CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. TERMINATION AND TRANSITION AGREEMENT
Exhibit 10.31
EXECUTION COPY
CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED.
TERMINATION AND TRANSITION AGREEMENT
THIS TERMINATION AND TRANSITION AGREEMENT (“Termination Agreement”) dated as of November 8, 2012 (“Termination Effective Date”), is entered into between XenoPort, Inc., a Delaware corporation having its principal place of business at 0000 Xxxxxxx Xxxxxxxxxx, Xxxxx Xxxxx, XX 00000 (“XenoPort”), and Glaxo Group Limited, a company existing under the laws of England and Wales, having its registered office at Glaxo Xxxxxxxx Xxxxx, Xxxxxxxx Xxxxxx, Xxxxxxxxx, Xxxxxxxxx, XX0 0XX, Xxxxxxx (“GSK”).
BACKGROUND
A. XenoPort has developed the Product (as hereinafter defined), which is a Transported Prodrug™ of gabapentin, for the treatment of restless legs syndrome (“RLS”) and the management of neuropathic pain (which includes post-herpetic neuralgia (“PHN”) and diabetic peripheral neuropathy), and which is currently marketed in the United States under the brand name “Horizant®” for the treatment of moderate-to-severe primary RLS in adults and for the management of PHN in adults;
B. The Parties (as hereinafter defined) entered into that certain Development and Commercialization Agreement dated as of February 7, 2007, as amended by that certain First Amendment to Development and Commercialization Agreement between XenoPort and GSK, dated as of May 4, 2007 (“First Amendment”), and as further amended by that certain Second Amendment to Development and Commercialization Agreement between XenoPort and GSK, dated as of February 13, 2009 (“Second Amendment”) (together with the First Amendment and the Second Amendment, the “Original Agreement”), pursuant to which XenoPort granted to GSK certain rights for the Product worldwide except for certain countries in Asia, and pursuant to which GSK and XenoPort were to co-develop and, in certain cases, co-commercialize the Product in the United States, all in accordance with the Original Agreement;
C. The Original Agreement was subsequently superseded and replaced by that certain Amended and Restated Development and Commercialization Agreement between XenoPort and GSK, dated as of November 7, 2010 (“Restated Agreement”), pursuant to which the Parties amended and restated the Original Agreement in its entirety to provide, among other matters, the reversion to XenoPort of all rights for the development and commercialization of the Product in all countries other than the United States and the right to conduct and control activities relating to the development of the Product for certain indications in the United States;
D. The Parties, and GlaxoSmithKline LLC and GlaxoSmithKline Holdings (Americas) Inc. are currently parties to a litigation pertaining to GSK’s performance of the Restated Agreement, filed in the United States District Court for the Northern District of California (originally filed in Santa Xxxxx County Superior Court, Case No. 1-12-CV-21937), and the Parties are currently parties to a declaratory judgment action pertaining to GSK’s performance of the Restated Agreement
EXECUTION COPY
in the United States District Court for the District of Delaware and styled XenoPort, Inc. v. Glaxo Group Limited, et al., Case No. CV 12-01544 EJD and Glaxo Group Limited v. XenoPort, Inc. et al., Case No. 1:12-CV00225-GMS, respectively (collectively, the “Litigation”); and
E. The Parties deem it to be in their best interests and to their mutual advantage to settle and dismiss the Litigation and terminate the Restated Agreement in its entirety, on the terms and conditions set forth in this Termination Agreement and the Stock Purchase Agreement to be entered into between XenoPort and GSK dated as of the Termination Effective Date (the “SPA”), to provide, among other matters, the reversion to XenoPort of all rights with respect to the Product, and for GSK to purchase certain shares of Common Stock of XenoPort pursuant to the SPA.
NOW, THEREFORE, in consideration of all of the terms and conditions of this Termination Agreement, the Parties agree as follows:
ARTICLE I
DEFINITIONS
The following capitalized terms will have the meanings set forth in this Article I when used in this Termination Agreement.
1.1 “Accounts Receivable” shall mean all trade accounts receivable and other similar rights to payment to GSK or its Affiliates from customers of GSK or its Affiliates, including all trade accounts receivable representing amounts receivable by GSK or its Affiliates, in each case with respect to Product sold by or on behalf of GSK or its Affiliates on or prior to the Transition Period End Date.
1.2 “ADAP” shall have the meaning set forth in Section 5.5(h) of this Termination Agreement.
1.3 “Additional Transferred Asset” shall have the meaning set forth in Section 3.6(c) of this Termination Agreement.
1.4 “Additional Licensed Asset” shall have the meaning set forth in Section 3.6(c) of this Termination Agreement.
1.5 “Affiliate” of a Party shall mean any Person that, directly or indirectly through one or more intermediaries, controls, is controlled by or is under common control with such Party, as the case may be, for as long as such control exists. As used in this Section 1.5, “control” shall mean: (a) to possess, directly or indirectly, the power to direct the management and policies of such Person, whether through ownership of voting securities or by contract relating to voting rights or corporate governance; or (b) direct or indirect beneficial ownership of at least fifty percent (50%) (or such lesser percentage that is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) of the voting share capital in such Person.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 2 -
EXECUTION COPY
1.6 “AMP” shall have the meaning set forth in Section 5.5(a) of this Termination Agreement.
1.7 “Ancillary Agreements” shall have the meaning set forth in Section 3.5(c) of this Termination Agreement.
1.8 “API” shall mean the gabapentin enacarbil active pharmaceutical ingredient used in the manufacture of Product.
1.9 “Applicable Laws” shall mean the applicable provisions of any and all national, supranational, regional, state and local laws, treaties, statutes, rules, regulations, administrative codes, guidances, ordinances, judgments, decrees, directives, injunctions, orders, permits (including the NDA) of, from or agreed to with any court, arbitrator, Regulatory Authority or governmental agency or authority having jurisdiction over or related to the subject item. In addition, with respect to either Party, Applicable Laws shall include the obligations of any corporate integrity agreement entered into by such Party with the Office of Inspector General of the Department of Health and Human Services, during the term of such obligations.
1.10 “Artwork” shall have the meaning set forth in Section 4.5(e) of this Termination Agreement.
1.11 “Assignment and Assumption Agreement” shall mean the assignment and assumption agreement, a form of which is attached hereto as Exhibit 1.11.
1.12 “Assumed Liabilities” shall have the meaning set forth in Section 3.7(b) of this Termination Agreement.
1.13 “Auditor” shall have the meaning set forth in Section 5.11(c) of this Termination Agreement.
1.14 “Xxxx of Sale” shall mean the xxxx of sale, a form of which is attached hereto as Exhibit 1.14.
1.15 “Bulk Manufacturer” shall have the meaning set forth in Section 5.13 of this Termination Agreement.
1.16 “Bulk Product” shall mean tablets containing, as and if applicable, 300mg or 600mg of gabapentin enacarbil, bulk packaged into large-scale intermediate containers (e.g., fiber drums) for use in the manufacture of commercial Product.
1.17 “Business Day(s)” shall mean a day (other than a Saturday or Sunday) on which banks are open for business in the State of Delaware but excluding each day in the period between December 24 and January 1 (inclusive) in any year.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 3 -
EXECUTION COPY
1.18 “cGMPs” shall mean the then-current requirements for current good manufacturing practices set forth in the United States Code of Federal Regulations 21 CFR Parts 210 and 211, and all related rules, regulations, guidance and regulatory requirements, together with the ICH Guidelines applicable to the manufacture, storage, transportation and testing of pharmaceutical drug products.
1.19 “Chargeback Contracts” shall have the meaning set forth in Section 5.8(b) of this Termination Agreement.
1.20 “Chargeback Product Termination Date” shall have the meaning set forth in Section 5.8(b) of this Termination Agreement.
1.21 “Claims” shall mean any and all claims, actions, causes of action, demands, costs, grievances, duties, obligations, rights, counterclaims, debts, damages, losses, liabilities, judgments, and charges of whatever nature, whether known or unknown, including but not limited to, claims asserted in the Litigation or that could have been asserted in the Litigation.
1.22 “CMS” shall have the meaning set forth in Section 5.5(b) of this Termination Agreement.
1.23 “Commercialization Transition Plan” shall have the meaning set forth in Section 4.4(a) of this Termination Agreement.
1.24 “Commercially Reasonable Efforts” shall mean that level of efforts and resources consistent with the usual practice followed by a Party in the exercise of reasonable business discretion relating to other pharmaceutical products owned by it or to which it has exclusive rights, which is of similar market potential and at a similar stage in development or product life, taking into account issues of patent coverage, safety and efficacy, product profile, the competitiveness of the marketplace, the proprietary position of the compound or product, the regulatory structure involved, the profitability of the products (including, without limitation, pricing and reimbursement status achieved), and other relevant factors, including without limitation technical, legal, scientific, and/or medical factors.
1.25 “Completion” shall mean, with respect to the Low Dose Study, GSK’s delivery of the second draft of the clinical study report to XenoPort as set forth under Section 4.3(a)(i), and with respect to each Ongoing Study other than the Low Dose Study, GSK’s delivery to XenoPort of a copy of the manuscript with respect to such Ongoing Study.
1.26 “Compound” shall mean that certain compound, referred to as gabapentin enacarbil, the structure of which is set forth on Schedule 1.26, and all esters, hydrates, metabolites [… * …]salts, solvates, isomers and/or mixtures of isomers thereof.
1.27 “Confidential Information” shall have the meaning set forth in Section 8.1 of this Termination Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 4 -
EXECUTION COPY
1.28 “Contracts” shall mean any contract, agreement or instrument, including, without limitation, supply contracts, market research agreements, ad agency agreements, quality agreements, clinical trial agreements, licenses, sale orders, bids, understandings or commitments, customer agreements, managed care agreements, distributor agreements, agreements with healthcare professionals, subcontracts or conditional sales agreements.
1.29 “Control” (including any variations such as “Controlled” and “Controlling”), in the context of the tangible and intangible assets of a Party (including, without limitation, intellectual property rights), shall mean that such Party or its Affiliate owns or possesses rights sufficient to assign, or grant the applicable license under or to such tangible or intangible assets, as provided herein without violating the terms of an agreement with a Third Party.
1.30 “Coverage Gap Program” shall have the meaning set forth in Section 5.5(a) of this Termination Agreement.
1.31 “Data” shall mean any and all research data, pharmacology data, preclinical data, clinical data and/or all regulatory documentation, manufacturing data, quality data, information and submissions pertaining to, or made in association with, a Regulatory Filing for, a pharmaceutical product.
1.32 “DDR” shall have the meaning set forth in Section 5.5(b) of this Termination Agreement.
1.33 “Development Plan” shall have the meaning set forth in Section 4.3(a)(i) of this Termination Agreement.
1.34 “Dispute” shall have the meaning set forth in Section 10.1 of this Termination Agreement.
1.35 “Direct Customer Contracts” shall have the meaning set forth in Section 5.8(c) of this Termination Agreement.
1.36 “Distributor Invoice” shall have the meaning set forth in Section 5.8(b) of this Termination Agreement.
1.37 “Domain Name(s)” shall mean any Internet domain names, whether in the form of an address for use in electronic mail transfer, a Universal Resource Locator (URL), a file transfer protocol (FTP) location, or other form suitable for specifying the location of an electronic data file over a distributed computer network, together with all translations, adaptations, derivations and combinations thereof.
1.38 “Domain Name Assignment Agreement” shall mean the domain name assignment agreement, a form of which is attached hereto as Exhibit 1.38.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 5 -
EXECUTION COPY
1.39 “Equipment” shall have the meaning set forth in Section 5.13 of this Termination Agreement.
1.40 “Excluded Assets” shall have the meaning set forth in Section 3.4(c).
1.41 “Excluded GSK Contracts” shall mean, subject to Sections 3.6(b) and 4.2(a) below, (i) any GSK Contracts between GSK and its Affiliates (i.e., inter-company agreements); (ii) any GSK Contracts between GSK or its Affiliate and a Third Party that relate (but not solely) to Compound or Product; (iii) any GSK Contracts between GSK or its Affiliate and a Third Party that solely relate to the Compound or Product but cannot be assigned by GSK or its Affiliate without such Third Party’s prior consent; and (iv) any other GSK Contracts so identified by GSK. The Excluded GSK Contracts referenced in (ii), (iii) and (iv) are identified on Schedule 1.41.
1.42 “Excluded GSK Inventory” shall mean the Inventory identified on Schedule 1.42, which specifies certain quantities of API that will be excluded from the Inventory transferred to XenoPort under this Termination Agreement.
1.43 “Excluded GSK Know-How” shall mean (i) any internal policies, processes and procedures used by GSK or its Affiliates from time to time prior to or after the Termination Effective Date that relate to the discovery, development, commercialization, promotion, marketing, sale, pricing and/or distribution of a pharmaceutical product; (ii) any internal policies and procedures used by GSK or its Affiliates from time to time prior to or after the Termination Effective Date that relate to the manufacture of a pharmaceutical product; and (iii) other GSK Know-How as identified on Schedule 1.43.
1.44 “Expired GSK Contracts” shall mean all Contracts between GSK or its Affiliate and a Third Party, which are solely related to the development, manufacture, marketing or commercialization of Compound or Product in the Territory and which have expired or been terminated prior to the Transition Period End Date, but under which GSK or its Affiliates have post-termination rights or obligations.
1.45 “FDA” shall mean the U.S. Food and Drug Administration, or any successor entity thereto performing similar functions.
1.46 “Final Transferred GSK Inventory Quantities” shall have meaning set forth in Section 4.2(f)(i)(A).
1.47 “Financial Information” shall mean all financial information solely relating to Compound or Product in the Territory that is Controlled by GSK or its Affiliates during the Original and Restated Term and/or the Transition Period, as identified in Schedule 1.47.
1.48 “Finished Product” shall have the meaning set forth in Section 5.4(a) of this Termination Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 6 -
EXECUTION COPY
1.49 “Firm Order” shall have the meaning set forth in Section 4.5(a) of this Termination Agreement.
1.50 “First Amendment” shall have the meaning set forth in paragraph B in the preamble of this Termination Agreement.
1.51 “FSS” shall mean federal supply schedule.
1.52 “Governmental Price Reports” shall have the meaning set forth in Section 5.5(a) of this Termination Agreement.
1.53 “GSK” shall have the meaning set forth in the preamble of this Termination Agreement.
1.54 “GSK Contracts” shall mean all Contracts in effect as of the Transition Period End Date to which GSK or its Affiliate is a party and which are related to the development, manufacture, marketing or commercialization of Compound or Product in or for the Territory, including all supply arrangements (including all related quality contracts) for Compound or Product (or any raw and pack materials, supplies and packaging materials therefor) and all clinical trial arrangements (including for the Ongoing Studies) for Compound or Product.
1.55 “GSK Distribution Center” shall mean the distribution center owned by GSK and located in Richmond, Virginia.
1.56 “GSK Indemnitees” shall have the meaning set forth in Section 9.1 of this Termination Agreement.
1.57 “GSK Housemarks” shall have the meaning set forth in Section 4.2(i) of this Termination Agreement.
1.58 “GSK Housemarks License” shall have the meaning set forth in Section 4.2(i) of this Termination Agreement.
1.59 “GSK Know-How” shall mean all Know-How relating to Compound or Product (including Financial Information) that are Controlled by GSK or its Affiliates and that prior to (i) the Transition Period End Date or (ii) the Completion of the Ongoing Studies (but only with respect to such Know-How arising out of the conduct of the Ongoing Studies) or (iii) the Supply Term (but only with respect to such Know-How arising out of the conduct of the manufacture and supply of Product during the Supply Term), were used in the development, manufacture or commercialization of Compound or Product in or for the Territory.
1.60 “GSK Patents” shall mean all Patents Controlled by GSK or its Affiliates and that, prior to (i) the Transition Period End Date, or (ii) the Completion of the Ongoing Studies (but only with respect to such Patents arising out of the conduct of the Ongoing Studies) or (iii) the Supply Term (but only with respect to such Patents arising out of the conduct of the manufacture and supply of Product during the Supply Term), are used in the development, manufacture or commercialization of Compound or Product.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 7 -
EXECUTION COPY
1.61 “GSK Safety Agreement” shall have the meaning set forth in Section 4.9 of this Termination Agreement.
1.62 “GSK’s Applicable Policies and Procedures” shall mean the then current applicable provisions of the policies or procedures of GSK in effect from time to time.
1.63 “HCERA” shall have the meaning set forth in Section 5.5(a) of this Termination Agreement.
1.64 “Know-How” shall mean all scientific, medical, technical, marketing, regulatory, manufacturing and other information, including Data and inventions for which no patent application has been filed.
1.65 “Improvement(s)” shall mean any inventions made by or under authority of a Party during the Original and Restated Term or Transition Period, and with respect to GSK, after the Transition Period with respect to the Ongoing Studies or manufacture and supply of Product during the Supply Term, in connection with manufacture, development and/or commercialization of Product and that solely relates, or was invented in the course of activities primarily directed, to Compound or Product, or the manufacture, storage, transport, use or formulation thereof.
1.66 “Indemnitee” shall have the meaning set forth in Section 9.3 of this Termination Agreement.
1.67 “Indemnitor” shall have the meaning set forth in Section 9.3 of this Termination Agreement.
1.68 “Interim Supply Notice” shall have the meaning set forth in Section 4.5 of this Termination Agreement.
1.69 “Inventory” shall mean API and related starting/intermediate material (if any), in each case, Controlled by GSK or its Affiliates directly relating to the manufacture and supply of Compound or Product, including, without limitation, clinical supplies (to the extent such clinical supplies are transferable under Applicable Laws). An initial schedule of the Inventory as of the Termination Effective Date (excluding the Excluded Inventory) is attached hereto as Schedule 1.69; provided that a list of clinical supplies shall be included in the Schedule 1.69 within thirty (30) Business Days.
1.70 “Inventory Transfer Date” shall have the meaning set forth in Section 4.2(f)(ii) of this Termination Agreement.
1.71 “JAMS” shall have the meaning set forth in Section 10.2 of this Termination Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 8 -
EXECUTION COPY
1.72 “Labels and/or Labeling” shall mean all labels and other written, printed or graphic matter on the Product or any container, carton or wrapper or other packaging utilized with the Product, or any written material accompanying the Product, including, without limitation, packaging inserts or outserts or medical information guides.
1.73 “Liabilities” shall have the meaning set forth in Section 9.1 of this Termination Agreement.
1.74 “Licensed Assets” shall have the meaning set forth in Section 3.2(a) of this Termination Agreement.
1.75 “Licensed GSK Know-How” shall mean all GSK Know-How other than the Transferred GSK Know-How and the Excluded GSK Know-How. Licensed GSK Know-How shall include any Additional Transferred Asset that is identified as Transferred GSK Know-How after the Termination Effective Date pursuant to Section 3.6 below before such Additional Transferred Asset is assigned and transferred from GSK to XenoPort pursuant to Section 3.6 below.
1.76 “Licensed GSK Patents” shall mean all GSK Patents other than the Transferred GSK Patents that claim an Improvement. The Licensed GSK Patents are identified on Schedule 1.76.
1.77 “Limited API” shall have the meaning set forth in Section 4.2(f)(vii) of this Termination Agreement.
1.78 “Litigation” shall have the meaning set forth in paragraph D in the preamble of this Termination Agreement.
1.79 “Low Dose Study” shall have the meaning set forth in Section 4.3(a)(i) of this Termination Agreement.
1.80 “Manufacturing Technology Transfer Plan” shall have the meaning set forth in Section 4.6 of this Termination Agreement.
1.81 “Material Adverse Effect” shall mean a [… * …] materially adverse effect on [… * …] but shall exclude any [… * …]
1.82 “MDRP” shall mean the Medicaid Drug Rebate Program.
1.83 “NDA” shall mean a New Drug Application or supplemental New Drug Application as defined in Title 21 of the U.S. Code of Federal Regulations, Section 314.50, et seq., which is filed with the FDA in order to gain the FDA’s approval to commercialize a pharmaceutical product in the United States for the indications set forth in the New Drug Application or supplemental New Drug Application.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 9 -
EXECUTION COPY
1.84 “NDC” shall mean the National Drug Code, as such term is used in the United States Federal Food, Drug and Cosmetic Act, as amended from time to time, including all regulations promulgated thereunder.
1.85 “New Safety Agreement” shall have the meaning set forth in Section 4.9 of this Termination Agreement.
1.86 “Non-Assignable Contract” shall have the meaning set forth in Section 4.2(a)(ii) of this Termination Agreement.
1.87 “Non-Assigned Contract” shall have the meaning set forth in Section 4.2(a)(iii) of this Termination Agreement.
1.88 “Non-Transferred GSK Know-How” shall have the meaning set forth in Section 4.2(c) of this Termination Agreement.
1.89 “On-Going Stability Studies” shall have the meaning set forth in Section 5.14(a) of this Termination Agreement.
1.90 “Ongoing Studies” shall mean those studies identified on Schedule 1.90, which includes the Low Dose Study.
1.91 “Original Agreement” shall have the meaning set forth in paragraph B in the preamble of this Termination Agreement.
1.92 “Party” shall mean XenoPort or GSK individually, and “Parties” shall mean XenoPort and GSK collectively.
1.93 “Patent(s)” shall mean any patented and unpatented inventions (including, inventions in patent applications for which claims have been filed and inventions in patent applications for which no claims have been filed; whether patentable or unpatentable and whether or not reduced to practice) and any patents and patent applications, together with all additions, divisions, continuations, continuations-in-part, substitutions, reissues, re-examinations, extensions, registrations, patent term extensions, supplemental protection certificates, renewals and foreign counterparts of any of the foregoing.
1.94 “Patent Assignment Agreement” shall mean the patent assignment agreement, a form of which is attached hereto as Exhibit 1.94.
1.95 “Permitted GSK Publications” shall have the meaning in Section 4.3(c) of this Termination Agreement.
1.96 “Person” shall mean any individual, corporation, partnership, firm, association, joint venture, joint stock company, trust or other entity, or any government or regulatory administrative or political subdivision or agency, department or instrumentality thereof.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 10 -
EXECUTION COPY
1.97 “PHN” shall have the meaning set forth in paragraph A in the preamble of this Termination Agreement.
1.98 “PHS” shall mean the U.S. Public Health Service.
1.99 “PPACA” shall have the meaning set forth in Section 5.5(a) of this Termination Agreement.
1.100 “Product” shall mean any pharmaceutical product containing a Compound, alone or in combination with one or more other active pharmaceutical ingredients, in any dosage form or formulation, including, as and if applicable, the 300 mg and 600 mg extended-release tablet formulations.
1.101 “Product Event” shall have the meaning set forth in Section 4.4(c) of this Termination Agreement.
1.102 “Promotional Material(s)” shall mean all written, printed, video or graphic advertising, promotional, educational and communication materials (other than the labeling for the Product) used solely for the marketing, advertising, promotion and sale of the Product in the Territory.
1.103 “Promotional Sample Inventory” shall mean the Product packaged as samples for use in the promotion of the Product in the Territory and maintained at the GSK Distribution Center, which will be identified on Schedule 1.103 as of the Transition Period End Date.
1.104 “Quality Agreement” shall have the meaning set forth in Section 4.4(d) of this Termination Agreement.
1.105 “Rebate Contracts” shall have the meaning set forth in Section 5.8(a) of this Termination Agreement.
1.106 “Regulatory Authority” shall mean the FDA, or any court, administrative agency or commission or other federal, state, or local governmental authority, body or other instrumentality with authority over the development, manufacture or commercialization of Compound or Product in the Territory, or any such instrumentality with similar authority in any other jurisdiction.
1.107 “Regulatory Filing(s)” shall mean any approvals, licenses, registrations, submissions, authorizations and related documentation and correspondence made to or received from a Regulatory Authority in a country necessary for the development, manufacture or commercialization of a pharmaceutical product.
1.108 “Released Matters” shall have the meaning set forth in Section 2.2 of this Termination Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 11 -
EXECUTION COPY
1.109 “Restated Agreement” shall have the meaning set forth in paragraph C in the preamble of this Termination Agreement.
1.110 “Retained Inventory Quantities” shall have the meaning set forth in Section 4.2(f)(i)(B).
1.111 “Retained Liabilities” shall have the meaning set forth in Section 3.7(a) of this Termination Agreement.
1.112 “RLS” shall have the meaning set forth in paragraph A in the preamble of this Termination Agreement.
1.113 “RLS Horizant Approval Letter” shall have the meaning set forth in Section 4.3(a)(i) of this Termination Agreement.
1.114 “Rules” shall have the meaning set forth in Section 10.3 of this Termination Agreement.
1.115 “Safety Data Exchange Transfer Plan” shall have the meaning set forth in Section 4.9 of this Termination Agreement.
1.116 “Second Amendment” shall have the meaning set forth in paragraph B in the preamble of this Termination Agreement.
1.117 “Social Security Act” shall have the meaning set forth in Section 5.5(a) of this Termination Agreement.
1.118 “SPA” shall have the meaning set forth in paragraph E in the preamble of this Termination Agreement. A form of the SPA is attached hereto as Exhibit 1.118.
1.119 “SPAP” shall have the meaning set forth in Section 5.5(h) of this Termination Agreement.
1.120 “Specifications” shall mean the specifications for Product set forth on Schedule 1.120.
1.121 “Stability Program Transfer” shall have the meaning set forth in Section 5.14 of this Termination Agreement.
1.122 “Supply Agreement” shall have the meaning set forth in Section 4.5 of this Termination Agreement.
1.123 “Supply Term” shall have the meaning set forth in Section 4.5 of this Termination Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 12 -
EXECUTION COPY
1.124 “Termination Agreement” shall have the meaning set forth in the preamble of this Termination Agreement.
1.125 “Termination Effective Date” shall have the meaning set forth in the preamble of this Termination Agreement.
1.126 “Territory” shall mean the United States.
1.127 “Third Party” shall mean any Person other than XenoPort, GSK and their respective Affiliates.
1.128 “Third Party Claim” shall have the meaning set forth in Section 9.1 of this Termination Agreement.
1.129 “Tolled API” shall have the meaning set forth in Section 4.2(h)(i) of this Termination Agreement.
1.130 “Trademark(s)” shall mean any registered and unregistered trademarks, trade names, service marks, service names, trade dress, logos, and slogans, whether registered or unregistered, and the goodwill associated therewith, together with any registrations and applications for registration thereof, and intellectual property rights residing in the foregoing, including copyrights and design rights.
1.131 “Trademark Assignment Agreement” shall mean the trademark assignment agreement, a form of which is attached hereto as Exhibit 1.131.
1.132 “Transfer Taxes” shall have the meaning set forth in Section 3.4(d) of this Termination Agreement.
1.133 “Transferred Assets” shall have the meaning set forth in Section 3.4(a) of this Termination Agreement.
1.134 “Transferred Contract Recovery Rights” shall have the meaning set forth in Section 3.7(a) of this Termination Agreement.
1.135 “Transferred GSK Contracts” shall mean all GSK Contracts, other than the Excluded GSK Contracts and Expired GSK Contracts, that are solely related to Compound or Product in or for the Territory and can be assigned by GSK or its Affiliate without a Third Party’s prior consent (or for which such consent to assignment from the applicable counterparty has been obtained), subject to Section 3.6(b), as identified on Schedule 1.135.
1.136 “Transferred GSK Domain Names” shall mean the Domain Names Controlled by GSK or its Affiliates as of the Termination Effective Date that relate to or are used with Compound and Product or related to RLS or PHN (but excluding any GSK Housemarks), as identified on Schedule 1.136.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 13 -
EXECUTION COPY
1.137 “Transferred GSK Inventory” shall mean the Inventory existing as of the Inventory Transfer Date (excluding Excluded GSK Inventory), which will be identified on Schedule 1.137 as of the Inventory Transfer Date as provided in Section 4.2(f)(i)(A).
1.138 “Transferred GSK Know-How” shall mean all GSK Know-How, other than the Excluded GSK Know-How, solely related to the Compound or Product in or for the Territory, as identified on Schedule 1.138.
1.139 “Transferred GSK Patents” shall mean all GSK Patents primarily related to the manufacture, use and/or sale of Compound, Product or Improvements Controlled by GSK or its Affiliates, as identified on Schedule 1.139.
1.140 “Transferred GSK Promotional Materials” shall mean all Promotional Materials Controlled by GSK or its Affiliate in the Territory, as identified on Schedule 1.140. For the avoidance of doubt, the Transferred GSK Promotional Materials shall not include or be deemed to include any rights in or to the GSK Housemarks.
1.141 “Transferred GSK Regulatory Filings” shall mean all Regulatory Filings solely related to the Compound or Product in the Territory Controlled by GSK or its Affiliates, as identified on Schedule 1.141.
1.142 “Transferred GSK Trademarks” shall mean all Trademarks Controlled by GSK or its Affiliates that relate to or are or were used with Compound or Product, prior to the Transition Period End Date (but excluding any rights in and to the Transferred GSK Promotional Materials), as identified on Schedule 1.142.
1.143 “Transition Period” shall mean the period commencing on the Termination Effective Date and ending on the Transition Period End Date.
1.144 “Transition Period End Date” shall have the meaning set forth in Section 4.1 of this Termination Agreement.
1.145 “Transfer Taxes” shall have the meaning set forth in Section 3.4(d) of this Termination Agreement.
1.146 “Transition Team” shall have the meaning set forth in Section 4.7 of the Termination Agreement.
1.147 [… * …]
1.148 “United States” or “U.S.” shall mean the fifty (50) states of the United States of America and the District of Columbia and the territories of the United States of America.
1.149 “VA” shall have the meaning set forth in Section 5.5(c) of this Termination Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 14 -
EXECUTION COPY
1.150 [… * …]
1.151 “WAC” shall have the meaning set forth in Section 5.5(f) of this Termination Agreement.
1.152 “XenoPort” shall have the meaning set forth in the preamble of this Termination Agreement.
1.153 “XenoPort Indemnitees” shall have the meaning set forth in Section 9.2 of this Termination Agreement.
1.154 “XenoPort Know-How” shall mean all Know-How relating to Compound or Product, in each case, that are Controlled by XenoPort or its Affiliates as of the Termination Effective Date or during the Transition Period. For the avoidance of doubt, all Transferred GSK Know-How and Transferred Regulatory Filings shall be deemed to be included in XenoPort Know-How as of and after the Termination Effective Date.
1.155 “XenoPort Patents” shall mean all Patents owned or Controlled by XenoPort that relate to the manufacture, use and/or sale of the Compound and Product. For the avoidance of doubt, all Transferred GSK Patents shall be deemed to be included in XenoPort Patents on and after the Termination Effective Date.
1.156 “XenoPort Trademarks” shall mean all Trademarks owned or Controlled by XenoPort that relate to or are used with the Compound and Product, including the tradenames, logos, brands, corporate names or similar items used by XenoPort or its Affiliates. For the avoidance of doubt, all Transferred GSK Trademarks shall be deemed to be included in XenoPort Trademarks as of and after the Termination Effective Date.
1.158 “300mg Product” shall have the meaning set forth in Section 4.11 of this Termination Agreement.
ARTICLE II
TERMINATION; RELEASE; DISMISSAL
2.1 Termination. On the terms set forth in this Termination Agreement, the Restated Agreement is hereby terminated in its entirety as of the Termination Effective Date, and shall be of no further force and effect from and after the Termination Effective Date. Notwithstanding any provision to the contrary in the Restated Agreement, including, without limitation, Section 15.5 of the Restated Agreement, no provisions of the Restated Agreement shall be deemed to survive or have any further force or effect. For clarity, and without limiting the foregoing, all licenses and rights granted by XenoPort to GSK under the Restated Agreement (including the licenses and rights granted by XenoPort to GSK pursuant to Sections 2.1 and 2.2 of the Restated Agreement) with respect to all Compound and Product (including all licenses in the Territory, and licenses to make or have made outside the Territory) shall terminate immediately, and all such licenses and rights with
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 15 -
EXECUTION COPY
respect to all Compound and Product shall revert back to XenoPort. GSK will have the limited license set forth in Section 3.1 below to conduct the activities set forth in ARTICLE IV of this Termination Agreement. Except as set forth in this Termination Agreement (or the Supply Agreement, if entered into by the Parties), GSK shall have no rights or obligations with respect to any Compound or Product. Further, all of XenoPort’s rights to co-promote the Product as set forth in Section 5.2 of the Restated Agreement shall terminate immediately and XenoPort shall have no right to co-promote or otherwise commercialize the Product during the Transition Period.
2.2 Release. Except with respect to the obligations created by, acknowledged in or arising out of this Termination Agreement, each Party does hereby for itself and its respective legal predecessors, successors and assigns, and its Affiliates (as to GSK, including without limitation GlaxoSmithKline LLC and GlaxoSmithKline Holdings (Americas) Inc.), and each of their respective current and former trustees, officers, directors, employees, agents, attorneys and representatives, unconditionally releases, covenants not to xxx, and absolutely and forever discharges the other Party, together with its respective legal predecessors, successors and assigns, and its Affiliates, and each of their respective current and former trustees, officers, directors, employees, agents, attorneys, and representatives, from any and all Claims relating to the Restated Agreement (including the Original Agreement), other than Claims based upon this Termination Agreement, whether known or unknown, anticipated or unanticipated, whether at law or in equity, which either Party may have or claim to have against the other Party or the other Persons identified above in the past, now or at any time in the future relating to the Restated Agreement (including the Original Agreement), other than Claims based upon this Termination Agreement, including any and all claims for attorneys’ fees and costs (all of which are hereinafter referred to as and included within the “Released Matters”). It is the intention of the Parties in executing this Termination Agreement that this Termination Agreement will be effective as a full and final accord and satisfaction and mutual general release of and from all Released Matters.
2.3 Unknown Claims. In furtherance of the intentions set forth herein, each of the Parties acknowledges that it is familiar with Section 1542 of the Civil Code of the State of California, which provides as follows:
“A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS WHICH THE CREDITOR DOES NOT KNOW OR SUSPECT TO EXIST IN HIS FAVOR AT THE TIME OF EXECUTING THE RELEASE, WHICH IF KNOWN BY HIM MUST HAVE MATERIALLY AFFECTED HIS SETTLEMENT WITH THE DEBTOR.”
Each Party expressly waives and relinquishes any right or benefit which it has or may have under Section 1542 of the Civil Code of the State of California and any and all provisions, rights and benefits to similar effect conferred by any law of any state or territory of the United States or foreign country or principle of common or civil law. In connection with such waiver and relinquishment, each of the Parties acknowledges that it is aware that it or its attorneys or accountants may hereafter discover claims or facts in addition to or different from those which it now knows or believes to exist with respect to the subject matter of this Termination Agreement or the other Party hereto, but that it is its intention hereby fully, finally and forever to settle and release all of the Released Matters,
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 16 -
EXECUTION COPY
disputes and differences known or unknown, suspected or unsuspected, which now exist, may exist or heretofore have existed between the Parties, except as otherwise expressly provided. In furtherance of this intention, the releases herein given will be and remain in effect as full and complete mutual releases notwithstanding the discovery or existence of any such additional or different claim or fact.
2.4 Dismissal. Within five (5) business days after the Termination Effective Date, the Parties shall cause to be completed, executed and filed with the United States District Court for the Northern District of California and with the United States District Court for the District of Delaware, stipulations for dismissal with prejudice of the Litigation consistent with this Termination Agreement and in the forms attached hereto as Exhibit 2.4A and Exhibit 2.4B, respectively.
ARTICLE III
LICENSE GRANTS; STOCK PURCHASE; TRANSFER OF ASSETS
3.1 License Grant to GSK.
(a) Subject to the terms and conditions of this Termination Agreement, XenoPort hereby grants to GSK the following licenses, with the right to grant sublicenses as provided in Section 3.1(b) below, under the XenoPort Patents, XenoPort Know-How and XenoPort Trademarks: (i) an exclusive license during the Transition Period to sell, market, distribute and otherwise commercialize Product in the Territory pursuant to Section 4.4, including developing Promotional Materials for use by GSK or its Affiliates in such commercialization of the Product in the Territory; (ii) a non-exclusive license solely for the purposes of permitting GSK to (i) perform other activities under ARTICLE IV below for the applicable periods described therein, including (A) using the Product in the Ongoing Studies as provided in Section 4.3(a), and (B) making, having made and importing Product for the Territory solely for sale by GSK pursuant to Section 4.4, use in Ongoing Studies pursuant to Section 4.3(a), inclusion in Inventory sold to XenoPort hereunder, [… * …] or for sale to XenoPort under the terms of the Supply Agreement; and (iii) a non-exclusive license to continue selling and distributing Product under GSK’s patient assistance programs as permitted under Section 5.8(d) during the time period described therein and distributing Products in accordance with Section 4.4(g) during the time period described therein.
(b) GSK shall have the right to sublicense its rights under the license granted in Section 3.1(a) above: (i) to its Affiliates; and (ii) to Third Parties to permit such Third Parties to provide services to and on behalf of GSK relating to the manufacturing or development of the Product solely as provided herein; in each case of (i) and (ii), without the consent of XenoPort. GSK may sublicense its rights as provided in this Section 3.1(b) to Affiliates solely for so long as such Person remains an Affiliate. GSK and its Affiliates shall be responsible for any actions of its sublicensees in exercising the rights under a sublicense of rights granted by XenoPort and its Affiliates under this Termination Agreement to the same extent as if such actions had been undertaken by GSK itself or its Affiliates.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 17 -
EXECUTION COPY
3.2 License Grant to XenoPort.
(a) GSK hereby grants to XenoPort a worldwide license, with the right to grant and authorize sublicenses as provided in Section 3.2(c) below, under the Licensed GSK Patents and the Licensed GSK Know-How (collectively, the “Licensed Assets”) to make, have made, use, develop, import, offer for sale, sell, distribute, market, promote and otherwise exploit Compound and Product. The foregoing license shall be non-exclusive, perpetual and irrevocable; provided, however, that during the period from the Termination Effective Date to the Transition Period End Date, the foregoing licenses shall be subject to the covenants of XenoPort set forth in Sections 4.4(f) and 7.2(a) below.
(b) GSK hereby grants to XenoPort a non-exclusive license, with the right to grant and authorize sublicenses as provided in Section 3.2(c) below under GSK’s rights and its Affiliate’s rights in the Transferred GSK Promotional Materials (including any copyrights therein, but excluding any rights in the GSK Housemarks), to prepare Promotional Materials not containing the GSK Housemarks (which Promotional Materials may otherwise be based, in whole or part, on the Transferred GSK Promotional Materials, in XenoPort’s discretion), and to use, reproduce, modify, and distribute such Promotional Materials and derivatives thereof in connection with the manufacture, use, development, importation, offer for sale, sale, distribution, marketing, promotion and other exploitation of Compound and Product. The foregoing license shall expire upon the assignment and transfer of such rights in the Transferred GSK Promotional Materials from GSK to XenoPort pursuant to Sections 3.5, 3.6, and 3.7 below and shall be subject to GSK’s exclusive right to commercialize, promote, market and sell the Product in the Territory during the Transition Period as set forth in Section 4.4 below and the covenants of XenoPort set forth in Sections 4.4(f) and 7.2(a) below.
(c) XenoPort shall have the right to grant and authorize sublicenses under the licenses granted under Sections 3.2(a) and 3.2(b) above, without the consent of GSK, to its Affiliates and Third Parties. XenoPort and its Affiliates shall be responsible for any actions of its sublicensees in exercising the rights under a sublicense of rights granted by GSK and its Affiliates under this Termination Agreement to the same extent as if such actions had been undertaken by XenoPort itself or its Affiliates.
3.3 Stock Purchase Agreement. Contemporaneously with the execution of this Termination Agreement, GSK and XenoPort shall enter into the SPA pursuant to which GSK shall purchase from XenoPort certain shares of common stock of XenoPort.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 18 -
EXECUTION COPY
3.4 Assignment of Transferred Assets.
(a) Subject to GSK’s license rights as set forth in Section 3.1 of this Termination Agreement, GSK shall convey, transfer and assign (or cause its Affiliates to convey, transfer and assign) to XenoPort all right, title and interest in and to the following (collectively, the “Transferred Assets”):
(i) on the Termination Effective Date, Transferred GSK Know-How;
(ii) on the Termination Effective Date, Transferred GSK Regulatory Filings;
(iii) on the Termination Effective Date, Transferred GSK Domain Names;
(iv) on the Termination Effective Date, Transferred GSK Trademarks;
(v) on the Termination Effective Date, Transferred GSK Patents;
(vi) on the Transition Period End Date, Transferred GSK Contracts;
(vii) on the Transition Period End Date, Transferred GSK Promotional Materials;
(viii) on the Transition Period End Date, the Promotional Sample Inventory; and
(ix) on the Inventory Transfer Date, the Transferred GSK Inventory.
(b) On the Transition Period End Date, GSK shall convey, transfer and assign (or cause its Affiliates to convey, transfer and assign) to XenoPort all right, title and interest in and to any Additional Transferred Asset not conveyed by GSK to XenoPort as set forth in Section 3.4(a) above, as further set forth under Section 3.6 below. Further, upon the Completion of each Ongoing Study and upon the end of the Supply Term, GSK shall convey, transfer and assign (or cause its Affiliates to convey, transfer and assign) to XenoPort all right, title and interest in and to any Additional Transferred Asset arising out of or identified in connection with the conduct of the applicable Ongoing Study or the manufacture and supply of Product during the Supply Term, respectively, as further set forth under Section 3.6 below.
(c) All properties, assets, and rights of GSK not specifically listed and identified in Section 3.4(a) above, including without limitation, any Accounts Receivable, Excluded GSK Contracts, Expired GSK Contracts (except as otherwise provided in Sections 3.6(b) and 4.2(a)), Excluded GSK Inventory and Excluded GSK Know-How (collectively, the “Excluded Assets”) are excluded from the Transferred Assets and shall not be conveyed, transferred or otherwise assigned by GSK or its Affiliates (and GSK and its Affiliates shall have no obligation hereunder to convey, transfer or otherwise assign) to XenoPort or any of its Affiliates. Further, neither XenoPort nor its
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 19 -
EXECUTION COPY
Affiliates shall have any right, title or interest in or to any of the Excluded Assets. For the avoidance of doubt, XenoPort shall not assume or be responsible or liable for any debts, losses, damages, liabilities and obligations associated with the Excluded Assets.
(d) All transfer, documentary, sales, use, valued-added, gross receipts, stamp, registration or other similar transfer taxes (collectively, “Transfer Taxes”) incurred in connection with the transfer and assignment of the Transferred Assets as contemplated by the terms of this Termination Agreement, including [… * …] The Parties hereto agree to reasonably cooperate with each other to claim any applicable exemption from, or reduction of, any applicable Transfer Taxes.
3.5 Deliverables.
(a) On the Termination Effective Date, GSK shall deliver or cause to be delivered the following documents, each duly executed by GSK, and XenoPort shall deliver or cause to be delivered the following documents, each duly executed by XenoPort:
(i) Assignment and Assumption Agreement with respect to the Transferred GSK Know-How and Transferred GSK Regulatory Filings;
(ii) Trademark Assignment Agreement with respect to the Transferred GSK Trademarks;
(iii) Domain Name Assignment Agreement with respect to the Transferred GSK Domain Names; and
(iv) Patent Assignment Agreement with respect to the Transferred GSK Patents.
(b) On the Transition Period End Date, GSK shall deliver or cause to be delivered, each duly executed by GSK, and XenoPort shall deliver or cause to be delivered, each duly executed by XenoPort, (i) the Assignment and Assumption Agreement with respect to the Transferred GSK Contracts and Transferred GSK Promotional Materials; and (ii) a Xxxx of Sale with respect to the Promotional Sample Inventory. Notwithstanding anything to the contrary contained in this Termination Agreement, for any Transferred GSK Contracts required by GSK to fulfill obligations following the Transition Period End Date, including without limitation GSK’s interim supply obligations set forth in Section 4.5, GSK’s assignment of each such Transferred GSK Contracts shall be a partial assignment so that GSK is able to continue to utilize such Transferred GSK Contracts to fulfill all such obligations.
(c) On the Inventory Transfer Date, GSK shall deliver or cause to be delivered, each duly executed by GSK, and XenoPort shall deliver or cause to be delivered, each duly executed by XenoPort, a Xxxx of Sale with respect to the Transferred GSK Inventory (all documents referred to in Section 3.5(a) (i) through (iv) inclusive, Section 3.5(b) and this Section 3.5(c) are collectively referred to as the “Ancillary Agreements”).
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 20 -
EXECUTION COPY
(d) On the first Business Day after the Termination Effective Date, GSK shall deliver to the FDA, with copies to XenoPort (i) the letter sent from GSK to the FDA, duly executed by GSK, transferring the rights to IND 071352 to XenoPort as of the Termination Effective Date and accepting the transfer of sponsor obligations to GSK, acting as a Contract Research Organization for the conduct of Low Dose Study under IND 071352 as of the Termination Effective Date, and (ii) the letters sent from GSK to the FDA, duly executed by GSK, transferring the rights to the other Transferred GSK Regulatory Filings to XenoPort as of the Termination Effective Date.
(e) On the second Business Day after the Termination Effective Date, GSK, on XenoPort’s behalf, shall deliver to the FDA, with copies to XenoPort (i) the letter sent from GSK on XenoPort’s behalf to the FDA, duly executed by GSK on XenoPort’s behalf, assuming responsibility for IND 071352 from GSK as of the Termination Effective Date and transferring sponsor obligations to GSK, acting as a Contract Research Organization for the conduct of the Low Dose Study under IND 071352 as of the Termination Effective Date, and (ii) the letters sent from GSK on XenoPort’s behalf to the FDA, duly executed by GSK on XenoPort’s behalf, assuming responsibility for the other Transferred GSK Regulatory Filings from GSK as of the Termination Effective Date.
3.6 Additional Transferred Assets; Additional Licensed Assets.
(a) The Parties recognize and acknowledge [… * …] to identify all the Transferred Assets and Licensed Assets [… * …] accordingly, the Parties shall [… * …] prior to the end of the Transition Period so that [… * …] Except as otherwise provided under Section 3.6(b) below with respect to GSK Contracts, either Party may notify the other Party during the Transition Period (or with respect to Transferred Assets or Licensed Assets arising out of or identified in connection with the Ongoing Studies or the manufacture and supply of Compound or Product during the Supply Term, within thirty (30) days after such activity concludes) of [… * …], and upon the provision or receipt of such notice, [… * …], as applicable, and assigned or licensed, as applicable, from GSK to XenoPort as further set forth under Sections 3.6(d) and 3.6(e) below. Except as provided hereunder with respect to Inventory and Transferred GSK Inventory and Transferred GSK Contracts, [… * …]
(b) During the Transition Period, either Party may notify the other Party of [… * …] that relate to Compound or Product (as such assets are defined without limitation to the referenced Schedule), and upon the provision or receipt of such notice, [… * …] XenoPort shall not be required to accept assignment of any proposed Transferred GSK Contract that is not listed on the referenced Schedule as of the Termination Effective Date until XenoPort has agreed in writing, in its discretion, whether to accept such assignment after reviewing a true and complete copy of such proposed Transferred GSK Contract [… * …] With respect to any Transferred GSK Contract that is listed on the referenced Schedule as of the Termination Effective Date, [… * …] whether XenoPort, in its discretion, wishes to have such Transferred GSK Contract assigned to XenoPort on the Transition Period End Date as set forth in Section 3.4(a)(vi), and in the event that XenoPort [… * …] for purposes of this Termination Agreement [… * …] With respect to each Transferred GSK Contract or proposed Transferred GSK Contract, GSK agrees to notify XenoPort, prior to the assignment of a contract to XenoPort as a Transferred GSK Contract under this Termination
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 21 -
EXECUTION COPY
Agreement, of any disputed amounts claimed or alleged by the applicable counterparty to such contract to be due, and XenoPort [… * …] In the event XenoPort requests such GSK Contract to be assigned and such GSK Contract can be assigned to XenoPort in accordance with the provisions set forth under Section 4.2(a) below, such GSK Contract will be deemed a Transferred GSK Contract and assigned and transferred from GSK to XenoPort as further set forth under Section 3.6(d) below; [… * …] For clarity, neither XenoPort nor any of its Affiliates [… * …] Further, nothing herein shall be construed or deemed as [… * …]
(c) On the Transition Period End Date (or, for Transferred GSK Inventory, the Inventory Transfer Date), (i) GSK shall deliver to XenoPort updated Schedules to include any assets identified as a Transferred Asset or Licensed Asset in accordance with Section 3.6(a) above, so that such Schedules set forth a true, complete and correct description of all such Transferred Assets and Licensed Assets (as such assets are defined without limitation to the referenced Schedule) as of the Transition Period End Date (or, for Transferred GSK Inventory, the Inventory Transfer Date), and (ii) GSK shall deliver to XenoPort updated Schedules to include any assets identified as a Transferred GSK Contract or Excluded GSK Contract in accordance with Section 3.6(b) above (and in accordance with Section 1.43 with respect to the Excluded GSK Contracts), so that such Schedule set forth a true, complete and correct description of all GSK Contracts as of the Transition Period End Date. Further, upon Completion of each Ongoing Study and upon the end of the Supply Term, GSK shall deliver to XenoPort updated Schedules to include any assets identified as a Transferred Asset or Licensed Asset (as such assets are defined without limitation to the referenced Schedule) arising out of or identified in the conduct of the applicable Ongoing Study or the manufacture and supply of Product during the Supply Term, respectively. All assets identified as a Transferred Asset or Licensed Asset and added to the referenced Schedule as provided under this Section 3.6(c) shall be deemed an “Additional Transferred Asset” or “Additional Licensed Asset,” respectively.
(d) Pursuant to Section 3.4(b) above, GSK shall convey, transfer and assign (or cause to be conveyed, transferred and assigned) to XenoPort all right, title and interest in and to any Additional Transferred Asset, and each Party shall deliver or cause to be delivered to the other Party, along with the updated Schedules as provided in Section 3.6(c) above, duly executed copies of the applicable Ancillary Documents in order to convey, assign and otherwise transfer the Additional Transferred Assets as contemplated herein. All provisions in this Termination Agreement applicable to the Transferred Assets shall apply, mutatis mutandis, to the Additional Transferred Assets such that each reference to the applicable Transferred Asset(s) shall be deemed to include the applicable Additional Transferred Asset(s); provided that XenoPort shall not assume any liability for any debts, losses, damages and/or obligations associated with any Additional Transferred Asset unless and until such Additional Transferred Asset is assigned or transferred from GSK to XenoPort and XenoPort assumes such liability in accordance with this Section 3.6.
(e) GSK hereby grants (and shall cause its Affiliates to hereby grant) to XenoPort a license under any Additional Licensed Asset as set forth under Section 3.2(a) above and all provisions in this Termination Agreement applicable to the Licensed Assets shall apply, mutatis mutandis, to the Additional Licensed Assets such that each reference to the applicable Licensed Asset(s) shall be deemed to include the applicable Additional Licensed Asset(s).
(f) For a period of [… * …] following the Transition Period End Date, in the event that [… * …] the Transferred Assets or Licensed Assets and that are necessary [… * …] such Party shall notify the other Party, and [… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 22 -
EXECUTION COPY
3.7 Assumed and Retained Liabilities.
(a) Except as otherwise set forth in Sections 5.3, 5.4, 5.5, 5.6, 5.7 and 5.8 and Articles VII and IX of this Termination Agreement, and subject to Section 3.7(c), GSK shall retain liability for any debts, losses, damages and/or obligations associated with any Transferred Assets that are incurred prior to the date on which the Transferred Assets are assigned and transferred from GSK to XenoPort or its designee as provided in Section 3.4(a), 3.4(b) and 3.6 above (the “Retained Liabilities”) and neither XenoPort nor any of its Affiliates shall have any liability for such Retained Liabilities; provided, however, that if, in accordance with this Termination Agreement, GSK has transferred a Transferred GSK Contract to XenoPort that allows for recovery of any debts, losses, damages and/or obligations associated with any Transferred Assets from the counterparty to such Transferred GSK Contract, then, [… * …], XenoPort shall assume the right to recover any such debts, losses, damages and/or obligations from the counterparty to such Transferred GSK Contract [… * …] (the “Transferred Contract Recovery Rights”).
(b) Except as otherwise set forth in Sections 5.3, 5.4, 5.5, 5.6, 5.7 and 5.8 and Articles VII and IX of this Termination Agreement, and subject to Section 3.7(c), XenoPort shall assume (i) liability for any debts, losses, damages, and/or obligations associated with any Transferred Assets that are incurred on or after the date on which such Transferred Assets are assigned and transferred from GSK to XenoPort or its designee as provided in Section 3.4(a), 3.4(b) and 3.6 above, and (ii) the Transferred Contract Recovery Rights (collectively, the “Assumed Liabilities”) and neither GSK nor any of its Affiliates shall have any liability for such Assumed Liabilities.
(c) Except to the extent that this Termination Agreement provides for a payment from one Party to the other or otherwise specifies that certain costs or expenses shall be borne by one Party or the other, each Party shall bear the cost and expense of conducting its (and its Affiliates’ and Third Party contractors’) activities under this Termination Agreement.
3.8 Further Assurances. The Parties further agree to execute such other documents and take such other actions as the other Party may reasonably request in order to convey, license, assign and otherwise transfer the Transferred Assets or Licensed Assets from GSK to XenoPort as contemplated in this Termination Agreement.
ARTICLE IV
TRANSITION ACTIVITIES
4.1 Transition Period. The “Transition Period” shall be that period of time commencing on the Termination Effective Date and continuing up to 11:59 p.m. (Eastern) on April 30, 2013 (the “Transition Period End Date”), after which time the Transition Period shall expire; provided,
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 23 -
EXECUTION COPY
however, that XenoPort may change the Transition Period End Date to an earlier date, and thus shorten the duration of the Transition Period, if XenoPort provides at least thirty (30) days’ prior written notice to GSK of the new Transition Period End Date, which new Transition Period End Date must occur on the last day of the calendar month (e.g., January 31, 2013, February 28, 2013, etc.).
4.2 Transfer of Assets.
(a) GSK Contracts.
(i) GSK shall deliver to XenoPort true and correct and complete [… * …] of the Transferred GSK Contracts, Expired GSK Contracts and Excluded GSK Contracts (other than GSK Contracts between GSK and its Affiliates and GSK Contracts between GSK or its Affiliate and a Third Party [… * …] within [… * …] Business Days of the Termination Effective Date for XenoPort’s review. To the extent not previously provided to XenoPort during the Transition Period, the Transition Team shall use Commercially Reasonable Efforts to identify the Transferred GSK Contracts, Expired GSK Contracts and Excluded GSK Contracts, and GSK shall deliver to XenoPort true and correct and complete electronic copies of any newly identified Transferred GSK Contracts, Expired GSK Contracts or Excluded GSK Contracts (other than GSK Contracts between GSK and its Affiliates and GSK Contracts between GSK or its Affiliate and a Third Party [… * …] as provided in Section 3.6, on the Transition Period End Date. For clarity, [… * …] Nothing herein shall be construed or deemed as obligating GSK to breach any provision in a GSK Contract, including, without limitation, any obligations of confidentiality with respect to such GSK Contract. Further, GSK [… * …]
(ii) To the extent that XenoPort requests in writing, no less than thirty (30) days prior to the Transition Period End Date, that an Excluded GSK Contract or Expired GSK Contract, in each case, [… * …] that is not assignable without the consent of another Person (a “Non-Assignable Contract”) be assigned to XenoPort, GSK shall [… * …] prior to the Transition Period End Date. GSK shall be deemed [… * …] If consent to assign a Non-Assignable Contract is not granted [… * …] prior to the Transition Period End Date, then [… * …] If, however, such Person consents to the assignment of a Non-Assignable Contract [… * …] prior to the Transition Period End Date, then such Non-Assignable Contract shall (subject to Section 3.6(b)) be deemed to be included within the Transferred GSK Contracts and assigned and transferred from GSK to XenoPort as set forth under Sections 3.4, 3.5, and 3.6 above; provided however, [… * …], or to obtain consent for assignment, of such Non-Assignable Contract. Further, nothing herein shall obligate or be deemed to obligate [… * …] If XenoPort notifies GSK in writing prior to the Transition Period End Date, and subject to Section 3.6(b), that XenoPort requests an Expired GSK Contract that is assignable without the consent of another Person be included as a Transferred GSK Contract and assigned to XenoPort, such Expired GSK Contract shall be included within the Transferred GSK Contracts and assigned and transferred from GSK to XenoPort as set forth under Sections 3.4, 3.5, and 3.6 above.
(iii) With respect to any Expired GSK Contract or any Non-Assignable Contract, in each case that has been identified by the Parties prior to the date [… * …] after the Transition Period End Date and unless and until such Expired GSK Contract or Non-Assignable Contract is assigned to XenoPort as set forth in Section 4.2(a)(ii) above (each a “Non-Assigned Contract”), at XenoPort’s written request, GSK shall [… * …] as reasonably necessary for the purposes of making, having made, using, developing, importing, offering for sale, selling, distributing, marketing, promoting and otherwise exploiting Compound or Product; provided, however, [… * …] and further provided, however, that such obligation shall continue [… * …] If, in order for GSK to fulfill any commitments under this Section 4.2(a)(iii), XenoPort [… * …] GSK’s exercise of its rights [… * …] Nothing herein shall [… * …] provided, however that XenoPort shall be [… * …] The Parties acknowledge and agree that, depending on the circumstances in a given case, GSK’s obligation [… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 24 -
EXECUTION COPY
(b) GSK Know-How.
(i) GSK shall deliver to XenoPort true and correct and complete [… * …] of the Transferred GSK Know-How and Licensed GSK Know-How (except as otherwise noted on Schedule 4.2B as Transferred GSK Know-How that has been previously delivered and assigned to XenoPort pursuant to the Restated Agreement) on timelines to be determined and agreed by the Transition Team, but no later than within [… * …] Business Days after the Termination Effective Date with respect to Transferred GSK Know-How and Licensed GSK Know-How that is related to distribution, shipping or safety, no later than [… * …] Business Days with respect to Transferred GSK Know-How and Licensed GSK Know-How related to manufacturing, and no later than within [… * …] Business Days after the Termination Effective Date for other Transferred GSK Know-How and Licensed GSK Know-How. In determining and agreeing to such timelines, the Transition Team may specify [… * …] for delivery of various portions of the Transferred GSK Know-How and Licensed GSK Know-How, and shall discuss and consider whether certain portions of the Transferred GSK Know-How and Licensed GSK Know-How may need to be [… * …] than other portions in order to facilitate XenoPort’s ability to assume commercialization of the Product on the Transition Period End Date but in any event taking into account resources required for GSK to meet its obligations hereunder. To the extent not previously provided to XenoPort during the Transition Period, GSK shall deliver to XenoPort true and correct and complete [… * …] electronic copies of any newly identified Transferred GSK Know-How or Licensed GSK Know-How (including with respect to an Ongoing Study), as provided in Section 3.6(a), on the Transition Period End Date or upon Completion of each Ongoing Study or the end of the Supply Term, as applicable. For clarity, [… * …] To the extent that the Parties agree that the delivery of such Transferred GSK Know-How or Licensed GSK Know-How as set forth herein [… * …] The Parties acknowledge that GSK has previously assigned and transferred to XenoPort pursuant to the Restated Agreement all GSK Know-How (other than the Excluded GSK Know-How) related to the Compound or Product outside the Territory, including the GSK Know-How identified on Schedule 4.2B.
(ii) To the extent any [… * …] GSK or its Affiliates, and except to the extent that [… * …] of GSK (or any of its Affiliates) in accordance with Applicable Laws, GSK shall provide (or cause its Affiliates to provide) such [… * …] to XenoPort. In the event that
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 25 -
EXECUTION COPY
XenoPort believes [… * …] and GSK believes that [… * …] the Parties shall discuss the matter to consider possible resolutions that would meet the needs of both Parties. Notwithstanding the foregoing, in the event that GSK [… * …] that are related to an Ongoing Study in order to perform such Ongoing Study, GSK may [… * …] the Completion of such Ongoing Study.
(c) To the extent that any copies of documents reflecting or containing Transferred GSK Know-How or Licensed GSK Know-How has not been provided by GSK or its Affiliates and is in possession of a Third Party (“Non-Transferred GSK Know-How”), GSK shall [… * …] and (ii) provide XenoPort (or its designees) with [… * …] GSK shall be deemed to have satisfied its obligation [… * …] If, in order for GSK to fulfill any commitments under this Section 4.2(c), XenoPort [… * …] with respect to access to any such Non-Transferred GSK Know-How. GSK shall not require [… * …] Further, nothing herein shall obligate or be deemed to obligate GSK or any of its Affiliates [… * …] Additionally, nothing contained herein shall [… * …]
(d) Transferred GSK Regulatory Filings. GSK shall deliver to XenoPort true and correct and complete electronic copies [… * …] of the Transferred GSK Regulatory Filings within [… * …] Business Days of the Termination Effective Date. For clarity, such electronic copies of the Transferred GSK Regulatory Filings shall be provided in [… * …]
(e) Transferred GSK Promotional Materials.
(i) [… * …] of the Transferred GSK Promotional Materials (including underlying layouts, typesets, print proofs, etc.) within [… * …] Business Days of the Termination Effective Date. To the extent not previously provided to XenoPort during the Transition Period, GSK shall deliver to XenoPort true and correct and complete electronic copies of any newly created or identified Transferred GSK Promotional Materials, as provided in Section 3.6, on the Transition Period End Date. For clarity, such electronic copies of the Transferred GSK Promotional Materials shall [… * …]
(ii) Subject to GSK’s exclusive right to commercialize, promote, market and sell the Product in the Territory during the Transition Period as set forth in Section 4.4 below and the covenant(s) of XenoPort set forth in Sections 4.4(f) and 7.2(a) below, XenoPort shall have the right to use and disclose all such Transferred GSK Promotional Materials following the Termination Effective Date for sole purpose of preparing (at any time after the Termination Effective Date) Promotional Materials not containing the GSK Housemarks (which Promotional Materials may otherwise be based, in whole or part, on the Transferred GSK Promotional Materials, in XenoPort’s discretion) for use by or under authority of XenoPort after the Transition Period End Date.
(iii) The Parties acknowledge and agree that such Transferred GSK Promotional Materials are being conveyed, transferred, assigned and provided by GSK to XenoPort “as is, where is, with all faults” and with no express or implied representations or warranties of any kind, including without limitation, whether such Transferred GSK Promotional Materials comply with Applicable Laws. XenoPort shall have sole responsibility for determining whether any promotional materials used by XenoPort to commercialize, promote or otherwise market Product in the Territory on and after the Transition Period End Date comply with Applicable Laws.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 26 -
EXECUTION COPY
(f) Transferred GSK Inventory.
(i) An initial schedule of the Inventory as of the Termination Effective Date (excluding the Excluded Inventory) is attached hereto as Schedule 1.69. The Parties acknowledge that items on the initial Schedule attached hereto will be used by GSK in its manufacture, development and commercialization activities under Sections 4.3(a) and 4.4(c) and/or in connection with manufacture of Product for XenoPort as provided under Section 4.5, and that accordingly the initial Schedule attached hereto as of the Termination Effective Date is not a statement of the Transferred GSK Inventory that will in fact be transferred to XenoPort on the Inventory Transfer Date as provided herein. An updated, final binding schedule identifying the Transferred GSK Inventory will be provided to XenoPort as of the Inventory Transfer Date as set forth below.
(A) By January 30, 2013, GSK shall deliver Schedule 1.137 to XenoPort, which shall identify the Transferred GSK Inventory to be transferred to XenoPort pursuant to this Termination Agreement (the “Final Transferred GSK Inventory Quantities”).
(B) The Final Transferred GSK Inventory Quantities shall exclude quantities of Inventory retained by GSK to fulfill its obligations (whether by or through GSK, its Affiliates or Third Party contractor(s)) to manufacture and supply Product in support of or to conduct GSK’s obligations set forth in Section 4.3(a), 4.4(a), 4.4(c) and 4.5 (collectively, the “Retained Inventory Quantities”). For the avoidance of doubt, GSK shall retain ownership of the Retained Inventory Quantities, except as such Retained Inventory Quantities may be subsequently transferred to XenoPort pursuant to Section 4.2(f)(iii) below.
(ii) To effect the transfer of Final Transferred GSK Inventory Quantities to XenoPort, GSK and XenoPort shall enter into customary documents, including the Xxxx of Sale as set forth in Section 3.5(c), which shall transfer right, title and ownership of such Final Transferred Inventory Quantities to XenoPort. Such transfer (and delivery) of Final Transferred GSK Inventory Quantities to XenoPort shall occur as promptly as possible following January 30, 2013 (the date of such transfer, the “Inventory Transfer Date”). Delivery of the Final Transferred GSK Inventory Quantities shall be made to XenoPort [… * …]; provided, however, [… * …]
(iii) If after the Transition Period End Date, Retained Inventory Quantities are still available and are no longer required by GSK to manufacture and supply Product in support of or to conduct its obligations set forth in Section 4.3(a), 4.4(a), 4.4(c) and 4.5, then GSK shall promptly transfer such remaining Retained Inventory Quantities to XenoPort. Any such transfer of additional Inventory shall be delivered under the same terms under Section 4.2(f)(ii) above for Final Transferred GSK Inventory Quantities without charge to XenoPort [… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 27 -
EXECUTION COPY
(iv) In the event that the Retained Inventory Quantities are not sufficient to manufacture and supply those quantities of Product required to support GSK’s obligations set forth in Sections 4.3(a), 4.4(a), 4.4(c) and 4.5 (whether due to unexpected increases in demand, rejected batches, or otherwise), GSK shall be permitted to place orders for additional API in accordance with the principles set forth in Section 4.2(h) below, and any such API supplied to GSK pursuant to this Section 4.2(f)(iv) shall be included within the meaning of “Tolled API” (and, if applicable, subject to the terms agreed in Section 4.2(h) below). [… * …]
(v) [… * …]
(vi) XenoPort acknowledges and agrees that API [… * …] XenoPort agrees that it shall comply with cGMP and Applicable Laws regarding [… * …] Such Final Transferred GSK Inventory Quantities is being transferred to XenoPort [… * …] set forth in Section 7.3(g), and subject to Section 7.4).
(vii) Notwithstanding the above, GSK acknowledges that XenoPort may require certain limited quantities of API prior to the Inventory Transfer Date (“Limited API”). GSK shall provide those Limited API quantities as reasonably requested by XenoPort, without additional charge for purchase thereof other than amounts to be paid pursuant to Section 6.2, with any such delivery of Limited API to be under the same terms set forth in Section 4.2(f)(ii) above for Final Transferred GSK Inventory Quantities; provided, however, that unless conflicting obligations or commitments are waived by XenoPort, any such request for Limited API shall not conflict with GSK’s obligations under this Termination Agreement or the Supply Agreement. Supply of Limited API shall be made on documentation reasonably agreed by the Parties to establish transfer of ownership and risk of loss to XenoPort, with such Limited API considered for purposes of this Termination Agreement “Transferred GSK Inventory” and included within “Final Transferred GSK Inventory Quantities”.
(viii) Notwithstanding anything to the contrary contained in this Termination Agreement, if pursuant to this Termination Agreement GSK assigns and transfers a Transferred GSK Contract to XenoPort that directly relates to the manufacture and supply of any Transferred GSK Inventory or Promotional Sample Inventory, [… * …]
(g) Promotional Sample Inventory. The Promotional Sample Inventory shall be transferred to XenoPort within thirty (30) days after the Transition Period End Date. Any inventory of sample Products held by GSK’s sales representatives as of the Transition Period End Date shall be destroyed by GSK. GSK shall notify XenoPort in writing of the quantity of Promotional Sample Inventory being transferred to XenoPort ten (10) Business Days prior to the Transition Period End Date.
(h) Tolled API.
(i) In the event XenoPort elects for GSK to provide interim supply pursuant to Section 4.5, then (i) for Product to be so supplied under a Firm Order provided from
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 28 -
EXECUTION COPY
XenoPort to GSK prior to the Inventory Transfer Date, GSK shall supply the API therefor from the API in GSK’s Inventory, and (ii) for Product so supplied under a Firm Order provided to GSK after the Inventory Transfer Date, GSK may from time to time place orders with XenoPort for those quantities of API required to manufacture and supply such Product (any such API supplied back to GSK after the Inventory Transfer Date referred to herein as the “Tolled API”). Such Tolled API shall be provided to GSK (or as directed by GSK) free of charge, in sufficient quantity and in accordance with the requested delivery time to allow GSK to meet any Firm Order commitments (and delivery dates agreed therein); provided that GSK shall in the normal course give at least [… * …] to XenoPort prior to the requested date of delivery and for any unexpected events (e.g., batch failure) as promptly as practicable. In the event that XenoPort fails to deliver (in whole or in part) the Tolled API (or fails to deliver in the time period requested by GSK), GSK shall make appropriate adjustments to the quantities (or delivery phasing) of Product included in the relevant Firm Order without such adjustment(s) deemed a breach by GSK of any Firm Order commitments (or a breach of any obligations under this Termination Agreement or the Supply Agreement by reason of GSK’s failure to supply, to the extent so prevented). Any such adjustment to quantities of Product (or timing of delivery) shall be based on those quantities of Tolled API that XenoPort is not able to provide (or based on the delayed receipt of Tolled API), which, for the avoidance of doubt, shall not prevent GSK’s use of Commercially Reasonable Efforts to supply those quantities of Product able to be supplied using Tolled API actually received (or based on reasonably adjusted delivery times).
(ii) XenoPort acknowledges and agrees that Tolled API will be delivered directly to GSK’s Third Party contractor and utilized at such Third Party contractor’s site in the manufacture of Bulk Product. In the event that any such Tolled API delivered by (or on behalf of) XenoPort in accordance with this Section [… * …] or for any failure to satisfy Firm Order quantities requested by XenoPort to the extent caused by the delivery of Tolled API [… * …] Any Tolled API will be supplied to such Third Party contractor under an existing contractual agreement with GSK, which provides assurance to GSK that materials (including Tolled API) will be stored in compliance with an existing quality agreement, cGMPs, and Applicable Laws. In the event of any loss or damage to Tolled API, GSK shall [… * …] for all such losses and/or damages, and GSK shall [… * …]
(iii) For the avoidance of doubt, neither GSK nor its Affiliates shall be responsible for any [… * …] delivered pursuant to a Firm Order to the extent any such [… * …] from shipping, handling, re-testing, or storage of such Tolled API after such API is transferred to XenoPort pursuant to Section 4.2(f) and prior to delivery to GSK’s Third Party contractor pursuant to Section 4.2(h)(ii).
(i) GSK Housemarks License. To the extent that the Transferred GSK Inventory of Finished Product (or any Product supplied pursuant to interim supply terms below) and Promotional Sample Inventory delivered to XenoPort is in packaging that bears (or contains inserts or other documentation bearing) trademarks, tradenames, logos, brands, corporate names or similar items used by GSK or any of GSK’s Affiliates other than the Transferred GSK Trademarks (collectively, the “GSK Housemarks”), then as of the Inventory Transfer Date with respect to the Finished Product and the Transition Period End Date with respect to the Promotional Sample
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 29 -
EXECUTION COPY
Inventory, GSK hereby grants to XenoPort, and XenoPort hereby accepts, a limited non-exclusive, royalty-free license, with the right to grant and authorize sublicenses, to use the GSK Housemarks, solely to the extent necessary to allow XenoPort to distribute and sell such Finished Product or distribute and use the Promotional Sample Inventory containing the GSK Housemarks in accordance with the terms of this Termination Agreement (the “GSK Housemarks License”). XenoPort acknowledges that the GSK Housemarks License is being granted solely for transitional purposes and that XenoPort shall use its Commercially Reasonable Efforts to cease its use of the GSK Housemarks and exhaust its inventory of such Finished Product and Promotional Sample Inventory labeled with GSK Housemarks as quickly as is reasonably possible after the Inventory Transfer Date or Transition Period End Date, as applicable. XenoPort shall not: (A) add any other labels or marks to, or otherwise alter, the GSK Housemarks as used by GSK as of the Inventory Transfer Date or Transition Period End Date, as applicable; (B) change in any way the style of the GSK Housemarks as used by GSK as of the Inventory Transfer Date or Transition Period End Date, as applicable; or (C) otherwise use the GSK Housemarks in any manner other than as specifically permitted in this Section 4.2(i). XenoPort acknowledges GSK’s (or its Affiliates’) ownership of the GSK Housemarks, and XenoPort shall not knowingly do anything inconsistent with such ownership, and agrees that all use of the GSK Housemarks by XenoPort shall inure to the benefit and be on behalf of GSK (or its Affiliates). Nothing in this Termination Agreement shall give XenoPort any right, title or interest in the GSK Housemarks other than the right to use the GSK Housemarks strictly in accordance with this Section 4.2(i). All use of the GSK Housemarks by XenoPort under this Section 4.2(i) shall conform to the standards followed by the GSK (or its Affiliates) prior to the Inventory Transfer Date or Transition Period End Date, as applicable, and upon reasonable notice to XenoPort, GSK (or its Affiliates) shall have the right to review XenoPort’s use of the GSK Housemarks after the Transition Period End Date or Transition Period End Date, as applicable, to ensure XenoPort’s compliance with this requirement related to the GSK Housemarks. XenoPort shall not have the right to, and shall not, sublicense, assign, pledge, grant or otherwise encumber or transfer to any Third Party any rights licensed by GSK (or its Affiliates) to XenoPort under this Section 4.2(i) without GSK’s prior written consent; provided, however, for the avoidance of doubt, the foregoing shall not be construed to require GSK’s consent for XenoPort or its Affiliate to engage and use distributors for the distribution and sale, on behalf of XenoPort or its Affiliate, of Products bearing the GSK Housemarks. The Parties understand and agree that, in addition to all other legal remedies, [… * …] If XenoPort shall breach this Section 4.2(i) (whether or not cured), GSK shall have the right to immediately terminate the GSK Housemarks License by providing written notice to XenoPort. Nothing in this Section 4.2(i), or any other provision of this Termination Agreement shall grant XenoPort any rights in any of GSK’s Domain Names, registrations or applications for registration, or renewals thereof, registered in the Territory or any other country or jurisdiction throughout the world, except for the Transferred GSK Domain Names.
4.3 Development Activities; Maintenance of Regulatory Filings; Publications.
(a) Conduct of the Ongoing Studies.
(i) Following the Termination Effective Date, GSK shall continue to conduct, and shall use Commercially Reasonable Efforts to conduct, the Ongoing Studies in
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 30 -
EXECUTION COPY
accordance with the development plan attached hereto as Exhibit 4.3A (the “Development Plan”), including the timelines therein, even if such Ongoing Studies continue after the Transition Period End Date. Promptly following transfer of the NDA for the Product to XenoPort, the Parties shall enter into a mutually agreed written Transfer of Obligations statement in connection with GSK’s performance of activities to complete such Ongoing Studies. With respect to the low-dose RLS efficacy study RXP114025 (the “Low Dose Study”), GSK agrees to use Commercially Reasonable Efforts to conduct and complete the Low Dose Study in accordance with the timeline as stipulated in the approval letter from the FDA to GSK, dated April 6, 2011, approving the NDA for Horizant (gabapentin enacarbil) Extended-Release Tablets as a treatment for moderate-to-severe primary restless legs syndrome (the “RLS Horizant Approval Letter”), attached hereto as Exhibit 4.3B (or a revised timeline proposed or approved by the FDA). Without limiting the foregoing, the Low Dose Study shall be conducted in accordance with the applicable protocols therefor, Applicable Laws and GSK’s Applicable Policies and Procedures. GSK shall have discretion regarding the manner in which it conducts the Low Dose Study; provided, however, that GSK shall keep XenoPort reasonably informed regarding the status and results of the Low Dose Study and all other Ongoing Studies, and GSK shall not materially amend or modify the protocol for the Low Dose Study, or conduct the Low Dose Study in a manner that is materially inconsistent with the protocol therefor, without the prior written approval of XenoPort. In the event that a material modification to a protocol for the Low Dose Study is reasonably required to comply with Applicable Laws, GSK’s Applicable Policies and Procedures or with the request or suggestion of an applicable Regulatory Authority, the Parties shall reasonably cooperate to agree upon mutually acceptable modifications to such protocol to accommodate such requirement, request or suggestion. GSK may in its discretion, but subject to advance consultation and discussion with XenoPort, terminate early or wind-down any such Low Dose Study for safety reasons or to comply with Applicable Laws or GSK’s Applicable Policies and Procedures. Subject to XenoPort’s prior consultation and approval, GSK shall interpret the results of the Low Dose Study, determine whether any additional analyses are required (provided that GSK shall not initiate any such additional analyses without the prior approval of XenoPort), and prepare and deliver to XenoPort a second draft of the clinical study report for the Low Dose Study promptly upon collection of all data for such Low Dose Study, or upon early termination or wind-down of such Low Dose Study as permitted in this Section 4.3(a)(i) above, but no later than [… * …] For the purposes of this Section 4.3(a), the term [… * …] With respect to each Ongoing Study other than the Low Dose Study, GSK shall provide to XenoPort a copy of the manuscript with respect to such Ongoing Study. As between the Parties, GSK shall be responsible for all costs (including wind-down or close-out costs) associated with the Ongoing Studies, even if such costs are incurred or paid following the Termination Effective Date. Further as between the Parties, XenoPort will be solely responsible, at XenoPort’s expense, for any additional clinical trials (i.e., beyond the Ongoing Studies) initiated and conducted after the Termination Effective Date (including, without limitation, any pediatric studies (PMRs 1588-1, 2, 3, and 4) and the adult driving simulation study (PMR 1588-8), as required by the FDA under the terms of the RLS Horizant Approval Letter); provided, however that XenoPort will not directly or indirectly, initiate or conduct any clinical trials relating to Compound or Product in the Territory prior to the Transition Period End Date, unless otherwise required or requested by a Regulatory Authority. For the avoidance of doubt, nothing herein shall restrict XenoPort from conducting development activities relating to Compound or
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 31 -
EXECUTION COPY
Product after the Transition Period End Date or outside the Territory. After the Termination Effective Date, GSK shall not undertake any clinical study of Product other than the Ongoing Studies as outlined in the Development Plan unless approved in writing by XenoPort prior to the commencement of any such clinical study.
(ii) GSK shall have the right to publish in its clinical trial register the results or summaries of the results of the Low Dose Study. The Parties shall review and discuss such results or summary of results of the Low Dose Study to be published prior to publication, provided, however that GSK shall have final say on what is published in its clinical trial register. Further, notwithstanding anything in this Termination Agreement to the contrary, GSK shall have the right to submit and publish information and data pertaining to the Low Dose Study to meet GSK’s obligations under Applicable Laws or under GSK’s Applicable Policies and Procedures; provided, however, that (A) as between the Parties, XenoPort shall determine and be responsible for publishing information pertaining to the Low Dose Study and the results thereof on xxxxxxxxxxxxxx.xxx (or any substitute website established by the FDA or another Regulatory Authority for similar purposes), including any such publication that may be required under Applicable Laws, and (B) GSK shall provide to XenoPort information for such publication on xxxxxxxxxxxxxx.xxx and discuss such information (including discussion of reasonable questions XenoPort may have regarding such information) with XenoPort.
(b) Responsibility For and Interactions with Regulatory Authorities Concerning the Transferred GSK Regulatory Filings.
(i) The Parties acknowledge and agree that as of the Termination Effective Date, XenoPort will have sole control and responsibility for maintaining the Transferred GSK Regulatory Filings and all other Regulatory Filings for the Compound and Product in the Territory. Notwithstanding the forgoing, XenoPort hereby designates and appoints GSK as an agent of XenoPort during the Transition Period for the purposes of filing and maintaining all Regulatory Filings in the Territory relating to the Compound and Product, including without limitation the Transferred GSK Regulatory Filings. During the Transition Period, GSK or its Affiliate shall use Commercially Reasonable Efforts to file and maintain, on behalf of XenoPort, all Regulatory Filings relating to the Compound and Product in the Territory, and in consultation with and at the direction of XenoPort, GSK shall be responsible for liaising with and managing all interactions with Regulatory Authorities in the Territory with respect to Product, except to the extent that XenoPort is required by Applicable Laws to liaise directly with any Regulatory Authority. For the avoidance of doubt, the foregoing shall not be construed to preclude XenoPort from participating in any such interactions with Regulatory Authorities in the Territory with respect to Product. As between the Parties, each Party shall bear the expense of its own and its Affiliates’ personnel and contractors in conducting such activities. XenoPort shall be responsible for fees paid to the FDA in connection with the Product on and after the Termination Effective Date; provided, however, that with respect to Establishment Fees, Product Fees and other fees paid to the FDA that are assessed annually or in respect to another set time period, GSK shall bear that portion (based on number of days) that is attributable to the part of the applicable time period up to the Transition Period End Date, and XenoPort shall bear that portion (based on number of days) that is attributable to the part of the
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 32 -
EXECUTION COPY
applicable time period that follows the Transition Period End Date. XenoPort shall, within thirty (30) days after receipt of an invoice from GSK or its Affiliate, reimburse GSK or its Affiliate for XenoPort’s portion of any such fees paid to the FDA by GSK or its Affiliate in filing and maintaining all Regulatory Filings relating to the Compound and Product in the Territory as provided herein, and GSK shall, within thirty (30) days after receipt of an invoice from XenoPort or its Affiliate, reimburse XenoPort or its Affiliate for GSK’s portion of any such fees paid to the FDA by XenoPort or its Affiliate in filing and maintaining all Regulatory Filings relating to the Compound and Product in the Territory as provided herein.
(ii) During the Transition Period, GSK shall use Commercially Reasonable Efforts to participate in all of XenoPort’s interactions with Regulatory Authorities (including the Office of Prescription Drug Promotion) related to Product in the Territory. Each Party shall provide the other Party reasonable advanced notice (and in no event less than [… * …] of substantive meetings with a Regulatory Authority in the Territory relating to the Compound or Product. GSK shall be entitled to have [… * …] representatives [… * …] attend any such meetings, and if requested by XenoPort GSK will actively participate in all substantive meetings with such Regulatory Authority. For the avoidance of doubt, XenoPort shall not be restricted hereunder with respect to the number of XenoPort representatives participating in such meetings. Each Party shall keep the other Party informed as to all material interactions with Regulatory Authorities in the Territory during the Transition Period. Any activities relating to Product Events arising during the Transition Period shall be handled as set forth in Section 4.4(c). During the Transition Period, and except to the extent expressly agreed otherwise in the last sentence of Section 4.4(c), each Party shall provide the other Party with electronic copies of any material documents, information and correspondence submitted to a Regulatory Authority in the Territory during the Transition Period relating to the Compound or Product as soon as reasonably practicable. Further, each Party shall promptly provide the other Party with electronic copies of all material documents, information and correspondence received from a Regulatory Authority in the Territory during the Transition Period directly related to any Compound or Product. During the Transition Period, all submissions to Regulatory Authorities in the Territory with respect to the Compound or Product shall be subject to XenoPort’s review and prior approval, and the Parties shall consult regarding matters to be discussed, positions to be taken and messaging before any scheduled meetings or teleconferences, and before initiating any unscheduled meetings or teleconferences, with Regulatory Authorities in the Territory with respect to the Compound or Product. In the event that GSK and XenoPort do not agree with respect to any such submissions to Regulatory Authorities, or positions to be taken or messaging in such meetings or teleconferences with Regulatory Authorities, and subject to Section 4.4(c), XenoPort’s view shall prevail and the Parties shall implement the course of action directed by XenoPort; provided, however, that if GSK determines in good faith in its sole discretion that such course of action would violate Applicable Laws or GSK’s Applicable Policies or Procedures, GSK may so notify XenoPort and GSK shall not be obligated to participate in implementing such course of action (and, for the avoidance of doubt, XenoPort may in its discretion proceed, on its own or with another agent, to institute such course of action without GSK’s participation).
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 33 -
EXECUTION COPY
(iii) Notwithstanding anything contained herein, GSK shall have no obligation under this Termination Agreement to take or abstain from taking any action with respect to the filing and/or maintenance of any Regulatory Filings in the Territory relating to the Compound and Product as provided herein or with respect to interactions with Regulatory Authorities in the Territory regarding Regulatory Filings relating to the Compound or Product that, in any case as determined by GSK in good faith in its sole discretion, violates Applicable Laws or GSK’s Applicable Policies or Procedures. Further, GSK shall have no liability to XenoPort or XenoPort’s Affiliates for taking or abstaining from taking any action with respect to the filing and/or maintenance of any Regulatory Filings in the Territory relating to the Compound and Product as provided herein or with respect to interactions with Regulatory Authorities in the Territory concerning Regulatory Filings relating to the Compound or Product in order to, in any case as determined by GSK in good faith in its sole discretion, to comply with Applicable Laws or GSK’s Applicable Policies or Procedures.
(iv) For the avoidance of doubt, after the Transition Period End Date, GSK shall have no further responsibilities on behalf of XenoPort or any of XenoPort’s Affiliates with respect to any Regulatory Filings in the Territory relating to the Compound and Product, including the Transferred GSK Regulatory Filings.
(c) Publications. After the Termination Effective Date, GSK shall not publish, present publicly, or submit for written or oral publication any manuscript, abstract presentation or the like that includes Transferred GSK Know-How other than those publications set forth on Schedule 4.3C attached hereto (“Permitted GSK Publications”). Prior to GSK publishing, publicly presenting and/or submitting for written or oral publication any Permitted GSK Publications, GSK shall provide XenoPort a copy thereof for review at least [… * …] in advance (unless GSK is required by Applicable Laws or GSK’s Applicable Policies and Procedures to publish such information sooner, in which event GSK shall provide a copy for review as far in advance as practicable). GSK shall consider in good faith, but shall in no event be obligated to incorporate, any comments provided by XenoPort during such [… * …] period. In the event that XenoPort fails to provide any comments during such [… * …] period (or such shorter period if required under Applicable Laws), XenoPort shall be deemed to have no comments to such Permitted GSK Publication. In addition, GSK shall, at the request of XenoPort within such [… * …]period, remove any Confidential Information of XenoPort therefrom, except GSK shall have the right to publicly disclose any information, including Confidential Information, pertaining to safety of Product that GSK reasonably believes in good faith it is obligated to disclose to comply with Applicable Laws or GSK’s Applicable Policies and Procedures. During the Transition Period, XenoPort may submit for publication a manuscript, abstract or the like in the Territory that includes data or other information relating to Compound or Product only with GSK’s consent, not to be unreasonably withheld (provided that such manuscript, abstract or like would not be published until after the Transition Period End Date), and after the Transition Period End Date, XenoPort may publish, publicly present or submit for publication a manuscript, abstract or the like that includes data or other information relating to Compound or Product. For the avoidance of doubt, XenoPort may at any time submit for publication a manuscript, abstract or the like outside the Territory that includes data or other
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 34 -
EXECUTION COPY
information relating to Compound or Product, but for any such publication a manuscript, abstract or the like submitted prior to the Transition Period End Date shall provide GSK a copy thereof for its clinical review for at least [… * …] (unless XenoPort is required by Applicable Laws to publish such information sooner), and shall consider in good faith any comments provided by GSK during such [… * …] period. In the event that GSK fails to provide any comments during such [… * …] period (or such shorter period if required under Applicable Laws), GSK shall be deemed to have no comments thereto. In addition, XenoPort shall, at the request of GSK within such [… * …] period, remove any Confidential Information of GSK therefrom, except XenoPort shall have the right to publicly disclose any information, including Confidential Information, pertaining to safety of Product that XenoPort reasonably believes in good faith it is obligated to disclose to comply with Applicable Laws. The contribution of each Party shall be noted in all publications or presentations by acknowledgment or co-authorship, whichever is appropriate. Further, nothing herein shall be deemed as preventing GSK or any of its Affiliates from publishing data or other information relating to the Compound or Product, or relating to RLS, PHN or any related medical condition, that is publicly known or that was generated independently of GSK’s program with respect to the Compound or Product prior to the Termination Effective Date and independently of GSK’s activities with respect to the Compound or Product under this Termination Agreement (including the Ongoing Studies), in any manuscript, abstract or the like.
4.4 Commercialization and Sale of the Product.
(a) During the Transition Period, GSK shall use Commercially Reasonable Efforts, and in a manner consistent with Applicable Laws and GSK’s Policies and Procedures, to exclusively (i.e., even as to XenoPort) sell, market, promote, distribute and otherwise commercialize the Product, solely in the Territory in accordance with the terms and conditions of the commercialization transition plan attached hereto as Exhibit 4.4 (the “Commercialization Transition Plan”). GSK shall have the exclusive right, using Commercially Reasonable Efforts and its sole discretion, to create and develop Promotional Materials for use in the commercialization and promotion of the Product in the Territory during the Transition Period. Notwithstanding the foregoing, GSK shall cease any particular activities set forth in the Commercialization Transition Plan prior to the Transition Period End Date in the event that XenoPort provides GSK with at least [… * …] prior written notice requesting that such activities or portion thereof be ceased prior to the Transition Period End Date; provided, however, that in no event shall XenoPort have the right to take over the conduct of such activities directly or through others prior to the Transition Period End Date in the event that GSK is engaging in any commercialization, promotion or marketing activities with respect to the Product during the Transition Period.
(b) GSK shall use the Transferred GSK Trademarks in accordance with past practice prior to the Termination Effective Date, and in accordance with Applicable Laws, in a manner that will not reflect adversely upon the goodwill and reputation associated with XenoPort or the Transferred GSK Trademarks. GSK acknowledges XenoPort’s ownership of the Transferred GSK Trademarks, and GSK shall not knowingly do anything inconsistent with such ownership and agrees that all use of the Transferred GSK Trademarks by GSK shall inure to the benefit and be on behalf of XenoPort. Nothing in this Termination Agreement shall give GSK any right, title or
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 35 -
EXECUTION COPY
interest in the Transferred GSK Trademarks or any other XenoPort Trademark other than the right to use the Transferred GSK Trademarks strictly in accordance with this Section 4.4(b). GSK shall not have the right to, and shall not, sublicense (except solely to the extent otherwise provided in Section 3.1(b)), assign, pledge, grant or otherwise encumber or transfer to any Third Party any rights licensed by XenoPort to GSK with respect to the Transferred GSK Trademark without XenoPort’s prior written consent; provided, however, for the avoidance of doubt, the foregoing shall not be construed to require XenoPort’s consent for GSK or its Affiliate to engage and use distributors for the distribution and sale, on behalf of GSK or its Affiliate, of Products bearing Transferred GSK Trademark. The Parties understand and agree that, in addition to all other legal remedies, [… * …] If GSK shall breach this Section 4.4(b) (whether or not cured), XenoPort shall have the right to immediately terminate the license to GSK with respect to the GSK Transferred Trademark by providing written notice to GSK. Nothing in this Section 4.4(b), or any other provision of this Termination Agreement shall grant XenoPort any rights in any of GSK’s Domain Names, registrations or applications for registration, or renewals thereof, registered in the Territory or any other country or jurisdiction throughout the world, except for the Transferred GSK Domain Names.
(c) GSK shall have the sole right and responsibility to manufacture and supply Product for use by GSK, its Affiliates, or their respective designees in connection with the commercialization and sale of Product in the Territory as set forth in this Section 4.4. During the Transition Period, GSK shall continue to have sole oversight over all aspects of the manufacturing relating to such Product to be sold by GSK and, in no event, will XenoPort interfere (or attempt to change) any manufacturing processes related to such Product; provided, however, that XenoPort shall have the right to review and consent to any change to manufacturing processes, if any, that may supplement, alter, or change the Regulatory Filings. GSK acknowledges that nothing in this Termination Agreement shall preclude XenoPort from manufacturing Compound or Product at any time following the Termination Effective Date for sale outside the Territory or for sale in the Territory following the Transition Period End Date. Notwithstanding anything to the contrary contained in this Termination Agreement, during the period of time prior to the Transition Period End Date, GSK shall have the sole right to determine whether any recall, withdrawal, field correction, field alert report, or comparable report with respect to any Product in the Territory (a “Product Event”) is required to be made, and shall develop (if applicable) appropriate correspondence and communications regarding such Product Event for submission to a Regulatory Authority, provided that GSK shall in good faith consult with XenoPort regarding any such decision and/or submission. [… * …] XenoPort, if required as the owner of the Regulatory Filings after the Termination Effective Date, shall submit any such correspondence and/or communications developed as part of a Product Event in accordance with this Section 4.4(c) (and within the time periods required by Applicable Laws); provided, however, that XenoPort shall not be obligated to take any action (including filing such correspondence or communication) which XenoPort determines in good faith in its sole discretion would not be in compliance with Applicable Laws; and further provided, however, that to the extent that GSK is able to make any such submissions to a Regulatory Authority as XenoPort’s agent (as set forth in Section 4.3(b)(i)), then GSK shall make such submissions on XenoPort’s behalf. XenoPort and GSK each agree [… * …] In the event that GSK is required to separately notify a Regulatory Authority regarding a Product Event to comply with Applicable Laws (including any corporate integrity agreement that may be in effect), GSK shall have the right to provide such notice without consultation with XenoPort; provided that [… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 36 -
EXECUTION COPY
(d) Within thirty (30) days after the Termination Effective Date, the Parties agree to enter into a quality agreement that outlines each Party’s responsibilities with respect to the manufacture of Product during the Transition Period (the “Quality Agreement”). Each Party shall comply with the terms and conditions of the Quality Agreement.
(e) Notwithstanding anything contained herein, GSK shall have no obligation under this Termination Agreement to commercialize, market, promote, sell and/or distribute the Product in the Territory (including using any Promotional Materials) in any manner that, in any case as determined by GSK in good faith in its sole discretion, violates Applicable Laws or GSK’s Applicable Policies or Procedures. Further, GSK shall have no liability to XenoPort or XenoPort’s Affiliates for taking or refraining from taking any action to commercialize, market, promote, sell and/or distribute the Product in the Territory in order to, in each case as determined in GSK’s sole discretion, comply with Applicable Laws or GSK’s Applicable Policies or Procedures.
(f) Without limiting Section 4.8, XenoPort acknowledges and agrees that during the Transition Period, it will not, directly or indirectly through Affiliates or Third Parties, commercialize, market or otherwise promote the Product in the Territory. Further, during the Transition Period, XenoPort [… * …] provided, however, that the foregoing shall [… * …] In the event of a breach by XenoPort of this Section 4.4(f), GSK shall have a right, upon providing written notice to XenoPort, to immediately cease commercializing, marketing, promoting and selling the Product in the Territory for the remainder of the Transition Period; provided, however, that GSK would continue using its Commercially Reasonable Efforts to distribute the Product in the Territory in accordance with Applicable Laws and GSK’s Policies and Procedures for the remainder of the Transition Period.
(g) In the event that Applicable Laws prevent XenoPort from distributing or selling the Product in the Territory after the Transition Period End Date and XenoPort requests GSK to continue distributing and selling Product, GSK shall, using Commercially Reasonable Efforts and in its sole discretion, distribute the Product in the Territory until such time as agreed to by the Parties (or, if the Parties do not or cannot mutually agree, no later than [… * …] days following written notice by XenoPort requesting GSK to discontinue such distribution and sales). GSK shall provide to XenoPort all payments (if any) received by GSK or its Affiliates with respect to Product sold by or on behalf of GSK or its Affiliates after the Transition Period End Date pursuant to this Section 4.4(g), and invoice XenoPort for all costs and expenses incurred by GSK, its Affiliates or their respective designees in distributing such Product after the Transition Period End Date, and XenoPort shall reimburse GSK for such costs and expenses within thirty (30) days after receipt of such invoice. Notwithstanding the foregoing, GSK shall have no obligation whatsoever to commercialize, market or otherwise promote the Product in the Territory after the Transition Period End Date.
(h) GSK agrees that it and its Affiliates shall not sell Product during the period from the Termination Effective Date to the Transition Period End Date in quantities that are materially higher than quantities of such Product sold by GSK and its Affiliates in the calendar quarter prior to the Termination Effective Date (or, if higher, the volumes specified in the mutually agreed Commercialization Transition Plan); provided, however, that the foregoing shall not be construed to prevent GSK and its Affiliates from selling such quantities of Product as are reasonably required to meet demand for Product in the Territory up to the Transition Period End Date.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 37 -
EXECUTION COPY
4.5 Supply Agreement. By written notice provided to GSK not later than [… * …] (the “Interim Supply Notice”), XenoPort may request that GSK manufacture and supply (or arrange for the manufacture and supply of) quantities of Product, for commercialization by or on behalf of XenoPort in the Territory, with such supply obligation to commence on the date XenoPort submits to GSK the Interim Supply Notice in accordance with this Section 4.5 and continue until October 30, 2013 (the “Supply Term”), at a supply price and upon terms to be agreed by the Parties in a separate supply agreement. Any such supply agreement entered into by the Parties shall be consistent with the terms set forth on Schedule 4.5 attached hereto (the “Supply Agreement”). The Parties shall use Commercially Reasonable Efforts to negotiate and execute the definitive written Supply Agreement promptly following the Termination Effective Date, [… * …] (provided, however, that the Parties shall have entered into an appropriate quality agreement covering responsibilities that will apply to the Product sold to XenoPort pursuant to this Section 4.5, including responsibilities for relevant quality assurance and quality control activities related thereto), [… * …]. If, in accordance with the above, XenoPort requests that GSK provide interim supply of Product, the following shall apply:
(a) XenoPort shall issue firm purchase orders (each, a “Firm Order”) for those quantities of Product to be supplied to XenoPort during the term of the Supply Agreement, which shall be provided to GSK [… * …] XenoPort may issue up to [… * …].
(b) The Firm Orders may be delivered electronically or by other means to such location as GSK shall designate and shall include (i) [… * …]. Product quantities shall be ordered by XenoPort in accordance with agreed minimum quantities and consistent with agreed forecasted demand in effect for such Product, in each case, as set forth on Schedule 4.5B.
(c) If after GSK’s review of XenoPort’s Firm Orders, questions arise based on [… * …] Once all questions relating to [… * …] GSK shall be deemed to have accepted such Firm Order; provided, however, that any delay in resolving such questions may impact the [… * …]. In the event that the Parties are not reasonably able to agree to quantities included in any Firm Order, [… * …] GSK shall proceed with supplying those quantities of Product that are agreed to by the Parties (and, therefore, not under review). XenoPort [… * …]
(d) [… * …]
(e) If XenoPort issues the Interim Supply Notice to GSK, then on that same date, XenoPort shall provide to GSK all required artwork to update Labels and/or Labeling (the “Artwork”) for use on Product supplied pursuant to the Supply Agreement, which shall include, among other things, XenoPort’s NDC number(s) for the Product, and the following terms of this
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 38 -
EXECUTION COPY
Section 4.5(e) shall apply. XenoPort shall ensure that all labels, packaging, or trade dress for use on Product supplied under the Supply Agreement shall be approved by the appropriate Regulatory Authority prior to commercial distribution of such Product. XenoPort shall be solely responsible for ensuring the accuracy of all information contained on the Labels and/or Labeling for the Product and for the compliance of all such Labels and/or Labeling with Applicable Laws and the specifications contained in the applicable NDA. If (i) XenoPort is required pursuant to Applicable Laws to revise or change Labels and/or Labeling or (ii) XenoPort requests a revision or change of Labels and/or Labeling for reasons other than compliance with Applicable Laws, [… * …]
(f) After the Transition Period End Date or, if effective, the expiration or termination of the Supply Agreement, XenoPort will be solely responsible for the manufacturing and packaging of Product (including, without limitation, API, Bulk Product, and Finished Product) and shall ensure, at its own cost and expense, that any approvals or certifications required by any Regulatory Authority are in place prior to the manufacture, distribution or sale of Products.
(g) Except to the extent otherwise directed by the FDA or required by Applicable Laws, GSK shall not knowingly make any material modifications to its manufacturing process for Product during the Transition Period without providing prior written notice to XenoPort (or as provided under mutually agreed terms, if any, of the definitive written Supply Agreement).
4.6 Manufacturing Technology Transfer. Following the Termination Effective Date, [… * …] to effect the technical transfer relating to the manufacturing and packaging of Product by (i) [… * …] and (ii) with [… * …] In the event that additional technical transfer support is required, and for a period not to exceed [… * …] following the Termination Effective Date, and in accordance with the technology transfer plan set forth on Exhibit 4.6 attached hereto (the “Manufacturing Technology Transfer Plan”), [… * …] with the object of transitioning the manufacture and packaging of commercial Product to XenoPort. [… * …] XenoPort will be responsible for all activities related to the development and preparation of data for use in regulatory submissions.
4.7 Transition Team. The Parties acknowledge and agree that effective upon the Termination Effective Date, all of the committees (and subcommittees, if applicable) established pursuant to Article III of the Restated Agreement shall be immediately disbanded. Within ten (10) days after the Termination Effective Date, the Parties shall form a team composed of five (5) representatives of each Party (the “Transition Team”) to facilitate the transition of the manufacture, development and commercialization of Compound and Product from GSK to XenoPort pursuant to this Termination Agreement and to facilitate communication of information to be exchanged under this Termination Agreement, in each case, during the Transition Period. The Transition Team shall coordinate and facilitate communications between personnel of the various operational groups of the Parties involved in the transfer of information and transition of activities under this Termination Agreement, which shall be consistent with (and subject to) any project plans agreed in this Termination Agreement (including, without limitation, the Manufacturing Technology Transfer Plan) and shall assist in the delivery and transfer of the Transferred Assets and Licensed Assets, including undertaking the duties set forth in Sections 4.2(a), 4.2(b) and 5.4(a). During the Transition Period, the Transition Team shall meet periodically (but not less than once every two (2) weeks), by
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 39 -
EXECUTION COPY
telephone or in person, to discuss the status of the transition (including any material modification or additions to the items or matters listed in the various Schedules that identify the Transferred Assets) and to attempt to amicably resolve any disagreements or miscommunications between the Parties that may arise in connection with information to be exchanged, timelines for transfer of documents or information, transition of Regulatory Filings or the like. The Transition Team is intended to facilitate the smooth transition of activities and responsibilities regarding Compound and Product pursuant to this Termination Agreement, and may serve as a contact point and initial forum for discussing any matters arising in connection with the performance of this Termination Agreement, and shall cooperate when necessary to facilitate referring any such matters to appropriate decision-makers of each Party for prompt resolution; however, the Transition Team shall not have authority to amend or modify the Commercialization Transition Plan, the Development Plan, the Manufacturing Technology Transfer Plan, or the terms of this Termination Agreement, the Supply Agreement or the GSK Safety Agreement (as defined below). The Transition Team may designate other personnel from the relevant operation group(s) of the Parties to be the primary contact to facilitate discussions or transfer of documents and information related to particular matters. The Parties shall make available to the Transition Team the applicable functional area experts and personnel necessary to further facilitate the communication of information to be exchanged under this Termination Agreement. [… * …] and GSK will reasonably cooperate with XenoPort to identify other GSK personnel with comparable familiarity with the Product and comparable experience to assist and participate with such transition matters in the event such an individual leaves employment or becomes unavailable.
4.8 XenoPort Activities During Transition.
(a) Notwithstanding the provisions of Section 4.4 (stating that GSK may sell, market, promote, distribute and otherwise commercialize Product “exclusively” in the Territory) or any other provision of this Termination Agreement, and except for activities that would interfere with GSK’s ability to comply with Applicable Laws or GSK’s Applicable Practices and Policies, XenoPort shall have the right during the Transition Period to perform such activities as XenoPort deems reasonably necessary or appropriate in connection with its preparation to take over the manufacture, development, commercialization, promotion and distribution of the Compound and Product in or for the Territory after the Transition Period End Date, and nothing in this Termination Agreement shall be construed to prohibit or preclude XenoPort from conducting such activities during the Transition Period: including (i) negotiating and entering into contracts for the manufacture, conduct of clinical trials, licensing, distribution, storage, or promotion of Product (provided, however, that XenoPort may not commence promoting the Product or selling Product prior to the Transition Period End Date); (b) manufacturing or obtaining inventories of Compound or Product in advance of the Transition Period End Date in preparation for sales of Product in the Territory by or under authority of XenoPort after the Transition Period End Date; (c) negotiating and entering into agreements with managed care organizations and others regarding Product to be sold in the Territory by or under authority of XenoPort after the Transition Period End Date; (d) contacting and negotiating with Regulatory Authorities and others with regard to reimbursement for Product sold in the Territory by or under authority of XenoPort after the Transition Period End Date; (e)
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 40 -
EXECUTION COPY
preparing and obtaining quantities of Promotional Materials and product labeling for Product that will be commercialized and sold by or under authority of XenoPort after the Transition Period End Date; (f) organizing a physician advisory board, and preparing and conducting meetings therefor, which would only occur after the Transition Period End Date; (g) planning and entering into commitments for medical congresses or trade shows that would only occur after the Transition Period End Date; and (h) planning of sales activities and medical education activities, and preparing related materials, and hiring and training sales representatives, medical liaisons and other personnel who will be involved in the commercialization of the Product in the Territory after the Transition Period End Date. For the avoidance of doubt, none of the foregoing, nor the covenants of XenoPort set forth in Sections 4.4(f) and 7.2(a), nor any other provision of this Termination Agreement shall be construed to limit or restrict XenoPort’s activities with respect to Compound or Product sold in, or manufactured or developed in or for, any country outside the Territory.
(b) Prior to the Transition Period End Date, XenoPort shall initiate the process to obtain an MDRP Agreement (“MDRP”), Pharmaceutical Pricing Agreement and FSS Agreement. In addition, XenoPort will ensure that the Government Price Reports under Section 5.5 will be administered by XenoPort or a Third Party consultant specializing in government price reporting on the Transition Period End Date.
4.9 Safety Data Exchange. Until the Transition Period End Date or, if earlier, such time as XenoPort has a functioning safety database, GSK shall continue to be responsible for pharmacovigilance and adverse event reporting within the Territory as provided in that certain Safety Data Exchange Agreement, dated as of February 13, 2012, between the Parties (the “GSK Safety Agreement”). Thereafter, the Parties will enter into a new safety data exchange agreement (the “New Safety Agreement”) with respect to the Low Dose Study which will set forth the procedure pursuant to which serious adverse events from the Low Dose Study will be forwarded by GSK to XenoPort and expedited to the FDA. Further, as between the Parties, pursuant to the New Safety Agreement and thereafter, XenoPort shall be responsible for pharmacovigilance and adverse event reporting within the Territory with respect to Compound and Product. The Parties shall each use Commercially Reasonable Efforts to cooperate such that XenoPort (or XenoPort’s designee) will be in a position to take over, as of the Transition Period End Date and pursuant to the New Safety Agreement, the maintenance of the worldwide safety database for the Product, including transferring all Transferred GSK Know-How pertaining to pharmacovigilance and adverse event reporting of Compound pursuant to the plan set forth in Exhibit 4.9 attached hereto (the “Safety Data Exchange Transfer Plan”). In the event that XenoPort is not in a position to take over, as of the Transition Period End Date and pursuant to the New Safety Agreement, the maintenance of the worldwide safety database for the Product after the Transition Period End Date, GSK shall continue to be responsible for pharmacovigilance and adverse event reporting within the Territory as provided in the GSK Safety Agreement, and shall invoice XenoPort for all costs and expenses incurred by GSK, its Affiliates and/or their respective designees in conducting such activities. XenoPort shall reimburse GSK for such costs and expenses within thirty (30) days after receipt of such invoice. The Parties shall promptly implement any appropriate amendments to the GSK Safety Agreement in accordance with this Section 4.9.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 41 -
EXECUTION COPY
4.10 Cooperation Regarding Third Party Contractors. The Parties acknowledge that GSK and its Affiliates have used or are using certain Third Party contractors for the manufacture of the Compound or Product (including without limitation [… * …]), the conduct of clinical trials and other development activities (including without limitation stability testing) related to the Compound or Product in the Territory, and storage and distribution of the Compound or Product in the Territory, and anticipate that XenoPort may contact one or more such Third Party contractors to negotiate potential agreements for such Third Party contractors to conduct similar activities with respect to Compound or Product in the Territory for XenoPort or its designee; accordingly, the Parties agree as follows with respect to such Third Party Contractors:
(a) During the Transition Period (and with respect to the contract research organization(s) involved with the conduct of the Low Dose Study, until Completion of the Ongoing Studies), if requested by XenoPort, GSK shall use Commercially Reasonable Efforts to identify to XenoPort the Third Party contractors so used by GSK or its Affiliates, to facilitate an introduction of XenoPort or its designee to such Third Party contractors. [… * …] If, in order for GSK to fulfill any commitments under this Section 4.10(a), XenoPort is required to agree to terms of confidentiality, provide certain assurances, or to take any other actions that may be required in order to obtain access to such Third Party contractors, then any failure by XenoPort to comply with these requirements will be deemed a waiver by XenoPort of its rights under this Section 4.10(a) with respect to access to any such Third Party contractors. XenoPort shall not be required to make any payment for access to any such Third Party contractor. Further, [… * …] Nothing herein shall be construed or deemed as obligating GSK to breach any provision in any Contract with a Third Party contractor, including, without limitation, any obligations of confidentiality with respect to such Contract. Further, neither GSK nor its Affiliates shall have any liability of any kind whatsoever under or with respect to any Contract that may be entered into by XenoPort, its Affiliates and/or their respective designees and a Third Party contractor; and
(b) In the event and to the extent that any such Third Party contractor as described in this Section 4.10 that is a contract manufacturer or contract research organization has possession of, or a right or license to use, any Know-How (including without limitation clinical Data or manufacturing Know-How) or Patent directly and primarily related to the Compound or Product, GSK hereby waives on behalf of itself and its Affiliates any restrictions or limitations (including, if applicable, licenses to GSK or its Affiliate) contained in any contract between such Third Party contractor and GSK or its Affiliate that (i) would restrict, limit or preclude such Third Party contractor from (A) disclosing or providing such Know-How or Patent to XenoPort or its designee and licensing or otherwise authorizing its use for the manufacture, development and commercialization of the Compound or Product, or (B) accessing and using such Know-How or Patent itself in conducting activities for the manufacture, development and commercialization of the Compound or Product for XenoPort or its designee, or (ii) would impose a requirement for payment from the applicable Third Party to GSK or its Affiliate for any actions described in the receding clause (i). If requested by XenoPort or the applicable Third Party contractor, GSK agrees that it or its Affiliate will confirm any waiver in writing to the applicable Third Party contractor.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 42 -
EXECUTION COPY
4.11 Validation Activities. XenoPort acknowledges that certain validation activities have been on-going with respect to the 300mg (gabapentin enacarbil) strength Product (the “300mg Product”) to allow for commercial supply of the 300mg Product. [… * …] With respect to any API required to [… * …] (i) for such quantities as are required prior to the Inventory Transfer Date to conduct such activities, GSK shall use quantities of API from the Inventory in GSK’s possession, and (ii) for such quantities as are required after the Inventory Transfer Date to conduct such activities, GSK may place orders for such quantities of additional API in accordance with the principles set forth in Section 4.2(h) above, and any such API supplied to GSK pursuant to this Section 4.11 shall be included within the meaning of “Tolled API” (and, if applicable, subject to the terms agreed in Section 4.2(h) above). For the avoidance of doubt, in the event that XenoPort is not able to provide additional quantities of API in accordance with this Section 4.11, and in such case, GSK is prevented from fulfilling its agreed obligations in this Section 4.11 (and only to the extent it is precluded from fulfilling these obligations), [… * …] Notwithstanding anything to the contrary contained in this Termination Agreement, XenoPort acknowledges and agrees that GSK will be under no further obligation to create (or attempt to create), or assist XenoPort in creating (or attempting to create) any Improvements with respect to the Product after the Termination Effective Date, including, without limitation, Improvements to the manufacturing processes of the Product.
ARTICLE V
ADDITIONAL AGREEMENTS BETWEEN THE PARTIES
5.1 Return of Materials. Within thirty (30) days after the Transition Period End Date and upon the written request by XenoPort, GSK shall use Commercially Reasonable Efforts to destroy (and certify in writing to XenoPort) all tangible items comprising Confidential Information of XenoPort that are in GSK’s possession (other than, for the avoidance of doubt, any Transferred Assets to be transferred to XenoPort under the terms of this Termination Agreement), except that GSK shall be permitted to retain one (1) copy of such tangible items including such Confidential Information of XenoPort as necessary to permit GSK to perform its obligations after the Transition Period End Date under this Termination Agreement.
5.2 Inspection.
(a) To the extent any Regulatory Authority requests an inspection or audit of, or XenoPort otherwise reasonably requests to inspect or audit, the clinical trial sites and/or manufacturing sites of GSK or its Affiliates or other applicable GSK facilities with respect to Compound or Product (including inspection or audit of any underlying raw or original source data at the clinical trial sites and/or manufacturing sites), or XenoPort or its licensee is otherwise required by Applicable Laws to conduct such audits or inspections, GSK shall permit such Regulatory Authority and XenoPort (together with its licensee, as applicable) to enter the relevant clinical trial sites and, if applicable, manufacturing sites or other applicable facilities of GSK and its Affiliates during normal business hours and upon reasonable advance notice to inspect such sites and to verify compliance with applicable regulatory requirements. GSK shall provide reasonable assistance for a full and correct carrying out of the inspection. Such inspection shall not relieve GSK of any of its obligations under this Termination Agreement. Notwithstanding the above, XenoPort acknowledges
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 43 -
EXECUTION COPY
and agrees that any inspection of GSK (or its Affiliate’s) manufacturing facility(ies) by XenoPort or its licensee (but not by any Regulatory Authority) shall be limited to those portions of the facility(ies) related to the manufacture of Product and for the sole purpose of ensuring such facility’s compliance with cGMP and Applicable Laws and [… * …]
(b) GSK shall use Commercially Reasonable Efforts to secure the rights of inspection set forth under Section 5.2(a) above from GSK’s trial sites and other contractors (including, but not limited to, manufacturers of Compound or Product) for Product. [… * …]
(c) Any inspection by XenoPort or its licensee (but not by any Regulatory Authority) of any manufacturing facility(ies) (whether pursuant to Section 5.2(a) or Section 5.2(b)) will be [… * …]
5.3 Product Responsibility.
(a) Except as otherwise set forth in this Termination Agreement or in the Supply Agreement, as applicable, from and after the Transition Period End Date, XenoPort shall have the sole control and responsibility for (i) all regulatory matters with respect to Product, including without limitation relating to communicating and corresponding, preparing and filing reports, making adverse drug experience reports, and paying applicable fees, with and to applicable Regulatory Authorities, under all Applicable Laws, including the United States Federal Food, Drug and Cosmetic Act, as amended from time to time, including all regulations promulgated thereunder, the Prescription Drug Marketing Act of 1987, and the Prescription Drug User Fee Act of 1992; (ii) taking all actions and conducting all communication with Third Parties in respect of Product (whether sold before or after the Transition Period End Date), including responding to all complaints in respect thereof (except for any complaints managed by GSK as set forth below in Section 5.14(b)) and all medical information requests; and (iii) investigating all complaints and adverse drug experiences in respect of Product (except for any complaints managed by GSK as set forth below in Section 5.14(b)).
(b) Notwithstanding anything to the contrary contained in this Termination Agreement or in the Supply Agreement, if applicable, during the period of time from and after the Transition Period End Date, XenoPort shall have the sole right to determine whether any Product Event is required to be made, and to develop (if applicable) appropriate correspondence and communications regarding such Product Event for submission to a Regulatory Authority, and shall have the sole control and be responsible for conducting, handling or processing all such Product Events (including withdrawals or voluntary and involuntary recalls of units of Product, regardless of whether such unit of Product was sold before or after the Transition Period End Date); provided, however, that if any such Product Event involves Product sold prior to the Transition Period End Date, then (i) XenoPort shall notify GSK of, and if requested consult with GSK regarding, any decision (or requirement) to withdraw or recall Product sold before the Transition Period End Date, (ii) GSK shall in good faith consult with XenoPort as XenoPort may reasonably request regarding any such matters, (iii) XenoPort and GSK each agree [… * …] and (iv) XenoPort shall destroy, or cause to be destroyed, in either case at XenoPort’s expense, all recalled Product in a manner consistent with Applicable Laws. [… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 44 -
EXECUTION COPY
(c) [… * …] following the Transition Period End Date, XenoPort shall use Commercially Reasonable Efforts to institute appropriate procedures (including any appropriate procedures for tracking of lot number information) to ensure that the Product sold by or on behalf of XenoPort can be distinguished from the Product sold by or on behalf of GSK.
(d) [… * …] XenoPort shall use Commercially Reasonable Efforts to establish new NDC numbers for Product and thereafter shall use Commercially Reasonable Efforts to cause such NDC numbers to be used on the labeling and packaging for Product.
5.4 Product Returns.
(a) During the Transition Period and for [… * …] after the Transition Period End Date, GSK shall be financially responsible for, and shall be responsible for processing, all returns of Product packaged for commercial sale in the Territory (“Finished Product”) labeled with GSK’s NDC number. During the Transition Period and for [… * …] thereafter, all returns of Finished Product labeled with GSK’s NDC number shall be sent to GSK or GSK’s return vendor. Returns of Finished Product will be processed GSK as provided in this Section 5.4(a) in accordance with GSK’s then-current returned goods policy, regardless of which Party made the corresponding original sale. XenoPort will provide to GSK an invoice for all out-of-pocket costs incurred by XenoPort and its Affiliates with respect to returns of any Finished Product during the Transition Period and for [… * …] after the Transition Period End Date, and GSK will pay to XenoPort such amount within thirty (30) days of receipt of such invoice.
(b) XenoPort shall be financially responsible for, and shall be responsible for processing, all returns of Finished Product labeled with XenoPort’s NDC number, and on and after the [… * …] after the Transition Period End Date, XenoPort shall be financially responsible for, and shall be responsible for processing, all returns of Finished Product labeled with GSK’s NDC number. Returns of Finished Product will be processed by XenoPort as provided in this Section 5.4(b) in accordance with XenoPort’s then-current returned goods policy, regardless of which Party made the corresponding original sale. GSK will provide to XenoPort an invoice for all out-of-pocket costs incurred by GSK and its Affiliates with respect to returns of any Finished Product on and after the [… * …] after the Transition Period End Date, and XenoPort will pay GSK such amount within thirty (30) days of receipt of such invoice.
(c) The Parties, through the Transition Team, shall agree upon a joint notice, which will notify all customers of the foregoing changes in trade return procedures as soon as practicable following the Transition Period End Date, but in any event no later than ten (10) Business Days prior to the Transition Period End Date. For any Finished Product that is returned to one Party but is the processing responsibility of the other Party pursuant to this Section 5.4, the Party receiving such returned Finished Product will destroy, or cause to be destroyed, all such Finished Product. Each of XenoPort and GSK shall destroy, or cause to be destroyed, all such returned Finished Product in a manner consistent with Applicable Laws and the costs of such destruction shall not be reimbursed.
(d) Neither XenoPort nor GSK shall instruct, recommend or attempt to induce customers who have previously purchased any Finished Product from it to (i) return such Finished Product when that would not otherwise have been the case but for such Party’s instructions, recommendations or inducement or (ii) delay the return of such Finished Product. For the avoidance of doubt, a Party’s shipment of units of Products to customers in the ordinary course will not be deemed to violate this Section 5.4.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 45 -
EXECUTION COPY
5.5 Governmental Price Reporting.
(a) “Governmental Price Reports” means (i) any and all data required to be reported relating to the Product under the MDRP, including without limitation the Average Manufacturer Price (as defined at 42 U.S.C. § 1396r-8(k)(1)) (“AMP”) and the Best Price (as defined at 42 U.S.C. § 1396r-8(c)(1)(C)); (ii) any and all data required to be reported relating to the Product(s) under state drug price reporting requirements; (iii) Federal Ceiling Prices required to be reported relating to the Product under 38 U.S.C. § 8126, and the master agreements issued thereunder; (iv) any and all data required to be reported relating to the Product under a Tricare retail rebate agreement or otherwise under 10 U.S.C. § 1074f(g); (v) any and all data required to be reported relating to the Product under a Pharmaceutical Pricing Agreement under section 340B of the PHS Act, 42 U.S.C. 256b; (vi) any and all data required to be reported relating to the Medicare Coverage Gap Discount Program (“Coverage Gap Program”) for purposes of sections 1860D-14A and 1860D-43 of the Social Security Act (the “Social Security Act”), as set forth in the Patient Protection and Affordable Care Act of 2010 (“PPACA”), Pub. L. 111-148, and the Health Care and Education Reconciliation Act of 2010, (“HCERA”) Pub. L. 111-152; and (vii) any and all data required to be reported relating to the Product under the Pharmaceutical Manufacturer Industry Fee Program pursuant to section 9008 of the PPACA, Public Law 111-148 (124 Stat. 119 (2010)), as amended by section 1404 of the HCERA, Public Law 111-152 (124 Stat. 1029 (2010)).
(b) GSK shall be responsible for submitting all Governmental Price Reports for all Product bearing an NDC of GSK for all reporting periods ending on or before the Transition Period End Date. Beginning with the reporting period in which the Transition Period End Date occurs, XenoPort shall be responsible for submitting all Governmental Price Reports for Product bearing an NDC of GSK. Effective with the reporting period in which the Transition Period End Date occurs, GSK shall name XenoPort as a designee, with certification access, in the Drug Data Reporting for Medicaid (“DDR”) system for purposes of reporting the AMP and the Best Price and other required pricing data for the Product bearing an NDC of GSK. If other Product is reported to the Centers for Medicare and Medicaid Services (“CMS”) by GSK under the labeler code of any Product, GSK shall remain as the technical, invoice and legal contacts for such Product with CMS.
(c) GSK acknowledges that XenoPort will require certain information from GSK in order to submit the Governmental Price Reports after the Transition Period End Date. Accordingly, GSK agrees that, (i) for the calendar month in which the Transition Period End Date
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 46 -
EXECUTION COPY
occurs, GSK shall provide to XenoPort, within [… * …] after the end of such calendar month, relevant Product information for use in calculating AMP, and if requested by XenoPort, GSK would provide 12 months of summary monthly historical commercial sales and sales deduction data; (ii) for the calendar quarter in which the Transition Period End Date occurs, GSK shall provide to XenoPort, within [… * …] after the end of such calendar quarter, the Best Price, customary prompt pay discounts, and sales at nominal price, and any additional data needed to comply with state price reporting requirements, and (iii) GSK shall provide to XenoPort, within [… * …] after the Transition Period End Date, baseline AMPs for the Product. GSK further agrees that, for Product, it shall provide to XenoPort, within [… * …] after the Transition Period End Date, the following: (i) if the Transition Period End Date occurs in calendar quarters 1, 2, or 3, GSK shall provide the Federal Ceiling Prices in effect during the calendar year in which the Transition Period End Date occurs; (ii) if the Transition Period End Date occurs in calendar quarter 4, GSK shall provide both the Federal Ceiling Price in effect during the calendar year in which the Transition Period End Date occurs and the calculated Federal Ceiling Price for the next calendar year. Per the Department of Veteran Affairs (“VA”) Product transfer rules, depending on the calendar quarter in which the Transition Period End Date occurs, XenoPort is required to sell at a price no greater than GSK’s calculated Federal Ceiling Prices in effect during the calendar year in which the Transition Period End Date occurs, or if the Transition Period End Date occurs in calendar quarter 4, XenoPort is required to sell at a price no greater than GSK’s calculated Federal Ceiling Prices for the next calendar year; (iii) all commercial sales and sales deduction data required for Non-FAMP calculation for the current calendar quarter through the Transition Period End Date, as well as such data for preceding calendar quarters within the Federal Fiscal Year (October 1 – September 30) in which the Transition Period End Date occurs; (iv) relevant Non-FAMPs necessary to complete Annual Public Law Update; (v) all commercial sales and sales deduction data for the current calendar quarter through the Transition Period End Date, as well as all such data for the preceding calendar quarters within the Federal Fiscal Year (October 1 – September 30) in which the Transition Period End Date occurs; and (vi) the most recent Tricare retail pharmacy dispensing data report for the previous four quarters made available by the Department of Defense either on or before the Transition Period End Date. All pricing data furnished by GSK pursuant to this subsection shall be provided in the same format that such data are provided by GSK to CMS and/or the VA in connection with GSK’s own Governmental Price Reports, or in a format otherwise prescribed by GSK. For pricing and sales data that are required in order to submit Governmental Price Reports, but that are not themselves provided to the CMS and/or the VA, GSK will furnish that data in a format prescribed by XenoPort. Without limiting the foregoing, any and all data furnished by GSK that are required for the reporting of the Product under the MDRP (and under Medicare Part B) pursuant to this subsection shall be certified in the same format provided by GSK to CMS.
(d) Without limiting the foregoing, GSK agrees that, from and after the Transition Period End Date until [… * …] bearing an NDC of GSK, GSK shall provide to XenoPort, within [… * …] after the end of each reporting period, all data or other information to the Governmental Price Reports for such Product reasonably requested by XenoPort for use by XenoPort in completing such Governmental Price Reports.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 47 -
EXECUTION COPY
(e) GSK shall promptly notify XenoPort upon discovery of any errors on or corrections to the data or other information provided to XenoPort pursuant to Sections 5.5(b), (c) or (d) above.
(f) XenoPort shall notify GSK of any change in the wholesale acquisition cost (“WAC”) of any Product(s) bearing GSK’s NDC no later than the same time that XenoPort notifies third party vendors of the change in such WAC.
(g) XenoPort shall notify GSK of the date(s) of last lot expiration of Product when the NDC(s) therefor are discontinued.
(h) For use in the payment of any and all Medicaid rebates (including Medicaid supplemental rebates, state pharmaceutical assistance program (“SPAP”) rebates, and AIDS drug assistance program (“ADAP”) rebates) payable for all Product bearing an NDC of GSK, XenoPort shall immediately notify GSK in writing of any quarterly AMP or Best Prices, including re-stated values, submitted to CMS bearing the NDC of GSK for periods in and after the Transition Period End Date occurs.
5.6 GSK Governmental Payments and Other Contractual Obligations.
(a) GSK shall be solely and exclusively responsible for processing and payment of any and all Medicaid rebates (including Medicaid supplemental rebates, SPAP rebates, and ADAP rebates) payable for all Product bearing an NDC of GSK for periods prior to and including the calendar quarter in which the Transition Period End Date takes place.
(b) GSK shall be solely and exclusively responsible for processing and payment of any and all Tricare retail refunds owed based on Tricare retail pharmacy dispensing of all Product(s) bearing an NDC of GSK for which utilization data is available or has been made available electronically or in writing for periods prior to and including the calendar quarter in which the Transition Period End Date takes place. In the event that XenoPort does not have an existing Tricare agreement, GSK shall continue to pay such Product retail refunds until such Product has been deleted from GSK’s Pricing Agreement with Tricare Management Activity and added to XenoPort’s Pricing Agreement with Tricare Management Activity (even if the due date for refund payment falls after the Transition Period End Date).
(c) GSK shall be solely and exclusively responsible for processing and payment of any and all rebates, chargeback claims and related fees payable under a PHS pharmaceutical pricing agreement for [… * …] after the Transition Period End Date and providing the initial PHS prices within [… * …] of the Transition Period End Date for the Product and the subsequent quarter’s PHS prices within [… * …] of the start of the quarter to XenoPort. In the event XenoPort does not have an existing PHS agreement, GSK shall continue to pay rebates/chargebacks for Product bearing an NDC of GSK. XenoPort shall reimburse GSK for all rebates, chargeback claims and related fees on distributor invoices dated [… * …] or more after the Transition Period End Date.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 48 -
EXECUTION COPY
(d) GSK shall be solely and exclusively responsible for processing and payment of any and all rebates, chargebacks claims and related fees (i.e., Industrial Funding Fee) payable under GSK’s FSS contract for all Product bearing an NDC of GSK issued in writing or electronically on or prior to the Transition Period End Date up through the effective date on which the VA National Acquisition Center approves the Request for Modification to add Product to XenoPort’s FSS contract; in the event XenoPort does not have an existing FSS agreement, GSK shall continue to pay rebates, chargeback claims and related fees for Product bearing an NDC of GSK. XenoPort shall reimburse GSK for all rebates, chargeback claims and related fees (i.e., Industrial Funding Fee) on invoices dated on or after the Transition Period End Date up through the effective date on which the VA National Acquisition Center approves the Request For Modification to add Product to XenoPort’s FSS contract, but GSK shall not issue an invoice pursuant to Section 5.6(h) with respect to such amounts sooner than [… * …] following the Transition Period End Date.
(e) GSK shall be solely and exclusively responsible for processing and payment of any and all payments required under the Medicare Coverage Gap Discount Agreement for all GSK NDCs that are dispensed before and after the Transition Period End Date under the Coverage Gap Program. Accordingly, with respect to discount claims under such program on or after the Transition Period End Date, XenoPort will reimburse GSK for one hundred percent (100%) of all such paid discounts with a Pharmacy Claim Payment Date on or after Transition Period End Date. In the event that XenoPort is not able to obtain a Medicare Coverage Gap labeler agreement until 2015, GSK will continue to utilize XenoPort’s labeler code under GSK’s current Medicare Coverage Gap Discount Agreement for the Products until December 31, 2014 and XenoPort will reimburse GSK for one hundred percent (100%) of such claims for XenoPort labeler paid discounts on behalf of GSK. XenoPort shall be responsible for transferring its labeler code for the Product(s) to XenoPort Medicare Coverage Gap Discount agreement effective January 1, 2015 and such payments with respect to such discount claims for the XenoPort’s labeler will thereafter be made by XenoPort. GSK shall provide quarterly corresponding utilization summary and state payment reports within [… * …] after the end of the applicable Calendar Quarter that describe the requested rebate payments in reasonable detail.
(f) GSK shall be solely and exclusively responsible for processing and payment of any and all payments required under the Pharmaceutical Manufacturer Industry Fee under the Affordable Care Act for all GSK NDCs dispensed before and after the Transition Period End Date. With respect to the Pharmaceutical Manufacturer Industry Fees invoiced to GSK during 2012, which are based on a prior year’s sales data (e.g., 2011), and subject to any true-ups and adjustments, GSK will be responsible for payment upon receipt of invoice for such fees. With respect to Pharmaceutical Manufacturer Industry Fees invoiced to GSK during 2013 and subsequent years, which are based on prior year’s sale data (e.g., 2012 and forward), and subject to any true-ups and adjustments, GSK will be responsible for payment upon receipt of invoice for such fees. To the extent that GSK is invoiced for Product labeled with GSK NDCs after the Transition Period End Date and subsequent years, that was sold by XenoPort in 2012 and subsequent years, GSK will xxxx XenoPort for such portion. The amount billed to XenoPort shall be equal to the proportion of the fee that XenoPort would pay, assuming such sales of Product with GSK’s NDC are included as part of
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 49 -
EXECUTION COPY
GSK’s total sales in the Secretary of Treasury’s calculation of the Pharmaceutical Manufacturer Industry Fee owed by GSK. GSK shall provide detailed support of the invoiced Pharmaceutical Manufacturer Industry Fee. The Parties acknowledge that the provisions of this Section are intended to allocate responsibilities for Pharmaceutical Manufacturer Industry Fees on the basis of their respective periods of ownership. In the event that the United States Department of Treasury subsequently issues regulatory guidance with respect to the determination of such fees that mandates a mechanism the effect of which a Party believes is materially and substantially different from that contemplated herein such that it would render the allocation method described herein impracticable, inequitable, or not in compliance with any Applicable Law, it shall so notify the other Party and propose an alternative allocation method, and the Parties shall meet in good faith to discuss and agree on the alternative method.
(g) GSK shall notify XenoPort within [… * …] of notice of any inspection, investigation or other inquiry by, or other material governmental notice or communication from CMS, the Department of Health and Human Services Office of the Inspector General, or any other Regulatory Authority directly relating to the manufacture, sale, marketing, promotion, distribution, or use of the Product or relating to any Governmental Price Reports submitted by GSK. This obligation extends to any subsequent revisions to such claims even if made after the Transition Period End Date.
(h) With respect to all payments due to GSK under this Section 5.6 or Section 5.7(b), GSK shall invoice XenoPort on a quarterly basis starting after the Transition Period End Date. Payment from XenoPort shall be due within 30 days of receipt.
5.7 XenoPort Governmental Payments and Other Contractual Obligations.
(a) XenoPort shall be solely and exclusively responsible for: (i) reimbursement to GSK of any and all Medicaid rebates (including Medicaid supplemental rebates, SPAP rebates, and ADAP rebates) paid for claim quarters beginning with the calendar quarter immediately following the calendar quarter during which the Transition Period End Date takes place for all Product bearing an NDC of GSK within 30 days of receipt of an invoice for such payments from GSK; (ii) payment of any and all Medicaid rebates (including Medicaid supplemental rebates, SPAP rebates, and ADAP rebates) payable for all Product bearing an NDC of XenoPort; and (iii) notifying GSK of any state supplemental contracts for the Product bearing a GSK NDC in effect after the Transition Period End Date. With respect to section 5.7(a)(i), XenoPort’s duty to pay GSK will begin on or after October 1, 2013.
(b) XenoPort shall be solely and exclusively responsible for Tricare retail refunds relating to Product (i) bearing an NDC of GSK for which utilization data are first made available by the United States Department of Defense for periods including and after the calendar quarter after the calendar quarter in which the Transition Period End Date takes place. In the event that XenoPort does not have an existing Tricare agreement and GSK continues to pay Product retail refunds as set forth in Section 5.6(b) for some portion of such periods, XenoPort shall reimburse such amounts to GSK.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 50 -
EXECUTION COPY
(c) XenoPort shall be solely and exclusively responsible for PHS rebates, chargeback claims and related fees on: (i) distributor invoices dated [… * …] or more after the Transition Period End Date for Product bearing an NDC of GSK; and (ii) any Product bearing an NDC of XenoPort.
(d) XenoPort shall be solely and exclusively responsible for Federal Supply Schedule rebates, chargeback claims and related fees (i.e., Industrial Funding Fee) for (i) distributor invoices dated [… * …] or more after the Transition Period End Date for Product bearing an NDC of GSK; and (ii) any Product bearing an NDC of XenoPort.
(e) XenoPort shall be solely and exclusively responsible for (i) reimbursement to GSK for any and all payments required under the Medicare Coverage Gap Discount Agreement for all GSK NDCs that are dispensed after the Transition Period End Date under the Coverage Gap Program; and (ii) all payments required under the Medicare Coverage Gap Discount Agreement for XenoPort NDCs.
(f) To the extent that GSK is invoiced for Product labeled with GSK NDCs, that was sold by XenoPort, XenoPort shall be solely and exclusively liable for reimbursement to GSK for any and all payments required after the Transition Period End Date under the Pharmaceutical Manufacturer Industry Fee bearing GSK NDC. XenoPort shall be solely and exclusively liable for reimbursement to GSK of any and all payments required under the Pharmaceutical Manufacturer Industry Fee under the Affordable Care Act for XenoPort NDC after the Transition Period End Date.
5.8 Processing and Payment of Commercial Rebates, Customer Contracts or Other Contractual Obligations.
(a) Commercial Rebates. GSK shall use Commercially Reasonable Efforts to remove the Product from all contracts providing for the payment of commercial rebates in the Territory (“Rebate Contracts”), effective [… * …] following the Transition Period End Date. For clarity, the term “Rebate Contract” shall include Medicare Part D prescription drug plans, but shall exclude the Coverage Gap Program. GSK shall not assign to XenoPort, and XenoPort shall not assume, any of the Rebate Contracts. GSK shall be solely responsible for the processing, handling and payment of all rebates owed under the Rebate Contracts with respect to Product bearing an NDC of GSK dispensed prior to and including the effective date of the Product termination for each such Rebate Contract. As soon as practicable following the Transition Period End Date, GSK shall notify all relevant Third Parties of the Product termination date for all Rebate Contracts and that any future Rebate Contracts for Product must be entered into with XenoPort or its distributors. For all commercial rebate claims that are submitted to one Party but are the processing responsibility of the other Party, the Party receiving the claim shall deny the claim.
(b) Customer Contracts (Commercial Chargebacks). GSK shall use Commercially Reasonable Efforts to remove the Product from all contracts providing for the payment of chargebacks and associated contracted fees with respect to the Product(s) in the Territory (“Chargeback Contracts”), effective [… * …] following the Transition Period End Date. GSK shall
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 51 -
EXECUTION COPY
not assign to XenoPort, and XenoPort shall not assume, any of the Chargeback Contracts. GSK shall process and be financially responsible for all chargeback claims and associated contracted fees related to the Product sold in the Territory by distributors with distributor invoice to the chargeback customer (“Distributor Invoice”) on dates up to and including the Product termination date for each such Chargeback Contract (the “Chargeback Product Termination Date”). GSK shall not process and shall not be financially responsible for any chargeback claims and associated contracted fees related to Product sold by distributors with Distributor Invoice dates after such Chargeback Product Termination Date, and XenoPort shall or shall cause its distributor to process and be financially responsible for all chargeback claims and associated contracted fees related to Product sold by distributors with Distributor Invoice dates after such Chargeback Product Termination Date.
(c) Other Direct Customer Discounts. GSK shall use Commercially Reasonable Efforts to remove the Product from all contracts with direct customers providing for the issuance of rebates and other discounts to such direct customers in or for the Territory (“Direct Customer Contracts”), effective on the Transition Period End Date. GSK shall not assign to XenoPort, and XenoPort shall not assume, any of the Direct Customer Contracts. GSK shall be solely responsible for the processing, handling and payment of all rebates and other discounts owed under the Direct Customer Contracts with respect to Product sold to direct customers up to the Transition Period End Date. As soon as practicable following the Transition Period End Date, GSK shall notify all relevant Third Parties of the Product termination date for all Direct Customer Contracts and that any future Direct Customer Contracts for any Product must be entered into with XenoPort or its distributors. For all direct customer discount program claims that are submitted to one Party but are the processing responsibility of the other Party, the Party receiving the claim shall deny the claim.
(d) Patient Assistance Programs. GSK shall be authorized to continue its patient assistance programs for the Product for all patients enrolled and active in such programs as of the Transition Period End Date. XenoPort hereby authorizes GSK to reserve and utilize Product and to pay claims for eligible patients for a minimum of [… * …] following the Transition Period End Date and up to [… * …] following the Transition Period End Date, after which time, patients will be removed from such patient assistance programs. GSK shall be responsible for all costs associated with such programs, and for ensuring that such programs comply with Applicable Laws. Any reasonable, documented costs incurred by XenoPort, at GSK’s request, in connection with GSK’s patient assistance programs shall be reimbursed by GSK.
5.9 GSK’s Accounts Receivable; Payments for XenoPort Sales. XenoPort agrees to promptly deliver to GSK any amounts received by or on behalf of XenoPort (including but not limited to negotiable instruments, which shall be endorsed to the order of GSK) with respect to any and all Accounts Receivable. GSK agrees to promptly deliver to XenoPort any amounts received by or on behalf of GSK or its Affiliates (including but not limited to negotiable instruments, which shall be endorsed to the order of XenoPort) with respect to any and all payments for Products sold by or under authority of XenoPort after the Transition Period End Date.
5.10 Cooperation. With respect to Sections 5.3 through 5.9, the Parties agree to use Commercially Reasonable Efforts to cooperate in good faith, and in consultation with each other, to
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 52 -
EXECUTION COPY
establish appropriate processes and procedures for implementing the foregoing and to facilitate and manage the smooth transition of responsibilities and activities with respect to reimbursement, pricing and payment matters for the Product and related government reporting, consistent with industry standards, GSK’s Applicable Policies and Procedures and Applicable Laws.
5.11 Financial Information; Records; Audit.
(a) In accordance with the timelines set forth in Schedule 1.47, GSK shall provide to XenoPort true and complete electronic copies of all Financial Information. Such copies of Financial Information shall be provided in an electronic format [… * …]
(b) Within [… * …] Business Days after the Termination Effective Date, GSK shall provide to XenoPort a true and complete copy of the final close-out of the detailed Joint P&L (as defined in the Restated Agreement) for the Product through the Termination Effective Date, prepared in accordance with Section 6.5(c) of the Restated Agreement, such final close-out of the detailed Joint P&L to be provided for informational purposes only. No payment from either Party to the other will be due in connection with such final close-out of the detailed Joint P&L.
(c) GSK acknowledges and agrees that it has kept, and has required its Affiliates to keep, complete, true and accurate books of accounts and records for the purpose of determining amounts payable to XenoPort pursuant to the Restated Agreement. Each Party shall keep, and require its Affiliates to keep, complete, true and accurate books of accounts and records for the purpose of determining amounts to be paid by either Party (or its Affiliate) to the other Party (or its Affiliate) under this Termination Agreement or the Supply Agreement. All such books and records shall be kept for at least three (3) years following the end of the calendar quarter to which they pertain and shall be open for inspection and audit by the other Party such three (3) year period on the terms of this Section 5.11(c). Upon not less than [… * …] prior written notice, such Party shall permit an independent, certified public accountant selected by the other Party and reasonably acceptable to the Party whose books and records are to be inspected, which acceptance will not be unreasonably withheld or delayed (for the purposes of this Section 5.11(c), the “Auditor”), to audit or inspect those books or records of such Party or its Affiliate that relate to the transition activities and obligations pursuant to this Termination Agreement. Such inspections may be made no more than once each calendar year and during normal business hours. Such records for any particular calendar quarter shall be subject to no more than one inspection. The Auditor shall be obligated to execute a reasonable confidentiality agreement prior to commencing any such inspection. Inspections conducted under this Section 5.11(c) shall be at the expense of the Party conducting such audit, unless non-compliance with the transition activities and obligations pursuant to this Termination Agreement is established or unless a variation or error producing an underpayment in amounts payable hereunder to the Party conducting the audit, or an overpayment in amounts paid hereunder by the Party conducting the audit, exceeding [… * …] of the amount paid for a period covered by the inspection is established. In any such case, all reasonable costs relating to the inspection for such period and any unpaid amounts that are discovered shall be paid by the audited Party, together with interest on such unpaid amounts or overpaid amounts at (i) the prime rate as reported by Citibank N.A., plus [… * …] per year; or (ii) if lower, the maximum rate permitted by
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 53 -
EXECUTION COPY
law; calculated on the number of days such payment is delinquent, compounded annually and computed on the basis of a three hundred sixty five (365) day year; provided, however that interest will not be charged in instances where payments are disputed or subject to interpretation resolution, payment has been delayed by reason of the Party to whom such payment is to be made (e.g., due to invalid or late changes to bank details, non-compliant invoices, etc.), or the Party to whom such payment is to be made has not responded to reasonable questions or queries from the other Party. The Parties will endeavor in such inspection to minimize disruption of the audited Party’s (and its Affiliates’) normal business activities to the extent reasonably practicable.
5.12 Ownership of Inventions. Except with respect to the Transferred GSK Patents (which all right, title and interest shall be assigned and transferred to XenoPort pursuant to Sections 3.4(a)(v), 3.4(b) and 3.6 above), title to all inventions and other intellectual property (other than Trademarks) made jointly by personnel of XenoPort and GSK in connection with the Restated Agreement (including the Original Agreement) or this Termination Agreement shall be jointly owned by XenoPort and GSK. Prosecution of any Patent with respect to such jointly-owned inventions and intellectual property shall be solely as mutually agreed. Except as expressly provided in this Termination Agreement, it is understood that neither Party shall have any obligation to obtain any approval of, nor pay a share of the proceeds to, the other Party to practice, enforce, license, assign or otherwise exploit such jointly-owned inventions or intellectual property, and each Party hereby waives any right it may have under the Applicable Laws of any jurisdiction to require such approval or accounting.
5.13 Equipment. As of the Termination Effective Date, GSK is the owner of the manufacturing equipment set forth on Schedule 5.13 attached hereto (the “Equipment”), which is currently located at the facility of GSK’s contract manufacturer for Bulk Product (the “Bulk Manufacturer”). [… * …]
5.14 Stability Program; Complaint Handling.
(a) GSK shall continue to manage the on-going stability studies to support Product distributed by GSK to the extent such studies were initiated before the Termination Effective Date or are subsequently initiated during the Transition Period (the “On-Going Stability Studies”). Any communications regarding the data generated from On-Going Stability Studies will be shared between the Parties as set forth in the Quality Agreement. For the avoidance of doubt, any additional or future stability studies for the Product that are required to be made or conducted beyond those studies supporting Product distributed by GSK prior to the Transition Period End Date shall be the responsibility of XenoPort.
(b) For those lots of Product distributed by GSK before or during the Transition Period, GSK shall manage any complaints arising with respect to such Product, including communication regarding such complaint and any related investigation of such complaint. Any communications regarding such complaints (and the related investigation) will be shared between the Parties as set forth in the Quality Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 54 -
EXECUTION COPY
ARTICLE VI
FINANCIAL TERMS
6.1 GSK and its Affiliates shall have no obligation to pay, and XenoPort and its Affiliates shall have no right to receive, any portion of amounts received by GSK or its Affiliates from sales of the Product during the Transition Period.
6.2 Payment for Inventory. Subject to the terms and conditions of this Termination Agreement, in consideration for the Inventory to be transferred and conveyed to XenoPort under the terms of this Termination Agreement, XenoPort shall pay GSK a total of Six Million Dollars (US$6,000,000), in six equal installments of One Million United States Dollars (US$1,000,000) each, as follows: The first such payment shall be due by June 30th of each of calendar years 2016 through 2021. For clarity, it is understood that no payment shall be due under this Section 6.2 prior to June 30, 2016 or after June 30, 2021, and that no more than a total of US$6,000,000 in the aggregate shall be due under this Section 6.2. XenoPort shall be solely responsible for the payment of all Transfer Taxes incurred in connection with the transfer and assignment of the Inventory as set forth in this Termination Agreement.
ARTICLE VII
REPRESENTATIONS AND WARRANTIES
7.1 General Representations. Each Party hereby represents and warrants to the other Party as follows as of the Termination Effective Date:
(a) Such Party is a company duly organized, validly existing and in good standing under the laws of the jurisdiction of its incorporation or organization, is qualified to do business and is in good standing as a foreign corporation in each jurisdiction in which the conduct of its business or the ownership of its properties requires such qualification and failure to have such would not prevent such Party from performing its obligations under this Termination Agreement.
(b) This Termination Agreement is a legal and valid obligation binding upon such Party and enforceable in accordance with its terms. The execution, delivery and performance of this Termination Agreement by such Party have been duly authorized by all necessary corporate action and do not and will not: (i) require any consent or approval of its stockholders; (ii) to such Party’s knowledge, violate any Applicable Laws; nor (iii) conflict with, or constitute a default under, any agreement, instrument or understanding, oral or written, to which such Party is a party or by which it is bound, except for any conflicts or defaults that (A) would not prevent such Party from performing its obligations under this Termination Agreement, (B) would not prevent the other Party from exercising its rights under this Termination Agreement, and (C) would not adversely affect the conveyance of all right title and interest in the Transferred Assets or the grant of rights and licenses hereunder. In particular, and without limiting the generality of the foregoing, each Party represents and warrants to the other Party that it is fully entitled to grant the releases, enter into the covenants, and undertake the obligations set forth herein.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 55 -
EXECUTION COPY
(c) Such Party has not sold, assigned, conveyed, pledged, encumbered, or otherwise in any way transferred to any Person any Claim released by such Party pursuant to this Termination Agreement.
(d) Such Party has not filed, and has no actual knowledge, that any Third Party has filed, any legal or administrative proceeding of any kind or nature against the other Party relating to the Restated Agreement (including the Original Agreement), other than the Claims filed in the Litigation.
(e) Such Party is not relying in any manner on any statement, promise, representation or omission, whether oral or written, express or implied, made by any Person or entity, not specifically set forth in this Termination Agreement.
(f) The Parties acknowledge that they have worked together and participated on joint committees with respect to the Compound and Product during the term of the Original Agreement and Restated Agreement, and in connection therewith have from time to time exchanged information concerning the development and commercialization of the Compound and Product.
7.2 Additional Covenant and Warranties of XenoPort.
(a) XenoPort covenants to GSK that during the Transition Period, it will not, directly or indirectly through Affiliates or Third Parties, promote, market or sell the Product in the Territory or engage in any of the activities set forth in Section 4.4(f) (other than, for the avoidance of doubt, activities permitted under Section 4.8).
(b) XenoPort covenants to GSK that it shall comply with Applicable Laws in connection with XenoPort’s handling and storage of Transferred Inventory and with respect to XenoPort’s activities relating to with the manufacture and sale of Products made therefrom, including any quality assurance or quality release activities that may not be delegated to GSK, its Affiliates, or a Third Party.
(c) XenoPort represents, warrants and covenants as follows to GSK:
(i) As of the Termination Effective Date, XenoPort has sufficient right, title and interest in and to the XenoPort Patents, XenoPort Trademarks and XenoPort Know-How to convey to GSK the licenses as provided in this Termination Agreement, free and clear of any liens or encumbrances (other than obligations and restrictions set forth in this Termination Agreement).
(ii) As of the Termination Effective Date, XenoPort has the full right and authority to grant the licenses under the XenoPort Patents, XenoPort Trademarks and XenoPort Know-How as provided herein. XenoPort and its Affiliates have not previously granted, and during the Transition Period (and during any portion of the periods described in Section 5.8(d) or 4.4(g) following the Transition Period End Date in which GSK continues to distribute and sell Product as provided under such Section(s)) XenoPort and its Affiliates shall not grant, any right, license or
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 56 -
EXECUTION COPY
interest in or to the XenoPort Patents, XenoPort Trademarks and XenoPort Know-How, or any portion thereof, that is in conflict with the licenses granted to GSK under this Termination Agreement.
(iii) To its knowledge as of the Termination Effective Date, there are no actual, pending, anticipated, alleged or threatened actions, suits, claims, interference or governmental investigations involving the XenoPort Patents, XenoPort Trademarks and XenoPort Know-How by or against XenoPort or any of its Affiliates.
(iv) All necessary consents, approvals and authorizations of all Regulatory Authorities, other governmental authorities and other persons or entities required to be obtained by XenoPort in order to enter into this Termination Agreement have been obtained.
(v) To its knowledge as of the Termination Effective Date, there is no actual, pending, anticipated, alleged or threatened infringement by a Third Party of any of the XenoPort Patents or misappropriation by a Third Party of any of the XenoPort Know-How.
7.3 Additional Warranties of GSK. In addition, GSK represents, warrants and covenants as follows to XenoPort:
(a) Title; Rights to Transfer and Assign or License.
(i) As of the Termination Effective Date (and, with respect to any Transferred Assets assigned or transferred to XenoPort after the Termination Effective Date, as of the date of such assignment or transfer), (A) GSK has good and marketable title, and owns all right, title and interest, in and to the Transferred Assets, and has the right to transfer and assign rights in or to each of the foregoing to XenoPort or its designee as provided in this Termination Agreement, free and clear of any liens or encumbrances, (B) no Person (including any Affiliate of GSK) other than GSK (or XenoPort) has any right, title or interest in or to such Transferred Assets (other than any Transferred GSK Contract), and (C) no Person (including any Affiliate of GSK) other than GSK has any right, title or interest in any Transferred GSK Contract other than the applicable counterparty thereto. To its actual knowledge as of the Termination Effective Date, GSK has sufficient right, title and interest in and to the Licensed Assets to convey to XenoPort the licenses as provided in this Termination Agreement, free and clear of any liens or encumbrances (other than obligations and restrictions set forth in this Termination Agreement).
(ii) As of the Termination Effective Date, GSK has the full right and authority to grant the rights and licenses as provided herein, and GSK and its Affiliates have not previously granted (and during the period from the Termination Effective Date until the Transition Period End Date, GSK and its Affiliates shall not grant) any right, license or interest in or to the Transferred GSK Patents, Transferred GSK Know-How, Transferred GSK Trademarks or, to its actual knowledge as of the Termination Effective Date, the Licensed Assets, or any portion thereof, that is in conflict with the rights or licenses granted to XenoPort under this Termination Agreement.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 57 -
EXECUTION COPY
(iii) To its knowledge as of the Termination Effective Date, there are no actual, pending, anticipated, alleged or threatened actions, suits, claims, interference or governmental investigations involving Compound, Product, Transferred GSK Patents or Transferred GSK Know-How by or against GSK or any of its Affiliates (other than the Litigation).
(iv) All necessary consents, approvals and authorizations of all Regulatory Authorities, other governmental authorities and other persons or entities required to be obtained by GSK in order to enter into this Termination Agreement have been obtained.
(v) To its knowledge as of the Termination Effective Date, there is no actual, pending, anticipated, alleged or threatened infringement by a Third Party of any of the Transferred GSK Patents or misappropriation by a Third Party of any of the Transferred GSK Know-How.
(vi) It has not, up through and including the Termination Effective Date, knowingly withheld any material information in its possession from XenoPort in response to XenoPort’s reasonable inquiries in connection with XenoPort’s due diligence relating to Compound or Product in or for the Territory, and to its knowledge, the information related to Compound or Product in or for the Territory that GSK has provided to XenoPort prior to the Termination Effective Date is up-to-date and accurate in all material respects.
(b) Schedules.
(i) To GSK’s knowledge and subject to each Party’s rights under Sections 3.6 and 4.7 of this Termination Agreement, the Schedules attached to this Termination Agreement that identify the Transferred Assets and Licensed Assets, both as of the Termination Effective Date and [… * …] XenoPort as of the Transition Period End Date pursuant to Section 3.6 above, set forth a true, complete and correct description of all Transferred Assets and Licensed Assets (as each such asset is defined without limitation to the referenced Schedule) as of the Termination Effective Date and as of the Transition Period End Date, respectively, except to the extent that the omission from any Schedule attached to this Termination Agreement of any Transferred Asset or Licensed Asset is not a material omission.
(ii) To GSK’s knowledge, the Schedules attached to this Termination Agreement as of the Termination Effective Date and [… * …] XenoPort as of the Transition Period End Date pursuant to Section 3.6 above that identify the Transferred GSK Contracts and Excluded GSK Contracts (other than GSK Contracts between GSK and its Affiliates (i.e., inter-company agreements)) set forth a true, complete and correct description of all GSK Contracts as of the Termination Effective Date and as of the Transition Period End Date, respectively, except to the extent that the omission from such Schedule attached to this Termination Agreement of any of any Transferred GSK Contracts or Excluded GSK Contracts is not a material omission.
(iii) The Transferred Assets, Licensed Assets, Excluded GSK Contracts, Expired GSK Contracts, Excluded GSK Inventory, Excluded GSK Know-How, and GSK
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 58 -
EXECUTION COPY
Housemarks (as each such asset is defined without limitation to the referenced Schedule) include all material tangible and intangible assets primarily related to, or used by or on behalf of GSK or its Affiliates in, the development, manufacture or commercialization of Compound or Product in or for the Territory prior to the Termination Effective Date and as of the Transition Period End Date, respectively. Schedule 1.90 attached hereto sets forth a true, complete and correct description of all Ongoing Studies.
(c) Compliance with Applicable Laws. Except to the extent that non-compliance would not have a Material Adverse Effect, as of the Termination Effective Date, GSK has conducted the development, manufacture and commercialization (including marketing, promotion and distribution by or on behalf of GSK and its Affiliates) of Compound and Product, as applicable, for and in the Territory in compliance with all applicable Transferred GSK Regulatory Filings and Applicable Laws, including cGMP, regulations, “Good Clinical Practice,” or GCP, regulations, “Good Laboratory Practice,” or GLP, regulations, “Informed Consent” and “Institutional Review Board” regulations, patient privacy and security regulations and all applicable requirements relating to the protection of human subjects for its clinical trials as required by the FDA. To the knowledge of GSK, as of the Termination Effective Date, GSK has not received any written notice from any Regulatory Authority relating to any violation of any applicable Transferred GSK Regulatory Filing or Applicable Laws in conducting the development, manufacture and commercialization of Compound and Product for and in the Territory.
(d) Regulatory Matters.
(i) To the knowledge of GSK, as of the Termination Effective Date, no notification, written or oral, has been received by GSK or any of its Affiliates from the FDA regarding the following that remains unresolved: (A) any FDA Form 483 Notice of Observation, or similar notice from any other Regulatory Authority, relating to Product or, during the period of time that such facilities manufactured Product, the facilities in which Product is manufactured; (B) any FDA Notices of adverse events, or similar notice from any other Regulatory Authority, with respect to Product; (C) any “warning letters,” or “untitled letters,” or other similar Regulatory Authority notice of inspectional observations or deficiencies or other written correspondence from the FDA or any other Regulatory Authority concerning Product or, during the period of time that such facilities manufactured Product, the facilities in which Product is manufactured or (D) any notice indicating that any Product is misbranded or adulterated as defined in 21 U.S.C. §321, et seq., as amended, and the rules and regulations promulgated thereunder.
(ii) Neither GSK nor any of its Affiliates (including their respective current and former officers and employees) has been convicted of any crime or engaged in any conduct that would reasonably be expected to result in exclusion under 42 U.S.C. Section 1320a-7 or any similar state law or regulation or been debarred by the FDA under Article 306 of the FDCA, 21 U.S.C. §335a(a) or (b), in each case, with respect to the development, manufacture or commercialization of Compound or Product.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 59 -
EXECUTION COPY
(iii) As of the Termination Effective Date, there has been no recall or market withdrawal of any Product in the Territory by, or on behalf of, GSK or any of its Affiliates, whether voluntary or involuntary, or any termination or suspension of the marketing, or seizure, of any Product sold by or on behalf of GSK or any of its Affiliates, or any material change in the U.S. package inserts for the Product that has not been communicated to XenoPort prior to the Termination Effective Date.
(iv) On or before the Termination Effective Date, GSK has not knowingly withheld any information from XenoPort concerning any (i) adverse drug experience information; and (ii) medical inquiries and complaints, in each case, relating to the Product in the Territory.
(e) Transferred Contracts. All of the Transferred GSK Contracts are valid and binding agreements of GSK or its Affiliates, enforceable as of Termination Effective Date (or, if later, the date of assignment of such Transferred GSK Contract to XenoPort) in accordance with their terms. Neither GSK nor its Affiliates is in breach or default of such Transferred GSK Contracts, and, to GSK’s knowledge, no event has occurred that with notice or lapse of time, or both, would constitute a default by GSK or its Affiliates under any Transferred GSK Contract. To the knowledge of GSK and its Affiliates, no other party to a Transferred GSK Contract is in breach or default of such Transferred GSK Contract, and no event has occurred that with notice or lapse of time, or both, would constitute a default by such other party under any Transferred GSK Contract. No party has repudiated in writing or expressed any intention in writing to repudiate any provision of a Transferred GSK Contract. Subject to each Party’s rights under Sections 3.6 and 4.7 of this Termination Agreement, GSK has disclosed and made available to XenoPort true and complete and accurate copies of all Transferred GSK Contracts (or written summaries of the material terms thereof, if such contracts are not in writing), including all amendments, supplements, modifications and waivers thereof, as in effect on the Termination Effective Date (or, if later, the date of assignment of such Transferred GSK Contract to XenoPort). GSK has paid, or shall pay when due (even if after the date of transfer), all undisputed payments under each Transferred GSK Contracts that have accrued on or prior to the date of assignment of such Transferred GSK Contract to XenoPort.
(f) Product Liability. GSK has not received any written notice concerning any legal proceedings or, to the knowledge of GSK and its Affiliates, investigations by Regulatory Authorities with respect to the safety of Product sold or advertised for sale by GSK or any of its Affiliates in the Territory, in each case during the last [… * …]. GSK has also not received any written notice concerning any product liability claims relating to the development, manufacture or commercialization of the Product involving amounts in excess of [… * …] (or, to the knowledge of GSK or any of its Affiliates, threatened) within the past five (5) years.
(g) Inventory. GSK and its Affiliates represent and warrant as of the date of delivery to or as directed by XenoPort hereunder that (i) each unit of Transferred GSK Inventory has been manufactured, stored, and handled materially in compliance with the applicable then-current Specifications, cGMPs and other Applicable Laws, [… * …]; (ii) to GSK’s knowledge, all facilities and equipment used for the manufacture of each unit of Transferred GSK Inventory have been and
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 60 -
EXECUTION COPY
are in material compliance with all Applicable Laws; and (iii) except to the extent title and interest may be impacted while payment to the relevant Third Party supplier is pending, title to each unit of Transferred GSK Inventory shall pass to XenoPort, free and clear of any liens or encumbrances.
(h) Promotional Sample Inventory. GSK and its Affiliates represent and warrant as of the Transition Period End Date that (i) each unit of Promotional Sample Inventory has been manufactured, stored, and handled materially in compliance with the applicable then-current Specifications, cGMPs and other Applicable Laws; (ii) to GSK’s knowledge, all facilities and equipment used for the manufacture of each unit of Promotional Sample Inventory have been and are in material compliance with all Applicable Laws; and (iii) except to the extent title and interest may be impacted while payment to the relevant Third Party supplier is pending, title to each unit of Promotional Sample Inventory shall pass to XenoPort, free and clear of any liens or encumbrances.
(i) GSK’s Applicable Policies and Procedures. To its knowledge as of the Termination Effective Date, GSK’s Applicable Policies and Procedures do not preclude GSK from materially performing its obligations hereunder or conducting the activities to be conducted by GSK hereunder (including without limitation conducting Ongoing Studies, interacting with Regulatory Authorities as provided in Section 4.3, transferring Inventory, or supplying Product pursuant to Section 4.5 and the Supply Agreement), as such activities are contemplated to be conducted as of the Termination Effective Date. During the Transition Period, GSK shall not enact any new policies or procedures where such policy or procedure is enacted with the sole intent of relieving GSK from the obligation to materially perform its obligations hereunder or conduct the activities to be conducted by GSK hereunder.
7.4 DISCLAIMER. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS TERMINATION AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTIES OF ANY KIND EITHER EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, (I) WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NONINFRINGEMENT OR VALIDITY OF ANY PATENTS ISSUED OR PENDING, (II) THE USE, MARKETING, DISTRIBUTION, OFFER FOR SALE, SALE, COMMERCIALIZATION OR MANUFACTURE OF COMPOUND AND/OR PRODUCT AFTER THE TRANSITION PERIOD END DATE BY XENOPORT, ITS AFFILIATES OR ANY THIRD PARTY IN ANY MANNER, (III) THE PROBABLE SUCCESS OR PROFITABILITY OF PRODUCT AFTER THE TERMINATION EFFECTIVE DATE OR (IV) AS TO THE CONDITION, VALUE OR QUALITY OF THE TRANSFERRED ASSETS. WITHOUT LIMITING THE FOREGOING, THE TRANSFERRED GSK PROMOTIONAL MATERIALS ARE BEING TRANSFERRED AS IS, WHERE IS, WITH ALL FAULTS. FURTHERMORE, NOTWITHSTANDING ANY REPRESENTATION OR WARRANTY MADE PURSUANT TO SECTION 7.3(G), FOR ANY TRANSFERRED GSK INVENTORY TO THE EXTENT COVERED BY A TRANSFERRED GSK CONTRACT ASSIGNED TO XENOPORT PURSUANT TO THIS TERMINATION AGREEMENT, GSK WILL BE DEEMED TO HAVE MADE NO REPRESENTATION OR WARRANTY WITH RESPECT TO SUCH TRANSFERRED GSK INVENTORY TO THE EXTENT APPLICABLE TO OR BASED ON ACTIVITIES BY THE COUNTERPARTY TO SUCH TRANSFERRED GSK CONTRACT OR DELIVERABLES
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 61 -
EXECUTION COPY
(INCLUDING, IF APPLICABLE, COMPOUND OR PRODUCT SUPPLIED) THEREUNDER FROM SUCH COUNTERPARTY, AND XENOPORT SHALL SOLELY RELY ON THE RELEVANT REPRESENTATIONS AND WARRANTIES INCLUDED IN SUCH TRANSFERRED GSK CONTRACT WITH RESPECT TO SUCH MATTERS IN CONNECTION WITH SUCH TRANSFERRED GSK INVENTORY.
ARTICLE VIII
CONFIDENTIALITY; PRESS RELEASE
8.1 Confidential Information. Except as expressly provided in this Termination Agreement, the Parties agree that the receiving Party shall not publish or otherwise disclose and shall not use for any purpose any information furnished to it by the other Party hereto pursuant to the Restated Agreement (including the Original Agreement) or this Termination Agreement (collectively, “Confidential Information”). Notwithstanding the foregoing, for purposes of this Article VIII, the Parties agree that from and after the Termination Effective Date, GSK shall treat as Confidential Information of XenoPort all information within the Transferred Assets in its possession or Control without regard to the exceptions under subsections (a) or (e) below, and shall not publish or otherwise disclose such information within the Transferred Assets other than as provided in Sections 4.3(c) and 8.2. Notwithstanding the foregoing, Confidential Information shall not include information that, in each case as demonstrated by written documentation:
(a) was already known to the receiving Party, other than under an obligation of confidentiality, at the time of disclosure or, as shown by written documentation, was developed by the receiving Party prior to its disclosure by the disclosing Party;
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of the Restated Agreement or this Termination Agreement;
(d) was subsequently lawfully disclosed to the receiving Party by a person other than the disclosing Party, and who did not directly or indirectly receive such information from disclosing Party; or
(e) is developed by the receiving Party without use of or reference to any Confidential Information disclosed by the disclosing Party.
8.2 Permitted Disclosures. Notwithstanding the provisions of Section 8.1 above, and subject to Section 8.3 below, each Party hereto may use and disclose the other Party’s Confidential Information to its Affiliates, licensees, contractors and any other Third Parties to the extent such use and/or disclosure is: (a) reasonably necessary to perform its obligations hereunder, (b) necessary to comply with Applicable Laws, including applicable court orders or other legal process or (c) in the
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 62 -
EXECUTION COPY
case of XenoPort, is made in connection with the development, manufacture, commercialization or other exploitation of Compound or Product in or outside the Territory. If a Party proposes to make a disclosure of Confidential Information under clause (b), above, of this Section 8.2, to the extent it may legally do so, it will give reasonable advance notice to the latter Party of such disclosure and will use its good faith efforts to secure confidential treatment of such Confidential Information prior to its disclosure (whether through protective orders or otherwise). For any other disclosures of the other Party’s Confidential Information, including to Affiliates, licensees, contractors and other Third Parties, a Party shall ensure that the recipient thereof is bound by appropriate confidentiality provisions consistent with the nature of the information disclosed.
8.3 Confidentiality of Terms. Each Party agrees not to disclose to any Third Party the terms of this Termination Agreement, without the prior written consent of the other Party hereto, except each Party may disclose the terms of this Termination Agreement: (a) to advisors (including financial advisors, attorneys and accountants), actual or potential acquisition partners or private investors, potential licensees of XenoPort, and others on a need to know basis, in each case under appropriate confidentiality provisions consistent with the nature of the information disclosed; (b) to the extent necessary to comply with Applicable Laws, including securities laws, regulations or guidances; provided that in the case of clause (b) the disclosing Party shall promptly notify the other Party and (other than in the case where such disclosure is necessary, in the reasonable opinion of the disclosing Party’s legal counsel, to comply with securities laws, regulations or guidances) allow the other Party a reasonable opportunity to oppose with the body initiating the process and, to the extent allowable by Applicable Laws, to seek limitations on the portion of the Termination Agreement that is required to be disclosed. Notwithstanding the foregoing, the Parties shall agree upon press releases of each Party to announce the execution of this Termination Agreement, drafts of which are attached in Exhibit 8.3; thereafter, each Party may each disclose to Third Parties the information contained in such press releases without the need for further approval by the other Party.
ARTICLE IX
INDEMNIFICATION
9.1 Indemnification of GSK. XenoPort shall indemnify and hold harmless each of GSK, and its Affiliates and the directors, officers and employees of GSK and its Affiliates and the successors and assigns of any of the foregoing (the “GSK Indemnitees”), from and against any and all liabilities, damages, penalties, fines, costs, expenses (including, reasonable attorneys’ fees and other expenses of litigation) (“Liabilities”) from any claims, actions, suits or proceedings brought by a Third Party (a “Third Party Claim”) incurred by any GSK Indemnitee, arising from, or occurring as a result of (a) gross negligence or willful misconduct in the conduct of the research and development activities conducted by XenoPort or its Affiliates related to Compound and/or any Product, (b) the use, marketing, distribution, or sale of any Product by or under authority of XenoPort, its Affiliates or licensees (other than by or under the authority of GSK, its Affiliates and sublicensees) inside or outside the Territory, (c) the manufacture of any Compound or Product by or on behalf of XenoPort, its Affiliates or licensees (other than by or on behalf of GSK, its Affiliates and sublicensees); or (d) any material breach of any representations, warranties or covenants by XenoPort in ARTICLE VII above, except to the extent such Third Party Claims arise from and are attributable to causes described in clauses (a)-(d) of Section 9.2 below or result from the negligence or fault of an GSK Indemnitee.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 63 -
EXECUTION COPY
9.2 Indemnification of XenoPort. GSK shall indemnify and hold harmless each of XenoPort, its Affiliates and the directors, officers, stockholders and employees of such entities and the successors and assigns of any of the foregoing (the “XenoPort Indemnitees”), from and against any and all Liabilities from any Third Party Claims incurred by any XenoPort Indemnitee, arising from, or occurring as a result of: (a) the use, marketing, distribution, importation or sale of any Product by or under authority of GSK (or its Affiliates); (b) the manufacture of any Compound or Product by or on behalf of GSK (or its Affiliates) for sale by GSK or its Affiliates prior to the Transition Period End Date, but (to the extent that Tolled API is supplied by XenoPort to GSK as set forth in Section 4.2(f)(iv)) excluding any Liabilities arising from the storage, handling, or alteration of such Tolled API after such Tolled API is transferred from GSK to XenoPort (or its designee) but before such Tolled API is subsequently transferred to GSK’s Third Party contractor pursuant to Section 4.2(h)(ii); (c) gross negligence or willful misconduct in the conduct of the research and development activities conducted by GSK, or any of its Affiliates, related to the Compound and/or any Product; and (d) any material breach of any representations, warranties or covenants by GSK in Article VII above; except to the extent such Third Party Claims arise from and are attributable to causes described in clauses (a)-(d) of Section 9.1 above or result from the gross negligence or fault of a XenoPort Indemnitee.
9.3 Procedure. A Party that intends to claim indemnification under this ARTICLE IX (the “Indemnitee”) shall promptly notify the other Party (the “Indemnitor”) in writing of the assertion or the commencement of Third Party Claim and will provide the Indemnitor such information with respect thereto that the Indemnitor may reasonably request. The Indemnitor shall be entitled to participate in the defense of any Third Party Claim and, subject to the limitations set forth in this Section 9.3, shall be entitled to control and appoint lead counsel for such defense, in each case at its expense. If the Indemnitor shall assume the control of the defense of any Third Party Claim in accordance with the provisions of this Section 9.3, the Indemnitor shall obtain the prior written consent of the Indemnitee (which shall not be unreasonably withheld) before entering into any settlement of such Third Party Claim. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim, if prejudicial to its ability to defend such action, shall relieve such Indemnitor of any liability to the Indemnitee under this Section 9.3, but the omission to so deliver written notice to the Indemnitor shall not relieve the Indemnitor of any liability that it may have to any Indemnitee otherwise than under this Section 9.3. The Indemnitee under this Section 9.3 shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action with respect to a Third Party Claim covered by this indemnification.
9.4 Limitation of Liability.
(a) Notwithstanding anything to the contrary contained herein, and except in the event of fraud or willful misconduct by GSK or its Affiliates, in no event shall GSK be responsible for any Liabilities from Third Party Claims or any other losses, expenses, costs, liabilities, damages,
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 64 -
EXECUTION COPY
penalties, or fines arising out of or relating to any Transferred GSK Inventory transferred by (or on behalf of) GSK to XenoPort, however arising, to the extent that such amounts (in the aggregate) exceed Six Million US Dollars ($6,000,000).
(b) EXCEPT IN THE EVENT OF FRAUD OR WILLFUL MISCONDUCT BY THE APPLICABLE PARTY OR ANY OF ITS AFFILIATES, IN NO EVENT SHALL EITHER PARTY (OR ANY OF ITS AFFILIATES) BE LIABLE TO THE OTHER (OR ANY OF ITS AFFILIATES) FOR ANY SPECIAL, INDIRECT, INCIDENTAL, CONSEQUENTIAL, OR PUNITIVE DAMAGES ARISING OUT OF, OR AS THE RESULT OF, THIS TERMINATION AGREEMENT OR ANY BREACH OF THIS TERMINATION AGREEMENT, OR THE EXERCISE BY EITHER PARTY OF RIGHTS CONVEYED HEREUNDER, OR THE SALE, TRANSFER, DELIVERY, USE, OR LOSS OF USE OF ANY INVENTORY, REGARDLESS OF WHETHER SUCH CLAIM IS BASED UPON BREACH OF WARRANTY, BREACH OF CONTRACT, NEGLIGENCE, STRICT LIABILITY, OR ANY LEGAL THEORY, AND REGARDLESS OF ANY NOTICE OF SUCH DAMAGES.
ARTICLE X
DISPUTE RESOLUTION
10.1 Dispute Resolution. Except as otherwise provided herein, any dispute, controversy or claim arising under, out of or in connection with this Termination Agreement, including any subsequent amendments, or the validity, enforceability, interpretation, performance or breach hereof (and including the applicability of this Article X to any such dispute, controversy or claim) and any dispute within the Transition Team (each a “Dispute”), shall first be presented to the Chief Executive Officer of XenoPort and the Chief Operating Officer of GSK, or their respective designees, for resolution. Any Party may give the other Party written notice of any Dispute not resolved in the normal course of business. If the Chief Executive Officer of XenoPort and the Chief Executive Officer of GSK, or their respective designees, cannot resolve the Dispute within [… * …] of the request to do so, either Party may, upon written notice to the other, then refer such Dispute be resolved by mediation in accordance with the provisions of Section 10.2 below or final, binding arbitration in accordance with the provisions of Section 10.3 below. All negotiations pursuant to this Section 10.1 are confidential and will be treated as compromise and settlement negotiations for purposes of applicable rules of evidence.
10.2 Mediation. If the Dispute has not been resolved by negotiation as provided in Section 10.1 above, the Parties will endeavor to settle the dispute by mediation conducted by the Judicial Arbitration and Mediation Services, Inc. (or any successor entity thereto) (“JAMS”) under its procedures regarding mediation then currently in effect; provided, however that if one Party fails to participate in the negotiation under Section 10.1 above within a reasonable period after delivery of the notice of Dispute, the other Party may initiate mediation prior to the expiration of the initial [… * …] period referenced above.
10.3 Arbitration. Except as otherwise provided in Section 10.3(c) with respect to Disputes involving the intellectual property rights of a Party, the Parties agree that any Dispute arising out of
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 65 -
EXECUTION COPY
or relating to this Termination Agreement, including the breach, termination or validity thereof, which has not been resolved by mediation as provided in Section 10.2 above within [… * …] after either Party provides notice to JAMS and the other Party submitting such matter for mediation as contemplated by Section 10.2 above, will be finally resolved by binding arbitration conducted by JAMS under its rules of arbitration then in effect (“Rules”), except as modified herein. If the amount in controversy is [… * …] there shall be [… * …] shall be agreed upon by the Parties within [… * …] of receipt by respondent of a copy of the demand for arbitration. If the amount in controversy is [… * …] there shall be [… * …] of the arbitral tribunal [… * …] If any arbitrator is not appointed within the time limit provided herein, such arbitrator shall be appointed in accordance with the Rules, or if the Rules do not provide for such selection, by the chief executive of JAMS. Notwithstanding the foregoing, if one Party fails to participate in either the negotiation or mediation as agreed herein, the other Party can commence arbitration pursuant to this Section 10.3 prior to the expiration of the time periods set forth therein. The arbitration will be governed by the Federal Arbitration Act, 9 U.S.C. §§1 et. seq. and judgment upon the award rendered by the arbitrator(s) may be entered by any court having jurisdiction thereof.
(a) The Parties agree that the decision of the arbitrator(s) shall be the binding remedy between them regarding the Dispute presented to the arbitrator(s). Any decision of the arbitrator(s) may be entered in a court of competent jurisdiction for judicial recognition of the decision and an order of enforcement. The arbitration proceedings and the decision of the arbitrator(s) shall not be made public without the joint consent of the Parties and each Party shall maintain the confidentiality of such proceedings and decision unless each Party otherwise agrees in writing; provided that either Party may make such disclosures as are permitted for Confidential Information of the other Party under Article VIII above.
(b) Unless otherwise mutually agreed upon by the Parties, the arbitration proceedings shall be conducted in [… * …] The Parties agree that they shall share equally the cost of the arbitration filing and hearing fees, the cost of any independent expert retained by the arbitrator(s), and the cost of the arbitrator(s) and administrative fees. Each Party shall bear its own costs and attorneys’ and witnesses’ fees and associated costs and expenses.
(c) Notwithstanding the foregoing provisions of this Section 10.3, either Party may initiate legal proceedings with respect to any Disputes related to intellectual property rights of a Party that are not resolved under Sections 10.1 or 10.2, including any such Disputes regarding the validity or enforceability of any Patent owned or Controlled by either Party.
(d) Nothing in this Termination Agreement shall limit the right of either Party to seek to obtain in any court of competent jurisdiction any equitable or interim relief or provisional remedy, including injunctive relief, that may be necessary to protect the rights or property of that Party pending resolution of a Dispute under this Section 10.3. Seeking or obtaining such equitable or interim relief or provisional remedy in a court shall not be deemed a waiver of the agreement to arbitrate. For clarity, any such equitable remedies shall be cumulative and not exclusive and are in addition to any other remedies that either Party may have under this Termination Agreement or Applicable Laws.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 66 -
EXECUTION COPY
10.4 Waiver of Jury Trial. EXCEPT AS OTHERWISE PROVIDED IN SECTION 10.3(C) WITH RESPECT TO DISPUTES INVOLVING THE INTELLECTUAL PROPERTY RIGHTS OF A PARTY, EACH PARTY HERETO WAIVES ITS RIGHT TO TRIAL OF ANY ISSUE BY JURY.
ARTICLE XI
GENERAL PROVISIONS
11.1 Governing Law. The validity or interpretation, or the breach or performance of this Termination Agreement, and any dispute, controversy or claim arising under, out of or in connection with this Termination Agreement, including any tort and statutory claims, shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without reference to conflict of law principles.
11.2 Waiver of Breach. Except as otherwise expressly provided in this Termination Agreement, any term of this Termination Agreement may be waived only by a written instrument executed by a duly authorized representative of the Party waiving compliance. The delay or failure of either Party at any time to require performance of any provision of this Termination Agreement shall in no manner affect such Party’s rights at a later time to enforce the same. No waiver by either Party of any condition or term in any one or more instances shall be construed as a further or continuing waiver of such condition or term or of another condition or term.
11.3 Modification. No amendment or modification of any provision of this Termination Agreement shall be effective unless in writing signed by a duly authorized representative of each Party. No provision of this Termination Agreement shall be varied, contradicted or explained by any oral agreement, course of dealing or performance or any other matter not set forth in an agreement in writing and signed by a duly authorized representative of each Party.
11.4 Severability. In the event any provision of this Termination Agreement should be held invalid, illegal or unenforceable in any jurisdiction, the Parties shall negotiate in good faith a valid, legal and enforceable substitute provision that most nearly reflects the original intent of the Parties and all other provisions of this Termination Agreement shall remain in full force and effect in such jurisdiction. Such invalidity, illegality or unenforceability shall not affect the validity, legality or enforceability of such provision in any other jurisdiction.
11.5 Entire Agreement. This Termination Agreement (including the Schedules and Exhibits attached hereto), together with the SPA, Ancillary Agreements, GSK Safety Agreement and Supply Agreement (if applicable), constitute the entire agreement between the Parties relating to its subject matter and supersedes all prior or contemporaneous agreements, understandings or representations, either written or oral, including the Original Agreement and the Restated Agreement, between XenoPort and GSK with respect to such subject matter.
11.6 Notices. Unless otherwise agreed by the Parties or specified in this Termination Agreement, all communications between the Parties relating to, and all written documentation to be
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 67 -
EXECUTION COPY
prepared and provided under, this Termination Agreement shall be in the English language. Any notice required or permitted under this Termination Agreement shall be in writing in the English language: (a) delivered personally; (b) sent by registered or certified mail (return receipt requested and postage prepaid); (c) sent by express courier service providing evidence of receipt, postage pre-paid where applicable; or (d) sent by facsimile (receipt verified and a copy promptly sent by another permissible method of providing notice described in paragraphs (b), (c) or (d) above), to the following addresses of the Parties or such other address for a Party as may be specified by like notice:
| To XenoPort: | To GSK: | |
| XenoPort, Inc. | Glaxo Group Limited | |
| 3410 Central Expressway | Glaxo Xxxxxxxx Xxxxx, Xxxxxxxx Xxxxxx | |
| Xxxxx Xxxxx, XX 00000 | Xxxxxxxxx, Xxxxxxxxx, XX0 0XX, Xxxxxxx | |
| Telephone: (000) 000-0000 | Telephone: | |
| Facsimile: (000) 000-0000 | Facsimile: | |
| Attention: Secretary | Attention: Company Secretary | |
| With a copy to: | With a copy to: | |
| Wilson, Sonsini, Xxxxxxxx & Xxxxxx | GlaxoSmithKline | |
| 000 Xxxx Xxxx Xxxx | 000 Xxxxxxxxx Xxxx | |
| Xxxx Xxxx, XX 00000 | X.X. Xxx 0000 Xxxx xx Xxxxxxx, XX 00000-0000 XXX | |
| Telephone: (000) 000-0000 | Telephone: | |
| Facsimile: (000) 000-0000 | Facsimile: | |
| Attention: Xxxxxxx X. Xxxxx | Attention : Senior Vice President, Worldwide Business Development | |
| and | ||
| GlaxoSmithKline | ||
| 0000 Xxxxxxxxxxx Xxxxxxxxx | ||
| Mailcode XX0000 | ||
| Xxxx xx Xxxxxxx, XX 00000-0000 Telephone: | ||
| Facsimile: | ||
| Attention: Vice President and Associate General Counsel, Business Development Transactions | ||
Any notice required or permitted to be given concerning this Termination Agreement shall be effective upon receipt by the Party to whom it is addressed or within [… * …] of dispatch whichever is earlier.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 68 -
EXECUTION COPY
11.7 Assignment. This Termination Agreement shall not be assignable by either Party to any Affiliate or Third Party hereto without the written consent of the other Party hereto; except XenoPort may assign this Termination Agreement without GSK’s consent to an entity that acquires substantially all of the business or assets of XenoPort to which this Termination Agreement relates, whether by merger, acquisition or otherwise, provided that the entity to whom this Termination Agreement is assigned assumes this Termination Agreement in writing or by operation of law. Subject to the foregoing, this Termination Agreement shall inure to the benefit of each Party, its successors and permitted assigns. Any assignment of this Termination Agreement in contravention of this Section 11.7 shall be null and void.
11.8 Compromise Agreement. This Termination Agreement is a compromise and settlement of disputed Claims and is not intended to be, nor shall be construed as, any admission of liability or wrongdoing by any Party. This Termination Agreement and any performance or conduct under this Termination Agreement shall not be admissible in any arbitration, lawsuit or other proceeding except as necessary to enforce the terms and conditions hereof. The Parties agree that this Termination Agreement fully resolves the Litigation. GSK and XenoPort each shall be responsible for their respective legal and other fees and expenses associated with all aspects of the Litigation, and the negotiation of this Termination Agreement and any other related agreements.
11.9 No Partnership, Joint Venture or Principal-Agent Relationship. Nothing in this Termination Agreement is intended, or shall be deemed, to establish a joint venture, partnership or principal-agent relationship between XenoPort and GSK, except as provided in Section 4.3(b)(i). Neither Party to this Termination Agreement shall have any express or implied right or authority to assume or create any obligations on behalf of, or in the name of, the other Party, or to bind the other Party to any contract, agreement or undertaking with any Third Party.
11.10 Force Majeure. If the performance of any part of this Termination Agreement (except for any payment obligation under this Termination Agreement) by either Party is prevented, restricted, interfered with or delayed by reason of force majeure (including, fire, flood, embargo, power shortage or failure, acts of war, insurrection, riot, terrorism, strike, lockout or other labor disturbance or acts of God), the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such prevention, restriction, interference or delay; provided that the affected Party shall use its reasonable efforts to avoid or remove such causes of non-performance and shall continue performance with the utmost dispatch whenever such causes are removed.
11.11 Interpretation. The captions to the several Articles and Sections of this Termination Agreement are not a part of this Termination Agreement, but are included for convenience of reference and shall not affect its meaning or interpretation. In this Termination Agreement: (a) the word “including” shall be deemed to be followed by the phrase “without limitation” or like expression; (b) the singular shall include the plural and vice versa; and (c) masculine, feminine and neuter pronouns and expressions shall be interchangeable. Each accounting term used herein that is not specifically defined herein shall have the meaning given to it under International Generally Accepted Accounting Principles. All references to a “business day” or “business days” in this Termination Agreement means any day other than a day which is a Saturday, a Sunday or any day banks are authorized or required to be closed in the United States or in the United Kingdom.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 69 -
EXECUTION COPY
11.12 Counterparts. This Termination Agreement may be executed in any number of counterparts, each of which shall be deemed an original, and all of which together shall constitute one and the same instrument.
[Remainder of This Page Intentionally Blank. Signature Page Follows.]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
- 70 -
IN WITNESS WHEREOF, the Parties have executed this Termination Agreement as of the date first set forth above.
| XENOPORT, INC. | ||
| BY: | /s/ Xxxxxx Xxxxxxx, PhD | |
| Xxxxxx Xxxxxxx, PhD | ||
| Chief Executive Officer | ||
| GLAXO GROUP LIMITED | ||
| BY: | /s/ Xxxx Xxxxxxxxxx | |
| NAME: | Xxxx Xxxxxxxxxx | |
| TITLE: | Authorized Signatory | |
| For and on behalf of | ||
| Edinburgh Pharmaceutical Industries Limited | ||
| Corporate Director | ||
[Signature Page for Termination and Transition Agreement]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
EXECUTION COPY
| SCHEDULES | ||
| Schedule 1.26 | Compound | |
| Schedule 1.41 | Excluded GSK Contracts | |
| Schedule 1.42 | Excluded GSK Inventory | |
| Schedule 1.43 | Excluded GSK Know-How | |
| Schedule 1.44 | Expired GSK Contracts | |
| Schedule 1.47 | Financial Information | |
| Schedule 1.69 | Inventory (as of Termination Effective Date) | |
| Schedule 1.75 | Licensed GSK Know-How | |
| Schedule 1.76 | Licensed GSK Patents | |
| Schedule 1.90 | Ongoing Studies | |
| Schedule 1.103 | Promotional Sample Inventory (as of the Transition Period End Date) | |
| Schedule 1.120 | Specifications | |
| Schedule 1.135 | Transferred GSK Contracts | |
| Schedule 1.136 | Transferred GSK Domain Names | |
| Schedule 1.137 | Transferred GSK Inventory (as of the Inventory Transfer Date) | |
| Schedule 1.138 | Transferred GSK Know-How | |
| Schedule 1.139 | Transferred GSK Patents | |
| Schedule 1.140 | Transferred GSK Promotional Materials | |
| Schedule 1.141 | Transferred GSK Regulatory Filings | |
| Schedule 1.142 | Transferred GSK Trademarks | |
| Schedule 4.2B | Transferred GSK Know-How (not delivered) | |
| Schedule 4.3C | Permitted GSK Publications | |
| Schedule 4.5 | Key Terms of Supply Agreement | |
| Schedule 4.5B | Minimum Quantities and Forecast Requirements | |
| Schedule 5.13 | Equipment | |
| EXHIBITS | ||
| Exhibit 1.11 | Form of Assignment and Assumption Agreement | |
| Exhibit 1.14 | Form of Xxxx of Sale | |
| Exhibit 1.38 | Form of Domain Name Assignment Agreement | |
| Exhibit 1.94 | Form of Patent Assignment Agreement | |
| Exhibit 1.118 | Form of Stock Purchase Agreement | |
| Exhibit 1.131 | Form of Trademark Assignment Agreement | |
| Exhibit 2.4A | California Dismissal Letter | |
| Exhibit 2.4B | Delaware Dismissal Letter | |
| Exhibit 3.5E | XenoPort FDA Letter | |
| Exhibit 4.3A | Development Plan | |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
1
EXECUTION COPY
| Exhibit 4.3B | RLS Horizant Approval Letter | |
| Exhibit 4.4 | Commercialization Transition Plan | |
| Exhibit 4.6 | Manufacturing Technology Transfer Plan | |
| Exhibit 4.9 | Safety Data Exchange Transfer Plan | |
| Exhibit 8.3 | Form of Press Releases |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
2
EXECUTION COPY
SCHEDULE 1.26
Compound
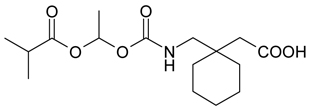
Gabapentin Enacarbil
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
3
EXECUTION COPY
SCHEDULE 1.41
Excluded GSK Contracts
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
4
EXECUTION COPY
SCHEDULE 1.42
Excluded GSK Inventory
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
5
EXECUTION COPY
SCHEDULE 1.43
Excluded GSK Know-How
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
6
EXECUTION COPY
SCHEDULE 1.44
Expired GSK Contracts
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
7
EXECUTION COPY
SCHEDULE 1.47
Financial Information
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
8
EXECUTION COPY
Attachment 1.47
Schedule Detailing RAR Expense Lines
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
9
EXECUTION COPY
SCHEDULE 1.69
Inventory
(as of the Termination Effective Date)
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
10
EXECUTION COPY
SCHEDULE 1.76
Licensed GSK Patents
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
11
EXECUTION COPY
SCHEDULE 1.90
Ongoing Studies
| • | RXP114025: A Randomized, Double-Blind, Placebo-Controlled, Fixed-Dose, Parallel-Group Study to Compare the Efficacy, Tolerability, and Safety of 3 Doses of Gabapentin Enacarbil (GSK1838262) With Placebo in the Treatment of Subjects With Moderate-to-Severe Primary Restless Legs Syndrome (RLS) |
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
12
EXECUTION COPY
SCHEDULE 1.103
Promotional Sample Inventory
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
13
EXECUTION COPY
SCHEDULE 1.120
Specifications
Commercial Product: Those product specifications listed in NDA 022399 for drug substance and for finished product.
Clinical/R&D Product: Those product specifications for material used in clinical studies listed in IND 71352 and/or IND 68341
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
14
EXECUTION COPY
SCHEDULE 1.135
Transferred GSK Contracts
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
15
EXECUTION COPY
SCHEDULE 1.136
Transferred GSK Domain Names
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
16
EXECUTION COPY
SCHEDULE 1.137
Transferred GSK Inventory
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
17
EXECUTION COPY
SCHEDULE 1.138
Transferred GSK Know-How
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
18
EXECUTION COPY
SCHEDULE 1.139
Transferred GSK Patents
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
19
EXECUTION COPY
SCHEDULE 1.140
Transferred GSK Promotional Materials
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
20
EXECUTION COPY
SCHEDULE 1.141
Transferred GSK Regulatory Filings
| • | US IND 071352 for the treatment of restless legs syndrome, including electronic filing and all correspondence and associated logs pertaining to this IND between GSK and the FDA |
| • | US NDA 022399 for the treatment of moderate-to-severe primary restless legs syndrome and for the management of post-herpetic neuralgia, including electronic filing and all correspondence and associated logs pertaining to this NDA between GSK and the FDA (e.g., promotional materials filed and correspondence with OPDP but excluding approval histories for such promotional materials which are maintained in the PromoNet history archives) |
| • | US IND 068341 for the treatment of neuropathic pain, including electronic filing and all correspondence and associated logs pertaining to this IND between GSK and the FDA |
| • | US Orphan Drug Application 10-3307 for the management of post-herpetic neuralgia in adults, including all correspondence |
| • | US Labeling files for NDA 022399, including label version and approval history |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
21
EXECUTION COPY
SCHEDULE 1.142
Transferred GSK Trademarks
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
22
EXECUTION COPY
SCHEDULE 4.2B
Transferred GSK Know-How (not delivered)
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
23
EXECUTION COPY
SCHEDULE 4.3C
Permitted GSK Publications
| • | Study XP083/RXP111463 – manuscript submission anticipated Nov 2012; may require revision based on reviewer comments |
| • | Study RXP114111 – manuscript submission anticipated Nov 2012; may require revision based on reviewer comments |
| • | Study RXP114112 – manuscript submission anticipated Jan 2013; may require revision based on reviewer comments |
| • | Study RXP115720 – manuscript submission anticipated Feb 2013; may require revision based on reviewer comments |
| • | Study RXP116672 – manuscript submission anticipated Jan 2013; may require revision based on reviewer comments |
| • | Study RXP116688 – manuscript submission anticipated Nov 2012; may require revision based on reviewer comments |
| • | Study RXP115391 – manuscript submitted 24 Sep 2012; may require revision based on reviewer comments |
| • | Study PXN110527 – manuscript submitted 10 Aug 2012; may require revision based on reviewer comments |
| • | Study XXX000000 – manuscript submitted 21 Aug 2012; may require revision based on reviewer comments |
| • | WEUSKOP4087 (Xxxxxx XX Study; disease specific, does not involve any mention of Product) |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
24
EXECUTION COPY
SCHEDULE 4.5
Key Terms of Supply Agreement
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
25
EXECUTION COPY
SCHEDULE 4.5B
Minimum Quantities and Forecast Requirements
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
26
EXECUTION COPY
SCHEDULE 5.13
Equipment
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
27
EXECUTION COPY
EXHIBIT 1.11
Form of Assignment and Assumption Agreement
THIS ASSIGNMENT AND ASSUMPTION AGREEMENT (this “Assignment and Assumption”) is made and entered into as of [ ,] 2012 between XenoPort, Inc., a Delaware corporation having its principal place of business at 0000 Xxxxxxx Xxxxxxxxxx, Xxxxx Xxxxx, XX 00000 (“Assignee” or “XenoPort”), and [Glaxo Group Limited, a company existing under the laws of England and Wales, having its registered office at Glaxo Wellcome House, Berkeley Avenue, Greenford, Middlesex, UB6 0NN, England]1 (“Assignor” or “GSK”).
BACKGROUND
A. Assignor and Assignee are parties to that certain Termination and Transition Agreement (“Termination Agreement”), dated as of November [ ], 2012 (“Termination Effective Date”), pursuant to which Assignor and Assignee have agreed to settle the Litigation and terminate the Restated Agreement, as set forth in, and subject to the terms and conditions of, the Termination Agreement and the Stock Purchase Agreement between XenoPort and GSK, dated as of the Termination Effective Date (the “SPA”), to provide for, among other matters, the reversion to XenoPort of all rights with respect to the Product, and for GSK to purchase certain shares of Common Stock of XenoPort pursuant to the SPA. Capitalized terms used herein and not otherwise defined herein shall have the meanings respectively ascribed to such capitalized terms in the Termination Agreement; and
B. Pursuant to Sections 3.4(a) and 3.4(b) of the Termination Agreement, Assignor and Assignee have agreed that Assignor will assign to Assignee, and Assignee will assume from Assignor, the [Transferred GSK Know-How / Transferred GSK Regulatory Filings / Transferred GSK Contracts / Transferred GSK Promotional Materials] set forth on Exhibits [A – D] attached.
NOW, THEREFORE, for and in consideration of the premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt, adequacy and legal sufficiency of which are hereby acknowledged, the parties do hereby agree as follows:
1. Assignment and Assumption of Contracts. In accordance with, and subject to the terms of, the Termination Agreement, effective as of the [Transition Period End Date] (the “Contract Assignment and Assumption Effective Date”), Assignor hereby assigns, transfers and conveys to Assignee, and Assignee hereby accepts, all of Assignor’s right, title and interest in, to and under the Transferred GSK Contracts, and Assignee hereby assumes and agrees to perform and discharge
| 1 | Note to draft: Appropriate entity to be confirmed. |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
28
EXECUTION COPY
when due, all of Assignor’s obligations and liabilities in, to and under the Transferred GSK Contracts; provided, however, that Assignee shall not assume and shall not be liable for any obligations or liabilities of any nature whatsoever of Assignor, whether known or unknown, accrued or not accrued, fixed or contingent, arising prior to the Contract Assignment and Assumption Effective Date (“Contract Excluded Liabilities”), and the parties hereto agree that all such Excluded Liabilities shall remain the sole responsibility of Assignor.
2. Assignment of Other Assets. In accordance with, and subject to the terms of, the Termination Agreement, effective as of the [Termination Effective Date / Transition Period End Date / Completion of the Ongoing Studies / end of the Supply Term] (the “Other Asset Assignment Effective Date”), Assignor hereby assigns, transfers, and conveys to Assignee, and Assignee hereby accepts, all of Assignor’s right, title and interest in, to and under [Transferred GSK Know-How / Transferred GSK Regulatory Filings / Transferred GSK Promotional Materials], including all intellectual property rights residing in the foregoing, including copyrights and trade secrets to the extent available (collectively, the “Transferred Asset”), for Assignee’s own use and enjoyment, and for the use and enjoyment of Assignee’s successors, assigns or other legal representatives, as fully and entirely as the same would have been held and enjoyed by Assignor if this assignment had not been made, together with all income, royalties or payments due or payable as of the Other Asset Assignment Effective Date or thereafter, including, without limitation, all claims for damages by reason of past, present or future infringement or misappropriation, with the right to xxx for and collect the same for Assignee’s own use and enjoyment and for the use and enjoyment of its successors, assigns or other legal representatives.
3. Terms of the Termination Agreement. Nothing herein will, or will be deemed to, modify or otherwise affect any provisions of the Termination Agreement or affect or modify any of the rights or obligations of the parties under the Termination Agreement, including, but not limited to, Assignor’s representations, warranties, covenants, agreements and indemnities relating to the [Transferred GSK Know-How / Transferred GSK Regulatory Filings / Transferred GSK Contracts / Transferred GSK Promotional Materials]. In the event of any conflict or inconsistency between the terms of the Termination Agreement and the terms hereof, the terms of the Termination Agreement shall govern.
4. Further Actions. Each of the parties hereto covenants and agrees, at its own expense, to execute and deliver, at the request of the other party hereto, such further instruments of transfer and assignment and to take such other action as such other party may reasonably request to more effectively consummate the assignments and assumptions contemplated by this Assignment and Assumption. [The Parties will cooperate to send applicable notices to the applicable counterparties to the various Transferred GSK Contracts regarding assignment of the applicable Transferred GSK Contract hereunder, as required under the terms of the applicable Transferred GSK Contract (or, if such notice is not required, as requested by Assignee).] [If consent of the counterparty to a given Transferred GSK Contract is required for assignment of such Transferred GSK Contract hereunder, Assignor agrees upon request of Assignee to use commercially reasonable efforts to obtain such consent.]2
| 2 | Note to draft: Bracketed text to be reviewed following identification and review of Transferred GSK Contracts. |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
29
EXECUTION COPY
5. Miscellaneous. This Assignment and Assumption and all questions regarding its validity or interpretation, or the breach or performance of this Assignment and Assumption, shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without reference to conflict of law principles. No amendment or modification of any provision of this Assignment and Assumption shall be effective unless in writing signed by a duly authorized representative of each party hereto. This Assignment and Assumption will bind and inure to the benefit of Assignor and Assignee and their respective successors and assigns. This Assignment and Assumption may be executed in any number of counterparts, each of which shall be deemed an original, and all of which together shall constitute one and the same instrument.
[The remainder of this page is intentionally left blank; signatures appear on the following page.]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
30
EXECUTION COPY
IN WITNESS WHEREOF, the parties have executed this Assignment and Assumption Agreement as of the date first set forth above.
| XENOPORT, INC. | ||
| BY: |
| |
| NAME: | Xxxxxx Xxxxxxx, PhD | |
| TITLE: | Chief Executive Officer | |
| [GLAXO GROUP LIMITED] | ||
| BY: |
| |
| NAME: |
| |
| TITLE: |
| |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
31
EXECUTION COPY
EXHIBIT [A – D]
[Transferred GSK Know-How / Transferred GSK Regulatory Filings / Transferred GSK Contracts / Transferred GSK Promotional Materials]
[To be completed]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
32
EXECUTION COPY
EXHIBIT 1.14
Form of Xxxx of Sale
THIS XXXX OF SALE (this “Xxxx of Sale”), is made an entered into as of [ ] 20 (“Effective Date”) between XenoPort, Inc., a Delaware corporation having its principal place of business at 0000 Xxxxxxx Xxxxxxxxxx, Xxxxx Xxxxx, XX 00000 (“XenoPort”) and [Glaxo Group Limited, a company existing under the laws of England and Wales, having it registered office at Glaxo Wellcome House, Berkeley Avenue, Greenford, Middlesex, UB6 0NN, England]3 (“GSK”).
BACKGROUND
A. GSK and XenoPort are parties to that certain Termination and Transition Agreement (“Termination Agreement”), dated as of November [ ], 2012, pursuant to which GSK and XenoPort have agreed to settle the Litigation and terminate the Restated Agreement, as set forth in, and subject to the terms and conditions of, the Termination Agreement and the Stock Purchase Agreement between XenoPort and GSK, dated as of the Termination Effective Date (the “SPA”), to provide for, among other matters, the reversion to XenoPort of all rights with respect to the Product, and for GSK to purchase certain shares of Common Stock of XenoPort pursuant to the SPA. Capitalized terms used herein and not otherwise defined herein shall have the meanings respectively ascribed to such capitalized terms in the Termination Agreement; and
B. Pursuant to Section [3.4(a)] of the Termination Agreement, GSK and XenoPort have agreed that GSK will sell, assign, transfer, convey and deliver, and XenoPort will purchase, accept and acquire from GSK, the Transferred GSK Inventory set forth on Schedule 1 attached to this Xxxx of Sale.
NOW, THEREFORE, for and in consideration of the premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt, adequacy and legal sufficiency of which are hereby acknowledged, the parties do hereby agree as follows:
1. Sale of Inventory. In accordance with, and subject to the terms of, the Termination Agreement, GSK hereby sells, assigns, transfers and conveys and delivers to XenoPort, and XenoPort hereby purchases, accepts and acquires from GSK, all of GSK’s right, title and interest in, to and under the Transferred GSK Inventory.
2. Terms of the Termination Agreement. Nothing herein will, or will be deemed to, modify or otherwise affect any provisions of the Termination Agreement or affect or modify any of the rights or obligations of the parties under the Termination Agreement, including, but not limited to, Assignor’s representations, warranties, covenants, agreements and indemnities relating to the Transferred GSK Inventory. In the event of any conflict or inconsistency between the terms of the Termination Agreement and the terms hereof, the terms of the Termination Agreement shall govern.
| 3 | Note to draft: Appropriate entity to be confirmed |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
33
EXECUTION COPY
3. Further Actions. Each of the parties hereto covenants and agrees, at its own expense, to execute and deliver, at the request of the other party hereto, such further instruments of transfer and assignment and to take such other action as such other party may reasonably request to effect the sale of the Transferred GSK Inventory contemplated by this Xxxx of Sale.
4. Miscellaneous. This Xxxx of Sale and all questions regarding its validity or interpretation, or the breach or performance of this Xxxx of Sale, shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without reference to conflict of law principles. No amendment or modification of any provision of this Xxxx of Sale shall be effective unless in writing signed by a duly authorized representative of each party hereto. This Xxxx of Sale will bind and inure to the benefit of GSK and XenoPort and their respective successors and assigns. This Xxxx of Sale may be executed in any number of counterparts, each of which shall be deemed an original, and all of which together shall constitute one and the same instrument.
[The remainder of this page is intentionally left blank; signatures appear on the following page.]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
34
EXECUTION COPY
IN WITNESS WHEREOF, the parties have executed this Xxxx of Sale as of the date first set forth above.
| XENOPORT, INC. | ||
| BY: |
| |
| NAME: | Xxxxxx Xxxxxxx, PhD | |
| TITLE: | Chief Executive Officer | |
| [GLAXO GROUP LIMITED] | ||
| BY: |
| |
| NAME: |
| |
| TITLE: |
| |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
35
EXECUTION COPY
SCHEDULE 1
[Insert list of Transferred GSK Inventory]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
36
EXECUTION COPY
EXHIBIT 1.38
Form of Domain Name Assignment Agreement
THIS DOMAIN ASSIGNMENT AGREEMENT (this “Domain Name Assignment”) is made and entered into as of [ ,] 2012 (“Effective Date”) between XenoPort, Inc., a Delaware corporation having its principal place of business at 0000 Xxxxxxx Xxxxxxxxxx, Xxxxx Xxxxx, XX 00000 (“Assignee” or “XenoPort”), and [Glaxo Group Limited, a company existing under the laws of England and Wales, having its registered office at Glaxo Wellcome House, Berkeley Avenue, Greenford, Middlesex, UB6 0NN, England]4 (“Assignor” or “GSK”).
BACKGROUND
A. Assignor and Assignee are parties to that certain Termination and Transition Agreement (“Termination Agreement”), dated as of November [ ], 2012 (“Termination Effective Date”), pursuant to which Assignor and Assignee have agreed to settle the Litigation and terminate the Restated Agreement, as set forth in, and subject to the terms and conditions of, the Termination Agreement and the Stock Purchase Agreement between XenoPort and GSK, dated as of the Termination Effective Date (the “SPA”), to provide for, among other matters, the reversion to XenoPort of all rights with respect to the Product, and for GSK to purchase certain shares of Common Stock of XenoPort pursuant to the SPA. Capitalized terms used herein and not otherwise defined herein shall have the meanings respectively ascribed to such capitalized terms in the Termination Agreement; and
B. Pursuant to Section [3.4(a)] of the Termination Agreement, Assignor and Assignee have agreed that Assignor shall assign to Assignee and Assignee shall acquire all right, title and interest in and to the Transferred GSK Domain Names, as set forth on Schedule 1 attached hereto, including all lower-level internet domain names for which such domain name is a root or parent, whether in the form of an address for use in electronic mail transfer, a Universal Resource Locator, a file transfer protocol location, or other form suitable for specifying the location of an electronic data file over a distributed computer network (collectively, the “Domain Names”).
NOW, THEREFORE, for and in consideration of the premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt, adequacy and legal sufficiency of which are hereby acknowledged, the parties do hereby agree as follows:
1. Assignment. Assignor hereby perpetually, irrevocably and unconditionally assigns, transfers, conveys and sets over to Assignee and its successors, assigns and other legal representatives all of Assignor’s rights, titles and interests in and to the Domain Names and the goodwill associated therewith, together with any registrations and applications for registration thereof for such Domain Names, for Assignee’s own use and enjoyment, and for the use and
| 4 | Note to draft: Appropriate entity to be confirmed. |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
37
EXECUTION COPY
enjoyment of Assignee’s successors, assigns or other legal representatives, as fully and entirely as the same would have been held and enjoyed by Assignor if this assignment had not been made, together with all income, royalties or payments due or payable as of the Effective Date or thereafter, including, without limitation, all claims for damages by reason of past, present or future infringement of the Domain Names, with the right to xxx for and collect the same for Assignee’s own use and enjoyment and for the use and enjoyment of its successors, assigns or other legal representatives.
2. Terms of the Termination Agreement. Nothing herein will, or will be deemed to, modify or otherwise affect any provisions of the Termination Agreement or affect or modify any of the rights or obligations of the parties under the Termination Agreement, including, but not limited to, Assignor’s representations, warranties, covenants, agreements and indemnities relating to the Domain Names. In the event of any conflict or inconsistency between the terms of the Termination Agreement and the terms hereof, the terms of the Termination Agreement shall govern.
3. Further Actions. Assignor agrees to execute such documents, render such assistance, and take such other action as Assignee may request, to apply for, register, perfect, confirm, and protect Assignee’s rights in the Domain Names. Without limiting the foregoing, within [10] days of the Effective Date, Assignor shall initiate the process of transferring registration for the Domain Names to Assignee with the applicable registrar, including filing a Registrant Name Change Agreement with the applicable registrar as necessary, in accordance with all registrar policies and requirements for domain transfer to effectuate the transfer of registration for the Domain Names to Assignee.
4. Miscellaneous. This Domain Name Assignment and all questions regarding its validity or interpretation, or the breach or performance of this Domain Name Assignment, shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without reference to conflict of law principles. No amendment or modification of any provision of this Domain Name Assignment shall be effective unless in writing signed by a duly authorized representative of each party hereto. This Domain Name Assignment will bind and inure to the benefit of Assignor and Assignee and their respective successors and assigns. This Domain Name Assignment may be executed in any number of counterparts, each of which shall be deemed an original, and all of which together shall constitute one and the same instrument.
[The remainder of this page is intentionally left blank; signatures appear on the following page.]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
38
EXECUTION COPY
IN WITNESS WHEREOF, the parties have executed this Domain Name Assignment as of the date first set forth above.
| XENOPORT, INC. | ||
| BY: |
| |
| NAME: | Xxxxxx Xxxxxxx, PhD | |
| TITLE: | Chief Executive Officer | |
| [GLAXO GROUP LIMITED] | ||
| BY: |
| |
| NAME: |
| |
| TITLE: |
| |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
39
EXECUTION COPY
SCHEDULE 1
[Insert list of Transferred GSK Domain Names]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
40
EXECUTION COPY
EXHIBIT 1.94
Form of Patent Assignment Agreement
THIS PATENT ASSIGNMENT AGREEMENT (this “Patent Assignment”) is made and entered into as of [ ,] 2012 (“Effective Date”) between XenoPort, Inc., a Delaware corporation having its principal place of business at 0000 Xxxxxxx Xxxxxxxxxx, Xxxxx Xxxxx, XX 00000 (“Assignee” or “XenoPort”), and [Glaxo Group Limited, a company existing under the laws of England and Wales, having its registered office at Glaxo Wellcome House, Berkeley Avenue, Greenford, Middlesex, UB6 0NN, England]5 (“Assignor” or “GSK”).
BACKGROUND
A. Assignor and Assignee are parties to that certain Termination and Transition Agreement (“Termination Agreement”), dated as of November [ ], 2012 (“Termination Effective Date”), pursuant to which Assignor and Assignee have agreed to settle the Litigation and terminate the Restated Agreement, as set forth in, and subject to the terms and conditions of, the Termination Agreement and the Stock Purchase Agreement between XenoPort and GSK, dated as of the Termination Effective Date (the “SPA”), to provide for, among other matters, the reversion to XenoPort of all rights with respect to the Product, and for GSK to purchase certain shares of Common Stock of XenoPort pursuant to the SPA. Capitalized terms used herein and not otherwise defined herein shall have the meanings respectively ascribed to such capitalized terms in the Termination Agreement; and
B. Pursuant to Section [3.4(a)] of the Termination Agreement, Assignor and Assignee have agreed that Assignor shall assign to Assignee and Assignee shall acquire all right, title and interest in and to the Transferred GSK Patents set forth on Schedule 1 attached hereto.
NOW, THEREFORE, for and in consideration of the premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt, adequacy and legal sufficiency of which are hereby acknowledged, the parties do hereby agree as follows:
1. Assignment. Assignor hereby perpetually, irrevocably and unconditionally assigns, transfers, conveys and sets over to Assignee and its successors, assigns and other legal representatives all of Assignor’s rights, titles and interests in and to the Transferred GSK Patents and the inventions disclosed therein, together with all additions, divisions, continuations, continuations-in-part, substitutions, reissues, re-examinations, extensions, registrations, patent term extensions, supplemental protection certificates, renewals and foreign counterparts of any of the foregoing (collectively, the “Transferred Patents”), for Assignee’s own use and enjoyment, and for the use and enjoyment of Assignee’s successors, assigns or other legal representatives, as fully and entirely as the same would have been held and enjoyed by Assignor if this assignment had not been made,
| 5 | Note to draft: Appropriate entity to be confirmed. |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
41
EXECUTION COPY
together with all income, royalties or payments due or payable as of the Effective Date or thereafter, including, without limitation, all claims for damages by reason of past, present or future infringement of the Transferred Patents, with the right to xxx for and collect the same for Assignee’s own use and enjoyment and for the use and enjoyment of its successors, assigns or other legal representatives.
2. Terms of the Termination Agreement. Nothing herein will, or will be deemed to, modify or otherwise affect any provisions of the Termination Agreement or affect or modify any of the rights or obligations of the parties under the Termination Agreement, including, but not limited to, Assignor’s representations, warranties, covenants, agreements and indemnities relating to the Transferred Patents. In the event of any conflict or inconsistency between the terms of the Termination Agreement and the terms hereof, the terms of the Termination Agreement shall govern.
3. Further Actions. Each of the parties hereto covenants and agrees, at its own expense, to execute and deliver, at the request of the other party hereto, such further instruments of transfer and assignment and to take such other action as such other party may reasonably request to effect the assignments contemplated by this Patent Assignment.
4. Authorization. Assignor authorizes and requests the United States Commissioner of Patents and Trademarks and any other applicable government authority to record Assignee as the assignee and owner of the Transferred Patents, and issue any and all registrations or patents thereon to Assignee, as assignee of the entire right, title and interest in, to and under the same, for the sole use and enjoyment of Assignee and its successors, assigns or other legal representatives.
5. Miscellaneous. This Patent Assignment and all questions regarding its validity or interpretation, or the breach or performance of this Patent Assignment, shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without reference to conflict of law principles. No amendment or modification of any provision of this Patent Assignment shall be effective unless in writing signed by a duly authorized representative of each party hereto. This Patent Assignment will bind and inure to the benefit of Assignor and Assignee and their respective successors and assigns. This Patent Assignment may be executed in any number of counterparts, each of which shall be deemed an original, and all of which together shall constitute one and the same instrument.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
42
EXECUTION COPY
IN WITNESS WHEREOF, the parties have executed this Patent Assignment as of the date first set forth above.
| XENOPORT, INC. | ||
| BY: |
| |
| NAME: | Xxxxxx Xxxxxxx, PhD | |
| TITLE: | Chief Executive Officer | |
| [GLAXO GROUP LIMITED] | ||
| BY: |
| |
| NAME: |
| |
| TITLE: |
| |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
43
EXECUTION COPY
SCHEDULE 1
[Insert list of Transferred GSK Patents]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
44
EXECUTION COPY
EXHIBIT 1.118
Form of Stock Purchase Agreement
See attached.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
45
EXECUTION COPY
EXHIBIT 1.131
Form of Trademark Assignment Agreement
THIS TRADEMARK ASSIGNMENT AGREEMENT (this “Trademark Assignment”) is made and entered into as of [ ,] 2012 (“Effective Date”) between XenoPort, Inc., a Delaware corporation having its principal place of business at 0000 Xxxxxxx Xxxxxxxxxx, Xxxxx Xxxxx, XX 00000 (“Assignee” or “XenoPort”), and [Glaxo Group Limited, a company existing under the laws of England and Wales, having its registered office at Glaxo Wellcome House, Berkeley Avenue, Greenford, Middlesex, UB6 0NN, England]6 (“Assignor” or “GSK”).
BACKGROUND
A. Assignor and Assignee are parties to that certain Termination and Transition Agreement (“Termination Agreement”), dated as of November [ ], 2012 (“Termination Effective Date”), pursuant to which Assignor and Assignee have agreed to settle the Litigation and terminate the Restated Agreement, as set forth in, and subject to the terms and conditions of, the Termination Agreement and the Stock Purchase Agreement between XenoPort and GSK, dated as of the Termination Effective Date (the “SPA”), to provide for, among other matters, the reversion to XenoPort of all rights with respect to the Product, and for GSK to purchase certain shares of Common Stock of XenoPort pursuant to the SPA. Capitalized terms used herein and not otherwise defined herein shall have the meanings respectively ascribed to such capitalized terms in the Termination Agreement; and
B. Pursuant to Section [3.4(a)] of the Termination Agreement, Assignor and Assignee have agreed that Assignor shall assign to Assignee and Assignee shall acquire all right, title and interest in and to the Transferred GSK Trademarks set forth on Schedule 1 attached hereto.
NOW, THEREFORE, for and in consideration of the premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt, adequacy and legal sufficiency of which are hereby acknowledged, the parties do hereby agree as follows:
1. Assignment. Assignor hereby perpetually, irrevocably and unconditionally assigns, transfers, conveys and sets over to Assignee and its successors, assigns and other legal representatives all of Assignor’s rights, titles and interests in and to the Transferred GSK Trademarks and the goodwill associated therewith, including the rights to apply for and maintain any applications, registrations or renewals therefor, together with any registrations and applications for registration thereof, and intellectual property rights residing in the foregoing, including copyrights and design rights (collectively, the “Transferred Trademarks”), for Assignee’s own use and enjoyment, and for the use and enjoyment of Assignee’s successors, assigns or other legal representatives, as fully and entirely as the same would have been held and enjoyed by Assignor if
| 6 | Note to draft: Appropriate entity to be confirmed. |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
46
EXECUTION COPY
this assignment had not been made, together with all income, royalties or payments due or payable as of the Effective Date or thereafter, including, without limitation, all claims for damages by reason of past, present or future infringement, dilution or other violation of the Transferred Trademarks by third parties, with the right to xxx for and collect the same for Assignee’s own use and enjoyment and for the use and enjoyment of its successors, assigns or other legal representatives. Assignor hereby waives and agrees not to enforce any rights of attribution and integrity and other moral rights Assignor may have in the Transferred Trademarks.
2. Terms of the Termination Agreement. Nothing herein will, or will be deemed to, modify or otherwise affect any provisions of the Termination Agreement or affect or modify any of the rights or obligations of the parties under the Termination Agreement, including, but not limited to, Assignor’s representations, warranties, covenants, agreements and indemnities relating to the Transferred Trademarks. In the event of any conflict or inconsistency between the terms of the Termination Agreement and the terms hereof, the terms of the Termination Agreement shall govern.
3. Further Actions. Each of the parties hereto covenants and agrees, at its own expense, to execute and deliver, at the request of the other party hereto, such further instruments of transfer and assignment and to take such other action as such other party may reasonably request to effect the assignments contemplated by this Trademark Assignment.
4. Authorization. Assignor authorizes and requests the United States Commissioner of Patents and Trademarks and any other applicable government authority to record Assignee as the assignee and owner of the Transferred Trademarks, and issue any and all registrations thereon to Assignee, as assignee of the entire right, title and interest in, to and under the same, for the sole use and enjoyment of Assignee and its successors, assigns or other legal representatives.
5. Miscellaneous. This Trademark Assignment and all questions regarding its validity or interpretation, or the breach or performance of this Trademark Assignment, shall be governed by, and construed and enforced in accordance with, the laws of the State of Delaware, without reference to conflict of law principles. No amendment or modification of any provision of this Trademark Assignment shall be effective unless in writing signed by a duly authorized representative of each party hereto. This Trademark Assignment will bind and inure to the benefit of Assignor and Assignee and their respective successors and assigns. This Trademark Assignment may be executed in any number of counterparts, each of which shall be deemed an original, and all of which together shall constitute one and the same instrument.
[The remainder of this page is intentionally left blank; signatures appear on the following page.]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
47
EXECUTION COPY
IN WITNESS WHEREOF, the parties have executed this Trademark Assignment as of the date first set forth above.
| XENOPORT, INC. | ||
| BY: |
| |
| NAME: | Xxxxxx Xxxxxxx, PhD | |
| TITLE: | Chief Executive Officer | |
| [GLAXO GROUP LIMITED] | ||
| BY: |
| |
| NAME: |
| |
| TITLE: |
| |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
48
EXECUTION COPY
SCHEDULE 1
[Insert list of Transferred GSK Trademarks]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
49
EXECUTION COPY
EXHIBIT 2.4A
California Dismissal Letter
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
50
EXECUTION COPY
XXXXXX X. XXXXXXXXX (BAR NO. 66781)
XXxXxxxxxx@xxxx.xxx
XXXX X. XXXXX (BAR NO. 147948)
XXxxxx@xxxx.xxx
XXXXXXXX & XXXXXXXX LLP
000 Xxxxxx Xxxxxx
Xxx Xxxxxxxxx, Xxxxxxxxxx 00000-0000
Telephone: 000.000.0000
Facsimile: 415.268.7522
XXXX X. XXX (BAR NO. 247604)
XXxx@xxxx.xxx
XXXXXXXX & XXXXXXXX LLP
000 Xxxx Xxxx Xxxx
Xxxx Xxxx, Xxxxxxxxxx 00000-0000
Telephone: 000.000.0000
Facsimile: 650.494.0792
Attorneys for Plaintiff
XENOPORT, INC.
[Counsel information for Defendants on signature page]
UNITED STATES DISTRICT COURT
NORTHERN DISTRICT OF CALIFORNIA
SAN XXXX DIVISION
| XENOPORT, INC., a Delaware corporation,
Plaintiff,
v.
GLAXO GROUP LIMITED, a U.K. corporation, GLAXOSMITHKLINE LLC, a Delaware limited liability company, and GLAXOSMITHKLINE HOLDINGS (AMERICAS) INC., a Delaware corporation,
Defendants. |
Case No. CV 12-01544 EJD
JOINT STIPULATION AND [PROPOSED] ORDER REGARDING DISMISSAL OF ACTION
Judge: Xxx. Xxxxxx X. Xxxxxx |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
51
EXECUTION COPY
Pursuant to Civil Local Rule 7-12, and Rule 41(a)(1)(A) of the Federal Rules of Civil Procedure, Plaintiff XenoPort, Inc. and Defendants Glaxo Group Limited, GlaxoSmithKline LLC, and GlaxoSmithKline Holdings (Americas) Inc., through their undersigned counsel of record, hereby stipulate to the dismissal with prejudice of the above-captioned action. All parties shall bear their own costs, expenses and attorneys’ fees.
Respectfully submitted,
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
52
EXECUTION COPY
| Dated: November , 2012 | XXXXXXXX & XXXXXXXX LLP | |||||
| By: | /s/ Xxxx X. Xxxxx | |||||
| Xxxx X. Xxxxx | ||||||
| Attorneys for Plaintiff | ||||||
| XENOPORT, INC. | ||||||
| Dated: November , 2012 | XXXX XXXXX LLP | |||||
| By: | /s/ Xxxxxxx X. Xxxxxxxxxx | |||||
| Xxxxxxx X. Xxxxxxxxxx | ||||||
| Xxxxxxx X. Xxxxxxxxxx (SBN 135976) xxxxxxxxxxx@xxxxxxxxx.xxx Xxxxxxxx Xxx Cheng (SBN 239711) xxxxxx@xxxxxxxxx.xxx Farah Tabibkhoei (SBN 266312) xxxxxxxxxxx@xxxxxxxxx.xxx XXXX XXXXX LLP 000 Xxxxx Xxxxx Xxxxxx, Xxxxx 0000 Xxx Xxxxxxx, XX 00000-0000 Telephone: x0 000 000 0000 Facsimile: x0 000 000 0000 | ||||||
| Xxxxx X. Xxxxx (SBN 84923) xxxxxx@xxxxxxxxx.xxx Xxxxxxx Xxxxxxx (SBN 173263) xxxxxxxx@xxxxxxxxx.xxx XXXX XXXXX LLP 000 0xx Xx. Xxx. 0000 Xxx Xxxxxxxxx, XX 00000 Telephone: x0 000 000 0000 Facsimile: x0 000 000 0000 | ||||||
| Attorneys for Defendants GLAXO GROUP LIMITED, GLAXOSMITHKLINE LLC, and GLAXOSMITHKLINE HOLDINGS (AMERICAS) INC. | ||||||
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
53
EXECUTION COPY
ORDER
PURSUANT TO STIPULATION, IT IS SO ORDERED.
Dated:
| Xxxxxxxxx Xxxxxx X. Xxxxxx |
| United States District Judge |
| Northern District of California |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
54
EXECUTION COPY
EXHIBIT 2.4B
Delaware Dismissal Letter
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
55
EXECUTION COPY
IN THE UNITED STATES DISTRICT COURT
FOR THE DISTRICT OF DELAWARE
| : | ||||
| GLAXO GROUP LIMITED, | : | |||
| : | C.A. No. 12-225-GMS | |||
| Plaintiff, |
: | |||
| : | ||||
| vs. | : | |||
| : | ||||
| XENOPORT, INC., and DOES 1-10, | : | |||
| : | ||||
| Defendants. |
: | |||
| : | ||||
| : |
STIPULATION AND ORDER OF VOLUNTARY DISMISSAL OF ACTION BETWEEN
PLAINTIFF GLAXO GROUP LIMITED AND DEFENDANT XENOPORT, INC.
Plaintiff Glaxo Group Limited and Defendant XenoPort, Inc., by their attorneys, hereby stipulate, subject to approval by the Court, that the above-captioned action shall be dismissed with prejudice pursuant to Federal Rule of Civil Procedure 41(a)(1)(A)(ii). Each party shall bear its own costs and attorneys’ fees.
Dated: November , 2012
| XXXX XXXXX LLP | YOUNG XXXXXXX STARGATT & XXXXXX, LLP | |||
|
|
| |||
| Xxxxxxxxx X. Xxxxxx (DE #5563) 0000 Xxxxxx Xxxxxx, Xxxxx 0000 Xxxxxxxxxx, XX 00000 Telephone: (000) 000-0000 Fax: (000) 000-0000 E-mail: xxxxxxx@xxxxxxxxx.xxx |
Xxxxx X. Xxxxxx (No. 4780) Xxxxx X. Xxxxxx (No. 4764) Xxxxxx Square 0000 Xxxxx Xxxx Xxxxxx Xxxxxxxxxx, Xxxxxxxx 00000 xxxxxxx@xxxx.xxx | |||
| xxxxxxx@xxxx.xxx (000) 000-0000 | ||||
| Attorneys for Plaintiff Glaxo Group Limited |
Attorneys for Defendant XenoPort, Inc. |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
56
EXECUTION COPY
IT IS SO ORDERED this day of , 2012.
|
|
| Chief, United States District Judge |
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
57
EXECUTION COPY
EXHIBIT 4.3A
Development Plan
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
58
EXECUTION COPY
EXHIBIT 4.3B
RLS Horizant Approval Letter
See Attached.
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
59
EXECUTION COPY
EXHIBIT 4.4
Commercialization Transition Plan
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
60
EXECUTION COPY
EXHIBIT 4.6
Manufacturing Technology Transfer Plan
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
61
EXECUTION COPY
EXHIBIT 4.9
Safety Data Exchange Transfer Plan
[… * …]
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
62
EXECUTION COPY
EXHIBIT 8.3
Press Releases
(See attached.)
| * | CERTAIN CONFIDENTIAL INFORMATION CONTAINED IN THIS DOCUMENT, MARKED BY BRACKETS, IS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 24B-2 OF THE SECURITIES EXCHANGE ACT OF 1934, AS AMENDED. |
63

