COMMERCIAL SUPPLY AGREEMENT
Exhibit 10.9
This Commercial Supply Agreement (this “Agreement”) is entered into as of October 16, 2008, (the “Effective Date”) by and between Enzon Pharmaceuticals, Inc., a Delaware corporation with an address of ▇▇▇ ▇▇▇▇▇ ▇▇▇-▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ (“Enzon”), and Savient Pharmaceuticals, Inc., a Delaware corporation, having its principal place of business at ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ (“Savient”). Enzon and Savient may be referred to individually as a “Party” or collectively as “Parties.”
R E C I T A L S
WHEREAS, Savient is engaged in the development and research of certain biologic products and requires manufacture of such a product for commercial distribution;
WHEREAS, Enzon is a contract manufacturer that possesses the necessary technical capabilities and operates facilities for the manufacture of pharmaceutical and biological products for commercial distribution;
WHEREAS, Savient desires Enzon to supply to it the Product on the terms and conditions set forth herein; and
WHEREAS, Enzon is willing to supply the Product to Savient on the terms and conditions set forth below.
NOW, THEREFORE, in consideration of the mutual promises contained herein, and for other good and valuable consideration, the receipt and adequacy of which each of the Parties does hereby acknowledge, the Parties, intending to be legally bound, agree as follows:
Section 1. DEFINITIONS
1.1 As used herein, the following terms shall have the following meanings:
1.2 “Affiliate” shall mean any business entity which directly or indirectly controls, is controlled by, or is under common control with any Party to this Agreement. A business entity shall be deemed to “control” another business entity if (i) it owns, directly or indirectly, at least fifty percent (50%) of the issued and outstanding voting securities, capital stock, or other comparable equity or ownership interest of such business entity, or (ii) it has the de facto ability to control or direct the management of such business entity.
1.3 “Applicable Laws” means all relevant federal, state and local laws, statutes, rules, and regulations which are applicable to a Party’s activities hereunder, including, without limitation, the applicable regulations of the Regulatory Authority, European Medicines Agency (EMEA) and United States and European Union cGMPs. The Parties may amend this section in writing to include additional countries.
Execution Copy
Page 1 of 44
1.4 “BLA” means a regulatory application filed with a governmental agency in a the United States, the European Union, or any other country that the Parties mutually agree upon in writing (e.g. FDA and EMEA) for the purpose of lawfully marketing, selling, distributing, importing, exporting, manufacturing, developing or using a therapeutic or prophylactic product for the treatment or prevention of a disease or physical condition. As used herein, BLA shall include, without limitation, a Marketing Authorization Application in the European Union, a Biologics License Application in the United States.
1.5 “Bulk Product” shall mean the bulk solution of methoxy-polyethylene glycol (m-PEG) conjugate of uricase supplied by Savient to Enzon pursuant to this Agreement.
1.6 “Commercially Reasonable Efforts” shall mean efforts in accordance with the standards of care and diligence Enzon practices with respect to its own products.
1.7 “cGMPs” shall mean current good manufacturing practices as promulgated by the FDA under the United States Food, Drug and Cosmetic Act, 21 C.F.R. Part 210 et seq., as amended from time to time, and the European Union.
1.8 “Field Alerts” shall have the definition of field alerts used by the FDA irrespective of the jurisdiction in which the acts or circumstances giving rise to such field alerts occur.
1.9 “Process Consumables” means media, resins, raw materials, filters, membranes, product contact materials or surfaces, disposable lab supplies and similar materials used in the manufacture of Product. Provided, however, that Process Consumables shall not include components of manufacture supplied by third parties such as labels (hereinafter referred to as “Manufacture Components”).
1.10 “Product” means pegloticase, a PEGylated recombinant mammalian uricase formulation in final drug product form ready for commercial sale.
1.11 “Quality Agreement” shall mean that certain Quality Agreement by and between the Parties hereto, dated as of the date hereof and attached to this Agreement
1.12 “Regulatory Authority” shall mean any governmental agency with jurisdiction over the regulation of drug and biological agents for use in man, including, but not limited to, the United States Food and Drug Administration and any foreign equivalents thereto.
1.13 “Savient-supplied Materials” shall mean those materials including, but not limited to Bulk Product, supplied by Savient for use in connection with the manufacture of the Product, as set forth in the Work Plan which is attached hereto as Exhibit A or any subsequent Work Plan signed by both parties.
1.14 “Savient Intellectual Property” shall mean (i) all valid patent claims owned or licensed by Savient and all converted provisionals, divisions, continuations, continuations-in-part, reissues, reexaminations or extensions thereof, as well as any corresponding foreign
Execution Copy
Page 2 of 44
counterparts and equivalents thereof, whether issued or pending as of the Effective Date or thereafter; (ii) trademarks which are owned, licensed or sublicensed by Savient and which are registered in the United States and, where applicable, foreign jurisdictions for use in association with the Product; and (iii) any Savient Know-How developed by Savient or any of its Affiliates during the Term relating to (a) the Bulk Product or the Product (including, without limitation, its pharmaceutical utility) or (b) the Services provided hereunder
1.15 “Savient Know-How” shall mean all technical information, data (including, without limitation, regulatory data) patentable and unpatentable inventions, developments, discoveries, methods and processes that are, in each case, not disclosed in a published patent application or patent or otherwise publicly available, which are developed or conceived of by Savient or any of its Affiliates or which is licensed to Savient or any of its Affiliates.
1.16 “Service” means those services described in any Work Plan which is made a part of this Agreement and those services described in any Quality Agreement pertaining to such services.
1.17 “Specifications” means the written specifications for the Product and Savient-supplied Materials attached hereto as Exhibit B, which may be amended from time to time by the mutual written agreement of the parties.
1.18 “Work Plan” means the schedule and detailed plans used to prepare formulated Bulk Product, fill Bulk Product into vials and package the resulting drug product thereby resulting in the ultimate deliverable which is the Product. The definition of Work Plan shall also include subsequent change orders to any Work Plan (as described in Section 3.3) . The first Work Plan is attached hereto as Exhibit A.
Section 2. SERVICES
2.1 Enzon shall perform the Services described in this Agreement and in the exhibits hereto, which are made part of this Agreement, or as described in any Work Plan, the Specifications, or change order pursuant to Section 3.3. Savient shall provide such Savient-supplied Materials and make such payments as are set forth therein. The Parties mutually acknowledge and agree that nothing contained in this Agreement or any Work Plan executed hereunder shall create an exclusive manufacture or supply arrangement between the Parties.
2.2 To the extent necessary to enable Enzon to provide the Services, Savient hereby grants to Enzon a royalty-free, non-exclusive license and, where appropriate, sublicense, to use the Savient Intellectual Property which pertains to the Product or the Services hereunder; provided, however, that any license, or sublicense, granted herein as the case may be, shall be used by Enzon or any permitted sublicensee solely for the purposes of carrying out the Services and no rights or title in or to the Savient Intellectual Property shall vest in Enzon. Upon the expiration or earlier termination of this Agreement the license or sublicense granted to Enzon to
Execution Copy
Page 3 of 44
use Savient Intellectual Property shall immediately cease and Enzon shall make no further use thereof and shall cause any permitted sublicensee to make no further use thereof.
2.3 Enzon agrees to provide the Services outlined in the Work Plan attached hereto as Exhibit A which is incorporated and made a part of this Agreement and any other Services that may be described in any future Work Plan or change order, or Quality Agreement which addresses the Services described in this Section 2, which shall be incorporated into this Agreement upon execution by both parties. Such Services shall be performed in accordance with Applicable Laws. Savient agrees to make payments in accordance with this Agreement and all Work Plans. In the event of a conflict between this Agreement and any Work Plan, this Agreement shall control.
2.4 Enzon shall provide Savient, at no additional charge, product support services, at Savient’s reasonable request, for the activities listed below:
| • | Meetings with Regulatory Authorities, whether in person or by phone |
| • | Routine documentation provided to Regulatory Authorities on behalf of Savient |
| • | Annual product reviews for commercial products, as required by Regulatory Authorities. |
| • | All audit correspondence including Savient-requested revisions to Enzon’s audit response. |
Savient may request from Enzon other product support services at its customary rate, as set forth on Exhibit C, including but not limited to:
| • | Preparation of documents in anticipation of a Pre-Approval Inspection. |
| • | Letters of reference from Enzon or Enzon’s vendors that are requested by Savient (e.g., Master file reference letters, rubber or glass vendor letters). |
| • | Documentation provided to Regulatory Authorities on behalf of Savient, other than routine documentation. |
| • | All time used for collecting and photocopying Savient documentation. One copy of a batch record is exempted from support charges. |
| • | Changes and revisions to artwork mandated by Regulatory Authorities or requested by Savient. |
| • | Any additional validation work requested by Savient beyond original Work Plan or outside current validation requirements. |
| • | Any analytical development and/or analyses beyond original Work Plan. |
For all requests under this Section 2.4, Savient shall provide Enzon a written request for product support services that describes the required services and/or documents/work product required. Enzon shall provide Savient an estimate based on its customary rate. Upon acceptance of such estimate by Savient, Savient shall issue a purchase order to Enzon and Enzon shall perform such services in accordance with the terms hereof.
Execution Copy
Page 4 of 44
2.5 Enzon shall prepare and effect the Product shipment in accordance with explict written instructions issued by Savient, which shall include the packaging instructions and Savient’s selected mode of transportation. All transportation costs shall be borne by Savient in accordance with the terms contained herein.
Section 3. MANAGEMENT/FORECASTING/MATERIALS
3.1 Account Management. Each party will appoint an account manager who will be the party responsible for overseeing the activities hereunder.
3.2 Content of Work Plans. Each Work Plan shall include a reasonably detailed description of the Services to be provided, relevant Specifications, a schedule for completion of the Work Plan, a fee and payment schedule, and such other information as is necessary for Enzon to perform the relevant Services.
3.3 Change Orders. In the event that Enzon is requested to perform services that are outside the scope of agreed-upon Work Plans such changes must be mutually agreed upon by the parties in a written change order prior to the provision of said services. Each such change order constitutes an amendment to the applicable Work Plan (which shall be explicitly referenced in such change order) and the services set forth therein shall be deemed to be part of such Work Plan. After receipt of the reasonably detailed description of the additional services from Savient, Enzon shall provide Savient with a cost estimate for performing the changed or additional services. Each change order shall be governed by the terms and conditions of this Agreement and by such supplementary written amendments of this Agreement or Work Plans as may be, from time to time, executed between the parties.
3.4 Forecasting And Savient-supplied Materials
(a) Upon execution of this Agreement and on the first day of each calendar quarter thereafter, Savient shall deliver to Enzon’s account manager an updated rolling forecast of Product requirements (in full-batch quantities) for the twenty four (24) month period commencing on the first day of the immediately following calendar month. Enzon shall, within ten (10) days of receipt of a forecast from Savient, confirm its receipt thereof in writing and shall advise Savient of whether such forecast is accepted in whole or in part; in the event that any part of the forecast is not accepted by Enzon then Enzon shall detail in writing the rationale for such non-acceptance. Within thirty (30) days of accepting each forecast, Enzon will provide Savient a projected manufacturing schedule indicating approximate dates of manufacturing which shall conform with the delivery dates specified in the forecast supplied by Savient. The foregoing notwithstanding, once a forecast (or any portion thereof) has been accepted by Enzon it shall be binding on both parties except as otherwise may be explicity set forth herein; in the event that Enzon neither accepts or rejects any forecast submitted by Savient within ten (10) days of receipt from Savient then the entire forecast as submitted by Savient shall be deemed accepted by Enzon. If accepted, the forecast for the first six (6) calendar months of each forecast (“Firm Forecast”) shall be 100% binding on both parties and the forecast for the next twelve (12) calendar months (“Planning Forecast”) shall be binding on both parties as set forth in the
Execution Copy
Page 5 of 44
following sentence. Product requirements within the Planning Forecast shall not be increased or decreased by Savient by more than one (1) batch per three month period, per forecast, provided that no month may be reduced to zero (0) batches unless the initial Planning Forecast for that particular month was initially set as one or zero batches; for purposes of clarification only, the parties agree that the intention of this provision is to allow Savient, in each subsequent forecast, to modify each three month period of the most recently supplied Planning Forecast by one (1) batch as follows: the first three month period of the most recently provided Planning Forecast (which becomes the final three month period of the Firm Forecast) may be modified by one batch, the second three month period of the Planning Forecast may be modified by one batch, the third three month period of the most recently provided Planning Forecast may be modified by one batch and the fourth three month period of the most recently provided Planning Forecast may be modified by one batch. Savient shall forecast Product requirements for the six (6) months following the Planning Forecast, and the forecast for those six months are non-binding (“Non-Binding Forecast”) on Savient. Savient shall place firm purchase orders for its requirement of the Product in full-batch quantities at least ninety (90) days prior to the requested date of delivery. Each firm written purchase order, signed by Savient’s duly authorized representative, shall authorize Enzon to manufacture the number of batches of the Product as are set forth therein. The number of purchase orders submitted by Savient shall not exceed one (1) per calendar month, unless otherwise agreed to by the parties in writing. Enzon shall have completed any and all activities which are required by the applicable Work Plan and all Applicable Laws so as to be able to deliver the Product on or before the delivery dates specified by Savient in the subject purchase order but in any event the Product shall not be delivered by Enzon more than one (1) month in advance of any specified delivery date. Provided, however, that Enzon shall use Commercially Reasonable Efforts to minimize the amount of time elapsing between the completion of manufacturing activities and delivery of the completed Product to Savient.
(b) Starting from inception of the manufacture of the Product, Savient shall supply to Enzon, and use Commercially Reasonable Efforts to ensure that Enzon has on hand, a sufficient stock of Savient-supplied Materials as is necessary to provide the Services. Enzon shall have no liability for any failure to supply Product to Savient in accordance with the delivery terms contained in a Savient purchase order if sufficient quantities of Savient-supplied Materials in light of the forecasting described above have not been supplied to Enzon at least four (4) weeks prior to the scheduled manufacture date, as communicated to Savient pursuant to Section 3.4(a) . In such case, manufacture of Product may be delayed until receipt of adequate supplies of Savient-supplied Materials and the availability of an appropriate manufacturing slot; provided, however, that Enzon shall use Commercially Reasonable Efforts to schedule the manufacture of the Product as soon as is possible subsequent to receiving the Savient-supplied Materials. If Savient provides Enzon with insufficient Savient-supplied Materials to produce the amount of Product requested in a particular purchase order, both sides may nonetheless agree in writing to have Enzon produce a lesser amount based on the amount of Savient-supplied Materials provided to Enzon, and all such batches shall be subject to the pricing listed in Exhibit C, including the minimum batch price, if applicable. Provided, however, that the Parties agree that any shortfall on the part of Enzon to produce at least ten thousand five hundred (10,500) vials of Product when provided with at least fifteen kilograms (15kg) of Bulk Product by Savient shall
Execution Copy
Page 6 of 44
not constitute a breach of this Agreement and that the the pricing for such shortfall below ten thousand five hundred (10,500 vials) shall be computed as set forth in Section 6.2(e) herein. Additionally, in the event that any scheduled manufacture of the Product is delayed due to the unavailability of adequate stores of Savient-supplied Materials, then any Firm Forecast then in effect shall be carried forward until such a time as the manufacture and delivery of the Product in accordance with the most recently supplied firm purchase order have been completed. Savient shall be responsible for verifying that all Savient-supplied Materials meet relevant Specifications. Title to Savient-supplied Materials shall not be transferred to Enzon. Savient will provide a signed, abbreviated Certificate of Analysis (“CofA”) which shall, at minimum, certify that Savient-supplied Materials meet the Specifications for such Savient-supplied Materials as defined on Exhibit B prior to the processing of Savient-supplied Materials by Enzon. Enzon shall store all Savient-supplied Materials and finished Product in accordance with instructions provided by Savient in the Quality Agreement.
(c) All costs associated with the selection and/or qualification of alternative suppliers for any materials required to perform the Services shall be borne by Savient. Any such activities will be defined by Savient in writing an accompanied by an appropriate purchase order to Enzon.
(d) Upon execution of this Agreement and along with every quarterly forecast, Savient shall pay Enzon a rolling, non-refundable reservation fee equal to 25% of the minimum batch (specifed on Exhibit C) price for batches included in the Firm Forecast to secure manufacturing capacity slots corresponding to the forecast provided. Savient shall pay such reservation fee to Enzon within ten (10) days of Enzon’s provision to Savient of the manufacturing schedule, as set forth in Section 3.4(a), which schedule sets forth the approximate dates of manufacturing for the Product. Such reservation fee shall be credited towards Product produced by Enzon on a batch-by-batch basis in a prorated amount. Additionally, upon payment of the reservation fee by Savient, Enzon warrants that manufacture of the Product shall occur on or before the dates specified in the manufacturing schedule which conforms to the Firm Forecast for which the reservation fee is paid. Upon shipment of each completed batch, Enzon will invoice Savient at a rate equivalent to the applicable unit price multiplied by the total number of vials produced less the applicable portion of any reservation fees paid. No less than two weeks prior to each quarterly update of the Firm Forecast, Enzon and Savient will reconcile the invoices against the above-mentioned reservation fee. In the event that Savient cancels any batch within the Firm Forecast, Enzon will charge Savient, and Savient agrees to pay to Enzon, a cancellation fee as set forth in the following sentence. For each batch canceled by Savient, Savient will pay Enzon an amount equal to the minimum batch price set forth on Exhibit C (less nineteen thousand eight hundred ninety dollars ($19,890) representing unused Process Consumables and Manufacturing Components), which amount shall represent liquidated damages resulting from unused manufacturing capacity. In the event that Savient postpones the manufacture of any batch scheduled during the Firm Forecast period for a period of more than thirty (30) days, then Enzon will charge Savient, and Savient agrees to pay to Enzon, a postponement fee as set forth in the following sentence. For each batch postponed by Savient, Savient will pay Enzon an amount equal to the minimum batch price set forth on Exhibit C (less nineteen thousand eight hundred ninety dollars ($19,890) representing unused Process Consumables and Manufacturing
Execution Copy
Page 7 of 44
Components) which amount shall represent liquidated damages resulting from unused manufacturing capacity. Only with respect to batches which are postponed beyond the Firm Forecast, Savient will remit to Enzon the amount drawn within 30 days, and that amount will be credited back to the reservation fee. Enzon will draw the cancellation and postponement fee amounts described above from the amounts previously remitted to Enzon as reservation fees. In the event that any amounts owing to Enzon pursuant to this Section exceed the amounts previously remitted to Enzon as reservation fees, Enzon shall submit an invoice to Savient for the difference and Savient shall submit payment for such invoiced amounts in accordance with the terms of this Agreement.
Section 4. COMPENSATION AND EXPENSES
4.1 As compensation for rendering the Services hereunder, Savient shall pay Enzon the amounts specified in Exhibit C and any subsequent additional Work Plans executed in writing by both parties. Except as otherwise specifically provided in the attached Work Plan or any subsequent additional Work Plan, all payments by Savient shall be made within thirty (30) days of the date of its receipt of the appropriate invoice from Enzon. Enzon will charge a late payment fee of 1 1⁄2% per month, or the maximum amount permitted by law if less than 1 1⁄2% per month, for any payment not received within thirty (30) days of the date of Savient’s receipt of the appropriate invoice from Enzon. Failure to invoice for interest due shall not be a waiver of Enzon’s right to charge interest. Savient will pay any sales, use, gross receipts or other taxes, licenses, or fees (excluding tax based on Enzon’s net income) required to be paid by Enzon to any state or tax jurisdiction in connection with the Services performed hereunder.
4.2 All invoices and/or other requests for payment shall be itemized with a reasonable degree of specificity to ensure accuracy in accounting for services and/or goods provided and invoiced for. All invoices and/or other requests for payment shall be sent to:
Accounts Payable
Savient Pharmaceuticals, Inc.
▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇
▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇
4.3 Enzon will adjust prices not more often than annually, commencing on January 1, 2010, based on normal and customary increases in costs, not greater than the pharmaceutical Producer Price Index (as published by Bureau of Labor Statistics, Industry Code 325412). Additionally, Enzon may revise the prices provided in an attached Work Plan either upward or downward with Savient’s prior written consent, such consent not to be unreasonably withheld, if (i) any information which the parties reasonably agree is material to the performance of the Services proves to be incomplete or inaccurate (including but not limited to a material reduction in volume or a material change in prices of Enzon’s raw materials), (ii) Savient revises Enzon’s manufacturing or packaging responsibilities, procedures, or assumptions in a way that would impact the cost of the Services, or (iii) unforeseen circumstances, which both parties reasonably agree were unforeseeable at the time of contracting and which are not directly attributable to Enzon, affect the activities required to complete the Work Plan. Enzon will notify Savient
Execution Copy
Page 8 of 44
immediately if the costs to complete Services materially differ, either positively or negatively, from the prices stated in the attached Work Plan. Enzon will not commence work involving charges in excess of those stated in the attached Work Plan without Savient’s written approval unless such advance notice was not possible due to the circumstances. Savient shall be responsible for all non-cancelable costs incurred by Enzon as a direct result of any change order or other variation in Services requested by Savient, including but not limited to, inventory rendered unusable under the Work Plan; provided, however, that Enzon shall use Commercially Reasonable Efforts to minimize any non-cancelable costs contemplated herein including, but not limited to, by maintaining on hand only such Manufacture Components which are reasonably required to manufacture such quantities of Product as are specified in the Firm Forecast.
4.4 Savient’s failure to pay for the amounts due under this Agreement (including but not limited to payments under 3.4(d)) shall constitute a material breach of this Agreement. Savient shall have 45 days from Enzon’s written notice to cure such breach; provided, however, that Savient’s failure to pay any amounts otherwise owing hereunder due to a good faith dispute relating to such amounts shall not constitute a material breach only with respect to such amounts. Upon the expiration of the stated cure period, Enzon shall have the right to suspend any Services under this Agreement. Any batch cancellations resulting from such actions will be billed to Savient in accordance with Section 3.4(d) .
Section 5. CERTAIN REPRESENTATIONS, WARRANTIES, AND COVENANTS
OF ENZON:
5.1 Authority. Enzon represents and warrants that it has full authority to enter into this Agreement.
5.2 Material/Supplies. Enzon shall use Savient-supplied Materials only to perform the Services hereunder.
5.3 Savient Intellectual Property. Enzon warrants that it shall use Savient Intellectual Property only for the purpose of manufacturing the Product on behalf of Savient in accordance with the terms of this Agreement.
5.4 MVP Confidential Information. Enzon hereby represents and warrants that, during the Term of this Agreement, it has not taken any action, nor failed to take any action, which would violate or cause to be violated the terms and conditions contained in the attached Exhibit E, which is incorporated herein by reference. The warranty contained herein shall survive the termination or expiration of the Agreement in accordance with the terms contained in the attached Exhibit E. Anything to the contrary contained in this Agreement notwithstanding, Enzon agrees that there shall be no limitation on the amount of liability for which Enzon may be liable to either Savient or Mountain View Pharmaceuticals, Inc., for breach of this Section 5.4.
Commercial Supply Agreement
Execution Copy
Page 9 of 44
5.5 Books and Records. Enzon shall maintain true and accurate books, records, test and laboratory data, reports and all other information relating to manufacture of Product as required by regulation and in accord with current good manufacturing practices (“cGMP”) and as set forth in the Quality Agreement.
5.6 Regulatory Inspections. Enzon shall make its facilities and all records relating to the Product manufacture available to the Regulatory Authorities at times agreed with such authorities and shall notify Savient if the Regulatory Authority begins or schedules an inspection of Enzon’s records, facilities, or manufacturing processes related to the manufacture of Product and provide Savient access to any documentation related to or resulting from the inspection as described in the Quality Agreement.
5.7 Debarment. Enzon hereby certifies it does not and shall not employ, contract with or retain any person directly or indirectly to perform services under this Agreement if such person is debarred under 21 U.S.C. 335a (a) or (b) or other equivalent laws, rules, regulations or standards of any other relevant jurisdiction.
5.8 Regulatory Filings. Enzon will cooperate in providing to Savient any non-confidential information in its control relating to this Agreement or the Product that Savient may reasonably require in connection with its regulatory or governmental filings, provided that such information shall be provided in whatever form held by Enzon. If applicable, Enzon will provide a letter permitting applicable Regulatory Authority to reference its relevant drug master file.
5.9 Product and Process. Enzon provides services to its customers on a contractual fee-for-service basis. Enzon warrants that it will perform the Services with due care and in accordance with agreed upon protocols and/or specifications, the terms of this Agreement and any Work Plan hereunder, generally prevailing industry standards and Applicable Laws. Enzon warrants that its fill/finish process does not and will not infringe on the rights of any third parties.
OF SAVIENT:
5.10 Authority. Savient represents and warrants that it has full authority to enter into this Agreement.
5.11 Savient-supplied Materials. Savient represents, warrants and covenants as follows: (i) all Savient-supplied Materials will be supplied not later than four (4) weeks prior to a scheduled manufacturing date, as communicated to Savient pursuant to Section 3.4(a), so as to enable Enzon to complete manufacture and delivery of the Product in accordance with all forecasts and firm purchase orders submitted by Savient and accepted by Enzon; (ii) all Savient-supplied Materials shall meet all relevant specifications, (iii) Savient shall take sole and exclusive responsibility for the quality and sufficient supply of all such Savient-supplied Materials, including responsibility for all testing and inspection of the same except to the extent (if any) that Savient and Enzon agree that Enzon shall perform any such testing and/or inspections in any Work Plan to this Agreement, and (iv) Enzon shall have no liability for a loss of Savient-supplied Materials except as set forth in Section 11.4.
Commercial Supply Agreement
Execution Copy
Page 10 of 44
5.12 IP Rights. Savient represents, warrants and covenants that Savient has all the rights necessary, including the rights to the Savient Trademarks, to permit Enzon to perform the Services hereunder without infringing the intellectual property rights of any third party.
5.13 Debarment. Savient hereby certifies it does not and shall not employ, contract with or retain any person directly or indirectly to perform services under this Agreement if such person is debarred under 21 U.S.C. 335a (a) or (b) or other equivalent laws, rules, regulations or standards of any other relevant jurisdiction.
Section 6. ADDITIONAL PRODUCT SUPPLY TERMS
6.1 Delivery. Delivery terms shall be FCA (Incoterms 2000) Enzon’s manufacturing facility in Indianapolis, Indiana (or such other facility as the Parties may agree upon); Product shall be delivered in accordance with the timeframe set forth in the applicable purchase order. Title to Product and Savient-supplied Materials shall remain vested with Savient at all times.
6.2 Rejected Goods; Failure of Supply.
(a) Except as provided for in Section 11.4, Savient’s sole remedy for breach of Enzon’s warranty in Section 5.9 shall be to require Enzon to re-perform the relevant services at Enzon’s cost.
(b) Concurrent with Enzon’s delivery to Savient of any Product contemplated hereunder, Enzon shall provide to Savient a written, executed CofA demonstrating compliance of Product with all relevant Specifications; such CofA may be transmitted to Savient via facsimile or electronic mail. Promptly following receipt of Product, Savient shall have the right but not the obligation to test such Product to determine compliance with the Specifications. Savient shall notify Enzon in writing of any rejection of Product based on any claim that the Product fails to meet Specifications within thirty (30) days of delivery, after which point all unrejected Product shall be deemed accepted. Any rightly rejected Product that does not meet the Specifications shall, at Enzon’s sole discretion and expense, either (i) be returned to Enzon within a reasonable period of time and relabeled or reworked as permitted in the Marketing Authorizations and Specifications, if permitted by the Applicable Laws, or (ii) be destroyed in accordance with Applicable Laws.
(c) In the event that Enzon believes that Product has been incorrectly rejected, Enzon may require that Savient provide to it Product samples for testing. Enzon may retain and test the samples of Product retained by it. In the event of a discrepancy between Savient’s and Enzon’s test results such that one Party’s test results fall within relevant Specifications and the other Party’s test results fall outside the relevant Specifications, or there exists a dispute between the Parties over the extent to which such failure is attributable to a given Party, the Parties shall cause an independent laboratory or appropriate expert promptly to review records, test data and perform comparative tests and/or analyses on samples of the alleged defective Product. Such independent laboratory or expert shall be mutually agreed upon by the Parties, and shall be of such national repute as to allow both Parties to reasonably agree that the independent laboratory
Commercial Supply Agreement
Execution Copy
Page 11 of 44
or expert is sufficiently qualified to perform such analyses. The independent laboratory’s results shall be in writing and shall be final and binding save for manifest error. Unless otherwise agreed to by the Parties in writing, the costs associated with such testing and review shall be borne by the Party against whom the independent laboratory rules.
(d) Enzon shall replace any rightly rejected Product as promptly as practicable, using Commercially Reasonable Efforts to make available manufacturing capacity, after the notice of such rejection, and in any case as soon as reasonably possible after receiving such notice, provided that Savient shall provide to Enzon sufficient quantities of Savient-supplied Materials at no additional cost to Enzon. However, if the failure to meet Specifications is due to defects in the Savient-supplied Materials (where such defects are not due to any failure on the part of Enzon), or any other cause except Enzon’s failure to perform the Services in accordance with this Agreement, Savient will pay the full cost of the rejected batch.
(e) The Parties agree that Savient shall supply variable amounts of Bulk Product to Enzon for purposes of allowing Enzon to provide Services to fill and finish such Bulk Product into Product; the Parties further agree that.if Savient supplies to Enzon fifteen kilograms (15kg) or more of Bulk Product for a single filling run that Enzon shall produce not less than ten thousand five hundred (10,500) vials of Product; if Enzon should fail to produce at least ten thousand five hundred (10,500) vials of Product as indicated herein, then Savient shall pay to Enzon an amount equal to the per-vial price indicated on the attached Exhibit C multiplied by the actual number of vials produced. In the event that Savient supplies less than fifteen kilograms (15kg) of Bulk Product to Enzon for a single filling run, then Savient shall pay to Enzon the minimum batch price indicated on Exhibit C attached hereto.
6.3 Recall; Withdrawal; Modification; Complaints. Savient shall be responsible for the cost of and all losses associated with any recall or product withdrawal or modification; provided, however, that to the extent that any such recall or product withdrawal is due to the gross negligence or willful misconduct on the part of Enzon, then Enzon shall reimburse Savient for all direct costs associated with such recall or withdrawal, in addition to any other rights or remedies Savient may have, but in any case only to the extent attributable to Enzon. Enzon shall reasonably cooperate with Savient in connection with any recall, withdrawal, or modification, at the expense of Savient except as otherwise provided for herein. Savient shall share with Enzon all relevant information relating to any such recall, withdrawal, or modification. In addition, Savient shall also promptly and fully detail for Enzon any Product complaints or Field Alerts it receives insofar as any such complaints relate to the Services rendered by Enzon hereunder. Enzon shall cooperate with Savient to report any adverse events of which it becomes aware in accordance with the terms of the Quality Agreement. Enzon shall only be responsible for the testing and protocols set forth in the Work Plan and Exhibits A and B, as applicable, and Savient is responsible for all other testing and protocols.
Section 7. TERM AND TERMINATION
7.1 Term. This Agreement shall commence on the Effective Date and shall remain in full force and effect unless terminated as provided herein.
Commercial Supply Agreement
Execution Copy
Page 12 of 44
7.2 Termination. Subsequent to the first (1st) anniversary of the Effective Date of this Agreement, this Agreement may be terminated by either party at any time by giving at least twenty-four (24) months prior written notice to the other party as follows: either party may give notice to the other party thirty (30) days prior to every such anniversary date. During the 24-month period between the notice of termination and the effectiveness of such termination, the Parties shall continue to cooperate with each other in good faith to effectuate the purpose of this Agreement; specifically, and without limitation, Savient may place, and Enzon shall accept and fulfill, forecasts and purchase orders for the manufacture of Product, all in accordance with the terms and conditions of this Agreement. During the pendency of the effective date of the termination notice, Savient shall not reduce the final six (6) months of any previously submitted forecast to zero (0) batches except if Enzon is the party which is terminating this Agreement. For the purposes of clarification only, the prohibition contained in the immediately preceding sentence shall not apply where the final six (6) months of the most recently supplied forecast were identified as having zero (0) batches at the time that the notice of termination was provided. Except as provided for herein, if, at any time subsequent to the tendering of a notice of termination pursuant to the terms herein, Savient reduces any of the final six months of the forecast to zero (0) batches, Savient shall pay Enzon a termination fee of $55,000 per batch. Enzon shall use Commercially Reasonable Efforts to minimize the incurrence of any additional charges, fees or expenses which will not be utilized in the manufacture of the Product on behalf of Savient prior to the effective date of termination of this Agreement. Within thirty (30) days of the effective date of the termination of this Agreement, Enzon shall provide to Savient any case reports, analyses and other deliverables which were prepared by Enzon, if any, prior to the date of termination and Enzon shall also provide Savient with a written itemized statement of all Services performed by it hereunder and all costs incurred or for which Enzon is obligated. In the event of termination pursuant to this Section 7.2, Enzon shall be entitled to full payment for the Services actually rendered by it hereunder and all non-cancelable costs incurred through the date of termination. In addition to the foregoing, if Savient terminates this Agreement or any Work Plan pursuant to this Section 7.2, Savient shall pay any cancellation or postponement amounts set forth in Section 3.4(d); provided, however, that Enzon shall use Commercially Reasonable Efforts to mitigate any such cancellation or postponement amounts by scheduling, to the extent practicable, additional third party manufacturing activities, with any such mitigation accruing to the benefit of Savient and proportionately reducing any cancellation or postponement amounts otherwise owing. If the amount already paid by Savient to Enzon exceeds such amounts payable hereunder, Enzon shall refund such excess to Savient and if such amounts payable are greater than the amounts already paid by Savient to Enzon, then Savient shall pay the amount of such shortfall to Enzon.
7.3 This Agreement may also be terminated by either party upon material default in performance of the other party, provided that any defaulting party shall be given not less than ninety (90) days’ prior written notice of default and the opportunity to cure the default during such period. In the event this Agreement is terminated pursuant to this Section 7.3, Enzon shall be entitled to full payment for the services provided by it hereunder (as set forth in any Work Plan(s) made a part hereof) and all costs incurred through the date of termination or for which Enzon is obligated as of the date of termination; provided, however, that if Savient terminates this Agreement pursuant to this Section 7.3 then, anything to the contrary notwithstanding,
Commercial Supply Agreement
Execution Copy
Page 13 of 44
Enzon shall be entitled only to payment for such Services which it actually rendered on behalf of Savient through the effective date of termination. In addition to the foregoing, if Enzon terminates this Agreement or any Work Plan pursuant to this Section 7.3, Savient shall pay any applicable cancellation or postponement amounts set forth in Section 3.4(d); provided, however, that Enzon shall use Commercially Reasonable Efforts to mitigate any such cancellation or postponement amounts by scheduling, to the extent practicable, additional third party manufacturing activities, with any such mitigation accruing to the benefit of Savient and proportionately reducing any cancellation or postponement amounts otherwise owing. If the amount previously paid by Savient exceeds such amount payable hereunder, the excess shall be refunded to Savient and if such amounts payable are greater than the amounts already paid by Savient to Enzon, then Savient shall pay the amount of such shortfall to Enzon.
7.4 In the event that Savient’s BLA for the Product is not approved by the FDA, and where such disapproval is final or otherwise not appealed by Savient, then either Party shall have the right, but not the obligation, to terminate this Agreement upon the provision of thirty (30) days notice to the other Party. In the event this Agreement is terminated pursuant to this Section 7.4, Savient shall pay Enzon for packaging and labeling materials, any unpaid amounts for manufactured batches, and any reservation fees or other applicable cancellation or termination fees, provided that Enzon shall use Commercially Reasonable Efforts to mitigate such fees.
7.5 In the event that Savient’s BLA for the Product has not been approved by April 2009, then this Agreement shall continue in force and effect but any deliverables and obligations of the parties shall be held in abeyance for up to 18 months so as to allow Savient to address any findings in such approvable letter issued by the FDA and resubmit the subject BLA. Savient shall pay any cancellation or postponement amounts set forth in Section 3; provided, however, that Enzon shall use Commercially Reasonable Efforts to mitigate any such postponement amounts by scheduling, to the extent practicable, additional third party manufacturing activities, with any such mitigation accruing to the benefit of Savient and proportionately reducing any cancellation or postponement amounts otherwise owing. Savient shall provide to Enzon notice of its receipt of an approvable letter from the FDA within five (5) business days of its receipt of same. After said 18 months lapses, Enzon shall have the right to terminate this agreement immediately and with no penalty, and Savient shall pay all applicable cancellation and postponement amounts as set forth .
7.6 This Agreement may be terminated immediately, upon written notice, upon either party’s bankruptcy (voluntary or involuntary), insolvency, or placing of either party’s business in the hands of a receiver.
7.7 Survival. The rights and obligations of each Party which by their nature survive the termination or expiration of this Agreement shall survive the termination or expiration of this Agreement, including Sections 4, 6, 7, 8, 9, 10, 11, 14, and 15 (to the extent relevant). In addition, Enzon hereby acknowledges that neither expiration nor termination of this Agreement shall affect in any manner Savient’s right to manufacture and sell, or have manufactured and sold, the Product.
Commercial Supply Agreement
Execution Copy
Page 14 of 44
Section 8. INTELLECTUAL PROPERTY
8.1 Subject to Section 8.2, all Savient Intellectual Property supplied to Enzon or developed by Enzon in the course of performing the Services hereunder are owned by Savient. All information developed by Enzon and related to the Bulk Product or the Product shall be disclosed to Savient promptly upon discovery or development by Enzon. Savient shall have the right to make any use of such information and Enzon agrees to execute any documents which may be reasonably required to effectuate any assignment of inventorship contemplated by this provision, at Savient’s expense. Following completion of the Services outlined in any Work Plan, Enzon will insure the return of all client data or other materials furnished to Enzon. Subject to Section 8.2, all intellectual property rights subsisting in or relating to any calculations, data, methods, specifications, papers, documents, and any other items, material or information arising from the performance of the Services by Enzon under this Agreement are vested in and are the sole property of Savient and Enzon shall execute any and all documents reasonably requested by Savient in order to effectuate the intent of this provision, at Savient’s expense.
8.2 Enzon shall own all rights to any invention (whether or not patentable) relating to manufacturing and analytical methods and processes developed by Enzon in connection with Services performed hereunder that have general use in biopharmaceutical manufacturing, to the extent not specific to Savient’s Product, and to the extent not directed to or derived from any pre-existing Savient Intellectual Property or MVP Confidential Information (“Process Invention”); provided that the provisions of this Section 8.2 shall not apply to manufacturing and analytical methods and processes developed by Enzon at the direction of Savient. Except as specifically prohibited with respect to MVP Confidential Information, Enzon reserves the right to use data developed during the course of performing Services hereunder to support applications, assignments or other instruments necessary to apply for and obtain patent or other intellectual property protection with respect to Process Inventions so long as no information which Enzon is required to keep confidential under this Agreement or any other previously executed agreement between the Parties relating to confidentiality of information is disclosed in any such application, assignment, or other instrument without the prior consent of Savient (not to be unreasonably withheld). For Process Inventions developed by Enzon in connection with performing services hereunder, Enzon will grant to Savient a perpetual, world-wide, royalty-free, non-exclusive license for Savient to use such Process Inventions in the development and manufacture of the Savient Products.
Section 9. CONFIDENTIALITY
9.1 For the duration of the Agreement and five (5) years thereafter with respect to Savient Confidential Information (as defined below), or, in the case of MVP Confidential Information (as defined below), for twenty (20) years from the Effective Date of the Agreement, Enzon will not disclose, without Savient’s written permission, any such Savient Confidential Information or MVP Confidential Information, unless such disclosure: (i) is to an Affiliate, agent, employee or consultant of Enzon that is under a similar obligation to keep such information confidential and such disclosure is reasonably necessary for the performance of the Services contemplated herein; (ii) is or becomes publicly available through no fault of Enzon;
Commercial Supply Agreement
Execution Copy
Page 15 of 44
(iii) is disclosed by a third party entitled to disclose it; (iv) is already known to Enzon as shown by its prior written records; or, (v) is required by any law, rule, regulation, order, decision, decree, subpoena or other legal process to be disclosed or in response to a request or order from a regulatory agency. If such disclosure is requested by a regulatory agency or legal process, Enzon will make all reasonable efforts to notify Savient of this request promptly prior to any disclosure to permit Savient to oppose such disclosure by appropriate legal action. Enzon shall use reasonable precautions to protect the confidentiality of both Savient Confidential Information and MVP Confidential Information in a manner that is comparable to precautions taken to protect is own proprietary information. As used herein, “MVP Confidential Information” means any Confidential Information that Savient provides, or has provided, to Enzon which is specifically identified in writing as containing Mountain View Pharmaceuticals, Inc.’s proprietary technology for the manufacture of PEGylated uricase (Puricase®/pegloticase), specifically including the documents referenced in Schedule A of the Second Amendment to the Agreement for Services between Savient and Enzon dated October 31, 2006, which the Parties have agreed to in a letter dated September 12, 2007, as containing Confidential Information belonging to Mountain View Pharmaceuticals, Inc; “Savient Confidential Information” means any Confidential Information provided by Savient to Enzon, with the sole exception of MVP Confidential Information, during the term of the Agreement.
9.2 For the duration of the Agreement and five (5) years thereafter with respect to Savient Confidential Information, or in the case of MVP Confidential Information, for twenty (20) years from the Effective Date of the Agreement, Enzon will not use such Confidential Information except in connection with the performance of Services under the Agreement or any other Agreement between Savient and Enzon related to Savient’s PEGylated uricase (Puricase®/pegloticase) product and in particular represents and warrants that it will not utilize such Confidential Information in the manufacturing of any other product.
9.3 For twenty (20) years from the Effective Date of the Agreement, Savient will not disclose, without Enzon’s written permission, any Confidential Information belonging to Enzon which is provided to Savient by Enzon during the Term of the Agreement (“Enzon Confidential Information”) unless such disclosure: (i) is to an affiliate, agent, employee or consultant of Savient that is under a similar obligation to keep such information confidential; (ii) is or becomes publicly available through no fault of Savient; (iii) is disclosed by a third party entitled to disclose it; (iv) is already known to Savient as shown by its prior written records; or, (v) is required by any law, rule, regulation, order decision, decree, subpoena or other legal process to be disclosed or in response to a request or order from a regulatory agency. If such disclosure is requested by a regulatory agency or legal process, Savient will make all reasonable efforts to notify Enzon of this request promptly prior to any disclosure to permit Enzon to oppose such disclosure by appropriate legal action. Savient shall use reasonable precautions to protect the confidentiality of Enzon Confidential Information in a manner that is comparable to precautions taken to protect its own proprietary information.
9.4 If either Party shall be obliged to provide testimony or records pertaining to the Confidential Information provided by the other in any legal or administrative proceeding, then the Party which supplied the Confidential Information shall reimburse the other Party for its out-
Commercial Supply Agreement
Execution Copy
Page 16 of 44
of-pocket costs therefore plus an hourly fee for its employees or representatives equal to the internal fully burdened costs of such employee or representative.
9.5 For both Parties, “Confidential Information” shall mean and include, without limitation, such types of information as: inventions, methods, plans, processes, specifications, characteristics, raw data, analyses, equipment design, trade secrets, costs, marketing, sales, and product performance information, including patents and patent applications, grant applications, notes, and memoranda, whether in writing or presented, stored or maintained electronically, magnetically or by other means, which are disclosed by the disclosing Party to the recipient Party in writing or in other tangible form and marked “confidential” or, if disclosed orally (or in some other non-tangible form), are identified as confidential to the recipient Party in writing within sixty (60) days of such disclosure; provided, however, that failure to reduce any verbal disclosure to writing shall not, in and of itself, vitiate the confidential nature of such Confidential Information and provided, further, that for the purposes of this Agreement, Confidential Information shall include any and all such information exchanged between the Parties prior to the Effective Date of this Agreement pursuant to the Confidentiality Agreement between the Parties dated July 24, 2006.”
Section 10. INSURANCE
Each Party shall for the term of this Agreement and for two (2) years after the last Product is delivered, obtain and maintain at its own cost and expense from a qualified insurance company, comprehensive general liability insurance including, but not limited to, contractual liability coverage and standard product liability coverage in an amount commensurate with industry standards. Savient shall for the term of this Agreement and for two (2) years after the last Product is delivered, obtain and maintain at its own cost and expense from a qualified insurance Savient, insurance coverage for losses of inventory at Enzon’s facility prior to, and following manufacture of the Product. At a Party’s request, the other Party shall provide it with proof of such coverage.
Section 11. INDEMNIFICATION AND LIMITS OF LIABILITY
11.1 Without limiting Enzon’s rights under law or in equity, Savient agrees to indemnify and hold harmless Enzon and its employees, directors and agents from and against any loss, damage, cost and expense (including without limitation attorneys’ fees and expenses) incurred in connection with any claims, proceedings or investigations arising directly or indirectly from (a) the manufacture, promotion, marketing, distribution or sale of the Product, (b) use or exposure to Product or any material provided to Enzon by Savient, (c) use of any Savient Intellectual Property provided by Savient to Enzon (but only in cases where Savient has provided such Savient Intellectual Property for Enzon’s use) or any infringement of the intellectual property rights of any third party related to the Product, or (d) any breach of Savient’s representations and/or warranties, except to the extent any such claim is the result of Enzon’s gross negligence or willful misconduct.
Commercial Supply Agreement
Execution Copy
Page 17 of 44
11.2 Without limiting Savient’s rights under law or in equity, Enzon agrees to indemnify and hold harmless Savient and its employees, directors and agents from and against any loss, damage, cost and expense (including without limitation attorneys’ fees and expenses) incurred in connection with any claims, proceedings or investigations arising out of or in connection with (a) this Agreement and the Product produced and the Services rendered hereunder to the extent that such claim, proceeding or investigation is based on the gross negligence or willful misconduct of Enzon or its employees, (b) any breach of Section 9.2 of this Agreement with respect to MVP Confidential Information, (c) any breach of the representations made by Enzon in the Letter Agreement between Enzon and Savient dated September 12, 2007, attached hereto as Exhibit E; but in any case only to the extent attributable to Enzon.
11.3 Any party seeking indemnification pursuant to this Section 11 (the “Indemnitee”) shall give notice within five (5) days to the party from whom indemnification is sought (the “Indemnitor”) of any claim, proceeding or investigation; provided, however, that any failure to notify the Indemnitor within such five (5) day period shall not negate the rights of indemnification granted hereunder except to the extent that the Indemnitor is actually prejudiced by such delay in notification. The Indemnitee shall cooperate in the defense of such claim, proceeding or investigation, subject to reimbursement by the Indemnitor for all reasonable out-of-pocket expenses. The Indemnitor shall, at its option, assume control of the defense of any such claim, proceeding or investigation. The indemnities set forth in Sections 11.1 and 11.2 shall include amounts paid in settlement provided, however, that no such settlement shall be entered into without the Indemnitor’s consent, which consent shall not be unreasonably withheld.
11.4 As Savient’s sole remedy, Enzon agrees to reimburse Savient up to a maximum of $25,000 per batch, pro-rated over the usable portion of the batch, if applicable, for any loss of Savient-supplied Materials for each batch that does not meet Specifications or was not manufactured in accordance with the Manufacturing Process or cGMP or the requirements of this Agreement, and therefore cannot be released or otherwise utilized for its intended purpose; provided that the loss of such materials can be shown after investigation to be caused solely and directly by: (a) the failure of Enzon to follow its SOP’s; or (b) Enzon’s negligence, gross negligence, willful misconduct, or breach of this Agreement. In addition to this payment, if due to Enzon’s gross negligence, willful misconduct, or breach of this Agreement, Enzon will re-perform the Services as provided in Section 6.2(a) .
11.5 SECTION 11.4 IS SAVIENT’S SOLE AND EXCLUSIVE REMEDY FOR ANY LOSSES OF SAVIENT-SUPPLIED MATERIAL AS A RESULT OF PRODUCT THAT DOES NOT COMPLY WITH THE SPECIFICATIONS OR THE OTHER REQUIRMENTS OF THIS AGREEMENT. UNDER NO CIRCUMSTANCES SHALL ENZON BE LIABLE TO SAVIENT OR ANY THIRD PARTY FOR ANY CONSEQUENTIAL, INDIRECT (INCLUDING LOST REVENUES OR PROFITS), SPECIAL, OR OTHER DAMAGES, AND THE WARRANTY SET FORTH IN SECTION 5.9 IS THE SOLE AND EXCLUSIVE WARRANTY AND IN LIEU OF ANY AND ALL OTHER WARRANTIES RELATING TO THE SERVICES TO BE PERFORMED, EXPRESS OR IMPLIED, INCLUDING, WITHOUT LIMITATION, ANY IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, OR FOR NON-INFRINGEMENT OF INTELLECTUAL
Commercial Supply Agreement
Execution Copy
Page 18 of 44
PROPERTY RIGHTS. ENZON’S MAXIMUM LIABILITY FOR DAMAGES IN CONNECTION WITH A CLAIM RELATED TO THIS AGREEMENT, REGARDLESS OF THE CAUSE OF ACTION, WILL NOT EXCEED THE SUM TOTAL OF THE AMOUNTS PAID BY SAVIENT TO ENZON IN THE PRECEEDING TWELVE (12) MONTHS.
EXCEPT AS EXPRESSLY STATED HEREIN, NEITHER PARTY PROVIDES TO THE OTHER PARTY HERETO ANY WARRANTIES, EXPRESS OR IMPLIED, WITH RESPECT TO THE SERVICES PROVIDED HEREUNDER, AND ALL SUCH WARRANTIES, EXPRESS OR IMPLIED, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTIES OF MERCHANTIBILITY OR FITNESS FOR A PARTICULAR PURPOSE ARE WAIVED, OTHER THAN AGREED HEREIN. WITHOUT LIMITING THE PROVISIONS OF SECTION 5.9 AND 6.2(e), ENZON MAKES NO WARRANTIES THAT THE EXECUTION OF THIS AGREEMENT WILL RESULT IN ANY SPECIFIC QUANTITY OR QUALITY OF PRODUCT.
Section 12. PUBLICITY AND PUBLICATIONS
Neither Savient nor Enzon shall make any news release or other public statement, whether to the press or otherwise, disclosing the existence of this Agreement, the terms thereof, or of any amendment thereto without the prior written approval of the other Party, except as required by Applicable Laws including, without limitation, those regulations promulgated by the United States Securities and Exchange Commission.
Section 13. FORCE MAJEURE AND CHANGE IN CIRCUMSTANCES
If either Party shall be delayed or hindered in or prevented from the performance of any act required hereunder by reason of strike, lockouts, labor troubles, restrictive governmental or judicial orders or decrees, riots, insurrection, war, terrorist acts, acts of God, inclement weather, or other reason or cause reasonably beyond such Party’s control (each a “Force Majeure”), then performance of such act shall be excused for the period of such Force Majeure. The Party affected by the Force Majeure shall provide notice to the other of the commencement and termination of the Force Majeure. Should a Force Majeure continue for more than three (3) months, the Party unaffected by the Force Majeure may terminate this Agreement upon prior written notice to the affected Party. If the Force Majeure equally affects the ability of each Party to perform under this Agreement, then such termination shall only be by mutual written agreement. In the event of any other type of unforeseen material change in circumstances (that does not qualify as force majeure), both parties agree to negotiate in good faith to find a commercially reasonable solution.
Section 14. NOTICES
14.1 All administrative communications provided for in this Agreement shall be sent via first class mail (subject to Section 14.2 below), postage prepaid, addressed to the respective parties as follows:
| To Enzon: | To Savient |
Commercial Supply Agreement
Execution Copy
Page 19 of 44
| Executive Vice President, Operations | Vice President, Technical Operations | |
| Enzon Pharmaceuticals, Inc. | Savient Pharmaceuticals, Inc. | |
| ▇▇▇ ▇▇▇▇▇ 202/206 | ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇ | |
| ▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ | ▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | |
| ▇▇▇▇▇▇ ▇▇▇▇▇▇ of America | United States of America | |
| With a copy to: | ||
| Legal Department | General Counsel | |
| Enzon Pharmaceuticals, Inc. | Savient Pharmaceuticals, Inc. | |
| ▇▇▇ ▇▇▇▇▇ 202/206 | ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇ | |
| ▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ | ▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | |
| ▇▇▇▇▇▇ ▇▇▇▇▇▇ of America | USA | |
14.2 Original documents and other than routine correspondence required under this Agreement shall be sent by certified mail and addressed to the respective parties at the addresses set forth in Section 14.1. All legal notices shall be in writing and sent by certified mail, return receipt requested. Such notices shall be effective on receipt. All routine correspondence between the Parties may be sent via electronic mail, facsimile or by regular mail.
Section 15. MISCELLANEOUS
15.1 Amendments; Assignment. This Agreement, including any Work Plans or other attachments, may not be altered, amended or modified except by a written document signed by both Parties. Enzon will not assign this Agreement without the prior written consent of Savient and any purported assignment in contravention of this Section shall be null and void; provided, however, that either Party may assign this Agreement in connection with the sale of all or substantially all of its assets related to this Agreement or the Services to be provided hereunder; provided, further, that any such successor or assignee assumes and accepts in writing all obligations of the purported assigning party hereunder.
15.2 Subcontracting. Enzon may subcontract or delegate any of its rights or obligations under this Agreement with the prior written authorization of Savient, such authorization not to be unreasonably withheld. Enzon shall cause any subcontractor to be subject by contract to the same restrictions, exceptions, obligations, reports, termination provisions and other provisions contained in this Agreement.
15.3 Successors; Assigns. This Agreement shall be binding upon and inure to the benefit of the Parties hereto and each of their respective successors and permitted assigns.
15.4 Severability. All agreements and covenants contained herein are severable, and in the event any of them shall be held to be invalid by any competent court, this Agreement shall be interpreted as if such invalid agreements or covenants were not contained herein.
Commercial Supply Agreement
Execution Copy
Page 20 of 44
15.5 Entire Agreement. This Agreement, including the attached Work Plans, constitutes the entire agreement between the Parties and supersedes all prior communications, representations, or agreements, either verbal or written between the Parties which are specifically related to the subject matter contemplated herein; anything to the contrary notwithstanding, any previously executed Confidentiality and Nondisclosure Agreement shall remain valid and enforceable in accordance with its terms. Each Party confirms that it is not relying on any representations or warranties of the other Party except as specifically set forth herein.
15.6 Independent Contractor. This Agreement shall not be deemed to create any partnership, joint venture, or agency relationship between the Parties. Each Party shall act hereunder as an independent contractor and its agents and employees shall have no right or authority under this Agreement to assume or create any obligation on behalf of, or in the name of, the other Party. All persons employed by a Party shall be employees of such Party and not of the other Party, and all costs and obligations incurred by reason of any such employment shall be for the account and expense of such Party.
15.7 Waiver. The waiver by either Party of any right hereunder shall not be deemed a waiver of any other right hereunder.
15.8 Counterparts. This Agreement may be executed in two (2) or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
15.9 Headings. The headings used in this Agreement are for convenience only and are not a part of this Agreement.
15.10 Governing Law. This Agreement shall be construed and enforced in accordance with the laws of the State of New Jersey, without application of its principles of conflict of laws.
15.11 Audits. Once each calendar year during the term of this Agreement, Savient and its agents and designees shall have the right to audit Enzon’s facilities, systems, records solely related to this Agreement or the Product. Such audits may be conducted upon reasonable notice during the term of this Agreement, so long as (i) all auditors have entered into confidentiality agreements relating to the materials to be reviewed, (ii) no materials are removed from the premises of Enzon, provided, however, that Savient may make and retain copies of Enzon materials as may be reasonably necessary solely for purposes of completing the contemplated audit and any such materials shall be considered confidential, and (iii) a copy of all findings is provided to Enzon. All costs for such audits shall be paid by Savient. For the avoidance of doubt, pre-approval inspections shall be considered an audit under this Section 15.11. Anything to the contrary notwithstanding, in the event that an audit is required due to batch failures or because the Services are not rendered in accordance with the terms of this Agreement (including any Work Plan), then such for-cause audit shall not count towards the annual audit provided for herein.
15.12 Nonsolicitation. For the term of this Agreement, and for twelve (12) months following termination of this Agreement, for any reason, neither Savient nor Enzon nor any of
Commercial Supply Agreement
Execution Copy
Page 21 of 44
their employees or agents shall, directly or indirectly, solicit any employees of the other, who have been involved in the Services, unless otherwise approved by the other party.
IN WITNESS WHEREOF, each of the Parties hereto has caused this Commercial Supply Agreement to be executed by its duly authorized representative as of the date written above.
| ENZON PHARMACEUTICALS, INC. | SAVIENT PHARMACEUTICALS, INC. | |||||||
| By: | /s/ ▇▇▇▇▇ del ▇▇▇▇▇ |
By: | /s/ ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ | |||||
| ▇▇▇▇▇ del ▇▇▇▇▇ | ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ | |||||||
| EVP - Operations | EVP & Chief Business Officer | |||||||
Commercial Supply Agreement
Execution Copy
Page 22 of 44
Exhibit A
Work Plan
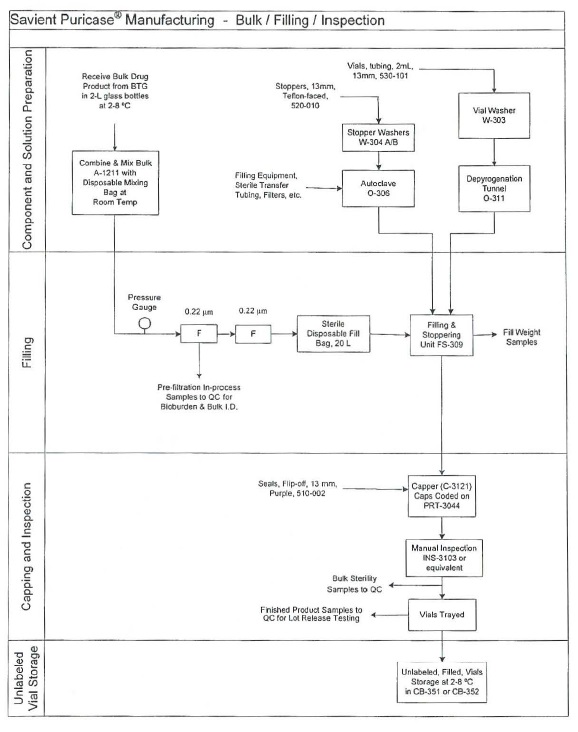
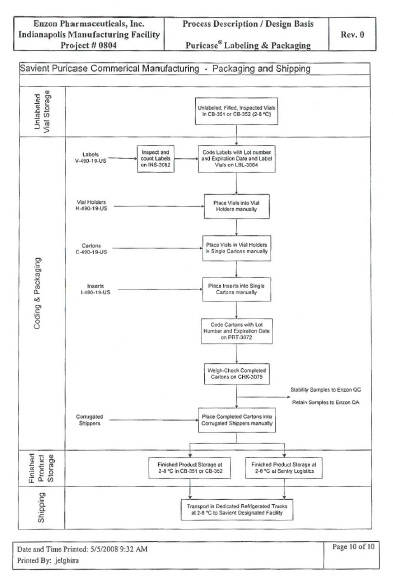
Enzon will fill, inspect, package and test the Product using the components defined below and the process as outlined on the following Process Flow Diagram.
COMPONENTS:
| Bulk Product |
Supplier | Form | Concentration in Bulk Formulation |
Storage Conditions |
Special Handling | |||||
| Bulk Product formulated, prefiltered peg-uricase |
Savient through contract manufacturers |
0.22 micron filtered liquid |
8 mg/mL | 2-8°C | Prevent from freezing |
CONTAINER CLOSURE COMPONENTS:
| Description |
Enzon Part # |
Manufacturer/Part # | Height, min | OD, mm | ||||||
| 2 mL, 13 mm Vial |
530-101 | Alcan/2702-B9BA | 31.5-32.5 mm | 14.5-15.00 mm | ||||||
| 13 mm Stopper |
520-010 | West Pharmaceutical Services/1012-4668 |
STOPPER, 13MM, 4416/50, GREY, FEP |
|||||||
| Seal, 13 mm, FO, AL Purple #0527 |
510-002 | 54130024 |
| Packaging Components | ||||
| Description |
Enzon Part # | Supplier Part # | ||
| Carton, Puricase USA |
C49011USA | C49011USA | ||
| Carton Label, Puricase USA |
CL49011USA | CL49011USA | ||
| ▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇ |
▇▇▇▇▇▇▇▇▇ | H49011USA | ||
| Package Insert, Puricase USA |
I49011USA | I49011USA | ||
| Packer, Puricase USA |
P49011USA | P49011USA | ||
| ▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇ | ▇▇▇▇▇▇▇▇▇ | V49011USA | ||
Commercial Supply Agreement
Execution Copy
Page 23 of 44
Commercial Supply Agreement
Execution Copy
Page 24 of 44
Commercial Supply Agreement
Execution Copy
Page 25 of 44
Exhibit B
Final Product Release Specifications
| Parameter |
Test Method | Specification | ||
| Endotoxin |
ACM-0073 | <80 EU/mL | ||
| Sterility |
ACM-0071 | Sterile | ||
| Particulates |
ACM-0070 (Light Obscuration method) |
Size >10 µm: <6000 particles per vial Size >25 µm: <600particles per vial |
In-Process Product Specifications
| Parameter |
Test Method | Specification | ||
| Bioburden (in-process only) |
ACM-0072 | <10 CFU/100 mL | ||
| Identity |
ACM-1900 | Positive for Urate Oxidase activity | ||
| Bulk Sterility |
ACM-007 | Sterile |
Commercial Supply Agreement
Execution Copy
Page 26 of 44
Exhibit C
Product Price
| Commercial Manufacturing |
Price | |
| Activitites Included
• Prepare Master Batch Records
• Materials to be packed with one vial and product insert per carton.
• Commercial Batch Prices are effective on 1 January 2008, and are effective through 31 December 2009.
• Enzon reserves the right to increase prices pursuant to the terms of the Supply Agreement.
• Release testing of batches; provision of CoA
• Supply temperature recorders to Drug Substance manufacturer for shipping. Download and provide temperature data to Savient. |
$20.80 per vial
The minimum price per batch of $218,400.00 shall apply in accordance with the terms of Section 6.2(e) of the Agreement. |
Puricase Final Drug Product Stability test Schedule at 5°C ±3°C
Endotoxin and Sterility At Each Pull Point
Price is per each lot tested
| Setup & initiation Fee |
Initial Test | 3 Months | 6 Months | 12 Months | 24 Months | 36 Months | Total Cost | |||||||||||||||||||||
| $1,900 |
$ | 5,150 | N/T | N/T | $ | 5,150 | $ | 5,150 | $ | 5,150 | $ | 22,500 | ||||||||||||||||
Puricase Final Drug Product Stability test Schedule at 25°C ±2°C and 70% ±5% Relative Humidity
Endotoxin and Sterility At Each Pull Point
Price is per each lot tested
| Setup & initiation Fee |
Initial Test | 1 Months | 2 Months | 3 Months | 6 Months | Total Cost | ||||||||||||||||||||||
| $1,900 |
$ | 5,150 | $ | 5,150 | $ | 5,150 | $ | 5,150 | $ | 22,500 | ||||||||||||||||||
Stability studies will be conducted on batches requested in advance by Savient. Prices will be in effect for stability studies initiated on 2008 or 2009 and subject to review at the end of 2009.
| Professional Services Fee Structure |
Price | |
| • One (1) man-hour |
$195.00
Enzon to update prices on or about January 1 of every year |
Terms: Purchase Orders are required for each scheduled batch.
| • | Invoice for vials produced will be sent upon shipment of materials. Payment due net 30 days. |
| • | Delivery terms are FCA Enzon’s manufacturing facility in Indianapolis, IN |
| • | Cancelled and postoponed batches shall be billed in accordance with Section 3.4(d). |
Commercial Supply Agreement
Execution Copy
Page 27 of 44
Exhibit D
Product Forecast
Savient has provided the following forecast for the 28 month period beginning October 2008.
(See following page.)
Commercial Supply Agreement
Execution Copy
Page 28 of 44


Exhibit E
Letter Agreement
(see attached)
Commercial Supply Agreement
Execution Copy
Page 30 of 44

| September 12, 2007 | Our Ref: pky/jmw |
Enzon Pharmaceuticals, Inc.
Attn: ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇▇ ▇▇▇/▇▇▇
▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇
| Re: | Second Amendment to Agreement for Services (“Agreement”) between Savient Pharmaceuticals, Inc. (“Savient”) and Enzon Pharmaceuticals, Inc. (“Enzon”) dated October 31, 2006 and as amended on June 15, 2007 |
Dear ▇▇. ▇▇▇▇▇▇:
Pursuant to Section 13,01 of the Agreement, Savient and Enzon hereby agree to amend the Agreement by repealing Section 4: Confidentiality and replacing it as follows:
“4.01: For the duration of the Agreement and five (5) years thereafter with respect to Savient Confidential Information (as defined below), or, in the case of MVP Confidential Information (as defined below), for twenty (20) years from the Effective Date of the Agreement, Enzon will not disclose, without Savient’s written permission, any such Savient Confidential Information or MVP Confidential Information, unless such disclosure: (i) is to an affiliate, agent, employee or consultant of Enzon that is under a similar obligation to keep such information confidential and such disclosure is reasonably necessary for the performance of the Services contemplated herein; (ii) is or becomes publicly available through no fault of Enzon; (iii) is disclosed by a third party entitled to disclose it; (iv) is already known to Enzon as shown by its prior written records; or, (v) is required by any law, rule, regulation, order decision, decree, subpoena or other legal process to be disclosed or in response to a request or order from a regulatory agency. If such disclosure is requested by a regulatory agency or legal process, Enzon will make all reasonable efforts to notify Savient of this request promptly prior to any disclosure to permit Savient to oppose such disclosure by appropriate legal action. Enzon shall use reasonable precautions to protect the confidentiality of both Savient Confidential Information and MVP Confidential Information in a manner that is comparable to precautions taken to protect its own proprietary information. As used herein, “MVP Confidential Information” means any Confidential Information that Savient provides, or has provided, to Enzon which is specifically identified in writing as containing Mountain View Pharmaceuticals, Inc.’s proprietary technology for the manufacture of PEGylated uricase
Cont.../…
(Puricase®/pegloticase), specifically including the documents referenced in the attached Schedule A, which the Parties have agreed to in a letter dated September 12, 2007, as containing Confidential Information belonging to Mountain View Pharmaceuticals, Inc; “Savient Confidential Information” means any Confidential Information provided by Savient to Enzon, with the sole exception of MVP Confidential Information, during the Term of the Agreement,
4.02 For the duration of the Agreement and five (5) years thereafter with respect to Savient Confidential Information, or in the case of MVP Confidential Information, for twenty (20) years from the Effective Date of the Agreement, Enzon will not use such Confidential Information except in connection with the performance of Services under the Agreement or any other Agreement between Savient and Enzon related to Savient’s PEGylated uricase (Puricase®/pegloticase) product and in particular represents and warrants that it will not utilize such Confidential Information in the manufacturing of any other product.
4.03 For twenty (20) years from the Effective Date of the Agreement, Savient will not disclose, without Enzon’s written permission, any Confidential Information belonging to Enzon which is provided to Savient by Enzon during the Term of the Agreement (“Enzon Confidential Information”) unless such disclosure: (i) is to an affiliate, agent, employee or consultant of Savient that is under a similar obligation to keep such information confidential; (ii) is or becomes publicly available through no fault of Savient; (iii) is disclosed by a third party entitled to disclose it; (iv) is already known to Savient as shown by its prior written records; or, (v) is required by any law, rule, regulation, order decision, decree, subpoena or other legal process to be disclosed or in response to a request or order from a regulatory agency. If such disclosure is requested by a regulatory agency or legal process, Savient will make all reasonable efforts to notify Enzon of this request promptly prior to any disclosure to permit Enzon to oppose such disclosure by appropriate legal action. Savient shall use reasonable precautions to protect the confidentiality of Enzon Confidential Information in a manner that is comparable to precautions taken to protect its own proprietary information.
| 4.04 | If either Party shall be obliged to provide testimony or records pertaining to the Confidential Information provided by the other in any legal or administrative proceeding, then the Party which supplied the Confidential Information shall reimburse the other Party for its out-of-pocket costs therefore plus an hourly fee for its employees or representatives equal to the internal fully burdened costs of such employee or representative. |
Cont.../…
2
4.05. For both Parties, “Confidential Information” shall mean and include, without limitation, such types of information as: inventions, methods, plans, processes, specifications, characteristics, raw data, analyses, equipment design, trade secrets, costs, marketing, sales, and product performance information, including patents and patent applications, grant applications, notes, and memoranda, whether in writing or presented, stored or maintained electronically, magnetically or by other means, which are disclosed by the disclosing Party to the recipient Party in writing or in other tangible form and marked “confidential” or, if disclosed orally (or in some other non-tangible form), are identified as confidential to the recipient Party in writing within sixty (60) days of such disclosure; provided, however, that failure to reduce any verbal disclosure to writing shall not, in and of itself, vitiate the confidential nature of such Confidential Information and provided, further, that for the purposes of this Agreement, Confidential Information shall include any and all such information exchanged between the Parties prior to the effective date of this Agreement pursuant to the Confidentiality Agreement between the Parties dated July 24, 2006.”
To signify your acceptance of this Amendment, kindly countersign and return one copy to my attention.
Should you have any questions, please do not hesitate to contact ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ at (▇▇▇) ▇▇▇-▇▇▇▇.
Very truly yours,
/s/ ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇
▇▇▇▇▇▇ ▇. Yechmetz
Executive Vice President
Chief Business Officer
I hereby agree to the terms and conditions contained herein.
Enzon Pharmaceuticals, Inc.
| By: | /s/ ▇▇▇▇▇ del Campo | |
| Name: | ▇▇▇▇▇ del ▇▇▇▇▇ | |
| Title: | EVP Technical Operations | |
| Date: | 9/17/07 |
3
SCHEDULE A
List of Confidential Documents identified pursuant to
Section 4.01 of this Agreement in the letter of September 12, 2007
| 1. | 01V398-3.pdf - Report on the validation of the kinetic turbidimetric method for measuring endotoxin. |
| 2. | 03V715-2.pdf - Report on the validation of the KT Endotoxin method using a plate reader |
| 3. | ▇▇-▇▇-▇▇▇.pdf SOP for the endotoxin method. |
| 4. | ▇▇-▇▇-▇▇▇ - UV Activity Assay. |
4
Exhibit F
QUALITY AGREEMENT
Quality Technical Agreement for:
PRODUCT: Puricase® (PEG-Uricase)
DOSAGE/FORM: 8 mg/ml per vial
This Quality Agreement shall be read in conjunction with a commercial Supply Agreement between ENZON and SAVIENT (“Supply Agreement”), dated as of October 16, 2008 and is incorporated into the Supply Agreement. Capitalized terms not defined herein shall have the respective meanings set forth in the Supply Agreement. The effective date of this Quality Agreement shall be the Effective Date of the Supply Agreement.
This Quality Agreement defines the duties of ENZON and SAVIENT for the contract pharmaceutical manufacture of Product. In particular this Quality Agreement clearly states who is responsible for the cGMP aspects of manufacturing and specifies the way in which the Party releasing Product for sale ensures that the Product complies with the approved Product Specifications (defined below) and the Marketing Authorizations (defined below).
This Quality Agreement takes the form of a detailed checklist of all the activities associated with pharmaceutical production, analysis, release, and distribution. Responsibility for each activity is assigned to either ENZON or SAVIENT in the appropriate box in the Delegation Responsibility Checklist which follows.
In order to provide better quality assurance, ENZON will perform the activities defined herein in accordance with its Standard Operating Procedures (defined below) to the extent that a Standard Operating Procedure is applicable to such activity.
This Agreement is subject to the terms of the Supply Agreement. A breach of this Quality Agreement shall be deemed a breach of the Supply Agreement. In the event of a conflict between this Quality Agreement and the Supply Agreement, the Supply Agreement shall control.
This Quality Agreement is intended to comply with the guidance and directives set forth in the current versions and effective amendments of (I) FDA Guidance for Industry, Cooperative Manufacturing Arrangements for Licensed Biologics, August 1999; (ii) 21 CFR 210 & 211 and applicable portions of 21 CFR 600 through 610; and (iii) European Commission Directive 91/356 down the principles and guidelines of good manufacturing practice for medicinal Products for human use. This will be made accessible to relevant regulatory authorities if required by them.
[signature page follows]
Commercial Supply Agreement
Execution Copy
Page 31 of 44
The Parties have caused their duly authorized representatives to executed this Quality Agreement, effective as of October 16, 2008.
| SAVIENT PHARMACEUTICALS, Inc. | ENZON PHARMACEUTICALS, Inc. | |||
| /s/ ▇▇▇▇▇▇ ▇▇▇▇ |
/s/ ▇▇▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ | |||
| Signature | Signature | |||
| ▇▇▇▇▇▇ ▇▇▇▇ |
▇▇▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ | |||
| Printed Name | Printed Name | |||
| SVP, QA, RA |
V.P. QOP | |||
| Title | Title | |||
| 10/16/08 |
10/21/08 | |||
| Date | Date | |||
Commercial Supply Agreement
Execution Copy
Page 32 of 44
For purposes of this Quality Agreement, the following definitions shall apply:
| A. | “FDA” shall mean the United States Food and Drug Administration, and any successor entity thereto. |
| B. | “Marketing Application” shall mean an application for Product marketing authorization which has not yet been approved by the FDA or other regulatory authority, including, without limitation, FDA New Drug Application, FDA Biologics License Application, and other similar marketing applications promulgated by regulatory authorities. |
| C. | “Marketing Authorizations” shall mean any approved application for Product marketing authorization, including, without limitation, FDA New Drug Application, FDA Biologics License Application, and other similar marketing authorizations promulgated by international regulatory authorities. |
| D. | “Process” or “Processing” shall mean the sterile compounding, filling, producing and/or packaging of the raw materials into Product in accordance with the Product Specifications and the terms and conditions set forth in the Supply Agreement and this Quality Agreement. |
| E. | “Product Specifications” shall mean the procedures, requirements, specifications, standards, quality control testing, other data and scope of Supply related to the Product, as set forth in the Project Plan and/or attached hereto. ENZON shall not release Product if these parameters are not met and investigation shows the non-complying parameter to be a valid test result. |
| F. | “Standard Operating Procedures” or “SOPS” shall mean the standard operating procedures in effect at ENZON which have been approved by ENZON Quality Assurance department and which are applicable to the Processing; provided that all Standard Operating Procedures applicable to the Processing or the Product shall also be approved by SAVIENT. |
| G. | “Bulk Product” shall mean the bulk solution of methoxy-polyethylene glycol (m-PEG) conjugate of uricase supplied by Savient to Enzon pursuant to this agreement. |
| H. | “Business Day” shall mean Monday through Friday excluding government holidays. |
| I. | “Component” shall mean all packaging materials utilized during manufacture, including all primary and secondary packaging materials. |
| J. | “Deviation” shall mean any planned or unplanned event or result that is different from the expected event or result defined in existing procedures or specifications. |
Commercial Supply Agreement
Execution Copy
Page 33 of 44
| K. | “Filled Product” shall mean in-process sterile Product that has been filled into its final primary package for further labeling and packaging. |
| L. | “Product” shall mean sterile Product in its final packaged and labeled form that is ready for disposition. |
| M. | “Manufacture” shall mean finished drug Product pooling, filling, packaging, and associated in-process and stability testing, as applicable. |
| N. | “Master Production Control Record (MPCR)” shall mean a master document that represents a detailed procedure and data record for the batch manufacturing process, pursuant to CFR 21 §211.186. |
| O. | “Out of Specification (OOS)” shall mean any in-process, intermediate, or finished Product test result that is outside of acceptable ranges defined in approved specifications or analytical test methods. |
| P. | “cGMPs’ shall mean current good manufacturing practices as promulgated by the FDA under the United States Food, Drug, and Cosmetic Act, 21 C.F.R. Part 210 et seq., as amended from time to time, and the European Union. |
The following Facilities shall be used by ENZON for Processing or provision of Supply (“Facilities”):
| Manufacturing, Packaging, Testing and Storage |
| ▇▇▇▇ ▇▇▇▇▇ ▇▇. ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ ▇▇▇ |
Section 16. RESPONSIBILITY DELEGATION CHECKLIST
| RESPONSIBILITIES |
SAVIENT | ENZON | ||||
| 1. | Regulatory Authorizations & cGMP Compliance | |||||
| 1.1 | Maintain all licenses, registrations and other authorizations as are required to operate a cGMP pharmaceutical manufacturing facility under the Applicable Laws. | X | ||||
| 1.2 | Maintain and operate the Facility in compliance with the cGMPs, Applicable Laws and all other Product-specific instructions and requirements agreed to by the Parties. | X | ||||
Commercial Supply Agreement
Execution Copy
Page 34 of 44
| RESPONSIBILITIES |
SAVIENT | ENZON | ||||
| 1.3 | Process the Product in accordance with the cGMPs, Applicable Laws and all other Product-specific instructions and requirements agreed to by the Parties. | X | ||||
| 1.4 | Prepare, maintain and update the Marketing Authorizations in accordance with cGMPs, Applicable Laws and all other Product-specific instructions and requirements agreed to by the Parties. | X | ||||
| 1.5 | Provide ENZON with copies of those portions of the Marketing Applications which are applicable to the Processing, prior to submission of such Marketing Applications to the applicable regulatory authorities. | X | ||||
| 1.6 | Provide ENZON with copies of updates of those portions of the Marketing Authorizations which are applicable to the Processing, prior to submission of such Marketing Applications to the applicable regulatory authorities. | X | ||||
| 1.7 | Satisfy all drug listing filing requirements for all Product and packaging configurations processed at the Facilities. | X | ||||
| 1.8 | Prepare and submit post-marketing annual reports to the FDA and other applicable regulatory authorities in accordance with cGMPs, Applicable Laws and all other Product-specific instructions and requirements agreed to by the Parties. | X | ||||
| 1.9 | Provide SAVIENT within 30 business days of their request with the following information to be included in the post-marketing annual reports:
• Change control information for all changes implemented during the preceding year relating to the Product.
• Applicable Product test data submitted in accordance with the requirements of the Supply Agreement, including any non-conforming data. |
X | ||||
| 1.10 | Conduct Annual Product Quality Review for the Product in accordance with cGMP’s, and Applicable Laws. | X | ||||
| 1.11 | Provide SAVIENT with the following information to be included in the Annual Product Quality Review:
• All requested data and information required per 21 CFR 211.180(e) |
X | ||||
Commercial Supply Agreement
Execution Copy
Page 35 of 44
| RESPONSIBILITIES |
SAVIENT | ENZON | ||||
| 2. | Regulatory Actions & Inspections | |||||
| 2.1 | Promptly (within 24 hours of receiving notice) notify SAVIENT of any FDA or other regulatory authority (a) notice of inspection or inspection of the Facilities directly relating to the Product, or (b) inspection or investigation relating to the Product; and promptly (within 3 days) notify SAVIENT of any regulatory authority request for Product samples or Product batch records. | X | ||||
| 2.2 | Promptly (within 24 hours of receiving notice) notify ENZON of any FDA or other regulatory authority inspection or investigation relating to the Product; and promptly (within 3 days) notify ENZON of any regulatory authority request for Product samples or Product batch records. | X | ||||
| 2.3 | Provide SAVIENT copies of any FDA Form 483’s, warning letters or the like from applicable regulatory authorities within 30 days of receipt and copies of all subsequent response(s) relating to the Product or Quality Systems. | X | ||||
| 2.4 | Approve inspection responses to observations relevant to Product. | X | ||||
| 2.5 | Other than a request(s) delivered in conjunction with an inspection, notify the other Party of any requests for information, notices of violations or other communications from a regulatory authority relating directly to the Product produced at the Facility. | X | ||||
| 3. | Specifications & Change Control | |||||
| 3.1 | Approve Product Specifications. | X | ||||
| 3.2 | Assume primary responsibility for ensuring that all Specifications (including Product Specifications) and batch records that specifically relate to the manufacture and release of Product comply with relevant portions of the Marketing Applications and Marketing Authorizations, as amended from time to time. | X | ||||
Commercial Supply Agreement
Execution Copy
Page 36 of 44
| 3.3 | Assume secondary responsibility for ensuring that all Specifications (including Product Specifications) and batch records that specifically relate to the manufacture and release of Product comply with relevant portions of the Marketing Applications and Marketing Authorizations, as amended from time to time. | X | ||||
| RESPONSIBILITIES | SAVIENT | ENZON | ||||
| 3.4 | Submit any proposed changes to the Specifications to SAVIENT for review and approval, prior to the implementation of such changes by ENZON. | X | ||||
| 3.5 | Submit any proposed changes to the Specifications to ENZON for review and comment, prior to the submission of any such changes by SAVIENT to the regulatory authorities. | X | ||||
| 3.6 | Discuss and reach agreement with SAVIENT regarding any proposed changes to the Facilities or the Processing that may impact the Product, prior to implementation of such proposed changes. | X | ||||
| 3.7 | Serve as sole communicator with regulatory authorities for the approval and any revisions of Product Specifications in the Market Applications and Marketing Authorizations. | X | ||||
| 4. | Materials | |||||
| 4.1 | Maintain Bulk Product according to cGMPs and Appplicable Laws | X | ||||
| 4.2 | Retain reference samples of Bulk Product, including samples for periodic re-tests, for 6 years beyond Product expiry date. | X | ||||
| 4.3 | Provide Bulk Product meeting the Specifications and cGMPs for manufacture, as well as a certificate of analysis for Bulk Product. | X | ||||
| 4.4 | Perform identification testing of Bulk Product. | X | ||||
| 4.5 | Source and qualify raw materials (excluding Bulk Product) used in Processing. | X | ||||
| 4.6 | Maintain Specifications for Components and procure, store, sample, test and release raw materials. | X | ||||
| 4.7 | Audit suppliers that provide Components and Process Consumables used in Processing in accordance with applicable SOPs to ensure full compliance with cGMPs and Applicable Laws. | X | ||||
Commercial Supply Agreement
Execution Copy
Page 37 of 44
| 4.8 | Store Bulk Product and Components in accordance with the Specifications, SOPs, cGMPs and Applicable Laws while at the Facilities. | X | ||||
| RESPONSIBILITIES | SAVIENT | ENZON | ||||
| 4.9 | Retain reference samples of raw materials in a quantity sufficient to perform periodic re-tests, for 1 year beyond Product expiry date in accordance with Specifications, SOPs, cGMPs and Applicable Laws. | X | ||||
| 4.10 | Notify SAVIENT of intent to dispose of material retains. | X | ||||
| 4.11 | At SAVIENT’s option, ship material retains to SAVIENT (at SAVIENT’s expense) or destroy. | X | ||||
| 4.12 | Dispose of Product waste and any special waste related to the Processing of the Product in accordance with Applicable Laws. | X | ||||
| 5. | Production, Investigations & Validations | |||||
| 5.1 | Provide personnel with appropriate education, training and/or experience for manufacturing, testing and disposition of Product that is suitable for human use, and for provision of Supply hereunder. | X | ||||
| 5.2 | Provide premises that are maintained and able to meet design and cleanliness requirements in accordance with Applicable Laws and industry standards. | X | ||||
| 5.3 | Test and maintain utilities and environment to the appropriate compendia or environmental standard to assure appropriateness for use in connection with Processing and the Product. | X | ||||
| 5.4 | Maintain, qualify and validate the Facility, equipment, utilities (air and water) and processes associated with Processing the Product in accordance with Applicable Laws and industry standards. | X | ||||
| 5.5 | Manufacture and test the Product at the Facilities in accordance with the Product master Production control record, the SOPs referenced therein, and the Specifications. | X | ||||
| 5.6 | Perform visual inspection of finished Product in accordance with the Product master Production control record, the SOPs referenced therein, and the Specifications. | X | ||||
| 5.7 | Label Product in accordance with the Product master Production control record, the SOPs referenced therein, and the Specifications. | X | ||||
Commercial Supply Agreement
Execution Copy
Page 38 of 44
| 5.8 | Prepare and approve all artwork, inserts, labeling and packaging in connection with the Product. | X | ||||
| 5.9 | Package the Product in accordance with the Product master Production control record, the SOPs referenced therein, and the Specifications. | X | ||||
| RESPONSIBILITIES | SAVIENT | ENZON | ||||
| 5.10 | Perform finished Product testing in accordance with the supply agreement and supply a certificate of analysis and a Certificate of Compliance to SAVIENT. | X | ||||
| 5.11 | Final release Product in accordance with the Product Specifications. | X | ||||
| 5.12 | Investigate, resolve and document deviations from the Master Production Control Record and OOS test results in accordance with the cGMPs. Investigations should be completed with 30 calendar days. Interim status reports must be provided to Savient periodically in writing for investigations remaining open beyond 30 business days. | X | ||||
| 5.13 | Obtain Quality Assurance approval of all investigations and corrective and preventive action plans. | X | ||||
| 5.14 | Provide equipment maintained and able to meet design and cleanliness requirements in accordance with Applicable Laws and industry standards, as applicable. | X | ||||
| 5.15 | Establish a validation master plan and maintain the validation program in accordance with plan requirements. | X | ||||
| 5.16 | Prepare and execute all Product related validation protocols, and complete validation reports. | X | ||||
| 5.17 | Review and approve validation protocols related to Product. | X | ||||
| 5.18 | Provide Quality Assurance review and approval of all validation packages. | X | ||||
| 6. | Audits | |||||
| 6.1 | SAVIENT will schedule and audit ENZON Facilities, records and documentation related to the Product manufactured by ENZON at a time mutually agreed by Enzon with a minimum advanced notice of 3 months and at a frequency of not more than once every 12 months. SAVIENT may request for-cause audits as needed. | X | ||||
| 6.2 | Conduct internal audits of Facilities, processes and quality systems, in accordance with cGMPs and applicable SOPs. | X | ||||
Commercial Supply Agreement
Execution Copy
Page 39 of 44
| 6.3 | SAVIENT shall provide ENZON with a written audit report containing audit observations within 30 business days of the audit. | X | ||||
| 6.4 | ENZON will respond to Savient audit report in writing within 15 business days. | X | ||||
| 6.5 | SAVIENT is entitled to inspect and audit suppliers, vendors and contractors used by ENZON in connection with the Product. | X | ||||
| 7. | Lot Codes & Expiration Dating | |||||
| 7.1 | Establish Product lot code. | X | ||||
| 7.2 | Establish Product expiry dating as per approved Product License/Marketing Authorization. | X | ||||
| 8. | Samples | |||||
| 8.1 | Perform Product sampling in accordance with the Supply Agreement, cGMP’s, and as otherwise agreed to by the Parties in the master Production control record for the Product. | X | ||||
| 8.2 | Retain Finished Product samples including Stability samples in accordance with cGMP’s and the Supply Agreement. | X | ||||
| 9. | Testing & Analysis | |||||
| 9.1 | Perform all applicable Product testing according to the Supply Agreement. | X | X | |||
| 9.2 | Track and investigate all deviations (including DOS’s) associated with the Product, and notify SAVIENT Quality and Manufacturing within 24 hours of discovery of any significant deviations (those that may affect the identity, strength, quality, or purity of the Product). | X | ||||
| 9.3 | Notify ENZON of any Product recall that might be attributed to Processing the Product. | X | ||||
| 9.4 | Notify SAVIENT Quality and Manufacturing via email within the business day followed by signed documents of any confirmed failure of the Product that might be attributed to Processing the Product. | X | ||||
| 10. | Release | |||||
| 10.1 | Provide initial QA dispostion of Product to SAVIENT. | X | ||||
| 10.2 | Provide final QA dispostion of Product. | X | ||||
| 11. | Records | |||||
Commercial Supply Agreement
Execution Copy
Page 40 of 44
| 11.1 | Review and approve the executed batch records. | X | X | |||
| RESPONSIBILITIES | SAVIENT | ENZON | ||||
| 11.2 | Provide the released, executed batch record documentation for each batch of Product, which shall include the following:
• A statement that the lot was manufactured, packaged and tested in accordance with cGMPs, identifies the master batch Record documents, and lists any incident reports and investigations associated with the batch.
• A certificate of analysis covering all regulatory authority and compendial tests, and a Certificate of Compliance.
• The signature of the QA Representative who released the batch.
• Copies of significant deviations (those that may materially affect the identify, strength, quality or purity of the Product).
• A list of other deviations that may affect the Product.
• A list of change control records that could impact the Product.
• Copies of summary Quality Assurance reviewed release test records. |
X | ||||
| 11.3 | Store the master record, batch records, manufacturing documentation and all other documentation related to the Product for the minimum period required by all Applicable Laws. | X | ||||
| 11.4 | Provide copies of all documentation necessary for SAVIENT to respond to inquiries by regulatory authorities. | X | ||||
| 12. | Storage | |||||
| 12.1 | Store and ship the Bulk Product in accordance with the Bulk Product Specifications, SOPs, cGMPs and Applicable Laws until manufacture of the Product. | X | ||||
| 12.2 | Receive and store the Bulk Product. Intermediates, and finished Product in accordance with the Specifications, SOPs, cGMPs and Applicable Laws pending release of the Product. | X | ||||
| 12.3 | Provide written instructions for shipping prior to Product release and shipment. | X | ||||
| 13. | Safety | |||||
| 13.1 | Maintain safety/hazard and handling data on the Product and Bulk Product. | X | X | |||
Commercial Supply Agreement
Execution Copy
Page 41 of 44
| RESPONSIBILITIES | SAVIENT | ENZON | ||||
| 13.2 | Maintain safety/hazard and handling data on the raw materials. | X | X | |||
| 14. | Complaints | |||||
| 14.1 | Upon request of SAVIENT, assist SAVIENT in investigating and resolving all medical, adverse events, and non-medical Product complaints. | X | ||||
| 14.2 | Collect and log all information relating to Product complaints and adverse drug events. | X | X | |||
| 14.3 | Investigate all Product complaints and adverse drug events. | X | X | |||
| 14.4 | Issue all reports and conduct follow up corrective action relating to Product complaints and adverse drug events. | X | X | |||
| 15. | Recall, Field Alerts and Product Withdrawal | |||||
| 15.1 | Inform the Quality Assurance contact from the other Party within 24 hours upon knowledge of all quality issues which might compromise the other Party’s quality requirements for Products already shipped, or about to be shipped. | X | X | |||
| 15.2 | Issue any decision to initiate Product recall or Product withdrawal. | X | ||||
| 15.3 | Communicate decision to initiate Product recall to ENZON. | X | ||||
| 15.4 | Notify appropriate regulatory authorities of any Product recall or Product withdrawal. | X | ||||
| 15.5 | Manage any Product recall or Product withdrawal. | X | ||||
| 15.6 | Reconcile returned Product following Product recall or Product withdrawal. | X | X | |||
| 15.7 | Issue and follow up on FDA Field Alerts (or other similar processes of other regulatory authorities). | X | ||||
| 15.8 | Perform mock recall and recall effectiveness checks. | X | ||||
| 16 | Quality Agreement Review | |||||
| 16.1 | On an as-needed basis (or once every two years), conduct a review to ensure that the Quality Agreement is in alignment with the current scope of the Project Plan and the then- current Supply Agreement. Update by mutual agreement of the Parties (if necessary). | X | X | |||
| 17 | Key Contacts | |||||
Commercial Supply Agreement
Execution Copy
Page 42 of 44
| 17.1 | Either party may change the following contact information by issuing a memo to the other party. Each party shall attach the memo to this original signed agreement. The updated information shall be incorporated into the next controlled revision of this agreement. | X | X |
Savient Pharmaceuticals Inc.
For All Product Concerns:
Savient Pharmaceutical’s Inc.
▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇ ▇▇▇▇▇
▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇
Enzon Pharmaceuticals, Inc.
For Manufacturing, Quality Assurance and Quality Control:
Enzon Pharmaceuticals, Inc.
▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇
▇▇▇
| For Regulatory Affairs: |
For Operations, Planning & Logistics: | |
| Enzon Pharmaceuticals, Inc. |
Enzon Pharmaceuticals, Inc. | |
| ▇▇▇ ▇▇▇▇▇ 202/206 |
▇▇▇ ▇▇▇▇▇ ▇▇▇/▇▇▇ | |
| ▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ |
▇▇▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇ | |
| ▇▇▇ |
▇▇▇ | |
| Enzon Contact |
Name/Title | Phone | Fax | |||||
| Quality Assurance |
▇▇▇▇▇ ▇▇▇▇▇▇, Vice President Quality Operations |
▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇.▇▇▇▇▇▇@▇▇▇▇▇.▇▇▇ | ||||
| QA – Product Release |
▇▇▇▇▇▇▇ ▇’▇▇▇▇▇▇▇▇▇▇▇, Associate Director, QA |
▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇▇.▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇.▇▇▇ | ||||
| Quality Control Lab |
▇▇▇▇▇▇ ▇▇▇▇, Director, Quality |
▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇.▇▇▇▇@▇▇▇▇▇.▇▇▇ |
Commercial Supply Agreement
Execution Copy
Page 43 of 44
| Operations |
Control | |||||||||||||
| Regulatory Affairs |
▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇, Vice President, Regulatory Affairs | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇.▇▇▇▇▇▇▇▇@▇▇▇▇▇.▇▇▇ | ||||||||||
| Operations – Planning |
▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, Director, Planning & Logistics | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇.▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇.▇▇▇ |
| Savient Contact |
Name/Title |
Phone | Fax | |||||
| Quality Assurance |
▇▇▇▇▇▇ ▇▇▇▇, Ph.D Senior Vice President, QA | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ | ||||
| Quality Assurance |
▇▇▇▇ ▇▇▇▇▇▇▇▇▇ Senior Director, QA | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ | ||||
| QA — Product Release |
▇▇▇▇▇▇▇ ▇▇▇▇▇ Manager, QA | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ | ||||
| Regulatory Affairs |
▇▇▇▇▇ ▇▇▇▇▇▇ Vice President Regulatory Affairs | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ | ||||
| Manufacturing |
▇▇▇▇▇ ▇▇▇▇▇▇, Ph.D Vice President, Technical Operations | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ | ||||
| Planning and Logistics |
▇▇▇▇ ▇▇▇▇▇▇ Manager, Manufacturing and Logistics | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇-▇▇▇-▇▇▇▇ | ▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ |
Commercial Supply Agreement
Execution Copy
Page 44 of 44

