Smith & Nephew Agreement to acquire Osiris Therapeutics, Inc Forward looking statements and non-IFRS measures Cautionary Statement Regarding Forward Looking Statements This document contains forward-looking information related to Smith & Nephew,...
Forward looking statements and non-IFRS measures Cautionary Statement Regarding Forward Looking Statements This document contains forward-looking information related to Xxxxx & Xxxxxx, Xxxxxx and the proposed transaction that involves substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. These forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and include words such as “believes,” “plans,” “anticipates,” “projects,” “estimates,” “expects,” “intends,” “strategy,” “future,” “opportunity,” “may,” “will,” “should,” “could,” “potential,” or similar expressions. Forward-looking statements in this document include, among other things, statements about the potential benefits of the proposed transaction, including expected synergies, the expected timing of completion of the proposed transaction, anticipated earnings accretion, as well as Xxxxx & Xxxxxx’s plans and expectations and Osiris’ financial condition, results of operations, products and businesses. Forward-looking statements involve known and unknown risks, uncertainties and other important factors that could cause actual results to differ materially from what is expressed or implied by the statements. These forward-looking statements may be affected by risks and uncertainties, including, without limitation, the risk that the proposed transaction will not close when expected or at all; the risk that the conditions to the tender offer will not be satisfied in the anticipated timeframe or at all, including uncertainties as to how many of Osiris’ stockholders will tender their shares in the tender offer; risks related to the ability to realize the anticipated benefits of the proposed transaction, including the possibility that its expected benefits and synergies will not be realized or will not be realized within the expected time period; negative effects of the announcement or consummation of the proposed transaction on the market price of Xxxxx & Nephew shares and its operating results; the risk that Xxxxx & Xxxxxx’s and Osiris’ business will be adversely impacted during the pendency of the proposed transaction; the risk that the operations of the two companies will not be integrated successfully; unknown liabilities; and the risk of litigation and regulatory actions related to the proposed transaction. Additionally, for Xxxxx & Nephew, these factors include: economic and financial conditions in the markets we serve, especially those affecting health care providers, payers and customers; price levels for established and innovative medical devices; developments in medical technology; regulatory approvals, reimbursement decisions or other government actions; product defects or recalls or other problems with quality management systems or failure to comply with related regulations; litigation relating to patent or other claims; legal compliance risks and related investigative, remedial or enforcement actions; disruption to our supply chain or operations or those of our suppliers; competition for qualified personnel; strategic actions, including acquisitions and dispositions, our success in performing due diligence, valuing and integrating acquired businesses and disruption that may result from transactions or other changes we make in our business plans or organisation to adapt to market developments; and numerous other matters that affect us or our markets, including those of a political, economic, business, competitive or reputational nature. Please refer to the documents that Xxxxx & Xxxxxx has filed with the U.S. Securities and Exchange Commission (the “SEC”) under the U.S. Securities Exchange Act of 1934, as amended, including Xxxxx & Xxxxxx's most recent annual report on Form 20-F, for a discussion of certain of these factors. Any forward-looking statement is based on information available to Xxxxx & Xxxxxx as of the date of the statement. All written or oral forward-looking statements attributable to Xxxxx & Xxxxxx are qualified by this caution. Xxxxx & Xxxxxx does not undertake any obligation to update or revise any forward-looking statement to reflect any change in circumstances or in Xxxxx & Xxxxxx's expectations. Additional Information about the Proposed Transaction and Where to Find It The tender offer described in this document has not yet commenced. This document is provided for informational purposes only and does not constitute an offer to purchase or the solicitation of an offer to sell any securities. At the time the tender offer is commenced, Xxxxx & Nephew, Xxxxx & Nephew Consolidated, Inc. and a wholly owned subsidiary of Xxxxx & Nephew intend to file with the SEC a Tender Offer Statement on Schedule TO containing an offer to purchase, a form of letter of transmittal and other documents relating to the tender offer, and Xxxxxx intends to file with the SEC a Solicitation/Recommendation Statement on Schedule 14D-9 with respect to the tender offer. Xxxxx & Xxxxxx and Xxxxxx intend to mail these documents to the Osiris stockholders. Investors and stockholders should read those filings carefully when they become available as they will contain important information about the tender offer. Those documents may be obtained without charge at the SEC’s website at xxx.xxx.xxx or by contacting the information agent for the tender offer. INVESTORS AND SECURITY HOLDERS ARE ADVISED TO READ THESE DOCUMENTS WHEN THEY BECOME AVAILABLE, INCLUDING THE SOLICITATION/RECOMMENDATION STATEMENT OF OSIRIS AND ANY AMENDMENTS THERETO, AS WELL AS ANY OTHER DOCUMENTS RELATING TO THE PROPOSED TRANSACTION THAT ARE FILED WITH THE SEC, CAREFULLY AND IN THEIR ENTIRETY PRIOR TO MAKING ANY DECISIONS WITH RESPECT TO WHETHER TO TENDER THEIR SHARES PURSUANT TO THE PROPOSED TRANSACTION BECAUSE THEY CONTAIN IMPORTANT INFORMATION, INCLUDING THE TERMS AND CONDITIONS OF THE PROPOSED TRANSACTION Non IFRS Measures Certain items included in ‘trading results’, such as trading profit, trading profit margin, tax rate on trading results, trading cash flow, trading profit to cash conversion ratio, EPSA, net debt to adjusted EBITDA ratio, and underlying growth are non-IFRS financial measures. The non-IFRS financial measures in this announcement are explained and reconciled to the most directly comparable financial measure prepared in accordance with IFRS in our Fourth Quarter and Full Year 2018 Results announcement dated 7 February 2019. 2

Xxxxx Xxxxxx Chief Executive Officer 3

Overview of Osiris transaction 4 Agreement to acquire for $19.00 per share in cash, or approximately $660m Company delivering regenerative medicine products; founded in 1992, with $102m of revenue in the first nine months of 2018 360 employees, of which >130 are sales reps; key products manufactured at Columbia, Maryland (U.S) site Expands Xxxxx & Nephew’s presence in high growth area of skin substitutes Synergy with existing AWM portfolio from common call points Increased access for products to wider Xxxxx & Nephew customer base

Xxxxx Xxxxxx President, Advanced Wound Management 5

Expanding in a fast growing segment 6 US Market Size Market CAGR1 $0.5bn 9% Allograft sub-segment 2 Osiris market share 22% $0.9bn 7% Skin substitute segment 12% 1 SmartTrak Market Research 2 Amniotic tissue and dermal allografts Osiris market position #2 Key allograft competitors:

Two principal products driving growth 7 Cryopreserved placental membrane for acute and chronic wounds 9M 2018 sales: $75m 74% of group sales 9M 2018 growth: +20.5% Cryopreserved placental tissue as wound covering or wrap for soft tissue repair Bone matrix for bone repair 9M 2018 growth: +16.8% 9M 2018 sales: $20m Distributed by Stryker Allograft for articular cartilage repair 9M 2018 growth: +6.8% 9M 2018 sales: $7m Distributed by Arthrex &

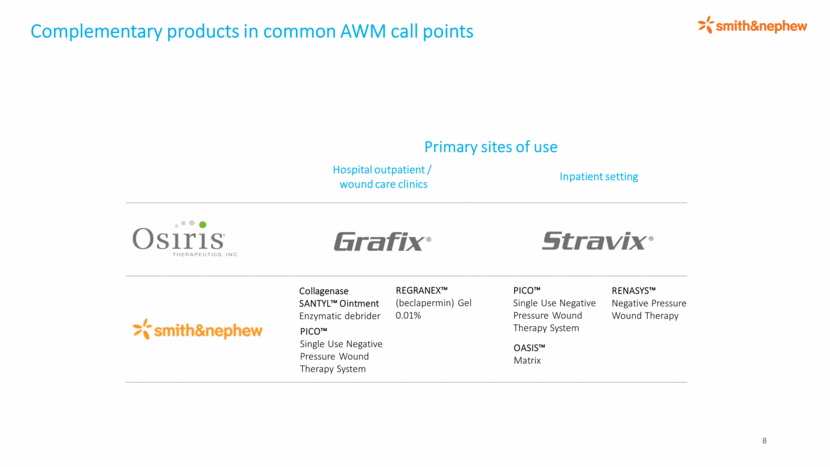
Complementary products in common AWM call points 8 Primary sites of use Hospital outpatient / wound care clinics Inpatient setting PICO™ Single Use Negative Pressure Wound Therapy System RENASYS™ Negative Pressure Wound Therapy REGRANEX™ (beclapermin) Gel 0.01% Collagenase SANTYL™ Ointment Enzymatic debrider OASIS™ Matrix PICO™ Single Use Negative Pressure Wound Therapy System
Xxxxxx Xxxxx Chief Financial Officer 9

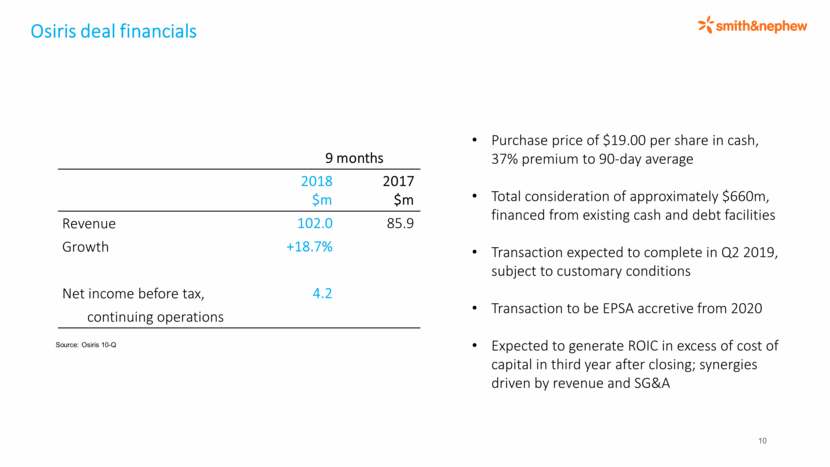
Osiris deal financials 10 9 months 2018 $m 2017 $m Revenue 102.0 85.9 Growth +18.7% Net income before tax, 4.2 continuing operations Source: Osiris 10-Q Purchase price of $19.00 per share in cash, 37% premium to 90-day average Total consideration of approximately $660m, financed from existing cash and debt facilities Transaction expected to complete in Q2 2019, subject to customary conditions Transaction to be EPSA accretive from 2020 Expected to generate ROIC in excess of cost of capital in third year after closing; synergies driven by revenue and SG&A
Questions 11


