DEVELOPMENT, COMMERCIALIZATION AND LICENSE AGREEMENT BY AND AMONG CELL THERAPEUTICS, INC., BAXTER INTERNATIONAL INC., BAXTER HEALTHCARE CORPORATION AND BAXTER HEALTHCARE SA DATED: NOVEMBER 14, 2013
Exhibit 10.32
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
DEVELOPMENT, COMMERCIALIZATION AND LICENSE AGREEMENT
BY AND AMONG
CELL THERAPEUTICS, INC.,
XXXXXX INTERNATIONAL INC.,
XXXXXX HEALTHCARE CORPORATION
AND
XXXXXX HEALTHCARE SA
DATED: NOVEMBER 14, 2013
TABLE OF CONTENTS
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Page | ||||||||
| ARTICLE I. | DEFINITIONS | 1 | ||||||
| 1.1. | Definitions | 1 | ||||||
| ARTICLE II. | GRANT OF LICENSES | 17 | ||||||
| 2.1. | License to Baxter | 17 | ||||||
| 2.2. | License to CTI | 18 | ||||||
| 2.3. | CTI Reservation of Rights | 18 | ||||||
| 2.4. | Sublicenses | 18 | ||||||
| 2.5. | No Other Licenses | 19 | ||||||
| 2.6. | Controlled IP | 19 | ||||||
| 2.7. | Co-Promotion | 19 | ||||||
| 2.8. | Subcontracting | 20 | ||||||
| ARTICLE III. | GOVERNANCE | 20 | ||||||
| 3.1. | General Committee Procedures | 20 | ||||||
| 3.2. | Joint Steering Committee | 22 | ||||||
| 3.3. | Joint Development Committee | 22 | ||||||
| 3.4. | Joint Manufacturing Committee | 23 | ||||||
| 3.5. | Joint Commercialization Committee | 24 | ||||||
| 3.6. | Alliance Managers | 25 | ||||||
| ARTICLE IV. | DEVELOPMENT RESPONSIBILITIES | 25 | ||||||
| 4.1. | Development Obligations | 25 | ||||||
| ARTICLE V. | DEVELOPMENT COOPERATION | 25 | ||||||
| 5.1. | Sharing of Development Updates and Related Information | 25 | ||||||
| 5.2. | Information Sharing Coordination | 26 | ||||||
| ARTICLE VI. | REGULATORY MATTERS | 26 | ||||||
| 6.1. | Regulatory Roles | 26 | ||||||
| 6.2. | Cooperation | 27 | ||||||
-i-
TABLE OF CONTENTS
(continued)
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Page | ||||||||
| ARTICLE VII. | LAUNCH AND COMMERCIALIZATION OF LICENSED PRODUCTS | 28 | ||||||
| 7.1. | Launch and Commercialization | 28 | ||||||
| 7.2. | Co-Promotion | 29 | ||||||
| 7.3. | Coordination of Marketing Efforts | 31 | ||||||
| 7.4. | Recalls | 32 | ||||||
| 7.5. | Safety Information; Adverse Events; Pharmacovigilance | 33 | ||||||
| 7.6. | Medical and Scientific Affairs | 34 | ||||||
| 7.7. | Springback Option | 35 | ||||||
| ARTICLE VIII. | MANUFACTURING AND SUPPLY | 36 | ||||||
| 8.1. | General Supply Terms | 36 | ||||||
| 8.2. | Supply Agreement | 36 | ||||||
| 8.3. | Fill/Finish Option | 37 | ||||||
| 8.4. | Quality Agreement | 37 | ||||||
| 8.5. | Manufacturing Licenses | 37 | ||||||
| ARTICLE IX. | PAYMENTS | 38 | ||||||
| 9.1. | Upfront Fee | 38 | ||||||
| 9.2. | Milestones | 39 | ||||||
| 9.3. | Royalty Payment; Audits | 40 | ||||||
| 9.4. | Development Costs and Expenses | 42 | ||||||
| 9.5. | Profit & Loss Share for Co-Promotion | 46 | ||||||
| 9.6. | Upstream Agreements | 46 | ||||||
| 9.7. | Exchange Rate; Manner and Place of Payment | 46 | ||||||
| 9.8. | Taxes | 46 | ||||||
| 9.9. | Late Payments | 47 | ||||||
| 9.10. | Reporting | 47 | ||||||
| ARTICLE X. | INVENTIONS; ACCESS TO IMPROVEMENTS; PATENTS | 47 | ||||||
| 10.1. | Improvements and Inventions | 47 | ||||||
| 10.2. | Ownership of Inventions | 48 | ||||||
-ii-
TABLE OF CONTENTS
(continued)
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Page | ||||||||
| 10.3. | Disclosure of Inventions | 48 | ||||||
| 10.4. | Prosecution of Patents | 48 | ||||||
| 10.5. | Infringement of Patents by Third Parties | 50 | ||||||
| 10.6. | Infringement of Third Party Rights in the Licensed Territory | 52 | ||||||
| 10.7. | Patent Oppositions and Other Proceedings | 52 | ||||||
| 10.8. | Patent Term Extensions in the Licensed Territory | 53 | ||||||
| 10.9. | Registration of License | 54 | ||||||
| 10.10. | Patent Marking | 54 | ||||||
| ARTICLE XI. | TRADEMARKS | 54 | ||||||
| 11.1. | Baxter Trademarks | 54 | ||||||
| 11.2. | Use of CTI Trademarks | 54 | ||||||
| 11.3. | Infringement of Baxter Trademarks by Third Parties | 56 | ||||||
| ARTICLE XII. | REPRESENTATIONS AND WARRANTIES | 56 | ||||||
| 12.1. | The Parties’ Representations and Warranties | 56 | ||||||
| 12.2. | CTI’s Representations and Warranties | 57 | ||||||
| 12.3. | Xxxxxx’x Representation and Warranties | 61 | ||||||
| 12.4. | The Parties’ Covenants | 62 | ||||||
| 12.5. | CTI’s Covenants | 62 | ||||||
| ARTICLE XIII. | CONFIDENTIALITY | 63 | ||||||
| 13.1. | Confidentiality Obligations of Baxter | 63 | ||||||
| 13.2. | Confidentiality Obligations of CTI | 65 | ||||||
| 13.3. | Press Releases; Publicity | 66 | ||||||
| ARTICLE XIV. | INDEMNIFICATION | 67 | ||||||
| 14.1. | CTI Indemnity | 67 | ||||||
| 14.2. | Baxter Indemnity | 68 | ||||||
| 14.3. | Indemnification Procedure | 68 | ||||||
| 14.4. | Limitation of Liability; Exclusion of Damages; Disclaimer | 70 | ||||||
-iii-
TABLE OF CONTENTS
(continued)
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Page | ||||||||
| ARTICLE XV. | TERM; TERMINATION | 70 | ||||||
| 15.1. | Term | 70 | ||||||
| 15.2. | Early Termination | 71 | ||||||
| 15.3. | Obligations upon Early Termination | 72 | ||||||
| 15.4. | Force Majeure | 73 | ||||||
| 15.5. | Survival | 74 | ||||||
| 15.6. | Other Remedies | 74 | ||||||
| ARTICLE XVI. | GENERAL PROVISIONS | 74 | ||||||
| 16.1. | Assignment | 74 | ||||||
| 16.2. | Headings | 74 | ||||||
| 16.3. | Waiver | 75 | ||||||
| 16.4. | Notices | 75 | ||||||
| 16.5. | Severability | 76 | ||||||
| 16.6. | Entire Agreement | 76 | ||||||
| 16.7. | Amendment; No Waiver | 76 | ||||||
| 16.8. | Counterparts | 76 | ||||||
| 16.9. | Agency | 76 | ||||||
| 16.10. | Further Actions | 76 | ||||||
| 16.11. | Compliance with Laws | 76 | ||||||
| 16.12. | Governing Law | 76 | ||||||
| 16.13. | Dispute Resolution; Jurisdiction | 77 | ||||||
| 16.14. | Bankruptcy Code | 77 | ||||||
| 16.15. | Joint and Several Obligations | 77 | ||||||
-iv-
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
| Exhibits |
||
| Exhibit 1.26 | Chemical Structure of Pacritinib Citrate | |
| Exhibit 1.34 | Co-Promotion Terms | |
| Exhibit 1.46 | CTI Patents | |
| Exhibit 1.47 | CTI Trademarks | |
| Exhibit 1.54 | Development Plan | |
| Exhibit 1.132 | Form of Registration Rights Agreement | |
| Exhibit 1.160 | Upstream Agreement | |
| Exhibit 8.2 | Manufacturing and Supply Agreement Terms | |
| Exhibit 9.1.2 | Preferred Stock Terms | |
| Exhibit 13.3.1 | Form of Press Release | |
| Exhibit 16.13 | Alternative Dispute Resolution | |
-v-
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
DEVELOPMENT, COMMERCIALIZATION AND LICENSE AGREEMENT
This DEVELOPMENT, COMMERCIALIZATION AND LICENSE AGREEMENT (this “Agreement”) is entered into on this 14th day of November, 2013 (the “Effective Date”), by and among CELL THERAPEUTICS, INC., a company organized under the laws of the State of Washington with its principal place of business at 0000 Xxxxxxx Xxxxxx, Xxxxxxx, XX 00000 (“CTI”), and XXXXXX INTERNATIONAL INC., a company organized under the laws of Delaware with its principal place of business at Xxx Xxxxxx Xxxxxxx, Xxxxxxxxx, XX 00000 (“BII”), XXXXXX HEALTHCARE CORPORATION, a company organized under the laws of Delaware with its principal place of business at Xxx Xxxxxx Xxxxxxx, Xxxxxxxxx, XX 00000 (“BHC”), and XXXXXX HEALTHCARE SA, a company organized under the laws of Switzerland with its principal place of business at Xxxxxxxx 0000, Xxxxxx, Xxxxxxxxxxx (“BHSA” and together with BII and BHC, “Baxter”). CTI and Baxter may each be referred to herein individually as a “Party” and collectively as the “Parties.”
RECITALS
WHEREAS, CTI is developing pacritinib for use in oncology and potentially additional therapeutic areas, and owns or controls certain patents, and proprietary technology, know-how and information relating to such compound;
WHEREAS, Baxter is engaged in the research, development and commercialization of pharmaceutical products, and desires to acquire a license in the Licensed Territory (as defined below) under CTI’s patents, technology, know-how and other information relating to such compound, and CTI desires to grant such license, on the terms and conditions of this Agreement; and
WHEREAS, the Parties wish to co-promote such product in the Co-Promotion Territory (as defined below), on the terms and conditions of this Agreement.
AGREEMENT
NOW, THEREFORE, in consideration of the mutual promises and covenants set forth below and other good and valuable consideration, the receipt and sufficiency of which is hereby acknowledged, the Parties agree as follows:
ARTICLE I.
The following terms as used in this Agreement shall have the meanings set forth in this Article I (which meanings shall be applicable both to the singular and the plural forms of such terms):
1.1. “Additional Indication” means any indication other than the Initial Indication.
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.2. “Affiliate” means with respect to each Party, any Person that directly or indirectly is controlled by, controls or is under common control with a Party. For the purposes of this definition only, the term “control” (including, with correlative meanings, the terms “controlled by” and “under common control with”) as used with respect to a Person means (a) in the case of a corporate entity, direct or indirect ownership of voting securities entitled to cast at least fifty percent (50%) of the votes in the election of directors, (b) in the case of a non-corporate entity, direct or indirect ownership of at least fifty percent (50%) of the equity interests or (c) with respect to any Person, the power to direct the management and policies of such entity.
1.3. “Alliance Manager” has the meaning set forth in Section 3.6.
1.4. “Bankruptcy Laws” has the meaning set forth in Section 16.14.
1.5. “Baxter Approved Supplier” has the meaning set forth in Section 12.5.2.
1.6. “Baxter Confidential Information” has the meaning set forth in Section 13.2.1(a).
1.7. “Baxter Exclusive Territory” means all of the Licensed Territory except for the Co-Promotion Territory, subject to the provisions of Section 7.1.4.
1.8. “Baxter Indemnitees” has the meaning set forth in Section 14.1.
1.9. “Baxter Know-How” means Information Controlled by Baxter or its Affiliates that is necessary for the Development, manufacture, use, Commercialization, sale, offer for sale and/or importation of the Licensed Products in the Licensed Field. Notwithstanding anything herein to the contrary, Baxter Know-How shall exclude Baxter Patents.
1.10. “Baxter Patents” means Patents Controlled by Baxter or its Affiliates that claim inventions necessary for the Development, manufacture, use, Commercialization, sale, offer for sale and/or importation of Licensed Products within the Licensed Field, including without limitation Xxxxxx’x interest in any Joint Patents.
1.11. “Baxter Trademarks” has the meaning set forth in Section 11.1.
1.12. “Business Day” means a day other than Saturday, Sunday or other day on which commercial banks in New York, New York are authorized or required by Law to close.
1.13. “Calendar Quarter” means the successive periods of three (3) consecutive calendar months ending on March 31, June 30, September 30 or December 31, for so long as this Agreement is in effect.
1.14. “Calendar Year” means any calendar year.
2
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.15. “Clinical Trial” means a clinical study conducted on certain numbers of human subjects (depending on the phase of the trial) that is designed to (a) establish that a pharmaceutical product is reasonably safe for continued testing, (b) investigate the safety and efficacy of the pharmaceutical product for its intended use, and to define warnings, precautions and adverse reactions that may be associated with the pharmaceutical product in the dosage range to be prescribed, and/or (c) support Marketing Approval or Reimbursement Approval of such pharmaceutical product or label expansion of such pharmaceutical product.
1.16. “CMO” means contract manufacturing organization.
1.17. “Combination Product” has the meaning set forth in the definition of Net Sales.
1.18. “Commercial Failure” means: **.
1.19. “Commercialization” means all activities related to the commercial exploitation of the Compound, the Drug Product and/or the Licensed Products, including, without limitation, importation, exportation, marketing, Promotion, distribution, pre-launch, launch, sale or offering for sale of the Licensed Products. When used as a verb, “Commercialize” or “Commercializing” means to engage in Commercialization.
1.20. “Commercialization Obligations” has the meaning set forth in Section 7.1.3.
1.21. “Commercialization Plan” has the meaning set forth in Section 7.1.5.
1.22. “Commercially Reasonable Efforts” means with respect to either Party, a level of efforts consistent with the efforts ** typically devotes to a product of similar market potential, ** at a similar stage in its development or product life, taking into account conditions then prevailing, including its safety and efficacy, product profile, cost to develop, cost and availability of supply, the time required to complete development, the competitiveness of the marketplace, the patent position with respect to such product (including the ability to obtain or enforce, or have obtained or enforced, such patent rights), the third-party patent landscape relevant to the product, the regulatory structure involved, the likelihood of regulatory approval, the anticipated or actual profitability of the applicable product and other technical, legal, scientific, regulatory and medical considerations.
1.23. “Committee” means the JSC, JDC, JCC and/or JMC as the context requires.
1.24. “Common Stock” has the meaning set forth in Section 9.1.2.
1.25. “Company SEC Documents” has the meaning set forth in Section 12.2.12.
1.26. “Compound” means 11-(2-Pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)] heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene (including any of its salt forms), the chemical structure of its citrate salt form of which is shown in Exhibit 1.26 attached hereto **.
3
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.27. “Confidential Information” means Baxter Confidential Information and/or CTI Confidential Information.
1.28. “Confidentiality Agreement” means that certain Mutual Confidential Disclosure Agreement between Baxter and CTI dated February 8, 2013.
1.29. “CONSOB” has the meaning set forth in Section 12.3.3.
1.30. “Control” means, with respect to Patents, know-how, trademarks, copyrights, trade secrets and Confidential Information, the possession of the ability to grant a license, sublicense or access as provided for under this Agreement without (a) violating the terms of any agreement or other arrangement with any Third Party or (b) increasing at any time the amount of any payments required under any such agreement or arrangement; provided that this clause (b) shall not apply to Upstream Agreements.
1.31. “Co-Promotion Agreement” has the meaning set forth in Section 2.7.
1.32. “Co-Promotion” means the activities conducted pursuant to Section 7.3, the Co-Promotion Terms and the Commercialization Plans.
1.33. “Co-Promotion Rights” means those rights retained by CTI pursuant to Section 2.7.
1.34. “Co-Promotion Terms” means the terms attached hereto as Exhibit 1.34.
1.35. “Co-Promotion Territory” means the U.S.
1.36. “Cost of Goods Sold” or “COGS” shall mean the cost of manufacturing of bulk drug substance, encapsulation, and packaging. The manufacturing costs shall include (a) the cost of material, labor, and appropriate administrative overhead of the contract manufacturer, (b) costs associated with logistics and shipping/transport and (c) costs for production line validation, quality assurance and quality control.
1.37. “CPR” has the meaning set forth in Section 16.13.
1.38. “CTI Assigned Joint Patents” has the meaning set forth in Section 10.2.
1.39. “CTI Assigned Patents” has the meaning set forth in Section 10.2.
1.40. “CTI Confidential Information” has the meaning set forth in Section 13.1.1(a).
1.41. “CTI Development Cost Budget” means the budget for CTI Development Costs included in an approved Development Plan.
1.42. “CTI Development Cost Threshold” has the meaning set forth in Section 9.4.2.1.
4
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.43. “CTI Development Costs” means expenses incurred by CTI in conducting Development activities in accordance with an approved Development Plan and the CTI Development Cost Budget approved by the JSC (with such JSC approval subject to the JDC governance provisions included in Section 3.1.6) or agreed by the Parties in writing, consisting of (a) the cost of actual direct FTEs, as recorded in CTI’s time reporting system, with costs calculated at the FTE Rate and (b) direct out-of-pocket expenses incurred in the performance of Development activities assigned to CTI under such Development Plan.
1.44. “CTI Indemnitees” has the meaning set forth in Section 14.2.
1.45. “CTI Know-How” means Information Controlled by CTI or its Affiliates that is necessary for the Development, manufacture, use, Commercialization, sale, offer for sale and/or importation of Licensed Products in the Licensed Field in the Licensed Territory. Notwithstanding anything herein to the contrary, CTI Know-How shall exclude CTI Patents.
1.46. “CTI Patents” means all Patents Controlled by CTI or its Affiliates that claim inventions necessary for the Development, manufacture, use, Commercialization, sale, offer for sale and/or importation of Licensed Products within the Licensed Field in the Licensed Territory, including CTI’s interest in any Joint Patents. CTI Patents include the Patents listed on Exhibit 1.46.
1.47. “CTI Trademarks” means the trademarks set forth on Exhibit 1.47 and such trademarks as CTI Controls during the Term and elects in its sole discretion to add to such Exhibit 1.47.
1.48. “Damages” has the meaning set forth in Section 14.1.
1.49. “Detail” means a face-to-face interaction with a targeted healthcare stakeholder where Licensed Product knowledge and/or information is exchanged.
1.50. “Development” means all activities related to the development of drug products and obtaining Regulatory Approval for drug products, including without limitation all activities related to research, development, preclinical testing, preclinical toxicology, preclinical pharmacokinetics, preclinical pharmacodynamics, stability testing, toxicology, formulation, Clinical Trials, regulatory affairs, statistical analysis, report writing, manufacturing process scale up (including without limitation, registration batches/process validation, engineering studies qualification and validation, process validation, characterization and stability, scale and technology transfer to CMOs), qualification and validation activities, quality assurance/quality control development and Regulatory Filing creation and submission related to obtaining Regulatory Approval for a Licensed Product. When used as a verb, “Develop” means to engage in Development.
1.51. “Development Account” has the meaning set forth in Section 9.4.4.
1.52. “Development Cost Summary” has the meaning set forth in Section 9.4.4.
5
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.53. “Development Milestone” has the meaning set forth in Section 9.2.1
1.54. “Development Plan” means the Development Plan attached hereto as Exhibit 1.54 setting forth the specific activities to be undertaken in connection with the Development of the Licensed Product including, but not limited to: **.
1.55. “Drug Approval Application” means an application for Marketing Approval required before commercial sale or use of a pharmaceutical product as a drug in a regulatory jurisdiction or country.
1.56. “Drug Product” means bulk drug product containing the Compound that is encapsulated, but excluding any final packaging, finishing and labeling. Once so packed, finished and labeled, a Drug Product is a Licensed Product.
1.57. “Election Time Period” has the meaning set forth in Section 14.3.1.
1.58. “EMA” means the European Medicines Agency or any successor agency or agencies thereto.
1.59. “Enforcing Party” has the meaning set forth in Section 10.5.3.
1.60. “Equity Consideration” has the meaning set forth in Section 9.1.2.
1.61. “European Union” or “EU” means all countries of the European Union, as may be included from time to time.
1.62. “Exchange Act” has the meaning set forth in Section 12.2.17.
1.63. “Executive Officers” has the meaning set forth in Section 3.1.6.2.
1.64. “FCPA” means the Foreign Corrupt Practices Act, as amended (15 U.S.C. §§ 78dd-1, et. seq.).
1.65. “FDA” means the U.S. Food and Drug Administration or any successor agency or agencies thereto.
1.66. “FDCA” means the United States Food, Drug and Cosmetic Act, as amended (21 U.S.C. §§ 301, et seq.).
1.67. “Field Correction” means any action taken or changes performed affecting a distributed product to mitigate a risk to health or correct issues with misbranded or non-conforming product.
1.68. “Field Representative” means a Sales Representative or a Medical Science Liaison, or both, as applicable.
6
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.69. “Fill/Finish Option” has the meaning set forth in Section 8.3.
1.70. “First Commercial Sale” means, with respect to a Licensed Product in any country in the Licensed Territory, the first sale of such Licensed Product under this Agreement for use in the Licensed Field to a Third Party in such country, after such Licensed Product has been granted Marketing Approval. ** be deemed to have occurred on such six (6) month anniversary.
1.71. “Force Majeure” has the meaning set forth in Section 15.4.1.
1.72. “FTE” means the full time equivalent effort of one CTI or Baxter (as applicable) employee who participates directly in the activities under an approved Development Plan or Commercialization Plan. For purposes of this definition, “full time equivalent effort” shall mean ** hours of work per year with reimbursement based on actual hours worked.
1.73. “FTE Rate” means the hourly rate of ** per hour for Development work pursuant to the Development Plan during Calendar Year 2013, increasing at the beginning of each subsequent Calendar Year over the prior year amount by the increase of the Consumer Price Index-All Urban Consumers during the prior year.
1.74. “Global Dossier” has the meaning set forth in Section 6.1.1.
1.75. “Governmental Authority” means any nation or government, any state, local or other political subdivision thereof, and any entity, department, commission, bureau, agency, authority, board, court, official or officer, domestic or foreign, exercising executive, judicial, regulatory or administrative governmental functions.
1.76. “Improvement” means any improvement or modification of a Licensed Product, Drug Product and/or the Compound that is developed by (a) CTI, (b) Baxter or, if rights thereto are obtained by Baxter pursuant to Section 10.1.3., its Sublicensees or (c) jointly by CTI and Baxter, for use in the Licensed Field.
1.77. “Indemnification Claim Notice” has the meaning set forth in Section 14.3.1.
1.78. “Indemnified Party” has the meaning set forth in Section 14.3.1.
1.79. “Indemnifying Party” has the meaning set forth in Section 14.3.1.
1.80. “Independently Active Pharmaceutical Ingredient” means an active pharmaceutical ingredient having a different target or mode of action, or is otherwise treated or designated by the applicable Regulatory Authority as a separate active ingredient, than the Compound.
1.81. “Information” means techniques, data, inventions, practices, methods, knowledge, know-how, skill, experience, test data including pharmacological, toxicological,
7
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
preclinical and clinical test data and analytical and quality control data or descriptions, including all proprietary information submitted to relevant Regulatory Authorities to support a Drug Approval Application.
1.82. “Initial Indication” means Myelofibrosis.
1.83. “Invention” means any and all discoveries, Developments, Improvements, modifications, formulations, compositions of matter, Processes and other inventions (whether patentable or not patentable) specifically related to the Compound, Drug Product and/or a Licensed Product or otherwise necessary or useful for the Development, manufacturing and/or Commercialization of the Compound, Drug Product and/or a Licensed Product made in the course of activities performed under this Agreement by or on behalf of either Party or both Parties.
1.84. “Investigator-Sponsored Trial” means any Clinical Trial of the Compound, Drug Product and/or Licensed Product for which either or both of the Parties provide Compound, Drug Product and/or Licensed Product and/or financial support, but do not sponsor the trial, it being understood and agreed that all such trials must be conducted in compliance with applicable Law and consistent with Baxter-specific requirements and policies for an investigator-sponsored trial, provided that in the case of conduct by CTI, such Baxter-specific requirements shall have been communicated to CTI in a timely manner.
1.85. “Joint Commercialization Committee” or “JCC” means the joint commercialization committee established pursuant to Section 3.5.
1.86. “Joint Development Committee” or “JDC” means the joint development committee established pursuant to Section 3.3.
1.87. “Joint Inventions” has the meaning set forth in Section 10.2.
1.88. “Joint Manufacturing Committee” or “JMC” means the joint manufacturing committee established pursuant to Section 3.4.
1.89. “Joint Patent” has the meaning set forth in Section 10.2.
1.90. “Joint Steering Committee” or “JSC” means the joint steering committee established pursuant to Section 3.2.
1.91. “Last Patient First Dose” means the administration of the first dose of Licensed Product or comparator to the last patient randomized in the PERSIST-1 or PERSIST-2 Clinical Trial (as applicable) as specified in the then-current protocol for such Clinical Trial.
1.92. “Law” means all applicable laws, statutes, rules, regulations, ordinances and other pronouncements having the effect of law of any federal, national, multinational, state, provincial, county, city or other political subdivision, domestic or foreign, including
8
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
without limitation all laws pertaining to the pharmaceutical industry or the healthcare industry and all anti-bribery or anti-corruption laws, including without limitation the FDCA and the FCPA and their implementing regulations and all foreign equivalents thereof.
1.93. “Licensed Field” means all human therapeutic indications.
1.94. “Licensed Product” means a pharmaceutical product in finished form that contains the Compound and all present and future formulations, dosages and dosage forms thereof.
1.95. “Licensed Rights” has the meaning set forth in Section 2.1.
1.96. “Licensed Territory” means worldwide, subject to the Co-Promotion Rights in the U.S.
1.97. “Litigation Conditions” has the meaning set forth in Section 14.3.1.
1.98. “Major Market Countries” means Australia, Canada, China, France, Germany, Italy, Japan, Spain, the United Kingdom and the U.S.
1.99. “Manufacturing Plan” has the meaning set forth in Section 3.4.1.
1.100. “Marketing Approval” means, with respect to a particular country or regulatory jurisdiction, the registrations, authorizations and approvals of the applicable Regulatory Authority or Governmental Authority in such country or regulatory jurisdiction (including, but not limited to, the EMA) that are necessary to market and sell a Licensed Product in such country or regulatory jurisdiction (e.g., a Marketing Authorization in the EMA) with respect to such Licensed Product in such country or region.
1.101. “Medical Affairs Representative” means a non-field-based medical science representative responsible for responding to unsolicited requests for medical information and otherwise engaging in the exchange of scientific information with healthcare stakeholders regarding Licensed Products.
1.102. “Medical Science Liaison” means a field-based medical science representative responsible for responding to unsolicited requests for medical information and otherwise engaging in the exchange of scientific information with healthcare stakeholders regarding Licensed Products.
1.103. “Myelofibrosis” means the disease or condition referred to and recognized by the FDA as myelofibrosis as of the date of this Agreement (whether or not such disease or condition continues to be known by such name in the U.S. after the date of this Agreement, and whether or not any Regulatory Authority outside of the U.S. refers to such disease or condition by that name or another name as of or after the date of this Agreement).
9
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.104. “Net Sales” means the gross amount invoiced for sales of Licensed Product by Xxxxxx and/or its Affiliates or Sublicensees to Third Parties, less the following deductions from such gross amounts to the extent attributable to such Licensed Product and to the extent actually incurred, allowed, accrued or specifically allocated:
(a) trade, cash and quantity discounts actually given, credits, price adjustments or allowances actually granted customers for damaged Licensed Products, returns or rejections of Licensed Products;
(b) chargeback payments and rebates (or the equivalent thereof) for the Licensed Products granted to group purchasing organizations, Third Party payors (including managed health care organizations) or federal, state/provincial, local and other governments, including their agencies, or to trade customers;
(c) reasonable and customary freight, shipping, insurance and other transportation charges directly related to the sale of the Licensed Products separately stated on the invoice to the Third Party; and
(d) sales, value added, excise taxes, tariffs and duties, and other taxes and government charges directly related to the sale, to the extent that such items are included in the gross invoice price of the Licensed Products and actually borne by Xxxxxx or its Affiliates, Sublicensees or distributors without reimbursement from any Third Party (but not including taxes assessed against the income derived from such sale);
all as determined in accordance with U.S. GAAP on a basis consistent with Xxxxxx’x annual audited financial statements.
The Parties agree that none of: (w) the transfer of Licensed Product between or among Xxxxxx and its Affiliates and Sublicensees for resale (which resale will give rise to Net Sales), (x) use of Licensed Product in a preclinical or Clinical Trial, (y) use of Licensed Product as free marketing samples or (z) the transfer of Licensed Product by Xxxxxx and/or its Affiliates to a Third Party in connection with the sale or donations for charitable, compassionate use or expanded access program purposes will be considered a sale for purposes of calculating any amounts due to CTI hereunder.
Upon the sale or other transfer of a Licensed Product, such sale or transfer will be deemed to constitute a sale with the consideration for the sale being the consideration for the relevant transaction and constituting Net Sales hereunder, or if the consideration is not a monetary amount, a sale will be deemed to have occurred for a price assessed on the value of whatever consideration has been provided in exchange for the sale. Disposal of a Licensed Product for or use of a Licensed Product in Clinical Trials or as free samples will not give rise to any deemed sale under this definition. For clarity, there will be no limit on the quantity of Licensed Products which may be used in Clinical Trials but the quantity of Licensed Products to be given away as free samples will be such quantities customary in the industry for this sort of Licensed Product. Such amounts will be determined from the books and records of Xxxxxx, its Affiliates and Sublicensees maintained in accordance with U.S. GAAP, consistently applied throughout the organization.
10
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
In the event a Licensed Product is sold in a finished dosage form containing the Compound in combination with one or more other Independently Active Pharmaceutical Ingredients (a “Combination Product”), Net Sales, for purposes of determining royalty payments under Section 9.3 on such Licensed Product, will be calculated by multiplying the Net Sales of the end user product by the fraction A over A+B, in which A is the net selling price of the Licensed Product portion of the Combination Product when such Licensed Products is sold separately during the applicable accounting period in which the sales of the Combination Product were made, and B is the net selling price of the other Independently Active Pharmaceutical Ingredients of the Combination Product sold separately during the accounting period in question. All net selling prices of the Licensed Product portion of the Combination Product and of the other Independently Active Pharmaceutical Ingredients of such Combination Product will be calculated as the average net selling price of the said ingredients during the applicable accounting period for which the Net Sales are being calculated in the particular country where the Combination Product is sold. In the event that, in any country or countries, no separate sale of either such above designated Licensed Product or such above designated other Independently Active Pharmaceutical Ingredients of the Combination Product are made during the accounting period in which the sale was made or if the net retail selling price for an Independently Active Pharmaceutical Ingredient cannot be determined for an accounting period, Net Sales allocable to the Licensed Product in each such country will be determined by mutual agreement reached in good faith by Xxxxxx and CTI prior to the end of the accounting period in question based on an equitable method of determining same that takes into account, on a country by country basis, variations in potency, the relative contribution of the Compound and each other Independently Active Pharmaceutical Ingredient in the combination, and relative value to the end user of the Compound and each such other Independently Active Pharmaceutical Ingredient. Xxxxxx and CTI agree that, for purposes of this paragraph, drug delivery vehicles, devices, adjuvants, half-life extenders, solubilizers and excipients will not be deemed to be Independently Active Pharmaceutical Ingredients.
1.105. “Operating Profit or Loss” has the meaning set forth in Exhibit 1.34.
1.106. “Other Joint Invention” has the meaning set forth in Section 10.4.2.
1.107. “Other Joint Patents” has the meaning set forth in Section 10.4.2.
1.108. “Outside Contractor” means any Person, other than a Sublicensee, contracted by a Party to provide products or services relating to the performance of such Party’s obligations under this Agreement, including without limitation CMOs and clinical research service providers.
11
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.109. “Pacritinib Citrate” means the citrate salt of 11-(2-Pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)] heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene, the chemical structure of which is shown in Exhibit 1.26 attached hereto.
1.110. “Patent” means (a) unexpired and currently in-force letters patent (or other equivalent legal instrument), including without limitation utility and design patents, and including without limitation any extension, substitution, registration, confirmation, reissue, re-examination or renewal thereof, (b) applications for letters patent, a reissue application, a continuation application, a continuation-in-part application, a divisional application or any equivalent of the foregoing applications, that are pending at any time during the term of this Agreement before a government patent authority and (c) all foreign or international equivalents of any of the foregoing in any country.
1.111. “Patent Term Extensions” has the meaning set forth in Section 10.8.
1.112. “Paying Party” has the meaning set forth in Section 9.8.
1.113. “PERSIST-1” means the Clinical Trial entitled “A Randomized Controlled Phase 3 Study of Oral Pacritinib versus Best Available Therapy in Patients with Primary Myelofibrosis, Post-Polycythemia Xxxx Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis,” CTI Protocol # PAC325, registered under EUDRA CT 0000-000000-00 and IND 78,406.
1.114. “PERSIST-2” means the Clinical Trial entitled “A Randomized Controlled Phase 3 Study of Oral Pacritinib versus Best Available Therapy in Patients with Thrombocytopenia and Primary Myelofibrosis, Post-Polycythemia Xxxx Myelofibrosis, or Post-Essential Thrombocythemia Myelofibrosis,” CTI Protocol # PAC326, registered under EUDRA CT 0000-000000-00 and IND 78,406.
1.115. “Person” means any individual, firm, corporation, partnership, limited liability company, trust, business trust, joint venture, Governmental Authority, association or other entity.
1.116. “Pharmacovigilance Agreement” has the meaning set forth in Section 7.5.2.
1.117. “Phase 3 Clinical Trial” means a Clinical Trial as defined in 21 C.F.R. 312.21(c), as may be amended from time to time, or the equivalent thereto in any jurisdiction other than the U.S. in the Licensed Territory.
1.118. “Post-Marketing Approval Study” means any Clinical Trial conducted following receipt of Marketing Approval of a Licensed Product in the applicable country or region, carried out for purposes of conducting safety surveillance, ongoing technical support of the Licensed Product, market access, or line extensions of existing approved indications but expressly excluding: (a) any safety registries mandated as a condition to the granting of any Marketing Approval and (b) any Investigator-Sponsored Trials.
12
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.119. “Preferred Stock” has the meaning set forth in Section 9.1.2.
1.120. “Principal Market” has the meaning set forth in Section 12.2.17.
1.121. “Product Infringement” has the meaning set forth in Section 10.5.2(a).
1.122. “Profit & Loss Share” has the meaning set forth in Section 9.4.10.
1.123. “Promote” means those activities, including without limitation Detailing and distributing samples of a product, normally undertaken by a pharmaceutical company’s sales force in accordance with applicable Laws to implement marketing plans and strategies aimed at encouraging the appropriate use of a particular prescription pharmaceutical product. When used as a verb, “Promote” shall mean to engage in such activities. “Promotion” and “Promotional” have correlative meanings.
1.124. “Prosecuting Party” has the meaning set forth in Section 10.4.2.
1.125. “Publications” has the meaning set forth in Section 13.1.3.
1.126. “Quality” has the meaning set forth in Section 8.4.
1.127. “Quality Agreement” has the meaning set forth in Section 8.4.
1.128. “Recall” means the removal or correction of a marketed product that the FDA or other Regulatory Authority considers to be in violation of the laws it administers and against which the agency would initiate legal action (e.g., seizure). A Recall may be conducted on a firm’s own initiative, by FDA or other Regulatory Authority request, or by FDA or other Regulatory Authority order under statutory authority. The words ‘Recalled,’ ‘Recalling,’ etc., shall have correlative meanings.
1.129. “Recall Costs” means all out-of-pocket expenses directly relating to or arising out of compliance with any order of a Regulatory Authority for a Recall, Withdrawal or Field Correction and out-of-pocket expenses incurred by either Party relative to notification, shipping, disposal and return of the Recalled or Withdrawn product and the notification and correction of any product subject to a Field Correction.
1.130. “Recipient Party” has the meaning set forth in Section 9.8.
1.131. “Registration Directed” means, with respect to a Clinical Trial, a Clinical Trial that is intended to serve as the basis for an application for Marketing Approval in the U.S. or the EU and would be successful if such Clinical Trial meets its primary endpoint or co-primary endpoints, as applicable.
13
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.132. “Registration Rights Agreement” means the agreement attached hereto substantially in the form of Exhibit 1.132.
1.133. “Regulatory Approval” has the meaning set forth in the definition of Regulatory Filings.
1.134. “Regulatory Authority” means any national, supra-national, regional, state or local regulatory authority, department, bureau, commission, council or other Governmental Authority in such country (including, but not limited to, the FDA and EMA) that is responsible for overseeing the development (including the conduct of Clinical Trials), manufacture, distribution, importation, exportation, transport, storage, marketing, Promotion, offer for sale, use or sale of the Compound, Drug Product and/or Licensed Product.
1.135. “Regulatory Exclusivity” means any rights or protections which are recognized, afforded or granted by any Regulatory Authority in any country or region of the Licensed Territory in association with the Marketing Approval of the Licensed Product, providing such Licensed Product: (a) a period of marketing exclusivity during which the applicable Regulatory Authority shall refrain from either reviewing or approving a Marketing Approval application or similar regulatory submission submitted by a Third Party seeking to market a competing product, or (b) a period of data exclusivity during which a Third Party seeking to market a competing product is precluded from either referencing or relying upon, without an express right of reference from the dossier holder, such Licensed Product’s clinical dossier or relying on previous Regulatory Authority findings of safety or effectiveness with respect to such Licensed Product to support the submission, review or approval of a Marketing Approval application or similar regulatory submission before the applicable Regulatory Authority. Regulatory Exclusivity shall include rights conferred in the European Union pursuant to Section 10.1(a)(iii) of Directive 2001/EC/83.
1.136. “Regulatory Filings” means any Drug Approval Application and any application for Reimbursement Approval, notification or other submission made to or with a Regulatory Authority that is necessary or reasonably desirable to Develop, manufacture or Commercialize the Licensed Product in the Licensed Field in a particular country or regulatory jurisdiction, whether made before or after receipt of Marketing Approval in the country or regulatory jurisdiction, and any approval resulting from any such application is a “Regulatory Approval”. The term ‘Regulatory Filings’ shall include all amendments and supplements to any of the foregoing and all proposed labels, labeling, package inserts, monographs and packaging for a Licensed Product in a particular country.
1.137. “Regulatory Role” has the meaning set forth in Section 6.1.
1.138. “Reimbursement Approval” means with respect to a particular country or regulatory jurisdiction, any pricing and reimbursement approvals of the applicable Regulatory Authority or Governmental Authority in such country or regulatory jurisdiction that are necessary or of material use in order to Commercialize a Licensed Product in such country or region at the relevant time.
14
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.139. “Rest of World” or “ROW” means the Xxxxxx Exclusive Territory, excluding the EU, Canada and Switzerland.
1.140. “Royalty Term” has the meaning set forth in Section 9.3.3.
1.141. “Sales Milestone” has the meaning set forth in Section 9.2.2.
1.142. “Sales Report” means with respect to each Calendar Quarter a report detailing
(a) the relevant gross amounts invoiced or billed in each local currency by Xxxxxx or its Affiliates or Sublicensees to Third Parties, including wholesalers, hospitals or other intermediate Third Parties, indicating the breakdown of sales by each type of the Licensed Product;
(b) the deductions from gross amounts invoiced or billed used to calculate Net Sales;
(c) the Net Sales in each local currency;
(d) the Bloomberg exchange rates and dates used; and
(e) the sum of royalties due pursuant to Section 9.3.
1.143. “Sales Representative” means a field-based sales representative charged with Detailing targeted healthcare stakeholder regarding Licensed Products.
1.144. “S*BIO Agreement” means the Asset Purchase Agreement dated May 31, 2012, between S*BIO Pte Ltd. (“S*BIO”) and CTI, as amended from time to time.
1.145. “SEC” has the meaning set forth in Section 12.2.12.
1.146. “Securities” has the meaning set forth in Section 9.1.2.
1.147. “Securities Act” has the meaning set forth in Section 9.1.2.
1.148. “Sole Inventions” has the meaning set forth in Section 10.2.
1.149. “Subject Country” has the meaning set forth in Section 7.1.4.
1.150. “Subject Patents” has the meaning set forth in Section 10.4.1.
1.151. “Sublicensee” means, with respect to Xxxxxx, any Affiliate or Third Party to which Xxxxxx sublicenses the Licensed Rights and, with respect to CTI, any Affiliate or Third Party to which CTI sublicenses the rights granted under Section 2.2.
15
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.152. “Supply Agreement” means a Manufacturing and Supply Agreement between CTI and Xxxxxx pursuant to which CTI will, by itself or through one or more Third Party CMOs selected by CTI and reasonably acceptable to Xxxxxx, supply to Xxxxxx its requirements of Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product) for sale and distribution in the Licensed Territory.
1.153. “Supply Cap” means, for Calendar Year 2014 the following price per capsule of Pacritinib Citrate in Drug Product form, which price shall be increased at the beginning of each subsequent Calendar Year over the prior year amount by the percentage increase of the Consumer Price Index-All Urban Consumers during the prior year or, for applicable European countries, by the percentage increase in the Harmonized Indices of Consumer Prices:
| Volume (number of capsules) |
Price Per Capsule | |||
| ** |
* | * | ||
| ** |
* | * | ||
| ** |
* | * | ||
| ** |
* | * | ||
For the avoidance of doubt, the prices are not incremental. That is, if Xxxxxx purchases ** capsules in a calendar year, the maximum supply price (excluding packaging) is ** (subject to the annual adjustment noted above). Should any backup supplier (as agreed by the JMC and/or the JSC) materially increase the expected cost per capsule, then the Parties shall negotiate in good faith to modify the Supply Cap to reflect the additional costs of such backup supplier.
1.154. “Supply Failure” shall have the meaning set forth in the Supply Agreement.
1.155. “Target Product Profile” or “TPP” means the written document with respect to a Licensed Product that: (a) specifies the labeling concepts that are the ‘base case’ goals of the Development program, (b) documents the specific studies intended to support the labeling concepts for such Licensed Product, and (c) forms the basis for a constructive dialogue with the FDA or EMA. The Target Product Profile sets forth the preferred version of what is requested to be claimed in labeling for such Licensed Product which then guide the design, conduct, and analysis of Clinical Trials to maximize the efficiency of the Development of such Licensed Product. The TPP shall be determined on an Indication-by-Indication basis by the JDC. It is expected but not certain that the final version of the TPP would be similar in content to the annotated draft labeling submitted with a new drug application or biologics license application; provided, however, that there shall be no consequences hereunder if the labeling meets at least the foregoing minimum acceptable labeling. The Parties acknowledge that the TPP attached hereto as part of the Exhibit 1.54 does not contain the ‘minimum acceptable labeling’ for the Licensed Product. The Parties shall, no later than March 31, 2015, agree on and document the minimum acceptable labeling which shall then become a part of the TPP and shall be incorporated therein; provided, however, that in developing such ‘minimum acceptable labeling’, Section 3.1.6.3(a) shall not apply.
16
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
1.156. “Term” has the meaning set forth in Section 15.1.
1.157. “Third Party” means any Person other than CTI and Xxxxxx and their respective Affiliates.
1.158. “Trademark Infringement” has the meaning set forth in Section 11.2.5.
1.159. “Upfront Fee” has the meaning set forth in Section 9.1.1.
1.160. “Upstream Agreement” means each agreement existing as of the Effective Date under which CTI obtains or has obtained a license or other right from a Third Party to Develop, make, use, sell, offer for sale or import the Compound, Drug Product and/or Licensed Product in the Licensed Territory, but excluding Clinical Trial agreements, material transfer agreements, or other agreements which do not grant material rights with respect to the Licensed Rights. Exhibit 1.160 sets forth a true and complete list of all Upstream Agreements.
1.161. “U.S.” means the United States of America, its territories and possessions.
1.162. “U.S. GAAP” means U.S. Generally Accepted Accounting Principles.
1.163. “USD” means U.S. Dollar.
1.164. “Valid Claim” means any claim within a pending, allowed or issued U.S. Patent application or Patent or pending, accepted or issued Patent application or Patent in a jurisdiction outside the U.S. that has not expired, lapsed, been cancelled or abandoned, or been held unenforceable, invalid, or cancelled by a court of competent jurisdiction in an order or decision from which no appeal has been or can be taken. For purposes of this definition, a “pending” Patent application will include any such Patent application that has been pending for seven (7) or fewer years in the case of U.S. Patent applications or eight (8) or fewer years in the case of all other Patent applications.
1.165. “Withdrawal” means a removal or correction of a distributed product which involves a minor violation that would not be subject to legal action by the FDA or other Regulatory Authority, or which involves no violation of a Law. The words ‘withdrawn’ ‘withdrawing’, etc. shall have correlative meanings.
1.166. “Withholding Taxes” has the meaning set forth in Section 9.8.
ARTICLE II.
GRANT OF LICENSES
2.1. License to Xxxxxx. Subject to the terms and conditions of this Agreement, CTI hereby grants to Xxxxxx, and Xxxxxx accepts, an exclusive (even as to CTI, except as provided in Section 2.3 and Section 2.7), royalty-bearing, non-transferable (except pursuant to Section 16.1) right and license under CTI Know-How and CTI Patents, with the right, subject to Section 2.4, to
17
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
grant sublicenses through multiple tiers of Sublicensees, to research, Develop, make, have made, import, export, use, sell, offer for sale, have sold and otherwise Commercialize Licensed Products in the Licensed Field in the Licensed Territory (the “Licensed Rights”). Notwithstanding the foregoing, Xxxxxx shall not have the right to exercise its right to “make or have made” with respect to the Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product) unless there has been a Supply Failure.
2.2. License to CTI. Subject to the terms and conditions of this Agreement, Xxxxxx hereby grants to CTI, and CTI accepts, a non-exclusive, royalty-free, non-transferable (except pursuant to Section 16.1 and for sublicenses to Affiliates ), non-sublicensable right and license under Xxxxxx Know-How and Xxxxxx Patents to: (a) research, Develop, import, export and use, in the Licensed Field in the Licensed Territory as contemplated under the Development Plan, as amended in accordance herewith, and as contemplated under Section 4.1, (b) manufacture and have manufactured the Licensed Product in the Licensed Territory to fulfill its obligations under the Supply Agreement and (c) import, sell, offer for sale, have sold and otherwise directly Commercialize Licensed Products in the Co-Promotion Territory.
2.3. CTI Reservation of Rights. CTI hereby retains the right under the CTI Patents and CTI Know-How to (a) conduct research and Development activities with respect to the Licensed Products in the Licensed Territory as contemplated under the Development Plan and as contemplated under Section 4.1, (b) subject to: (i) the rights granted to Xxxxxx to manufacture or have manufactured Licensed Products and/or Drug Product in the event of a Supply Failure and (ii) Xxxxxx’x exercise of its option to perform the final packaging pursuant to Section 8.3, manufacture and have manufactured Licensed Product and/or Drug Product (as applicable) in the Licensed Territory to fulfill its obligations under the Supply Agreement and (c) exercise the Co-Promotion Rights set forth in Section 2.7.
2.4. Sublicenses. Xxxxxx shall have the right to sublicense its rights under Section 2.1 to its Affiliates and, excluding the Co-Promotion Territory, to Third Parties; provided that if Xxxxxx proposes to grant a sublicense to a Third Party granting the Third Party the right to Commercialize the Licensed Product in a Major Market Country in the Xxxxxx Exclusive Territory, Xxxxxx shall provide CTI with written notice of such sublicense at least thirty (30) days in advance of executing such sublicense, which notice shall include a reasonable level of detail regarding the terms of the proposed sublicense, and CTI shall have the right to approve or reject such sublicense (such approval not to be unreasonably withheld). Each agreement between Xxxxxx and a Sublicensee (a) shall be in writing and subject and subordinate to, and consistent with, the terms and conditions of this Agreement; (b) shall not diminish, reduce or eliminate any of Xxxxxx’x obligations under this Agreement; (c) shall require the Sublicensee(s) to comply with all applicable terms of this Agreement (except for payment obligations, for which Xxxxxx shall remain financially responsible); and (d) shall prohibit further sublicensing except on terms consistent with this Section 2.4. Xxxxxx shall provide written notice to CTI of any agreement entered into with a Sublicensee, and shall provide a complete copy of such agreement to CTI within thirty (30) days of its execution, which copy may be redacted by Xxxxxx to the extent that such redactions do not reasonably impair CTI’s ability to ensure compliance with this
18
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Agreement. Xxxxxx shall be responsible for the performance of each Sublicensee and shall ensure that each Sublicensee complies with all relevant provisions of this Agreement. In the case of any such Sublicense **, Xxxxxx shall pay to CTI ** an amount equal to ** of any Sublicensee fee, upfront license fee or milestone (whether regulatory, based on sales or otherwise) or other payment related to such Sublicense not based on a royalty on net sales paid to Xxxxxx by or on behalf of such Sublicensee, it being understood that if such payment is made in a form other than in cash, the Parties shall use good faith reasonable efforts to agree on the fair market value thereof and the cash equivalent shall be paid to CTI.
2.5. No Other Licenses. Neither Party grants to the other Party any rights, licenses or covenants in or to any intellectual property, whether by implication, estoppel, or otherwise, other than the license rights that are expressly granted under this Agreement or the Supply Agreement. Notwithstanding anything to the contrary herein, neither Party grants to the other Party any license or other right to manufacture, Develop or Commercialize any active ingredients other than the Compound, including without limitation any method of making any active ingredients other than the Compound, any composition or formulations of any active ingredients other than the Compound, or any method of using or administering any active ingredients other than the Compound.
2.6. Controlled IP. Subject to Section 9.3.2, if, after the Effective Date, either Party enters into an agreement or other arrangement to obtain a license or other rights to or under Information or Patents that are owned or controlled by a Third Party and that would, solely but for the operation of subsection (b) of the definition of Control, in the case of CTI be included in the CTI Information or CTI Patents or, in the case of Xxxxxx, be included in the Xxxxxx Information or Xxxxxx Patents, then the Party obtaining such license or rights shall promptly notify the other Party and shall specify in such notice the type and amount of payments that would be due to such Third Party by reason of the practice or use of, or access to, such Information or Patents by the other Party pursuant to the license set forth in Section 2.1 or Section 2.2, as applicable (but not by reason of the practice or use of, or access to, such Information or Patents outside the scope of such license). The Party receiving such notice may elect in writing to bear the responsibility for such additional payments, and upon such receiving Party’s written election to bear such responsibility, the Information or Patents as applicable, shall thereafter be deemed “Controlled” by the Party originally obtaining such license or rights (notwithstanding subsection (b) of the definition of Control), and shall be subject to the license under Section 2.1 or 2.2, as applicable.
2.7. Co-Promotion. CTI hereby retains the co-exclusive right (with Xxxxxx) under the CTI Know-How and CTI Patents, and Xxxxxx hereby grants to CTI, the co-exclusive right (with Xxxxxx) under the Xxxxxx Know-How and Xxxxxx Patents, to Promote the Licensed Products in the Co-Promotion Territory, in accordance with the terms and conditions of the Co-Promotion Terms attached hereto as Exhibit 1.34, the Commercialization Plan and Section 7.2. It is anticipated that at an appropriate time following the Effective Date, the Parties will enter into a separate agreement (a “Co-Promotion Agreement”) that more precisely details the Co-Promotion activities of the Parties in the Co-Promotion Territory and this Agreement shall be appropriately
19
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
amended to defer all Co-Promotion matters to the Co-Promotion Agreement. Unless otherwise mutually agreed by the Parties, the Co-Promotion Agreement shall be consistent with the terms of this Agreement and the Co-Promotion Terms.
2.8. Subcontracting. Xxxxxx acknowledges that in connection with CTI’s obligations to perform the Development of the Licensed Product and to manufacture and supply Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product) to Xxxxxx pursuant to the Supply Agreement, CTI may need to use certain Outside Contractors; provided, however, that CTI shall notify Xxxxxx in writing prior to engaging any Outside Contractor for any material activities (excluding activities conducted under Clinical Trial agreements and other less significant agreements) for which it is obligated under this Agreement and each agreement with an Outside Contractor: (a) shall be in writing and be consistent with the terms and conditions of this Agreement; (b) shall not diminish, reduce or eliminate any of CTI’s obligations under this Agreement; (c) shall require the Outside Contractor to comply with all applicable terms of this Agreement (except for payment obligations, for which CTI shall remain financially responsible); and (d) shall prohibit further subcontracting. For the avoidance of doubt, CTI may not subcontract its Co-Promotion Rights in the Co-Promotion Territory.
ARTICLE III.
GOVERNANCE
3.1. General Committee Procedures.
3.1.1. Authority. Each Committee shall have the responsibilities and authority allocated to it in this Article III and elsewhere in this Agreement. The following procedures shall apply to the Committees under this Agreement.
3.1.2. Composition. Within ** after the Effective Date, each Party shall appoint ** of its employees to serve on the JSC and ** employees to the JDC. Within ** after the effective date of the Supply Agreement, each Party shall appoint ** of its employees to serve on the JMC, and at least ** prior to filing any application for Marketing Approval, each Party shall appoint ** of its employees to serve on the JCC. Each Party may replace its representatives to the JSC by written notice to the other Party. Each Party may replace representatives of Committees at their own discretion.
3.1.3. Chairperson; Qualifications. For the JSC initial meeting, the chairperson shall be appointed by CTI, and thereafter shall alternate between the Parties annually. The chairperson will be responsible for calling meetings and, after seeking input from the other Party, setting the agenda (which shall include a list of all participants expected at a meeting) and circulating such agenda at least ** prior to each meeting and distributing minutes of the meetings within ** following such meeting, but will not otherwise have any greater power or authority than any other member of the JSC. The Committee members of the JDC, JMC and JCC shall have such expertise as appropriate to the activities of the Committee from time to time, and the Committee may invite personnel of the Parties having non-clinical safety and animal pharmacology, pharmaceutical development, clinical, biostatistical, regulatory affairs,
20
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
pharmacovigilance, formulation, manufacturing, commercial, marketing, legal and other expertise to participate in discussions of the Committee from time to time as appropriate to assist in the activities of the Committee.
3.1.4. Meetings. The JSC and each Committee shall, after appointment of its initial members, meet at least once every ** at times mutually agreed upon by the Parties. At least two (2) meetings each year shall be held in person. The location of the meetings of the Committee to be held in person shall be agreed upon by the Parties (with the intent that it should alternate between the Parties’ respective headquarters locations and/or be held at the time and sites of major medical conferences attended by both Parties such as ASH annually in December, EHA annually in June and ASCO annually in June). Each Party shall use reasonable efforts to cause its representatives to attend the meetings of the Committee. If a representative of a Party is unable to attend a meeting, such Party may designate an alternate to attend such meeting in place of the absent representative. Each Party shall bear all the expenses of its representatives on the Committee.
3.1.5. Minutes. The minutes of each Committee meeting shall be distributed to the members within ** after the completion of the relevant Committee meeting and shall provide a description in reasonable detail of the discussions held at the meeting and a list of any actions, decisions or determinations approved by the Committee. Minutes of each Committee meeting shall be approved or disapproved, and revised as necessary, within ** after the applicable Committee meeting.
3.1.6. Matter Resolution; Escalation.
3.1.6.1 Each Party shall have one (1) vote on all matters that are within the responsibility of a Committee, regardless of the number of such Party’s representatives on such Committee. The members of each Committee will use best reasonable efforts to reach unanimous consensus on all decisions.
3.1.6.2 In the event that the members of the JDC, JCC or the JMC are unable to reach consensus on a particular issue within ** after such issue is first presented to the JDC, JCC or the JMC (as applicable), such issue shall be referred to the JSC. If the members of the JSC are unable to reach consensus on a particular issue (including any payments or amounts due from one Party to the other) within ** after such issue is first presented to the JSC, such issue shall be referred to an executive officer of each Party or their designees selected for such purpose and notified to other Party (the “Executive Officers”) for resolution. Each Party shall use good faith efforts to resolve issues at the lowest level possible within the governance structure established by this Article III and, notwithstanding the escalation procedures set forth herein, to limit any such escalations.
3.1.6.3 If the Executive Officers are unable to resolve such issue within ** after such issue is referred to them, then the following will control: **.
21
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
3.1.6.4 In the event an issue arises which is not covered by the foregoing, including without limitation the fulfilling of the obligation to use Commercially Reasonable Efforts in any respect, either Executive Officer may invoke the dispute resolution provisions of Section 16.13.
3.1.6.5 For the avoidance of doubt, no Committee shall have the power to amend or waive compliance with this Agreement nor, without the consent of the affected Party, to materially increase or reduce the obligations of the Parties under this Agreement.
3.2. Joint Steering Committee. The JSC shall have the responsibility for the overall strategic and operational direction of the Parties’ collaboration under this Agreement and shall have the authority (but not the obligation) in connection therewith, to establish certain sub-Committees or other Committees in addition to the JDC, JCC and/or JMC. The JSC shall have responsibility for overseeing the activities of the JDC, JCC, JMC and any additional sub-Committees established during the course of this Agreement (with all JSC responsibilities subject to the JDC, JCC and JMC governance provisions included in Section 3.1.6). The JSC’s responsibilities shall also include:
3.2.1. reviewing and evaluating progress under the Development Plan and the Manufacturing Plan;
3.2.2. reviewing and approving each Development Plan, Manufacturing Plan and Commercialization Plan, including amendments thereto, submitted by the JDC, JMC and JCC (with such approvals subject to the JDC, JCC and JMC governance provisions included in Section 3.1.6);
3.2.3. identifying, prioritizing and approving Additional Indications for the Licensed Product beyond the Initial Indication; and
3.2.4. appointing additional Committees and sub-Committees as desired, such Committees and sub-Committees to have equal representation from the Parties and subject to governance by the JSC (such committees would be sub-committees of either the JDC, JCC or JMC and would be subject to the JDC, JCC and JMC governance provisions included in Section 3.1.6).
3.3. Joint Development Committee. The JDC shall oversee and direct the Development and regulatory strategy of the Licensed Product in the Licensed Field in the Licensed Territory, and shall coordinate communications among the Parties and the exchange of Information regarding the Development and regulatory activities pursuant to the Agreement. The JDC’s responsibilities shall include:
3.3.1. discussing, preparing and approving for submission to the JSC the Development Plan for each Licensed Product in the Licensed Territory, including any amendments thereto (subject to the JDC governance provisions included in Section 3.1.6);
22
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
3.3.2. discussing, preparing and approving for submission to the JSC budgets for the activities contemplated by the Development Plan, as amended, including without limitation the CTI Development Cost Budget (which shall be deemed part of the Development Plan, as amended) (subject to the JDC governance provisions included in Section 3.1.6);
3.3.3. overseeing implementation of each Development Plan, including monitoring progress of preclinical, pharmaceutical and clinical studies of the Licensed Product, proposing additional studies for the Licensed Product, and reviewing and commenting on regulatory submissions in the Licensed Territory relating to the Licensed Product;
3.3.4. reviewing and commenting on worldwide publication strategy with respect to the Compound, Drug Product and/or Licensed Product;
3.3.5. establishing procedures regarding the collection, sharing and reporting of Adverse Event information related to the Licensed Products consistent with the Pharmacovigilance Agreement to be entered into in accordance with the terms of Section 7.5 and reviewing and assessing compliance therewith;
3.3.6. evaluating and establishing the patent prosecution and maintenance strategy together with appropriate legal counsel for each Party;
3.3.7. coordinate and confer concerning Investigator-Sponsored Trials;
3.3.8. coordinate and confer concerning Regulatory Filings by the Parties as provided in Article VI, including establishing procedures for preparing, coordinating and approving such Regulatory Filings which shall be based to the extent reasonably possible on Xxxxxx’x templates and processes;
3.3.9. overseeing and coordinating the Parties’ activities regarding medical and scientific affairs under Section 7.6; and
3.3.10. performing such other functions as appropriate to further the purposes of this Agreement, as directed by the JSC (for the avoidance of doubt, all JDC responsibilities included in this Section 3.3 are subject to the governance provisions included in Section 3.1.6).
3.4. Joint Manufacturing Committee. The JMC shall oversee and direct the manufacturing strategy of the Licensed Products in the Licensed Field in the Licensed Territory, and shall coordinate communications among the Parties and the exchange of Information regarding the manufacturing activities pursuant to this Agreement as further specified in Article VIII. The JMC’s responsibilities shall include:
3.4.1. discussing, preparing and approving for submission to the JSC a manufacturing plan for each Licensed Product in the Licensed Territory, including any amendments thereto (each, a “Manufacturing Plan”);
23
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
3.4.2. overseeing implementation of each Manufacturing Plan;
3.4.3. meeting, discussing and using good faith efforts to resolve issues which arise with respect to manufacturing due to circumstances out of the control of the Parties (e.g. implementation of regional or global directives or regulations of Governmental Authorities which require changes to the manufacturing process or specifications for the Licensed Product) or the occurrence of any Force Majeure event, including without limitation changes to be made to either the supply timelines and/or Supply Cap, as necessary or appropriate; and
3.4.4. performing such other functions as appropriate to further the purposes of this Agreement, as directed by the JSC (for the avoidance of doubt, all JMC responsibilities included in this Section 3.4 are subject to the governance provisions included in Section 3.1.6).
3.5. Joint Commercialization Committee. The JCC shall oversee and direct the Commercialization strategy for the Licensed Products in the Licensed Field in the Licensed Territory, taking into consideration CTI’s Co-Promotion Rights, and shall coordinate communications among the Parties and the exchange of Information regarding the Commercialization activities pursuant to this Agreement as further specified in Article VII. The JCC’s responsibilities shall include:
3.5.1. discussing and reviewing the Commercialization Plans prepared by Xxxxxx and amendments thereto;
3.5.2. overseeing implementation of each Commercialization Plan (including discussion and implementation of approaches to minimize the risk of enforcement actions by Regulatory Authorities in connection with the Promotion of the Licensed Product);
3.5.3. discussing and preparing amendments to the Co-Promotion Terms (or, if a separate Co-Promotion Agreement is executed by the Parties, discussing and preparing amendments to the Co-Promotion Agreement);
3.5.4. assessing performance on each Commercialization Plan, including forecasting relevant matters including without limitation sales and expenses, and assessing the accuracy of such forecasts;
3.5.5. coordinating branding on a global basis for Licensed Products;
3.5.6. discussing and submitting to the JSC for approval, Third Party vendors to be engaged to perform marketing research and other marketing services; and
3.5.7. performing such other functions as appropriate to further the purposes of this Agreement, as directed by the JSC (for the avoidance of doubt, all JCC responsibilities included in this Section 3.5 are subject to the governance provisions included in Section 3.1.6).
24
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
3.6. Alliance Managers. Promptly after the Effective Date, each Party shall appoint an individual to act as alliance manager for such Party, which may be one of the representatives of such Party on the JSC (each, an “Alliance Manager”). The Alliance Managers shall be the primary point of contact for the Parties regarding the activities contemplated by this Agreement and shall facilitate all such activities hereunder. The Alliance Managers shall attend all meetings of the JSC and shall be responsible for assisting the JSC in performing its oversight responsibilities. The name and contact information for each Party’s Alliance Manager, as well as any replacement(s) chosen by such Party, in its sole discretion, from time to time, shall be promptly provided to the other Party in accordance with Section 16.4.
ARTICLE IV.
DEVELOPMENT RESPONSIBILITIES
4.1. Development Obligations.
4.1.1. CTI shall, subject to the oversight of the JSC, use its Commercially Reasonable Efforts to direct, coordinate and manage the Development of Licensed Products for the Initial Indication and Additional Indications in accordance with this Agreement and the Development Plan (as such may be amended from time to time). Through the JDC and/or the JSC, each Party shall have the right to propose changes and additions to the Development Plan on an ongoing basis as necessary. The JSC shall have the authority to review and approve such changes and additions, provided that the Development Plan shall at all times contain terms that are consistent with this Article IV. Each Party may suggest to the other changes and additions to the Development Plan and the other Party shall reasonably consider including such changes and additions when developing amendments to the Development Plan in accordance with this Section 4.1.
4.1.2. ** The Parties shall, through the JDC, coordinate efforts to ensure that for any Development costs and expenses incurred prior to the achievement of the CTI Development Cost Threshold (and in particular for those Development costs and expenses for Additional Indications beyond those set forth in the Development Plan as of the Effective Date), priority shall be given to spending for the Initial Indication; provided, however, that the Parties may alter the priority if a Commercial Failure has occurred with respect to the Initial Indication or the Parties have otherwise determined to de-prioritize the Initial Indication.
ARTICLE V.
DEVELOPMENT COOPERATION
5.1. Sharing of Development Updates and Related Information.
5.1.1. Whenever any material event occurs in the course of the Development of a Licensed Product by either Party of which such Party becomes aware, but in no event less than once every Calendar Quarter until such time as Marketing Approval for the Licensed Product has been obtained in each country of the Licensed Territory, such Party shall disclose to the other Party Information regarding and resulting from all Development and Commercialization
25
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
activities with respect to the Compound, Drug Product and/or Licensed Product conducted by such Party, which Information is necessary or useful for Development or Commercialization of the Licensed Product in the Licensed Territory, and a description of the status of such Development efforts.
5.1.2. Each Party shall have the right, at no additional expense, to use all Information disclosed to it pursuant to Section 5.1.1 for the Development and Commercialization of the Licensed Product, and to grant to its Sublicensees the right to use such Information for the Development and Commercialization of the Licensed Product, without any obligation to the other Party except as otherwise set forth in this Agreement.
5.2. Information Sharing Coordination. The JSC shall coordinate the Parties’ sharing of Information as required under this Agreement or under applicable Laws:
5.2.1. Each Party shall share with the other Party, free of charge (except for amounts becoming due and payable to CTI pursuant to Article IX), any and all Information generated or compiled during the term of this Agreement for use in Development and Commercialization of the Licensed Product in the Licensed Territory.
5.2.2. Xxxxxx agrees that all Information delivered by CTI or any of its Affiliates hereunder shall, as between the Parties, at all times be and remain sole and exclusive property of CTI or its Affiliates, respectively.
5.2.3. CTI agrees that all Information delivered by Xxxxxx or any of its Affiliates or Sublicensees hereunder shall, as between the Parties, at all times be and remain sole and exclusive property of Xxxxxx or its Affiliates or Sublicensees, respectively.
ARTICLE VI.
REGULATORY MATTERS
6.1. Regulatory Roles. Subject to the obligations set forth in Article XV, the Parties shall have responsibility for the following activities with respect to Regulatory Filings for the Licensed Product (each Party’s activities, its “Regulatory Role”):
6.1.1. CTI will be responsible (in collaboration with Xxxxxx through the JDC) for the creation of a global dossier (the “Global Dossier”) that will be the basis for all Regulatory Filings in the Licensed Territory.
6.1.2. CTI shall (in collaboration with Xxxxxx through the JDC) prepare all Regulatory Filings (which shall be reviewed in a timely manner by Xxxxxx) necessary for obtaining Marketing Approval in the name of CTI with respect to Licensed Products in the Co-Promotion Territory for the Initial Indication, such Regulatory Filings to be based on the Global Dossier. CTI shall promptly (but in any event within ten (10) Business Days of receipt thereof) assign such Marketing Approval to Xxxxxx upon receipt of such Marketing Approval. In addition, CTI will transfer all the source documentation, analysis files, source program files (e.g.,
26
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
SAS files, etc.), Clinical Trial master files, case report forms, validation reports, study reports and all documentation supporting the Drug Approval Application to Xxxxxx in a reasonable time-frame (as determined by the JSC) following receipt of Marketing Approval in the first country or geography, provided that CTI may retain copies of all of the foregoing for use in connection with its activities under this Agreement. Final approval for all such Regulatory Filings shall be in accord with the governance provisions of Section 3.3.
6.1.3. CTI shall be responsible (in coordination with Xxxxxx through the JDC) for responding to all requests and for managing all inspections/audits from any Regulatory Authority in the Territory related to the PERSIST-1 and/or PERSIST-2 Clinical Trials until receipt of Marketing Authorization in the applicable country. CTI shall also be responsible for implementing any commitments resulting from such inspections/audits. All costs resulting from any such inspections/audits shall be considered Development expenses.
6.1.4. Xxxxxx shall (in collaboration with CTI through the JDC) prepare all Regulatory Filings necessary for obtaining Marketing Approvals and Reimbursement Approvals in the name of Xxxxxx with respect to Licensed Products in the Xxxxxx Exclusive Territory for all Additional Indications, such Regulatory Filings to be based on the Global Dossier. Further, Xxxxxx shall also be responsible for submitting the Regulatory Filings necessary for obtaining Marketing Approvals (as prepared by CTI based upon the Global Dossier) and Reimbursement Approvals in the name of Xxxxxx with respect to Licensed Products in the Xxxxxx Exclusive Territory for the Initial Indication. Xxxxxx may, as necessary, appoint CTI as its agent for the purposes of the foregoing in this Section 6.1.4.
6.1.5. If at any time Reimbursement Approval becomes necessary or advisable in the Co-Promotion Territory, the Parties shall cooperate and work together to obtain such Reimbursement Approval.
6.1.6. Xxxxxx shall own and be the license holder for all Marketing Approvals. Subject to the cost allocation provisions set forth in Section 9.4, Xxxxxx shall be responsible for complying with all requirements of Regulatory Authorities in the Licensed Territory after receipt of the applicable Marketing Approval(s); provided, however, that with respect to the Co-Promotion Territory, the Parties shall be jointly responsible for such compliance including the cost thereof.
6.1.7. Except as set forth in Section 9.4 with respect to the filing fees for the Regulatory Filings for Licensed Products, each Party shall bear its own costs and expenses for effecting its respective Regulatory Role.
6.2. Cooperation.
6.2.1. The Parties will cooperate in providing technical regulatory expertise for assistance in developing the submission strategy for Regulatory Filings and defining technical content. Each Party shall designate a global regulatory affairs representative (who shall be a member of the JDC) to represent it in connection with Regulatory Filings. Each such
27
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
representative will be invited to attend critical meetings with Regulatory Authorities associated with the submission of Regulatory Filings for Licensed Products. Each Party shall provide required support to the other through the JDC to ensure timely regulatory submissions during the review cycle for the Marketing Approval in each of the geographies/countries within the Licensed Territory and for any Post-Marketing Approval regulatory submission on an as-needed basis. All significant regulatory correspondence will be reviewed in a timely manner by Xxxxxx prior to submission to the regulatory authorities in each of the geographies/countries within the Licensed Territory. Final approval for all such Regulatory Filings shall be in accord with the governance provisions of Section 3.3.
6.2.2. Through the JDC, each Party shall have the right to review the content of all Regulatory Filings for the Licensed Product prepared by such other Party and each Party agrees to consider in good faith the comments of the other Party with respect to each such Regulatory Filing.
6.2.3. Each Party shall, within their respective Regulatory Roles, use Commercially Reasonable Efforts to obtain Marketing Approvals and, where applicable, Reimbursement Approvals for the Licensed Products in the Licensed Territory.
6.2.4. Without limiting the foregoing, each Party shall endeavor to keep the other Party well informed with respect to the contents of any Regulatory Filings related to the Licensed Products in the Licensed Territory and all discussions with the applicable Regulatory Authorities.
6.2.5. CTI shall inform Xxxxxx of all changes (including both changes in process as well as new contracts with CMOs to be entered into after December 1, 2013) that are being considered with respect to the manufacturing process for the Licensed Product that may reasonably be expected to impact the manufacture of the Licensed Product or the Commercialization thereof, and shall obtain Xxxxxx’x consent (which consent shall not be unreasonably withheld, conditioned or delayed) prior to the implementation of any such manufacturing changes.
ARTICLE VII.
LAUNCH AND COMMERCIALIZATION
OF LICENSED PRODUCTS
7.1. Launch and Commercialization.
7.1.1. Xxxxxx shall have full responsibility for and control of all Commercialization activities for the Licensed Products in the Xxxxxx Exclusive Territory, at Xxxxxx’x sole cost and expense, and shall use Commercially Reasonable Efforts to market, Promote, sell and otherwise Commercialize Licensed Products in the Xxxxxx Exclusive Territory in accordance with the terms of this Agreement and in compliance with all applicable Laws. Notwithstanding anything to the contrary, the provisions of this Article VII are all subject to CTI’s Co-Promotion Rights pursuant to Sections 2.7 and 7.2.
28
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
7.1.2. After receiving Marketing Approval and Reimbursement Approval of a Licensed Product for a country in the Xxxxxx Exclusive Territory, Xxxxxx shall use Commercially Reasonable Efforts to market, Promote and sell the Licensed Product in such country. ** Xxxxxx shall notify CTI promptly of the date of First Commercial Sale of a Licensed Product in each country of the Xxxxxx Exclusive Territory.
7.1.3. Xxxxxx shall use Commercially Reasonable Efforts to (a) launch commercially a Licensed Product in a given country as set forth in Section 7.1.2 above and (b) not withdraw a Licensed Product from sale or abandon the sale of a Licensed Product after First Commercial Sale; **.
7.1.4. **.
7.1.5. In connection with Xxxxxx’x Commercialization Obligations, Xxxxxx shall undertake appropriate activities related to pre-launch, launch and Commercialization of a Licensed Product, which activities may include the development of: (a) a market access strategy including Licensed Product pricing and Reimbursement Approval, (b) a pre-launch and post-launch publication strategy and the scheduling of targeted meetings for presentations of clinical data, (c) a product supply chain distribution strategy, (d) a patient education plan for the Licensed Products, (e) an overall sales and marketing strategy for the Licensed Product, (f) patient support programs, (g) a physician and hospital targeting plan, and (h) a sales execution plan. At least one (1) year prior to the first anticipated approval of an application for Marketing Approval in any Major Market Country in the Xxxxxx Exclusive Territory, Xxxxxx shall have prepared and delivered to the JCC a preliminary, non-binding commercialization plan documenting the activities, plans and strategies that will be implemented to market and commercialize such Licensed Product, which shall be reviewed by the JCC. Xxxxxx shall provide to the JCC a final, binding version of such commercialization plan (the “Commercialization Plan”) ** in the applicable country within the Licensed Territory. The Parties shall review and discuss each version of the Commercialization Plan through the JCC, and Xxxxxx will, in good faith, consider any changes proposed by CTI to each version of the Commercialization Plan. Xxxxxx shall use Commercially Reasonable Efforts to implement and execute the Commercialization Plan. The Commercialization Plan shall also cover Co-Promotion activities consistent with the Co-Promotion Terms in order to reflect developments subsequent to the initial preparation thereof; provided, however, that no amendment to the Co-Promotion Terms may be made without the unanimous approval of the Parties.
7.2. Co-Promotion. Pursuant to the rights granted under Section 2.7, the following provisions, in addition to the Co-Promotion Terms, shall govern the Parties’ activities regarding Commercialization of Licensed Products in the Co-Promotion Territory:
7.2.1. The obligations of Xxxxxx in Section 7.1 for the Xxxxxx Exclusive Territory shall apply to each Party in the Co-Promotion Territory.
29
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
7.2.2. Xxxxxx and CTI will jointly direct the training of both Parties’ Sales Representatives for the Co-Promotion Territory (together with the first line managers who oversee such Sales Representatives) and will prepare and implement a training program and training materials for such Sales Representatives. Both Parties will cause its Sales Representatives assigned to Promote Licensed Products to attend and complete a training program developed by Xxxxxx and CTI for the Licensed Product in the Co-Promotion Territory to assure a consistent, focused on-label Promotional strategy and message as and to the extent consistent with applicable Law.
7.2.3. Each Party will be solely responsible for recruiting, hiring and maintaining its sales force of Sales Representatives for Promotion of Licensed Products in the Co-Promotion Territory in accordance with its standard procedures and the requirements of this Agreement. Each Party will be responsible for the activities of its Sales Representatives, including compliance by its Sales Representatives with training and Detailing requirements. In particular, each Party will provide its Sales Representatives assigned to Promote Licensed Products in the Co-Promotion Territory with the level of oversight, management, direction and sales support with respect to the Promotion of the Licensed Product necessary to effectively and efficiently Promote Licensed Product in accordance with the terms of this Agreement and applicable Law. If either Party or a knowledgeable Third Party, such as a health care professional or representative of a competitor, raises any concern with the other Party regarding the performance or fitness of such other Party’s Sales Representative, such other Party will appropriately address such concerns in a manner consistent with the provisions of this Agreement, the Commercialization Plan, such Party’s policies and procedures and applicable Law, including removal of such Field Representative from the Promotion of Licensed Products and shall inform the notifying Party of the resolution of the concern.
7.2.4. Each Party’s Sales Representatives assigned to Promote the Licensed Product in the Co-Promotion Territory will utilize only Promotional materials that have been approved by the JCC and Xxxxxx’x and CTI’s promotional review committees. Neither Party, including without limitation, their Sales Representatives, is permitted to create “home-made” promotional pieces, and each Party shall maintain policies that prohibit such activity. All Detailing activities conducted by each Party’s Sales Representatives will be consistent in all material respects with the Promotional materials so approved. Each Party will train and instruct their respective Sales Representatives to make only those statements and claims regarding the Licensed Product, including as to efficacy and safety, that are consistent with the Licensed Product labeling and accompanying inserts and the approved Promotional materials, as well as applicable Laws and regulations.
7.2.5. For the purposes of cost allocation, and to the extent a Sales Representative is Detailing additional products beyond the Licensed Product, such FTE cost will reflect the time actually spent on the Licensed Product, and not any other additional products.
7.2.6. If either Party is not at any particular time able to provide, for any reason, the number of Sales Representatives specified in the Commercialization Plan, Co-Promotion Terms
30
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
or Co-Promotion Agreement (as applicable), then the other Party will have the right to make up such shortfall using its Sales Representatives until such time as such Party is able to provide its agreed upon number of Sales Representatives, and, for clarity, appropriate costs related to such shortfall incurred by the other Party shall be included in the calculation of Operating Profit or Loss. CTI and Xxxxxx will engage Sales Representatives having the minimum qualifications set forth in the Co-Promotion Terms. Each Party agrees that any of its Sales Representatives conducting activities with respect to the Licensed Product will not have any legal or regulatory disqualifications, bars or sanctions, and if a Party learns that a Sales Representative is subject to such disqualifications, bars or sanctions, such Sales Representative shall be terminated immediately (subject to applicable Law).
7.2.7. Each Party will maintain records and otherwise establish procedures designed to ensure compliance with all applicable Laws and professional requirements that apply to the Promotion and marketing of Licensed Product in the Co-Promotion Territory, including compliance with the PhRMA Code on Interactions with Healthcare Professionals.
7.2.8. Each Commercialization Plan shall include a section planning and updating the Co-Promotion activities of the Parties.
7.3. Coordination of Marketing Efforts. Sales and Marketing efforts within the Co-Promotion Territory will be under the direction of the JCC and subject to the principles described below:
7.3.1. The JCC shall establish policies and procedures to ensure coordinated branding on a global basis for the Licensed Products. Each Party shall share with the other Party free of charge any and all of marketing information in the control of the Party with respect to the Licensed Products, including without limitation, complete Promotion plans and strategies, as well as all Promotional and sales activities and materials used for the launch and marketing of the Licensed Product in the Licensed Territory.
7.3.2. If practicable, the Parties shall conduct joint, and not separate, sales training and education events such as Plan of Action meetings, annual sales meetings, and other such usual and customary commercial activities that are limited to and specific to the Licensed Products. This section does not prohibit either Party from having independent training and education events that are not limited to and specific to the Licensed Product. For example, Xxxxxx may continue to hold compliance training or its annual sales meetings for its businesses and CTI personnel shall only be able to attend at the sole discretion of Xxxxxx. Such activities shall be coordinated by the JCC.
7.3.3. Xxxxxx shall share with CTI free of charge any and all of marketing information in the control of Xxxxxx with respect to the Licensed Products, including without limitation copies of complete Promotion plans and strategies, as well as all Promotional and sales activities and materials used for the launch and marketing of the Licensed Product in the Licensed Territory, whether generated by Xxxxxx or its Sublicensees. All such information (other than materials used or to be used for such launch and marketing) shall be treated as Xxxxxx Confidential Information.
31
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
7.4. Recalls.
7.4.1. Generally. In the event that either Party:
7.4.1.1 determines that an event, incident, or circumstance has occurred which may result in the need for a Recall, Withdrawal, Field Correction or other removal of any Licensed Product or any lot or lots thereof from the market in the Licensed Territory;
7.4.1.2 determines that an event, incident, or circumstance that could reasonably adversely affect the Licensed Product in the Licensed Territory has occurred which is reasonably likely to result in the need for a Recall, Withdrawal, Field Correction or other removal of any Licensed Product, or any lot or lots thereof from the market;
7.4.1.3 becomes aware that a Regulatory Authority is threatening or has initiated an action to remove the Licensed Product from the market in the Licensed Territory or, if such event could reasonably adversely affect the Licensed Product in the Licensed Territory, any Regulatory Authority is threatening or has initiated an action to remove the Licensed Product from the market; or
7.4.1.4 is required by any Regulatory Authority to distribute a “Dear Doctor” letter or its equivalent regarding use of the Licensed Product in the Licensed Territory,
it shall promptly advise the other Party in writing with respect thereto, and shall provide to the other Party copies of all relevant correspondence, notices, and the like in the possession or control of such Party. In such event, Xxxxxx shall have the sole authority to determine if a Recall or other removal of the Licensed Product is required in the Licensed Territory, and shall be responsible for conducting any such Recall, Withdrawal, Field Correction or other removal of any Licensed Product from the Licensed Territory, whether voluntary or involuntary, or taking such other remedial action required by applicable Laws in the Licensed Territory.
7.4.2. CTI Assistance. At Xxxxxx’x reasonable request, CTI shall assist Xxxxxx with respect to any such Recall, Withdrawal or Field Correction, and shall provide Xxxxxx with all information that Xxxxxx may reasonably request in connection with its dealings with a Regulatory Authority in connection with such Recall, Withdrawal or Field Correction.
32
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
7.4.3. Recall Costs. Notwithstanding anything else contained in this Agreement to the contrary, if a Recall, Withdrawal, Field Correction or other removal of any Licensed Product or any lot or lots thereof from the market in the Licensed Territory:
7.4.3.1 results solely from a breach by CTI or its Affiliates or agents of the terms of this Agreement or the Supply Agreement, then CTI shall bear all Recall Costs and shall bear all costs for any assistance provided by CTI to Xxxxxx or its Affiliates or agents in connection with such Recall, Withdrawal, Field Correction or other removal;
7.4.3.2 results solely from a breach by Xxxxxx or its Affiliates or agents of the terms of this Agreement or the Supply Agreement, then Xxxxxx shall bear all Recall Costs and shall bear all costs for any assistance provided by CTI to Xxxxxx or its Affiliates or agents in connection with such Recall, Withdrawal, Field Correction or other removal;
7.4.3.3 results both from a breach by Xxxxxx or its Affiliates or agents of the terms of this Agreement or the Supply Agreement and a breach by CTI or its Affiliates or agents of the terms of this Agreement or the Supply Agreement, then all Recall Costs and all costs for any assistance provided by CTI to Xxxxxx or its Affiliates or agents in connection with such Recall, Withdrawal, Field Correction or other removal shall be allocated to each of Xxxxxx and CTI in an amount proportional to the contribution of each Party’s breach to the necessity of the Recall, Withdrawal, Field Correction or other removal; and
7.4.3.4 does not result from a breach by either Party or its Affiliates or agents of the terms of this Agreement or Supply Agreement, then ** of all ** in connection with such Recall, Withdrawal, Field Correction or other removal.
7.5. Safety Information; Adverse Events; Pharmacovigilance.
7.5.1. During the Term, each Party shall provide to the other Party, all information of which it becomes aware relating to the occurrence of any serious adverse event or any adverse event related to the Licensed Product in accordance with the Pharmacovigilance Agreement contemplated in Section 7.5.2. In addition, all information learned in connection with the Development of the Licensed Product shall be exchanged by the Parties per procedures developed by the Joint Development Committee in accordance with Section 3.3.5. The Parties will mutually agree upon, and the JSC (or a sub Committee thereof) will coordinate the preparation and maintenance of, safety databases, periodic safety update reports, global safety reports, etc.
7.5.2. Ninety (90) days prior to the submission of a Regulatory Filing for Marketing Approval in any country in the Licensed Territory, but in no event later than twelve (12) months after the Effective Date, the Parties shall enter into a Pharmacovigilance Agreement substantially in the form of the Xxxxxx Pharmacovigilance Agreement template previously made available to CTI (the “Pharmacovigilance Agreement”) concerning all matters relating to pharmacovigilance and the exchange of safety information for the Licensed Product, including, but not limited to: (a) the maintenance by the Parties of an adverse event database for the Licensed Product
33
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
following FDA Marketing Approval, (b) AE or ADR reporting (as such acronyms are commonly understood in the industry) including literature review and associated reporting, (c) AE or ADR follow-up reporting, (d) preparation and submission of all safety reports to the Regulatory Authorities as required by local laws and/or regulations in the Licensed Territory, (e) global safety database maintenance, (f) management of all interactions with health authorities relating to the safety of the Licensed Product, including establishment and maintenance of a formal process to adequately communicate urgent issues regarding safety and labeling, (g) periodic submissions, (h) labeling modifications, (i) safety monitoring and detection, and (j) safety measures (e.g., “Dear Doctor” letter, restriction on distribution).
7.5.3. All costs and expenses related to the establishment and maintenance of any safety registry mandated as part of Marketing Approvals shall be shared as follows: (a) if the safety registry is applicable solely to the Co-Promotion Territory, **, (b) if the safety registry is applicable to the Co-Promotion Territory and one or more countries outside the Co-Promotion Territory, Xxxxxx shall be responsible for ** of the costs and expenses and CTI shall be responsible for ** of the costs and expenses and (c) in all other cases, **.
7.6. Medical and Scientific Affairs.
7.6.1. Subject to Section 3.1.6 and Section 3.5, Xxxxxx and CTI shall discuss and approve a medical affairs plan within three (3) months of the Effective Date and Xxxxxx and CTI shall jointly develop and execute medical and scientific affairs and programs (including supporting professional symposia and other educational activities, and medical affairs studies based upon approved protocols) within the Co-Promotion Territory. All materials must be reviewed and approved by the Parties’ respective Medical Affairs and Legal departments (as applicable). Xxxxxx will have the exclusive authority to execute medical and scientific affairs and programs (including professional symposia and other educational activities, and medical affairs studies based upon approved protocols) for all other geographies within the Licensed Territory.
7.6.2. Subject to Section 3.1.6 and Section 3.5, Xxxxxx and CTI shall jointly develop guidelines for market access approach within three (3) months of the Effective Date and thereafter shall jointly develop and execute market access activities (including wholesaler and distributor management, local contracting and pricing, payor relations and patient support programs and the designation of any personnel for any of the foregoing) in the Co-Promotion Territory. Xxxxxx will have exclusive authority to develop and execute market access activities (including wholesaler and distributor management, local contracting and pricing, payor relations and patient support programs and the designation of any personnel for any of the foregoing) for all other geographies within the Licensed Territory.
7.6.3. All questions and requests for information about Licensed Products received by CTI or Xxxxxx with respect to the Co-Promotion Territory that: (a) warrant a clinical response beyond the competence of a Sales Representative, or (b) are beyond the scope of labels and package inserts for the Licensed Product (whether within or outside of the Co-Promotion Territory) shall be referred to Xxxxxx’x or CTI’s Medical Affairs Representatives or to the
34
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
relevant Third Party vendor, it being understood that the Parties’ goal and intent is a coordinated response. Should such request require more interaction than simply providing reprints of published research (in which case Xxxxxx’x medical information department or Third Party vendor may so provide such reprint), such referral will result in either a CTI or a Xxxxxx Medical Affairs Representative having the exclusive right to respond to such questions and requests for information about Licensed Products, depending on which Party is responsible for servicing the account asking such question or making such request for information and other relevant considerations.
7.6.4. Both Parties for the Co-Promotion Territory and Xxxxxx for the Xxxxxx Exclusive Territory shall be solely responsible for recruiting, hiring and maintaining its own staff of Medical Affairs Representatives and Medical Science Liaisons for responding to questions and requests for information about Licensed Products, in accordance with its standard procedures and the requirements of this Agreement. Each Party shall be responsible for the activities of its Medical Affairs Representatives and Medical Science Liaisons, including compliance by its Medical Affairs Representatives and Medical Science Liaisons with training requirements. In particular, each Party shall provide its Medical Affairs Representatives and Medical Science Liaisons assigned to respond to questions and requests for information about Licensed Products with the level of oversight, management, direction and support with respect to activities related to the Licensed Product in accordance with the terms of this Agreement and applicable Law.
7.6.5. Each Party’s Medical Affairs Representatives and Medical Science Liaisons for the Co-Promotion Territory and Xxxxxx’x Medical Affairs Representatives and Medical Science Liaisons for the Xxxxxx Exclusive Territory assigned to respond to questions and requests for information about Licensed Products may use available clinical information that has been approved by the JCC. All activities conducted by each Party’s Medical Affairs Representatives will be consistent in all material respects with the materials so approved. Each Party shall train and instruct its respective Medical Affairs Representatives and Medical Science Liaisons to make only those statements and claims regarding the Licensed Product, including as to efficacy and safety, that are consistent with the Licensed Product labeling and accompanying inserts, applicable Laws and regulations and the materials approved by the JCC unless responding to an unsolicited request for off-label information.
7.7. Springback Option.
7.7.1. Upon the one (1) year anniversary of receipt of the first Regulatory Approval of a Licensed Product in a Major Market Country, and on each yearly anniversary thereafter, Xxxxxx shall provide to CTI a report setting forth, for each Major Market Country (other than those which are members of the EU) and each country within the Xxxxxx Exclusive Territory with a population greater than ** people (as identified by the CIA World Fact Book) ** (collectively, the “Springback Countries”), whether or not Regulatory Approval has been received for a Licensed Product. If Regulatory Approval has been received for a given Springback Country, the report shall specify whether Xxxxxx is actively Commercializing the Licensed Product in such country. If Regulatory Approval has not been received for any such country, the report shall specify whether Xxxxxx, in its own good-faith determination, is actively pursuing Regulatory Approval of the Licensed Product in such country.
35
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
7.7.2. Following the one (1) year anniversary of receipt of the first Regulatory Approval in a Major Market Country, if CTI receives a bona fide offer from any Third Party with respect to the potential Commercialization of the Licensed Product in a Springback Country in which Xxxxxx is not actively pursuing Regulatory Approval or Commercialization (as applicable), CTI shall refer such inquiry to Xxxxxx in writing. If, within a period of ** following such referral, Xxxxxx has not: (a) entered into a sublicense with such Third Party with respect to such Springback Country or (b) undertaken reasonable efforts directed toward obtaining Regulatory Approval for or, if Regulatory Approval has been received, Commercialization of, the Licensed Product in such Springback Country, CTI may, upon written notice to Xxxxxx, terminate the Agreement with respect to such Springback Country. Upon termination of the Agreement with respect to such country, CTI shall have the right, at its sole cost and expense, either on its own or through a Third Party, to Develop and Commercialize the Licensed Product in such Springback Country. **
ARTICLE VIII.
MANUFACTURING AND SUPPLY
8.1. General Supply Terms. As between the Parties, and subject to the provisions of the Supply Agreement: CTI will be solely responsible, by itself or through one or more CMOs, for the manufacture and supply of Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product). CTI shall be responsible for all capital expenditures associated with the engagement of any CMOs by CTI for the manufacture and supply of Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product) to Xxxxxx. Unless otherwise agreed to by the Parties, CTI shall not be responsible for capital expenditures associated with the engagement of any CMOs by Xxxxxx or otherwise incurred by Xxxxxx for the manufacture and supply of Licensed Product to Xxxxxx and/or CTI hereunder. If Xxxxxx exercises its Fill/Finish Option (as defined below), Xxxxxx will be solely responsible, by itself or through one or more CMOs, for the manufacture and supply of the fill and finish (such as blistering and package inserts), freight, insurance and other subsequent supply chain expenses to produce Licensed Product from Drug Product. The Parties will use Commercially Reasonable Efforts to perform their respective obligations under this Article VIII.
8.2. Supply Agreement. On or before a date to be established by the JSC but in no event later than one hundred eighty (180) days after the Effective Date, the Parties shall enter into the Supply Agreement. The terms of such Supply Agreement shall be negotiated in good faith by the Parties and will contain customary terms and conditions that are consistent with this Agreement and the terms attached hereto as Exhibit 8.2. The purchase price for the Drug Product under the Supply Agreement will be CTI’s actual Cost of Goods Sold through manufacture of the Drug Product in capsule form, subject to the Supply Cap. Fill and finish (such as blistering and package inserts), freight, insurance and other subsequent supply chain expenses are not subject to the Supply Cap. The Supply Agreement will provide appropriate
36
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
technology transfer provisions (including provision of drug master files and other customary terms in the industry) sufficient to enable Xxxxxx or CTI, as appropriate, to manufacture the Licensed Product in the event that there is a Supply Failure.
8.3. Fill/Finish Option. During the Term of this Agreement, Xxxxxx shall have the option, exercisable by providing written notice to CTI to perform, by itself or through one or more CMOs, the packaging, finishing and labeling activities (such as blistering and package inserts), to produce Licensed Product from Drug Product (the “Fill/Finish Option”). Upon exercise of the Fill/Finish Option the Parties (a) shall cooperate to execute any technology transfer that is necessary to allow Xxxxxx or its CMO(s) to perform such activities and (b) enter into a second supply agreement pursuant to which Xxxxxx will agree to convert the Drug Product supplied by CTI pursuant to the Supply Agreement into Licensed Product and will thereafter supply such Licensed Product to CTI for sale by CTI in the Co-Promotion Territory. The terms of such second supply agreement will be negotiated by the Parties in good faith.
8.4. Quality Agreement. In connection with the negotiation and execution of the Supply Agreement, the Parties shall also, within one hundred eighty (180) days of the effective date of the Supply Agreement, enter into a separate agreement governing the quality control, quality assurance and validation (the “Quality”) of any Drug Product and/or Licensed Product (as applicable) delivered to Xxxxxx under the Supply Agreement (the “Quality Agreement”). The Quality Agreement shall be negotiated in good faith by the Parties, will contain customary terms and conditions that are consistent with this Agreement, and shall set forth the respective requirements, roles and responsibilities of the Parties which shall be consistent with the following:
8.4.1. Prior to receipt of Marketing Approval in the United States: (a) CTI shall have final functional Quality decision-making authority with respect to the Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product) and the manufacture thereof and (b) CTI shall have responsibility for Quality oversight of all Third Party manufacturers for the Licensed Product that are engaged by CTI for the manufacture and supply of Licensed Product and/or Drug Product (as applicable) to Xxxxxx (including, as applicable, any Third Party vendors).
8.4.2. Following receipt of Marketing Approval in the United States: (a) Xxxxxx shall have final functional Quality decision-making authority with respect to the Licensed Product and the manufacture thereof and (b) Xxxxxx shall have responsibility for Quality oversight of the Third Party manufacturers for Licensed Product (including, as applicable, any Third Party vendors).
8.5. Manufacturing Licenses. Notwithstanding anything contained in this Agreement to the contrary, but subject to Xxxxxx’x right to manufacture or have manufactured the Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product) in the event of a CTI Supply Failure as provided in the Supply Agreement, the Parties acknowledge and agree that CTI or its CMO shall hold all manufacturing licenses for the Licensed Product and/or Drug Product (as applicable).
37
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
ARTICLE IX.
PAYMENTS
9.1. Upfront Fee.
9.1.1. License Fee. In consideration of the licenses and other rights granted to Xxxxxx under this Agreement, in addition to the payments specified in Section 9.2 and Section 9.3, Xxxxxx shall pay to CTI or an Affiliate designated by CTI, upon the Effective Date or the next Business Day, a one-time, non-refundable, non-creditable license fee equal to sixty million U.S. dollars (USD 60,000,000) (the “Upfront Fee”), provided that, in the event that Xxxxxx purchases any Preferred Stock (defined below) pursuant to Section 9.1.2, said Upfront Fee shall be reduced by the aggregate stated value of the dollar amount of Equity Consideration paid by Xxxxxx for such Preferred Stock.
9.1.2. Equity Investment. At Xxxxxx’x option, Xxxxxx shall purchase from CTI, and CTI shall issue and sell to Xxxxxx on the Effective Date, up to 30,000 shares of convertible preferred stock of CTI (any such shares actually purchased by Xxxxxx, the “Preferred Stock”) for up to thirty million U.S. dollars (USD 30,000,000) at a purchase price of $1,000 per share of Preferred Stock (the aggregate purchase price of such Preferred Stock, the “Equity Consideration”), such Preferred Stock to have terms substantially as set forth as Exhibit 9.1.2, in accordance with the following provisions of this Section 9.1.2; provided, however, that at CTI’s option, CTI may, in lieu of issuing shares of Preferred Stock, issue a number of shares of CTI common stock equal to the quotient obtained by dividing the Equity Consideration by an amount equal to one hundred ten percent (110%) of the last consolidated closing bid price per share of CTI common stock on NASDAQ immediately prior to the effectiveness of this Agreement, rounded to the nearest whole share. The purchase and sale of the applicable Securities (defined below) for the Equity Consideration shall occur as soon as practicable following the Effective Date (and in no event later than three (3) Business Days following the Effective Date). The Preferred Stock shall be convertible into CTI common stock (such common stock into which the Preferred Stock is convertible, together with any CTI common stock issued in lieu of the Preferred Stock, the “Common Stock,” and together with the Preferred Stock, the “Securities”) and have a stated value per share of $1,000, and the conversion price for the purpose of determining the number of shares of Common Stock to be issued upon conversion of the shares of Preferred Stock shall be equal to one hundred ten percent (110%) of the last consolidated closing bid price per share of CTI common stock on NASDAQ immediately prior to the effectiveness of this Agreement. The Securities will not initially be registered under the Securities Act of 1933, as amended (“Securities Act”) and will accordingly bear applicable restricted securities legends, but CTI has agreed to register the Common Stock pursuant to the terms of the Registration Rights Agreement, to be executed and delivered on the Effective Date. Notwithstanding any other provision of this Agreement to the contrary, CTI shall in no event be required to issue any Securities to Xxxxxx pursuant to this Section 9.1.2 to the extent that such issuance (a) in the aggregate constitutes, or is convertible into, CTI common stock that constitutes, greater than 13.9% of CTI common stock outstanding after giving effect to any Preferred Stock issuance hereunder, (b) in the aggregate constitutes, or is convertible into
38
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Common Stock that constitutes, greater than 19.9% of the common stock or voting power of CTI outstanding as of the date of this Agreement prior to the issuance of the Securities, (c) would constitute a change of control under NASDAQ rules, (d) would otherwise trigger a shareholder approval requirement under NASDAQ rules or (e) would cause Xxxxxx, together with its Affiliates, to become a Beneficial Owner (as defined in the Shareholder Rights Agreement between CTI and Computershare Trust Company, N.A. as rights agent dated December 28, 2009, as amended or as may be amended from time to time) of 20% or more of CTI’s outstanding common stock, except in the case of the foregoing clauses (b), (c) and (d), with the prior approval of CTI’s stockholders. In the event that Xxxxxx purchases any Securities under this Section 9.1.2, CTI shall deliver to Xxxxxx, on the Effective Date, copies of the certificate(s) representing such Securities, and shall, promptly thereafter, deliver to Xxxxxx the original certificate(s).
9.2. Milestones. In addition to the payments specified in Section 9.1 and Section 9.3, Xxxxxx shall make the following payments to CTI in consideration of the licenses and other rights granted to Xxxxxx under this Agreement:
9.2.1. Development Milestones. Xxxxxx shall pay to CTI or an Affiliate designated by CTI the following non-creditable, non-refundable amounts ** (each, a “Development Milestone”):
| Events with respect to the Initial Indication | Milestone Payment |
|||
| ** |
$ | * | * | |
| ** |
$ | * | * | |
| ** |
$ | * | * | |
| ** |
$ | * | * | |
| ** |
$ | * | * | |
| Event with respect to an Additional Indication | Milestone Payment |
|||
| ** Registration Directed ** Clinical Trial ** |
$ | 15,000,000 | ||
39
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
9.2.2. Sales Milestones. Xxxxxx shall pay to CTI or an Affiliate designated by CTI the following non-creditable, non-refundable amounts ** (each, a “Sales Milestone”):
| First time Calendar Year Net Sales exceed the following amounts: | Milestone Payment |
|||
| ** |
$ | * | * | |
| ** |
$ | * | * | |
| ** |
$ | * | * | |
| ** |
$ | * | * | |
For purposes of clarity, Xxxxxx shall be obligated to make a Sales Milestone payment corresponding to each of the foregoing events only once, regardless of whether such Sales Milestone event occurs during more than one Calendar Year. If more than one Sales Milestone event is first achieved in the same Calendar Year, Xxxxxx shall be obligated to pay the corresponding milestone payment for all such events.
9.2.3. No Set-Off. Subject to Section 9.6, all payments under this Section 9.2 shall be made without setoff or deduction of any kind.
9.3. Royalty Payment; Audits.
9.3.1. Royalty Payments. In addition to the payments specified in Section 9.1 and Section 9.2, in consideration of the rights granted to Xxxxxx under this Agreement, Xxxxxx shall pay to CTI or an Affiliate designated by CTI (a) a royalty of ** of Net Sales of the Licensed Product in the Xxxxxx Exclusive Territory for Licensed Products for all Additional Indications, and (b) the following royalties on annual Net Sales of the Licensed Product in the Xxxxxx Exclusive Territory for the Initial Indication:
| Calendar Year Net Sales | Royalty Rate |
|||
| ** |
* | * | ||
| ** |
* | * | ||
9.3.2. Royalty Stacking. If Xxxxxx, in the opinion of reputable patent counsel, is required to obtain a license under any Patent from any Third Party in order to fulfill its obligations under this Agreement, and if Xxxxxx is required to pay a royalty under such license, and in such opinion the infringement of such Patent cannot be avoided by Xxxxxx absent such license, then Xxxxxx’x obligation to pay royalties to CTI with respect to such Licensed Product shall be reduced by fifty percent (50%) of the amount of the royalty paid to such Third Party with respect to such Licensed Product, provided the following conditions are met: (a) the
40
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
royalties payable to CTI shall not be reduced to less than fifty (50%) of the royalty payment that would otherwise be due to CTI in the absence of the reduction hereunder and (b) Xxxxxx shall provide CTI with written documentation regarding the payments to Third Parties for such Patent rights.
9.3.3. Royalty Term. Xxxxxx’x obligation to pay royalties pursuant to Section 9.3.1 shall expire, on a country-by-country and Licensed Product-by-Licensed Product basis, upon the last to expire of (a) the last to expire Valid Claim of a CTI Patent, Subject Patent or Joint Patent in such country that covers the manufacture, use, sale, offer for sale or importing of such Licensed Product in the Licensed Field in such country, taking into consideration all applicable patent extensions, (b) all applicable Regulatory Exclusivity for such Licensed Product in the Licensed Field in such country, and (c) ** from the First Commercial Sale of such Licensed Product in such country (the “Royalty Term”); provided, however, that on a country-by-country basis, if no Valid Claim or Regulatory Exclusivity remains during any portion of the Royalty Term in a particular country, the royalties due during such portion of the Royalty Term for such country shall be reduced to fifty percent (50%) of the amounts specified in Section 9.3.1 (subject to any other deductions provided for herein including, without limitation, those set forth in Section 9.3.2).
9.3.4. Royalty Payment Timing; Royalty Reports. ** following the end of each Calendar Quarter during the period in which royalties accrue, Xxxxxx shall provide CTI with a Sales Report and any other information reasonably required by CTI for the purpose of calculating royalties and Sales Milestone payments due under this Agreement. Any royalty payments due to CTI will be paid on the date of delivery of such report. In the event that either Party determines that the calculation of Net Sales for a Calendar Quarter deviates from the amounts previously reported to CTI for any reason (such as, on account of additional amounts collected or Licensed Product returns), Xxxxxx and CTI shall reasonably cooperate to reconcile any such deviations to the extent necessary under applicable legal or financial reporting requirements.
9.3.5. Audit. Until the expiration of all royalty payment obligations hereunder and for a period of three (3) years thereafter, Xxxxxx shall keep complete and accurate records pertaining to the sale or other disposition of Licensed Products by Xxxxxx, its Affiliates and Sublicensees in sufficient detail to permit CTI to confirm the accuracy of the royalties and Sales Milestone payments due hereunder. CTI shall have the right to cause an independent, certified public accountant reasonably acceptable to Xxxxxx to audit such records to confirm Net Sales and royalties for a period covering not more than the preceding three (3) fiscal years. Xxxxxx may require such accountant to execute a reasonable confidentiality agreement with Xxxxxx prior to commencing the audit. Such audits may be conducted during normal business hours upon reasonable prior written notice to Xxxxxx, but no more than frequently than once per year. No accounting period of Xxxxxx shall be subject to audit more than one time by CTI, unless after an accounting period has been audited by CTI, Xxxxxx restates its financial results for such accounting period, in which event CTI may conduct a second audit of such accounting period in accordance with this Section 9.3.5. Prompt adjustments (including remittances of
41
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
underpayments or overpayments disclosed by such audit) shall be made by the Parties to reflect the results of such audit. CTI shall bear the full cost of such audit unless such audit discloses an underpayment by Xxxxxx of five percent (5%) or more of the amount of royalties due under this Agreement, in which case Xxxxxx shall bear the full cost of such audit.
9.4. Development Costs and Expenses.
9.4.1. CTI Development Costs Generally. CTI shall be responsible for and shall pay all CTI Development Costs incurred prior to January 1, 2014. CTI shall pay for all CTI Development Costs incurred by it as part of the Development under the Development Plan, subject to reimbursement for costs incurred on or after January 1, 2014 by Xxxxxx in accordance with the remainder of this Section 9.4.
9.4.2. CTI Development Cost Threshold. CTI Developments Costs incurred on or after January 1, 2014 will be allocated as follows:
9.4.2.1 CTI shall be responsible for the first ninety-six million U.S. dollars (USD 96,000,000) of such CTI Development Costs applicable to the Licensed Territory (such amount, the “CTI Development Cost Threshold”); provided, however, that if any such CTI Development Costs relate exclusively to the Co-Promotion Territory, the Parties will confer and agree on an upward adjustment of the CTI Development Cost Threshold commensurate with the corresponding increase in costs for the Co-Promotion Territory, and provided, further, and notwithstanding any other provision of this Agreement, that (a) one hundred percent (100%) of those CTI Development Costs which are applicable primarily or solely to obtaining Marketing Approval for countries outside of the European Union and the Co-Promotion Territory and (b) if paid initially by CTI, all fees for Regulatory Filings in the Xxxxxx Exclusive Territory (except with respect to the EU prior to the first Marketing Approval in the EU of a Licensed Product) shall be reimbursed promptly by Xxxxxx to CTI and shall not be counted toward the CTI Development Cost Threshold.
9.4.2.2 For all CTI Development Costs which exceed the CTI Development Cost Threshold: (a) subject to Section 9.4.2.3, those CTI Development Costs which are generally applicable to the Licensed Territory shall be shared by the Parties such that seventy-five percent (75%) shall be borne by Xxxxxx and reimbursed to CTI as set forth in Section 9.4.4, with the remaining twenty-five percent (25%) to be borne by CTI, (b) those CTI Development Costs which are applicable exclusively to the Xxxxxx Exclusive Territory, one hundred percent (100%) shall be borne by Xxxxxx and reimbursed to CTI as set forth in Section 9.4.4, and (c) those CTI Development Costs which are applicable exclusively to the Co-Promotion Territory shall be borne fifty percent (50%) by Xxxxxx and reimbursed to CTI as provided in Section 9.4.4, with the remaining fifty percent (50%) to be borne by CTI. For the avoidance of doubt, no CTI Development Costs shall be taken into consideration for purposes of calculating the Profit & Loss
42
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Share for the Co-Promotion Territory. For purposes of Section 9.4, costs are considered “incurred” upon the delivery and acceptance of the relevant services or goods.
9.4.2.3 Notwithstanding anything contained in this Agreement to the contrary and in particular Section 9.4.2.1, if, at any time prior to receipt of the first Regulatory Approval of the Licensed Product in a country or region for Myelofibrosis, the CTI Development Costs associated with the Development of the Licensed Product for Myelofibrosis (collectively, the “MF Development Costs”) exceed **, the obligations of the Parties with respect to the CTI Development Costs as set forth in Sections 9.4.2.1 and 9.4.2.2 above shall be modified such that ** of the MF Development Costs shall be borne by **, with the remaining ** of MF Development Costs **. Any Development Costs related to manufacturing activities not currently contemplated, including without limitation scale-up beyond current lot size of ** and qualification of a backup supplier, shall be excluded from such reallocation. For the avoidance of doubt, such reallocation shall only apply with respect to any CTI Development Costs that are associated with the Development of the Licensed Product for Myelofibrosis. This re-allocation of responsibility shall continue until the receipt of the first Regulatory Approval of the Licensed Product. At the point of receipt of first Regulatory Approval of the Licensed Product, the obligations of the Parties shall revert to the allocation set forth in Sections 9.4.2.1 and 9.4.2.2 such that if the first Regulatory Approval of the Licensed Product is received in the EU first, ** of the MF Development Costs shall be borne ** and if the first Regulatory Approval of the Licensed Product is received in the Co-Promotion Territory, ** of the MF Development Costs shall be borne **.
9.4.3. Adjustment of the CTI Development Cost Threshold.
9.4.3.1 If the first Marketing Approval for a Licensed Product in the Co-Promotion Territory is obtained on or before October 1, 2016, then the CTI Development Cost Threshold shall be reduced by ** to ** (the “Reduced Threshold”) and for all purposes of this Agreement, the Reduced Threshold shall be deemed to be the CTI Development Cost Threshold. To the extent that the Reduced Threshold is lower than the aggregate of CTI Development Costs which have been incurred at the time of such Marketing Approval, such that ** incurred expenses in excess of the Reduced Threshold, then ** shall thereafter pay ** of ongoing CTI Development Costs (and not **, as the case may be), until such excess has been offset completely.
9.4.3.2 There shall be no change in the CTI Development Cost Threshold if the first Marketing Approval for a Licensed Product is obtained after October 1, 2016 but before February 1, 2017.
9.4.3.3 If the first Marketing Approval for a Licensed Product in the Co-Promotion Territory is obtained on or after February 1, 2017, then
43
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
the CTI Development Cost Threshold shall be increased by ** to ** (the “Increased Threshold”) and for all purposes of this Agreement, the Increased Threshold shall be deemed to be the CTI Development Cost Threshold. To the extent that the Increased Threshold is higher than the aggregate CTI Development Costs which have been incurred at the time of such Marketing Approval, such that Xxxxxx payments will have been made commencing prior to the attainment of the Increased Threshold resulting in an overpayment by Xxxxxx, then CTI shall thereafter pay ** of ongoing CTI Development Costs (and not **, as the case may be), until such overpayment has been offset completely.
9.4.4. Reimbursement. CTI shall charge all CTI Development Costs incurred by it or its Affiliates to a separate account created by CTI on its books and records solely for the purpose of tracking CTI Development Costs (the “Development Account”). As soon as reasonably practicable following the end of each calendar month, CTI shall provide to Xxxxxx a report setting forth all CTI Development Costs incurred during the immediately ended calendar month and charged to the Development Account together with reasonable supporting documentation for such expenses and, to the extent that Xxxxxx is responsible for any portion thereof pursuant to this Section 9.4, an invoice setting forth the portion of such CTI Development Costs for which Xxxxxx is responsible) (the invoice, summary and documentation, collectively, the “Development Cost Summary”). Unless Xxxxxx disputes any of the costs set forth therein or the apportionment of the Development costs, Xxxxxx shall reimburse CTI for Xxxxxx’x portion of the CTI Development Costs **, provided that Xxxxxx shall pay any undisputed costs within such time period.
9.4.5. Employee Efforts. The efforts of the employees of CTI or its Affiliates in performing activities under this Agreement shall be charged to CTI’s Development Account at the FTE Rate. Only those efforts that are contemplated by the applicable Development Plan shall be chargeable by CTI to its Development Account. All costs incurred by CTI owing to a Third Party in connection with the performance of its activities under a Development Plan shall be charged to CTI’s Development Account at CTI’s actual out-of-pocket cost. At the time of the annual update of the Development Plan for the Licensed Products, the CTI Development Cost Budget shall also be approved, as determined by the JSC. If the JSC is unable to agree on any changes to the CTI Development Cost Budget, then CTI shall be entitled to continue its spending for activities budgeted in the most recent CTI Development Cost Budget approved by the JSC or agreed by the Parties for which Xxxxxx shall reimburse CTI in accordance with Section 9.4.2 and Section 9.4.4.
9.4.6. Records and Audit. CTI shall keep and maintain accurate and complete records showing the expenses incurred by it in performing its activities under the Development Plan during the three (3) preceding Calendar Years, which books and records shall be in sufficient detail such that CTI Development Costs can accurately be determined. Upon ** prior written notice from Xxxxxx, on an audit date as mutually agreed by the Parties, CTI shall permit an independent certified public accounting firm of nationally recognized standing, selected by Xxxxxx and reasonably acceptable to CTI, to examine, at Xxxxxx’x sole expense, the relevant
44
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
books and records of CTI and its Affiliates as may be reasonably necessary to verify the reports submitted by CTI in accordance with Section 9.4.4. An examination by Xxxxxx under this Section 9.4.6 shall occur not more than once in any Calendar Year and shall be limited to the pertinent books and records for any Calendar Year ending not more than two (2) years before the date of the request. The accounting firm shall be provided access to such books and records at CTI’s facility(ies) where such books and records are normally kept and such examination shall be conducted during CTI’s normal business hours. CTI may require the accounting firm to sign a standard non-disclosure agreement before providing the accounting firm access to CTI’s facilities or records. Upon completion of the audit, the accounting firm shall provide both CTI and Xxxxxx a written report disclosing whether the reports submitted by CTI are correct or incorrect and the specific details concerning any discrepancies. No other information shall be provided to Xxxxxx. If the accounting firm concludes that CTI overstated the CTI Development Costs and Xxxxxx overpaid CTI for its portion of the CTI Development Costs as a result, CTI shall promptly pay Xxxxxx the amount of such overpayment plus interest, which shall be calculated at the average of the **. ** Xxxxxx shall not reveal to such accounting firm the conditions under which the audit expenses are to be reimbursed hereunder. If the accounting firm concludes that CTI understated the Development costs incurred by Xxxxxx and Xxxxxx underpaid CTI for its portion of the CTI Development Costs as a result, **.
9.4.7. Financial Information. All financial information of CTI which is subject to review under this Section 9.4 shall be deemed to be CTI Confidential Information subject to the provisions of Article XIII hereof, and Xxxxxx shall not disclose such CTI Confidential Information to any Third Party or use such CTI Confidential Information for any purpose other than reviewing progress made or verifying payments to be made hereunder; provided, however, that such CTI Confidential Information may be disclosed by Xxxxxx to Third Parties only to the extent necessary to enforce Xxxxxx’x rights under this Agreement.
9.4.8. Post-Marketing Approval Studies. For any Post-Marketing Approval Studies, costs shall be allocated as follows: (a) those costs which are applicable to both the Co-Promotion Territory and the Xxxxxx Exclusive Territory shall be shared by the Parties such that ** shall be borne ** pursuant to Section 9.4.4, with the remaining ** to be borne **, (b) those Costs which are applicable exclusively to the Xxxxxx Exclusive Territory, ** shall be **, and (c) those Costs which are applicable exclusively to the Co-Promotion territory shall be paid and borne **. All such expenses borne by CTI but reimbursed by Xxxxxx shall be independent of the provisions of Section 9.4.2. The above subsections (a) and (c) are applicable only after the CTI Development Cost Threshold has been surpassed (i.e., Post-Marketing Approval Studies are included in the CTI Development Cost Threshold).
9.4.9. Investigator-Sponsored Trials. Notwithstanding anything contained in this Agreement to the contrary, CTI shall bear all costs and expenses for Investigator-Sponsored Trials for the Licensed Product (for either the Initial Indication or any Additional Indications or both) up to an annual cap of ** per calendar year. Any costs and expenses for Investigator-Sponsored Trials for the Licensed Product in excess of this initial cap and up to a secondary cap of ** per calendar year **. Neither Party may approve, initiate or fund any
45
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Investigator-Sponsored Trials which would result in either Party incurring any Investigator-Sponsored Trial costs and/or expenses in excess of such secondary cap unless mutually agreed by the Parties.
9.4.10. Regulatory Filings Fees. All fees for Regulatory Filings for: (a) the Co-Promotion Territory shall be considered ** and shall be borne by the Parties in accordance with **, (b) the First Marketing Authorization in the EU in the Xxxxxx Exclusive Territory shall be considered ** and shall be borne by the Parties in accordance with ** and (c) all other geographies and Regulatory Approvals (including any associated with Reimbursement Approvals) shall be borne **.
9.5. Profit & Loss Share for Co-Promotion. The Parties will share ** with respect to Licensed Product for the Co-Promotion Territory as more specifically set forth in the Co-Promotion Terms (the “Profit & Loss Share”). Procedures for reporting of actual results on a Calendar Quarter basis, review and discussion of potential discrepancies, reconciliation on a Calendar Quarter basis, reasonable forecasting, and other finance and accounting matters, are set forth in Exhibit 1.34, and to the extent not set forth in Exhibit 1.34, will be established by the JCC, provided that Xxxxxx’x Executive Officer shall not have the right to make final decisions regarding any modifications or additions to Exhibit 1.34, but in the case of disagreement between the Parties within the JCC, the matter shall be decided pursuant to the dispute resolution procedures set forth in Section 16.13. Notwithstanding the foregoing, to the extent expenses are incurred as a result of a lawsuit or settlement by or with a Governmental Authority or Third Party payor (e.g. resulting from a corporate integrity agreement) that are unrelated to a Licensed Product, such expenses associated therewith ** from the Profit & Loss Share with respect to the Co-Promotion Territory.
9.6. Upstream Agreements. CTI shall be solely responsible for paying all amounts due pursuant to the Upstream Agreements, including the S*BIO Agreement. **
9.7. Exchange Rate; Manner and Place of Payment. All payments to be made by either Party hereunder shall be made in U.S. dollars. With respect to each quarter, for countries other than the U.S., whenever conversion of payments from any foreign currency shall be required, such conversion shall be made at the historical NY close rate (spot price) for the relevant day or date range as reported by Bloomberg in its Historical Price Table. All payments owed under this Agreement shall be made in USD by wire transfer to a bank and account designated in writing by CTI, unless otherwise specified in writing by CTI.
9.8. Taxes. All payments under this Agreement shall be made without any deduction or withholding for or on account of any tax, except as set forth in this Section 9.8. The Parties agree to cooperate with one another and use reasonable efforts to minimize under applicable Law obligations for any and all income or other taxes required by Law to be withheld or deducted from any of the royalty and other payments made by or on behalf of a Party hereunder (“Withholding Taxes”). The applicable paying Party under this Agreement (the “Paying Party”) shall, if required by Law, deduct from any amounts that it is required to pay to the recipient Party hereunder (the “Recipient Party”) an amount equal to such Withholding Taxes; provided that the
46
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Paying Party shall give the Recipient Party reasonable notice prior to paying any such Withholding Taxes. Such Withholding Taxes shall be paid to the proper taxing authority for the Recipient Party’s account and, if available, evidence of such payment shall be secured and sent to Recipient Party within thirty (30) days of such payment. The Paying Party shall, at the Recipient Party’s cost and expense, as mutually agreed by the Parties, do all such lawful acts and things and sign all such lawful deeds and documents as the Recipient Party may reasonably request to enable the Paying Party to avail itself of any applicable legal provision or any double taxation treaties with the goal of paying the sums due to the Recipient Party hereunder without deducting any Withholding Taxes.
9.9. Late Payments. If CTI does not receive payment of any sum due to it under this Agreement on or before the due date, interest shall thereafter accrue on the sum due to CTI from the due date until the date of payment, such interest to be calculated at the average of the prime rate reported by JPMorgan Chase, New York City, each month during the period from the time any payment was due until paid in full, plus two percent (2%) per annum.
9.10. Reporting. All financial reporting hereunder shall be, if applicable, on the basis of U.S. GAAP, consistently applied.
ARTICLE X.
INVENTIONS; ACCESS TO IMPROVEMENTS; PATENTS
10.1. Improvements and Inventions.
10.1.1. The Parties acknowledge that Xxxxxx and its Sublicensees may improve or modify the Licensed Products or otherwise make Inventions in the course of exercising Xxxxxx’x rights and performing its obligations under this Agreement, and that CTI may improve or modify the Licensed Products and otherwise make Inventions as CTI continues to Develop the Licensed Product and to manufacture Licensed Product (or, if Xxxxxx exercises its Fill/Finish Option, Drug Product) for supply for the Licensed Territory.
10.1.2. Any Improvements made by CTI shall be included in the CTI Know-How, and all Patents claiming such Improvements shall be included in the CTI Patents. Any Improvements made by Xxxxxx shall be included in the Xxxxxx Know-How, and all Patents claiming such Improvements shall be included in the Xxxxxx Patents, subject to the provisions of Section 10.2.
10.1.3. Xxxxxx will use Commercially Reasonable Efforts to obtain from its Sublicensees Control of Improvements made or developed by or on behalf of such Sublicensees, as well as Control of all intellectual property rights therein that are owned or controlled by such Sublicensees, in each case as necessary to provide CTI access to rights to such Improvements as part of the Xxxxxx Information, Xxxxxx Know-How or Xxxxxx Patents, as applicable, pursuant to this Agreement. Any such Improvements made by or on behalf of Xxxxxx’x Sublicensees of which Xxxxxx obtains Control shall be included in the Xxxxxx Know-How, and all Patents claiming such Improvements of which Xxxxxx obtains Control shall be included in the Xxxxxx Patents.
47
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
10.2. Ownership of Inventions. Inventorship shall be determined in accordance with U.S. patent laws. Any Invention made solely by employees, agents, or independent contractors of a Party or its Affiliates in the course of performing activities under this Agreement, together with all intellectual property rights therein (“Sole Inventions”), shall be owned by such Party; provided that CTI shall own, and Xxxxxx (on behalf of itself and its Affiliates) hereby assigns to CTI, all Sole Inventions specifically related to the composition of matter or use of Licensed Products and all other Sole Inventions otherwise specifically related to Licensed Products (in each case, solely to the extent of such specific relationship) (such Sole Inventions assigned to CTI together with all intellectual property rights therein, “CTI Assigned Patents”). Any Invention made jointly by at least one (1) employee, agent, or independent contractor of each Party or such Party’s Affiliate, together with all intellectual property rights therein (“Joint Inventions”, and all Patents covering such Joint Inventions, hereinafter, “Joint Patents”), shall be owned jointly by the Parties in accordance with joint ownership interests of co-inventors under U.S. patent laws, with each joint Party having, unless otherwise set forth in this Agreement, the unrestricted right to license and grant rights to sublicense any such Joint Invention; provided that CTI shall own, and Xxxxxx (on behalf of itself and its Affiliates) hereby assigns to CTI, all of its interest in Joint Inventions specifically related to the composition of matter or use of the Licensed Product and all other Joint Inventions otherwise specifically related to the Licensed Product (in each case, solely to the extent of such specific relationship) (such interests in Joint Inventions assigned to CTI together with all intellectual property rights therein, “CTI Assigned Joint Patents”). Each Party hereby grants to the other Party a nonexclusive, royalty-free, worldwide license, with the right to grant sublicenses, under such Party’s interest in Joint Inventions, solely for the purpose of performing its obligations under this Agreement; provided that the foregoing shall not apply to any uses of such Joint Inventions for which the receiving Party otherwise retains an exclusive license pursuant to this Agreement, with such proviso to apply only for so long as such receiving Party retains such exclusive license.
10.3. Disclosure of Inventions. Each Party shall promptly disclose to the other Party in writing any Inventions and any written Invention disclosures, or other similar documents, submitted to it by its employees, agents, or independent contractors describing each and every Invention that may be either a Sole Invention or a Joint Invention, and all Information relating to such Invention.
10.4. Prosecution of Patents.
10.4.1. CTI Patents Other than Joint Patents. CTI shall have the first right and authority to file, prosecute, and maintain CTI Patents, CTI Assigned Patents and CTI Assigned Joint Patents (collectively, the “Subject Patents”), on a worldwide basis at its sole discretion, subject to this Section 10.4.1. ** CTI will submit quarterly invoices for such costs and expenses (which must be accompanied by complete copies of the supporting invoices from CTI’s outside counsel) to Xxxxxx and Xxxxxx will pay each such invoice within **. CTI shall provide
48
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Xxxxxx with the opportunity to review and comment on any and all prosecution efforts, but in no case less than thirty (30) days prior to any filing deadlines, regarding the Subject Patents within the Licensed Territory; provided that CTI shall have final control over such prosecution efforts after reasonably considering Xxxxxx’x comments, if any. CTI shall provide Xxxxxx with a copy of material communications from Patent authorities in the Licensed Territory regarding the Subject Patents, and shall provide drafts of any material filings or responses to be made to such Patent authorities in a timely manner. Notwithstanding the foregoing, if CTI determines in its sole discretion to abandon or not maintain in the Licensed Territory any Subject Patents, other than a Joint Patent not included in the Subject Patents, CTI shall provide Xxxxxx with at least forty-five (45) days prior written notice of such determination and, if Xxxxxx so requests, shall provide Xxxxxx with the opportunity to prosecute and maintain such CTI Patent in the Licensed Territory in the name of CTI.
10.4.2. Other Joint Patents. With respect to any potentially patentable Joint Invention not covered by a Subject Patent (“Other Joint Inventions”), the Parties shall meet and agree upon which Party shall file, prosecute and maintain Patent applications covering such Other Joint Invention (any such Patent application and any Patents issuing therefrom, an “Other Joint Patent”) in particular countries and jurisdictions throughout the world. Unless otherwise agreed by the Parties, Xxxxxx will file, prosecute and maintain any Other Joint Patents in the Licensed Territory, subject to the Parties coordinating their efforts as appropriate to make such prosecution activities as efficient, convenient, and harmonious as possible. The Party that prosecutes an Other Joint Patent (the “Prosecuting Party”) shall be responsible for all expenses of filing, prosecuting and maintaining such Other Joint Patents. The Prosecuting Party shall provide the other Party the opportunity to review and comment on any and all such prosecution efforts regarding the applicable Joint Patent in the particular jurisdictions, and such other Party shall provide the Prosecuting Party reasonable assistance in such efforts; provided that the Prosecuting Party shall have final control over such prosecution efforts after reasonably considering the other Party’s comments, if any. The Prosecuting Party shall provide the other Party with a copy of all material communications from any Patent authority in the applicable jurisdictions regarding the Other Joint Patent being prosecuted by such Party, and shall provide drafts of any material filings or responses to be made to such Patent authorities a reasonable amount of time, but in no event less than thirty (30) days, in advance of submitting such filings or responses. In particular, each Party agrees to provide the other Party with all information necessary or desirable to enable the other Party to comply with any duty of candor and/or duty of disclosure requirements of any Patent authority. Except to the extent a Party is restricted by the licenses granted by such Party to the other Party under the terms of this Agreement, and/or the other covenants contained in this Agreement, each Party shall be entitled to practice, and grant licenses to Third Parties and Affiliates of such Third Parties to practice, the Other Joint Patents and all Other Joint Inventions without restriction or an obligation to account to the other Party, and the other Party shall consent and hereby consents, without additional consideration, to any and all such licenses. Notwithstanding anything contained herein to the contrary, if CTI determines that it shall no longer prosecute a Patent application directed to an Other Joint Invention or maintain an Other Joint Patent, Xxxxxx shall have the right to do so and Xxxxxx shall thereafter own such Patent or Application and CTI shall execute appropriate documentation reflecting Xxxxxx’x ownership thereof.
49
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
10.4.3. Cooperation in Prosecution. Each Party shall provide the other Party all reasonable assistance and cooperation in the Patent prosecution efforts described above in this Article X, including providing any necessary powers of attorney and executing any other required documents or instruments for such prosecution.
10.5. Infringement of Patents by Third Parties.
10.5.1. Notification. Each Party shall promptly notify the other Party in writing of any existing or threatened infringement of the Subject Patents of which it becomes aware in the Licensed Territory, and shall provide to the other Party any and all evidence and information available to such Party regarding such alleged infringement.
10.5.2. Product Infringement of Subject Patents in the Licensed Territory.
(a) If a Party becomes aware of any actual or alleged existing or threatened infringement by a Third Party of any Subject Patent, by making, using, importing, offering for sale, or selling the Compound, Drug Product and/or Licensed Product (such activities, “Product Infringement”) in the Licensed Territory, such Party shall notify the other Party as provided in Section 10.5.1. CTI shall have the first right, but not the obligation, to bring an appropriate suit or other action against any person or entity engaged in such Product Infringement in the Licensed Territory, subject to Section 10.5.2(b). CTI shall have a period of thirty (30) days after such notification to or by Xxxxxx, to elect to so enforce such Subject Patent in the Licensed Territory. If CTI does not so elect, CTI shall so notify Xxxxxx in writing during such thirty (30) day period, or ten (10) days prior to any deadline relating to loss of any rights with respect to the Product Infringement, whichever is earlier, and Xxxxxx shall have the right, but not the obligation, to commence a suit or take action to enforce such Subject Patent against such Third Party allegedly perpetrating such Product Infringement. Each Party shall provide to the Party enforcing any such rights under this Section 10.5.2(a) reasonable assistance in such enforcement, including joining an action as a party plaintiff if so required by Laws to pursue such action, at the Enforcing Party’s sole expense. The Enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts, and shall reasonably consider the other Party’s comments on any such efforts. Except as set forth above, the Enforcing Party shall bear and be responsible for all costs incurred in connection with each Party’s activities under this Section 10.5.2(a).
(b) The Party not bringing an action with respect to Product Infringement under this Section 10.5.2 shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, but such Party shall at all times cooperate fully with the Party bringing such action. Additionally, the Party not bringing an action under this Section 10.5.2 may have an opportunity to participate in such action to the extent that the Parties may mutually agree at the time the other Party elects to bring such action hereunder.
50
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
10.5.3. Non-Product Infringement of Joint Patents Anywhere in the Licensed Territory. For infringement of the Joint Patents that is not Product Infringement, the Parties shall confer to determine which Party shall have the first right to bring an appropriate suit or other action against any person or entity engaged in such infringement, and the manner in which they shall bear costs and share related recoveries of such suit or action. The Party that brings such suit or actions (the “Enforcing Party”) shall keep the other Party regularly informed of the status and progress of such enforcement efforts, and shall reasonably consider the other Party’s comments on any such efforts. The other Party shall cooperate with the enforcing Party in enforcing Joint Patents against such infringement, without limitation joining an action as a party plaintiff if so required by Laws to pursue such action. If the Parties are unable to reach agreement upon which Party shall bring an appropriate suit or other action against any person or entity engaged in such infringement of such Joint Patent within thirty (30) days, or ten (10) days prior to any deadline relating to loss of any rights with respect to such infringement, whichever is earlier, then Xxxxxx shall have the first right, but not the obligation, to bring such suit or other actions against such infringement in the Licensed Territory at its sole expense. The Party that does not bring such suit or action shall have the right to participate in such actions at its expense upon written notice to the other Party.
10.5.4. Settlement. Xxxxxx shall not settle any claim, suit, or action that it brings under this Section 10.5 involving Subject Patents in any manner that would, in CTI’s reasonable judgment, negatively impact CTI, including settlements involving the ownership, validity or enforceability of any of the CTI Patents, or that do not include a full and unconditional release from all liability of CTI, without the prior written consent of CTI, which shall not be unreasonably withheld, conditioned or delayed. CTI shall not settle any claim, suit, or action that it brings under this Section 10.5 involving Subject Patents in the Licensed Territory in any manner that would, in Xxxxxx’x reasonable judgment, negatively impact Xxxxxx, without the prior written consent of Xxxxxx, which shall not be unreasonably withheld, conditioned or delayed. Moreover, any settlement by Xxxxxx involving Subject Patents in the Licensed Territory, that (a) results in cross-licensing or (b) results in sublicenses to Third Parties shall require CTI’s prior written consent. Neither Party shall settle any claim, suit, or action that it brings under this Section 10.5 involving Joint Patents (other than Subject Patents) in any manner that would negatively impact the other Party, including the ownership, validity or enforceability of any of such Joint Patents, or if the settlement does not include a full and unconditional release from all liability of the other Party, without the prior written consent of such other Party.
10.5.5. Allocation of Proceeds. Except as otherwise provided in this Section 10.5, if either Party recovers monetary damages from any Third Party in a suit or action brought under this Section 10.5, whether such damages result from the infringement of Xxxxxx Patents or CTI Patents, such recovery shall be allocated as follows:
(a) First to the repayment of expenses with respect to the action;
(b) ** of the remainder to the Party which brought the action (except in the Co-Promotion Territory, in which such Party shall receive ** of the remainder); and
(c) ** of the remainder to the other Party (except in the Co-Promotion Territory, in which such Party shall receive ** of the remainder).
51
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
10.6. Infringement of Third Party Rights in the Licensed Territory.
10.6.1. Notice. If the development, manufacture, use, sale, offer for sale, import or export of the Licensed Product in the Licensed Field and in the Licensed Territory results in a claim for Patent infringement by a Third Party, the Party first having notice of such claim shall promptly notify the other Party in writing of such a claim. Following such notice, the Parties agree to enter into either a joint defense or common interest agreement, under which agreement the Parties can share the known facts of such infringement in reasonable detail, if they are advised to do so by counsel.
10.6.2. Third Party Claims. Xxxxxx shall assume control of the defense of any claims brought by Third Parties alleging infringement of Third Party intellectual property rights in connection with the development, manufacture, use, sale, offer for sale, import or export of the Licensed Product in the Licensed Field in the Licensed Territory, represented by its own counsel. If requested by Xxxxxx, CTI agrees to join in any litigation, and in any event shall reasonably cooperate with Xxxxxx, at Xxxxxx’x expense. Xxxxxx shall have the exclusive right to settle any such claim without the consent of CTI, unless such settlement shall negatively impact on CTI, including without limitation on the ownership, validity or enforceability of any CTI Patents. Any expenses incurred in defending any such claims shall be solely the responsibility of Xxxxxx. CTI shall be entitled to separate representation in the defense of any such claims brought by Third Parties using counsel of its own choice and at its own expense.
10.6.3. Potential Third Party Claims. In the event that either Party becomes aware of a Third Party intellectual property right that might reasonably be expected to give rise to a claim of infringement under this Section 10.6, that Party shall promptly notify the other Party of such intellectual property right and all relevant facts and circumstances known to the discovering Party. Following such notice, the Parties agree to enter into either a joint defense or common interest agreement, under which agreement the Parties can share the known facts of such infringement in reasonable detail, if they are advised to do so by counsel, and to consult thereafter regarding any corrective or preventive action to be taken to address such potential claim.
10.7. Patent Oppositions and Other Proceedings.
10.7.1. By the Parties. If either Party desires to bring an opposition, action for declaratory judgment, nullity action, interference, declaration for non-infringement, reexamination, or other attack upon the validity, title, or enforceability of a Patent owned or controlled by a Third Party that covers, in the Licensed Territory, the Compound, Drug Product and/or Licensed Product, or the manufacture, use, sale, offer for sale, or importation of the Compound, Drug Product and/or Licensed Product (except insofar as such action is a counterclaim to or defense of, or accompanies a defense of, a Third Party’s claim or assertion of infringement under Section 10.6, in which case the provisions of Section 10.6 shall govern), such
52
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Party shall so notify the other Party, and the Parties shall promptly confer to determine whether to bring such action or the manner in which to settle such action. Xxxxxx shall have the first right, but not the obligation, to bring in its sole control and at its sole expense such action in the Licensed Territory. If Xxxxxx does not bring such action within thirty (30) days of notification thereof pursuant to this Section 10.7 (or earlier, if required by the nature of the proceeding), then CTI shall have the right, but not the obligation, to bring, in CTI’s sole control and at its sole expense, such action. The Party not bringing an action under this Section 10.7 shall join the action as a joint party plaintiff if required to enable the other Party to bring such action, at the other Party’s expense. Additionally, if appropriate, the Party not bringing an action under this Section 10.7 shall be entitled to separate representation, at its sole expense, in such proceeding by counsel of its own choice, and shall cooperate fully with the Party bringing such action. Any awards or amounts received in bringing any such action shall be first allocated to reimburse the Parties’ expenses in such action, and any remaining amounts shall be retained by the Party bringing such action.
10.7.2. By Third Parties. If a CTI Patent (including a Joint Patent) becomes the subject of any proceeding commenced by a Third Party in the Licensed Territory in connection with an opposition, reexamination request, action for declaratory judgment, nullity action, interference, or other attack upon the validity, title or enforceability thereof (except insofar as such action is a counterclaim to or defense of, or accompanies a defense of, an action for infringement against a Third Party under Section 10.5, in which case the provisions of Section 10.5 shall govern), then CTI shall have the first right, but not the obligation, to control such defense at its sole cost. CTI shall permit Xxxxxx to participate in the proceeding to the extent permissible under Laws, and to be represented by its own counsel in such proceeding, at Xxxxxx’x sole expense. If CTI decides that it does not wish to defend against such action, then Xxxxxx shall have a backup right to assume defense of such Third-Party action. Except as set forth above, all expenses incurred by the Parties in such an action shall be borne by the Party controlling the defense of the Third-Party action. Any awards or amounts received in defending any such Third-Party action, if any, shall be allocated between the Parties as provided in Section 10.5.5 as if the Party controlling the defense of the Third-Party Action were the Party that brought an action against an alleged infringer.
10.8. Patent Term Extensions in the Licensed Territory. The patent counsel of each Party shall discuss and recommend for which, if any, of the Subject Patents in the Licensed Territory the Parties should seek any term extensions, supplementary protection certificates, and equivalents thereof offering Patent protection beyond the initial term with respect to any issued Patents (“Patent Term Extensions”) licensed to Xxxxxx hereunder in the Licensed Territory. Xxxxxx shall have the final decision-making authority with respect to applying for any such Patent Term Extensions in the Licensed Territory; provided that Xxxxxx shall not unreasonably fail or refuse to do so, and shall have the sole right to apply for any such Patent Term Extensions Xxxxxx decides to seek, at its expense; provided, however, that to the extent any such application for Patent Term Extension must be filed in the name of CTI, CTI hereby grants Xxxxxx the power to file such application on behalf of and/or as agent for CTI. CTI shall cooperate fully with Xxxxxx, at Xxxxxx’x expense, in making such filings or taking any related actions, for example and without limitation, making available all required regulatory data and information and executing any required authorizations to apply for such Patent Term Extension.
53
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
10.9. Registration of License. CTI agrees that Xxxxxx may, if applicable, register its license under the Subject Patents with the Patent authorities in the Licensed Territory. Xxxxxx shall, at its expense, prepare and deliver to CTI such instruments and other documents reasonably necessary and in proper form for such registration. The Parties shall mutually agree on the form of documents to be used for such purpose, and shall cooperate to preserve confidentiality of this Agreement to the extent permitted under applicable Laws in the relevant country. CTI shall execute and return to Xxxxxx such instruments and documents within fifteen (15) days from the receipt thereof.
10.10. Patent Marking. Xxxxxx agrees to xxxx or have marked with the Subject Patents to the extent consistent with applicable Laws any Licensed Product sold by Xxxxxx or by its Sublicensees in accordance with the statutes of any country within the Licensed Territory relating to the marketing of patented articles.
ARTICLE XI.
TRADEMARKS
11.1. Xxxxxx Trademarks. Xxxxxx shall be responsible for the selection, registration and maintenance of all trademarks which it employs solely in connection with the Commercialization of any Licensed Product in the Licensed Territory under this Agreement, other than the CTI Trademarks (the “Xxxxxx Trademarks”). Xxxxxx shall solely own the Xxxxxx Trademarks and pay all relevant costs thereof. Xxxxxx shall not select, register or otherwise use any trademark that is the same as or confusingly similar to, misleading or deceptive with respect to or that dilutes any of the CTI Trademarks. CTI shall not use any trademark that is the same as or confusingly similar to, misleading or deceptive with respect to, or that dilutes, any of the Xxxxxx Trademarks. Xxxxxx shall have the sole right to initiate at its own discretion legal proceedings against any infringement or threatened infringement of any Xxxxxx Trademark. Xxxxxx shall and hereby does grant to CTI a non-exclusive royalty-free license to use the Xxxxxx Trademarks on or in connection with the Commercialization of Licensed Products in the Co-Promotion Territory.
11.2. Use of CTI Trademarks. Licensed Products Commercialized in the Co-Promotion Territory shall be co-branded with CTI and Xxxxxx Trademarks, with equal prominence given to each. Each Party shall approve any labeling or promotional materials containing its trademarks, such approval not to be unreasonably withheld or delayed. Licensed Products Commercialized in the Xxxxxx Exclusive Territory shall have the following statement on all packaging, in legible size (but such size to be subject to Xxxxxx’x control of such packaging): “Under license from Cell Therapeutics, Inc.”
11.2.1. CTI shall and hereby does grant to Xxxxxx a non-exclusive royalty-free license, with the right to grant sublicenses through multiple tiers of Sublicensees (subject to Section 2.4), to use the CTI Trademarks solely for purposes of the immediately preceding paragraph of this Section 11.2.
54
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
11.2.2. Xxxxxx shall not use any trademark that is confusingly similar to, misleading or deceptive with respect to, or that dilutes, any CTI Trademark.
11.2.3. Xxxxxx shall properly designate the CTI Trademarks on the packaging of the final Licensed Product, to the extent required or permissible by the applicable Marketing Approvals and Xxxxxx agrees that all Licensed Products with which the CTI Trademarks are used shall conform to all requirements of any Applicable Laws and any Regulatory Authorities in the Licensed Territory and shall be no less than a reasonable level of quality.
11.2.4. Except as otherwise provided in this Section 11.2, CTI shall have the first right and authority, but not an obligation, to register and maintain the CTI Trademarks in the Licensed Territory (subject to this Section 11.2). CTI shall be responsible for all costs and expenses of such registration and maintenance. CTI shall provide Xxxxxx reasonable opportunity to review and comment on such registration efforts regarding the CTI Trademarks. CTI shall provide Xxxxxx with a copy of material communications from any Governmental Authority in the Licensed Territory regarding the CTI Trademark, and shall provide drafts of any material filings or responses to be made to such authorities in a timely manner.
11.2.5. If a Party becomes aware of any actual or alleged existing or threatened infringement by a Third Party of any CTI Trademark in the Licensed Territory (such activities, “Trademark Infringement”), such Party shall notify the other Party, and shall provide to the other Party any and all evidence and information available to such Party regarding such alleged infringement. CTI shall have the first right, but not the obligation, to bring an appropriate suit or other action against any person or entity engaged in such Trademark Infringement, at its sole expense, subject to this Section 11.2.5. CTI shall have a period of thirty (30) days after such notification to or by Xxxxxx, to elect to so enforce such CTI Trademark. If CTI does not so elect, CTI shall so notify Xxxxxx in writing during such thirty (30) day period, or ten (10) days prior to any deadline relating to loss of any rights with respect to the Trademark Infringement, whichever is earlier, and Xxxxxx shall have the right, but not the obligation, to commence a suit or take action to enforce such CTI Trademark against such Third Party, at its sole expense. Each Party shall provide to the Party enforcing any such rights under this Section 11.2.5 reasonable assistance in such enforcement, at such enforcing Party’s request and expense, including without limitation joining an action as a party plaintiff if so required by Laws to pursue such action. The enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts, and shall reasonably consider the other Party’s comments on any such efforts.
11.2.6. The Party not bringing an action with respect to Trademark Infringement under Section 11.2.5 shall be entitled to separate representation in such matter by counsel of its own choice and at its expense, but such Party shall at all times cooperate fully with the Party bringing such action. Additionally, the Party not bringing an action under Section 11.2.5 may have an opportunity to participate in such action to the extent that the Parties may mutually agree at the time the other Party elects to bring an action hereunder.
55
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
11.3. Infringement of Xxxxxx Trademarks by Third Parties. With respect to any Xxxxxx Trademarks associated with Licensed Products in the Licensed Territory, each Party shall notify the other Party promptly upon learning of any actual or alleged threatened or existing infringement of any trademark or of any unfair trade practices, trade dress imitation, passing off of counterfeit goods or like offenses, against such trademark. Xxxxxx shall have the sole right, in its own discretion and at its own expense, to bring an action to address such infringement.
ARTICLE XII.
REPRESENTATIONS AND WARRANTIES
12.1. The Parties’ Representations and Warranties. Each Party hereby represents and warrants to the other Party, as of the Effective Date, as set forth below. In the case of Xxxxxx, “Party” shall mean and refer to each of BII, BHC and BHSA.
12.1.1. Such Party (a) is a corporation duly organized and subsisting under the applicable Laws of its jurisdiction of organization, and (b) has full power and authority and the legal right to own and operate its property and assets and to carry on its business as it is now being conducted and as it is contemplated to be conducted by this Agreement.
12.1.2. Such Party has the power, authority and legal right, and is free to enter into this Agreement and, in so doing, will not violate any other agreement to which such Party is a party as of the Effective Date.
12.1.3. This Agreement has been duly executed and delivered on behalf of such Party and constitutes a legal, valid, and binding obligation of such Party and is enforceable against it in accordance with its terms, subject to the effects of bankruptcy, insolvency or other applicable Laws of general application affecting the enforcement of creditor rights and judicial principles affecting the availability of specific performance and general principles of equity.
12.1.4. Such Party has taken all corporate action necessary to authorize the execution and delivery of this Agreement.
12.1.5. Except with respect to Marketing Approvals and Reimbursement Approvals for Licensed Products or as otherwise described in this Agreement, such Party has obtained all necessary consents, approvals, and authorizations of all Regulatory Authorities and other Third Parties required to be obtained by such Party in connection with the execution and delivery of this Agreement and the performance of its obligations hereunder.
12.1.6. The execution and delivery of this Agreement and the performance of such Party’s obligations hereunder (a) do not conflict with or violate any requirement of applicable Laws or any provision of the articles of incorporation, bylaws, limited partnership agreement, or any similar instrument of such Party, as applicable, in any material way, and (b) do not conflict with, violate, or breach or constitute a default or require any consent under, any applicable Laws or any contractual obligation or court or administrative order by which such Party is bound.
56
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
12.1.7. All of such Party’s employees, officers, independent contractors, consultants, and agents have executed agreements requiring assignment or licensing to such Party of all Inventions made during the course of and as a result of their association with such Party and obligating the individual to maintain as confidential the confidential information of such Party; provided, however, that for employees of a Party’s Affiliates based in Germany, Austria, or any other jurisdiction where a prior obligation to assign is not permitted, the obligation under this paragraph will be deemed satisfied if (a) each such employee is obligated to notify his employer of such Inventions and (b) the employer has an established program for receiving such notifications and timely claiming ownership of such Inventions after notification.
12.1.8. Neither such Party, nor any of such Party’s employees, independent contractors, consultants, agents or officers: (a) has ever been debarred or is subject to debarment or, to such Party’s knowledge, convicted of a crime for which a Person could be debarred before a Regulatory Authority under applicable Laws, or (b) to such Party’s knowledge, has ever been under indictment for a crime for which a Person could be debarred under such Laws.
12.1.9. All documents, information and know-how furnished or transferred by such Party to the other Party under this Agreement shall be, to its knowledge, free of errors in any material respect.
12.1.10. Neither such Party is a party to or bound by any corporate integrity agreement or similar compliance agreement to which any Governmental Authority or Third Party payor is a counterparty, and in the event either Party becomes aware of any potential or actual investigation or violation of applicable Law or regulations that may result in the entry by such Party into any such agreement, such Party shall promptly notify the other Party of such action in accordance with Section 14.3.1.
12.2. CTI’s Representations and Warranties. CTI hereby represents and warrants to Xxxxxx, as of the Effective Date, as set forth below:
12.2.1. A complete listing of all of the Upstream Agreements is attached hereto as Exhibit 1.160 and a true and complete copy (with certain financial terms redacted) of each of the Upstream Agreements together with all exhibits, schedules, appendices, attachments and amendments thereto has been provided to Xxxxxx prior to the Effective Date. The Upstream Agreements have not been modified, supplemented or amended since such copy was provided to Xxxxxx. The Upstream Agreements are, to CTI’s knowledge, in full force and effect, all payments to date required to be made thereunder by CTI have been made, and CTI is in compliance in all material respects with its obligations thereunder.
12.2.2. CTI has not received or provided any notice of termination of any of the Upstream Agreements, or any notices of breach of any of the Upstream Agreements.
12.2.3. CTI has sufficient legal and/or beneficial title under its intellectual property rights necessary to grant the licenses contained in this Agreement.
57
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
12.2.4. There is no pending or, to CTI’s knowledge, threatened claim, litigation or any other proceeding brought by a Third Party against CTI challenging the validity of the CTI Patent Rights in the Licensed Territory, or claiming that the Development, manufacture or Commercialization of the Licensed Product in the Licensed Field in any portion of the Licensed Territory constitutes or would constitute infringement of such Third Party’s intellectual property right(s), and CTI has no present knowledge of any Third Party intellectual property right that would reasonably be expected to give rise to any such claim, litigation or proceeding. To CTI’s knowledge as of the Effective Date, the use of the CTI Know-How and the CTI Patents in existence on the Effective Date for the Development, manufacture and Commercialization of Licensed Products as contemplated by this Agreement does not infringe the intellectual property rights of any Third Party.
12.2.5. There is no pending or, to CTI’s knowledge, threatened claim, litigation or any other proceeding brought by a Third Party against CTI claiming that the Development of the Licensed Product or Drug Product or the manufacture of the Licensed Product or Drug Product at the location where CTI currently manufactures such Licensed Product or Drug Product constitutes or would constitute infringement of such Third Party’s intellectual property right(s), and CTI has no present knowledge of any Third Party intellectual property right that would reasonably be expected to give rise to any such claim, litigation or proceeding.
12.2.6. CTI has not received any written communications alleging that it has violated or that it would violate, through the manufacture, use, import, export, sale, and/or offer for sale of the Licensed Product in the Licensed Field and in the Licensed Territory or any portion thereof, any intellectual property rights of any Third Party.
12.2.7. To CTI’s knowledge, no invention claimed by the CTI Patents was made or reduced to practice using any funding of the U.S. Government; provided that CTI does not make any representation or warranty with respect to inventions licensed under the Upstream Agreements.
12.2.8. CTI is not in possession of any in-licensed Third Party know-how, technology, trade secrets or patent rights that are necessary to manufacture a Licensed Product or Drug Product but are not Controlled by CTI.
12.2.9. The issuance of the Securities has been duly authorized and, when issued and paid for in accordance with the terms of this Agreement, will be duly and validly issued, fully paid and nonassessable, free and clear of all liens imposed by CTI other than restrictions on transfer provided for in this Agreement. At the Effective Time, CTI has reserved the Preferred Stock and Common Stock for issuance from its duly authorized capital stock.
12.2.10. CTI has the requisite corporate power to issue and sell the Securities. The issuance and sale of the Securities will not (a) result in any violation of, be in conflict with, or constitute a default under, with or without the passage of time or the giving of notice: (i) any provision of CTI’s or its subsidiaries’ articles of incorporation or bylaws as in effect on the date hereof, (ii) any provision of any judgment, arbitration ruling, decree or order to which CTI or its
58
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
subsidiaries are a party or by which they are bound, (iii) any bond, debenture, note or other evidence of indebtedness, or any lease, contract, mortgage, indenture, deed of trust, loan agreement, joint venture or other agreement, instrument or commitment to which CTI or any subsidiary is a party or by which they or their respective properties are bound, or (iv) any statute, rule, law or governmental regulation applicable to CTI, or (b) result in the creation or imposition of any lien, encumbrance, claim, security interest or restriction whatsoever upon any of the properties or assets of CTI or any subsidiary or any acceleration of indebtedness pursuant to any obligation, agreement or condition contained in any bond, debenture, note or any other evidence of indebtedness or any indenture, mortgage, deed of trust or any other agreement or instrument to which CTI or any subsidiary are a party or by which they are bound or to which any of the property or assets of CTI or any subsidiary is subject. No consent, approval, authorization or other order of, or registration, qualification or filing with, any regulatory body, administrative agency, or other governmental body is required for the valid issuance or sale of the Securities by CTI pursuant to this Agreement, other than such as have been made or obtained and that remain in full force and effect, and except for the filing of a Form D or any filings required to be made under state securities laws.
12.2.11. CTI has made available to Xxxxxx true, correct and complete copies of the articles of incorporation and bylaws of CTI, as in effect on the date hereof.
12.2.12. The consolidated financial statements included in CTI’s Annual Report on Form 10-K for the year ended December 31, 2012 and its quarterly reports on Form 10-Q for the year ending December 31, 2013 (such reports, including all exhibits and documents incorporated by reference therein, together with any 8-Ks or proxy statements filed since January 1, 2013 and any exhibits and documents incorporated by reference therein, the “Company SEC Documents”) filed by CTI with the Securities and Exchange Commission (the “SEC”): (a) complied as to form in all material respects with the published rules and regulations of the SEC applicable thereto when filed and were timely filed, (b) were prepared in accordance with generally accepted accounting principles applied on a consistent basis throughout the periods covered, except as may be indicated in the notes to such financial statements and (in the case of unaudited statements) as permitted by Form 10-Q , and except that unaudited financial statements may not contain footnotes and are subject to year-end audit adjustments, and (c) fairly present in all material respects the consolidated financial position of CTI and its subsidiaries as of the respective dates thereof and the consolidated results of operations cash flows and the changes in shareholders’ equity of CTI and its subsidiaries for the periods covered thereby.
12.2.13. Except as set forth in the financial statements included in the Company SEC Documents, neither CTI nor its subsidiaries has incurred any liabilities, contingent or otherwise, other than (a) trade payables, accrued expenses, and other liabilities incurred in the ordinary course of business subsequent to September 30, 2013, and (b) liabilities of the type not required under generally accepted accounting principles to be reflected in such financial statements.
12.2.14. Except as disclosed in the Company SEC Documents, CTI has not issued any capital stock since its most recently filed periodic report filed with the SEC, other than pursuant
59
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
to the exercise and granting of employee stock options and stock awards pursuant to CTI’s equity incentive plans, or the issuance of shares of common stock pursuant to employee stock purchase plans or pursuant to the conversion or exercise of outstanding warrants or other convertible securities as of the date of the most recently filed periodic report filed with the SEC. All issued and outstanding shares of common stock have been duly authorized and validly issued, are fully paid and nonassessable, have been issued and sold in compliance with the registration requirements of federal and state securities laws or the applicable statutes of limitation have expired, and were not issued in violation of any preemptive rights or similar rights to subscribe for or purchase securities.
12.2.15. Except as set forth herein or in the Company SEC Documents, there are no (a) outstanding rights (including, without limitation, preemptive rights), warrants or options to acquire, or instruments convertible into or exchangeable for, any unissued shares of capital stock or other equity interest in CTI, or any contract, commitment, agreement, understanding or arrangement of any kind to which CTI or any subsidiary is a party and relating to the issuance or sale of any capital stock or convertible or exchangeable security of CTI or any subsidiary, other than options or stock awards granted to directors and employees of CTI pursuant to existing equity incentive plans or (b) obligations of CTI to purchase, redeem or otherwise acquire any of its outstanding capital stock or any interest therein or to pay any dividend or make any other distribution in respect thereof. Except as disclosed in the Company SEC Documents, there are no anti-dilution or price adjustment provisions, co-sale rights, registration rights, rights of first refusal or other similar rights contained in the terms governing any outstanding security of CTI that will be triggered by the issuance of the Securities.
12.2.16. Assuming the accuracy of the representations of Xxxxxx in Section 12.3 of this Agreement on the date hereof, the offer, issue and sale of the Securities to Xxxxxx under the terms of this Agreement are exempt from the registration and prospectus delivery requirements of the Securities Act and have been or will be registered or qualified (or are or will be exempt from registration and qualification) under the registration, permit or qualification requirements of all applicable state securities laws. Neither CTI, nor any of its affiliates, nor any person acting on its or their behalf, has directly or indirectly made any offers or sales of any security or solicited any offers to buy any security under circumstances that would require registration under the Securities Act of the issuance of the Securities to Xxxxxx.
12.2.17. CTI’s common stock is registered pursuant to Section 12(b) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and is listed on the Nasdaq Capital Market (the “Principal Market”), and CTI has taken no action designed to, or likely to have the effect of, terminating the registration of the Common Stock under the Exchange Act or delisting the Common Stock from the Principal Market. CTI is in compliance with all of the presently applicable requirements for continued listing of the Common Stock on the Principal Market. The issuance of the Securities does not require shareholder approval including, without limitation, pursuant to the rules and regulations of the Principal Market.
60
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
12.2.18. CTI is eligible to register the Common Stock for resale by the holder thereof using Form S-3 promulgated under the Securities Act.
12.2.19. All stock transfer or other taxes (other than income taxes) that are required to be paid in connection with the issuance and sale of the Securities by CTI to Xxxxxx will be, or will have been, fully paid or provided for by CTI and CTI will have complied with all applicable laws imposing such taxes.
12.2.20. CTI is not required to register as an “investment company” under the Investment Company Act of 1940 and will not be required to register as an “investment company” as a result of the issuance of the Securities pursuant to the terms of this Agreement and the receipt of the proceeds therefrom.
12.2.21. The representations and warranties contained in this Section 12.2 do not contain any untrue statement of a material fact or omit a material fact necessary to make the statements in this Section 12.2, in light of the circumstances in which they are made, not misleading. To the knowledge of CTI, there is no circumstance that has specific application to CTI (other than general economic or industry conditions) that has had or CTI can reasonably foresee would have a material adverse effect on the assets, business, prospects, financial condition or results of operations of CTI and its subsidiaries taken as a whole, except in each case that has been described in this Section 12.2 or any Company SEC Document.
12.3. Xxxxxx’x Representations and Warranties.
Xxxxxx hereby represents and warrants to CTI, as of the Effective Date, that Xxxxxx is (a) an “accredited investor” within the meaning of Rule 501 of Regulation D under the Exchange Act, as presently in effect and is not an entity formed for the sole purpose of acquiring the Securities, and has a total in assets of at least €20 million and net revenues of at least €40 million. Xxxxxx has such knowledge, sophistication and experience in business and financial matters so as to be capable of evaluating the merits and risks of the prospective investment in the Securities. Xxxxxx has had access to such information as it deemed necessary in order to conduct any due diligence it has determined is necessary or appropriate in connection with the purchase and sale of the Securities and its decision to participate in such purchase and sale. Xxxxxx is able to bear the economic risk of an investment in the Securities and, at the present time, is able to afford a complete loss of such investment. Xxxxxx understands that nothing in the Agreement or any other materials presented to Xxxxxx in connection with the transactions contemplated hereby constitutes legal, tax or investment advice. Xxxxxx acknowledges that it must rely on legal, tax and investment advisors of its own choosing in connection with its purchase of the Securities. Xxxxxx’x decision to purchase the Securities was based solely upon the representations and warranties set forth herein, and Xxxxxx has not relied upon any other information or representations made by or on behalf of the Securities.
12.3.1. Immediately prior to the execution of this Agreement, neither Xxxxxx nor any of its Affiliates beneficially own any CTI equity securities or any securities convertible into CTI equity securities. Xxxxxx has no present actual intent to seek to effect, or to assist others in effecting, a hostile acquisition of CTI.
61
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
12.3.2. Xxxxxx understands that any Securities issued hereunder shall be characterized as “restricted securities” under the federal securities Laws inasmuch as they are being acquired from CTI in a transaction not involving a public offering and that under such Laws and applicable regulations such securities may be resold with registration under the Securities Act, only in certain limited circumstances. Xxxxxx understands that any Securities issued as part of the Equity Consideration may bear the following or similar legend:
“THE SECURITIES HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, THEY MAY NOT BE SOLD, OFFERED FOR SALE, PLEDGED, HYPOTHECATED, OR OTHERWISE TRANSFERRED EXCEPT PURSUANT TO AN EFFECTIVE REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR AN OPINION OF COUNSEL SATISFACTORY TO THE COMPANY THAT REGISTRATION IS NOT REQUIRED UNDER SUCH ACT OR UNLESS SOLD PURSUANT TO RULE 144 UNDER SUCH ACT.”
12.3.3. Xxxxxx understands that the issuance or delivery of any Securities has not been registered with or cleared by the Commissione Nazionale per le Societa e la Borsa (“CONSOB”) pursuant to the European Union Directive 2003/71/EC (so-called Prospectus Directive) and Italian securities laws and regulations and no prospectus has been or will be distributed in the Republic of Italy.
12.4. The Parties’ Covenants. Each Party hereby covenants throughout the Term as set forth below:
12.4.1. Such Party shall not enter into any agreement with a Third Party that will conflict with the rights granted to the other Party under this Agreement.
12.4.2. If during the term of this Agreement, a Party has reason to believe that it or any of its employees, officers, independent contractors, consultants, or agents rendering services relating to the Licensed Product: (x) is or will be debarred or convicted of a crime for which such Person could be debarred before a Regulatory Authority under applicable Laws, or (y) is or will be under indictment under such Laws, then such Party promptly shall notify the other Party of same in writing.
12.5. CTI’s Covenants.
12.5.1.Upstream Agreements. CTI hereby covenants that throughout the Term it shall not agree to amend any terms or conditions of the Upstream Agreements in any manner that
62
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
would adversely affect the rights granted to Xxxxxx under this Agreement in any respect without the prior written consent of Xxxxxx. CTI further agrees that it shall not terminate the Upstream Agreements, shall not cease to exercise its rights under the Upstream Agreements, and shall not amend the Upstream Agreements in any manner that would adversely affect Xxxxxx, in each case without the prior written consent of Xxxxxx. CTI shall comply with the terms of the Upstream Agreements in all material respects; provided that CTI shall not be in breach of the foregoing obligations to the extent that CTI’s failure to comply results from Xxxxxx’x non-compliance with the terms of this Agreement, and further provided that if Section 15.4 applies, then CTI shall not be deemed in breach of this Section 12.5 and the Parties shall have the rights and obligations set forth in Section 15.4. CTI shall notify Xxxxxx of any anticipated termination of the Upstream Agreements by CTI prior to such termination. CTI shall further notify Xxxxxx when it has received any notice from any Third Party that is a party to an Upstream Agreement stating that such Third Party intends to terminate or is terminating such Upstream Agreement.
12.5.2. Xxxxxx Approved Supplier Status. CTI will (in cooperation with Xxxxxx) undertake such activities (including an obligation by such parties to undertake any remediation efforts) as are required to ensure that CTI and its applicable Third Party manufacturers for Licensed Product and/or Drug Product shall, no later than one hundred eighty (180) days prior to the projected First Commercial Sale of a Licensed Product, become, and shall throughout the Term remain, Xxxxxx Approved Suppliers, it being understood and agreed that any Xxxxxx judgments in connection with audit assessments in relation to CTI becoming a Xxxxxx Approved Supplier shall be commercially reasonable and made in good faith. For purposes hereof, “Xxxxxx Approved Supplier” shall mean a supplier approved in accordance with written criteria provided in advance to CTI.
ARTICLE XIII.
CONFIDENTIALITY
13.1. Confidentiality Obligations of Xxxxxx.
13.1.1. Subject to Section 13.3, during the term of this Agreement and for a period of ** thereafter, or ** from the Effective Date, whichever is longer, Xxxxxx:
(a) shall hold in strict confidence any and all information disclosed to it by CTI, including CTI Information (together “CTI Confidential Information”), and shall not use, nor disclose or supply to any Third Party, nor permit any Third Party, to have access to the CTI Confidential Information, without first obtaining the written consent of CTI, except as expressly permitted in this Agreement;
(b) shall take all reasonable precautions necessary or prudent to prevent material in its possession or control that contains or refers to CTI Confidential Information from being destroyed or lost, or discovered, received, used, intercepted or copied by any Third Party; and
63
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
(c) may disclose the CTI Confidential Information only to its employees, consultants, independent contractors, agents, Affiliates, actual and potential Sublicensees and actual and potential acquirers; provided that such employees, consultants, independent contractors, agents, Affiliates, actual and potential Sublicensees and actual and potential acquirers are bound by terms and conditions of confidentiality no less protective than the terms and conditions that bind Xxxxxx hereunder.
For the avoidance of doubt, it is understood that Xxxxxx shall be liable for any breach of the confidentiality obligation under this Section 13.1 by any person or corporation to whom the CTI Confidential Information is disclosed by Xxxxxx.
13.1.2. Xxxxxx’x obligations of confidentiality and non-use under this Section 13.1 shall not apply and Xxxxxx shall have no further obligations with respect to any of the CTI Confidential Information, to the extent that such CTI Confidential Information:
(a) is or becomes part of the public domain without breach by Xxxxxx of this Agreement;
(b) was rightfully in Xxxxxx’x possession before disclosure by CTI and was not acquired directly or indirectly from CTI;
(c) is obtained from a Third Party with no obligation of confidentiality to CTI, who has a right to disclose it to Xxxxxx;
(d) is developed independently by Xxxxxx without use of the CTI Confidential Information, as evidenced by Xxxxxx’x written records; or
(e) is required to be revealed in response to a court decision or administrative order, or to comply with Laws of a Governmental Authority or rules of a securities exchange, in which case Xxxxxx shall inform CTI immediately by written notice and cooperate with CTI using its Commercially Reasonable Efforts either to seek protective measures for such CTI Confidential Information, or to seek confidential treatment of such CTI Confidential Information, and in any case Xxxxxx shall disclose only such portion of the CTI Confidential Information which is so required to be disclosed.
13.1.3. Nothing herein shall prevent Xxxxxx from disclosing any CTI Confidential Information to the extent that such CTI Confidential Information is required to be used or disclosed for the purposes of seeking or obtaining Marketing or Reimbursement Approvals of Licensed Products in the Licensed Territory or seeking patent protection for Inventions it owns or has responsibility for prosecuting under Article X. Xxxxxx shall further have the right to present CTI Confidential Information at conferences or to publish CTI Confidential Information in journals (collectively “Publications”); provided that if the CTI Confidential Information concerned has not been previously published, Xxxxxx shall give CTI reasonable notice thereof and such Publication shall be subject to CTI’s prior written consent, not to be unreasonably withheld, conditioned or delayed.
64
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
13.2. Confidentiality Obligations of CTI.
13.2.1. Subject to Section 13.3, during the term of this Agreement and for a period of ** thereafter, or ** from the Effective Date, whichever is longer, CTI:
(a) shall hold in strict confidence any and all information disclosed to it by Xxxxxx, including Xxxxxx Information (together “Xxxxxx Confidential Information”), and shall not use, nor disclose or supply to any Third Party nor permit any Third Party to have access to the Xxxxxx Confidential Information, without first obtaining the written consent of Xxxxxx, except as expressly permitted in this Agreement;
(b) shall take all reasonable precautions necessary or prudent to prevent material in its possession or control that contains or refers to Xxxxxx Confidential Information from being destroyed or lost, or discovered, received, used, intercepted or copied by any Third Party; and
(c) may disclose the Xxxxxx Confidential Information to its employees, consultants, independent contractors, agents, Affiliates, and actual and potential acquirers; provided that such employees, consultants, independent contractors, agents, Affiliates, and actual and potential acquirers are bound by terms and conditions of confidentiality no less protective than the terms and conditions that bind CTI hereunder.
For the avoidance of doubt, it is understood that CTI shall be liable for any breach of the confidentiality obligation under this Section 13.2 by any person or corporation to whom the Xxxxxx Confidential Information is disclosed by CTI.
13.2.2. CTI’s obligations of confidentiality and non-use under this Section 13.2 shall not apply and CTI shall have no further obligations with respect to any of the Xxxxxx Confidential Information to the extent that such Xxxxxx Confidential Information:
(a) is or becomes part of the public domain without breach by CTI of this Agreement;
(b) was rightfully in CTI’s possession before disclosure by Xxxxxx to CTI and was not acquired directly or indirectly from Xxxxxx;
(c) is obtained from a Third Party with no obligation of confidentiality to Xxxxxx, who has a right to disclose it to CTI;
(d) is developed independently by CTI without use of the Xxxxxx Confidential Information, as evidenced by CTI’s written records; or
(e) is required to be revealed in response to a court decision or administrative order, or to comply with Laws of a Governmental Authority or rules of a securities exchange, in which case CTI shall inform Xxxxxx immediately by written notice and
65
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
cooperate with Xxxxxx using its Commercially Reasonable Efforts either to seek protective measures for such Xxxxxx Confidential Information, or to seek confidential treatment of such Xxxxxx Confidential Information, and in any case CTI shall disclose only such portion of the Xxxxxx Confidential Information which is so required to be disclosed.
13.2.3. Nothing herein shall prevent CTI from disclosing any Xxxxxx Confidential Information to the extent that such Xxxxxx Confidential Information is required to be used or disclosed for the purposes of seeking or obtaining Marketing Approvals of Licensed Products or seeking patent protection for Inventions it owns or has responsibility for prosecuting under Article X. CTI shall further have the right to disclose any Xxxxxx Confidential Information in a Publication; provided that if the Xxxxxx Confidential Information concerned has not been previously published, CTI shall give Xxxxxx reasonable notice thereof and such Publication shall be subject to Xxxxxx’x prior written consent, not to be unreasonably withheld, delayed or conditioned.
13.2.4. Other Initiatives. Each Party understands that the other Party may have present or future initiatives, including initiatives with third parties, involving similar products, technologies or processes that compete with a product, technology or process contemplated or offered by the other Party. Accordingly, the Parties agree that nothing in this Agreement other than its agreement to use Commercially Reasonable Efforts with respect to certain obligations hereunder shall be construed as a representation or inference that either Party will not develop for itself, or enter into business relationships with other Third Parties that involve products, technologies or processes that are similar to or compete with any product, technology or process contemplated or offered by the other Party, provided that Confidential Information is not used or disclosed in breach of this Agreement or is otherwise in contravention of this Agreement.
13.3. Press Releases; Publicity.
13.3.1. Except with respect to the press release attached hereto as Exhibit 13.3.1 (or any press release, public disclosure or any other disclosure to a Third Party with content substantially similar thereto) which may be issued by either or both of the Parties upon execution of this Agreement, no disclosure shall be made by either Party concerning the execution of this Agreement or the terms and conditions hereof without the prior written consent of the other Party, which shall not be unreasonably withheld, conditioned or delayed; provided, however, that neither Party will be prevented from complying on a timely basis with any duty of disclosure it may have pursuant to Law or pursuant to the rules of any recognized stock exchange or quotation system. Notwithstanding the foregoing, either Party may disclose the existence of this Agreement and the terms and conditions hereof without the prior written consent of the other pursuant to Section 13.1.2(e) or Section 13.2.2(e), as applicable, or in connection with a due diligence process associated with any future financing by either Party or the negotiation or exploration of a possible strategic transaction involving such Party; provided that such disclosure is made in the course of such diligence, negotiation or exploration pursuant to confidentiality obligations consistent with those set forth in this Agreement. Each Party may issue a press release or public announcement concerning the development of the Product, provided that such
66
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Party shall provide the other Party with a copy of such press release or public announcement at least ten (10) days in advance of its intended publication or release thereof and shall consider in good faith the comments of the other Party which comments shall be provided as promptly as reasonably practicable following receipt of the press release or public announcement from the Party desiring to make the disclosure.
13.3.2. Each Party agrees that it shall cooperate fully and in a timely manner with the other Party with respect to all disclosures required by the Securities and Exchange Commission and any other Governmental Authority or Regulatory Authority, including requests for confidential treatment of confidential information of either Party included in any such disclosure. Notwithstanding any other provision of this Agreement, either Party may issue any public announcement or other disclosure that it is advised by legal counsel is required under applicable Laws or the rules of any recognized stock exchange or quotation system, provided that the Parties shall coordinate with each other with respect to the timing, form and content of such required disclosure to the extent practicable under the circumstances, and the Party seeking such disclosure shall use Commercially Reasonable Efforts to provide the other Party with reasonable notice thereof in advance of any disclosure. Any request for revision to the content of said disclosure by the non-disclosing Party shall be furnished to the disclosing Party as promptly as necessary for the disclosing Party to comply with such requirements in a timely manner. Without limiting the foregoing, each Party shall consult with the other Party on the provisions of this Agreement, together with exhibits or other attachments attached hereto, to be redacted in any filings made by CTI and/or Xxxxxx with the Securities and Exchange Commission or as otherwise required by Law.
13.3.3. Once a disclosure is disclosed in accordance with the terms of this Agreement, the content of such disclosure (or any portion thereof) may be repeated in a subsequent disclosure by either Party without regard to the notification or other requirements of this Section 13.3.
ARTICLE XIV.
INDEMNIFICATION
14.1. CTI Indemnity. CTI shall defend, indemnify and hold harmless Xxxxxx and its Affiliates and their respective directors, officers, agents, successors, assignees and employees (the “Xxxxxx Indemnitees”) from and against any and all claims, liabilities, losses, costs, actions, suits, damages and expenses, including reasonable attorneys’ fees (collectively “Damages”), to the extent arising from any claim, action or proceeding made or brought against Xxxxxx Indemnitees by a Third Party in connection with (a) the gross negligence, recklessness, or intentional wrongful acts or omissions of CTI or its Affiliates or their respective employees, officers, independent contractors, consultants, or agents, in connection with the performance by or on behalf of CTI of CTI’s obligations or exercise of its rights under this Agreement, (b) any breach by CTI, or its Affiliates or their respective independent contractors of any representation, warranty, covenant, or obligation of CTI set forth in this Agreement, **; except in any such case to the extent such Damages are reasonably attributable to any gross negligence, recklessness, willful misconduct, or breach of this Agreement by Xxxxxx or a Xxxxxx Indemnitee.
67
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
14.2. Xxxxxx Indemnity. Xxxxxx shall defend, indemnify and hold harmless CTI and its Affiliates, directors, officers, agents, successors, assignees and employees (the “CTI Indemnitees”) from and against any and all Damages to the extent arising from any claim, action or proceeding made or brought against CTI Indemnitees by a Third Party in connection with (a) the gross negligence, recklessness, or intentional wrongful acts or omissions of Xxxxxx or its Affiliates or their respective employees, officers, independent contractors, consultants, or agents, in connection with the performance by or on behalf of Xxxxxx of Xxxxxx’x obligations or exercise of its rights under this Agreement, (b) any breach by Xxxxxx or its Affiliates or their respective independent contractors of any representation, warranty, covenant, or obligation of Xxxxxx set forth in this Agreement, or **; except in any such case to the extent such Damages are reasonably attributable to any gross negligence, recklessness, willful misconduct, or breach of this Agreement by CTI or a CTI Indemnitee.
14.3. Indemnification Procedure.
14.3.1. Each Party shall notify the other in the event it becomes aware of a claim for which indemnification may be sought pursuant to this Article XIV. In case any proceeding (including any governmental investigation) shall be instituted involving any Party in respect of which indemnity may be sought pursuant to this Article XIV, such Party (the “Indemnified Party”) shall promptly notify the other Party (the “Indemnifying Party”) in writing (an “Indemnification Claim Notice”). The Indemnifying Party and Indemnified Party shall promptly meet to discuss how to respond to any claims that are the subject matter of such proceeding. At its option, the Indemnifying Party may assume the defense of any Third Party claim subject to indemnification as provided for in this Section 14.3 by giving written notice to the Indemnified Party within thirty (30) days or until such time provided in any applicable extension to appropriately answer any complaint, if any, but no longer than sixty (60) days (the “Election Time Period”); with the Indemnified Party being obligated to make all reasonable efforts to obtain any such extension after the Indemnifying Party’s receipt of an Indemnification Claim Notice, solely for claims (a) that solely seek monetary damages and (b) as to which the Indemnifying Party expressly agrees in writing that, as between the Indemnifying Party and the Indemnified Party, the Indemnifying Party shall be solely obligated to satisfy and discharge the claim in full (the matters described in (a) and (b), the “Litigation Conditions”). The Indemnified Party may assume responsibility for such defense if the Litigation Conditions are not satisfied, by written notice to the Indemnifying Party within the Election Time Period. If the Indemnified Party fails to promptly provide an Indemnification Claim Notice, and such failure materially prejudices the defense of such claim, then the Indemnifying Party shall be relieved of its responsibility to indemnify the Indemnified Party.
14.3.2. Upon assuming the defense of a Third Party claim in accordance with this Section 14.3, the Indemnifying Party shall be entitled to appoint lead and any local counsel in the defense of the Third Party claim. Should the Indemnifying Party assume and continue the
68
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
defense of a Third Party claim, except as otherwise set forth in this Section 14.3, the Indemnifying Party will not be liable to the Indemnified Party for any legal expenses subsequently incurred by such Indemnified Party after the date of assumption of defense in connection with the analysis, defense, countersuit or settlement of the Third Party claim. Without limiting this Section 14.3, any Indemnified Party will be entitled to participate in, but not control, the defense of a Third Party claim for which it has sought indemnification hereunder and to engage counsel of its choice for such purpose; provided, however, that such engagement will be at the Indemnified Party’s own expense unless (a) the engagement thereof has been specifically requested by the Indemnifying Party in writing, or (b) the Indemnifying Party has failed to assume and actively further the defense and engage counsel in accordance with this Section 14.3 (in which case the Indemnified Party will control the defense), or (c) the Indemnifying Party no longer satisfies the Litigation Conditions.
14.3.3. Subject to the Litigation Conditions being satisfied, the Indemnifying Party will have the sole right to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Damages, on such terms as the Indemnifying Party, in its reasonable discretion, will deem appropriate (provided, however, that such terms shall include a complete and unconditional release of the Indemnified Party from all liability with respect thereto), and will transfer to the Indemnified Party all amounts which said Indemnified Party will be liable to pay pursuant to such settlement or disposal of such claim prior to the time such payments become due by the Indemnified Party. With respect to all other Damages in connection with Third Party claims, where the Indemnifying Party has assumed the defense of the Third Party claim in accordance with this Section 14.3, the Indemnifying Party will have authority to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Damages; provided it obtains the prior written consent of the Indemnified Party, not to be unreasonably withheld, conditioned or delayed.
14.3.4. The Indemnifying Party that has assumed the defense of the Third Party claim in accordance with this Section 14.3 will not be liable for any settlement or other disposition of any Damages by an Indemnified Party that is reached without the written consent of such Indemnifying Party. The Indemnified Party will not admit any liability with respect to, or settle, compromise or discharge, any Third Party claim without first offering to the Indemnifying Party the opportunity to assume the defense of the Third Party claim in accordance with this Section 14.3. If the Indemnifying Party chooses to defend or prosecute any Third Party claim, the Indemnified Party will cooperate in the defense or prosecution thereof and will furnish such records, information and testimony, provide such witnesses including to the extent possible, former employees and attend such conferences, discovery proceedings, hearings, trials and appeals as may be reasonably requested in connection with such Third Party claim. Such cooperation will include access during normal business hours afforded to the Indemnifying Party to, and reasonable retention by the Indemnified Party of, records and information that are reasonably relevant to such Third Party claim, and making employees and agents available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder. The Indemnifying Party will reimburse the Indemnified Party for all its reasonable out-of-pocket expenses incurred in connection with such cooperation.
69
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
14.3.5. Each Party shall maintain, at its cost, a program of insurance and/or self-insurance against liability and other risks associated with its activities and obligations under this Agreement, including its Clinical Trials, the Commercialization of any Licensed Products and its indemnification obligations hereunder, in such amounts, subject to such deductibles and on such terms as are customary for the activities to be conducted by it under this Agreement. All insurance required by this Section 14.3.5 shall be maintained during the Term and each Party shall, from time to time, provide copies of certificates of such insurance to the other Party upon request. Further, each Party shall list the other Party as an additional insured on all insurance policies. All insurance required by this Section 14.3.5 shall be maintained for at least three (3) years following expiration or termination of this Agreement.
14.4. Limitation of Liability; Exclusion of Damages; Disclaimer.
14.4.1. EXCEPT WITH RESPECT TO DAMAGES AWARDED TO A THIRD PARTY BY A COURT OF COMPETENT JURISDICTION THAT ARE REQUIRED TO BE INDEMNIFIED UNDER SECTION 14.1 OR SECTION 14.2, AND EXCEPT IN THE CASE OF A BREACH OF ARTICLE XIII, AND WITHOUT LIMITING THE LIABILITY OF A PARTY FOR INFRINGEMENT OR MISAPPROPRIATION OF THE INTELLECTUAL PROPERTY RIGHTS OF THE OTHER PARTY OR FOR FRAUD OR WILLFUL MISCONDUCT, NEITHER PARTY SHALL BE LIABLE TO THE OTHER PARTY FOR SPECIAL, INDIRECT, INCIDENTAL, PUNITIVE, OR CONSEQUENTIAL DAMAGES (INCLUDING WITHOUT LIMITATION DAMAGES RESULTING FROM LOSS OF USE, LOSS OF PROFITS, INTERRUPTION OR LOSS OF BUSINESS, DIMINUTION OF VALUE, OR OTHER ECONOMIC LOSS) ARISING OUT OF THIS AGREEMENT OR WITH RESPECT TO A PARTY’S PERFORMANCE OR NON-PERFORMANCE HEREUNDER.
14.4.2. EXCEPT AS EXPRESSLY PROVIDED IN THIS AGREEMENT, NEITHER PARTY PROVIDES ANY WARRANTIES, WHETHER WRITTEN OR ORAL, EXPRESS OR IMPLIED, REGARDING THE LICENSED PRODUCT AND EACH PARTY HEREBY DISCLAIMS ALL OTHER WARRANTIES, WHETHER WRITTEN OR ORAL, EXPRESS AND IMPLIED, INCLUDING WITHOUT LIMITATION THE IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, AND FREEDOM FROM INFRINGEMENT OF THIRD PARTY RIGHTS.
ARTICLE XV.
TERM; TERMINATION
15.1. Term. This Agreement shall expire in its entirety upon the expiration of all applicable Royalty Terms in the Licensed Territory and satisfaction of the applicable payment obligations (including Milestone payments) under this Agreement with respect to all Licensed Products in all countries in the Licensed Territory (the “Term”).
70
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
15.1.1. Effect of Expiration. Upon expiration of this Agreement in accordance with Section 15.1:
(a) the Licensed Rights shall continue in full force and effect and be considered to be fully paid-up; and
(b) Subject to the Confidentiality obligations contained in Article XIII, Baxter and CTI shall each have the right to freely use all Information disclosed to it by the other Party hereunder solely in connection with the development, manufacture and commercialization of Licensed Products.
15.2. Early Termination.
15.2.1. By Either Party for Breach. Without prejudice and in addition to any other contractual remedy the non-defaulting Party may have under this Agreement, either Party may terminate this Agreement in writing forthwith, if the other Party commits a material breach of any provision of this Agreement and such breach is not cured within sixty (60) days after written notice of the breach is received by the other Party, which notice shall specify the nature of the breach and demand its cure. Notwithstanding the foregoing, if a material breach is not susceptible to cure within the cure period specified above, the non-breaching Party’s right of termination shall be suspended only if, and for so long as, (a) the breaching Party has provided to the non-breaching Party a written plan that is reasonably calculated to effect a cure, (b) such plan is reasonably acceptable to the non-breaching Party and (c) the breaching Party commits to and does carry out such plan; provided, however, that, unless otherwise mutually agreed by the Parties in such plan, in no event shall such suspension of the non-breaching Party’s right to terminate extend beyond sixty (60) days after the original cure period.
15.2.2. By Either Party for Force Majeure. The Agreement may be terminated by either Party in the event of a Force Majeure (as hereinafter defined) pursuant to Section 15.4.3.
15.2.3. By Either Party for Insolvency. Either Party may terminate this Agreement upon written notice if the other Party is dissolved or liquidated, files or has filed against it a petition under any bankruptcy or insolvency law that is not dismissed within sixty (60) days, makes an assignment for the benefit of its creditors or has a receiver or trustee appointed for all or substantially all of its property.
15.2.4. By CTI for Baxter Patent Challenge. In the event that Baxter or any of its Affiliates commences or otherwise, directly or indirectly, pursues (or, other than as required by Law or legal process, voluntarily assists any Third Party to pursue in any material respect where Baxter has knowledge that its assistance will be used by the Third Party to pursue) any proceeding seeking to have any of the CTI Patents revoked or declared invalid, unpatentable, or unenforceable, CTI may declare a material breach hereunder, terminate this Agreement on written notice to Baxter and shall then have the right to exercise the remedies available under Section 15.3 with immediate effect.
15.2.5. By Baxter for CTI Development Costs. In the event Baxter determines in good faith based on documentary evidence (including without limitation information contained in the Development Account) that the CTI Development Costs that are reasonably projected to
71
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
be incurred on or after January 1, 2014 with respect to the Initial Indication that are (A) applicable to both the Baxter Exclusive Territory and the Co-Promotion Territory, or (B) exclusive to the Co-Promotion Territory, in the case of either (A) or (B), exceed or will exceed **, Baxter may terminate this Agreement. If Baxter determines that it desires to so terminate, it shall notify CTI in writing and such notification shall be accompanied by a reasonably detailed description of the CTI Development Costs involved, including without limitation the territory or territories with respect to which such CTI Development Costs were or are expected to be incurred, and such other information as CTI may reasonably request. Unless CTI, within thirty (30) days of receipt of Xxxxxx’x notification pursuant to this Section 15.2.5, delivers to Baxter a written objection setting forth in reasonable detail its determination as to why it disagrees with Xxxxxx’x calculations, the Agreement shall terminate on the expiration of such thirty (30) day period. If CTI responds in writing with such a written objection within such thirty (30) day period, and Baxter disagrees with such response, the Parties shall follow the dispute resolution procedures set forth in Section 16.13. In the event the matter is decided in Xxxxxx’x favor, then Baxter shall have the right to terminate this Agreement effective immediately upon the decision rendered pursuant to Section 16.13.
15.2.6. By Baxter for Commercial Failure. In the event of a Commercial Failure, Baxter shall have the right to terminate this Agreement on a country-by-country basis or in its entirety upon ninety (90) days’ prior written notice to CTI.
15.2.7. By Baxter for Convenience. Baxter may terminate this Agreement without cause upon written notice to CTI at any time following the one year anniversary of the Effective Date; provided, however, that such termination shall not be effective for a period of six (6) calendar months following delivery to CTI of the termination notice, provided that during such 6-month period Baxter continues to perform its obligations hereunder, including without limitation participation in Committees, payment of amounts due and using Commercially Reasonable Efforts to perform its Commercialization Obligations .
15.3. Obligations upon Early Termination.
15.3.1. In the event of termination of this Agreement by either Party in accordance with Section 15.2:
(a) all Licensed Rights shall revert to CTI without any compensation to be paid by CTI;
(b) Baxter shall promptly return to CTI any and all CTI Information;
(c) Baxter shall promptly transfer to CTI or its designee any and all Marketing Approvals and all other filings and submissions with and to Regulatory Authorities owned by Baxter or its Affiliates with respect to the Licensed Product. To this end Baxter shall make Commercially Reasonable Efforts to file for transfer with the relevant Regulatory Authorities and to give all other notifications and approvals necessary under law for the transfer of Marketing Approvals and such other filings and submissions;
72
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
(d) Baxter shall grant to CTI a worldwide license, with the right to sublicense, through multiple tiers of Sublicensees, to use any Baxter Trademarks (including, without limitation, the goodwill symbolized by such Baxter Trademarks) used to brand Licensed Products, and a license to reproduce, distribute, perform, display and prepare derivative works of Xxxxxx’x copyrights used to brand or Promote the Licensed Product, in each case solely to the extent necessary or useful for Commercializing the Licensed Product; provided that such license shall bear a commercially reasonable royalty to be agreed by the Parties in good faith and provided further that the term of the license shall be no greater than ** unless agreed to by Baxter;
(e) The licenses granted by Baxter to CTI pursuant to Section 2.2 and other provisions of this Agreement shall continue in effect, but shall also from such time forward include the right to grant sublicenses through multiple tiers of Sublicensees, but solely to the extent necessary or useful for Developing or Commercializing the Licensed Product, in addition to those sections that also survive pursuant to Section 15.5;
(f) Baxter shall furnish CTI with reasonable cooperation, at Xxxxxx’x expense, to assure a smooth transition of any clinical or other studies in progress then being conducted by Baxter or its Affiliates related to the Compound, Drug Product and/or Licensed Product which CTI determines to continue in compliance with applicable Laws and ethical guidelines applicable to the transfer or termination of any such studies. In the event that CTI informs Baxter that it does not intend to continue specific development activities then in progress, each Party shall bear its own costs incurred in closing out such activities; and
(g) Baxter shall not withdraw or cancel any Marketing Approval or Reimbursement Approval or application for either, unless expressly instructed so by CTI in writing; provided that CTI shall be responsible for all costs and expenses for the maintenance of all Marketing Approvals and Reimbursement Approvals following receipt of notice of termination.
15.4. Force Majeure.
15.4.1. No failure or delay by either Party in the performance of any obligation hereunder shall be deemed a breach of this Agreement nor create any liability for any damages, increased cost or losses which the other Party may sustain by reason of such failure or delay of performance, if the same shall arise from any cause or causes beyond the control of that Party, such as earthquake, storm, flood, fire, other acts of nature, epidemic, war, riot, hostility, public disturbance, cessation of transport, act of public enemies, prohibition or act by a Government Authority or public agency, strike or other labor dispute or work stoppage (collectively “Force Majeure”); provided, however, that the Party so prevented shall continue to take all commercially reasonable actions within its power to comply with its obligations hereunder as fully as possible and to mitigate possible damages.
15.4.2. The Party so prevented shall without undue delay notify the other Party in writing thereof.
73
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
15.4.3. Should the event of Force Majeure continue for more than ninety (90) days, the Parties shall promptly discuss their further performance under this Agreement and whether to modify or terminate this Agreement in view of the effect of the event of Force Majeure. If no agreement can be reached within sixty (60) days after expiration of such ninety (90) day period, either Party may terminate this Agreement effective immediately upon written notice to the other Party.
15.5. Survival. For the avoidance of doubt, it is understood that the following Sections of this Agreement shall survive expiration or termination of this Agreement for any reason: 1.1 (Definitions), 7.4 (Recalls), 9.3.4 (Royalty Payment Timing; Royalty Reports), 9.3.5 (Audit), 10.2 (Ownership of Inventions), 13 (Confidentiality), 14 (Indemnification), 15 (Term; Termination), and 16 (General Provisions). The following Sections of this Agreement shall survive expiration or termination of this Agreement with respect to payments accrued before such expiration or termination: 9.2 (Milestones), 9.3.1 (Royalty Payments), 9.7 (Exchange Rate; Manner and Place of Payment) and 9.8 (Taxes).
15.6. Other Remedies. Termination or expiration of this Agreement for any reason shall not release any Party from any liability or obligation that has accrued prior to such expiration or termination, nor affect the survival of any provision hereof to the extent it is expressly stated to survive termination. Termination or expiration of this Agreement for any reason shall not constitute a waiver or release of, or otherwise be deemed to prejudice or adversely affect, any rights, remedies, or claims, whether for damages or otherwise, that a Party may have hereunder or that may arise out of or in connection with such termination or expiration.
ARTICLE XVI.
GENERAL PROVISIONS
16.1. Assignment. This Agreement is binding upon and will inure to the benefit of the Parties and their respective permitted assignees or successors in interest, including without limitation those that may succeed by assignment, transfer or otherwise to the ownership of either of the Parties or of the assets necessary to the conduct of the business to which this Agreement relates. This Agreement may not be assigned or otherwise transferred by either Party without the prior written consent of the other Party, which consent shall not be unreasonably withheld, conditioned or delayed; provided, however, that either Party may, without such consent, assign this Agreement together with all of its rights and obligations hereunder to its Affiliates, or to a successor in interest in connection with the transfer or sale of all or substantially all of its business to which this Agreement relates, or in the event of its merger or consolidation or similar transaction, subject to the assignee agreeing to be bound by the terms of this Agreement. Any purported assignment in violation of the preceding sentences shall be void. Any permitted successor shall assume and be bound by all obligations of its assignor or predecessor under this Agreement.
16.2. Headings. Headings are inserted for convenience and shall not affect the meaning or interpretation of this Agreement.
74
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
16.3. Waiver. No waiver of any default hereunder by either Party or any failure to enforce any rights hereunder shall be deemed to constitute a waiver of any subsequent default with respect to the same or any other provision hereof.
16.4. Notices. Any and all notices given by one Party to the other Party under this Agreement must be in writing and shall be delivered by hand, sent by registered or certified air mail (postage prepaid), international courier service, email or fax to the other Party’s address as set forth below or to the latest address of such Party as shall have been communicated to the other Party.
If to CTI:
CELL THERAPEUTICS, INC.
0000 Xxxxxxx Xxxxxx
Xxxxx #000
Xxxxxxx, XX 00000
Attention: Executive Vice President, Corporate Development
Telephone: (000 000-0000
Facsimile: (000) 000-0000
Email: **
with a copy to CTI Legal Affairs at the above address.
with a copy (which copy shall not constitute legal notice to CTI) to:
O’Melveny & Xxxxx LLP
Xxx Xxxxxxxxxxx Xxxxxx
Xxx Xxxxxxxxx, XX 00000
Facsimile: x0 (000) 000-0000
Email: **
Attention: X. Xxxxxx Xxxxxxxxxxx, Esq.
If to Baxter:
Xxxxxx Healthcare SA
Xxxxxxxx
0000 Xxxxxx, Xxxxxxxxxxx
Telephone: x00 00 000 00 00
Facsimile: x00 00 000 00 00
Attention: Legal Department
75
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
with a copy (which copy shall not constitute legal notice to Baxter) to counsel:
Xxxxxx Healthcare Corporation
Xxx Xxxxxx Xxxxxxx
Xxxxxxxxx, Xxxxxxxx 00000
Telephone: (000) 000-0000
Facsimile: (000) 000-0000
Email: **
Attention: General Counsel
16.5. Severability. Should any part of this Agreement be held unenforceable or in conflict with the applicable Laws of any jurisdiction, the invalid or unenforceable part or provision shall be replaced with a provision which accomplishes, to the extent possible, the original business purpose of such part or provision in a valid and enforceable manner, and the remainder of this Agreement shall remain binding upon the Parties hereto.
16.6. Entire Agreement. This Agreement constitutes the whole agreement between the Parties and shall cancel and supersede any and all prior and contemporaneous negotiations, correspondence, understandings and agreements, whether oral or written, between the Parties respecting the subject matter hereof, including without limitation the Confidentiality Agreement.
16.7. Amendment; No Waiver. Any amendment or modification to this Agreement shall only be made in writing and shall only be valid when signed by the due representatives of the Parties. No provision of this Agreement may be waived except by a writing signed by the Party against which such waiver is sought to be enforced.
16.8. Counterparts. This Agreement may be executed in more than one counterpart (including by electronic transmission), each of which shall be deemed an original, but all of such counterparts taken together shall constitute one and the same agreement.
16.9. Agency. Neither Party is, nor shall be deemed to be, an employee, agent, co-venturer, or legal representative of the other Party for any purpose. Neither Party shall be entitled to enter into any contracts in the name of, or on behalf of the other Party, nor shall either Party be entitled to pledge the credit of the other Party in any way or hold itself out as having the authority to do so.
16.10. Further Actions. Each Party agrees to execute, acknowledge, and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
16.11. Compliance with Laws. Each Party will comply with all Laws in performing its obligations and exercising its rights hereunder, including without limitation all Laws relating to the export, re-export or other transfer of any Information transferred pursuant to this Agreement or the Licensed Product.
16.12. Governing Law. The construction, validity and performance of this Agreement shall be governed in all respects by the laws of the state of New York, excluding its provisions regarding conflicts of law. The UNCITRAL Convention on the International Sale of Goods shall not apply.
76
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
16.13. Dispute Resolution; Jurisdiction. Any disputes under this Agreement shall be submitted initially by either Party for resolution by the Executive Officers. The Executive Officers shall meet and discuss such matter within fifteen (15) days after a Party proposes that such Executive Officers meet to discuss the dispute. In the event the Executive Officers of each Party are unable to resolve the dispute within thirty (30) days after receiving notice of the dispute (or such longer period as the Parties may mutually agree upon), then such dispute shall, after expiration of the thirty (30) day period, be submitted to the International Institute for Conflict Prevention & Resolution (“CPR”) for final and binding arbitration pursuant to the arbitration clause set forth in Exhibit 16.13. Notwithstanding the foregoing, no matters relating to breach or alleged breach of the provisions of Article XIII or the ownership of intellectual property or rights in intellectual property (including without limitation Improvements and Inventions) or the validity or enforceability thereof shall be subject to resolution by the CPR, but rather shall be determined by a U.S. federal court of appropriate jurisdiction.
16.14. Bankruptcy Code. All rights and licenses granted under or pursuant to this Agreement by CTI or Baxter are, and shall otherwise be deemed to be, for purposes of Section 365(n) of the U.S. Bankruptcy Code and of any similar provisions of applicable Laws under any other jurisdiction (collectively, the “Bankruptcy Laws”), licenses of right to “intellectual property” as defined under the Bankruptcy Laws. CTI agrees that Baxter, as a licensee of rights under this Agreement, shall retain and may fully exercise all of its rights and elections under the Bankruptcy Laws.
16.15. Joint and Several Obligations. As among the entities comprising Baxter hereunder, consisting of BII, BHC and BHSA, all obligations and liabilities of Baxter under or in connection with this Agreement shall be joint and several.
[Signature Page Follows]
77
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
[Signature Page to Development, Commercialization and License Agreement]
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be executed in duplicate by their respective duly authorized officers or representatives.
| CELL THERAPEUTICS, INC. | ||
| By: | /s/ Xxxxx Xxxxxx | |
| Name: | Xxxxx Xxxxxx | |
| Title: | President & CEO | |
| XXXXXX INTERNATIONAL INC. | ||
| By: | /s/ Xxxxxx X. Xxxxxxx | |
| Name: | Xxxxxx X. Xxxxxxx | |
| Title: | CVP/President BioScience | |
| XXXXXX HEALTHCARE CORPORATION | ||
| By: | /s/ Xxxxxx X. Xxxxxxx | |
| Name: | Xxxxxx X. Xxxxxxx | |
| Title: | CVP/President BioScience | |
| XXXXXX HEALTHCARE SA | ||
| By: | /s/ Xxxxxxxx Xxxxxxxx | |
| Name: | Xxxxxxxx Xxxxxxxx | |
| Title: | Vice President - Supply Chain | |
| By: | /s/ Pier Pollini | |
| Name: | Pier Pollini | |
| Title: | Senior Director - Supply Chain | |
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.26
Chemical Structure of Pacritinib Citrate
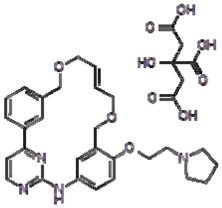
Chemical name: 11-(2-Pyrrolidin-1-yl-ethoxy)-14,19-dioxa-5,7,26-triaza-tetracyclo[19.3.1.1(2,6).1(8,12)] heptacosa-1(25),2(26),3,5,8,10,12(27),16,21,23-decaene citrate
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.34
Co-Promotion Terms
****
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.46
CTI Patents
| “OXYGEN LINKED PYRIMIDINE DERIVATIVES” | ||||
| Jurisdiction |
Application No. |
Patent No. (if any) | ||
| Australia | 0000000000 | 0000000000 | ||
| Brazil | PI0618552-5 | |||
| Canada | 2,629,443 | |||
| China | 200680050676.5 | ZL200680050676.5 | ||
| Europe | 06813132.5 | |||
| Hong Kong | 09100412.1 | |||
| India | 0000/XXXXX/0000 | |||
| Xxxxxxxxx | WO200801506 | |||
| Japan | 2008-541128 | 5225856 | ||
| Xxxxxxxx | XX 00000000 | |||
| Xxxxxx | MX/a/2008/006432 | MX298029 | ||
| New Zealand | 568325 | 568325 | ||
| PCT | XXX/XX0000/000000 | |||
| Xxxxxxxxxxx | 0-0000-000000 | |||
| Philippines | 0-0000-000000 | |||
| Philippines | 0-0000-000000 | |||
| Singapore | 200803992-7 | 142892 | ||
| Xxxxx Xxxxxx | 0000/00000 | 0000/00000 | ||
| Xxxxx Xxxxx | 00-0000-0000000 | |||
| Taiwan | 095142296 | |||
| Thailand | 0601005660 | |||
| USA | 12/093,867 | 8,153,632 | ||
| USA | 13/438,989 | 8,415,338 | ||
| USA | 13/771,546 | |||
| Vietnam | 0-0000-00000 | |||
|
“11-(2-PYRROLIDIN-1-YL-ETHOXY)-14,19-DIOXA-5,7,26-TRIAZA-TETRACYCLO[19.3.1.1(2,6).1(8,12)]HEPTACOSA-1(25),2(26), | ||||
| Jurisdiction |
Application No. |
Patent No. (if any) | ||
| Argentina | X000000000 | |||
| Australia | 2009325147 | |||
| Brazil | PI 0922736-9 | |||
| Canada | 2,746,058 | |||
| China | 200980150325.5 | |||
| Europe | 09788621.2 | |||
| GCC | 14895 | |||
| Hong Kong | 12102375.7 | |||
| India | 2306/KOLNP/2011 | |||
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
|
“11-(2-PYRROLIDIN-1-YL-ETHOXY)-14,19-DIOXA-5,7,26-TRIAZA-TETRACYCLO[19.3.1.1(2,6).1(8,12)]HEPTACOSA-1(25),2(26), | ||||
| Jurisdiction |
Application No. |
Patent No. (if any) | ||
| Indonesia | W00201102475 | |||
| Israel | 000000 | |||
| Xxxxx | 2011-540664 | |||
| Malaysia | PI 2011002647 | |||
| Mexico | MX/A/2011/006206 | |||
| Xxx Xxxxxxx | 000000 | |||
| XXX | XXX/XX0000/000000 | |||
| Xxxxxxxxxxx | 0-0000-000000 | |||
| Russia | 2011126173 | |||
| Singapore | 201104006-0 | 171907 | ||
| Xxxxx Xxxxxx | 0000/00000 | 0000/00000 | ||
| Xxxxx Xxxxx | 00-0000-0000000 | |||
| Taiwan | 098142605 | |||
| Thailand | 0901005538 | |||
| USA | 13/133,297 | |||
| Vietnam | 0-0000-00000 | |||
|
“11-(2-PYRROLIDIN-1-YL-ETHOXY)-14,19-DIOXA-5,7,26-TRIAZA-TETRACYCLO[19.3.1.1(2,6).1(8,12)]HEPTACOSA-1(25),2(26), | ||||
| Jurisdiction |
Application No. |
Patent No. (if any) | ||
| Argentina | P090104835 | |||
| Europe | 09793626.4 | |||
| GCC | 14896 | |||
| PCT | PCT/SG2009/000474 | |||
| Taiwan | 098142603 | |||
| Thailand | 0901005537 | |||
| USA | 13/133,288 | |||
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.47
CTI Trademarks
CTI
CELL THERAPEUTICS
CELL THERAPEUTICS, INC.

| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.54
Development Plan
****
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.54
Target Product Profile
****
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.54
Development Plan Xxxxx Chart and Budget
****
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.132
Form of Registration Rights Agreement
REGISTRATION RIGHTS AGREEMENT
This REGISTRATION RIGHTS AGREEMENT (this “Agreement”) is made as of November 14, 2013, by and among (i) Cell Therapeutics, Inc., a Washington corporation (the “Company”), (ii) Xxxxxx Healthcare SA, a company organized under the laws of Switzerland (the “Initial Holder”), and (iii) each person or entity that subsequently becomes a party to this Agreement pursuant to, and in accordance with, the provisions of Section 12 hereof (collectively, the “Holder Permitted Transferees,” and each individually, a “Holder Permitted Transferee”).
WHEREAS, pursuant to the terms and conditions set forth in that certain Development, Commercialization and License Agreement, dated as of November 14, 2013, between the Company and the Initial Holder (the “DCLA”), the Initial Holder has agreed to purchase from the Company, and the Company has agreed to issue and sell to Xxxxxx, shares of the Company’s Series 19 Preferred Stock and/or shares of the Company’s common stock, all upon the terms and conditions set forth in the DCLA.
WHEREAS, the terms of the DCLA provide for the Company and the Initial Holder to execute and deliver this Agreement.
NOW, THEREFORE, in consideration of the premises and mutual covenants contained herein, the parties hereto hereby agree as follows:
1. Definitions. The following terms shall have the meanings provided therefor below or elsewhere in this Agreement as described below:
“Agreement” has the meaning set forth in the Preamble.
“Board” means the board of directors of the Company.
“Business Day” means any day other than a Saturday, a Sunday or a day on which banks in New York are authorized or obligated by law or executive order to close.
“Company” has the meaning set forth in the Preamble.
“DCLA” has the meaning set forth in the Preamble.
“Effective Date” has the meaning set forth in the DCLA.
3
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
“Exchange Act” means the Securities Exchange Act of 1934, as amended, and all of the rules and regulations promulgated thereunder.
“Holder Indemnified Person” has the meaning set forth in Section 9.1.
“Holder Permitted Transferee” and “Holder Permitted Transferees” have the meanings set forth in the Preamble.
“Holders” means, collectively, the Initial Holder and the Holder Permitted Transferees; provided, however, that the term “Holders” shall not include the Initial Holder or any of the Holder Permitted Transferees if such Holder ceases to own or hold any Registrable Shares.
“Initial Holder” has the meaning set forth in the Preamble.
“Loss” has the meaning set forth in Section 9.1.
“Mandatory Registration Termination Date” has the meaning set forth in Section 3.2.
“Majority Holders” means, at the relevant time of reference thereto, those Holders holding more than fifty percent (50%) of the Registrable Shares held by all of the Holders.
“Qualifying Holder” has the meaning set forth in Section 12.
“Registrable Shares” means (i) any shares of common stock of the Company issued to the Initial Holder, (ii) any shares of common stock of the Company issuable upon conversion of any shares of Series 19 Preferred Stock issued to the Initial Holder or (iii) any common stock issued as (or issuable upon the conversion or exercise of any warrant, right, or other security that is issued as) a dividend or other distribution with respect to, or in exchange for or in replacement of, such common stock or Series 19 Preferred Stock. For the avoidance of doubt, a security shall cease to be a Registrable Share once it has been sold in an SEC-registered transaction or pursuant to Rule 144.
“Registration Statement” has the meaning set forth in Section 3.1.
“Rule 144” means Rule 144 promulgated under the Securities Act and any successor or substitute rule, law or provision.
“SEC” means the U.S. Securities and Exchange Commission.
“Securities Act” means the Securities Act of 1933, as amended, and all of the rules and regulations promulgated thereunder.
“Suspension Period” has the meaning set forth in Section 11.
2. Effectiveness. This Agreement shall become effective and legally binding on the Effective Date.
4
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
3. Mandatory Registration.
3.1. Within thirty (30) calendar days after the Effective Date, the Company will prepare and file with the SEC a registration statement on Form S-3, or any other available form if the Company is not eligible to use Form S-3, for the purpose of registering under the Securities Act all of the Registrable Shares for resale by, and for the account of, the Holders as selling stockholders thereunder (the “Registration Statement”). The Registration Statement shall permit the Holders to offer and sell, on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, any or all of the Registrable Shares. The Company agrees to use commercially reasonable efforts to cause the Registration Statement to become effective as soon as practicable after the filing thereof, and in no event later than the earlier of (i) ninety (90) calendar days following the Effective Date (subject to reasonable extension to the extent necessary to accommodate a delay resulting from unresolved SEC comments or the need to file financial statements within the time periods prescribed by the SEC) and (ii) the fifth calendar day following the date on which the Company is notified by the SEC that (a) such Registration Statement will not be reviewed or is no longer subject to further review and comments and that (b) the SEC is willing to declare the Registration Statement effective. The Registration Statement filed pursuant to this Section 3.1 (and each amendment or supplement thereto, and each request for acceleration of effectiveness thereof) shall be provided to each Holder and its counsel prior to its filing or other submission.
3.2. The Company shall be required to keep the Registration Statement effective until such date that is the earlier to occur of (i) the date as of which all of the Holders may sell all of the Registrable Shares to the public without restriction pursuant to Rule 144 (or the successor rule thereto) promulgated under the Securities Act, and (ii) the date when all of the Registrable Shares registered thereunder shall have been sold pursuant to the Registration Statement or Rule 144 (such date is referred to herein as the “Mandatory Registration Termination Date”). Thereafter, the Company shall be entitled to withdraw the Registration Statement and the Holders shall have no further right to offer or sell any of the Registrable Shares pursuant to the Registration Statement (or any prospectus relating thereto). The Company shall not be required to register the offer and sale of the Registrable Shares pursuant to the Registration Statement in an underwritten offering.
3.3. The Company shall not, and shall not agree to (i) allow the holders of any securities of the Company, other than holders of the Registrable Shares, to include any of their securities in the Registration Statement under Section 3.1 hereof or any amendment or supplement thereto without the consent of the Holders or (ii) offer any securities for its own account or the account of others in the Registration Statement under Section 3.1 hereof or any amendment or supplement thereto without the consent of the Holders, in each such case, subject to, and other than with respect to, any registration obligations of the Company under any agreement entered into prior to the Effective Date; provided, however, that the Company at all times reserves the right to provide registration rights, pursuant to a separate registration statement, to the holders of any securities of the Company.
5
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
4. Notification of Effectiveness. Company shall notify the Holders by facsimile or e-mail (as provided by Holders) as promptly as practicable, and in any event, within twenty-four (24) hours, after the Registration Statement is declared effective and shall simultaneously provide the Holders with copies of any related prospectus to be used in connection with the sale or other disposition of the securities covered thereby.
5. Obligations of the Company. In connection with the Company’s obligation under Sections 3 and 4 hereof to file the Registration Statement with the SEC and to use commercially reasonable efforts to cause the Registration Statement to become effective as soon as practicable, the Company shall, as expeditiously as reasonably possible:
5.1. Prepare and file with the SEC such amendments and supplements to the Registration Statement and the prospectus used in connection therewith as may be necessary to comply with the provisions of the Securities Act with respect to the disposition of all Registrable Shares covered by the Registration Statement, and furnish to each Holder and the single firm of counsel designated by the Holders to the extent requested by such Holder or counsel, without charge, at least one conformed copy of each Registration Statement and each amendment thereto, including financial statements and schedules, all documents incorporated or deemed to be incorporated therein by reference, and all exhibits (including those previously furnished or incorporated by reference) promptly after the filing of such documents with the SEC;
5.2. Furnish to the Holders, without charge, such number of copies of a prospectus, including a preliminary prospectus, in conformity with the requirements of the Securities Act, and such other documents (including, without limitation, prospectus amendments and supplements as are prepared by the Company in accordance with Section 5.1 above) as the Holders may reasonably request in order to facilitate the disposition of such Holders’ Registrable Shares. The Company hereby consents to the use of such prospectus and each amendment or supplement thereto by each of the selling Holders in connection with the offering and sale of the Registrable Shares covered by such prospectus and any amendment or supplement thereto.
5.3. Notify the Holders as promptly as reasonably possible, at any time when a prospectus relating to the Registration Statement is required to be delivered under the Securities Act, of the happening of any event as a result of which the prospectus included in or relating to the Registration Statement contains an untrue statement of a material fact or omits any fact necessary to make the statements therein not misleading; and, thereafter, the Company will promptly prepare (and, when completed, give notice to each Holder) a supplement or amendment to such prospectus so that, as thereafter delivered to the purchasers of such Registrable Shares, such prospectus will not contain an untrue statement of a material fact or omit to state any fact necessary to make the statements therein not misleading; upon such notification by the Company, the Holders will not offer or sell Registrable Shares until the Company has notified the Holders that it has prepared a supplement or amendment to such prospectus and delivered copies of such supplement or amendment to the Holders (it being understood and agreed by the Company that the foregoing clause shall in no way diminish or otherwise impair the Company’s obligation to promptly prepare a prospectus amendment or supplement as above provided in this Section 5.3 and deliver copies of same as above provided in Section 5.2 hereof);
6
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
5.4. Promptly respond to any and all comments received from the SEC, with a view towards causing the Registration Statement or any amendment thereto to be declared effective by the SEC as soon as practicable, and file an acceleration request as soon as practicable, but no later than five (5) Business Days, following the resolution or clearance of all SEC comments or, if applicable, notification by the SEC that any such Registration Statement or any amendment thereto will not be subject to review;
5.5. Use commercially reasonable efforts to register and qualify the Registrable Shares covered by the Registration Statement under such other securities or Blue Sky laws of such states where such registration and/or qualification is required as shall be reasonably requested by a Holder, provided that the Company shall not be required in connection therewith or as a condition thereto to qualify to do business or to file a general consent to service of process in any such states or jurisdictions, and provided further that (notwithstanding anything in this Agreement to the contrary with respect to the bearing of expenses) if any jurisdiction in which any of such Registrable Shares shall be qualified shall require that expenses incurred in connection with the qualification therein of any such Registrable Shares be borne by the Holders, then the Holders shall, to the extent required by such jurisdiction, pay their pro rata share of such qualification expenses;
5.6. Subject to the terms and conditions of this Agreement, use commercially reasonable efforts to (i) prevent the issuance of any stop order or other suspension of effectiveness of a Registration Statement, or the suspension of the qualification of any of the Registrable Shares for sale in any jurisdiction in the United States, and (ii) if such an order or suspension is issued, obtain the withdrawal of such order or suspension at the earliest practicable moment and notify each holder of Registrable Shares of the issuance of such order and the resolution thereof or its receipt of notice of the initiation or threat of any proceeding such purpose;
5.7. Permit a single firm of counsel designated by the Holders to review the Registration Statement and all amendments and supplements thereto (as well as all requests for acceleration or effectiveness thereof), at Holders’ own cost, a reasonable period of time prior to their filing with the SEC (not less than five (5) Business Days) and use commercially reasonable efforts to reflect in such documents any comments as such counsel may reasonably propose (so long as such comments are provided to the Company at least (2) Business Days prior to the expected filing date) and will not request acceleration of such Registration Statement without prior notice to such counsel, provided, however that the Company shall not file the Registration Statement or any amendments or supplements thereto to which the Majority Holders and the counsel designated by the Holders shall reasonably object in good faith;
5.8. Cooperate with the Holders to facilitate the timely preparation and delivery of certificates representing Registrable Shares to be delivered to a transferee pursuant to the Registration Statement, which certificates shall be free, to the extent permitted by law, of all restrictive legends, and to enable such Registrable Shares to be in such denominations and registered in such names as any such Holders may request;
7
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
5.9. Comply with all applicable rules and regulations of the SEC;
5.10. Use commercially reasonable efforts to cause all the Registrable Shares covered by the Registration Statement to be listed on the NASDAQ National Market, or such other securities exchange on which the Company’s common stock is then listed; and
5.11. Comply with all requirements of the Financial Industry Regulatory Authority, Inc. with regard to the issuance of the Registrable Shares and the listing thereof on the NASDAQ National Market, and engage a transfer agent and registrar to maintain the Company’s stock ledger for all Registrable Shares covered by the Registration Statement not later than the effective date of the Registration Statement.
6. Furnish Information. It shall be a condition precedent to the obligations of the Company to take any action pursuant to this Agreement that the Holders shall furnish to the Company such information regarding them and the securities held by them as the Company shall reasonably request and as shall be required in order to effect any registration by the Company pursuant to this Agreement. Each Holder shall promptly notify the Company of any changes in the information furnished to the Company.
7. Expenses of Registration. All fees and expenses incurred by the Company in connection with the registration of the Registrable Shares pursuant to this Agreement, including, without limitation, all registration and qualification and filing fees, printing (including, without limitation, expenses of printing certificates for Registrable Shares and of printing prospectuses if the printing of prospectuses is reasonably requested by the holders of a majority of the Registrable Shares included in the Registration Statement), fees and disbursements of counsel for, or other persons retained by, the Company, and fees and expenses incident to the performance of or compliance with this Agreement by the Company, shall be borne by the Company. In addition, the Company shall be responsible for all of its internal expenses incurred in connection with the consummation of the transactions contemplated by this Agreement (including, without limitation, all salaries and expenses of its officers and employees performing legal or accounting duties), the expense of any annual audit and the fees and expenses incurred in connection with the listing of the Registrable Shares on any securities exchange as required hereunder. Any expenses incurred by a Holder, including, without limitation, fees and disbursements of counsel for such Holder or any brokerage and other selling commissions and discounts shall be borne by such Holder.
8. Delay of Registration. The Holders shall not take any action with the primary intent to restrain, enjoin or otherwise delay any registration as the result of any controversy which might arise with respect to the interpretation or implementation of this Agreement. In the event such a delay occurs because of such action, the dates by which the Registration Statement is required to be filed and become effective pursuant to this Agreement shall be extended by the same number of days of such delay.
8
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
9. Indemnification.
9.1. The Company will, notwithstanding any termination of this Agreement, indemnify and hold harmless each Holder, the officers, directors, agents and employees of each of them, each person who controls each Holder within the meaning of the Securities Act or the Exchange Act, if any, and the officers, directors, agents and employees of each such controlling person (in each case, a “Holder Indemnified Person”), to the fullest extent permitted by applicable law, from and against any loss, claim, damage, cost (including, without limitation, reasonable costs of preparation and reasonable attorneys’ fees), expenses, or liability (“Loss”), to which such Holder Indemnified Person may become subject under the Securities Act or otherwise, insofar as such Loss arises out of or is based upon (i) any untrue or alleged untrue statement of any material fact contained in the Registration Statement, in any preliminary prospectus or final prospectus relating thereto or in any amendments or supplements to the Registration Statement or any such preliminary prospectus or final prospectus, or (ii) the omission or alleged omission to state therein a material fact required to be stated therein, or necessary to make the statements therein not misleading; provided, however, that the indemnity agreement contained in this Section 9.1 shall not apply to amounts paid in settlement of any such Loss if such settlement is effected without the consent of the Company (such consent not to be unreasonably withheld, conditioned or delayed), nor shall the Company be liable in any such case for any such Loss to the extent, but only to the extent, that it arises solely out of or is based solely upon (1) an untrue statement or alleged untrue statement or omission or alleged omission made in connection with the Registration Statement, any preliminary prospectus or final prospectus relating thereto or any amendments or supplements to the Registration Statement or any such preliminary prospectus or final prospectus, based upon and in conformity with written information furnished expressly for use in connection with the Registration Statement or any such preliminary prospectus or final prospectus by a Holder, any underwriter for such Holder or controlling person with respect to such Holder, or (2) any breach by any Holder of this Agreement or the failure of such Holder to comply with the covenants and agreements contained in this Agreement respecting sales of the Registrable Shares.
9.2. Each Holder will severally and not jointly indemnify and hold harmless the Company, each of its directors, each of its officers who have signed the Registration Statement, each person, if any, who controls the Company within the meaning of the Securities Act, and all other Holders against any Loss to which the Company or any such director, officer, controlling person, or such other Holder may become subject to, under the Securities Act or otherwise, insofar as such Loss arises solely out of or is based solely upon any untrue or alleged untrue statement of any material fact contained in the Registration Statement or any preliminary prospectus or final prospectus, relating thereto or in any amendments or supplements to the Registration Statement or any such preliminary prospectus or final prospectus, or arises out of or is based upon the omission or alleged omission to state therein a material fact required to be stated therein or necessary to make the statements therein not misleading, in each case to the extent and only to the extent that such untrue statement or alleged untrue statement or omission or alleged omission was made in the Registration Statement, in any preliminary prospectus or final prospectus relating thereto or in any amendments or supplements to the Registration
9
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Statement or any such preliminary prospectus or final prospectus, in reliance upon and in conformity with written information furnished by the Holder expressly for use in connection with the Registration Statement, or any preliminary prospectus or final prospectus; and provided, further, however, that the indemnity agreement contained in this Section 9.2 shall not apply to amounts paid in settlement of any such Loss if such settlement is effected without the consent of those Holder(s) against which the request for indemnity is being made. In no event shall the liability of any selling Holder hereunder be greater in amount than the dollar amount of the net proceeds received by such Holder upon the sale of the Registrable Shares giving rise to such indemnification obligation.
9.3. Promptly after receipt by an indemnified party under this Section 9 of notice of the commencement of any action, such indemnified party will, if a claim in respect thereof is to be made against any indemnifying party under this Section 9, notify the indemnifying party in writing of the commencement thereof and the indemnifying party shall have the right to participate in and, to the extent the indemnifying party desires, jointly with any other indemnifying party similarly noticed, to assume at its expense the defense thereof with counsel mutually satisfactory to the indemnifying parties and the indemnified parties. In the event that the indemnifying party assumes any such defense, the indemnified party may participate in such defense with its own counsel and at its own expense, provided, however, that the counsel for the indemnifying party shall act as lead counsel in all matters pertaining to such defense or settlement of such claim. The failure to notify an indemnifying party promptly of the commencement of any such action shall not relieve such indemnifying party of any liability to the indemnified party under this Section 9, except (and only) to the extent the indemnifying party is actually and materially adversely prejudiced in its ability to defend such action.
9.4. If the indemnification provided for in this Section 9 is held by a court of competent jurisdiction to be unavailable to an indemnified party with respect to any Loss referred to therein, then the indemnifying party, in lieu of indemnifying such indemnified party hereunder, shall, to the extent permitted by applicable law, contribute to the amount paid or payable by such indemnified party as a result of such Loss in such proportion as is appropriate to reflect the relative fault of the indemnifying party on the one hand and of the indemnified party on the other in connection with the statements or omissions that resulted in such Loss as well as any other relevant equitable considerations. The relative fault of the indemnifying party and of the indemnified party shall be determined by reference to, among other things, whether the untrue or alleged untrue statement of a material fact or the omission to state a material fact relates to information supplied by the indemnifying party or by the indemnified party and the parties’ relative intent, knowledge, access to information, and opportunity to correct or prevent such statement or omission. The indemnity and contribution agreements contained in this Agreement are in addition to any liability that the indemnifying parties may have to the indemnified parties.
10. Reports Under The Exchange Act. With a view to making available to the Holders the benefits of Rule 144 and any other rule or regulation of the SEC that may at any time permit the Holders to sell the Registrable Shares to the public without registration until the Mandatory Registration Termination Date, the Company agrees to use commercially reasonable efforts: (i) to make and keep public information available as those terms are understood in Rule 144, (ii) to file with the SEC in a timely manner all reports and other documents required to be filed by an issuer of securities registered under the Securities Act or the Exchange Act, (iii) as long as any Holder owns any Registrable Shares, to furnish in writing upon such Holder’s request a written statement by the Company that it has complied with the reporting requirements of Rule 144 and of the Securities Act and the Exchange Act, and (iv) undertake any additional actions reasonably necessary to maintain the availability of the Registration Statement or the use of Rule 144.
10
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
11. Suspension. Notwithstanding anything in this Agreement to the contrary, if the Company shall furnish to the Holders a certificate signed by the President or Chief Executive Officer of the Company stating that the Board has made the good faith determination (i) that continued use by the Holders of the Registration Statement for purposes of effecting offers or sales of Registrable Shares pursuant thereto would require, under the Securities Act, premature disclosure in the Registration Statement (or the prospectus relating thereto) of material, nonpublic information concerning the Company, its business or prospects or any proposed material transaction involving the Company, (ii) that such premature disclosure would be materially adverse to the Company, its business or prospects or any such proposed material transaction or would make the successful consummation by the Company of any such material transaction significantly less likely and (iii) that it is therefore essential to suspend the use by the Holders of such Registration Statement (and the prospectus relating thereto) for purposes of effecting offers or sales of Registrable Shares pursuant thereto, then the right of the Holders to use the Registration Statement (and the prospectus relating thereto) for purposes of effecting offers or sales of Registrable Shares pursuant thereto shall be suspended for a period (the “Suspension Period”) of not more than forty-five (45) days after delivery by the Company of the certificate referred to above in this Section 11; provided that the Company shall be entitled to no more than two (2) such Suspension Periods during any twelve (12) month period. During the Suspension Period, none of the Holders shall offer or sell any Registrable Shares pursuant to or in reliance upon the Registration Statement (or the prospectus relating thereto). The Company shall use commercially reasonable efforts to terminate any Suspension Period as promptly as practicable.
12. Transfer of Registration Rights. None of the rights of any Holder under this Agreement shall be transferred or assigned to any person unless (i) such person is a Qualifying Holder (as defined below), and (ii) such person agrees to become a party to, and bound by, all of the terms and conditions of, this Agreement by duly executing and delivering to the Company an Instrument of Adherence in the form attached as Exhibit A hereto. For purposes of this Section 12, the term “Qualifying Holder” shall mean, with respect to any Holder, (a) any corporation, partnership controlling, controlled by, or under common control with, such Holder or any partner thereof, or (b) any other direct transferee from such Holder of at least 25% of those Registrable Shares held by such Holder. None of the rights of any Holder under this Agreement shall be transferred or assigned to any Person (including, without limitation, a Qualifying Holder) that acquires Registrable Shares in the event that and to the extent that such Person is eligible to immediately resell such Registrable Shares pursuant to Rule 144 of the Securities Act or any
11
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
other exemption from the registration provisions of the Securities Act. After any transfer in accordance with this Section 12, the rights and obligations of a Holder as to any transferred Registrable Shares shall be the rights and obligations of the Holder Permitted Transferee holding such Registrable Shares.
13. Confidentiality of Records. Each Holder agrees not to disclose any material non-public information provided by the Company in connection with a registration (including, without limitation, the contemplated filing and timing of filing of a Registration Statement).
14. Entire Agreement. This Agreement constitutes and contains the entire agreement and understanding of the parties with respect to the subject matter hereof, and it also supersedes any and all prior negotiations, correspondence, agreements or understandings with respect to the subject matter hereof.
15. Miscellaneous.
15.1. This Agreement may not be amended, modified or terminated, and no rights or provisions may be waived, except with the written consent of the Majority Holders and the Company.
15.2. This Agreement shall be governed by and construed and enforced in accordance with the laws of the State of New York, and shall be binding upon and inure to the benefit of the parties hereto and their respective heirs, personal representatives, successors or assigns, provided that the terms and conditions of Section 12 hereof are satisfied.
15.3. This Agreement shall be binding upon and inure to the benefit of any transferee of any of the Registrable Shares provided that the terms and conditions of Section 12 hereof are satisfied. Notwithstanding anything in this Agreement to the contrary, if at any time any Holder shall cease to own any Registrable Shares, all of such Holder’s rights under this Agreement shall immediately terminate.
15.4. All notices and communications hereunder shall be deemed to have been duly given and made if in writing and if served by personal delivery upon the party for whom it is intended or delivered by registered or certified mail, return receipt requested, or if sent by facsimile or email, provided that the facsimile or email is promptly confirmed by telephone confirmation thereof, to the person at the address set forth below, or such other address as may be designated in writing hereafter, in the same manner, by such person:
If to the Company:
Cell Therapeutics, Inc.
0000 Xxxxxxx Xxxxxx, Xxxxx 000
Xxxxxxx, Xxxxxxxxxx 00000
Telephone: (000) 000-0000
Facsimile: (000) 000-0000
Email:
Attention: Xxxxx X. Xxxxxx, M.D., Chief Executive Officer
12
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
with copies to:
O’Melveny & Xxxxx LLP
Xxx Xxxxxxxxxxx Xxxxxx
Xxx Xxxxxxxxx, XX 00000-0000
Telephone: (000) 000-0000
Facsimile: (000) 000-0000
Email:
Attention: X. Xxxxxx Xxxxxxxxxxx, Esq. and Xxxx Xxxxxxx, Esq.
and
CTI Legal Affairs
Attention: Xxxx Xxxxxxx, Director, Legal Corporate Development & Securities
If to the Initial Holder:
Xxxxxx Healthcare SA
Xxxxxxxx
0000 Xxxxxx, Xxxxxxxxxxx
Telephone: x00 00 000 00 00
Facsimile: x00 00 000 00 00
Attention: Legal Department
with a copy to counsel, provided that such copy shall not constitute legal notice to the Initial Holder:
Xxxxxx Healthcare Corporation
Xxx Xxxxxx Xxxxxxx
Xxxxxxxxx, Xxxxxxxx 00000
Telephone: (000) 000-0000
Facsimile: (000) 000-0000
Email:
Attention: General Counsel
15.5. Any person may change the address to which correspondence to it is to be addressed by notification as provided for herein.
15.6. The parties acknowledge and agree that in the event of any breach of this Agreement, remedies at law may be inadequate, and each of the parties hereto shall be entitled to seek specific performance of the obligations of the other parties hereto and such appropriate injunctive relief as may be granted by a court of competent jurisdiction, without the necessity of showing economic loss and without any bond or other security being required.
13
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
15.7. This Agreement may be executed in a number of counterparts, including by electronic transmission, any of which together shall for all purposes constitute one Agreement, binding on all the parties hereto notwithstanding that all such parties have not signed the same counterpart.
[REMAINDER OF PAGE INTENTIONALLY LEFT BLANK]
14
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
IN WITNESS WHEREOF, each Holder and the Company have caused their respective signature pages to this Registration Rights Agreement to be duly executed as of the day and year first above written.
| CELL THERAPEUTICS, INC. |
|
|
| Name: |
| Title: |
[SIGNATURE PAGE TO REGISTRATION RIGHTS AGREEMENT]
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
IN WITNESS WHEREOF, each Holder and the Company have caused their respective signature pages to this Registration Rights Agreement to be duly executed as of the day and year first above written.
| XXXXXX HEALTHCARE SA |
|
|
| Name: |
| Title: |
[SIGNATURE PAGE TO REGISTRATION RIGHTS AGREEMENT]
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
EXHIBIT A
Instrument of Adherence
Reference is hereby made to that certain Registration Rights Agreement, dated as of November 14, 2013, among Cell Therapeutics, Inc., a Washington corporation (the “Company”), the Initial Holder and the Holder Permitted Transferees, as amended and in effect from time to time (the “Registration Rights Agreement”). Capitalized terms used herein without definition shall have the respective meanings ascribed thereto in the Registration Rights Agreement.
The undersigned, in order to become the owner or holder of [ ] shares of Series 19 Preferred Stock (the “Preferred Stock”) or shares of common stock of the Company issuable upon conversion of the Preferred Stock (collectively, “Subject Securities”), of the Company, hereby agrees that, from and after the date hereof, the undersigned has become a party to the Registration Rights Agreement in the capacity of a Holder Permitted Transferee, and is entitled to all of the benefits under, and is subject to all of the obligations, restrictions and limitations set forth in, the Registration Rights Agreement that are applicable to Holder Permitted Transferees. The notice information for purposes of the Registration Rights Agreement is provided below. This Instrument of Adherence shall take effect and shall become a part of the Registration Rights Agreement immediately upon execution.
Executed as of the date set forth below under the laws of New York.
| Signature: |
| |
| Name: | ||
| Title: | ||
| Telephone: | ||
| Facsimile: | ||
| Email: | ||
| Attention: |
| Accepted: | ||
| CELL THERAPEUTICS, INC. | ||
| By: |
| |
| Name: | ||
| Title: | ||
Date: , 2013
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 1.160
Upstream Agreement
| 1. | The Asset Purchase Agreement, dated May 31, 2012, between S*BIO Pte Ltd. and CTI, as amended from time to time. |
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 8.2
Manufacturing and Supply Agreement Terms
The following is a summary of certain material terms relating to the manufacture and supply of Licensed Product from CTI to Baxter that will be contained in a definitive Manufacturing and Supply Agreement (the “Supply Agreement”). An initial draft of the Supply Agreement was prepared by Baxter and commented upon by CTI to reflect the Parties initial positions on certain manufacturing and supply related matters (the “Draft Supply Agreement”). The length of time of the negotiation with respect to the Supply Agreement and the efforts and manner in which the Parties shall negotiate are as set forth in the Development, Commercialization and License Agreement (“DCLA”) to which this Exhibit is attached. All terms not defined herein shall have the respective meanings attached to such terms in the DCLA. The following material terms of the Supply Agreement shall be binding on the Parties and shall not be subject to modification unless mutually agreed by the Parties.
| Supply Agreement Term |
Details | |
| Term | Supply Agreement will be coterminous with the DCLA; provided, however that upon expiration (but not termination) of the DCLA the Parties shall discuss in good faith an extension of the Supply Agreement and/or a technology transfer to ensure that Baxter (and CTI as applicable) will continue to have a supply of Licensed Product and/or Drug Product (as applicable). | |
| Fill/Finish Option | The Parties acknowledge that the DCLA provides that Baxter shall have an option to perform, by itself or through one or more CMOs, the packaging, finishing and labeling activities to produce Licensed Product from Drug Product, and that upon exercise of this Fill/Finish Option the Supply Agreement will be appropriately modified and that the Parties will enter into a second supply agreement which shall, to the extent practicable and in accordance with industry custom and the circumstances, ensure that the Parties will be is comparable positions with respect to such second supply agreement as with respect to the Supply Agreement. In the event that Baxter exercises the Fill/Finish Option and supplies finished Licensed Product to CTI, it shall be at Xxxxxx’x COGS therefor. | |
| Supply Price | The purchase price for the Licensed Product under the Supply Agreement will be ** through manufacture of the Licensed Product in Drug Product form, subject to the Supply Cap. For purposes of clarification, fill & finish (such as blistering and package inserts), freight, insurance and other subsequent supply chain expenses are not subject to the Supply Cap but shall **. | |
| Supply Cap | Notwithstanding ** for Licensed Product referenced above, ** of Licensed Product (the “Supply Cap Prices”) shall apply to all Pacritinib Citrate ** delivered to Baxter under the Supply Agreement. The ** shall be increased at the beginning of each subsequent Calendar Year over the prior year amount by ** | |
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
| Volume |
2014 Supply Cap Price Per Capsule |
|||
| ** |
** | |||
| ** |
** | |||
| ** |
** | |||
| ** |
** | |||
| For the avoidance of doubt, the above prices are not incremental. That is, if Baxter purchases ** capsules in a calendar year, the maximum supply price (excluding packaging) is ** (subject to the annual adjustment noted above).
As noted above, ** will be charged for each unit of Licensed Product delivered under the Supply Agreement (subject to the Supply Cap). However the Supply Agreement will also contain a “true-up” mechanism to ensure that, based upon the above Supply Cap, upon the completion of the applicable calendar year, any payment overages for the purchase of Licensed Product are refunded to Baxter or any payment deficits are paid to CTI, in either case, within 60 days following the end of the applicable calendar year.
The Parties agree that the foregoing Supply Cap Prices are based upon the assumption of one (1) Drug Substance manufacturing run per Calendar Year, produced by a single Drug Substance manufacturer. Should the Parties agree to greater than one Drug Substance manufacturing run per Calendar Year, then the JSC shall adjust the Supply Cap Prices to reflect any expected incremental expense. The Supply Cap Prices only apply to Drug Product by a single primary supplier. Should Drug Product be produced under any Back-Up Manufacturing provisions, then the Supply Prices Cap shall not apply to any such Drug Product. | ||
| Binding Orders | The timeframe for forecasting and any binding order provisions in the Supply Agreement shall be aligned with the contractual provisions with any CMOs engaged to perform such work, as well as any provisions for Safety Stock, for which Baxter will have approval authority according to the terms of the DCLA. | |
| Supply Failure | The Supply Agreement will include appropriate technology transfer provisions (including provision of drug master files and other customary terms in the industry) sufficient to enable Baxter to manufacture (or have manufactured) the Licensed Product in the event that there is a Supply Failure (as defined in the Draft Supply Agreement). ** | |
| Back-up Manufacturing | The Supply Agreement will include a covenant from CTI requiring CTI, within an agreed upon period of time (as determined by the JMC but no | |
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
| later than 30 months following first commercial sale), to have engaged and qualified a CMO to provide back-up manufacturing and supply for the Licensed Product (a “Secondary Supply Source”). Expenses with any scale-up, tech transfer and validation of Back-up Manufacturing are Development expenses. Expenses associated with producing any Licensed Product under Back-up Manufacturing shall be provided at actual COGS but not subject to the Supply Cap. With notice suitable for any contemplated contracts, Baxter may waive or extent the timeframe for this provision for Back-up Manufacturing. | ||
| Safety Stock | Until such time as CTI has engaged and qualified a Secondary Supply Source, CTI will agree to maintain, a safety stock of Licensed Product as forecast and ordered by Baxter sufficient to supply Baxter with its requirements of Licensed Product for the Territory for a period of not less than six (6) months. The cost for the safety stock will be shared by the Parties such that Baxter is responsible for ** of the cost and CTI shall be responsible for ** of the cost. For the avoidance of doubt, as such safety stock of Licensed Product is depleted, Baxter shall be responsible under the Supply Agreement only for the remaining ** that was previously borne by CTI, until aforementioned 6-month requirement is fulfilled. | |
| Representations/ Warranties/ Indemnification |
The Supply Agreement will contain customary representations and warranties relating to the manufacture and supply of the Licensed Product including each Party’s obligation to comply with all applicable Laws. Further, the Supply Agreement will include appropriate indemnification language (a draft of which is set forth in the Draft Supply Agreement). | |
| Recalls | Section 7.4 of the Agreement shall govern the Parties’ obligations with respect to Recalls, Withdrawals and/or Field Corrections. | |
| Confidentiality | Article XIII of the Agreement shall govern the Parties’ obligations with respect to confidentiality. | |
| Dispute Resolution | Section 16.13 of the Agreement shall govern the Parties’ rights and obligations with respect to the resolution of any disputes under the Supply Agreement. | |
| CTI CMO Agreement(s) | Baxter has the right to approve in writing any supply agreement CTI enters into with CTI’s CMOs before execution thereof. | |
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 9.1.2
Preferred Stock Terms
ARTICLES OF AMENDMENT TO
AMENDED AND RESTATED ARTICLES OF
CELL THERAPEUTICS, INC.
DESIGNATION OF PREFERENCES,
RIGHTS AND LIMITATIONS
OF
SERIES 19 PREFERRED STOCK
Pursuant to the Washington Business Corporation Act, Chapter 23B.10, the undersigned officer of Cell Therapeutics, Inc., a Washington corporation (the “Corporation”), does hereby submit for filing these Articles of Amendment:
FIRST: The name of the Corporation is Cell Therapeutics, Inc.
SECOND: This amendment to the Corporation’s Amended and Restated Articles of Incorporation, as amended to date (the “Restated Articles”), was adopted by the Board of Directors of the Corporation (the “Board”) on November 9, 2013 as set forth in resolutions adopted by the Board on November 9, 2013. Shareholder action was not required on this amendment pursuant to Article II.2 of the Restated Articles.
THIRD: A new Section 2(z) of Article II is added to the Restated Articles to add the designations, rights and preferences of a new series of preferred stock as follows, such Section to be effective as of November 15, 2013:
“(z) Series 19 Preferred Stock
TERMS OF PREFERRED STOCK
Section 1. Definitions. For the purposes hereof, the following terms shall have the following meanings:
“Affiliate” means any person or entity controlling, controlled by or under common control with a Holder.
“Alternate Consideration” has the meaning set forth in Section 7(d).
“Business Day” means any day except Saturday, Sunday, any day which shall be a federal legal holiday in the United States or any day on which banking institutions in the State of New York are authorized or required by law or other governmental action to close.
“Change of Control Transaction” means the occurrence after the date hereof of any of (i) an acquisition by an individual, legal entity or “group” (as described in Rule 13d-5(b)(1) promulgated under
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
the Exchange Act) of effective control (whether through legal or beneficial ownership of capital stock of the Corporation, by contract or otherwise) of in excess of 33% of the voting securities of the Corporation (other than by means of conversion of shares of Series 19 Preferred Stock), or (ii) the Corporation merges into or consolidates with any other person, or any person merges into or consolidates with the Corporation and, after giving effect to such transaction, the shareholders of the Corporation immediately before such transaction own less than 66% of the aggregate voting power of the Corporation or the successor entity of such transaction, or (iii) the Corporation sells or transfers all or substantially all of its assets to another person and the shareholders of the Corporation immediately before such transaction own less than 66% of the aggregate voting power of the acquiring entity immediately after the transaction, or (iv) a replacement at one time or within a one-year period of more than one-half of the members of the Board which is not approved by a majority of those individuals who are members of the Board on the date hereof (or by those individuals who are serving as members of the Board on any date whose nomination to the Board was approved by a majority of the members of the Board who are members on the date hereof), or (v) the execution by the Corporation of an agreement to which the Corporation is a party or by which it is bound, providing for any of the events set forth in clauses (i) through (iv) herein.
“Common Stock” means the Corporation’s common stock, no par value per share, and stock of any other class of securities into which such securities may hereafter be reclassified or changed into.
“Common Stock Equivalents” means any securities which would entitle the holder thereof to acquire at any time Common Stock, including, without limitation, any preferred stock, rights, options, warrants or other instrument that is at any time convertible into or exercisable or exchangeable for, or otherwise entitles the holder thereof to receive, Common Stock; provided, however, that Common Stock Equivalents shall not include any debt securities of the Corporation.
“Conversion Amount” means the sum of the Stated Value at issue.
“Conversion Date” has the meaning set forth in Section 6(a).
“Conversion Price” has the meaning set forth in Section 6(c).
“Conversion Shares” means, collectively, the shares of Common Stock issuable upon conversion of the shares of Series 19 Preferred Stock in accordance with the terms hereof.
“Exchange Act” means the Securities Exchange Act of 1934 and the rules and regulations promulgated thereunder.
“Fundamental Transaction” means, at any time while the Series 19 Preferred Stock is outstanding, (i) the Corporation effects any merger or consolidation of the Corporation with or into another person in which the Corporation is not the surviving person, (ii) the Corporation effects any sale of all or substantially all of its assets in one transaction or a series of related transactions, (iii) any tender offer or exchange offer (whether by the Corporation or another person) is completed pursuant to which holders of Common Stock are permitted to tender or exchange a material portion of the Corporation’s shares for other securities, cash or property, or (iv) the Corporation effects any reclassification of the Common Stock or any compulsory share exchange pursuant to which the Common Stock is effectively converted into or exchanged for other securities, cash or property; provided, however, that for the purposes of clause (ii) above, a “Fundamental Transaction” shall not include the Corporation entering into a license or other agreement that licenses any intellectual property to an unaffiliated and unrelated person so long as the Corporation and its subsidiaries continue to have bona fide, substantial and continuing
2
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
business operations and activities after such license or other agreement is entered into; provided, further, however, that a “Fundamental Transaction” shall not include a reverse stock split with respect to the Common Stock.
“Holder” means a holder of shares of Series 19 Preferred Stock.
“Junior Securities” means (i) the Common Stock and all other Common Stock Equivalents of the Corporation other than those securities which are explicitly senior to or pari passu with the Series 19 Preferred Stock as to dividend rights or liquidation preference and (ii) the Series ZZ Junior Participating Cumulative Preferred Stock of the Corporation.
“Liquidation” has the meaning set forth in Section 5.
“Notice of Conversion” has the meaning set forth in Section 6(a).
“Non-Senior Securities” means (i) the Common Stock and all other Common Stock Equivalents of the Corporation other than those securities which are explicitly senior to the Series 19 Preferred Stock as to dividend rights or liquidation preference and (ii) the Series ZZ Junior Participating Cumulative Preferred Stock of the Corporation.
“Original Issue Date” means the date of the first issuance of any shares of Series 19 Preferred Stock regardless of the number of transfers of any particular shares of Series 19 Preferred Stock and regardless of the number of certificates which may be issued to evidence such Series 19 Preferred Stock.
“Series 19 Preferred Stock” has the meaning set forth in Section 2.
“Stated Value” has the meaning set forth in Section 2, as the same may be increased pursuant to Section 3(a).
“Trading Day” means a day on which the New York Stock Exchange is open for business.
“Trading Market” means the following markets or exchanges on which the Common Stock is listed or quoted for trading on the date in question: The NYSE Amex, The NASDAQ Capital Market, The NASDAQ Global Market, The NASDAQ Global Select Market, the New York Stock Exchange or the Mercato Telematico Azionario (MTA) organized and managed by Borsa Italiana S.p.A.
“Transfer” has the meaning set forth in Section 10.
“VWAP” means, for any date, the price determined by the first of the following clauses that applies: (i) if the Common Stock is then listed or quoted on a national securities exchange, the daily volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the national securities exchange on which the Common Stock is then listed or quoted for trading as reported by Bloomberg L.P. (based on a Trading Day from 9:30 a.m. (New York City time) to 4:02 p.m. (New York City time)); (ii) if the Common Stock is then listed or traded on the OTC Bulletin Board and the OTC Bulletin Board is not a Trading Market, the volume weighted average price of the Common Stock for such date (or the nearest preceding date) on the OTC Bulletin Board; (iii) if the Common Stock is not then quoted for trading on a national securities exchange or the OTC Bulletin Board and if prices for the Common Stock are then reported in the “Pink Sheets” published by Pink OTC Markets, Inc. (or a similar organization or agency succeeding to its functions of reporting prices), the most recent bid price per share
3
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
of the Common Stock so reported; or (iv) in all other cases, the fair market value of a share of Common Stock as determined by an independent appraiser selected in good faith by a majority in interest of the Holders and reasonably acceptable to the Corporation, the fees and expenses of which shall be paid by the Corporation.
Section 2. Designation, Amount, Par Value and Rank. The series of preferred stock shall be designated as the Corporation’s Series 19 Preferred Stock (the “Series 19 Preferred Stock”) and the number of shares so designated shall be 30,000. Each share of Series 19 Preferred Stock shall have no par value per share and a stated value equal to $1,000, subject to increase as set forth in Section 3(a) below (the “Stated Value”).
Section 3. Dividends.
(a) Dividends. Holders shall be entitled to receive, and the Corporation shall pay, dividends on shares of Series 19 Preferred Stock equal (on an as-if-converted-to-Common-Stock basis) to and in the same form as dividends (other than dividends in the form of Common Stock) actually paid on shares of the Common Stock or other Non-Senior Securities when, as and if such dividends (other than dividends in the form of Common Stock) are paid on shares of the Common Stock or other Non-Senior Securities. Other than as set forth in the previous sentence, no other dividends shall be paid on shares of Series 19 Preferred Stock; and the Corporation shall pay no dividends (other than dividends in the form of Common Stock) on shares of the Common Stock or other Non-Senior Securities unless it simultaneously complies with the previous sentence. All declared but unpaid dividends on shares of Series 19 Preferred Stock shall increase the Stated Value of such shares, but when such dividends are actually paid any such increase in the Stated Value shall be rescinded.
(b) So long as any shares of Series 19 Preferred Stock remain outstanding, neither the Corporation nor any subsidiary thereof shall redeem, purchase or otherwise acquire directly or indirectly any material amount of Non-Senior Securities except as expressly permitted by Section 9(b).
Section 4. Voting Rights. Except as otherwise expressly provided herein or as otherwise required by law, Holders of shares of Series 19 Preferred Stock shall have no voting rights.
Section 5. Liquidation. Upon any liquidation, dissolution or winding-up of the Corporation, whether voluntary or involuntary (a “Liquidation”), the Holders shall be entitled to receive out of the assets, whether capital or surplus, of the Corporation an amount equal to the Stated Value for each outstanding share of Series 19 Preferred Stock before any distribution or payment shall be made to the holders of any Junior Securities, and if the assets of the Corporation shall be insufficient to pay in full such amounts, then the entire assets to be distributed to the Holders shall be ratably distributed among the Holders and the holders of all securities which are pari passu with the Series 19 Preferred Stock as to liquidation in accordance with the respective amounts that would be payable on all such securities if all amounts payable thereon were paid in full. A Fundamental Transaction or Change of Control Transaction shall not be deemed a Liquidation unless the Corporation expressly declares that such Fundamental Transaction or Change of Control Transaction shall be treated as if it were a Liquidation. The Corporation shall mail written notice of any such Liquidation, not less than 25 days before the payment date stated therein, to each Holder.
4
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Section 6. Conversion and Exchange Rights.
(a) Conversions at Option of Holder.
(i) Each share of Series 19 Preferred Stock shall be convertible at any time and from time to time from and after the Original Issue Date, at the option of the Holder thereof, into that number of shares of Common Stock determined by dividing the Stated Value of such share of Series 19 Preferred Stock by the Conversion Price. Holders shall effect conversions by providing the Corporation or its designated conversion agent with the form of conversion notice attached hereto as Annex A (a “Notice of Conversion”), which may be delivered before the date of conversion. Each Notice of Conversion shall specify the number of shares of Series 19 Preferred Stock to be converted, the number of shares of Series 19 Preferred Stock owned before the conversion at issue, the number of shares of Series 19 Preferred Stock owned subsequent to the conversion at issue and the date on which such conversion is to be effected, which date must be on or after the Original Issue Date and may not be before the date the applicable Holder delivers such Notice of Conversion to the Corporation in accordance with Section 11(a) (such date, the “Conversion Date”); provided, however, that in the case of an automatic conversion pursuant to Section 6(b), the “Conversion Date” shall be the first to occur of the dates set forth in clauses (A) through (C) of Section 6(b). If no Conversion Date is specified in a Notice of Conversion, the Conversion Date shall be the date that such Notice of Conversion to the Corporation is deemed delivered hereunder (or the first date thereafter that conversion is permitted pursuant to this Section 6(a) or Section 6(b), as applicable). The calculations and entries set forth in the Notice of Conversion shall control in the absence of manifest or mathematical error. To effect conversions of shares of Series 19 Preferred Stock, a Holder shall be required to (and by delivering a Notice of Conversion shall thereby be deemed to agree to) forthwith surrender the certificate(s) representing such shares of Series 19 Preferred Stock to the Corporation. Notwithstanding anything to the contrary set forth herein, upon conversion of shares of Series 19 Preferred Stock in accordance with the terms hereof, no Holder thereof shall be required to physically surrender the certificate representing such Holder’s shares of Series 19 Preferred Stock to the Corporation unless (A) the full or remaining number of shares of Series 19 Preferred Stock represented by such certificate are being converted or (B) such Holder has provided the Corporation with prior written notice (which notice may be included in a Notice of Conversion) requesting reissuance of a certificate representing the remaining shares of Series 19 Preferred Stock upon physical surrender of any certificate representing the shares of Series 19 Preferred Stock being converted. Each Holder and the Corporation shall maintain records showing the number of shares of Series 19 Preferred Stock so converted by such Holder and the dates of such conversions or shall use such other method, reasonably satisfactory to such Holder and the Corporation, so as not to require physical surrender of the certificate representing the shares of Series 19 Preferred Stock upon each such conversion. In the event of any dispute or discrepancy, such records of the Corporation establishing the number of shares of Series 19 Preferred Stock to which the record holder is entitled shall be controlling and determinative in the absence of manifest error.
(ii) Notwithstanding the foregoing, no shares of Series 19 Preferred Stock shall be convertible by a Holder to the extent (but only to the extent) that such conversion would result in such Holder and its affiliates beneficially owning more than 19.99% of the Common Stock (the “Beneficial Ownership Limitation”). To the extent the Beneficial Ownership Limitation applies, the determination of whether the shares of Series 19 Preferred Stock held by such Holder shall be convertible (vis-à-vis other convertible, exercisable or exchangeable securities owned by such Holder) shall, subject to such Beneficial Ownership Limitation, be determined on the basis of the first submission to the Corporation for conversion, exercise or exchange (as the case may be). No prior inability of a Holder to convert shares of Series 19 Preferred Stock pursuant to this paragraph shall have any effect on the applicability of the provisions of this paragraph with respect to any subsequent determination of convertibility or issuance (as the case may be). For purposes of this paragraph, beneficial ownership and all determinations and calculations (including, without limitation, with respect to calculations of percentage ownership) shall be determined in accordance with Section 13(d) of the Exchange Act and the rules and regulations promulgated thereunder; provided, however, that in effecting any conversion, the Corporation shall be entitled to assume that no Holder, together with its affiliates, beneficially owns more than 19.99% of the Common Stock unless written notice specifying the number of shares of Common Stock beneficially held by such Holder and its affiliates is sent to the Corporation by the Holder within the three Business Day period before the date of the automatic conversion.
5
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
The provisions of this paragraph shall be implemented in a manner otherwise than in strict conformity with the terms of this paragraph to correct this paragraph (or any portion hereof) which may be defective or inconsistent with the intended Beneficial Ownership Limitation herein contained or to make changes or supplements necessary or desirable to properly give effect to such Beneficial Ownership Limitation. The limitations contained in this paragraph shall apply to a successor Holder. The holders of Common Stock shall be third party beneficiaries of this paragraph and the Corporation may not waive this paragraph without the consent of holders of a majority of its Common Stock. For any reason at any time, upon the written or oral request of a Holder, the Corporation shall within two Business Days confirm orally and in writing to such Holder the number of shares of Common Stock then outstanding, including by virtue of any prior conversion or exercise of convertible or exercisable securities into Common Stock.
(b) Automatic Conversion. Upon the earliest to occur of the events described in the following clauses (A), (B) and (C) of this Section 6(b), each outstanding share of Series 19 Preferred Stock shall automatically convert into that number of shares of Common Stock determined by dividing the Stated Value of such share of Series 19 Preferred Stock by the Conversion Price (A) on the 30th day after the Original Issue Date, (B) on the date on which 5,000 or less shares of Series 19 Preferred Stock remain outstanding, or (C) immediately upon the adoption by the Board of a resolution that it intends to adopt an amendment to the Restated Articles without shareholder approval to effect a reverse stock split of the outstanding Common Stock and the number of authorized shares of Common Stock in the same proportions in order to achieve compliance with the listing rules of The NASDAQ Capital Market or for other good-faith business reasons.
Upon a Conversion Date, a Holder shall be required to forthwith surrender any certificate(s) representing such shares of Series 19 Preferred Stock to the Corporation within two Trading Days of the date established for such conversion and set forth in a written notice from the Corporation; provided, however, that the failure by a Holder to surrender the certificate(s) representing such converted shares of Series 19 Preferred Stock shall not prevent the Corporation from delivering the shares of Common Stock issuable upon automatic conversion thereof and, upon receipt of such consideration by such Holder, such shares of Series 19 Preferred Stock shall be converted for all purposes hereunder.
(c) Conversion Price. The conversion price for the Series 19 Preferred Stock shall equal $1.914, subject to adjustment as provided herein (the “Conversion Price”).
(d) Mechanics of Conversion.
(i) Delivery of Certificate upon Conversion. Not later than three Trading Days after each Conversion Date, whether pursuant to Section 6(a) or (b), the Corporation shall deliver, or cause to be delivered, to the converting Holder a certificate or certificates, which shall be free of restrictive legends and issuer-imposed trading restrictions (provided that a registration statement covering resales of the Conversion Shares is then in effect), representing the number of shares of
6
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Common Stock being acquired upon the conversion of shares of Series 19 Preferred Stock. The Corporation shall use its best efforts to, if the Holder is not an affiliate of the Corporation, deliver any certificate(s) required to be delivered by the Corporation under this Section 6 electronically through The Depository Trust Company or its nominee (“DTC”) or another established clearing corporation performing similar functions (provided that a registration statement covering resales of the Conversion Shares is then in effect). If, in the case of any Notice of Conversion, such certificate(s) are not delivered to or as directed by the applicable Holder by the seventh Trading Day after the Conversion Date, then (without limiting the Holder’s other rights and remedies hereunder for the Corporation’s failure to comply with its obligations under the preceding portion of this paragraph) the applicable Holder shall be entitled to elect to rescind such Conversion Notice by written notice to the Corporation at any time on or before its receipt of such certificate(s), in which event the Corporation shall promptly return to such Holder any original Series 19 Preferred Stock certificate delivered to the Corporation and such Holder shall promptly return any Common Stock certificates representing the shares of Series 19 Preferred Stock tendered for conversion to the Corporation.
(ii) Obligation Absolute. The Corporation’s obligation to issue and deliver the Conversion Shares upon conversion of shares of Series 19 Preferred Stock in accordance with the terms hereof is absolute and unconditional, irrespective of any action or inaction by a Holder to enforce the same, any waiver or consent with respect to any provision hereof, the recovery of any judgment against any person or any action to enforce the same, or any setoff, counterclaim, recoupment, limitation or termination, or any breach or alleged breach by such Holder or any other person of any obligation to the Corporation or any violation or alleged violation of law by such Holder or any other person, and irrespective of any other circumstance which might otherwise limit such obligation of the Corporation to such Holder in connection with the issuance of such Conversion Shares; provided, however, that such delivery shall not operate as a waiver by the Corporation of any such action that the Corporation may have against such Holder. In the event a Holder shall elect to convert any or all of the Stated Value of its Series 19 Preferred Stock, the Corporation may not refuse conversion based on any claim that such Holder or anyone associated or affiliated with such Holder has been engaged in any violation of law, agreement or for any other reason, unless an injunction from a court, on notice to Holder, restraining and/or enjoining conversion of all or part of the Series 19 Preferred Stock of such Holder shall have been sought and obtained. In the absence of such an injunction, the Corporation shall issue Conversion Shares upon a properly noticed conversion. Nothing herein shall limit a Holder’s right to pursue actual damages for the Corporation’s failure to deliver Conversion Shares within the period specified herein and such Holder shall have the right to pursue all remedies available to it hereunder, at law or in equity, including, without limitation, a decree of specific performance and/or injunctive relief. The exercise of any such rights shall not prohibit a Holder from seeking to enforce damages pursuant to any other Section hereof or under applicable law.
(iii) Reservation of Shares Issuable upon Conversion. The Corporation covenants that it will at all times use reasonable best efforts to reserve and keep available out of its authorized and unissued shares of Common Stock, for the sole purpose of issuance upon conversion of the Series 19 Preferred Stock, as herein provided, free from preemptive rights or any other actual contingent purchase rights of persons other than the Holders of the Series 19 Preferred Stock, not less than such aggregate number of shares of the Common Stock as shall be issuable (taking into account the adjustments and restrictions of Section 7) upon the conversion of all outstanding shares of Series 19 Preferred Stock. The Corporation covenants that all shares of Common Stock that shall be so issuable shall, upon issue, be duly authorized, validly issued, fully paid and nonassessable.
7
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
(iv) Fractional Shares. Upon a conversion of the Series 19 Preferred Stock hereunder, the Corporation shall not be required to issue fractions of shares of Common Stock, but shall instead, if otherwise permitted, round the total number of Conversion Shares for such conversion up or down to the nearest whole number of shares of Common Stock.
(v) Transfer Taxes. The issuance of certificates for shares of the Common Stock issued upon conversion of shares of Series 19 Preferred Stock shall be made without charge to any Holder for any documentary stamp, issuance or similar taxes that may be payable in respect of the issue or delivery of such certificates, provided that the Corporation shall not be required to pay any tax that may be payable in respect of any transfer involved in the issuance and delivery of any such certificate upon conversion in a name other than that of the Holder of such shares of Series 19 Preferred Stock so converted and the Corporation shall not be required to issue or deliver such certificates unless or until the person or persons requesting the issuance thereof shall have paid to the Corporation the amount of such tax or shall have established to the satisfaction of the Corporation that such tax has been paid.
Section 7. Certain Adjustments.
(a) Stock Dividends and Stock Splits. If the Corporation, at any time while the Series 19 Preferred Stock is outstanding: (A) pays a stock dividend or otherwise makes a distribution or distributions payable in shares of Common Stock on shares of Common Stock or any other Common Stock Equivalents (which, for avoidance of doubt, shall not include any shares of Common Stock issued by the Corporation upon conversion of the Series 19 Preferred Stock); (B) subdivides outstanding shares of Common Stock into a larger number of shares; (C) combines (including by way of a reverse stock split) outstanding shares of Common Stock into a smaller number of shares; or (D) issues, in the event of a reclassification of shares of the Common Stock, any shares of capital stock of the Corporation, then the Conversion Price shall be multiplied by a fraction of which the numerator shall be the number of shares of Common Stock outstanding immediately before such event and of which the denominator shall be the number of shares of Common Stock outstanding immediately after such event and any other adjustments to the Holders’ conversion rights necessary to reflect such event shall be made. Any adjustment made pursuant to this Section 7(a) shall become effective immediately after the record date for the determination of shareholders entitled to receive such dividend or distribution and shall become effective immediately after the effective date in the case of a subdivision, combination or reclassification.
(b) Subsequent Rights Offerings. If the Corporation, at any time while the Series 19 Preferred Stock is outstanding, shall issue rights, options or warrants to all holders of Common Stock (and not proportionately to the Holders) entitling them to subscribe for or purchase shares of Common Stock at a price per share that is lower than the VWAP on the record date for such issuance, and do not offer the same rights to the Holders, then the Conversion Price shall be adjusted to reflect such rights, options or warrants offering by multiplying the Conversion Price by a fraction, the numerator of which shall be the number of shares of Common Stock outstanding before the record date for such issuance plus the number of shares of Common Stock which the aggregate offering price of the total number of shares of Common Stock so offered (assuming delivery to the Corporation in full of all consideration payable upon exercise of such rights, options or warrants) would purchase at such VWAP on the record date for such issuance and the denominator of which shall be the number of shares of the Common Stock outstanding on such record date plus the aggregate number of additional shares of Common Stock offered
8
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
for subscription or purchase. Such adjustment shall be made whenever such rights, options or warrants are issued, and shall become effective immediately after the record date for the determination of shareholders entitled to receive such rights, options or warrants.
(c) Pro Rata Distributions. If the Corporation, at any time while the Series 19 Preferred Stock is outstanding, distributes (other than as a dividend) to all holders of Common Stock (and not proportionately to the Holders) evidences of its indebtedness or assets or rights or warrants to subscribe for or purchase any security (other than Common Stock, which shall be subject to Section 7(b)), then in each such case the Conversion Price shall be adjusted by multiplying such Conversion Price in effect immediately before the record date fixed for determination of shareholders entitled to receive such distribution by a fraction of which the denominator shall be the VWAP determined as of the record date mentioned above, and of which the numerator shall be such VWAP on such record date less the then fair market value at such record date of the portion of such assets, evidence of indebtedness or rights or warrants so distributed applicable to one outstanding share of the Common Stock as determined by the Board in good faith. In either case the adjustments shall be described in a statement delivered to the Holders describing the portion of assets or evidences of indebtedness so distributed or such subscription rights applicable to one share of Common Stock. Such adjustment shall be made whenever any such distribution is made and shall become effective immediately after the record date mentioned above. For avoidance of doubt, distributions that are dividends shall be subject to Section 3(a) and not subject to this Section 7(c).
(d) Fundamental Transaction. If, at any time while the Series 19 Preferred Stock is outstanding, a Fundamental Transaction occurs, then, upon any subsequent conversion of the Series 19 Preferred Stock, the Holders shall have the right to receive, for each Conversion Share that would have been issuable upon such conversion immediately before the occurrence of such Fundamental Transaction, the same kind and amount of securities, cash or property as it would have been entitled to receive upon the occurrence of such Fundamental Transaction if it had been, immediately before such Fundamental Transaction, the holder of one share of Common Stock (the “Alternate Consideration”); and the Holders shall no longer have the right to receive Conversion Shares per se upon such conversion. For purposes of any such conversion, the determination of the Conversion Price shall be appropriately adjusted to apply to such Alternate Consideration based on the amount of Alternate Consideration issuable in respect of one share of Common Stock in such Fundamental Transaction, and the Corporation shall apportion the Conversion Price among the Alternate Consideration in a reasonable manner reflecting the relative value of any different components of the Alternate Consideration. If holders of Common Stock are given any choice as to the securities, cash or property to be received in a Fundamental Transaction, then the Holders shall be given the same choice as to the Alternate Consideration it receives upon any conversion of the Series 19 Preferred Stock following such Fundamental Transaction. To the extent necessary to effectuate the foregoing provisions, any successor to the Corporation or surviving entity in such Fundamental Transaction shall adopt articles of incorporation or an amendment to its articles of incorporation with the same terms and conditions and issue to the Holders new preferred stock consistent with the foregoing provisions and evidencing the Holders’ right to convert such preferred stock into Alternate Consideration. Unless the Corporation elects to treat such Fundamental Transaction as a Liquidation, the terms of any agreement pursuant to which a Fundamental Transaction is effected shall include terms requiring any such successor or surviving entity to comply with the provisions of this Section 7(d) and ensuring that the Series 19 Preferred Stock (or any such replacement security) will be similarly adjusted upon any subsequent transaction analogous to a Fundamental Transaction.
(e) Calculations. All calculations under this Section 7 shall be made to the nearest cent or the nearest 1/100th of a share, as the case may be.
9
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
(f) Notice to the Holders.
(i) Adjustment to Conversion Price. Whenever the Conversion Price is adjusted pursuant to any provision of this Section 7, the Corporation shall promptly deliver to each Holder a notice setting forth the Conversion Price after such adjustment and setting forth a brief statement of the facts requiring such adjustment.
(ii) Notice to Allow Conversion by Holder. If (A) the Corporation shall declare a dividend (or any other distribution in whatever form) on the Common Stock, (B) the Corporation shall declare a special nonrecurring cash dividend on or a redemption of the Common Stock, (C) the Corporation shall authorize the granting to all holders of the Common Stock of rights or warrants to subscribe for or purchase any shares of capital stock of any class or of any rights, (D) the approval of any shareholders of the Corporation shall be required in connection with any reclassification of the Common Stock, any consolidation or merger to which the Corporation is a party, any sale or transfer of all or substantially all of the assets of the Corporation, of any compulsory share exchange whereby the Common Stock is converted into other securities, cash or property, or (E) the Corporation shall authorize the voluntary or involuntary dissolution, liquidation or winding up of the affairs of the Corporation, then, in each case, the Corporation shall cause to be filed at each office or agency maintained for the purpose of conversion of the Series 19 Preferred Stock, and shall cause to be delivered to each Holder at its last address as it shall appear upon the stock books of the Corporation, at least 20 calendar days before the applicable record or effective date hereinafter specified, a notice stating (x) the date on which a record is to be taken for the purpose of such dividend, distribution, redemption, rights or warrants, or if a record is not to be taken, the date as of which the holders of the Common Stock of record to be entitled to such dividend, distributions, redemption, rights or warrants are to be determined or (y) the date on which such reclassification, consolidation, merger, sale, transfer or share exchange is expected to become effective or close, and the date as of which it is expected that holders of the Common Stock of record shall be entitled to exchange their shares of the Common Stock for securities, cash or other property deliverable upon such reclassification, consolidation, merger, sale, transfer or share exchange, provided that the failure to deliver such notice or any defect therein or in the delivery thereof shall not affect the validity of the corporate action required to be specified in such notice. To the extent that any notice provided hereunder constitutes, or contains, material, non-public information regarding the Corporation or any of its subsidiaries, the Corporation shall simultaneously file such notice with the Commission pursuant to a Current Report on Form 8-K. The Holder is entitled to convert the Stated Value of its Series 19 Preferred Stock during the 20-day period commencing on the date of such notice through the effective date of the event triggering such notice.
Section 8. [Reserved.]
Section 9. Negative Covenants. As long as at least 20% of the aggregate number of originally issued shares of Series 19 Preferred Stock are outstanding (as appropriately adjusted for share splits and similar transactions), the Corporation shall not, without the Corporation obtaining the affirmative written consent of Holders of a majority of the then outstanding shares of the Series 19 Preferred Stock:
(a) amend these articles of incorporation, its bylaws or other charter documents so as to materially, specifically and adversely affect any rights of any Holder with respect to Series 19 Preferred Stock;
10
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
(b) repay, repurchase or offer to repay, repurchase or otherwise acquire any material amount of its Junior Securities (other than securities described in clause (ii) of the definition of “Junior Securities”); provided, however, that this restriction shall not apply to the repurchase of up to 5,750,000 shares of Common Stock in any 12-month period (subject to adjustment for reverse and forward stock splits, stock dividends, stock combinations and other similar transactions of the Common Stock that occur after the Original Issue Date) from employees, officers, directors, consultants or other persons performing services for the Corporation or any subsidiary pursuant to agreements approved by a majority of the Board or under which the Corporation has the option to repurchase such shares at cost or at cost upon the occurrence of certain events, such as termination of employment;
(c) authorize or create any class or series of stock ranking senior to the Series 19 Preferred Stock as to dividend rights or liquidation preference; or
(d) enter into any agreement or understanding with respect to any of the foregoing.
Notwithstanding the foregoing, this Section 9 shall not prohibit the issuance of additional series of preferred stock that do not rank senior to the Series 19 Preferred Stock as to dividend rights or liquidation preference.
Section 10. Transferability. The Series 19 Preferred Stock may only be sold, transferred, assigned, pledged or otherwise disposed of (any of the foregoing, a “Transfer”) in accordance with U.S. state and federal securities laws. The Corporation shall keep at its principal office, or at the offices of the transfer agent, a register of the Series 19 Preferred Stock. In connection with any such permitted Transfer, upon the surrender of any certificate representing Series 19 Preferred Stock at such place, the Corporation, at the request of the record Holder of such certificate, shall execute and deliver (at the Corporation’s expense) a new certificate or certificates in exchange therefor representing in the aggregate the number of shares represented by the surrendered certificate; provided that the Corporation shall not be required to pay any tax that may be payable in respect of any such Transfer involved in the issuance and delivery of any such new certificate in a name other than that of Holder and the Corporation shall not be required to issue or deliver such new certificate(s) unless or until the person or persons requesting the issuance thereof shall have paid to the Corporation the amount of such tax or shall have established to the satisfaction of the Corporation that such tax has been paid. Each such new certificate shall be registered in such name and shall represent such number of shares as is requested by the Holder of the surrendered certificate and shall be substantially identical in form to the surrendered certificate.
Section 11. Miscellaneous.
(a) Notices. Any and all notices or other communications or deliveries to be provided by the Holders hereunder shall be in writing and delivered personally, by facsimile or by email, or sent by a nationally recognized overnight courier service, addressed to the Corporation, at 0000 Xxxxxxx Xxxxxx, Xxxxx 000, Xxxxxxx, Xxxxxxxxxx 00000, facsimile number (000) 000-0000, or email xxxxxxx@xxxxxxxxxx.xxx, Attention: Xxxxx Xxxxxx, or such other street address, facsimile number or email address as the Corporation may specify for such purposes by notice to the Holders delivered in accordance with this Section 11(a). Any and all notices or other communications or deliveries to be provided by the Corporation hereunder shall be in writing and delivered personally, by facsimile, by email or sent by a nationally recognized overnight courier service addressed to each Holder at the facsimile number, email address or street address of such Holder appearing on the books of the Corporation, or if no such facsimile number, email address or street address appears on the books of the Corporation, at the principal place of business of the Holder. Any notice or other communication or deliveries hereunder shall be deemed given and effective on the earliest of (i) the date of transmission, if such notice or communication is delivered via facsimile or email to the facsimile number or email address specified in this Section 11(a) before 5:30 p.m. (New York City time) on any date, (ii) the date immediately following the date of transmission, if such notice or communication is delivered via facsimile or email to the facsimile number or email address specified in this Section 11(a) between 5:30 p.m. and 11:59 p.m. (New York City time) on any date, (iii) the second Business Day following the date of dispatch, if sent by nationally recognized overnight courier service, or (iv) upon actual receipt by the party to whom such notice is required to be given.
11
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
(b) Lost or Mutilated Series 19 Preferred Stock Certificate. If a Holder’s Series 19 Preferred Stock certificate shall be mutilated, lost, stolen or destroyed, the Corporation shall execute and deliver, in exchange and substitution for and upon cancellation of a mutilated certificate, or in lieu of or in substitution for a lost, stolen or destroyed certificate, a new certificate for the shares of Series 19 Preferred Stock so mutilated, lost, stolen or destroyed, but only upon receipt of evidence of such loss, theft or destruction of such certificate, and of the ownership thereof reasonably satisfactory to the Corporation.
(c) Governing Law. All questions concerning the construction, validity, enforcement and interpretation of this instrument shall be governed by and construed and enforced in accordance with the internal laws of the State of Washington, without regard to the principles of conflict of laws thereof.
(d) Waiver. Any waiver by the Corporation or a Holder of a breach of any provision of this instrument shall not operate as or be construed to be a waiver of any other breach of such provision or of any breach of any other provision of this instrument or a waiver by any other Holders. The failure of the Corporation or a Holder to insist upon strict adherence to any term of this instrument on one or more occasions shall not be considered a waiver or deprive that party (or any other Holder) of the right thereafter to insist upon strict adherence to that term or any other term of this instrument. Any waiver by the Corporation or a Holder must be in writing.
(e) Severability. If any provision of this Article II.2(z) is invalid, illegal or unenforceable, the balance of this Article II.2(z) shall remain in effect, and if any provision is inapplicable to any person or circumstance, it shall nevertheless remain applicable to all other persons and circumstances.
(f) Next Business Day. Whenever any payment or other obligation hereunder shall be due on a day other than a Business Day, such payment shall be made on the next succeeding Business Day.
(g) Headings. The headings contained herein are for convenience only, do not constitute a part of this Article II.2(v) and shall not be deemed to limit or affect any of the provisions hereof.
(h) Status of Converted or Redeemed Series 19 Preferred Stock. If any shares of Series 19 Preferred Stock are converted, redeemed or reacquired by the Corporation, such shares shall resume the status of authorized but unissued shares of preferred stock and shall no longer be designated as Series 19 Preferred Stock.
(i) Remedies, Characterizations, Other Obligations, Breaches and Injunctive Relief. The remedies provided herein shall be cumulative and in addition to all other remedies available hereunder, at law or in equity (including a decree of specific performance and/or other injunctive relief), and no remedy contained herein shall be deemed a waiver of compliance with the provisions giving rise to such remedy. Nothing herein shall limit a Holder’s right to pursue actual damages for any failure by the Corporation to
12
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
comply with the terms hereof. The Corporation covenants to each Holder that there shall be no characterization concerning this instrument other than as expressly provided herein. Amounts set forth or provided for herein with respect to payments, conversion and the like (and the computation thereof) shall be the amounts to be received by a Holder and shall not, except as expressly provided herein, be subject to any other obligation of the Corporation (or the performance thereof). The Corporation acknowledges that a breach by it of its obligations hereunder will cause irreparable harm to the Holders and that the remedy at law for any such breach may be inadequate. The Corporation therefore agrees that, in the event of any such breach or threatened breach, the Holders shall be entitled, in addition to all other available remedies, to an injunction restraining any breach, without the necessity of showing economic loss and without any bond or other security being required.
[Signature page follows.]
13
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
I certify that I am a duly appointed and incumbent officer of the above named Corporation and that I am authorized to execute these Articles of Amendment on behalf of the Corporation.
EXECUTED, this 15th day of November, 2013.
| CELL THERAPEUTICS, INC., a Washington corporation | ||
| By: |
| |
| Name: Xxxxx X. Xxxxxx, M.D. | ||
| Title: President and Chief Executive Officer | ||
[Articles of Amendment (Series 19)]
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
ANNEX A
NOTICE OF CONVERSION
(TO BE EXECUTED BY THE HOLDER IN ORDER TO CONVERT SHARES
OF SERIES 19 PREFERRED STOCK)
The undersigned hereby elects to convert the number of shares of Series 19 Preferred Stock, no par value per share (the “Preferred Stock”), of Cell Therapeutics, Inc., a Washington corporation (the “Corporation”), indicated below into shares of common stock, no par value per share (the “Common Stock”), of the Corporation, according to the conditions hereof, as of the date written below. If shares of Common Stock are to be issued in the name of a person other than the undersigned, the undersigned will pay all transfer taxes payable with respect thereto and is delivering herewith such certificates and opinions as may be reasonably required by the Corporation. No fee will be charged to the Holders for any conversion of Preferred Stock, except for any such transfer taxes.
Conversion calculations:
| Date to Effect Conversion: |
|
| Number of shares of Preferred Stock owned before Conversion: | CUSIP 150934 859 | |||
| Number of shares of Preferred Stock to be Converted: |
| |||
| Stated Value of shares of Preferred Stock to be Converted: |
| |||
| Number of shares of Common Stock to be Issued: | CUSIP 150934 883 | |||
| Applicable Conversion Price per share of Common Stock: |
| |||
| Number of shares of Preferred Stock subsequent to Conversion: |
| |||
| Address of Record: | ||||
| By: |
| |||
| Name: |
| |||
| Title: |
| |||
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 13.3.1
Form of Press Release
(See attached.)
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |

FOR IMMEDIATE RELEASE
Baxter Media Contacts
Xxxxx Xxxxx or Xxxxxxx Xxxx
(000) 000-0000
xxxxx@xxxxxx.xxx
Baxter Investor Contact
Xxxx Xxx Xxxxxx, (000) 000-0000
CTI Contact
Xxxxxxx Xxxxx
000-000-0000
xxxxxx@xxxxxxxxxx.xxx
BAXTER AND CELL THERAPEUTICS ANNOUNCE WORLDWIDE STRATEGIC
COLLABORATION TO DEVELOP AND COMMERCIALIZE PACRITINIB
Pacritinib Pivotal Phase III Clinical Program for Myelofibrosis Underway
DEERFIELD, Ill., and SEATTLE, Wash., November 15, 2013 – Xxxxxx International Inc. (NYSE:BAX) and Cell Therapeutics, Inc. (CTI) (NASDAQ and MTA: CTIC) today jointly announced that they have entered into an exclusive worldwide licensing agreement to develop and commercialize pacritinib. Pacritinib is a novel investigational JAK2/FLT3 inhibitor with activity against genetic mutations linked to myelofibrosis, leukemia and certain solid tumors. Pacritinib is currently in Phase III development for patients with myelofibrosis, a chronic malignant bone marrow disorder.
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
BAXTER AND CELL THERAPEUTICS ANNOUNCE WORLDWIDE STRATEGIC COLLABORATION TO DEVELOP AND COMMERCIALIZE PACRITINIB – PAGE TWO
Under the terms of the agreement, Baxter gains exclusive commercialization rights for all indications for pacritinib outside the United States and Baxter and CTI will jointly commercialize pacritinib in the United States. Baxter will make an upfront payment of $60 million, including an equity investment in CTI of $30 million. In addition, CTI is eligible to receive clinical, regulatory, and commercial launch milestone payments of up to $112 million, $40 million of which relates to clinical milestones that may be achieved in 2014. Assuming regulatory approval and commercial launch, CTI may receive additional sales milestone payments. CTI will receive royalties on net sales of pacritinib in ex-US markets, and the companies will share U.S. profits equally. Baxter will record a special pre-tax in-process research and development charge in the fourth quarter of 2013 of approximately $30 million.
“The collaboration will complement Xxxxxx’x existing oncology business and growing oncology pipeline, and will leverage our global commercialization capacity to extend the availability of pacritinib, which we believe has the potential to address a significant unmet medical need,” said Xxxxxx Xxxxxxx, Ph.D., president of Baxter BioScience. “As an established leader in hematology and rare diseases, we are committed to advancing novel therapies in an effort to broaden patient access to care.”
“We believe Baxter represents the ideal strategic partner to achieve the full potential of pacritinib,” said Xxxxx X. Xxxxxx, M.D., President and CEO of CTI. “Our two companies share a dedication to oncology and a vision for bringing this unique oral JAK2/FLT3 inhibitor to patients with certain blood cancers and solid tumors. This collaboration will provide additional financial resources and commercial expertise to position us to pursue the development, commercialization and market potential of pacritinib.”
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
BAXTER AND CELL THERAPEUTICS ANNOUNCE WORLDWIDE STRATEGIC COLLABORATION TO DEVELOP AND COMMERCIALIZE PACRITINIB – PAGE THREE
Pacritinib is an oral tyrosine kinase inhibitor (TKI) with dual activity against JAK2 and FLT3. The JAK family of enzymes is a central component in signal transduction pathways, which are critical to normal blood cell growth and development as well as inflammatory cytokine expression and immune responses. Mutations in these kinases have been shown to be directly related to the development of certain blood related cancers including myeloproliferative neoplasms, leukemia and lymphoma.
About the Pacritinib Phase III Program
Results from earlier Phase I and II studies of pacritinib demonstrated encouraging results, including meaningful clinical benefits coupled with good tolerability among patients with advanced myelofibrosis. Based on the efficacy and tolerability profile demonstrated to date, the pivotal program for pacritinib includes two Phase III clinical trials: PERSIST-1 is studying efficacy and safety in a broad set of patients with myelofibrosis without limitations on blood platelet counts, and PERSIST-2 will evaluate efficacy and safety in patients with low platelet counts, and is expected to begin in the fourth quarter of 2013.
About Baxter in Oncology
Baxter continues to expand its portfolio of branded products and R&D programs in oncology. Current products include chemotherapy agents used alone or in
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
combination with other products to treat cancers such as non-Hodgkin’s lymphoma, as well as anti-emetic products designed to relieve the side effects of cancer therapeutics. Recent collaborations and development programs have further expanded Xxxxxx’x footprint in hematology/oncology, building upon the company’s expertise in treating patients with life-threatening medical conditions.
About Xxxxxx International Inc.
Xxxxxx International Inc., through its subsidiaries, develops, manufactures and markets products that save and sustain the lives of people with hemophilia, immune disorders, cancer, infectious diseases, kidney disease, trauma and other chronic and acute medical conditions. As a global, diversified healthcare company, Baxter applies a unique combination of expertise in medical devices, pharmaceuticals and biotechnology to create products that advance patient care worldwide.
About Cell Therapeutics
Cell Therapeutics Inc. (NASDAQ and MTA: CTIC) is a biopharmaceutical company committed to the development and commercialization of an integrated portfolio of oncology products aimed at making cancer more treatable. CTI is headquartered in Seattle, WA. For additional information and to sign up for email alerts and get RSS feeds, please visit xxx.XxxxXxxxxxxxxxxx.xxx.
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
BAXTER AND CELL THERAPEUTICS ANNOUNCE WORLDWIDE STRATEGIC COLLABORATION TO DEVELOP AND COMMERCIALIZE PACRITINIB – PAGE FOUR
This press release includes forward-looking statements concerning a collaboration agreement between Xxxxxx International Inc. and Cell Therapeutics Inc., which are within the meaning of the Safe Harbor provisions of the Private Securities Litigation Reform Act of 1995. Such statements are subject to a number of risks and uncertainties, the outcome of which could materially and/or adversely affect actual future results and the trading price of the issuers’ securities. Such statements include, but are not limited to, expectations with respect to milestone and royalty payments, the expected benefits of the collaboration and the ability to maximize the potential of pacritinib and extend its applicability to additional malignancies, including solid tumors. The statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: satisfaction of regulatory and other requirements; actions of regulatory bodies and other governmental authorities; clinical trial results; changes in laws and regulations; product quality or patient safety issues; product development risks; the impact of competitive products and pricing and reimbursement; and other risks identified in each issuer’s most recent filings on Form 10-K and other Securities and Exchange Commission filings. Neither Baxter nor CTI undertakes to update its forward-looking statements.
###
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
Exhibit 16.13
Alternative Dispute Resolution
(a) In accordance with and subject to the procedures set forth in Section 3.1.6 and Section 16.13, and without adding any additional time to such procedures, the Parties shall attempt to resolve any and all disputes, claims or controversies arising out of or relating to this Agreement promptly by negotiation between executives who have authority to settle the controversy. If such disputes, claims or controversies are not resolved through such negotiation or the Section 3.1.6 and Section 16.13 procedures, then they shall be submitted for final and binding arbitration pursuant to the arbitration clause set forth below. Either Party may initiate arbitration with respect to the matters submitted to negotiation by filing a written demand for arbitration at any time following the initial negotiation session.
(b) To the extent not resolved by mediation, any dispute, claim or controversy arising out of or relating to this Agreement or the breach, termination, enforcement, interpretation or validity thereof, including the determination of the scope or applicability of this agreement to arbitrate, shall be determined by arbitration in New York, NY, in the language in which the contract was written. The arbitration shall be administered by the CPR pursuant to its Arbitration Rules and Procedures. References herein to any arbitration rules or procedures mean such rules or procedures as amended from time to time, including any successor rules or procedures, and references herein to the CPR include any successor thereto. The arbitration shall be before three (3) arbitrators. Each Party shall designate one arbitrator in accordance with the “screened” appointment procedure provided in Rule 5.4 of the CPR Rules. The two (2) Party-appointed arbitrators will select the third, who will serve as the panel’s chair or president. All three (3) arbitrators shall have experience in biotechnology licensing, development and/or commercialization matters. This arbitration provision, and the arbitration itself, shall be governed by the laws of the state of New York and the Federal Arbitration Act, 9 U.S.C. §§ 1-16.
(c) Consistent with the expedited nature of arbitration, each Party will, upon the written request of the other Party, promptly provide the other with copies of documents on which the producing Party may rely in support of or in opposition to any claim or defense. At the request of a Party, the arbitrators shall have the discretion to order examination by deposition of witnesses to the extent the arbitrator deems such additional discovery relevant and appropriate. Depositions shall be limited to a maximum of five per Party and shall be held within forty-five (45) days of the grant of a request. Additional depositions may be scheduled only with the permission of the arbitrators, and for good cause shown. Each deposition shall be limited to a maximum of one day’s duration. All objections are reserved for the arbitration hearing except for objections based on privilege and proprietary or confidential information. The Parties shall not utilize any other discovery mechanisms, including international processes and U.S.
| ** | Indicates that certain information contained herein has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. | |
| **** | Indicates that the amount of information omitted was a page or more in length, and such information has been filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions |
federal statutes, to obtain additional evidence for use in the arbitration. Any dispute regarding discovery, or the relevance or scope thereof, shall be determined by the arbitrators, which determination shall be conclusive. All discovery shall be completed within sixty (60) days following the appointment of the arbitrators. All costs and/or fees relating to the retrieval, review and production of electronic discovery shall be paid by the Party requesting such discovery.
(d) The arbitrators will have no authority to award punitive or other damages not measured by the prevailing Party’s actual damages, except as may be required by statute. Each Party expressly waives and foregoes any right to consequential, punitive, special, exemplary or similar damages or lost profits. The arbitrators shall have no power or authority, under the CPR Rules for Non-Administered Arbitration or otherwise, to relieve the Parties from their agreement hereunder to arbitrate or otherwise to amend or disregard any provision of this Agreement. The award of the arbitrators shall be final, binding and the sole and exclusive remedy to the Parties. Either Party may seek to confirm and enforce any final award entered in arbitration, in any court of competent jurisdiction. The cost of the arbitration, including the fees of the arbitrators, shall be borne by the Party the arbitrator determines has not prevailed in the arbitration.
(e) If an arbitral award does not impose an injunction on the losing Party or contain a money damages award in excess of five million dollars USD ($5,000,000), then the arbitral award shall not be appealable and shall only be subject to such challenges as would otherwise be permissible under the Federal Arbitration Act, 9 U.S.C. §§ 1-16. In the event that the arbitration does result in an arbitral award, which imposes an injunction or a monetary award in excess of five million dollars USD ($5,000,000), such award may be appealed to a tribunal of appellate arbitrators via the CPR Arbitration Appeal Procedure.
(f) Except as may be required by law, neither a Party nor an arbitrator may disclose the existence, content, or results of any arbitration hereunder without the prior written consent of both Parties.

