonging to COLLABORATOR. COLLABORATOR may take such steps as it may decide from time to time, and at its own expense, to register and maintain any protection for the Foreground Intellectual Property, including filing and |
18
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| prosecuting patent applications. Catapult shall ensure that its employees involved in the creation of the Foreground Intellectual Property give COLLABORATOR such assistance as COLLABORATOR may reasonably request in connection with the registration and protection of the Foreground Intellectual Property, including filing and prosecuting patent applications, and taking any action in respect of any alleged or actual infringement of the Foreground Intellectual Property (and for clarity, any out of pocket costs of Catapult associated with such assistance will be reimbursed by COLLABORATOR). |
| 12.2 | All Foreground Intellectual Property that constitutes an improvement to the Catapult Background Intellectual Property shall be owned by Catapult (“Catapult Foreground Intellectual Property”). Catapult grants to COLLABORATOR a non-exclusive, fully paid-up, royalty-free, worldwide, sublicensable licence under the Catapult Foreground Intellectual Property to undertake the Project. From the Termination Date, such license will extend to permit COLLABORATOR to replicate the Module, and to such extent as required to enable COLLABORATOR to otherwise replicate, utilise and develop the COLLABORATOR Process, and/or to produce and exploit the COLLABORATOR Product, provided that it is acknowledged that any licence to any Catapult Background Intellectual Property forming part of, or that is required to use such licensed Catapult Foreground Intellectual Property will remain subject to the restrictions and conditions of use in Clause 11.3 regarding Third Party Intellectual Property. |
| 12.3 | To the extent that any Catapult Foreground Intellectual Property is capable of prospective assignment, COLLABORATOR now hereby assigns the Catapult Foreground Intellectual Property to Catapult; and to the extent any Catapult Foreground Intellectual Property cannot prospectively be assigned, COLLABORATOR shall assign such Catapult Foreground IP to Catapult as and when they are created, at the request of Catapult (and for clarity, any out of pocket costs of COLLABORATOR associated with such assignment will be reimbursed by Catapult). |
| 12.4 | To the extent that any COLLABORATOR Foreground Intellectual Property is capable of prospective assignment, Catapult now hereby assigns COLLABORATOR Foreground Intellectual Property to COLLABORATOR; and to the extent any COLLABORATOR Foreground Intellectual Property cannot prospectively be assigned, Catapult shall assign such COLLABORATOR Foreground Intellectual Property to COLLABORATOR as and when they are created, at the request of COLLABORATOR (and for clarity, any out of pocket costs of Catapult associated with such assignment will be reimbursed by COLLABORATOR). |
| 13. | CONFIDENTIAL INFORMATION |
| 13.1 | The Receiving Party undertakes: |
| 13.1.1 | to maintain as secret and confidential all Confidential Information of the Disclosing Party and its Affiliates; |
| 13.1.2 | where the Receiving Party is Catapult, to use such Confidential Information only for the purposes of making the Centre, Module and Inputs available to COLLABORATOR (and other Collaborators, solely with respect to their Confidential Information) in accordance with this Agreement and the QTA and for exercising its rights under this Agreement and the QTA, or where the Receiving Party is COLLABORATOR, for the purpose of exercising its rights, and complying with its obligations, under this Agreement and the QTA (in each case, a “Permitted Purpose”, and together, the “Permitted Purposes” as it applies to each Party); and |
| 13.1.3 | Subject to Clause 13.3, to disclose such Confidential Information only to those of its employees, officers, contractors, consultants, advisors, legal counsel, Affiliates and sublicensees pursuant to this Agreement (if any) to whom and to the extent that such disclosure is reasonably necessary for the Permitted Purposes. For clarity, notwithstanding the foregoing, Catapult shall not disclose COLLABORATOR’s or its Affiliates’ Confidential Information (or the Confidential Information of any other entity with which COLLABORATOR or any of its Affiliates is in business negotiations or has contracted or to which it owes a duty of confidence) to any other Collaborator, without (i) COLLABORATOR’s express prior written permission, on a case by case basis, and (ii) Catapult complying with Clause 13.7 and ensuring that such collaborator is made aware of the confidential nature of the Confidential Information. |
| 13.2 | The provisions of Clause 13.1 shall not apply to Confidential information which the Receiving Party can demonstrate by reasonable, written evidence: |
| 13.2.1 | was, prior to its receipt by the Receiving Party from the Disclosing Party or Alternative Disclosing Party, in the possession of the Receiving Party and at its free disposal; |
19
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 13.2.2 | is subsequently disclosed to the Receiving Party without any obligations of confidence by a Third Party who has not derived it directly or indirectly from the Disclosing Party or Alternative Disclosing Party; |
| 13.2.3 | is or becomes generally available to the public through no act or default of the Receiving Party or its agents, officers, employees, Affiliates, advisors, contractors, consultants, legal counsel or sub-licensees; |
| 13.2.4 | is independently developed by the Receiving Party by individuals who have not had any direct or indirect access to the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information; or |
| 13.2.5 | the Receiving Party is required to disclose (and so discloses) to the courts of any competent jurisdiction, or to any government regulatory agency or financial authority, provided that the Receiving Party shall (i) inform the Disclosing Party or Alternative Disclosing Party as soon as is reasonably practicable, and (ii) at the Disclosing Party’s or Alternative Disclosing Party’s request seek to persuade the court, agency or authority to have the information treated in a confidential manner, where this is possible under the court, agency, or authority’s procedures, in which case, if the Confidential Information is treated in a confidential manner by the applicable court, agency or authority, such that it retains its confidential nature, Clause 13.1 shall continue to apply to such Confidential Information. |
| 13.3 | The Receiving Party shall procure that all of its employees, officers, contractors, consultants, advisors, legal counsel, Affiliates and sub licensees pursuant to this Agreement (if any) who have access to any of the Disclosing Party’s or Alternative Disclosing Party’s information to which Clause 13.1 applies, shall be made aware of and subject to these obligations and shall be subject to undertakings of confidentiality at least as restrictive as Clause 13.1 and which apply to the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information before being given access to the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information, and the Receiving Party shall be liable to the Disclosing Party or Alternative Disclosing Party for any breach of its employees, officers, contractors, consultants, advisors, legal counsel, Affiliates or sub licensees of the terms of this Clause 13. |
| 13.4 | Upon any termination or expiry of this Agreement, the Receiving Party shall return to the Disclosing Party or Alternative Disclosing Party any documents or other materials that contain the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information, including all copies made, and make no further use or disclosure thereof save that the Receiving Party shall not be obliged to purge or delete Confidential Information of the Disclosing Party or Alternative Disclosing Party from its IT systems that is stored by any automated back-up system, and shall be permitted to retain one (1) copy of all such Confidential information in its legal files solely for purposes of ensuring compliance with the terms of this Agreement, and shall not otherwise use or disclose such Confidential Information. |
| 13.5 | For the avoidance of doubt, and in light of Catapult’s objective to disseminate best practices and xxxxxx the development of the regenerative medicine sector in the UK, Catapult shall be entitled to publish or otherwise disclose any of its Confidential Information (but excluding the terms of this Agreement), including the Catapult Background Intellectual Property and Catapult Foreground Intellectual Property. |
| 13.6 | Catapult (a) will procure that each other Collaborator, and any other Third Party contractor or other business partner working under contract with Catapult or any other Collaborator that has access to the Centre, agrees written obligations of confidence equivalent to those set out in this Agreement with Catapult in relation to COLLABORATOR’S Confidential Information (and COLLABORATOR’S Affiliates’ Confidential Information (and the Confidential Information of any other entity with which COLLABORATOR or any of its Affiliates is in business negotiations or has contracted or to which it owes a duty of confidence)) and (b) will procure that (wherever possible with respect to such Third Parties other than Collaborators, but in all cases for Collaborators) COLLABORATOR will be given a third party right to enforce such confidentiality provisions. |
| 13.7 | For the purposes of this Agreement, Confidential Information shall also include any confidential information disclosed between COLLABORATOR and Catapult under (a) the confidentiality agreement between the Parties dated 27th May 2016, and (b) the heads of terms relating to the Collaboration between the Parties dated 10th November 2017. In relation to confidential information disclosed under these prior agreements, the terms of this Agreement shall supercede the terms in those prior agreements. |
20
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 14. | WARRANTIES |
| 14.1 | All Party Warranties |
| Each Party warrants, represents and undertakes to the other that: |
| 14.1.1 | it has full capacity and authority to enter into and to perform this Agreement; |
| 14.1.2 | there are no: |
| (a) | actions, suits or proceedings pending or, to its knowledge, threatened against or affecting it before any court or administrative body or arbitration tribunal; or |
| (b) | investigations by any Regulatory Authority pending or, to its knowledge, threatened against or affecting it; |
| 14.1.3 | once duly executed, this Agreement will constitute its legal, valid and binding obligations; and |
| 14.1.4 | it is not aware of any matters which might adversely affect its ability to perform its obligations pursuant to this Agreement. |
| 14.2 | Catapult Warranties |
| Catapult warrants, represents and undertakes to COLLABORATOR that from the Effective Date until the Termination Date: |
| 14.2.1 | it will use reasonable commercial endeavours to ensure it will always have the ability and all rights, titles and Necessary Consents to perform its obligations under this Agreement; |
| 14.2.2 | it will comply with its obligations under the QTA; |
| 14.2.3 | it will ensure that all other Collaborators are contractually obliged to comply with the operating requirements of the Centre so as to comply with GMP (as defined in the QTA) and Applicable Law, and will exercise appropriate diligence to ensure that all other Collaborators comply with these obligations; |
| 14.2.4 | it will maintain the Lease and perform all its obligations thereunder; |
| 14.2.5 | it will provide the inputs with all due care and skill and in any event in accordance with Applicable Law; and |
| 14.2.6 | it will make available the Centre and the Module in accordance with the provisions of this Agreement and in any event in accordance with all Applicable Law. |
| 14.3 | COLLABORATOR Warranties |
| COLLABORATOR warrants, represents and undertakes to Catapult that from the Effective Date until the Termination Date it will: |
| 14.3.1 | use reasonable commercial endeavours to ensure it will at all times have the ability and all rights, titles and Necessary Consents to perform its obligations under this Agreement; |
| 14.3.2 | perform both its obligations under this Agreement and all activities in respect of the Project in accordance with all Applicable Law and the Code of Conduct; and |
| 14.3.3 | perform COLLABORATOR Responsibilities. |
| 14.4 | Intellectual Property Warranties |
| 14.4.1 | Each Party represents, warrants, and undertakes to the other Party that: |
21
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| (a) | it has all right, title, and interest in and to its Background Intellectual Property or has, in the case of licensed Background Intellectual Property, the right to use such licensed Background Intellectual Property for the Project (subject to the Third Party Intellectual Property Rights in the categories of Catapult Background in Clause 11.3); and |
| (b) | it has not done, and will not do nor agree to do during the continuation of this Agreement, anything that would be inconsistent with the exercise by the other Party of the rights granted to it under this Agreement. |
| 14.4.2 | Without limiting the scope of Clause 14.4.1 and except as expressly provided in Clause 11.3, neither Party makes any representation nor gives any warranty or undertaking: |
| (a) | as to the efficacy or usefulness of its Background Intellectual Property; |
| (b) | that the use of any of its Background Intellectual Property or the exercise of any of the rights granted under this Agreement will not infringe any other Intellectual Property or other rights of any other person; |
| (c) | that the use of any of its Background Intellectual Property under or in connection with this Agreement will produce Products of satisfactory or merchantable quality or fit for their intended purpose or that any Product will not have any latent or other defects, whether or not discoverable; or |
| (d) | as imposing any obligation on it to bring or prosecute actions or proceedings against third parties for infringement. |
| 15. | INDEMNITY |
| 15.1 | COLLABORATOR agrees to indemnify, and hold Catapult harmless from and against Liabilities that Catapult suffers or incurs arising out of or in connection with: |
| 15.1.1 | any claim or proceedings made, brought or threatened against Catapult by a Third Party in respect of the COLLABORATOR Product or Process, or when in respect of the Project, whenever a Third-Party claim or proceeding is made, brought or threatened against Catapult because of the negligence, omission or wilful misconduct of COLLABORATOR, its employees, agents, contractors, visitors or subcontractors; |
| 15.1.2 | any loss of or damage to the tangible property or equipment belonging to a Third Party caused by or resulting from the negligence, omission or wilful misconduct of COLLABORATOR, its visitors, employees, agents, contractors, or subcontractors; and/or |
| 15.1.3 | any costs relating to an investigation, action or proceeding by a Regulatory Authority which arises because of COLLABORATOR’s material breach of this Agreement. |
| 15.2 | Catapult agrees to indemnify, and hold COLLABORATOR harmless from and against Liabilities that COLLABORATOR suffers or incurs arising out of or in connection with: |
| 15.2.1 | any claim or proceedings made, brought or threatened against COLLABORATOR by a Third Party whenever a Third-Party claim or proceeding is made, brought or threatened against COLLABORATOR because of the negligence, omission or wilful misconduct of Catapult, its employees, agents, visitors, contractors, or subcontractors; |
| 15.2.2 | any loss of or damage to the tangible property or equipment belonging to a Third Party caused by or resulting from the negligence, omission or wilful misconduct of Catapult, its visitors, employees, agents, contractors or subcontractors; and/or |
| 15.2.3 | any costs relating to an investigation, action or proceeding by a Regulatory Authority which arises because of Catapult’s material breach of this Agreement. |
| 15.3 | Indemnification of either party under this Clause 15 is conditional upon: (a) the extent that the indemnified claim is caused by or resulting from the negligence, omission or wilful misconduct of the indemnified party, its employees, agents or subcontractors, or their failure to take any measures as are reasonable in the relevant circumstances to mitigate the loss or damage that has occurred or may occur (in which case any portion of the claim not caused by or resulting from the negligence, omission or wilful |
22
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| misconduct of the indemnified party, its employees, agents or subcontractors or failure to mitigate will remain valid for indemnification); (b) the indemnified party promptly, on becoming aware of such claim, notifying the indemnifying party of the existence of the relevant claim; (c) the indemnified party refraining from making any admissions in respect of the relevant claim; and (d) the indemnifying party having sole control over the defence and/or settlement of the relevant claim. |
| 16. | LIMITATION OF LIABILITY |
| 16.1 | Collaborators occupying the Centre generally, and COLLABORATOR and Catapult in particular with respect to this Agreement, in choosing to employ the Centre as a base for GMP manufacturing activities accept and acknowledge a degree of risk inherent in any multi-mode, shared occupancy manufacturing facility and the nature of the biological processes undertaken within. Occasional unforeseen situations may arise associated with, for example utilities, equipment and associated processes that have the potential to disrupt or have a detrimental impact on processing, including on the products manufactured and developed at the Centre, and/or the manufacturing process(es) utilised at the Centre, by Collaborators and COLLABORATOR. |
| 16.1.1 | In light of this, Catapult will procure from each Collaborator, prior to their occupation of the Centre, contractual agreement not to commence or sustain legal proceedings against COLLABORATOR (or any other Collaborators or Catapult) for damages, or any other financial reimbursement (“Agreement Not to Xxx”), as a consequence of any unexpected and unintended consequences as a result of such a situation at the Centre as described in this Clause 16.1 (“Unforeseen Risks”) unless it is a result of attributable gross negligence or wilful misconduct of COLLABORATOR (or any other Collaborators or Catapult, as applicable), breach of confidence, material breach of any obligation under the relevant Collaborator’s (or, COLLABORATOR’S, or Catapult’s) collaboration agreement or quality agreement for the Centre, or material breach of Catapult SOPs or breach of Applicable Laws, by COLLABORATOR (or any other Collaborators or Catapult, as applicable) and shall procure a direct right of enforcement of such Agreement Not to Xxx by COLLABORATOR against each such Collaborator or Catapult pursuant to the Contracts (Rights of Third Parties) Xxx 0000. |
| 16.1.2 | With respect to each Collaborator that Catapult has obtained an enforceable Agreement Not to Xxx in accordance with Clause 16.1.1, which COLLABORATOR has a direct right to enforce against such Collaborator pursuant to the Contracts (Rights of Third Parties) Xxx 0000, COLLABORATOR agrees that it shall not commence or sustain legal proceedings against such a Collaborator for damages, or any other financial reimbursement, as a consequence of any Unforeseen Risks unless it is a result of attributable gross negligence or wilful misconduct of, or a breach of confidence, material breach of any obligation under the relevant Collaborator’s collaboration agreement or quality agreement for the Centre, or material breach of Catapult SOPs or breach of Applicable Laws by, such a Collaborator. |
| 16.1.3 | Catapult and COLLABORATOR, each agree not to commence or sustain legal proceedings against the other Party for damages, or any other financial reimbursement, as a consequence of any Unforeseen Risks unless it is a result of attributable gross negligence or wilful misconduct of the other Party, or is a breach of confidence, material breach of any obligation under this Agreement or the QTA, or a material breach of Catapult SOPs or breach of Applicable Laws, by the other Party. |
| 16.1.4 | This Clause 16.1 is not intended to qualify, and is subject to and without prejudice to, each Party’s rights and obligations under Clause 15 (Indemnity). |
| 16.2 | Catapult, COLLABORATOR, and all Collaborators are obliged to abide by the operating requirements of the Centre so as to comply with GMP (as defined in the Quality Technical Agreement) and exercise an appropriate duty of care such as to minimise the frequency and severity of events alluded to in Clause 16.1, thus offering each other mutual protection. In order to ensure that Catapult, COLLABORATOR and all Collaborators do not expose themselves to unreasonable financial risk as a result of such a situation, Catapult and COLLABORATOR (and Catapult will procure this of each other Collaborator) will ensure the continued and uninterrupted maintenance of an appropriate level of public liability, business continuity and professional indemnity insurances. |
| 16.3 | Without prejudice to Clauses 16.1, 16.5, 16.6 and 16.7, the maximum aggregate Liability of COLLABORATOR which arises from any single event which occur in any Year will be limited to [**] for any single event, with no limit on the number of events. |
23
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 16.4 | Without prejudice to Clauses 16.1, 16.5, 16.6 and 16.7, the maximum aggregate Liability of Catapult which arises from events which occur in any Year will be limited to [**] for any single event, with no limit on the number of events. In no circumstance will any Party have any Liability for: |
| 16.4.1 | any indirect, special or consequential loss; or |
| 16.4.2 | any loss of profits, revenue, business opportunity, data, or goodwill (in each case whether such loss is direct or indirect). |
| 16.6 | Nothing in this Agreement limits or excludes any person’s liability to the extent that it may not be so limited or excluded by law, including any such liability for death or personal injury caused by that person’s negligence, or liability for fraud or fraudulent misrepresentation. |
| 16.6 | Without prejudice to Clause 16.5, nothing in this Agreement will operate to exclude or restrict COLLABORATOR’s Liability: |
| 16.6.1 | under the indemnity contained in Clause 15.1; or (in each of Clauses 16.6.2 and 16.6.3 below, other than when the specific conditions stipulated in the Agreement are met so as to justify otherwise); |
| 16.6.2 | to pay the Financial Contributions; or |
| 16.6.3 | to pay the Compensations. |
| 16.7 | The Parties agree that they have negotiated this Clause 16 and the allocation of risk in this Clause is a fair and equitable position. |
| 17. | DURATION AND TERMINATION |
| 17.1 | This Agreement, and the licences granted hereunder, shall come into effect on the Effective Date and, unless terminated earlier in accordance with this Clause 17 or unless specified in the continuing obligations provisions of this Agreement as having continued effect, shall continue in force for [**] from the Actual Occupation Date (“Initial Period”) after which date the Term will terminate automatically by expiry. |
| 17.2 | COLLABORATOR will have an option to extend the Initial Period on a rolling basis for further periods of up to [**] on condition: |
| 17.2.1 | COLLABORATOR provides 12 months’ advance notice of the end of the Initial Period (or any extension thereof) of its intention to extend the Term; |
| 17.2.2 | COLLABORATOR has consistently materially complied with its material obligations (but this does not preclude multiple, repeated breaches of minor obligations, which have been raised by Catapult with COLLABORATOR, constituting a material breach so as to prevent COLLABORATOR’S right to this option) in the Collaboration Agreement throughout the term of the Collaboration Agreement up to the date the option is exercised; |
| 17.2.3 | Catapult intends to continue the operation of the Centre beyond the Initial Period (or any extension thereof); and |
| 17.2.4 | the terms of any extensions are mutually agreed, with both Parties acting reasonably and in good faith to negotiate such terms, although it is agreed such terms will be substantially the same as those in this Agreement, save for any changes required because of changes in law or regulation, or required in order to enable compliance with GMP or to maintain the Centre’s licence. The Financial Contributions to be made by COLLABORATOR for the period of the applicable extended term will be based on those prevailing on the date the option is exercised. |
| 17.3 | COLLABORATOR shall be able to terminate on the provision of [**] written notice to Catapult. |
| 17.4 | The Parties may terminate this Agreement at any time by agreement to do so in writing signed by the authorised signatories of the Parties and the provisions of Clauses 18.1 shall not apply. |
24
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 17.5 | For a period of 12 months from the date that Catapult announces that the second set of standard modules (“Phase 2”) is available for reservation, on condition COLLABORATOR is in continuing occupation of the Module and has been in continuing material compliance with its obligations and warranties under this Agreement at the time the option is exercised, Catapult grants to COLLABORATOR the non-transferrable option to: |
| 17.5.1 | negotiate (with both Parties acting reasonably and in good faith), for occupation of one additional module in Phase 2 on terms to be agreed in an amendment to this Agreement (but with the financial contributions relative to manufacturing area in any additional module in Phase 2 not to be materially different from the financial contributions prevailing that apply to modules available in Phase 2 on the date the option is exercised); and |
| 17.5.2 | negotiate (with both Parties acting reasonably and in good faith), to transfer the Module to a module in Phase 2 on terms of occupation, relative to manufacturing space area, to be unchanged from those of the Module (but with the financial contributions relative to manufacturing area not to be materially different from the financial contributions prevailing that apply to modules available in Phase 2 on the date the option is exercised), save that COLLABORATOR shall be responsible for any reasonable costs of relocation including Catapult inputs for Manufacturing Space de-contamination and qualification costs. Any such transfer to Phase 2 will take into consideration any reasonable location and timing requirements of COLLABORATOR. |
| 17.6 | If either option is exercised by COLLABORATOR, the Facility, Integral and Business Rates Contributions attributable to Phase 2 will be equally apportioned between all Collaborators occupying Phase 2 modules. For the avoidance of doubt, COLLABORATOR will be responsible for no more than a [**] share of all Facility, Integral and Business Rates Contributions attributable to Phase 2. |
| 17.7 | If either option is not exercised during the period set out above, then that option will lapse and Catapult will owe no further obligation to COLLABORATOR regarding Phase II modules. |
| 17.8 | Either Party may elect to terminate this Agreement at any time by notice in writing to the other Party, such notice to take effect as specified in the notice: |
| 17.8.1 | if the other Party is in material breach of this Agreement (including any breach of Clause 20) and, in the case of a breach capable of remedy within 90 days, the breach is not remedied within 90 days of the party receiving notice specifying the breach and requiring its remedy; or |
| 17.8.2 | if (A) the other Party becomes insolvent or unable to pay its debts as and when they become due; or (B) an order is made or a resolution is passed for the winding up of the other Party (other than voluntarily for the purpose of solvent amalgamation or reconstruction); or (C) a liquidator, administrator, administrative receiver, receiver, or trustee is appointed in respect of the whole or any part of the other party’s assets or business; or (D) the other Party makes any composition with its creditors; or (E) the other Party ceases to continue its business; or (F) as a result of debt and/or maladministration the other party takes or suffers any similar or analogous action in any jurisdiction |
| 17.9 | COLLABORATOR may elect to terminate this Agreement by notice in writing if, a party that is not an Affiliate obtains Control of Catapult. |
| 17.10 | A Party’s right of termination under this Agreement, and the exercise of any such right, shall be without prejudice to any other right or remedy (including any right to claim damages) that such Party may have in the event of a breach of contract or other default by the other Party. |
| 17.11 | If there is destruction or damage to the Centre that leaves the whole or substantially the whole of the Centre unfit for occupation and use or inaccessible, and the Centre and Module have not been made fit for occupation and use and accessible within 5 months of such damage or destruction, and within that 5 month period it has been agreed that there is no possibility that the Centre and Module can be made fit for occupation and GMP operation within another 12 months from the end of that 5 month period, then either Party may terminate this Agreement immediately by written notice to the other. Such right of termination, If not exercised, shall continue until such time as the Centre and Module are made fit for occupation and use, and accessible, again. |
| 17.12 | If there is destruction or damage to the Centre by any of the Insured Risks that leaves the whole or substantially the whole of the Centre and / or the Module unfit for occupation and use or inaccessible, |
25
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| then, save to the extent that the Catapult’s insurance has not been vitiated or policy moneys refused because of any act or default of COLLABORATOR then the Facility Contribution, the Activity Related Inputs Contributions, the Integral Contributions, and the Business Rates or a fair proportion of them shall not be payable from and including the date of such damage or destruction until the earlier of the date that the Module is once again fit for occupation end use and accessible end the date 2 years from and including the date of such damage or destruction. |
| 18. | CONSEQUENCES OF TERMINATION |
| 18.1 | COLLABORATOR recognises that considerable planning and advanced preparation is required to ensure timely COLLABORATOR occupation of the Module. In recognition of the opportunity cost of reserving a Module for COLLABORATOR, If COLLABORATOR serves notice to terminate this Agreement in any circumstances other than as set out in Clauses 17.3 or 17.4, COLLABORATOR will compensate Catapult as follows: Following notice to terminate, COLLABORATOR will pay to Catapult the sum equivalent [**]. |
| 18.2 | The provisions of Clause 18.1 shall not apply where this Agreement is terminated in accordance with Clause 20. The Compensations will be payable by COLLABORATOR in the event Catapult terminates under Clause 17.8.1 because of a material breach committed by COLLABORATOR that is either not remediable, or that is not remedied within 90 days or less where a remedy is possible. |
| 18.3 | Upon termination of this Agreement for any reason (and unless otherwise agreed by the Parties in a subsequent, written agreement, including any agreement entered into in accordance with the provisions of Clause 9(c)): |
| 18.3.1 | the provisions of Clauses 1, 11,3, 11.4, 12.1 to 12.4 inclusive, 13, 14, 15, 16, 18, 19, 21, 22, 23, 24, 28, 31, 32, 33 and 36 shall remain in force; and |
| 18.3.2 | the Collaboration will terminate, subject to any subsisting and continuing obligations. |
| 19. | INSURANCE |
| 19.1 | Catapult shall take out with a reputable insurance company and maintain at all times during the Term of this Agreement buildings, employers liability, professional indemnity, public and product liability insurance including against all loss of and damage to the Module, the Centre, and Injury to persons including death arising out of or in connection with this Agreement. Such insurances may be limited in respect of one claim provided that such limit must be at least [**]. Product liability insurance shall continue to be maintained for a further [**] from the end of the term of this Agreement. |
| 19.2 | COLLABORATOR shall take out with a reputable insurance company, and maintain at all times during the Term of this Agreement professional indemnity, public and product liability insurance including against all loss of and damage to the Module and Centre, injury to persons including death arising out of or in connection with this Agreement, and against all loss of and damage to any COLLABORATOR owned equipment, or COLLABORATOR personnel personal effects within the Module or in the Centre generally. Copies of COLLABORATOR insurance certificates will be provided for Catapult’s records annually on request. Such insurances may be limited in respect of one claim provided that such limit must be at least [**]. Product liability insurance shall continue to be maintained for a further [**] from the end of the Term. COLLABORATOR acknowledges that Catapult will have no responsibility for any COLLABORATOR owned equipment or any COLLABORATOR personnel personal effects located in the Module or any other part of the Centre save where any damage to such is caused directly by Catapult negligence or intentional misconduct. |
26
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 20. | ANTI-BRIBERY AND ANTI-CORRUPTION |
| 20.1 | Each Party agrees that, in connection with this Agreement and the Project, they shall each, (and shall procure that their respective officers, employees, agents and any other persons who perform services for them or on their behalf in connection with this Agreement shall): |
| 20.1.1 | not commit any act or omission which causes or could cause the other Party to breach, or commit an offence under, any laws relating to anti-bribery and/or anti-corruption including Foreign Corrupt Practices Act in the United States and the UK Anti-Bribery Act; |
| 20.1.2 | keep accurate and up to date records showing all payments made and received and all other advantages given and received in connection with this Agreement and the steps taken to comply with this Clause 20, and permit the other Party to inspect those records as reasonably required; |
| 20.1.3 | promptly notify the other Party of: |
| 20.1.3.1 | any request or demand for any financial or other advantage received by it (or that person); and |
| 20.1.3.2 | any financial or other advantage it (or that person) give or intend to give whether directly or indirectly in connection with this Agreement; and |
| 20.1.4 | promptly notify the other Party of any breach of this Clause 20. |
| 20.1.5 | in the case of Catapult, ensure that all other collaborators are contractually obliged to comply with equivalent obligations as set out above. |
| 20.2 | Any breach of this Clause 20 shall constitute a material breach. |
| 21. | PUBLICITY |
The Parties agree consent is required in before use of the other’s name, or any adaptation of their name, or any of their logo(s), trademark(s), or other of their device(s) in any advertising, promotional, or sales materials (however, they also agree that such consent is not to be unreasonably withheld).
| 22. | STATE AID |
| 22.1 | The parties acknowledge that Catapult is a ‘Research Organisation’ as defined under European Union legislation and has an obligation to ensure, and is subject to audits to demonstrate, that all activities it undertakes are compliant with EU state aid rules, including its activities under this Agreement. The parties therefore agree that, notwithstanding any other provision of this Agreement: |
| 22.1.1 | Catapult shall be entitled to cooperate fully with any investigation by any grant funder of Catapult or by the European Commission or any court of law with respect to this Agreement regarding the grant/alleged grant of state aid and the provision of inputs hereunder and COLLABORATOR shall, if so requested by Catapult, promptly provide to Catapult all reasonable and necessary assistance in connection with any such investigation(s); |
| 22.1.2 | Catapult shall keep COLLABORATOR informed of any active and specific investigation into this Agreement and, where possible, liaise with COLLABORATOR concerning any response to the European Commission; and |
| 22.1.3 | the parties shall comply with any ruling of the European Commission or court of law in relation to the application of the EU state aid rules to this Agreement. |
| 22.2 | The obligations set out in Clause 22.1 above shall subsist for a period of 10 years from the date of this Agreement, notwithstanding any earlier termination of this Agreement. |
27
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 23. | NOTICES |
| 23.1 | Any notice required to be given under this Agreement shall be given in writing and sent by prepaid airmail post or courier, delivered personal, or sent by email to the following addresses or such other address as may be notified by the relevant party from time to time in writing: |
| To Catapult | To COLLABORATOR: | |
| If sent by post to:
Cell Therapy Catapult 12th Floor Tower Wing Guy’s Hospital Great Xxxx Xxxx Xxxxxx XX0 0XX Xxxxxx Xxxxxxx |
If sent by post to:
Stevenage Bioscience Catalyst Xxxxxxx Xxxx Xxxx Xxxxxxxxx Xxxxx XX0 0XX | |
| For the attention of: | For the attention of: | |
| [**] | [**] Chief Development Officer | |
| If sent by email, to: | If sent by email, to: | |
| [****] | [****] | |
| 23.2 | Any notice so sent shall be deemed to have been duly given: |
| 23.2.1 | if sent by personal delivery or courier, on delivery at the address of the relevant party; |
| 23.2.2 | if sent by prepaid airmail post, five days after the date of posting; and |
| 23.2.3 | if sent by email, only on acknowledgement of receipt, such acknowledgement not being an automated message. |
| 24. | FURTHER ASSURANCES |
Each Party shall, as and when requested by the other Party and without charge, do all such acts and execute all such documents as may be reasonably necessary to give full effect to the provisions of this Agreement.
| 26. | ENTIRE AGREEMENT |
| 25.1 | This Agreement constitutes the entire agreement between the Parties and supersedes and replaces any and all previous agreements, understandings or arrangements between the parties, whether oral or in writing, relating to its subject matter. |
| 25.2 | The Parties acknowledge that in entering into this Agreement they do not rely on any statement, representation (including, without limitation, any negligent misrepresentation but excluding any fraudulent misrepresentation), warranty, course of dealing, custom, understanding or promise except for those expressly set out in this Agreement. |
| 25.3 | The Parties irrevocably and unconditionally waive any rights and/or remedies they may have to the fullest extent permitted by law (including without limitation the right to claim damages and/or to rescind this Agreement) in respect of any misrepresentation (including, without limitation, any negligent misrepresentation but excluding any fraudulent misrepresentation). |
| 25.4 | Except as expressly set forth in this Agreement, neither Party grants to the other by implication, estoppel or otherwise, any right, title, licence or interest in any Intellectual Property right. |
28
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 26. | VARIATION |
| 26.1 | Subject to Clause 26.2, no variation or amendment to this Agreement shall be effective unless it is made in writing and signed by the duly authorised representatives of both Parties. |
| 26.2 | The following principles will be adhered to in the event a change is proposed by Catapult to Schedule 8 (Warehouse and Procurement Management Provisions), Schedule 9 (Quality Control), Schedule 10 (IT Infrastructure), and Schedule 12 (Module and Centre Specifications) only: |
| 26.2.1 | If the proposed change has no material impact on COLLABORATOR Product(s) or Process(es), or COLLABORATOR’S compliance with GMP guidelines or would not require COLLABORATOR to amend or change any regulatory filing or regulated procedure, Catapult may enact the change by a written notification (signed by a member of Catapult’s Quality team) to COLLABORATOR, such written notification forming an amendment to this Agreement. Catapult will provide at least 30 days notification to enable COLLABORATOR to assess impact ahead of any impact occurring; |
| 26.2.2 | If the proposed change has a material impact on the COLLABORATOR Product(s) or Process(es), or COLLABORATOR’S compliance with GMP guidelines, or would require COLLABORATOR to amend or change any regulatory filing or regulated procedure, such change will require the mutual written consent of the Parties in the form of an amendment including the authorised signatories of their respective quality assurance teams where relevant to quality procedures to this Agreement in accordance with Clause 26.1; |
| 26.2.3 | If COLLABORATOR does not agree that a change proposed by Catapult to fall under Clause 26.2.1 has no material impact, the matter will be referred to the Steering Committee for resolution, and if no agreement is reached within a reasonable period, then resolved through the use of the Expert Determination Procedure under Schedule 13. |
| 26.2.4 | This section shall not override the Quality Technical Agreement in relation to changes relating to quality. |
| 27. | ASSIGNMENT AND SUB-CONTRACTING |
| 27.1 | Except as provided in Clause 27.2, neither Party shall assign, sub-contract, mortgage, charge, or otherwise transfer any rights or obligations under this Agreement, without the prior written consent of the other Party. |
| 27.2 | Catapult will be entitled to sub-contract any of its obligations under this Agreement, provided that it shall ensure any relevant obligations are passed on to such sub-contractor and Catapult shall be responsible for the performance of such sub-contractor. |
| 28. | WAIVER |
No failure or delay by a party to exercise any right or remedy provided under this Agreement or by law shall constitute a waiver of that or any other right or remedy, nor shall any single or partial exercise of any right or remedy preclude the further exercise of such right or remedy.
| 29. | SEVERABILITY |
If any provision (or part of any provision) of this Agreement is held to be invalid, void or otherwise unenforceable by a court of competent jurisdiction from whose decision no appeal is available, or from whose decision no appeal is made within the applicable time limit, then the provision (or relevant part of the provision) shall be re-written to be compliant where possible or omitted and the remaining provisions of this Agreement (and parts of the relevant provision, as applicable) shall continue in full force and effect. Should a material provision be rendered void, unenforceable or invalid by a court, either Party may terminate this Agreement within 30 days of the relevant court finding of voidness, unenforceability or invalidity.
| 30. | RELATIONSHIP OF THE PARTIES |
Nothing in this Agreement is intended to, or shall be deemed to, establish or imply any agency, partnership or joint venture between the Parties. Neither Party shall act or describe itself as the agent of the other Party and neither Party shall have, or hold itself out as having any authority to make commitments for or on behalf of the other Party.
29
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 31. | THIRD PARTY RIGHTS |
This Agreement does not create any right enforceable by any person who is not a Party to it save for (i) with respect to Clause 13.6 which the Parties agree may be directly enforceable by any other Collaborator that has occupied the Centre concurrently with the COLLABORATOR, provided that COLLABORATOR is able to directly enforce provisions equivalent to Clause 13 with respect to COLLABORATOR’s Confidential Information in a collaboration agreement between such Collaborator and Catapult, and (ii) as provided in Clause 16.1.
| 32. | GOVERNING LAW AND JURISDICTION |
This Agreement and any dispute or claim arising out of or in connection with it or its subject matter or formation (including non-contractual disputes or claims) shall be governed by and construed in accordance with the laws of England and Wales.
The Parties to this Agreement irrevocably agree that the courts of England shall have exclusive jurisdiction to settle any dispute or claim that arises out of or in connection with this Agreement or its subject matter or formation (including non-contractual disputes or claims), except that a Party may seek an interim injunction in any court of competent jurisdiction.
| 33. | DISPUTE RESOLUTION PROCEDURE |
| 33.1 | If a dispute arises out of or in connection with this Agreement or the performance, validity or enforceability of it (a “Dispute”), then, except as expressly provided in Clauses 7.2 and 26.2.3, the Parties shall follow the procedure set out in this clause: |
| (a) | either Party shall give to the other written notice of the Dispute, setting out its nature and full particulars (“Dispute Notice”), together with relevant supporting documents. On service of the Dispute Notice, the Steering Committee shall attempt in good faith to resolve the Dispute within 20 days, and if they’re unable to do so, then the Dispute shall be referred to the Chief Business Officer of Catapult, and Chief Operating Officer of COLLABORATOR, who shall attempt in good faith to resolve the Dispute; |
| (b) | if the Chief Business Officer of Catapult and Chief Operating Officer of COLLABORATOR are for any reason unable to resolve the Dispute within 30 days of the matter being referred to them from the Steering Committee under paragraph (a) above, then unless the Parties mutually agree to enter into mediation in good faith to settle the Dispute in accordance with the CEDR Model Mediation Procedure (but with no obligation on either Party to do so), then either Party shall be free to commence legal proceedings and the Dispute shall be finally resolved by the courts of England and Wales in accordance with Clause 32 (Governing Law and Jurisdiction). |
| 34. | COUNTERPARTS |
This Agreement may be executed in any number of counterparts, each of which when executed and delivered shall constitute a duplicate original of this agreement, but all the counterparts shall together constitute the same agreement. If this Agreement is executed in counterparts, it shall not be effective unless and until each Party has executed and delivered a counterpart to the other Party.
| 35. | FORCE MAJEURE |
Neither Party shall have any liability or be deemed to be in breach of this Agreement for any delays or failures in performance of this Agreement that result from circumstances beyond the reasonable control of that Party (each Party having in place appropriate disaster recovery and fail-safe measures to minimise the risk of force majeure), including without limitation labour disputes involving that Party. The Party affected by such circumstances shall promptly notify the other Party in writing when such circumstances cause a delay or failure in performance and when they cease to do so. To the extent any force majeure continues for more than 3 continuous months, the affected Party may serve 14 days written notice to terminate this Agreement. Such termination shall be automatic on expiry of the 14 day period provided the force majeure event has not ceased.
| 36. | DATA PROTECTION |
| 36.1 | In this Agreement the terms “Personal Data”, “Data Processor”, “Data Subject”, “Process” and “Data Controller” are as defined in the Data Protection Act 1988 (“Act”) or the GDPR or other data protection legislation in force in the UK from time to time. Each Party shall comply with its respective obligations under the provisions of the Act. |
30
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 36.2 | COLLABORATOR shall act as the Data Controller in respect of any Personal Data Processed by Catapult relating to this Agreement, the Project and/or Services and in compliance with COLLABORATOR’s obligations as such under the Act. |
| 36.3 | Insofar as COLLABORATOR provides or otherwise makes available Personal Date to Catapult and such Personal Data is Processed by Catapult, or if Catapult is required to Process Personal Data in connection with this Agreement; Catapult shall (a) keep such Personal Data strictly confidential; (b) only distribute to employees of Catapult to the extent such employees require access to such Personal Data for the performance of the Agreement; (c) not transfer such Personal Data to any third party (including any sub-contractor) without the prior written approval of COLLABORATOR; (d) only Process the Personal Data for purposes authorised by COLLABORATOR and in accordance with any instructions provided by COLLABORATOR (and for clarity, any purpose set out in this Agreement will be deemed to meet this requirement to the extent processing is require for the performance of that purpose); and (g) keep such Personal Data secure in accordance with the requirements of the Act and the principles articulated in the Act. Should Catapult receive any request from a Data Subject in relation to any Personal Data provided by COLLABORATOR, Catapult shall immediately pass on such Data Subject request to COLLABORATOR. |
| 36.4 | To the extent required under data protection legislation, each Party will permit and assist the other to carry out any privacy impact assessments or other data protection assessments reasonably required under data protection legislation. |
AGREED by the parties through their duly authorised representatives on the date written at the start of this Agreement:
|
|
For and on behalf of: Freeline Therapeutics Limited |
For and on behalf of Cell Therapy Catapult Limited | ||||||||
| Signed: | [**] |
Signed: | [**] | |||||||
| Full Name: | [**] | Full Name: | [**] | |||||||
| Job Title: | CEO | Job Title: | CEO | |||||||
31
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 1
THE PROJECT
[**]
32
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 2
| Part 1 | Occupation of Module |
| 1. | Definitions |
| In this Schedule 2: |
| 1.1 | “Co-location Fee” means the aggregate of the Facility Contribution and proportion of business rates payable under Clauses 8.4.1 and 8.4.2 of this Agreement. |
| 1.2 | “end of the Licence Period” means the expiry of the Term or its earlier termination pursuant to Clause 17 of this Agreement. |
| 2. | Occupation of the Module |
| 2.1 | Catapult permits COLLABORATOR to occupy the Non-Manufacturing Office from the Effective Date until the Actual Occupation Date for general administration and project management purposes connected with the Project. |
| 2.2 | Catapult permits COLLABORATOR to occupy the Module for the Permitted Use from the Actual Occupation Date until the end of the Licence Period together with the rights mentioned in Part 2 of this Schedule 2 and subject to the rights reserved to Catapult in Part 3 of this Schedule 2 and subject further to payment of the Co-location Fee in accordance with Clause 8 of this Agreement. |
| 2.3 | Subject to reasonable notice to Catapult, COLLABORATOR’s subcontractors may enter and use the Module and Common Access Areas (as necessary only to gain access to the Module) under COLLABORATOR’s supervision, for the purposes of the Project, provided that COLLABORATOR shall ensure that any relevant obligations are passed on to such subcontractors and COLLABORATOR shall be responsible for the actions of such subcontractors while at the Centre. |
| 2.4 | Catapult permits COLLABORATOR to access the Module at any time (24 hours a day, 7 days a week, 365 days per year), except in situations of Centre shutdown / an emergency. |
| 3. | COLLABORATOR’s Covenants and Acknowledgement |
| 3.1 | COLLABORATOR covenants with Catapult as follows: |
| 3.1.1 | to keep the Module clean, tidy and clear of rubbish; |
| 3.1.2 | not to use the Module other than for the Permitted Use; |
| 3.1.3 | not to make any alteration or addition to the Module or the Centre without the prior written consent of Catapult (such consent not to be unreasonably withheld, delayed or conditioned); |
| 3.1.4 | not to display any advertisement, signboards, nameplate, inscription, flag, banner, placard, poster, signs or notices at the Module or elsewhere in the Centre (that is not on agreed signage areas) without the prior written consent of Catapult, such consent not to be unreasonably withheld, delayed or conditioned; |
| 3.1.5 | not to de or permit to be done in the Module anything which is illegal or which may be or become a disruption, nuisance (whether actionable or not), annoyance, inconvenience, or disturbance to Catapult, or to other occupiers of the Centre or to the owner or occupier of neighbouring property; |
| 3.1.6 | not to cause or permit to be caused any damage (other than general wear and tear as would be expected from general usage of the Module over time for the Permitted Use) to: |
| 3.1.6.1 | the Module, Centre or any neighbouring property; or |
| 3.1.6.2 | any property of the owners or occupiers of any neighbouring property; |
33
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 3.1.7 | not to obstruct the Common Access Areas, make them dirty or untidy or leave any rubbish on them and to otherwise keep the Common Access Areas free and clear of any equipment, materials or personal property of COLLABORATOR; |
| 3.1.8 | not to apply for any planning permission in respect of the Module unless agreed in advance in writing with Catapult; |
| 3.1.9 | (In as much as this applies to COLLABORATOR as the end user of any such Supplies) to comply with all Applicable Laws and with any recommendations of the relevant suppliers relating to the supply and removal of electricity, gas, water, sewage, telecommunications and data and other services and utilities to or from the Module; |
| 3.1.10 | to observe any reasonable rules and regulations Catapult makes and notifies to COLLABORATOR from time to time in writing governing COLLABORATOR’s use of the Module and the Common Access Areas; and |
| 3.1.11 | not to do anything on or in relation to the Module and the Centre that would or might cause Catapult to be In breach of Catapult’s covenants and the conditions contained in the Lease; and |
| 3.1.12 | to comply with Catapult’s reasonable requests for cooperation with respect to any further development of the Centre and the Module and not to raise any objection to any noise and disturbance resulting from such further development on condition Catapult uses reasonable endeavours to minimise any disruption to the COLLABORATOR’s activities within the Module and the Centre. |
| 3.2 | COLLABORATOR acknowledges that Catapult is entitled to exclusive control and possession of the Centre and the Module and nothing contained in this Agreement creates any relationship of landlord and tenant or any other relationship other than that of a licensor and licensee between Catapult and COLLABORATOR. |
| 4. | Relocation of Module |
| Catapult shall be entitled, upon provision of as much written notice as possible (target notice period will be 12 months, but it will not be less than 6 months) to COLLABORATOR, from time to time, to relocate COLLABORATOR to a different location within the Centre provided that: (a) Catapult has first considered all reasonable alternatives to relocation (taking into account the costs that may be incurred by COLLABORATOR due to any programme delays resulting from such relocation) while discussing such alternatives with COLLABORATOR; (b) there is made available to COLLABORATOR a Module which is in all material respects is the same as the Module; and (c) the Collaborator is permitted to continue occupation of the Module for 3 months in tandem with that of the proposed replacement module for the latter 3 months of the 6 month notice period to enable a smooth handover. The costs and expenses incurred in relocating COLLABORATOR shall be borne by Catapult, and, for the avoidance of doubt, (i) for such period as COLLABORATOR occupies two modules pursuant to this paragraph 4, COLLABORATOR shall, nonetheless, be charged Contributions only for occupation of one module, and (ii) the relocation shall not cause any increase in COLLABORATOR’s Contributions as compared to those payable before the relocation. |
| 5. | Termination |
| 5.1 | At the end of the Licence Period: |
| 5.1.1 | COLLABORATOR’s rights to occupy the Module will automatically terminate; |
| 5.1.2 | COLLABORATOR will leave the Module in the same state and condition (taking into account normal “wear and tear” usage and excluding any damage caused by Catapult or any Third Party authorised to access the Module by Catapult or any Insured Risks), with all fixtures, fittings and equipment as were provided to it by Catapult as recorded in the Schedule of Condition and Inventory referred to in Schedule 7. |
| 5.2 | The termination of COLLABORATOR’s rights to occupy the Module will be without prejudice to any subsisting breach of COLLABORATOR’s obligations contained in this Schedule 2. |
34
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
Part 2 A – Rights granted to COLLABORATOR
The following rights are granted to COLLABORATOR in common with Catapult, any person authorised by Catapult and all other Collaborators but subject to Catapult’s rights:
| 1. | Running of services |
| To connect to and use the existing service media at the Centre for the passage of Supplies to and from and to the Module. |
| 2. | Access and servicing |
| 2.1 | Access to and from the Module on foot only over the Common Access Areas from time to time designated by Catapult for COLLABORATOR’s use. For the avoidance of doubt, other Collaborators shall not have access to or use of the Module. |
| 2.2 | To use any service area from time to time designated by Catapult for COLLABORATOR’s use for loading and unloading and otherwise servicing the Module and the service roads with or without vehicles to come and go to and from that service area. |
| 3. | Refuse disposal |
| To deposit rubbish in any receptacles or waste compactors within the Common Access Areas provided by Catapult for that purpose and designated by Catapult for the use of COLLABORATOR. |
| 4. | Support and shelter |
| Support and shelter for the Module from the Centre. |
| 5. | Parking |
| Use of up to 6 parking spaces designated by Catapult, from time to time, as available for COLLABORATOR’S use. |
| 6. | Signage |
| To exhibit COLLABORATOR’s name in such form, shape and size as Catapult approves (acting reasonably) on any appropriate directory board within the Centre. |
| 7. | Toilet facilities |
| To use any toilet facilities Within the Common Access Areas designated by Catapult as facilities for the use of COLLABORATOR. |
| 8. | Escape |
| On foot only, in emergencies and for fire escape drills, to use all fire escape routes in the Centre designated by Catapult for the use of COLLABORATOR whether or not forming part of the Common Access Areas. |
| 9. | QC Lab Space |
| The Centre has Catapult-operated quality control laboratories and three additional QC laboratories of 15 to 16 square meters. From time to time COLLABORATOR may request access to, and use of one of these additional QC laboratory spaces. Catapult may, reserving its sole discretion, grant access to, and use of such QC laboratory space as may be available at the time of request under the terms of an Additional Input Agreement. |
Part 2 B – Rights granted to COLLABORATOR II
The Parties acknowledge that certain licences to perform alterations to the Module contained within the On-boarding Project may be granted as Additional Inputs are agreed between the Parties in accordance with Clause 9(c) that may require further modifications to the Module to be carried out.
35
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
Part 3 - Rights reserved to Catapult
The following rights are excepted and reserved to Catapult and all those authorised by Catapult:
| 1. | Support, shelter, light and air |
| 1.1 | Support and shelter for the remainder of the Centre from the Module. |
| 1.2 | All rights of light or air to the Module that now exist or that might (but for this reservation) be acquired over any other land. |
| 2. | Running of services |
The passage and running of Supplies from and to the remainder of the Centre through existing Conducting Media (if any) within the Module.
| 3. | Entry on to the Module |
| 3.1 | To enter the Module during regular business hours and with maximum possible notice to COLLABORATOR and on not less than 24 hours prior notice, but excluding any period in which such access would disrupt preparations for manufacturing or when COLLABORATOR Products are being manufactured In the Manufacturing Space for any purpose including to: |
| 3.1.1 | perform any action required under the QTA or to maintain GMP compliance within the Centre— however with respect to this Clause 3.1.1 only, no notice will be required (unless notice is required under the QTA, and providing the visits do not pose a material risk to the quality of Products being manufactured at the time or expose Catapult staff to safety risks)); |
| 3.1.2 | estimate the current value or rebuilding cost of the Centre for Insurance or any other purpose; |
| 3.1.3 | install, Inspect, clean, maintain, replace and to take readings from metering equipment, heat cost allocators and thermostatic radiator valves within or relating to the Module and to prepare an energy performance certificate; |
| 3.1.4 | do anything that Catapult is expressly entitled or required to do under this Agreement or the Lease or for any other reasonable purpose in connection with this Agreement Including to Inspect the state of repair and condition of the Module; |
| 3.1.5 | carry out any works to the Module to improve their environmental performance; |
| 3.1.6 | build on or into any boundary or party walls on or adjacent to the Module (but only to the extent this cannot be carried out without entry to the Module); |
| 3.1.7 | inspect, clean, maintain, repair, alter, decorate, rebuild or carry out works upon the Centre (but only to the extent this cannot be carried out without entry to the Module); |
| 3.1.8 | carry out any of the necessary Inputs (but only to the extent this cannot be carried out without entry to the Module); or |
| 3.1.9 | for any other reasonable management purpose (but only to the extent that the required action is agreed with COLLABORATOR first and that this action cannot be carried out without entry to the Module). |
| 3.2 | Where reasonably possible, Catapult, or its contractors, will undertake any works described in this paragraph 3 in a manner which causes the least disruption to the COLLABORATOR and its use of the Module as possible. |
| 3.3 | Subject to Clause 3.1.1, right of entry to the Module under this paragraph 3 shall only be permitted to the extent that it does not disrupt the activities of COLLABORATOR within the Module. In the event that such entry would disrupt the activities of the COLLABORATOR within the Module, the Parties shall, acting reasonably, agree appropriate times and dates at which such entry and works may take place in order to minimise any such disruption. |
36
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 4. | Common Access Areas and Conducting Media |
| 4.1 | In an emergency, or when works are being carried out to them, to close off or restrict access to the Common Access Areas, so long as (except an emergency) alternative facilities are provided that are not materially less convenient. |
| 4.2 | To change, end the use of or reduce the extent of any Common Access Areas or Conducting Media so long as alternative facilities are provided that are not materially less convenient or, if no alternative is provided, the use and enjoyment of the Module is not adversely affected. In such event, Catapult shall provide all Collaborators and COLLABORATOR with a reasonable amount of advance notice as part of the Collaborator Forums at Schedule 15. |
| 5. | Adjoining Property |
To carry out works of construction, demolition, alteration or redevelopment on Centre and any adjoining property (and to permit others to do so) as Catapult in its absolute discretion considers fit (whether or not these works interfere with the flow of light and air to the Module). Catapult shall use all reasonable efforts to ensure that any such works do not interfere with COLLABORATOR’s use of the Module and undertaking of the Project. Catapult shall provide all Collaborators and COLLABORATOR with reasonably advanced notice of any such works through the Collaborator Forums.
| 6. | Plant, equipment and scaffolding |
The right, where necessary, to bring plant and equipment onto the Module and to place scaffolding and ladders upon the exterior of or outside any buildings on the Centre (Including the Module) on not less than 24 hours prior notice.
37
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
Part 4 Plans of the Module and the Centre
[**]
38
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 3
Financial Contributions
| Contribution per module | ||
| Facility Contribution per year from the Actual Occupation Date | [**] | |
| Business Rates per year from the Actual Occupation Date | [**] | |
| Integral Inputs Contribution per year from the Actual Occupation Date* (estimated) | [**] | |
| Activity Related Input Contribution* (estimated) | These contributions are dependent on Collaborator readiness, activity, and level of operation. This means that the precise nature of how these services will be provided, and the pricing attributable to them will not be confirmed until the Formal Agreement to Collaborate is entered into between the Parties | |
| Establishment Input Contributions* | Establishment Input Contributions are set out in paragraph 7 of the Establishment Input Contributions Statement entered into by the Parties setting out the Parties’ activities with respect to the On-boarding Project. The Establishment Input Contributions Statement is included in Schedule 6. | |
| * | Note that these costs are subject to a [**] and capital charge, per Clause 8.1 |
39
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 4
Catapult Background Intellectual Property
[**]
40
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 5
Code of Conduct
Both Parties agree to:
| (a) | Operate in a manner consistent with EU GMP to maintain a compliant multiproduct environment; |
| (b) | Operate in the spirit of the Collaboration; |
| (c) | Respect the confidentiality, privacy and operations of collaborators; |
| (d) | Wherever possible utilise the common infrastructure offered by the collection of cell and gene therapy related organisations in the local area, and nationally (the “Cluster”) in order to increase the benefits of the collaboration, and augment Cluster development, including the development of infrastructure connected with the Cluster; |
| (e) | Adhere to facility quality policies and protocol; |
| (f) | Adhere to roles and responsibilities as detailed in the Quality Technical Agreement and this Agreement; and |
| (g) | operate in compliance with all appropriate environment, health and safety requirements (both national and local). |
COLLABORATOR also agrees to:
| (i) | Maintain an environment within its Module in accordance with any procedures governing the Centre’s operation and the terms of occupation as stipulated in this Agreement and the QTA; and |
| (ii) | Abide by reasonable incident reporting requirements communicated by Catapult. |
41
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 6
New Business Introduction and On-boarding
Introduction
| 1. | The Catapult is committed to providing excellence in new business introduction for our collaborators. The following objectives underpin the Catapult’s new business introduction philosophy: |
| (a) | to guarantee that new business is introduced in accordance with our collaborators’ expectations; |
| (b) | to manage the new business introduction process to add value for our collaborators and for Catapult; |
| (c) | to ensure that new business introductions meet the requirements of the Catapult Quality and EHS policies; |
| (d) | to be open, honest and accurate in all of our new business introduction communications; and |
| (e) | to develop excellence in our employees through the development of world class Project Management skills. |
| 2. | The new business introduction process for the Centre is divided into (i) a selection process, (ii) a negotiation phase on a Heads of Terms basis and a subsequent Collaboration Agreement and (iii) the On-boarding phase. The flow chart at the bottom of this schedule gives an overview of the different phases. |
Summary of the Selection process
The new business introduction process starts with the Interest of a potential new collaborator in joining the Centre. After signing a CDA (Confidential Disclosure Agreement) Catapult will provide the COLLABORATOR with a ‘Pre-screen questionnaire’ to fill in. This document should allow Catapult to assess if the company and the planned manufactured product(s) will meet the requirements of the Centre’s standards. The process should ensure a smooth transition to the negotiation phase on the Collaboration Agreement from a quality and operational perspective.
The Establishment Input Contributions Statement
The following Establishment Input Contributions Statement was entered into prior to signature of this Collaboration Agreement between COLLABORATOR and Catapult setting out the required Establishment Inputs and associated Contributions required to establish the GMP manufacture of cell and gene therapy medicinal products at the Centre:
42
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
ESTABLISHMENT INPUT CONTRIBUTIONS STATEMENT
Between:
| (1) | Freeline Therapeutics Limited a company incorporated and registered in England & Wales with company number 09500073 and whose registered office is at Stevenage Bioscience Catalyst, Xxxxxxx Xxxx Xxxx, Xxxxxxxxx, XX0 0XX (“COLLABORATOR”); and |
| (2) | Cell Therapy Catapult Limited, trading as Cell and Gene Therapy Catapult, a company incorporated and registered in England & Wales with company number 07964711 whose registered office is at 12th Floor Tower Wing B, Guys Hospital, Great Xxxx Xxxx, Xxxxxx, XX0 0XX, Xxxxxx Xxxxxxx (“Catapult”) |
| Date: |
|
| 1. | BACKGROUND |
This Statement refers to the Collaboration Agreement to be entered into between COLLABORATOR and Catapult (“Collaboration Agreement”) in respect of the project to establish the GMP manufacture of cell and gene therapy medicinal products at the Cell and Gene Therapy Catapult Manufacturing Centre, Xxxxxxx Xxxx Xxxx, Xxxxxxxxx, Xxxxx XX0 0XX, Xxxxxx Xxxxxxx (“Centre”). Unless explained otherwise, capitalised terms used in this Statement have the meanings given in the Collaboration Agreement from the date it is executed by the parties and becomes effective.
This Statement sets out the activities to be performed by both COLLABORATOR and Catapult to support the set-up of COLLABORATOR’S manufacturing processes and products at the Centre (“On-boarding Project”). These activities form part of the Establishment Inputs referred to in the Collaboration Agreement.
43
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
The Contributions due from COLLABORATOR in relation to the On-boarding Project are set out in paragraph 7 below.
| 2. | OVERVIEW |
COLLABORATOR acknowledges that
| • | The activities comprising Work Package 1 may be identified, planned and agreed prior to the Actual Occupation Date and prior to COLLABORATOR and Catapult entering into the Collaboration Agreement (CA) and Quality Technical Agreement (QTA). This will include providing the On-boarding documentation package to COLLABORATOR, reviewing the returned documentation and developing a detailed project plan, identifying any critical activities which are required such as facility modifications. |
| • | Final execution and approval of the activities of Work Package 1 cannot take place until execution of the CA and QTA, at which point Catapult can establish COLLABORATOR within the Catapult quality system and raise a change control. COLLABORATOR can be established within the other IT systems and Catapult can apply for any required license updates plus commence any other activities that can take place prior to Module occupation, |
| • | Those activities requiring Module occupation (operator training, gowning qualification and equipment installation) cannot take place until the after the Actual Occupation Date and execution of the CA and QTA. For the avoidance of doubt, the parties acknowledge that, depending on the timing of the Actual Occupation Date, the activities of Work Package 1 may continue following the Actual Occupation Date. |
| • | There may be a short period of time (which will be defined during the On-boarding process) between the Actual Occupation Date and the point at which COLLABORATOR will be trained and qualified to enable them to take responsibility for Module cleaning and environmental monitoring. During this period, Catapult will retain responsible for this activity, with COLLABORATOR paying for consumables only (catapult gowning and environmental monitoring plates). |
| • | Up until the date that the CA is executed by the parties, the term “Actual Occupation Date” when used in this Statement shall mean the date of COLLABORATOR’s occupation of the manufacturing space in COLLABORATOR’s Module in the Centre. Following the execution of the Collaboration Agreement, the term “Actual Occupation Date” shall have the meaning ascribed to it in the Collaboration Agreement. |
| 3. | WORK PACKAGE 1 – STANDARD ON-BOARDING PACKAGE |
Administration and Control
The activity comprises a quality risk-based process that will be documented in the Centre’s Quality Management System following execution of the QTA. Catapult QA team will guide the process to ensure the GMP compliant Introduction of COLLABORATOR and its new manufacturing process into the Centre.
Project Management
The Standard On-boarding Package is led by a Catapult project manager, to a mutually agreed project schedule and a project plan determined by different work streams. It is anticipated that the majority of the Standard On-boarding Package will be performed prior to the Actual Occupation Date, except for operator training and gowning qualification plus equipment installation, which will take place after the Actual Occupation Date, however it may be the case that the Actual Occupation Date falls earlier in the Standard On-boarding Package.
Scope
On-boarding subject areas. Catapult will provide to COLLABORATOR the following Establishment Inputs:
| • | PROCESS – Process mapping, incorporating, where requested by COLLABORATOR, use of the Hak0bio software and virtual reality visualisation if required (managed by a Catapult Senior Scientist). Process mapping will identify: |
| • | optimised equipment layout and work flow in the manufacturing space (optional, if requested by COLLABORATOR) |
| • | initial and peak production estimates |
| • | material and equipment list |
| • | environmental monitoring, in-process and release testing requirements during a manufacturing run that require testing by Catapult |
| • | waste streams (large scale/small scale) |
As part of the On-Boarding Project, including process mapping, COLLABORATOR will provide information relating to its product and manufacturing process only to the extent that the information materially relates to the anticipated interactions between COLLABORATOR and Catapult at the Centre. No other product or manufacturing process specific information will need to be provided to Catapult by COLLABORATOR.
| • | QUALITY CONTROL – Establishing COLLABORATOR analytical and environmental monitoring requirements. Establishing environmental monitoring framework within the LIMS software framework (It should be noted that the LIMS may not be available for configuration until January 2018) and training on the execution of routine environmental monitoring (managed by Catapult Quality Control) |
44
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| • | ENVIRONMENTAL HEALTH & SAFETY – for example Genetically Modified Organism HSE update notification, trade effluent license changes (managed by the Catapult Health & Safety officer) |
| • | MATERIALS and Product – Warehouse Management System set up (managed by the Catapult Warehouse and Logistic manager), including: |
| • | Entering and review of Raw Material Specifications into the warehouse management system |
| • | Location set up in the warehouse at different storage temperatures |
| • | Product storage schedule, including Cryohub relationship if applicable |
| • | Generation of xxxx of materials (BoMs) |
| • | WASTE MANAGEMENT – set up of solid, liquid and clinical waste management system (managed by the Catapult Facilities management) |
| • | EQUIPMENT – asset log, assessment and installation of equipment including linkage to the 24/7 monitoring/alarm system (to be agreed) (managed by the Catapult Facilities management) |
| • | TRAINING – Centre Induction and basic GMP Training (managed by the Catapult Quality Assurance team) plus gowning qualification (managed by the Catapult QA & QC teams), for up to 6 operators |
| • | FINANCE & IT – systems set up and training (managed by the Catapult IT manager) |
| • | COMMUNICATIONS – Site Induction & Communications (managed by Catapult administrative staff) |
| • | MANAGEMENT – Project management, QA oversight & governance and site senior management review process |
| • | QUALITY ASSURANCE – Training in facility procedures, setting up the Master Control electronic Quality Management System (eQMS) roles and access rights, providing access to the supporting paper QMS |
Additional Requirements
Additional COLLABORATOR requirements may be identified. These may include but will not be limited to: facility modifications, establishing systems for large volume waste inactivation and disposal and configuration of the LIMS for Additional QC Inputs or for connection of COLLABORATOR equipment. Such requirements will be managed by the Parties following the procedure governing Additional Inputs in the Collaboration Agreement.
| 4. | WORK PACKAGE 2 – ON-BOARDING: MANUFACTURING SPACE QUALIFICATION |
Catapult responsibilities
Catapult will perform an Operation Qualification (OQ) of the Manufacturing Space (as-built) prior to the Actual Occupation Date.
Catapult will define the minimum requirements for the at-rest OQ (Operation Qualification) and room PQ (Performance Qualification). Catapult will provide a template for the ‘at rest’ room OQ and room PQ protocols and reports for use by COLLABORATOR. Should COLLABORATOR elect not to use the Catapult templates, any templates proposed by COLLABORATOR must be approved in advance by Catapult.
Catapult will perform all analysis of the environmental monitoring outputs produced by COLLABORATOR according to the agreed study design, including specification if required.
Catapult will provide a final report of the environmental monitoring analysis.
The executed ‘et rest’ room OQ and room PQ reports will be approved by both COLLABORATOR and Catapult.
Catapult will provide all environmental monitoring consumables and 3 mobile volumetric air samplers and non-viable particle counters for use by COLLABORATOR for the initial manufacturing space qualification. Catapult will train COLLABORATOR in the relevant environmental monitoring sampling methods.
Collaborator responsibilities
COLLABORATOR is responsible for the execution of the ‘at rest’ OQ and PQ of its Module, in accordance with Catapult’s defined minimum requirements. This protocol will include environmental sampling within the Module and also a subcontracted Cleanroom Performance Study incorporating air pattern assessment employing smoke visualisation techniques, room clean-up rate assessment and non-viable particle analysis.
COLLABORATOR will perform the ‘at rest’ OQ and room PQ, the environmental monitoring sampling outputs being transferred to the Catapult for analysis.
Subcontractors
Cleanroom Performance testing will be performed by Clean Air Technology (CAT).
45
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
Assumptions
Contributions (as set out in paragraph 6 below) are based on a standard study design, with a single performance of the OQ and PQ (incorporating a minimum of three separate sampling events). Study designs involving additional analysis or repetitions will incur additional Contributions to those set out in paragraph 6 below. Contributions Include no operator monitoring.
| 5. | COOPERATION AND GOVERNANCE |
Catapult and COLLABORATOR will work together to complete the On-boarding Project.
COLLABORATOR acknowledges that its regular input is key to the successful delivery of the On-boarding Project. Specific COLLABORATOR responsibilities are listed under Work Package 2 at paragraph 4 above.
Information will be shared by COLLABORATOR and Catapult and project plans will be generated and maintained during regular steering meetings attended by representatives of both COLLABORATOR and Catapult.
| 6. | TIMEFRAME |
It is estimated that (subject to COLLABORATOR making all necessary information available to Catapult and providing timely responses to enquiries from Catapult), the section of the Standard On-boarding Package which can occur prior to the Actual Occupation Date should commence approximately 6 to 8 weeks before the Actual Occupation Date. Following the Occupation Date, the remainder of the Standard On-boarding Package plus the On-boarding Manufacturing Space Qualification is expected be completed within 2 to 3 months following the Actual Occupation Date.
| 7. | CONTRIBUTIONS |
| Inputs |
Contribution | |||
| Work Package 1 Standard On-boarding Package |
[**] | |||
| Work Package 2 On-boarding: |
||||
| Manufacturing Space Qualification |
[**] | |||
| Subtotal: |
[**] | |||
| [**] capital and risk charge |
[**] | |||
|
|
|
|||
| Total Input Contribution |
[**] | |||
|
|
|
|||
| Indicative consumable cost for WP1 |
[**] | |||
|
|
|
|||
| Indicative consumable cost for WP2 |
[**] | |||
| Indicative subcontracting costs for WP2 |
[**] | |||
|
|
|
|||
| TOTAL |
[**] | |||
|
|
|
|||
All Contributions are subject to vat
| * | Consumable prices are provided as an estimate and will be charged as a pass-through cost. Any significant variation from the estimate will be communicated to COLLABORATOR promptly. |
| ** | Subcontract costs will be passed directly to COLLABORATOR at the cost charged by the subcontractor. |
| *** | Contributions are subject to [**] Capital and Risk charge, which has been included above. |
46
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
The “Total Input Contribution” set out in this paragraph 7 will be invoiced by Catapult on the Actual Occupation Date. Consumables and subcontracting costs will be invoiced as used. COLLABORATOR will pay accurate, complete and valid vat invoices within 30 days of receipt.
| 8. | LIABILITY |
[**] relation to the On-boarding Project shall be limited to [**]. In no circumstances shall Catapult or COLLABORATOR have any liability for any indirect, special or consequential loss or for any loss of profits, revenue, business opportunity, data, or goodwill (in each case whether such loss is direct or indirect). Nothing herein limits or excludes any person’s liability to the extent that it may not be so limited or excluded by law, including any such liability for death or personal injury caused by that person’s negligence, or liability for fraud or fraudulent misrepresentation.
Once and provided that COLLABORATOR and Catapult enter into the Collaboration Agreement then this paragraph 8 shall be deemed deleted and replaced by the relevant provisions of the Collaboration Agreement with effect from the date the Collaboration Agreement takes effect in accordance with its terms.
| 9. | TERMINATION |
COLLABORATOR may, with or without cause, terminate this Statement with immediate effect on written notice to Catapult. Upon such termination, except as agreed to by Catapult and COLLABORATOR in writing, Catapult shall not undertake further work, or incur additional expenses or enter into further commitments, under this Statement. Following such termination, Catapult shall invoice COLLABORATOR, and COLLABORATOR shall pay for, all inputs properly performed, and consumables used under this Statement up to its termination, and non-cancellable subcontractor costs under this Statement. This paragraph 9 shall survive termination of this Statement pursuant to paragraph 9.
This paragraph 9 shall cease to have any effect on execution of the Collaboration Agreement by the parties.
| 10. | CONFIDENTIALITY |
Neither COLLABORATOR nor Catapult will make any further public disclosure relating to this Statement or the underlying work without the other’s prior written consent.
Any confidential information supplied by either COLLABORATOR or Catapult in relation to this Statement will be subject (i) to the terms of the Confidentiality Agreement, dated 5 April 2013, entered into between COLLABORATOR and Catapult, before such time as the Collaboration Agreement is entered into by the parties, and then (ii) by the Collaboration Agreement, once it is executed by the parties.
AGREED by the parties through their duly authorised representatives on the date set out at the top of this Statement.
| For and on behalf of: |
For and on behalf of: | |||||||||
| Freeline Therapeutics Limited |
Cell Therapy Catapult Limited | |||||||||
| Signed: |
Signed: | [**] | ||||||||
| Name: |
Name: | [**] | ||||||||
| Position: |
Position: | CEO | ||||||||
47
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
New Business Introduction process flow: set out on the following page
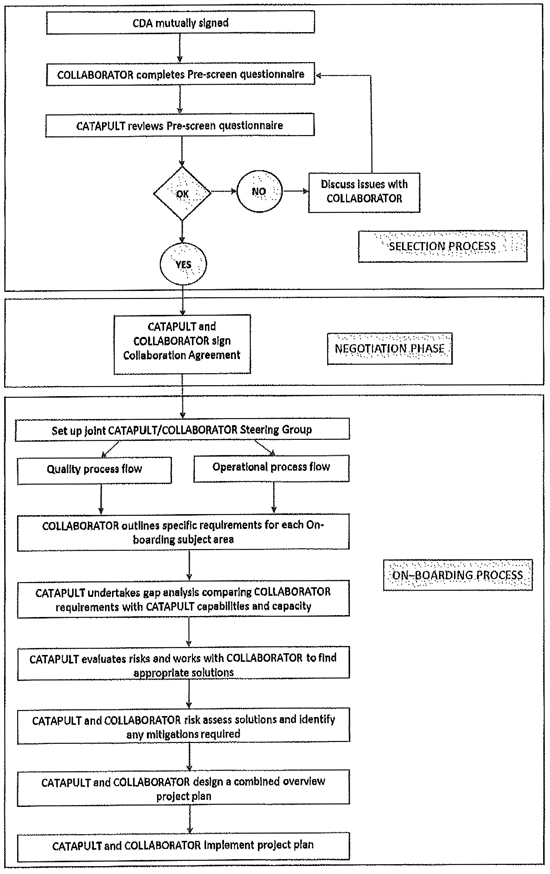
48
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 7
Schedule of Condition and Inventory of Module Fixtures and Fittings
To be provided under cover of a separate document, signed by both Parties immediately prior to occupation, but incorporated into this Schedule 7 by reference.
49
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 8
Warehouse and Procurement Management Provisions
| 1. | General |
| 1.1 | Catapult is responsible for operating the warehouse area & processes at the Centre in a GMP compliant manner. In summary this includes goods in, common consumables stock and COLLABORATOR owned stock, storage, picking, delivery and final product storage. |
| 1.2 | Catapult is responsible for the EHS within the warehouse and monitoring storage temperatures. |
| 1.3 | The Centre warehouse is an access controlled area limited to authorised Catapult personnel. COLLABORATOR personnel can only access the warehouse when accompanied by Catapult personnel. |
| 1.4 | Catapult will man the warehouse during business hours each week day (excluding bank holidays in England). |
| 1.5 | Catapult will provide a 24/7 call-out system for out of hours’ deliveries, as an Additional input. |
| 1.6 | All COLLABORATOR equipment, samples and materials must enter the Centre through the Centre’s goods in warehouse entrance. Samples and Materials will be booked onto the Catapult Warehouse management system. |
| 1.7 | Transfer to the Manufacturing Space of all COLLABORATOR equipment, samples and materials must be formally authorised by Catapult personnel. |
| 1.8 | The Centre is considered a forward picking area and as such warehouse space is limited. |
| (i) | Catapult will maintain e stock of common consumables. |
| (ii) | Each collaborator will have allocated storage at ambient temperature (15°C to 25°C), 0-0xX, -00xX, -00xX, and LN2 for their raw materials, product contact equipment, drug product, references and standards, and excipients. Catapult will be responsible for the qualification (IQ and OQ plus equipment-specific PQ with the units under load), monitoring, maintenance and functioning of the storage equipment and areas. Any COLLABORATOR-specific PQ will be an Additional Input. |
| (iii) | Visibility of the COLLABORATOR’s inventory is through the warehouse management system (Initially a paper based system). Each collaborator will only have visibility of their inventory items. |
| 2. | Warehouse Space |
COLLABORATOR will be allocated a minimum amount of storage within the Centre’s warehouse shown in the plans at Schedule 4 [**]
| [**] |
50
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 2.2 | Subsequent to occupation of modules in Phase 2 of the Centre, the minimum storage allocation above within the Centre’s warehouse shown in the plans at Schedule 4 will be halved, with the only exception to this being [**] |
| 2.3 | Catapult undertakes to provide and manage, at the COLLABORATOR’S expense, additional warehouse space suitable for the operation of GMP manufacture. Catapult agrees to implement this development in consultation with COLLABORATOR and agrees not to implement this Additional input without agreement of COLLABORATOR to its design and costs. Subject to timely COLLABORATOR agreement to the design and costs Catapult undertakes to use reasonable efforts to have the additional warehouse space available before commencement of Phase 2. Where the additional warehouse space is agreed in the Operational Forum as an additional Integral Input it will be paid for through the Integral Input Contribution. Where the Operational Forum does not agree it to be an Integral Input, the cost will be equitably spilt among the collaborators benefitting from it and paid for as an Additional Input Contribution. |
| 3. | Common consumables stock |
| 3.1 | CATAPULT will maintain a stock of an agreed list of commonly used consumables. |
| 3.2 | CATAPULT will be responsible for purchasing this stock, determining minimum stock levels required by collaborators, maintaining stock levels, undertaking the appropriate QC & putting the stock away. |
| 3.3 | CATAPULT will invoice the COLLABORATOR for the stock used by COLLABORATOR. |
| 3.4 | CATAPULT personnel will transfer the consumables to the COLLABORATOR’s Grade C MAL staging area once a day or as agreed. |
| 3.5 | Common consumables stock will not be segregated between Collaborators and COLLABORATOR. |
| 4. | COLLABORATOR owned inventory |
| 4.1 | COLLABORATOR is responsible for the management of its own inventory supply chain including sourcing and auditing suppliers, price negotiation, purchasing and insurance. |
| 4.2 | Before purchasing any stock to be stored in the Centre, COLLABORATOR is responsible for providing a list of the inventory they will store and uae within the Centre. Catapult reserves the right to reject any Inventory Item that is not in compliance with CATAPULT policies & procedures e.g. EHS. |
| 4.3 | COLLABORATOR is responsible for the completion & submission of a material specification for each item which will include such information as product container size, weight, required stock level, minimum stock level, QC sampling and testing regime. |
| 4.4 | Catapult will be responsible for notifying COLLABORATOR when the stock has reached its minimum stock level. The stock may then be re-ordered by COLLABORATOR. |
| 4.5 | COLLABORATOR must give at least 48 hours’ notice of a delivery of their items, and must be delivered during the warehouse opening hours unless by prior agreement. |
| 4.6 | Catapult personnel will book the goods into the Warehouse management system, attach appropriate labels, undertake the initial goods inspection, notify COLLABORATOR of the goods receipt (and promptly notify COLLABORATOR of any issues identified on initial goods inspection) and place the goods in a location. Catapult will store COLLABORATOR inventory in a separate location from that of other Collaborators. |
| 4.7 | Catapult will make available storage (including LN2 storage) for retention samples, for which COLLABORATOR is responsible for the management of all of COLLABORATOR’s retention samples stored by Catapult. Storage of retention samples for longer than 30 days will be available as an Additional Input. |
| 4.8 | COLLABORATOR will be responsible for the Quality Control (QC) of their inventory & pass labelling. |
| 4.9 | If the COLLABORATOR’s products fail QC then COLLABORATOR personnel will be responsible for attaching reject labels, CATAPULT personnel will transfer these reject products to the relevant Reject product storage area. CATAPULT will store these Reject products for up to 30 days during which time it is expected that the COLLABORATOR will arrange appropriate disposal. If this is not arranged CATAPULT will manage the appropriate disposal with additional costs being charged to the COLLABORATOR. |
51
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 5. | Picking & delivery of stock for collaborators |
| 5.1 | For non-batch related common consumables stock such as clean room clothing Catapult manage the supply of these items. |
| 5.2 | For batch specific common consumables and for COLLABORATOR owned materials, COLLABORATOR will provide Catapult with a Xxxx of Materials (BoM) and a schedule identifying when the full or part BoMs are required. |
| 5.3 | A member of the COLLABORATOR staff will formally receive the BoM. If there are any queries or discrepancies with the BoM these will be highlighted & addressed at this time. |
| 5.4 | For biological material or cold-stored items, through prior arrangement, the COLLABORATOR with a Catapult representative will pick and transfer these items to the Manufacturing Space. |
| 6. | Final product storage |
| 6.1 | The Centre will provide final product, and product intermediate, storage at the following temperatures: |
| 6.1.1 | -20°C |
| 6.1.2 | -80°C |
| 6.1.3 | LN2 |
| 6.1.4 | Controlled rate freezer |
Rejected material will be stored in a segregated, multi-collaborator quarantine area in -80°C and LN2 storage.
| 6.2 | The final product (or any intermediate thereof as requested by COLLABORATOR) will be stored in multi-collaborator storage equipment. |
| 6.3 | COLLABORATOR can store final product (or any intermediate thereof as requested by COLLABORATOR) in these storage areas for up to 44 days (and occasionally for additional periods by agreement with Catapult, at Catapult’s discretion) |
| 6.4 | Access to the product storage areas will be strictly controlled and will only be possible when accompanied by an appropriately trained and authorised Catapult representative. |
| 6.5 | Catapult will be responsible for the qualification and maintenance of all equipment in this area including the temperature monitoring system and 24/7 emergency cover. |
| 7. | Drug substance (DS) or drug product (DP) (or product intermediate) packing area |
| 7.1 | Catapult will provide either a supervised GMP packing area or perform GMP packing. |
| 7.2 | Catapult will provide an area to charge dry shippers with liquid nitrogen. |
| 7.3 | Catapult will provide storage area for a reasonable supply of packing materials and boxes. |
| 8. | Drug substance (DS) or drug product (DP) (or product intermediate) shipping |
| 8.1 | If required by COLLABORATOR Catapult will provide access to cold chain GMP compliant courier service. |
| 9. | Examples of the warehouse & logistics additional services, available as Additional Inputs which are not included within Integral or Activity Related Inputs |
| 9.1 | Out of hours support. |
52
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 9.2 | Sampling of COLLABORATOR raw materials. |
| 9.3 | QC analysis of COLLABORATOR raw materials. |
| 9.4 | Auditing the COLLABORATOR supply chain. |
| 9.5 | Purchasing the COLLABORATOR raw materials. |
| 9.6 | Storing COLLABORATOR raw materials or starting materials for longer than the specified period. |
| 9.7 | Managing and storing retention samples. |
| 9.8 | GMP packing. |
| 9.9 | Arranging GMP shipping service through a GMP compliant logistics service provider. |
| 9.10 | Offsite additional storage space. |
53
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 9
Environmental Monitoring Schedule
Introduction
Catapult is committed to providing and maintaining manufacturing and manufacturing support environments that are fit for their intended purpose with regard to air quality. These environments will be appropriately controlled and monitored based on the room classification requirements defined in Eudralex Volume 4 Annex 1. This will be achieved by:
| • | The regular application of qualified oleaning procedures to all GMP environments within the Centre. |
| • | The training and qualification of personnel to assure the consistent and appropriate execution of gowning and de-gowning procedures. |
| • | The development of and adherence to an appropriate environmental monitoring program. |
| • | Regular reporting and trending of data generated by the program of Centre EM monitoring. |
| • | The creation and dissemination of procedures for the appropriate handling of starting materials, raw materials, consumables, samples, In-process and final product and waste within the manufacturing facility. |
Summary of the environmental monitoring process
| • | The environmental monitoring (EM) program shall be established by Catapult to comply with the requirements of Eudralex Volume 4 Annex 1 – Manufacture of sterile medicinal products. |
| • | Catapult Quality will establish and periodically reassess (based on historical data) action and alert limits for EM test result values or all types of monitoring. |
| • | Catapult will supply and perform routine calibration and servicing of the following calibrated EM sampling and measuring equipment per module for COLLABORATOR use, unless COLLABORATOR elects to employ their own monitoring equipment within isolators. |
| • | 3 portable active air samplers (for ‘in-operation’ viable air monitoring). |
| • | 3 portable non-viable particulate monitors. |
| • | 5 fixed sampling points and associated non-viable particulate monitors. |
| • | Catapult will maintain a stock in the Catapult warehouse of all necessary consumables to facilitate collaborators to undertake viable ‘in-operation’ environmental monitoring (including sufficient TSA & SDA settle plates and contact plates to cover monitoring of the entire daily processing period). |
| • | Responsibility for the execution of the Centre environmental monitoring program will be shared between Catapult and the collaborators per the QTA. |
| • | When an order is placed by COLLABORATOR, Catapult Technical Services staff are responsible for the delivery of EM consumables to the relevant Materials Air Lock of the COLLABORATOR’S Manufacturing Space. |
| • | COLLABORATOR collected EM samples should be appropriately labelled and packaged Immediately subsequent to exposure. |
| • | When requested by COLLABORATOR staff, Catapult Technical Services staff are responsible for the collection of exposed EM samples from the PrAL, their transportation to Catapult QC Microbiology, documentation of their receipt and transfer to QC staff for storage prior to testing. |
54
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| • | Catapult QC Microbiology staff are responsible for the appropriate processing of EM samples (incubation, enumeration and speciation as required), documenting the results and providing trended data to COLLABORATOR. |
| • | All Manufacturing Space specific EM data and that collected from sampling of the common Centre areas will be made available to individual collaborators. |
| • | Data will be presented per specific EM session and as a trend graph on a mutually agreed frequency. Data will include the results of any speciation undertaken as a result of an action or alert limit breach. |
| • | Alert limit breach trends and any action limit breaches will result in Catapult QC staff raising a record in the Quality Management System to document the event, investigate root cause (with COLLABORATOR assistance if appropriate) and identify the appropriate preventative and corrective actions necessary to mitigate the risk of recurrence. |
55
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 10
IT Infrastructure
The Centre will accommodate several collaborators consecutively, each of whom could potentially use the Centre in a different way.
The underlying IT infrastructure has been configured for each Module to have its own self-contained secure network. This will allow independent network scenarios, the configuration of these requirements will be carried out, administered and monitored by Catapult IT staff.
COLLABORATOR will have its own dedicated secure virtual local areas networks (VLANs).
All collaborators will however be subject to the Catapult’s Information security policy.
Internet provision is not provided as standard however we can provide the following:
| • | Synchronous fibre broadband provided by Catapult at current market rates. |
| • | COLLABORATOR supplies their own internet connectivity subject to wayleave. |
| • | COLLABORATOR can also organise their own lease Line (PPTP) connectivity between their own sites end the Centre, subject to wayleave. |
Server Infrastructure can also be provided by Catapult, the options that are available are as follows:
| • | Physical server on premises – this will be located in one of the Centre’s communications rooms with restricted access (all access will be accompanied by Catapult IT staff) |
| • | Virtual server on premises – this will be located on the Catapult virtual environment, remote access will be provided to the COLLABORATOR – SSL VPNs will be provided for access from external sites. |
| • | Virtual server in privete cloud – this will be located on Catapult’s own private cloud, remote access will be provided to the COLLABORATOR – SSL VPNs will be provided for access from external sites. |
If COLLABORATOR does not wish to use the options above, Catapult may offer, subject to availability and feasibility, the following option: physical/virtual server on COLLABORATOR’s own site – A SSL site to site tunnel will be provided, access controlled by dedicated virtual local area networks VLANs.
56
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 11
(NOT USED)
57
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 12
Module and Centre Specifications
| 1. | Part A: Manufacturing Space Specification |
It will:
| (a) | be part of a GMP-compliant facility developed in close relationship with, and licensed by, the Medicines and Healthcare Products Regulatory Agency; |
| (b) | be of a design, construction, fit and finish in compliance with governing environment health and safety legislation; |
| (c) | be designed, and built fitted and finished in compliance with Applicable Law including 2001/83/EC and 2001/20/EC; |
| (d) | have individual personnel access control; |
| (e) | include a positive pressure maintained cleanroom with production area of not less than 86m2 and a culture room of not less than 15m2; |
| (f) | have appropriate pressure cascades with negative pressure sinks in all entry and exit routes to minimise the risk of ingress and/or egress of contamination; |
| (g) | have walk-on ceilings and a technical corridor; |
| (h) | have a high-efficiency particulate arrestance (“HEPA”) filtered HVAC supplying segregated air as single pass through, with heat recovery; |
| (i) | have a gas supply delivered through services plates (details of the service plates are set out in Schedule 7); |
| (j) | have single and three phase power supply, partly with UPS and emergency generator back-up, supplied through service plates (details of the service plates are set out in Schedule 7); and |
| (k) | have dedicated adjacent material air locks (‘MALs”), dedicated adjacent personnel air locks (“PALs”). |
| 2. | Part B: Manufacturing Office and Non-Manufacturtng Office Specification |
| (a) | one Manufacturing Office of not less than 15m2 as set out in the Plans with direct access from the controlled, non-classsified corridor and designed for occupation by 4 people; |
| (b) | one Non-Manufacturing Office of not less than 28m2 on the first or second floor of the Centre, as set out in the Plans and designed for occupation by 2 people; |
| (c) | Both of these offices will; |
| (i) | be equipped for normal administrative functions only; |
| (ii) | be equipped with lighting in line with British standards; |
| (iii) | have lockable doors compliant with insurers requirements; |
| (iv) | be furnished with desks, chairs and storage as agreed with the Centre staff, to accommodate the number of occupants they are designed to house; |
| (v) | have small power outlets suitable for normal small office equipment use. The electricity usage will be measured and recharged as appropriate; |
58
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| (vi) | have service media outlets for IT and Telephones. Internet services can be provided on request and recharged as appropriate as an Activity Related Contribution; |
| (vii) | a telephone for communication with Catapult. Outgoing calls will be charged at the service provider’s rate; and |
| (d) | be heated and cooled from the central Centre systems. Temperature control will be only via the Building Management System (BMS) under the control of Catapult. |
| 3. | Part C: Centre Specifications |
Facilities will include:
| (a) | a warehouse packing and dispatch area; operated in accordance with Schedule 8; |
| (b) | a solid waste staging area; |
| (c) | a liquid waste staging disposal area; |
| (d) | male and female changing areas to access the controlled non classified (CNC) corridor; |
| (e) | PALs in and MALs in to access the grade C corridor; |
| (f) | PrALs to access the CNC corridor; |
| (g) | PALs out and MALs out to access the CNC corridor; |
| (h) | a reception area; |
| (i) | a rest/kitchen area |
| (j) | a meeting room for COLLABORATOR use; and |
| (k) | parking as set out in Schedule 2, Part 2A |
59
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 13
Expert Determination
EXPERT
| 1. | An Independent “Expert” is a person appointed in accordance with this Schedule 13 to resolve a disagreement under Clause 26.2 in connection with a change to the schedules listed at that Clause. |
| 2. | The Parties shall agree on the appointment of an Independent Expert and shall agree with the Expert the terms of their appointment. |
| 3. | If the Parties are unable to agree on an Expert or the terms of their appointment within 7 days of either Party serving details of a suggested expert on the other, either Party shall then be entitled to request the Centre for Effective Dispute Resolution (CEDR) to appoint an Expert of professional repute and for the CEDR to agree with the Expert the terms of appointment. |
| 4. | The Expert is required to prepare a written decision including reasons and give notice (including a copy) of the decision to the Parties within a maximum of 3 months of the matter being referred to the Expert. |
| 5. | If the Expert dies or becomes unwilling or incapable of acting, or does not deliver the decision within the time required by this Schedule 13 then: |
| (a) | either Party may apply to the London Court of International Arbitration to discharge the Expert and to appoint a replacement Expert with the required expertise; and |
| (b) | this Schedule 13 shall apply to the new Expert as if they were the first Expert appointed. |
| 6. | All matters under this Schedule 13 must be conducted, and the Expert’s decision shall be written, in the English language. |
| 7. | The Parties are entitled to make submissions to the Expert including oral submissions and will provide (or procure that others provide) the Expert with such assistance and documents as the Expert reasonably requires for the purpose of reaching a decision. |
| 8. | Each Party shall with reasonable promptness supply each other with all information and give each other access to all documentation and personnel and/or things as the other Party may reasonably require to make a submission under this Schedule. |
| 9. | The Expert shall act as an expert and not as an arbitrator. The Expert shall determine the matter under this Agreement. The Expert’s written decision on the matters referred to them shall be final and binding on the Parties in the absence of manifest error or fraud. |
| 10. | The Expert’s fees and any costs properly incurred by them in arriving at their determination (including any fees and costs of any advisers appointed by the Expert) shall be borne by the Parties equally or in such other proportions as the Expert shall direct. |
| 11. | All matters concerning the process and result of the determination by the Expert shall be kept confidential among the Parties and the Expert. |
| 12. | Each Party shall act reasonably and co-operate to give effect to the provisions of this Schedule and otherwise do nothing to hinder or prevent the Expert from reaching their determination. |
| 13. | The Expert and CEDR shall have no liability to the Parties for any act or omission in relation to this appointment; save in the case of bad faith. |
60
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 14
List of Centre Utilities
| Utility |
Provider | |
| Natural Gas |
Natural Grid (Supply)
Npower (Meter and Usage)
| |
| Electricity |
UK Power Networks (Supply and Infrastructure)
Npower (Meter and Usage)
| |
| Water |
Affinity (Supply)
| |
| Sewage |
Thames Watar(Supply)
|
61
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 15
Collaborator Forums
The purposes of the collaborator forums (the “Forums” or “Collaborator Forums”) are:
| • | to facilitate open and transparent exchange of Information between Catapult and collaborators, and between collaborators; |
| • | to enable all parties to contribute to the safe, efficient, and successful operation of the Centre, and of the collaborators’ manufacturing activity, and |
| • | to enable the standards of the centre to be maintained at an appropriate cost. |
The Forums will be supplemented by regular informal ad-hoc meetings and weekly/bi-weekly surgeries involving Catapult and any collaborator as is necessary.
| 1. | Key objectives of the Forums include: |
| (a) | updating any requirements needed to continue to maintain a suitable level of services for operation of a licensed facility suitable for late stage clinical and commercial manufacture of ATMPs in the most economical way; |
| (b) | considering collaborator input into the relevant aspects of the management and operation of the Centre; |
| (c) | ensuring Catapult and collaborator compliance with all relevant Quality, Health & Safety and legal requirements; |
| (d) | discussing any modifications to any module or the Centre with the potential to impact any collaborator (prior to being raised in the relevant Forum, Catapult will consider all collaborator requests for facility modifications that require any other collaborator’s manufacturing space to be non-operational for any period of time, or that affect the Centre license and will discuss feasibility with the all collaborators. For clarity, such modifications should remain part of the notification to collaborators of the agenda for any Collaborator Forum); |
| (e) | having formal two-way communications between Catapult and collaborators to discuss common issues; |
| (f) | raising awareness of issues and incidents with potential for impact on the Catapult and collaborators; |
| (g) | encouraging and facilitating the sharing of best practice between collaborators; and |
| (h) | examining appropriate ways of managing costs. |
| 2. | The Forums will be advisory in their nature and initially take place monthly, with their frequency being reviewed/varied as required. However, the frequency of Forums will be no less than quarterly. |
| 3. | The agenda, format, time and venue will be set and reasonable notice given in advance by Catapult, with the agenda being subject to change based on operational experience and input from collaborators. |
| 4. | Relevant issues will be discussed and appropriate recommendations made during the Forums. Outputs of any key decisions that need to be made separately outside of the Forums will be communicated prior to the following meeting. |
| 5. | Key issues and follow-up actions will be summarised and circulated to all collaborators by Catapult after each Forum. |
| 6. | Collaborators will be fully consulted prior to any key decisions being made. Subject to the terms of this Agreement, in recognition of the fact that the Catapult has overall responsibility for the operation of the Centre, Catapult reserves the right to make the final decision in the best interests of all collaborators and the Catapult regarding matters put before the Forums. |
62
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| 7. | There will be 3 Forums covering 3 key areas, with the relevant Catapult chairs, Catapult leads, and Terms of Reference being summarised in the table below. |
The Forums will be separated into 3 key areas, with the relevant Catapult chairs, Catapult leads or their representatives, and Terms of Reference being summarised in the table below.
| FORUM |
CATAPULT CHAIR |
CATAPULT LEAD |
TERMS OF REFERENCE | |||
| Quality Forum | Director of Quality | Head of QA | • Environmental Monitoring Trends
• Collective discussion of recent deviations or changes with a shared impact
• Audit findings (internal and external
• Audit findings of shared Vendors
• Regulatory Trends
• Best Practise Information
• Training Requirements
• Updates to Facility Management Procedures
• Updates to Foundation Documents
• Quality Agreement Compliance
| |||
| Health & Safety | Manufacturing Centre Director |
H&S Representative |
• Review of Catapult & collaborator accidents, Incidents and near misses since last meeting
• Review of accident, incident and near miss trends
• Review of collaborators’ EHS concerns
• Catapult H&S update as it relates to:
• People
• Facilities, offices & equipment
• Facility modifications
• Biological & chemical
• Contractor management
• Catapult Environmental update
• New or updated EHS legislation
| |||
| Operational Forum |
Manufacturing Centre Director | Operations Lead(s) |
• Summary of current key discussions in the Quality and H&S forum, to ensure that any business critical topics receive broad attention
• Catapult general operational updates
• Collaborator general operational updates
• Area specific issues/updates
• Welfare
• Process
• Equipment
• Materials & Product
• Waste Management
• IT
• Communications
• Proposed Facility modifications – requirements and costs
• Schedule for any planned shutdowns
• People & Training
• Budgetary and resource issues/updates
• Proposed capital expenditure
• Update on any expected changes in Integral, Activity Related Input Contributions and Additional Input Contributions
• Staff resources |
63
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
SCHEDULE 16
| ADDITIONAL INPUT AGREEMENT |
This Additional lnput Agreement number [**] is entered into between the Collaborator and Catapult in accordance
with, and in relation to, the Collaboration Agreement entered between Collaborator and Catapult on [**].
| Collaborator:
|
Freeline Therapeutics Ltd.
|
Collaborator’s Manager:
|
||||
| Catapult: | Cell Therapy Catapult Limited
|
Catapult’s Manager: | ||||
| Date of this Additional Input Agreement:
|
||||||
Additional Inputs Required:
[List all items required with description]
Changes to the Contributions associated with the changes to be made under this Additional Input Agreement (Including changes to invoicing provisions):
[Price list – For facility modifications, this may include contributions for project scoping and also [**] of the agreement value may be charged for Project Management. [**]% capital and risk and vat will also be charged]
Additional terms required as a result of the changes to be made under this Additional Input Agreement:
[Any additional terms, if different to those in the CA]
This Additional Input Agreement is accepted:
| For and an behalf of: Freeline Therapeutics Ltd. |
For and an behalf of: Cell Therapy Catapult Limited |
|||||||||
| Signed: |
|
Signed: |
|
|||||||
| Full Name: |
|
Full Name: |
|
|||||||
| Job Title: |
|
Job Title: |
|
|||||||
64
Certain confidential information contained in this document, marked by [**], has been omitted because the information (i) is not material and (ii) would likely cause competitive harm to the Company if publicly disclosed.
| For and an behalf of: Freeline Therapeutics Ltd. |
For and an behalf of: Cell Therapy Catapult Limited |
|||||||||
| Signed: |
|
Signed: |
|
|||||||
| Full Name: |
|
Full Name: |
|
|||||||
| Job Title: |
|
Job Title: |
|
|||||||
END OF DOCUMENT
65

