RESEARCH, OPTION AND LICENSE AGREEMENT
Exhibit 10.18
RESEARCH, OPTION AND LICENSE AGREEMENT
This Research, Option and License Agreement (hereinafter “Agreement”), effective as of April 27, 2015 (the “Effective Date”), is made by and between Spark Therapeutics, Inc., a Delaware corporation with corporate offices at ▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ (“Spark”) and Clearside Biomedical, Inc., a Delaware corporation with corporate offices at ▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇., ▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ (“Clearside”) (each, a “Party” and collectively, the “Parties”).
Whereas, Spark is a biopharmaceutical company specializing in the development of gene therapies.
Whereas, Clearside is a biopharmaceutical company with proprietary technology designed to administer drugs to the targeted tissue of the eye using a microneedle injection into the suprachoroidal space and related formulation technology (the “Clearside Technology”).
Whereas, Spark desires to collaborate with Clearside on researching the use of Clearside Technology for the delivery of gene therapies, and, if such efforts are successful, Spark desires to have the right to further develop and commercialize gene therapy products delivered using the Clearside Technology.
Now, therefore, in consideration of the mutual covenants and agreements provided herein below and other consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereby agree as follows:
ARTICLE 1
DEFINITIONS AND INTERPRETATION
| 1.1 | Definitions. Unless the context otherwise requires, the terms in this Agreement, when used with initial capital letters, shall have the meanings set forth below or at their first use in this Agreement: |
“Affiliate” means, with respect to a Party, any person, corporation, firm, joint venture or other entity which, directly or indirectly through one or more intermediaries, controls, is controlled by or is under common control with such Party. As used in this definition, “control” means the possession of the majority of the ownership, or the power to direct or cause the direction of the management and policies, of an entity, whether through the ownership of the outstanding voting securities thereof, by contract or otherwise. Notwithstanding the foregoing, CHOP shall be deemed to not be an Affiliate of Spark.
“Annual Net Sales” means, with respect to a particular calendar year, all Net Sales of a Licensed Product or multiple Licensed Products in the Field in the Territory during such calendar year.
“Bankruptcy Laws” is defined in Section 3.6.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
1
“CHOP” means The Children’s Hospital of Philadelphia.
“Clearside Background IP” means all Patents and Know-How relating to the Clearside Technology (a) that are Controlled by Clearside or its Affiliates as of the Effective Date, including the Patents set forth on Exhibit B or (b) that become Controlled by Clearside or its Affiliates on or after the Effective Date independent of the activities undertaken hereunder and that claim or embody Clearside Technology or improvements thereto. In no event shall the term “Clearside Background IP” include Patents or Know-How of any Person that becomes an Affiliate of Clearside after the Effective Date, provided that such excluded Patents and Know-How of such future Affiliate of Clearside claim and embody only technology that is conceived and reduced to practice independently from the Clearside Technology and do not comprise improvements thereto.
“Clearside Collaboration IP” is defined in Section 8.1(b)(i).
“Clearside IP” means Clearside Background IP, Clearside Collaboration IP, and Clearside’s interest in Joint Collaboration IP.
“Clearside Technology” is defined in the Preamble.
“Clinical Trial” means any study of a product in human subjects.
“Collaboration IP” means the Clearside Collaboration IP, the Spark Collaboration IP and the Joint Collaboration IP.
“Commercialization” means activities directed to marketing, promoting, distributing or selling a Licensed Product, including all activities directed to obtaining pricing approval in the Territory; and excluding Development, Manufacturing and supply of such product. “Commercialize” and “Commercializing” shall have their correlative meanings.
“Commercially Reasonable Efforts” means (a) with respect to the efforts to be expended by a Party with respect to an agreed objective, except as otherwise provided in clause (b), such reasonable, diligent and good faith efforts as such Party would normally use to accomplish a similar objective under similar circumstances, and (b) with respect to Spark’s obligations relating to the Development or Commercialization of Licensed Product(s) pursuant to Section 4.1 or Section 5.1, the efforts and resources normally used by a company in the biopharmaceutical industry for a product that is of similar market potential at a similar stage in its Development or product life, taking into account all relevant factors, including the potential profitability of the product, the costs and risks of Developing, Manufacturing and Commercializing the product, scientific, safety and regulatory concerns, product profile, the competitiveness of the marketplace and the proprietary position of the product. Commercially Reasonable Efforts under the foregoing clause (b) shall be determined on a country-by-country or market-by-market basis (as most applicable) for a Licensed Product, and it is anticipated that the level of effort will change over time, including to reflect changes in the status of the Licensed Product and the countries (or markets) involved.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
2
“Confidential Information” means any confidential information disclosed in any form whatsoever by one Party to the other Party, including the content of the transactions contemplated herein, all technology belonging to the disclosing Party and any improvements thereto, any information relating to a Party’s interests, business, finances, products, operations, sales, marketing, customers, suppliers and suppliers’ bills of materials, trade secrets, Know-How, data, processes, methods, techniques, formulas, test data, presentations, analyses, studies, patent applications (as long as unpublished and/or undisclosed), financial data, product development, assays, strategic and market research information, other relevant marketing information, clinical data and any other information, whether developed in connection with this Agreement or not.
“Control” means with respect to any Know-How, Patent or other tangible or intangible intellectual property right, the possession (whether by ownership or license, other than licenses granted pursuant to this Agreement) by a Party or its Affiliate of the ability to grant to the other Party access to, ownership of, or a license or sublicense under, such Know-How, Patent, or other intellectual property, in each case as provided under this Agreement, without violating the terms of any agreement or other arrangement with any Third Party.
“Development” means, with respect to a product, research (other than Research) and any and all processes and activities conducted to obtain and maintain Marketing Authorization for a product, including pre- and post-marketing approval clinical studies and activities relating to development or preparation of such product for Commercialization. Development includes performance of Clinical Trials. “Develop” and “Developing” shall have their correlative meanings.
“Dollar” or “$” means the legal currency of the United States.
“Early Stage Sublicense” means a sublicense under Clearside Background IP that includes the right to Commercialize in the Field a Licensed Product as to which Spark has not, prior to the grant of such sublicense, dosed an aggregate of ten (10) or more subjects in one or more clinical study(-ies) with the applicable Gene Therapeutic (i.e., the applicable combination of Vector and nucleic acid(s)).
“Early Stage Sublicense Revenue” shall mean, with respect to any Licensed Product that is the subject of an Early Stage Sublicense, the aggregate consideration received by Spark or its Affiliates in consideration for granting the Early Stage Sublicense with respect to such Licensed Product, including license fees, sales and other milestone fees and minimum royalties (in excess of earned royalties), but excluding (i) royalties on Net Sales, (ii) proceeds from the issuance of debt or equity securities of Spark or its Affiliates up to the fair market value of such securities at the time of their issuance to the Early Stage Sublicensee (with any excess proceeds over such fair market value to be included
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
3
in Early Stage Sublicense Revenue) and (iii) payments received by Spark or its Affiliates to fund or reimburse the actual costs of its research, development and similar services (including such amounts calculated on a commercially reasonable full-time equivalent, or FTE, rate basis, which FTE rates may include normal and customary allocations of overhead) for such Licensed Product performed for such Early Stage Sublicensee during the term of the Early Stage Sublicense.
“Early Stage Sublicensee” means any Third Party that is a sublicensee under an Early Stage Sublicense granted in accordance with Section 3.4(b).
“Emory License Agreement” means the License Agreement between Emory University, the Georgia Tech Research Corporation and Clearside, executed on July 4, 2012, as amended as of April 2, 2014, and otherwise from time to time.
“Field” means delivery of any ocular Gene Therapeutic for all ophthalmic therapeutic, diagnostic, prophylactic, palliative and veterinary purposes.
“First Commercial Sale” means, with respect to a Licensed Product and a country, the first sale of such Licensed Product in such country for use or consumption in commerce made by Spark, its Affiliates or sublicensees after all required Marketing Authorizations have been received from the applicable Regulatory Authority for such country. Sales for Clinical Trial purposes or compassionate, named patient or similar use shall not constitute a First Commercial Sale.
“Gene Therapeutic” means any product incorporating a Vector and a nucleic acid that confers a therapeutic benefit.
“Government Authority” means any multi-national, federal, state, local, municipal or other government authority of any nature (including any governmental division, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal).
“IND” means an Investigational New Drug application as defined in the U.S. Federal Food, Drug, and Cosmetic Act, as amended, and applicable regulations promulgated thereunder by the U.S Food and Drug Administration (“FDA”), or an equivalent application submitted to an equivalent Regulatory Authority in any other country or jurisdiction in the Territory, the filing of which is necessary to initiate Clinical Trials in such country or jurisdiction, including a clinical trial application.
“Invention” means any discovery, development, innovation, modification, update, enhancement, improvement or invention (whether or not patentable) that is conceived, made, developed or reduced to practice in activities undertaken under this Agreement.
“Joint Collaboration IP” is defined in Section 8.1(b)(iii).
“Joint Data” is defined in Section 8.1(b)(iii).
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
4
“Know-How” means any tangible and intangible information, data, results (including pharmacological, research and development data, reports and batch records), and materials, discoveries, improvements, inventions, compositions of matter, cell lines, assays, sequences, processes, methods, knowledge, protocols, formulas, utility, formulations, inventions (whether patentable or not), strategy, know-how and trade secrets, and all other scientific, pre-clinical, clinical, regulatory, manufacturing, marketing, financial and commercial information or data, in each case that either Party has treated as confidential or proprietary information.
“Law” means the applicable laws, rules and regulations, including any rules, regulations, guidelines or other requirements of any Government Authorities (including any Regulatory Authorities) that may be in effect from time to time in any country or jurisdiction of the Territory.
“Licensed Product” means any product that incorporates a Gene Therapeutic delivered with the use of a Microinjector.
“Major EU Country” means France, Germany, Italy, Spain or the United Kingdom.
“Manufacture” means activities directed to the manufacture, receipt, incoming inspections, storage and handling of raw materials and the manufacture, processing, formulation, packaging, labeling, warehousing, quality control testing (including in-process release and stability testing), supplying, shipping and release of a product, as the case may be and to the extent applicable, including manufacturing process development, scale-up and validation. “Manufacturing” shall have the correlative meaning.
“Marketing Authorization” means the technical, medical and scientific licenses, registrations, authorizations and approvals (including supplements and amendments, pre- and post-approvals, pricing approvals, and labeling approvals) of any Regulatory Authority necessary for the Commercialization of a product in the Field in such Regulatory Authority’s jurisdiction in the Territory.
“Microinjector” means any device containing a microneedle for injecting material into the suprachoroidal space that comprises Clearside Technology.
“Net Sales” of a Licensed Product in a particular period means the amount calculated by deducting from invoiced sales of such Licensed Product made by or on behalf of Spark or its Affiliates or sublicensees (other than Early Stage Sublicensees) (a “Selling Party”) to Third Parties for such period: (a) normal, customary trade discounts (including volume discounts), credits, chargebacks, reductions and rebates; (b) allowances and adjustments for rejections, recalls, outdated products or returns (in each event whether voluntary or required); (c) freight, shipping, insurance, sales, use, excise, value-added, consumption and similar tariffs, taxes or duties imposed on such sale; (d) credits actually given or allowances actually made for wastage replacement, Medicare/Medicaid or other governmental rebates, indigent patient, compassionate use and similar programs to
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
5
provide Licensed Product on at-cost (or lower) basis, to the extent actually deducted from the gross amount invoiced and either not required to be paid by or refunded to the customer or other payor; (e) annual fees due under Section 9008 of the United States Patient Protection and Affordable Care Act of 2010 (Pub. L. No. 111-48) allocable to sales of such Licensed Product; (f) compensation paid to Third Party distributors and wholesalers for maintaining agreed inventory levels; and (g) uncollectible amounts included in Net Sales on previously sold Licensed Products. Each of the foregoing deductions shall be determined on a basis consistent with the Selling Party’s audited consolidated financial statements and consistently applied across all products of the Selling Party. Even if there is overlap between any of deductions described in (a) through (g), each individual item shall only be deducted once in the overall Net Sales calculation.
Licensed Products transferred between Selling Parties and Early Stage Sublicensees shall not count toward Net Sales unless the Early Stage Sublicensee is an end-user of such Licensed Product.
In the event that a Licensed Product under this Agreement is sold in combination (a “Combination Product”) with active ingredient(s) other than Gene Therapeutics delivered via Microinjector (“Supplemental Ingredient(s)”), then “Net Sales” of the Combination Product shall be calculated using one of the following methods:
(x) By multiplying the Net Sales of the Combination Product (calculated prior to the application of this formula) by the fraction A/A+B, where A is the average gross selling price, during the applicable quarter in the country concerned, of the Licensed Product when sold separately, and B is the average gross selling price, during the applicable quarter in the country concerned, of the Supplemental Ingredient(s) when sold separately; or
(y) In the event that no such separate sales are made of the Licensed Product or any of Supplemental Ingredients in such Combination Product during the applicable quarter in the country concerned, Net Sales shall be calculated using the above formula where A is the reasonably estimated commercial value of the Licensed Product sold separately and B is the reasonably estimated commercial value of the Supplemental Ingredient(s) sold separately. Any such estimates shall be determined using criteria to be mutually agreed upon by the Parties. Such estimates shall be reported to Clearside in the reports to be provided pursuant to Section 7.7(b). If the Parties are unable to agree on the criteria for determining such estimates, either Party may submit such dispute for resolution pursuant to the provisions of Article 13.
For the avoidance of doubt, active ingredients that are Gene Therapeutics delivered via Microinjectors do not constitute Supplemental Ingredients, and no license is granted to Spark hereunder with respect to the use of Microinjectors to deliver active ingredients other than Gene Therapeutics.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
6
“New Microinjector” means a New Spark Microinjector, a New Clearside Microinjector or a Third Party-Funded New Microinjector, each as defined or described in Section 4.6.
“Option” is defined in Section 3.1.
“Option Exercise Date” is defined in Section 3.1.
“Option Exercise Period” means the Option Period #1 Exercise Period or the Option Period #2 Exercise Period, as applicable.
“Option Period #1” shall, unless otherwise mutually agreed by the Parties, be a period of six (6) months from the Effective Date, and is subject to extension pursuant to Section 2.4.
“Option Period #1 Exercise Period” means the period commencing upon the Effective Date and ending thirty (30) days after the later to occur of (i) Spark’s receipt of all data from the Research conducted pursuant to the Option Period #1 Workplan or (ii) the expiration of Option Period #1.
“Option Period #1 Workplan” means the initial Option Period #1 Workplan attached hereto as Exhibit A-1, as such workplan may be modified by the Parties in accordance with Section 2.2.
“Option Period #2” shall, unless otherwise mutually agreed by the Parties, be a period of twelve (12) months commencing upon Spark’s election to initiate Option Period #2 in accordance with Section 2.3, and is subject to extension pursuant to Section 2.4.
“Option Period #2 Exercise Period” means the period commencing upon Spark’s election to commence Option Period #2 in accordance with Section 2.3 and ending ninety (90) days after the later of (i) Spark’s receipt of all data from the Research conducted pursuant to the Option Period #2 Workplan or (ii) the expiration of Option Period #2 .
“Option Period #2 Workplan” means the initial Option Period #2 Workplan attached hereto as Exhibit A-2, as such workplan may be modified by the Parties in accordance with Section 2.3.
“Option Periods” means Option Period #1 and Option Period #2.
“Patent” means (a) any patent, re-examination, reissue, renewal, extension, supplementary protection certificate and term restoration, any confirmation patent or registration patent or patent of addition based on any such patent, (b) any pending application for patents, including provisional, converted provisional, continuations, continuations-in-part, divisional and substitute applications, and inventors’ certificates, (c) all foreign counterparts of any of the foregoing, and (d) all applications claiming priority to any of the foregoing.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
7
“Person” means any individual, incorporated or unincorporated organization or association, Government Authority, or other entity.
“Pivotal Clinical Study” means a well-controlled, randomized pivotal study in the Field in human patients of a Licensed Product designed to ascertain efficacy and safety of such Licensed Product for the purposes of enabling the preparation and submission of applications for Marketing Authorization to the competent Regulatory Authorities in a country of the Territory and that is adequate to satisfy the requirements of 21 C.F.R. § 312.21(c) or its equivalent in that country.
“Prosecution and Maintenance” means, with respect to a Patent, the preparing, filing, prosecuting and maintenance of such Patent, as well as re-examinations, reissues, requests for Patent term extensions and the like (including Supplementary Protection Certificates and other ex-US equivalents) with respect to such Patent, together with the conduct of interferences, the defense of oppositions and other similar post-grant proceedings with respect to the particular Patent; and “Prosecute and Maintain” shall have the correlative meaning.
“Regulatory Authority” means, in a particular country or jurisdiction in the Territory, any applicable Governmental Authority involved in granting (a) approval to initiate or conduct clinical testing in humans, (b) the authorizations, approvals, licenses, permits, consents, registrations and filings necessary for the Commercialization of a product in a country in the Territory including Marketing Authorizations and manufacturing licenses, or (c) to the extent required in such country or jurisdiction, pricing approval for a product in such country or jurisdiction.
“Regulatory Exclusivity” means, with respect to a Licensed Product and a country in the Territory, any exclusive marketing rights or data exclusivity rights conferred by a Regulatory Authority or other applicable Government Authority in such country, other than a Patent, including biological reference product exclusivity, orphan drug exclusivity, pediatric exclusivity and other relevant exclusivity rights, including those conferred in the European Union under Directive 2001/EC/83 and rights similar thereto in any country in the Territory.
“Regulatory Materials” means regulatory applications, submissions, notifications, registrations, Marketing Authorizations or other submissions made to or with a Regulatory Authority that are necessary or reasonably desirable in order to Develop, Manufacture or Commercialize the Licensed Products in the Field in a particular country.
“Research” means the activities to be performed by the Parties pursuant to the Workplans.
“Royalty Term” means, as to a Licensed Product and a country, the period commencing on the First Commercial Sale of such Licensed Product in such country and terminating upon the expiration of the last-to-expire Valid Claim included in the Clearside IP that
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
8
covers the manufacture, use, offer for sale or sale of the Microinjector contained in such Licensed Product in such country (including as such Microinjector is incorporated into such Licensed Product).
“Spark Background IP” means all Patents and Know-How relating to Gene Therapeutics that are Controlled by Spark or its Affiliates as of the Effective Date or become Controlled by Spark or its Affiliates on or after the Effective Date independent of the activities undertaken hereunder.
“Spark Collaboration IP” is defined in Section 8.1(b)(ii).
“Spark IP” means the Spark Background IP, the Spark Collaboration IP and Spark’s interest in the Joint Collaboration IP.
“Standard Microinjector” means a Microinjector meeting the technical specifications set forth on Exhibit D.
“Territory” means worldwide.
“Third Party” means any Person other than Clearside, Spark or any Affiliate of either Party.
“Valid Claim” means (a) an issued and unexpired claim of a Patent that has not been revoked or held unenforceable, unpatentable or invalid by a decision of a court or other governmental agency of competent jurisdiction, which is not appealable or has not been appealed within the time allowed for appeal, and which has not been abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination or disclaimer or otherwise, or (b) pending claim of a Patent that has been pending for less than five (5) years from the filing of the earliest patent application from which such pending claim derives priority and that has not been cancelled, withdrawn or abandoned or finally rejected.
“Vector” means a DNA molecule used to deliver foreign genetic material to a cell.
“Workplans” mean the Option Period #1 Workplan and, if applicable, the Option Period #2 Workplan.
| 1.2 | Interpretation. The captions and headings to this Agreement are for convenience only, and are to be of no force or effect in construing or interpreting any of the provisions of this Agreement. Unless specified to the contrary, references to Sections or Exhibits shall refer to the particular Sections or Exhibits of or to this Agreement and references to this Agreement include all Exhibits hereto. Unless context otherwise clearly requires, whenever used in this Agreement: |
(a) the words “include” or “including” shall be construed as incorporating, also, “but not limited to” or “without limitation;”
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
9
(b) the word “day,” “quarter” or “year” (and derivatives thereof, e.g., “quarterly”) shall mean a calendar day, calendar quarter or calendar year unless otherwise specified (and “annual” or “annually” refer to a calendar year);
(c) the word “notice” shall mean notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement;
(d) the word “hereof,” “herein,” “hereby” and derivative or similar word refers to this Agreement (including any Exhibits);
(e) the word “or” shall have its inclusive meaning identified with the phrase “and/or;”
(f) the words “will” and “shall” shall have the same obligatory meaning;
(g) provisions that require that a party or the parties hereunder “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise;
(h) words of any gender include the other gender; and
(i) words using the singular or plural number also include the plural or singular number, respectively.
ARTICLE 2
RESEARCH COLLABORATION
| 2.1 | Scope of Collaboration. Clearside and Spark shall, in accordance with the terms and conditions of this Agreement, collaborate during Option Period #1 and, if Spark elects to initiate Option Period #2 pursuant to Section 2.3 in Spark’s sole discretion, during Option Period #2, on research relating to the application of Clearside Technology to gene therapy, as further set forth in this Article 2. During Option Period #1 and, if Spark elects to initiate Option Period #2 pursuant to Section 2.3 in Spark’s sole discretion, during Option Period #2, Clearside shall not participate in any human clinical study, primate study or GLP safety study conducted hereunder associated with Gene Therapeutics beyond providing Standard Microinjectors for such studies. |
| 2.2 | Option Period #1. Clearside shall collaborate exclusively with Spark during Option Period #1 to conduct initial proof-of-principle experiments, including in vivo studies of Clearside Technology and its application to gene therapy, in accordance with the Option Period #1 Workplan. Following the conduct of initial experiments under the Option Period #1 Workplan, the Parties will agree in good faith on appropriate modifications, if any, to the Option Period #1 Workplan. Each Party shall use Commercially Reasonable Efforts to carry out the responsibilities under the Option Period #1 Workplan assigned to such Party. |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
10
| 2.3 | Option Period #2. During Option Period #1, the Parties shall agree on an initial workplan for follow-on studies consistent with Exhibit A-2 including a budget therefor (the “Option Period #2 Workplan”) that would (if Spark does not exercise the Option during the Option Period #1 Exercise Period and elects to initiate Option Period #2 as set forth below) be undertaken to establish the utility of Clearside Technology and its application to gene therapy. If Spark does not exercise the Option during the Option Period #1 Exercise Period, then, within thirty (30) days thereafter, Spark shall notify Clearside in writing as to whether or not Spark intends to initiate Option Period #2. If Spark elects to initiate Option Period #2, the Parties shall collaborate on the conduct of follow-on studies in accordance with the initial Option Period #2 Workplan and thereafter will agree in good faith on appropriate modifications, if any, to the Option Period #2 Workplan. If Option Period #2 is initiated by Spark as set forth above, each Party shall use Commercially Reasonable Efforts to carry out the responsibilities under the Option Period #2 Workplan assigned to such Party. |
| 2.4 | Option Period Extensions. Clearside will not unreasonably withhold its agreement to extensions of Option Period #1 or Option Period #2 to the extent such extensions are necessary to complete the scope of work contemplated in the Option Period #1 Workplan or the Option Period #2 Workplan, respectively. |
| 2.5. | Research Supplies; Costs. Spark shall provide Gene Therapeutics at no charge, and Clearside shall provide the Standard Microinjectors at no charge, in each case as needed for Research conducted as part of Option Period #1 and, if applicable, Option Period #2. All other expenses incurred in the conduct of the Research shall be borne by the Parties as set forth in Section 7.2. |
ARTICLE 3
OPTION AND LICENSES
| 3.1 | Option. Spark shall have the right to exercise an option to obtain the exclusive license set forth in Section 3.3(b) (the “Option”) at any time during the Option #1 Period Exercise Period or, if Spark initiates Option Period #2, during the Option #2 Period Exercise Period, in either case by providing written notice of exercise to Clearside within the applicable Option Exercise Period (the date of such notice, the “Option Exercise Date”). If Spark does not exercise the Option within the Option #1 Period Exercise Period and does not elect to initiate Option Period #2 in accordance with Section 2.3, or if Spark elects to initiate Option Period #2 in accordance with Section 2.3 and does not exercise the Option within the Option #2 Period Exercise Period, then this Agreement shall terminate at the end of the applicable Option Exercise Period in accordance with Section 10.1. |
| 3.2 | Grants to Spark. |
(a) Research License. Subject to the terms and conditions of this Agreement and, in the case of intellectual property rights licensed to Clearside pursuant to the Emory
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
11
License Agreement, the applicable terms of the Emory License Agreement set forth in Exhibit C, Clearside hereby grants Spark a royalty-free, non-exclusive license, without any right to grant sublicenses, under the Clearside IP solely to the extent necessary for Spark to perform activities allocated to Spark under the Workplans.
(b) Commercialization License. Subject to the terms and conditions of this Agreement and, in the case of intellectual property rights licensed to Clearside pursuant to the Emory License Agreement, the applicable terms of the Emory License Agreement set forth in Exhibit C, effective upon timely payment of the option exercise fee provided for in Section 7.3, Clearside hereby grants to Spark an exclusive license, with the right to grant sublicenses in accordance with Section 3.4, under the Clearside IP to Research, Develop, have Developed, Manufacture, have Manufactured, Commercialize and have Commercialized Licensed Products in the Field in the Territory, provided that Clearside shall retain the exclusive right to manufacture and supply to Spark the Microinjector component of Licensed Products subject to and in accordance with Article 6.
| 3.3 | Grant to Clearside. Subject to the terms and conditions of this Agreement, Spark hereby grants Clearside (i) a royalty-free, non-exclusive license, without any right to grant sublicenses, under the Spark IP solely to the extent necessary for Clearside to perform activities allocated to Clearside under the Workplans and (ii) a royalty-free, non-exclusive worldwide license outside the Field, with the right to grant sublicenses, under any Spark IP related to improvements to Clearside IP, in each case as necessary or helpful to research, Develop, have Developed, Manufacture, have Manufactured, Commercialize and have Commercialized products that incorporate Microinjectors. If Clearside requests a royalty-bearing, exclusive license outside the Field under Spark IP related to improvements to Clearside IP as described (other than its royalty-bearing and exclusive nature) in clause (ii) above, and Spark is willing in its discretion to grant such license, Clearside and Spark will negotiate a mutually acceptable royalty therefor and document such license and royalty in a separate agreement. |
| 3.4 | Sublicenses. |
(a) Spark-Developed Products. Spark may grant sublicenses (other than Early Stage Sublicenses) under the rights granted to it in Section 3.2(b); provided that each such sublicense is subject to the applicable terms of the Emory License Agreement set forth in Exhibit C.
(b) Early Stage Sublicenses. Spark shall notify Clearside in writing within thirty (30) days following Spark’s grant of any Early Stage Sublicense. Any such Early Stage Sublicense shall be subject to the applicable terms of the Emory License Agreement set forth in Exhibit C. If any Early Stage Sublicensee requires any customization, R&D, training, commercial support or other services to be provided by Clearside related to the Standard Microinjector, such services shall be subject to a separate services agreement with Clearside, which agreement shall include reimbursement of Clearside’s actual direct costs plus 50% and shall otherwise be made available by Clearside on reasonable terms.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
12
For clarification, Clearside shall use Commercially Reasonable Efforts to make such services available but shall have no obligation to render services that would require hiring or training additional staff or that would materially interfere with Clearside’s other business activities. In addition, any Standard Microinjector supply required by the Early Stage Sublicensee shall be provided pursuant to separate supply agreement between Clearside and the Sublicensee, royalties due Clearside shall be payable as set forth in Section 7.6(a) and Spark shall pay Clearside [***] of any Early Stage Sublicense Revenue received by Spark and its Affiliates from the Early Stage Sublicensee as set forth in greater detail in Section 7.10.
(c) Early Stage Sublicense Opportunities. If Clearside becomes aware that a Third Party desires to obtain an Early Stage Sublicense, Clearside shall provide Spark with written notice. Spark shall consider any such request in good faith and shall keep Clearside apprised of the status of discussions. When negotiations cease, Spark shall notify Clearside and advise Clearside the reason or reasons for the termination of negotiations.
| 3.5 | No Implied Rights. Except as specifically set forth in this Agreement, neither Party shall acquire any license, intellectual property interest or other rights, by implication or otherwise, in any Know-How disclosed to it under this Agreement or under any Patents Controlled by the other Party or its Affiliates. |
| 3.6 | Rights Upon Bankruptcy. All rights and licenses granted under or pursuant to this Agreement are, and shall otherwise be deemed to be, for purposes of Section 365(n) of Title 11 of the United States Code and other similar laws in any jurisdiction outside the U.S. (collectively, the “Bankruptcy Laws”), licenses of rights to “intellectual property” as defined under the Bankruptcy Laws. If a case is commenced during the term of this Agreement by or against a Party under Bankruptcy Laws then, unless and until this Agreement is rejected as provided in such Bankruptcy Laws, such Party (in any capacity, including debtor-in-possession) and its successors and assigns (including a trustee) shall perform all of the obligations provided in this Agreement to be performed by such Party. If a case is commenced during the term of this Agreement by or against a Party under the Bankruptcy Laws, this Agreement is rejected as provided in the Bankruptcy Laws and the other Party elects to retain its rights hereunder as provided in the Bankruptcy Laws, then the Party subject to such case under the Bankruptcy Laws (in any capacity, including debtor-in-possession) and its successors and assigns (including a Title 11 trustee), shall provide to the other Party copies of all information necessary for such other Party to prosecute, maintain and enjoy its rights under the terms of this Agreement promptly upon such other Party’s written request therefor. All rights, powers and remedies of the non-bankrupt Party as provided herein are in addition to and not in substitution for any and all other rights, powers and remedies now or hereafter existing at law or in equity (including the Bankruptcy Laws) in the event of the commencement of a case by or against a Party under the Bankruptcy Laws. |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
13
| 3.7 | Information Rights. During the Option Period, Clearside shall notify Spark within five (5) business days following each time that the cash Clearside has on hand falls below [***]. |
| 3.8 | Exclusivity. Notwithstanding that Clearside’s obligation to supply Microinjectors and related services to Spark, its Affiliates and sublicensees is limited to Standard Microinjectors and related services, Clearside and its Affiliates shall not, and shall not grant any license to or otherwise assist any Third Party, directly or indirectly, to research, develop, manufacture or commercialize any Microinjector in or for use in the Field. |
ARTICLE 4
DEVELOPMENT
| 4.1 | Development. |
(a) Diligence by Spark. At Spark’s sole discretion, Spark may Develop Licensed Products and shall be solely responsible to undertake Development of Licensed Products in the Field in the Territory; provided that Spark shall use Commercially Reasonable Efforts to (a) Develop a Licensed Product in the Field, and (b) seek Marketing Authorization in the United States and Europe for a Licensed Product in the Field. In addition, Spark shall conduct preclinical research with respect to Licensed Products directed to at least two (2) biological targets in the sixty (60) months following the Option Exercise Date. Clearside may provide Spark with written notice if Spark fails to conduct such preclinical research on [***] during such sixty (60) month period, in which event, if Spark does not commence such preclinical research within one hundred eighty (180) days after receipt of such notice, as Spark’s sole and exclusive liability and Clearside’s sole and exclusive remedy for such failure, Spark’s license under Section 3.2(b) shall thereafter be limited to Licensed Products directed to the biological target, if any, as to which Spark has conducted preclinical research as of the end of such one hundred eighty (180) day period. The foregoing due diligence obligations may be modified as provided for in Section 14.3(b).
| 4.2 | Regulatory Activities. |
(a) By Clearside. Clearside shall use all reasonable efforts to secure from Regulatory Authorities or other Government Authorities and maintain all licenses, waivers and approvals solely related to Standard Microinjectors (and any New Microinjectors supplied to Spark by Clearside) that are required under Law for Spark to fully exercise its rights hereunder to Develop, Manufacture or Commercialize Licensed Products in the Field in the United States and Europe and for Clearside to perform its manufacturing and supply obligations pursuant to Article 6. Without limiting the foregoing, as between Spark and Clearside, Clearside shall be solely responsible for obtaining and shall use all reasonable efforts to secure from all national, state and local Regulatory Authorities and other Government Authorities all approvals required for the Development, Manufacture and Commercialization of Standard Microinjectors (and any New Microinjectors
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
14
supplied to Spark by Clearside) for use with Gene Therapeutics, including such approvals from (i) institutional review boards, (ii) hospital formularies, (iii) pharmacy and therapeutics committees and (iv) other hospital governing bodies.
(b) By Spark. Except as otherwise set forth in Section 4.2(a), Spark shall prepare and file all INDs and applications and otherwise obtain and maintain approvals from Regulatory Authorities (including Marketing Authorizations) that are necessary for Development and Commercialization of the Licensed Products in the Field in the Territory, and otherwise interact with Regulatory Authorities as appropriate with respect to the Licensed Products. Spark will own all such INDs, applications and other Regulatory Materials for Licensed Products.
(c) Safety Information Regarding Microinjectors. Clearside shall promptly, and in all cases within timeframes that enable Spark to meet its safety reporting obligations to Regulatory Authorities, provide Spark with all adverse event and other material safety information relating to Standard Microinjectors (and any New Microinjectors supplied to Spark by Clearside) that is or becomes known to Clearside. Spark shall promptly, and in all cases within timeframes that enable Clearside to meet its safety reporting obligations to Regulatory Authorities, provide Clearside with all adverse event and other material safety information relating to Microinjectors using Clearside Technology that is or becomes known to Spark.
| 4.3 | Assistance by Clearside. Clearside shall assist Spark (including by taking actions or providing data, documents and other information in accordance with Spark’s reasonable request) as required by any of the following: (a) a Regulatory Authority, (b) an investigational review board, (c) a hospital formulary, (d) a pharmacy and therapeutics committee, or (e) other hospital governing authority, in each case for the use of the Clearside Technology for the conduct of Clinical Trials or Commercialization of Licensed Products in the Field. Clearside shall also provide any support reasonably requested by Spark in any FDA and EMA meetings and correspondence conducted pursuant to Section 4.2(b). |
| 4.4 | Progress Reports. Within sixty (60) days after the end of each June and December of each year prior to the First Commercial Sale of a Licensed Product in the Field in the United States or Europe, Spark shall provide to Clearside a reasonably detailed written report describing the progress made with respect to the Development of Licensed Products in the Field in the Territory and, if requested by Clearside, shall participate in a telephone conference with Clearside to discuss the contents of the report. Unless otherwise agreed, each such telephone conference shall include an officer with appropriate decision-making authority (e.g., the CEO or another officer who reports directly to the CEO) from each Party. |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
15
| 4.5 | Development Costs. |
(a) Except as otherwise set forth in Sections 2.5, 4.5(b) and 7.2(b), Spark shall bear all costs and expenses relating to its Development of Licensed Products in the Field.
(b) Except as otherwise set forth in Sections 2.5, 4.6(b) and 7.2(b), Clearside shall bear all costs and expenses relating to Development of Standard Microinjectors (and any New Microinjectors supplied to Spark by Clearside) for use in Licensed Products and obtaining necessary approvals from Regulatory Authorities to support the use, Manufacture and Commercialization of Standard Microinjectors (and any New Microinjectors supplied to Spark by Clearside) in connection with the Development and Commercialization of Licensed Products as set forth in Section 4.2(a).
| 4.6 | Reservation of Rights; New Microinjectors. |
(a) Clearside expressly reserves the right to research, Develop, and Commercialize Microinjectors for all purposes outside the Field.
(b) Spark shall not seek to develop a Microinjector incorporating any proprietary Clearside Technology without first presenting the proposed modification to Clearside for development as provided in this section. Any Microinjector incorporating any proprietary Clearside Technology developed based on Spark’s proposed modifications is referred to as a “New Spark Microinjector”. During the term of this Agreement, the Parties shall discuss in good faith whether the Parties wish to collaborate on the New Spark Microinjector and, if so, how to allocate development responsibilities and costs between the Parties and a supply price for the New Spark Microinjector. Such allocation shall be reflected in an agreed-upon written Workplan. Neither Party shall be bound to go forward with any new Workplan unless the new Workplan has been signed by an authorized signatory of each Party.
(c) If Clearside independently develops a Microinjector which is not a Standard Microinjector, a New Spark Microinjector or a Third Party-Funded New Microinjector (as described in the next paragraph) (such independently developed Microinjector, a “New Clearside Microinjector”), the license granted pursuant to Section 3.2(b) shall extend to the right to practice Clearside Technology incorporated into such New Clearside Microinjector.
(d) If Clearside develops any New Microinjector in a program funded by or otherwise the subject of a bona fide collaboration with one or more Third Parties, the resulting Microinjector shall be referred to as a “Third Party-Funded New Microinjector”. Clearside shall use Commercially Reasonable Efforts to acquire “Control” over any Patents or Know-How developed in such collaboration sufficient to enable Clearside to grant a license in the Field to Spark to practice such inventions. Clearside shall not, without Spark’s prior written consent, grant any sublicense to any Third Party under, use, incorporate or permit any Third Party to do any of the foregoing, any Joint Collaboration IP for use in or in connection with any Third Party-Funded New Microinjector if such Third Party does not grant Clearside rights sufficient to enable Clearside to permit Spark to Commercialize the Third Party-Funded New Microinjector in the Field in accordance with the terms of this Agreement.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
16
ARTICLE 5
COMMERCIALIZATION
| 5.1 | Licensed Products. Spark shall in its sole discretion determine whether or not to Commercialize any Licensed Product, and which, if any, Licensed Products to Commercialize; provided that, following receipt of Marketing Authorization for a Licensed Product in the Field in the United States or Europe, Spark shall use Commercially Reasonable Efforts to Commercialize such Licensed Product in the Field in the United States or Europe, respectively. |
| 5.2 | Pricing. Spark shall be solely responsible for determining the pricing of each Licensed Product in the Field in the Territory. |
ARTICLE 6
MANUFACTURE
| 6.1 | General. Spark shall retain all rights to Manufacture Licensed Products and Clearside shall retain all rights to Manufacture clinical and commercial supplies of Microinjectors that use Clearside Technology to be included in Licensed Products, subject to Section 6.3, and shall supply Spark’s clinical and commercial requirements of Standard Microinjectors. Clearside shall supply at its own expense all Standard Microinjectors required to perform the Research in accordance with the Workplans. In consideration of clinical and commercial supplies of Standard Microinjectors that are Manufactured by or on behalf of Clearside (after an initial 250 Standard Microinjectors to be provided by Clearside at no charge with respect to each Licensed Product during the post-Research Development period) and supplied to Spark, Spark shall pay Clearside [***] per Standard Microinjector, which price shall be included as term of the Supply Agreement described in Section 6.2 below. |
| 6.2 | Supply. All Manufacturing and supply by Clearside of Microinjectors using Clearside Technology for the clinical Development and Commercialization by Spark of Licensed Products shall be covered by a mutually acceptable supply agreement to include the terms set forth on Exhibit E and such other terms and conditions as are reasonable and customary for an agreement governing the Manufacturing and supply of a product similar to a Standard Microinjector to be entered into by the Parties concurrent with exercise by Spark of the Option or at such later date as may be agreed by Clearside and Spark, pursuant to which Clearside shall supply exclusively to Spark Spark’s requirements for clinical and commercial supply of the Standard Microinjector solely for inclusion in Licensed Products (the “Supply Agreement”). The Supply Agreement shall provide for authorization by Clearside, including any rights and licenses necessary, for Spark to |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
17
| obtain supply of Standard Microinjectors directly from Clearside’s Third Party contract manufacturer in the event that Clearside fails to supply as required thereunder and shall also include provisions that permit Spark to qualify another Third Party contract manufacturer designated by Spark and that is reasonably acceptable to Clearside as a second source manufacturer for Standard Microinjectors. Concurrently with the Supply Agreement, the Parties shall negotiate and enter into a quality agreement (the “Quality Agreement”). |
| 6.3 | Manufacturing Transfer. If the Parties fail to agree upon and execute the Supply Agreement within the time period therefor set forth in Section 6.2, or if a “Failure to Supply” occurs under the Supply Agreement (as defined therein), then Spark shall have the option of obtaining the right to have Manufactured clinical and commercial supplies of Standard Microinjectors (or any New Microinjectors supplied to Spark by Clearside prior to such Failure to Supply) solely for inclusion in Licensed Products upon written notice to Clearside. If Spark exercises such option, the Parties will promptly enter into a technology transfer agreement pursuant to which Clearside shall transfer to a Third Party contract manufacturer designated by Spark and reasonably acceptable to Clearside Clearside’s Know-How concerning the Manufacture of such Standard Microinjectors (or any New Microinjectors supplied to Spark by Clearside prior to such Failure to Supply), grant Spark an exclusive (in the Field), worldwide license under Clearside IP to have Manufactured such Microinjectors for inclusion in Licensed Products in the Field, and provide Spark with reasonable assistance in Spark’s preparations to have Manufactured such Microinjectors. Spark acknowledges that Manufacture of Licensed Product for sale in the United States may be required to take place in the United States to the extent required by the Emory License Agreement. Such technology transfer agreement shall include provisions under which Spark shall reimburse Clearside for its reasonable costs and expenses in conducting such technology transfer and assistance, which shall be Spark’s only payment obligations thereunder. In addition, such technology transfer agreement shall include reasonable provisions necessary for the protection of Clearside’s rights in the transferred Know-How. |
ARTICLE 7
PAYMENTS
| 7.1 | Initial Payments. |
(a) In consideration of the rights granted hereunder with respect to Option Period #1 , Spark shall reimburse Clearside for costs incurred in the development of Licensed Products by making an upfront option payment of Five Hundred Thousand Dollars ($500,000), which payment shall be payable by Spark within five (5) days after the Effective Date.
(b) If Spark elects to initiate Option Period #2 pursuant to Section 2.3, Spark shall pay Clearside an additional option payment of One Million Dollars ($1,000,000), which payment shall be payable by Spark within thirty (30) days after Spark’s notice that it is initiating Option Period #2.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
18
| 7.2 | Research and Development Expenses and Payments. |
(a) Each Party shall bear the expense of its own human resources necessary for the conduct of the Research during each Option Period.
(b) Subject to Sections 2.5 and 7.2(a), each Party shall share equally in the out-of-pocket cost of the studies conducted hereunder during each Option Period in accordance with the applicable Workplan and budget set forth therein, excluding the cost of Gene Therapeutics and Standard Microinjectors. Notwithstanding the foregoing, Clearside’s cost-sharing obligations (i) for vendors shall be limited to agreed-upon vendors associated with maximizing gene expression using suprachoroidal dosing, and (ii) for studies shall be limited to studies other than human clinical studies, primate studies and GLP safety studies associated with Gene Therapeutics. Following the Option Exercise Date, Clearside shall use Commercially Reasonable Efforts to make trained personnel available to assist with training professionals on the use of Standard Microinjectors as reasonably requested by Spark, subject to reimbursement of direct personnel costs plus 50% for personnel time following the training of the first five (5) retinal surgeons designated by Spark and out of pocket expenses. Clearside shall not invoice Spark for personnel time for (a) the training (at an ophthalmic medical conference or other location reasonably acceptable to Clearside) of the first five (5) retinal surgeons designated by Spark or (b) incidental calls and correspondence which calls and correspondence, in aggregate, do not exceed eight hours per month.
(c) All other costs relating to Development of Microinjectors and Licensed Products hereunder shall be borne by the Parties as set forth in Section 4.5.
| 7.3 | Option Exercise Payments. |
(a) If Spark exercises the Option during the Option Period #1 Exercise Period, Spark shall pay Clearside an option exercise payment of Two Million Dollars ($2,000,000), which payment is payable by Spark thirty (30) days after Spark’s notice that it is so exercising the Option. For clarity, if Spark exercises the Option during the Option Period #1 Exercise Period, Spark shall not be obligated to pay Clearside the additional option payment under Section 7.1(b).
(b) If Spark exercises the Option during the Option Period #2 Exercise Period, Spark shall pay Clearside an option exercise payment of Three Million Dollars ($3,000,000), which payment is payable by Spark thirty (30) days after Spark’s notice that it is so exercising the Option.
(c) For clarity, if Spark exercises the Option, only one of the two option exercise payments set forth in Sections 7.3(a) and 7.3(b) above shall become payable.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
19
| 7.4 | Development and Launch Milestones. Spark shall pay Clearside a milestone payment upon the first achievement with a Licensed Product by Spark, its Affiliate or a sublicensee (other than an Early Stage Sublicensee) of the applicable milestone event set forth in the table below. Each such milestone payment shall be paid no more than once and the maximum aggregate amount that can become payable under this Section 7.4 is $13,500,000. |
| Milestone Event (for a Licensed Product) |
Milestone Payment | |||
| [***] |
[*** | ] | ||
| [***] |
[*** | ] | ||
| [***] |
[*** | ] | ||
| [***] |
[*** | ] | ||
| 7.5 | Annual Net Sales Milestones. Spark shall pay Clearside the corresponding one-time milestone payment upon the first occurrence in a calendar year of Annual Net Sales for a Licensed Product or multiple Licensed Products achieving an Annual Net Sales milestone set forth in the table below. Each such milestone payment shall be paid no more than once and the maximum aggregate amount that can become payable under this Section 7.5 is $12,000,000. |
| Annual Net Sales Milestone |
Milestone Payment | |||
| [***] |
[*** | ] | ||
| [***] |
[*** | ] | ||
| [***] |
[*** | ] | ||
| 7.6 | Royalties. |
(a) Base Rates. Subject to Section 7.7, Spark shall pay to Clearside royalties on a Licensed Product-by-Licensed Product basis and country-by-country basis, in respect of Net Sales of Licensed Products in the Field in the Territory during the applicable Royalty Term, at a royalty rate of (a) [***] of Net Sales of the first Licensed Product to obtain Marketing Authorization in such country and (b) [***] of Net Sales of each subsequent Licensed Product to obtain Marketing Authorization in such country.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
20
(b) Bonus Royalty. In addition to the royalty under Section 7.6(a), subject to Section 7.7(b), Spark shall pay Clearside a bonus royalty of [***] of Net Sales of a Licensed Product in a country during any portion of the Royalty Term when, at the time of sale, the only Valid Claim(s) covering the manufacture, use, offer for sale or sale of such Licensed Product in such country are Valid Claim(s) in the Clearside Background IP, and such Licensed Product is not covered in such country by any Regulatory Exclusivity at the time of sale.
(c) Expiration of Royalty Term. Upon expiration (but not following earlier termination) of the Royalty Term for a Licensed Product in a country, all licenses granted to Spark hereunder with respect to such Licensed Product in such country shall become royalty-free, fully paid-up, perpetual, irrevocable and will survive any termination or expiration of this Agreement.
| 7.7 | Royalty Reductions. |
(a) Cost of Microinjectors. Spark may deduct from royalties payable to Clearside under Section 7.6 amounts paid to Clearside pursuant to Article 6 for Microinjectors included in the Licensed Products the Net Sales of which were used to calculate such royalties. For example, if Net Sales of the first Licensed Product in a given period during the applicable Royalty Term are $100,000 and the cost to Spark of the Microinjectors incorporated into such Licensed Product sold during such period is [***], Spark would pay Clearside royalties on such Net Sales equal to ($100,000 [***] minus the [***] paid for the Microinjector supply x the number of units sold), assuming Section 7.6(b) and the other reductions under this Section 7.7 are not applicable.
(b) Competitive Products. In the event a Third Party Gene Therapeutic product that uses a non-surgical delivery to the suprachoroidal space receives Marketing Authorization from the applicable Regulatory Authority in a country to treat the same indication as a Licensed Product, and such product competes with a Licensed Product in such country, then the additional royalty bonus of [***] described in Section 7.6(b), if otherwise applicable, shall cease to apply to Net Sales of such Licensed Product in such country and the royalty rate for Net Sales of such Licensed Product in such country shall thereafter be reduced by [***] from the applicable base royalty rate set forth in Section 7.6(a) (i.e., shall be reduced from [***] or from [***], as applicable).
(c) If Clearside elects not to take an Enforcement Action with respect to Competitive Infringement pursuant to Section 8.4(b), Spark’s obligation to pay royalties on Net Sales of the affected Licensed Product in such country shall be limited to that amount that Clearside owes to its upstream licensor(s) and which is attributable to such Net Sales as documented by Clearside to Spark’s reasonable satisfaction, not to exceed an aggregate of [***] of Net Sales.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
21
| 7.8 | Reports and Payments. |
(a) Research Expenses. Spark shall enter into services agreements and purchase orders with, and pay invoices from, Third Party contractors engaged to perform Research in accordance with the Workplans. Within thirty (30) days after the end of each quarter in which Spark has incurred out-of-pocket costs in connection with the Research that the Parties are required to share pursuant to Section 7.2(b), Spark shall submit to Clearside a statement detailing such costs and will prepare and provide to Clearside an invoice for Clearside’s share of such costs as contemplated under Section 7.2(b). Clearside shall pay such amount to Spark within thirty (30) days after the provision of such invoice, provided that in no event shall Clearside be obligated to pay more than the amount allocated to Clearside in a mutually agreed budget that is consistent with the Workplans and Section 7.2(b).
(b) Milestones. Spark shall promptly notify Clearside of the achievement of any milestone event for a Licensed Product in the Field achieved in accordance with Sections 7.4 and 7.5. Each milestone payment pursuant to Section 7.4 shall be due within thirty (30) days after achievement of the applicable milestone event, and each milestone payment under Section 7.5 shall be paid pursuant to Section 7.8(b) below concurrently with royalties for the quarter during which such milestone was achieved.
(c) Royalties. Within forty-five (45) days after the end of each quarter, Spark shall deliver to Clearside a report setting forth for such quarter the following information: (i) the Net Sales for Licensed Products, and the basis for the calculation of Net Sales; (ii) the applicable royalty rate; (iii) the royalty amount due hereunder for the sale of Licensed Products; and (iv) any Annual Net Sales milestone achieved during such quarter pursuant to Section 7.5. No such reports shall be due for any Licensed Product before the First Commercial Sale of a Licensed Product in the Territory. The total royalty and any Annual Net Sales milestone payment(s) due for the sale of Licensed Products and/or the achievement of Annual Net Sales milestone(s) during such quarter shall be remitted no later than forty-five (45) days after the end of each such quarter.
| 7.9 | Third Party Licenses for Microinjectors. |
(a) Clearside shall be responsible for all upfront payments, milestone payments, royalties or other payments due to the licensors under the Emory License Agreement and to any other licensor of any Clearside IP for rights to use the Standard Microinjector (and, subject to Section 7.9(c), any New Microinjector supplied to Spark by Clearside) in Licensed Products, whether the agreements with such licensors are entered into before or after the Option Exercise Date. For clarification, Clearside’s responsibility under this Section 7.9(a) shall not extend to rights held by any Third Party which rights are not necessary for the Commercialization of the Standard Microinjector (or any New Microinjector supplied to Spark by Clearside) contained in Licensed Products (including as such Microinjector is incorporated into Licensed Products, but excluding rights of any Third Party that are necessary based on specific Gene Therapeutics included in Licensed Products).
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
22
(b) If Spark believes that any intellectual property rights of a Third Party are necessary for the Commercialization of a Licensed Product incorporating a Standard Microinjector, then Spark shall promptly notify Clearside of such belief and the Parties shall discuss in good faith whether a license to such Third Party intellectual property is needed or advisable. If (a) Spark determines that a license from a Third Party is needed or advisable for the Commercialization of a Licensed Product incorporating a Standard Microinjector in the Territory, (b) Clearside does not enter into an appropriate license agreement for such technology within a reasonably period not to exceed one hundred eighty (180) days after Spark’s written request, (c) Spark enters into such a license agreement, and (d) upfront payments, milestone payments, royalties or other payments are owed to such Third Party for rights to incorporate a Standard Microinjector into a Licensed Product pursuant to the Third Party license, then Spark may deduct, on a country-by-country basis from royalties owed to Clearside under this Agreement one hundred percent (100%) of any such payment not made by Clearside to such Third Party licensor, provided that in no event shall the amount payable to Clearside for any calendar quarter be reduced by more than [***] by operation of this Section 7.9(b). For clarification, Clearside’s responsibility under this Section 7.9(b) shall not extend to rights held by any Third Party which rights are not necessary for the Commercialization of the Standard Microinjector contained in such Licensed Product (including as such Microinjector is incorporated into such Licensed Product, but excluding rights of any Third Party that are necessary based on the specific Gene Therapeutic included in such Licensed Product).
(c) If, during the Term, Clearside obtains Control in the Field over Third Party intellectual property rights that are necessary for the Commercialization of a New Microinjector supplied by Clearside to Spark, then Clearside shall notify Spark in writing and include in such notification a summary of such Third Party intellectual property rights, the commercial and sublicensing terms of the license and other relevant information. Spark will have ninety (90) days thereafter to notify Clearside of its desire to obtain a sublicense to such Third Party intellectual property rights. Upon receipt of such written notice from Spark, Clearside shall grant to Spark a sublicense of such Third Party property rights, which shall include terms that require payment by Spark of [***] of the royalties due to the Third Party attributable to the manufacture, use or sale of Licensed Products incorporating the New Microinjector by Spark, its sublicensees and their respective Affiliates as well as any terms that Clearside is required to impose on its sublicensees pursuant to the relevant in-license. If Spark believes that any intellectual property rights of a Third Party are necessary for the Commercialization of a Licensed Product incorporating a New Microinjector supplied by Clearside to Spark, then Spark shall promptly notify Clearside of such belief and the Parties shall discuss in good faith whether a license to such Third Party intellectual property is needed or advisable. If (a) Spark determines that a license from a Third Party is needed or advisable for the Commercialization of a Licensed Product incorporating the New Microinjector in the
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
23
Territory, (b) Clearside does not enter into an appropriate license agreement for such technology within a reasonably period not to exceed one hundred eighty (180) days after Spark’s written request, (c) Spark enters into such a license agreement, and (d) upfront payments, milestone payments, royalties or other payments are owed to such Third Party for rights to incorporate the New Microinjector into a Licensed Product pursuant to the Third Party license, then Spark may deduct, on a country-by-country basis from royalties owed to Clearside under this Agreement [***] of any such payment not made by Clearside to such Third Party licensor, provided that in no event shall the amount payable to Clearside for any calendar quarter be reduced by more than [***] by operation of this Section 7.9(c). For clarification, Clearside’s responsibility under this Section 7.9(c) shall not extend to rights held by any Third Party which rights are not necessary for the Commercialization of the New Microinjector contained in such Licensed Product (including as such Microinjector is incorporated into such Licensed Product, but excluding rights of any Third Party that are necessary based on the specific Gene Therapeutic included in such Licensed Product).
| 7.10 | Early Stage Sublicense Revenue. When Spark receives Early Stage Sublicense Revenue with respect to an Early Stage Sublicense, Spark will pay [***] of Early Stage Sublicense Revenue to Clearside; provided that in the event any such Sublicensee is the first to achieve any development or launch-based milestone event set forth in Section 7.4 above, Spark shall pay Clearside the greater of (i) [***] of Early Stage Sublicense Revenue received from such Sublicensee upon the occurrence of such milestone or (ii) the milestone payment set forth in Section 7.4; provided further that with regard to sales-based milestones received from such Sublicensee, Spark shall pay Clearside the greater of (i) [***] of such sales-based milestone fees received from Sublicensees or (ii) the aggregate sales-based milestone payments as set forth in Section 7.5 above for all sales-based milestone events achieved (based on the combined sales of Spark and all Sublicensees). |
| 7.11 | Payment Method; Late Payments. Payments hereunder shall be paid by wire transfer, or electronic funds transfer (EFT) in immediately available funds to a bank account designated by the receiving Party at least ten (10) days in advance of such payment. Royalties and other payments, including patent expense reimbursements, required to be paid by Spark pursuant to this Agreement shall, if overdue, bear interest until payment at a rate equal to the London Interbank Offered Rate plus two hundred basis points. The interest payment shall be due from the day the original payment was due until the day that the payment was received by Clearside. The payment of such interest shall not restrict Clearside from exercising any other rights it may have because any payment is overdue. |
| 7.12 | Currency. All amounts payable and calculations hereunder shall be in Dollars. Conversion of sales recorded in local currencies to Dollars will first be determined in the foreign currency of the country in which such Licensed Products are sold and then converted to Dollars at a ninety (90)-day trailing average published by the Wall Street Journal (U.S. editions) for conversion of the foreign currency into dollars on the last day of the quarter for which such payment is due. |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
24
| 7.13 | Taxes and Withholding. All payments due under this Agreement will be made without any deduction or withholding for or on account of any tax unless such deduction or withholding is required by Law to be assessed against the receiving Party. If the paying Party is so required to deduct or withhold, the paying Party will (a) promptly notify the receiving Party of such requirement, (b) pay to the relevant authorities the full amount required to be deducted or withheld promptly upon the earlier of determining that such deduction or withholding is required or receiving notice that such amount has been assessed against the receiving Party, and (c) promptly forward to the receiving Party an official receipt (or certified copy) or other documentation reasonably acceptable to the receiving Party, to the extent available, evidencing such payment to such authorities. Spark shall reasonably cooperate with Clearside in any lawful action to claim exemption from such deductions or withholdings and otherwise to minimize the amount required to be so withheld or deducted. |
| 7.14 | Maintenance of Records. Each Party shall keep accurate books and accounts of record in connection with the calculation of payments to be made by such Party under this Agreement in sufficient detail to permit accurate determination of all figures necessary for verification of payments to be paid under this Agreement. Each Party shall maintain such records for a period of at least five (5) years after the end of the year in which they were generated or longer if and to the extent required by applicable law or regulation |
| 7.15 | Audits. Each Party shall have the right, at its own expense and no more than once per year, to have an independent, certified public accountant of national standing, selected by such Party and reasonably acceptable to the other Party, review all records maintained in accordance with Section 7.14 upon reasonable notice and during regular business hours and under obligations of strict confidence, for the sole purpose of verifying the basis and accuracy of payments required and made under this Agreement within the prior thirty six (36) month period. No quarter may be audited more than one time. The audited Party shall receive a copy of each audit report promptly from the auditing Party. Should the inspection lead to the discovery of a discrepancy to the auditing Party’s detriment, the audited Party shall pay the amount of the discrepancy in the audited Party’s favor within thirty (30) days after being notified thereof. The auditing Party shall pay the full cost of the inspection unless the discrepancy is greater than ten percent (10%) of the amount paid for the applicable year that is the subject of such inspection, in which case the audited Party shall pay to the auditing Party the reasonable and documented cost charged by such accountant for such inspection. If such audit shows a discrepancy in the auditing Party’s favor, then the auditing Party shall pay the audited Party the amount of the discrepancy within thirty (30) days after being notified thereof. |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
25
ARTICLE 8
INTELLECTUAL PROPERTY
| 8.1 | Ownership. |
(a) Background IP. As between the Parties, Clearside shall solely own the Clearside Background IP, and Spark shall solely own the Spark Background IP.
(b) Inventions.
(i) Any Invention arising from the Research that is solely invented by or on behalf of Clearside or its Affiliates (“Clearside Collaboration IP”) shall be solely owned by Clearside. Spark shall assign and transfer, and hereby assigns and transfers, to Clearside, without further consideration, Spark’s entire right title and interest in and to any such Clearside Collaboration IP.
(ii) Any Invention arising from the Research that is solely invented by or on behalf of Spark or its Affiliates and all data arising from the Research whether generated by or on behalf of Clearside or its Affiliates or by or on behalf of Spark or its Affiliates and all data arising from the Research other than Joint Data (as defined in Section 8.1(b)(iii)) (such inventions and data, the “Spark Collaboration IP”) shall be owned by Spark. Clearside shall assign and transfer, and hereby assigns and transfers, to Spark, without further consideration, Clearside’s entire right title and interest in and to any such Spark Collaboration IP.
(iii) Any (a) Inventions that are jointly invented by or on behalf of Spark or its Affiliates, on the one hand, and by or on behalf of Clearside or its Affiliates, on the other hand and (b) data arising from the portion of the Research that Clearside co-funds pursuant to Section 7.2(b) (such data, the “Joint Data”) (such Inventions and Joint Data, the “Joint Collaboration IP”) shall be jointly owned. Each Party shall assign and transfer, and hereby assigns and transfers, to the other Party, without further consideration, sufficient of its right title and interest in and to Joint Collaboration IP such that each Party has one-half of an undivided interest in the whole of such Joint Collaboration IP. Each Party shall have the right to freely exploit and license its interest in Joint Collaboration IP, without any duty to account or obtain consent from the other Party for such exploitation and licensing.
(iv) Each Party shall promptly and fully disclose to the other Party any and all Inventions to the extent related to Licensed Product made by its employees, agents, consultants or sub-contractors. In order to effect the intent of Sections 8.1(b)(i), 8.1(b)(ii) and 8.1(b)(iii), each Party shall ensure and hereby represents and warrants that all Persons performing Research or Development hereunder (1) have in writing assigned to such Party all right, title and interest in and to
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
26
Inventions, including all Intellectual Property in Inventions conceived by such persons; and (2) have agreed in writing to assist such Party in the same manner that such Party shall assist the other Party as set forth in this Section 8.1(b)(iv).
(v) Inventorship as to Inventions shall be determined in accordance with applicable United States Law.
| 8.2 | Prosecution and Maintenance of Patents. |
(a) Subject to Section 8.2(b), each Party shall have the right, but not the obligation, at its sole expense to Prosecute and Maintain Patents solely owned by such Party in accordance with Section 8.1(a), Section 8.1(b)(i) or Section 8.1(b)(ii), including, except as otherwise set forth in Section 8.2(e), filing and pursuing any valid request for a patent term adjustment or extension.
(b) Clearside shall keep Spark apprised of the status of each Patent application and Patent within the Clearside Collaboration IP and shall seek the advice of Spark with respect to patent strategy and draft patent applications and shall give reasonable consideration to any suggestions or recommendations promptly provided by Spark concerning the preparation, filing, Prosecution and Maintenance thereof. Clearside shall cooperate reasonably in the prosecution of all such Patent applications and Patents within the Clearside Collaboration IP and shall share all material information relating thereto promptly after receipt of such information. If, during the term of this Agreement, Clearside intends to allow any Patent or Patent application within the Clearside Collaboration IP to lapse or become abandoned without having first filed a substitute, (e.g., a continuation, continuation-in-part, or divisional application), Clearside shall notify Spark of such intention at least sixty (60) days prior to the date upon which such Patent application or Patent shall lapse or become abandoned, and Spark shall thereupon have the right, but not the obligation, to assume responsibility for the Prosecution and Maintenance thereof at its own expense.
(c) In the event, as to a Patent solely owned by a Party in accordance with Section 8.1(b)(i) or Section 8.1(b)(ii), such Party is unable for any reason to secure the signature of the relevant other Party’s employees to any document required to file, prosecute, register, or memorialize the assignment, the other Party does hereby irrevocably designate and appoint such Party and such Party’s duly authorized officers and agents as such other Party’s agents and attorneys-in-fact to act for and on such other Party’s behalf and instead for such Party to do all lawfully permitted acts to further the Prosecution and Maintenance of Clearside Collaboration IP or Spark Collaboration IP, as applicable, all with the same legal force and effect as if executed by such other Party.
(d) Subject to Sections 8.2(b) and 8.2(e), unless otherwise agreed, Spark shall be responsible for Prosecuting and Maintaining Patents within the Joint Collaboration IP and the Parties shall each pay fifty percent (50%) of Spark’s out-of-pocket costs incurred for such Prosecution and Maintenance.
(e) For clarity, Spark shall have the right to file and pursue any valid request for a patent term adjustment or extension as to any Patent within the Spark IP, the Clearside Collaboration IP or the Joint Collaboration IP in connection with any Marketing Authorization of a Licensed Product hereunder.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
27
| 8.3 | Defense of Third Party Infringement Claims. Subject to the Parties’ respective indemnification rights and obligations pursuant to Article 12, if a Licensed Product becomes the subject of a Third Party’s claim or assertion of infringement of a Patent relating to Development, Manufacture or Commercialization of the Licensed Product in the Field in the Territory (each, an “Infringement Claim”), the Party first having notice of the claim or assertion shall promptly notify the other Party, and the Parties shall promptly confer to consider the claim or assertion and the appropriate course of action. Unless the Parties otherwise agree in writing, Spark shall have the right to defend any Infringement Claim, and Clearside shall reasonably assist Spark and cooperate in any such litigation at Spark’s request and expense. Spark shall keep Clearside reasonably informed with respect to the progress of any such litigation. Spark shall not enter into any settlement of any claim described in this Section 8.3 that adversely affects Clearside’s rights and interests without Clearside’s written consent, which consent shall not be unreasonably conditioned, withheld or delayed. |
| 8.4 | Enforcement; Patent Challenges. |
(a) If a Party reasonably believes that any Patent covering a Licensed Product is being infringed by a Third Party in the Field (including through notification of a Paragraph IV certification) (“Third Party Infringement”) or is subject to a declaratory judgment action arising from such infringement (“Declaratory Judgment Action”) or becomes aware of any actual or threatened challenge by a Third Party with respect to the scope, validity or enforceability of any such Patent in the Territory, whether through opposition, inter partes dispute or otherwise (“Third Party Challenge”), then such Party shall promptly notify the other Party.
(b) If any Patent included in the Clearside Background IP is implicated by Third Party Infringement, a Declaratory Judgment Action or a Third Party Challenge, Clearside shall have the sole right (but not the obligation) to enforce such Patent with respect to such Third Party Infringement and to defend any such Declaratory Judgment Action or Third Party Challenge as to such Patent (each, an “Enforcement Action”), at its sole expense. Clearside shall consult with Spark and shall reasonably consider Spark’s views regarding the desirability and conduct of any such Enforcement Action. If Clearside does not take an Enforcement Action against any Third Party that is manufacturing or commercializing a Gene Therapeutic product in the Field in a country that competes or is likely to compete with a Licensed Product in the Field in such country (“Competitive Infringement”) within sixty (60) days after Spark has notified Clearside of the Third Party Infringement, Declaratory Judgment Action or Third Party Challenge and requested that Clearside bring such Enforcement Action, then Spark’s obligation to pay royalties thereafter on Net Sales of such Licensed Product shall be limited as set forth in Section 7.7(c).
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
28
If Clearside determines not to undertake a proposed Enforcement Action with respect to Competitive Infringement for purely financial reasons (i.e., that anticipated costs do not justify potential returns to Clearside), Clearside shall grant Spark the right to undertake the Enforcement Action. However, Clearside reserves the right in its sole discretion to not pursue or permit any Enforcement Action if such action could, in Clearside’s determination, jeopardize Clearside’s interests outside the Field.
(c) If any Patent included in the Collaboration IP is implicated by the Third Party Infringement, Declaratory Judgment Action or Third Party Challenge, Spark shall have the sole right (but not the obligation) to take an Enforcement Action as to such Patent, at its sole expense. If Spark determines not to undertake a proposed Enforcement Action with respect to Collaboration IP for purely financial reasons (i.e., that anticipated costs do not justify potential returns to Spark), Spark shall grant Clearside the right to undertake the Enforcement Action. However, Spark reserves the right in its sole discretion to not pursue or permit any Enforcement Action if such action could, in Spark’s determination, jeopardize Spark’s interests in the Field.
(d) Each Party shall reasonably cooperate, at the other Party’s expense, with the Party taking an Enforcement Action including joining as a party to such Enforcement Action as may be necessary or desirable for purposes of standing or establishing damages.
| 8.5 | Recoveries. Any recovery received as a result of any Enforcement Action pursuant to Section 8.4(b) shall be used first to reimburse the Party taking the Enforcement Action for the costs and expenses (including attorneys’ and professional fees) incurred in connection with such Enforcement Action, then any amount payable to Clearside’s licensor(s) under the Emory License Agreement based on such recovery shall be paid to such licensor(s), and finally [***] of the remainder of the recovery shall be retained by or paid to Spark and the remaining [***] shall be retained by or paid to Clearside. Any recovery received as a result of any Enforcement Action pursuant to Section 8.4(c) shall be used first to reimburse the Party taking the Enforcement Action for the costs and expenses (including attorneys’ and professional fees) incurred in connection with such Enforcement Action, and then [***] of the remainder of the recovery shall be retained by or paid to Spark and the remaining [***] shall be retained by or paid to Clearside. |
| 8.6 | ▇▇▇▇▇-▇▇▇▇▇▇ Act Litigation. Notwithstanding anything herein to the contrary, should a Party receive a certification as to a Licensed Product pursuant to the Drug Price Competition and Patent Term Restoration Act of 1984 (Public Law 98-417), as amended (the “▇▇▇▇▇-▇▇▇▇▇▇ Act”), or its equivalent with respect to biologics in the United States or in a country other than the United States, then such Party shall immediately provide the other Party with a copy of such certification. The Party with the right to take an Enforcement Action pursuant to Section 8.4 with respect to the applicable Patent(s) shall, within thirty (30) days from the date on which it receives or provides a copy of such certification provide written notice to the other Party (“H-W Suit Notice”) stating whether it will bring suit, at its expense, within a forty-five (45) day period from the date of such certification, and if not, whether the applicable step-in right of the other Party pursuant to Section 8.4(b) or 8.4(c) will apply (i.e., whether such Party is exercising its right pursuant to such Section not to permit the other Party to exercise such step-in right). |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
29
ARTICLE 9
CONFIDENTIALITY
| 9.1 | Confidentiality; Exceptions. Except to the extent expressly authorized by this Agreement or otherwise agreed by the Parties in writing, during the term of this Agreement and for ten (10) years thereafter, the Parties agree that the receiving Party shall keep confidential and shall not publish or otherwise disclose or use for any purpose other than as provided for in this Agreement any Confidential Information furnished to it by the other Party pursuant to this Agreement. For clarity, Confidential Information of a Party shall include all information and materials disclosed by such Party or its designee that (x) if disclosed in writing or other tangible form, is marked as “Confidential,” “Proprietary” or with similar designation at the time of disclosure, (y) if disclosed verbally or in other intangible form, is indicated upon first disclosure as being confidential or (z) by its nature can reasonably be expected to be considered Confidential Information by the recipient. Notwithstanding the foregoing, Confidential Information shall not be deemed to include information or materials to the extent that it can be established by written documentation by the receiving Party that such information or material: |
(a) was already known to or possessed by the receiving Party, other than under an obligation of confidentiality (except to the extent such obligation has expired or an exception is applicable under the relevant agreement pursuant to which such obligation established), at the time of disclosure;
(b) was generally available to the public or otherwise part of the public domain at the time of its first disclosure to the receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement;
(d) was independently developed by the receiving Party as demonstrated by documented evidence prepared contemporaneously with such independent development; or
(e) was disclosed to the receiving Party, other than under an obligation of confidentiality, by a Third Party who had no obligation to the disclosing Party not to disclose such information to others.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
30
| 9.2 | Authorized Use and Disclosure. Each Party may use and disclose Confidential Information of the other Party as follows: |
(a) under appropriate confidentiality provisions substantially equivalent to those in this Agreement, in connection with the performance of its obligations or exercise of rights granted to such Party in this Agreement; and
(b) to the extent such disclosure is reasonably necessary in Prosecuting and Maintaining Patents, copyrights and trademarks (including applications therefor) in accordance with this Agreement, prosecuting or defending litigation, complying with applicable governmental regulations, filing for, conducting Development hereunder, obtaining and maintaining Marketing Authorizations, or otherwise required by Law, the rules of a recognized stock exchange or automated quotation system applicable to such Party; provided, however, that if a Party is required by Law, the rules of a recognized stock exchange or automated quotation system (collectively, “Securities Laws”) applicable to such Party to make any such disclosure of the other Party’s Confidential Information it will, except where prohibited by Law or impracticable for necessary disclosures (for example, in the event of medical emergency), give reasonable advance notice to the other Party of such disclosure requirement and, where practicable, will use its reasonable efforts to secure confidential treatment of such Confidential Information required to be disclosed.
| 9.3 | Injunctive Relief. Given the nature of the Confidential Information and the competitive damage that would result to a Party upon unauthorized disclosure, use or transfer of its Confidential Information to any Third Party, the Parties agree that monetary damages may not be a sufficient remedy for any breach of this Article 9. In addition to all other remedies, a disclosing Party shall be entitled to seek specific performance and injunctive and other equitable relief as a remedy for any breach or threatened breach of this Article 9. The receiving Party waives its right to have bond posted for the injunctive relief in a court of law. |
| 9.4 | Terms of Agreement. |
(a) The Parties shall treat the existence and material terms of this Agreement as confidential and shall not disclose such information to Third Parties without the prior written consent of the other Parties or except as provided in Section 9.2 or Section 9.4(b) below. With respect to complying with the disclosure requirements of Securities Laws applicable to a Party, the Parties shall consult with each other concerning which terms of this Agreement shall be requested to be redacted in any public disclosure of the Agreement by the agency, and each Party shall seek confidential treatment, to the extent available, from the agency in public disclosure of the Agreement for all sensitive commercial, financial and technical information, including any dollar amounts set forth herein.
(b) Either Party may disclose to bona fide potential investors, lenders and acquirors/acquirees, and to such Party’s consultants and advisors, the existence and terms of this Agreement to the extent necessary in connection with a proposed equity or debt financing of such Party, or a proposed acquisition or business combination, or to bona fide potential sublicensees, so long as such recipients are bound in writing to maintain the confidentiality of such information in accordance with the terms of this Agreement.
(c) Clearside may provide, subject to confidentiality obligations, a copy of this Agreement (and any Sublicense) to the licensors under the Emory License Agreement as required by the terms of such Agreement.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
31
| 9.5 | Publications. During the Option Periods, neither party nor its Affiliates shall publish or publicly disclose the results generated in the course of performing any of the Research without the prior written consent of the other party, which shall not be unreasonably withheld. After Spark’s exercise of the Option, Spark and its Affiliates shall have the right to publish or publicly disclose the results generated in the course of performing any of the Research, provided that Spark submits the proposed publication or disclosure to Clearside for its review at least thirty (30) days prior to the scheduled submission of such proposed publication or public disclosure (including, without limitation, to any journal for review). If, during its thirty (30) day review period, Clearside notifies Spark that it desires changes to the publication or public disclosure reasonably necessary to protect proprietary information of Clearside specifically related to Microinjectors or Clearside’s Background IP, Spark shall use reasonable efforts to accommodate such request. Following Spark’s exercise of the Option, all publications and presentations of the results generated in the course of performing the Research shall be made in a manner and have content consistent with the publication strategy developed by Spark and Clearside shall not publish or publicly disclose the results generated in the course of performing any of the Research without the prior written consent of Spark. If Spark does not exercise the Option, Clearside shall thereafter have the right to publish or publicly disclose the results generated in the course of performing any of the Research, provided that such publications do not disclose the identity of any Gene Therapeutic or information from which the identity of any Gene Therapeutic can be deduced. |
| 9.6 | Publicity; Press Releases. |
(a) The Parties shall issue the initial press release set forth on Exhibit F hereto following the Effective Date.
(b) Except as otherwise mutually agreed by the Parties or as required by Law or the rules of any stock exchange, no Party shall issue or cause the publication of any other press release or public announcement regarding the terms of this Agreement without the express prior approval of the other Party, which approval shall not be unreasonably withheld or delayed, provided that if any such publication, press release or public announcement is required by Law, the Party obligated to make such publication, press release or public announcement shall, if practicable, notify the other Party in advance thereof and reasonably consider any timely comments from such other Party, including any reasonable request to limit such publication, press release or public announcement. Without limiting the generality of the foregoing, the achievement of an event giving rise to a payment obligation under Section 7.3, 7.4 or 7.5 shall be deemed to be an event required to be disclosed pursuant to Securities Laws if so determined by either Party.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
32
ARTICLE 10
TERM AND TERMINATION
| 10.1 | Term. This Agreement is effective as of the Effective Date and, following the Option Exercise Date, shall continue in full force and effect unless earlier terminated by a Party in accordance with Section 10.2 and shall expire on a Licensed Product-by-Licensed Product and country-by-country basis upon the expiration of the Royalty Term with respect to such Licensed Product in such country. Notwithstanding the foregoing, only the following provisions of this Agreement shall be in effect from and after the Effective Date and prior to the Option Exercise Date: Articles 1, 2, 9, 11, 12, 13 and 14, and Sections 3.2(a), 3.3, 3.5, 7.1, 7.2, 7.8-7.14 (solely as applicable to amounts payable prior to the Option Exercise Date), 8.1, 8.2, 10.1 and 10.2. If Spark does not exercise the Option as provided in Section 2.1, this Agreement shall terminate upon expiration of the last applicable Option Exercise Period. |
| 10.2 | Termination. |
(a) Convenience. At any time following the Option Exercise Date, Spark may terminate this Agreement in its entirety or with respect to any Licensed Product or country upon written notice to Clearside, for any reason or for no reason, without liability to Clearside.
(b) Material Breach. Subject to Section 4.1, either Party may terminate the Agreement, on a Licensed Product-by-Licensed Product, at any time upon an uncured material breach by the other Party of its obligations hereunder with respect to such Licensed Product by giving written notice to the other Party specifying the nature of the material breach not less than ninety (90) days prior to the date the non-breaching Party intends to terminate the Agreement. If such material breach has been cured by such breaching Party within such ninety (90) day period, no such termination shall occur. If such material breach has not been cured by the breaching Party within such ninety (90) day period, then the non-breaching Party shall be entitled to terminate this Agreement with respect to such Licensed Product with immediate effect upon delivery to the breaching Party of a written notice terminating the Agreement; provided, however, that if the Party accused of materially beaching notifies the accusing Party in writing (i) within such ninety (90) day cure period, that the accused Party disputes that it is in material breach, or (ii) within thirty (30) days after delivery of a termination notice for failure to cure a material breach, that the accused Party contends it cured such material breach, then in either such case no such termination shall become effective until (1) a final, binding determination pursuant to Article 13 that the accused Party was in material breach and failed to cure such material breach during the ninety (90) day cure period, and (2) the accusing Party’s delivery to the accused Party, after such determination, of a written notice terminating the Agreement with respect to the applicable Licensed Product(s).
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
33
Any breach involving a disputed failure to make a payment when due may be cured by the breaching Party by paying such amount within fifteen (15) days following final resolution of such dispute.
(c) Bankruptcy. Either Party may terminate the Agreement if the other Party makes a voluntary or involuntary general assignment of its assets for the benefit of creditors, a petition in bankruptcy is filed by or against the other Party and is not dismissed in ninety (90) days, or a receiver or trustee is appointed for all or any part of the other Party’s property.
| 10.3 | Consequences of Termination. |
(a) Accrued Obligations. Expiration or termination of this Agreement for any reason shall not release any Party of any obligation or liability which, at the time of such expiration or termination, has already accrued or which is attributable to a period prior to such expiration or termination. Upon expiration or earlier termination of this Agreement (including due to Spark not exercising the Option). Clearside shall have a royalty-free, fully paid-up, perpetual, irrevocable, transferable and sublicensable license to all of Spark’s interest in Collaboration IP to the extent necessary or useful for the commercialization of products incorporating Microinjectors, but excluding any such rights relating to any specific Gene Therapeutic.
(b) Ancillary Agreements. Unless otherwise agreed in writing by the Parties, the termination of this Agreement shall cause the automatic termination of the Supply Agreement and the Quality Agreement, to the extent such agreement(s) are in force as of the termination of this Agreement, with respect to the terminated Licensed Product(s).
(c) Non-Exclusive Remedy. Notwithstanding anything herein to the contrary, but subject to Section 4.1, termination of this Agreement by a Party shall be without prejudice to other remedies such Party may have at law or equity.
(d) Survival. The following provisions shall survive expiration or termination of this Agreement and continue to be enforceable: Section 3.3(ii), Section 7.6(c) (Expiration of Royalty Term), Article 9 (Confidentiality), Section 11.3 (Disclaimer), Article 12 (Indemnification, Insurance and Liability), Article 13 (Dispute Resolution), and Article 14 (Miscellaneous); and Sections 8.1 (Ownership) and 10.3 (Consequences of Termination).
ARTICLE 11
REPRESENTATIONS AND WARRANTIES
| 11.1 | Representations, Warranties and Covenants By Both Parties. Each Party hereby severally represents, warrants and covenants to the other Party as of the Effective Date: |
(a) it is duly organized and validly existing under the laws of the jurisdiction of its incorporation or continuance, as the case may be, and has full corporate power and authority to enter into this Agreement and to carry out the provisions hereof;
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
34
(b) it is duly authorized to execute and deliver this Agreement and to perform its obligations hereunder, and the individual executing this Agreement on its behalf has been duly authorized to do so by all requisite corporate action;
(c) this Agreement is legally binding upon it and enforceable in accordance with its terms;
(d) the execution, delivery and performance of this Agreement by it does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, nor violate any material Law;
(e) it has not granted, and shall not grant during the Term, any right to any Third Party which would conflict with the rights granted to the other Party hereunder;
(f) it is not aware of any action, suit or inquiry or investigation instituted by any Person which questions or threatens the validity of this Agreement; and
(g) no consent or approval from any Third Party (including any governmental or administrative body or court) is necessary to consummate this Agreement or, to its knowledge, to conduct the activities contemplated hereunder.
| 11.2 | Clearside Representations, Warranties and Covenants. Clearside hereby represents and warrants that as of the Effective Date: |
(a) it shall perform the activities allocated to it under the Workplans in a timely and professional manner, with due care and in accordance with industry standards;
(b) it has full legal rights and authority to grant the licenses and rights under the Clearside Background IP granted under this Agreement and has not assigned, transferred, conveyed or licensed its right, title and interest in the Clearside Background IP in any manner inconsistent with such license grant or the other terms of this Agreement;
(c) there is no pending litigation or written threat of litigation that has been received by Clearside (and has not been resolved by taking a license or otherwise), which alleges that Clearside’s activities with respect to the Clearside Background IP have infringed or misappropriated any of the intellectual property rights of any Third Party;
(d) the performance by Clearside of the activities allocated to it under the Workplans and its other obligations under this Agreement shall not infringe or otherwise violate the intellectual property rights of any Third Party related to Standard Microinjectors; and
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
35
(e) neither Clearside nor any of its Affiliates, nor, to its knowledge, any other Person that will be involved in activities under this Agreement has been debarred or is subject to debarment, and neither Clearside nor any of its Affiliates will knowingly use in any capacity, in connection with this Agreement, any Person who has been debarred pursuant to Section 306 of the United States Federal Food, Drug, and Cosmetic Act, or who is the subject of a conviction described in such section. Clearside agrees to inform Spark in writing immediately if it or any Person who is performing activities hereunder is debarred or is the subject of a conviction described in Section 306, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to the best of Clearside’s knowledge, is threatened, relating to the debarment or conviction of Clearside or any Person used in any capacity by Clearside or any of its Affiliates in connection with this Agreement.
(f) Clearside covenants that Clearside shall not, without Spark’s prior written consent, (i) waive, amend, cancel or terminate any material provision of, or fail to maintain, the Emory License Agreement in any manner that would be materially detrimental to the rights granted to Spark hereunder or that would impose additional or more onerous obligations on Spark, or (ii) take or fail to take any action that would give any counterparty to the Emory License Agreement the right to terminate the Emory License Agreement.
(g) Clearside has been advised by the United States (US) Food and Drug Administration (FDA) that products comprised of a Standard Microinjector used to deliver an active pharmaceutical ingredient to the suprachoroidal space (SCS) will be regulated as a drug subject to a new drug application (NDA) regulatory pathway, without any requirement for 510(k) or premarket approval of the Microinjector, and to Clearside’s knowledge, a biologics license application (BLA) regulatory pathway will apply to Licensed Products comprising a Standard Microinjector hereunder.
(h) The Patents set forth on Exhibit B are all of the Patents relating to the Clearside Technology that are Controlled by Clearside or its Affiliates as of the Effective Date. If any Patents relating to the Clearside Technology that are Controlled by Clearside or its Affiliates as of the Effective Date are determined to have been omitted from Exhibit B, Exhibit B shall automatically be updated to include such omitted Patents.
| 11.3 | Spark Representations, Warranties and Covenants. Spark hereby represents and warrants that as of the Effective Date: |
(a) it shall perform the activities allocated to it under the Workplans in a timely and professional manner, with due care and in accordance with industry standards;
(b) the performance by Clearside of the activities allocated to it under the Workplans and its other obligations under this Agreement shall not infringe or otherwise violate the intellectual property rights of any Third Party related to Gene Therapeutics; and
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
36
(c) neither Spark nor any of its Affiliates, nor, to its knowledge, any other Person that will be involved in activities under this Agreement has been debarred or is subject to debarment, and neither Spark nor any of its Affiliates will knowingly use in any capacity, in connection with this Agreement, any Person who has been debarred pursuant to Section 306 of the United States Federal Food, Drug, and Cosmetic Act, or who is the subject of a conviction described in such section. Spark agrees to inform Clearside in writing immediately if it or any Person who is performing activities hereunder is debarred or is the subject of a conviction described in Section 306, or if any action, suit, claim, investigation or legal or administrative proceeding is pending or, to the best of Spark’s knowledge, is threatened, relating to the debarment or conviction of Spark or any Person used in any capacity by Spark or any of its Affiliates in connection with this Agreement.
(d) Spark covenants that Spark shall comply with the terms of the Emory License Agreement set forth in Exhibit C. Spark covenants that any Licensed Products made or sold by or on behalf of Spark, its Affiliates and Sublicensees pursuant to this Agreement shall comply with all applicable federal and state law regulations, including but not limited to regulations of the Federal Drug Administration, the Environmental Protection Agency, and their state equivalents.
| 11.4 | Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN SECTIONS 11.1, 11.2 AND- 11.3, THE PARTIES MAKE NO REPRESENTATIONS AND EXTEND NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, AND PARTICULARLY THAT LICENSED PRODUCTS WILL BE SUCCESSFULLY DEVELOPED OR COMMERCIALIZED HEREUNDER, AND IF LICENSED PRODUCTS ARE DEVELOPED, WITH RESPECT TO SUCH LICENSED PRODUCTS, AND TO THE EXTENT PERMITTED BY LAW, THE PARTIES EXCLUDE ALL IMPLIED WARRANTIES OF TITLE, NON-INFRINGEMENT, MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE. |
ARTICLE 12
INDEMNIFICATION, INSURANCE AND LIABILITY
| 12.1 | Indemnification by Clearside. Clearside shall defend, indemnify and hold harmless Spark and its officers, directors, employees, agents, representatives, successor and assigns (“Spark Indemnitee”) from and against any liability or expense (including reasonable legal expenses, costs of litigation and attorneys’ fees), damages, or judgments, whether for money or equitable relief (collectively, “Losses”) resulting from suits, proceedings, claims, actions, demands, or threatened claims, actions or demands, in each case brought by a Third Party (each, a “Claim”) against a Spark Indemnitee arising out of: (a) any negligent act or omission, or willful wrongdoing by Clearside or its Affiliates in the performance of this Agreement, (b) the failure by Clearside to comply with any Law, (c) any breach of any representation or warranty or covenant of Clearside under this Agreement, except, in each case, to the extent any such Losses result from the gross negligence or willful misconduct of a Spark Indemnitee or from the breach of any representation or warranty or obligation under this Agreement by Spark. |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
37
| 12.2 | Indemnification by Spark. Spark shall defend, indemnify and hold harmless Clearside and its Affiliates, the “Indemnitees” (under and as such term is defined in the Emory License Agreement), and its and their officers, directors, employees, agents, representatives, successor and assigns (“Clearside Indemnitee”) from and against any and all Losses resulting from Claims, including, bodily, injury, risk of bodily injury, death, property damage and product liability, against an Clearside Indemnitee arising out of or relating to, directly or indirectly: (a) any negligent act or omission, or willful wrongdoing by Spark in the performance of this Agreement, (b) the failure by Spark to comply with any Law, (c) any alleged personal injuries or death resulting from, arising out of or relating to any Clinical Trials or use of a Licensed Product, or (d) any breach of any representation or warranty or covenant of Spark under this Agreement; except, in each case, to the extent any such Losses result from the gross negligence or willful misconduct of a Clearside Indemnitee or from the breach of any representation or warranty or obligation under this Agreement by Clearside. |
| 12.3 | Limitations on Indemnification. The obligations to indemnify, defend, and hold harmless set forth in Sections 12.1 and 12.2 shall be contingent upon the Party seeking indemnification (the “Indemnitee”): (a) notifying the indemnifying Party of a claim, demand or suit within ten (10) days of receipt of same; provided, however, that Indemnitee’s failure or delay in providing such notice shall not relieve the indemnifying Party of its indemnification obligation except to the extent the indemnifying Party is prejudiced thereby; (b) allowing the indemnifying Party or its insurers the right to assume direction and control of the defense of any such claim, demand or suit; (c) using its best efforts to cooperate with the indemnifying Party or its insurers, at the indemnifying Party’s expense, in the defense of such claim, demand or suit; and (d) agreeing not to settle or compromise any claim, demand or suit without prior written authorization of the indemnifying Party. The Indemnitee shall have the right to participate in the defense of any such claim, demand or suit referred to in this Section utilizing attorneys of its choice, at its own expense, provided, however, that the indemnifying Party shall have full authority and control to handle any such claim, demand or suit. |
| 12.4 | Limitation on Liability. IN NO EVENT SHALL ANY PARTY BE LIABLE TO THE OTHER PARTY FOR ANY INDIRECT, SPECIAL, INCIDENTAL, EXEMPLARY OR CONSEQUENTIAL DAMAGES OF ANY KIND ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY (WHETHER IN CONTRACT, TORT (INCLUDING NEGLIGENCE), STRICT LIABILITY OR OTHERWISE), EVEN IF SUCH PARTY WAS ADVISED OR OTHERWISE AWARE OF THE LIKELIHOOD OF SUCH DAMAGES. The limitations set forth in this Section 12.4 shall not apply with respect to (a) the Party’s indemnification obligations under Sections 12.1 and 12.2, as applicable, (b) breach of Article 9, or (c) intentional misconduct of a Party. Nothing in this Section 12.4 shall exclude a Party’s liability for death or injury caused by that Party’s negligence, or fraud or fraudulent misrepresentation. |
| 12.5 | Insurance. During the term of this Agreement and for a period of five (5) years after termination or expiration, each Party shall obtain or maintain, at its sole cost and expense, insurance policies, including product liability insurance, adequate to cover its obligations hereunder and which are consistent with normal business practices of prudent companies similarly situated (which may include programs of self insurance). |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
38
ARTICLE 13
DISPUTE RESOLUTION
| 13.1 | General. Any controversy, claim or dispute arising out of or relating to this Agreement shall be settled, if possible, through good faith negotiations between the Parties. If, however, the Parties are unable to settle such dispute after good faith negotiations, the matter shall be referred to executive officers of the Parties to be resolved by negotiation in good faith as soon as is practicable but in no event later than thirty (30) days after referral. |
| 13.2 | Failure of Executive Officers to Resolve Dispute. If the executive officers are unable to settle the dispute after good faith negotiation in the manner set forth above, either Party may submit the matter for resolution by binding arbitration in accordance with Section 13.3. |
| 13.3 | Arbitration. A Party submitting a dispute for resolution by binding arbitration pursuant to this Section 13.3 shall provide the other Party with written notice thereof (an “Arbitration Notice”). Any arbitration hereunder shall be conducted under the Commercial Arbitration Rules of the American Arbitration Association, except as modified herein, and the arbitration proceeding shall be governed by the United States Federal Arbitration Act (the “FAA”). Except as specified below, such arbitration shall be conducted by a single arbitrator and the arbitrator shall be either mutually acceptable or, if the parties cannot agree on an arbitrator within fifteen (15) days after the matter is referred to arbitration, the single arbitrator shall be a person selected by the applicable rules. Within ten (10) business days after the arbitrator is selected, each party shall submit to the arbitrator that party’s proposed resolution of the dispute and justification therefor. The arbitrator shall, within ten (10) business days after receiving the proposed resolution from each party, select one of the proposals, and such selection shall be deemed to be the arbitrator’s conclusive decision and shall be binding on the parties. Notwithstanding the foregoing, in the case of a dispute relating to an alleged material breach, then the arbitration shall be conducted by a panel of three arbitrators, in which case each Party shall select one arbitrator within thirty (30) days after the date of the Arbitration Notice and the two (2) arbitrators so selected shall choose a third arbitrator to resolve the dispute within thirty (30) days after the date the second of the initial two (2) arbitrators is selected. All arbitrators shall be independent of the Parties and shall not have any present or past relationship with either Party. An arbitration decision shall be rendered in writing and shall be binding and not be appealable to any court in any jurisdiction except pursuant to the FAA. The arbitration proceedings shall be conducted in the English language and shall be held in: (a) Philadelphia, PA, if brought by Spark, and (b) Atlanta, |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
39
| Georgia, if brought by Clearside. The arbitrators shall have the authority to grant specific performance, and to allocate between the Parties the costs and legal fees of arbitration in such equitable manner as they determine. Judgment upon the award so rendered may be entered in any court having jurisdiction and application may be made to such court for judicial acceptance of any award and an order of enforcement, as the case may be. |
ARTICLE 14
MISCELLANEOUS
| 14.1 | Governing Law. This Agreement and any non-contractual obligations arising out of or in connection with it shall be governed by and interpreted in accordance with the laws of the State of Delaware without regard to conflict of law principles thereof, and excluding the United National Convention on Contracts for the International Sales of Goods. |
| 14.2 | Compliance with Laws. Each Party shall conduct its activities under this Agreement in accordance with Law and good business practices. Furthermore, each Party represents, warrants and agrees that it has been at all times and will continue to be in compliance with all potentially applicable anti-bribery and anti-corruption laws, including the U.S. Foreign Corrupt Practices Act of 1977. Each Party represents, warrants and agrees that no bribes, payments, kickbacks, gifts, hospitality, donations, loans, or anything of value have been or will be made or received, offered, promised, or authorized, directly or indirectly, to improperly influence any act or decision of any person or entity, induce any person or entity to do or omit to do any act in violation of any person’s or entities’ lawful duties, or secure any improper advantage. |
| 14.3 | Assignment of Rights and Obligations. |
(a) General Rule. This Agreement and its rights or obligations may not be assigned or otherwise transferred by either Party without the prior written consent of the other Party.
(b) Permitted Assignments to Affiliates and in Case of Sale of Business Transactions. Notwithstanding Section 14.3(a), either Party may, even without the consent of the other Party, assign this Agreement or any of its rights or obligations (i) to any of its Affiliates, or (ii) in connection with a sale or transfer of all or substantially all of such Party’s business or assets relating to the subject matter of this Agreement, whether by merger, sale of assets or otherwise; provided, however, that such Party’s rights and obligations under this Agreement shall be assumed in writing by its successor in interest in any such transaction. In the event of an assignment of this Agreement by Spark, the successor or assignee shall be obligated, in addition to and without limiting the provisions of Section 4.1(a), if an IND clearance is obtained by Spark (or such successor or assignee) with respect to a second Licensed Product, to use Commercially Reasonable Efforts to Develop a second Licensed Product in the Field if warranted by safety, efficacy,
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
40
commercial opportunity and the other considerations included in the definition of “Commercially Reasonable Efforts.” In addition, either Party may assign its rights hereunder, subject to any applicable defenses or set offs, as collateral security to its senior secured creditor(s).
| 14.4 | Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of the Agreement. |
| 14.5 | Force Majeure. Except with respect to payment of money, no Party shall be liable to the other Party for failure or delay in the performance of any of its obligations under this Agreement for the time and to the extent such failure or delay is caused by earthquake, riot, civil commotion, war, terrorist acts, strike, flood, or governmental acts or restriction, or other cause that is beyond the reasonable control of the respective Party (“Force Majeure”). The Party affected by such Force Majeure will provide the other Party with full particulars thereof as soon as it becomes aware of the same (including its best estimate of the likely extent and duration of the interference with its activities), and will use Commercially Reasonable Efforts to overcome the difficulties created thereby and to resume performance of its obligations as soon as practicable. If the performance of any such obligation under this Agreement is delayed owing to an event of Force Majeure for any continuous period of more than ninety (90) days, the Parties will consult with respect to an equitable solution, including the possibility of the termination of this Agreement. |
| 14.6 | Representation by Legal Counsel. Each Party hereto represents that it has been represented by legal counsel in connection with this Agreement and acknowledges that it has participated in the drafting hereof. In interpreting and applying the terms and provisions of this Agreement, the Parties agree that no presumption shall exist or be implied against the Party which drafted such terms and provisions. |
| 14.7 | Notices. Any notice, request, delivery, approval or consent required or permitted to be given under this Agreement shall be in writing and shall be deemed to have been sufficiently given if delivered in person, transmitted by facsimile (receipt verified) or by express courier service (signature required) or five (5) days after it was sent by registered letter, return receipt requested (or its equivalent), provided that no postal strike or other disruption is then in effect or comes into effect within two (2) days after such mailing, to the Party to which it is directed at its address or facsimile number shown below or such other address or facsimile number as such Party will have last given by notice to the other Party. |
| If to Spark: | Spark Therapeutics, Inc. ▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | |
| Attention: General Counsel | ||
| Facsimile: (▇▇▇) ▇▇▇-▇▇▇▇ |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
41
| If to Clearside: | ▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇., ▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | |
| Attention: Chief Executive Officer | ||
| With a copy to: | ▇▇▇▇▇▇▇▇▇ PLLC ▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ | |
| Attn: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇ | ||
| 14.8 | Entire Agreement. The Parties hereto acknowledge that this Agreement, together with the Exhibits attached hereto, set forth the entire agreement and understanding of the Parties hereto as to the subject matter hereof, and supersedes all prior and contemporaneous discussions, agreements and writings in respect. Except as required by statute, no terms shall be implied (whether by custom, usage or otherwise) into this Agreement. |
| 14.9 | Amendment. No amendment, modification or supplement of any provision of this Agreement shall be valid or effective unless made in writing and signed by a duly authorized officer of each Party. |
| 14.10 | Waiver. No provision of the Agreement shall be waived by any act, omission or knowledge of a Party or its agents or employees except by an instrument in writing expressly waiving such provision and signed by a duly authorized officer of the waiving Party. The waiver by any of the Parties of any breach of any provision hereof by another Party shall not be construed to be a waiver of any succeeding breach of such provision or a waiver of the provision itself. |
| 14.11 | Severability. If any clause or portion thereof in this Agreement is for any reason held to be invalid, illegal or unenforceable, the same shall not affect any other portion of this Agreement, as it is the intent of the Parties that this Agreement shall be construed in such fashion as to maintain its existence, validity and enforceability to the greatest extent possible. In any such event, this Agreement shall be construed as if such clause of portion thereof had never been contained in this Agreement, and there shall be deemed substituted therefor such provision as will most nearly carry out the intent of the Parties as expressed in this Agreement to the fullest extent permitted by Law. |
| 14.12 | Relationship of the Parties. The Parties agree that the relationship of Spark and Clearside established by this Agreement is that of independent contractors. Furthermore, the Parties agree that this Agreement does not, is not intended to, and shall not be construed to, establish an employment, agency, partnership or any other relationship. Except as may be specifically provided herein, no Party shall have any right, power or authority, nor shall they represent themselves as having any authority to assume, create or incur any expense, liability or obligation, express or implied, on behalf of any other Party, or otherwise act as an agent for any other Party for any purpose. |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
42
| 14.13 | Third Party Beneficiaries. Except for the rights to indemnification provided for a Party’s Indemnitees pursuant to Article 12, all rights, benefits and remedies under this Agreement are solely intended for the benefit of the Parties (including any successor in interest or permitted assigns), and except rights to indemnification expressly provided pursuant to Article 12, no Third Party shall have any rights whatsoever to (a) enforce any obligation contained in this Agreement, (b) seek a benefit or remedy for any breach of this Agreement, or (c) take any other action relating to this Agreement under any legal theory, including actions in contract, tort (including negligence, gross negligence and strict liability), or as a defense, setoff or counterclaim to any action or claim brought or made by the Parties. |
| 14.14 | Nonsolicitation. During the term of this Agreement and for a period of twelve (12) months thereafter, neither Party shall solicit an employee of the other Party who is or has been involved in the performance or oversight of any of the Research hereunder to terminate his or her employment and accept employment or work as a consultant with the soliciting Party. Notwithstanding the foregoing, nothing herein shall restrict or preclude the Parties’ right to make generalized searches for employees by way of a general solicitation for employment placed in a trade journal, newspaper or website. |
| 14.15 | Counterparts. This Agreement may be executed in any number of counterparts, each of which need not contain the signature of more than one Party but all such counterparts taken together shall constitute one and the same agreement. Any signature page delivered by facsimile or electronic image transmission shall be binding to the same extent as an original signature page. |
[Signature page follows]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
43
IN WITNESS WHEREOF, the Parties have executed this Agreement by their duly authorized representatives as of the dates set forth below.
| SPARK THERAPEUTICS, INC. | CLEARSIDE BIOMEDICAL, INC. | |||||||
| By: | /s/ ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ |
By: | /s/ ▇▇▇▇▇▇ ▇. ▇▇▇▇▇ | |||||
| Name: | ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ |
Name: | ▇▇▇▇▇▇ ▇. ▇▇▇▇▇ | |||||
| Title: | CEO |
Title: | President & CEO | |||||
| Date: |
|
Date: | 4-27-2015 | |||||
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
44
Exhibits:
Exhibit A-1 – Initial Option Period #1 Workplan
Proposal Summary
| Study Title |
Price (USD) | |||
| Ocular Distribution of AAV Vector by Suprachoroidal or Subretinal Injection in Pigmented Rabbits with a Tolerability Phase |
$ | [*** | ] | |
Assumptions
In the preparation of this quotation, certain assumptions have been made which are presented below:
The study information presented within this document is based on standard Covance practices and the study outline/assumptions included.
This document is not a contract. A contract will typically be issued at study plan finalization.
Price estimate has been prepared according to the information provided in the study outline/assumptions listed.
Price estimate is subject to change should there be any alteration to the study outline/assumptions.
The final study price will be calculated on the authorized definitive protocol.
Prices are valid for 60 days from the document date.
Where costs of shipping are included in this quotation, these are for convenience only and may vary depending upon the type and volume of material shipped. All shipping is provided by a third party vendor and is not considered part of the services provided by Covance.
One-year archive period included (can be extended if required). After retention has ended, Archives will request disposition decisions and communicate the applicable charges.
Ocular Distribution of AAV Vector by Suprachoroidal or Subretinal Injection in Pigmented Rabbits with a Tolerability Phase
| Quote No.: | 98777 | |
| Quote (USD): | $[***] | |
| Study Location: | Madison, WI, USA | |
| Estimated Study Start: | Late March/Early April 2015 – based on study outline and current capacity. No schedule reserved at this time. | |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
45
Client Authorization
If Clearside Biomedical, Inc. would like Covance Laboratories Inc. to proceed with the scheduling and initial setup of the following project:
| Study Title |
Price (USD) | |||
| Ocular Distribution of AAV Vector by Suprachoroidal or Subretinal Injection in Pigmented Rabbits with a Tolerability Phase |
$ | [*** | ] | |
Please sign below and e-mail as a .pdf to the Client Manager noted on Page 1.
Please issue a Purchase Order Number for the project.
Purchase Order Number (if applicable):
Covance Laboratories Inc. and Clearside Biomedical, Inc. agree that the final contract values of the above-mentioned project will be determined upon completion of the definitive protocol before study start.
Upon receipt of this authorization, the appropriate resources will be reserved for the project, and you will be notified of the scheduled start date. Any subsequent delay or cancellation of the study may be subject to additional charges as noted below.
In the event of a delay or cancellation of a study contracted between the parties, Delay/Cancellation costs will be in accordance with the policy as established and mutually agreed upon under the Master Laboratory Services Agreement or Laboratory Services Agreement.
| Signed for and on behalf of Clearside Biomedical, Inc. |
|
|
| (Name & Title) |
|
|
| (Date) |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
46
Exhibit A-2
Preliminary Work Plan #2
Following the successful completion of the first rabbit study as evidenced by the expression of GFP from an AAV5 vector in RPE and photoreceptor cells, the additional studies are anticipated. Based on the data from the initial study Clearside and Spark may recommend revision of this proposal.
| 1. | Dose Optimization Study |
[***]
| 2. | Efficacy study |
[***]
| 3. | Non-human primate study |
[***]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
Exhibit B – Existing Clearside Background IP
Owned by Emory University and/or Georgia Tech Research Corporation and subject to Emory License Agreement
| Patent No. |
Issue date |
Docket No. | ||
| U.S. Patent No. 7,918,814 |
April 5, 2011 | CLRS-004/02US | ||
| U.S. Patent No. 8,197,435 |
June 12, 2012 | CLRS-004/03US | ||
| U.S. Patent No. 8,636,713 |
January 28, 2014 | CLRS-004/04US | ||
| U.S. Patent No. 8,808,225 |
August 19, 2014 | CLRS-004/05US | ||
| Application No. |
Filing date |
Docket No. | ||
| International Patent Application No. PCT/US14/71623 |
December 19, 2014 | CLRS-023/01WO | ||
| U.S. Provisional Patent Application No. 61/918,992 |
December 20, 2013 | CLRS-023/00US | ||
| U.S. Provisional Patent Application No. 60/746,237 |
May 2, 2006 | CLRS-004/00US | ||
| U.S. Provisional Patent Application No. 61/172,409 |
April 24, 2009 | CLRS-004/01US | ||
| U.S. Provisional Patent Application No. 61/698,254 |
September 7, 2012 | CLRS-005/00US | ||
| U.S. Patent Application Serial No. 14/136,657 |
December 20, 2013 | CLRS-004/06US | ||
| International Patent Application No. PCT/US2011/033987 |
April 26, 2011 | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇▇▇▇▇ Patent Application No. ▇▇▇▇▇▇▇▇▇▇ |
April 26, 2011 | CLRS-004/03AU | ||
| Brazil Patent Application No. 11 2012 027416-3 |
April 26, 2011 | CLRS-004/03BR | ||
| Canada Patent Application No. 2797258 |
April 26, 2011 | CLRS-004/03CA | ||
| Chinese Patent Application No. 201180024176.5 |
April 26, 2011 | CLRS-004/03CN | ||
| European Patent Application No. 11777924.9 |
April 26, 2011 | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇ Patent Application ▇▇. ▇▇▇▇▇/▇▇▇▇▇/▇▇▇▇ |
▇▇▇▇▇ ▇▇, ▇▇▇▇ | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇▇ Patent Application ▇▇. ▇▇▇▇▇▇ |
▇▇▇▇▇ ▇▇, ▇▇▇▇ | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇ Patent Application No. 2013-508168 |
April 26, 2011 | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇▇ Patent Application No. MX/a/2012/012495 |
April 26, 2011 | CLRS-004/03MX | ||
| New Zealand Patent Application No. 603185 |
April 26, 2011 | CLRS-004/03NZ | ||
| New Zealand Patent Application No. 623752 |
April 26, 2011 | CLRS-004/04NZ | ||
| Russia Patent Application No. 2012147341 |
April 26, ▇▇▇▇ | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇▇▇▇▇ Patent Application No. 201207910-9 |
April 26, ▇▇▇▇ | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇ ▇▇▇▇▇▇ Application ▇▇. ▇▇▇▇/▇▇▇▇▇ |
▇▇▇▇▇ ▇▇, ▇▇▇▇ | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇▇▇▇▇ ▇▇▇▇▇▇ Application No. 2014/00616 |
April 26, 2011 | CLRS-004/04ZA | ||
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
48
Owned jointly by Clearside and Emory University and/or Georgia Tech Research Corporation and subject to Emory License Agreement
| Application No. |
Filing date |
Docket No. | ||
| U.S. Provisional Patent Application No. 61/693,542 |
August 27, 2012 | CLRS-006/00US | ||
| U.S. Provisional Patent Application No. 61/754,495 |
January 18, 2013 | CLRS-006/01US | ||
| U.S. Provisional Patent Application No. 61/784,817 |
March 14, 2013 | CLRS-006/02US | ||
| PCT Patent Application No. PCT/US2013/056863 |
August 27, 2013 | CLRS-006/03WO | ||
| U.S. Patent Application No. 14/424,685 |
August 27, 2013 | CLRS-006/03US | ||
| EU national phase of PCT Patent Application No. PCT/US2013/056863 |
August 27, 2013 | CLRS-006/00EP | ||
| CA national phase of PCT Patent Application No. PCT/US2013/056863 |
August 27, 2013 | CLRS-006/03CA | ||
Independently developed and owned solely by Clearside
| Application No. |
Filing date |
Docket No. | ||
| U.S. Provisional Patent Application No. 61/759,771 |
February 1, 2013 | CLRS-011/00US | ||
| U.S. Provisional Patent Application No. 61/724,144 |
November 8, 2012 | CLRS-007/00US | ||
| U.S. Provisional Patent Application No. 61/734,872 |
December 7, 2012 | CLRS-008/00US | ||
| U.S. Provisional Patent Application No.61/745,237 |
December 21, 2012 | CLRS-010/00US | ||
| U.S. Provisional Patent Application No. 61/773,124 |
March 5, 2013 | CLRS-012/00US | ||
| U.S. Provisional Patent Application No. 61/785,229 |
March 14, 2013 | CLRS-012/01US | ||
| U.S. Provisional Patent Application No. 61/819,388 |
May 3, 2013 | CLRS-012/02US | ||
| U.S. Provisional Patent Application No. 61/873,660 |
September 4, 2013 | CLRS-017/00US | ||
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
49
| Application No. |
Filing date |
Docket No. | ||
| U.S. Provisional Patent Application No. 61/898,926 |
November 1, 2013 | CLRS-017/01US | ||
| International Application No. PCT/US2013/069156 |
November 8, 2013 | CLRS-007/01WO | ||
| U.S. Provisional Patent Application No. 61/830,324 |
June 3, 2013 | CLRS-013/00US | ||
| International Application No. PCT/US2014/040254 |
May 30, 2014 | CLRS-013/01WO | ||
| U.S. Provisional Patent Application No. 61/819,048 |
May 3, 2013 | CLRS-014/00US | ||
| U.S. Provisional Patent Application No. 61/819,052 |
May 3, 2013 | CLRS-015/00US | ||
| U.S. Provisional Patent Application No. 61/827,371 |
May 24, 2013 | CLRS-016/00US | ||
| U.S. Provisional Patent Application No. 61/944,214 |
February 25, 2014 | CLRS-014/01US | ||
| U.S. Provisional Patent Application No. 61/953,147 |
March 14, 2014 | CLRS-018/00US | ||
| International Application ▇▇. ▇▇▇/▇▇▇▇▇▇/▇▇▇▇▇▇ |
▇▇▇ ▇, ▇▇▇▇ | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇.▇. Patent Application No. 14/268,687 |
May 2, 2014 | CLRS-018/01US | ||
| U.S. Patent Application No. 14/523,243 |
May 2, 2014 | CLRS-018/02US | ||
| U.S. Provisional Patent Application No. 62/014,766 |
June 20, 2014 | CLRS-021/00US | ||
| U.S. Provisional Patent Application No. 62/035,682 |
August 11, 2014 | CLRS-014/02US | ||
| U.S. Design Patent Application No. 29/506,275 |
October 14, 2014 | CLRS-022/00US | ||
| U.S. Provisional Patent Application No. 62/063,792 |
October 14, 2014 | CLRS-022/01US | ||
Acquired from iScience and owned solely by Clearside
| Case Numbers |
Title |
Application Information |
Status | |||
| 641-008BR (ISSCP008BR) |
Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Filed: 02/22/2007 App. #: PI0708133-2 |
[***] | |||
| 641-008C (ISSCP008C1US) |
Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Filed: 03/15/2013 App. #: 13/842,288 |
[***] |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
50
| 641-008CN (ISSCP008CN) |
Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Issued: 06/8/2011 ▇▇▇. #: ZL200780014501.3 Filed: 2/22/2007 App. #: 200780014501.3 |
[***] | |||
| 641-008CN-DV1 (ISSCP008CND1) |
Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Issued: 10/30/2013 ▇▇▇. #: ZL2001110093644.6 Filed: 2/22/2007 App. #: 201110093644.6 |
[***] | |||
| 641-008DV (ISSCP008D1US) |
Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Filed: 03/15/2013 App. #: 13/842,218 |
[***] | |||
| 641-008EP (ISSCP008EP) |
Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Filed: 02/22/2007 App. #: 07751620.1 |
[***] | |||
| 641-008US (ISSCP008US) |
Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Filed: 02/21/2007 App. #: 11/709,941 |
[***] | |||
| 641-015BR (ISSCP015BR) |
Title: DEVICE FOR OCULAR ACCESS | Filed: 04/15/2013 App. #: 1120130092050 |
[***] | |||
| 641-015CN (ISSCP015CN) |
Title: DEVICE FOR OCULAR ACCESS | Filed: 10/14/2011 App. #: 201180060268.9 |
[***] | |||
| 641-015CN-DV | Title: DEVICE FOR OCULAR ACCESS | Filed: Not yet App. #: |
[***] | |||
| 641-015EP (ISSCP015EP) |
Title: DEVICE FOR OCULAR ACCESS | Filed: 05/09/2013 App. #: 11776049.6 |
[***] | |||
| 641-015JP (ISSCP015JP) |
Title: DEVICE FOR OCULAR ACCESS | Filed: 4/12/2012 App. #: 2013-534049 |
[***] |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
51
| 641-015US (ISSCP015US) |
Title: DEVICE FOR OCULAR ACCESS | Filed: 10/14/2011 App. #: 13/273,775 |
[***] | |||
| Title: APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | Filed: February 22, 2006 U.S. Provisional Patent Application Serial No.: 60/776,903 |
|||||
| APPARATUS AND FORMULATIONS FOR SUPRACHOROIDAL DRUG DELIVERY | International Patent Application Serial No.:PCT/US2007/004874 | [***] | ||||
| DEVICE FOR OCULAR ACCESS | U.S. Provisional Patent Application Serial No.: 61/393,741 | [***] | ||||
| DEVICE FOR OCULAR ACCESS | International Patent Application Serial No.: PCT/US2011/056433 | [***] |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
52
Developed and owned solely by Clearside
| Application No. |
Filing date |
Docket No. | ||
| U.S. Provisional Patent Application No. 61/759,771 | February 1, 2013 | CLRS-011/00US | ||
| U.S. Provisional Patent Application No. 61/724,144 | November 8, 2012 | CLRS-007/00US | ||
| U.S. Provisional Patent Application No. 61/734,872 | December 7, 2012 | CLRS-008/00US | ||
| U.S. Provisional Patent Application No. 61/745,237 | December 21, 2012 | CLRS-010/00US | ||
| U.S. Provisional Patent Application No. 61/773,124 | March 5, 2013 | CLRS-012/00US | ||
| U.S. Provisional Patent Application No. 61/785,229 | March 14, 2013 | CLRS-012/01US | ||
| U.S. Provisional Patent Application No. 61/819,388 | May 3, 2013 | CLRS-012/02US | ||
| U.S. Provisional Patent Application No. 61/873,660 | September 4, 2013 | CLRS-017/00US | ||
| U.S. Provisional Patent Application No. 61/898,926 | November 1, 2013 | CLRS-017/01US | ||
| International Application No. PCT/US2013/069156 | November 8, 2013 | CLRS-007/01WO | ||
| U.S. Provisional Patent Application No. 61/830,324 | June 3, 2013 | CLRS-013/00US | ||
| International Application No. PCT/US2014/040254 | May 30, 2014 | CLRS-013/01WO | ||
| U.S. Provisional Patent Application No. 61/819,048 | May 3, 2013 | CLRS-014/00US | ||
| U.S. Provisional Patent Application No. 61/819,052 | May 3, 2013 | CLRS-015/00US | ||
| U.S. Provisional Patent Application No. 61/827,371 | May 24, 2013 | CLRS-016/00US | ||
| U.S. Provisional Patent Application No. 61/944,214 | February 25, 2014 | CLRS-014/01US | ||
| U.S. Provisional Patent Application No. 61/953,147 | March 14, 2014 | CLRS-018/00US | ||
| International Application ▇▇. ▇▇▇/▇▇▇▇▇▇/▇▇▇▇▇▇ | ▇▇▇ ▇, ▇▇▇▇ | ▇▇▇▇-▇▇▇/▇▇▇▇ | ||
| ▇.▇. Patent Application No. 14/268,687 | May 2, 2014 | CLRS-018/01US | ||
| U.S. Patent Application No. 14/523,243 | May 2, 2014 | CLRS-018/02US | ||
| U.S. Provisional Patent Application No. 62/014,766 | June 20, 2014 | CLRS-021/00US | ||
| U.S. Provisional Patent Application No. 62/035,682 | August 11, 2014 | CLRS-014/02US | ||
| U.S. Design Patent Application No. 29/506,275 | October 14, 2014 | CLRS-022/00US | ||
| U.S. Provisional Patent Application No. 62/063,792 | October 14, 2014 | CLRS-022/01US | ||
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
53
Exhibit C – Provisions of Emory License Agreement
The following provisions of the Emory License Agreement shall be included in any Sublicense:
2.5.1. Spark or Sublicensee shall indemnify Emory/GTRC and maintain liability coverage to the same extent that Clearside is so required pursuant to Section 10.3 of the Emory License Agreement.
2.5.2. Emory/GTRC has the right to audit Spark or the Sublicensee as described below.
2.5.3. Spark shall provide Clearside with copies of all sublicense agreements within thirty (30) days of their execution date, which, if redacted, must include the relevant provisions under this Article 2 and a disclosure of the financial terms of the sublicense;
2.5.4. Sublicense to Spark or to a Sublicensee is subject to automatic termination in the event that Spark, a Sublicensee or distributor challenges, either directly or indirectly, the validity, enforceability or scope of any claim within the patent rights licensed under the Emory License Agreement in a court or other governmental agency of competent jurisdiction, including in a reexamination or opposition proceeding.
2.5.5. If the Emory License Agreement terminates for any reason, Spark or any Sublicensee shall, unless the sublicense agreement also terminates, from the effective date of such termination, automatically become a direct licensee of Emory/GTRC with respect to the rights originally sublicensed to it by Clearside, provided Spark or such Sublicensee did not cause the termination of the Agreement, agrees to comply with all the terms of the Emory License Agreement and assumes the responsibilities of Clearside, to the extent applicable to the sublicense originally granted to it.
2.7 Any Licensed Products used or sold in the United States will be manufactured substantially in the United States unless any waivers required are obtained from the United States Government by COMPANY
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
54
| 4.3. | Right to Audit. Emory/GTRC shall have the right, upon prior notice, not more than once in each calendar year and the calendar year immediately following termination of the Agreement, through an independent certified public accountant selected by Emory/GTRC, to have access during normal business hours as may be reasonably necessary to examine the records of Spark or Sublicensee to include, but not be limited to, sales invoice registers, sales analysis reports, original invoices, inventory records, price lists, sublicense and distributor agreements, accounting general ledgers, and sales tax returns, in order to verify the accuracy of the of the calculation of any payment due under the Emory License Agreement. Such audit shall be limited in scope to the preceding thirty-six (36) months from the date of the notice of audit. If such independent public accountant’s report shows any underpayment of royalties by Spark, its Affiliates or Sublicensees, within thirty (30) days after receipt of such report, Spark or Sublicensee shall remit to Emory/GTRC: |
| i. | the amount of such underpayment; and |
| ii. | if such underpayment exceeds five (5%) percent of the total royalties owed for the fiscal year then being reviewed, the reasonably necessary fees and expenses of such independent public accountant performing the audit. Otherwise, Emory/GTRC’s accountant’s fees and expenses shall be borne by Emory/GTRC’s. |
9.3 FDA and Other Regulations. Any Sublicensee shall represent and warrant that any Licensed Products made or sold by it or its Affiliates under this license shall comply with all applicable federal and state law regulations, including but not limited to regulations of the Federal Drug Administration, the Environmental Protection Agency, and their state equivalents.
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
55
Exhibit D – Standard Microinjector Technical Specifications
As of the Effective Date, the Standard Microinjector is a single needle, single-use device sterilized with ethylene oxide and designed to be filled at the point of use with a vial adapter tube and used at room temperature. See accompanying image.
| Length of microneedle: | [***] | |
| Needle gauge: | [***] | |
| Size of Bevel opening: | [***] | |
| Volume: | [***] | |
| Force: | [***] | |
For the sake of clarification, improved versions of the Standard Microinjector made after the Effective Date (including improved versions that include changes to the above parameters) shall also constitute Standard Microinjectors, but in no event shall a Microinjector constitute a “Standard Microinjector” if such device incorporates any of the following components or characteristics:
| 1. | Battery power |
| 2. | Thermodynamic modulation (i.e., insulation, heating or cooling elements) |
| 3. | Glass barrel |
| 4. | Multiple ▇▇▇▇▇▇▇▇ |
| 5. | Multi-pronged needle(s) |
| 6. | Sterilization with autoclave/heat or radiation |
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
56
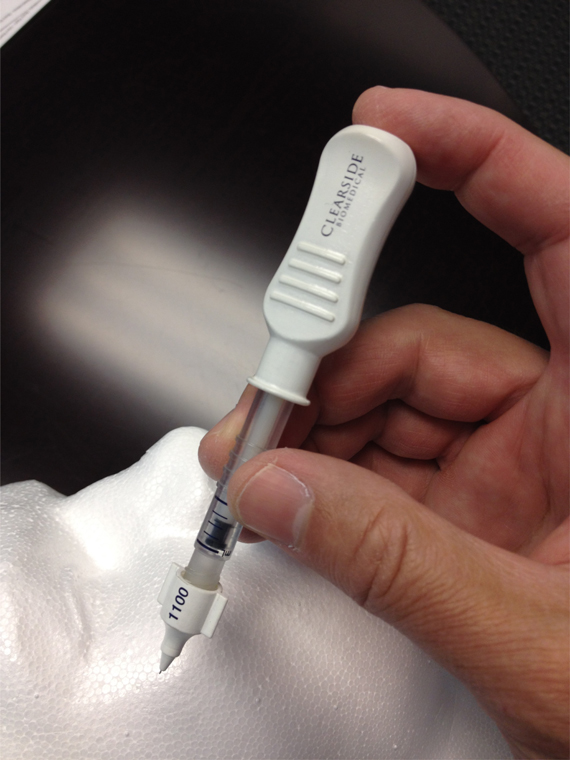
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
57
Exhibit E – Supply Agreement Terms
| Pricing: | [***] (subject to inflation adjuster 5 years after first commercial sale) | |||
| If the parties agree for Clearside to supply New Microinjectors, unit pricing would be at the greater of [***]. | ||||
| Delivery: | F.O.B., Clearside’s designated manufacturing facility in the US | |||
| Product Warrants: | Microinjectors to be manufactured to GMP standards and to meet specifications | |||
| Forecast; Lead times: | Firm Period | [tbd] | ||
| Semi-firm Period | [tbd] | |||
| Non-binding forecast | [tbd] | |||
| “Failure to Supply” | [Failure to meet at least [tbd]% of firm orders in consecutive calendar quarters] | |||
| “Most Favored Nation” | Clearside will commit to performance, warranty, indemnification, priority allocation, quality and similar terms no less favorable to Spark than comparable terms offered by Clearside to Third Party customers and no less favorable to Spark than comparable terms obtained by Clearside from its Third Party contract manufacturer. The Parties acknowledge that, due to the relatively modest quantities that Spark anticipates requiring, the Parties will need to cooperate with respect to forecasting and ordering to enable Spark to obtain timely supply, which cooperation may include joint planning to enable Spark’s orders to be fulfilled concurrently with larger production runs but without undue delay. | |||
| Production Ready Date: | Timeline for commercial scale production readiness to be negotiated. | |||
| Payment Terms: | Payment for supplies of commercial Standard Microinjector will be due net thirty (30) days after the later of the date of invoice or the date of delivery of such Microinjectors. | |||
| Late Payments: | Overdue amounts will carry an annual interest charge, calculated monthly, at London Interbank Offered Rate plus two hundred basis points. | |||
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
58
Exhibit F – Initial Press Release
[To be provided]
CONFIDENTIAL TREATMENT HAS BEEN REQUESTED FOR PORTIONS OF THIS EXHIBIT. THE COPY FILED HEREWITH OMITS THE INFORMATION SUBJECT TO A CONFIDENTIALITY REQUEST. OMISSIONS ARE DESIGNATED [***]. A COMPLETE VERSION OF THIS EXHIBIT HAS BEEN FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION.
59

