AMENDED AND RESTATED LICENSE AGREEMENT BY AND BETWEEN INFINITY PHARMACEUTICALS, INC. AND VERASTEM, INC.
CONFIDENTIAL
Execution Version
Execution Version
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Double asterisks denote omissions.
Exhibit 10.4
AMENDED AND RESTATED LICENSE AGREEMENT
BY AND BETWEEN
AND
VERASTEM, INC.
CONFIDENTIAL
This Amended and Restated License Agreement (this “Agreement”) is entered into as the 1st day of November, 2016 and made effective as of the 29th day of October, 2016 (the “Effective Date”), by and between Infinity Pharmaceuticals, Inc., a corporation organized and existing under the laws of the State of Delaware and having a principal office located at 000 Xxxxxxxx Xxxxx, Xxxxxxxxx, Xxxxxxxxxxxxx 00000 (“INFI”), and Verastem, Inc., a corporation organized and existing under the laws of Delaware, having a principal office located at 000 Xxxxxxxx Xxxxxx, Xxxxx 000, Xxxxxxx, Xxxxxxxxxxxxx 00000 (“Licensee”). INFI and Licensee are each referred to herein by name or as a “Party” or, collectively, as “Parties.”
RECITALS
WHEREAS, Licensee and INFI are parties to that certain License Agreement, dated October 29, 2016 (the “Superseded Agreement”) which Licensee and INFI wish to replace and supersede in its entirety with this Agreement;
WHEREAS, Licensee possesses expertise in the Development and Commercialization (each as defined below) of pharmaceutical products;
WHEREAS, INFI controls certain intellectual property related to the IPI-145 Product (as defined below); and
WHEREAS, Licensee is interested in obtaining a license under such intellectual property to Develop, Manufacture and Commercialize the IPI-145 Product in the Field in the Territory (each as defined below), and INFI is willing to grant Licensee such license on the terms and conditions set forth in this Agreement.
NOW, THEREFORE, in consideration of the premises and mutual covenants herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
DEFINITIONS
As used in this Agreement, the following terms will have the meanings set forth in this Article 1 unless context dictates otherwise:
1.1 “Affiliate” means any entity that directly or indirectly controls or is controlled by or is under common control with a Person. For purposes of this definition, “control” or “controlled” means ownership, directly or indirectly, of more than fifty percent (50%) of the shares of stock entitled to vote for the election of directors, in the case of a corporation, or more than fifty percent (50%) of the equity interest in the case of any other type of legal entity (or if the jurisdiction where such corporation or other entity is domiciled prohibits foreign ownership of such entity, the maximum foreign ownership interest permitted under such laws, provided, that such ownership interest provides actual control over such entity), status as a general partner in any partnership, or
- 1 -
CONFIDENTIAL
any other arrangement whereby a Person controls or has the right to control the board of directors or equivalent governing body of a corporation or other entity.
1.2 “Annual Net Sales” means aggregate Net Sales of IPI-145 Products by Licensee, its Affiliates and/or the Sublicensees during a given Calendar Year.
1.3 “Business Day” means any day other than Saturday or Sunday on which the banks in New York, New York, United States are open for business.
1.4 “Calendar Quarter” means the respective periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31.
1.5 “Calendar Year” means a period of time commencing on January 1 and ending on the following December 31.
1.6 “Change of Control” means, with respect to a Party, any of the following: (a) the sale or disposition of all or substantially all of the assets of such Party or its direct or indirect controlling Affiliate to a Third Party, other than to an entity of which more than fifty percent (50%) of the voting capital stock are owned after such sale or disposition by the Persons that were shareholders of such Party or its direct or indirect controlling Affiliate (in either case, whether directly or indirectly through any parent entity) immediately prior to such transaction; or (b) (i) the acquisition by a Third Party, alone or together with any of its Affiliates, other than an employee benefit plan (or related trust) sponsored or maintained by such Party or any of its Affiliates, of more than fifty percent (50%) of the outstanding shares of voting capital stock of such Party or its direct or indirect controlling Affiliate, or (ii) the acquisition, merger or consolidation of such Party or its direct or indirect controlling Affiliate with or into another Person, other than, in the case of this clause (b), an acquisition or a merger or consolidation of such Party or its controlling Affiliate in which the holders of shares of voting capital stock of such Party or its controlling Affiliate, as the case may be, immediately prior to such acquisition, merger or consolidation will beneficially own, directly or indirectly, at least fifty percent (50%) of the shares of voting capital stock of the acquiring Third Party or the surviving corporation in such acquisition, merger or consolidation, as the case may be, immediately after such acquisition, merger or consolidation.
1.7 “Combination Product” means any pharmaceutical Product which contains two or more active pharmaceutical ingredients, at least one of which is an IPI-145 Compound.
1.8 “Commercial Sale” means any sale of a Product to a Third Party in any country in the Territory after the receipt of the Marketing Authorization for that country, if such Marketing Authorization is required.
1.9 “Commercialization” or “Commercialize” means any and all activities directed to the preparation for sale of, offering for sale of, or sale of a Compound or Product, including activities to secure and maintain market access (including any phase IV/post-approval clinical study that is not required to obtain or maintain Regulatory Approval) market, promote, distribute, and import a Product.
- 2 -
CONFIDENTIAL
1.10 “Compound” means a compound and any references to a Compound shall include all of its various chemical forms, including acids, bases, salts, metabolites, esters, isomers, enantiomers, pro-drug forms, hydrates, solvates, polymorphs and degradants thereof in crystal, powder or other form.
1.11 “Confidential Information” means (a) subject to clause (c) below, any Know-How and other proprietary scientific marketing, financial or commercial information or data, in any form (written, oral, photographic, electronic, magnetic, or otherwise) that is disclosed, supplied or made available to a Party (the “Receiving Party”) or any of its Affiliates by the other Party (the “Disclosing Party”) or any of its Affiliates or otherwise received or accessed by the Receiving Party or any of its Affiliates in the course of performing the Receiving Party’s obligations or exercising the Receiving Party’s rights under this Agreement; (b) subject to clause (c) below, any information that was disclosed by INFI to Licensee or any Affiliate of Licensee prior to the Effective Date pursuant to the Confidential Disclosure Agreement between INFI and Licensee, dated [**] (the “Existing Confidentiality Agreement”), which shall be treated as INFI’s Confidential Information, with INFI considered the Disclosing Party and Licensee considered the Receiving Party; (c) any Duvelisib Know-How Controlled by INFI as of the Effective Date that is solely and specifically related to the IPI-145 Compound or IPI-145 Product, which shall be treated as INFI’s and Licensee’s Confidential Information, with each of INFI and Licensee considered the Disclosing Party and each of Licensee and INFI considered the Receiving Party; (d) any Know-How with respect to which INFI is subject to any confidentiality or non-use obligations to any Third Party Grantor pursuant to an INFI Third Party Agreement, which shall be treated as INFI’s Confidential Information, with INFI considered the Disclosing Party and Licensee considered the Receiving Party; (e) any reports or other information (including any information made available in connection with any audit) delivered, disclosed or made available by Licensee, its Affiliates or its Sublicensees to INFI, its Affiliates or any Third Party Grantor in connection with this Agreement, which shall be treated as Licensee’s Confidential Information; and (f) the terms and conditions of this Agreement, which shall be treated as the Confidential information of both INFI and Licensee.
1.12 “Control” or “Controlled” means, with respect to any Know-How, Patent Right, other intellectual property right or any Compound, the legal authority or right (whether by ownership, license or otherwise, but without taking into account any rights granted by one Party to the other Party under the terms of this Agreement) of a Party or, as set forth herein, its relevant Affiliate, to grant access to, a license or a sublicense of or under such Know-How, Patent Right, intellectual property right or Compound to the other Party, or to otherwise disclose proprietary or trade secret information to the other Party, without breaching the terms of any agreement with a Third Party, or misappropriating the proprietary or trade secret information of a Third Party.
1.13 “Counterpart” means (a) with respect to a patent, collectively, any patent applications from which such patent issued, and all patents and patent applications described in clause (b) with respect to each such patent application; and (b) with respect to a patent application (including any provisional application), the following items, collectively: (i) all divisionals, continuations and continuations-in-part of such patent application; (ii) any patents (including certificates of correction) issuing from such patent application or any patent application described in clause (i); (iii) all patents and patent applications based on, corresponding to or claiming the priority date(s) of such patent
- 3 -
CONFIDENTIAL
application or any of the patents and patent applications described in clauses (i) or (ii); (iv) all rights derived from any of the items described in clauses (i), (ii) or (iii) including any substitutions, extensions (including supplemental protection certificates), registrations, confirmations, reissues, re-examinations and renewals of any of the patents described in clauses (ii) or (iii); and (v) foreign counterparts of any of the foregoing.
1.14 “Development” or “Develop” means, with respect to a Compound, all development activities starting with the initiation of the first IND-enabling GLP toxicology study for such Compound, excluding Research, medicinal chemistry and Commercialization.
1.15 “Diligent Efforts” means the efforts that [**]; provided, however, that a Person required to use “Diligent Efforts” under this Agreement will not be thereby required to take actions that [**]. Without limiting the generality of the foregoing, in determining Diligent Efforts with respect to the Development and Commercialization of the IPI-145 Compound or IPI-145 Product, the Parties shall take into account the following: [**].
1.16 “Dollars” or “$” means the legal tender of the United States.
1.17 “Duvelisib IP” means the Duvelisib Know-How, the Duvelisib Patent Rights and INFI’s and its Affiliates’ interest in any Joint IP.
1.18 “Duvelisib Know-How” means, subject to Section 12.6, Know-How that is (a) Controlled by INFI or any of its Affiliates on the Effective Date or thereafter during the Term (including INFI’s and its Affiliates’ interest in Joint Know-How), and (b) necessary or useful to Research, Develop, Manufacture or Commercialize any IPI-145 Compound or IPI-145 Product.
1.19 “Duvelisib Patent Rights” means, subject to Section 12.6, Patent Rights that (a) are Controlled by INFI or any of its Affiliates on the Effective Date or thereafter during the Term (including INFI’s and its Affiliates’ interest in Joint Patent Rights), and (b) claim or otherwise cover the Research, Development, Manufacture or Commercialization of any IPI-145 Compound or IPI-145 Product. Duvelisib Patent Rights include the INFI Prosecution Patent Rights, the INK Prosecution Patent Rights, the INK Non-Prosecution Patent Rights and the INFI Other Patent Rights.
1.20 “EMA” means the European Medicines Agency and any successor agency.
1.21 “FDA” means the U.S. Food and Drug Administration and any successor agency.
1.22 “FD&C Act” means the United States Federal Food, Drug, and Cosmetic Act, as amended.
1.23 “Field” means the treatment, prevention, palliation or diagnosis of any oncology Indication in humans or animals.
1.24 “Good Clinical Practices” or “GCP” means the then-current standards, practices and procedures (a) promulgated or endorsed by the FDA as set forth in the guidelines entitled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” including related regulatory requirements imposed by the FDA; (b) set forth in Directive 2001/20/EC of the European Parliament
- 4 -
CONFIDENTIAL
and of the Council of 4 April 2001 and Commission Directive 2005//28/EC of 8 April 2005; (c) ICH Guideline for Good Clinical Practice E6; (d) equivalent Laws of an applicable Regulatory Authority; and (e) all additional Regulatory Authority documents or regulations that replace, amend, modify, supplant or complement any of the foregoing.
1.25 “Good Laboratory Practices” or “GLP” means the then-current good laboratory practice standards promulgated or endorsed by the FDA as defined in 21 C.F.R. Part 58, as such regulations may be amended from time to time, and the equivalent regulations promulgated by the equivalent Regulatory Authority in the jurisdiction where the relevant Research or Development activities are performed.
1.26 “Good Manufacturing Practices” or “GMP” means then-current standards for the manufacture of pharmaceutical products, pursuant to (a) the FD&C Act (21 U.S.C. 321 et seq.); (b) relevant United States regulations in Title 21 of the United States Code of Federal Regulations (including Parts 11, 210, and 211); (c) European Community Directives 2003/94 and 91/356/EC; (d) the European Community Guide to Good Manufacturing Practice for Medicinal Intermediate Products; (e) ICH Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients; (f) equivalent Laws of an applicable Regulatory Authority at the time of Manufacture; and (g) all additional Regulatory Authority documents or regulations that replace, amend, modify, supplant or complement any of the foregoing.
1.27 “Governmental Authority” means any multinational, federal, state, county, local, municipal or other entity, office, commission, bureau, agency, political subdivision, instrumentality, branch, department, authority, board, court, arbitral or other tribunal, official or officer, exercising executive, judicial, legislative, police, regulatory, administrative or taxing authority or functions of any nature pertaining to government.
1.28 “Headlicense Termination Event” means the termination of the INK Agreement by INK for a material breach thereof and such material breach is the direct result of Licensee’s, its Affiliates’ or Sublicensees’ acts or omissions in breach of Licensee’s obligations under this Agreement that has not been cured in a timely manner; provided, that INFI has not received notice from INK that INFI is otherwise in material breach of the INK Agreement as of the time of such termination.
1.29 “ICH” means the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use.
1.30 “IND” an investigational new drug application filed with the FDA or the corresponding application for the investigation of a Product in any other country or group of countries, as defined in the applicable Laws and regulations and filed with the Regulatory Authority of such country or group of countries.
1.31 “Indication” means a disease, condition, disorder or syndrome.
1.32 “INFI Indemnitees” means INFI, its Affiliates and their respective directors, officers, employees and agents.
- 5 -
CONFIDENTIAL
1.33 “INFI Other Patent Rights” means, subject to Section 12.6, the Patent Rights Controlled by INFI as of the Effective Date or during the Term that are necessary or useful to Research, Develop, Manufacture or Commercialize the IPI-145 Product, but excluding the INFI Prosecution Patent Rights, INK Prosecution Patent Rights and INK Non-Prosecution Patent Rights.
1.34 “INFI Prosecution Patent Rights” means, subject to Section 12.6, the Patent Rights Controlled by INFI or any of its Affiliates that are set forth on Exhibit A, and including any Counterparts thereof.
1.35 “INFI Product Related Contracts” means (a) the agreements identified in Exhibit F-1 and (b) any agreement between INFI (or any of its Affiliates) and any Third Party that is a clinical trial site or investigator with respect to the Development of the IPI-145 Compound or IPI-145 Product (a “Clinical Site Agreement”).
1.36 “INFI Third Party Agreements” means the INK Agreement and the MICL Agreements.
1.37 “INK Agreement” means the Amended and Restated Development and License Agreement, dated December 24, 2012, as amended, by and between INFI and Intellikine LLC (“INK”), as may be amended from time to time to the extent permitted by this Agreement.
1.38 “INK Prosecution Patent Rights” means, subject to Section 12.6, the Patent Rights Controlled by INFI or any of its Affiliates that are set forth on Exhibit B, and including any Counterparts thereof.
1.39 “INK Non-Prosecution Patent Rights” means, subject to Section 12.6, the Patent Rights Controlled, but not owned, by INFI or any of its Affiliates pursuant to a license or sublicense granted to INFI pursuant to the INK Agreement, and including all Counterparts thereof, but excluding the INFI Prosecution Patent Rights and INK Prosecution Patent Rights.
1.40 “Internal Personnel Expenses” means with respect to INFI personnel or Licensee personnel, $[**] per FTE year, prorated to reflect the reasonable estimated percentage of such personnel’s time spent performing activities under this Agreement based on an 1800 hour FTE year.
1.41 “IPI-145 Compound” means the Compound known as IPI-145 or Duvelisib and described in Exhibit C, or, for clarity, any of its various chemical forms, including acids, bases, salts, metabolites, esters, isomers, enantiomers, pro-drug forms, hydrates, solvates, polymorphs and degradants thereof, in each case that has substantially the same pharmacological effect, in crystal, powder or other form.
1.42 “IPI-145 Product” means any Product which is, or which contains or comprises, the IPI-145 Compound.
1.43 “IPI-443 Product” means any Product which is, or which contains or comprises the Compound set forth in Exhibit D.
- 6 -
CONFIDENTIAL
1.44 “Joint IP” means Joint Know-How and Joint Patent Rights and other intellectual property rights (other than Patent Rights) covering Joint Know-How.
1.45 “Know-How” means all technical information, know-how and data, including inventions, discoveries, trade secrets, specifications, instructions, processes, formulae, materials, expertise and other technology applicable to formulations, compositions or products or to their manufacture, development, registration, use or marketing or to methods of assaying or testing them or processes for their manufacture, formulations containing them or compositions incorporating or comprising them, and including all biological, chemical, pharmacological, biochemical, toxicological, pharmaceutical, physical and analytical, safety, quality control, manufacturing, nonclinical and clinical data, regulatory data and filings, instructions, processes, formulae, expertise and information, relevant to the research, development, manufacture, use, importation, offering for sale or sale of, or which may be useful in studying, testing, developing, producing or formulating, products, or intermediates for the synthesis thereof. Know-How excludes the Patent Rights covering any inventions.
1.46 “Knowledge” means the actual knowledge, without any duty to investigate, of the INFI employee with the specified title as of the Effective Date.
1.47 “Law” means any provision of any then-current multinational, federal, national, state, county, local, municipal or foreign law, statute, ordinance, order, writ, code, rule or regulation, promulgated or issued by any Governmental Authority, as well as with respect to either Party any binding judgments, decrees, stipulations, injunctions, determinations, awards or agreements issued by or entered into by such Party with any Governmental Authority.
1.48 “Licensee Indemnitees” means Licensee, its Affiliates and their respective directors, officers, employees and agents.
1.49 “Licensee IP” means the Licensee Know-How and the Licensee Patent Rights, in each case, solely to the extent arising from the Research, Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Product using any Duvelisib IP.
1.50 “Licensee Know-How” means, subject to Section 12.6, Know-How that is (a) Controlled by Licensee or any of its Affiliates during the Term but not on the Effective Date; and (b) necessary or useful to Research, Develop, Manufacture or Commercialize any Compound that is a Target Inhibitor, or any Product containing such a Compound, in the Territory. Licensee Know-How includes Licensee’s and its Affiliates’ rights in Joint Know-How.
1.51 “Licensee Patent Rights” means, subject to Section 12.6, Patent Rights Controlled by Licensee during the Term but not on the Effective Date (and not prior to the Effective Date) and claiming Licensee Know-How. Licensee Patent Rights includes Licensee’s and its Affiliates’ interest in any Joint Patent Rights.
1.52 “MAA” means an application for the authorization for marketing of a Product in any country or group of countries outside the United States, and all supplements, including all
- 7 -
CONFIDENTIAL
documents, data and other information concerning the Product, as defined in the applicable laws and regulations and filed with the Regulatory Authority of a given country or group of countries.
1.53 “Manufacture” or “Manufacturing” means any activities directed to producing, manufacturing, scaling up, processing, filling, finishing, packaging, labeling, quality assurance testing and release, shipping and storage of a Compound or Product or component thereof (including production of drug substance and drug product, in bulk form, for preclinical and clinical studies and for Commercialization).
1.54 “Marketing Authorization” means the grant of all necessary permits, registrations, authorizations, licenses and approvals (or waivers) required for the manufacture, promotion, marketing, storage, import, export, transport, distribution, use, offer for sale, sale or other commercialization of a Product in any country.
1.55 “MHLW” means the Japanese Ministry of Health, Labour and Welfare and any successor agency.
1.56 “MICL Agreements” means (a) the Termination and Revised Relationship Agreement by and between INFI and Mundipharma International Corporation Limited (“MICL”), entered into as of July 17, 2012; and (b) the Termination and Revised Relationship Agreement by and between INFI and Purdue Pharmaceutical Products L.P. (“Purdue”), entered into as of July 17, 2012; each ((a) and (b)) as may be amended from time to time to the extent permitted by this Agreement.
1.57 “NDA” means with respect to a Product, a new drug application and all supplements filed with the FDA with respect to such Product, including all documents, data and other information concerning such Product which are necessary for, or included in, a Marketing Authorization to use, sell, supply or market such Product in the United States.
1.58 “Net Sales” means (I) with respect to an IPI-145 Product (subject to clause (II) below, for a Combination Product) in a particular period, the gross amount invoiced by Licensee, its Affiliates and/or the Sublicensees on sales or other dispositions (excluding sales or dispositions for use in clinical trials or other scientific testing, in either case for which Licensees, its Affiliates and/or the Sublicensees receive no revenue) of such IPI-145 Product to unrelated Third Parties during such period, less the following deductions (to the extent included in the gross amount invoiced or otherwise directly paid or incurred by Licensee, its Affiliates and/or its Sublicensees):
(a) trade, cash and quantity discounts actually allowed and taken directly with respect to such sales or other dispositions;
(b) tariffs, duties, excises, sales taxes or other taxes imposed upon and paid directly with respect to the delivery, sale or use of the IPI-145 Product and included and separately stated in the applicable invoice (excluding national, state or local taxes based on income);
(c) allowances for amounts repaid or credited by reason of rejections, defects, recalls or returns or because of reasonable and customary chargebacks, refunds, coupons, patient
- 8 -
CONFIDENTIAL
co-pay savings cards, rebates (including related administration fees), wholesaler fee for service, reasonable amounts of physician samples, reasonable amounts of free products given to indigent patients, retroactive price reductions or any other items substantially similar in character and substance to the foregoing, with equitable adjustments to be made from time to time for any differences between these allowances and actual amounts;
(d) amounts previously included in Net Sales of IPI-145 Products that are written-off by Licensee as uncollectible in accordance with Licensee’s standard practices for writing off uncollectible amounts consistently applied; and
(e) freight, insurance and other transportation charges incurred in shipping an IPI-145 Product to Third Parties, included and separately stated in the applicable invoice;
and (II) with respect to an IPI-145 Product that is a Combination Product in a particular period, Net Sales of such Combination Product during such period (as determined in accordance with clause (I)) multiplied by (a) the fraction, A/(A+B), where A is the average sale price of the IPI-145 Product when sold separately in finished form and B is the average sale price of the other active pharmaceutical ingredients included in the Combination Product when sold separately in finished form or (b) where the average sale price cannot be determined for both the IPI-145 Product and all other active pharmaceutical ingredients included in such Combination Product, the fraction, C/(C+D), where C is the fair market value of the IPI-145 Product and D is the fair market value of all other active pharmaceutical ingredients included in the Combination Product (and in such event, Licensee will in good faith make a determination of the respective fair market values of the IPI-145 Product and all other active pharmaceutical ingredients included in the Combination Product).
There shall be no double-counting in determining the foregoing deductions.
Such amounts shall be determined from the books and records of Licensee, its Affiliates and/or the Sublicensees, maintained in accordance with applicable accounting principles (such as U.S. generally accepted accounting principles (“U.S. GAAP”) and/or International Financial Reporting Standards), consistently applied.
1.59 “Out-of-Pocket Expenses” means, with respect to a Party or any of its Affiliates, direct expenses paid or payable by such Party or its Affiliates to any Third Party.
1.60 “Patent Expenses” means reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses (including attorney’s fees, disbursements to agents in foreign jurisdictions, and government filing fees and annuity fees) incurred by or invoiced to a Party at any time on or after November 1, 2016 in connection with the Prosecution and Maintenance, enforcement or defense of, or seeking Patent Term Extension with respect to, any of the Prosecution Patent Rights.
1.61 “Patent Right” means all patents and patent applications (including provisional applications), including all divisionals, continuations, substitutions, continuations-in-part, re-examinations, re-issues, additions, renewals, extensions, confirmations, registrations, any confirmation patent or registration patent or patent of addition based on any such patent, patent
- 9 -
CONFIDENTIAL
term extensions, and supplemental protection certificates or requests for continued examinations, foreign counterparts, and the like of any of the foregoing.
1.62 “Person” means any natural person, corporation, general partnership, limited partnership, joint venture, proprietorship or other business organization or a governmental agency or a political subdivision thereto.
1.63 “Product” means a preparation, kit, article of manufacture, composition of matter, material, compound, component or product which is, or which contains or comprises a Compound, including all formulations, modes of administration and dosage forms thereof.
1.64 “Prosecution and Maintenance” or “Prosecute and Maintain” means, with regard to a Patent Right, the preparation, filing, prosecution and maintenance of such Patent Right, as well as re-examinations, reissues, appeals, together with the initiation or defense of interferences, the initiation or defense of oppositions and other similar proceedings with respect to such Patent Right, and any appeals therefrom, including any nullity or revocation proceeding, or any of the foregoing, as applicable; provided, however, that “Prosecution and Maintenance” or “Prosecute and Maintain” shall not include any request for Patent Term Extension, any post-grant review or any other defense or enforcement action taken with respect to a Patent Right.
1.65 “Regulatory Approval” means, with respect to a Product, the approval of the applicable Regulatory Authority necessary for the marketing and sale of such Product for a particular indication in a country. Regulatory Approval shall also include any “orphan drug” or similar designation.
1.66 “Regulatory Authority” means a federal, national, multinational, state, provincial or local regulatory agency, department, bureau or other governmental entity with authority over the testing, manufacture, use, storage, import, promotion, marketing or sale of a pharmaceutical product in a country or territory, including the FDA, EMA and MHLW.
1.67 “Regulatory Documentation” means, with respect to any Compound or Product, all INDs, NDAs, and other regulatory applications submitted to any Regulatory Authority, copies of Regulatory Approvals, regulatory materials, drug dossiers, master files (including Drug Master Files, as defined in 21 C.F.R. §314.420 and any non-United States equivalents), and any other reports, records, regulatory correspondence, meeting minutes, telephone logs, and other materials relating to Regulatory Approval of such Compound or Product (including any underlying safety and effectiveness data whether or not submitted to any Regulatory Authority), or required to Research, Develop, Manufacture or Commercialize such Compound or Product, including any information that relates to pharmacology, toxicology, chemistry, manufacturing and controls data, batch records, safety and efficacy, and any safety database required to be maintained for Regulatory Authorities.
1.68 “Regulatory Exclusivity” means the ability to exclude Third Parties from Manufacturing or Commercializing a product that could compete with a Product in a country, either through data exclusivity rights, orphan drug designation, or such other rights conferred by a Regulatory Authority in such country other than through Patent Rights.
- 10 -
CONFIDENTIAL
1.69 “Reimbursement Event” means the DUO Reimbursement Event or the Approval Reimbursement Event.
1.70 “Reimbursement Payment” means a payment to be made pursuant to Section 3.1.2(c)(i) upon achievement of the DUO Reimbursement Event or the Approval Reimbursement Event, as applicable.
1.71 “Research” means, with respect to a Compound, any activities prior to the initiation of the first IND-enabling GLP toxicology study for such Compound, excluding any medicinal chemistry activities.
1.72 “Royalty Term” means, with respect to an IPI-145 Product in a particular country, the period of time commencing on the first Commercial Sale of such IPI-145 Product in such country and ending on the last to occur of (a) the date on which all Duvelisib Patent Rights containing a Valid Claim covering the composition, formulation, preparation, Manufacture, Commercialization or other use of such IPI-145 Product in the country of sale have expired, (b) the date on which all Duvelisib Patent Rights containing a Valid Claim covering the Manufacture in the country of actual Manufacture of such IPI-145 Product have expired, or (c) the expiration of any Regulatory Exclusivity with respect to such IPI-145 Product in such country.
1.73 “Senior Executive” means, in the case of INFI, the Chief Executive Officer of INFI (or a senior executive officer designated by the Chief Executive Officer of INFI), and in the case of Licensee, the Chief Executive Officer of Licensee (or a senior executive officer designated by the Chief Executive Officer of Licensee).
1.74 “Sublicensee” means a Third Party to whom Licensee, or any of its Affiliates or any other Sublicensee, grants a sublicense as permitted under this Agreement, under any of the Duvelisib IP.
1.75 “Target Inhibitor” means any Compound which meets the criteria set forth in Exhibit I.
1.76 “Territory” means worldwide.
1.77 “Third Party” means any Person other than INFI, Licensee or any Affiliate of INFI or Licensee.
1.78 “Third Party Grantor” means INK, MICL or Purdue.
1.79 “United States” or “U.S.” means the United States of America and all of its territories and possessions.
1.80 “U.S. Bankruptcy Code” means of Title 11 of the United States Code, as amended.
1.81 “Valid Claim” means a claim of any issued, unexpired patent that has not been revoked or held unenforceable or invalid by a decision of a court or governmental agency of competent jurisdiction from which no appeal can be taken, or with respect to which an appeal is
- 11 -
CONFIDENTIAL
not taken within the time allowed for appeal, and that has not been disclaimed or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise.
1.82 Additional Definitions. Each of the following definitions is set forth in the section of this Agreement indicated below:
Definition: | Section: |
AAA | 12.2.3 |
Agreement | Preamble |
Arbitration Request | 12.2.1 |
Approval Reimbursement Event | 3.1.2.(c)(i)(2) |
Audit Opinion | 6.6 |
Audited Financial Statements | 6.6 |
Breaching Party | 11.2 |
Clinical Site Agreement | 1.3.6 |
Development Plan | 3.1.1 |
Disclosing Party | 1.10 |
DUO Reimbursement Event | 3.1.2.(c)(i)(1) |
Effective Date | Preamble |
Existing Confidentiality Agreement | 1.10 |
Existing IPI-145 Product | 6.1.1(d) |
Existing Patents | 9.2.4 |
Headlicense Breach | 2.5.5 |
Holdback Payment | 3.1.2(b) |
Indemnified Party | 10.3 |
Indemnifying Party | 10.3 |
Independent Auditor | 6.6 |
INFI | Preamble |
INFI Acquirer | 12.6.1 |
INFI Pre-Existing Affiliates | 12.6.1 |
Initiating Party | 7.6 |
INK | 1.37 |
INK Xxxx | 7.8.1 |
Infringed Patent Right | 6.1.1(d) |
Insurance Period | 10.6.1 |
Joint Know-How | 7.2 |
Joint Patent Rights | 7.2 |
Licensee | Preamble |
Licensee Common Stock | 3.1.2(c)(ii) |
Licensee Acquirer | 12.6.2 |
Licensee Pre-Existing Affiliates | 12.6.2 |
Losses | 10.1 |
MICL | 1.56 |
- 12 -
CONFIDENTIAL
Definition: | Section: |
MICL Repayment Amount | 6.1.3(b)(i) |
MICL Royalty Payment | 6.1.3(b)(i) |
MICL Trailing Royalty Payment | 6.1.3(c) |
Non-Breaching Party | 11.2 |
Paragraph IV Certification | 7.4 |
Party or Parties | Preamble |
Patent Term Extensions | 7.9.1 |
Product Xxxx | 2.5.1 |
Prosecution Patent Rights | 7.3.1(a) |
Purdue | 1.56 |
Purdue Repayment Amount | 6.1.3(b)(ii) |
Purdue Royalty Payment | 6.1.3(b)(ii) |
Receiving Party | 1.11 |
Registration Statement | 3.1.2(c)(iv) |
Reimbursable Amount | 3.1.2(c)(i) |
Reimbursement Announcement Date | 3.1.2(c)(i) |
Reimbursement Notice | 3.1.2(c)(i) |
Representatives | 8.2.1 |
Reviewing Party | 8.5 |
Royalty Termination Date | 6.1.1(b) |
SEC | 3.1.2(c)(iv) |
SEC Financial Statements | 6.6 |
Securities Act | 3.1.2(c)(ii) |
Superseded Agreement | Preamble |
Term | 11.1 |
Third Party Infringement | 7.4 |
Transition Plan | 3.2.1 |
Transition Period | 3.2.1 |
Unaudited Financial Statements | 6.6 |
U.S. GAAP | 1.58 |
ARTICLE 2
GRANT OF RIGHTS
GRANT OF RIGHTS
2.1 License Grant to Licensee. During the Term, subject to the terms and conditions of this Agreement, INFI hereby grants Licensee an exclusive (exclusive even with respect to INFI), royalty-bearing, non-transferable (except in accordance with Section 12.5) license, with the right to sublicense (subject to Section 2.2), under the Duvelisib IP to Research, Develop, Manufacture, Commercialize and import the IPI-145 Compound and IPI-145 Products in the Territory in the Field. For the avoidance of doubt, the license set forth in this Section 2.1 includes exclusive rights with respect to IPI-145 Products that are Combination Products; provided, however, that nothing set
- 13 -
CONFIDENTIAL
forth in this this Agreement shall grant Licensee the right to Research, perform medicinal chemistry on, Develop, Manufacture, Commercialize or import any Compound (other than the IPI-145 Compound) that is claimed or covered by, or embodies, any Patent Right or Know-How owned by or licensed to INFI or any of its Affiliates. With respect to any exclusive license granted to Licensee under this Agreement, “exclusive” means exclusive to Licensee (even with respect INFI and its Affiliates), except for (a) non-exclusive licenses granted by INFI to Third Parties under INFI Product Related Contracts that will not adversely affect Licensee’s ability to Research, Develop, Manufacture and Commercialize the IPI-145 Product in accordance with this Agreement, and (b) any limitations on the rights granted to INFI by any applicable Third Party Grantor in the INFI Third Party Agreements as of the Effective Date (or as amended thereafter to the extent permitted by this Agreement).
2.2 Sublicenses.
2.2.1 Licensee shall have the right to grant sublicenses within the scope of the license under Section 2.1; provided, that any sublicense agreement shall be in writing and shall be consistent with the relevant restrictions and limitations set forth in this Agreement.
2.2.2 Licensee shall be liable for the failure of any of the Sublicensees to comply with the relevant obligations under this Agreement and shall, at its own cost, use Diligent Efforts to enforce compliance by the Sublicensees with the terms of the sublicense agreement.
2.3 License Grant to INFI. Subject to the terms and conditions of this Agreement, Licensee hereby grants to INFI a non-exclusive, perpetual, sublicensable (through multiple tiers), fully-paid up, worldwide, royalty-free license under the Licensee IP to Research (including to perform medicinal chemistry), Develop, Manufacture and Commercialize Compounds that are Target Inhibitors and Products that contain one or more of such Compounds, except that, (a) such license does not extend to any Compound or Product that is Controlled by Licensee, its Affiliates, licensees or Sublicensees as of the Effective Date, and (b) during the Term, such license does not extend to the IPI-145 Compound or IPI-145 Products.
2.4 INFI Third Party Agreements.
2.4.1 Licensee acknowledges and agrees, subject to the accuracy of the representations and warranties contained in Section 9.2.9, that (a) it has received a copy of the INFI Third Party Agreements existing as of the Effective Date and (b) all rights granted to and obligations of Licensee hereunder are subject to the terms and conditions of the INFI Third Party Agreements. Licensee acknowledges that the Third Party Grantors retain, and the activities conducted by Licensee, its Affiliates and the Sublicensees pursuant to this Agreement shall not limit, the Third Party Grantors’ rights with respect to the Know-How and Patent Rights as set forth in the INFI Third Party Agreements.
2.4.2 Licensee shall, and shall cause its Affiliates and Sublicensees to, comply in all material respects with the INFI Third Party Agreements and take any action reasonably requested by INFI to prevent any potential material breach by Licensee, its Affiliates or Sublicensees of any applicable term of any INFI Third Party Agreements.
- 14 -
CONFIDENTIAL
2.4.3 INFI shall not, without Licensee’s prior written consent (which shall not be unreasonably withheld), terminate, or enter into any amendment to, any INFI Third Party Agreement which termination or amendment would have an adverse effect, in any material respect, on Licensee’s rights or obligations under this Agreement or on the Research, Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Products as contemplated hereunder. To the extent permitted under the relevant INFI Third Party Agreement, INFI shall provide Licensee with a copy of all modifications to or amendments of the INFI Third Party Agreements, regardless of whether Licensee’s consent was required with respect thereto.
2.4.4 Each Party shall, and shall cause its Affiliates and licensees or sublicensees to, use Diligent Efforts not to perform any acts or omissions that would constitute a breach of any of the INFI Third Party Agreements which breach would have an adverse effect, in any material respect, on the Research, Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Products as contemplated hereunder. Licensee shall and shall cause its Affiliates and licensees or sublicensees to use Diligent Efforts not to perform any acts or omissions that would constitute a breach of any of the INFI Third Party Agreements which breach would have an adverse effect, in any material respect, on the Research, Development, Manufacture or Commercialization of the Target Inhibitors as contemplated under such INFI Third Party Agreement. Each Party shall provide the other promptly with notice of the occurrence of any such breach (or receipt of notice of an allegation of any such breach).
2.4.5 If INFI receives a notice from INK alleging that INFI has materially breached its obligations under the INK Agreement and such material breach is a result of Licensee’s, its Affiliates’ or Sublicensees’ acts or omissions in breach of Licensee’s obligations under this Agreement (such alleged material breach, a “Headlicense Breach”), then INFI shall promptly forward such notice of the Headlicense Breach to Licensee. Licensee shall have an opportunity to cure such Headlicense Breach in accordance with the terms set forth in Section 11.2 (but without any extension of the cure period therein), so long as Licensee provides evidence to INFI during such cure period of its actions to cure such breach. If Licensee fails to cure its Headlicense Breach or to provide evidence of such actions in accordance with the preceding sentence, then Licensee’s Headlicense Breach shall be considered a material breach of this Agreement by Licensee, which material breach shall not be subject to any further cure periods under Section 11.2 of this Agreement.
2.4.6 [**]
2.4.7 Licensee acknowledges and agrees that (a) INFI may provide a copy of this Agreement, and any amendment to this Agreement, to any Third Party Grantor and (b) INFI may provide to any Third Party Grantor any information required to be provided to such Third Party Grantor in accordance with the applicable INFI Third Party Agreement. INFI acknowledges and agrees that Licensee may provide to any Affiliate or Sublicensee a copy of the INFI Third Party Agreements; provided, that such Affiliate or Sublicensee is subject to confidentiality and non-use obligations no less stringent than those set forth in Article 8.
2.4.8 Termination of the INK Agreement.
- 15 -
CONFIDENTIAL
(a) Subject to the terms of this Section 2.4.8, the licenses granted to Licensee hereunder with respect to the Patent Rights and Know-How licensed to INFI pursuant to the INK Agreement shall terminate upon termination of the INK Agreement (except as provided in Section 15.1(b) therein) and the provisions of Section 15.2 or Section 15.3, as applicable, of the INK Agreement shall, to the extent applicable to Licensee, apply, except that any such license to Licensee of the rights granted to INFI under Section 2.1 of the INK Agreement to Research, Develop, Manufacture or Commercialize the IPI-145 Compound or the IPI-145 Products shall not terminate upon termination of the INK Agreement but instead shall remain in full force and effect if Licensee is not then in material breach of this Agreement and Licensee provides to INK within thirty (30) days after termination of the INK Agreement a written agreement to be bound as the licensee under the terms and conditions of the INK Agreement as to the field and territory in which Licensee has been granted rights under this Agreement.
(b) If the INK Agreement is terminated by INK solely as a direct result of Licensee’s or any Affiliate’s or Sublicensee’s breach of this Agreement and INFI has not received notice from INK that INFI is otherwise in material breach of the INK Agreement as of the time of such termination, then Licensee and its Affiliates shall not directly or indirectly acquire or license rights from INK or any of its Affiliates permitting Licensee or any of its Affiliates to Research, perform medicinal chemistry on, Develop, Manufacture or Commercialize any Compound that is a Target Inhibitor or any Product containing such a Compound, in each case to the extent that such Compound or Product is licensed to INFI under the INK Agreement as of the date of the termination of the INK Agreement.
2.5 Trademark License.
2.5.1 Subject to the terms and conditions of this Agreement, INFI hereby grants Licensee an exclusive (even as to INFI), worldwide, royalty-free right and license to use and sublicense to its Affiliates and Sublicensees INFI’s trademarks set forth on Exhibit E (each a “Product Xxxx”), solely during the Term, solely for the purpose of Commercializing IPI-145 Products.
2.5.2 Licensee shall ensure that the quality of the IPI-145 Product, and the Manufacture and Commercialization thereof, marketed under the Product Marks shall be consistent with the quality of any IPI-145 Product Manufactured by or on behalf of INFI prior to the Effective Date and with the standards of quality customary in the pharmaceuticals industry. Licensee shall, and shall cause its Affiliates and the Sublicensees to, at Licensee’s expense, submit a sample of each proposed use of a Product Xxxx to INFI for approval, which approval shall not be unreasonably withheld, conditioned or delayed. If INFI reasonably objects to a proposed usage of a Product Xxxx, it shall give written notice of such objection to Licensee within [**] days of receipt of such sample, specifying the way in which such usage of the Product Xxxx fails to meet the quality standards, or quality control, style or usage guidelines for such Product or Product Xxxx. If Licensee, any of its Affiliates or any Sublicensee wishes to use the Product Xxxx in the manner included in such sample, it must remedy the failure and submit further samples to INFI for approval.
2.5.3 Licensee shall be responsible for all of INFI’s reasonable and documented Out-of-Pocket Expenses and Internal Personnel Expenses incurred on or after November 1, 2016 associated with registering, prosecuting, maintaining and enforcing the Product Xxxx and shall
- 16 -
CONFIDENTIAL
reimburse INFI within [**] days of Licensee’s receipt of an invoice therefor. Licensee shall have the first right to control the registration, prosecution, maintenance and enforcement of the Product Xxxx, in INFI’s name. INFI shall, at Licensee’s request, reasonably assist Licensee with respect thereto, and Licensee shall reimburse INFI for its reasonable and documented Out-of-Pocket Expenses and Internal Personnel Expenses related thereto.
2.5.4 If Licensee does not wish to register, prosecute, maintain or enforce a Product Xxxx in a country, Licensee shall notify INFI thereof.
2.5.5 If INFI determines in good faith that Licensee has not registered, prosecuted, maintained or enforced a Product Xxxx in a country in a timely manner, and in any event if INFI reasonably believes it is in danger of losing any rights in such Product Xxxx, then INFI shall have the right to register, prosecute, maintain or enforce such Product Xxxx in such country, at INFI’s expense, and Licensee shall reasonably assist INFI with respect thereto.
2.5.6 As between the Parties and except as set forth in Section 2.5.7, and subject to the licenses set forth in this Section 2.5, INFI will own the Product Marks. Subject to Section 2.5.7, Licensee, its Affiliates and Sublicensees will not contest, oppose or challenge INFI’s ownership of any Product Xxxx.
2.5.7 At any time following Licensee’s filing of an NDA in the United States or an MAA in any other country in the Territory with respect to an IPI-145 Product, Licensee may request that INFI transfer ownership of the Product Xxxx and any goodwill associated therewith (but not any of the Duvelisib IP or any assets of INFI or any of its Affiliates, other than the Product Xxxx and the Internet domain names described hereafter) and any Internet domain names incorporating any Product Xxxx, or any variation or part of any Product Xxxx. Promptly following such request, INFI shall assign ownership of the Product Xxxx and any goodwill associated therewith (but not any of the Duvelisib IP or any assets of INFI or any of its Affiliates, other than the Product Xxxx and such Internet domain names) and any Internet domain names incorporating any Product to Licensee or its designee, and Licensee shall reimburse INFI for its reasonable and documented Out-of-Pocket Expenses and Internal Personnel Expenses related thereto.
2.6 Rights Retained by the Parties.
2.6.1 Any rights of INFI not expressly granted to Licensee pursuant to this Agreement shall be retained by INFI. Any rights of Licensee not expressly granted to INFI pursuant to this Agreement shall be retained by Licensee. Licensee agrees not to practice any Duvelisib IP except pursuant to the licenses expressly granted to Licensee in this Agreement (it being agreed that no such license grants any right to Research, perform medicinal chemistry on, Develop, have Developed, Manufacture, have Manufactured, use, sell, offer to sell, otherwise Commercialize or import any Compound, or any Product containing or comprising any Compound, other than the XXX-000 Xxxxxxxx, XXX-000 Product or a Combination Product to the extent set forth herein).
2.6.2 INFI shall not directly or indirectly, Research, perform medicinal chemistry on, Develop, Manufacture or Commercialize the IPI-145 Compound or any IPI-145 Product for the treatment, prevention, palliation or diagnosis of any Indication in humans or animals in the Territory,
- 17 -
CONFIDENTIAL
nor collaborate with, license, sell to or enable or otherwise authorize, permit or grant any right to any Third Party to Research, perform medicinal chemistry on, Develop, Manufacture or Commercialize the IPI-145 Compound or any IPI-145 Product for the treatment, prevention, palliation or diagnosis of any Indication in humans or animals in the Territory.
2.7 Section 365(n) of the U.S. Bankruptcy Code.
2.7.1 All rights and licenses now or hereafter granted by a Party to the other Party under or pursuant to any section of this Agreement constitute rights to “intellectual property” (as defined in the U.S. Bankruptcy Code). The Parties hereto acknowledge and agree that the payments provided for in the Agreement by Licensee to INFI hereunder, other than royalty payments pursuant to Section 6.1.1, do not constitute royalties within the meaning of Section 365(n) of the U.S. Bankruptcy Code or relate to licenses of intellectual property hereunder.
2.7.2 If (a) a case under the U.S. Bankruptcy Code is commenced by or against INFI, (b) this Agreement is rejected as provided in the U.S. Bankruptcy Code and (c) Licensee elects to retain its rights hereunder as provided in Section 365(n) of the U.S. Bankruptcy Code, then INFI (in any capacity, including debtor-in-possession) and its successors and assigns (including any trustee) shall provide to Licensee all intellectual property licensed hereunder, and agrees to grant and hereby grants to Licensee and its Affiliates a right to access and to obtain possession of and to benefit from and, in the case of any chemical or biological material or other tangible item of which there is a fixed or limited quantity, to obtain a pro rata portion of, such articles and materials which were to have been, but were not, transferred as part of the Transition Plan.
2.7.3 The Party against which a case under the U.S. Bankruptcy Code is commenced shall not interfere with the exercise by the other Party or its Affiliates of rights and licenses to intellectual property licensed hereunder and embodiments thereof in accordance with this Agreement and agrees to use Diligent Efforts to assist the other Party and its Affiliates to obtain such intellectual property and embodiments thereof in the possession or control of Third Parties as reasonably necessary or desirable for the other Party or its Affiliates or licensee or sublicensees to exercise such rights and licenses in accordance with this Agreement.
2.8 Infinity Exclusivity Covenants. During the Term, except pursuant to and in accordance with the terms of this Agreement, neither INFI nor any of its Affiliates shall directly or indirectly conduct clinical trials of the IPI-443 Product as a therapeutic, or Commercialize the IPI-443 Product, in each case in the Field in the Territory, nor collaborate with, license, sell to, or enable or otherwise authorize, permit or grant any right to, any Third Party to Commercialize or conduct such clinical trials of the IPI-443 Product in the Field in the Territory. For the purposes of this Section 2.8 only, Field shall not include (a) immunotherapy treatments that treat T-cells ex-vivo or (b) any other ex-vivo uses.
ARTICLE 3
RESEARCH AND DEVELOPMENT
RESEARCH AND DEVELOPMENT
- 18 -
CONFIDENTIAL
3.1 Diligence. Licensee (itself or through its Affiliates and the Sublicensees) shall use Diligent Efforts to Develop, Manufacture and Commercialize one IPI-145 Product in the Field in the Territory.
3.1.1 Development Plan. The initial plan for Development activities to be conducted by Licensee (itself or through its Affiliates and the Sublicensees) with respect to the IPI-145 Product during the Term is set forth in Exhibit G (the “Development Plan”). The Development Plan may be updated or amended by Licensee from time to time during the Term; provided that such updated or amended Development Plan shall be sufficient to permit INFI to comply with its obligations under this Agreement and the INK Agreement. Licensee shall provide to INFI any such updated or amended Development Plan concurrently with the delivery of Development reports pursuant to Section 3.3. To the extent that any provision of the Development Plan conflicts with or is inconsistent with the provisions of this Agreement, the provisions of this Agreement shall control.
3.1.2 Expenditures.
(a) Licensee’s Diligent Efforts to Develop one IPI-145 Product will include demonstration that it, its Affiliates and the Sublicensees, [**]
(b) Notwithstanding anything to the contrary in this Agreement (other than the provisions of Section 3.1.4(b)), Licensee will be responsible for all reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses related to the IPI-145 Compound or IPI-145 Product in the Territory incurred by INFI on or after November 1, 2016, including all costs related to the Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Product and all Patent Expenses; provided, however, that Licensee shall not have any obligation to reimburse INFI for any such costs incurred by INFI after the Effective Date except for those costs incurred in accordance with this Agreement or as directed by Licensee; provided, that Licensee shall be permitted to holdback [**] of all such payments incurred by INFI after the date a Key Item is to have been completed (as set forth in the Transition Plan) and such Key Item has not been completed (other than through any action or inaction of Licensee) (such payments actually withheld by Licensee, the “Holdback Payments”); further, provided, that within [**] days following the completion of such Key Item that entitled Licensee to holdback the Holdback Payment, Licensee shall pay the amount of such Holdback Payment to INFI. Subject to the foregoing, Licensee shall reimburse INFI for all such expenses within [**] days following Licensee’s receipt of an invoice therefor.
(c) Reimbursement for Pre-Effective Date Costs and Expenses.
(i) The Parties agree and acknowledge that, INFI’s and its Affiliates’ aggregate internal costs and Out-of-Pocket Expenses related to the IPI-145 Product between July 1, 2016 and October 31, 2016, and INFI’s and its Affiliates’ costs related to the clinical studies described in Section 3.1.4(b), are estimated at [**] (the “Reimbursable Amount”). Subject to the terms and conditions of this Agreement, Licensee shall reimburse such costs by paying to INFI the following amounts:
- 19 -
CONFIDENTIAL
(1) Six Million Dollars ($6,000,000) upon the determination that the DUO clinical trial has met its [**], each as defined in the DUO clinical trial protocol, attached as Exhibit H (such event, the “DUO Reimbursement Event”) and;
(2) Twenty-Two Million Dollars ($22,000,000) upon the approval of an NDA or MAA for an IPI-145 Product (such event, the “Approval Reimbursement Event”).
To the extent that the payments made to INFI under Sections 3.1.2(c)(1) and 3.1.2(c)(2) are less than the Reimbursable Amount, the remainder of the Reimbursable Amount shall be reimbursed to INFI through the payment of royalties pursuant to Section 6.1.1.
Licensee shall pay the amounts set forth in Section 3.1.2(c)(i)(1) and Section 3.1.2(c)(i)(2) within [**] days after the achievement of the relevant Reimbursement Event; provided, however, that Licensee shall have no obligation to make the relevant Reimbursement Payment upon the achievement of the applicable Reimbursement Event until INFI shall have completed the items marked as “Key Items” on the Transition Plan that were to have been completed (as set forth in the Transition Plan) prior to the date on which such Reimbursement Event is achieved. Within [**] calendar days after Licensee becomes aware that a Reimbursement Event has been achieved, it shall notify INFI thereof in writing (the “Reimbursement Notice”) and shall issue a public announcement of such achievement, which announcement shall have been subject to written approval by INFI, such approval not to be unreasonably withheld, conditioned or delayed. The date of such public announcement is hereinafter referred to as the “Reimbursement Announcement Date.”
(ii) Form of Payment. Within [**] days after the achievement of the relevant Reimbursement Event set forth in Section 3.1.2(c)(i)(1) or Section 3.1.2(c)(i)(2), Licensee shall make a Reimbursement Payment (1) in Dollars in immediately available funds, or (2) in lieu of (or as partial consideration with) making the Reimbursement Payment in Dollars, by issuing shares of its common stock, $0.0001 par value per share (“Licensee Common Stock”), such shares constituting “restricted securities” within the meaning of Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”). As part of any Reimbursement Notice, Licensee shall inform INFI of its form of payment election, whether in Dollars, shares of Licensee Common Stock or a combination of any of the foregoing.
(iii) Calculating Reimbursement Payments. For any portion of any Reimbursement Payment in which Licensee elects to issue shares of Licensee Common Stock, the number of shares of Licensee Common Stock to be so issued will be determined by multiplying (1) 1.025 by (2) the number of shares of Licensee Common Stock equal to (a) the amount of the Reimbursement Payment to be paid in shares of Licensee Common Stock, divided by (b) the average closing price of a share of Licensee Common Stock as quoted on NASDAQ for the twenty (20) day period following the Reimbursement Announcement Date.
(iv) Registration Rights. If Licensee issues shares of Licensee Common Stock to INFI to satisfy all or a portion of a Reimbursement Payment, Licensee shall as promptly as possible, but no later than [**] Business Days following the issuance of such shares, file a registration statement on Form S-3 (or such other registration statement then available to
- 20 -
CONFIDENTIAL
Licensee, each, a “Registration Statement”) with the Securities and Exchange Commission (the “SEC”) registering all such shares of Licensee Common Stock issued as consideration for all or a portion of such Reimbursement Payment. Licensee shall use commercially reasonable efforts to have the applicable Registration Statement and the related prospectuses declared effective by the SEC as soon as possible thereafter and to prepare and file with the SEC such amendments and supplements to the registration as may be necessary to keep such Registration Statement effective until the first anniversary of the effective date of such Registration Statement. The obligations of the Licensee to maintain an effective Registration Statement under this Section 3.1.2(c)(iv) for any issuance of Licensee Common Stock shall cease on the first anniversary of the effective date of such Registration Statement.
(v) Resale Limitations. In any resales within the first three months after the effective date of the applicable Registration Statement, regardless of whether conducted pursuant to the Registration Statement, INFI shall effect such sales only through [**] or another broker to be mutually agreed upon between INFI and Licensee.
(vi) Legends. All Licensee Common Stock issued as consideration for all or a portion of a Reimbursement Payment shall bear the following legend:
THESE SECURITIES HAVE NOT BEEN REGISTERED WITH THE SECURITIES AND EXCHANGE COMMISSION OR THE SECURITIES COMMISSION OF ANY STATE IN RELIANCE UPON AN EXEMPTION FROM REGISTRATION UNDER THE SECURITIES ACT OF 1933, AS AMENDED (THE “SECURITIES ACT”), AND, ACCORDINGLY, MAY NOT BE OFFERED OR SOLD EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OR PURSUANT TO AN AVAILABLE EXEMPTION FROM, OR IN A TRANSACTION NOT SUBJECT TO, THE REGISTRATION REQUIREMENTS OF THE SECURITIES ACT AND IN ACCORDANCE WITH APPLICABLE STATE SECURITIES LAWS AS EVIDENCED BY A LEGAL OPINION OF COUNSEL TO THE TRANSFEROR TO SUCH EFFECT, THE SUBSTANCE OF WHICH SHALL BE REASONABLY ACCEPTABLE TO THE COMPANY.
(vii) Authorizations; Approvals; Timing. The Parties acknowledge and agree that it shall be a condition to the closing of the sale of any issuance of shares of Licensee Common Stock that: (i) all material authorizations, consents, orders or approvals of, or regulations, declarations or filings with, or expirations of applicable waiting periods imposed by, any Governmental Authority necessary for the consummation of the sale of such shares shall have been obtained or filed or shall have occurred (as applicable), and (ii) INFI shall have received such customary certificates, instruments or other similar closing deliverables as it may reasonably request. Notwithstanding anything herein to the contrary, in no event will Licensee issue any shares of Licensee Common Stock without first obtaining approval from its stockholders to the extent that such approval is then required as a condition to such issuance of such shares pursuant to NASDAQ Listing Rule 5635 or any successor rule. The right of Licensee to pay all or a portion of a Reimbursement Payment in shares of Licensee Common Stock shall immediately terminate if the
- 21 -
CONFIDENTIAL
closing of the sale of such shares shall not have taken place within [**] calendar days after the Reimbursement Announcement Date. In the event of such termination, Licensee shall, within a period of [**] Business Days thereafter, make such Reimbursement Payment to INFI in Dollars in immediately available funds.
3.1.3 Subcontractors. Licensee may perform its Development, Manufacturing or Commercialization rights or obligations under this Agreement through one or more subcontractors or consultants, provided, that: (a) Licensee shall remain responsible for the work allocated to, and payment to, such subcontractors and consultants as it selects to the same extent it would if it had done such work itself; and (b) each such subcontractor or consultant shall undertake in writing obligations of confidentiality and non-use regarding INFI’s Confidential Information that are no less restrictive than those undertaken by Licensee pursuant to ARTICLE 8 hereof.
3.1.4 Continuation of Clinical Trials.
(a) Licensee shall assume all costs associated with the [**] clinical trials as of [**] (unless Licensee provides INFI with written notice prior to [**] that the [**] clinical trial will be wound down, in which case it shall be wound down under Section 3.1.4(b)).
(b) INFI shall be responsible for winding down the [**] clinical trials (and the [**] clinical trial if Licensee elects to wind down the [**] clinical trial pursuant to Section 3.1.4(a)) until December 31, 2016, including the costs thereof, and shall use Diligent Efforts to wind down such clinical trials in accordance with the Transition Plan. After [**], Licensee shall become responsible for all activities and costs to wind down such clinical trials; provided, however, that INFI shall reimburse Licensee for Licensee’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses for winding down such clinical trials (such reimbursements to be made within [**] days of INFI’s receipt of invoice therefor from Licensee). In any event, INFI’s aggregate expenditures under this Section 3.1.4(b), including INFI’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses and INFI’s reimbursement of Licensee’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses, shall be capped at Four Million Five Hundred Thousand Dollars ($4,500,000).
(c) Following Licensee’s assumption of responsibility for the DUO clinical trial, Licensee shall continue the DUO clinical trial in accordance with the DUO clinical trial protocol attached as Exhibit H until it is complete; provided, however, that in the event that Licensee, a Regulatory Authority, an institutional review board or independent safety board determines that the DUO clinical trial would pose an unacceptable safety risk for subjects or patients participating in it, then Licensee shall not be obligated to continue the DUO clinical trial and Licensee shall provide INFI with an explanation of the safety issue concerns, including those raised by such Regulatory Authority, institutional review board or independent safety board and, if requested by INFI, reasonable documentation thereof.
3.2 Transfer of INFI Know-How.
3.2.1 Each Party shall perform its respective obligations under the transition plan attached hereto as Exhibit F (the “Transition Plan”). Except for those obligations specified in the
- 22 -
CONFIDENTIAL
Transition Plan or in this Agreement to endure past the end of the Transition Period, by the end of the Transition Period, (a) each Party shall have performed all of its obligations under the Transition Plan, (b) INFI shall have disclosed and transferred to Licensee the process used by INFI as of the Effective Date for the Manufacture of IPI-145 Product and such other Manufacturing specifications set forth in Exhibit F-2, (c) INFI shall have provided Licensee with copies of such other relevant material and information included in the Duvelisib Know-How with respect to the IPI-145 Product as set forth in the Transition Plan, and (d) INFI shall have transferred control and ownership to Licensee of the materials and inventory of the IPI-145 Compound and IPI-145 Product identified in the Transition Plan in such amounts as set forth in Exhibit F-3. “Transition Period” means the period beginning on the Effective Date and ending on [**]. Prior to the end of the Transition Period, INFI shall provide to Licensee a copy all of all Clinical Site Agreements.
3.2.2 INFI Product Related Contracts.
(a) Within thirty (30) days after the Effective Date, (A) to the extent not previously provided to Licensee, INFI will provide Licensee with electronic copies of each INFI Product Related Agreement and (B) the Parties will, in good faith, mutually determine in writing which INFI Product Related Contracts will be assigned to Licensee and which will be wound down or terminated. INFI shall use Diligent Efforts to assign to Licensee, in accordance with the schedule determined in accordance with Section 3.2.2(c), the rights and obligations under the applicable INFI Product Related Contracts (through a novation, except that if a novation cannot be secured for an INFI Product Related Contract, INFI shall use Diligent Efforts to assign such INFI Product Related Contract to Licensee and Licensee shall indemnify, defend and hold harmless the INFI Indemnitees from and against any and all Losses arising from such INFI Product Related Contract after the Effective Date except to the extent such Losses are caused by INFI’s or its Affiliate’s failure to comply with the terms of such INFI Product Related Contract, breach of any terms or conditions of this Agreement, or failure to follow Licensee’s reasonable instructions with respect to INFI’s and its Affiliates’ activities in connection therewith), and Licensee shall accept such rights and obligations and accept all liability with respect to INFI’s obligations under such INFI Product Related Contracts other than those payment obligations (i) incurred by INFI prior to November 1, 2016, or (ii) that do not relate to the IPI-145 Compound or IPI-145 Product; provided, however, that INFI shall have no obligation to incur any costs or payment obligations in order to effect such assignment, unless Licensee agrees to any bear all such costs and payment obligations.
(b) With respect to each applicable INFI Product Related Contract (i.e., an INFI Product Related Contract that Licensee and INFI determined should be assigned to Licensee), until the earlier of the date on which such INFI Product Related Contract (i) is so assigned to Licensee, (ii) expires or (iii) is terminated, INFI shall use Diligent Efforts to provide to Licensee the benefits of such INFI Product Related Contract to the extent that such benefits relate to the IPI-145 Compound or IPI-145 Product and enforce, at the request and expense of and for the account and benefit of Licensee, any rights of INFI arising thereunder against any counterparty to the INFI Product Related Contracts, including the right to seek any available remedies or to elect to terminate such INFI Product Related Contracts in accordance with the terms thereof upon the direction of Licensee. In connection with the foregoing, Licensee shall assume responsibility for payments incurred after the Effective Date under each such INFI Product Related Contract and Licensee shall
- 23 -
CONFIDENTIAL
perform the obligations of INFI under each such INFI Product Related Contract, in each case, to the extent related to the IPI-145 Compound or IPI-145 Product; provided, however, that Licensee shall reimburse INFI for any amounts pre-paid by INFI under any INFI Product Related Contract as of the Effective Date, provided that such prepayments relate to the IPI-145 Compound or the IPI-145 Product.
(c) With respect to each applicable INFI Product Related Contract, INFI will use Diligent Efforts to cooperate with Licensee on determining the preferred effective dates of assignment for key INFI Product Related Contracts and the accounting groups of each Party will cooperate with Licensee in the assessment of proper accounting treatment of the applicable INFI Product Related Contracts.
3.2.3 During the Transition Period, INFI shall make its relevant and available scientific and technical personnel reasonably available to Licensee to answer questions or provide instruction as reasonably requested by Licensee concerning the Duvelisib Know-How delivered pursuant to this Section 3.2 in order to facilitate the transfer of such Duvelisib Know-How to Licensee. Notwithstanding the foregoing, INFI shall have no obligation to (i) maintain any personnel or (ii) following the disclosure or transfer, as applicable, of information and materials as described in Section 3.2.1, maintain any records, files or other materials, related to the IPI-145 Product or any of the information or materials disclosed or transferred hereunder.
3.2.4 Licensee shall reimburse INFI for any reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses incurred by INFI pursuant to Sections 3.2.1, 3.2.2, and 3.2.3 within [**] days following receipt by Licensee of an invoice providing reasonable documentation of such expenses.
3.3 Reports. Licensee shall submit semi-annual written progress reports by December 20 and June 20 of each year, summarizing Licensee’s (and its Affiliates’ and the Sublicensees’) activities related to the development of the IPI-145 Product in the Field, including Development activities and an overview of future Development activities reasonably contemplated, including the status of obtaining Marketing Authorization for each of the United States, Europe and Japan, and planning for Commercialization in such territories (including a projection of all such activities for the next thirty days). Such reports shall be submitted, with respect to activities for the United States, until first Commercial Sale of the IPI-145 Product in the United States, and with respect to activities for countries or regions outside the United States, until first Commercial Sale of the IPI-145 Product in any country outside the United States.
ARTICLE 4
REGULATORY MATTERS
REGULATORY MATTERS
4.1 Licensee Regulatory Responsibility.
4.1.1 INDs. Subject to this Section 4.1.1, INFI shall own and be responsible for preparing, filing and maintaining all INDs for the IPI-145 Compound and IPI-145 Product in the Field in the Territory as of the Effective Date and Licensee shall reimburse INFI’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses related thereto. Promptly
- 24 -
CONFIDENTIAL
after the Effective Date and in any event no later than the end of the Transition Period, INFI and Licensee, as applicable, shall make the necessary filings with the Regulatory Authorities in the Territory necessary to transfer the INDs for the IPI-145 Compound and IPI-145 Product to Licensee, and following the approval of such transfer by the applicable Regulatory Authorities (if applicable) or other effectuated transfer, Licensee shall own all such INDs and be the IND holder for the IPI-145 Compound and IPI-145 Product in the Territory.
(a) Until such time as the INDs have been transferred to Licensee, INFI shall act as Licensee’s agent to maintain the INDs and communicate with Regulatory Authorities in the Territory relating to the IPI-145 Compound and IPI-145 Product and Licensee shall reimburse INFI’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses related thereto. Except with respect to non-substantive administrative correspondence with Regulatory Authorities, (i) INFI shall act on Licensee’s behalf as instructed by Licensee with respect to submissions related to the INDs for the IPI-145 Compound and IPI-145 Product and receiving and submitting correspondence with Regulatory Authorities in the Territory related thereto and (ii) INFI will provide to Licensee copies of all correspondence received from Regulatory Authorities within [**] Business Days of receipt or such earlier date as required by applicable Law or the relevant Regulatory Authority or if necessary given the circumstances of the correspondence, and INFI shall not respond to such correspondences or otherwise interact with the Regulatory Authorities except as instructed by Licensee.
(b) With respect to the INDs for the IPI-145 Compound and IPI-145 Product, Licensee will provide INFI with copies of all submissions in advance of filing so that INFI may submit such submissions on behalf of Licensee. INFI will provide to Licensee copies of any material written communications to or from Regulatory Authorities related to the IPI-145 Compound and the IPI-145 Product within [**] Business Days of receipt or delivery of such communication, as the case may be, or such earlier date as required by applicable Law or the relevant Regulatory Authority or if necessary given the circumstances of the correspondence. In addition, except for submissions which are required to meet the “Sponsor” obligations under 21 C.F.R. 312 and analogous regulations in non-U.S. jurisdictions, during such period INFI will be responsible for all communications and other dealings with Regulatory Authorities in the Territory with respect to the IPI-145 Compound and the IPI-145 Product, provided, however, that INFI will only communicate with the Regulatory Authorities as instructed by Licensee. To the extent permitted by applicable Law, INFI will arrange all meetings with Regulatory Authorities such that representatives of Licensee are able to attend and participate. Licensee shall reimburse INFI’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses related to the activities set forth in this Section 4.1.1.
4.1.2 Marketing Authorizations. Licensee shall, at its sole cost, use Diligent Efforts, itself or through its Affiliates and the Sublicensees, to prepare, file, prosecute and maintain all applications for Marketing Authorization for the marketing, use, promotion, import, sale, distribution or commercialization of the IPI-145 Product in the Field in the Territory.
4.1.3 Regulatory Documentation. Except as otherwise set forth in this Section 4.1, Licensee shall own and be responsible for preparing, filing and maintaining all Regulatory
- 25 -
CONFIDENTIAL
Documentation and Regulatory Approvals that are required for the Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Product in the Field in the Territory; and Licensee shall be responsible for all other submissions to, and communications and interactions with, Regulatory Authorities in the Territory with respect to the IPI-145 Compound or IPI-145 Product in the Field.
4.2 Safety Data Reporting. As set forth in the Transition Plan, until INFI has transferred all INDs to Licensee, INFI shall be responsible for reporting all adverse drug reactions/experiences with respect to the IPI-145 Product in the Field to the appropriate Regulatory Authorities in the Territory in accordance with all applicable Laws. INFI shall ensure that its Affiliates comply with such reporting obligations in the Territory. Following the transfer of all INDs to Licensee, Licensee shall be responsible for reporting all adverse drug reactions/experiences with respect to the IPI-145 Product in the Field to the appropriate Regulatory Authorities in the Territory in accordance with all applicable Laws. Licensee shall ensure that its Affiliates and the Sublicensees comply with such reporting obligations in the Territory. Licensee shall be responsible for each Party’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses with respect the activities under this Section 4.2.
ARTICLE 5
COMMERCIALIZATION
COMMERCIALIZATION
5.1 Overview. As between the Parties, and subject to the terms and conditions of this Agreement, Licensee shall control, and bear all responsibility, costs and expenses associated with, the Commercialization of the IPI-145 Product in the Field in the Territory.
5.2 Commercial Diligence. Licensee shall, at its sole cost, use Diligent Efforts, itself or through its Affiliates and the Sublicensees, to Commercialize the IPI-145 Product that receives Marketing Authorization in the Field in the Territory.
5.3 Standards of Conduct. In Commercializing the IPI-145 Product under this Agreement, Licensee shall, and shall ensure that its Affiliates and the Sublicensees, comply in all respects with the INFI Third Party Agreements and with all applicable Laws and applicable guidelines, including those concerning the advertising, sales and marketing of prescription drug products, the Foreign Corrupt Practices Act of 1977, as amended, and any applicable local anti-bribery Laws.
5.4 Progress Reports. Within [**] days after the first Commercial Sale of IPI-145 Product by Licensee or any of its Affiliates or any Sublicensee, and by each January 20th thereafter, Licensee shall provide a forward-looking, non-binding forecast, for the relevant Calendar Year (or, with respect to the first such forecast, the remainder of the current Calendar Year), of anticipated Annual Net Sales (as defined in this Agreement) of the IPI-145 Product; provided, however, that if the first Commercial Sale of the IPI-145 Product by Licensee or any of its Affiliates or any of the Sublicensees occurs between October 1st and December 31st, the first such forecast shall cover the remainder of the current Calendar Year (if applicable) and the next Calendar Year, and no forecast shall be due by January 20th of such next Calendar Year. By way of example and without limitation, if the first Commercial Sale of the IPI-145 Product by Licensee, any of its Affiliates or any
- 26 -
CONFIDENTIAL
Sublicensee occurs on November 1, 2017, the first such forecast shall be due by November 20, 2017 and shall cover the period from November 20, 2017 through December 31, 2018 and no forecast shall be due by January 20, 2018.
ARTICLE 6
PAYMENTS
PAYMENTS
6.1 Payments.
6.1.1 Royalties to INFI.
(a) Licensee will pay royalties to INFI on Annual Net Sales of IPI-145 Product at the applicable rates set forth below, subject to the provisions of this Section 6.1 and Section 6.2. For the avoidance of doubt, royalties shall be payable only once with respect to the same unit of IPI-145 Product.
Annual Net Sales of IPI-145 Product | Royalty Rate |
The portion less than US$[**] | [**]% |
The portion greater than or equal to US$[**] and less than US$[**] | [**]% |
The portion greater than or equal to US$[**] and less than US$[**] | [**]% |
The portion greater than or equal to US$[**] | [**]% |
(b) Royalties will be payable to INFI [**] on an IPI-145 Product-by-IPI-145 Product and country-by-country basis until the later of (i) expiration of the applicable Royalty Term or (ii) ten (10) years after the first Commercial Sale of such IPI-145 Product in such country (such later date, the “Royalty Termination Date”).
(c) Solely with respect to Net Sales in the United States, following the expiration of the last to expire Valid Claim of any Duvelisib Patent Right claiming or covering the composition, formulation, preparation or method of manufacture or use of the applicable IPI-145 Product (or the IPI-145 Compound therein) in the United States, the applicable royalty under Section 6.1.1(a) with respect to Net Sales of such IPI-145 Product in the United States shall be reduced by fifty percent (50%), with the Net Sales for such IPI-145 Product in the United States allocated pro rata across each of the relevant royalty tiers. [**]
(d) If Licensee (i) reasonably determines in good faith that it is required to obtain a license from a Third Party to any Patent Right that, in the absence of such license, would be infringed by the Commercialization in a particular country of the IPI-145 Product in the form in which the IPI-145 Product exists as of the Effective Date (the “Existing IPI-145 Product”), which Patent Right (A) is not licensed or sublicensed hereunder, (B) claims the composition of matter of the IPI-145 Compound contained in the Existing IPI-145 Product or the method of use of such composition of matter in hematologic malignancies, and (C) is necessary (and not just useful) to Commercialize the Existing IPI-145 Product (the relevant “Infringed Patent Right”), or (ii) shall
- 27 -
CONFIDENTIAL
be subject to a final court or other binding order or ruling that such Commercialization of the Existing IPI-145 Product infringed an Infringed Patent Right requiring any payments, including a payment of a royalty to the applicable Third Party Patent Right holder in respect of future sales of the Existing IPI-145 Product in a country in the Territory, then the amount of Licensee’s royalty payments to INFI under Section 6.1.1(a) shall be reduced by fifty percent (50%) of the amount paid by Licensee to such Third Party with respect to such Infringed Patent Right in each applicable Calendar Quarter that is reasonably and appropriately allocable to the Existing IPI-145 Product in such country in each Calendar Quarter; provided, however, that in no event will a deduction or deductions under this Section 6.1.1(d) reduce any royalty payment made by Licensee in respect of Net Sales (or Combination Product Net Sales) of the Existing IPI-145 Product in such country in such Calendar Quarter by more than fifty percent (50%) of the royalties otherwise payable by Licensee to INFI under Section 6.1.1(a) with respect to IPI-145 Product.
(e) Notwithstanding any provision of this Agreement to the contrary, in no event will the deductions or adjustments under Sections 6.1.1(c) or 6.1.1(d) cause the royalties due to INFI in any applicable Calendar Quarter with respect to any IPI-145 Product in such country to be less than fifty percent (50%) of the royalties otherwise payable by Licensee to INFI under Section 6.1.1(a) (without taking into account Sections 6.1.1(c) or 6.1.1(d)) with respect to IPI-145 Product.
6.1.2 Paid Up License Following Royalty Termination Date. Except with respect to the payments owed to INFI pursuant to Section 6.1.3, following the Royalty Termination Date on an IPI-145 Product-by-IPI-145 Product and country-by-country basis, Licensee’s licenses with respect to such IPI-145 Product shall continue in effect, but become fully paid-up and royalty-free and shall become perpetual and irrevocable upon expiration of this Agreement or termination by Licensee for INFI’s breach; provided, however, that, following the last Royalty Termination Date with respect to all IPI-145 Products, on a country-by-country basis, Licensee’s licenses with respect to all IPI-145 Compounds and IPI-145 Products shall continue in effect, but become fully paid-up, royalty-free, and shall become perpetual and irrevocable upon expiration of this Agreement or termination by Licensee for INFI’s breach.
6.1.3 Payments to Third Party Grantors.
(a) Payments to INK. INFI shall be responsible for making all payments owed to INK on INFI’s Qualifying Transaction Revenue (as defined in the INK Agreement) in accordance with the terms of the INK Agreement. Licensee shall have no obligation to make any such payments to INK and the royalties and other amounts paid under this Agreement to INFI shall not be increased to cover such amounts owed to INK by INFI under the INK Agreement. INFI hereby covenants to make all payments owed to INK on INFI’s Qualifying Transaction Revenue.
(b) Payments to Mundipharma and Purdue.
(i) In addition to the royalty owed to INFI pursuant to Section 6.1.1, Licensee shall pay to INFI (for payment to MICL) an amount equal to 3.756% of Net Sales (the “MICL Royalty Payment”) of IPI-145 Product. For purposes of this Section 6.1.3(b)(i) only, Net Sales of Combination Products will be calculated in accordance with Section 1.59(I) and will
- 28 -
CONFIDENTIAL
not be reduced by the multiplication factor reflected in Section 1.59(II). INFI shall provide Licensee with prompt notice if the Securities Purchase Agreement (as defined in the MICL Agreements) is terminated pursuant to Section 8.1 thereof, and in such case, the MICL Royalty Payment shall be reduced to 2.817% of Net Sales. Licensee shall only be obligated to make the MICL Royalty Payment on Net Sales of IPI-145 Product until such time as MICL has received an aggregate amount equal to $244,547,850 (the “MICL Repayment Amount”) from the combination of MICL Royalty Payments made by Licensee with respect to IPI-145 Product and other royalties paid by INFI or its Affiliates or licensees or sublicensees with respect to any other Products subject to the royalty payments under the MICL Agreement with MICL. On an annual basis, INFI shall inform Licensee of the remaining balance of the MICL Repayment Amount. INFI shall provide Licensee with prompt notice when such MICL Repayment Amount has been paid in full, in which case, (a) Licensee shall no longer be required to make the MICL Royalty Payment to INFI and (b) Licensee will be required to make the MICL Trailing Royalty Payment to INFI pursuant to Section 6.1.3(c). In the event the rate of the MICL Royalty Payment has changed from the initial rate, as set forth in this Section 6.1.3(b)(i), and, as a result, Licensee has overpaid to INFI the MICL Royalty Payment with respect thereto, such overpaid amount shall be credited toward Licensee’s MICL Royalty Payments or MICL Trailing Royalty Payment until fully credited.
(ii) In addition to the royalty owed to INFI pursuant to Section 6.1.1, Licensee shall pay to INFI (for payment to Purdue) an amount equal to 0.244% of Net Sales (the “Purdue Royalty Payment”) of an IPI-145 Product. For purposes of this Section 6.1.3(b)(ii) only, Net Sales of Combination Products will be calculated in accordance with Section 1.59(I) and will not be reduced by the multiplication factor reflected in Section 1.59(II). INFI shall provide Licensee with prompt notice if the Securities Purchase Agreement (as defined in the MICL Agreements) is terminated pursuant to Section 8.1 thereof, and in such case, the Purdue Royalty Payment shall be reduced to 0.183% of Net Sales. Licensee shall only be obligated to make the Purdue Royalty Payment on Net Sales of IPI-145 Product until such time as Purdue has received an aggregate amount equal to $15,908,706 (the “Purdue Repayment Amount”) from the combination of Purdue Royalty Payments made by Licensee with respect to IPI-145 Product and other royalties paid by INFI or its Affiliates or licensees or sublicensees with respect to any other Products subject to the royalty payments under the MICL Agreement with Purdue. On an annual basis, INFI shall inform Licensee of the remaining balance of the Purdue Repayment Amount. INFI shall provide Licensee with prompt notice when such Purdue Repayment Amount has been paid in full, in which case, Licensee shall no longer be required to make the Purdue Royalty Payment to INFI. In the event the rate of the Purdue Royalty Payment has changed from the initial rate, as set forth in this Section 6.1.3(b)(ii), and, as a result, Licensee has overpaid to INFI the Purdue Royalty Payment with respect thereto, such overpaid amount shall be credited toward Licensee’s Purdue Royalty Payments or, if the Purdue Repayment Amount has been paid in full, any other payment owed by Licensee to INFI.
(iii) On an IPI-145 Product-by-IPI-145 Product and country-by-country basis, if the sole basis for the continuance of a Royalty Term is the existence of Regulatory Exclusivity, the MICL Royalty Payment and the Purdue Royalty Payment shall be reduced by fifty percent (50%).
- 29 -
CONFIDENTIAL
(iv) INFI shall promptly pay the full amount of all MICL Royalty Payments and Purdue Royalty Payments received from Licensee to MICL, subject only to the last sentence of Section 6.1.3(b)(i) and the last sentence of Section 6.1.3(b)(ii).
(c) Mundipharma Trailing Royalty. Once the MICL Repayment Amount has been paid in full, Licensee shall no longer be required the pay the MICL Royalty Payment. Instead, Licensee will be required to pay MICL an amount equal to one percent (1%) of Net Sales of IPI-145 Product in the United States (the “MICL Trailing Royalty Payment”). For purposes of this Section 6.1.3(c) only, Net Sales of Combination Products will be calculated in accordance with Section 1.59(I) and will not be reduced by the multiplication factor reflected in Section 1.59(II). The MICL Trailing Royalty Payment shall be paid on an IPI-145 Product-by-IPI-145 Product basis until the expiration of the applicable Royalty Term in the United States. Thereafter, no further amounts shall be payable by Licensee to INFI for payment to MICL with respect to the MICL Agreements.
(i) On an IPI-145 Product-by-IPI-145 Product basis, if the sole basis for the continuation of a Royalty Term in the United States is the existence of Regulatory Exclusivity, then the MICL Trailing Royalty Payment shall be reduced by fifty percent (50%).
(ii) If Licensee (i) reasonably determines in good faith it, in order to avoid infringement of any patent not licensed hereunder, it is reasonably necessary to obtain a license from a Third Party in order to Manufacture or Commercialize an IPI-145 Product in a country in the Territory and to pay a royalty or other consideration under such license (including in connection with the settlement of a patent infringement claim), or (ii) shall be subject to a final court or other binding order or ruling requiring any payments, including a payment of a royalty to a Third Party patent holder in respect of future sales of any IPI-145 Product in a country in the Territory, then the amount of the MICL Trailing Royalty Payment shall be reduced by fifty percent (50%) of the amount paid by Licensee to such Third Party that is reasonably and appropriately allocable to, as applicable, such IPI-145 Product; provided, however, that in no event will a deduction or deductions under this Section 6.1.3(c) reduce the MICL Trailing Royalty Payment by more than fifty percent (50%).
6.1.4 Payment Terms. Except as otherwise set forth in this Agreement, all payments by or on behalf of Licensee under this Agreement shall be non-creditable (except pursuant to Section 6.5, the last sentence of Section 6.1.3(b)(i), or the last sentence of Section 6.1.3(b)(ii))) and non-refundable.
6.2 Methods of Payment.
6.2.1 All payments due under this Agreement shall be paid in Dollars, except as expressly set forth in Section 3.1.2(c)(ii). All payments to INFI under this Agreement shall be paid by electronic wire transfer of immediately available funds to a bank account in the United States designated in writing by INFI. All payments to INFI pursuant to Section 6.1.1 for a [**] shall be due [**] days after the end of each [**]. With respect to all payments to INFI pursuant to Sections 6.1.3(b) or 6.1.3(c), Licensee shall deliver such payments to INFI within [**] days after the end of each [**] during the applicable Royalty Term, reasonably detailed written accountings of Net Sales of IPI-145 Products that are subject to payments due to MICL or Purdue, as applicable for such
- 30 -
CONFIDENTIAL
[**]. Such accountings shall be Confidential Information of Licensee and subject to the confidentiality provisions set forth in the MICL Agreements. Such [**] reports shall indicate (i) gross sales and Net Sales (including reasonable detail for deductions from gross sales to Net Sales) on a country-by-country and IPI-145 Product-by-IPI-145 Product basis, and (ii) the calculation of the MICL Royalty, the Purdue Royalty, the MICL Trailing Royalty Payment from such gross sales and Net Sales; provided, however, such reports shall include (i) the actual information specified in this Section 6.2.1 for all [**] other than the [**] and (ii) a good faith estimate of the information specified in this Section 6.2.1 for the [**], which estimates shall be promptly reconciled with the actual information for such month in the next [**]. On the date on which Licensee is required to have delivered such accounting to INFI, Licensee shall also deliver the payments due for such [**]; provided, however, that such payments will be based on (a) the actual information specified in this Section 6.2.1 for all weeks in such [**] other than the last month of such [**] and (b) a good faith estimate of the information specified in this Section 6.2.1 for the last month of such [**], which estimates shall be promptly reconciled with the actual information for such month in the next [**].
6.2.2 For the purposes of calculating any sums due under this Agreement, Licensee shall convert any amount expressed in a foreign currency into US Dollar equivalents, calculated using the applicable currency conversion rate as published in [**], (a) for sales, on the last Business Day of the applicable Calendar Quarter for the Calendar Quarter in which the relevant sales were made or (b) for calculations of all other payments payable under this Agreement, on the day the payment obligation accrued.
6.3 Late Payments. Without limiting any other rights or remedies available to INFI hereunder, interest shall be payable by Licensee on any amounts payable to INFI, Purdue or MICL under this Agreement which are not paid by the due date for payment. All interest shall accrue and be calculated on a daily basis (both before and after any judgment) at a rate per annum equal to [**] percentage points above the then current “prime rate” in effect published in [**] (but in no event in excess of the maximum rate permissible under applicable Law), for the period from the due date for payment until the date of actual payment.
6.4 Taxes.
6.4.1 All payments due and payable under this Agreement will be made without any deduction or withholding for or on account of any tax except to the extent otherwise required by applicable Laws. If Licensee is so required to withhold, Licensee will (a) promptly notify INFI of such requirement; (b) deduct from each payment to which such requirement relates and pay to the relevant Governmental Authority the full amount required to be withheld promptly upon the earlier of (i) determining that such withholding is required or (ii) receiving notice that such amount has been assessed against INFI or any Third Party Grantor; and (c) promptly forward to INFI an official receipt (or certified copy) or other documentation reasonably acceptable to INFI evidencing such payment to such authorities.
6.4.2 [**]
- 31 -
CONFIDENTIAL
6.5 Books and Records; Audit Rights.
6.5.1 Licensee shall keep, and shall require its Affiliates and the Sublicensees to keep, complete and accurate records of the latest [**] years relating to gross sales, Annual Net Sales, and all revenue and expense data relating to the calculations of any payment due under this Agreement. For the sole purpose of verifying amounts payable to INFI, MICL or Purdue under Article 6, INFI shall have the right, [**], at INFI’s expense (except as set forth below), to retain an independent certified public accountant selected by INFI and reasonably acceptable to Licensee, to review such records in the location(s) where such records are maintained by Licensee, its Affiliates and the Sublicensees upon reasonable notice and during regular business hours. Such representatives shall execute a suitable confidentiality agreement reasonably acceptable to Licensee prior to conducting such audit. Such representatives shall disclose to each of INFI and Licensee only their conclusions regarding the accuracy of payments hereunder and of records related thereto. The right to audit any records underlying any royalty report shall extend for [**] from the end of the Calendar Year in which the royalty report was delivered. Licensee shall, within [**] days after the Parties’ receipt of the audit report, pay INFI the amount of any underpayment revealed by such audit together with interest calculated in the manner provided in Section 6.3. If the underpayment is equal to or greater than [**] of the amount that was otherwise due, Licensee shall reimburse INFI’s reasonable Out-of-Pocket Expenses of such review. If the audit demonstrates that Licensee has made an overpayment to INFI, Licensee shall be entitled to credit such amount against future payments due to INFI.
6.5.2 Upon the expiration of the [**] years following the end of any Calendar Year, the calculation of amounts payable under Article 6 with respect to such Calendar Year shall be binding and conclusive upon the Parties, and the Parties shall be released from any liability or accountability with respect to payments for such Calendar Year.
6.5.3 The Third Party Grantors shall have the same rights of audit and inspection with respect to Licensee and its Affiliates and Sublicensees as granted by INFI to such Third Party Grantor pursuant to the applicable INFI Third Party Agreement, provided, however, that any audit conducted by a Third Party Grantor shall constitute an audit conducted by INFI for purposes of this Section 6.5 and any such audit shall be limited to the scope set forth in this Section 6.5.
6.6 Financial Statements Required by Rule 3-05 of Regulation S-X. If Licensee determines in good faith that it would be required to file with the SEC pursuant to Rule 3-05 of Regulation S-X audited annual financial statements of the business related to the IPI-145 Product (the “Audited Financial Statements”) and/or unaudited quarterly financial statements of the business related to the IPI-145 Product (the “Unaudited Financial Statements”) for the periods specified by Rule 3-05 of Regulation S-X (any Audited Financial Statements together with any Unaudited Financial Statements, the “SEC Financial Statements”), then (X) Licensee will notify INFI of such determination no later than [**] days after the Effective Date and (Y) INFI will deliver to Licensee as soon as reasonably practicable, but in any event no later than [**] days after the Effective Date, the SEC Financial Statements. The SEC Financial Statements will be (a) prepared in accordance with the books and records of the business related to the IPI-145 Product, (b) prepared in accordance with Regulation S-X and U.S. GAAP and (c) in the case of the Audited Financial Statements,
- 32 -
CONFIDENTIAL
accompanied by an opinion (the “Audit Opinion”) of Ernst & Young (the “Independent Auditor”), which opinion complies with Regulation S-X. INFI will use its commercially reasonable efforts to cause the Independent Auditor to provide to Licensee the consents requested by Licensee no later than [**] Business Days prior to the required filing date of the SEC Financial Statements to permit the inclusion of the Audit Opinion with respect to the Audited Financial Statements in Licensee’s reports and registration statements filed with the SEC for periods required under applicable Law. Licensee will reimburse INFI for INFI’s costs incurred by INFI supported by reasonable documentation for INFI’s activities pursuant to this Section 6.6.
6.7 Other INFI Financial Deliverables. INFI will deliver to Licensee (a) within [**] after the Effective Date, a statement of assets acquired and liabilities assumed of the business related to the IPI-145 Product as of the Effective Date and (b) as soon as reasonably practicable, but in any event no later than [**] Business Days after the Effective Date, a statement of direct revenues and expenses of the business related to the IPI-145 Product (i) for the year ended December 31, 2015 and (ii) for the nine (9) months ended September 30, 2016 that includes information by Calendar Quarter for each of the first three (3) Calendar Quarters of fiscal year 2016. The financial information described in this Section 6.7 will be prepared in accordance with (X) the books and records of the business related to the IPI-145 Product and (Y) U.S. GAAP; provided, however, that all such information is unaudited and may be subject to change.
ARTICLE 7
OWNERSHIP OF INTELLECTUAL PROPERTY RIGHTS
OWNERSHIP OF INTELLECTUAL PROPERTY RIGHTS
7.1 Inventorship. For purposes of determining ownership of inventions pursuant to Section 7.2, inventorship for patentable inventions conceived or reduced to practice during the course of the performance of activities pursuant to this Agreement shall be determined in accordance with United States patent laws for determining inventorship.
7.2 Ownership. Subject to the licenses and rights granted to Licensee under this Agreement, INFI shall own the entire right, title and interest in and to any Know-How first made, authored, discovered, conceived or reduced to practice solely by employees, consultants, contractors or subcontractors of INFI, or acquired solely by INFI, any Patent Rights claiming patentable inventions therein and any other intellectual property rights (other than Patent Rights) covering such Know-How. Licensee shall solely own the entire right, title and interest in and to any Know-How first made, authored, discovered, conceived or reduced to practice solely by employees, consultants, contractors or subcontractors of Licensee or acquired solely by Licensee, any Patent Rights claiming patentable inventions therein and any other intellectual property rights (other than Patent Rights) covering such Know-How. Subject to the licenses and rights granted under this Agreement, all Know-How first made, authored, discovered, conceived or reduced to practice jointly by (i) employees, consultants, contractors or subcontractors of Licensee or any of its Affiliates and (ii) employees, consultants, contractors or subcontractors of INFI or any of its Affiliates, (“Joint Know-How”), Patent Rights claiming patentable inventions therein (“Joint Patent Rights”) and other intellectual property rights (other than Patent Rights) covering such Know-How, shall be jointly owned by the Parties without any duty to account, and each Party shall have the right to grant licenses and otherwise exploit the Joint IP, subject to the licenses granted hereunder. Each
- 33 -
CONFIDENTIAL
Party shall, and shall ensure that its Affiliates and its and its Affiliates’ employees, consultants, contractors, subcontractors and any other agents, execute all documents necessary, and otherwise reasonably cooperate with the other Party, to effectuate this Section 7.2.
7.3 Prosecution and Maintenance of Patent Rights.
7.3.1 Prosecution Patent Rights.
(a) Licensee shall have the first right, at its sole expense, to Prosecute and Maintain the INFI Prosecution Patent Rights, the INK Prosecution Patent Rights and the Joint Patent Rights (collectively, the “Prosecution Patent Rights”) using Xxxxx Day, Xxxxx & Xxxxxxxx or other legal counsel reasonably acceptable to INFI. Licensee shall (i) provide INFI and, with respect to the INK Prosecution Patent Rights, INK, copies of all prosecution filings related to the Prosecution Patent Rights sent to or received from patent offices in the Territory, unless otherwise directed by INFI, (ii) provide INFI with a draft of each such filing reasonably in advance of submission, (iii) provide INFI an opportunity to provide comments on and make requests of Licensee concerning such filings, (iv) consider in good faith any comments regarding such draft application that INFI may timely provide, (v) keep INFI and, with respect to the INK Prosecution Patent Rights, INK, regularly and reasonably informed of the status of the Prosecution Patent Rights as may be requested from time to time by INK, and (vi) provide INFI and, with respect to the INK Prosecution Patent Rights, INK, such other information related to Prosecution and Maintenance of the Prosecution Patent Rights in the Territory as INFI or, with respect to the INK Prosecution Patent Rights, INK, may from time to time reasonably request to allow INFI and, with respect to the INK Prosecution Patent Rights, INK, to track Prosecution and Maintenance of such Patent Rights.
(b) Licensee shall bear one hundred percent (100%) of all Patent Expenses during the Term with respect to the Prosecution and Maintenance of the Prosecution Patent Rights in accordance with Section 7.3.1(a).
(c) If INK objects to Licensee’s Prosecution and Maintenance of any of the INK Prosecution Patent Rights, then, upon Licensee’s request, (i) INFI shall Prosecute and Maintain such Patent Right, in accordance with Licensee’s reasonable direction with respect thereto using mutually acceptable counsel which, as of the Effective Date includes Xxxxx Day and Lando & Xxxxxxxx; and (ii) INFI shall resolve such dispute with INK in accordance with the INK Agreement. Licensee shall pay, or reimburse INFI, for all reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses associated with such Prosecution and Maintenance and such dispute resolution.
(d) In the event Licensee decides to cease to Prosecute or Maintain any claim of a Prosecution Patent Right in a country of the Territory, decides to not otherwise Prosecute and Maintain any Prosecution Patent Right in a country of the Territory, or does not wish to bear the costs or expenses with respect to the Prosecution or Maintenance of any Prosecution Patent Right in a country of the Territory:
(i) Licensee shall give INFI prior written notice sufficiently in advance thereof, but not less than [**] days before any action would be required to be taken by
- 34 -
CONFIDENTIAL
INFI to avoid a loss of rights in such Prosecution Patent Right, in order to allow INFI (at its discretion) to assume such Prosecution or Maintenance without a loss of rights in such Prosecution Patent Right. If INFI determines not to assume such Prosecution or Maintenance with respect to such INK Prosecution Patent Right, then INFI shall give written notice to INK in sufficient time (but no less than [**] days before any applicable statutory bar) to permit INK to Prosecute and Maintain such INK Prosecution Patent Right;
(ii) INFI or, with respect to the INK Prosecution Patent Rights, INK (to the extent set forth in the INK Agreement), shall thereafter have the sole right to Prosecute and Maintain such Prosecution Patent Right, in INFI’s name or, with respect to the INK Prosecution Patent Rights, INK’s name, in such country. Licensee shall use reasonable efforts to make available to INFI and, with respect to the INK Prosecution Patent Rights, INK, Licensee’s employees, authorized attorneys, agents or representatives as are reasonably necessary to assist INFI and, with respect to the INK Prosecution Patent Rights, INK, in Prosecuting and Maintaining, such Prosecution Patent Rights. Licensee shall sign, or have signed, all legal documents necessary to Prosecute and Maintain such patent applications or patents in respect of such Patent Rights; and
(iii) such Patent Right shall no longer be included in the INFI Prosecution Patent Rights or INK Prosecution Patent Rights, as applicable, and all licenses and rights granted to Licensee hereunder with respect to such Patent Right, including the licenses granted under Section 2.1, shall automatically terminate.
7.3.2 Non-Prosecution Patent Rights. Licensee shall have no right to Prosecute or Maintain, and Licensee shall not be required to bear any costs associated with the Prosecution and Maintenance of, any of the INFI Other Patent Rights or any of the INK Non-Prosecution Patent Rights.
7.4 Third Party Infringement. Each Party will promptly notify the other Party and, with respect to the INK Prosecution Patent Rights, the notifying Party will promptly notify INK (in accordance with the notice provision in the INK Agreement), in writing of (a) any actual or threatened infringement or misappropriation by a Third Party of any Prosecution Patent Right of which it becomes aware, as a result of such Third Party’s Research, Development, Manufacture, use, sale, offer for sale, other Commercialization or importation of the IPI-145 Compound or any IPI-145 Product in the Territory, including any certification filed by a Third Party pursuant to 21 U.S.C. §355(b)(2)(A)(iv) or 355(j)(2)(A)(vii)(IV) or any notice under comparable U.S. or foreign law (a “Paragraph IV Certification”) which references the foregoing; or (b) an actual or threatened challenge to any Prosecution Patent Right by a Third Party (any such infringement or challenge in clause (a) or (b), a “Third Party Infringement”). The Parties will consult with each other through each Party’s patent attorneys (and, with respect to any INK Prosecution Patent, INK, through its patent attorney, may consult with respect to any INK Prosecution Patent) to determine the response to any such infringement or challenge by a Third Party of any Prosecution Patent Right, including any Paragraph IV Certification which references the foregoing.
7.5 Enforcement Rights.
- 35 -
CONFIDENTIAL
7.5.1 With respect to the INFI Prosecution Patent Rights and the INK Prosecution Patent Rights, Licensee shall have the first right, but not the obligation, to initiate a proceeding or take other appropriate action in connection with the Third Party Infringement to the extent that such Third Party Infringement involves the Research, Development, Manufacture, use or Commercialization of the IPI-145 Compound or any IPI-145 Product in the Territory. Notwithstanding the foregoing sentence, Licensee shall not initiate any lawsuit or other enforcement action asserting any such Patent Rights without first consulting with INFI and giving good faith consideration to any reasonable objection from INFI regarding Licensee’s proposed course of action. INFI shall have the right, at INFI’s sole expense, to be represented in any such action by counsel of its own choice; provided, however, that Licensee shall bear all of INFI’s costs and expenses with respect to any activities undertaken by INFI at Licensee’s request. With respect to any INK Prosecution Patent, INK shall have the right to be represented in any such action by counsel of its own choice, at INK’s sole expense. Licensee shall not, through any court action or proceeding, any settlement arrangement or any proceeding, filing or communication with any patent office, admit the invalidity of, or otherwise impair INFI’s or INK’s rights in, any Duvelisib Patent Right without the prior written consent of INFI and, with respect to the INK Prosecution Patent Rights or INK Non-Prosecution Patent Rights, INK. Any recoveries resulting from such an action brought by Licensee in accordance with this Section 7.5.1 shall be applied as follows:
(a) First, to reimburse (i) INK’s out-of-pocket expenses and (ii) each Party for all Out-of-Pocket Expenses in connection with such proceeding (on a pro rata basis, based on each Party’s respective litigation costs, to the extent the recovery was less than all such litigation costs);
(b) Second, any portion of the remainder that is attributable to lost profits with respect to sales of the IPI-145 Product outside the Field shall be subject to a royalty payment to INK in accordance with the INK Agreement equal to the amount that would be due if such amount were Net Sales (as defined in the INK Agreement) under the INK Agreement, and Licensee shall promptly pay such royalty payment to INK; and
(c) Third, the remainder shall be retained by Licensee, shall be considered Net Sales under this Agreement and shall be subject to the royalty obligations under this Agreement.
7.5.2 If Licensee decides not to, or fails to, initiate proceedings or take other appropriate action pursuant to Section 7.5.1 with respect to a Third Party Infringement of any such Prosecution Patent Right within the shorter of (a) [**] days following Licensee’s becoming aware of the alleged infringement (which shall be [**] days with respect to the INK Prosecution Patent Rights) or (b) solely with respect to a Paragraph IV Certification, [**] days following the earlier of Licensee’s or INFI’s receipt of notice thereof (which shall be [**] days with respect to the INK Prosecution Patent Rights), then (y) Licensee shall promptly notify INFI thereof and (z) INFI or, with respect to the INK Prosecution Patent Rights, INK (to the extent set forth in the INK Agreement), shall have the right, but not the obligation, to bring and control any such action at its own expense and by counsel of its own choice. Licensee shall notify INFI and, with respect to the INK Prosecution Patent Rights, Licensee shall notify INK (in accordance with the notice provision in the INK
- 36 -
CONFIDENTIAL
Agreement), as soon as Licensee is aware that it will not initiate such proceedings or take such action within such time periods. Any recoveries resulting from such an action brought by INFI or INK in accordance with this Section 7.5.2 will be retained by INFI or, with respect to the INK Prosecution Patent Rights, INK (to the extent set forth in the INK Agreement).
7.6 Conduct of Certain Actions; Costs. The Party initiating legal action under Section 7.5 with respect to Prosecution Patent Rights (the “Initiating Party”) shall have the sole and exclusive right to select counsel for any suit initiated by it. At the request of the Initiating Party or, with respect to the INK Prosecution Patent Rights, INK (if it is then controlling the relevant action), the other Party shall provide reasonable assistance and cooperation in connection therewith. If Licensee is the Initiating Party, Licensee shall [**]. The other Party shall reasonably cooperate in the prosecution of such suit as may be reasonably requested by the Initiating Party or, with respect to the INK Prosecution Patent Rights, INK (if it is then controlling the relevant action), including by agreeing to be joined to such legal action to the extent required in order to maintain such legal action; provided, that if Licensee is the Initiating Party, Licensee shall [**]. The other Party and INK (where applicable pursuant to Section 10.3(b) of the INK Agreement) shall have the right to participate and be represented in any such legal action (in cases where such other Party has standing) by its own counsel at its own cost.
7.7 Defense of Actions. In the event that a declaratory judgment or similar action alleging the invalidity or non-infringement, or any request for, or filing or declaration of, any interference, opposition, reissue or reexamination, of any Prosecution Patent Right is initiated by any Third Party, each Party will promptly notify the other and the rights and responsibilities for defending against any such action shall be determined in the same manner as Prosecution and Maintenance of the relevant Prosecution Patent Right pursuant to Section 7.4. INK shall have the sole right to defend against any declaratory judgment or similar action alleging the invalidity or non-infringement, or any request for, or filing or declaration of, any interference, opposition, reissue or reexamination, of any INK Non-Prosecution Patent Right.
7.8 Trademarks.
7.8.1 Licensee shall have the right to brand IPI-145 Products using Licensee related trademarks and trade names and any other trademarks and trade names it determines appropriate for the IPI-145 Product, which may vary by country or within a country. Licensee and, if applicable, certain Licensee Affiliates or Sublicensees, shall own all right, title and interest in and to such marks and all goodwill associated therewith and Licensee or such Affiliates or Sublicensees may file, seek registration and maintain such marks in the countries and regions they determine reasonably necessary, in each case solely to the extent such marks are not Product Marks or the INK Xxxx licensed to Licensee pursuant to this Agreement. Notwithstanding the foregoing, unless INK waives its relevant rights under the INK Agreement, (a) with respect to any IPI-145 Product sold in the United States after receipt of Marketing Authorization for such IPI-145 Product in the United States, Licensee shall and shall ensure that its applicable Affiliates and the applicable Sublicensees, to the extent permitted under applicable Law and if reasonably practicable, include the INK name or logo (“INK Xxxx”) on the commercial packaging for such IPI-145 Product, and a disclosure that such IPI-145 Product is licensed from INK, and (b) Licensee and its applicable Affiliates or the applicable
- 37 -
CONFIDENTIAL
Sublicensees may otherwise include the INK Xxxx on the IPI-145 Product or any packaging, labels, containers, advertisements and other materials related thereto; provided, however, that any use of the INK Xxxx shall be in compliance with INK’s then-current reasonable trademark guidelines provided to Licensee (whether by INFI or by INK).
7.8.2 Subject to the terms and conditions of this Agreement, INFI hereby grants Licensee a non-exclusive, sublicenseable, royalty-free, transferrable (in accordance with Section 12.5) right to use the INK Xxxx in connection with the foregoing.
7.8.3 INK or an Affiliate of INK shall retain the ownership of the entire right, title and interest in and to the INK Xxxx, and all goodwill associated with or attached to the INK Xxxx arising out of the use thereof by Licensee, its Affiliates and the Sublicensees shall inure to the benefit of INK. Licensee shall not, and shall ensure that its Affiliates and the Sublicensees shall not, contest, oppose or challenge INK’s ownership of the INK Xxxx. Licensee shall not, and shall ensure that its Affiliates and the Sublicensees shall not, at any time do or suffer to be done any act or thing that will in any way impair INK’s ownership of or rights in and to the INK Xxxx or any registration thereof or that may depreciate the value of the INK Xxxx or the reputation of INK.
7.9 Drug Price Competition and Patent Term Restoration Act.
7.9.1 The Parties shall cooperate with each other in an effort to avoid loss of any Prosecution Patent Rights which may otherwise be available under the provisions of the Drug Price Competition and Patent Term Restoration Act of 1984 or comparable United States or foreign laws, including by executing any documents as may be reasonably required. In particular, the Parties shall, at Licensee’s sole expense, cooperate in obtaining patent term restoration or supplemental protection certificates or their equivalents in any country and region (“Patent Term Extensions”), where applicable to the Prosecution Patent Rights. INFI shall provide all reasonable assistance to Licensee, including permitting Licensee to proceed with applications for such in the name of INFI, if so required.
7.9.2 After consultation by Licensee with INFI and INK, Licensee shall have the sole right to determine, if applicable, for which, if any, of the Prosecution Patent Rights the Parties will attempt to seek Patent Term Extensions for the IPI-145 Product. INFI shall provide reasonable assistance to Licensee, at Licensee’s sole expense, including by executing any required documents and providing any relevant patent information and other relevant information to Licensee, so that Licensee can obtain such extensions and additional protection and inform the FDA or other Regulatory Authority of such intended Patent Term Extension.
7.9.3 Licensee shall have no right to seek Patent Term Extension for any INK Non-Prosecution Patent Right or INFI Other Patent Right.
7.10 Orange Book Information. Licensee shall have the sole right, but not the obligation, to select and submit to all applicable Governmental Authorities patent information pertaining to each IPI-145 Product pursuant to 21 U.S.C. § 355(b)(1)(G) (or any amendment or successor statute thereto), or any similar statutory or regulatory requirement in any non-U.S. country or other regulatory jurisdiction.
- 38 -
CONFIDENTIAL
7.11 Patent Marking. Licensee shall, and shall ensure that its Affiliates and the Sublicensees, comply with the patent marking statutes in each country in which a IPI-145 Product is sold by Licensee, its Affiliates or the Sublicensees.
ARTICLE 8
CONFIDENTIALITY
CONFIDENTIALITY
8.1 Confidentiality; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed to by the Parties in writing, the Receiving Party and its Affiliates shall keep confidential, and shall not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement, any Confidential Information of the Disclosing Party or any of its Affiliates, except to the extent that it can be established by the Receiving Party that such Confidential Information:
8.1.1 was in the lawful knowledge and possession of the Receiving Party or any of its Affiliates prior to the time it was disclosed to the Receiving Party or any of its Affiliates by the Disclosing Party or any of its Affiliates;
8.1.2 was developed by the Receiving Party or any of its Affiliates without the aid, use, or access of or to Confidential Information of the Disclosing Party or any of its Affiliates, as evidenced by written records kept in the ordinary course of business or other documentary proof of actual use by the Receiving Party or any of its Affiliates;
8.1.3 was generally available to the public or otherwise part of the public domain at the time of its disclosure to the Receiving Party or any of its Affiliates by the Disclosing Party or any of its Affiliates;
8.1.4 became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the Receiving Party or any of its Representatives in breach of this Agreement; or
8.1.5 was disclosed to the Receiving Party or any of its Affiliates, other than under an obligation of confidentiality, by a Third Party who had no obligation to the Disclosing Party or any of its Affiliates not to disclose such information to others.
8.2 Authorized Disclosure. Except as expressly provided otherwise in this Agreement, a Receiving Party or any of its Affiliates may use and disclose Confidential Information of the Disclosing Party or any of its Affiliates as follows:
8.2.1 to its Affiliates, its Sublicensees (solely with respect to Licensee), and its and their respective employees, consultants, contractors, subcontractors, agents, legal advisors and financial advisors (all the foregoing, collectively, “Representatives”) who need to know such Confidential Information for purposes of the Receiving Party performing its obligations or exercising its rights under this Agreement, each of which Representatives shall, prior to such disclosure, be subject to written obligations, or professional ethical obligations, substantially similar
- 39 -
CONFIDENTIAL
to those in the Agreement, and the Receiving Party shall remain responsible for any failure by its Representatives to treat such Confidential Information as required under this ARTICLE 8;
8.2.2 except as set forth in Section 8.2.1, in connection with the performance of its obligations or exercise of rights granted or reserved in this Agreement, provided, that such Confidential Information is disclosed under appropriate confidentiality provisions substantially similar to those in this Agreement and the Receiving Party shall remain responsible for any failure by any such recipient to treat such Confidential Information as required under this ARTICLE 8;
8.2.3 to the extent such disclosure is reasonably necessary in Prosecution and Maintenance of Patent Rights in a manner not inconsistent with this Agreement, prosecuting or defending litigation, complying with applicable Law (including the rules and regulations of any stock exchange or NASDAQ), preparing and submitting filings to Regulatory Authorities consistent with this Agreement or is otherwise required by Law; except that if the Receiving Party or any of its Affiliates is required by Law to make any such disclosure of a Disclosing Party’s (or any of its Affiliates’) Confidential Information (other than a disclosure to a Regulatory Authority in a filing required by Law) the Receiving Party will, to the extent practicable, give reasonable advance notice to the Disclosing Party of such disclosure requirement and shall furnish only that portion of the Disclosing Party’s (or its Affiliate’s) Confidential Information that the Receiving Party or its Affiliate is legally required to furnish;
8.2.4 by INFI to any Third Party Grantor in order to exercise INFI’s rights or comply with INFI’s obligations under the INFI Third Party Agreement, and Licensee agrees and acknowledges that such Third Parties shall not be bound to any confidentiality or non-use information with respect to Licensee’s Confidential Information other than as set forth in the relevant INFI Third Party Agreement;
8.2.5 by INFI to any counterparty to any INFI Product Related Contract to the extent reasonably necessary to comply with INFI’s obligations under this Agreement with respect to such INFI Product Related Contract, and Licensee agrees and acknowledges that such Third Parties shall not be bound to any confidentiality or non-use information with respect to Licensee’s Confidential Information other than as set forth in the relevant INFI Product Related Contract;
8.2.6 except as set forth in Section 8.2.1 or Section 8.2.4, in communications with existing or prospective acquirers, merger partners, investors, financing sources, advisors, licensees, sublicensees or collaborators or others on a need to know basis, in each case under appropriate confidentiality provisions substantially equivalent to those of this Agreement, and the Receiving Party shall remain responsible for any failure by any of the foregoing to treat such Confidential Information as required under this ARTICLE 8; or
8.2.7 to the extent agreed to in writing by the Disclosing Party.
8.3 Press Release; Disclosure of Agreement.
8.3.1 Neither Party shall issue any press release or make other disclosures regarding this Agreement or the Parties’ activities hereunder, or any results or data arising hereunder, except
- 40 -
CONFIDENTIAL
(a) that either Party may issue a press release agreed to in writing by the other Party, such agreement not to be unreasonably withheld, conditioned or delayed; (b) with the other Party’s prior written consent; (c) in accordance with Section 8.5; or (d) for any disclosure that is reasonably necessary to comply with applicable securities exchange listing requirements or other applicable Laws. Notwithstanding the foregoing, to the extent information regarding this Agreement has already been publicly disclosed, either Party may subsequently disclose the same information to the public without the consent of the other Party. Each Party shall be permitted to disclose the terms of this Agreement, in each case subject to Section 8.2.6 and under appropriate confidentiality provisions substantially equivalent to those of this Agreement, to any actual or potential acquirers, merger partners, licensees, sublicensees, licensors, investors, financing sources and professional advisors on a need to know basis.
8.3.2 Each Party shall, if practicable, give the other Party a reasonable opportunity to review applications for confidential treatment of this Agreement filed with the United States Securities and Exchange Commission (or any stock exchange, including NASDAQ, or any similar regulatory agency in any country other than the United States) prior to submission of such filings, and shall give due consideration to any reasonable comments by the non-filing Party relating to such filing.
8.4 Remedies. In the event a Party breaches any of the confidentiality or non-use obligations set forth in this ARTICLE 8, the other Party shall be entitled to seek, in addition to any other right or remedy it may have, at law or in equity, a temporary injunction, without the posting of any bond or other security, enjoining or restraining the breaching Party from any violation or threatened violation of this ARTICLE 8.
8.5 Publications. Licensee may publish the scientific results of activities undertaken by either Party, any of its Affiliates or any Sublicensee (or, with respect to INFI, any licensee or sublicensee) with respect to the Research, Development, Manufacture and Commercialization of the IPI-145 Compound or IPI-145 Product. Except to the extent required by applicable Law, INFI shall not publish scientific or other results of activities undertaken by INFI with respect to the Research, Development, Manufacture and Commercialization of the IPI-145 Compound or the IPI-145 Product without the prior written consent of Licensee.
8.6 Existing Third Party Agreements. The provisions of this ARTICLE 8 are subject to the terms of each applicable INFI Third Party Agreement or INFI Product Related Contract and shall be interpreted in a manner that is consistent with the rights of the relevant Third Party under the relevant INFI Third Party Agreement or INFI Product Related Contract. Notwithstanding anything to the contrary in this ARTICLE 8, Licensee shall comply with all applicable restrictions in the relevant INFI Third Party Agreements with respect to Licensee’s publication or disclosure of the results of any of the activities conducted by Licensee under this Agreement.
8.7 Survival. The confidentiality and non-use obligations set forth in this ARTICLE 8 shall survive for the longer of (a) [**] years after the Term or (b) with respect to any Confidential Information subject to any obligations to the relevant Third Party under any INFI Third Party Agreement or INFI Product Related Contract, such longer period as may be required under such agreement.
- 41 -
CONFIDENTIAL
ARTICLE 9
REPRESENTATIONS AND WARRANTIES
REPRESENTATIONS AND WARRANTIES
9.1 Representations and Warranties of Both Parties. Each Party hereby represents and warrants to the other Party, as of the Effective Date, that:
9.1.1 such Party is duly organized, validly existing and in good standing under the Laws of the jurisdiction of its incorporation and has full corporate power and authority to enter into this Agreement and to carry out the provisions hereof;
9.1.2 such Party has taken all necessary action on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder;
9.1.3 this Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, binding obligation, enforceable against it in accordance with the terms hereof;
9.1.4 the execution, delivery and performance of this Agreement by such Party do not conflict with any agreement or any provision thereof, or any instrument or understanding, oral or written, to which it is a party or by which it is bound, nor violate any Law of any Governmental Authority having jurisdiction over such Party;
9.1.5 no government authorization, consent, approval, license, exemption of or filing or registration with any court or governmental department, commission, board, bureau, agency or instrumentality, domestic or foreign, under any applicable Laws currently in effect, is or will be necessary for, or in connection with, the transaction contemplated by this Agreement, or for the performance by it of its obligations under this Agreement, except as necessary to conduct clinical studies, to transfer the INDs and other Regulatory Documentation in accordance with Section 3.2 or to seek or obtain Regulatory Approvals; and
9.1.6 neither it nor any of its or its Affiliates’ employees or agents performing hereunder has ever been, or is currently: (a) debarred under 21 U.S.C. § 335a; (b) excluded, debarred, suspended, or otherwise ineligible to participate in Federal health care programs or in Federal procurement or non-procurement programs; (c) listed on the FDA’s Disqualified and Restricted Lists for clinical investigators; or (d) convicted of a criminal offense that falls within the scope of 42 U.S.C. § 1320a-7(a), even if not yet excluded, debarred, suspended, or otherwise declared ineligible. If Licensee becomes aware that it or any of its or its Affiliates’ employees or agents performing hereunder is the subject of any investigation or proceeding that could lead to such Person becoming a debarred entity or individual, an excluded entity or individual or a convicted entity or individual, Licensee shall immediately notify INFI, and INFI shall have the right to immediately terminate this Agreement.
9.2 Representations and Warranties of INFI. INFI hereby represents and warrants to Licensee, as of the Effective Date:
- 42 -
CONFIDENTIAL
9.2.1 INFI is the sole and exclusive owner of the entire right, title and interest in the INFI Prosecution Patent Rights. INFI is the sole and exclusive licensee of the INK Prosecution Patent Rights. INFI is a non-exclusive licensee of the INK Non-Prosecution Patent Rights. With respect to the INFI Prosecution Patent Rights and the INK Prosecution Patent Rights, such Patent Rights are (i) subsisting and in good standing, and (ii) being diligently prosecuted in the respective patent offices in the Territory in accordance with Law, and have been filed and maintained properly and correctly and all applicable fees have been paid on or before the due date for payment.
9.2.2 To the Knowledge of INFI’s General Counsel and INFI’s Chief Patent Counsel, all Know-How being used by INFI to Research, Develop, Manufacture and Commercialize the IPI-145 Compound and IPI-145 Products as of the Effective Date (a) constitutes Duvelisib Know-How and is being licensed to Licensee hereunder or (b) is generally known to the public.
9.2.3 To the Knowledge of INFI’s Vice President, Regulatory Affairs and Quality Assurance, true, complete, and correct copies of: (a) all Regulatory Documentation existing as of the Effective Date relating to the IPI-145 Product in the Field, that is to be transferred to Licensee pursuant to the Transition Plan; and (b) all material adverse information with respect to the safety and efficacy of the IPI-145 Compound known to INFI as of the Effective Date, to be transferred to Licensee pursuant to the Transition Plan, in each case ((a) and (b)) have been or will be provided or made available to Licensee prior to the end of the Transition Period.
9.2.4 There are no claims, judgments, or settlements against, or amounts with respect thereto, owed by INFI or any of its Affiliates relating to the INFI Prosecution Patent Rights or INK Prosecution Patent Rights existing as of the Effective Date (the “Existing Patents”) or the Duvelisib Know-How. No claim or litigation has been brought or, to the Knowledge of INFI’s General Counsel and INFI’s Chief Patent Counsel, threatened by any Person (a) alleging that Existing Patents are invalid or unenforceable, (b) asserting the misuse, or non-infringement of any of the Existing Patents, (c) challenging INFI’s Control of the Existing Patents or (d) alleging misappropriation of the Duvelisib Know-How.
9.2.5 Except as set forth in the INFI Third Party Agreements, the Existing Patent Rights are free and clear of any liens, charges, encumbrances or, to the Knowledge of INFI’s General Counsel and INFI’s Chief Patent Counsel, claims of ownership by an Third Party, other than (a) non-exclusive licenses granted by Infinity to Third Parties, which grants are not in conflict with, or do not preclude Licensee from exercising, the licenses granted to Licensee hereunder, or of the nature of material transfer agreements, clinical trial agreements and manufacturing agreements, which will not adversely affect Licensee’s ability to Develop, Manufacture or Commercialize the IPI-145 Products in accordance with this Agreement and (b) the rights of the relevant Third Party Grantor and their licensors. INFI is entitled to grant the licenses specified in this Agreement.
9.2.6 No written claim of infringement of the Patent Rights or misappropriation of the Know-How of any Third Party has been made, or to the Knowledge of INFI’s General Counsel and INFI’s Patent Counsel, threatened, against INFI or any of its Affiliates with respect to the Research, Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Products.
- 43 -
CONFIDENTIAL
9.2.7 There are no judgments or settlements against or owed by INFI or, to the Knowledge of INFI’s General Counsel and INFI’s Patent Counsel, pending litigation against INFI or litigation threatened against INFI in writing, in each case related to the IPI-145 Product, including any relating to any Regulatory Documentation Controlled by INFI as of the Effective Date.
9.2.8 Neither INFI nor any of its Affiliates is or has been a party to any agreement with the U.S. federal government or an agency thereof pursuant to which the U.S. federal government or such agency provided funding for the Development of the IPI-145 Compound or IPI-145 Product, and the inventions claimed or covered by the Existing Patents are not a “subject invention” as that term is described in 35 U.S.C. Section 201(f).
9.2.9 (a) The INFI Third Party Agreements are the only agreements between INFI and any Third Party pursuant to which INFI has in-licensed any Patent Rights or pursuant to which INFI owes any Third Party any royalties with respect to IPI-145 Compound or IPI-145 Products; (b) prior to the Effective Date, INFI has provided Licensee with an opportunity to review complete and correct copies of the INFI Third Party Agreements; (c) to the Knowledge of INFI’s General Counsel and INFI’s Patent Counsel, such INFI Third Party Agreements remain in full force and effect as of the Effective Date; (d) as of the Effective Date, INFI is in material compliance with the terms of such INFI Third Party Agreements and, to the Knowledge of INFI’s General Counsel and INFI’s Patent Counsel, the Third Party Grantors are in material compliance with the terms of the applicable INFI Third Party Agreements; and (e) INFI has obtained any and all consents required under the INFI Third Party Agreements as may be necessary to perform its obligations under this Agreement. Without limiting this Section 9.2.9, the terms of this Agreement do not materially breach or constitute a material default under the terms of any INFI Third Party Agreement.
9.2.10 To the Knowledge of INFI’s Vice President, Regulatory Affairs and Quality Assurance, INFI and its Affiliates have generated, prepared, maintained, and retained all Regulatory Documentation that are required to be maintained or retained pursuant to and in material compliance with applicable Law, and have conducted in material compliance with applicable Law, including GLP and GCP, (a) all Development of the IPI-145 Compound or the IPI-145 Products in the Field that they have conducted prior to the Effective Date and (b) all Research activities that are material to the receipt of Regulatory Approval for the IPI-145 Product.
9.2.11 To the Knowledge of INFI’s General Counsel and INFI’s Chief Patent Counsel, no material breach of confidentiality has been committed by any Third Party with respect to the Duvelisib Know-How and INFI has used reasonable measures to protect the confidentiality thereof.
9.2.12 (a) INFI has obtained from each of its Affiliates, employees and agents, and from the employees and agents of its Affiliates, who have participated in the Research, Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Products, rights to any and all Know-How created by such employees and agents that relates to the IPI-145 Compound or IPI-145 Products, such that Licensee shall, by virtue of this Agreement, receive from INFI, without payments beyond those required by ARTICLE 6, the licenses and other rights granted to Licensee hereunder, except with respect to those Persons from whom obtaining such rights is not customary, such as academic and non-profit Persons; (b) each Person who has or has had any ownership rights
- 44 -
CONFIDENTIAL
in or to any issued Existing Patents purported to be owned solely by INFI, has assigned and has executed an agreement assigning its entire right, title, and interest in and to such Existing Patent to INFI; and (c) to the Knowledge of INFI’s General Counsel and INFI’s Patent Counsel, no current officer, employee, agent, or consultant of INFI or any of its Affiliates is in violation of any term of any assignment or other agreement, in each case, regarding the protection of Patents Rights or other intellectual property or proprietary information of INFI or such Affiliate.
9.2.13 To the Knowledge of INFI’s Vice President, Regulatory Affairs and Quality Assurance, neither INFI nor any of its Affiliates, nor any of its or their respective officers, employees, or agents has made an untrue statement of material fact or fraudulent statement to the FDA or any other Regulatory Authority with respect to the Development of the IPI-145 Compound or the IPI-145 Products, failed to disclose a material fact required to be disclosed to the FDA or any other Regulatory Authority with respect to the Development of the IPI-145 Compound or the IPI-145 Products, or committed an act, made a statement, or failed to make a statement with respect to the Development of the IPI-145 Compound or the IPI-145 Products that could reasonably be expected to provide a basis for the FDA to invoke its policy respecting “Fraud, Untrue Statements of Material Facts, Bribery, and Illegal Gratuities”, set forth in 56 Fed. Reg. 46191 (September 10, 1991) and any amendments thereto or any analogous laws or policies in the Territory.
9.2.14 Any shares of Licensee Common Stock acquired by INFI in accordance with the terms of this Agreement will be acquired for investment for INFI’s own account, not as a nominee or agent, and not with a view to the resale or distribution of any part thereof, and INFI has no present intention of selling, granting any participation in, or otherwise distributing the same. INFI is aware of the Licensee’s business affairs and financial condition and has acquired sufficient information about the Licensee to reach an informed and knowledgeable decision to acquire such shares of Licensee Common Stock. INFI is an accredited investor as defined in Rule 501(a) of Regulation D promulgated under the Securities Act.
9.3 Mutual Covenants. Each Party hereby covenants to the other Party that it shall not, during the Term, grant any right or license to any Third Party relating to any of the intellectual property rights it owns or Controls which would conflict with any of the rights or licenses granted or to be granted to the other Party hereunder.
9.4 Licensee Covenants. Licensee hereby covenants to INFI that:
9.4.1 Licensee shall comply, and shall ensure that its Affiliates and the Sublicensees comply, with all applicable Laws in connection with their activities under this Agreement and the transactions contemplated hereby, including GCP, GLP and GMP and ICH guidelines;
9.4.2 All employees, consultants, contractors and subcontractors of Licensee or its Affiliates working under this Agreement are and will be under the obligation to automatically assign all right, title and interest in and to their inventions, discoveries and other Know-How, whether or not patentable, and all Patent Rights and other intellectual property rights therein, to Licensee or its Affiliate as the sole owner thereof and waive all moral rights therein;
- 45 -
CONFIDENTIAL
9.4.3 Neither Licensee nor any of its Affiliates is subject to any non-compete or other restrictions that would impair its ability to Develop, Manufacture or Commercialize the IPI-145 Product in the Field in the Territory;
9.5 Disclaimer. Except as otherwise expressly set forth in this Agreement, (a) NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY THAT ANY PATENT RIGHTS ARE VALID OR ENFORCEABLE, AND (b) EACH PARTY EXPRESSLY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NONINFRINGEMENT. Without limiting the generality of the foregoing, except as otherwise set forth in this Agreement, INFI disclaims any warranties with regards to: (x) the success of the IPI-145 Compound or IPI-145 Product under this Agreement; (y) the safety or usefulness for any purpose of the technology or materials, including any compounds, it provides or discovers under this Agreement; and (z) the validity, enforceability, or non-infringement of any intellectual property rights or technology it provides or licenses to Licensee under this Agreement.
ARTICLE 10
INDEMNIFICATION; LIMITATION OF LIABILITY; INSURANCE
INDEMNIFICATION; LIMITATION OF LIABILITY; INSURANCE
10.1 Indemnification by Licensee. Licensee shall defend, indemnify and hold harmless the INFI Indemnitees from and against any and all losses, damages, fees, expenses, settlement amounts or costs (including reasonable legal expense, attorneys’ fees and witness fees) (“Losses”) relating to or in connection with a Third Party claim to the extent arising out of (a) the research, development, manufacture or commercialization of the IPI-145 Compound or the IPI-145 Product by Licensee, any Licensee Affiliate, any Sublicensee, INFI (to the extent properly acting in accordance with Licensee’s express direction) or any of their respective employees, consultants, contractors, subcontractors or agents after the Effective Date, including any actual or alleged death, personal bodily injury or damage to real or tangible personal property, or other product liability claimed to result from the IPI-145 Product Researched, Developed, Manufactured or Commercialized by or on behalf of Licensee or any of its Affiliates or any Sublicensee, (b) any breach by Licensee of any of its representations, warranties, covenants or obligations under this Agreement, or (c) any negligent act or omission or willful misconduct of Licensee, any of its Affiliates or any Sublicensee, or any of their respective employees, consultants, contractors, subcontractors or agents, in performing Licensee’s obligations or exercising Licensee’s rights under this Agreement; except that the foregoing indemnity shall not apply with respect to any INFI Indemnitee to the extent that any such Losses (x) are caused by the gross negligence or willful misconduct of any INFI Indemnitee, or (y) are otherwise subject to an obligation by INFI to indemnify the Licensee Indemnitees under Section 10.2.
10.2 Indemnification by INFI. INFI shall defend, indemnify and hold harmless the Licensee Indemnitees from and against any and all Losses relating to or in connection with a Third Party claim to the extent arising out of (a) the research, development, manufacture or commercialization of the IPI-145 Compound or the IPI-145 Product by INFI, any INFI Affiliate, any sublicensee of INFI (other than Licensee, any of its Affiliates or any Sublicensee) or any of their respective employees, consultants, contractors, subcontractors or agents prior to the Effective
- 46 -
CONFIDENTIAL
Date, including any actual or alleged death, personal bodily injury or damage to real or tangible personal property, or other product liability claimed to result from the IPI-145 Product Researched, Developed, Manufactured or Commercialized by or on behalf of INFI, any INFI Affiliate, any sublicensee of INFI (other than Licensee, any of its Affiliates or any Sublicensee), (b) any breach by INFI of its representations, warranties, covenants or obligations under this Agreement, or (c) any negligent act or omission or willful misconduct of INFI or any of its Affiliates, or any of their respective employees, consultants, contractors, subcontractors or agents, in performing INFI’s obligations or exercising INFI’s rights under this Agreement; except that that the foregoing indemnity shall not apply with respect to any Licensee Indemnitee to the extent that any such Losses (x) are caused by the negligence or willful misconduct of any of the Licensee Indemnitees, or (y) are otherwise subject to an obligation by Licensee to indemnify any of the INFI Indemnitees under Section 10.1.
10.3 Procedure. In the event of a claim by a Third Party against any Person entitled to indemnification under this Agreement, the Party claiming indemnification on behalf of such Person (in such capacity, the “Indemnified Party”) shall promptly notify the other Party (in such capacity, the “Indemnifying Party”) in writing of the claim (it being understood that the failure by the Indemnified Party to give prompt notice of a Third Party claim as provided in this Section 10.3 shall not relieve the Indemnifying Party of its indemnification obligation under this Agreement except and only to the extent that such Indemnifying Party is actually prejudiced as a result of such failure to give prompt notice). Within [**] days after delivery of such notification, the Indemnifying Party may, upon written notice thereof to the Indemnified Party, undertake and solely manage and control, at its sole expense and with counsel reasonably satisfactory to the Indemnified Party, the defense of the claim. If the Indemnifying Party does not undertake such defense, the Indemnified Party may control such defense but shall not be entitled to indemnification hereunder if it does not then control such defense. The Party not controlling such defense shall cooperate with the other Party and may, at its option and expense, participate in such defense; provided, that if the Indemnifying Party assumes control of such defense and the Indemnified Party in good faith concludes, based on advice from counsel, that the Indemnifying Party and the Indemnified Party (or the relevant INFI Indemnitee or Licensee Indemnitee seeking indemnification) have conflicting interests with respect to such action, suit, proceeding or claim, the Indemnified Party’s counsel may fully participate in such defense and the Indemnifying Party shall be responsible for the reasonable fees and expenses of counsel to the indemnified Persons solely in connection therewith. The Party controlling such defense shall keep the other Party advised of the status of such action, suit, proceeding or claim and the defense thereof and shall consider recommendations made by the other Party with respect thereto. Except if the Indemnifying Party did not undertake defense of the claim or if the Indemnifying Party and the Indemnified Party (or the relevant INFI Indemnitee or Licensee Indemnitee seeking indemnification) have conflicting interests with respect to such action, suit, proceeding or claim and the Indemnified Party engages separate counsel, as provided above, the Indemnifying Party shall not be liable for any litigation costs or expenses incurred by the Indemnified Party (or the relevant INFI Indemnitee or Licensee Indemnitee seeking indemnification) without the Indemnifying Party’s written consent. The Indemnified Party and any Person seeking indemnification under this Agreement shall not settle any such action, suit, proceeding or claim without the prior written consent of the Indemnifying Party, which shall not be unreasonably withheld, delayed or conditioned. The Indemnifying Party shall not settle, without the prior written
- 47 -
CONFIDENTIAL
consent of the Indemnified Party, any such action, suit, proceeding or claim, or consent to any judgment in respect thereof, that (a) does not include a complete and unconditional release of the Indemnified Party (and the relevant INFI Indemnitees or Licensee Indemnitees seeking indemnification) from all liability with respect thereto, (b) imposes any liability or obligation on the Indemnified Party (or any relevant INFI Indemnitee or Licensee Indemnitee seeking indemnification), (c) permits any injunction, declaratory judgment, other order or other non-monetary relief to be entered, directly or indirectly against the Indemnified Party (or any relevant INFI Indemnitee or Licensee Indemnitee seeking indemnification), or (d) acknowledges fault by the Indemnified Party (or any relevant INFI Indemnitee or Licensee Indemnitee seeking indemnification).
10.4 Allocation. In the event a claim is based partially on an indemnified claim and partially on a non-indemnified claim or based partially on a claim indemnified by one Party and partially on a claim indemnified by the other Party, any payments in connection with such claims are to be apportioned between the Parties in accordance with the degree of cause attributable to each Party.
10.5 EXCLUSION OF CONSEQUENTIAL DAMAGES. EXCEPT WITH RESPECT TO [**], NEITHER INFI NOR LICENSEE, NOR ANY OF THEIR RESPECTIVE AFFILIATES, WILL BE LIABLE FOR ANY INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, EXEMPLARY, MULTIPLE OR PUNITIVE DAMAGES, COSTS OR EXPENSES (INCLUDING LOST PROFITS, LOST REVENUES OR LOST SAVINGS), ARISING OUT OF THIS AGREEMENT OR RELATING TO ANY BREACH OF THIS AGREEMENT, WHETHER LIABILITY IS ASSERTED IN CONTRACT, TORT (INCLUDING NEGLIGENCE AND STRICT PRODUCT LIABILITY), INDEMNITY OR CONTRIBUTION, AND IRRESPECTIVE OF WHETHER SUCH PARTY OR ANY REPRESENTATIVE OF SUCH PARTY HAS BEEN ADVISED OF, OR OTHERWISE MIGHT HAVE ANTICIPATED THE POSSIBILITY OF, ANY SUCH LOSS OR DAMAGE.
10.6 Insurance.
10.6.1 Licensee’s Insurance Requirement. During the Term and thereafter for a period of at least [**] years after the later of the expiration or termination of this Agreement or the last commercial sale of the IPI-145 Product under this Agreement (the “Insurance Period”), Licensee shall maintain on an ongoing basis with a reputable, solvent insurer, comprehensive general liability insurance in the minimum amount of $[**] per occurrence and $[**] annual aggregate combined single limit for bodily injury and property damage liability; clinical trial coverage with limits and policy terms required by applicable Law in the territories where applicable clinical trials are taking place (and in any event not less than $[**]), which coverage shall include clinical trials using inventory or other materials manufactured by INFI or at INFI’s direction or otherwise provided to Licensee by INFI; and products liability insurance (including contractual liability coverage on Licensee’s indemnification obligations under this Agreement) in the amount of at least $[**] per occurrence and as an annual aggregate combined single limit for bodily injury and property damage liability; provided, however, that, (a) Licensee will not be required to procure or maintain the clinical trial coverage described above until ten days prior to transfer of the INDs for IPI-145 Product from
- 48 -
CONFIDENTIAL
INFI to Licensee pursuant to Section 4.1.1 and (b) commencing not later than [**] days prior to the reasonably anticipated first Commercial Sale of the IPI-145 Product by Licensee or any of its Affiliates or any Sublicensee, and thereafter during the Insurance Period, Licensee shall obtain and maintain on an ongoing basis products liability insurance (including contractual liability coverage on Licensee’s indemnification obligations under this Agreement) in the amount of at least $[**] per occurrence and as an annual aggregate combined single limit for bodily injury and property damage liability. All of such insurance coverage may be maintained through a self insurance plan that substantially complies with the foregoing limits and requirements and may be satisfied through one or more policies, including an umbrella policy. INFI and INK shall each be named as an additional insured on such policy and Licensee shall provide INFI with written evidence of such insurance on the Effective Date and at any other times upon request. Licensee shall provide INFI with written notice at least [**] days prior to the cancellation or non-renewal of such insurance; provided, that the provision of such notice shall not permit Licensee to cancel or not renew such insurance contrary to the provisions of this Section 10.6.1.
10.6.2 INFI’s Insurance Requirement. For a period of at least [**] years after the Effective Date, INFI shall maintain on an ongoing basis with a reputable, solvent insurer, comprehensive general liability insurance in the minimum amount of $[**] per occurrence and $[**] annual aggregate combined single limit for bodily injury and property damage liability; and products liability insurance (including contractual liability coverage on INFI’s indemnification obligations under this Agreement) in the amount of at least $[**] per occurrence and as an annual aggregate combined single limit for bodily injury and property damage liability. All of such insurance coverage may be maintained through a self insurance plan that substantially complies with the foregoing limits and requirements and may be satisfied through one or more policies, including an umbrella policy. Licensee shall be named as an additional insured on such policy and INFI shall provide Licensee with written evidence of such insurance on the Effective Date and at any other times upon request. INFI shall provide Licensee with written notice at least [**] days prior to the cancellation or non-renewal of such insurance; provided, that such notice shall not permit INFI to cancel or not renew such insurance contrary to the provisions of this Section 10.6.2.
10.6.3 Additional INFI Insurance Requirement. From the Effective Date until the date that all of the INDs for the IPI-145 Compound and IPI-145 Product have transferred to Licensee, INFI shall maintain on an ongoing basis with a reputable, solvent insurer, comprehensive general liability insurance, product liability insurance and clinical trial insurance covering the DUO clinical trial consistent with the amount and coverage INFI had prior to the Effective Date. Licensee shall reimburse INFI the premiums of such insurance.
ARTICLE 11
TERM AND TERMINATION
TERM AND TERMINATION
11.1 Term; Expiration. This Agreement shall become effective as of the Effective Date, and, shall continue in full force and effect until the Parties have no further obligations to each other hereunder, unless and until earlier terminated as provided herein (the “Term”). The Parties acknowledge and agree that this Agreement cannot be terminated except as expressly set forth herein.
- 49 -
CONFIDENTIAL
11.2 Termination for Cause. Either Party (the “Non-Breaching Party”) may, without prejudice to any other remedies available to it at law or in equity, terminate this Agreement if the other Party (the “Breaching Party”) shall have materially breached or defaulted in the performance of its obligations hereunder, and such default shall have continued for sixty (60) days (or, in the case of a payment breach, thirty (30) days) following the Breaching Party’s receipt of notice of such breach from the Non-Breaching Party. Any such termination of this Agreement under this Section 11.2 shall become effective at the end of such sixty (60) day or thirty (30) day (as applicable) cure period, unless the Breaching Party has cured such breach or default prior to the expiration of such cure period. The right of either Party to terminate this Agreement as provided in this Section 11.2 shall not be affected in any way by such Party’s waiver or failure to take action with respect to any previous default. Notwithstanding the foregoing, (a) if such material breach (other than a payment breach), by its nature, is curable, but is not reasonably curable within the sixty (60) day cure period, then such cure period shall be extended if the Breaching Party provides a written plan for curing such breach to the Non-Breaching Party and uses Diligent Efforts to cure such breach in accordance with such written plan; provided, that no such extension shall exceed sixty (60) days without the consent of the Non-Breaching Party; and (2) if the Breaching Party disputes that it has materially breached this Agreement, the dispute shall be resolved pursuant to Section 12.1 and Section 12.2, as applicable. If, as a result of the application of such dispute resolution procedures, the Breaching Party is determined to be in material breach of this Agreement (an “Adverse Ruling”), then if the Breaching Party fails to cure such material breach within sixty (60) days after such ruling (whether or not such actions are specified by the Adverse Ruling) (or thirty (30) days after such ruling in the case of a payment breach), then the Non-Breaching Party may terminate this Agreement upon written notice to the Breaching Party as provided in this Section 11.2.
11.3 Termination for Patent Challenge. If Licensee or any of its Affiliates or any Sublicensee (a) commences or otherwise voluntarily determines to participate in any action or proceeding (including any patent opposition or re-examination proceeding), challenging or denying the validity or enforceability of any INFI Prosecution Patent Right, INFI Other Patent Right or INK Prosecution Patent Right or any claim thereof, or (b) actively assists any other Person in bringing or prosecuting any action or proceeding (including any patent opposition or re-examination proceeding) challenging or denying the validity or enforceability of any INFI Prosecution Patent Right, INFI Other Patent Right or INK Prosecution Patent Right or any claim thereof, then INFI shall have the right to terminate this Agreement upon thirty (30) days written notice to Licensee unless Licensee, its Affiliates and Sublicensees have withdrawn such action before the end of the above notice period.
11.4 Licensee’s Termination for Convenience. At any time during the Term following the earlier of (a) determination whether the DUO clinical trial has or has not met its pre-specified primary endpoint as defined in the DUO clinical trial protocol, as amended, attached as Exhibit H, and (b) a determination by Licensee to discontinue the DUO clinical trial under Section 3.1.4(c), Licensee shall have the right to terminate this Agreement in its entirety upon not less than one hundred eighty (180) days prior written notice thereof to INFI.
11.5 Termination for Insolvency. In the event that either Party (a) files for protection under bankruptcy or insolvency laws, (b) makes an assignment for the benefit of creditors, (c) appoints
- 50 -
CONFIDENTIAL
or suffers appointment of a receiver or trustee over substantially all of its property that is not discharged within ninety (90) days after such filing, (d) proposes a written agreement of composition or extension of its debts, (e) proposes or is a party to any dissolution or liquidation, (f) files a petition under any bankruptcy or insolvency act or has any such petition filed against that is not discharged within sixty (60) days of the filing thereof, or (g) admits in writing its inability generally to meet its obligations as they fall due in the general course, then the other Party may terminate this Agreement in its entirety effective immediately upon written notice to such Party.
11.6 Effect of Termination by INFI Pursuant to Section 11.2, 11.3 or 11.5 or Licensee pursuant to Section 11.4. Upon INFI’s termination of this Agreement pursuant to Section 11.2, 11.3 or 11.5 or Licensee’s termination of this Agreement pursuant to Section 11.4, all rights and licenses granted by INFI to Licensee hereunder shall terminate and Licensee shall not have any rights to use, or exercise any rights under, the Duvelisib IP. If, within thirty (30) days following the effective date of such termination, Licensee receives from INFI a written waiver of any and all claims for damages that INFI or any of its Affiliates may have against Licensee, its Affiliates or its Sublicensees arising from or relating to this Agreement (except, to the extent that such termination results from a Headlicense Termination Event, such waiver will not be required to waive any direct damages INFI suffers as a result of such Headlicense Termination Event, which may include (i) any payments INFI is required to make to INK resulting from termination of the INK Agreement, (ii) any reasonable costs associated with INFI’s obtaining a replacement for the INK Agreement, or (iii) the difference between the economic terms of such new agreement with INK and the economic terms of the INK Agreement (provided that INFI uses commercially reasonable efforts to mitigate any such difference), then at Licensee’s sole cost:
11.6.1 INFI, within thirty (30) days after the date of such notice or waiver, shall promptly prepare, with Licensee’s reasonable cooperation, and the Parties shall negotiate, a termination and wind-down plan that will include, at a minimum, a plan for accomplishing the activities described in this Section 11.6.
11.6.2 Licensee shall, at INFI’s request, promptly provide to INFI a fair and accurate detailed written description of the status of the Development, Manufacture and Commercialization of the IPI-145 Compound and the IPI-145 Product in the Territory as of the effective date of the termination;
11.6.3 To the extent requested by INFI, Licensee shall, at its own expense, promptly transfer and assign to INFI all of Licensee’s, each of its Affiliates’ and each Sublicensee’s rights in any INDs, Marketing Authorizations and Regulatory Documentation necessary or useful for the Research (including to perform medicinal chemistry), Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Product in the Territory; except that Licensee may retain a single copy of such items for its records, and such Regulatory Documentation shall become the Confidential Information of INFI (with INFI considered the Disclosing Party and Licensee considered the Receiving Party), and Licensee may not rely on the exceptions enumerated in Sections 8.1.1, 8.1.2 or 8.1.5 with respect to its obligations regarding the confidentiality and non-use of such Confidential Information under this Agreement;
- 51 -
CONFIDENTIAL
11.6.4 To the extent requested by INFI, Licensee shall, at its own expense, promptly transfer and assign to INFI all of Licensee’s, each of its Affiliates’ and each Sublicensee’s rights to other technical and other information or materials that are necessary or useful for the Research (including to perform medicinal chemistry), Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Product in the Territory and all promotional materials, customer data, competitive intelligence data, market research and other materials, information or data related to the marketing, promotion or sale of the IPI-145 Compound or IPI-145 Product in the Territory in its possession or control as of the effective date of such termination; except that Licensee may retain a single copy of such items for its records, and such technical and other information or materials shall become the Confidential Information of INFI (with INFI considered the Disclosing Party and Licensee considered the Receiving Party), and Licensee may not rely on the exceptions enumerated in Sections 8.1.1, 8.1.2 or 8.1.5 with respect to its obligations regarding the confidentiality and non-use of such Confidential Information under this Agreement;
11.6.5 Within thirty (30) days after the effective date of expiration or termination of this Agreement, the Receiving Party shall, and shall cause its Affiliates to, (a) destroy all tangible items solely comprising, bearing or containing any Confidential Information of the Disclosing Party or any of its Affiliates that are in the Receiving Party’s or its Affiliates’ possession or control, and provide written certification of such destruction, or (b) ship such tangible items of the Disclosing Party’s (or any of its Affiliates’) Confidential Information to the Disclosing Party, as the Disclosing Party may direct, at the Receiving Party’s expense; provided, that in any event, (x) each Party may retain one copy of the Confidential Information of the other Party or any of its Affiliates to the extent necessary to perform its obligations that survive expiration or termination of this Agreement; (y) the Receiving Party may retain one copy of such Confidential Information of the Disclosing Party or any of its Affiliates for its legal archives; and (z) INFI may retain Licensee’s (or any of its Affiliates’) Confidential Information to the extent necessary for INFI to exercise its rights that survive expiration or termination of this Agreement. Any Confidential Information that is subject to the exceptions enumerated in Sections 8.1.1, 8.1.2, 8.1.3, 8.1.4 or 8.1.5 shall not be subject to the obligations imposed on the Receiving Party pursuant to clause (a) or (b) of this Section 11.6.5;
11.6.6 At INFI’s request, Licensee shall, at its own expense, promptly transfer and assign to INFI all of Licensee’s, each of its Affiliates’ and each Sublicensee’s rights, title and interests in and to the IPI-145 Product-specific trademark(s) (for the avoidance of doubt, not including any Licensee housemarks) used for the IPI-145 Product in the Territory, including the Product Xxxx, and all goodwill therein;
11.6.7 Promptly upon request by INFI, but in no event commencing later than [**] days after the effective date of termination and in no event lasting longer than [**] days following the effective date of termination, Licensee shall provide such assistance as may be reasonably necessary or useful for INFI to commence or continue Developing, Manufacturing or Commercializing the IPI-145 Compound or IPI-145 Product in the Territory, to the extent Licensee, any of its Affiliates or any Sublicensee is then performing or having performed such activities, including transferring (by novation) or amending as appropriate and where permitted by applicable contractual restriction, upon request of INFI, any agreements or arrangements with Third Party vendors to Develop, Manufacture, distribute, sell or otherwise Commercialize the IPI-145
- 52 -
CONFIDENTIAL
Compound or IPI-145 Product in the Territory. To the extent that any such contract is not assignable to INFI, Licensee shall reasonably cooperate with INFI to arrange to continue to provide such services for a reasonable time after termination;
11.6.8 If there are any clinical studies being conducted by or under the authority of Licensee or any of its Affiliates or any Sublicensee at the time of notice of termination, Licensee shall, as INFI may request, (a) at Licensee’s expense, promptly transition to INFI or its designee some or all of such on-going clinical studies and the activities related to or supporting such clinical studies, (b) at INFI’s expense, continue to conduct such on-going clinical studies for a period requested by INFI up to a maximum of [**] months after the effective date of such termination, or (c) at Licensee’s expense, terminate such on-going clinical studies in a manner consistent with applicable Law; provided, however, that in the event that INFI, Licensee, an institutional review board or independent safety board determines that an on-going clinical study being run by Licensee or any of its Affiliates or any Sublicensee would pose an unacceptable safety risk for subjects or patients participating in such on-going clinical study, Licensee shall not be obligated to continue such clinical study and Licensee shall provide INFI with a full explanation of the safety issue concerns raised by such institutional review board or independent safety board and, if requested by INFI, reasonable documentation thereof; and
11.6.9 At INFI’s request, Licensee shall provide INFI written notice of the quantity of the IPI-145 Compound or IPI-145 Product that Licensee or any of its Affiliates has in inventory in the Territory and permit INFI, at INFI’s option, to take ownership and control of all or any part of such inventory.
Notwithstanding any provision of this Agreement to the contrary, Licensee shall have no obligations under Sections 11.6.1 through 11.6.9 unless and until INFI executes the waiver of damages described in Section 11.6 and delivers such executed waiver of damages to Licensee.
11.7 Effect of Termination by Licensee Pursuant to Section 11.2 or 11.5. Upon Licensee’s termination of this Agreement pursuant to Sections 11.2 or 11.5, all rights and licenses granted by INFI to Licensee hereunder shall terminate and Licensee shall not have any rights to use, or exercise any rights under, the Duvelisib IP and all rights and license granted by Licensee to INFI under Section 2.3 shall terminate (except as otherwise set forth in Section 6.1.2) and INFI shall not have any rights to use, or exercise any rights under, the Licensee IP. At INFI’s sole cost and request, the Parties shall perform the following actions and in such an event, INFI shall pay to Licensee a royalty of [**] on Net Sales (applied to INFI in the same manner as applied to Licensee):
11.7.1 To the extent requested by INFI, Licensee shall, at INFI’s own expense, promptly transfer and assign to INFI all of Licensee’s, each of its Affiliates’ and each Sublicensee’s rights in any INDs, Marketing Authorizations and Regulatory Documentation necessary or useful for the Research (including to perform medicinal chemistry), Development, Manufacture or Commercialization of the IPI-145 Compound or IPI-145 Product in the Territory; except that Licensee may retain a single copy of such items for its records, and such Regulatory Documentation shall become the Confidential Information of INFI (with INFI considered the Disclosing Party and Licensee considered the Receiving Party), and Licensee may not rely on the exceptions enumerated
- 53 -
CONFIDENTIAL
in Sections 8.1.1, 8.1.2 or 8.1.5 with respect to its obligations regarding the confidentiality and non-use of such Confidential Information under this Agreement;
11.7.2 At INFI’s request, Licensee shall, at INFI’s expense, promptly transfer and assign to INFI all of Licensee’s, each of its Affiliates’ and each Sublicensee’s rights, title and interests in and to the IPI-145 Product-specific trademark(s) (for the avoidance of doubt, not including any Licensee housemarks) used for the IPI-145 Product in the Territory, including the Product Xxxx, and all goodwill therein;
11.7.3 If there are any clinical studies being conducted by or under the authority of Licensee or any of its Affiliates or any Sublicensee at the time of notice of termination, Licensee shall, as INFI may request, (a) at INFI’s expense (including the reimbursement of Licensee’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses in connection therewith), promptly transition to INFI or its designee some or all of such on-going clinical studies and the activities related to or supporting such clinical studies, (b) at INFI’s expense (including the reimbursement of Licensee’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses in connection therewith), and to the extent possible given the resources Licensee has available to it at the relevant time, continue to conduct such on-going clinical studies for a period requested by INFI up to a maximum of [**] months after the effective date of such termination, or (c) at INFI’s expense (including the reimbursement of Licensee’s reasonable and documented Internal Personnel Expenses and Out-of-Pocket Expenses in connection therewith), terminate such on-going clinical studies in a manner consistent with applicable Law
11.8 Accrued Rights; Surviving Provisions of the Agreement.
11.8.1 Termination or expiration of this Agreement for any reason shall be without prejudice to any rights that shall have accrued to the benefit of any Party or any Third Party Grantor prior to such termination or expiration, including the payment obligations under this Agreement or any INFI Third Party Agreement (including Licensee’s payment obligations for sales of the IPI-145 Product made during the Term and including Licensee’s payment obligations with respect to any milestone payment or Reimbursement Event achieved during the Term), and any and all damages or remedies arising from any breach hereunder. Such termination or expiration shall not relieve any Party from obligations which are expressly indicated to survive termination of this Agreement.
11.8.2 The provisions of Sections 2.1 (to the extent such license survives pursuant to Section 6.1.2), 2.2 (to the extent the license in Section 2.1 survives pursuant to Section 6.1.2), 2.3 (except if such license is terminated as a result of INFI’s breach), 2.4.6 (as applicable), 2.4.7, 2.4.8 (as applicable), 2.6.1, 2.7 (to the extent the relevant license survives in accordance with this Agreement), 3.1.2(c) (to the extent any portion of the Reimbursement Payments are made in Licensee Common Stock and such restrictions still apply at the time of termination of this Agreement), 3.1.4(b), 6.1.2 (to the extent the grant of such licenses is triggered prior to the effective date of termination), 6.1.4, 6.2 (to the extent related to a Calendar Quarter prior to the termination of this Agreement), 6.3, 6.4, 6.5 (for [**]), 6.6 (to the extent there are remaining obligations at the time of termination of this Agreement), 6.7 (to the extent there are remaining obligations at the time of termination of this Agreement), 7.1, 7.2, 7.9.3, 8 (for the survival term specified in Section 8.7), 9.5, 10.1 through 10.4 (solely with respect to indemnifiable events that occur prior to the effective date of termination),
- 54 -
CONFIDENTIAL
10.5, 10.6 (for the survival periods specified therein), 11.6, 11.7, 11.8, 11.9, 12.1, 12.2, 12.3, 12.4, 12.5, 12.6, 12.7, 12.9, 12.11, 12.12, 12.13, 12.14, 12.15, 12.16, 12.17, 12.18 and 12.19, any applicable definitions in ARTICLE 1 and any other definitions or provisions necessary to interpret such surviving provisions, shall survive the termination of this Agreement in its entirety or expiration of this Agreement for any reason, in accordance with their respective terms and conditions, and for the duration stated, and where no duration is stated, shall survive indefinitely.
11.9 Damages; Relief. Except to the extent INFI executes and delivers a waiver of damages described in Section 11.6, termination of this Agreement shall not preclude either Party from claiming any other damages, compensation or relief that it may be entitled to upon such termination.
ARTICLE 12
MISCELLANEOUS
MISCELLANEOUS
12.1 Disputes. In the event any dispute arises out of or in relation to or in connection with this Agreement, including failure to perform under or breach of this Agreement, or any issue relating to the interpretation or application of this Agreement or any INFI Third Party Agreement, the Parties shall use good faith efforts to resolve such dispute within [**] days after a Party notifies the other Party of such dispute. If the Parties are unable to resolve such dispute within such [**] day period, either Party may, by written notice to the other Party, refer such dispute to the Senior Executives for resolution, and the Senior Executives shall attempt in good faith to resolve such dispute within [**] days after such notice.
12.2 Arbitration. If the Senior Executives are unable to resolve a given dispute referred to it pursuant to Section 12.1 within [**] days following such referral of such dispute, either Party may have such dispute settled by binding arbitration in the manner described below:
12.2.1 Arbitration Request. If a Party intends to begin an arbitration to resolve a dispute arising under this Agreement, such Party shall provide written notice (the “Arbitration Request”) to the other Party of such intention and the issues for resolution.
12.2.2 Additional Issues. Within [**] days after the receipt of the Arbitration Request, the other Party may, by written notice, add additional issues for resolution.
12.2.3 Arbitration Rules; Location. Except as expressly provided herein, the sole mechanism for resolution of any claim, dispute or controversy arising out of or in connection with or relating to this Agreement or the breach or alleged breach thereof shall be arbitration by the American Arbitration Association (“AAA”), in accordance with the Commercial Arbitration Rules and Supplementary Procedures for Large Complex Disputes of the AAA as then in effect. The arbitration shall take place in Boston, Massachusetts.
12.2.4 English Language. All proceedings shall be held in English and a transcribed record prepared in English. Documents submitted in the arbitration (the originals of which are not in English) shall be submitted together with a reasonably complete and accurate English translation.
- 55 -
CONFIDENTIAL
12.2.5 Selection of Arbitrators. Each Party shall choose one arbitrator within [**] days after receipt of notice of the intent to arbitrate and the said two arbitrators shall select by mutual agreement a third arbitrator within [**] days after they have been selected as arbitrators. If one or more arbitrators are not appointed within the times herein provided or any extension of time that is mutually agreed on, the AAA shall make such appointment within [**] days after such failure.
12.2.6 Experience. If the issues in dispute involve scientific or technical matters, any arbitrators chosen hereunder shall have educational training or experience sufficient to demonstrate a reasonable level of knowledge in the pharmaceutical and biotechnology fields.
12.2.7 Time Schedule. Within [**] days after initiation of arbitration, the Parties shall reach agreement upon and thereafter follow procedures directed at ensuring that the arbitration will be concluded and the final award rendered within no more than [**] months from selection of the three arbitrators or as soon thereafter as practicable. Failing such agreement, the AAA will design and the Parties will follow procedures directed at meeting such a time schedule.
12.2.8 Powers of Arbitrators. The arbitrators shall be limited in the scope of their authority to resolving only the specific matter which the Parties have referred to arbitration for resolution and shall not have authority to render any decision or award on any other issues. Without limiting the foregoing, the arbitrators:
(a) shall not have any power or authority to add to, alter, amend or modify the terms of this Agreement but shall specify rules sufficient to allow reasonable discovery by the Parties;
(b) shall establish and enforce appropriate rules to ensure that the proceedings, including the decision, be kept confidential and that all Confidential Information of any Party disclosed during such proceedings be kept confidential in accordance with this Agreement and be used for no purpose other than the arbitration unless otherwise permitted in accordance with ARTICLE 8; and
(c) shall issue all preliminary awards and the final award in writing.
12.2.9 Injunctive Relief. Nothing in this Agreement shall be deemed as preventing either Party from seeking injunctive relief (or any other provisional remedy such as temporary restraining order, preliminary injunction or other interim equitable relief) from the arbitrators or from any court having jurisdiction over the Parties (and prior to or during any arbitration if necessary to protect the interests of such Party in avoiding irreparable harm or to preserve the status quo pending the arbitration proceeding) and the subject matter of the dispute, as necessary to protect such Party’s name, Confidential Information, Know-How or any other proprietary right or otherwise to avoid irreparable harm. Without limiting the generality of the foregoing, either Party may seek such injunctive relief (or any other such provisional remedy) if it reasonably believes that the other Party has breached this Agreement.
12.2.10 Costs; Exclusion from Award. The award rendered by the arbitrators shall not include costs of arbitration, attorneys’ fees or costs for expert and other witnesses, which
- 56 -
CONFIDENTIAL
shall be the responsibility of each Party (i.e., each Party shall bear its own costs and expenses), except that the Parties shall share equally the fees of the arbitrators.
12.2.11 Judgment. Judgment on the award rendered by the arbitrators may be entered in any court having jurisdiction thereof.
12.2.12 Survivability. Any duty to arbitrate under this Agreement shall remain in effect and be enforceable after termination of this Agreement.
12.3 Timing. Resolution of any disputes shall be subject to the relevant Third Party Grantor’s rights under the applicable INFI Third Party Agreement and any time frames set forth in Sections 12.1 or 12.2 shall, to the extent necessary to comply with such rights, be modified to accommodate the time-frames for dispute resolution under the relevant INFI Third Party Agreement.
12.4 Governing Law. This Agreement and any dispute arising from the performance or breach hereof shall be governed by and construed and enforced in accordance with the Laws of the State of New York without giving effect to conflicts of the laws provisions thereof. The provisions of the United Nations Convention on Contracts for the International Sale of Goods shall not apply to this Agreement or any subject matter hereof.
12.5 Assignment. Neither this Agreement nor any right or obligation hereunder may be assigned or otherwise transferred by any Party without the consent of the other Party; except that any Party may, without such consent, assign this Agreement, in whole or in part: (a) to any of its respective Affiliates, provided, that the assigning Party shall remain jointly and severally liable with such Affiliate in respect of all obligations so assigned; or (b) to any successor in interest by way of merger, acquisition or sale of all or substantially all of its assets to which this Agreement relates, provided, that such successor agrees in writing to be bound by the terms of this Agreement as if it were the assigning party. Any assignment or transfer of this Agreement not in accordance with this Section 12.5 shall be void and unenforceable.
12.6 No Reach Through to Acquirer IP.
12.6.1 Notwithstanding anything in this Agreement to the contrary, following the closing of a Change of Control of INFI, Licensee shall not obtain rights or access to the Patent Rights or Know-How controlled by the INFI Acquirer (as defined below) or any of the Affiliates of INFI (other than INFI and its Affiliates which exist immediately prior to the closing of such Change of Control (such Affiliates, the “INFI Pre-Existing Affiliates”)). For clarity but without limitation, Licensee’s rights in all Patent Rights and Know-How Controlled by INFI or any of its INFI Pre-Existing Affiliates, which Patent Rights and Know-How exist as of the date of the closing of such Change of Control and are then licensed hereunder to Licensee, and all Counterparts of such Patent Rights, shall remain licensed to Licensee after the date of the closing of such Change of Control in accordance with and subject to the terms and conditions of this Agreement and shall not be affected in any manner by virtue of such Change of Control. “INFI Acquirer” means the Third Party that acquires INFI or its direct or indirect controlling Affiliate, or that acquires all or substantially all of the assets of INFI or its direct or indirect controlling Affiliate.
- 57 -
CONFIDENTIAL
12.6.2 Notwithstanding anything in this Agreement to the contrary, following the closing of a Change of Control of Licensee, INFI shall not obtain rights or access to the Patent Rights or Know-How controlled by the Licensee Acquirer (as defined below) or any of the Affiliates of Licensee (other than Licensee and its Affiliates which exist immediately prior to the closing of such Change of Control (such Affiliates, the “Licensee Pre-Existing Affiliates”)). For clarity but without limitation, INFI’s rights in all Patent Rights and Know-How Controlled by Licensee or any of its Licensee Pre-Existing Affiliates, which Patent Rights and Know-How exist as of the date of the closing of such Change of Control and are then licensed hereunder to INFI, and all Counterparts of such Patent Rights, shall remain licensed to INFI after the date of the closing of such Change of Control in accordance with and subject to the terms and conditions of this Agreement and shall not be affected in any manner by virtue of such Change of Control. “Licensee Acquirer” means the Third Party that acquires Licensee or its direct or indirect controlling Affiliate, or that acquires all or substantially all of the assets of Licensee or its direct or indirect controlling Affiliate.
12.7 Licensee Acquisition of Third Party Grantor. In the event that (a) Licensee or any of its Affiliates acquires any Third Party Grantor or any of its Affiliates, by merger, purchase of assets or otherwise, and (b) a breach by Licensee, any of its Affiliates or any Sublicensee of this Agreement results in a breach by INFI of the applicable INFI Third Party Agreement, then: (x) such breach shall not be cited by Licensee or its Affiliates against INFI as a breach of such INFI Third Party Agreement and INFI shall have a reasonable period of time to cure such breach that is no less than the longer of (i) the time that Licensee had to perform such activity or to cure such breach or (ii) one hundred eighty (180) days; (y) if such breach relates to Licensee’s failure to make any payment due hereunder which amount is owed to such Third Party Grantor under such INFI Third Party Agreement, INFI shall have no obligation to make the corresponding payment to such Third Party Grantor; and (z) if such breach is incapable of cure using commercially reasonable efforts, it shall not be deemed a breach of either this Agreement or such INFI Third Party Agreement, and neither Licensee nor its Affiliates shall be entitled to take any further action against INFI with respect to such breach.
12.8 Force Majeure. No Party shall be held liable or responsible to the other Party nor be deemed to be in default under, or in breach of any provision of, this Agreement for failure or delay in fulfilling or performing any obligation (other than a payment obligation) of this Agreement when such failure or delay is due to force majeure, and without the fault or negligence of the Party so failing or delaying. For purposes of this Agreement, force majeure is defined as causes beyond the reasonable control of the failing or delaying Party, which may include strike, fire, flood, earthquake, accident, war, act of terrorism, act of God or of the government of any country or of any local government or by other cause unavoidable or beyond the reasonable control of such Party. In such event the affected Party shall immediately notify the other Party of such inability and of the period for which such inability is expected to continue. The Party giving such notice shall thereupon be excused from such of its obligations under this Agreement as it is thereby disabled from performing for so long as it is so disabled for up to a maximum of ninety (90) days, after which time INFI and Licensee shall promptly meet to discuss in good faith how to best proceed in a manner that maintains and abides by the Agreement. The failing or delaying Party shall use commercially reasonable efforts to minimize the duration of any force majeure and to resume performance of its obligations. Notwithstanding the foregoing, Licensee may not rely on this Section 12.8, or any
- 58 -
CONFIDENTIAL
comparable provision at law or in equity, (a) to excuse, or extend any cure period without respect to, any breach or failure to perform by Licensee that may cause INFI to be in breach of any INFI Third Party Agreement, except to the extent permitted by the applicable INFI Third Party Agreement or (b) to extend any period for performance of any obligation of Licensee (whether to be performed directly or through any of its Affiliates or any Sublicensee) that, if breached, may cause INFI to be in breach of any INFI Third Party Agreement, except to the extent permitted by the applicable INFI Third Party Agreement.
12.9 Notices. All notices, consents, waivers, and other communications under this Agreement must be in writing and will be deemed to have been duly given when (a) delivered by hand (with written confirmation of receipt) or (b) when received by the addressee, if sent by an internationally recognized overnight delivery service (receipt requested), in each case to the appropriate addresses set forth below (or to such other addresses as a Party may designate by notice):
If to INFI, addressed to:
Infinity Pharmaceuticals, Inc.
000 Xxxxxxxx Xxxxx
Xxxxxxxxx, Xxxxxxxxxxxxx 00000
Attention: General Counsel
000 Xxxxxxxx Xxxxx
Xxxxxxxxx, Xxxxxxxxxxxxx 00000
Attention: General Counsel
with a copies to:
Infinity Pharmaceuticals, Inc.
000 Xxxxxxxx Xxxxx
Xxxxxxxxx, Xxxxxxxxxxxxx 00000
Attention: Chief Executive Officer
000 Xxxxxxxx Xxxxx
Xxxxxxxxx, Xxxxxxxxxxxxx 00000
Attention: Chief Executive Officer
and
Xxxxxx Xxxxxx Xxxxxxxxx Xxxx and Xxxx LLP
00 Xxxxx Xxxxxx
Xxxxxx, XX 00000
Attention: Xxxxxxx X. Xxxxx, Esq.
00 Xxxxx Xxxxxx
Xxxxxx, XX 00000
Attention: Xxxxxxx X. Xxxxx, Esq.
If to Licensee, addressed to:
Verastem, Inc.
000 Xxxxxxxx Xxxxxx, Xxxxx 000
Xxxxxxx, Xxxxxxxxxxxxx 00000
Attention: Chief Operating Officer
Attention: Chief Operating Officer
with a copy to:
Verastem, Inc.
000 Xxxxxxxx Xxxxxx, Xxxxx 000
- 00 -
XXXXXXXXXXXX
Xxxxxxx, Xxxxxxxxxxxxx 00000
Attention: Senior Corporate Counsel
Attention: Senior Corporate Counsel
and
Ropes & Xxxx LLP
Prudential Tower, 000 Xxxxxxxx Xxxxxx
Xxxxxx, XX 00000-0000
Attention: Xxxxx Xxxxxxx
12.10 Export Clause. Each Party acknowledges that the Laws of the United States restrict the export and re-export of commodities and technical data of United States origin. Each Party agrees that it will not export or re-export restricted commodities or the technical data of the other Party in any form without the appropriate United States and non-U.S. United States government licenses.
12.11 Waiver. Neither Party may waive or release any of its rights or interests in this Agreement except in writing. The failure of either Party to assert a right hereunder or to insist upon compliance with any term of this Agreement shall not constitute a waiver of that right or excuse a similar subsequent failure to perform any such term or condition. No waiver by either Party of any condition or term in any one or more instances shall be construed as a continuing waiver of such condition or term or of another condition or term.
12.12 Severability. If any provision hereof should be held invalid, illegal or unenforceable in any jurisdiction, (a) such provision shall be deemed stricken from this Agreement, (b) the Parties shall negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and (c) all other provisions hereof shall remain in full force and effect in such jurisdiction and shall be liberally construed in order to carry out the intentions of the Parties as nearly as may be possible. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction.
12.13 Entire Agreement. This Agreement, together with the Exhibits hereto, sets forth all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties as to the subject matter of this Agreement and supersedes and terminates all prior agreements and understanding between the Parties with respect to the subject matter hereof. In particular, and without limitation, this Agreement supersedes and replaces the Superseded Agreement which is hereby terminated in its entirety effective as of the Effective Date, the Existing Confidentiality Agreement and any and all term sheets relating to the transactions contemplated by this Agreement and exchanged between the Parties or any of their Affiliates prior to the Effective Date. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties as to the subject matter of this Agreement other than as set forth herein and therein. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by the respective authorized officers of the Parties.
- 60 -
CONFIDENTIAL
12.14 Independent Contractors. Nothing herein shall be construed to create any relationship of employer and employee, agent and principal, partnership or joint venture between the Parties. Each Party is an independent contractor. Neither Party shall assume, either directly or indirectly, any liability of or for the other Party. Neither Party shall have the authority to bind or obligate the other Party and neither Party shall represent that it has such authority.
12.15 Headings; Construction; Interpretation.
12.15.1 Headings used herein are for convenience only and shall not in any way affect the construction of or be taken into consideration in interpreting this Agreement.
12.15.2 The terms of this Agreement represent the results of negotiations between the Parties and their representatives, each of which has been represented by counsel of its own choosing, and neither of which has acted under duress or compulsion, whether legal, economic or otherwise. Accordingly, the terms of this Agreement shall be interpreted and construed in accordance with their usual and customary meanings, and each of the Parties hereby waives the application in connection with the interpretation and construction of this Agreement of any rule of Law to the effect that ambiguous or conflicting terms or provisions contained in this Agreement shall be interpreted or construed against the Party whose attorney prepared the executed draft or any earlier draft of this Agreement.
12.15.3 Any reference in this Agreement to an Article, Section, subsection, paragraph, clause, Schedule or Exhibit shall be deemed to be a reference to any Article, Section, subsection, paragraph, clause, Schedule or Exhibit, of or to, as the case may be, this Agreement.
12.15.4 Except where the context otherwise requires, (a) any definition of or reference to any agreement, instrument or other document refers to such agreement, instrument other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein or therein), (b) any reference to any Law refers to such Law as from time to time enacted, repealed or amended, (c) the words “herein,” “hereof” and “hereunder,” and words of similar import, refer to this Agreement in its entirety and not to any particular provision hereof, (d) the words “include,” “includes,” and “including” shall be deemed to be followed by the phrase “but not limited to,” “without limitation” or words of similar import, (e) the word “or” is used in the inclusive sense (and/or), (f) words denoting the singular shall include the plural and vice versa and words denoting any gender shall include all genders, (g) a capitalized term not defined herein but reflecting a different part of speech than a capitalized term which is defined herein shall be interpreted in a correlative manner, (h) the word “will” will be construed to have the same meaning and effect as the word “shall”, (i) any reference herein to any Person will be construed to include such Person’s successors and/or permitted assignees, (j) the word “notice” means notice in writing (whether or not specifically stated) and no inference or conclusions of any sort shall be drawn from the fact that in some instances in this Agreement, the word “notice” is actually preceded or followed by “in writing” or the equivalent while in other instances they are not, and (k) provisions that require a Party or the Parties to “agree”, “consent”, “approve” or the like, or to inform the other Party, will require that such agreement, consent, approval or the like, or such notice informing the other Party, be specific and in a writing signed by an authorized officer of such Party(ies), and no inferences or conclusions of any sort shall
- 61 -
CONFIDENTIAL
be drawn from the fact that in some instances in this Agreement, the words “agree”, “consent”, “approve” or the like, or the requirement to inform the other Party, are actually preceded or followed by “in writing” or the equivalent while in other instances they are not.
12.16 Further Actions. Each Party shall execute, acknowledge and deliver such further instruments as may be necessary or appropriate in order to carry out the expressly stated purposes and the clear intent of this Agreement.
12.17 Parties in Interest. All of the terms and provisions of this Agreement shall be binding upon, and shall inure to the benefit of and be enforceable by the Parties and their respective and permitted assigns.
12.18 Performance by Affiliates. To the extent that this Agreement imposes obligations on Affiliates of a Party, such Party agrees to cause its Affiliates to perform such obligations and a breach by such Affiliate shall be considered a breach by such Party.
12.19 Counterparts. This Agreement may be signed in counterparts, each and every one of which shall be deemed an original, notwithstanding variations in format or file designation which may result from the electronic transmission, storage and printing of copies from separate computers or printers. Facsimile signatures and signatures transmitted via PDF shall be treated as original signatures.
- 62 -
CONFIDENTIAL
IN WITNESS WHEREOF, and intending to be legally bound hereby, the Parties have caused this Agreement to be executed by their duly authorized representatives to be effective as of the Effective Date.
By: _/s/ Xxxxxxx X. Perkins_____________ Name:___ Xxxxxxx X. Perkins___________ Title: __CEO and Chair________________ | VERASTEM, INC. By: __/s/ Xxxxxx Forrester_______________ Name: __ Xxxxxx Xxxxxxxxx ______________ Title: __CEO_________________________ |
[Signature Page to Amended and Restated License Agreement]
CONFIDENTIAL
Exhibit A
INFI PROSECUTION PATENT RIGHTS
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. A total of 13 pages were omitted. [**]
CONFIDENTIAL
Exhibit B
INK PROSECUTION PATENT RIGHTS
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. A total of 17 pages were omitted. [**]
CONFIDENTIAL
Exhibit C
IPI-145 OR DUVELISIB
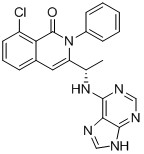
Exhibit C-1
CONFIDENTIAL
Exhibit D
IPI-443
Exhibit D-1
CONFIDENTIAL
Exhibit E
PRODUCT XXXXX
Xxxx (Class) | Country | Status | Filing No. | Filing Date | Reg. No. | Reg. Date | |
[**] | [**] | [**] | [**] | [**] | |||
[**] | [**] | [**] | [**] | [**] | |||
[**] | [**] | [**] | [**] | [**] | |||
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |
[**] | [**] | [**] | [**] | [**] | |||
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |
Exhibit E-1
CONFIDENTIAL
EXHIBIT F
TRANSITION PLAN
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 9 pages were omitted. [**]
CONFIDENTIAL
Exhibit F-1
INFI PRODUCT RELATED CONTRACTS
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 7 pages were omitted. [**]
CONFIDENTIAL
Exhibit F-2
SPECIFICATION FOR DUVELISIB DRUG SUBSTANCE AND RSMS
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 8 pages were omitted. [**]
CONFIDENTIAL
EXHIBIT F-3
Inventory
Confidential Materials omitted and filed separately with the Securities and Exchange Commission.
A total of 3 pages were omitted. [**]
CONFIDENTIAL
Exhibit G
DEVELOPMENT PLAN
[**]
CONFIDENTIAL
Exhibit H
DUO CLINICAL TRIAL PROTOCOL
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. A total of 111 pages were omitted. [**]
CONFIDENTIAL
Exhibit I
TARGET INHIBITOR CRITERIA
[**]
CONFIDENTIAL
Exhibit I-1
[**] DESCRIPTION
[**]

