MARKETING AND DISTRIBUTION AGREEMENT
Exhibit 10.72
*** TEXT OMITTED AND SUBMITTED PURSUANT TO CONFIDENTIAL TREATMENT REQUEST
MARKETING AND DISTRIBUTION AGREEMENT
This MARKETING AND DISTRIBUTION AGREEMENT (this “Agreement”) is made as of April 14, 2016 (the “Effective Date”), by and among Napo Pharmaceuticals, Inc., a Delaware corporation, with its principal place of business at 000 Xxxx Xxxxxx # 00X, Xxx Xxxxxxxxx, Xxxxxxxxxx 00000 (“Napo”), and BexR Logistix, LLC, a Texas limited liability company (“BexR”) with its principal place of business at 00000 Xxxxxxxxxx Xxxxxxx 00 Xxxx, Xxxxx 0000, Xxx Xxxxxxx, XX 00000-0000. (Napo and BexR are also sometimes referred to herein as the “parties” or individually as a “party”).
RECITALS
A. Napo is in the business of developing and commercializing pharmaceuticals, including the drug Fulyzaq® (Crofelemer).
B. BexR provides logistical support and product consignment services to a broad range of industries, including pharmaceutical companies.
C. Napo wishes to engage BexR, and BexR wishes to be so engaged, to provide certain telemarketing, consignment and distribution services pursuant to the terms and conditions set forth in this Agreement.
NOW, THEREFORE, IN CONSIDERATION of the mutual covenants and agreements herein contained, Napo and BexR hereby agree as follows:
1. Appointment
1.1 Distributor. Subject to the terms and conditions of this Agreement, Napo appoints BexR as its distributor with the right to telemarked and sell (as described in Section 4.9 of this agreement), and the exclusive right to distribute the prescription drug Fulyzaq® (Crofelemer) (the “Product”) during the Term of this Agreement in the United States and its Territories (the “U.S.”), and BexR accepts such appointment.
2. Napo’s Agreements
2.1 Product. Napo agrees to make, or to cause to be made, the Product available to BexR for sale to customers in a manner consistent with the written forecasts of Product sales prepared and provided by BexR and delivered to and accepted in writing by Napo. Napo shall be responsible for planning and coordinating the relabeling of existing Product inventory, including modification of bottle labels and package inserts. Should Napo wish to engage Mission in coordination of repacking Product, there will be a pass-thru cost plus coordination fee to be determined. Napo shall be responsible for the creation of a sample unit for the Product. Napo shall be the owner of the Product paying BexR for the consignment services including storage, shipping and distribution services and telemarketing services it will also provide.
2.2 Marketing and Marketing Strategy. Napo shall develop a marketing plan (“Marketing Plan”) for the Product, including a budget for advertising and promotion expenses, based on net revenue benchmarks. Napo will prepare, at Napo’s sole expense, all
documentation, advertising and sales promotion materials, instructions, and manuals relating to the Product (collectively, “Product Materials”). Napo agrees to spend a minimum of $[***] (which shall include any fees paid to SmartPharma for its marketing services and the Advance from BexR which will be detailed in Exhibit A “the Marketing Plan” upon approval by Napo) for Product Materials and commercialization of the Product during the first twelve (12) months of the Agreement. In connection with the marketing strategy and unless agreed otherwise by BexR, Napo shall purchase and analyze market data (IMS or Symphony), including national and physician-level data. Napo shall develop web site content for promotion of the Product. In coordination with BexR, Napo shall develop a prescriber target list.
2.3 Intellectual Property. Napo shall maintain all patents, trademarks, URLs and other intellectual property related to the Product, as determined in Napo’s sole business judgment.
2.4 Reimbursement and Managed Care Strategy. In cooperation with and based on input from BexR, Napo shall develop a managed care strategy for sales to customers, including pricing discounts and rebates. Napo shall analyze current co-payment assistance programs and develop and negotiate new or additional co-payment assistance programs as needed based upon the Marketing Plan.
2.5 Regulatory Approvals. Napo shall own the NDA for the Product and shall be responsible for obtaining and maintaining all required regulatory approvals, and for all communications with federal, state and local authorities in the U.S. (“Governmental Entities”). Napo shall be responsible for obtaining all approvals from Governmental Entities for labeling and relabeling of the Product.
2.6 Training. Napo shall develop speaker development and training for key opinion leaders, including development of training content, training materials and event planning. Napo shall review and revise sales training materials and develop new training materials as needed specific to the Product. Napo shall conduct Product specific sales training for BexR telemarketing representatives.
3. Term. The Term of this Agreement shall be four (4) years commencing as of the Effective Date (“Initial Term”). The Agreement will renew automatically for successive one (1) year terms (“Subsequent Terms”) unless written notice of termination is provided by either party to the other party not less than 90 days prior to the expiration of the then current Initial or Subsequent Term (the Initial Term, plus any Subsequent Terms, collectively, the “Term”).
*** Confidential Treatment Requested
4. BexR’s Agreements
4.1 NDA and NDC. BexR shall assist Napo with transfer of the Product New Drug Application (“NDA”) from Valeant Pharmaceuticals International, Inc., as successor to Salix Pharmaceuticals, Inc. (“Valeant”) and shall coordinate with Napo to obtain a new National Drug Code (“NDC”) for the Product, including assistance with interactions with the FDA regarding the foregoing, and thereafter, assist Napo with the maintenance of the NDA in the U.S. as defined in Exhibit B.
4.2 Sunshine Act and Prescription Drug Promotion Reporting Compliance. Upon request, BexR shall assist Napo with the Compilation of data under Section 6002 of the Patient Protection and Affordable Care Act “Sunshine Act” (a.k.a. “Open Payments”) expense reporting. Such data shall include all information reasonably necessary to permit Napo to complete any transparency or “Sunshine” reporting requirements which may be applicable under state or federal laws, including assistance with the completion of Form 2253 submissions to the Office of Prescription Drug Promotion Reporting.
4.3 Pharmacovigilance. BexR shall assist Napo in the development and implementation of policies, standard operating procedures and applicable infrastructure for compliance with drug safety and consumer protection. BexR shall provide call center staffing and assistance with professional and consumer inquiries. BexR shall prepare the appropriate documentation in compliance of annual reports and of reported adverse events, and will provide these to Napo for FDA submission. Further, BexR shall provide support for all product inquiries received by healthcare professionals and consumers regarding Product, and assist Napo in the development of all medical communications as set forth in a separate pharmacovigilance agreement which the Parties agree to finalize within thirty (30) days of Closing.
4.4 Warehousing and Distribution of Finished Product. BexR shall store and distribute the finished Product, BexR shall provide inventory management to facilitate finished Product supply, including communication with Napo and other contract development, supply chain, and finished product manufacturers. BexR shall arrange for and coordinate all trade services, including contract management with wholesalers, group purchasing organizations and others, handling sales transactions, and management of wholesale incentives, rebates and returns. BexR shall provide retail trade support, as needed, and any other post-finished Product supply chain requirements.
4.5 Managed Care Contracts and Rebates. BexR shall assist Napo in transitioning managed care contracts, including the structure and management of rebates. Upon request, BexR shall assist Napo in the negotiation of new managed care contracts on terms and conditions, including discount and rebate terms, provided by Napo. BexR shall assist Napo with preparation, negotiation and implementation of managed care contracts with Governmental Entities, including contracts under the federal and state Medicare and Medicaid programs and VA pricing agreements and the like, and with managing communications with such Governmental Entities.
4.6 Promotional Review. BexR shall provide regulatory, medical, and legal recommendations of all promotional materials prepared by Napo with respect to the Product. The final decision on all labeling and promotional materials shall be made by Napo.
4.7 Delegation. BexR shall use subcontractors as needed to complete these and other tasks in the Agreement and shall maintain a written contract with third parties for these services, including a Nondisclosure Agreement with each subcontractor.
4.8 Non-Personal Promotion and Marketing Support. As agreed upon, BexR shall provide the following non-personal promotion services:
(a) Selling and tele-detailing to an agreed upon target list (the “Non-Personal Promotion Target List”);
(b) Distribution of samples to the Non-Personal Promotion Target List;
(c) Implementation of co-pay assistance program for non-personal prescribers.
5. Fees
5.1 Advance. Upon execution of this Agreement, BexR shall place Two Hundred Fifty Thousand Dollars ($250,000) (the “Advance”) in a designated account (the “Account”) to cover necessary and reasonable expenses of Napo relating to commercialization of the Product.
5.2 Expenditures from the Account. Napo may draw funds from the Account to pay reasonable commercialization expenses, including but not limited to, costs related to the following:
(a) Transfer of inventory from Valeant to Napo’s or BexR’s warehouse;
(b) Relabeling of inventory, including printing new package inserts and bottle labels, purchase of bottles, and repackaging;
(c) Packaging of portions of Product inventory as samples;
(d) Purchase of market data, including prescriber-level data for the HIV antiretroviral market and for Product prescribers;
(e) Reasonable honoraria and out of pocket expenses for market research and development of training for key opinion leaders related to the Product, to be conducted by SmartPharma, LLC or another vendor mutually agreed upon by the parties;
(f) Out of pocket costs for design and printing of promotional materials;
(g) Out of pocket costs for development of copay assistance program;
(h) Out of pocket costs for revision of sales training materials; and
(i) Additional expenses as agreed upon by Napo and BexR.
Napo shall provide monthly reports to BexR within ten (10) days of the end of each month, detailing the expenses paid from the Account during the prior month.
5.3 Repayment of Advance. Napo shall repay the entire Advance to BexR within the first twenty-four (24) months when Napo has access to the Advance in the Account (the “Payment Period”). Napo shall make quarterly payments to repay the Advance commencing on the first day of the third quarter after the first sale of the Product under a Napo NDC code. The quarterly payments shall be equal to $0.10 per dollar of Net Sales for the first quarter payment, $0.25 per dollar of Net Sales for the second quarter payment and $0.15 per dollar of Net Sales for the remaining quarterly payments. In the event that Napo has not repaid the Advance to BexR by the end of the Payment Period, Napo shall pay the entire outstanding balance to BexR within ten (10) days of the end of the Payment Period.
5.4 Consignment Revenue Share. As consideration for the provision of services under this Agreement, Napo shall pay BexR a share of Napo’s Net Sales equal to: [***] percent ([***]%) of Net Sales for the first twelve (12) months following the first sale of the Product under a Napo NDC code; [***] percent ([***]%) of Net Sales for the second twelve (12) month period; and [***] percent ([***]%) for each twelve (12) month period thereafter until the Agreement is terminated. Beginning in the third twelve (12) month period, Napo shall pay BexR the [***] ([***]) percent of Net Sales up to and including a cap of $[***]for any twelve (12) month period. BexR will pay Napo its share of Net Sales forty-five (45) days after month-end for the first sixty (60) days of the Agreement. Thereafter, BexR will pay Napo its share of Net Sales sixty (60) days after month-end. BexR may reduce any amount currently owed to BexR prior to distributing the amounts due to Napo. For purposes of this Agreement, Net Sales means the gross amount invoiced by BexR for the sale of the Product, less deductions for (a) trade discounts, returns, allowances, fees, reimbursements, rebates, and chargebacks to wholesalers and distributors, buying groups, pharmacy benefit managers, insurance carriers, and distributors; (b) freight, postage, shipping, and insurance expenses; (c) customs and excise duties; (d) rebates and payment to Governmental Entities, under government programs, such as Medicare and Medicaid; (e) sales and other taxes and duties; (f) any other similar or customary deductions consistent with GAAP; and (g) any invoiced amounts not collected by BexR.
6. Compliance with Laws. Each party shall, and shall cause its customers to comply with all foreign, federal, state and local rules, regulations, ordinances and laws applicable to their respective businesses and the use, operation, handling, storage, shipment and distribution of the Product, including without limitation, the U.S. Food, Drug and Cosmetics Act, the federal and state anti-kickback laws, Section 34013 of the Public Health Services Act, and the Sunshine Act. BexR does not assume any obligation for Napo’s compliance with law. BexR and BexR’s customers are solely responsible for the use and operation of the Product in accordance with all applicable laws and regulations, and medical and treatment guidelines, and for ensuring that each operator of the Product is adequately trained and qualified to use and operate each Product safely and properly in a clinical setting and to perform medical procedures in accordance with such laws, regulations and guidelines. Each party represents and warrants that it is aware of and
*** Confidential Treatment Requested
currently in compliance with, and covenants that it will at all times comply with, all anti-bribery and anti-corruption laws applicable to doing business in the areas in which the parties do business, including, but not limited to, the U.S. Foreign Corrupt Practice Act (the “FCPA”) and, if applicable, anti-bribery legislation enacted in accordance with the Organization for Economic Co-operation and Development Convention on Combating Bribery of Foreign Public Officials in International Business Transactions. Each party agrees to comply with all other regulations or industry codes of conduct applicable to its business.
7. Mutual Governance. Mission shall assign an Account Manager, who shall serve as a key point of contact to Napo for all issues arising and related to the Business.
8. Proceedings. If either party receives notice of an actual or threatened inspection, investigation, field action, warning, inquiry, import or export ban, product seizure, enforcement proceeding or similar action by a Governmental Entity with respect to the Product or either party’s activities in connection with the Product, the party receiving such notice shall notify the other in writing within two (2) business days after its receipt of notice of the action and will promptly deliver to the other party copies of any relevant documents received from the government authority. Napo and BexR shall cooperate in response to the action, including but not limited to providing information and documentation as requested by the Government Entity. If the action primarily concerns one party’s activities, that party shall have primary responsibility to respond to the Governmental Entity and to pay all expenses related thereto. In either case, upon request of the responding party, the other party shall reasonably cooperate and provide consulting advice and assistance with the response.
9. Indemnification. Each party shall indemnify the other against all claims, damages, losses, and expenses, including reasonable attorneys’ fees (collectively, “Losses”), arising out of (i) such party’s performance or failure to perform in accordance with the terms and conditions of this Agreement, whether caused by such party’s action or inaction, negligence or the negligence of its employees or agents; and (ii) such party’s failure to comply with the laws, statutes, rules, regulations or orders of any governmental authority having jurisdiction over such party or its business. However, the parties agree that under no circumstances shall BexR be liable for any losses alleged due to lower than forecasted sales amounts, ineffective marketing campaigns, or alleged lost sales of the Product.
10. Insurance. Each party will maintain commercial general liability insurance, including blanket contractual liability insurance covering the obligations (within the scope of insurance) of that party under this Agreement through the term of this Agreement and for a period of three (3) years thereafter, which insurance will afford limits of not less than (i) $5,000,000 for each occurrence for personal injury or property damage liability; and (ii) $5,000,000 in the aggregate with respect to product and completed operations liability. The above limit requirements may be satisfied with the combination of an Umbrella or Excess Liability policy. Napo’s commercial general liability and product and completed operations liability policies shall be endorsed to (i) name Provider as an additional insured; (ii) waive all rights of subrogation against Provider and; (iii) act as primary/non-contributory coverage over any other valid and collectable coverage available to Provider. Each party will provide the other with a certificate of insurance evidencing the above and showing the name of the issuing company, the policy number, the effective date, the expiration date and the limits of liability. The insurance certificate will further
provide for a minimum of thirty (30) days’ written notice (except ten (10) days for non-payment of premium) to the insured of a cancellation of the insurance. If a party is unable to maintain the insurance policies required under this Agreement through no fault on the part of such party, then such party will forthwith notify the other party in writing and the parties will in good faith negotiate appropriate amendments to the insurance provision of this Agreement in order to provide adequate assurances.
11. Adverse Event Reporting. Each party shall notify the other immediately of (a) any adverse comments or complaints by prescribers, users or Governmental Entities regarding the Product, including comments regarding the Product’s quality, stability, contamination, potency, condition, packaging, or any other attributes or defects, and (b) any adverse events that may be attributable to use of the Product, whether or not such party can confirm that the event is actually associated with the Product, and whether or not such party can confirm that the event was due to improper dosing or other negligence on the part of any party. In the event of an actual or alleged defect of the Product, neither party nor its representatives or agents shall make any statement as to the cause of the event until the parties have agreed upon a proposed communication strategy.
12. Product Recall. Napo has the exclusive right to determine if a recall of Product is required and shall bare all expense related to all recalls.
13. Further Obligations. During the Term, each party shall:
(a) not without the other party’s prior written consent make any promises or guarantees about the Product beyond those contained in the Product Materials agreed upon by the parties;
(b) employ a sufficient number of suitably qualified personnel to ensure the proper fulfillment of its obligations under this Agreement;
(c) keep full and proper books of account and records clearly showing all inquiries, quotations, transactions and proceedings relating to the Product;
(d) not make any modifications to the Product, Product labeling or Product instructions for use or packaging except as contemplated by this Agreement;
(e) maintain and store the Product in accordance with all applicable laws and regulations, in a manner that will prevent any compromise of or change in the Product and at a facility that is operated in compliance with all applicable laws and regulations;
(f) present the Product fairly to potential prescribers and users and not disparage the Product or the other party in any way;
(g) not authorize, promote or permit any unapproved use of the Product;
(h) not offer, pay or arrange for any rebate, kickback, bribe or other remuneration to potential prescribers or users in exchange for agreement to prescribe, purchase or use the Product;
(i) not market or distribute the Product for any off-label use;
(j) ensure that all sales and marketing personnel involved in promoting and marketing the Product are adequately trained with respect to the Product;
(k) ensure that no personnel employed or under contract with BexR are or have been subject to debarment or exclusion under any federal or state program; and
(l) make available, and use its best efforts to provide, to any prescribers and other users of the Product all training and continuing education necessary to ensure the proper usage of the Product.
14. Proprietary Information
14.1 Confidentiality. Each party shall treat as confidential and proprietary all data and information disclosed by the other party that is proprietary to, or a trade secret of, such disclosing party (collectively, “Proprietary Information”) and shall not disclose such Proprietary Information to any person not in the disclosing party’s employment or to any person in the disclosing party’s employment not having a specific need to know such Proprietary Information in connection with his or her employment, and shall use such Proprietary Information only in connection with its proper performance of this Agreement. Each party agrees to be liable for any use or disclosure of Proprietary Information in violation of this Agreement by any of its employees, consultants and professional advisors who have access to or are otherwise provided such Proprietary Information. Proprietary Information shall include, but is not limited to, data and information labeled as such by a party, hut shall not include any data or information that is in the public domain through no fault or breach of this Agreement by a party. Neither party shall cause its employees or agents to, reverse engineer, decompile, disassemble or copy any Proprietary Information, including, without limitation, any data or information relating to the Product.
14.2 Injunctive Relief. Each party acknowledges that there is no adequate remedy at law for any breach of this Section 14. Accordingly, each party agrees that the other party shall be entitled to seek injunctive relief, without necessity of posting a bond, to restrain any actual or threatened breach of this Section 14.
15. Termination
15.1 Termination. This Agreement may be terminated prior to the end of any Term as follows:
(a) Breach. Either party may terminate this Agreement immediately upon written notice to the other in the event the other party breaches this Agreement;
(b) With Notice. Either party may terminate this Agreement without cause upon 90 days prior written notice to the other party.
(c) Bankruptcy/Insolvency. Either party may terminate this Agreement immediately upon written notice to the other party in the event that proceedings in bankruptcy or
insolvency are instituted by or against the other party, or a receiver is appointed for the other party, or if any substantial part of the assets of the other party is the object of attachment, sequestration or other type of comparable proceeding, and such proceeding is not vacated or terminated within thirty (30) days after its commencement or institution.
15.2 Payment of Advance on Termination. In the event that either party elects to terminate this Agreement prior to the end of the Term pursuant to this Section 15, Napo shall immediately pay to BexR the entire outstanding balance of the Advance owed to BexR pursuant to Section 5.3 of this Agreement.
15.3 Option. Upon termination of this Agreement pursuant to this Section 15, BexR shall have the first right of negotiation to license the Product if Napo decides to out-license any remaining commercial rights of the Product
15.4 Termination Fee. If Napo terminates this Agreement during the first two years, a Termination Fee shall be paid by Napo to Mission:
(a) The Termination Fee for termination during the first year of the Agreement shall be equal to twenty percent (20%) of Napo’s Net Sales for the last thirty (30) days prior to termination, multiplied by the number of months remaining in the first year of the Agreement. A partial month shall be pro-rated. The Termination Fee for termination during year two of the Agreement shall be equal to fifteen percent (15%) of Napo’s Net Sales for the last thirty (30) days prior to termination, multiplied by six (6).
16. General Provisions
16.1 Notices. All notices and other communications in connection with this Agreement shall be in writing and shall be sent to the respective parties at the addresses set forth in the Preamble hereof, or to such other addresses as may be designated by the respective parties in writing from time to time in accordance with this Section 18.1, by personal delivery, or by registered or certified air mail, postage prepaid, or by express courier service, service fee prepaid, or by facsimile with a hard copy to follow via air mail or express courier service, service fee prepaid. All notices shall be deemed received (i) if given by hand, immediately, (ii) if given by air mail, ten (10) business days after posting, (iii) if given by express courier service, the next business day in the jurisdiction of the recipient or (iv) if given by facsimile, upon delivery of the facsimile transmittal, provided that a confirmation copy is sent by regular mail.
16.2 Independent Contractor. It is expressly agreed and understood that BexR is, and at all times shall be, an independent contractor and not an employee or agent of Napo. Napo shall have no right to direct the time, manner and method by which BexR shall accomplish its work nor shall Napo have the right to control the manner in which any services to be rendered by BexR are to be performed. Nothing in this Agreement shall be construed to characterize the relationship between the parties as that of employer/employee, principal/agent, franchisor/franchisee, partnership or co-venture. All expenses and disbursements that either party may incur in connection with this Agreement shall be borne wholly and completely by such party. Neither party shall have any right or authority to commit or legally bind the other
party in any way. All financial obligations associated with each party’s business are the sole responsibility of such party.
16.3 Entire Agreement; Amendments. This Agreement sets forth the full understanding and entire agreement between the parties concerning its subject matter, and supersedes all prior oral and written understandings and agreements relating thereto. No terms, conditions, understandings or agreements purporting to modify or amend this Agreement shall be effective unless made in writing and signed by both parties. Neither party has relied upon any representation or statement of the other except as stated in this Agreement. All amendments of this Agreement, notices and communications between the parties, and all materials supplied under this Agreement by either party to the other, shall be in the English language.
16.4 Severability. In the event that any one or more of the provisions contained herein, or the application thereof in any circumstances, is held invalid, illegal or unenforceable in any respect for any reason in any jurisdiction, the validity, legality and enforceability of any such provision in every other respect and of the remaining provisions hereof shall not be in any way impaired or affected, it being intended that each of party’s rights and privileges shall be enforceable to the fullest extent permitted by law.
16.5 Governing Law; Consent to Jurisdiction. This Agreement shall be governed by and interpreted in accordance with the laws of the State of Texas. The parties hereby submit to the jurisdiction and venue of any state or federal court located within the State of Texas for resolution of any and all claims, causes of action and disputes arising out of, related to or concerning this Agreement.
16.6 Assignments; Subcontracting. Neither party may assign, transfer or otherwise dispose of this Agreement or any interest therein, or subcontract any of its obligations hereunder, in whole or in part to any individual, firm or corporation without the prior written consent of the other party. Notwithstanding the foregoing, BexR shall entitled to appoint subcontractors or representatives, contract with affiliates and assign this Agreement to its affiliates, provided that BexR shall promptly inform Napo of the appointment of any such subcontractor or representative or an assignment. BexR shall cause any subcontractor, representative or assignee so appointed to comply with all requirements of this Agreement.
16.7 Waiver of Compliance. Any failure by any party hereto to enforce at any time any term or condition under this Agreement shall not be considered a waiver of that party’s right thereafter to enforce each and every term and condition of this Agreement.
16.8 Force Majeure. Neither party shall be liable for failure or delay in performance of any obligation under this Agreement, other than payment of any amount due and payable, if such failure or delay is caused by circumstances beyond the control of the party concerned, including, without limitation, failures resulting from fires, explosion, flood, riot, accidents, labor stoppages, war, inability to secure materials or labor, government acts or regulations or acts of God.
16.9 No Third Party Beneficiaries. Nothing in this Agreement is intended to confer any rights or remedies under or by reason of this Agreement on any persons other than the
parties and their respective successors and permitted assigns. Nothing in this Agreement is intended to relieve or discharge the obligations or liability of any third persons to any of the parties. No provision of this Agreement shall give any third persons any right of subrogation or action over or against any of the parties.
16.10 Expenses. Each of the parties hereto shall pay the fees and expenses of its respective counsel, accountants and other advisors and shall pay all other expenses incurred by it in connection with the negotiation, preparation and execution of this Agreement and the consummation of the transactions contemplated by this Agreement.
16.11 Headings. The headings of the sections and paragraphs of this Agreement have been inserted for convenience of reference only and shall not be deemed to be part of this Agreement.
16.12 Counterparts. This Agreement may be executed in any number of counterparts and by the different parties hereto on separate counterparts, each of which when so executed and delivered shall be an original, but all of which together shall constitute one and the same instrument, and it shall not be necessary in making proof of this Agreement to produce or account for more than one such counterpart.
[signature page follows]
IN WITNESS WHEREOF, the parties have executed this Agreement as an instrument under seal on the day and year first above written by their representatives duly authorizes for such purposes.
|
|
NAPO PHARMACEUTICALS, INC. | |
|
|
a Delaware corporation | |
|
|
| |
|
|
By: |
/s/Xxxx X Xxxxx |
|
|
Name: Xxxx X. Xxxxx | |
|
|
Title: Chief Executive Officer | |
|
|
| |
|
|
| |
|
|
BEXR LOGISTIX, LLC, a Texas limited liability company | |
|
|
| |
|
|
By: |
/s/Xxx Xxxxxx |
|
|
Name: Xxx Xxxxxx | |
|
|
Title: General Manager, BexR Logistix, LLC | |
Crofelemer 2016 Marketing Plan April 2016 Exhibit A

Sep Oct Nov Dec Product Transfer Jan-Apr Onboard partners Feb-Apr Re-Launch Preparation Feb-May Napo Distribution of Fulyzaq Crofelemer New Name Re-Launch Testing and Assessment Crofelemer 2016 Marketing Plan Overview Objectives: Product: Establish supply chain and distribution of crofelemer by Napo Data: Develop key opinion leaders and begin publication of supplemental data analyses in HIV-D Promotion: Test promotional strategies and tactics to determine optimal spend level and mix for 2017 May Jun Jul Aug Jan Feb Mar Apr 2016 May-Jun

Crofelemer 2016 Marketing Plan Re-Launch Preparation Slide 3 Re-Launch Preparation Feb-May 2016 Market Preparation Prescriber analyses – create target lists Fulyzaq prescribers (n=588) HIV writers (n=1,200 – 2,700) Identify potential new brand names – conduct safety testing Identify and develop core of KOLs Meet with HIV advocates Train telesales team for May distribution CMS registration Assess current status of copay assistance program Promotional Activity Review market research to date with HCPs and consumers Conduct supplemental efficacy data analyses of ADVENT and CFHD3092 Develop new messages and data layout for testing Refresh Napo web site Refresh Fulyzaq web site Operations Establish supply chain for trade and samples Establish wholesaler contacts; assess inventory and take orders Package drug at Patheon with Napo label Obtain state licenses necessary for product distribution by Napo Assure compliance readiness for product distribution by Napo Assure regulatory readiness for product distribution by Napo

Crofelemer 2016 Marketing Plan Napo Distribution of Fulyzaq® Slide 4 Napo Distribution of Fulyzaq May-Jun Market Preparation [***] Select new brand name and register trademark Identify reimbursement strategies Prepare HCP and Patient new name communication Train telesales team for launch Design copay assistance program (if required based on assessment) Develop sample distribution plan Promotional Activity Meetings/calls to KOLs and Top 55 Fulyzaq Prescribers One-on-ones / focus groups / teleconferences Telesales to current Fulyzaq writers (Dec 3-10, n=247) Determine managed care formulary position and reimbursement in major plans and state Medicaid programs Testing of new messages/data with target prescribers Consumer market research and testing of new messages Develop new logo for new brand name on crofelemer Develop new promotional materials for HCPs and consumers Create new product web site with new name and new data/messages Operations Fulfill orders with Napo-labeled product Package drug with new name label Notify trade of new name Package samples with new name Conduct Pharmacovigilance Assure inventory management and adequate supply chain Develop standard Medical Affairs responses to information requests ________________________ ***Confidential Treatment Requested

Crofelemer 2016 Marketing Plan Re-Launch with New Name Slide 5 Launch with New Name Jul - Dec Market Preparation PR around Napo re-launch of product / [***] Regulatory strategy – target FDA meeting re: pediatric plan, PReP, etc. Attend key conventions and conduct KOL meetings and advocates events Develop and submit publications with new efficacy data Promotional Activity Conduct test detailing to target HIV prescribers in select cities Telesales outbound promotion to target HIV prescribers Product sampling through telesales team Conduct medical education programs with Fulyzaq writers and target HIV prescribers Small group live meetings Teleconferences / web conferences Focus groups Conduct patient experience trial and gather feedback data Develop consumer education/awareness through HIV Advocacy community Test consumer digital marketing and social media Assess and develop strategy for managed care contracting Operations Continue Pharmacovigilance Continue Medical Affairs / drug information support Gather data to assure Sunshine Act reporting Manage inventory and supply chain Process returns/rebates/chargebacks ________________________ ***Confidential Treatment Requested

Crofelemer Performance Metrics - 2016 Measure Source Frequency/Timing % Unaided and aided awareness among target prescribers Quantitative market research to crofelemer target prescriber list 4Q2016 Goal (among Napo-detailed prescribers): Unaided >70% Aided >90% Salix baseline (2014), included 55% of MDs detailed: Unaided = 48% Aided = 70% Number of Key Opinion Leaders (KOLs) met with by Napo Crofelemer investigators HIV and GI opinion leaders Publications, congresses Thought Leader Select list Goals: 15 KOLs by end-Jun 30 KOLs by end-Dec % of Top 55 Fulyzaq writers met with by Napo IMS Exponent data and records of contacts Goal: >[***]% participate in live meeting or telecon by end-Sep Audited NRx, TRx, and sales IMS NPA and NSP data Quarterly Actual sales Invoiced sales Monthly Slide 6 ________________________ ***Confidential Treatment Requested

Total Fulyzaq Prescribers in 2015 Prescriber Count by Decile (Total = 588) Decile Definition Each decile = 10% of Rx’s From highest to lowest prescribers 6 10 12 9 1 2 3 4 5 6 7 8 261 80 80 47 30 16 21 35 Decile Cumulative % of Rx Cumulative # of Prescribers 10 10% 6 9 20% 18 8 30% 34 7 40% 55 6 50% 90 5 60% 120 4 70% 167 3 80% 247 2 90% 327 1 100% 588 Avg. Rx/MD 81 38 21 16 13 10 7 5 2 28

Group Practice Prescriber Individual Prescriber Each Digit = 1 Prescriber/Decile Top Fulyzaq Prescribers by Location (Total Decile 7-10, n=55) 80% of Fulyzaq Prescriptions in 2015 came from 10 States 7 4 1 7 1 1 7 7 9 8 8 5 3 2 2 1 10 San Xxxx 9 8 8 1 9 3 1 8 7 10 9 1 1 1 9 5 Palm Springs 7 5 7 7 8 6 6 7 Chicago 9 10 7 6 3 3 2 1 7 2 Dallas 7 8 8 9 2 Houston 10 9 8 8 St. Louis 8 6 2 9 7 6 7 9 9 7 6 7 6 7 4 3 8 4 Miami 9 10 2 2 8 7 7 8 10 NYC 8 7 5 1 1 7 Witchita 7 1 1

Fulyzaq Prescribers by Specialty Specialty Number of Prescribers Total Rx Avg. Rx/MD ID 190 1,511 8.0 IM 144 1,406 9.8 NP/PA 121 884 7.3 FP 84 474 5.6 GI 24 174 7.3 Other 25 104 4.2 Total 588 4,553 % of Total Fulyzaq Prescriptions By Specialty FP 11% GI 4% ID 33% IM 31% NP/PA 19% Other 2%

Top Fulyzaq Prescribers by Specialty Fulyzaq Prescribers in Decile 7-10 (2015) Specialty Number of Prescribers Total Rx Avg. Rx/MD IM 20 726 36.3 ID 18 677 37.6 NP/PA 12 332 27.7 FP 3 59 19.7 GI 1 23 23.0 Other 1 22 22.0 Total 55 1,839 Top Fulyzaq Prescribers (Dec 7-10) By Specialty (n=55) 20 18 12 3 1 1 0 5 10 15 20 25 IM ID NP/PA FP GI Other # of Prescribers

Fulyzaq Metrics – Salix 2013-2014 Unaided & Aided Awareness In 2014, 55% of physicians in the study had been detailed on Fulyzaq Historical Data (Salix) Unaware Aware no Rx Rx, some Rx, often 32% 62% 48% 70% 0% 10% 20% 30% 40% 50% 60% 70% 80% Unaided Aided % of Physicians 2013 (n=50) 2014 (n=60) Unaware Aware no Rx Rx, some Rx, often Fulyzaq 30% 40% 22% 8% 0% %of Physicians 100%

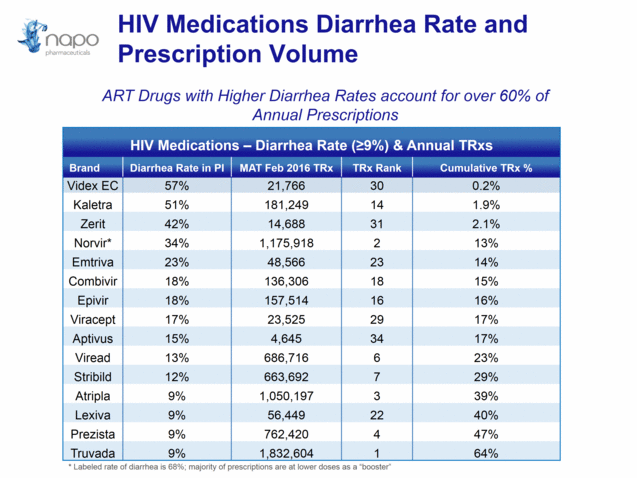
HIV Medications Diarrhea Rate and Prescription Volume HIV Medications – Diarrhea Rate (>9%) & Annual TRxs Brand Diarrhea Rate in PI MAT Feb 2016 TRx TRx Rank Cumulative TRx % Videx EC 57% 21,766 30 0.2% Kaletra 51% 181,249 14 1.9% Zerit 42% 14,688 31 2.1% Norvir* 34% 1,175,918 2 13% Emtriva 23% 48,566 23 14% Combivir 18% 136,306 18 15% Epivir 18% 157,514 16 16% Viracept 17% 23,525 29 17% Aptivus 15% 4,645 34 17% Viread 13% 686,716 6 23% Stribild 12% 663,692 7 29% Atripla 9% 1,050,197 3 39% Lexiva 9% 56,449 22 40% Prezista 9% 762,420 4 47% Truvada 9% 1,832,604 1 64% ART Drugs with Higher Diarrhea Rates account for over 60% of Annual Prescriptions * Labeled rate of diarrhea is 68%; majority of prescriptions are at lower doses as a “booster”
Thank You

Exhibit B: Regulatory Responsibilities
Transfer of IND Documents
|
Project Activity/Task |
|
Napo |
|
BexR |
|
Advyzom |
|
Information |
|
|
|
Transfer of IND/NDA Documents | ||||||
|
Transfer of IND/NDA Documents |
|
X |
|
|
|
X |
|
Advyzom will work with Napo to coordinate the transfer of all IND/NDA documents. Regulatory Operations will also prepare the submission documents for the change in US Agent role to be assumed by US authorized agent. |
|
|
|
Regulatory Strategic Advice | ||||||
|
Regulatory Strategic Advice |
|
X |
|
|
|
X |
|
Advyzom is providing regulatory and development advice for any regulatory issues that may arise including clinical, nonclinical, CMC, manufacturing, promotion and any other IND/NDA related activities for Napo products. |
|
|
|
US Agent Services | ||||||
|
US Agent |
|
X |
|
|
|
X |
|
Advyzom will serve as Napo’s US Regulatory Agent. This designated US agent is the official point of contact for the FDA for all communications regarding the assigned application. He/she will also assist in the review of regulatory submissions. |
|
|
|
US Agent: Post-IND/NDA Submission Support and IND/NDA Maintenance | ||||||
|
IND/NDA Annual Report Preparation, Assembly, and Submission |
|
Review |
|
Prepare Safety Sections |
|
Prepare, Review, Submit |
|
Advyzom will provide a content outline for the Annual Report. BexR will organize, prepare, and format the safety portion of the annual report (b)(2)(i), if applicable. Napo and Advyzom will prepare, review, format, publish, and submit the annual report. |
|
IND Information Amendments |
|
Review |
|
|
|
Prepare, Submit |
|
Advyzom will prepare, review and submit general amendments. |
|
NDA Submissions / Maintenance |
|
Review |
|
|
|
Prepare, Review, Submit |
|
Advyzom will prepare, review and make NDA submissions / maintain the NDA |
|
Protocols or Amendments |
|
Prepare |
|
|
|
Review, Submit |
|
Advyzom will review and submit new protocols or protocol amendments. |
|
CMC Amendments |
|
Prepare |
|
|
|
Review, Submit |
|
Advyzom will review and submit Client-prepared CMC amendments. Advyzom can prepare CMC amendments, if Client requests this service. |
|
Investigator 1572s and CVs of Principal Investigators |
|
Prepare |
|
|
|
Review, Submit |
|
Advyzom will perform review, publishing and submission of Investigator 1572s and CVs of Principal Investigators. Per regulations, US Agent will prepare investigator submissions for submission to FDA every 30 days. |
|
NDA Safety Reporting |
|
|
|
Duties as per Appendix 2 |
|
Duties as per Appendix 2 |
|
BexR and Advyzom will perform Pharmacovigilance Duties as listed in Appendix 2. |
|
Promotion |
|
Prepare |
|
Review |
|
Review, Submit |
|
BexR will review and provide recommendations on content of promotional materials. Advyzom will perform promotional material review and submission. |
|
Labeling |
|
Prepare, Review |
|
Review |
|
Review, Submit |
|
Advyzom will perform submission of labeling. |
|
Provision of FDA Liaison Services |
|
Prepare |
|
|
|
Prepare |
|
Advyzom will document all dialogue with the FDA on written FDA contact reports. |
FIRST AMENDMENT TO MARKETING AND DISTRIBUTION AGREEMENT
THIS FIRST AMENDMENT TO THE MARKETING AND DISTRIBUTION AGREEMENT (this “Amendment”) is made and entered into as of July 19, 2016, and made effective on July 19, 2016 by and between BexR Logistix, LLC (“BexR”), with its principal business office located at 00000 XX-00 Xxxx, Xxxxx 0000, Xxx Xxxxxxx, Xxxxx 00000, and Napo Pharmaceuticals, Inc. (“Napo”), with its principal business office located at 000 Xxxxxxx Xxxxxx, Xxx. 0000, Xxx Xxxxxxxxx, Xxxxxxxxxx 00000.
W I T N E S S E T H:
A. Napo and Mission originally entered into that certain Marketing and Distribution Agreement dated April 14, 2016 (the “Agreement”).
B. Napo and Mission agree to the change of the trademarked Product name in the Agreement from Fulyzaq® to MytesiTM
C. Napo and Mission desire to amend the Agreement as more fully set forth herein.
NOW, THEREFORE, in consideration of the mutual promises and covenants and agreements more fully set forth herein, and other good and valuable consideration the receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
Except as provided herein, all terms and conditions of the Agreement and any prior amendments shall remain in full force and effect without change through the Term of the Agreement. The amended Recitals and Sections of the Agreement set forth below shall replace in its entirety the same numbered Recitals and Sections in the Agreement and is incorporated into the Agreement as binding Recitals and Sections of the Agreement on the Parties.
D. The Recitals A, B, and C in the Agreement shall be replaced with the following recitals:
RECITALS
A. Napo is in the business of developing and commercializing pharmaceuticals, including the drug MytesiTM (Crofelemer).
B. BexR provides logistical support and product consignment services to a broad range of industries, including pharmaceutical companies.
C. Napo wishes to engage BexR, and BexR wishes to be so engaged, to provide certain telemarketing, consignment and distribution services pursuant to the terms and conditions set forth in this Agreement
E. Section 1(a), “Appointment,” shall be replaced with the following Section 1(a) to reflect the agreed change of the trademarked name of the Product:
1. Appointment
a. Distributor. Subject to the terms and conditions of this Agreement, Napo appoints BexR as its distributor with the right to telemarket and sell (as described in Section 4.9 of this agreement), and the exclusive right to distribute the prescription drug MytesiTM (Crofelemer) (the “Product”) during the Term of this Agreement in the United States and its Territories (the “U.S.”), and BexR accepts such appointment.
IN WITNESS WHEREOF, the parties have executed this First Amendment to Marketing and Distribution Agreement as of the date set forth above.
|
|
NAPO PHARMACEUTICALS, INC. | |
|
|
a Delaware corporation | |
|
|
| |
|
|
By: |
/s/Xxxx X Xxxxx |
|
|
Name: Xxxx X. Xxxxx | |
|
|
Title: CEO | |
|
|
| |
|
|
| |
|
|
BEXR LOGISTIX, LLC, a Texas limited liability company | |
|
|
| |
|
|
By: |
/s/Xxxxxx X. Xxxxxx |
|
|
Name: Xxxxxx X. Xxxxxx | |
|
|
Title: Treasurer | |
SECOND AMENDMENT TO MARKETING AND DISTRIBUTION AGREEMENT
THIS SECOND AMENDMENT TO THE MARKETING AND DISTRIBUTION AGREEMENT (this “Amendment”) is made and entered into as of February 27, 2017, by and between BexR Logistix, LLC (“BexR”), with its principal business office located at 00000 XX-00 Xxxx, Xxxxx 0000, Xxx Xxxxxxx, Xxxxx 00000, and Napo Pharmaceuticals, Inc. (“Napo”), with its principal business office located at 000 Xxxxxxx Xxxxxx, Xxx. 0000, Xxx Xxxxxxxxx, Xxxxxxxxxx 00000.
W I T N E S S E T H:
Napo and BexR entered into a Marketing and Distribution Agreement (the “Agreement”) dated as April 14, 2016. Napo and BexR entered into a First Amendment to the Marketing and Distribution Agreement dated July 19, 2016.
NOW, THEREFORE, in consideration of the mutual promises and covenants and agreements more fully set forth herein, and other good and valuable consideration the receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
Except as provided herein, all terms and conditions of the Agreement and any prior amendments shall remain in full force and effect without change through the Term of the Agreement. The amended Section of the Agreement set forth below shall replace in its entirety the same numbered Section in the Agreement and is incorporated into the Agreement as a binding Section of the Agreement on the Parties.
Section 5.3, “Advance,” shall be replaced with the following Section 5.3:
5.3 Repayment of Advance
Amended loan amount shall be $267,500.00 payable to Mission Pharmacal Company to start July 2017 on a payment schedule set forth below:
|
Deferral |
|
Dec |
|
Jan |
|
Feb |
|
March |
|
Apr |
|
May |
|
Jun |
|
Jul |
|
Aug |
|
Sep |
|
Oct |
|
Nov |
|
Dec |
|
Jan |
|
Total |
| |||||||||||||||
|
New Deferral |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
32,100 |
|
$ |
32,100 |
|
$ |
34,775 |
|
$ |
40,125 |
|
$ |
40,125 |
|
$ |
42,800 |
|
$ |
45,475 |
|
$ |
267,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12.00 |
% |
12.00 |
% |
13.00 |
% |
15.00 |
% |
15.00 |
% |
16.00 |
% |
17.00 |
% |
100.00 |
% | |||||||||||||||
In the event that Napo has not repaid the Advance to BexR by the end of the Payment Period, Napo shall pay the entire outstanding balance to BexR within ten (10) days of the end of the Payment Period.
IN WITNESS WHEREOF, the parties have executed this Second Amendment to Marketing and Distribution Agreement as of the date set forth above.
[Signature Page follows]
|
|
NAPO PHARMACEUTICALS, INC. | |
|
|
a Delaware corporation | |
|
|
| |
|
|
By: |
/s/Xxxx X Xxxxx |
|
|
Name: Xxxx X. Xxxxx | |
|
|
Title: Chief Executive Officer | |
|
|
| |
|
|
| |
|
|
BEXR LOGISTIX, LLC, a Texas limited liability company | |
|
|
| |
|
|
By: |
/s/Xxxxxx X. Xxxxxx |
|
|
Name: Xxxxxx X. Xxxxxx | |
|
|
Title: Treasurer | |
THIRD AMENDMENT TO MARKETING AND DISTRIBUTION AGREEMENT
THIS THIRD AMENDMENT TO THE MARKETING AND DISTRIBUTION AGREEMENT (this “Amendment”) is made and entered into as of March 31, 2017, by and between BexR Logistix, LLC (“BexR”), with its principal business office located at 00000 XX-00 Xxxx, Xxxxx 0000, Xxx Xxxxxxx, Xxxxx 00000, and Napo Pharmaceuticals, Inc. (“Napo”), with its principal business office located at 000 Xxxxxxx Xxxxxx, Xxx. 0000, Xxx Xxxxxxxxx, Xxxxxxxxxx 00000.
W I T N E S S E T H:
Napo and BexR entered into a Marketing and Distribution Agreement (the “Agreement”) dated as April 14, 2016. Napo and BexR entered into a First Amendment to the Marketing and Distribution Agreement dated July 19, 2016. Napo and BexR entered into a Second Amendment to the Marketing and Distribution Agreement dated February 27, 2017.
NOW, THEREFORE, in consideration of the mutual promises and covenants and agreements more fully set forth herein, and other good and valuable consideration the receipt and sufficiency of which is hereby acknowledged, the parties hereto agree as follows:
Except as provided herein, all terms and conditions of the Agreement and any prior amendments shall remain in full force and effect without change through the Term of the Agreement. The amended Section of the Agreement set forth below shall replace in its entirety the same numbered Section in the Agreement and is incorporated into the Agreement as a binding Section of the Agreement on the Parties.
Section 5.3, “Advance,” shall be replaced with the following Section 5.3:
5.3 Repayment of Advance
Amended loan amount shall be $267,500.00 payable to BexR to start July 2017 on a payment schedule set forth below:
|
Deferral |
|
Dec |
|
Jan |
|
Feb |
|
March |
|
Apr |
|
May |
|
Jun |
|
Jul |
|
Aug |
|
Sep |
|
Oct |
|
Nov |
|
Dec |
|
Jan |
|
Total |
| |||||||||||||||
|
New Deferral |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
32,100 |
|
$ |
32,100 |
|
$ |
34,775 |
|
$ |
40,125 |
|
$ |
40,125 |
|
$ |
42,800 |
|
$ |
45,475 |
|
$ |
267,500 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12.00 |
% |
12.00 |
% |
13.00 |
% |
15.00 |
% |
15.00 |
% |
16.00 |
% |
17.00 |
% |
100.00 |
% | |||||||||||||||
In the event that Napo has not repaid the Advance to BexR by the end of the Payment Period, Napo shall pay the entire outstanding balance to BexR within ten (10) days of the end of the Payment Period.
IN WITNESS WHEREOF, the parties have executed this Second Amendment to Marketing and Distribution Agreement as of the date set forth above.
[Signature Page follows]
|
|
NAPO PHARMACEUTICALS, INC. | |
|
|
a Delaware corporation | |
|
|
| |
|
|
By: |
/s/Xxxx X Xxxxx |
|
|
Name: Xxxx X. Xxxxx | |
|
|
Title: Chief Executive Officer | |
|
|
| |
|
|
| |
|
|
BEXR LOGISTIX, LLC, a Texas limited liability company | |
|
|
| |
|
|
By: |
/s/Xxxxxx X. Xxxxxx |
|
|
Name: Xxxxxx X. Xxxxxx | |
|
|
Title: Treasurer | |

