ROYALTY PARTICIPATION AGREEMENT between THERAVANCE, INC. and ELAN CORPORATION PLC Dated as of May 12, 2013
Exhibit 10.1
ROYALTY PARTICIPATION AGREEMENT
between
THERAVANCE, INC.
and
ELAN CORPORATION PLC
Dated as of May 12, 2013
TABLE OF CONTENTS
Article I
DEFINITIONS AND INTERPRETATION
Article II
SALE OF BENEFICIAL INTEREST
|
Section 2.1 |
|
Sale of Beneficial Interest |
|
1 |
|
Section 2.2 |
|
Exclusion of GGL Agreement Rights |
|
2 |
|
Section 2.3 |
|
No Obligations Transferred |
|
3 |
|
Section 2.4 |
|
Purchase Price |
|
3 |
|
| ||||
|
Article III | ||||
|
| ||||
|
CLOSING | ||||
|
| ||||
|
Section 3.1 |
|
Closing |
|
3 |
|
Section 3.2 |
|
Payment of Purchase Price; Delivery of ▇▇▇▇ of Sale |
|
4 |
|
Section 3.3 |
|
Closing Conditions of Each Party |
|
4 |
|
| ||||
|
Article IV | ||||
|
| ||||
|
REPRESENTATIONS AND WARRANTIES OF SELLER | ||||
|
| ||||
|
Section 4.1 |
|
Organization |
|
5 |
|
Section 4.2 |
|
Power, Authorization and Enforceability |
|
5 |
|
Section 4.3 |
|
No Conflicts or Violations |
|
6 |
|
Section 4.4 |
|
Proceedings |
|
7 |
|
Section 4.5 |
|
GGL Agreements |
|
7 |
|
Section 4.6 |
|
Contractual Rights to Royalty Interest |
|
7 |
|
Section 4.7 |
|
Intellectual Property |
|
7 |
|
Section 4.8 |
|
UCC Matters |
|
8 |
|
Section 4.9 |
|
No Implied Representations and Warranties |
|
8 |
|
| ||||
|
Article V | ||||
|
| ||||
|
REPRESENTATIONS AND WARRANTIES OF PURCHASER | ||||
|
| ||||
|
Section 5.1 |
|
Organization |
|
9 |
|
Section 5.2 |
|
Power, Authorization and Enforceability |
|
9 |
|
Section 5.3 |
|
No Conflicts or Violations |
|
10 |
|
Section 5.4 |
|
Proceedings |
|
10 |
|
Section 5.5 |
|
Financing |
|
10 |
|
Section 5.6 |
|
No Implied Representations and Warranties |
|
10 |
|
| ||||
|
Article VI | ||||
|
| ||||
|
COVENANTS | ||||
|
| ||||
|
Section 6.1 |
|
Payment of Beneficial Interest |
|
10 |
|
Section 6.2 |
|
Tax Matters |
|
12 |
|
Section 6.3 |
|
GGL Agreements |
|
14 |
|
Section 6.4 |
|
Confidentiality |
|
14 |
|
Section 6.5 |
|
Information Rights |
|
16 |
|
Section 6.6 |
|
Books and Records |
|
16 |
|
Section 6.7 |
|
Public Announcement |
|
16 |
|
Section 6.8 |
|
Required Shareholder Approval |
|
17 |
|
Section 6.9 |
|
Royalty Pharma Offer |
|
17 |
|
Section 6.10 |
|
Reasonable Best Efforts |
|
18 |
|
Section 6.11 |
|
UCC Matters |
|
18 |
|
Section 6.12 |
|
Royalty Interest Disclosures |
|
18 |
|
Section 6.13 |
|
Minimum Seller Percentage of Royalty Interest |
|
18 |
|
Section 6.14 |
|
Non-Cash Consideration |
|
19 |
|
Section 6.15 |
|
Bankruptcy |
|
19 |
|
Section 6.16 |
|
Compliance with Trust Agreement |
|
22 |
|
| ||||
|
Article VII | ||||
|
| ||||
|
TERM AND TERMINATION | ||||
|
| ||||
|
Section 7.1 |
|
Term |
|
22 |
|
Section 7.2 |
|
Termination Prior to Closing |
|
22 |
|
Section 7.3 |
|
Effect of Termination |
|
23 |
|
| ||||
|
Article VIII | ||||
|
| ||||
|
INDEMNIFICATION | ||||
|
| ||||
|
Section 8.1 |
|
Indemnification by Seller |
|
24 |
|
Section 8.2 |
|
Indemnification by Purchaser |
|
24 |
|
Section 8.3 |
|
Indemnification Procedure |
|
24 |
|
Section 8.4 |
|
Claims Period for Breach of Representations and Warranties |
|
26 |
|
Section 8.5 |
|
Limitations on Indemnification |
|
26 |
|
Section 8.6 |
|
Exclusive Remedy |
|
27 |
|
Section 8.7 |
|
Disclaimer of Consequential Damages |
|
27 |
|
| ||||
|
Article IX | ||||
|
| ||||
|
MISCELLANEOUS | ||||
|
| ||||
|
Section 9.1 |
|
Assignment |
|
28 |
|
Section 9.2 |
|
Further Assurances |
|
29 |
|
Section 9.3 |
|
Notices |
|
29 |
|
Section 9.4 |
|
Specific Performance |
|
31 |
|
Section 9.5 |
|
Title; Headings; Captions |
|
31 |
|
Section 9.6 |
|
Independent Contractors |
|
31 |
|
Section 9.7 |
|
Third-Party Beneficiaries |
|
31 |
|
Section 9.8 |
|
Entire Agreement |
|
31 |
|
Section 9.9 |
|
Amendment and Supplementation |
|
32 |
|
Section 9.10 |
|
No Waiver |
|
32 |
|
Section 9.11 |
|
Severability |
|
32 |
|
Section 9.12 |
|
Governing Law |
|
32 |
|
Section 9.13 |
|
Submission to Jurisdiction |
|
32 |
|
Section 9.14 |
|
Waiver of Jury Trial |
|
32 |
|
Section 9.15 |
|
Counterparts |
|
32 |
|
|
|
|
|
|
|
Appendix A |
|
Definitions |
|
A-1 |
|
Exhibit A |
|
Press Release |
|
A-1 |
|
Exhibit B |
|
Form of ▇▇▇▇ of Sale |
|
B-1 |
|
Exhibit C |
|
Form of UCC Financing Statement |
|
C-1 |
|
Exhibit D |
|
Form of Assignment Agreement |
|
D-1 |
|
Exhibit E |
|
Form of Trust Agreement |
|
E-1 |
ROYALTY PARTICIPATION AGREEMENT
ROYALTY PARTICIPATION AGREEMENT, dated as of May 12, 2013 (this “Agreement”), between THERAVANCE, INC., a Delaware corporation (“Seller”), and ELAN CORPORATION PLC, an Irish public limited company (“Purchaser”; and each of Seller and Purchaser, a “Party” and together, the “Parties”).
BACKGROUND
A. Seller is party to certain drug research, development and commercialization agreements with GGL, referred to herein as the GGL Agreements (each as defined herein), pursuant to which Seller is entitled to certain royalties, milestone payments and other payments in connection with the development and commercialization of products under the GGL Agreements.
B. Seller wishes to sell to Purchaser, and Purchaser wishes to purchase, a participation interest in certain royalty payments when, as and if received by Seller from GGL, but not assign or otherwise transfer to Purchaser any rights to royalty payments, or any other rights, under any of the GGL Agreements.
C. To effect such intent, Purchaser and Seller intend for Seller to sell to Purchaser the beneficial interest in a trust, the corpus of which is a fractional undivided beneficial interest in amounts representing certain royalty payments, when, as and if received (but excluding (i) any rights of any kind under the GGL Agreements and (ii) any milestone payments and other payments) that Seller may receive from GGL pursuant to the GGL Agreements with respect to certain products, subject to the terms and conditions of this Agreement and in compliance with the terms and conditions of the GGL Agreements.
DEFINITIONS AND INTERPRETATION
Capitalized terms used but not otherwise defined in this Agreement are defined in Appendix A to this Agreement. Appendix A also contains rules of interpretation applicable to this Agreement.
SALE OF BENEFICIAL INTEREST
Section 2.1 Sale of Beneficial Interest.
(a) Effective as of the Closing:
(i) Pursuant to the terms of the Assignment Agreement, Seller shall sell, grant, assign, put over, transfer and convey to Newco the Assignment Interest, free and clear of all Encumbrances other than Permitted Encumbrances (the “Assignment”);
(ii) Pursuant to the terms of the Trust Agreement, Newco agrees to enter into the Trust Agreement in consideration for the Assignment, whereby Newco appoints the Trustee to hold in trust the Trust Property (as defined in the Trust Agreement) in favor of the Seller (as beneficiary) (“Declaration of Trust”); and
(iii) In consideration of the payment of the Purchase Price and subject to the terms and conditions of this Agreement, Seller shall sell, grant, assign, put over, transfer and convey to Purchaser, and Purchaser shall purchase, acquire and accept, the Beneficial Interest, free and clear of all Encumbrances, other than Permitted Encumbrances.
(b) For the avoidance of doubt, Purchaser is not acquiring any right or interest, including any royalty payment rights, under any GGL Agreement; Purchaser is instead acquiring the beneficial interest in a trust, the corpus of which is certain amounts when, as and if received by Newco pursuant to the Assignment Interest in its fractional undivided beneficial interest equal to the Percentage of the Royalty Interest payments that Seller may receive from GGL pursuant to the GGL Agreements (but excluding (i) any contractual rights of Seller to royalty payments, and (ii) any milestone payments and other payments that Seller may receive, in each case, under the GGL Agreements). In no circumstances will the Assignment Interest be based on:
(i) any royalty or other payments received by Seller pursuant to the GGL Agreements based on or relating to sales of Royalty Interest Products which occur prior to the Closing Date;
(ii) any royalty or other payments received by Seller pursuant to any of the GGL Agreements based on or relating to sales of Royalty Interest Products which occur at any time after the end of the Royalty Interest Term with respect to any Royalty Interest Product in any country;
(iii) any royalty or other payments received by Seller pursuant to any of the GGL Agreements based on or relating to sales of any Excluded Products, whether before, during or after the Royalty Interest Term; or
(iv) any non-monetary consideration provided to Seller pursuant to the terms of the GGL Agreements with respect to the Royalty Interest Products, subject to the terms of Section 6.14 of this Agreement.
Section 2.2 Exclusion of GGL Agreement Rights. It is understood and agreed by the Parties that, notwithstanding anything to the contrary in the Transaction Documents, Seller shall retain all of its rights and interests in, and no rights or interests are being sold, granted, assigned, put over, transferred or conveyed by Seller to Newco (other than the
Assignment Interest) or to Purchaser, under any of the GGL Agreements. Without limiting the foregoing, pursuant to the Trust Agreement, no contractual rights to any payments (whether to royalties or otherwise) under the GGL Agreements or the Assignment Agreement are being sold, granted, assigned, put over, transferred or conveyed, and no security interest or other Encumbrance is being granted or imposed on the GGL Agreements or the Assignment Agreement or on any rights to any payments under the GGL Agreements or the Assignment Agreement (whether to royalties or otherwise). It is the intent of the Parties that the transfer of the Assignment Interest shall constitute a true sale of the Percentage of the undivided beneficial interest in the Royalty Interest to Newco. The Seller hereby authorizes the filing under the UCC of a financing statement in the form attached hereto as Exhibit C with respect to the sale of the Assignment Interest by Seller to Newco, and any continuation statements (including amendments to effect such continuation) required under the UCC with respect thereto reasonably requested by Purchaser (subject to confidentiality obligations under the GGL Agreements) in order to evidence such sale.
Section 2.3 No Obligations Transferred. Notwithstanding anything to the contrary in the Transaction Documents, the sale, grant, put over, assignment, transfer and conveyance of the Assignment Interest and the Beneficial Interest shall not: (i) transfer to Newco, or make Newco subject to, any obligation or liability of Seller under the GGL Agreements, except as required to comply with the GGL Agreements (including the confidentiality obligations therein) and (ii) transfer to Purchaser, or make Purchaser subject to, any obligation or liability of Seller under the GGL Agreements. Such obligations and liabilities, including the VI Reverse Milestone Payment Obligation, are, and from and after the Closing shall remain, the obligations and liabilities of Seller. Purchaser understands and agrees that, under the Trust Agreement, neither it nor the Trustee shall have any rights (whether as a third party beneficiary or otherwise) to enforce (or in the case of Purchaser), to request or require that the Trustee enforce any rights or obligations under the Assignment Agreement, and for the avoidance of doubt, Purchaser hereby disclaims any and all such claims. For the avoidance of doubt, the foregoing shall not (a) affect Seller’s or Purchaser’s rights under this Agreement or modify the obligations Purchaser and Seller have under this Agreement or (b) affect any liability of either Party to the other under the provisions of Article VIII hereof.
Section 2.4 Purchase Price. The Purchase Price to be paid by Purchaser for the Beneficial Interest is One Billion United States Dollars (US$1,000,000,000) (the “Purchase Price”).
CLOSING
Section 3.1 Closing. Subject to the satisfaction or, if permissible, waiver of the conditions set forth in Section 3.3, the closing of the Transactions (the “Closing”) will take place on the Business Day following the satisfaction or waiver of the conditions set forth in Section 3.3 at the offices of Skadden, Arps, Slate, ▇▇▇▇▇▇▇ & ▇▇▇▇ LLP; ▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇; ▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, unless another time, date or place is agreed to in writing by the Parties (such date being the “Closing Date”).
Section 3.2 Payment of Purchase Price; Delivery of ▇▇▇▇ of Sale. At the Closing, (a) Purchaser shall deliver to Seller payment of the Purchase Price in its entirety, by wire transfer (same day) of immediately available funds, to the account(s) notified in writing by Seller, without any reduction, deduction, offset or exclusion, whether for withholding or other taxes, or otherwise and (b) Seller shall deliver to Purchaser the executed ▇▇▇▇ of Sale duly executed on behalf of Seller and shall instruct the Trustee to change the beneficiary of the Trust to Purchaser.
Section 3.3 Closing Conditions of Each Party.
(a) The respective obligations of each Party to consummate the Transactions are subject to the satisfaction or (to the extent permitted by Law) waiver by Seller and Purchaser at or prior to the Closing of the following conditions:
(i) if required pursuant to Rule 21 of the Irish Takeover Rules (“Rule 21”), the Required Shareholder Approval approving the Transactions shall have been obtained (it being understood and agreed that if such approval is no longer required pursuant to Rule 21, then this condition shall be deemed satisfied); and
(ii) no Governmental Authority shall have enacted, issued, promulgated, enforced or entered any Law or Order which is then in effect and has the effect of prohibiting the consummation of the Transactions.
(b) The obligations of Seller to consummate the Transactions are subject to the satisfaction at or prior to the Closing of the following further conditions:
(i) (A) the representations and warranties of Purchaser contained in Sections 5.1 and 5.2 (1) that are qualified by materiality or Purchaser Material Adverse Effect shall be true in all respects at and as of the Closing Date as if made at and as of such date, and (2) that are not qualified by materiality or Purchaser Material Adverse Effect shall be true in all material respects at and as of the Closing Date as if made at and as of such time, and (B) Seller shall have received a certificate signed by an appropriate executive officer of Purchaser to the foregoing effect.
(c) The obligations of Purchaser to consummate the Transactions are subject to the satisfaction at or prior to the Closing of the following further conditions:
(i) the Initial Transactions shall have been consummated; and
(ii) (A) the representations and warranties of Seller contained in Sections 4.1, 4.2, 4.5(a), 4.5(c), 4.5(d) and 4.6 (1) that are qualified by materiality or Seller Material Adverse Effect shall be true in all respects at and as of the Closing Date as if made at and as of such date, and (2) that are not qualified by materiality or Seller Material Adverse Effect shall be true in all material respects at and as of the Closing Date as if made at and as of such time,
and (B) Purchaser shall have received a certificate signed by an appropriate executive officer of Seller to the foregoing effect.
REPRESENTATIONS AND WARRANTIES OF SELLER
Except as disclosed in the separate disclosure letter which has been delivered by Seller to Purchaser prior to the execution of this Agreement (the “Seller Disclosure Schedule”) (each of which disclosures shall be effective with respect to (i) the corresponding sections and subsections of this Article IV, and (ii) any other section or subsection of this Article IV, but only if the relevance of that reference as an exception to (or a disclosure for purposes of) such section or subsection would be reasonably apparent to a reasonable person who has read that reference and such representations and warranties), Seller hereby represents and warrants to Purchaser as of the date of this Agreement and as of the Closing Date:
Section 4.1 Organization. Seller is a corporation duly organized, validly existing and in good standing, under the Laws of the state of Delaware. As of the Closing, Newco shall be a limited liability partnership duly organized and validly existing under the laws of England and Wales. Seller has made available to Purchaser true, correct and complete copies of its charter and bylaws, in each case as amended to the date hereof (the “Seller Charter Documents”), and shall have made available to Purchaser prior to Closing true, correct and complete copies of Newco’s organizational documents (the “Newco Charter Documents”) and Delaware Sub’s organizational documents (the “Delaware Sub Charter Documents”). All Seller Charter Documents are in full force and effect, and the Newco Charter Documents shall, as of the Closing, be in full force and effect. Seller is not in violation of the Seller Charter Documents, and, as of the Closing, (i) Newco shall not be in violation of the Newco Charter Documents, and (ii) Delaware Sub shall not be in violation of the Delaware Sub Charter Documents, except, in each case, as would not have, individually or in the aggregate, a Seller Material Adverse Effect.
Section 4.2 Power, Authorization and Enforceability.
(a) Seller has all necessary corporate power and authority to execute and deliver the Transaction Documents to which it is a party, to perform its obligations hereunder and thereunder, and to consummate the Transactions to which it is a party. The execution, delivery and performance by Seller of the Transaction Documents to which it is a party and the consummation by Seller of the Transactions to which it is a party have been duly and validly authorized by all necessary corporate action of Seller, and no other corporate proceedings on the part of Seller are necessary to authorize the execution, delivery and performance of this Agreement or to consummate such Transactions. This Agreement has been duly and validly executed and delivered by Seller and, assuming it is duly and validly executed and delivered by Purchaser, shall be a legal and valid binding obligation of Seller, enforceable in accordance with its terms, subject to applicable Laws affecting creditors’ rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
(b) As of the Closing, Newco shall have all necessary partnership power and authority, and Delaware Sub shall have all necessary limited liability company power and authority, to execute and deliver the Transaction Documents to which it is a party, to perform its respective obligations thereunder, and to consummate the Transactions to which it is a party. As of the Closing, the execution, delivery and performance by each of Newco and Delaware Sub of the Transaction Documents to which it is party and the consummation by each of Newco and Delaware Sub of the Transactions to which it is a party shall have been duly and validly authorized by all necessary partnership action of Newco and all necessary limited liability company action of Delaware Sub, and no other partnership proceedings on the part of Newco or limited liability company proceedings on the part of Delaware Sub shall be necessary to authorize the execution, delivery and performance of such Transaction Documents or to consummate such Transactions. As of the Closing, such Transaction Documents shall have been duly and validly executed and delivered by each of Newco and Delaware Sub and, assuming such Transaction Documents are duly and validly executed and delivered by the other parties thereto, shall be a legal and valid binding obligation of each of Newco and Delaware Sub, enforceable in accordance with their respective terms, subject to applicable Laws affecting creditors’ rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
Section 4.3 No Conflicts or Violations.
(a) None of the execution and delivery by Seller of the Transaction Documents to which it is a party, the consummation by Seller of the Transactions to which it is a party, or Seller’s compliance with such Transaction Documents will (i) conflict with or violate the Seller Charter Documents, (ii) constitute a breach or default by Seller under any of the GGL Agreements, (iii) constitute a breach or default under any agreement (other than the GGL Agreements) to which Seller or any of its Subsidiaries is a party or by which any of its or their property or assets are bound, (iv) require Seller to make any filing with or obtain any permit, authorization, consent or approval of any Governmental Authority or other Person, or (v) violate any Law or Order to which Seller or the Beneficial Interest is subject, except, in the case of Sections 4.3(a)(ii), (iii), (iv) and (v), as would not reasonably be expected to result in a Seller Material Adverse Effect.
(b) As of the Closing, none of the execution and delivery by each of Newco and Delaware Sub of ,the Transaction Documents to which it is a party, the consummation by each of Newco and Delaware Sub of the Transactions to which it is a party, or the compliance by each of Newco and Delaware Sub with such Transaction Documents will (i) conflict with or violate the Newco Charter Documents or the Delaware Sub Charter Documents, respectively, (ii) constitute a breach or default by Seller under any of the GGL Agreements, (iii) constitute a breach or default under any agreement (other than the GGL Agreements) to which Seller or any of its Subsidiaries is a party or by which any of its or their property or assets are bound, (iv) require Newco or Delaware Sub to make any filing with or obtain any permit, authorization, consent or approval of any Governmental Authority or other Person, or (v) violate any Law or Order to which Newco, Delaware Sub, or the Assigned Interest will be subject, except, in the case of Sections 4.3(b)(ii), (iii), (iv) and (v), as would not reasonably be expected to result in a Seller Material Adverse Effect.
Section 4.4 Proceedings. As of the date hereof, there is no action, suit, proceeding or, to the Knowledge of Seller, investigation before any Governmental Authority, court or arbitrator pending or, to the Knowledge of Seller, threatened in writing against Seller or its Subsidiaries relating to the GGL Agreements or the Beneficial Interest, except as would not reasonably be expected to result in a Seller Material Adverse Effect.
Section 4.5 GGL Agreements.
(a) Validity and Enforceability. Each of the GGL Agreements is a legal, valid and binding obligation, in full force and effect, enforceable in accordance with its terms in all material respects, of Seller and, to the Knowledge of Seller, of GGL, subject to applicable Laws affecting creditors’ rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
(b) Breach. Seller is not in breach of any of the GGL Agreements in any material respect.
(c) Assignment. Seller has not assigned to any Person any of the GGL Agreements since their initial dates of execution, in whole or in part, except as contemplated herein.
(d) Other.
(i) GGL does not have any right to unilaterally amend any GGL Agreement in any manner which would or would reasonably be expected to result, individually or in the aggregate, in a Seller Material Adverse Effect.
(ii) The information publicly disclosed, as publicly amended and supplemented, by Seller in the Seller Exchange Act Reports regarding the GGL Agreements and the Royalty Interest Products, does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make such information, in light of the circumstances in which it was disclosed, not misleading.
Section 4.6 Contractual Rights to Royalty Interest. Other than in connection with the Initial Transactions, Seller (i) is the exclusive owner of Seller’s entire right and interest in, to and under the GGL Agreements and the Royalty Interest, free and clear of all Encumbrances other than Permitted Encumbrances and (ii) has not assigned or granted to any Third Party any right or interest (including payments or proceeds) in or to any Royalty Interest beneficially or otherwise.
Section 4.7 Intellectual Property. To the Knowledge of Seller, except as would not reasonably be expected to have a Seller Material Adverse Effect, as of the date hereof:
(a) there are no pending litigations, interferences, reexaminations, post-grant proceedings or oppositions in any Major Market Country involving any patents or patent
applications owned by Seller and that cover the composition of matter of any of the Royalty Interest Products (collectively, the “Key Patents”);
(b) the Key Patents that have been granted are valid and enforceable in all material respects;
(c) there is no Person who is or claims to be an inventor under any of the Key Patents who is not a named inventor thereof;
(d) Seller has not received written notice of any claim by any Person challenging the ownership or the validity or enforceability of the Key Patents, or asserting that the manufacture, importation, sale, offer for sale or use of any of the Royalty Interest Products infringes such Person's patents or other intellectual property rights;
(e) no Third Party patent rights have been, are or currently would be, but for an infringement safe harbor, infringed by the manufacture, importation, sale, offer for sale or use of any of the Royalty Interest Products;
(f) no Person is infringing any of the Key Patents in any material respect; and
(g) all fees necessary to maintain in effect the issued Key Patents have been paid as and when due.
Section 4.8 UCC Matters. The Seller's exact legal name is, and during the five (5) years prior to the date hereof has been, "Theravance, Inc.". The principal place of business of the Seller is, and during the five (5) years prior to the date hereof has been, located in South San Francisco. The jurisdiction of organization of the Seller is, and has been during the five (5) years prior to the date hereof, located in Delaware. During the five (5) years prior to the date hereof, the Seller has not been the subject of any merger or limited partnership or other reorganization in which its identity or status was materially changed, except in each case when it was the surviving or resulting entity.
Section 4.9 No Implied Representations and Warranties. Purchaser acknowledges and agrees that: (i) other than the representations and warranties of Seller specifically contained in this Article IV, there are no representations or warranties of Seller, Newco or any other Person, and Seller hereby disclaims all other representations and warranties, whether express, statutory or implied, in connection with this Agreement or the Transactions, including with respect to the Beneficial Interest, the Initial Transactions, the GGL Agreements, the Royalty Interest Products, the Key Patents or any other Intellectual Property or data relating to the Royalty Interest Products including patents and patent applications and other Intellectual Property owned by GGL, or the Transactions, and (ii) Purchaser does not rely on, and Seller shall have no liability in respect of, any representation or warranty not specifically set forth in this Article IV. Without limiting the foregoing, but subject to Section 4.5(d)(ii), Purchaser acknowledges and agrees that (a)(i) the GGL Agreements generally impose confidentiality obligations on information relating to or generated in connection with those agreements and performance thereunder, and, accordingly, Purchaser has made its own investigation and assessment of the Beneficial Interest, the Royalty Interest Products, the GGL Agreements, the
Key Patents and any other Intellectual Property related to the Royalty Interest Products, including patents and patent applications and other Intellectual Property owned by GGL, and the Transactions, that Purchaser is entering into this Agreement based on such investigation and assessment and that Purchaser has not relied on and specifically waives any representation, warranty, description or statement, express or implied, set forth in any advertising, marketing literature or other documentation or materials concerning the GGL Agreements, the Beneficial Interest, the Royalty Interest Products, the Key Patents and any other Intellectual Property related to the Royalty Interest Products including patents and patent applications and other Intellectual Property owned by GGL, and (ii) Purchaser is not relying on, and shall have no remedies in respect of, any implied warranties whatsoever, including as to the future amount or potential amount of the Beneficial Interest, the creditworthiness of GGL or any of its Affiliates or any other matter, and (b) except as expressly set forth in any representation or warranty in this Article IV (as modified by the Seller Disclosure Schedule), Seller shall have no liability for losses or damages pursuant to this Agreement (or otherwise) with respect to any information, documents or materials furnished or made available to Purchaser or any of its Affiliates in any data room, presentation, interview or in any other form or manner relating to the Transactions or the GGL Agreements.
REPRESENTATIONS AND WARRANTIES OF PURCHASER
Except as disclosed in the separate disclosure letter which has been delivered by Purchaser to Seller prior to the execution of this Agreement (the "Purchaser Disclosure Schedule") (each of which disclosures shall be effective with respect to (i) the corresponding sections and subsections of this Article V, and (ii) any other section or subsection of this Article V, but only if the relevance of that reference as an exception to (or a disclosure for purposes of) such section or subsection would be reasonably apparent to a reasonable person who has read that reference and such representations and warranties), Purchaser hereby represents and warrants to Seller as of the date of this Agreement and as of the Closing Date:
Section 5.1 Organization. Purchaser is a public limited company duly organized and validly existing under the Laws of Ireland. Purchaser has made available to Seller true, correct and complete copies of its memorandum and articles of association or other relevant organizational and governance documents, in each case as amended to the date hereof (the "Purchaser Charter Documents"). All Purchaser Charter Documents are in full force and effect. Purchaser is not in violation of the Purchaser Charter Documents, except as would not have, individually or in the aggregate, a Purchaser Material Adverse Effect.
Section 5.2 Power, Authorization and Enforceability. Purchaser has, all necessary power and authority to execute and deliver the Transaction Documents to which it is a party, to perform its obligations hereunder and thereunder, and to consummate the Transactions to which it is a party. The execution, delivery and performance by Purchaser of the Transaction Documents to which it is a party and the consummation by Purchaser of the Transactions to which it is a party have been duly and validly authorized by all necessary corporate action of Purchaser, other than the Required Shareholder Approval, and no other corporate proceedings on the part of Purchaser are necessary to authorize the execution, delivery and performance of this
Agreement or to consummate such Transactions. This Agreement has been duly and validly executed and delivered by Purchaser and, assuming it is duly and validly executed and delivered by Seller, shall be a legal and valid binding obligation of Purchaser, enforceable in accordance with its terms, subject to applicable Laws affecting creditors' rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
Section 5.3 No Conflicts or Violations. None of the execution and delivery by Purchaser of the Transaction Documents to which it is a party, the consummation by Purchaser of the Transactions to which it is a party, or Purchaser's compliance with such Transaction Documents will (a) conflict with or violate the Purchaser Charter Documents, (b) constitute a breach or default under any agreement to which Purchaser or any of its Subsidiaries is a party or by which any of its or their property or assets is bound, (c) require Purchaser to make any filing with or obtain any permit, authorization, consent or approval of any Governmental Authority or other Person, or (d) violate any Law or Order to which Purchaser is subject, except in the case of Sections 5.3(b), (c) and (d), as would not reasonably be expected to result in a Purchaser Material Adverse Effect.
Section 5.4 Proceedings. There is no action, suit, proceeding or, to the Knowledge of Purchaser, investigation before any Governmental Authority, court or arbitrator pending or, to the Knowledge of Purchaser, threatened in writing against Purchaser or its Affiliates relating to the Transactions, except as would not reasonably be expected to result in a Purchaser Material Adverse Effect.
Section 5.5 Financing. Purchaser has and, at the time of the Closing will have, sufficient funds in cash available to consummate the Transactions, including payment of the Purchase Price. Purchaser acknowledges and agrees that its obligations hereunder are not subject to any conditions regarding Purchaser's or any other Person's ability to obtain funding or financing for the consummation of the Transactions.
Section 5.6 No Implied Representations and Warranties. Seller acknowledges and agrees that, other than the representations and warranties of Purchaser specifically contained in this Article V, there are no representations or warranties of Purchaser or any other Person either expressed or implied with respect to the Beneficial Interest or the Transactions, and that it does not rely on, and Purchaser shall have no liability in respect of, any representation or warranty not specifically set forth in this Article V.
COVENANTS
Section 6.1 Payment of Beneficial Interest.
(a) Seller shall irrevocably instruct GGL to make any and all payments in respect of the Assignment Interest into the Account or, if the Lock Box Arrangement is in place, into the Lock Box Account (and from the Lock Box Account into the Account in accordance with the Lock Box Arrangement) when, as and if any such amounts become payable. Payments in the Account shall be released to Purchaser on the earlier to occur of (i) the next
Business Day following the delivery to the Trustee of an Earnings Release Notification (as such term is defined in the Trust Agreement) and (ii) sixty (60) days after receipt into the Account of such payments. Pursuant to the Trust Agreement, the Settlor can make additional contributions to the Trust for the benefit of the Beneficiary at any time in its own discretion. At any ▇▇▇▇ ▇▇▇▇▇▇ releases its quarterly or annual public financial statements, Seller shall promptly thereafter deliver to the Trustee an Earnings Release Notification (as such term is defined in the Trust Agreement) regarding such financial statements. Failure to so notify the Trustee shall be without prejudice to Purchaser delivering to the Trustee an Earnings Release Notification (as such term is defined in the Trust Agreement). Pursuant to the Trust Agreement, Seller may deliver a certificate setting forth the amount of any Cost-Sharing Deductions to be remitted to Seller pursuant to the Trust Agreement. The Parties shall share equally the out-of-pocket costs and expenses associated with the Trust, the Lock Box Account and the Account. Notwithstanding the foregoing, to the extent Seller receives any amounts in respect of the Assignment Interest from GGL, Seller shall, within two (2) Business Days after such receipt remit, by wire transfer of immediately available funds in United States Dollars to the Account. All such payments are subject to withholding for Taxes, levies and other duties as applicable under the GGL Agreement.
(b) In the event that GGL reduces, deducts, offsets or excludes from royalty payments made to Seller in respect of sales of Royalty Interest Products any amounts that GGL claims Seller owes to GGL (i) related to the Royalty Interest, the Transactions or any of the Transaction Documents (including any deduction or withholding for Taxes), and Seller disputes any such reductions, deductions, offsets or exclusions, Seller shall notify Purchaser (to the extent not prohibited under the GGL Agreements) of such dispute and, upon resolution (if any) of such dispute and payment of such disputed amount by GGL to Seller, Seller shall instruct GGL to make a payment in respect of the Assignment Interest into the Account with respect to such disputed amount, (ii) related to the Royalty Interest and (A) there is no dispute between GGL and Seller as to the amount of the royalty payment due in respect of a Royalty Interest Product under the GGL Agreements, (B) there is no dispute between GGL and Seller as to the appropriate amount of the reductions, deductions, offsets or exclusions from such royalty payment and (C) Seller nonetheless consents to a reduction, deduction, offset or exclusion from such royalty payment of an amount in excess of the appropriate reduction, deduction, offset or exclusion amount, then Seller shall pay to the Account an amount equal to such agreed to reduction, deduction, offset or exclusion in excess of such appropriate amount of reductions, deductions, offsets and exclusions multiplied by the Percentage, or (iii) unrelated to the Royalty Interest, the Transactions or any of the Transaction Documents, then, Seller shall pay to the Account an amount equal to the amount of such reduction, deduction, offset or exclusion multiplied by the Percentage.
(c) To the extent that any amount other than payments of the Assignment Interest is deposited by GGL in the Account, Purchaser shall, within two (2) Business Days after becoming aware thereof (but in no event prior to receipt of such amount by Purchaser from the Account), remit such amount, by wire transfer of immediately available funds in United States Dollars to Seller.
(d) Purchaser acknowledges and agrees that neither Seller nor Newco is guaranteeing the performance or payment by GGL of its obligations under the GGL Agreements,
and without limiting the foregoing, Purchaser shall have no recourse to Seller or Newco under this Agreement or otherwise due to GGL's failure to perform its obligations under or breach of the GGL Agreements.
(e) To the extent that Seller incurs any out-of-pocket costs and expenses under the GGL Agreements in connection with the Royalty Interest or brings suit or other action against GGL to enforce Seller's rights or GGL's obligations under the GGL Agreements with respect to or to the extent affecting the Royalty Interest, Seller shall be entitled to receive from the Trustee pursuant to Section 7.1 of the Trust Agreement an amount equal to the product of (x) the Percentage multiplied by (y) such out-of-pocket costs and expenses actually incurred by Seller and not reimbursed by GGL pursuant to the GGL Agreements ("Cost-Sharing Deductions") without duplication (after giving effect to all actions taken under the other Transaction Documents). Without prejudice to Seller’s right to receive such amount under Section 7.1 of the Trust Agreement, Purchaser reserves the right to object to any claim by Seller with respect to any such Cost-Sharing Deduction, and following the resolution of any such objection, Seller shall pay Purchaser such portion (if any) of any such Cost-Sharing Deduction that is determined to not have been owed to Seller, it being understood and agreed that any restrictions on Seller's right to provide information under the GGL Agreements in connection with such claim shall not be used to prejudice Seller's position in any such dispute. In the event that any amounts then in the Account are insufficient to pay in full the Cost-Sharing Deductions, then Purchaser shall, promptly upon demand from Seller, pay Seller the extent of any such insufficiency. To the extent Seller is subsequently reimbursed by GGL for such Cost-Sharing Deductions, Seller shall deposit into the Account an amount equal to the product of (x) the Percentage, multiplied by (y) the amount of such reimbursement.
(f) If at any time, Seller and GGL (or their successors under the GGL Agreements) merge, consolidate or otherwise become one and the same entity and the Royalty Interest ceases to be payable in whole or in part, then Seller, or if Seller is not the surviving entity, the surviving entity shall pay to Purchaser at such times as would have been payable an amount equal to the amounts Purchaser would otherwise have been entitled to receive under the Transaction Documents as if the GGL Agreements were in full force and effect and the Royalty Interest remained outstanding.
(g) Seller shall not, and shall cause Newco not to, amend or terminate the Assignment Agreement or the Trust Agreement without Purchaser's prior written consent.
Section 6.2 Tax Matters.
(a) Seller shall be entitled to deduct and withhold from the amounts paid or delivered in connection with the Transactions any amounts that Seller is required to deduct and withhold with respect to any such deliveries and payments under the Internal Revenue Code of 1986, as amended, or any provision of state, local, or non-U.S. Law. If (A) Seller assigns or otherwise transfers this Agreement or the GGL Agreements, (B) Seller, Newco or a Newco Partner changes its jurisdiction of incorporation, residence or organizational structure for tax purposes, (C) Seller violates Section 6.2(b) or Section 6.3 or (D) Seller ceases to be a company described in Article 23(2)(c) of the Treaty, and the amount that is deducted or withheld pursuant to the previous sentence or any amount deducted or withheld by GGL on a
Royalty Interest payment under the GGL Agreement is greater than the amount that otherwise would have been deducted or withheld, then Seller (or such assignees or transferees) shall pay to the Account such additional amounts so that after all such deduction and withholding have been paid, Purchaser receives an amount equal to the amount it would have received had such event described in clause (A), (B), (C) or (D) not occurred, provided that in the event that (W) Purchaser assigns or otherwise transfers this Agreement, (X) Purchaser changes its jurisdiction of incorporation, residence, or organizational structure for tax purposes, (Y) Purchaser violates Section 6.2(c), or (Z) such requirement to deduct and withhold arises from a change in Law occurring after the date of this Agreement, the amount payable by Seller (or its assignees or transferees) pursuant to this sentence shall be reduced to an amount equal to the payment that would have been required by Seller (or its assignees or transferees) had such event described in clause (W), (X), (Y) or (Z) not occurred. To the extent that Seller withholds such amounts and remits such withheld amounts to the applicable Governmental Authority, such withheld amounts shall be treated as having been paid to or on behalf of Purchaser. If Seller determines that it is required by Law to deduct and withhold any amount as described in this Section 6.2(a), Seller shall notify the Trustee and Purchaser of any such requirement as soon as reasonably practical after such determination is made by Seller. Assuming the accuracy of all representations and covenants made by Purchaser in this Agreement, Seller does not intend, as of the date of this Agreement and under Law at such time, to deduct and withhold any amount as described in this Section 6.2(a), provided that Purchaser complies in all respects with its obligations under this Agreement. Seller makes no guarantees, however, as to whether such deduction and withholding may ultimately be required.
(b) To the extent not prohibited by the GGL Agreements, Seller agrees to use its commercially reasonable efforts to provide in a timely manner such cooperation or assistance (including by providing any necessary tax forms or other documents) as may be reasonably requested by Purchaser or the Trustee (at the sole cost of Purchaser) at any time after the date of this Agreement, so long as the cooperation or assistance could not reasonably be expected to prejudice in any material respect the legal or commercial position of Seller, in order to reduce or eliminate any amounts deducted or withheld pursuant to Section 6.2(a) or any amounts deducted or withheld by GGL on a Royalty Interest payment under the GGL Agreements.
(c) Purchaser agrees to use its commercially reasonable efforts to provide in a timely manner such cooperation or assistance (including by providing any necessary tax forms or other documents) as may be reasonably requested by Seller or the Trustee at any time after the date of this Agreement, so long as the cooperation or assistance could not reasonably be expected to prejudice in any material respect the legal or commercial position of Purchaser, in order to reduce or eliminate any amounts deducted or withheld pursuant to Section 6.2(a) or any amounts deducted or withheld by GGL on a Royalty Interest payment under the GGL Agreements.
(d) In the event that Seller or any Seller Affiliate receives from any Tax Authority any refund or repayment of any amount which has been deducted or withheld pursuant to Section 6.2(a) or any amount which has been deducted or withheld by GGL on a Royalty Interest payment under the GGL Agreements, Seller shall pay such refund or repayment to Purchaser as soon as reasonably practicable.
Section 6.3 GGL Agreements.
(a) Seller shall comply in all material respects with its obligations under the GGL Agreements.
(b) Seller agrees that it will not, without Purchaser’s prior written consent, (i) consent to any termination of the GGL Agreements with respect to the Royalty Interest Products, (ii) consent to any amendment of the provisions of the GGL Agreements that implement the formula for calculating the royalties payable under the Royalty Interest or (iii) impose an Encumbrance on the Assignment Interest or Beneficial Interest.
(c) Purchaser acknowledges and agrees that, except for the express covenants contained in the Transaction Documents, neither Seller nor Newco shall have any obligation or liability, whether express or implied, in connection with the exercise of Seller’s rights and performance of its obligations under the GGL Agreements and that neither Purchaser nor any other person shall have any claim of any kind against Seller or Newco based on or arising out of Seller’s performance under or in connection with the GGL Agreements, it being understood that Seller shall not be liable or have any obligation with respect to allocations of resources, scope, intensity and duration of efforts, and decisions and judgments made in connection with development and commercialization (including any acts or omissions that result in, or increase the likelihood of, greater or lesser commercial success) (i) with respect to, or as among, any Royalty Interest Products, or (ii) as among any one or more Royalty Interest Products, on the one hand, and any Excluded Products, other products or therapeutically active components, on the other hand.
Section 6.4 Confidentiality.
(a) Definition. “Confidential Information” means all information (whether written or oral, or in electronic or other form, and whether furnished before or after the date of this Agreement) disclosed or made available to Purchaser by or on behalf of Seller concerning, or relating in any way, directly or indirectly, to this Agreement, the Transactions or the Royalty Interest, including (i) this Agreement, including all terms and conditions hereof, (ii) any royalty information, assignments, notices, requests, correspondence or other information furnished pursuant to this Agreement, and any other reports, data, materials, notices, correspondence or documents of any kind relating in any way, directly or indirectly, to this Agreement, the Royalty Interest or the intellectual property, compounds or products giving rise to the Royalty Interest, (iii) any inventions, devices, improvements, formulations, discoveries, compositions, ingredients, patents, patent applications, know-how, processes, trial results, research, developments or any other intellectual property, trade secrets or information involving or relating in any way, directly or indirectly, to the Royalty Interest or the compounds or products giving rise to the Royalty Interest, and (iv) any other material proprietary and confidential information, in whatever form.
(b) Exclusions. Confidential Information will not include information which:
(i) at or prior to the time of disclosure by Seller was known to Purchaser or its Affiliates through lawful means and is not under any obligation of confidentiality, as evidenced by Purchaser’s written records;
(ii) at or after the time of disclosure by Seller becomes generally available to the public through no act or omission on the part of Purchaser or its Affiliates in violation of any obligation of confidentiality or any other legal, contractual or fiduciary obligation to Seller, GGL or any of their respective Affiliates, as evidenced by documents that were generally published prior to any disclosure of such information by Seller;
(iii) is developed by Purchaser independent of, and without any use of or access or reference to Confidential Information, as evidenced by Purchaser’s written records; or
(iv) Purchaser receives from a Third Party free to make such disclosure free of any obligation of confidentiality and without breach of any other legal obligation; provided that such Third Party, had the right to disclose such information to Purchaser (free of any obligation of confidentiality and without any legal, contractual or fiduciary obligation to Seller, GGL or any of their respective Affiliates) as evidenced by Purchaser’s written records.
(c) Requirements. Purchaser acknowledges the confidential and proprietary nature of the Confidential Information and agrees that it shall not discuss, reveal, or disclose the Confidential Information to any other Person, or use any Confidential Information for any purpose, other than in connection with the performance of this Agreement, in each case, without the prior written consent of Seller. Purchaser agrees to take all reasonable precautions (no less rigorous than Purchaser takes with respect to its own comparable confidential and proprietary information) to prevent unauthorized or inadvertent disclosure of the Confidential Information.
(d) Disclosure, Legal Obligations. Notwithstanding the foregoing, Purchaser may disclose such Confidential Information to its officers, directors, employees, accountants, counsel, consultants, advisors and agents who need to know such information in connection with Purchaser’s evaluation and performance of the Transactions and to its lenders or other financing sources in connection with obtaining financing for Purchaser’s operations, so long as (i) such Persons are informed by Purchaser of the confidential nature of such Confidential Information and agree to treat such information confidentially in accordance with the requirements of this Section 6.4 for the benefit of Seller and (ii) Purchaser shall be liable for any breach or violation of such obligations by any such Person. Purchaser may also disclose Confidential Information to the extent required by Law, including the applicable rules of any stock exchange that are binding on Purchaser, provided that Purchaser shall use its reasonable best efforts to notify Seller of such requirement (to the extent permitted by applicable Law) promptly and sufficiently in advance of any such disclosure so that Seller may seek an appropriate protective order or other appropriate remedy. Purchaser shall use its reasonable best efforts to limit such disclosures to that portion of the information legally required to be disclosed and shall use its reasonable best efforts to seek confidential treatment for such information if so
requested by Seller. In addition, the Parties will coordinate in advance with each other in connection with the redaction of certain provisions of this Agreement with respect to any SEC or foreign equivalent filings of this Agreement, and each Party shall use its reasonable best efforts to seek confidential treatment for such terms if so requested by the other Party.
(e) Return of Information. Purchaser will, at the request of Seller, after the expiration of the Term or effective termination of this Agreement (i) promptly return all Confidential Information held or used by Purchaser in whatever form, or (ii) at the discretion of Seller, promptly destroy all such Confidential Information, including all copies thereof, and those portions of all documents (e.g., analyses, summaries, etc.) that incorporate or are based on Confidential Information; provided, however, that (x) Purchaser’s legal department may retain one copy of such information for legal compliance purposes only for so long as required by applicable Law or Purchaser’s bona fide pre-existing record-keeping policy, and (y) notwithstanding anything else in this Agreement to the contrary, the confidentiality restrictions imposed hereby shall continue to apply to such information, and shall survive any termination of this Agreement, for so long as such information is retained. At the request of Seller, Purchaser will promptly certify that it has complied in full with this Section 6.4.
Section 6.5 Information Rights. Purchaser hereby acknowledges the confidentiality obligations imposed on Seller by the GGL Agreements. Notwithstanding any other provision of this Agreement, Purchaser agrees that Seller shall not, and shall not be required to, disclose any information in violation of its obligations under the GGL Agreements. Unless prohibited under the GGL Agreements (as determined in Seller’s sole reasonable discretion), Seller shall use its reasonable efforts to provide to Purchaser information reasonably requested by Purchaser that is material to the Beneficial Interest (it being understood that Seller shall not be required to undertake litigation or incur significant expense pursuant to this Section 6.5).
Section 6.6 Books and Records. For accounting purposes only, each of the Parties will treat the sale and assignment to Purchaser of the Beneficial Interest pursuant to Section 2.1 as a sale and assignment on its books and records to the extent permitted under generally accepted accounting principles in the United States.
Section 6.7 Public Announcement.
(a) On the date of execution of this Agreement or such later date as is mutually agreed by the Parties (the “Announcement Date”), the Parties shall release the mutually agreed upon press release attached as Exhibit A hereto announcing the Transactions. Upon the occurrence of the Closing, the Parties shall release a mutually agreed upon press release announcing the closing of the Transactions. These press releases may be modified after the date of this Agreement by the mutual written consent of both Parties.
(b) Except as required by applicable Law and subject to the final two (2) sentences of this Section 6.7(b), neither Party will make any public announcement of any information regarding this Agreement without the prior written approval of the other Party, which approval shall not be unreasonably withheld, conditioned or delayed; provided that the foregoing restriction shall not restrict Seller from making any public announcement of any
information regarding the Royalty Interest Products. Once any statement is approved for disclosure by the Parties or information is otherwise made public in accordance with the preceding sentence, either Party may make a subsequent public disclosure of the contents of such statement without further approval of the other Party. Notwithstanding the foregoing, within sixty (60) days following the Closing, appropriate representatives of the Parties will meet and agree upon a process and principles for reaching timely consensus on how the Parties will make public disclosure concerning this Agreement.
Section 6.8 Required Shareholder Approval.
(a) Within ten (10) days of the Announcement Date, Purchaser shall (i) give notice in compliance with applicable Law and otherwise in a form to be reasonably agreed by the Parties, of the extraordinary general meeting of its shareholders to be held to approve the Transactions (the “EGM”) to all shareholders of Purchaser entitled to such notice, (ii) distribute the documents required in connection with the EGM to its shareholders, in a form to be reasonably agreed by the Parties (the “EGM Documents”), and (iii) promptly take any other actions necessary to comply with applicable Law to convene the EGM and conduct a valid shareholder vote within thirty-five (35) days of the Announcement Date (the “Meeting Deadline”). To the extent that Purchaser proposes to distribute any other documents or make any other communications to its shareholders with respect to the Transactions, Purchaser shall first provide copies thereof to Seller for review and approval, such approval not to be unreasonably withheld or delayed.
(b) Purchaser confirms and undertakes that the EGM Documents and any other documentation with disclosure in respect of the merits of the Transactions sent to Purchaser’s shareholders in connection with the Transactions will contain a recommendation from Purchaser’s board of directors to such shareholders to vote in favor of the Transactions and such recommendation shall not be withdrawn, modified or altered in any way without Seller’s prior written consent.
(c) Following distribution of the EGM Documents, the EGM shall be held on a date prior to the Meeting Deadline to be reasonably agreed by the Parties, subject to the requirements of applicable Law. Purchaser shall not postpone or delay the EGM or the shareholder vote for any reason without Seller’s prior written consent.
Section 6.9 Royalty Pharma Offer
(a) To the extent permitted by Law, Purchaser shall give Seller reasonable prior written notice of any proposed discussion or correspondence between it or its representatives with the Irish Takeover Panel with respect to the Transactions and shall give Seller the opportunity to participate in any such proposed discussion or comment on the correspondence.
(b) Purchaser agrees to complete the Transactions without the need for the approval of its shareholders if the offer period in respect of the offer by Royalty Pharma (to be made through Echo Pharma Acquisition Limited) for Purchaser announced on April 15, 2013
pursuant to Rule 2.5 of the Irish Takeover Rules ends or Purchaser is not otherwise required to seek shareholder approval of the Transactions.
Section 6.10 Reasonable Best Efforts. The Parties hereto will use their respective reasonable best efforts to consummate and make effective the Transactions and to cause the conditions to the Closing set forth herein to be satisfied, including (a) the obtaining of all necessary actions or non-actions, consents and approvals from Governmental Authorities or other Persons necessary in connection with the consummation of the Transactions, including the shareholder vote with respect to the Required Shareholder Approval, and the making of all necessary registrations and filings (including filings with Governmental Authorities, if any) and the taking of all reasonable steps as may be necessary to obtain an approval from, or to avoid an action or proceeding by, any Governmental Authority or other Persons necessary in connection with the consummation of the Transactions, provided that neither Party shall seek or obtain, or be obligated to seek or obtain, any consent or approval under any GGL Agreement, (b) the defending of any lawsuits or other legal proceedings, whether judicial or administrative, challenging this Agreement or the consummation of the Transactions, performed or consummated by such Party in accordance with the terms and conditions of this Agreement, including seeking to have any stay or temporary restraining order entered by any court or other Governmental Authority vacated or reversed, and (c) the execution and delivery of any additional instruments necessary to consummate the Transactions.
Section 6.11 UCC Matters. The Seller shall file and maintain in effect all UCC filings, and shall take such other actions under the UCC, as may be necessary to protect the validity and perfection of Newco’s Assignment Interest under the UCC. The Seller will not change its location (within the meaning of Sections 9-301 and 9-307 of the applicable UCC) or its legal name without at least ten (10) days’ prior written notice to the Purchaser. In the event that the Seller desires to so change its location or change its legal name, the Seller will make any required filings and will deliver to the Purchaser copies of all such required filings with the filing information duly noted thereon by the office in which such filings were made.
Section 6.12 Royalty Interest Disclosures. Seller shall comply with its obligations under applicable Law to make prompt disclosure to the public of material information concerning the Royalty Interest.
Section 6.13 Minimum Seller Percentage of Royalty Interest. Seller hereby agrees that, with respect to each of the BREO/RELVAR/VI Monotherapy Royalty Interest and the ANORO Royalty Interest, during the Holding Period, either (x) Seller and its Affiliates or (y) the Applicable Successor of Seller and its Affiliates, shall hold, in the aggregate, the Minimum Percentage of such Royalty Interest; provided, however, that in the event Seller or an Applicable Successor is acquired (or all or substantially all of the assets of Seller or such Applicable Successor are acquired), then, in each case, the obligations under this Section 6.13 shall apply to such acquiror (provided that, for purposes of this Section 6.13, such acquiror may take into account the holding period of its predecessors-in-interest). For the avoidance of doubt, nothing in this Section 6.13 shall restrict the rights of Seller, its Affiliates or an Applicable Successor to pledge, encumber or otherwise ▇▇▇▇▇ ▇ ▇▇▇▇ or security interest over all or any portion of the Royalty Interest (other than the Assignment Interest) to a lender in connection with the bona fide borrowing of money by Seller or its Affiliates or such Applicable Successor from such lender
(other than a monetization or other transaction designed to hedge or transfer the economic interest in the Minimum Percentage of the Royalty Interest that Seller, its Affiliates or any such Applicable Successor may be required to hold at such time pursuant to this Section 6.13).
Section 6.14 Non-Cash Consideration. In the event a Royalty Interest is paid other than in cash, Seller shall pay to the Account an amount in cash equal to the fair market value, as reasonably determined by a third party mutually agreed by Purchaser and Seller, of the Percentage of such non-cash consideration as promptly as reasonably practicable following the determination of the fair market value of such non-cash consideration.
Section 6.15 Bankruptcy.
(a) Seller shall, during the term of the Trust, cause (i) Newco’s organizational documents to include the provisions substantially similar to the provisions set forth in Exhibit F hereto, and (ii) Delaware Sub’s limited liability agreement to be substantially in the form attached hereto as Exhibit G.
(b) Seller agrees to cause Newco, during the term of the Trust, to:
(i) keep proper books of account in accordance with its obligations under English law;
(ii) maintain its registered office in the United Kingdom, and not to establish a branch, agency or place of business or register as a partnership, company or body corporate within the United States or any other country or jurisdiction other than the United Kingdom;
(iii) do all such things as are necessary to maintain its limited liability partnership existence;
(iv) not:
(1) amend the Trust Agreement or the Assignment Agreement;
(2) incur any indebtedness for borrowed money;
(3) amend its partnership agreement in a manner adverse to Purchaser;
(4) unless required by applicable Law, have any employees (for the avoidance of doubt, the directors of Newco do not constitute employees);
(5) enter into any reconstruction, amalgamation, merger or consolidation or take any voluntary steps with a view to its winding-up;
(6) otherwise than as contemplated in the Transaction Documents, release from or terminate the appointment of the Account Bank (as that term is defined in the Trust Agreement); or
(v) subject to any requirement under applicable Law to maintain a registered office, enter into any lease for, or own, any real property.
(c) Seller agrees to cause Delaware Sub, during the term of the Trust, to:
(i) hold itself out to the public and all other Persons as a legal entity separate from the members and any other person and conduct its own business in its own name and require that all full-time employees of Delaware Sub, if any, identify themselves as such and not as employees of Seller or any of its subsidiaries;
(ii) allocate fairly and reasonably any taxes and any overhead expenses that are shared with an affiliate, including for shared office space and for services performed by an employee of an Affiliate;
(iii) use separate stationery, invoices and checks bearing its own name;
(iv) conduct all transactions with Seller and each such subsidiary strictly on an arm’s-length basis;
(v) at all times have a board of managers and not less than two independent managers, each of who (A) (1) has prior experience as an independent director, independent manager or independent member with at least three years of employment experience with one or more entities that provide, in the ordinary course of their respective businesses, advisory, management or placement services to issuers of securitization or structured finance instruments, agreements or securities and (2) is provided by CT Corporation, Corporation Service Company, National Registered Agents, Inc., Wilmington Trust Company SP Services, Inc., ▇▇▇▇▇▇▇ Management Company, Lord Securities Corporation or, if none of those companies is then providing professional Independent Managers, another nationally-recognized company reasonably approved by Seller and by the Purchaser, in each case that is not an Affiliate of Delaware Sub and that provides professional Independent Managers and other corporate services in the ordinary course of its business, (B) is not, and has not been for a period of five years prior to his or her appointment as an independent manager of Delaware Sub: (1) a stockholder (whether direct, indirect or beneficial), counterparty under a contract for commercial services, advisor or supplier of Seller or any of its Affiliates (the “Parent Group”), (2) a director, officer, employee, partner, attorney or consultant of the Parent Group, (3) a person related to any person referred to in clauses (1) or (2) above, (4) a person or other entity controlling or under common control with any such stockholder, partner, counterparty under a contract for
commercial services, supplier, employee, officer or director or (5) a trustee, conservator or receiver for any member of the Parent Group and (D) shall not at any time serve as a trustee in bankruptcy for Delaware Sub, Seller or any Affiliate thereof (each such individual meeting the requirements set forth above, the “Independent Manager”), and insure that (v) no resignation or removal of an Independent Manager shall be effective until (1) Delaware Sub has provided Purchaser and Purchaser with two Business Days’ prior written notice of such resignation or removal, and (2) a successor Independent Manager is appointed and such successor shall have accepted his or her appointment as an Independent Manager by a written instrument, (w) at least two members of Delaware Sub’s board of managers shall be Independent Managers, (x) Delaware Sub’s board of managers shall not approve, or take any other action to cause the filing of, a voluntary bankruptcy petition with respect to Delaware Sub or consent to an involuntary bankruptcy petition with respect to Delaware Sub unless a unanimous vote of Delaware Sub’s board of managers (which vote shall include the affirmative vote of the Independent Managers) shall approve the taking of such action in writing prior to the taking of such action, (y) Delaware Sub’s board of managers shall not vote on any matter requiring the vote of its Independent Managers under its limited liability company agreement unless and until each Independent Manager is then serving on Delaware Sub’s board of managers and (z) the provisions requiring Independent Managers and the provisions described in clauses (x) and (y) of this paragraph (v) cannot be amended without the prior written consent of Seller and Purchaser (for the avoidance of doubt, the Delaware Sub is permitted to have as many additional managers as Seller may determine in its sole and absolute discretion);
(vi) observe all organizational formalities as a distinct entity, and ensure that all actions relating to (A) the dissolution or liquidation of Delaware Sub or (B) the initiation of, participation in, acquiescence in or consent to any bankruptcy, insolvency, reorganization or similar proceeding involving Delaware Sub, are duly authorized by unanimous vote of its managers (including the Independent Managers);
(vii) maintain Delaware Sub’s books, records and bank accounts separate from those of Seller and each other subsidiary of Seller and otherwise in such a manner so that such books and records are readily identifiable as its own assets rather than assets of Seller or any such subsidiary;
(viii) maintain separate financial statements, showing its assets and liabilities separate and apart from those of any other person and not have its assets listed on any financial statement of any other person; provided, however, that Delaware Sub’s assets may be included in a consolidated financial statement of its Affiliates; provided that (i) appropriate notation shall be made on such consolidated financial statements to indicate the separateness of Delaware Sub from such Affiliates and to indicate that Delaware Sub’s assets and credit are not available to satisfy the debts and other obligations of such Affiliates or any
other company and (ii) such assets shall also be listed on Delaware Sub’s own separate balance sheet;
(ix) except as specifically permitted herein or in the Transaction Documents, not commingle its assets with assets of any other person;
(x) pay all of Delaware Sub’s expenses and liabilities out of its own funds;
(xi) except as otherwise provided herein, maintain its certificate of formation and limited liability company agreement in conformity with the Transaction Documents, such that it does not amend, restate, supplement or otherwise modify its certificate of formation and limited liability company agreement in any respect that would impair its ability to comply with the terms or provisions of any of the Transaction Documents;
(xii) maintain its separateness such that it does not merge or consolidate with or into, or convey, transfer, lease or otherwise dispose of (whether in one transaction or in a series of transactions, and except as otherwise contemplated herein) all or substantially all of its assets (whether now owned or hereafter acquired) to, or acquire all or substantially all of the assets of, any Person, nor at any time create, have, acquire, maintain or hold any interest in any subsidiary; and
(xiii) maintain at all times adequate capital in light of its contemplated business operations and liabilities and refrain from making any distributions or other payments in respect of its membership interests (including any repurchase of membership interests or return of capital) that would cause it to have inadequate capital.
Section 6.16 Compliance with Trust Agreement. Purchaser shall not, and shall instruct the Trustee not to, request any information regarding the payments made into the Account before the time specified in Section 2.5 of the Trust Agreement.
TERM AND TERMINATION
Section 7.1 Term. The term of this Agreement shall commence on the date hereof and end, with respect to each Royalty Interest Product, upon expiration or termination (including any early termination) of the Royalty Interest Term with respect to such Royalty Interest Product (the “Term”).
Section 7.2 Termination Prior to Closing. This Agreement may be terminated and the Transactions abandoned prior to the Closing:
(a) by Seller if the condition in Section 3.3(a)(ii) shall have become incapable of fulfillment, and shall not have been waived by Seller; provided, that, Seller may
only seek termination pursuant to this Section 7.2(a), if Seller has not breached in any material respect its representations and warranties or covenants in this Agreement, including, without limitation, its obligations under Section 6.10;
(b) by Seller if the Required Shareholder Approval is not obtained as required by the terms and conditions of this Agreement by the Meeting Deadline or at the EGM or if the Closing has not occurred by the Meeting Deadline and the conditions set forth in in Section 3.3(b)(i) are not then satisfied;
(c) by Seller if the EGM is not held before the Meeting Deadline or Purchaser otherwise breaches its obligations under Section 6.8;
(d) subject to Purchaser’s compliance with Section 7.3(b)(ii), by Purchaser if the condition in Section 3.3(a)(ii) shall have become incapable of fulfillment or if the Required Shareholder Approval is not obtained at the EGM, and, in each case, such condition shall not have been waived by Purchaser; provided, that, Purchaser may only seek termination pursuant to this Section 7.2(d), if Purchaser has not breached in any material respect its representations and warranties or covenants in this Agreement, including, without limitation, its obligations under Sections 6.8 and 6.10; or
(e) by Purchaser if the Closing has not occurred by the Meeting Deadline and the conditions set forth in Section 3.3(c) are not then satisfied.
In the event of termination by Seller or Purchaser pursuant to this Section 7.2, written notice thereof shall forthwith be given to the other and this Agreement shall be terminated, without further action by any Party.
Section 7.3 Effect of Termination.
(a) If this Agreement is terminated and the Transactions are abandoned as described in Section 7.2, this Agreement shall be of no further force and effect, except for the provisions of Section 6.4 (Confidentiality), this Section 7.3 and 0. Notwithstanding the foregoing, nothing in this Section 7.3 shall be deemed to release any Party from any liability for any prior breach by such Party of the terms and conditions of this Agreement or to impair the right of any Party to compel specific performance by the other Party of its obligations under this Agreement.
(b) If this Agreement is terminated pursuant to Section 7.2, Purchaser shall pay to Seller Ten Million United States Dollars (US$10,000,000) by wire transfer of immediately available funds to the bank account notified by Seller to Purchaser in writing: (i) if this Agreement is terminated pursuant to Section 7.2(a), 7.2(b) or 7.2(c), within two (2) Business Days of such termination or (ii) if terminated pursuant to Section 7.2(d) (other than due to the condition in Section 3.3(a)(ii) having become incapable of fulfillment in connection with a Specified Claim), contemporaneously with such termination.
INDEMNIFICATION
Section 8.1 Indemnification by Seller. Subject to the terms and conditions of this Article VIII, Seller shall indemnify Purchaser, its Affiliates and each of their respective officers, directors, managers, agents and representatives (collectively “Purchaser Indemnitees”) from and against any and all direct damages, losses, claims, costs, liabilities, demands or expenses (including reasonable attorneys’ fees and expenses in connection with any action, suit or proceeding) (collectively, “Losses”) suffered or incurred by any such Purchaser Indemnitee arising from:
(a) any breach of any representation or warranty made by Seller in this Agreement (provided, however, that for purposes of determining whether a breach of the representations and warranties set forth in Sections 4.1, 4.3, 4.4, 4.5(a), 4.5(b), 4.5(d)(i) and 4.7 has occurred for purposes of this Section 8.1(a) only, a violation, conflict, breach, default, failure or the existence of a state of facts or circumstances shall be deemed to be material or to have a Seller Material Adverse Effect if it changes the royalty rate payable in respect of the Royalty Interest Products in a manner adverse to Purchaser); and
(b) any breach of any covenant of Seller contained in this Agreement.
Section 8.2 Indemnification by Purchaser. Subject to the terms and conditions of this Article VIII, Purchaser shall indemnify Seller, its Affiliates and each of their respective officers, directors, managers, agents and representatives (collectively “Seller Indemnitees”) from and against any and all Losses suffered or incurred by any such Seller Indemnitee arising from:
(a) any breach of any representation or warranty made by Purchaser in this Agreement; and
(b) any breach of any covenant of Purchaser contained in this Agreement.
Section 8.3 Indemnification Procedure.
(a) Third-Party Claims.
(i) Notice of Third-Party Claims. A Party seeking indemnification (the “Indemnified Party”) with respect to any claim or demand made by any Person against the Indemnified Party (a “Third-Party Claim”) shall promptly notify the other Party (the “Indemnifying Party”) in writing (including in such notice a brief description of the claim for indemnification and the Loss, including damages sought or estimated, to the extent actually known or reasonably capable of estimation by the Indemnified Party) of any claim for indemnification, provided that failure to give such notice shall not relieve the Indemnifying Party of any liability hereunder except to the extent the Indemnifying Party is materially prejudiced by such failure.
(ii) Defense of Third-Party Claims. The Indemnifying Party shall be entitled to participate in the defense of the Third-Party Claim and, if it so chooses, to assume the defense thereof, at its own expense, with counsel selected by the Indemnifying Party; provided that such counsel is not reasonably objected to by the Indemnified Party. If the Indemnifying Party assumes the defense thereof, the Indemnifying Party (1) shall not be liable to the Indemnified Party for legal expenses subsequently incurred by the Indemnified Party in connection with the defense thereof, and (2) shall permit the Indemnified Party to participate in, but not control, the defense of any such action or suit through counsel chosen by the Indemnified Party, provided that such counsel is not reasonably objected to by the Indemnifying Party and the fees and expenses of such counsel shall be borne by the Indemnified Party. The Indemnifying Party shall be liable for the fees and expenses of counsel employed by the Indemnified Party in the defense of a Third-Party Claim for any period during which the Indemnifying Party has not assumed the defense thereof (other than during the period prior to the time the Indemnified Party shall have notified the Indemnifying Party of such Third-Party Claim).
(iii) Cooperation. The Indemnified Party shall, if requested by the Indemnifying Party, give reasonable assistance to and cooperate with the Indemnifying Party in defense of any claim. The Indemnifying Party shall reimburse the Indemnified Party for any Third Party reasonable legal expenses directly incurred from providing such assistance and cooperation. Such assistance and cooperation shall include (1) the retention of and the provision to the Indemnifying Party of records and information that are reasonably relevant to such Third-Party Claim and (2) the making available of employees on a mutually convenient basis for providing additional information and explanation of any material provided hereunder. The Indemnifying Party shall have the right to consent to the entry of judgment with respect to, or otherwise settle, a Third-Party Claim with the prior written consent of the Indemnified Party, which consent shall not be unreasonably withheld; provided that the Indemnified Party may withhold its consent if any such judgment or settlement imposes an unreimbursed monetary or continuing non-monetary obligation on such Indemnified Party or does not include an unconditional release of such Indemnified Party and its Affiliates from all liability in respect of claims that are the subject matter of the indemnified claim. Regardless of whether the Indemnifying Party shall have assumed defense of a Third-Party Claim, the Indemnified Party shall not be entitled to be indemnified pursuant to this Article VIII if the Indemnified Party shall settle such Third-Party Claim without the prior written consent of the Indemnifying Party.
(b) Claims by a Party. In order for an Indemnified Party to be entitled to any indemnification under this Article VIII in respect of Losses that do not arise out of or involve a Third Party Claim, the Indemnified Party must notify the Indemnifying Party promptly in writing (including in such notice a brief description of the claim for indemnification and the Loss, including damages sought or estimated, to the extent actually known or reasonably capable of estimation by the Indemnified Party); provided, that, the failure to promptly provide such
notice shall not affect the indemnification provided under this Article VIII except to the extent that the Indemnifying Party has been actually prejudiced as a result of such failure.
Section 8.4 Claims Period for Breach of Representations and Warranties. The representations and warranties contained in this Agreement shall not survive the Closing; however, claims for breach of the: (a) representations and warranties contained in this Agreement (other than any claims for breach of the Fundamental Representations) may be made at any time following the Closing and up to and including the date that is the first anniversary of the Closing Date; and (b) Fundamental Representations may be made at any time following the Closing and prior to the expiration of the applicable statute of limitations. In no event shall any Party hereto have any liability for any breach of a representation or warranty set forth in this Agreement unless the other Party hereto shall have delivered a notice to such Party, pursuant to Section 8.3 prior to the deadline applicable to the making of such claim set forth in the preceding sentence.
Section 8.5 Limitations on Indemnification.
(a) Notwithstanding anything herein to the contrary, Seller shall have no liability to Purchaser under this Agreement for any action taken by Seller or any failure by Seller to take any action pursuant to Article VI in compliance with any written instructions of Purchaser or with the prior written consent of Purchaser.
(b) Seller or Purchaser, as applicable, shall not be obligated to indemnify any Purchaser Indemnitee or Seller Indemnitee, as applicable, if the Losses incurred by such Person arise from the gross negligence, fraud or willful misconduct of Purchaser or Seller, as applicable, or their respective Affiliates, officers, directors, managers, agents and representatives (acting in their capacity as such).
(c) Notwithstanding anything in this Agreement to the contrary, Seller shall not have any liability under: (i) Section 8.1 with respect to any individual item (or any series of related items) if the Loss related thereto is less than US$750,000, and such items shall not be aggregated for purposes of Section 8.5(c)(ii); (ii) Section 8.1(a) unless the aggregate liability for all Losses suffered by the Purchaser Indemnitees thereunder exceeds US$15,000,000, and then only to the extent of such excess; or (iii) Section 8.1 (except to the extent of any amounts Seller fails to pay or have paid Purchaser in breach of Section 6.1 or Section 6.2(a) or for breach of Section 6.1(g)) in excess of the amount by which (x) the Purchase Price exceeds (y) the aggregate amount of payments made to Purchaser in respect of the Beneficial Interest theretofore received or receivable by Purchaser, in the aggregate; provided, however, that the cap set forth in clause (iii) shall not be less than Two Hundred Million Dollars (US$200,000,000) except with respect to any breach for which indemnification is sought that arises from or relates to a Specified Claim. Notwithstanding anything to the contrary in any Transaction Document, for any Loss for which Seller must indemnify any Purchaser Indemnitee under this Agreement, the amount paid by Seller shall count toward the liability cap under both this Agreement and under the Assignment Agreement and in no event shall Seller have any liability for Losses under the Assignment Agreement arising out of the same matter or set of facts or circumstances as any claims made, or for which a claim could be made, under this Agreement.
(d) Notwithstanding anything in this Agreement to the contrary, Purchaser shall not have any liability under: (i) Section 8.2 with respect to any individual item (or any series of related items) if the Loss related thereto is less than US$750,000, and such items shall not be aggregated for purposes of Section 8.5(d)(ii); (ii) Section 8.2(a) unless the aggregate liability for all Losses suffered by the Seller Indemnitees thereunder exceeds US$15,000,000, and then only to the extent of such excess; or (iii) after the Closing, Section 8.2 in excess of US$1,000,000,000.
(e) The amount of any Losses payable under this Article VIII by an Indemnifying Party shall be reduced by the amount, if any, of any tax benefit available to the Indemnified Party by reason of such Losses or the payment thereof and by any insurance proceeds or other recovery from a Third Party received by the Indemnified Party (net of any expenses incurred by such Indemnified Party in procuring such recovery) in connection with the matter out of which such Losses arise. If the Indemnified Party receives any proceeds subsequent to an indemnification payment by the Indemnifying Party, then such Indemnified Party shall reimburse the Indemnifying Party as promptly as practicable thereafter for any payment made by such Indemnifying Party pursuant to such Indemnifying Party’s indemnification obligations hereunder up to the amount received pursuant to this Article VIII by the Indemnified Party from such Indemnifying Party (net of any expenses incurred by such Indemnified Party in procuring such recovery).
(f) For purposes of determining the amount of any Losses payable pursuant to Sections 8.1 or 8.2 (but not, for the avoidance of doubt, for the purposes of determining the existence of a breach), any qualification as to materiality, Seller Material Adverse Effect or Purchaser Material Adverse Effect contained in the applicable underlying representation or warranty shall be disregarded.
Section 8.6 Exclusive Remedy. Notwithstanding anything to the contrary in the Transaction Documents the Parties hereto acknowledge and agree that this Article VIII (including Section 8.4 and Section 8.5) shall provide the Parties’ sole and exclusive remedy with respect to any matter or claim arising out of, relating to, or in connection with, the Transaction Documents and the Transactions, except (i) for fraud or willful misconduct, (ii) injunctive relief or claims for specific performance pursuant to Section 9.4 (Specific Performance) and (iii) Purchaser’s indemnification obligation pursuant to the last sentence of Section 9.1(b).
Section 8.7 Disclaimer of Consequential Damages.
(a) EXCEPT WITH RESPECT TO (i) A PARTY’S FRAUD OR WILLFUL MISCONDUCT, OR (ii) LOSSES ACTUALLY PAID TO A THIRD PARTY PURSUANT TO A THIRD-PARTY CLAIM IN ACCORDANCE WITH ARTICLE VIII, TO THE MAXIMUM EXTENT PERMITTED BY LAW, IN NO EVENT SHALL EITHER PARTY BE LIABLE TO THE OTHER PARTY OR ANY THIRD PARTIES FOR ANY INDIRECT, SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES, INVESTMENT LOSSES, LOST PROFITS, LOSS OF DATA, OR LOSS OF USE ARISING IN ANY MANNER OUT OF OR IN CONNECTION WITH THE TRANSACTION DOCUMENTS HOWEVER CAUSED WHETHER BY NEGLIGENCE, BREACH OF CONTRACT, TORT OR OTHERWISE AND WHETHER OR NOT SUCH PARTY HAS
BEEN ADVISED OF OR OTHERWISE MIGHT HAVE ANTICIPATED THE POSSIBILITY OF ANY SUCH DAMAGES.
(b) THE PARTIES HAVE AGREED THAT THE LIABILITY LIMITATIONS SPECIFIED IN SECTION 8.5 AND THIS SECTION 8.7 WILL SURVIVE AND APPLY EVEN IF ANY LIMITED REMEDY SPECIFIED IN THIS AGREEMENT IS FOUND TO HAVE FAILED ITS ESSENTIAL PURPOSE.
MISCELLANEOUS
Section 9.1 Assignment.
(a) Assignment Prior to Closing. Prior to the Closing, neither Party may assign or otherwise transfer this Agreement, in whole or in part, without the prior written consent of the other Party, except that (i) Seller may assign this Agreement in its entirety to an Affiliate of Seller to which Seller’s interests under the GGL Agreements may be assigned for so long as such Affiliate remains an Affiliate of Seller and if Seller guarantees the performance of this Agreement by such Affiliate; (ii) Purchaser may assign this Agreement in its entirety to an Affiliate of Purchaser for so long as such Affiliate remains an Affiliate of Purchaser and if Purchaser guarantees the performance of this Agreement by such Affiliate, and (iii) each of Seller and Purchaser may and shall assign this Agreement in its entirety to a successor to all or substantially all of the assets of such Party whether by merger, sale of stock, sale of assets or other similar transaction.
(b) Assignment Post-Closing. Following the Closing, (i) Seller may not assign or otherwise transfer this Agreement without the prior written consent of Purchaser except that Seller may assign this Agreement, in whole or in part, to (A) an acquirer of Seller or a successor to all or substantially all of the assets of Seller, whether by merger, sale of stock, sale of assets or other similar transaction, (B) with respect to any Royalty Interest Product, an Applicable Successor of Seller relating to such Royalty Interest Product, whether by merger, sale of stock, sale of assets or other similar transaction or (C) an Affiliate of Seller for so long as such Affiliate remains an Affiliate of Seller and if Seller guarantees the performance of this Agreement by such Affiliate; (ii) Purchaser may not assign or transfer this Agreement or the Beneficial Interest, in whole or in part, without the prior written consent of Seller except that Purchaser may assign this Agreement in its entirety to (A) an Affiliate of Purchaser for so long as such Affiliate remains an Affiliate of Purchaser and if Purchaser guarantees the performance of this Agreement by such Affiliate or (B) a Third Party that has acquired Purchaser or all or substantially all of the business or assets of Purchaser; and (iii) Purchaser may pledge, mortgage or otherwise grant a security interest over all or any part of the Beneficial Interest to a Third Party lender in connection with the borrowing of money by Purchaser from such Third Party lender without the consent of Seller, provided that (A) Purchaser shall remain obligated to comply with all of its obligations under this Agreement and (B) such Third Party assignee agrees in writing to comply with this Agreement, including the confidentiality obligations set forth in Section 6.4 of this Agreement. For the avoidance of doubt, the restrictions on assignment contained in this Section 9.1(b)(ii) shall not restrict Purchaser from selling a participation interest
in the Beneficial Interest as, if and when received, it being understood and agreed that such sale will not involve any assignment or transfer, in whole or in part, of this Agreement or any rights or obligations under this Agreement and the purchaser of such an interest will not have any rights to enforce this Agreement against Seller or Newco, whether directly, as a third party beneficiary or otherwise and Purchaser shall indemnify and hold harmless Seller and Newco from any such claim brought in violation of this provision.
(c) Seller Corporate Reorganization. Notwithstanding anything to the contrary herein, Seller shall be permitted to assign this Agreement and the rights and obligations hereunder in respect of the MABA ‘081 Royalty Interest, in whole or in part, without Purchaser’s consent, to a Third Party pursuant to the Seller Corporate Reorganization.
(d) Successors and Assigns. Subject to the terms and conditions of this Section 9.1, this Agreement shall be binding upon and inure to the benefit of the Parties hereto and their permitted successors and assigns. Any purported assignment or other transfer in violation of this Section 9.1 shall be void ab initio and of no force or effect.
Section 9.2 Further Assurances. After the Closing, from time to time, as and when reasonably requested by any Party, each Party shall execute and deliver, or cause to be executed and delivered, all such documents, certificates, agreements and instruments, and shall take, or cause to be taken, all such further or other actions, as are reasonably necessary and appropriate to carry out all of the provisions of this Agreement, or to consummate the Transactions.
Section 9.3 Notices. All notices, requests, demands, consents, waivers or other communications to or from either Party to the other Party must be in writing and will be deemed to have been given
(a) upon delivery, including upon confirmation by the carrier of delivery (which may be in electronic form), if delivered by hand or mailed via overnight or express mail service or by courier,
(b) in the case of a fax, when receipt is confirmed by telephone, reply email or reply fax from the recipient or other fax confirmation of receipt, or
(c) in the case of an email, when receipt is confirmed by telephone or reply email from the recipient,
addressed as follows (or to such other address or addresses as the Seller or the Purchaser may from time to time designate by notice given in accordance with this Section 9.3:
if to Seller:
Theravance, Inc.
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇
▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention of: ▇▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
Senior Vice President & General Counsel
Fax No.: (▇▇▇) ▇▇▇-▇▇▇▇
E-mail: ▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇.▇▇▇
with a copy to:
Skadden, Arps, Slate, ▇▇▇▇▇▇▇ & ▇▇▇▇, LLP
▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇
▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Attention of: Amr ▇▇▇▇▇▇; ▇▇▇▇ ▇▇▇▇▇▇▇
Fax No.: (▇▇▇) ▇▇▇-▇▇▇▇; (▇▇▇) ▇▇▇-▇▇▇▇
E-Mail: ▇▇▇.▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇; ▇▇▇▇.▇▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇
with a copy to:
▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ & ▇▇▇▇▇▇▇▇▇, LLP
▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇.
▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Attention of: ▇▇▇▇▇▇ ▇▇▇▇▇▇; ▇▇▇▇▇ ▇▇▇▇▇
Fax No.: (▇▇▇) ▇▇▇-▇▇▇▇
E-Mail: ▇▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇; ▇▇▇▇▇▇@▇▇▇▇▇▇.▇▇▇
if to the Purchaser:
Elan Corporation, plc
▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇
▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇
Attention of: ▇▇▇▇ ▇▇▇▇▇
General Counsel
Fax No.: ▇▇▇▇ ▇ ▇▇▇ ▇▇▇▇
E-mail: ▇▇▇▇.▇▇▇▇▇@▇▇▇▇.▇▇▇
with a copy to:
Cadwalader, ▇▇▇▇▇▇▇▇▇▇ & ▇▇▇▇ LLP
▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention of: ▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇
Fax No.: (▇▇▇) ▇▇▇-▇▇▇▇
E-mail: ▇▇▇▇@▇▇▇.▇▇▇
with a copy to:
A&L Goodbody Solicitors
25/28 International Financial Services ▇▇▇▇▇▇
▇▇▇▇▇ ▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇ ▇, ▇▇▇▇▇▇▇
Attention of: ▇▇▇▇ ▇▇▇▇▇
Fax No.: + 353 1 649 2649
E-mail: ▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇.▇▇▇
Section 9.4 Specific Performance. Each of the Parties hereto acknowledges that the other Party may have no adequate remedy at law if the first Party fails to perform any of its non-monetary obligations under this Agreement. In such event, each of the Parties agrees that the other Party shall have the right, in addition to any other rights it has (whether at law or in equity), to pursue equitable remedies such as an injunction or specific performance of this Agreement without necessity of posting any bond or undertaking in connection therewith. This remedy is separate and apart from any other remedy the Parties may have under this Agreement.
Section 9.5 Title; Headings; Captions. The title, headings and captions in this Agreement are included for convenience only and will not affect the meaning or interpretation of any provision of this Agreement.
Section 9.6 Independent Contractors. The Parties recognize and agree that each is operating as an independent contractor and not as an agent or fiduciary of the other. Notwithstanding any provision of this Agreement which may indicate otherwise, it is the specific intent of the Parties that this Agreement and the relationship created hereby is not, nor shall it be deemed to be, a separate entity, joint venture or partnership or any similar arrangement, nor shall any master/servant, employer/employee or principal/agent relationship be created between the Parties.
Section 9.7 Third-Party Beneficiaries. Except to the extent expressly contemplated with respect to Seller Indemnitees and Purchaser Indemnitees in Article VIII, this Agreement is for the sole benefit of Seller and Purchaser and their permitted successors and assigns, and nothing herein expressed or implied shall give or be construed to give to any Person, other than the parties hereto and such permitted successors and assigns, any legal or equitable rights hereunder. Notwithstanding anything herein to the contrary, with respect to any indemnification claim made pursuant to Article VIII, Seller or Purchaser shall make such claim on behalf of the applicable Seller Indemnitee or Purchase Indemnitee, as applicable, and such Person (unless such Person is Seller or Purchaser, itself) shall not be entitled to directly make such claim against Purchaser or Seller, as applicable.
Section 9.8 Entire Agreement. This Agreement, along with the Appendices, Exhibits, Schedules, Seller Disclosure Letter and Purchaser Disclosure Letter and other attachments hereto, sets forth the entire agreement and understanding between the Parties hereto with respect to the subject matter hereof and supersedes all prior and contemporaneous agreements and understandings relating to such subject matter.
Section 9.9 Amendment and Supplementation. This Agreement may be amended, modified or supplemented only by a written agreement signed by both Parties.
Section 9.10 No Waiver. Any waiver of, or consent to depart from, the requirements of any provision of this Agreement shall be effective only if it is in writing and signed by the Party giving it, and only in the specific instance and for the specific purpose for which it has been given. No failure or delay of either Party in exercising any power, right or remedy under this Agreement will operate as a waiver. No single or partial exercise of any power, right or remedy precludes any other or further exercise of such power, right or remedy or the exercise of any other power, right or remedy.
Section 9.11 Severability. If any of the covenants, agreements or terms of this Agreement is held invalid, illegal or unenforceable, then it will be deemed severable from the remaining covenants, agreements or terms of this Agreement and will in no way affect the validity, legality or enforceability of the remaining Agreement. Upon such determination that any covenant, agreement or term is invalid, illegal or unenforceable, this Agreement shall be deemed modified so as to effect the original intent of the Parties as closely as possible in order that the Transactions be consummated as originally contemplated to the fullest extent consistent with applicable Law.
Section 9.12 Governing Law. THIS AGREEMENT AND ALL ACTIONS, PROCEEDINGS OR COUNTERCLAIMS (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT WILL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF NEW YORK, WITHOUT GIVING EFFECT TO THE PRINCIPLES OF CONFLICTS OF LAW THEREOF EXCEPT AS SET FORTH IN SECTIONS 5-1401 OF THE NEW YORK GENERAL OBLIGATIONS LAW.
Section 9.13 Submission to Jurisdiction. The parties submit to the exclusive jurisdiction of the United States District Court for the Southern District of New York and of any New York State Court sitting in New York, New York for purposes of all legal proceedings arising out of or relating to this Agreement. The parties irrevocably waive, to the fullest extent they may do so, any objection that they may now or hereafter have to the laying of the venue of any such proceeding brought in such a court and any claim that any such proceeding brought in such a court has been brought in an inconvenient forum.
Section 9.14 Waiver of Jury Trial. Each Party irrevocably waives, to the fullest extent permitted by applicable Law, any and all right to trial by jury in any legal proceeding directly or indirectly arising out of or relating to this Agreement or the Transactions.
Section 9.15 Counterparts. This Agreement may be executed in any number of counterparts. Each counterpart will be an original, and all counterparts will together constitute one and the same instrument. Delivery of an executed counterpart via PDF or other electronic delivery shall be considered the same as delivery of a manually executed counterpart.
[Signature Page Follows]
IN WITNESS WHEREOF, the Parties hereto have set their hands by their authorized representatives as of the year and date first indicated above.
|
|
THERAVANCE, INC. | ||
|
|
| ||
|
|
| ||
|
|
By: |
/s/ ▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇▇▇ | |
|
|
|
Name: |
▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇▇▇ |
|
|
|
Title: |
Chief Executive Officer |
|
|
| ||
|
|
| ||
|
|
ELAN CORPORATION PLC | ||
|
|
| ||
|
|
| ||
|
|
By: |
/s/ ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ | |
|
|
|
Name: |
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇ |
|
|
|
Title: |
Executive Vice President and |
|
|
|
|
Secretary |
APPENDIX A
DEFINED TERMS
A. DEFINITIONS.
For the purposes of this Agreement, unless otherwise indicated, the following terms shall have the respective meanings specified below, and grammatical variations of such terms shall have corresponding meanings:
1. “Account” has the meaning set forth in the Trust Agreement.
2. “Affiliate” means, with respect to either Party, any other Person directly or indirectly controlling, controlled by or under common control with, such Party. For the purposes of this definition “control” means the possession, directly or indirectly, of (a) the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities or other equity interest, representation on its board of directors or body performing similar functions, by contract or otherwise, together with (b) ownership of the related equity interest in such Person; and the terms “controlling” and “controlled” have corollary meanings.
3. “Agreement” has the meaning set forth in the introductory paragraph of this Agreement.
4. “Announcement Date” has the meaning set forth in Section 6.7 of this Agreement.
5. “ANORO” means (a) the combination medicine comprising UMEC with VI, with no other therapeutically active component, and explicitly excluding either component as a monotherapy, and which is proposed, as of the date hereof, to be sold under the brand name “ANORO™ ELLIPTA™”, and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect to only such combination medicine set forth in subsection (a) comprising UMEC with VI, with no other therapeutically active component (and explicitly excluding either component as a monotherapy).
6. “ANORO Royalty Interest”:
(a) Means Seller’s entitlement under the Collaboration Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
(i) royalty payments on any net sales worldwide of ANORO by or on behalf of GGL, pursuant to Section 6.3 of the Collaboration Agreement;
(ii) any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Collaboration Agreement;
(iii) any Infringement Payments with respect to ANORO in lieu of the payments referred to in clause (a)(i) immediately above; and
(iv) any Other Amounts with respect to ANORO in lieu of the payments referred to in clause (a)(i) immediately above.
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets contemplated by Sections 6.3, 6.4.1 and 6.9 of the Collaboration Agreement. The VI Reverse Milestone Payment Obligation shall remain the obligation of Seller, and shall not be a reduction, deduction, offset or exclusion from the royalty payments under subsection (a) above of this definition.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Collaboration Agreement), (ii) one-time fees for new compounds contributed to the collaboration (including any one-time fee payments from GGL to Seller pursuant to Sections 6.1.3 or 6.1.4 of the Collaboration Agreement), (iii) milestone payments (including any milestone payments pursuant to Section 6.2 of the Collaboration Agreement), or (iv) any other payments (other than those expressly identified in subsection (a) above of this definition), in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to ANORO.
7. “Applicable Successor” means with respect to Seller and any of its Affiliates,with respect to each Royalty Interest Product, the assignee or successor-in-interest to all or substantially all of Seller’s interest under the applicable GGL Agreement with respect to such Royalty Interest Product (other than GGL).
8. “Assignment” has the meaning set forth in Section 2.1(a)(i) of this Agreement.
9. “Assignment Agreement” means the Assignment Agreement to be entered into on the Closing Date by Seller and Newco substantially in the form attached hereto as Exhibit D.
10. “Assignment Interest” means (i) a twenty-one percent (21%) undivided beneficial interest in the Royalty Interest and (ii) the assignment of a non-exclusive right to enforce payment in respect thereof under the GGL Agreements.
11. “Beneficial Interest” means 100% of the beneficial interest in the Trust.
12. “▇▇▇▇ of Sale” means that certain ▇▇▇▇ of Sale substantially in the form attached to this Agreement as Exhibit B.
13. “BREO/RELVAR” means (a) the combination medicine comprising FF and VI, with no other therapeutically active component, and explicitly excluding either component as a monotherapy, and which is proposed, as of the date hereof, to be sold under the brand name “BREO™ ELLIPTA™” in the United States and “RELVAR™ ELLIPTA™” in the European Union and Japan, and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect only to such combination medicine set forth in subsection (a) comprising FF and VI, with no other therapeutically active component (and explicitly excluding either component as a monotherapy).
14. “BREO/RELVAR Royalty Interest”:
(a) Means Seller’s entitlement under the Collaboration Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
(i) royalty payments on any net sales worldwide of BREO/RELVAR by or on behalf of GGL, pursuant to Section 6.3 of the Collaboration Agreement;
(ii) any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Collaboration Agreement;
(iii) any Infringement Payments with respect to BREO/RELVAR in lieu of the payments referred to in clause (a)(i) immediately above; and
(iv) any Other Amounts with respect to BREO/RELVAR in lieu of the payments referred to in clause (a)(i) immediately above.
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets contemplated by Sections 6.3, 6.4.1 and 6.9 of the Collaboration Agreement. The VI Reverse Milestone Payment Obligation shall remain the obligation of Seller, and shall not be a reduction, deduction, offset or exclusion from the royalty payments under subsection (a) above of this definition.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Collaboration Agreement), (ii) one-time fees for new compounds contributed to the collaboration (including any one-time fee payments from GGL to Seller pursuant to Sections 6.1.3 or 6.1.4 of the Collaboration Agreement), (iii) milestone payments (including any milestone payments pursuant to Section 6.2 of the Collaboration Agreement), or (iv) any other payments (other than those expressly identified in subsection (a) above of
this definition), in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to BREO/RELVAR.
15. “BREO/RELVAR/VI Monotherapy Royalty Interest” means the BREO/RELVAR Royalty Interest together with the VI Monotherapy Royalty Interest.
16. “Business Day” means any day that is not a Saturday, Sunday or other day on which banks are required or expressly authorized to be closed in London, England, New York, New York, San Francisco, California or Dublin, Ireland.
17. “Closing” has the meaning set forth in Section 3.1 of this Agreement.
18. “Closing Date” has the meaning set forth in Section 3.1 of this Agreement.
19. “Collaboration Agreement” means that certain Collaboration Agreement by and between Theravance, Inc. and Glaxo Group Limited dated November 14, 2002, as amended from time to time.
20. “Confidential Information” has the meaning set forth in Section 6.4(a) of this Agreement.
21. “Cost-Sharing Deductions” has the meaning set forth in Section 6.1(e) of this Agreement.
22. “Declaration of Trust” has the meaning set forth in Section 2.1(a)(i).
23. “Delaware Sub” means the general partner of Newco.
24. “Delaware Sub Charter Documents” shall have the meaning set forth in Section 4.1 of this Agreement.
25. “EGM” has the meaning set forth in Section 6.8(a).
26. “EGM Documents” has the meaning set forth in Section 6.8(a).
27. “Encumbrance” means any lien, charge, security interest, mortgage or pledge.
28. “Excluded Products” means any therapeutically active component or product, whether as a monotherapy, combination product or otherwise, and any delivery devices (whether stand-alone or with any one or more therapeutically active components or products), other than the Royalty Interest Products. “Excluded Products” includes, by way of example:
(a) UMEC, as a monotherapy or in a combination product (other than UMEC and VI, with no other therapeutically active component), including a combination product that includes (i) UMEC, (ii) VI and (iii) at least one other therapeutically active component;
(b) FF, as a monotherapy or in a combination product (other than FF and VI, with no other therapeutically active component), including a combination product that includes (i) FF, (ii) VI and (iii) at least one other therapeutically active component;
(c) MABA ‘081 other than as a monotherapy (i.e. excluding MABA ‘081 in combination with any one or more other therapeutically active component(s));
(d) VI, in any product other than (i) as a monotherapy, (ii) ANORO and (iii) BREO/RELVAR; and
(e) any other therapeutically active component or product (including a combination product of two or more therapeutically active components not expressly specified in the definition of each of ANORO, BREO/RELVAR, MABA ‘081 and VI Monotherapy).
29. “FF” means the inhaled corticosteroid fluticasone furoate (with the chemical structure as set forth in Attachment A-1) or an ester, salt or other noncovalent derivative thereof.
30. “Fundamental Representations” means the representations and warranties set forth in Sections 4.1, 4.2, 4.6, 5.1 and 5.2 of this Agreement.
31. “GGL” means Glaxo Group Limited and, with respect to any GGL Agreement, any successor in interest under such GGL Agreement.
32. “GGL Agreements” means the Collaboration Agreement and the Strategic Alliance Agreement.
33. “Governmental Authority” means any United States (federal, state or local), Irish or other foreign government, or any governmental, regulatory, judicial or administrative authority, agency or commission.
34. “Holding Period” means the eight (8) year period commencing on the Closing Date.
35. “Indemnified Party” has the meaning set forth in Section 8.3(a) of this Agreement.
36. “Indemnifying Party” has the meaning set forth in Section 8.3(a) of this Agreement.
37. “Infringement Payment” means any net collections, recoveries, damages, awards or settlement payments that are actually paid to Seller as a result of any settlement discussion, litigation, arbitration or other legal proceeding brought against third-party infringers by Seller pursuant to Section 13 of the Collaboration Agreement or Section 13 of Strategic Alliance Agreement, as applicable.
38. “Initial Transactions” means the Assignment and the Declaration of Trust.
39. “Intellectual Property” means all worldwide intellectual property and rights arising from or in respect of the following: all (a) inventions, discoveries, industrial designs,
business methods, patents and patent applications (including provisional and Patent Cooperation Treaty applications), including continuations, divisionals, continuations-in-part, reexaminations and reissues, extensions, renewals and any patents that may be issued with respect to the foregoing; (b) trademarks, service marks, certification marks, collective marks, trade names, business names, assumed names, d/b/a’s, fictitious names, brand names, trade dress, logos, symbols, Internet domain names and corporate names, and general intangibles of a like nature and other indicia of origin or quality, whether registered, unregistered or arising by Law, and all applications, registrations, and renewals for any of the foregoing, together with the goodwill associated with and symbolized by each of the foregoing; (c) published and unpublished works of authorship in any medium, whether copyrightable or not (including databases and other compilations of information, computer software, source code, object code, algorithms, and other similar materials and Internet website content), copyrights and moral rights therein and thereto, and registrations and applications therefor, and all issuances, renewals, extensions, restorations and reversions thereof; and (d) confidential and proprietary information, trade secrets, and know-how, including methods, processes, business plans, schematics, concepts, software and databases (including source code, object code and algorithms), formulae, drawings, prototypes, models, designs, devices, technology, research and development and customer information and lists.
40. “Irish Takeover Rules” means the Rules in the Takeover Panel Act, 1997, 2007 (as amended).
41. “Key Patents” has the meaning set forth in Section 4.7(a) of this Agreement.
42. “Knowledge of Purchaser” means the knowledge, after reasonable inquiry, of the following individuals: ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇▇ and ▇▇▇▇▇ ▇▇▇▇▇▇▇.
43. “Knowledge of Seller” means the knowledge, after reasonable inquiry, of the following individuals: ▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇▇ and ▇▇▇▇ ▇▇▇▇▇▇▇.
44. “Law” means any and all domestic (federal, state or local) or foreign laws, rules, regulations, statutes, orders, ordinance, judgments or decrees or other pronouncements by any Governmental Authority.
45. “Lock Box Account” means a lock box account that Seller may elect to establish from time to time, to receive the Royalty Interest payments from GGL, and from which the Beneficial Interest in Royalty Interest payments received would be paid into the Account.
46. “Lock Box Arrangement” means the contractual terms and conditions that govern the Lock Box Account.
47. “Losses” has the meaning set forth in Section 8.1 of this Agreement.
48. “MABA” means a bifunctional muscarinic antagonist-beta2 agonist.
49. “MABA ‘081” means (a) the MABA compound known as GSK961081, solely as a monotherapy (i.e., excluding GSK961081 in combination with any one or more other
therapeutically active component(s)), and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect to only GSK961081 solely as a monotherapy (i.e., excluding GSK961081 in combination with any one or more other therapeutically active component(s)).
50. “MABA ‘081 Royalty Interest”:
(a) Means Seller’s entitlement under the Strategic Alliance Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
(i) royalty payments on any net sales worldwide of MABA ‘081 by or on behalf of GGL, pursuant to Section 6.3 of the Strategic Alliance Agreement;
(ii) any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Strategic Alliance Agreement;
(iii) any Infringement Payments with respect to MABA ‘081 in lieu of the payments referred to in clause (a)(i) immediately above;
(iv) any Other Amounts with respect to MABA ‘081 in lieu of the payments referred to in clause (a)(i) immediately above.
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets in Sections 6.3, 6.4.1 and 6.9 of the Strategic Alliance Agreement.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Strategic Alliance Agreement), (ii) opt-in fees or payments for equity investments (including any payments from GGL to Seller pursuant to Sections 6.1.2 or 6.1.4 of the Strategic Alliance Agreement), (iii) milestone payments (including any milestone payments pursuant to Section 6.2 of the Strategic Alliance Agreement), or (iv) any other payments (other than those expressly identified in subsection (a) above of this definition), in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to MABA ‘081.
51. “Major Market Country” means each of Canada, France, Germany, Italy, Japan, Spain, the United Kingdom and the United States.
52. “Material Action” means to file any insolvency, or reorganization case or proceeding, to institute proceedings to have Delaware Sub be adjudicated bankrupt or insolvent, to institute proceedings under any applicable insolvency law, to seek any relief under any law relating to relief from debts or the protection of debtors, to consent to the filing or institution of bankruptcy or insolvency proceedings against Delaware Sub, to file a petition seeking, or consent to, reorganization or relief with respect to Delaware Sub under any applicable federal or state law relating to bankruptcy or insolvency, to seek or consent to the appointment of a receiver, liquidator, assignee, trustee, sequestrator, custodian, or any similar official of or for Delaware Sub or a substantial part of its property, to make any assignment for the benefit of creditors of Delaware Sub, to admit in writing Delaware Sub’s inability to pay its debts generally as they become due, or to take action in furtherance of any of the foregoing.
53. “Meeting Deadline” shall have the meaning set forth in Section 6.8 of this Agreement.
54. “Minimum Percentage” means twenty-one percent (21%).
55. “Newco” means an English limited partnership, the limited partner of which shall initially be Seller and the general partner of which shall be an Affiliate of Seller.
56. “Newco Charter Documents” shall have the meaning set forth in Section 4.1 of this Agreement.
57. “Newco Partners” means the partners of Newco, each of which shall initially be United States persons or disregarded entities of a United States person for U.S. federal income tax purposes.
58. “Order” means any decree, order, judgment, ruling, writ, injunction, temporary restraining order or other formal order in any suit or proceeding by or with any Governmental Authority which has become final and nonappealable.
59. “Other Amounts” means (i) in respect of each of the ANORO Royalty Interest, BREO/RELVAR Royalty Interest, MABA ‘081 Royalty Interest and VI Monotherapy Royalty Interest, any proceeds (whether cash proceeds or otherwise) other than those in clauses (a)(ii) and (a)(iii) of the definition of each of the foregoing Royalty Interests as, if and when received by Seller in lieu of the royalty payments under clause (a)(i) of the definition of each of the foregoing Royalty Interests, including without limitation amounts received by Seller relating to any damages or penalties or following any court action or out-of-court settlement between Seller and GGL, and in all cases, subject to the reductions, deductions, offsets and exclusions set forth in clauses (b), (c) and (d) of the definition of each Royalty Interest, (ii) any amounts paid or payable to Newco pursuant to the first sentence of Section 2.4(c) of the Assignment Agreement, and (iii) if a Recharacterization Event (as defined in the Assignment Agreement) has occurred pursuant to the second sentence of Section 2.4(c) of the Assignment Agreement, the proceeds thereof, including any proceeds of assets realized upon foreclosure in connection therewith.
60. “Party” has the meaning set forth in the introductory paragraph of this Agreement.
61. “Percentage” means twenty-one percent (21%).
62. “Permitted Encumbrances” means any of the following: (a) statutory Encumbrances for current taxes not yet due and payable, and (b) landlords’, carriers’, warehousemen’s, mechanics’, suppliers’, materialmen’s or other like Encumbrances arising in the ordinary course of business with respect to amounts not yet overdue or being contested in good faith by appropriate proceedings.
63. “Person” means any individual, corporation, limited-liability company, partnership, firm, joint venture, association, joint-stock company, trust, unincorporated organization, governmental body, or other entity.
64. “Purchase Price” shall have the meaning set forth in Section 2.4 of this Agreement.
65. “Purchaser” has the meaning set forth in the introductory paragraph of this Agreement.
66. “Purchaser Charter Documents” has the meaning set forth in Section 5.1 of this Agreement.
67. “Purchaser Disclosure Schedule” has the meaning set forth in Article V of this Agreement.
68. “Purchaser Material Adverse Effect” means any one or more of (a) a material adverse effect on the ability of Purchaser to consummate the Transactions and perform its obligations under this Agreement and (b) a material adverse effect on the validity or enforceability of this Agreement or the rights of Seller hereunder.
69. “Purchaser Indemnitees” has the meaning set forth in Section 8.1 of this Agreement.
70. “Required Shareholder Approval” means the affirmative vote of the majority of outstanding ordinary shares of Purchaser represented at a shareholders’ meeting where a quorum is present, as required pursuant to Rule 21.
71. “Royalty Interest” means (a) the ANORO Royalty Interest, (b) the BREO/RELVAR Royalty Interest, (c) the MABA ‘081 Royalty Interest, and (d) the VI Monotherapy Royalty Interest.
72. “Royalty Interest Product” means each of (a) ANORO, (b) BREO/RELVAR, (c) MABA ‘081 and (d) VI Monotherapy, and excluding, in each case, all Excluded Products.
73. “Royalty Interest Term” means, with respect to each Royalty Interest Product, the time period commencing on the Closing Date and ending, on a country-by-country and product-by-product basis, on the date that the “Term” (as defined in the Collaboration Agreement, with respect to ANORO, BREO/RELVAR and VI, and as defined in the Strategic Alliance Agreement, with respect to MABA ‘081) expires or terminates with respect to such Royalty Interest Product; provided that in the event that either the Collaboration Agreement or the Strategic Alliance Agreement is terminated and Seller remains entitled to Royalty Interest payments under Section 14.6 of the Collaboration
Agreement or Section 14.5 of the Strategic Alliance Agreement, the Royalty Interest Term with respect to the relevant Royalty Interest Product shall be extended until the expiration or termination of such rights under Section 14.6 of the Collaboration Agreement and Section 14.5 of the Strategic Alliance Agreement, respectively.
74. “Rule 21” has the meaning set forth in Section 3.3(a)(i).
75. “Seller” has the meaning set forth in the introductory paragraph of this Agreement.
76. “Seller Charter Documents” has the meaning set forth in Section 4.1 of this Agreement.
77. “Seller Corporate Reorganization” means the separation of Seller’s businesses as described in Seller’s press release and related materials dated 25 April 2013, as it may be amended.
78. “Seller Disclosure Schedule” has the meaning set forth in Article IV of this Agreement.
79. “Seller Indemnitees” has the meaning set forth in Section 8.2 of this Agreement.
80. “Seller SEC Reports” means all reports, schedules, forms and statements filed by Seller with the United States Securities and Exchange Commission pursuant to the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
81. “Seller Material Adverse Effect” means any one or more of: (a) a material adverse effect on the ability of Seller to consummate the Transactions and perform its obligations under this Agreement, (b) a material adverse effect on the validity or enforceability of this Agreement or the rights of Purchaser hereunder, or (c) a material adverse effect on the Beneficial Interest.
82. “Specified Claim” means any allegation, claim, announcement, finding or determination that the Transaction Documents, Transactions or Seller’s or Newco’s negotiation, performance or disclosure of the Transaction Documents or the Transactions is or might be a breach of the GGL Agreements.
83. “Strategic Alliance Agreement” means that certain Strategic Alliance Agreement by and between Theravance, Inc. and Glaxo Group Limited dated March 30, 2004, as amended from time to time.
84. “Taxation” or “Tax” means all (i) forms of taxation whether direct or indirect and whether levied by reference to income, profits, gains, net wealth, net worth, equity, asset values, turnover, gross receipts, added value or other reference, and statutory, governmental, state, provincial, local government or municipal impositions, duties, contributions, rates and levies, whenever and wherever imposed (whether imposed by way of a deduction or withholding for or on account of tax or otherwise) and in respect of any Person, and all penalties, fines, levies, charges, costs, interest and other additional amounts relating thereto.
85. “Tax Authority” means any taxing or other Governmental Authority (whether within or outside the United Kingdom) competent to impose, administer or collect any Taxation.
86. “Term” has the meaning set forth in Section 7.1 of this Agreement.
87. “Territory” means the entire world.
88. “Third Party” means any Person other than either Party or any of their respective Affiliates.
89. “Third-Party Claim” has the meaning set forth in Section 8.3(a) of this Agreement.
90. “Transactions” means the transactions contemplated by the Transaction Documents.
91. “Transaction Documents” means this Agreement, the ▇▇▇▇ of Sale, the Trust Agreement and the Assignment Agreement.
92. “Treaty” means the Convention between the Government of the United Kingdom of Great Britain and Northern Ireland and the Government of the United States of America for the avoidance of double taxation and prevention of fiscal evasion with respect to taxes on income and capital gains of 24 July 2001.
93. “Trust” means the trust declared pursuant to the Trust Agreement.
94. “Trust Agreement” means the Declaration of Trust to be entered into on the Closing Date among Seller, Newco and the other parties thereto substantially in the form attached hereto as Exhibit E.
95. “Trustee” means the trustee under the Trust Agreement.
96. “UCC” means the Uniform Commercial Code as in effect from time to time in the State of New York or in any other state or the District of Columbia to the extent applicable to the perfection of security interests or filings in respect thereof.
97. “UMEC” means the long-acting muscarinic antagonist umeclidinium bromide (with the chemical structure as set forth in Attachment A-2) or an ester, salt or other noncovalent derivative thereof.
98. “VI” means the long-acting beta2 agonist vilanterol (with the chemical structure as set forth in Attachment A-3) or an ester, salt or other noncovalent derivative thereof.
99. “VI Monotherapy” means (a) VI, solely as a monotherapy (i.e., excluding VI in combination with any one or more other therapeutically active component(s)), and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect to only VI solely as a monotherapy (i.e., excluding VI in combination with any one or more other therapeutically active component(s)).
100. “VI Monotherapy Royalty Interest”:
(a) Means Seller’s entitlement under the Collaboration Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
(i) royalty payments on any net sales worldwide of VI Monotherapy by or on behalf of GGL, pursuant to Section 6.3 of the Collaboration Agreement;
(ii) any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Collaboration Agreement;
(iii) any Infringement Payments with respect to VI Monotherapy in lieu of the payments referred to in clause (a)(i) immediately above; and
(iv) any Other Amounts with respect to VI Monotherapy in lieu of the payments referred to in clause (a)(i) immediately above.
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets contemplated by Sections 6.3, 6.4.1 and 6.9 the Collaboration Agreement. The VI Reverse Milestone Payment Obligation shall remain the obligation of Seller, and shall not be a reduction, deduction, offset or exclusion from the royalty payments under subsection (a) above of this definition.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Collaboration Agreement), (ii) one-time fees for new compounds contributed to the collaboration (including any one-time fee payments from GGL to Seller pursuant to Sections 6.1.3 or 6.1.4 of the Collaboration Agreement), (iii) milestone payments (including any milestone payments pursuant to Section 6.2 of the Collaboration Agreement), or (iv) any other payments (other than those expressly identified in subsection (a) above of this definition) in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to VI Monotherapy.
101. “VI Reverse Milestone Payment Obligation” means Seller’s obligation to pay to GGL milestone payments in the event a product containing VI is successfully developed and commercialized, pursuant to Section 6.2.3 of the Collaboration Agreement.
B. INTERPRETATION.
(a) When a reference is made in this Agreement to an “Article”, “Section” or “Schedule”, such reference shall be to an Article or Section of or Schedule to this Agreement unless otherwise indicated.
(b) The words “include,” “includes” and “including” when used herein shall be deemed in each case to be followed by the words “without limitation” and shall not be construed to limit any general statement which it follows to the specific or similar items or matters immediately following it.
(c) Neither Party shall be or be deemed to be the drafter of this Agreement for the purposes of construing this Agreement against the other Party.
(d) All uses of the words “hereto”, “herein”, “hereof”, “hereby” and “hereunder” and similar expressions refer to this Agreement as a whole and not to any particular provision of this Agreement.
(e) Unless otherwise specified in this Agreement, words in the singular include the plural and vice versa and words importing one gender include all genders.
(f) Any reference to a Person shall also be its permitted successors and assigns.
(g) Any references to a Law shall include any amendment or modification to such Law and any rules and regulations issued thereunder, whether such amendment or modification is made, or issuance of such rules and regulations occurs, before or after the date of this Agreement.
▇▇▇▇▇▇▇▇▇▇ ▇-▇
FF CHEMICAL STRUCTURE
STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL:
|
USAN |
FLUTICASONE FUROATE |
|
|
|
|
PRONUNCIATION |
floo tik’ a sone fur’ oh ate |
|
|
|
|
THERAPEUTIC CLAIM |
Asthma, allergy, and chronic obstructive pulmonary disease |
CHEMICAL NAMES
1. Androsta-1,4-diene-17-carbothioic acid, 6,9-difluoro-17-[(2-furanylcarbonyl)oxy]-11-hydroxy-16-methyl-3-oxo-, S-(fluoromethyl) ester, (6α,11β,16α,17α)-
2. 6α,9-difluoro-17-[[(fluoromethyl)sulfanyl]carbonyl]-11β-hydroxy-16α-methyl-3-oxoandrosta-1,4-dien-17α-yl furan-2-carboxylate
3. (6α,11β,16α,17α)-6,9-difluoro-17-(((fluoromethyl)thio)carbonyl)-11-hydroxy-16-methyl-3-oxoandrosta-1,4-dien-17-yl-2-furancarboxylate
STRUCTURAL FORMULA
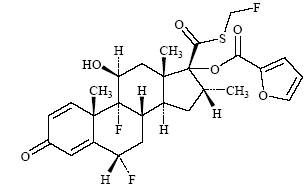
|
MOLECULAR FORMULA |
C27H29F3O6S |
|
|
|
|
MOLECULAR WEIGHT |
538.6 |
|
|
|
|
TRADEMARK |
None as yet |
|
|
|
|
MANUFACTURER |
GlaxoSmithKline |
|
|
|
|
CODE DESIGNATION |
GW685698X |
|
|
|
|
CAS REGISTRY NUMBER |
397864-44-7 |
▇▇▇▇▇▇▇▇▇▇ ▇-▇
UMEC CHEMICAL STRUCTURE
STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL
|
USAN |
UMECLIDINIUM |
|
|
|
|
PRONUNCIATION |
ue mek” li din’ ee um |
|
|
|
|
THERAPEUTIC CLAIM |
Treatment of COPD |
|
|
|
|
CHEMICAL NAMES |
|
1. 1-Azoniabicyclo[2.2.2]octane, 4-(hydroxydiphenylmethyl)-1-[2-(phenylmethoxy)ethyl]-
2. 1-[2-(benzyloxy)ethyl]-4-(hydroxydiphenylmethyl)-1-azoniabicyclo[2.2.2]octane
STRUCTURAL FORMULA

|
MOLECULAR FORMULA |
C29H34NO2+ |
|
|
|
|
MOLECULAR WEIGHT |
428.6 |
|
|
|
|
TRADEMARK |
None as yet |
|
|
|
|
SPONSOR |
GlaxoSmithKline |
|
|
|
|
CODE DESIGNATION |
GSK573719 |
|
|
|
|
CAS REGISTRY NUMBER |
869185-19-3 |
▇▇▇▇▇▇▇▇▇▇ ▇-▇
VI CHEMICAL STRUCTURE
STATEMENT ON A NONPROPRIETARY NAME ADOPTED BY THE USAN COUNCIL
|
USAN |
VILANTEROL TRIFENATATE |
|
|
|
|
PRONUNCIATION |
vye lan’ ter ol trye fen’ a ▇▇▇▇ |
|
|
|
|
THERAPEUTIC CLAIM |
Treatment of COPD and asthma |
|
|
|
|
CHEMICAL NAMES |
|
1. Benzeneacetic acid, α,α-diphenyl-, compd. with (α1R)-α1-[[[6-[2-[(2,6-dichlorophenyl)methoxy]ethoxy]hexyl]amino]methyl]-4-hydroxy-1,3-benzenedimethanol (1:1)
2. 4-{(1R)-2-[(6-{2-[(2,6-dichlorophenyl)methoxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol triphenylacetate
3. Triphenylacetic acid-4-{(1R)-2-[(6-{2-[(2,6-dichlorobenzyl)oxy]ethoxy}hexyl)amino]-1-hydroxyethyl}-2-(hydroxymethyl)phenol (1:1)
STRUCTURAL FORMULA
|
MOLECULAR FORMULA |
C24H33Cl2NO5 . C20H16O2 |
|
|
|
|
MOLECULAR WEIGHT |
774.8 |
|
|
|
|
TRADEMARK |
None as yet |
|
|
|
|
MANUFACTURER |
GlaxoSmithKline |
|
|
|
|
CODE DESIGNATION |
GW642444M |
|
|
|
|
CAS REGISTRY NUMBER |
503070-58-4 |
EXHIBIT A
PRESS RELEASE
|
|
|
Theravance and Elan Enter Into a $1.0 Billion Royalty Participation Agreement
Theravance to receive $1.0 billion upfront payment upon closing
Elan to receive a 21% participation interest in potential future royalty payments associated with four respiratory programs
▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇ — May 13, 2013 — Theravance, Inc. (NASDAQ: THRX) and Elan Corporation, plc (NYSE: ELN) today announced that they have entered into a royalty participation agreement wherein Elan will purchase a participation interest in potential future royalty payments related to four respiratory programs partnered with GlaxoSmithKline plc (GSK): RELVAR™ ELLIPTA™/BREO™ ELLIPTA™, ANORO™ ELLIPTA™, MABA (Bifunctional Muscarinic Antagonist-Beta2 Agonist) monotherapy (GSK961081, or MABA ‘081), and vilanterol (VI) monotherapy. Under the terms of the agreement, Elan will make a one-time cash payment of $1.0 billion to Theravance in exchange for a 21% participation interest in the potential future royalty payments from the four programs when, as and if received by Theravance.
“We are very excited to partner with Elan in a transaction that recognizes the significant value of four programs from our GSK collaborations targeted at respiratory disease,” said ▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇▇▇, Theravance’s Chief Executive Officer. “This agreement complements our strategy to facilitate and accelerate the return of capital to our stockholders and build value, consistent with our recently announced plan to separate Theravance into two entities, Royalty Management Company and Theravance Biopharma.”
Mr ▇▇▇▇▇ ▇▇▇▇▇▇, Chief Executive Officer of Elan commented, “This transaction, upon closing, will immediately diversify our business with an investment in four high quality and late stage clinical assets within a large and growing therapeutic area. This diversification should benefit our shareholders by spreading the inherent risk embedded in any one specific asset. In addition, the long term and future potential cash flow streams and net income will be shared with investors both directly - through a dividend pass through - and indirectly through overall after tax earnings.”
▇▇ ▇▇▇▇▇▇ added, “Being involved, even indirectly, with an important therapeutic area that addresses the needs of millions of patients who suffer from respiratory disease is particularly meaningful to all of us at Elan.”
RELVAR™ ELLIPTA™/BREO™ ELLIPTA™ (fluticasone furoate (FF)/vilanterol (VI)), ANORO™ ELLIPTA™ (umeclidinium bromide (UMEC)/VI) and VI monotherapy have been developed under the LABA collaboration with GSK. For RELVAR™ ELLIPTA™/BREO™ ELLIPTA™ and VI, Theravance is entitled to receive royalties from GSK of 15% of the first $3.0 billion of combined annual global net sales, and 5% of combined annual global net sales above $3.0 billion. If ANORO™ ELLIPTA™ is approved and commercialized, royalties on annual global net sales are upward tiering and range from the mid-single digits to 10%. The transaction does not include any royalty participation interest associated with UMEC/VI/FF, an investigational medicine also in development under the LABA collaboration with GSK.
MABA ‘081 is an investigational medicine in development under the strategic alliance between Theravance and GSK. If MABA ‘081 is successfully developed and commercialized as monotherapy, Theravance is entitled to receive royalties from GSK of between 10% and 20% of the first $3.5 billion of annual global net sales, and 7.5% of all annual global net sales above $3.5 billion. The transaction does not include any royalty participation interest associated with MABA ‘081 in combination with any other therapeutically active component, including an inhaled corticosteroid, or any other MABA compound as monotherapy or in combination.
The transaction is not subject to any material conditions, other than approval by Elan’s shareholders. Elan plans to promptly prepare the required documentation to enable a shareholder vote, which Elan has agreed to hold within 35 days. If approved by Elan’s shareholders, the parties expect the transaction to be consummated by the end of June 2013.
Theravance Tax Treatment and Use of Proceeds
Theravance does not expect to pay significant income taxes associated with the transaction.
As previously disclosed, Theravance intends to separate its biopharmaceutical operations and its late stage partnered respiratory assets into two independent publicly traded companies, referred to as Theravance Biopharma and Royalty Management Co, respectively. We intend for Royalty Management Co to be the primary vehicle for the return of capital and that the proceeds from this transaction will facilitate and accelerate returns to its stockholders following the separation. Theravance Biopharma will be primarily focused on the discovery, development and commercialization of small-molecule medicines in areas of significant unmet medical need. This transaction does not change the overall structure of the planned separation, including which assets are expected to be in each company. Additionally, we do not plan to increase 2013 research and development spending above what was included in our 2013 expense guidance. Theravance is currently evaluating the optimal strategies to return capital to stockholders of Royalty Management Co following completion of the separation, including through dividends or the repurchase of shares and/or convertible debt.
Centerview Partners LLC is acting as financial advisor and Skadden, Arps, Slate, ▇▇▇▇▇▇▇ & ▇▇▇▇ LLP and ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ & ▇▇▇▇▇▇▇▇▇, LLP are acting as legal advisors to Theravance in connection with the transaction.
Evercore Partners and Ondra Partners are acting as financial advisors to Elan. Cadwalader, ▇▇▇▇▇▇▇▇▇▇ & ▇▇▇▇ LLP and A&L Goodbody are acting as legal advisors to Elan in connection with the transaction.
Conference Call and Webcast Information
Theravance will discuss this announcement at 8:30 a.m. Eastern Daylight Time today. To participate in the live call by telephone, please dial (▇▇▇) ▇▇▇-▇▇▇▇ from the U.S., or (▇▇▇) ▇▇▇-▇▇▇▇ for international callers. Those interested in listening to the conference call live via the internet may do so by visiting Theravance’s web site at ▇▇▇.▇▇▇▇▇▇▇▇▇▇.▇▇▇. To listen to the live call, please go to the web site 15 minutes prior to its start to register, download, and install any necessary audio software.
A replay of the conference call will be available on Theravance’s web site for 30 days through June 12, 2013. An audio replay will also be available through 11:59 p.m. Eastern Daylight Time on May 20, 2013 by dialing (▇▇▇) ▇▇▇-▇▇▇▇ from the U.S., or (▇▇▇) ▇▇▇-▇▇▇▇ for international callers, and entering confirmation code 71715858.
For more information, please visit Theravance’s website at ▇▇▇.▇▇▇▇▇▇▇▇▇▇.▇▇▇.
About Four Respiratory Programs
RELVAR™ ELLIPTA™/BREO™ ELLIPTA™, ANORO™ ELLIPTA™, VI monotherapy and MABA monotherapy (GSK961081, or MABA ‘081), are assets developed in collaboration with GlaxoSmithKline plc (GSK).
In November 2002, Theravance entered into its LABA collaboration with GSK to develop and commercialize once-daily long-acting beta2 agonist (LABA) products for the treatment of chronic obstructive pulmonary disease (COPD) and asthma. For the treatment of COPD, the collaboration is developing two combination products: (1) RELVAR™ ELLIPTA™ or BREO™ ELLIPTA™ (FF/VI), an investigational once-daily combination medicine consisting of a LABA, vilanterol (VI), and an inhaled corticosteroid (ICS), fluticasone furoate (FF) and (2) ANORO™ ELLIPTA™ (UMEC/VI), a once-daily investigational medicine combining a long-acting muscarinic antagonist (LAMA), umeclidinium
bromide (UMEC), with a LABA, VI. For the treatment of asthma, the collaboration is developing FF/VI. BREO™ ELLIPTA™ 100/25 mcg is approved in the United States as an inhaled long-term, once-daily maintenance treatment of airflow obstruction in patients with COPD, including chronic bronchitis and/or emphysema. It is also indicated to reduce exacerbations of COPD in patients with a history of exacerbations. RELVAR™ ELLIPTA™ for the treatment of COPD and asthma patients is currently under review by the European Medicines Agency (EMA) and Japan. FF/VI is not approved or licensed in the European Union or anywhere outside of the United States. ANORO™ ELLIPTA™ for the treatment of COPD patients is currently under review by the U.S. Food and Drug Administration, the EMA and Japan. The Prescription Drug User Fee Act goal date for ANORO™ ELLIPTA™ is December 18, 2013. ANORO™ ELLIPTA™ (UMEC/VI) is an investigational medicine and is not currently approved anywhere in the world.
In March 2004, Theravance entered into its strategic alliance with GSK. Under this alliance, GSK received an option to license exclusive development and commercialization rights to product candidates from certain of Theravance’s discovery programs on predetermined terms and on an exclusive, worldwide basis. In 2005, GSK licensed Theravance’s MABA program for the treatment of COPD. GSK961081 (‘081), the lead MABA, is an investigational, single molecule bifunctional bronchodilator with both muscarinic antagonist and beta2 receptor agonist activities. Based on the results from a Phase 2b study, GSK and Theravance plan to advance ‘081 monotherapy into Phase 3 and the ‘081/FF combination into Phase 3-enabling studies, later in 2013.
Important Safety Information
BREO ELLIPTA is contraindicated in patients with severe hypersensitivity to milk proteins or who have demonstrated hypersensitivity to either fluticasone furoate, vilanterol, or any of the excipients.
BREO ELLIPTA should not be initiated in patients during rapidly deteriorating or potentially life-threatening episodes of COPD, or as rescue therapy for the treatment of acute episodes of bronchospasm, which should be treated with an inhaled, short-acting beta2-agonist.
BREO ELLIPTA should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABAs, as an overdose may result.
Oropharyngeal candidiasis has occurred in patients treated with BREO ELLIPTA.
An increase in the incidence of pneumonia has been observed in subjects with COPD receiving the fluticasone furoate/vilanterol combination, including BREO ELLIPTA 100 mcg/25 mcg, in clinical trials. There was also an increased incidence of pneumonias resulting in hospitalization. In some incidences these pneumonia events were fatal.
Patients who use corticosteroids are at risk for potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. A more serious or even fatal course of chickenpox or measles may occur in susceptible patients.
Particular care is needed for patients who have been transferred from systemically active corticosteroids to inhaled corticosteroids because deaths due to adrenal insufficiency have occurred in patients with asthma during and after transfer from systemic corticosteroids to less systemically available inhaled corticosteroids.
Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage of inhaled corticosteroids in susceptible individuals.
Caution should be exercised when considering the coadministration of BREO ELLIPTA with long-term ketoconazole and other known strong CYP3A4 inhibitors because increased systemic corticosteroid and cardiovascular adverse effects may occur.
Inhaled medicines can produce paradoxical bronchospasm, which may be life-threatening. Vilanterol, the LABA in BREO ELLIPTA, can produce clinically significant cardiovascular effects in some patients. Decreases in bone mineral density have been observed with long-term administration of products containing inhaled corticosteroids, as have glaucoma, increased intraocular pressure, and cataracts.
The most common adverse reactions (>3% and more common than in placebo) reported in two 6-month clinical trials with BREO ELLIPTA (and placebo) were nasopharyngitis, 9% (8%); upper respiratory tract infection, 7% (3%); headache, 7% (5%); and oral candidiasis, 5% (2%). In addition to the events reported in the 6-month studies, adverse reactions occurring in >3% of the subjects treated with BREO ELLIPTA in two 1-year studies included COPD, back pain, pneumonia, bronchitis, sinusitis, cough, oropharyngeal pain, arthralgia, hypertension, influenza, pharyngitis, diarrhea, peripheral edema, and pyrexia.
BREO ELLIPTA is not indicated for the relief of acute bronchospasm or the treatment of asthma. The safety and efficacy of BREO ELLIPTA in patients with asthma have not been established. Long-acting beta2-adrenergic agonists (LABAs), such as vilanterol, one of the active ingredients in BREO ELLIPTA, increase the risk of asthma-related death. A placebo-controlled trial with another LABA (salmeterol) showed an increase in asthma-related deaths in subjects receiving salmeterol. This finding with salmeterol is considered a class effect of all LABAs, including vilanterol.
About Elan
Elan Corporation, plc is a biotechnology company, headquartered in Ireland, committed to making a difference in the lives of patients and their families by dedicating itself to bringing innovations in science to fill significant unmet medical needs that continue to exist around the world. Elan shares trade on the New York and Irish Stock Exchanges. For additional information about Elan, please visit ▇▇▇.▇▇▇▇.▇▇▇.
As required by the Irish Takeover Rules, the Directors of Elan accept responsibility for the information contained in this announcement. To the best of their knowledge and belief (having taken all reasonable care to ensure such is the case); the information contained in this announcement is in accordance with the facts and does not omit anything likely to affect the import of such information.
Any holder of 1% or more of any class of relevant securities of Elan may have disclosure obligations under Rule 8.3 of the Irish Takeover Panel Act, 1997, Takeover Rules 2007 (as amended).
About Theravance
Theravance is a biopharmaceutical company with a pipeline of internally discovered product candidates and strategic collaborations with pharmaceutical companies. Theravance is focused on the discovery, development and commercialization of small molecule medicines across a number of therapeutic areas including respiratory disease, bacterial infections, and central nervous system (CNS)/pain. Theravance’s key programmes include: RELVAR™ ELLIPTA™ or BREO™ ELLIPTA™ (FF/VI), ANORO™ ELLIPTA™ (UMEC/VI) and MABA (Bifunctional Muscarinic Antagonist-Beta2 Agonist), each partnered with GlaxoSmithKline plc, and its oral Peripheral Mu Opioid Receptor Antagonist program. By leveraging its proprietary insight of multivalency to drug discovery, Theravance is pursuing a best-in-class strategy designed to discover superior medicines in areas of significant unmet medical need. For more information, please visit Theravance’s web site at ▇▇▇.▇▇▇▇▇▇▇▇▇▇.▇▇▇.
THERAVANCE®, the Theravance logo, and MEDICINES THAT MAKE A DIFFERENCE® are registered trademarks of Theravance, Inc.
RELVAR™, BREO™, ANORO™ and ELLIPTA™ are trademarks of the GlaxoSmithKline group of companies. The use of the brand names ANORO™ and RELVAR™ has not yet been approved by any regulatory authority.
Theravance Forward-Looking Statements
This press release contains and the conference call will contain certain “forward-looking” statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Theravance intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. Examples of such statements include statements relating to the expected timing of the Elan shareholder vote on the transaction and the outcome of such vote, the expected timing for consummating the transaction if Elan shareholder approval is obtained, the effect of the transaction if it is consummated on the strategies, plans and objectives of Theravance, the timing, manner and amount of anticipated potential returns of capital to stockholders if the transaction and/or Theravance’s previously announced planned separation is consummated, the timing, plans and objectives of and the possible impact of the transaction on Theravance’s previously announced, planned separation, the status and timing of clinical studies, data analysis and communication of results, statements regarding the potential benefits and mechanisms of action of drug candidates, statements concerning the timing of seeking regulatory approval of product candidates, statements concerning the enabling capabilities of Theravance’s approach to drug discovery and its proprietary insights, statements concerning expectations for product candidates through development and commercialization and projections of the tax effects of the transaction and other financial items. These statements are based on the current estimates and assumptions of the management of Theravance as of the date of this press release and the conference call and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance to be materially different from those reflected in the forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to delays or difficulties encountered in obtaining, or the failure to obtain, the approval of Elan’s shareholders for the transaction, the possibility that intervening events could arise which could alter the timing, or the ability to consummate, the transaction, the anticipated separation of Theravance into two independent companies or the intended return of capital to stockholders, the risk that third parties could challenge the transaction, the risk that Theravance’s net operating loss may not be available to offset taxes from the transaction, the potential that results of clinical or non-clinical studies indicate product candidates are unsafe or ineffective, our dependence on third parties in the conduct of our clinical studies, delays or failure to achieve regulatory approvals for product candidates and risks of collaborating with third parties to develop and commercialize products. These and other risks are described in greater detail under the heading “Risk Factors” contained in Theravance’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on May 1, 2013 and the risks discussed in our other periodic filings with the SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance assumes no obligation to update its forward-looking statements.
Elan Forward-Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties. You can identify these statements by the fact that they use words such as “anticipate”, “estimate”, “project”, “target”, “intend”, “plan”, “will”, “believe”, “expect” and other words and terms of similar meaning in connection with any discussion of future financial performance or events. Among the factors that could cause actual results to differ materially from those described or projected herein are the following: risks related to delays or difficulties encountered in obtaining, or the failure to obtain, the approval of Elan’s shareholders for the transaction; the possibility that intervening events could arise which could alter the timing, or the ability to consummate the transaction if Elan shareholder approval is obtained; the risk that third parties could challenge the transaction, even if the transaction is approved by Elan shareholders and consummated; risks related to the development, approval and commercialization of the products or potential products that underlie the royalty participation interest; whether this agreement will provide diversification benefits to Elan shareholders; whether any cash flow streams will result from this agreement; as Elan’s principal source of revenue may remain a royalty on sales of Tysabri, the potential of Tysabri, which may be severely constrained by increases in the incidence of serious adverse events (including death) associated with Tysabri (in particular, by increases in the incidence rate for cases of PML), or by competition from existing or new therapies (in particular, oral therapies), and the potential for the successful development and commercialization of
products, whether internally or by acquisition, especially given the separation of the Prothena business which left Elan with no material pre-clinical research programs or capabilities; Elan’s ability to maintain sufficient cash, liquid resources, and investments and other assets capable of being monetized to meet its liquidity requirements; the success of our development activities, and research and development activities in which Elan retains an interest, including, in particular, the impact of the announced discontinuation of the development of bapineuzumab intravenous in mild to moderate Alzheimer’s disease; failure to comply with anti-kickback, bribery and false claims laws in the United States, Europe and elsewhere; difficulties or delays in manufacturing and supply of Tysabri; trade buying patterns; the impact of potential biosimilar competition, the trend towards managed care and health care cost containment, including Medicare and Medicaid; legislation and other developments affecting pharmaceutical pricing and reimbursement (including, in particular, the dispute in Italy with respect to Tysabri sales), both domestically and internationally; failure to comply with Elan’s payment obligations under Medicaid and other governmental programs; exposure to product liability (including, in particular, with respect to Tysabri) and other types of lawsuits and legal defense costs and the risks of adverse decisions or settlements related to product liability, patent protection, securities class actions, governmental investigations and other legal proceedings; Elan’s ability to protect its patents and other intellectual property; claims and concerns that may arise regarding the safety or efficacy of Elan’s product candidates; interest rate and foreign currency exchange rate fluctuations and the risk of a partial or total collapse of the euro; governmental laws and regulations affecting domestic and foreign operations, including tax obligations; whether Elan is deemed to be an Investment Company or a Passive Foreign Investment Company; general changes in United States and International generally accepted accounting principles; growth in costs and expenses; and the impact of acquisitions, divestitures, restructurings, product withdrawals and other unusual items. A further list and description of these risks, uncertainties and other matters can be found in Elan’s Annual Report on Form 20-F for the fiscal year ended December 31, 2012, and in its Reports of Foreign Issuer on Form 6-K filed with the SEC. Elan assumes no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise.
(THRX-G)
Contact Information:
Theravance, Inc.
▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇
Senior Vice President and Chief Financial Officer
▇▇▇-▇▇▇-▇▇▇▇
▇▇▇▇▇▇▇▇.▇▇▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇.▇▇▇
Elan Corporation, plc
Investor Relations:
▇▇▇▇▇ ▇▇▇▇▇, ▇▇-▇▇▇-▇▇▇-▇▇▇▇
▇▇▇▇▇ ▇▇▇▇▇▇▇▇, ▇▇▇▇-▇-▇▇▇-▇▇▇▇
or
Media Relations:
▇▇▇▇ ▇▇▇▇▇▇▇▇, ▇▇▇▇-▇-▇▇▇-▇▇▇▇
▇▇▇▇▇▇▇▇ ▇▇▇▇/FTI Consulting, ▇▇▇-▇▇▇-▇▇▇-▇▇▇▇
▇▇▇▇▇ ▇▇▇▇▇/Sard Verbinnen & Co, ▇▇-▇▇▇-▇▇▇-▇▇▇▇
EXHIBIT B
FORM OF ▇▇▇▇ OF SALE
▇▇▇▇ OF SALE (this “▇▇▇▇ of Sale”), is made and effective as of [ ] [ ], 2013, between THERAVANCE, INC., a Delaware corporation (“Seller”), and ELAN CORPORATION PLC, an Irish public limited company (“Purchaser”). Capitalized terms used but not otherwise defined herein shall have the meanings specified in the Participation Agreement (as defined below).
WITNESSETH
WHEREAS, Seller and Purchaser have entered into a Royalty Participation Agreement dated as of May 12, 2013 (the “Participation Agreement”), pursuant to which Seller has agreed to sell, grant, assign, put over, transfer and convey to Purchaser, and Purchaser has agreed to purchase, acquire and accept from Seller, all right, title and interest in and to the Beneficial Interest.
NOW, THEREFORE, in consideration of the representations, warranties, covenants and agreements contained herein and in the Participation Agreement, and other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, and as contemplated by Section 3.2 of the Participation Agreement, the Parties hereto agree as follows:
1. Conveyance, Assignment and Acceptance of Assigned Rights. Seller hereby sells, grants, assigns, puts over, transfers and conveys to Purchaser all right, title and interest in the Beneficial Interest, and Purchaser hereby purchases, acquires and accepts such sale, grant, assignment, put over, transfer and conveyance.
2. Governing Law. THIS ▇▇▇▇ OF SALE AND ALL ACTIONS, PROCEEDINGS OR COUNTERCLAIMS (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS ▇▇▇▇ OF SALE WILL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF NEW YORK, WITHOUT GIVING EFFECT TO THE PRINCIPLES OF CONFLICTS OF LAW THEREOF EXCEPT AS SET FORTH IN SECTIONS 5-1401 OF THE NEW YORK GENERAL OBLIGATIONS LAW.
3. Counterparts. This ▇▇▇▇ of Sale may be executed in any number of counterparts. Each counterpart will be an original, and all counterparts will together constitute one and the same instrument. Delivery of an executed counterpart via PDF or other electronic delivery shall be considered the same as delivery of a manually executed counterpart.
4. Successors and Assigns. This ▇▇▇▇ of Sale shall be binding upon and inure to the benefit of Seller and Purchaser and their permitted successors and assigns.
5. Severability. If any of the covenants, agreements or terms of this ▇▇▇▇ of Sale is held invalid, illegal or unenforceable, then it will be deemed severable from the remaining
covenants, agreements or terms of this ▇▇▇▇ of Sale and will in no way affect the validity, legality or enforceability of the remaining ▇▇▇▇ of Sale. Upon such determination that any covenant, agreement or term is invalid, illegal or unenforceable, this ▇▇▇▇ of Sale shall be deemed modified so as to effect the original intent of the Parties as closely as possible in order that the Transactions be consummated as originally contemplated to the fullest extent consistent with applicable Law.
6. Entire Agreement. This ▇▇▇▇ of Sale is subject to all of the terms, conditions and limitations set forth in the Participation Agreement, and together with the Participation Agreement, and the other documents and agreements referenced in Section 9.8 of the Participation Agreement, sets forth the entire agreement and understanding between the Parties hereto with respect to the subject matter hereof and supersedes all prior and contemporaneous agreements and understandings relating to such subject matter.
7. Conflicts. Notwithstanding anything to the contrary contained in this ▇▇▇▇ of Sale, in the event of any conflict between the terms of this ▇▇▇▇ of Sale and the terms of the Participation Agreement, the terms of the Participation Agreement shall control.
[Signature Page Follows]
IN WITNESS WHEREOF, the Parties hereto have set their hands by their authorized representatives as of the year and date first indicated above.
|
|
THERAVANCE, INC. | |
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
|
|
|
|
|
|
|
|
|
|
ELAN CORPORATION PLC | |
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
EXHIBIT C
FORM OF UCC FINANCING STATEMENT
EXHIBIT A TO UCC1 FINANCING STATEMENT
|
Debtor: |
[Taurus] |
|
|
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇ |
|
|
▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇ |
|
|
▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ |
|
|
|
|
Secured Party: |
[Newco] |
I. This FINANCING STATEMENT covers the following collateral:
TWENTY-ONE PERCENT (21%) UNDIVIDED BENEFICIAL INTEREST IN (1) CERTAIN PAYMENT OBLIGATIONS UNDER SECTIONS 6.3, 6.8 AND 13.4 OF THAT CERTAIN COLLABORATION AGREEMENT, DATED NOVEMBER 14, 2002, BETWEEN TAURUS AND GOLIATH (2) CERTAIN PAYMENT OBLIGATIONS UNDER SECTIONS 6.3, 6.8 AND 13.4 OF THAT CERTAIN STRATEGIC ALLIANCE AGREEMENT, DATED MARCH 30, 2004, BETWEEN TAURUS AND GOLIATH, AND ALL PROCEEDS THEREOF (THE “COLLATERAL”):
It is intended by the Debtor and the Secured Party that all right, title and interest of the Debtor in and to the Collateral is being conveyed to the Secured Party under the transactions between such parties, and the use of the terms “Debtor,” “Secured Party” and “Collateral” shall not be construed to evidence a contrary intent, nor shall this FINANCING STATEMENT constitute an admission or acknowledgment by Debtor, or Secured Party or any other person that the transactions between Debtor and Secured Party create only a security interest in the foregoing property.
EXHIBIT D
FORM OF ASSIGNMENT AGREEMENT
|
|
|
ASSIGNMENT AGREEMENT
between
THERAVANCE, INC.
and
[NEWCO]
Dated as of [ ] [ ], 2013
|
|
|
|
TABLE OF CONTENTS |
| |
|
|
| |
|
Article I |
| |
|
|
|
|
|
DEFINITIONS AND INTERPRETATION |
| |
|
|
|
|
|
Article II |
| |
|
|
|
|
|
ASSIGNMENT OF ASSIGNMENT INTEREST |
| |
|
|
|
|
|
Section 2.1 |
Assignment of Assignment Interest |
1 |
|
Section 2.2 |
Exclusion of GGL Agreement Rights |
2 |
|
Section 2.3 |
No Obligations Transferred |
2 |
|
Section 2.4 |
Intent; Savings Clause |
2 |
|
|
|
|
|
Article III |
| |
|
|
| |
|
CLOSING |
| |
|
Section 3.1 |
Closing |
3 |
|
Section 3.2 |
Delivery of Trust Agreement |
3 |
|
|
| |
|
Article IV |
| |
|
|
| |
|
REPRESENTATIONS AND WARRANTIES OF SELLER |
| |
|
|
| |
|
Section 4.1 |
Organization |
4 |
|
Section 4.2 |
Power, Authorization and Enforceability |
4 |
|
Section 4.3 |
No Conflicts or Violations |
4 |
|
Section 4.4 |
Proceedings |
4 |
|
Section 4.5 |
GGL Agreements |
4 |
|
Section 4.6 |
Contractual Rights to Royalty Interest |
5 |
|
Section 4.7 |
Intellectual Property |
5 |
|
Section 4.8 |
UCC Matters |
6 |
|
Section 4.9 |
No Implied Representations and Warranties |
6 |
|
|
|
|
|
Article V |
| |
|
|
|
|
|
REPRESENTATIONS AND WARRANTIES OF NEWCO |
| |
|
|
|
|
|
Section 5.1 |
Organization |
7 |
|
Section 5.2 |
Power, Authorization and Enforceability |
7 |
|
Section 5.3 |
No Conflicts or Violations |
7 |
|
Section 5.4 |
Proceedings |
8 |
|
Section 5.5 |
No Implied Representations and Warranties |
8 |
|
Article VI |
| |
|
|
| |
|
COVENANTS |
| |
|
|
|
|
|
Section 6.1 |
Payment of Assignment Interest |
8 |
|
Section 6.2 |
Tax Matters |
9 |
|
Section 6.3 |
GGL Agreements |
10 |
|
Section 6.4 |
Confidentiality |
11 |
|
Section 6.5 |
Information Rights |
12 |
|
Section 6.6 |
Books and Records |
13 |
|
Section 6.7 |
Public Announcement |
13 |
|
Section 6.8 |
UCC Matters |
13 |
|
Section 6.9 |
Royalty Interest Disclosures |
13 |
|
Section 6.10 |
Non-Cash Consideration |
13 |
|
|
|
|
|
Article VII |
| |
|
|
|
|
|
TERM |
| |
|
|
|
|
|
Section 7.1 |
Term |
13 |
|
|
|
|
|
Article VIII |
| |
|
|
| |
|
INDEMNIFICATION |
| |
|
|
| |
|
Section 8.1 |
Indemnification by Seller |
14 |
|
Section 8.2 |
Indemnification by Newco |
14 |
|
Section 8.3 |
Indemnification Procedure |
14 |
|
Section 8.4 |
Claims Period for Breach of Representations and Warranties |
16 |
|
Section 8.5 |
Limitations on Indemnification |
16 |
|
Section 8.6 |
Exclusive Remedy |
17 |
|
Section 8.7 |
Disclaimer of Consequential Damages |
17 |
|
|
|
|
|
Article IX |
| |
|
|
|
|
|
MISCELLANEOUS |
| |
|
Section 9.1 |
Assignment |
18 |
|
Section 9.2 |
Further Assurances |
18 |
|
Section 9.3 |
Notices |
19 |
|
Section 9.4 |
Specific Performance |
20 |
|
Section 9.5 |
Title; Headings; Captions |
20 |
|
Section 9.6 |
Independent Contractors |
20 |
|
Section 9.7 |
Third-Party Beneficiaries |
20 |
|
Section 9.8 |
Entire Agreement |
20 |
|
Section 9.9 |
Amendment and Supplementation |
21 |
|
Section 9.10 |
No Waiver |
21 |
|
Section 9.11 |
Severability |
21 |
|
Section 9.12 |
Governing Law |
21 |
|
Section 9.13 |
Submission to Jurisdiction |
21 |
|
Section 9.14 |
Waiver of Jury Trial |
21 |
|
Section 9.15 |
Counterparts |
21 |
|
Appendix A |
Definitions |
A-1 |
|
Exhibit A |
Form of UCC Financing Statements |
A-1 |
|
Exhibit B |
Form of Trust Agreement |
B-1 |
ASSIGNMENT AGREEMENT
ASSIGNMENT AGREEMENT, dated as of [ ] [ ], 2013 (this “Agreement”), between THERAVANCE, INC., a Delaware corporation (“Seller”), and [NEWCO], an English limited partnership (“Newco”; and each of Seller and Newco, a “Party” and together, the “Parties”).
BACKGROUND
A. Seller is party to certain drug research, development and commercialization agreements with GGL, referred to herein as the GGL Agreements (each as defined herein), pursuant to which Seller is entitled to certain royalties, milestone payments and other payments in connection with the development and commercialization of products under the GGL Agreements.
B. Seller wishes to sell to Newco, and Newco wishes to acquire, a participation interest in certain royalty payments (but excluding (i) any rights of any kind under the GGL Agreements (other than the Assignment Interest) and (ii) any milestone payments and other payments) that Seller may receive from GGL pursuant to the GGL Agreements with respect to certain products, subject to the terms and conditions of this Agreement and in compliance with the terms and conditions of the GGL Agreements, but not assign or otherwise transfer to Newco any rights to royalty payments, or any other rights, under any of the GGL Agreements (other than the Assignment Interest).
ARTICLE I
DEFINITIONS AND INTERPRETATION
Capitalized terms used but not otherwise defined in this Agreement are defined in Appendix A to this Agreement. Appendix A also contains rules of interpretation applicable to this Agreement.
ARTICLE II
ASSIGNMENT OF ASSIGNMENT INTEREST
Section 2.1 Assignment of Assignment Interest.
(a) Seller hereby sells, grants, assigns, puts over, transfers and conveys to Newco the Assignment Interest, free and clear of all Encumbrances other than Permitted Encumbrances (the “Assignment”).
(b) In consideration for Assignment, and simultaneously with the execution of this Agreement, Newco is entering into the Trust Agreement, pursuant to which Newco is appointing the Trustee to hold in trust the Trust Property (as defined in the Trust Agreement) in favor of the Seller (as beneficiary) (“Declaration of Trust”).
(c) For the avoidance of doubt, Newco is not acquiring any right or interest, including any royalty payment rights (other than the Assignment Interest) under any GGL Agreement. In no circumstances will the Assignment Interest be based on:
(i) any royalty or other payments received by Seller pursuant to the GGL Agreements based on or relating to sales of Royalty Interest Products which occur prior to the date hereof;
(ii) any royalty or other payments received by Seller pursuant to any of the GGL Agreements based on or relating to sales of Royalty Interest Products which occur at any time after the end of the Royalty Interest Term with respect to any Royalty Interest Product in any country;
(iii) any royalty or other payments received by Seller pursuant to any of the GGL Agreements based on or relating to sales of any Excluded Products, whether before, during or after the Royalty Interest Term; or
(iv) any non-monetary consideration provided to Seller pursuant to the terms of the GGL Agreements with respect to the Royalty Interest Products, subject to the terms of Section 6.10 of this Agreement.
Section 2.2 Exclusion of GGL Agreement Rights. It is understood and agreed by the Parties that, notwithstanding anything to the contrary in the Transaction Documents, Seller shall retain all of its rights and interests in, and no rights or interests are being sold, granted, assigned, put over, transferred or conveyed by Seller to Newco (other than the Assignment Interest) under, any of the GGL Agreements or this Agreement.
Section 2.3 No Obligations Transferred. Notwithstanding anything to the contrary in this Agreement, the sale, grant, put over, assignment, transfer and conveyance of the Assignment Interest shall not transfer to Newco, or make Newco subject to, any obligation or liability of Seller under the GGL Agreements, except as required to comply with the GGL Agreements (including the confidentiality obligations therein). For the avoidance of doubt, the foregoing shall not (a) affect Seller’s or Newco’s rights under this Agreement or modify the obligations Newco and Seller have under this Agreement or (b) affect any liability of either Party to the other under the provisions of Article VIII hereof.
Section 2.4 Intent; Savings Clause.
(a) This Agreement is intended to effect a sale of the Assignment Interest by Seller to Newco and, immediately after giving effect to the transfer contemplated by Section 2.1, Seller will have no further interest (legal or equitable) in any of the Assignment Interest. The Seller hereby authorizes the filing under the UCC of a financing statement in the form attached hereto as Exhibit A with respect to the sale of the Assignment Interest by Seller to
Newco, and any continuation statements (including amendments to effect such continuation) required under the UCC with respect thereto reasonably requested by Newco (subject to confidentiality obligations under the GGL Agreements) in order to evidence such sale.
(b) If, notwithstanding Section 2.4(a), the transfer of the Assignment Interest pursuant to this Agreement (taken together with the Declaration of Trust) is characterized as a collateral transfer for security or as a financing transaction (a “Recharacterization Event”), Seller intends that Newco have a first priority, perfected security interest in, and lien on, the Assignment Interest to secure an obligation of Seller to pay to Newco an amount (the “Seller Secured Amount”) equal to the Assignment Interest. Accordingly, if a Recharacterization Event occurs, Seller shall be deemed to have granted, and Seller does hereby grant, a security interest in, to and under the Assignment Interest and all proceeds thereof, whether now owned or existing or hereafter acquired, in each case to secure the obligation of the Seller set forth in Section 2.4(c).
(c) If a Recharacterization Event has occurred, Seller agrees to pay or cause to be paid to Newco all amounts that would have been required to be paid to Newco if the Recharacterization Event had not occurred; such payments to be made when, as and if payments of the Assignment Interest are received from GGL. The maximum amount payable by Seller to Newco pursuant to this Section 2.4 shall be the Seller Secured Amount. If Seller fails to pay to Newco any such amounts, (i) Newco may exercise all rights and remedies of a secured party under the relevant UCC (including the rights of a secured party obtaining a lien under Section 9-608 of the relevant UCC) and (ii) Seller may exercise all of the rights of a debtor granting a lien under the relevant UCC (including the rights of a debtor granting a lien under Section 9-623).
ARTICLE III
CLOSING
Section 3.1 Closing. The closing of the Transactions (the “Closing”) will take place simultaneously with the execution of this Agreement by Seller and Newco, at the offices of Skadden, Arps, Slate, ▇▇▇▇▇▇▇ & ▇▇▇▇ LLP; ▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇; ▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇.
Section 3.2 Delivery of Trust Agreement. At the Closing, Newco and Seller shall deliver to each other the executed Trust Agreement duly executed on behalf of Seller, Newco and the other parties thereto.
ARTICLE IV
REPRESENTATIONS AND WARRANTIES OF SELLER
Except as disclosed in the separate disclosure letter which has been delivered by Seller to Newco prior to the execution of this Agreement (the “Seller Disclosure Schedule”) (each of which disclosures shall be effective with respect to (i) the corresponding sections and subsections of this Article IV, and (ii) any other section or subsection of this Article IV, but only if the relevance of that reference as an exception to (or a disclosure for purposes of) such section
or subsection would be reasonably apparent to a reasonable person who has read that reference and such representations and warranties), Seller hereby represents and warrants to Newco as of the date of this Agreement:
Section 4.1 Organization. Seller is a corporation duly organized, validly existing and in good standing, under the Laws of the state of Delaware. Seller has made available to Newco true, correct and complete copies of its charter and bylaws, in each case as amended to the date hereof (the “Seller Charter Documents”). All Seller Charter Documents are in full force and effect. Seller is not in violation of the Seller Charter Documents, except as would not have, individually or in the aggregate, a Seller Material Adverse Effect.
Section 4.2 Power, Authorization and Enforceability. Seller has all necessary corporate power and authority to execute and deliver the Transaction Documents to which it is a party, to perform its obligations hereunder and thereunder, and to consummate the Transactions to which it is a party. The execution, delivery and performance by Seller of the Transaction Documents to which it is a party and the consummation by Seller of the Transactions to which it is a party have been duly and validly authorized by all necessary corporate action of Seller, and no other corporate proceedings on the part of Seller are necessary to authorize the execution, delivery and performance of this Agreement or to consummate such Transactions. This Agreement has been duly and validly executed and delivered by Seller and, assuming it is duly and validly executed and delivered by Newco, shall be a legal and valid binding obligation of Seller, enforceable in accordance with its terms, subject to applicable Laws affecting creditors’ rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
Section 4.3 No Conflicts or Violations. None of the execution and delivery by Seller of the Transaction Documents to which it is a party, the consummation by Seller of the Transactions to which it is a party, or Seller’s compliance with such Transaction Documents will (a) conflict with or violate the Seller Charter Documents, (b) constitute a breach or default by Seller under any of the GGL Agreements, (c) constitute a breach or default under any agreement (other than the GGL Agreements) to which Seller or any of its Subsidiaries is a party or by which any of its or their property or assets are bound, (d) require Seller to make any filing with or obtain any permit, authorization, consent or approval of any Governmental Authority or other Person, or (e) violate any Law or Order to which Seller or the Assignment Interest is subject, except, in the case of Sections 4.3(b), (c), (d) and (e), as would not reasonably be expected to result in a Seller Material Adverse Effect.
Section 4.4 Proceedings. As of the date hereof, there is no action, suit, proceeding or, to the Knowledge of Seller, investigation before any Governmental Authority, court or arbitrator pending or, to the Knowledge of Seller, threatened in writing against Seller or its Subsidiaries relating to the GGL Agreements or the Assignment Interest, except as would not reasonably be expected to result in a Seller Material Adverse Effect.
Section 4.5 GGL Agreements.
(a) Validity and Enforceability. Each of the GGL Agreements is a legal, valid and binding obligation, in full force and effect, enforceable in accordance with its terms in
all material respects, of Seller and, to the Knowledge of Seller, of GGL, subject to applicable Laws affecting creditors’ rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
(b) Breach. Seller is not in breach of any of the GGL Agreements in any material respect.
(c) Assignment. Seller has not assigned to any Person any of the GGL Agreements since their initial dates of execution, in whole or in part, except as contemplated herein.
(d) Other.
(i) GGL does not have any right to unilaterally amend any GGL Agreement in any manner which would or would reasonably be expected to result, individually or in the aggregate, in a Seller Material Adverse Effect.
(ii) The information publicly disclosed, as publicly amended and supplemented, by Seller in the Seller Exchange Act Reports regarding the GGL Agreements and the Royalty Interest Products, does not contain any untrue statement of a material fact or omit to state any material fact necessary in order to make such information, in light of the circumstances in which it was disclosed, not misleading.
Section 4.6 Contractual Rights to Royalty Interest. Seller (i) is the exclusive owner of Seller’s entire right and interest in, to and under the GGL Agreements and the Royalty Interest, free and clear of all Encumbrances other than Permitted Encumbrances and (ii) has not assigned or granted to any Third Party any right or interest (including payments or proceeds) in or to any Royalty Interest beneficially or otherwise.
Section 4.7 Intellectual Property. To the Knowledge of Seller, except as would not reasonably be expected to have a Seller Material Adverse Effect:
(a) there are no pending litigations, interferences, reexaminations, post-grant proceedings or oppositions in any Major Market Country involving any patents or patent applications owned by Seller and that cover the composition of matter of any of the Royalty Interest Products (collectively, the “Key Patents”);
(b) the Key Patents that have been granted are valid and enforceable in all material respects;
(c) there is no Person who is or claims to be an inventor under any of the Key Patents who is not a named inventor thereof;
(d) Seller has not received written notice of any claim by any Person challenging the ownership or the validity or enforceability of the Key Patents, or asserting that
the manufacture, importation, sale, offer for sale or use of any of the Royalty Interest Products infringes such Person’s patents or other intellectual property rights;
(e) no Third Party patent rights have been, are or currently would be, but for an infringement safe harbor, infringed by the manufacture, importation, sale, offer for sale or use of any of the Royalty Interest Products;
(f) no Person is infringing any of the Key Patents in any material respect; and
(g) all fees necessary to maintain in effect the issued Key Patents have been paid as and when due.
Section 4.8 UCC Matters. The Seller’s exact legal name is, and during the five (5) years prior to the date hereof has been, “Theravance, Inc.”. The principal place of business of the Seller is, and during the five (5) years prior to the date hereof has been, located in South San Francisco. The jurisdiction of organization of the Seller is, and has been during the five (5) years prior to the date hereof, located in Delaware. During the five (5) years prior to the date hereof, the Seller has not been the subject of any merger or limited partnership or other reorganization in which its identity or status was materially changed, except in each case when it was the surviving or resulting entity.
Section 4.9 No Implied Representations and Warranties. Newco acknowledges and agrees that: (i) other than the representations and warranties of Seller specifically contained in this Article IV, there are no representations or warranties of Seller or any other Person, and Seller hereby disclaims all other representations and warranties, whether express, statutory or implied, in connection with this Agreement or the Transactions, including with respect to the Assignment Interest, the GGL Agreements, the Royalty Interest Products, the Key Patents or any other Intellectual Property or data relating to the Royalty Interest Products including patents and patent applications and other Intellectual Property owned by GGL, or the Transactions, and (ii) Newco does not rely on, and Seller shall have no liability in respect of, any representation or warranty not specifically set forth in this Article IV. Without limiting the foregoing, but subject to Section 4.5(d)(ii), Newco acknowledges and agrees that (a)(i) the GGL Agreements generally impose confidentiality obligations on information relating to or generated in connection with those agreements and performance thereunder, and, accordingly, Newco has made its own investigation and assessment of the Assignment Interest, the Royalty Interest Products, the GGL Agreements, the Key Patents and any other Intellectual Property related to the Royalty Interest Products, including patents and patent applications and other Intellectual Property owned by GGL, and the Transactions, that Newco is entering into this Agreement based on such investigation and assessment and that Newco has not relied on and specifically waives any representation, warranty, description or statement, express or implied, set forth in any advertising, marketing literature or other documentation or materials concerning the GGL Agreements, the Assignment Interest, the Royalty Interest Products, the Key Patents and any other Intellectual Property related to the Royalty Interest Products including patents and patent applications and other Intellectual Property owned by GGL, and (ii) Newco is not relying on, and shall have no remedies in respect of, any implied warranties whatsoever, including as to the future amount or potential amount of the Assignment Interest, the creditworthiness of GGL or
any of its Affiliates or any other matter, and (b) except as expressly set forth in any representation or warranty in this Article IV (as modified by the Seller Disclosure Schedule), Seller shall have no liability for losses or damages pursuant to this Agreement (or otherwise) with respect to any information, documents or materials furnished or made available to Newco or any of its Affiliates in any data room, presentation, interview or in any other form or manner relating to the Transactions or the GGL Agreements.
ARTICLE V
REPRESENTATIONS AND WARRANTIES OF NEWCO
Except as disclosed in the separate disclosure letter which has been delivered by Newco to Seller prior to the execution of this Agreement (the “Newco Disclosure Schedule”) (each of which disclosures shall be effective with respect to (i) the corresponding sections and subsections of this Article V, and (ii) any other section or subsection of this Article V, but only if the relevance of that reference as an exception to (or a disclosure for purposes of) such section or subsection would be reasonably apparent to a reasonable person who has read that reference and such representations and warranties), Newco hereby represents and warrants to Seller as of the date of this Agreement:
Section 5.1 Organization. Newco is a limited liability partnership duly organized and validly existing under the laws of England and Wales. Newco has made available to Seller true, correct and complete copies of Newco’s organizational documents (the “Newco Charter Documents”). All Newco Charter Documents are in full force and effect. Newco is not in violation of the Newco Charter Documents, except as would not have, individually or in the aggregate, a Newco Material Adverse Effect.
Section 5.2 Power, Authorization and Enforceability. Newco has all necessary partnership power and authority to execute and deliver the Transaction Documents to which it is a party, to perform its obligations thereunder, and to consummate the Transactions to which it is a party. The execution, delivery and performance by Newco of the Transaction Documents to which it is party and the consummation by Newco of the Transactions to which it is a party has been duly and validly authorized by all necessary partnership action of Newco, and no other partnership proceedings on the part of Newco are necessary to authorize the execution, delivery and performance of such Transaction Documents or to consummate such Transactions. This Agreement has been duly and validly executed and delivered by Newco and, assuming this Agreement is duly and validly executed and delivered by Seller, shall be a legal and valid binding obligation of Newco, enforceable in accordance with their respective terms, subject to applicable Laws affecting creditors’ rights generally and, as to enforcement, to general principles of equity, regardless of whether applied in a proceeding at law or in equity.
Section 5.3 No Conflicts or Violations. None of the execution and delivery by Newco of the Transaction Documents to which it will be a party, the consummation by Newco of the Transactions to which it is a party, or Newco’s compliance with such Transaction Documents will (a) conflict with or violate the Newco Charter Documents, (b) constitute a breach or default under any agreement to which Newco or any of its Subsidiaries is a party or by which any of its or their property or assets are bound, (c) require Newco to make any filing with or obtain any
permit, authorization, consent or approval of any Governmental Authority or other Person, or (d) violate any Law or Order to which Newco is subject, except, in the case of Sections 5.3(b), (c) and (d), as would not reasonably be expected to result in a Newco Material Adverse Effect.
Section 5.4 Proceedings. There is no action, suit, proceeding or, to the Knowledge of Newco, investigation before any Governmental Authority, court or arbitrator pending or, to the Knowledge of Newco, threatened in writing against Newco or its Affiliates relating to the Transactions, except as would not reasonably be expected to result in a Newco Material Adverse Effect.
Section 5.5 No Implied Representations and Warranties. Seller acknowledges and agrees that, other than the representations and warranties of Newco specifically contained in this Article V, there are no representations or warranties of Newco or any other Person either expressed or implied with respect to the Assignment Interest or the Transactions, and that it does not rely on, and Newco shall have no liability in respect of, any representation or warranty not specifically set forth in this Article V.
ARTICLE VI
COVENANTS
Section 6.1 Payment of Assignment Interest.
(a) The Parties shall work in good faith to negotiate the terms of the Lock Box Arrangement that are reasonably acceptable to the parties and shall cooperate fully in implementing the Lock Box Arrangement. The parties anticipate that the Lock Box Arrangement will provide for the establishment of a bank account not within the dominion and control of the Seller into which GGL shall be instructed to make payments in respect of the Assignment Interest and from which the financial institution in control of the Lock Box Account will disburse such amounts to the Account when, as and if any such amounts become payable. Notwithstanding the foregoing, the Parties hereby expressly agree that Seller shall have fully satisfied the requirements of this Assignment Agreement by delivering to GGL a written instruction to make all payments in respect of the Assignment Interest into the Account; such instruction to be revocable only upon completion of the Lock Box Arrangement. Upon implementation of the Lock Box Arrangement and the establishment of the Lock Box Account, the Seller shall irrevocably instruct GGL to make any and all payments in respect of the Assignment Interest into the Lock Box Account. Notwithstanding the foregoing, to the extent Seller receives any amounts in respect of the Assignment Interest from GGL, Seller shall, within two (2) Business Days after such receipt remit, by wire transfer of immediately available funds in United States Dollars to the Account. All such payments are subject to withholding for Taxes, levies and other duties as applicable under the GGL Agreement.
(b) In the event that GGL reduces, deducts, offsets or excludes from royalty payments made to Seller in respect of sales of Royalty Interest Products any amounts that GGL claims Seller owes to GGL (i) related to the Royalty Interest, the Transactions or any of the Transaction Documents (including any deduction or withholding for Taxes), and Seller disputes any such reductions, deductions, offsets or exclusions, Seller shall notify Newco (to the extent not prohibited under the GGL Agreements) of such dispute and, upon resolution (if any) of such
dispute and payment of such disputed amount by GGL to Seller, Seller shall instruct GGL to make a payment in respect of the Assignment Interest into the Account with respect to such disputed amount, (ii) related to the Royalty Interest and (A) there is no dispute between GGL and Seller as to the amount of the royalty payment due in respect of a Royalty Interest Product under the GGL Agreements, (B) there is no dispute between GGL and Seller as to the appropriate amount of the reductions, deductions, offsets or exclusions from such royalty payment and (C) Seller nonetheless consents to a reduction, deduction, offset or exclusion from such royalty payment of an amount in excess of the appropriate reduction, deduction, offset or exclusion amount, then Seller shall pay to the Account an amount equal to such agreed to reduction, deduction, offset or exclusion in excess of such appropriate amount of reductions, deductions, offsets and exclusions multiplied by the Percentage, or (iii) unrelated to the Royalty Interest, the Transactions or any of the Transaction Documents, then, Seller shall pay to the Account an amount equal to the amount of such reduction, deduction, offset or exclusion multiplied by the Percentage.
(c) To the extent that any amount other than payments of the Assignment Interest is deposited by GGL in the Account, Newco shall, within two (2) Business Days after becoming aware thereof (but in no event prior to receipt of such amount by Newco from the Account), remit such amount, by wire transfer of immediately available funds in United States Dollars to Seller.
(d) Newco acknowledges and agrees that Seller is not guaranteeing the performance or payment by GGL of its obligations under the GGL Agreements, and without limiting the foregoing, Newco shall have no recourse to Seller under this Agreement or otherwise due to GGL’s failure to perform its obligations under or breach of the GGL Agreements.
(e) To the extent that Seller incurs any out-of-pocket costs and expenses under the GGL Agreements in connection with the Royalty Interest or brings suit or other action against GGL to enforce Seller’s rights or GGL’s obligations under the GGL Agreements with respect to or to the extent affecting the Royalty Interest, Seller shall be entitled to receive from the Trustee pursuant to Section 7.1 of the Trust Agreement an amount equal to the product of (x) the Percentage multiplied by (y) such out-of-pocket costs and expenses actually incurred by Seller and not reimbursed by GGL pursuant to the GGL Agreements (“Cost-Sharing Deductions”) without duplication (after giving effect to all actions taken under the other Transaction Documents and the Participation Agreement). In the event that any amounts then in the Account are insufficient to pay in full the Cost-Sharing Deductions, then Newco shall, promptly upon demand from Seller, pay Seller the extent of any such insufficiency. To the extent Seller is subsequently reimbursed by GGL for such Cost-Sharing Deductions, Seller shall deposit into the Account an amount equal to the product of (x) the Percentage multiplied by (y) the amount of such reimbursement.
Section 6.2 Tax Matters.
(a) Seller shall be entitled to deduct and withhold from the amounts paid or delivered in connection with the Transactions any amounts that Seller is required to deduct and withhold with respect to any such deliveries and payments under the Internal
Revenue Code of 1986, as amended, or any provision of state, local, or non-U.S. Law. To the extent that Seller withholds such amounts and remits such withheld amounts to the applicable Governmental Authority, such withheld amounts shall be treated as having been paid to or on behalf of Newco. If Seller determines that it is required by Law to deduct and withhold any amount as described in this Section 6.2(a), Seller shall notify Newco of any such requirement as soon as reasonably practical after such determination is made by Seller.
(b) To the extent not prohibited by the GGL Agreements, Seller agrees to use its commercially reasonable efforts to provide in a timely manner such cooperation or assistance, (including by providing any necessary tax forms or other documents) as may be reasonably requested by Newco (at the sole cost of Newco) at any time after the date of this Agreement, so long as the cooperation or assistance could not reasonably be expected to prejudice in any material respect the legal or commercial position of Seller, in order to reduce or eliminate any amounts deducted or withheld pursuant to Section 6.2(a) or any amounts deducted or withheld by GGL on a Royalty Interest payment under the GGL Agreements.
Section 6.3 GGL Agreements.
(a) Seller shall comply in all material respects with its obligations under the GGL Agreements.
(b) Seller agrees that it will not, without Newco’s prior written consent, (i) consent to any termination of the GGL Agreements with respect to the Royalty Interest Products, (ii) consent to any amendment of the provisions of the GGL Agreements that implement the formula for calculating the royalties payable under the Royalty Interest or (iii) impose an Encumbrance on the Assignment Interest.
(c) Newco acknowledges and agrees that, except for the express covenants contained in the Transaction Documents, Seller shall have any obligation or liability, whether express or implied, in connection with the exercise of Seller’s rights and performance of its obligations under the GGL Agreements and that neither Newco nor any other person shall have any claim of any kind against Seller based on or arising out of Seller’s performance under or in connection with the GGL Agreements, it being understood that Seller shall not be liable or have any obligation with respect to allocations of resources, scope, intensity and duration of efforts, and decisions and judgments made in connection with development and commercialization (including any acts or omissions that result in, or increase the likelihood of, greater or lesser commercial success) (i) with respect to, or as among, any Royalty Interest Products, or (ii) as among any one or more Royalty Interest Products, on the one hand, and any Excluded Products, other products or therapeutically active components, on the other hand.
(d) The Parties hereto acknowledge and agree (and expressly and irrevocably disclaim any right to the contrary) that, notwithstanding any other provision of this Agreement or any other Transaction Document to the contrary, Newco has the nonexclusive right, but no obligation, to bring any enforcement action against GGL under any of the GGL Agreements, and neither the Trustee nor the Beneficiary shall have any third party beneficiary rights (expressed, implied or otherwise) in connection with the GGL Agreements. Any such enforcement action by Newco requires the consent of the general manager of Newco.
Section 6.4 Confidentiality.
(a) Definition. “Confidential Information” means all information (whether written or oral, or in electronic or other form, and whether furnished before or after the date of this Agreement) disclosed or made available to Newco by or on behalf of Seller concerning, or relating in any way, directly or indirectly, to this Agreement, the Transactions or the Royalty Interest, including (i) this Agreement, including all terms and conditions hereof, (ii) any royalty information, assignments, notices, requests, correspondence or other information furnished pursuant to this Agreement, and any other reports, data, materials, notices, correspondence or documents of any kind relating in any way, directly or indirectly, to this Agreement, the Royalty Interest or the intellectual property, compounds or products giving rise to the Royalty Interest, (iii) any inventions, devices, improvements, formulations, discoveries, compositions, ingredients, patents, patent applications, know-how, processes, trial results, research, developments or any other intellectual property, trade secrets or information involving or relating in any way, directly or indirectly, to the Royalty Interest or the compounds or products giving rise to the Royalty Interest, and (iv) any other material proprietary and confidential information, in whatever form.
(b) Exclusions. Confidential Information will not include information which:
(i) at or prior to the time of disclosure by Seller was known to Newco through lawful means and is not under any obligation of confidentiality, as evidenced by Newco’s written records;
(ii) at or after the time of disclosure by Seller becomes generally available to the public through no act or omission on the part of Newco in violation of any obligation of confidentiality or any other legal, contractual or fiduciary obligation to Seller, GGL or any of their respective Affiliates, as evidenced by documents that were generally published prior to any disclosure of such information by Seller;
(iii) is developed by Newco independent of, and without any use of or access or reference to Confidential Information, as evidenced by Newco’s written records; or
(iv) Newco receives from a Third Party free to make such disclosure free of any obligation of confidentiality and without breach of any other legal obligation; provided that such Third Party, had the right to disclose such information to Newco (free of any obligation of confidentiality and without any legal, contractual or fiduciary obligation to Seller, GGL or any of their respective Affiliates) as evidenced by Newco’s written records.
(c) Requirements. Newco acknowledges the confidential and proprietary nature of the Confidential Information and agrees that it shall not discuss, reveal, or disclose the Confidential Information to any other Person, or use any Confidential Information for any purpose, other than in connection with the performance of this Agreement, in each case,
without the prior written consent of Seller. Newco agrees to take all reasonable precautions (no less rigorous than Newco takes with respect to its own comparable confidential and proprietary information) to prevent unauthorized or inadvertent disclosure of the Confidential Information.
(d) Disclosure, Legal Obligations. Notwithstanding the foregoing, Newco may disclose such Confidential Information to its officers, directors, employees, accountants, counsel, consultants, advisors and agents who need to know such information in connection with Newco’s evaluation and performance of the Transactions and to its lenders or other financing sources in connection with obtaining financing for Newco’s operations, so long as (i) such Persons are informed by Newco of the confidential nature of such Confidential Information and agree to treat such information confidentially in accordance with the requirements of this Section 6.4 for the benefit of Seller and (ii) Newco shall be liable for any breach or violation of such obligations by any such Person. Newco may also disclose Confidential Information to the extent required by Law, including the applicable rules of any stock exchange that are binding on Newco, provided that Newco shall use its reasonable best efforts to notify Seller of such requirement (to the extent permitted by applicable Law) promptly and sufficiently in advance of any such disclosure so that Seller may seek an appropriate protective order or other appropriate remedy. Newco shall use its reasonable best efforts to limit such disclosures to that portion of the information legally required to be disclosed and shall use its reasonable best efforts to seek confidential treatment for such information if so requested by Seller. In addition, the Parties will coordinate in advance with each other in connection with the redaction of certain provisions of this Agreement with respect to any SEC or foreign equivalent filings of this Agreement, and each Party shall use its reasonable best efforts to seek confidential treatment for such terms if so requested by the other Party.
(e) Return of Information. Newco will, at the request of Seller, after the expiration of the Term or effective termination of this Agreement (i) promptly return all Confidential Information held or used by Newco in whatever form, or (ii) at the discretion of Seller, promptly destroy all such Confidential Information, including all copies thereof, and those portions of all documents (e.g., analyses, summaries, etc.) that incorporate or are based on Confidential Information; provided, however, that (x) Newco’s legal department may retain one copy of such information for legal compliance purposes only for so long as required by applicable Law or Newco’s bona fide pre-existing record-keeping policy, and (y) notwithstanding anything else in this Agreement to the contrary, the confidentiality restrictions imposed hereby shall continue to apply to such information, and shall survive any termination of this Agreement, for so long as such information is retained. At the request of Seller, Newco will promptly certify that it has complied in full with this Section 6.4
Section 6.5 Information Rights. Newco hereby acknowledges the confidentiality obligations imposed on Seller by the GGL Agreements. Notwithstanding any other provision of this Agreement, Newco agrees that Seller shall not, and shall not be required to, disclose any information in violation of its obligations under the GGL Agreements. Unless prohibited under the GGL Agreements (as determined in Seller’s sole reasonable discretion), Seller shall use its reasonable efforts to provide to Newco information reasonably requested by Newco that is material to the Assignment Interest (it being understood that Seller shall not be required to undertake litigation or incur significant expense pursuant to this Section 6.4).
Section 6.6 Books and Records. For accounting purposes only, each of the Parties will treat the sale and assignment to Newco of the Assignment Interest pursuant to Section 2.1 as a sale and assignment on its books and records to the extent permitted under generally accepted accounting principles in the United States.
Section 6.7 Public Announcement. Except as required by applicable Law and subject to the final two (2) sentences of this Section 6.5, neither Party will make any public announcement of any information regarding this Agreement without the prior written approval of the other Party, which approval shall not be unreasonably withheld, conditioned or delayed; provided that the foregoing restriction shall not restrict Seller from making any public announcement of any information regarding the Royalty Interest Products. Once any statement is approved for disclosure by the Parties or information is otherwise made public in accordance with the preceding sentence, either Party may make a subsequent public disclosure of the contents of such statement without further approval of the other Party. Notwithstanding the foregoing, within sixty (60) days following the Closing, appropriate representatives of the Parties will meet and agree upon a process and principles for reaching timely consensus on how the Parties will make public disclosure concerning this Agreement.
Section 6.8 UCC Matters. The Seller shall file and maintain in effect all UCC filings, and shall take such other actions under the UCC, as may be necessary to protect the validity and perfection of Newco’s Assignment Interest under the UCC. The Seller will not change its location (within the meaning of Sections 9-301 and 9-307 of the applicable UCC) or its legal name without at least ten (10) days’ prior written notice to the Newco. In the event that the Seller desires to so change its location or change its legal name, the Seller will make any required filings and will deliver to the Newco copies of all such required filings with the filing information duly noted thereon by the office in which such filings were made.
Section 6.9 Royalty Interest Disclosures. Seller shall comply with its obligations under applicable Law to make prompt disclosure to the public of material information concerning the Royalty Interest.
Section 6.10 Non-Cash Consideration. In the event a Royalty Interest is paid other than in cash, Seller shall pay to the Account an amount in cash equal to the fair market value, as reasonably determined by Seller, of the Percentage of such non-cash consideration as promptly as reasonably practicable following the determination of the fair market value of such non-cash consideration.
ARTICLE VII
TERM
Section 7.1 Term. The term of this Agreement shall commence on the date hereof and end, with respect to each Royalty Interest Product, upon expiration or termination (including any early termination) of the Royalty Interest Term with respect to such Royalty Interest Product (the “Term”).
ARTICLE VIII
INDEMNIFICATION
Section 8.1 Indemnification by Seller. Subject to the terms and conditions of this Article VIII, Seller shall indemnify Newco and its officers, directors, managers, agents and representatives (but expressly excluding any Trustee or Beneficiary) (collectively “Newco Indemnitees”) from and against any and all direct damages, losses, claims, costs, liabilities, demands or expenses (including reasonable attorneys’ fees and expenses in connection with any action, suit or proceeding) (collectively, “Losses”) suffered or incurred by any such Newco Indemnitee arising from:
(a) any breach of any representation or warranty made by Seller in this Agreement (provided, however, that for purposes of determining whether a breach of the representations and warranties set forth in Sections 4.1, 4.3, 4.4, 4.5(a), 4.5(b), 4.5(d)(i) and 4.7 has occurred for purposes of this Section 8.1(a) only, a violation, conflict, breach, default, failure or the existence of a state of facts or circumstances shall be deemed to be material or to have a Seller Material Adverse Effect if it changes the royalty rate payable in respect of the Royalty Interest Products in a manner adverse to Newco); and
(b) any breach of any covenant of Seller contained in this Agreement.
Section 8.2 Indemnification by Newco. Subject to the terms and conditions of this Article VIII, Newco shall indemnify Seller, its Affiliates and each of their respective officers, directors, managers, agents and representatives (collectively “Seller Indemnitees”) from and against any and all Losses suffered or incurred by any such Seller Indemnitee arising from:
(a) any breach of any representation or warranty made by Newco in this Agreement; and
(b) any breach of any covenant of Newco contained in this Agreement.
Section 8.3 Indemnification Procedure.
(a) Third-Party Claims.
(i) Notice of Third-Party Claims. A Party seeking indemnification (the “Indemnified Party”) with respect to any claim or demand made by any Person against the Indemnified Party (a “Third-Party Claim”) shall promptly notify the other Party (the “Indemnifying Party”) in writing (including in such notice a brief description of the claim for indemnification and the Loss, including damages sought or estimated, to the extent actually known or reasonably capable of estimation by the Indemnified Party) of any claim for indemnification, provided that failure to give such notice shall not relieve the Indemnifying Party of any liability hereunder except to the extent the Indemnifying Party is materially prejudiced by such failure.
(ii) Defense of Third-Party Claims. The Indemnifying Party shall be entitled to participate in the defense of the Third-Party Claim and, if it so chooses, to assume the defense thereof, at its own expense, with counsel selected by the Indemnifying Party; provided that such counsel is not reasonably objected to by the Indemnified Party. If the Indemnifying Party assumes the defense thereof, the Indemnifying Party (1) shall not be liable to the Indemnified Party for legal expenses subsequently incurred by the Indemnified Party in connection with the defense thereof, and (2) shall permit the Indemnified Party to participate in, but not control, the defense of any such action or suit through counsel chosen by the Indemnified Party, provided that such counsel is not reasonably objected to by the Indemnifying Party and the fees and expenses of such counsel shall be borne by the Indemnified Party. The Indemnifying Party shall be liable for the fees and expenses of counsel employed by the Indemnified Party in the defense of a Third-Party Claim for any period during which the Indemnifying Party has not assumed the defense thereof (other than during the period prior to the time the Indemnified Party shall have notified the Indemnifying Party of such Third-Party Claim).
(iii) Cooperation. The Indemnified Party shall, if requested by the Indemnifying Party, give reasonable assistance to and cooperate with the Indemnifying Party in defense of any claim. The Indemnifying Party shall reimburse the Indemnified Party for any Third Party reasonable legal expenses directly incurred from providing such assistance and cooperation. Such assistance and cooperation shall include (1) the retention of and the provision to the Indemnifying Party of records and information that are reasonably relevant to such Third-Party Claim and (2) the making available of employees on a mutually convenient basis for providing additional information and explanation of any material provided hereunder. The Indemnifying Party shall have the right to consent to the entry of judgment with respect to, or otherwise settle, a Third-Party Claim with the prior written consent of the Indemnified Party, which consent shall not be unreasonably withheld; provided that the Indemnified Party may withhold its consent if any such judgment or settlement imposes an unreimbursed monetary or continuing non-monetary obligation on such Indemnified Party or does not include an unconditional release of such Indemnified Party and its Affiliates from all liability in respect of claims that are the subject matter of the indemnified claim. Regardless of whether the Indemnifying Party shall have assumed defense of a Third-Party Claim, the Indemnified Party shall not be entitled to be indemnified pursuant to this Article VIII if the Indemnified Party shall settle such Third-Party Claim without the prior written consent of the Indemnifying Party.
(b) Claims by a Party. In order for an Indemnified Party to be entitled to any indemnification under this Article VIII in respect of Losses that do not arise out of or involve a Third Party Claim, the Indemnified Party must notify the Indemnifying Party promptly in writing (including in such notice a brief description of the claim for indemnification and the Loss, including damages sought or estimated, to the extent actually known or reasonably capable of estimation by the Indemnified Party); provided, that, the failure to promptly provide such
notice shall not affect the indemnification provided under this Article VIII except to the extent that the Indemnifying Party has been actually prejudiced as a result of such failure.
Section 8.4 Claims Period for Breach of Representations and Warranties. The representations and warranties contained in this Agreement shall not survive the Closing; however, claims for breach of the: (a) representations and warranties contained in this Agreement (other than any claims for breach of the Fundamental Representations) may be made at any time following the Closing and up to and including the date that is the first anniversary of the date hereof; and (b) Fundamental Representations may be made at any time following the Closing and prior to the expiration of the applicable statute of limitations. In no event shall any Party hereto have any liability for any breach of a representation or warranty set forth in this Agreement unless the other Party hereto shall have delivered a notice to such Party, pursuant to Section 8.3 prior to the deadline applicable to the making of such claim set forth in the preceding sentence.
Section 8.5 Limitations on Indemnification.
(a) Notwithstanding anything herein to the contrary, Seller shall have no liability to Newco under this Agreement for any action taken by Seller or any failure by Seller to take any action pursuant to Article VI in compliance with any written instructions of Newco or with the prior written consent of Newco.
(b) Seller or Newco as applicable, shall not be obligated to indemnify any Newco Indemnitee or Seller Indemnitee, as applicable, if the Losses incurred by such Person arise from the gross negligence, fraud or willful misconduct of Newco or Seller, as applicable, or their respective Affiliates, officers, directors, managers, agents and representatives (acting in their capacity as such).
(c) Notwithstanding anything in this Agreement to the contrary, Seller shall not have any liability under: (i) Section 8.1 with respect to any individual item (or any series of related items) if the Loss related thereto is less than US$750,000, and such items shall not be aggregated for purposes of Section 8.5(c)(ii); (ii) Section 8.1(a) unless the aggregate liability for all Losses suffered by the Newco Indemnitees thereunder exceeds US$15,000,000, and then only to the extent of such excess; or (iii) Section 8.1 in excess of the amount by which (x) the One Billion United States Dollars (US$1,000,000,000) exceeds (y) the aggregate amount of payments made to Newco in respect of the Assignment Interest theretofore received or receivable by Newco, in the aggregate; provided, however, that the cap set forth in clause (iii) shall not be less than Two Hundred Million Dollars (US$200,000,000) except with respect to any breach for which indemnification is sought that arises from or relates to a Specified Claim. Notwithstanding anything to the contrary in any Transaction Document, for any Loss for which Seller must indemnify any Newco Indemnitee under this Agreement, the amount paid by Seller shall count toward the liability cap under both this Agreement and under the Participation Agreement and in no event shall Seller have any liability for Losses under the this Agreement arising out of the same matter or set of facts or circumstances as any claims made, or for which a claim could be made, under the Participation Agreement.
(d) Notwithstanding anything in this Agreement to the contrary, Newco shall not have any liability under: (i) Section 8.2 with respect to any individual item (or any series of related items) if the Loss related thereto is less than US$750,000, and such items shall not be aggregated for purposes of Section 8.5(d)(ii); (ii) Section 8.2(a) unless the aggregate liability for all Losses suffered by the Seller Indemnitees thereunder exceeds US$15,000,000, and then only to the extent of such excess or (iii) after the Closing, Section 8.2 in excess of US$1,000,000,000.
(e) The amount of any Losses payable under this Article VIII by an Indemnifying Party shall be reduced by the amount, if any, of any tax benefit available to the Indemnified Party by reason of such Losses or the payment thereof and by any insurance proceeds or other recovery from a Third Party received by the Indemnified Party (net of any expenses incurred by such Indemnified Party in procuring such recovery) in connection with the matter out of which such Losses arise. If the Indemnified Party receives any proceeds subsequent to an indemnification payment by the Indemnifying Party, then such Indemnified Party shall reimburse the Indemnifying Party as promptly as practicable thereafter for any payment made by such Indemnifying Party pursuant to such Indemnifying Party’s indemnification obligations hereunder up to the amount received pursuant to this Article VIII by the Indemnified Party from such Indemnifying Party (net of any expenses incurred by such Indemnified Party in procuring such recovery).
(f) For purposes of determining the amount of any Losses payable pursuant to Sections 8.1 or 8.2 (but not, for the avoidance of doubt, for the purposes of determining the existence of a breach), any qualification as to materiality, Seller Material Adverse Effect or Newco Material Adverse Effect contained in the applicable underlying representation or warranty shall be disregarded.
(g) To the extent Seller is obligated to indemnify Purchaser for any Losses arising out of the same occurrence or omission in respect of which Seller is liable to Newco under the provisions of this Article VIII, Seller shall indemnify Purchaser pursuant to the Participation Agreement with respect to such occurrence or omission, and Newco’s claims with respect to such occurrence or omission shall be deemed to have been satisfied in respect thereof following such indemnification of Purchaser.
Section 8.6 Exclusive Remedy. Notwithstanding anything to the contrary in the Transaction Documents, the Parties hereto acknowledge and agree that this Article VIII (including Section 8.4 and Section 8.5) shall provide the Parties’ sole and exclusive remedy with respect to any matter or claim arising out of, relating to, or in connection with, the Transaction Documents and the Transactions, except (i) for fraud or willful misconduct and (ii) injunctive relief or claims for specific performance pursuant to Section 9.4 (Specific Performance).
Section 8.7 Disclaimer of Consequential Damages.
(a) EXCEPT WITH RESPECT TO (i) A PARTY’S FRAUD OR WILLFUL MISCONDUCT, OR (ii) LOSSES ACTUALLY PAID TO A THIRD PARTY PURSUANT TO A THIRD-PARTY CLAIM IN ACCORDANCE WITH ARTICLE VIII, TO THE MAXIMUM EXTENT PERMITTED BY LAW, IN NO EVENT SHALL EITHER
PARTY BE LIABLE TO THE OTHER PARTY OR ANY THIRD PARTIES FOR ANY INDIRECT, SPECIAL, INCIDENTAL, CONSEQUENTIAL OR PUNITIVE DAMAGES, INVESTMENT LOSSES, LOST PROFITS, LOSS OF DATA, OR LOSS OF USE ARISING IN ANY MANNER OUT OF OR IN CONNECTION WITH THE TRANSACTION DOCUMENTS HOWEVER CAUSED WHETHER BY NEGLIGENCE, BREACH OF CONTRACT, TORT OR OTHERWISE AND WHETHER OR NOT SUCH PARTY HAS BEEN ADVISED OF OR OTHERWISE MIGHT HAVE ANTICIPATED THE POSSIBILITY OF ANY SUCH DAMAGES.
(b) THE PARTIES HAVE AGREED THAT THE LIABILITY LIMITATIONS SPECIFIED IN SECTION 8.5 AND THIS SECTION 8.7 WILL SURVIVE AND APPLY EVEN IF ANY LIMITED REMEDY SPECIFIED IN THIS AGREEMENT IS FOUND TO HAVE FAILED ITS ESSENTIAL PURPOSE.
ARTICLE IX
MISCELLANEOUS
Section 9.1 Assignment.
(a) Assignment by Seller. Seller may not assign or otherwise transfer this Agreement without the prior written consent of Newco, except that Seller may assign this Agreement, in whole or in part, to (A) an acquiror of Seller or a successor to all or substantially all of the assets of Seller, whether by merger, sale of stock, sale of assets or other similar transaction, (B) with respect to any Royalty Interest Product, an Applicable Successor to Seller relating to such Royalty Interest Product, whether by merger, sale of stock, sale of assets or other similar transaction or (C) an Affiliate of Seller for so long as such Affiliate remains an Affiliate of Seller and if Seller guarantees the performance of this Agreement by such Affiliate.
(b) Assignment by Newco. Newco may not assign or transfer this Agreement or the Assignment Interest, in whole or in part, without the prior written consent of Seller.
(c) Seller Corporate Reorganization. Notwithstanding anything to the contrary herein, Seller shall be permitted to assign this Agreement and the rights and obligations hereunder in respect of the MABA ‘081 Royalty Interest, in whole or in part, without Newco’s consent, to a Third Party pursuant to the Seller Corporate Reorganization.
(d) Successors and Assigns. Subject to the terms and conditions of this Section 9.1, this Agreement shall be binding upon and inure to the benefit of the Parties hereto and their permitted successors and assigns. Any purported assignment or other transfer in violation of this Section 9.1 shall be void ab initio and of no force or effect.
Section 9.2 Further Assurances. From time to time, as and when reasonably requested by any Party, each Party shall execute and deliver, or cause to be executed and delivered, all such documents, certificates, agreements and instruments, and shall take, or cause to be taken, all such further or other actions, as are reasonably necessary and appropriate to carry out all of the provisions of this Agreement, or to consummate the Transactions.
Section 9.3 Notices. All notices, requests, demands, consents, waivers or other communications to or from either Party to the other Party must be in writing and will be deemed to have been given
(a) upon delivery, including upon confirmation by the carrier of delivery (which may be in electronic form), if delivered by hand or mailed via overnight or express mail service or by courier,
(b) in the case of a fax, when receipt is confirmed by telephone, reply email or reply fax from the recipient or other fax confirmation of receipt, or
(c) in the case of an email, when receipt is confirmed by telephone or reply email from the recipient,
addressed as follows (or to such other address or addresses as the Seller or the Newco may from time to time designate by notice given in accordance with this Section 9.3:
if to Seller:
Theravance, Inc.
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇
▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attention of: ▇▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇, Senior Vice President & General Counsel
|
Fax No.: |
(▇▇▇) ▇▇▇-▇▇▇▇ |
|
|
|
|
E-mail: |
▇▇▇▇▇▇▇@▇▇▇▇▇▇▇▇▇▇.▇▇▇ |
with a copy to:
Skadden, Arps, Slate, ▇▇▇▇▇▇▇ & ▇▇▇▇, LLP
▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇
▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Attention of: Amr ▇▇▇▇▇▇
|
Fax No.: |
(▇▇▇) ▇▇▇-▇▇▇▇ |
|
|
|
|
E-Mail: |
▇▇▇.▇▇▇▇▇▇@▇▇▇▇▇▇▇.▇▇▇ |
if to the Newco:
[Newco]
[Address]
Attention of:
Fax No.:
E-mail:
with a copy to:
[Address]
Attention of:
Fax No.:
E-Mail:
Section 9.4 Specific Performance. Each of the Parties hereto acknowledges that the other Party may have no adequate remedy at law if the first Party fails to perform any of its non-monetary obligations under this Agreement. In such event, each of the Parties agrees that the other Party shall have the right, in addition to any other rights it has (whether at law or in equity), to pursue equitable remedies such as an injunction or specific performance of this Agreement without necessity of posting any bond or undertaking in connection therewith. This remedy is separate and apart from any other remedy the Parties may have under this Agreement.
Section 9.5 Title; Headings; Captions. The title, headings and captions in this Agreement are included for convenience only and will not affect the meaning or interpretation of any provision of this Agreement.
Section 9.6 Independent Contractors. The Parties recognize and agree that each is operating as an independent contractor and not as an agent or fiduciary of the other. Notwithstanding any provision of this Agreement which may indicate otherwise, it is the specific intent of the Parties that this Agreement and the relationship created hereby is not, nor shall it be deemed to be, a separate entity, joint venture or partnership or any similar arrangement, nor shall any master/servant, employer/employee or principal/agent relationship be created between the Parties.
Section 9.7 Third-Party Beneficiaries. Except to the extent expressly contemplated with respect to Seller Indemnitees and Newco Indemnitees in Article VIII, this Agreement is for the sole benefit of Seller and Newco and their permitted successors and assigns, and nothing herein expressed or implied shall give or be construed to give to any Person (including Trustee or Beneficiary), other than the parties hereto and such permitted successors and assigns, any legal or equitable rights hereunder. Notwithstanding anything herein to the contrary, with respect to any indemnification claim made pursuant to Article VIII, Seller or Newco shall make such claim on behalf of the applicable Seller Indemnitee or Newco Indemnitee, as applicable, and such Person (unless such Person is Seller or Newco, itself) shall not be entitled to directly make such claim against Newco or Seller, as applicable.
Section 9.8 Entire Agreement. This Agreement, along with the Appendices, Exhibits, Schedules, Seller Disclosure Letter and Newco Disclosure Letter and other attachments hereto, sets forth the entire agreement and understanding between the Parties hereto with respect
to the subject matter hereof and supersedes all prior and contemporaneous agreements and understandings relating to such subject matter.
Section 9.9 Amendment and Supplementation. This Agreement may be amended, modified or supplemented only by a written agreement signed by both Parties.
Section 9.10 No Waiver. Any waiver of, or consent to depart from, the requirements of any provision of this Agreement shall be effective only if it is in writing and signed by the Party giving it, and only in the specific instance and for the specific purpose for which it has been given. No failure or delay of either Party in exercising any power, right or remedy under this Agreement will operate as a waiver. No single or partial exercise of any power, right or remedy precludes any other or further exercise of such power, right or remedy or the exercise of any other power, right or remedy.
Section 9.11 Severability. If any of the covenants, agreements or terms of this Agreement is held invalid, illegal or unenforceable, then it will be deemed severable from the remaining covenants, agreements or terms of this Agreement and will in no way affect the validity, legality or enforceability of the remaining Agreement. Upon such determination that any covenant, agreement or term is invalid, illegal or unenforceable, this Agreement shall be deemed modified so as to effect the original intent of the Parties as closely as possible in order that the Transactions be consummated as originally contemplated to the fullest extent consistent with applicable Law.
Section 9.12 Governing Law. THIS AGREEMENT AND ALL ACTIONS, PROCEEDINGS OR COUNTERCLAIMS (WHETHER BASED ON CONTRACT, TORT OR OTHERWISE) ARISING OUT OF OR RELATING TO THIS AGREEMENT WILL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH THE LAWS OF THE STATE OF NEW YORK, WITHOUT GIVING EFFECT TO THE PRINCIPLES OF CONFLICTS OF LAW THEREOF EXCEPT AS SET FORTH IN SECTIONS 5-1401 OF THE NEW YORK GENERAL OBLIGATIONS LAW.
Section 9.13 Submission to Jurisdiction. The parties submit to the exclusive jurisdiction of the United States District Court for the Southern District of New York and of any New York State Court sitting in New York, New York for purposes of all legal proceedings arising out of or relating to this Agreement. The parties irrevocably waive, to the fullest extent they may do so, any objection that they may now or hereafter have to the laying of the venue of any such proceeding brought in such a court and any claim that any such proceeding brought in such a court has been brought in an inconvenient forum.
Section 9.14 Waiver of Jury Trial. Each Party irrevocably waives, to the fullest extent permitted by applicable Law, any and all right to trial by jury in any legal proceeding directly or indirectly arising out of or relating to this Agreement or the Transactions.
Section 9.15 Counterparts. This Agreement may be executed in any number of counterparts. Each counterpart will be an original, and all counterparts will together constitute one and the same instrument. Delivery of an executed counterpart via PDF or other electronic delivery shall be considered the same as delivery of a manually executed counterpart.
IN WITNESS WHEREOF, the Parties hereto have set their hands by their authorized representatives as of the year and date first indicated above.
|
|
THERAVANCE, INC. | |
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
|
|
|
|
|
|
|
|
|
|
[NEWCO] | |
|
|
| |
|
|
|
|
|
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
APPENDIX A
DEFINED TERMS
A. DEFINITIONS.
For the purposes of this Agreement, unless otherwise indicated, the following terms shall have the respective meanings specified below, and grammatical variations of such terms shall have corresponding meanings:
1. “Account” has the meaning set forth in the Trust Agreement.
2. “Affiliate” means, with respect to either Party, any other Person directly or indirectly controlling, controlled by or under common control with, such Party. For the purposes of this definition “control” means the possession, directly or indirectly, of (a) the power to direct or cause the direction of the management and policies of a Person, whether through the ownership of voting securities or other equity interest, representation on its board of directors or body performing similar functions, by contract or otherwise, together with (b) ownership of the related equity interest in such Person; and the terms “controlling” and “controlled” have corollary meanings.
3. “Agreement” has the meaning set forth in the introductory paragraph of this Agreement.
4. “ANORO” means (a) the combination medicine comprising UMEC with VI, with no other therapeutically active component, and explicitly excluding either component as a monotherapy, and which is proposed, as of the date hereof, to be sold under the brand name “ANORO™ ELLIPTA™”, and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect to only such combination medicine set forth in subsection (a) comprising UMEC with VI, with no other therapeutically active component (and explicitly excluding either component as a monotherapy).
5. “ANORO Royalty Interest”:
(a) Means Seller’s entitlement under the Collaboration Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
(i) royalty payments on any net sales worldwide of ANORO by or on behalf of GGL, pursuant to Section 6.3 of the Collaboration Agreement;
(ii) any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Collaboration Agreement;
(iii) any Infringement Payments with respect to ANORO in lieu of the payments referred to in clause (a)(i) immediately above; and
(iv) any Other Amounts with respect to ANORO in lieu of the payments referred to in clause (a)(i) immediately above.
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets contemplated by Sections 6.3, 6.4.1 and 6.9 of the Collaboration Agreement. The VI Reverse Milestone Payment Obligation shall remain the obligation of Seller, and shall not be a reduction, deduction, offset or exclusion from the royalty payments under subsection (a) above of this definition.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Collaboration Agreement), (ii) one-time fees for new compounds contributed to the collaboration (including any one-time fee payments from GGL to Seller pursuant to Sections 6.1.3 or 6.1.4 of the Collaboration Agreement), (iii) milestone payments (including any milestone payments pursuant to Section 6.2 of the Collaboration Agreement), or (iv) any other payments (other than those expressly identified in subsection (a) above of this definition), in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to ANORO.
6. “Applicable Successor” means with respect to Seller and any of its Affiliates with respect to each Royalty Interest Product, the assignee or successor-in-interest to all or substantially all of Seller’s (or Seller’s Affiliate’s) interest under the applicable GGL Agreement with respect to such Royalty Interest Product (other than GGL).
7. “Assignment” has the meaning set forth in Section 2.1(a) of this Agreement.
8. “Assignment Interest” means (i) a twenty-one percent (21%) undivided beneficial interest in the Royalty Interest and (ii) the assignment of a non-exclusive right to enforce payment in respect thereof under the GGL Agreements.
9. “Beneficiary” means the beneficiary under the Trust Agreement
10. “BREO/RELVAR” means (a) the combination medicine comprising FF and VI, with no other therapeutically active component, and explicitly excluding either component as a monotherapy, and which is proposed, as of the date hereof, to be sold under the brand name “BREO™ ELLIPTA™” in the United States and “RELVAR™ ELLIPTA™” in the European Union and Japan, and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect to only such combination medicine set forth in subsection (a) comprising FF and VI, with
no other therapeutically active component (and explicitly excluding either component as a monotherapy).
11. “BREO/RELVAR Royalty Interest”:
(a) Means Seller’s entitlement under the Collaboration Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
|
(i) |
|
royalty payments on any net sales worldwide of BREO/RELVAR by or on behalf of GGL, pursuant to Section 6.3 of the Collaboration Agreement; |
|
|
|
|
|
(ii) |
|
any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Collaboration Agreement; |
|
|
|
|
|
(iii) |
|
any Infringement Payments with respect to BREO/RELVAR in lieu of the payments referred to in clause (a)(i) immediately above; and |
|
|
|
|
|
(iv) |
|
any Other Amounts with respect to BREO/RELVAR in lieu of the payments referred to in clause (a)(i) immediately above. |
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets contemplated by Sections 6.3, 6.4.1 and 6.9 of the Collaboration Agreement. The VI Reverse Milestone Payment Obligation shall remain the obligation of Seller, and shall not be a reduction, deduction, offset or exclusion from the royalty payments under subsection (a) above of this definition.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Collaboration Agreement), (ii) one-time fees for new compounds contributed to the collaboration (including any one-time fee payments from GGL to Seller pursuant to Sections 6.1.3 or 6.1.4 of the Collaboration Agreement), (iii) milestone payments (including any milestone payments pursuant to Section 6.2 of the Collaboration Agreement), or (iv) any other payments (other than those expressly identified in subsection (a) above of this definition), in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to BREO/RELVAR.
12. “Business Day” means any day that is not a Saturday, Sunday or other day on which banks are required or expressly authorized to be closed in London, England, New York, New York, San Francisco, California or Dublin, Ireland.
13. “Closing” has the meaning set forth in Section 3.1 of this Agreement.
14. “Collaboration Agreement” means that certain Collaboration Agreement by and between Theravance, Inc. and Glaxo Group Limited dated November 14, 2002, as amended from time to time.
15. “Confidential Information” has the meaning set forth in the Section 6.4(a) of this Agreement.
16. “Cost-Sharing Deductions” has the meaning set forth in Section 6.1(e) of this Agreement.
17. “Declaration of Trust” has the meaning set forth in Section 2.1(a).
18. “Encumbrance” means any lien, charge, security interest, mortgage or pledge.
19. “Excluded Products” means any therapeutically active component or product, whether as a monotherapy, combination product or otherwise, and any delivery devices (whether stand-alone or with any one or more therapeutically active components or products), other than the Royalty Interest Products. “Excluded Products” includes, by way of example:
(a) UMEC, as a monotherapy or in a combination product (other than UMEC and VI, with no other therapeutically active component), including a combination product that includes (i) UMEC, (ii) VI and (iii) at least one other therapeutically active component;
(b) FF, as a monotherapy or in a combination product (other than FF and VI, with no other therapeutically active component), including a combination product that includes (i) FF, (ii) VI and (iii) at least one other therapeutically active component;
(c) MABA ‘081 other than as a monotherapy (i.e. excluding MABA ‘081 in combination with any one or more other therapeutically active component(s));
(d) VI, in any product other than (i) as a monotherapy, (ii) ANORO and (iii) BREO/RELVAR; and
(e) any other therapeutically active component or product (including a combination product of two or more therapeutically active components not expressly specified in the definition of each of ANORO, BREO/RELVAR, MABA ‘081 and VI Monotherapy).
20. “FF” means the inhaled corticosteroid fluticasone furoate (with the chemical structure as set forth in Attachment A-1) or an ester, salt or other noncovalent derivative thereof.
21. “Fundamental Representations” means the representations and warranties set forth in Sections 4.1, 4.2, 4.6, 5.1 and 5.2 of this Agreement.
22. “GGL” means Glaxo Group Limited and, with respect to any GGL Agreement, any successor in interest under such GGL Agreement.
23. “GGL Agreements” means the Collaboration Agreement and the Strategic Alliance Agreement.
24. “Governmental Authority” means any United States (federal, state or local), Irish or other foreign government, or any governmental, regulatory, judicial or administrative authority, agency or commission.
25. “Indemnified Party” has the meaning set forth in Section 8.3(a) of this Agreement.
26. “Indemnifying Party” has the meaning set forth in Section 8.3(a) of this Agreement.
27. “Infringement Payment” means any net collections, recoveries, damages, awards or settlement payments that are actually paid to Seller as a result of any settlement discussion, litigation, arbitration or other legal proceeding brought against third-party infringers by Seller pursuant to Section 13 of the Collaboration Agreement or Section 13 of Strategic Alliance Agreement, as applicable.
28. “Intellectual Property” means all worldwide intellectual property and rights arising from or in respect of the following: all (a) inventions, discoveries, industrial designs, business methods, patents and patent applications (including provisional and Patent Cooperation Treaty applications), including continuations, divisionals, continuations-in-part, reexaminations and reissues, extensions, renewals and any patents that may be issued with respect to the foregoing; (b) trademarks, service marks, certification marks, collective marks, trade names, business names, assumed names, d/b/a’s, fictitious names, brand names, trade dress, logos, symbols, Internet domain names and corporate names, and general intangibles of a like nature and other indicia of origin or quality, whether registered, unregistered or arising by Law, and all applications, registrations, and renewals for any of the foregoing, together with the goodwill associated with and symbolized by each of the foregoing; (c) published and unpublished works of authorship in any medium, whether copyrightable or not (including databases and other compilations of information, computer software, source code, object code, algorithms, and other similar materials and Internet website content), copyrights and moral rights therein and thereto, and registrations and applications therefor, and all issuances, renewals, extensions, restorations and reversions thereof; and (d) confidential and proprietary information, trade secrets, and know-how, including methods, processes, business plans, schematics, concepts, software and databases (including source code, object code and algorithms), formulae, drawings, prototypes, models, designs, devices, technology, research and development and customer information and lists.
29. “Key Patents” has the meaning set forth in Section 4.7(a) of this Agreement.
30. “Knowledge of Newco” means the knowledge, after reasonable inquiry, of the following individuals: [ ].
31. “Knowledge of Seller” means the knowledge, after reasonable inquiry, of the following individuals: ▇▇▇▇ ▇ ▇▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇▇▇▇ and ▇▇▇▇ ▇▇▇▇▇▇▇.
32. “Law” means any and all domestic (federal, state or local) or foreign laws, rules, regulations, statutes, orders, ordinance, judgments or decrees or other pronouncements by any Governmental Authority.
33. “Losses” has the meaning set forth in Section 8.1 of this Agreement.
34. “MABA” means a bifunctional muscarinic antagonist-beta2 agonist.
35. “MABA ‘081” means (a) the MABA compound known as GSK961081, solely as a monotherapy (i.e., excluding GSK961081 in combination with any one or more other therapeutically active component(s)), and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect to only GSK961081 solely as a monotherapy (i.e., excluding GSK961081 in combination with any one or more other therapeutically active component(s)).
36. “MABA ‘081 Royalty Interest”:
(a) Means Seller’s entitlement under the Strategic Alliance Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
|
(i) |
|
royalty payments on any net sales worldwide of MABA ‘081 by or on behalf of GGL, pursuant to Section 6.3 of the Strategic Alliance Agreement; |
|
|
|
|
|
(ii) |
|
any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Strategic Alliance Agreement; |
|
|
|
|
|
(iii) |
|
any Infringement Payments with respect to MABA ‘081 in lieu of the payments referred to in clause (a)(i) immediately above; |
|
|
|
|
|
(iv) |
|
any Other Amounts with respect to MABA ‘081 in lieu of the payments referred to in clause (a)(i) immediately above. |
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets in Sections 6.3, 6.4.1 and 6.9 of the Strategic Alliance Agreement.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Strategic Alliance Agreement), (ii) opt-in fees or payments for equity investments (including any payments from GGL to Seller pursuant to Sections 6.1.2 or 6.1.4 of the Strategic Alliance Agreement), (iii)
milestone payments (including any milestone payments pursuant to Section 6.2 of the Strategic Alliance Agreement) or (iv) any other payments (other than those expressly identified in subsection (a) above of this definition), in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to MABA ‘081.
37. “Major Market Country” means each of Canada, France, Germany, Italy, Japan, Spain, the United Kingdom and the United States.
38. “Newco Charter Documents” shall have the meaning set forth in Section 4.1 of this Agreement.
39. “Newco Disclosure Schedule” has the meaning set forth in Article V of this Agreement.
40. “Newco Indemnitees” has the meaning set forth in Section 8.1 of this Agreement.
41. “Newco Material Adverse Effect” means any one or more of (a) a material adverse effect on the ability of Newco to consummate the Transactions and perform its obligations under this Agreement and (b) a material adverse effect on the validity or enforceability of this Agreement or the rights of Seller hereunder.
42. “Order” means any decree, order, judgment, ruling, writ, injunction, temporary restraining order or other formal order in any suit or proceeding by or with any Governmental Authority which has become final and nonappealable.
43. “Other Amounts” means in respect of each of the ANORO Royalty Interest, BREO/RELVAR Royalty Interest, MABA ‘081 Royalty Interest and VI Monotherapy Royalty Interest, any proceeds (whether cash proceeds or otherwise) other than those in clauses (a)(ii) and (a)(iii) of the definition of each of the foregoing Royalty Interests as, if and when received by Seller in lieu of the royalty payments under clause (a)(i) of the definition of each of the foregoing Royalty Interests, including without limitation amounts received by Seller relating to any damages or penalties or following any court action or out-of-court settlement between Seller and GGL, and in all cases, subject to the reductions, deductions, offsets and exclusions set forth in clauses (b), (c) and (d) of the definition of each Royalty Interest.
44. “Participation Agreement” means the Royalty Participation Agreement dated as of May [ ], 2013 between Seller and Elan Corporation PLC.
45. “Party” has the meaning set forth in the introductory paragraph of this Agreement.
46. “Percentage” means twenty-one percent (21%).
47. “Permitted Encumbrances” means any of the following: (a) statutory Encumbrances for current taxes not yet due and payable, and (b) landlords’, carriers’, warehousemen’s, mechanics’, suppliers’, materialmen’s or other like Encumbrances arising in the ordinary course of business with respect to amounts not yet overdue or being contested in good faith by appropriate proceedings.
48. “Person” means any individual, corporation, limited-liability company, partnership, firm, joint venture, association, joint-stock company, trust, unincorporated organization, governmental body, or other entity.
49. “Purchaser” has the meaning given to such term in the Participation Agreement.
50. “Recharacterization Event” has the meaning set forth in Section 2.4(b).
51. “Royalty Interest” means (a) the ANORO Royalty Interest, (b) the BREO/RELVAR Royalty Interest, (c) the MABA ‘081 Royalty Interest, and (d) the VI Monotherapy Royalty Interest.
52. “Royalty Interest Product” means each of (a) ANORO, (b) BREO/RELVAR, (c) MABA ‘081 and (d) VI Monotherapy, and excluding, in each case, all Excluded Products.
53. “Royalty Interest Term” means, with respect to each Royalty Interest Product, the time period commencing on the date hereof and ending, on a country-by-country and product-by-product basis, on the date that the “Term” (as defined in the Collaboration Agreement, with respect to ANORO, BREO/RELVAR and VI, and as defined in the Strategic Alliance Agreement, with respect to MABA ‘081) expires or terminates with respect to such Royalty Interest Product; provided that in the event that either the Collaboration Agreement or the Strategic Alliance Agreement is terminated and Seller remains entitled to Royalty Interest payments under Section 14.6 of the Collaboration Agreement or Section 14.5 of the Strategic Alliance Agreement, the Royalty Interest Term with respect to the relevant Royalty Interest Product shall be extended until the expiration or termination of such rights under Section 14.6 of the Collaboration Agreement and Section 14.5 of the Strategic Alliance Agreement, respectively.
54. “Seller SEC Reports” means all reports, schedules, forms and statements filed by Seller with the United States Securities and Exchange Commission pursuant to the Securities Exchange Act of 1934, as amended, and the rules and regulations promulgated thereunder.
55. “Seller” has the meaning set forth in the introductory paragraph of this Agreement.
56. “Seller Charter Documents” has the meaning set forth in Section 4.1 of this Agreement.
57. “Seller Corporate Reorganization” means the separation of Seller’s businesses as described in Seller’s press release and related materials dated 25 April 2013, as it may be amended.
58. “Seller Disclosure Schedule” has the meaning set forth in Article IV of this Agreement.
59. “Seller Indemnitees” has the meaning set forth in Section 8.2 of this Agreement.
60. “Seller Material Adverse Effect” means any one or more of: (a) a material adverse effect on the ability of Seller to consummate the Transactions and perform its obligations under this Agreement, (b) a material adverse effect on the validity or enforceability of
this Agreement or the rights of Newco hereunder, or (c) a material adverse effect on the Assignment Interest.
61. “Seller Secured Amount” has the meaning set forth in Section 2.4(b).
62. “Specified Claim” means any allegation, claim, announcement, finding or determination that the Transaction Documents, Transactions or Seller’s or Newco’s negotiation, performance or disclosure of the Transaction Document or the Transactions is or might be a breach of the GGL Agreements.
63. “Strategic Alliance Agreement” means that certain Strategic Alliance Agreement by and between Theravance, Inc. and Glaxo Group Limited dated March 30, 2004, as amended from time to time.
64. “Taxation” or “Tax” means all (i) forms of taxation whether direct or indirect and whether levied by reference to income, profits, gains, net wealth, net worth, equity, asset values, turnover, gross receipts, added value or other reference, and statutory, governmental, state, provincial, local government or municipal impositions, duties, contributions, rates and levies, whenever and wherever imposed (whether imposed by way of a deduction or withholding for or on account of tax or otherwise) and in respect of any Person, and all penalties, fines, levies, charges, costs, interest and other additional amounts relating thereto.
65. “Tax Authority” means any taxing or other Governmental Authority (whether within or outside the United Kingdom) competent to impose, administer or collect any Taxation.
66. “Term” has the meaning set forth in Section 7.1 of this Agreement.
67. “Territory” means the entire world.
68. “Third Party” means any Person other than either Party or any of their respective Affiliates.
69. “Third-Party Claim” has the meaning set forth in Section 8.3(a) of this Agreement.
70. “Transactions” means the transactions contemplated by the Transaction Documents.
71. “Transaction Documents” means this Agreement and the Trust Agreement.
72. “Trust” means the trust declared pursuant to the Trust Agreement.
73. “Trust Agreement” means the Declaration of Trust to be entered into simultaneously with this Agreement among Seller, Newco and the other parties thereto substantially in the form attached hereto as Exhibit B.
74. “Trustee” means the trustee under the Trust Agreement.
75. “UCC” means the Uniform Commercial Code as in effect from time to time in the State of New York or in any other state or the District of Columbia to the extent applicable to the perfection of security interests or filings in respect thereof.
76. “UMEC” means the long-acting muscarinic antagonist umeclidinium bromide (with the chemical structure as set forth in Attachment A-2) or an ester, salt or other noncovalent derivative thereof.
77. “VI” means the long-acting beta2 agonist vilanterol (with the chemical structure as set forth in Attachment A-3) or an ester, salt or other noncovalent derivative thereof.
78. “VI Monotherapy” means (a) VI, solely as a monotherapy (i.e., excluding VI in combination with any one or more other therapeutically active component(s)), and (b) any and all product improvements, additional claims, line extensions, dosage changes and alternate delivery systems, in each case, with respect to only VI solely as a monotherapy (i.e., excluding VI in combination with any one or more other therapeutically active component(s)).
79. “VI Monotherapy Royalty Interest”:
(a) Means Seller’s entitlement under the Collaboration Agreement to receive the following payments during the Royalty Interest Term, subject to subsections (b), (c) and (d) of this definition:
|
(i) |
|
royalty payments on any net sales worldwide of VI Monotherapy by or on behalf of GGL, pursuant to Section 6.3 of the Collaboration Agreement; |
|
|
|
|
|
(ii) |
|
any interest on amounts referred to in clause (a)(i) immediately above that is paid pursuant to Section 6.8 of the Collaboration Agreement; |
|
|
|
|
|
(iii) |
|
any Infringement Payments with respect to VI Monotherapy in lieu of the payments referred to in clause (a)(i) immediately above; and |
|
|
|
|
|
(iv) |
|
any Other Amounts with respect to VI Monotherapy in lieu of the payments referred to in clause (a)(i) immediately above. |
(b) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to the reductions, deductions and offsets contemplated by Sections 6.3, 6.4.1 and 6.9 the Collaboration Agreement. The VI Reverse Milestone Payment Obligation shall remain the obligation of Seller, and shall not be a reduction, deduction, offset or exclusion from the royalty payments under subsection (a) above of this definition.
(c) The entitlement to the payments and amounts set forth in subsection (a) above of this definition is subject to reduction, deduction, offset and exclusion from such royalties of any Cost-Sharing Deductions (without double counting).
(d) The entitlement to the payments and amounts set forth in subsection (a) above of this definition does not include any (i) signing payments (including any signing payments pursuant to Section 6.1.1 of the Collaboration Agreement), (ii) one-time fees for new compounds contributed to the collaboration (including any one-time fee payments from GGL to Seller pursuant to Sections 6.1.3 or 6.1.4 of the Collaboration Agreement), (iii) milestone payments (including any milestone payments pursuant to Section 6.2 of the Collaboration Agreement) or (iv) any other payments (other than those expressly identified in subsection (a) above of this definition), in the case of clauses (i), (ii), (iii) and (iv) of this clause (d), paid to Seller relating to VI Monotherapy.
80. “VI Reverse Milestone Payment Obligation” means Seller’s obligation to pay to GGL milestone payments in the event a product containing VI is successfully developed and commercialized, pursuant to Section 6.2.3 of the Collaboration Agreement.
B. INTERPRETATION.
(a) When a reference is made in this Agreement to an “Article”, “Section” or “Schedule”, such reference shall be to an Article or Section of or Schedule to this Agreement unless otherwise indicated.
(b) The words “include,” “includes” and “including” when used herein shall be deemed in each case to be followed by the words “without limitation” and shall not be construed to limit any general statement which it follows to the specific or similar items or matters immediately following it.
(c) Neither Party shall be or be deemed to be the drafter of this Agreement for the purposes of construing this Agreement against the other Party.
(d) All uses of the words “hereto”, “herein”, “hereof”, “hereby” and “hereunder” and similar expressions refer to this Agreement as a whole and not to any particular provision of this Agreement.
(e) Unless otherwise specified in this Agreement, words in the singular include the plural and vice versa and words importing one gender include all genders.
(f) Any reference to a Person shall also be its permitted successors and assigns.
(g) Any references to a Law shall include any amendment or modification to such Law and any rules and regulations issued thereunder, whether such amendment or modification is made, or issuance of such rules and regulations occurs, before or after the date of this Agreement.
EXHIBIT E
FORM OF TRUST AGREEMENT
NEWCO
as Settlor
[Parent]
as Beneficiary
and
[·]
as Trustee and Account Bank
DECLARATION OF TRUST
Cadwalader, ▇▇▇▇▇▇▇▇▇▇ & ▇▇▇▇ LLP
Dashwood House
▇▇ ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇, ▇▇▇▇ ▇▇▇
Tel: ▇▇▇ (▇) ▇▇ ▇▇▇▇ ▇▇▇▇
Fax: ▇▇▇ (▇) ▇▇ ▇▇▇▇ ▇▇▇▇
TABLE OF CONTENTS
|
|
|
Page |
|
|
|
|
|
1 |
INTERPRETATION |
1 |
|
2 |
DECLARATION OF TRUST |
5 |
|
3 |
COVERED PAYMENTS |
6 |
|
4 |
THE ACCOUNT |
6 |
|
5 |
PAYMENTS AND APPLICATION OF MONEYS |
7 |
|
6 |
ACCOUNT SERVICES |
7 |
|
7 |
REMOVAL OR RESIGNATION OF THE ACCOUNT BANK |
9 |
|
8 |
REMUNERATION AND INDEMNIFICATION OF TRUSTEE |
10 |
|
9 |
TRUSTEE’S POWERS AND LIABILITY |
11 |
|
10 |
APPOINTMENT, RETIREMENT AND REMOVAL OF TRUSTEE |
12 |
|
11 |
TRANSFER OF BENEFICIAL INTEREST |
12 |
|
12 |
NOTICES |
12 |
|
13 |
GOVERNING LAW |
13 |
|
14 |
MISCELLANEOUS |
13 |
THIS DECLARATION OF TRUST has been executed as a Deed by the parties set out below on [ ] 2013
(1) NEWCO of [·], an English limited liability partnership (the “Settlor”);
(2) [Parent] of [·] and any transferee thereof pursuant to Section 11 hereof (the “Beneficiary”);
(3) [·] of [·] as “Trustee”; and
(4) [·] of [·] “Account Bank”.
WHEREAS:
(A) Pursuant to the Assignment Agreement, Beneficiary has sold, granted, assigned, put over, transferred and conveyed (collectively, “Transferred” and “Transfer” shall be interpreted accordingly) to the Settlor the Assignment Interest. The Settlor holds the Assignment Interest, and accordingly the beneficial title to the corresponding Covered Payments.
(B) Pursuant to this Deed, the Settlor wishes to declare a trust in favour of the Beneficiary by which the Trustee shall hold upon such trust as bare trustee: the Trust Property (as defined below) and subject at all times to the trust established by this Deed.
(C) The Settlor, the Trustee and the Beneficiary intend that nothing in this Deed shall result in the disposal, sale or transfer by the Settlor of any interest in, or ownership of, the Royalty Interest (or any part thereof) or any payments on, or in respect of, the GGL Agreements.
(D) Nothing in this Deed is intended to give rise to any obligations on the part of the Settlor that would be in breach of, or provide the Trustee or the Beneficiary with any rights under, or any right to enforce any provisions of, the GGL Agreements or the Assignment Agreement.
NOW THIS DEED WITNESSETH AND IT IS HEREBY DECLARED as follows:
1 INTERPRETATION
1.1 In this Deed:
Capitalized terms used herein without a definition shall have the meaning ascribed thereto in the Royalty Participation Agreement between [·] and [·], dated as of May [·], 2013 (the “Participation Agreement”).
“Account” means the account established by the Account Bank at the request of the Settlor in accordance with clause 4 of this Deed.
“Additional Amounts” is defined in clause 2.1(c).
“Aggregate Payment” is defined in clause 2.1.
“Beneficiary” means, initially, Seller and thereafter, the Person designated in accordance with clause 11.1 hereof.
“Business Day” means a day (other than Saturday or Sunday) which is a day on which commercial banks and foreign exchange markets settle payments and are open for general business in London, England, New York, New York, San Francisco, California, and Dublin, Ireland.
“Company” means Glaxo Group Limited, including any successor entity under the GGL Agreements.
“Consideration” means the Transfer of the Assignment Interest to the Settlor pursuant to the terms of the Assignment Agreement.
“Cost-Sharing Deduction” has the meaning set forth in the Participation Agreement.
“Cost-Sharing Instruction” means a written notice from the Settlor or Seller to the Trustee and the Account Bank setting forth the amount of a Cost-Sharing Deduction.
“Covered Payments” means the Paid Amounts, when, as and if received by the Account Bank.
“Deed” means this deed and “this Deed” shall be construed accordingly.
“Earnings Release Notification” means (i) a written notification by the Settlor to the Account Bank and the Trustee that Parent has publicly announced its financial statements with respect to the most recently ended quarter or year (as applicable), or (ii) a written notification by the Beneficiary to the Account Bank and the Trustee (together with a copy of the applicable public filing containing the Seller’s financial statements for the relevant period) that Parent’s financial statements with respect to the most recently ended quarter or year (as applicable) are available for review on the U.S. Securities and Exchange Commission’s “Electronic Data Gathering, Analysis, and Retrieval” (▇▇▇▇▇) system.
“Fitch” means Fitch Ratings Ltd.
“Goliath” means Glaxo Group Limited, including any successor entity under the GGL Agreements.
“Insolvency Event” means, in respect of any entity, that such entity is (1) is dissolved (other than pursuant to a consolidation, amalgamation or merger); (2) becomes insolvent or is unable to pay its debts or fails or admits in writing its inability generally to pay its debts as they become due or is deemed under applicable law to, or is declared to, be unable to pay its debts; (3) makes a general assignment, arrangement or composition with or for the benefit of its creditors; (4) institutes or has instituted against it a proceeding seeking a judgment of insolvency or bankruptcy or any other relief under any bankruptcy or insolvency law or other similar law affecting creditors’ rights, or a petition is presented for its winding-up or liquidation, and, in the case of any such proceeding or petition instituted or presented against it, such proceeding or petition (A) results in a judgment of insolvency or bankruptcy or the entry of an order for relief or the making of an order for its winding-up or liquidation or (B) is not
dismissed, discharged, stayed or restrained in each case within 60 days of the institution or presentation thereof; (5) has a resolution passed for its winding-up, official management, examination or liquidation (other than pursuant to a consolidation, amalgamation or merger); (6) seeks or becomes subject to the appointment of an administrator, provisional liquidator, conservator, receiver, trustee, custodian, examiner or other similar official for it or for all or substantially all its assets; (7) has a secured party take possession of all or substantially all its assets or has a distress, execution, attachment, sequestration or other legal process levied, enforced or sued on or against all or substantially all its assets and such secured party maintains possession, or any such process is not dismissed, discharged, stayed or restrained, in each case within 60 days thereafter; (8) causes or is subject to any event with respect to it which, under the applicable laws of any jurisdiction, has an analogous effect to any of the events specified in clauses (1) to (7) (inclusive); or (9) takes any action in furtherance of, or indicating its consent to, approval or, or acquiescence in, any of the foregoing acts.
“Liability” means any loss, damage, cost, charge, claim, demand, expense, judgment, action, proceedings or other liability whatsoever (including without limitation, taxes, duties, levies, imposts and other charges, any value added tax or similar tax charged or chargeable in respect thereof) and the reasonable fees and expenses of any legal advisers or accounting or investment banking firms employed by the Trustee pursuant to this Deed.
“Lock Box Account” means a lock box account that Seller or Newco may elect to establish from time to time, to receive the Royalty Interest payments from GGL, and from which Paid Amounts would be paid into the Account.
“Lock Box Arrangement” means the contractual terms and conditions that govern the Lock Box Account, which shall be in form and substance reasonably satisfactory to the Beneficiary.
“Moody’s” means ▇▇▇▇▇’▇ Investors Service Limited.
“Paid Amounts” means, in relation to the Assignment Interest, (i) 100 per cent of the cash payments, when, as and if received under the Assignment Agreement, (ii) any amounts paid or payable to Settlor pursuant to the first sentence of Section 2.4(c) of the Assignment Agreement, and (iii) if a Recharacterization Event (as defined in the Assignment Agreement) has occurred pursuant to the second sentence of Section 2.4(c) of the Assignment Agreement, the proceeds thereof, including any proceeds of assets realized upon foreclosure in connection therewith.
“Related Rights” means all powers, rights, claims, discretions, title, interest and benefit whatsoever whether now or in the future which the Settlor has under or otherwise in connection with the Account including without limitation all and any right of the Settlor to require the Account Bank to deliver title to and possession of any or all monies standing to the credit of the Account.
“Relevant Rating Agencies” means S&P, ▇▇▇▇▇’▇ and Fitch.
“Relevant Required Rating” means at least [·] by S&P, [·] by Moody’s, [·] by Fitch and [·] by DBRS.
“Seller” has the meaning set forth in the Assignment Agreement.
“S&P” means Standard & Poor’s Rating Services, a division of The ▇▇▇▇▇▇-▇▇▇▇ Companies, Inc.
“Termination Date” is defined in clause 2.6.
“Trust Property” means, collectively: (i) the Covered Payments; (ii) the cash standing to the credit of the Account; (iii) any Related Rights and (iv) and the amounts deposited to the Account pursuant to clause 2.1(c) and pursuant to clause 2.1(d).
“Trustee Acts” means the Trustee ▇▇▇ ▇▇▇▇ and the Trustee ▇▇▇ ▇▇▇▇ of England and Wales.
“Withholding Instruction” means a written notice from the Settlor or Seller to the Trustee and the Account Bank setting forth the amount of any amount the Settlor or Seller is required to deduct and withhold for Taxes as set forth in Section 6.2 of the Participation Agreement or Section 6.2 of the Assignment Agreement.
“Written Instructions” shall mean written communications received by the Trustee via S.W.I.F.T., tested telex, certified letter, facsimile transmission, or any other method or system specified by the Trustee as available for use in connection with this Deed.
1.2 Interpretation
(a) All references in this Deed to any statute or any provision of any statute shall be deemed also to refer to any statutory modification or re-enactment thereof or any statutory instrument, order or regulation made thereunder or under such modification or re-enactment.
(b) In this Deed references to clauses, schedules and paragraphs to the schedules (unless otherwise specified) shall be construed as references to the clauses, schedules and paragraphs to the schedules to this Deed and of this Deed respectively.
(c) Save where the contrary is indicated, any reference in this Deed to the Deed or any other agreement or document shall be construed as a reference to this Deed or, as the case may be, such other agreement or document as the same may from time to time be amended, supplemented, varied, novated or replaced.
(d) Words denoting the singular number shall include the plural number also and vice versa.
(e) References to any person shall include references to its permitted successors, transferees and assigns.
(f) A “person” includes any individual, firm, company, corporation, government, state or agency of a state or any association, trust, joint venture, consortium, partnership or other entity (whether or not having separate legal personality.
2 DECLARATION OF TRUST
2.1 With effect from the date of this Deed, the Settlor in consideration of the receipt of the Consideration hereby declares a bare trust absolutely in favour of the Beneficiary over the Settlor’s beneficial interest in:
(a) the Covered Payments;
(b) any cash standing to the credit of the Account, and any Related Rights;
(c) any other amounts deposited to the Account by the Settlor from time to time for the benefit of the Beneficiary (the “Additional Amounts”); and
(d) any amounts paid by Seller to the Account pursuant to Section 6.1(b) of the Participation Agreement or the last sentence of Section 6.1(e) of the Participation Agreement
(together, the “Aggregate Payment”).
2.2 The parties to this Deed acknowledge and agree that, notwithstanding anything to the contrary in this Deed or in connection with the trust set out herein, Newco has no obligation to bring any enforcement action against GGL under any of the GGL Agreements. Any such enforcement action by Newco requires the consent of the general partner of Newco. Notwithstanding anything to the contrary in this Deed, nothing in this Deed is intended to give rise to any obligation on the part of the Settlor that would be in breach of, or provide the Trustee or the Beneficiary with any rights or interests under, or any right to enforce any provisions of, the Royalty Interests, the GGL Agreements, the Assignment Agreement, the Participation Agreement (whether as an express or implied third party beneficiary or otherwise). The trust set out in clause 2.1 above is not intended by the Settlor to, and shall not, result in the disposal of any interest in, or ownership by the Beneficiary or Trustee in any payment right, or part of any right, that the Settlor holds in, or in respect of, the Royalty Interest, the GGL Agreements, the Assignment Agreement or the Participation Agreement. Each of the Beneficiary and the Trustee hereby expressly disclaims any such interest or right.
2.3 The Settlor hereby appoints the Trustee as a trustee of the bare trust created under this Deed and the Trustee hereby acknowledges and declares it shall at all times hold the Trust Property on trust for the Beneficiary.
2.4 The Trustee further acknowledges and agrees that it holds the Trust Property upon the terms and subject to the conditions set forth in this Deed and agrees to be bound by the same. The Trustee holds the bare legal title to the Trust Property upon trust absolutely and unconditionally for the Beneficiary.
2.5 The Account Bank shall maintain any information regarding any Covered Payments, including the amounts of any such Covered Payments, as strictly confidential (including by not disclosing such information to the Trustee or the Beneficiary), except that the Account Bank shall disclose the amount of, the Aggregate Payment (but not the Covered Amount in the event that any Earnings Release Notification for the relevant period has not been delivered) to the Trustee and the Beneficiary
promptly following the payment of such Aggregate Payment or any portion thereof to the Beneficiary. For the avoidance of doubt, the Account Bank may not, and shall be under no obligation to, disclose any information with respect to any Covered Payment in respect of which it has not received an Earnings Release Notification. Each of the Trustee and the Beneficiary expressly acknowledges and agrees to the foregoing confidentiality obligations of the Account Bank, and expressly waives any duty or obligation of the Account Bank to the Trustee and the Beneficiary in connection with the foregoing in this clause 2.5.
2.6 The trust created under this Deed shall cease to have effect upon the date on which all Paid Amounts arising under the Assignment Agreement have been collected by the Trustee and paid to the Beneficiary in full (such date being the “Termination Date”).
3 COVERED PAYMENTS
3.1 The Settlor shall procure and take all necessary steps to ensure that when, as and if any Paid Amounts are paid by Company or any other person in accordance with the GGL Agreements the corresponding Covered Payments shall be paid promptly into the Account or, if the Lock Box Account is in place, promptly into the Lock Box Account and from the Lock Box Account to the Account in accordance with the Lock Box Arrangement.
3.2 References in this clause 3 to Covered Payments are to the amount actually paid by the Company, or any other person in accordance with the GGL Agreements.
3.3 All actions required by the Settlor under this clause 3 shall be subject to all applicable fiscal and other tax, legal or regulatory requirements.
3.4 All payments under this clause 3 shall be made in the currency in which the relevant Covered Payment amount is actually paid.
4 THE ACCOUNT
4.1 The Account Bank shall establish the Account for the purpose of receiving, holding and distributing the Covered Payments in accordance with the provisions of this Deed.
4.2 The Trustee shall hold the Trust Property in the Account and shall identify the Trust Property on its books and records as being held subject to the trusts contained in this Deed. The Trustee will not comingle the Trust Property with any other property.
4.3 Pursuant to this Deed, but subject to the terms and conditions herein (including clauses 2.5 and 5.1), the Beneficiary shall enjoy the same rights to the Account as the Settlor would have enjoyed had the transactions contemplated in this Deed not taken place and had the Settlor retained the beneficial ownership of the Account and the contents of the Account, including the right to demand payment from the Account Bank of any sums held in the Account in accordance with the terms of the Account and of this Deed.
4.4 In accordance with the declaration of trust set out at clause 2.1 but subject to the termination provisions set out in clause 2.6, the Settlor shall deliver an irrevocable,
written instruction to the Company that all payments in respect of the Royalty Interest shall be paid into the Lock Box Account (or if there is no Lock Box Account, the Account) when, as and if any such amounts become payable. Covered Payments shall be paid out of the Lock Box Account into the Account in accordance with the terms of the Lock Box Arrangement. This obligation is without prejudice to any obligation Seller may have to deliver similar written instructions to the Company pursuant to the Participation Agreement and/ or Assignment Agreement.
5 PAYMENTS AND APPLICATION OF MONEYS
5.1 Immediately following the earlier of (i) the receipt by the Trustee of an Earnings Release Notice, and (ii) 60 days following the receipt into the Account of a Covered Payment, the Trustee shall procure that the following amounts are paid in the following order:
first, pursuant to clause 8.1 of this Deed;
second, to an account of the Settlor, an amount equal to the Cost-Sharing Deductions set forth in any Cost-Sharing Instruction theretofore received by the Trustee and not previously paid to the Settlor and an amount equal to the amount specified in any Withholding Instruction, and
third, to an account of the Beneficiary, the balance of any amounts (including any and all interest thereon) then standing in the Account.
6 ACCOUNT SERVICES
6.1 It is the intent and explicit agreement of the parties to this Deed that the Trustee has control of the Account in the manner set out in this Deed.
6.2 The Account Bank shall not honour or otherwise comply with any instructions originated by or on behalf of the Settlor to withdraw, transfer, sell, redeem, pledge, rehypothecate or otherwise deliver or dispose of any Trust Property or re-register title of the Account, other than as permitted by the terms of this Deed.
6.3 From (and including) the date of this Deed to and including the Termination Date, the Settlor hereby authorises and instructs the Account Bank to comply with all Written Instructions originated by the Trustee and the Beneficiary from time to time with respect to the Trust Property without further consent or direction from the Settlor or any other party, in accordance with, and subject to the following terms and conditions:
(a) the Account Bank shall not honour any Written Instructions or other instructions or requests received from the Trustee or the Beneficiary that:
(i) would require disclosure of information to the Trustee, Beneficiary or any other Person regarding any Covered Payment (including the amount of such Covered Payment) other than in accordance with clause 2.5;
(ii) would require the Account Bank to make any payment not in conformity with clause 5.1; or
(iii) prohibit the Account Bank from providing any information to the Settlor regarding the Account and amounts deposited therein or withdrawn therefrom in accordance with Section 6.3(c).
(b) The Account Bank shall not honour any instructions or requests received from the Settlor concerning the Account and the Settlor shall have no right or ability to access or withdraw or transfer funds from the Account. Without limiting the generality of the foregoing, the Account Bank shall:
(i) subject to clause 6.3(a), follow the Written Instructions of the Trustee and the Beneficiary regarding the Account;
(ii) subject to clauses 2.5 and 5.1, if directed by the Trustee and the Beneficiary, (A) transfer any or all of the proceeds standing to the credit of the Account to any account or accounts designated by the Beneficiary, or (B) otherwise deal with the Trust Property as directed by the Trustee and the Beneficiary, in each case without the consent of the Settlor or any other person; and
(iii) subject to clauses 2.5 and 5.1, otherwise comply with instructions originated by the Trustee and the Beneficiary with respect to financial assets held in or to be credited to the Account without further consent of the Settlor or any other person.
(c) Upon the reasonable request of the Trustee and the Beneficiary, but subject to clause 2.5, the Account Bank shall furnish, make available or otherwise provide the Trustee and the Beneficiary with copies of all account statements, and, subject to clause 2.5, the Account Bank shall make available to the Trustee and the Beneficiary online reports and other information that is in the Account Bank’s possession that relates to the Account regarding the transactions affecting the Account and account statements periodically. The Trustee and/or, as applicable, the Beneficiary shall execute all account documentation, if any, that the Account Bank may from time to time require in order to establish the Trustee’s and /or the Beneficiary’s authority to have access to all such statements, reports and information. The Account Bank shall furnish, make available or otherwise provide the Settlor with copies of all account statements and the Account Bank shall make available to the Settlor online reports and other information that is in the Account Bank’s possession that relates to the Account regarding the transactions affecting the Account and account statements periodically.
(d) As of the date of this Deed, the Account Bank has not received notice of any liens, claims or encumbrances with respect to the Account, nor has the Trustee confirmed as at the date of execution of this Deed any interest in any Account to any persons or entities other than the Beneficiary.
(e) Upon receipt of written notice of any lien, encumbrance or adverse claim against the Account or any portion of the Trust Property contained therein, the Account Bank shall use all reasonable efforts to notify the Trustee and Beneficiary as soon as commercially practicable under the circumstances.
(f) The Account Bank agrees that: (i) all Trust Property which is a financial asset or monetary amount delivered to the Account Bank will (as far as possible) be promptly credited to the Account; and (ii) in no case will any financial asset credited to the Account be registered in the name of the Settlor or, except as expressly provided in clause 5.1 with respect to Cost-Sharing Instructions, payable to the order of the Settlor.
(g) The Account Bank hereby waives any lien, charge or other security or right of set-off that it may now have or hereafter acquire in or with respect to the Account or any cash or financial asset held therein or credited thereto. None of the cash or financial assets held in or credited to the Account in respect thereof will be subject to deduction, set-off, banker’s lien or any other right in favour of any person (subject to clause 5.1).
(h) Beyond the exercise of reasonable care in the custody and preservation of the Trust Property and the performance of its obligations hereunder, the Account Bank shall have no duty to the Settlor or Beneficiary. The Account Bank shall be deemed to have exercised reasonable care in the custody and preservation of the Trust Property if the Trust Property is accorded treatment substantially equal to that which the Account Bank accords to its own property, and the Account Bank shall not be liable for any loss or damage to, or diminution in the value of, any Trust Property except to the extent that such liability arises from the Account Bank’s gross negligence, wilful misconduct or breach of this Deed. The Account Bank may rely and shall be protected in acting upon any notice, instruction, or other communication from the Trustee which it reasonably believes to be genuine and authorised.
(i) The Account Bank hereby represents and warrants it has been duly incorporated and is validly existing as [·] under the laws [·]; it has the corporate power and authority and all applicable regulatory consents, to conduct its activity and to execute, deliver and perform its obligations under this Deed.
7 REMOVAL OR RESIGNATION OF THE ACCOUNT BANK
7.1 Subject to as provided below, the Account Bank may, at any time, cease to operate the Account on giving not less than 60 days’ prior written notice thereof to each of the parties hereto. The Settlor undertakes that, in the event of the Account Bank giving notice under this clause 7.1, it shall as soon as possible use reasonable best efforts to procure a new bank that is reasonably acceptable to the Trustee and the Beneficiary to operate the Account on the terms of this Deed. The Account Bank shall not cease to operate the Account unless and until a new bank has been appointed and the Account has been transferred to that new bank.
7.2 If there is any change in the identity of the Account Bank, the Settlor, Trustee and Beneficiary shall execute such documents and take such actions as the new Account Bank and the outgoing Account Bank may reasonably require for the purpose of vesting into the new Account Bank the rights and obligations of the outgoing Account Bank.
7.3 The Settlor shall be obliged to terminate the appointment of the Account Bank and appoint a new Account Bank if any of the following events shall occur and Settlor has received written notice thereof:
(a) the Account Bank fails to make when due a payment required to be made to the Beneficiary under this Deed;
(b) the Account Bank fails to perform or observe its duties, obligations, covenants and services under this Deed and such default continues unremedied for a period of [10] Business Days after the earlier of the Account Bank becoming aware of such default or receipt by the Account Bank of written notice from the Trustee or Settlor requiring the same to be corrected;
(c) an Insolvency Event occurs in respect of the Account Bank; or
(d) the unsecured and unsubordinated long-term debt rating of the Account Bank ceases to be rated by any two of the Relevant Rating Agencies at the Relevant Required Rating, or any such rating is withdrawn.
7.4 The Account Bank agrees to use all reasonable efforts to cooperate with the Settlor, Beneficiary and the successor Account Bank in effecting the termination of the responsibilities and rights of the Account Bank under this Deed, including, without limitation, to transfer to such successor the Account and the Trust Property and take such further action as the Trustee or Beneficiary may reasonably direct.
8 REMUNERATION AND INDEMNIFICATION OF TRUSTEE
8.1 The Trustee shall receive remuneration for its services as trustee as from the date of this Deed upon each [quarterly date] such remuneration to be at such rate as may from time to time be agreed among the Settlor, Beneficiary and the Trustee, with the Settlor paying one-half of such remuneration and the Trustee authorized to withdraw one-half of such remuneration from the Account. Such remuneration shall accrue from day to day and be payable up to and including the date when all amounts owing to the Beneficiary under this Deed have been paid in full or otherwise duly provided for to the satisfaction of the Trustee.
8.2 In the event of the Trustee considering it expedient or necessary or on being requested by the Settlor or the Beneficiary to undertake duties which the Trustee agrees to be of an exceptional nature or otherwise outside the scope of the normal duties of the Trustee under this Deed, the Trustee shall be paid such additional remuneration as shall be agreed among the Trustee, the Settlor and the Beneficiary, with the Settlor paying one-half of such remuneration and the Trustee authorized to withdraw one-half of such remuneration from the Account.
8.3 Without prejudice to the right of indemnity by law given to trustees, the Settlor and Beneficiary shall indemnify the Trustee and keep it indemnified against all Liabilities to which it may be or become subject or which may be incurred by it in the execution or purported execution of any of its trusts, powers, authorities and discretions under this Deed or in respect of any other matter or thing done or omitted in any way relating to this Deed. The Settlor and the Beneficiary hereby agree, as between them,
that each is responsible for one-half of the indemnification obligation under this clause 8.3.
8.4 With respect to any remuneration under this clause 8 to be funded by withdrawing funds from the Account, if insufficient funds are in the Account on the due date of such remuneration to satisfy such one half of such Trustee remuneration, the Beneficiary promptly shall pay the unsatisfied portion of such one-half of such Trustee remuneration.
8.5 Nothing in this Deed shall, in any case in which the Trustee has failed to show the degree of care and diligence required of it as trustee having regard to the provisions of this Deed conferring on it any trusts, powers, authorities or discretions, exempt the Trustee from, or indemnify it against, any Liability for breach of trust or any liability which by virtue of any rule of law would otherwise attach to it in respect of any negligence, wilful default, breach of duty or breach of trust of which it may be guilty in relation to its duties under this Deed.
9 TRUSTEE’S POWERS AND LIABILITY
9.1 The Trustee shall have all powers conferred upon trustees by the Trustee Acts subject to the express terms set out in this Deed.
9.2 The Trustee may in relation to this Deed act on the advice or opinion of or any information obtained from any lawyer, valuer, accountant, banker, broker, auctioneer or other expert whether obtained by the Settlor, the Trustee or otherwise and shall not be responsible for any Liability occasioned by so acting. Any such advice, opinion or information may be sent or obtained by letter, telex, facsimile transmission or cable and the Trustee shall not be liable for acting in good faith on any advice, opinion or information purporting to be conveyed by any such letter, telex, facsimile transmission or cable although the same shall contain some error or shall not be authentic.
9.3 Save as expressly otherwise provided in this Deed, the Trustee shall have absolute and uncontrolled discretion as to the exercise of its trusts, powers, authorities and discretions under this Deed and shall not be responsible for any Liability which may result from their exercise or non-exercise.
9.4 Any consent or approval given by the Trustee for the purposes of this Deed may be given on such terms and subject to such conditions (if any) as the Trustee thinks fit and notwithstanding anything to the contrary in this Deed may be given retrospectively.
9.5 The Trustee hereby represents and warrants on the date of this Deed that it has been duly incorporated and is validly existing as [·] under the laws [·]; it has the corporate power and authority to conduct its activity and to execute, deliver and perform its obligations under this Deed.
9.6 In no event shall the Trustee or the Beneficiary (i) bring, or have any right to bring, (or encourage or assist any other Person in brining) any claim or action against the Settlor under or in connection with the Assignment Agreement or (ii) require, or seek to require, Settlor to bring any claim or action against Seller under the Assignment
Agreement. If the Trustee or the Beneficiary breaches the foregoing covenant, without limiting any rights or remedies of the Settlor or any of its Affiliates (including Seller), then the Beneficiary shall indemnify and hold harmless the Settlor, Seller and their respective Affiliates. The Trustee and the Beneficiary acknowledge and agree that they are subject to injunctive relief for any breach of this Section 9.6. The Beneficiary hereby irrevocably and perpetually waives any duty or obligation of the Trustee to the Beneficiary with respect to the foregoing.
10 APPOINTMENT, RETIREMENT AND REMOVAL OF TRUSTEE
10.1 The power to appoint a new trustee of this Deed shall be vested in the Settlor, subject to the Beneficiary’s prior written consent (not to be unreasonably withheld, conditioned or delayed).
10.2 A trustee of this Deed may retire at any time on giving not less than 60 days’ prior written notice to the Settlor without assigning any reason and without being responsible for any Liabilities incurred by reason of such retirement.
10.3 The Settlor undertakes that in the event of the Trustee giving notice under this clause or being removed, it will use its reasonable best endeavours to procure that a new trustee is appointed as soon as reasonably practicable thereafter.
10.4 The resignation of the Trustee shall not be effective unless and until the Settlor has appointed a new trustee.
10.5 The Trustee agrees to use all reasonable efforts to cooperate with the Settlor, Beneficiary and the successor trustee in effecting the termination of the responsibilities and rights of the Trustee under this Deed, including, without limitation, to transfer to such successor the Trust Property and take such further action as the Settlor and/or Beneficiary may reasonably direct.
10.6 The Settlor will be obliged to remove the Trustee promptly upon the Settlor becoming aware that there has in fact occurred an Insolvency Event in relation to the Trustee and shall, as soon as reasonably possible following such removal, find and appoint a new trustee.
11 TRANSFER OF BENEFICIAL INTEREST
11.1 Upon receipt by the Trustee of written notice from the Beneficiary that the Beneficiary has transferred its interests under this Deed to another Person (the “Transferee”) in compliance with the provisions of Section 9.1 of the Participation Agreement, the Trustee shall immediately treat the Transferee as the beneficiary of trusts created under this Deed. The Settlor and the Beneficiary agree to take reasonable steps necessary to effectuate any such permitted transfer to the Transferee.
12 NOTICES
All notices or other communication under or in connection with this Deed shall be given in writing or by email to the addresses or email addresses set out below (unless the sender of the notice or other communication is otherwise notified in writing):
[·]
13 GOVERNING LAW
13.1 This Deed and all matters arising from or connected to it are to be construed in accordance with, and governed by, English law. The courts of England shall have exclusive jurisdiction to adjudicate on any disputes or claims that may arise out of or in connection with this Deed. Each party to this Deed irrevocably submits to the jurisdiction of the courts of England and waives any objection to proceedings in such courts whether on the ground of venue or on the ground that the proceedings have been brought in an inconvenient forum.
13.2 [The Settlor hereby irrevocably appoints [·], to receive service of process on its behalf as its respective authorised agent for service of process in England. If for any reason such agent shall cease to be such agent for service of process, the Settlor shall forthwith appoint a new agent for service of process in England and deliver to the parties to this Deed a copy of the new agent’s acceptance of appointment within 30 days.]
13.3 The Beneficiary hereby irrevocably appoints [·], to receive service of process on its behalf as its respective authorised agent for service of process in England. If for any reason such agent shall cease to be such agent for service of process, the Beneficiary shall forthwith appoint a new agent for service of process in England and deliver to the parties to this Deed a copy of the new agent’s acceptance of appointment within 30 days.
13.4 The Trustee and the Account Bank hereby irrevocably appoints [·], to receive service of process on its behalf as its respective authorised agent for service of process in England. If for any reason such agent shall cease to be such agent for service of process, the Trustee or, as applicable, the Account Bank shall forthwith appoint a new agent for service of process in England and deliver to the parties to this Deed a copy of the new agent’s acceptance of appointment within 30 days.
13.5 Nothing in this Deed shall affect the right to serve process in any other manner permitted by law.
14 MISCELLANEOUS
14.1 [The perpetuity period for the trusts in this Deed is eighty (80) years from the date of this Deed.]
14.2 This Deed may be executed in any number of counterparts, each of which shall be deemed to be an original, but such counterparts shall, together, constitute only one instrument.
14.3 The parties hereto shall perform, execute and deliver such further acts and documents as may be required by law or reasonably requested by each other to implement the purpose of and to perfect this Deed.
14.4 Any person who is not a party to this Deed has no right under the Contracts (Rights of Third Parties) Ac▇ ▇▇▇▇ ▇o enforce any term of this Deed.
14.5 If, at any time, any provision of this Deed is or becomes illegal, invalid or unenforceable in any respect under any law of any jurisdiction, neither the legality, validity or enforceability of the remaining provisions nor the legality, validity or enforceability of such provision under the law of any other jurisdiction will, in any way, be affected or impaired.
14.6 The headings used in this Deed are for convenience of reference only and are not to affect the construction of or to be taken in consideration in interpreting this Deed.
IN WITNESS whereof the above has been executed as a Deed on the day written at the beginning hereof.
SIGNATORIES
|
EXECUTED as a deed and delivered by a |
) |
| |
|
duly authorised signatory of |
) |
| |
|
NEWCO: |
| ||
|
|
| ||
|
|
| ||
|
By: |
|
|
|
|
|
Name: |
| |
|
|
Title: |
| |
|
|
| ||
|
|
| ||
|
EXECUTED as a deed and delivered by a |
) |
| |
|
duly authorised signatory of |
) |
| |
|
[·]: |
| ||
|
|
| ||
|
|
| ||
|
By: |
|
|
|
|
|
Name: |
| |
|
|
Title: |
| |
|
|
| ||
|
|
| ||
|
EXECUTED as a deed and delivered by a |
) |
| |
|
duly authorised signatory of |
) |
| |
|
TRUSTEE AND ACCOUNT BANK |
| ||
|
|
| ||
|
|
| ||
|
By: |
|
|
|
|
|
Name: |
| |
|
|
Title: |
| |
EXHIBIT F
1. The Initial Members will be Seller and Delaware Sub.
2. No other persons shall become Members without the prior written agreement of [ ].
3. The Designated Members shall be all the Members [or such of the Members as are designated in accordance with the Limited Liability Partnership Agreement (“LPA”)]. The Designated Members shall be responsible for ensuring compliance with all registration and other requirements of the Limited Liability Partnerships Act 2000 and any amendments to that it (the “Act”) and other applicable legislation.
4. The Members will agree that the relationship between them does not constitute any form of co-ownership of the assets and liabilities of the LLP or any form of trust under any applicable law or any form of trust relationship or equitable relationship under any applicable law.
5. [The LLP shall carry on the Business with a view to profit.](1)
6. The Members shall not cause or permit the LLP, during the term of the Trust, to (and the LLP shall not):
(a) incur any indebtedness for borrowed money;
(b) form, acquire or hold any subsidiary (whether corporate, partnership, limited liability company or other) or own any equity interest in any other entity;
(f) amend the LPA in a manner adverse to the Purchaser;
(g) unless required by applicable Law, have any employees (for the avoidance of doubt, the Members do not constitute employees);
(h) enter into any reconstruction, amalgamation, merger or consolidation or take any voluntary steps with a view to its winding-up;
(i) otherwise than as contemplated in the Transaction Documents, release from or terminate the appointment of the Account Bank; or
(j) subject to any requirement under applicable Law to maintain a registered office, enter into any lease for, or own, any real property.
7. [To the extent not prohibited by the GGL Agreements, Newco agrees to use its commercially reasonable efforts to provide in a timely manner such cooperation or
(1) To be discussed between the parties.
assistance (including by providing any necessary tax forms or other documents) as may be reasonably requested by Purchaser or the Trustee (at the sole cost of Purchaser) at any time after the date of this Agreement, so long as the cooperation or assistance could not reasonably be expected to prejudice in any material respect the legal or commercial position of Newco or Seller, in order to reduce or eliminate any amounts deducted or withheld pursuant to Section 6.2(a) of the Royalty Participation Agreement or any amounts deducted or withheld by GGL on a Royalty Interest payment under the GGL Agreements.](2)
8. [No initial Member in Newco shall be a person that is not a United States person or a disregarded entity of a United States person for U.S. federal income tax purposes.](3)
9. [Newco will initially be a disregarded entity for U.S. federal income tax purposes.](4)
(2) To be discussed between the parties.
(3) To be discussed between the parties.
(4) To be discussed between the parties.
EXHIBIT G
FORM OF LIMITED LIABILITY COMPANY AGREEMENT
OF
[TAURUS], LLC
THIS LIMITED LIABILITY COMPANY AGREEMENT, dated as of May [ ], 2013 (as the same may be further amended or otherwise modified from time to time, together with the schedules attached hereto, the “Agreement”) of [TAURUS], LLC, a Delaware limited liability company (the “Company”), is entered into by [TAURUS], a Delaware corporation, as the sole member (the “Member”) of the Company.
RECITAL
The Member has formed the Company as a limited liability company under the laws of the State of Delaware and desires to enter into a written agreement, in accordance with the provisions of the Delaware Limited Liability Company Act (6 Del. C. § 18-101 et seq.) and any successor statute, as amended from time to time (the “Act”), governing the affairs of the Company and the conduct of its business. Capitalized terms used herein and not otherwise defined have the meanings set forth on Schedule A hereto.
Section 1. Formation.
The Member has previously formed the Company as a limited liability company pursuant to the provisions of the Act. A certificate of formation for the Company as described in Section 18-201 of the Act (the “Certificate of Formation”) has been filed in the Office of the Secretary of State of the State of Delaware in conformity with the Act under the name “[TAURUS], LLC” on May , 2013, by [NAME OF AUTHORIZED PERSON] as an “authorized person” within the meaning of the Act. The Member, by execution of this Agreement, hereby ratifies and approves the execution, delivery and filing of the Certificate of Formation of the Company in such manner. Upon the execution of this Agreement, [NAME OF AUTHORIZED PERSON]’s authority shall cease and the Member shall be designated as an “authorized person” within the meaning of the Act to execute, deliver and file, or cause the execution, delivery and filing of, all certificates (and any amendments and/or restatements thereof) required by the Act. In connection therewith, the Company and, if required, the Member, in its capacity as the “authorized person” within the meaning of the Act to take such actions, shall execute or cause to be executed from time to time all other instruments, certificates, notices and documents and shall do or cause to be done all such acts and things (including keeping books and records and making publications or periodic filings) as may now or hereafter be required for the formation, valid existence and, when appropriate, termination of the Company as a limited liability company under the laws of the State of Delaware.
Section 2. Name.
The name of the Company shall be “[TAURUS], LLC” and its business shall be carried on in such name with such variations and changes as the Board (as
hereinafter defined) shall determine or deem necessary to comply with requirements of the jurisdictions in which the Company’s operations are conducted.
Section 3. Principal Business Office.
The principal business office of the Company shall be located at [PRINCIPAL BUSINESS ADDRESS], or such other location as may hereafter be determined by the Board from time to time. The Board may, from time to time, change the Company’s principal business office and may establish such other place or places of business within or without the State of Delaware as the Board may deem advisable.
Section 4. Registered Office.
The address of the registered office of the Company in the State of Delaware is c/o [NAME OF REGISTERED AGENT], [ADDRESS], [CITY], [COUNTY], Delaware [ZIP CODE]. The Board may, from time to time, change the Company’s registered office and shall forthwith amend the Certificate of Formation to reflect such change.
Section 5. Registered Agent.
The name and address of the registered agent of the Company for service of process on the Company in the State of Delaware is [NAME OF REGISTERED AGENT] [ADDRESS], [CITY], [COUNTY], Delaware [ZIP CODE]. The Board may, from time to time, change the Company’s registered agent and shall forthwith amend the Certificate of Formation to reflect such change.
Section 6. Member; No Liability of Member.
(a) The name and the mailing address of the Member as of the date hereof are set forth on Schedule B attached hereto.
(b) Subject to Section 10(j), the Member may act by written consent.
(c) All debts, obligations and liabilities of the Company, whether arising in contract, tort or otherwise, shall be solely the debts, obligations and liabilities of the Company, and the Member shall not be obligated personally for any such debt, obligation or liability of the Company solely by reason of being the Member.
Section 7. Authorized Person; Filings; Term of Existence; Fiscal Year.
The Member shall be the designated “authorized person” within the meaning of the Act. The Member or an Officer shall execute, deliver and file any certificates (and any amendments and/or restatements thereof) necessary for the Company to qualify to do business in any jurisdiction in which the Company may wish to conduct business. The existence of the Company as a separate legal entity shall continue until the cancellation of its Certificate of Formation as provided in the Act. The “Fiscal Year” of the Company shall be from [January 1] to [December 31] of each year.
Section 8. Purposes.
(a) Subject to Section 10(j), the purposes of the Company are to engage in any activity that Delaware limited liability company may engage in under Delaware Law.
(b) The Company, by or through any Manager or any Officer on behalf of the Company, may execute, deliver, enter into and perform the Transaction Documents, and all documents, instruments, agreements, certificates, or financing statements contemplated thereby or related thereto, all without any further act, vote or approval of the Member or any Manager or Officer notwithstanding any other provision of this Agreement, the Act or applicable law, rule or regulation. The foregoing authorization shall not be deemed a restriction on the powers of any Manager or any Officer to enter into other documents, instruments, agreements, certificates or financing statements on behalf of the Company.
(c) The Company will cause [NEWCO]’s organizational documents to at all times during the term of the Trust include the provisions set forth in Exhibit I attached hereto.
(d) The Company will become a member of [NEWCO] and in such capacity cause [NEWCO] to comply with the provisions set forth in Exhibit II attached hereto.
Section 9. Powers.
Subject to Section 10(j), the Company (a) shall have and exercise all powers and rights necessary, convenient or incidental to accomplish its purposes as set forth in Section 8 and (b) shall have and exercise all of the powers and rights conferred upon limited liability companies formed pursuant to the Act.
Section 10. Management.
(a) Board of Managers. Subject to Section 10(j), the business and affairs of the Company shall be managed by or under the direction of a Board of two or more Managers. The Member shall designate, appoint and elect each Manager. Subject to Section 11, the Member may determine at any time in its sole and absolute discretion the number of Managers to constitute the Board. The authorized number of Managers may be increased or decreased by the Member at any time in its sole and absolute discretion, upon notice to all Managers, and subject in all cases to Section 11. The initial number of Managers shall be five, two of which shall be Independent Managers pursuant to Section 11. Each Manager elected, designated or appointed shall hold office until a successor is elected and qualified or until such Manager’s earlier death, resignation or removal. Each Manager shall execute and deliver the Management Agreement. Managers need not be Members. The initial Managers hereby designated by the Member are listed in Schedule B hereto.
(b) Powers. Subject to Section 10(j), the Board of Managers shall have the power to do any and all acts necessary, convenient or incidental to or for the furtherance of the purposes described herein, including all powers, statutory or otherwise. Subject to Section 10(j), the Board of Managers has the authority to bind the Company pursuant to a resolution expressly authorizing any matter which resolution is duly adopted by an affirmative vote of a majority of the Board or as otherwise required by this Agreement. An individual Manager shall not have the authority to bind the Company to any matter involving a third party without the affirmative vote of a majority of the Board or as otherwise required pursuant to this Agreement.
(c) Meeting of the Board of Managers. The Board of Managers may hold meetings, both regular and special, within or outside the State of Delaware. Regular meetings of the Board may be held without notice at such time and at such place as shall from time to time be determined by the Board. Special meetings of the Board may be called by the President on not less than one day’s notice to each Manager by telephone, facsimile, mail, telegram or any other means of communication, and special meetings shall be called by the President or Secretary in like manner and with like notice upon the written request of any one or more of the Managers.
(d) Quorum; Acts of the Board. At all meetings of the Board, a majority of the Managers shall constitute a quorum for the transaction of business and, except as otherwise provided in any other provision of this Agreement, the act of a majority of the Managers present at any meeting at which there is a quorum shall be the act of the Board. If a quorum shall not be present at any meeting of the Board, the Managers present at such meeting may adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum shall be present. Any action required or permitted to be taken at any meeting of the Board or of any committee thereof may be taken without a meeting if all members of the Board or committee, as the case may be, consent thereto in writing, and the writing or writings are filed with the minutes of proceedings of the Board or committee.
(e) Electronic Communications. Members of the Board, or any committee designated by the Board, may participate in meetings of the Board, or any committee, by means of telephone conference or similar communications equipment that allows all persons participating in the meeting to hear each other, and such participation in a meeting shall constitute presence in person at the meeting. If all the participants are participating by telephone conference or similar communications equipment, the meeting shall be deemed to be held at the principal place of business of the Company.
(f) Committees of Managers.
(i) The Board may, by resolution passed by a majority of the whole Board, designate one or more committees, each committee to consist of one or more of the Managers of the Company. The Board may designate one or more Managers as alternate members of any committee, who may replace any absent or disqualified member at any meeting of the committee.
(ii) In the absence or disqualification of a member of a committee, the member or members thereof present at any meeting and not disqualified from voting, whether or not such members constitute a quorum, may unanimously appoint another member of the Board to act at the meeting in the place of any such absent or disqualified member.
(iii) Any such committee, to the extent provided in the resolution of the Board, shall have and may exercise all the powers and authority of the Board in the management of the business and affairs of the Company. Such committee or committees shall have such name or names as may be determined from time to time by resolution adopted by the Board. Each committee shall keep regular minutes of its meetings and report the same to the Board when required.
(g) Compensation of Managers; Expenses. The Board shall have the authority to fix the compensation of Managers. The Managers may be paid their expenses, if any, of attendance at meetings of the Board, which may be a fixed sum for attendance at each meeting of the Board or a stated salary as Manager. No such payment shall preclude any Manager from serving the Company in any other capacity and receiving compensation therefor. Members of special or standing committees may be allowed like compensation for attending committee meetings.
(h) Remova1 of Managers. Unless otherwise restricted by law, any Manager or the entire Board of Managers may be removed, with or without cause, at any time by the Member, and, subject to Section 11, any vacancy caused by any such removal may be filled by action of the Member. Except as provided in this Agreement, a Manager may not bind the Company.
(i) Managers as Agents. To the extent of their powers set forth in this Agreement and subject to Section 10(j), the Managers are agents of the Company for the purpose of the Company’s business, and the actions of the Managers taken in accordance with such powers set forth in this Agreement shall bind the Company.
(j) Limitations on the Company’s Activities.
(i) This Section 10(j) is being adopted in order to comply with certain provisions required in order to qualify the Company as a “special purpose entity.”
(ii) The Member shall not amend, alter, change or repeal the definition of “Independent Manager” or Section 8, 9, 10, 11, 19, 20, 22, 23, 24 or the definition of “Independent Manager” set forth in Schedule A of this Agreement or engage in any business or activity other than as set forth in Section 8(a) without the unanimous written consent of the Board (including both Independent Managers). Subject to this Section 10(j), the Member may amend, alter, change or repeal any provisions contained in this Agreement in accordance with Section 29.
(iii) Notwithstanding any other provision of this Agreement and any provision of law that otherwise so empowers the Company, the Member or the Board, neither the Member nor the Board shall be authorized or empowered, nor shall they permit the Company, without the prior unanimous written consent of the Member and the Board (including both Independent Managers), to take any Material Action.
(iv) The Board and the Member shall cause the Company to do or cause to be done all things necessary to preserve and keep in full force and effect its existence, rights (charter and statutory) and franchises; provided, however, that the Company shall not be required to preserve any such right or franchise if the Board shall determine that the preservation thereof is no longer desirable for the conduct of its business. The Board shall cause the Company to be operated in such a manner as the Board deems reasonable and necessary or appropriate to preserve the limited liability of the Member, the separateness of the Company from the business and affairs of the Member or any Affiliate of the Member, and until one year and one day after any indebtedness and any other liabilities of the Company have been paid in full, the special purpose, bankruptcy remote status of the Company. The Board also shall cause the Company to:
(1) hold itself out to the public and all other Persons as a legal entity separate from the members and any other person and conduct its own business in its own name and require that all full-time employees of the Company, if any, identify themselves as such and not as employees of the Member or any of its subsidiaries;
(2) maintain the Company’s books, records and bank accounts separate from those of the Member and each other subsidiary of the Member and otherwise in such a manner so that such books and records are readily identifiable as its own assets rather than assets of the Member or any such subsidiary;
(3) have a Board composed differently from that of the Member and any other Person;
(4) file its own tax returns, if any, as may be required under applicable law, to the extent (A) not part of a consolidated group filing a consolidated return or returns or (B) not treated as a division for tax purposes of another taxpayer, and pay any taxes so required to be paid under applicable law;
(5) allocate fairly and reasonably any taxes and any overhead expenses that are shared with an affiliate, including
for shared office space and for services performed by an employee of an Affiliate;
(6) except as specifically permitted herein or in the Transaction Documents, not commingle its assets with assets of any other Person;
(7) maintain separate financial statements, showing its assets and liabilities separate and apart from those of any other person and not have its assets listed on any financial statement of any other person; provided, however, that the Company’s assets may be included in a consolidated financial statement of its Affiliate; provided that (A) appropriate notation shall be made on such consolidated financial statements to indicate the separateness of the Company from such Affiliate and to indicate that the Company’s assets and credit are not available to satisfy the debts and other obligations of such Affiliate or any other company and (B) such assets shall also be listed on the Company’s own separate balance sheet;
(8) pay its indebtedness and other liabilities out of its own funds and assets;
(9) maintain an arm’s-length relationship with the Member and its other Affiliates;
(10) pay the salaries of its own officers and employees, if any;
(11) not hold out its credit as being available to satisfy the obligations of others;
(12) allocate fairly and reasonably any overhead for office space or other expenses incurred by itself or any Affiliate on its behalf;
(13) use stationery, invoices, checks and other business forms separate from those of any other Person;
(14) not pledge its assets for the benefit of any other Person (except as contemplated by the Transaction Documents to which it is a party);
(15) correct any known misunderstanding regarding its separate identity;
(16) maintain at all times adequate capital in light of its contemplated business operations and liabilities and refrain
from making any distributions or other payments in respect of its membership interests (including any repurchase of membership interests or return of capital) that would cause it to have inadequate capital;
(17) cause its Board of Managers to meet at least annually or act pursuant to written consent and keep minutes of such meetings and actions and observe all other Delaware limited liability company formalities;
(18) cause its officers and managers, to the extent permitted by law, until one year and one day after any indebtedness and any other liabilities of the Company are paid in full, to make decisions with respect to the business and daily operations of the Company independent of, and not dictated by, the Member or any Affiliate of the Member;
(19) not acquire any obligations or securities of the Member; and
(20) except as otherwise provided herein, maintain its Certificate of Formation and this Agreement in conformity with the Transaction Documents, such that it does not amend, restate, supplement or otherwise modify its Certificate of Formation and this Agreement in any respect that would impair its ability to comply with the terms or provisions of any of the Transaction Documents.
(v) The Board shall not cause or permit the Company to:
(1) guarantee any obligation of any Person, including any Affiliate of the Company;
(2) engage, directly or indirectly, in any business other than as contemplated by this Agreement;
(3) incur, create or assume any indebtedness other than as contemplated by this Agreement or the Transaction Documents to which the Company is a party;
(4) take any action relating to (A) the dissolution or liquidation of the Company or (B) the initiation of, participation in, acquiescence in or consent to any bankruptcy, insolvency, reorganization or similar proceeding involving the Company, that is not duly authorized by unanimous vote of its managers (including the Independent Managers);
(5) maintain its separateness such that it does not merge or consolidate with or into, or convey, transfer, lease or otherwise dispose of (whether in one transaction or in a series of transactions, and except as otherwise contemplated herein) all or substantially all of its assets (whether now owned or hereafter acquired) to, or acquire all or substantially all of the assets of, any Person, nor at any time create, have, acquire, maintain or hold any interest in any subsidiary; or
(6) observe all organizational formalities as a distinct entity, and ensure that all actions relating to (A) the dissolution or liquidation of the Company or (B) the initiation of, participation in, acquiescence in or consent to any bankruptcy, insolvency, reorganization or similar proceeding involving the Company, are duly authorized by unanimous vote of its managers (including the Independent Managers).
Failure of the Company to comply with any of the covenants in this Section 10(j) shall not affect the status of the Company as a separate legal entity or the limited liability of the Member and Managers.
Section 11. Independent Managers.
The Member shall cause the Company at all times to have at least two Independent Managers, each of whom will be appointed by the Member. To the fullest extent permitted by Section 18-1101(c) of the Act, each of the Independent Managers shall consider only the interests of the Company and its creditors in acting or otherwise voting on matters subject to the vote of the Board of Managers, including those matters referred to in Sections 10(j)(iii) and (iv). No resignation or removal of an Independent Manager, and no appointment of a successor Independent Manager, shall be effective until the successor Independent Manager shall have accepted its appointment by a written instrument, which may be a counterpart signature page to the Management Agreement. All right, power and authority of the Independent Managers shall be limited to the extent necessary to exercise those rights and perform those duties specifically set forth in this Agreement. Except as provided in the second sentence of this Section 11, in exercising their rights and performing their duties under this Agreement, the Independent Managers shall have a fiduciary duty of loyalty and care to the Company and its creditors similar to that of a director of a business corporation organized under the General Corporation Law of the State of Delaware. Except as provided in this Agreement, an Independent Manager shall not bind the Company. The Member shall provide not less than ten (10) days’ prior written notice to the Company of the replacement or appointment of any Manager that is to serve as an Independent Manager for purposes of this Agreement. As a condition to the effectiveness of any such replacement or appointment, the Member shall certify to the Company that the designated Person satisfied the criteria set forth in the definition of “Independent Manager” and the Board shall acknowledge in writing, that in the Board’s reasonable judgment, the designated Person satisfies the criteria set forth in the definition of “Independent Manager.”
Section 12. Officers.
(a) Officers. The Officers of the Company shall consist of at least a President, a Secretary and a Treasurer. The Officers of the Company as of the date hereof are listed in Schedule B hereto. The Board of Managers or the Member may also choose a Chief Financial Officer and a General Counsel (or an Associate General Counsel) and one or more Vice Presidents, Assistant Secretaries, Assistant Treasurers and Assistant General Counsel in addition to the initial Officers appointed by the Member. Any number of offices may be held by the Member or the same person. The Board or the Member shall choose a President, a Secretary and a Treasurer following the resignation or removal of the initial Officers appointed by the Member. The Board or the Member may appoint such other Officers and agents as it shall deem necessary or advisable who shall hold their offices for such terms and shall exercise such powers and perform such duties as shall be determined from time to time by the Board. The salaries of all Officers and agents of the Company, if any, shall be fixed by or in the manner prescribed by the Board. The Officers of the Company shall hold office until their successors are chosen and qualified. Any Officer elected or appointed by the Member or the Board may be removed at any time, with or without cause, by the affirmative vote of a majority of the Board. Any vacancy occurring in any office of the Company shall be filled by the Board.
(b) President. The President shall be the chief executive officer of the Company, shall preside at all meetings of the Member, if any, and the Board, shall be responsible for the general and active management of the business of the Company and shall see that all orders and resolutions of the Board are carried into effect. The President shall execute all bonds, mortgages and other contracts, except: (i) where required or permitted by law or this Agreement to be otherwise signed and executed, including Section 8(b); (ii) where signing and execution thereof shall be expressly delegated by the Board to some other Officer or agent of the Company; and (iii) as otherwise permitted in Section 12(c).
(c) Vice President. In the absence of the President or in the event of the President’s inability to act, the Vice President, if any (or in the event there be more than one Vice President, the Vice Presidents in the order designated by the Managers, or in the absence of any designation, then in the order of their election), shall perform the duties of the President, and when so acting, shall have all the powers of and be subject to all the restrictions upon the President. The Vice Presidents, if any, shall perform such other duties and have such other powers as the Board may from time to time prescribe.
(d) Secretary and Assistant Secretary. The Secretary shall be responsible for filing legal documents and maintaining records for the Company except to the extent that such duties are allocated to the Chief Financial Officer, General Counsel, Associate General Counsel and/or Assistant General Counsel, if any. The Secretary shall attend all meetings of the Board and all meetings of the Company, if any, and record all the proceedings of the meetings of the Company and of the Board in a book to be kept for that purpose and shall perform like duties for the standing committees when required. The Secretary shall give, or cause to be given, notice of all meetings of the Company, if
any, and special meetings of the Board, and shall perform such other duties as may be prescribed by the Board or the President, under whose supervision the Secretary shall serve. The Assistant Secretary, or if there be more than one, the Assistant Secretaries in the order determined by the Board (or if there be no such determination, then in order of their election), shall, in the absence of the Secretary or in the event of the Secretary’s inability to act, perform the duties and exercise the powers of the Secretary and shall perform such other duties and have such other powers as the Board may from time to time prescribe.
(e) Treasurer and Assistant Treasurer. The Treasurer shall have the custody of the Company funds and securities and shall keep full and accurate accounts of receipts and disbursements in books belonging to the Company and shall deposit all moneys and other valuable effects in the name and to the credit of the Company in such depositories as may be designated by the Board. The Treasurer shall disburse the funds of the Company as may be ordered by the Board, taking proper vouchers for such disbursements, and shall render to the President and to the Board, at its regular meetings or when the Board so requires, an account of all of the Treasurer’s transactions and of the financial condition of the Company. The Assistant Treasurer, or if there shall be more than one, the Assistant Treasurers in the order determined by the Board (or if there be no such determination, then in the order of their election), shall, in the absence of the Treasurer or in the event of the Treasurer’s inability to act, perform the duties and exercise the powers of the Treasurer and shall perform such other duties and have such other powers as the Board may from time to time prescribe. Any of the duties of the Treasurer and Assistant Treasurer described in this clause (e) may also be performed by the Chief Financial Officer, if any.
(f) Officers as Agents. The Officers, to the extent of their powers set forth in this Agreement or otherwise vested in them by action of the Board not inconsistent with this Agreement, are agents of the Company for the purpose of the Company’s business, and, subject to Section 10(j), the actions of the Officers taken in accordance with such powers shall bind the Company.
(g) Duties of Board and Officers. Except to the extent otherwise provided herein, each Manager and Officer shall have a fiduciary duty of loyalty and care similar to that of directors and officers of business corporations organized under the General Corporation Law of the State of Delaware.
Section 13. Limited Liability.
Except as otherwise expressly provided by the Act, the debts, obligations and liabilities of the Company, whether arising in contract, tort or otherwise, shall be the debts, obligations and liabilities solely of the Company, and none of the Member or any Manager shall be obligated personally for any such debt, obligation or liability of the Company solely by reason of being the Member or a Manager of the Company.
Section 14. Capital Contributions.
The Member will be deemed admitted as the Member of the Company upon the execution and delivery of this Agreement. The Member has made a capital contribution to the Company listed on Schedule B attached hereto.
Section 15. Additional Contributions.
The Member is not required to make any additional capital contribution to the Company. The Member may make additional capital contributions to the Company at any time. The Member shall maintain records setting forth in reasonable detail any additional capital contributions made by the Member to the Company. The provisions of this Agreement, including this Section 15, are intended solely to benefit the Member and, to the fullest extent permitted by law, shall not be construed as conferring any benefit upon any creditor of the Company (and no such creditor of the Company shall be a third-party beneficiary of this Agreement) and the Member shall not have any duty or obligation to any creditor of the Company to make any contribution to the Company or to issue any call for capital pursuant to this Agreement.
Section 16. Allocation of Profits and Losses; Tax Treatment.
(a) For financial accounting and tax purposes, the Company’s net profits or net losses shall be determined on an annual basis in accordance with the manner determined by the Member. Net income of the Company for federal income tax purposes (and each item of income, gain, loss and deduction entering into the calculation thereof) shall be allocated to the Member.
(b) Unless otherwise determined by the Member, the Company shall be a disregarded entity for U.S. federal income tax purposes (as well as for any analogous state or local tax purposes), and the Member and the Company shall timely make any and all necessary elections and filings for the Company in connection with treatment as a disregarded entity for U.S. federal income tax purposes (as well as for any analogous state or local tax purposes).
Section 17. Distributions.
Unless otherwise directed by the Board, distributions on the Company’s property shall be distributed to the Member at the direction of the Member, which direction may be standing instructions. In the absence of any direction to the contrary by the Member, distributions shall be made to the Member at the times and in the aggregate amounts determined by the Board. Notwithstanding any provision to the contrary contained in this Agreement, the Company shall not be required to make a distribution to the Member on account of its interest in the Company if such distribution would violate Section 18-607 of the Act or any other applicable law.
Section 18. Books and Records; Bank Accounts.
(a) The Board shall keep or cause to be kept complete and accurate books of account and records with respect to the Company’s business. The books of the Company shall at all times be maintained by the Board. The Member and its duly
authorized representatives shall have the right to examine the Company books, records and documents during normal business hours. The Company’s books of account shall be kept using the method of accounting determined by the Member. The Company’s independent auditor shall be an independent public accounting firm selected by the Member.
(b) The Member may authorize any Manager or Officer to open and maintain one or more bank accounts; rent safety deposit boxes or vaults; sign checks, written directions, or other instruments to withdraw all or any part of the funds belonging to the Company and on deposit in any savings account or checking account; negotiate and purchase certificates of deposit, obtain access to the Company safety deposit box or boxes, and generally sign such forms on behalf of the Company as may be required to conduct the banking activities of the Company.
Section 19. Other Business; Business Transactions of the Member with the Company; Company Property.
(a) The Member and any Affiliate thereof may engage in or possess an interest in other business ventures (unconnected with the Company) of every kind and description, independently or with others. The Company shall not have any rights in or to such independent ventures or the income or profits therefrom by virtue of this Agreement.
(b) In accordance with Section 18-107 of the Act, the Member or any Affiliate thereof may lend money to, borrow money from, act as surety, guarantor or endorser for, guarantee or assume one or more obligations of, provide collateral for, and transact other business with, the Company and, subject to applicable law, shall have the same rights and obligations with respect to any such matter as a Person who is not the Member or an Affiliate thereof.
(c) No real or other property of the Company shall be deemed to be owned by the Member individually, but shall be owned by and title shall be vested solely in the Company.
Section 20. Exculpation and Indemnification.
(a) The Member shall not, and no Officer, Manager, employee or agent of the Company and no employee, representative, agent or Affiliate of the Member (collectively, the “Covered Persons”) shall, be liable to the Company or any other Person who has an interest in or claim against the Company for any loss, damage or claim incurred by reason of any act or omission performed or omitted by such Covered Person in good faith on behalf of the Company and in a manner reasonably believed to be within the scope of the authority conferred on such Covered Person by this Agreement, except that a Covered Person shall be liable for any such loss, damage or claim incurred by reason of such Covered Person’s gross negligence or willful misconduct.
(b) To the fullest extent permitted by applicable law, a Covered Person shall be entitled to indemnification from the Company for any loss, damage or claim
incurred by such Covered Person by reason of any act or omission performed or omitted by such Covered Person in good faith on behalf of the Company and in a manner reasonably believed to be within the scope of the authority conferred on such Covered Person by this Agreement, except that no Covered Person shall be entitled to be indemnified in respect of any loss, damage or claim incurred by such Covered Person by reason of such Covered Person’s gross negligence or willful misconduct with respect to such acts or omissions; provided, however, that any indemnity under this Section 20 shall be provided out of and to the extent of Company assets only, and the Member shall not have personal liability on account thereof.
(c) To the fullest extent permitted by applicable law, expenses (including legal fees) incurred by a Covered Person defending any claim, demand, action, suit or proceeding shall, from time to time, be advanced by the Company prior to the final disposition of such claim, demand, action, suit or proceeding upon receipt by the Company of an undertaking by or on behalf of the Covered Person to repay such amount if it shall be determined that the Covered Person is not entitled to be indemnified as authorized in this Section 20.
(d) A Covered Person shall be fully protected in relying in good faith upon the records of the Company and upon such information, opinions, reports or statements presented to the Company by any Person as to matters the Covered Person reasonably believes are within such other Person’s professional or expert competence and who has been selected with reasonable care by or on behalf of the Company, including information, opinions, reports or statements as to the value and amount of the assets, liabilities, or any other facts pertinent to the existence and amount of assets from which distributions to the Member might properly be paid.
(e) To the extent that, at law or in equity, a Covered Person has duties (including fiduciary duties) and liabilities relating thereto to the Company or to any other Covered Person, a Covered Person acting under this Agreement shall not be liable to the Company or to any other Covered Person for its good faith reliance on the provisions of this Agreement or any approval or authorization granted by the Company or any other Covered Person. The provisions of this Agreement, to the extent that they restrict the duties and liabilities of a Covered Person otherwise existing at law or in equity, are agreed by the Member to replace such other duties and liabilities of such Covered Person.
(f) The foregoing provisions of this Section 20 shall survive any termination of this Agreement.
Section 21. Transfer.
The Member shall be entitled to sell, convey, assign, transfer, pledge, grant a security interest in or otherwise dispose of all or any part of its interest in the Company subject to the execution and delivery of such documentation required by the Board in connection therewith; provided, however, that no such assignment shall relieve the Member from its obligations hereunder.
Section 22. Dissolution.
(a) Subject to Section 10(j), the Company shall be dissolved and its affairs shall be wound up upon the first to occur of the following: (i) the Member votes for dissolution or (ii) the entry of a decree of judicial dissolution under Section 18-802 of the Act.
(b) No other event, including the retirement, insolvency, liquidation, dissolution, insanity, expulsion, Bankruptcy, death, incapacity or adjudication of incompetency of the Member, shall cause the existence of the Company to terminate. Upon the Bankruptcy of the Member, the retirement or dissolution of the Member or the occurrence of any other event which terminates the continued membership of the Member in the Company, each Independent Manager shall become a special member of the Company and the business of the Company shall continued in a manner permitted by the Act.
(c) The Bankruptcy of the Member shall not cause the Member to cease to be a member of the Company and upon the occurrence of such an event, the business of the Company shall continue without dissolution. Notwithstanding any other provision of this Agreement, the Member waives any right that it might have under Section 18-80l (b) of the Act to agree in writing to dissolve the Company upon the Bankruptcy of the Member or the occurrence of any event that causes the Member to cease to be a member of the Company.
(d) In the event of dissolution, the Company shall conduct only such activities as are necessary to wind up its affairs (including the sale of the assets of the Company in an orderly manner), and the assets of the Company shall be applied in the manner, and in the order of priority, set forth in Section 18-804 of the Act.
Section 23. Waiver of Partition; Nature of Interest.
Except as otherwise expressly provided in this Agreement, to the fullest extent permitted by law, the Member hereby irrevocably waives any right or power that the Member might have to cause the Company or any of its assets to be partitioned, to cause the appointment of a receiver for all or any portion of the assets of the Company, to compel any sale of all or any portion of the assets of the Company pursuant to any applicable law or to file a complaint or to institute any proceeding at law or in equity to cause the dissolution, liquidation, winding up or termination of the Company. The Member shall not have any interest in any specific assets of the Company, and the Member shall not have the status of a creditor with respect to any distribution pursuant to Section 17 hereof. The interest of the Member in the Company is personal property.
Section 24. Benefits of Agreement; No Third-Party Rights.
None of the provisions of this Agreement shall be for the benefit of or enforceable by any creditor of the Company or by any creditor of the Member. Subject to Section 27, nothing in this Agreement shall be deemed to create any right in any Person (other than Covered Persons) not a party hereto, and this Agreement shall not be
construed in any respect to be a contract in whole or in part for the benefit of any third Person.
Section 25. Severability of Provisions.
Each provision of this Agreement shall be considered severable and if for any reason any provision or provisions herein are determined to be invalid, unenforceable or illegal under any existing or future law, such invalidity, unenforceability or illegality shall not impair the operation of or affect those portions of this Agreement which are valid, enforceable and legal.
Section 26. Entire Agreement.
This Agreement constitutes the entire agreement of the parties with respect to the subject matter hereof.
Section 27. Binding Agreement.
Notwithstanding any other provision of this Agreement, the Member agrees that this Agreement constitutes a legal, valid and binding agreement of the Member, and is enforceable against the Member by each Independent Manager in accordance with its terms. Each Independent Manager is an intended beneficiary of this Agreement.
Section 28. Governing Law.
THIS AGREEMENT SHALL BE GOVERNED BY AND CONSTRUED UNDER THE LAWS OF THE STATE OF DELAWARE (WITHOUT REGARD TO CONFLICTS OF LAW PRINCIPLES), ALL RIGHTS AND REMEDIES BEING GOVERNED BY SUCH LAWS.
Section 29. Amendments.
(a) This Agreement may be amended, supplemented or otherwise modified by the Member to cure any ambiguity, to correct or supplement any provisions in this Agreement, to add any provisions to this Agreement, to change this Agreement in any manner or to eliminate any of the provisions in this Agreement subject in each case to Section 10(j).
(b) Any amendment, supplement or other modification to this Agreement shall be in writing. Promptly after the execution of any amendment, supplement or other modification to this Agreement, the Company shall furnish a copy of such amendment, supplement or other modification to the Member.
(c) Promptly after the execution of any amendment, supplement or other modification to the Certificate of Formation, the Company shall cause its filing with the Secretary of State of the State of Delaware.
Section 30. Counterparts.
This Agreement may be executed in any number of counterparts, including by telefacsimile or other electronic means of communication, each of which shall be deemed an original of this Agreement and all of which together shall constitute one and the same instrument.
Section 31. Notices.
Any notices required to be delivered hereunder shall be in writing and personally delivered, mailed or sent by electronic mail, telefacsimile or other similar form of rapid transmission, and shall be deemed to have been duly given upon receipt (a) in the case of the Company, to the Company at its address in Section 3, (b) in the case of the Member, to the Member at its address as listed on Schedule B attached hereto and (c) in the case of either of the foregoing, at such other address as may be designated by written notice to the other party.
IN WITNESS WHEREOF, the undersigned, intending to be legally bound hereby, has duly executed this Limited Liability Company Agreement of [TAURUS], LLC as of the date first above written.
|
|
MEMBER: | |
|
|
| |
|
|
[TAURUS], LLC | |
|
|
|
|
|
|
|
|
|
|
By: |
|
|
|
|
Name: |
|
|
|
Title: |
[TAURUS], LLC
LIMITED LIABILITY COMPANY AGREEMENT
SCHEDULE A
DEFINITIONS
A. Definitions
When used in this Agreement, the following terms not otherwise defined herein have the following meanings:
“Act” has the meaning set forth in the preamble to this Agreement.
“Affiliate” means, with respect to any Person, any other Person directly or indirectly Controlling or Controlled by or under direct or indirect common Control with such Person.
“Agreement” means this Limited Liability Company Agreement of the Company, dated as of May , 2013 and entered into by the Member as the sole member of the Company, together with the schedules attached hereto, as amended, supplemented or otherwise modified from time to time in accordance with the terms hereof.
“Assignment Agreement” means the Assignment Agreement, dated as of May , 2013, by and between [TAURUS] and [NEWCO].
“Bankruptcy” means, with respect to any Person, if (i) such Person makes an assignment for the benefit of creditors, (ii) such Person files a voluntary petition in bankruptcy, (iii) such Person is adjudged a bankrupt or insolvent, or has entered against it an order for relief, in any bankruptcy or insolvency proceeding, (iv) such Person files a petition or answer seeking for itself any reorganization, arrangement, composition, readjustment, liquidation, or similar relief under any statute, law or regulation, (v) such Person files an answer or other pleading admitting or failing to contest the material allegations of a petition filed against it in any proceeding of this nature, (vi) such Person seeks, consents to or acquiesces in the appointment of a trustee, receiver or liquidator of the Person or of all or any substantial part of its properties, or (vii) 120 days after the commencement of any proceeding against the Person seeking reorganization, arrangement, composition, readjustment, liquidation, dissolution or similar relief under any statute, law or regulation, the proceeding has not been dismissed, or within 90 days after the appointment without such Person’s consent or acquiescence of a trustee, receiver or liquidator of such Person or of all or any substantial part of its properties, the appointment is not vacated or stayed, or within 90 days after the expiration of any such stay, the appointment is not vacated. With respect to the Member, the foregoing definition of “Bankruptcy” is intended to replace and shall supersede and replace the definition of “Bankruptcy” set forth in Sections 18-101 (1) and 18-304 of the Act.
“Board” or “Board of Managers” means the Board of Managers of the Company.
“Certificate of Formation” means the Certificate of Formation of the Company, filed with the Secretary of State of the State of Delaware on May , 2013, as the same may be amended, supplemented or otherwise modified from time to time.
“Company” means [TAURUS], LLC, a Delaware limited liability company.
“Control” means the possession, directly or indirectly, or the power to direct or cause the direction of the management or policies of a Person, whether through the ownership of voting securities or general partnership or managing member interests, by contract or otherwise. “Controlling” and “Controlled” shall have correlative meanings. Without limiting the generality of the foregoing, a Person shall be deemed to Control any other Person in which it owns, directly or indirectly, a majority of the ownership interests.
“Covered Persons” has the meaning set forth in Section 20(a) of this Agreement.
“[EUROPA]” means [EUROPA], an Irish public limited company.
“Fiscal Year” has the meaning set forth in Section 7 of this Agreement.
“Independent Manager” means a natural person who, (A) (1) has prior experience as an independent director, independent manager or independent member with at least three years of employment experience with one or more entities that provide, in the ordinary course of their respective businesses, advisory, management or placement services to issuers of securitization or structured finance instruments, agreements or securities and (2) is provided by CT Corporation, Corporation Service Company, National Registered Agents, Inc., Wilmington Trust Company SP Services, Inc., ▇▇▇▇▇▇▇ Management Company, Lord Securities Corporation or, if none of those companies is then providing professional Independent Managers, another nationally-recognized company reasonably approved by the Member and by [EUROPA], in each case that is not an Affiliate of the Company and that provides professional Independent Managers and other corporate services in the ordinary course of its business, (B) is not, and has not been for a period of five years prior to his or her appointment as an independent manager of the Company: (1) a stockholder (whether direct, indirect or beneficial), counterparty under a contract for commercial services, advisor or supplier of the Member or any of its Affiliates (the “Parent Group”), (2) a director, officer, employee, partner, attorney or consultant of the Parent Group, (3) a person related to any person referred to in clauses (1) or (2) above, (4) a person or other entity controlling or under common control with any such stockholder, partner, counterparty under a contract for commercial services, supplier, employee, officer or director or (5) a trustee, conservator or receiver for any
member of the Parent Group and (D) shall not at any time serve as a trustee in bankruptcy for the Company, the Member or any Affiliate thereof, and insure that (v) no resignation or removal of an Independent Manager shall be effective until (1) the Company has provided [EUROPA] with two Business Days’ prior written notice of such resignation or removal, and (2) a successor Independent Manager is appointed and such successor shall have accepted his or her appointment as an Independent Manager by a written instrument, (w) at least two members of the Company’s Board of Managers shall be Independent Managers, (x) the Company’s Board of Managers shall not approve, or take any other action to cause the filing of, a voluntary bankruptcy petition with respect to the Company or consent to an involuntary bankruptcy petition with respect to the Company unless a unanimous vote of the Company’s Board of Managers (which vote shall include the affirmative vote of the Independent Managers) shall approve the taking of such action in writing prior to the taking of such action, (y) the Company’s Board of Managers shall not vote on any matter requiring the vote of its Independent Managers under its limited liability company agreement unless and until each Independent Manager is then serving on the Company’s Board of Managers and (z) the provisions requiring Independent Managers and the provisions described in clauses (x) and (y) of this paragraph (v) cannot be amended without the prior written consent of the Member and [EUROPA].
“Management Agreement” means the agreement of the Managers in the form attached hereto as Schedule C, as the same may be amended, supplemented or otherwise modified from time to time in accordance with the terms thereof, which Management Agreement shall be deemed incorporated into, and part of, this Agreement.
“Managers” means the managers elected to the Board of Managers from time to time by the Member, including the Independent Managers. Each Manager is hereby designated as a “manager” of the Company within the meaning of Section 18-101(10) of the Act.
“Material Action” means to consolidate or merge the Company with or into any Person, or to convey or transfer the property and assets of the Company substantially as an entirety to any entity other than in the ordinary course of engaging in the transactions contemplated by the Transaction Documents, or to institute or participate in proceedings to have the Company be adjudicated bankrupt or insolvent, or to consent to the institution of bankruptcy or insolvency proceedings against the Company or to file a petition seeking, or to consent to, reorganization, liquidation or relief with respect to the Company under any applicable federal or state law relating to bankruptcy, insolvency, reorganization or dissolution or to consent to the appointment of a receiver, liquidator, assignee, trustee, sequestrator (or other similar official) of the Company or a substantial part of its property, or to make any assignment for the benefit of creditors of the Company, or to admit in writing the Company’s inability to pay its debts generally as they become due, or, to the fullest extent permitted by law, take action in furtherance of any such action, or to dissolve or liquidate the Company, in whole or in part, or to increase or reclassify the membership interests of the Company or to issue any additional membership interests of the Company.
“Member” means [TAURUS], as the sole Member of the Company, and its successors in interest.
“[NEWCO]” means [NEWCO], an English limited partnership.
“Officer” means an officer of the Company described in Section 12(a).
“Person” means any individual, corporation, partnership, joint venture, limited liability company, limited liability partnership, association, joint-stock company, trust, unincorporated organization, or other organization, whether or not a legal entity, and any governmental authority.
“Royalty Participation Agreement” means the Royalty Participation Agreement, dated as of May , 2013, by and between [TAURUS] and [EUROPA].
“[TAURUS]” means [TAURUS], a Delaware corporation.
“Transaction Documents” means this Agreement, the Assignment Agreement, the Royalty Participation Agreement, the Trust Agreement and all other documents and certificates delivered in connection herewith or therewith, as the same may be amended, supplemented or otherwise modified from time to time.
“Trust Agreement” means the Declaration of Trust, as of May , 2013, by and among [TAURUS], [NEWCO] and the other parties thereto.
B. Rules of Construction
Definitions in this Agreement apply equally to both the singular and plural forms of the defined terms. The words “include” and “including” shall be deemed to be followed by the phrase “without limitation.” The terms “herein,” “hereof’ and “hereunder” and other words of similar import refer to this Agreement as a whole and not to any particular Section, paragraph or subdivision. The Section titles appear as a matter of convenience only and shall not affect the interpretation of this Agreement. All Section, paragraph, clause, Exhibit or Schedule references not attributed to a particular document shall be references to such parts of this Agreement.
SCHEDULE B
|
MEMBER |
|
|
|
Name |
|
Mailing Address |
|
|
|
|
|
[TAURUS] |
|
|
|
|
|
|
|
MANAGERS |
|
|
|
Name |
|
Mailing Address |
|
|
|
|
|
1. |
|
|
|
|
|
|
|
2. |
|
|
|
|
|
|
|
3. |
|
|
|
|
|
|
|
4. |
|
|
|
|
|
|
|
5. |
|
|
|
|
|
|
|
OFFICERS |
|
|
|
Name |
|
Title |
|
|
|
|
|
|
|
[President] |
|
1. |
|
|
|
|
|
[Vice Presidents] |
|
1. |
|
|
|
2. |
|
|
|
3. |
|
|
|
|
|
[Chief Financial Officer and Treasurer] |
|
1. |
|
|
|
|
|
[General Counsel and Secretary] |
|
1. |
|
|
|
|
|
[Associate General Counsel and Assistant Secretary] |
|
1. |
|
|
|
Initial Capital Contribution of the Member: $1 |
|
|
SCHEDULE C
FORM OF MANAGEMENT AGREEMENT
May , 2013
[TAURUS], LLC
[PRINCIPAL BUSINESS ADDRESS]
Re: Management Agreement
[TAURUS], LLC
Ladies and Gentlemen:
For good and valuable consideration, each of the undersigned persons, who have been designated as managers of [TAURUS], LLC, a Delaware limited liability company (the “Company”), in accordance with the Limited Liability Company Agreement of the Company, dated as of May , 2013 (as the same may be amended, supplemented or otherwise modified from time to time, the “LLC Agreement”), hereby agree as follows:
1. Each of the undersigned accepts such person’s rights and authority as a Manager under the LLC Agreement and agrees to perform and discharge such person’s duties and obligations as a Manager under the LLC Agreement, and further agrees that such rights, authorities, duties and obligations under the LLC Agreement shall continue until such person’s successor as a Manager is designated or until such person’s resignation or removal as a Manager in accordance with the LLC Agreement. Each of the undersigned agrees and acknowledges that it has been designated as a “manager” of the Company within the meaning of Section 18-101(10) of the Delaware Limited Liability Company Act.
2. Each of the undersigned agrees, solely in its capacity as a creditor of the Company on account of any indemnification or other payment owing to the undersigned by the Company, not to acquiesce, petition or otherwise invoke or cause the Company to invoke the process of any court or governmental authority for the purpose of commencing or sustaining a case against the Company under any federal or state bankruptcy, insolvency or similar law or appointing a receiver, liquidator, assignee, trustee, custodian, sequestrator or other similar official of the Company or any substantial part of the property of the Company, or ordering the winding up or liquidation of the affairs of the Company.
3. THIS MANAGEMENT AGREEMENT SHALL BE GOVERNED BY AND CONSTRUED IN ACCORDANCE WITH, THE LAWS OF THE STATE OF DELAWARE, AND ALL RIGHTS AND REMEDIES SHALL BE GOVERNED BY SUCH LAWS, WITHOUT REGARD TO PRINCIPLES OF CONFLICTS OF LAW.
Capitalized terms used herein that are not otherwise defined shall have the meanings ascribed thereto in the LLC Agreement.
IN WITNESS WHEREOF, the undersigned have executed this Management Agreement as of the day and year first above written.
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
|
Name: |
|
|
|
|
|
|
|
|
Name: |
Exhibit I
PROVISIONS TO BE INCLUDED IN [NEWCO]’S ORGANIZATIONAL DOCUMENTS
(Exhibit F of Royalty Participation Agreement(1))
1. The Initial Members will be Seller and Delaware Sub.
2. No other persons shall become Members without the prior written agreement of [ ].
3. The Designated Members shall be all the Members [or such of the Members as are designated in accordance with the LPA(2)]. The Designated Members shall be responsible for ensuring compliance with all registration and other requirements of the Limited Liability Partnerships Act 2000 and any amendments to that it (the “Act”) and other applicable legislation.
4. The Members will agree that the relationship between them does not constitute any form of co-ownership of the assets and liabilities of the LLP or any form of trust under any applicable law or any form of trust relationship or equitable relationship under any applicable law.
5. The business of the LLP (the “Business”) shall consist solely of the business determined in accordance with the Trust Agreement and the Assignment Agreement; provided that the Business shall also include the acquisition of rights to receive royalty payments or other payments derived directly or indirectly from the exploitation of intellectual property related to pharmaceutical products and to sell or otherwise monetize such rights.
6. The Members shall not cause or permit the LLP during the term of the Trust to (and the LLP shall not):
(a) incur any indebtedness for borrowed money;
(b) form, acquire or hold any subsidiary (whether corporate, partnership, limited liability company or other) or own any equity interest in any other entity;
(1) Note that defined terms in this Exhibit I are incorporated by reference from the corresponding definitions in the Royalty Participation Agreement and not from this Agreement.
(2) Note that “LPA” is not defined in the Royalty Participation Agreement.
(i) amend the LPA in a manner adverse to Purchaser;
(j) unless required by applicable Law, have any employees (for the avoidance of doubt, the Members do not constitute employees);
(k) enter into any reconstruction, amalgamation, merger or consolidation or take any voluntary steps with a view to its winding-up;
(m) otherwise than as contemplated in the Transaction Documents, release from or terminate the appointment of the Account Bank;
(n) subject to any requirement under applicable Law to maintain a registered office, enter into any lease for, or own, any real property.
Exhibit II
[NEWCO] BANKRUPTCY REMOTENESS PROVISIONS
(Section 6.15(b) of the Royalty Participation Agreement(3))
(i) keep proper books of account in accordance with its obligations under English law;
(ii) maintain its registered office in the United Kingdom, and not to establish a branch, agency or place of business or register as a partnership, company or body corporate within the United States or any other country or jurisdiction other than the United Kingdom.
(iii) do all such things as are necessary to maintain its limited liability partnership existence; and
(iv) not:
(1) amend Trust Agreement or the Assignment Agreement;
(2) incur any indebtedness for borrowed money;
(3) amend its partnership agreement in a manner adverse to Purchaser;
(4) unless required by Law, have any employees (for the avoidance of doubt, the directors of [NEWCO] do not constitute employees);
(5) enter into any reconstruction, amalgamation, merger or consolidation or take any voluntary steps with a view to its winding-up;
(3) Note that defined terms in this Exhibit II are incorporated by reference from the corresponding definitions in the Royalty Participation Agreement and not from this Agreement.
(6) otherwise than as contemplated in the Transaction Documents, release from or terminate the appointment of the Account Bank (as that term is defined in the Trust Agreement); or
(v) subject to any requirement under applicable Law to maintain a registered office, enter into any lease for, or own, any real property.



