INHIBIKASE THERAPEUTICS FIRST AMENDMENT TO COLLABORATIVE RESEARCH AND DEVELOPMENT AGREEMENT
Exhibit 10.4
INHIBIKASE THERAPEUTICS
FIRST AMENDMENT TO
COLLABORATIVE RESEARCH AND DEVELOPMENT AGREEMENT
THIS FIRST AMENDMENT TO THE COLLABORATIVE RESEARCH AND DEVELOPMENT AGREEMENT (“Agreement”) is entered into with an effective date as of the 5th day of October 2012 (the “Amendment Effective Date”) by and among, on the one hand, Inhibikase Therapeutics, Inc., a Delaware corporation, with offices located at 0000 Xxxxxxxxx Xxxxxxx, Xxxxx 0000, Xxxxxxx, Xxxxxxx (the “Company”) and, on the other hand, Sphaera Pharma Pte. Ltd., a company incorporated under the laws of Singapore with its registered office at 0 Xxxxxxx Xxxxxxxxx, #00-00 Xxxxxx Xxxxx 0, Xxxxxxxxx 000000 (“Sphaera Singapore”) and Sphaera Pharma Pvt. Ltd., with its registered office at X-000, Xxxxx Xxxxx, Xxxxxxx Xxxxxxx-XX, Xxx Xxxxx-000000, XXXXX (“Sphaera India”)(together with Sphaera Singapore, hereinafter referred to as “Sphaera Pharma”). (Company and Sphaera Pharma shall be referred to individually as a “Party” and collectively as the “Parties.”) Except as otherwise provided in this Agreement, capitalized terms and phrases shall have the meaning ascribed thereto in the Original Agreement (as defined below)
RECITALS
|
Table 1: Imatinib derivatives |
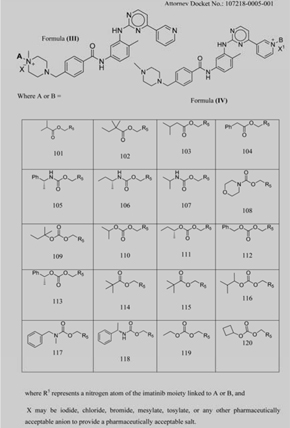
WHEREAS, Sphaera Pharma and Company entered into that certain Collaborative Research and Development Agreement as of the 29 day of February 2012 (the “Original Agreement”) to address, among other things, certain issues relating to the Company Compounds (as defined below);
WHEREAS, since having entered into the Original Agreement, each of Sphaera Pharma and Company agreed to file the Joint Applications (as defined below) on the Company Compounds and Sphaera Compounds (as defined below);
WHEREAS, each of the Parties desire to enter into this Agreement for the purpose of amending the Original Agreement to address, among other things, the Parties’ relative rights to the Joint Applications;
NOW THEREFORE, in consideration of the mutual covenants and promises herein, the receipt and sufficiency of which are hereby acknowledged, Sphaera Pharma and Company agree as follows:
| Page 1 |
1. Amendment. Each of Sphaera Pharma and Company hereby agree that the Original Agreement is and shall be amended by adding at the end of Section 4 the following provisions in sequential order thereto:
d. The Joint Applications. Sphaera Pharma and Company jointly filed intellectual property under application number 61/709704 in the United States on 4 October, 2012 and under application number 3105/DEL/2012 in India on 4 October, 2012 (“the Joint Applications”). The intellectual property covered by the Joint Applications as filed in the United States and India includes a series of novel compounds. The representative novel compounds covered by the Joint Applications appear in Table 1 thereto (“Table 1”).
e. The Company Compounds. Under and for purposes of the Original Agreement, specifically compounds 101 thru 113 of the isomer represented by Formula III described on Table 1 (hereinafter the “Company Compounds”) constitute Project Results and/or Project Improvements (as such phrases are defined under the Original Agreement) and, as a result thereof, the sole and exclusive property of Company, with any and all right title and interest in and to the Company Compounds and all methods and compositions relating specifically to such Company Compounds being both subject to the terms of the Original Agreement and hereby assigned to Company.
f. The Sphaera Compounds. All other compounds envisioned, implied or subsumed within the Joint Application as isomers represented by Formula III or Formula IV, including compounds 114 thru 120 described on Table 1, are not subject to the Original Agreement, but are owned by Sphaera (hereinafter, the “Sphaera Compounds”), with any and all right, title, and interest to the Sphaera Compounds being held by Sphaera Pharma.
g. Patent Ownership and Cooperation.
i. The Mixed Patents. The Parties agree that the Mixed Patents (as defined below), including, without limitation, the Joint Applications, will be jointly owned by both Parties.
ii. Cooperation. Sphaera Pharma and Company therefore agree to cooperate in good faith to conduct prosecution of any patent applications claiming priority to the Joint Applications to obtain patents, to be solely owned by Company, that specifically claim inventions related to the Company Compounds (as represented by, for example, claim 22 of 61/709704, solely to the extent that A is a moiety selected from 101-113, and claims 25-27 and 29-36 solely to the extent they depend from claim 22 and where A is a moiety selected from 101-113), to the extent requested by Company and to the extent reasonably practicable, on a jurisdiction-by-jurisdiction basis, without negatively impacting Sphaera Pharma’s ability to protect inventions related to the Sphaera Compounds. Ownership of any other patent applications claiming priority to the Joint Applications shall be determined according to inventorship as determined under U.S. law. With respect to the Joint Applications and any patents and patent applications arising from or claiming priority to the Joint Applications and whose claims encompass both Company Compounds and Sphaera Compounds (“Mixed Patents”), the Parties agree to cooperate in good faith in prosecution, enforcement, and other activities relating to the Mixed Patents and to the commercialization of Company Compounds and Sphaera Compounds. Sphaera Pharma and Company agree to exchange materials, know-how and to act jointly to collect data useful to support the specification of the Joint Applications prior to September 15, 2013.
| Page 2 |
h. Cross Licenses.
i. By Sphaera Pharma. Sphaera Pharma grants to Company an exclusive (even as to Sphaera), worldwide, royalty-free, fully paid-up license to the Mixed Patents and any and all know how and materials controlled by Sphaera Pharma relating thereto and useful in the practice thereof to make, Develop, use, sell, offer to sell, and otherwise exploit Company Compounds and related compositions and methods for the period commencing with the Amendment Effective Date and ending on the later to occur of the expiration of the last valid claim covering Company Compounds and related compositions and methods or the 15th anniversary of the Amendment Effective Date; provided, however, that such license is subject to Section 10 of the Original Agreement.
ii. By Company. Company grants to Sphaera Pharma an exclusive (even as to Company), worldwide, royalty-free, fully paid-up license to the Mixed Patents and any and all know how and materials controlled by Company relating thereto and useful in the practice thereof to make, Develop, use, sell, offer to sell, and otherwise exploit Sphaera Compounds and related compositions and methods for the period commencing with the Amendment Effective Date and ending on the later to occur of the expiration of the last valid claim covering Company Compounds and related compositions and methods or the 15th anniversary of the Amendment Effective Date (the “Term”); provided, however, that Sphaera Pharma hereby grants to Company a right of first offer to license for the Term exclusively under the Mixed Patents and know-how and materials relating thereto and useful in the practice thereof from Sphaera Pharma the right to make, use, sell, offer to sell, and otherwise exploit any such Sphaera Compound and related compositions and methods only within the field of infectious disease, the terms of which license both parties agree to use commercially reasonable efforts to negotiate in good faith and which shall be on customary terms and conditions for licenses of similar intellectual property. To the extent Company wishes to obtain a license to the Mixed Patents to make, use, sell, or offer to sell any of the Sphaera Compounds (i.e., compounds 114-120 of Table 1, or any other compound envisioned, implied or subsumed within the applications 61/709704 in the United States and 3105/DEL/2012 in India filed on 4 October, 2012, other than the Company Compounds), such a license will require an agreement and acceptance by Sphaera Pharma; for purposes of such license, the financial terms and conditions thereof shall be negotiated by the parties, with Article 2, entitled “Consideration of Services,” of the Original Agreement having applicability to only the Company Compounds. For the purpose of clarity, except as otherwise provided in this Section (e)(ii), Sphaera Pharma may use the Sphaera Compounds outside the scope of the Original Agreement without restriction and without any further approval or agreement from Company.
| Page 3 |
i. No Overlap in Commercial Pursuits.
i. Section 10(e) of the Original Agreement provides, in pertinent part, “that Sphaera Pharma will have the right to [D]evelop the [Company Compounds] for use in the treatment of cancer in humans,” but as provided in Section 10(a) may not commercialize any of the Company Compounds until and unless Company delivers an Abandonment Notice (as defined in the Original Agreement) and otherwise complies with Section 10 thereof. Subject to the foregoing, Section 10(e) is hereby amended (but is not otherwise modified) to add as the last sentence thereof the following:
“(a) Company shall neither undertake to Develop nor Develop or, if in the process of Developing, shall cease developing any one of the Company Compound for which Company is notified in writing by Sphaera Pharma that Sphaera Pharma has filed an IND on such Company Compound for a cancer indication in humans, and (b) Sphaera Pharma shall neither undertake to Develop nor Develop or, if it in the process of Developing, shall cease Developing any Company Compound for which Sphaera Pharma is notified in writing that an IND has been filed for such Company Compound, which limitation shall remain in effect for the period during which such Development remains continuously active. As such, each of the Parties agrees to provide to the other written notice of it determining to both (y) choose as a lead candidate for Development one of the Company Compounds and, as a result thereof, (z) commence IND-enabling studies for the purpose of filing an IND, which notice shall be delivered to the other Party within ten (10) consecutive calendar days of any such determination. For purposes of this section, “Development” or “Develop” shall mean those activities relating to non-clinical and clinical research and drug development, including, without limitation, toxicology, pharmacology and other discovery efforts, test method development and stability testing, process development, formulation development, delivery system development, quality assurance and quality control development, statistical analysis, clinical studies or trials (including pre-approval studies and investigator sponsored clinical studies or trials), regulatory affairs and clinical study or trial regulatory activities (excluding, however, regulatory activities directed to obtaining pricing and reimbursement approvals and activities constituting manufacturing, marketing, sales, distribution or other commercialization); and “IND” shall mean an investigational new drug application filed with the U.S. Food and Drug Administration (“FDA”) or any equivalent filing with any governmental agency having regulatory authority similar to that of the FDA for a jurisdiction other than the U.S.”
| Page 4 |
ii. Unless and until expansion of the Original Agreement is executed between the parties, Company cannot and will not pursue any development of the Sphaera Compounds 114-120 of Table 1.
2. Binding and Enforceable Agreement; Entirety of Agreement. The terms of this Agreement shall be binding upon, and shall inure to the benefit of each of the Parties hereto and their respective successors, heirs and assigns. This Agreement shall be considered an integral part of the Original Agreement and shall be binding upon each Party from the date first above written. Subject only to the modifications referred to in this Agreement, the Original Agreement shall remain in full force and effect and where necessary shall be read and construed and be enforceable as if the terms of this Agreement were inserted therein.
3. Fulfillment of Original Agreement.
a. Each of the Parties hereby acknowledges that the Services as contemplated under the Original Agreement have been completed and as of the Amendment Effective Date are terminated; except, however, that Company remains obligated to fulfill its financial obligations agreed to under Section 2 of the Original Agreement. More specifically, as of the date hereof, the Project Fixed Fee (as defined under the Original Agreement) equals in total the amount of $160,000 for the synthesis and analysis of Company Compounds remain due and payable by Company. Each Party hereby acknowledges that the Project Variable Fees have been paid in full.
b. Failure by Company to pay the Project Fixed Fees by the first anniversary of the Amendment Effective Date may constitute grounds for termination of the Original Agreement in accordance with Article 12 thereof, but shall not otherwise affect the rights of the Parties as described thereunder or under this Agreement.
4. Counterparts. This Agreement may be executed in several counterparts, each of which shall be deemed an original, but together they shall constitute one and the same instrument.
[Signatures continued on next page.]
| Page 5 |
IN WITNESS WHEREOF, the parties have caused this Agreement to be executed by their duly authorized representatives.
| Sphaera Singapore | Inhibikase Therapeutics, Inc. | |
| Sphaera Pharma Pte. Ltd. | ||
| /s/ Xxxxxxx Xxxxx | /s/ Xxxxxx X. Xxxxxx | |
| Signature | Signature | |
| Xxxxxxx Xxxxx, PhD | Xxxxxx X. Xxxxxx, PhD | |
| Printed Name | Printed Name | |
| President & CEO | President & CEO | |
| Title | Title | |
| April 12, 2013 | 04/05/2013 | |
| Date | Date | |
| Sphaera India | Read and acknowledged by | |
| Sphaera Pharma Pvt. Ltd. | Project Coordinator, Sphaera Pharma: | |
| /s/ Xxxxxxx Xxxxxxx | /s/ Xxxxx X. Xxxxxxxxx | |
| Signature | Signature | |
| Xxxxxxx Xxxxxxx | Xxxxx X. Xxxxxxxxx, PhD | |
| Printed Name | Printed Name | |
| Assoc. Dir - Corporate | ||
| Affairs & Business Dev. | Vice President | |
| Title | Title | |
| April 11, 2013 | 4/6/2013 | |
| Date | Date |
| Page 6 |

