Confidential Materials omitted and filed separately with the Securities and Exchange Commission. Double asterisks denote omissions. ASSET PURCHASE AGREEMENT by and between BIOGEN MA INC. and KARYOPHARM THERAPEUTICS INC. DATED AS OF JANUARY 24, 2018
Exhibit 10.1
Confidential Materials omitted and filed separately with the
Securities and Exchange Commission. Double asterisks denote omissions.
by and between
BIOGEN MA INC.
and
DATED AS OF JANUARY 24, 2018
TABLE OF CONTENTS
| Page | ||||||
| ARTICLE I DEFINITIONS AND TERMS |
1 | |||||
| Section 1.1. |
Definitions |
1 | ||||
| Section 1.2. |
Other Definitional Provisions |
16 | ||||
| ARTICLE II PURCHASE AND SALE OF ASSETS |
17 | |||||
| Section 2.1. |
Purchase and Sale of Assets |
17 | ||||
| Section 2.2. |
Excluded Assets |
18 | ||||
| Section 2.3. |
Assumed Liabilities |
18 | ||||
| Section 2.4. |
Excluded Liabilities |
18 | ||||
| Section 2.5. |
Licenses and Sub-Licenses |
18 | ||||
| Section 2.6. |
Purchase Price |
19 | ||||
| Section 2.7. |
Closing |
19 | ||||
| Section 2.8. |
Purchased Assets Not Transferred at Closing |
19 | ||||
| Section 2.9. |
Taxes |
20 | ||||
| Section 2.10. |
Transition Plan |
21 | ||||
| Section 2.11. |
Wrong Pockets |
21 | ||||
| ARTICLE III MILESTONES, NET SALES PAYMENTS AND OTHER FINANCIAL OBLIGATIONS |
21 | |||||
| Section 3.1. |
Development Milestone Payments |
21 | ||||
| Section 3.2. |
Commercial Milestone Payments |
23 | ||||
| Section 3.3. |
Net Sales Payments |
25 | ||||
| Section 3.4. |
Milestones and Net Sales Payment Adjustments |
26 | ||||
| Section 3.5. |
Net Sales Audit Rights |
27 | ||||
| Section 3.6. |
Currency Exchange |
28 | ||||
| Section 3.7. |
Taxes |
28 | ||||
| Section 3.8. |
Diligence |
28 | ||||
| Section 3.9. |
Termination for Convenience |
29 | ||||
| Section 3.10. |
Non-Competition. |
30 | ||||
| Section 3.11. |
Term |
32 | ||||
| ARTICLE IV REPRESENTATIONS AND WARRANTIES OF SELLER |
32 | |||||
| Section 4.1. |
Organization and Good Standing |
32 | ||||
| Section 4.2. |
Authority |
32 | ||||
i
| Section 4.3. |
No Conflict |
33 | ||||
| Section 4.4. |
Required Filings and Consents |
33 | ||||
| Section 4.5. |
Purchased Assets |
33 | ||||
| Section 4.6. |
Contracts |
33 | ||||
| Section 4.7. |
Intellectual Property |
34 | ||||
| Section 4.8. |
Inventory |
36 | ||||
| Section 4.9. |
Compliance with Laws; Regulatory Compliance |
37 | ||||
| Section 4.10. |
Brokers |
38 | ||||
| Section 4.11. |
Non-Reliance by Seller |
38 | ||||
| ARTICLE V REPRESENTATIONS AND WARRANTIES OF PURCHASER |
38 | |||||
| Section 5.1. |
Organization and Good Standing |
38 | ||||
| Section 5.2. |
Authority |
38 | ||||
| Section 5.3. |
No Conflict |
39 | ||||
| Section 5.4. |
Required Filings and Consents |
39 | ||||
| Section 5.5. |
Litigation |
39 | ||||
| Section 5.6. |
Sufficient Funds |
39 | ||||
| Section 5.7. |
Brokers |
39 | ||||
| Section 5.8. |
Non-Reliance by Purchaser |
40 | ||||
| ARTICLE VI INTELLECTUAL PROPERTY; KNOW-HOW TRANSFER |
40 | |||||
| Section 6.1. |
Prosecution and Maintenance |
40 | ||||
| Section 6.2. |
Cooperation |
41 | ||||
| Section 6.3. |
Defense of Claims Brought by Third Parties |
41 | ||||
| Section 6.4. |
Notice of Infringement and Paragraph IV type Notices |
42 | ||||
| Section 6.5. |
Enforcement of IP |
42 | ||||
| Section 6.6. |
Settlement |
44 | ||||
| Section 6.7. |
Costs and Recoveries |
44 | ||||
| Section 6.8. |
Other Actions by Third Parties |
45 | ||||
| Section 6.9. |
References to Seller IP |
45 | ||||
| Section 6.10. |
Patent Challenges by Seller of the Transferred IP |
46 | ||||
| Section 6.11. |
Unblocking License |
46 | ||||
| Section 6.12. |
Know-How Transfer |
46 | ||||
| ARTICLE VII INDEMNIFICATION |
47 | |||||
| Section 7.1. |
Indemnification by Seller |
47 | ||||
| Section 7.2. |
Indemnification by Purchaser |
47 | ||||
ii
| Section 7.3. |
Notice of Claims |
47 | ||||
| Section 7.4. |
Indemnification Procedures |
48 | ||||
| Section 7.5. |
Survival of Representations and Warranties |
48 | ||||
| Section 7.6. |
Limitations |
49 | ||||
| Section 7.7. |
Exclusive Remedy |
49 | ||||
| ARTICLE VIII MISCELLANEOUS |
50 | |||||
| Section 8.1. |
Further Assurances and Post-Closing Covenants |
50 | ||||
| Section 8.2. |
Notices |
50 | ||||
| Section 8.3. |
Amendment; Waiver |
51 | ||||
| Section 8.4. |
Assignment |
51 | ||||
| Section 8.5. |
Entire Agreement |
52 | ||||
| Section 8.6. |
No Third-Party Beneficiaries |
52 | ||||
| Section 8.7. |
Public Disclosure |
53 | ||||
| Section 8.8. |
Publishing and Use of Trademarks |
53 | ||||
| Section 8.9. |
Confidentiality; Return of Information |
54 | ||||
| Section 8.10. |
Equitable Relief |
54 | ||||
| Section 8.11. |
Expenses |
54 | ||||
| Section 8.12. |
Governing Law; Jurisdiction; Venue and Service |
54 | ||||
| Section 8.13. |
Counterparts |
55 | ||||
| Section 8.14. |
Headings |
55 | ||||
| Section 8.15. |
Severability |
55 | ||||
| Section 8.16. |
Force Majeure |
56 | ||||
EXHIBITS
| A |
Form of Instrument of Assignment and Assumption | |
| B |
Form of Patent Assignment | |
| C |
Form of Tax Certificate |
iii
This Asset Purchase Agreement, dated as of January 24, 2018, is made by and between Karyopharm Therapeutics Inc., a Delaware corporation (with its principal corporate offices at 00 Xxxxx Xxxxxx, Xxxxxx, XX 02459) (“Seller”) and Biogen MA Inc., a Massachusetts corporation (with its principal corporate offices at 000 Xxxxxx Xxxxxx, Xxxxxxxxx, XX 02142) (“Purchaser”). Seller and Purchaser are collectively referred to herein as the “Parties” and individually as a “Party.”
W I T N E S S E T H:
WHEREAS, Purchaser desires to purchase from Seller, and Seller desires to sell to Purchaser, the Purchased Assets (as defined below) on the terms and conditions set forth in this Agreement.
NOW, THEREFORE, in consideration of the foregoing and the representations, warranties, covenants and agreements contained herein, the Parties hereby agree as follows:
ARTICLE I
DEFINITIONS AND TERMS
Section 1.1. Definitions. As used in this Agreement, the following terms shall have the meanings set forth or as referenced below:
“Affiliate” shall mean, with respect to any Person, any other Person which controls, is controlled by or is under common control with such Person, for as long as such control exists. For purposes of this definition, “control” shall mean the direct or indirect ownership of more than fifty percent (50%) of the voting or economic interest of a Person, or the power, whether pursuant to contract, ownership of securities or otherwise, to direct the management and policies of a Person. For clarity, once a Person ceases to be an Affiliate of a Party, then, without any further action, such Person shall cease to have any rights or obligations under this Agreement by reason of being an Affiliate of such Party.
“Agreement” shall mean this Asset Purchase Agreement, including all Schedules and Exhibits attached hereto, as the same may be amended, modified or supplemented from time to time in accordance with the terms hereof.
“Allocation” shall have the meaning set forth in Section 2.9(b).
“Ancillary Agreements” shall mean the Instrument of Assignment and Assumption and the Patent Assignments.
“Assumed Contracts” shall have the meaning set forth in Section 2.1(a)(i).
1
“Assumed Liabilities” shall have the meaning set forth in Section 2.3.
“Background IP” shall mean the Background Know-How and Background Patents.
“Background Know-How” shall mean all Know-How owned or Controlled by Seller or an Affiliate, other than the Transferred IP, that is necessary or useful for the purpose of enabling Purchaser to Exploit the Transferred IP.
“Background Patents” shall mean all Patents owned or Controlled by Seller or an Affiliate as of the Closing Date, other than the Transferred IP, that Cover Background Know-How.
“Business Day” shall mean any day other than a Saturday, a Sunday or a United States Federal Holiday.
“Cap” shall have the meaning set forth in Section 7.6(c).
“CDER” shall mean the Center for Drug Evaluation and Research of the United States Food and Drug Administration.
“Challenge” shall mean, with respect to any IP Rights under the Seller IP, to contest the validity or enforceability of any such IP Rights, in whole or in part, in any court, arbitration proceeding or other tribunal, including the United States Patent and Trademark Office, the European Patent Office, and the United States International Trade Commission. As used in this term “Challenge”, the term “contest” includes (a) filing an action under 28 U.S.C. §§ 2201-2202 seeking a declaration of invalidity or unenforceability of any such IP Rights; (b) filing, or joining in, a petition under 35 U.S.C. § 311 to institute inter partes review of any such IP Rights, or any portion thereof; (c) filing, or joining in, a petition under 35 U.S.C. § 321 to institute post-grant review of any such IP Rights, or any portion thereof; (d) any foreign equivalent of clauses (a), (b) or (c) in any country outside of the United States; or (e) filing or commencing any opposition, nullity or similar proceedings challenging the validity of any such IP Rights in any country outside the United States; but excludes (i) filing a request under 35 U.S.C. § 302 for re-examination of any such IP Rights, (ii) filing a request under 35 U.S.C. § 251 for a reissue of any such IP Rights, or (iii) any foreign equivalents of clause (i) or (ii) applicable in a country outside of the United States.
“Challenged IP Right” shall have the meaning set forth in Section 6.10.
“Closing” shall mean the consummation of the transactions contemplated by this Agreement pursuant to the terms of this Agreement.
“Closing Date” shall mean the date hereof.
“Code” shall mean the United States Internal Revenue Code of 1986, as amended.
2
“Combination Product” shall mean (a) any single product in finished form containing as active ingredients both a Royalty Product and one or more other pharmaceutically active compounds or substances, whether co-formulated or co-packaged (i.e., within a single box or sales unit); or (b) any Royalty Product sold in combination with one or more other products (such as devices) or services for a single invoice price; or (c) any Royalty Product sold where the sale of such Royalty Product is only available with the purchase of other products or services.
“Commercial Milestone Event” shall have the meaning set forth in Section 3.2(a).
“Commercial Milestone Payment” shall have the meaning set forth in Section 3.2(a).
“Commercially Reasonable Efforts” shall mean, with respect to Purchaser’s obligations under this Agreement to Develop a Product, the level of efforts that are similar to the efforts normally deployed by Purchaser and its Affiliates to the development of a product of a similar market potential or profit potential or strategic importance and that is at a similar stage in development or product life as such Product, based on conditions then prevailing and taking into consideration all payments due under this Agreement and all other potentially relevant factors that may affect the deployment of efforts to Development, including actual and potential issues of safety and efficacy; stage of Development or product lifecycle status; the nature and extent of market exclusivity (including regulatory exclusivity and the patent and other proprietary position of such Product); Product profile; all costs, budgets and expenses associated with the Development of such Product (including both actual or projected costs) based on conditions then prevailing; any expected issues relating to the Manufacture of such Product; timing and likelihood of success of technology transfer, process development and Manufacturing validation and scale-up; the competitiveness of alternative products in the marketplace; the likely timing of such Product’s entry into the market; the likelihood and cost of obtaining Regulatory Approval and of the anticipated or actual approved labeling; the timing of such approvals; the regulatory environment and the current and projected regulatory status; legal considerations; the anticipated overall commercial success of such Product and other relevant scientific, technical and commercial factors. The level of effort with respect to Commercially Reasonable Efforts will be determined on a country-by-country basis and is expected to change over time, reflecting changes in the status of such Product and the markets or countries involved. Seller expressly understands and accepts that the use of Commercially Reasonable Efforts may result in ceasing Development of a Product (in whole or in part), and that once Development for a Product has ceased in compliance with this Agreement, Commercially Reasonable Efforts does not require the continued re-evaluation of whether Development must be re-initiated for such Product.
“Competitive Infringement” shall have the meaning set forth in Section 6.4(a).
“Confidentiality Agreement” shall mean that certain confidentiality agreement, dated May 1, 2013 between Seller and Purchaser, as amended by that certain Amendment No. 1, dated March 16, 2015 and as amended by that certain Amendment No. 2, dated April 15, 2016 and as may be further amended from time to time.
3
“Confidential Information” shall mean (i) with respect to Purchaser, any and all proprietary or nonpublic information, including information that is written, electronic, oral or visual, that is directly or indirectly related to any of the Transferred Compounds, the Purchased Assets, the Assumed Liabilities, Licensed IP, Purchaser or any of its Affiliates’ present or future business, operations, services, products, research, inventions, discoveries, drawings, designs, plans, processes, models, technical information, facilities, methods, trade secrets, Copyrights, software, source code, systems, Patents, procedures, manuals, specifications, any other intellectual property, confidential reports, price lists, pricing formulas, customer lists, financial information (including the revenues, costs or profits associated with Purchaser or any its Affiliates’ products or services), business plans, lease structures, projections, prospects, opportunities or strategies, acquisitions or mergers, advertising or promotions, personnel matters, legal matters, or any other confidential and proprietary information and (ii) with respect to Seller, any and all proprietary and nonpublic information related to the Background IP, Manufacturing IP or Seller’s or its Affiliates’ present or future business, operations, services, products, research, inventions, discoveries, drawings, designs, plans, processes, models, technical information, facilities, methods, trade secrets, Copyrights, software, source code, systems, patents, procedures, manuals, specifications, any other intellectual property, confidential reports, financial information, business plans, projections, prospects, opportunities or strategies, acquisitions or mergers, personnel matters, legal matters, or any other confidential and proprietary information (but excluding information set forth in clause (i)). Confidential Information shall also include all notes, analyses, compilations, collections, forecasts or other documents or materials to the extent such documents or materials contain, reflect or are based on Confidential Information. The terms and conditions of this Agreement and the Parties’ discussions regarding this Agreement will constitute Confidential Information of each of Purchaser and Seller.
“Contract” shall mean any agreement, contract, subcontract, settlement agreement, lease, sublease, legally binding understanding, note, option, bond, mortgage, indenture, trust document, loan or credit agreement, license, sublicense, insurance policy or legally binding commitment or undertaking of any nature, as in effect as of the date hereof or as may hereinafter be in effect.
“Control” or “Controlled by” shall mean, with respect to any intellectual property right (including any Patent or Know-How), the possession of (whether by ownership or license, other than by a license granted pursuant to this Agreement) the ability of a Person or its Affiliates to assign, transfer, or grant access to, or to grant a license or sublicense of, such right as provided for herein without violating (a) the terms of any agreement or other arrangement with any Third Party existing as of the time a Person or its Affiliates would be required hereunder to grant such access, ownership, license or sublicense and (b) any applicable Law.
“Copyrights” shall mean all copyrightable works and all copyrights and applications, including in and to works of authorship and all other rights corresponding thereto throughout the world, whether published or unpublished, including rights to prepare, reproduce, perform, display and distribute copyrighted works and copies, compilations and derivative works thereof (including all unregistered copyrights).
4
“Cover” shall mean, with respect to a given compound, product, or material and a given Patent, that the Manufacture, use, sale, offer for sale, or importation of such compound, product, or material would infringe one or more claims of such Patent absent ownership of or a license under such Patent (or, in the case of a Patent application, would infringe one or more claims thereof if such claims were to issue).
“Development” shall mean, together with all correlative meanings, pre-clinical and clinical drug development activities, conducted before or after obtaining Regulatory Approval, that are reasonably related to or leading to the development, preparation, and submission of data and information to a Regulatory Authority for the purpose of obtaining, supporting or expanding Regulatory Approval, including without limitation, all activities related to preclinical testing, assay development and validation, in vivo testing, biomarker development and validation, toxicology, pharmacokinetic profiling, design and conduct of clinical trials or studies, regulatory affairs, statistical analysis, report writing, and Regulatory Materials creation and submission (including the services of outside advisors and consultants in connection therewith). “Develop” has a correlative meaning.
“Development Milestone Event” shall have the meaning set forth in Section 3.1.
“Development Milestone Payment” shall have the meaning set forth in Section 3.1.
“Encumbrance” shall mean, with respect to any Purchased Asset, any pledges, liens, licenses, charges, encumbrances and security interests of any kind or nature whatsoever.
“Excluded Assets” shall have the meaning set forth in Section 2.2.
“Excluded Liabilities” shall have the meaning set forth in Section 2.4.
“Exhibits” shall have the meaning set forth in Section 1.2(g).
“Exploit” shall mean, together with all correlative meanings, any or all of the following: research, Develop, commercialize, design, test, modify, Manufacture, have Manufactured, service, have serviced, make, have made, use, have used, sell, have sold, offer for sale, have offered for sale, import, have imported, export, have exported, reproduce, promote, market and distribute, including commercial activities conducted in preparation for a product launch, such product. “Exploitation” has a correlative meaning.
“FDA” shall mean the United States Food and Drug Administration and any successor agency.
“First Commercial Sale” means, on a Royalty Product-by-Royalty Product and country-by-country basis, the first sale of such Royalty Product for end use or consumption of such Royalty Product in such country following receipt of Regulatory Approval for such Royalty Product in such country. A First Commercial Sale excludes any sale or distribution for use in any clinical trial or other Development activity, promotional use (including samples) or for compassionate use or on a named patient basis.
5
“First Reimbursed Sale” means, on a Royalty Product-by-Royalty Product and country-by-country basis, the first sale of such Royalty Product for end use or consumption of such Royalty Product in such country following receipt of Pricing Approval for such Royalty Product in such country. First Reimbursed Sale excludes any sale or other distribution for use in any clinical trial or other Development activity, promotional use (including samples), or for compassionate use or on a named patient basis. If, with respect to a particular country or jurisdiction at any given time, the applicable governmental authority in such country or jurisdiction does not require reimbursement for pharmaceutical products to be marketed or sold, then the First Reimbursed Sale for a Royalty Product in such country or jurisdiction shall be deemed to have occurred upon the First Commercial Sale of such Royalty Product in such country or jurisdiction.
“Fiscal Year” shall mean Purchaser’s fiscal year.
“Force Majeure” shall mean any occurrence beyond the reasonable control of a Party that (a) prevents or substantially interferes with or delays the performance by such Party of any of its obligations hereunder and (b) occurs by reason of any act of God, flood, fire, explosion, earthquake, strike, lockout, labor dispute, casualty or accident, or war, revolution, civil commotion, act of terrorism, blockage or embargo, or enactment of any mandatory applicable Laws after the date of this Agreement prohibiting the nonperforming party from performing its obligations under this Agreement.
“Fraud” shall mean actual and intentional common law fraud.
“Fundamental Representations” shall have the meaning set forth in Section 7.5.
“GAAP” shall mean accounting principles generally accepted in the United States, as in effect as of the date hereof.
“Generic Product” shall have the meaning set forth in Section 3.4(c).
“Governmental Entity” shall mean any United States federal, state or local or any foreign government or any court, administrative or other governmental or government-authorized authority or agency, domestic or foreign, including any Regulatory Authority.
“IND” shall mean (i) an Investigational New Drug Application as defined in the United States Federal Food, Drug and Cosmetics Act, as amended, and all regulations promulgated thereunder, or (ii) an analogous application, filing or submission to the analogous Regulatory Authority in a regulatory jurisdiction outside the United States, the filing of which is necessary to initiate or conduct clinical testing of a pharmaceutical product in humans in such jurisdiction.
“Indemnified Party” shall have the meaning set forth in Section 7.3.
6
“Indemnifying Party” shall have the meaning set forth in Section 7.3.
“Independent Accounting Firm” shall mean an independent accounting firm mutually agreed to by Purchaser and Seller.
“Indication” shall mean a separate and distinct disease or medical condition in humans that a Product is intended to treat, prevent, diagnose, monitor or ameliorate, as set forth in the IND or label for such Product, as applicable, as approved by the applicable Regulatory Authority. The Parties agree and acknowledge that (a) a disease or medical condition and all primary symptoms associated with the disease or medical condition shall be the same Indication, (b) the use of a Product to treat an expanded set of patients or a sub-population of patients for a disease or medical condition, when such Product has already received Regulatory Approval in a different patient population or sub-population of patients with respect to such disease or medical condition, shall not constitute a separate Indication with respect to such Product, and (c) to qualify as an Indication, Regulatory Approval of such Indication must require completion of a human clinical trial sufficient to obtain Regulatory Approval and may not be a mere extension of an existing labeled Indication.
“Instrument of Assignment and Assumption” shall mean the instrument of assignment and assumption to be executed by the Parties on the Closing Date in the form attached hereto as Exhibit A.
“IP Contracts” shall have the meaning set forth in Section 4.7(c).
“IP Rights” shall mean all rights in and to intellectual property and intangible industrial property rights anywhere in the world, including, without limitation, (i) Patents, Know-How, Copyrights, Trademarks, software, and internet domain names, (ii) any rights similar, corresponding or equivalent to any of the foregoing, (iii) all other proprietary rights, and (iv) all copies and tangible embodiments of the foregoing, if applicable (in whatever form or medium).
“Know-How” shall mean all trade secrets and other know-how and confidential or proprietary information, including new developments, inventions, processes, ideas or other proprietary information and documentation thereof (including related papers, invention disclosures, blueprints, drawings, research data and results, flowcharts, diagrams, chemical compositions, formulae, diaries, notebooks, specifications, designs, methods of Manufacture, methods of service, processing techniques, data processing techniques, compilations of information, customer and supplier lists, pricing and cost information, and business and marketing plans and proposals) and all claims and rights related thereto.
“KPT-350 Molecule” shall mean the molecule presently identified as KPT-350 and described with additional specificity on Schedule 1.1(b).
“KPT-420 Molecule” shall mean the molecule presently identified as KPT-420 and described with additional specificity on Schedule 1.1(b).
7
“Knowledge of Seller” shall mean the actual knowledge of the individuals listed on Schedule 1.1(a), after reasonable inquiry of the Seller employees (or, solely in the case of Seller IP, outside legal counsel) with responsibility for the applicable subject matter.
“Law” shall mean, as applicable, any United States federal, state or local or any foreign statute, law, rule, regulation, ordinance, code or any other requirement or rule of law including Regulatory Laws.
“Liabilities” shall mean any and all debts, liabilities, costs, guarantees, commitments, assessments, expenses, claims, penalties, Losses, damages, deficiencies and obligations, whether accrued or fixed, known or unknown, liquidated or unliquidated, asserted or unasserted, absolute or contingent, matured or unmatured, determined or determinable, accrued or not accrued, due or to become due, direct or indirect, whenever or however arising (including whether arising out of any Contract, common law or tort based on negligence or strict liability) and whether or not the same would be required by GAAP to be reflected in financial statements or disclosed in the notes thereto.
“Licensed IP” shall mean the IP Rights, whether filed or unfiled, registered or unregistered, licensed to Seller pursuant to the Assumed Contracts, including the Patents and registered Trademarks or registered Copyrights included in the Licensed IP set forth on Schedule 4.7(a)(ii).
“Licensed or Manufacturing IP Action” shall have the meaning set forth in Section 6.8(a).
“Litigation” shall have the meaning set forth in Section 4.9(b).
“Losses” shall have the meaning set forth in Section 7.1.
“Manufacture” shall mean to make, produce, manufacture, process, fill, finish, package, label, perform quality control and quality assurance testing, release, ship or store a compound or product or any component thereof.
“Manufacturing Enforcement Proceeding” shall have the meaning set forth in Section 6.5(b)(ii).
“Manufacturing IP” shall mean the Manufacturing Know-How and the Manufacturing Patents.
“Manufacturing Intermediate” shall mean any molecule or compound that is included within the Manufacturing IP.
“Manufacturing Know-How” shall mean any Know-How that is (a) owned or Controlled by Seller or any of its Affiliates as of the Closing Date or during the Term and (b) necessary or useful to Manufacture any of the Transferred Compounds (including any Royalty Product).
8
“Manufacturing Patents” shall mean any Patents that (a) are owned or Controlled by Seller or any of its Affiliates as of the Closing Date or during the Term and (b) Cover any Manufacturing Know-How.
“NDA” means, with respect to a pharmaceutical product, a New Drug Application submitted to the FDA in accordance with the United States Federal Food, Drug and Cosmetic Act, as amended, and the rules and regulations promulgated thereunder, or any analogous application or submission to market a pharmaceutical product filed with any Regulatory Authority outside of the United States.
“Net Sales” shall mean, with respect to a Royalty Product, the gross amount invoiced in a country by Purchaser, its Affiliates or licensees, other than any distributors, for the sale or other disposition of such Royalty Product in such country to Third Parties (including distributors, wholesalers and end-users), less the following deductions:
(a) sales returns and allowances actually paid, granted or accrued on such Royalty Product, including trade, quantity, prompt pay and cash discounts and any other adjustments, including those granted on account of price adjustments or billing errors;
(b) credits or allowances given or made for rejection, recall, return or wastage replacement of, and for uncollectible amounts on, such Royalty Product or for rebates or retroactive price reductions (including Medicare, Medicaid, copay assistance, managed care and similar types of rebates and chargebacks);
(c) taxes, duties or other governmental charges levied on or measured by the billing amount for such Royalty Product, as adjusted for rebates and refunds, including without limitation pharmaceutical excise taxes (such as those imposed on a Royalty Product by the United States Patient Protection and Affordable Care Act of 2010 and other comparable laws), but which shall not include any tax, duty, or other charge imposed on or measured by net income (however denominated) or any franchise taxes, branch profits taxes, or similar tax;
(d) charges for freight, customs and insurance directly related to the distribution of such Royalty Product and wholesaler and distributor administration fees; and
(e) other future similar deductions, taken in the ordinary course of business or in accordance with GAAP and Purchaser’s standard practices;
in each case (clauses (a) through (e)) to the extent such deductions: (i) are reasonable and customary, (ii) are included in the gross invoiced sales price for such Royalty Product or otherwise directly paid, allowed, accrued, or incurred by Purchaser, its Affiliates or licensees with respect to the sale of such Royalty Product (iii) are applicable and in accordance with standard allocation procedures, (iv) have not already been deducted or excluded, (v) are incurred in the ordinary course of business in type and amount consistent with good industry practice, and (vi) except with respect to the uncollectible amounts and pharmaceutical excise taxes described in clauses (b) and (c) above, are determined in accordance with, and as recorded in revenues
9
under, GAAP. Net Sales shall not be imputed to transfers of such Royalty Product without consideration or for nominal consideration for use in any clinical trial, or for any bona fide charitable, compassionate use or indigent patient program purpose where such Royalty Product is sold at or below cost of goods sold or as a sample. In the case of any transfer of any Royalty Product between or among Purchaser and its Affiliates or licensees for resale, Net Sales shall be determined based on the sales made by such Affiliate or licensee to a Third Party.
Notwithstanding the foregoing, in the event a Royalty Product is sold as a component of a Combination Product in any country in any Fiscal Year, Net Sales shall be calculated by multiplying the Net Sales of the Combination Product (calculated in the same manner as set forth above as if the Combination Product were a Royalty Product) in such country during such Fiscal Year by the fraction A/(A+B), where A is the average Net Sales of the Royalty Product when sold separately in such country during such Fiscal Year and B is the average Net Sales of the Other Components included in the Combination Product (calculated in the same manner as set forth above as if the Other Components were a Royalty Product) when sold separately in such country during such Fiscal Year. In the event that no separate sales of the Royalty Product or any Other Components included in a Combination Product are made by Purchaser, its Affiliates or licensees in a country during a Fiscal Year in which such Combination Product is sold in such country, the average Net Sales in the above described equation shall be replaced with reasonable good faith estimates of the fair market value, as mutually determined by the Parties, of the Royalty Product and each of the Other Components included in such Combination Product.
“Net Sales Payment Rates” shall have the meaning set forth in Section 3.3(a).
“Net Sales Payment Term” shall have the meaning set forth in Section 3.3(c).
“Net Sales Payments” shall have the meaning set forth in Section 3.3(a).
“Net Sales Statement” shall have the meaning set forth in Section 3.3(b).
“Non-Assigned Assets” shall have the meaning set forth in Section 2.8(b).
“Non-Prosecuting Party” shall have the meaning set forth in Section 6.2(b).
“Non-Responsible Party” shall have the meaning set forth in Section 6.7(a).
“OFAC” shall have the meaning set forth in Section 4.9(g).
“Ono Agreement” shall have the meaning set forth in Section 3.10(a)(ii).
“Order” shall mean any charge, temporary restraining order or other order, writ, injunction (whether preliminary, permanent or otherwise), judgment, doctrine, decree, ruling, determination, directive, corporate integrity agreement or similar agreement, award or settlement, in each case by any Governmental Entity, whether civil, criminal or administrative.
10
“Other Components” shall mean any other pharmaceutically active compounds or substances, or such other products or services referred to in clauses (a) through (c) of the definition of a Combination Product, other than a Royalty Product.
“Party” shall have the meaning set forth in the preamble of this Agreement.
“Patent Assignment” shall mean a Patent assignment, substantially in the form of Exhibit A attached hereto, for all of the Patents listed on Schedule 4.7(a)(i).
“Patents” shall mean all United States and foreign patents and utility models and applications therefor and all reissues, divisions, re-examinations, revisions, renewals, extensions, provisionals, continuations and continuations-in-part thereof, and equivalent or similar rights anywhere in the world in inventions and discoveries, including invention disclosures.
“Permitted Encumbrance” shall mean any (i) landlord’s, mechanics’, carriers’, warehousemens’, workmens’ and other similar Encumbrance arising in the ordinary course of business and securing a Liability that is not yet overdue or that is overdue and is being contested in good faith by appropriate proceedings; (ii) Encumbrance for Taxes, assessment and other governmental charge securing a Liability that is not yet due and payable or that is due but not delinquent or that is being contested in good faith by appropriate proceedings; (iii) Encumbrance arising by operation of law on an insurance policy and proceeds thereof to secure premiums thereunder and that are not delinquent; (iv) Encumbrance arising under worker’s compensation, unemployment insurance, social security, retirement and similar legislation; (v) Encumbrance arising under applicable securities laws; and (vi) Encumbrance arising solely by action of Purchaser.
“Permitted Third-Party Assignee” shall have the meaning set forth in Section 8.4(b).
“Person” shall mean any individual, corporation, partnership (general or limited), limited liability company, limited liability partnership, trust, joint venture, joint-stock company, syndicate, association, entity, unincorporated organization or government or any political subdivision, agency or instrumentality thereof.
“Phase I Clinical Trial” shall mean a clinical trial of a product that generally provides for the first introduction into humans of a pharmaceutical product with the primary purpose of determining safety, metabolism and pharmacokinetic properties and clinical pharmacology of such product, in a manner that is generally consistent with 21 CFR § 312.21(a), as amended (or its successor regulation) or a similar clinical study prescribed by a Regulatory Authority outside the United States; provided, however, a Phase I Clinical Trial does not include any clinical trial or study generally characterized by the FDA as an “exploratory IND study” as described in CDER’s Guidance for Industry, Investigators, and Reviewers: Exploratory IND Studies, dated as of January 2006, irrespective of whether or not such study is actually performed in the United States or under an IND .
11
“Phase I Multiple Ascending Dose Trial” shall mean a multiple ascending dose escalation study on human subjects conducted as part of a Phase I Clinical Trial.
“Phase II Clinical Trial” shall mean a human clinical trial of a product, the principal purpose of which is to make a preliminary determination about such pharmaceutical product’s efficacy, conducted in a manner that is generally consistent with 21 CFR § 312.21(b), as amended (or its successor regulation), or a similar clinical study prescribed by a Regulatory Authority outside the United States, and that is intended to explore one or more doses, dose response and duration of effect in the target patient population, and to generate initial evidence of clinical activity and safety in such patient population; provided that the treatment of patients for compassionate use, including in an expanded access program, single patient program or named patient program shall not be included in determining whether or not a clinical trial is a Phase II Clinical Trial or whether a patient has been dosed thereunder.
“Phase III Clinical Trial” shall mean a clinical trial in a human patient population that is sufficient to support Regulatory Approval in the proposed indication, as more fully defined in 21 C.F.R. §312.21(c), as amended (or its successor regulation) and that is designed to obtain data determining efficacy and safety of a pharmaceutical product to support such Regulatory Approval, or a similar clinical study prescribed by a Regulatory Authority outside the United States; provided, however, that the FDA permits the treatment of patients in the U.S. under an open IND in such clinical trial; and provided, further, that treatment of patients for compassionate use, including in an expanded access program, single patient program or named patient program shall not be included in determining whether or not a clinical trial is a Phase III Clinical Trial or whether a patient has been dosed thereunder.
“Primary Indication” shall mean the treatment of amyotrophic lateral sclerosis or traumatic brain injury (however such treatment is denominated in a Regulatory Approval).
“Pricing Approval” shall mean, with respect to a product, in any country or jurisdiction where the applicable governmental authority authorizes reimbursement for, or approves or determines pricing for, pharmaceutical products, receipt (or, if required to make such authorization, approval or determination effective, publication) of reimbursement authorization or pricing approval or determination (as the case may be) for such product in such country.
“Product” shall mean a product containing the KPT-350 Molecule.
“Product Registrations” shall mean all authorizations, approvals, registrations, clearances, consents, qualifications, certifications, licenses, permits and other rights from the FDA and other Regulatory Authorities that are necessary for the research, Development, clinical testing, commercialization, Manufacture, service, distribution, marketing, promotion, offer for sale, use, import, export and sale of a Royalty Product.
“Prosecute and Maintain” shall have the meaning set forth in Section 6.1(a).
“Prosecuting Party” shall have the meaning set forth in Section 6.2(b).
12
“Purchased Assets” shall have the meaning set forth in Section 2.1(a).
“Purchased Inventory” shall mean the inventory owned by Seller or any of its Affiliates of works in progress, precursors, intermediates (including any Manufacturing Intermediate), active pharmaceutical ingredients and finished goods (including any packaging) of any of the Transferred Compounds (including any Product) and all rights to market and sell such inventory.
“Purchaser” shall have the meaning set forth in the preamble of this Agreement.
“Purchaser Indemnified Party” shall have the meaning set forth in Section 7.1.
“Records” shall have the meaning set forth in Section 2.1(a)(v).
“Reduced Payment Product” shall mean a product containing the KPT-420 Molecule (unless such product also contains a KPT-350 Molecule, in which case, it shall be a Product).
“Reference Product” shall have the meaning set forth in Section 3.4(c).
“Regulatory Approval” shall mean all approvals necessary for the Manufacture, marketing, importation and sale of a product for one or more indications in a country or regulatory jurisdiction, which may include satisfaction of all applicable regulatory and notification requirements, including any pricing and reimbursement approvals. Regulatory Approvals include approvals by Regulatory Authorities of INDs.
“Regulatory Authority” shall mean the FDA, the Federal Trade Commission, the United States Department of Health and Human Services, Centers for Medicare and Medicaid Services or any other federal, state, local or foreign Governmental Entity that is concerned with or regulates the Development, testing, packaging, labeling, storage, sale, quality, safety, efficacy, reliability or Manufacturing and servicing of medical devices, federal or state health care programs, or the provision of health care or similar services.
“Regulatory Laws” shall mean the following Laws: (i) the federal Food, Drug, and Cosmetic Act, as amended, and all regulations promulgated thereunder, (ii) the federal False Claims Act (42 U.S.C. § 1320a-7b(a)), as amended, (iii) the Physician Payments Sunshine Act, (iv) the Patient Protection and Affordable Care Act, (v) the federal Medicare and Medicaid statutes, (vi) the federal Xxxx-Xxxxxxxx Xxxxxxx, 00 X.X.X. § 0000x-0x, (xxx) the federal Physician Self-Referral (Xxxxx) Law, 42 U.S.C. § 0000xx, (xxxx) the federal Civil Monetary Penalties Law, 42 U.S.C. § 1320a-7a, (ix) the Federal Trade Commission Act, (x) any other Laws governing research, Development, clinical testing, investigational use, marketing clearance, marketing approval, Manufacturing, servicing, packaging, labeling, promotion, sale, import or export of medical devices, and (xi) all Laws similar to the foregoing within any other federal, state, local or foreign jurisdiction.
“Regulatory Materials” shall mean copies of the Product Registrations and any applications therefor, including currently pending, previously denied or previously withdrawn
13
applications, together with copies of related correspondence between Seller and the applicable Regulatory Authority, and any other existing files and dossiers relating to the Product Registrations, and the underlying data or information used to support, maintain or obtain Product Registrations.
“Repeated Clinical Trial” shall have the meaning set forth in Section 3.1(a).
“Representatives” shall mean, with respect to a Party, such Party’s Affiliates and their respective members, principals, officers, directors, shareholders, trustees, employees, agents, consultants and advisors.
“Responsible Party” shall have the meaning set forth in Section 6.7(a).
“Rights Transfer Event” shall have the meaning set forth in Section 8.4(b).
“ROFR” shall have the meaning set forth in Section 3.10(b)(i).
“ROFR Window” shall have the meaning set forth in Section 3.10(b)(ii).
“Royalty Product” means a Product and/or a Reduced Payment Product.
“Schedules” shall have the meaning set forth in Section 1.2(g).
“Seller” shall have the meaning set forth in the preamble of this Agreement.
“Seller Disclosure Schedules” shall have the meaning set forth in Section 1.2(f).
“Seller Indemnification Threshold” shall have the meaning set forth in Section 7.6(b).
“Seller Indemnified Party” shall have the meaning set forth in Section 7.2.
“Seller IP” shall mean all Patents and Know-How set forth on Schedule 4.7(a)(i) and any other IP Rights, whether filed or unfiled, registered or unregistered, which are both (a) owned by Seller or an Affiliate and (b) used exclusively or held exclusively for use by Seller or an Affiliate in connection with the Exploitation of any of the Transferred Compounds (including the KPT-350 Molecule and the KPT-420 Molecule), or included within the Product Registration and Regulatory Materials included within the Purchased Assets, together with all rights to xxx or recover and retain damages, costs and attorneys’ fees for past, present and future infringement, misappropriation or other violation of any such IP Rights.
“Seller IP Action” shall have the meaning set forth in Section 6.8(a).
“Selling Affiliate” shall have the meaning set forth in Section 4.1.
“Skipped Milestone” shall have the meaning set forth in Section 3.1(a).
14
“Specified Indication” shall mean any of the following Indications: any Primary Indication, Multiple Sclerosis, Huntington’s Disease, Epilepsy, Duchenne Muscular Dystrophy, Alzheimer’s Disease, Xxxxxxxxx’x Disease and Stroke.
“Step-In Proceeding” shall have the meaning set forth in Section 6.5(b)(iii).
“Tax Return” shall mean any return, report, declaration, information return, statement or other document filed or required to be filed with any Taxing Authority in connection with the determination, assessment or collection of any Tax or the administration of any Laws relating to any Tax, including any attachment or schedule thereto and including any amendments thereof.
“Taxes” shall mean all taxes, charges, duties, fees, levies or other assessments, including income, excise, real property and personal property, sales or use, value added, profits, license, withholding (with respect to compensation or otherwise), payroll, employment, unemployment, disability, net worth, capital gains, transfer, documentary, stamp, social security, environmental, occupation, and franchise, gross receipts, premium, escheat or unclaimed property obligation, ad valorem, alternative or add-on minimum, custom duty, and estimated taxes, imposed by any Taxing Authority, and including any interest, penalties and additions attributable thereto, and all amounts payable pursuant to an agreement or arrangement with respect to taxes or payable with respect to taxes as successor or transferee.
“Taxing Authority” shall mean any Governmental Entity exercising any authority to impose, regulate or administer the imposition of Taxes.
“Term” shall have the meaning set forth in Section 3.11.
“Third Party” shall mean any Person other than Purchaser or Seller or their respective Affiliates.
“Third-Party Claim” shall have the meaning set forth in Section 7.3.
“Third-Party Claim Notice” shall have the meaning set forth in Section 7.3.
“Trademarks” shall mean any and all trademarks, service marks, trade dress, logos, slogans, trade names, all material unregistered trademarks, together with all adaptations, derivations and combinations thereof, and all goodwill associated with any of the foregoing throughout the world.
“Transfer Taxes” shall mean any federal, state, county, local, foreign or other sales, use, transfer, value added, conveyance, documentary transfer, stamp duty, recording or other similar tax, fee or charge imposed in connection with the transactions contemplated by this Agreement, provided that Transfer Taxes shall not include any income taxes, withholding taxes or any taxes imposed in connection with the recording of any sale, transfer or assignment of any IP Rights (or any interest therein) effected pursuant to this Agreement.
15
“Transferred Compounds” shall mean all compounds disclosed in or claimed by any of the IP Rights contained in the Seller IP, including the KPT-350 molecule and the KPT-420 molecule.
“Transferred IP” shall mean (i) all Seller IP (ii) all Licensed IP and (iii) all Manufacturing Know-How and Manufacturing Patents.
“United States” or “U.S.” shall mean the United States of America and its territories, commonwealths and possessions.
“Valid Claim” shall mean a claim of (a) an issued and unexpired patent, which claim has not been revoked or held unenforceable, unpatentable or invalid by a decision of a court or other governmental agency of competent jurisdiction which is not appealable or has not been appealed within the time allowed for appeal, and which has not been abandoned, disclaimed, denied or admitted to be invalid or unenforceable through reissue, re-examination, inter-partes review, post-grant review, disclaimer, opposition procedure, nullity suit, or otherwise, or (b) a patent application for a patent that has been pending less than six (6) years from the earliest date on which such patent application claims priority and which claim has not been cancelled, withdrawn, abandoned or finally rejected by an administrative agency action from which no appeal can be taken.
Section 1.2. Other Definitional Provisions.
(a) The words “hereof,” “herein,” “hereto” and “hereunder” and words of similar import, when used in this Agreement, shall refer to this Agreement as a whole and not to any particular provision of this Agreement.
(b) The terms defined in the singular shall have a comparable meaning when used in the plural, and vice versa.
(c) The terms “United States Dollars,” “dollars” and “$” shall mean lawful currency of the United States.
(d) The words “include,” “includes” and “including” and words of similar import will be by way of example rather than by limitation.
(e) Time periods based on a number of days within or following which any payment is to be made or act is to be done shall be calculated by excluding the day on which the period commences and including the day on which the period ends and, if applicable, by extending the period to the next Business Day following if the last day of the period is not a Business Day.
(f) When a reference is made in this Agreement to an Article, a Section, an Exhibit or a Schedule, such reference shall be to an Article or a Section of, or an Exhibit or a Schedule to, this Agreement unless otherwise indicated. All references herein to a Schedule or Schedules, shall be to Seller’s disclosure schedules delivered by Seller contemporaneously with the execution and delivery of this Agreement (the “Seller Disclosure Schedules”).
16
(g) All schedules and exhibits (the “Schedules” and “Exhibits,” respectively) annexed hereto or referred to herein are hereby incorporated in and made a part of this Agreement as if set forth in full herein and are an integral part of this Agreement.
ARTICLE II
PURCHASE AND SALE OF ASSETS
Section 2.1. Purchase and Sale of Assets.
(a) Upon the terms and subject to the conditions set forth herein, at the Closing, Seller shall, and shall cause any of its Affiliates who own or Control Purchased Assets to, sell, convey, assign and transfer to Purchaser, and Purchaser shall purchase, acquire and accept from Seller and its Affiliates, all of Seller and its Affiliates’ right, title and interest in, to and under the following (collectively, the “Purchased Assets”), in each case free and clear of all Encumbrances:
(i) all Contracts set forth on Schedule 2.1(a)(i) or, whether or not set forth on Schedule 2.1(a)(i), any other Contracts to which Seller or any of its Affiliates is a party used exclusively or held exclusively for use by Seller or an Affiliate in connection with the Exploitation of any Royalty Product (collectively, the “Assumed Contracts”); provided that any lease of real property, any Contracts in the nature of employee benefit plans, any Contracts evidencing indebtedness and any Contracts for general administrative services and office supplies, in each case, that are not set forth in Schedule 2.1(a)(i), shall not be included in the Assumed Contracts;
(ii) the Seller IP;
(iii) the Purchased Inventory;
(iv) the Product Registrations and Regulatory Materials set forth on Schedule 2.1(a)(iv) or, whether or not set forth on Schedule 2.1(a)(iv), all Product Registrations and Regulatory Materials used or held for use by Seller or an Affiliate exclusively in connection with the Exploitation of any Royalty Product; and
(v) all books and records to the extent exclusively relating to the other Purchased Assets or any Royalty Product (the “Records”), including correspondence with the FDA or other Regulatory Authorities, product drawings, work instructions and bills of materials, customer lists and vendor lists; provided, that Seller shall be entitled to redact from such Records any information to the extent that it is not conveyed to Purchaser hereunder.
17
Section 2.2. Excluded Assets. Purchaser acknowledges and agrees that it is not acquiring any right, title or interest in, to or under any assets, property, rights and interests of Seller or any of its Affiliates other than the Purchased Assets (such assets collectively, the “Excluded Assets”).
Section 2.3. Assumed Liabilities. Upon the terms and subject to the conditions set forth herein, Purchaser shall, effective at the Closing, assume, satisfy and thereafter discharge, all Liabilities of Seller and its Affiliates arising after the Closing under the Assumed Contracts, other than any Liability in respect of (a) any breach of an Assumed Contract prior to or at the Closing or (b) any indemnification obligation to the extent arising out of any act or omission by Seller or any Affiliate in connection with the ownership or Exploitation by Seller or an Affiliate of the Purchased Assets prior to or at the Closing (collectively, the “Assumed Liabilities”).
Section 2.4. Excluded Liabilities. Seller acknowledges and agrees that Purchaser will not assume any Liability of Seller or any of its Affiliates other than the Assumed Liabilities (such Liabilities collectively, the “Excluded Liabilities”).
Section 2.5. Licenses and Sub-Licenses.
(a) Seller hereby grants to Purchaser a worldwide, exclusive, sublicensable (through multiple tiers, subject to Section 2.5(c)), transferrable (in whole or in part), right and license to use the Manufacturing IP to make, have made, use, sell, have sold, import or have imported any Transferred Compound (including any Royalty Product). Subject to Section 3.9, the foregoing license shall be irrevocable and perpetual.
(b) To the extent any of the Manufacturing IP is Controlled by an Affiliate of Seller, then as of the Closing Date, Seller shall cause such Affiliate to take all necessary actions to give effect to the licenses granted under Section 2.5(a).
(c) Purchaser may sublicense (in whole or in part) any of the Background IP and/or Manufacturing IP (i) to any of its Affiliates or (ii) to a Third Party that Purchaser has contracted with to support Purchaser’s Development and/or commercialization of any Royalty Product, the rights granted to it by Seller under this Section 2.5 without Seller’s approval, provided that in the case of Section 2.5(c)(ii), Purchaser may not sublicense any of the Background IP or Manufacturing IP to a Third Party where such sublicense is, in Purchaser’s good faith determination, for the purpose of Developing or commercializing a product that is directly competitive with Seller’s products. All sublicense agreements shall be subject to and consistent with the applicable terms and conditions of this Agreement and shall preclude the granting of further sublicenses in contravention with the terms and conditions of this Agreement. Subject to Section 8.4, a sublicense of the Background IP or Manufacturing IP will not otherwise relieve Purchaser of any of its obligations under this Agreement.
(d) Purchaser hereby grants Seller a license to use Seller IP to support the ongoing research projects listed in Schedule 2.5(d)(i), provided that such license shall not entitle Seller or any of its Affiliates to Manufacture any Royalty Product. Within [**] days of the Closing Date,
18
Purchaser and Seller shall each negotiate in good faith with the other to agree upon a supply agreement with the terms set forth in that certain letter agreement, dated the date hereof, between the Parties and other customary terms and conditions for supply agreements of this nature.
Section 2.6. Purchase Price. As consideration for the conveyance of the Purchased Assets and subject to the terms and conditions set forth in this Agreement, Purchaser shall (i) within [**] Business Days following the Closing Date, deliver to Seller, in immediately available funds by wire transfer and in accordance with written instructions given by Seller to Purchaser reasonably in advance of the Closing, an amount equal to ten million dollars ($10,000,000) provided that Purchaser and Seller shall allocate [**] dollars ($[**]) of such amount as the sole amount to be paid in respect of the licenses granted hereunder, (ii) make the payments described in Article III, if, as and when due and payable thereunder, and (iii) assume the Assumed Liabilities.
Section 2.7. Closing.
(a) The Closing shall take place on the date hereof, simultaneously with the execution and delivery of this Agreement by the parties hereto, virtually through the exchange of signatures by email. All proceedings to take place at the Closing shall be deemed to take place simultaneously. Within [**] Business Days after the Closing Date, Seller shall deliver the Purchased Assets to Purchaser or otherwise put Purchaser in control of them.
(b) At the Closing, each of Purchaser and Seller shall, as applicable, execute and deliver to each other the Ancillary Agreements, along with such other instruments, certificates, affidavits of title as Purchaser may reasonably request or as may be otherwise necessary to evidence the sale, assignment, transfer, conveyance and delivery of the Purchased Assets to Purchaser pursuant to this Agreement and the other transactions contemplated by this Agreement and the Ancillary Agreements and to carry out the obligations of the Parties hereunder and thereunder
(c) Seller will deliver a certification, substantially in the form of Exhibit C attached hereto, conforming to the requirements of Treasury Regulations 1.1445-2(b)(2) and stating that Seller is not a “foreign person” for purposes of Section 1445 of the Code. If Seller fails to or is unable to deliver this certification on or before the Closing Date, Purchaser will be permitted to withhold or cause to be withheld from the payments to be made pursuant to this Agreement any required withholding tax under Section 1445 of the Code.
Section 2.8. Purchased Assets Not Transferred at Closing.
(a) Notwithstanding anything in this Agreement to the contrary, this Agreement shall not constitute an agreement to assign or transfer any Purchased Asset that is not assignable or transferable without the consent of any Person, other than Seller, Purchaser or any of their respective Affiliates, to the extent that such consent shall not have been given prior to the Closing. Seller shall, and shall cause its Affiliates to use their respective commercially reasonable efforts to obtain, and Purchaser shall cooperate with Seller in connection therewith,
19
all necessary consents to the assignment and transfer thereof, provided, that neither Seller nor any of its Affiliates shall be required to pay any material amount of money to any Third Party, commence any Litigation or offer or grant any accommodation (financial or otherwise) to any Third Party in connection with such efforts.
(b) With respect to any Purchased Asset that is not transferred, licensed or assigned to Purchaser at the Closing by reason of Section 2.8(a) (the “Non-Assigned Assets”), after the Closing and until any requisite consent is obtained and the foregoing is transferred and assigned to Purchaser, Seller shall, and shall cause its Affiliates to, to the extent practicable, use commercially reasonable efforts to provide to Purchaser the benefits thereof and shall assert, at the request of and for the account of Purchaser, any rights of Seller arising thereunder against any Person, including the right to elect to terminate in accordance with the terms thereof upon the request of Purchaser. To the extent that Purchaser is provided with benefits of any Non-Assigned Asset, Purchaser shall perform, at the direction of Seller, the obligations of Seller thereunder. Notwithstanding anything to the contrary set forth herein, to the extent that Seller fails to provide any benefits of any Non-Assigned Asset to Purchaser, any Assumed Liability arising from such Non-Assigned Asset shall be deemed to be an Excluded Liability until such Non-Assigned Asset is transferred and assigned to Purchaser or the benefits thereof are provided to Purchaser, at which point such Excluded Liability shall automatically become an Assumed Liability.
Section 2.9. Taxes.
(a) Any Transfer Taxes incurred in connection with this Agreement and the transactions contemplated hereby shall be [**]. Any fees or other costs imposed in connection with the recording of any sale, transfer or assignment of any IP Rights (or any interest therein) shall be [**]. Purchaser and Seller shall cooperate in timely filing all Tax Returns as may be required to comply with the provisions of such Transfer Tax laws. All property Taxes and assessments on the Purchased Assets for any taxable period commencing prior to the Closing Date and ending after the Closing Date shall be prorated on a per diem basis between Purchaser and Seller as of the Closing Date.
(b) Within [**] days after the Closing Date, Purchaser shall prepare and deliver to Seller a statement allocating the purchase price and Assumed Liabilities in accordance with the principles of Section 1060 of the Code (as finally determined pursuant to this Section 2.9(b), the “Allocation”), provided that Purchaser and Seller shall allocate [**] dollars ($[**]) of such amount as the sole amount to be paid in respect of the licenses granted hereunder with such allocation being a portion of the payment of ten million dollars ($10,000,000) due to Seller on the Closing Date as described in Section 2.6, as paid in respect of the licenses granted hereunder. Seller shall have the right to review and comment on the allocation provided by Purchaser, and the Parties shall work together in good faith to agree upon the Allocation. If the Parties are unable to reach agreement regarding the Allocation, all unresolved items will be referred to the Independent Accounting Firm for resolution, the costs of which will be [**]. The Parties each agree to (i) be bound by the Allocation, (ii) act in accordance with the Allocation in the filing of
20
all Tax Returns (including filing Form 8594 with its federal income Tax Return for the taxable year that includes the Closing Date) and (iii) take no position inconsistent with the Allocation for all Tax purposes, provided that this shall not limit a Party’s ability to settle audits or other proceedings. In the event that any Taxing Authority disputes the Allocation, Seller or Purchaser, as the case may be, shall promptly notify the other Party in writing of the nature of such dispute.
(c) Each Party shall cooperate, to the extent reasonably requested by the other Party, in connection with any Tax matters relating to the Purchased Assets (including by the provision of reasonably relevant records or information). Notwithstanding the foregoing, no Party shall have an obligation to provide any copies of its consolidated, combined or unitary Tax Returns to the other Party.
(d) Seller shall cause all Tax sharing or allocation agreements or arrangements and all powers of attorney with respect to the Purchased Assets to be terminated as of the Closing such that after the Closing Purchaser will not be bound thereby or have any liability thereunder.
Section 2.10. Transition Plan. Seller and Purchaser shall reasonably cooperate with each other to transition to Purchaser or Purchaser’s designee any ongoing research, Development and Manufacturing activities, related to any Royalty Product, including taking the actions specified in the transition plan attached hereto as Schedule 2.10, as may be updated from time to time upon written agreement of the Parties. If there is an inconsistency between the transition plan and this Agreement, then the terms of this Agreement shall prevail.
Section 2.11. Wrong Pockets.
(a) If, after the Closing, Purchaser determines that it or any of its Affiliates possesses any Excluded Asset, Purchaser shall notify Seller and, at Seller’s request, shall, or shall cause its Affiliates to, use commercially reasonable efforts to transfer such asset, at no cost, to Seller.
(b) If, after the Closing, Seller determines that it or any of its Affiliates possesses any Purchased Asset, Seller shall notify Purchaser and, at Purchaser’s request, shall, or shall cause its Affiliates to, use commercially reasonable efforts to transfer such asset, at no cost, to Purchaser.
ARTICLE III
MILESTONES, NET SALES PAYMENTS AND OTHER FINANCIAL
OBLIGATIONS
Section 3.1. Development Milestone Payments. Purchaser shall make the payments described in Table 1 below (each, a “Development Milestone Payment”) with respect to the indicated Product following achievement of the corresponding event (each, a “Development Milestone Event”) described in the row to the left of such payment in Table 1, provided that such Product is Covered by a Valid Claim of an IP Right included within the Seller IP at the time of achievement of the indicated event.
21
| Table 1 | ||||
| No. |
Development Milestone Event |
Development Milestone Payment | ||
| 1. | [**] | [**] | ||
| 2. | [**] | [**] | ||
| 3. | [**] | [**] | ||
| 4. | [**] | [**] | ||
| 5. | [**] | [**] | ||
| 6. | [**] | [**] | ||
| 7. | [**] | [**] | ||
| 8. | [**] | [**] | ||
(a) Each Development Milestone Payment shall be payable only on the first Product that achieves the corresponding Development Milestone Event. In no event shall any of the Development Milestone Payments be paid more than once (regardless of the number of times a Product achieves each Development Milestone Event, the number of Indications for which any such Product is Developed or commercialized or the number of Products that achieve a Development Milestone Event). If Purchaser shall have conducted more than one [**] or [**] (a “Repeated Clinical Trial”) related to the achievement of Development Milestone Events [**], respectively, the Development Milestone Payment due for the achievement of the subsequent Development Milestone Event immediately following the Development Milestone Event related to such Repeated Clinical Trial shall be reduced by [**] percent ([**]%) of the Development Milestone Payment previously made for the Repeated Clinical Trial. For example, if Purchaser conducts more than one [**] and has paid the Development Milestone Payment set forth for Development Milestone Event [**], then the Development Milestone Payment for Development Milestone Event [**] shall be reduced by $[**]. Subject to any reduction in payment pursuant to Section 3.4, if Development Milestone Event 4 is achieved, and Development Milestone Events 1, 2 and/or 3 was not previously achieved (a “Skipped Milestone”), the Development Milestone Payment corresponding to such Skipped Milestone shall become due and payable at the time Development Milestone Event 4 becomes due and payable.
22
(b) Purchaser shall pay to Seller the applicable Development Milestone Payment within [**] days after achievement (or, in the event of a Rights Transfer Event pursuant to Section 8.4(b) following which Purchaser remains the relevant payment party, within [**] days after Purchaser’s receipt of payment from a Third Party). Each such payment will be made by wire transfer of immediately available funds into an account designated by Seller reasonably in advance of the due date of the payment.
(c) In the event that the first [**] or [**] of a Product is conducted in a Specified Indication that is not a Primary Indication (e.g., Stroke), then [**] percent ([**]%) of the applicable Development Milestone Payment (Development Milestone Events [**]) shall be payable by Purchaser to Seller upon achievement of such Development Milestone Event, and the remaining [**] percent ([**]%) of the applicable Development Milestone Payment shall be payable at such time, if any, as the related Development Milestone Event is achieved in a Primary Indication.
Section 3.2. Commercial Milestone Payments.
(a) Purchaser shall make the payments described in Table 2 below (each, a “Commercial Milestone Payment”) with respect to a Product when worldwide Net Sales of such Product in a given Fiscal Year first exceed the indicated dollar value (each, a “Commercial Milestone Event”), provided that such Product is Covered by a Valid Claim of an IP Right included within the Seller IP at the time of achievement of the indicated event.
23
| Table 2 | ||||
| No. |
Commercial Milestone Event |
Commercial Milestone Payment | ||
| 1. | First achievement of annual worldwide aggregate Net Sales of a Product exceeding $[**] in a Fiscal Year | [**] | ||
| 2. | First achievement of annual worldwide aggregate Net Sales of a Product exceeding $[**] in a Fiscal Year | [**] | ||
(b) Each Commercial Milestone Payment is payable only once and only for the first Product that achieves the corresponding Commercial Milestone Event. In no event shall any of the Commercial Milestone Payments be paid more than once (regardless of the number of times a Product achieves the corresponding Commercial Milestone Event or the number of Products that achieve such Commercial Milestone Event). Each such Commercial Milestone Payment will be made by wire transfer of immediately available funds into an account designated by Seller reasonably in advance of the due date of the payment.
(c) Each of the Commercial Milestone Payments, if earned, are non-refundable, shall be paid only once and shall be paid within [**] days after the close of the Fiscal Year in which the corresponding Commercial Milestone Event is achieved. Notwithstanding the foregoing, if either of the Commercial Milestone Events is achieved on or after [**], then the applicable Commercial Milestone Payment shall be paid in two (2) equal installments, the first installment of which shall be paid within [**] days after the close of the Fiscal Year in which the corresponding Commercial Milestone Event was achieved, and the second installment of which shall be paid within [**] days after the close of the subsequent Fiscal Year, provided that the payment of such second installment shall be subject to the following: (i) such second installment shall be paid in full if, in such subsequent Fiscal Year, the amount of annual worldwide aggregate Net Sales of a Product is equal to or exceeds the amount that would be required to achieve such Commercial Milestone Event; (ii) if, in such subsequent Fiscal Year, the amount of annual worldwide aggregate Net Sales of a Product is less than the amount that would be required to achieve such Commercial Milestone Event but is equal to or greater than [**] percent ([**]%) of such amount, then the amount of such second installment shall be equal to the initial amount of such second installment multiplied by a fraction, the numerator of which is equal to the actual annual worldwide aggregate Net Sales of a Product in such subsequent Fiscal Year and the denominator of which is equal to the amount that would be required to achieve the applicable Commercial Milestone Event; and (iii) if, in such subsequent Fiscal Year, the annual worldwide aggregate Net Sales of a Product is less than [**] percent ([**]%) of the amount that would be required to achieve the applicable Commercial Milestone Event, then such second installment shall not be payable. For example, if Commercial Milestone Event 1 is achieved on or after [**]
24
and annual worldwide aggregate Net Sales of the applicable Product in the subsequent Fiscal Year equals $[**], the second installment of Commercial Milestone Payment 1 (which, without adjustment, would be $[**]) will be adjusted to $[**].
Section 3.3. Net Sales Payments.
(a) Net Sales Payments. Purchaser will pay to Seller net sales payments (the “Net Sales Payments”) on a Royalty Product-by-Royalty Product basis during the applicable Net Sales Payment Term at the rates (“Net Sales Payment Rates”) set forth in Table 3 below for Products and at [**]% of the Net Sales Payment Rates set forth in Table 3 below for Reduced Payment Products.
| Table 3 | ||||
| No. |
Annual Worldwide Net Sales of a Product |
Net Sales Payment Rate for Products | ||
| 1. | Portion of annual worldwide Net Sales of a Product up to and including US$[**] in a Fiscal Year | [**]% | ||
| 2. | Portion of annual worldwide Net Sales of a Product exceeding $[**] up to and including $[**] in a Fiscal Year | [**]% | ||
| 3. | Portion of annual worldwide Net Sales of a Product exceeding $[**] in a Fiscal Year | [**]% | ||
(b) Net Sales Statement. Commencing on the First Reimbursed Sale of a Royalty Product, Purchaser shall furnish to Seller a good faith estimate within [**] Business Days after the end of each calendar quarter of Net Sales of each Royalty Product in such calendar quarter and the amount of Net Sales Payment payable with respect to such Net Sales. On or prior to the [**] day following a calendar quarter, Purchaser shall deliver to Seller a written report (a “Net Sales Statement”) detailing the Net Sales Payments earned by Seller during the preceding calendar quarter. Such report will include the aggregate gross sales of each Royalty Product during such calendar quarter, the corresponding Net Sales, the Net Sales Payment Rates applied, and the amount of the Net Sales Payment payable with respect to such Net Sales. Each Net Sales Statement shall be accompanied by payment of the aggregate amount due to Seller pursuant to this Section 3.3(b) in United States Dollars by wire transfer to an account designated in writing by Seller to Purchaser reasonably in advance of the due date of the payment.
25
(c) Net Sales Payment Term. Net Sales Payments shall be payable on a country-by-country and Royalty Product-by-Royalty Product basis on Net Sales of Royalty Products from the date of the First Commercial Sale of a particular Royalty Product in an applicable country until the later of (A) the expiration of the last to expire Valid Claim of an IP Right in respect of such Royalty Product included within the Seller IP that Covers such Royalty Product in such country and (B) ten (10) years following the date of First Commercial Sale of such Royalty Product in such country (the “Net Sales Payment Term”). The Net Sales Payment Term with respect to each Royalty Product shall be determined individually with respect to such Royalty Product.
Section 3.4. Milestones and Net Sales Payment Adjustments.
(a) No Valid Claim. Subject to Section 3.4(d), on a country-by-country and Royalty Product-by-Royalty Product basis, if a Royalty Product is not Covered by a Valid Claim of an IP Right included within the Seller IP for the use or sale of such Royalty Product at any time during the Net Sales Payment Term, any Net Sales Payment otherwise payable to Seller under this Agreement from and after the date such Royalty Product ceases to be covered by such a Valid Claim shall be reduced by [**] percent ([**]%).
(b) Anti-Stacking Adjustment. Subject to Section 3.4(d), Purchaser shall have the right to deduct from any Development Milestone Payment, Commercial Milestone Payment or Net Sales Payment owed to Seller with respect to a Royalty Product, [**] percent ([**]%) of any payments that Purchaser has become obligated to make to a Third Party in consideration (including as a result of settlement or dispute resolution) for any acquisition of rights (whether by purchase, license or otherwise), or other access to, intellectual property that Purchaser reasonably determines is necessary and/or useful to Exploit any Royalty Product and that has not previously been deducted pursuant to this Section 3.4(b).
(c) Generic Competition Adjustment. Subject to Section 3.4(d), if (A) a Generic Product is introduced in a country and (B) in the then-current Fiscal Year or a later occurring Fiscal Year, revenue, sales unit volume, net selling price or market share of a Royalty Product in such country is at least [**] percent ([**]%) lower as compared to the same metric in the Fiscal Year immediately preceding the Fiscal Year in which such Generic Product was introduced, then commencing in such Fiscal Year and thereafter during the Net Sales Payment Term for the applicable Royalty Product in such country, each Development Milestone Payment, Commercial Milestone Payment or Net Sales Payment due and payable with respect to such Fiscal Year in such country with [**] percent ([**]%) lower sales shall be reduced by [**] percent ([**]%). The term “Generic Product” means, with respect to a Royalty Product (the “Reference Product”), any pharmaceutical product that (a) is sold by a Third Party that is not an Affiliate or licensee of Purchaser and (b) (i) contains the same active pharmaceutical ingredient as the Reference Product or (ii) is approved in reliance, in whole or in part, on a prior Regulatory Approval of the Reference Product.
(d) Payment Floor. The payment reductions set forth in this Section 3.4 shall be applied on a cumulative basis in the order listed above, provided that the Net Sales Payment
26
payable to Seller for any Royalty Product in a given Fiscal Year shall not be reduced by more than [**] percent ([**]%) of the amount otherwise payable to Seller as a result of the payment reductions set forth in this Section 3.4. Purchaser may carry unused adjustment credits forward to any future Development Milestone Payment, Commercial Milestone Payment or Net Sales Payment.
Section 3.5. Net Sales Audit Rights.
(a) Seller may engage, at its own cost and expense (except as otherwise provided below), subject to this Section 3.5, an Independent Accounting Firm to conduct an audit of Purchaser for the purposes of confirming Purchaser’s compliance with the Net Sales Payment provisions of this Agreement.
(b) Not earlier than [**] days following Seller’s request of an audit pursuant to this Section 3.5, Purchaser shall afford the Independent Accounting Firm access to and an opportunity to examine such books and records of Purchaser as it reasonably requests, during regular business hours, in a manner designed to avoid disruption to Purchaser’s business and subject to execution and delivery to Purchaser of a reasonable confidentiality agreement for the sole purpose of determining compliance with the Net Sales Payment provisions of this Agreement.
(c) Each of Seller and Purchaser will be entitled to receive (substantially simultaneously) a full written report of the Independent Accounting Firm with respect to its findings directly from the Independent Accounting Firm.
(d) Within [**] days after completion of the Independent Accounting Firm’s audit, Purchaser will pay to Seller any deficiency in the Net Sales Payment amount determined by the Independent Accounting Firm. If the amount of the deficiency exceeds [**] percent ([**]%) of the total Net Sales Payment made for the audited period, then Purchaser shall also pay the fees and expenses of the Independent Accounting Firm incurred in such audit. If the report of the Independent Accounting Firm shows that Purchaser overpaid, then Purchaser will be entitled to off-set such overpayment against any Development Milestone Payments, Commercial Milestone Payments or Net Sales Payments then or thereafter owed to Seller. If no such amount is then owed to Seller, then Seller will remit such overpayment to Purchaser.
(e) In the event of any dispute between Seller and Purchaser regarding the findings of an audit under this Section 3.5, the Parties will initially attempt in good faith to resolve the dispute amicably between themselves, and if the Parties are unable to resolve such dispute within [**] days after delivery to both Parties of the Independent Accounting Firm’s report, Purchaser will select, subject to Seller’s consent, such consent not to be unreasonably withheld or delayed, an internationally recognized independent certified public accounting firm (other than the Independent Accounting Firm), and such accounting firm’s determination will be binding on both Parties absent manifest error by such accounting firm.
27
(f) Seller’s exercise of its audit rights under this Section 3.5 may not (A) be conducted for any Fiscal Year more than [**] years after the end of such Fiscal Year to which such books and records pertain, (B) be conducted more than [**] period (unless a previous audit during such [**] period revealed a material underpayment with respect to such period), or (C) be repeated for any Fiscal Year.
Section 3.6. Currency Exchange. Purchaser’s then current standard exchange rate methodology will be employed for the translation of foreign currency sales into United States Dollars, provided that such methodology is used by Purchaser in the translation of its foreign currency operating results, is consistent with GAAP, and is audited by Purchaser’s independent certified public accountants in connection with the audit of the consolidated financial statements of Purchaser, and is used for Purchaser’s external reporting of foreign currency operating results.
Section 3.7. Taxes. Where required by applicable Law, Purchaser shall have the right to withhold applicable Taxes from any payments to be made by Purchaser to Seller pursuant to this Agreement; provided that, to the extent allowed by applicable Law, prior to such withholding, Purchaser shall give written notice of its intention to withhold and allow Seller sufficient time to furnish any documentation or forms to the applicable Governmental Entity to minimize or eliminate such withholding. Where applicable, Purchaser shall provide Seller with receipts from the appropriate taxing authority for all payments of Taxes withheld and paid by Purchaser to such authorities on behalf of Seller. To the extent that any such amounts are so deducted and withheld, such amounts shall be treated for all purposes of this Agreement as having been paid to Seller.
Section 3.8. Diligence.
(a) Purchaser shall itself, or through its Affiliates and licensees, use Commercially Reasonable Efforts to Develop a Product in a Primary Indication in any of the United States, United Kingdom, France, Spain, Germany or Italy.
(b) The parties acknowledge that Seller has entered into this Agreement with the anticipation of receiving the payments potentially payable pursuant to Section 3.1, Section 3.2 and Section 3.3. Seller acknowledges and agrees, however, that (i) subject to Section 3.8(a) above, Purchaser has total and absolute discretion in the Exploitation of, and operation of its business and the Development of any Product, (ii) such potential payments are contingent upon satisfaction of events that may not occur and may therefore never be paid, (iii) Purchaser has made no representation or warranty to Seller that the conditions to any such payments will be satisfied, (iv) Seller has not relied on any such statement by Purchaser or any Third Party and (v) except as set forth in Section 3.8(a) above, Purchaser is under no obligation to use any other standard of diligence with respect to satisfying the conditions to any payment hereunder, and Seller hereby waives any such potential other standard of diligence.
(c) Before signing this Agreement the Parties have had numerous conversations and have generated correspondence and other writings, in which the Parties discussed the transaction which is the subject of this Agreement and their aspirations for success. In such conversations
28
and writings, the Parties and individuals representing them may have expressed their judgments and beliefs concerning the intentions, capabilities, and practices of the Parties, and may have forecasted future events. The Parties recognize that such conversations and writings often involve an effort by both sides to be positive and optimistic about the prospects for the transaction. However, each Party acknowledges that all business transactions contain an element of risk, as do the transactions contemplated by this Agreement, and that it is normal business practice to limit the legal obligations of contracting parties to only those promises and representations which are essential to their transaction so as to provide certainty as to their respective future rights and remedies. Accordingly, other than the Confidentiality Agreement entered into between the Parties, this Agreement is intended to define the full extent of the legally enforceable undertakings of the Parties hereto, and no promise or representation, written or oral, which is not set forth explicitly in this Agreement or such letter agreement is intended by either Party to be legally binding. Each of the Parties acknowledge that in deciding to enter into this Agreement and to consummate the transaction contemplated hereby none of them has relied upon any statements or representations, written or oral, other than those explicitly set forth herein or therein.
(d) From and after the Closing and until Purchaser has filed an NDA for a Product with the FDA, Purchaser shall provide Seller [**], within [**] days following [**], with a high-level progress report.
(e) If Purchaser materially fails to comply with its obligations under Section 3.8(a), Seller shall provide written notice of the alleged material breach by the earlier of (i) [**] months of learning of such breach, or (ii) [**] months after the breach has occurred, such written notice including in reasonable detail (A) a description of the steps Purchaser took or omitted that committed a breach of its obligations under Section 3.8(a), (B) a description of the steps Seller alleges that Purchaser should have taken or omitted to take such that Purchaser would have complied, and (C) if curable, the actions Purchaser should take to cure the alleged material breach. Representatives of Seller and Purchaser with authority to resolve the dispute shall meet and discuss in good faith to resolve the dispute, and if the dispute remains unresolved after [**] days after the delivery of the written notice by Seller, Purchaser or Seller may seek a judicial determination of the question in accordance with this Agreement, provided that, notwithstanding anything to the contrary in this Agreement, Purchaser shall only be liable for a breach for which timely written notice under this Section 3.8(e) has been provided.
(f) Purchaser shall have the sole right, such right to be exercised in its absolute discretion, to sponsor any IND for any Royalty Product.
Section 3.9. Termination for Convenience.
Purchaser may, in its absolute discretion, terminate this Agreement on a Royalty Product-by-Royalty Product, country-by-country basis, at any time, for any reason or for no reason at all by delivery of written notice to Seller of its election to do so. Upon any termination with respect to any Royalty Product pursuant to this Section 3.9, at the option of the Seller, the Parties shall negotiate in good faith (i) an assignment by Purchaser to Seller of the Purchased Assets relating
29
to the Royalty Products for which Purchaser terminated this Agreement, and/or (ii) the grant by Purchaser to Seller of a license or sub-license to Exploit such Purchased Assets in the applicable country, under any IP Rights, Product Registrations and Regulatory Materials (exclusive to the extent within Purchaser’s Control to do so and non-exclusive otherwise) used or held for use by Purchaser or an Affiliate to Exploit the applicable Royalty Product in the applicable country and (iii) the financial terms relating to such assignment and license grant, provided that, if Purchaser enters into any agreement with a Third Party to transfer rights related to such terminated Royalty Product at or following such termination (and subject to compliance with the foregoing provisions of this Section 3.9) , whether by an assignment, sale, license, sublicense or otherwise, Purchaser’s obligations under this Agreement shall, with respect to such terminated Royalty Product, only terminate if assumed by such Third Party in a writing enforceable by Seller. Following a termination of this Agreement pursuant to this Section 3.9 with respect to all Products on a worldwide basis, Purchaser shall be relieved from all its obligations under Section 3.8 (subject to the preceding sentence, which shall survive any termination of this Agreement under this Section 3.9).
Section 3.10. Non-Competition. Except in accordance with the terms and conditions of this Agreement and the Ancillary Agreements and subject to applicable Law,
(a) On a Transferred Compound-by-Transferred Compound and country-by-country basis, Purchaser shall not, and Purchaser shall cause its Affiliates, licensees and assignees including any Permitted Third Party Assignees to not,
(i) for so long as a Transferred Compound is Covered by a Valid Claim of an IP Right included within the Seller IP, sell, offer for sale, or commercialize such Transferred Compound for the prevention, diagnosis or treatment of cancer; and
(ii) with respect to Japan, Republic of Korea, Republic of China (known as Taiwan), Hong Kong, Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, Philippines, Singapore, Thailand, and Vietnam, develop, use, and/or commercialize any Transferred Compound for the prevention, diagnosis or treatment of cancer, provided that, with respect to any country covered by this Section 3.10(a)(ii), if Seller shall no longer be bound by the non-competition obligations in that certain license agreement (the “Ono Agreement”) by and between Seller and Ono Pharmaceutical Co., Ltd., dated October 11, 2017, for any reason, including, but not limited to, the termination of the Ono Agreement, waiver of the non-competition obligations in their entirety or in part by Ono Pharmaceutical Co. Ltd., a judgment by any court of competent jurisdiction, or otherwise, such country shall then become subject to Section 3.10(a)(i) only. In the event that Seller is no longer bound by the non-competition obligations in the Ono Agreement with respect to any such country, Seller shall notify Purchaser within [**] days.
(b) Until the earlier of fifteen (15) years after the Closing Date or two (2) years following Regulatory Approval of a Product in any of the United States, France, Italy, Germany, Spain, the United Kingdom, or Japan, Seller shall not, and shall cause its Affiliates to not, Exploit any of their respective assets in humans or animals in connection with a Specified Indication; provided that, subject to Seller providing Purchaser with a high-level progress report within [**] days following [**], Seller and its Affiliates shall not be prohibited from Exploiting eltanexor (KPT-8602) in Duchenne Muscular Dystrophy.
30
(i) Before Seller may grant any license to any Third Party or otherwise transfer rights that permit any Third Party to Exploit eltanexor (KPT-8602) in Duchenne Muscular Dystrophy, Seller shall provide Purchaser with a right of first refusal (the “ROFR”) to obtain a license or transfer of such rights to Purchaser on the same terms proposed to such Third Party, provided that Purchaser shall be required to exercise its ROFR by providing written notice to Seller of its election to do so within [**] days of Purchaser’s receipt of the proposed economic terms between Seller and such Third Party, along with all due diligence materials made available to such Third Party. During such [**] day period pursuant to the ROFR, Seller shall provide to Purchaser such other due diligence materials as reasonably requested by Purchaser.
(ii) If Purchaser does not exercise its ROFR, then for [**] days (the “ROFR Window”) after the earlier of (A) the date that Seller receives written notice of Purchaser’s election not to exercise its ROFR or (B) the expiry of the [**] day period provided under Section 3.10(b)(i), Seller shall be permitted to execute a license or other agreement for the transfer of such rights to a Third Party, provided that the terms of such agreement are not materially more favorable to the Third Party, taken as a whole, than the terms which Purchaser reviewed pursuant to its ROFR. Purchaser’s ROFR shall (A) renew after the expiry of the ROFR Window, provided that the ROFR Window may be extended by Purchaser in its absolute discretion, and (B) apply to all transactions where Seller intends to grant the rights of eltanexor (KPT-8602) in DMD to any Third Party, subject to Section 3.10(b)(iii).
(iii) The provisions of Section 3.10(b) shall not apply to any transaction (or to the successor of Seller in such a transaction) involving a merger, sale of all or substantially all of Seller’s assets, sale of at least a majority of Seller’s stock with the intent to change ownership, or other transaction intended to be an acquisition of Seller.
(c) Upon termination of this Agreement pursuant to Section 3.9 with respect to all Purchased Assets on a worldwide basis, the non-compete obligations under this Section 3.10 shall terminate.
(d) Following the Closing Date and during the Term, unless Purchaser provides its prior written consent, Seller shall not, and shall cause its Affiliates and licensees not to, submit any Manufacturing Intermediate (including, but not limited to, the molecule presently identified as [**]) for Regulatory Approval in any Indication in any country, nor shall it market or sell any Manufacturing Intermediate for the treatment of any Indication or otherwise Exploit any Manufacturing Intermediate as a final therapeutic product for clinical use in humans in any country; provided, however, that this Section 3.10(d) shall not limit Seller’s use of any Manufacturing Intermediate to Manufacture a final therapeutic product for clinical use in humans where such final therapeutic product is Covered under the Background Patents. Seller shall not limit Purchaser’s use of any Manufacturing Intermediate to Manufacture any Royalty Product.
31
Section 3.11. Term. The term (“Term”) of this Agreement shall commence as of (and including) the Closing Date and shall ultimately expire with respect to all Royalty Products in all countries upon the date that all Net Sales Payment Terms with respect to all Royalty Products have expired. In the event of a material breach of Section 3.10 (Non-competition) or Section 6.10 (Patent Challenges by Seller of the Transferred IP) of this Agreement by Seller, the Purchaser, its Affiliates, or in the case of a Rights Transfer Event pursuant to Section 8.4(b), a Permitted Third-Party Transferee, shall have the right to (i) suspend the reporting obligations under Section 3.8(d) until such material breach is cured, if such breach is curable, and (ii) reduce any amounts due and payable to Seller, or that become due or payable, on or after the date of such material breach and the subsequent failure to cure, by [**] percent ([**]%). Any such reduction shall be in addition to any other reductions or adjustments set forth in this Agreement. Any reduction of payments under this Section 3.11 may only commence after Purchaser, its Affiliates or the respective Third Party, as the case may be, has provided written notice to Seller specifying the alleged material breach, and Seller shall have a reasonable time (of at least [**] days but not more than [**] days) to cure such breach, if such breach is curable; if such material breach is cured within such time period, no reduction in payments shall be made.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF SELLER
Except as set forth on the Seller Disclosure Schedules (it being understood that an item included on a Schedule corresponding to any Section or subsection of this Article IV shall be deemed to relate to such Section or subsection and each other Section or subsection of this Article IV to the extent such relationship is reasonably apparent from a reading of the disclosure or the applicable representation), Seller hereby represents and warrants to Purchaser as follows:
Section 4.1. Organization and Good Standing. Seller is a corporation duly organized, validly existing and in good standing under the Laws of the State of Delaware. Seller’s Affiliates who own Purchased Assets (each, a “Selling Affiliate”) are duly organized, validly existing and in good standing under the laws of their respective jurisdiction of organization. Seller and each Selling Affiliate is duly qualified or licensed to do business and is in good standing in each jurisdiction in which the nature or conduct of its business or the ownership, leasing or operation of its properties or assets requires it to be so qualified, licensed or in good standing, except where the failure to be so qualified, licensed or in good standing would not have a material adverse effect on the consummation of the transactions contemplated by this Agreement.
Section 4.2. Authority. Seller, and each Selling Affiliate, has all requisite corporate power and authority to own and operate their respective properties and assets, and to carry on its business as it is now being conducted. Seller, and each Selling Affiliate, has all requisite corporate power and authority to execute and deliver this Agreement and the Ancillary Agreements and to perform their respective obligations hereunder and thereunder. The execution and delivery by Seller and any Selling Affiliate of this Agreement and the Ancillary
32
Agreements and the performance by Seller and each Selling Affiliate of their respective obligations hereunder and thereunder have been duly authorized by all requisite corporate action on the part of Seller and such Selling Affiliate. This Agreement and each Ancillary Agreement has been duly executed and delivered by Seller and, as applicable, each Selling Affiliate, and, assuming the valid execution and delivery by Purchaser, constitute a legal, valid and binding obligation of Seller and each Selling Affiliate, as applicable, enforceable against Seller and each Selling Affiliate, as applicable, in accordance with its terms, except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is sought in a proceeding in equity or law).
Section 4.3. No Conflict. The execution, delivery and performance by Seller and each Selling Affiliate of this Agreement and each of the Ancillary Agreements and the consummation of the transactions contemplated hereby and thereby, do not and will not (a) violate any provision of the certificate of incorporation, the by-laws or limited liability company operating agreement, as applicable, of Seller or such Selling Affiliate; (b) require any material action by (including any material authorization, consent or approval) or in respect of (including notice to), any Person under any Assumed Contract; (c) violate or conflict with in any material respect, or result in a material breach of, constitute a material default under, or create material rights of acceleration, termination or cancellation under any Assumed Contract, or (d) result in the creation or imposition of a material Encumbrance upon, or the material forfeiture of, any Purchased Asset; or (e) violate, in any material respect, or result in a material breach of or constitute a material default under any Law or other restriction of any Governmental Entity to which Seller or any Selling Affiliate is subject.
Section 4.4. Required Filings and Consents. The execution and delivery of this Agreement by Seller and each Selling Affiliate, if any and as applicable, and the consummation of the transactions contemplated hereby, do not require any material consents, approvals, notices or filings with any Governmental Entities.
Section 4.5. Purchased Assets.
(a) Seller has good, valid and marketable title to all the Purchased Assets free and clear of all Encumbrances other than Permitted Encumbrances.
(b) The Purchased Assets, together with the licenses set forth in Section 2.5 and Section 6.11, comprise all material assets (other than human resources and administrative overhead) used by Seller and its Affiliates in the Exploitation of the Transferred Compounds.
Section 4.6. Contracts.
(a) Seller has delivered to Purchaser a true, correct and complete copy of each Assumed Contract, as amended. Each Assumed Contract is, in all material respects in full force and effect and is a legal, valid and binding agreement of Seller and, to the Knowledge of Seller, is a legal, valid and binding agreement of each other party thereto, enforceable against Seller and
33
each other party thereto in accordance with its terms, subject, as to enforcement of remedies, to bankruptcy, insolvency, reorganization, moratorium or similar Laws affecting the rights and remedies of creditors generally and subject to general principles of equity (regardless of whether such enforceability is considered in a proceeding at law or in equity). Seller has performed or is performing in all material respects its obligations required to be performed by it under the Assumed Contracts and is not in material breach or default thereunder, and to the Knowledge of Seller, no other party to any of the Assumed Contracts is in material breach or default thereunder, and no event has occurred which, with or without notice, lapse of time, or both, would constitute a material default under the provisions of such Assumed Contract or would give to others any material right of termination, amendment or cancellation of any Assumed Contract.
(b) Schedule 4.6(b) sets forth a list of each Assumed Contract that contains (i) a non-competition or exclusive dealing covenant, (ii) a license that would, immediately after the Closing, effect an out-license of any IP Right of Purchaser or (iii) a right of first refusal, preemptive right or other similar provision that would reasonably be expected to materially adversely affect the marketability of any Purchased Asset.
Section 4.7. Intellectual Property.
(a) Schedule 4.7(a)(i) sets forth an accurate, correct and complete list of all Seller IP. Seller owns the entire right, title and interest in, under and to the Seller IP, free and clear of any Encumbrances other than Permitted Encumbrances. Seller Controls all of the Licensed IP and either owns or Controls all of the Manufacturing IP. Schedule 4.7(a)(ii) sets forth an accurate, correct and complete list of all Patents and other registered IP included in Licensed IP. All registered Transferred IP is registered in the name of Seller and, with respect to the Licensed IP, Seller possesses the right to use the Licensed IP. On the Closing Date, Seller shall transfer to Purchaser all of its right, title, and interest in, under and to all Seller IP.
(b) To the Knowledge of Seller, the Transferred IP is valid, enforceable, subsisting and in full force and effect. With respect to registered Transferred IP, Seller has not taken or failed to take any action the taking or failure to take of which reasonably would be expected to result in the abandonment, cancellation, forfeiture, relinquishment, invalidity or unenforceability of any of such Transferred IP. With respect to any of the Transferred IP that is owned by a Third Party, to the Knowledge of Seller, such owner has not taken or has failed to take, as the case may be, any of the actions in the preceding sentence.
(c) Schedule 4.7(c) lists (i) all Assumed Contracts that restrict Seller’s use, transfer, delivery or licensing of any material Transferred IP, (ii) all Assumed Contracts involving the licensing of any material Transferred IP to or from a Third Party and (iii) all Assumed Contracts that provide any Third Party with any right, title or interest in any future IP Rights that Cover a Royalty Product (collectively, the “IP Contracts”). Except as set forth in Schedule 4.7(c), Seller has not entered into any Contract with respect to the Transferred IP requiring Seller to indemnify any Person against infringement, misappropriation or violation of any Third Party IP Rights. There are no outstanding or threatened disputes with respect to any IP Contract.
34
(d) To the Knowledge of Seller, there have been no violations of any confidentiality or assignment agreement relating to the Transferred IP or any unauthorized disclosure of any material trade secret that are included in the Transferred IP. Seller has taken commercially reasonable measures to protect and safeguard the proprietary nature of the Transferred IP and to maintain in confidence all material Confidential Information and material Know-How that are included in the Transferred IP. No material Confidential Information of Seller has been disclosed or authorized to be disclosed to any Third Party not subject to confidentiality obligations to Seller, and, to the Knowledge of Seller, no party to a nondisclosure agreement with Seller with respect to Transferred IP is in breach or default thereof.
(e) To the Knowledge of Seller, the Exploitation by Seller and its Affiliates of the KPT-350 Molecule does not, and would not reasonably be expected, following commercialization to, misappropriate, infringe, dilute or otherwise violate any Person’s IP Rights. (i) To the Knowledge of Seller, no Person has misappropriated, infringed, diluted, or otherwise violated, either directly or indirectly, any material Transferred IP (ii) neither Seller nor any Affiliate has brought or threatened to bring any claim, suit or proceeding against any Person alleging any such misappropriation, infringement, dilution or violation, and (iii) to the Knowledge of Seller, neither of the foregoing clauses (i) or (ii) have been asserted in any cease and desist letter or other written notice, including in the nature of offering a license or covenant not to xxx, in each case, relating to Transferred IP.
(f) Neither Seller nor any Affiliate has been notified of any proceedings before the United States Patent and Trademark Office (USPTO) or the European Patent Office (EPO), and there are no proceedings pending before any other Governmental Entity anywhere in the world related to any of the Transferred IP. Seller is not undertaking any interference, reissue, reexamination, opposition, proceeding, or other post-grant proceeding with respect to IP Rights of any third Person that Cover any Royalty Product.
(g) There has not been any claim, suit or proceeding asserted or threatened in writing, including in the form of an offer or invitation to obtain a license, against Seller or any Affiliate relating to the Transferred IP (i) alleging misappropriation, infringement, dilution or other violation of any Person’s IP Rights, (ii) challenging Seller’s or any Affiliate’s, as applicable, ownership of, right, title or interest in, under or to, use of, or the registrability or maintenance of, any Transferred IP, (iii) adversely affecting the ownership rights of Seller or any Affiliate in, under or to any Seller IP, (iv) challenging the scope, duration, validity or enforceability of any Transferred IP, and, to the Knowledge of Seller, there is no basis for any such claim, suit or proceeding, (v) alleging that Seller or any Affiliate is in breach of any applicable grant, license, agreement, instrument or other arrangement pursuant to which Seller or any Affiliate acquired the right to use such Transferred IP, or (vi) alleging misuse or antitrust violations arising from the use or other Exploitation by Seller or any Affiliate of any Transferred IP, in each case, that would result in material liability to Seller or any Affiliate. To the Knowledge of Seller, no material Transferred IP has been, or is being, used or enforced by Seller or any Affiliate in a manner that, individually or in the aggregate, is reasonably likely to result in the cancellation, invalidity or unenforceability of such material Transferred IP.
35
(h) Neither Seller nor any Affiliate has granted any Person, and except as expressly set forth in an Assumed Contract, any right to control the prosecution or registration of any Transferred IP or to bring, defend, or otherwise control any Litigation with respect to any Transferred IP. Neither Seller nor any Affiliate has entered into, or is subject to, any consents, indemnifications, forbearances to xxx, licenses or other arrangements in connection with the resolution of any disputes or Litigation that (i) restrict Seller or an Affiliate with respect to the use, registration or maintenance of any Transferred IP or (ii) permit any Person to use any Transferred IP (other than the Manufacturing Know-How and Manufacturing Patents).
(i) No funding, facilities or personnel of any Governmental Entity were used, directly or indirectly, to Develop or create, in whole or in part, any Transferred IP. No current or former partner, director, stockholder, officer, contractor or employee of Seller or of any Affiliate will, after giving effect to the transactions contemplated by this Agreement, own or retain any rights to use any of the Transferred IP, and no royalty or other payment is payable, or will become payable, to any such Person or to a Third Party for the use of any of the Transferred IP.
(j) All Patents, Trademarks, trade names, service marks, trade dress and domain names contained in the Transferred IP, and all IP Rights to any inventions claimed or disclosed therein, have been properly assigned to Seller, and all such assignments have been properly recorded in the USPTO, the European Patent Office or any other patent or trademark office, to the extent such patent or trademark office requires or permits such recording.
(k) All fees required to be paid by Seller in any jurisdiction in order to maintain the IP Rights included in the Transferred IP and, to the Knowledge of Seller, the Licensed IP, due and payable prior to the date hereof or within the [**] days following the date hereof have been timely paid.
(l) Except as set forth on Schedule 4.7(l), the Transferred IP has not been created pursuant to, and is not subject to, any funding agreement with any government or government agency or any Third Party, and is not subject to the requirements of the Xxxx-Xxxx Act or any similar provision of any applicable law. With respect to any IP Right set forth on Schedule 4.7(l) that is subject to the requirements of the Xxxx-Xxxx Act or any similar provision of any applicable law, to the Knowledge of Seller, the prosecution and maintenance of such IP Right has been conducted in all respects in compliance with such requirements.
Section 4.8. Inventory. All of the Purchased Inventory is set forth on Schedule 4.8 and (a) meets, and was Manufactured in accordance with its specifications and all applicable legal and regulatory requirements (including cGMP where applicable) in all material respects, (b) has a shelf life in accordance with the aging schedule set forth in Schedule 4.8, and (c) is free from contamination, dilutents and defects in materials and workmanship and (d) is not adulterated in any material respect within the meaning of the United States Federal Food, Drug and Cosmetics Act.
36
Section 4.9. Compliance with Laws; Regulatory Compliance.
(a) The Exploitation by Seller and its Affiliates of any Royalty Product and the ownership by Seller and its Affiliates of the Purchased Assets have been, since January 1, 2015, in material compliance with all applicable Law, including Regulatory Laws. Neither Seller nor any Affiliate has received any written communication from a Governmental Entity that alleges that Seller or any Affiliate is not in compliance in any material respect with any Law or Order with respect to their respective Exploitation of any Royalty Product or ownership of the Purchased Assets. Seller and its Affiliates have and have had, since January 1, 2015, all material Product Registrations necessary for its lawful Exploitation of any Royalty Product, and each of such Product Registrations, if any, is valid and in full force and effect.
(b) There has not been any material claim or investigation of which Seller or any Affiliate has received notice, suit, action, indictment, administrative proceeding, arbitration, alternative dispute resolution or other similar proceeding (“Litigation”) pending or, to the Knowledge of Seller, threatened against Seller or an Affiliate relating to any Royalty Product or the Purchased Assets. There is no Litigation pending by Seller or any Affiliate, or which Seller intends to initiate against any Third Party, in each case relating to any Royalty Product or the Purchased Assets.
(c) Seller and its Affiliates have filed all material reports, statements, documents, registrations, filings, amendments, supplements and submissions required to be filed by it with respect to any Royalty Product and the Purchased Assets under applicable Regulatory Laws, and such filings have been timely in all material respects. Each such filing was, in all material respects, true, complete and correct as of the date of submission, and any material and legally necessary or required updates, changes, corrections, amendments, supplements or modifications to such filings have been submitted to the applicable Governmental Entity.
(d) Neither Seller nor any Affiliate has, regarding or related to any Royalty Product or the Purchased Assets, (i) received notice of or, to the Knowledge of Seller, been subject to any action, notice, warning, administrative proceeding, review or investigation by a Regulatory Authority that alleges or asserts that Seller or any Affiliate has violated any applicable Regulatory Laws in any material respect or (ii) been subject to a corporate integrity agreement, deferred prosecution agreement, consent decree, monitoring agreement, settlement agreement or other similar agreement or Order mandating or prohibiting future or past activities.
(e) The Manufacturing and servicing operations conducted by or on behalf of Seller and its Affiliates with respect to any Royalty Product are conducted in material compliance with applicable Regulatory Laws, including the provisions of the FDA’s current good manufacturing practice regulations at 21 C.F.R. Part 820 and similar federal, state, local or foreign requirements for the Manufacture of any Royalty Product.
(f) Neither Seller, nor any Affiliate, nor any officer, employee or agent of Seller or of any Affiliate, in each case who has been materially involved in the Exploitation of any Royalty Product (i) has been convicted of any crime or engaged in any conduct in the operation of
37
Seller’s business for which debarment is mandated or authorized by 21 U.S.C. § 335a or any similar applicable Law, nor has any such Person been so debarred; (ii) has been convicted of any crime or engaged in any conduct which would reasonably be expected to cause Seller to be excluded from participating in federal health care programs under Section 1128 of the Social Security Act of 1935, as amended, or any similar applicable Law or been so excluded; or (iii) is subject to an investigation or proceeding by any Regulatory Authority that could result in such a suspension, exclusion or debarment.
(g) Neither Seller nor any Affiliate has, in connection with its Exploitation of a Royalty Product, conducted any material business or engaged in any material transaction with any Person with whom transactions were, at the time of such transaction, prohibited as to U.S. Persons by any applicable sanctions Laws administered by the U.S. Treasury Department’s Office of Foreign Assets Control (“OFAC”), including persons appearing on the List of Specially Designated Nationals and Blocked Persons published by OFAC.
Section 4.10. Brokers. No broker, finder or investment banker is entitled to any brokerage, finders or other fee or commission in connection with the transactions contemplated by this Agreement based upon arrangements made by or on behalf of Seller.
Section 4.11. Non-Reliance by Seller. Seller acknowledges, agrees, represents and warrants that none of Purchaser, its Affiliates, agents or Representatives or any other Person acting on behalf of or in concert with Purchaser, has made or provided any representations, warranties, facts or information of any kind or nature, express or implied, oral or written, relating to Purchaser or otherwise relating in any way to the subject matter of this Agreement, except as specifically set forth in Article V. Seller also acknowledges, agrees, represents and warrants that it is not relying, and has not relied, on any representations, warranties, facts or information whatsoever except as specifically set forth in Article V, in its decision to enter into this Agreement and to proceed with the transactions contemplated by this Agreement.
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF PURCHASER
Purchaser hereby represents and warrants to Seller as follows:
Section 5.1. Organization and Good Standing. Purchaser is a corporation duly organized, validly existing and in good standing under the Laws of the Commonwealth of Massachusetts. Purchaser is duly qualified or licensed to do business and is in good standing in each jurisdiction in which the nature or conduct of its business or the ownership, leasing or operation of its properties and other assets requires it to be so qualified, licensed or in good standing.
Section 5.2. Authority. Purchaser has all requisite corporate power and authority to own and operate its properties and assets, to carry on its business as it is now being conducted and to execute and deliver this Agreement and the Ancillary Agreements and to perform its
38
obligations hereunder and thereunder. The execution and delivery by Purchaser of this Agreement and the Ancillary Agreements and the performance by Purchaser of its obligations hereunder and thereunder have been duly authorized by all requisite corporate action on the part of Purchaser and no additional authorization on the part of Purchaser is necessary in connection with the execution, delivery and performance of this Agreement or of the Ancillary Agreements. This Agreement and each Ancillary Agreement to be executed on the date hereof has been, and each other Ancillary Agreement to be executed on the Closing Date will be, duly executed and delivered by Purchaser and, assuming the valid execution and delivery by Seller, constitute a legal, valid and binding obligation of Purchaser, enforceable against Purchaser in accordance with its terms, except as enforcement may be limited by bankruptcy, insolvency, reorganization, fraudulent conveyance, moratorium or similar Laws affecting creditors’ rights generally or by general principles of equity (regardless of whether enforcement is sought in a proceeding in equity or law).
Section 5.3. No Conflict. The execution, delivery and performance of this Agreement and each of the Ancillary Agreements by Purchaser and the consummation of the transactions contemplated hereby and thereby, do not and will not (a) violate any provision of the certificate of incorporation or bylaws of Purchaser, (b) violate or conflict with in any respect, or result in a breach of, constitute a default under, or create rights of acceleration, termination or cancellation under, or to a loss of any benefit to which Purchaser or any of its Affiliates is entitled under, any agreement, to which Purchaser or any of its Affiliates is a party or to which its properties or assets are subject where any of the listed items, individually or in the aggregate, would reasonably be expected to prevent or materially delay the consummation of the transactions contemplated by this Agreement or (c) violate, in any material respect, or result in a material breach of or constitute a material default under any Law or other restriction of any Governmental Entity to which Purchaser is subject.
Section 5.4. Required Filings and Consents. The execution and delivery of this Agreement by Purchaser and the consummation of the transactions contemplated hereby, do not require any consents, approvals, notices and filings.
Section 5.5. Litigation. There is no Litigation pending or threatened against Purchaser which, individually or in the aggregate, would reasonably be expected to prevent or materially delay or interfere with the consummation of the transactions contemplated by this Agreement. There are no Orders of any Governmental Entity or arbitrator outstanding against or investigation by any Governmental Entity involving Purchaser or any of its assets which, individually or in the aggregate, would reasonably be expected to prevent or materially delay or interfere with the consummation of the transactions contemplated by this Agreement.
Section 5.6. Sufficient Funds. Purchaser has, and, at Closing, will have, sufficient funds available as and when needed to consummate the transactions contemplated hereby and to perform its obligations hereunder.
Section 5.7. Brokers. No broker, finder or investment banker is entitled to any brokerage, finders or other fee or commission in connection with the transactions contemplated by this Agreement based upon arrangements made by or on behalf of Purchaser or any of its Affiliates.
39
Section 5.8. Non-Reliance by Purchaser. Purchaser acknowledges, agrees, represents and warrants that none of Seller, its Affiliates, agents or Representatives or any other Person acting on behalf of or in concert with Seller, has made or provided any representations, warranties, facts or information of any kind or nature, express or implied, oral or written, relating to Seller, any Royalty Product or the Purchased Assets, or otherwise relating in any way to the subject matter of this Agreement, except as specifically set forth in Article IV. Purchaser also acknowledges, agrees, represents and warrants that it is not relying, and has not relied, on any representations, warranties, facts or information whatsoever except as specifically set forth in Article IV, in its decision to enter into this Agreement and to proceed with the transactions contemplated by this Agreement.
ARTICLE VI
INTELLECTUAL PROPERTY; KNOW-HOW TRANSFER
Section 6.1. Prosecution and Maintenance.
(a) Seller IP. Purchaser shall have the sole right, responsibility and discretion to file, prosecute (including the defense of any oppositions, interferences, reissue proceedings, re-examinations and other post-grant proceedings originating in a patent office, including the filing of any patent term extensions) and maintain Patents included in the Seller IP at its sole cost and expense (“Prosecute and Maintain”), provided, however, that for any Royalty Product that Purchaser commercializes, Purchaser shall use commercially reasonable efforts to Prosecute and Maintain IP Rights Covering such Royalty Product.
(b) Manufacturing Patents. Seller shall have the first right, responsibility and discretion to Prosecute and Maintain the Manufacturing Patents at its sole cost and expense. If Seller determines to abandon or not to Prosecute and Maintain any Manufacturing Patent in any country or jurisdiction, then Seller will provide Purchaser with written notice promptly after any such determination to allow Purchaser a reasonable period of time to determine, on a country-by-country basis in its sole discretion, its interest in Prosecuting and Maintaining such Manufacturing Patent (which notice by Seller will be given at least [**] days prior to the abandonment of Seller’s Prosecution and Maintenance of such Manufacturing Patent by Seller). If Purchaser provides written notice to Seller expressing its interest in maintaining such Manufacturing Patent then, with respect to such Manufacturing Patent in such country, (i) Purchaser may, in its sole discretion and at Purchaser’s cost and expense, Prosecute and Maintain or abandon such Manufacturing Patent; (ii) Seller will promptly provide to Purchaser or counsel designated by Purchaser all files related to Prosecuting and Maintaining such Manufacturing Patent; and (iii) Seller will provide to Purchaser a report detailing the status of all Manufacturing Patents that Seller is Prosecuting and Maintaining as of the applicable date of such notice by Seller.
40
Section 6.2. Cooperation.
(a) Seller IP. Seller agrees to cooperate with Purchaser with respect to Purchaser’s efforts to Prosecute and Maintain, and enforce the Seller IP, and to execute any documents necessary or desirable in connection with the Prosecution and Maintenance, and enforcement of the Seller IP or to secure and perfect any of Purchaser’s rights in the Seller IP.
(b) Manufacturing Patents. The Party that is not responsible for the Prosecution and Maintenance of the Manufacturing Patents (the “Non-Prosecuting Party”) agrees to cooperate with the Party that is responsible for the Prosecution and Maintenance of the Manufacturing Patents (the “Prosecuting Party”) with respect to the Prosecuting Party’s efforts to Prosecute and Maintain, and enforce, the Manufacturing Patents, and provide additional information and execute any documents necessary or desirable in connection with the Prosecution and Maintenance, and enforcement, of the Manufacturing Patents. Without limiting the generality of the foregoing, with respect to the Manufacturing Patents, the Prosecuting Party shall (i) provide to the Non-Prosecuting Party copies of all correspondence with patent authorities in a jurisdiction; (ii) provide to the Non-Prosecuting Party copies of all material correspondence with patent counsel for such jurisdiction; (iii) provide to the Non-Prosecuting Party copies of, and reasonable time (but in no event less than [**] days, except where such document or submission to such patent authority must be provided in fewer than [**] days under requirements of such patent authority, in which case no less than [**] days) to review and comment upon any documents intended for submission to any patent authority in a jurisdiction, including any patent applications, prior to submission; (iv) furnish to the Non-Prosecuting Party copies of documents related to any filing, prosecution, and maintenance of such Manufacturing Patents; (v) provide the Non-Prosecuting Party copies of each patent application as filed, together with notice of its filing date and serial number; (vi) incorporate in good faith all comments and requests of the Non-Prosecuting Party on documents to be filed with any patent authority in a jurisdiction that would affect such Non-Prosecuting Party’s rights in such Manufacturing Patent hereunder unless the Prosecuting Party reasonably determines in good faith that such comments or requests would adversely affect such Prosecuting Party’s rights hereunder; and (vii) keep the other Party reasonably informed in writing of progress regarding such Manufacturing Patents.
Section 6.3. Defense of Claims Brought by Third Parties.
(a) If Seller becomes aware of any actual or potential claim that the Exploitation of any Royalty Product infringes the intellectual property rights of any Third Party, then Seller shall promptly notify Purchaser and shall share with Purchaser all information available to it with respect to such alleged infringement. If Purchaser becomes aware of any actual or potential claim that the Manufacture of any Royalty Product infringes the intellectual property rights of any Third Party, then Purchaser shall promptly notify Seller and shall share with Seller all information available to it with respect to such alleged infringement. Purchaser shall have the sole right, but not the obligation, to defend and dispose (including through settlement or license) such claim.
41
(b) The costs and expenses incurred in connection with defense of any claim described in Section 6.3(a) shall be borne by Purchaser, provided that Purchaser shall not be responsible for any costs incurred by Seller, including any internal costs or attorneys’ fees, unless agreed to in writing by Purchaser in advance of the incurrence of any such costs.
(c) Subject to the terms and conditions of this Agreement, in connection with any of the rights or obligations under Section 6.1 to Section 6.8, neither Purchaser, nor any of its Affiliates, employees, agents or Representatives, will be liable to Seller in respect of any act, omission, default or neglect on the part of itself or any such Affiliate, employee, agent or representative in connection with activities undertaken in good faith.
Section 6.4. Notice of Infringement and Paragraph IV type Notices.
(a) If Seller learns of an infringement or threatened infringement by a Third Party (i) of any Patents included in the Seller IP or (ii) of any Manufacturing Patents or Licensed IP involving the Exploitation of compounds or products that are substantially the same as or otherwise competitive with any Royalty Product, in each case ((i) or (ii)) including actual or alleged infringement under 35 USC § 271(e)(1) (each, a “Competitive Infringement”), then Seller shall promptly notify Purchaser and shall provide Purchaser with available evidence of such Competitive Infringement. For any Competitive Infringement, Seller shall share with Purchaser all information available to it regarding such alleged infringement.
(b) Notwithstanding any provision of this Agreement to the contrary, Seller shall immediately (but in no event later than [**] following receipt or discovery, whichever occurs first) give written notice to Purchaser of any certification of which it becomes aware filed pursuant to any statutory or regulatory requirement in any country similar to 21 U.S.C. § 355(b)(2)(A)(iv) or § 355(j)(2)(A)(vii)(IV) (or any amendment or successor statute thereto) claiming that the Transferred IP claiming or Covering any Royalty Product is invalid or that infringement will not arise from the Exploitation of such Royalty Product by a Third Party. Upon the giving or receipt of such notice, Purchaser will have the sole right, but not the obligation, to bring an infringement action against such Third Party. Seller shall, upon Purchaser’s request, cooperate with Purchaser in any such action and shall, upon Purchaser’s request, timely commence or join in any such action, including but not limited to, to establish standing in connection with any action brought by Purchaser under this Section 6.4(b)
Section 6.5. Enforcement of IP.
(a) Seller IP. Purchaser shall have the exclusive right, but not the obligation, to institute, prosecute, and control any action or proceeding with respect to any Competitive Infringement of any Royalty Product by counsel of its own choice, in Purchaser’s own name and under Purchaser’s direction and control. The foregoing right of Purchaser shall include the right to perform all actions of a Reference Product sponsor set forth in the U.S. Xxxxx-Xxxxxx Act or Public Health Service Act, and any equivalent of such laws in a foreign jurisdiction.
42
(b) Manufacturing IP.
(i) Seller shall have the first right, but not the obligation, to institute, prosecute, and control any action or proceeding with respect to any Competitive Infringement of any Royalty Product with respect to any Manufacturing Patent using counsel of its own choice, in Seller’s own name and under Seller’s direction and control; provided that the foregoing right of Seller shall not include the right to perform any actions of a Reference Product sponsor set forth in the U.S. Xxxxx-Xxxxxx Act or Public Health Service Act, or any equivalent of such laws in a foreign jurisdiction.
(ii) Seller will have a period of [**] days after its receipt of notice and evidence or receipt of written notice from Purchaser with respect to any Competitive Infringement with respect to any Manufacturing Patent (a “Manufacturing Enforcement Proceeding”), to elect to so enforce such Manufacturing Patents in the applicable jurisdiction (or to settle or otherwise secure the abatement of such Competitive Infringement), provided, however, that such period will be:
(A) More than [**] days to the extent applicable law prevents earlier enforcement of such Manufacturing Patents.
(B) Less than [**] days to the extent that a delay in bringing such Manufacturing Enforcement Proceeding against such alleged Third Party infringer would limit or compromise the remedies (including monetary relief, and stay of Regulatory Approval) available against such alleged Third Party infringer.
(iii) If Seller does not so elect (or settle or otherwise secure the abatement of such Competitive Infringement) before the first to occur of (A) the expiration of the applicable period of time set forth above in Section 6.5(b)(ii)(A) or Section 6.5(b)(ii)(B), as applicable, or (B) [**] days before the expiration of any time period under applicable law, that would, if a Manufacturing Enforcement Proceeding was not filed within such time period, limit or compromise the remedies available from such Manufacturing Enforcement Proceeding, it shall so notify Purchaser in writing. In such event, if Purchaser desires to commence a suit or take action to enforce the applicable Manufacturing Patents with respect to such Competitive Infringement in the applicable jurisdiction, Purchaser will thereafter have the right to commence such a suit or take such action to enforce the applicable Manufacturing Patents (such action, a “Step-In Proceeding”), at Purchaser’s cost and expense, provided, however, that Purchaser agrees to work with Seller to minimize the risk to claims which cover subject matter which is relevant outside of the Royalty Products.
(iv) If Seller elects to institute, prosecute or control any action or proceeding under this Section 6.5(b), Seller shall provide Purchaser with a reasonable opportunity to review and comment on any documents, including any legal briefs or motions, that will be filed with or provided to any court, arbitrator, Governmental Entity or other tribunal, including the United States Patent and Trademark Office, the European Patent Office and the United States International Trade Commission, prior to the filing with or provision of such document to the applicable tribunal.
43
Section 6.6. Settlement.
(a) Purchaser may, in its absolute discretion, enter into any settlement, consent judgment or other voluntary final disposition of a suit with respect to the Seller IP provided, however, that any such settlement, consent judgment or other disposition of any action or proceeding by a Purchaser, shall not on its terms, directly impose any liability or obligation on Seller without Seller’s prior written consent.
(b) With respect to the Licensed IP and Manufacturing IP, any settlement, consent judgment or other voluntary final disposition of a suit under this ARTICLE VI, may be entered into without the consent of the Party not bringing suit; provided, however, that any such settlement, consent judgment or other disposition of any action or proceeding by a Party, shall not, without the consent of the Party not bringing suit, (a) impose any liability or obligation on the Party not bringing suit, (b) include the grant of any license, covenant or other rights to any Third Party that would conflict with or reduce the scope of the subject matter included under this Agreement, (c) conflict with or reduce the scope of the subject matter claimed in any Transferred IP, or (d) adversely affect the interest of the Party not bringing suit in any material respect or impair its rights under this Agreement, provided that such consent shall not be unreasonably withheld or delayed.
Section 6.7. Costs and Recoveries. Each Party shall bear all of its own internal costs incurred in connection with its activities under Section 6.1 to Section 6.8. If a Party commences a Manufacturing Enforcement Proceeding or a Step-In Proceeding, then it shall bear all external costs and expenses for such action. Any damages or other monetary awards recovered in any action, suit or proceeding under Section 6.1 to Section 6.8 shall be shared as follows:
(a) Such damages or other sums recovered shall be applied to all out-of-pocket costs and expenses incurred by the Party responsible for any activities under Section 6.1 to Section 6.8 (the “Responsible Party” and the other Party, the “Non-Responsible Party”) directly in connection with such action (including expenses of outside counsel), and if agreed to in writing by the Responsible Party, to the reasonable actual and documented out-of-pocket costs and expenses incurred by the Non-Responsible Party. If such recovery is insufficient to Cover all such costs and expenses incurred by the Responsible Party and the agreed-upon costs and expenses incurred by the Non-Responsible Party, then the recovery shall be shared in proportion to the total of such costs and expenses incurred or agreed-upon, as the case may be.
(b) Any remaining proceeds shall be allocated such that Seller shall receive an amount calculated by applying the applicable Net Sales Payment Rates to such proceeds as of the Fiscal Year that the Responsible Party received the proceeds, provided that such proceeds allocated to Seller shall not be included toward the calculation of the achievement of any Commercial Milestone Event.
44
Section 6.8. Other Actions by Third Parties.
(a) Notice. Seller shall promptly notify Purchaser in the event of any legal or administrative action by any Third Party involving any Seller IP of which it becomes aware, including any nullity, revocation, interference, reexamination or compulsory license proceeding (“Seller IP Action”). Each Party shall promptly notify the other Party in the event of any legal or administrative action by any Third Party involving any Licensed IP or Manufacturing IP of which it becomes aware, including any nullity, revocation, interference, reexamination or compulsory license proceeding (“Licensed or Manufacturing IP Action”).
(b) Seller IP. Purchaser shall have the sole right, but not the obligation, to defend against any Seller IP Action involving any Royalty Product, in its own name (to the extent permitted by applicable law), and any such defense will be at Purchaser’s expense. Seller, upon Purchaser’s request, agrees to join in any such Seller IP Action at Purchaser’s expense and in any event to cooperate with Purchaser at Purchaser’s expense.
(c) Licensed IP and Manufacturing IP.
(i) Seller shall have the first right, but not the obligation, to defend against any Licensed or Manufacturing IP Action involving any Royalty Product, in its own name (to the extent permitted by applicable law), and any such defense will be at Seller’s expense. Purchaser, upon Seller’s request, agrees to cooperate with Seller at Seller’s expense. If Seller fails to defend against any Licensed or Manufacturing IP Action involving a Royalty Product, then Purchaser will have the right to defend such action, in its own name, and any such defense shall be at Purchaser’s expense. In such event, Seller, upon Purchaser’s request, agrees to join in any such Licensed or Manufacturing IP Action at Purchaser’s expense and in any event to cooperate with Purchaser at Purchaser’s expense.
(ii) If Seller defends any Licensed or Manufacturing IP Action under this Section 6.8(c), Seller shall provide Purchaser with a reasonable opportunity to review and comment on any documents, including any legal briefs or motions, that will be filed with or provided to any court, arbitrator, Governmental Entity or other tribunal, including the United States Patent and Trademark Office, the European Patent Office and the United States International Trade Commission, prior to the filing with or provision of such document to the applicable tribunal.
Section 6.9. References to Seller IP. In connection with any activities by Seller, any of its Affiliates or licensees to Prosecute and Maintain, enforce or defend any IP Rights (including, but not limited to, any IP Rights that disclose or claim KPT-330 and KPT-335) Covered under the Background IP, including by instituting, prosecuting, controlling, or defending any action or proceeding in any country, Seller shall (i) promptly notify Purchaser in writing prior to including any language that refers to, relates to, makes an argument based on, describes, discloses, cites to or otherwise mentions any of the Transferred IP, (ii) provide Purchaser with a reasonable opportunity to comment on any such language relating to the Transferred IP and (iii) in the case of language relating to the Seller IP, obtain Purchaser’s prior written approval regarding the language relating to the Seller IP, such approval not to be unreasonably withheld or delayed.
45
Section 6.10. Patent Challenges by Seller of the Transferred IP. If Seller or any of its Affiliates Challenges (or assists or enables any Third Party in Challenging) an IP Right under the Seller IP in any country (such IP Right, a “Challenged IP Right”), then Purchaser may, in its sole discretion, take any or all of the following actions: (a) assign or license such Challenged IP Right to Seller, (b) withhold all payment obligations under this Agreement that become due after the date that Seller or Affiliate Challenges the Challenged IP Right until the validity or enforceability of such Challenged IP Right has been finally judicially determined, and (c) reduce each of the Development Milestone Payments, Commercial Milestone Payments and Net Sales Payment Rates payable under this Agreement by [**] percent ([**]%), in each case (a) to (c), by providing written notice to Seller, and, if Purchaser so chooses, suing Seller for infringement in any forum of competent jurisdiction of Purchaser’s choosing, provided further that Purchaser may reduce its payments to Seller in accordance with Section 3.11.
Section 6.11. Unblocking License. Seller, on behalf of itself and its Affiliates, hereby grants to Purchaser and its Affiliates a perpetual, non-exclusive, royalty-free, fully paid-up, worldwide license (with the right to grant sublicenses through multiple tiers) under the Background IP that is necessary or useful to Exploit all Royalty Products and the Purchased Assets. Subject to Section 2.5(c), this license is assignable or sub-licensable through multiple tiers, in whole or in part, to any Third Party in a Rights Transfer Event pursuant to Section 8.4(b).
Section 6.12. Know-How Transfer. To enable Purchaser to Exploit Royalty Products, Seller shall, as promptly as reasonably practicable, enable Purchaser, and/or, at Purchaser’s election, contract manufacturers or other service providers to Purchaser, with respect to the Transferred IP. Without limiting the generality of the foregoing, as described with additional specificity on Schedule 6.12, Seller will deliver to or as directed by Purchaser all books and records included in the Purchased Assets and all copies of Regulatory Materials, documents, files, diagrams, specifications, designs, schematics, reports, records, laboratory notebooks, data, materials, prototypes, test devices, models and simulations, or other written, graphic, biologic, or other tangible material in the possession or under the control of Seller or any Affiliate in any media, to the extent it discloses or embodies Transferred IP or Manufacturing IP. After the Closing Date, as requested by Purchaser from time to time, qualified personnel from Seller or its Affiliates familiar with the books and records included within the Purchased Assets, the Transferred IP and/or the Manufacturing IP will meet or participate in telephone conference calls with personnel from Purchaser or Purchaser’s designee at such times, and in the case of in-person meetings, at such venues, to be agreed upon by the Parties as reasonably necessary to exchange knowledge necessary to fully transfer all of the Purchased Assets, Licensed IP and Manufacturing IP and to enable Purchaser or its designee to Manufacture all Transferred Compounds. Seller shall designate a transition manager to act as the primary contact person with respect to all chemistry, Manufacturing and control (CMC) and Manufacturing-related matters and a transition manager to act as the primary contact person with respect to all other matters
46
relating to this Section 6.12. Purchaser shall designate one transition manager to act as the primary contact person with respect to all matters relating to this Section 6.12. The names and contact information of each Party’s initial transition managers are set forth in Schedule 6.12. Each Party may replace its transition manager at any time upon written notice to the other Party.
ARTICLE VII
INDEMNIFICATION
Section 7.1. Indemnification by Seller. Seller shall defend, indemnify and hold harmless Purchaser and its Affiliates and, if applicable, their respective directors and officers, successors and permitted assigns (a “Purchaser Indemnified Party”), from and against any and all damages, losses, Liabilities or other amounts payable to a Third Party claimant, as well as any reasonable attorneys’ fees and costs of Litigation (collectively, the “Losses”) incurred by such Purchaser Indemnified Party to the extent arising from:
(a) any breach of or inaccuracy in any representation or warranty of Seller set forth in this Agreement (ignoring for this purpose any materiality qualifiers set forth in such representation or warranty);
(b) any nonfulfillment or breach of any covenant or agreement on the part of Seller set forth in this Agreement; and
(c) any Excluded Liability.
Section 7.2. Indemnification by Purchaser. Purchaser agrees to defend, indemnify and hold harmless Seller and its Affiliates and, if applicable, their respective directors and officers, successors and permitted assigns (a “Seller Indemnified Party”), from and against any and all Losses incurred by such Seller Indemnified Party to the extent arising from :
(a) any breach of or inaccuracy in any representation or warranty of Purchaser set forth in this Agreement (ignoring for this purpose any materiality qualifiers set forth in such representation or warranty):
(b) any nonfulfillment or breach of any covenant or agreement on the part of Purchaser set forth in this Agreement; and
(c) any Assumed Liability.
Section 7.3. Notice of Claims. If any Litigation (in equity or at law) is instituted by a Third Party (a “Third-Party Claim”) with respect to which any of the Persons to be indemnified under this Article VII (the “Indemnified Party”) intends to claim any Loss under this Article VII, the Indemnified Party shall promptly notify the Party from whom indemnification is sought (the “Indemnifying Party”) of such Third-Party Claim (the “Third-Party Claim Notice”). A failure by the Indemnified Party to give notice of any Third-Party Claim in a timely manner pursuant to this Section 7.3 shall not limit the obligation of the Indemnifying Party under this Article VII, except to the extent such Indemnifying Party is actually prejudiced thereby.
47
Section 7.4. Indemnification Procedures.
(a) The Indemnifying Party under this Article VII shall have the right, but not the obligation, exercisable by written notice to the Indemnified Party, to assume the conduct and control, at the expense of the Indemnifying Party and through counsel of its choosing that is reasonably acceptable to the Indemnified Party, any Third-Party Claim, and the Indemnifying Party may compromise or settle the same; provided, that the Indemnifying Party shall give the Indemnified Party advance written notice of any proposed compromise or settlement and shall not, without the prior written consent of the Indemnified Party (which consent shall not be unreasonably withheld or delayed), consent to or enter into any compromise or settlement that commits the Indemnified Party to take, or to forbear to take, any action or does not provide for a full and complete written release by the applicable Third Party of the Indemnified Party. No Indemnified Party may compromise or settle any Third-Party Claim for which it is seeking indemnification hereunder without the consent of the Indemnifying Party (which consent shall not be unreasonably withheld or delayed). No Indemnifying Party may consent to the entry of any judgment that does not relate solely to monetary damages arising from any such Third-Party Claim without the prior written consent of the Indemnified Party (which consent shall not be unreasonably withheld or delayed). The Indemnifying Party shall permit the Indemnified Party to participate in, but not control, the defense of any such Third-Party Claim through counsel chosen by the Indemnified Party; provided, that the fees and expenses of such counsel shall be borne by the Indemnified Party. If the Indemnifying Party elects not to control or conduct the defense of a Third-Party Claim, the Indemnifying Party nevertheless shall have the right to participate in the defense of any Third-Party Claim and, at its own expense, to employ counsel of its own choosing for such purpose.
(b) The Parties shall cooperate in the defense of any Third-Party Claim, with such cooperation to include (i) the retention and the provision to the Indemnifying Party of records and information that are reasonably relevant to such Third-Party Claim and (ii) reasonable access to employees on a mutually convenient basis for providing additional information and explanation of any material provided hereunder.
Section 7.5. Survival of Representations and Warranties. The representations and warranties of Seller and of Purchaser set forth in this Agreement shall survive the Closing and, except in the case of Fraud, shall expire (a) with respect to representations and warranties contained in Section 4.1 (Organization and Good Standing), Section 4.2 (Authority), Section 4.3(a) (No Conflicts with Organizational Documents), Section 4.5 (Purchased Assets), Section 4.10 (Brokers), Section 5.1 (Organization and Good Standing), Section 5.2 (Authority), Section 5.3(a) (No Conflict with Organizational Documents) and Section 5.7 (Brokers) (the “Fundamental Representations”), upon the expiration of the applicable statute of limitations and (b) with respect to all other representations and warranties, eighteen (18) months after the Closing Date; provided, however, that claims validly asserted prior to the relevant expiry date provided for above shall survive such expiry until final resolution.
48
Section 7.6. Limitations.
(a) Notwithstanding Section 7.1, there shall be no liability for indemnification under Section 7.1(a) or Section 7.2(a) for any individual claim or series of related claims where the Losses related to such claim or series of related claims is less than $[**]; provided that this Section 7.6(a) shall not apply in the case of any Fundamental Representation or in the case of Fraud.
(b) Notwithstanding Section 7.1, there shall be no liability for indemnification under Section 7.1(a) or Section 7.2(a) unless the aggregate amount of Losses suffered by the Purchaser Indemnified Parties or the Seller Indemnified Parties, as applicable, under this Agreement (excluding claims subject to Section 7.6(a)) exceeds $[**] (the “Seller Indemnification Threshold”), at which xxxx Xxxxxx or Purchaser, as applicable, will be obligated to indemnify the Purchaser Indemnified Parties or the Seller Indemnified Parties, as applicable, with respect to the aggregate amount of all Losses described in Section 7.1(a), without regard to the Seller Indemnification Threshold; provided that the Seller Indemnification Threshold shall not apply in the case of any Fundamental Representation or in the case of Fraud.
(c) The indemnification obligations of Seller or Purchaser under Section 7.1(a) or Section 7.2(a) shall be subject to a limit (the “Cap”) of (i) [**] percent ([**]%) of the aggregate of payments made to Seller until such payments aggregate [**] dollars ($[**]) and [**]%) of any additional payments made to Seller, provided, however, that the Cap shall not apply in the case of Fraud or in the case of indemnification obligations in respect of any breaches of or inaccuracies in any Fundamental Representation, which obligations (when aggregated with Seller’s or Purchaser’s other indemnification obligations under Section 7.1(a) or Section 7.2(a), as applicable), together with Seller’s and Purchaser’s respective indemnification obligations under Section 7.1(b) and Section 7.2(b), as applicable, shall be limited to an amount equal to an amount, in the aggregate, equal to the total amount of all payments made to Seller hereunder.
(d) The amount of Losses recoverable by the Indemnified Party under this Article VII shall be reduced, on a dollar-for-dollar basis, by the amount of any insurance proceeds or other third party recoveries received by the Indemnified Party in connection with a claim under this Article VII (net of any costs of obtaining such recovery and increases in premiums resulting from such Losses which are borne by the Indemnified Party).
Section 7.7. Exclusive Remedy. Absent Fraud, the Parties’ sole and exclusive remedy under this Agreement after the Closing with respect to the transactions contemplated hereby shall be indemnification provided in this Article VII, provided that, Section 7.7 shall not affect the Parties’ rights to specific performance or other equitable remedies available pursuant to Section 8.10. Seller and Purchaser each hereby waive any provision of applicable Law to the extent that it would conflict with this Section 7.7.
49
ARTICLE VIII
MISCELLANEOUS
Section 8.1. Further Assurances and Post-Closing Covenants. From time to time after the Closing, and for no further consideration, each of the Parties shall, and shall cause its Affiliates to, execute, acknowledge and deliver such assignments, transfers, consents, assumptions and other documents and instruments and take such other commercially reasonable actions as may reasonably be requested to more effectively assign, convey or transfer to or vest in Purchaser the Purchased Assets and the Assumed Liabilities contemplated by this Agreement to be transferred or assumed at the Closing (including transferring, at no additional cost to Purchaser, any Purchased Asset contemplated by this Agreement to be transferred to Purchaser at the Closing and that was not so transferred at the Closing).
Section 8.2. Notices. All notices or other communications hereunder shall be deemed to have been duly given and made if in writing and if served by personal delivery upon the Party for whom it is intended, delivered by registered or certified mail, return receipt requested, or by a national overnight courier service, or sent by electronic means or facsimile (provided, that notice by electronic means or facsimile is promptly confirmed by telephone confirmation thereof and , in the case of facsimile, receipt is promptly confirmed by the sending facsimile machine), to the Person at the address or facsimile number set forth below, or such other address as may be designated in writing hereafter, in the same manner, by such Person:
(a) If to Seller, to:
00 Xxxxx Xxxxxx, Xxx 000
Xxxxxx, XX 00000
Facsimile: [**]
Attention: Chief Executive Officer
with a copy to:
00 Xxxxx Xxxxxx, Xxx 000
Xxxxxx, XX 00000
Facsimile: [**]
Attention: General Counsel
with a copy to:
WilmerHale LLP
00 Xxxxx Xxxxxx
Xxxxxx, XX 00000
Facsimile: 617-526-5000
Attention: Xxxxxx X. Xxxxxx, Esq.
50
(b) If to Purchaser, to:
Biogen MA Inc.
000 Xxxxxx Xxxxxx
Xxxxxxxxx, XX 00000
Facsimile: [**]
Attention: Executive Vice President, Chief Legal Corporate Services and Secretary
with a copy to:
Ropes & Xxxx LLP
Prudential Tower, 000 Xxxxxxxx Xxxxxx
Xxxxxx, XX 00000-0000
Telephone: (000) 000-0000
Facsimile: [**]
Attention: Xxxxxxxxxxx X. Xxxxxx, Esq.
All notices and other communications under this Agreement shall be deemed to have been received (i) when delivered by hand, if personally delivered, (ii) three (3) Business Days after being delivered by registered or certified mail, return receipt requested, (iii) one (1) Business Day after being delivered to a national overnight courier service or (iv) on the date of receipt, if sent by facsimile, with a telephonic acknowledgment of sending and confirmation of receipt by the sending facsimile machine.
Section 8.3. Amendment; Waiver. Any provision of this Agreement may be amended or waived if, and only if, such amendment or waiver is in writing and signed (a) in the case of an amendment, by Purchaser and Seller and (b) in the case of a waiver, by the Party against whom the waiver is to be effective. No failure or delay by any Party in exercising any right, power or privilege hereunder shall operate as a waiver thereof, nor shall any single or partial exercise thereof preclude any other or further exercise thereof or the exercise of any other right, power or privilege.
Section 8.4. Assignment.
(a) Neither Party may assign or transfer (whether by operation of law or otherwise) this Agreement or any rights (including any rights to payments) or obligations hereunder without the prior written consent of the other, except (i) in connection with a Rights Transfer Event pursuant to Section 8.4(b), (ii) Seller may make an assignment, in whole or in part, without Purchaser’s consent to an Affiliate or to a successor of Seller, whether in a merger, sale of all or substantially all of Seller’s assets, sale of at least a majority of Seller’s stock with the intent to
51
change ownership or other transaction intended to be an acquisition of Seller, and (iii) Purchaser may make an assignment, in whole or in part, on a product-by-product, country-by-country basis without Seller’s consent to an Affiliate or to a successor to substantially all of the business to which this Agreement relates, whether in a merger, sale of stock, sale of assets, reorganization or other transaction; provided that, in the cases of (ii) and (iii) such permitted successor or assignee of obligations hereunder has expressly assumed performance of such obligations (and in any event, any Party assigning this Agreement will remain bound by the terms and conditions hereof, subject to Section 8.4(c)). Any permitted assignment will be binding on and inure to the benefit of the successors of the assigning Party. Any assignment or attempted assignment by either Party in violation of the terms of this Section 8.4 will be null, void and of no legal effect.
(b) If Purchaser determines in good faith that a Third Party has the capabilities and access to financial resources required to Develop a Royalty Product and make the payments required to be made to Seller under this Agreement (a “Permitted Third Party Assignee”), Purchaser may consummate a transaction (a “Rights Transfer Event”) that enables such Permitted Third Party Assignee to research, Develop, market and/or sell such a Royalty Product (if applicable, following further Development and Regulatory Approval) through the transfer, assignment, license, sub-license, sale or other disposition of any Transferred IP from Purchaser to such Permitted Third Party Assignee. In connection with a Rights Transfer Event, if requested by Purchaser, Seller shall engage in a good faith negotiation to amend the definition of “Net Sales,” Section 3.3, Section 3.5 and other provisions of this Agreement to align such provisions with corresponding concepts set forth in the definitive documentation for such Rights Transfer Event, in furtherance of harmonizing the provisions of this Agreement with the definitive agreement effecting the Rights Transfer Event, provided that that foregoing shall not be construed to require that Seller agree to any terms that materially differ from the provisions of this Agreement.
(c) Purchaser’s obligations hereunder shall terminate, and Purchaser shall be relieved of any liability or obligation thereafter with respect thereto, to the extent such obligations are assumed by a Permitted Third-Party Assignee in connection with a Rights Transfer Event and such Permitted Third-Party Assignee executes an enforceable agreement acknowledging its obligations under this Agreement and agreeing that Seller shall have the right to enforce this Agreement directly against such Permitted Third-Party Assignee.
Section 8.5. Entire Agreement. This Agreement, together with all other documents and instruments referenced herein, including the Ancillary Agreements, contains the entire agreement among the Parties with respect to the subject matter hereof and supersedes all prior agreements and understandings, oral or written, with respect to such matters, but excluding the Confidentiality Agreement, which shall remain in full force and effect for the term provided for therein.
Section 8.6. No Third-Party Beneficiaries. This Agreement shall inure to the benefit of and be binding upon the Parties and their respective successors and permitted assigns. Nothing in this Agreement, express or implied, is intended to confer upon any Person other than Purchaser, Seller or their successors or permitted assigns, any rights or remedies under or by reason of this Agreement.
52
Section 8.7. Public Disclosure.
(a) Purchaser and its Affiliates shall have the right to issue press releases at any time and in any form, provided that, for the initial press release following the execution of this Agreement, both Purchaser and Seller and their respective Affiliates may issue a press release as long as (i) such press release is provided to the other Party for its review and comment, and (ii) the other Party approves the issuance of such initial press release, such approval not to be unreasonably withheld or delayed. Thereafter, Seller agrees not to issue any press release or other public statement, whether written, electronic, oral or otherwise, disclosing the existence of this Agreement, the terms hereof, the transactions contemplated therein, or any information relating to or development made under this Agreement without the prior written consent of Purchaser, provided that Seller shall not be required to seek the permission of Purchaser to repeat any such information that has already been publicly disclosed by Purchaser or its Affiliates or licensees so long as such information remains true, correct and consistent with the most recent information publicly disclosed by Purchaser or its Affiliates or licensees on the applicable topic as of such time of disclosure by Seller.
(b) However, the provisions of this Section 8.7 shall not prohibit (i) any disclosure required to comply with the requirements of any applicable Law or the rules of any nationally recognized stock exchange (in which case such Party shall notify the other Party promptly and shall use commercially reasonable efforts to provide the other Party with a copy of the contemplated disclosure prior to submission or release, as the case may be), and (ii) any disclosure made in connection with the enforcement of any right or remedy relating to this Agreement or the transactions contemplated herein.
Section 8.8. Publishing and Use of Trademarks.
(a) Neither Party (nor any of its Affiliates or agents) shall use the registered or unregistered Trademarks, service marks, trade dress, trade names, logos, insignia, domain names, symbols or designs of the other Party or its Affiliates in any press release, publication or other form of promotional disclosure without the prior written consent of the other Party in each instance.
(b) The Parties recognize the desirability of publishing and publicly disclosing the results of, and scientific information regarding, activities under this Agreement. Accordingly, during the Term, Purchaser will be free to publish and present the results of and information regarding activities under this Agreement, provided that if Purchaser reasonably determines that the proposed publication contains Confidential Information of Seller, Purchaser shall deliver to Seller a copy of the proposed publication or presentation at least [**] days prior to submission for publication or presentation to enable Seller to identify any Confidential Information of Seller which Purchaser shall be required to delete. If Seller wishes to publish or present any information or results relating to any asset under the Transferred IP, Seller shall (a) deliver to
53
Purchaser a copy of the proposed written publication or an outline of the proposed presentation at least [**] days prior to submission for publication or presentation in order to give Purchaser the opportunity to comment on such publication or presentation; (b) agree to all reasonable comments and proposed changes of Purchaser to the proposed publication or presentation; and (c) obtain the prior written consent of Purchaser, such consent to be given in the absolute discretion of the Purchaser.
Section 8.9. Confidentiality; Return of Information.
(a) The Confidentiality Agreement, to the extent it restricts disclosure or use of Purchased Assets by Purchaser, is hereby amended such that is ceases to do so.
(b) Each Party shall, and shall cause its Affiliates to, keep confidential and not use, all Confidential Information, including information received from the other Party or a designee in the context of such Party’s performance of its obligations under this Agreement that constitutes Confidential Information, including all Know-How included in the Seller IP, except (A) as required by Law, including under the Order of a Governmental Entity, (B) as necessary to arbitrate, defend or prosecute any indemnification claim or any Litigation or dispute or (C) for information that is available to the public on the Closing Date, or thereafter becomes available to the public other than as a result of a breach of this Section 8.9(b), or is furnished to the other Party after the Closing by a Third Party that, to the knowledge of such Party, is under no obligation of confidentiality to the other Party with respect to such information.
Section 8.10. Equitable Relief. The Parties agree that irreparable damage would occur in the event that any of the provisions of this Agreement is not performed in accordance with its specific terms or is otherwise breached. It is accordingly agreed that the non-breaching Party shall be entitled to an injunction or injunctions to prevent breaches of this Agreement and to enforce specifically the terms and provisions of this Agreement in any court of the United States or any state having jurisdiction, this being in addition to any other remedy to which Purchaser is entitled at law or in equity. Each Party hereby waives (i) any requirement that the other Party post a bond or other security as a condition for obtaining any such relief and (ii) any defenses in any action for specific performance, including the defense that a remedy at Law would be adequate. Notwithstanding the foregoing, Seller acknowledges that damages would be an adequate remedy for any breach by Purchaser of Section 3.8(a), and hereby waives any right to equitable relief in respect of any breach of such provision.
Section 8.11. Expenses. Except as otherwise provided in this Agreement, whether or not the Closing takes place, all costs and expenses incurred in connection with this Agreement and the transactions contemplated hereby shall be paid by the Party incurring such costs and expenses.
Section 8.12. Governing Law; Jurisdiction; Venue and Service.
(a) Governing Law. This Agreement shall be governed by and construed in accordance with the Laws of the Commonwealth of Massachusetts, excluding any conflicts or choice of Law rule or principle that might otherwise refer construction or interpretation of this Agreement to the substantive law of another jurisdiction.
54
(b) Jurisdiction. The Parties hereby irrevocably and unconditionally consent to the exclusive jurisdiction of the courts of the Commonwealth of Massachusetts and the United States District Court for the District of Massachusetts for any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement and agree not to commence any action, suit or proceeding (other than appeals therefrom) related thereto except in such courts. The Parties irrevocably and unconditionally waive their right to a jury trial.
(c) Venue. The Parties further hereby irrevocably and unconditionally waive any objection to the laying of venue of any action, suit or proceeding (other than appeals therefrom) arising out of or relating to this Agreement in the courts of the Commonwealth of Massachusetts or in the United States District Court for the District of Massachusetts, and hereby further irrevocably and unconditionally waive and agree not to plead or claim in any such court that any such action, suit or proceeding brought in any such court has been brought in an inconvenient forum.
(d) Service. Each Party further agrees that service of any process, summons, notice or document by registered mail to its address set forth in Section 8.2 shall be effective service of process for any action, suit or proceeding brought against it under this Agreement in any such court.
Section 8.13. Counterparts. This Agreement may be executed in counterparts, and by the Parties hereto in separate counterparts, each of which when executed shall be deemed to be an original but all of which taken together shall constitute one and the same agreement. Delivery of an executed counterpart of a signature page to this Agreement by facsimile transmission or by e-mail of a .pdf attachment shall be effective as delivery of a manually executed counterpart of this Agreement.
Section 8.14. Headings. The heading references herein and the table of contents hereto are for convenience purposes only, do not constitute a part of this Agreement and shall not be deemed to limit or affect any of the provisions hereof.
Section 8.15. Severability. The provisions of this Agreement shall be deemed severable and the invalidity or unenforceability of any provision shall not affect the validity or enforceability of the other provisions hereof. If any term or other provision of this Agreement, or the application thereof to any Person or any circumstance, is invalid, illegal or unenforceable, (a) a suitable and equitable provision shall be substituted therefor in order to carry out, so far as may be valid and enforceable, the intent and purpose of such invalid or unenforceable provision and (b) the remainder of this Agreement and the application of such provision to other Persons or circumstances shall not be affected by such invalidity, illegality or unenforceability, nor shall such invalidity, illegality or unenforceability affect the validity or enforceability of such provision, or the application thereof, in any other jurisdiction.
55
Section 8.16. Force Majeure. Except for the obligation to pay money when due, neither Party shall be liable to the other for failure or delay in the performance of any of its obligations under this Agreement for the time and to the extent such failure or delay is caused by a Force Majeure. The Party affected by the Force Majeure shall provide the other Party in writing with full particulars thereof as soon as it becomes aware of the same (including its best estimate of the likely extent and duration of the interference with its activities), and will use commercially reasonable efforts to overcome the difficulties created thereby and to resume performance of its obligations as soon as practicable. For the avoidance of doubt, under no circumstances shall the alleged or actual inability to pay money be considered a Force Majeure.
[REMAINDER OF PAGE LEFT BLANK INTENTIONALLY]
56
IN WITNESS WHEREOF, the Parties have executed or caused this Agreement to be executed as of the date first written above.
| PURCHASER: | ||||
| BIOGEN MA INC. | ||||
| By: | /s/ Xxxxxxx Xxxxxx | |||
| Name: | Xxxxxxx Xxxxxx | |||
| Title: | EVP, Research & Development | |||
[Signature Page to Asset Purchase Agreement]
| SELLER: | ||||
| KARYOPHARM THERAPEUTICS INC. | ||||
| By: | /s/ Xxxxxxx X. Xxxxxxxx | |||
| Name: | Xxxxxxx X. Xxxxxxxx | |||
| Title: | Chief Executive Officer | |||
[Signature Page to Asset Purchase Agreement]
58
Schedule 1.1(a)
Knowledge of Seller
Karyopharm Representatives:
Chief Executive Officer
Chief Scientific Officer
General Counsel
Chief Financial Officer
Head of Business Development
Head of Pharmaceutical Sciences
59
Schedule 1.1(b)
Description of Molecules
KPT-350
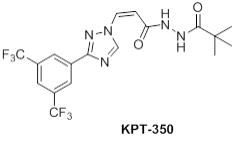
KPT-420
[**]
60
Schedule 2.1(a)(i)
Assumed Contracts
(currently active)
[**]
61
Schedule 2.1(a)(iv)
Product Registrations & Regulatory Materials
[**]
62
Schedule 2.5(d)(i)
Research Projects
| Active Industry Collaborations | ||||||||||
| Company |
Source and Amount of Funding |
Indication/model | Contact person | Study status | Amount of compound | |||||
| Sensorion |
N/A | [**] | [**] | Will begin Dec 1st.2017 |
1.3 gram | |||||
| Active Academia Collaborations
| ||||||||||
| Institute |
Source and Amount of Funding |
Indication/model | Contact person | Study status | Amount of compound | |||||
| [**] |
N/A | [**] | [**] | On-going | 100mg | |||||
| [**] |
N/A | [**] | [**] | On-going | 100mg | |||||
| [**] |
N/A | [**] | [**] | On-going | 100mg | |||||
| [**] |
ALSA $[**] total
2 years |
[**] | [**] | Will end by July 2018 |
1 gram | |||||
| [**] |
ALSA $[**] total
3 years |
[**] | [**] | Will begin Dec 1, 2017 |
5 gram | |||||
| [**] |
MDA $[**] total
3 years |
[**] | [**] | Will begin Feb 1, 2018 |
TBD | |||||
| [**] |
NT$[**] (about US $[**]) total
1 year |
[**] | [**] | Started on Aug 2017 |
100 mg | |||||
63
Schedule 2.10
Transition Plan
| 1. | Technology Transfer |
| a. | Within [**] of the date of the Agreement, the Seller shall transfer to the Purchaser all materials identified in Schedule 6.12 of the Agreement. |
| b. | For a period of [**] following the date of the Agreement, the Seller will make available to the Purchaser as reasonably requested for transition purposes the Seller’s transition manager that will act as the primary contact person with respect to all chemistry, Manufacturing and control (CMC) and Manufacturing-related matters. |
| 2. | Non-Oncology Program |
| a. | For a period of [**] following the date of the Agreement, the Seller will make available to the Purchaser as reasonably requested for transition purposes one or more of Seller’s employees responsible for the Seller’s non-oncology program. |
64
Schedule 4.6(b)
Assumed Contracts Containing Covenants that Affect Marketability
N/A
65
Schedule 4.7(a)(i)
Seller IP
| Country |
Application Status |
Application No. | Filing Date | Publication No./ Publication Date |
Patent No./ Patent Date | |||||
| [**] |
[**] | [**] | [**] |
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 2 pages were omitted. [**]
Last Updated: December 18, 2017
66
Schedule 4.7(a)(ii)
Licensed IP
[**]
| Country |
Application Status |
Application No. |
Filing Date |
Publication No. |
Publication Date |
Patent No. | Patent Date | |||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | |||||||||
| [**] |
[**] | [**] | [**] | [**] | [**] | [**] | [**] | |||||||
| [**] |
[**] | [**] | [**] | [**] | [**] |
67
Schedule 4.7(c)
IP Contracts that Provide Rights to Third Parties
N/A
68
Schedule 4.7(l)
Government and Third Party IP Rights
N/A
69
Schedule 4.8
Inventory
|
Material Description |
Manufacturer’s Lot Number |
Quantity | ||
| [**] |
[**] | [**] |
[**]
| Material Description |
Manufacturer’s Lot Number |
Quantity (No. of bottles) | ||
| [**] |
[**] | [**] | ||
| [**] |
[**] | [**] | ||
| [**] |
[**] | [**] | ||
| [**] |
[**] | [**] | ||
| [**] |
[**] | [**] | ||
| [**] |
[**] | [**] |
Drug Product Expiry: [**]
|
Material Description |
Manufacturer’s Lot Number |
Quantity | ||
| [**] |
[**] | [**] |
[**]
70
Schedule 6.12
Know-How Transfer
KPT-350 Documents
Confidential Materials omitted and filed separately with the Securities and Exchange Commission. A total of 4 pages were omitted. [**]
71
Exhibit A
Instrument of Assignment and Assumption
This Instrument of Assignment and Assumption is made, executed and delivered as of January [—], 2018 by and between Karyopharm Therapeutics Inc., a Delaware corporation, with its principal corporate offices at 00 Xxxxx Xxxxxx, Xxxxxx, XX 00000 (“Seller”) and Biogen MA Inc., a Massachusetts corporation, with its principal corporate offices at 000 Xxxxxx Xxxxxx, Xxxxxxxxx, XX 00000 (“Purchaser”).
For good and valuable consideration, the receipt, adequacy and legal sufficiency of which are hereby acknowledged, and as contemplated by Section 2.1 of that certain Asset Purchase Agreement, dated as of the date hereof, by and between Purchaser and Seller (the “Asset Purchase Agreement”) (capitalized terms used but not defined herein shall have the meanings ascribed to them in the Asset Purchase Agreement), Seller hereby sells, transfers, assigns, conveys, grants and delivers to Purchaser, effective as of Closing, all of Seller’s right, title and interest in and to the assets and rights described on Schedule A hereto (the “Purchased Assets”), and, as contemplated by Section 2.3 of the Asset Purchase Agreement and subject to the terms therein, Purchaser hereby assumes, effective as of the Closing, from Seller and agrees to perform and discharge the Assumed Liabilities.
This Instrument of Assignment and Assumption shall be binding upon and shall inure to the benefit of the respective successors and assigns of Purchaser and its Affiliates and the Seller and its Affiliates.
This Instrument of Assignment and Assumption is not intended to in any way conflict with the rights and obligations of the Purchaser and Seller set forth in the Asset Purchase Agreement, and in the event of any conflict or inconsistency between the terms of the Asset Purchase Agreement and the terms of this Instrument of Assignment and Assumption, the terms of the Asset Purchase Agreement shall control.
This Instrument of Assignment and Assumption may be executed in any number of counterparts, including by facsimile copies or by electronic copies delivered by email, each of which will be deemed an original, with the same effect as if the signatures were upon the same instrument.
[Remainder of Page Intentionally Left Blank]
IN WITNESS WHEREOF, Seller and Purchaser have executed this Instrument of Assignment and Assumption as of the date first above written.
| KARYOPHARM THERAPEUTICS INC. | ||
| By: |
| |
| Name: | ||
| Title: | ||
| BIOGEN MA, INC. | ||
| By: |
| |
| Name: | ||
| Title: | ||
[SIGNATURE PAGE TO INSTRUMENT OF ASSIGNMENT AND ASSUMPTION]
Schedule A
Purchased Assets
| 1. | Assumed Contracts; |
| 2. | Seller IP; |
| 3. | Purchased Inventory; |
| 4. | Product Registrations and Regulatory Materials set forth on Schedule 2.1(a)(iv) of the Asset Purchase Agreement or, whether or not set forth on such Schedule 2.1(a)(iv), all Product Registrations and Regulatory Materials used or held for use by Seller or an Affiliate exclusively in connection with the Exploitation of any Royalty Product; |
| 5. | All books and records to the extent exclusively relating to the other Purchased Assets or any Royalty Product (the “Records”), including correspondence with the FDA or other Regulatory Authorities, Product drawings, work instructions and bills of materials, customer lists and vendor lists; provided, that Seller shall be entitled to redact from such Records any information to the extent that it is not conveyed to Purchaser hereunder. |
Exhibit B
PATENT ASSIGNMENT
This PATENT ASSIGNMENT (the “Assignment”) is made and entered into as of this [—] (the “Closing Date”), by and between Karyopharm Therapeutics Inc., having its principal place of business at 00 Xxxxx Xxxxxx, Xxxxxx, XX 00000 Xxxxxx Xxxxxx of America (hereinafter “Assignor”), and Biogen MA Inc., having its principal place of business at 000 Xxxxxx Xxxxxx, Xxxxxxxxx, Xxxxxxxxxxxxx 00000, Xxxxxx Xxxxxx of America (hereafter “Assignee”).
For good and valuable consideration, the receipt, adequacy and legal sufficiency of which is hereby acknowledged, Assignor does hereby sell, assign and transfer unto said Assignee, its successors and assigns, the entire interest for the United States of America, and its possessions and territories, and all foreign countries jurisdictions, in, to, and for (1) certain inventions or improvements described in the patent application and patents identified in Schedule A to this Patent Assignment, which Schedule A is attached hereto and incorporated herein by reference (collectively, the “Patents and Patent Applications”), (2) the Patents and Patent Applications, (3) all patents of the United States and its possessions and territories and of all foreign countries and jurisdictions which may or shall be granted on said invention(s), on said improvement(s), or on said Patent Application(s), (4) all provisional, divisional, continuation, reissue, PCT, national phase, or other applications based on said Patents and Patent Applications, and (5) all rights of priority, including the right to claim priority under the Paris Convention for the Protection of Industrial Property, the Patent Cooperation Treaty, the European Patent Convention, and any other treaty relating thereto, in each case that may arise from the Patents and Patent Applications, together with all rights to xxx for past, present, or future infringement thereof, including the right to collect damages for any past infringement thereto.
Assignor agrees with said Assignee, but at Assignee’s expense, hereafter to execute all applications, amended specifications, deeds or other instruments, and to do all acts necessary or proper to secure the grant of patents in the United States and its possessions and territories and in all other foreign countries and jurisdictions to said Assignee, and to vest and confirm in said Assignee, its successors and assigns, the legal title to all such patents.
Assignor hereby authorizes and requests the Commissioner of Patents and Trademarks of the United States and equivalent authorities in all patent offices worldwide to issue all patents that shall be granted on the Patent Applications or to transfer all Patents to said Assignee, its successors and assigns. This Assignment may be executed in any number of counterparts, including by facsimile copies or by electronic copies delivered by email, each of which will be deemed an original, with the same effect as if the signatures were upon the same instrument.
[Signature page follows]
| Karyopharm Therapeutics Inc. | ||
| By |
| |
| Title |
| |
| Date |
| |
| Witness Signature |
| |
| Print Witness Name |
| |
| Address |
| |
|
| ||
| Witness Signature |
| |
| Print Witness Name |
| |
| Address |
| |
| Biogen MA Inc. | ||
| By |
| |
| Title |
| |
| Date |
| |
| Witness Signature |
| |
| Print Witness Name |
| |
| Address |
| |
|
| ||
| Witness Signature |
| |
| Print Witness Name |
| |
| Address |
| |
- 2 -
Schedule A
[Same as Schedule 4.7(a)(i) from the APA]
- 3 -
Exhibit C
CERTIFICATION OF NON-FOREIGN STATUS
PURSUANT TO TREASURY REGULATION SECTION 1.1445-2(b)
Section 1445 of the Internal Revenue Code of 1986, as amended (the “Code”), provides that a transferee of a U.S. real property interest must withhold tax if the transferor is a foreign person. For U.S. tax purposes (including section 1445), the owner of a disregarded entity (which has legal title to a U.S. real property interest under local law) will be the transferor of the property and not the disregarded entity. To inform the transferee that withholding of tax is not required upon the disposition of a U.S. real property interest by Karyopharm Therapeutics Inc. (the “Transferor”), the undersigned certifies the following on behalf of Transferor:
| 1. | Transferor is not a foreign corporation, foreign partnership, foreign trust, or foreign estate (as those terms are defined in the Code and Treasury Regulations); |
| 2. | Transferor is not a disregarded entity as defined in Treasury Regulation section 1.1445-2(b)(2)(iii); |
| 3. | Transferor’s U.S. employer identification number is [—]; and |
| 4. | Transferor’s office address is 00 Xxxxx Xxxxxx, Xxx 000 Xxxxxx, XX 00000. |
Transferor understands that this certification may be disclosed to the Internal Revenue Service by the transferee and that any false statement contained herein could be punished by fine, imprisonment, or both.
Under penalties of perjury I declare that I have examined this certification and to the best of my knowledge and belief it is true, correct, and complete, and I further declare that I have authority to sign this document on behalf of Transferor.
| Dated as of January [—], 2018 | ||
|
| ||
| Name: [—] | ||
| Title: [—] | ||

