Certain information in this document has been omitted from this exhibit because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed. CO-DEVELOPMENT AGREEMENT
Exhibit 10.35
[***] Certain information in this document has been omitted from this exhibit because it is both (i) not material and (ii) would be competitively harmful if publicly disclosed.
This Co-Development Agreement (“Agreement”), made effective as of February ___, 2021 (the “Effective Date”), is by and between Nektar Therapeutics, a Delaware corporation (“Nektar”), and SFJ Pharmaceuticals XII, L.P. (“SFJ”), a Delaware limited partnership (each, a “Party” and collectively, the “Parties”).
WHEREAS, SFJ is in the business of facilitating, among other things, the development and approval of pharmaceutical products and desires to fund and conduct the clinical trial for the development of the Product for the use, in combination with pembrolizumab as a first line treatment of patients with squamous cell carcinoma of the head and neck (the “HNC Indication”); and
WHEREAS, Nektar has rights to the Product, is conducting clinical trials of the Product globally for various indications and would like to enter into an agreement with SFJ to conduct and fund the HNC Clinical Trials of the Product.
NOW THEREFORE, in consideration of the mutual agreements contained herein and other good and valuable consideration, the sufficiency of which is hereby acknowledged, the Parties agree as follows:
ARTICLE 1
DEFINITIONS
1.1 Defined Terms. Initially capitalized terms will have the meaning ascribed to such terms in this Agreement, including the following terms which will have the following respective meanings:
1.1.1 “AAA” has the meaning ascribed to such term in Section 14.10.1.
1.1.2 “Accelerated Regulatory Approval” means (i) an accelerated approval of an indication by the FDA based on a surrogate or an intermediate clinical endpoint under Section 901 of the Food and Drug Administration Safety Innovations Act (FDASIA), and (ii) that the prescribing label states “that the drug was approved based upon accelerated approval and that continued approval for the drug (or indication) may be contingent upon verification and description of clinical benefit in a confirmatory trial or trials.”
1.1.3 “Adverse Patent Impact” has the meaning ascribed to such term in Section 13.2.7.
1.1.4 “Affiliate” means, with respect to a party, a business entity under common control with, or controlling or controlled by, such party, with “control” meaning direct or indirect
ACTIVE/105681617.22
ownership of 50% or more of the voting interest in such other entity, and in the case of a partnership, control of the general partner. Notwithstanding the foregoing, (a) neither The Blackstone Group Inc. (“Blackstone”) nor any of its divisions, including Blackstone Life Sciences Advisors L.L.C. (“BXLS”), nor any funds managed by Blackstone, BXLS or their respective Affiliates shall be deemed to be an “Affiliate” of SFJ, (b) neither Abingworth LLP nor any of its subsidiaries or divisions, nor any funds managed by the foregoing, shall be deemed to be an “Affiliate” of SFJ and (c) SFJ Pharmaceuticals, Inc. shall not be deemed to be an “Affiliate” of SFJ.
1.1.5 “Alliance Manager” has the meaning ascribed to such term in Section 5.1.5.
1.1.6 “Anti-Corruption Laws” means the U.S. Foreign Corrupt Practices Act, as amended, the UK ▇▇▇▇▇▇▇ ▇▇▇ ▇▇▇▇, as amended, and any other applicable anti-corruption laws and laws for the prevention of fraud, racketeering, money laundering or terrorism.
1.1.7 “Applicable Law” means the applicable laws, rules and regulations, including any rules, regulations, guidelines, or other requirements of any Governmental Authorities (including any Regulatory Authorities), to the extent legally binding, that may be in effect from time to time. For clarity, Applicable Laws will include the FFDCA, the PHSA, the Anti-Corruption Laws, and all laws, regulations and legally binding guidelines applicable to the Clinical Trials, including GCP, GLP, GMP and ICH guidelines.
1.1.8 “Approval Buy-Out Payment” has the meaning ascribed to such term in Section 6.7.1.
1.1.9 “Approved CRO” has the meaning ascribed to such term in Section 2.4.1.
1.1.10 “Approved Third Party Vendor Costs” has the meaning ascribed to such term in Section 5.2.2.2.
1.1.11 “Approved Vendor” has the meaning ascribed to such term in Section 2.4.2.
1.1.12 [***]
1.1.13 “BEMPEG” means bempegaldesleukin.
1.1.14 “BLA” means: (a) a biologics license application submitted to the FDA pursuant to Section 351(a) of the PHSA and the regulations promulgated thereunder, or its successor application; or (b) an application for authorization to market and/or sell a biological product in any country or regulatory jurisdiction other than the US submitted to the applicable Regulatory Authority in such country or regulatory jurisdiction, including, with respect to the EU, a marketing authorization application submitted either (i) to the EMA pursuant to the centralized EU filing procedure or (ii) to the applicable national Regulatory Authority in an individual EU member state if the centralized EU filing procedure is not used.
2
ACTIVE/105681617.22
1.1.15 “BMS” means ▇▇▇▇▇▇▇-▇▇▇▇▇ Squibb Company, a Delaware corporation.
1.1.16 “BMS Strategic Collaboration Agreement” means the Strategic Collaboration Agreement between BMS and Nektar dated February 13, 2018, as amended by that certain First Amendment to the Strategic Collaboration Agreement dated January 9, 2020.
1.1.17 “Business Day” means a day that is not a Saturday, Sunday or a US federal holiday.
1.1.18 “Buy-Out Payment” means an Approval Buy-Out Payment or a Change of Control Payment.
1.1.19 “Calendar Quarter” means each successive period of three (3) consecutive calendar months ending on March 31, June 30, September 30 and December 31; provided, that, the (a) the first Calendar Quarter shall begin on the Effective Date and end on the last day of the Calendar Quarter in which the Effective Date falls, and (b) the final Calendar Quarter shall end on the last day of the Term.
1.1.20 “Calendar Year” means each successive period of twelve (12) months commencing on January 1 and ending on December 31; provided, that, (a) the first Calendar Year shall begin on the Effective Date and end on December 31 of the Calendar Year in which the Effective Date falls, and (b) the final Calendar Year shall end on the last day of the Term.
1.1.21 “Case Report Form” or “CRF” means the collection of documents designed specifically for recording data pursuant to the Protocol. A CRF is completed for each Subject and will be in electronic form, validated and in compliance with all Applicable Laws.
1.1.22 “Change of Control” means, with respect to Nektar, at any time prior to the date of the payment by Nektar of the final Success Payment hereunder, (a) a merger, reorganization or consolidation with a Third Party which results in the voting securities of Nektar outstanding immediately prior thereto ceasing to represent, or being converted into or exchanged for voting securities that do not represent, at least fifty percent (50%) of the combined voting power of the voting securities of the surviving entity or the parent corporation of the surviving entity immediately after such merger, reorganization or consolidation, (b) a transaction in which a Third Party becomes the beneficial owner of fifty percent (50%) or more of the combined voting power of the outstanding securities of Nektar, other than through the issuance of voting securities for the purpose of raising financing to one or more financial or institutional investors that are not then controlled by an entity engaged in the development or commercialization of pharmaceutical or biotechnology products, or (c) the sale or other transfer of all or substantially all of Nektar’s business or assets. For avoidance of doubt, a Licensing Transaction shall not constitute a Change of Control.
1.1.23 “Change of Control Payment” has the meaning ascribed to such term in Section 6.7.2.
3
ACTIVE/105681617.22
1.1.24 “Claim” means any Third Party claim, demand, suit and/or cause of action.
1.1.25 “Clinical Investigator” means the principal investigator at each Site.
1.1.26 “Clinical Investigator Meeting” has the meaning ascribed to such term in Section 3.2.2.1.
1.1.27 “Clinical Trials” means the Melanoma Clinical Trial and the HNC Clinical Trials.
1.1.28 “Clinical Trial Activity” has the meaning ascribed to such term in Section 2.3.1.
1.1.29 “Clinical Trial Agreement” has the meaning ascribed to such term in Section 3.2.1.2.
1.1.30 “Clinical Trial Success Criteria” means the Melanoma Clinical Trial Success Criteria or the HNC Clinical Trial Success Criteria, as the case may be.
1.1.31 “Clinical Trials Database” has the meaning ascribed to such term in Section 3.5.3.1.
1.1.32 “Clinical Trials Master File” has the meaning ascribed to such term in Section 3.5.4.
1.1.33 “CMC” means chemistry, manufacturing and controls.
1.1.34 “CMC Information” means the CMC information intended or required for the submission of an IND or BLA.
1.1.35 “CMO” means contract manufacturing organization or contract development and manufacturing organization.
1.1.36 “Code” means the Internal Revenue Code of 1986, as amended, and the Treasury Regulations adopted thereunder.
1.1.37 “Commercial Launch” means, with respect to the Product, the first sale to a Third Party of such Product in the United States after the applicable Regulatory Approval.
1.1.38 “Commercialization” or “Commercialize” means the commercial manufacture, marketing, promotion, sale and/or distribution of the Product. For clarity, Commercialization excludes all activities associated with development and seeking Regulatory Approval for the Product.
1.1.39 “Commercially Reasonable Efforts” means with respect to the performance of activities under this Agreement by Party (and (x) in the case of SFJ as pertains to
4
ACTIVE/105681617.22
its role in conducting the HNC Clinical Trials, and (y) in the case of Nektar as pertains to its role in conducting or monitoring the Melanoma Clinical Trial): reasonable, diligent, good-faith efforts to accomplish such objective [***].
1.1.40 “Competing Product” means an IL-2 conjugate used for the treatment of HNC.
1.1.41 “Completion Date” means, as to a particular Clinical Trial, the earlier of (a) the date of the final CSR for such Clinical Trial and (b) the date such Clinical Trial or this Agreement is terminated.
1.1.42 “Confidential Information” of a Party means all information and materials provided and/or disclosed (including in written form, electronic form or otherwise) by, or on behalf of, such Party or its Affiliates or their Representatives to the other Party, its Affiliates, or its of their Representatives in connection with this Agreement, including, technical, scientific, regulatory and other information, results, knowledge, techniques, data, analyses, inventions, invention disclosures, plans, processes, methods, know-how, ideas, concepts, test data (including pharmacological, toxicological and clinical test data), analytical and quality control data, formulae, specifications, marketing, pricing, distribution, cost, sales, and manufacturing data and descriptions. In addition, the terms and conditions of this Agreement shall be deemed to be Confidential Information of both SFJ and Nektar. In addition, the Research Results shall at all times be deemed to be Confidential Information of Nektar, and Nektar and SFJ shall be deemed the disclosing Party and the receiving Party, respectively, with respect thereto.
1.1.43 “Contingent Obligation” means, for any Person, any direct or indirect liability, contingent or not, of that Person for (a) any indebtedness, letter of credit or other Indebtedness of another Person, in each case, directly or indirectly guaranteed, endorsed or co-made by that Person, or for which that Person is directly or indirectly liable; (b) any obligations for undrawn letters of credit for the account of that Person; and (c) all obligations from any interest rate, currency or commodity swap agreement, interest rate cap or collar agreement, or other agreement or arrangement designated to protect a Person against fluctuation in interest rates, currency exchange rates or commodity prices. The amount of a Contingent Obligation is the stated or determined amount of the primary obligation for which the Contingent Obligation is made or, if not determinable, the maximum reasonably anticipated liability for it determined by the Person in good faith; but the amount may not exceed the maximum of the obligations under any guarantee or other support arrangement.
1.1.44 “Control” or “Controlled” means (a) for Intellectual Property, a Party’s ability to grant applicable licenses, sublicenses and/or other rights thereunder and (b) for materials and documents, a Party’s ability to provide, or provide access to, such materials and/or documents, each without violating any contractual obligations to a Third Party. For clarity, if a Party only can grant a license or sublicense and/or provide rights and/or access of limited scope, for a specific purpose or under certain conditions due to an encumbrance, “Control” or “Controlled” will be construed to so limit such license, sublicense, provision of rights and/or access.
5
ACTIVE/105681617.22
1.1.45 “Copyrights” means, collectively, all works of authorship, mask works and any and all other registered and unregistered copyrights and copyrightable works, and all applications, registrations, extensions, and renewals thereof.
1.1.46 “Cover,” “Covered” or “Covering” means, with respect to the applicable Intellectual Property, in the absence of the applicable rights and licenses granted, would be infringed, misappropriated, or otherwise violated by.
1.1.47 “CRO” means contract research organization as defined in 21 C.F.R. 312.3(b).
1.1.48 “CRO Agreement” has the meaning ascribed to such term in Section 2.4.1.
1.1.49 “CSR” means, for with respect to a Clinical Trial, a clinical study report, or other equivalent document or series of materials, constituting a summary report of the clinical and medical data resulting from such Clinical Trial and prepared for incorporation into submissions seeking Regulatory Approval for the Product, and includes all statistical analyses of such data per the Statistical Analysis Plan.
1.1.50 “Data Room” means that certain electronic data room established by Nektar and to which SFJ and/or its advisors were granted access.
1.1.51 “Develop” or “Development” means all clinical and non-clinical research and development activities conducted for the Product, including toxicology, pharmacology test method development and stability testing, process development, formulation development, quality assurance and quality control development, statistical analysis, conducting Clinical Trials, regulatory affairs, and obtaining and maintaining Regulatory Approval, and related digital system and general and administrative support therefor.
1.1.52 “Development Costs” means the following internal and external costs, associated with completing the HNC Clinical Trials: (a) Approved Third Party Vendor Costs, (b) the SFJ Quarterly Labor Fees as outlined in Exhibit G-1, (c) the Nektar Quarterly Labor Fees as outlined in Exhibit G-2, and (d) such other costs that are deemed to be Development Costs as provided for in this Agreement.
1.1.53 “Development Cost Reconciliation Procedures” has the meaning ascribed to such term in Section 5.5.6.1.
1.1.54 “Development Program” means a clinical and regulatory development program to be undertaken by the Parties to Develop the Product for the Indication, carry out the Clinical Trials, and seek Regulatory Approval for the Product.
1.1.55 “Development Term” means the period commencing on the Effective Date and ending on the later of (a) the latest of the Completion Dates of the Clinical Trials, and (b) the date on which all efforts in pursuit of Regulatory Approval of the Product for any Indication have been concluded or terminated.
6
ACTIVE/105681617.22
1.1.56 “Development Termination” has the meaning ascribed to such term in Section 13.2.2.2(b).
1.1.57 “Disclosing Party” has the meaning ascribed to such term in Section 9.1.
1.1.58 “Discount Rate” has the meaning ascribed to such term in Section 6.7.1.1.
1.1.59 “Dispute” has the meaning ascribed to such term in Section 14.10.
1.1.60 “Effective Date” has the meaning ascribed to such term in the Preamble.
1.1.61 “EU” means the European Union or any successor union of European states thereto having a substantially similar function.
1.1.62 “Excess Development Costs” has the meaning ascribed to such term in Section 4.1.
1.1.63 “Exclusive Period” means (a) in the case of the conduct of human clinical trials with respect to a Competing Product, the period beginning on the Effective Date and ending on December 31, 2025.
1.1.64 “Executive Officers” means the executive officers of each of Nektar and SFJ identified on Exhibit E.
1.1.65 “Existing Nektar Intellectual Property” has the meaning ascribed to such term in Section 10.1.1.1.
1.1.66 “FDA” means the US Food and Drug Administration and any successor agency thereto in the US having substantially the same function.
1.1.67 “FFDCA” means the US Federal Food, Drug, and Cosmetic Act, as amended from time to time, together with any rules, regulations and requirements promulgated thereunder (including all additions, supplements, extensions and modifications thereto).
1.1.68 “First Regulatory Approval” means the first to occur of the HNC Regulatory Approval and the Melanoma Regulatory Approval.
1.1.69 “GAAP” means generally accepted accounting principles in the US, as consistently applied by the applicable Party.
1.1.70 “GMP Manufacturer” means the Party that is responsible for ensuring that the Product is manufactured in accordance with GMP.
1.1.71 “Good Clinical Practices” or “GCP” means all applicable good clinical practice standards for the design, conduct, performance, monitoring, auditing, recording, analyses and reporting of clinical trials, including, as applicable, (a) the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human
7
ACTIVE/105681617.22
Use (“ICH”) Harmonised Tripartite Guideline for Good Clinical Practice (CPMP/ICH/135/95) and any other guidelines for good clinical practice for clinical trials on medicinal products; (b) the Declaration of Helsinki (2004) as last amended at the 52nd World Medical Association in October 2000 and any further amendments or clarifications thereto; and (c) the equivalent Applicable Laws in any relevant country, each as may be amended and applicable from time to time and in each case, that provide for, among other things, assurance that the clinical data and reported results are credible and accurate and protect the rights, integrity, and confidentiality of clinical trial Subjects.
1.1.72 “Good Manufacturing Practices” or “GMP” means all applicable good manufacturing practices including, as applicable, (a) the applicable part of quality assurance to ensure that products are consistently produced and controlled in accordance with the quality standards appropriate for their intended use, as defined in European Commission Directive 2003/94/EC laying down the principals and guidelines of good manufacturing practice; (b) the principles detailed in the US Current Good Manufacturing Practices, 21 C.F.R. Sections 210, 211, 601 and 610; (c) the Rules Governing Medicinal Product in the European Community, Volume IV Good Manufacturing Practice for Medicinal Product; (d) the principles detailed in the ICH Q7A guidelines; and (e) the equivalent Applicable Laws in any relevant country, each as may be amended and applicable from time to time.
1.1.73 “Government Official” is broadly defined as and includes: (a) any elected or appointed government official (e.g., a member of a ministry of health); (b) any employee or person acting for or on behalf of a government official, agency, or enterprise performing a governmental function; (c) any non-US political party officer, employee, or person acting for or on behalf of a non-US political party or candidate for public office; (d) any employee or person acting for or on behalf of a public international organization; (e) all government employees and employees of state-owned enterprises; or (f) any person otherwise categorized as a government official under local law; where “government” is meant to include all levels and subdivisions of non-US governments (i.e., local, regional, or national and administrative, legislative, or executive).
1.1.74 “Governmental Authority” means any supranational, federal, national, state or local court, agency, authority, department, regulatory body or other governmental instrumentality.
1.1.75 “HNC Clinical Trials” has the meaning ascribed to such term in Section 2.1.1.
1.1.76 “HNC Clinical Trial Futility” means that the HNC Main Clinical Trial meets the end of Phase 2 futility criteria set forth in the HNC Main Clinical Trial Protocol.
1.1.77 “HNC Clinical Trial Protocols” has the meaning ascribed to such term in Section 2.1.1.
1.1.78 “HNC Main Clinical Trial Protocol” has the meaning ascribed to such term in Section 2.1.1.
8
ACTIVE/105681617.22
1.1.79 “HNC Clinical Trial Success Criteria” means that, following database lock, the results of the HNC Main Clinical Trial meet the overall survival primary endpoint set forth in the HNC Clinical Trial Protocol.
1.1.80 “HNC Indication” has the meaning ascribed to such term in the preamble of this Agreement.
1.1.81 “HNC Main Clinical Trial” means the clinical trial of the Product for the HNC Indication in accordance with the HNC Main Clinical Trial Protocol, as such protocol may be amended from time to time in accordance with this Agreement.
1.1.82 “HNC Regulatory Approval” means accelerated or full approval of a BLA for the Product for the HNC Indication by the FDA in the US. For clarity, “HNC Regulatory Approval” excludes any pricing or reimbursement approval that may be necessary or useful for marketing or sale of the Product in the US.
1.1.83 “ICH” has the meaning ascribed to such term in Section 1.1.74
1.1.84 “IDMC” means the independent data monitoring committee, which will be established pursuant to Section 3.9.1.
1.1.85 “IDMC Charter” has the meaning ascribed to such term in Section 3.9.1.
1.1.86 “IND” means an investigational new drug application, clinical trial application, clinical trial exemption, or similar application or submission filed with or submitted to a Regulatory Authority in a jurisdiction that is necessary to initiate human clinical testing of a pharmaceutical product in such jurisdiction, including any such application filed with the FDA pursuant to 21 C.F.R. Part 312.
1.1.87 “Indebtedness” means (a) indebtedness for borrowed money or the deferred price of property or services (excluding accounts payable incurred in the ordinary course of business, earn-out or similar obligations with respect to deferred purchase price and deferred compensation), (b) obligations evidenced by notes, bonds, debentures or similar instruments, (c) capital lease obligations (as such term is understood under GAAP as in effect on the date of this Agreement, but excluding obligations treated as operating leases prior to adoption of changes described by ASC Topic 842) and (d) Contingent Obligations.
1.1.88 “Indemnification Claim Notice” has the meaning ascribed to such term in Section 11.2.1.
1.1.89 “Indemnified Party” has the meaning ascribed to such term in Section 11.2.1.
1.1.90 “Indemnifying Party” has the meaning ascribed to such term in Section 11.2.1.
9
ACTIVE/105681617.22
1.1.91 “Indications” means (a) the HNC Indication and (b) the Melanoma Indication.
1.1.92 “Information” means technical or scientific know-how, trade secrets, methods, processes, formulae, designs, specifications and data, including biological, chemical, pharmacological, toxicological, pre-clinical, clinical, safety, manufacturing and quality control data and assays; in each case, whether or not confidential, proprietary, patented or patentable.
1.1.93 “Informed Consent” has the meaning ascribed to such term in Section 3.3.2.1.
1.1.94 “Initial Payment Date” has the meaning ascribed to such term in Section 6.1.1.
1.1.95 “Initial Payment Trigger Date” means (a) if the Melanoma Regulatory Approval occurs before the HNC Regulatory Approval, the earliest of (i) the date on which the database lock for the primary endpoint of median overall survival in the Phase 3 portion of the HNC Main Clinical Trial used for a full approval of a BLA for the Product for the HNC Indication occurs and (ii) the 18-month anniversary of the last patient-in for the HNC Main Clinical Trial [***], or (b) if the HNC Regulatory Approval occurs on or before the Melanoma Regulatory Approval, the day following the HNC Regulatory Approval.
1.1.96 “Initial Schedule A Success Payment” has the meaning ascribed to such term in Section 6.1.1.
1.1.97 “Initial Schedule B Success Payment” has the meaning ascribed to such term in Section 6.1.2.
1.1.98 “Intellectual Property” means all intellectual property and industrial property rights of any kind or nature throughout the world, including all US and foreign, (a) Patents; (b) Trademarks; (c) Copyrights; (d) rights in computer programs (whether in source code, object code, or other form), algorithms, databases, compilations and data, technology supporting the foregoing, and all documentation, including user manuals and training materials, related to any of the foregoing; (e) trade secrets and all other Confidential Information, know-how, inventions, proprietary processes, formulae, models, and methodologies; (f) rights of publicity, privacy, and rights to personal information; (g) all rights in the foregoing and in other similar intangible assets; and (h) all applications and registrations for the foregoing.
1.1.99 “Investigator’s Brochure” means the written document containing a brief description of the drug substance and formulation of the Product, a summary of the pharmacological and toxicological effects of the Product in animals and human nonclinical models, a summary of the pharmacokinetics and biological disposition of the Product in animals and humans, a summary of information relating to safety and effectiveness of the Product in humans obtained from prior clinical studies, and a description of possible risks and side effects to be anticipated on the basis of prior experience with the Product under investigation or with related drugs.
10
ACTIVE/105681617.22
1.1.100 “IRB” means institutional review board, or its equivalent.
1.1.101 “ISC” has the meaning ascribed to such term Section 3.9.1.
1.1.102 “JOC” has the meaning ascribed to such term in Section 5.4.1.
1.1.103 “JOC Chairperson” has the meaning ascribed to such term in Section 5.4.2.
1.1.104 “JOC Representative(s)” has the meaning ascribed to such term in Section 5.4.1.
1.1.105 “JFC” has the meaning ascribed to such term in Section 5.5.1.
1.1.106 “JFC Chairperson” has the meaning ascribed to such term in Section 5.5.2.
1.1.107 “JFC Representatives” has the meaning ascribed to such term in Section 5.5.1.
1.1.108 “JPT” has the meaning ascribed to such term in Section 5.4.6.
1.1.109 “JSC” has the meaning ascribed to such term in Section 5.1.1
1.1.110 “JSC Chairperson” has the meaning ascribed to such term in Section 5.1.2.
1.1.111 “JSC Representative(s)” has the meaning ascribed to such term in Section 5.1.1.
1.1.112 “Knowledge of Nektar” means [***].
1.1.113 “Leftover Funding” has the meaning ascribed to such term in Section 4.2.1.3.
1.1.114 “Licensing Transaction” means: a license or sublicense to a Third Party under any of the Nektar Intellectual Property to Commercialize the Product or an equivalent sale or other transfer of Nektar Intellectual Property that conveys the right to Commercialize the Product (other than, (a) in the case of a Third Party contract testing, development, research and/or manufacturing organization, a license or sublicense to commercially manufacture the Product on behalf of Nektar or its Affiliates, without any license or sublicense to engage in any other Commercialization activities with respect to the Product and (b) in the case of a Third Party wholesaler, distributor or distribution logistics services provider, a license or sublicense to distribute the Product (and/or conduct other typical distribution activities) on behalf of Nektar or its Affiliates, without any license or sublicense to engage in any other Commercialization activities with respect to the Product), in each case, other than in conjunction with a permitted assignment of this Agreement pursuant to Section 14.6 in connection with the sale or other transfer of all or substantially all of its business or assets to which this Agreement relates.
11
ACTIVE/105681617.22
1.1.115 “Lien” means a mortgage, deed of trust, levy, charge, pledge, security interest or other encumbrance of any kind, whether voluntarily incurred or arising by operation of law or otherwise against any property.
1.1.116 “Losses” means liabilities, losses, costs, damages, fees and/or expenses (including reasonable legal expenses and attorneys’ fees) payable to a Third Party.
1.1.117 “Major European Markets” means [***].
1.1.118 “Material Anti-Corruption Law Violation” means a violation by a Party or its Affiliate of an Anti-Corruption Law relating to the subject matter of this Agreement that would, if it were publicly known, have a material adverse effect on the other Party or its Affiliate because of its relationship with such Party.
1.1.119 “Maximum Development Costs” has the meaning ascribed to such term in Section 4.1.
1.1.120 “Melanoma Clinical Trial” means the clinical trial of the Product for the Melanoma Indication with a protocol title of “A Study of NKTR-214 Combined with Nivolumab vs. Nivolumab Alone in Participants with Previously Untreated Inoperable or Metastatic Melanoma” with the ▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇ identifier of NCT03635983, as such protocol may be amended from time to time in accordance with the BMS Strategic Agreement.
1.1.121 “Melanoma Clinical Trial Research Results” means all Information arising out of, or resulting from, the Melanoma Clinical Trial and/or the CMC activities contemplated by the Development Program, including the Clinical Trials database for the data collected from each study site for the Melanoma Clinical Trial; but excluding Trial Inventions (including Intellectual Property in or to Trial Inventions).
1.1.122 “Melanoma Clinical Trial Success Criteria” means that the results of the Melanoma Clinical Trial meet the primary endpoint of progression free survival as set forth in the protocol of the Melanoma Clinical Trial.
1.1.123 “Melanoma Indication” means first-line metastatic or untreated, unresectable melanoma.
1.1.124 “Melanoma Regulatory Approval” means accelerated or full approval of a BLA for the Product for the Melanoma Indication by the FDA in the US. For clarity, “Melanoma Regulatory Approval” excludes any pricing or reimbursement approval that may be necessary or useful for marketing or sale of the Product in the US.
1.1.125 “Merck” mean MSD International GmbH.
1.1.126 “Merck CTCSA” means the Clinical Trial Collaboration and Supply Agreement between Merck and Nektar dated February 11, 2021.
1.1.127 “Merck Product” means Keytruda (pembrolizumab).
12
ACTIVE/105681617.22
1.1.128 “Nektar” has the meaning ascribed to such term in the Preamble.
1.1.129 “Nektar Compliance Breach” has the meaning specified in Section 13.2.6.1.
1.1.130 “Nektar Confidential Information” means all Confidential Information provided and/or disclosed by or on behalf of Nektar or its Affiliates, agents or representatives to SFJ or its Affiliates, agents or representatives hereunder. For clarity, Nektar Confidential Information will include any and all CMC Information.
1.1.131 “Nektar Indemnified Parties” has the meaning ascribed to such term in Section 11.1.1.
1.1.132 “Nektar Intellectual Property” means all Intellectual Property owned or Controlled by Nektar that is necessary or useful for the Development, manufacture, use, sale or import of the Product, including Trial Inventions.
1.1.133 “Nektar Obligations” means all Indebtedness, liabilities and other obligations of Nektar to SFJ under or in connection with this Agreement and any other documents executed in connection herewith, including, without limitation, all amounts payable to SFJ pursuant to Article 6 hereof, all interest accrued thereon, all fees and all other amounts payable by Nektar to SFJ thereunder or in connection therewith, whether now existing or hereafter arising, and whether due or to become due, absolute or contingent, liquidated or unliquidated, determined or undetermined, and including interest that accrues after the commencement by or against Nektar of any bankruptcy or insolvency proceeding naming such individual or entity as the debtor in such proceeding, and including performing the Nektar Services.
1.1.134 “Nektar Quarterly Labor Fees” means the quarterly fees outlined in Exhibit G-2.
1.1.135 “Nektar Services” means performing or managing all Commercialization, publications, regulatory activities, investigational drug branch (IDB) and study reporting, including those responsibilities set forth on Exhibit H, in each case with respect to the HNC Clinical Trials and unless the HNC Clinical Trials have terminated.
1.1.136 “Nektar SOPs” has the meaning ascribed to such term in Section 3.1.4.
1.1.137 “Other Indication” means any oncology indication, other than the Indications, either alone or in combination with another drug.
1.1.138 “Other Indication Regulatory Approval” means accelerated or full approval of a BLA for the Product for an Other Indication by the FDA in the US. For clarity, “Other Indication Regulatory Approval” excludes any pricing or reimbursement approval that may be necessary or useful for marketing or sale of the Product in the US.
1.1.139 [***]
13
ACTIVE/105681617.22
1.1.140 “Party” or “Parties” has the meaning ascribed to such term in the Preamble.
1.1.141 “Patent” will mean patents, patent applications, patent disclosures, and all related continuations, continuations-in-part, divisionals, reissues, re-examinations, substitutions, and extensions thereof.
1.1.142 “Permitted Third Party” means any CRO, Site, Clinical Investigator and/or Vendor to whom Nektar or SFJ has delegated responsibility or whom Nektar or SFJ has engaged in connection with the HNC Clinical Trials or any CMO whom Nektar has engaged to perform CMC related activities (including supply of Product for use in the Clinical Trials). For clarity, Third Parties that have been delegated responsibility by or engaged by a Permitted Third Party will be considered Permitted Third Parties.
1.1.143 “Person” means any individual, corporation, general or limited partnership, limited liability company, joint venture, estate, trust, association, organization, labor union, or other entity or Governmental Authority.
1.1.144 “Personally Identifiable Information” means any information relating to an identified or, in combination with other information, identifiable person or persons captured in an electronic or hardcopy format, including such information as it relates to clinical trials subjects (including key-coded patient data), physicians, clinicians, healthcare professionals, consultants, or other persons participating in the Clinical Trials, and any equivalent definition in the Applicable Laws to the extent that such definition is broader than that provided here.
1.1.145 “PHSA” means the Public Health Service Act as set forth at 42 U.S.C. Chapter 6A, as may be amended from time to time, together with any rules, regulations and requirements promulgated thereunder (including all additions, supplements, extensions and modifications thereto).
1.1.146 “Pre-Approval Commercialization Activities” has the meaning ascribed to such term in Section 4.3.
1.1.147 “Product” means the product containing BEMPEG described on Exhibit A.
1.1.148 “Product Filings” means INDs, BLAs and Regulatory Approvals (including all amendments and supplements to any of the foregoing) and other filings with, and formal submissions to, the FDA or other applicable Regulatory Authorities, in each case with respect to the Product.
1.1.149 “Quarterly Labor Fees” means, collectively, the Nektar Quarterly Labor Fees and the SFJ Quarterly Labor Fees.
1.1.150 “Receiving Party” has the meaning ascribed to such term in Section 9.1.
1.1.151 “Recipient Party” has the meaning ascribed to such term in Section 6.5.2.
14
ACTIVE/105681617.22
1.1.152 “Regulatory Approval” means any of the HNC Regulatory Approval and the Melanoma Regulatory Approval.
1.1.153 “Regulatory Authority” means in a particular country or regulatory jurisdiction, any applicable Governmental Authority involved in granting approval to initiate or conduct clinical testing in humans, for Regulatory Approval.
1.1.154 “Representatives” has the meaning ascribed to such term in Section 12.1.3.
1.1.155 “Research Results” means all Information arising out of, or resulting from, the HNC Clinical Trials and/or the CMC activities contemplated by the Development Program, including the Clinical Trials Database; but excluding Trial Inventions (including Intellectual Property in or to Trial Inventions).
1.1.156 “Safety Concern” has the meaning ascribed to such term in Section 13.2.5.
1.1.157 “Schedule A Success Payment” has the meaning ascribed to such term in Section 6.1.1.
1.1.158 “Schedule B Success Payment” has the meaning ascribed to such term in Section 6.1.2.
1.1.159 “Second Regulatory Approval” means, following or concurrent with the First Regulatory Approval, the occurrence of an HNC Regulatory Approval (if the First Regulatory Approval was a Melanoma Regulatory Approval) or a Melanoma Regulatory Approval (if the First Regulatory Approval was an HNC Regulatory Approval).
1.1.160 “Second Regulatory Approval Date” has the meaning ascribed to such term in Section 6.1.2.
1.1.161 “Serious Safety Issue” means any SUSAR or series of SUSARs directly related to or caused by the administration of the Product in the conduct of the Clinical Trials where such SUSAR or series of SUSARs substantially diminishes the probability of receiving Regulatory Approval for the Product, or results in a Regulatory Authority imposing a clinical hold on further development of the Product which clinical hold is not lifted or removed within [***].
1.1.162 “SFJ” has the meaning ascribed to such term in the Preamble.
1.1.163 “SFJ Indemnified Parties” has the meaning ascribed to such term in Section 11.1.2.
1.1.164 “SFJ Services” means the provision of clinical development and others services by SFJ in accordance with the RACI as described in Exhibit H.
1.1.165 “SFJ SOPs” has the meaning ascribed to such term in Section 3.1.2.
15
ACTIVE/105681617.22
1.1.166 “SFJ Quarterly Labor Fees” means the quarterly fees outlined in Exhibit G-1.
1.1.167 “Site” has the meaning ascribed to such term in Section 3.2.1.2.
1.1.168 “SOPs” means the Nektar SOPs, SFJ SOPs, or CRO SOPs as agreed to by the Parties.
1.1.169 “Specified Investors” means (a) The Blackstone Group Inc., its divisions and Affiliates including Blackstone Life Sciences, and any funds or investment vehicles managed by the foregoing, and (b) Abingworth LLP, its divisions and Affiliates, and any funds or investment vehicles managed by the foregoing.
1.1.170 “Specified Regulatory Approval” has the meaning ascribed to such term in Section 6.1.3.
1.1.171 “Statistical Analysis Plan” has the meaning ascribed to such term in Section 3.5.6.
1.1.172 “Subject” has the meaning ascribed to such term in Section 3.3.2.1.
1.1.173 “Subject Recruitment Plan” has the meaning ascribed to such term in Section 3.3.1.
1.1.174 “Subsequent Regulatory Approval” has the meaning ascribed to such term in Section 6.1.3.
1.1.175 “Success Payment Default” has the meaning ascribed to such term in Section 13.2.1.2.
1.1.176 “Success Payments” has the meaning ascribed to such term in Section 6.1.
1.1.177 “SUSAR” means a suspected unexpected serious adverse reaction, without regard to causality, that is life-threatening (i.e., causes an immediate risk of death) or that results in any of the following outcomes: death; in-patient hospitalization or prolongation of existing hospitalization; persistent or significant disability or incapacity (i.e., substantial disruption of the ability to conduct normal life functions); or a congenital anomaly or birth defect. For clarity, a planned medical or surgical procedure is not, in itself, a SUSAR.
1.1.178 “Term” has the meaning ascribed to such term in Section 13.1.
1.1.179 “Third Party” means any Person other than Nektar, SFJ and their Affiliates.
1.1.180 “Third Party Infringement” means any actual or threatened infringement, misappropriation, or other violation by a Third Party of any Intellectual Property Controlled by Nektar that relates to this Agreement and/or the Product, including the Trial Inventions.
16
ACTIVE/105681617.22
1.1.181 “Third Party Intellectual Property” means any Intellectual Property owned or Controlled by a Third Party that is necessary or useful for, or could reasonably be expected to be relevant to, the development, manufacture, use, sale or import of the Product, including Trial Inventions.
1.1.182 “Timeline” has the meaning ascribed to such term in Section 2.3.1.
1.1.183 “Timeline Remediation Plan” has the meaning ascribed to such term in Section 2.3.2.
1.1.184 “Trademarks” means, collectively, all registered and unregistered marks, trade dress rights, logos, taglines, slogans, Internet domain names, web addresses, and other indicia of origin, together with the goodwill associated with any of the foregoing, and all applications, registrations, extensions and renewals thereof, selected for use on the Product.
1.1.185 “Trial Data Package” means all Information, in any form, generated or developed by or on behalf of a Party or any of its Affiliates (including by any of their respective Permitted Third Parties) in the conduct of the HNC Clinical Trials during the Development Term, including the Clinical Trial Database and other data and reports arising out of the HNC Clinical Trials, any Clinical Trial Agreements or any Vendor Agreements or CRO Agreements related to the conduct of the HNC Clinical Trials, including the Research Results; but, in each case, excluding Trial Inventions.
1.1.186 “Trial Invention” means: (a) any invention or discovery, whether or not patentable, made, developed, generated, conceived, or reduced to practice by or on behalf of a Party or any of its Affiliates or Permitted Third Parties, or jointly by or on behalf of the Parties or any of their respective Affiliates or Permitted Third Parties, in the course or as a result of the conduct of any Clinical Trial or any other activity conducted pursuant to this Agreement, including, without limitation, any improvement to any Existing Nektar Intellectual Property; and (b) all Intellectual Property in any of the items described in the preceding clause (a).
1.1.187 “US,” “U.S.” or “USA” means the United States of America, its territories and possessions, including Puerto Rico.
1.1.188 “Vendor Agreement” has the meaning ascribed to such term in Section 2.4.2.
1.1.189 “Withholding Party” has the meaning ascribed to such term in Section 6.5.2.
1.2 Construction. For purposes of this Agreement: (a) words in the singular will be held to include the plural and vice versa as the context requires; (b) the words “including” and “include” will mean “including, without limitation,” unless otherwise specified; (c) the terms “hereof,” “herein,” “herewith,” and “hereunder,” and words of similar import will, unless otherwise stated, be construed to refer to this Agreement as a whole and not to any particular
17
ACTIVE/105681617.22
provision of this Agreement; and (d) all references to “Section” and “Exhibit,” unless otherwise specified, are intended to refer to a Section or Exhibit of or to this Agreement.
1.3 Conflicts. In the event of any conflict between the terms of this Agreement, the Protocol and/or any other Exhibit, the Protocol will control (as applicable), followed by the terms of this Agreement, and followed by any applicable other Exhibit.
ARTICLE 2
THE clinical trials
2.1 The HNC Clinical Trial Protocols.
2.1.1 HNC Clinical Trial Protocols. The protocol for the HNC Main Clinical Trial (the “HNC Main Clinical Trial Protocol”) will be finalized by Nektar based on the draft protocol attached hereto as Exhibit D and approved by the JOC within [***] days after the Effective Date. If any additional regional trials are needed in order to supplement the HNC Main Clinical Trial, then the protocol for each such additional clinical trial of the Product (such additional trials, “Additional HNC Clinical Trials” and, together with the HNC Main Clinical Trial, the “HNC Clinical Trials” and such protocols, “Additional HNC Clinical Trial Protocols” and, together with the HNC Main Clinical Trial Protocol, the “HNC Clinical Trial Protocols”) will be prepared by SFJ in consultation with Nektar and approved by the JOC.
2.1.2 Changes to the Protocols.
2.1.2.1 Any changes to the HNC Main Clinical Trial Protocol, including any country-specific appendices required by Applicable Law and changes made in response to any communications with any Regulatory Authorities, that require a submission to a Regulatory Authority, an IRB or other ethics committee, will be prepared by SFJ, with consultation from Nektar, and will require the JOC’s approval, which will not be unreasonably withheld or delayed and which will be communicated to the Parties as soon as reasonably practicable following the JOC’s receipt of the draft amendment from Nektar. Any changes to any Additional HNC Clinical Trial Protocol, including any country-specific appendices required by Applicable Law and changes made in response to any communications with any Regulatory Authorities, that require a submission to a Regulatory Authority, an IRB or other ethics committee, will be prepared by SFJ, with consultation from Nektar, and will require the JOC’s approval, which will not be unreasonably withheld or delayed and which will be communicated to the Parties as soon as reasonably practicable following the JOC’s receipt of the draft amendment from SFJ.
2.1.2.2 If either Party believes that a HNC Clinical Trial Protocol requires an amendment (for example, to comply with any Applicable Laws or based on any communications from any Regulatory Authorities), such Party will inform the JOC. If the JOC agrees that such an amendment is required, the JOC will provide each Party with written notice thereof as soon as reasonably practicable, and SFJ, with consultation from Nektar, will prepare a draft amendment to such Protocol, which will only be effective and part of such Protocol upon
18
ACTIVE/105681617.22
approval by the JOC pursuant to Section 5.4.5.1, which approval will not be unreasonably withheld and which will be communicated to the Parties as soon as reasonably practicable following the JOC’s receipt of the draft amendment from such Party.
2.1.3 Protocol Approval. Nektar will be responsible for obtaining all necessary approvals of each HNC Clinical Trial Protocol (including as required by Applicable Laws) on a global basis, in each case prior to the commencement of the applicable HNC Clinical Trial. SFJ will reasonably co-operate with Nektar in such regard.
2.2 Sponsor.
2.2.1 Sponsorship and Responsibilities. Nektar will be the sponsor of the HNC Clinical Trials. SFJ, together with one or more Permitted Third Parties, will have all responsibilities of a sponsor as specified in Applicable Laws. The responsibilities of SFJ and Nektar are set forth in Exhibit H. For purposes of 21 C.F.R. 312.52, SFJ shall be a CRO, and the document set forth on Exhibit K is the written transfer of Nektar’s sponsor obligations to SFJ as required by 21 C.F.R.312.52(a) and an assumption of such sponsor obligations by SFJ as required by 21 C.F.R. 312.52(b).
2.2.2 Compliance with the Protocol and Applicable Laws. SFJ will conduct the HNC Clinical Trials and perform all other responsibilities assigned to it hereunder in compliance with the applicable Protocol, all Applicable Laws and the terms hereof.
2.2.3 Diligence. SFJ will conduct due diligence with respect to each Permitted Third Party used by SFJ to ensure that such Permitted Third Party can comply with all applicable terms and obligations of this Agreement and Applicable Laws.
2.3 Compliance with the Timeline.
2.3.1 The Timeline. The timeline for conducting the HNC Clinical Trials are attached as Exhibit I hereto (the “Timeline”). In conducting the HNC Clinical Trials, the Parties will use Commercially Reasonable Efforts to complete each activity specified on the Timeline (each, a “Clinical Trial Activity”) by the date specified for such Clinical Trial Activity on the Timeline. The Parties will notify the JOC in writing upon completion or achievement of each of their designated Clinical Trial Activities.
2.3.2 Failure to Complete a Clinical Trial Activity. If a Party fails to, or reasonably believes that it will not, complete (a) any Clinical Trial Activity within [***] days of the date for completion of any Clinical Trial Activity on the Timeline or (b) the final Clinical Trial Activity within [***] of the date for the final Clinical Trial Activity on the Timeline, the Party will provide the JOC with a written remediation plan detailing the means by which, and the date on which, that Party expects to be able to complete the relevant Clinical Trial Activities (each, a “Timeline Remediation Plan”). Following receipt thereof, the JOC Representatives will discuss and consider in good faith such Timeline Remediation Plan. If the JOC approves such Timeline Remediation Plan (such approval not to be unreasonably withheld or delayed), the JOC will provide the appropriate Party with written notice thereof, specifying the dates on which, and
19
ACTIVE/105681617.22
the detail with which, the Party will be required to update the JOC of its progress with respect thereto. If the JOC is unable to approve such Timeline Remediation Plan, the matter will be decided by the JSC in accordance with Section 5.2. After approval of a Party’s Timeline Remediation Plan, if such Party believes in good faith that any modification to such Timeline Remediation Plan is necessary or appropriate, such Party may propose such modification to the JOC and shall disclose to the JOC any additional information or circumstances that have become known to such Party that form the basis for its request for modification. The JOC will discuss and consider such in good faith such modification, which shall be subject to JOC approval (such approval not to be unreasonably withheld or delayed) as described above.
2.3.3 Failure to Complete a Timeline Remediation Plan. If Nektar fails to complete a Clinical Trial Activity it is responsible for as outlined in an approved Timeline Remediation Plan, then SFJ has the right to withhold any payments due to Nektar pursuant to Section 4.2, suspend the SFJ Services and cease paying Approved Third Party Vendor Costs and incurring other Development Costs until the Clinical Trial Activity is completed, in which event SFJ will not be considered in breach of this Agreement for withholding any such amounts due to Nektar pursuant to this Section 2.3.3, suspending the HNC Clinical Trials, ceasing to pay Approved Third Party Costs and incurring other Development Costs. If SFJ fails to complete a Clinical Trial Activity it is responsible for as outlined in an approved Timeline Remediation Plan, then Nektar, at its sole discretion, may assume responsibility for completing such Clinical Trial Activity, in which event (a) SFJ shall reimburse Nektar for the costs incurred by Nektar in completing such Clinical Activity, and (b) such costs incurred by Nektar shall be included in actual Development Costs for purposes of Section 6.2, and (c) in no event shall any failure or delay by Nektar in performing any of its obligations hereunder that are dependent upon the completion of such Clinical Trial Activity constitute a breach of this Agreement, or entitle SFJ (i) to withhold any payments due to Nektar or other amounts SFJ is obligated to pay or incur pursuant to Section 4.2, (ii) to terminate this Agreement or (iii) to exercise any other remedy available to it under this Agreement.
2.4 Approved CROs and Approved Vendors.
2.4.1 Approved CROs. Except as otherwise provided herein, a Party may delegate any of its responsibilities described in Section 2.2 to (a) any of its Affiliates (subject to Section 14.1), (b) any CRO that is listed on Exhibit B or to SFJ Pharmaceuticals, Inc., or (c) any CRO that is identified by a Party from time to time during the Development Term (provided that prior to such delegation, such Party must provide notice to the JOC of the identity of the CRO to which it desires to delegate its responsibilities, the JOC shall discuss the same, and such Party shall take into reasonable consideration any comments from the JOC) (any Person listed on Exhibit B or any Person identified in accordance with clause (c) from time to time, an “Approved CRO”); provided that SFJ may not delegate any of its responsibilities to any CRO other than those identified on Exhibit B or to SFJ Pharmaceuticals, Inc. without the prior written consent of Nektar. Each Party will be required to enter into a written agreement with each Approved CRO utilized by such Party (each, a “CRO Agreement”) on commercially reasonable and customary terms, consistent with industry standards for similar agreements and sufficient to enable such Party to comply with its obligations hereunder with respect to the delegated responsibilities,
20
ACTIVE/105681617.22
including, but not limited to, Section 2.2.2, and the terms pertaining to ownership of Intellectual Property and publications, and treatment of Confidential Information.
2.4.2 Approved Vendors. Except with respect to manufacturing and supply of the Product as provided in Section 3.14.1, a Party will be permitted to contract for services, equipment, tools, materials and/or supplies required for the HNC Clinical Trials or Regulatory Approval with (a) any Person that is either listed on Exhibit C or (b) any Person that is identified by a Party from time to time during the Development Term (provided that prior to such delegation, such Party must provide notice to the JOC of the identity of the Person with which it desires to contract, the JOC shall discuss the same, and such Party shall take into reasonable consideration any comments from the JOC) (any Person listed on Exhibit C or any Person identified in accordance with clause (b) from time to time, an “Approved Vendor”); provided that SFJ may not contract for services, equipment, tools, materials and/or supplies required for the HNC Clinical Trials or Regulatory Approval with any Person other than those identified on Exhibit B and SFJ Pharmaceuticals, Inc. without the prior written consent of Nektar. Each Party will be required to enter into a written agreement with each Approved Vendor utilized by such Party (each, a “Vendor Agreement”) on commercially reasonable and customary terms, consistent with industry standards for similar agreements and sufficient to enable such Party to comply with its obligations hereunder with respect to the contracted activities, including, but not limited to, the terms pertaining to publications and ownership of Intellectual Property, and treatment of Confidential Information.
2.4.3 Responsibility. For clarity, each Party will remain responsible for all of its obligations under this Agreement, notwithstanding any delegation to an Affiliate or an Approved CRO or any contracting with an Approved Vendor. Each Party shall use Commercially Reasonable Efforts to oversee the services of its Affiliates and any Approved CRO or Approved Vendor it utilizes to provide services hereunder.
2.4.4 [***]
2.5 [***] Reasonable Assistance.
2.5.1 [***]
2.5.2 Questions Pertaining to the HNC Clinical Trials. Promptly following the Effective Date during the Development Term, Nektar will identify one (1) individual with knowledge of the HNC Clinical Trials Protocol, the Product and the Merck Product who will be made available at reasonable times during normal business hours in such employee’s country of residence upon reasonable advance notice to answer SFJ’s questions directly pertaining to such Protocol.
ARTICLE 3
CLINICAL TRIALS ACTIVITIES, REGULATORY APPROVAL AND RESPONSIBILITIES
21
ACTIVE/105681617.22
3.1 Parties’ Roles and Responsibilities.
3.1.1 Nektar Responsibilities.
3.1.1.1 Nektar will have sole responsibility for the Melanoma Clinical Trial and the clinical trials for all Other Indications.
3.1.1.2 In regards to the HNC Clinical Trials, Nektar will have primary responsibility for all Nektar Services, provided that SFJ will provide operational support for and assist with these activities as specified on Exhibit H.
3.1.1.3 If the HNC Main Clinical Trial meets the HNC Clinical Trial Success Criteria, Nektar shall submit or cause to be submitted a BLA with the FDA and use Commercially Reasonable Efforts to do so [***] of the data read-out indicating that the Clinical Success Criteria were achieved for the HNC Indication, and will use Commercially Reasonable Efforts to perform all activities associated with submitting BLAs and seeking Regulatory Approval for the HNC Indication in the US.
3.1.1.4 If the Melanoma Clinical Trial meets the Melanoma Clinical Trial Success Criteria, Nektar shall (a) submit, cause of its controlled Affiliates to submit, or use Commercially Reasonable Efforts to cause BMS to submit a BLA with the FDA, (b) use Commercially Reasonable Efforts to do so (and cause BMS to use Commercially Reasonable Efforts (as that term is defined in the BMS Strategic Collaboration Agreement) to do so) [***] of the data read-out indicating that the Melanoma Clinical Success Criteria were achieved for the Melanoma Indication, and (c) will use Commercially Reasonable Efforts to perform or cause BMS to use Commercially Reasonable Efforts (as that term is defined in the BMS Strategic Collaboration Agreement) to perform all activities associated with submitting BLAs and seeking Regulatory Approval for the Melanoma Indication in the US.
3.1.1.5 If Nektar terminates the BMS Collaboration Agreement as a result of an uncured material breach by BMS under Section 16.2 of the BMS Collaboration Agreement, and the Melanoma Trial is transitioned to Nektar pursuant to Section 16.6(b)(i) of the BMS Collaboration Agreement, then Nektar must use Commercially Reasonable Efforts to (a) complete the Melanoma Trial and (b) if the Melanoma Trial meets the Success Criteria, file a BLA with FDA.
3.1.1.6 Following the achievement of Regulatory Approval of the Product in any country, Nektar shall use Commercially Reasonable Efforts to commercialize the Product in such country for the applicable Indication.
3.1.2 SFJ Responsibilities. SFJ will have primary responsibility for conducting the HNC Clinical Trials globally. Subject to the terms hereof, SFJ will use Commercially Reasonable Efforts to conduct, or ensure that the applicable Approved CRO conducts, the HNC Clinical Trials in accordance with SFJ’s standard operating procedures (the “SFJ SOPs”) that will be provided to Nektar [***] following the selection of the Approved CRO for Nektar’s review and comment, as well as CRO SOPs, to the extent applicable, and SFJ will reasonably
22
ACTIVE/105681617.22
consider incorporating such comments. Following the Effective Date, SFJ may amend any SFJ SOPs; provided that with respect to material amendments to SFJ SOPs that pertain to Clinical Trial Activities and/or other obligations that are, or will be, performed by SFJ or any Permitted Third Party utilized by SFJ during the remainder of the Term or any time thereafter as set forth in this Agreement, SFJ will provide the JOC with a copy of each such amendment to permit the JOC Representatives to review and comment on such amendments and SFJ will reasonably consider incorporating such comments.
3.1.3 Compliance. Each Party will conduct its portion of the Development Program and perform all other of its duties and responsibilities hereunder in accordance with the HNC Main Clinical Trial Protocol and any other approved Additional HNC Clinical Trial Protocols and in material compliance with all Applicable Laws. Nektar will use Commercially Reasonable Efforts to oversee the Manufacture of the Product, and Nektar will materially comply, and Nektar will require that all Permitted Third Parties of Nektar materially comply, with all Applicable Laws with respect to the analysis, storage, handling, disposal and transfer of the Product. SFJ will materially comply, and SFJ will require that all Permitted Third Parties of SFJ materially comply, with all Applicable Laws with respect to the storage, handling, disposal and transfer of all quantities of Product and Merck Product supplied by or on behalf of Nektar for use in the conduct of HNC Clinical Trials.
3.1.4 Nektar SOPs. Subject to the terms hereof, Nektar will, use Commercially Reasonable Efforts to conduct, or ensure that the applicable Approved CRO conducts, the Melanoma and Other Clinical Trials in accordance with Nektar’s standard operating procedures (the “Nektar SOPs”). Nektar will use Commercially Reasonable Efforts to conduct its Clinical Trial Activities on the HNC Clinical Trials in accordance with Nektar SOPs that will be provided to SFJ [***] following the Effective Date for SFJ’s review and comment. Following the Effective Date, Nektar may amend any Nektar SOPs; provided that with respect to material amendments to Nektar SOPs that pertain to Clinical Trial Activities and/or other obligations that are, or will be, performed by Nektar or any Permitted Third Party utilized by Nektar during the remainder of the Term or any time thereafter as set forth in this Agreement, Nektar will provide the JOC with a copy of each such amendment to permit the JOC Representatives to review and comment on such amendments and Nektar will reasonably consider incorporating such comments.
3.2 Sites and Clinical Investigators.
3.2.1 Selection of Sites and Investigators.
3.2.1.1 SFJ will select the study sites to conduct the HNC Clinical Trials and will inform the JOC in advance of SFJ’s choice of each study site; the JOC will have the right to reject any such site(s) which the JOC will determine in its reasonable judgment are not appropriate. The study sites for the HNC Clinical Trials will be located in one or more of the countries listed on Exhibit J.
3.2.1.2 SFJ will enter, and will ensure that its Affiliates enter, and each Approved CRO will enter, into an agreement with each study site; such an agreement will
23
ACTIVE/105681617.22
be substantially in the form agreed upon by the Parties [***] following approval by the JOC of the final Protocol (the “Clinical Trial Agreement”) (upon execution of such Clinical Trial Agreement, such study site will be deemed a “Site”). If a study site requires any material changes to such form Clinical Trial Agreement, SFJ will inform the JOC and seek JOC approval of such change, and the JOC will not unreasonably withhold such approval. For clarity, each Clinical Trial Agreement will be on commercially reasonable and customary terms, consistent with industry standards for similar agreements and sufficient to enable SFJ to comply with its obligations hereunder with respect to such HNC Clinical Trial, including, but not limited to, Section 2.2.2, the terms pertaining to ownership of Intellectual Property and publications, and treatment of Confidential Information.
3.2.2 Obligations During the Clinical Trials Conduct.
3.2.2.1 During the Development Term, SFJ will conduct meetings with the Clinical Investigators (each, a “Clinical Investigator Meeting”), of which the JOC will be provided with reasonable advance notice and in which Nektar will have the right (but not the obligation) to attend and participate. Minutes of Clinical Investigator Meetings will be made available to the JOC upon request.
3.2.2.2 SFJ will provide the JOC with copies of all communications relevant to the HNC Clinical Trials and provided to all Sites, and upon request of the JOC, provide the JOC with copies of any other communications between SFJ and any individual Sites and/or any Affiliate or Approved CRO and any individual Sites.
3.2.2.3 If SFJ terminates a Site, SFJ will inform the JOC with the reason for such termination and if reasonably practicable, such notice will be provided reasonably in advance of such termination.
3.2.2.4 Nektar will be responsible for preparing and submitting any INDs and amendments thereto to Regulatory Authorities as required by Applicable Laws in the countries for which Sites have been selected, [***]. Nektar will prepare the CMC Information and any updates to this information and submit it to the applicable Regulatory Authority as required by Applicable Laws. [***].
3.2.3 Transparency Reports. [***], SFJ will provide Nektar access to all records of SFJ related to any direct or indirect payments or other transfers of value made by or on behalf of SFJ (such as by a CRO or other vendor) to health care professionals, IDEC members, consultants or others in connection with the HNC Clinical Trials, which payments or other transfers of value, including all such details as are reasonably required [***] to determine whether any such payment requires the filing of a report with any Governmental Authority [***] pursuant to Applicable Law (such as the Sunshine Act in the United States and similar transparency laws in foreign countries). In the event that Nektar is or becomes aware that any such reports are required to be filed, Nektar shall provide prompt written notice to SFJ providing particulars as to the information to be reported and the time deadlines for the filing of reports.
24
ACTIVE/105681617.22
3.3 Subjects and Informed Consent.
3.3.1 Subject Recruitment Plan. SFJ will comply with the subject recruitment plan for the HNC Clinical Trials, which will be established by SFJ and communicated to the JOC, for approval by the JOC not to be unreasonably withheld, within a reasonable period of time after the Effective Date not to [***] of the Effective Date (the “Subject Recruitment Plan”) in recruiting subjects to participate in the HNC Clinical Trials. For clarity, prior to engaging in any recruiting activities, SFJ will ensure that the applicable IRBs and/or other ethics committees approve any related materials and activities as required by the JOC and all Applicable Laws.
3.3.2 Informed Consent.
3.3.2.1 SFJ, with consultation from Nektar, will prepare the informed consent document(s) for use in the HNC Clinical Trials. SFJ will ensure that the informed consent of each subject participating in a HNC Clinical Trial be obtained in accordance with all Applicable Laws, including completion of the informed consent document. Such informed consent document for a HNC Clinical Trial will be substantially in the form to be approved by the JOC [***] following approval by the JOC of the final Protocol for such HNC Clinical Trial (collectively, “Informed Consent”) (upon obtaining such Informed Consent, a prospective subject will be deemed a “Subject”). For clarity, the Informed Consent document that each Subject signs will expressly state that each Subject understands that Nektar is the Sponsor and SFJ is conducting the HNC Clinical Trials and will authorize disclosure of data and results related to the HNC Clinical Trials to Nektar and SFJ, for any purpose, subject to all Applicable Laws.
3.3.2.2 SFJ will ensure that the Informed Consent has been obtained by a Permitted Third Party from each Subject prior to administration of the Product and/or Merck Product to such Subject in accordance with the HNC Clinical Trials Protocol.
3.3.3 Inclusion and Exclusion Criteria. SFJ will not waive, and SFJ will require that its Permitted Third Parties do not waive, any exclusion or inclusion criteria specified in the HNC Clinical Trials Protocol.
3.4 Investigator’s Brochure.
3.4.1 Investigator’s Brochure. Nektar will maintain the Investigator’s Brochure for the Product. SFJ will, promptly following receipt of written notice from Nektar of the need for an Investigator’s Brochure update, provide Nektar with all information regarding the HNC Clinical Trials that is necessary to enable Nektar to update the Investigator’s Brochure.
3.4.2 Parties’ Responsibilities. Promptly following the Effective Date, Nektar will provide SFJ with the most recent version of the Investigator’s Brochure. Nektar will also promptly provide SFJ with any updated versions of the Investigator’s Brochure. SFJ will ensure that each Site and all applicable IRBs and other ethics committees receive a copy of, and promptly receive any updates to, the Investigator’s Brochure.
25
ACTIVE/105681617.22
3.5 Data Collection and Data Management.
3.5.1 CRF. Nektar, with consultation from SFJ, will be responsible for preparing the form of CRF for the HNC Clinical Trials in accordance with the HNC Clinical Trials Protocol and Nektar’s CRF standards.
3.5.2 Data Management Plan.
3.5.2.1 SFJ, with consultation from Nektar, will be responsible for preparing the data management plan (the “Data Management Plan”) for the HNC Clinical Trials. Each Party will use Commercially Reasonable Efforts to comply with the Data Management Plan to be agreed upon by the Parties [***] following approval by the JOC of the final Protocol. For clarity, the Data Management Plan will be agreed upon by the Parties prior to recruitment of subjects for the HNC Clinical Trials.
3.5.2.2 With respect to any data collected in connection with the HNC Clinical Trials, SFJ will ensure that such data is held in one or more appropriate facilities with information security protections in accordance with all Applicable Laws including (a) unique accounts for all operators; (b) cancellation of an account when an employee or other personnel terminates employment; (c) deactivation of an account when an employee or other personnel ceases working on the Clinical Trials; (d) required password changes at frequent intervals; and (e) regular backups of electronic data.
3.5.3 Clinical Trials Database.
3.5.3.1 Nektar, with consultation from SFJ, will use Commercially Reasonable Efforts to establish a Clinical Trials database for the data collected from each Site for the HNC Clinical Trials (the “Clinical Trials Database”) [***] following approval by the JOC of the Final HNC Clinical Trial Protocol. Nektar, with consultation from SFJ, will promptly update the Clinical Trials Database upon any final amendments to the Protocol, as necessary, and in accordance with Section 3.5.3.3. SFJ will promptly update the Clinical Trials Database upon receiving data for the HNC Clinical Trials from any Site and any other applicable Permitted Third Party, and SFJ will ensure that the Sites and such other Permitted Third Parties promptly following collection thereof, provide data in connection with the HNC Clinical Trials to such Party.
3.5.3.2 SFJ will provide the JOC with electronic copies of such data requested by the JOC at JOC meetings and in accordance with Applicable Laws.
3.5.3.3 If, at any time during the Development Term, SFJ decides to change the format of the database for the HNC Clinical Trials, SFJ will so notify the JOC and the Parties will cooperate to ensure that the format that SFJ selects permits SFJ to incorporate the data from the HNC Clinical Trials into its relevant systems and is in compliance with all Applicable Laws.
26
ACTIVE/105681617.22
3.5.3.4 The Vendor responsible for the database will provide SAS datasets to the Parties in accordance with specifications as defined by Nektar [***].
3.5.3.5 SFJ will ensure that information included in the Clinical Trials Database is accurate and up-to-date. Nektar will be responsible for registering, maintaining and updating any registries pertaining to the HNC Clinical Trials to the extent required by any Applicable Laws, including ▇▇▇.▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇, ▇▇▇.▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇▇.▇▇▇, and the PHRMA Website Synopsis.
3.5.4 Clinical Trials Master File. Promptly following the Effective Date, SFJ will establish and maintain a Clinical Trials master file for each Clinical Trial in the format as agreed upon by the JOC (each, a “Clinical Trials Master File”). Notwithstanding anything to the contrary herein, SFJ will not be permitted to delegate its rights and obligations pursuant to this Section 3.5.4 to any Permitted Third Parties without the prior approval of the JOC, except SFJ may delegate its rights and obligations pursuant to this Section 3.5.4 to any of its Affiliates.
3.5.5 Source Data Verification. SFJ will be responsible for source verification of data records. At Nektar’s request, SFJ will provide Nektar with copies of any reports relating to source data verification and other types of HNC Clinical Trials audits.
3.5.6 Statistical Analysis. Nektar statisticians will work with SFJ programmers to perform any statistical analysis required in accordance with the statistical analysis plan (“Statistical Analysis Plan”) for the HNC Clinical Trials. The Statistical Analysis Plan will initially be developed by Nektar and agreed upon by the Parties [***].
3.6 Audits.
3.6.1 SFJ will conduct quality oversight inspections and audits of the facilities and services of the Permitted Third Parties utilized by SFJ in accordance with its standard operating procedures and will provide Nektar with copies of such audit reports upon request.
3.6.2 During the Development Term, Nektar will conduct quality oversight inspections and audits of the manufacturing facilities for the Product in accordance with its internal policies and Nektar will provide SFJ with copies of such audit reports, subject to SFJ’s compliance with confidentiality requirements imposed by Nektar’s contract manufacturers.
3.6.3 Audits; Inspections.
3.6.3.1 Upon [***] prior written notice and during normal business hours, no more frequently than once per Calendar Year (the “Annual Limitation”), Nektar, or an external auditor appointed by Nektar and reasonably acceptable to SFJ, may inspect and/or audit all records created by SFJ and its Affiliates in the conduct of the HNC Clinical Trials for the purpose of confirming SFJ’s compliance with this Agreement and Applicable Laws in the conduct of the HNC Clinical Trials. SFJ agrees to make available employees of SFJ and its Affiliates as reasonably requested by Nektar to answer Nektar’s reasonable questions in connection with such inspection and/or audit.
27
ACTIVE/105681617.22
3.6.3.2 Notwithstanding the foregoing, if Nektar has a reasonable good faith belief that SFJ has materially failed to comply with this Agreement or with Applicable Laws in the conduct of the HNC Clinical Trials, Nektar shall have the right to conduct an audit and/or inspection described in 3.6.3.1 upon [***] prior written notice and without regard to the Annual Limitation.
3.6.3.3 All expenses of any inspection or audit requested by Nektar pursuant to Section 3.6.3 (including the fees and expenses of any external auditor engaged by Nektar for such purpose) shall be borne by Nektar. All information obtained by Nektar as a result of such inspection or audit shall be Confidential Information subject to Article 9. Any external auditor appointed by Nektar pursuant to this Section 3.6.3 shall be subject to confidentiality obligations no less restrictive than those set forth in Article 9 with respect to such Confidential Information.
3.7 Monitoring. SFJ will monitor the HNC Clinical Trials, and share information with the JOC pertaining to monitoring the HNC Clinical Trials, in accordance with the monitoring plan for the Clinical Trials to be agreed upon by the Parties [***] following the Effective Date.
3.8 IRBs and Other Ethics Committees.
3.8.1 SFJ will be responsible for obtaining the approval of the IRBs and other ethics committees required prior to commencing, and during, the HNC Clinical Trials at every Site.
3.8.2 SFJ will ensure that IRBs and such other relevant ethics committees have current registrations and accreditations as required by Applicable Law and will provide all ethics committees, including all IRBs, and Regulatory Authorities, with all necessary documentation prior to, and during the course of, the HNC Clinical Trials as required by Applicable Law.
3.8.3 SFJ will be responsible for responding to all queries from the IRBs and other ethics committees; provided that (a) Nektar will make itself reasonably available to assist with any such queries and (b) if such query relates solely to the CMC Information, the Manufacturing Dossier, and/or preclinical studies, Nektar will prepare the applicable response and provide SFJ with a copy thereof.
3.9 IDMC.
3.9.1 Nektar, in consultation with SFJ, will establish an IDMC for the Clinical Trials, which will be governed by a charter substantially in the form to be agreed upon by the Parties [***] of the Effective Date (the “IDMC Charter”). For clarity, the IDMC Charter will specify the number of members of the IDMC, which shall be appointed by Nektar, the qualifications of such members, the roles and responsibilities of IDMC members, the role and responsibilities of the IDMC chair, who shall be appointed from among the IDMC members, independent statistical center (“ISC”), the study team, the sponsor executive committee, rules of
28
ACTIVE/105681617.22
conduct of and procedures for IDMC meetings including open sessions and closed sessions, communication of IDMC recommendations, and other subjects mutually agreed by the Parties.
3.9.2 Nektar, via the ISC, will ensure that the IDMC is provided with all required information and data in a timely manner as specified in the IDMC Charter, and SFJ will reasonably cooperate with Nektar in such regard.
3.10 Environmental Health and Safety.
3.10.1 In conducting the Clinical Trials, each Party will comply with all Applicable Laws relating to environmental, health and/or safety matters and will be solely responsible for establishing material and specimen handling guidelines and for ensuring use of controls, including appropriate personal protective equipment, that minimize potential worker exposure, obtaining the material safety data sheets and providing the appropriate training for workers who will be potentially exposed to the Product.
3.10.2 Each Party will promptly notify the JOC, in writing, of any worker claims of suspected occupational illnesses related to working with the Product, regardless of whether such claims are received during the Development Term or any time thereafter. After termination of this Agreement for whatever reasons, or expiration of this Agreement, each Party will promptly notify the other Party of any worker claims of suspected occupational illnesses related to working with the Product during the Development Term, of which it has Knowledge.
3.11 Completion of the Clinical Trials.
3.11.1 SFJ will use Commercially Reasonable Efforts to keep the Sites participating in the HNC Clinical Trials, operational, including continuing to dose Subjects with the Product and/or Merck Product in accordance with the Protocol, and conducting any follow-up work required, until the Completion Date for such HNC Clinical Trial. As a HNC Clinical Trial is completed or otherwise terminated at each Site, SFJ will close out such HNC Clinical Trial as specified in the Protocol, including performing all Subject follow-up and providing Nektar with all HNC Clinical Trial data not provided as of such date. For clarity, copies of documents, including any CRFs and the Clinical Trials Master File will be transferred to Nektar upon the completion of the HNC Clinical Trials and at Nektar’s request, or at Nektar’s option, destroyed (provided that such destruction is in compliance with ICH guidelines). Notwithstanding the foregoing, SFJ will not provide Nektar with any Personally Identifiable Information.
3.11.2 Upon the Completion Date of a HNC Clinical Trial, SFJ will return to the location specified by Nektar at such time, or, at Nektar option, destroy, any unused Product and/or Merck Product from such HNC Clinical Trial (SFJ’s expenses in doing so will be included in Development Costs), and will comply with all Applicable Laws in so returning or destroying such Product.
3.11.3 The CSR, and any interim CSRs, for each HNC Clinical Trial will be prepared by Nektar, with support from SFJ, in compliance with all Applicable Laws, including
29
ACTIVE/105681617.22
ICH E3 guidelines. The final, signed CSR for each HNC Clinical Trial (the “Final HNC Clinical Trial CSR”) will be provided to SFJ promptly following the Completion Date of the HNC Clinical Trials and any interim CSRs will be provided to SFJ promptly after it is completed. In the event that there are any additional safety or efficacy data pertaining to a HNC Clinical Trial that come into the possession of Nektar after it has provided SFJ with the Final HNC Clinical Trial CSR for such HNC Clinical Trial, Nektar will prepare and promptly provide SFJ with a supplement to such CSR.
3.12 Commercially Reasonable Efforts.
3.12.1 Timely performance of the Clinical Trials and receipt of Regulatory Approvals are important to the success of this Agreement. Each Party will use Commercially Reasonable Efforts to complete the Melanoma and HNC Clinical Trials, as appropriate, according to the Timeline and, if the Melanoma and/or HNC Clinical Trials are successful, Nektar shall comply with the provisions of Sections 3.1.1.3, 3.1.1.4 and 3.1.1.5 hereof. In the event that either Party fails to complete its responsibilities according to the Timeline, following discussion by the JSC that such Party failed to use Commercially Reasonable Efforts, the other Party may assume the roles and responsibilities of such Party; provided that in the event of such failure by SFJ and the assumption by Nektar of any of SFJ’s roles or responsibilities, SFJ will remain obligated to pay Development Costs under Section 4.2, and provided further that in the event of such failure by Nektar and the assumption by SFJ of any of Nektar’s roles or responsibilities, SFJ’s costs for the assumption of Nektar’s roles and responsibilities shall be deemed to be Development Costs. In the event that Nektar fails to use Commercially Reasonable Efforts to so obtain Regulatory Approval for the Product for the Indications, including the obligation to file a BLA for the Product for the Indications with the FDA by the dates set forth in Article 3, and this failure is not cured [***] after receipt of written notice from SFJ requesting such cure, SFJ may terminate this Agreement pursuant to Section 13.2.1.
3.12.2 Regulatory Approvals. The Parties acknowledge that regulatory matters with respect to the Product will reasonably require coordination with regulatory matters with respect to the Product, and SFJ agrees to cooperate in good faith with Nektar and Merck as reasonably necessary for and in relation to each of Nektar and SFJ, on the one hand, and Merck, on the other hand, to obtain and maintain regulatory approvals (including Regulatory Approvals) with respect to the Product in the case of Nektar and SFJ and with respect to the Merck Product in the case of Merck. Prior to submitting any written or electronic communication to a Regulatory Authority in a particular country with respect to Merck Product that would reasonably be expected to require a change to the Regulatory Authorityapproved full prescribing information for the Product for such country, SFJ shall cooperate with Nektar in Nektar’s consultation with Merck. Nektar shall keep SFJ reasonably informed of its efforts to obtain and maintain Regulatory Approvals and any Other Indication Regulatory Approvals and developments with respect thereto, including Nektar’s expected timing with respect to submission and receipt of any and all Regulatory Approvals and Other Indication Regulatory Approvals.
3.13 Pharmacovigilance and Safety Information Exchange.
30
ACTIVE/105681617.22
3.13.1 The safety reporting units from each of the Parties shall meet and shall [***] of the Effective Date agree upon a written agreement for exchanging adverse event and other safety information relating to the Product (the “Pharmacovigilance Agreement”). SFJ agrees to provide Nektar with a draft of the Pharmacovigilance Agreement [***] of the Effective Date. The Pharmacovigilance Agreement will ensure that adverse event and other safety information are exchanged upon terms that will permit (a) Nektar to comply with Section 10.8 of the BMS Strategic Collaboration Agreement and the terms of the BMS Pharmacovigilance Agreement, and (b) each Party to comply with Applicable Laws and requirements of Regulatory Authorities. In particular the Pharmacovigilance will include: (i) Nektar’s responsibility for establishing and maintaining the Clinical Trials Database pursuant to Section 3.5.3 of this Agreement, and (ii) Nektar’s responsibility for establishing and maintaining the Global Drug Safety Database (“ARGUS”) and reconciling information between ARGUS and the Clinical Trials Database
3.13.2 SFJ agrees not to enter into any clinical activity implicating pharmacovigilance obligations for the Product prior to execution of the Pharmacovigilance Agreement.
3.14 Product.
3.14.1 Supply of the Product.
3.14.1.1 Nektar will be the GMP Manufacturer of the Product for the Clinical Trials, either directly or through a CMO. In particular, with respect to the Clinical Trials, Nektar will maintain in force a clinical supply agreement with a CMO that has sufficient capacity to manufacture and supply GMP-compliant Product for the HNC Clinical Trials in a timely manner in accordance with a clinical supply schedule approved by the JOC (as amended by the JOC from time to time, the “Clinical Supply Schedule”).
3.14.1.2 During the Development Term, Nektar will supply, as determined by the JOC, or cause to be supplied, as determined by the JOC, to SFJ GMP-compliant Product and Merck Product manufactured in compliance with the then-current CMC Information included in the IND submitted to the applicable Regulatory Authority for the HNC Clinical Trials, in accordance with the Clinical Supply Schedule as set forth in a clinical supply agreement to be entered into between the Parties [***] after the Effective Date. The costs for the supply of the Product for the HNC Clinical Trials and the costs for the supply of the Merck Product for the HNC Clinical Trials will be borne by Nektar. SFJ will provide the JOC at each JOC meeting with quarterly reports regarding inventory of the Product and Merck Product and the reasonably anticipated needs for the Product and Merck Product to ensure that Nektar can supply the Product and Merck Product in accordance with the Clinical Supply Schedule.
3.14.2 Use of the Product.
3.14.2.1 SFJ will (a) in conducting the HNC Clinical Trials, only use Product and Merck Product supplied by Nektar or such Third Parties designated by Nektar; (b) only use the Product and Merck Product supplied by Nektar or Third Parties designated by
31
ACTIVE/105681617.22
Nektar, and require that its Permitted Third Parties that receive any of the Product and Merck Product supplied by Nektar or Third Parties designated by Nektar only use such Product and Merck Product, for the sole purpose of conducting the HNC Clinical Trials in accordance with the respective HNC Clinical Trials Protocols; and (c) ensure subject dosing compliance per the respective HNC Clinical Trials Protocols for the HNC Clinical Trials. Dosage and Administration Instructions will be provided to SFJ by Nektar sufficiently in advance of the HNC Clinical Trials’ commencement.
3.14.2.2 SFJ, will be responsible for ensuring that the Product and Merck Product is administered solely to the Subjects in HNC Clinical Trials conducted by SFJ in accordance with the respective Protocols. For each dose administered to a Subject in a HNC Clinical Trial conducted by SFJ, SFJ will implement procedures and ensure that records are maintained specifying the date and time that such dose of the Product and/or Merck Product is administered, the amount of the Product and/or Merck Product administered to such Subject, the lot number of the Product and/or Merck Product from which such dosage came, and the number of the Subject to which such dosage was administered. SFJ shall provide copies of such records to Nektar upon Nektar’s reasonable request.
3.15 Complaints Related to the Product. During the Development Term, SFJ will promptly forward to Nektar any complaints that it receives related to the Product and/or Merck Product. SFJ will respond to any complaints of which SFJ becomes aware relating to the Product and/or Merck Product provided that Nektar will provide reasonable cooperation in connection therewith. Notwithstanding the foregoing, if a complaint pertains to the manufacturing, appearance or general physical characteristics of the Product and/or Merck Product or other processes at the manufacturing facility, Nektar will be solely responsible for responding to such complaint.
3.16 Recall of the Product in Connection with Study Prior to Approval. If the Product and/or Merck Product is recalled for safety reasons or GMP noncompliance prior to Regulatory Approval, SFJ will be responsible for the operational execution of such recall. Nektar will cooperate with SFJ in connection with any such recall. The costs for such any such recall will be at Nektar’s expense and not be a Development Cost, unless such recall and/or costs were based on the material breach of this Agreement, intentional misconduct, or gross negligence of SFJ or any of its Affiliates or Permitted Third Parties, in which case, SFJ will bear the expense of any such recall and such expense will not be a Development Cost.
3.17 Compliance with Laws. SFJ and its Affiliates and Nektar and its Affiliates will comply, and each Party will use Commercially Reasonable Efforts to ensure that all Permitted Third Parties utilized by such Party comply, with all Applicable Laws with respect to the storage, handling, disposal and transfer of the Product and Merck Product, and each Party assumes sole responsibility for the violation of such Applicable Laws by such Party or any of its Affiliates or its Permitted Third Parties.
3.18 Disclosures.
3.18.1 [***]
32
ACTIVE/105681617.22
3.18.2 Nektar shall (a) promptly notify SFJ of achieving the Clinical Trial Success Criteria, and (b) promptly notify SFJ of achieving Regulatory Approval in the Indications and the first Other Indication. [***].
3.19 Exclusivity Commitment of SFJ. During the applicable Exclusive Period, SFJ shall not, and shall cause its Affiliates not to, either by itself or through a Third Party, conduct human clinical trials of, or sell, offer for sale or have sold:
3.19.1 any Competing Product (other than Product) alone or in combination (whether fixed dose or co-packaged) with one (1) or more other active ingredients; or
3.19.2 any combination (whether fixed dose or co-packaged) with one (1) or more other active ingredients of the Product and a Competing Product.
ARTICLE 4
DEVELOPMENT COSTS
4.1 Development Costs. SFJ will be obligated to pay up to One Hundred Fifty Million U.S. Dollars ($150,000,000.00) of Development Costs (the “Maximum Development Costs”) in accordance with Section 4.2. Any Development Costs in excess of the Maximum Development Costs (the “Excess Development Costs”) will be borne by Nektar unless otherwise agreed in writing by SFJ and Nektar, and provided that Nektar may request that SFJ pay some or all of the Excess Development Costs and SFJ shall have the sole discretion to decide whether or not to pay any of such Excess Development Costs, and provided further that the failure of SFJ to agree to pay any Excess Development Costs shall not excuse Nektar from its obligation to pay the Excess Development Costs. Any failure by Nektar to bear any necessary Excess Development Costs that SFJ did not agree to pay shall be deemed to be a material breach of this Agreement by Nektar.
4.2 Funding Schedule; Quarterly Reconciliation of Development Costs.
4.2.1 SFJ will pay up to a total of One Hundred Fifty Million U.S. Dollars ($150,000,000.00) of Development Costs as set forth as follows:
4.2.1.1 SFJ shall promptly pay all Approved Third Party Vendor Costs incurred by SFJ in connection with the HNC Clinical Trials during the Development Term.
4.2.1.2 Development Costs will include the Quarterly Labor Fees. Payment by SFJ to Nektar of the Quarterly Labor Fees shall be made on a quarterly basis, with each payment made at least fifteen days prior to the end of the Calendar Quarter to which it relates.
4.2.1.3 If (a) the Development Costs paid by SFJ after paying all required payments under the preceding provisions of this Section 4.2.1 shall be less than the Maximum Development Costs and (b) the HNC Clinical Trial has not terminated prior to
33
ACTIVE/105681617.22
completion due to the occurrence of HNC Clinical Trial Futility or a Safety Concern, then any remaining balance (such amount, the “Leftover Funding”) shall be paid to Nektar by SFJ [***] of the last payment under Section 4.2.1.1 and Section 4.2.1.2, to be used by Nektar for commercialization activities, and such amount paid by SFJ shall be deemed to be included in Development Costs, it being understood that the provisions of this Section 4.2.1.3 shall not apply as a result of or during any period in which SFJ’s obligations to make payments to Nektar or pay or incur other Development Costs are suspended pursuant to Section 2.3.1. If the HNC Clinical Trial is completed, but the HNC Clinical Trial Success Criteria is not achieved, SFJ shall not have the option of paying the Leftover Funding, if any, to Nektar.
4.2.2 Notwithstanding anything else contained herein to the contrary, in no event shall SFJ be required to pay or incur Development Costs in excess of $150 million. If the Development Costs exceed $150 million, Nektar shall pay or incur all such excess Development Costs including SFJ’s ongoing internal costs and continuing to provide the Nektar Services at the expense of Nektar unless otherwise agreed to in writing by SFJ. Notwithstanding anything else contained herein to the contrary, if the HNC Clinical Trial is terminated prior to completion due to the occurrence of (a) HNC Clinical Trial Futility or (b) a Safety Concern, then SFJ shall not pay the Leftover Funding to Nektar nor shall it pay any other Development Costs except for those incurred in the orderly close-out of the HNC Clinical Trials.
4.2.3 Development Costs initially shall be borne by SFJ as provided herein. Each Party shall comply with the Development Cost Reconciliation Procedures. With respect to each HNC Clinical Trial, each Party will provide the other Party, [***] of the end of each Calendar Quarter, the estimated Development Costs incurred in accordance with GAAP during such Calendar Quarter by such Party. A final report of actual Development Costs incurred in accordance with GAAP during such Calendar Quarter by such Party will be provided [***] of the end of each Calendar Quarter. Such report shall specify in reasonable detail all expenses included in such Development Costs during such Calendar Quarter. If reasonably requested by the other Party, each Party shall make every reasonable attempt to accommodate the request to provide copies of any applicable invoices within the Development Costs that individually exceed one million dollars ($1,000,000.00).
4.3 PreCommercialization Costs. During the Term, Nektar will be solely responsible at its own cost (subject to Sections 4.2) for performing those activities reasonably necessary to prepare for Commercial Launch of the Product (the “Pre-Approval Commercialization Activities”). Such Pre-Approval Commercialization Activities may include at Nektar’s sole discretion creating educational or marketing materials, establishing distribution channels and designing packaging and labeling, in each case as reasonably necessary to Commercialize the Product.
ARTICLE 5
GOVERNANCE
34
ACTIVE/105681617.22
5.1 Joint Steering Committee.
5.1.1 Representatives. Within sixty (60) days after the Effective Date, the Parties will establish a joint steering committee to oversee and manage the collaboration (the “JSC”). Each Party initially will appoint three (3) individuals to serve as representatives to the JSC (the “JSC Representatives”), with each JSC Representative having knowledge and expertise regarding developing products similar to the Product and sufficient decision-making authority within the applicable Party to make decisions on behalf of such Party within the scope of the JSC’s decisionmaking authority and, if any such individual is not an employee of the appointing Party, such individual shall execute a confidentiality agreement in form and substance acceptable to the other Party (and, for the avoidance of doubt, the appointing Party shall remain responsible to the other Party for any noncompliance by such individual with such confidentiality obligations). Each Party may replace its JSC Representatives at any time upon written notice to the other Party, which notice may be provided by email from one Party’s Alliance Manager to the other Party’s Alliance Manager.
5.1.2 Chairperson. The JSC chairperson (“JSC Chairperson”) shall be designated from the Parties’ JSC Representatives and shall serve for a term of one (1) year. SFJ shall appoint the first JSC Chairperson and subsequent appointments will rotate on an annual basis between Nektar and SFJ. The JSC Chairperson will be responsible for drafting and circulating the draft agenda and ensuring minutes are prepared.
5.1.3 Meetings. From the Effective Date, through the Development Term the JSC will meet at least once every two months (and for clarity, such meetings are intended to be conducted via teleconference) unless the Parties mutually agree otherwise. Either Party may call a special meeting of the JSC (by videoconference or teleconference) during the Term by providing at least five (5) Business Days prior written notice to the other Party, which notice shall include a reasonably detailed description of the matter, in the event such Party reasonably believes that a significant matter must be addressed prior to the next scheduled meeting.
5.1.4 Participants. The JSC may invite individuals who are not JSC Representatives to participate in JSC meetings; provided that (a) all JSC Representatives of both Parties consent to such non-member’s participation; and (b) such non-member has executed a confidentiality agreement in form and substance acceptable to the non-inviting Party (and, for the avoidance of doubt, the inviting Party shall remain responsible to the non-inviting Party for any noncompliance by such individual with such confidentiality obligations). For clarity, such non-members will have no voting rights at the JSC.
5.1.5 Alliance Managers. Each Party shall appoint an individual to act as an alliance manager for such Party (each, an “Alliance Manager”) by providing the name and contact information for the Alliance Manager to the JSC. Each Party may change its Alliance Manager from time to time in its sole discretion upon written notice to the JSC. The Alliance Managers shall be the primary point of contact for the Parties regarding the activities contemplated by the Agreement, and the Parties shall use reasonable efforts to ensure that any requests for information and data made outside of the JSC are made through the Alliance
35
ACTIVE/105681617.22
Managers. The Alliance Managers shall attend all meetings of the JSC. For clarity, the Alliance Managers may also be members of the JSC.
5.1.6 Costs. Each Party will bear its own expenses relating to the meetings and activities of the JSC.
5.2 JSC Responsibilities and Decision-Making.
5.2.1 Responsibilities (Review and Discuss). The JSC’s responsibilities will include reviewing and discussing (but not approving) the following:
5.2.1.1 Oversight of the Parties’ collaboration including (a) overall strategic direction, (b) developing strategies to maximize the value of the Product for the Indication, and (c) reviewing and commenting on the Development Program and Regulatory Approval strategies;
5.2.1.2 material changes in the Development Program, including changes required by, or made to respond to comments from, a Regulatory Authority, that do not require approval pursuant to Section 5.2.2;
5.2.1.3 the activities related to, the progress of, and the costs incurred in connection with, the Development Program;
5.2.1.4 summaries of the Research Results;
5.2.1.5 forecast of the estimated timeline (on at least a quarterly basis) for its development activities with respect to the Product for any of the Indications;
5.2.1.6 the addition to the Development Program of any new HNC Clinical Trials testing the efficacy of the Product for the HNC Indication; and
5.2.1.7 any other matters the Parties mutually agree in writing will be, or are expressly provided in this Agreement to be, reviewed and discussed by the JSC.
5.2.2 Responsibilities (Review and Approve). The JSC’s responsibilities will include reviewing and approving (in each case, such approval not to be unreasonably withheld, conditioned or delayed) the following:
5.2.2.1 any change in the Development Program that is not required by, or made to respond to comments from, a Regulatory Authority, that would materially decrease the likelihood of obtaining or materially increase the timeline for obtaining a Regulatory Approval for the HNC Indication or the Melanoma Indication in the US, and that is:
(a) a change to the Indication,
36
ACTIVE/105681617.22
(b) a change to any primary or secondary endpoint or the ordering of secondary endpoints of the HNC Main Clinical Trial as set forth on Exhibit D or any other HNC Clinical Trial,
(c) a material change to the Statistical Analysis Plan or material reduction of the statistical powering of a HNC Clinical Trial as set forth in the Protocol for such HNC Clinical Trial,
(d) the substitution or addition of any arms in any HNC Clinical Trial,
(e) any material change to the inclusion criteria or exclusion criteria with respect to a HNC Clinical Trial as set forth in the applicable Protocol, or
(f) any change from the CMO that is then engaged, or any material changes to the manufacturing process for either (i) the drug substance utilized in the Product or (ii) the final Product that, in either case, will be used in the HNC Clinical Trials;
5.2.2.2 commercially reasonable budgets of CRO and Third Party Vendor costs (the “Approved Third Party Vendor Costs”); and
5.2.2.3 any other matters the Parties mutually agree in writing will be, or are expressly provided in this Agreement to be, reviewed and approved by the JSC.
5.2.3 Limitation on Authority. Notwithstanding anything to the contrary set forth in this Agreement, the JSC will have no authority to (x) amend, modify or waive compliance with this Agreement, or (y) resolve any dispute concerning the validity, interpretation, construction of, or breach of this Agreement.
5.2.4 Decision-Making. Nektar shall retain sole decision-making authority over all matters within the scope of the JSC’s oversight other than the matters described in the foregoing Section 5.2.2. The unanimous approval of the JSC will be required with respect to all matters within its decision-making authority as described in the foregoing Section 5.2.2. The JSC Representatives of each Party will collectively have one (1) vote. The presence of at least one of each Party’s JSC representatives constitutes a quorum for the conduct of business at any JSC meeting, and no vote of the JSC may be taken without a quorum present. If the JSC cannot reach consensus on an issue for which it has decision-making authority, then Nektar shall have the final decision-making authority, provided that if SFJ disagrees with any such Nektar decision with regard to any of the matters set forth in Section 5.2.2, then, at SFJ’s request, the matter shall be escalated to the Executive Officers for attempted resolution by good faith negotiations during a period of [***]. If, notwithstanding such good faith negotiations, the Executive Officers fail to resolve such matter prior to expiration of such [***] period, and SFJ in good faith continues to disagree with such Nektar decision and such decision materially delays or reduces the probability of achieving Regulatory Approval of any of the Indications, then SFJ shall have the right to terminate this Agreement as provided in Section 13.2.8 upon written notice to Nektar delivered [***].
37
ACTIVE/105681617.22
5.3 Reports to be Provided to the JSC. Except as may otherwise be agreed by the Parties, at each JSC meeting Nektar with regard to the Melanoma Clinical Trial, the Other Indications and CMC, and SFJ with regard to the HNC Clinical Trials, will provide an update on the progress of their respective responsibilities and Nektar with regard to the U.S. will report on progress toward obtaining Regulatory Approvals in the Indications.
5.4 Joint Operations Committee.
5.4.1 Representatives. Within sixty (60) days of the Effective Date, the Parties will establish a joint operations committee to oversee the conduct of the Clinical Trials (the “JOC”). Each Party initially will appoint four (4) individuals to serve as representatives to the JOC (the “JOC Representatives”), with each JOC Representative having knowledge and expertise regarding developing products similar to the Product and sufficient seniority within the applicable Party to make decisions within the scope of the JOC’s decision-making authority. Each Party may replace its JOC Representatives at any time upon written notice to the other Party, which notice may be provided by email from one Party’s Alliance Manager to the other Party’s Alliance Manager.
5.4.2 Chairperson. The JOC chairperson (“JOC Chairperson”) shall be designated from SFJ’s JOC Representatives and shall serve throughout the Term. The JOC Chairperson will be responsible for drafting and circulating the draft agenda and ensuring minutes are prepared.
5.4.3 Meetings.
5.4.3.1 Timing.
(a) From the Effective Date through the Development Term, the JOC will meet at least once every month (and for clarity, such meetings are intended to be conducted via teleconference) unless the Parties mutually agree otherwise.
(b) Either Party may call a special meeting of the JOC (by videoconference or teleconference) during the Development Term by at least five (5) Business Days prior written notice to the other Party in the event such Party reasonably believes that a significant matter must be addressed prior to the next scheduled meeting.
5.4.3.2 Participants. The JOC may invite individuals who are not JOC Representatives to participate in JOC meetings; provided that (a) the JOC Representatives of both Parties consent to such non-member’s participation; and (b) such non-member is subject to confidentiality obligations consistent with those described in Article 10 of this Agreement. For clarity, such non-members will have no voting rights at the JOC.
5.4.3.3 Costs. For clarity, each Party will bear its own expenses relating to the meetings and activities of the JOC and such costs will not be Development Costs hereunder.
38
ACTIVE/105681617.22
5.4.4 Notices and Reports to be Provided to the JOC.
5.4.4.1 Unusual or Unforeseen Events. Each Party will promptly notify the JOC of any unforeseen or unusual events that occur in connection with the HNC Clinical Trials that may affect the quality, integrity, or timeliness of the HNC Clinical Trials. Nektar will promptly notify the JOC of any unforeseen or unusual events that occur in connection with the Melanoma Clinical Trial that may affect the quality, integrity, or timeliness of the Melanoma Clinical Trial.
5.4.4.2 Urgent Safety Measures or Serious Breaches. If SFJ becomes aware of (a) any urgent safety measures taken by a Clinical Investigator to protect Subjects against immediate hazard or (b) any serious breaches of the Protocol or any Applicable Laws (including ICH GCP guidelines), SFJ will immediately inform the JOC.
5.4.4.3 Regulatory Inspections.
(a) SFJ will promptly notify the JOC within twenty-four (24) hours of any inspection by any Governmental Authority, including any Regulatory Authority, in connection with the HNC Clinical Trials. SFJ will promptly forward to the JOC copies of any inspection findings that a Site receives from any Regulatory Authority.
(b) Nektar will promptly notify the JOC within twenty-four (24) hours of any inspection by any Governmental Authority, including any Regulatory Authority, (i) at any CMC facility associated with any of the Clinical Trials or (ii) in connection with the Melanoma Clinical Trial. Nektar will promptly forward to the JOC copies of any inspection findings that a CMC facility or a study site for the Melanoma Clinical Trial receives from any Regulatory Authority.
5.4.4.4 Government Investigations. SFJ will promptly notify the JOC upon learning of any investigations by any Governmental Authority in connection with the HNC Clinical Trials. Nektar will promptly notify the JOC upon learning of any investigations by any Governmental Authority in connection with the Melanoma Clinical Trial.
5.4.4.5 Notification of Error. If either Party learns of an error or omission in the conduct of the HNC Clinical Trials or Melanoma Clinical Trial that could call into question the validity, or otherwise compromise the quality and/or integrity, of part or all of the HNC Clinical Trials or Melanoma Clinical Trial, or activities conducted in connection therewith, such Party will inform the JOC in writing within twenty-four (24) hours of either Party learning of such error and/or omission. In the event that such error and/or omission involves any of the HNC Clinical Trials, the members of the JOC will discuss in good faith a remediation plan to address such error within thirty (30) days of such written notification. Such remediation plan will not be effective unless and until approved by the JOC (such approval not to be unreasonably withheld or delayed). If the JOC approves such remediation plan, the JOC will provide each Party with written notice thereof, specifying the dates on which, and the detail with which the Party responsible for such Clinical Trial Activity will be required to update the JOC of
39
ACTIVE/105681617.22
its progress with respect thereto. If the JOC is not able to approve such remediation plan, the matter will be decided by the JSC pursuant to the procedure described in Section 5.2.4.
5.4.4.6 Compliance with Laws. With respect to each of the foregoing Sections 5.4.4.1 through 5.4.4.5, the Party responsible for notifying the JOC will notify the Person to whom notice is required to comply with all Applicable Laws.
5.4.4.7 Progress Reports. Except as may otherwise be agreed to by the Parties, at each JOC meeting (a) SFJ will provide an update on the progress and cost of the HNC Clinical Trials and Regulatory Approval as measured against the Timeline and (b) Nektar will provide an update on the progress and cost of the Melanoma Clinical Trial and Regulatory Approval as measured against the Timeline.
5.4.4.8 IP Reports. In addition to the progress report to be provided by Nektar described in Section 5.4.4.7, at each JOC meeting Nektar shall provide on request an update on (a) any developments relating to or affecting the Nektar Intellectual Property (including, without limitation, any known Third Party Infringements), (b) any developments regarding Third Party Intellectual Property and (c) whether Nektar has licensed or has determined that it will be necessary to license any Intellectual Property in connection with the development, manufacture, use, sale or import of the Product.
5.4.4.9 Post-Development Term Notices. Following completion of the Development Term and through the end of the Term, any and all notices required pursuant to this Section 5.4 will be provided to the JSC instead of the JOC.
5.4.5 Responsibilities and Decision-Making.
5.4.5.1 Responsibilities. The JOC’s responsibilities will include: (a) approving the initial HNC Clinical Trial Protocol (b) approving any changes to any HNC Clinical Trial Protocol that requires a submission to a Regulatory Authority, an IRB or other ethics committees; (c) discussing the activities in connection with, the progress of, and the costs incurred in connection with, the HNC Clinical Trials, including updates from any Clinical Investigator Meetings; (d) reviewing and discussing any notices that it receives pursuant to the foregoing Section 5.4.4; (e) discussing and reviewing the Research Results; (f) reviewing and discussing on at least a quarterly basis the forecast Development Costs and Timeline, and reviewing and approving budgets for Development Costs prepared by the JFC; (g) reviewing and discussing (as necessary) proof of submission of any safety reports to the Regulatory Authorities, Clinical Investigators, IRBs and any other ethics committees; (h) reviewing certain data to be provided by each Party at each JOC meeting as requested by the other Party and in accordance with all Applicable Laws; (i) reviewing performance and progress of the HNC Clinical Trials and Regulatory Approval process; and (j) any other matters the Parties mutually agree will be, or are expressly provided in this Agreement to be, within the responsibilities of the JOC.
5.4.5.2 Decision-Making. The unanimous approval of the JOC will be required with respect to all matters within its decision-making authority as described in the foregoing Section 5.4.5.1. The JOC Representatives of each Party will collectively have one (1)
40
ACTIVE/105681617.22
vote. The presence of at least one of each Party’s JOC representatives constitutes a quorum for the conduct of business at any JOC meeting, and no vote of the JOC may be taken without a quorum present. If the JOC cannot reach consensus on an issue for which it has decision-making authority, then such matter will be escalated to the JSC.
5.4.6 Appointment of Joint Project Team. Within thirty (30) days of the establishment of the JOC, the JOC will establish a joint project team (the “JPT”) to be responsible for the day-to-day execution of the HNC Clinical trials. The responsibilities, leadership, decision-making authority and meeting schedule for the JPT will be specified by the JOC when the JPT is established. Each Party initially will appoint four (4) employees with knowledge and experience in operational matters relating to the conduct of multi-national clinical studies to serve as representatives to the JPT. Each Party may replace its JPT Representatives at any time upon written notice to the other Party, which notice may be provided by email from one Party’s Alliance Manager to the other Party’s Alliance Manager. For clarity, each Party shall bear its own expenses relating to the meetings of the JPT and such costs will not be deemed Development Costs hereunder.
5.5 Joint Finance Committee.
5.5.1 Representatives. Within five (5) days of the Effective Date, the Parties will establish a joint finance committee (“JFC”) to manage the financial interactions of the Parties under this Agreement, including preparation and review of budgets, monitoring performance of HNC Clinical Trials vs budgets, reconciliation of costs incurred on a Calendar Quarter basis, and to act as the primary point of contact between the Parties with respect to all financial matters arising under this Agreement. Each Party initially will appoint one (1) individual with knowledge and experience in financial matters relating to the conduct of multi-national clinical studies to serve as representatives to the JFC (“JFC Representatives”). On reasonable advance notice to the other Party’s JFC Representative and Alliance Manager (which notice may be provided by email), each JFC Representative may appoint a designee to attend any JFC meeting that the JFC Representative is unable to attend. Each Party may replace its JFC Representatives at any time upon written notice to the other Party, which notice may be provided by email from one Party’s Alliance Manager to the other Party’s Alliance Manager.
5.5.2 Chairperson. The JFC chairperson (“JFC Chairperson”) shall be Nektar’s JFC Representative and shall serve throughout the Term.
5.5.3 Meetings. The JFC shall meet at least once per Calendar Quarter unless otherwise mutually agreed by the Parties, at dates and times to be mutually agreed. Either Party may request a special meeting of the JFC on prior written notice to the other Party, which special meeting will be held within a reasonable time of the request, at a date and time to be mutually agreed. The JFC may invite individuals who are not JFC Representatives to participate in JFC meetings; provided that (a) the JFC Representatives of both Parties consent to such non-member’s participation; and (b) such non-member is subject to confidentiality obligations consistent with those set forth in Article 10 of this Agreement.
41
ACTIVE/105681617.22
5.5.4 Attendance at Other Meetings. The JFC Representatives of both Parties shall have the right to attend any meetings of the JSC, JOC or JPT, on reasonable advance notice to the other Party’s Alliance Manager, which notice may be provided by email from one Party’s Alliance Manager to the other Party’s Alliance Manager.
5.5.5 Costs. For clarity, each Party shall bear its own expenses relating to the meetings of the JFC and such costs will not be deemed Development Costs hereunder.
5.5.6 Responsibilities of the JFC (Review and Discuss). The JFC’s responsibilities will include the following:
5.5.6.1 development of procedures for: the maintenance of records by SFJ of Development Costs incurred by it; Calendar Quarterly information sharing; reporting of actual financial results; Calendar Quarterly reconciliation; reasonable cost forecasting; other finance and accounting matters related to Development Costs for HNC Clinical Trials; and such other procedures as are necessary or desirable to enable each Party to achieve and maintain finance and accounting compliance with Applicable Law (collectively, the “Development Cost Reconciliation Procedures”) for approval by the JOC;
5.5.6.2 preparation of initial Development Costs budgets, of which the JFC Representative designated by SFJ shall prepare the initial draft that the full JFC will then review, discuss and revise (including to include any additional information as the JFC may agree), for all initial HNC Clinical Trials anticipated to commence during Calendar Year 2021, on a Calendar Year basis, promptly following the establishment of the JFC by the JOC, for approval by the JOC;
5.5.6.3 by October 15, 2021 and by October 15th of each Calendar Year thereafter while HNC Clinical Trials are being conducted, preparation and presentation of initial draft versions of Development Costs budgets for all HNC Clinical Trials to be conducted during the following Calendar Year, and presentation of final draft versions of such Development Costs budgets for all HNC Clinical Trials to be conducted during the following Calendar Year to the JOC for approval by November 30th of each Calendar Year (unless a different date is mutually agreed by the JFC Representatives);
5.5.6.4 periodic review of the status of Development Cost budgets, the progress of HNC Clinical Trials, and performance of HNC Clinical Trials against Development Cost budgets, including actual Development Costs incurred to date, forecast of remaining Development Costs, and costs contracted with Third Parties,
5.5.6.5 periodic review and, if deemed necessary by the JFC, revision of the Quarterly Labor Fees, including any revisions reasonably necessary for Additional HNC Clinical Trial Protocols (if any);
5.5.6.6 overseeing Development Cost Reconciliation Procedures;
42
ACTIVE/105681617.22
5.5.6.7 providing support to the JSC, JOC and JPT with respect to financial, accounting, budgeting, reporting and other matters that may arise in connection with a Development Costs budget and other activities under this Agreement;
5.5.6.8 review and discuss any Success Payments that may become due from Nektar to SFJ pursuant to Article 6; and
5.5.6.9 discussing any other topics or issues relating to any Development Costs budget, other activities of the JFC, or other activities under this Agreement as either Party’s JFC Representative or the JOC may request.
ARTICLE 6
PAYMENTS TO SFJ
6.1 Regulatory Approval. Nektar will pay to SFJ, in US Dollars, the amounts set forth in this Section 6.1.
6.1.1 Upon the latest to occur of (a) the First Regulatory Approval and (b) the Initial Payment Trigger Date (such latest date, the “Initial Payment Date”), and subject to Section 6.1.3, Nektar will pay SFJ the amounts set forth in Success Payment Schedule A below (in the column entitled “Amount of Payment”) on the dates set forth in the column entitled “Date of Payment” (each payment payable pursuant to this Section 6.1.1, a “Schedule A Success Payment” and the first payment payable pursuant to this Section 6.1.1 no later than thirty (30) days following the Initial Payment Date, the “Initial Schedule A Success Payment”).
Success Payment Schedule A
| Date of Payment | Amount of Payment | ||||
| No later than 30 days following the Initial Payment Date | $30,000,000 | ||||
| 1-Year Anniversary of Initial Payment Date | [***] | ||||
| 2-Year Anniversary of Initial Payment Date | [***] | ||||
| 3-Year Anniversary of Initial Payment Date | [***] | ||||
| 4-Year Anniversary of Initial Payment Date | [***] | ||||
| 5-Year Anniversary of Initial Payment Date | [***] | ||||
| Total | $450,000,000 | ||||
6.1.2 Upon the notification to Nektar of a Second Regulatory Approval (the date such notification, the “Second Regulatory Approval Date”), and subject to Section 6.1.3, Nektar will pay SFJ the amounts set forth in Success Payment Schedule B below (in the column entitled “Amount of Payment”) on the dates set forth in the column entitled “Date of Payment” (each payment payable pursuant to this Section 6.1.2, a “Schedule B Success Payment” and the first
43
ACTIVE/105681617.22
payment payable pursuant to this Section 6.1.2 no later than thirty (30) days following the Second Regulatory Approval Date, the “Initial Schedule B Success Payment”).
Success Payment Schedule B
| Date of Payment | Amount of Payment | ||||
| No later than 30 days following the Second Regulatory Approval Date | [***] | ||||
| 1-Year Anniversary of Second Regulatory Approval Date | [***] | ||||
| 2-Year Anniversary of Second Regulatory Approval Date | [***] | ||||
| 3-Year Anniversary of Second Regulatory Approval Date | [***] | ||||
| 4-Year Anniversary of Second Regulatory Approval Date | [***] | ||||
| 5-Year Anniversary of Second Regulatory Approval Date | [***] | ||||
| 6-Year Anniversary of Second Regulatory Approval Date | [***] | ||||
| 7-Year Anniversary of Second Regulatory Approval Date | [***] | ||||
| Total | $150,000,000 | ||||
6.1.3 If the Regulatory Approval giving rise to the requirement for Nektar to make payments to SFJ pursuant to Section 6.1.1 or 6.1.2 (the “Specified Regulatory Approval”) is an Accelerated Regulatory Approval, and such Accelerated Regulatory Approval is later withdrawn by the FDA for any reason (other than to issue a full Regulatory Approval), then (a) Nektar shall have no obligation to make any additional payments pursuant to Section 6.1.1 or 6.1.2, as applicable, during the period after withdrawal of such Accelerated Regulatory Approval and before such time (if ever) as the Specified Regulatory Approval is again obtained (a “Subsequent Regulatory Approval”) and (b) Nektar shall be obligated to use Commercially Reasonable Efforts to (i) resolve any manufacturing issues, if the withdrawal is related to a manufacturing related issue, [***], and (ii) obtain Subsequent Regulatory Approval unless such withdrawal is due to safety or efficacy reasons; provided that in no event shall Nektar be required to conduct any additional clinical study to demonstrate the safety and/or efficacy of the Product following withdrawal by the FDA of such Accelerated Regulatory Approval, except as set forth in clause (i) above. If Subsequent Regulatory Approval is obtained, Nektar’s obligation to make Success Payments shall resume and the due date for payments pursuant to Section 6.1.1 or 6.1.2, as applicable, following such Subsequent Regulatory Approval shall be extended by the number of days between the withdrawal of the Accelerated Regulatory Approval and the applicable Subsequent Regulatory Approval.
6.1.4 Within ninety (90) days following receipt by Nektar of an Other Indication Regulatory Approval, Nektar shall pay SFJ an amount equal to $37,500,000. Nektar shall not be responsible for making more than one payment to SFJ pursuant to this Section 6.1.4.
6.1.5 Each Schedule A Success Payment, Schedule B Success Payment and the payment set forth in Section 6.1.4 is referred to as a “Success Payment” and collectively as the “Success Payments,” and shall be subject to adjustment as provided in Section 6.2.
44
ACTIVE/105681617.22
6.2 Payment Adjustments. In the event that the actual Development Costs paid or incurred by SFJ hereunder are lower or greater than One Hundred Fifty Million U.S. Dollars ($150,000,000.00) as of the date of payment of any Success Payment, Approval Buyout Payment or Buy-Out Payment, such payment will be multiplied by a fraction, the numerator of which is equal to the actual amount of Development Costs paid or incurred by SFJ hereunder and the denominator of which is One Hundred Fifty Million U.S. Dollars ($150,000,000.00). In the event that Nektar becomes obligated to make a Success Payment to SFJ while Development Costs are still being paid or incurred, SFJ shall recalculate the applicable adjustment as of the end of each Calendar Year and as of such time as the final amount of actual Development Costs is known and determine any true-up payments required to be made by Nektar with respect to any payment made pursuant to Section 6.1 prior to such time, and Nektar shall pay any such true-up payment to SFJ within thirty (30) days after receipt of invoice from SFJ. For purposes of clarity, in the event that the amount funded by SFJ is less than $150,000,000 as a result of an early termination of the HNC Clinical Trials due to the occurrence of HNC Clinical Trial Futility or Safety Concern, the foregoing pro rata funding provision based on actual SFJ investment shall apply.
6.3 Method and Timing of Payment. The Success Payments to SFJ will be due as of the applicable dates set forth in Sections 6.1.1, 6.1.2 or 6.1.4, as may be adjusted pursuant to Sections 6.1.3 or 6.2, and shall be paid by wire transfer of immediately available funds to an account specified by SFJ from time to time. Nektar will provide SFJ with written notice of each wire transfer to SFJ’s account. All amounts payable and calculations under this Agreement shall be in US dollars.
6.4 Late Payments. If Nektar fails to pay any undisputed amount due under this Agreement on the due date therefore, then, without prejudice to any other remedies that SFJ may have, that amount will bear interest from the due date until payment of such amount is made, both before and after any judgment, [***].
6.5 Taxes.
6.5.1 Notwithstanding anything to the contrary, on or prior to the Effective Date, SFJ shall deliver to Nektar a duly completed and valid (A) IRS Form W-9 certifying that SFJ is a United States person, as such term is defined in Section 7701(a)(30) of the IRC, (B) applicable IRS Form W-8BEN-E claiming treaty benefits under a double taxation treaty with respect to each of “royalties,” “interest” and “other income,” (C) IRS Form W-8IMY to which the forms set forth in the preceding (A) and (B) are attached, or (D) other applicable IRS Form W-8 that indicates no withholding is required in respect of the Product Payments, (in each case ((A) through (D)), the “IRS Withholding Form”), and SFJ shall provide an updated IRS Withholding Form to Nektar throughout the Term whenever required in order for SFJ to have on file a duly completed and valid IRS Withholding Form.
6.5.2 The Parties hereby acknowledge and agree that payments made under this Agreement will be made without reduction for withholding or similar taxes if Nektar, at the time of any such payment, has on file a duly completed and valid IRS Withholding Form in respect of SFJ, unless such withholding or similar tax is required (x) by a taxing authority as a result of an
45
ACTIVE/105681617.22
audit or examination, (y) due to the assignment of this Agreement or any payment or other obligation or responsibility hereunder (to the extent permitted) by either Party to an Affiliate or Third Party, the re-domiciling of either Party outside the US or any other circumstance that results in either Party no longer being a United States person for U.S. federal income tax purposes, or that results in SFJ no longer being able to provide an IRS Withholding Form, or (z) as a result of a change in Applicable Laws at any time during the Term. In any such case, the Parties shall use commercially reasonable and legal efforts to mitigate the amount of such taxes that would need to be withheld and/or paid. Either Party may request a meeting of the JFC to further discuss any conclusion that a payment should be made with reduction for withholding or similar taxes. Any amounts withheld pursuant to this Section 6.5 will be timely paid over to the appropriate taxing authority, and will be treated for purposes of this Agreement as having been paid to the Party that otherwise would have received such amounts. If a Party (the “Withholding Party”) is required to withhold any taxes on the amounts payable to the other Party (the “Recipient Party”) hereunder as a result of any actions described in clause (y) above by such Withholding Party (or its Affiliates), the Withholding Party shall pay the Recipient Party such additional amounts as are necessary to ensure receipt by the Recipient Party of the full amount which the Recipient Party would have received but for the deduction on account of such withholding. The paying party shall be liable for and shall pay any sales, use, value-added or other similar taxes imposed on payments required to be made hereunder.
6.6 Tax Cooperation. The Parties will cooperate and produce on a timely basis any tax forms or reports, including any IRS Forms W-8BEN or W-9, as applicable, reasonably requested by the other Party in connection with any payment made under this Agreement. Each Party will provide to the other Party any other tax forms that may be reasonably necessary in order for such Party not to withhold tax or to withhold tax at a reduced rate under an applicable bilateral income tax treaty. Each Party will provide, upon request, to the other Party any tax forms at least thirty (30) days prior to the due date for any such payments; provided that the request for such forms was made in a timely manner. Each Party will provide the other with commercially reasonable assistance to enable the recovery, as permitted by law, of withholding taxes, VAT, or similar obligations resulting from payments made under this Agreement, such recovery to be for the benefit of the Party bearing such withholding tax or VAT. Each Party will provide commercially reasonable cooperation to the other Party, at the other Party’s expense, in connection with any official or unofficial tax audit or contest relating to tax payments made with respect to amounts paid or payable to such other Party under this Agreement.
6.7 Buy-Out Option.
6.7.1 Approval Buy-Out Option.
6.7.1.1 Within one hundred and eighty (180) days following the Initial Payment Date (in the case of Schedule A Success Payments) or one hundred and eighty (180) days following the Second Regulatory Approval Date (in the case of Schedule B Success Payments), Nektar shall have the right to make a one-time payment (each, an “Approval Buy-Out Payment”) in lieu of all (but not less than all) Schedule A Success Payments or Schedule B Success Payments, as applicable (in each case as adjusted in accordance with
46
ACTIVE/105681617.22
Section 6.2) (other than the Initial Schedule A Success Payment or Initial Schedule B Success Payment, as applicable, payable pursuant to Section 6.1, in each case, as adjusted in accordance with Section 6.2, which shall remain due and payable if not previously paid) by written notice delivered to SFJ no later than ninety (90) days after the date of the Initial Payment Date or Second Regulatory Approval Date, as applicable, which written notice shall set forth the amount of the applicable Approval Buy-Out Payment, the proposed date of closing (which shall occur within one hundred and eighty (180) days after the Initial Payment Date or Second Regulatory Approval Date, as applicable), and the calculation of the applicable Approval Buy-Out Payment in reasonable detail based upon the proposed closing date. The Approval Buy-Out Payment will be calculated as follows:
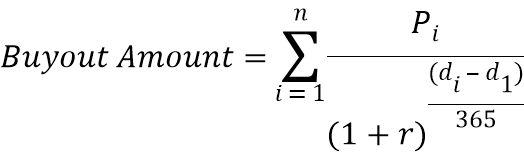
Where:
Pi = Success Payments that the Approval Buy-Out Amount applies to, as adjusted in accordance with Section 6.2
r = the Discount Rate
di = payment date per schedule
d1 = closing date of buyout
Each Approval Buy-Out Payment will be payable in one installment in cash at the closing to an account specified by SFJ. The discount rate used to calculate each Approval Buy-Out Payment shall be [***] (the “Discount Rate”). If Nektar elects to make an Approval Buy-Out Payment in lieu of the Schedule A Success Payments, then following such payment Nektar shall no longer be subject to the provision of Section 7.1.
6.7.1.2 In the event that Nektar becomes obligated to make Schedule A Success Payments or Schedule B Payments as a result of an Accelerated Regulatory Approval, then the time period for Nektar to elect to buy out the Schedule A Success Payments or Schedule B Payments, as applicable, shall be extended to the date which is ninety (90) days after such Accelerated Regulatory Approval becomes a full Regulatory Approval, with the applicable Approval Buy-Out Payment to be paid within one hundred eighty (180) days after the date of such full Regulatory Approval.
6.7.2 Change of Control Payment. If a Change of Control is consummated, then within forty-five (45) days following the closing of such Change of Control, Nektar shall pay SFJ an amount in cash equal to one hundred and fifty percent (150%) of actual Development Costs paid or incurred by SFJ hereunder prior to such Change of Control (such payment, the “Change of Control Payment”), provided that no Change of Control Payment shall be payable to SFJ if either (a) the HNC Clinical Trial has been completed but the HNC Clinical Trial Success Criteria was not achieved or (b) HNC Clinical Trial Futility has occurred. The
47
ACTIVE/105681617.22
Change of Control Payment, if any, shall be credited toward future Success Payments in chronological order.
6.7.3 [***].
ARTICLE 7
CERTAIN COVENANTS
7.1 Negative Covenants.
7.1.1 Encumbrances. Nektar shall not, without SFJ’s prior written consent, create, incur, allow, or suffer any Lien on any of the Nektar Intellectual Property, or assign or convey any right to receive income with respect to the Nektar Intellectual Property (other than royalty and other license fee obligations to licensors thereof in accordance with the applicable license agreement), including the sale of any Nektar Intellectual Property, or permit any of its subsidiaries to do so; provided, however, that nothing in this provision or this Agreement shall require the consent of SFJ (a) for Nektar to issue senior secured debt secured by all or substantially all of the assets of Nektar including the Nektar Intellectual Property; or (b) for any transaction between Nektar and one or more of its Affiliates so long as such Affiliates are bound by the same restrictions as Nektar set forth in this Section 7.1.
7.1.2 Licenses. Without SFJ’s prior approval, Nektar shall not enter into a Licensing Transaction in the U.S. or Major European Markets to any Third Party; provided that this consent provision will not apply to any transaction between Nektar and one or more Nektar Affiliates so long as Nektar guarantees the obligations and performance of such Nektar Affiliates.
7.1.3 Sales of Royalty Streams. Nektar shall not, without SFJ’s consent, sell, transfer or assign, directly or indirectly, in whole or in part, any rights to receive payments of royalties or license fees with respect to the Product or the Nektar Intellectual Property (including any Accounts with respect to such royalties or license fees), other than to a wholly owned direct or indirect subsidiary of Nektar.
7.1.4 Restriction on Acquiring or Transacting in Nektar Securities. SFJ agrees that, unless otherwise agreed in writing by Nektar, until the earliest of (a) the expiration of the Term and (b) the date on which the top line results of the last remaining Clinical Trial are published or otherwise become publicly available, neither it nor any of its Affiliates, and its and their respective officers, directors, managers, employees, agents, or representatives will acquire beneficial ownership of or transact in any securities (including any derivative securities or any other instrument that provides the holder thereof with the economic equivalent of ownership of an amount of securities of Nektar) of Nektar.
48
ACTIVE/105681617.22
7.2 Affirmative Covenants.
7.2.1 Government Compliance. Nektar shall maintain its and all its subsidiaries’ legal existence and good standing in their respective jurisdictions of formation and maintain qualification in each jurisdiction in which the failure to so qualify would reasonably be expected to have a material adverse effect on the Development or Commercialization of the Product, provided that any subsidiary may liquidate or dissolve so long as such liquidation or dissolution would not reasonably be expected to have a material adverse effect on the Development or Commercialization of the Product. Nektar shall comply, and shall cause each subsidiary to comply, in all material respects, with all laws, ordinances and regulations to which it is subject, noncompliance with which would reasonably be expected to have a material adverse effect on the Development or Commercialization of the Product.
7.2.2 Regulatory Compliance. Nektar shall not become an “investment company” or a company “controlled” by an “investment company” under the Investment Company Act of 1940, as amended. Nektar shall not become engaged as one of its important activities in extending credit for margin stock (under Regulations X, T and U of the Federal Reserve Board of Governors). Neither Nektar’s nor any of its Subsidiaries’ properties or assets shall be used by Nektar or any Subsidiary in disposing, producing, storing, treating, or transporting any hazardous substance other than legally. Nektar and each of its subsidiaries shall obtain all consents, approvals and authorizations of, make all declarations or filings with, and give all notices to, all Governmental Authorities that are necessary to continue their respective businesses as currently conducted, unless such failure could not reasonably be expected to have a material adverse effect on the Development or Commercialization of the Product.
7.2.3 Protection of Intellectual Property Rights. Nektar shall use Commercially Reasonable Efforts in the exercise of its business judgment to prosecute, protect, defend and maintain the validity and enforceability of the Nektar Intellectual Property.
7.2.4 Acceleration. In the event that, following an applicable Regulatory Approval, (a) Nektar shall fail to make any Success Payment associated with such Regulatory Approval on or before its due date, (b) Nektar has received written notice from SFJ of such failure, specifying in reasonable detail the particulars of such failure, (c) Nektar has not cured such failure [***] following receipt of such notice and (d) the amount of such Success Payment and/or whether such Success Payment is due and payable has not been disputed, all remaining unpaid Success Payments that are based on such Regulatory Approval shall become immediately due and payable; provided that, in the event of any such acceleration, SFJ’s rights to receive such Success Payments, if any, shall be adjusted as set forth in Section 6.2 and reduced by any amounts previously paid to SFJ
7.2.5 Audit Rights. Upon at least [***] and during normal business hours, [***], Nektar may cause an inspection and/or audit by an independent public accounting firm, to be compensated on the basis of time spent and to be reasonably acceptable to SFJ, to be made of the books and records of SFJ and its Affiliates, and the Approved CROs and Approved Vendors with which it contracts, for the [***] purpose of determining the correctness of Development Costs paid and/or incurred under this Agreement. Upon Nektar’s reasonable request not more
49
ACTIVE/105681617.22
than once in any Calendar Year, SFJ shall use commercially reasonable efforts to exercise any rights it may have under any agreement with a Permitted CRO or a Permitted Vendor to cause an inspection and/or audit by an independent public accounting firm, to be compensated on the basis of time spent, to be made of the books of account of such Permitted CRO or Permitted Vendor for the purpose of determining the correctness of payments made to such Permitted CRO or Permitted Vendor and the correctness of Development Costs paid and/or incurred under this Agreement. All of the expenses of any inspection or audit requested by Nektar hereunder (including the fees and expenses of such independent public accounting firm designated for such purpose) shall be borne by (i) Nektar, if the independent public accounting firm determines that Development Costs were underpaid by an amount less than or equal to [***] of the Development Costs actually paid or (ii) Nektar, if the independent public accounting firm determines that the Development Costs previously paid were incorrect by an amount greater than [***] of the Development Costs actually paid. All information obtained by Nektar as a result of any such inspection or audit shall be Confidential Information subject to Article 9.
ARTICLE 8
RECORDS
8.1 Accounting. Each Party will maintain materially complete and accurate accounting records related to this Agreement in accordance with GAAP. Each Party will retain such records for two (2) years after the earlier of expiration or early termination of this Agreement.
8.2 Clinical Trials-Related Records. Each Party shall, and shall cause its Affiliates and its and their Permitted Third Parties conducting Development of the Product to, maintain, in good scientific manner, complete and accurate books and records pertaining to Development of the Product hereunder, in sufficient detail to verify compliance with its obligations under this Agreement. Such books and records shall (a) be appropriate for patent and regulatory purposes, (b) be in compliance with Applicable Law, (c) properly reflect all work done and results achieved in the performance of its Development activities hereunder, and (d) be retained by such Party for such period as may be required by Applicable Law.
ARTICLE 9
CONFIDENTIAL INFORMATION
9.1 Confidentiality. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing by the Parties, each Party (each, a “Receiving Party”) agrees that, during the Term and for the five (5)-year period following the expiration or termination of this Agreement (except that the obligations will survive thereafter with respect to any Confidential Information that constitutes a trade secret under Applicable Law) or such longer periods for which such Confidential Information may be maintained pursuant to Article 8, it will keep confidential and will not publish or otherwise disclose and will not use for any purpose other than as provided for in this Agreement (which includes the exercise of any rights or the performance of any obligations hereunder) any Confidential Information furnished to it by or on
50
ACTIVE/105681617.22
behalf of the other Party (each, a “Disclosing Party”) or its Affiliates in connection with this Agreement. The foregoing obligations will not apply to any portion of such information or materials that the Receiving Party can demonstrate:
9.1.1 was publicly disclosed by the Disclosing Party before or after such Confidential Information becomes known to the Receiving Party;
9.1.2 was already known to the Receiving Party or any of its Affiliates, other than under an obligation of confidentiality or non-use, prior to when it was received from the Disclosing Party;
9.1.3 is subsequently disclosed to the Receiving Party or any of its Affiliates by a Third Party lawfully in possession thereof without obligation to keep such Confidential Information confidential;
9.1.4 has been published by a Third Party or otherwise enters the public domain through no fault of the Receiving Party or any of its Affiliates in breach of this Agreement; or
9.1.5 has been independently developed by the Receiving Party or any of its Affiliates, without the aid, application or use of any Confidential Information of the other Party.
9.2 Authorized Disclosure. Each Party may disclose Confidential Information belonging to the other Party to the extent such disclosure is reasonably necessary for complying with Applicable Laws, including regulations promulgated by securities exchanges, provided that the Party required to disclose such information promptly notifies the Disclosing Party prior to making any such disclosure and cooperates with the Disclosing Party’s efforts to seek confidential treatment or to otherwise limit disclosure. Each Receiving Party may disclose the other Party’s Confidential Information to its Affiliates and its and their Representatives, advisors and independent contractors (including Permitted Third Parties) engaged by such Receiving Party, and with respect to SFJ, the Specified Investors, in each case (a) only to the extent such Persons need to know the Confidential Information solely in connection with the performance of this Agreement or in the case of the Specified Investors, as necessary to monitor and manage its investment in SFJ, and (b) provided that each Person receiving Confidential Information must be bound by obligations of confidentiality and non-use at least as stringent as an equivalent in scope to those set forth in this Article 9 prior to any such disclosure and the Party making such disclosure to such Person shall be liable to the other Party for any breach of such obligations by such disclosee. [***]. In any event, each Party agrees to take all reasonable action to avoid unauthorized use or disclosure of Confidential Information of the other Party hereunder.
9.2.1 [***].
9.2.2 [***].
9.3 Return of Confidential Information. Except as otherwise provided herein, upon expiration or earlier termination of this Agreement, all Confidential Information (including any copies thereof) in written or other tangible form will, at the Disclosing Party’s direction, be
51
ACTIVE/105681617.22
returned to the Disclosing Party or destroyed by the Receiving Party, and any Person(s) to whom the Receiving Party disclosed (with such destruction being certified in writing by an authorized officer of the Receiving Party), except (i) to the extent such Confidential Information is necessary to exercise any license and/or rights hereunder that survive such expiration or earlier termination; and (ii) one (1) copy of each document may be retained by the Receiving Party solely to the extent necessary to permit it to comply with any ongoing rights and responsibilities with respect to such Confidential Information.
9.4 Confidential Status of the Agreement. Subject to Section 9.2 and Section 9.5, the terms of this Agreement are deemed to be Confidential Information and will be subject to the confidentiality requirements of this Article 9, with each Party being deemed a Receiving Party for such purposes. The Parties each acknowledge that it will be necessary for Nektar to file this Agreement with the U.S. Securities and Exchange Commission and to make other required public disclosures regarding the terms of this Agreement, and accordingly Nektar shall prepare a confidential treatment request in connection with such filing and provide SFJ a reasonable opportunity to review and comment on such filing as well as on such other required public disclosures and thereafter use Commercially Reasonable Efforts to obtain confidential treatment as to the terms of this Agreement; provided that Nektar shall not be required to provide SFJ the opportunity to review and comment on any disclosure previously reviewed and commented upon by SFJ.
9.5 Publicity. The Parties recognize that following the Effective Date the Parties (either individually or jointly) shall issue mutually agreed press release(s) announcing the execution of this Agreement, and thereafter each Party may from time to time desire to issue additional press releases and make other public statements or disclosures regarding the subject matter of this Agreement, and hereby agree that such additional press releases, public statements and disclosures regarding the terms of this Agreement will be permitted only with the other Party’s written consent (which shall not be unreasonably withheld, conditioned or delayed). Any publication, news release or other public announcement relating to the terms of this Agreement will first be reviewed and approved in writing by both Parties; provided, however, that any disclosure of the minimum information which is required by Applicable Law (including the rules of a securities exchange), as reasonably advised by the disclosing Party’s counsel, may be made without the prior consent of the other Party, although the other Party will be given prompt notice of any such legally required disclosure and to the extent practicable will be provided an opportunity to comment on the proposed disclosure and the disclosing Party will consider in good faith any comments provided by the other Party on such proposed disclosure. For avoidance of doubt, this Section 9.5 shall not restrict Nektar from releasing public statements or disclosures regarding Nektar’s development and Commercialization activities with respect to the Product.
9.6 Use of Name. Unless otherwise expressly permitted herein, Nektar will (i) obtain the written consent of SFJ prior to making any public reference to any of SFJ’s investors (including without limitation the Specified Investors), including without limitation in any filings with the SEC, and (ii) obtain SFJ’s written consent (which consent will not unreasonably be withheld, conditioned or delayed) prior to referring to SFJ or any of its investors (including
52
ACTIVE/105681617.22
without limitation the Specified Investors) in any correspondence with any Regulatory Authority or Governmental Authority (whether or not such references would be expected to become publicly available) or in any press release except as may be required by Applicable Law. This Section 9.6 shall also apply vice versa to any use by SFJ of Nektar’s name.
ARTICLE 10
INTELLECTUAL PROPERTY AND PERSONALLY
IDENTIFIABLE INFORMATION
10.1 Ownership and Rights.
10.1.1 Ownership.
10.1.1.1 Existing Intellectual Property. Subject to Section 10.1.1.2, it is agreed between the Parties that each Party will retain all right, title and interest in, to and under all Intellectual Property that is Controlled by such Party as of the Effective Date.
(a) Without limiting the generality of the foregoing, as between the Parties, Nektar shall be and remain the sole and exclusive owner of all right, title and interest in and to all Nektar Intellectual Property existing as of the Effective Date (“Existing Nektar Intellectual Property”), including, in the case of Patents within the Existing Nektar Intellectual Property (“Existing Nektar Patents”), all patent applications filed after the Effective Date that claim priority to, or are foreign counterparts of, patent applications within the Existing Nektar Patents (“Corresponding Nektar Patent Applications”) and all Patents that may issue or be granted from any patent application within the Existing Nektar Patents or any Corresponding Nektar Patent Application after the Effective Date. In addition, Nektar shall be and remain the sole and exclusive owner of all right, title and interest in and to all Nektar Intellectual Property arising during the term of this Agreement independent of the conduct of the activities contemplated by this Agreement.
10.1.1.2 Trial Inventions.
(a) Nektar shall be the exclusive and sole owner of, and retain all right, title and interest in and to, all Trial Inventions (which shall constitute Nektar Intellectual Property), regardless of inventorship. SFJ will promptly disclose, and will cause its Affiliates and all Permitted Third Parties engaged by SFJ or its Affiliates to perform any of SFJ’s obligations hereunder promptly to disclose, to Nektar in writing in reasonable detail each Trial Invention made, developed, created, generated, conceived or reduced to practice in whole or in part by or on behalf of SFJ, such Affiliate or such Permitted Third Party, which written disclosure shall include all available information and data necessary to support the filing of patent applications Covering such Trial Invention. SFJ, for itself and on behalf of its Affiliates, hereby assigns, and shall cause such other Permitted Third Parties to assign (subject to Section 10.1.1.2(c)), to Nektar all its right, title and interest in and to Trial Inventions and all information and data necessary to support the filing of patent applications Covering such Trial Inventions. SFJ will cooperate, and will cause the foregoing Persons to cooperate, with Nektar
53
ACTIVE/105681617.22
to effectuate and perfect the foregoing ownership, including by promptly executing and recording assignments and other documents consistent with such ownership.
(b) SFJ shall cause each employee and individual consultant of SFJ or its Affiliates (but excluding Permitted Third Parties of SFJ and its Affiliates, which are separately addressed in Section 10.1.1.2(c)) who conceives, discovers, develops or otherwise makes any Trial Invention to be subject to a present obligation to assign to Nektar all right, title and interest in and to in any such Trial Invention. In the case of any individual consultant of SFJ or its Affiliates (excluding SFJ’s and its Affiliates’ Permitted Third Parties), if SFJ is unable to cause such consultant to agree to such assignment obligation despite SFJ’s using Commercially Reasonable Efforts to negotiate such assignment obligation, then SFJ shall inform Nektar of such inabiltiy to cause such consultant to agree to such assignment obligation and at Nektar’s sole discretion either: (A) cause such consultant to grant an exclusive, worldwide, royalty-free, fully-paid, freely-assignable license, with the right to sublicense through multiple tiers, under their rights in such Trial Invention to develop, make, have made, use, sell, have sold, offer for sale and import the Product for any and all uses, except where prohibited by Applicable Law and except in the case of consultants who are employed by governmental, not-for-profit, or public institutions that have standard policies against such an assignment (in which case, SFJ shall use Commercially Reasonable Efforts to obtain a suitable license, or right to obtain such a license); or (B) refrain from using such consultant to conduct activities pursuant to this Agreement.
(c) SFJ shall obtain from each Third Party contractor that SFJ or its Affiliate proposes to engage to conduct activities under or in connection with this Agreement on behalf of SFJ or its Affiliates (i) an assignment, (ii) an exclusive, worldwide, royalty-free, fully-paid, freely-assignable license, with the right to sublicense through multiple tiers, or (iii) a nonexclusive, worldwide, royalty-free, fully-paid, freely-assignable license, with the right to sublicense through multiple tiers ((i) through (iii) in order of preference), to Nektar of any Trial Invention that such Third Party contractor conceives, discovers, develops or otherwise makes in connection with activities conducted relating to this Agreement. The Parties acknowledge that it may not be possible to obtain such assignment or license from any such Third Party contractor with respect to technology of broad applicability to the operation of such Third Party contractor’s business or improvements, or improvements to such Third Party contractor’s own proprietary technology used in the performance of services on behalf of SFJ or its Affiliate, in each case, on acceptable terms or at all, and accordingly, following notice by SFJ to Nektar, the Parties agree that the inability of SFJ or its Affiliate to obtain such assignment or license from a Third Party contractor on acceptable terms or at all shall not constitute a breach of SFJ’s obligations under this Agreement.
10.1.1.3 Trial Data Package. Nektar shall be the sole and exclusive owner of the Trial Data Package including the Research Results included therein. SFJ, for itself and on behalf of its Affiliates, hereby assigns, and shall cause such other Permitted Third Parties to assign (subject to Section 10.1.1.2(c)), to Nektar all its right, title and interest in and to the Trial Data Package, including the Research Results included therein. SFJ will cooperate, and will cause the foregoing Persons to cooperate, with Nektar to effectuate and perfect the foregoing
54
ACTIVE/105681617.22
ownership, including by promptly executing and recording assignments and other documents consistent with such ownership.
10.1.1.4 Inventorship; Further Assurances. Inventorship of Trial Inventions will be determined according to the principles of US patent law. SFJ agrees to cooperate fully, to cause its Affiliates to cooperate fully, and to use Commercially Reasonable Efforts to cause its and their respective Permitted Third Parties to cooperate fully with Nektar in the preparation, filing, prosecution and maintenance of Patents Covering Trial Inventions filed by or on behalf of Nektar claiming any Licensed Know-How. Such cooperation includes executing all papers and instruments, or requiring its employees, consultants and Permitted Third Parties, to execute such papers and instruments, so as to (a) effectuate the ownership of Trial Inventions set forth in Section 10.1.1.2(a), including Patents claiming or disclosing Trial Inventions, and (b) enable Nektar to apply for and to prosecute patent applications claiming Trial Inventions in any country.
10.1.1.5 No Other Rights. The delivery or disclosure by or on behalf of Nektar to SFJ of any information or materials hereunder will not be construed to grant SFJ any rights or license to use any Intellectual Property Controlled by Nektar other than as necessary to comply with its obligations hereunder or as expressly set forth herein. Except as otherwise expressly permitted in this Agreement, SFJ may not use, publish or otherwise disclose any Intellectual Property Controlled by Nektar without Nektar’s prior written consent.
10.2 Patent Prosecution. As between SFJ and Nektar, Nektar will have sole and exclusive right to prepare, file, prosecute and maintain all Patents within the Nektar Intellectual Property, including all Patents that cover the Trial Inventions, at its own expense (provided that Nektar shall use Commercially Reasonable Efforts to prosecute and maintain such Patents). At Nektar’s request and expense (for reasonable out-of-pocket expenses), SFJ will reasonably cooperate with Nektar in preparing, filing, prosecuting, and maintaining such Patents.
10.3 Intellectual Property Enforcement.
10.3.1 Nektar Intellectual Property. Nektar will use Commercially Reasonable Efforts to enforce the Nektar Intellectual Property, including Intellectual Property that covers the Trial Inventions, against Third Party Infringements.
10.3.2 Infringement of Third Party Rights. If either Party learns of Third Party allegations that it or the other Party or any of its or the other Party’s Affiliates or Permitted Third Parties, have infringed, misappropriated or otherwise violated, or are infringing, misappropriating or otherwise violating, any Intellectual Property of a Third Party in connection with either the Clinical Trials or performing its obligations or duties hereunder, such Party will promptly notify the other Party. Nektar will have sole control and responsibility of, and discretion with respect to, such allegations and any related actions and/or litigation.
55
ACTIVE/105681617.22
10.4 Personally Identifiable Information.
10.4.1 In conducting the Clinical Trials and its other obligations under this Agreement, each Party will comply, and will use Commercially Reasonable Efforts to require each applicable Permitted Third Party of such Party to comply, with Applicable Laws relating to privacy or data protection applicable to such Party or the Clinical Trials being conducted by or on behalf of such Party, including ensuring that all necessary (a) consents from Clinical Investigators, Subjects and any others from whom Personally Identifiable Information will be received are obtained; (b) regulatory notifications are filed in all countries for which Sites have been selected; and (c) approvals are obtained in all countries for which Sites have been selected, prior to collection or transfer of such Personally Identifiable Information. Without prejudice to the generality of the foregoing, each Party shall (i) work together with the other Party in good faith to ensure the information referred to in applicable laws and, if applicable, in particular Articles 13 and 14 of the General Data Protection Regulation (2016/679) (“GDPR”) is made available to data subjects (as defined in the GDPR) in relation to the processing of their Personally Identifiable Information by either Party when acting as a data controller (as defined in the GDPR), and the information is in a concise, transparent, intelligible and easily accessible form, using clear and plain language as required by Article 12 of the GDPR; (ii) if either Party (the “Data Receiving Party”) receives any complaint, notice or communication from a supervisory authority (as defined in the GDPR) which relates directly or indirectly to the other Party’s (A) processing of the Personally Identifiable Information; or (B) potential failure to comply with the provisions of the GDPR, the Data Receiving Party shall, to the extent permitted by law, promptly forward the complaint, notice or communication to the other Party and provide the other Party with reasonable co-operation and assistance in relation to the same; (iii) if a data subject makes a written request to a Party to exercise their rights in relation to their Personally Identifiable Information that concerns processing in respect of which the other Party is the data controller, that Party shall forward the request to the other Party promptly and in any event within five (5) Business Days from the date on which it received the request and, upon the other Party’s reasonable written request, provide that other Party with reasonable co-operation and assistance in relation to that request to enable the other to respond to such request and meet applicable timescales set out under the GDPR; (iv) if either Party becomes aware of a personal data breach (as defined in the GDPR), it shall notify the other Party without undue delay, and each Party shall co-operate with the other, to the extent reasonably requested, in relation to any notifications to supervisory authorities or to data subjects which either Party is required to make under the GDPR.
10.4.2 Each Party will not process, and will use Commercially Reasonable Efforts to require each applicable Permitted Third Party of such Party to not process, any Personally Identifiable Information in a way that is contrary to Applicable Laws or any Informed Consent.
10.4.3 Each Party will use Commercially Reasonable Efforts to maintain, and will use Commercially Reasonable Efforts to require each applicable Permitted Third Party of such Party to maintain, appropriate and sufficient technical and organizational security measures to maintain the confidentiality of Personally Identifiable Information and to protect such data
56
ACTIVE/105681617.22
against accidental or unlawful destruction or accidental loss, damage, alteration, unauthorized disclosure or access, in particular where such data is transmitted over a network. These technical and organizational security measures shall ensure a level of security appropriate to the risk, including, as appropriate, (a) pseudonymisation and encryption; (b) the ability to ensure the ongoing confidentiality, integrity, availability and resilience of processing systems and services; (c) the ability to restore the availability and access to the Personally Identifiable Information in a timely manner in the event of a physical or technical incident; and (d) a process for regularly testing, assessing and evaluating the effectiveness of those measures.
10.4.4 Each Party shall notify the other Party of: (a) any unauthorized use or disclosure or breach of any Personally Identifiable Information promptly upon discovery of such occurrence; and (b) the transmittal of any related breach notification to any affected person, Governmental Authority or the media. Each Party will use Commercially Reasonable Efforts to require each applicable Permitted Third Party of such Party to notify the such Party of: (i) any unauthorized use or disclosure or breach of any Personally Identifiable Information promptly upon discovery of such occurrence and (ii) the transmittal of any related breach notification to any affected person, Governmental Authority or the media.
ARTICLE 11
INDEMNIFICATION AND INSURANCE
11.1 Indemnification by Each Party.
11.1.1 By SFJ. SFJ will indemnify and hold Nektar; its Affiliates and their respective officers, directors, employees and agents (the “Nektar Indemnified Parties”), harmless from any and all Losses arising or resulting from any Claims by a Third Party against any Nektar Indemnified Parties to the extent arising from (a) the gross negligence or willful misconduct of SFJ or any of its Affiliates or any of its or their respective Permitted Third Parties in performing SFJ’s obligations under this Agreement; (b) SFJ’s material breach of this Agreement; (c) any material breach of a Protocol by SFJ, or its Affiliate, or any of its or their respective Permitted Third Parties; except to the extent that any of the foregoing clauses (a) through (c) was caused by (i) the gross negligence or willful misconduct of any Nektar Indemnified Party, or (ii) material breach of this Agreement by Nektar.
11.1.2 By Nektar. Nektar will indemnify and hold SFJ, its Affiliates, SFJ’s investors (including the Specified Investors) and their respective officers, directors, employees and agents (the “SFJ Indemnified Parties”), harmless from any and all Losses arising or resulting from any Claims by a Third Party against any SFJ Indemnified Parties to the extent arising from (a) a Product supplied by Nektar; (b) a physical injury or death of a Subject that is caused by the Subject’s participation in the HNC Clinical Trials whether or not directly attributable to the Product; (c) Nektar’s gross negligence or willful misconduct in performing its obligations under this Agreement; (d) Nektar’s material breach of this Agreement (e) any material breach of a Protocol by Nektar, or its Affiliate, or of its or their respective Permitted Third Parties, (f) actual or alleged infringement of any Third Party’s Intellectual Property by the Product or by either Party in performing its duties or obligations hereunder with respect to the Product; and
57
ACTIVE/105681617.22
(g) injuries sustained by Subjects in connection with the HNC Clinical Trials or the Melanoma Clinical Trial, including Claims arising prior to the Effective Date based upon physical injury or death of a Subject in connection with the HNC Clinical Trials or the Melanoma Clinical Trial, or from the Commercialization of the Product; except to the extent that any of the foregoing (a) through (g) were caused by (i) the gross negligence or willful misconduct of any SFJ Indemnified Party, or (ii) material breach of this Agreement by, SFJ.
11.2 Indemnification Procedure.
11.2.1 Notice of Claim. A Party believing that it is entitled to indemnification under Section 11.1.1 or 11.1.2 (an “Indemnified Party”) will give prompt written notice (each, an “Indemnification Claim Notice”) to the other Party (the “Indemnifying Party”) upon receipt of notice of the commencement of any Claim for which indemnification may be sought, or if earlier, upon the assertion of any such Claim by a Third Party (it being understood and agreed, however, that the failure by an Indemnified Party to give notice of a Claim of a Third Party as provided in this Section 11.2.1 will not relieve the Indemnifying Party of its indemnification obligation under this Agreement except and only to the extent that such Indemnifying Party is actually prejudiced as a result of such failure to give notice). Each Indemnification Claim Notice will contain a description of the Claim and the nature and amount of the Loss (to the extent that the nature and amount of such Loss are known at such time). The Indemnified Party will furnish promptly to the Indemnifying Party copies of all papers and official documents received in respect of any Losses.
11.2.2 Control of Defense. At its option, the Indemnifying Party may assume the defense of any Claim by giving written notice to the Indemnified Party within thirty (30) days after the Indemnifying Party’s receipt of an Indemnification Claim Notice. The assumption of the defense of a Claim by the Indemnifying Party will not be construed as an acknowledgment that the Indemnifying Party is liable to indemnify the Indemnified Party in respect of the Claim, nor will it constitute a waiver by the Indemnifying Party of any defenses it may assert against the Indemnified Party’s claim for indemnification. Upon assuming the defense of a Claim, the Indemnifying Party may appoint as lead counsel in the defense of the Claim any legal counsel selected by the Indemnifying Party that is reasonably satisfactory to the Indemnified Party. In the event the Indemnifying Party assumes the defense of a Claim, the Indemnified Party will promptly deliver to the Indemnifying Party all original notices and documents (including court papers) received by the Indemnified Party in connection with the Claim. Should the Indemnifying Party assume the defense of a Claim, the Indemnifying Party will not be liable to the Indemnified Party for any legal expenses subsequently incurred by such Indemnified Party in connection with the analysis, defense or settlement of such Claim.
11.2.3 Right to Participate in Defense. Without limiting Section 11.2.2, the Indemnified Party will be entitled to (a) participate in, but not control, the defense of such Claim and to engage counsel of its choice for such purpose; provided, however, that such engagement will be at the Indemnified Party’s own expense unless the engagement thereof has been specifically authorized by the Indemnifying Party in writing, and (b) control its defense of such Claim and to engage counsel of its choice for such purpose, at the expense of the Indemnifying
58
ACTIVE/105681617.22
Party, if the Indemnifying Party has failed to assume the defense and engage counsel in accordance with Section 11.2.2.
11.2.4 Settlement. With respect to any Losses related solely to payment of money damages in connection with a Claim and that includes a complete and unconditional release of the Indemnified Party, will not result in the Indemnified Party admitting liability, becoming subject to injunctive or other equitable relief that will otherwise adversely affect the business of the Indemnified Party in any manner, and as to which the Indemnifying Party will have acknowledged in writing the obligation to indemnify the Indemnified Party hereunder, the Indemnifying Party will have the sole right to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Loss, on such terms as the Indemnifying Party, in its sole discretion, will deem appropriate. With respect to all other Losses in connection with Claims, where the Indemnifying Party has assumed the defense of the Claim in accordance with Section 11.2.2, the Indemnifying Party will have authority to consent to the entry of any judgment, enter into any settlement or otherwise dispose of such Loss provided it obtains the prior written consent of the Indemnified Party (which consent will not be unreasonably withheld, conditioned or delayed). The Indemnifying Party will not be liable for any settlement or other disposition of a Loss by the Indemnified Party that is reached without the written consent of the Indemnifying Party (which consent will not be unreasonably withheld, conditioned or delayed). Regardless of whether the Indemnifying Party chooses to defend or prosecute any Claim, the Indemnified Party will not admit any liability with respect to, or settle, compromise or discharge, any Claim without the prior written consent of the Indemnifying Party, not to be unreasonably withheld or delayed.
11.2.5 Cooperation. Regardless of whether the Indemnifying Party chooses to defend or prosecute any Claim, the Indemnified Party will reasonably cooperate in the defense or prosecution thereof and will furnish such records, information and testimony, provide such witnesses and attend such conferences, discovery proceedings, hearings, trials and appeals as may be reasonably requested in connection therewith. Such cooperation will include access during normal business hours afforded to the Indemnifying Party to, and reasonable retention by the Indemnified Party of, records and information that are reasonably relevant to such Claim, and making employees and agents available on a mutually convenient basis to provide additional information and explanation of any material provided hereunder, and the Indemnifying Party will reimburse the Indemnified Party for all its reasonable out-of-pocket expenses in connection therewith.
11.3 Insurance.
11.3.1 Generally. Commencing as of the Effective Date and thereafter during the Development Term, and subject to Section 11.3.2 below, each Party will carry and maintain, at its own expense, insurance coverage of the kind and with liability limits that, at a minimum, satisfy the requirements of Section 11.3.2, to protect itself and the other Party against any claims or liabilities that may arise from the conduct of the HNC Clinical Trials and all other rights and obligations hereunder with insurers with a minimum “A-VII” or better A.M. Best rating. Any deductibles for such insurance policies will be assumed by the insuring Party. Prior to the
59
ACTIVE/105681617.22
Effective Date, and annually, at each anniversary of the Effective Date (unless, during such year, expiration of the applicable policy occurs first, in which case, on such expiration date), at a Party’s written request the other Party will supply documentation of such insurance coverage via certificates of insurance, if applicable. Each Party shall endeavor to provide the other Party a minimum of thirty (30) days’ notice of cancellation or non-renewal, 10 days for non-payment of premium prior written notice if it is unable to obtain appropriate insurance coverage or if its coverage is canceled, unable to be renewed or changed. For clarity, any insurance coverage or the failure to maintain adequate insurance coverage does not limit or reduce a Party’s liability under this Agreement. Each Party will ensure that no subcontractor, including any Permitted Third Party, will continue to perform the work unless such subcontractor is insured as deemed appropriate by the Party engaging the Permitted Third Party.
11.3.2 Minimum Requirements. Commencing as of the start of the Clinical Trials and thereafter, during the Term (or longer if otherwise stated below), at a minimum, each Party will maintain the following types of insurance coverage at a minimum level that is the greater of (a) the highest minimum level required by Applicable Law in the countries in which the HNC Clinical Trials and other obligations hereunder are being performed or (b) the following (to the extent different).
11.3.2.1 Commercial General Liability: Commercial General Liability, [***] per occurrence; [***] aggregate, including Premises & Operations and Personal Injury.
11.3.2.2 Umbrella Excess Liability: [***] per occurrence.
11.3.2.3 Clinical Trials Liability: [***] per occurrence. Nektar will obtain such Clinical Trials Liability insurance on a global basis, and, if required, supplemented Clinical Trials Liability Insurance in the US, at its expense and SFJ will obtain supplemental Clinical Trials Liability insurance on a country specific basis as required by Applicable Law at its expense, which will be considered Development Costs. Coverage must be maintained for as long as required by Applicable Law in each country after release of the last Subject from the Clinical Trials or where there is no legal requirement at least three (3) years after the termination of this Agreement.
11.3.2.4 Professional Liability: For SFJ, any subcontractor, including any Permitted Third Party, who provides professional services to a Party for the Clinical Trials, will obtain Professional Liability Insurance in lieu of Clinical Trial Insurance, with a minimum limit of [***] per occurrence. Coverage must be maintained for at least three (3) years after the later of (a) expiration or early termination of this Agreement and (b) release of the last Subject from the HNC Clinical Trials.
11.3.3 Additional Insured. Each Party will include the other Party and its Affiliates as additional insured parties on such Party’s Clinical Trial Liability insurance, as set forth in Section 11.3.2.3 for five (5) years after the later of termination of this Agreement or release of the last Subject from the Clinical Trials.
60
ACTIVE/105681617.22
11.3.4 Product Liability Insurance. Nektar will be responsible for maintaining product liability insurance related to the Development and Commercialization of the Product at its expense with SFJ to be named as an additional insured party.
ARTICLE 12
REPRESENTATIONS AND WARRANTIES
12.1 Representations, Warranties and Covenants of Both Parties.
12.1.1 Each Party hereby represents and warrants that it has the requisite corporate power and authority to enter into this Agreement and that this Agreement constitutes a legal and valid obligation binding upon such Party, enforceable in accordance with its terms.
12.1.2 Each Party hereby represents and warrants that it is not a party to any agreement that would prevent it from fulfilling its obligations under this Agreement.
12.1.3 Each Party agrees, on behalf of itself and its Affiliates, that for the performance of its obligations hereunder, it shall (and shall use Commercially Reasonable Efforts to cause its Permitted Third Parties engaged in connection with the subject matter of this Agreement and its and their respective officers, directors, employees, agents, representatives and consultants (“Representatives”) to):
12.1.3.1 comply with the Anti-Corruption Laws and shall not take any action that will, or would reasonably be expected to, cause the other Party or its Affiliates to be in violation of any Anti-Corruption Laws; and
12.1.3.2 promptly provide the other Party with written notice of the following events: (a) upon becoming aware of any breach or violation by such Party, its Affiliate or any of its or their respective Representatives of any representation, warranty or undertaking set forth in Section 12.1.3.1, or (b) upon receiving a formal notification that it is the target of a formal investigation by a Governmental Authority for a Material Anti-Corruption Law Violation or upon receipt of information from any of its Representatives connected with this Agreement that any of them is the target of a formal investigation by a governmental authority for a Material Anti-Corruption Law Violation.
12.1.4 Each Party represents that neither it, nor any of its Affiliates, nor to its Knowledge any Permitted Third Parties engaged by it to perform activities in relation to the Product as of the Effective Date is debarred, and each Party covenants that it shall use reasonable efforts to ensure that neither it, nor any of its Affiliates is debarred, in each case under subsections 306(a) or (b) of the US Federal Food, Drug, and Cosmetic Act (US Generic Drug Enforcement Act of 1992; 21 U.S.C. 335a (a) or (b)), and represents that it has not and covenants that it will not, in each case, to its Knowledge, use in any capacity the services of any Person or Permitted Third Party debarred under this law to conduct the HNC Clinical Trials. Each Party further represents that as of the Effective Date, neither it, nor any of its Affiliates are excluded from any federal health care program, including but not limited to Medicare and Medicaid. Each
61
ACTIVE/105681617.22
Party will notify the JSC immediately if any of the representations contained in this Section 12.1.4 becomes untrue to such Party’s Knowledge in any respect.
12.1.5 Each Party hereby represents and warrants that it is licensed, registered, or otherwise qualified in all material respects under all Applicable Laws to do business in each jurisdiction where such licenses, registrations or other qualifications are required. Each Party further covenants that it and its Permitted Third Parties have, or will have at the required times, such certifications, permits, and authorizations as are required to conduct the HNC Clinical Trials and perform any and all of their obligations in connection with the HNC Clinical Trials supervised by it.
12.2 Additional Nektar Representations, Warranties and Covenants.
12.2.1 Licensure, Registration and Accreditation. Nektar hereby represents and warrants that it is licensed, registered, or otherwise qualified in all material respects under all Applicable Laws to do business in each jurisdiction where such licenses, registrations or other qualifications are required. Nektar further represents and warrants that there has not been and covenants that there will not be during the Term any breach or default by Nektar under the BMS Strategic Collaboration Agreement or the Merck CTCSA which has not been or will not be, as applicable, timely cured as permitted thereunder, and that the BMS Strategic Collaboration Agreement and Merck CTCSA are and shall continue to be in full force and effect during the Term, except to the extent that such a breach, default or failure as to the BMS Strategic Collaboration Agreement or Merck CTCSA, as applicable, would not have a material adverse effect on Nektar’s ability to satisfy its obligations under this Agreement.
12.2.2 [***].
12.2.3 CRO Inquiry. Nektar hereby represents and warrants that, up to and as at the Effective Date, after due inquiry to its CRO responsible for conducting the Clinical Trials, Nektar has not received any verbal or written notice of the occurrence of any Serious Safety Issue in the Clinical Trials.
12.2.4 Compliance. Nektar hereby represents and warrants that, prior to the Effective Date, (a) it has conducted all preclinical and clinical activities related to the development of the Product for the Indications in material compliance with Applicable Laws, and (b) to the Knowledge of Nektar, all Third Parties utilized by Nektar to perform any portion of the preclinical and clinical activities have conducted such portion of such preclinical activities in material compliance with Applicable Laws. Nektar will manufacture or contract with a Third Party to manufacture the Product for the Clinical Trials in accordance with GMP.
12.2.5 Intellectual Property. Nektar owns or possesses sufficient legal rights to all patents, trademarks, service marks, trade names, copyrights, trade secrets, information, proprietary rights and processes necessary for the Development, manufacture and Commercialization of the Product without, to the Knowledge of Nektar, any known conflict with or known infringement of the rights of others. To the Knowledge of Nektar, the Development, manufacture and Commercialization of the Product by Nektar does not violate and will not
62
ACTIVE/105681617.22
violate any license or infringes or will infringe any intellectual property rights of any Third Party. There are no pending or, to the Knowledge of Nektar, threatened claims or proceedings by any Person with respect to the ownership, validity or enforceability of the Nektar Intellectual Property. Except as set forth in Schedule 12.2.5, there are no outstanding options, licenses or agreements of any kind granted by Nektar relating to the Development, manufacture and Commercialization of the Product. Nektar has not received any written communications alleging that Nektar has violated or that the Development, manufacture and Commercialization of the Product would violate any of the patents, trademarks, service marks, trade names, copyrights, trade secrets or other proprietary rights of any Third Party. To the Knowledge of Nektar, all issued Patents included in the Nektar Intellectual Property as of the Effective Date are valid and enforceable.
12.2.6 [***].
12.3 SFJ Representations, Warranty and Covenant.
12.3.1 SFJ hereby represents, warrants and covenants that it will have, as and when needed, sufficient funds to satisfy its obligations hereunder as they become due.
12.3.2 Licensure, Registration and Accreditation. SFJ hereby represents and warrants that it is licensed, registered, or otherwise qualified in all material respects under all Applicable Laws to do business in each jurisdiction where such licenses, registrations or other qualifications are required, except as would not reasonably be expected to have a material adverse effect on the Development or Commercialization of the Product.
12.4 DISCLAIMER OF REPRESENTATIONS AND WARRANTIES.
12.4.1 Each Party hereby agrees and understands that because the Clinical Trials and the Product are experimental in nature, the outcome is inherently uncertain and unpredictable. Each Party hereby agrees and understands that the other Party makes no representation, guarantee or warranty, express or implied, regarding the outcome of the Clinical Trials (including achievement of the applicable Clinical Trial Success Criteria), any Research Results generated after the Effective Date, the ability to obtain Regulatory Approval or the patentability, legal protectability or usefulness of any Intellectual Property arising from the Clinical Trials.
12.4.2 EXCEPT AS OTHERWISE SET FORTH IN THIS ARTICLE 12, NEITHER PARTY MAKES, AND EACH PARTY EXPRESSLY DISCLAIMS, ANY REPRESENTATION OR WARRANTY OF ANY KIND WITH RESPECT TO THE SUBJECT MATTER OF THIS AGREEMENT, EITHER ORAL OR WRITTEN, EXPRESS, IMPLIED, STATUTORY OR OTHERWISE, INCLUDING ANY REPRESENTATION OR WARRANTY REGARDING THE USE, RESULTS OR EFFICACY OF THE PRODUCT.
ARTICLE 13
63
ACTIVE/105681617.22
TERM AND TERMINATION
13.1 Term. The term of this Agreement (the “Term”) will commence on the Effective Date and will expire upon the earliest of (i) termination of this Agreement in accordance with Section 13.2, (ii) the date of payment of the last Success Payment due based on all applicable Regulatory Approvals which have been received and (iii) the date of Development Termination, if any.
13.2 Termination.
13.2.1 Termination for Material Breach.
13.2.1.1 Material Breach.
(a) Either Party may terminate this Agreement immediately in the event of a material breach of this Agreement by the other Party (including, for the avoidance of doubt, a Success Payment Default) provided that the breaching Party has received written notice from the non-breaching Party of such breach, specifying in the reasonable detail the particulars of the alleged breach and such breach has not been cured [***] after the date of the relevant notice. For the avoidance of doubt, (i) it will not be a material breach by Nektar if Nektar elects not to file for Regulatory Approval in the event a Clinical Trial does not achieve its Clinical Trial Success Criteria, (ii) a material breach by Nektar under the BMS Agreement that materially and adversely impacts the completion of the Melanoma Clinical Trial or the submission of a filing for Regulatory Approval will be deemed to be a material breach of this Agreement by Nektar and (iii) a failure by Nektar to use Commercially Reasonable Efforts to enforce the BMS Agreement in the event of a material breach by BMS under the BMS Agreement that materially and adversely impacts the completion of the Melanoma Clinical Trial or the submission of a filing for Regulatory Approval will be deemed to be a material breach of this Agreement by Nektar. The non-breaching Party shall have the right to pursue remedies it may have at law or equity for such breach, including the right to seek damages from the breaching Party.
(b) In the event this Agreement is terminated pursuant to this Section 13.2.1 then Nektar may elect to continue development of the Product. If any Regulatory Approval or Other Indication Regulatory Approval is obtained following such termination, Nektar shall remain obligated to pay to SFJ any Success Payments that become due and payable pursuant to Article 6 at the time such payments become due and payable pursuant to Article 6 (if ever) (except to the extent of the amount of any Buy-Out Payment pursuant to Section 6.7) as a result of such Regulatory Approval or Other Indication Regulatory Approval, provided that such Success Payments (or Buy-Out Payment paid by Nektar, as applicable) shall be adjusted as set forth in Section 6.2.
13.2.1.2 Material Breach for Success Payment Default. SFJ may terminate this Agreement immediately in the event that Nektar fails to make any Success Payment on or before its due date (a “Success Payment Default”), provided that Nektar has received written notice from SFJ of such breach, specifying in the reasonable detail the particulars of the Success Payment Default and such Success Payment Default has not been
64
ACTIVE/105681617.22
cured [***] after the date of the relevant notice. In the event that SFJ terminates this Agreement pursuant to this Section 13.2.1.2, Nektar shall pay SFJ, [***] following such termination, an amount equal to all remaining unpaid Success Payments arising from Regulatory Approvals or Other Indication Regulatory Approval that occurred prior to such termination, as adjusted pursuant to Section 6.2. Additionally, if Nektar obtains any Regulatory Approval or Other Indication Regulatory Approval following such termination, Nektar shall remain obligated to pay to SFJ all Success Payments arising from such Regulatory Approval or Other Indication Regulatory Approval in one lump sum payable within thirty (30) days of such Regulatory Approval or Other Indication Regulatory Approval (except to the extent of the amount of any Buy-Out Payment pursuant to Section 6.7), provided that each lump-sum Success Payment (or Buy-Out Payment paid by Nektar, as applicable) shall be adjusted as set forth in Section 6.2.
13.2.2 Termination for Failure to Achieve Clinical Trial Success Criteria.
13.2.2.1 SFJ may terminate this Agreement at any time upon written notice to Nektar in the event that any HNC Clinical Trial does not achieve its Clinical Trial Success Criteria. In the event of such termination by SFJ, or in the event of any termination pursuant to Section 13.2.5 due to a Safety Concern, then SFJ shall have a post-termination obligation to fund the wind down the HNC Clinical Trials (including, for the avoidance of doubt, any non-cancellable obligations, but excluding any costs associated with the continued conduct of the HNC Clinical Trials) and conduct operational wind-down of the HNC Clinical Trials in accordance with good clinical practice guidelines, provided that SFJ’s aggregate post-termination monetary obligation to fund and conduct the operational wind-down of HNC Clinical Trials (which for the avoidance of doubt shall be deemed to be Development Costs paid by SFJ), together with all other Development Costs funded by SFJ prior to such termination, shall in no event exceed the Maximum Development Costs.
13.2.2.2 In the event this Agreement is terminated by SFJ pursuant to Section 13.2.2.1, then:
(a) Nektar may elect to continue development of the Product and obtain Regulatory Approval for the Melanoma Indication or the HNC Indication following such termination.
(b) If, within thirty (30) days following such termination Nektar notifies SFJ that it has elected not to continue development of the Product and obtain Regulatory Approval for the HNC Indication following such termination as contemplated by clause (i) of Section 13.2.2.2(a) (a “Development Termination”), SFJ shall have a post-termination obligation to fund the wind down the HNC Clinical Trials (including, for the avoidance of doubt, any non-cancellable obligations but excluding any costs associated with continued conduct of the HNC Clinical Trials) and conduct operational wind-down of the HNC Clinical Trials in accordance with good clinical practice guidelines, provided that SFJ’s aggregate post-termination monetary obligation to fund and conduct the operational wind-down of HNC Clinical Trials (which for the avoidance of doubt shall be deemed to be Development Costs paid by SFJ), together with all other Development Costs funded by SFJ prior to such termination, shall in no event exceed the Maximum Development Costs.
65
ACTIVE/105681617.22
13.2.3 Termination for Failure to Receive Regulatory Approval.
13.2.3.1 This Agreement will, upon written notice from either Party to the other Party, terminate with no further action from either Party if the Product has not received Regulatory Approval from the FDA for any of the Indications after completion of the Clinical Trials, submission by Nektar or BMS, as applicable, of applications for Regulatory Approvals to the FDA, and after Commercially Reasonable Efforts by Nektar to obtain such Regulatory Approvals based on such submitted applications as may be amended from time to time. For the avoidance of doubt, if Regulatory Approval for any Indication or any Other Indication is received from the FDA, then his Agreement may not thereafter be terminated pursuant to this Section 13.2.3.1.
13.2.3.2 This Agreement will, upon written notice from either Party to the other Party, terminate with no further action from either Party, if each of the Clinical Trials is completed or terminated and, with respect to each Clinical Trial, (a) a primary endpoint in such Clinical Trial sufficient for regulatory filing in the U.S. for either full Regulatory Approval or Accelerated Regulatory Approval is not achieved and (b) SFJ reasonably determines that the Research Results of such Clinical Trial do not support Regulatory Approval. For avoidance of doubt, if an application for Regulatory Approval is submitted for any of the Indications or any Other Indication, then this Agreement may not thereafter be terminated pursuant to this Section 13.2.3.2. In the event of any such termination related to the Melanoma Clinical Trial, if (i) the HNC Clinical Trial has been completed but the HNC Clinical Trial Success Criteria was not achieved or (ii) HNC Clinical Trial Futility has occurred, then SFJ shall have a post-termination obligation to fund the wind down the HNC Clinical Trials (including, for the avoidance of doubt, any non-cancellable obligations, but excluding any costs associated with the continued conduct of the HNC Clinical Trials) and conduct operational wind-down of the HNC Clinical Trials in accordance with good clinical practice guidelines, provided that SFJ’s aggregate post-termination monetary obligation to fund and conduct the operational wind-down of HNC Clinical Trials (which for the avoidance of doubt shall be deemed to be Development Costs paid by SFJ), together with all other Development Costs funded by SFJ prior to such termination, shall in no event exceed the Maximum Development Costs.
13.2.3.3 In the event that this Agreement is terminated pursuant to this Section 13.2.3, then, if Nektar elects to continue development of the Product for any Indication or Other Indication, obtains any Regulatory Approval or Other Indication Regulatory Approval following such termination, and the Trial Data Package, Research Results or the Melanoma Clinical Trial Research Results are utilized to demonstrate the efficacy of the Product in support of such Regulatory Approval or Other Indication Regulatory Approval, Nektar shall remain obligated to make any Success Payments that become due and payable pursuant to Article 6 at such time that such payments become due and payable (if ever) pursuant to Article 6 as a result of such Regulatory Approval or Other Indication Regulatory Approval (except to the extent of the amount of any Buy-Out Payment paid by Nektar pursuant to Section 6.7), provided that such Success Payments (or Buy-Out Payment, as applicable) shall be adjusted as set forth in Section 6.2.
66
ACTIVE/105681617.22
13.2.4 Termination for Bankruptcy. Either Party may terminate this Agreement upon written notice to the other Party if the other Party makes an assignment for the benefit of creditors, or commences a case or proceeding under any bankruptcy, reorganization, insolvency, or similar laws, has a trustee or receiver or similar officer of any court appointed for such Party, or for substantial part of the property of such Party, or bankruptcy, reorganization, insolvency, or liquidation proceedings are instituted by or against such Party without such proceedings being dismissed, in each of the foregoing cases for a period of at least [***].
13.2.4.1 In the event SFJ terminates this Agreement pursuant to this Section 13.2.4 at any time prior to the First Regulatory Approval, then Nektar will pay SFJ within sixty (60) days of the date of termination an amount equal to one hundred percent (100%) of Development Costs paid or incurred by SFJ prior to such termination.
13.2.4.2 In the event SFJ terminates this Agreement pursuant to this Section 13.2.4 at any time on or after the First Regulatory Approval and prior to the Second Regulatory Approval, then (a) Nektar will pay SFJ within sixty (60) days of the date of termination an amount equal to all remaining unpaid Schedule A Success Payments (as adjusted pursuant to Section 6.2) plus (b) the amount, if any, by which one hundred percent (100%) of Development Costs paid or incurred by SFJ prior to such termination exceeds the amount set forth in clause (a).
13.2.4.3 In the event SFJ terminates this Agreement pursuant to this Section 13.2.4 at any time on or after the Second Regulatory Approval, then Nektar will pay SFJ within sixty (60) days of the date of termination an amount equal to all remaining unpaid Schedule A Success Payments and all remaining Schedule B Success Payments (in each case as adjusted pursuant to Section 6.2).
13.2.5 Termination for Safety Concerns. Either Party may terminate this Agreement upon written notice to the other Party if (a)(i) the IDMC recommends termination of the HNC Clinical Trial for reasons pertaining to the health or safety of the Subjects or as a result of HNC Clinical Trial Futility and (ii) Nektar in good faith reasonably believes there to be a basis for termination of the HNC Clinical Trial based upon such IDMC recommendation for reasons pertaining to the health or safety of the Subjects or for HNC Clinical Trial Futility, or (b) the Parties mutually agree a material health or safety concern with respect to the Subjects of the HNC Clinical Trial exists (either (a) or (b) being a “Safety Concern”). In the event that this Agreement terminates pursuant to this Section 13.2.5 due to a Safety Concern, then Nektar will not be obligated to pay SFJ any Success Payments arising from an HNC Regulatory Approval or reimburse SFJ for any Development Costs incurred by SFJ in connection with the HNC Clinical Trials (provided, that for the avoidance of doubt, Nektar will remain obligated to pay SFJ for any Success Payments arising from a Melanoma Regulatory Approval or an Other Regulatory Approval, regardless of whether such Melanoma Regulatory Approval or Other Regulatory Approval occurs before or after such termination of this Agreement). Notwithstanding the foregoing, if this Agreement terminates pursuant to this Section 13.2.5: (A) if (i) such termination was due to a Safety Concern that was Known by Nektar as being material as of the Effective Date, (ii) the material data Known to Nektar as of the Effective Date that show,
67
ACTIVE/105681617.22
demonstrate, or identify such material Safety Concern were not included in the Data Room, disclosed in writing to SFJ or otherwise publicly known prior to the Effective Date and (iii) SFJ was not otherwise aware of such Safety Concern as of the Effective Date, then Nektar will pay SFJ within sixty (60) days of the date of termination an amount equal to three hundred percent (300%) of Development Costs paid or incurred by SFJ through the date of termination, with all Success Payments previously made to SFJ to be credited against such termination payment, and (B) if following such termination Nektar elects to continue development of the Product pursuant to the HNC Clinical Trials and obtains HNC Regulatory Approval, Nektar will remain obligated to pay any Success Payments that become due and payable pursuant to Article 6 at such time as such Success Payments become due and payable (if ever) pursuant to Article 6 as a result of such Regulatory Approval or Other Indication Regulatory Approval (except to the extent of the amount of any Buy-Out Payment paid by Nektar pursuant to Section 6.7), provided that such Success Payments (or Buy-Out Payment, as applicable) shall be adjusted as set forth in Section 6.2 and shall be reduced by the amount previously paid by Nektar to SFJ pursuant to this Section 13.2.5.
13.2.6 Termination for Certain Breaches/Actions.
13.2.6.1 SFJ may terminate this Agreement if (a) Nektar has breached by its own actions, or by the actions of any of its Representatives, either of Section 12.1.3 or Section 12.1.4 in any material respect, (b) a Representative of Nektar has breached the policy attached as Exhibit F1 in any material respect and such breach results in a Material Anti-Corruption Law Violation, or (c) (i) Nektar or any of its Representatives on behalf of Nektar makes improper payments to Government Officials or any other person by or (ii) Nektar or any of its Representatives with respect to services performed on behalf of Nektar accepts any payment, item, or benefit, regardless of value, as an improper inducement to award, obtain or retain business or otherwise gain or grant an improper business advantage from or to any other person or entity, in each case of (i) or (ii) that constitutes a Material Anti-Corruption Law Violation (in any such case ((a), (b) or (c)), a “Nektar Compliance Breach”), unless such Nektar Compliance Breach can be cured without materially delaying or reducing the probability of completing the Clinical Trials or achieving Regulatory Approval of any of the Indications. In the event of such termination, Nektar will not be entitled to any further payments under Article 4, regardless of any activities undertaken or agreements with additional Third Parties entered into prior to termination. In the event that SFJ terminates this Agreement pursuant to this Section 13.2.6.1, then (a) Nektar will pay SFJ, within sixty (60) days of the date of termination, an amount equal to one hundred fifty percent (150%) of Development Costs paid or incurred to Nektar by SFJ prior to such termination and (b) if Nektar elects to continue development of the Product and obtains any Regulatory Approval or Other Indication Regulatory Approval following such termination, Nektar shall remain obligated to pay to SFJ any Success Payments that become due and payable pursuant to Article 6 at such time that such payments become due and payable (if ever) pursuant to Article 6 as a result of such Regulatory Approval or Other Indication Regulatory Approval (except to the extent of the amount of any Buy-Out Payment paid by Nektar pursuant to Section 6.7), provided that such Success Payments (or Buy-Out Payment, as applicable) shall be adjusted as set forth in Section 6.2, and reduced by the amount previously paid by Nektar to SFJ pursuant to this Section 13.2.6.1.
68
ACTIVE/105681617.22
13.2.6.2 Nektar may terminate this Agreement if (a) SFJ has breached by its own actions, or by the actions of any of its Representatives, either of Section 12.1.3 or Section 12.1.4 in any material respect, (b) a Representative of SFJ has breached the policy attached as Exhibit F2 in any material respect and such breach results in a Material Anti-Corruption Law Violation, or (c) (i) SFJ or any of its Representatives on behalf of SFJ makes improper payments to Government Officials or any other person or (ii) SFJ or any of its Representatives with respect to services performed on behalf of SFJ accepts any payment, item, or benefit, regardless of value, as an improper inducement to award, obtain or retain business or otherwise gain or grant an improper business advantage from or to any other person or entity, in each case of (i) or (ii) that constitutes a Material Anti-Corruption Law Violation (in any such case ((a), (b) or (c)), an “SFJ Compliance Breach”), unless such SFJ Compliance Breach can be cured without materially delaying or reducing the probability of completing the Clinical Trials or achieving Regulatory Approval of any of the Indications. In the event of such termination, SFJ will not be entitled to any further payments hereunder except as set forth below. In the event that Nektar terminates this Agreement pursuant to this Section 13.2.6.2, then Nektar shall pay to SFJ an amount equal to the 100% of the Development Costs paid or incurred by SFJ as of the date of such termination, less the amount of all documented out-of-pocket expenses incurred by or on behalf of Nektar as a result or arising out of such violation by SFJ or any of its Representatives (including any and all amounts paid by Nektar as penalties or fines for such violation, in settlement of legal or administrative proceedings relating to such violation, or otherwise).
13.2.6.3 If a Party learns that any of its Permitted Third Parties has materially breached Section 12.1.3 or Section 12.1.4, or Exhibit F1 or Exhibit F2, as applicable, or that improper payments are being or have been made to Government Officials by any of its Permitted Third Parties with respect to services performed on behalf of such Party or in connection with the Clinical Trials, such Party will notify the other Party and, at the other Party’s option, such Party will terminate its relationship with such Permitted Third Party with respect to the Clinical Trials.
13.2.7 Termination Because of Adverse Patent Impact. SFJ may terminate this Agreement if (a) Nektar is prevented, by final and non-appealable judgment of a court of competent jurisdiction, from further developing or commercializing the Product for any of the Indications, (b) the future value of the Product would likely be materially adversely affected due to (i) a final and non-appealable judgment of a court of competent jurisdiction that Third Party patents that were not publicly disclosed or known to SFJ at the Effective Date are infringed by the manufacture, use, sale, offer for sale or import of the Product for any of the Indications (an “Adverse Patent Impact”), (c) SFJ provides written notice to Nektar of such Adverse Patent Impact and (d) Nektar does not cure such Adverse Patent Impact within [***] from the date of SFJ’s notice to Nektar of an Adverse Patent Impact. In the event that SFJ terminates this Agreement pursuant to this Section 13.2.7, then Nektar shall pay to SFJ, within sixty (60) days of the date of termination, an amount equal to all Development Costs paid or incurred by SFJ as of the date of termination.
13.2.8 Termination for JSC Decision. SFJ may, in its sole discretion, terminate this Agreement in its entirety at any time prior to the date of receipt of the first Regulatory
69
ACTIVE/105681617.22
Approval for any Indication or Other Indication in the event (a) Nektar exercises its decision-making authority under Section 5.2.4 to approve a matter set forth in Section 5.2.2 and, after escalation to the Executive Officers in accordance with Section 5.2.4, SFJ continues in good faith to disagree with such decision and such decision would materially delay or reduce the probability of achieving Regulatory Approval of any of the Indications. In the event that SFJ terminates this Agreement pursuant to this Section 13.2.8, then Nektar will pay to SFJ, within sixty (60) days of the date of termination, an amount equal to the 100% of the Development Costs paid or incurred by SFJ as of the date of such termination plus interest at the annual rate of twenty-five percent (25%) from the date such Development Costs were paid or incurred by SFJ.
13.3 Certain Additional Consequences of Termination. In the event of any termination of this Agreement pursuant to Section 13.2, then:
13.3.1 effective as of such termination, SFJ shall, and it hereby does, assign to Nektar all of SFJ’s and its Affiliates’ right, title and interest in and to all Product Filings, if any, then owned or Controlled by SFJ or any of its Affiliates; provided that if any such Product Filing is not immediately transferable in a country, SFJ shall provide Nektar with all benefit of such Product Filing and such assistance and cooperation as necessary or reasonably requested by Nektar to timely transfer such Product Filing to Nektar or its designee or, at Nektar’s option, to enable Nektar to obtain a substitute for such Product Filing without disruption to Nektar’s development or Commercialization of the Product in such country;
13.3.2 within thirty (30) days after assignment of the Product Filings, if any, pursuant to Section 13.3.1, SFJ shall deliver to Nektar: (a) true, correct and complete copies of all Product Filings in such country (in each case, whether held in the name of SFJ or any of its Affiliates), and disclose to Nektar in writing all previously-undisclosed Research Results within the Trial Data Package; (b) formally transfer or assign, or cause to be formally transferred or assigned, into the name of Nektar or its designee all Product Filings in such country (in each case, whether held in the name of SFJ or any of its Affiliates); and (c) take such other actions and execute such other instruments, assignments and documents as may be necessary to effect, evidence, register and record the transfer, assignment or other conveyance of such rights to Nektar or its designee;
13.3.3 at Nektar’s written request and election in Nektar’s sole discretion, SFJ shall and hereby does, and shall cause its Affiliates to either: (i) wind down in accordance with Applicable Law and observing applicable ethical and regulatory guidelines any or all Clinical Trials being conducted by or on behalf of SFJ or its Affiliate as of the effective date of termination, at SFJ’s cost and expense (which for the avoidance of doubt shall be deemed to be Development Costs paid by SFJ); or (ii) (x) transfer control to Nektar of any or all Clinical Trials being conducted by or on behalf of SFJ or its Affiliate as of the effective date of termination and (y) continue to conduct such Clinical Trials being conducted by or on behalf of SFJ or an Affiliate as of the effective date of termination for up to six (6) months to enable such transfer to be completed without interruption of any such Clinical Trial, in each case ((x) and (y)), at Nektar’s cost and expense; and
70
ACTIVE/105681617.22
13.3.4 SFJ shall, and shall cause its Affiliates to, promptly assign to Nektar or its designee any and all Clinical Trial Agreements, CRO Agreements and other Vendor Agreements to which any of them is a party and cooperate in good faith with Nektar to provide appropriate notice and new contact information to the applicable Sites, Clinical Investigators, CROs and other Vendors and Nektar shall accept such assignment of all obligations of SFJ and its Affiliates thereunder without recourse to SFJ other than any indemnification obligations which SFJ may be liable for thereunder.
13.4 Surviving Obligations.
13.4.1 Accrued Rights and Obligations. Except as expressly set forth in Section 13.4.2, expiration or termination of this Agreement for any reason will not release either Party from any obligation or liability which, at the time of such expiration or termination, has already accrued to the other Party or which is attributable to a period prior to such expiration or termination.
13.4.2 Exclusive Remedy. Notwithstanding anything herein to the contrary, termination of this Agreement by a Party will be without prejudice to other remedies such Party may have at law or equity; provided that the payment by Nektar to SFJ of the amounts specified as being payable upon a given termination in Section 13.2 shall be in lieu of any claim for damages that SFJ may have arising out of or in connection with the circumstances that formed the basis for such termination..
13.4.3 Surviving Obligations. The following provisions of this Agreement, together with any other provisions that expressly specify that they survive, will survive expiration or earlier termination of this Agreement:
13.4.3.1 Article 1, Article 8, Article 9, Article 10, Article 11, Section 12.1, Section 12.4, Section 13.4 and Article 14; and
13.4.3.2 in addition, solely in the case of termination of this Agreement after payment by SFJ to Nektar, or incurrence by SFJ, of any Development Costs, but not in the case of expiration of this Agreement, Sections 6.1–6.7, 7.1–7.2 (in the case of such Sections 7.1–7.2, such provisions shall terminate only after all Nektar Obligations, other than contingent indemnity obligations, have been paid to SFJ or otherwise satisfied in accordance with this Agreement in full), 13.2 and 13.3.
ARTICLE 14
MISCELLANEOUS
14.1 Relationship with Affiliates. Each Party will be responsible for any breach by its Affiliates of its obligations in connection with this Agreement, and each such Party will remain responsible for any responsibilities that it has delegated to an Affiliate as though such Party had performed (or failed to perform) such responsibilities itself.
71
ACTIVE/105681617.22
14.2 Prior Agreements. The Parties agree on behalf of themselves and their respective Affiliates that any prior Confidentiality Agreement, by and between Nektar and SFJ (the “Prior CDA”) is hereby terminated and superseded by this Agreement and that all Information disclosed under or pursuant to the Prior CDAs will constitute Confidential Information disclosed pursuant to this Agreement and will be subject to the terms of Article 9, with the confidentiality and non-use provisions of Article 9 applying retroactively to such Confidential Information from the date of disclosure.
14.3 Notices. Any notice or other communication required or permitted to be given by either Party under this Agreement will be in writing and will be effective when delivered if delivered by hand, reputable courier service, or five (5) days after mailing if mailed by registered or certified mail, postage prepaid and return receipt requested, addressed to the other Party at the following addresses or such other address as may be designated by notice pursuant to this Section 14.3:
14.3.1 If to Nektar:
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
▇▇▇
Attn: Chief Executive Officer
with a copy to:
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
▇▇▇
Attn: General Counsel
with another copy, which shall not constitute notice, to:
▇▇▇▇▇▇▇ Procter LLP
▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇
Attn: ▇▇▇▇▇ ▇▇▇▇▇▇▇▇
72
ACTIVE/105681617.22
14.3.2 If to SFJ:
SFJ Pharmaceuticals XII, L.P.
c/o SFJ Pharmaceuticals XII GP LLC
SIX, ▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇ ▇▇▇ ▇▇▇▇
▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇-▇▇▇▇
▇▇▇▇▇▇ ▇▇▇▇▇▇▇
Attn: ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇
with a copy to:
▇▇▇▇▇▇▇▇ & ▇▇▇▇▇▇▇▇ LLP
▇▇▇ ▇▇▇▇ ▇▇▇▇ ▇▇▇▇
▇▇▇▇ ▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
Attn: ▇▇▇▇▇▇▇ ▇’▇▇▇▇▇▇▇
14.4 Force Majeure. Neither Party will be liable for any breach or delay in performance of any obligation under this Agreement to the extent caused by any of the following: war, terrorism, riot, fire, explosion, pandemic, government prescribed shut-downs, accident, flood, sabotage, changes in Applicable Laws, actions of Governmental Authorities, or any other event beyond the reasonable control of such Party. The Party invoking this Section 14.4 must provide prompt written notice and full particulars of such event to the other Party and will use diligent and commercially reasonable efforts to mitigate the effects of any such force majeure event on such Party’s compliance with and performance under this Agreement.
14.5 Use of Names. Neither Party will use the other Party’s nor any of its Affiliates’ (and, in the case of SFJ and its Affiliates, their respective investors including the Specified Investors) names or trademarks in any promotional materials or advertising without the prior written consent of the other Party except as otherwise expressly permitted in this Agreement.
14.6 Assignment. Without the prior written consent of the other Party hereto, neither Party will sell, transfer, assign, pledge or otherwise dispose of, whether voluntarily, involuntarily, by operation of law or otherwise, this Agreement or any of its rights or duties hereunder; provided, however, that either Party may assign, sublicense or transfer this Agreement and all of its rights and obligations hereunder, in their entirety, to any of its Affiliates or to a successor in connection with the sale or other transfer of all or substantially all of its business or assets to which this Agreement relates, whether by merger, sale of stock, sale of assets or otherwise, and whether this Agreement is actually assigned or is assumed by a Third Party acquirer or the surviving corporation resulting from such transaction by operation of law (e.g., in the context of a reverse triangular merger). Notwithstanding the foregoing, any assignment of the rights or obligations under this Agreement by a Party (i) to an Affiliate shall require such Party to guarantee the performance of such Affiliate’s financial and performance obligations hereunder or (ii) in connection with the sale or other transfer of all or substantially all
73
ACTIVE/105681617.22
of such Party’s business or assets to which this Agreement relates shall require the ultimate Affiliate controlling the other party in such transaction to guarantee such Party’s financial and performance obligations hereunder and such Party shall remain liable for such financial and performance obligations notwithstanding such sale or other transfer of all or substantially all of such Party’s business or assets to which this Agreement relates. Furthermore, notwithstanding any of the foregoing, SFJ may assign its right to receive Success Payments to (a) the limited partners of SFJ or its parent entities, provided that such limited partners notify Nektar of a single account to which Nektar can make all payments that may become due hereunder and assume sole responsibility for distributing all such payments, or to a liquidating trust or similar entity that is established to receive and distribute Success Payments for the benefit of the limited partners in SFJ, that is required to carry out such responsibilities as a single entity, (and in any case under this clause (a), Nektar shall have the unconditional right to follow any instruction it receives or rely on any actions, consents and communications received from or taken by such limited partners or liquidating trust or similar entity without any duty to verify or otherwise determine the validity thereof), (b) an other Third Party to which SFJ assigns this Agreement in its entirety, as permitted by the preceding provisions of this Section 14.6, or (c) an other Third Party to which SFJ sells, assigns or pledges some or all of the Success Payments as permitted by Section 6.7.3. This Agreement is binding upon and will inure to the benefit of each of the Parties, its successors and permitted assigns.
14.7 Further Assurances. The Parties will execute such further reasonable documents and perform such further reasonable acts as may be necessary to comply with or more fully effectuate the terms of this Agreement.
14.8 Fees and Expenses. Each Party to this Agreement will bear its own costs and expenses, including attorneys’ fees and expenses, in connection with the closing of the transactions contemplated hereby.
14.9 Governing Law. The construction and validity of this Agreement and the provisions hereof, and the rights and obligations of the Parties hereunder, will be governed by the internal laws of the State of New York, USA, and, to the extent applicable to Intellectual Property, the applicable federal laws of the US, in each instance without regard to conflict of laws principles.
14.10 Dispute Resolution. The Parties recognize that disputes as to certain matters relating to this Agreement may arise from time to time. It is the objective of the Parties to establish procedures to facilitate the resolution of disputes in an expedient manner by mutual cooperation and without resort to litigation. Accordingly, the Parties agree that any dispute, controversy or claim arising under, out of or in connection with this Agreement, including any subsequent amendments, or the validity, enforceability, construction, performance or breach hereof (and including the applicability of this Section 14.10 to any such dispute, controversy or claim) (each a “Dispute”) shall be resolved as follows:
14.10.1 Either Party shall have the right to refer such Dispute to the Executive Officers for attempted resolution by good faith negotiations for a period of [***]. Any final decision mutually agreed to by the Executive Officers in writing shall be conclusive and binding
74
ACTIVE/105681617.22
on the Parties. With respect to any Dispute that remains unresolved after the expiration of [***] after a Dispute is notified to the Executive Officers, then such Dispute be submitted to the American Arbitration Association (“AAA”) for final and binding arbitration pursuant as set forth in Section 14.10.2 in accordance with its Commercial Arbitration Rules then in force. Notwithstanding the foregoing, no matters relating to breach or alleged breach of the ownership of intellectual property or rights in intellectual property or the validity or enforceability thereof shall be resolved by arbitration, but rather shall be determined by a U.S. federal court of appropriate jurisdiction. Notwithstanding anything in this Agreement to the contrary, either Party shall be entitled to seek preliminary injunctive relief in any court of competent jurisdiction immediately if necessary to prevent irreparable harm to that Party.
14.10.2 Arbitration Process.
14.10.2.1 Either Party shall have the right to initiate arbitration at any time after the expiration of [***] after a Dispute is notified to the Executive Officers. Questions of jurisdiction and arbitration shall be finally settled by the arbitral tribunal.
14.10.2.2 The seat, or legal place, of arbitration shall be New York, New York, and the language of the arbitration shall be English. References herein to any arbitration rules or procedures mean such rules or procedures as amended from time to time, including any successor rules or procedures, and references herein to the AAA include any successor thereto. The arbitration shall be before a tribunal comprised of three (3) arbitrators. Each Party shall select one arbitrator and within fifteen (15) days of the second arbitrator’s appointment, the two (2) Party-appointed arbitrators shall select the third, who shall serve as the tribunal’s chair or president. Any arbitrator(s) not timely selected shall by selected by the AAA. All three (3) arbitrators shall be professionals with substantial experience in development and Commercialization of biopharmaceutical products and related legal principles. An arbitrator shall be deemed to meet these qualifications unless a Party objects within fifteen (15) days after the arbitrator is appointed. The Parties acknowledge that this Agreement evidence a transaction involving interstate commerce. Notwithstanding the provision in Section 14.9 with respect to the applicable substantive law, this arbitration provision, and the arbitration itself, shall be governed by the Federal Arbitration Act, 9 U.S.C. §§ 1 et. seq.
14.10.2.3 Consistent with the expedited nature of arbitration, each Party will, upon the written request of the other Party, promptly provide the other with copies of documents on which the producing Party may rely in support of or in opposition to any claim or defense. There shall be no right to depositions of witnesses unless the arbitrators find good cause to conclude that such additional discovery is necessary, in which case each Party shall be entitled to take up to a maximum of three (3) depositions, which shall be held within forty-five (45) days after such finding of good cause. Additional depositions may be scheduled only with the permission of the arbitrators, and for good cause shown. Each deposition shall be limited to a maximum of one (1) day’s duration. The arbitrators shall also have the discretion to allow requests for production of documents, upon a showing of good cause. All objections are reserved for the arbitration hearing except for objections based on privilege and proprietary or Confidential Information. The Parties shall not utilize any other discovery mechanisms,
75
ACTIVE/105681617.22
including international processes, U.S. federal statutes and third party discovery, to obtain additional evidence for use in the arbitration. Any Dispute regarding discovery, or the relevance or scope thereof, shall be determined by the arbitrators, which determination shall be conclusive. All discovery shall be completed within sixty (60) days following the appointment of the arbitrators. The arbitrators will conduct any evidentiary hearing or final hearing on the merits within one hundred twenty (120) days of confirmation of the Panel, and shall issue a reasoned decision within forty-five (45) days of the conclusion of the evidentiary hearing or final hearing on the merits..
14.10.2.4 The arbitrators shall have no authority to award punitive, enhanced, multiple or other damages not measured by the prevailing Party’s actual damages, except as may be required by statute. Each Party expressly waives and foregoes any right to consequential, punitive, special, exemplary or similar damages or lost profits, and the arbitrators will have no authority to award the same. The arbitrators shall have no power or authority, under the AAA’s Commercial Arbitration Rules and procedures or otherwise, to relieve the Parties from their agreement hereunder to arbitrate or otherwise to amend or disregard any provision of this Agreement. The cost of the arbitration, including the fees of the arbitrators and reasonable attorneys’ fees of the prevailing Party, shall be borne by the Party the arbitrator determines has not prevailed in the arbitration.
14.10.2.5 If an arbitral award does not impose an injunction on the losing Party or contain a money damages award in excess of five million dollars ($5,000,000), then the arbitral award shall be final and binding and shall only be subject to such challenges as would otherwise be permissible under the Federal Arbitration Act, 9 U.S.C. § 1 et. seq. Judgment on such an award may be entered in any court of competent jurisdiction and the Parties undertake to carry out the award without delay. In the event that an arbitral award imposes an injunction or contains a monetary award in excess of five million dollars ($5,000,000), the Parties agree that such award may be appealed pursuant to the AAA’s Optional Appellate Arbitration Rules (“Appellate Rules”) and should not be considered to be final and binding until after the time for filing the notice of appeal under the Appellate Rules has expired. Appeals must be initiated within thirty (30) days of receipt of the award, as defined by the Appellate Rules, by filing a Notice of Appeal within any AAA office. Following the appeal process, the decision rendered by the appeal tribunal shall be final and binding and judgment on that award may be entered in any court of competent jurisdiction and the Parties undertake to carry out the award without delay.
14.10.2.6 Except as may be required by law, or to protect or pursue a legal right to enforce or challenge an award in legal proceedings, where needed for the preparation or presentation of a claim or defense in this arbitration, or by order of the arbitral tribunal upon application of a Party, neither a Party nor an arbitrator may disclose the existence, content, or results of any arbitration hereunder without the prior written consent of both Parties.
14.11 Limitation of Liability. TO THE MAXIMUM EXTENT PERMITTED BY LAW AND NOTWITHSTANDING ANY PROVISION IN THIS AGREEMENT TO THE CONTRARY, NEITHER PARTY WILL BE LIABLE TO THE OTHER PARTY FOR ANY
76
ACTIVE/105681617.22
INDIRECT, INCIDENTAL, CONSEQUENTIAL, SPECIAL, RELIANCE OR PUNITIVE DAMAGES OR LOST OR IMPUTED PROFITS OR ROYALTIES OR COST OF PROCUREMENT OF SUBSTITUTE GOODS OR SERVICES, WHETHER LIABILITY IS ASSERTED IN CONTRACT, TORT (INCLUDING NEGLIGENCE AND STRICT PRODUCTS LIABILITY), INDEMNITY OR CONTRIBUTION, AND IRRESPECTIVE OF WHETHER THAT PARTY OR ANY REPRESENTATIVE OF THAT PARTY HAS BEEN ADVISED OF, OR OTHERWISE MIGHT HAVE ANTICIPATED THE POSSIBILITY OF, ANY SUCH LOSS OR DAMAGE. THE PARTIES AGREE THAT THE LIMITATIONS SPECIFIED IN THIS SECTION 14.11 WILL APPLY EVEN IF ANY LIMITED REMEDY SPECIFIED IN THIS AGREEMENT IS FOUND TO HAVE FAILED OF ITS ESSENTIAL PURPOSE. WITHOUT LIMITING THE GENERALITY OF THE FOREGOING, “CONSEQUENTIAL DAMAGES” WILL BE DEEMED TO INCLUDE, AND NEITHER PARTY WILL BE LIABLE TO THE OTHER PARTY OR ANY OF SUCH OTHER PARTY’S AFFILIATES, REPRESENTATIVES OR STOCKHOLDERS FOR ANY DAMAGES BASED ON OR MEASURED BY LOSS OF PROJECTED OR SPECULATIVE FUTURE SALES OF THE PRODUCT, ANY PAYMENT DUE UPON ANY UNACHIEVED EVENT UNDER ARTICLE 6, OR ANY OTHER UNEARNED, SPECULATIVE OR OTHERWISE CONTINGENT PAYMENTS PROVIDED FOR IN THIS AGREEMENT. FOR THE AVOIDANCE OF DOUBT, THIS SECTION 14.11 IS NOT MEANT TO LIMIT NEKTAR’S OBLIGATION TO PAY SFJ THE AMOUNTS SET FORTH IN ARTICLE 6 OR SECTION 14.2.
14.12 Covenant Not to ▇▇▇. Nektar shall not, and shall cause its Affiliates to not, commence or pursue, or aid any other Person in any action, claim, or other legal proceeding (whether in contract, tort, or otherwise) against the Specified Investors with respect to the transactions contemplated by this Agreement.
14.13 Cumulative Remedies. Unless expressly set forth in this Agreement, all rights and remedies of the Parties, including all rights to payment, rights of termination, rights to injunctive relief, and other rights provided under this Agreement, will be cumulative and in addition to all other remedies provided for in this Agreement, in law, and in equity.
14.14 Relationship of the Parties.
14.14.1 Independent Contractors. Nothing contained herein will be deemed to create a partnership, joint venture, or similar relationship between the Parties, including for tax purposes. Neither Party is the agent, employee, joint venturer, partner, franchisee, or representative of the other Party. Each Party specifically acknowledges that it does not have the authority to, and will not, incur any obligations or responsibilities on behalf of the other Party. Notwithstanding anything to the contrary in this Agreement, each Party (and its officers, directors, agents, employees, and members) will not hold themselves out as employees, agents, representatives, or franchisees of the other Party or enter into any agreements on such Party’s behalf.
14.14.2 Direction. Neither Party will be subject to the supervisory direction of the other Party in regard to the conduct of the Clinical Trials.
77
ACTIVE/105681617.22
14.15 No Third Party Beneficiaries. This Agreement and the provisions herein are for the benefit of the Parties only, and are not intended to confer any rights or benefits to any Third Party other than the Specified Investors and their successors and assigns, which are express third party beneficiaries of Sections 9.2, 9.6, 11.1.2, 14.5 and 14.12 hereunder.
14.16 Rights Reserved. No license or any other right is granted to either Party, by implication or otherwise, except as specifically set forth in this Agreement. All rights not exclusively granted to SFJ are reserved to Nektar and its Affiliates. Notwithstanding any other provision of this Agreement to the contrary, and for clarity, no Intellectual Property or other proprietary rights Controlled by Nektar or its Affiliates will be assigned or licensed to SFJ in connection with this Agreement.
14.17 Nonsolicitation. During the Term and for a period of twelve (12) months thereafter, neither Party shall solicit an employee of the other Party who is or has been involved in the performance or oversight of any of the development activities hereunder to terminate his or her employment and accept employment or work as a consultant with the soliciting Party. Notwithstanding the foregoing, nothing herein shall restrict or preclude the Parties’ right to make generalized searches for employees by way of a general solicitation for employment placed in a trade journal, newspaper or website.
14.18 Amendments; No Waiver. Unless otherwise specified herein, no amendment, supplement, or modification of this Agreement will be binding on either Party unless it is in writing and signed by both Parties. No delay or failure on the part of a Party in the exercise of any right under this Agreement or available at law or equity will be construed as a waiver of such right, nor will any single or partial exercise thereof preclude any other exercise thereof. All waivers must be in writing and signed by the Party against whom the waiver is to be effective. Any such waiver will constitute a waiver only with respect to the specific matter described in such writing and will in no way impair the rights of the Party granting such waiver in any other respect or at any other time.
14.19 Severability. If any provision (or portion thereof) of this Agreement is determined by a court or arbitration to be unenforceable as drafted by virtue of the scope, duration, extent, or character of any obligation contained herein, it is the Parties’ intention that such provision (or portion thereof) will be construed in a manner designed to effectuate the purposes of such provision to the maximum extent enforceable under such Applicable Law. The Parties will enter into whatever amendment to this Agreement as may be necessary to effectuate such purposes.
14.20 Entire Agreement. This Agreement, including all Exhibits hereto and the Disclosure Letter, contains the entire understanding of the Parties and supersedes, revokes, terminates, and cancels any and all other arrangements, understandings, agreements, term sheets, or representations and warranties, whether oral or written, between the Parties relating to the subject matter of this Agreement.
14.21 Counterparts; Electronic Signatures. This Agreement will be executed in two (2) counterparts, one (1) for either Party, which, taken together, will constitute one and the same
78
ACTIVE/105681617.22
agreement. This Agreement will not be binding on the Parties or otherwise effective unless and until executed by both Parties. This Agreement may be executed electronically, such as by DocuSign.
14.22 Construction. This Agreement has been negotiated by the Parties and their respective counsel. This Agreement will not be construed in favor of or against either Party by reason of the authorship of any provisions hereof.
[Signature Page Follows]
79
ACTIVE/105681617.22
IN WITNESS WHEREOF, the Parties, intending to be legally bound hereby, have caused this Agreement to be executed in duplicate by their duly authorized representatives as of the Effective Date.
By: /s/ ▇▇▇▇▇▇ ▇. ▇▇▇▇▇
Name: ▇▇▇▇▇▇ ▇. ▇▇▇▇▇
Title: President and Chief Executive Officer
Date: February 12, 2021
By: /s/ ▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇▇
Name: ▇▇▇ ▇. ▇▇▇▇▇▇▇▇▇▇▇
Title: Senior Vice President, Chief Operating Officer and Chief Financial Officer
Date: February 12, 2021
IN WITNESS WHEREOF, the Parties, intending to be legally bound hereby, have caused this Agreement to be executed in duplicate by their duly authorized representatives as of the Effective Date.
SFJ Pharmaceuticals XII, L.P.
By: SFJ XII GP LLC,
a Caymans Islands limited liability company
Its: General Partner
By: /s/ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Name: ▇▇▇▇▇▇▇▇ ▇▇▇▇▇
Title: Manager
Date: February 12, 2021
EXHIBIT LIST
Exhibit A The Product
Exhibit B Current Approved CROs
Exhibit C Current Approved Vendors
Exhibit D HNC Main Clinical Draft Trial Protocol
Exhibit E Executive Officers
Exhibit F1 Nektar Anti-Bribery and Anti-Corruption Practices
Exhibit F2 SFJ Anti-Bribery and Anti-Corruption Practices
Exhibit G-1 SFJ Quarterly Labor Fee
Exhibit G-2 Nektar Quarterly Labor Fee
Exhibit H RACI
Exhibit I Timeline
Exhibit J Study Site Countries
Exhibit K TORO
ACTIVE/105681617.22

