DATED 19 OCTOBER 2012 TRANSLATION AWARD FUNDING AGREEMENT
| Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Double asterisks denote omissions. |
Exhibit 10.3 |
DATED 19 OCTOBER 2012
| (1) | THE WELLCOME TRUST LIMITED |
| (2) | SUMMIT CORPORATION PLC |
TRANSLATION AWARD FUNDING AGREEMENT
| 1 | INTERPRETATION |
4 | ||||
| 2 | AWARD TO BE ADVANCED BY THE TRUST |
20 | ||||
| 3 | REPAYMENT |
26 | ||||
| 4 | INTEREST |
27 | ||||
| 5. | EXPLOITATION |
27 | ||||
| 6. | AWARD AUDIT |
28 | ||||
| 7. | WARRANTIES |
29 | ||||
| 8. | WARRANTY CLAIMS |
31 | ||||
| 9. | LIMITS ON LIABILITY |
32 | ||||
| 10. | REPORTING |
32 | ||||
| 11. | THIRD PARTY COLLABORATIONS AND SUBCONTRACTING |
34 | ||||
| 12. | TERMINATION |
34 | ||||
| 13. | EVENTS OF DEFAULT |
35 | ||||
| 14. | OBLIGATIONS OF THE COMPANY |
38 | ||||
| 15. | SITE VISIT GROUP |
40 | ||||
| 16. | UNEXPLOITED IPRS |
41 | ||||
| 17. | REVENUE SHARING |
43 | ||||
| 18. | ACCOUNTING STATEMENTS AND PAYMENTS |
46 | ||||
| 19. | FURTHER FUNDING |
49 | ||||
| 20. | DISPUTE RESOLUTION |
49 | ||||
| 21. | WAIVER |
51 | ||||
| 22. | ENTIRE AGREEMENT/VARIATIONS |
51 | ||||
| 23. | ANNOUNCEMENTS |
52 | ||||
| 24. | CONFIDENTIALITY |
53 | ||||
| 25. | NOTICES |
55 | ||||
| 26. | ASSIGNMENT |
56 | ||||
| 27. | SEVERANCE OF TERMS |
56 | ||||
| 28. | COSTS |
57 | ||||
| 29. | FURTHER ASSURANCES |
57 | ||||
| 30. | GENERAL |
57 | ||||
| 31. | GOVERNING LAW |
58 | ||||
| SCHEDULE 1 DRAWDOWN NOTICE | 60 | |||||
| SCHEDULE 2 DRAWDOWN NOTICE | 62 | |||||
| SCHEDULE 3 DETAILS OF SUMMIT CORPORATION PLC | 64 | |||||
| SCHEDULE 4A THE APPLICATION | 66 | |||||
| SCHEDULE 4B THE PLAN | 120 | |||||
| SCHEDULE 5 MILESTONES, TRANCHES AND INSTALMENTS | 130 | |||||
| SCHEDULE 6 TREASURY POLICY | 132 | |||||
| SCHEDULE 7 CONDITIONS | 133 | |||||
| SCHEDULE 8 REVENUE SHARING AGREEMENT | 134 | |||||
| SCHEDULE 9 COSTS SCHEDULE (INCLUDING COMPANY CONTRIBUTION) | 147 | |||||
| SCHEDULE 10 COMPANY PATENTS | 154 | |||||

THIS AGREEMENT is made and entered into as of the 19th day of October 2012.
BETWEEN:
| (1) | THE WELLCOME TRUST LIMITED a company registered in England & Wales with company no. 2711000 with registered address at 000 Xxxxxx Xx Xxxxxx XX0 0XX XX, as Trustee of the Wellcome Trust, a charity registered in England under no. 210183 (the “Trust”); and |
| (2) | SUMMIT CORPORATION PLC a public limited company registered in England and Wales under number 05197494 whose registered office is at 00 Xxxxxx Xxxx, Xxxxxxxx, Xxxxxxxxxxx XX00 0XX (the “Company”). |
RECITALS:
| (A) | The Company is a public limited company incorporated on 4 August 2004 in England and Wales under the provisions of the Companies Act 1985. |
| (B) | Pursuant to a funding agreement between the Trust and the Company dated 30 October 2009, the Trust made a programme-related investment by way of an award of two million, two hundred and eighty eight thousand, two hundred and twenty one pounds sterling (£2,288,221) to the Company to progress the development of a novel class of antibiotics for the targeted treatment of Clostridium difficile infection in consideration of a share of any resulting revenue. |
| (C) | In order to further its charitable objects, the Trust wishes to make a further programme-related investment by way of an award of a maximum amount of four million pounds sterling (£4,000,000) to the Company to support a first-in-human Phase I and Phase II clinical trial for the novel Clostridium difficile antibiotic SMT19969 in consideration of a share of any resulting revenue. |
| 1 | INTERPRETATION |
| 1.1 | In this Agreement, unless the context otherwise requires: |
| 1.2 | “Accounts Date” | means: | ||||
| (a) | as at the Effective Date, the date to which the most recent set of Audited Accounts immediately preceding the Effective Date are made up, and | |||||
| (b) | after the Effective Date, means the date to which the most recent Audited Accounts immediately preceding the date upon which the Warranties are repeated are made up; | |||||
| 1.3 | “Accrued Interest” | means interest payable and accrued in respect of the Award as calculated in accordance with Clause 4.1; | ||||

| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.4 | “Advance” | means the payment of tranches of the Award Amount by the Trust to the Company from time to time in accordance with Clause 2; | ||
| 1.5 | “Affiliate” | means, with respect to a given entity, any person, corporation, partnership or other entity, that Controls, is Controlled by, or is under common Control with such entity; | ||
| 1.6 | “Agreement” | means this agreement; | ||
| 1.7 | “Anniversary Date” | means each anniversary of the Effective Date or, if such date is not a Business Day, the next following Business Day; | ||
| 1.8 | “Application” | means the application as set out at Schedule 4A as amended by the Plan; | ||
| 1.9 | “Audited Accounts” | means the audited Group and Parent Company financial statements of the Company, for the relevant financial year of the Company, together with the related cash flow statements, notes, directors’ reports and Auditors’ reports; | ||
| 1.10 | “Auditors” | means BDO LLP or such other firm of chartered accountants as may be appointed as auditors of the Company from time to time; | ||
| 1.11 | “Award” | means the award made available by the Trust to the Company on the terms and conditions of this Agreement; | ||
| 1.12 | “Award Amount” | means up to four million pounds sterling (£4,000,000): | ||
| 1.13 | “Background IPRs” | means any IPRs created, devised, generated, owned by or licensed to the Company or which the Company had rights to prior to 30 October 2009, which are necessary or useful for undertaking the Project or for the protection or Exploitation of the Project IPRs or the Project Patents including without limitation the Company patents; | ||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 5 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.14 | “Board” | means the Company’s board of directors from time to time; | ||||
| 1.15 | “Business Day” | means a day on which banks are normally open for business and which is not a Saturday or Sunday or a bank or public holiday in England and Wales; | ||||
| 1.16 | “Calendar Quarter” | means each successive period of three (3) calendar months commencing on 1st January, 1st April, 1st July and 1st October or such other quarterly dates as may be agreed between the parties, and “Quarterly” shall be construed accordingly; | ||||
| 1.17 | “Change of Control” | means, in relation to the Company, where a person (or persons acting in concert) directly or indirectly, including through any Subsidiary or Holding Company or Subsidiary of such Holding Company: | ||||
| (a) | has beneficial ownership over more than 50 per cent of the total voting rights conferred by all the issued shares in the capital of the Company which are ordinarily exercisable in general meeting; or | |||||
| (b) | has the right to appoint or remove a majority of its directors; or | |||||
| (c) | has power to direct that the affairs of the Company are conducted in accordance with its wishes; | |||||
| in each case where such person or persons did not have such beneficial ownership, right or power at the Effective Date; | ||||||
| 1.18 | “Claim” | means any claim by the Trust for breach of any of the Warranties; | ||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 6 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.19 | “Clinical Trial Cover” | means either: | ||||
| (i) | a clinical trial liability insurance policy which will cover the clinical trial due to take place as part of the Project; and/or | |||||
| (ii) | (ii) an NHS indemnity for clinical trials which will cover the trial due to take place as part of the Project together with evidence that the clinical trial due to take place as part of the Project shall be conducted with the permission of the NHS; | |||||
| 1.20 | “Close Down Activities” | means all activities reasonably required pursuant to ICH GCP in order to close down any clinical trial that is the subject of the Project; | ||||
| 1.21 | “Companies Acts” | means Companies Act 1985, Companies Act 1989, Companies Act 2006, Business Names Act 1985 and Enterprise Act 2002; | ||||
| 1.22 | “Company Contribution” | means the contributions (financial and otherwise) to be made by the Company to the Project as set out in Schedule 9; | ||||
| 1.23 | “Company Patents” | means the patent and patent applications registered or filed in the name of the Company set out at Schedule 10; | ||||
| 1.24 | “Company TPC” | means the Company’s Treasury Policy contact as set out in Clause 2.14 or such other contact as may be notified by the Company to the Trust in writing from time to time in accordance with Clause 2.14; | ||||
| 1.25 | “Conditions” | means the conditions described in Schedule 7; | ||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 7 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.26 | “Confidential Information” | means any and all data, results, Know-How, software, plans, details of research work, discoveries, inventions, intended publications, intended or pending patent applications, designs, technical information, business plans, budgets and strategies, business or financial information or other information in any medium and in any form, and any physical items, prototypes, compounds, samples, components or other articles or materials disclosed by one Party to another Party whether orally or in writing or in any other form; | ||||
| 1.27 | “Connected Persons” | means a person connected with the Company or any director or any former director or any shareholder of the Company within the meaning of Section 839, Income and Corporation Taxes Act 1988; | ||||
| 1.28 | “Control” | means the direct or indirect ownership of more than fifty percent (50%) of the outstanding voting securities of an entity, or the right to receive more than fifty percent (50%) of the profits or earnings of an entity. Any other relationship which in fact results in one entity having a decisive influence over the management, business and affairs of another entity shall also be deemed to constitute Control; | ||||
| 1.29 | “Cost Schedule” | means the Cost Schedule at Schedule 9 setting out the Trust’s approved budget for the Project; | ||||
| 1.30 | “CTSC” | means the clinical trial steering committee for the clinical trial that is the subject of the Project; | ||||
| 1.31 | “Direct Costs” | means any costs and expenses incurred or allowed from time to time in accordance with this Agreement by or for the account of the Trust or the Company (as appropriate) in prosecuting, maintaining, enforcing or defending any of the Exploitation IPRs, marketing the Exploitation IPRs and negotiating, concluding or enforcing agreements for the licensing or other Exploitation of the Exploitation IPRs (including by way of acquisition of equity in a company), including without limitation: | ||||
| (a) | all reasonable legal, accounting and other professional fees and charges; | |||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 8 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| (b) | official filing, prosecution, maintenance and renewal fees; | |||||
| (c) | travelling and other out-of-pocket expenditure; and | |||||
| (d) | Cost of Goods; | |||||
| 1.32 | “Disclosure Letter” | means: | ||||
| (a) | as at the Effective Date, the disclosure letter dated the same date as this Agreement and accepted by the Trust, and | |||||
| (b) | after the Effective Date, the disclosure letter as subsequently amended and agreed by the Parties on each Anniversary Date and on the date of each Advance; | |||||
| 1.33 | “Drawdown Date” | means a Business Day on which an Advance is made; | ||||
| 1.34 | “Drawdown Notice” | means a notice in writing signed by the Company as detailed in Schedules 1 or 2 of this Agreement; | ||||
| 1.35 | “Drawdown Period” | means the period starting on the Effective Date and ending on the date which is the earlier of the Repayment Date or thirty six (36) months from the date of this Agreement; | ||||
| 1.36 | “DSMB” | means the Data Safety Monitoring Board for the clinical trial that is the subject of the Project; | ||||
| 1.37 | “Effective Date” | means the date of this Agreement as set out at the top of page 4 of this Agreement; | ||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 9 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.38 | “Encumbrance” | means any claim, charge, mortgage, security, lien, option, equity, power of sale, hypothecation or other third party rights, retention of title, right of pre-emption, right of first refusal or security interest of any kind; | ||||
| 1.39 | “Event of Default” | means any event or circumstance listed in Clause 13; | ||||
| 1.40 | “Exploitation” | means development and exploitation activities after completion of or during the Project including any further clinical trials, obtaining Marketing Approvals in any country, the commercialisation, licensing, promotion, marketing, distribution and sales of any Project Compounds and/or Licensed Products and “Exploited”, “Exploiting” and “Exploitation” shall be construed accordingly; | ||||
| 1.41 | “Exploitation IPRs” | means the Background IPRs, Project IPRs, Project Compounds and Licensed Products; | ||||
| 1.42 | “Exploiting Party” | means the Party(ies) undertaking Exploitation pursuant to Clauses 5 and 16. For clarity any Third Party to whom the Company or its Affiliates licences the Exploitation of the Exploitation IPRs shall not be considered an Exploiting Party for the purposes of this Agreement; | ||||
| 1.43 | “Fair Value” | means the fair market value of any asset taking into account the following assumptions: | ||||
| (a) |
the sale is between a willing seller and a willing purchaser on an arms length basis; | |||||
| (b) | the relevant asset is sold free of all restrictions, liens, charges and other encumbrances; | |||||
| or as determined by the Expert in accordance with Clause 19; | ||||||
| 1.44 | “Financial Terms” | shall have the meaning given to it in Clause 17.1; | ||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 10 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.45 | “Grant Conditions” | means the conditions under which the Trust makes grants to Organisations (currently version Cond 10/11) as amended from time to time; | ||
| 1.46 | “Group” | means, in relation to any Party, its Holding Companies, its Subsidiaries and the Subsidiaries of those Holding Companies; | ||
| 1.47 | “Holding Company” | shall have the definition given to it in the definition of Subsidiary; | ||
| 1.48 | “Indication” | means a disease indication manifested by a characteristic set of pathological symptoms and signs. Such disease indication is not manifested by reference to either patient sub-populations or to drug formulations, dosage regimes or other product line extensions, which relate to the characteristics of the product rather than the characteristics of the disease; | ||
| 1.49 | “IND” | means an investigational new drug application filed with the FDA or any equivalent from another regulatory authority(ies) including but not limited to a Clinical Trials Application to the EMA; | ||
| 1.50 | “IPRs” | means (i) patents, designs, trade marks and trade names (whether registered or unregistered), copyright and related rights, database rights, Know-How and Confidential Information; (ii) all other intellectual property rights and similar or equivalent rights anywhere in the world which currently exist or are recognised in the future; (iii) applications, extensions and renewals in relation to any such rights; and (iv) rights in the nature of data exclusivity relating to any IND; | ||
| 1.51 | “Know-How” | means any technical and other information which is not in the public domain at the date created, including information comprising or relating to concepts, discoveries, data, designs, formulae, ideas, inventions, methods, models, assays, research plans, | ||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 11 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| procedures, designs for experiments and tests and results of experimentation and testing (including results of research or development), processes (including manufacturing processes, specifications and techniques), laboratory records, chemical, pharmacological, toxicological, clinical, analytical and quality control data, clinical trial data, case report forms, data analyses, reports, manufacturing data or summaries and information contained in submissions to and information from ethical committees, regulatory authorities and/or Marketing Authorities; | ||||||
| 1.52 | “Licensed-In IPRs” | means all IPRs licensed by the Company from a Third Party which are being used or have been used by the Company at any time in the course of undertaking the Project; | ||||
| 1.53 | “Licensed Products” | means any product developed or Exploited by or on behalf of the Exploiting Party that is derived from or incorporates any Background IPRs, Project IPRs and/or any Project Compound(s); | ||||
| 1.54 | “Major Markets” | means the United States of America and the European Union; | ||||
| 1.55 | “Management Accounts” | means: | ||||
| (a) | as at the Effective Date, the most recent unaudited monthly management accounts of the Company prior to the Effective Date, and | |||||
| (b) | after the Effective Date, the most recent management accounts prior to the date upon which the Warranties are given; | |||||
| 1.56 | “Marketing Approval” | means all approvals, licences, registrations or authorisations of any federal, state or local regulatory agency, department or bureau or other governmental entity, required for the manufacturing, use, storage, distribution, promotion, marketing, import, transport and/or sale of Project Compounds and/or Licensed | ||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 12 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| Products in any jurisdiction (including in the case of countries where no national agency exists for the approval of drugs, approval by the World Health Organisation) | ||||||
| 1.57 | “Milestones” | means the Milestones as described in Schedule 5, and “Milestone” means any one of them; | ||||
| 1.58 | “Milestone Date” | means a date set out in Schedule 5 for the achievement of a Milestone; | ||||
| 1.59 | “MRC Guidelines” | means the Medical Research Council’s Guidelines for Good Clinical Practice in Clinical Trials set out at xxxx://xxx.xxx.xx.xx/Xxxxxxxxx/Xxxxxxxxxxxxxx/xxxxx.xxx?xxXXX000000; | ||||
| 1.60 | “Net Revenue” | means Revenue less: | ||||
| (a) | any Direct Costs; | |||||
| (b) | any applicable VAT on Revenue and/or Direct Costs; | |||||
| (c) | amounts repaid or credited and allowances including cash, credit or free goods allowances, given by reason of billing errors, discounts, actually allowed or paid or accrued; | |||||
| (d) | amounts refunded or credited for Licensed Products which were rejected or damaged or recalled or by reason of reasonable purchase chargebacks or rebates; | |||||
| (e) | freight, postage and shipping insurance invoiced to the Third Party; | |||||
| (f) | taxes, tariffs, customs duties and surcharges and other governmental charges incurred in connection with the sale, exportation or importation of Licensed Products; and | |||||
| (g) | government mandated and other reasonable rebates (such as those in respect of any state or federal Medicare or Medicaid or similar programs). | |||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 13 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| The transfer of Licensed Products between the Company and any of its Affiliates shall not be considered a sale for the purposes of calculating Revenue. In such cases, Revenue shall be determined on the gross invoiced price levied by the Affiliate on a Third Party, less the aforementioned deductions to the extent they are allowed, paid or accrued. | ||||||
| Any Licensed Product which is transferred by the Company or its Affiliates to a Third Party on less than arms length terms shall be deemed for the purposes of calculation of Revenue to be a sale at the list price of Licensed Product provided always that the use of Licensed Product in clinical trials shall not give rise to any deemed sale under this definition. | ||||||
| Transfers or dispositions of Licensed Product free of charge and in line with normal industry practice (a) for charitable purposes; (b) for non-commercial manufacturing purposes; (c) as free promotional samples of Licensed Product; or (d) for regulatory or governmental purposes shall not in each case be deemed “sales” for the purposes of calculating Revenue; | ||||||
| 1.61 | “Non-Cancellable Commitments” | means the reasonable costs of: | ||||
| (i) | the Close Down Activities; | |||||
| (ii) | final assessment of any clinical trial subjects; | |||||
| (iii) | data compilation and analysis in relation to the any clinical trial; and | |||||
| (iv) | preparation of publications including the clinical study report; | |||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 14 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.62 | “Non-Exploiting Party” | means the Party(ies) that are not undertaking Exploitation pursuant to Clauses 5 and 16; | ||
| 1.63 | “Parties” | means the parties to this Agreement, or either of them, as the context may require, and “Party” shall be interpreted accordingly; | ||
| 1.64 | “Plan” | means the plan set out in Schedule 4B; | ||
| 1.65 | “Policies and Positions” | means the Trust’s policies and positions set out at xxxx://xxx.xxxxxxxx.xx.xx/Xxxxx-xx/Xxxxxx/Xxxxxx-xxx-xxxxxxxx-xxxxxxxxxx/xxxxx.xxx as amended from time to time; | ||
| 1.66 | “Principal Scientific Contact” | means Xx Xxxxxxx Xxxx Xxxxxxx or such other person appointed by the Company to oversee the Project reasonably acceptable to and agreed with the Trust; | ||
| 1.67 | “Project” | means a first-in-human Phase I and Phase II clinical trial for the novel Clostridium difficile antibiotic SMT19969 in accordance with the Application and the Protocol; | ||
| 1.68 | “Project Compound” | means any compound in respect of which activities are undertaken by the Company in the course of the SDD Project and/or the Project, and shall include the chemical compound as well as all esters, salts, hydrates, solvates, polymorphs and isomers thereof, and shall include compositions comprising such compound, or esters, salts, hydrates, solvates, polymorphs, isomers and enantiomers; | ||
| 1.69 | “Project Inventions” | means any inventions created, devised or arising out of the Company’s undertaking and performance of the SDD Project, the Project or any part of them; | ||
| 1.70 | “Project IPRs” | means any IPRs (including the Project Patents, Project Inventions, and Project Compounds) created, | ||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 15 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| devised or arising out of the Company’s undertaking and performance of the SDD Project, the Project or any part of them; | ||||
| 1.71 | “Project Patents” | means any patent applications that may be made by the Company which claim any Project Inventions or parts thereof, and any patents resulting from any such applications, utility certificates, improvement patents and models and certificates of addition and all foreign counterparts of them in all countries, including any divisional applications and patents, refiling, renewals, continuations, continuations-in-part, patents of addition, extensions, (including patent term extensions,) reissues, substitutions, confirmations, registrations, revalidations, pipeline and administrative protections and additions, and any equivalents of the foregoing in any and all countries of or to any of them, as well as any supplementary protection certificates and equivalent protection rights in respect of any of them; | ||
| 1.72 | “Protocol” | means the description of any clinical trial comprising part of the Project signed by the Principal Scientific Contact; | ||
| 1.73 | “PubMed Central” | means an archive of life science journal literature operated by the National Center for Biotechnology Information, a division of the US National Library of Medicine accessible at xxxx://xxx.xxxxxxxxxxxxx.xxx.xxx/; | ||
| 1.74 | “Repayment Date” | means the date which is [**] Business Days following the date of any notice for repayment served by the Trust on the Company pursuant to Clause 3.1; | ||
| 1.75 | “Revenue” | means the pre-tax gross receipts actually received by the Exploiting Party and its Affiliates from time to time in respect of the Exploitation of Exploitation Project IPRs and/or Licensed Products, whether by grant of a licence or an option thereto in respect of any Exploitation IPRs and/or Licensed Products, the | ||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 16 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| assignment of the Exploitation IPR or otherwise, including, without limitation, gross receipts representing sales of the Licensed Products, cash sums, other monetary sums, royalties, licences fees, signature fees, lump sum payments or otherwise and/or any other consideration actually received by the Exploiting Party and/or its Affiliates such as the provision of premises, equipment or cross licences. Where any consideration comprising Revenue is received other than in money the value of the consideration shall be determined by reference to the Fair Value of the goods, services, licence or other benefit to the Exploiting Party as at the date of receipt by the Exploiting Party and/or its Affiliates. The Exploiting Party shall pay to the Non-Exploiting Party an amount in cash as required to satisfy the Non-Exploiting Party’s share of the Fair Value at the time it converts the non-cash consideration into cash. If the Parties are unable to agree on the Fair Value such dispute shall be referred to an expert under Clause 20 of the Funding Agreement. For the avoidance of doubt, Revenues shall include any award of damages received by the Exploiting Party and/or its Affiliates in respect of enforcement of the Exploitation IPRs, less the costs of such action. For the further avoidance of doubt, where the Company is the Exploiting Party, Revenue shall not include any equity investment made in the Company by a Third Party or money paid to the Company by way of a grant; | ||||
| 1.76 | “Revenue Sharing Agreement” | means the Template Equity and Revenue Sharing Agreement to be entered into between the Parties in the form attached at Schedule 8 as amended from time to time on the mutual agreement of the Parties; | ||
| 1.77 | “Sale” | means (i) the acquisition by any person of more than fifty percent (50%) of the shares of the Company or all of the shares not already owned by the acquirer; or (ii) the acquisition by any person of the business or assets of the Company or any material part thereof (including the Project IPRs); | ||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 17 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.78 | “Secondary Markets” | means the following countries namely Japan, Brazil, India, China, Russia and the following regions namely Africa and Oceania; | ||||
| 1.79 | “Site Visit Group” | shall have the meaning given to it in Clause 15; | ||||
| 1.80 | “SDD Funding Agreement” | means the funding agreement between the Trust and the Company dated 30 October 2009 in respect of the SDD Project; | ||||
| 1.81 | “SDD Project” | means the Company’s project to progress the development of a novel class of antibiotics for the targeted treatment of Clostridium difficile infection funded by the Trust pursuant to the SDD Funding Agreement; | ||||
| 1.82 | “Subsidiary” | means a company of which another company, its “Holding Company”: | ||||
| (a) | holds a majority of the voting rights in; | |||||
| (b) | is a member of and has the right to appoint or remove a majority of its board of directors; or | |||||
| (c) | is a member of and controls alone, pursuant to an agreement with other members, a majority of the voting rights; | |||||
| and shall include companies which are the subsidiary of a company that is itself a subsidiary of the Holding Company; | ||||||
| 1.83 | “Tax” or “Taxes” | means all forms of taxation, duties, imposts, levies and rates whenever created or imposed and whether of the United Kingdom or elsewhere and all penalties and interest payable in respect thereof including VAT; | ||||
| 1.84 | “Third Party” | means an entity or person other than the Parties or an Affiliate of any of the Parties: | ||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 18 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 1.85 | “Trust CP Position” | means the Trust’s position statement and guidance note about research involving people living in low and middle income countries as set out at xxxx://xxx.xxxxxxxx.xx.xx/Xxxxx-xx/Xxxxxx/Xxxxxx-xxx-xxxxxxxx-xxxxxxxxxx/XXX000000.xxx; | ||
| 1.86 | “Trust TPC” | means the Trust’s Treasury Policy contact as set out at Clause 2.15 or such other contact as may be notified by the Trust to the Company in writing from time to time in accordance with Clause 2.15; | ||
| 1.87 | “VAT” | means goods, sales, value added or any similar tax; | ||
| 1.88 | “Warranties” | means the representations and warranties contained in Clause 7 and each and any of them; and | ||
| 1.89 | “Year” | means a period of twelve (12) months starting on the Repayment Date or the Effective Date as the case may require and ending on the date twelve (12) months thereafter and each subsequent period of twelve (12) months. | ||
| 1.90 | References in this Agreement to any statutory provisions shall be construed as references to those provisions as respectively amended consolidated or re-enacted (whether before or after the Effective Date) from time to time and shall include any provisions of which they are consolidations or re-enactments (whether with or without amendment). |
| 1.91 | Reference to any statute, statutory instrument, regulation, by law or other requirement of English law and to any English legal term for any actions, remedy, method of judicial proceeding, legal document, legal status, court, official or any legal concept or doctrine shall, in respect of any jurisdiction other than England, be deemed to include that which most nearly approximates in that jurisdiction to the relevant English term. |
| 1.92 | The Schedules and Recitals form part of this Agreement and any reference to this Agreement shall include the Schedules and Recitals. |
| 1.93 | In this Agreement: |
| (a) | the masculine gender shall include the feminine and neuter and the singular number shall include the plural and vice versa; |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 19 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| (b) | references to persons shall include bodies corporate, unincorporated associations, partnerships and individuals; |
| (c) | except where the contrary is stated, any reference in this Agreement to a Clause or Schedule is to a Clause of or Schedule to this Agreement, and any reference within a Clause or Schedule to a sub-Clause, paragraph or other sub-division is a reference to such sub-Clause, paragraph or other sub-division so numbered or lettered in that Clause or Schedule. |
| 1.94 | The headings in this Agreement are inserted for convenience only and shall not affect the construction of the provision to which they relate. |
| 1.95 | References to the winding-up of a person include the amalgamation, reconstruction, reorganisation, administration, dissolution, liquidation, bankruptcy, merger or consolidation of such person and an equivalent or analogous procedure under the law of any jurisdiction in which that person is incorporated, domiciled or resident or carries on business or has assets. |
| 1.96 | Any reference to books, records or other information includes books, records or other information in any format or medium including paper, electronically stored data, video or audio recordings and microfilm. |
| 1.97 | Any phrase introduced by the terms “including”, “include”, “in particular” or any similar expression shall be construed as illustrative and shall not limit the sense of the words preceding those terms. |
| 1.98 | Where reference is made in this Agreement to the prior written consent of the Trust being required in respect of any matter, the Company shall give not less than [**] Business Days notice to the Trust of the matter for which such consent is required. |
| 2 | AWARD TO BE ADVANCED BY THE TRUST |
| 2.1 | In consideration of the rights and obligations of the Parties as set out in this Agreement, the Trust shall grant the Award to the Company on the terms and conditions set out in this Agreement. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 20 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 2.2 | The Trust and the Company agree that the Project will be carried out in accordance with this Agreement in addition to the Grant Conditions and the Policies and Positions. If there is any conflict between the provisions of this Agreement and the Grant Conditions or the Policies and Positions, then the provisions of this Agreement shall prevail. |
| 2.3 | The Award shall be used by the Company for the sole purpose of providing funding and support for the Project as described in the Application and shall be used for no other purpose without the prior written consent of the Trust. |
| 2.4 | The first instalment of the first tranche of the Award may be drawn down by the Company at any time after the Effective Date by providing to the Trust a Drawdown Notice in the form set out in Schedule 1. The Trust shall release the first instalment of the first tranche of the Award within [**] Business Days of the later of: |
| (a) | the date of receipt by the Trust of such Drawdown Notice (subject to the satisfaction of the conditions set out in Clause 2.10); and |
| (b) | the date of written confirmation from the Trust to the Company of acceptance of the then current Treasury Policy. |
The obligations under Clauses 2, 3, 4, 5, 6, 11, 15, 16, 17 and 18 shall not come into effect unless and until such Drawdown Notice is submitted. If no Drawdown Notice is received within [**] Business Days of the Effective Date, the Award shall be cancelled unless agreed otherwise in writing by the Trust. The Company may draw down subsequent tranches (or instalments) of the Award on the dates specified in Schedule 5. For the avoidance of doubt, the Trust shall not pay any part of the Award to the Company unless and until the Company’s then current Treasury Policy has been accepted by the Trust in writing and the Company is in compliance with such Treasury Policy.
| 2.5 | When the Company considers that any Milestone has been achieved by the relevant Milestone Date: |
| (a) | The Company shall as soon as reasonably practicable provide the Trust with a report of how the Milestone was achieved, a signed Drawdown Notice in the form set out in Schedule 2 and an updated Disclosure Letter; and |
| (b) | The Trust shall confirm to the Company in writing, within [**] Business Days of receipt by the Trust of notification pursuant to Clause 2.5 either that: |
| (i) | the Milestone has been achieved by the relevant Milestone Date the contents of the Disclosure Letter are reasonably acceptable to the Trust and |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 21 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| (subject to the satisfaction of the conditions set out in Clause 2.9) the first instalment of the next tranche of the Award will be released, in which case, within [**] Business Days of the later of: |
| (A) | receipt by the Company of the Trust’s confirmation pursuant to this Clause 2.5(a); or |
| (B) | where the Company has amended its Treasury Policy, the date of written acceptance from the Trust to the Company of the amended Treasury Policy, |
the Company shall draw down the next tranche of the Award in the instalments set out in Schedule 5; or
| (ii) | the Milestone has not been achieved by the relevant Milestone Date and the relevant tranche of the Award will not be released, in which case the Trust shall provide the Company with reasonable details of the grounds on which it has reached this decision. Any disagreement between the Parties as to whether or not a Milestone has been achieved shall be referred to the Dispute Resolution Procedure. The Trust shall grant the Company a reasonable period of time (“Milestone Extension”), in order to address the reasons why the Trust has judged that a particular Milestone has not been met. In the event of a dispute between the Parties regarding the achievement of a Milestone the Trust shall grant a Milestone Extension for the duration of the resolution of the Dispute. Any dispute between the Parties concerning the achievement of a Milestone arising from to one set of facts may only be referred through the Dispute Resolution Procedure once, and, for clarity, it is not intended that this clause creates a perpetual right to refer a dispute between the Parties concerning the achievement of a Milestone arising from to one set of facts through the Dispute Resolution Procedure. Upon the expiry of a Milestone Extension, the Trust shall, at its sole discretion, decide whether or not to release the relevant instalment of the next tranche of the Award to the Company, but the Trust shall not be obliged to do so; or |
| (iii) | the contents of the Disclosure Letter are not reasonably acceptable to the Trust and the relevant tranche of the Award will not be released, in which case the Trust shall provide the Company with reasonable details of the grounds on which it has reached this decision. For the avoidance of doubt, the Trust must have reasonable grounds for not accepting the contents of the relevant Disclosure Letter. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 22 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 2.6 | The Company shall complete and submit a detailed report on the work done and outcomes of the Project (“End of Award Report”) in the prescribed form to the Trust within [**] months of completion of analysis of the results of the Project. The Trust will evaluate the End of Award Report and will notify the Company within [**] Business Days of receipt whether the report is acceptable to the Trust, such approval not to be unreasonably withheld. If the End of Award Report is not acceptable to the Trust, it shall notify the Company of its reasons at the same time, which may include that the report is incomplete or insufficiently detailed. |
| 2.7 | Up to [**] sterling (£[**]) (the “Retained Amount”) shall be retained by the Trust until receipt of an End of Award Report acceptable to the Trust in accordance with Clause 2.6 above. The Retained Amount may be drawn down by the Company [**] Business Days following notification of the Trust’s acceptance of the End of Award Report by submitting a signed Drawdown Notice. |
| 2.8 | If any Milestones have not been achieved by the last day of the Drawdown Period, the Award shall be cancelled to the extent not drawn down, unless agreed otherwise in writing by the Trust or where there are outstanding Non-Cancellable Commitments. |
| 2.9 | The Company undertakes to use commercially reasonable efforts to ensure that the Conditions will be satisfied at all times throughout the duration of the Project and that the Milestones will be achieved by the Milestone Dates. |
| 2.10 | The Trust will only be obliged to make an Advance if on the date of the Drawdown Notice and on the proposed Drawdown Date: |
| (a) | no breach or default is subsisting or would result from the proposed Advance; |
| (b) | the Warranties are true and correct in all respects, subject to the matters set out in the relevant Disclosure Letter; |
| (c) | the Trust has received the relevant Disclosure Letter and the contents of such Disclosure Letter are reasonably acceptable to the Trust; |
| (d) | no written demand for repayment has been issued by the Trust pursuant to Clauses 3 and/or 12; |
| (e) | other than in the case of a drawdown of the first tranche of the Award or the Retained Amount, the Trust has provided confirmation to the Company in accordance with Clause 2.5 that the relevant Milestone has been met; |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 23 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| (f) | in the case of the Retained Amount, the End Of Award Report has been accepted by the Trust in accordance with Clause 2.7; |
| (g) | the Company’s then current Treasury Policy includes provisions ensuring maintenance of the Project funds (including the Award) in banks with at least the minimum credit rating (e.g. Standard & Poor’s) required by the Trust from time to time and such Treasury Policy has been accepted in writing by the Trust; and |
| (h) | the Company operates such Treasury Policy accepted in writing by the Trust. |
| 2.11 | The Company shall ensure that it holds a bank account in the currency in which the Award Amount shall be advanced. All payments made by the Company to the Trust or by the Trust to the Company as the case may be under this Agreement shall be made in pounds sterling. Payment shall be made by electronic wire transfer of immediately available funds directly to the account of the relevant Party designated below or to any other account which the relevant Party may specify by written notice. |
| (a) | Bank Account for the Company: |
| Account Name: | [**] | |
| Account No.: | [**] | |
| Bank: | [**] | |
| Sort code: | [**] | |
| SWIFT code: | [**] | |
| Branch: | [**] |
| (b) | Bank Account for the Trust: |
| Bank name: | [**] | |
| Bank Address: | [**] | |
| Account Name: | [**] | |
| Sort Code: | [**] | |
| Account No: | [**] | |
| IBAN: | [**] | |
| BIC / SWIFT: | [**] |
| 2.12 | Written confirmation of such transfer shall be sent by the Party sending the funds to the individual at the Party receiving the funds at the address provided in Clause 24.2. |
| 2.13 | Each Party shall pay any and all Taxes levied in respect of all payments it receives or makes under this Agreement. Any withholding or other taxes that any Party is required by law to withhold or pay on behalf of any other Party, with respect to any payments to it under this Agreement, shall be deducted from such payments and paid contemporaneously |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 24 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| with the remittance to such other Party, together with evidence of such withholding or payment. Such withholding and payment shall fully discharge the Party making the payment and no further payment shall be required by the payor to the payee. The Party withholding or making such payment shall furnish the other Party with appropriate documents to secure application of the most favourable rate of withholding tax under applicable law. |
| 2.14 | The contact details of the Company TPC are set out below. The Company shall promptly notify the Trust TPC in writing of any changes to the identity and/or contact details of the Company TPC. |
| Company TPC: | ||
| Name: | The Chief Financial Officer and the Financial Controller of the Company who, at the Effective Date are Xxxxxxx Xxxxxxx and Xxxxxxx Xxxxxxx | |
| Position: | Chief Financial Officer, Financial Controller | |
| Address: | 00 Xxxxxx Xxxx, Xxxxxxxx, Xxxxxxxxxxx, XX00 0XX | |
| Phone number: | [**] | |
| Email: | [**] | |
| 2.15 | The contact details of the Trust TPC are set out below. The Trust shall promptly notify the Company TPC in writing of any changes to the identity and/or contact details of the Trust TPC. |
| Trust TPC: | ||
| Name: | [**] | |
| Position: | Financial Account Manager / Financial Controller / Head of Financial Accounting | |
| Address: | Wellcome Trust | |
| Xxxxx Building | ||
| 000 Xxxxxx Xxxx | ||
| London NW1 2BE, UK | ||
| Phone number: | [**] | |
| Email: | [**] | |
| 2.16 | In the event that the Company makes any amendments to the Treasury Policy most recently accepted in writing by the Trust, the Company shall prior to such changes taking effect: |
| (a) | notify the Trust TPC in writing of the amendments to the Treasury Policy; and |
| (b) | provide a copy (in English) of the amended Treasury Policy to the Trust TPC for acceptance by the Trust. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 25 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
The Trust shall notify the Company in writing within [**] Business Days of receipt of such amended Treasury Policy whether such amended Treasury Policy has been accepted by the Trust.
| 2.17 | In the event that the credit rating of the Company’s bank holding the bank account for the Project funds (including the Award) falls to a credit rating below the Trust’s minimum required credit rating, the Trust shall not be under any obligation to pay any part of the Award to the Company unless and until the Company operates a bank account for the Project funds with a bank with at least the minimum credit rating required by the Trust from time to time. For the avoidance of doubt, the Trust may require the Company to open and operate a bank account with an alternative bank where the Company’s original bank’s credit rating falls below the minimum credit rating required by the Trust from time to time. |
| 3 | REPAYMENT |
| 3.1 | The Trust may, in its absolute discretion, serve a written demand on the Company requiring that the Company repay the part of the Award Amount that has actually been paid to the Company or, in the event the whole of the Award Amount has been paid to the Company, the whole of the Award Amount together with Accrued Interest in the following circumstances: |
| (i) | where the Company has used the Award Amount for purposes other than those reasonably related to the Project; or; |
| (ii) | if the Company has been fraudulent, has engaged in wilful misconduct or has knowingly withheld material information from the Trust. |
| 3.2 | The Trust may, in its absolute discretion, serve a written notice on the Company requiring the Company to repay to it any unused part of any of the tranches of the Award Amount actually paid to the Company together with Accrued Interest thereon less the costs of the Close Down Activities in the following circumstances:- |
| (i) | an Event of Default has occurred and the Trust has elected to terminate the Project; or |
| (ii) | a Sale or the assignment of the Exploitation IPR to a Third Party falling within the scope of Clause 13.1.11. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 26 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 3.3 | Any written demand served by the Trust on the Company requiring repayment of the Award shall state the amount of the Award and Accrued Interest that shall be repaid (the “Repayment Amount”). |
| 3.4 | Upon payment by the Company to the Trust of the outstanding amount of the Award and Accrued Interest the Company shall have no further obligation to the Trust with respect to any revenue received by the Company from the Exploitation of the Exploitation IPRs, Background IPRs and/or Licensed Products, whether under this Agreement, any Revenue Sharing Agreement between the Parties, or otherwise howsoever. |
| 4 | INTEREST |
| 4.1 | The Accrued Interest payable by the Company on a repayment of the Award shall be deemed to have accrued on a daily basis on the amount of the Award from time to time outstanding at the rate of [**] percent ([**]%) per annum above the three month sterling LIBOR from time to time. Such interest shall have accrued from day to day by reference to a year of three hundred and sixty five (365) days and such interest shall be deemed to have been added to the principal amount of the Award annually on each Anniversary Date and on each Repayment Date (if the relevant Repayment Date is not an Anniversary Date). |
| 4.2 | If the Company fails to pay any amount payable by it under this Agreement on the relevant due date, interest shall accrue on the overdue amount from the due date up to the date of actual payment (both before and after judgement) at a rate which is the sum of [**] percent ([**]%) per annum and the rate which would have been payable if the overdue amount had constituted a Award in an amount equal to such overdue amount on the same terms as the Award. Any interest accruing under this Clause 4.2 shall be immediately payable by the Company on demand. |
| 5. | EXPLOITATION |
| 5.1 | The Company shall be the first Exploiting Party under this Agreement. |
| 5.2 | Prior to any member of the Group (whether itself or through any other member of the Group or by granting a licence or in collaboration with any Third Party) commencing the Exploitation of the Exploitation IPRs, the Company or the relevant member of the Group shall obtain the prior written consent of the Trust to such Exploitation by sending written notice to the Trust and the following information: reasonable details of the relevant Exploitation IPRs and the activity proposed. The grant of the Trust’s consent shall be conditional on the parties promptly entering into a Revenue Sharing Agreement incorporating the Financial Terms. The Trust shall notify the Company or the relevant |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 27 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| member of the Group as the case may be as to whether it consents (such consent not to be unreasonably withheld or delayed) to such Exploitation and, as far as is reasonably practicable, within [**] Business Days of receiving the written notice from the Company as provided for in this clause 5.2. The Company shall use reasonable endeavours to keep the Trust informed of any proposed Exploitation transactions prior to presenting the final agreement for an Exploitation for approval of the Trust pursuant to this Clause 5.2. |
| 5.3 | Following the receipt of such consent the Company or the relevant member of the Group shall be free to Exploit the relevant Exploitation IPRs in accordance with the consent given by the Trust without further consent or approval from the Trust. If, in respect of any Exploitation IPRs, the Trust does not give its consent, the Parties shall meet to discuss the Trust’s concerns and if they are unable to resolve those concerns that matter shall be referred to the dispute resolution procedure set out in Clause 20. All agreements entered into by the Company or Group shall be consistent with the terms of this Agreement. |
| 6. | AWARD AUDIT |
| 6.1 | The Company shall procure that the control of expenditure to be funded under this Agreement is governed by the normal standards and procedures of the Company and is covered by the formal audit arrangements that exist in the Company. |
| 6.2 | The Trust (at its own expense) shall have the right to ask for confirmation from the Auditors that the Auditors have signed their opinion on the annual accounts of the Company without qualification and that any management letter(s) raises no matters that have, or could, significantly affect the administration of the award made by the Trust. |
| 6.3 | The Trust shall have the right, at its discretion, to audit (either directly or via Third Parties engaged by it) any expenditure of the Award Amount and any amounts due to the Trust under this Agreement. To this end, the Company shall, and shall procure that its Affiliates and sublicensee’s shall [**], provide access (during normal business hours) to all or any part of the Company’s or its Affiliates’ or sublicensees’ accounting and other financial and corporate records and books necessary to check the accuracy of the expenditure of the Award Amount, any share of Revenue paid to the Trust under this Agreement and any other amounts due to the Trust under this Agreement. The Parties agree to settle any discrepancies promptly. The costs of such audit shall be paid by the Trust, except in the case of a discrepancy in favour of the trust of more than [**] per cent ([**]%) of any sums paid or payable to the Trust under this Agreement where the charges shall be paid by the Company. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 28 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 7. | WARRANTIES |
| 7.1 | The Company represents and warrants to the Trust that: |
| (a) | It has the requisite authority to enter into this Agreement; |
| (b) | It has the full power and authority to assume all of its obligations under this Agreement; |
| (c) | No consent, approval, authorisation, or order of any court or governmental agency or body is required for the consummation of the transactions contemplated by this Agreement; and |
| (d) | The execution, delivery, and performance of this Agreement will not result in a breach or violation of, or constitute a default under, any statue, regulation, or other law or agreement or instrument to which it is a party or by which it is bound, or any order, rule or regulation of any court or governmental agency of body having jurisdiction over it or any of its properties. |
| (e) | to the best of the Group’s actual knowledge, information and belief that, on the Effective Date and on the date of any Advance, each of the statements below are true and accurate in all respects, subject to matters fairly and accurately disclosed in the Disclosure Letter or otherwise through information disclosed in writing or by email to the Trust prior to the date of the relevant Disclosure Letter): |
| (i) | all facts and information reasonably believed by the Group to be material for disclosure to the Trust in connection with the grant of the Award (including in relation to the Company and/or Group) have been fairly and accurately disclosed to the Trust in writing, by email or in the Disclosure Letter; |
| (ii) | the Company is the sole legal and beneficial owner and, where registered, the sole registered proprietor of all the Background IPRs and Project IPRs free from all Encumbrances; |
| (iii) | no material Third Party IPRs are required for the Project and/or Exploitation of the Exploitation IPRs; |
| (iv) | all agreements, arrangements and obligations relating to material licensed-in IPRs are in writing, valid and in force and have not been the subject of any breach or default by any party or of any event which, with the giving of notice or lapse of time, would constitute a default and no notice has been given by any relevant party to terminate any of them; |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 29 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| (v) | the Company and all counterparties have complied with their respective obligations under all agreements, arrangements and obligations relating to any material licensed-in IPRs, and no disputes or claims are pending or threatened in respect of any of them; |
| (vi) | there are no grounds for invalidity, termination, avoidance or repudiation of any agreements, arrangements or obligations in respect of any material licensed-in IPRs; |
| (vii) | no Third Party has given notice of its intention to terminate, or has sought to repudiate or disclaim any agreement, arrangement or obligation in respect of any material licensed-in IPRs; |
| (viii) | the Background IPRs and Project IPRs created pursuant to the SDD Project are not subject to any pending or threatened claims, challenges or proceedings save for examinations of the applications by patent offices; |
| (ix) | no Third Party has made unauthorised use of any Background IPRs and/or Project IPRs nor threatened to do so; and |
| (x) | the Company has not received notice of any allegation that the activities of the Company in relation to the Background IPRs infringe, any Third Party IPRs and the Company is not in receipt of actual knowledge that the activities of the Company in relation to the Background IPRs infringe any Third Party IPRs. |
| 7.2 | The Company acknowledges that the Company has given the Warranties with the intention of inducing the Trust to enter into this Agreement and, as the case may be, to the make the Advances on the achievement of each of the Milestones and that the Trust has been induced to enter into this Agreement and make available the Award on the basis of and in full reliance on them. |
| 7.3 | Each of the Warranties shall be construed as a separate and independent warranty and (save where expressly provided to the contrary) shall not be limited or restricted by reference to or inference from any other term of this Agreement or other Warranty save for the Disclosure Letter. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 30 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 7.4 | Where any statement in the Warranties is qualified by reference to the knowledge, awareness or belief of the Company and/or Group, the Company and/or Group shall be deemed to be aware of all matters of which it had actual knowledge. |
| 7.5 | The Company will immediately cause to be disclosed in writing to the Trust any fact, matters, circumstances or other information which may become known to any of them which is a breach of or can reasonably be expected to be, or be likely to cause, a breach of the Warranties. |
| 8. | WARRANTY CLAIMS |
| 8.1 | The maximum liability of the Company under this Agreement in respect of the aggregate of all Claims shall not exceed any unused part of any of the tranches of the Award Amount that have actually been paid to the Company together with Accrued Interest thereon less the costs of the Close Down Activities save in the circumstances set forth in Clause 3.1 and in such circumstances the maximum liability of the Company under this Agreement shall not, save in the event of fraud, wilful misconduct or the withholding of material information, exceed the part of the Award Amount that has actually been paid to the Company or, in the event the whole of the Award Amount has been paid to the Company, the whole of the Award Amount together with Accrued Interest thereon The Company shall not be liable and no Claim or Claims shall be made against the Company: |
| (a) | if the fact, omission, circumstances or occurrence giving rise to the Claim has been fairly and accurately disclosed to the Trust in the Disclosure Letter or through information provided in writing or by email to the Trust prior to the date of the relevant Disclosure Letter; |
| (b) | if the matter giving rise to the Claim is provided for under the terms of this Agreement; |
| (c) | if the Claim arises from any act, matter or thing done by Company at and in accordance with the written request of the Trust; or |
| (d) | if the Claim occurs as a result of the passing of any legislation not in force at the Effective Date, or which takes effect retroactively, or occurs as a result of any increase in the tax rate in force on the Effective Date or any change in the generally established practice of the relevant tax authority; and |
| (e) | unless the Trust has given the Company notice in writing of the Claim, summarising the nature of the Claim as far as it is known to the Trust and, if known, the amount claimed. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 31 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 8.2 | To the extent that any breach of the Warranties is in the reasonable opinion of the Trust capable of remedy, the Trust shall afford the Company an opportunity to remedy the matter complained of within [**] Business Days of receipt of written notice from the Trust specifying the breach and requiring its remedy. |
| 9. | LIMITS ON LIABILITY |
| 9.1 | Except in circumstances of fraud or wilful misconduct by a Party or its Affiliates, no Party nor any of its Affiliates shall be liable to another Party or any Affiliate of another Party for special, indirect, incidental or consequential damages (including loss of profit, whether arising directly or indirectly), whether in contract, warranty, negligence, tort, strict liability or otherwise, arising out of any breach of or failure to perform any of the provisions of this Agreement. |
| 9.2 | Nothing in this Agreement shall limit the liability of any Party in respect of: |
| 9.2.1 | personal injury or death arising out of that Party’s negligence or wilful misconduct, or |
| 9.2.2 | fraud or fraudulent misrepresentation. |
| 9.3 | The maximum liability of the Trust under this Agreement in respect of the aggregate of all claims shall not exceed the Award Amount less the amount of the Award Amount spent by the Company in undertaking the Project. |
| 9.4 | The maximum liability of the Company under this Agreement shall not exceed any unused part of any of the tranches of the Award Amount that has actually been paid to the Company together with Accrued Interest thereon less the costs of the Close Down Activities save in the circumstances set forth in Clause 3.1 and in such circumstances the maximum liability of the Company under this Agreement shall not, save in the event of fraud, wilful misconduct or the withholding of material information exceed the part of the Award Amount that has actually been paid to the Company or, in the event the whole of the Award Amount has been paid to the Company, the whole of the Award Amount together with Accrued Interest thereon |
| 10. | REPORTING |
| 10.1 | The Trust (or its representative) shall have the right to: |
| 10.1.1 | attend meetings of the CTSC and meetings of the DSMB for the Project as an observer; |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 32 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 10.1.2 | receive all papers that a member of the CTSC and/or DSMB would be entitled to receive under the Project; and |
| 10.1.3 | with the permission of the chair of the CTSC and/or DSMB (as the case may be), attend meetings of the CTSC and/or DSMB regarding the Project by telephone or other electronic means rather than in person, provided that persons attending the meeting can hear and be heard for all parts of the meeting. |
| 10.2 | The Company shall supply to the Trust and/or its representative, subject at all times to its obligations to the London Stock Exchange in respect of price sensitive information: |
| 10.2.1 | reports on the progress of the Project and Exploitation in such form and in such intervals as agreed with the Trust; and |
| 10.2.2 | Audited Accounts (if it is legally required to prepare audited accounts, and otherwise annual accounts for the previous financial year), together with any management letters relating to them, as soon as they are available and in any event, within [**] Business Days of the end of each financial year; |
| 10.2.3 | copies of all documents the Company discloses to its creditors generally, and such documents as may be available in the public domain; |
| 10.2.4 | details of any material litigation, arbitration or administrative proceedings which are current, threatened or pending against the Company or any of its directors as soon as it becomes aware of them; and |
| 10.2.5 | such additional financial or corporate information relating to the Company as the Trust may reasonably require. |
| 10.3 | Subject at all times to the Company’s obligation to the London Stock Exchange in respect of price sensitive information, the Company shall keep the Trust reasonably informed and report at least [**] all matters relating to the Exploitation of the Exploitation IPRs by or on behalf of the Company. |
| 10.4 | The Company shall ensure that data reported to the Trust and/or its representatives are reliable, accurate and not misleading. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 33 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 10.5 | Following the end of the Project, the Trust (or its representative) and the Company shall meet within [**] Business Days to discuss and report on the outcomes of the Project. |
| 11. | THIRD PARTY COLLABORATIONS AND SUBCONTRACTING |
| 11.1 | The Company shall ensure in all cases that any collaborations or sub-contracts shall be on the following terms: |
| 11.1.1 | that the Third Party shall not have any rights to any results emerging from such work, and all such results shall as between the parties and the Third Party be deemed to be Project IPRs and owned in accordance with the provisions of this Agreement; |
| 11.1.2 | that the Third Party shall be under obligations of confidence concerning such results on terms equivalent to those set out under this Agreement; |
| 11.1.3 | that the Third Party shall keep detailed records including scientific notebooks of all of its activities and upon request shall make available copies to the Trust; |
| 11.1.4 | that the Third Party will upon reasonable request make available its employees and/or consultants for discussion with the Site Visit Group; and |
| 11.1.5 | that the provisions of such sub-contract or collaboration agreement shall be consistent with the milestoned nature of the award and the termination provisions of this Agreement, and shall terminate if this Agreement terminates. |
| 12. | TERMINATION |
| 12.1 | This Agreement shall terminate on the earlier of: |
| 12.1.1 | full repayment of the Award and any Accrued Interest in cash pursuant to Clauses 3 and/or 13; or |
| 12.1.2 | the expiration of all payment obligations under this Agreement and/or the Revenue Sharing Agreement with respect to Revenue. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 34 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 12.2 | The Trust may terminate or suspend the Project on the occurrence of an Event of Default and where, following receipt of written notice from the Trust requiring remedy of any such circumstances, the Company fails to remedy those circumstances within [**] Business Days from receipt of such written notice save that such notice shall not be required in the circumstances set forth in Clauses 13.1.3 to 13.1.10. |
| 12.3 | On such termination, all payment obligations under this Agreement and/or the Revenue Sharing Agreement with respect to Revenue shall cease. |
| 12.4 | On Termination, the following provisions of this Agreement shall survive termination 6, 7, 9, 21 to 31 inclusive. |
| 13. | EVENTS OF DEFAULT |
| 13.1 | The following events or circumstances set out in this Clause 13 shall each constitute an Event of Default: |
| 13.1.1 | any material breach of this Agreement, including any material breach of a Warranty by the Company, subject to the matters fairly and accurately set out in any Disclosure Letter which has been accepted by the Trust; |
| 13.1.2 | the provision by the Company of a Disclosure Letter the contents of which indicate that completion of the Project and/or Exploitation are unlikely due to an adverse intellectual property position held by a Third Party; |
| 13.1.3 | the Company is unable or admits inability to pay its debts as they fall due, suspends making payments on any of its debts or, by reason of actual or anticipated financial difficulties commences negotiations with one or more of its creditors with a view to rescheduling any of its indebtedness; |
| 13.1.4 | a proposal is made or a nominee or supervisor is appointed for a composition in satisfaction of the debts of the Company or a scheme or voluntary arrangement of its affairs within the meaning of the relevant bankruptcy or insolvency laws, or the Company enters into any composition or voluntary arrangement for the benefit of its creditors, or proceedings are commenced in relation to the Company under any law, regulation or procedure relating to the re-construction, deferment or re-adjustment of all or substantially all of the Company’s debts; |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 35 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 13.1.5 | the Company takes any action, or any legal proceedings are started whether by a third party or not, for the purpose of the winding up or dissolution of the Company, other than for a solvent reconstruction or amalgamation; |
| 13.1.6 | the appointment of a liquidator, trustee, receiver, administrative receiver, receiver and manager, interim receiver custodian, sequestrator, administrator or similar officer, in respect of all or a substantial part of the assets of the Company; |
| 13.1.7 | an effective resolution being passed for the winding-up or entering into administration (whether out of court or otherwise) of the Company; |
| 13.1.8 | a distress, execution or other legal process being levied against all or substantially all of the assets of the Company, and not being discharged or paid out in full within ten (10) Business Days of the commencement of each process; |
| 13.1.9 | the occurrence in respect of the Company of any event in any jurisdiction to which it is subject having an effect similar to that of any of the events referred to in Clauses 13.1.2 to 13.1.8 above; |
| 13.1.10 | the Company ceases or threatens to cease to carry on all or a substantial part of its business or operations necessary for the completion of its obligations under this Agreement; |
| 13.1.11 | the Company takes any action, or omits to take any action, or is subject to any action the consequences of which, in the reasonable opinion of the Trust, would be incompatible with or have an adverse effect (i) on the Trust’s charitable objectives or reputation, or (ii) on the ability of the Company to comply with its obligations under this Agreement, including where such incompatibility or adverse effect is a consequence of undergoing a Change of Control. For clarity and by way of examples, the Trust cannot be involved in a business relationship with any tobacco company, any arms manufacturing, dealing or selling company, any company that does not comply with MHRA or equivalent guidelines for clinical trials, companies that do not comply with the Trust’s policies regarding to the use of animals in medical research, animal health or undertaking clinical trials in low income populations and any other material policies of the Trust.; |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX UK | 36 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 13.1.12 | the Trust and the Company are unable, having used bona fide reasonable efforts, to agree upon a replacement for Xx Xxxxxxx Xxxx Xxxxxxx as Principal Scientific Contact |
| 13.1.13 | the Company or any Group company enters into any transactions involving any of Exploitation IPRs and/or any of the Licensed-In IPRs necessary or useful for undertaking the Project or for the protection or exploitation of the Exploitation IPRs without the prior written consent of the Trust; |
| 13.1.14 | the Site Visit Group recommends to the Trust’s Technology Transfer Division that the Trust terminates the Project under Clause 15.1 and the Company fails to correct any identified failings within the time period granted by the Trust (if any) under Clause 15.2; or |
| 13.1.15 | the Company fails to comply with any of the Conditions. |
| 13.2 | On the occurrence of an Event of Default the Trust may in its absolute discretion serve written notice on the Company (“Default Notice”) and shall permit the Company [**] Business Days from the date of receipt of the Default Notice to remedy any such Event of Default (if such Event of Default is capable of remedy) to the satisfaction of the Trust. For clarity, the rights of the Trust to demand repayment of all or any part of the Award Amount are set forth in Clause 3. |
| 13.3 | If the Trust requires repayment of any part of the Award and Accrued Interest pursuant to Clause 3, the relevant sumsshall be repayable as follows: |
| 13.3.1 | Within [**] Business Days of the date on which the Trust notifies the Company that repayment is required pursuant to Clause 3, (the “Notification Date”), the Company shall refund to the Trust any portion of the Award advanced by the Trust but not yet spent (other than any amount which the Company has irrevocably committed to pay to a third party, provided that the Company shall use all reasonable endeavours to minimise any further payments that it is required to pay) and shall provide to the Trust such information as the Trust may reasonably require to enable the Trust to verify compliance with this paragraph; and |
| 13.3.2 | the balance of the Award and Accrued Interest not repaid pursuant to Clause 13.3.1 shall be repaid by the Company to the Trust by the next [**] following the Notification Date. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 37 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 13.4 | For the avoidance of doubt following the service of a Default Notice by the Trust pursuant to Clause 13.2, the Trust shall not be required to make any further Advance of the Award. |
| 14. | OBLIGATIONS OF THE COMPANY |
Company Contribution
| 14.1 | The Company undertakes to make, or to procure that other members of the Group make, the Company Contribution available to finance and support the Project in accordance with Schedule 9. |
| 14.2 | The Company shall provide written statements to the Trust setting out in reasonable detail the Company Contribution provided by the Company to the Project at [**] monthly intervals. Each such statement shall include details of the funding provided, the date on which the funding was provided and the cash amount attributed to such Company Contribution. |
Company undertakings
| 14.3 | The Company undertakes to the Trust that the Company shall not, and none of the Company’s Subsidiaries or Holding Company (if any) shall, do any of the following without the prior written consent of the Trust: |
| 14.3.1 | enter into any transactions involving any of the Exploitation IPRs and/or any of the Licensed-In IPRs necessary or useful for undertaking the Project or for the protection or Exploitation of the Exploitation IPRs, without the prior written consent of the Trust save that the Company may assign or otherwise transfer any of the foregoing from the Company to a Subsidiary or Holding Company provided such Subsidiary or Holding Company agrees in writing with the Trust to be bound by the term of this Agreement; |
| 14.3.2 | make any material change to the general nature of the business of the Company as carried on as at the Effective Date; and/or |
| 14.3.3 | create any new security, or increase any existing security over any of the Exploitation IPRs, Project IPRs and/or any of the Licensed-In IPRs necessary or useful for undertaking the Project or for the protection or Exploitation of the Exploitation IPRs, (other than any netting or set-off arrangement entered into in the ordinary course of the Company’s banking or financing arrangements for the purpose of netting debit and credit balances; or any lien arising by operation of law and in the ordinary course of business). |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 38 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
Project Inventions
| 14.4 | The Company shall procure that the Principal Scientific Contact monitors the Project for material that may be the subject of Project Inventions and shall promptly notify the Trust of any such Project Invention. |
Project Manager
| 14.5 | The Company shall appoint a project manager from its employees who shall be responsible on a day-to-day basis for co-ordinating the internal and external components of the Project |
Use of Trust name
| 14.6 | The Company shall not use the “Wellcome Trust” name or logo except with the prior written consent of the Trust and in the manner approved by the Trust except where the Company is legally required to disclose the source of funding for the Project. |
Management of research work
| 14.7 | The Company shall be responsible for the management, monitoring and control of all research work undertaken by it. This shall include, as appropriate, the requirements of all applicable laws and regulatory authorities, including but not limited to those governing the use of radioactive isotopes, animals, pathogenic organisms, diagnostic tools, medical devices, genetically modified organisms, toxic and hazardous substances, research on human subjects and human embryos, and include appropriate ethical approvals and consents, including for example but not limited to, such approvals and consents for obtaining tissues and other human samples. |
Clinical trials
| 14.8 | Any clinical trial which is undertaken by the Company, its collaborators, sub-contractors or service providers under the Project: |
| 14.8.1 | Where the clinical trial is to be undertaken in the UK and/or other high income economies (as defined by the World Bank), shall comply with the MRC Guidelines, insofar as it is reasonable to do so; and |
| 14.8.2 | Where the clinical trial is to be undertaken in low or middle income economies (as defined by the World Bank), shall comply with: |
| 14.8.2.1 | The Trust CT Position; and |
| 14.8.2.2 | The MRC Guidelines, provided that the MRC Guidelines are not inconsistent with the Trust CT Position, and only insofar as it is reasonable to comply with the MRC Guidelines under the circumstances. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 39 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
Clinical trial insurance
| 14.9 | Prior to the commencement of any clinical trial under the Project, the Company shall take out and maintain, or procure adequate Clinical Trial Cover which must be effective from the commencement date of the clinical trial until at least [**] years after the completion of the clinical trial. The adequacy of such Clinical Trial Cover shall be considered in relation to generally accepted industry standards at the time of the clinical trial |
Trust clinical trial register
| 14.10 | For any clinical trial carried out pursuant to the Project the Company shall on the Trust’s written request supply details of such clinical trial for publication on the Trust’s clinical trial register, such details not to include Company Confidential Information. |
Principal Scientific Contact
| 14.11 | If the Principal Scientific Contact ceases to be involved with the Project, ceases to be employed by or provide services to the Company or is prevented through illness or injury from promptly fulfilling his obligations in respect of the Project, the Company shall propose a replacement Principal Scientific Contact and shall notify the Trust of such person for approval by the Trust, such consent not to be unreasonably withheld. The Trust shall confirm to the Company within [**] Business Days of receipt of such notification whether or not it approves such appointment. If the Trust and the Company are unable to agree upon a replacement, the Trust may terminate the Agreement pursuant to Clauses 12 and 13. |
| 15. | SITE VISIT GROUP |
| 15.1 | The Trust may appoint a Site Visit Group, made up of a small team of independent experts and observers from the Trust’s Technology Transfer Division. The Company shall have [**] Business Days from notification by the Trust to notify the Trust of any concerns regarding the qualifications or independence of the designated members of the Site Visit Group, which concerns the Trust shall consider in good faith. The Site Visit Group shall have reasonable access during normal working hours and at mutually agreed times to visit the premises where the Project is being conducted to consult informally with the Company’s researchers, consultants or contractors working on the Project, to evaluate progress, performance and key issues and to report back to the Trust on their findings. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX UK | 40 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 15.2 | The Site Visit Group may recommend that the Trust terminates the Project due to a serious failure in the progress, management or conduct of the Project (including a finding that the Project will be unable to achieve the next Milestone within a reasonable time period after the relevant Milestone Date, or due to a major external scientific, technical or commercial barrier which the Site Visit Group considers will mean that the Project is unlikely to succeed in its objectives). If the Site Visit Group makes such a recommendation pursuant to this Clause 15.1, the Trust may terminate this Agreement pursuant to Clause 13.1.14. |
| 15.3 | The Trust may, in its sole discretion, allow the Company a reasonable period of time to take corrective action to address any failings identified by the Site Visit Group (if such failings are capable of correction). If the Trust grants the Company a period of time (such period of time to be no less than [**] Business Days) to correct such failings and the Company does not correct such failings within the period specified by the Trust (if any), the Trust shall retain the right to terminate this Agreement pursuant to Clause 13.1.13. |
| 16. | UNEXPLOITED IPRS |
| 16.1 | If any Exploitation IPRs remain unExploited or not further developed by the Company in any Major Market or in respect of any Indication where such Indication is identified in the Application or by the CTSC, in each case within [**] years following completion of the Project, the Trust shall have the option in its sole discretion by giving written notice to the Company to become the Exploiting Party and take responsibility on behalf of the Company for the commercialisation and Exploitation of such Exploitation IPRs in that country or in respect of that Indication as the case may be, which includes discretion to make any and all decisions regarding the negotiation, acceptance and conclusion of terms for any agreement regarding the commercial development and Exploitation of such unexploited Exploitation IPRs (including development and Exploitation by way of licence, sale, assignment, materials transfer or other transfer of rights, as well as any transaction which involves placing such unexploited Exploitation IPRs into a separate corporate vehicle) in such country and, if applicable, in such Indication. For clarity, if a further clinical trial is being undertaken anywhere in the World with a view to providing data that will enable applications for Marketing Approvalss to be made in either or both of the Major Markets, the Company shall be considered to be fulfilling its further development and Exploitation obligations. |
| 16.2 | If any Exploitation IPRs remain unExploited or not further developed by the Company in any Secondary Market or in respect of any Indication where such Indication is identified in the Application or by the CTSC, in each case within [**] years following completion of the Project, the Trust shall have the option in its sole discretion by giving written notice to the Company to become the Exploiting Party and take responsibility on behalf of the Company for the |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 41 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| commercialisation and Exploitation of such Exploitation IPRs in those countries or regions or in respect of that Indication as the case may be, which includes discretion to make any and all decisions regarding the negotiation, acceptance and conclusion of terms for any agreement regarding the commercial development and Exploitation of such unexploited Exploitation IPRs (including development and Exploitation by way of licence, sale, assignment, materials transfer or other transfer of rights, as well as any transaction which involves placing such unexploited Exploitation IPRs into a separate corporate vehicle) in such country and, if applicable, in such Indication. For clarity and without limitation, (i) if a further clinical trial is being undertaken or is pending anywhere in the World with a view to providing data that will enable applications for Marketing Approvals to be made in any of the Secondary Markets or (ii) applications for Marketing Approvals have been made within all of the countries or regions of the Secondary Markets, in either case in the timeline set forth in this Clause 16.2, the Company shall be considered to be fulfilling its further development and Exploitation obligations in the Secondary Markets. |
| 16.3 | If the Trust exercises its right to become the Exploiting Party and to exploit on behalf of the Company under Clause 16.1 or 16.2, the Company agrees that it shall pass to the Trust immediately any or all Exploitation opportunities in the applicable country and with respect to the applicable Indication that it becomes aware of from time to time in connection with the Exploitation IPRs, The Company further undertakes that it shall not engage in any activities (including in relation to the Background IPRs) that could reasonably lead to the loss of an Exploitation opportunity in the applicable country and with respect to the applicable Indication without the prior written consent of the Trust. |
| 16.4 | If the Trust exercises its right to become the Exploiting Party and to exploit on behalf of the Company under Clause 16, the Company will license or assign the Exploitation IPRs to the Trust or its nominee and provide the Trust with access to any associated data, documents, materials, regulatory approvals or information as required for the Trust to exploit such rights. At the Trust’s request, the Company shall grant to the Trust or its nominee a licence to the Background IPRs to the extent that they are required to exploit the Exploitation IPRs. Any such licence grant shall be non-exclusive and free of charge other than for reasonable costs that are incurred in respect of necessary Third-Party licences. |
| 16.5 | If the Trust exercises its right to Exploit under Clause 16, the Trust shall share Net Revenue received in respect of Exploitation of the Exploitation IPRs in accordance with the Financial Terms. |
| 16.6 | Notwithstanding anything to the contrary set forth in Clause 16.1 or 16.2, in the event that the Company licenses a third party to exploit the Exploitation IPRs (whether alone or |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 42 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| together with other IPRs of the Company) in any country in any indication then the Trust shall have no rights under Clause 16.1 or 16.2 with respect to such Exploitation IPRs in such countries or indications, provided that either (a) under a written agreement with the Company such licensee is required to use diligent efforts to exploit the licensed Exploitation IPRs in such country or indication, and such written agreement provides for a reversion to the Company of the Exploitation IPRs in such country or indication if the licensee materially breaches this diligence obligation; or (b) the Trust has approved such licence in writing (a decision on the granting of such approval to be provided by the Trust, as far as is reasonably practicable, within [**] Working Days). The Company shall use reasonable endeavours to keep the Trust informed of any proposed Exploitation transactions prior to presenting the final agreement for an Exploitation for approval of the Trust pursuant to this Clause 16.6. |
| 17. | REVENUE SHARING |
| 17.1 | Except where the Company has repaid the Repayment Amount pursuant to Clauses 3 and/or 13, as a condition of granting Exploitation consent pursuant to Clause 5 the Trust will require the Company to enter into the Revenue Sharing Agreement pursuant to which the Parties shall share cumulative Net Revenue received in respect of Exploitation of the Exploitation IPRs in accordance with the following financial terms (the “Financial Terms”): |
| 17.1.1 | Revenue Share as at Effective Date (i.e. where there has been no drawdown under the Agreement): |
| Cumulative Net Revenue (Pounds Sterling): |
Percentage of Net Revenue due to the Trust (excluding Taxes): |
|||
| Up to [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 43 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 17.1.2 | Revenue Share where the Company has drawdown the total Award Amount under the Agreement and the Company has made a Company Contribution of £[**] to Phase I and Phase II development of SMT19969: |
| Cumulative Net Revenue (Pounds Sterling): |
Percentage of Net Revenue due to the Trust (excluding Taxes): |
|||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
In addition to the percentage of cumulative Net Revenue due to the Trust set out at Clause 17.1.2 above, where the cumulative Net Revenue from the Exploitation of the Exploitation IPRs exceeds [**] pounds Sterling (£[**]) the Trust shall be entitled to a one-off Exploitation milestone payment of [**] Pounds Sterling (£[**]).
| 17.1.3 | Revenue Share where the Company has drawdown the total Award Amount under the Agreement and the Company has contributed at least £[**] to Phase I and Phase II development of SMT19969: |
| Cumulative Net Revenue (Pounds Sterling): |
Percentage of Net Revenue due to the Trust (excluding Taxes): |
|||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| [**] |
[** | ] | ||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX UK | 44 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
In addition to the percentage of cumulative Net Revenue due to the Trust set out at 17.1.3 above, where the cumulative Net Revenue from the Exploitation of the Exploitation IPRs exceeds [**] pounds Sterling (£[**]) the Trust shall be entitled to a one-off Exploitation milestone payment of [**] Pounds Sterling (£[**]).
| 17.2 | For clarity, all of the Net Revenue shares in all of the situations set forth in Clause 17.1 above are tiered. By way of example where the Company has drawdown the total Award Amount under the Agreement and the Company has contributed at least £[**] to Phase I and Phase II development of SMT19969 (see Clause 17.1.3) and the cumulative Net Revenue is GBP [**] (£[**]), the percentage of Net Revenue due to the Trust shall be [**] percent ([**]%) for the first GBP [**] (£[**]) and [**] percent ([**]%) for the next GBP [**] (£[**]) being a total sum of GBP [**] (£[**]). |
| 17.3 | If, following the end of the Project, the Company, the Trust or any Third Party contributes any funding to further develop any of the Exploitation IPRs (including general working capital of the Company used to fund such development), the Parties shall promptly negotiate in good faith modifications to the Financial Terms to reflect such further funding based on the change in the Parties’ respective proportion of the overall development cost of the Licensed Products. In the event that the Parties are unable to agree the share of Net Revenue due to the Trust and the Group, the matter shall be referred to an Expert pursuant to Clause 20. |
| 17.4 | If, during the Project or thereafter the Trust: |
contributes any funding in addition to the SDD Award and this Award; in order to enable the Company to achieve a particular Milestone or complete a relevant development phase, the share of Net Revenue due to the Trust shall be increased based on the change in the Parties’ respective proportions on the overall development cost of the Licensed Products so as to give the Trust an equitable share of such Net Revenue with regard to the contribution made by the Trust to the development of the Exploitation IPRs.
| 17.5 | Where any Net Revenue is received by the Exploiting Party as consideration for the grant of rights under the Exploitation IPRs in one or more Indications and other rights, then the consideration shall be apportioned by the Exploiting Party between, on the one hand, the Exploitation IPRs and on the other hand, any other rights granted, in such manner as is fair and reasonable. If the Parties are unable to agree on such apportionment, the matter shall be referred to an Expert pursuant to Clause 20. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 45 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 17.6 | Where Direct Costs incurred/allowed in a given accounting year exceed the Revenue from Exploitation of Exploitation IPRs (if any) for that year, then such excess costs shall be carried forward and offset against future Revenue until such time as they have been fully recovered. |
| 18. | ACCOUNTING STATEMENTS AND PAYMENTS |
| 18.1 | Within [**] days of the end of each Calendar Quarter, the Exploiting Party shall deliver a statement to the Non-Exploiting Party setting out for the relevant Calendar Quarter: |
| 18.1.1 | Revenue and Net Revenue received; |
| 18.1.2 | deductible Direct Costs and taxes; |
| 18.1.3 | sales of Licensed Products made by any member of the Exploiting Party’s Group or any Third Party; |
| 18.1.4 | cumulative Net Revenue, cumulative Revenue and cumulative Direct Costs; and |
| 18.1.5 | the share of Net Revenue due to the Non-Exploiting Party pursuant to Clause 17 above; |
(the “Quarterly Statement”).
| 18.2 | The Non-Exploiting Party shall deliver to the Exploiting Party an invoice for the amount due to it as set out in the Quarterly Statement in Pounds Sterling. |
| 18.3 | The share of Net Revenue due to the Non-Exploiting Party and any other amount invoiced shall be payable to the Non-Exploiting Party within [**] days of receipt of the invoice. |
| 18.4 | All payments of Net Revenue made by the Company to the Trust or by the Trust to the Company as the case may be under this Agreement shall be made in Pounds Sterling. Payment shall be made by electronic wire transfer of immediately available funds directly to the account of the relevant Party designated below or to any other account which the relevant Party may specify by written notice in accordance with Clause 23. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 46 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 18.5 | Bank Account for the Company: |
| Account Name: | [**] | |
| Account No.: | [**] | |
| Bank: | [**] | |
| Sort code: | [**] | |
| SWIFT code: | [**] | |
| Branch: | [**] |
| 18.6 | Bank Account for the Trust: |
| Account Name: | [**] | |
| Account No.: | [**] | |
| Bank: | [**] | |
| Sort code: | [**] | |
| SWIFT code: | [**] | |
| Branch: | [**] |
| 18.7 | Written confirmation of such transfer shall be sent by the Party sending the funds to the individual at the Party receiving the funds at the address provided in Clause 25. |
| 18.8 | Where any Revenue and Direct Costs in respect of the Exploitation IPRs is received or made in a currency other than sterling, the sterling equivalent of the sum shall be: |
| 18.8.1 | where such sum has been converted into sterling prior to preparation of the Quarterly Statement, the actual sterling sum on conversion; or |
| 18.8.2 | where such conversion has not taken place prior to preparation of the Quarterly Statement, calculated using the average of the buying and selling rates quoted by [**]. at the date the sum is received or paid by the Exploiting Party as applicable, or at such other date as the paying Party may reasonably specify having regard to the circumstances. |
| 18.9 | If the paying Party fails to pay any amount payable by it under this Agreement on the relevant due date, interest shall accrue on the overdue amount from the due date up to the date of actual payment (both before and after judgement) at the rate equivalent to [**] percent ([**]%) per annum above the three month sterling LIBOR from time to time. |
| 18.10 | All Net Revenue payments under this Agreement are expressed to be exclusive of VAT howsoever arising. Set out below are the VAT registration details for the Company and the Trust: |
| 18.10.1 | VAT registration details for the Company: 876331407 |
| 18.10.2 | VAT registration details for the Trust: 744495211 |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX UK | 47 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 18.11 | Each Party shall promptly inform the other Party in writing of any changes to the VAT registration details set out above. Neither Party shall charge VAT to the other Party on the supply of rights made or deemed to be made for VAT purposes pursuant to this Agreement provided however that if the VAT registration details provided are otherwise not acceptable to the competent authority, each Party may charge VAT appropriately as necessary in order to comply with that Party’s obligations under applicable law. In such event the paying Party shall pay the receiving Party, in addition to any payment due hereunder, all VAT for which the receiving Party is liable to account to any competent authority in relation to any supply made or deemed to be made for VAT purposes pursuant to this Agreement. The paying Party shall pay any payments due to the receiving Party at the same time as the relevant payment is due under this Agreement. |
| 18.12 | If any paying Party is required by law to make any withholding or similar Tax payment on behalf of the receiving Party, with respect to any of the payments to be made to the receiving Party under this Agreement, the paying Party shall pay to the receiving Party such amount as shall after the deduction of any withholding tax, result in the receiving Party receiving such amount as it would have been entitled to had no withholding been made. |
| 18.13 | The Exploiting Party shall keep such records as are reasonably necessary to enable a proper assessment to be made of the following for at least [**] years: |
| 18.13.1 | the sums payable under this Agreement; |
| 18.13.2 | Revenue and Net Revenue received; |
| 18.13.3 | deductible Direct Costs and taxes on the Exploitation IPRs; and |
| 18.13.4 | sales of Licensed Products made by any member of the Exploiting Party’s Group or any Third Party; |
| 18.13.5 | cumulative Revenue, cumulative Direct Costs and cumulative Net Revenue. |
(the “Records”).
| 18.14 | The Exploiting Party shall allow an independent accountant duly authorised on behalf of and at the expense of the Non-Exploiting Party to inspect the Records by prior written appointment during normal business hours and not more than [**]. Such accountant shall not |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 48 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| disclose to any Third Party or use for any unauthorised purpose any information not relevant to the verification of the sums due to the Non-Exploiting Party that is obtained as a result of any such inspection. The Exploiting Party shall procure that these inspection and audit rights extend to the records of the Exploiting Party’s Group and any sub-licensees thereof. |
| 18.15 | The Party arranging for the audit shall pay for the audit as well as its own legal expenses associated with enforcing its rights with respect to any payments due under this Agreement except where the audit reveals a discrepancy of [**] percent ([**]%) or more of any sums paid or payable, in which case the costs of the audit shall be paid by the audited Party. |
| 19. | FURTHER FUNDING |
| 19.1 | Save as provided in Clause 17, the Company undertakes that it will not, without the prior written consent of the Trust (such consent not to be unreasonably withheld, delayed or conditioned), accept any further funding to complete the Phase II trial of SMT 19969 by way of loan, grant or other funding the conditions of which would materially prejudice the Trust’s position under this Agreement with respect to its share of Net Revenues or its rights to become the Exploiting Party set forth in Clause 16. For clarity the Company is not restricted from raising general working capital. |
| 20. | DISPUTE RESOLUTION |
| 20.1 | Any question, difference or dispute which may arise concerning the construction meaning or effect of this Agreement or concerning the rights and liabilities of the Parties hereunder or any other matter arising out of or in connection with this Agreement shall first be submitted to the senior officers of the Parties set out below (or their nominees): |
| 20.1.1 | In the case of the Trust, the Director of the Technology Transfer Division; and |
| 20.1.2 | In the case of the Company, Xx Xxxx Xxxxxxx, Chief Executive Officer |
(together, the “Senior Officers”).
| 20.2 | The Senior Officers may call on others to advise them as they see fit. If the Senior Officers are unable to resolve the dispute within [**] days of the date on which the matter is referred to them, the dispute may be referred by one of the Parties for resolution by an Expert pursuant to the procedure set out below. |
| 20.3 | Any expert (the “Expert”) appointed to resolve any matter arising under this Agreement shall be an independent expert whose appointment is agreed between the Parties. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 49 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 20.4 | If the Parties are unable to agree on an Expert within [**] Business Days of either Party serving notice that it wishes to seek an expert determination, then the expert shall be (i) in the event of a determination of Fair Value, an accountant nominated at the request of either Party by the President for the time being of the Institute of Chartered Accountants in England and Wales or (ii) in the case of determination as to whether or not a Milestone has been achieved, an experienced independent consultant drug development professional nominated at the request of the Parties by the Chairman of the BioIndustry Association or (iii) in all other cases an independent experienced arbitrator nominated at the request of the Parties by the President of the Chartered Institute of Arbitrators in England. |
| 20.5 | The Expert shall be required to deliver a notice setting out their determination within [**] Business Days of her appointment. The Expert shall adopt a valuation method which they consider, in their absolute discretion, to be the most appropriate method for the matter upon which determination is required. |
| 20.6 | The Parties shall be entitled to make submissions to the Expert and shall provide (or procure that others provide) the Expert with such assistance and documents as they shall reasonably require for the purposes of making their determination. |
| 20.7 | The Parties shall provide each other with such reasonable information concerning the affairs of the Company as will enable them to make submissions under Clause 20.6. |
| 20.8 | The Expert shall act as an expert and not as an arbitrator and their written opinion on the matters referred to them shall, save for manifest error, be final and binding. |
| 20.9 | The costs of any reference to an Expert under Clause 20 shall be borne by the Parties equally unless the Expert shall decide otherwise in which case the costs shall be borne by the Parties in the proportions indicated by the Expert. |
| 20.10 | If the Expert dies or becomes unwilling or incapable of acting, or does not deliver the decision within the time required by this Clause 20 then: |
| 20.10.1 | either Party may apply to the president of the relevant body (as set forth in Clause 20.4 above) to discharge the Expert and to appoint a replacement Expert with the required expertise; and |
| 20.10.2 | this Clause 20 shall apply in relation to the new Expert as if he were the first Expert appointed. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 50 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 20.11 | The Expert shall determine any dispute, which may include any issue involving the interpretation of any provision of this Agreement, his jurisdiction to determine the matters and issues referred to him or his terms of reference. |
| 20.12 | If the Expert resolution procedure set out above should fail to resolve the question, difference or dispute the Parties agree to try in good faith to settle the matter by mediation in accordance with the Centre for Effective Dispute Resolution (“CEDR”) Model Mediation Procedure prior to any reference of the matter to the courts of England. Unless otherwise agreed by the Parties, the mediator will be nominated by CEDR. Any mediation under this Clause 19 shall take place in London, England. |
| 20.13 | If the mediation under Clause 20.12 should fail to resolve the question, difference or dispute within [**] days of commencement of the discussions under Clause 20, the Parties shall submit to the exclusive jurisdiction of the English courts. |
| 20.14 | Nothing in this Clause 20 shall prevent a Party from seeking injunctive relief in any court of competent jurisdiction for any reason including but not limited to in respect of a breach or threatened breach of Clause 24 (Confidentiality). |
| 21. | WAIVER |
No Party shall be deemed to have waived any of its rights or remedies under this Agreement unless the waiver is expressly made in writing and signed by a duly authorised representative of that Party. In particular, no delay or failure of any Party in exercising or enforcing any of its rights or remedies under this Agreement shall operate as a waiver of those rights or remedies nor shall any single or partial exercise or enforcement of any right or remedy by any Party preclude or impair any other exercise or enforcement of that right or remedy by that Party.
| 22. | ENTIRE AGREEMENT/VARIATIONS |
| 22.1 | This Agreement, together with the Application and any agreement between the Parties entered into pursuant to the Agreement constitutes the entire agreement and understanding between the Parties relating to the subject matter hereof and together they supersede and replace all prior drafts, previous understandings, arrangements, representations or agreements, whether in writing or oral, between the Parties relating to the subject matter of this Agreement. |
| 22.2 | That Parties agree that the terms and condition of this Agreement shall govern the relationship between the two Parties and that, except for Clause 7.3 (in respect of audit of any expenditure of the Award Amount) and Clause 21 of the SDD Funding Agreement, the SDD Funding Agreement and the revenue sharing agreement that formed part of the SDD Funding Agreement are hereby terminated. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 51 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 22.3 | No variation, amendments, modification or supplement to this Agreement shall be valid unless and until it is made in writing and signed by a duly authorised representative of each Party. |
| 23. | ANNOUNCEMENTS |
| 23.1 | Subject to ensuring it does not put the Company in breach of its obligations to the London Stock Exchange regarding price sensitive information, (which for clarity means the Trust must have the agreement of the Company before releasing the following statement) the Trust may publish summary details of the Project including the name of the Principal Scientific Contact, the name of the Company, the title of the Project, the Award Amount and the following description of the Project: |
“First in Human clinical trials for SMT19969: A novel antibiotic for the treatment of Clostridium difficile infection.
Hospital acquired bacterial infections continue to be a significant burden to the healthcare system and to patient welfare due to ever increasing rates of antibiotic resistance and the rise in prevalence of emerging and hard to treat infections. One of the most important of these, Clostridium difficile, is a typically harmless bacteria that under certain conditions can cause a life-threatening infection of the colon. In particular, C. difficile infection (CDI) is associated with antibiotic use, which can cause an imbalance in the healthy bacterial population of the gut resulting in an overgrowth of C. difficile.
There are estimated to be around 900,000 cases of CDI each year across North America and the EU and the infection now accounts for >80% of deaths due to gastroenteritis. Of particular concern are outbreaks due to hyper-virulent strains of the bacteria that are responsible for more severe forms of the disease. Antibiotic choices for CDI are limited and of sub-optimal efficacy with up to 30 per cent of patients suffering at least one recurrence of the infection. Each recurrence tends to be more severe and is associated with increased risk of further infection. Combatting recurrent disease remains the central issue in achieving effective therapy for this life threatening infection.
SMT19969 is a novel antibiotic being developed by Summit Corporation PLC for the specific treatment of CDI. SMT19969 shows high levels of selectivity for C. difficile
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 52 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
whilst having minimal effect on the normal healthy gut bacteria, which is expected to result in a significant healthcare benefit by reducing rates of recurrent disease. Preclinical development by a team led by Xx Xxxxxxx Xxxxxxx was funded by a Wellcome Trust Seeding Drug Discovery award. With continuing support via a Translation Award to Summit Corporation PLC the company is now undertaking Phase I first-in-man safety studies and Phase II efficacy trials.”
| 23.2 | Except as provided in Clause 23.1, required by law or any competent regulatory authority, no announcement concerning this Agreement or its subject matter shall be made by a Party without the prior written approval of the other Party. For clarity the requirements of law include the rules of the London Stock Exchange and the Company shall make such statements regarding this Agreement and/or its subject matter as are necessary to comply with the rules of the London Stock Exchange. |
| 23.3 | The Trust’s contribution must be acknowledged in all scientific publications concerning the Project, quoting the Award reference number. |
| 23.4 | A copy of the final manuscript of all research publications that relate to the Project must be deposited into PubMed Central (or UK PubMed Central) upon acceptance for publication, to be made freely available as soon as possible and in any event no later than [**] months after the journal publisher’s official date of final publication. |
| 24. | CONFIDENTIALITY |
| 24.1 | The Parties shall keep confidential and ensure that their respective Connected Persons, and their respective officers, employees, consultants, agents and professional and other advisers shall keep confidential any of the following information save that nothing herein shall prevent the Company using any information relating to the Project for its business purposes including the development of SMT 19969, any Exploitation and including obtaining any further funding: |
| 24.1.1 | relating to the customers, business, assets or affairs of the Company; |
| 24.1.2 | relating to the customers, business, assets or affairs of the Trust; or |
| 24.1.3 | which relates to the Project or the contents of this Agreement or any agreement or arrangement entered into pursuant to this Agreement |
(the “Confidential Information”).
| 24.2 | Save as set out below, neither Party may use for its own business purposes or disclose to any third party any Confidential Information of the other Party without the prior consent of the Party to whom the Confidential Information relates. Confidential Information does not include: |
| 24.2.1 | information which is or becomes publicly available (otherwise than as a result of a breach of this Agreement or any other agreement between the Parties); |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 53 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 24.2.2 | information which is independently developed by the relevant Party or acquired from a third party, to the extent that it is acquired with the right to disclose it; |
| 24.2.3 | information which was lawfully in the possession of the relevant Party prior to or on the Effective Date, free of any restriction on disclosure as can be shown by that Party’s written records or other reasonable evidence; |
| 24.2.4 | the disclosure of information to the extent required to be disclosed by law, including any requirements for disclosure under the Freedom of Information Act 2000 or any court of competent jurisdiction, any governmental official, any tax or regulatory authority (including any Recognised Investment Exchange and the Panel on Takeovers and Mergers) or any binding judgement, order or requirement of any other competent authority; |
| 24.2.5 | the disclosure to a Party’s professional advisers or to the Trust’s Site Visit Group of information reasonably required to be disclosed for purposes relating to this Agreement; |
| 24.2.6 | any announcement made, or information provided in relation to the Company with the approval of the Trust in accordance with Clause 23; and |
| 24.2.7 | the disclosure of information by the Trust for the purposes of publishing summary details of awards made by the Trust including the name of the Company, the name of the Principal Scientific Contact, the title of the Project and the amount of the Award Amount and (in the event that the Project includes a clinical trial) for the purpose of registering a clinical trial on the Trust’s clinical trial register. |
| 24.3 | Each Party shall inform any officer, employee, consultant or agent or any professional or other adviser advising it in relation to matters relating to this Agreement, or to whom it provides Confidential Information, that such information is confidential and shall instruct them: |
| 24.3.1 | to keep it confidential; and |
| 24.3.2 | not to disclose it to any third party (other than those persons to whom it has already been or may be disclosed in accordance with the terms of this Agreement), |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX UK | 54 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
provided that the disclosing Party shall remain responsible for any breach of Clause 23 by the person to whom that Confidential Information is disclosed.
| 24.4 | Without prejudice to any other rights or remedies which a Party may have, the Parties acknowledge and agree that damages would not be an adequate remedy for any breach of Clause 24 and the remedies of injunction, specific performance and other equitable relief are appropriate for any threatened or actual breach of any such provision. |
| 25. | NOTICES |
| 25.1 | Any notice to be given pursuant to this Agreement shall be in writing in the English language and shall be delivered by overnight courier, by registered, recorded delivery or certified mail (postage prepaid) or by facsimile confirmed by registered, recorded delivery or certified mail (postage prepaid) to the address or facsimile number of the recipient Party set out below or such other address or facsimile number as a Party may from time to time designate by written notice to the other Parties. Any notice by facsimile shall be confirmed by the sender sending a confirmatory copy of the notice by registered, recorded delivery or certified mail (postage prepaid). |
| 25.2 | Address of Company |
Summit plc
00 Xxxxxx Xxxx
Abingdon
Oxfordshire
OX14 4RY
| Fax No: | 00000 000000 | |
| For the attention of: | The Company Secretary (currently Xxxxxxx Xxxxxxx) | |
| 25.3 | Address of the Trust |
Technology Transfer Division
The Wellcome Trust Limited,
000 Xxxxxx Xxxx
London NW1 2BE
| Fax No: | x00 (0) 00 0000 0000 | |
| For the attention of: | The Contracts Officer | |
| with a copy to: | [**] | |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 55 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 25.4 | Any notice given pursuant to Clause 25.1 shall be deemed to have been received: |
| 25.4.1 | in the case of delivery by courier or sending by certified mail, on the day of receipt, provided receipt occurs on a Business Day or otherwise on the next following Business Day; or |
| 25.4.2 | in the case of facsimile, on acknowledgement by the recipient facsimile receiving equipment on a Business Day if the acknowledgement occurs before 5:00 pm local time of the recipient Party and in any other case on the following Business Day. |
| 25.5 | Any notice that is required in this Agreement may be validly given if transmitted by fax or sent by post in accordance with Clause 25.1. For the avoidance of doubt, email is not a valid method of giving notice under this Agreement. |
| 26. | ASSIGNMENT |
Save as set forth herein, no Party shall without the prior written consent of the other Parties assign, transfer, convey or declare a trust over this Agreement or make any other disposition (whether in whole or in part) of any of its rights and obligations hereunder to any third party. The aforementioned consent of the other Party shall not be unreasonably withheld, delayed or conditioned save that the Company acknowledges that in the case of an assignment by it at any time prior to the payment of the full Award Amount by the Trust, the Trust will have to undertake due diligence on the intended assignee. The Company may assign the benefit and burden of this agreement to any of its Group companies without the prior written consent of the Trust provided the assignee agrees with the Trust to be bound by the terms of this Agreement.
| 27. | SEVERANCE OF TERMS |
| 27.1 | If the whole or any part of this Agreement is or becomes or is declared illegal, invalid or unenforceable in any jurisdiction for any reason (including both by reason of the provisions of any legislation and also by reason of any court or competent authority which either has jurisdiction over this Agreement or has jurisdiction over any of the Parties): |
| 27.1.1 | in the case of the illegality, invalidity or un-enforceability of the whole of this Agreement it shall terminate only in relation to the jurisdiction in question; or |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 56 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 27.1.2 | in the case of the illegality, invalidity or un-enforceability of part of this Agreement that part shall be severed from this Agreement in the jurisdiction in question and that illegality, invalidity or un-enforceability shall not in any way whatsoever prejudice or affect the remaining parts of this Agreement, which shall continue in full force and effect. |
| 27.2 | If in the reasonable opinion of any Party any severance under Clause 16 materially affects the commercial basis of this Agreement, the Parties shall discuss, in good faith, ways to eliminate the material effect. |
| 28. | COSTS |
Each Party shall bear its own legal costs, legal fees and other expenses incurred in the preparation and execution of this Agreement.
| 29. | FURTHER ASSURANCES |
Each Party shall perform such acts and execute such documents as may be reasonably required for securing to or vesting in another Party the rights agreed to be granted to it under or pursuant to this Agreement.
| 30. | GENERAL |
| 30.1 | If any provisions of the Memorandum or Articles of the Company at any time conflict with any of the provisions of this Agreement, the provisions of this Agreement shall prevail. |
| 30.2 | Nothing in this Agreement shall be taken to constitute a partnership between the Parties. Except as specifically provided in this Agreement, none of the Parties shall by reason of this Agreement be empowered to act as agent for any other party nor to pledge the credit of any other party nor shall any Party be held liable for or incur liability in respect of the acts or defaults of any other Party to this Agreement. |
| 30.3 | This Agreement may be executed in any number of counterparts and by the Parties on separate counterparts, but shall not be effective until each Party has executed at least one counterpart. Each counterpart shall constitute an original of this Agreement, but all the counterparts shall together constitute one and the same instrument. |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 57 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| 30.4 | A person who is not a Party has no right under the Contracts (Rights of Third Parties) Act 1999 to enforce or to enjoy the benefit of any term of this Agreement. |
| 31. | GOVERNING LAW |
This Agreement (and any dispute, controversy, proceedings or claim of whatever nature arising out of this Agreement or its formation) shall be governed by and construed in accordance with the laws of England.
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 58 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
IN WITNESS of which this Agreement has been signed as follows:
| Signed for and on behalf of | ) | |||||
| THE WELLCOME TRUST | ) | /s/ Xx. Xxxxxx Xxxxx | ||||
| LIMITED as trustee of | ) | Authorised Signatory | ||||
| The Wellcome Trust by its | ) | Name: | Xx. Xxxxxx Xxxxx | |||
| Authorised signatory(ies) |
) | Position: | Head of Legal & Operations, Technology Transfer | |||
| ) | Date: | 30th October 2012 | ||||
| ) | ||||||
| Signed for and on behalf of | ) | |||||
| SUMMIT CORPORATION | ) | |||||
| PLC | ) | /s/ Xxxx Xxxxxxx | ||||
| ) | Name: | Xxxx Xxxxxxx | ||||
| ) | Position: | Chief Executive Officer | ||||
| ) | Date: | 19/10/2012 | ||||
| ) | ||||||
| ) | /s/ XX Xxxxxxx | |||||
| ) | Name: | XX Xxxxxxx | ||||
| ) | Position: | Chief Financial Officer | ||||
| ) | Date: | 19/10/2012 | ||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 59 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
SCHEDULE 1
Drawdown Notice
Summit Corporation plc
00 Xxxxxx Xxxx
Abingdon
Oxfordshire
OX14 4RY
The Contracts Officer
Technology Transfer Division
The Wellcome Trust Limited
000 Xxxxxx Xxxx
London NW1 2BE
[Date]
Dear Sirs
The Funding Agreement made on [ ] 2012 between Summit Corporation plc and The Wellcome Trust Limited (the “Award Agreement”)
We hereby give you irrevocable notice that, pursuant to Clause 2.5 of the Award Agreement we wish to drawdown [—] Pounds Sterling (£[—]) of the Award Amount upon the terms and subject to the conditions of the Award Agreement.
We confirm that each condition specified in Clause 2.10 is satisfied on the date of the proposed drawdown and enclose an amended Disclosure Letter.
Terms and expressions defined in the Award Agreement shall have the same meanings in this Letter.
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX UK | 60 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
Yours faithfully
|
|
| For and on behalf of |
| Summit Corporation plc |
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 61 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
SCHEDULE 2
Drawdown Notice
Summit Corporation plc
00 Xxxxxx Xxxx
Abingdon
Oxfordshire
OX14 4RY
The Contracts Officer
Technology Transfer Division
The Wellcome Trust Limited
000 Xxxxxx Xxxx
London NW1 2BE
[Date]
Dear Sirs
The Funding Agreement made on [ ] 2012 between Summit Corporation plc and The Wellcome Trust Limited (the “Award Agreement”)
We refer to Milestone [number] as described in the Award Letter and hereby confirm the completion of the achievement of such Milestone. A report detailing achievement of Milestone [number] [is attached to this letter]/[has been provided to the Trust]. Please confirm that Milestone [number] has been achieved to your reasonable satisfaction and that we may proceed to drawdown [—] Pounds Sterling (£[—]) in respect of the [number] tranche of the Award Amount.
Subject to receipt of your confirmation that we may proceed to drawdown the next tranche of the Award Amount, we hereby give you irrevocable notice that, pursuant to the Funding Agreement we wish to draw down [—] Pounds Sterling (£[—]) of the Award Amount upon the terms and subject to the conditions of the Funding Agreement.
We confirm that each condition specified in Clause 2.10 is satisfied on the date of the proposed drawdown and enclose an amended Disclosure Letter.
Terms and expressions defined in the Award Agreement shall have the same meanings in this Letter.
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 62 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
Yours faithfully
|
| ||
For and on behalf of
Summit Corporation plc
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 63 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
SCHEDULE 3
DETAILS OF SUMMIT CORPORATION PLC
| (1) | Company Number: | 05197494 | ||||||||||
| (2) | Date and Place of Incorporation: | 04/08/2004, England and Wales | ||||||||||
| (3) | Share Capital: | |||||||||||
| (i) Authorised: |
- No upper limit | |||||||||||
| (ii) Issued: |
- 354,088,450 new Ordinary shares of 1p each | |||||||||||
| - 524,702,133 deferred shares of 1p each | ||||||||||||
| (4) | Registered Office: | 00 Xxxxxx Xxxx | ||||||||||
| Abingdon | ||||||||||||
| Oxfordshire | ||||||||||||
| OX14 4RY | ||||||||||||
| (5) | Directors: | Xxxxx Xxxxx PhD | ||||||||||
| Xxxx Xxxxxxx MBE | ||||||||||||
| Professor Xxxxxxx Xxxxxx MA, X.Xxxx | ||||||||||||
| Xxxxxx Xxxxxxx XX, CA | ||||||||||||
| Xxxxxx Xxxxxxxx PhD | ||||||||||||
| Xxxxxxx Xxxxxx DPhil | ||||||||||||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 64 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
(6) Secretary: Xxxxxxx Xxxxxxx ACA
(7) Accounting Reference Date: 31/01
(8) Stock Exchange: LSE (AIM)
(9) Symbol: SUMM
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 65 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
SCHEDULE 4A
THE APPLICATION
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 66 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
TA 02/12
FULL APPLICATION FOR A TRANSLATION AWARD
PLEASE READ THE ACCOMPANYING TRANSLATION AWARD NOTES FOR GUIDANCE IN CONJUNCTION WITH THIS APPLICATION FORM.
Non-company applicants:
PLEASE READ THE TRUST’S GRANT CONDITIONS IN CONJUNCTION WITH THIS APPLICATION FORM (Wellcome Trust Grant Conditions).
Company applicants:
PLEASE READ THE TRUST’S CONVERTIBLE LOAN AGREEMENT IN CONJUNCTION WITH THIS APPLICATION FORM (Convertible loan agreement).
If you have any questions or comments about the completion of this form, including submission of the final version, please contact Technology Transfer.
| Tel: | (0)00 0000 0000 | |
| Fax: | (0)00 0000 0000 | |
| E-mail: | xxxxxxxxxxxx@xxxxxxxx.xx.xx |
Macintosh users can save this document in .rtf format before completing the application form.
Applicants experiencing any technical difficulties with the form should call: x00 (0)00 0000 0000.
Index to sections of the form:
Undertakings
Front page
Contact details
Science
Curriculum Vitae
Financial details
Justification of costs requested
Administration
Subject classification
Collaboration form
Equal opportunities monitoring form
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 67 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX
| TA 09/11
FULL APPLICATION FOR |

| |
|
A Translation Award |
||
| XXXXX BUILDING 000 XXXXXX XXXX XXXXXX XX0 0XX XX | 68 |
T x00 (0)00 0000 0000 F x00 (0)00 0000 0000 XXX.XXXXXXXX.XX.XX
REGISTERED CHARITY NO. 210183 TRUSTEE: THE WELLCOME TRUST LIMITED REGISTERED IN ENGLAND NO. 2711000 REGISTERED OFFICE: 000 XXXXXX XXXX XXXXXX XX0 0XX

APPLICATION FOR A TRANSLATION AWARD
WELLCOME TRUST DATA PROTECTION STATEMENT
| 1. | This statement is a “fair processing notice” under the Data Protection Act 1998 (the “Act”) and sets out what the Wellcome Trust will do with the information that it collects from you during the grant/award application process and throughout the life of the grant/award (including all information relating to the grant/award application and, as applicable, any subsequent grant/award made). |
| 2. | Information (including “personal data” as defined under the Act) that you supply to the Wellcome Trust, including in any application and progress or update report, may be used by the Wellcome Trust to process applications and administer grants/awards, for the purposes of audit and evaluation and to monitor the fairness of and trends in application decisions. Your information may be disclosed for these purposes only to individuals and organisations connected with the Wellcome Trust, including funding partners, external peer reviewers and external committee members. Your information may also be shared with selected third parties for the purpose of independent audit, evaluation and assessment of activities funded by the grant/award and their outputs and outcomes. Some of these third parties may be based outside the European Economic Area. All personal data will be stored and used by or on behalf of the Wellcome Trust in accordance with the Act. |
| 3. | The Wellcome Trust may anonymise your personal data and use it for research and statistical purposes. |
| 4. | The Wellcome Trust may publish details of successful grants/awards and their outputs, including your name, employing organisation, project title, a summary of the grant/award and its value and, in the case of grants/awards funding research, scientific/academic abstracts and lay summaries of research (e.g. via the internet or via publicly accessible databases or other publications, some of which may be accessible from outside the EEA). |
| 5. | The Wellcome Trust may contact you about its activities and events, or to help inform or evaluate these activities and events, Wellcome Trust application processes and policy work. |
| 6. | The Wellcome Trust also has a more general Privacy Statement on its website at xxx.xxxxxxxx.xx.xx. Please contact the Wellcome Trust Data Protection Compliance Officer by email at xxxxxxxxxxxxxx@xxxxxxxx.xx.xx or by post at The Wellcome Trust, 000 Xxxxxx Xxxx, Xxxxxx XX0 0XX if you have any queries about the use of your personal data. |
| 7. | The Wellcome Trust may amend this Statement from time to time. Any material changes in how the Wellcome Trust collects, uses or shares your personal data will be posted on the Wellcome Trust website at xxx.xxxxxxxx.xx.xx. |
UNDERTAKINGS
| 1. | I confirm that I (and all those providing personal information in the application) have read and understood the Wellcome Trust Data Protection statement above. |
| 2. | To the best of my knowledge, the information provided in this application is accurate and complete and I agree to inform the Wellcome Trust of any material changes to this information during the period of the grant/award. |
| 3. | I have read the conditions under which grants/awards are made and agree to abide by the conditions should a grant/award be made. |
| 4. | The necessary facilities will be made available to conduct the research/activities funded by the Wellcome Trust’s grant/award, and will continue to be available for the duration of the grant/award. |
| Signature of Principal Applicant |
|
Date: |
| |||||
| Signature of Coapplicant (1) |
|
Date: |
| |||||


| Signature of Coapplicant (2) |
|
Date: |
| |||||
| Signature of Coapplicant (3) |
|
Date: |
| |||||
| FRONT PAGE | 70 |

| Signature of Head Technology Transfer Office/Group or Business Officer |
|
Date: |
| |||||
| Signature of Chief Scientific Officer |
|
Date: |
| |||||
|
For and on behalf of the Institution:
|
||||||||
| Signature of Secretary of Institution/Finance Officer/Company Official: |
|
Date: |
| |||||
| Position: | CEO | Company/ Institution: | Summit PLC | |||||
| Q1 Applicants |
Principal Applicant |
Coapplicant (1) | ||
| Surname | Xxxxxxx | |||
| Forenames | Xxxxxxx Xxxx | |||
| Title (Dr etc.) | Dr |
| Coapplicant (2) |
Coapplicant (3) |
Technology Transfer Officer | ||||
| Surname | ||||||
| Forenames | ||||||
| Title (Dr etc.) |
| Q2 | Title of project: (no more than 220 characters) |
| A First-in-Human Phase I Clinical Trial for the Novel Clostridium difficile Antibiotic SMT19969 |
| Q3 | Company name and address or department name and address at administering institution: |
| Summit PLC, 00 Xxxxxx Xxxx, Xxxxxxxx, Xxxxxxxxxxx, XX00 0XX |
| Q4 | Type of Translation Award requested:
(Strategic Awards by prior agreement only) |
TRANSLATION AWARD | ||
| Q5 | Period for which support is sought: (state in months) | 12 | ||
| Q6 | Proposed start date: (dd/mm/yy) | 06/08/2012 | ||
| FRONT PAGE | 71 |
TA 02/12
Principal Applicant
| Name | Xxxxxxx Xxxxxxx | Telephone numbers: | ||||
| Contact address | 00 Xxxxxx Xxxx Xxxxxxxx Xxxxxxxxxxx XX00 0XX |
Day | [**] | |||
| Mobile | [**] | |||||
| Fax. | [**] | |||||
| [**] | ||||||
Coapplicant (1)
| Name | Telephone numbers: | |||||
| Contact address | Day | |||||
| Mobile | ||||||
| Fax. | ||||||
Coapplicant (2)
| Name | Telephone numbers: | |||||
| Contact address | Day | |||||
| Mobile | ||||||
| Fax. | ||||||
Coapplicant (3)
| Name | Telephone numbers: | |||||
| Contact address | Day | |||||
| Mobile | ||||||
| Fax. | ||||||
| CONTACT DETAILS | 72 |
TA 02/12
Technology Transfer Officer
| Name | Telephone numbers: | |||||
| Contact address | Day | |||||
| Mobile | ||||||
| Fax. | ||||||
| CONTACT DETAILS | 73 |
TA 02/12
If a company please provide the following:
| Company number: |
05197494 | |
| Date and place of incorporation: |
England and Wales 04/08/2004 | |
| Shared capital: |
354,088,450 | |
| Authorised: |
N/A | |
| Issued: |
354,088,450 | |
| Registered holders (name, number and type): |
Publically listed company – multiple shareholders | |
| Registered office: |
00 Xxxxxx Xxxx Xxxxxxxx Xxxxxxxxxxx XX00 0XX | |
| Directors: |
Dr Xxxxx Xxxxx – Non-executive Chairman Xx Xxxx Xxxxxxx - CEO Xx Xxxxxxx Xxxxxx - CSO Prof Xxxxx Xxxxxx - XXX Dr Xxxxxx Xxxxxxxx – XXX Mr Xxxxxx Xxxxxx - XXX | |
| Secretary: |
Xxxxxxx Xxxxxxx - CFO | |
| Accounting reference date: |
31st January | |
| Previous source of funding and amount: |
Public Markets IPO - £15mill Secondary placings - £10.45mill (2006); £5.4mill (2009); £1.35mill (2011); £5.0mill (2012) | |
| Cash in bank and other investments: |
£2.0mill. Year end 31/1/2012 | |
| Average monthly expenditure: |
£275K | |
| Board of Directors: |
Dr Xxxxx Xxxxx – Non-executive Chairman Xx Xxxx Xxxxxxx - CEO Xx Xxxxxxx Xxxxxx - CSO Prof Xxxxx Xxxxxx - XXX Dr Xxxxxx Xxxxxxxx – XXX Mr Xxxxxx Xxxxxx - XXX | |
| Scientific advisory Board: |
N/A – independent advisors engaged for relevant projects | |
| Number of Employees: |
31 | |
| SCIENCE | 74 |
TA 02/12
| SCIENCE | 75 |
TA 02/12
Please enclose a copy of the current Business Plan Executive Summary with application.
Summit is an Oxford (UK) based drug discovery Company with an innovative Seglin™ technology platform for the discovery of new medicines and a portfolio of drug programme assets. Summit’s programme portfolio consists of a number of drug programmes targeting high-value areas of unmet medical need, including rare/orphan diseases and infectious disease. Summit is listed on the AIM market of the London Stock Exchange and trades under the ticker symbol SUMM. Further information is available at xxx.xxxxxxxxx.xxx.
Summit’s value proposition stems from four key value drivers: (i) SMT C1100, a first-in-class, potentially disease-modifying, small molecule utrophin upregulator for Duchenne Muscular Dystrophy; (ii) SMT 19969, a novel, potentially front-line, small molecule antibiotic for Clostridium difficile infection; (iii) OGA Inhibitors as potentially disease-modifying therapies for Alzheimer’s Disease and related Tauopathies and (iv) Seglin™ Technology - an innovative drug discovery platform that aims to prosecute new targets emerging from advances in the understanding of the role played by carbohydrate recognition and processing in disease pathogenesis.
Summit’s strategy is to monetise its small molecule assets at key value inflexion points, up to and following pivotal, proof-of-concept clinical trials, thereby mitigating the risk and costs of large-scale, late-stage registration trials and providing a favourable return on investment through securing of pre-marketing payments (up-fronts, development and regulatory milestone payments) in addition to sales milestones and royalties on product sales.
(i) SMT C1100: A Utrophin Upregulator for Duchenne Muscular Dystrophy (DMD).
DMD is an X-linked, fatal, progressive neuromuscular disease affecting one in 3,500 male births, with an estimated prevalence of 50,000+ patients in the seven major markets. DMD is caused by mutations which adversely affect the expression of dystrophin, a key musculoskeletal protein. Based on the orphan pricing model, a disease-modifying therapy for all DMD patients would be expected to achieve annual revenues in excess of $1bn.
Utrophin is a naturally occurring protein that has a similar function to dystrophin. Utrophin is produced during foetal development and in regenerating muscle fibres but downregulated in adult muscle. Maintaining the expression of utrophin has been shown to be able to function in place of the aberrantly regulated dystrophin to maintain the healthy function of muscles in animal models of DMD. Utrophin upregulation will be beneficial to all DMD patients regardless of their specific genetic mutation and is also expected to be complimentary to other therapeutic approaches in development. This approach is based on the ground-breaking work of Summit Co-founder, Dame Xxxx. Xxx Xxxxxx, FRS.
Discovered and developed by Summit scientists, SMT C1100 has demonstrated exciting potential as a disease-modifying drug in non-clinical efficacy studies. SMT C1100 disengages normal utrophin control such that utrophin RNA and protein is expressed continually in muscle. It has received orphan drug designation in the US and Europe. In December 2011 Summit signed a $1.5 million funding agreement with a group of US-based DMD organisations to support the development of SMT C1100. Summit intends to commence a randomised, double-blind, placebo-controlled Phase 1 study to investigate safety, tolerability and pharmacokinetic of single and multiple oral escalating doses in healthy volunteers in Q2 2012 with top-line results expected in Q3 2012.
(ii) SMT 19969: A Novel, Front-line Antibiotic for C. difficile Infection.
Clostridium difficile infection (‘CDI’) is a significant healthcare issue in hospitals, long-term care homes and there is growing concern about its spread to the wider community. It is a serious illness caused by infection of the inner lining of the colon by the C. difficile bacteria, which produces toxins that cause inflammation of the colon, severe diarrhea and, in the most serious cases, death. Patients typically develop CDI following the use of broad-spectrum antibiotics (e.g. cephalosporins and fluoroquinolones) that disrupt the normal gastrointestinal (gut) flora and so allow C. difficile bacteria to flourish. Broad spectrum antibiotics are associated with recurrent disease which represents the major clinical issue in treating CDI because repeat episodes of infection are often more severe. The severity of the disease is also increasing due to infection from hyper-virulent strains such as BI/NAP1/027. The limited treatment options currently available are failing to address these clinical challenges.
SMT 19969 is a small molecule, novel antibiotic that is being developed for the treatment of CDI. Results from in vivo and in vitro non-clinical efficacy studies have shown that SMT 19969 has a superior profile
| SCIENCE | 76 |
TA 02/12
compared to antibiotics that are currently on the market to treat CDI. The molecule combines potent activity against C. difficile, including hyper-virulent, endemic and emerging strains, with exceptionally high levels of antibacterial selectivity. This selectivity results in a lack of disruption to the healthy gut bacteria and this is important in naturally preventing recurrence of CDI to improve the prognosis for patients. In addition, SMT 19969 is targeted exclusively to the site of infection by being retained in the GI tract. It also shows exceptionally low levels of resistance development and has an excellent safety profile. SMT 19969 has recently completed preclinical development and a CTA application for a Phase I study will be submitted in Q3 2012. SMT 19969 has been developed under a prestigious Seeding Drug Discovery Award in conjunction with the Wellcome Trust.
(iii) OGA Inhibitor Programme for Alzheimer’s Disease and related Tauopathies.
Alzheimer’s disease is the most common form of dementia and is a progressive, debilitating disorder with symptoms including memory loss, change in mood and personality, and a decline in cognitive abilities. One of the main characteristics of the disease is the formation of neurofibrillary tangles (‘NFT’), which are toxic aggregates of tau protein that contribute to the death of nerve cells in the brains of Alzheimer’s patients. Recent independent scientific publications have placed greater emphasis on the importance of tau and NFTs in the cause and spread of Alzheimer’s disease. Additional studies have highlighted how inhibiting the enzyme O-linked N-acetylglucosaminidase (‘OGA’) can prevent tau from aggregating and forming NFTs, confirming it as a target for the development of potential disease modifying drugs to treat Alzheimer’s and related Tauopathies. OGA is a hexoseaminidase – a hydrolytic enzyme responsible for removing post-translational, terminal N-acetyl-D-hexosamine moieties from serine and threonine residues of proteins. As a carbohydrate processing enzyme, OGA was identified as a prime candidate for Summit Seglin Technology.
Using Seglin™ Technology, Summit has identified potent and highly selective Seglin inhibitors of OGA and has established in vitro efficacy in human cell models of Alzheimer’s disease. Recent in vivo, non-clinical studies evaluated the pharmacokinetic properties of these potent Seglin OGA inhibitors and the results demonstrated to have rapid oral bioavailability and CNS penetration. Summit is seeking to establish proof of concept for its OGA inhibitor programme in transgenic tauopathy models.
(iv) Seglin™ Technology.
Recent advances in glycobiology have led to a dramatically improved understanding of the role that carbohydrate processing and recognition plays in disease pathogenesis. However, Xxxxxx has identified that in order to capitalise on these new insights to address serious unmet medical need, requires access to new uncharted chemical space. Summit has therefore developed Seglin™ technology to comprehensively cover carbohydrate diversity space. Seglins are orally available, small molecule carbohydrate mimetics with intrinsic biological activity and excellent drug properties that renders them highly attractive for the development of novel, oral therapeutics. The Seglin™ Technology is expected to have broad use with applicability across all main therapeutic areas. Summit intends to exploit the Seglin™ Technology to address serious unmet need, with a particular emphasis on infectious and rare/orphan diseases. The wider potential of the technology will be realised through discovery collaborations with Pharma and biotech partners.
| SCIENCE | 77 |
TA 02/12
| Q7 | TIME SPENT BY APPLICANTS ON RESEARCH |
| (a) | How many hours per week do the Principal Applicant and Coapplicant(s) spend on research? |
| Principal Applicant |
Coapplicant 1 | Coapplicant 2 | Coapplicant 3 | Coapplicant 4 | ||||
| 40 |
| (b) | How many hours per week will be spent on this project by the Principal Applicant and Coapplicant(s)? |
| Principal Applicant |
Coapplicant 1 | Coapplicant 2 | Coapplicant 3 | Coapplicant 4 | ||||
| 40 |
| Q8 | RELATED APPLICATIONS |
| (a) Is this or a related application currently being submitted elsewhere? |
YES ¨ NO x |
| If yes, to which organisation? |
||
| By what date is a decision expected? (dd/mm/yy) |
||
| (b) Has this, or a similar, application been submitted elsewhere over the past year? |
YES ¨ NO x |
| If yes, to which organisation? |
||
| What was the result? |
||
| (c) Is this application a resubmission or has it been previously considered under another Wellcome Trust scheme? |
YES ¨ NO x |
| If yes, when was it originally considered? |
||||
| Please give the Wellcome Trust’s reference number: |
||||
| Briefly state how this application differs from the original (no more than 250 words) | ||||
| SCIENCE | 78 |
TA 02/12
| Q9 | SUMMARY OF PROPOSED RESEARCH INCLUDING KEY GOALS |
| (a) | For scientifically qualified assessors: (no more than 200 words) |
| SMT19969 is a novel antibiotic under development for the specific treatment of C. difficile infection (CDI) with a unique profile that clearly differentiates it from all other CDI antibiotics in clinical use and development. In particular, the exceptionally narrow spectrum of activity is expected to significantly reduce rates of recurrent disease which remains the main issue in CDI management. Up to 30% of patients will experience at least one recurrent episode with each recurrent period associated with significantly increased risk of further recurrence, disease severity, risk of life threatening complications and mortality.
Wellcome Trust funding will allow completion of a First-in-Human (FIH) Phase I trial on SMT19969. In addition to data on safety and tolerability in healthy volunteers a comprehensive package of scientific data supporting efficacy being achieved in patients will be generated. In particular, the absence of gut flora damage by SMT19969 following repeat dosing will be examined which will add significant support to a reduction in recurrent disease being achieved in efficacy trials. Once successfully complete, the package of work described in this proposal will result in a significantly de-risked Phase II ready asset for commercialisation. |
| (b) | For lay readers: (no more than 200 words) |
| The bacterium Clostridium difficile is often a harmless resident of the human intestine with levels kept in check by the complex community of other microorganisms resident in the gut. However, following disruption to the healthy balance of the gut flora it can cause a potentially fatal infection of the colon. Recent years have seen a dramatic increase in both the number of cases and severity of C. difficile infection (CDI) and the disease is now firmly established as a significant healthcare issue. Current therapy options are limited and of sub-optimal efficacy with up to 30% of patients experiencing at least one additional episode of CDI following initial treatment. The primary goal of this proposal is to complete a first-in-human (FIH) Phase I clinical trial on SMT19969 which is a novel antibiotic specifically targeted to the treatment of CDI that is expected to significantly reduce rates of recurrent disease. In addition to basic safety and tolerability data in man, an extensive package of additional scientific data will be generated that will support high levels of efficacy being achieved in CDI patients. |
| SCIENCE | 79 |
TA 02/12
Q10 DETAILS OF PROJECT
Provide over 4 pages, using the box below, in the following order:
(a) What is the total requested cost from the Trust?
| Funding dependent milestones |
Cumulative Costs | |||||||||||
| Cost of Milestone Period 1 (M1): |
£ | 263,342 | ||||||||||
| Cost of Milestone Period 2 (M2): |
£ | 1,076,670 | £ | 1,340,012 | =M1+M2 | |||||||
(b) The plan of investigation proposed to be funded by the Trust including: the specific aims and objectives; at least 2 milestones, (m1, m2 and/or m3) for the Trust-funded component during the course of the project (funding maybe dependent on achieving milestones); and how this proposal will ultimately lead to a healthcare benefit.
(c) A description of the validation or proof of concept to date. (Guide 1⁄2 page)
(d) A Xxxxx chart or similar graphical overview of the tasks to be undertaken, their sequence and duration for the entire project including those (marked separately) that will be undertaken in parallel but without Trust funding (if applicable) and key development steps after Trust funding. What other funds (if any) are contributing to related project tasks in the Xxxxx chart. Please give a brief description of the work to be undertaken with these alternative funds.
(e) How the project will be managed to deliver the milestones and key objectives, describing in-house expertise and any to be accessed externally.
Graphs, figures and supporting unpublished data may be embedded in the text or included as an appendix. This data must not exceed the equivalent of 5 A4 pages in length.
| SCIENCE | 80 |
TA 02/12
CDI remains a significant clinical issue (see Appendix C) and is a leading cause of morbidity and mortality in the healthcare system. In the USA, mortality increased 400% between 2000 and 2007 and although the dramatic rise in prevalence witnessed since the late 1990s has slowed, US and UK prevalence now seems to have stabilised at around 500,000 and 20,000 cases p.a respectively. However, prevalence rates are increasing in many other Western EU countries and the disease is now becoming established in Eastern Asia and Australia. In addition to suboptimal and eroding efficacy, the limited choice of mainstay antibiotics (vancomycin and metronidazole) are associated with up to a 30% rate of recurrent disease which remains the central clinical problem in CDI, especially in patients infected with hyper-virulent strains such as BI/NAP1/027. Recurrent disease is frustratingly difficult to treat and results in a significant detrimental impact on patient welfare and disease progression and is associated with major economic and resource repercussions to the healthcare system. SMT19969 is a novel, narrow spectrum antibiotic specifically under development for the treatment of CDI that is clearly differentiated from clinical and competitor products. Of particular importance is an exceptionally narrow spectrum of activity which is expected to address recurrent disease whilst effectively treating the initial infection. C. difficile spore outgrowth and toxin production invariably occurs following disruption to the healthy balance of the gut flora, typically as a result of prior antibiotic use. CDI antibiotics, such as vancomycin and metronidazole, continue to suppress significant components of the gut flora during treatment, thereby rendering patients susceptible to recurrent disease. By targeting the offending pathogen whilst allowing gut flora to recover during dosing, it is expected that SMT19969 will reduce rates of recurrent disease by allowing natural restoration of the gut flora to normal healthy levels thereby allowing colonisation resistance to re-establish. This restoration of gut flora in preventing recurrent disease is supported by the remarkable success (³ 90% long term cure rates) of faecal biotherapy which acts to completely restore a healthy ecological balance to the gut flora (Q12e).
Q10(b). The project has two clear Milestones (M1 & M2) with the overall aim of the programme being successful completion of a Phase I human clinical trial. Xxxxxx believes the studies described in this application are the optimal package of work to ensure that a comprehensive picture of SMT19969’s profile continues to be built and ensure expedient clinical development of the compound. All non-clinical studies on the compound are complete (Appendix B) and the work proposed in this application can be initiated immediately on confirmation of funding. A Xxxxx chart showing the timing and order of activities is included in Appendix A. The proposed studies described in this application are designed to add as much scientific, and therefore commercial, value to SMT19969 as possible by generating a data package that not only answers key safety and tolerability questions but also continues to support efficacy being achieved in Phase II studies. As such, this will make the asset highly attractive to potential commercial and development partners.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of three pages were omitted. [**]
Once all data from the clinical trial and supporting studies has been analysed an out-licensing package will be prepared for discussion with potential partners (Q12f). Translational award funding of the work outlined above will not only allow a comprehensive and compelling package of scientific data to be generated but will also allow Summit to differentiate and position SMT19969 in the competitive landscape making the asset attractive to development partners.
Q10(c). Validation and Proof of Concept to Date: A brief synopsis of the validation data surrounding SMT19969 is described here with a more extensive review found in Appendix B. In addition to potent growth inhibition of C. difficile (MIC90=0.125µg/mL), SMT19969’s key differentiator is an exceptionally narrow spectrum of activity with no significant growth inhibition of gut flora components. MIC90 values against C. difficile were typically >1,000 fold lower than those recorded against key members of the GI microflora including Gram negative and, importantly, Gram positive anaerobic and facultative bacteria. It is this sparing of gut flora that is expected to result in clinical superiority by significantly reducing rates of recurrent disease. C. difficile has been shown to have a very low propensity to develop SMT19969 resistance. Spontaneous resistant mutants could not be isolated and no resistance was observed following 14 serial passages at 0.5xMIC. Superior efficacy to vancomycin has been shown in the hamster model of CDI with SMT19969 (20mg/Kg SID) conferring complete protection from CDI with a 100% survival rate through to day 21. Regulatory GLP toxicology studies are now complete and data have shown SMT19969 to be very well tolerated. Following 28 days repeat oral dosing (route of clinical administration) at 1,000mg/Kg in both rat and dog (>150 fold higher than the maximum likely clinical dose) there were no adverse effects, post-dosing observations or any findings from in-life endpoints or from histopathology of tissues. Importantly, there was no systemic exposure of the compound with SMT19969 not detected in any plasma sample confirming retention in the GI tract. As such, the No Observed Adverse Effect Limit (NOAEL) from oral dosing is set at 1,000mg/Kg.
| SCIENCE | 81 |
TA 02/12
Although SMT19969 is to be dosed orally in the clinic and will likely be retained in the GI tract, the regulatory bodies will expect a thorough understanding of any potential systemic toxicity and therefore IV dosing arms were included in the 28 day repeat dose GLP studies. During the course of IV dosing SMT19969 was generally well tolerated and although findings were recorded in the high and mid dose groups following histopathology of tissue, these were mostly consistent with lack of solubility (e.g. granuloma formation) rather than compound specific toxicity. Based on these studies, the systemic NOAEL (Appendix B) demonstrates at least a 450 fold window between the oral and systemic peak plasma concentrations at their respective NOAELs. In addition, no effects were seen in the rat Xxxxx test, no genotoxicity was observed in standard Xxxx and chromosomal aberration assays and no significant inhibition of hERG tail current recorded. All chemistry, manufacturing and control (CMC) activities to support an FIH study have been completed, including manufacture of a single GMP batch of SMT19969 used in the pivotal GLP toxicology studies that will be used in the PI human trial.
Q10(d-e): Project Management and Expertise: All suppliers/collaborators for the proposed studies have been identified and draft work plans/protocols have been agreed. Overall timelines for each phase of the programme are shown in the Xxxxx chart (Appendix A). The programme of work will be coordinated and managed by the same Summit team that successfully delivered the Wellcome Trust SDDI funded discovery phases. The project will make use of external groups with leading expertise for each of the sections of work. Long-term successful relationships have been built up with these collaborators and suppliers during the course of SMT19969’s preclinical development thereby ensuring the maximum chance of successfully delivering the project as anticipated. In particular, Professor Xxxx Xxxxxx, a world-leading clinician in CDI, will continue to be a close advisor to the programme and will carry out faecal flora analysis and additional efficacy studies. Covance (Summit’s on-going and preferred supplier for all preclinical toxicology and PI studies) will conduct the PI trial and any associated regulatory work. Continuing mode-of-action studies and supporting microbiology will be carried out by Quotient Bioresearch and Euprotec – leading UK microbiology CROs who perform all microbiology on Summit’s portfolio of anti-infective drug discovery programmes.
| SCIENCE | 82 |
TA 02/12
| Q11 | REFERENCES (Research project) |
Please give citation in full, including title of paper and all authors.
Leading Reviews on C. difficile Infection:
| • | Xxxxxxxxxxxxxxx, A. N.; Clostridium difficile Infection: Epidemiology, Risk Factors and Management. Nature Reviews Gastroenterology and Hepatology, 2011, 8, 17-26. |
| • | Xxxxxx, M.; Xxxxxx, X. X.; Xxxxxxx, X. X.; Clostridium difficile Infection: New Developments in Epidemiology and Pathogenesis. Nature Reviews Microbiology, 2009, 7, 526-536. |
| • | Xxxxx, X. X.; Clostridium difficile Associated Disease: An Emerging Threat to Patient Safety. Pharmacotherapy, 2006, 26(3), 299-311. |
Current Epidemiology:
| • | Xxxxxx, X.; Xxxxx, X. X.; Xxxxxxx, X. X.; Xxxxxx, P. P.; Xxxxxxxxx, R.; Xx Xxxxxx, X. X.; Xxxxxxx, S.; Xxxxxxxxxx, A. R.; The Epidemiology of Community-Acquired Clostridium difficile Infection: A Population Based Study. The American Journal of Gastroenterology, 2012, 107, 89-95. |
| • | Xxxxxx, X.; Xxxxx, X.; Xxxxxxxxxx, A.; Clostridium difficile Infections (CDI) in Hospital Stays, 2009. HCUP Statistical Brief #124. January 2012. Agency for Healthcare Research and Quality, Rockville, MD. xxxx://xxx.xxxx-xx.xxxx.xxx/xxxxxxx/xxxxxxxxxx/xx000.xxx |
| • | Centers for Disease Control and Prevention; Preventing Clostridium difficile Infections; Morbidity and Mortality Weekly Report. Xxxxx Xxxxx. 2012, 61, March 6. |
| • | Bauer. M. P.; Xxxxxxxxx, X. X.; xxx Xxxxxxx, B. H. B.; Xxxxxxx, X. X.; Xxxxxx, X. X.; Xxxxxx, M.; Xxxxxx, X. X.; xxx Xxxxxx X. X.; Clostridium difficile Infection in Europe: a Hospital-based Survey. Lancet, 2011, 377, 63-73. |
| • | Xxxxxxx, X.; Xxxxx, X. X.; Xxxxxx, S. D.; Xxxxxx, J.; Fawley, W. N.; Xxxxxxxx, B.; Xxxxxxx, X. X. Xxxxxx, X. X.; The Changing Epidemiology of Clostridium difficile Infections. Clinical Microbiology Reviews, 2010, 23 (3), 529-549. |
Marketed and Development CDI Therapies:
General Reviews:
| • | Xxxxxxx, X. X.; Xxxxxxx, S.; Management of Clostridium difficile Infection: Thinking Inside and Outside the Box. Clinical Infectious Diseases. 2010, 51, 1306-1313. |
| • | Xxxxxxx, X. X.; Xxxx, C. A.; Xxxxx Xx, R.; Treatment of Clostridium difficile Infection. Clinical Infectious Disease, 2008, 46 (S1), S32-S42. |
| • | Xxxxxx, X. X.; Clinical Management of Clostridium difficile-Associated Disease. Clinical Infectious Disease, 2007, 45 (S2), S122-S128. |
| • | Xxxxxxxx, X. X.; New Drugs for Clostridium difficile Infection. Clinical Infectious Diseases, 2006, 43 (4), 428-431. |
| • | XxXxxxxxx, X. X.; Alternative treatments for Clostridium difficile disease: what really works? Journal of Medical Microbiology, 2005, 54, 101-111. |
Optimer Pharmaceuticals – Dificid (fidaxomicin):
| • | Xxxxx, X. X.; Xxxxxx, M. A.; Xxxxxxx, X. X.; Xxxxx, K.; Xxxxxxx, A.; Xxxxx, Y.; Xxxxxxx, S.; Xxxxx, P.; Xxxx, X. X.; Fidaxomicin versus Vancomycin for Clostridium difficile Infection. New England Journal of Medicine, 2011, 364(5), 422-431. |
| • | Xxxxxxx, X. X.; Xxxxx, X. X.; Xxxxxxxx, R.; Xxxxxxx, A.; Xxxxxx, M. S.; Xxxxx, K.; Sears, P.; Xxxxxxx, S.; Fidaxomicin versus Vancomycin for Infection with Clostridium difficile in Europe, Canada, and the USA: a Double-blind, non-inferiority, Randomised Controlled Trial. Lancet Infectious Diseases, 2012, 12, 281-289. |
Merck – CDI Antibodies:
| • | Xxxx, X.; Xxxxxxx, D. C.; Xxxx. B. A.; Xxxxx, X. X.; Xxxxxx, R.; Xxxxxxx, X. X.; Xxxxxx, G.; Xxxxxx, Xx. X. X.; Xxxxx, M. ; Xxxxx, S.; Xxx, C. A.; Xxxxxxxxx, X. X.; Treatment with Monoclonal Antibodies against Clostridium difficile Toxins, New England Journal of Medicine, 2010, 362(3), 197-205. |
| • | Xxxxxxx, X.X.; Xxxxxxxx, X.X.; Xxxxxxxxx, X.X.; Xxxxxxx, X.X.; Xxxxxxx, K.; Xxxxxxxxx, N.; Xxxxx, A.M.; Xxxx, I.; Xxxxxxxx, R.; Xxxxxxx, D.; Xxxxxxxxx, X.X.; Xxxxxx, X.X.Xx.; Human monoclonal antibodies directed against toxins A and B prevent Clostridium difficile-induced mortality in hamsters. Infection and Immunity. 2006, 74(11), 6339-6347. |
| SCIENCE | 83 |
TA 02/12
Faecal Biotherapy:
| • | Xxxxx, X.; Xxxxx, Xxxxxx.; Xxxxxx, A. R.; Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium difficile Infection. Clinical Infectious Diseases, 2011, 53, 994-1002. |
| • | xxx Xxxx, X., Xxxxxxxx, P.; Kuijper, E, X.; Xxxxxx, J, X. Xxxxxxxxxx with Recurrent Clostridium difficile Infections: Is Donor Faeces the Solution? Eurosurveillance, 2009, 14 (34), 1-6. |
| SCIENCE | 84 |
TA 02/12
| Q12 | COMMERCIAL MATTERS |
[**]
| REF |
TITLE |
FAMILY |
APPLN NO. |
XXXX.XXXX | ||||
| [**] | [**] | [**] | [**] | [**] | ||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | |||||
| [**] | [**] | [**] | [**] | [**] | ||||
| [**] | [**] | [**] | [**] | [**] | ||||
| [**] | [**] | [**] | [**] | [**] | ||||
| [**] | [**] | [**] | [**] | [**] | ||||
| [**] | [**] | [**] | [**] | [**] | ||||
| [**] | [**] | [**] | [**] | [**] |
Table 1: Summary of Summit’s Patent Estate Covering its Novel Class of C. difficile Antibiotics
| SCIENCE | 85 |
TA 02/12
| (ai) | Patent information (continue on another sheet if necessary) |
| Application number: | [**] | |
| Priority date: | [**] | |
| Inventors: | [**] | |
| Applicant: | [**] | |
| Funding source: | [**] | |
| Title | [**] | |
| (bi) | How do these patent(s) or patent application(s) relate to the proposal? (Max. 1 page) |
[**].
| (ci) | Describe any freedom to operate issues that have been identified or that might arise and how these will be tackled. Include the type and date of any searches that have been conducted. (Max. 1⁄2 page) |
[**].
| (di) | Describe any new types of intellectual property that can be anticipated including how the identification of these inventions will be managed. (Max. 1⁄2 page) |
[**]
| SCIENCE | 86 |
TA 02/12
(aii) Patent information (continue on another sheet if necessary)
| Application number: | [**] | |
| Priority date: | [**] | |
| Inventors: | [**] | |
| Applicant: | [**] | |
| Funding source: | [**] | |
| Title | [**] | |
| (bii) | How do these patent(s) or patent application(s) relate to the proposal? |
[**].
| (cii) | Describe any freedom to operate issues that have been identified or that might arise and how these will be or have been addressed. Include the type and date of any searches that have been conducted. |
[**].
| (dii) | Describe any new types of intellectual property that can be anticipated including how the identification of these inventions will be managed. |
[**].
(aiii) Patent information (continue on another sheet if necessary)
| Application number: |
[**] | |
| Priority date: | [**] | |
| SCIENCE | 87 |
TA 02/12
| Inventors: | [**] | |
| Applicant: | [**] | |
| Funding source: | [**] | |
| Title | [**] | |
| (biii) | How do these patent(s) or patent application(s) relate to the proposal? |
[**].
| (ciii) | Describe any freedom to operate issues that have been identified or that might arise and how these will be or have been addressed. Include the type and date of any searches that have been conducted. |
[**].
| (diii) | Describe any new types of intellectual property that can be anticipated including how the identification of these inventions will be managed. |
[**].
| SCIENCE | 88 |
TA 02/12
| (e) | Please describe any competing technologies identifying those groups carrying out research and development in the field. In addition, please provide a description of the competitive advantage of this technology over currently accepted methods. (Max. 1 page) |
As discussed in detail in Appendix C, CDI is a significant and difficult to manage infection with an estimated 900,000 cases p.a. across the EU, US and Canada. Disease severity and mortality rates are increasing and CDI prevalence is continuing to rise in may EU countries. The disease is now emerging as a significant issue in other territories such as Eastern Asia and there are increasing reports of CDI as an emerging community problem. However, the most significant hurdle to effectively treating CDI is recurrent disease and any novel CDI therapy that is able to address this aspect of the disease more effectively than its competitors will be expected to become a front line agent. In the context of both approved CDI agents and those in clinical development, the profile of SMT19969 offers several significant competitive advantages. In particular, the narrow spectrum of activity is expected to result in significantly less collateral damage to the patients gut flora than other antibiotics and this is expected to translate to significantly reduced rates of recurrent disease (Q10). In addition, the very low resistance development potential will extend clinical utility and, as a synthetic heterocyclic small molecule, SMT19969’s low cost-of-goods and simple, reliable manufacture (especially when compared to antibiotics generated from fermentation broths and biologics such as antibodies and vaccines) offers significant clinical and commercial appeal. Given this encouraging profile it is expected that SMT19969 would be positioned as a front line agent for CDI for use in all patients suffering initial infection rather than a sub-population of patients who are, for example, treatment failures following other therapies. In addition, eventual prophylactic use in select groups of high risk patients could also be considered Overall, the competitive landscape in CDI can be broken down into antibiotic and non-antibiotic approaches.
1. Antibiotic Approaches: The only FDA approved CDI antibiotics are Vancocin (oral vancomycin) and Dificid (fidaxomicin) and although reasonably effective at treating initial infection, neither agent is truly effective at reducing recurrent disease. Although Dificid does show an overall improvement over vancomycin, 30% of Dificid patients are still either treatment failures or suffer recurrent disease. In addition, and in common with all current development antibiotics, Dificid does not show any improvement over vancomycin in rates of recurrent disease for patients infected with hyper-virulent strains. All CDI antibiotics in both regular clinical use and in development cause on-going collateral damage to gut flora during the course of CDI therapy as described below (Table 4, Appendix D). As discussed previously (Q10), this continues to hold open the niche that C. difficile can exploit allowing subsequent periods of spore outgrowth and recurrent disease. As SMT19969 is sparing of both the Gram positive and Gram negative anaerobic and facultative components of the gut flora, this on-going collateral damage should be minimised during the course of CDI therapy allowing colonisation resistance to re-establish thereby reducing rates of recurrent disease.
2. Non-Antibiotic Approaches: Alternatives to antibiotics such as pro/prebiotics, antibodies, vaccines and faecal biotherapy have shown either limited efficacy or are appropriate only as second line therapies, especially as many of them must be dosed in conjunction with antibiotics and are associated with high cost and inconvenient administration.
To date, there is little or no evidence to show any clinical benefit in the use of toxin sequestering agents, prebiotics and IV immunoglobulin G. However, recent PII data on the Merck antibodies (CDA1 and CDB1) targeting the C. difficile toxins A and B showed a 70% reduction in relapse rates when used in combination with either vancomycin or metronidazole. In addition, a reduction in the severity of disease symptoms was also observed and the antibodies were well tolerated. However, despite these encouraging results, such an approach suffers from certain drawbacks. The antibodies must be used in combination with standard antibiotics, must be dosed IV and only target Toxins A and B and not the binary toxin produced by certain strains. Antibody approaches to CDI are also most likely positioned for use in severe disease only and in patients who have suffered multiple recurrent episodes.
The only approach to CDI that has shown significant success is faecal biotherapy which achieves >90% cure rates in treating patients suffering multiple recurrent episodes of CDI who are completely refractory to all other treatment options. Indeed, many of these patients are facing colectomy as the only remaining course of action. The procedure involves transplantation by enema or nasogastric tube of a liquid suspension of faeces from a healthy individual, usually a close relative. This acts to recreate a healthy balance to the intestinal microbiota of the diseased individual and therefore prevents active CDI. Despite the obvious limitations of this approach it does clearly demonstrate the vital role played by the gut flora in CDI pathogenesis and the requirement for a narrow spectrum of activity in any CDI antibiotic.
| SCIENCE | 89 |
TA 02/12
| (f) | Describe the commercialisation strategy for the technology, including (where appropriate) factors such as potential sources of further funding, relevant licensing partners and potential to create early revenue streams to support development. (Max. 1 page) |
Commercialisation Strategy: SMT19969 constitutes a highly differentiated, first-in-class antibiotic being developed for use as a front line CDI therapy with genuine potential to minimise rates of recurrent disease. Summit’s commercialisation strategy is to monetise its small molecule assets at key value inflexion points through licensing to partners with specific clinical development expertise. In the case of SMT19969, the value inflexion point would be a package of human safety and tolerability data in healthy volunteers and a differentiated package of in vitro and in vivo discovery data on efficacy, pharmacokinetics and microbiology. Wellcome Trust funding will be used for the translation of SMT19969 through to this value inflexion point.
Project Funding: Adequate funding to allow the full programme of work described in this proposal to proceed in a timely manner is critical to the expeditious progression of SMT19969. It is our view that this is appropriate to provide the necessary data to ensure engagement with licensing partners who would take the project through subsequent pivotal trials and ultimately to the market. Inability to commit to and complete the full work package would compromise this position. Whilst Summit might in future be in a position to undertake a more modest programme of work, Wellcome Trust funding will be instrumental in allowing the full package of work to be undertaken. Discussions between Summit and the Trust regarding the equity stakes in the programme following the SDDI award are currently on-going and successful application for this translational award would be factored into those discussions as appropriate.
[**].
| SCIENCE | 90 |
TA 02/12
| (g) | What are the key current and downstream regulatory considerations or risks that need to be addressed to achieve approval for this technology? (Max. 1⁄2 page) |
[**].
| (h) | Describe the R&D strategy and portfolio/pipeline, in the case of companies. (Max 1⁄2 page) |
[**].
| Q13 | OUTLINE OF PUBLIC ENGAGEMENT PLANS (max. 1⁄2 page) |
Summit will, where appropriate, present data on the programme at leading global scientific meetings specialising in anti-infectives. This would include events such as ECCMID (European Congress of Clinical Microbiology and Infectious Diseases) and ICAAC (Interscience Conference on Antimicrobial Agents and Chemotherapy). In addition, Summit and its collaborators will publish selected data in the primary scientific literature when this does not impinge on patent protection of data or on commercialisation of the project.
In addition to publication and presentation of data at scientific meetings, Summit will also discuss its programme, when appropriate, at specialist pharmaceutical partnering conferences such as BIO in North America and BIO Europe.
The primary purpose of all these publicity activities would be raise commercial interest in the programme and to help identify and meet with suitable licensing partners.
| Please note that we provide support for researchers in the UK and Republic of Ireland to engage with the lay public. To receive information about training, funding and other public engagement opportunities, please tick the box. | ¨ |
| SCIENCE | 91 |
TA 02/12
Q14 CURRICULUM VITAE OF APPLICANT(S)
This section should be completed by the Principal Applicant and all Coapplicants and can be duplicated if required.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of one page was omitted. [**]
| (h) | Summary of career to date, including key achievements (no more than 700 words) |
[**].
| (i) | Publications |
Applicants should provide references or examples to 5 key achievements over the past 5 years which may include any of the following:
Inventions protected by intellectual property rights that have been developed commercially and or adopted; ii) patents that have issued; iii) peer-reviewed publications.
Please append to the application in PDF format any references that are particularly relevant.
| • | Xxxxxx, S., Xxxxxx. X., Xxxxxxx. J., Xxxxxx. X., Xxxxxxx. J., Xxxxxxx. R., Xxxxxx. X., Xxxxxx. F., Xxxx. S. SMT19969: A novel antibiotic for C. difficile infection; C. difficile growth inhibition, spectrum of activity and resistance development.; Interscience Conference on Antimicrobial Agents and Chemotherapy; 2011, Abstract B-1194. |
| • | Publication appended to application |
| • | Xxxxxx, X., Pulse. X., Xxxxxx, X., Xxxxxxx, X., Xxxxxxx, X., Xxxxx, W.; Efficacy of SMT19969 and SMT21829 in a hamster model of Clostridium difficile associated disease. Interscience Conference on Antimicrobial Agents and Chemotherapy; 2011 Abstract B-1195 |
| • | Publication appended to application |
| • | Xxxxxx, X., Xxxxxxx, X., Xxxxxxxx, X., Xxxxxxxxx, X., Xxxxxxx, R., Xxxxxx, M.; Efficacy of novel antimicrobial agent SMT19969 against simulated Clostridium difficile infection in an in vitro human gut model. Interscience Conference on Antimicrobial Agents and Chemotherapy; 2011, Abstract B-1193 |
| • | Publication appended to application |
| • | Xxxxxx, Xxxxxxx, Xxxxxx; Xxxxxxx, Xxxxx, Xxxxx; Xxxxxxx, Xxxxxxx; Xxxxxx, Xxxxxxx; Xxxxx, Xxxxxx, Xxxxxxx; Xxxxx, Xxxx, Xxxxxxxx; Xx Xxxx, Xxxxxxx; Xxxxxx, Xxxxx, Xxxxxxx; Xxxxx, Xxxx, Xxxxx; Antibacterial Agents; WO2010/063996 (C. difficile SUM35 patent family – see Q12) |
| • | Xxxxxxxx IM, Xxxxxx S, Xxxxxxx XX, Xxxxxx E, Xxxxxxxx MC, Xxxxxxxx A, Xxxxxxxx XX, Xxxxxxx XX, Xxxxxxxx V, Xxxxx XX, Xxxxxxx A, Xxxxxx XX, Xxx E; Identification of Arylamine N-acetyltransferase Inhibitors as an Approach Towards Novel Anti-tuberculars. Protein Cell. 2010, 1(1), 82-95. |
| (j) | Financial support |
Please list all key forms of financial support in the last 5 years (commercial, research grant etc). Please state the name of the funder, title of project or enterprise, amounts awarded and start to end dates of support. For any current grants indicate the proportion of time spent on each project. Please identify with a (*) those sources of support that have contributed to the background of this proposal.
| * | Wellcome Trust SDDI – Development of a Novel Class of Antibiotics for the Targeted Treatment of Clostridium difficile Infection - £2.2mill – Jan 2010 to March 2012. |
| Q15 | PREVIOUS APPLICATIONS TO THE WELLCOME TRUST |
| (a) | Is this the Principal Applicant’s first application to the Wellcome Trust? | YES ¨ NO x | ||||
| (b) | Give details of all previous applications to the Wellcome Trust over the last five years. This information should be provided for the Principal Applicant and all Coapplicant(s). Please include name of xxxxx xxxxxx, xxxxx number (if known) and, if application was successful, the amount and period of award. | |||||
| CURRICULUM VITAE OF APPLICANT | 92 |
TA 02/12
| • | Successful application made to Wellcome Trust under the Seeding Drug Discovery Initiative. |
| • | Principal Investigator was Xx Xxxxxxx Xxxxxxx (principal applicant on this proposal). |
| • | Project Title: Development of a Novel Class of Antibiotics for the Targeted Treatment of Clostridium difficile Infection. |
| • | Total Award was £2.2mill. |
| • | Funding period was Jan 2010 to March 2012. |
| CURRICULUM VITAE OF APPLICANT | 93 |
TA 02/12
| Q16 | CURRICULUM VITAE OF NAMED RESEARCH ASSISTANT | |||
| This page may be duplicated if more than one research assistant is required. | ||||
| (a) | Surname: | Forenames: | ||
| Date of birth: | ||||
| (b) | Degrees, diplomas etc: (subject, class, university and dates) | |||
| (c) | Current post: (if not currently in employment, please give details of most recent post) | |||
| Position and grade: | ||||
| Department: | ||||
| Institution: | ||||
| Funding body: | ||||
| Termination date of support: | ||||
| Current basic salary and incremental date: | ||||
| Basic salary must be shown separately from any salary enhancements or other allowances. | ||||
| If currently funded by a Wellcome Trust grant, please give grant reference number: | ||||
| (d) | Previous posts: (with dates) | |||
| (e) | Most recent publications: (no more than five; please give citation in full, including title of paper and all authors) | |||
| CURRICULUM VITAE OF RESEARCH ASSISTANT | 94 |
TA 02/12
| Q17 | CURRENCY REQUESTED | |||
| (a) | What currency is being used for the costings in this application? | Sterling | ||
| (b) | Is the chosen currency your local currency? | Yes | ||
| (c) | What is your local currency if the chosen currency is not your local currency? | N/A | ||
| (d) | Please specify the exchange rate to GBP£ with your local currency that has been used to provide the costings in this application. | N/A | ||
| (e) | Please state clearly the reasons for requesting costs in the chosen currency (no more than 150 words) | |||
| Xxxxxxxx is the functional currency of Summit and of all the collaborators, suppliers and service providers involved with the programme. | ||||
| Q18 | SUMMARY OF FINANCIAL SUPPORT REQUESTED | |||
| Duration of grant (state in months): | 12 months | |||
| Total cost | ||||
| (a) Salaries (Summit staff only) |
£ | [** | ] | |
| (b) Materials and consumables |
N/A | |||
| (c) Animals |
N/A | |||
| (d) Equipment |
N/A | |||
| (e) Miscellaneous (outsourced costs for clinical development etc.) |
£ | [** | ] | |
|
|
|
|||
| GRAND TOTAL |
£ | 1,340,012 | ||
|
|
|
|||
| FINANCIAL DETAILS | 95 |
TA 02/12
| Q19 | DETAILS OF FINANCIAL SUPPORT AND RESOURCES REQUESTED |
| (a) | Salaries |
Please refer to Guidance Notes and definition of terms for further details. Expand table as necessary.
| EFFORT ON |
||||||||||||||||||||||||||||||||||||
| Post no. |
Staff category |
Name (if known) |
Starting salary |
Grade/ Scale |
Increment date (dd/mm) |
Start date (dd/mm/yy) |
Period on project (months) |
% of full time |
London Allowance |
Total of other allowances |
Employer’s contributions |
Total cost on grant |
||||||||||||||||||||||||
| 1 |
Principal Investigator |
[**] | [** | ] | [**] | [**] | [**] | [**] | [** | ] | [** | ] | [** | ] | [** | ] | [** | ] | ||||||||||||||||||
| 2 |
Senior Management |
[**] | [** | ] | [**] | [**] | [**] | [**] | [** | ] | [** | ] | [** | ] | [** | ] | [** | ] | ||||||||||||||||||
| FINANCIAL DETAILS | 96 |
TA 02/12
| Q19 | DETAILS OF FINANCIAL SUPPORT AND RESOURCES REQUESTED (cont.) |
Expand table as necessary.
| Costs | ||||
| (b) Materials and consumables (description) |
||||
|
|
|
|||
| Subtotal |
||||
|
|
|
|||
| (c) Animals |
||||
| Total purchase cost |
||||
| Total maintenance cost |
||||
| Total procedures cost |
||||
|
|
|
|||
| Total associated cost |
||||
|
|
|
|||
| Subtotal |
||||
|
|
|
|||
The table below should be duplicated for each different species.
| (i) Animal species to be used, and strain if relevant |
||||
| (ii) Source of supply |
||||
| (iii) Purchase |
||||
| Purchase price per animal |
||||
|
|
|
|||
| Total number of animals to be purchased |
||||
|
|
|
|||
| Total purchase cost |
||||
|
|
|
|||
| (iv) Maintenance |
||||
| Total number of animals to be maintained |
||||
|
|
|
|||
| Total number of weeks’ maintenance required |
||||
|
|
|
|||
| Cost per animal per week |
||||
|
|
|
|||
| Total maintenance cost |
||||
|
|
|
|||
| (v) Experimental procedures |
||||
| Types of procedure(s) |
||||
| Cost per procedure(s) |
||||
|
|
|
|||
| Total procedures cost |
||||
|
|
|
|||
| (vi) Associated costs |
||||
| Staff training costs |
||||
| FINANCIAL DETAILS | 97 |
TA 02/12
|
|
|
|||
| Total staff training costs |
||||
|
|
|
|||
| Animal environment, training and enrichment costs |
||||
|
|
|
|||
| Total animal environment, training and enrichment costs |
||||
|
|
|
|||
| Animal licence costs |
||||
|
|
|
|||
| Total animal licence costs |
||||
|
|
|
|||
| Total associated cost |
||||
|
|
|
| FINANCIAL DETAILS | 98 |
TA 02/12
| Q19 | DETAILS OF FINANCIAL SUPPORT AND RESOURCES REQUESTED (cont.) |
| (d) | Equipment |
Please provide contact details for the Institution’s Director of Procurement/Head of Purchasing (or equivalent).
| Name: | Tel: | |||||
| Address: | E-mail: | |||||
| (i) | Request for equipment. Expand table as necessary. |
| Type of equipment |
Equipment specification |
Preferred manufacturer/ supplier (if known) |
Duration & total cost of purchased |
Number of items |
Cost per item |
Total cost | ||||||||||||
|
|
|
|||||||||||||||||
| Total: |
||||||||||||||||||
|
|
|
|||||||||||||||||
| Contribution from other sources: |
||||||||||||||||||
| Amount requested: |
||||||||||||||||||
| FINANCIAL DETAILS | 99 |
TA 02/12
| Q19 | DETAILS OF FINANCIAL SUPPORT AND RESOURCES REQUESTED (cont.) |
| (d) | Equipment (cont.) |
| (ii) | Request for equipment maintenance. Expand table as necessary. |
Maintenance of existing Wellcome Trust-funded equipment
The Wellcome Trust will only consider providing maintenance funds for equipment more than five years old if the applicant can demonstrate it is cost-effective to do so.
| Details of equipment/facility |
Wellcome Trust grant reference number and start/end dates of original award |
Date of purchase |
Start/end dates of any current maintenance contract, length and total cost |
% of time/hours of use for this project |
Maintenance cost requested based on time used for this project | |||||
| (iii) | Request for access charges. Expand table as necessary. |
Access charges
| Details of equipment/facility |
Original source of funding (provide Wellcome Trust grant reference number if applicable) |
Standard access charge per hour/day |
% of time/hours of use for this project |
Access charge requested based on time used for this project | ||||
| FINANCIAL DETAILS | 100 |
TA 02/12
| Q19 | DETAILS OF FINANCIAL SUPPORT AND RESOURCES REQUESTED (cont.) |
Expand table as necessary.
| Costs | ||||||
| (e) Miscellaneous |
||||||
| • |
All outsourced costs are included in this section and this covers all programme costs except for Summit staff costs detailed in Q19a. | |||||
| Chemistry Manufacture and Control |
[** | ] | ||||
| Bioanalysis |
[** | ] | ||||
| Regulatory |
[** | ] | ||||
| Mode of Action |
[** | ] | ||||
| Clinical |
[** | ] | ||||
| CDARO Service |
[** | ] | ||||
| Independent Clinical Monitoring |
[** | ] | ||||
| ADMET |
[** | ] | ||||
| Supporting Microbiology |
[** | ] | ||||
| Patent Costs |
[** | ] | ||||
| Consultancy (Clinical and regulatory – Q21a) |
[** | ] | ||||
| Misc (travel etc.) |
[** | ] | ||||
|
|
|
|||||
| Subtotal |
£ | 1,215,147 | ||||
|
|
|
|||||
| FINANCIAL DETAILS | 101 |
TA 02/12
| Q20 | ACCESS TO RADIATION SOURCES | |||
| (a) | Will the proposed research require access to either the Synchrotron Radiation Source (SRS) at Daresbury or the European Synchrotron Radiation Facility (ESRF) at Grenoble? | YES ¨ NO x | ||
| If yes, please complete the table below, providing details of beam time requested and scheduling information (anticipated usage must be specified in whole days). | ||||
| Special requirements (single bunch, |
Total number |
Number of days per annum | ||||||||||||||
| Synchrotron |
Station |
other specify) |
of days |
Year 1 |
Year 2 |
Year 3 |
Year 4 |
Year 5 | ||||||||
| (b) | Please justify the stations and beam time requested (no more than 500 words). | |||
| (c) | Will the proposed research require access to a neutron source? | YES ¨ NO x | ||
| If yes, complete Q19 (a) and (b) above indicating that it is a neutron source that is required, and Q18 (d)(iii) Access charges, detailing the costs required. | ||||
| FINANCIAL DETAILS | 102 |
TA 02/12
| Q21 | REASONS FOR SUPPORT REQUESTED |
In this section, justify:
| (a) | Staff requested specifying their roles, responsibilities and location, if appropriate, with respect to the proposed project (no more than 700 words) |
Project Leader - Xx Xxxxxxx Xxxxxxx
| • | Xx Xxxxxxx Xxxxxxx is based at Summit’s facilities in Oxford and has specific overall responsibility for the programme and ensuring successful delivery against the agreed project plan. He has acted as project leader from the project’s inception, acted as principal investigator during the Wellcome Trust SDDI funded sections and will continue this role through the programme of work described in this application. Xxxxxxx will act as the primary point of contact between all the various parties involved in the project (including Wellcome Trust) and will be responsible for the overall strategy. |
Consultants:
| • | Dr Xxxx Xxxxxxxx: Xx Xxxxxxxx will continue as a long term consultant to the project and will offer expert guidance and review of the regulatory and clinical documents and will work closely with Xx Xxxxxxx on the day to day management of the programme. Xxxx has been an advisor to the programme during preclinical development, monitoring study protocols and reports across all sections (CMC, toxicology, regulatory). Xx Xxxxxxxx also fulfils this role on Summit’s other preclinical and clinical programmes. |
| • | Regulatory and Clinical: Although the day-to-day management of the programme will be carried out by Xx Xxxxxxx, expert consultants on the regulatory and clinical development of antibiotics will be engaged. The role of these consultants will be to ensure that an appropriate overall clinical development strategy is in place, especially with respect to FDA/EMEA guidelines on the design of antibiotic and CDI trials. The use of such consultants will not only ensure that the correct studies are being carried out but will also add a significant level of credibility during any licensing discussions with potential commercial partners. A short list of suitable candidates is being reviewed and the consultants will be in place shortly. |
Summit Senior Management:
| • | Xx Xxxxxxx Xxxxxx – Chief Scientific Officer; Xx Xxx Xxxxxxx – Senior Director R&D; Xx Xxxxxx Xxxxxxxx – Director Business Development |
A proportion of Summit’s senior management time (located at Summit’s Oxford facility) will be required to oversee the scientific strategy and programme progression in line with the overall Summit portfolio. This will also include significant involvement from the commercial department to ensure that a robust licensing package is prepared, that potential commercial partners are engaged in a timely manner and to lead any commercial discussions and negotiations.
| (b) | Materials and consumables (no more than 300 words) |
N/A
| (c) | Animals (numbers and species) (no more than 300 words) |
N/A
| (d) | Equipment, equipment maintenance and access charges (no more than 700 words) For access charges, please show how they have been calculated on a cost-recovery basis. This can include (i) a maintenance or service contract providing a basic level of service; (ii) running costs; (iii) materials and consumables; and (iv) staff time. Please also state the percentage of time/number of hours the equipment/facility will be used for the project. |
N/A
| (e) | Miscellaneous costs (no more than 300 words) |
The programme of work detailed in the proposal will primarily rely on outsourced studies with overall coordination and management of the programme by Summit. Summit has found this to be the most appropriate, efficient and cost effective means of accessing specialist services.
All companies to which studies will be outsourced are long-term suppliers of services to Summit and have been associated with the programme during the Wellcome Trust funded SDDI discovery phase.
All costs described in the proposal are based on accurate quotations provided by each supplier.
| JUSTIFICATION OF COSTS REQUESTED | 103 |
02/12
| Q22 | FULL ECONOMIC COSTING (UK applicants only) |
|||
| The Wellcome Trust would like to monitor the full economic cost of research proposals. If your institution is calculating the full economic costs of this proposal, the table below should be completed.
Please note that the Wellcome Trust will not fund the full economic cost of research and the actual costs sought from the Wellcome Trust should be detailed in the ‘DETAILS OF FINANCIAL SUPPORT AND RESOURCES REQUESTED’ section of the form.
This information is being gathered for monitoring purposes only and will have no bearing on the peer review and decision-making process for your application. | ||||
| (a) | Does the host institution use TRAC or an alternative methodology validated by the UK Research Councils to calculate full economic costs? | YES ¨ NO ¨ | ||
| (b) | If yes, please complete the following table: | |||
| Full Economic Cost (£) |
Contribution requested from the Wellcome Trust (£) | |||
| Directly Incurred Costs |
||||
| Staff |
||||
| Travel and subsistence |
||||
| Other costs |
||||
| Equipment |
||||
| Subtotal |
||||
| Directly Allocated Costs |
||||
| Principal Applicant salary costs |
||||
| Coapplicant salary costs |
||||
| Estates costs |
||||
| Other directly allocated costs |
||||
| Subtotal |
||||
| Indirect Costs |
||||
| TOTAL |
| ADMINISTRATION | 104 |
02/12
| Q23 | RESEARCH INVOLVING HUMAN PARTICIPANTS, BIOLOGICAL SAMPLES AND PERSONAL DATA | |||
| (a) | Does your project involve human participants? | YES x NO ¨ | ||
| If yes, refer to notes. | ||||
| (b) | Will personal data be used? | YES x NO ¨ | ||
| (c) | Will your project involve use of biological samples? | YES x NO ¨ | ||
| (d) | Please state by whom the project will be, or has been, ethically reviewed, and specify any other regulatory approvals that have been, or will be, obtained. | |||
| A Clinical Trial Application (CTA), supported by the Investigational Medicinal Product Dossier (IMPD), Investigator’s Brochure (IB) and Clinical Trial Protocol, will be submitted to the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) and National Research Ethics Service for review prior to initiation of the proposed trial. | ||||
| (e) | In the course of your project: | |||
| (i) | Do you propose to use facilities within the National Health Service (NHS)? | YES ¨ NO x | ||
| (ii) | Does your research involve patients being cared for by the NHS? | YES ¨ NO x | ||
| (iii) | If the answer is yes to (i) or (ii) above, please indicate which organisation has agreed to be the sponsor for the project under the Research Governance Framework for Health and Social Care, published by the Department of Health in England or the corresponding departments in Northern Ireland, Scotland or Wales.
Please note that the Wellcome Trust cannot act as sponsor. | |||
| (f) | If your project involves a clinical trial: | |||
| (i) | Please state whether it is covered by The Medicines for Human Use (Clinical Trials) Regulations. | YES x NO ¨ | ||
| (ii) | Please indicate which organisation has agreed to be the sponsor for the project. | |||
| Please note that the Wellcome Trust cannot act as sponsor. | ||||
| Summit PLC | ||||
| Q24 | EXPERIMENTS ON ANIMALS | |||
| Please note, this question is mandatory for all applications for funding that propose research using animals. Applications may be referred to the National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) for review. Where animal work is sub-contracted, this question must be completed by the organization conducting the animal studies. | ||||
| (a) | Do your proposals involve the use of animals or animal tissue? | YES [**] NO [**] | ||
| (b) | Do your proposals include procedures to be carried out on animals in the UK which require a Home Office licence?
If yes, refer to notes. |
YES [**] NO [**] | ||
| (c) | Does the institution where the animal work is to be carried out hold a certificate of designation under the Animals (Scientific Procedures) Act 1986? | YES [**] NO [**] | ||
| (d) | Do your proposals involve the use of animals or animal tissue outside the UK?
If yes, refer to notes. |
YES [**] NO [**] | ||
| ADMINISTRATION | 105 |
02/12
| (e) | If your project does involve the use of animals, what would be the severity of the procedures? | Mild | [**] | |||||
| Moderate | [**] | |||||||
| Substantial | [**] | |||||||
| (f) | Please provide details of any procedures of substantial or moderate severity (no more than 250 words). | |||||||
| [**] | ||||||||
| (g) | Why is animal use necessary: are there any other possible approaches? (no more than 250 words) | |||||||
| [**]. | ||||||||
| (i) | Will the following species to be used? | |||||||
| Primate | [**] | |||||||
| Cat | [**] | |||||||
| Dog | [**] | |||||||
| Equidae | [**] | |||||||
| Genetically Altered Animals | [**] | |||||||
| Other animals | [**] | |||||||
| (j) | Why is the species to be used the most appropriate? (no more than 250 words) | |||||||
| [**]. | ||||||||
| (l) | Primates | |||||||
| (i) | Do you expect facilities and practices, and the proposed research will comply with the principles set out in the ‘National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3Rs) Guidelines: Primate accommodation, care and use’ (xxxx://xxx.xx0xx.xxx.xx/xxxxxxxxxxx.xxx?xxx000)? | |||||||
| If not, please explain why. | ||||||||
| (ii) | Will it be necessary to transport the non-human primates (i.e from breeding facility and within the host institution environment)? | |||||||
| If so, indicate approximate journey times and the measures that will be taken to minimise the potential stress during transport. | ||||||||
| (iii) | Will single housing of the non-human primates be necessary at any time? | |||||||
| ADMINISTRATION | 106 |
02/12
| If so, please provide details in terms of the justification for single housing, its duration, and what additional resources will be provided to the animals to minimise the impact on animal welfare. | ||
| (iv) | Describe the experimental procedures involved and how any pain, suffering, distress and/or lasting harm will be minimised. Have the procedures been recently reviewed by the Named Veterinary Surgeon (NVS), Named Animal Care and Welfare Officer (NACWO) and ethical review process (ERP)? | |
| (v) | Will any of the experimental procedures involve food and/or water restriction? | |
| If so, justify why this is necessary and outline what alternatives have been considered. | ||
| (vi) | Will any of the experimental procedures involve restraint? | |
| What alternatives have been considered? Describe the nature of the restraint, its duration and frequency, and what will be done to avoid distress? | ||
| (vii) | What prior experience and training in non-human primate use, care and welfare have the staff named in the application had? What provision is made for continuing professional development in these areas? | |
| (viii) | Will any of the staff involved require specific training for any of the procedures concerned? | |
| Please provide details of the training needed and where it will be undertaken. | ||
| (l) | Cats and Dogs | |
| (i) | From where will the animals be sourced? | |
| (ii) | Will it be necessary to transport the animals? | |
| ADMINISTRATION | 107 |
02/12
| If so, indicate approximate journey times and the measure that will be taken to minimise the potential stress during transport. | ||
| (iii) | Are animals to be imported? | |
| Where animals are to be imported, what journey times have been agreed with the Home Office? Describe the conditions for the animals at the breeding establishment and how the potential stress during transport will be minimised. | ||
| (iv) | Please provide details of the housing for the animals, e.g. enclosure size, environmental enrichment. | |
| (v) | Will single housing of the animals be necessary at any time? | |
| If so, please provide details in terms of the justification for single housing, its duration, and what additional resources will be provided to the animals to minimise the impact of the single housing. | ||
| (vi) | Describe the experimental procedures involved and how any pain, suffering, distress and/or lasting harm will be minimised. Have the procedures been recently reviewed by the Named Veterinary Surgeon (NVS), Named Animal Care and Welfare Officer (NACWO) and ethical review process (ERP)? | |
| (vii) | Will any of the experimental procedures involve restraint? | |
| What alternatives have been considered? Describe the nature of the restraint, its duration and frequency, and what will be done to avoid distress? | ||
| (viii) | What prior experience and training in animal use, care and welfare will be required of the staff named in the application? What provision is made for continuing professional development in these areas? | |
| (ix) | Will any of the staff involved require specific training for any of the procedures concerned? | |
| Please provide details of the training needed and where it will be undertaken. | ||
| ADMINISTRATION | 108 |
02/12
| Q25 | RISKS OF RESEARCH MISUSE | |||||
| (a) | It is the responsibility of institutions in receipt of Wellcome Trust funding to ensure that any risks that research could be misused for harmful purposes are managed in an appropriate manner. | |||||
|
Please tick the box to confirm that you have considered whether your proposed research could generate outcomes that could be misused for harmful purposes. |
x | |||||
| (b) | If you have identified any tangible risks of this type, please briefly describe these risks and the steps that you and your institution will take to manage them (no more than 250 words). | |||||
| Q26 | LOCATION OF RESEARCH | |||||
| (a) | Will the research project be undertaken in a Wellcome Trust Clinical Research Facility? | YES ¨ NO x | ||||
| If yes, please specify: | ||||||
| (b) | Will the research project be undertaken in the Wellcome Trust Sanger Institute or a Wellcome Trust Centre? | YES ¨ NO x | ||||
| If yes, please specify: | ||||||
| Please provide a letter of support from the Director of the Centre/Clinical Research Facility specified. | ||||||
| Q27 | CONSULTANCIES AND EQUITIES | |||||
| Do any of the applicants have consultancies or any equity holdings in companies or other organisations that might have an interest in the results of the proposed research? |
YES ¨ NO x | |||||
| If yes, refer to notes and give brief details (no more than 200 words). | ||||||
| Q28 | COMMERCIAL EXPLOITATION | |||||
| (a) | Will the proposed research use technology, materials or other invention that, as far as you are aware, are subject to any patents or other form of intellectual property protection? | YES ¨ NO x | ||||
| If yes, give brief details (no more than 200 words). | ||||||
| (b) | Is the proposed research, in whole or in part, subject to any agreements with commercial, academic or other organisations? | YES ¨ NO x | ||||
| If yes, give brief details (no more than 200 words). | ||||||
| (c) | Is the proposed research likely to lead to any patentable or commercially exploitable results? | YES x NO ¨ | ||||
| If yes, give brief details (no more than 200 words). | ||||||
| ADMINISTRATION | 109 |
02/12
| At the end of the proposed work plan, it is expected that a successful Phase I clinical trial on SMT19969 will have been completed. At this time, Summit intends to seek a commercial partner to continue the later stage clinical development of the compound and this would be subject to the usual out-license deal structures discussed in Section Q12. | ||
| (d) | If any potentially commercially exploitable results may be based upon tissues or samples derived from human participants, please confirm that there has been appropriate informed consent for such use. | |
| N/A | ||
| ADMINISTRATION | 110 |
02/12

SUBJECT CLASSIFICATION
1. Systems and processes
Choose one primary (compulsory) and up to three secondary (optional).
Infection
Drug and vaccine development
2. Disease
Choose one primary (compulsory) and up to three secondary (optional).
Bacterial
3. Discipline
Choose one primary (compulsory) and up to three secondary (optional).
Clinical research
Microbiology - bacteriology
4. Technique
Choose up to three (optional).
5. Other identifier
Choose up to six (optional).
Antibiotic
6. Tick all relevant boxes (compulsory).
| BASIC | ¨ | |||
| CLINICAL | x | |||
| TROPICAL | ¨ | |||
| VETERINARY | ¨ | |||
| TRANSLATION | ¨ | |||
| ADMINISTRATION | 111 |

COLLABORATION ON A GRANT FORM
| Reference Number: |
Collaborators, i.e. scientific/medical colleagues, who are associated with a research proposal and named in the body of the application, but are not Coapplicants, are asked to complete this form.
| Name of grant applicant: | ||||
| Department and institution: | ||||
| Name of collaborator: | ||||
| Full address: | ||||
| Title of research project: | ||||
| Extent and nature of collaboration:
|
||||
| (A brief paragraph providing details of:
|
||||
| • | The role and contribution of the collaborator, with an indication of the time the collaborator will spend on the project.
|
|||
| • | Any reagents the collaborator will provide. Please indicate if there are any Intellectual Property issues or restrictions arising from Material Transfer Agreements.) | |||
I confirm that I am willing to collaborate as stated above with on this research project
| Signed: |
|
Date: |
|
(if more than one copy of this form is required, duplicate as necessary)
| COLLABORATION FORM | 112 |

EQUAL OPPORTUNITIES MONITORING FORM
CONFIDENTIAL
The Commission for Racial Equality and the Equal Opportunities Commission recommend collecting data to monitor the fairness of selection decisions. There is no obligation to provide this information but the Wellcome Trust would be grateful if the Principal Applicant would complete this form to assist with this process. On receipt, this form will be separated from the completed application form. The information provided will be regarded as strictly confidential and will be held on a secure database; it will not be shown to anybody involved in the processing of the application. The Wellcome Trust will anonymise these data (i.e. remove the applicant’s name) when using them for statistical and research purposes.
| Name: | [**] | |||||
| 1. Sex: | x Male ¨ Female | |||||
| 2. Date of birth: | [**] | |||||
| 3. Ethnic origin: | [**] White | |||||
| [**] Chinese | ||||||
| [**] Black African | ||||||
| [**] Black Caribbean | ||||||
| [**] Black – Other Please describe: | ||||||
| [**] Indian | ||||||
| [**] Irish | ||||||
| [**] Pakistani | ||||||
| [**] Bangladeshi | ||||||
| [**] Other Please describe: | ||||||
| 4. Disability: | The Disability Discrimination Act 1995 states a person has a disability for the purposes of the Act if he/she: “Has a physical or mental impairment which has a substantial and long-term adverse effect on his/her ability to carry out normal day to day activities”. | |||||
| Do you consider yourself to be disabled within the definition of this Act? | YES ¨ NO x | |||||
| EQUAL OPPORTUNITIES MONITORING FORM | 113 |

Appendix A: Programme Xxxxx Chart.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of one pages was omitted. [**]
| APPENDIX | 114 |

Appendix B: Validation and Proof of Concept to Date.
B1: Spectrum of activity. SMT19969’s key differentiator, especially when compared to all other CDI antibiotics in clinical use or development, is an exceptionally narrow spectrum of activity with no significant growth inhibition of gut flora components. MIC90 values against C. difficile were typically >1,000 fold lower than those recorded against key members of the GI microflora including Gram positive and negative anaerobic and facultative bacteria such as Bacteroides, Bifidobacterium and Lactobacillus spp., as well as fungal organisms (both yeasts and moulds). This sparing of gut flora is expected to result in superiority over other CDI antibiotics by significantly reducing rates of recurrent disease (Q10) which remains the key clinical hurdle to achieving effective treatment of CDI. SMT19969 showed potent growth inhibition of C. difficile (MIC90=0.125µg/mL; range=0.06-0.25µg/mL) when tested against a comprehensive panel of 82 C. difficile clinical isolates, covering major ribotypes of clinical significance including the hyper-virulent ribotype 027 (BI/NAP1), endemic EU ribotypes such as 106 and 001 and emerging strains such as ribotype 078. All C. difficile isolates tested to date are susceptible to SMT19969 and no significant differences in MICs against different ribotypes have been observed (Table 2).
| C. difficile Group (No Isolates) |
SMT19969 |
Metronidazole |
Vancomycin | |||||
| MIC Range |
MIC90 |
MIC90 |
MIC90 | |||||
| Overall Total (82/82) |
0.06 - 0.25 | 0.125 | 8 | 2 | ||||
| Genotypically distinct group (30) |
0.06 - 0.125 | 0.125 | 2 | 2 | ||||
| • Ribotype 001 (10) |
0.06 - 0.125 | 0.125 | 1 | 4 | ||||
| • Ribotype 027 (11) |
0.125 - 0.25 | 0.125 | 2 | 2 | ||||
| • Ribotype 106 (10) |
0.125 - 0.25 | 0.125 | 2 | 2 | ||||
| Reduced MET susceptibility (21) |
0.06 - 0.125 | 0.125 | 8 | 2 | ||||
Table 2: Minimum Inhibitory Concentrations of SMT19969, Metronidazole and Vancomycin against 82 C. difficile Clinical Isolates. MICs recorded in µg/mL.
| B2: Efficacy Studies. In the gold standard hamster model of CDI, SMT19969 showed superior efficacy to vancomycin (Figure 1). SMT19969 (20mg/Kg SID) showed complete protection from CDI with a 100% survival rate through to day 21 compared to a 40% survival rate in vancomycin controls (20mg/Kg SID). In the vehicle control group, 0% survival was recorded by day 2. SMT19969 has also been assessed in the human gut model of CDI (developed by Professor Xxxx Xxxxxx) which monitors effect of drug against C. difficile in the context of the entire human gut microbiome. SMT19969 showed a rapid reduction in toxin titres, approximately a 3 log reduction in C. difficile total viable counts and, most importantly, was highly sparing of the indigenous gut flora which was at normal healthy levels by the end of SMT19969 dosing. |
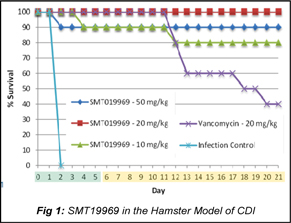
|
B3: Resistance Development. Resistance development studies have demonstrated the very low propensity for C. difficile to develop SMT19969 resistance which is an important aspect in the development of any new antibiotic. Spontaneous resistant mutants could not be isolated (Fres<6.99x10-9) and no increase in SMT19969 C. difficile MIC was observed following 14 serial passages at 0.5xMIC. In fact, generation of C. difficile mutants resistant to SMT19969 required the aggressive use of mutagens on gradient plates. Importantly these mutants showed no cross resistance to known classes of antibiotics including the current front-line CDI antibiotics vancomycin and metronidazole.
| APPENDIX | 115 |

B4: Preclinical Development. Regulatory GLP toxicology studies are now complete and data generated support moving the compound into clinical trials. No effects were seen in the rat Xxxxx test, no genotoxicity was observed (Xxxx and chromosomal aberration +/-S9) and no significant inhibition of hERG tail current was observed. 28 days of repeat oral dosing (clinical route of administration) in both rat and dog at 1,000mg/Kg/day resulted in no in-life or necropsy findings and the No Observed Adverse Effect Limit (NOAEL) for oral dosing is set at 1,000mg/Kg which is at least 150 fold higher than the likely maximum clinical dose. Importantly, no systemic exposure is seen following oral dosing with no SMT19969 detected in any plasma sample (LOQ=1ng/mL). This retention in the GI tract and therefore the site of infection, is supported by QWBA and excretion mass balance studies using 14C labelled SMT19969 where no radioactivity was detected in any tissue outside of the GI tract and >99.8% of radioactivity was excreted in faeces. Although SMT19969 will likely be retained in the GI tract and no plasma exposure is expected in patients the regulatory bodies will expect an understanding of any systemic toxicity associated with XXX000000 be developed should systemic exposure be seen and also allows the stopping criteria to be set for the Phase I trials (Q10). As such, the 28 day repeat dose toxicology studies in rat and dog included IV administration arms and although compound solubility issues hampered administration by IV, 28 days of repeat administration was successfully completed and NOAELs set that demonstrate a significant window between oral bioavailability and systemic adverse effects and these NOAELs will form the basis for setting the hard stop in the PI trial discussed in Q10. Finally, the NOAEL in the anaesthetised dog cardiovascular and respiratory study was set at 0.5mg/Kg (IV administration) and all chemistry, manufacturing and control (CMC) activities to support an FIH study have been completed, including manufacture of a single GMP batch of SMT19969 that is being used in the pivotal GLP toxicology studies and will be used in the proposed PI human trials.
| Summary of NOAEL limits from the 28 Day Repeat Dose Studies | ||||||
| Rat |
IV |
PO |
Fold Difference at NOAELs | |||
| Dose (mg/Kg) |
0.1 | 1,000 | — | |||
| Cmax/Co (ng/mL) |
1170 | <1 | >1170 | |||
| AUC0-t (ng.h/mL) |
35.3 | <<1 | >>35 | |||
| Dog |
IV |
PO |
Fold Difference at NOAELs | |||
| Dose (mg/Kg) |
0.1 | 1,000 | — | |||
| Cmax/Co (ng/mL) |
481 | <1 | >480 | |||
| AUC0-t (ng.h/mL) |
32.7 | <<1 | >>33 | |||
Table 3: Summary of the NOAELs following 2 8days repeat IV and oral administration in rat and dog.
Appendix C: CDI and the Medical Need
| C1: Epidemiology. Clostridium difficile infection (CDI) is now firmly established as a significant healthcare issue and is the leading cause of infectious nosocomial diarrhoea in the developed world. The unprecedented rise in prevalence in recent years, starting in the late 1990s with outbreaks in the US and Canada, has resulted in CDI becoming endemic in the North American and European healthcare systems. In the USA, the CDC reported a total of 350,000 cases in 2010 (Figure 2: Data from HCUP Statistical Brief #124 and CDC National Vital Statistic Reports) although reliable estimates put the annual number of cases at around 500,000. Although the rise in prevalence seems to have halted in the US, figures over the last few years suggest that a plateau has been reached at around 4 fold more cases than reported in 1993. Overall, this has placed an enormous financial and human welfare burden on the healthcare system. Healthcare costs in the US are |
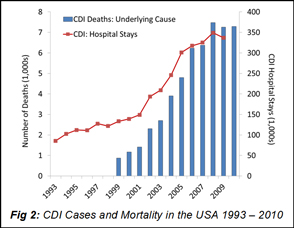
|
| APPENDIX | 116 |

estimated at >$1bn p.a and the individual cost of each CDI case in the EU is €33,840. The management of patients with CDI often requires isolation and environmental decontamination and in the case of outbreaks may necessitate cohort isolation and xxxx closure. Although CDI is a disease that disproportionally affects the elderly or immunocompromised, increasing numbers of cases are being reported in previously low risk groups such as the young. There is increasing awareness of CDI as an emerging community issue with community onset CDI now being linked with higher risk of associated colectomy. A similar picture has been reported in the UK where a dramatic rise in prevalence resulted in a peak of >55,000 cases in 2006 and although significant efforts in the UK have reduced the number of cases, recent data suggests a stabilisation at around 20,000 p.a. The wider European picture continues to show an increasing number of cases in Denmark, Finland, Germany and Spain and an on-going north to south spread of the disease across the continent. Although now endemic in the EU and USA, CDI in Eastern Asia and Australia has recently started to emerge as a significant issue. Although Australia has historically had a relatively well controlled level of CDI with few cases progressing to severe disease in 2011 the first cases of CDI due to hypervirulent BI/NAP1/027 strains were encountered and a similar picture has been emerging in Japan.
C2: Hyper-virulent Strains. One of the most significant drivers for the emergence of CDI as a worldwide problem is the emergence of hypervirulent strains of C. difficile and in particular the BI/NAP1/027 strain. The key issues presented by hyper-virulent BI/NAP1/027 infection are:
| • | Mutations in the negative regulatory gene tcdC result in production of up to 20-fold more toxin than non-hypervirulent strains resulting in more severe forms of the disease, increased risk of life threatening complications and a 2.5-3.5 fold increase in associated mortality rates. |
| • | Increased rates of recurrent disease when compared to infection by non-hypervirulent strains. This is likely due to factors such as an increased sporulation capability that allows BI/NAP1/027 to effectively exploit any niche held open by previous rounds of antibiotic use. |
Although BI/NAP1/027 remains a significant issue in North America (approx. 30% of cases) the number of 027 cases in the UK and EU has fallen over recent years. However, new strains continue to emerge and in particular ribotype 078, which was first reported in The Netherlands in 2005 is now the 3rd most common ribotype in the EU. Ribotype 078 displays all the characteristics of a hyper-virulent strain with mutations in tcdC resulting in increased toxin production and increased rates of severe disease. In addition, new ribotypes continue to emerge that are associated with increased disease severity.
| C4: Antibiotic Treatment Options. Despite this, therapy options are limited with the FDA approved Vancocin® (oral vancomycin) and off label metronidazole being the only two mainstay antibiotics available. Whilst reasonably effective in treating initial infection, the major clinical problem with CDI antibiotics is recurrent disease. Up to 30% of patients receiving vancomycin or metronidazole will experience at least one episode of recurrent CDI with each episode associated with an increased risk of further recurrence and severe disease. In addition, treatment failure rates and rates of recurrent disease have increased over the last three decades (Figure 3; Adapted from Lancet Infectious Dis. 2005: 5, 549–557). In the case of metronidazole the recent emergence of isolates with reduced susceptibility is likely to significantly erode efficacy further in the coming years and could result in metronidazole no longer being considered a realistic treatment option. Dificid™ (fidaxomicin) has recently been approved in the US |
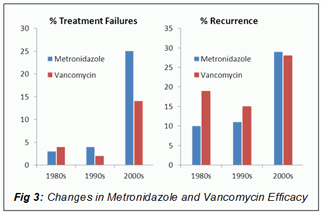
| |
| and EU with the US launch in Q3 2011. Although fidaxomicin has shown an improvement in preventing recurrent disease when compared to vancomycin, only 70% of fidaxomicin patients showed a sustained clinical response | ||
| APPENDIX | 117 |

during PIII trials (compared to vancomycin at 58%) – i.e. 30% of fidaxomicin patients are either treatment failures or suffer recurrent disease. In addition, PIII subgroup analysis has shown that fidaxomicin shows no improvement in rates of recurrent disease for BI/NAP1/027 infections when compared to vancomycin. Alternative approaches (see Q12e) to CDI therapy, such as the use of probiotics, toxin sequestering agents, antibodies to the C. difficile toxins and faecal biotherapy have shown either limited efficacy or are impractical as first choice therapy options for the effective management of CDI.
C5: Summary. Overall, these data show that although prevalence increases have generally slowed or halted in the US and UK, CDI is a continually shifting and dynamic disease with the continued emergence of new and difficult to treat strains set against a backdrop of inadequate therapies. The profile of SMT19969 presents a genuine opportunity to develop an effective and economically viable CDI therapy that would address recurrent disease and hyper-virulent infections and would offer significant healthcare benefits by reducing morbidity and mortality rates and relieving the economic and resource pressures of CDI on the healthcare system.
| APPENDIX | 118 |

Appendix D: Supporting Tables of Data
| Agent and |
Current Status |
Spectrum of |
C. difficile |
PII Study Design Summary | ||||||||||||
| Study Design |
Dosing Regimen |
No
of |
No
of |
Optimal | ||||||||||||
| Vancomycin ViroPharma |
Approved | Broad spectrum Gram+ve activity Activity against B. fragilis group |
1.0 | N/A | N/A | N/A | N/A | 500mg (125mg QID) | ||||||||
| Metronidazole Generic |
Off-label for CDI | Broad spectrum anaerobe activity | 1.0 | N/A | N/A | N/A | N/A | 1500mg (500mg TID) | ||||||||
| Fidaxomicin Optimer Pharmaceuticals |
Approved (CDI only) | Inhibits growth of most Gram+ve bacteria | 0.125-0.25 | Open label Randomised No comparator |
10 days 50mg BID 100mg BID 200 mg BID |
48 | 5 (US) | 400mg (200mg BID) | ||||||||
| CB-183,315 Cubist Pharmaceuticals |
PII/III | Broad spectrum Gram+ve activity | 0.25 | Double blind Randomised Vancomycin comparator |
10 days 125mg BID 250mg BID |
209 | 32 (US) | 500mg (250mg BID) | ||||||||
| LFF571 Novartis |
PI/II | Broad spectrum Gram+ve activity | 0.5 | Single blind Randomised Vancomycin comparator |
Unknown | 88 | 18 (US) | N/A | ||||||||
| Cadazolid Actelion |
PII | Unknown Likely broad spectrum |
Unknown | Double blind Randomised Unknown comparator |
Unknown | Unknown | 92 | 19 (US and EU) | ||||||||
| CDA1/CDB1 (MK3415A) Merck |
PIII | N/A – toxin antibody | Double blind Randomised Placebo |
Single IV dose of antibodies + standard of care | 200 | 29 (US) | N/A | |||||||||
Table 4: Leading Marketed and Development CDI Agents.
| 119 |

SCHEDULE 4B
THE PLAN
SMT19969: A Selective Antibiotic for C. difficile Infection
Project Proposal to Complete a CDI Patient Trial
Confidential
Prepared by
Summit PLC
for the
Wellcome Trust
FINAL
Version 2.0
9th October 2012
| 120 |

Table of Contents
| Introduction |
122 | |||
| Milestone 1 |
123 | |||
| 1.0 Introduction |
123 | |||
| 2.0 Phase 1 Trial Design Summary |
123 | |||
| 2.1 Part 1 – Single Ascending Dose Phase |
123 | |||
| 2.2 Part 2 – Multiple Ascending Dose Phase |
124 | |||
| 3.0 Supporting Studies |
126 | |||
| 3.1 Microbiology - Introduction |
133 | |||
| [**] |
||||
| Milestone 2 |
126 | |||
| 4.0 Introduction |
126 | |||
| 4.1 CMC Activities Introduction |
127 | |||
| [**] |
||||
| 5.0 Regulatory - Introduction |
127 | |||
| [**] |
||||
| 6.0 Staff Recruitment |
127 | |||
| Milestone 3 |
127 | |||
| 7.0 Introduction |
127 | |||
| 7.1 Comments on Trial Protocols and Budgets |
128 | |||
| 8.0 Smaller Phase 2 - Introduction |
128 | |||
| [**] |
||||
| 9.1 Trial Design |
128 | |||
| [**] |
||||
| Budget |
129 | |||
| 10.0 Summary |
129 | |||
| 121 |

Introduction
Following submission of a Translation Award application to Wellcome Trust by Summit PLC, Wellcome Trust has made a conditional offer of funding subject to:
| • | Mutual agreement, by the end of September 2012, of a revised plan and budget for a SMT19969 Phase I study in healthy volunteers and a Phase 2a efficacy study in a patient cohort |
| • | Due diligence on project cost |
| • | Both parties agreeing and executing the funding agreement |
This document describes a plan to take SMT19969 from the current stage of development through to completion of a Phase 2 patient trial. The programme of work is divided into 3 Milestones as highlighted in Table 1. Milestone 1 will essentially be the package of work described in the original Translation Award application (re-summarised in this document) although additional microbiology and CMC activities have been included to support the proposed patient trial. Milestones 2 and 3 will be work required to complete the revised plan requested by Wellcome Trust. Costs and timelines are based, where possible, on competing quotations from various CROs and service providers.
| [**] |
[**] |
[**] |
[**] |
|||||||||
| [**] |
[**] | [**] | [**] | |||||||||
| [**] |
[**] | [**] | [**] | |||||||||
| [**] |
[**] | [**] | [**] | |||||||||
Table 1: Summary of Revised Project Milestones
Discussions have been held between Wellcome and Summit on the design of the first CDI patient trials with SMT19969. [**]
Following the meeting of 19th September between Summit and Wellcome Trust, it was agreed that Wellcome would contribute £4million towards funding of the smaller of the two trial designs described below and that Summit would commit to the remaining £2.4million. Both trial designs were discussed and it was agreed, following some modification to the designs initially presented, that the smaller of the Phase 2 trials would generate meaningful data on the clinical efficacy and commercial differentiation of SMT19969. However, should additional funding beyond that agreed at this stage between Wellcome and Summit be secured, then a larger trial design will be considered which is also described in this document.
| 122 |

| Smaller Phase 2 |
Larger Phase 2 | |||||
| Summary Design |
Objectives |
Summary Design |
Objectives | |||
| • Three groups
• Total of 100 patients
• Vancomycin comparator
• Three SMT19969 regimens |
• Efficacy
• Early recurrence rates
• Microbiology
• Gut flora analysis
• Selected immune/inflammatory markers |
• Four groups
• Total of 200 patients
• Vancomycin comparator
• Three SMT19969 regimens |
• Efficacy
• Dose selection for Phase 3
• Early recurrence rates
• Long term recurrence rates
• Microbiology
• Gut flora analysis
• Immune/inflammatory biomarkers | |||
Table 2: Summary of Phase 2 Trial Options
Milestone 1
1.0 Introduction
The objective of Milestone 1 will be the completion of a Phase 1 healthy volunteer study. In parallel to the clinical trial a series of supporting studies are to be run that will continue to build on the data package surrounding the compound and to allow initiation of a CDI patient trial.
Documentation for regulatory and ethics review of the trial has been submitted and it is expected that the first volunteer will be dosed on 8th October 2012.
2.0 Phase 1 Trial Design Summary
The trial will be a randomised, double-blind, placebo controlled study to investigate the safety, tolerability and pharmacokinetics of single and multiple ascending oral doses of SMT19969 in healthy male volunteers. The trial is conducted in two parts as described below and the full trial protocol is included in Appendix A.
| • | 2.1 Part 1 – Single Ascending Dose Phase |
| Objectives | ||
| Primary objective |
• Determine safety and tolerability of ascending single oral doses | |
| Secondary objectives |
• Determine concentrations of SMT19969 in plasma and faeces
• Protocol allows for metabolite, degradant or biomarkers analysis from plasma and faecal samples if required
• Assess the effect of food on systemic exposure | |
| 123 |

Table 3: Phase 1 Part 1 Trial Objectives
| • | Part 1 will use 4 groups (A-D) of healthy male volunteers aged 18-55 years as summarised in Table 4. |
| • | Group C will take part in two treatment periods (TP1 – fasted and TP2 – fed) to assess effect of dietary state on systemic exposure. |
| • | Starting dose (group A) will be 100mg based on review of data from preclinical toxicology studies. |
| • | Anticipated dose escalations are described in Table 4 but will be determined following review of safety and PK data from previous group. Protocol allows for a maximum single dose of 2,000mg. |
| • | There will be a minimum of 7 days between each dose escalation to review safety, tolerability and PK data. |
| • | Faecal samples will be analysed for levels of SMT19969 to 72 hours post dose (Groups A, B, CTP1, D) and this will form an important part of determining dose selection in Part 2 of the study. |
| • | Table 5 summarises the timeline and sampling points for Part 1 of the study. |
| Group | Dose | N |
Dietary Sate | Residence at Unit | ||||||
| SMT19969 |
Placebo |
|||||||||
| A |
100mg | 6 | 2 | Fasted | Day -1 to Day 4 | |||||
| B |
400mg a | 6 | 2 | Fasted | Day -1 to Day 4 | |||||
| C (TP1) |
1,000mg a | 6 | 2 | Fasted | Day -1 to Day 4 | |||||
| D |
2,000mg a | 6 | 2 | Fasted | Day -1 to Day 4 | |||||
| C (TP2) |
1,000mg a | 6 | 2 | Fed | Day -1 to Day 2 | |||||
Table 4: Phase 1 Part 1 Summary
| a: | Dose escalations to be confirmed following review of previous group data. |
| Time point – hours post dose | ||||||||||||||||||||
| Group |
Sample |
Pre- dose |
1 |
2 |
4 |
8 |
12 |
24 |
48 |
72 | ||||||||||
| A, B,C (TP1), D |
Plasma | X | X | X | X | X | X | X | X | X | ||||||||||
| Faeces | X | Xa | Xa | Xa | ||||||||||||||||
| C (TP2) |
Plasma | X | X | X | X | X | X | X | — | — | ||||||||||
| Faeces | X | Xa | — | — | ||||||||||||||||
Table 5: Sample collection for Part 1
| a: | Samples voided over each 24 hour period to be pooled for analysis. |
| • | 2.2 Part 2 – Multiple Ascending Dose Phase |
| Objectives | ||||||||
| Primary objective | • | Determine safety and tolerability of ascending multiple oral doses | ||||||
| Secondary objectives | • | Determine concentrations of SMT19969 in plasma and faeces. | ||||||
|
• |
Protocol allows for metabolite, degradant or biomarkers analysis from plasma and faecal samples if required. | |||||||
| • | Determine effects of repeat oral dosing on gut flora composition | |||||||
| 124 |

Table 6: Phase 1 Part 2 Trial Objectives
| • | Part 2 will use 2 groups (E, F) of healthy male volunteers aged 18-55 years as summarised in Table 7. |
| • | The protocol allows for an additional Group G to be included should data from Groups E and F warrant examination of alternative dose levels or regimens. |
| • | Starting dose for group E will be determined following review of data from Part 1 but will likely be 200mg BID. Dose escalations will be determined following review of safety and PK data from previous group. |
| • | Table 8 summarises the timeline and sampling points for Part 2 of the study. |
| • | Samples collected on Days (±1 day) 1, 5 and 10 to be analysed for levels of SMT19969. |
| • | Samples collected on Days (±1 day) 0, 4, 9 and 24 will be assessed for effects of repeat administration of SMT19969 on the composition of the gut flora. |
| Group | Dose | Regimen | N |
Residence at Unit | ||||||
| SMT19969 |
Placebo |
|||||||||
| E |
200mg a | BID on days 1-9, Single dose day 10 | 6 | 2 | Day -1 to Day 12 | |||||
| F |
500mg b | BID on days 1-9, Single dose day 10 | 6 | 2 | Day -1 to Day 12 | |||||
| Gc |
TBC | TBC | 6 | 2 | Day -1 to Day 12 | |||||
Table 7: Phase 1 Part 2 Summary
a: Starting dose to be confirmed following review of data from Part 1. b: Dose escalations to be confirmed following review of previous group data. c: Inclusion of Group G to be confirmed following review of Group E and F data
| Time point – days post dose | ||||||||||||||||||||||||||||||
| Group |
Sample |
Pre- dose |
1 |
2 |
3 |
4 |
5 |
6 |
7 |
8 |
9 |
10 |
11 |
12 |
24 | |||||||||||||||
| E, F | Plasma analysed for SMT19969 | — | Xa | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xb | Xc | — | — | — | |||||||||||||||
| Faeces collected | Xd | Xd | Xd | Xd | Xd | Xd | Xd | Xd | Xd | Xd | Xd | Xd | Xd | X | ||||||||||||||||
| Faeces analysed for SMT19969 | — | X | — | — | — | X | — | — | — | — | X | — | — | — | ||||||||||||||||
| Faeces analysed for gut flora | X | — | — | — | X | — | — | — | — | X | — | — | — | X | ||||||||||||||||
Table 8: Sample Collection for Part 2
a: 0, 1, 2, 4, 8, 12 hours post dose. b: Before morning dose. c: 0, 1, 2, 4, 8, 12, 24, 48 hours post dose. d: Samples voided over each 24 hour period to be pooled for analysis
| 125 |

Although feedback from the Translation Award committee is that assessment of faecal samples for gut flora composition should not be included, Summit recommends that this remain a part of the Phase 1 trial for the following reasons:
| • | The Phase 1 is the only opportunity to assess the effects of SMT19969 on a healthy and complete gut microbiota since the gut flora in CDI patients is significantly damaged. |
| • | Should any deleterious effects against gut flora be observed during the MAD phase at higher doses then a preliminary dose response relationship between GI concentrations and gut flora inhibition will be established. This will be valuable data in dose selection for Phase 2. |
| • | The cost of this analysis is minimal as all faecal samples voided during the course of the MAD phase are to be collected anyway and low cost, simple culture methods to analyse gut flora are to be used rather than the 16S RNA sequencing methods to be used in the proposed patient trial. |
| • | Data from the human gut model (detailed in SDDi Milestone 1 report) showed that total Clostridia were reduced during SMT19969 treatment. As Clostridia are often resident in the human GI tract, observation of a similar reduction would give a surrogate endpoint for an antibiotic effect in humans. |
3.0 Supporting Studies
[**]
Table 9: Summary of Supporting Studies
3.1 Microbiology - Introduction
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 2 pages were omitted. [**]
Milestone 2
4.0 Introduction
The objective of Milestone 2 will be to file an IND to support a Phase 2 clinical trial with SMT19969. In addition to completion and submission of the requisite regulatory documents, the drug product will be manufactured (following development work carried out in Milestone 1), finalisation of clinical trial protocols and engagement of trial centres. As summary of activities is shown in Table 10 with further detail in Sections 4.1-6.0.
| [**] |
||
| [**] | [**] | |
| [**] | [**] | |
| [**] |
||
| [**] | [**] | |
| [**] | [**] | |
| 126 |

Table 10: Summary of Milestone 2 Activities
| • | 4.1 CMC Activities Introduction |
CMC quotes have been requested from Covance as they have the most experience of SMT19969 and they have provided a reasonably detailed proposal. Additional CMC suppliers will be contacted shortly for competing quotes.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 1 page was omitted. [**]
5.0 Regulatory - Introduction
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 1 page was omitted. [**]
| • | 6.0 Staff Recruitment |
[**]
Milestone 3
7.0 Introduction
As discussed in Sections 1.0 two possible options for Phase 2 patient trials with SMT19969 are presented in this document. These options have been prepared following discussions with independent experts and a summary of the trial designs is shown below (Table 11) with specific discussions on endpoints presented in Section 8 and 9. Draft trial synopses are included in Appendix B and C and budget in Section 10.
Following the meeting of 19th September between Summit and Wellcome Trust, it was agreed that Wellcome would contribute £4million towards funding of the smaller of the two trial designs described below and that Summit would commit to the remaining £2.4million. Both trial designs were discussed and it was agreed, following some modification to the designs initially presented, that the smaller of the Phase 2 trials would generate meaningful data on the clinical efficacy and commercial differentiation of SMT19969. However, should additional funding beyond that agreed at this stage between Wellcome and Summit be secured, then a larger trial design will be considered.
| 127 |

[**]
Table 11: Summary Designs of Phase 2 Options
| • | 7.1 Comments on Trial Protocols and Budgets |
This proposal reflects Summits projections and trial designs based on current knowledge. As the project progresses Summit, in conjunction with Wellcome, expects the project plan to be modified and refined as further discussions are held with experts and as additional information becomes available.
Quotes have been requested from INC Clinical, Parexel and Covance for the clinical trials. Costs described in this document are those provided by INC Clinical as they have the most recent and in-depth experience of CDI trials having carried out Optimer’s Phase 3, Cubist’s Phase 2 and are currently running Cubist’s Phase 3 CDI trials. The costs provided by Parexel are Covance broadly in-line with the INC quote.
It should be noted that the costs provided at this stage are ball-park figures and will be refined in line with the project plan’s development but should be sufficient for these early planning phases. In addition, no negotiation on costs has been held yet.
For the purposes of this document timelines for both the Phase 2 options are the same (See Table 24).
8.0 Proposed Smaller Phase 2 – Introduction
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 4 pages were omitted. [**]
| • | 9.1 Trial Design |
The study will be comprised of up to 4 groups, each of 50 CDI patients, as described in Table 16. An N=50 per arm would provide sufficient data to allow for selection of SMT19969 dosing regimen for Phase 3 and would also provide more robust data on rates of recurrent CDI. Vancomycin will be the comparator since it is recommended as the best treatment for the full range of disease severity that is likely to be encountered in the trial.
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 3 pages were omitted. [**]
| 128 |

Budget
10.0 Summary
Budgets for both the Phase2A and Phase 2 options are described with summary costs for each Milestone under each option shown below in Table 19.
[**]
| 129 |

SCHEDULE 5
MILESTONES, TRANCHES AND INSTALMENTS
| Tranche |
Amount of Advance (Pounds Sterling) |
Released on: | ||
| 1 | One million, two hundred and sixty two thousand and fifty seven (£1,262,057) | [**] | ||
| 2 | [**] | Within [**] Business Days of achievement of Milestone One | ||
| 3 | [**] | [**] | ||
| 4 (Retained Amount) |
[**] | Within [**] Business Days of receipt of the Clinical Study Report and an End of Award Report which is acceptable to the Trust | ||
MILESTONES AND MILESTONE DATES
| Milestone 1 |
Activity |
Milestone date | ||
| Phase 1 Trial | [**] | [**] | ||
| [**] | [**] | |||
| [**] | [**] | |||
| [**] | [**] | |||
| Supporting Studies | [**] | [**] | ||
| [**] | [**] | |||
| [**] | [**] | |||
| [**] | [**] | |||
| CMC | [**] | [**] | ||
| Toxicology | [**] | [**] | ||
| Milestone 1 Complete | [**] | |||
| 130 |

| Milestone 2 | ||||
| CMC | [**] | [**] | ||
| [**] | [**] | |||
| Regulatory | [**] | [**] | ||
| [**] | [**] | |||
| [**] | [**] | |||
| [**] | [**] | |||
| Milestone 2 Complete | [**] | |||
| Milestone 3 | ||||
| Clinical | [**] | [**] | ||
| [**] | [**] | |||
| [**] | [**] | |||
| [**] | [**] | |||
| Milestone 3 Complete | [**] | |||
| Milestone 4 | ||||
| End of award report and End of Grant Spend report and Final Clinical Study Report | [**] | |||
| Milestone 4 Complete | [**] | |||
| 131 |
SCHEDULE 6
TREASURY POLICY
Treasury Policy (for cash balances up to £[**])
| 1. | The company will maintain a balance representing up to [**] weeks operational requirements (currently c.£[**]) with its principal clearing bankers, currently HSBC. Overnight surplus balances will be “swept” to a Special Interest Bearing Account. |
| 2. | Balances of up to £[**] equivalent may also be held in currency accounts with its principal clearing bank in order to meet anticipated payments in foreign currency over a [**] month period. |
| 3. | The balance of remaining cash will be deposited with UK banks, or UK subsidiaries of European banks on immediate notice withdrawal or on fixed term between [**]. |
| 4. | Only banks with a Xxxxx’x short term ratings of P1 or S&P AA- (or better) will be considered. |
| 5. | The maximum amount to be placed with any one institution will be the higher of [**]% of total cash balances or £[**]. |
X X Xxxxxxx
Chief Financial Officer
| 132 |

SCHEDULE 7
CONDITIONS
The satisfaction of the following throughout the duration of the Project:
| 1. | submission of [**] monthly reports on Project progress to the Trust within [**] Business Days of the [**] anniversary of the Effective Date; |
| 2. | co-operation with the Site Visit Group prior to and during visits in accordance with Clause 14; and |
| 3. | compliance with any other agreements between the Company and the Trust relating to the Project. |
| 133 |

SCHEDULE 8
REVENUE SHARING AGREEMENT
EQUITY AND REVENUE SHARING AGREEMENT
DATED 20[ ]
TEMPLATE EQUITY AND REVENUE
SHARING AGREEMENT
(Seeding Drug Discovery Initiative)
BETWEEN
(1) SUMMIT CORPORATION PLC
and
(2) THE WELLCOME TRUST LIMITED
| 134 |

THIS AGREEMENT is made the day of 20[ ]
BETWEEN:
| (1) | THE WELLCOME TRUST LIMITED a company registered in England & Wales with company no. 2711000 with registered address at 000 Xxxxxx Xx Xxxxxx XX0 0XX XX, as Trustee of the Wellcome Trust, a charity registered in England under no. 210183 (the “Trust”); and |
| (2) | SUMMIT CORPORATION PLC a public limited company registered in England and Wales under number 05197494 whose registered office is at 00 Xxxxxx Xxxx, Xxxxxxxx, Xxxxxxxxxxx XX00 0XX (the “Company”). |
WHEREAS:
| (A) | Pursuant to a funding agreement between the Trust and the Company dated 30 October 2009, the Trust made a programme-related investment by way of an award of two million, two hundred and eighty eight thousand, two hundred and twenty one pounds sterling (£2,288,221) to the Company to progress the development of a novel class of antibiotics for the targeted treatment of Clostridium difficile infection in consideration of a share of any resulting revenue (the “SDD Award”). |
| (B) | The Trust has approved a Translation Award (award no. WT099444) to the Company to support a first-in-human Phase I and Phase II clinical trial for the novel Clostridium difficile antibiotic SMT19969 in consideration of a share of any resulting revenue (the “TA Award”). |
| (C) | To facilitate management and commercialisation of the technology arising under the SDD Award and the TA Award, the Parties have agreed that the Exploitation IPRs (as defined in the TA Award funding agreement) shall be exploited in accordance with the terms of this Agreement. |
IT IS XXXXXX AGREED as follows:
| 1. | INTERPRETATION |
| 1.1 | Capitalised terms in this Agreement shall be interpreted in accordance with the definitions as set out in the Funding Agreement or above. Where a capitalised term is defined in both this Agreement and the Funding Agreement, the definition in this Agreement shall apply. |
| 1. | 1.2 In this Agreement, unless the context otherwise requires: |
| 1.3 | “Accounting Standard” | means IFRS (International Financial Reporting Standards) as generally and consistently applied throughout each Party’s organization; | ||
| 1.4 | “Effective Date” | means [ ]; and | ||
| 1.5 | “TA Funding Agreement” | means the Translation Award Funding Agreement between the Parties dated [insert date]; | ||
| 135 |

| 1.6 | Capitalised terms in this Agreement shall be interpreted in accordance with the definitions as set out in the Funding Agreement or above. Where a capitalised term is defined in both this Agreement and the Funding Agreement, the definition in this Agreement shall apply. |
| 1.7 | References in this Agreement to any statutory provisions shall be construed as references to those provisions as respectively amended consolidated or re-enacted (whether before or after the Effective Date) from time to time and shall include any provisions of which they are consolidations or re-enactments (whether with or without amendment). |
| 1.8 | The Schedules and Recitals form part of this Agreement and any reference to this Agreement shall include the Schedules and Recitals. |
| 1.9 | In this Agreement: |
| (a) | the masculine gender shall include the feminine and neuter and the singular number shall include the plural and vice versa; |
| (b) | references to persons shall include bodies corporate, unincorporated associations, partnerships and individuals; and |
| (c) | except where the contrary is stated, any reference in this Agreement to a Clause or Schedule is to a Clause of or Schedule to this Agreement, and any reference within a Clause or Schedule to a sub-Clause, paragraph or other sub-division is a reference to such sub-Clause, paragraph or other sub-division so numbered or lettered in that Clause or Schedule. |
| 1.10 | The headings in this Agreement are inserted for convenience only and shall not affect the construction of the provision to which they relate. |
| 1.11 | References to the winding-up of a person include the amalgamation, reconstruction, Company, administration, dissolution, liquidation, bankruptcy, merger or consolidation of such person and an equivalent or analogous procedure under the law of any jurisdiction in which that person is incorporated, domiciled or resident or carries on business or has assets. |
| 1.12 | Any reference to books, records or other information includes books, records or other information in any format or medium including paper, electronically stored data, video or audio recordings and microfilm. |
| 1.13 | Any phrase introduced by the terms “including”, “include”, “in particular” or any similar expression shall be construed as illustrative and shall not limit the sense of the words preceding those terms. |
| 1.14 | Reference to any statute, statutory instrument, regulation, by law or other requirement of English law and to any English legal term for any actions, remedy, method of judicial proceeding, legal document, legal status, court, official or any legal concept or doctrine shall, in respect of any jurisdiction other than England, be deemed to include that which most nearly approximates in that jurisdiction to the relevant English term. |
| 136 |

| 2 | REVENUE SHARING ARRANGEMENTS |
| 2.1 | The Trust and the Company shall share cumulative Net Revenue received in respect of Exploitation of the Exploitation IPRs in accordance with the Financial Terms. |
| 3 | RECOVERY OF COSTS |
| 3.1 | Where Direct Costs incurred/allowed in a given accounting year exceed the Revenue from Exploitation of Exploitation IPRs for that year, then such excess costs shall be carried forward and offset against future Revenue until such time as they have been fully recovered. |
| 4 | ACCOUNTING STATEMENTS AND PAYMENTS |
| 4.1 | Within [**] days of the end of each Calendar Quarter, the Exploiting Party shall deliver a statement to the Non-Exploiting Party setting out for the relevant Calendar Quarter: |
| (a) | Revenue and Net Revenue received; |
| (b) | deductible Direct Costs and taxes; |
| (c) | sales of Licensed Products made by any member of the Exploiting Party’s Group or any Third Party; |
| (d) | the share of Net Revenue due to the Non-Exploiting Party pursuant to Clause 2.1 above; and |
| (e) | cumulative Revenue, cumulative Net Revenue and cumulative Direct Costs; |
(the “Quarterly Statement”).
| 4.2 | The Non-Exploiting Party shall deliver to the Exploiting Party an invoice for the amount due to it as set out in the Quarterly Statement in [pounds sterling]. |
| 4.3 | The share of Net Revenue due to the Non-Exploiting Party and any other amount invoiced shall be payable to the Non-Exploiting Party within [**] days of receipt of the invoice. |
| 4.4 | All payments of Net Revenue made by the Company to the Trust or by the Trust to the Company as the case may be under this Agreement shall be made in [pounds sterling]. Payment shall be made by electronic wire transfer of immediately available funds directly to the account of the relevant Party designated below or to any other account which the relevant Party may specify by written notice in accordance with Clause 7.3. |
| 4.5 | Bank Account for the Company: |
| Account Name: | [ ] | |
| Account No.: | [ ] | |
| Bank: | [ ] | |
| Sort code: | [ ] | |
| SWIFT code: | [ ] | |
| Branch: | [ ] |
| 137 |

| 4.6 | Bank Account for the Trust: |
| Account Name: | [ ] | |
| Account No.: | [ ] | |
| Bank: | [ ] | |
| Sort code: | [ ] | |
| SWIFT code: | [ ] | |
| Branch: | [ ] |
| 4.7 | Written confirmation of such transfer shall be sent by the Party sending the funds to the individual at the Party receiving the funds at the address provided in Clause 25 of the TA Funding Agreement. |
| 4.8 | Where any Revenue and Direct Costs in respect of the Exploitation IPRs is received or made in a currency other than sterling, the sterling equivalent of the sum shall be: |
| (a) | where such sum has been converted into sterling prior to preparation of the Quarterly Statement, the actual sterling sum on conversion; or |
| (b) | where such conversion has not taken place prior to preparation of the Quarterly Statement, calculated using the average of the buying and selling rates quoted by [**]. at the date the sum is received or paid by the Exploiting Party as applicable, or at such other date as the paying Party may reasonably specify having regard to the circumstances. |
| 4.9 | If the paying Party fails to pay any amount payable by it under this Agreement on the relevant due date, interest shall accrue on the overdue amount from the due date up to the date of actual payment (both before and after judgement) at the rate equivalent to [**] percent ([**]%) per annum above the three month sterling LIBOR from time to time. |
| 4.10 | All Net Revenue payments under this Agreement are expressed to be exclusive of VAT howsoever arising. Set out below are the VAT registration details for the Company and the Trust: |
| (a) | VAT registration details for the Company: |
[Company to insert]
| (b) | VAT registration details for the Trust: |
[Trust to insert]
| 4.11 | Each Party shall promptly inform the other Party in writing of any changes to the VAT registration details set out above. Neither Party shall charge VAT to the other Party on the supply of rights made or deemed to be made for VAT purposes pursuant to this Agreement provided however that if the VAT registration details provided are otherwise not acceptable to the competent authority, each Party may charge VAT appropriately as necessary in order to comply with that Party’s obligations under applicable law. In such event the |
| 138 |

| paying Party shall pay the receiving Party, in addition to any payment due hereunder, all VAT for which the receiving Party is liable to account to any competent authority in relation to any supply made or deemed to be made for VAT purposes pursuant to this Agreement. The paying Party shall pay any payments due to the receiving Party at the same time as the relevant payment is due under this Agreement. |
| 4.12 | If any paying Party is required by law to make any withholding or similar Tax payment on behalf of the receiving Party, with respect to any of the payments to be made to the receiving Party under this Agreement, the paying Party shall pay to the receiving Party such amount as shall after the deduction of any withholding tax, result in the receiving Party receiving such amount as it would have been entitled to had no withholding been made. |
| 4.13 | The Exploiting Party shall keep such records as are reasonably necessary to enable a proper assessment to be made of the following for at least [**] years: |
| (a) | the sums payable under this Agreement; |
| (b) | Revenue and Net Revenue received; |
| (c) | deductible Direct Costs and taxes on the Exploitation IPRs; |
| (d) | sales of Licensed Products made by any member of the Exploiting Party’s Group or any Third Party; and |
| (e) | cumulative Revenue, cumulative Net Revenue and cumulative Direct Costs; |
(the “Records”).
| 4.14 | The Exploiting Party shall allow an independent accountant duly authorised on behalf of and at the expense of the Non-Exploiting Party to inspect the Records by prior written appointment during normal business hours and not more than [**]. Such accountant shall not disclose to any Third Party or use for any unauthorised purpose any information not relevant to the verification of the sums due to the Non-Exploiting Party that is obtained as a result of any such inspection. The Exploiting Party shall procure that these inspection and audit rights extend to the records of the Exploiting Party’s Group and any sub-licensees thereof. |
| 4.15 | The Party arranging for the audit shall pay for the audit as well as its own legal expenses associated with enforcing its rights with respect to any payments due under this Agreement except where the audit reveals a discrepancy of [**] percent ([**]%) or more of any sums paid or payable, in which case the costs of the audit shall be paid by the audited Party. |
| 5 | DURATION AND TERMINATION |
| 5.1 | This Agreement shall commence on the Effective Date and shall continue for whichever is the longer of: |
| (a) | the last to expire of the Project Patents; |
| 139 |

| (b) | the expiry of any agreement entered into for the Exploitation of the Project IPRs; or |
| (c) | the expiry of any payment obligation relating to the Exploitation of the Project IPRs. |
| 5.2 | Either Party (“Terminating Party”) shall have the right to terminate this Agreement forthwith at any time upon giving written notice of termination to the other Party (“Defaulting Party”), upon the occurrence of any of the following events: |
| (a) | the Defaulting Party commits a breach of a material obligation set out in this Agreement which is not capable of remedy; |
| (b) | the Defaulting Party commits a breach of a material obligation set out in this Agreement which is capable of remedy but has not been remedied within [**] days of the receipt by it of a notice from the other Party identifying the breach and requiring its remedy; |
| (c) | the Defaulting Party is unable or admits inability to pay its debts as they fall due, suspends making payments on any of its debts or, by reason of actual or anticipated financial difficulties commences negotiations with one or more of its creditors with a view to rescheduling any of its indebtedness; |
| (d) | a proposal is made or a nominee or supervisor is appointed for a composition in satisfaction of the debts of the Defaulting Party or a scheme or voluntary arrangement of its affairs within the meaning of the relevant bankruptcy or insolvency laws, or the Defaulting Party enters into any composition or voluntary arrangement for the benefit of its creditors, or proceedings are commenced in relation to the Defaulting Party under any law, regulation or procedure relating to the re-construction, deferment or re-adjustment of all or substantially all of the Defaulting Party’s debts; |
| (e) | the Defaulting Party takes any action, or any legal proceedings are started whether by a Third Party or not, for the purpose of the winding up or dissolution of the Defaulting Party, other than for a solvent reconstruction or amalgamation; |
| (f) | the appointment of a liquidator, trustee, receiver, administrative receiver, receiver and manager, interim receiver custodian, sequestrator, administrator or similar officer, in respect of all or a substantial part of the assets of the Defaulting Party; |
| (g) | an effective resolution being passed for the winding-up or entering into administration (whether out of court or otherwise) of the Defaulting Party; |
| (h) | a distress, execution or other legal process being levied against all or substantially all of the assets of the Defaulting Party, and not being discharged or paid out in full within ten (10) Business Days of the commencement of each process; |
| 140 |

| (i) | the Funding Agreement has expired or terminated; and/or |
| (j) | the occurrence in respect of the Defaulting Party of any event in any jurisdiction to which it is subject having an effect similar to that of any of the events referred to in Clauses 6.2(c) to 6.2(h) above. |
| 6 | GENERAL |
| 6.1 | This Agreement is in addition to the TA Funding Agreement (as may be amended from time to time), which will continue to apply unless terminated on its terms. Should there be any conflict between this Agreement and the TA Funding Agreement, then this Agreement shall prevail. |
| 6.2 | Nothing in this Agreement shall give rise to any partnership or the relationship of principal and agent between the Trust and the Company. |
| 6.3 | All notices and communications shall be in writing and addressed to the Parties at the relevant address stated at the beginning of this Agreement (or such other address as may be notified from time to time). |
| 6.4 | None of the rights or obligations under this Agreement may be assigned or transferred without the prior written consent of the other Party. This Agreement shall be binding on and enure for the benefit of the successors in title of the Parties. |
| 6.5 | No waiver of any breach or default under this Agreement or any of the terms herein shall be effective unless such waiver is in writing and has been signed by the Parties. No waiver of any such breach or default shall constitute a waiver of any other or subsequent breach or default. |
| 6.6 | If any provisions of this Agreement are held to be invalid, illegal or unenforceable (in whole or in part) such provisions or parts shall to that extent be deemed not to form part of this Agreement but the remainder of this Agreement shall continue in full force and effect. |
| 6.7 | Each Party shall do and execute or arrange for the doing or executing of all acts, documents and things as may be necessary in order to implement this Agreement. |
| 6.8 | This Agreement (and any dispute, controversy, proceedings or claim of whatever nature arising out of this Agreement or its formation) shall be governed by and construed in accordance with the laws of England. The Parties irrevocably submit to the exclusive jurisdiction of the Courts of England. |
| 141 |

IN WITNESS whereof the Parties or their duly authorised representatives have executed this Agreement on the date hereinbefore written.
| Signed for and on behalf of
SUMMIT CORPORATION PLC
by its duly authorised representative: |
Signed for and on behalf of
SUMMIT CORPORATION PLC
by its duly authorised representative: | |||
| Signature: | Signature: | |||
| Name: | Name: | |||
| Title: | Title: | |||
| Date: | Date: | |||
| Signed for and on behalf of
THE WELLCOME TRUST LIMITED as trustee of the Wellcome Trust
by its duly authorised representative: |
Signed for and on behalf of
THE WELLCOME TRUST LIMITED as trustee of the Wellcome Trust
by its duly authorised representative: | |||
| Signature: | Signature: | |||
| Name: | Name: | |||
| Title: | Title: | |||
| Date: | Date: | |||
| 142 |

SCHEDULE 1 TO THE REVENUE SHARING AGREEMENT
COST OF GOODS
For the purpose of calculating the cost of Licensed Product a standard costing approach is to be applied. The following types of expenses shall be included:
| (i) | direct materials; |
| (ii) | direct labour; |
| (iii) | indirect manufacturing costs; |
| (iv) | quality assurance, and |
| (v) | certain variances as set out in 5 below, but not including other production costs, as identified below. |
| Each | of these categories of expenses are further specified below. |
In any event, the Cost of Goods shall include all cost elements appropriate under the Accounting Standard.
| 1. | Direct Materials |
Materials used in the manufacturing process that are traced directly to the completed Licensed Product, such as:
| • | Inert raw materials or excipients |
| • | Active substances/ingredients |
| • | Packaging components such as bottles, caps, labels, etc. |
| 2. | Direct Labour |
The cost of employees engaged in production activities that are directly identifiable with Licensed Product costs. This shall exclude supervision, which is included in indirect labour, and production support activities such as inspection, plant and equipment maintenance labour, and material handling personnel.
Direct labour cost includes:
| • | Base pay, overtime, vacation and holidays, illness, personal time with pay and shift differential. |
| • | Cost of employee fringe benefits such as health and life insurance, payroll taxes, welfare, pension and profit sharing. |
| 143 |

| 3. | Indirect Manufacturing Costs |
Costs for plant and equipment are to be applied to standard costs taking normal capacity utilization as a reference.
Costs which are ultimately allocated to product based on standard direct labour hours of the operating departments. These costs include:
| • | Indirect Production Labour - salaries of employees engaged in production activities who are not classified as direct labour, including supervision, clerical, etc. |
| • | Costs of Direct Labour - employees not utilized for the manufacturing of product such as training and general duties. |
| • | Indirect Materials - supplies and chemicals which are used in the manufacturing process and are not assigned to specific products but are included in manufacturing overhead costs. Includes supplies for which direct assignment to products is not practical. |
| • | Utilities - expenses incurred for fuel, electricity and water in providing power for production and other plant equipment and waste disposal. |
| • | Maintenance and Repairs - amount of expense incurred in-house or purchased to provide services for plant maintenance and repairs of facilities and equipment. |
| • | Other Services - purchased outside services and rentals such as the cost of security, ground maintenance, etc. |
| • | Depreciation - of plant and equipment utilizing the straight-line method of calculation. |
| • | Insurance - cost of comprehensive and other insurance necessary for the safeguard of manufacturing plant and equipment. |
| • | Taxes - expense incurred for taxes on real and personal property (manufacturing site, buildings and the fixed assets of equipment, furniture and fixtures, etc.) If manufacturing site includes other operations (marketing, R&D, etc.), taxes are allocated to manufacturing on the basis of total real and personal property. |
| • | Cost of manufacturing, service departments - such as: |
(where applicable)
| • | Packaging Engineering |
| • | Manufacturing Maintenance |
| • | Industrial Engineering |
| • | Receiving and Warehousing |
| 144 |

| • | Purchasing and Accounting |
| • | Production Scheduling |
| • | Inventory Management |
| • | Plant Materials Management |
| • | Central Weigh |
| • | Manufacturing Administration |
| • | Allocated costs of services provided to manufacturing including: (where applicable) |
| • | Cafeteria |
| • | Personnel Operations |
| • | Health and Safety Services |
| • | Division Engineering and Operations Services |
| • | Plant Services (housekeeping) |
| • | Manufacturing Information Systems |
| • | Plant Power |
| • | Office of V.P. Manufacturing |
Various bases are used for allocating these costs to manufacturing operating departments including headcount, square feet, metered utilities use, estimated services rendered, EDP computer hours, etc.
| 4. | Quality Assurance Costs |
Direct labour and indirect costs for Quality Assurance departments testing and approving materials used in manufacturing and completed manufacturing batches and finished products. This includes all manufacturing in-process testing and testing of finished materials. Excluded from product costs are Quality Assurance costs related to research and development, stability testing, and other costs customarily excluded from such Quality Assurance costs.
| 5. | Variance Costs |
| • | Standard Cost of Goods include cost elements which are set at so-called standard costs. They serve as a norm on how much typically a product costs. Deviations from such standard costs are captured in variances. |
| • | Inventory re/devaluation shall mean the gain or loss as a result of the inventory value adjustment due to changes in the standard costs. |
| 145 |

| • | Non-product related production costs shall contain Technical Operations Corporate Headquarter overhead costs, non product allocated QA costs, validation costs, directly expensed IT project costs, and other costs that cannot be attributed to specific products. |
| • | Warehousing & Distribution costs are costs related to warehousing and distribution activities for Finished Goods to be shipped to 3rd parties. |
| • | Write-offs are captured for the destruction of products that cannot be used anymore due to expiration of shelf-life, spoilage in the production process, and transportation mishaps. |
| • | Third Party royalties for manufacturing or marketing, and/or supply royalties paid to third parties |
| • | Product liability and/or business interruption insurance expenses, and |
| • | Patent maintenance costs |
The following expenses are not included in production costs:
| a) | Inventory Carrying Costs |
| b) | Regulatory Affairs Costs |
| c) | Significant idle capacity is eliminated from factory overhead and product cost. |
| d) | Intracompany profit. |
| 146 |

SCHEDULE 9
COSTS SCHEDULE
(including Company Contribution)
Milestone 1 budget – [**]
| Staff | ||||||
| Person |
Role |
Trust |
Company Contribution | |||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
|
|
| |||||
| [**] | [**] | |||||
|
|
| |||||
| Phase 1 Trial | ||||||||
| Section |
Primary Activities |
Service Provider |
Trust |
Company Contribution | ||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
|
|
| |||||||
| £[**] | £[**] | |||||||
|
|
| |||||||
| 147 |

| Supporting Studies |
||||||||||||||
| Section |
Activity |
Service Provider |
Trust |
Company Contribution |
||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] |
[**] | [**] | [**] | |||||||||||
| [**] | [**] | |||||||||||||
|
|
|
|
|
|||||||||||
| TOTAL | £[**] | |||||||||||||
|
|
|
|||||||||||||
| 148 |

Milestone 2 budget – [**]
| Staff |
||||||||
| Person |
Role |
Trust |
Company Contribution |
|||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
| [**] |
[**] | [**] | ||||||
|
|
|
|
||||||
| [**] | [**] | |||||||
|
|
|
|
||||||
Primary Activities
| Section |
Primary Activities |
Service Provider |
Trust |
Company Contribution | ||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] |
[**] | [**] | [**] | |||||
| [**] | [**] | |||||||
|
|
| |||||||
| TOTAL | [**] | |||||||
|
| ||||||||
| 149 |

Milestone 3 budget – [**]
| Person |
Role |
Trust |
Company Contribution | |||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
|
|
| |||||
| £[**] | [**] | |||||
|
|
|
| 150 |

| Section |
Primary Activities |
Trust |
Company Contribution | |||
| [**] |
[**] | [**] | ||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | |||||
| [**] | [**] | [**] | ||||
| [**] |
[**] | [**] | [**] | |||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] | [**] | |||||
|
|
| |||||
| TOTAL | £[**] | |||||
|
| ||||||
| 151 |

Milestone 3 budget – [**]
| Person |
Role |
Trust |
Company | |||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
| [**] |
[**] | [**] | ||||
|
|
| |||||
| [**] | [**] | |||||
|
|
|
Primary Activities
| Primary Activities |
Primary Activities |
Trust |
Company Contribution |
|||||
| [**] |
[**] | [**] | ||||||
| [**] | [**] | |||||||
| [**] | [**] | |||||||
| [**] | [**] | [**] | ||||||
| [**] | [**] | |||||||
| 152 |

| Primary Activities |
Primary Activities |
Trust |
Company Contribution |
|||||||
| [**] | [**] | |||||||||
| [**] | [**] | |||||||||
| [**] | [**] | |||||||||
| [**] | [**] | |||||||||
| [**] | [**] | |||||||||
| [**] | [**] | |||||||||
| [**] | [**] | |||||||||
| [**] | [**] | |||||||||
| [**] |
[**] | [**] | ||||||||
| [**] |
[**] | [**] | ||||||||
| [**] |
[**] | [**] | ||||||||
| [**] | [**] | |||||||||
|
|
|
|
|
|||||||
| TOTAL | £[**] | |||||||||
|
|
|
|||||||||
| 153 |

SCHEDULE 10
COMPANY PATENTS
| • |
| REF |
TITLE |
FAMILY |
APPLN NO. |
PRIO. DATE* |
FILING DATE | |||||
| [**] |
[**] | [**] | [**] | [**] | [**] |
Confidential Materials omitted and filed separately with the Securities and Exchange Commission
| 154 |

