[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Exhibit 10.48
SUPPLY AGREEMENT
This Supply Agreement (the “Supply Agreement”) is entered into as of October 29, 2018 (the “Effective Date”) by and between Rigel Pharmaceuticals, Inc., a Delaware company having an address at 0000 Xxxxxxxx Xxxx., Xxxxx Xxx Xxxxxxxxx, XX 00000, XXX (“Rigel”) and Kissei Pharmaceutical Co. Ltd., a Japanese company having an address at 00-00 Xxxxxxx, Xxxxxxxxx, Xxxxxx 000-0000, Xxxxx (“Kissei”). Rigel and Kissei may be referred to herein individually as a “Party” or collectively as the “Parties”.
RECITALS
Whereas, Rigel, a biopharmaceutical company, has developed its proprietary compound fostamatinib disodium hexahydrate, also known as TAVALISSE™ in the United States, which has been approved by the FDA for the treatment of chronic immune thrombocytopenia and is under development for the treatment of autoimmune hemolytic anemia, IgA nephropathy, and potentially other indications;
Whereas, Rigel and Kissei are parties to a certain Collaboration and License Agreement of even date hereof (the “Collaboration and License Agreement”), under which Rigel has granted Kissei the right to develop and commercialize fostamatinib disodium hexahydrate in the Kissei Territory; and
Whereas, the Collaboration and License Agreement contemplates that Rigel will manufacture, or have manufactured, and supply fostamatinib disodium hexahydrate to Kissei for development and commercial use, and Rigel is willing to manufacture and supply fostamatinib disodium hexahydrate to Kissei, on the terms and conditions set forth below.
Now, Therefore, in consideration of the foregoing premises and the mutual covenants contained herein, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties agree as follows:
Capitalized terms used in this Supply Agreement but not defined herein shall have the meanings set forth in the Collaboration and License Agreement.
|
1.1
“Batch” means the quantity of a Product produced in a single production run of such Product. |
|
1.2
“Business Day” means a day that is not a Saturday, Sunday, or a day on which banking institutions in [*] are authorized by Applicable Law to remain closed. |
|
1.3
“Claim” had the meaning set forth in Section 9.3. |
|
1.4
“Collaboration and License Agreement” has the meaning set forth in the Recitals. |
|
1.5
“Compound” means fostamatinib disodium hexahydrate, having the chemical structure set forth in Exhibit A. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
1
|
1.6
“Finish Manufacture” means the manufacture of Finished Product from bulk Drug Product. |
|
1.7
“Finished Product” means the Product in appropriate final form, packaged and labeled and ready for its intended use (i.e., sale to the end-user, use in any Clinical Trial or other Development work, or use as a sample). |
|
1.8
“GMP” means the current minimum standards for methods to be used in, and the facilities or controls to be used for, the manufacture, processing, packing, or holding of a drug as specified by applicable laws of the relevant countries at the time of manufacturing conducted in accordance with this Supply Agreement, defined under (a) 21 C.F.R. Part 210 and 211, and (b) equivalent law or regulations in any other applicable jurisdiction in the Territory. |
|
1.9
“Indemnitee” has the meaning set forth in Section 9.3. |
|
1.10
“Indemnitor” has the meaning set forth in Section 9.3. |
|
1.11
“Information” means any data, results, technology, business, or financial information, or information of any type whatsoever, in any tangible or intangible form, including know-how, trade secrets, practices, techniques, methods, processes, inventions, developments, specifications, formulae, software, algorithms, marketing reports, expertise, technology, test data (including pharmacological, biological, chemical, biochemical, clinical test data, and data resulting from non-clinical studies), CMC information, stability data, and other study data and procedures. |
|
1.12
“Kissei Indemnitee” has the meaning set forth in Section 9.1. |
|
1.13
“Losses” has the meaning set forth in Section 9.1. |
|
1.14
“Manufacture” means all activities related to the manufacturing of the Drug Product in fill and finished form but without final packaging or labeling, including quality assurance activities related to manufacturing and release of product, ongoing stability tests, and regulatory activities related to any of the foregoing. “Manufacturing” has a correlative meaning. |
|
1.15
“Order Forecast” has the meaning set forth in Section 2.2(a). |
|
1.16
“Quality Agreement” has the meaning set forth in Section 2.6. |
|
1.17
“Rigel Indemnitee” has the meaning set forth in Section 9.2. |
|
1.18
“Specification” means the written specifications for the Product. Specifications may be required to be different for a Product for use in different countries due to individual Regulatory Authority requirements in such countries. |
|
1.19
“Term” has the meaning set forth in Section 10.1. |
|
1.20
“Transfer Price” has the meaning set forth in Section 3.1. |
|
2.1
Purchase and Sale. Pursuant to the terms and conditions of this Supply Agreement, Rigel (either itself or through its Affiliates or Third Party subcontractors) shall Manufacture and supply the Product and its placebo |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
2
(if applicable) to Kissei in such quantities as Kissei shall order pursuant to and in accordance with this Article 2, and Kissei shall purchase from Rigel all of Kissei’s and its Affiliates’ and Sublicensees’ requirements for Products for development and commercialization in the Field in the Kissei Territory pursuant to and in accordance with the Collaboration and License Agreement. For clarity, Rigel may perform its obligations under this Supply Agreement through one or more Third Party subcontractors, provided that Rigel remains responsible for the work allocated to, and payment to, such subcontractors as it selects, to the same extent it would if it had done such work itself. Notwithstanding the following Sections 2.2 and 2.3, the Parties agree that Kissei may amend Order Forecasts (as defined below) and Purchase Orders (as defined below) from time to time during the [*] from the Effective Date with the prior mutual consent of the Parties via the JSC and Rigel shall supply the Product to Kissei in such agreed quantities. |
|
(a)
Rolling Forecast. On or before the [*] of each Calendar Quarter during the Term of this Supply Agreement, Kissei shall provide Rigel a rolling forecast of the quantity of Products to be used for (i) Development purposes that Kissei plans to order during the [*] period commencing the following Calendar Quarter and (ii) Commercial use that Kissei plans to order during the [*] period commencing the following Calendar Quarter (“Order Forecast”). For clarity, each Order Forecast shall itemize the applicable quantity of Drug Product for each of Development and Commercial use. Each Order Forecast shall be made in good faith for budget and capacity planning purposes only and shall be non-binding on Kissei and Rigel, except as provided in Section 2.2(b). The Parties shall discuss and review the Order Forecast at each regularly scheduled meeting of the JSC established by the Parties under the Collaboration and License Agreement (or by a subcommittee established by the JSC to oversee the manufacture and supply of the Product). The Order Forecast will be in substantially the form attached hereto as Exhibit B. |
|
(b)
Binding Commitment. The first [*] of each Order Forecast shall constitute a binding commitment for Kissei to purchase, pursuant to Section 2.3(a), [*] of the quantities of Drug Product specified therein and Kissei shall be required to order such quantities pursuant to Section 2.3(a). For clarity, the numbers set out in the following [*] of the Order Forecast constitute the non-binding forecast of Kissei’s expected requirements. |
|
2.3
Purchase Orders; Delivery Terms. |
|
(a)
Purchase Orders. On or before the [*] of each Calendar Quarter during the Term of this Supply Agreement, Kissei shall submit to Rigel a binding purchase order (a “Purchase Order”) for Drug Product to be delivered during the next Calendar Quarter of the most recent Order Forecast for Development use and/or Commercial use in quantities [*] those set forth for such Calendar Quarter in the most recent Order Forecast. Rigel shall accept or reject each Purchase Order in writing within [*] after its receipt of such Purchase Order; provided, however, that Rigel shall accept such Purchase Order if the quantities of Drug Product ordered for each of Development and Commercial uses in such Purchase Order are [*] the quantities for such use set forth in the most recent Order Forecast, as applicable. |
|
(b)
Additional Quantities. In the event Kissei desires to obtain quantities of Drug Product in a particular Calendar Quarter in excess of the quantities specified in the Order Forecast after such forecast became binding, Kissei shall notify Rigel in writing and the Parties will discuss in good faith whether Rigel may be able to supply Kissei with such additional quantities, provided that Rigel shall use Commercially Reasonable Efforts to accept such order for such additional quantities, and provided further that Kissei shall be solely responsible for any additional cost incurred in supplying such additional quantities. For clarity, Rigel shall not be obligated to accept any such order for additional quantities if accepting such order would result in or is reasonably likely to result in a Drug Product shortfall in the Rigel Territory. |
|
(c)
Delivery and Shipping Terms. Purchase Orders submitted for quantities of Product that are in accordance with Section 2.3(a) and/or Section 2.3(b) will be binding on both Parties after acceptance in writing |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
3
by Rigel. The Purchase Order will specify delivery dates for such order to be delivered in such Calendar Quarter, but will in no event be a date sooner than [*] following the Purchase Order date. By way of example, a Purchase Order submitted on [*] would specify the quantity of Product ordered for delivery in [*], with a delivery date no sooner than [*]. Notwithstanding the foregoing, Rigel’s delivery schedule under this Supply Agreement shall be subject to any change in the delivery schedule under the supply agreements between Rigel and its contract manufacturers. The Parties agree to discuss in good faith any adjustment of the minimum delivery time of [*] from Purchase Order submission if a Party deems such an adjustment necessary. Rigel shall inform Kissei in advance of any such change. Rigel shall deliver all Product [*], and title and risk of loss shall pass from Rigel to Kissei upon the Product’s being placed at the disposal of Kissei [*]. Rigel shall be responsible for obtaining all licenses or other authorizations for the exportation of such shipments and shall supply Kissei with the documentation required for filing or claiming credit or deduction for any applicable taxes and/or duties. Kissei shall be responsible for [*], and shall be the importer of record and responsible for [*], and shall be responsible for obtaining all distribution licenses for the Product. Notwithstanding the foregoing, Rigel shall [*], and cooperates with Kissei on such shipment. |
|
(d)
Separate Contracts. Each Purchase Order will constitute a separate contract for the supply of Drug Product on the terms of this Supply Agreement (and excluding all other terms and conditions including any set out or referred to in any Purchase Order). In the event of a conflict between a Purchase Order and the terms of this Supply Agreement, the terms of this Supply Agreement will govern. |
|
(a)
Documentation. Rigel shall establish and maintain any necessary drug master files, standard operating procedures, protocols, and master batch records for the Manufacture of the Product. Rigel shall, in connection with each shipment of Product to Kissei, provide to Kissei the certificate of compliance, certificate of analysis, completed batch records, and any other documentation as may be required in the Quality Agreement with respect to such shipment. |
|
(b)
Traceability. Rigel shall xxxx the Drug Product shipment supplied to Kissei with a lot number for the purposes of traceability. Kissei shall record the lot number of each Drug Product used for each Clinical Trial, promotion and marketing event, distributed to each patient in an expanded access program, or sold to each customer, and shall retain all such records for at least [*] after the date of termination or expiration of this Supply Agreement to facilitate in the event of a Recall under Section 5.7 of the Collaboration and License Agreement. |
|
(c)
Form of Supply. Rigel shall supply Kissei with Drug Product and Kissei shall perform the Finish Manufacture of the Drug Product, including final packaging and labeling, for Development uses. Kissei shall perform the tablet appearance test with the appearance testing machine and the Finish Manufacture of the Drug Product, including final packaging and labeling, for Commercial uses. Kissei shall be responsible for ensuring that the Finished Product conforms with all Applicable Laws and Regulatory Approvals for each applicable jurisdiction within Kissei Territory. |
|
(d)
Finished Product Release. Kissei (by itself or through its contract manufacturer) shall conduct release tests of the Product, and the Parties will agree to a mechanism in the Quality Agreement for the shipment of test samples of each Batch of the Drug Product to Kissei for local release testing purposes. |
|
(f)
Product Shelf Life. The Product supplied by Rigel to Kissei hereunder shall have a remaining shelf life of [*]. |
|
(g)
Inventory Management; Safety Stock. Each Party shall manage its inventory in a manner that maximizes the remaining shelf life of its inventory. Kissei shall carry a reasonable quantity of inventory of the Finished Product, and Rigel shall carry a reasonable quantity of raw materials, including the Compound, which may be used in the event of an interruption to the supply chain. The quantity of such safety stock shall be sufficient to cover the quantity set forth in the Order Forecast for [*]. The Parties shall replace and replenish the safety stock |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
4
continuously on a first to expire, first out basis. Each Party shall be responsible for the cost of maintaining its own safety stock. |
|
2.5
Inspection and Acceptance. |
|
(a)
Non-Conforming Product. |
|
(i)
Kissei shall inspect all shipments of Product promptly upon receipt, and shall notify Rigel in writing in reasonable detail within [*] of receipt if Kissei is rejecting any Product that fails to conform to Rigel’s warranties set forth in Sections 8.2(a) or 8.2(b). All Product not rejected within such [*] period will be deemed accepted. |
|
(ii)
If Kissei notifies Rigel of any nonconformity of any Product in accordance with Section 2.5(a)(i), Rigel shall have the right to inspect the Product in question and Kissei shall cooperate with Rigel’s inspection, including providing Rigel with samples of the Product in question for testing upon request. If Rigel agrees with such notice of nonconformity and that such nonconformity was not caused by occurrences after the delivery of the Product to Kissei, Rigel shall, at its discretion and expense, either: (A) replace such Product, [*], as soon as reasonably practicable after receipt of notification of such nonconformity or (B) refund any portion of the applicable amount that has already been paid for such Product; provided, however, that if Rigel is required to make a payment to any contract manufacturer (or is not entitled to a refund from such contract manufacturer) in connection with any such non-conforming Product caused by Kissei or while under Kissei’s control, Kissei shall be required to pay Rigel under this Supply Agreement with respect to such non-conforming Product unless and until Rigel is relieved of its payment obligation (or is refunded its payment) for such non-conforming Product under its agreements with such contract manufacturers. |
|
(iii)
In the event that Rigel disagrees with Kissei that a Product does not conform to Rigel’s warranties set forth in Section 8.2(a) or 8.2(b), as applicable, or considers that the defect was caused by occurrences after the delivery of the Product to Kissei, it may require a sample of the allegedly nonconforming Product to be delivered to a mutually acceptable independent testing laboratory for testing or, in the case of a dispute concerning compliance with GMP, an independent consultant for evaluation. Except in the case of manifest error, the determination of the laboratory or consultant as to whether the Product is nonconforming will be final and binding on the Parties. The fees and expenses of such laboratory testing or consultant, as the case may be, shall be borne entirely by the Party against whom such laboratory’s or consultant’s determination is made. If, as the case may be, such determination is against Kissei, then such Product shall be deemed accepted by Kissei. If, as the case may be, such determination is against Rigel, then Rigel shall, subject to the instruction of Kissei, either refund any portion of the applicable amount that has already been paid by Kissei for such Product or replace such Product, at no additional cost to Kissei, as soon as reasonably possible, but in no event later than [*] if replacement Drug Product stock is available, or if replacement Drug Product stock is unavailable at such time, as soon as reasonably practical after it becomes available; provided, however, that if Rigel is required to make a payment to any contract manufacturer (or is not entitled to a refund from such contract manufacturer) in connection with any such non-conforming Product caused by Kissei or while under Kissei’s control, Kissei shall be required to pay Rigel under this Supply Agreement with respect to such non-conforming Product unless and until Rigel is relieved of its payment obligation (or is refunded its payment) for such non-conforming Product under its agreements with such contract manufacturers. |
|
(b)
Sole Remedy. Notwithstanding anything to the contrary in this Supply Agreement, the remedy set forth in this Section 2.5 will be Kissei’s sole and exclusive remedy and recourse with respect to the shortages that are not also nonconforming Product delivered to Kissei by Rigel hereunder. |
|
(c)
Damage after Delivery. Kissei shall bear the risk of damage to the Product after delivery to Kissei pursuant to Section 2.3(c). If the Product is damaged after delivery to Kissei pursuant to Section 2.3(c) and Kissei intends to order replacement Product, Kissei shall promptly notify Rigel of the damage and any orders for replacement Product, and Rigel may, at its sole discretion but in good faith, accept or reject all or a portion of the order for the replacement Product. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
5
|
2.6
Quality Agreement. As soon as reasonably practicable after the Effective Date, the Parties shall agree to the terms and conditions of a quality agreement (the “Quality Agreement”) setting forth in detail the quality assurance arrangements and procedures for the Manufacture of the Product, which Quality Agreement shall be incorporated herein by reference. For clarity, the Parties shall agree to the terms and conditions of (a) the Quality Agreement for Drug Product for Development use as soon as reasonably practicable after the Effective Date, and (b) the Quality Agreement for Drug Product for Commercial uses, if different than the Quality Agreement specified in subsection (a), as soon as reasonably practicable after the first MAA for the Product is submitted in the Kissei Territory. To the extent that the terms of this Supply Agreement and those of the Quality Agreement are in conflict, the terms of this Supply Agreement shall control except with respect to quality issues, which shall be governed by the Quality Agreement. For clarity, if there are any financial terms in the Quality Agreement that are in conflict with this Supply Agreement, this Supply Agreement shall control with respect to such financial terms. |
|
2.7
Backup Supplier. In the event that for a period of [*], Rigel has failed to supply [*] of the quantity of the Product [*], Kissei shall have the right to manufacture the Compound and the Product by itself or a Third Party manufacturer in the Kissei Territory (a “Backup Manufacturer”). In preparation for manufacturing at the Backup Manufacturer, upon Kissei’s reasonable request, Rigel shall [*] transfer to Kissei the technology concerning the manufacture of the Compound and the Product after the Effective Date hereof. The costs and expenses associated with the engagement of the Backup Manufacturer, including the costs for transferring the Manufacturing process to such Backup Manufacturer, shall be borne [*]. |
|
2.8
Allocation in the Event of Product Shortages. |
|
(a)
This Section 2.8 shall apply in the event that Rigel is unable to supply, with respect to a Calendar Quarter, [*] (i) Product ordered by Kissei pursuant to Sections 2.2 and 2.3 for delivery in such Calendar Quarter, plus (ii) Product required by Rigel or its Affiliates or other licensees for their own use with respect to such Calendar Quarter (such event, a “Shortfall”). The purpose of these allocation rules is to permit Kissei (with respect to the Kissei Territory) and Rigel (with respect to the Rigel Territory) to independently make their respective long-term purchase decisions for the Product, with the benefits and risks of such purchase decisions to be allocated to Kissei or Rigel, as the case may be. |
|
(b)
If Rigel is unable to supply [*] (i) Product ordered by Kissei pursuant to a Purchase Order plus (ii) Product required by Rigel or its Affiliates or other licensees for their own use, then the available Product in each Calendar Quarter in which a Shortfall occurs shall be [*]. |
|
(c)
The allocation rules set forth in this Section 2.8 shall restart for each Calendar Quarter, without any carryover of a Shortfall realized by either Kissei or Rigel in the prior Calendar Quarter. |
|
(d)
If Rigel determines that it will not be able to deliver the quantities of the Product specified in the Purchase Order on the requested delivery date, or Rigel is made aware of any future anticipated shortages, then Rigel shall promptly notify Kissei of such determination, and in any event, no later than [*] following such determination. Such notification shall include the reasons for and the expected duration of Rigel’s anticipated inability to deliver such quantities of the Product. Promptly thereafter, but in no event more than [*] after such notification, the Parties shall discuss in good faith the matters set forth in such notification and begin good faith negotiations with respect to an alternative delivery schedule or alternative sourcing for such Product; provided that any such negotiations shall not relieve Rigel of its obligations hereunder. |
|
2.9
Supply Contacts. Each Party shall designate one (1) qualified and experienced supply chain professional to serve as that Party’s primary supply contact regarding the supply of Product within this Supply Agreement (“Supply Contacts”) and under the direction of the JSC. Each Party may replace its Supply Contact with an alternative representative at any time with prior written notice to the other Party. Supply Contacts shall be responsible for facilitating information exchange and discussion between the Parties regarding the supply of Product |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
6
under this Supply Agreement. Supply Contact shall have decision-making authority within the guidance and subject to the review and approval of the JSC. Each Party shall bear its own costs of its Supply Contact, [*]. |
|
(a)
Development Use. All Drug Product supplied by Rigel to Kissei for use for Development purposes shall be at the applicable price set forth in Section 7.1 of the Collaboration and License Agreement. |
|
(b)
Commercial Use. All Drug Product supplied by Rigel to Kissei for use for Commercial purposes shall be equal to the Transfer Price calculated in accordance with Section 8.5 of the Collaboration and License Agreement. |
|
3.2
Invoice and Payment. Concurrently with delivery of Product to Kissei, Rigel shall submit to Kissei an invoice for payment, in U.S. Dollars, of the payment for such delivery, which invoice shall be prepared accordingly: |
|
(a)
for Product and its placebo supplied for Development purposes, in accordance with Section 7.1 of the Collaboration and License Agreement, |
|
(b)
for Product supplied for Commercial purposes during the Commercialization Term, in accordance with Section 8.5(c) of the Collaboration and License Agreement, and |
|
(c)
for Product supplied for Commercial purposes during the Extended Commercialization Term, Kissei shall pay to Rigel a Transfer Price equal to [*]. |
Kissei shall pay each invoice, in U.S. Dollars, within [*] Kissei receives such invoice by wire transfer of immediately available funds into an account designated by Rigel. Financial audits shall be conducted in accordance with Section 9.4 of the Collaboration and License Agreement, and late payments shall bear interest as set forth in Section 9.5 of the Collaboration and License Agreement.
|
3.3
Other Manufacture Related Costs. Kissei shall be responsible for the costs and expenses of Manufacture-related work that is performed by or on behalf of Rigel at Kissei’s reasonable request, which costs and expenses are not included in the calculation of Cost of Goods, including internal costs, but excluding, for clarity, any costs and expenses specifically for capital investment that should generally be required by a pharmaceutical manufacturing facility. Within [*], Rigel shall submit to Kissei a reasonably detailed invoice, in U.S. Dollars, setting forth the costs and expenses incurred by Rigel in connection with such work. Kissei shall pay to Rigel the amount invoiced, in U.S. Dollars, within [*] Kissei receives such invoice by wire transfer of immediately available funds into an account designated by Rigel. Late payments shall bear interest as set forth in Section 9.5 of the Collaboration and License Agreement. |
|
3.4
Tax. Kissei shall pay any and all taxes (other than taxes based on Rigel’s income), duties, assessments, and other charges and expenses imposed by any Governmental Authority in connection with the supply and transfer of Product to Kissei. If a withholding or deduction obligation occurs, then the sum payable by Kissei (in respect of which such deduction or withholding is required to be made) shall be increased to the extent necessary to ensure that Rigel receives a sum equal to the sum which it would have received had no such withholding or deduction occurred. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
7
|
4.1
Regulatory Inspections. Rigel shall cooperate with any inspection of its facilities by any Regulatory Authority overseeing the Manufacture of the Product for use in the Kissei Territory. Each Party shall notify the other Party of any such inspection and shall permit the other Party’s representative to observe such inspection to the extent such inspection is scheduled at least [*] in advance and such observation is permitted by Applicable Laws and any applicable agreement between Rigel and a Third Party (such as a contract manufacturing organization) in the event such facility is owned and/or operated by such Third Party. |
|
4.2
GMP, Quality Assurance, and Other Audits. Kissei shall have the right to conduct GMP, quality assurance, and other audits (e.g., Environment, Health & Safety) pursuant to the terms and conditions of the Quality Agreement, but subject to any applicable agreement between Rigel and a Third Party (such as a contract manufacturing organization) in the event such facility is owned and/or operated by such Third Party. |
|
4.3
Inquiries and Customer Complaints. Kissei shall comply with the Pharmacovigilance Agreement and Section 5.4 of the Collaboration and License Agreement with respect to all inquiries, complaints, and adverse events regarding the Products in the Kissei Territory. |
|
4.4
Notification of Potential Recall; Recalls. Each Party will act in accordance with the notice requirements set forth in Section 5.7 of the Collaboration and License Agreement. In the event that any Recall with respect to a Product is the direct result of a breach of any warranty of Rigel set forth in Section 8.2 and is not the result of Kissei’s, its Affiliates’, or its sublicensees’ Finish Manufacture, transportation, storage, marketing, use, sale, or distribution of the Product, then Rigel shall bear (and reimburse Kissei for) all of the costs and expenses of such recalled Product and the destruction of such recalled Product. To the extent that the reason for any Recall with respect to the Product hereunder is in part the direct result of the breach of any warranty of Rigel set forth in Section 8.2 and in part the result of Kissei’s, its Affiliates’, or its sublicensees’ Finish Manufacture, transportation, storage, marketing, use, sale, or distribution of the Product, then the expenses of such Recall shall be allocated in an equitable manner between the Parties. |
|
Article 5
CONFIDENTIALITY |
|
5.1
Confidentiality. Any and all Information disclosed by a Party to the other Party under this Supply Agreement shall be deemed Confidential Information of such Party under the Collaboration and License Agreement and subject to the confidentiality provisions set forth in Article 13 of the Collaboration and License Agreement. |
|
Article 6
Intellectual Property |
|
6.1
Intellectual Property. Any and all inventions, whether patentable or not and including all intellectual property rights therein, generated by either Party in the course of conducting their activities under this Supply Agreement shall be deemed to be generated under the Collaboration and License Agreement and subject to the rights and obligations of the Parties as set forth therein. |
|
7.1
Force Majeure. Notwithstanding anything to the contrary in this Supply Agreement, both Parties shall be excused from the performance of their obligations under this Supply Agreement to the extent that (a) force majeure prevents such performance or, with respect to Rigel’s supply obligations pursuant to Article 2, prevents the |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
8
combined supply of (i) Product specified in accepted orders placed by Kissei in accordance with Section 2.3(a) and (ii) Product required by Rigel and its Affiliates, and (b) the nonperforming Party promptly provides notice of the force majeure to the other Party. Such excuse shall continue so long as the condition constituting force majeure continues and the nonperforming Party takes reasonable efforts to remove the condition. For purposes of this Supply Agreement, force majeure shall include conditions beyond the reasonable control of the applicable Party, including an act of God, war, civil commotion, terrorist act, labor strike or lock-out, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm, or like catastrophe. Notwithstanding the foregoing, a Party will not be excused from making payments owed hereunder because of a force majeure affecting such Party. If a force majeure persists for more than [*], then the Parties will discuss in good faith the modification of the Parties’ obligations under this Supply Agreement in order to mitigate the delays caused by such force majeure. |
|
Article 8
REPRESENTATIONS AND WARRANTIES |
|
8.1
Mutual Representations and Warranties. Each Party represents and warrants to the other that, as of the Effective Date: (i) it is duly organized and validly existing under the laws of its jurisdiction of incorporation or formation, and has full corporate or other power and authority to enter into this Supply Agreement and to carry out the provisions hereof, (ii) it is duly authorized to execute and deliver this Supply Agreement and to perform its obligations hereunder, and the person or persons executing this Supply Agreement on its behalf has been duly authorized to do so by all requisite corporate or partnership action, and (iii) this Supply Agreement is legally binding upon it, enforceable in accordance with its terms, and does not conflict with any agreement, instrument, or understanding, oral or written, to which it is a Party or by which it may be bound, nor violate any material law or regulation of any court, governmental body, or administrative or other agency having jurisdiction over it. |
|
8.2
Product Warranties. Rigel represents and warrants to Kissei that: |
|
(a)
all Product supplied to Kissei pursuant to this Supply Agreement shall be Manufactured in conformity with GMPs; |
|
(b)
each Product supplied to Kissei pursuant to this Supply Agreement, at the time of shipment of such Product to Kissei pursuant to Section 2.3(c), shall conform to the applicable Specifications for such Product; and |
|
(c)
all Product supplied to Kissei pursuant to this Supply Agreement shall, at the time of shipment of such Product to Kissei pursuant to Section 2.3(c), be free and clear of all liens, security interests, and other encumbrances; provided, however, that Rigel shall retain a security interest in such Product until Kissei pays for it in full pursuant to Section 3.2 of this Supply Agreement and Section 8.5 of the Collaboration and License Agreement. |
|
8.3
Disclaimers. Except as expressly stated in this Supply Agreement, no representations or warranties whatsoever, whether express or implied, including warranties of merchantability, AND fitness for a particular purpose, are made or given by or on behalf a Party, and all representations and warranties, whether arising by operation of law or otherwise, are hereby expressly excluded. |
|
Article 9
INDEMNIFICATION |
|
9.1
Indemnification by Rigel. Rigel hereby agrees to defend, indemnify, and hold harmless Kissei and its Affiliates and their respective directors, officers, employees, and agents (each, a “Kissei Indemnitee”) from and against any and all liabilities, expenses, and losses including any product liability, personal injury, property damage, |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
9
including reasonable legal expenses and attorneys’ fees (collectively, “Losses”), to which any Kissei Indemnitee may become subject as a result of any claim, demand, action, or other proceeding by any Third Party to the extent such Losses arise out of or result from: (a) the negligence or willful misconduct of any Rigel Indemnitee, or (b) the breach by Rigel of any warranty, representation, covenant, or agreement made by Rigel in this Supply Agreement; except, in each case (a)-(b), to the extent such Losses arise out of any activities set forth in Section 9.2(a), (b), (c), or (d) for which Kissei is obligated to indemnify any Rigel Indemnitee(s) under Section 9.2. |
|
9.2
Indemnification by Kissei. Kissei hereby agrees to defend, indemnify, and hold harmless Rigel, its Affiliates, and licensees and their respective directors, officers, employees, and agents (each, a “Rigel Indemnitee”) from and against any and all Losses to which any Rigel Indemnitee may become subject as a result of any claim, demand, action, or other proceeding by any Third Party to the extent such Losses arise out of: (a) the negligence or willful misconduct of any Kissei Indemnitee, (b) the breach by Kissei of any warranty, representation, covenant, or agreement made by Kissei in this Supply Agreement, (c) the Finish Manufacture, export, import, storage, packaging, or labeling, by or on behalf of Kissei or its Affiliates or sublicensees, of any Product supplied by Rigel hereunder, or (d) the commercialization of any Product supplied by Rigel hereunder; except, in each case (a)-(d), to the extent such Losses arise out of any activities set forth in Section 9.1(a) or (b) for which Rigel is obligated to indemnify any Kissei Indemnitee(s) under Section 9.1. |
|
9.3
Indemnification Procedures. A party that intends to claim indemnification under this Article 9 (the “Indemnitee”) shall promptly notify the indemnifying Party (the “Indemnitor”) in writing of any Third Party claim, demand, action, or other proceeding (each, a “Claim”) in respect of which the Indemnitee intends to claim such indemnification, and the Indemnitor shall have sole control of the defense or settlement thereof. The Indemnitee may participate at its expense in the Indemnitor’s defense of and settlement negotiations for any Claim with counsel of the Indemnitee’s own choice. The indemnity arrangement in this Article 9 shall not apply to amounts paid in settlement of any action with respect to a Claim if such settlement is effected without the consent of the Indemnitor, which consent shall not be unreasonably withheld or delayed. The failure to deliver written notice to the Indemnitor within a reasonable time after the commencement of any action with respect to a Third Party Claim shall only relieve the Indemnitor of its indemnification obligations under this Article 9 if and to the extent the Indemnitor is actually prejudiced thereby. The Indemnitee shall cooperate fully with the Indemnitor and its legal representatives in the investigation of any action with respect to a Claim covered by this indemnification. |
|
9.4
Insurance. Each Party, at its own expense, shall maintain insurance as set forth in Section 12.4 of the Collaboration and License Agreement. |
|
9.5
Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, LOST PROFITS, PUNITIVE, OR INDIRECT DAMAGES ARISING FROM OR RELATING TO ANY BREACH OF THIS SUPPLY AGREEMENT, REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. NOTWITHSTANDING THE FOREGOING, NOTHING IN THIS SECTION 9.5 IS INTENDED TO OR SHALL LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF ANY PARTY UNDER SECTIONS 9.1 OR 9.2, OR DAMAGES AVAILABLE FOR A PARTY’S BREACH OF CONFIDENTIALITY OBLIGATIONS IN Article 5. Without limiting the generality of the foregoing, Rigel’s obligationS and liability in connection with its supply obligations under this Supply Agreement (including in connection with any supply shortage, delays, and quality and other matters and Rigel’s indemnification obligations to Kissei under this Supply Agreement) shall be limited to the extent of the remedies actually obtained and recovered by Rigel from its contract manufacturers under the supply agreements between Rigel and the applicable contract manufacturer. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
10
|
Article 10
TERM AND TERMINATION |
|
10.1
Term. This Supply Agreement shall commence on the Effective Date and shall continue until terminated as provided in this Section 10.2 (the “Term”). |
|
(a)
Material Breach. A Party’s material breach of this Supply Agreement will constitute such Party’s material breach of the Collaboration and License Agreement, and each Party shall have the right to terminate this Supply Agreement and the Collaboration and License Agreement for the other Party’s uncured material breach of this Supply Agreement as set forth in Section 14.2(a) of the Collaboration and License Agreement. |
|
(b)
Due to Early Termination of the Collaboration and License Agreement. This Supply Agreement shall automatically terminate upon termination of the Collaboration and License Agreement pursuant to Section 14.2, 14.3(a), or 14.3(b) of the Collaboration and License Agreement. |
|
(c)
After the Commercialization Term. Either Party shall have the right to terminate this Supply Agreement, on a Product-by-Product and country-by-country basis, without cause upon [*] prior written notice to the other Party so long as such termination becomes effective on or after the end of the Commercialization Term for such Product in such country. |
|
10.3
Effects of Termination; Survival. Termination or expiration of this Supply Agreement shall not affect the rights or obligations of the Parties under this Supply Agreement that have accrued prior to the date of termination or expiration. Upon termination of this Supply Agreement for any reason: (a) Products Manufactured pursuant to Purchase Orders will be delivered on the scheduled delivery dates and Kissei shall pay Rigel not later than [*] after the delivery date (provided, however, that Kissei makes advance payment prior to shipment in the event of termination due to payment default by Kissei); and (b) all costs of unused and unusable by Rigel raw materials, labels, and packaging incurred by Rigel shall be paid by Kissei in the event that Rigel terminates this Supply Agreement pursuant to Section 10.2(a) or that this Supply Agreement is terminated pursuant to Section 10.2(b) as a result of termination of the Collaboration and License Agreement by Kissei pursuant to Sections 14.3(a) or (b) of the Collaboration and License Agreement. Notwithstanding anything to the contrary, the following provisions shall survive any expiration or termination of this Supply Agreement: Sections 5 (Confidentiality), 6 (Intellectual Property), 9 (Indemnification), 10.3 (Effects of Termination; Survival), and 11 (General Provisions). |
|
Article 11
GENERAL PROVISIONS |
|
11.1
Governing Law; Dispute Resolution. This Supply Agreement, and all questions regarding the existence, validity, interpretation, breach, or performance of this Supply Agreement, shall be governed by, and construed and enforced in accordance with, the laws of the State of New York, United States, without reference to its conflicts of law principles. The application of the U.N. Convention on Contracts for the International Sale of Goods (1980) is excluded. Any controversy or claim arising out of, relating to, or in connection with any provision of this Supply Agreement shall be resolved in accordance with Article 15 of the Collaboration and License Agreement. |
|
11.2
Entire Agreement; Amendment. This Supply Agreement, including the Exhibits, together with the Collaboration and License Agreement, is both a final expression of the Parties’ agreement and a complete and exclusive statement with respect to all of its terms. This Supply Agreement supersedes all prior and contemporaneous agreements and communications, whether oral, written, or otherwise, concerning any and all matters contained herein. This Supply Agreement may only be modified or supplemented in a writing expressly stated for such purpose and signed by the Parties to this Supply Agreement. No modification to this Supply Agreement will be effected by the |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
11
acknowledgment or acceptance of any Purchase Order or shipping instruction forms or similar documents containing terms or conditions at variance with or in addition to those set forth herein. |
|
11.3
Notices. Any notice to be given under this Supply Agreement must be in writing and delivered either in person, by (a) air mail (postage prepaid) requiring return receipt, (b) overnight courier, or (c) facsimile confirmed thereafter by any of the foregoing, to the Party to be notified at its address(es) given below, or at any address such Party may designate by prior written notice to the other in accordance with this Section 11.3. Notice shall be deemed sufficiently given for all purposes upon the earliest of: (i) the date of actual receipt, (ii) if air mailed, [*] after the date of postmark, (iii) if delivered by overnight courier, the next day the overnight courier regularly makes deliveries, or (iv) if sent by facsimile, the date of confirmation of receipt if during the recipient’s normal business hours, otherwise the next business day. |
If to Kissei, notices must be addressed to:
Kissei Pharmaceutical Co., Ltd
0-0-0 Xxxxxxxxxx-Xxxxxxxxx,
Xxxx-xx, Xxxxx 000-0000 Xxxxx
Attention: [*]
Facsimile: [*]
【cc.】
Kissei Pharmaceutical Co., Ltd.
19-48 Yoshino, Matsumoto-City
Nagano-prefecture, 399-8710 Japan
Attention: [*]
Facsimile: [*]
If to Rigel, notices must be addressed to:
Rigel Pharmaceuticals, Inc.
0000 Xxxxxxxx Xxxx.
Xxxxx Xxx Xxxxxxxxx, XX 00000
XXX
Attention: [*]
Facsimile: [*]
|
11.4
Interpretation. The headings of clauses contained in this Supply Agreement preceding the text of the sections, subsections, and paragraphs hereof are inserted solely for convenience and ease of reference only and shall not constitute any part of this Supply Agreement, or have any effect on its interpretation or construction. All references in this Supply Agreement to the singular shall include the plural where applicable. Unless otherwise specified, references in this Supply Agreement to any Article shall include all Sections, subsections, and paragraphs in such Article, references to any Section shall include all subsections and paragraphs in such Section, and references in this Supply Agreement to any subsection shall include all paragraphs in such subsection. The word “including” and similar words means including without limitation. The word “or” means “and/or” unless the context dictates otherwise because the subjects of the conjunction are, or are intended to be, mutually exclusive. The words “herein”, “hereof”, and “hereunder” and other words of similar import refer to this Supply Agreement as a whole and not to any particular Section or other subdivision. All references to days in this Supply Agreement mean calendar days, unless |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
12
otherwise specified. Ambiguities and uncertainties in this Supply Agreement, if any, shall not be interpreted against either Party, irrespective of which Party may be deemed to have caused the ambiguity or uncertainty to exist. This Supply Agreement has been prepared in the English language and the English language shall control its interpretation. In addition, all notices required or permitted to be given hereunder, and all written, electronic, oral, or other communications between the Parties regarding this Supply Agreement shall be in the English language. |
|
11.5
Assignment. Except as expressly provided hereunder, neither this Supply Agreement nor any rights or obligations hereunder may be assigned or otherwise transferred by either Party without the prior written consent of the other Party (which consent shall not be unreasonably withheld); provided, however, that either Party may assign or otherwise transfer this Agreement and its rights and obligations hereunder without the other Party’s consent: |
|
(a)
in connection with the assignment of the Collaboration and License Agreement to a Third Party as set forth in Section 16.5 of the Collaboration and License Agreement; or |
|
(b)
to an Affiliate, provided that the assigning Party shall remain liable and responsible to the non-assigning Party hereto for the performance and observance of all such duties and obligations by such Affiliate. |
The rights and obligations of the Parties under this Supply Agreement shall be binding upon and inure to the benefit of the successors and permitted assigns of the Parties specified above, and the name of a Party appearing herein will be deemed to include the name of such Party’s successors and permitted assigns to the extent necessary to carry out the intent of this Section 11.4. Any assignment not in accordance with this Section 11.4 shall be null and void.
|
11.6
Performance by Affiliates. Each Party may discharge any obligations and exercise any right hereunder through any of its Affiliates. Each Party hereby guarantees the performance by its Affiliates of such Party’s obligations under this Supply Agreement, and shall cause its Affiliates to comply with the provisions of this Supply Agreement in connection with such performance. Any breach by a Party’s Affiliate of any of such Party’s obligations under this Supply Agreement shall be deemed a breach by such Party, and the other Party may proceed directly against such Party without any obligation to first proceed against such Party’s Affiliate. |
|
11.7
Further Actions. Each Party agrees to execute, acknowledge, and deliver the Quality Agreement. |
|
11.8
Compliance with Applicable Laws. Each Party shall comply in all material respects with all Applicable Laws, including, but not limited to, those concerning drugs, drug manufacture regulatory requirements, or exportation or importation of Products, including but not limited to proper declaration of dutiable values. Except as provided in Section 2.3(c), Kissei shall be responsible for obtaining all exportation and importation licenses or other authorizations. |
|
11.9
Severability. If, for any reason, any part of this Supply Agreement is adjudicated invalid, unenforceable, or illegal by a court of competent jurisdiction, such adjudication shall not, to the extent feasible, affect or impair, in whole or in part, the validity, enforceability, or legality of any remaining portions of this Supply Agreement. All remaining portions shall remain in full force and effect as if the original Supply Agreement had been executed without the invalidated, unenforceable, or illegal part. |
|
11.10
No Waiver. The failure of a Party to insist upon strict performance of any provision of this Supply Agreement or to exercise any right arising out of this Supply Agreement shall neither impair that provision or right nor constitute a waiver of that provision or right, in whole or in part, in that instance or in any other instance. Any waiver by a Party of a particular provision or right shall be in writing, shall be as to a particular matter and, if applicable, for a particular period of time and shall be signed by such Party. |
|
11.11
Relationship Between the Parties. The Parties’ relationship, as established by this Supply Agreement together with the Collaboration and License Agreement, is solely that of independent contractors. This Supply Agreement does not create any partnership, joint venture, or similar business relationship between the Parties. |
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
13
Neither Party is a legal representative of the other Party and neither Party can assume or create any obligation, representation, warranty, or guarantee, express or implied, on behalf of the other Party for any purpose whatsoever. |
|
11.12
Counterparts; Electronic or Facsimile Signatures. This Supply Agreement may be executed in any number of counterparts, each of which shall be an original, but all of which together shall constitute one instrument. This Supply Agreement may be executed and delivered electronically or by facsimile and upon such delivery such electronic or facsimile signature will be deemed to have the same effect as if the original signature had been delivered to the other Party. |
{Signature Page Follows}
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
14
In Witness Whereof, the Parties hereto have caused this Supply Agreement to be executed and entered into by their duly authorized representatives as of the Effective Date.
|
|
|
|
Rigel Pharmaceuticals, Inc.
By: /s/ Xxxx X. Xxxxxxxxx
Name: Xxxx X. Xxxxxxxxx
Title: President and CEO
|
Kissei Pharmaceutical Co. Ltd.
By: /s/ Xxxxxx Xxxxxxx
Name: Xxxxxx Xxxxxxx
Title: Chairman and CEO
|
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
15
List of Exhibits
Exhibit A:Compound
Exhibit B: Form of Order Forecast
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
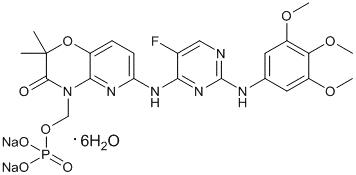
Exhibit A:Compound
fostamatinib disodium hexahydrate (“Compound”)
Chemical Name: disodium (6-[[5-fluoro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]-2,2-dimethyl-3-oxo-pyrido[3,2-b][1,4]oxazin-4-yl)methyl phosphate hexahydrate
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
2
Exhibit B: Form of Order Forecast
[*]
[*] = Certain confidential information contained in this document, marked by brackets, has been omitted and filed separately with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
3


