EXCLUSIVE OPTION AGREEMENT
Exhibit 10.3
THIS EXCLUSIVE OPTION AGREEMENT (“Agreement”) is entered into this 16th day of March 2020 (“Effective Date”) by and between CEDARS-SINAI MEDICAL CENTER, a California nonprofit public benefit corporation (“CSMC”), with offices at ▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇, and ENVIRO THERAPEUTICS, INC., a C corporation (hereinafter, “Licensee”), with offices at ▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇. #▇▇▇, ▇▇▇ ▇▇▇▇▇▇▇ ▇▇ ▇▇▇▇▇ (each, a “Party,” and together, the “Parties”).
R E C I T A L S
A. CSMC owns and is entitled to grant license rights with respect to certain Patent Rights and Technical Information (as such terms are defined in Section 1) invented by ▇▇▇▇ ▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇-▇▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇, and ▇▇▇▇▇▇ ▇▇▇▇▇▇ (“Inventors”).
B. CSMC desires to have the Patent Rights and Technical Information developed, used and commercialized, and the Parties desire to enter into discussions regarding an exclusive license to Licensee to develop, manufacture, use and sell products utilized or derived from the Patent Rights and Technical Information in the Territory (“Exclusive License”).
C. CSMC desires to grant to Licensee and Licensee desires to accept from CSMC, subject to the terms and conditions herein, an exclusive option to obtain the Exclusive License. Other than the rights expressly granted by CSMC hereunder, Licensee acknowledges that CSMC shall retain all other rights with respect to the Patent Rights and Technical Information.
D. CSMC and Licensee intend that the execution, delivery and performance of this Agreement by each Party, and the consummation of the transactions contemplated hereunder, shall not at any time threaten CSMC’s tax-exempt status under Section 501(c)(3) of the Internal Revenue Code and Section 23701d of the California Revenue and Taxation Code, or cause CSMC to be in default under any of CSMC’s issued and outstanding tax-exempt bonds.
NOW, THEREFORE, in consideration of the mutual covenants and premises herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties hereto hereby agree as follows:
| 1. | Definition of “Patent Rights” and “Technical Information.” For purposes of this Agreement, the term “Patent Rights” shall mean the patents and/or patent applications existing on the Effective Date which are described on Schedule A attached hereto, and all patents and/or patent applications (including provisional patent applications) existing as of the Effective Date in any other country corresponding to any of the foregoing, and all divisions, continuations, reissues, reexaminations, supplementary protection certificates and extensions thereof, whether domestic or foreign, all claims of continuations-in-part that are entitled to the benefit of the priority date of any of the foregoing, and any patent that issues thereon. For purposes of this Agreement, the term “Technical Information” shall mean the following information in the Field of Use which is described in the Patent Rights or otherwise provided to Licensee: know-how, trade secrets, unpublished patent applications, software, bioinformatics, unpatented technology, technical information, statistical information and analyses, biological materials, chemical reagents, preclinical and clinical information, in each case which has been conceived or reduced to practice prior to the Effective Date, in the conduct by CSMC of the Sensitization of Solid Tumor programs at CSMC under the direction of one or any of the Inventors. The Technical Information shall further include information in the Field of Use described in Schedule B hereto and which has been reduced to practice prior to the Effective Date in the conduct of the aforementioned research programs at CSMC under the direction of one or any of the Inventors. The Patent Rights and Technical Information are all owned by CSMC. |
| 2. | Option Fee. In consideration of the execution and delivery by CSMC of this Agreement, Licensee agrees to pay to CSMC a non-refundable fee of Three Thousand Dollars ($3,000.00) (“Option Fee”), payable in cash, no later than thirty (30) days after the execution of this Agreement. The Option Fee may be credited against any license fees payable under the Exclusive License in the event that the Parties enter into the Exclusive License. |
| 3. | Development Plan. In consideration of the execution and delivery by CSMC of this Agreement, Licensee agrees to perform the development activities described in the Development Plan attached as Schedule C. |
| 4. | Grant of Option. Except as provided hereinafter and subject to the terms of this Agreement, Licensee shall have until the expiration of nine (9) months from the Effective Date as an exclusive option period (“Option Period”) to notify CSMC of its desire to enter into an Exclusive License (“Option Notice”). Upon receipt of the Option Notice within the Option Period, the Parties shall enter into the Exclusive License in substantially the form attached as Schedule D hereto within ninety (90) days following the Option Notice. If, after such ninety (90) day period, the Licensee does not consummate the Exclusive License, or if an Option Notice is not delivered within the Option Period, then this Agreement and the exclusive option herein shall terminate, CSMC shall have no further obligation whatsoever to Licensee with respect to the Patent Rights and Technical Information, and CSMC may freely dispose of the Patent Rights and Technical Information as it sees fit in its own discretion. |
| 5. | No Warranties. CSMC makes no representation or warranty other than those expressly specified in this Agreement. |
| 6. | Indemnification. Licensee shall hold harmless, defend and indemnify CSMC and each of its officers, directors, employees and agents and Inventors (each, an “Indemnified Party”, and collectively, the “Indemnified Parties”) from and against any and all claims, damages, losses, liabilities, costs and expenses (including reasonable attorneys’ fees and expenses and costs of investigation, whether or not suit is filed) suffered or incurred by any of the Indemnified Parties in any action, suit, litigation, arbitration or dispute of any kind (“Action”) arising or resulting from any negligence or willful acts or omissions on the part of Licensee in connection with this Agreement. As part of its obligations hereunder, Licensee shall defend any Action brought against any of the Indemnified Parties with counsel of its own choosing and reasonably acceptable to CSMC, and neither CSMC nor any other Indemnified Party shall enter into any settlement of any such Action without first obtaining prior approval of Licensee. Should CSMC or any other Indemnified Party not afford Licensee the right to defend any such Action, or should CSMC or any other Indemnified Party not obtain the approval of Licensee to any such settlement, Licensee shall have no obligation to indemnify CSMC or any other Indemnified Party hereunder. Should Licensee fail to provide a defense for the Indemnified Parties as required hereunder, then Licensee shall reimburse CSMC for its out-of-pocket expenses (including reasonable attorneys’ fees and expenses and costs of investigation) which are incurred as a result of any investigation, defense or settlement relating to an indemnified matter, which reimbursement shall be made to CSMC upon receipt by Licensee of invoices reflecting in reasonable detail such expenses incurred by CSMC. Licensee shall obtain and maintain insurance policies or a program of self-insurance (including products liability and general liability policies at such time as is appropriate) which are reasonable and necessary to cover its activities and to comply with the indemnification obligations set forth above. CSMC shall promptly notify Licensee in writing of any claim or Action or material threat thereof brought against any Indemnified Party in respect of which indemnification may be sought and, to the extent allowed by law, shall reasonably cooperate with Licensee in defending or settling any such claim or Action. |
| 2 |
| 7. | Prosecution Costs. CSMC will prepare, file, prosecute and maintain the Patent Rights during the Option Period and Licensee shall reimburse CSMC for the costs and expenses, including reasonable attorneys’ fees, filing fees and translation fees actually incurred by CSMC (including but not limited to any costs and expenses related to foreign filings) in the prosecution of the Patent Rights (“Prosecution Costs”) during the Option Period. Licensee shall reimburse CSMC within thirty (30) days of receiving an invoice from CSMC for such Prosecution Costs. Further, the Exclusive License shall provide that Licensee will reimburse CSMC for Prosecution Costs actually incurred by CSMC in the prosecution of any Patent Rights prior to the Option Period. The Exclusive License shall provide that Licensee shall control patent prosecution and be responsible for all Prosecution Costs related to the Patent Rights following its exercise of the Option. |
| 8. | Use of Names. Licensee shall not make any written use of or reference to the name of CSMC and/or any of its trademarks, service marks, trade names or fictitious business names without the prior written consent of CSMC, which consent may be withheld or granted in CSMC’s sole and absolute discretion. Further, prior to any reference by Licensee to the names or marks of CSMC in any manner, Licensee shall provide CSMC with a writing reflecting the proposed reference so that CSMC can review the reference within a reasonable period of time prior to the proposed use thereof by Licensee. This limitation includes, but is not limited to, use by Licensee in any regulatory filing, advertising, offering circular, prospectus, sales presentation, news release or trade publication. |
| 9. | Confidentiality. The terms of this Agreement and any information disclosed by either Party hereunder shall be maintained in the strictest of confidence by the Parties hereto and their respective officers, directors, employees and agents, including any proprietary, non-public information designated as such and disclosed by one Party to the other in connection with the transactions proposed in this Agreement. Furthermore, the Patent Rights and Technical Information are understood by Licensee to be the Confidential Information of CSMC to the extent “unpublished” as such term is construed under the United States Patent Laws. As such, Licensee’s confidentiality obligations hereunder automatically extend to any and all patent applications of CSMC relating to any Patent Rights. Any public announcements, notices or other communications regarding such matters to third parties, other than the Parties’ respective professional advisors or potential investors or business partners, including, without limitation, any disclosure regarding the transactions contemplated hereby, shall require the prior written approval of both Licensee and CSMC. |
| 10. | Notices. Any notice, request, instruction or other document required by this Agreement, including the Option Notice, shall be in writing and shall be deemed to have been given: (a) if mailed with the United States Postal Service by prepaid, first class, certified mail, return receipt requested, at the time of receipt by the intended recipient, (b) if sent by Federal Express®, Airborne®, or other overnight carrier, signature of delivery required, at the time of receipt by the intended recipient, or (c) if sent by facsimile transmission, when so sent and when receipt has been acknowledged by appropriate telephone or facsimile receipt, addressed as follows: |
in the case of CSMC, to:
Cedars-Sinai Medical Center
▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇
Los Angeles, CA 90048-1865
Attention: Executive Vice President for Finance & CFO
Copy to: Vice President, Intellectual Property
or in the case of Licensee, to:
Enviro Therapeutics, Inc.
▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇. #▇▇▇
Los Angeles CA 90064
Attention: CEO
or to such other address or to such other person(s) as may be given from time to time under the terms of this Section.
| 11. | Wire Transfer Instructions. All payments due hereunder shall be made by Licensee to CSMC in accordance with the following wire transfer instructions: |
City National Bank
▇▇▇ ▇. ▇▇▇▇▇▇▇ ▇▇▇▇▇
Beverly Hills, CA 90210
ABA# 122 016 066
Account Number: 210-132260
Account Name: Cedars-Sinai Medical Center – Tech Transfer
Reference: Tech Transfer – Enviro Therapeutics, Inc.
| 3 |
| 12. | Miscellaneous. |
| a. | Compliance with Laws. Each Party shall comply with all applicable federal, state and local laws and regulations in connection with its activities pursuant to this Agreement. |
| b. | Governing Law. This Agreement shall be construed and enforced in accordance with the laws of the United States of America and of the State of California, irrespective of choice of laws provisions. |
| c. | Waiver. Failure of any Party to enforce a right under this Agreement shall not act as a waiver of that right or the ability to assert that right relative to the particular situation involved. |
| d. | Enforceability. If any provision of this Agreement shall be found by a court of competent jurisdiction to be void, invalid or unenforceable, the same shall be reformed to comply with applicable law or stricken if not so conformable, so as not to affect the validity or enforceability of the remainder of this Agreement. |
| e. | Modification. No change, modification, or addition or amendment to this Agreement, or waiver of any term or condition of this Agreement, is valid or enforceable unless in writing and signed and dated by the authorized officers of the Parties to this Agreement. |
| f. | Entire Agreement. This Agreement and the Confidentiality Agreement currently in effect between the Parties constitute the entire agreements between the Parties with respect to the subject matter hereof and thereof, and replace and supersede as of the date hereof and thereof any and all prior agreements and understandings, whether oral or written, between the Parties with respect to the subject matter of such agreements. |
| g. | Construction. This Agreement has been prepared, examined, negotiated and revised by each Party and their respective attorneys, and no implication shall be drawn and no provision shall be construed against any Party to this Agreement by virtue of the purported identity of the drafter of this Agreement or any portion thereof. |
| h. | Counterparts. This Agreement may be executed simultaneously in one or more counterparts, each of which shall constitute one and the same instrument. This Agreement may be executed by facsimile. |
| i. | Assignment. Except as otherwise expressly provided in this Agreement, this Agreement shall be binding upon, inures to the benefit of, and is enforceable by, the Parties and their respective heirs, legal representatives, successors and permitted assigns. This Agreement shall not be assignable by Licensee absent the prior written consent of CSMC unless to a successor to Licensee or a purchaser of all or substantially all of the assets of Licensee relating to the subject matter of this Agreement. |
| j. | Further Assurances. At any time and from time to time after the Effective Date, each Party shall do, execute, acknowledge and deliver, and cause to be done, executed, acknowledged or delivered, all such further acts, transfers, conveyances, assignments or assurances as may be reasonably required to consummate the transactions contemplated by this Agreement. |
| k. | Survival. The following sections shall survive any expiration or earlier termination of this Agreement: Section 5 (“No Warranties”), Section 6 (“Indemnification”), Section 7 (“Prosecution Costs”) and Section 8 (“Use of Names”). |
| 4 |
IN WITNESS WHEREOF, the Parties have caused their duly authorized representatives to execute this Agreement as of the Effective Date.
| “CSMC”: | “Licensee” | |||
| Cedars-Sinai Medical Center | Enviro Therapeutics, Inc. | |||
| A California Nonprofit Public Benefit Corporation | A Corporation | |||
| By: | /s/ ▇▇▇▇▇ ▇. ▇▇▇▇ | By: | /s/ ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇ | |
| ▇▇▇▇▇ ▇. ▇▇▇▇, ▇▇ | ▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇, PhD | |||
| Vice President, Intellectual | CEO | |||
| By: | ▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇ | |||
| Executive Vice President for Finance & CFO | ||||
| 5 |
Schedule A
Patent Rights
1. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 2017286561 |
| Country: | Australia |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621AU00 |
| CSMC Tech ID: | bho000961 |
2. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 3026066 |
| Country: | Canada |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621CA00 |
| CSMC Tech ID: | bho000961 |
3. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 201780000000 |
| Country: | China |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621CN00 |
| CSMC Tech ID: | bho000961 |
4. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 17814046.3 |
| Country: | Europe |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621EP00 |
| CSMC Tech ID: | bho000961 |
5. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 2018-565397 |
| Country: | Japan |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621JP00 |
| CSMC Tech ID: | bho000961 |
6. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 16/305,821 |
| Country: | United States |
| Filing Date: | Nov 29, 2018 |
| Docket No.: | 065472.000621US00 |
| CSMC Tech ID: | bho000961 |
| 6 |
Schedule B
Technical Information
All lab notes, methods, protocols, SOPs, and technical know-how developed by inventors, including
▇▇▇▇▇▇ et al (2018) Antagonizing CD105 enhances radiation sensitivity in prostate cancer.
Oncogene. 2018 Aug;37(32):4385-4397
| 7 |
Schedule C
Development Plan
See attached.
| 8 |

Business Plan
I. Executive summary
Our mission is to develop therapeutics targeting the tumor microenvironment to complement conventional and targeted therapies designed against the cancer cells. The focus on the non-tumor supportive cells of solid tumors has emerged from the ▇▇▇▇▇▇▇▇ laboratory, where multiple viable therapeutic targets have been identified in the last 15 years. One such target for the endoglin receptor is the lead therapeutic interest for Enviro Therapeutics. This target has a partially humanized neutralizing antibody that been in the laboratory and clinical trials by Tracon Pharmaceuticals Inc. for many years. Most recently, we performed a Phase 2 clinical trial (IND134672) for castrate resistant prostate cancer patients that were failing androgen signaling inhibitor therapy with a drug combination including the endoglin inhibitor (ENV105). In this heavily pre-treated population, the 43% progression free survival rate was extraordinary. In addition, a three gene panel was identified to serve as a companion biomarker for patient selection. Enviro Therapeutics will strive to co-develop companion biomarkers with all drugs in its portfolio.
The intellectual property owned by Cedars-Sinai Medical Center will be licensed by Enviro Therapeutics. The current preclinical data suggest efficacy of ENV105 for combinations with androgen signaling inhibitors for prostate cancer and as a radiation sensitizer for multiple solid tumors having intact p53 activity.
ENV105 will be aggressively pursued in pre-clinical and clinical trials. Future therapeutic targets will be developed based on the overriding mission to complement traditional cancer-targeted drugs with those that address the tumor microenvironment.
II. Leadership team
Enviro Therapeutics Inc. was incorporated on November 15, 2019 in the state of California. ▇▇▇▇ ▇▇▇▇▇▇▇▇ is the CEO and Secretary. ▇▇▇▇ ▇▇ is the Treasurer and Director.
III. Market analysis
The initial market for ENV105 will be prostate cancer. More than 2.9 million men in the US live diagnosed with prostate cancer today. The market of prostate cancer is highest in the Americas ($12 billion by 2025). The disease is significant in the Europe and rapidly growing in east Asia. The main drivers of growth will be an aging population, continued uptake of second-generation hormonal agents in the metastatic and non- metastatic castration resistant prostate cancer settings.
In the spectrum of prostate cancer progression, 26,730 fatalities will occur primarily of metastatic castration- resistant prostate cancer (mCRPC) annually in the US. Historically the median survival for men with mCRPC has been less than two years. The goal will be to address this patient population with a combination of ENV105 and androgen signaling inhibitors to extend overall survival. However, ENV105 is an effective radiation therapy sensitizer for breast and prostate cancer. The potential market share for ENV105 in combination external beam irradiation will be vast. 70% of the estimated 268,600 new cases of invasive breast cancer patients expected to be diagnosed in the U.S. this year (being p53 positive) would be eligible for this therapeutic strategy, as would nearly 100% of the 174,650 prostate cancer patients diagnosed yearly in the US. ENV105 use enable significantly lower doses of the irradiation for otherwise frail subjects to be eligible for irradiation therapy than otherwise possible. Other solid tumors will be explored in preclinical models to further expand market share.
IV. Value Proposition
| - | No sensitizers to irradiation, androgen signaling inhibition, or chemotherapy for prostate cancer patients exist (apart from androgen signaling inhibitors themselves) | |
| - | There are no companies that have the goal of making cancer therapeutics that target the non-vascular tumor microenvironment. | |
| - | Having companion biomarkers for ENV105 provide the advantage of improved clinical success. | |
Biomarkers provide an 18% advantage for phase 2 trials moving to phase 3 and 21% improvement in gaining an NDA for drugs in phase 3 trials. | ||
| - | ENV105 has a proven safety record in 4 independent clinical trials employed in single agent and combination therapy strategies. | |
| - | Formulation and capacity to scale up ENV105 production is in hand (Lonza Group) | |
| - | Partnership with Tracon Pharmaceuticals Inc. provide experience in therapeutic development. |
| 9 |
V. Milestones and Timeline
| - | Phase 2 clinical trial will be initiated for castrate resistant prostate cancer patients – based on the results of IND134672. The new trial will be executed combining an androgen signaling inhibitor (e.g. abiraterone [Zytiga; ▇▇▇▇▇▇▇]) with ENV105. This involves obtaining FDA approval for the ENV105 Phase 2 trial following regulatory approval by Cedars-Sinai Medical Center. | 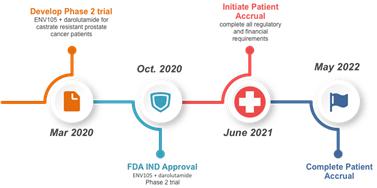 |
| - | Study biomarkers for ENV105 |
| - | Develop Phase 1 clinical trial for ENV105 combination with external beam irradiation for localized ER+ breast cancer. |
| 10 |

Enviro Therapeutics Inc. Development Plan
ENV105 will be aggressively pursued in pre-clinical and clinical trials. Future therapeutic targets will be developed based on the overriding mission to complement traditional cancer-targeted drugs with those that address the tumor microenvironment. Enviro Therapeutics will strive to co-develop companion biomarkers with all drugs in its portfolio.
Preclinical Plan:
| - | Year 1: Identify other cancer targets for ENV105 | |
| - | Year 2: Study biomarkers for ENV105 |
| - | Clinical Plan: |
| - | Year 1: Develop Phase 2 clinical trial for ENV105 combination with darolutamide for castrate resistance prostate cancer patients | |
| - | Year 2: Begin accrual for ENV105 clinical trial | |
| - | Year 2: Develop Phase 1 clinical trial for external beam irradiation for localized ER+ breast cancer with ENV105. | |
| - | Year 3: Complete accrual for ENV105 clinical trial |
| 11 |
Schedule D
Exclusive License Agreement
THIS EXCLUSIVE LICENSE AGREEMENT (“Agreement”) is entered into as of this 16th day of March 2020 (“Effective Date”) by and between CEDARS-SINAI MEDICAL CENTER, a California nonprofit public benefit corporation (“CSMC”), with offices at ▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇ ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇-1865, and ENVIRO THERAPEUTICS, INC.., a C Corporation (“Licensee”), with offices at ▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇. #▇▇▇, ▇▇▇ ▇▇▇▇▇▇▇ ▇▇ ▇▇▇▇▇.
R E C I T A L S
A. CSMC owns and/or is entitled to grant license rights with respect to certain Patent Rights and Technical Information (as defined below) invented or developed in the course of the Sensitization of Solid Tumor programs at CSMC conducted under the direction of ▇▇▇▇ ▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇-▇▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇, and ▇▇▇▇▇▇ ▇▇▇▇▇▇ (hereinafter collectively referred to as the “Inventors”).
B. CSMC and Licensee entered into that certain Exclusive Option Agreement dated March 16, 2020 (“Option Agreement”), under which CSMC granted to Licensee an exclusive option to negotiate and obtain an exclusive license to the Patent Rights and Technical Information.
C. Licensee has exercised its option under the Option Agreement to negotiate and obtain an exclusive license to the Patent Rights and Technical Information, under which CSMC desires to have the Patent Rights and the Technical Information developed, used and commercialized in the Field of Use (as defined below) by Licensee, and Licensee desires to obtain an exclusive, worldwide license to conduct research in the Field of Use, and to develop, manufacture, use and sell Products (as defined below) in the Field of Use, using the Patent Rights and Technical Information in accordance with the terms of this Agreement. Other than the rights expressly granted by CSMC hereunder within the Field of Use, Licensee acknowledges that CSMC shall retain all other rights with respect to the Patent Rights and the Technical Information.
D. CSMC and Licensee intend that the execution, delivery and performance of this Agreement by each party, and the consummation of the transactions contemplated hereunder, shall not at any time threaten CSMC’s tax-exempt status under Section 501(c)(3) of the Internal Revenue Code and Section 23701d of the California Revenue and Taxation Code, or cause CSMC to be in default under any of CSMC’s issued and outstanding tax-exempt bonds.
NOW, THEREFORE, in consideration of the mutual covenants and premises herein contained, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the parties hereto hereby agree as follows:
| 1. | DEFINITIONS |
1.1 “Affiliate” or “Affiliates” shall mean any corporation, person or entity, which controls, is controlled by, or is under common control with, a party to this Agreement without regard to stock or other equity ownership. For purposes hereof, the terms “control” and “controls” mean the possession, direct or indirect, of the power to direct or cause the direction of the management and policies of a corporation, person or entity, whether through the ownership of voting securities, by contract or otherwise.
| 12 |
1.2 “Confidential Information” shall mean any confidential or proprietary information furnished by one party (the “Disclosing Party”) to the other party (the “Receiving Party”) in connection with this Agreement, including, without limitation, all specifications, know-how, trade secrets, technical information, drawings, software, models, business information and patent applications pertaining to the Patent Rights and Technical Information, and as further provided in Section 10 hereof.
1.3 “FDA” shall mean the United States Food and Drug Administration, or any successor agency thereof.
1.4 “Field of Use” shall mean the treatment of cancer.
1.5 “Funding Agencies” shall mean any public or private granting agencies which have provided funding to CSMC or to any of the Inventors for the development of any of the Patent Rights or Technical Information prior to the Effective Date.
1.6 “Improvements” shall mean all improvements or enhancements to the Patent Rights that, after the Effective Date, are conceived and reduced to practice if patentable, or reduced to practice if not patentable, by at least one Inventor (other than by an Inventor that conceived or reduced to practice such Improvement in his or her role as a paid consultant or employee of Licensee).
1.7 “Licensee Developments” shall mean any and all processes, uses, designs, applications, methods and compositions-of-matter, indications, improvements, enhancements and modifications in the Field of Use directly based upon or directly created using the Patent Rights and/or Technical Information and which were discovered or developed by or on behalf of Licensee (exclusive of work performed by CSMC or the Inventors on behalf of CSMC) during the term of this Agreement. The Licensee Developments are all owned by Licensee.
1.8 “Net Sales” shall mean the gross amount invoiced by or on behalf of Licensee and Permitted Sublicensees to third parties for all sales of Products less (a) discounts actually allowed;
(b) credits for claims, allowances, retroactive price reductions or returned goods; (c) prepaid freight; and (d) sales taxes or other governmental charges actually paid in connection with sales of Products (but excluding what are commonly known as income taxes and value-added taxes). Sales of Products by Licensee, or a Permitted Sublicensee of Licensee, to any Affiliate or Permitted Sublicensee which is a reseller thereof shall be excluded, and only the subsequent sale of such Products by Affiliates or Permitted Sublicensees of Licensee to unrelated parties shall be deemed Net Sales hereunder. If a Product is sold or provided as part of a system, package, or combination product or service that involve one or more products or services not covered by the definition of Product (each, a “Combination Product”), Net Sales shall be calculated by multiplying the Net Sales of such Combination Product, by the fraction A/B, where “A” is the price of the Product included in such Combination Product when sold separately from any other products or services not covered by the definition of Product and “B” is the price of the Combination Product. In the event that no market price is available for the Product included in such Combination Product when supplied or priced separately, CSMC and Licensee shall determine in good faith the fair market value thereof.
| 13 |
1.9 “Non-Royalty Sublicense Revenues” shall mean, but is not limited to, upfront fees, license maintenance fees, and milestone payments, or other payments, including the fair market value of any non-cash consideration, received by Licensee in consideration for any rights granted to Patent Rights under a sublicense agreement, and excludes any and all sales-based royalties paid to Licensee.
1.10 “Other Product” or “Other Products” shall mean any products and/or services in the Field of Use, other than a Patent Product, derived in any manner whatsoever from any of the Technical Information and/or Licensee Developments.
1.11 “Patent Rights” shall mean the patents and/or patent applications existing on the Effective Date which are described on Schedule A attached hereto, and all patents and/or patent applications (including provisional patent applications) existing as of the Effective Date in any other country corresponding to any of the foregoing, and all divisions, continuations, reissues, reexaminations, supplementary protection certificates and extensions thereof, whether domestic or foreign, all claims of continuations-in-part that are entitled to the benefit of the priority date of any of the foregoing, and any patent that issues thereon. The Patent Rights are all owned by CSMC.
1.12 “Patent Product” or “Patent Products” shall mean any products and/or services in the Field of Use that, except for the license granted hereunder, would infringe a Valid Claim.
1.13 “Product” or “Products” shall mean a Patent Product and/or an Other Product.
1.14 “Technical Information” shall mean, as of the Effective Date, the following information in the Field of Use which is described in the Patent Rights or otherwise provided to Licensee: know-how, trade secrets, unpublished patent applications, software, bioinformatics, unpatented technology, technical information, statistical information and analyses, biological materials, chemical reagents, preclinical and clinical information, in each case which has been conceived or reduced to practice prior to the Effective Date, in the conduct by CSMC of the Sensitization of Solid Tumor programs at CSMC under the direction of one or any of the Inventors. The Technical Information shall further include information in the Field of Use described in Schedule B hereto and which has been reduced to practice prior to the Effective Date in the conduct of the aforementioned research programs at CSMC under the direction of one or any of the Inventors. Technical Information is all owned by CSMC.
1.15 “Territory” shall mean worldwide.
1.16 “Valid Claim” shall mean a claim of an issued patent included within the Patent Rights and/or Licensee Developments, which claim has not (a) lapsed, been canceled or become abandoned, (b) been declared invalid or unenforceable by a non-appealable decision or judgment of a court or other appropriate body or authority of competent jurisdiction, or (c) been admitted to be invalid or unenforceable through reissue, disclaimer or otherwise. The term Valid Claim shall also include the claims of a pending patent application within the Patent Rights and/or Licensee Developments for a period of seven (7) years from the actual filing date of that patent application.
| 14 |
| 2. | LICENSE |
2.1 Grant of Exclusive Rights. Subject to the terms of this Agreement, CSMC hereby grants to Licensee, and Licensee hereby accepts from CSMC, the exclusive license, with the right to grant sublicenses (subject to the terms of Section 2.2 hereof), under and to the Patent Rights and Technical Information during the term of this Agreement (as provided in Section 6 hereof) to conduct research in the Field of Use and to make, have made, use, import, offer for sale, sell and/or have sold Products in the Field of Use in the Territory. The foregoing grant of exclusivity is made expressly subject to the following:
(a) All applicable laws and regulations, including, without limitation, the requirements of federal law as pertains to the manufacture of products within the United States;
(b) All applicable rules of the Funding Agencies which have provided funding to CSMC or to any of its employees (including any of the Inventors) for the development of the Patent Rights and Technical Information; and
(c) The following non-exclusive rights to the Patent Rights and Technical Information, which are retained by CSMC within the Field of Use:
(i) the right to submit for publication the scientific findings from research conducted by or through CSMC or its investigators (including the Inventors) related to the Patent Rights and the Technical Information; and
(ii) the right (A) to use any tangible or intangible information contained in the Patent Rights or the Technical Information or any Improvements (so long as CSMC shall treat such information as Confidential Information and maintain its confidentiality in accordance with Section 10 hereof), for CSMC’s research, internal teaching and other educationally-related and non-commercial (except for charges to its own patients) clinical purposes, where clinical use does not involve a third party funding grant to commercialize such information, and (B) to obtain research funding for further study and development thereof from governmental and other nonprofit organizations (including grant applications).
(d) Notwithstanding any other provision hereof to the contrary, all rights to the Patent Rights, Technical Information and Improvements outside of the Field of Use are retained by CSMC. Furthermore, this Agreement confers no license or rights by implication, estoppel, or otherwise under any patent applications or patents of CSMC other than Patent Rights regardless of whether such patents are dominant or subordinate to Patent Rights.
2.2 Sublicensing. Licensee shall have the right to grant sublicenses or to assign any or all of the rights granted hereunder only to an entity which has been approved in writing by CSMC (each, “Permitted Sublicensee”). Any such Permitted Sublicensee shall be subject in all respects to the provisions contained in this Agreement and Licensee will remain primarily liable to CSMC for, and shall be responsible for monitoring and enforcing, performance of all of Licensee’s obligations hereunder by any such Permitted Sublicensee. Without limiting the generality of the foregoing, as an express condition of any such sublicense, any such Permitted Sublicensee shall be required to agree in writing to be bound by commercially reasonable reporting and record keeping, indemnification and inspection provisions, and the applicable provisions of this Agreement, including, without limitation, those pertaining to the use of CSMC’s name and marks, indemnification of CSMC and the use of CSMC’s Confidential Information. Permitted Sublicensees may not further sublicense without CSMC’s prior written consent, which consent shall not be unreasonably withheld. Licensee shall promptly forward to CSMC a copy of any and all fully executed sublicense agreements, any subsequent amendments, and all copies of Permitted Sublicensees’ profit sharing or royalty reports, in no event more than thirty (30) days following execution or receipt thereof, as applicable. Licensee shall also keep CSMC reasonably informed with respect to the progress of any relations entered into with any Permitted Sublicensees. If Licensee shall conduct one or more audits of its Permitted Sublicensees hereunder during the term hereof, Licensee shall provide copies of all audit reports to CSMC on a timely basis. The covenants pertaining to the use of CSMC’s name and marks, the indemnification of CSMC and the use of CSMC’s Confidential Information in any sublicense or assignment shall run for the benefit of CSMC, who shall be expressly stated as being a third-party beneficiary thereof with respect to the covenants set forth in this Agreement. Licensee understands and agrees that none of its permitted sublicenses hereunder shall reduce in any manner any of its obligations set forth in this Agreement.
| 15 |
(a) Royalty-Free Sublicenses. If, and only if, Licensee pays all royalties due CSMC from a Permitted Sublicensee’s Net Sales, Licensee may grant that Permitted Sublicensee a royalty-free or non-cash sublicense or cross-license.
2.3 Improvements. Subject to the rights and applicable rules of the Funding Agencies, Licensee shall have, for a period of sixty (60) days after receipt by Licensee of written notice from CSMC disclosing an Improvement, the exclusive first right to negotiate with CSMC to obtain one or more licenses to the Improvement in the Field of Use upon such terms and conditions as shall be agreed by the parties hereto, which terms and conditions shall include provisions for fair market value consideration for the grant of any such licenses. If Licensee declines or fails to pursue, or if the parties fail to conclude negotiations for a license to, such Improvement in the Field of Use during the sixty (60) day period specified above, then CSMC shall have the right to commence discussions with any other party concerning such Improvement. Subject to the provisions of this Section 2.3, Licensee acknowledges and agrees that CSMC expressly retains and reserves any and all right, title and interest in and to the Improvement, whether or not in the Field of Use and, accordingly, no license to any Improvement is granted to Licensee under this Agreement.
2.4 Licensee Developments. Licensee hereby grants, and shall require its Permitted Sublicensee(s) to grant to CSMC a nonexclusive, royalty-free, irrevocable, paid-up license, with the right to grant sublicenses to non-profit research institutions and governmental agencies, to practice and use Licensee Developments for non-commercial research purposes, which license shall include, without limitation, the rights to: (a) publish the scientific findings from research conducted by or through CSMC or on its behalf, (b) use any tangible or intangible information contained in the Licensee Developments (so long as CSMC shall treat such information as Confidential Information and maintain its confidentiality in accordance with Section 10 hereof), for CSMC’s internal teaching and other educationally-related and non-commercial (except for charges to its own patients) clinical purposes, where clinical use does not involve a third party funding grant to commercialize such information, and (c) obtain research funding from governmental and other nonprofit organizations (including grant applications).
| 16 |
2.5 Diligent Commercialization. Licensee acknowledges that it is important to CSMC, and a requirement of the United States Government under Title 35, Section 203 of the United States Code, that Licensee pursue the development, commercialization and marketing of Products and otherwise exercise commercially reasonable efforts to maximize the value of this Agreement to CSMC. Without limiting the foregoing, Licensee shall maintain a bona fide, funded, ongoing and active research, development, manufacturing, regulatory, marketing or sales program (all as commercially reasonable) to make Products commercially available to the public as soon as commercially practicable in the Territory. Should CSMC determine that Licensee fails to use commercially reasonable efforts to maximize the Patent Rights in any national political jurisdiction in the Territory at any time following the one (1) year anniversary of the Effective Date of this Agreement, CSMC shall provide Licensee with notice of such determination and give Licensee a period of ninety (90) days to provide written evidence satisfactory to CSMC that Licensee or its Permitted Sublicensee(s) has sales of Products in such jurisdiction in question or an effective, ongoing and active research, development, manufacturing, marketing or sales program, as appropriate, directed toward obtaining regulatory approval, and/or production and/or sales of Products in such jurisdiction in accordance with Licensee’s business, legal, medical and scientific judgment and Licensee’s normal practices and procedures for products having similar technical and commercial potential. Should Licensee fail to provide such written evidence satisfactory to CSMC within such ninety (90) day period, CSMC shall have the right, at CSMC’s sole and absolute discretion, to either: (i) terminate this Agreement with respect to the applicable national political jurisdiction within the Territory or (ii) require Licensee to negotiate in good faith a sublicense with a Permitted Sublicensee(s) in the applicable national jurisdiction to maximize the Patent Rights in such jurisdiction.
2.6 Milestones. As part of Licensee’s diligent commercialization efforts under Section 2.5, CSMC and Licensee have agreed on the Milestones set forth on Schedule C, with each such Milestone being deemed a separate and independent condition. Within sixty (60) days after each anniversary of the Effective Date, Licensee shall prepare and deliver to CSMC an annual written report (to be certified by an executive officer of Licensee) indicating its compliance with the Milestones. If Licensee believes that it is or will be unable to achieve such Milestones despite its diligent efforts, Licensee may request amendments or reasonable extensions to Schedule C in writing for CSMC’s consideration. Licensee agrees to provide any additional information reasonably required by CSMC to evaluate Licensee’s performance under this Agreement. If Licensee fails to meet any annual Milestone designated in Schedule C hereto, and has not obtained an extension or amendment to such Milestone(s), CSMC may, at its option and as its sole remedy for Licensee’s breach of this Section 2.6, upon written notice to Licensee, convert the exclusive license granted under Section 2.1 hereof to a non-exclusive license or to a co-exclusive license, or terminate the license.
2.7 Preference for United States Industry. To the extent that the Patent Rights and Technical Information have been developed using funding from the United States Government, Licensee agrees that any Products made, used or sold in the United States shall be manufactured substantially in the United States.
| 17 |
| 3. | REPRESENTATIONS AND WARRANTIES |
3.1 Rights to Technology. Except for the rights, if any, of the Funding Agencies or the United States Government, CSMC represents and warrants to Licensee that, to the best of its actual, current knowledge (without investigation outside of CSMC as to such representations and warranties) (a) it has the right to grant the licenses in this Agreement, (b) it has not granted licenses to the Patent Rights or Technical Information to any other party that would restrict the rights granted hereunder except as stated herein and (c) there are no claims, judgments or settlements to be paid by CSMC with respect the Patent Rights or Technical Information or pending claims or litigation relating to the Patent Rights or Technical Information. Except for any potential or actual rights of Funding Agencies or the United States Government, CSMC is not aware that any additional rights or licenses are necessary for Licensee to exercise its licensed rights granted by CSMC under this Agreement.
3.2 Limited Warranty; Certain Damages.
(a) Limited Warranty. CSMC makes no representation or warranty other than those expressly specified in this Agreement. Licensee accepts the Patent Rights and the Technical Information on an “AS-IS” basis. CSMC MAKES NO OTHER WARRANTIES CONCERNING PATENT RIGHTS OR TECHNICAL INFORMATION COVERED BY THIS AGREEMENT, INCLUDING WITHOUT LIMITATION, ANY EXPRESS OR IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE AS TO PATENT RIGHTS, TECHNICAL INFORMATION OR ANY PRODUCT. CSMC MAKES NO WARRANTY OR REPRESENTATION AS TO THE VALIDITY OR SCOPE OF PATENT RIGHTS, OR THAT ANY PRODUCT WILL BE FREE FROM AN INFRINGEMENT ON PATENTS OR OTHER INTELLECTUAL PROPERTY RIGHTS OF THIRD PARTIES, OR THAT NO THIRD PARTIES ARE IN ANY WAY INFRINGING PATENT RIGHTS COVERED BY THIS AGREEMENT. LICENSEE HEREBY AGREES THAT LICENSEE WILL NOT GIVE, AND SHALL NOT PERMIT ANY AFFILIATES OR SUBLICENSEES OR AFFILIATES THEREOF TO GIVE, ANY SUCH WARRANTY OR REPRESENTATION TO THIRD PARTIES ON BEHALF OF CSMC.
(b) Certain Damages. EXCEPT FOR THE BREACH OF THE CONFIDENTIALITY PROVISIONS IN SECTION 10 OR IN ACCORDANCE WITH THE OBLIGATION TO INDEMNIFY SET FORTH IN SECTION 8, IN NO EVENT SHALL EITHER PARTY BE LIABLE FOR ANY INDIRECT, SPECIAL OR CONSEQUENTIAL DAMAGES (INCLUDING, WITHOUT LIMITATION, DAMAGES FOR LOSS OF PROFITS OR EXPECTED SAVINGS OR OTHER ECONOMIC LOSSES, OR FOR INJURY TO PERSONS OR PROPERTY) ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT OR ITS SUBJECT MATTER, REGARDLESS OF WHETHER SUCH PARTY KNOWS OR SHOULD KNOW OF THE POSSIBILITY OF SUCH DAMAGES. THE FOREGOING EXCLUSIONS AND LIMITATIONS SHALL APPLY TO ALL CLAIMS AND ACTIONS OF ANY KIND, WHETHER BASED ON CONTRACT, TORT (INCLUDING, BUT NOT LIMITED TO NEGLIGENCE), OR ANY OTHER GROUNDS.
3.3 Rights Retained by Funding Agencies. Licensee acknowledges that to the extent that the Patent Rights and Technical Information have been developed in part under one or more funding agreements (“Funding Agreements”) with one or more Funding Agencies, such Funding Agencies have certain statutory, non-exclusive rights relative thereto for use for government purposes as well as regulatory or statutory “march-in rights” (collectively, “Statutory Rights”). Licensee also acknowledges that to the extent that the Improvements may be developed in part under one or more Funding Agreements with one or more Funding Agencies, such Funding Agencies may have certain Statutory Rights relative thereto. This Agreement is explicitly made subject to such Statutory Rights and, to the extent of any conflict between any such Statutory Rights and this Agreement, such Statutory Rights shall prevail.
| 4. | CONSIDERATION |
In consideration of the execution and delivery by CSMC of this Agreement, Licensee agrees as follows:
4.1 License Fee.
(a) Upfront Fee. Licensee shall pay to CSMC a non-refundable license fee in two (2) installments in accordance with the following: (i) an amount equal to $10,000 shall be payable within thirty (30) days of the Effective Date, and (ii) an amount equal to $25,000 shall be payable within thirty (30) days of the date on which Licensee has raised at least $250,000 in capital company-wide for any program or purpose; provided, that if Licensee has paid CSMC the second installment of the license fee described in clause (ii) of this Section 4.1(a), then Licensee shall not be required to pay CSMC the second installment of the license fee described in the license agreement between Licensee and CSMC with respect to CSMC’s technology titled “Depletion of mitochondrial DNA from circulation” (bho001234) (the “Mitochondrial DNA License.”). For the avoidance of doubt, upon raising at least $250,000 in capital company-wide for any program or purpose, Licensee shall only be required to pay CSMC one (1) $25,000 license fee installment in total, and such payment shall satisfy the requirements to make a second license fee installment payment which are set forth in both this Agreement and the Mitochondrial DNA License.
(b) Patent Cost Reimbursement. Within thirty (30) days of the execution of this Agreement, Licensee shall reimburse CSMC for the costs, including attorneys’ fees and filing fees, actually incurred to date by CSMC in the prosecution of the Patent Rights (which have not already been reimbursed by Licensee under the Option Agreement), which are [$ ] as of ______________, 202_.
| 18 |
4.2 Equity Grant. Licensee shall issue to CSMC that number of shares of Licensee’s common stock as is equal, in the aggregate, to 1.5% of the outstanding shares of the capital stock of Licensee on a Fully Diluted Basis (as defined hereafter), as of the Effective Date, subject to execution of a Stock Issuance Agreement of the form attached hereto as Schedule C. Failure of Licensee to issue such shares shall render this Agreement null and void (ab initio). Such shares issued hereunder shall be of the same class and series as founders’ shares. A true and complete copy of Licensee’s capitalization table as of the Effective Date of the Agreement is set forth in Schedule E hereto. For the purposes of this Agreement, “Fully Diluted Basis” shall include all of the issued and outstanding shares of common stock, preferred stock and other capital stock of the Licensee (calculated on an as-converted to common stock basis) plus any then issued and outstanding options and warrants to purchase common stock (calculated on an as-exercised, as converted to common stock basis) plus any unissued but reserved for shares of common stock for future issuance as options or stock grants under the Licensee’s stock plan.
(a) Anti-Dilution Protection. Licensee will issue to CSMC, without further consideration, any additional shares of stock of the class issued pursuant to Section 4.2 necessary to ensure that the number of shares issued CSMC pursuant to Section 4.2 does not represent less than 1.5% of the shares issued and outstanding on a Fully-Diluted Basis at any time until the earlier of (i) Licensee’s initial public offering or (ii) Licensee having raised at least $20,000,000 in capital.
(b) Future Offerings. In any private offering of Licensee’s equity securities in exchange for cash (or in satisfaction of debt issued for cash), CSMC may purchase for cash that number of the securities issued in such offering as is necessary for CSMC to maintain its pro rata ownership interest in the Licensee on a Fully-Diluted Basis. This right is in addition to CSMC’s rights under Section 4.2(a). In any offering subject to this Section 4.2(b), (i) CSMC’s purchase right shall be on the same terms as the other investors in the financing in question, except that CSMC shall not have any board representation or board meeting attendance rights, (ii) Licensee will give CSMC notice of the terms of the offering, including the names of the investors and the amounts to be invested by each, and CSMC may elect to exercise its right of purchase, in whole or in part, by notice given to Licensee within thirty (30) days after receipt of Licensee’s notice, and (iii) if CSMC elects not to purchase, or fails to give an election notice within such period, CSMC’s purchase right will not apply to the offering if (and only if and to the extent) it is consummated within ninety (90) days on the same or less favorable (to the investor) terms as stated in Licensee’s notice to CSMC. CSMC’s rights under this Section 4.2(b) will not apply to the issuance of stock to employees and other service providers pursuant to a plan approved by Licensee’s board of directors, to shares issued in connection with an acquisition, joint venture or other strategic transaction, or to shares issued as additional consideration in lending or leasing transactions. The rights granted in this Section 4.2(b) will terminate (in addition to any earlier termination pursuant to their terms) immediately before Licensee’s initial public offering or a merger, sale or consolidation with a company that is subject to the periodic reporting requirements of Section 12(g) or 15(d) of the Securities Exchange Act of 1934, as amended.
4.3 Royalties and Non-Royalty Revenue.
(a) Running Royalties for Products. Licensee agrees to pay and shall pay to CSMC the following non-refundable running royalties (each, a “Royalty”) in the amounts set forth below:
(i) a running royalty of four percent (4%) on Net Sales of Patent Products made, used, sold or otherwise distributed by Licensee or any Permitted Sublicensee hereunder (“Patent Product Royalty Rate”) and
(ii) a running royalty of one-half percent (0.5%) on Net Sales of Other Products made, used, sold or otherwise distributed by Licensee or any Permitted Sublicensee hereunder (“Other Product Royalty Rate”).
(b) Minimum Royalties. Beginning on the third (3rd) anniversary of the Effective Date, Licensee shall pay CSMC a minimum royalty of $10,000 per year (“Minimum Royalty”). Each annual payment of the Minimum Royalty shall be credited against Royalties payable such that no Royalties shall be payable unless the Royalties for such annual period exceed $10,000; provided, that excess amounts paid in respect of the Minimum Royalty may not be carried over to the following calendar year.
| 19 |
(c) Permitted Deductions. If Licensee is required to make any payment to a third party to obtain a license for the manufacture, use, sale or import of a Product or otherwise exploit the Patent Rights, Licensee shall be entitled to deduct up to fifty percent (50%) of such third party payments made in a particular calendar year against Royalties payable to CSMC for that year; provided, however, that in no event shall the Royalties payable to CSMC hereunder for any one-year period be reduced by operation of this section by more than fifty percent (50%); and provided, further, that such third party payments shall only be creditable against Royalties payable to CSMC for the calendar year in which the third party payment was actually made by Licensee.
(d) Highest Royalty Due. If a Product is covered by both the definition of Patent Product and Other Product, CSMC shall be entitled to the Patent Product Royalty Rate on the Product as defined in Section 4.3(a)(i), but shall not be entitled to also receive the Other Product Royalty Rate on the same Product under Section 4.3(a)(ii). To the extent a Product ceases being a Patent Product, but is still an Other Product, CSMC shall be entitled to receive a Royalty on such Product at the Other Product Royalty Rate, but only for such time as specified in Section 4.3(g).
(e) Licensee Challenge of Patent Rights. Should Licensee bring, directly or through a third party indirectly, an action challenging the validity, scope or enforceability of any Patent Rights, Licensee will first provide CSMC with at least ninety (90) days’ prior written notice that it intends so to do before filing such a challenge. Following the giving of such notice, Licensee will pay to CSMC the Royalties and Non-Royalty Sublicense Revenue due hereunder at the rate of two times the applicable rate during the pendency of such action. Should the outcome of such action determine that any claim of a patent challenged by Licensee is valid and/or infringed and/or enforceable, as applicable, Licensee will thereafter pay to CSMC the Royalties and Non-Royalty Sublicense Revenue due hereunder at the rate of three times the applicable rate for all Products sold that would infringe such claim and/or transactions that include a grant of rights to such claim. Such increased royalty reflects the increased value of the Patent Rights upheld in such action. In the event that a challenge to the Patent Rights brought by Licensee is partially or entirely successful, Licensee will have no right to recoup any Royalties or other amounts paid before or during the period of the challenge. Additionally, Licensee agrees to disburse any and all proceeds received from any sublicense of the applicable Patent Rights throughout the duration of any such challenge to CSMC, and agrees to reimburse CSMC for all costs actually incurred by CSMC in connection with the applicable legal proceedings. In the event that all or any portion of this Section 4.3(e) is invalid, illegal or unenforceable, then the parties will use their best efforts to replace the invalid, illegal or unenforceable provision(s) with valid, legal and enforceable provision(s) which, insofar as practical, gives effect to the intent of this Section 4.3(e).
(f) Arm’s-Length Transactions. On sales of Products which are made in other than an arms’-length transaction, the value of the Net Sales attributed under this Section 4.3 to such a transaction shall be that which would have been received in an arms’-length transaction, based on sales of like quality and quantity products on or about the time of such transaction.
| 20 |
(g) Duration of Royalty Obligations. The royalty obligations of Licensee as to each Product shall terminate on a country-by-country and product-by-product basis concurrently with the expiration of:
(i) For Patent Products, the last to expire of a Valid Claim that covers such Patent Product, including any term extensions thereof, and
(ii) For Other Products, the longer of twenty (20) years after the first bona fide commercial sale of such Other Product in a country or expiration of any market exclusivity period granted by a regulatory agency for such Other Product.
(h) Non-Royalty Revenues.
(i) Non-Royalty Sublicense Revenues. Any and all “Non-Royalty Sublicense Revenues” shall be reported and paid to CSMC by Licensee as set forth below within sixty (60) days of receipt by Licensee. Licensee shall pay to CSMC a percentage of these Non- Royalty Sublicense Revenues according to the following schedule:
Date of Receipt of Non-Royalty Sublicense Revenue |
Percent of Non-Royalty Sublicense Revenues Payable to CSMC | |
Prior to receipt of FDA authorization of an Investigational New Drug (“IND”) application or equivalent regulatory agency authorization in another jurisdiction for a Product |
35% | |
After receipt of FDA authorization of an IND application for a Product or equivalent regulatory agency authorization in another jurisdiction, but prior to submission of a New Drug Application (“NDA”) or Biologics License Applications (BLA) to the FDA or equivalent regulatory agency in another jurisdiction |
10% | |
After submission of an NDA/BLA for a Product but prior to receipt of FDA approval of an NDA/BLA or equivalent regulatory agency approval in another jurisdiction for a Product |
8% | |
After receipt of FDA approval of an NDA/BLA or equivalent regulatory agency approval in another jurisdiction for a Product |
5% |
Any non-cash consideration received by Licensee from Permitted Sublicensees shall be valued at its fair market value as of the date of receipt and such amount shall be paid in cash to CSMC in accordance with the schedule above. In the event that the Patent Rights are sublicensed in combination with one or more patented technologies that are not covered under this Agreement, Non-Royalty Sublicense Revenues for the purposes of this Section 4.3(g) shall be calculated on a pro-rata basis in a manner to be mutually agreed by CSMC and Licensee (which agreement may be a condition of approval under Section 2.2).
| 21 |
(ii) Product Development Milestones. Licensee agrees to pay and shall pay to CSMC the following non-creditable, non-refundable product development milestone payments within sixty (60) days of the first occurrence of the following milestones (or their equivalents):
| Milestone Event | Milestone Payment | |||
| Successful completion of a Phase II clinical trial for a product and receipt of FDA or equivalent regulatory agency in another jurisdiction and approval for a Phase III clinical trial | $ | 300,000 | ||
| Receipt of FDA approval of a New Drug Application (“NDA”) or Biologics License Applications (“BLA”) or equivalent foreign regulatory approval in a non-United States major commercial market | $ | 1,500,000 | ||
| Cumulative Net Sales have exceeded $50,000,000 | $ | 2,500,000 | ||
(i) Payment and Accounting.
(i) Reports. Each payment of Royalties shall be accompanied by a report in the form attached as Schedule F hereto, which sets forth in reasonable detail the number and each type of Product sold and the calculation of Net Sales applicable thereto, and such additional details as may be reasonably requested by CSMC for the determination of Royalties payable hereunder. Products shall be considered as being sold for the purpose of the calculation of Royalties under this Agreement when the Products have been invoiced. Each payment of Non- Royalty Sublicense Revenue shall be accompanied by a report in the form attached as Schedule F hereto setting forth in reasonable detail the basis for the calculation of such amounts, and such additional details as may be reasonably requested by CSMC for the determination of Non-Royalty Sublicense Revenue payable hereunder. All copies of reports under this Section shall be sent by electronic mail to ▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇.▇▇▇. Except as otherwise provided herein, all amounts due hereunder shall be paid in United States dollars and shall be made without set off and free and clear of (and without any deduction or withholding for) any taxes, duties, levies, imposts or similar fees or charges. Royalties shall be payable by Licensee quarterly, within forty-five (45) days after the end of each calendar quarter, based upon revenues accrued during the immediately preceding calendar quarter. Licensee agrees to pay and shall pay to CSMC, or cause its Permitted Sublicensees to pay to CSMC, all Royalties resulting from the activities of its Permitted Sublicensees, within forty-five (45) days after the end of each calendar quarter.
(ii) Notice of Payment. Licensee shall provide prompt written notice to CSMC that it has paid the licensee fee required by Section 4.1 or any product development milestone payment required by Section 4.3(h)(ii) by electronic mail to ▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇.▇▇▇.
| 22 |
(iii) Wire Transfer Instructions. All payments due hereunder shall be made by Licensee to CSMC in accordance with the following wire transfer instructions:
City National Bank
▇▇▇ ▇. ▇▇▇▇▇▇▇ ▇▇▇▇▇
Beverly Hills, CA 90210
ABA# 122 016 066
Account Number: 210-132260
Account Name: Cedars-Sinai Medical Center – Tech Transfer
Reference: Tech Transfer – Enviro Therapeutics, Inc.
(iv) Records and Audits. Licensee shall create and maintain complete and accurate records and documentation concerning all sales of Products by Licensee, its Affiliates and Permitted Sublicensees as well as transactions based upon which Non-Royalty Sublicense Revenue is due, in sufficient detail to enable the Royalties and Non-Royalty Sublicense Revenue, respectively, that is payable hereunder to be determined. Licensee shall retain such records and documentation for not less than seven (7) years from the date of their creation. During the term of this Agreement and for a period of three (3) years thereafter, CSMC and its representatives shall have the right to audit such records and documentation as shall pertain to the determination and payment of Royalties and Non-Royalty Sublicense Revenue. Such examiners shall have reasonable access during regular business hours to Licensee’s offices and the relevant records, files and books of account, and shall have the right to examine any other records reasonably necessary to determine the accuracy of the calculations provided by Licensee. The costs of any such audit shall be borne by CSMC, unless as a result of such inspection it is determined that the amounts payable by Licensee for any period are in error by greater than five percent (5%), in which case the costs of such audit shall be borne by Licensee. CSMC shall report the results of any such audit to Licensee within forty-five (45) days of completion. Thereafter, Licensee shall promptly pay to CSMC the amount of any underpayment discovered in such audit, or CSMC shall credit to Licensee against future Royalty payments the amount of any overpayment discovered in such audit, as the case may be. In addition, Licensee shall pay interest on any underpayment at the rate that is the lower of (i) two percent (2%) over the rate of interest announced by Bank of America in Los Angeles, California (or any successor in interest thereto or any commercially equivalent financial institution if no such successor exists) to be its “prime rate”, or (ii) the highest rate permitted by applicable law, from the date such amount was underpaid to the date payment is actually received.
(v) Currency Transfer Restrictions. If any restrictions on the transfer of currency exist in any country or other jurisdiction so as to prevent Licensee from making payments to CSMC, Licensee shall take all commercially reasonable steps to obtain a waiver of such restrictions or to otherwise enable Licensee to make such payments. If Licensee is unable to do so, Licensee shall make such payments to CSMC in a bank account or other depository designated by CSMC in such country or jurisdiction, which payments shall be in the local currency of such country or jurisdiction, unless payment in United States dollars is permitted. Any payment by Licensee to CSMC in currencies other than United States dollars shall be calculated using the appropriate foreign exchange rate for such currency quoted in the California edition of The Wall Street Journal for the close of business of the last banking day of the calendar quarter in which such payment is being made.
| 23 |
(vi) Late Charges. A service charge of two percent (2%) per month, not to exceed the maximum rate allowed by applicable law, shall be payable by Licensee on any portion of Licensee’s outstanding Royalty or Non-Royalty Sublicense Revenue balance or any other amount payable by Licensee hereunder (including, without limitation, reimbursement for Prosecution costs as set forth in Section 4.1 hereof) that is not paid to CSMC within thirty (30) days past the due date.
(vii) Taxes. Licensee shall pay, or cause to be paid, any and all taxes required to be paid or withheld on any sales, licenses or other transfers for value of Products or Patent Rights (other than taxes imposed on the income or revenues of CSMC); provided, however, that under no circumstances shall the amounts of such taxes be deducted from the total amount of payments otherwise due to CSMC hereunder. Upon CSMC’s request, Licensee shall secure and send to CSMC proof of any such taxes withheld and paid by Licensee, its Affiliates or Permitted Sublicensees.
(viii) No Escrow. Licensee shall pay all Royalties and Non-Royalty Sublicense Revenue directly to CSMC and shall not pay royalties into any escrow or other similar account, including in the event of a validity or non-infringement challenge to the Patent Rights.
| 5. | PATENT RIGHTS |
5.1 Prosecution. Commencing on the Effective Date, Licensee shall assume, in coordination with CSMC, full responsibility for the application, maintenance, reexamination, reissue, opposition and prosecution of any kind (collectively “Prosecution”) relating to the Patent Rights in the Territory, including, but not limited to, payment of all costs, fees and expenses related thereto. Subject to the approval of CSMC (which approval shall not be unreasonably withheld), Licensee shall have the right to select counsel with respect to the responsibility assumed by Licensee in this Section 5.1, and Licensee shall diligently pursue the Prosecution of the Patent Rights to the benefit of CSMC. For all purposes of the patent Prosecution, CSMC shall be the named “client” of such patent counsel. Each party shall provide the other with copies of any and all material or communications with the United States Patent and Trademark Office, or any foreign patent office, and CSMC shall be afforded the opportunity of prior review and comment on such action or paper.
(a) Foreign Filings. Should Licensee elect not to file for patent protection for the Patent Rights in a certain national jurisdiction in the Territory, Licensee shall notify CSMC of such election at least forty-five (45) days before a final due date which would result in the bar of patent protection for the Patent Rights in such national jurisdiction. In such event, CSMC may, at its sole option and expense, file for patent protection for the Patent Rights in the applicable national jurisdiction, and Licensee’s license under this Agreement with respect to such national jurisdiction shall terminate, allowing CSMC to freely dispose of its Patent Rights in the applicable national jurisdiction as it sees fit in its sole discretion.
5.2 Abandonment, Disclaimers, etc. Licensee shall obtain the prior written consent of CSMC (which consent shall not be unreasonably withheld), prior to abandoning, disclaiming, withdrawing, seeking reissue, seeking reexamination or allowing to lapse any patent or patent application within the Patent Rights. In the event that Licensee shall elect to abandon the Prosecution (including the payment of maintenance fees or annuities) of any patent or patent application included in the Patent Rights, Licensee shall notify CSMC of such election at least forty-five (45) days before a final due date which would result in abandonment or bar of patentability of the patent or patent application. In such event, CSMC may, at its sole option and expense, continue Prosecution of the patent application or patent. Licensee further agrees that it shall not file any continuation-in-part application relating to the Patent Rights unless the additional disclosure or material to be included in the continuation-in-part application is necessary or appropriate to support the patentability of a claim recited in a parent application on which the continuation-in-part application is based. Licensee shall not file any continuation-in-part application without CSMC’s prior written consent, which shall not be unreasonably withheld.
| 24 |
5.3 Expenses. Licensee shall pay all expenses resulting from its obligations in Section 5.1 hereof. CSMC shall exercise reasonable efforts to cause the Inventors (to the extent they are available and on CSMC’s staff as employees) to cooperate fully with Licensee with respect to the Prosecution of the Patent Rights, and CSMC shall be reimbursed for all reasonable out-of-pocket expenses as such expenses are incurred.
5.4 CREATE Act. Licensee shall not invoke the Cooperative Research and Technology Enhancement Act of 2004, as set forth under Title 35, Section 102(c) of the United States Code (the “CREATE Act”), with respect to the Patent Rights without first obtaining the prior written consent of CSMC.
| 6. | TERM AND TERMINATION |
6.1 Term. Unless earlier terminated as provided in Section 6.2 hereof, the term of this Agreement shall commence on the Effective Date and shall expire, on a country-by-country and product-by-product basis, on the later of (i) the date upon which the last to expire Valid Claim shall expire, (ii) twenty (20) years after the first bona fide commercial sale of an Other Product in the country in question or (iii) the expiration of any market exclusivity period granted by a regulatory agency.
6.2 Termination. Except as provided by Section 6.3 hereof, and in addition to the termination provisions of Sections 2.5, 2.6 and 5.1(a), this Agreement shall terminate upon the earliest to occur of the following:
(a) Automatically if Licensee shall enter into a liquidating bankruptcy, be adjudged insolvent, liquidate, dissolve and/or if the business of Licensee shall be placed in the hands of a receiver, assignee, or trustee, whether by voluntary act of Licensee or otherwise; provided, however, that if any such action is involuntary, termination shall not take place unless the action is not reversed within thirty (30) days. Further, Licensee shall give CSMC at least forty- five (45) days’ prior written notice before Licensee initiates any bankruptcy proceeding, and CSMC shall have the right to terminate this Agreement immediately upon receipt of such notice;
(b) Automatically if the performance by either party to this Agreement of any term, covenant, condition or provision hereof (i) shall jeopardize (A) the licensure of CSMC, (B) CSMC’s participation in the Medicare, Medi-Cal or other reimbursement or payment programs, (C) the full accreditation of CSMC by the Joint Commission of Accreditation of Healthcare Organizations or any other state or nationally recognized accreditation organization, or (D) CSMC’s tax-exempt status; or (ii) is deemed illegal or unethical by any recognized governmental agency or body. Upon the occurrence of any of the items set forth in this subparagraph (b), CSMC shall provide written notice to Licensee setting forth the reason for such termination (which termination shall be effective immediately);
| 25 |
(c) Upon thirty (30) days’ written notice from CSMC if, within such thirty (30) day period (i) Licensee shall fail to pay fully any Royalty or Non-Royalty Sublicense Revenue payment required by Section 4.3 hereof, or (ii) Licensee shall fail to undertake commercially reasonable efforts to exploit the Patent Rights in the Field of Use in the Territory, regardless of Licensee’s satisfaction of the Milestones provided in Schedule D hereto;
(d) Upon sixty (60) days’ written notice from CSMC if, within such sixty (60) day period, Licensee shall fail to cure fully any breach or default of any material obligation under this Agreement as described in such written notice detailing the facts of such breach with reasonable specificity; provided, however, that Licensee may avoid such termination if, before the end of such 60-day period, such breach or default has been cured by Licensee to the reasonable satisfaction of CSMC;
(e) Upon ninety (90) days’ written notice from Licensee if, within such ninety (90) day period, CSMC shall fail to cure fully any breach or default of any material obligation under this Agreement as described in such written notice detailing the facts of such breach with reasonable specificity; provided, however, that CSMC may avoid such termination if, before the end of such 90-day period, such breach or default has been cured by CSMC to the reasonable satisfaction of Licensee; and
(f) Upon the mutual written agreement of the parties hereto (such termination to be effective as of the date mutually agreed upon in such written agreement).
6.3 Obligations Upon Termination. Upon any termination of this Agreement pursuant to Section 6.2 hereof, nothing herein shall be construed to release any party from any liability for any obligation incurred through the effective date of termination (e.g., confidentiality, reimbursement of patent expenses incurred prior to such date, etc.) or for any breach of this Agreement prior to the effective date of such termination. Licensee may, for a period of one (1) year after the effective date of such termination, sell all tangible Products customarily classified as “inventory” that it has on hand at the date of termination, subject to payment by Licensee to CSMC of the applicable Royalty and Non-Royalty Sublicense Revenue; provided, however, that any such action by Licensee does not subject CSMC to any of the occurrences set forth in Section 6.2(b) hereof.
6.4 Effect of Termination. In the event of any termination of this Agreement pursuant to Section 6.2 hereof, where such termination has not been caused by any action or inaction on the part of any Permitted Sublicensee of Licensee or by any breach by such Permitted Sublicensee of its obligations under its sublicense from Licensee, such termination of this Agreement shall be without prejudice to the rights of each non-breaching Permitted Sublicensee of Licensee and each non-breaching Permitted Sublicensee shall be deemed to be a licensee of CSMC thereunder, and CSMC shall be entitled to all rights, but shall not be subject to any obligations (other than the grant of license and appurtenant obligations under this Agreement to the extent provided for in such sublicense) of Licensee thereunder. This Section 6.4, however, shall not be applicable if this Agreement has been terminated under Section 6.2(b) under circumstances where the application of this Section 6.4 would subject CSMC to any of the occurrences set forth in Section 6.2(b).
| 26 |
6.5 Right to Institute Legal Actions. Notwithstanding the provisions of Section 6.2 hereof, CSMC, on the one hand, and Licensee, on the other hand, may institute any other legal action or pursue any other remedy against the other party permitted by applicable law if the other party does not substantially cure any breach or default of any material obligation as provided herein.
6.6 Reversion of Rights. Notwithstanding anything to the contrary set forth herein (including, but not limited to, Section 5 hereof), full responsibility for Prosecution of the Patent Rights shall, at the option of CSMC (exercisable in its sole and absolute discretion), and at its sole expense from the date of reversion, revert to CSMC upon any termination of this Agreement.
6.7 Return of Data. In the event of any termination or expiration of this Agreement, Licensee shall promptly provide CSMC with copies of all data, information and materials obtained or generated by or on behalf of Licensee in the course of conducting research and developing Products using the Patent Rights and the Technical Information.
| 7. | INFRINGEMENT BY THIRD PARTIES |
7.1 Enforcement. Licensee shall have the first right and the obligation to enforce, at its sole expense, any Patent Rights to the extent licensed hereunder against infringement by third parties and shall notify CSMC in writing in advance of all such enforcement efforts. Upon Licensee’s undertaking to pay all expenditures reasonably incurred by CSMC, CSMC shall reasonably cooperate in any such enforcement and, as necessary, join as a party therein. Licensee shall reimburse CSMC for all expenses, including reasonable attorneys’ fees, incurred in connection with any such enforcement. In the event that Licensee does not file suit against or commence and conclude settlement negotiations with a substantial infringer of Patent Rights within ninety (90) days of receipt of a written demand from CSMC that Licensee bring suit, then the parties will consult with one another in an effort to determine whether a reasonably prudent licensee would institute litigation to enforce the patent in question in light of all relevant business and economic factors (including, but not limited to, the projected cost of such litigation, the likelihood of success on the merits, the probable amount of any damage award, the prospects for satisfaction of any judgment against the alleged infringer, the possibility of counterclaims against the parties hereto, the impact of any possible adverse outcome on Licensee and the effect any publicity might have on the parties’ respective reputations and goodwill). If, after such process, it is determined that a suit should be filed and Licensee does not file suit or commence settlement negotiations forthwith against the infringer, then CSMC shall have the right, at its own expense, to enforce any Patent Rights licensed hereunder on behalf of itself and Licensee. Any damages or other recovery from an infringement action undertaken by Licensee shall first be used to reimburse the parties, on a pro rata pari passu basis, for the costs and expenses incurred in such action, and shall thereafter be allocated between the parties as follows: (i) fifty percent (50%) to CSMC and (ii) fifty percent (50%) to Licensee. If Licensee fails to prosecute any such action to completion, then any damages or other recovery net of the parties’ costs and expenses incurred in such infringement action shall be the sole property of CSMC.
| 27 |
7.2 Defense Of Patent Rights. In the event that any Patent Rights are the subject of a legal action seeking declaratory relief or of any reexamination, inter partes review, post grant review, opposition, or similar proceeding instituted by a third party, the parties agree to promptly consult with each other concerning the defense of such actions or proceedings. If the parties agree that such defense should be undertaken, then Licensee shall bear the expenses, including attorneys’ fees, associated with such defense and in any recoupment of expenses. If the parties disagree, then the party desiring to defend the action or proceeding may proceed with such defense and will bear its own expenses, and be entitled to all sums recovered.
| 8. | INDEMNIFICATION |
8.1 Indemnification by Licensee. Subject to Section 8.2 hereof, Licensee shall hold harmless, defend and indemnify CSMC and each of its officers, directors, employees (including the Inventors), agents and sponsors of the research (except Licensee) (each, an “Indemnified Party”, and collectively, the “Indemnified Parties”) from and against any and all claims, damages, losses, liabilities, costs and expenses (including reasonable attorneys’ fees and expenses and costs of investigation, whether or not suit is filed) suffered or incurred by any of the Indemnified Parties in any action, suit, litigation, arbitration or dispute of any kind (“Action”) arising or resulting from any negligence or willful acts or omissions on the part of Licensee, its Affiliates or Permitted Sublicensees in connection with (a) their use of the Patent Rights or Technical Information and/or (b) the exercise of their rights hereunder or under any sublicense, including, but not limited to (i) the preclinical development and clinical testing of Products, and (ii) the manufacture, sale, use, marketing, or other disposition of Products developed, manufactured, sold, marketed, used or otherwise disposed of under this Agreement. As part of its obligations hereunder, Licensee shall defend any Action brought against any of the Indemnified Parties with counsel of its own choosing and reasonably acceptable to CSMC, and neither CSMC nor any other Indemnified Party shall enter into any settlement of any such Action without first obtaining prior approval of Licensee. Licensee shall pay all costs, including attorney’s fees, incurred in enforcing this indemnification provision. Should CSMC or any other Indemnified Party not afford Licensee the right to defend any such Action, or should CSMC or any other Indemnified Party not obtain the approval of Licensee to any such settlement, Licensee shall have no obligation to indemnify CSMC or any other Indemnified Party hereunder. Should Licensee fail to provide a defense for the Indemnified Parties as required hereunder, then Licensee shall reimburse CSMC for its out-of-pocket expenses (including reasonable attorneys’ fees and expenses and costs of investigation) which are incurred as a result of any investigation, defense or settlement relating to the foregoing, which reimbursement shall be made to CSMC upon receipt by Licensee of invoices reflecting in reasonable detail such expenses incurred by CSMC. Licensee shall obtain and maintain insurance policies (including products liability and general liability policies at such time as is appropriate) which are reasonable and necessary to cover its activities and to comply with the indemnification obligations set forth above. Such insurance policies shall name CSMC as an additional insured party and shall provide a minimum of $3,000,000 in coverage per occurrence. Upon initiation of any human clinical studies of Products, Licensee shall have first increased its insurance coverage to a minimum of $10,000,000 in the aggregate. Licensee shall provide CSMC with prompt written notice of any material change in coverage under such policies. If the parties determine that evidence of Licensee’s insurance coverage is necessary and appropriate, within thirty (30) days of the Effective Date (subject to extension if reasonably required) and annually thereafter, Licensee shall provide CSMC with a certificate of insurance issued by the appropriate insurance company evidencing the insurance coverage required by this Section 8.1, together with copies of the endorsement which specifies CSMC as an additional insured and the declarations page for each such insurance policy. The certificate of insurance, endorsements and declarations pages (and any renewals or replacements thereof), if required, shall be sent to CSMC’s Technology Transfer Office by electronic mail at ▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇.▇▇▇.
| 28 |
8.2 Notice of Claim. CSMC shall promptly notify Licensee in writing of any claim or Action or material threat thereof brought against any Indemnified Party in respect of which indemnification may be sought and, to the extent allowed by law, shall reasonably cooperate with Licensee in defending or settling any such claim or Action. No settlement of any claim, Action or threat thereof received by CSMC and for which CSMC intends to seek indemnification (for itself or on behalf of any other Indemnified Party) shall be made without the prior joint written approval of Licensee and CSMC.
| 9. | USE OF NAMES |
9.1 CSMC Names and Marks. Licensee shall not, unless as required by any law or governmental regulation, use the name of CSMC, and/or any of its trademarks, service marks, trade names or fictitious business names or the name of any CSMC officer, faculty member, employee, student or volunteer (collectively, “CSMC Names and Marks”) without express prior written consent of the Vice President for Public Relations and Marketing of CSMC. Further, prior to any reference by Licensee to CSMC Names and Marks in any manner, Licensee shall provide CSMC with a writing reflecting the proposed reference so that CSMC can review the reference within a reasonable period of time prior to the proposed use thereof by Licensee. This limitation includes, but is not limited to, use by Licensee in any regulatory filing, advertising, offering circular, prospectus, sales presentation, news release or trade publication. Subject to compliance by Licensee with the foregoing, which shall be deemed conditions precedent to any use of CSMC Names and Marks by Licensee, Licensee shall ensure that the CSMC Names and Marks are used as scientifically or academically appropriate in the “byline” of any article, abstract, manuscript or any other publication related to the subject matter hereof.
9.2 No Endorsements by CSMC. By entering into this Agreement, CSMC does not directly or indirectly endorse any product or service provided, or to be provided, by Licensee whether directly or indirectly related to this Agreement. Licensee shall not state or imply that this Agreement is an endorsement by CSMC.
| 10. | CONFIDENTIALITY |
10.1 Non-Disclosure. The parties hereto shall keep the terms of this Agreement and all business and scientific discussions relating to the business of the parties strictly confidential. All patient information to which a party is given access by the other party shall be subject to the provisions of the Confidentiality of Medical Information Act (Cal. Civ. Code §§56, et seq.) and the Health Insurance Portability and Accountability Act of 1996, and all regulations promulgated thereunder. It may, from time to time, be necessary for the parties, in connection with performance under this Agreement, to disclose Confidential Information (including know-how) to each other. The Receiving Party (as defined in Section 1.2 hereof) shall keep in strictest confidence the Confidential Information of the Disclosing Party (as defined in Section 1.2 hereof), using the standard of care it normally uses for information of like character, and shall not disclose the Confidential Information to any third party or use it except as expressly authorized by the prior written consent of the Disclosing Party or as otherwise permitted by this Agreement; provided, however, that Licensee may disclose the Confidential Information received from CSMC to its Affiliates and Permitted Sublicensees as shall be reasonably necessary to carry out the intent of this Agreement or any sublicense granted by Licensee as contemplated by this Agreement if, but only if, such Affiliates and/or Permitted Sublicensees each execute a confidentiality agreement containing confidentiality provisions no less restrictive than those confidentiality provisions contained in this Section 10. The Receiving Party’s obligation hereunder shall not apply to Confidential Information that the Receiving Party can show:
(a) Is or later becomes part of the public domain through no fault or neglect of the Receiving Party;
| 29 |
(b) Is received in good faith from a third party having no obligations of confidentiality to the Disclosing Party, provided that the Receiving Party complies with any restrictions imposed by the third party;
(c) Is independently developed by the Receiving Party without use of the Disclosing Party’s Confidential Information; or
(d) Is required by law or regulation to be disclosed (including, without limitation, in connection with FDA filings, filings with another government agency or as required under the California Public Records Act), provided that the Receiving Party uses reasonable efforts to restrict disclosure and to obtain confidential treatment.
10.2 Limits on Permitted Disclosures. Each party agrees that any disclosure or distribution of the other party’s Confidential Information within its own organization shall be made only as is reasonably necessary to carry out the intent of this Agreement. The parties further agree that all of their respective officers, employees, agents, representatives or approved sublicensees to whom any Confidential Information is disclosed or distributed shall have agreed to maintain its confidentiality. In such event, the Receiving Party shall identify with reasonable particularity, upon request by the Disclosing Party, each person within the Receiving Party’s organization to whom the Receiving Party has disclosed or distributed Confidential Information.
10.3 Legally Required Disclosures. If a subpoena or other legal process concerning Confidential Information is served upon any party hereto pertaining to the subject matter hereof, the party served shall notify the other party immediately, the other party shall cooperate with the party served, at the other party’s expense, in any effort to contest the validity of such subpoena or other legal process. This Section 10.3 shall not be construed in any way to limit any party’s ability to satisfy any disclosure of its relationship with the other party required by any governmental authority.
| 30 |
10.4 Patent Rights as Confidential Information. The Patent Rights are understood by Licensee to be the Confidential Information of CSMC to the extent “unpublished” as such term is construed under the United States Patent Laws. As such, Licensee’s confidentiality obligations hereunder automatically extend to any and all Technical Information and to any and all patent applications of CSMC relating to any Patent Rights, Technical Information and Improvements and to any and all communications with the United States Patent Office, and any foreign patent office relating to any Patent Rights, Technical Information or Improvements.
10.5 Return of Confidential Information. In the event of any termination of this Agreement, the Receiving Party shall promptly return all Confidential Information and any copies made thereof previously made available to the Receiving Party by the Disclosing Party.
10.6 Remedies. Both parties acknowledge and agree that it would be difficult to measure damages for breach by either party of the covenants set forth in this Section 10, and that injury from any such breach would be incalculable, and that money damages would therefore be an inadequate remedy for any such breach. Accordingly, either party shall be entitled, in addition to all other remedies available hereunder or under law or equity, to injunctive or such other equitable relief as a court may deem appropriate to restrain or remedy any breach of such covenants.
| 11. | INFORMATION EXCHANGE |
In addition to the Patent Rights and Technical Information, the parties shall cooperate to exchange such non-confidential information as may be appropriate and necessary to facilitate Licensee’s development and commercialization of Products incorporating any Patent Rights or Technical Information.
| 12. | PATENT MARKING |
Licensee shall actually or virtually mark all Patent Products made, sold or otherwise disposed of by or on behalf of it or any of its Permitted Sublicensees as set forth under Title 35, Section 287(a) of the United States Code and shall respond to any request or disclosure under Title 35, Section 287(b)(4)(B) of the United States Code by only notifying CSMC of the request for disclosure.
| 13. | MISCELLANEOUS |
13.1 Notices. Any notice, request, instruction or other document required by this Agreement shall be in writing and shall be deemed to have been given (a) if mailed with the United States Postal Service by prepaid, first class, certified mail, return receipt requested, at the time of receipt by the intended recipient, (b) if sent by Federal Express or other overnight carrier, signature of delivery required, at the time of receipt by the intended recipient, or (c) if sent by facsimile transmission, when so sent and when receipt has been acknowledged by appropriate telephone or facsimile receipt, addressed as follows:
In the case of CSMC to:
Cedars-Sinai Medical Center
▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇
Los Angeles, California 90048-1865
Attention: Executive Vice President for Finance & CFO
with a copy to Vice President, Intellectual Property
or in the case of Licensee to:
Enviro Therapeutics, Inc.
▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇. #▇▇▇
Los Angeles CA 90064
Attention: CEO
or to such other address or to such other person(s) as may be given from time to time under the terms of this Section 13.1.
13.2 Compliance with Laws. Each party shall comply with all applicable federal, state and local laws and regulations in connection with its activities pursuant to this Agreement.
13.3 Governing Law. For any dispute between the parties to this Agreement which arises from or relates to this Agreement, the Agreement shall be construed and enforced in accordance with the laws of the United States of America and of the State of California, irrespective of choice of laws provisions. The parties agree that Los Angeles County, California shall be the situs of any legal proceeding arising out of or relating to this Agreement. Each party hereby waives any right it may have to assert the doctrine of forum non conveniens or similar doctrine or to object to venue with respect to any proceeding brought in accordance with this Section, and stipulates that the state and federal courts located in Los Angeles, California shall have in personam jurisdiction and venue over each of them for the purpose of litigating any dispute, controversy, or proceeding arising out of or related to this Agreement. Each party hereby authorizes and accepts service of process sufficient for personal jurisdiction in any action against it as contemplated by this Section by registered or certified mail, return receipt requested, postage prepaid, to its address for the giving of notices as set forth in this Agreement.
13.4 Waiver. Failure of any party to enforce a right under this Agreement shall not act as a waiver of that right or the ability to assert that right relative to the particular situation involved.
13.5 Enforceability. If any provision of this Agreement shall be found by a court of competent jurisdiction to be void, invalid or unenforceable, the same shall be reformed to comply with applicable law or stricken if not so conformable, so as not to affect the validity or enforceability of the remainder of this Agreement.
13.6 Modification. No change, modification, or addition or amendment to this Agreement, or waiver of any term or condition of this Agreement, is valid or enforceable unless in writing and signed and dated by the authorized officers of the parties to this Agreement.
| 31 |
13.7 Entire Agreement. This Agreement and the Schedules hereto (which are incorporated herein by this reference as if fully set forth herein) constitute the entire agreement between the parties with respect to the subject matter hereof and thereof, and replace and supersede as of the date hereof and thereof any and all prior agreements and understandings, whether oral or written, between the parties with respect to the subject matter of such agreements.
13.8 Construction. This Agreement has been prepared, examined, negotiated and revised by each party and their respective attorneys, and no implication shall be drawn and no provision shall be construed against any party to this Agreement by virtue of the purported identity of the drafter of this Agreement or any portion thereof.
13.9 Counterparts. This Agreement may be executed simultaneously in one or more counterparts, each of which shall constitute one and the same instrument. This Agreement may be executed by facsimile or in .pdf format.
13.10 Attorneys’ Fees. In the event of any action at law or in equity between the parties hereto to enforce any of the provisions hereof, the unsuccessful party to such litigation shall pay to the successful party all reasonable costs and expenses, including reasonable attorneys’ fees, incurred therein by such successful party; and if such successful party shall recover a judgment in any such action or proceeding, such reasonable costs, expenses and attorneys’ fees may be included in and as part of such judgment.
13.11 Assignment; Successors.
(a) Subject to Section 13.11(b), Licensee may assign this Agreement as part of a sale, regardless of whether such a sale occurs through an asset sale, stock sale, merger or other combination, or any other transfer of Licensee’s entire business, or that part of Licensee’s business that exercises all rights granted under this Agreement. Any other attempt to assign this Agreement by Licensee is null and void. In the event of a bankruptcy, assignment is permitted only to a party that can provide adequate assurance of future performance, including diligent development and sales, of Products.
(b) Prior to any assignment, the following conditions must be met: (i) Licensee must give CSMC thirty (30) days prior written notice of the assignment, including the new assignee’s contact information, (ii) the new assignee must agree in writing to CSMC to be bound by this Agreement, and (iii) CSMC must have received a $25,000.00 assignment fee.
(c) Subject to the limitations on assignment herein, this Agreement shall be binding upon and inure to the benefit of any successors in interest and assigns of CSMC and Licensee. CSMC shall have the right to assign its rights hereunder as part of any reorganization or bond financing.
13.12 Further Assurances. At any time and from time to time after the Effective Date, each party shall do, execute, acknowledge and deliver, and cause to be done, executed, acknowledged or delivered, all such further acts, transfers, conveyances, assignments or assurances as may be reasonably required to consummate the transactions contemplated by this Agreement.
13.13 Survival. The following sections shall survive any expiration or earlier termination of this Agreement: 4.3(e), 6.3, 8, 9, 10, 12 and 13.
[signature page follows]
| 32 |

| 33 |
Schedule A
Patent Rights
1. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 2017286561 |
| Country: | Australia |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621AU00 |
| CSMC Tech ID: | bho000961 |
2. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 3026066 |
| Country: | Canada |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621CA00 |
| CSMC Tech ID: | bho000961 |
3. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 201780000000 |
| Country: | China |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621CN00 |
| CSMC Tech ID: | bho000961 |
4. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 17814046.3 |
| Country: | Europe |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621EP00 |
| CSMC Tech ID: | bho000961 |
5. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 2018-565397 |
| Country: | Japan |
| Filing Date: | June 14, 2017 |
| Docket No.: | 065472.000621JP00 |
| CSMC Tech ID: | bho000961 |
6. Sensitization of Tumors to Therapies Through Endoglin Antagonism
| Inventor(s): | ▇▇▇▇▇▇▇▇ ▇▇, ▇▇▇▇▇ B, ▇▇▇▇▇▇▇▇▇ V, ▇▇▇▇▇▇, ▇. |
| Application No.: | 16/305,821 |
| Country: | United States |
| Filing Date: | Nov 29, 2018 |
| Docket No.: | 065472.000621US00 |
| CSMC Tech ID: | bho000961 |
Schedule B
Technical Information
All lab notes, methods, protocols, SOPs, and technical know-how developed by inventors, including
▇▇▇▇▇▇ et al (2018) Antagonizing CD105 enhances radiation sensitivity in prostate cancer.
Oncogene. 2018 Aug;37(32):4385-4397
Schedule C
Stock Issuance Agreement
[to be completed]
Schedule D
Milestones
| 1. | Licensee shall obtain an IND for a Product within one (1) year of the Effective Date | |
| 2. | Licensee shall commence a Phase II trial for a Product within two (2) years of the Effective Date | |
| 3. | Licensee shall submit an NDA/BLA to the FDA or equivalent regulatory agency in another jurisdiction for a Product within seven (7) years of the Effective Date |
Schedule E
Licensee Capitalization Table
[to be completed]
Schedule F
Royalty Reporting Form
Licensee name:
Reporting period:
Date of report:
Date of first commercial sale:
Royalty Report
| Product (list products by name) | No. units sold | Invoiced price per unit | Gross sales | Allowable deductions (attached itemized detail) |
Country of sale/foreign currency/ conversion rate |
Net sales | ||||||
Product name |
||||||||||||
Product name |
||||||||||||
Product name |
||||||||||||
| Total |
| Total net sales | $ | |
| Royalty rate | ||
| Royalty due | $ |
Total royalty due: $
Non-Royalty Sublicense Revenue Report
Total Non-Royalty Sublicense Revenue received |
$ | |
| Date received | ||
| Applicable percentage payable to CSMC | ||
| Total Non-Royalty Sublicense Revenue payable to CSMC | $ |
Report prepared by:
Title:
Date:
Please send electronic copy of report to ▇▇▇▇▇▇▇▇▇▇▇▇▇▇@▇▇▇▇.▇▇▇.

