AMENDED AND RESTATED CANCER LICENSE AGREEMENT
Exhibit 10.16
|
Confidential Materials omitted and filed separately with the |
|
Securities and Exchange Commission. Double asterisks denote omissions. |
AMENDED AND RESTATED CANCER LICENSE AGREEMENT
THIS AMENDED AND RESTATED CANCER LICENSE AGREEMENT (this “Agreement”) dated as of April 28, 2016 (the “Restatement Date”) is entered into between TMRC Co., Ltd., a Japanese corporation having a place of business at 1-12-12, Xxxx Xxxxxxxx, Xxxxxxxx-xx, Xxxxx 000-0000, Xxxxx (“TMRC”) and Syros Pharmaceuticals, Inc., a Delaware corporation having a place of business at 000 Xxxxxxxx Xxxxx, Xxxxx 000, Xxxxxxxxx XX 00000 XXX (“Syros”).
WHEREAS, TMRC and Syros previously entered into a Cancer License Agreement (the “Original Agreement”) dated as of September 11, 2015 (the “Original Agreement Effective Date”).
WHEREAS, TMRC owns or has rights in the Technology (as defined below).
WHEREAS, pursuant to the Original Agreement, Syros obtained an exclusive license under TMRC’s rights in the Technology on the terms and conditions set forth therein.
WHEREAS, the parties desire to amend and restate the Original Agreement in its entirety as set forth herein.
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, the parties hereby agree as follows:
|
1. |
AMENDMENT AND RESTATEMENT |
The Original Agreement is hereby amended and restated in its entirety as set forth herein.
|
2. |
DEFINITIONS |
For purposes of this Agreement, the terms defined in this Section 2 shall have the respective meanings set forth below:
2.1.“Affiliate” means, with respect to any Person, any other Person which directly or indirectly controls, is controlled by, or is under common control with, such Person. A Person shall be regarded as in control of another Person if it owns, or directly or indirectly controls, at least fifty percent (50%) of the voting stock or other ownership interest of the other Person, or if it directly or indirectly possesses the power to direct or cause the direction of the management and policies of the other Person by any means whatsoever.
2.2.“API” means active pharmaceutical ingredient in bulk form that is manufactured from the Technology.
2.3.“Business Day” means a day that is not a Saturday, Sunday or a day on which banks in Boston, Massachusetts are authorized to remain closed.
2.4.“Clinical Trial” means a Phase I Clinical Trial, a Phase II Clinical Trial or a Phase III Clinical Trial.
2.5.“Competent Authority” means, collectively, the governmental authorities in each country responsible for (a) the regulation of any Product intended for use in the Field, including the FDA, (b) the establishment, maintenance or protection of rights related to the Patent Rights licensed hereunder, or (c) any other applicable regulatory or administrative agency in any country in the Territory that is comparable to, or a counterpart of, the foregoing.
2.6.“Confidential Information” means any proprietary or confidential Know-how of a party which is disclosed (whether in written, graphic, oral, electronic or other form) by or on behalf of such party (the “Disclosing Party”) to the other party (the “Receiving Party”) pursuant to this Agreement, including: information regarding the Disclosing Party’s technology, products, programs, business, financial status, biological or chemical substances, formulations, techniques, methodology, equipment, sources of supply, patent positioning or business plans. In addition, the terms of this Agreement shall be deemed Confidential Information of each party, and neither party may rely on clause (ii) to exclude the terms of this Agreement from Confidential Information. Confidential Information excludes any information that: (i) is or becomes generally known to the public other than by fault of the Receiving Party or its Personnel or by breach of this Agreement, (ii) is demonstrated by documentation to have been in the Receiving Party’s possession at the time of disclosure, (iii) was disclosed to the Receiving Party on an unrestricted basis from a source not under a duty of confidentiality to the Disclosing Party or (iv) is independently developed by the Receiving Party without reference or reliance upon any of the Disclosing Party’s Confidential Information.
2.7.“Control” or “Controlled” means, with respect to a Person and an item of or right under Know-how or Patent Rights, the ability of such Person or its Affiliates, whether by ownership or a license (other than pursuant to a license granted under this Agreement), to assign, transfer or grant access to, or a license or sublicense under, such item or right as provided for herein without violating the terms of any agreement or other arrangement with any Third Party.
2.8.“Data Room” means the electronic data room by which TMRC shared certain materials with Syros under the Memorandum between the parties dated as of July 16, 2015.
2.9.“EMA” means the European Medicines Agency of the European Union, or the successor thereto.
2.10.“FDA” means the Food and Drug Administration of the United States, or the successor thereto.
2.11.“Field” means any and all uses for the treatment of human cancer indications, excluding supportive care.
-2-
2.12.“First Commercial Sale” shall mean, with respect to any Product, the first sale of such Product after all applicable marketing and pricing approvals (if any) have been granted by the applicable Competent Authority for the applicable country.
2.13.“IND” or “Investigational New Drug Application” means an Investigational New Drug Application filed with the FDA in the United States or any equivalent counterpart in any country other than the United States, including any supplements or amendments thereto.
2.14.“Know-how” means any information, inventions, know-how, data, results or materials, whether patentable or not, including: (a) ideas, discoveries, improvements or trade secrets, (b) pharmaceutical, chemical or biological materials, products, compositions, formulae or processes, (c) tests, assays, techniques, methods, procedures, formulas or processes, (d) technical, medical, clinical, toxicological or other data or other information relating to any of the foregoing, and (e) drawings, plans, designs, diagrams, sketches, specifications or other documents containing or relating to such information, inventions, know-how, data, results or materials; including any intellectual property rights therein except for Patent Rights covering the foregoing.
2.15.“Know-how Royalty Term” means, with respect to each Product, the term commencing on the First Commercial Sale of such Product anywhere in the Territory and ending on the seventh (7th) anniversary of such First Commercial Sale.
2.16.“Law” means any laws (including common law), statutes, regulations, rules, executive orders, supervisory requirements, licensing requirements, export requirements, directives, circulars, opinions, decrees, interpretive letters, guidance or other official releases of or by any Competent Authority or other governmental authority.
2.17.“Licensed Know-how Rights” means Know-how that is within the TMRC IP Rights.
2.18.“Licensed Patent Rights” means Patent Rights that is within the TMRC IP Rights, including the Patent Rights set forth on Exhibit A.
2.19.“NDA” means a New Drug Application, or similar application for marketing approval of a Product for use in the Field submitted to the FDA, or its foreign equivalent.
2.20.“NDA Acceptance” means acceptance for filing of an NDA by the applicable Competent Authority in the applicable jurisdiction.
2.21.“NDA Approval” means (a) in the case of the United States, approval by FDA of an NDA granting marketing approval for a Product; and (b) in the case of the European Union, approval by the EMA of an NDA granting marketing approval and pricing approval by the applicable Competent Authorities in any one (1) of the following countries: the United Kingdom, Germany, France, Italy or Spain.
2.22.“Net Sales” means, with respect to any Product, the gross sales price of such Product invoiced by Syros and its Affiliates and sublicensees to Third Party customers (who are not Affiliates) less, to the extent actually paid or accrued by Syros or its Affiliates or sublicensees (as applicable), (a) credits, allowances, discounts and rebates to, and chargebacks from the account of, such customers for nonconforming, damaged, outdated or returned Product; (b) freight and insurance costs incurred by Syros or its Affiliates or sublicensees (as applicable)
-3-
in transporting such Product to such customers; (c) cash, quantity and trade discounts, rebates and other price reductions for such Product given to such customers under price reduction programs; (d) sales, use, value-added and other direct taxes incurred on the sale of such Product to such customers; (e) customs duties, tariffs, surcharges and other governmental charges incurred in exporting or importing such Product to such customers; (f) sales commissions incurred on the sale of such Product to such customers; and (g) an allowance for uncollectible or bad debts determined in accordance with generally accepted accounting principles.
2.23.“Patent Family 3” means the Patent Rights identified as “Family 3” in Exhibit A.
2.24.“Patent Rights” mean patents, patent applications, utility models, and certificates of invention and other governmental grants for the protection of inventions (including any provisional application, continuation, continuation-in-part, divisional, reissue, renewal, re-examination, extension, or supplementary protection certificate granted in relation thereto, as well as any utility model, innovation patent, xxxxx patent, patent of addition, inventor’s certificate or equivalent in any country or jurisdiction).
2.25.“Patent Royalty Term” means, with respect to each Product in each country, the term, if any, during which (a) a Valid Claim remains in effect and would be infringed but for the license granted by this Agreement, by the use, offer for sale, sale or import of such Product in such country in the Field and (b) no Third Party generic product that has received marketing approval pursuant to an abbreviated regulatory process under which the applicable Third Party or Competent Authority has relied on data or other information in any Registration for the applicable Product is commercially available in such country.
2.26.“Person” means an individual, corporation, partnership, limited liability company, trust, business trust, association, joint stock company, joint venture, pool, syndicate, sole proprietorship, unincorporated organization, governmental authority or any other form of entity not specifically listed herein.
2.27.“Phase I Clinical Trial” means any human clinical trial (whether including a phase 1a or a phase 1b trial) in any country, the principal purpose of which is a preliminary determination of safety in individuals or patients, that would satisfy the requirements of 21 C.F.R. §312.21(a), or an equivalent clinical study required by a Competent Authority outside of the United States.
2.28.“Phase II Clinical Trial” means any human clinical trial conducted in any country, intended to explore multiple doses, dose response and duration of effect to generate initial evidence of safety and activity in a target patient population, that would satisfy the requirements of 21 C.F.R. §312.21(b), or an equivalent clinical study required by a Competent Authority outside of the United States.
2.29.“Phase III Clinical Trial” means any human clinical trial in any country that would satisfy the requirements of 21 C.F.R. §312.21(c), or an equivalent clinical study required by a Competent Authority outside of the United States.
2.30.“Product” means any pharmaceutical product made from or comprising API for human use in the Field.
-4-
2.31.“Registration” means any and all permits, licenses, authorizations, registrations or regulatory approvals (including NDAs) required or granted by any Competent Authority as a prerequisite to the development, manufacturing, packaging, marketing or selling of any Product.
2.32.“Technology” means the compound known as AM80 (Tamibarotene) listed on Exhibit B, in all formulations.
2.33.“Territory” means (a) Xxxxx Xxxxxxx (xxx Xxxxxx Xxxxxx xx Xxxxxxx, Xxxxxx, and Mexico); (b) Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Liechtenstein, Lithuania, Luxembourg, Malta, Netherlands, Norway, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden, Switzerland and the United Kingdom; and (c) any other country to which the EMA’s regulatory jurisdiction extends.
2.34.“Third Party” means any Person other than TMRC, Syros and their respective Affiliates.
2.35.“TMRC IP Rights” means TMRC’s own rights and rights TMRC has the right to license consisting of: (1) (i) the patents and patent applications listed in Exhibit A; (ii) the foreign equivalent and national phase patent applications of (i); (iii) the patents proceeding from (i) and (ii); (iv) all claims of continuations-in-part that are entitled to the benefit of the priority date of (i) or any applications to which they make a claim of priority; and (v) divisionals, continuations, reissues, reexaminations, and extensions of any patent or application set forth in (i)-(iv) above, so long as said patents have not been held invalid and/or unenforceable by a court of competent jurisdiction from which there is no appeal or, if appealable, from which no appeal has been taken; (2) clinical data and preclinical data for Technology controlled by, or belonging to, TMRC; (3) Letter(s) of Authorization to reference the contents of the drug master file of TMRC’s licensor for Technology and all rights associated with and contents of the manufacturing records and analytical records of Technology which TMRC has rights to license; and (4) Know-how rights and Patent Rights that are Controlled by TMRC as of the Original Agreement Effective Date or come into the Control of TMRC at any time after the Original Agreement Effective Date that related to AM80 (Tamibarotene).
2.36.“Toko” means Toko Pharmaceutical Ind. Co., Ltd., a Japanese corporation.
2.37.“Toko Agreement” means the agreement between Toko and TMRC entitled “License Agreement for the development et al. of retinoid based drug in foreign countries” dated August 15, 2008.
2.38.“Toko Information” means information which is (a) owned, controlled or disclosed by Toko; and (b) subject to the provisions of the Toko Agreement.
2.39.“Toko Letter Agreement” means a Consent and Stand-by License Agreement between Toko and Syros that is substantially in the form of Exhibit C and reasonably satisfactory to Syros.
-5-
2.40.“Valid Claim” means (a) any claim in any issued and unexpired patent within the Licensed Patent Rights that has not been disclaimed, revoked or held invalid or unenforceable by a decision of a court or other governmental authority of competent jurisdiction from which no appeal can be taken, or with respect to which an appeal is not taken within the time allowed for appeal, and that has not been disclaimed or admitted to be invalid or unenforceable through reissue, disclaimer or otherwise; or (b) any claim of the Licensed Patent Rights in (a) that has not been finally abandoned or finally rejected and which has been pending for no more than [**] years from the date of filing of the earlier priority patent application.
|
3. |
REPRESENTATIONS AND WARRANTIES |
Each party hereby represents and warrants to the other party as follows:
3.1.Corporate Existence. Such party is a corporation duly organized, validly existing and in good standing under the Laws of the jurisdiction in which it is incorporated.
3.2.Authorization and Enforcement of Obligations. Such party (a) has the corporate power and authority and the legal right to enter into this Agreement and to perform its obligations hereunder, and (b) has taken all necessary corporate action on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder. This Agreement has been duly executed and delivered on behalf of such party, and constitutes a legal, valid, binding obligation, enforceable against such party in accordance with its terms.
3.3.No Consents. All necessary consents, approvals and authorizations of all governmental authorities and other Persons required to be obtained by such party in connection with license of this Agreement have been obtained.
3.4.No Conflict. The execution and delivery of this Agreement and the performance of such party’s obligations hereunder (a) do not conflict with or violate any requirement of applicable Laws, and (b) do not conflict with, or constitute a default under, any contractual obligation of such party.
In addition, TMRC hereby represents and warrants to Syros as follows:
3.5.IP Rights. TMRC (a) is the sole owner or exclusive licensee of, and Controls, the TMRC IP Rights, and except as set forth in Exhibit D, TMRC has not granted to any Third Party any license or other interest in the TMRC IP Rights in the Territory, (b) is not aware of any Third Party patent, patent application or other intellectual property rights that would be infringed (i) by practicing any process or method or by making, using or selling any composition which is claimed or disclosed in the Licensed Patent Rights or which constitutes Licensed Know-how Rights, or (ii) by making, using or selling Products, (c) Controls and has the right to license all TMRC IP Rights to Syros as provided herein (d) is not aware of any infringement or misappropriation by a Third Party of the TMRC IP Rights and (e)(i) all agreements relating to the Technology or any of the TMRC IP Rights between TMRC and Innovive Pharmaceuticals, Inc. (or its successors or assigns) (collectively, “CytRx”) have been terminated, except for a [**], terminating all other such agreements, (ii) [**], TMRC has unfettered rights to use all data and documents generated by CytRx or TMRC [**] relating to the Technology or any of the TMRC IP Rights, including the unfettered right to license and transfer such data and documents to Syros
-6-
as provided herein, (iii) all litigation between CytRx and TMRC has been settled and dismissed with prejudice and all claims between CytRx and TMRC have been released, in each case that pertain to the Technology or any of the TMRC IP Rights, and (iv) CytRx does not retain any right [**] or otherwise to terminate any right of TMRC to use or license data or documents generated by CytRx and TMRC pursuant to any agreement between CytRx and TMRC relating to the Technology or any of the TMRC IP Rights.
3.6.DISCLAIMER. EXCEPT AS EXPRESSLY STATED IN THIS AGREEMENT NOTHING IN THIS AGREEMENT IS OR SHALL BE CONSTRUED AS (i) A WARRANTY OR REPRESENTATION BY TMRC AS TO THE VALIDITY OR SCOPE OF ANY LICENSED PATENT RIGHTS; (ii) A WARRANTY OR REPRESENTATION THAT ANYTHING MADE, USED, SOLD OR OTHERWISE DISPOSED OF UNDER ANY LICENSE GRANTED IN THIS AGREEMENT IS OR WILL BE FREE FROM INFRINGEMENT OF PATENTS OR OTHER INTELLECTUAL PROPERTY OF THIRD PARTIES; OR (iii) A REPRESENTATION OR WARRANTY BY TMRC OF THE ACCURACY, SAFETY OR USEFULNESS FOR ANY PURPOSE OF ANY TMRC KNOW-HOW AT ANY TIME MADE AVAILABLE BY TMRC. TMRC SHALL HAVE NO LIABILITY WHATSOEVER TO SYROS OR ANY OTHER PERSON FOR OR ON ACCOUNT OF ANY INJURY, LOSS OR DAMAGE, OF ANY KIND OR NATURE, SUSTAINED BY, OR ANY DAMAGE ASSERTED OR ASSERTED AGAINST, OR ANY OTHER LIABILITY INCURRED BY OR IMPOSED ON SYROS OR ANY OTHER PERSON, ARISING OUT OF IN CONNECTION WITH OR RESULTING FROM (A) THE PRODUCTION, USE OR SALE OF ANY PRODUCT BY SYROS, OR THE PRACTICE OF THE LICENSED PATENT RIGHTS BY SYROS; OR (B) THE USE BY SYROS OF ANY TMRC KNOW-HOW, AND SYROS SHALL HOLD TMRC, OR ITS OFFICERS, EMPLOYEES OR AGENTS, HARMLESS IN THE EVENT TMRC, OR ITS OFFICERS, EMPLOYEES OR AGENTS, IS HELD LIABLE THEREFOR, EXCEPT TO THE EXTENT RESULTING FROM A BREACH BY TMRC OF ITS REPRESENTATIONS, WARRANTIES OR OBLIGATIONS HEREUNDER OR THE NEGLIGENCE OR WILLFUL MISCONDUCT OF TMRC OR ITS OFFICERS, EMPLOYEES OR AGENTS.
|
4. |
LICENSES AND OTHER GRANTS OF RIGHTS; OTHER AGREEMENTS |
4.1.TMRC IP Rights.
4.1.1.Grant. TMRC hereby grants to Syros an exclusive license (with the right to grant sublicenses) under the TMRC IP Rights (excluding Patent Rights as to which Syros has opted out of pursuant to Section 10.2) to conduct research and to develop, make, have made, use, offer for sale, sell and import Products in the Territory for use in the Field.
4.1.2.Ex-Territory Development. TMRC hereby grants to Syros a non-exclusive license (with the right to grant sublicenses) under the TMRC IP Rights to conduct research, to develop and to use Products outside the Territory (excluding all Asian countries) solely for the purpose of developing and commercializing Products for use in the Field in the Territory. For clarity, Syros shall not use TMRC IP Rights for the purpose of researching, developing or commercializing Products for use in the Field outside the Territory.
-7-
4.1.3.Manufacture. TMRC hereby grants to Syros a non-exclusive license (with the right to grant sublicenses) under the TMRC IP Rights to make and have made Products inside and outside the Territory solely for the purpose of manufacturing Products for use in the Field in the Territory.
4.2.Information Transfer. As of the Original Agreement Effective Date, TMRC has provided Syros with all information that was made available to Syros in the Data Room and any other Licensed Know-how Rights possessed by TMRC or its Affiliates or in respect of which TMRC or its Affiliates otherwise have the right to grant licenses as of the Original Agreement Effective Date. After the Restatement Date, on at least a [**] basis or more frequently as may be requested by Syros, TMRC shall provide Syros with updates to any chemistry, manufacturing and controls information relating to the Technology possessed by or otherwise available to TMRC or its Affiliates, if any, and with copies of or access to any other information within the Licensed Know-how Rights that Syros may request to satisfy requirements for Registrations or other required submissions to Competent Authorities, including source data and documents underlying any Registration and/or other regulatory documents to which Syros’s Right of Reference under Section 4.3 applies.
4.3.Registrations; Right of Reference. TMRC acknowledges and agrees that Syros shall own all Registrations for Products for use in the Field in each country in the Territory. TMRC hereby grants to Syros a fully paid-up, royalty-free right to reference and use and have full access to all Registrations held by TMRC or its Affiliates as of the Original Agreement Effective Date and all other regulatory documents held by TMRC or its Affiliates after the Original Agreement Effective Date that relate to chemistry, manufacturing and controls information for the manufacture of Products, and any supplements, amendments or updates to any of the foregoing (collectively, the “Right of Reference”) which TMRC or its Affiliates have the right to grant. Syros shall have the right to license or sublicense the Right of Reference to its Affiliates or sublicensees. TMRC shall promptly notify Syros of any written or oral notices received from, or inspections by any Competent Authority relating to any such Registrations, and shall promptly inform Syros of any responses to such written notices or inspections and the resolution of any issue raised by such Competent Authority.
4.4.Information Sharing Regarding Planned Clinical Trials. Each party will use commercially reasonable efforts to share information regarding Clinical Trials to be conducted by such party or any of its Affiliates or (sub)licensees, which information shall be maintained as Confidential Information of the Disclosing Party and used solely for development, manufacture and commercialization of Products as permitted hereunder.
4.5.Pharmacovigilance Agreement. The parties’ responsibilities concerning adverse drug reactions, safety information and compliance with regulatory requirements with respect thereto will be detailed in a separate pharmacovigilance agreement to be mutually agreed upon by the parties as soon as practicable after the Restatement Date, and in any event no later than [**] days after the Restatement Date.
-8-
5.1.Royalties.
5.1.1.Subject to Section 5.2, in consideration for the licenses granted to Syros herein, Syros shall pay to TMRC Patent Rights-based royalties on annual Net Sales of each Product by Syros, its Affiliates and its sublicensees, equal to:
(i)[**] percent ([**]%) of calendar year Net Sales of such Product up to US$[**] in such Net Sales, and
(ii)[**] percent ([**]%) of calendar year Net Sales of such Product over US$[**] in such Net Sales;
provided, however, that during any period within the applicable Patent Royalty Term in which the only applicable Valid Claim(s) of Licensed Patent Rights that are in effect are pending claims, the foregoing royalty rates shall be [**].
5.1.2.Subject to Section 5.2, in consideration for the licenses granted to Syros herein, Syros shall pay to TMRC, for TMRC’s payment to Dr. Koichi Shudo and/or to Toko as required by agreement between such parties, Know-how-based royalties on Net Sales of each Product by Syros, its Affiliates and its sublicensees, equal to [**] percent ([**]%) of Net Sales of such Product.
5.2.Royalty Terms. Royalties as calculated pursuant to Section 5.1.1 shall be payable on Net Sales during the applicable Patent Royalty Term for the applicable Product in the applicable country in the Field. For purposes of determining the applicable royalty tier specified in Sections 5.1.1 for calendar year Net Sales, only Net Sales of the applicable Product in the Field made during the applicable Patent Royalty Term therefor shall be counted. Royalties as calculated pursuant to Section 5.1.2 shall be payable on Net Sales during the applicable Know-how Royalty Term for the applicable Product in the applicable country in the Field. Following expiration of the Patent Royalty Term and the Know-how Royalty Term applicable to a Product in the Field in a country, Syros’ licenses under Section 4.1 shall become fully paid up, non-royalty-bearing and perpetual.
5.3.Combination Products. If a Product comprises both AM80 (Tamibarotene) and another active pharmaceutical ingredient, then for purposes of the royalty payments under Section 5.1 for Net Sales of such Product in the Field, such Net Sales, prior to the royalty calculation set forth in Section 5.1, shall first shall be multiplied by the fraction A/(A+B), where A is the value of the AM80 (Tamibarotene) component, and B is the value of the other active pharmaceutical ingredient, each as reasonably determined by Syros, and such resulting amount shall be the “Net Sales” for purposes of the royalty calculation in Section 5.1 for such Product.
-9-
5.4.Upfront and Milestone Payments. Syros has, prior to the Restatement Date, paid TMRC the one-time and non-refundable upfront payment of US$500,000 set forth as #1 below, and, after the Restatement Date, shall pay to TMRC the following one-time and non-refundable upfront payment set forth as #2 below and one-time (per indication) and non-refundable milestone payments (#3-#9 or #5-#9, as applicable) for each cancer indications within, in the case of upfront payment #2, and the milestone payments, [**] days following the first achievement of the corresponding milestones:
|
1. Upfront payment |
US$500,000 |
|
2. Execution of Toko Letter Agreement |
US$500,000 |
The following milestone payments apply to the first indication and any subsequent indication, except for [**].
|
3. Dosing of a first subject in a Phase I Clinical Trial of a Product for an indication in the Field
|
US$500,000 |
|
4. Dosing of a first subject in a Phase II Clinical Trial of a Product for an indication in the Field |
US$500,000 |
|
[**] |
[**] |
|
[**] |
[**] |
|
[**] |
[**] |
|
[**] |
[**] |
|
[**] |
[**] |
-10-
The following milestone payments apply solely to a second indication where the [**] and, in the event that such milestones apply, are in lieu of the milestones set forth in the table above with respect to the second indication:
|
[**] |
[**] |
|
[**] |
[**] |
|
[**] |
[**] |
|
[**] |
[**] |
|
[**] |
[**] |
For the purposes of this Section 5.4, an “indication” means a distinct tumor type (i.e., irrespective of patient subpopulations) that is tested in a separate Clinical Trial.
5.4.1.No Skipped Milestones. If a milestone is not achieved for a Product for an indication, then the corresponding milestone payment will become payable upon the achievement of the next milestone for such Product for such indication in the applicable region (i.e., [**], as applicable).
5.6Full Compensation. The consideration set forth in this Section 5 is the only compensation due from Syros to TMRC, and TMRC shall be responsible for all of its costs and expenses for performance hereunder, including any payments made to any Third Party licensors.
|
6. |
ROYALTY REPORTS AND ACCOUNTING |
6.1.Royalty Reports. Within [**] days after the end of each calendar quarter during the term of this Agreement following the First Commercial Sale of a Product, Syros shall furnish to TMRC a quarterly written report showing in reasonably specific detail, on a Product-by-Product and country-by-country basis: (a) Net Sales during such calendar quarter; (b) the calculation of the royalties, if any, that shall have accrued based upon such Net Sales; (c) the withholding taxes, if any, required by applicable Law to be deducted with respect to such sales; and (d) the exchange rates, if any, used in determining Net Sales in United States dollars. With respect to sales of Products invoiced in United States dollars, the gross sales, Net Sales and royalties payable shall be expressed in United States dollars. With respect to Net Sales invoiced in a currency other than United States dollars, all such amounts shall be expressed both in the currency in which the distribution is invoiced and in the United States dollar equivalent. The United States dollar equivalent shall be calculated using the average of the exchange rate (local currency per US$1) published in The Wall Street Journal, Western Edition, under the heading “Currency Trading” on the last business day of each month during the applicable calendar quarter.
-11-
6.2.1.Upon the written request of TMRC and not more than [**], Syros shall permit an independent certified public accounting firm of internationally recognized standing selected by TMRC and reasonably acceptable to Syros, at TMRC’s expense, to have access during normal business hours to such of the financial records of Syros as may be reasonably necessary to verify the accuracy of the payment reports hereunder for the [**] immediately prior to the date of such request (other than records for which TMRC has already conducted an audit under this Section).
6.2.2.If such accounting firm concludes that additional amounts were owed during the audited period, Syros shall pay such additional amounts within [**] days after the date TMRC delivers to Syros such accounting firm’s written report so concluding. The fees charged by such accounting firm shall be paid by TMRC; provided, however, that if the audit determines that the royalties payable by Syros for such period are more than [**] percent ([**]%) of the royalties actually paid for such period, then Syros shall pay the reasonable fees and expenses charged by such accounting firm.
6.2.3.TMRC shall cause its accounting firm to retain all financial information subject to review under this Section 6.2 in strict confidence; provided, however, that Syros shall have the right to require that such accounting firm, prior to conducting such audit, enter into an appropriate non-disclosure agreement with Syros regarding such financial information. The accounting firm shall disclose to TMRC only whether the reports are correct or not and the amount of any discrepancy. No other information shall be shared. TMRC shall treat any such financial information as Syros’s Confidential Information
|
7. |
PAYMENTS |
7.1.Payment Terms. Royalties shown to have accrued by each royalty report provided for under Section 6.1 above shall be due on the date such royalty report is due. Payment of royalties in whole or in part may be made in advance of such due date.
7.2.Exchange Control. If at any time legal restrictions prevent the prompt remittance of part or all royalties with respect to any country in the Territory where the Product is sold, Syros shall have the right, in its sole discretion, to make such payments by depositing the amount thereof in local currency to TMRC’s account in a bank or other depository institution in such country. If the royalty rate specified in this Agreement should exceed the permissible rate established in any country, the royalty rate for sales in such country shall be adjusted to the highest legally permissible or government-approved rate.
-12-
7.3.Withholding Taxes. Syros shall be entitled to deduct the amount of any withholding taxes, value-added taxes or other taxes, levies or charges with respect to amounts payable hereunder to TMRC by Syros, its Affiliates or sublicensees, and the amount of any taxes required to be withheld by Syros, its Affiliates or sublicensees with respect to amounts payable hereunder to TMRC, to the extent Syros, its Affiliates or sublicensees pay to the appropriate governmental authority on behalf of TMRC such taxes, levies or charges. Syros shall use reasonable efforts to minimize any such taxes, levies or charges required to be paid on behalf of TMRC by Syros, its Affiliates or sublicensees. Syros promptly shall deliver to TMRC proof of payment of all such taxes, levies and other charges, together with copies of all communications from or with such governmental authority with respect thereto.
|
8. |
DEVELOPMENT OBLIGATIONS |
8.1.Development Efforts. As between the parties, Syros shall solely control and fund all Product development activities as to Products in the Field for any of the Territory. Syros, together with its Affiliates and sublicensees, shall use commercially reasonable efforts to: (a) commence development activities in the Field within one (1) year after the Original Agreement Effective Date; (b) to develop a Product for at least one indication in the Field; and (c) following marketing approval, market the Product. Following the Original Agreement Effective Date, Syros shall promptly begin preparation for an IND filing. Such commercially reasonable efforts shall require Syros to use the level of efforts and resources that Syros generally uses for products of comparable market potential at comparable stage of development, taking into account relevant costs and risks of development and commercialization, including relevant legal, regulatory, scientific, competitive and commercial factors.
8.2.Records. Syros shall maintain records, in sufficient detail and in good scientific manner, which shall reflect all work done and results achieved in the performance of its development regarding the Products.
8.3.Reports. Within [**] days following the end of each quarter during the term of this Agreement prior to the first marketing approval of a Product in each Field in the Territory, Syros shall prepare and deliver to TMRC a written summary report which shall describe (a) the progress of the development and testing of Product(s) in the Field together with a summary of the enrollment of then-active Clinical Trials, and (b) the status of obtaining regulatory approvals to market Product(s) in the Territory in each Field, and Syros shall inform TMRC of the commencement of Clinical Trials and receipt of regulatory approvals from time to time. The parties shall hold a conference call on a quarterly basis at a mutually agreed upon time after TMRC’s receipt of the report mentioned above.
-13-
9.1.Confidential Information. During the term of this Agreement, and for a period of [**] years following the expiration or earlier termination hereof (or, for Toko Information, for such longer period as may be required by the Toko Agreement), each Receiving Party shall maintain in confidence all Confidential Information of the Disclosing Party, and shall not use, disclose or grant the use of the Confidential Information except on a need-to-know basis to those directors, officers, affiliates, employees, to the extent such disclosure is reasonably necessary in connection with performing its obligations or exercising its rights, including its rights to develop and commercialize the Product, under this Agreement. To the extent that disclosure is authorized by this Agreement, prior to disclosure, each Receiving Party shall obtain agreement of any such Person to hold in confidence and not make use of the Confidential Information for any purpose other than those permitted by this Agreement. Each Receiving Party shall notify the Disclosing Party promptly upon discovery of any unauthorized use or disclosure of the Disclosing Party’s Confidential Information.
9.2.Permitted Disclosures. The confidentiality obligations contained in Sections 9.1 above and 9.3 below shall not apply to the extent that any Receiving Party is required (a) to disclose information by Law, including disclosure obligations under applicable securities Laws, or (b) to disclose information to any governmental authority for purposes of obtaining approval to test or market a product, provided that in either case that the Receiving Party shall provide written notice thereof to the Disclosing Party and sufficient opportunity to object to any such disclosure or to request confidential treatment thereof. Notwithstanding any other provision of this Agreement, Syros may disclose Confidential Information of TMRC relating to information developed pursuant to this Agreement: (x) to any Person who is permitted licensees, permitted assignees or agents, consultants, clinical investigators or clinical contractors, to the extent such disclosure is reasonably necessary in connection with performing its obligations or exercising its rights to develop and commercialize the Product under this Agreement as long as such Person has entered into a confidentiality agreement with Syros; (y) in connection with the normal course of development and commercialization of Products for the Territory, including for the filing of Registrations and satisfaction of reporting obligations to Competent Authorities and for the filing and prosecution of Patent Rights; and (z) TMRC may disclose Confidential Information of Syros, including the terms of this Agreement, to Toko, Dr. Koichi Shudo and Itsuu Laboratory to the extent such disclosure is reasonably necessary in connection with performing its obligations hereunder as long as Toko, Dr. Koichi Shudo and Itsuu Laboratory have entered into a confidentiality agreement with TMRC.
9.3.Press Releases and Other Public Announcements.
9.3.1.The parties previously cooperated in the release of a mutually agreed press release following the Original Agreement Effective Date. The parties also recognize that each party may from time to time desire to issue additional press releases and make other public statements or disclosures regarding specific terms of this Agreement, which may be made pursuant to Section 9.3.3 below.
-14-
9.3.2.Syros may from time to time issue additional press releases and make other public statements or disclosures regarding the progress of its development or commercialization of Technology and Products hereunder without the need to obtain TMRC’s prior consent, so long as such press releases, other public statements or disclosures do not reference TMRC or disclose specific terms of this Agreement that have not previously been made public pursuant to this Section 9.3.
9.3.3.Notwithstanding the foregoing provisions of this Section 9.3, (a) a party may make any disclosure or public announcement if the contents of such disclosure or public announcement have been agreed by the parties; (b) a party may make any disclosure or public announcement if the contents of such disclosure or public announcement have previously been made public other than through a breach of this Agreement by the issuing party; (c) if a party reasonably determines that a public disclosure shall be required by Law, including in a public filing with the U.S. Securities and Exchange Commission or applicable stock exchange (including in connection with an initial public offering), such party may disclose specific terms of this Agreement where so required; provided that such party shall, to the extent practicable and permitted by applicable Law, notify the other party and allow the other party to comment on the proposed disclosure, which comments shall be considered by the party obligated to make such public disclosure in good faith; and (d) a party may disclose the terms of this Agreement to bona fide potential or actual advisors, consultants, investors, acquirers, lenders, investment bankers or other potential financial partners in connection with such party’s proposed financing or business combination activities, and to bona fide potential or actual sublicensees, as reasonably necessary in connection with a permitted sublicense or license under the rights granted in this Agreement, in each case of (c) provided that such person is under an appropriate obligation of confidentiality.
|
10. |
PATENTS |
10.1.Patent Prosecution and Maintenance. Except for Patent Family 3 (as to which responsibility for the preparation, filing, prosecution and maintenance shall remain with TMRC, at TMRC’s sole cost), Syros shall, subject to Section 10.2, control, at Syros’ cost, the preparation, filing, prosecution and maintenance of all patents and patent applications within the Licensed Patent Rights in the Territory. Syros shall give TMRC an opportunity to review and comment on the text of each such patent application, and the preparation, filing and prosecution thereof, and shall supply TMRC with a copy of such patent application as filed, together with notice of its filing date and serial number. TMRC shall cooperate with Syros, execute all lawful papers and instruments and make all rightful oaths and declarations as may be necessary for Syros’ preparation, prosecution and maintenance of patents and other filings referred to in this Section 10.1.
10.2.Abandonment; Reverted Patents. If Syros, in its sole discretion, decides to abandon or not pursue the preparation, filing, prosecution or maintenance of any patent or patent application in the Licensed Patent Rights (other than Patent Family 3), then Syros shall notify TMRC in writing thereof and following the date of such notice TMRC may control, at TMRC’s cost, the preparation, filing, prosecution and maintenance of such patents and patent applications. If Syros decides to abandon or not pursue the preparation, filing, prosecution or maintenance of any patent or patent application within any of the Licensed Patent Rights in any country of the Territory, Syros’s rights to prosecute, maintain and enforce such patent in such country will
-15-
revert to TMRC, and such reverted patent in such country shall be excluded from the Licensed Patent Rights thereafter. In the event that, as a result of such a reversion, Syros infringes a patent of TMRC, then TMRC shall promptly inform Syros thereof, and Syros and TMRC shall negotiate regarding TMRC granting a license with respect to such reverted patent, as long as TMRC still Controls such reverted Patent Rights. If such reverted Patent Rights are still Controlled by TMRC, Syros has the option to obtain a license from TMRC to such Patent Rights by paying TMRC [**] times TMRC’s out-of-pocket patent prosecution and maintenance expenses for such Patent Rights in the Field and in the applicable country in the Territory.
10.3.IP Representations. Subject to Section 10.1, TMRC represents and warrants to Syros that as of the Original Agreement Effective Date and throughout the term of this Agreement TMRC has and shall maintain, together with its Affiliates and licensors, Licensed Patent Rights in the Territory to control the preparation, filing, prosecution and maintenance of all patents and patent applications within the Licensed Patent Rights and also to grant to Syros the right to prepare, file, prosecute and maintain the Licensed Patent Rights in accordance with Section 10.1.
10.4.Patent Family 3. TMRC shall control, at TMRC’s cost, the preparation, filing, prosecution and maintenance of all patents and patent applications in Patent Family 3.
10.5.Notification of Infringement. Each party shall notify the other party of any infringement in the Territory known to such party of any Licensed Patent Rights by a product that is competitive with the Products and shall provide the other party with the available evidence, if any, of such infringement.
10.6.Enforcement of Patent Rights. Syros, at Syros’ expense, shall have the right to determine the appropriate course of action to enforce Licensed Patent Rights (except for any Patent Rights that have reverted to TMRC in accordance with Section 10.2) in the Territory or otherwise xxxxx the infringement thereof, to take (or refrain from taking) appropriate action to enforce Licensed Patent Rights in the Territory, to defend any declaratory judgments seeking to invalidate or hold the Licensed Patent Rights unenforceable in the Territory, to control any litigation or other enforcement action and to enter into, or permit, the settlement of any such litigation, declaratory judgments or other enforcement action with respect to Licensed Patent Rights in the Territory, in each case in Syros’ own name and, if required by Law, in the name of TMRC and shall consider, in good faith, the interests of TMRC in so doing. If Syros does not, within [**] days of receipt of notice from TMRC, xxxxx the infringement or file suit to enforce the Licensed Patent Rights against at least one infringing party in the Territory, TMRC shall have the right to take whatever action it reasonably deems appropriate to enforce the Licensed Patent Rights; provided that TMRC shall consider, in good faith, the interests of Syros in so doing. The party controlling any such enforcement action shall not settle the action or otherwise consent to an adverse judgment in such action that diminishes the rights or interests of the non-controlling party without the prior written consent of the other party. All monies recovered upon the final judgment or settlement of any such suit to enforce the Licensed Patent Rights shall be shared, after reimbursement of expenses, in relation to the damages suffered by each party.
-16-
10.7.Cooperation. In any suit to enforce or defend the License Patent Rights pursuant to this Section 10, the party not in control of such suit shall, at the request and expense (for out-of-pocket costs only) of the controlling party, cooperate in all respects and, to the extent possible, have its employees testify when requested and make available relevant records, papers, information, samples, specimens, and the like.
|
11. |
TERMINATION |
11.1.Expiration. Subject to Sections 11.2, 11.3, 11.4, 11.5, 11.6 and 11.7 below, this Agreement shall expire on the expiration of the last to expire Patent Royalty Term or fifteen (15) years from the date of the First Commercial Sale of the Product in the Territory, whichever is later.
11.2.Termination by Syros. At any time after the first anniversary of the Original Agreement Effective Date, Syros shall have the right at its sole discretion to terminate this Agreement upon ninety (90) days advanced written notice to TMRC for reasons such as data or results of development, issues of safety or efficacy, costs and risks of development, competitive dynamics, market dynamics, government regulation and regulatory issues or pricing strategy and expected profitability from the commercialization of Products.
11.3.Termination for Toko Letter Agreement. In the event that Toko, TMRC and Syros have not executed and delivered to one another the Toko Letter Agreement within sixty (60) days after the Restatement Date, Syros shall have the right to terminate this Agreement by providing written notice to TMRC, and: (x) Syros shall have no further commitment or restriction after the effectiveness of such termination; (y) TMRC shall be entitled to retain such payments as have been made by Syros prior to the effectiveness of such termination; and (z) TMRC shall not license any TMRC IP Rights in the Field in the Territory to a Third Party or Affiliate of TMRC for a period of [**] months after the effective date of such termination.
11.4.Termination by Syros for Breach. Except as otherwise provided in Section 13, Syros may terminate this Agreement upon or after the material breach of this Agreement by TMRC if TMRC has not cured such breach in all material respects within [**] days after notice thereof by Syros; provided, however, that if any default is not capable of being cured within such [**] day period and TMRC is diligently undertaking to cure such default as soon as commercially feasible thereafter under the circumstances, Syros shall have no right to terminate this Agreement pursuant to this Section 11.4.
11.5.Termination by TMRC. Except as otherwise provided in Section 13, TMRC may terminate this Agreement upon or after the material breach of this Agreement by Syros if Syros has not cured such breach in all material respects within [**] days after notice thereof by TMRC; provided, however, that if any default is not capable of being cured within such [**] day period and Syros is diligently undertaking to cure such default as soon as commercially feasible thereafter under the circumstances, TMRC shall have no right to terminate this Agreement pursuant to this Section 11.5. Notwithstanding termination by TMRC under this Section 11.5, any royalties or milestone payments that have accrued prior to such termination shall survive such termination.
-17-
11.6.Insolvency or Bankruptcy. To the extent permitted by applicable Laws, either party may, in addition to any other remedies available to it by Law or in equity, terminate this Agreement, in whole or in part, by written notice to the other party in the event the other party shall have become insolvent or bankrupt, or shall have made assignment for the benefit of its creditors, or there shall have been appointed a trustee or receiver of the other party. The parties agree that, in the event of the commencement of a bankruptcy proceeding by or against TMRC or Syros under applicable Laws that has not been dismissed or resolved within [**] days, the other party shall be entitled to a complete duplicate of (or complete access to, as appropriate) any intellectual property obtained in the course of development of the Product and all embodiments of such intellectual property shall be promptly delivered to it upon any such termination of this Agreement, upon expiration of such [**] day period upon its written request therefor, unless the insolvent or bankrupt party elects to continue to perform all of its obligations under this Agreement.
11.7.Effect of Expiration or Termination.
11.7.1.Expiration or termination of this Agreement shall not relieve the parties of any right or obligation accruing prior to such expiration or termination, and the provisions of Sections 5, 9, 11.3, 11.7, 12 and 14 shall survive the expiration or termination of this Agreement.
11.7.2.Upon any termination of this Agreement, at Syros’s request, TMRC shall grant a direct license to any sublicensee of Syros hereunder having the same scope as the sublicense granted to such sublicensee by Syros and on terms and conditions no less favorable to such sublicensee than the terms and conditions of this Agreement, provided that such sublicensee is not in default of any applicable obligations under this Agreement and agrees in writing to be bound by the terms and conditions of such direct license.
11.7.3.Upon a termination of this Agreement under Sections 11.2 or 11.5 or a termination by TMRC under Section 11.6, (a) Syros shall, within [**] days after the effective date of such termination, provide TMRC with any data generated by Syros or its Affiliates as part of a Clinical Trial of the Technology; and (b) Syros hereby grants TMRC, effective as of the effective date of such termination, a Right of Reference to Registrations held by Syros or its affiliates as of the effective date of such termination that relate to the Technology, which TMRC may sublicense to its Affiliates or sublicensees.
|
12. |
INDEMNIFICATION |
12.1.Indemnification. Each party shall defend, indemnify and hold harmless the other party from all losses, liabilities, damages and expenses (including attorneys’ fees and costs) incurred as a result of any Third Party claim, demand, action or proceeding arising out of any breach of this Agreement by the indemnifying party, or the gross negligence or willful misconduct of the indemnifying party in the performance of its obligations under this Agreement, except in each case to the extent arising from the gross negligence or willful misconduct of the other party or the breach of this Agreement by the other party.
-18-
12.2.Procedure. The indemnified party shall promptly notify the indemnifying party of any liability or action in respect of which the indemnified party intends to claim such indemnification and the indemnifying party shall have the right to assume the defense thereof with counsel selected by the indemnifying party. The indemnity agreement in this Section 12 shall not apply to amounts paid in settlement of any loss, claim, damage, liability or action if such settlement is effected without the consent of the indemnifying party, which consent shall not be withheld unreasonably. The failure to deliver notice to the indemnifying party within a reasonable time after the commencement of any such action, if prejudicial to its ability to defend such action, shall relieve the indemnifying party of any liability to the indemnified party under this Section 12, but the omission so to deliver notice to the indemnifying party will not relieve it of any liability that it may have to the indemnified party otherwise than under this Section 12. The indemnified party under this Section 12, and its employees and agents, shall cooperate fully with the indemnifying party and its legal representatives in the investigation and defense of any action, claim or liability covered by this indemnification.
12.3.Insurance. Syros shall maintain product liability insurance with respect to the research, development, manufacture and sales of Products by Syros in such amount as Syros customarily maintains with respect to the research, development, manufacture and sales of its similar products. Syros shall maintain such insurance for so long as it continues to research, develop, manufacture or sell any Products, and thereafter for so long as Syros customarily maintains insurance covering the research, development, manufacture or sale of its similar products.
|
13. |
FORCE MAJEURE |
Neither party shall be held liable or responsible to the other party nor be deemed to have defaulted under or breached this Agreement for failure or delay in fulfilling or performing any term of this Agreement to the extent, and for so long as, such failure or delay is caused by or results from causes beyond the reasonable control of the affected party including fire, floods, embargoes, war, acts of war (whether war be declared or not), acts of terrorism, insurrections, riots, civil commotions, strikes, lockouts or other labor disturbances, acts of God or acts, omissions or delays in acting by any governmental authority or the other party.
|
14. |
MISCELLANEOUS |
14.1.Notices. Any consent, notice or report required or permitted to be given or made under this Agreement by one of the parties hereto to the other party shall be in writing, delivered by any lawful means to such other party at its address indicated below, or to such other address as the addressee shall have last furnished in writing to the addressor and (except as otherwise provided in this Agreement) shall be effective upon receipt by the addressee.
-19-
1-12-12 Kita Shinjuku
Xxxxxxxx-xx, Xxxxx, 000-0000, Xxxxx
Attention: Research & Development Department
If to Syros:Syros Pharmaceuticals, Inc.
Attention: Chief Business Officer
14.2.Governing Law. This Agreement shall be governed by and construed in accordance with the Laws of the State of California, without regard to the conflicts of Law principles thereof.
14.3.Arbitration. Any dispute, controversy or claim initiated by either party arising out of or relating to this Agreement, its negotiations, execution or interpretation, or the performance by either party of its obligations under this Agreement (other than (a) any dispute, controversy or claim regarding the validity, enforceability, claim construction or infringement of any patent rights, or defenses to any of the foregoing, or (b) any bona fide third party action or proceeding filed or instituted in an action or proceeding by a Third Party against a party to this agreement), whether before or after termination of this Agreement, shall be finally resolved by binding arbitration. Whenever a party shall decide to institute arbitration proceedings, it shall give prompt written notice to that effect to the other party. Any such arbitration shall be conducted in the English language under the International Dispute Resolution Procedures and Arbitration Rules of the American Arbitration Association (the “Rules”) by a panel of three (3) arbitrators appointed in accordance with such Rules. Any such arbitration shall be held in Los Angeles, California. The method and manner of discovery in any such arbitration proceedings shall be governed by the Rules. The arbitrators shall have the authority to grant specific performance and to allocate between the parties the costs of arbitration (including attorneys’ fees and expenses of the parties) in such equitable manner as they determine.
Judgment upon the award so rendered may be entered in any court having jurisdiction or application may be made to such court for judicial acceptance of any award and an order of enforcement, as the case may be. In no event shall a demand for arbitration be made after the date when institution of a legal or equitable proceeding based upon such claim, dispute or other matter in question would be barred by the applicable statute of limitations. Notwithstanding the foregoing, either party shall have the right, without waiving any right or remedy available to such party under this Agreement or otherwise, to seek and obtain from any court of competent jurisdiction any interim or provisional relief that is necessary or desirable to protect the rights or property of such party, pending the selection of the arbitrators hereunder or pending the arbitrators’ determination of any dispute, controversy or claim hereunder.
-20-
14.4.Assignment. Syros shall not assign its rights or obligations under this Agreement without the prior written consent of TMRC; provided, however, that Syros may, without such consent, assign this Agreement and its rights and obligations hereunder (a) to any Affiliate, or (b) in connection with the sale or transfer of all or substantially all of its business or assets relating to the subject matter of this Agreement, or in the event of its merger, consolidation, change in control or similar transaction. Any permitted assignee shall assume all obligations of its assignor under this Agreement.
14.5.Waivers and Amendments. No change, modification, extension, termination or waiver of this Agreement, or any of the provisions herein contained, shall be valid unless made in writing and signed by duly authorized representatives of the parties hereto.
14.6.Entire Agreement. This Agreement embodies the entire agreement between the parties and supersedes any prior representations, understandings and agreements, including the Original Agreement, between the parties regarding the subject matter hereof except the Confidential Disclosure and Non-Use Agreement dated as of February 24, 2015 between TMRC and Syros (the “Existing Confidentiality Agreement”). There are no representations, understandings or agreements, oral or written, between the parties regarding the subject matter hereof that are not fully expressed herein except the Existing Confidentiality Agreement. Nothing in this Agreement removes or overrides any right of action by any party in respect of any fraudulent misrepresentation, fraudulent concealment or other fraudulent action.
14.7.No Benefit to Third Parties. The provisions of this Agreement are for the sole benefit of the parties and their successors and permitted assigns, and shall not be construed as conferring any rights in any other Persons.
14.8.Severability. Any of the provisions of this Agreement which are determined to be invalid or unenforceable in any jurisdiction shall be ineffective to the extent of such invalidity or unenforceability in such jurisdiction, without rendering invalid or unenforceable the remaining provisions hereof and without affecting the validity or enforceability of any of the terms of this Agreement in any other jurisdiction.
14.9.Waiver. The waiver by either party of any right hereunder or the failure to perform or of a breach by the other party shall not be deemed a waiver of any other right hereunder or of any other breach or failure by said other party whether of a similar nature or otherwise.
14.10.Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
[Signature page follows]
-21-
IN WITNESS WHEREOF, the parties have executed this Amended and Restated Cancer License Agreement effective as of the Restatement Date.
|
TMRC CO., LTD. |
||
|
|
||
|
By: |
|
/s/ Xxxxx Xxxxxxx |
|
Name: |
|
Xxxxx Xxxxxxx |
|
Title: |
|
President & CEO |
|
April 28, 2016 |
||
|
|
||
|
By: |
|
/s/ Xxxxx Xxxxxxxx |
|
Name: |
|
Xxxxx Xxxxxxxx |
|
Title: |
|
CEO |
|
April 28, 2016 |
||
[Signature page to Amended and Restated Cancer License Agreement]
-22-
Certain Licensed Patent Rights
|
Series |
Country |
Serial No. |
Filing Date |
Publicat-ion |
Patent |
Priority |
Subject Matter/Other Info |
|
[**] |
[**] |
|
[**] |
|
[**] |
[**] |
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
|
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
|
|
|
|
|
|
|
|
[**] |
[**] |
|
[**] |
|
[**] |
[**] |
[**] |
|
|
[**] |
|
[**] |
|
[**] |
|
|
|
|
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
|
|
|
|
|
|
|
|
[**] |
[**] |
|
[**] |
|
[**] |
|
[**] |
|
|
|
|
|
|
|
|
|
-23-
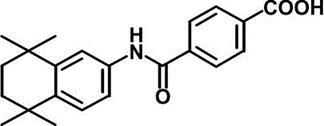
AM80 (Tamibarotene)
Consent and Stand-by License Agreement
This CONSENT AND STAND-BY LICENSE AGREEMENT (this “Agreement”) dated as of April 28, 2016 (the “Effective Date”) is entered into among TOKO PHARMACEUTICAL IND. CO., LTD., a Japanese corporation having its principal office at 0-0-00, Xxxxxxx, Xxxxxx-xx, Xxxxx 000-0000, Xxxxx (“Toko”), SYROS PHARMACEUTICALS, INC., a Delaware corporation having its principal office at 000 Xxxxxxxx Xxxxx, Xxxxx 000, Xxxxxxxxx, Xxxxxxxxxxxxx 00000, XXX (“Syros”) and TMRC Co., Ltd., a Japanese corporation having its principal office at 1-12-12, Xxxx Xxxxxxxx, Xxxxxxxx-xx, Xxxxx 000-0000, Xxxxx (“TMRC”).
WHEREAS, Toko is a party to an agreement with TMRC entitled “License Agreement for the development et al. of retinoid based drug in foreign countries for cancer” dated August 15, 2008 and License Agreement for the development et al. of retinoid based drug in foreign countries for neutropenia dated June 13, 2014 (collectively, the “Toko-TMRC License Agreement”);
WHEREAS, Syros and TMRC have negotiated and executed an Amended and Restated Cancer License Agreement dated April 28, 2016 and may in the future negotiate a license agreement for neutropenia pursuant to which, upon execution, TMRC will grant to Syros certain rights and licenses under patent rights, know-how and data controlled by TMRC as to AM80 (Tamibarotene), including under certain rights and licenses therein granted to TMRC by Toko in the Toko-TMRC License Agreement (collectively, the “TMRC-Syros License Agreement”); and
WHEREAS, Syros has requested that Toko provide the assurances and make the undertakings set forth herein;
NOW, THEREFORE, in consideration of the foregoing premises and the mutual covenants herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Toko, Syros and TMRC hereby agree as follows:
|
|
2. |
Toko and TMRC will provide prompt written notice to Syros if the Toko-TMRC License Agreement or any right or license granted to TMRC thereunder terminates or otherwise ceases to be in effect for any reason. |
-25-
|
|
6. |
This Consent shall be governed by the laws of Japan, excluding the conflicts of laws provisions thereof. |
-26-
IN WITNESS WHEREOF, Toko, Syros and TMRC have executed this Consent and Stand-by License Agreement as of the Effective Date.
|
TOKO PHARMACEUTICAL IND. CO., LTD. |
||
|
|
||
|
By: |
|
/s/ illegible |
|
|
|
|
|
Title: |
|
President & CEO |
|
|
|
|
|
Date: |
|
April 28, 2016 |
|
|
||
|
By: |
|
/s/ Xxxxx Xxxxxxxx |
|
|
|
|
|
Title: |
|
CEO |
|
|
|
|
|
Date: |
|
April 28, 2016 |
|
TMRC CO., LTD. |
||
|
|
||
|
By: |
|
/s/ Xxxxx Xxxxxxx |
|
|
|
|
|
Title: |
|
President and CEO |
|
|
|
|
|
Date: |
|
April 28, 2016 |
-27-
TMRC Grants of Rights to Third Parties
The rights granted by TMRC to CHILDREN’S HOSPITAL LOS ANGELES in the COLLABORATION AGREEMENT as of July 18, 2014.


