CORPORATE RESEARCH AGREEMENT
Exhibit 10.1
THIS CORPORATE RESEARCH AGREEMENT (this “Agreement”) is effective as of the 8th day of July, 2021 (the “Effective Date”), by and between Avalon GloboCare Corp. (“AVALON”), a DELAWARE corporation having its administrative headquarters at 0000 Xxxxx 0 Xxxxx, Xxxxx 0000, Xxxxxxxx, XX 00000, XXX (the “Sponsor”), and the UNIVERSITY OF PITTSBURGH – OF THE COMMONWEALTH SYSTEM OF HIGHER EDUCATION, a non-profit, state-related educational institution incorporated under the laws of the Commonwealth of Pennsylvania and having an office at Office of Sponsored Programs, 0000 Xxxxxx Xxxxxx, 000 Xxxxxxx X Xxxxxxxx, Xxxxxxxxxx, Xxxxxxxxxxxx 00000 (the “University”). For the avoidance of doubt, any activities undertaken in connection with this Agreement at or in connection with the facilities of the University of Pittsburgh, the University of Pittsburgh School of Medicine (UPSOM), the Immunologic Monitoring and Cellular Products Laboratory (IMCPL), the University of Pittsburgh Medical Center (UPMC) or the Xxxxxxx Cancer Center (HCC) at UPMC shall be deemed to be covered under this Agreement (all such facilities, collectively “Research Facilities”).
WHEREAS, Sponsor desires to fund the University’s research with respect to a specific research project and research plan agreed upon by University and Sponsor as set forth in further detail below, and the University agrees to perform such research with Sponsor’s funding;
WHEREAS, Yen Xxxxxxx X. Xxx, M.D., Ph.D. (“Xx. Xxx”), Associate Professor of Medicine, Division of Hematology/Oncology at the University of Pittsburgh School of Medicine (UPSOM), Director of Immunologic Monitoring and Cellular Products Laboratory (IMCPL) and Co-Director of Cellular Therapeutics program at the University of Pittsburgh Medical Center (UPMC) Xxxxxxx Cancer Center (HCC) with an address at 000 Xxxxxxxxxx Xxxxx, Xxxxx 000, Xxxxxxxxxx, XX 00000, XXX shall be the University’s principal investigator in conducting the research for Sponsor (the “Principal Investigator”);
WHEREAS, Sponsor and Xx. Xxx entered into that certain Preliminary Agreement, dated 25 March 2021, and Sponsor hereby acknowledges and agrees that the Preliminary Agreement did not bind the University under the terms thereof; provided, that notwithstanding the foregoing, the University and Sponsor hereby acknowledge and agree that any activities undertaken at the Research Facilities in connection with the Preliminary Agreement shall be deemed to be governed by the terms and conditions of this Agreement as of the Effective Date;
NOW, THEREFORE, in consideration of the mutual promises and covenants contained herein, and intending to be legally bound hereby, the parties agree as follows:
| 1. | Scope of Work. |
| 1.1 | University agrees to use its reasonable efforts to perform academic research funded by Sponsor in accordance with the Research Plan entitled “Development of Point-of-care Modular Autonomous Processing System (“PMAPsysTM” or “PMAPsys”) for Cell and Gene Therapies” as set out in Exhibit A as attached hereto and made a part hereof (the “Project”). The purpose of this Agreement is for University to conduct the Project for the design, assembly and validation of Sponsor’s PMAPsysTM platform and system as well as the completion of the development and validation of the production and manufacturing processes for clinical-grade AVA-011 (the “Purpose”). AVA-011 is Sponsor’s FLASH- CAR™ platform therapeutic candidate that is the subject of pre-clinical research and development by Sponsor as of the Effective Date. Notwithstanding anything otherwise to the contrary, University acknowledges and agrees that it shall not modify AVA-011 in any manner without the express prior written authorization of Sponsor. |
| 1.2 | University shall perform the Project in accordance with academic standards and all laws and regulations that apply to (a) such standards, (b) the conduct of the Project and (c) the activities undertaken by University and Principal Investigator hereunder (all such standards, laws and regulations collectively, “Applicable Laws and Standards”). Sponsor recognizes that the University will not conduct the Project in accordance with U.S. Food and Drug Administration Good Laboratory Practice regulatory standards (“GLP”), and Sponsor understand and agrees that any Project results generated may not be used by Sponsor for any filings that require a certification of GLP compliance. |
| 1.3 | University certifies that University and Principal Investigator shall maintain and fully comply with all applicable Accreditations (as defined in Exhibit D) to the extent necessary for University and Principal Investigator to conduct the Project and fulfill their obligations hereunder as set forth in further detail in Exhibit D attached hereto and incorporated herein by reference. |
| 2. | Term. This Agreement shall commence on the Effective Date of this Agreement and shall continue until the second anniversary of the Effective Date; provided, that this Agreement may be (a) earlier terminated by a party in accordance with Section 10 below or (b) extended for such additional period of time as may be agreed upon in writing by the parties pursuant to a duly executed amendment hereto (the duration of this Agreement, the “Term”). Notwithstanding the foregoing, if during any period of the Term, the University is required to temporarily cease its normal operations due to a federal, state, or local government declaration, the Term and University’s performance obligations hereunder shall be extended to a time that is commensurate with the University’s temporary cessation of normal operations, plus 10 business days; and Avalon’s payment obligations and payment terms hereunder shall be similarly tolled and extended for the corresponding time period. |
| 3. | Payment. |
| 3.1 | Sponsor agrees to pay to University the amounts set forth in the budget in accordance with the payment schedule and other terms set forth in the budget attached hereto and incorporated herein as Exhibit B (the “Budget”). Such payments shall clearly identify the Principal Investigator and the Project. |
The parties acknowledge and agree that the amounts set forth in the Budget shall not be deemed installment payments; and in the event that this Agreement is terminated for any reason, Sponsor shall have no further payment obligations to University following the effective date of such termination.
| 3.2 | If, at any time during the Term, expenditures are expected to exceed the total Budget, the University may request additional funds from Sponsor, which Sponsor may elect to provide at its discretion. In the event that Sponsor elects not to provide additional funds, Sponsor shall have no rights to utilize such portion of the research results to the extent arising solely from such portion of the research activities that Sponsor has expressly declined to fund by written notification thereof to the University. |
| 3.3 | The University may reasonably reallocate funds within categories of the Budget to meet the primary objectives of the Project; provided, that the University shall notify Sponsor, and seek Sponsor’s written approval, thereto prior to any such reallocation. In the event that the Project is completed and there are funds remaining in the Budget, such unexpended funds shall be refunded to Sponsor upon submission of the University’s final financial statement. |
| 4. | Materials Provided. |
| 4.1 | All materials provided for the Project under this Agreement (the “Materials”) shall be listed on Exhibit C, which shall include the name and amount of the Materials, and the party providing such Materials (the “Provider”). If no Materials are being provided, Exhibit C shall indicate “none.” |
2
| 4.2 | All Materials and unmodified derivatives thereof shall remain the property of the Provider and will be used by the receiving party (the “Recipient”) solely for the Project. Notwithstanding anything otherwise to the contrary, University acknowledges and agrees that Sponsor Materials existing at the Effective Date or otherwise provided by Sponsor to University during the term of this Agreement and any intellectual property rights therein or thereto are and shall be the exclusive property of Sponsor and Sponsor’s preservation of exclusive rights therein are and shall be essential to the business of Sponsor. The Materials will be returned to the Provider or destroyed by the Recipient, as requested in writing by Provider, at the end of the Term of this Agreement or upon earlier termination of this Agreement. |
| 4.3 | The Materials shall be used with prudence and appropriate caution in any experimental work. THE MATERIALS ARE PROVIDED WITHOUT WARRANTY OF MERCHANTABILITY OR FITNESS FOR ANY PARTICULAR PURPOSE OR ANY OTHER WARRANTY, EXPRESS OR IMPLIED. Recipient agrees to defend and indemnify the Provider, its trustees, directors, officers, employees and agents from any and all claims and damages in any way arising from the acquisition, use, storage or disposal of the Materials by Recipient, unless such claim is solely due to negligence on the part of the Provider. |
| 4.4 | Subject to the University limitations set forth in Section 1.2, the Materials will be used in compliance with all applicable statutes and regulations, including the National Institutes of Health guidelines on the use of animals and recombinant DNA. The Materials may not be used for in vivo testing in human subjects. Materials derived from human donors may not be transferred with any individual donor-identifying information. |
| 4.5 | No option, license, or conveyance of rights, express or implied, is granted by one party to the other party in connection with any Materials, except the right to use the Materials strictly in accordance with the terms of this Agreement for the Project. |
| 5. | Equipment. |
| 5.1 | Subject to the terms and conditions of this Agreement and the Sponsor Equipment Loan Agreement (as defined below) relating to the use of Sponsor Equipment (as defined below) by University, Sponsor will be responsible, at its cost, for the procurement (whether by purchase or lease, at Sponsor’s sole discretion), installation, regular maintenance and repair of Sponsor’s PMAPsys equipment. Sponsor Equipment will be provided on loan for University to conduct the sponsored research in accordance with the Research Plan and this Agreement and the Sponsor Equipment Loan Agreement. Upon agreement of Sponsor, Principal Investigator and University and as set forth in the written Research Plan and the Sponsor Equipment Loan Agreement, Sponsor Equipment shall include the following instrumentation and equipment: the Maxcyte GTx electroporator, Terumo Quantum, and Cytiva Sepax. Sponsor and University shall enter into a separate, ancillary agreement governing the loan by Sponsor of Sponsor Equipment to University and University’s use thereof (the “Sponsor Equipment Loan Agreement”) within 60 days following the Effective Date of this Agreement to set forth the terms and conditions pursuant to which Sponsor may install and support Sponsor Equipment at University’s facilities and University may access and use such Sponsor Equipment. |
| 5.2 | Except as otherwise provided in this Agreement, the Research Plan and the Sponsor Equipment Loan Agreement, title to any equipment or supplies (other than Materials provided by or on behalf of Sponsor) purchased or manufactured by University in the performance of the Project funded under this Agreement shall vest in the University upon acquisition. Notwithstanding the foregoing, (a) any equipment or hardware provided directly or indirectly by or on behalf of Sponsor to the University shall be deemed “Sponsor Equipment” and (b) as between the parties, Sponsor Equipment shall be exclusively owned by Sponsor. Sponsor Equipment shall include without limitation the PMAPsys equipment (including any components thereof) provided by Sponsor. University shall have no rights to any Sponsor Equipment after the expiration or termination of this Agreement. |
3
| 6. | Principal Investigator. The parties acknowledge and agree that Sponsor’s election to fund the Project is dependent on the appointment of Xx. Xxx Xxxxxxx X. Xxx as Principal Investigator for the Project and his assumption of responsibilities to direct and undertake the design, development and performance of all Project-related obligations of University hereunder. If, for any reason, the Principal Investigator is unable to continue to serve as Principal Investigator, the University may, upon the written request of Sponsor, designate another faculty member who is acceptable to Sponsor to serve as Principal Investigator of the Project; provided, that Sponsor may elect at its discretion to terminate the Project immediately upon written notice to University. If a substitute Principal Investigator has not been designated by the University and approved in writing by Sponsor within thirty(30) days after the original Principal Investigator ceases his or her services under this Agreement, either party may terminate this Agreement upon written notice thereof to the other party, subject to the provisions of Section 10 (Termination). |
| 7. | Independent Contractor. |
| 7.1 | University is an independent contractor of Sponsor. Sponsor shall not attempt at any time to exercise any significant degree of control to impede the integrity of the University’s research efforts and research independence in connection with this Agreement. |
| 7.2 | Nothing in this Agreement shall be construed to create a partnership or joint venture between University and Sponsor, nor shall either party’s employees, servants, agents or representatives be considered the employees, servants, agents or representatives of the other. Neither party shall have any express or implied right or authority to assume or create any obligation on behalf of, or in the name of, the other party; or to bind the other party to any contract, agreement or undertaking with any third party. |
| 7.3 | University shall withhold and pay all statutory payroll taxes and provide any and all employment related insurances, including Workers’ Compensation and Employer’s Liability, for its employees involved in the Project. |
| 8. | Reports and Records. |
| 8.1 | The University shall provide Sponsor with periodic progress reports on the Project, as specified in the Research Plan attached hereto as Exhibit A; provided, that University shall provide such periodic progress reports no less frequently than once per calendar quarter during the term of this Agreement. Such progress reports shall include a detailed description of all Project activities undertaken as of the date of such report, the progress made with respect to all Project activities with respect to the Research Plan, the rate of spend of the Project funding against the budgeted amounts set forth in Exhibit B (Budget) hereto and any changes to Project timelines or Project scope or activities anticipated or undertaken by University. Within thirty (30) days after the expiration of every six-month period during the Term of this Agreement, Principal Investigator shall provide Sponsor with a written report summarizing all Project data and results obtained during the preceding six-month period. |
| 8.2 | University shall provide Sponsor with a final written report at no additional cost upon completion of the Project or upon earlier termination of this Agreement. |
| 8.3 | University shall, at mutually agreed upon times, meet with Sponsor’s representatives to discuss Project results and reports. |
| 9. | Intellectual Property Rights; ROFO |
| 9.1 | Ownership. Ownership of inventions and/or discoveries developed under this Agreement shall follow inventorship under United States patent laws except as otherwise expressly provided herein. For purposes of this Section 9.1, (1) “solely” shall mean, with respect to intellectual property made by a party hereto, without involvement or contribution of any kind by the other party or such other party’s employees or other personnel (including students) or agents or other third parties contracted by such other party or engaged in a business relationship of any kind with such other party and (2) “jointly” shall mean, with respect to intellectual property made by the parties hereto, joint involvement or contribution by both parties or the respective employees (including students) or other personnel or agents or contractors of each party. |
| 9.2 | University shall not file any patent applications or otherwise seek patent protection claiming any (a) Materials provided by or on behalf of Sponsor or (b) Sponsor Project IP or Sponsor Independent IP. |
| 9.3 | University shall own all rights, title and interest in and
to inventions and/or discoveries developed solely by University employees, students or agents in the performance of the Project under
this Agreement during the term of this Agreement (“University Project IP”); provided, that University hereby grants
to Sponsor a worldwide, irrevocable, non-exclusive, royalty-free, fully paid-up, perpetual right (with the right to sublicense to collaborators
and contractors working on Sponsor’s behalf)
to use University Project IP for commercial purposes research activities and other purposes. The University shall disclose all University
Project IP to Sponsor in writing before the end of the Term of this Agreement. For the avoidance of doubt, University Project IP shall
exclude Sponsor Project IP and Sponsor Independent IP.
|
4
| (a) | Notwithstanding anything otherwise to the contrary, Sponsor shall exclusively own all rights, title and interest in and to all inventions (whether or not patentable), discoveries and other intellectual property of any kind conceived, discovered, created, generated, reduced to practice, developed or otherwise made, solely by Sponsor in the performance of the Project during this Agreement (collectively, “Sponsor Project IP”); provided, that the University shall retain a worldwide, irrevocable, non- exclusive, royalty-free right to use such Sponsor Project IP to the extent specifically relating to the Project only for non-commercial, academic research purposes and for no other purposes. |
| (b) | Notwithstanding anything otherwise to the contrary, Sponsor shall exclusively own, as between the parties, all rights, title and interest in and to Sponsor Independent IP (as defined below). “Sponsor Independent IP” shall mean any and all inventions (whether or not patentable), discoveries, know-how, data, information, technologies and other intellectual property and intellectual property rights therein or thereto owned or controlled by Sponsor prior to the commencement of the Project or at any time thereafter to the extent made independently by Sponsor outside the scope of this Project including any intellectual property or intellectual property rights therein or thereto owned or controlled by Sponsor relating to Sponsor’s therapeutic agents, compounds, cells, tissues, reagents, standards, samples, materials, products, product candidates, formulations, formulation technologies, delivery technologies, therapies, services, methodologies, procedures, production or manufacturing processes, other processes, data or know-how. |
| (c) | University and Sponsor shall each own an undivided, one-half interest in and to any inventions (whether or not patentable), data, discoveries and other intellectual property and intellectual property rights therein or thereto developed jointly by both parties in the performance of the Project during the term of this Agreement (“Co-developed IP”). Sponsor shall have a right of first offer to exclusively license the University’s interest in Co-developed IP in accordance with the provisions of this Section 9; provided, that Sponsor as a joint owner of Co-developed IP shall have the right to freely use, make, sell or otherwise exploit (including without limitation the right to license or sublicense through multiple tiers of sublicense) Co-developed IP on a non-exclusive basis anywhere in the world without further accounting to or requiring consent from the University. For the avoidance of doubt, Co-developed IP shall exclude any University Project IP, Sponsor Project IP or any Sponsor Independent IP. |
| 9.2 | Right of First Offer. During the ROFO Period (as defined below), subject to Public Law 96-517 (codified in 35 U.S.C. §200-212 and implemented in 37 C.F.R. 401) and the terms of this Agreement, the University hereby grants to Sponsor an exclusive right of first offer (an “ROFO”) to an exclusive royalty- bearing license to (a) University Project IP and (b) University’s rights, title and interest in and to any Co-developed IP. Such right of first offer shall remain in effect for ninety (90) days after the date of disclosure to Sponsor (“ROFO Period”). If Sponsor has not notified University in writing of its desire to enter into license negotiations within such ROFO Period, University shall have the right, but not the obligation, to license such rights to a third party. The right of first offer granted to Sponsor hereunder shall not apply to University patents or patent applications issued or filed before the Effective Date of this Agreement. University shall not offer to a third party more favorable terms when considered in the aggregate than the terms last offered to Sponsor with respect to such exclusive, royalty-bearing license for a period of one-year following the expiration or termination of this Agreement. |
| 9.3 | Should Sponsor notify University of its desire to enter into license negotiations in accordance with Section 9.2 hereof, the University shall provide Sponsor with a license agreement which shall include the following negotiable terms: |
| (a) | Sponsor shall pay to University an execution fee, annual maintenance payments/minimum royalties, milestones payments (where applicable) and/or reasonable royalty rates on terms to be negotiated; |
| (b) | University shall retain a royalty-free, non-exclusive right to use the applicable inventions and/or discoveries for non-commercial education and academic research purposes; |
5
| (c) | Sponsor shall reimburse the University for all applicable patent prosecution and maintenance out-of-pocket costs actually incurred by University as mutually agreed upon in writing in advance by the parties in connection with University Project IP or Co-developed IP; |
| (d) | such license agreement must be finalized and fully executed within ninety (90) days following Sponsor’s written notification to University of Sponsor’s exercise of the applicable ROFO pursuant to Section 9.2 or such other longer period of time as may be mutually agreed upon by the parties in the event both parties are using reasonable efforts to undertake and continue active negotiations with respect to such agreement (the “Exclusive Option Negotiation Period”); |
| (e) | Such other reasonable terms and conditions as agreed upon by the parties. |
| 9.4 | Should a mutually acceptable license agreement not be executed and delivered within the Exclusive Option Negotiation Period, Sponsor shall have no further rights with respect to the ROFO. |
| 9.5 | Nothing contained herein shall affect, or be construed to grant to the other party by implication or otherwise, any rights, title, or interest in or to any (a) pre- existing rights of a party hereto in intellectual property developed prior to the Effective Date of this Agreement or (b) the rights of a party hereto with respect to any intellectual property developed independently of this Agreement. |
| 10. | Termination; Effects of Termination. |
| 10.1 | This Agreement may be terminated prior to the expiration of the Term should any one or more of the following events occur: |
| (a) | either party provides the other party with ninety (90) days advance written notice of termination at will for any or no reason; |
| (b) | a party materially breaches this Agreement, and the non-breaching party provides the breaching party with thirty (30) days advance written notice of termination and such breach is not remedied within such thirty (30) day period; or |
| (c) | immediately by either party should conditions outside of its control due to a Force Majeure Event (as defined in Section 23 below) render performance an impossibility or impracticability and result in a cessation of the research Project hereunder for a period of 90 consecutive days or longer (the “Force Majeure Period”). |
| 10.2 | Sponsor shall reimburse the University for any non-cancelable obligations of University, if any, that have been pre-approved in writing by Sponsor and specifically and solely allocable to Sponsor’s Project that the University will be contractually required to pay thereafter, notwithstanding the termination (such non-cancellable obligations, if any, “Sponsor-Specific Non-Cancellable Costs”); provided, that (a) the University shall notify Sponsor of any such Sponsor- Specific Non-Cancellable Costs prior to committing to assume such non- cancellable obligations and seek Sponsor’s prior written approval thereof; and (b) upon termination or expiration of this Agreement, University shall confer with Sponsor and the applicable third party vendor to mitigate and cancel any such outstanding Sponsor-Specific Non-Cancellable Costs. |
| 10.2 | Upon any expiration or termination of this Agreement (a) Principal Investigator and University shall immediately cease use of all materials, equipment and Confidential Information of Sponsor and Sponsor’s intellectual property then in University’s possession, unless otherwise instructed in writing by Sponsor and (b) University shall promptly return to Sponsor at Sponsor’s expense, or destroy at Sponsor’s written request, all Materials, Confidential Information and Sponsor IP then in University’s possession. |
| 10.3 | Sections 9, 10, 11, 12, 13 (as set forth therein), 14, 15, and 18-25 shall survive any expiration or termination of this Agreement. |
6
| 11. | Limitation of Liability. |
| 11.1 | Sponsor agrees to indemnify, defend and hold harmless the University, its trustees, officers, employees and agents, against any third party claims of loss, damage or costs (including attorneys’ fees) to the extent actually incurred and arising out of Sponsor’s use of the University’s research data or results generated from the Project; provided, that the foregoing obligation shall not apply to the extent any such third party claims, losses, damages or costs result or arise from University’s negligence or willful misconduct. University agrees to indemnify, defend and hold harmless Sponsor, its Affiliates, directors, officers, employees and agents, against any third party claims of loss, damage or costs (including attorneys’ fees) to the extent actually incurred and arising out of University’s gross negligence and willful misconducted in connection with this Agreement. |
| 11.1 | University shall not be liable to Sponsor, its officers, employees, servants or agents or to any third party for injuries or losses arising out of use of the Project’s research data or results except to the extent of University’s gross negligence, willful misconduct or fraud. As between the parties, each party shall be responsible for its own acts and omissions relating to the Project and its use of the Project results. |
| 11.3 | Limitation of Liability. Except with respect to a party’s indemnification obligations hereunder or a party’s material breach of its confidentiality obligations hereunder or the misuse, misappropriation or other material violation of a party’s intellectual property rights, neither party will be liable to the other party with respect to any subject matter of this Agreement under any contract, negligence, strict liability or other legal or equitable theory for any indirect, incidental, consequential or punitive damages or lost profits. |
| 12. | Warranty Disclaimers. UNIVERSITY DISCLAIMS AND MAKES NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AS TO ANY MATTER, INCLUDING BUT NOT LIMITED TO WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE, MERCHANTABILITY, PATENTABILITY OR THAT SPONSOR’S USE OF THE PROJECT RESEARCH RESULTS WILL BE FREE FROM INFRINGEMENT OF PATENTS, COPYRIGHTS, TRADEMARKS OR OTHER RIGHTS OF THIRD PARTIES. SPONSOR DISCLAIMS AND MAKES NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AS TO ANY MATTER, INCLUDING BUT NOT LIMITED TO WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE, MERCHANTABILITY, PATENTABILITY OR THAT UNIVERSITY’S USE OF ANY MATERIALS, DOCUMENTATION, SOFTWARE, HARDWARE, EQUIPMENT OR OTHER TECHNOLOGY PROVIDED BY SPONSOR IN CONNECTION WITH THE PROJECT OR THIS AGREEMENT WILL BE FREE FROM INFRINGEMENT OF PATENTS, COPYRIGHTS, TRADEMARKS OR OTHER RIGHTS OF THIRD PARTIES. |
| 13. | Confidentiality. |
| 13.1 | University and Sponsor acknowledge that it may be necessary to disclose information to each other which each party considers proprietary or confidential in order for the University to perform the Project. To preserve the proprietary or confidential nature of such information, University and Sponsor agree to use commercially reasonable efforts to either: (a) clearly xxxx the term “Confidential Information” upon the information and forward it only to the other party in writing; or (b) orally disclose to the other party the proprietary or confidential nature of the information, subsequently indicate the nature of such information in a writing addressed to the other party, and clearly xxxx the writing or information with the term “Confidential Information” and deliver it to the other party within sixty (60) days of disclosure; provided, that in each of the foregoing cases in the event either party declines to so xxxx such information, the absence of such markings shall not relieve the other party from its confidentiality obligations under this Agreement with respect to such unmarked information. |
| 13.2 | Neither party shall disclose nor cause to be disclosed any Confidential Information of the other party without the other party’s prior written consent. |
7
| 13.3 | The parties’ obligation of non-disclosure shall not apply to any or all of information which: |
| (a) | is in the public domain at the time of disclosure; |
| (b) | becomes part of the public domain after disclosure through no fault of the other party; |
| (c) | is in the other party’s possession at the time of disclosure or is properly obtained by recipient from a third party with a valid legal right to disclose such information, and such third party is not under a confidentiality obligation to the disclosing party; or |
| (d) | is required to be disclosed due to applicable law, regulation or order of court; provided, that the disclosing party shall use reasonable efforts to obtain confidential treatment thereof by the applicable governmental entity to which such information is disclosed; or |
| (e) | is independently developed by a party without direct or indirect reliance on any Confidential Information of the other party or any Materials of Sponsor. |
| 13.4 | The parties’ obligations regarding Confidential Information shall survive the expiration or termination of this Agreement for a period of ten (10) years. |
| 14. | Publicity. The Sponsor shall not use the name of the University or any member of the University’s staff, in connection with any products, promotion, or advertising without the prior written approval of the University. University shall not use the name of Sponsor in connection with any products, promotion, or advertising without the prior written approval of the Sponsor. |
| 15. | Publication Rights. |
| 15.1 | University shall have the right to publish the results of and disseminate information pertaining to the University’s research for the Project conducted under this Agreement, subject to (a) University’s confidentiality obligations with respect to any Confidential Information of Sponsor and (b) the rights of Sponsor to seek and obtain protection for its intellectual property rights as set forth in Section 15.2. Sponsor acknowledges the University’s strong institutional policy favoring the retention of publication rights and dependence upon publication as an essential means of intellectual exchange. |
| 15.2 | University agrees to submit any proposed publication or presentation of Project results sufficiently in advance to Sponsor for review and approval prior to publication. University shall identify Sponsor in any publication and may include Sponsor as a co-author on any publication or presentation of Project data or results when mutually agreeable to the parties and in accordance with journal publication standards, upon prior written notification thereof to Sponsor and providing Sponsor with the opportunity to review and comment on any such draft sufficiently prior to publication or presentation thereof. Within thirty (30) days of its receipt, Sponsor shall advise University in writing of any proprietary or patentable information contained therein and may, as necessary, formally request the University to delay disclosure of such information. University agrees to refrain from publishing any such information categorized by Sponsor as proprietary or patentable for a period not to exceed ninety (90) days from the date of such written request or such additional reasonable amount time as may be mutually agreed to by the parties in writing, to enable Sponsor to appropriately coordinate with the University to file for the protection of any intellectual or proprietary property interests. University and Sponsor shall reasonably cooperate in good faith to perfect intellectual or other proprietary property interests in any Project results or other intellectual property generated in connection with the activities undertaken herein. Except as set forth in this Agreement, University shall have no other legal obligation to delay publication of results. Unless otherwise agreed to by Sponsor in writing, University will not disclose Sponsor’s Confidential Information (as defined above) in any proposed publication or presentation in accordance with Section 13, Confidentiality. |
8
| 16. | Independent Inquiry. Nothing in this Agreement shall be construed to limit the freedom of researchers who are participants in this Agreement, whether paid under this Agreement or not, from engaging in similar research inquiries made independently under other grants, contracts or agreements with parties other than the Sponsor; provided, that such University researchers shall not utilize Sponsor’s Confidential Information, Materials and other intellectual property owned or controlled by Sponsor in connection with such research inquiries with such third parties.. University shall ensure that all researchers and other personnel who perform the Project or otherwise undertake any activities in connection with this Agreement, whether or not paid for such research or activities, shall comply with all obligations of University under this Agreement including without limitation all obligations of University with respect to Sponsor’s Confidential Information, Materials and other intellectual property. For purposes of clarity, University shall not use Sponsor’s Confidential Information for any purposes other than Sponsor’s use. |
| 17. | Export Control Regulations. Notwithstanding any other provision of this Agreement, the parties understand and agree that they are subject to, and agree to abide by, any and all applicable United States laws and regulations controlling the export of technical data, computer software, laboratory prototypes and other commodities. The University’s and Sponsor’s obligations hereunder are contingent on each party’s ability to comply with applicable United States export and embargo laws and regulations. It is the expectation of the University and Sponsor that the work done pursuant to this Agreement will constitute fundamental research and be exempt from export control licensing requirements under the applicable export control laws and regulations. As an institution of higher learning, the University does not wish to take receipt of export-controlled information except as may be knowingly and expressly agreed to in writing signed by an authorized representative of each of the University and Sponsor, and for which the University and Sponsor have each made specific arrangements. Sponsor agrees that it will not knowingly provide or make accessible to University any export-controlled materials (including, without limitation, equipment, information and/or data) without first informing University of the export-controlled nature of the materials and obtaining from University’s Office of Sponsored Programs its prior written consent to accept such materials as well as any specific instructions regarding the mechanism pursuant to which such materials should be passed to University; provided, that University shall promptly provide all such information in the possession of University to enable Sponsor to make such determination. Sponsor and University each agree to comply with any and all applicable U.S. export control laws and regulations, as well any and all embargoes and/or other restrictions imposed by the Treasury Department’s Office of Foreign Asset Controls. |
| 18. | Notice. Any notice or communication pursuant to this Agreement shall be sufficiently made or given if (a) sent by certified or registered mail, postage prepaid, or by overnight courier, with proof of delivery by receipt, addressed to the address below or as either party shall designate by written notice to the other party, (b) faxed to other party if the sender retains evidence of successful transmission and if the sender promptly sends the original by ordinary mail, in any event, to the addresses below or as either party shall designate by written notice to the other party or (c) sent via e-mail to the following e-mail addresses: |
In the case of University:
| For administrative matters: | Office of Sponsored Programs | |
| University of Pittsburgh | ||
0000 Xxxxxx Xxxxxx, 000 Xxxxxxx X Xxxxxxxx Xxxxxxxxxx, XX 00000 | ||
| Attention: | Clinical/Corporate Contracts SRA00001518 | |
| Telephone: | (000) 000-0000 | |
| For scientific matters: | Yen Xxxxxxx X. Xxx, M.D., Ph.D. | |
| UPMC Xxxxxxx Cancer Center | ||
University of Pittsburgh School of Medicine 000 Xxxxxxxxxx Xxxxx, Xxxxx 000 Xxxxxxxxxx, XX 00000 | ||
| Email: | xxx00@xxxx.xxx, xxxxx@xxxx.xxx | |
| Office Phone: | (000) 000-0000 | |
| Office Fax: | (000) 000-0000 | |
9
| In the case of Sponsor: | ||
| Avalon GloboCare Corp. | ||
| 0000 Xxxxx 0 Xxxxx, Xxxxx 0000 | ||
| Xxxxxxxx, XX 00000, XXX | ||
| Attention: | Xxxxx Xxx, M.D., Ph.D. | |
| Email: | xxxxx@xxxxxx-xxxxxxxxx.xxx | |
| Office Phone: | (000) 000-0000 | |
| Office Fax: | (000) 000-0000 | |
By such notice, either party may change its address or e-mail address for future notices. Notices mailed shall be deemed given on the date postmarked on the envelope. Notices sent by overnight courier shall be deemed given on the date received by such courier, as indicated on the shipping manifest or waybill. Notices sent by fax shall be deemed given on the date faxed. Notices sent via e-mail shall be deemed given on the date the e-mail was received in the recipient’s e-mail account.
| 19. | Governing Law. This Agreement shall be governed by and construed in accordance with the laws of the Commonwealth of Pennsylvania. |
| 20. | Entire Agreement. This Agreement, together with all attachments and exhibits, constitutes the entire agreement and understanding between the parties and supersedes any prior or contemporaneous negotiations, agreements, understandings, or arrangements of any nature or kind with respect to the subject matter herein. In the event of any inconsistency between this Agreement or any attachments and exhibits, the terms of this Agreement shall govern. |
| 21. | Waiver. Neither party waives its right to enforce any and all provisions of the Agreement at any time during the Term. Either party’s failure to enforce any provision shall not prejudice such party from later enforcing or exercising the same or any other provision of the Agreement. |
| 22. | Modifications. This Agreement may not be changed, altered, modified, amended, rescinded, canceled or waived except by a writing executed by authorized representatives the parties. |
| 23. | Force Majeure. No Party will be liable to the other for any failure or delay in the performance of its obligations to the extent such failure or delay is caused by fire, flood, earthquakes, other elements of nature, acts of war, terrorism, riots, civil disorders, rebellions or revolutions, disease, epidemics, quarantines, pandemics, acts of government, a declared state of emergency, delays in visas, changes in laws and governmental policies, or other conditions beyond its reasonable control following execution of this Agreement. If the performance by either party of any of its obligations under this Agreement (including making a payment) is prevented by any such circumstances, then such party shall communicate the situation to the other as soon as possible, and the parties shall endeavor to limit the impact to the Project. The parties agree to mitigate risks to the Project and personnel, and to amend Project period of performance and milestones if necessary to mitigate such risks as mutually determined by the parties. Nothing herein shall limit the rights of either party to terminate this Agreement as indicated in Section 10 hereunder. |
| 24. | Binding Agreement on Successors. This Agreement shall be binding upon each party’s successors and assigns. |
| 25. | Headings. Headings are for convenience of reference only, and not for interpreting the provisions of the Agreement. |
| 26. | Counterparts. This Agreement may be executed in counterparts, and by either party on separate counterpart, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument. |
[Signature page follows.]
10
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their respective duly authorized representatives as of the date first above written.
| SPONSOR: Avalon GloboCare Corp. | ||
| By: | /s/ | |
| Name: | ||
| Title: | ||
| Date: | ||
UNIVERSITY OF PITTSBURGH – OF THE COMMONWEALTH SYSTEM OF HIGHER EDUCATION | ||
| By: | /s/ | |
| Name: | ||
| Title: | ||
| Date: | ||
| Principal Investigator confirms that he has read and understands this Agreement and agrees to abide by its terms. | ||
| /s/ | ||
| Principal Investigator | ||
| Date: | ||
11
EXHIBIT A
RESEARCH PLAN/RESEARCH PROTOCOL
Within 30 days following the Effective Date (or such other reasonable period of time as may be agreed by the Parties following the Effective Date), the Parties shall update the Research Plan, as applicable, and amend this Exhibit A in this Agreement accordingly with the updated Research Plan pursuant to Section 22 (Modifications) of the Agreement.
Title of Research Project:
Development of Point-of-care Modular Autonomous Processing System (PMAPsysTM) for Cell and Gene Therapies.
Objective:
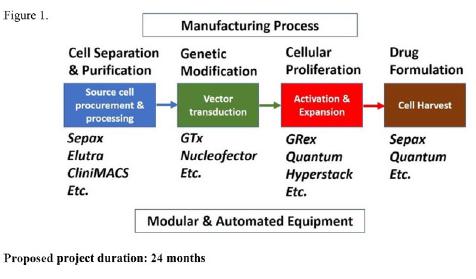
This sponsored research project will evaluate the optimal combination of bioreactors and equipment used in clinical manufacturing for RNA anti-CD19 and anti-CD22 CAR-T (Figure 1). The ultimate goal is to determine the most cost-efficient pre-cGMP workflow to produce experimental anti-CDl9 and anti-CD22 CAR-T for future development of cGMP manufacturing and IND applications.
12
Development of Point-of-care Modular Autonomous Processing System (PMAPsysTM) for Cell and Gene Therapies.
Avalon’s PMAPsysTM is a proprietary laboratory platform currently under development. The technology is a multi-purpose modular autonomous clinical-grade laboratory system intended to develop, optimize, and manufacture cell and gene therapies at point-of-use clinical settings. It consists of assembly and integration of different functional cell processing, production and validation components for generation of specific clinical-grade re-engineered cell and gene products.
The objective of this strategic collaboration and co-development research project is to establish the PMAPsysTM and bio-production for (not limited to) Avalon’s cell and gene therapy candidate “AVA-011”, a RNA-based chimeric antigen receptor (CAR)-T for clinical studies. A proposed work-flow diagram is demonstrated in Exhibit A.
The location of this strategic collaboration and co-development project will be conducted within Principal Investigator’s research area at IMCPL, a registered and accredited facility to conduct innovative product development and production.
13
EXHIBIT B
BUDGET
University and Principal Investigator shall comply with the budget principles for the Project as set forth in this Exhibit B:
| Expense Items | Expense Details | Year 1 | Year 2 | Total | ||||||||||
| Personnel | PI (partial salary support) & hiring researcher(s) | $ | 102,700 | $ | 104,909 | $ | 207,609 | |||||||
| Reagents | Supplies, Reagents & Core Facility Costs | $ | 197,330 | $ | 195,121 | $ | 392,451 | |||||||
| Other Expenses | Conferences, publications, computers, software, etc. | $ | 3,000 | $ | 3,000 | $ | 6,000 | |||||||
| F&A Expenses | 65% | $ | 196,970 | $ | 196,970 | $ | 393,941 | |||||||
| Total Budget | $ | 500,000 | $ | 500,000 | $ | 1,000,000 | ||||||||
During the term of this Agreement, Sponsor shall pay to University the following amounts for use by Principal Investigator solely to conduct the activities set forth in the Research Plan in accordance with the following payment schedule:
| Payment Number | Amount Payable by Sponsor to University |
Due Date of Payment | ||
| Payment #1 | US$125,000 |
Within 10 business days following the Effective Date | ||
| Payment #2 | US$125,000 | 1 October 2021 | ||
| Payment #3 | US$125,000 | 1 January 2022 | ||
| Payment #4 | US$125,000 | 1 April 2022 | ||
| Payment #5 | US$125,000 | 1 July 2022 | ||
| Payment #6 | US$125,000 | 1 October 2022 | ||
| Payment #7 | US$125,000 | 1 January 2023 | ||
| Payment #8 | US$125,000 | 1 April 2023 |
Notwithstanding anything otherwise to the contrary (i) the amounts listed above shall not be deemed installment payments and (ii) in the event this Agreement is earlier terminated by either party for any reason, Sponsor shall have no obligation to pay any amounts due after the effective date of such termination or otherwise any amounts for research activities undertaken after the terminating party provides notice of termination to the other party.
14
EXHIBIT C
SPONSOR MATERIALS
Sponsor Materials: AVA-011 Chimeric Antigen Receptor gene expression vector and other materials provided by Sponsor to University in connection with the Project during the term of this Agreement.
15
EXHIBIT D
UNIVERSITY ACCREDITATIONS
Accreditations maintained as of the Effective Date by University and Principal Investigator including without limitation in connection with University’s facilities, equipment, systems, infrastructure, processes, methodologies, procedures, personnel and other resources to the extent relating to the Project and University’s research and undertakings hereunder (such accreditations, collectively the “University Accreditations”):
| 1. | FACT: Foundation for the Accreditations of Cellular Therapy | |
| 2. | CAP: College of American Pathologists | |
| 3. | CLIA: Clinical Laboratory Improvement Amendments | |
| 4. | PA DOH: Pennsylvania Department of Health | |
| 5. | FDA: United States Food and Drug Administration (cGMP, cGTP, cGXP and drug master file and another applicable industry standards and regulations) |
University shall acquire and maintain, and fully comply at all times with all requirements and standards relating to, all Accreditations listed above and all additional Accreditations required by Sponsor in connection with University’s undertakings under this Agreement. Without limiting any other rights or remedies of Sponsor, Sponsor shall have the right to terminate this Agreement immediately if University fails to comply with the foregoing obligations.

16

