DEVELOPMENT AND LICENSE AGREEMENT BY AND BETWEEN BIOCRYST PHARMACEUTICALS, INC. AND AND HOFFMANN-LA ROCHE INC.
EXECUTION COPY
Exhibit 10.2
NOTE: THIS DOCUMENT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST PURSUANT TO RULE 24B-2
UNDER THE SECURITIES EXCHANGE ACT OF 1934. PORTIONS OF THIS DOCUMENT FOR WHICH CONFIDENTIAL
TREATMENT HAS BEEN REQUESTED HAVE BEEN REDACTED AND ARE MARKED HEREIN BY “***”. SUCH REDACTED
INFORMATION HAS BEEN FILED SEPARATELY WITH THE COMMISSION PURSUANT TO THE CONFIDENTIAL TREATMENT
REQUEST.
BY AND BETWEEN
BIOCRYST PHARMACEUTICALS, INC.
AND
X.XXXXXXXX-XX XXXXX LTD
AND
XXXXXXXX-XX XXXXX INC.
EXECUTION COPY
TABLE OF CONTENTS
ARTICLE 1 DEFINITIONS |
1 | |||
1.1 Actively Developing |
1 | |||
1.2 Adjusted Gross Sales |
1 | |||
1.3 Affiliate |
1 | |||
1.4 Autoimmune Indications |
2 | |||
1.5 Backup Compounds |
2 | |||
1.6 BioCryst Know-How |
2 | |||
1.7 BioCryst Patents |
2 | |||
1.8 Calendar Quarter |
2 | |||
1.9 Closing Date |
2 | |||
1.10 Compound |
2 | |||
1.11 Commercially Reasonable Efforts |
2 | |||
1.12 Commercialization |
2 | |||
1.13 Confidential Information |
3 | |||
1.14 Control |
3 | |||
1.15 Dermatology |
3 | |||
1.16 Detail |
3 | |||
1.17 Develop |
3 | |||
1.18 Development Costs |
4 | |||
1.19 Development Plan |
4 | |||
1.20 Effective Date |
4 | |||
1.21 Field |
4 | |||
1.22 First Commercial Sale |
4 | |||
1.23 Fodosine |
4 | |||
1.24 Generic Licensed Product |
4 | |||
1.25 IND |
4 | |||
1.26 Insolvency Event |
4 | |||
1.27 Invoice Price |
5 | |||
1.28 Joint Development Committee |
5 | |||
1.29 Joint Steering Committee |
5 | |||
1.30 Law |
5 | |||
1.31 Licensed Product |
5 | |||
1.32 Major Market Countries |
5 | |||
1.33 NDA Approval |
5 | |||
1.34 Net Sales |
5 | |||
1.35 Neurology |
5 | |||
1.36 Party |
5 | |||
1.37 Phase II |
6 | |||
1.38 Phase IIa |
6 | |||
1.39 Phase IIb |
6 | |||
1.40 Phase III |
6 | |||
1.41 Pre-Existing Third Party License |
6 | |||
1.42 Promotional Material |
6 | |||
1.43 Regulatory Authority |
6 | |||
1.44 Roche Compound |
6 | |||
1.45 Roche Group |
6 | |||
1.46 Roche Know-How |
6 | |||
1.47 Roche Patents |
6 | |||
1.48 Roche Products |
6 | |||
1.49 ROW Country |
7 | |||
1.50 Royalty Bearing Products |
7 | |||
1.51 Sale, Sold or Sell |
7 |
EXECUTION COPY
1.52 Special Indication Product |
7 | |||
1.53 Special Indications |
7 | |||
1.54 Start of Phase IIa |
7 | |||
1.55 Start of Phase IIb |
7 | |||
1.56 Start of Phase III or Pivotal Trial |
7 | |||
1.57 Stem-Cell Transplantation |
7 | |||
1.58 Sublicensee |
7 | |||
1.59 Term of the Agreement |
7 | |||
1.60 Third Party |
7 | |||
1.61 Transplantation Indications |
7 | |||
1.62 Valid Claim |
7 | |||
1.63 Western Europe |
8 | |||
ARTICLE 2 GRANT OF RIGHTS |
8 | |||
2.1 License Grants |
8 | |||
2.2 Backup Compounds |
9 | |||
2.3 First Right of Negotiation of Backup Compounds |
9 | |||
2.4 Roche Compounds |
10 | |||
2.5 Fodosine |
10 | |||
2.6 Pre-Existing Third Party License |
10 | |||
ARTICLE 3 BIOCRYST RIGHT TO PROMOTE |
10 | |||
3.1 Co-Promotion |
10 | |||
ARTICLE 4 GOVERNANCE |
10 | |||
4.1 Steering Committee |
10 | |||
4.2 Development Committee |
11 | |||
4.3 Committee Membership |
12 | |||
4.4 Committee Chairs |
12 | |||
4.5 Committee Meetings |
12 | |||
4.6 Decision Making |
12 | |||
4.7 Interactions Between Committees |
13 | |||
4.8 Performance of Representatives |
13 | |||
ARTICLE 5 DILIGENCE AND SPECIAL INDICATIONS |
13 | |||
5.1 Diligence |
13 | |||
5.2 Special Indications |
13 | |||
5.3 Development Plan |
14 | |||
5.4 Reports |
14 | |||
5.5 End of Xxxxx XXx |
00 | |||
5.6 Registration |
14 | |||
5.7 Sequencing of Licensed Products |
15 | |||
ARTICLE 6
CLINICAL TRIALS, REGULATORY MATTERS AND MANUFACTURING |
15 | |||
6.1 Clinical Trials and Regulatory Matters |
15 | |||
6.2 Adverse Event Reporting |
16 | |||
6.3 Manufacturing and Technical Assistance |
16 | |||
ARTICLE 7 CONSIDERATION |
17 | |||
EXECUTION COPY
7.1 Lump Sum Payments |
17 | |||
7.2 Development Event Payments |
17 | |||
7.3 Commercial Event Payments |
19 | |||
7.4 Royalties |
19 | |||
7.5 Patent Coverage Adjustment |
19 | |||
7.6 Combination Licensed Products |
20 | |||
7.7 Payments to Third Parties |
20 | |||
7.8 Generic Sales Adjustment |
20 | |||
7.9 Royalties Due Once |
20 | |||
ARTICLE 8 PAYMENTS; RECORDS; AUDIT |
20 | |||
8.1 Payment of Royalties |
20 | |||
8.2 Currency |
21 | |||
8.3 Payments Nonrefundable |
21 | |||
8.4 Roche Records and Audit |
21 | |||
8.5 BioCryst Records and Audit |
21 | |||
8.6 Withholding Taxes |
22 | |||
ARTICLE 9 INTELLECTUAL PROPERTY |
22 | |||
9.1 Prosecution of BioCryst Patent Rights |
22 | |||
9.2 Infringement |
23 | |||
9.3 Xxxxx-Xxxxxx |
24 | |||
ARTICLE 10 CONFIDENTIAL INFORMATION |
24 | |||
10.1 Non-Use and Xxx-Xxxxxxxxxx |
00 | |||
10.2 Authorized Disclosure |
24 | |||
ARTICLE 11 TERMINATION |
25 | |||
11.1 Commencement and Term |
25 | |||
11.2 Termination for Breach |
25 | |||
11.3 Termination by Roche Without Cause |
25 | |||
11.4 Consequences of Termination |
25 | |||
11.5 Royalty and Payment Obligations |
27 | |||
ARTICLE 12 INDEMNIFICATION |
27 | |||
12.1 Indemnification by BioCryst |
27 | |||
12.2 Indemnification by Roche |
27 | |||
12.3 Procedure |
27 | |||
12.4 Limitation on Damages |
28 | |||
ARTICLE 13 REPRESENTATIONS AND WARRANTIES |
28 | |||
13.1 Mutual Representations and Warranties |
28 | |||
13.2 BioCryst Representations and Warranties |
28 | |||
13.3 Roche Representations and Warranties |
29 | |||
13.4 HSR Act and Other Applicable Governmental Filings |
29 | |||
13.5 No Debarment |
30 | |||
13.6 No PNP Inhibitors |
30 | |||
13.7 No Other Representations |
30 |
EXECUTION COPY
ARTICLE 14 CONDITIONS TO CLOSING |
30 | |||
14.1 Conditions Subsequent |
30 | |||
ARTICLE 15 DISPUTE RESOLUTIONS AND GOVERNING LAW |
30 | |||
15.1 Disputes |
30 | |||
15.2 Arbitration |
31 | |||
15.3 Arbitration Procedure |
31 | |||
15.4 Governing Law |
31 | |||
ARTICLE 16 PUBLICITY |
32 | |||
16.1 Initial Press Release |
32 | |||
16.2 Subsequent Press Releases |
32 | |||
16.3 Disclosures Required by Law |
32 | |||
16.4 Publications |
32 | |||
16.5 Use of Name |
33 | |||
ARTICLE 17 MISCELLANEOUS |
33 | |||
17.1 Pre-Existing Third Party License |
33 | |||
17.2 Extended Benefits |
33 | |||
17.3 Survival |
33 | |||
17.4 Agency |
33 | |||
17.5 Entire Agreement |
33 | |||
17.6 Counterparts |
33 | |||
17.7 Amendment |
33 | |||
17.8 Assignment |
33 | |||
17.9 Requirement to Divest |
34 | |||
17.10 Notices |
34 | |||
17.11 Force Majeure |
35 | |||
17.12 Affiliates |
35 | |||
17.13 Bankruptcy |
35 | |||
17.14 Severability |
35 |
EXECUTION COPY
This Development and License Agreement (this “Agreement") dated as of November 29, 2005 (“Effective
Date") is made by and between BioCryst Pharmaceuticals, Inc., a corporation organized and existing
under the laws of the State of Delaware having an office and place of business at 0000 Xxxxxxx Xxxx
Xxxxx, Xxxxxxxxxx, Xxxxxxx 00000 (“BioCryst"), on one hand, and on the other hand X.Xxxxxxxx-Xx
Xxxxx Ltd, a corporation of Switzerland having its principle office at Xxxxxxxxxxxxxxxxx 000,
XX-0000, Xxxxx, Xxxxxxxxxxx (“Roche-Basel") and Xxxxxxxx-Xx Xxxxx Inc., a New Jersey corporation
having its principle place of business at 000 Xxxxxxxxx Xxxxxx, Xxxxxx, Xxx Xxxxxx 00000
(“Xxxxx-Xxxxxx") (Xxxxx-Xxxxxx and Xxxxx-Xxxxx are collectively referred to as “Roche").
RECITAL
WHEREAS, BioCryst owns or controls patents and know-how related to a series of proprietary
compounds which act as purine nucleoside phosphorylase inhibitors (“PNP Inhibitors"), including the
compound known as BCX-4208.
WHEREAS, Roche has expertise in the discovery, development, manufacture and sale of pharmaceutical
products.
WHEREAS, Roche wishes to obtain, and BioCryst wishes to grant, rights and licenses under certain
patents and know-how owned or controlled by BioCryst.
NOW, THEREFORE, in consideration of the mutual promises and covenants contained in this Agreement,
the parties agree as follows:
ARTICLE 1 DEFINITIONS
1.1 “Actively Developing” means, with respect to a particular indication as of a given time, that
(i) Roche is then undertaking active human clinical trials for a Licensed Product in such
indication, or (b) the then-current Development Plan indicates that, within two years of such time,
active human clinical trials for a Licensed Product in such indication shall be commenced. For the
purposes of clarity, Roche shall not be deemed to be Actively Developing a Licensed Product for a
particular indication which is then not part of the Development Plan even if the Licensed Product
has been previously tested in human clinical trials in such indication.
1.2 “Adjusted Gross Sales” means (a) the Invoice Price of Licensed Products multiplied by the
quantity of Licensed Products Sold in the Territory, less (b) without duplication of any of those
sales related deductions which are accounted for in the calculation of Net Sales, (i) returns and
return reserves (including allowances actually given for spoiled, damaged, out-dated, rejected,
returned Licensed Products sold, withdrawals and recalls), (ii) rebates (for example, price
reductions, rebates to social and welfare systems, charge backs or reserves for charge backs, cash
sales incentives), (iii) government mandated rebates and similar types of rebates (e.g., P.P.R.S,
Medicaid), (iv) volume (quantity) discounts, (v) cash discounts and (vi) value added or sales
taxes, government mandated exceptional taxes and other taxes directly linked to the gross sales
amount (it being understood that income, withholding and capital gains taxes are not the type of
taxes contemplated as a deduction in this definition of Adjusted Gross Sales), each of clauses (i)
through (vi) as consistently applied by Roche to its products.
1.3 “Affiliate” means (a) an entity that owns directly or indirectly, a controlling interest in a
Party, by stock ownership or otherwise, (b) any entity in which a Party owns a controlling
interest, by stock ownership or otherwise, or (c) any entity under common control with a Party,
directly or indirectly. For purposes of this paragraph, “controlling interest” and “control” mean
ownership of fifty percent (50%) or more of the voting stock permitted to vote for the election of
the board of directors or any other arrangement resulting in control of or the right to control the
management and the affairs of the entity or Party in question. For purposes of this Agreement,
Genentech, Inc., 0 XXX Xxx, Xxxxx Xxx Xxxxxxxxx, Xxxxxxxxxx shall not be deemed an Affiliate of
Roche, unless approved by BioCryst in its sole discretion. For purposes of this Agreement, Chugai
Pharmaceutical Co. Ltd., 1-9 Kyobashi 2-chome, Chuo-ku,
EXECUTION COPY
Tokyo, 104-8301, Japan (“Chugai"), shall not be deemed an Affiliate of Roche unless Roche provides
written notice to BioCryst of its desire to include Chugai as an Affiliate of Roche.
1.4 “Autoimmune Indications” means all indications that involve pathogenic consequences, including
tissue injury, produced by autoantibodies or autoreactive T lymphocytes interacting with self
epitopes, i.e., autoantigens. Autoimmune Indications shall include, without limitation, asthma,
psoriasis, rheumatoid arthritis, systemic lupus erythematosus, scleroderma, juvenile rheumatoid
arthritis, polymyositis, ankylosing spondylitis, Type I diabetes, sarcoidosis, Sjogrens syndrome,
chronic active non-pathogenic hepatitis, non-infectious uveitis (Behcet’s), aplastic anemia,
hemolytic anemia, idiopathic thrombocytopenia purpura, vasculitis, Hashimoto’s thyroiditis, atopic
dermatitis, regional non-pathogenic enteritis (including ulcerative colitis, Crohn’s Disease and
inflammatory bowel disease), Kawasaki’s disease, post-infectious encephalitis, myasthenia gravis,
multiple sclerosis, alopecia, and tropic spastic paraparesis.
1.5 “Backup Compounds” shall mean any PNP Inhibitor which (i) as of the Effective Date is owned or
Controlled by BioCryst, and (ii) has completed Phase IIb related to an indication in the Field
during the time period between the Effective Date and the five year anniversary of the Closing
Date. Backup Compounds shall exclude (a) the Compound, and (b) Fodosine. For the purposes of this
Agreement, in no event shall Fodosine be deemed to be a Backup Compound.
1.6 “BioCryst Know-How” means all knowledge and proprietary information, including trade secrets,
owned or Controlled by BioCryst during the Term of this Agreement, directly related to the
manufacture, formulation, sale or use of the Compound. BioCryst Know-How includes without
limitation materials, samples, chemical manufacturing data, toxicological data, pharmacological
data, clinical data, formulations, specifications, quality control testing data, and submissions
and correspondence to and from governmental agencies, with regard to Licensed Product.
1.7 “BioCryst Patents” means those patents and patent applications or portions thereof that are
necessary for the manufacture, use or sale of the Compound or Licensed Product(s) and which are
owned or Controlled by BioCryst during the Term of the Agreement, To the extent a BioCryst Patent
is necessary for the making, using or selling of the Compound, BioCryst Patents shall include all
patents and patent applications based upon or claiming priority to any of the foregoing, and any
extensions, supplementary protection certificates, continuations, continuations-in-part, divisions,
reissues, re-examinations, additions, substitutions, confirmations, registrations, or
re-validations of or to any of the foregoing. Notwithstanding the definition above, BioCryst
Patents shall not include any patent rights relating to Fodosine. Exhibit 1.7 lists all BioCryst
Patents as of the Effective Date.
1.8 “Calendar Quarter” means each three month period beginning on each of January 1, April 1, July
1 and October 1 during the Term of this Agreement.
1.9 “Closing Date” means the date of approval of the transaction by the Federal Trade Commission or
the appropriate US anti-trust authorities or the expiration or termination of all applicable
waiting periods and requests for information (and any extensions thereof) under the
Xxxx-Xxxxx-Xxxxxx Antitrust Improvements Act of 1976 (the
“HSR Act”).
1.10 “Compound” means the PNP Inhibitor known as BCX-4208 ***.
1.11 “Commercially Reasonable Efforts” means a level of resources and efforts to Develop and
Commercialize a Licensed Product applied by Roche consistent with Roche’s practices in pursuing the
Development and commercialization of its other pharmaceutical products at a similar stage of
product life, safety, efficacy and commercial potential. It is understood that such efforts may
change from time to time based upon changing safety, efficacy, scientific, business and commercial
considerations.
1.12 “Commercialization” means, with respect to a particular Licensed Product, any and all
processes and activities necessary to be conducted to establish and maintain sales for such
Licensed
2
EXECUTION COPY
Product, including negotiating and obtaining pricing approvals, offering for sale, Detailing
(including launch), promoting, manufacturing, storing, transporting, supporting, distributing, and
importing such Licensed Product, and all studies and tests of such Licensed Product, but in all
cases excluding Development. “Commercialize” and “Commercializing” shall have their correlative
meanings.
1.13 “Confidential Information” means any and all information, data or know-how of a confidential
nature (including BioCryst Know-How and Roche Know-How), whether technical or non-technical (such
as financial, business and legal information), oral or written, related to Compound or Licensed
Product or a Party’s business that is disclosed by one Party or
its Affiliates (“Disclosing Party”)
to the other Party or its Affiliates (“Receiving Party”). For the purposes of this Agreement, all
information related to BioCryst’s licensors and the Pre-Existing Third Party License shall be
deemed to be BioCryst Confidential Information.
Confidential Information shall not include any information, data or know-how which:
(i) either before or after disclosure to the Receiving Party, was or becomes published or
generally known to the public through no fault or omission on the part of the Receiving Party or
its Affiliates; or
(ii) was known or used by the Receiving Party or its Affiliates prior to its disclosure by the
Disclosing Party to the Receiving Party; or
(iii) either before or after disclosure to the Receiving Party, is provided to the Receiving
Party by a Third Party having the legal right to do so;
(iv) is independently developed by the Receiving Party or its Affiliates without access to our
use of the Disclosing Party’s Confidential Information; or
(v) is required to be disclosed by the Receiving Party or its Affiliates to comply with
applicable Laws, to defend or prosecute litigation or to comply with governmental regulations,
provided that, the Receiving Party provides prior written notice of such disclosure to the
Disclosing Party and, to the extent practicable, takes reasonable and lawful actions to minimize
the degree of such disclosure.
1.14 “Control” means, with respect to any intellectual property right, that the Party has a license
to such intellectual property right and has the ability to grant to the other Party a sublicense to
such intellectual property right as provided herein without violating the terms of any agreement or
other arrangements with any Third Party existing at the time such Party would be first required
hereunder to grant the other Party such sublicense. “Controlled” shall have a correlative meaning.
1.15 “Dermatology” means the treatment of disorders of the skin in which the skin is the primary
site of the disorder (e.g., psoriasis).
1.16 “Detail” means, with respect to a particular Licensed Product, a face-to-face presentation
(or other means of contact) by a Party’s sales representative, to one or several medical
professional(s) having prescribing authority in the United States in the Field, as well as to other
individuals or entities that have significant impact or influence on prescribing decisions in the
United States in the Field, in which the principal objective of such presentation is to emphasize
the features and function of such Licensed Product in accordance with Law. For clarity, Detail
shall exclude discussions at conventions, tradeshows and similar forums, meetings where only a
sample drop or a reminder occurs or other incidental contact. When used as a verb, the terms
“Detail” or “Detailing” means to perform a Detail.
1.17 “Develop” means with respect to a particular Licensed Product or Special Indication Product
(as the case may be), any and all processes and activities which are necessary to be conducted to
obtain NDA Approval for such product, including NDA Approval applications, clinical trial
application enabling studies and all other activities conducted thereafter, which may involve
preclinical testing, toxicology,
3
EXECUTION COPY
clinical trials, quality of life assessments, pharmacoeconomics, post-marketing studies required to
maintain approval of the label, label expansion studies, and further activities related to
development of such product to a stage ready for commercialization
thereof. “Develop”,
“Development” and “Developing” shall have their correlative meanings.
1.18 “Development Costs” means all out of pocket and internally allocated costs, expenses and
liabilities incurred by BioCryst in connection with the Development of a Special Indication
Product, including but not limited to (i) fees paid under the Pre-Existing Third Party License (ii)
the costs of base salaries and benefits of personnel for the time devoted to, or in furtherance of,
the Development of the Special Indication Product, (iii) costs of and in obtaining supplies to be
used in conducting the Development of Special Indication Product, and (iv) costs of retaining third
party consultants or other independent contractors to assist with the Development of a Special
Indication Product.
1.19 “Development Plan” means a plan for the Development of Licensed Products in the Field approved
from time to time by the JSC pursuant to Section 4.1(a). The initial Development Plan, reflecting
the mutual objectives of the Parties with respect to the sequence of events for the Development of
the Compound is attached hereto as Exhibit 1.19.
1.20 “Effective Date” means the date set forth in the first paragraph of the Agreement.
1.21 “Field” means the prevention or treatment of Autoimmune Indications and Transplantation
Indications.
1.22 “First Commercial Sale” means, for a given country, the first Sale of Licensed Product after
NDA Approval in the given country for the Licensed Product (including authorizations for
reimbursement).
1.23 “Fodosine” means the compound known as “BCX-1777”
(7-((2S,3S,4R,5R)-3,4-dihydroxy-5-hydroxymethyl)pyrrolidin-2-yl)-3H-pyrrolo[3,2-d]pyrimidin-4(5H)-on
e hydrochloride)
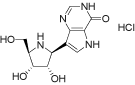
1.24 “Generic Licensed Product” means a product that contains Compound which is offered for sale in
a country by a Third Party.
1.25 “IND” means Investigational new Drug Applications or corresponding filings in any country in
the world.
1.26 “Insolvency Event” means, in relation to the Party in question, any of the following: (i) that
Party proposes or makes any arrangement or composition with or any assignment for the benefit of
its creditors generally; (ii) a petition is filed that is not dismissed within sixty (60) days or a
court order is made or a resolution is passed for the bankruptcy, liquidation or winding up of that
Party (save for the purposes of a solvent reconstruction the terms of which have been approved in
writing by the other Party) or for the appointment of a provisional liquidator or a judicial factor
or similar officer in relation to that Party; (iii) an encumbrancer takes possession of or a
trustee, receiver, liquidator, provisional liquidator, administrator, manager ad interim,
administrative receiver, judicial factor or similar officer is appointed in respect of all or a
material part of that Party’s intellectual property rights which are the subject of this Agreement
and such appointment prejudices the other Party’s rights under this Agreement; (iv) that Party is
unable to pay its debts as they become due in the ordinary course of business; (v) that Party gives
written notice to its creditors that it has ceased to pay its debts in the ordinary course of
business; or (vi) that Party does, or
4
EXECUTION COPY
suffers to be done in relation to it, any analogous action or proceeding in any jurisdiction
anywhere in the world (including without limitation any actions or proceedings relating to
bankruptcy Law of any nature in the United States of America).
1.27 “Invoice Price” means, with respect to a Licensed Product, the unit price, without deduction,
actually invoiced by the Roche Group for the Sale of such Licensed Product. If any Sales of
Licensed Products are made in transactions that are not at arm’s length, then the Gross Price for
such Licensed Products shall be the amount that would have been invoiced had the transaction been
conducted at arm’s length. Such amount that would have been invoiced shall be determined, wherever
possible, by reference to the average selling price of the Licensed Product in contemporaneous
similar transactions which are at arm’s length. If, in addition to or in lieu of cash
consideration, other consideration is paid in an arm’s length transaction to the Roche Group in
connection with the Licensed Product or the customer’s rights or relationship with the Roche Group
in relation thereto, then the amount or fair market value of such other consideration shall be
included in the calculation of Invoice Price in the period in which the Sales of Licensed Products
occur.
1.28 “Joint Development Committee” or “JDC” means that committee comprised of an equal number of
representatives of BioCryst and Roche, but not less than three (3) representatives from each
company, which shall have the responsibilities set forth in Section 4.2.
1.29 “Joint Steering Committee” or “JSC” means that committee comprised of equal number of
representatives of BioCryst and Roche, but not less than three (3) representatives from each
company, which shall have the responsibilities set forth in Section 4.1.
1.30
“Law” means, individually and collectively, any and all laws, ordinances, rules, directives
and regulations of any kind whatsoever of any governmental or regulatory authority within the
applicable jurisdiction, including any present and future supra-national, national, state and local
laws (including rules and regulations having the force of law); requirements under permits; orders,
decrees, judgments and directives; requirements of the FDA and other regulatory authorities,
including without limitation cGMPs, other requirements imposed under the Federal Food, Drug and
Cosmetic Act and the Public Health Service Act, each as amended from time to time, requirements in
21 C.F.R. Part 312, applicable requirements in 21 C.F.R. Parts 600-680 and similar requirements of
Regulatory Authorities in countries outside the United States.
1.31 “Licensed Product” means any and all pharmaceutical products containing Compound.
1.32 “Major Market Countries” means the United States of America, Western Europe, and with respect
to Autoimmune Indications only, also Japan.
1.33 “NDA Approval” means all approvals (including pricing and reimbursement approvals), licenses,
registrations or authorizations of any national or international or local regulatory authority,
department, bureau or other governmental entity, necessary for the Development and
Commercialization of Licensed Product in a regulatory jurisdiction.
1.34 “Net Sales” means, for the US, the amount calculated by subtracting from the amount of
Adjusted Gross Sales a lump sum deduction of ***. Notwithstanding the foregoing, amounts received
by Roche, its Affiliates and sublicensees for the sale of Licensed Product among Roche, its
Affiliates or sublicensees for resale shall not be included in the computation of Adjusted Gross
Sales and Net Sales.
1.35 “Neurology” means the treatment of central nervous system autoimmune disorders or diseases
wherein the disorder or disease is specific to the central nervous system (e.g., multiple
sclerosis).
1.36 “Party” means Roche and/or BioCryst, and “Parties” means both Roche and BioCryst.
5
EXECUTION COPY
1.37 “Phase II” means a human clinical trial in the Field performed to evaluate the efficacy of a
Licensed Product for a particular indication or indications in patients with the disease or
condition under study and/or to determine the common short-term side effects and risks associated
with the drug, as described in 21 C.F.R. Part 312, as it may be amended.
1.38 “Phase IIa” means a human clinical trial performed to estimate the biologic or clinical effect
of a pharmaceutical product in a target population.
1.39 “Phase IIb” means a placebo or active drug controlled, randomized human clinical trial
performed to gain evidence of the efficacy of a pharmaceutical product in a target population,
and/or to establish the optimal dosing regimen for such product.
1.40 “Phase III” means a human clinical trial required by the FDA or other equivalent regulatory
authority to gain evidence of efficacy in the target population and obtain expanded evidence of
safety for Licensed Product, as described in 21 C.F.R. Part 312, as it may be amended.
1.41 “Pre-Existing Third Party License” means the agreement dated June 27, 2000 by and between on
the one hand Xxxxxx Xxxxxxxx College of Medicine of Yeshiva University, a division of Yeshiva
University and Industrial Research Ltd., and on the other hand BioCryst, as amended from time to
time.
1.42 “Promotional Material” means all materials for training commercial personnel and all written,
printed, graphic, electronic, audio or video matter, including journal advertisements, sales visual
aids, leave behind items, formulary binders, reprints, direct mail, direct-to consumer advertising,
Internet postings, broadcast advertisements and sales reminder aids (for example, scratch pads,
pens and other like items), in each case created by a Party or on its behalf and used or intended
for use in connection with any promotion of a Licensed Product, but excluding Licensed Product
labeling.
1.43 “Regulatory Authority” means the United States Food and Drug Administration (“FDA”), or in the
case of a country other than the United States, such other appropriate regulatory authority with
similar responsibilities, including, without limitation, the EMA.
1.44 “Roche Compound” means any PNP Inhibitor other than the Compound which (i) is owned or
Controlled by the Roche Group, and (ii) has completed Phase IIb for an indication in the Field
during the time period between the Effective Date and the five year anniversary of the Closing
Date.
1.45 “Roche Group” means, individually and collectively, Roche and its Affiliates.
1.46 “Roche Know-How” means all knowledge and proprietary information, including trade secrets,
owned or Controlled by the Roche Group during the Term of this Agreement, which is an improvement
on the BioCryst Know-How or which directly relates to the manufacture, formulation or use of the
Compound, Licensed Products, Roche Compound or Roche Products. Roche Know-How includes without
limitation materials, samples, chemical manufacturing data, toxicological data, pharmacological
data, clinical data, formulations, specifications, quality control testing data, and submissions
and correspondence to and from governmental agencies.
1.47 “Roche Patents” means all patent applications and patents which claim improvements upon or
modification to the inventions and discoveries claimed in any of the BioCryst Patents or which
claim inventions directly related to the manufacture, use or sale of Compound, Backup Compound,
Roche Compound, Roche Products, or Licensed Products, which patent applications and patents are
owned or Controlled by the Roche Group during the Term of this Agreement, and any extensions,
supplementary protection certificates, continuations, continuations-in-part, divisions, reissues,
re-examinations, additions, substitutions, confirmations, registrations, or re-validations of or to
any of the foregoing.
1.48 “Roche Products” means any and all pharmaceutical products containing a Roche Compound.
6
EXECUTION COPY
1.49 “ROW Country” shall mean a country which is not included in the Major Market Countries. “ROW”
means all of the non-Major Market Countries.
1.50 “Royalty Bearing Products” means Licensed Products and Roche Products.
1.51 “Sale”, “Sold” or “Sell” means the sale, transfer or disposition of a Licensed Product (or
Roche Product) for commercial purposes (excluding compassionate use where no consideration is
received by the Roche Group) for value to a Third Party (whether an end user, wholesaler or
otherwise) by a member of the Roche Group (or, with respect to a Roche Product, sublicensees). For
the avoidance of doubt, sales, transfers or dispositions of a Licensed Product among the Roche
Group shall not be subject to a royalty, but shall become subject to a royalty when Sold to a Third
Party.
1.52 “Special Indication Product” means a Licensed Product for Commercialization in a Special
Indication.
1.53 “Special Indication” means an Autoimmune Indication with respect to which, at the time
BioCryst desires to commence development work pursuant to Section 5.2, Roche is not Actively
Developing a Licensed Product for such Autoimmune Indication.
1.54 “Start of Phase IIa” means the date that a patient is first dosed with Royalty-Bearing Product
in a Phase IIa clinical trial conducted by or on behalf of the Roche Group.
1.55 “Start of Phase IIb” means the date that a patient is first dosed with Royalty-Bearing Product
in a Phase IIb clinical trial conducted by or on behalf of the Roche Group
1.56 “Start of Phase III or Pivotal Trial” means the date that a patient is first dosed with
Royalty-Bearing Product in a Phase III trial conducted by or on behalf of the Roche Group.
1.57 “Stem-Cell Transplantation” means a procedure in which healthy stem cells are infused to help
restore normal bone marrow function.
1.58 “Sublicensee” means any Third Party to whom a sublicense has been granted pursuant to Section
2.1(d).
1.59 “Term of the Agreement” means the period commencing upon the Effective Date and, unless this
Agreement is terminated sooner as provided herein, expiring on the date when no royalty or other
payment obligations under this Agreement are or will become due.
1.60 “Third Party” means an entity other than a Party to this Agreement or its Affiliates.
1.61 “Transplantation Indications” means all indications that involve the suppression of rejection
of transplanted organs, bone marrow or other tissue, including, without limitation, solid organ
transplantation (including tolerance induction and xenotransplantation), bone marrow
transplantation, graft versus host disease and cell transplantation. In any event, if a given
indication satisfies the criteria for both an Autoimmune Indication and a Transplantation
Indication, such indication shall be deemed a Transplant Indication and not an Autoimmune
Indication, provided that an Autoimmune Indication shall not be deemed a Transplant Indication
merely because it may cause the need for a transplant (e.g., Type I diabetes, even if it causes the
need for an organ transplant). For the avoidance of doubt, Transplantation Indications does not
include the treatment or prevention of cancer but does include bone marrow transplantation.
1.62 “Valid Claim” means a claim in any unexpired and issued BioCryst Patent that has not been
revoked or held invalid by a final unappealable decision of a court or governmental agency of
competent jurisdiction.
7
EXECUTION COPY
1.63 “Western Europe” means the United Kingdom, Italy, France, Germany, and Spain.
In these definitions, the singular shall include the plural and vice versa.
ARTICLE 2 GRANT OF RIGHTS
2.1 License Grants.
(a) By BioCryst. BioCryst hereby grants to the Roche Group a sole and exclusive (even as to
BioCryst), royalty-bearing, worldwide right and license under the BioCryst Patents and BioCryst
Know-How to make, have made, use, offer for sale, sell and import Licensed Products in the Field.
The foregoing license to the BioCryst Patents and BioCryst Know-How is limited to the exercise of
the rights granted under the previous sentence and is sublicensable solely as set forth in Section
2.1(d).
(b) Retained Rights. All rights not specifically granted are reserved to BioCryst. BioCryst
further retains (i) all rights under the BioCryst Patents and BioCryst Know-How outside the Field;
and (ii) under the BioCryst Patents and BioCryst Know-How to develop, conduct preclinical and
clinical activities, make, have made, use, and otherwise exercise all rights in accordance with the
terms hereof with respect to Special Indications and Special Indication Products. The license
grant under Section 2.1(a) is also subject to (aa) the rights retained by BioCryst’s licensors
under the Pre-existing Third Party License Agreement, (bb) the rights retained by the United States
government as set forth in 35 U.S.C. § 200, et seq. (as more fully set forth in Sections 2.01 and
2.02 of the Pre-Existing Third Party License Agreement), (cc) the right to permit Industrial
Research, Ltd. to perform research sponsored in part by the New Zealand Foundation for Research,
Science and Technology and related to the “Field” as defined in the Pre-Existing Third Party
License Agreement (as more fully set forth in Section 2.03 of the Pre-Existing Third Party License
Agreement), (dd) the right to permit Xxxxxx Xxxxxxxx College of Medicine of Yeshiva University, a
division of Yeshiva University through the “Investigators” as defined in the Pre-Existing Third
Party License Agreement to conduct research in collaboration with the National Cancer Institute and
with contractors hired by the National Cancer Institute (as more fully set forth in Section 2.04 of
the Pre-Existing Third Party License Agreement), (ee) the rights retained by BioCryst’s licensors
to make, use and practice those BioCryst Patents that are “Agreement Patents” as defined in the
Pre-Existing Third Party License Agreement in their own laboratories solely for non-commercial
scientific purposes and for continued non-commercial research (as more fully set forth in Section
4.02 of Pre-Existing Third Party License Agreement), (ff) the rights retained by BioCryst’s
licensors to make available to not-for-profit scientific institutions and non-commercial
researchers small quantities of biological materials covered under those BioCryst Patents that are
“Agreement Patents” in the Pre-Existing Third Party License Agreement solely for non-commercial
scientific and research purposes, provided this is done pursuant to the material transfer
agreement, set forth in Appendix B of the Pre-Existing Third Party License Agreement (as more fully
set forth in Section 4.02 of the Pre-Existing Third Party License Agreement), and (gg) the rights
retained by BioCryst’s licensors to grant licenses to third parties under “Agreement Patents” for
all uses outside of the “Field” as such terms are defined in the Pre-Existing Third Party License
Agreement (as more fully set forth in Section 4.03 of the Pre-Existing Third Party License
Agreement). Other than as set forth above, BioCryst warrants that it is not aware of any retained
rights related to making, using or selling the Compound or Licensed Product. If it becomes known
to BioCryst that there have been inventions by the licensors under the Pre-Existing Third Party
License Agreement relating to the retained rights that are necessary to allow Roche’s exercise of
its rights under this Agreement, BioCryst shall use commercially reasonable efforts to obtain a
license on behalf of Roche to use such improvements in accordance with the terms and conditions of
this Agreement.
(c) By Roche Group. Roche Group hereby grants to BioCryst a co-exclusive (with the Roche
Group only), royalty-free, fully paid up worldwide right and license, without the right to
sublicense (other than to service providers on BioCryst’s behalf), under the Roche Patents and
Roche Know-How to make, have made, and use Backup Compounds and Special Indication Product in the
Field. BioCryst shall not commercially launch or otherwise sell, distribute or supply, or enable
any Affiliate of BioCryst, any licensor of the Pre-Existing Third Party License, or any or Third
Party, to commercially launch or
8
EXECUTION COPY
otherwise sell, distribute or supply (other than to BioCryst), Licensed Products or Compound
in the Field under the BioCryst Patents, Roche Patents, Roche Know-How or BioCryst Know-How.
(d) Sublicensing. The following license grant is subject to all retained rights set forth in
Section 2.1(b).
(i) Roche shall have the right to grant sublicenses under the license granted to it in Section
2.1(a) of this Agreement to an Affiliate or to engage any contract manufacturer, distributor,
subcontractor or outsourced service provider for the benefit of Roche. Notwithstanding the rights
granted above, Roche shall not be entitled to sublicense the entirety of its rights or obligations
hereunder in any country (except for in Japan to Chugai) without BioCryst’s prior written approval,
which shall not be unreasonably withheld. In Japan, Roche shall have the right to sublicense the
entirety of its rights only to Chugai, and such sublicense shall not require the prior written
consent of BioCryst. All obligations set forth in this Agreement which apply to Roche shall be
deemed to apply to any permitted Sublicensee, but Roche shall continue to remain liable for all
obligations hereunder.
(ii) Roche shall ensure that each permitted Sublicensee hereunder shall consent to be bound by
the terms of this Agreement as a Sublicensee and to the same extent as Roche. At BioCryst’s
request, Roche shall inform BioCryst, in confidence, of any sublicense granted, and any
modification or termination thereof. At BioCryst’s request, Roche shall also provide BioCryst with
an executed copy of the written agreement between Roche and such Sublicensee.
2.2 Backup Compounds. While no rights to any Backup Compounds are licensed hereunder, upon
completion of a Phase IIb clinical trial of a Backup Compound (if any), BioCryst shall give prompt
written notice thereof to Roche. Roche shall have *** (***) days after receipt of such notice from
BioCryst to request in writing that BioCryst provide Roche with a data package for that Backup
Compound. Commencing on the date that Roche receives the data package, Roche shall have the right
to conduct due diligence related to the Backup Compound for a period of up to *** (***) days (the
“Backup Compound Diligence Period”). BioCryst shall cooperate with Roche during the Backup
Compound Diligence Period. Roche’s due diligence may include, but is not limited to, the
following: (i) a full clinical and manufacturing audit of BioCryst with respect to the Backup
Compound, at Roche’s expense, (ii) the right to request all data, including raw data, obtained to
date relating to the Backup Compound, (iii) the right to inspect BioCryst’s facilities and, to the
extent within the reasonable control of BioCryst, the facilities of its clinicians and
manufacturers, each with respect to the Backup Compound (and BioCryst agrees to use good faith and
commercially reasonable efforts so that Roche may inspect the facilities of such third parties),
and (iv) a complete detailed report of the Development Costs actually incurred with respect to the
Backup Compound. The data package, all information learned by Roche during any audit pursuant to
this Section 2.2 and any and all other information provided by BioCryst to Roche relating to the
Backup Compound shall be treated by Roche as the Confidential Information of BioCryst in accordance
with the provisions of ARTICLE 10 of this Agreement. Roche agrees that BioCryst shall have no
obligations hereunder to develop any Backup Compounds.
2.3 First Right of Negotiation of Backup Compounds. Roche shall have the right to negotiate with
BioCryst to have any Backup Compound licensed to Roche for use in the Field. Such license shall be
upon mutually agreeable financial and other terms. BioCryst and Roche shall exclusively negotiate
such offer in good faith for *** (***) days from the expiration of the Backup Compound Diligence
Period. If the parties reach agreement on the material terms of such a license during the ***
(***) day period, then the parties shall promptly execute a license which shall include all
additional terms and conditions. If, at the end of the *** (***) day period, the Parties have been
unable to reach agreement on the material terms of such a license, then BioCryst shall be free to
offer such rights to any Third Party. If BioCryst does sell a product having the same mechanism of
action as the Licensed Product in the Field in the United States, then the parties shall meet and
confer in good faith as to the appropriate modifications, if any, related to joint actions of the
Parties related to Licensed Products (e.g., modifications to the JDC, JSC and co-promotion);
provided, however, in no event shall BioCryst be required to waive its right to participate in the
activities of any Committees as set forth in this Agreement.
9
EXECUTION COPY
2.4 Roche Compounds. Roche shall promptly notify BioCryst in writing in the event of the
identification of a Roche Compound, and such Roche Compound and related Roche Product shall be a
Royalty-Bearing Product subject to the terms and conditions of this Agreement. In the event any
Development Event Payments (as described in Section 7.2) or Commercial Event Payments (as described
in Section 6.3) are triggered by Roche Products achieving the associated development or commercial
event, such payments shall be made to BioCryst as if such payments were due for a Licensed Product.
Additionally, Royalties (as set forth in Section 7.3) shall be payable to BioCryst on Roche
Products.
2.5 Fodosine. *** for the purposes of clarity, it is understood and agreed that no rights related
to Fodosine are granted to Roche in this Agreement.
2.6 Pre-Existing Third Party License. BioCryst shall use its good faith and commercially
reasonable efforts to maintain the Pre-Existing Third Party License. If BioCryst receives a notice
of breach under Section 10.03 from a licensor of the Pre-Existing Third Party License, then
BioCryst shall promptly inform Roche of the notice and of the plan to cure the breach. If BioCryst
does not plan to, or can not, cure the breach within the time period allowed, then Roche shall have
the right to cure the breach on BioCryst’s behalf. If the Pre-Existing Third Party License
terminates under Section 10.03 of the Pre-Existing Third Party License as a result of Roche’s
failure to cure such breach, this Agreement shall terminate and such termination shall be treated
as any other termination under Section 11.4(b) and the provisions of Sections 11.4(b)-11.4(e), and
11.4(g) shall apply.
ARTICLE 3 BIOCRYST RIGHT TO PROMOTE.
3.1 Co-Promotion. No later than *** (***) years prior to the anticipated Regulatory Approval in
the U.S. of a Licensed Product for an indication in the area of Neurology or Stem Cell
Transplantation or no later than *** (***) years prior to the anticipated Regulatory Approval in
the U.S. of a Licensed Product for an indication in the area of Dermatology, Roche shall notify
BioCryst of the anticipated Regulatory Approval date
(“Anticipated Launch”). After such notice and
prior to the date which is *** (***) months prior to the Anticipated Launch in the U.S. of a
Licensed Product for an indication in the area of Neurology or Stem Cell Transplantation or prior
to the date which is *** (***) years prior to the Anticipated Launch in the U.S. of a Licensed
Product for a Dermatology indication, BioCryst shall have the right to provide written notice to
Roche that it shall Detail Licensed Product in the U.S. as set forth below. BioCryst shall have the
right to use BioCryst employees (i.e., not a Third Party) to provide (i) all Details in the U.S.
with respect to all Licensed Products for the area of Stem-Cell Transplantation, and (ii) all
Details for all Licensed Products in the U.S. in the areas of Neurology and/or Dermatology in those
indications for which Roche does not have a full time employed sales force to cover all Details for
all Licensed Products in such indications. If Roche is Detailing Licensed Products in the areas of
Neurology and/or Dermatology, BioCryst shall have the right to provide up to *** percent (***%) of
all Details with respect to all sales of Licensed Products for indications in such areas where
Roche is providing Details. Notwithstanding the foregoing, for the *** (***) month period
immediately following the actual commercial launch of each Licensed Product for a particular
indication, BioCryst shall have the right to discharge its obligations to Detail through a Third
Party (i.e., contract sales force), provided that BioCryst will transition a significant portion of
such contract sales force to become BioCryst employees.
(a) The Details described in Section 3.1 shall be performed as directed by, and in a manner
determined by, the JSC and/or a joint marketing committee constituted by the JSC, and shall be
performed in accordance with the terms of this Agreement.
(b) Roche shall compensate BioCryst on a Detail-by-Detail basis at current market rates for
contract sales force FTEs for Details in those indications for which Roche does not have a full
time employed sales force to cover all Details for all Licensed Products in such indications.
10
EXECUTION COPY
ARTICLE 4 GOVERNANCE
4.1 Steering Committee.
(a) The Parties shall establish a Joint Steering Committee (“JSC”) to oversee and review the
research and development activities of the Parties with respect to Licensed Product. The JSC in
turn may establish additional committees to achieve this result (“JSC Subcommittees"). Upon notice
by BioCryst to co-promote pursuant to ARTICLE 3, the JSC shall establish a Co-Promotion Committee
to oversee and address issues related to the parties’ activities under ARTICLE 3. All committees
established under this Agreement, including the JDC and all JSC Subcommittees, shall be subordinate
to the JSC.
(b) The JSC shall consist of an equal number of representatives of each Party, which shall be
at least three (3), who are experts in their field and who shall not serve on the JDC or any other
group or committee, including a Subcommittee established pursuant to this Agreement. The size of
the JSC may be changed by agreement of the Parties but shall always have an equal number of
representatives from each Party. Each Party may select representatives to replace the initial JSC
members selected by such Party as necessary. The JSC shall meet at least four (4) times per
calendar year, and more often as mutually agreed by the Parties as appropriate for the continued
Development of Licensed Products.
(c) The JSC shall be responsible for overseeing, managing and providing strategic direction to
the Parties in Development of Licensed Products and otherwise carrying out their obligations under
this Agreement, including: (i) discussing all matters of strategic relevance or other importance to
the Parties under this Agreement, (ii) quarterly reviewing and approving all Development Plans
(including setting key efficacy endpoints of clinical studies, clinical and regulatory plans) and
changes in Development Plans recommended by the JDC; (iii) reviewing and monitoring the activities
and verbally communicating the progress of the JDC and Subcommittees, if any; (iv) considering
disputes, disagreements and deadlocks that are not resolved by the other committees established
under this Agreement pursuant to Section 4.1(a) or Section 4.2(a); (v) overseeing the integration
and coordination of the Development of the Licensed Products in accordance with the terms and
conditions of this Agreement; (vi) undertaking and/or approving such other matters as are
specifically provided for the JSC under this Agreement, (vii) reviewing and approving a global
clinical trial program prior to the conduct of any clinical trials for Licensed Products (including
Special Indication Products) and the plans for any individual clinical trial of Licensed Product in
advance of the anticipated commencement thereof, (viii) updating BioCryst on Roche’s sublicensing
activities, and (ix) updating Roche on the activities of BioCryst or the licensors of the
Pre-Existing Third Party License, the U.S. government, the National Cancer Institute or the New
Zealand Foundation for Research, Science and Technology in connection with the Compound or Backup
Compounds in the Field to the extent BioCryst is aware of such activities. At Roche’s request,
BioCryst shall use its commercially reasonable efforts to obtain from its licensors under the
Pre-Existing Third Party License and provide to Roche requested information related to the
activities of the licensors of the Pre-Existing Third Party License, the U.S. government, the
National Cancer Institute or the New Zealand Foundation for Research, Science and Technology in
connection with the Compound or Backup Compounds in the Field.
(d) The Parties shall report to the JSC on all significant clinical and regulatory issues
related to Licensed Product, and the JSC shall make recommendations and provide strategic guidance
with respect to such issues.
(e) Neither Party shall commence any clinical trial of Licensed Product until the JSC has
approved plans therefor.
(f) Each Party shall keep the JSC informed of the progress and results of activities for which
it is responsible under this Agreement through its members on the JSC and as otherwise provided
herein. BioCryst shall keep the JSC informed regarding its plans for developing any Special
Indication Product, including sufficient information to determine potential safety issues and
target population. Once per calendar year, Roche shall advise the JSC of its Commercialization
plans and activities. Once per calendar year, Roche shall provide BioCryst with a written
Commercialization plan, to the extent one exists.
11
EXECUTION COPY
4.2 Development Committee.
(a) The Parties shall establish a Joint Development Committee (“JDC”; the JDC, JSC, JDC
Subcommittees and JSC Subcommittees, each a “Committee”) to coordinate the conduct and progress of
the Development of Licensed Products. The JDC may establish committees to achieve its results
(“JDC Subcommittees”). The JDC and JDC Subcommittees shall be subordinate to the JSC.
(b) The JDC shall consist of an equal number of representatives of each Party, which shall be
at least three (3), who are experts in their field and who are not members of the JSC or any
Subcommittee or other team established pursuant to this Agreement. The JDC is responsible for,
among other things: (i) devising the strategy and plans for the Development of Licensed Products
including regulatory strategies; (ii) annually reviewing and updating the Development Plans, as
needed, but at a minimum once per year and recommending changes for consideration by the JSC,
including, without limitation, changes in the strategy for Developing the Licensed Products,
seeking approval of particular indications, or expanding the markets being targeted; (iii)
reviewing and approving clinical study endpoints, clinical methodology and monitoring requirements
for the clinical studies described in the Development Plans; (iv) reviewing, coordinating and
ensuring compliance with the Development Plans; and (v) undertaking and/or approving such other
matters as are specifically provided for the JDC under this Agreement.
(c) The JDC shall meet at least four (4) times per calendar year, and more often as mutually
agreed by the Parties as appropriate for the continued Development of Licensed Products.
4.3 Committee Membership. In the event a Committee member from either Party is unable to attend or
participate in a meeting of its Committee, the Party who designated such representative may
designate a substitute representative for the meeting in its sole discretion.
4.4 Committee Chairs. For each Committee, a Party shall appoint one (1) of its members to such
Committee to chair the meetings for such Committee (a
“Chair”). The JSC Chair shall serve for a
one (1) year period. Roche shall have the right to designate the first Chair of the JSC, whose
term shall run until December 31, 2006, and the right to designate the Chair of the JSC shall
thereafter alternate between the Parties on a calendar year basis. Notwithstanding the above,
Roche shall have the right to designate all Chairs for the JDC, JDC Subcommittees, and Co-Promotion
Committee. The Chair for each Committee shall (i) coordinate and issue the agenda of such
Committee’s meetings, (ii) attend each meeting of such Committee, and (iii) issue written minutes
of each meeting within thirty (30) days thereafter accurately reflecting the discussions and
decisions of such Committee. Minutes from each Committee’s meeting shall not be finalized until
the other Party has reviewed and confirmed the accuracy of such minutes in writing. The agendas
for the JSC and JDC shall be prepared jointly with input from both Parties, provided that if either
Party requests an addition to an agenda, it will be added. In the event the Chair is unable to
attend or participate in its Committee’s meeting, the Party who designated such Chair may designate
a substitute Chair for the meeting.
4.5 Committee Meetings. Committees may meet in person or by video conference or telephonically as
agreed by the Parties; provided that the JSC and the JDC shall meet at least once annually in
person. Each Party shall be responsible for its own expenses relating to such meetings. As
appropriate, other employee representatives of the Parties may attend Committee meetings as
nonvoting observers, subject to the reasonable approval of the other Party. Each Party may also
call for special meetings of a Committee to resolve particular matters requested by such Party and
within the areas of responsibility of such Committee. Each Committee’s Chair shall ensure that its
Committee members receive adequate notice of such meetings.
4.6 Decision Making. Decisions of each Committee shall be made by consensus of the members present
in person or by other means (e.g., teleconference) at any meeting, with each Party having one vote.
In order to make any decision, any Committee established under this Agreement must have present
(in person or telephonically) at least one representative of each Party. In the event that a JDC
Subcommittee does not reach consensus with respect to a particular matter endeavoring in good faith
for *** (***) days to do so, then the Parties shall refer such issue to the JDC for resolution. In
the event that any JSC Subcommittee or the JDC does not reach consensus with respect to a
particular matter after
12
EXECUTION COPY
endeavoring in good faith for *** (***) days to do so, then the Parties shall refer the issue to
the JSC for resolution. In the event that the JSC does not reach consensus with respect to a
particular matter after endeavoring in good faith for *** (***) days to do so, then the matter
shall be submitted to the respective executive officers of the Parties designated below or their
successors. The designated executive officers of the Parties are as follows:
| For Roche: | Head of Global Development/Pharma Division | |||
| For BioCryst: | Chairman and CEO |
If the positions of the designated executive officers listed above are vacant or no longer exist,
then the person having the most nearly equivalent position (or such individual’s designee) shall be
deemed to be the designated executive officer of the relevant Party. In the event that such
executives do not reach consensus with respect to such matter after endeavoring in good faith for
*** (***) days to do so, then Roche shall have the right to cast a deciding vote on such matter.
Notwithstanding anything herein to the contrary, no Committee shall have any authority to amend,
modify or waive compliance with this Agreement.
4.7 Interactions Between Committees. The Parties recognize that while they will establish various
Committees for the purposes hereof, each Party maintains internal structures (including its own
committees, teams and review boards) that will be involved in administering such Party’s activities
under this Agreement. The Parties shall establish procedures to facilitate communications between
the various Committees hereunder and the relevant internal committee, team or board within the
Party in order to maximize the efficiency of the Parties’ activities pursuant to this Agreement.
In addition, each Committee shall coordinate with each other as appropriate.
4.8 Performance of Representatives. BioCryst and Roche shall cause each of their representatives
on a Committee established under this Agreement to vote, and shall otherwise perform their
respective activities under this Agreement, in a good faith manner.
ARTICLE 5 DILIGENCE AND SPECIAL INDICATIONS
5.1 Diligence. During the Term of the Agreement, Roche, directly or through its Affiliates, shall
use Commercially Reasonable Efforts in the Major Market Countries in order to Develop, manufacture
and Commercialize Licensed Product in the Field. ***. Roche shall fulfill its obligations
hereunder in compliance with all Laws.
(a) ***.
(b) ***.
(c) ***.
(d) ***.
(e) ***.
(f) ***.
(g) ***.
(h) ***.
5.2 Special Indications. BioCryst shall have the right, at its sole cost and expense, to develop
the Compound for use in any Special Indication.
13
EXECUTION COPY
5.3 Development Plan. BioCryst shall, to the extent it exists, deliver to Roche its Development
plan for any Special Indication with respect to which it desires to conduct development. BioCryst
shall update this plan by delivering a written supplement to Roche from time to time during the
term of this Agreement. BioCryst shall also present the development plans to the JSC.
5.4 Reports. BioCryst will provide the JSC within *** (***) days of each June 30 and December 31
during the Term of this Agreement, a written report summarizing for the preceding semi-annual
period all BioCryst development activities, including publications, related to any relevant Special
Indication Product.
5.5 End of Phase IIb.
(a) BioCryst shall notify in writing Roche in the event that BioCryst determines that any
clinical trial results in a Special Indication would justify commencing a pivotal trial directed to
obtaining Regulatory Approval in the U.S. for such indication
(“End of Phase IIb Notice”). The
Notice shall be accompanied by (i) data and information that BioCryst reasonably believes is
sufficient to justify commencing such trial, including without limitation the clinical trial
results, (ii) the proposed end points with respect to which BioCryst proposes such pivotal trial,
and (iii) accrued BioCryst Development Costs through the most recent Calendar Quarter for which
BioCryst financial information is available prior to the date of the Notice. Roche shall have the
right to request, and BioCryst shall endeavor to timely supply any existing additional data and
information from BioCryst that Roche believes to be reasonably required or useful to make its
decision to file or not to file hereunder.
(b) Within *** (***) days after receipt by Roche of the End of Phase IIb Notice and the
accompanying information requested by Roche or set forth in Section 5.5(a)(i) — (iii), Roche shall
notify BioCryst in writing of whether Roche (i) elects to commence such pivotal trial, or (ii) will
not commence such a trial (in which case BioCryst shall be entitled to commence the trial and
continue to develop the Special Indication Product). If Roche notifies BioCryst pursuant to (i) of
the foregoing sentence, then Roche shall, simultaneous with Roche’s notice, (i) reimburse BioCryst
*** percent (***%) of BioCryst’s Development Costs, excluding material supplied by Roche, for such
Special Indication Product, (ii) bear the costs of making the pivotal trial, and thereafter
exercise Commercially Reasonable Efforts in accordance with the terms and conditions of this
Agreement to Develop and Commercialize such Special Indication Product (which, for all purposes
hereunder, shall be deemed to be a Licensed Product).
5.6 Registration.
(a) BioCryst shall notify in writing Roche in the event that BioCryst determines that any
clinical trial results in a Special Indication would justify filing in the U.S. for Regulatory
Approval for such indication (“Filing Notice”). The Filing Notice shall be accompanied by (i) data
and information that BioCryst reasonably believes is sufficient to justify such filing, including
without limitation the clinical trial results, (ii) the proposed labeling with respect to which
BioCryst proposes such filing, and (iii) accrued BioCryst Development Costs through the most recent
Calendar Quarter for which BioCryst financial information is available prior to the date of the
Filing Notice. Roche shall have the right to request, and BioCryst shall endeavor to timely
supply, any existing additional data and information from BioCryst that Roche believes to be
reasonably required or useful to make its decision to file or not to file hereunder.
(b) Within *** (***) days after receipt by Roche of the Filing Notice and accompanying
information requested by Roche or set forth in Section 5.6(a)(i) — (iii), Roche shall notify
BioCryst in writing of whether Roche (i) elects to make such a filing, or (ii) will not make such a
filing. If Roche notifies BioCryst pursuant to (i) of the foregoing sentence, then Roche shall,
simultaneous with Roche’s notice, (i) reimburse BioCryst *** percent (***%) of BioCryst’s
Development Costs, excluding material supplied by Roche, of such Special Indication Product and
(ii) bear the costs of making any filing. In addition, royalties otherwise due hereunder from
Roche to BioCryst with respect to such Special Indication Product shall be increased by *** (***%)
percent with respect to sales of such Special Indication Product. BioCryst shall not be entitled
to make a filing for registration of a Special Indication Product with any Regulatory Authority and
shall not be entitled to commercialize any Special Indication Product.
14
EXECUTION COPY
5.7 Sequencing of Licensed Products. The Parties acknowledge that, even within the Major Market
Countries, Roche and its Affiliates do not seek to obtain regulatory approval for every potential
indication or every compound that has potential for an indication. The parties agree that the JSC
shall be responsible for determining the sequencing of Licensed Products on an indication by
indication basis.
ARTICLE 6 CLINICAL TRIALS, REGULATORY
MATTERS AND MANUFACTURING
MATTERS AND MANUFACTURING
6.1 Clinical Trials and Regulatory Matters. Roche shall be responsible for all prospective costs
by or on behalf of the Roche Group that are associated with the Development and Commercialization
of Licensed Product in the Field, including clinical trials and regulatory submissions.
(a) The IND for Licensed Product shall be transferred to Roche as soon as practicable after
the Effective Date. Roche shall be responsible for all regulatory affairs in all countries related
to Licensed Product, provided that with respect to any activities conducted by BioCryst in the
United States that Roche will, to the extent permitted by Law, provide all drug master files or
other regulatory dossiers containing information necessary or useful to BioCryst in connection with
such activities. In addition, Roche shall promptly provide BioCryst with copies of all clinical
data (upon request by BioCryst) and with all material correspondence with Regulatory Authorities
and all annual reports and summaries provided to any Regulatory Authority relating to Licensed
Products or Compound, to the extent permitted by Law.
(b) Roche shall be responsible for reporting to the appropriate regulatory authorities all
adverse events related to the use of Licensed Product worldwide, except that prior to the transfer
of the IND to Roche as provided herein, BioCryst shall be responsible.
(c) BioCryst shall have the right to file in BioCryst’s name INDs for any Special Indication.
Roche shall, at no cost to BioCryst, provide permission to allow BioCryst to cross-reference Roche
filings to allow BioCryst to carry out without delay any related clinical trial. BioCryst shall
advise and consult with Roche with respect to any significant issues or questions raised by any
regulatory authorities with respect to any such IND or related clinical trial. BioCryst shall
provide copies to Roche of any such IND and any other records of interactions with regulatory
authorities (e.g., correspondence, minutes or notes of telephone conferences or meetings, etc.)
with respect to any such IND or related clinical trials in a Major Market Country.
(d) To the extent Roche is required under applicable Law, rule or regulation, Roche shall make
all filings necessary to permit the use of the clinical materials supplied by Roche pursuant to
Section 6.3(a). BioCryst and Roche shall each supply the other copies of all regulatory filings
and/or the appropriate data related to the use of the clinical materials for such development
promptly after the time of such filings.
(e) To the extent permitted by applicable Law, Roche agrees to seek approval from the
Regulatory Authorities in the Major Market Countries to prominently include on all packaging,
labeling, inserts, Web sites, Promotional Material and other written materials used with Licensed
Products for Sale and all samples thereof (collectively,
“Product Materials”) that BioCryst is
licensor of the Licensed Product. BioCryst shall provide the text and style to Roche of the
BioCryst trademark (the “BioCryst Trademark”) prior to submission to the applicable Regulatory
Authority in a Major Market Country. To the extent such approval is received (and in any event in
situations where approval is not necessary) Roche agrees to include the BioCryst Trademark on all
of the foregoing materials. BioCryst shall also use its reasonable efforts to assist Roche in any
submission to the applicable Regulatory Authority in a Major Market Country pursuant to this
Section 6.1(e).
(f) During the Term hereof, BioCryst grants to Roche and its Affiliates a nonexclusive,
nontransferable, limited right to use the BioCryst trademark designated by BioCryst on such
Product Materials to identify BioCryst as the licensor of the Licensed Product. Roche acknowledges
that (i) BioCryst is the sole and exclusive owner of the BioCryst Trademark and all combinations,
forms and derivatives thereof which may hereafter be approved by BioCryst for use by Roche or its
Affiliates
15
EXECUTION COPY
hereunder, (ii) Roche’s right to use the BioCryst Trademark shall be governed exclusively by
this Agreement, and (iii) all good will from use of the BioCryst Trademark by Roche or its
Affiliates shall inure solely to the benefit of BioCryst. Roche further acknowledges the great
value of the goodwill associated with the BioCryst Trademark and acknowledges that the BioCryst
Trademark and all the rights therein, and goodwill attached thereto, belong exclusively to
BioCryst. Roche shall cooperate fully and in good faith with BioCryst for the purpose of securing,
preserving and protecting BioCryst’s rights in and to the BioCryst Trademark and any secondary
trademarks that may be used by Roche or its Affiliates with the approval of BioCryst. Roche shall
not use the BioCryst Trademark except on the Product Materials in accordance with Section 6.1(e),
and shall use the BioCryst Trademark strictly in compliance with all applicable Laws. Roche shall
duly display all other notices with respect to the BioCryst Trademark on the Product Materials, as
are or may be required by the trademark Laws and regulations applicable in each applicable
jurisdiction. BioCryst shall have the right to approve all use of the BioCryst Trademark on the
Product Materials.
6.2 Adverse Event Reporting. Each Party shall notify the other of all information coming into its
possession (including information of the BioCryst licensors to the Pre-Existing Third Party License
Agreement) concerning any and all side effects, injury, toxicity, pregnancy or sensitivity reaction
associated with commercial or clinical uses, studies, investigations or tests with Compound, Backup
Compounds, or Licensed Product, throughout the world, whether or not determined to be attributable
to Compound, Backup Compounds or Licensed Product (“Adverse
Event Reports”). The Parties shall
enter into a pharmacovigilance agreement prior to commencing development of a Special Indication
Product.
6.3 Manufacturing and Technical Assistance.
(a) Provided that Roche uses good faith efforts in order to assist BioCryst in fulfilling its
obligations hereunder, BioCryst shall: (1) during the first twenty-four (24) months after the
Effective Date supply (in accordance with the guidelines set forth on Exhibit 6.3(a)(1)) at its own
cost all Compound required to be used for Development of Licensed Product in the Field; provided,
however, that notwithstanding anything to the contrary contained in Exhibit 6.3(a)(1), BioCryst
shall not be obligated to provide Roche more than *** of Compound, and (2) within sixty (60) days
of the Effective Date, commence a Know-How transfer (in accordance with the guidelines set forth on
Exhibit 6.3(a)(2)), to Roche that will enable Roche to manufacture Licensed Product. The clinical
supply of Compound shall meet current good manufacturing practices of the FDA (as set forth in 21
C.F.R. Parts 210 and 211) and the ICH guidelines of the European Union and all other applicable
rules, regulations, guides and guidances. Promptly after the Closing Date, BioCryst shall deliver
to Roche: (i) *** of all intermediates pure enough to calibrate analytical instruments, (ii)
analytical methods, (iii) batch records of the whole chemical synthesis, to the extent they exist,
(iv) safety investigation reports for all chemical steps, and (v) a list of key suppliers including
agreements (if any) and all respective lead times. After transition of manufacturing to Roche as
contemplated in this paragraph, Roche shall be responsible to supply, at its own cost, all clinical
supply of Compound required for Development of Licensed Product in the Field, as approved by the
JSC. In consideration for BioCryst’s agreement to supply Roche with Compound as set forth herein,
Roche agrees to prepay BioCryst’s costs and expenses incurred in manufacturing such Compound in the
amount of five million dollars ($5,000,000) (the “Contract Manufacturing and Manufacturing Transfer
Fee”), which shall be due within *** (***) days of (i) the Closing Date of this Agreement and (ii)
receipt by Roche of an invoice (in BioCryst’s standard invoice format) for such sum.
(b) The Contract Manufacturing and Manufacturing Transfer Fee shall be nonrefundable and
noncreditable.
(c) Roche shall be solely and exclusively responsible at its own expense for the technical
development, manufacture and supply of Licensed Product during Development and for sale in the
Field.
(d) BioCryst shall be allowed to manufacture Compound for its use in connection with the
development of Special Indication Products or, after ***, request supply of Compound and/or
Licensed Product from Roche for use by BioCryst in connection with the development of Special
Indication
16
EXECUTION COPY
Products. Provided there is no impact on Roche’s Development and Commercialization of
Licensed Product or other products, Roche shall supply BioCryst with Compound and/or Licensed
Product for use by BioCryst in connection with the development of Special Indication Products at
Roche’s ***.
(e) If Roche requests that BioCryst provide Roche with technical assistance in transferring
technology required for the manufacture of Compound at a Roche Group manufacturing facility,
BioCryst shall provide one visit of up to five days in duration of one full time employee’s time to
provide such services. Thereafter, Roche shall pay BioCryst on a time and materials basis at
commercially competitive hourly consulting rates for technical personnel time. In connection with
all of the foregoing, Roche shall also reimburse BioCryst for BioCryst’s reasonable out-of-pocket
expenses, including travel and lodging expenses.
ARTICLE 7 CONSIDERATION
7.1 Lump Sum Payments. In consideration for the licenses granted herein, Roche shall pay BioCryst
a non-refundable, non-creditable payment in the amount of twenty five million dollars ($25,000,000)
within *** (***) days after (i) the Closing Date of this Agreement and (ii) receipt by Roche of an
invoice (in BioCryst’s standard invoice format) for such sum.
7.2 Development Event Payments. Roche shall promptly notify BioCryst after an event in this
Section 7.2 has occurred. If any Royalty-Bearing Product reaches a following event for an
Autoimmune Indication, then Roche shall pay BioCryst the corresponding payment within *** (***)
days of achievement of such event and receipt by Roche of an invoice from BioCryst (in BioCryst’s
standard invoice format):
Start Phase IIb: |
$ | * | ** | |
Start Phase III or Pivotal: |
$ | * | ** | |
First NDA filing or Foreign Equivalent: |
||||
USA |
$ | * | ** | |
Western Europe |
$ | * | ** | |
Japan |
$ | * | ** | |
NDA Approval or Foreign Equivalent: |
||||
USA |
$ | * | ** | |
Western Europe |
$ | * | ** | |
Japan |
$ | * | ** |
If any Royalty-Bearing Product reaches a following event for up to two (2) additional Autoimmune
Indications, then Roche shall pay BioCryst the corresponding payment within *** (***) days of
achievement of such event and receipt by Roche of an invoice from BioCryst (in BioCryst’s standard
invoice format):
| 1st OTHER | 2NDOTHER | |||||||
| INDICATION | INDICATION | |||||||
Start Phase IIa: |
$ | * | ** | $ | * | ** | ||
Start Phase IIb: |
$ | * | ** | $ | * | ** | ||
Start Phase III or Pivotal: |
$ | * | ** | $ | * | ** | ||
First NDA filing or
Foreign Equivalent: |
||||||||
USA |
$ | * | ** | $ | * | ** | ||
17
EXECUTION COPY
| 1st OTHER | 2NDOTHER | |||||||
| INDICATION | INDICATION | |||||||
Western Europe |
$ | * | ** | $ | * | ** | ||
Japan |
$ | * | ** | $ | * | ** | ||
NDA Approval or Foreign
Equivalent: |
||||||||
USA |
$ | * | ** | $ | * | ** | ||
Western Europe |
$ | * | ** | $ | * | ** | ||
Japan |
$ | * | ** | $ | * | ** | ||
The above payments for Autoimmune Indications are payable for each achievement by any
Royalty-Bearing Product of the above-mentioned event; provided, however, no more than one payment
can ever be payable for a given Autoimmune Indication, regardless of the number of times of
achievement of the event for the given Indication.
By way of example and not limitation, if a first Royalty-Bearing Product in an Autoimmune
Indication (the “First Royalty-Bearing Product”) reaches the Start of Phase IIb, then the event
payment would be *** dollars ($***). If after the First Royalty-Bearing Product reaches the Start
of Phase IIb, any Royalty-Bearing Product in an Autoimmune Indication different than the Autoimmune
Indication of the First Royalty-Bearing Product reaches the Start of Phase IIa (the “Second
Royalty-Bearing Product”), then the event payment would be *** dollars ($***). If the Second
Royalty-Bearing Product reaches the Start of Phase III prior to the time that the First Royalty
Bearing Product reaches the Start of Phase III, then the event payment would be *** dollars ($***)
because the Second Royalty-Bearing Product is the first Autoimmune Indication to reach the Start of
Phase III. If after the Second Royalty-Bearing Product reaches the Start of Phase III, the First
Royalty-Bearing Product reaches the Start of Phase III, then the event payment would be *** dollars
($***), because the First Royalty-Bearing Product is the second Autoimmune Indication to reach the
Start of Phase III.
If any Royalty-Bearing Product reaches a following event for a Transplantation Indication, then
Roche shall pay BioCryst the corresponding payment within *** (***) days of achievement of such
event and receipt by Roche of an invoice from BioCryst (in BioCryst’s standard invoice format):
START PHASE
IIa |
$ | * | ** | |
Start Phase IIb |
$ | * | ** | |
Start Phase III Pivotal: |
$ | * | ** | |
First NDA filing or Foreign Equivalent: |
||||
USA |
$ | * | ** | |
Western Europe |
$ | * | ** | |
ROW |
$ | * | ** | |
NDA Approval or Foreign Equivalent: |
||||
USA |
$ | * | ** | |
Western Europe |
$ | * | ** | |
ROW |
$ | * | ** |
The above payments for Transplantation Indications are payable only one time for the given event
(e.g. the first time the event is achieved only).
Notwithstanding anything to the contrary above, in examples or otherwise, for any indication for
any Royalty-Bearing Product for which a Phase IIa event payment is payable, if a Phase IIa event
payment would otherwise be due and Roche decides not to conduct a Phase IIa trial, then the Start
of Phase IIa event payment that would otherwise be due shall be paid at the same time as the Phase
IIb event payment. If a Phase IIb event payment would otherwise be due and Roche decides not to
conduct a
18
EXECUTION COPY
Phase IIb trial, then the Start of Phase IIb event payment that would otherwise be due shall be
paid at the same time as the Phase III event payment.
7.3 Commercial Event Payments. If aggregate Net Sales for all Royalty-Bearing Products exceed ***
dollars ($***) in any given calendar year, then Roche shall pay BioCryst a one-time payment of ***
dollars ($***) within *** (***) days after achievement of such event. If aggregate Net Sales for
all Royalty-Bearing Products exceed *** dollars ($***) in any given calendar year, then Roche shall
pay BioCryst a one-time payment of *** dollars ($***) within *** (***) days of achievement of such
event. If aggregate Net Sales for all Royalty-Bearing Products exceed *** dollars ($***) in any
given calendar year, then Roche shall pay BioCryst a one-time payment of *** dollars ($***) within
*** (***) days after achievement of such event. For the purposes of clarity, each of the above
payments is payable only the first time the associated commercial event is triggered.
7.4 Royalties. Roche shall pay BioCryst, on a country-by-country basis, and on a Royalty-Bearing
Product by Royalty-Bearing Product basis a royalty payment on incremental Net Sales according to
the following rates for the following ranges of Net Sales:
| CUMULATIVE NET SALES ($ MILLION) | INCREMENTAL ROYALTY RATE (%) | |
*** |
*** | |
*** |
*** | |
*** |
*** |
Notwithstanding the preceding, if no Royalty-Bearing Product has received NDA Approval in a Major
Market Country for an Autoimmune Indication, then Roche shall pay BioCryst, on a country-by-country
basis, a royalty payment on Net Sales of Royalty-Bearing Products according to the following rates:
| NET SALES ($ MILLION) | INCREMENTAL ROYALTY RATE (%) | |
*** |
*** | |
*** |
*** | |
*** |
*** |
By way of example, if, in the year 2013, total Net Sales equals $*** and Licensed Product has
received NDA Approval in a major Market Country for at least one Autoimmune Indication, then the
royalty payable to BioCryst hereunder shall equal $***, calculated in the following manner:
| AMOUNT OF NET SALES | ROYALTY RATE | ROYALTY PAYMENT | ||||||
First $*** |
*** | % | $ | *** | ||||
Next $*** |
*** | % | $ | *** | ||||
Total Royalty |
$ | *** | ||||||
7.5 Patent Coverage Adjustment. For a given country, if there is no Valid Claim in any BioCryst
Patent in such country that, but for this Agreement would be infringed by the manufacture, use or
sale of Licensed Product in such country, then the royalty obligations from Roche to BioCryst for
such country shall be reduced by *** percent (***%) until the end of *** (***) years after First
Commercial Sale of Licensed Product in such country, and thereafter by *** percent (***%), for so
long as the Roche Group sells Licensed Product in such country. If the royalty obligations in this
Section 7.5 are prohibited by applicable Law in any country, then the royalty obligations shall
continue on a country-by-country basis until such time as the obligation is prohibited by
applicable Law. If after *** (***) years after First Commercial Sale of Licensed Product in a
given country in any given year: (i) there is no Valid Claim in any BioCryst Patent in such country
that, but for this Agreement would be infringed by the manufacture, use or sale of Licensed Product
in such country, and (ii) the global Net Sales of Licensed Product are less than *** dollars
($***), then the royalty obligations from Roche to BioCryst for such country shall be reduced by
*** percent (***%).
19
EXECUTION COPY
7.6 Combination Licensed Products. For a Licensed Product containing (i) one or more
pharmaceutically active ingredients which are a Compound and (ii) one or more pharmaceutically
active ingredients which are not a Compound, then the Parties shall, on a country-by-country basis,
agree to an appropriate adjustment to Net Sales to reflect a good faith determination of the
relative value of each pharmaceutically active ingredient, based on the estimated fair market value
of each such therapeutically active ingredient, as follows:
(a) In the case of a combination product for which a Licensed Product and each of the other
therapeutically active ingredients contained in the combination product are sold separately in such
country by the Roche Group, the Adjusted Gross Sales shall be determined by multiplying actual
Adjusted Gross Sales of the combination product by a fraction, the numerator of which shall be the
Gross Price of the Licensed Product as sold separately, and the denominator of which shall be the
sum of the Gross Price of the Licensed Product as sold separately and the gross amount invoiced for
all other active ingredients contained in the combination product, if sold separately.
(b) In the case of a combination product for which the Licensed Product is sold separately in
such country but the non-Licensed Product therapeutically active ingredients contained in the
combination product are not sold separately by the Roche Group in such country, Adjusted Gross
Sales of the Licensed Product forming a part of the combination product shall be calculated by
multiplying actual Adjusted Gross Sales of such combination product by the fraction of A/C where A
is the Gross Price for the Licensed Product used in the combination if sold separately by the Roche
Group and C is the gross amount invoiced for the combination product.
(c) If in a country neither the Licensed Product nor the therapeutically active ingredients
contained in the combination product are sold separately in said country by the Roche Group,
Adjusted Gross Sales of the Licensed Product forming part of the combination product shall be
reasonably determined by allocating value between the Licensed Product and the other
therapeutically active ingredients based on their relative value as determined by the Parties in
good faith. In the case where the parties are unable to agree on the relative value, the Parties
shall agree upon an internationally recognized independent certified public accountant who shall
make such determination and whose determination shall be final and binding on the Parties.
7.7 Payments to Third Parties. BioCryst shall maintain the Pre-Existing Third Party License at its
own cost. If BioCryst’s licensor claims a payment is due under the Pre-Existing Third Party
License and BioCryst does not agree to make such payment, then BioCryst shall promptly advise Roche
in writing of such fact. Other than with respect to the Pre-Existing Third Party License, the
Roche Group shall obtain all licenses from any Third Party necessary to make, have made, use, offer
for sale, sell or import Royalty-Bearing Products, at its own cost.
7.8 Generic Sales Adjustment. If there is no Valid Claim, then Roche may reduce the royalties
otherwise due for Licensed Product in a given country by *** percent (***%) if, in the country,
sales of units of Generic Licensed Product in aggregate total at least *** percent (***%) of the
aggregate sales of units of (i) Licensed Product sold by the Roche Group and (ii) Generic
Licensed Products.
7.9 Royalties Due Once. The obligation to pay royalties to BioCryst under this Agreement is
imposed only once with respect to the same unit of Licensed Product.
ARTICLE 8 PAYMENTS; RECORDS; AUDIT
8.1 Payment of Royalties. Following the First Commercial Sale in a country, within *** (***) days
after the end of each Calendar Quarter during the Term of this Agreement, Roche shall (i) provide
BioCryst with a report detailing, on a Royalty-Bearing Product and country-by-country basis, the
Adjusted Gross Sales and Net Sales for the Calendar Quarter and (ii) pay royalties to BioCryst
under this Agreement for the Calendar Quarter. Within *** (***) days after the end of each
calendar year, Roche shall provide BioCryst with ***. Roche shall provide reports to BioCryst at
the address listed below:
20
EXECUTION COPY
BioCryst Pharmaceuticals, Inc.
0000 Xxxxxxx Xxxx Xxxxx
Xxxxxxxxxx, Xxxxxxx 00000
Attention: Chief Financial Officer
0000 Xxxxxxx Xxxx Xxxxx
Xxxxxxxxxx, Xxxxxxx 00000
Attention: Chief Financial Officer
(a) Roche shall pay royalties to BioCryst under this Agreement by wire transfer to the bank
account listed below:
***
8.2 Currency. All Royalties shall be paid by Roche in U.S. Dollars. Roche shall convert the amount
of all Sales in currencies other than U.S. Dollars into U.S. Dollars using Roche’s then current
standard practices actually used on a consistent basis.
8.3 Payments Nonrefundable. Except as provided for in this Agreement, all payments made hereunder
shall be nonrefundable and non-creditable.
8.4 Roche Records and Audit. Roche and its Affiliates shall keep full, true and accurate books of
account containing all particulars that may be necessary for the purpose of calculating all
royalties and other payments payable under this Agreement.
(a) Such books of accounts shall be kept at the maintaining Party’s principal place of
business. At the expense of BioCryst, BioCryst has the right to engage *** to perform, on behalf of
BioCryst an audit of such books and records of Roche and its Affiliates to report on Net Sales of
Licensed Product for the period or periods requested by BioCryst and the correctness of any report
or payments made under this Agreement.
(b) Upon not less than *** (***) days’ prior written notice from BioCryst, such audit shall be
conducted in the countries specifically requested by BioCryst, during regular business hours in
such a manner as to not unnecessarily interfere with Roche’s normal business activities, and shall
be limited to results in the two (2) calendar years prior to audit notification. Such audit shall
not be performed more frequently than once per calendar year.
(c) All information, data documents and abstracts herein referred to shall be used only for
the purpose of verifying royalty statements or compliance with this Agreement, shall be treated as
Roche Confidential Information subject to the obligations of this Agreement, and (provided that
there is at that time no request or an audit or an ongoing audit), need neither be retained more
than one (1) year after completion of an audit hereof, if an audit has been requested; nor more
than two (2) years from the end of the calendar year to which each shall pertain; nor more than two
(2) years after the date of termination of this Agreement, unless a longer period is required by
Law.
(d) Audit results and findings shall be shared by Roche and BioCryst. If the audit reveals an
overpayment, BioCryst shall reimburse Roche for the amount of the overpayment within *** (***)
days. If the audit reveals an underpayment, Roche shall make up such underpayment within *** (***)
days, ***. If the audit reveals an underpayment in the amount of *** percent (***%) or more, then
Roche shall reimburse BioCryst for the costs of the audit. The failure of BioCryst to request
verification of any royalty calculation within the period during which corresponding records must
be maintained will be deemed to be acceptance of the royalty reporting.
8.5 BioCryst Records and Audit. BioCryst and its Affiliates shall keep full, true and accurate
books of account containing all particulars that may be necessary for the purpose of calculating
all sums invoiced under this Agreement.
(a) Such books of accounts shall be kept at the maintaining Party’s principal place of
business. At the expense of Roche, Roche has the right to engage *** to perform, on behalf of Roche
an
21
EXECUTION COPY
audit of such books and records of BioCryst to verify the correctness of any Development Costs
invoiced under this Agreement.
(b) Upon not less than *** (***) days’ prior written notice from Roche, such audit shall be
conducted in the countries specifically requested by Roche, during regular business hours in such a
manner as to not unnecessarily interfere with BioCryst’s normal business activities, and shall be
limited to results in the two (2) calendar years prior to audit notification. Such audit shall not
be performed more frequently than once per calendar year.
(c) All information, data documents and abstracts herein referred to shall be used only for
the purpose of verifying invoices under this Agreement, shall be treated as BioCryst Confidential
Information subject to the obligations of this Agreement, and (provided that there is at that time
no request for an audit or an ongoing audit), need neither be retained more than one (1) year after
completion of an audit hereof, if an audit has been requested; nor more than two (2) years from the
end of the calendar year to which each shall pertain; nor more than two (2) years after the date of
termination of this Agreement, unless a longer period is required as a matter of Law.
(d) Audit results and findings shall be shared by Roche and BioCryst. If the audit reveals an
overpayment of Development Costs by Roche, BioCryst shall reimburse Roche for the amount of the
overpayment within *** (***) days, ***. If the audit reveals an overpayment of Development Costs in
the amount of *** percent (***%) or more, then BioCryst shall reimburse Roche for the costs of the
audit. If the audit reveals an underpayment, then Roche shall promptly pay all amounts due.
8.6 Withholding Taxes. BioCryst shall pay all sales, turnover, income, revenue, value added, and
other taxes levied on account of event payments, royalties and any other payments accruing or made
to BioCryst under this Agreement. If provision is made in Law of any country for withholding of
taxes of any type, levies or other charges with respect to any royalty or other amounts payable
under this Agreement to BioCryst; then (i) Roche shall promptly notify BioCryst of such Law in
advance of the payment requiring the withholding; and (ii) Roche shall promptly pay such tax, levy
or charge for and on behalf of BioCryst to the proper governmental authority, and shall promptly
furnish BioCryst with receipt of payment. Roche shall be entitled to deduct any such tax, levy or
charge actually paid from royalty or other payment due BioCryst or be promptly reimbursed by
BioCryst if no further payments are due BioCryst. Each Party agrees to assist the other Party in
claiming exemption from such deductions or withholdings under double taxation or similar agreement
or treaty from time to time in force and in minimizing the amount required to be so withheld or
deducted (including by maintaining or changing, as reasonably necessary and in accordance with
applicable Law, the payor of amounts under this Agreement) or obtaining any available refund of
amounts so withheld or deducted.
| ARTICLE 9 INTELLECTUAL PROPERTY |
9.1 Prosecution of BioCryst Patent Rights.
(a) BioCryst Patents. BioCryst shall prepare, file, prosecute and maintain (hereinafter
“Patent Activities”) the BioCryst Patents, at its expense. BioCryst shall consult with Roche as to
the Patent Activities, and furnish to Roche copies of all substantive documents relevant to the
Patent Activities. BioCryst shall furnish such documents and consult with Roche in sufficient time
(at least one week) before any action by BioCryst is due, to allow Roche to provide comments
thereon, which comments BioCryst will consider in good faith. BioCryst will not reduce the scope
of any claim related to the Compound in a given patent application without the prior written
approval of Roche, which approval shall not be unreasonably withheld or delayed. At BioCryst’s
expense and reasonable request, Roche shall cooperate in all reasonable ways in connection with the
Patent Activities. Should BioCryst decide that it does not desire to continue the Patent
Activities in relation to a BioCryst Patent in any given country, or in relation to any pending
claim, it shall promptly advise Roche thereof. At the written request of Roche, BioCryst shall
continue at Roche’s expense and direction to prosecute and/or maintain such
22
EXECUTION COPY
BioCryst Patent or pending claim in such country. At Roche’s expense and reasonable request,
BioCryst shall cooperate in all reasonable ways in connection with the Patent Activities of such
BioCryst Patent or pending claim.
(b) Roche Patents. Roche shall be responsible for the Patent Activities relating to the Roche
Patents. Roche shall consult with BioCryst as to the Patent Activities, and furnish to BioCryst
copies of all substantive documents relevant to the Patent Activities. Roche shall furnish such
documents and consult with BioCryst in sufficient time (at least one week) before any action by
Roche is due to allow BioCryst to provide comments thereon, which comments Roche must consider. At
Roche’s expense and reasonable request, BioCryst shall cooperate, in all reasonable ways in
connection with the Patent Activities. Should Roche decide that it does not desire to continue the
Patent Activities related to a Roche Patent(s) in a country, it shall promptly advise BioCryst
thereof. At the written request of BioCryst, Roche shall then, at no cost to BioCryst, assign to
BioCryst such Roche Patent in such country or countries, and BioCryst may thereafter handle the
Patent Activities at BioCryst’s own cost, to the extent that BioCryst desires to do so. At
BioCryst’s expense and reasonable request, Roche shall cooperate, in all reasonable ways in
connection with the Patent Activities of such Roche Patents.
9.2 Infringement. Each Party shall promptly provide written notice to the other Party during the
Term of this Agreement of any (i) known infringement or suspected infringement by a Third Party of
any BioCryst Patent or Roche Patent in the Field, (ii) known or suspected unauthorized use or
misappropriation by a Third Party of any BioCryst Know-How or Roche Know-How in the Field, or (iii)
receipt by the Party of a paragraph iv certification for a Licensed Products pursuant to the Drug
Price Competition and Patent Term Restoration Act of 1984 (Public Law 98-417), as amended, or its
equivalent in a country other than the United States of America and shall discuss with the other
Party all evidence in its possession supporting such infringement or unauthorized use or
misappropriation.
(a) Within a period of *** (***) days after Roche provides or receives such written notice
(“Decision Period”), Roche, in its sole discretion, shall decide whether or not to initiate a suit
or take other appropriate action in the Field and shall notify BioCryst in writing of its decision
(“Suit Notice”).
(b) Upon BioCryst’s receipt of Suit Notice, BioCryst and Roche shall meet and confer within
*** (***) days regarding the suit or action proposed by Roche. At this meeting, BioCryst shall
present any objections or concerns regarding Roche’s proposed suit or action, especially as the
proposed suit or action relates to BioCryst Patents that have applicability outside the Field, and
Roche shall consider BioCryst’s objections or concerns in good faith. Notwithstanding the
foregoing, Roche need not obtain BioCryst’s consent to proceed with a suit or other action in the
Field.
(c) In the event that Roche (i) does not in writing advise BioCryst within the Decision Period
that Roche will commence suit or take action in the Field, or (ii) fails to commence suit or take
action in the Field within a reasonable time after providing Suit Notice, then BioCryst shall
thereafter have the right to commence suit or take action and shall provide written notice to Roche
prior to commencement of any such suit or action taken by BioCryst.
(d) Upon
written request, the Party bringing suit or taking action
(“Initiating Party”) shall
keep the other Party informed of the status of any such suit or action and shall provide the other
Party with copies of all substantive documents and communications filed in such suit or action that
are not protected by the attorney-client privilege or work product protection. The Initiating
Party shall have the sole and exclusive right to select counsel for any such suit or action.
(e) The Initiating Party shall, except as provided below, pay all expenses of the suit or
action, including, without limitation, the Initiating Party’s attorneys’ fees and court costs. Any
damages, settlement fees or other consideration received as a result of a suit or action initiated
by Roche shall be treated as Net Sales pursuant to this Agreement after the Initiating Party
deducts from the damages, settlement fees or other consideration received its actual counsel fees
and out-of-pocket expenses. Any damages, settlement fees or other consideration received as a
result of a suit or action initiated by BioCryst shall be given *** percent (***%) to *** after
BioCryst deducts from the damages, settlement fees
23
EXECUTION COPY
or other consideration received, its actual counsel fees and out-of-pocket expenses and the
actual counsel fees and out-of-pocket expenses of Roche.
(f) If the Initiating Party believes it reasonably necessary, upon written request the other
Party shall join as a Party to the suit or action but shall be under no obligation to participate
except to the extent that such participation is required as the result of its being a named Party
to the suit or action. At the Initiating Party’s written request, the other Party shall offer
reasonable assistance to the Initiating Party in connection therewith at no charge to the
Initiating Party except for reimbursement of reasonable out-of-pocket expenses incurred by the
other Party in rendering such assistance. The other Party shall have the right to participate and
be represented in any such suit or action by its own counsel at its own expense.
(g) When Roche is the Initiating Party, Roche shall not settle, consent to judgment or
otherwise voluntarily dispose of the suit or action without discussing such action with BioCryst
and considering any objection by BioCryst in good faith. Notwithstanding the foregoing, Roche need
not obtain BioCryst’s consent to settle, consent to judgment or otherwise voluntarily dispose of
the suit or action in the Field with the exception of any settlement, consent judgment, or other
voluntary disposal of the suit or action that would have the effect of rendering any of the patent
claims invalid, unenforceable, or disclaimed, in which instance Roche must first obtain BioCryst’s
consent, and said consent shall not be unreasonably withheld or delayed.
9.3 Xxxxx-Xxxxxx. Notwithstanding anything herein to the contrary, should a Party receive a
certification for a Product pursuant to the Drug Price Competition and Patent Term Restoration Act
of 1984 (Public Law 98-417), as amended, or its equivalent in a country other than the United
States of America, then such Party shall immediately provide the other Party with a copy of such
certification. Roche shall have thirty (30) days from date on which it receives or provides a copy
of such certification to provide written notice to BioCryst
(“H-W Suit Notice”) whether Roche will
bring suit, at its expense, within a forty-five (45) day period from the date of such
certification. Should such thirty (30) day period expire without Roche bringing suit or providing
such H-W Suit Notice, then BioCryst shall be free to immediately bring suit in its name.
ARTICLE 10 CONFIDENTIAL INFORMATION
10.1 Non-Use and Non-Disclosure. During the Term of this Agreement and for *** (***) years
thereafter (or, with respect to trade secrets, indefinitely), a Receiving Party shall (i) treat
Confidential Information provided by Disclosing Party as it would treat its own information of a
similar nature, (ii) take all reasonable precautions not to disclose such Confidential Information
to Third Parties, except to Affiliates, without the Disclosing Party’s prior written consent, and
(iii) not use such Confidential Information other than for fulfilling its obligations under this
Agreement. In the event this Agreement is terminated, all Confidential Information assigned by
Roche to BioCryst shall be deemed to be BioCryst Confidential Information. The Confidential
Information disclosed pursuant to the Non-Disclosure Agreement between Xxxxx-Xxxxxx and BioCryst
dated April 5, 2005 shall be considered Confidential Information hereunder.
10.2 Authorized Disclosure. Nothing in this Agreement shall prevent either Party from conducting
the Patent Activities relating to a patent application or its resulting patents related to a
Licensed Product. Nothing in this Agreement shall prevent the Roche Group from disclosing
Confidential Information to (i) governmental agencies of any country to the extent required or
desirable to secure government approval for the Development, manufacture or sale of Licensed
Product, (ii) Third Parties acting on behalf of the Roche Group, to the extent reasonably necessary
for the Development, manufacture or sale of Licensed Product (and provided that Roche has a written
confidentiality agreement with such Third Party which is as protective of such Confidential
Information as the terms of this Agreement), or (iii) Third Parties to the extent reasonably
necessary to market Licensed Product (and provided that Roche has a written confidentiality
agreement with such Third Party which is as protective of such Confidential Information as the
terms of this Agreement). To the extent provided for in this Agreement, BioCryst may disclose
Confidential Information to its licensors pursuant to the Pre-Existing Third Party License,
provided that
24
EXECUTION COPY
such information shall be treated as Licensee Confidential Information under the Pre-Existing Third
Party License.
ARTICLE 11 TERMINATION
11.1 Commencement and Term. The Term of this Agreement shall commence upon the Effective Date and,
unless this Agreement is terminated sooner as provided in this Article, expire on the date when no
royalty or other payment obligations under this Agreement are or will become due, at which time all
the rights and licenses granted to Roche by BioCryst under this Agreement shall automatically
become irrevocable and fully-paid.
11.2 Termination for Breach.
(a) A Party (“Non-Breaching Party”) shall have the right to terminate this Agreement in
accordance with this Section 11.2(a), as follows: (i) ***; and/or (ii) ***; and/or (iii) ***;
and/or (iv) ***, in the event the other Party (“Breaching Party”) is in breach of any of its
material obligations under this Agreement. The Non-Breaching Party shall provide written notice to
the Breaching Party, which notice shall identify the breach and the countries in which the
Non-Breaching Party intends to have this Agreement terminate. The Breaching Party shall have a
period of *** (***) days after such written notice is provided to cure such breach. If such breach
is not cured within the *** (***) day period, this Agreement shall effectively terminate in such
countries.
(b) Either Party shall have the right to terminate this Agreement in its entirety by giving
written notice to the other Party, in the event of an Insolvency Event of the other Party, such
termination to become effective upon delivery of a notice of termination to such Party. Termination
pursuant to this Section 11.2(b) shall be considered termination for “cause” for the purposes of
this Agreement.
(c) The waiver by either Party of any breach of any term or condition of this Agreement shall
not be deemed a waiver as to any subsequent or similar breach.
11.3 Termination by Roche Without Cause.
(a) Prior to the Commercial Launch of the First Licensed Product. Prior to the commercial
launch of the first Licensed Product, Roche shall have the right to terminate this Agreement (i)
***, and/or (ii) ***, and/or (iii) ***, and/or (iv) ***. The effective date of termination shall be
*** (***) days after the date Roche provides such written notice.
(b) After FDA or EMEA Approval For A Licensed Product. After FDA or EMEA approval for a
Licensed Product, Roche shall have the right to terminate this Agreement on *** (i) ***, and/or
(ii) ***, and/or (iii) ***, and/or (iv) ***. The effective date of termination shall be *** (***)
days after the date Roche provides such written notice.
(c) ***.
11.4 Consequences of Termination.
(a) Partial Termination. Upon any partial termination of this Agreement by a Party for breach
or by Roche without cause, all rights granted to Roche by BioCryst under this Agreement with
respect to such terminated countries shall terminate. Roche shall, to the extent Roche has the
right to do so, and hereby does, assign, and shall ensure that its Affiliates assign, and transfer
to BioCryst, at no expense to BioCryst, all trademarks and applications used or to be used in
connection with Licensed Products in such countries, along with all regulatory filings and
approvals and all data, including clinical data, materials (including raw materials and
manufactured Licensed Product) and information, along with all INDs, and NDAs submitted by Roche
for the Licensed Product in such countries, and to the extent possible, all contract and other
rights in Roche’s possession or control related to Licensed Products in
25
EXECUTION COPY
such countries, along with tangible embodiments of all of the foregoing. Roche may keep one
copy of the foregoing information for archival purposes and to comply with this Agreement. Roche
shall deliver to BioCryst all of the foregoing information promptly after the effective date of
termination. Upon such transfer and assignment, BioCryst shall have the right to use, disclose and
dispose of such materials, rights and licenses in its sole discretion. Roche shall assign or, to
the extent such an assignment is prohibited by Law or third party rights, grant an exclusive, fully
paid up, sublicensable and transferable license for the Licensed Product in the Field) to BioCryst
under Roche Patent Rights and Roche Know-How (with each of the foregoing to include Roche’s
interest in any joint inventions created by the Parties) in such terminated countries. The parties
agree that in the event of a partial termination, BioCryst shall be free to (on its own or with one
or more Third Parties) develop and commercialize Licensed Products in any country with respect to
which rights under this Agreement have been terminated. To the extent any Roche Patent Rights or
Roche Know-How do not solely relate to the Licensed Product, then Roche may grant BioCryst an
exclusive license under such rights as set forth above, rather than assign such rights to BioCryst.
(b) Termination of the Agreement in its Entirety. Upon any termination of this Agreement by a
Party for the other Party’s uncured material breach or by Roche without cause, (i) all licenses
granted to BioCryst by Roche under this Agreement shall become irrevocable, perpetual and
fully-paid, (ii) all licenses granted to Roche by BioCryst shall terminate; (iii) Roche shall
return to BioCryst all BioCryst Confidential Information and tangible examples and copies thereof;
and (iv) Roche shall and hereby does assign or, to the extent such an assignment is prohibited by
Law or third party rights, grant an exclusive, fully paid up, sublicensable and transferable
license for the Licensed Product in the Field), and shall ensure that its Affiliates assign or
exclusively license, to BioCryst all right, title and interest in and to all Roche Know-How and
Roche Patents (with each of the foregoing to include Roche’s interest in any joint inventions
created by the Parties). Roche shall, to the extent Roche has the right to do so, assign and
transfer to BioCryst, at no expense to BioCryst, all trademarks and applications used or to be used
in connection with Licensed Products in such countries, along with all regulatory filings and
approvals and all data, including clinical data, materials (including raw materials and
manufactured Licensed Product) and information, along with all INDs, and NDAs submitted by Roche
for a Licensed Product and all contract and other rights in Roche’s possession or control related
to Licensed Products, along with tangible embodiments of all of the foregoing. Roche may keep one
copy of the foregoing information for archival purposes and to comply with this Agreement. Roche
shall use its commercially reasonable efforts to deliver to BioCryst all of the foregoing in all
forms as soon as practicable but no later than *** (***) days of the effective date of termination.
Upon such transfer and assignment, BioCryst shall have the right to use, disclose and dispose of
such materials, rights and licenses in its sole discretion. To the extent any Roche Patent Rights
or Roche Know-How do not solely relate to the Licensed Product, then Roche may grant BioCryst an
exclusive license under such rights as set forth above, rather than assign such rights to BioCryst.
(c) Further Assurances. Roche and its Affiliates shall execute and deliver any additional
documents or instruments that BioCryst reasonably requests to give effect to the foregoing
assignments and licenses under (a) and (b) above.
(d) Contract Rights. Promptly after notice of any termination under (a) or (b) above, Roche
shall provide BioCryst with copies of all relevant sublicenses, agreements with clinical research
organizations and other Third Party agreements relating to Licensed Products hereunder, and allow
BioCryst *** (***) days from the date of such delivery to choose whether to assume any or all of
such contracts to the extent allowed by the applicable contract or Law. Roche shall, subject to its
ability to do so, assign to BioCryst those Third Party agreements BioCryst chooses to assume.
(e) Transition. In all cases of termination, Roche shall ensure a swift and orderly
transition to BioCryst of all Licensed Products and work in progress, including ongoing clinical
trials, in connection with Licensed Products, with the continuing costs for such work assumed by
BioCryst as of the effective date of termination.
26
EXECUTION COPY
(f) Supply. In such event, Roche shall supply BioCryst with Compound or with Licensed
Product (at BioCryst’s option) at *** for a period of *** years, and during such period BioCryst
shall use its good faith and commercially reasonable efforts to obtain an alternate supply of
Compound or Licensed Product. If prior to the end of *** years BioCryst has obtained an alternate
supply of Compound or Licensed Product, then Roche shall be relieved of its supply obligations
hereunder.
(g) Royalties on Roche Products. Royalties payable on Roche Products will survive termination
or expiration of this Agreement.
11.5 Royalty and Payment Obligations. Roche shall continue to make all payments accrued under this
Agreement in respect of Net Sales in the terminated countries (for a partial termination) and in
all countries (for a complete termination).
ARTICLE 12 INDEMNIFICATION
12.1 Indemnification by BioCryst. BioCryst shall indemnify, hold harmless and defend Roche and its
Affiliates and Roche’s and its Affiliates’ directors, officers, employees and agents from and
against any and all losses, expenses, cost of defense (including without limitation attorneys’
fees, witness fees, damages, judgments, fines and amounts paid in settlement) and any amounts Roche
becomes legally obligated to pay because of any claim or claims against it to the extent that such
claim or claims arise out of or relates to: (i) activities by or on behalf of BioCryst related to
Backup Compounds or Special Indication Products that were performed prior to any license to Roche
relating to any such Backup Compound or Special Indication Product (provided that all
indemnification obligations under this clause (i) with respect to any Backup Compound or Special
Indication Product shall terminate immediately upon Roche’s license to develop and/or commercialize
such Backup Compound or Special Indication Product), and/or (ii) breach of BioCryst’s
representations and warranties in ARTICLE 13, except to the extent such losses, expenses, costs and
amounts are due to the negligence or misconduct or failure to act of Roche.
12.2 Indemnification by Roche. Roche shall indemnify, hold harmless and defend BioCryst and its
Affiliates and BioCryst’s and its Affiliates’ licensors, directors, officers, employees and agents
from and against any and all losses, expenses, cost of defense (including without limitation
attorneys’ fees, witness fees, damages, judgments, fines and amounts paid in settlement) and any
amounts BioCryst becomes legally obligated to pay because of any claim or claims against it to the
extent that such claim or claims arise out of or relates to (i) the making, having made, using,
offering for sale, selling or importing Royalty-Bearing Products, Special Indication Products or
Backup Compounds (including, with respect to each of the foregoing, Development and/or
Commercialization), by or on behalf of Roche or its Affiliates (provided that all indemnification
obligations under this clause with respect to any Backup Compound or Special Indication Product
shall not include any actions by or on behalf of BioCryst prior to Roche’s license to develop
and/or commercialize such Backup Compound or Special Indication Product), (ii) any breach of
Roche’s representations and warranties set forth in ARTICLE 13, and/or (iii) any breach of the
Pre-Existing Third Party License Agreement (as read with the Consent and Waiver) arising out of any
action or inaction of the Roche Group, or by BioCryst as a result of any action or inaction by or
on behalf of the Roche Group or as a result of any direction or request by Roche, except to the
extent such losses, expenses and costs are due to the negligence or misconduct or failure to act of
BioCryst.
12.3 Procedure. In the event of a claim by a Third Party against a Party entitled to
indemnification under this Agreement (“Indemnified Party”), the Indemnified Party shall promptly
notify the other Party (“Indemnifying Party”) in writing of the claim and the Indemnifying Party
shall undertake and solely manage and control, at its sole expense, the defense of the claim and
its settlement. The Indemnified Party shall cooperate with the Indemnifying Party and may, at its
option and expense, be represented in any such action or proceeding by counsel of its choice. The
Indemnifying Party shall not be liable for any litigation costs or expenses incurred by the
Indemnified Party without the Indemnifying Party’s written consent. The Indemnifying Party shall
not settle any such claim unless such settlement fully and unconditionally releases the Indemnified
Party from all liability relating thereto, unless the Indemnified Party otherwise agrees in
writing.
27
EXECUTION COPY
12.4 Limitation on Damages. In no event shall either Party be liable to the other Party for
special, indirect, incidental or consequential damages arising out of this Agreement other than in
connection with such Party’s indemnification obligations under this Agreement.
ARTICLE 13 REPRESENTATIONS AND WARRANTIES
13.1 Mutual Representations and Warranties. Each Party hereby represents and warrants:
(a) Authority. Such Party has the full right and authority to enter into this Agreement, and
that it is not aware of any impediment that would inhibit its ability to perform its obligations
under this Agreement.
(b) Material Facts. Such Party has made available to the other Party information in its
possession or control which it reasonably deems material to the other Party’s decision whether to
enter into this Agreement, and to such Party’s knowledge, information disclosed does not contain
any untrue statement of material fact or omit to state a material fact.
13.2 BioCryst Representations and Warranties. BioCryst warrants and represents that:
(a) Safety Data. BioCryst has allowed Roche access to all material information containing,
and will continue to allow Roche access to information containing (i) the results of all
preclinical testing and human clinical testing of Licensed Product in its possession or control and
(ii) all material information in its possession or control concerning side effects, injury,
toxicity or sensitivity reaction and incidents or severity thereof with respect to Licensed
Product.
(b) Patents. BioCryst has no knowledge of the existence of any patent or patent application
owned by or licensed to the licensors of the Pre-Existing Third Party License or any other Third
Party which would prevent the Roche Group from making, having made, using, offering for sale,
selling or importing Licensed Product. BioCryst has in the past and will continue in the future to
comply with its duty of candor to the United States Patent & Trademark Office in connection with
the Patent Activities of any BioCryst Patent. Exhibit 1.7 is a complete listing of all patents and
patent applications that are necessary for the manufacture, use or sale of the Compound or Licensed
Product(s) and that are owned or Controlled by BioCryst as of the Effective Date.
(c) Grants. BioCryst has the right to xxxxx Xxxxx the rights and licenses described in this
Agreement.
(d) Authorization. The execution, delivery and performance of this Agreement by BioCryst and
all instruments and documents to be delivered by BioCryst hereunder: (i) are within the corporate
power of BioCryst; (ii) have been duly authorized by all necessary or proper corporate action;
(iii) are not in contravention of any provision of the certificate of formation or limited
liability company agreement of BioCryst; (iv) to the knowledge of BioCryst, will not violate any
Law or regulation or any order or decree of any court of governmental instrumentality; (v) will not
violate the terms of any indenture, mortgage, deed of trust, lease, agreement, or other instrument
to which BioCryst is a Party or by which BioCryst or any of its property is bound, which violation
would have an adverse effect on the financial condition of BioCryst or on the ability of BioCryst
to perform its obligations hereunder; and (vi) do not require any filing or registration with, or
the consent or approval of, any governmental body, agency, authority or any other Person, which has
not been made or obtained previously (other than approvals required under the HSR Act, Regulatory
Approvals required for the Sale of Licensed Products and filings with regulatory authorities
required in connection with Licensed Products).
(e) Due Execution. This Agreement has been duly executed and delivered by BioCryst and
constitutes a legal, valid and binding obligation of BioCryst, enforceable against BioCryst in
accordance with its terms, except as such enforceability may be limited by the availability of
equitable remedies.
28
EXECUTION COPY
(f) No Claims. There are no claims or investigations (other than with respect to the Parties’
HSR Filings), pending or threatened against BioCryst or any of its Affiliates, at Law or in equity,
or before or by any governmental authority relating to the matters contemplated under this
Agreement or that would materially adversely affect BioCryst’s ability to perform its obligations
hereunder.
(g) No Conflict. Neither BioCryst nor any of its Affiliates is or will be under any
obligation to any person, contractual or otherwise, that is conflicting with the terms of this
Agreement or that would impede the fulfillment of BioCryst’s obligations hereunder.
13.3 Roche Representations and Warranties.
(a) Authorization. The execution, delivery and performance of this Agreement by Roche and all
instruments and documents to be delivered by Roche hereunder: (i) are within the corporate power
of Roche; (ii) have been duly authorized by all necessary or proper corporate action; (iii) are not
in contravention of any provision of the certificate of formation or limited liability company
agreement of Roche; (iv) to the knowledge of Roche, will not violate any Law or regulation or any
order or decree of any court of governmental instrumentality; (v) will not violate the terms of any
indenture, mortgage, deed of trust, lease, agreement, or other instrument to which Roche is a Party
or by which Roche or any of its property is bound, which violation would have an adverse effect on
the financial condition of Roche or on the ability of Roche to perform its obligations hereunder;
and (vi) do not require any filing or registration with, or the consent or approval of, any
governmental body, agency, authority or any other Person, which has not been made or obtained
previously (other than approvals required under the HSR Act, Regulatory Approvals required for the
Sale of Licensed Products and filings with regulatory authorities required in connection with
Licensed Products).
(b) Due Execution. This Agreement has been duly executed and delivered by Roche and
constitutes a legal, valid and binding obligation of Roche, enforceable against Roche in accordance
with its terms, except as such enforceability may be limited by the availability of equitable
remedies.
(c) No Claims. There are no claims or investigations (other than with respect to the Parties’
HSR Filings), pending or threatened against Roche or any of its Affiliates, at Law or in equity, or
before or by any governmental authority relating to the matters contemplated under this Agreement
or that would materially adversely affect Roche’s ability to perform its obligations hereunder.
(d) No Conflict. Neither Roche nor any of its Affiliates is or will be under any obligation
to any person, contractual or otherwise, that is conflicting with the terms of this Agreement or
that would impede the fulfillment of Roche’s obligations hereunder.
(e) Insurance. Roche represents and warrants that during the Term of this Agreement and for a
period of ten years thereafter, it has and shall maintain adequate insurance and/or financial
resources to cover all liability for any failure of such Licensed Product including, without
limitation, failure in design, manufacture, production and/or operation. BioCryst acknowledges that
Roche may choose to be self-insured.
(f) Investigation. Roche has received all information that Roche or any of its Affiliates has
requested from BioCryst with respect to the transactions contemplated hereby, and has had a
reasonable opportunity to ask questions of BioCryst and its representatives; and BioCryst has
answered all inquiries made by Roche, its Affiliates and their representatives. Roche has had the
opportunity to evaluate the merits and risks of the transactions as contemplated by this Agreement.
Furthermore, no representations or warranties have been made by BioCryst to Roche upon which Roche
is relying in connection with the transactions contemplated by this Agreement, other than as set
forth in this Agreement..
13.4 HSR Act and Other Applicable Governmental Filings. BioCryst and Roche each covenant to timely
make any required application pursuant to the HSR Act. Each Party shall cooperate fully and
promptly in the HSR notification process as well as any other applicable governmental or regulatory
filing.
29
EXECUTION COPY
13.5 No Debarment. None of the Roche Group has been debarred or is the subject of debarment
proceedings by any Regulatory Authority. Roche shall not knowingly use in connection with its
performance of its obligations or duties or its exercise of its rights under this Agreement
(including, without limitation, the Development of any Licensed Products) any employee, consultant
or investigator that has been debarred or the subject or debarment proceedings by any Regulatory
Authority. BioCryst and its Affiliates have not been debarred and are not the subject of debarment
proceedings by any Regulatory Authority. BioCryst and its Affiliates shall not knowingly use in
connection with its performance of its obligations or duties or its exercise of its rights under
this Agreement (including, without limitation, the Development of any Licensed Products) any
employee, consultant or investigator that has been debarred or the subject or debarment proceedings
by any Regulatory Authority.
13.6 No PNP Inhibitors. As of the Effective Date, the Roche Group does not have clinical programs
relating to, and does not have any current plans to develop, any PNP Inhibitor other than Licensed
Product.
13.7 No Other Representations. EXCEPT AS OTHERWISE PROVIDED IN THIS AGREEMENT, THE FOREGOING
REPRESENTATIONS AND WARRANTIES ARE IN LIEU OF ALL OTHER REPRESENTATIONS AND WARRANTIES, EXPRESS OR
IMPLIED, INCLUDING WITHOUT LIMITATION, WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR
PURPOSE OF COMPOUND AND LICENSED PRODUCTS. SPECIFICALLY, BIOCRYST MAKES NO OTHER REPRESENTATIONS OR
WARRANTIES IN RELATION TO THE BIOCRYST PATENTS, THE BIOCRYST KNOW-HOW, THE COMPOUND (INCLUDING
COMPOUND SUPPLIED HEREUNDER), THE BACKUP COMPOUNDS OR THE LICENSED PRODUCTS. SPECIFICALLY ROCHE
MAKES NO OTHER REPRESENTATIONS OR WARRANTIES IN RELATION TO THE ROCHE PATENTS, THE ROCHE KNOW-HOW,
THE COMPOUND OR THE BACKUP COMPOUNDS OR THE LICENSED PRODUCTS. BIOCRYST SHALL HAVE NO LIABILITY
WHATSOEVER ARISING OUT OF OR RELATING TO COMPOUND SUPPLIED TO ROCHE HEREUNDER.
ARTICLE 14 CONDITIONS TO CLOSING
14.1 Conditions Subsequent.
(a) The effectiveness of this Agreement and the transactions contemplated hereunder shall be
subject to and contingent upon the satisfaction under the following condition subsequent to the
execution of this Agreement. The condition subsequent shall be the earlier to occur of (i)
approval of the transaction by the Federal Trade Commission or any other applicable governmental
authority, or (ii) expiration or termination of all applicable waiting periods, and requests for
information (and any extensions thereof) under the HSR Act or other applicable Law. Subject to the
terms and conditions of this Agreement, each Party shall use all reasonable efforts to take, or
cause to be taken, all reasonable actions and to do, or cause to be done, all things necessary and
appropriate to satisfy the condition subsequent and to consummate the transactions contemplated by
this Agreement. Each Party shall cooperate with the other Party in the preparation, execution and
filing of all documents that are required or permitted to be filed on or before the Closing Date
for the purpose of consummating this transaction, including, filings pursuant to the HSR Act or
other governmental filing. Each Party shall bear its own costs (including counsel or other expert
fees) with respect to preparing, executing and filing such documents; provided that Roche shall be
obligated to pay all filing fees under the HSR Act.
(b) Either Party may terminate this Agreement in its entirety, upon ten (10) days prior
written notice to the other Party if the condition subsequent under Section 14.1(a) has not been
fulfilled within six (6) months after the Signing Date, in which case, upon termination, there
shall be no liabilities for obligations on the part of either Party except if there has been a
breach of this Section 14.1.
ARTICLE 15 DISPUTE RESOLUTIONS AND GOVERNING LAW
15.1 Disputes. Unless otherwise set forth in this Agreement, in the event of a dispute arising
under or relating to this Agreement between the Parties and/or their Affiliates, such dispute
shall be referred to
30
EXECUTION COPY
the respective executive officers of the Parties designated below, or their successors, for good
faith negotiations attempting to resolve the dispute. The designated executive officers are as
follows:
| For Roche: | CEO of the Pharma Division | |||
| For BioCryst: | Chairman and CEO |
If the positions of the designated executive officers listed above are vacant or no longer exist,
then the person having the most nearly equivalent position (or such individual’s designee) shall be
deemed to be the designated executive officer of the relevant Party.
15.2 Arbitration. Following the Parties’ attempt to resolve a given dispute pursuant to the above
Section 15.1, either Party as its exclusive recourse subject to Sections 15.2(c) and 15.3(c) may
have the given dispute settled by binding Arbitration in the manner described below:
(a) Arbitration Request. If a Party intends to begin arbitration to resolve a dispute arising
under this Agreement, such Party shall provide written notice (the “Arbitration Request”) to the
other Party of such intention and the issues to be resolved. From the date of the Arbitration
Request and until such time as any matter has been finally settled, the running of the time periods
as to which Party must cure a breach of this Agreement shall be suspended as to the subject matter
of the dispute.
(b) Additional Issues. Within *** (***) business days after the receipt of the Arbitration
Request, the other Party may, by written notice, add additional issues to be resolved.
(c) No Arbitration of Intellectual Property Issues. Notwithstanding anything to the contrary
in this Agreement, unless otherwise agreed by the Parties, disputes relating to intellectual
property shall not be subject to arbitration, and shall be submitted to a court of competent
jurisdiction.
15.3 Arbitration Procedure. The Arbitration (including with respect to discovery) shall be
conducted pursuant to the rules of the American Arbitration Association. If Roche is the Party
initiating Arbitration, the Arbitration shall be held in Birmingham, Alabama. If BioCryst is the
Party initiating Arbitration, the Arbitration shall be held in Newark, New Jersey. The Arbitration
shall be conducted in English by three arbitrators. Each Party shall select one arbitrator within
*** (***) days of the Arbitration Request. Should a Party not select an independent arbitrator
within *** (***) days of the Arbitration Request, the American Arbitration Association shall select
an arbitrator on behalf of the Party. The two (2) arbitrators selected by the Parties shall select
a third, neutral, arbitrator within *** (***) days after the Arbitration Request. Should the
arbitrators not select a third, neutral, arbitrator within such time period, the American
Arbitration Association shall select an arbitrator on their behalf. The third neutral arbitrator
shall not be associated with either Party.
(a) The arbitrators may award any remedy allowed by Law, excluding punitive damages and
attorneys’ fees. Promptly after rendering a decision, the arbitrators shall issue to both parties
a written opinion of the findings of fact and conclusions of Law. The decision of the arbitrators
shall be binding upon the parties without the right of appeal, and judgment upon the decision
rendered by the arbitrator may be confirmed and enforced in any court of competent jurisdiction.
(b) The parties shall share equally the reasonable documented cost of such Arbitration
procedure. Each Party shall bear its own cost in participating in such proceeding.
(c) Nothing herein limits in any way either Party’s right to seek injunctive relief.
15.4 Governing Law. This Agreement is made in accordance with and shall be governed and construed
under the Laws of Delaware, without regard to its choice of law principles.
31
EXECUTION COPY
ARTICLE 16 PUBLICITY
16.1 Initial Press Release. The Parties will issue the initial press release(s) attached hereto as
Exhibit 16.1 on the Effective Date.
16.2 Subsequent External Communications. Each Party shall only issue external media and investor
communications, including press releases related to the activities contemplated by this Agreement
that have either (i) been approved by the other Party or (ii) are required to be issued by such
Party as a matter of Law as determined by such Party’s legal counsel. In all circumstances, the
Party issuing the external media or investor communication shall provide the other Party with a
copy of the press release or external communication at least *** prior to its intended publication
or communication for the other Party’s review. During such period, the other Party shall (i)
approve the draft press release or communication and permit the party issuing the press release to
issue the press release, (ii) contact the Party issuing the press release or communication to
discuss modification to the draft press release or communication, or (iii) contact the Party
issuing the press release or communication and disapprove the press release or communication. If
the other Party asks for modification, then the Party issuing the press release or communication
shall either make such modification or work with the other Party to arrive at a press release or
communication that the other Party approves.
16.3 Disclosures Required by Law. Nothing in this Agreement shall impair either Party’s compliance
with any requirements of: (i) governmental agencies to the extent required or desirable to secure
government approval for the development, manufacture or sale of Licensed Product in the Territory
(ii) the Securities and Exchange Commission or the national securities exchange or other stock
market on which such Party’s securities are traded, (iii) or any other applicable Law. In
connection with any filing by either Party of a copy of this Agreement with the Securities and
Exchange Commission (or the national securities exchange or other stock market on which such
Party’s securities are traded), the filing Party shall endeavor to obtain confidential treatment of
economic and trade secret information. Reasonably in advance of any filing under this Section
(whether or not this Agreement is included in the filing), the filing Party shall provide to the
other Party a copy of the proposed filing and the Parties shall work cooperatively in good faith,
taking into consideration the other Party’s suggestions, regarding the information for which the
filing Party will seek to obtain confidential treatment. However, in the event of any
disagreements that cannot be amicably resolved, the Party which is making the filing shall,
together with input from their own legal counsel, have the ultimate authority to make the filing in
the fashion in which it feels the filing must be made.
16.4 Publications. During the Term of this Agreement, the following restrictions shall apply with
respect to disclosure by any Party of Confidential Information relating to the Product in any
publication or presentation:
(a) Both Parties acknowledge that it is their policy for the studies and results thereof to be
registered and published in accordance with their internal guidelines. Roche, in accordance with
its internal policies and procedures, shall have the right to publish all studies, clinical trials
and results thereof on the clinical trial registries which are maintained by or on behalf of Roche.
BioCryst shall not publish any studies, clinical trials or results thereof on its clinical trial
registry, provided however, that Roche’s clinical trial registry can be accessed via a link from
BioCryst’s clinical trial registry.
(b) A Party (“Publishing Party”) shall provide the other Party with a copy of any proposed
publication or presentation at least *** (***) days (or at least *** days in the case of oral
presentations) prior to submission for publication so as to provide such other party with an
opportunity to recommend any changes it reasonably believes are necessary to continue to maintain
the Confidential Information disclosed by the other party to the Publishing party in accordance
with the requirements of this Agreement. The incorporation of such recommended changes shall not be
unreasonably refused; and If such other party notifies (“Notice”) the Publishing Party in writing,
within *** (***) days after receipt of the copy of the proposed publication or presentation (or at
least *** (***) days in the case of oral presentations), that such publication or presentation in
its reasonable judgment (i) contains an invention, solely or jointly conceived and/or reduced to
practice by the other party, for which the other party
32
EXECUTION COPY
reasonably desires to obtain patent protection or (ii) contains any Confidential Information
disclosed by the other party to the Publishing Party, the Publishing Party shall prevent such
publication or delay such publication for a mutually agreeable period of time. In the case of
inventions, a delay shall be for a period reasonably sufficient to permit the timely preparation
and filing of a patent application(s) on such invention, and in no event less than *** (***) days
from the date of the Notice.
16.5 Use of Name. Neither Party shall use the other Party’s or its Affiliates name or trademarks
for publicity or advertising purposes, except with the prior written consent of the other Party.
Subject to Section 16.3, Roche shall not use any of BioCryst’s licensors’ names or trademarks for
publicity or advertising purposes (or any other information relating to the Pre-Existing Third
Party License), except with the prior written consent of BioCryst.
ARTICLE 17 MISCELLANEOUS
17.1 Pre-Existing Third Party License. Roche acknowledges and agrees that the terms of this
Agreement are subject in all respects to the terms and conditions of the Pre-Existing Third Party
License, as amended, ***, which have been previously provided to Roche. Roche further agrees that
(i) the licensors under the Pre-Existing Third Party License retained certain rights as described
in Section 2.1(b) above, which are not granted to Roche hereunder; (ii) such licensors shall be
deemed to be third party beneficiaries of this Agreement; (iii) all Confidential Information
provided to BioCryst hereunder may be shared with such licensors under the terms and conditions of
***; and (iv) Roche shall fully cooperate with BioCryst to assist BioCryst in complying with its
obligations (including but not limited to recordkeeping and information sharing) under the
Pre-Existing Third Party License ***.
17.2 Extended Benefits. Roche may extend any benefit and assign any right under this Agreement to
its Affiliates and Roche guarantees the performance of all obligations imposed on such Affiliates
by such extension or assignment. Each of Roche Nutley and Roche Basel shall be jointly and
severally liable for the obligations of Roche hereunder.
17.3 Survival. Any provisions which by their nature are intended to survive termination of this
Agreement shall survive termination of this Agreement for any reason, including, without
limitation, Articles 1, 8, 10, 12 and 15 and Sections 7.3, 7.4, 7.5, 7.6, 7.8, 7.9, 11.4, 11.5,
17.3, 17.5, 17.10 and 17.12.
17.4 Agency. Neither Party is, nor will be deemed to be, an employee, agent or representative of
the other Party for any purpose. Each Party is an independent contractor, not an employee or
partner of the other Party. Neither Party shall have the authority to speak for, represent or
obligate the other Party in any way without prior written authority from the other Party.
17.5 Entire Agreement. This Agreement, including all Exhibits and Schedules, embodies the entire
understanding of the Parties with respect to the subject matter hereof and supersedes all previous
communications, representations or understandings, and agreements, whether oral or written, between
the Parties relating to the subject matter hereof.
17.6 Counterparts. This Agreement may be executed in one or more counterparts, each of which shall
be deemed an original and all of which, taken together, shall constitute one and the same
instrument. Original signatures hereto may be delivered by facsimile which shall be deemed
originals.
17.7 Amendment. No amendment or modification hereof shall be valid or binding upon the parties
unless made in writing and signed by both Parties.
17.8 Assignment. This Agreement shall not be assignable in part or in whole, by operation of Law
or otherwise, by any Party without the prior written consent of the other; provided, however, that
either Party, without notice and at any time for any reason, may assign this Agreement in whole or
in part to (i) any of its Affiliates who agree to be bound by the terms and conditions of this
Agreement or (ii) any successor of
33
EXECUTION COPY
such Party by merger or sale of all or substantially all of its pharmaceutical business assets. In
the event of assignment to an Affiliate, the Party making the assignment will remain liable and
responsible for the performance and observance of all its duties and obligations hereunder.
17.9 Requirement to Divest. If Roche is required by a relevant Regulatory Authority in a Major
Market Country to divest rights to a Licensed Product with respect to which Roche has not commenced
the Start of Phase III trial prior to the order to divest, then Roche shall use its commercially
reasonable efforts to obtain authority to fulfill such requirements by returning rights to BioCryst
in and to such Licensed Product in accordance with the procedures specified in Section 11.4(b). If
Roche is required by a relevant Regulatory Authority in a Major Market Country to divest rights to
a Licensed Product with respect to which Roche has commenced the Start of Phase III trial prior to
the order to divest, then Roche shall afford BioCryst a reasonable opportunity to acquire such
rights on terms no less advantageous than those granted to other potential acquirers of such
rights, including in bidding or other acquisition processes.
17.10 Notices. Any notice or other communication to be given under this Agreement, unless
otherwise specified, shall be in writing and shall be deemed to have been provided when delivered
to the addressee at the address listed below (i) on the date of delivery if delivered in person or
(ii) one (1) day after mailing to the other Party by express mail or overnight delivery service,
which obtains a signed receipt, or (iii) three (3) days after mailing by registered or certified
mail, postage paid:
In the case of BioCryst:
BioCryst Pharmaceuticals, Inc.
0000 Xxxxxxx Xxxx Xxxxx
Xxxxxxxxxx, Xxxxxxx 00000
0000 Xxxxxxx Xxxx Xxxxx
Xxxxxxxxxx, Xxxxxxx 00000
Attention: Chairman and CEO
Telephone: 000-000-0000
Fax: 000-000-0000
Fax: 000-000-0000
With a copy to:
Proskauer Rose LLP
0000 Xxxxxxxx
Xxx Xxxx, Xxx Xxxx 00000
0000 Xxxxxxxx
Xxx Xxxx, Xxx Xxxx 00000
Attention: Xxxxx Xxxxxxxx, Esq.
Telephone: (000) 000-0000
Fax: (000) 000-0000
Fax: (000) 000-0000
In the case of Roche:
Xxxxxxxx-Xx Xxxxx Inc.
000 Xxxxxxxxx Xxxxxx
Xxxxxx, Xxx Xxxxxx 00000
000 Xxxxxxxxx Xxxxxx
Xxxxxx, Xxx Xxxxxx 00000
Attention: Corporate Secretary
With a copy to:
X.Xxxxxxxx-Xx Xxxxx Ltd.
Xxxxxxxxxxxxxxxxx 000
Xxxxxxxxxxxxxxxxx 000
00
EXECUTION COPY
XX-0000 Xxxxx, Xxxxxxxxxxx
Attention: Law Department
Either Party may change its address for communications by a notice in writing to the other Party in
accordance with this Section.
17.11 Force Majeure. Any prevention, delay or interruption of performance (collectively “Delay”)
by any Party under this Agreement shall not be considered a breach of this Agreement if and to the
extent caused by occurrences beyond the reasonable control of the Party affected by the force
majeure, including but not limited to acts of God, embargoes, governmental restrictions, strikes or
other concerted acts of workers, fire, flood, earthquake, explosion, riots, wars, terrorism, civil
disorder, rebellion or sabotage. The affected Party shall immediately notify the other Party upon
the commencement and end of the Delay and any time for performance hereunder shall be extended by
the actual time of Delay. If the Delay resulting from the force majeure exceeds six (6) months, the
other Party, upon written notice to the affected Party, may elect to (i) treat such Delay as a
material breach, or (ii) extend the term of this Agreement for an amount of time equal to the
Delay.
17.12 Affiliates. Roche shall cause its Affiliates to comply with Roche’s obligations hereunder.
17.13 Bankruptcy. All licenses (and to the extent applicable rights) granted under or pursuant to
this Agreement by BioCryst to Roche are, and shall otherwise be deemed to be, for purposes of
Section 365(n) of Title 11, US Code (the “Bankruptcy Code”) licenses of rights to “intellectual
property” as defined under Section 101(60) of the Bankruptcy Code. Unless Roche elects to
terminate this Agreement, the Parties agree that Roche, as a licensee or sublicensee of such rights
under this Agreement, shall retain and may fully exercise all of its rights and elections under the
Bankruptcy Code, subject to the continued performance of its obligations under this Agreement.
17.14 Severability. If any term or condition of this Agreement is held by a court of competent
jurisdiction to be unenforceable for any reason, it shall, if possible, be interpreted to achieve
the intent of the Parties to this Agreement rather than voided. If not capable of such
interpretation, the Parties shall in good faith seek to agree on an alternative provision
reflecting the intent of the Parties which is enforceable. In any event, all other terms,
conditions and provision of this Agreement shall be deemed valid and enforceable to the full
extent.
[REMAINDER OF PAGE INTENTIONALLY BLANK]
35
EXECUTION COPY
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the day and year first above
written.
| BIOCRYST PHARMACEUTICALS, INC. | XXXXXXXX-XX XXXXX INC. | |||||||||
By:
|
/s/ Xxxxxxx X. Xxxx | By: | /s/ Xxxxxxxxx X. Xxxxx III | |||||||
| Name: Xxxxxxx X. Xxxx | Name: Xxxxxxxxx X. Xxxxx III | |||||||||
| Title: Chairman and CEO | Title: Vice President | |||||||||
| X. XXXXXXXX-XX XXXXX LTD | ||||||||||
| By: | /s/ Xxxxx Xxx | |||||||||
| Name: Xx. Xxxxx Xxx | ||||||||||
| Title: Executive Vice President Pharma Partnering |
||||||||||
| By: | /s/ Xxxxxxx Xxxx Xxxx | |||||||||
| Name: Xx. Xxxxxxx Xxxx Wick | ||||||||||
| Title: Authorized Signatory | ||||||||||
36

