EXCLUSIVE LICENSE AGREEMENT between Monogram Orthopedics and Icahn School of Medicine at Mount Sinai EXECUTION COPY
Exhibit 10.5
BLU-0122
between
Monogram Orthopedics
and
Icahn School of Medicine at Mount Sinai
EXECUTION COPY
The submission of this draft for review or negotiation, or the negotiation of the transaction described herein, does not constitute an offer and the execution of this agreement by the Icahn School of Medicine at Mount Sinai does not constitute a binding contract until such time as it has been executed by authorized officers of the Icahn School of Medicine at Mount Sinai and Monogram Orthopedics.
TABLE OF CONTENTS
| | Page | |
| | | |
1. | DEFINITIONS | 1 | |
| | | |
2. | LICENSE GRANT | 10 | |
| | | |
3. | DUE DILIGENCE | 13 | |
| | | |
4. | FEES, ROYALTIES, MILESTONES, AND PAYMENTS | 14 | |
| | | |
5. | REPORTS AND PAYMENTS | 17 | |
| | | |
6. | EQUITY OWNERSHIP | 20 | |
| | | |
7. | CONFIDENTIALITY; PUBLICITY; USE OF NAME | 21 | |
| | | |
8. | PATENT PROSECUTION AND REIMBURSEMENT | 23 | |
| | | |
9. | INFRINGEMENT | 24 | |
| | | |
10. | REPRESENTATIONS; DISCLAIMER OF WARRANTIES; LIMITATION OF LIABILITIES | 25 | |
| | | |
11. | INDEMNIFICATION | 27 | |
| | | |
12. | INSURANCE | 28 | |
| | | |
13. | TERM AND TERMINATION | 29 | |
| | | |
14. | EFFECT OF TERMINATION | 30 | |
| | | |
15. | ADDITIONAL PROVISIONS | 31 | |
Exhibit A: | Licensed Patents |
Exhibit B: | Software |
Exhibit C: | Initial Company Business/Development Plan |
Exhibit D: | Form of Quarterly Royalty and Sublicense Income Report |
Exhibit E: | Client and Billing Agreement |
Exhibit F: | Capitalization Table for Monogram Orthopedics |
This Exclusive License Agreement (this “Agreement”) is by and between Icahn School of Medicine at Mount Sinai, a New York not-for-profit education corporation, with a principal place of business at Xxx Xxxxxxx X. Xxxx Xxxxx, Xxx Xxxx, XX 00000 (“Mount Sinai”, also referred to herein as “Licensor”) and Monogram Orthopedics, a Delaware corporation, with a principal place of business at New Lab, Studio 000, 00 Xxxxxx Xxxxxx, Xxxxxxxx, XX 00000 (referred to herein as “Monogram” or “Licensee”). This Agreement will become effective on October 3, 2017, (the “Effective Date”).
WHEREAS, Licensor has created and owns certain intellectual property relating to customizable bone implants;
WHEREAS, Licensee wishes to obtain from Licensor certain rights to such intellectual property and to Develop and Commercialize Licensed Products (as defined below); and
WHEREAS, Licensor has determined that the exploitation of the intellectual property by Licensee subject to the terms and conditions of this Agreement is in the best interest of Licensor, consistent with Licensor’s educational, research, and public health missions and goals.
NOW THEREFORE, in consideration of the mutual rights and obligations contained in this Agreement, and intending to be legally bound, Licensor and Licensee (individually, a “Party”, and together, the “Parties”) agree as follows:
1.DEFINITIONS
1.1. “Affiliate” means any Entity that is controlled by Licensee. For purposes of this definition, “control” and its various forms means the possession of the power to direct or cause the direction of the management and policies of such Entity, whether through ownership of voting securities, by contract or otherwise. Licensee will be deemed to control another Entity if the Licensee owns or controls more than fifty percent (50%) of the voting stock or other securities of the Entity.
1.2. “Business Day” means a day other than Saturday, Sunday, or any day indicated by 5 USC Section 6103 to be a legal public holiday. As of the Effective Date such legal public holidays are: 1st day in January (New Year’s Day), the 3rd Monday in January (Xxxxxx Xxxxxx Xxxx Xx. Day); the 3rd Monday in February (Washington’s Birthday); the last Monday in May (Memorial Day); July 4th (Independence Day); the first Monday in September (Labor Day); the 2nd Monday in October (Columbus Day); November 11th (Veterans Day); the 4th Thursday in November (Thanksgiving Day); and December 25th (Christmas Day).
1.3. “Calendar Year” means January 1 through December 31 of a given year.
1.4. “Commercial Sale” means any bona fide transaction with a Third Party for which consideration is received for the sale, use, lease, transfer or other disposition of a Licensed Product by or on behalf of Licensee, its Affiliate or Sublicensee, and a Commercial Sale is deemed completed at the time that Licensee, its Affiliate or Sublicensee invoices, ships, or receives payment for a Licensed Product, whichever occurs first.
1
1.5. “Commercialization” means any and all activities related to the manufacturing, promotion, distribution, marketing, offering for sale and selling of or otherwise granting rights to a product, including advertising, educating, planning, obtaining, supporting and maintaining pricing and reimbursement approvals and Regulatory Authorizations, managing and responding to adverse events involving the product, pricing, price reporting, marketing, promoting, detailing, storing, handling, shipping, distributing, importing, exporting, using, offering for sale, or selling a product anywhere in the world. Commercialization excludes Development activities. When used as a verb, “Commercialize” means to engage in Commercialization.
1.6. “Commercially Reasonable Efforts” means, with respect to a Licensed Product, those efforts utilizing those resources that would be employed by the Licensee, or a company of similar size and resources, for the development of a product or compound of similar market potential at a similar stage in its development or product life of such Licensed Product taking into account, without limitation, issues of safety and efficacy, product profile, intellectual property situation, regulatory environment and other relevant scientific and commercial factors. At a minimum, Commercially Reasonable Efforts shall be based upon the Development Plan to be submitted to Licensor by Licensee as shall be required hereunder. In determining Commercially Reasonable Efforts with respect to a particular Licensed Product, Licensee may not reduce such efforts due to the competitive, regulatory or other impact of any other product or method that it owns, licenses or is developing or commercializing.
1.7. “Confidential Information” shall have the meaning assigned in Section 7.1.
1.8. “Control” or “Controlled” shall mean, with respect to any Patent, other intellectual property right or other intangible property, an Entity’s ownership or the possession (whether by ownership, license or “control” (as defined in the definition of “Affiliate” above) over an Affiliate having possession by ownership or license) of the ability to grant access to, or a license or sublicense to, such Patent, rights or property.
1.9. “Derivative Work” means any work created by or for Licensee that qualifies as a “derivative work” of the Software under the United States Copyright Act of 1976, as amended, as interpreted by U.S. courts sitting in the Second Circuit, specifically including, but not limited to, translations, abridgments, condensations, recastings, transformations, or adaptations of the Software, or works comprising editorial revisions, annotations, elaborations, or other modifications of the Software. Notwithstanding the foregoing, the term Derivative Work shall not include any derivative works that are developed by or for Licensor.
1.10. “Development” means any and all activities related to researching or developing a product or process or service, including preclinical and clinical research, testing and development activities relating to the discovery and/or development of device, product or process candidates and submission of information and applications to a Regulatory Authority, including toxicology, pharmacology, and other discovery, optimization, and preclinical efforts, test method development and stability testing, manufacturing process development, formulation development, upscaling, validation, delivery system development, quality assurance and quality control development, statistical analysis, managing and responding to adverse events involving a product, any clinical studies (including pre and post Regulatory Approval studies), and activities relating to obtaining Regulatory Approvals, but excluding Commercialization activities. When used as a verb, “Develop” means to engage in Development.
2
1.11. “Development Plan” means the then-current version of the plan for the Exploitation by Licensee of the Licensed Patents, Software, and Know-How attached hereto as Exhibit C, as such plan may be adjusted or updated from time to time e.g. as contemplated by Section 3.1. For clarity no updated Development Plan will be effective until agreed to by both Parties.
1.12. “EMA” means the European Medicines Agency or any successor Entities thereto.
1.13. “End User” means a Third Party that is granted rights by Licensee or a Sublicensee pursuant to this Agreement to use, reproduce, perform or display the Licensed Product pursuant to an end user license agreement, without any right to sublicense or distribute the Licensed Product. For avoidance of doubt, End Users are distinct from Sublicensees.
1.14. “Entity” means a corporation, an association, a joint venture, a partnership, a trust, a business, an institution, an individual, a government or political subdivision thereof, including an agency, or any other organization that can exercise independent legal standing.
1.15. “Exploit” means, collectively, to Develop, have Developed, Commercialize, have Commercialized, Manufacture, have Manufactured, Research, use, rent, have rented, lease, have leased, sublicense, including to Manufacture, to have Developed, to have Manufactured, to have Commercialized, and otherwise to commercially exploit. “Exploitation” has a correlative meaning.
1.16. “Fair Market Value” means (a) in the case of arm’s length sale of a Licensed Product, (i) the cash consideration that Licensee, its Affiliate, or Sublicensee has realized from such sale, or (ii) if there have been no such sales or such sales have been insufficient, the cash consideration that Licensee, its Affiliate, or Sublicensee would have realized from an unaffiliated, unrelated buyer in an arm’s length sale of Licensed Product in the same quantity, under the same terms, and at the same time and place as the sale for which Fair Market Value is being determined; (b) in the case of non-cash consideration received in a sale of a Licensed Product or in a transaction giving rise to Sublicense Income, the cash value of such consideration; or (c) in the case of determining the portion of proceeds from an issuance of equity to be included in Sublicense Income, the value of the issued equity as then most recently determined under U.S. Internal Revenue Code § 409A for purposes of the Licensee’s equity grants (or, if the class of equity issued has not then been so valued, then a value based on the value of a class of equity that has been so valued, taking into account differences between the rights and preferences of the class of equity issued and those of the class of equity then most recently valued).
1.17. “FDA” means the United States Food and Drug Administration or any successor Entities thereto.
3
1.18. “Field of Use” means the design, fabrication, and/or implantation of orthopedic implants, along with the robotic design, and/or robotic preparation of the surgical site for such implants.
1.19. “First Commercial Sale” means, on a Jurisdiction-by-Jurisdiction basis, the first time a Commercial Sale is made.
1.20. “Good Clinical Practices” means the then-current standards, practices and procedures for good clinical practices in the conduct of clinical trials, including adequate human subject protections, as promulgated or endorsed by the FDA and other applicable Governmental Authorities, such as set forth in, “International Conference on Harmonization - Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” or as otherwise required by applicable Law.
1.21. “Good Laboratory Practices” means the then-current standards, practices and procedures for good laboratory practices by facilities that perform non-clinical (including pre-clinical) laboratory studies, as promulgated or endorsed by the FDA and other applicable Governmental Authorities, including as set forth in 21 C.F.R. Part 58, or as otherwise required by applicable Law.
1.22. “Good Manufacturing Practices” means the then-current standards, practices and procedures for the manufacture of drugs or medical devices, as applicable to the Licensed Products (including the practices of and methods to be used in, and the facilities or controls to be used for, the manufacture, processing, packaging, sterilizing, labeling, testing or holding of the Licensed Products), as promulgated or endorsed by the FDA and other applicable Governmental Authorities, including, as applicable, as set forth in 21 C.F.R. Parts 210, 211, and 820, or as otherwise required by applicable Law.
1.23. “Governmental Authority” means any supranational, national, federal, state, provincial, local or foreign Entity of any nature exercising executive, legislative, judicial, regulatory or administrative functions of or pertaining to government, including any governmental authority, agency, department, board, commission, court, tribunal, judicial body or instrumentality of any union of nations, federation, nation, state, municipality, county, locality or other political subdivision thereof.
1.24. “Gross Sales” means the greater of the gross invoice or contract price charged to a Third Party by Licensee, its Affiliates, or Sublicensees, as applicable, for Commercial Sales, prior to any discounts or other list price reductions granted. A Licensed Product shall be considered sold for purposes of calculating Gross Sales when it is shipped, invoiced or paid for, whichever occurs earlier. In the event Licensee, its Affiliate, or Sublicensee transfers a Licensed Product to a Third Party in a bona fide arm’s length transaction, for any consideration other than cash, then the Gross Sales price for such Licensed Product shall be deemed to be the standard invoice price then being invoiced by Licensee, its Affiliate, or Sublicensee, as applicable, in an arm’s length transaction with similar companies. In the absence of such standard invoice price, then the Gross Sales price shall be the Fair Market Value of the Licensed Product.
4
1.25. “Health Care Law” means all applicable Laws relating in any way to patient care and human health and safety, including such Laws pertaining to: (a) the Development, Manufacture and Commercialization of drugs and medical devices, including, without limitation, the United States Food, Drug and Cosmetic Act, the Public Health Service Act, the regulations promulgated thereunder (including with respect to Good Clinical Practices, Good Laboratory Practices and Good Manufacturing Practices), and equivalent applicable Laws of other Governmental Authorities; and (b) the reimbursement and payment for health care products and services, including any United States federal health care program (as such term is defined in 42 U.S.C. § 1320a-7b(f)), and programs and arrangements pertaining to providers of health care products or services that are paid for by any Governmental Authority or other Entity, including the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)), the civil False Claims Act (31 U.S.C. § 3729 et seq.), the administrative False Claims Law (42 U.S.C. § 1320a-7b(a)), 42 U.S.C. § 1320a-7 and 42 U.S.C. § 1320a-7a, and the regulations promulgated pursuant to such statutes, Medicare (Title XVIII of the Social Security Act) and the regulations promulgated thereunder, Medicaid (Title XIX of the Social Security Act) and the regulations promulgated thereunder, and equivalent applicable Laws of other Governmental Authorities; and (c) the privacy and security of patient-identifying information, including, without limitation, the Health Insurance Portability and Accountability Act of 1996 (42 U.S.C. § 1320d et seq.) and the regulations promulgated thereunder and equivalent applicable Laws of other Governmental Authorities; in each of the foregoing (a) through (c), as may be amended from time to time.
1.26. “Indication” means a separate and distinct disease, disorder, syndrome or other medical condition in humans that a Licensed Product is marketed to treat, prevent, diagnose, monitor or ameliorate, for which any prior Regulatory Approval is insufficient for such marketing (i.e., which required a new Regulatory Approval for such marketing).
1.27. “Infringement Action” means any threatened, pending, or ongoing action, claim, litigation, or proceeding (other than oppositions, cancellations, interferences, reissue proceedings, or reexaminations), respecting any Licensed Patent, whether initiated by or against a Party, its Affiliate or Sublicensee.
1.28. “Initial Company Business/Development Plan” means the initial Company business/development plan for the Exploitation by Licensee of the Licensed Patents, Software, and Know-How, attached hereto and incorporated herein as Exhibit C, which is hereby incorporated into and made part of this Agreement.
1.29. “Interruption Royalty” means the amount that would have been payable as an earned royalty if Gross Sales during the Substantial Interruption were the greater of (a) the Gross Sales that were forecasted with respect to such period in the ordinary course of Licensee’s business prior to commencement of the Substantial Interruption Event, or (b) the average Gross Sales of Licensed Product in the two (2) Quarters with the highest Gross Sales in the eight (8) Quarters preceding such Substantial Interruption.
1.30. “Jurisdiction” means a geographic area (e.g. country or region) in which patent or other exclusive intellectual property rights or market exclusivity exist.
5
1.31. “Know-How” means any and all technical, scientific, and other information, information architecture, drawings, diagrams, processes, knowledge, methods, processes, practices, formulae, assays, instructions, skills, techniques, procedures, technical assistance, designs, drawings, specifications, related to Software, the Licensed Patents, or Licensed Products in all cases, whether or not confidential, developed in the Principal Investigators’ laboratory, or by the Principal Investigator(s), prior to the Effective Date.
1.32. “Laws” means all active governmental constitutions, laws, statutes, ordinances, treaties, rules, common laws, rulings, regulations, orders, charges, directives, determinations, executive orders, writs, judgments, injunctions, decrees, restrictions or similar legally effective pronouncements of any Governmental Authority.
1.33. “Licensed Patents” means the Patents owned or Controlled by Licensor prior to the Effective Date and listed in Exhibit A hereto, which is hereby incorporated into and made part of this Agreement. Notwithstanding the preceding definition, Licensed Patents shall not include any Patent based on research conducted after the Effective Date, except as otherwise agreed in a separate legally enforceable writing executed by the Parties.
1.34. “Licensed Product” will mean any product or service, including but not limited to hardware or software, Exploited by Licensee, any Sublicensee, or any of their respective Affiliates or agents as permitted hereunder (a) the Development, Manufacturing, Commercialization, use, rental or lease of which would, in the absence of the licenses granted to Licensee hereunder, infringe at least one Valid Claim or (b) that is based on, derived from, incorporates, arises from or otherwise makes use of the Software, any Derivative Work, any Licensee Modified Product, or any Know-How, whether in whole or in part.
1.35. “Licensed Product Data” means data (including clinical data) that is possessed, owned or Controlled by Licensee, its Affiliate, or Sublicensee directly relating to any Licensed Product and generated after the Effective Date.
1.36. “Licensee Modified Product” means computer software created by or for Licensee that is not a Derivative Work, but that incorporates, is based on, interoperates with, or shares functionality with the Software or any Derivative Work, in whole or in part.
1.37. “Manufacturing” means all activities directed to sourcing of necessary raw materials, producing, processing, packaging, labeling, quality assurance testing, release of a Licensed Product or Licensed Product candidate, whether for Development or Commercialization. When used as a verb, “Manufacture” means to engage in Manufacturing.
1.38. “Net Sales” means all Gross Sales of Licensed Product less the total of the following deductions to the extent they are included in the gross invoiced sale price of the Licensed Product or otherwise directly paid or incurred by Licensee, its Affiliates or its Sublicensees with respect to such sale of the Licensed Product:
(a) trade, cash and/or quantity discounts, retroactive price reductions, chargeback payments and rebates actually allowed to and/or taken by purchasers of a Licensed Product or Third Party payors, including discounts and rebates to governmental payors or managed care organizations, their agencies, purchasers and reimbursers, and allowances or credits to Third Parties for rejections or returns that do not exceed the original invoice amount;
6
(b) taxes, tariffs, duties and governmental charges applicable to the sale, transportation or delivery of Licensed Product that Licensee, its Affiliates, or Sublicensees have to pay on such sales, transportation or delivery of Licensed Product (including annual fees due under Section 9008 of the United States Patient Protection and Affordable Care Act of 2010 (Pub. L. No. 111-48));
(c) outbound transportation and insurance charges prepaid or allowed, but not separately reimbursed by the purchaser; and
(d) invoiced amounts written off as uncollectible not to exceed two percent (2%) of Gross Sales.
In no event will the above deductions in clauses (b), (c), and (d) of this Section in aggregate exceed 12% of Gross Sales.
Sales or other transfers of Licensed Products between Licensee and its Affiliates or Sublicensees shall be excluded from the computation of Net Sales (and therefore no payments will be payable to Licensor on such sales or transfers) except where such Affiliates or Sublicensees are end users or consumers of Licensed Products in which event, notwithstanding anything herein to the contrary, Licensed Product transfers to such Affiliates and Sublicensees shall be included in Net Sales. For avoidance of doubt, the sale of Licensed Product by Affiliates or Sublicensees to Third Parties shall be considered as part of Net Sales. In the event Licensee, its Affiliate, or Sublicensee transfers a Licensed Product to a Third Party in a bona fide arm’s length transaction, for any consideration other than cash, then the Net Sales price for such Licensed Product shall be deemed to be the standard invoice price then being invoiced by Licensee, its Affiliate, or Sublicensee, as applicable, in an arm’s length transaction with similar companies. In the absence of such standard invoice price, then the Net Sales price shall be the Fair Market Value of the Licensed Product. Components of Net Sales shall be determined in the ordinary course of business using the accrual method of accounting in accordance with generally accepted accounting principles, consistently applied.
No deductions shall be made from Net Sales for commissions paid to individuals whether they are (i) with independent sales agents or agencies or (ii) regularly employed by Licensee, its Affiliates, or Sublicensees on its or their payroll, or (iii) for the cost of collections.
For the avoidance of doubt, disposal of any Licensed Product without charge for use in any clinical trials, as free samples, or under compassionate use, patient assistance, named patient or test marketing programs or non-registrational studies or other similar programs or studies where Licensed Product is supplied or delivered without charge, shall not result in any Net Sales. No Licensed Product donated by Licensee, its Affiliate, or Sublicensee to non-profit institutions or government agencies for a non-commercial purpose shall result in any Net Sales.
If Licensee, its Affiliate, or Sublicensee sells, leases or otherwise Commercializes any Licensed Product at a reduced fee or price for the purpose of promoting other products, goods or services or for the purpose of facilitating the sale, license or lease of other products, goods or services, then notwithstanding anything herein to the contrary, Licensor shall be entitled to payments under Article 4 based upon the Fair Market Value of the Licensed Product.
7
1.39. “Patents” means (i) the United States and foreign patents and/or patent applications; (ii) any and all patents issuing from the foregoing; (iii) any and all claims of continuation-in-part applications that claim priority to the United States patent applications, but only where such claims are directed to inventions disclosed in the manner provided in the first paragraph of 35 U.S.C. § 112 in such United States patent applications, and such claims in any patents issuing from such continuation-in-part applications; (iv) any and all foreign patent applications, foreign patents, or related foreign patent documents that claim priority to the patents and/or patent applications; and (v) any and all divisionals, continuations, reissues, re-examinations, renewals, substitutions, and extensions of the foregoing.
1.40. “Principal Investigator(s)” means Douglas Unis, Xxxxxxx Xxxxx and Xxxxxxxx Xxxxxx.
1.41. “Prosecution” means the filing, preparation, prosecution (including any interferences, reissue proceedings, reexaminations, and oppositions), extension, term adjustment, and maintenance of Licensed Patents. When used as a verb, “Prosecute” means to engage in Prosecution.
1.42. “Quarter” means each three-month period beginning on January 1, April 1, July 1 and October 1 of each Calendar Year; provided, however, that as it relates to the Commercial Sale of Licensed Products, the first Quarter shall be comprised of the time period beginning on the date of First Commercial Sale and ending at the end of the Quarter during which such First Commercial Sale occurs. “Quarterly” means once during each Quarter.
1.43. “Quarterly Reports” shall have the meaning assigned in Section 5.2.
1.44. “Regulatory Approval” means all approvals from the relevant Regulatory Authorities necessary to market a Licensed Product in a Jurisdiction (not including any applicable pricing and governmental reimbursement approvals unless legally required to market the Licensed Product in a Jurisdiction).
1.45. “Regulatory Authority” means any applicable Governmental Authority involved in granting Regulatory Approval for, and responsible for the regulation of, the Licensed Product in any Jurisdiction, including the FDA and any corresponding Governmental Authority.
1.46. “Royalty Term” means, on a Licensed Product-by-Licensed Product and Jurisdiction-by-Jurisdiction basis, the period from the First Commercial Sale of such Licensed Product in such Jurisdiction until the later of: (a) expiration of the last Valid Claim of a Licensed Patent covering such Licensed Product in such Jurisdiction or (b) twelve (12) years from First Commercial Sale of such Licensed Product in such Jurisdiction.
8
1.47. “Significant Transaction” means the first to occur of a single transaction, or series of related transactions, consisting of or resulting in any of the following: (i) an assignment of the License; (ii) an exclusive worldwide sublicense of all or substantially all of the Mount Sinai Patent Rights; (iii) an initial public offering of securities by Company (or its successor) or other transaction resulting in either (A) Company becoming a public company or (B) any of Company's securities being traded on a nationally recognized stock exchange or automated quotation system; (iv) a sale, license or other disposition of all or substantially all of Company's assets; or (v) a reorganization, consolidation or merger of Company, or sale or transfer of the securities of Company, where the holders of Company's outstanding voting securities before the transaction beneficially own less than fifty percent (50%) of the outstanding voting securities, or hold less than fifty percent (50%) of the voting power of the voting security holders of the surviving entity after the transaction. Notwithstanding anything above to the contrary, a Significant Transaction shall not be deemed to occur as a result of a bona fide, arms-length equity financing for cash in which Company issues securities representing more than fifty percent (50%) of the voting power of its security holders to venture capital or other similar or strategic professional investors who do not actively manage day-to-day operations of Company.
1.48. “Software” means that certain software, software code, and software documentation developed in Principal Investigators’ laboratory prior to the Effective Date (and all copyright protections therein) that is listed in Exhibit B.
1.49. “Sublicense Income” means consideration Licensee receives, directly or indirectly, from any Sublicensee or other Third Party in consideration of a Sublicense or otherwise in consideration of any of the rights granted to Licensee under this Agreement (including any option or contingent right to obtain a sublicense or other right), that is not an earned royalty a portion of which will be payable to Licensor as provided in Section 4.3, including but not limited to any fixed fee, option fee, license fee, maintenance fee, milestone payment, unearned portion of any minimum royalty payment, equity, joint marketing fee, intellectual property cross license, settlement agreement, research and development funding in excess of Licensee’s cost of performing such research and development, and any other property, consideration or thing of value given or exchanged for a sublicense or otherwise in consideration of any of the rights granted to Licensee under this Agreement, regardless of how Licensee and Sublicensee characterize such payments or consideration.
1.50. “Sublicensee” means any Entity, other than an End User, that enters into an agreement or arrangement with Licensee, or receives from Licensee a license grant or option for license grant under any of the rights granted to Licensee by Licensor hereunder (such agreement, arrangement, or license herein referred to as a “Sublicense”).
1.51. “Substantial Interruption Event” means (a) the applicable Licensed Product has previously had its First Commercial Sale in the relevant Jurisdiction; (b) Gross Sales of Licensed Product in any given Quarter are at least fifty percent (50%) less than the average Gross Sales of Licensed Product in the two (2) Quarters with the highest Gross Sales in the eight (8) Quarters preceding such interruption, which reduced (or, as applicable, ceased) Gross Sales persist or are anticipated to persist for a period of at least three (3) months (“Substantial Interruption”); (c) such Substantial Interruption is caused by the negligence of Licensee, its Affiliates, or Sublicensees in the Manufacturing, Commercialization, distribution or sale of Licensed Product; and (d) the control or prevention of the Substantial Interruption was in the reasonable control of Licensee, its Affiliates, or Sublicensees.
9
1.52. “Term” means the term of this Agreement which will commence on the Effective Date and expire upon the expiration of the last Royalty Term for the last Licensed Product, unless terminated earlier pursuant to Article 13.
1.53. “Territory” means worldwide.
1.54. “Third Party” means any Entity other than a Party or its Affiliates.
1.55. “Valid Claim” means (a) an unexpired claim of an issued Patent within the Licensed Patents that has not been ruled unpatentable, invalid or unenforceable by a final and unappealable decision of a court or other competent authority in the subject Jurisdiction; or (b) a pending claim of a Patent application within the Licensed Patents.
2.LICENSE GRANT
2.1. Exclusive License to Licensee. Subject to the terms and conditions set forth herein, Licensor hereby grants to Licensee a royalty-bearing exclusive, sublicensable, license under the Licensed Patents to Exploit any Licensed Product in the Field of Use, during the Term and throughout the Territory.
2.2. Non-Exclusive Know-How License to Licensee. Subject to the terms and conditions set forth herein, Licensor hereby grants to Licensee a royalty-bearing non-exclusive, sublicensable license to use the Know-How, solely to the extent Licensor agrees necessary for Exploitation of any Licensed Product in the Field of Use, during the Term and throughout the Territory.
2.3. Software License. Subject to the terms and conditions set forth herein, Licensor hereby grants to Licensee a royalty-bearing, exclusive license, under Licensor’s copyright in the Software (i) to create Derivative Works in the Field of Use, and (ii) to use, reproduce, market, distribute, publicly display, and publicly perform the Software and the Derivative Works for the sole purpose of Developing and Commercializing Licensed Products in the Field of Use during the Term and throughout the Territory, including by way of End User licenses. The license in Section 2.3(ii) above includes the right to grant limited Sublicenses, but only in accordance with the terms set forth in this Agreement. For the avoidance of doubt, the right to sublicense shall not include the right for Licensee to grant Sublicensees the right to create derivative works of either the Software or Licensee’s Derivative Works, and any Sublicense must expressly prohibit the creation of such derivative works by Sublicensee.
2.4. Grant-Back Software License. Subject to the terms and conditions set forth herein, Licensee hereby grants to Licensor a perpetual royalty-free, irrevocable license, for Licensor and its sublicensees to create derivative works of the Derivative Works of Licensee, and to use, reproduce, publicly display, and publicly perform the Derivative Works and Licensor’s derivative works thereof. This license shall be and is hereby, exclusive, fully paid-up, perpetual, fully sublicenseable and limited to (a) commercial activities outside the Field of Use and (b) teaching, patient care, and non-commercial academic research purposes within the Field of Use (including publication of any such research results).
10
2.5. Sublicensing. Subject to the terms and conditions set forth herein, Licensor hereby grants to Licensee the right to grant Sublicenses, provided that:
(a)Any and all such Sublicenses shall:
(i) expressly identify Licensor as a third party beneficiary
(ii) obligate the Sublicensee to abide by and be subject to all of the terms, conditions, and limitations of this Agreement applicable to the Licensee;
(iii) expressly prohibit the Sublicensee from granting further sublicenses and declare any such purported grant of a further sublicense to be invalid and unenforceable;
(iv) prohibit Sublicensee from making payments in exchange for receipt of Sublicense rights, e.g. royalty payments, into an escrow or similar account or to any Third Party;
(v) cause the Sublicensee to comply with the provisions of Sections 2.1 and 2.2 to the same extent as Licensee is required to comply and include a provision providing for the termination of the Sublicense, upon written request by Xxxxxxxx, in the event that the Sublicensee does not so comply;
(vi) provide that, in the event of any inconsistency between the Sublicense and this Agreement, this Agreement shall control;
(vii) obligate the Sublicensee to submit annual, Quarterly, and interim reports to Licensor consistent with the reporting provisions of Article 5 and all other relevant provisions herein;
(viii) be written in the English language (for clarity, this is a reference to the original Sublicense as executed; provision of a translation to Licensor shall not satisfy this requirement); and
(ix) specify that New York law shall control any dispute arising under such sublicense, and that jurisdiction for resolving any such dispute shall New York City, New York State.
(x) Each Sublicense granted by Licensee under this Agreement shall provide for its termination upon termination of this Agreement. Each Sublicense shall automatically terminate upon any termination of this Agreement unless Licensee previously has assigned its rights under the Sublicense to Licensor and Licensor has expressly agreed in writing, in Licensor’s sole discretion, to accept such assignment.
11
(b) If Licensee enters into any agreement, arrangement, or license purporting to grant rights to any Licensed Patents, Software or Know-How, that does not comport with the requirements of Section 2.5, or is otherwise inconsistent with the terms and conditions of this Agreement, such agreement, arrangement, or license shall be null and void. Licensee acknowledges and agrees that entering into such an agreement, arrangement, or license constitutes a material breach of this Agreement.
(c) Licensee shall notify Licensor in writing of any proposed grant of a Sublicense and provide to Licensor a copy of any proposed Sublicense at least twenty (20) Business Days prior to execution thereof for review and comment by Licensor, which comments Licensee shall incorporate therein.
(d) Licensee hereby agrees to remain fully liable under this Agreement to Licensor for the performance or non-performance under this Agreement and the relevant Sublicense by any party to those agreements. Licensee shall enforce all such Sublicenses against its Sublicensees, ensuring its Sublicensees’ performance in accordance with the terms of this Agreement and the relevant Sublicense. No such Sublicense or attempt to obtain a Sublicense shall relieve Licensee of its obligations hereunder to exercise its Commercially Reasonable Efforts, directly or through a Sublicensee, to Develop and Commercialize Licensed Products, nor relieve Licensee of its obligations to pay Licensor any and all license fees, royalties and other payments due under the Agreement.
2.6. Retained Rights. The grants provided hereunder are subject to and contingent upon Licensee’s compliance with all of its obligations hereunder including, but not limited to, the payment by Licensee to Licensor of all consideration required under this Agreement, and further subject to rights retained by Licensor to: (a) practice the Licensed Patents, and permit other Entities to practice the Licensed Patents, outside of the Field of Use for any purpose; (b) practice the Licensed Patents, and permit other non-commercial Entities to practice the Licensed Patents, within the Field of Use for teaching, non-commercial academic research (including publication of any such research results), and patient care purposes; and (c) create derivative works of the Software, and to use, reproduce, publicly display, and publicly perform the Software and Licensor’s derivative works thereof subject to Section 2.4 (Grant-Back Software License). For clarity, industry sponsored research shall be considered non-commercial academic research for the purposes of this Article 2.
2.7. Government Rights. All rights and licenses granted by Licensor to Licensee under this Agreement are subject to (a) any limitations imposed by the terms of any grant, contract or cooperative agreement by any Governmental Authority applicable to the technology that is the subject of this Agreement, and (b) applicable requirements of 35 U.S.C. § 200 et seq., as amended, and implementing regulations and policies. Without limitation of the foregoing, Licensee agrees that, to the extent required under 35 U.S.C. § 204, any Licensed Product used, sold, distributed, rented or leased by Licensee, its Affiliates, or Sublicensees in the United States will be Manufactured substantially in the United States. In addition, Licensee agrees that, to the extent required by Law including under 35 U.S.C. § 202(c)(4), the United States government is granted a nonexclusive, nontransferable, irrevocable, paid-up license to practice or have practiced for or on behalf of the United States any Licensed Patent throughout the world.
12
2.8. No Implied Licenses. Except as expressly provided under this Article 2, no right or license is granted under this Agreement (expressly or by implication or estoppel) by Licensor to Licensee, its Affiliates, or Sublicensees under any tangible or intellectual property, materials, Patent, Patent application, trademark, software, know-how copyright, technical information, data, or other proprietary right.
3.DUE DILIGENCE
3.1. Development Plan. The Initial Company Business/Development Plan as attached hereto has been agreed upon by the Parties. With respect to each Calendar Year following the Effective Date, Licensee shall deliver to Licensor an annual updated Development Plan in accordance with Section 5.4, which shall set forth in reasonable detail the planned Development activities for such Calendar Year and the subsequent Calendar Year, as well as the anticipated timeline and budget for such activities. Such updated Development Plan shall replace the prior Development Plan and become incorporated into and a part of this Agreement only upon both Parties signing such updated Development Plan.
3.2. Commercially Reasonable Efforts. Throughout the Term and at Licensee’s sole cost and expense, Licensee shall use no less than Commercially Reasonable Efforts to Develop and Commercialize the Licensed Products in the Field and Territory as soon as reasonably practicable. Licensee shall maintain such active diligent Commercially Reasonable Efforts to Develop and Commercialize the Licensed Products at all times throughout the Term.
3.3. Due Diligence Events. In addition, Licensee shall perform at least the following obligations as part of its Commercially Reasonable Efforts to Develop and Commercialize the Licensed Products required under this Article 3:
(a) Perform the activities set forth in the applicable Development Plan/Business Plan as first set out in Exhibit C.
(b) Within four (4) years from the Effective Date, Licensee will have a First Commercial Sale.
3.4 Failure to Achieve Due Diligence Events. If Licensee fails to exercise Commercially Reasonable Efforts to achieve the above due diligence obligation or, if despite consistent use of Commercially Reasonable Efforts, Licensee is unable to achieve the due diligence events set forth in Section 3.3 above, then Licensor at its option, in its sole discretion, may: (a) terminate this License in whole or in part immediately upon provision of written notice to Licensee; (b) convert the License in whole or in part to non-exclusive license status immediately upon providing notice to such effect to Licensee (in such event no amendment or further writing will be required to convert the License to non-exclusive status); or (c) meet with License to arrange for revision of the due diligence events. It is agreed and understood that in the event Licensee fails to achieve the due diligence events set forth in Section 3.3 above and has not consistently used Commercially Reasonable Efforts to do so, then Licensor may exercise any and all remedies available at law or otherwise. To exercise its rights under this Section 3.4, Licensor shall provide Licensee with written notice of breach of Section 3.3. Licensee thereafter has ninety (90) days to cure such breach which may include providing a mutually agreed upon development plan for initiating diligent Development. If Licensor does not receive within the ninety (90) day period satisfactory tangible evidence that Licensee has cured the failure, or has a bona fide plan to cure such failure in a commercially reasonable timeframe, then Licensor may, at its option, pursue the remedies provided in this Section 3.4. Notwithstanding the foregoing, Licensee retains the right to petition Licensor to waive its right under this Section 3.4 upon written request to Licensor and provision of sufficient documentation to support that Licensee reasonably believes that Licensor’s exercise of such right would have a bona fide negative impact on Licensee’s Exploitation of Licensed Products currently in Development and/or on the market and such waiver may be granted or denied at the sole discretion of Licensor. For clarity, any grant of such waiver by Licensor shall not be deemed a waiver of any future rights under this Section 3.4 that Licensor may have.
13
4.FEES, ROYALTIES, MILESTONES, AND PAYMENTS
4.1. License Maintenance Fee. As additional consideration for the license and other rights granted under the Agreement, Licensee shall pay to Licensor an annual non-refundable, non-creditable license maintenance fee, payable no later than the first Business Day of each Calendar Year beginning on the third anniversary of the Effective Date through the expiration of the Royalty Term, according to the following schedule:
YEAR |
| ANNUAL FEE |
| |
Beginning on the third anniversary of the Effective Date of the Agreement and each Calendar Year through First Commercial Sale of a Licensed Product | | $ | 10,000 | |
Each Calendar Year following First Commercial Sale until the expiration of the Royalty Term | | $ | 30,000 | |
4.2. Milestone Payments. As additional consideration for the license and other rights granted under this Agreement, with respect to each Licensed Product, Licensee shall make the following non-creditable milestone payments to Licensor within forty-five (45) days after the occurrence of each of the following events, whether Licensee, its Affiliate, or Sublicensee achieves the events:
14
MILESTONE EVENT |
| MILESTONE PAYMENT (USD) |
| |||||||
|
| 1st Indication |
| 2nd Indication |
| 3rd & 4th Indications | | |||
FDA Clearance/Approval for the custom implant | | $ | 100,000 | | $ | 75,000 | | $ | 50,000 | |
CE Mark or other foreign equivalent of Regulatory Approval for the custom implant | | $ | 100,000 | | $ | 75,000 | | $ | 50,000 | |
FDA Clearance/Approval for the orthopedic robot | | $ | 100,000 | | $ | 75,000 | | $ | 50,000 | |
CE Mark or other foreign equivalent of Regulatory Approval for the orthopedic robot | | $ | 100,000 | | $ | 75,000 | | $ | 50,000 | |
First Year of annual Net Sales of Licensed Products of at least $10,000,000 | | $ | 400,000 | | | | | | | |
First Year of annual Net Sales of Licensed Products of at least $50,000,000 | | $ | 2,000,000 | | | | | | | |
If at the time of a Significant Transaction the Company has a valuation of $150,000,000 or greater | | | 1% of the Fair Market Value of Company at the time of completion of the Significant Transaction | | ||||||
The milestones set forth in this Section 4.2 are successive and not creditable against any other obligations of Licensee. If a particular Licensed Product is not required to undergo the event associated with a particular milestone for a Licensed Product (“Skipped Milestone”), such Skipped Milestone will be deemed to have been achieved upon the occurrence of the next successive milestone that is achieved with respect to such Licensed Product (“Achieved Milestone”). Payment for any Skipped Milestone that is owed in accordance with the provisions of this Section shall be due within forty-five (45) days after the occurrence of the Achieved Milestone.
4.3. Running Royalties. As additional consideration for the license and other rights granted under the Agreement, during the Royalty Term, Licensee shall pay to Licensor the annual percentage of Net Sales on a Licensed Product-by-Licensed Product basis as follows:
Net Sales for the Applicable Calendar Year in the Jurisdiction |
| Running Royalty |
| Running Royalty |
|
Where there is a Valid Claim in the country of sale | | 5 | % | 3 | % |
Where there is no Valid Claim in the country of sale | | 3 | % | 1.5 | % |
15
4.4. Commercial Interruptions. In the event that Licensee has a Substantial Interruption Event, then the provisions of this Section 4.4 shall apply. Licensee shall notify Licensor in writing promptly in the event that a Substantial Interruption Event occurs or is anticipated to occur, and such notice shall include a reasonably detailed description and projected timeline and plan for curing any such Substantial Interruption Event. For the period that such Substantial Interruption Event is in effect (including, for clarity, any applicable period prior to a determination that a Substantial Interruption Event is in effect, during which the conditions of a Substantial Interruption Event were met), Licensee shall pay to Licensor the Interruption Royalty. Running royalties under Section 4.3 are fully creditable against such Interruption Royalty. In no event shall such Interruption Royalty be less than the running royalty owed to Licensor over any commensurate time period during the previous Calendar Year.
4.5. Stacking Protection. If Licensee becomes obligated to pay royalties to Third Parties for any patents necessary to Develop or Manufacture a particular Licensed Product, then during the period of time Licensee is so obligated, Licensee may deduct fifty percent (50%) of the documented amount of such Third Party royalties paid by Licensee solely attributable to Sales of those particular Licensed Products during a Quarter (and not attributable to any other products or processes) from the royalty amounts otherwise due to Licensor on Net Sales of those particular Licensed Products during such Quarter, provided that notwithstanding anything to the contrary herein, no deduction shall reduce the amount otherwise due to Licensor in royalties on Net Sales in any Quarter by more than fifty percent (50%) and no excess deductions may be carried forward into subsequent Quarters.
4.6. Sublicense Fees. In accordance with this Section, Licensee shall pay to Licensor a percentage of all Sublicense Income within fifteen (15) days after receipt of such Sublicense Income. All consideration received by Licensee from any Sublicensee shall be fully auditable by Licensor pursuant to the audit right in Section 5.10. Licensee shall not receive from any Sublicensee anything of value in lieu of cash payments in consideration for any Sublicense without the express prior written consent of Licensor. Any non-cash consideration, including, without limitation, equity in other companies or equity investments in Licensee, received by Licensee from any Sublicensee will be valued at its Fair Market Value as of the date of receipt by Licensee for purposes of calculating Sublicense Income. Licensee shall not sell or transfer, voluntarily or involuntarily, to a Third Party any of Licensee’s interest in any portion of any future sublicensing revenues under any Sublicense without the prior written consent of Licensor.
The percentage of consideration of Sublicense Income paid to Licensor by Licensee shall be:
Date of Sublicense |
| Percentage of Sublicense Income |
|
Prior to the first successful implantation or use of the Licensed Product in a sawbone model | | 60 | % |
On or after the first successful implantation or use of the Licensed Product in a sawbone model | | 45 | % |
On or after the first successful implantation or use of the Licensed Product in a cadaveric model | | 35 | % |
On or after FDA Clearance/Approval of the Licensed Product | | 20 | % |
From first commercial sale of the Licensed Product and thereafter | | 15 | % |
16
5.REPORTS AND PAYMENTS
5.1. Reporting of First Commercial Sale. In addition to the Quarterly Reports required under Section 5.2, Licensee shall provide a written report to Licensor setting forth the date of First Commercial Sale in each Jurisdiction within sixty (60) days of the occurrence thereof.
5.2. Quarterly Royalty and Sublicense Income Report. Within sixty (60) days after the Quarter in which any First Commercial Sale occurs, and within sixty (60) days after each Quarter thereafter, Licensee shall provide Licensor with a written report detailing the amount of Gross Sales from Commercial Sales of Licensed Products during the preceding Quarter, the amount of Net Sales made during such Quarter and the royalty payments due to Licensor for such Quarter pursuant to Article 4 (each such report, a “Quarterly Report”). Each Quarterly Report shall include at least the following:
(a) accounting for Net Sales, detailing the Gross Sales and specifying the deductions taken to arrive at Net Sales, listed by Licensed Product and by Jurisdiction;
(b) total royalty payments due to Licensor by Licensed Product and by Jurisdiction;
(c) names and addresses of all Sublicensees, all Sublicense Income received by Licensee from such Sublicensees and all amounts payable under Section 4.6, as applicable; and
(d) milestones achieved as provided in Section 4.2, as applicable, and any fees associated therewith.
5.3. Each Quarterly Report shall be in substantially similar form as Exhibit D attached hereto (which is hereby incorporated into and made a part of this Agreement), or to such other form as Licensor may provide from time to time. Each Quarterly Report shall be certified as true and correct by an officer of Licensee. With each Quarterly Report submitted, Licensee shall pay to Licensor the royalties and fees due and payable under this Agreement, to the extent not already paid pursuant to Article 4. If no royalties or fees are due and payable, Licensee shall so report. Licensee’s failure to timely submit to Licensor payment or a Quarterly Report substantially in the required form will constitute a material breach of this Agreement permitting Licensor to terminate this Agreement in full pursuant to Section 13.2 hereof. Licensee will continue to deliver payment and Quarterly Reports to Licensor after the termination or expiration of this Agreement with respect to any Quarter during which this Agreement remained in effect and until such time as all Licensed Product(s) permitted to be sold after termination have been sold or destroyed.
17
5.4. Annual Progress Report and Development Plan. On the first Business Day of each Calendar Year following the Effective Date, Licensee shall submit to Licensor (a) an updated Development Plan, and (b) a written report covering Licensee’s, its Affiliate’s and/or Sublicensee’s, as applicable, progress evidencing no less than Commercially Reasonable Efforts regarding: (i) Development and testing of all Licensed Products; (ii) achieving the due diligence events specified in Section 3.3; (iii) preparing, filing, and obtaining and maintaining of any Regulatory Approvals; (iv) plans for the upcoming year related to Commercializing the Licensed Product(s); and (v) copies of annual financial statements and any business plans or quarterly internal reports of financial condition (an “Annual Progress Report”).
5.5. Annual Milestone Reports. On the first Business Day of each Calendar Year following the Effective Date, Licensee shall submit to Licensor a written report in which Licensee certifies, with respect to each milestone event set forth in Section 4.2, whether such milestone event has occurred as of the date of the report and, if so, (a) the date upon which such milestone event occurred, (b) the gross amount of the milestone payment due to Licensor, and (c) an amount and description of any applicable fees, credits or deductions (a “Milestone Report”). In addition, Licensee shall submit an interim Milestone Report no later than forty-five (45) days after the occurrence of any milestone event. After any First Commercial Sale has occurred, Licensee’s obligation to provide Milestone Reports shall be satisfied by providing Quarterly Reports pursuant to Section 5.2. For clarity, in each Milestone Report, Licensee shall identify the milestone(s) being reported on by reference to its description in this Agreement, shall certify as to the occurrence of each milestone event and whether Licensee, its Affiliate, or Sublicensee achieved the event.
5.6. Annual Sublicense Reports. On the first Business Day of each Calendar Year following the Effective Date, Licensee shall submit to Licensor a written report setting forth: (a) the names and addresses of all Sublicensees, (b) all Sublicense Income received by Licensee from each Sublicensee during the preceding Calendar Year, and (c) all amounts payable or paid to Licensor under Section 4.6 during the preceding Calendar Year. In addition, within fifteen (15) days of Licensee’s receipt of any Sublicense Income, Licensee shall submit to Licensor the amount payable to Licensor under Section 4.6, together with a written report describing the triggering event, the gross amount of Sublicense Income received, any applicable fees, credits or deductions, and the net amount of Sublicense Income payable to Licensor.
5.7. Payment and Currency. All dollar amounts referred to in this Agreement are expressed in United States dollars and Licensee shall make all payments due to Licensor in U.S. Dollars, without deduction of exchange, collection, wiring fees, bank fees, or any other charges, within thirty (30) days following the Quarter in which Net Sales occur. Each payment will reference Agreement BLU-0122. All payments to Licensor will be made in U.S. Dollars by wire transfer or check payable to the Icahn School of Medicine at Mount Sinai and sent to:
18
By Electronic Transfer: | By Check: |
| |
Bank Name: JPMorgan Chase Manhattan Bank | Mount Sinai Innovation Partners |
Account #: 134691296 | Icahn School of Medicine at Mount Sinai |
Account Name: Icahn School of Medicine at Mount Sinai | Xxx Xxxxxxx X. Xxxx Xxxxx Box 1675 |
ABA # (routing): 000000000 | New York, NY 10029 |
IBAN #: XXXXXX00 (For International Transfers) | |
Bank Contact Person: Xxxxxx Xxxxxxxx | |
Telephone: 000-000-0000 | |
Fax: 000-000-0000 | |
Address: 0 Xxxxxxxxx Xxxxxx, 00xx Xxxxx, Xxxxxxxx, XX 00000 | |
5.8. Currency Exchange; Taxes. For converting any Net Sales made in a currency other than United States Dollars, the Parties will use the conversion rate published in the Wall Street Journal/Telegraphic Transfer Selling conversion rate reported by the Sumitomo Bank, Tokyo, or other industry standard conversion rate approved in writing by Licensor for the last day of the Quarter for which such royalty payment is due or, if the last day is not a Business Day, the closest preceding Business Day. All applicable taxes and other charges such as duties, customs, tariffs, imposts and government imposed surcharges shall be borne by Licensee and will not be deducted from payments due to Licensor.
5.9. Late Payments. In the event royalty payments or other fees are not received by Licensor when due hereunder, Licensee shall pay to Licensor interest charges that will accrue interest until paid at a rate equal to two (2) percentage points above the U.S. Prime Rate, as reported in the Wall Street Journal, Eastern Edition from time-to-time (or the maximum allowed by Law, if less), calculated on the number of days such payment is overdue.
5.10. Records and Audit Rights. Licensee shall keep, and cause its Affiliates and Sublicensees to keep, complete, true and accurate records and books containing all particulars that may be necessary for the purpose of showing the amounts payable to Licensor hereunder. Copies of all such records and books shall be kept at Licensee’s principal place of business or the principal place of business of the appropriate division of Licensee to which this Agreement relates. The records for each Quarter will be maintained for at least five (5) years after the Calendar Year in which the applicable report was submitted to Licensor. Such books and the supporting data shall be open to inspection by Licensor, its contractors or agents at all reasonable times for a term of five (5) years following the end of the Calendar Year to which they pertain, for the purpose of verifying Licensee’s royalty statement or compliance in other respects with this Agreement. Such access will be available to Licensor, its contractors or agents upon not less than seven (7) calendar days written notice to Licensee, its Affiliate, or Sublicensee, as applicable, not more than twice each Calendar Year during the Term and once per Calendar Year after the expiration or termination of this Agreement. Should such inspection lead to the discovery of at least a five percent (5%) or Five Thousand dollars ($5,000) discrepancy in reporting to Licensor’s detriment (whichever is greater), Licensee agrees to pay the full cost of such inspection. Whenever Licensee, its Affiliate, or Sublicensee has its books and records audited by an independent certified public accountant with respect to any Quarter in which amounts are payable to Licensor hereunder, Licensee, its Affiliate, or Sublicensee, as applicable, will, within thirty (30) days of the conclusion of such audit, provide Licensor with a written statement, certified by said auditor, setting forth the calculation of royalties, fees, and other payments due to Licensor over the time period audited as determined from the books and records of such Entity, together with the payment of any outstanding amounts due to Licensor.
19
6.EQUITY OWNERSHIP
6.1. Within sixty (60) days after the Effective Date, Licensee shall issue to Licensor that number of shares of the Licensee’s Equity Securities (as defined below), representing twelve percent (12%) of the Pro Forma Fully-Diluted Equity (as defined below) (the “Initial Issuance”). In addition, the Licensee shall from time to time, if necessary, issue to the Licensor additional shares of Common Stock (the “Additional Shares”), so that the Licensor’s ownership of Licensee’s Fully Diluted Equity (as defined below) shall not fall below twelve percent (12%), as calculated after giving effect to such issuance of Additional Shares; provided, that such issuances of Additional Shares shall continue after the Initial Issuance only through the receipt by Licensee of an aggregate of ten million dollars ($10,000,000) in cash in exchange for its Equity Securities (the “Threshold”). Beyond the Threshold, no Additional Shares shall be due to Licensor pursuant to this Section and the percentage of Licensee’s Fully Diluted Equity represented by the Common Stock issued to Licensee may be diluted below twelve percent (12%).
6.2. Licensee hereby represents and warrants to Licensor that the capitalization table attached hereto as Exhibit F completely and accurately reflects the Pro Forma Fully-Diluted Equity of Licensee as of the date hereof.
6.3. At all times, common stock shall be subject to a customary stock purchase agreement (the “Purchase Agreement”), which Licensor shall enter into upon the Initial Issuance. Under the Purchase Agreement, the Licensor shall agree to enter into reasonable or customary agreements reasonably required by any future institutional equity investors with respect to the voting of its common stock, and regarding subjecting the common stock held by Licensor to rights of first refusal and co-sale, on substantially the same terms as all other institutional investors and subject to customary exceptions for such institutional investors.
6.4. The Purchase agreement shall provide for a right of participation such that if Licensee proposes to offer and sell any Equity Securities (excluding customary exceptions, such as (but not limited to) the grant of Equity Securities to service providers to Licensee for compensatory purposes, issuances of Equity Securities in connection with strategic transactions, vendor financings or debt financings), then Licensor and or its designees or assignees will have the right to purchase Licensor’s Pro Rata Share of the Equity Securities so offered on the same terms and conditions as such Equity Securities are offered to the other purchasers thereof. Such right of participation shall provide, among other things, for at least 10 days’ prior notice of such offering, including reasonable detail regarding the terms of the Equity Securities and the purchasers thereof.
6.5. The following definitions shall be applicable to this Article 6:
20
(a) “Equity Financing” means a bona fide financing transaction or series of related transactions in which Licensee sells capital stock for the sole purpose of raising capital.
(b) “Equity Securities” means the capital stock of Licensee (including common and preferred shares) and any other securities of Licensee that are convertible into capital stock of Licensee (including, without limitation, options, warrants and convertible debt securities).
(c) “Fully Diluted Equity” means the total outstanding Equity Securities as of a given date subsequent to the Effective Date after giving effect to the conversion into capital stock of all outstanding convertible securities of Licensee (assuming the issuance of Equity Securities authorized and reserved for issuance under the employee incentive compensation and other stock option plans of Licensee).
(d) “Pro Forma Fully-Diluted Equity” means the total outstanding Equity Securities as of the Effective Date after (i) giving effect to the conversion into capital stock of all outstanding convertible securities of Licensee as of the Effective Date and (ii) assuming the issuance of all Equity Securities authorized and reserved for issuance under the employee incentive compensation and other stock option plans of Licensee.
(e) “Pro Rata Share” means the ratio of (i) the number of Equity Securities owned by Licensor immediately prior to such proposed new issuance of Equity Securities (assuming full conversion of all Equity Securities held by Licensor) to (ii) the Fully Diluted Equity immediately prior to such proposed new issuance of Equity Securities).
7.CONFIDENTIALITY; PUBLICITY; USE OF NAME
7.1. “Confidential Information” means any and all information of a Party (the “Disclosing Party”), or such information of such Party’s Affiliates or of Third Parties provided on behalf of such Party to the other Party (“Receiving Party”), that is disclosed in tangible form marked as “confidential” upon disclosure or, if disclosed in oral or other intangible form, is identified as confidential at the time of disclosure and summarized in a writing that is marked as “confidential” and provided to the Receiving Party within thirty (30) days of the intangible disclosure, provided however that failure to so mark, identify, or summarize shall not alter the status of such information as Confidential Information if a reasonable person would, based on the content and/or context of the disclosure, recognize such disclosure was intended as confidential. Notwithstanding the foregoing, Confidential Information shall not include information that the Receiving Party can demonstrate by written and/or electronic records: (i) is available to the public at the time of disclosure hereunder or, after disclosure, becomes a part of the public domain by publication or otherwise, through no breach by the Receiving Party; (ii) is already properly possessed by the Receiving Party prior to receipt from the Disclosing Party; (iii) was received by the Receiving Party without obligation of confidentiality or limitation on use from a Third Party who had the lawful right to disclose such information; or (iv) was independently developed by or for the Receiving Party by any person or persons who had no knowledge or benefit of the Disclosing Party’s Confidential Information, where the written or electronic records demonstrating such exception were created contemporaneously with such independent development.
21
7.2. Confidentiality. The Receiving Party shall maintain in confidence and not disclose to any Third Party any of Disclosing Party’s Confidential Information, using the same degree of care it uses to protect its own confidential information of a similar nature but in no event using less than a reasonable degree of care. The Receiving Party will use Disclosing Party’s Confidential Information solely as required to undertake its rights and obligations under this Agreement (the “Purpose”) and only during the Term. For clarity, the Purpose expressly excludes any use of Disclosing Party’s Confidential Information for regulatory or patent filing purposes, or for initiation or pursuit of any proceeding to challenge the patentability, validity, or enforceability of any patent application or issued patent (or any portion thereof) that is owned or Controlled by Disclosing Party (including, e.g., via pre-issuance submissions, post grant review, or inter partes review). Any such excluded use is hereby deemed a material breach of this Agreement and in such event, notwithstanding anything to the contrary herein, the non-breaching Party shall have the right to terminate this Agreement immediately upon notice to the breaching Party and seek resolution of such dispute in any court of competent jurisdiction notwithstanding any provisions herein regarding resolution of disputes between the Parties; in addition to any other relief granted to the non-breaching Party, the breaching Party shall pay to the non-breaching Party all costs such non-breaching Party incurs in such proceeding including in defense of such patent application or patent. Any such payment shall be made within thirty (30) days of written demand. The Receiving Party will ensure that its employees, independent contractors, Affiliates, and Sublicensees (“Recipient Individuals”) have access to Disclosing Party’s Confidential Information only on a need to know basis, are informed of all the obligations attaching to such Confidential Information in advance of being given access to it, and are required to comply with such Receiving Party’s obligations under this Agreement Receiving Party shall be fully responsible to Disclosing Party for such compliance by its Recipient Individuals. If such Recipient Individual is not an employee of a Party hereto, then Recipient will enter into a legally binding confidentiality agreement with provisions at least as strict as the confidentiality obligations and use restrictions herein, with such Recipient Individual prior to disclosing Disclosing Party’s Confidential Information to such Recipient Individual, and Receiving Party will be fully responsible to Disclosing Party for compliance with such obligations and restrictions by such Recipient Individual.
7.3. Notwithstanding the above Section 7.2, the Receiving Party may disclose Disclosing Party’s Confidential Information to the limited extent required by Law, court order, other governmental authority with jurisdiction, provided that the Receiving Party (a) promptly provides the Disclosing Party, to the extent legally permissible, with written notice of such requirement, (b) uses no less than reasonable efforts to obtain confidential treatment of such Disclosing Party’s Confidential Information by such court or governmental authority, and (c) cooperates, at the Disclosing Party’s written request and expense, with the Disclosing Party’s legal efforts to prevent or limit the scope of such required disclosure; the Receiving Party shall in all other respects continue to hold such Confidential Information as confidential and subject to all obligations of this Article 7. The Receiving Party’s obligations of confidentiality and non-use restrictions as set forth in this Article 7 shall remain in effect for a period of five (5) years from receipt of the Confidential Information from the Disclosing Party.
22
7.4. Each Party agrees to treat the terms and conditions of this Agreement as the Confidential Information of the other Party, provided however that in addition to the above exceptions, each Party shall be free to disclose any of the terms of this Agreement (i) to the extent that a Party is advised by its counsel that it is required to do so by the regulations or rules of any relevant stock exchange, (ii) to actual or prospective Licensees, (iii) to its accountants, attorneys and other professional advisors, or (iv) in connection with a financing, merger, consolidation, acquisition or a permitted assignment of this Agreement; provided that (l) in the case of any disclosure under clause (ii), (iii), or (iv) above, the recipient(s) are obligated and do so undertake to keep such terms of this Agreement confidential to the same extent as said Party (said Party being fully responsible to the other Party for such recipients’ compliance), and (2) in the case of disclosure under clause (i), such disclosure shall be in accordance with Section 7.3.
7.5. Publicity. The Parties may issue a press release only upon mutual written agreement and, if so, will cooperate to determine the timing and content of such press release.
7.6. Use of Licensor’s Name. Licensee and its Affiliates, Sublicensees, employees and agents may not use the name, logo, seal, trademark, or service mark of Licensor or any school, organization of Licensor, or, any faculty member, student, employee, officer, director, trustee, or other representative of Licensor or of any school or organization of Licensor (or any adaptation of any of the foregoing) without the prior written consent of Licensor, which consent will be granted or denied in Licensor’s sole discretion by the Vice President of the Office of Marketing and Communications of the Licensor Health System.
8.PATENT PROSECUTION AND REIMBURSEMENT
8.1. Patent Prosecution. Licensor shall control the Prosecution of Licensed Patents and the selection of patent counsel. Licensor will require that copies of all documents prepared by patent counsel be provided to Licensee for review and comment prior to filing. Licensor will consider any comments from Licensee in good faith; provided, however, that Licensor shall have final authority regarding all Prosecution decisions. All Licensed Patents will be in Licensor’s name, and Licensee acknowledges that Licensor shall remain the client of such patent counsel and in every case shall retain the right to remand. Licensee shall pay, within thirty (30) days of invoice, all future expenses for Prosecuting the Licensed Patents, including without limitation, any taxes, annuities or maintenance fees on such Licensed Patents. Licensee agrees to receive such invoices directly from patent counsel, with Licensor receiving a copy of such invoice, pursuant to a Client and Billing Agreement with patent counsel substantially in the form of Exhibit E, which will be incorporated into and made a part of the Agreement. Licensee shall pay such invoices directly to patent counsel with written confirmation of payment to Licensor.
8.2. Patent Reimbursement. Within thirty (30) days after the Effective Date, Licensee will reimburse Licensor for all historically accrued, un-reimbursed attorneys’ fees, expenses, official fees and all other charges accumulated prior to the Effective Date incident to the Prosecution of the Licensed Patents, which amount is currently estimated at Nineteen Thousand and One U.S. Dollars ($19,001.00) as of August 18th, 2017.
23
8.3. Patent Extension. Licensee shall promptly notify Licensor of any Regulatory Approval for any Licensed Product for which an application for Patent term extension may be based, including with respect to any Third Party product, or any other event in any Jurisdiction that would enable Licensor or Licensee as appropriate to apply for Patent term extension or other regulatory or marketing exclusivity or extension thereof in any Jurisdiction. For clarity, Licensee will notify Licensor of an opportunity to apply for Patent term extensions or regulatory or marketing exclusivity or extension thereof as soon as the event triggering the opportunity for application has occurred and in no event later than three (3) Business Days following the occurrence of the triggering event. Licensee agrees to cooperate fully with Licensor to provide any information or documentation necessary to support an application for Patent term extension or other regulatory or marketing exclusivity.
8.4. Abandonment. If Licensee decides that it does not wish to pay for the Prosecution of any Licensed Patent in a particular Jurisdiction (“Abandoned Patent”), Licensee shall provide Licensor with prompt written notice of such election. Upon receipt of such notice by Licensor, Licensee shall be released from its obligation to reimburse Licensor for the expenses incurred thereafter as to such Abandoned Patent; provided, however, that expenses incurred or authorized prior to the receipt by Licensor of such notice shall be deemed incurred prior to the notice. If any Licensed Patent becomes an Abandoned Patent hereunder, any license granted by Licensor to Licensee hereunder with respect to such Abandoned Patent will terminate, and Licensee will have no rights whatsoever to Exploit such Abandoned Patent. Licensor will then be free, without further notice or obligation to Licensee, to grant rights in and to such Abandoned Patent to any Third Parties. Should Licensee decline or fail to pay by the deadline set forth herein the costs and legal fees for the Prosecution of any Licensed Patents payable under this Agreement, Licensor may, at its sole discretion, elect to (a) exclude by written notice the particular Licensed Patent from this Agreement, without terminating the Agreement in its entirety, and such Licensed Patent shall be deemed an Abandoned Patent under this Agreement upon such notice, or (b) Licensor may terminate this Agreement in full pursuant to Section 13.2(a) hereof.
9.INFRINGEMENT
9.1. Notice. In the event that either Party becomes aware of any suspected infringement of any Licensed Patent or of any Infringement Action, such Party shall promptly notify the other Party thereof. Licensee and Licensor will consult each other in a timely manner concerning any appropriate response to such suspected infringement or Infringement Action.
9.2. Procedure.
(a) (i) As between the Parties, if Licensor consents in writing, then Licensee will have the first right to prosecute any Infringement Action against an infringing Third Party at its own expense. (ii) If, within sixty (60) days after becoming aware of any suspected infringement or Infringement Action, Licensor has not so consented to Licensee prosecuting such Infringement Action, or if Licensee has elected not to initiate, defend, or otherwise resolve such Infringement Action, then Licensor shall have the right, but not the obligation, to initiate, control, prosecute, and/or defend such Infringement Action at its own expense.
24
(b) The Party controlling any Infringement Action shall use reasonable efforts to: (i) inform the other Party of the status of such Infringement Action on a regular basis; (ii) provide to the other Party copies of any documents relating to the Infringement Action promptly upon receipt from any Third Party and/or, if practicable, prior to filing such documents; (iii) consult with the other Party regarding the advisability of any contemplated course of action; and (iv) consider any comments from the other Party in good faith, including with respect to the infringement, claim construction, or defense of the validity or enforceability of any claim in the involved Licensed Patent. The Party without primary control of an Infringement Action shall cooperate at its own expense with the Party controlling such Infringement Action to the extent reasonably possible, including joining the Infringement Action if necessary or desirable.
(c) No Party may enter into a settlement of any Infringement Action that restricts the scope or adversely affects the enforceability of, or grants a license to, any Licensed Patent, or includes admission of fault or wrongdoing on behalf of the other Party, without the prior written consent of such other Party. For clarity, if the settlement of any Infringement Action includes granting a Sublicense, Licensee shall pay to Licensor royalties on any Net Sales by such Sublicensee and a percentage of Sublicense Income, if applicable, in accordance with Article 4 in addition to any other share of recoveries due to Licensor under this Section.
9.3. Recoveries.
(a) Any recovery obtained by Licensee under 9.2(a)(i) as a result of any Infringement Action, by settlement or otherwise, shall be applied in the following order of priority: (i) first, to reimburse the Parties for all litigation costs (including attorneys’ fees) incurred in connection with such proceeding and not otherwise recovered; and (ii) second, the remainder of the recovery shall be shared equally between the Parties.
(b) Any recovery obtained by Licensor under 9.2(a)(ii) as a result of any Infringement Action, by settlement or otherwise, shall be applied in the following order of priority: (i) first, to reimburse the Parties for all litigation costs (including attorneys’ fees) incurred in connection with such proceeding and not otherwise recovered; and (ii) second, the remainder of the recovery shall be retained by Licensor.
10.REPRESENTATIONS; DISCLAIMER OF WARRANTIES; LIMITATION OF LIABILITIES
10.1. Certain Representations. Each Party represents to the other Party that, as of the Effective Date:
(a) it has the full right, power and authority to enter into this Agreement and to perform its obligations hereunder; and
(b) this Agreement has been duly authorized and executed by it and is legally binding upon it, enforceable in accordance with its terms, and does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, nor violate any applicable Law or applicable regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
25
10.2. Health Care Law. Licensee represents, warrants, and covenants to Licensor that:
(a) it, and its Affiliates, agents, and employees who are or shall be involved in the performance of this Agreement, have not been, and during the Term of this Agreement shall not be, debarred, excluded or disqualified (or convicted of any crime or engaged in any conduct for which debarment, exclusion or disqualification is mandated) under any Health Care Law, including pursuant to 21 U.S.C. § 335a;
(b) to its reasonable knowledge, no Third Party that, on behalf of Licensee, has been or during the Term of this Agreement will be, involved in the Development, Manufacture or Commercialization of the Licensed Products (each a “Licensee Partner”), has been or will be debarred, excluded or disqualified (or convicted of any crime or engaged in any conduct for which debarment, exclusion or disqualification is mandated) under any Health Care Law, including pursuant to 21 U.S.C. § 335a;
(c) Licensee, and its Affiliates, agents, and employees involved in the performance of this Agreement, and Licensee Partners, shall perform this Agreement in full compliance with all applicable Health Care Laws; and
(d) Licensee shall notify Licensor in writing immediately in the event of a violation of any of the foregoing, and shall, with respect to any Entity involved in such violation, promptly remove such Entity from performing any role under this Agreement.
10.3. DISCLAIMER OF WARRANTIES. THE LICENSED PATENTS, SOFTWARE, KNOW-HOW, LICENSED PRODUCTS, AND ANY OTHER TECHNOLOGY OR INFORMATION PROVIDED OR LICENSED UNDER THIS AGREEMENT ARE PROVIDED ON AN “AS IS” BASIS. LICENSOR MAKES NO REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO ANY WARRANTY OF ACCURACY, COMPLETENESS, PERFORMANCE, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, COMMERCIAL UTILITY, SCOPE, OR TITLE WITH RESPECT THERETO.
10.4. DISCLSIMER OF LIABILITIES. LICENSOR WILL NOT BE LIABLE TO LICENSEE, ITS SUCCESSORS OR ASSIGNS, OR TO ANY THIRD PARTY WITH RESPECT TO ANY CLAIM ARISING FROM OR ATTRIBUTABLE TO USE BY LICENSEE, ITS AFFILIATES, OR SUBLICENSEES OF THE LICENSED PATENTS, SOFTWARE, KNOW-HOW, LICENSED PRODUCTS, LICENSEE MODIFIED PRODUCTS OR ANY OTHER TECHNOLOGY OR INFORMATION PROVIDED OR LICENSED UNDER THIS AGREEMENT, OR ARISING FROM THE DEVELOPMENT, TESTING, MANUFACTURE, USE OR SALE OF LICENSED PRODUCTS, LICENSEE MODIFIED PRODUCTS OR FOR LOST PROFITS, BUSINESS INTERRUPTION, INCIDENTIAL, INDIRECT, SPECIAL, CONSEQUENTIAL OR OTHER DAMAGES OF ANY KIND.
10.5. LIMITATION OF LIABILITY. NOTWITHSTANDING ANY PROVISION IN THIS AGREEMENT TO THE CONTRARY, LICENSOR’S AGGREGATE LIABILITY UNDER THIS AGREEMENT SHALL NOT EXCEED AN AMOUNT EQUAL TO THE MAXIMUM AMOUNT OF ALL PAYMENTS MADE BY LICENSEE TO LICENSOR PURSUANT TO ARTICLE 4 DURING THE FULL CALENDAR YEAR DURING WHICH LICENSEE PAID TO LICENSOR THE HIGHEST AGGREGATE AMOUNT PURSUANT TO THAT ARTICLE 4.
26
10.6. WITHOUT LIMITING THE GENERALITY OF ANYTHING IN THIS ARTICLE 10, NOTHING IN THIS AGREEMENT SHALL BE CONSTRUED AS:
(a) A WARRANTY OR REPRESENTATION BY LICENSOR THAT ANYTHING MADE, USED, SOLD, OFFERED FOR SALE, DISTRIBUTED, OR AS APPLICABLE PUBLICLY PERFORMED, PUBLICLY DISPLAYED, DERIVED FROM, OR OTHERWISE DISPOSED OF PURSUANT TO ANY LICENSE GRANTED UNDER THIS AGREEMENT IS OR WILL BE FREE FROM INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES;
(b) AN OBLIGATION BY LICENSOR TO BRING OR PROSECUTE ACTIONS OR SUITS AGAINST THIRD PARTIES FOR INFRINGEMENT, MISAPPROPRIATION, OR OTHER SIMILAR CAUSES OF ACTION RELATED TO THE LICENSED PATENTS, SOFTWARE, OR KNOW-HOW; OR
(c) CONFERRING BY IMPLICATION, ESTOPPEL OR OTHERWISE ANY LICENSE OR RIGHTS UNDER ANY INTELLECTUAL PROPERTY RIGHTS OF LICENSOR OTHER THAN AS AND TO THE EXTENT EXPRESSLY SET FORTH HEREIN
11.INDEMNIFICATION
11.1. Indemnification. Licensee will indemnify, hold harmless, and at Licensor’s option, shall defend Licensor, and its trustees, officers, faculty, agents, employees and students (each, an “Indemnified Party”) from and against any and all claims, actions, liabilities, losses, damages, judgments, costs or expenses suffered or incurred by the Indemnified Parties, including attorneys’ fees and related costs (collectively, “Liabilities”), arising out of or resulting from:
(a) the exercise of any license granted under this Agreement, whether by Licensee, its Affiliates, Sublicensees, assignees, vendors or associated Third Parties;
(b) any breach of this Agreement or any Sublicense by Licensee, its Affiliates, or Sublicensees;
(c) the enforcement of this Article 11 by any Indemnified Party; and/or
(d) any act, error, or omission of Licensee, its Affiliates, or Sublicensees, or any of the officers, directors, employees or agents of any of the foregoing, with respect to its obligations hereunder or with respect to applicable law or regulation;
except in each case to the extent such Liabilities result solely from the gross negligence or willful misconduct of an Indemnified Party. Liabilities under this Section include, but are not limited to, Liabilities arising in connection with: (i) the use by a Third Party of a Licensed Product that was Developed, Manufactured or Commercialized by Licensee, Sublicensees, assignees, vendors or Third Parties; (ii) a claim by a Third Party that the Licensed Patents, Know-How, Software, or the design, composition, or Exploitation of any Licensed Product infringes or violates or appropriates any Patent, copyright, trade secret, trademark or other intellectual property right of such Third Party; (iii) clinical trials or studies conducted by or on behalf of Licensee, its Affiliates, Sublicensees, assignees, vendors or associated Third Parties relating to the Licensed Patents, Software, Know-How, or Licensed Products, such as claims by or on behalf of a human subject of any such trial or study; or (iv) a failure to perform under this Agreement or any Sublicense in material compliance with all applicable Laws, including, without limitation, all Health Care Laws.
27
11.2. Indemnification Procedure. An Indemnified Party will promptly provide Licensee with written notice of any Liability that is indemnifiable under this Article 11; provided, however, that the failure to so notify shall not relieve Licensee of its indemnification obligations hereunder except to the extent of any material prejudice to Licensee as a direct result of such failure. Except as otherwise provided in this Section 11.2, Licensor shall control such defense and all negotiations relative to the settlement of any indemnifiable claim or action, except that Licensor shall not settle or compromise any claim or action in any manner that may impose restrictions or obligations on any Indemnified Party, or that concedes any fault or wrongdoing on the part of Licensee, without Licensee’s prior written consent. If Licensor so directs in writing, Licensee shall control such defense and all negotiations relative to the settlement of any indemnifiable claim or action, except that Licensee shall not settle or compromise any claim or action in any manner that may impose restrictions or obligations on any Indemnified Party, or that grants any rights to the Licensed Patents, Software, Know-How or Licensed Products, or that concedes any fault or wrongdoing on the part of Licensor, without Licensor’s prior written consent. If, after receipt of written direction from Licensor, Licensee fails or declines to assume the defense against any claim or action within thirty (30) days after notice thereof, then Licensor may assume and control the defense of such claim or action for the account and at the risk of Licensee, and any Liabilities related to such claim or action will be conclusively deemed a liability of Licensee. The indemnification rights of the Indemnified Parties under this Article 11 are in addition to all other rights that an Indemnified Party may have at law, in equity or otherwise.
00.XXXXXXXXX
12.1. Coverages. Licensee will procure and maintain insurance policies for the following coverages with respect to personal injury, bodily injury, property damage and contractual liability arising out of Licensee’s performance under this Agreement as follows: (a) during the Term, comprehensive general liability, including broad form and contractual liability, in a minimum amount of Two Million U.S. Dollars ($2,000,000 USD) combined single limit per occurrence and in the aggregate, written on an occurrence-basis, with no deductible, containing a separation of insureds provision, with additional coverage for broad form and contractual liability, completed operations; (b) prior to the commencement of clinical trials involving Licensed Products, clinical trials coverage in a minimum amount of Five Million U.S. Dollars ($5,000,000 USD) combined single limit per occurrence and in the aggregate; and (c) prior to the sale of the first Licensed Product, product liability coverage, in a minimum amount of Ten Million U.S. Dollars ($10,000,000 USD) combined single limit per occurrence and in the aggregate. Licensor may review periodically the adequacy of the minimum amounts of insurance for each type of coverage required by this Article 12, and Licensor reserves the right to require Licensee to adjust the limits accordingly. Upon request, Licensee shall provide certificates of insurance and applicable endorsements evidencing the required insurance coverages noted herein. The failure of Licensor to request said evidence of coverage shall not constitute or be construed as a waiver of Licensee’s insurance obligations. Licensor, including its affiliates, shall be named as additional insureds under all applicable policies of insurance. Licensee’s comprehensive general liability insurance shall be primary and non-contributory to any insurance maintained by Licensor. The required minimum amounts of insurance do not constitute a limitation on Licensee’s liability or indemnification obligations to Licensor under this Agreement.
28
12.2. Other Requirements. Any policies of insurance required by Section 12.1 will be issued by an insurance carrier with an A.M. Best rating of “A” or better and will name Licensor as an additional insured, on a primary and non-contributory basis, with respect to Licensee’s performance under this Agreement. Licensee will provide Licensor with insurance certificates evidencing the required coverage within thirty (30) days after the commencement of each policy period and any renewal periods. Each certificate will provide that the insurance carrier will notify Licensor in writing at least thirty (30) days prior to the cancellation or material change in coverage.
13.TERM AND TERMINATION
13.1. Expiration of Royalty Term. Upon expiration of the Royalty Term with respect to a Licensed Product in any Jurisdiction and payment in full of all amounts owed hereunder with respect to such Licensed Product in such Jurisdiction, Licensee will have a non-exclusive, fully paid up license for such Licensed Product in such Jurisdiction.
13.2. Termination by Licensor.
(a) For Cause. Licensor may give written notice of default to Licensee, if Licensee: (i) materially breaches any obligation, covenant, condition, or undertaking of this Agreement to be performed by it hereunder (including e.g. if Licensee should cease or fail to undertake Commercially Reasonable Efforts with respect to Licensed Products, fail to make any payment at the time such payment is due, or fail to maintain the insurance coverage required hereunder); (ii) fails to make the first First Commercial Sale of Licensed Product(s) within four (4) years after the Effective Date; or (iii) experiences an Event of Bankruptcy. If Licensee should fail to cure such default within sixty (60) days of such notice, this Agreement, and all of the rights, privileges, and license granted hereunder, shall automatically terminate. For purposes of this provision, the term “Event of Bankruptcy” means, with respect to a Party: (a) filing by such Party in any court or agency pursuant to any statute or regulation of any state or country, a petition in bankruptcy or insolvency or for reorganization or for an arrangement or for the appointment of a receiver or trustee of the Party or of its assets; (b) such Party being served with an involuntary petition against such Party, filed in any insolvency proceeding, where such petition has not been dismissed within sixty (60) days after the filing thereof; (c) such Party proposing or being a party to any dissolution or liquidation of such Party; or (d) such Party making a general assignment for the benefit of creditors.
29
(b) Challenge of Patents. Licensee acknowledges and agrees that nothing herein shall be construed as preventing it from challenging the validity or enforceability of the Licensed Patents at any time. In the event that Licensee, its Affiliate or Sublicensee shall, however, challenge the validity or enforceability of any of the Licensed Patents in any forum through any means, or otherwise indicate the payment of any royalty due under this Agreement is made under protest or with any objection, Licensee agrees that Licensor shall have the right, but not the obligation, in addition to any other remedy it may have available to it at law and/or in equity, to terminate this Agreement immediately upon providing written notice of the same to Licensee. For clarity, Licensor in response to such challenge by Licensee or following termination by Licensor under this subsection may seek redress in any court of competent jurisdiction in its sole discretion notwithstanding Section 15.11 or any other provision of this Agreement.
14.EFFECT OF TERMINATION
14.1. Continuing Obligations. Termination shall not relieve Licensee of any monetary or any other obligation or liability accrued hereunder prior to the effective date of such termination, or rescind or give rise to any right to rescind any payments made or other consideration given to Licensor hereunder prior to the effective date of such termination; nor shall such termination affect in any manner any rights of Licensor arising under this Agreement prior to the date of such termination. Licensee shall pay all attorneys’ fees and costs incurred by Licensor in enforcing any obligation of Licensee or accrued right of Licensor.
14.2. Survival of Terms. In addition to any provision which by its terms contemplates performance after the Term, the following provisions shall survive the expiration or termination of this Agreement: Articles 1 (Definitions), 4 (Fees, Royalties, Milestones, and Payments), 5.4 (Records and Audit Rights), 7 (Confidentiality; Publicity; Use of Name), 10 (Representations; Disclaimer of Warranties; Limitation of Liabilities), 11 (Indemnification), 12 (Insurance), 14 (Effect of Termination), and 15 (Additional Provisions).
14.3. Licensed Product on Hand. Upon expiration or termination of this Agreement by either Party, Licensee shall provide Licensor with a written inventory of all Licensed Products in process of manufacture, in use, or in stock. Licensee may dispose of any such Licensed Products within the ninety (90) day period following such expiration or termination; provided, however, that Licensee shall pay royalties and render reports to Licensor thereon in the manner specified herein.
14.4. Licensed Product Data. A copy of all Licensed Product Data must be transferred to Licensor within forty-five (45) days of termination of this Agreement for any reason, and shall become the sole property of Licensor. Licensee shall retain no right or license with respect to Licensed Product Data. Further, upon termination of this Agreement for any reason, Licensee shall (and, as applicable, shall procure that its Affiliates and Sublicensees shall): (a) transfer or assign to Licensor or its designee (or have reissued in the name of Licensor or its designee, if applicable) all regulatory filings (including any regulatory applications, Regulatory Approvals and product dossiers) that relate to Licensed Products, and (b) grant, and hereby grants, to Licensor an exclusive, fully-paid, royalty-free, worldwide, perpetual, fully sublicenseable (through multiple tiers), transferable license under all improvements to the Licensed Patents, Software, and Know-How (and related intellectual property rights) in all fields of use and for all purposes. Licensee shall take such other actions and execute such other documents as may be necessary to effect the transfer and assignment of rights hereunder to Licensor or its designee.
30
14.5 Sublicense Survival. In the event of any termination of this Agreement by Licensor under Section 13.2(a), each Sublicense granted by Licensee under this Agreement shall survive as a direct license between Licensor and such Sublicensee on the same terms and conditions as those set forth in this Agreement, to the extent applicable to the rights granted by Licensee to such Sublicensee, provided that i) such Sublicense was granted in accordance with the terms of this Agreement; ii) such Sublicense does not impose on Licensor any obligations in excess of those contained in this Agreement; and iii) that such Sublicensee is in compliance with the terms of such Sublicense. Licensor and the Sublicensee shall promptly enter into an agreement granting such sublicense under the terms, including financial terms, of this Agreement, to the extent applicable to the scope of the sublicense granted to such Sublicensee.
15.ADDITIONAL PROVISIONS
15.1. Independent Contractors. The Parties are independent contractors. Nothing contained in this Agreement is intended to create an agency, partnership or joint venture between the Parties. At no time will either Party make commitments or incur any charges or expenses for or on behalf of the other Party.
15.2. Provision of Software; Derivative Works; Licensee Modified Products. During the term of this Agreement, Licensee will provide to Licensor one complete copy of any and all commercial versions, including revised versions, of the Software, any Derivative Works, and any Licensee Modified Products, including but not limited to executable code, source code, programmer documentation and End User documentation at the time it is made available to End Users directly or indirectly by Licensee or any Sub-Licensee. Prior to Commercialization, on a semi-annual basis, Licensee will provide to Licensor the most updated prototype versions of the Software, any Derivative Works and any License Modified Products, without any implied reps or warranties; provided that, where no such improvements have been made since the version last provided to Licensor, Licensee will instead provide to Licensor written certification that no improved versions of the Software, Derivative Works or Licensee Modified Products exist.
15.3. Compliance with Laws. Licensee must comply with all prevailing Laws that apply to its activities or obligations under this Agreement. For example, Licensee will comply with applicable United States export Laws. The transfer of certain technical data and commodities may require a license from the applicable agency of the United States government and/or written assurances by Licensee that Licensee will not export data or commodities to certain foreign countries without prior approval of the agency. Licensor does not represent that no license is required, or that, if required, the license will issue.
15.4. Marking. Licensee shall, and agrees to require its Affiliates and Sublicensees to, comply with any marking requirements of the intellectual property Laws of the applicable countries in the Territory to the extent any failure to do so would materially and adversely affect the Licensed Patents or any Licensed Product, or either Party’s ability to avail itself of all potential remedies for any infringement of the Licensed Patents, and particularly agrees to permanently and legibly mark all Licensed Products made, used, reproduced, or sold under the terms of this Agreement, or their respective containers, in accordance with the applicable provisions set forth in the Patent marking and notice provisions under Title 35 of the United States Code. Any Sublicense shall impose on the Sublicensee obligations substantially similar to those imposed in this paragraph.
31
15.5. Modification, Waiver and Remedies. This Agreement may only be modified by a written amendment that is executed by an authorized representative of each Party. Any waiver must be express and in writing. No waiver by either Party of a breach by the other Party will constitute a waiver of any different or succeeding breach. Unless otherwise specified, all remedies are cumulative.
15.6. Assignment. Licensee may not assign this Agreement or any part of it, either directly or by merger or operation of Law, without the prior written consent of Licensor. Any such assignment will be valid only if: (a) at least thirty (30) days before the closing of the proposed transaction, Licensee has given Licensor written notice and such background information as may be reasonably necessary to enable Licensor to give an informed consent; (b) the assignee agrees in writing to be legally bound by this Agreement; and (c) the assignee agrees to deliver to Licensor an updated Development Plan within forty-five (45) days after the closing of the proposed transaction. Any permitted assignment will not relieve Licensee of responsibility for performance of any obligation of Licensee that has accrued at the time of the assignment. Any assignment granted, or purported to be granted, contrary to this provision will be null and void.
15.7. Notices. Except as otherwise expressly set forth herein, any notice or other required communication under this Agreement (each, a “Notice”) must be in writing, addressed to the Party’s respective Notice Address, and delivered personally or by globally recognized express delivery service, charges prepaid. A Notice will be deemed delivered and received: (a) in the case of personal delivery, on the date of such delivery; and (b) in the case of a globally recognized express delivery service, on the Business Day that receipt by the addressee is confirmed pursuant to the service’s systems. The “Notice Address” of each Party is as follows:
if to Licensor, to: | Icahn School of Medicine at Mount Sinai |
| Mount Sinai Innovation Partners |
| One Xxxxxxx X. Xxxx Place, Box 1675 |
| New York, NY 10029 |
| Attention: Senior Vice President |
| |
and a copy of legal notices only to: | Icahn School of Medicine at Mount Sinai Place, Xxx Xxxxxxx X. |
| Attention: Office of General Counsel |
| |
If to Licensee, to: | Monogram Orthopedics |
| New Lab, Suite 105 |
| 00 Xxxxxx Xxxxxx |
| Brooklyn, NY 11205 |
32
15.8. Severability and Reformation. If any provision of this Agreement is held to be invalid or unenforceable by a court of competent jurisdiction, then the remaining provisions of this Agreement will remain in full force and effect. Such invalid or unenforceable provision will be automatically revised to be a valid or enforceable provision that comes as close as permitted by Law to the Parties’ original intent.
15.9. Headings and Counterparts. The headings of the articles and sections included in this Agreement are inserted for convenience only and are not intended to affect the meaning or interpretation of this Agreement. This Agreement may be executed in several counterparts, and execution signatures may be exchanged electronically including by facsimile or as scanned e-mail attachments, and signatures so exchanged shall be considered as original for all purposes and taken together will constitute one and the same instrument.
15.10. Governing Law. This Agreement will be governed in accordance with the Laws of the State of New York, without giving effect to the conflict of law provisions of any jurisdiction.
15.11. Dispute Resolution. Except as set forth in Section 13.2(b), if a dispute arises between the Parties concerning any right or duty under this Agreement, then the Parties will confer, as soon as practicable, in an attempt to resolve the dispute amicably. If the Parties are unable to resolve the dispute amicably, the Parties hereby consent to sole jurisdiction and venue in the state or federal courts located in New York, New York with respect to any dispute arising hereunder
15.12. Integration. This Agreement, together with all attached Exhibits, contains the entire agreement between the Parties with respect to the Licensed Patents, Software and Know-How, and supersedes all other oral or written representations, statements, or agreements with respect to such subject matter, including but not limited to, the term sheet exchanged prior to this Agreement.
15.13. Force Majeure. Neither Party will be responsible for nonperformance caused by forces beyond the reasonable control of such Party, including fire, explosion, natural disaster, war (whether declared or not), act of terrorism, strike, or riot, provided that the nonperforming Party uses reasonable efforts to avoid or remove such causes of nonperformance and continues performance under this Agreement with reasonable dispatch whenever such causes are removed, and notifies the other Party of such cause as promptly as is reasonably practical given the circumstances.
33
15.14. Certain Conventions. Any reference in this Agreement to an Article, Section, subsection, paragraph, clause or Exhibit shall be deemed to be a reference to an Article, Section, subsection, paragraph, clause or Exhibit, of or to, as the case may be, this Agreement, unless otherwise indicated. Unless the context of this Agreement otherwise requires, (a) all definitions set forth herein shall be deemed applicable whether the words defined are used herein with initial capital letters in the singular or the plural, (b) the word “will” shall be construed to have the same meaning and effect as the word “shall,” (c) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (d) any reference herein to any Party shall be construed to include the Party’s successors and assigns, (e) the word “notice” shall mean notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement, (f) provisions that require that a Party or the Parties “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing, whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging), (g) references to any specific Law, rule or regulation, or article, section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement or successor Law, rule or regulation thereof, (h) words of any gender include each other gender, (j) words such as “herein,” “hereof” and “hereunder” refer to this Agreement as a whole and not merely to the particular provision in which such words appear, (i) the words “include,” “includes” and “including” shall be deemed to be followed by the phrase “but not limited to,” “without limitation,” “inter alia” or words of similar import, and (j) unless “Business Days” is specified, “days” shall mean “calendar days.” In the event an ambiguity or question of intent or
interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement.
15.15. Business Day Requirements. In the event that any notice or other action or omission is required to be taken by a Party under this Agreement on a day that is not a Business Day, then such notice or other action or omission shall be deemed to be required to be taken on the next occurring Business Day.
15.16. Global Social Responsibility. Company and Mount Sinai shall take into consideration the principle of "Global Social Responsibility". “Global Social Responsibility" means facilitating the availability of Licensed Products in developing countries (i.e. The World Bank’s listing of “Low Income Economies”) at locally affordable prices to improve access to such Licensed Products in developing countries.
[Signature Page Follows]
34
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the Effective Date.
LICENSEE: | ICAHN SCHOOL OF MEDICINE AT MOUNT SINAI: | ||
| | | |
BY: | /s/ Xxxxxx X. Xxxxxx | BY: | /s/ Xxxx Xxxx |
| | | |
NAME: | Xxxxxx X. Xxxxxx | NAME: | Xxxx Xxxx |
| | | |
TITLE: | President and CEO | TITLE: | Senior Vice President |
| | | |
| | | 10/10/2017 | 5:38 PM EDT |
Exhibit A
Country |
| App Type |
| Serial No. |
| Status |
| File Date |
| Title |
| Publication Date |
| Publication No. |
|
United States | | Provisional | | 62/319,710 | | Expired | | 2016-04-07 | | APPARATUS, METHOD AND SYSTEM FOR PROVIDING CUSTOMIZABLE BONE IMPLANTS | | | | | |
United States | | PCT | | PCT/US2017/26681 | | Pending | | 2017-04-07 | | APPARATUS, METHOD AND SYSTEM FOR PROVIDING CUSTOMIZABLE BONE IMPLANTS | | | | | |
Exhibit B
Software
Xxxxxxxx Xxxxxx’x automated segmentation and surgical planning pipelines captured as Mount Sinai TechID: 170304.
Exhibit C
Initial Company Business/Development Plan
Monogram Orthopaedics, Inc
Business Plan
June 2017

Strictly Confidential
Executive Summary
Monogram Orthopaedics, Inc ("Monogram") was spun out of Mount Sinai School of Medicine ("MSSM"), based on ideas formulated by Xx. Xxxxxxx Unis, an Associate Professor of Orthopaedic Surgery at Mount Sinai, and technology developed by Dr Xxxx, Professor Xxxxxxx Xxxxx, the head of the Neurosurgery Simulation Core at Mount Sinai, and Xxxxxxxx Xxxxxx, a medical student.
Joint degeneration resulting from osteoarthritis led to 378,000 hip replacement surgeries and 925,000 knee replacement surgeries in the US in 2015. The global market for orthopaedic surgery is estimated to be $15.5Bln and is estimated to be growing at just under 4% per year. By most measures joint replacement surgery is successful. Nevertheless, about 30% of patients who have hip replacement surgery report complications which can be explained in part by the way the surgery is carried out. In hip replacement surgery, after dislocating the hip, the head of the femur is sawed off. A cavity is then manually prepared in the neck of the femur using what are essentially xxxxxxxxx'x tools to receive the metal stem upon which the new metallic ball joint will sit. The acetabular cup may be repaired and a new ceramic or metal lining inserted.
Stems come in a small range of sizes and a smaller range of neck angles. These currently available products would be adequate if internal femoral anatomy was invariant. However, our studies show a wide range of internal femoral anatomies. We believe that the complication rate results from the relatively crude manual preparation methods for the receiving cavity and the limited range of stem sizes and shapes available.
The Monogram solution has three major components
· A set of proprietary algorithms which automatically segment the patient's femur from a conventional CT scan and automatically and within minutes defines a unique cutting path for the cavity and a matching unique shape for the stem.
· A programmable robotically assisted bone cutting mill that registers accurately with the patient's femur and xxxxx out a precisely shaped cavity.
· A 3D printed custom stem designed to maximize implant cortical bone interactions and which matches the size and shape of the robotically milled cavity
A number of the large orthopaedics companies have recently moved into robotically assisted surgery through acquiring smaller robotics companies. However, none of these robots can be considered essential as they can only do what surgeons do manually now.
Similarly, investment in 3D printing or Additive Manufacturing facilities by the large orthopaedics companies has been accelerating in recent years. However, it is not clear that any of them are printing custom implants.
Strictly Confidential
To the best of our knowledge, no company other than Monogram is combining the use of 3D printing and robotic milling to scalably drive mass customization of hip implants manufacturing unique implants that match the anatomy of individual patients.
Monogram will adopt an optimally capital efficient approach. Monogram
· Will not develop its own robot from scratch but will instead use the Kuka iiwaa medical robot
· Will not invest in manufacturing plants initially or inventory as each implant will be made in a batch size of one
· Will work with experienced OEMs to develop and manufacture the cutting and milling tools
· Will work extensively with experienced CROs and consultants on an as needed basis.
The FDA has published guidance on surgical robots and 3D printing as a manufacturing technology. We are confident that the regulatory path for both the stem and the robot will be via the 510(k) approval process.
Monogram is raising $12-13mm to hire additional staff, develop and gain 510(k) approval for the robot and the stem and to conduct a post-approval clinical trial to examine the frequency of post-surgical complications.
Strictly Confidential
Introduction, Markets and Opportunity
Joint Replacement
In 2015 there were a reported 378,000 hip replacement surgeries in the US and the market is expected to grow to 510,000 by 2020. Globally hip replacement surgery generated $7 billion in revenue for suppliers of stems, cups, liners and associated surgical tools. The major reason for such surgery is osteoarthritic degeneration of the hip joint which is a major load-bearing joint. Two principal reasons for growth in the market are the aging of the population and the increased incidence of obesity. The more obese a person is the more weight the hips have to bear and the more they are subject to deterioration. In a study published by the CDC looking at the demographics of patients who had their hips replaced the fastest growing age group between 2001 and 2010 was those between 45 and 54 years of age. Such individuals want to remain active and are more demanding than much older more sedentary patients. 925,000 knees were replaced in the US in 2015 and worldwide the knee replacement market generated $8.5 billion in revenue.
Current Practice and Market Opportunity
There are two major approaches to surgery for hip replacement. One involves an anterior approach and incision and the other involves a posterior incision and approach. Minimally invasive approaches are also possible. After making the incision and exposing the hip joint from the surrounding muscles the hip is dislocated and the ball at the head of the femur is sawn off. The cavity into which the stem will be placed is prepared using devices referred to as broaches which are similar to carpenters' files or chisels. The surgeon then hammers the stem into the prepared cavity and attaches the new ceramic or metal head. The diseased bone tissue in the acetabulum or cup in the pelvis is removed and a porous coated cup placed into the acetabulum. The ball is relocated into the socket and the incisions closed.
Hip replacement surgery works very well but it is not without its problems. Complications and adverse events with hip replacement surgery are relatively common, with up to 30% of patients having at least a single unwanted outcome. The most common adverse events are: uneven legs, >20%; early loosening, 5%; peri-operative fractures, >5%; and early dislocation, 2-5%. One source for these problems is inter-surgeon variability. As Figure 1 (below) shows, surgeons vary widely in the force they apply when forcing home the implanted stem.
Surgeons' Variable Impact Force
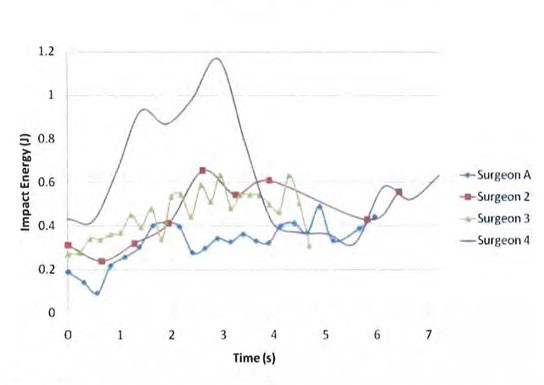
Figure 1
The images on the following page (Figure 2) illustrate two other factors that contribute to these complications. First, most surgical planning is still done manually from two-dimensional x-ray images which do not reveal all the nuances of geometry within the hip joint. Second, manual tools are still very widely used and intra-surgeon variability in technique as well as in force as well as the great variability in patient's anatomy all contribute to less than ideal outcomes
Figure 2
The Comprehensive Care for Joint Replacement Model (also known as "Bundling")
The Comprehensive Care for Joint Replacement Model was introduced on April 1, 2016 by the Centers for Medicare and Medicaid Services ("CMS") replacing the former, more traditional, fee for service reimbursement model. Its goal is to support better and more efficient care for Medicare beneficiaries undergoing hip or knee replacement surgery, the most common surgeries provided for these beneficiaries. This model was proposed because CMS observed that complication rates varied over a threefold range among hospitals while Medicare expenditures showed a twofold variation between different geographic regions. The model is being implemented in 67 Metropolitan Statistical Areas ("MSAs") and about 800 hospitals are participating.
Under this new model, an episode of care begins when a patient is admitted for joint replacement surgery and ends 90 days after the patient is discharged. It holds hospitals financially accountable for the quality and cost of a joint replacement. Each year participating hospitals are provided with a target price for lower joint replacement surgery and care. At the end of the model year hospitals that deliver quality care for less than the target price are eligible to receive a payment for the difference in actual episode spending and the target price. Hospitals whose costs exceed the target price will be financially responsible for paying Medicare a set fraction of the excess costs.
It is thus in a participating hospital's interests to maximize the quality of outcomes and minimize costs beyond those for the initial surgery and rehabilitation. It might also be expected that hospitals will keep a keen eye on how an individual surgeon's patients respond to and recover from surgery. Monogram believes that its surgical procedure which minimizes inter-surgeon variability and which does away with major factors that lead to complications will be looked upon favourably by surgeons, hospitals and payers.
The Monogram Solution
Rather than surgically modifying the patient's anatomy so that the off the shelf stem will fit Monogram believes it should be the other way round – the stem should be designed to fit the patient's anatomy. Monogram believes that complication and failure rates can be much reduced by acknowledging that patients have varied internal femoral anatomy and by designing and manufacturing patient specific implants. This is now possible because of advances in robotic surgery and additive manufacturing. Robotic surgery allows precise milling of the femoral cavity in a way that is much more precise than currently used manual methods. Additive manufacturing allows the economic manufacturing of products in a batch size of one. Thus, we can develop the concept of mass customization. Monogram's major contribution to this process is a cloud based software process (see figure 3, the final workflow slide) which from a standard CT scan
· | automatically produces a segmented 3D image of the femur within minutes |
· | automatically designs the optimum implant for that femur |
· | develops a file for the robot that encodes the milled cavity and cut path |
· | develops a file for the 3D printer that encodes the precisely shaped replacement stem that fits precisely in the robotically milled cavity. |
The Monogram workflow: from CT scan to custom stem and matched cavity
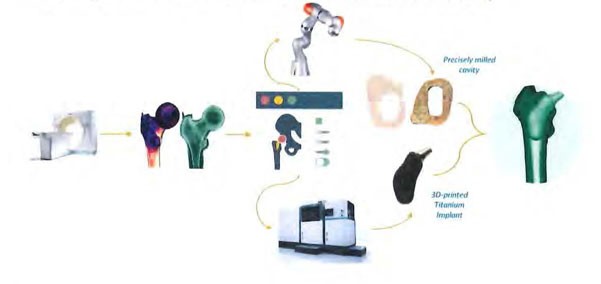
Figure 3
Several features are taken into account in designing the implant and, by extension the cavity. The major goal is to design an implant that maximizes outer cortical bone-implant contact and which in any case provides a minimum of at least 75% surface area contact. Minor modifications may be made to this optimal design to ensure that the cavity can be cut by the robot and that the implant can be inserted. Figure 4 (left) shows two views of an initial stem (in red) design that cannot be inserted. Note the small lateral volume at the left defined by the yellow line which impedes insertion. Figure 4 (right) show the modified stem with the problematic volume removed and then inserted in the original cavity.
Figure 4
Figure 5a (left) shows a cavity which has barriers preventing the complete cutpath from being traced. The milling head hits the area highlighted in yellow which have been removed in the cutpath in figure 5b (right) where the desired cutpath is unimpeded.
Figure 5
{Here we will insert the data from the technical POC experiments demonstrating i) less micromotion with our stem than with conventional manual methods; ii) more implant- cortical bone surface area contact than with conventional implants; and iii) far higher forces applied to the implant are needed to fracture the femur bearing a custom stem than are required to fracture a femur with a conventional implant. The two major causes of revision hip surgery are looseness and fractures. We believe that Monogram's solution will overcome both of these problems.}
3D printing allows not only custom patient specific implants to be manufactured it also allows the design of surface features on the implant that are not possible with conventional manufacturing methods. Figure 6a (left)show the surface of a monogram implant and Figure 6b (right)shows the surface at higher resolution. A number of studies have demonstrated that cortical bone ingrowth is much enhanced with microtrabecular structures like this. Thus, In addition to the cortical bone conforming shape we also believe that the enhanced bone ingrowth will further stabilize the implant and lead to better outcomes. {We should try to demonstrate that there is enhanced bone ingrowth.}

Figure 6a (left) and 6b (right) show a prototype Monogram stem and a higher resolution image of its surface to show the microtrabecular lattice
Monogram expects that its approach to hip replacement surgery will result in better outcomes and better restoration of each patient's anatomy providing the following benefits:
1) | The length, width and angles will be faithfully reproduced fully restoring the natural biomechanics resulting in what is referred to as a "forgotten hip". |
2) | The perfect match of the cavity and the implant dependent on the precise mapping of the inner cortical bone of the femur will result in high cortical bone-stem contact area, more bone ingrowth into the stem and increased stability. Because of this, bone conserving stems substantially shorter than conventional stems will be usable. |
3) | The functional anatomy of the patient's femur will also be matched so that the stiffness of the implant matches the stiffness of the patient's own bone resulting in less pain and less bone-wasting. |
Monogram's business model
Monogram has adopted an extremely capital efficient business model. Unlike other surgical robotics companies, Monogram will not invent its own robot but instead will partner with an established robotics company with a large installed base of robots both in manufacturing and in the medical setting. Similarly, Monogram will partner with an established medical device manufacturer with experience in designing and manufacturing handpieces for surgical robots. Finally, since each implant is made in a batch size of one for an individual patient, Monogram will have no major investments in plant, equipment or inventory.
Kuka, which has its headquarters just outside Munich, Germany, has an installed base of over 80,000 robots making it the 8th largest robotics company in the world. Currently, most of these robots are used in manufacturing settings but Kuka has begun moving aggressively into the medical space. Medical robots comprise just over 5% of the installed base now. Many of these robots are used for moving or positioning patients but importantly, Kuka adheres to IS0123456 and is already familiar with FDA requirements for medical devices.
Pro-Dex, based in Irvine, Ca is a high growth innovative technology company which designs and manufactures high value devices especially for the orthopedic sector. It lists among its customers such industry giants as Stryker, DePuy, Xxxxx & Xxxxxx, and Medtronic. Pro-Dex is recognized as a leader in powered surgical tools with over 40,000 handpieces manufactured by them sold by multiple large global distributors.
{EOS and 3D Systems Still deciding which one. Will know soon.}
Competition
The hip and knee replacement market is dominated by three companies, Stryker, ZimmerBiomet and Depuy Synthes, a division of Xxxxxxx and Xxxxxxx. Two other significant companies are Conformis because of its use of 3D printing and Xxxxx & Nephew because of its acquisition of Blue Belt Technologies.
Stryker operates in several business segments and generated $11.3 billion in revenue in 2016. $4.41 billion came from the orthopaedic sector and within that sector, $1.47 billion came from knee replacement surgery and $1.24 billion from hip replacement surgery. The company spent about $7.5mm on R&D in 2016 and about $4 billion on acquisitions. They have been investing heavily in 3D printing which they say minimizes manufacturing waste, allows for faster development times and more reproducible manufacturing of parts. They have introduced 3D printed components in the spine and knee replacement product lines. Recently they also announced a partnership with GE Additive, the 3D printing division of GE. In the press release announcing the partnership Stryker stated "Additive manufacturing allows Stryker to address design complexity and achieve previously unmanufacturable geometries."
Stryker acquired Mako Surgical in 2013 for $1.65 billion. At the time Xxxx had an installed base of about 174 robotic systems. That number has barely doubled since the time of acquisition. The list price of the Mako robotic system for use in total knee replacement surgery is $1mm.
Xxxxxx Biomet had total revenue in 2016 of $7,7 billion of which $2.75 billion came from knee replacement surgery and $1.87 billion came from hip replacement surgery. In 2016 ZimmerBiomet acquired Medtech SA the developer of the XXXX surgical platform for minimally invasive surgery in the brain and spine. They have stated that they intend to use the XXXX platform to migrate to other anatomies and at the AAOS meeting earlier this year unveiled the XXXX total knee replacement system concept. It is not clear if ZimmerBioMet has committed any resources to 3D printing.
Depuy Synthes is a division of Xxxxxxx &Johnson which had a total of $71.9 billion in global revenue in 2016. Medical devices accounted for $25.1 billion. Of that, orthopaedics accounted for $9.3 billion with knee replacement surgery accounting for $1.36 billion and hip replacement surgery for $1.52 billion. It is not clear if Depuy has made any investments in 3D printing or robotics.
Xxxxx & Xxxxxx had total revenue of $4.67 billion in 2016 with $932 million conning from knee replacements and $597 million from hip replacements. They acquired Blue Belt Technologies in 2016 for its NAVIO system which provides robotic assistance to surgeons carrying out partial knee replacements and more recently, total knee replacements. The NAVIO system is a hand held robotic system designed to assist surgeons with implant alignment, ligament balancing and bone preparation. Xxxxx & Nephew uses 3D printing in the manufacture of acetabular cups.
Conformis uses a proprietary algorithm to convert CT scans into 3D models by mapping the articular surface of the knee joint, allowing diseased areas to be defined. This 3D model is used to design the implant and instruments for each patient. Conformis has been engaged in knee replacement for several years and in June of this year received FDA clearance for the use of its system in hip replacement. Earlier in 2017 the company announced the publication of an outcomes and economics study which demonstrated that patients with knees replaced using the Conformis system had lower adverse event rates, were less likely to be discharged to a high cost post-acute facility, had lower average costs of care and lower average costs of follow up care. Conformis' revenues for 2016 were $79.9 million.
What stands out from this brief overview is that these companies seem to consider 3D printing and robotics as functioning in discrete silos. Robotic tools allow surgeons to be more precise or perform surgery more quickly but do not allow them to do anything fundamentally novel. 3D printing allows faster development times and savings in manufacturing, but, again it is not being developed for anything fundamentally novel. None of them appears to have thought of combining the two technologies and maximizing the value obtainable from both technologies working synergistically by taking a novel approach to joint replacement surgery.
Intellectual Property and Licenses
Monogram has an exclusive world-wide license from Mount Sinai School of Medicine to the patent application entitled "Apparatus, Method and System for providing Customizable Bone Implants". The listed inventors are Xxxxxxx X Unis, Xxxxxxx Xxxxx and Xxxxxxxx Xxxxxx.
Regulatory
We expect both the robot and the implant to be approved in the US via the 510(k) pathway. The FDA held a workshop on robotically assisted surgery in 2015 (see link) xxxxx://xxx.xxx.xxx/XxxxxxxXxxxxxx/XxxxXxxxxx/XxxxxxxxxXxxxxxxxxxx/xxx000000.xxx at which Xxxxxx Xxxxxx, Acting Deputy Director Division of Surgical Devices stated “Robotically Assisted Surgical Devices (RASD) are technically not robots, since they are guided by direct user control. Milling RASD are currently regulated as 510K Class II devices under 21 CFR 882.4560 product code OLO "Orthopaedic Stereotaxic Instrument. Indicated for orthopedic joint or spine surgery."
In May 2016 the FDA issued draft guidance on additive manufacturing or 3D printing for medical devices, "Technical Considerations for Additive Manufactured Devices. Draft Guidance for Industry and Food and Drug Administration Staff. May 10, 2016" available at the link below (xxxxx://xxx.xxx.xxx/xxxxxxxxx/XxxxxxXXxxxxxx/XxxxxxXxxxxxxxxxxxxXxxxxxxx/Xx idanceDocuments/UCM499809.pdf). Some pertinent language from the draft guidance is quoted below.
"For medical devices, AM has the advantage of facilitating the creation of anatomically matched devices and surgical instrumentation by using a patient's own medical imaging."
"It is anticipated that AM devices will follow the same regulatory requirements as the classification and/or regulation to which a non-AM device of the same type is subject to. In rare cases, AM may raise different questions of safety and/or effectiveness."
This language noted above suggests the FDA is likely to consider 3d printing to be just another manufacturing method and will not impose novel requirement on devices manufactured in this way. More importantly, the FDA recognizes the potential of 3D printing to allow customization of devices for individual patients.
Reimbursement
Hip replacement surgery is covered by DRG 469 which covers major joint replacement of a lower extremity with major comorbidities or complications and DRG470 which covers the same procedure without complications or comorbidities. Payments for situations where the hip is also fractured are considerably higher. CMS introduced a five year pilot bundled payment plan effective April 1 2016 in an effort to determine if such an approach would lead to increased quality of outcomes and less variation in outcomes across different hospital settings. The pilot covers 800 hundred hospitals in 67 MSAs (Metropolitan Statistical Areas) which include most major population centers.
Founders and Management
Xxxxxxx X Xxxx, Founder and interim Chief Medical Officer of Monogram, is an Associate Professor of Orthopaedics and Director of the Surgical Robotics Laboratory at Mount Sinai school of Medicine, Dr Xxxx is board certified in orthopaedic surgery and received his MD from Case Western Reserve University and completed his training at Northwestern Memorial Hospital and Rush University Medical Center. Xxx Xxxxxx, President and CEO of Monogram, was educated at the Universities of Glasgow and Oxford in cell and molecular biology and obtained his MBA from The Xxxxxxx School of the University of Pennsylvania. He has been a practicing scientist and venture capitalist focusing on investments in seed and early stage companies in the life sciences. He has served on multiple boards and has been involved in the founding of seven other spin-outs from academic centers. Xxxx Xxxxxxx is VP Robotics. He received his undergraduate degree from Carnegie Mellon and his master's degree from MIT, Xxxxx to joining Monogram he was at the Jet Propulsion Laboratory and Rethink Robotics. Xxxxxxxx Xxxxxx is Director, Software Development and is currently on a scholarly leave of absence from Mount Sinai School of Medicine where he is a medical student, Xxxx Xxxxxxxx is a Senior Research Associate and was previously with the Laboratory for Orthopaedic Implant Design at NYU Hospital for Joint Diseases. Xxxxxxx Xxxxx is a consultant to Monogram and an Assistant Professor in the Department of Neurosurgery at Mount Sinai and Director of the Neurosurgery Simulation Core. He received his PhD from Purdue University. Xxxxxxx is an accomplished computational scientist. Xxxx Xxxxx is a consultant to Monogram offering advice and guidance on bone mechanics. Xxxx obtained his PH D from SUNY Downstate.
The company is in the process of establishing a Scientific Advisory Board and a Clinical Advisory Board.
Use of Proceeds, Budgets, Timelines and Milestones
Monogram is seeking to raise $12-13mm in a Series A financing to:
o develop and gain FDA approval for its custom hip stent
o develop and gain FDA approval for its milling robot
o complete a post-approval multi-center clinical trial to demonstrate the improved outcomes and reduced rate of complication when Monogram's approach to hip replacement surgery is used
o engage with acquirers with the goal of obtaining maximum financial returns for Monogram's investors.
The tasks to be accomplished and their expected completion dates are listed below.
Series A Milestones ($12-13mm, 2-2.5 years)
Year 1
Software
o Revise or rewrite, verify and validate imaging, design and surgical planning software including links to 3D printer and robot Ongoing
o Develop segmentation protocol for distal femur Q1
o Develop anatomic registration point algorithm (proximal and distal) Q2
o Refinement of implant contours and shape of removed bone volume to ensure implant can be inserted while maintaining the desired level of bone-implant contact Q1-2
o Identify "keep out" zones of soft tissue and essential bone from CT scans and 3D segmentation Q1-2
o Develop registration and navigation system to avoid "keep out" zones and keep them safe Q3-4
o Design GUI for surgical planning software to allow surgeon to create surgical plan and change implant design within accepted parameters (with consultants and CRO) Q3-4
Implant
o Select predicate implant(s) for 510(k) process Q1
o Create algorithm to determine proximal femoral stiffness and flexibility based on CT images, published data and Finite Element Analysis (FEA) Ql-3
o Based on the known mechanical properties of medical grade Titanium alloys use FEA to predict how altering the porosity of a given implant shape will change its stiffness and flexibility Q1-3
o Determine how to vary flexibility and stiffness of implants by means of varying size of solid core, extent of macroporous interior and extent of surface microporous components Q3-4
o Manufacture multiple implants at prototyping site with a programmed range of degrees of stiffness and flexibility that match the range of properties for human bone Q3-4 (concurrent with bullets 2 and 3 above on FEA efforts)
o Establish post-printing processing to remove residual Titanium dust Q3-4
o Establish sterilization and other post-printing procedures Q3-4
Robot
o Select predicate robot(s) for 510(k) process Q1
o Determine functional parameters of robot/user interface (e.g., active, passive, active assist, haptics vs no haptics, visual, audible feedback, etc.) with robotic design consultants and IP attorneys to maximize "space to operate” Q1-2
o Complete integration of navigation system with robot, i.e., register bone, then cut precise cavity while perturbing the bone to model conditions that may arise during surgery Q4
o Freeze design of bur and end effector Q3
o Configure robot with necessary safety and ergonomic features (in collaboration with regulatory and design consultants) Q3 and ongoing
Regulatory, etc.
o File patents broadly on custom implants, acetabular cups, methods, etc. Q1 and ongoing
o Scope out possible second product opportunities Q3-4
Post-approval clinical trial
o Identify investigators Q2-3
o design trial - number of sites, number of patients, outcomes, protocols - in conjunction with CRO Q2-3
o seek IRB approvals Q3-4
Year 2
Software
o Design GUI for surgical planning software to allow surgeon to create surgical plan and change implant design within accepted parameters (with consultants and CRO) Q1-2
o Prepare and submit 510(k) for elements of software package Q1-3
o Receive FDA clearance for software package Q4
Implant
o Carry out mechanical strength and fatigue testing according to FDA protocol; (outsourced to CRO) Q1-2
o Incorporate industry standard 12/14 taper trunion with appropriate post-processing (Contract Manufacturer/Designer) Q1
o Demonstrate substantial equivalence of Monogram implants to predicate implants (outsourced to CRO) Q1-2
o Prepare and submit 510(k) for implants (outsourced to regulatory consultants) Q1-3
o Receive FDA 510(k) clearance for implants Q4
Second Product (knee)
o Investigate requirements for custom knee implants Q1-2
o Obtain CT scans of patients' knees for algorithm development/refinement Q1
o Develop knee specific software algorithm and initiate work with foam bones Q2-3
o Develop regulatory path and protocol Q2-3
Robot
o Prepare and submit 510(k) for robot (outsourced to regulatory consultants) Q1-3
o Receive FDA clearance for robot Q4
Regulatory, etc.
o Run validating cadaveric trials to show safety and accuracy of system (design with regulatory/clinical consultants) Ql-2
o Repeat experiments to determine the amount of bone-implant contact in robot/custom and manual/standard implants (outsourced to a CRO) Q1
o Calculate and compare contact with cortical and cancellous bone and ratio of total surface contact area to total implant surface area (outsourced to a CRO) Q2
o Repeat determination of range of micromotion for custom and standard implants in cadaveric bone (outsourced to a CRO) Q1
o Carry out MicroCT of stems to confirm no Ti powder residue (outsourced to a CRO) Q2
o File patents broadly Ongoing
o Draw up development plans for knee implants or another second- generation product Q3-4
Post-approval clinical trial
o Idventify investigators Q2
o design trial - number of sites, number of patients, outcomes, protocols - in conjunction with CRO Q2
o seek IRB approvals Q3-4
Exit/Financing
o Decide: go it alone or hire bankers. If the latter, interview select group and choose? Q2
o Create list of possible investors for Series B and of likely acquirers Q2
o Introductory meetings with potential investors and acquirers Q2 and ongoing
Year 3
Post-approval clinical trial
o Initiate trial Q1
o Complete trialQ2
o Analyze data and prepare manuscript for publication Q3-4
Exit/Financing
o Solicit bids/interest from potential acquirers Q1
o Final bids from top three candidates Q2
o Complete acquisition Q3
The pro Forma budgets for years 1-3 are shown on the following page
| Monogram Expenses in $000s) | |||||||||
| Year 1 | Year 2 | ||||||||
| Q1 | Q2 | Q3 | Q4 | Xxx. | Q1 | Q2 | Q3 | Q4 | Xxx. |
Payroll | 175 | 175 | 345 | 345 | 1040 | 300 | 300 | 375 | 375 | 1350 |
Benefits | 26.25 | 26.25 | 38.25 | 38.25 | 129 | 45 | 45 | 56.25 | 56.25 | 202.5 |
HR (cloud) | 0.5 | 0.5 | 0.5 | 0.5 | 2 | 1 | 1 | 1 | 1 | 4 |
Controller | 10 | 10 | 10 | 10 | 40 | 25 | 25 | 25 | 25 | 100 |
Rent | 12 | 12 | 12 | 12 | 48 | 25 | 25 | 25 | 25 | 100 |
Supplies | 45 | 45 | 65 | 65 | 220 | 75 | 75 | 95 | 95 | 340 |
Consulting | 15 | 15 | 15 | 15 | 60 | 50 | 50 | 50 | 50 | 200 |
CROs | 0 | 0 | 0 | 0 | 0 | 75 | 75 | 75 | 75 | 300 |
Regulatory | 25 | 25 | 25 | 25 | 100 | 250 | 250 | 250 | 250 | 1000 |
Clinical | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Legal | 12.5 | 12.5 | 50 | 50 | 125 | 25 | 25 | 25 | 25 | 100 |
IP | 21.5 | 12.5 | 25 | 25 | 84 | 25 | 25 | 25 | 25 | 100 |
T&E | 5 | 7.5 | 7.5 | 5 | 25 | 10 | 10 | 10 | 10 | 40 |
Robot | 90 | 0 | 0 | 0 | 90 | 180 | 0 | 180 | 0 | 360 |
Computers | 6 | 0 | 5 | 0 | 11 | 10 | 10 | 10 | 10 | 40 |
Furniture | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
Total | 445.75 | 341.25 | 598.25 | 590.75 | 1976 | 1096 | 916 | 1202.25 | 1022.25 | 4236.5 |
Notes: | Notes: |
4 FTEs Ql-2 | 10 FTEs Q1-2 add engineers |
6FTEs Q3-4 add 2 engineers | 11FTEs Q3-add CMO |
Supplies = 25% of payroll | |
| Year 3 | | |||||
| Q1 | Q2 | Q3 | Q4 | Xxx. | | |
Payroll | 375 | 375 | 375 | 375 | 1500 | | |
Benefits | 63 | 63 | 63 | 63 | 252 | | |
HR (cloud) | 1 | 1 | 1 | 1 | 4 | | |
Controller | 45 | 45 | 45 | 45 | 180 | | |
Rent | 25 | 25 | 25 | 25 | 100 | | |
Supplies | 84 | 84 | 84 | 84 | 336 | | |
Consulting | 50 | 50 | 50 | 50 | 200 | | |
CROs | 25 | 25 | 25 | 25 | 100 | | |
Regulatory | 500 | 250 | 100 | 100 | 950 | | |
Clinical | 1000 | 1000 | 500 | 0 | 2500 | | |
Legal | 25 | 25 | 25 | 25 | 100 | | |
IP | 50 | 50 | 50 | 50 | 200 | | |
T&E | 15 | 15 | 10 | 10 | 50 | | |
Robot | 0 | 0 | 0 | 0 | 0 | | |
Computers | 3 | 3 | 3 | 3 | 12 | | |
Furniture | 2 | 2 | 2 | 2 | 8 | | |
Total | 2263 | 2013 | 1358 | 858 | 6492 | 12704.5 | |
Notes: | |
12 FTEs Q1-add CFO | |
Notes: | |
12 FTEs Q1-add CFO | |
Should the Company decide to begin a sales and marketing effort by itself that would begin in 2019 and will require significant external financing. Revenue projections with assumptions are shown in the table below.
Monogram Revenue Projections (in $ '000s)
| 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 2026 |
Robots # | 5 | 25 | 40 | 65 | 100 | 100 | 100 | 100 |
Robots $ | 750 | 3,750 | 6,000 | 9,750 | 15,000 | 15,000 | 15,000 | 15,000 |
Robots Cumulative | 5 | 30 | 70 | 135 | 235 | 335 | 435 | 535 |
Service | 38 | 225 | 525 | 1,013 | 1,763 | 2,513 | 3,263 | 4,013 |
Stems # | 100 | 1,000 | 4,275 | 11,425 | 34,675 | 42,375 | 66,750 | 95,000 |
Stems $ | 250 | 2,500 | 10,688 | 28,563 | 86,688 | 105,938 | 166,875 | 237,500 |
Heads, etc. $ | 200 | 250 | 8,550 | 22,850 | 69,350 | 84,750 | 133,500 | 190,000 |
Handpieces $ | 50 | 500 | 2,138 | 5,713 | 17,338 | 21,188 | 33,375 | 47,500 |
Burs $ | 15 | 150 | 641 | 1,714 | 5,201 | 6,356 | 10,013 | 14,250 |
Saws $ | 15 | 150 | 641 | 1,714 | 5,201 | 6,356 | 10,013 | 14,250 |
Reg/Nav $ | 25 | 250 | 1,069 | 2,856 | 8,669 | 10,594 | 16,688 | 23,750 |
Total $ | 1,343 | 7,775 | 30,252 | 74,172 | 209,209 | 252,694 | 388,727 | 546,263 |
Assumptions: Robot sells for $150,000; Stem for $2,500; Annual Service for Robot $7,500;
One each of handpiece, bur and saw per stem priced at $500, $150 and $150 respectively;
One each of head, socket and liner per stem priced at $2,000;
Reg/Nav: Registration and Navigation disposables priced at $250 per stem.
Annual Stem use per Robot | | | | | | | |
Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | Year 6 | Year 7 | Year 8 |
20 | 100 | 175 | 250 | 300 | 300 | 300 | 300 |
For comparison, initial revenues and Sales & Marketing expenses for Mako Surgical and Conformis are shown in the table below.
Mako Historical Revenues (in $'000s)
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 (9mos) |
Revenue | 62 | 335 | 2,944 | 34,208 | 44,296 | 84,507 | 102,719 | 75,800 |
SG&A | | | | | | 67,965 | 76,992 | |
| | | | | | | | |
Net Income | | | (37,647) | (34,023) | (38,687) | (36,143) | (32,551) | |
Conformis Historical Revenues (in $'000s) | | | | | ||
| 2012 | 2013 | 2014 | 2015 | 2016 | |
Revenue | 24,644 | 34,597 | 48,186 | 66,867 | 79,899 | |
S&M | 26,070 | 26,149 | 29,367 | 37,588 | 41,086 | |
| | | | | | |
Net Income | (47,501) | (47,889) | (45,722) | (57,246) | (57,588) | |
Exhibit D
Form of Quarterly Royalty and Sublicense Income Report

Exhibit E
Client and Billing Agreement
The Icahn School of Medicine at Mount Sinai (“Mount Sinai”), a New York not-for-profit education corporation, organized under the laws of New York, and having an address at Xxx Xxxxxxx X. Xxxx Xxxxx, Xxx Xxxx, XX
10029; and Monogram Orthopedics (“Company”), a Delaware corporation, with a principal place of business at New Lab, Building 000, Xxxxxxxx Xxxx Xxxx, Xxxxxxxx, XX 00000, have entered into a License Agreement with respect to certain inventions which are the subject of the patent applications and patents listed in Appendix A hereto, including any continuations, divisions, extensions thereof, and any foreign counterpart patents, applications, or registrations (“Patent Rights”);
Mount Sinai has retained the services of [LAW FIRM NAME] (“Law Firm”), with offices [LAW FIRM ADDRESS], to prepare, file and prosecute the pending patent applications constituting the Patent Rights and to maintain the patents that issue thereon;
Law Firm represents Mount Sinai, not Company and has no duties to Company which has its own independent counsel. Law Firm’s client, Mount Sinai, has agreed to share Law Firm’s communications with Company, and has asked Law Firm to communicate with and send its bills directly to Company with copies to Mount Sinai. Pursuant to this Agreement, Company has agreed to pay those bills in accordance with the terms set forth below in this Client and Billing Agreement. Mount Sinai and Company have concluded that they share a common legal interest regarding the Patent Rights, and that their common interest would be served by exchanging information regarding the Patent Rights.
Mount Sinai, Company and Law Firm, intending to formalize their business relationships, agree as follows:
1. | Mount Sinai is the owner of the Patent Rights. |
2. | Company is the licensee of Mount Sinai’s interest in the Patent Rights. |
3. | Mount Sinai shall maintain its existing attorney-client relationship with Law Firm in furtherance of efforts to secure and maintain the Patent Rights. |
4. | Law Firm will interact directly with Company on all patent prosecution and patent maintenance matters related to the Patent Rights and will copy Mount Sinai on all correspondence related thereto. Law Firm agrees to notify Mount Sinai and Company in writing reasonably in advance of the due date or deadline for any action in connection with any patent application within the Patent Rights, and of Mount Sinai’s right to file any continuing application or foreign counterpart application based on the Patent Rights, and extension of patent term. In any case, Company shall give Mount Sinai written notice of any final decision regarding the action to be taken or not to be taken prior to or at the same time as instructing Law Firm to implement the decision. For Patent Rights that are solely owned by Mount Sinai, Mount Sinai reserves the right to countermand any instruction given by Company to Law Firm, so long as doing so does not adversely affect the scope of any claim covering a Company product and provided that a) the countermand is notified to Company before the due date or deadline for any action, b) is based on reasonable grounds and c) that Mount Sinai pays any additional costs incurred as a result of countermanding Company’s decision. |
5. | Law Firm’s legal services relating to the Patent Rights will be performed on behalf of Mount Sinai. Law Firm shall invoice Company directly for all work relating to the filing, prosecution and maintenance of the Patent Rights and shall provide copies of all invoices to Mount Sinai. Law Firm shall provide annual estimates to Company for all anticipated work to be carried out in advance of carrying out such work, and shall notify Company and provide updated cost estimates in the event the cost of the work exceeds the original cost estimate. Company is responsible for the payment of all charges and fees so invoiced by Law Firm. Company will pay invoices due by Company directly to Law Firm and copy Mount Sinai on each payment. |
6. | To clarify each party’s position with regard to prosecution and maintenance of the Patent Rights, Company will notify Law Firm in writing of all decisions to authorize the performance of any desired service(s), which shall be subject to Mount Sinai’s right to countermand, as provided in paragraph 4, above for Patent rights solely owned by Mount Sinai. In the event Mount Sinai countermands any decision or instruction of Company, such countermand shall be promptly communicated in writing to Law Firm and Company. |
7. | This agreement represents the complete understanding of each of the undersigned parties as to the client and billing arrangements defined herein. Additions or deletions of dockets identified in Appendix A will become effective only by written addendum to Appendix A. All such additions or deletions of individual patents or applications filed in the US, or as foreign counterparts thereof are considered to be within the terms of this client and billing agreement. |
8. | Notices and copies of all correspondence relating to the Patent Rights should be sent to the following: |
To MOUNT SINAI: | To COMPANY: |
Mount Sinai Innovation Partners | Monogram Orthopedics |
Icahn School of Medicine at Mount Sinai | New Lab, Building 128 |
One Xxxxxxx X. Xxxx Place, Box 1675 | Brooklyn Navy Yard |
New York, NY 10029 | Brooklyn, NY 11251 |
| |
Attn: Senior Vice President | |
To LAW FIRM:
ACCEPTED AND AGREED TO: |
|
|
| |
|
|
|
| |
ICAHN SCHOOL OF MEDICINE |
| MONOGRAM ORTHOPEDICS | ||
AT MOUNT SINAI |
|
|
| |
By: |
|
| By: |
|
Name: |
|
| Name: |
|
Title: |
|
| Title: |
|
Date: |
|
| Date: |
|
|
|
|
|
|
LAW FIRM |
|
|
| |
By: |
|
|
|
|
Name: |
|
|
|
|
Title: |
|
|
|
|
Date: |
|
|
|
|
Exhibit F:
Capitalization Table for Monogram Orthopedics
Monogram Pro Forma Cap tables 2-27-17
Seed Round | | | Post Series A | |
Owner | Number | % | Number | % |
Unis | 950,000 | 28.82 | 950,000 | 5 |
Costa | 285,000 | 8.65 | 285,000 | 1.5 |
XxXxxxx | 285,000 | 8.65 | 285,000 | 1.5 |
Xxxxx | 47,500 | 1.44 | 47,500 | 0.25 |
Sumani | 285,000 | 8.65 | 285,000 | 1.5 |
Xxxxxx | 950,000 | 28.82 | 950,000 | 5 |
MSSM | 395,617 | 12 | 1,692,308 | 12 |
Pro-Dex | 800,000 | 4.2 | | |
unallocated | 98,691 | 2.99 | 1,800,192 | 9.48 |
Investors | 0 | 0 | 12,000,000 | 63.2 |
Total | 3,296,808 | 100 | 19,095,000 | 104 |
Assumes $12,000,000 at $1/share
MSSM has anti-dilution protection to 12% stake until $10mm is raised Pro-Dex gets rights now to receive 800,000 Series A at closing of Series A
Everyone except Xxxxx: 25% vested, remainder vesting over 3 years Xxxxx: 100% vested


