LICENSE AGREEMENT
Exhibit 6.1
NOTE: Portions of this Exhibit are the subject of a Confidential Treatment Request by the Registrant to the Securities and Exchange Commission (the “Commission”). Such portions have been redacted and are marked with a “[***]” in the place of the redacted language. The redacted information has been filed separately with the Commission.
THIS AGREEMENT is made and entered into this 8th day of January, 2009 (“EFFECTIVE DATE”), by and between THE CURATORS OF THE UNIVERSITY OF MISSOURI, a public corporation of the State of Missouri having a principal office at The Office of Technology Management and Industry Relations, 000X Xxxx Xxxx Xxxxxxxx Xxxxxx, Xxxxxxxx, XX 00000, (“UNIVERSITY”) and CrossCart, Inc. having offices at 0000 Xxxxxxxx Xxxxxx, Xxx Xxxxxxxxx, XX 00000 (“LICENSEE”).
WHEREAS, UNIVERSITY has an ownership interest in PATENT RIGHTS related to LICENSED SUBJECT MATTER all as defined below; and
WHEREAS, LICENSEE is desirous of obtaining a license to practice the LICENSED SUBJECT MATTER; and
WHEREAS, UNIVERSITY is desirous of granting such a license to LICENSEE in accordance with the terms of this Agreement.
NOW, THEREFORE, in consideration of the foregoing premises and the covenants, representations and warranties contained herein, the Parties agree as follows:
ARTICLE
I
DEFINITIONS
1.01 “AFFILIATE” means any business entity more than fifty percent (50%) owned by LICENSEE, any business entity which owns more than fifty percent (50%) of LICENSEE, or any business entity that is more than fifty percent (50%) owned by a business entity that owns more than fifty percent (50%) of LICENSEE.
1.02 “LICENSED FIELD” means each of the following business areas:
(a) xenotransplantation.
1.03 “LICENSED PRODUCT” means any product or part thereof made using alpha -galactosidase within the scope of one or more claims of PATENT RIGHTS and/or made using a method or process within the LICENSED SUBJECT MATTER.
1.04 “LICENSED SUBJECT MATTER” means inventions and discoveries within the scope of the TECHNOLOGY and PATENT RIGHTS, if any, within LICENSED FIELD.
1.05 “LICENSED TERRITORY” means worldwide.
1.06 “NET SALES” means the amount received for the Sale of LICENSED PRODUCTS, less:
(a) Customary trade, quantity or cash discounts;
(b) Amounts repaid or credited by reason of rejection or return;
(c) Charges for transportation or delivery to be paid by or on behalf of LICENSEE’s customer, to the extent such charges are separately stated on purchase orders, invoices or other documents of Sale; and/or
e) Samples, demonstration or units provided at no cost for R&D and clinical studies.
| 2 |
1.07 “PATENT RIGHTS” means UNIVERSITY’s aggregate rights in the US patents identified in Appendix A, made a part of this Agreement, ; and continuing applications thereof including divisions, substitutions, continuations, but not including continuations-in-part; and any patents issuing on said continuing applications, including reissues, reexaminations and extensions; and any corresponding foreign applications or patents. All of the foregoing will be automatically incorporated in and added to this Agreement and shall periodically be added to Appendix A attached to this Agreement and made part thereof.
1.08 “Sale”, Sell”, or “Sold” means the, transfer, distribution or disposition of a product for monetary value to a party other than LICENSEE, or SUBLICENSEE as the case may be.
1.09 “SUBLICENSEE” means any person or entity to whom LICENSEE transfers any right or interest granted to LICENSEE by UNIVERSITY under this Agreement.
1.10 “TECHNOLOGY” means the information or discoveries developed prior to the date of this Agreement as disclosed in University of Missouri (UM) Disclosure No. 94UMC039 entitled “Buffer System for Increasing Seroconversion Efficiency” dated 04/26/1994, copy attached hereto as Exhibit A.
ARTICLE
II
GRANT
2.01 University hereby grants to LICENSEE and LICENSEE accepts, subject to the terms and conditions hereof, a royalty-bearing, exclusive license under LICENSED SUBJECT MATTER to make, have made, use, Sell, have Sold, import, distribute, or otherwise transfer LICENSED PRODUCT within the LICENSED TERRITORY for use within LICENSED FIELD for a term extending to the end of the term of the last to expire patent within the PATENT RIGHTS.
| 3 |
2.02 The license granted in Section 2.01 above shall include the right to grant sublicenses, but without the right of SUBLICENSEE to grant further sublicenses, subject to UNIVERSITY’s approval, which approval shall not be unreasonably withheld. In determining whether to approve a sublicense, UNIVERSITY will consider, among other things, whether the provisions of the proposed sublicense are consistent with and similar to those required of the LICENSEE by this Agreement. LICENSEE must deliver to UNIVERISTY a true and correct copy of each fully executed sublicense granted by LICENSEE, and any modification or termination thereof, within thirty (30) days after execution, modification, or termination. LICENSEE shall, at such times as UNIVERSITY directs and at UNIVERSITY’s expense, request the inspection of the sublicensee’s records by an independent certified public accountant.
2.03 Notwithstanding the exclusivity of the license granted in Section 2.01 above, UNIVERSITY shall have the right to make and to use the LICENSED SUBJECT MATTER for research and educational purposes only.
2.04 LICENSEE agrees that UNIVERSITY shall have a right to publish the research results related to the LICENSED SUBJECT MATTER in accordance with UNIVERSITY’s general policies and that this Agreement shall not restrict, in any fashion, UNIVERSITY’s right to publish.
ARTICLE
III
PAYMENTS
3.01 License Payments: In consideration of rights granted by UNIVERSITY to LICENSEE under this Agreement, LICENSEE will pay UNIVERSITY the following:
| a. | A nonrefundable license fee in the amount of fifteen thousand ($15,000.00) dollars, due and payable when this Agreement is fully executed; |
| 4 |
| b. | A SALES ROYALTY of: |
| (a) | 1.9% (one and nine-tenths percent) on Net Sales of less than $10 million, and |
| (b) | 1.6% (one and six-tenths percent) on Net Sales greater than $10 million |
of LICENSED PRODUCTS made by or for LICENSEE and/or are sold by LICENSEE, in a country in which there is a valid patent within the PATENT RIGHTS and
| (c) | 1.5% (one and five-tenths percent) on Net Sales less than $10 million, and |
| (d) | 1.2% (one and two-tenths percent) on Net Sales greater than $10 million, of any LICENSED PRODUCT made or sold in a country by LICENSEE in which there is no valid patent within the PATENT RIGHTS at the time of such making or Sale, |
of LICENSED PRODUCTS made by or for LICENSEE and/or are sold by LICENSEE, in a country in which there is no valid patent within the PATENT RIGHTS.
| c. | A minimum annual royalty of thirty-five thousand dollars ($35,000) for each year beginning eighteen (18) months from the EFFECTIVE DATE of this Agreement, due and payable within thirty (30) days from the end of each such one year period. Each minimum annual royalty payment shall be creditable against SALES ROYALTY due the UNIVERSITY during Calendar Year of payment. For the avoidance of doubt, such minimum annual royalty shall be considered a payment in advance of royalties yet to accrue. |
*** CONFIDENTIAL PORTIONS OMITTED AND FILED SEPARATELY WITH THE COMMISSION
| 5 |
| d. | Upon payment of an amount pursuant to paragraphs 3.01a - d above and paragraph 3.02 below which is cumulatively equal to three million, five hundred thousand dollars ($3,500,000) (Maximum Payment), then the license granted hereunder to LICENSEE will automatically become fully paid-up, royalty free, perpetual and irrevocable. Upon payment by LICENSEE of the Maximum Payment, LICENSEE shall have no further obligation to make any further payments, provide any reports or provide access to any records as described in Article IV. |
3.02 Sublicense Payments: In consideration of rights granted by UNIVERSITY to LICENSEE under this Agreement, LICENSEE further agrees to pay UNIVERSITY the following after the execution of a sublicense hereunder:
Within thirty (30) days from LICENSEE’s receipt, LICENSEE shall pay to UNIVERSITY an additional royalty of [***] percent ([***]%) of all revenue received from any SUBLICENSEE. Such revenue shall include, but not be limited to, all option fees, license issue fees (up-front payments), license maintenance fees, equity, and all royalty payments. Such revenue shall not include research funding provided to LICENSEE by SUBLICENSEE.
3.03 Payment of taxes and/or other governmental charges or fees on SALES ROYALTY payments made to UNIVERSITY shall be the responsibility of UNIVERSITY and shall not be deducted from SALES ROYALTY payments due UNIVERSITY.
*** CONFIDENTIAL PORTIONS OMITTED AND FILED SEPARATELY WITH THE COMMISSION
| 6 |
3.04 All payments to the UNIVERSITY pursuant to this Agreement shall be paid in U.S. Dollars. Conversion of foreign currency to U. S. Dollars shall be made at the conversion rate existing in the United States (as reported in the in the Wall Street Journal) on the last working day of each royalty period. Such payments shall be without deduction of exchange, collection or other charges. Such payments shall be made payable to The Curators of the University of Missouri and shall be mailed to The Office of Technology Management and Industry Relations, 000X Xxxx Xxxx Xxxxxxxx Xxxxxx, Xxxxxxxx, XX 00000.
3.05 Unless stipulated otherwise, all payments due the University hereunder for SALES ROYALTIES for a calendar quarter, shall be made within forty-five (45) days after the end of such calendar quarter. Late payments shall be subject to an interest charge of one and one half percent (1 1/2%) per month.
ARTICLE
IV
REPORTING
4.01 Prior to signing this Agreement, LICENSEE has provided to UNIVERSITY a written plan (hereinafter “COMMERCIALIZATION PLAN”) for LICENSED PRODUCT within the respective LICENSED FIELD and within the respective country or countries of the LICENSED TERRITORY to be introduced by LICENSEE into commercial use. The COMMERCIALIZATION PLAN includes, 1) planned research and development activities, 2) milestones and evidence of sufficient financial resources to successfully implement the COMMERCIALIZATION PLAN and ensure that LICENSED PRODUCT will be kept reasonably available to the public, and 3) projection of Sales of LICENSED PRODUCTS and proposed marketing efforts, Such COMMERCIALIZATION PLAN is incorporated as Appendix B.
| 7 |
4.02 LICENSEE shall report to UNIVERSITY the date of first Sale of LICENSED PRODUCTS in each country of LICENSED TERRITORY within forty-five (45) days of occurrence.
4.03 Within 45 days after each March 31, June 30, September 30, and December 31 following the first Sale of LICENSED PRODUCT, whether Sold by LICENSEE or its SUBLICENSEE, if any exists, LICENSEE shall deliver to UNIVERSITY a true and accurate written report, even if no payments are due UNIVERSITY, giving the particulars of the business conducted by LICENSEE and its SUBLICENSEE(s), during the preceding three (3) calendar months under this Agreement as are pertinent to calculating payments hereunder. This report will include at least:
a. total NET SALES;
b. offsets of minimum annual royalties or other offsets allowed under this Agreement; and
c. total SALES ROYALTY computed and due UNIVERSITY.
LICENSEE shall provide sufficient data for UNIVERSITY to verify the calculations, and any reasonable additional information UNIVERSITY requires to determine LICENSEE’s satisfaction of the reporting requirements hereunder or to clarify the information contained in reports provided by LICENSEE. LICENSEE shall provide such additional information within thirty (30) days of receiving a request from UNIVERSITY. Simultaneously with the delivery of each report, LICENSEE must pay to UNIVERSITY the amount, if any, due for the period of each report.
| 8 |
4.04 On or before each anniversary of the EFFECTIVE DATE, irrespective of having a first Sale or offer for Sale, LICENSEE must deliver to UNIVERSITY a written annual report as to LICENSEE’s (and any SUBLICENSEE’s) efforts and accomplishments during the preceding year in diligently commercializing LICENSED PRODUCT in the LICENSED FIELD, including but not limited to, progress on research and development, regulatory approvals, manufacturing, sublicensing, marketing and Sales and LICENSEE’s (and, if applicable, SUBLICENSEE’s) commercialization plans for the upcoming year. LICENSEE shall also provide any reasonable additional information UNIVERSITY requires to evaluate LICENSEE’S performance under this Agreement.
4.05 LICENSEE agrees to keep records for a period of three (3) years following termination of this Agreement showing the manufacturing, Sales, use, sublicense, and other disposition of LICENSED PRODUCT, Sold or otherwise disposed of under the license herein granted in sufficient detail to enable the royalties payable hereunder by LICENSEE to be determined. LICENSEE agrees to permit UNIVERSITY or its representatives, at UNIVERSITY’s expense, to periodically examine its books, ledgers, and records during regular business hours for the purpose of and to the extent necessary to verify any report required under this Agreement. If the amounts due to UNIVERSITY are determined to have been underpaid, LICENSEE will pay the amount of such underpayment and interest on the amount of such underpayment, calculated in accordance with Section 3.05 with interest accruing from the date such payment was originally due the UNIVERSITY. Such examination is to be made by UNIVERSITY at the expense of UNIVERSITY, except in the event that the results of the audit reveal a discrepancy in UNIVERSITY’S favor of five percent (5%) or more, then the audit fees shall be paid by LICENSEE.
| 9 |
ARTICLE
V
DUE DILIGENCE
5.01 LICENSEE shall use reasonable efforts to effect introduction of the LICENSED PRODUCT into the commercial market as soon as practicable, consistent with sound and reasonable business practices and judgment; thereafter, until the expiration of this Agreement, LICENSEE shall keep LICENSED PRODUCT reasonably available to the public.
5.02 UNIVERSITY shall have the right, at UNIVERSITY’s sole discretion, to either terminate or render this license nonexclusive in an individual LICENSED FIELD and/or individual country or countries within the LICENSED TERRITORY if LICENSEE or its SUBLICENSEE:
(a) Has not within six (6) months of the EFFECTIVE DATE presented to and obtained UNIVERSITY’S approval, which approval shall not be unreasonably withheld, a new COMMERCIALIZATION PLAN for LICENSED PRODUCT within the respective LICENSED FIELD and within the respective country or countries of the LICENSED TERRITORY not previously introduced by LICENSEE into commercial use,
(b) Has not within five (5) years of EFFECTIVE DATE obtained necessary regulatory approval to put LICENSED PRODUCT into commercial use in the respective LICENSED FIELD and within one or more country or countries of the LICENSED TERRITORY, or
*** CONFIDENTIAL PORTIONS OMITTED AND FILED SEPARATELY WITH THE COMMISSION
| 10 |
(c) Unless prevented by failure to obtain necessary regulatory approvals, has not within two (2) years of the EFFECTIVE DATE put LICENSED PRODUCT into commercial use in the respective LICENSED FIELD and within the respective country or countries of the LICENSED TERRITORY and is not keeping the LICENSED PRODUCT reasonably available to the public.
ARTICLE
VI
LIABILITY, WARRANTIES AND INSURANCE
6.01 LICENSEE shall at all times during the term of this Agreement and thereafter, indemnify, defend and hold UNIVERSITY, its current or former Curators, officers, employees and affiliates, harmless from any judgments and against all claims and expenses, including legal expenses and reasonable attorneys’ fees, arising out of the death of or injury to any person or persons or out of any damage to property and against any other claim, proceeding, demand, expense and liability of any kind whatsoever resulting from 1) the development, manufacture, use, or Sale of LICENSED PRODUCT by LICENSEE, its subsidiaries, and SUBLICENSEEs, or 2) from the use by the end users of LICENSED PRODUCT, or 3) arising from any obligation of LICENSEE hereunder. If any such claims or causes of action are made, UNIVERSITY shall be defended by counsel selected by LICENSEE, subject to UNIVERSITY’s approval, which shall not be unreasonably withheld. UNIVERSITY reserves the right to be represented by its own counsel at its own expense.
| 11 |
6.02 At such time as any product, process, or service relating to, or developed pursuant to, this Agreement is being commercially distributed or Sold (other than for the purpose of obtaining regulatory approvals) by LICENSEE, a SUBLICENSEE, or a subsidiary or agent of LICENSEE, LICENSEE shall at its sole cost and expense, procure and maintain comprehensive general liability insurance in amounts not less than $1,000,000 per incident and naming the UNIVERSITY, its Curators, trustees, officers, agents, employees and affiliates, as additional insureds. Such commercial general liability insurance shall provide (i) product liability coverage and (ii) broad form contractual liability coverage for LICENSEE’s indemnification under this Agreement. Such insurance will be considered primary as to any other valid and collectible insurance, but only as to acts of the named insured. Any carrier providing coverage shall have a minimum “Best” rating of “A-XII”. The minimum amounts of insurance coverage required shall not be construed to create a limit of LICENSEE’s liability with respect to its indemnification under this Agreement.
LICENSEE shall maintain such commercial general liability insurance beyond the expiration or termination of this Agreement during (i) the period that any product, process, or service, relating to, or developed pursuant to this Agreement is being commercially distributed or Sold by LICENSEE, or its SUBLICENSEE, subsidiary or agent of LICENSEE and (ii) a reasonable period after the period referred to in (i) above which in no event shall be less than 10 (ten) years.
LICENSEE shall provide UNIVERSITY with written evidence of the insurance requirements of this Section 6.02 within thirty (30) days after execution of this Agreement. LICENSEE shall provide UNIVERSITY with written notice at least fifteen (15) days prior to the cancellation, non-renewal or material change in such insurance; if LICENSEE does not obtain replacement insurance providing comparable coverage within such fifteen (15) day period, UNIVERSITY shall have the right to terminate this Agreement effective at the end of such fifteen (15) day period without notice or any additional waiting periods. It is agreed that the insurance required is required in the public interest and the UNIVERSITY does not assume any liability for acts of LICENSEE, their officers, agents, and employees or of a SUBLICENSEE, their officers, agents, and employees, in connection with the granting of this Agreement.
| 12 |
LICENSEE shall require in any sublicense in which LICENSEE grants to a third party the right to make, have made, use, import, offer to Sell or Sell any LICENSED PRODUCT, provisions that provide the UNIVERSITY, its Curators, trustees, officers, agents, employees and affiliates, comparable protections as those provided the UNIVERSITY in this Article VI.
6.03 University warrants that patents listed in Appendix A are current and have had all United States Patent and Trademark Office (USPTO) maintenance fees paid as of the EFFECTIVE DATE.
6.04 EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS LICENSE, UNIVERSITY MAKES NO REPRESENTATIONS AND EXTENDS NO WARRANTIES OF ANY KIND, EITHER EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO WARRANTIES OF MERCHANTABILITY, FITNESS FOR ANY PARTICULAR PURPOSE, AND VALIDITY OF PATENT RIGHTS CLAIMS, ISSUED OR PENDING, OR THAT THE MANUFACTURE, USE, OR SALE OF THE LICENSED SUBJECT MATTER WILL NOT INFRINGE ANY PATENT, COPYRIGHT, TRADEMARK, OR OTHER RIGHTS.
| 13 |
ARTICLE
VII
DOMESTIC AND FOREIGN PATENT FILING AND MAINTENANCE
7.01 LICENSEE shall reimburse UNIVERSITY for all out-of-pocket expenses UNIVERSITY has incurred for the preparation, filing, prosecution and maintenance of PATENT RIGHTS (hereinafter “PATENT EXPENSES”) and for all such future PATENT EXPENSES as a separate payment apart from any royalties or other revenues owed UNIVERSITY. To enable payment of such expenses, UNIVERSITY shall report to LICENSEE any and all payments already made as of the date of this agreement (listed in Exhibit B) for the preparation, filing, prosecution and maintenance of PATENT RIGHTS related to those listed in Appendix A and on a monthly basis, the current unpaid balance of such expenses, in such detail, so that LICENSEE can verify the accuracy of such report, including copies of invoices from counsel related to the same, such reimbursement shall be made within thirty (30) days of the end of each Calendar Year at a rate of $50,000 per Calendar Year until the balance of such expenses is zero. The initial reimbursement by LICENSEE for Patent Rights and Expenses incurred by the University shall be due June 30, 2010. Late payment of invoices of PATENT EXPENSES received by LICENSEE from UNIVERSITY shall be subject to interest charges of one and one-half percent (1 1/2%) per month. A payment due under this Section 7.01 is considered late if payment is not received by UNIVERSITY within thirty (30) days from the end of each calendar year.
7.02 UNIVERSITY shall be solely responsible for the preparation, filing, prosecution and maintenance of any and all U. S. and foreign patent applications and patents included in PATENT RIGHTS mutually agreed upon by UNIVERSITY and LICENSEE. UNIVERSITY shall first consult with LICENSEE as to the preparation, filing, prosecution, and maintenance of such patent applications and patents and shall furnish to LICENSEE copies of documents relevant to any such preparation, filing, prosecution or maintenance.
7.03 If LICENSEE elects not to continue paying future PATENT EXPENSES, for a patent within the PATENT RIGHTS, LICENSEE shall notify UNIVERSITY immediately in writing, and, provided UNIVERSITY provides written notice to LICENSEE more than ninety (90) days before any deadline for such patent(s), such notice by LICENSEE shall be not less than sixty (60) days prior to any deadline which should or must be met in order to maintain the patent or patent application in force (a “deadline” includes a date by which an action must be taken to avoid payment of a late fee). Such notice by LICENSEE shall constitute a waiver of and relinquishment of all of LICENSEE’s rights under this Agreement related to such patent or patent application.
| 14 |
ARTICLE
VIII
INFRINGEMENT
8.01 LICENSEE, at its expense, shall have the right to enforce PATENT RIGHTS against infringement by third parties and it is entitled to retain the recovery from such enforcement, including any cash or other consideration received by way of judgment, settlement or compromise (hereinafter RECOVERY). However, any RECOVERY, other than enhanced damages arising from intentional infringement, or punative damages, less direct out-of-pocket legal expenses incurred by LICENSEE for such enforcement (“NET RECOVERY”), shall be considered lost Sales and LICENSEE shall pay UNIVERSITY an amount equal to the applicable percentage of NET RECOVERY as specified in Section 3.01b above, in lieu of any SALES ROYALTY which might otherwise be due as a result of said NET RECOVERY.. Before LICENSEE commences a formal legal proceeding with respect to any infringement of PATENT RIGHTS, LICENSEE shall consult with UNIVERSITY regarding the potential effects such legal proceeding may have on the public interest. LICENSEE shall keep UNIVERSITY informed on all actions taken by LICENSEE in its enforcement against an infringer and shall furnish to UNIVERSITY copies of all documents related thereto.
8.02 In any infringement suit or dispute, UNIVERSITY agrees to cooperate reasonably with LICENSEE. At the request and expense of LICENSEE, the UNIVERSITY will permit access to all relevant personnel, records, papers, information, samples, specimens, etc., during regular business hours on UNIVERSITY premises as reasonably necessary for LICENSEE to vigorously conduct such proceeding. In the event that travel is required, LICENSEE agrees to reimburse UNIVERSITY for such travel.
| 15 |
8.03 In the event that LICENSEE elects not to exercise its right to prosecute an infringement of the PATENT RIGHTS pursuant to the above paragraphs, UNIVERSITY may do so at its own expense, controlling such action and retaining all RECOVERY therefrom. LICENSEE agrees to cooperate reasonably with UNIVERSITY in any such infringement suit or dispute.
ARTICLE
IX
CONFIDENTIALITY
9.01 LICENSEE agrees that all patent prosecution information and all other information contained in documents marked “confidential” received from UNIVERSITY shall (i) be received in strict confidence, (ii) be used only for the purposes of this Agreement, and (iii) not be disclosed by LICENSEE, its employees, agents, successors or assigns, without the prior written consent of UNIVERSITY, except to the extent that the LICENSEE can establish competent written proof that such information:
| a. | was in the public domain at the time of disclosure; |
| b. | later became part of the public domain through no act or omission of LICENSEE, its employees, agents, successors or assigns; |
| c. | was lawfully disclosed to LICENSEE by a third party having the right to disclose it; |
| d. | was already known by LICENSEE at the time of disclosure; |
| e. | was independently developed by LICENSEE; or |
| f. | is required by law or regulation to be disclosed. |
9.02 LICENSEE’s obligation of confidence hereunder shall be fulfilled by using at least the same degree of care with UNIVERSITY’s confidential information as LICENSEE uses to protect its own confidential information, but not less than reasonable care. This obligation shall exist during the term of this Agreement and for a period of five (5) years thereafter.
| 16 |
9.03 UNIVERSITY agrees that all reports received from LICENSEE pursuant to this Agreement and all other information contained in documents marked “confidential” received from LICENSEE shall (i) be received in strict confidence, (ii) be used only for the purposes of this Agreement, and (iii) not be disclosed by UNIVERSITY, its employees, agents, successors or assigns, without the prior written consent of LICENSEE, except to the extent that the UNIVERSITY can establish competent written proof that such information:
| a. | was in the public domain at the time of disclosure; |
| b. | later became part of the public domain through no act or omission of UNIVERSITY, its employees, agents, successors or assigns; |
| c. | was lawfully disclosed to UNIVERSITY by a third party having the right to disclose it; |
| d. | was already known by UNIVERSITY at the time of disclosure; |
| e. | was independently developed by UNIVERSITY; or |
| f. | is required by law or regulation to be disclosed. |
9.04 UNIVERSITY’s obligation of confidence hereunder shall be fulfilled by using at least the same degree of care with LICENSEE’s confidential information as UNIVERSITY uses to protect its own confidential information, but not less than reasonable care. This obligation shall exist during the term of this Agreement and for a period of five (5) years thereafter.
ARTICLE
X
TERM AND TERMINATION
10.01 This Agreement shall become effective upon the EFFECTIVE DATE and, unless sooner terminated in accordance with any of the provisions herein, shall remain in full force in the LICENSED TERRITORY during the life of the last to expire patents under PATENT RIGHTS.
| 17 |
10.02 In the event that either Party defaults or breaches any of the provisions of this Agreement, the other Party shall have the right to terminate this Agreement by giving written notice to the defaulting Party; provided, however, that if the said defaulting Party cures said default within sixty (60) days after said notice shall have been given, this Agreement shall continue in full force and effect. The failure on the part of either of the Parties hereto to exercise or enforce any right conferred upon it hereunder shall not be deemed to be a waiver of any such right nor operate to bar the exercise or enforcement thereof at any time or times thereafter.
10.03 Upon termination of this Agreement, LICENSEE’s interest in sublicenses granted by it under this Agreement shall at UNIVERSITY’s option, terminate or be assigned to UNIVERSITY. LICENSEE shall make provision for the UNIVERSITY’s rights under the preceding sentence to be included in all sublicenses granted by it under this Agreement.
10.04 In the event that LICENSEE shall become insolvent, shall make an assignment for the benefit of creditors, or shall have a petition in bankruptcy filed for or against it, this Agreement shall automatically terminate.
10.05 Termination of this Agreement for any reason shall not release either Party from any obligation theretofore accrued. Articles III, VI, and IX and Sections 4.03, 4.05, 10.03, 11.07, and 11.13 shall survive the termination of this Agreement.
ARTICLE
XI
GENERAL
11.01 Prior to the issuance of patents under PATENT RIGHTS, LICENSEE agrees to xxxx LICENSED PRODUCTS (or their containers or labels) Sold by LICENSEE, or a SUBLICENSEE, under the license granted in this Agreement with the numbers of the patent(s) within the PATENT RIGHTS covering the LICENSED PRODUCTS.
| 18 |
11.02 LICENSEE agrees to comply with all applicable federal, state, and local laws and regulations. In particular, it is understood and acknowledged that the transfer of certain commodities and technical data is subject to United States laws and regulations controlling the export of such commodities and technical data, including all Export Administration Regulations of the United States Department of Commerce. These laws and regulations among other things, prohibit or require a license for the export of certain types of technical data to certain specified countries. LICENSEE hereby agrees and gives written assurance that it will comply with all United States laws and regulations controlling the export of commodities and technical data, that it will be solely responsible for any violation of such by LICENSEE, its AFFILIATE, or SUBLICENSEES, and that it will defend and hold UNIVERSITY harmless in the event of any legal action of any nature occasioned by such violation.
11.03 LICENSEE agrees not to identify UNIVERSITY in any promotional advertising or other promotional materials to be disseminated to the public or any portion thereof or to use the name of any UNIVERSITY faculty member, employee, or student or any trademark, service xxxx, trade name, or symbol of UNIVERSITY, without UNIVERSITY’S prior written consent.
11.04 Except in connection with the sale of substantially all of LICENSEE’s assets to a third party, this Agreement may not be assigned by LICENSEE without the prior written consent of UNIVERSITY, which will not be unreasonably withheld.
11.05 If LICENSEE desires UNIVERSITY participation in performing research and development activities directed towards PATENT RIGHTS, negotiation for such assistance shall be separate and apart from this Agreement, and shall be performed according to UNIVERSITY’S procedures related to research grant and contract activities.
| 19 |
11.06 In the event LICENSEE wishes to engage the inventors as consultants, such an arrangement shall be separate and apart from this Agreement, but shall be in keeping with UNIVERSITY’S policy on consulting and ownership of intellectual property developed by UNIVERSITY employees.
11.07 Any payment, notice, or other communication given under this Agreement (except for correspondence relating to patent filing, prosecution and/or maintenance matters under Article VII herein) shall be in writing and shall be deemed delivered when sent by certified first class mail, registered mail, or overnight courier, or by facsimile, provided that a copy of such facsimile is promptly sent by certified first class mail, registered or overnight courier, addressed to the Parties as follows (or at such other addresses as the Parties may notify each other in writing):
If to UNIVERSITY:
The Office of Technology Management & Industry Relations
000 X, Xxxx Xxxx Xxxxxxxx Xxxxxx
Xxxxxxxx, XX 00000
If to LICENSEE:
Chief Executive Officer
CrossCart, Inc.
0000 Xxxxxxxx Xxxxxx
Xxx Xxxxxxxxx, XX 00000
| 20 |
11.08 This Agreement constitutes the entire and only agreement between the Parties for LICENSED SUBJECT MATTER and all other prior negotiations, representations, agreements, and understandings are superseded hereby. No agreements altering or supplementing the terms hereof may be made except by a written document signed by both Parties.
11.09 None of the terms, covenants, and conditions of this Agreement can be waived except by the written consent of the Party waiving compliance.
11.10 A failure by one of the Parties to this Agreement to assert its rights for or upon any breach or default of this Agreement shall not be deemed a waiver of such rights nor shall any such waiver be implied from acceptance of any payment. No such failure or waiver in writing by any one of the Parties hereto with respect to any rights, shall extend to or affect any subsequent breach or impair any right consequent thereon.
11.11 If any sentence, paragraph, clause or combination of the same is found by a court of competent jurisdiction to be in violation of any applicable law or regulation, or is unenforceable or void for any reason whatsoever, such sentence, paragraph, clause or combinations of the same shall be severed from the Agreement and the remainder of the Agreement shall remain binding upon the Parties.
11.12 The headings of the paragraphs of this Agreement are inserted for convenience only and shall not constitute a part hereof.
11.13 This Agreement shall be construed, interpreted, and applied in accordance with the laws of the State of Missouri. Any action to enforce the provisions of the Agreement shall be brought in a court of competent jurisdiction and proper venue in the State of Missouri.
| 21 |
IN WITNESS WHEREOF, the Parties hereto have executed this Agreement in duplicate originals by their duly authorized officers or representatives.
| THE CURATORS OF THE | LICENSEE: CrossCart, Inc. | |||
| UNIVERSITY OF MISSOURI | ||||
| BY: | BY: | |||
| NAME: Xxxxxxxxxxx X. Xxxxxx | NAME: Xxxxxx X. Xxx | |||
| TITLE: Interim Director, OTMIR | TITLE: CEO | |||
| DATE | DATE |
| 22 |
Appendix A
(list of technologies licensed)
US Patent No.: 5,606,042 - Glycine a.-D-galactosidases
US Patent No.: 5,633,130 – Buffer system for seroconversion efficiency
US Patent No.: 5,731,426 - Phaseolus a-D-galactosidases
US Patent No.: 6,184,017 – Glycine and phaseolus a-D-galactosidases
US Patent No.: 6,630,339 - Glycine and phaseolus a-D-galactosidases
Appendix B
Commercialization Plan
Z-Lig Development Status: Milestones
| · | Preclinical testing (completed) |
| - | Toxicology, sterility and biomechanics |
| - | Viral inactivation and biomaterials safety |
| - | Functional primate testing |
| · | Pilot clinical study initiated mid-2003 (completed) |
| - | 10 patient, non controlled, single investigator |
| - | Study focused on device safety and implantability |
| - | Completed two year follow-up (August 2005) |
| · | FDA approval for pivotal clinical trial (design approved) |
Z-Lig Development Status
| · | Pilot study strongly supports safety of Z-Process tissue treatment |
| - | No acute or chronic immune response/rejection |
| - | Device characteristics similar to allografts |
| - | Strong evidence of graft remodeling |
| · | 5 of 6 evaluable subjects successful and passed all functional assessments at 2-years |
| · | Objectives of technical feasibility and implantability met |
| · | Pilot study clinical outcomes (efficacy) impacted by complex patient etiologies and post operative trauma |
Z-Lig Development Status: Clinical Plans
| · | OUS Sell In Trial |
| - | 00 X-Xxx patients |
| - | One or two sites with Independent Investigator |
| - | Data for EU market release |
| · | US Pivotal Trial: Unconditional FDA Approval |
| - | 326 patients (163 Z-Lig and 163 allograft control) |
| - | Blinded Independent Evaluator |
| - | 7 to 10 sites |
| - | Non-inferiority design with power: 0.80 and margin: 0.15 |
| - | Additional statistics include Xxxxxx-Xxxxx & Xxx Models |
| - | Design approved by FDA and will be filed for PMA |
| · | Fostering S. American/S. African Clinical Relationships |
| - | No current allograft use in S. America/S. Africa |
| - | Growing Z-Lig Market Opportunity |
| - | Surgeons anxious for an off the shelf ACL device |
Z-Lig Development Status: Manufacturing Plans
| · | Contract Manufacturing Discussions |
| - | OEM contract manufacturing and co-development partner |
| - | Experience with xenograft products and processing |
| - | Experience with EU regulatory pathways |
| - | Market rollout to large-scale manufacturing facility plans |
| - | Viable distribution partner |
| · | Manufacturing Improvement and Cost Containment |
| - | Manufacturing validation plan for PMA filing launched |
| - | Process cost containment program initiated |
| - | Cost of Goods projections complete |
| - | Critical reagent supplier discussions initiated |
Z-Lig Development Status: Manufacturing Plans
PORCINE TISSUE PROCESSING FACILITY
| - | 4500 sq ft dedicated facility (nom) |
| - | ISO / CE xxxx compliant with class 10K – 100 air handling |
| - | Staffed by full time operations, project management and process technicians |
CrossCart Development Time Line (proposed)
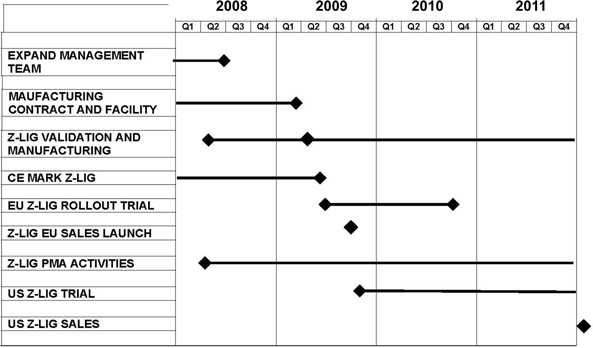
EXHIBIT A
University of Missouri (UM) Disclosure No. 94UMC039 entitled “Buffer System for Increasing Seroconversion Efficiency”
dated 04/26/1994


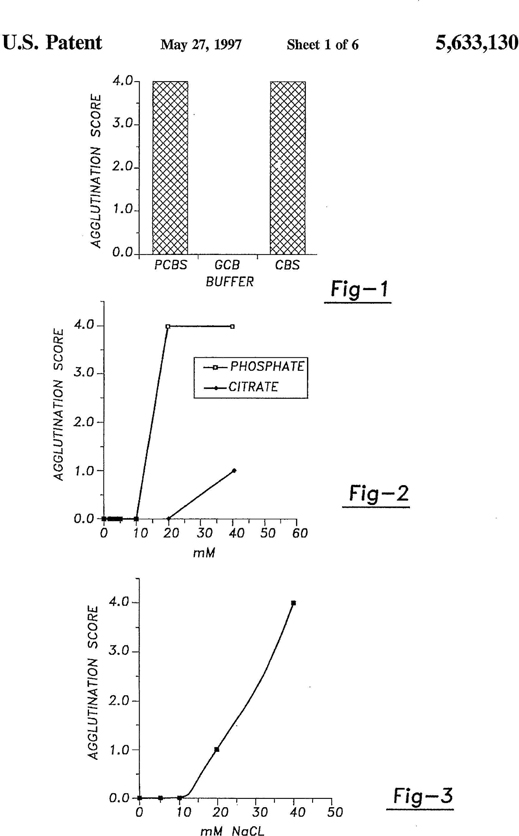

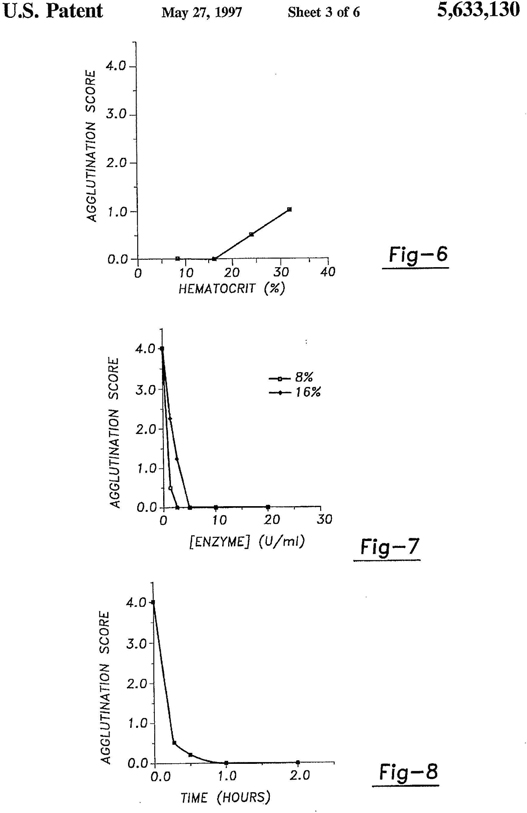










EXHIBIT B
(as of 1/8/2009)
Patent Expenses for US Patents under UM Disclosure No.
94UMC039
| Patent Number | Total Expense | |||
| 5,633,130 (08/303,156) Attny Ref #00014 (P-311) | $ | 18,689.29 | ||
| 6,184,017 (08/973,297) Attny Ref #00077 | $ | 10,446.58 | ||
| 6,630,339 (08/303,156) Attny Ref #00127 | $ | 11,255.84 | ||
| 5,731,426 (08/654,246) Attny Ref #00052 | $ | 6,489.01 | ||
| 5,606,042 (08/488,961) Attny Ref #00022 (P-319) | $ | 12,515.50 | ||
| $ | 59,396.22 | |||

