Azedra Commercial Facility and Supply Agreement THIS AGREEMENT made in duplicate as of this 6th day of August, 2009
Exhibit 10.3
Azedra Commercial Facility and Supply Agreement
THIS AGREEMENT made in duplicate as of this 6th day of August, 2009
| BETWEEN: | ||
| MDS NORDION, a division of MDS (Canada) Inc. | ||
| having a place of business at | ||
| 000 Xxxxx Xxxx | ||
| Xxxxxx, Xxxxxxx, Xxxxxx | ||
| (“Nordion”) | ||
| AND: | ||
| MOLECULAR INSIGHT | ||
| PHARMACEUTICALS, INC. | ||
| having a place of business at | ||
| 000 Xxxxxx Xxxxxx | ||
| Xxxxxxxxx Xxxxxxxxxxxxx, 00000 XXX | ||
| (“Molecular Insight Pharmaceuticals”) | ||
WHEREAS:
| I. | Molecular Insight Pharmaceuticals is the owner or licensee of a certain compound known as Azedra, a therapeutic radiopharmaceutical agent for the treatment of certain neuroendocrine cancers; |
| II. | MIP has expertise in the production and development of radiopharmaceuticals, processes, and in the radiolabelling of compounds; |
| III. | Nordion has expertise in the production of radiochemicals, in the development of radiopharmaceuticals, processes, and in the radiolabelling of compounds; |
| IV. | Nordion has expertise in designing and overseeing the construction of facilities for the radiolabelling of compounds; |
| V. | Nordion and Molecular Insight Pharmaceuticals agreed to a proposal for the Phase III Clinical Trial and Commercial Supply Facility Agreement dated February 5, 2009 (“Letter of Agreement”) and Molecular Insight Pharmaceuticals paid Nordion a sum of ****** for the Phase 1 inception which was due upon execution of the Letter of Agreement; |
* Confidential Treatment Requested *
| VI. | Nordion and Molecular Insight Pharmaceuticals entered into (i) a Development Agreement dated March 22, 2006 (the “Development Agreement”) for the purpose of undertaking a development program to establish a process permitting Molecular Insight Pharmaceutical’s precursor (unlabelled MIBG Ultrace™ resin) to be labeled with I-131 to form a radiopharmaceutical (i) a Clinical Supply and Interim Facility Agreement dated July 12, 2007 (“Interim Facility Agreement”) for the purpose of interim clinical supply of Azedra, and (ii) a Scale-up Agreement dated March 18, 2008 (the “Scale-up Agreement”) for the purpose of establishing a process to manufacture a scaled-up batch size of Azedra. |
| VII. | Molecular Insight Pharmaceuticals desires that Nordion establish a Facility at its site in Ottawa, Ontario to accommodate growth and expansion of the process developed under the Development Agreement for the manufacture and supply of Azedra in sufficient quantities for use in the Clinical Trial Phase and the Commercial Phase. |
| VIII | Molecular Insight Pharmaceuticals desires that upon completion of the Facility established under this Agreement that Nordion transition the manufacture of Azedra from the existing facility currently located in room 1215 at Nordion’s site in Ottawa, Ontario to the Facility established under this Agreement. |
NOW THEREFORE in consideration of the mutual covenants and agreements herein contained, and subject to the terms and conditions hereinafter set out, the parties hereto agree as follows:
ARTICLE 1 – DEFINITIONS
For the purposes of this Agreement:
| 1.1 | “Affiliate” shall mean an entity or person which controls, is controlled by or is under common control with either party. For purposes of this section 1.1 control shall mean (a) in the case of corporate entities, the direct or indirect ownership of more than one-half of the stock or participating shares entitled to vote for the election of directors, and (b) in the case of a partnership, the power to direct the management and policies of such partnership. |
| 1.2 | “Azedra” shall mean Molecular Insight Pharmaceuticals’s proprietary drug product, a high specific activity I-131 labeled meta-iodobenguane in therapeutic (“Tx”) and diagnostic (“Dx”) dosage forms also known as high specific activity I-131 MIBG, for use in treating certain neuroendocrine cancers. |
| 1.3 | “Batch” shall mean a production batch of Azedra Manufactured under this Agreement and shall be of the size set forth on Schedule D attached hereto. |
| 1.4 | “Batch Production Date” shall mean the date on which Isotope is added to Precursor during the Manufacture of Azedra. |
| 1.5 | “Clinical Trials” shall mean human trials for clinical development of Azedra for the purpose of seeking pharmaceutical regulatory approval. |
| 1.6 | “Clinical Trial Phase” shall mean human trials for clinical development of Azedra for use in Clinical Trials. |
| 1.7 | “Commercial Phase” shall mean the period of supply of Azedra commencing after NDA regulatory approval has been received in the United States from the FDA by Molecular Insight Pharmaceuticals. |
| 1.8 | “Current Good Manufacturing Practices” or “cGMP(s)” shall mean the good manufacturing practices required by the FDA and as set forth in the FD&C or FDA rules and regulations for the manufacturing, testing and quality control of pharmaceutical materials as applied to compounds, which practices are current on the Effective Date of this Agreement and may be supplemented, amended or modified from time to time. To the extent that standards in other countries for the manufacture, testing, and quality control of pharmaceutical materials are higher or more stringent than those required by the FDA, the more stringent standards if agreed to between the parties shall be delineated in subsequent Quality Agreements. |
| 1.9 | “Data” shall mean Azedra formulation data (under the predecessor agreements), and data summaries, data reports, and results generated under this Agreement, included in the following documents: stability testing reports, Azedra Batch records and validation reports. Data shall exclude Nordion Background Technology and Nordion Confidential Information. |
| 1.10 | “Effective Date” shall mean the date first appearing above. |
| 1.11 | “Equipment” means any equipment or machinery, and in the case of cGMP manufacturing hereunder, qualified equipment or machinery, used by Nordion in the development and/or Manufacture of Azedra, or the holding, processing, testing, or release of Azedra. |
| 1.12 | “FDA” shall mean the United States Food and Drug Administration. |
| 1.13 | “FD&C” shall mean the United States Federal Food, Drug and Cosmetic Act, as amended. |
| 1.14 | “Facility” shall mean the new facility and Equipment to be established by Nordion at its manufacturing site in Ottawa, Ontario as described in Schedule A and pursuant to cGMPs, to be used for the production of Azedra for Clinical Trials and Commercial Phase supply. |
| 1.15 | “Facility Milestone(s)” shall mean the milestones relating to the establishment of the Facility as described in Schedule A. |
| 1.16 | “Facility Program” shall mean the program by which Nordion shall establish the Facility in accordance with the Facility Milestones as described in Schedule A. |
| 1.17 | “IND” shall mean an Investigational New Drug Application as defined by the rules and regulations promulgated under the FD&C and U.S. Public Health Service Act and any supplements, modifications or amendments thereunder. |
| 1.18 | “Isotope” or “I-131” shall mean tellurium derived Iodine 131 (I-131). |
| 1.19 | “Manufacture(d)” means any processes and activities necessary to produce Azedra, including without limitation, the manufacturing, processing, packaging, labeling, quality control testing, stability testing, release or storage of Precursor and Azedra, but excluding sterilization. |
| 1.20 | “Master Batch Record” or “MBR” shall mean a formal set of instructions for the Manufacture of each Batch, developed and maintained in Nordion’s standard format by Nordion, using Molecular Insight Pharmaceuticals’ master formula and technical support. |
| 1.21 | “Master Validation Plan” shall mean the program by which documented evidence provides assurance that the Process will consistently produce Azedra that meets Specifications. |
| 1.22 | “Molecular Insight Pharmaceuticals Background Technology” shall mean technology conceived, created, developed or reduced to practice by Molecular Insight Pharmaceuticals prior to or during the Term of this Agreement or independently of this Agreement, and independently of Nordion Background Technology including, without limitation, patents, know-how, techniques, methods, processes, drawings, schematics, procedures, protocols, parameters, engineering details, functional descriptions, data and database content, technical or scientific information, manuals and trade secrets, which Molecular Insight Pharmaceuticals owns, uses, conceives, creates, develops, reduces to practice or provides in performing under this Agreement, or which is licensed to Molecular Insight Pharmaceuticals and which is in existence in the form of a writing, prototype or can otherwise be demonstrated to be the property of Molecular Insight Pharmaceuticals, or to which Molecular Insight Pharmaceuticals has the rights. |
| 1.23 | “Nordion Background Technology” shall mean technology, conceived, created, developed or reduced to practice by Nordion prior to or during the Term of this Agreement or independently of this Agreement, including, without limitation, patents, know-how, techniques, methods, processes, drawings, schematics, procedures, protocols, parameters, engineering details, functional descriptions, data and database content, technical or scientific information, manuals and trade secrets, which Nordion owns, uses, conceives, creates, develops, reduces to practice or provides in performing under this Agreement, or which is licensed to Nordion and which is in existence in the form of a writing, prototype or can otherwise be demonstrated to be the property of Nordion or to which Nordion has the rights. |
| 1.24 | “NDA” shall mean a new drug application as defined in the rules and regulations promulgated under the FD&C and U.S. Public Health Service Act, as supplemented, modified or amended from time to time. |
| 1.25 | “Precursor” shall mean Ultratrace™resin as specified in Schedule F and produced pursuant to cGMPs. |
| 1.26 | “Process” shall mean the method of radiolabeling of Precursor with I-131, formulation, dispensing and testing of Azedra developed pursuant to the Scale-up Agreement and in compliance with cGMPs, which shall be adjusted only to accommodate the physical set up and aseptic conditions of the Facility and shall be documented in the applicable Master Batch Record |
| 1.27 | “Quality Agreement” shall mean the agreement set out in Schedule “E”. |
| 1.28 | “Regulatory Authority” shall mean the United States Food and Drug Administration, the European Medicinal Evaluation Agency (EMEA), or respective Regulatory Authorities in other countries or any successor entity thereto. |
| 1.29 | “Reference Standards” shall mean the cGMP compliant compounds supplied by Molecular Insight Pharmaceuticals as specified in Schedule F. |
| 1.30 | “Specification(s)” shall mean those final conditions, characteristics and specifications for Azedra as set out in Schedule B, as amended by mutual written agreement of the parties from time to time. |
| 1.31 | “Term” shall mean the Initial Term and each Renewal Term as defined in section 17.1. |
The following Schedules attached to this Agreement are hereby incorporated by reference:
| Schedule A: | Description of Facility Program, Facility Milestones, Facility Schedule |
| Schedule B: | Azedra Specifications |
| Schedule C: | Facility Construction Fees |
| Schedule D: | Price of Batches for Clinical Trial and Commercial Phase Supply |
| Schedule E: | Quality Agreement |
| Schedule F: | Specifications for Precursor and Reference Standards |
| Schedule G: | Change in Scope Form |
ARTICLE 2 – PURPOSE
| 2.1 | Scope and Object |
| The scope and object of this Agreement is to establish a new facility at Nordion’s manufacturing site in Ottawa, Ontario in which to perform the Process for the Manufacture and supply of Azedra for Clinical Trials and Commercial Phase supply in accordance with the responsibilities and obligations attributed to each of the parties as set out in this Agreement. Both parties shall use commercially reasonable efforts to meet their respective milestones set out in Schedule A. If either party, acting in good faith, materially fails to satisfy any milestone, such party shall provide written notice thereof to the other party and the parties shall determine a reasonable corrective action plan and revised milestone schedule. |
ARTICLE 3 – FACILITY PROGRAM
| 3.1 | Facility Construction |
| In consideration of Nordion establishing the Facility, Molecular Insight Pharmaceuticals will pay to Nordion the fees as set out in Schedule C, provided that the respective milestone(s) for establishing the Facility as set forth on Schedule A attached hereto are materially met by Nordion. Unless otherwise set out (see Schedule C) amounts owing by Molecular Insight Pharmaceuticals during the Facility program shall be paid within thirty (30) days of receipt of Nordion’s invoice. |
| Nordion shall establish the Facility in accordance with its obligations described and attributed in Schedule A, it being understood that some activities may be reasonably delayed to the extent that such activity is premised on the work or provision of data, information, equipment or technology by Molecular Insight Pharmaceuticals, and provided that such activities do not materially interfere or could be reasonably expected to interfere with other established Nordion production activities. Molecular Insight Pharmaceuticals acknowledges that there may be risks and/or unforeseen circumstances that are associated with the implementation of Facility Program that may impact Nordion’s overall achievement of the Milestone Schedule and may result in delays and/or increased Facility costs (from those originally estimated). In that the aggressive timeline for implementation of the project was prepared at the request of Molecular Insight Pharmaceuticals, it is acknowledged by Molecular Insight Pharmaceuticals that such timeline may not, in the ordinary course, be achievable. It is understood and acknowledged that due to the nature of the activities to be carried out during the establishment of the Facility, the time for completion and sequence for carrying out the activities as set out in Schedule A shall therefore serve only as a guide. |
| Subject to the foregoing each party shall use their commercially reasonable efforts in order to carry out their respective obligations and responsibilities set out in Schedule A. |
| The Process established under the Scale-up Agreement being transferred to the Facility and validated under this Agreement has a 15 Ci starting activity of Isotope. It is assumed this Process and related methods have been fully validated and utilized in the current interim facility established under the Interim Facility Agreement. Yields are as described in Schedule D. Molecular Insight Pharmaceuticals accepts that the success of work detailed in this Agreement is dependant upon the successful completion of the 15 Ci process developed under the Scale-up Agreement. |
| Nordion shall, in consultation with Molecular Insight Pharmaceuticals, develop and implement a Master Validation Plan that will allow the production of Azedra under cGMPs for Clinical Trial supply and for Commercial Phase supply. Prior to implementation, both parties shall in writing approve the Master Validation Plan, which approval will not be unreasonably or untimely withheld. |
| 3.2 | Program Manager |
| The parties, upon signing this Agreement, shall each designate a program manager, who shall be responsible for coordinating communication and monitoring performance under this Agreement. The program manager for Nordion shall respond to Molecular Insight Pharmaceutical’s reasonable inquiries regarding the status of Nordion’s activities under this Agreement and |
| shall keep Molecular Insight Pharmaceuticals informed as to interim progress. The program managers shall meet either monthly or more frequently than monthly if agreed to in advance by the parties, in person or by telephone, for the purpose of reviewing the status of the project and assessing progress against the milestones and activities set forth in Schedule A. Minutes of meetings shall be prepared, maintained and provided to each of the parties. Prior to the scheduled monthly meetings, Nordion shall prepare and submit to Molecular Insight Pharmaceuticals a monthly written report that sets forth in reasonable detail the progress of the Facility construction and indicates any problems that are known to Nordion or reasonably anticipated by Nordion to occur that either may impact the program schedule for the Facility or may result in a cost increase to be borne by Molecular Insight Pharmaceuticals. |
| 3.3 | Scientific and Technical Dispute Resolution |
| Except as otherwise set out, in the event that at any time during the Term of this Agreement, a disagreement, dispute, controversy or claim should arise relating to the scientific or technical issues in connection with Nordion or Molecular Insight Pharmaceuticals performance under this Agreement, the Program Managers will attempt, in good faith, to resolve their differences within a period of ten (10) business days. With respect to the scientific or technical issues, if after ten (10) business days the Program Managers are unable to resolve such dispute, the Program Managers shall refer the matter to a joint Steering Committee comprised of three (3) senior members of each party. The Steering Committee shall meet as required to resolve the matter. |
| 3.4 | Scope Change |
| The parties acknowledge and agree that Schedule A may require amendment during the course of establishing the Facility. All such changes to Schedule A shall be made by written agreement of the parties in the form of the attached Change in Scope Form (Schedule G). If any change to Schedule A impacts the scope of work to be provided by Nordion, Nordion will provide a written estimate of the increased cost, which must be approved by Molecular Insight Pharmaceuticals in advance of implementation. Changes in scope shall be charged to Molecular Insight Pharmaceuticals based on the rates set out in Schedule C which rates shall be subject to escalation in accordance with section 4.6. No work on such scope change shall be carried out or implemented by Nordion prior to Nordion’s receipt of Molecular Insight Pharmaceuticals’ written approval of such change. |
| 3.5 | Use of the Facility |
| During the Term of this Agreement after transition of the Process to the Facility, subject to section 9.1, Nordion shall ensure that the Facility is available for the Manufacture of Azedra for supply to Molecular Insight Pharmaceuticals on an exclusive basis and Nordion shall only use the Facility for the Manufacture of Azedra or any other product at the election of Molecular Insight Pharmaceuticals and subject to the mutual agreement of the parties. Nordion further agrees that it shall not (i) use other facilities for the Manufacture of Azedra unless Molecular Insight Pharmaceuticals consents to the use of such other facilities in writing, such consent not to be unreasonably withheld, and (ii) Manufacture Azedra for any third party unless directed to do so in writing by Molecular Insight Pharmaceuticals. For the sake of clarity, it is the intent of the parties that the Facility may be used for the clinical or commercial manufacture of one or more Molecular Insight Pharmaceuticals products the manufacture of which shall be the subject of a separate agreement. |
| 3.6 | Additional Compensation for Molecular Insight Pharmaceuticals Audits |
| During the Facility Program, upon ten (10) days prior written notice to Nordion, Molecular Insight Pharmaceuticals and any third-party consultant or auditor appointed by Molecular Insight Pharmaceuticals, such consultant and/or auditor being subject to a confidentiality agreement with and reasonably acceptable to Nordion, shall have reasonable access to observe and inspect the Facility for the purpose of determining the progress against Facility Milestones. |
| In the event that Molecular Insight Pharmaceuticals elects to audit Nordion following a warning or similar citation to Nordion with respect to the Facility and Azedra from any applicable Regulatory Authority, Nordion shall not charge Molecular Insight Pharmaceuticals in connection with such an audit unless such warning or similar citation arises from the acts or omissions of Molecular Pharmaceuticals. |
| Molecular Insight Pharmaceuticals, upon thirty (30) days prior written notice to Nordion shall have the right to conduct one annual surveillance Quality Assurance audit, the duration of which shall not exceed two (2) business days. |
| Notwithstanding the foregoing, in the event that Molecular Insight Pharmaceuticals requests an audit for any other reason, Molecular Insight Pharmaceuticals will compensate Nordion based on the rate of ****** (subject to escalation in accordance with section 4.6) per required Nordion person per hour for the time spent by Nordion on the following audit activities including but not limited to: |
| • | preparing and hosting Facility audits requested by Molecular Insight Pharmaceuticals including FDA preaudit inspections; |
* Confidential Treatment Requested *
| • | preparing responses to FDA inquiries if requested by Molecular Insight Pharmaceuticals and preparation by Nordion of any other information requested by Molecular Insight Pharmaceuticals necessary to conduct any audit; and |
| • | attending meetings with the FDA at Molecular Insight Pharmaceuticals request. |
| Molecular Insight Pharmaceuticals shall reimburse Nordion for all reasonable costs incurred for travel incurred by and accommodation for Nordion personnel in carrying out the foregoing activities. Molecular Insight Pharmaceuticals shall not compensate Nordion for any travel time for Nordion employees. Nordion shall provide an estimate of all such activities to Molecular Insight Pharmaceuticals prior to incurring the expenditure. |
| 3.7 | Ownership of Physical Assets, Precursor, Reference Standards and Data |
| At all times (both during the Term and after expiration or termination of this Agreement for any reason) Nordion will retain all right and title in and to the physical assets and real property that comprise and/or are used and/or employed in the Facility. |
| Molecular Insight Pharmaceuticals shall at all times (both during the Term and after expiration or termination of this Agreement for any reason) retain title to and ownership of Precursor, Reference Standards and Data, provided further, that both during the Term and after termination and/or expiration of this Agreement (i) Nordion may retain an archival copy of the Data for the purpose of management of its obligations and (ii) Nordion may use the Data for the purposes of increasing its general knowledge and experience with respect to its radiopharmaceutical capabilities. Nordion will not use Data in any regulatory submission to any national regulatory authority for the purpose of clinical investigation or licensure of any Nordion produced MIBG dose form. Nordion shall at all times take such reasonable measures as are required to store, in accordance with applicable specifications, the Precursor, Reference Standards and Azedra. Nordion shall ensure that Azedra, and any work in process are free and clear of any liens or encumbrances. Nordion shall immediately notify Molecular Insight Pharmaceuticals if at any time it believes any Precursor, Reference Standards and/or Azedra has been damaged, lost or stolen. |
| 3.8 | Repairs and Maintenance |
| After completion of the Facility Program, Nordion shall, subject to section 14.7, maintain such Facility in satisfactory operating condition as required by the FDA, Specifications, Process and cGMPs, and all other applicable laws, regulations, rules or orders. |
| The cost of routine repairs, preventive maintenance and service contracts for the Facility and Equipment shall be borne by Nordion. Nordion may periodically require the Facility to be unavailable for Azedra production due to repair or unforeseen maintenance activities. In addition, Nordion may shut down the Facility annually for a two (2) week maintenance period. Such outages shall be made only with eight (8) weeks advance written notice to Molecular Insight Pharmaceuticals and, to the extent possible and reasonable in the circumstance, be planned in conjunction with Molecular Insights Pharmaceuticals so as to minimize the impact on supply capability. |
| 3.9 | Alternative Manufacturing Sites - Nordion Obligation to Assist |
| Nordion shall be the sole manufacturer of Azedra in the initial Azedra NDA filed with the FDA. Molecular Insight Pharmaceuticals may establish additional Azedra manufacturing sites and include such sites in the NDA once it has received regulatory approval from the FDA, provided that: |
| (a) Nordion shall, during the Term of this Agreement, upon written notification from Molecular Insight Pharmaceuticals of its desire to establish an additional manufacturing site, shall have a right of negotiation for the establishment of any such additional Azedra third party manufacturing site(s) that is not owned directly or indirectly, by Molecular Insight Pharmaceuticals. Molecular Insight Pharmaceuticals shall be entitled to discuss, negotiate and enter into an agreement with third parties for the establishment of additional Azedra manufacturing sites, provided further however, that the terms and conditions of establishment of such site(s) shall not be less favorable to Molecular Insight Pharmaceuticals than those last offered by MDS Nordion, and |
| (b) the establishment of any new manufacturing site by Molecular Insight Pharmaceuticals shall not reduce the then-current purchase levels (based on the average monthly purchase levels in the preceding four (4) months prior to exercise of the right of negotiation) of Azedra by Molecular Insight Pharmaceuticals as supplied by Nordion under this Agreement. |
| Molecular Insight Pharmaceuticals recognizes that Nordion has used and employed Nordion Background Technology in the Facility. Such Nordion Background Technology shall remain the exclusive property of Nordion. Molecular Insight Pharmaceuticals shall have no right to adopt or exploit Nordion Background Technology without Nordion’s prior written consent. Nordion’s obligation to assist Molecular Insight Pharmaceuticals (if any) in the establishment of any third party manufacturing site shall be limited to the provision of chemistry manufacturing and controls documents (“CMC”) excluding Nordion specific documents, Nordion Background Technology and Nordion Confidential Information. |
ARTICLE 4 – TRANSITION, PRICING, PURCHASE COMMITMENT AND PAYMENT
| 4.1 | Azedra Supply and Transition |
| Nordion shall notify Molecular Insight Pharmaceuticals in writing of the completion of the Facility. After written notice by Nordion of completion of the Facility pursuant to this Agreement and provided that Nordion is capable of Manufacture of Azedra in the Facility, and subject to Molecular Insight Pharmaceutical’s approval, such approval not to be unreasonably or untimely withheld, Nordion shall transition Manufacture of Azedra to the Facility within thirty (30) days. After transition to the Facility Nordion agrees to (i) use the Process to produce Batches of Azedra (in quantities specified on Schedule D) that meet the Specifications, FDA requirements and are manufactured in conformance with cGMPs and (ii) make arrangements for shipment of Azedra to third parties on behalf of Molecular Insight Pharmaceuticals as directed by Molecular Insight Pharmaceuticals. All Batches shall be shipped in regulatory approved lead xxxxxxx. Nordion reserves the right to withhold from shipment any Batch, or portion thereof, which does not conform to Specifications. Nordion, at the time of execution of this Agreement, will be supplying Azedra from an alternative facility established under the Interim Facility Agreement. |
| Azedra production runs will be performed on Mondays and up to two other weekdays each week subject to Nordion staff availability. In the event that the market demand for Azedra attains the level whereby the three production days are fully utilized, and that market demand is reasonably sustained for a period of at least three (3) consecutive months, Nordion will use commercially reasonable efforts to accommodate Azedra production on additional days. If thereafter such market demand is not sustained for any period of two consecutive months, Nordion may reduce the days on which it carries out production runs. |
| 4.2 | Clinical Trial and Commercial Phase Orders and Pricing |
| The purchase price for a Batch (and additional vials) supplied to Molecular Insight Pharmaceuticals for Clinical Trials and during the Commercial Phase shall be as set out in Schedule D and shall be payable as set forth in Section 4.7 herein. All orders for Azedra shall be forwarded by Molecular Insight Pharmaceuticals and received by Nordion a minimum of fourteen (14) business days for Clinical Trials and twenty (20) business days during the Commercial Phase prior to the week in which Azedra is to be Manufactured. |
| 4.3 | Minimum Purchase Obligation |
| Subject to section 4.4, commencing with the first full month after completion of the Facility (as notified by Nordion to Molecular Insight Pharmaceuticals pursuant to section 4.1), Molecular Insight Pharmaceuticals shall, during the remainder of the Term, purchase or otherwise pay Nordion for a minimum of two (2) Batches of Azedra per calendar month. In the event the minimum purchase obligations described in this section 4.3 are not met by Molecular Insight Pharmaceuticals, as averaged over a rolling two (2) month period, Nordion may without liability, in addition to any other remedy available, at its sole option and unfettered discretion, suspend Manufacture of Azedra until full payment is made by Molecular Insight Pharmaceuticals (during which period the minimum purchase commitment shall continue to accrue), or notwithstanding any other remedial or cure provision contained in this Agreement, terminate this Agreement upon ten (10) days prior written notice. The provisions of section 4.5 shall apply to cancellation of a Batch (and additional vials) however section 4.5 shall in no way limit Molecular Insight Pharmaceuticals’ minimum purchase commitment under this Agreement and any cancellation fee due and payable in a given month (and paid) shall be applied against amounts owing by Molecular Insight Pharmaceuticals with respect to its minimum purchase commitment in such month. |
| In the event that Nordion elects to suspend Manufacture of Azedra due to Molecular Insight Pharmaceuticals failure to meet its minimum purchase obligation pursuant to this Section 4.3 and Molecular Insight Pharmaceuticals subsequently cures such failure, Nordion agrees to resume Manufacture of Azedra within fifteen (15) business days of such cure. |
| 4.4 | Facility Reservation Fees |
| (i) | If at the time of completion of the Facility (as notified by Nordion to Molecular Insight Pharmaceuticals pursuant to section 4.1) use of the Facility to Manufacture Azedra be delayed due to a regulatory reason, the commencement of the minimum purchase commitment in section 4.3 shall on a one time basis, be temporarily suspended until such time as the delay preventing such use is resolved by Molecular Insight Pharmaceuticals, provided further however, that if such delay endures for a continuous period of six (6) months (from the date of Nordion’s notification of completion of the Facility pursuant to section 4.1), for the remaining duration of the delay Molecular Insight Pharmaceuticals in lieu of the minimum purchase |
| commitments set out in section 4.3, shall (subject to the terms and conditions of this Agreement), pay to Nordion a facility reservation fee of ****** per month, which payment shall be due and payable on the first day of each month. This facility reservation fee shall not be refundable or reimbursable. The temporarily suspended minimum purchase commitment shall resume immediately upon resolution of the delay preventing use of the Facility for the Manufacture of Azedra. |
| (ii) | During the period of FDA review of Molecular Insight Pharmaceuticals’ submission of the NDA for Azedra, the minimum purchase commitment in section 4.3 shall be temporarily suspended and the facility reservation fee shall be ****** per month for each full month or part thereof during which the NDA is under review. The temporarily suspended minimum purchase commitment in section 4.3 shall resume immediately upon FDA approval of the NDA. This facility reservation fee shall not be refundable or reimbursable, but shall be credited in a given month only towards amounts owed by Molecular Insight Pharmaceuticals for the purchase of Batches (and additional vials) in such month under this Agreement. Payment of the purchase price for Batches (and additional vials) delivered in excess of this facility reservation fee in a given month shall be invoiced to Molecular Insight Pharmaceuticals. The provisions of section 4.5 shall apply to cancellation of a Batch (and additional vials), and in the event that facility reservations fees are applicable, such cancellation fees payable (and paid) by Molecular Insight Pharmaceuticals in a given month, shall be applied against the facility reservation fees paid in such month. |
| 4.5 | Batch Cancellation |
| (a) Subject to section 4.5 (b) and the terms and conditions of this Agreement, Molecular Insight Pharmaceuticals shall be entitled to cancel any Batch (and additional vials) ordered from Nordion during the Term of this Agreement to be entirely used solely for the purpose of Clinical Trials) at no charge by providing to Nordion at least seven (7) or more business days written notice of cancellation prior to the scheduled Batch Production Date. If an order for a Batch is cancelled with less than seven (7) business days and more than two (2) business days written notice prior to the scheduled Batch production date, ****** of the full purchase price of such Batch (and additional vials) shall be due and payable by Molecular Insight Pharmaceuticals. For a Batch (and additional vials) cancelled upon two (2) or less business days notice prior to the scheduled Batch production date, ****** of the full purchase price of the Batch (and additional vials) shall be due and payable by Molecular Insight Pharmaceuticals. |
* Confidential Treatment Requested *
| (b) Subject to the terms and conditions of this Agreement, Molecular Insight Pharmaceuticals shall be entitled to cancel any Batch (and additional vials) ordered from Nordion during the Commercial Phase at no charge by providing to Nordion at least fifteen (15) or more business days written notice of cancellation prior to the scheduled Batch Production Date. If an order for a Batch is cancelled with less than fifteen (15) business days and more than two (2) business days written notice prior to the scheduled Batch production date, ****** of the full purchase price of such Batch (and additional vials) shall be due and payable by Molecular Insight Pharmaceuticals. For a Batch (and additional vials) cancelled upon two (2) or less business days notice prior to the scheduled Batch production date, ****** of the full purchase price of the Batch (and additional vials) shall be due and payable by Molecular Insight Pharmaceuticals. |
| 4.6 | Price Escalation |
| During the Term of this Agreement the Batch Fee (including additional vials) (pursuant to Schedule D item A (i)), Waste Disposal Fees (pursuant to Schedule D item A (iii)), facility reservation fees (section 4.4), Scope Change labour rates (Schedule C) and those fees set out in section 3.6, shall be subject to price escalation on January 1st of each contract year during the Term of this Agreement, in accordance with the increase in the Canadian Consumer Price Index (CPI) as published by the Government of Canada. The CPI will be determined based on the percentage increase in the CPI for the twelve (12) month period ending three (3) months prior to January 1st of each contract year during the Term of this Agreement. |
| 4.7 | Compensation |
| Molecular Insight Pharmaceuticals has paid Nordion a lump sum of ****** under the terms of the Letter of Agreement, of which payment Nordion hereby acknowledges receipt, and which amount shall be non-refundable as attributed as set out in Schedule C “Advance Payment”. |
| For the purposes of certainty all sums expressed in this Agreement shall be in Canadian or United States currency, as specified. |
| Nordion shall invoice Molecular Insight Pharmaceuticals for the purchase price of Batches (and additional vials) of Azedra. All undisputed payments for such Batches (and additional vials) will be paid by Molecular Insight Pharmaceuticals within thirty (30) days of receipt of the invoice or if the invoice is issued by e-mail payment shall be due within (30) days of the date of sending the e-mail. Any undisputed amounts which remain unpaid after the aforementioned thirty (30) day period shall bear annual interest at the rate twelve percent (12%) annually and calculated at the rate of one percent (1%) monthly. |
* Confidential Treatment Requested *
| In the event Molecular Insight Pharmaceuticals disputes any particular invoiced item or amount, Molecular Insight Pharmaceuticals shall pay the non-disputed portion of the invoice and the parties shall attempt in good faith to resolve the dispute pertaining to the balance within fifteen (15) days of receipt of the notice of dispute issued by either party. In the event that the parties cannot resolve the dispute within the aforementioned fifteen (15) day period either party may pursue any remedy available under this Agreement or available at law. |
ARTICLE 5 – PRECURSOR AND REFERENCE STANDARDS
| 5.1 | Precursor and Reference Standards |
| Molecular Insight Pharmaceuticals or, at Molecular Insight Pharmaceuticals’ discretion, its designee, shall provide to Nordion, at no charge, Precursor and Reference Standards which meet the specifications in Schedule F in sufficient quantities to permit Nordion to meet its obligations hereunder. Nordion shall only use Precursor and Reference Standards provided hereunder for the Manufacture of Azedra pursuant to this Agreement. Molecular Insight Pharmaceuticals shall at all times retain title in and to Precursor and Reference Standards in Nordion’s possession. |
| 5.2 | Unavailability or Scarcity of Precursor and/or Reference Standards |
| Molecular Insight Pharmaceuticals will notify Nordion upon Molecular Insight Pharmaceuticals becoming aware of a shortage of supply of Precursor or Reference Standards if such shortage will impact the manufacture of the Azedra. Molecular Insight Pharmaceuticals shall not be liable for any delays or shortages in the supply of Precursor or Reference Standards; provided, however, that notwithstanding anything to the contrary in this Agreement, that any such shortages or delays for any reason in Precursor or Reference Standards supply, will not affect any amounts payable by Molecular Insight Pharmaceuticals pursuant to sections 4.4 (facility reservation fees), section 4.3 (minimum purchase commitments) and 4.5 (Batch cancellation fees) and shall excuse Nordion’s performance of activities related to such Batch of Azedra to the extent that Nordion’s non-performance was caused by the Precursor or Reference Standards supply delay or shortage and only for a period of time equal to the delay. |
| 5.3 | Compliance with Law, Handling |
| While Precursor, Reference Standards, Isotope and Azedra are in its possession or under its control, Nordion shall be responsible for compliance in all material respects with applicable statutory and regulatory requirements in the United States and Canada. |
ARTICLE 6 – AZEDRA SHIPMENTS
| 6.1 | Orders and Shipments |
| During the Term of this Agreement, Molecular Insight Pharmaceuticals will forward orders to Nordion at its Ottawa, Ontario facility by facsimile or such other method as agreed by the parties. Each order will set forth the quantity of Azedra to be produced and prepared for shipment, the identity of the recipient, delivery destination protocol number, IND/NDA number, applicable USNRC materials license number and IRS number. Delivery of Azedra to Molecular Insight Pharmaceuticals or as otherwise directed by Molecular Insight Pharmaceuticals shall be Ex Works (Incoterms 2000) at Nordion’s facility in Ottawa, Ontario. Risk of loss of Azedra shall pass to Molecular Insight Pharmaceuticals at point of delivery at Nordion’s facility in Ottawa, Ontario. |
| During the Term of this Agreement Nordion shall use commercially reasonable efforts to meet Molecular Insight Pharmaceuticals’ orders and delivery requirements. Prior to the first shipment of Azedra to any third party site, Molecular Insight Pharmaceuticals shall obtain from such third party and provide to Nordion such third party’s license evidencing proper legal authority for the receipt and possession of Azedra by such third party. Molecular Insight Pharmaceuticals shall obtain all approvals, licenses and permits required to import Azedra into the United States. Nordion shall make shipping arrangements with FedEx or such other carrier designated by Nordion and reasonably approved by Molecular Insight Pharmaceuticals. All Azedra shipping costs incurred from the Ex Works point of delivery shall be borne by Molecular Insight Pharmaceuticals. |
ARTICLE 7 – PRODUCT WARRANTY and LIMITED LIABILITY
| 7.1 | Limited Product Warranty |
| Nordion provides a product warranty, and does warrant for each Batch, that Azedra will (i) conform with the Specifications, and (ii) be Manufactured, and prepared for shipment in accordance with cGMPs. |
| If either party discovers that a Batch of Azedra does not meet the Specifications, then the discovering party shall promptly communicate with the other party. All warranty obligations of Nordion with respect to a particular Batch shall cease and have no effect to the extent that any defect in such Batch arises from accident, abuse, misuse, alteration or negligence of Molecular Insight Pharmaceuticals or its third party suppliers or customers. If Molecular Insight Pharmaceuticals determines that the failure to meet Specifications results from an act, failure to act or other fault of Nordion, or agent of Nordion, Nordion will promptly: |
| (i) | replace such batch of Azedra; and |
| (ii) | pay for shipping costs of replacement of Azedra. |
| In the event that Nordion reasonably disputes Molecular Insight Pharmaceuticals’ determination that the fault is due to Nordion and/or its agent, the parties will select a mutually acceptable outside consulting firm which will be instructed to review the applicable information and data and confirm or dissent from Molecular Insight Pharmaceuticals’ determination. If the consulting firm confirms Molecular Insight Pharmaceuticals’ determination, Nordion will have the obligations set out in this section and Nordion will pay the fees of such consulting firm. If the consulting firm dissents from Molecular Insight Pharmaceuticals’ determination or determines that the failure to meet Specifications was due to products, information or services supplied by Molecular Insight Pharmaceuticals, Nordion will not have the obligations set out in this section with respect to the disputed Batch and Molecular Insight Pharmaceuticals will pay the fees for such consulting firm. |
| 7.2 | Acknowledgement |
| MOLECULAR INSIGHT PHARMACEUTICALS ACKNOWLEDGES THAT NORDION IS CARRYING OUT THE MANUFACTURE AND SUPPLY OF AZEDRA TO MEET SPECIFICATIONS. EXCEPT AS EXPRESSLY SET OUT IN THIS AGREEMENT, NORDION HEREBY DISCLAIMS ALL OTHER WARRANTIES OR CONDITIONS, WHETHER EXPRESS OR IMPLIED, STATUTORY OR OTHERWISE INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTIES OR CONDITIONS OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. |
| 7.3 | Disclaimer |
| EXCEPT IN THE CASE OF THEIR RESPECTIVE INDEMNIFICATION OBLIGATIONS HEREIN, IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER FOR INDIRECT, CONTINGENT, INCIDENTAL, SPECIAL OR CONSEQUENTIAL DAMAGES, WHETHER IN CONTRACT, TORT, OR ANY OTHER CAUSE OF ACTION, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. |
ARTICLE 8 – NONCOMPETE
| During the Term of this Agreement, Nordion will not produce or sell I-131 MIBG (Azedra or generic) for any oncology indication in North America except for Molecular Insight Pharmaceuticals. Nordion shall not be restricted in any way from developing, producing or selling generic I-131 MIBG for markets outside North America but is prohibited from using the Facility or any Data for any such production or development. Nordion shall not be restricted in any way from developing, producing or selling I-131 radiochemical or any other I-131 radiopharmaceutical product. |
ARTICLE 9 – MARKET ACCESS
| In the event that Nordion desires to market and distribute Azedra in a geographic market not actively being developed by Molecular Insight Pharmaceuticals or should Molecular Insight Pharmaceuticals desire that a third party market and distribute Azedra in a geographic market not actively being developed by Molecular Insight Pharmaceuticals, the parties agree to discuss potential access to such markets in good faith. |
ARTICLE 10 – LICENSE/OWNERSHIP
| 10.1 | Royalty-Free License |
| Molecular Insight Pharmaceuticals hereby provides to Nordion a non-exclusive, nontransferable, royalty-free license during the Term of this Agreement to use Molecular Insight Pharmaceuticals Background Technology relating to Azedra and the radiolabelling of Precursor with I-131 for the sole purpose of assisting Nordion in carrying out its obligations set out in this Agreement. |
| 10.2 | Ownership |
| Molecular Insight Pharmaceuticals Background Technology shall remain the sole and exclusive property of Molecular Insight Pharmaceuticals. Nordion Background Technology shall remain the sole and exclusive property of Nordion. The parties acknowledge and agree that if and to the extent that the Process is jointly developed by Nordion and Molecular Insight Pharmaceuticals during the Term of this Agreement then each party may continue to use and exploit such jointly developed technology as their own technology both during and after the Term of this Agreement. |
| Nothing in this Agreement transfers or licenses any right, title or interest to Molecular Insight Pharmaceuticals Background Technology or Nordion Background Technology, except as expressly set out herein. |
ARTICLE 11 – MOLECULAR INSIGHT PHARMACEUTICALS REPRESENTATIONS AND WARRANTIES
| 11.1 | Molecular Insight Pharmaceuticals’ Representations and Warranties |
| Molecular Insight Pharmaceuticals represents, warrants and covenants that: |
| (i) | it has full right, power and authority to enter into this Agreement; |
| (ii) | it is the owner or has the right of use of the Molecular Insight Pharmaceuticals Background Technology supplied to Nordion by Molecular Insight Pharmaceuticals to assist Nordion in Manufacture of Azedra and in carrying out its obligations hereunder; |
| (iii) | to Molecular Insight Pharmaceuticals’ knowledge, there is no action or proceeding pending or, to its knowledge, threatened against Molecular Insight Pharmaceuticals before any court, administrative agency or other tribunal which would have an adverse material effect on its business or its ability to perform its obligations hereunder; |
| (iv) | it has the right to grant the license in section 10.1 and right to permit Nordion to use Molecular Insight Pharmaceuticals Background Technology to the extent required to assist Nordion in carrying out its obligations under this Agreement; |
| (v) | it has not received any written notice of adverse claim or infringement of any patent or other intellectual property right, or misappropriation of trade secrets in connection with, the Molecular Insight Pharmaceuticals Background Technology or the use and exploitation of the Precursor, Reference Standard or Azedra; |
| (vi) | to Molecular Insight Pharmaceuticals’ best knowledge and belief, making, using, offering for sale or selling of Precursor, Reference Standards and Azedra, and the data, information, technology and know how used in the Process and manufacture of Azedra contributed by Molecular Insight Pharmaceuticals, do not infringe any valid third party patent, pending published patent application or other intellectual property right; |
| (vii) | it is not under any obligations, contractual or otherwise, to any other entity that might conflict, interfere or be inconsistent with any of the provisions of this Agreement; |
| (viii) | it shall obtain and maintain at its expense, all licenses, permits and approvals necessary for it to perform its obligations under this Agreement; |
| (ix) | as of the Effective Date, it is not currently subject to any proceeding pending or, to its knowledge, threatened for reorganization, liquidation or dissolution for the benefit of its creditors or otherwise; |
| (x) | Azedra shall not be misbranded within the meaning of the FD&C as a result of the application of the label content supplied by Molecular Insight Pharmaceuticals to Nordion pursuant to this Agreement, and Azedra is not an article which may not be introduced into interstate commerce under the provisions of section 505 of the FD&C. |
ARTICLE 12 – NORDION’S REPRESENTATIONS AND WARRANTIES
| 12.1 | Representations and Warranties |
| Nordion represents, warrants and covenants that: |
| (i) | it has full right and authority to enter into this Agreement; |
| (ii) | it is the owner or has the right to use the Nordion Background Technology and other Nordion proprietary technology used during the Term of this Agreement; |
| (iii) | the Nordion Background Technology does not, to Nordion’s best information and belief, infringe any patents, copyright or other industrial or intellectual property rights of third parties; |
| (iv) | it has not received any notice of adverse claim of infringement of any patent or other intellectual property right, or of misappropriation of trade secrets, in connection with the use and exploitation of the Nordion Background Technology; |
| (v) | there is no action or proceeding pending or, to its knowledge, threatened against Nordion before any court, administrative agency or other tribunal which would have an adverse material effect on Nordion’s business or its ability to perform its obligations hereunder; |
| (vi) | as of the Effective Date, Nordion is not currently subject to any proceeding pending or, to its knowledge, threatened for reorganization, liquidation or dissolution for the benefit of its creditors or otherwise; |
| (vii) | it shall obtain and maintain, at its expense, all Facility licenses, permits and approvals necessary for it to manufacture Azedra under this Agreement and shall provide Molecular Insight Pharmaceuticals with copies of such licenses, permits and approvals upon request; |
| (viii) | the Azedra delivered pursuant to this Agreement shall, at the time of delivery by Nordion to Molecular Insight Pharmaceuticals, not be misbranded (to the extent branded by Nordion) or adulterated within the meaning of FD&C and effective at the time of delivery of the Azedra. |
ARTICLE 13 – INDEMNITY
| 13.1 | Indemnification by Molecular Insight Pharmaceuticals |
| Molecular Insight Pharmaceuticals agrees to indemnify, defend and hold Nordion and its Affiliates and their respective directors, officers, employees and agents, harmless from and against any damages, claims, liabilities and expenses (including, |
| but not limited to, reasonable attorney’s fees) resulting from any third party claims or suits (“General Claims Against Nordion”) arising out of (a) Molecular Insight Pharmaceuticals’ or a third party’s use, handling or shipping of Reference Standards, Precursor or Azedra (including in the event that Nordion makes shipping arrangements on behalf of Molecular Insight Pharmaceuticals), (b) Molecular Insight Pharmaceuticals’ breach of any of its material obligations, warranties or representations hereunder, (c) Molecular Insight Pharmaceuticals’ negligent acts or omissions or willful misconduct, or (d) failure of the Reference Standards or Precursor to meet applicable specification. Notwithstanding the foregoing, Molecular Insight Pharmaceuticals will not be required to indemnify, defend and hold Nordion and its Affiliates and their respective directors, officers, employees and agents harmless from and against any General Claims Against Nordion to the extent that such claims arise out of (i) Nordion’s breach of any of its obligations, warranties or representations hereunder; (ii) Nordion’s negligent acts or omissions or willful misconduct; (iii) any failure of Nordion to Manufacture, (except to the extent labels and/or content thereof are provided by Molecular Insight Pharmaceuticals) or prepare for shipment Azedra in accordance with this Agreement, cGMPs or any other applicable laws, rules, regulations or other requirements of any applicable governmental entity; or (iv) any failure of Nordion to Manufacture Azedra consistent with the Specifications and requirements set forth herein. Notwithstanding anything in this section 13.1, “General Claims Against Nordion” shall not include “IP Claims Against Nordion” as described in section 13.3. |
| 13.2 | Indemnification by Nordion |
| Nordion agrees to indemnify, defend and hold Molecular Insight Pharmaceuticals and its Affiliates and their respective directors, officers, employees and agents, harmless from and against any damages, claims, liabilities and expenses (including, but not limited to, reasonable attorney’s fees) resulting from any third party claims or suits (“General Claims Against Molecular Insight Pharmaceuticals”) arising out of (a) Nordion’s Manufacture (except to the extent that labels and/or content thereof is provided by Molecular Insight Pharmaceuticals) or preparation for shipment of Azedra; (b) Nordion’s breach of any of its material obligations, warranties or representations hereunder; (c) Nordion’s negligent acts or omissions or willful misconduct; or (d) any failure of the Azedra to meet the Specifications. Notwithstanding the foregoing, Nordion will not be required to indemnify, defend and hold Molecular Insight Pharmaceuticals and its Affiliates and their respective directors, officers, employees and agents harmless from and against any General Claims Against Molecular Insight Pharmaceuticals to the extent that such claims arise out of (i) Molecular Insight Pharmaceuticals’ breach of any of its obligations, warranties or representations hereunder; (ii) Molecular Insight Pharmaceuticals’ negligent acts or omissions or willful misconduct; (iii) any |
| defect or failure of Reference Standards or Precursor to meet applicable specifications or (iv) Molecular Insight Pharmaceuticals’ or third party’s use, labeling, handling or shipment of Reference Standards, Precursor or, Azedra. Notwithstanding anything in this section 13.2, “General Claims Against Molecular Insight Pharmaceuticals” shall not include “IP Claims Against Molecular Insight Pharmaceuticals” as described in section 13.4. |
| 13.3 | Intellectual Property Claims Against Nordion |
| Molecular Insight Pharmaceuticals agrees to indemnify, defend and hold Nordion and its Affiliates and their respective directors, officers, employees and agents, harmless from and against any damages, claims, liabilities and expenses (including, but not limited to, reasonable attorneys’ fees) resulting from any third party claims or suits arising out of any proceeding instituted by or on behalf of a third party based upon a claim that Molecular Insight Pharmaceuticals Background Technology, the Process (only to the extent contributed by Molecular Insight Pharmaceuticals), use or sale of the Reference Standards, Precursors or Azedra infringes a patent or any other intellectual property right of a third party (“IP Claims Against Nordion”). To the extent the Process or method of manufacture is developed or contributed by Nordion, Molecular Insight Pharmaceuticals will not be required to indemnify, defend or hold harmless Nordion or its Affiliates, and their respective directors, officers, employees and agents from and against IP Claims Against Nordion. |
| 13.4 | Intellectual Property Claims Against Molecular Insight Pharmaceuticals |
| Nordion agrees to indemnify, defend and hold harmless Molecular Insight Pharmaceuticals and its Affiliates, and their respective directors, officers, employees and agents from and against any damages, claims, liabilities and expenses (including, but not limited to, reasonable attorney’s fees) resulting from any third party claims or suits arising out of any proceeding instituted by or on behalf of a third party based upon a claim that the Nordion Background Technology, the method of Manufacture of Azedra or the Process to the extent developed or contributed by Nordion, infringes a patent or other intellectual property right of a third party (“IP Claims Against Molecular Insight Pharmaceuticals”). |
| 13.5 | Nordion Infringement |
| In the event that any portion of the Nordion Background Technology, required in the Manufacture of Azedra, in the opinion of independent counsel mutually selected by the parties, becomes the subject of a valid claim for a patent, copyright or other intellectual property right infringement then Nordion shall, for a period of at least sixty (60) days after issuance of the opinion, use its commercially reasonable efforts to: |
| i) | procure the right to continue using the technology, or |
| ii) | modify the Nordion Background Technology to become non-infringing (with all Nordion costs, including any commercially reasonable increase to the cost of the manufacturing of Azedra, to be borne by Nordion). |
| After expiry of the sixty (60) day period from the issuance of the aforementioned opinion, if Nordion has been unable to remedy the infringement in accordance with subparagraph i) or ii) above, Molecular Insight Pharmaceuticals or Nordion, may upon written notice, suspend the Manufacture of Azedra and/or terminate this Agreement. The provisions of this section 13.5 are in addition to, and do not in any way limit, the indemnification obligations of Nordion set forth in this Article 13. |
| 13.6 | Molecular Insight Pharmaceuticals Infringement |
| In the event that any portion of the Molecular Insight Pharmaceuticals Background Technology required in the Manufacture of Azedra, in the opinion of independent counsel mutually selected by the parties, becomes the subject of a valid claim for a patent, copyright or other intellectual property right infringement, then Molecular Insight Pharmaceuticals shall, for a period of at least sixty (60) days after issuance of the opinion, use its commercially reasonable efforts to: |
| i) | procure the right to continue using the technology, or |
| ii) | modify the Molecular Insight Pharmaceuticals Background Technology to become non-infringing provided such modification does not increase the cost of Manufacture of Azedra by Nordion (with all Molecular Insight Pharmaceuticals’ costs including any increase to the cost of the Manufacture of Azedra by Nordion to be borne by Molecular Insight Pharmaceuticals). |
After expiry of the sixty (60) day period from the issuance of the aforementioned opinion, if Molecular Insight Pharmaceuticals has been unable to remedy the infringement in accordance with subparagraph i) or ii) above, Nordion or Molecular Insight Pharmaceuticals may upon written notice, suspend Manufacture activities and/or terminate this Agreement. The provisions of this section 13.6 are in addition to, and do not in any way limit, the indemnification obligations of Molecular Insight Pharmaceuticals set forth in this Article 13.
| 13.7 | Indemnification Procedures |
A party (the “indemnitee”) intending to claim indemnification under this Agreement shall promptly notify the other party (the “Indemnitor”) in writing of any action, claim or other matter in respect of which the Indemnitee or any of its directors, officers, employees or agents intend to claim such indemnification; provided, however, the failure to provide such notice within a reasonable period of time shall not relieve the Indemnitor of any of its obligations hereunder except to the extent the Indemnitor is materially prejudiced by such failure. The Indemnitor shall be entitled to control the defense of and/or settle any such action, claim or other matter. The Indemnitee agrees to the complete control of such defense or settlement by the Indemnitor, provided, however, any settlement of such claims shall require the Indemnitee’s prior written consent unless such settlement includes a full release of the Indemnitee, in which case no consent shall be required. The Indemnitee and its directors, officers, employees and agents shall co-operate fully with the Indemnitor and its legal representatives in the investigation and defence of any action, claim or other matter covered by this indemnification. The Indemnitee shall have the right, but not the obligation, to be represented by counsel of its own selection and at its own expense.
ARTICLE 14 – REGULATORY MATTERS
| 14.1 | Regulatory Status |
| Upon Nordion’s reasonable request, Molecular Insight Pharmaceuticals shall provide updates to Nordion on submissions to the FDA and other jurisdictions and regulatory agencies for marketing authorization with respect to Azedra. |
| 14.2 | Molecular Insight Pharmaceuticals Responsibilities |
| It shall be the responsibility of Molecular Insight Pharmaceuticals or its designee to file, obtain and maintain an IND/NDA, registrations, listings, authorizations and approvals as the FDA or any other applicable governmental entity may require to enable use of Azedra in Clinical Trials and the Commercial Phase in the United States. Nordion shall provide directly to Molecular Insight Pharmaceuticals, or at Nordion’s discretion for the purpose of protection of its proprietary technology with respect to the manufacture of the Isotope, directly to the regulatory authority (with a copy to Molecular Insight Pharmaceuticals purged of Nordion proprietary technology) all required information in its possession necessary to assist Molecular Insight Pharmaceuticals in filing, obtaining and maintaining all licenses, registrations, listings, authorizations and approvals of any governmental entities necessary for the use of Azedra in support of Molecular Insight Pharmaceuticals’ Azedra IND/NDA submission. Molecular Insight Pharmaceuticals will provide Nordion with copies of any submissions made describing activities that occur at Nordion. |
| 14.3 | Nordion Responsibilities |
Nordion shall be responsible for obtaining and maintaining all necessary Facility licenses, registrations, authorizations and approvals which are necessary to Manufacture, and prepare for shipment Azedra under cGMP conditions and other regulatory requirements including, but not limited to, the use and handling of radioactive materials.
At Nordion’s expense, Nordion shall update and maintain its existing I-131 bulk chemical Drug Master File (“DMF”) with the FDA as may be required for Molecular Insight Pharmaceuticals’ IND/NDA for Azedra. Nordion hereby grants Molecular Insight Pharmaceuticals for the purpose of this Agreement, a right of reference to such DMF, and upon request shall provide a letter of access to the DMF allowing regulatory review of the DMF by the FDA in conjunction with Molecular Insight Pharmaceuticals’ Azedra submissions.
| 14.4 | Government Inspections, Compliance Review and Inquiries |
Upon request of any governmental entity or any third party entity authorized by a governmental entity, such entity shall, for the purpose of regulatory review, have access to observe and inspect the (i) Facility, (ii) procedures used for the storage of Reference Standards and Precursor and (iii) manufacturing, testing, storage and preparation for shipment of Azedra, including Process development operations, and auditing the Facility for compliance with cGMP and/or other applicable regulatory standards. Nordion shall give Molecular Insight Pharmaceuticals prompt written notice of any upcoming inspections or audits by a governmental entity of the Facility or any of the foregoing and shall allow Molecular Insight Pharmaceuticals to participate in such audits by being present at any FDA close-out meeting and shall provide Molecular Insight Pharmaceuticals with a written summary of such inspection or audit following completion thereof. Notwithstanding the foregoing Molecular Insight Pharmaceuticals shall be entitled to have a representative in attendance at the Facility as an observer for the pre-approval inspection. Nordion agrees to use commercially reasonable efforts promptly to rectify or resolve any deficiencies noted by a government entity in a report or correspondence issued to Nordion. Subject to any specific arrangements agreed upon by the parties, Molecular Insight Pharmaceuticals shall be responsible for communicating with any governmental authority concerning the Azedra or the marketing, distribution or sale of Azedra, and Nordion shall in accordance with the compensation rates set forth in section 3.6 provide Molecular Insight Pharmaceuticals with whatever assistance Molecular Insight Pharmaceuticals may reasonably require to assist it in such communications. Nordion shall have no such communications specifically related to Azedra, except to the extent that they relate to Nordion’s Manufacture of Azedra under this Agreement, in which case Nordion
shall be responsible for such communications. Notwithstanding the foregoing and except to the extent that an immediate communication is necessary under the circumstances or required by law, Nordion in good faith shall consult in advance with Molecular Insight Pharmaceuticals regarding all communications that relate to Azedra or to Nordion’s ability to Manufacture Azedra pursuant to this Agreement.
| 14.5 | Complaints and Adverse Reactions |
Nordion or Molecular Insight Pharmaceuticals shall provide to each other prompt notice of any information either of them receives regarding the safety of the Precursor, Reference Standards, Azedra or Isotope, including any confirmed or unconfirmed information regarding adverse, serious or unexpected events associated with Azedra that may implicate the Manufacture of Azedra or one of its components; provided, however, that Molecular Insight Pharmaceuticals shall not be required to provide clinical trial status reporting to Nordion. For serious or adverse events, notice must be given by telephone within one (1) business day after receipt of the information, followed immediately with written notice, advising the other of any adverse reaction or safety issues with respect to Azedra of which it becomes aware, regardless of the origin of such information. Any other complaints shall be reported in writing to the other party on a weekly basis. Nordion agrees to co-operate with Molecular Insight Pharmaceuticals and any governmental entity in evaluating any complaint, claim, safety or adverse use report related to Azedra. Nordion will provide timely assistance in responding to any complaints, including reviews of Batch records and retained samples as well as any necessary testing.
| 14.6 | Recalls |
Molecular Insight Pharmaceuticals shall notify Nordion promptly if Azedra is the subject of a recall or correction (a “Recall”), and Molecular Insight Pharmaceuticals and/or its designee shall have sole responsibility for the handling and disposition of such Recall. Molecular Insight Pharmaceuticals and/or its designee shall bear the costs of any Recall of Azedra unless and to the extent such Recall shall have been the result of Nordion’s or its agents or employees negligent acts or omissions or any product defects for which Nordion is responsible in which case Nordion shall to such extent be responsible for all of Molecular Insight Pharmaceuticals’ reasonable out-of-pocket costs incurred for:
| (i) | notification of recall to Nordion and third parties; |
| (ii) | return shipment of any defective Azedra to Nordion; and |
| (iii) | replacement of Azedra. |
In the event that Nordion disputes Molecular Insight Pharmaceuticals’ determination that the fault is due to Nordion and/or to its employees or agents, the parties will select a mutually agreeable outside consulting firm which will be instructed to review the applicable information and data and to confirm or dissent from Molecular Insight Pharmaceuticals’ determination. If the consulting firm confirms Molecular Insight Pharmaceuticals’ determination, Nordion will pay the fees of such consulting firm. If the consulting firm dissents from Molecular Insight Pharmaceuticals’ determination Nordion will not have the obligations set forth herein with respect to the Recall and Molecular Insight Pharmaceuticals will pay the fees of such consulting firm. Molecular Insight Pharmaceuticals and/or its designee shall maintain records of all sales, shipping records of Azedra and customers in sufficient detail to adequately administer a Recall for the period of time as required by applicable regulation.
| 14.7 | New Regulatory Requirements |
Each party shall promptly notify the other of new regulatory requirements of which it becomes aware which are relevant to the manufacture of Azedra under this Agreement and which are required by the FDA and other applicable governmental entities. The parties shall confer with each other with respect to the best means to implement and comply with such requirements.
If after a NDA submission is made to the FDA or EMEA, any Facility investment above ****** in aggregate required to maintain cGMP or FDA compliance, and the need for such investment is not due to the negligence or fault of Nordion, such investment shall be treated as a scope change pursuant to Section 3.3, shall be the subject of a Change in Scope Form (Schedule G) and paid for by Molecular Insight Pharmaceuticals.
| 14.8 | Records |
Nordion shall maintain all records necessary to evidence compliance in all material respects with (i) all applicable laws, rules, regulations and other requirements of applicable governmental entities in the United States and Canada relating to the Manufacture and supply of Azedra; (ii) the Specifications; and (iii) material obligations under this Agreement. All such records shall be maintained by Nordion for at least two (2) years after termination or expiration of this Agreement. Nordion shall provide to Molecular Insight Pharmaceuticals reasonable access to such records upon request. Prior to destruction of any record after such time, Nordion shall give written notice to Molecular Insight Pharmaceuticals. Molecular Insight Pharmaceuticals shall have the right within thirty (30) days of receipt of such notice to request that Nordion maintain such records in an off site storage facility for such longer periods as Molecular Insight Pharmaceuticals requests, provided that Molecular Insight Pharmaceuticals pays all costs associated with such off site storage.
* Confidential Treatment Requested *
| 14.9 | Labels |
| Molecular Insight Pharmaceuticals shall be solely responsible for, and shall provide and approve the form and content of, the labels (except lot number and expiry date which shall be the responsibility of Nordion) to be applied to Azedra. Label form and content (other than lot number and expiry date) shall remain the liability and exclusive property of Molecular Insight Pharmaceuticals. Such labels shall not be used by Nordion after termination or expiration of this Agreement and the label provided by Molecular Insight Pharmaceuticals may only be used by Nordion for the purpose of performing its obligations under this Agreement. |
| 14.10 | Testing, Documentation, and Quality Assurance |
| Nordion shall maintain accurate and complete production records with respect to the Process, Batches and shipments, and Molecular Insight Pharmaceuticals shall have access upon not less than three (3) days prior written notice to such records in order to determine that each Batch was produced, tested and prepared for shipment in compliance with all applicable laws, rules, regulations, as well as the Specifications and cGMP requirements. |
| The tests and analyses provided in the Specifications as well as the nature and form of records may be amended by Nordion from time to time, subject to the consent of Molecular Insight Pharmaceuticals, which shall not be unreasonably or untimely withheld after Nordion shall have delivered to Molecular Insight Pharmaceuticals, in writing, an explanation of such changes and why they are necessary or advisable. The parties agree to execute the Quality Agreement in substantially the form attached as Schedule “E”. Nordion shall Manufacture and prepare for shipment, Azedra in conformance with cGMPs and regulations applicable to the shipment of radioactive materials and subject to the Quality Agreement. |
| Molecular Insight Pharmaceuticals shall be entitled to audit Nordion’s quality assurance processes in accordance with the Quality Agreement. |
ARTICLE 15 – CONFIDENTIALITY
| 15.1 | Confidentiality and Exceptions |
During the Term of this Agreement and for a period of ten (10) years thereafter, each party hereto shall maintain in confidence and not use or disclose to others for any purpose, other than to its employees or agents (which agents shall enter into a confidentiality agreement incorporating similar terms as set forth herein or be otherwise reasonably acceptable to the other party) with a need to know such information to perform such party’s obligations under this Agreement or other than as expressly authorized in this Agreement, the content of the transactions contemplated herein, all technology including Molecular Insight Pharmaceuticals Background Technology, Nordion Background Technology and improvements thereto, and other information disclosed to such party by the other party which is identified as “Confidential Information” by the disclosing party (collectively “Confidential Information”). This obligation of confidentiality shall not apply to the extent that it can be established by the party in receipt of such information, that the information:
| (i) | was already known to the receiving party at the time of disclosure; |
| (ii) | was generally available to the public or otherwise part of the public domain at the time of its disclosure; |
| (iii) | became generally available to the public or otherwise part of the public domain after its disclosure to the receiving party through no act or omission of the receiving party; |
| (iv) | was disclosed to the receiving party by a third party who was not known to the receiving party to have obligations restricting disclosure of such information; or |
| (v) | was independently developed by the receiving party without any use of Confidential Information of the disclosing party. |
Each party agrees that it will take the same degree of care to protect the confidentiality of the other party’s Confidential Information as it takes to protect its own proprietary and confidential information, which shall in no event be less than commercially reasonable. Each party, and its employees and agents shall protect and keep confidential and shall not use, publish or otherwise disclose to any third party, except as permitted by this Agreement, or with the other party’s written consent, the other party’s Confidential Information.
All Confidential Information supplied by one party to the other to assist in carrying out the obligations hereunder shall remain the property of such party and shall be returned to the other party upon termination or expiration of this Agreement.
ARTICLE 16 – DISCLOSURE OF INFORMATION
| 16.1 | Authorized Disclosure |
Notwithstanding section 15.1 each party may disclose Confidential Information to the extent such disclosure is reasonably necessary for prosecuting or defending litigation and/or complying with applicable government laws, rules or regulations, provided that if a party is required by law or regulation to make any such disclosure of the other party’s Confidential Information, except where impracticable for necessary disclosure, for example in the event of medical emergency, it will give reasonable notice to the other party of such disclosure requirement and will use its reasonable efforts to secure a protective order or limit the extent of the information to be disclosed.
ARTICLE 17 – TERM AND TERMINATION
| 17.1 | Term |
This Agreement shall commence upon the Effective Date and, unless terminated earlier pursuant to this Agreement or extended upon mutual agreement of the parties, shall expire five (5) years after the Effective Date (the “Initial Term”). Thereafter, this Agreement shall automatically be renewed for successive additional three (3) year terms (each a “Renewal Term”). Either party may terminate this Agreement by providing two (2) years prior written notice to the other party, such termination to be effective upon expiry of the Initial Term or any Renewal Term as is the case.
| 17.2 | Termination for Regulatory Delay |
In the event of any of the following:
| (i) | Molecular Insight Pharmaceuticals has not filed the NDA for regulatory approval with the FDA within one (1) year of the last patient having been treated in the clinical program, or |
| (ii) | the NDA has not received regulatory approval from the FDA within two (2) years from the filing date, or |
| (iii) | FDA regulatory approval is not received within three (3) years after the Effective Date, |
whichever is earlier, then Nordion may, without liability at its sole option and unfettered discretion terminate this Agreement. Should Nordion exercise this option, the Exit Fee defined in section 17.6 (v) shall not apply, however all other remedies shall remain in full force and effect.
| 17.3 | Termination for Breach |
This Agreement may be terminated by a party in the event of breach by the other party of a material term or condition hereof; provided, however, the other party shall first give to the breaching party written notice of the proposed termination of this
Agreement (a “Breach Notice”), specifying the grounds therefore. Upon receipt of such Breach Notice, the breaching party shall have such time as necessary, but in any event not more than sixty (60) days to cure such breach (or thirty (30) days with respect to a failure by Molecular Insight Pharmaceuticals to pay any amounts hereunder when due other than with respect to amounts which Molecular Insight Pharmaceuticals’ in good faith, disputes are due to Nordion). Notwithstanding the foregoing, if the breaching party does not cure such breach within such cure period, the other party may terminate the Agreement without prejudice to any other rights or remedies which may be available to the non-breaching party.
| 17.4 | Termination for Nordion Failure To Supply |
With respect to the supply of Batches of Azedra by Nordion pursuant to purchase orders placed pursuant to this Agreement, Nordion’s failure to supply Batches in a timely manner and consistent with such orders and the Specifications shall not be considered a material breach by Nordion unless and until Nordion has failed, in any one contract year period, to fulfill more than four (4) Batch orders consistent with the Specifications (a “Supply Breach”). It shall not be considered a Supply breach, in the event that (i) the failure to supply is attributable, in whole or in part, directly or indirectly, to Molecular Insight Pharmaceuticals, (ii) Nordion is able to supply an additional Batch meeting the Specifications in accordance with this Agreement within one (1) week of the delivery date of the originally scheduled Batch, or (iii) if the Batch failure is the result of conducting the Process under a deviation at the request of Molecular Insight Pharmaceuticals. In the event of a Supply Breach, Molecular Insight Pharmaceuticals may terminate this Agreement upon thirty (30) days prior written notice to Nordion provided it gives written notice of termination to Nordion within sixty (60) days of the Supply Breach. Any failure by Nordion to Manufacture or supply Azedra due to a Force Majeure shall not be a material breach or Supply Breach under this Agreement.
| 17.5 | Bankruptcy |
This Agreement may be terminated by a party in the event the other party files a petition in bankruptcy, is adjudicated a bankrupt, makes an assignment for the benefit of its creditors, or otherwise seeks relief under or pursuant to any bankruptcy, insolvency or reorganization statute or proceeding, or if a petition in bankruptcy is filed against it which is not dismissed within ninety (90) days or proceedings are taken to liquidate the assets of such party (each a “Bankruptcy Event”). In the event of a Bankruptcy Event or in the event that it can be reasonably be determined that a party cannot meet its debts and liabilities as they become due or make the required payments under this Agreement, the other party may modify the payment terms hereunder such that payment is required in advance.
| 17.6 | Remedies Upon Termination |
In the event of termination or expiration of this Agreement, in addition to any other remedies available to either of the parties:
| (i) | Nordion shall use reasonable efforts to terminate all activities under this Agreement immediately; |
| (ii) | within forty-five (45) days of such termination, at Molecular Insight Pharmaceuticals’ request and expense (subject to a quotation provided by Nordion and payable in advance at the request of Nordion) Nordion shall deliver to Molecular Insight Pharmaceuticals all data, documentation and other information belonging to Molecular Insight Pharmaceuticals pursuant to the terms of this Agreement; |
| (iii) | all licenses granted by Molecular Insight Pharmaceuticals to Nordion under this Agreement shall immediately terminate, |
| (iv) | within sixty (60) days of such termination all Precursor and Reference Standards remaining in inventory shall at Molecular Insight Pharmaceuticals’ option and expense (subject to a quotation provided by Nordion and payable in advance at the request of Nordion) be disposed of or returned by Nordion to Molecular Insight Pharmaceuticals, and |
| (v) | upon the termination of this Agreement for any reason, except a material breach by Nordion, Molecular Insight Pharmaceuticals shall reimburse Nordion for any reasonable expenses incurred in employee(s) severance as a result of or arising from discontinuation of the Manufacture of Azedra, inventory write-down, facility decommissioning and any related waste disposal costs (the “Exit Fee”). This provision will not apply in the event Molecular Insight Pharmaceuticals meets its minimum purchase obligations during the first three (3) years of the Commercial Phase, , in which case Nordion, as owner of the Facility, shall be responsible for any and all costs, expenses and actions necessary in connection with the reconditioning, dismantling or disposing of the Facility or any parts thereof. |
| (vi) | Molecular Insight Pharmaceuticals shall pay to Nordion any amounts otherwise due within thirty (30) days of the date of termination. |
ARTICLE 18 – SURVIVAL
| 18.1 | Consequences or Termination or Expiration |
All sections which by their nature must survive in order to give effect to their intent and meaning shall survive termination or expiration of this Agreement, including, without limitation, sections 3.7, 7.1-7.3, 10.2, 11.1, 12.1, 13.1-13.7, 14.8, 15.1, 16.1, 17.6, 18.1, 22.1, 23.1, 24.1, 25.1 and 28.1.
ARTICLE 19 – NOTICES
| 19.1 | Any notice to be sent to a party hereunder shall be forwarded to: |
| Nordion at: | MDS Nordion | |
| 000 Xxxxx Xxxx | ||
| Xxxxxx, XX X0X 0X0 | ||
| Attention: | Vice President, Marketing and Sales | |
| Fax: 000-000-0000 | ||
| with copy to : | Associate General Counsel | |
| Molecular Insight Pharmaceuticals at: | ||
| Molecular Insight Pharmaceuticals, Inc. | ||
| 000 Xxxxxx Xxxxxx | ||
| Xxxxxxxxx, XX | ||
| Attention: | Chief Executive Officer | |
| Fax: 000-000-0000 | ||
Any notice required or authorized to be given by a party to the other in accordance with the provisions of this Agreement shall, unless otherwise specifically stipulated, be in writing and delivered personally, by a nationally recognized overnight courier, or if by electronic facsimile confirmed by certified or registered mail. Notice shall be deemed delivered upon receipt.
ARTICLE 20 – ASSIGNMENT
| 20.1 | No Assignment |
This Agreement shall enure to the benefit of and shall be binding upon te heirs, executors, administrators, successors and permitted assigns of the parties. Neither Nordion nor Molecular Insight Pharmaceuticals shall assign this Agreement or any portion of this Agreement without the written approval of the other party, which approval shall not be unreasonably or untimely withheld; provided, however, that Molecular Insight Pharmaceuticals or Nordion may assign this Agreement without the other’s consent in connection with the sale of all or substantially all of its assets or the business to which this Agreement pertains, to a third party (provided such third party has agreed to accept assignment and assume the obligations and liabilities under this Agreement) or in connection with a merger, consolidation or similar transaction.
ARTICLE 21 – COMPLIANCE
| 21.1 | Compliance with Laws |
This Agreement and Nordion’s and Molecular Insight Pharmaceuticals’ obligations hereunder shall be carried out in all material respects in compliance with all applicable laws, by-laws, rules, regulations and orders of all applicable Federal, State, Provincial and Municipal governments.
ARTICLE 22 – NON-WAIVER
| 22.1 | Non-Waiver of Rights |
Failure by either party to enforce at any time any of the provisions of this Agreement shall not be construed as a waiver of its rights hereunder. Any waiver of a breach of any provision hereof shall not be effective unless in writing and shall not affect either party’s rights in the event of any additional breach.
| 22.2 | Force Majeure |
Neither party shall be liable to the other for failure to perform or delay in performing its obligations under this Agreement by virtue of the occurrence of an event of Force Majeure. In the event such Force Majeure affecting either party continues for more than ninety (90) days the party not subject of the Force Majeure may, upon thirty (30) days written notice terminate this Agreement. In the event such Force Majeure affecting either party continues for more than six (6) months either party, may upon thirty (30) days written notice terminate this Agreement. “Force Majeure” shall mean an occurrence arising from unforeseen circumstances beyond a party’s reasonable control which prevents, delays or interferes with the performance by such party of
any of its obligations hereunder including without limitation an event that occurs by reason of any act of God, flood, power failure, fire, explosion, casualty or accident, failure of suppliers or usual suppliers to have available for supply sufficient raw materials, equipment or machinery, or war, revolution, civil commotion, acts of public enemies, act of terrorism, blockage or embargo, interruption of or delay in transportation, strike or labor disruption.
In the event of Force Majeure, the party affected shall promptly notify the other and shall exert commercially reasonable efforts to eliminate, cure or overcome such event and to resume performance of its obligations.
In the event that such Force Majeure affects Isotope Nordion shall use commercially reasonable efforts to identify a then existing alternative source of Isotope supply. Any incremental cost incurred by Nordion subsequent to entering into this Agreement in obtaining Isotope from a then existing alternative supplier for Manufacture of Azedra, shall be borne on a pass through basis by Molecular Insight Pharmaceuticals. All costs and expenses, including but not limited to qualifying and validating an existing alternative supplier of Isotope for use in the Process shall be borne by Molecular Insight Pharmaceuticals. If Molecular Insight Pharmaceuticals elects not to bear the incremental cost, Manufacture of Azedra , shall be suspended during the period of Force Majeure, without liability to either party.
Molecular Insight Pharmaceuticals acknowledges and agrees that at the time of entering into this Agreement Nordion’s usual supplier Atomic Energy of Canada Ltd. and as such Nordion, due to Force Majeure (and for reasons beyond its reasonable control), may be unable to supply Isotope to Manufacture Azedra.
ARTICLE 23 – INSURANCE
| 23.1 | Product Liability Insurance |
During the Term of this Agreement and for a period of one (1) year thereafter Molecular Insight Pharmaceuticals at its own expense shall provide and maintain a Comprehensive General Liability insurance policy issued by a reputable insurance company with respect to Azedra. Such policy shall add Nordion as an additional insured and shall have a limit of liability of not less than ****** per occurrence and in the aggregate. Molecular Insight Pharmaceuticals shall be solely responsible for any
* Confidential Treatment Requested *
deductible or retention associated with this policy and such deductible or retention amounts shall not affect Nordion’s interests. The policy shall contain a cross liability clause and shall provide for severability of interest such that breach of a policy condition committed by any one insured shall not adversely affect the rights of the other insured and the insurance shall apply to each insured as though a separate policy were issued to each party. Nordion shall be provided thirty (30) days’ prior written notice of any material change to the policy and such change shall be subject to Nordion’s prior written consent, which consent shall not be unreasonably withheld. Nothing contained in this section shall be deemed to limit in any way the indemnification provisions contained in this Agreement.
ARTICLE 24 – PUBLICATION
| 24.1 | Publicity |
The parties agree that, except as may otherwise be required by applicable laws, regulations, rules or orders or in connection with obtaining regulatory approvals for Azedra, no information concerning this Agreement and the transactions contemplated herein shall be made public by either party without the prior written consent of the other, which consent shall not be unreasonably withheld or delayed. In the event either party decides to issue a press release announcing the execution of this Agreement, it shall not do so without the prior written approval of the other party.
A copy of any proposed press release shall be provided to the other party for approval at least three (3) business days prior to any proposed release.
In the event that this Agreement or any portion of its contents is required to be disclosed by Molecular Insight Pharmaceuticals or Nordion pursuant to Security Exchange Commission rules or regulations, the FDA, or other federal or state authorities, Molecular Insight Pharmaceuticals or Nordion, as the case may be, shall provide reasonable notice to the other prior to any such disclosure in order that, to the extent possible permitting such party to purge the Agreement of sensitive or confidential information while enabling the other party to comply with the applicable laws, rules and regulations.
ARTICLE 25 – INDEPENDENT CONTRACTOR
| 25.1 | No Joint Venture |
The parties agree that with respect to the transactions contemplated herein that they shall both be acting as independent contractors and nothing herein shall constitute the parties as entering into a joint venture or partnership, nor shall anything herein constitute either party as an agent of the other for any purpose whatsoever.
ARTICLE 26 – SEVERABILITY
| 26.1 | Invalid Provisions |
If any provision or term of this Agreement is found unenforceable under any of the laws or regulations applicable thereto, all other conditions and provisions of this Agreement shall nevertheless remain in full force and effect. Upon such determination that any term or other provision is invalid, illegal or incapable of being enforced, the parties hereto shall negotiate in good faith to modify this Agreement to effect the original intent of the parties as closely as possible in a mutually acceptable manner, in order that the transaction contemplated hereby be consummated as originally contemplated to the greatest extent possible.
ARTICLE 27 – AGREEMENT
| 27.1 | Entire Agreement |
This Agreement, including the Schedules hereto which are incorporated herein, constitute the entire agreement of the parties with respect to the subject matter hereof and supersedes all proposals, oral or written, and all negotiations, conversations, or discussions, including, without limitation, the Interim Facility Agreement which is terminated upon transition of the manufacture of Azedra under this Agreement pursuant to section 4.1. This Agreement may not be modified, amended, rescinded, canceled or waived, in whole or in part, except by written amendment signed by both parties hereto. In the event of a conflict between a term of the Agreement and any Schedule or Quality Agreement, the terms of this Agreement shall govern.
ARTICLE 28 – LAW
| 28.1 | Applicable Law |
This Agreement shall be governed and construed in accordance with the laws of the Province of Ontario, without reference to its principles on conflict of laws. The application of the United Nations Convention for the International Sale of Goods is expressly excluded.
IN WITNESS WHEREOF the parties hereto have executed this Agreement as of the date first above written.
| MDS Nordion, a division of MDS (Canada) Inc. | ||
| By: | /s/ Xxxxx Xxxxxx | |
| Molecular Insight Pharmaceuticals, Inc. | ||
| By: | /s/ Xxxxxx Xxxxxxxx | |
SCHEDULE A
Description of Facility Program, Facility Milestones and Facility Schedule
Facility Description:
MDS Nordion will modify an existing facility to establish a dedicated facility suitable for cGMP Manufacture of Molecular Insight Pharmaceuticals’ Azedra (I-131 MIBG) for clinical supply and Commercial Phase supply upon FDA approval. This will involve the deconstruction of some existing facility components, and as Nordion determines and in accordance with the Facility Program reuse of some of the existing components, modifications to the current room layout and construction of new walls and anterooms. The Azedra facility will be located in Room 1531 within Nordion’s Kanata Radiopharmaceutical Manufacturing Facility. The facility is purposely designed to comply with the current FDA and EMEA requirements for the aseptic manufacture of radiopharmaceuticals. The Class 10,000 hot cell will be used for product formulation and the aseptic Azedra cell will house the Class 100 dispensing system. The cells will be capable of safely holding up to 50 Ci of I131 and will be connected to an appropriate nuclear ventilation system including charcoal / HEPA filters.
Key Deliverables:
| 1. | Facility set-up and establishment of a validated 15 Ci process capable of yielding a minimum of 15 shippable vials per Batch of therapeutic, diagnostic or any combination thereof. |
| 2. | Commissioning and Validation of Facility and Process Equipment: |
| a. | A qualified Class 10,000 Production Suite Clean room housing the Formulation and Dispensing Cells |
| b. | A qualified Class 100,000 Service area to access to the Formulation and Dispensing Cells from the rear. |
| c. | A class 10,000 Formulation cell and a Class 100 Dispensing cell for related MIBG production |
| d. | A gowning anteroom (class 10,000). |
| e. | Bio-decontamination equipment |
| f. | Environmental Monitoring Equipment |
| g. | Filter Integrity Test Equipment |
| h. | Formulation Equipment |
| i. | Dispensing, Capping and Crimping equipment |
| j. | A dose calibrator. |
| k. | A visual inspection station. |
| l. | A pot loading and extraction system (manual) |
| 3. | Note that QC test methods are already validated for the current process and the move of the process to a new manufacturing facility has no impact on their validated state. |
| 4. | Validation Summary Reports: Facility, Aseptic Media Fills, Process Equipment, Process and Stability. |
Commercial Production Facility Layout:
FACILITY MILESTONES AND SCHEDULE
Phase 1 – Inception
| • | Creating a detailed project and quality plan |
| • | Validate project schedule and budget |
| • | Identifying and engaging the project team and key Operations Project representatives |
| • | Defining and implementing project management mechanisms |
| • | Identifying and aligning required 3rd party contractors |
| • | Creating the detailed design drawings for the facility and room and the detailed design specification for the hot cell. |
Phase 2 – Construction and Installation
| • | Hot Cell Factory Acceptance Testing |
| • | Deconstruct and remove all components from the existing facility that are not being reused |
| • | Obtain and install new facility components like hot cells, ventilation components, waste containers |
| • | Engage 3rd party contractors for civil construction, electrical, ventilation, plumbing etc. |
| • | Complete “As Built” Facilities drawing set |
Phase 3 – Process Configuration and Design
| 1. | Adapt existing 15 Ci scale process equipment, flow and layout to a 2 hot cell operation |
| 2. | Design updates to process equipment for a 2 cell process |
| 3. | Modify process documents and drawings as required |
| 4. | Complete set of Standard Operating Procedures |
Phase 4 – Facility and Process Equipment Commissioning and Qualification
| • | Final Master Validation Plan |
| • | Complete Site Acceptance Tests for all major components |
| • | Room and Ventilation Commissioning |
| • | Process Equipment Commissioning |
| • | Two (2) water runs to verify process flow |
| • | One (1) - 15Ci hot commissioning run to verify active process and flow |
| • | Establish routine maintenance / calibration / environmental monitoring programs |
| • | Final Commissioning Report |
| • | IQ / OQ / PQ for Facility and Process Equipment |
| • | Radiation safety verification |
| • | Facility & Process Equipment Validation Summary Report |
| • | Three (3) aseptic media fills to verify process sterility |
| • | Aseptic Media Fills Validation Summary Report |
Phase 5 – Process Validations
| • | Validate any procedures related to the manufacturing, and safety that were changed as a result of the transfer of the existing 15 Ci process to the new facility (the testing parameters and specifications will be mutually agreed upon by both parties) |
| • | Execution of three (3) Process Validation runs with full release as per the product specifications |
| • | Stability Validation Summary Report |
| • | Process Validation Summary Report |
Phase 6 – Transition and Project Close
| • | Complete Operations Training |
| • | Coordinated transition from project team to operations staff |
| • | Establish inventory of critical spare parts |
| • | Set-up of all Facility and Process Equipment into the maintenance and calibration program |
| • | All validation reports and project documents finalized |
| • | Final information for the Chemical, Manufacturing and Controls (CMC) section of the IND/NDA being prepared by Molecular Insight Pharmaceuticals (Molecular Insight Pharmaceuticals) will be available at this time |
| • | Final Safety Analysis Report |
| • | Initiate routine production in the new facility |
Timeline
Estimated 13 months based on commercially reasonable efforts, provided that such activities do not materially interfere or could be reasonably anticipated to interfere with established Nordion production activities Should a key element ,such as the delivery of the hot cell differ from the estimated lead time, the schedule will be affected.
SCHEDULE B
Azedra Specifications

|
UNCONTROLLED COPY | |
| 910700.SPE (4) | ||
| Effective Date: 09-05-12 CF 2881 |
Page 1 of 3 | |
| Ultratrace™ lobenguane I-131 Therapeutic Dose | ||
Signatures
| Prepared by: | signature on file |
Date: |
| |||
| X. Xxxxxx, Development | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| X. Xxxxxxxxx, Production | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| X. Xxxxxxxx, Quality Control | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| A. Warbick Xxxxxx, Regulatory Affairs | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| X. Xxxxxx, Global Sales & Marketing | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| X. Xxxxxxxx, Quality Assurance | yy/mm/dd | |||||
Document History
| Effective |
Version |
Comments |
Prepared By |
Reviewed By |
Approved By | |||||
| 06-12-21 |
1 | CF 61 | C. Bensimon | X. Xxxxxxxxx | ||||||
| F. Xxxxxxx | ||||||||||
| X. Xxxxxxxx | ||||||||||
| X. Xxxxxxxxx | ||||||||||
| X. Xxxxxxxx | ||||||||||
| 07-04-26 |
2 | CF 650 | X. Xxxxx | X. Xxxxxxxxx | ||||||
| X. Xxxxxxxx | ||||||||||
| X. Xxxxxxxx | ||||||||||
| X. Xxxxxxxxx | ||||||||||
| X. Xxxxxxxx | ||||||||||
| 08-05-16 |
3 | CF 1402 | X. Xxxxxxx | R. Pickeing | ||||||
| X. Xxxxxxxx | ||||||||||
| A. Warbick Xxxxxx | ||||||||||
| X. Xxxxxxxxx | ||||||||||
| X. Xxxxxxxx |
NOTE: A vertical line in the margin (tracking bar), denotes change. For complete rewrites, tracking bars are not used.
This document contains information proprietary to MDS Nordion.
Any disclosure or use of this information or any reproduction of this document other than the specified purpose for which it is intended is expressly prohibited except as MDS Nordion may otherwise agree in writing.
910700.SPE (4)
Page 2 of 3
Ultratrace™ lobenguane I-131 Therapeutic Dose
| PRODUCT | : Ultratrace™ lobenguane X-000 (X-000 XXXX) Therapeutic Dose contract manufacturing for Molecular Insight Pharmaceuticals Inc. | |
| MANUFACTURER | : Each I-131 MIBG Therapeutic Dose is manufactured by MDS Nordion according to 910700.SOP | |
| pH | : 4.5 - 5.5 per 910803.STM | |
| BACTERIAL ENDOTOXIN | : £ 3 EU/mL as per 910809.STM | |
| STERILITY | : Pass USP test for sterility as per 910808.STM | |
| RADIOCHEMICAL IDENTITY OF I131-IOBENGUANE |
: R1 ± 10% of reference MIBG standard as per 910801. STM | |
| UNBOUND IODINE-131 | : £ 5% as per 910801. STM | |
| I131-MIBG | : ³ 94% as per 910801 .STM | |
| OTHER RADIOCHEMICAL IMPURITIES |
: £ 1% as per 910801.STM | |
| TOTAL VOLUME | : 20 mL - 25 mL as per 910700E.PCR | |
| TOTAL ACTIVITY | : 270 - 405 mCi @ time of calibration as per 910802. STM | |
| ACTIVITY CONCENTRATION |
: 13.5 - 16.5 mCi/mL @ time of calibration as per 910805.STM | |
| MIBG CONCENTRATION | : 1 -10 µg/mL as per 910801.STM | |
| ASCORBIC CONCENTRATION (as sodium ascorbate) |
: 54 - 66 mg/mL as per 910804.STM | |

|
UNCONTROLLED COPY | |
| 910701.SPE (1) | ||
| Effective Date: 09-05-12 CF 2484 |
Page 1 of 3 | |
| Ultratrace™ lobenguane I-131 Dosimetry Dose | ||
Signatures
| Prepared by: | signature on file |
Date: |
| |||
| X. Xxxxxx, Development | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| X. Xxxxxxxxx, Production | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| X. Xxxxxxxx, Quality Control | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| A. Warbick Xxxxxx, Regulatory Affairs | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| A. Marius, Global Sales & Marketing | yy/mm/dd | |||||
| Approved by: | signature on file |
Date: |
| |||
| X. Xxxxxxxx, Quality Assurance | yy/mm/dd | |||||
Document History
| Effective |
Version |
Comments |
Prepared By |
Reviewed By |
Approved By |
NOTE: A vertical line in the margin (tracking bar), denotes change. For complete rewrites, tracking bars are not used.
This document contains information proprietary to MDS Nordion.
Any disclosure or use of this information or any reproduction of this document other than the specified purpose for which it is intended is expressly prohibited except as MDS Nordion may otherwise agree in writing.
910701.SPE (1)
Page 2 of 3
Ultratrace™ lobenguane I-131 Dosimetry Dose
| PRODUCT | : Ultratrace™ lobenguane X-000 (X-000 XXXX) Dosimetry Dose contract manufacturing for Molecular Insight Pharmaceuticals Inc. | |
| MANUFACTURER | : Each I-131 MIBG Dosimetry Dose is manufactured by MDS Nordion according to 910700.SOP | |
| pH | : 4.5 - 5.5 per 910803.STM | |
| BACTERIAL ENDOTOXIN | : £ 3 EU/mL as per 910809.STM | |
| STERILITY | : Pass USP test for sterility as per 910808.STM | |
| RADIOCHEMICAL IDENTITY OF I131-IOBENGUANE |
: R1 ± 10% of reference MIBG standard as per 910801.STM | |
| UNBOUND IODINE-131 | : £ 5% as per 910801. STM | |
| I131-MIBG | : ³ 94% as per 910801.STM | |
| OTHER RADIOCHEMICAL IMPURITIES |
: £ 1% as per 910801.STM | |
| TOTAL VOLUME | : 1.5 mL-2.5 mL as per 910700F.PCR | |
| TOTAL ACTIVITY | : 24 - 36 mCi @ time of calibration as per 910802.STM | |
| ACTIVITY CONCENTRATION |
: 13.5 - 16.5 mCi/mL @ time of calibration as per 910805.STM | |
| MIBG CONCENTRATION | : 1 - 10 µg/mL as per 910801. STM | |
| ASCORBIC CONCENTRATION (as sodium ascorbate) |
: 54 - 66 mg/mL as per 910804.STM | |
910701.SPE (1)
Page 3 of 3
Ultratrace™ lobenguane I-131 Dosimetry Dose
| GENTISATE CONCENTRATION (as sodium gentisate) |
: 20 - 25 mg/mL as per 910804.STM | |
| VISUAL INSPECTION | : Clear solution, free of visible particulates as per 910802.STM | |
| EXPIRY DATE (TOE) | : 8 days post production @ 12:00 ET | |
| CALIBRATION DATE (TOC) | : 2 days post production @ 12:00 ET | |
| STORAGE | : Frozen at £ -70°C | |
| SHIPPING CONDITIONS | : On Dry Ice | |
| CATEGORY | : Reserve | |
| PACKAGING | : Lead Pot F-517 (910207.SPE) Shipping Box (900201.SPE) Box Insert (Lid 900202.SPE/9T900202), (Bottom 900203.SPE/9T900203) Dry Ice Pellets (900208.SPE/10010201) Waxed Cardboard Tubes (900204.SPE/9T900204) | |
SCHEDULE C – Facility Fees
The total estimated cost of the facility portion of the project is ******. Materials and 3rd party services will be billed at actual Nordion ***** to cover administrative expenses. Costs incurred in currencies other than Canadian dollars will be converted to Canadian dollars based on the Bank of Canada closing rate on the day prior to effecting the purchase commitment. Nordion will provide copies of actual invoices. Nordion will allow Molecular Insight Pharmaceuticals interaction on choices of materials and equipment to be purchased, however the final decisions shall be made by Nordion. Labour has been estimated and is billed in Canadian dollars on a fixed cost basis.
Any deviation in:
| (i) | the Canadian dollar exchange rate against the US dollar (or other relevant currency) in excess of ******, or |
| (ii) | final invoice price for purchased materials and services, |
between the date of the notification by Nordion to Molecular Insight Pharmaceuticals of a purchase commitment being required and the date of payment by Nordion to the vendor with respect to such purchase, shall be subject to full adjustment between the parties.
Payments will be made in advance of Nordion making a purchase commitment for material, equipment, and third party services. Payment for Nordion labor shall be as set out below. The detailed spend profile will be determined as part of the detailed project planning phase and will then determine the final payment schedule. Based on the preliminary project plan, the payment schedule is as follows:
| Due on: |
Firm cost for Nordion labour ($CDN) |
Estimated cost for materials & 3rd party contracts ($CDN) |
Project Total ($CDN) | |||||
| Phase 1 |
Start | ****** | ||||||
| During Complete |
****** | ****** | ||||||
| Phase 2&3 |
Start | ****** | ||||||
| During Complete |
****** | ****** | ||||||
| Phase 4 |
Start | ****** | ||||||
| During Complete |
****** | ****** | ||||||
| Phase 5 |
Start | ****** | ||||||
| During Complete |
****** | ****** | ||||||
| Phase 6 |
Start | ****** | ||||||
| During Complete |
****** | |||||||
| Total |
****** | ****** | ****** |
The above material and third party contract payments may be broken down and staged for large capital purchases (i.e. payments related to large capital expenditures can be received just before Nordion makes the purchase commitment).
* Confidential Treatment Requested *
Advance Payment
Nordion acknowledges receipt of an advance payment of ****** from Molecular Insight Pharmaceuticals pursuant to the Letter of Agreement dated February 5, 2009, such advance payment to be applied by Nordion as a credit against Nordion labor costs incurred under this Agreement, as such labor charges become due and payable, provided further that notwithstanding anything to the contrary in this Agreement, such advance payment is nonrefundable.
Non-refundable Payments
Once a Phase is commenced:
| (i) | all amounts payable and to be paid by Molecular Insight Pharmaceuticals for labour with respect to such Phase are payable and non-reimbursable. |
| (ii) | all materials consumed and third party services rendered (and for purchase and service commitments to the extent such commitments cannot be terminated), are payable and non-reimbursable. |
* Confidential Treatment Requested *
Scope Change
Scope changes shall be charged to Molecular insight Pharmaceuticals as follows:
| (i) | ****** for any materials, equipment, third party services obtained via a Nordion purchase order, or Nordion inventory obtained via inventory requisition |
| (ii) | Nordion labour rate is ****** per hour |
| (iii) | Nordion staff travel and accommodation shall be reimbursed to Nordion at cost. Time incurred during travel to destinations shall be paid to Nordion for each person half day or less of travel at the rate of ****** ; and a full day travel at the rate of ****** |
The price of the Facility includes all equipment, material and labour Nordion deems appropriate and within scope for the proper execution of the project. Should Molecular Insight Pharmaceuticals request additional equipment such as back-up instruments, or additional work such as method development, then additional charges shall apply as scope change under this Agreement.
Third party services and materials were estimated by Nordion based on the available information at the time the estimate was being prepared. Actual costs or exchange rates may vary as against original cost projections as firm quotations are obtained from vendors, favorably or unfavorably affecting the cost thereof.
* Confidential Treatment Requested *
SCHEDULE D
Prices of Batches for Clinical Trial and Commercial Supply
| A. | Azedra Batch Pricing 2009 |
The Batch price shall be calculated as follows:
(i) The Batch Fee (excluding Isotope and Waste Disposal Fee): ******
A Batch shall yield a minimum of 15 shippable vials. Nordion shall use commercially reasonable efforts to increase the yield to 22 shippable vials / batch, in any combination of dosimetric or therapeutic doses. In the event a Batch should yield less than 15 shippable vials, the minimum Batch price will be adjusted on a pro rata basis.
Any vials produced above 22 shall be paid for as follows:
| • | For vials 23-40 in the same batch, ******/ treatment (max 2 Tx vials, 1 Dx vial) (above minimum batch fee). |
| • | For all vials > 40 in the same batch, ******/ treatment (2 Tx vials, 1 Dx vial) (above minimum batch fee). |
| • | There is no charge for additional Dx vials |
(ii) Isotope Fee per Batch: ******/ Ci x 15 Ci of activity (as at Batch Production Date): *****
(iii) Waste Disposal Fee per Batch: ******.
Total Batch price: ******
| B. | Price Increases 2010 and following: |
1 The Batch Fee (excluding Isotope and Waste Disposal Fee) will escalate in accordance with Section 4.6.
2. The Isotope Fee will increase each calendar year during the Term of the Agreement by an amount equal to the ***** by Nordion to customers in North America as at November 1 as compared to the average selling price as at October 31 of the previous year.
* Confidential Treatment Requested *
3. The Waste Disposal Fee will increase each calendar year by the greater of CPI as described in Section 4.6, or the increase in Nordion’s actual costs as certified by a duly authorized officer of Nordion.
Price increases under this Item B shall be provided in writing to Molecular Insight Pharmaceuticals on or about December 1 of each calendar year during the Term of this Agreement. Nordion shall provide an Officer’s Certificate with respect to the price increases in B2 and B3.
SCHEDULE E
Quality Agreement
To be reviewed by QA and MIP
INTERCOMPANY QUALITY AGREEMENT
MOLECULAR INSIGHT PHARMACEUTICALS, INC.
having a place of business at
000 Xxxxxx Xxxxxx
Xxxxxxxxx Xxxxxxxxxxxxx, 00000 XXX
(hereinafter called “Molecular Insight Pharmaceuticals”)
AND
MDS Nordion
Ottawa, Ontario, Canada K2K 1X8
(hereafter called “Nordion”)

|
MIPI / MDS Nordion Azedra Quality Agreement |
1. QUALITY AGREEMENT
This Quality Agreement (“Quality Agreement”) is entered into as of , 20 (“Effective Date”) by and between MDS Nordion (“NORDION”) and Molecular Insight Pharmaceuticals, Inc., (“MIPI”).
This Quality Agreement is entered into pursuant to and is expressly subject to the terms of that certain Azedra Manufacturing and Clinical Supply Agreement entered into as of August 6th, 2009 by and between NORDION and MIPI (the “Commercial Facility and Supply Agreement”).
| Note: | In the event of a conflict between the terms and conditions of this Quality Agreement and the terms and conditions of the Commercial Facility and Supply Agreement, the terms and conditions of the Commercial Facility and Supply Agreement shall govern and control. |
This Quality Agreement defines the duties of NORDION and MIPI with respect to the contracted clinical supply pharmaceutical formulation, manufacture, labeling, packaging and testing of AZEDRA drug product for the markets identified in the Commercial Facility and Supply Agreement. .
In particular this Quality Agreement clearly states which party is responsible for the cGMP aspects of Product formulation, manufacturing, packaging and testing and specifies the manner in which the party releasing Product ensures that the Product complies with the approved Product Specifications and the Marketing Authorizations.
This Quality Agreement takes the form of a detailed checklist of all the activities associated with pharmaceutical formulation, manufacture, production, packaging and testing, analysis, release, and distribution. Responsibility for each activity is assigned to either NORDION or MIPI in the appropriate box in the Delegation Responsibility Checklist, which follows.
In order to provide better quality assurance, NORDION will perform the activities defined herein in accordance with Standard Operating Procedures (defined below), to the extent that a Standard Operating Procedure is applicable to such activity. In the event of a conflict between the terms and conditions of this Quality Agreement and the terms and conditions of a Standard Operating Procedure, the terms and conditions of such Standard Operating Procedure shall govern and control, <upon mutual agreement between NORDION and MIPI>.
This Quality Agreement is intended to comply with the guidance and directives set forth in
| (i) | FDA: 21 CFR Parts 210 & 211 |
| (ii) | Health Canada guidance documents |
| Molecular Insight PHARMACEUTICALS, INC. (MIPI) | MDS Nordion | |||||||
| 000 Xxxxxx Xxx | 000 Xxxxx Xxxx | |||||||
| Xxxxxxxxx, XX 00000 | Xxxxxx, Xxxxxxx | |||||||
| X0X 0X0 | ||||||||
| By: |
|
By: |
| |||||
| Name: |
|
Name: |
| |||||
| Title: |
|
Title: |
| |||||
Page 1 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| 1. |
QUALITY AGREEMENT |
1 | ||
| 2. |
KEY TERMS |
3 | ||
| 3. |
MANAGEMENT CONTROLS |
5 | ||
| 4. |
PRODUCTION |
6 | ||
| 5. |
FACILITIES & EQUIPMENT |
8 | ||
| 6. |
MATERIALS MANAGEMENT |
9 | ||
| 7. |
PACKAGING AND LABELING |
10 | ||
| 8. |
LOT RELEASE |
11 | ||
| 9. |
VALIDATION |
13 | ||
| 10. |
ENVIRONMENTAL AND CRITICAL UTILITIES CONTROL |
14 | ||
| 11. |
TESTING |
15 | ||
| 12. |
STABILITY |
16 | ||
| 13. |
DEVIATION MANAGEMENT |
16 | ||
| 14. |
OUT OF SPECIFICATION ( OOS) |
17 | ||
| 15. |
CHANGE CONTROL |
17 | ||
| 16. |
DOCUMENT CONTROL |
19 | ||
| 17. |
TRAINING |
21 | ||
| 18. |
REGULATORY |
21 | ||
| 19. |
RECALLS |
24 | ||
| 20. |
COMPLAINTS |
25 | ||
| 21. |
ADVERSE EVENTS |
25 | ||
| 22. |
AUDITS |
26 | ||
| 23. |
MANAGEMENT OF RELATIONSHIPS AND COMMUNICATION STRATEGY |
26 | ||
Page 2 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
2. Key Terms
For purposes of this Quality Agreement, the following definitions shall apply (any capitalized terms not defined below or elsewhere herein shall have the meaning as such term has under the Commercial Facility and Supply Agreement):
| A. | “ACR” shall mean NORDION analytical control record. |
| B. | “Applicable Laws” means all laws, ordinances, rules and regulations applicable to the Processing of Product and to the obligations of NORDION or MIPI in connection therewith, as the context requires, including, without limitation, (i) all applicable federal, state and local laws and regulations of the Territory; (ii) the U.S. Federal Food, Drug and Cosmetic Act of 1941, as amended; and (iii) cGMP. |
| C. | “Deviations” or “deviation” shall have the meaning as set forth in the Deviation Management Section of this Quality Agreement. |
| D. | “Facilities” means the following NORDION facilities located in Ottawa, Canada. |
| E. | “FDA” shall mean the United States Food and Drug Administration, and any successor entity thereto. |
| F. | “cGMP” shall mean the current Good Manufacturing Practice regulations for finished pharmaceuticals, as promulgated by FDA and EU Regulatory Authorities, both as amended from time to time. cGMP shall also include Good Manufacturing Practice regulations promulgated by a Regulatory Authority in a Territory and internationally accepted standards for current Good Manufacturing Practices. |
| G. | Critical System shall be those equipment, software, facilities, utilities which have a direct impact on product SQUIPP |
| H. | “Marketing Application” shall mean an application for marketing authorization which has not yet been approved by the FDA or other Regulatory Authority, including without limitation, FDA Investigational New Drug Application, FDA New Drug Application, FDA Abbreviated New Drug Application, and other similar marketing applications promulgated by Regulatory Authorities. |
| I. | “Marketing Authorizations” shall mean any approved application for marketing authorization, including without limitation, FDA Investigational New Drug Application, FDA New Drug Application, FDA Abbreviated New Drug Application, and other similar marketing authorizations promulgated by a Regulatory Authority. |
| J. | “Out of Specifications” or “OOS” shall mean nonconformance with the Specifications, cGMP or Applicable Laws. |
| K. | “Process” or “Processing” means the sterile production, filling, producing and/or packaging of the Raw Materials into Product, in accordance with the Specifications and the terms and conditions set forth in the Commercial Facility and Supply Agreement and this Quality Agreement. |
| L. | “PCR” shall mean NORDION process control records. |
| M. | “Product” shall mean the Azedra, which is further defined in the Commercial Facility and Supply Agreement. |
| N. | “PRF” shall mean NORDION product release form. |
| O. | “Raw Materials” shall mean the materials listed in any Marketing Authorization. |
| P. | “Regulatory Authority” shall mean the FDA and any other regulatory authority, within a Territory, involved in regulating any aspect of the development, manufacture, market approval, sale, distribution, packaging or use of the Product. |
| Q. | “Specifications” means the specifications for the Product and the procedures, requirements, standards, quality control testing, other data and scope of services, each as set forth in the Commercial Facility and Supply Agreement. |
| R. | “Standard Operating Procedures” or “SOPs” shall mean the standard operating procedures in effect at NORDION, which have been approved by NORDION’s Quality Assurance department and which are applicable to the Processing. |
Page 3 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| S. | “SQUIPP” shall mean the safety, quality, identity, potency and purity of Product, as required by the Code of Federal Regulations (CFR) and other Applicable Laws. |
| T. | “Territory” shall mean the United States of America and any country in which Product where product is supplied as identified by the Commercial Facility and Supply Agreement. |
Page 4 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
DELEGATION RESPONSIBILITY CHECKLIST
3. Management Controls
| MANAGEMENT CONTROLS RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Quality System Program
• Assure the quality system is established and functioning properly and in accordance with cGMP requirements.
• Monitor the quality system and make necessary adjustments to maintain substantial compliance with cGMP requirements.
• Perform all obligations in accordance with:
• any applicable Product registrations;
• this Quality Agreement;
• Applicable cGMP guidelines;
• Any conditions registered in the manufacturing authorizations (IND), and
• The validated process as described in applicable approved SOPs, and the manufacturing Batch Records. |
X | ||||
| 2. |
Debarment
Warrant that it is not debarred under the U.S. Generic Drug Enforcement Act of 1992 and to its knowledge, after reasonable inquiry, that it does not employ or use the services of any individual (i) who is debarred or (ii) who has engaged in activities that could lead to being debarred. |
X | ||||
| 3. |
Quality Assurance
Maintain adequate quality assurance presence and oversight in the manufacturing facility during the manufacture of the Products to ensure compliance with cGMP requirements:
• NORDION Quality Assurance (QA) will have the responsibility and authority to approve and reject all components, containers, materials with the manufacture of Product.
• NORDION QA will be responsible for the review of batch documentation, investigating deviations, nonconformances, out of specification results associated with the manufacture of the Product.
• NORDION QA will be responsible for reviewing and approving or rejecting all procedures or specifications that may have adverse impact on the identity, strength, quality or purity of the Product. |
X | ||||
| MIPI, as outlined in the following sections of this agreement, has the ultimate authority for the dispositioning of Product. | X | |||||
| NORDION will notify MIPI regarding any instance that may adversely effect the Product and work with MIPI Quality Assurance to take a mutually agreed upon appropriate action within the mutually agreed upon timeframes outlined in the following sections of this agreement. | X | |||||
| 4. |
Audits
Perform periodic auditing of all the Quality System to assure compliance with Standard Operating Procedures, cGMP requirements, and MIPI expectations as defined in this quality agreement). |
X | X | |||
Page 5 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| MANAGEMENT CONTROLS RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 5. |
Access
• Provide access to procedures, methods, documentation, data as well as the records for those systems and facilities outlined in this Quality Agreement, supporting the manufacture, testing, storage or control of MIPI Product.
• Given the process is carried out in a highly controlled environment, access to the process will be limited to areas that are not environmentally controlled. MIPI personnel may periodically observe manufacture of the Product, from locations that are not environmentally controlled or by video tape. |
X | ||||
| 6. |
Revisions to this Quality Agreement
Any changes to be made to the this Quality Agreement which encompass the activities and elements contained herein, may only be made upon the mutual written agreement of both MIPI & NORDION. |
X | X | |||
4. Production
| PRODUCTION RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Production Program
• Ensure that Product is manufactured in accordance with manufacturing to approved instructions that are executed within manufacturing limits, and in accordance with the Quality system requirements in this Quality Agreement as well as regulatory requirements.
• Perform manufacturing tasks so as to not adversely impact the SQUIPP of the Product. |
X | ||||
| 2. |
Execution of Production
Training:
• Ensure personnel are trained per NORDION related procedures and methods. Training documentation will be provided to MIPI upon request during audits or on site visits |
X | ||||
|
Production:
• Perform manufacturing in accordance with preapproved batch documentation and instructions
• Document completion of instructions in accordance with cGMP documentation practices
• Perform in-process sampling, testing, and sample submission per approved instructions
• Perform and respond appropriately to in-process monitoring
• Maintain usage logs in accordance with established procedures and cGMP requirements.
• Materials used in production are approved for manufacturing. |
||||||
| Facilities & Equipment
• Equipment, systems, instruments, and facilities are maintained, calibrated, qualified, and/or validated per intended use. Use only equipment, facilities, instruments, methods, and materials that are approved for use. |
||||||
| Deviations
• Report any deviations (e.g., Deviations, Non-Conforming Material) in accordance with the deviation program |
||||||
| Performance Monitoring
• Perform routine inspection & approval of equipment & processing areas & related documentation. |
||||||
Page 6 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| PRODUCTION RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 3. |
Prevention of Contamination and Cross Contamination
Establish contamination mitigation program that ensures contamination is mitigated within and between facilities, systems, personnel during production processes applicable to Product manufacture as well as previous / subsequent customer production runs (i.e., contamination is mitigated during Product manufacturing runs and changeover between customer production runs and Product manufacture)
• Maintain equipment and facility cleaning per established procedures.
• Establish and comply with specified materials and personnel flow.
• Maintain, as appropriate, segregation and dedication of equipment, facilities and materials.
• Perform tasks under specified environmental conditions. |
X | ||||
| 4. |
Lot Numbers
Assign unique lot numbers as defined by NORDION procedure. |
X | ||||
| 5. |
Date of Manufacture
Assign the date of Product manufacture and expiry when issuing the batch production document, |
X | ||||
| 6. |
PRODUCT Expiration Date
• Changes to the expiration date will be handled in accordance with the requirements of this Quality Agreement, entitled Change Control. |
X | ||||
| 7. |
Certificate of Analysis (i.e., NORDION product release form)
NORDION will provide a certificate of analysis with the following information at a minimum:
• Name of the Product;
• Lot or Batch Identification Number;
• Date(s) of manufacture;
• Date of expiration;
• Name of each test method performed;
• Respective test specification;
• Respective test results expressed in numerical value, unless designated as pass/fail or not detectable in the specification limit;
• Identification and the dated signature of the authorized NORDION qualified person; and
• Batch size. |
X | ||||
| 8. |
Certificate of Compliance
The NORDION product release form will serve as a signed certificate of compliance confirming that the Products have been manufactured, tested, and stored in accordance with defined requirements, the Master Production Record, and cGMP criteria. |
X | ||||
Page 7 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
5. Facilities & Equipment
| FACILITIES AND EQUIPMENT RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Facilities and Equipment Program
MIPI and NORDION agree that Product facilities, equipment and supporting utilities are of suitable size, design, construction, location, and state to facilitate cleaning and proper operation through review of design of the commercial facility
• NORDION will ensure the same are maintained in a good state of repair to comply with applicable requirements associated with sanitation, housekeeping, pest control, security, and safety.
• Facilities and equipment used for MIPI Product manufacture are identified and are qualified and validated per intended use. |
X | X | |||
| 2. |
Facility Access
Controlled access to the premises is maintained.
• All visitors shall comply with NORDION’s security and safety requirements.
• NORDION will maintain confidentiality of Product manufacture related activities for visitors to the site. |
X | ||||
| 3. |
Maintenance, Calibration
Maintenance and calibration of the equipment used to manufacture and test and store the components and Products will be performed.
• Periodically evaluate trending data from calibration activities to assuring proper calibration activities and durations
• Ensure that all critical systems within the scope of the facilities and equipment program are calibrated prior to validation and Product manufacture / cGMP use.
• Non-conforming instruments shall be identified by NORDION and addressed via the applicable NORDION quality system |
X | ||||
| 4. |
Other operations at site
• MIPI will be provided with prior written notice of any addition or expansion of the range of products manufactured in the same facility as the Product, that could affect the SQUIPP of the Product as a Change Control. |
X | ||||
Page 8 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
6. Materials Management
| MATERIALS MANAGEMENT RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Materials Management Program
Manufacturing materials will be obtained through a documented program to accurately receive, test, track, inventory and store all manufacturing materials. |
X | ||||
| 2. |
Scope
At a minimum, include materials management of the materials used in the manufacture, testing and storage of MIPI Product and related components. Program elements shall include:
• Supplier qualification;
• Receiving;
• Tracking of usage and distribution;
• Quarantine and;
• Warehousing and storage |
X | ||||
| 3. |
Material Usage
Maintain product specifications. |
X | X | |||
| Retain samples of active ingredient(s) and raw materials, including samples for periodic re-tests, for a suitable period of time beyond Product expiry date. | X | |||||
| Use only chemical materials, packaging and labeling components approved and tested and/or confirmed in accordance with validated or compendial methods. | X | |||||
| Dispose of Product waste and any special waste related to the Processing of the Product in accordance with Applicable Laws. | X | |||||
| Non-conforming materials are identified by NORDION, addressed via the applicable NORDION quality system | X | |||||
| Non-conforming materials affecting MIPI related Product SQUIPP and/or Product disposition shall be communicated by NORDION to MIPI within 24 hours of NORDION’s observance. | X | |||||
| 4. |
Tracking, Inventory and Status
Materials shall be tracked using a documented system throughout their receipt and usage lifecycle, encompassing, but not limited to:
• Identity;
• Status (accepted, rejected, quarantine, pending);
• Quantity;
• Location;
• Lot genealogy and traceability; and
• Expiry. |
X | ||||
| 5. |
Supplier Program
Maintain a quality review program for materials vendors/suppliers according to defined policies and procedures, which shall be available for review by MIPI upon request.
• Maintain procedures that are a part of the Materials Management Quality System.
• Define audit requirements for qualifying key suppliers.
• Develop specific acceptance criteria for supplier materials or services.
• Execute audits of key suppliers.
• Monitor corrective actions for suppliers that are conditionally accepted.
• Maintain a list of qualified suppliers. |
X | X | |||
Page 9 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| MATERIALS MANAGEMENT RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 6. |
Suppliers Chosen by NORDION
Ensure suppliers chosen by NORDION meet requirements.
• Provide listing of approved NORDION suppliers whose component/materials are used in Product.
• Provide demonstration of approval of NORDION approved suppliers during MIPI audit of NORDION. |
X | ||||
| 7. |
Suppliers Chosen by MIPI
For suppliers designated by MIPI and which are not NORDION approved vendors, MIPI shall be responsible for meeting requirements of section 5 above. MIPI will provide adequate documentation to NORDION to demonstrate the material has been appropriately controlled, tested and released in accordance to approved specifications and procedures.
• Provide details of any labeling requirements, container sealing and integrity, and storage and shipping conditions for the material. |
X | ||||
| 8. |
Re-Testing Original Results / Specifications
• Re-testing will be performed within defined time intervals for a limited number of customer supplied materials to ensure the chemical, microbiological, and physical stability of the Raw Materials, unless MIPI provides an official expiration date (expired materials may not be re-tested).
• Testing may be conducted by a MIPI approved third-party contract testing laboratory |
X
X |
X | |||
| 9. |
Conformance Verification
• Prior to NORDION’s disposition of Product for release by MIPI, all materials used in the Product shall meet NORDION’s requirements for production use. |
X | ||||
| 10. |
Storage and Warehouse of Products
Materials, components and Products are stored under appropriate environmental conditions. |
X | ||||
| 11. |
Shipment of PRODUCT
The Products will be labeled and packaged for transit pursuant to regulatory filing and requirements.
• Only approved, finished, labeled Products will be shipped by NORDION to MIPI or a MIPI designated site, unless requested in writing by MIPI.
• NORDION will only ship Product according to procedure, which comply with all applicable regulatory requirements |
X | ||||
7. Packaging and Labeling
| PACKAGING AND LABELING RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Packaging and Labeling Program
Product will be packaged and labeled according to procedure that meets all applicable requirements. |
X |
Page 10 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| PACKAGING AND LABELING RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 2. |
Scope
System shall control the acceptance, issuance, use, and reconciliation of labels & labeling to assure that correct & accurate labeling of PRODUCT occurs.
• Ensure that only approved suitable containers and packaging materials are used for PRODUCT in accordance with written specifications. |
X | ||||
| 3. |
Label Content
Provide PRODUCT specific information for development of PRODUCT label copy. |
X | X | |||
| Review and approve master label copy and artwork | X | X | ||||
| MIPI will perform necessary regulatory applications for introduction or changes in product labeling. | X | |||||
8. Lot Release
| LOT RELEASE RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Lot Release Program
Product will be released to an established program, that ensures conformance to cGMP requirements. |
X | X | |||
| 2. |
Batch Record Review
Perform review of batch records to assure Product was made in accordance with approved procedures.
• Review batch records & any data, documents or test results that support the batch records for Product acceptance, including but not limited to:
• Laboratory testing records;
• Records of deviations occurring during manufacturing including environmental monitoring deviations; and
• Documentation of quality control testing & inspections conducted during manufacturing.
• Document deviations and out of specifications (“OOS’s”) in accordance with the Deviation Management and OOS Section of this Quality Agreement:
• Review deviations prior to release of Product for commercial distribution, deviations must not have an impact on the SQUIPP of the Product; and
• Ensure batch record issues related to deviations, non-conformances and OOS’s, which will potentially result in changes, are sufficiently linked to the Change Control system as outlined in the Change Control Section of this Quality Agreement.
• Ensure all OOS findings, occurring during manufacture of a given lot are satisfactorily resolved before the material is released.
• Notify MIPI upon observance of deviations or OOS which may affect Product, as outlined in accordance with the Deviations Section |
X | ||||
Page 11 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| LOT RELEASE RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 3. |
Product Lot Disposition
Note: Reference Appendix III for MIPI / NORDION Product Lot Release process map
Promptly notify MIPI on a timely basis (within 1 day) if any problems are discovered that could impact Product lots previously shipped to MIPI and /or MIPI designated locations, to assure that regulatory reporting guidelines can be met.
• Promptly notify MIPI of the disposition for every batch of rejected Product.
• Provide MIPI’s quality unit with (1) copies of all required batch record documentation
• Batch record documentation to be provided shall be reviewed and approved by NORDION’s Quality unit and shall include batch record documents, test data (analytical, chemistry and environmental), test reports and test records.
• For each lot of Product, NORDION shall provide the required documentation as shown in Appendix IV
• Prior to Product being shipped to Europe, an appropriate BSE / TSE Statement will be provided (pending MIP approval of a scope change to complete the study) |
X | ||||
| LOT RELEASE RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 4. |
Lot Review & Release
MIPI will review and approve Product Master Batch Record prior to release of the product for distribution |
X | ||||
| MIPI will review and approve the executed Product batch record and subsequent lot disposition.
• MIPI will approve the batch record OR notify NORDION of issues which require clarification, additional information and action by NORDION as soon as possible. MIPI will provide an “on-call” individual during lot disposition activities to ensure rapid communication through the process. |
X | |||||
| • Product lot release shall be based on MIPI’s and NORDION internal procedures (i.e., Product that does not meet defined specifications can be rejected by either party) |
X | X | ||||
| In the event that there is required follow-up by NORDION, NORDION shall perform such actions in a timely manner:
• Complete review and disposition of MIPI issues, where applicable, for Product release as soon as possible
• Provide response and/or additional information to MIPI for lot release |
X | |||||
| Upon receipt of additional NORDION provided information, MIPI will review the information and notify NORDION of issues which require clarification, additional information and action by NORDION and determine batch disposition (e.g., approve, quarantine, rejection). | X | |||||
| • MIPI shall delay or refuse approval for Product release in the event of unapproved changes and or unacceptable deviations or any other issues that MIPI determines would adversely affect the product quality, purity, identity, integrity or potency.
• NORDION would also reject product that does not meet specification, or from manufacturing or testing deviations that could adversely affect the product quality, purity, identity, integrity or potency . |
X | X
| ||||
Page 12 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| 5. |
Product & Batch Record Documentation Problems
Any problem discovered by MIPI likely to cause rejection of the approved Product will be communicated to NORDION as soon as reasonably possible. |
X |
9. Validation
| VALIDATION RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Validation Program
Establish, execute and maintain and periodically assess and audit a documented validation program that establishes documented evidence to provide a high degree of assurance that a specific process consistently produces a result meeting its predetermined specifications.
• The validation program must ensure that verification activities are performed in a controlled environment, with trained individuals using approved procedures and protocols, & with approved materials & components. |
X | ||||
| 2. |
Scope
The NORDION validation program will encompass those facilities, equipment, systems, utilities, processes, analytical testing and monitoring activities associated with the manufacturing, storage, testing and control of critical processes/data for MIPI Product.
• Qualifications and validations are to be completed prior to the manufacture of cGMP Product. |
X | ||||
| 3. |
Specifications and Acceptance Criteria | |||||
| NORDION will review and approve executed protocols/protocol amendments and associated reports. | X | |||||
| NORDION will provide MIPI specific documented specifications, requirements, and acceptance criteria where applicable | X | |||||
| NORDION shall provide MIPI with summaries of all validations and qualifications related to specific Product manufacture. Full validation reports are available for review at NORDION during MIPI audit. | X | |||||
| MIPI will review and approve the Master Validation Plan associated with Product manufacture. | X | |||||
| NORDION shall make available the executed qualification protocol reports associated with Product manufacture for MIP review on site. | X | |||||
| 4. |
Equipment, Computer, Facility, and Utilities Qualification:
Generate and execute protocols for qualification to ensure with a high degree of certainty that the protocol subject is installed and operates and performs as specified.
• Computer Qualification: establish and maintain procedures to ensure the integrity of electronic data. |
X | ||||
| 5. |
Process Validation
Process Validation for MIPI Process will be carried out.
• Protocols will be designed and carried out to ensure, with a high degree of certainty, the process can perform effectively and reproducibly to produce Product meeting predetermined specifications and quality attributes. |
X | ||||
Page 13 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| VALIDATION RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 6. |
Analytical Method Validation
Analytical methods are designed with detection limits to ensure they are able to test the Product to its specification. All methods used in the testing and release of the Product will be validated in accordance with pre-approved protocols.
Carry out any methods transferred to NORDION from MIPI to the pre-approved protocol. NORDION will ensure an adequate method transfer protocol is carried out prior to adoption of the method at NORDION
MIPI will ensure all methods transferred to NORDION are validated and will provide the validation package to NORDION for purposes of regulatory inspection |
X
X |
X
X | |||
| 7. |
Laboratory Qualification
Ensure that laboratories at NORDION that perform testing on the Product are in compliance with cGMP guidelines. |
X | ||||
| 8. |
Validation Maintenance
• Periodically review and assess the validated state of the subjects in the scope of the system.
• Address any deficiencies in the validated state via the supporting quality systems.
• Generate and execute revalidation, requalification and verification protocols and respective reports.
• Provide MIPI updates to validation plan. |
X | ||||
10. Environmental and Critical Utilities Control
| ENVIRONMENTAL and CRITICAL UTILITIES RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Environmental and Critical Utilities Program
Establish, execute, maintain and periodically audit a documented program for the control and monitoring of the environment and utilities to ensure that these meet:
• Applicable cGMP guidelines;
• Any conditions registered in the manufacturing authorizations; and
• The validated process as described in applicable approved SOPs, and the manufacturing batch records. |
X | ||||
| 2. |
Scope
• Critical utilities and associated equipment, systems and instruments are maintained, calibrated, qualified and validated for their intended use. |
X | ||||
| 3. |
Notification
Promptly notify MIPI within twenty-four (24) hours of any environmental and critical utilities data applicable to MIPI Product that may indicate adverse impact. |
X | ||||
| 4. |
Changes and Corrective Actions
• Ensure the impact of proposed changes on the environmental controls & monitoring are reviewed, mitigated & approved and & that changes which have an adverse impact, that cannot be mitigated, are denied. |
X | ||||
Page 14 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
11. Testing
| TESTING RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Testing Program
• Ensure the testing program conforms to cGMP requirements.
• Establish testing program that includes sampling, sample retention, testing identification, test evaluation, test performance & reporting and testing method validation, OOS/atypical evaluation. |
X | ||||
| 2. |
Scope
Test methods associated with MIPI containers and closures, components, drug substance, in-process materials and packaging and labeling materials shall be available for review by MIPI during an audit. |
X | ||||
| Review & approve all Standard Operating Procedures, test methods & proficiency testing procedures | X | |||||
| 3. |
Analytical methods provided by MIPI | X | ||||
| Provide to NORDION approved copies of the current and complete regulatory filed methods relating to the Product, for application by NORDION in the production process. | X | |||||
| 4. |
Transfer of Methods | |||||
| MIPI will collaborate with NORDION to transfer any methods required by NORDION. | X | |||||
| 5. |
Reference Standards
Supply qualified reference standards accompanied by a Certificate of Analysis listing the expiration date, storage conditions and any correction factors that need to be applied. |
X | ||||
| Maintain reference standards in accordance with specifications. | X | |||||
| 6. |
Release Testing
In the event that MIPI performs, or contracts a MIPI approved supplier to perform any release testing, MIPI will supply NORDION with a certificate of analysis containing the testing data and the specifications. |
X | ||||
| NORDION shall evaluate all testing data supplied by MIPI in order to assign final disposition of Product. | X | |||||
| For any testing which MIPI completes, the results shall be made available to NORDION in a timely manner, not to exceed (30) days, so as not to delay release procedures. | X | |||||
| 7. |
PRODUCT Process Samples
• NORDION or a site designated by MIPI will retain PRODUCT process samples for at least 3 months beyond the expiration of the PRODUCT.
• The amount of sample retained will be at least twice the quantity required to carry out the release tests, with the exception of sterility and endotoxin testing. |
X | ||||
| 8. |
Raw Materials Samples
NORDION will retain samples of Raw Materials for a minimum of one (1) year beyond the date of manufacture of the Product. |
X | ||||
Page 15 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
12. Stability
| STABILITY RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Stability System
The Product shall undergo stability testing to an established stability program.
• The stability program will include the establishment of written program and stability indicating methodology, the test program and associated documentation.
• MIPI will identify the critical stability indicating parameters for the product & for inclusion into the stability program.
• Establish trending data to identify changes or concerns that can be associated with the Product.
• Review and evaluate any trending data developed from the stability testing program. |
X
X
X |
X
X
X |
13. Deviation Management
| DEVIATION MANAGEMENT RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Deviation Management Program
• Apply the NORDION deviation system to the manufacture and testing of the Product. |
X | ||||
| 2. |
Scope
Definition of Deviations
• An excursion from approved operating, manufacturing, testing instructions, or procedure.,
• A deviation does not permanently change an existing procedure; it is typically a specific or one time excursion. If the corrective action plan includes a permanent change, initiate a change proposal through the change management system.
• Deviations that may affect MIPI’s Product or regulatory status are within the scope. |
X | ||||
| 3. |
Notification of Deviations
• Notify MIPI immediately, within 24 hours, where those deviations may have an effect on MIPI drug Product and Drug Substance. |
X | ||||
| 4. |
Investigation Documentation of Deviations
The deviation shall be documented and investigated as per the NORDION deviation procedure. |
X | ||||
| NORDION and MIPI shall work together to determine the impact of the deviation on MIPI Product and associated fillings. | X | X | ||||
| 5. |
Resolution of Deviations
• Corrective actions shall be identified as part of the deviation resolution.
• Corrective actions that require changes to approved equipment or documentation shall be governed by the Change Control Section of this Quality Agreement.
• Those batches with deviations will not be released until both parties have evaluated the impact of the deviations and agree that they do not affect the SQUIPP. |
X | X | |||
Page 16 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
14. Out of Specification (OOS)
| OUT OF SPECIFICATION RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Out of Specification Program
Testing results will be subject to the established OOS program, which has been designed to ensure conformance to cGMP requirements. |
X | ||||
| 2. |
Scope
• Perform investigations to determine the root cause of the OOS result in instances where an analytical result fails to conform to pre-established Specifications.
• NORDION shall provide OOS information related to MIPI Product |
X | ||||
15. Change Control
| CHANGE CONTROL RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Change Control Program
Ensure MIPI related activities for the Product occur within the established control program. All changes must be in compliance with cGMP requirements, and MIPI product registrations. |
X | ||||
| 2. |
Scope
The scope of such a change management process includes chemical manufacturing, pharmaceutical manufacturing and packaging processes that may affect the Product SQUIPP or the documentation thereof. The associated changes may relate to:
• Master Production Control Records;
• Standard Operating Procedures, analytical standards and test methods (for Raw Materials and finished Product);
• Material Specifications (for Raw Materials and packaging components); and
• MIPI specific validated/qualified process, equipment, facilities, utilities or computer systems. |
X | ||||
| 3. |
Prospective Changes
• The change program shall apply to prospective changes approved prior to implementation only.
• Excursions shall be in the scope of the deviation program. MIPI reserves the right to reject any batch when changes have been made without prior approval.
• Notify MIPI of any proposed changes to the Facilities or the Processing that may materially impact the Product. Such proposed changes must be approved by MIPI in writing in advance of their implementation. |
X | ||||
Page 17 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| CHANGE CONTROL RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 4. |
Proposal of Changes
All proposals shall include a detailed description of the proposed change and the rationale [supporting data, and timeline for implementation]. |
X | ||||
| In the event that proposed change will result in change to established regulatory filing:
• MIPI is responsible for assessing the impact of the change on the filing and supplementing the filing to include the change where required; and
• For any NORDION initiated change, NORDION is responsible for providing detailed description of change, rationale for change and documentation supporting the change. |
X |
X | ||||
| 5. |
Impact Assessment
All changes to the Product and related documents shall proceed through both a technical and a cGMP impact assessment by NORDION’s quality and change management personnel. The assessment shall consider impact to:
• Product safety quality identity and purity;
• Specifications;
• Documentation;
• Equipment, facilities, utilities, computerized systems;
• Materials;
• Validation. |
X | ||||
| 6. |
Implementation Plan
The assessment must include a plan for implementing the changes. In the event that additional supporting data is required, the plan must include the agreed upon conditional dispositioning of the Product. |
X | ||||
| 7. |
Approval and Implementation of Changes
All changes must be approved prior to implementation. Implementation can occur after formal or written approval from MIPI. |
X | X | |||
| 8. |
Supporting Data for Success of Change
In the case where the success of the change is evaluated by subsequent data the completed change package must include or cross-reference this data. |
X | ||||
Page 18 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
16. Document Control
| DOCUMENTATION CONTROL RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Documentation Control Program
• Product shall be manufactured using documentation from an established, documentation control program that ensures that only approved controlled and accurate documentation is used to execute and document regulated activities.
• The program shall provide a consistent document lifecycle, which includes:
• identification of new/changed documentation;
• preparation, review, approval methodology and responsibilities;
• implementation and training;
• version control;
• issuance and accountability;
• regular assessment for adequacy;
• archiving and security; and
• obsolescing and destruction. |
X | ||||
| 2. |
Scope
• Provide all documents generated specific to MIPI Product, manufacturing, processing, and testing:
• Documents used in the chemical & pharmaceutical, production, manufacturing, packaging, testing, and control of Product; and
• SOP’s, forms, specification and other batch documentation to support the manufacturing process.
• Provide NORDION’s generic and quality system related procedures, at time of on-site visit and/or audit which govern quality system execution. |
X | ||||
| 3. |
Control
Ensure that only authorized, officially issued copies of approved documentation are used for the execution and documentation of any regulated activity. |
X | ||||
| 4. |
Master Production Records
The Master Product Record will have sections to record the summaries of all Product testing data, lot release data, manufacturing data and deviations, as applicable. |
X | ||||
| MIPI will provide NORDION with sufficient MIPI specific Product and process information to generate the master production record for execution. | X | |||||
| MIPI reserves the right to transcribe the manufacturing information into MIPI’s format for MIPIs use | X | |||||
| The Master Production Record will be submitted to MIPI for written approval before use in manufacturing.
• Updates of the Master Batch Record will be part of the change control program requiring MIPIs approval for any changes before use in manufacturing |
X | |||||
Page 19 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| DOCUMENTATION CONTROL RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 5. |
Protection of Proprietary Information
NORDION shall have the right to remove its or its client’s proprietary information from documentation before supplying to MIPI for review. |
X | ||||
| MIPI shall have the right to remove its or its contractors’ proprietary information from the documentation before supplying to NORDION for review. | X | |||||
| 6. |
Changes
• MIPI shall not make any changes to NORDION-owned or NORDION-controlled cGMP documentation without the prior written consent of NORDION, in order to ensure that all cGMP documentation, which is maintained at NORDION and subject to regulatory review, matches or is consistent with information filed with regulatory authorities.
• NORDION shall not make any changes to the manufacturing and non-generic testing documentation directly supporting Product, without the prior written consent of MIPI through the change control program. |
X |
X
| |||
| 7. |
Records Retention Program
Responsible for maintaining all records pertaining to the manufacture of the Product in accordance to a documented record retention program. |
X | ||||
| 8. |
Record Retention Scope and Period
• Records subject to the retention program shall include but not be limited to manufacturing data, incoming, in process and final test data, stability records, labeling, customer complaints, validation records, preventative maintenance and calibration, facility, environmental and equipment monitoring, and customer, vendor and regulatory audits.
• Retain lot records of the clinical trial materials for a maximum of ten (10) year past the stop use date and or in accordance with the mutually agreed upon duration as will be established in a separate document mutually agreed upon by MIPI and NORDION.
• Store the Master Production Record, Master Batch Record, batch records and all other documentation related to the Product for a period of at least ten (10) years; provided, however, that NORDION may maintain such records for a shorter period of no less than two (2) years from the relevant finished Product expiration date if NORDION’s Standard Operating Procedures provide for such a shorter period; provided, further that, notwithstanding the foregoing, in no event shall NORDION retain such records for less than the period of time otherwise required under Applicable Laws. If NORDION plans to discontinue such storage prior to expiration of the ten (10) year period, NORDION shall provide MIPI with sufficient prior written notice so that MIPI can arrange for alternative storage, and NORDION shall cooperate with MIPI to have any such documentation transferred to an alternative storage facility. NORDION shall use best efforts to protect such records from fire, water or any and all other type of damage. |
X | ||||
Page 20 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| DOCUMENTATION CONTROL RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 9. |
Record Destruction
Notify MIPI prior to the destruction of any of the retained documentation. |
X | ||||
| Notify NORDION of desire to retain documentation slated for destruction. | X | |||||
| Arrange for secure transportation of retained documentation. | X | |||||
| Ensure secure destruction of documentation. | X | |||||
17. Training
| TRAINING RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Training Program
Establish, execute and maintain a documented training program. |
X | ||||
| 2. |
Scope
Train all personnel performing the manufacturing, validation, testing, and quality obligations of this Quality Agreement in accordance with a documented training program and the cGMP Authority requirements.
• Training shall be conducted and assessed on a routine basis by qualified personnel.
• Provide a sufficient number of qualified personnel to perform the manufacturing, validation, testing, and quality obligations of this Quality Agreement |
X | ||||
18. Regulatory
| REGULATORY RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Regulatory Program
• Establish regulatory program, Standard Operating Procedures, methods and facilities to include all Drug Substance to ensure conformance to cGMP requirements.
• Establish regulatory program to include Product inventory, submissions to Regulatory Authorities, quality assurance review, government regulatory agency actions (approval, suspension, revocation).
• Review & approve all licensing submission data to assure cGMP compliance.
• Ensure that all submissions to regulatory agencies are approvable, thereby assuring timely permission to distribute Product. |
X | ||||
| 2. |
Marketing Applications
• Prepare, maintain and update the Marketing Authorizations in accordance with all Applicable Laws.
• Provide NORDION with copies of those portions of the Marketing Applications which are applicable to the Processing or contain any information for activities that are the responsibility of or carried out by NORDION prior to submission of such Marketing Applications to the applicable Regulatory Authority. |
X | ||||
Page 21 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| REGULATORY RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 3. |
Filings
Ensure all appropriate regulatory filings and import/export documentation are filed with regulatory agencies and that approvals have been granted prior to shipment/human administration. |
X | ||||
| Review & approve all licensing submission data to assure cGMP compliance (NORDION is responsible for NORDION data only). | X | X | ||||
| Identify any cGMP concerns relating to a submission that have a cGMP impact & to approve of any changes or corrective actions to assure cGMP compliance. | X | |||||
| Provide point of contact between MIPI’s regulatory affairs staff or consultants regarding issues that impact the Chemistry Manufacturing and Controls (“CMC”) registration information for the Drug Substance and/or drug Product. | X | |||||
| Provide MIPI with agreed finalized CMC documentation covered in this Quality Agreement. | X | |||||
| Perform a technical/regulatory review of all documentation provided to MIPI to support regulatory submission (NORDION data only). | X | X | ||||
| Provide support for MIPI with respect to proposing appropriate CMC regulatory strategies and identifying potential regulatory consequences for issues involving drug products. | X | |||||
| MIPI will be responsible for making final decisions regarding CMC regulatory strategy and will document such decisions for NORDION. | X | |||||
| MIPI will provide a copy of applicable sections of MIPI’s final regulatory submissions, for reference during inspections. | X | |||||
| 4. |
Regulatory Inspections
Upon notification to Nordion, Nordion will in turn inform MIP of any regulatory inspections that may involve the Product and permit a representative from MIPI’s quality unit to be present. |
X | ||||
| Note: | During the regulatory inspection, MIPI will not be involved in the audit room with the regulatory inspection, unless MIPI is requested by NORDION to attend such session, | |||||||
| Consult and closely cooperate with MIPI prior to making any commitment to any regulatory agency regarding MIPI’s Product. | X | |||||
| Upon receipt of an notice of adverse findings Nordion will provide to MIP any regulatory correspondence related to the Product or other system related issues that support manufacturing, packaging or testing of MIPI Product, redacted of or summarized to remove any NORDION commercial confidential information not directly related to the Product. | X | |||||
| MIPI, within 24 hours, will inform NORDION in writing of any regulatory issue that impacts NORDION’s ability to manufacture the Product. | X | |||||
| 5. |
Regulatory Actions
MIPI will notify NORDION of any regulatory actions on the Product that may impact NORDION. |
X | ||||
| MIPI will promptly forward any regulatory correspondence on the Product to NORDION. | X | |||||
| NORDION is responsible for supporting all lot record investigations associated with regulatory actions. | X | |||||
Page 22 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| REGULATORY RESPONSIBILITIES |
MIPI |
NORDION | ||||
| NORDION agrees to supply MIPI with any manufacturing, testing, or storage data relating to the Product within two (2) business days, if requested, as the result of a regulatory inspection, or a potential regulatory exposure, such as a recall or significant Product complaint. | X | |||||
| Immediately notify MIPI of any FDA or other Regulatory Authority notice of inspection or inspection of the Facilities directly relating to the Product, and in no event later than 48 hours after the schedule has been established. | X | |||||
| Immediately, and in no event longer than 48 hours, notify MIPI of any FDA or other Regulatory Authority investigation that NORDION has a reasonable basis to believe is likely to impact the Processing of the Product. | X | |||||
| Provide copies of any FDA Form 483’s, Warning Letters or the like from applicable Regulatory Authorities, no later than 48 hours after receipt, including any subsequent response(s) relating to the Product, either redacted or summarized to remove any NORDION commercial confidential information. | X | |||||
| Notify the other party of any Regulatory Authority request for Product samples or Product batch records, no later than 48 hours after receipt of the request. | X | X | ||||
| Make available during audit notices of violations or other communication from a Regulatory Authority relating to environmental, occupational health and safety compliance, relating directly to Product. Information of a personnel confidential nature will not be provided. | X | X | ||||
| 6. |
Annual Product Review Generation
• Perform an annual product review for the Product, including but not limited to, those CFR requirements encompassing:
• Review critical in-process controls and results;
• Review of batches that failed to meet Specifications;
• Review critical deviations/non conformances and related investigations;
• Review changes carried out to process and/or analytical methods;
• Review of stability monitoring (as applicable);
• Review of adequacy of previous corrective actions;
• List of validated procedures and revalidation dates; and
• List of qualified equipment and requalification dates.
• Issue a report to MIPI. It will contain a review of any changes at NORDION in the manufacturing, testing, storage or validation of the Product in the previous calendar year and a summary of lots made, released, investigated and rejected.
• MDSN will provide data for MIPI to compile their annual product review.
• Control charting or trend analysis of key product parameters will be performed and reported. |
X | X | |||
| 7. |
Annual Report
MIPI will prepare any annual report as required by applicable regulations and Applicable Laws, including 21 CFR 314.70, 314.81, and/or 601.12. |
X | ||||
| Provide data and information related to batch records, complaints, recalls, deviations, investigations and non-conforming materials. | X | X | ||||
Page 23 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
| REGULATORY RESPONSIBILITIES |
MIPI |
NORDION | ||||
| The annual report cycle will be established at approval, and the specific request for information will be made at least ninety (90) calendar days before the annual report due date, MIPI shall request in writing, the chemistry, manufacturing and controls data required for preparation of the annual report. | X | |||||
| NORDION will provide the requested information to MIPI within thirty (30) days of the original request date. | X | |||||
| 8. |
Drug Listing
MIPI is responsible for drug listing as the manufacturer of the Product for MIPI. |
X | ||||
| MIPI is responsible for drug listing as the distributor of the Products. | X | |||||
| MIPI will provide NORDION with all required information needed to register the Product. | X | |||||
| MIPI will notify NORDION of the scheduled Product launches, as applicable. | X | |||||
19. Recalls
| RECALL RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Recall, Field Alerts, & Product Withdrawal Program
Establish documented complaints program and process necessary to assure effective, efficient & reliable management of complaints (other than reports of adverse events) associated with all Drug Substance and Product to ensure conformance to cGMP Authority requirements. |
X | X | |||
| 2. |
Scope
MIPI, with data and assistance provided by NORDION, is responsible for filing Field Alerts and initiating product recalls due to any defect considered sufficiently serious. |
X | ||||
| Provide NORDION with a copy of any regulatory correspondence related to field alerts or recalls. | X | |||||
| In the event that NORDION has reason to believe that any Product should be recalled or withdrawn from distribution, NORDION shall promptly inform MIPI in writing. | X | |||||
| MIPI shall notify the FDA and any foreign regulatory agencies, as required. | X | |||||
| MIPI shall be responsible for coordinating all necessary activities regarding the action taken. | X | |||||
| MIPI acknowledges and understands that NORDION, as manufacturer of the Product, has significant regulatory obligations if any recall or withdrawal would be necessary. | X | |||||
| NORDION and MIPI agree to cooperate fully regarding any proposed recall, product withdrawal, or field correction. | X | X | ||||
| Agree to keep each other advised, to exchange copies of documentation as required, and to assure regulatory compliance. | X | X | ||||
| Manage any recall or product withdrawal. | X | |||||
| Reconcile returned product following recall or product withdrawal. | X | |||||
| Issue and follow-up on FDA field alerts. | X | |||||
Page 24 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
20. Complaints
| COMPLAINT RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Complaint Program
Note: MIPI is responsible for complaints related to Product (“Complaints”). This section outlines MIPI expectations that NORDION will have an established Complaint handling program
• All complaints related to the Product will be processed adhering to the NORDION Complaints procedure to assure conformance to cGMP requirements. |
X | X | |||
| 2. |
Scope
NORDION complaint assessment focuses on possible failure of drug Product to meet any of its Specifications related to product quality & issues associated with manufacturing technique & equipment. |
X | X | |||
| 3. |
Receiving & Investigating Product Complaints.
Notify NORDION of any problems thought to be due to NORDION manufacturing on receipt of the Complaint (within forty-eight (48) hours). |
X | ||||
| Promptly perform investigations. | X | X | ||||
| Generate investigation report and forward to MIPI within thirty (30) days of receipt by NORDION. | X | |||||
| Perform review & approval of any corrective action plans proposed to eliminate the causes of a Complaint. | X | X | ||||
| Report any Complaint to the appropriate regulatory authority including adverse drug events reports. | X | |||||
| Any Quality Complaint directly related to product received by NORDION from a source other than MIPI will be immediately forwarded to MIPI. | X | |||||
21. Adverse Events
| ADVERSE EVENT RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Adverse Event System
Note: MIPI is responsible for handling of adverse events related to Product (“Adverse Events” or “AE”). This section outlines MIPI expectations that NORDION will have an established Adverse Event handling program
• Reports of adverse events are handled in NORDION complaint system. Any such report will be communicated to MIPI within 48 hours. |
X | X | |||
| Establish Adverse Events system to include activities associated with receipt, documentation, investigation, hazard evaluation, reporting and Annual Product Review. | X | X | ||||
| Provide support to MIPI in regulatory reporting of Adverse Events where required, providing support in analysis of manufacturing and testing data as it might relate to a reported event. | X | |||||
Page 25 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
22. AUDITS
| AUDIT RESPONSIBILITIES |
MIPI |
NORDION | ||||
| 1. |
Internal Auditing Program
• Establish, execute and maintain and internal auditing program for the quality systems identified in this Quality Agreement.
• Perform periodic auditing of facilities, processes, and systems outlined in this Quality Agreement to assure standards are established, NORDION’s performance and compliance to cGMP, and in the event of issues/deviations are observed, NORDION shall establish a corrective action plan to mitigate the outlined issues. NORDION will monitor the execution of the corrective action plan activities and completion thereof. |
X | ||||
| 2. |
Access
Provide access to MIPI quality representatives to manufacturing, warehousing, laboratory premises and program documents and records, relating to MIPI’s Product, for audit purposes. |
X | ||||
| Escort MIPI representatives at all times. | X | |||||
| Give reasonable notice for audits (i.e., 1 month for routine GMP Audits). | X | X | ||||
| Schedule audits upon mutual agreement. | X | |||||
| 3. |
Periodic Audits
Conduct a standard quality cGMP compliance audit at least once every two (2) years. |
X | ||||
| 4. |
Preparatory Audits
Perform a preparatory audit either for initiation of cGMP manufacture of the Products or for pre-approval inspections (PAI). |
X | ||||
| 5. |
Audit for Cause Product Quality or Safety Audits
Perform audits for cause in the event of significant Product quality or safety problems. |
X | ||||
| For cause audits can be scheduled as required, MIPI will provide at least 10 days notice when scheduling a for cause audit. | X | X | ||||
| 6. |
Audit Closeout
Conduct audit closeout meeting discuss significant audit observations. |
X | ||||
| Provide a written audit report to NORDION of all observations within thirty (30) days to NORDION. | X | |||||
| Provide a written response and corrective action plan to MIPI within thirty (30) days of the audit report receipt. | X | |||||
| As requested, provide status of corrective action plan and written confirmation of completion of corrective actions. | X | |||||
23. Management of Relationships and Communication Strategy
MIP and NORDION will designate senior quality staff to act as points of contact for the purpose of this Quality Agreement, Refer to Appendix I
Page 26 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
Appendix I – MIPI / MDS Nordion Quality Agreement Points of Contact.
| MIPI |
NORDION | |||
| Quality Assurance | Xxxxxx Xxxxx Associate Director, Quality Assurance 617.871.6681
Xxx Xxxxxxxx Vice President, Regulatory and Quality 000-000-0000 |
Xxxxx Xxx Director, Quality Assurance MDS Nordion 613.592.3400 ext 2689
Xxx XxXxxxxx Senior Vice President, Quality and Regulatory Affairs 000-000-0000 xxx 0000 | ||
|
Manufacturing |
Xxx Xxxxxxxx Vice President, Development 617.871.6619 |
|||
| Regulatory Affairs | Xxx Class Senior Director, Regulatory Affairs 617.871.6644 |
Xxxx Warbick-Xxxxxx Director, Regulatory Affairs 613.592.3400 ext 2033 | ||
| Program Management | Xxxxx Xxxxxx Coco Vice President, Program Management 617.871.6633 |
Xxxxx Xxxxx Director, Project Management Office 613.592.3400 ext 2018 | ||
Page 27 of 32

|
MIPI / MDS Nordion Azedra Quality Agreement |
Appendix II
Azedra Production Documentation & Flow
Page 28 of 32

|
MIPI / MDS Nordion Quality Agreement |
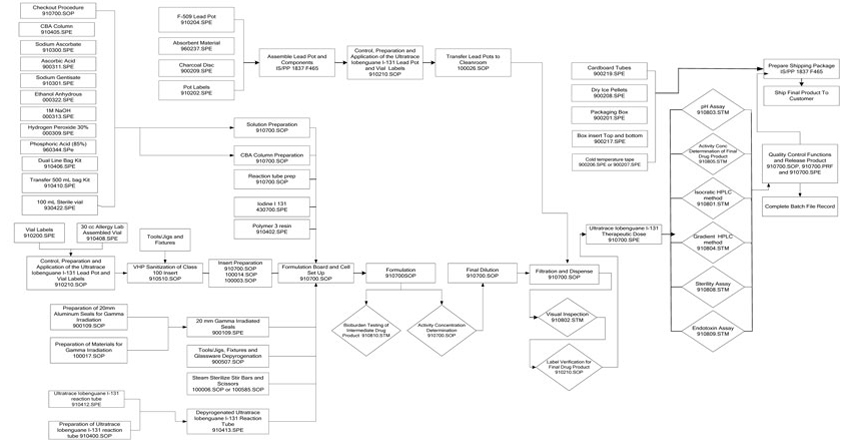
To be updated when commercial flow chart is available
Page 29 of 32

|
MIPI / MDS Nordion Quality Agreement |
Page 30 of 32

|
MIPI / MDS Nordion Quality Agreement |
Appendix IV
Lot Release Documentation
Page 31 of 32

|
MIPI / MDS Nordion Quality Agreement |
Ultratrace Iobenguane I-131
Documents sent to MIP on release day
910700.PRF – Complete
910700.PCR – Section E & F – Formulation
910210.PCR – Sample Labels Only – Enlarged 155% – Pot & Vial
Copies of pages from ACR’s where values are transcribed to PRF:
910802.ACR – Visual Inspection – Pages 2, 3 & 6 of 8 only
910809.ACR – Complete Document
910804.ACR – HPLC – Page 6 & 7 of 8 only
910803.ACR – pH – Complete document
000036.SOP – Radioactivity Measurement Sheet – Page 1 only
910801.ACR – HPLC – Page 5 & 7 of 10 only
Any applicable deviations/CFs/Lab Investigations
Documents sent within 48 hours of release
C of A – I-131 iodine
910210.PCR – Complete Labelling document
910510.PCR – STERIS VHP 1000 Biodecontamination Unit
910700.PCR – A, B, C and D
MetOne Excursion Log Sheet + Printouts
Accountability Report
910801.ACR – Identity, Radiochemical by HPLC & printouts
910802.ACR – Visual Inspection
910804.ACR – HPLC * printouts
000036.SOP – Radioactivity Measurement Sheet
Documents sent when completed post release
910810.ACR – Bioburden Testing
910808.ACR – Sterility Testing
100014.PCR – Non-Viable – Rooms 1226, 1217
040800.SOP – Product Inventory Sheets – TX and DX
910806.PCR – Shipping Documents
900835.PCR – Room 1226 EM
900834.PCR – Room 1217 EM
900838.PCR – Room 1215 EM
900510.PCR – AMSCO VHP 1000
Product Release Form – When batch is closed out
Completed Deviations, OOSs
All Environmental Notices
Page 32 of 32
SCHEDULE F
Specifications For Precursor and Reference Standards

|
UNCONTROLLED COPY | |
| 910402.SPE (1) | ||
| Effective Date: 06-12-19 CF 61 |
Page 1 of 2 | |
| Polymer 3 Resin | ||
Signatures
| Prepared by: |
Signature on file |
Date: |
| |||
| C. Bensimon | yy/mm/dd | |||||
| Senior Research Chemist | ||||||
| Approved by: |
Signature on file |
Date |
| |||
| X. Xxxxxxxxx | yy/mm/dd | |||||
| Production | ||||||
| Approved by: |
Signature on file |
Date: |
| |||
| X. Xxxxxxx | yy/mm/dd | |||||
| Quality Control | ||||||
| Approved by: |
Signature on file |
Date: |
| |||
| J Xxxxxxxx | yy/mm/dd | |||||
| Quality Assurance | ||||||
Document History
| Effective |
Version |
Comments |
Prepared By |
Reviewed By |
Approved By |
NOTE: A vertical line in the margin (tracking bar), denotes change. For complete rewrites, tracking bars are not used.
This document contains information proprietary to MDS Nordion.
Any disclosure or use of this information or any reproduction of this document other than the specified purpose for which it is intended is expressly prohibited except as MDS Nordion may otherwise agree in writing.
910402.SPE (1)
Page 2 of 2
Polymer 3 Resin
| PRODUCT* | : Polymer 3 Resin- (Polymer supported 3-benzyl guanidium acetate) (PN# 12920) | |
| MANUFACTURER* | : Polysciences Inc | |
| EXPIRY DATE* | : As per Quality Control Incoming Material Testing Specification provided by Molecular Insight Pharmaceuticals (MIP) | |
| STORAGE* | : Frozen (-20°C) | |
| SAMPLING PLAN | : Not required- Customer released product | |
| SHIPPING REQUIREMENT | : Room temperature shipment. Complete 000017.PCR, “Verification, Storage and Maintenance of Customer Supplied Product” | |
| CATEGORY | : Critical | |
| OTHER* | : Certificate of Analysis supplied by Polysciences.
Incoming Material Testing Specification supplied by Molecular Insight Pharmaceuticals | |
| * | To be verified by QC. |
910304.SPE (2)
Page 2 of 2
lobenguane Standard
| PRODUCT* | : m-lodobenzylguanidine hemisulfate salt or iobenguane hemisulfate or MIBG hemisulfate (PN# 700) | |
| MANUFACTURER* | : ABX advanced biochemical compounds | |
| EXPIRY DATE* | : As per Incoming Material Testing Specification from MIP | |
| STORAGE* | : 2-8°C | |
| SAMPLING PLAN | : Not required- Customer released product | |
| SHIPPING REQUIREMENT | : Room temperature shipment. Complete 000017. PCR, “Verification, Storage and Maintenance of Customer Supplied Product” | |
| CATEGORY | : Critical | |
| OTHER* | : Certificate of Analysis supplied by ABX Incoming Material Testing Specification supplied by MIP | |
| * | To be verified by QC. |
SCHEDULE G
CHANGE IN SCOPE ORDER
Effective Date: , 2009
This amendment shall form a change in scope order (“Change in Scope Order”) to the existing (INSERT SCHEDULE WHICH IS BEING MODIFIED) executed on , 2009 between Molecular Insight Pharmaceuticals, Inc. (“Molecular”) and MDS Nordion, a division of MDS (Canada) Inc. (hereinafter referred to as “Nordion”). All Articles and provisions of the (INSERT SCHEDULE WHICH IS BEING MODIFIED) remain in effect and will apply to all subsequent amendments to that (INSERT SCHEDULE WHICH IS BEING MODIFIED), unless specifically stated as otherwise herein.
The purpose of this Change in Scope Order is set forth below.
| Change in Scope Order Description for Azedra
¡
¡
Note to draft; Please insert columns | ||||||||
| Total |
||||||||
|
Original Project Budget: Change Order No. 1 Value: Revised Project Total: | ||||||||
|
See Attachment _ (Revised Schedule _ ) and _ (Revised Project Budget) incorporated herein by reference. | ||||||||
AGREED TO AND ACCEPTED BY:
| Molecular Insight Pharmaceuticals, Inc. | MDS Nordion, a division of MDS (Canada) Inc. | |||
|
|
| |||
| By: | By: | |||
|
|
| |||
| Name (Printed): | Name (Printed): | |||
|
|
| |||
| Title: | Title: | |||
|
|
| |||
| Date: | Date: |
Attachment A
Amended Schedule
Attachment B
Revised Project Budget

