DEVELOPMENT SERVICES AGREEMENT BETWEEN ACHAOGEN INC. AND ARK DIAGNOSTICS, INC. August 19, 2013
Exhibit 10.6
DEVELOPMENT SERVICES AGREEMENT
BETWEEN
ACHAOGEN INC.
AND
ARK DIAGNOSTICS, INC.
August 19, 2013
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
TABLE OF CONTENTS
| 1. | Definitions | 2 | ||||
| 2. | Services to be Provided | 7 | ||||
| 3. | Fees and Payments | 11 | ||||
| 4. | Joint Development Committee | 12 | ||||
| 5. | Proprietary Rights | 13 | ||||
| 6. | Patent Prosecution and Defense | 16 | ||||
| 7. | BARDA Requirements | 18 | ||||
| 8. | Commercialization | 19 | ||||
| 9. | Confidentiality | 23 | ||||
| 10. | Representations, Warranties and Covenants | 24 | ||||
| 11. | Testing of the Assays and Other Work Products | 26 | ||||
| 12. | Indemnities | 26 | ||||
| 13. | Limitation of Liability | 27 | ||||
| 14. | Term and Termination of this Agreement | 27 | ||||
| 15. | Notices | 28 | ||||
| 16. | Miscellaneous | 29 | ||||
| EXHIBITS | ||
| Exhibit A |
Statement of Work | |
| Exhibit B |
U.S. Government Rights | |
| Exhibit C |
Applicable Prime Contract Provisions | |
| Exhibit D |
Animal Use Provisions | |
| ATTACHMENT | ||
| Attachment A |
Background IP |
- i -
DEVELOPMENT SERVICES AGREEMENT
This Development Services Agreement (this “Agreement”), effective as of August 19, 2013 (the “Effective Date”), is made by and between Achaogen Inc. (“Achaogen”), a corporation with offices at ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇, ▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, and ARK Diagnostics, Inc. (“Ark”), a corporation with offices at ▇▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ (each a “Party,” and collectively the “Parties”).
WHEREAS, Achaogen is a biopharmaceutical company focused on the discovery and development of new antibacterials for the treatment of multi-drug resistant (“MDR”) gram-negative bacterial infections;
WHEREAS, Achaogen’s lead compound, ACHN-490 or plazomicin, is a novel aminoglycoside antibiotic, and Phase 1 and 2 studies of plazomicin support its progression to Phase 3 clinical development;
WHEREAS, plazomicin, like other aminoglycosides, is cleared through the kidneys and dose adjustment may be required in patients with moderate or severe renal impairment;
WHEREAS, to facilitate plazomicin dose monitoring and adjustment, Achaogen wishes to develop an Assay (as defined herein);
WHEREAS, Achaogen receives Federal funding from BARDA in support of Achaogen’s discovery and development of new antibacterial for the treatment of MDR gram-negative bacterial infections pursuant to the Prime Contract (as defined herein)
WHEREAS, Ark is a diagnostics company focused on, among other things, homogenous enzyme immunoassays for therapeutic drug monitoring; and
WHEREAS, Achaogen and Ark have previously entered into a Material Transfer Agreement (as defined herein) pursuant to which Achaogen provided selected materials to Ark and Ark began selected Assay preparatory work;
WHEREAS, Achaogen wishes to retain Ark (in part as a subcontractor under the Prime Contract) to further develop the Assay and to provide clinical supply of Assays for the Trial (as defined herein).
NOW, THEREFORE, in consideration of the mutual promises contained in this Agreement and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties, intending to be legally bound, hereby agree as follows.
1. Definitions
(a) “Achaogen” means Achaogen Inc., as identified in the opening paragraph of this Agreement.
(b) “Achaogen Background IP” means all Background IP owned or controlled by Achaogen, including, but not limited to, all such Background IP identified on Attachment A hereof, all Intellectual Property [***], and all Intellectual Property [***].
(c) “Achaogen Improvements” means all Foreground IP which constitutes any improvements or modifications to Achaogen Background IP.
(d) “Achaogen Materials” means all Specifications, and all other technology, processes, equipment, software, documentation and other materials provided to Ark by or on behalf of Achaogen under this Agreement, including any Antibodies provided to Ark by Achaogen.
(e) “Antibody” means any antibody [***] under this Agreement or the Material Transfer Agreement which [***], including but not limited to[***].
(f) “Applicable Laws” means all applicable federal, state, and local laws, statutes, ordinances, rules, and regulations, and any applicable orders, injunctions, or decrees of any court, administrative agency, or similar authority, and the rules and regulations of the FDA or any other regulatory authority in any jurisdiction in the world having jurisdiction over the development, manufacture or supply of the Assay, including without limitation the U.S. Federal Food, Drug, and Cosmetic Act, as amended from time to time (21 U.S.C. § 301 et seq.), all requirements related to the methods used in, and the facilities and controls for, developing, manufacturing, testing, packaging, labeling, storing, shipping and distributing drugs, as addressed in the FDA’s Quality System Regulation (21 CFR Part 820), and all requirements pertaining to the lawful marketing of the Assay.
(g) “Ark” means ARK Diagnostics, Inc., as identified in the opening paragraph of this Agreement.
(h) “Ark Background IP” means all Background IP owned or controlled by Ark, including but not limited to all such Background IP identified on Attachment A hereof and [***].
(i) “Ark Improvements” means all Foreground IP which constitutes any improvements or modifications to Ark Background IP, specifically excluding all Antibodies and the Assay.
(j) “Ark Materials” means all technology, processes, equipment, software, documentation and other materials provided to Achaogen by or on behalf of Ark under this Agreement.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 2 -
(k) “Assay” means a homogenous therapeutic drug monitoring immunoassay developed under this Agreement in accordance with the Specifications, including calibrators and controls, for the quantitative measurement of plazomicin in serum or plasma.
(l) “Assay Components” means proprietary components of the Assay sufficient to allow a Third Party manufacturer to package, label and finish (collectively, “Finish”) into the Assay using commercially available reagents and materials.
(m) “Background IP” means all Intellectual Property (i) developed or owned by a Party prior to the Effective Date, or (ii) developed or owned by a Party after the Effective Date but not developed (x) in the course of performing under this Agreement, or (y) directly or indirectly based on any work with or information received from the other Party.
(n) “BARDA” means the Biomedical Advanced Research and Development Authority within the Office of the Assistant Secretary for Preparedness and Response in the U.S. Department of Health and Human Services.
(o) “[***] Antibody” means that certain antibody which [***].
(p) “Claims” has the meaning set forth in Section 12(a) of this Agreement.
(q) “Commercialization Agreement” means the agreement governing the commercial sale and marketing of Assays by the Parties, and/or the supply of Assays or Assay Components by Ark, to be negotiated between the Parties in accordance with Section 8(a) of this Agreement.
(r) “Compound” means Achaogen’s lead compound, ACHN-490, also known as plazomicin.
(s) “Confidential Information” means any information or material disclosed by either Party to the other Party, directly or indirectly, in writing, orally, visually or by inspection of tangible objects, including, without limitation, any and all information relating to such Party’s or its business partners’ research, development, know-how, products, product plans, services, customers, customer lists, markets, software, developments, inventions, processes, formulas, technology, designs, drawings, marketing, finances, or other business information or trade secrets, that is designated as “confidential”, “proprietary” or the like, or that should reasonably be understood to be confidential or proprietary based on the nature of such information or the circumstances of its disclosure. For purposes of this Agreement, the terms of this Agreement shall be deemed to be Confidential Information of both Parties. All [***] shall be deemed to be Confidential Information of Achaogen. [***] shall be deemed Confidential Information of Ark.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 3 -
(t) “Effective Date” has the meaning set forth in the opening paragraph of this Agreement.
(u) “European Union” means all member states of the European Union as may be updated from time to time.
(v) “FDA” means the U.S. Food and Drug Administration.
(w) “Finish” or “Finishing” has the meaning set forth in Section 1(l) of this Agreement.
(x) “Foreground IP” means any Intellectual Property that is conceived, reduced to practice, discovered, developed or otherwise made by or on behalf of a Party after the Effective Date (i) [***], or (ii) [***].
(y) “IDE Approval” means approval of the Assay by the FDA of an Investigational Device Exemption.
(z) “Indemnitee” has the meaning set forth in Section 12(c) of this Agreement.
(aa) “Intellectual Property” means all new or improved apparatuses, designs, processes, formulae, information, products, inventions, discoveries, ideas, suggestions, materials, data, equipment, designs, drawings, prototypes, molds, masks, tooling, reports, computer software, documentation, proprietary information and other intellectual property and know-how.
(bb) “Intellectual Property Rights” means all patent, trade secret, copyright, know-how and other proprietary rights with respect to any present or future Intellectual Property that may be secured in any place under laws now or hereafter in effect.
(cc) “Interim Supply Date” has the meaning set forth in Section 8(c)(1) of this Agreement.
(dd) “Interim Supply Period” has the meaning set forth in Section 8(c)(1) of this Agreement.
(ee) “Joint Development Committee” has the meaning set forth in Article 4 of this Agreement.
(ff) “Joint Patents” has the meaning set forth in Section 6(c)(1) of this Agreement.
(gg) “Losses” has the meaning set forth in Section 12(a) of this Agreement.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 4 -
(hh) “Marketing Registrations” means any Registration Applications and other regulatory filings and approvals necessary for marketing the Assay.
(ii) “Material Transfer Agreement” means that certain Material Transfer Agreement by and between Ark and Achaogen as of October 24, 2012.
(jj) “MDR” has the meaning set forth in the first WHEREAS clause of this Agreement.
(kk) “NDA” means a new drug application filed with the FDA as more fully defined in U.S. 21 C.F.R. §314.50 et. seq. or any similar application or submission filed with or submitted to any Regulatory Authority outside of the U.S. to obtain permission to commence marketing and sales of the Compound.
(ll) “Nondisclosure Agreement” means that certain Mutual Nondisclosure Agreement by and between Ark and Achaogen as of July 24, 2012.
(mm) “Party” or “Parties” has the meaning set forth in the opening paragraph of this Agreement.
(nn) “Patents” means any U.S. provisional or nonprovisional patent application, including any continuations, divisionals, and continuations-in-part, and any substitute patents, any reissues or re-examinations of any such applications, as well as any foreign equivalent of any of the foregoing or Patent Cooperation Treaty (“PCT”) filings, and any patents issuing from any of the foregoing, and any renewal or extension of the term of any such patent.
(oo) “Patent Expenses” means all [***]costs for patent filing, prosecution, and maintenance of [***] that are incurred pursuant to this Agreement. [***].
(pp) “Prime Contract” means Contract No. HHSO 100201000046C between Achaogen and BARDA (as modified).
(qq) “Registration Application” means any filing(s) made with the Regulatory Authority in any country in the Territory for regulatory approval or clearance (including pricing approval when applicable) necessary to commence marketing and sale of the Assay in such country.
(rr) “Regulatory Authority” means the FDA or the authority(ies) in each country in the world that are comparable to the FDA and have responsibility for granting regulatory approval or clearance for the manufacture, use and sale of medical products, including the Assay in such country, including but not limited to pricing and reimbursement approvals.
(ss) “Resolution Officers” has the meaning set forth in Section 4(c) of this Agreement.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 5 -
(tt) “ROW Marketing Registrations” has the meaning set forth in Section 2(c)(2)(iv) of this Agreement.
(uu) “Services” has the meaning set forth in Section 2(a)(1) of this Agreement.
(vv) “Selected Antibody” means any [***].
(ww) “Side Letter” has the meaning set forth in Section 8(d)(1) of this Agreement.
(xx) “Specifications” has the meaning set forth in Section 2(a)(1) of this Agreement.
(yy) “Statement of Work” or “SOW” has the meaning set forth in Section 2(a)(1) of this Agreement.
(zz) “Technology Transfer and License Agreement” has the meaning set forth in Section 8(d)(1) of this Agreement.
(aaa) “Term” has the meaning set forth in Section 14(a) of this Agreement.
(bbb) “Third Party” means any individual, corporation, partnership, company or similar entity who or which is neither a Party nor an Affiliate of a Party.
(ccc) “Third Party Material” has the meaning set forth in Section 5(g) of this Agreement.
(ddd) “Third Party Supplier” has the meaning set forth in Section 8(d)(1) of this Agreement.
(eee) “Trial” means Achaogen’s global Phase 3 clinical trial program for the Compound, including a randomized Phase 3 study and an open-label safety study.
(fff) “Trial Registrations” means any Registration Applications and other regulatory filings and approvals necessary for clinical use of the Assay in the Trial.
(ggg) “United States” means the United States of America and its possessions and territories.
(hhh) “US/EU Marketing Registrations” has the meaning set forth in 2(c)(2)(i)
(iii) “Verification” means a determination that [***].
(jjj) “Work Products” means all documentation, works of authorship, and data generated as part of the Agreement, including clinical data, and all other work
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 6 -
products created or made by or on behalf of Ark pursuant to this Agreement as provided in the applicable SOW, but not including [***].
2. Services to be Provided
(a) General
(1) Ark shall provide services to support the development and regulatory approval of the Assay, based on designs and specifications developed in collaboration by the Parties (collectively, the “Specifications”), pursuant to this Agreement and each Statement of Work attached hereto as Exhibit A as agreed from time to time by the Parties (each, a “Statement of Work” or “SOW”). The term “Services” refers to all work performed by Ark pursuant to this Agreement, each Statement of Work and the Material Transfer Agreement, including, without limitation, the design, development and delivery of all Work Products and the Assay.
(2) In the event of a conflict between the terms and conditions of this Agreement and the terms and conditions of any Statement of Work or any other documentation exchanged between the Parties, the terms and conditions of this Agreement shall prevail.
(3) Notwithstanding any provision of this Agreement to the contrary, it is understood that (i) neither Party guarantees that the development of the Assay will be successful or the Assay will be approved by any Regulatory Authority for use in the Trial or for commercial sale in any territory, and (ii) there are uncertainties and potentially technical challenges beyond the control of the Parties in the development of the Assay that could adversely affect the successful and/or timely completion of the Assay according to the Specifications, which could require good faith discussions between the Parties to revise the Specification, Statement of Work, and/or terms of this Agreement.
(b) Development and Clinical Supply Services
(1) Ark shall develop the Assay according to the Specifications. Ark shall be responsible for [***], including [***]. Ark shall perform analytical Verification studies of the Assay to support IDE Approval, and shall timely provide all necessary information and materials and provide other cooperation reasonably required for the IDE preparation, submission, and Approval, including without limitation, those responsibilities allocated to Ark as set forth in the SOW. Achaogen shall timely provide all necessary information and materials and provide other cooperation reasonably required for the development and analytical Verification studies of the Assay.
(2)Ark shall manufacture and supply to Achaogen the Assay in sufficient quantities but in no event exceed [***] for use by Achaogen in the Trial in accordance with the Statement of Work, and according to a schedule agreed upon with
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 7 -
Achaogen based on projected shelf life of kits and length of Trial. In the event that more than [***] are required for the Trial, Ark and Achaogen will [***].
(3) The rights and obligations of the Parties with respect to any Antibodies owned or controlled by Achaogen and provided by Achaogen to Ark in connection with Assay development, validation, manufacture or supply shall be governed by the terms and conditions of the Material Transfer Agreement, except as expressly otherwise provided herein.
(4) ARK shall provide additional services to support Achaogen’s conduct of the Trial as defined in the SOW, including but not limited to training personnel at a Contract Research Organization designated by Achaogen on how to train clinical laboratory site(s) in performance of the Assay, and providing ongoing support to clinical laboratory sites in operation of the assay, including troubleshooting for out-of-specification assay results. ARK will also perform specimen testing and primary analysis for the method comparison between the ARK immunoassay and the reference method in support of market registrations. ARK will provide the aforementioned support at ARK’s facility in Fremont, CA or by teleconference and/or electronically.
(c) Regulatory Services. The Parties shall consult and collaborate on the most effective and expeditious plan for obtaining regulatory clearance for investigational use and commercial marketing of the Assay.
(1) Trial Registrations
(i) Ark and Achaogen shall be responsible for formulating regulatory strategy, preparing, filing and obtaining IDE Approval, with Achaogen being the lead party and formal holder of the IDE.
(ii) ARK is responsible for preparing and providing all technical and manufacturing information required for IDE approval
(iii) Achaogen is responsible for preparing and providing all information related to the investigational plan for IDE approval
(iv) Achaogen is responsible for compiling and submitting the IDE applications, and scheduling and managing meetings and teleconferences with the FDA.
(v) Ark shall be included in all meetings with the FDA and in all scheduled teleconferences with the FDA and, to the extent practicable, unscheduled teleconferences with the FDA.
(vi) Except as provided in Section 2(c)(1)(i), and unless otherwise expressly agreed by the Parties under any SOW, Achaogen shall be responsible for formulating regulatory strategy, preparing, filing and obtaining all Trial Registrations necessary for use of the Assay in the Trial throughout the world with Ark’s support and
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 8 -
involvement as reasonably required or as otherwise agreed by the Parties from time to time.
(vii) Achaogen shall own all Trial Registrations (including IDE Approval). Any expenses [***].
(2) Marketing Registrations
(i) In the event Ark will be commercializing the Assay in the US and EU, Ark shall be responsible for formulating regulatory strategy, preparing, filing and obtaining all Marketing Registrations necessary for marketing the Assay in the United States and European Union, with Achaogen’s active support and involvement as reasonably required or as otherwise agreed by the Parties from time to time. Ark shall own all such Marketing Registrations for the Assay in the United States and European Union (collectively, the “US/EU Marketing Registrations”), and shall be responsible for and conduct all interactions with such United States and European Union Regulatory Authorities involving such US/EU Marketing Registrations. Ark shall [***]. Achaogen shall be included in all meetings with the US and EU regulatory authorities and in all planned teleconferences. To the extent possible, Achaogen will be included in impromptu teleconferences with US and EU regulatory authorities.
(ii) In the event Achaogen will be commercializing the Assay in the US and EU, and Ark remains the manufacturer/supplier of the Assay, Achaogen shall be responsible for formulating regulatory strategy, preparing, filing and obtaining all Marketing Registrations necessary for marketing the Assay in the United States and European Union, with Ark’s support and involvement as reasonably required or as otherwise agreed by the Parties from time to time. Achaogen shall own all such Marketing Registrations for the Assay in the United States and European Union (collectively, the “US/EU Marketing Registrations”), and shall be responsible for and conduct all interactions with such United States and European Union Regulatory Authorities involving such US/EU Marketing Registrations. Achaogen shall [***]. Ark shall be included in all meetings with the US and EU regulatory authorities and in all planned teleconferences. To the extent possible, Ark will be included in impromptu teleconferences with US and EU regulatory authorities.
(iii)In the event Achaogen will be commercializing the Assay in the US and EU, and Ark will remain the manufacturer/supplier of the Assay, Ark shall be responsible for ensuring that all applicable manufacturing regulations are met, including but not limited to the manufacture of the Assay under FDA current Good Manufacturing Practices and maintenance of Ark’s certification under ISO 13485. Ark shall support Achaogen’s ongoing maintenance of the Market Registrations (e.g., the filing of annual reports or audits by regulatory agencies), including but not limited to the provision of materials and information, as reasonably required or as otherwise agreed by the Parties from time to time. In this case, Ark will work with Achaogen to ensure the Assay manufacturing conforms to Achaogen’s Quality System for ensuring compliance with Quality System Regulations.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 9 -
(iv) Achaogen shall be responsible for formulating regulatory strategy, preparing, filing and obtaining all Marketing Registrations necessary for marketing the Assay in all jurisdictions and territories in the world other than the United States and the European Union, with Ark’s support and involvement as reasonably required or as otherwise agreed by the Parties from time to time. For clarity, Ark shall not be responsible for conducting any additional studies for obtaining any ROW Marketing Registrations, provided that, [***], Ark will provide reasonable cooperation to Achaogen and its designees in performing any such additional studies required for obtaining ROW Marketing Registrations, as well as supporting Achaogen’s ongoing maintenance of the ROW Market Registrations (e.g., the filing of periodic reports or audits by regulatory agencies). Achaogen shall own all such Marketing Registrations for the Assay (collectively, the “ROW Marketing Registrations”), and shall be responsible for and conduct all interactions with Regulatory Authorities involving such ROW Marketing Registrations. Achaogen shall [***].
(3) License to Registrations. Ark hereby grants to Achaogen a nonexclusive, sublicensable and transferable license under any US/EU Marketing Registrations and data required for any ROW Marketing Registrations for the purpose of (i) applying for and obtaining ROW Marketing Registrations and (ii) Finishing and commercializing the Assay in all jurisdictions and territories in the world (excluding United States and the European Union in the event Ark has exercised its right to commercialize the Assay in the United States and European Union pursuant to the Commercialization Agreement). Achaogen hereby grants to Ark a nonexclusive, sublicensable and transferable license under any ROW Marketing Registrations and data required for any US/EU Marketing Registrations for the purpose of (i) applying for and obtaining US/EU Marketing Registrations and (ii) Finishing and commercializing the Assay in United States and European Union in the event Ark has exercised its right to commercialize the Assay in the United States and European Union pursuant to the Commercialization Agreement.
(d) Delivery of Work Products and Assays. Ark shall prepare the Work Products and the Assays identified in each Statement of Work, and shall provide to Achaogen the Work Products and the Assays in accordance with the schedule for performance and delivery set forth in the applicable Statement of Work. During the course of performing the Services, Ark shall consult with Achaogen as requested by Achaogen, and shall address feedback received from Achaogen relating to each project.
(e) Conformance with Specifications. If Achaogen reasonably determines that the Assay or any Work Products do not materially conform to the applicable Specifications or other requirements set forth in a Statement of Work, Ark promptly shall, subject to Section 2(a)(3), correct the nonconformance to Achaogen’s reasonable satisfaction, at no cost to Achaogen. In the event Achaogen requests any change to the Specification or other requirement for the Assay, Ark agrees to use commercially reasonable efforts to accept such request to the extent reasonable and practicable, provided that in no event shall Ark be obligated to incur any additional costs and expenses required for implementing any such change.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 10 -
3. Fees and Payments. The following payments shall be made for the Services:
(a) Prepaid Fee. Ark acknowledges that on [***], Achaogen paid to Ark a fee of [***] Dollars ($[***]) in [***], which amount is nonrefundable and noncreditable.
(b) Development and Regulatory Milestones
(1) Achaogen shall pay to Ark nonrefundable, noncreditable milestone payments in consideration for the achievement of the following one-time development and regulatory milestone events for the Assay:
| No. |
Event |
Payment | ||
| [***] |
| * | For clarity, the determination as to whether milestones [***] shall be paid [***] as mutually agreed by the Parties. Also, for clarity, [***]. |
(2) Achaogen shall pay all milestone payments due to Ark under Section 3(b)(1) within [***] days of receiving notice of the occurrence of such milestone, except that the Parties acknowledge and agree that [***] has been achieved prior to the execution of this Agreement and the corresponding milestone payment in the amount of $[***] shall be payable upon execution of this Agreement.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 11 -
(c) Complete Compensation; Currency. Without limiting Achaogen’s payment obligation under Section 14(d), the payments required by Sections 3(a) and 3(b) shall constitute [***]. All amounts payable under this Agreement shall be invoiced to and paid by Achaogen in U.S. dollars.
(d) Books and Records; Audit. Ark shall maintain complete and accurate records relating to its performance under this Agreement. Ark shall retain such records for the longer of [***] years after the termination or expiration of this Agreement or the period of time required by Applicable Laws and shall make such records available to Achaogen or its representative upon request during the record-keeping period set forth herein. Ark shall cooperate in any audit of such records that Achaogen or its representative may undertake, at no additional cost to Achaogen; provided, however, that Achaogen shall be responsible for any expenses directly incurred by Achaogen or its representative in connection with the audit.
4. Joint Development Committee. Within thirty (30) days from the Effective Date, the Parties shall establish a joint development committee (the “Joint Development Committee”) to coordinate and oversee the development of the Assay in the Territory.
(a) Composition of the Joint Development Committee. The Joint Development Committee is, and at all times during the Term shall be, comprised of [***] representatives from each Party, each of whom has relevant experience and skill appropriate for service on the Joint Development Committee, such as heads of clinical development and manufacturing. The Parties may establish and later change the number of representatives that each Party has on the Joint Development Committee, as long as [***] voting representatives from [***] is maintained (unless a Party desires to have fewer representatives). Each Party may change any of its representatives on the Joint Development Committee at any time upon notice to the other Party.
(b) Activities of the Joint Development Committee. The Joint Development Committee shall be responsible for establishing and approving plans and activates relating to development of the Assay, evaluating Ark’s progress under such plans, discussing any changes to such plans, and determining the successful completion of milestone events associated with the development of the Assay. The Joint Development Committee may also provide a forum for discussion of [***]. For clarity, the Joint Development Committee shall not have the authority to amend any terms or conditions of this Agreement or any SOW.
(c) Decisions of the Joint Development Committee. It is understood that Achaogen shall have final authority to approve the Specifications or any modifications thereto, in each case which are initially proposed by Ark. For any other matter within the jurisdiction of the Joint Development Committee as to which the Joint Development Committee cannot reach a decision, such matter under dispute shall first be referred to the Vice-President of Corporate Development of Achaogen and the president or chief executive officer of Ark (together, the “Resolution Officers”), for attempted resolution by good faith negotiations within [***] of notice thereof. Except as otherwise
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 12 -
provided in this Agreement, any dispute, other than one for which Achaogen has final decision-making authority, that cannot be resolved by the Resolution Officers may be resolved pursuant to Section 16(c).
(d) Meetings of the Joint Development Committee. The Joint Development Committee shall hold its first meeting within [***] after the Effective Date and shall meet thereafter on a schedule and at locations mutually determined by the Parties. Ad hoc meetings of the Joint Development Committee may be called by either Party upon reasonable advance notice to the other. Subject to the Parties’ mutual agreement, regular and ad hoc meetings may be face-to-face or by teleconference or videoconference.
(e) Joint Development Committee Expenses. Each Party shall bear the expense of the participation of its representatives on the Joint Development Committee and in Joint Development Committee meetings.
5. Proprietary Rights
(a) Background IP
(1) All right, title and interest, including all Intellectual Property Rights, in and to all Achaogen Background IP and all Achaogen Materials shall be and remain the exclusive property of Achaogen.
(2) All right, title and interest, including all Intellectual Property Rights, in and to all Ark Background IP and all Ark Materials shall be and remain the exclusive property of Ark.
(b) Improvements, Work Products, Antibodies and the Assay
(1) Achaogen shall have sole and exclusive ownership of all right, title and interest in all Achaogen Improvements, and any and all Intellectual Property Rights with respect thereto, regardless of whether such Achaogen Improvements were conceived, reduced to practice, discovered, developed or otherwise generated by Ark, by Achaogen, or jointly by the Parties.
(2) As between the Parties, Achaogen shall have sole and exclusive ownership of all right, title and interest in all Antibodies developed or acquired by Ark in the course of providing Services, and any and all Intellectual Property Rights with respect thereto, regardless of whether such Antibodies were conceived, reduced to practice, discovered, developed or otherwise generated by or on behalf of Ark, by or on behalf of Achaogen, or jointly by or on behalf of the Parties.
(3) Ark shall have sole and exclusive ownership of all right, title and interest in all Ark Improvements, and any and all Intellectual Property Rights with respect thereto, regardless of whether such Ark Improvements were conceived,
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 13 -
reduced to practice, discovered, developed or otherwise generated by Ark, by Achaogen, or jointly by the Parties.
(4) The Parties shall jointly own all right, title and interest in all [***], and any and all Intellectual Property Rights with respect thereto, regardless of whether such [***] was conceived, reduced to practice, discovered, developed or otherwise generated by Ark, by Achaogen, or jointly by the Parties. Ark shall disclose to Achaogen any subject inventions, as further described in Exhibits B and C, within [***] days of their conception or reduction to practice, so as to permit Achaogen to comply with its obligations under Article 7.
(5) Ark shall disclose all Achaogen Improvements and all Ark improvements to Achaogen, to the extent they are conceived, reduced to practice, discovered, identified or otherwise created in whole or in part by Ark, promptly and, in any event, within [***] of such conception or reduction to practice, so as to permit Achaogen to comply with its obligations under Article 7.
(c) Other Foreground IP
(1) All Foreground IP that is not otherwise owned as specifically provided in Section 5(b), and that is conceived, reduced to practice, discovered, developed or otherwise generated solely by employees or consultants of a Party, shall be owned by such Party.
(2) All Foreground IP that is not otherwise owned as specifically provided in Section 5(b), and that is conceived, reduced to practice, discovered, developed or otherwise generated by employees or consultants of both Parties, shall be owned jointly by the Parties.
(d) Assignment
(1) To the extent, if any, that [***] or any of its employees or others providing Services has rights in any [***], then, notwithstanding Section 5(b)(1) and Section 5(b)(2), [***] hereby irrevocably assigns to [***]) all right, title and interest in and to such [***], as applicable, including without limitation all Intellectual Property Rights therein. Upon the request of [***], [***] shall sign and deliver, and shall cause its personnel to sign and deliver, any assignments or other necessary documents and otherwise assist [***] to obtain, maintain, perfect or enforce any of [***] rights hereunder.
(2) To the extent, if any, that [***]or any of its employees has rights in any Ark Improvements, then, notwithstanding Section 5(b)(3), [***] hereby irrevocably assigns to [***] all right, title and interest in and to such [***], including without limitation all Intellectual Property Rights therein. Upon the request of [***], [***] shall sign and deliver, and shall cause its personnel to sign and deliver, any assignments or other necessary documents and otherwise assist Ark to obtain, maintain, perfect or enforce any of [***] rights hereunder.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 14 -
(e) Licenses
(1) Achaogen hereby grants to Ark a nonexclusive, worldwide, royalty-free, nonsublicensable and nontransferable (except in connection with the assignment permitted under Section 16(g) license to use the Achaogen Materials to the limited extent necessary to perform the Services in accordance with the terms and conditions of this Agreement. Ark shall not have the right to use the Achaogen Materials for any other purpose, and shall return to Achaogen all Achaogen Materials following the earlier of completion of the Services or expiration or termination of this Agreement, or earlier at Achaogen’s request.
(2) Achaogen hereby grants to Ark a nonexclusive, worldwide, royalty-free, nonsublicensable and nontransferable (except in connection with the assignment permitted under Section 16(g)) license under the Achaogen Background IP, the Achaogen Improvements, Antibodies, and any other Foreground IP owned or controlled by Achaogen to the limited extent necessary to perform the Services in accordance with the terms and conditions of this Agreement.
(3) Ark hereby grants to Achaogen a nonexclusive, worldwide, royalty-free, irrevocable, perpetual, fully paid up, sublicensable and nontransferable (except in connection with the assignment permitted under Section 16(g)) license under the Ark Background IP, the Ark Materials and the Ark Improvements solely to the extent necessary to use the Assays and any Work Products for Achaogen’s (i) development of the Compound, and (ii) use of the Assay in the Trial.
(4) Ark hereby grants to Achaogen a nonexclusive, worldwide, royalty-free, irrevocable, perpetual, fully paid up, sublicensable and nontransferable (except in connection with the assignment permitted under Section 16(g)) license under any Foreground IP owned or controlled by Ark and which is not an Ark Improvement, solely to the extent necessary to use the Assays and any Work Products for Achaogen’s (i) development of the Compound, (ii) use of the Assay in the Trial, and (iii) development, manufacture, use or sale of any other assays which may be used in connection with the Compound.
(5) Any rights granted to Achaogen under this Agreement that are sublicensable pursuant to this Agreement, including any rights granted to Achaogen under this Article 5 and Article 6 shall also inure to the benefit of, and be enforceable by, any sublicensee of any rights granted to Achaogen under this Agreement, provided that such sublicensee agrees in writing to be bound by the applicable terms and conditions set forth herein.
(f) Reservation of Rights. Neither Party shall acquire, directly, indirectly or by estoppel or implication, any rights in or under any Intellectual Property or Intellectual Property Rights of the other Party except as expressly provided herein or in any other written agreement between the Parties.
- 15 -
(g) Original Work. All Work Products shall be the original work of Ark and its employees or others providing Services, except for any portion of any Work Products that consist of material created by a Third Party and with respect to which Ark has advised Achaogen in writing of such material’s Third Party origin (“Third Party Material”). Ark shall not disclose to Achaogen or knowingly induce Achaogen to use any confidential, proprietary or trade secret information of any Third Party unless it has a sublicensable or assignable right to such Third Party information. Ark shall not knowingly incorporate any Third Party Material into any Work Product without obtaining Achaogen’s prior written approval. To the extent that Third Party Material is included or incorporated into any Work Products, Ark hereby grants to Achaogen a nonexclusive, worldwide, royalty-free, irrevocable, perpetual, fully paid up, sublicensable and nontransferable (except in connection with the assignment permitted under Section 16(g)) license under Ark’s rights in the Intellectual Property Rights in such Third Party Materials (i) to use the Assay and any Work Products for Achaogen’s development of the Compound and use of the Assay in the Trial; and (ii) to use, make, have made, offer for sale, sell and import the Assays and the Work Products in accordance with the terms and conditions of the Commercialization Agreement, if and when entered into.
(h) Covenant Not to ▇▇▇. Ark covenants that it shall not enforce its Intellectual Property Right against Achaogen or any of its designees for any proper exercise of any license of Intellectual Property Rights granted by Ark to Achaogen pursuant to this Agreement.
6. Patent Prosecution and Defense
(a) Achaogen IP. As between the Parties, Achaogen shall have the sole right, at its expense, for managing the filing, prosecution and maintenance of all Patents directed to [***] in accordance with Section 5(c)(1) of this Agreement.
(b) Ark IP. As between the Parties, Ark shall have the sole right, at its expense, for managing the filing, prosecution and maintenance of all Patents directed to [***] in accordance with Section 5(c)(1) of this Agreement.
(c) Jointly-Owned IP
(1) Achaogen shall be responsible for managing the filing, prosecution and maintenance of all Patents directed to any [***] in accordance with Section 5(c)(2) of this Agreement (collectively, the “Joint Patents”). Achaogen shall consult with Ark on a timely basis on all significant matters relating to the Joint Patents (including providing a copy of any patent application and any material correspondence with the applicable patent office reasonably in advance of its filing or submission) and consider in good faith Ark’s reasonable comments or suggestions with respect thereto. Achaogen shall not [***] without prior written notice to Ark, which notice shall be given sufficiently in advance of any statutory bar or other deadline that would cause such Joint Patents to be abandoned or otherwise lapse.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 16 -
(2) Achaogen shall, or shall instruct patent counsel to, promptly provide copies to Ark of all Joint Patents, as well as patents, patent documents and correspondence pertaining to the Joint Patents. Ark shall be allowed to review and provide comment thereon. Achaogen will consider any comments provided by Ark in good faith.
(3) Subject to Section 6(c)(4) below, Achaogen shall be responsible for [***] percent ([***]%) of the Patent Expenses, and Ark shall be responsible for [***] percent ([***]%) of the Patent Expenses. In the event Achaogen has paid a Patent Expense without receiving a prepayment from Ark of its proportionate share of such Patent Expense, Achaogen shall provide written notification to Ark of the nature, amount and date of payment of such Patent Expense, together with the receipt or other appropriate documentation, and subject to Section 6(c)(4) below, Ark shall reimburse Achaogen its share of the Patent Expenses within thirty (30) days of receipt of such notification.
(4) [***], shall determine the countries or jurisdictions where Joint Patents will be filed, prosecuted, and maintained. If, during the term of this Agreement, either Party [***] with respect to any Joint Patent in any country or jurisdiction, the other Party may pay all such expenses but thereafter, the paying Party shall have sole authority over licensing and patent prosecution of the Joint Patents in any such country or jurisdiction and the non-paying Party shall assign to the paying Party all of its right, title and interest in and to such Joint Patents in such country or jurisdiction.
(d) Patent Infringement and Defense
(1) In the event either Party learns of the actual or threatened infringement of a Joint Patent by a Third Party, the Party that learned of the infringement shall promptly notify the other Party in writing and will provide the other Party with all available evidence of such infringement. Achaogen, in cooperation with Ark, will have the first right, but not the obligation, to use commercially reasonable efforts to eliminate the infringement without litigation. If Achaogen does not within a reasonable period of time notify the infringer of the infringement, then Ark, after providing notice to Achaogen of its intent to do so, may use commercially reasonable efforts to eliminate the infringement without litigation.
(2) If the infringement has not ceased within [***] of the infringer being notified of the infringement, Achaogen and Ark shall confer regarding possible courses of action.
(3) Neither Party has an obligation under this Agreement to bring an infringement action; provided, however, that as between Achaogen and Ark, Achaogen shall have the first right to bring such an action. If Achaogen has not brought such an action within [***] after conferring with Ark as provided in Section 6(d)(2), then Ark shall have the right to bring such an action after providing notice to Achaogen of its intent to do so. Unless otherwise mutually agreed by the Parties in writing, (i) the Party
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 17 -
controlling the enforcement action shall [***], and (ii) any recovery resulting from a settlement or judgment on an infringement action will be first used to reimburse the controlling Party of the costs of litigation and the remainder of the recovery, if any, shall be divided between the controlling Party and non-controlling party on a [***] basis.
(4) In the event that a Third Party commences an action challenging any of the Joint Patents, Achaogen shall have the first right to defend such Joint Patents against such challenge. If Achaogen does not commence the defense against such challenge within [***] of Ark’s request to do so, then Ark shall have the right to defend such challenge after providing notice to Achaogen of its intent to do so; provided, however, that if Ark reasonably believes it must [***]. The controlling Party shall bear its own costs and expenses associated with any defense undertaken pursuant to this Section 6(d)(4).
(5) At the controlling Party’s request and expense, the non-controlling Party shall cooperate fully with the controlling Party in any action brought to enforce the Joint Patents against an infringer and in any defense of the Joint Patents against a challenge by a Third Party.
7. BARDA Requirements
(a) U.S. Government Rights. The rights and obligations of the Parties in Articles 5 and 6 of this Agreement are subject to the terms and conditions of Exhibit B, which are included herewith in compliance with Achaogen’s obligations to BARDA under the Prime Contract. In accordance with the terms of the Prime Contract, the language in Exhibit B has been reproduced verbatim from the Prime Contract except as otherwise instructed therein, including all defined terms and related conventions.
(b) Applicable Prime Contract Provisions. The rights and obligations of the Parties in this Agreement are subject to the Prime Contract terms and conditions listed in Exhibit C, which are included herewith in compliance with Achaogen’s obligations to BARDA under the Prime Contract.
(c) Animal Use Provisions. The rights and obligations of Ark in this Agreement are subject to the animal use provisions listed in Exhibit D, which are included herewith in compliance with Achaogen’s obligations to BARDA under the Prime Contract.
(d) Correspondence. Ark shall provide Achaogen and BARDA with electronic copies of all written materials submitted to any Regulatory Authority upon submission, and of all written correspondence sent to or received from any Regulatory Authority upon delivery. To the extent reasonably practicable, Ark shall share with Achaogen drafts of any written materials in advance of submitting such materials to a Regulatory Authority so as to allow for comment by Achaogen and/or BARDA.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 18 -
(e) Meetings. Ark may, from time to time, schedule meetings between Ark and a Regulatory Authority to discuss issues relevant to Ark’s performance of the Services hereunder. Ark shall notify Achaogen of the date and time of any such scheduled meeting as soon as possible, targeting within [***] within scheduling meeting. In addition, with respect to FDA meetings, Ark shall coordinate with FDA to enable at least [***] personnel total from Achaogen and BARDA to attend as observers.
8. Commercialization
(a) Terms. On or before October 31, 2013, the Parties shall commence good faith negotiations of a Commercialization Agreement consistent and in coordination with Achaogen’s plans for global commercialization of the Compound. The Commercialization Agreement shall include the following:
(1) Ark shall have the first right, but not the obligation, to commercialize (itself or through one or more designees) the Assay in the United States and European Union. Achaogen shall have the first right, but not the obligation, to commercialize (itself or through one or more designees) the Assay in any countries and territories other than the United States and European Union. If, for any reason, Ark elects not to exercise its right to commercialize the Assay in the United States and/or the European Union, Achaogen shall have the right to commercialize or appoint a distributor to commercialize the Assay in those territories;
(2) Subject to Section 8(a)(10), Ark shall have [***] right to manufacture and supply the Assay and Assay Components for commercialization. It is understood that with respect to any territory in which Ark does not commercialize the Assay (itself or through one or more designees), Ark will manufacture (or have manufactured) and supply either Assays or Assay Components which may be Finished by Achaogen or its designees (the provision of Assays or Assay Components to be determined in Ark’s reasonable discretion, unless otherwise outlined herein) such that Achaogen or its designees in the applicable territory may commercialize the Finished Assay in the applicable territory;
(3) A license under [***] solely to the extent necessary for [***] to commercially market and sell directly the Assay, or to sublicense the marketing and sale of the Assay, to a commercial partner, wherever the Compound is approved for such sale and marketing;
(4) A license under [***], solely to the extent necessary for Achaogen to develop, manufacture, use and sell any other assays which may be used in connection with the Compound;
(5) A provision that each Party and its sublicensees shall retain [***];
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 19 -
(6) Terms addressing Ark’s [***] Assays by reference to agreed-upon conditions, including the [***];
(7) Terms for the supply of Assays for sale by Achaogen or a co-promotion partner of Achaogen on [***], taking into consideration all relevant factors including without limitation, market size and volume of orders;
(8) Appropriate protections for continuity of supply, including [***];
(9) [***];
(10) In the event that (x) for specified reasons, including, but not limited to, [***], or (y) [***], Achaogen would have the limited right to elect to make or have made the Assays for sale in any jurisdiction in the world, and Ark would have the obligation to provide to Achaogen or its designated Third Party manufacturer [***];
(11) To mitigate the risk of supply interruption of Assays for any reason, commencing with commercialization activities involving the Assays, Ark would maintain and store, at its expense, a stockpile of Assays that the Parties agree is reasonably sufficient to supply the global market for a period equal to a conservative, good faith estimate of the time necessary to establish, qualify and start up a new, Third Party Assay manufacturing capacity. The extent of this stockpile would be reassessed by the Parties every [***], based on a [***]; and
(12) Necessary and appropriate terms for development and regulatory activities in jurisdictions where Regulatory Approvals for the Assay have not been received.
(b) Process for Entering Into Commercialization Agreement. If, by April 1, 2014, the Parties have been unable to agree on the terms and conditions of a Commercialization Agreement pursuant to Section 8(a), either Party may send the other Party a written notice that such Party wishes to appoint an expert panel made up of [***] to establish any unresolved terms for a Commercialization Agreement consistent with Section 8(a).
(1) In furtherance of this Subsection 8(b), each Party shall, within [***] of receipt of the notice seeking to establish such a panel, appoint [***] member of the panel who is not affiliated with either Party, and the [***] appointed members] shall, within [***] days of such notice, appoint the [***] member of the panel, who also shall [***]. Each appointed member shall also have demonstrated [***].
(2)Each appointed member shall enter into a written agreement providing for such member’s compensation, and binding such member to confidentiality and non-use provisions no less stringent than those contained in this Agreement. Each panel member shall be compensated at a rate to be agreed to by the Parties, and the fees and disbursements of the panel shall be [***].
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 20 -
(3) The Parties may submit such materials and arguments to the panel at a hearing to be held within [***] days of the appointment of the [***] of the panel, and the panel shall render its judgment [***] days of such hearing. Such terms shall be [***] and shall be [***].
(4) The [***] decision of the panel shall be binding upon the Parties, and the Parties shall [***].
(c) Interim Supply Period
(1) If, by [***], 2016 (the “Interim Supply Date”), the Parties have been unable to agree on the terms and conditions of a Commercialization Agreement pursuant to Section 8(a), Ark shall supply the Assay or Assay Components to Achaogen and its designees at a price that [***], for a period of five (5) years after the date Achaogen receives approval of the NDA for the Compound (the “Interim Supply Period”). Ark to supply Assays instead of Assay Components in the US and EU for at least the first two (2) years of the five (5) year supply period.
(2) The Parties may extend the Interim Supply Period by mutual agreement in writing.
(3) As of the Interim Supply Date, if no Commercialization Agreement has been entered into, Ark hereby grants to Achaogen and its designees a nonexclusive, nontransferable (except in connection with the assignment permitted under Section 16(g)), royalty-free, fully paid up license under the Ark Background IP, Ark Improvements and any Foreground IP owned or controlled by Ark solely to the extent necessary for Achaogen or such designee to Finish and commercialize the Assay during the Interim Supply Period.
(4) As of the Interim Supply Date, if no Commercialization Agreement has been entered into, Achaogen hereby grants to Ark and its designees a nonexclusive, nontransferable (except in connection with the assignment permitted under Section 16(g)), royalty-free, fully paid up license under Achaogen Background IP, Achaogen Improvements, Antibodies, and any other Foreground IP owned or controlled by Achaogen solely to the extent necessary for Ark or its designee to manufacture and supply the Assay to Achaogen and its designees during the Interim Supply Period.
(5) It is understood that prior to or after the Interim Supply Date the Parties may negotiate additional terms and conditions for the supply of Assays and/or Assay Components during the Interim Supply Period, provided, however, that such additional terms and conditions shall be customary, commercially reasonable and otherwise consistent with the applicable terms and conditions of this Agreement, and further provided that Ark’s obligations to supply Assays and/or Assay Components in accordance with this Section 8(c)(5) shall take effect as of the Interim Supply Date regardless of whether the Parties agree to any such additional terms and conditions. Unless and until such other terms are negotiated, the applicable terms and conditions of
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 21 -
this Agreement shall continue to apply, to the extent practicable, including but not limited to the provisions of Articles 7, 9, 10, 12 and 14.
(6) During the Interim Supply Period, the Parties shall continue to use good faith efforts to negotiate and execute the Commercialization Agreement.
(d) Third Party Supplier
(1) If, by January 1, 2018, the Parties have been unable to agree on the terms and conditions of a Commercialization Agreement pursuant to Section 8(b), at Achaogen’s request, Ark, Achaogen and a Third Party manufacturer that is reasonably acceptable by Ark (a “Third Party Supplier”) shall negotiate and enter into a technology transfer and license agreement (the “Technology Transfer and License Agreement”) pursuant to which, subject to commercially reasonable terms and conditions, Ark grants such Third Party Supplier a nonexclusive, nontransferable license under Ark Background IP and Foreground IP owned by Ark hereunder and provide reasonable know-how transfer to such Third Party Supplier; in each case to the extent necessary and solely for the purpose of manufacturing and supplying the Assay to Achaogen for use or commercialization by Achaogen and its designees. With respect to the Parties’ choice of Third Party Supplier, [***], provided that such Third Party Supplier has agreed to the requirements of confidentiality and the restrictions contained in the license as provided in this Section 8(d).
(2) Such Third Party Supplier would enter into a supply agreement directly with Achaogen on such terms as the Third Party Supplier and Achaogen may agree.
(3) If the Parties and such Third Party Supplier cannot agree on the terms and conditions of the Technology Transfer and License Agreement prior to [***], then either Party may send the other Party a written notice that such Party wishes to appoint an expert panel as provided pursuant to Section 8(b). For purposes of this Section 8(d), all of the provisions of Section 8(b) shall apply under such circumstances, but references to Commercialization Agreement shall constitute references to such Technology Transfer and License Agreement and references to 8(a) shall constitute references to this Section 8(d).
(4) For the avoidance of doubt, the Third Party Supplier shall be bound by reasonable confidentiality obligations with respect to any information, materials and know-how provided by Ark pursuant to the Technology Transfer and License Agreement. Without limiting the foregoing, the Technology Transfer and License Agreement shall provide the right for Ark to [***]. Achaogen agrees to reasonably cooperate with Ark to [***].
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 22 -
9. Confidentiality
(a) Effectiveness of the Nondisclosure Agreement. The terms and conditions of the Nondisclosure Agreement are hereby superseded by this Agreement and all Confidential Information furnished by one Party to the other under the Nondisclosure Agreement prior to the Effective Date shall be deemed Confidential Information under this Agreement and subject to the terms and conditions of this Article 9.
(b) Use of Confidential Information. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, the Parties agree that, during the Term and for [***] years thereafter, the receiving Party shall keep, and shall ensure that its employees, officers and directors keep, completely confidential and shall not publish or otherwise disclose and shall not use for any purpose any Confidential Information of the other Party, except to the extent that it can be established by the receiving Party by competent proof that such Confidential Information: (i) was already known to the receiving Party, other than under an obligation of confidentiality, at the time of disclosure by the other Party; (ii) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party; (iii) became generally available to the public or was otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party in breach of this Agreement; (iv) was disclosed to the receiving Party, other than under an obligation of confidentiality, by a Third Party who had no obligation to the disclosing Party not to disclose such information to others; or (v) was developed by the receiving Party independent of any disclosure received under this Agreement as shown by written records prepared by the receiving Party contemporaneously with such independent development.
(c) Authorized Disclosures. Each Party may disclose Confidential Information of the other Party to the extent such disclosure is reasonably necessary in the following instances: (i) is submitted by the recipient to governmental authorities to facilitate the issuance of Trial Registrations and/or Marketing Registrations for the Assay, provided that reasonable measures shall be taken to assure confidential treatment of such information; (ii) is provided by the recipient to Third Parties under confidentiality agreements having provisions at least as stringent as those in this Article 9, for consulting, manufacturing development, manufacturing, external testing, marketing trials, in each case to the extent necessary to perform its obligations or exercise its rights under this Agreement; (iii) to its actual or prospective investors or collaborators, or its accountants, attorneys and other professional advisors, in each case under confidentiality agreements having provisions at least as stringent as those in this Article 9; or (iv) is otherwise required to be disclosed in compliance with Applicable Laws (including any securities laws or rules of any recognized stock exchange) order by a court or other regulatory body having competent jurisdiction; provided that if a Party is required to make any such disclosure of the other Party’s Confidential Information such Party will give reasonable advance written notice to the other Party of such disclosure requirement and, except to the extent inappropriate in the case of patent applications, will use its reasonable efforts in assisting the disclosing Party to secure confidential treatment of such Confidential Information required to be disclosed.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 23 -
(d) Limitations on Use. Each Party shall use any Confidential Information obtained by such Party from the other Party and its Affiliates pursuant to this Agreement or otherwise, solely in connection with the activities or transactions contemplated by this Agreement.
(e) Publication. Neither Party shall publish any manuscript, abstract, text or any other material containing any Work Product or Foreground IP without the other Party’s prior written approval, with such approval not to be unreasonably withheld.
(f) Injunctive Relief. Each Party acknowledges and agrees that its actual or threatened breach of this Article 9 would cause the other irreparable injury for which it may not have an adequate remedy at law. Each Party shall be entitled, in addition to any other right or remedy it may have, at law or in equity, to seek an injunction, without the posting of any bond or other security, enjoining or restraining the other Party from any violation or threatened violation of this Article 9.
(g) Return or Destruction of Confidential Information. Upon the disclosing Party’s written request at any time or upon expiration or termination of this Agreement, the receiving Party shall:
(1) immediately cease use of use of Confidential Information of the disclosing Party as well as any information or materials that contain, incorporate or are derived from such Confidential Information; and
(2) return to the disclosing Party or shall destroy, at the disclosing Party’s discretion, any and all Confidential Information of the disclosing Party (including all copies and reproductions thereof), provided, however, that the receiving Party may retain one (1) copy of such Confidential Information solely for archival purposes. Notwithstanding the return or destruction of the Confidential Information, the receiving Party and its Representatives will continue to be bound by their obligations under the Agreement.
10. Representations, Warranties and Covenants
(a) Authority Relative to this Agreement. Each Party represents and warrants to the other Party, as of the Effective Date, that:
(1) such Party is duly organized and validly existing under the laws of the jurisdiction of its incorporation and has full corporate power and authority to enter into this Agreement and to carry out the provisions hereof;
(2) such Party is free to enter into this Agreement;
(3) in entering into this Agreement, such Party will not violate any other agreement to which it is a party; and
- 24 -
(4) such Party has taken all corporate action necessary to authorize the execution and delivery of this Agreement and the performance of its obligations under this Agreement.
(b) Standard of Performance. Ark represents, warrants and covenants that it shall provide the Services in a good and workmanlike manner, in accordance with (i) applicable industry standards, (ii) the standards of care and diligence and the level of skill, knowledge and judgment customarily practiced by companies in the same industry in performing services of a similar nature, (iii) all quality control requirements that Achaogen provides to Ark at the time of this Agreement or as specified in any Statement of Work and (iv) all terms and conditions of this Agreement and each Statement of Work.
(c) Conformance with Specifications. Ark represents, warrants and covenants that the Work Products, including the Assay, shall conform to the applicable Specifications or other requirements set forth in a Statement of Work, and free of defects in material and workmanship.
(d) No Infringement of Third Party Rights. Ark represents, warrants and covenants that to its actual knowledge, its performance of the Services, and all Third Party Material and Ark Materials that Ark incorporates into any Work Products, and Achaogen’s use of any such Work Products, shall not infringe or misappropriate any Intellectual Property Rights of any Third Party or otherwise conflict with the rights of any Third Party. Achaogen represents, warrants and covenants that to its actual knowledge, the performance of the Services in accordance with the Specifications and the use of any Achaogen Materials in accordance with the terms and conditions of this Agreement shall not infringe or misappropriate any Intellectual Property Rights of any Third Party or otherwise conflict with the rights of any Third Party.
(e) Compliance With Applicable Laws
(1) The Parties represent, warrant and covenant that they shall perform all activities hereunder in accordance with, and shall develop, manufacture, supply and market all Assays in accordance with, the terms and conditions of this Agreement and all Applicable Laws.
(2) Ark shall obtain and maintain, at its expense, all licenses, permits and approvals necessary for it to provide the Services. Ark shall provide Achaogen with copies of any such licenses, permits and approvals upon request.
(f) Debarment. Each Party represents and warrants to the other Party that it has not used, and covenants that they shall not use, the services of any Person or entity in any capacity who has been debarred, disqualified, excluded, suspended, or is otherwise ineligible to: (i) participate in any federal health care program as provided under 42 U.S.C. § 1320a-7, or in any other federal payment or procurement program, or (ii) engage in activities subject to regulation by U.S. government (including the FDA) relating to any drug product. Each Party shall notify the other Party in writing if any Person or entity in any capacity connected with the development, manufacture, supply or
- 25 -
marketing of the Assay pursuant to this Agreement is or becomes debarred, disqualified, excluded, suspended, or is otherwise ineligible to participate in any federal health care program.
11. Testing of the Assays and Other Work Products. Except as otherwise provided herein or in an applicable Statement of Work, Achaogen acknowledges and agrees that Ark is not responsible for testing the Assays or the other Work Products provided by Ark or for ensuring that it is tested, manufactured, packaged, labeled (including adequate warnings and users manuals), sold, or used in a safe and careful manner.
12. Indemnities
(a) Indemnification by Ark. Ark shall defend, indemnify and hold harmless Achaogen, its affiliates, and its and their respective directors, officers, attorneys, agents, employees and assigns from and against all liabilities, losses, damages and expenses, including without limitation costs and reasonable attorneys’ fees (collectively, “Losses”) resulting from any claims, suits, demands or proceedings brought by any Third Party, including without limitation, Third Party claims for [***] (collectively, “Claims”) arising from (i) [***]; (ii) [***] acts or omissions of Ark or its employees or other Ark personnel in providing the Services; or (iii) Ark’s breach of any of its representations, warranties or obligations under this Agreement, including, without limitation, Ark’s failure to comply with all laws and regulations applicable to its provision of the Services, failure to obtain and maintain all licenses, permits and approvals necessary for it to provide the Services or otherwise required hereunder or failure to comply with the terms of Exhibit B; in each case, except to the extent any such Losses result from any Claim for which Achaogen is obligated to indemnify Ark pursuant to Section 12(b).
(b) Indemnification by Achaogen. Achaogen shall defend, indemnify and hold harmless Ark, its affiliates, and its and their respective directors, officers, attorneys, agents, employees and assigns from and against all Losses resulting from Claims arising from (i) [***]; (ii) [***]; or (iii) [***]; (iv) [***] acts or omissions of Achaogen or its employees or other Achaogen personnel in performing its activities under this Agreement; or (v) Achaogen’s breach of any of its representations, warranties or obligations under this Agreement; in each case, except to the extent any such Losses result from any Claim for which Ark is obligated to indemnify Achaogen pursuant to Section 12(a).
(c) Notice. In the event that any individual, corporation, partnership, company or similar entity (an “Indemnitee”) entitled to indemnification under Section 12(a) or 12(b), or under any other indemnity in this Agreement, is seeking such indemnification, such Indemnitee shall inform the indemnifying Party of the claim as soon as reasonably practicable after such Indemnitee receives notice of such claim, shall permit the indemnifying Party to assume direction and control of the defense of the claim (including the sole right to settle it at the sole discretion of the indemnifying Party,
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 26 -
provided that such settlement does not impose any obligation on the Indemnitee) and shall cooperate as reasonably requested (and at the expense of the indemnifying Party) in the defense of the claim.
(d) Complete Indemnification. As the Parties intend complete indemnification, [***].
13. Limitation of Liability. Except for (i) [***], (ii) each Party’s breach of any provision of Article 9, and (iii) any indemnity claim under Article 12, to the extent permitted by law, neither Party shall be liable for any indirect, incidental or consequential losses. In no event shall Ark’s total liabilities under this Agreement exceed [***]. These exclusions and limitations on damages apply to any loss or damage however caused and on any theory of liability, whether in contract, tort or otherwise.
14. Term and Termination of this Agreement
(a) Term. The term of this Agreement shall commence on the Effective Date and shall continue until the later of the completion of the Services and January 1, 2020 (the “Term”). The Term may be extended by the mutual written agreement of the Parties.
(b) Termination for Breach. If either Party materially breaches this Agreement and fails to remedy that breach within thirty (30) days of receiving written notice of that breach from the other Party, the non-breaching Party may terminate this Agreement by providing written notice of termination to the breaching Party; provided, however, that in the event of a good faith dispute with respect to the existence of a material breach, this Agreement shall not be terminated unless it is finally determined by a court of competent jurisdiction or through any other mutually agreed dispute resolution mechanism that this Agreement was materially breached, and the breaching Party fails to cure such breach within thirty (30) days after such determination.
(c) Termination for Convenience. Achaogen may terminate this Agreement at any time, for any reason or no reason (such as, for example, on cancellation of the Trial), upon sixty (60) days’ written notice to Ark.
(d) Effect of Expiration or Termination
(1) Upon the expiration or termination of this Agreement, Ark shall cease providing Services under all Statements of Work in effect and promptly deliver to Achaogen all Work Products and Assays created pursuant to this Agreement up to the date of expiration or termination, whether completed or work in progress.
(2) In the event of termination by Achaogen pursuant to Section 14(b), Ark shall:
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 27 -
(i) assist Achaogen by [***]. Any information so provided by Ark shall be deemed Ark’s Confidential Information and may only be used by Achaogen for the sole purpose of [***]; and
(ii) immediately renounce in favor of Achaogen or its nominee and surrender automatically to Achaogen or its nominee any import permits, registrations, licenses, exemptions from customs duties and government consents of any nature whatsoever which Ark may have or retain directly or indirectly in connection with the Assay.
(3) In the event of termination pursuant to Section 14(c), Achaogen shall pay to Ark an amount equal (i) [***] of the [***] of this Agreement pursuant to Section 14(c), minus (ii) [***]; provided that no such amount shall be payable unless [***]. Achaogen shall pay such amount within [***] of receipt from Ark of an invoice containing reasonable supporting detail concerning such expenses.
(e) Survival. The provisions of Articles 1, 6, 7, 9, 12, 13, 14, 15 and 16, and Sections 2(c)(3), 3(d), 7(e), 8(c) and 8(d) shall survive the termination or expiration of this Agreement.
15. Notices
(a) Delivery of Notices. All notices sent under this Agreement shall be in writing and (i) hand delivered; (ii) transmitted by legible facsimile with a copy sent concurrently by certified mail, return receipt requested; or (iii) delivered by prepaid priority delivery service.
(b) Addresses for Notices. Notices shall be sent to the Parties at the following addresses or such other addresses as the Parties subsequently may provide:
| If to Achaogen: |
▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ |
|||||
| Attention: |
[***] | |||||
| Telephone: |
[***] | |||||
| Fax: |
[***] | |||||
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 28 -
| If to Ark: |
▇▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ |
|||||
| Attention: |
[***] | |||||
| Telephone: |
[***] | |||||
| Fax: |
[***] | |||||
16. Miscellaneous
(a) Use of Names. Neither Party shall, without the prior written approval of the other Party, (i) advertise or otherwise publicize the existence or terms of this Agreement or any other aspect of the relationship between Achaogen and Ark or (ii) use the other Party’s name or any trade name, trademark or service ▇▇▇▇ belonging to such other Party in press releases or in any form of advertising. Notwithstanding the foregoing, each Party shall have the right to disclose this Agreement, without the other Party’s approval, (a) to any existing or potential investors or collaborators, or its advisors (including financial advisors, lawyers and accountants) who are bound by confidentiality obligations no less restrictive than those provided in this Agreement, each on a need-to-know basis, or (b) to comply with any Applicable Laws including securities laws and rules of recognized stock exchange; provided that prior to such disclosure the disclosing Party shall notify the other Party and reasonably assist the other Party to limit such disclosure.
(b) Force Majeure. Neither Party shall have any liability to the other due to a delay or failure to perform under this Agreement which results without fault or negligence on the part of the Party involved and which is due to causes beyond its control including, without being limited to, acts of God or of the public enemy, any preference, priority or allocation order issued by the state or federal government or any other act of state or federal government, fires, floods, epidemics, quarantine restrictions, freight embargoes, and unusually severe weather. Each Party shall promptly notify the other in writing of any such delay and the cause thereof.
(c) Governing Law; Jurisdiction. This Agreement shall be governed by and construed in accordance with the law of the State of California, without regard to its conflict of laws principles. For the adjudication of any disputes arising under this Agreement, the Parties hereby consent to personal jurisdiction in the applicable state or federal courts in the State of California.
(d) Severability. The provisions of this Agreement are severable, and the unenforceability of any provision of this Agreement shall not affect the enforceability of the remainder of this Agreement. The Parties acknowledge that it is their intention that if any provision of this Agreement is determined by a court to be unenforceable as drafted, that provision should be construed in a manner designed to effectuate the purpose of that provision to the greatest extent possible under Applicable Laws.
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
- 29 -
(e) Construction of Agreement. The Parties acknowledge that they thoroughly have reviewed this Agreement and bargained over its terms. Accordingly, this Agreement shall be construed without regard to the Party or Parties responsible for its preparation, and shall be deemed to have been prepared jointly by the Parties.
(f) Cumulative Rights and Remedies. The rights and remedies provided in this Agreement and all other rights and remedies available to either Party at law or in equity are, to the extent permitted by law, cumulative and not exclusive of any other right or remedy now or hereafter available at law or in equity. Neither asserting a right nor employing a remedy shall preclude the concurrent assertion of any other right or employment of any other remedy, nor shall the failure to assert any right or remedy constitute a waiver of that right or remedy.
(g) Assignment. Neither Party may assign any of its rights or obligations under this Agreement without the prior written consent of the other Party; provided that each Party may assign this Agreement to a successor of all or substantially all of its business or assets to which this Agreement pertains, whether by merger, sale, reorganization, reincorporation, operations of law or otherwise. Any purported assignment in violation of the foregoing shall be void. For clarity, each Party may subcontract or otherwise delegate any of its rights, obligations, or duties to any Third Party by providing prior written notice to the other Party; provided that each Party shall be fully responsible for any breach of or noncompliance with the applicable terms and conditions of this Agreement by such Third Party.
(h) Binding Effect. This Agreement shall be binding upon and inure to the benefit of the Parties and their respective successors, permitted assigns and legal representatives.
(i) Headings. All headings in this Agreement are included solely for convenient reference, are not intended to be full and accurate descriptions of the contents of this Agreement, shall not be deemed a part of this Agreement, and shall not affect the meaning or interpretation of this Agreement.
(j) Relationship of the Parties. The Parties are independent contractors, and nothing in this Agreement shall be construed as creating a partnership, joint venture, employment or agency relationship between the Parties, or between a Party and any employee of the other Party, or as authorizing either Party to act as agent for the other or to enter into contracts on behalf of the other.
(k) Amendments. This Agreement may be modified or amended only by written agreement of the Parties.
(l) Entire Agreement. This Agreement, each Statement of Work (all of which are incorporated herein by reference), all exhibits attached hereto or thereto, the Material Transfer Agreement and the Side Letter constitute the entire agreement between the Parties concerning the subject matter of this Agreement and each Statement of Work, and, except as otherwise provided herein, supersede all prior agreements between the
- 30 -
Parties concerning the subject matter hereof. To the extent there is any conflict between any provision of this Agreement, a Statement of Work or an exhibit attached hereto or thereto and any provision of the Material Transfer Agreement, this Agreement, Statement of Work or exhibit (as appropriate) shall govern and supersede such term of the Material Transfer Agreement.
END OF PAGE
[signatures appear on following page]
- 31 -
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed by their respective duly authorized officers as of the Effective Date.
| ACHAOGEN INC. | ARK DIAGNOSTICS, INC. | |||||||
| By: | /s/ ▇▇▇▇▇▇ ▇▇▇ | By: | /s/ ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||||
| Name: | ▇▇▇▇▇▇ ▇▇▇ | Name: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||||
| Title: | VP Finance & Corporate Development | Title: | President & CEO | |||||
[signature page to DEVELOPMENT SERVICES AGREEMENT]
EXHIBIT A
STATEMENT OF WORK
Achaogen Inc. (“Achaogen”) and Ark Diagnostics, Inc. (“Ark”) are entering into this Statement of Work pursuant to the Development Services Agreement (the “Agreement”) between them dated as of August 19, 2013. Capitalized terms used and not otherwise defined in this Statement of Work shall have the meanings given to them in the Agreement. In accordance with the Agreement, Ark shall provide the Services and deliverables specified below in accordance with the terms and conditions set forth below.
A-1
PURPOSE OF PLAN
ARK Diagnostics, Inc. develops, manufactures, and distributes in vitro diagnostic products in the field of therapeutic drug management, or monitoring, (TDM). Therapeutic Drug Management is a branch of clinical chemistry that specializes in the measurement of medication levels in biological fluids, such as plasma. ARK will collaborate with Achaogen Inc., to develop a TDM enzyme immunoassay for plazomicin.
This document outlines the project plan for the development of the ARK™ Plazomicin Assay. The Project Plan provides a description of the project, objectives, resources, specifications, timeline and risks.
BACKGROUND
Achaogen is a biopharmaceutical company focused on the discovery and development of new antibiotics for the treatment of serious multi-drug resistant gram-negative bacterial infections. Plazomicin is an aminoglycoside antibiotic that met all objectives in a multi-national Phase 2 study. Achaogen is developing plazomicin, a next-generation aminoglycoside that overcomes common bacterial resistance mechanisms, as an intravenous treatment for serious Gram-negative bacterial infections, including those caused by multi-drug resistant Escherichia coli and Klebsiella pneumoniae.
Plazomicin (Fig. 1) is a broad-spectrum aminoglycoside with differentiated activity against multidrug resistant Enterobacteriaceae and is being developed by Achaogen, Inc. as a first line treatment for resistant bacterial pathogens. The molecular formula for plazomicin is C25H48N6O10. Its molecular weight is 592.7 and the CAS Registry Number is 1154757-24-0. The chemical structure is shown below:
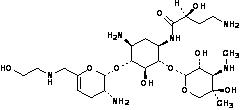
Figure 1. Plazomicin structure.
PROJECT ROLES
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-2
[***]
PROPOSED PACKAGE INSERT – INTENDED USE AND INDICATION FOR USE
[***]
TECHNOLOGY – PRINCIPLES OF THE PROCEDURE
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-3
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-4
KIT CONFIGURATIONS
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-5
[***]
HANDLING AND STORAGE
[***]
CLINICAL CHEMISTRY ANALYZER
[***]
DESIGN CHARACTERISTICS
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-6
[***]
| [***] | Eight pages have been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
[***]
REPORTING REQUIREMENTS
[***]
PROJECT TIMELINE
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-15
[***]
IDE REGULATORY TIMELINE
[***]
MARKET CLEARANCE, TRIAL PERFORMANCE, AND REGULATORY TIMELINE
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-16
[***]
TECHNICAL RISK MANAGEMENT
[***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-17
[***]
IN WITNESS WHEREOF, the Parties have caused this Statement of Work to be executed by their respective duly authorized officers as of August 19, 2013.
| ACHAOGEN INC. | ARK DIAGNOSTICS, INC. | |||||||
| By: | /s/ ▇▇▇▇▇▇ ▇▇▇ | By: | /s/ ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||||
| Name: | ▇▇▇▇▇▇ ▇▇▇ | Name: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |||||
| Title: | VP Finance & Corporate Development | Title: | President | |||||
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
A-18
EXHIBIT B
U.S. GOVERNMENT RIGHTS
This Exhibit B to the Development Services Agreement between Achaogen Inc. and ARK Diagnostics, Inc. contains the language referenced in Section 7(a), in compliance with Achaogen’s obligations to BARDA under the Prime Contract. In accordance with the terms of the Prime Contract, the language in this Exhibit B has been reproduced verbatim from the Prime Contract except as otherwise instructed therein, including all defined terms and related conventions.
Ark understands and agrees that the obligations created by this Exhibit B constitute a contract between Ark and BARDA with respect to the matters covered by the clause; provided, however, that nothing in this paragraph is intended to confer any jurisdiction under the Contract Disputes Act in connection with proceedings under paragraph (h) of this clause.
To the extent any provisions of Articles 5 (“Proprietary Rights”) or 6 (“Patent Prosecution and Defense”) of this Agreement could be interpreted to contradict any of (i) Ark’s obligations under this Exhibit B or (ii) Achaogen’s obligations under the Prime Contract, the Parties shall first perform their obligations to BARDA under this Exhibit B or the Prime Contract, as applicable, and then shall perform any obligations to the other Party remaining under Articles 5 and 6.1
(a) As used in this clause—
“Invention” means any invention or discovery that is or may be patentable or otherwise protectable under title 35 of the U.S. Code, or any variety of plant that is or may be protectable under the Plant Variety Protection Act (7 U.S.C. § 2321 et seq.)
“Made” means—
(1) When used in relation to any invention other than a plant variety, the conception or first actual reduction to practice of the invention; or
(2) When used in relation to a plant variety, that Ark has at least tentatively determined that the variety has been reproduced with recognized characteristics.
| 1 | In order to assure each party retains the IP rights contemplated under this Agreement, each party will need to fully comply with their Government obligations first, and then provide ownership or licenses as provided herein. For example, if Ark employees invent something during the term of the Agreement, but Achaogen would own it under this agreement, Ark should first comply with its obligations to the Government to ensure that Ark retains title (versus the Government), and then should execute any necessary assignments to Achaogen. |
B-1
“Nonprofit organization” means a university or other institution of higher education or an organization of the type described in section 501(c)(3) of the Internal Revenue Code of 1954 (26 U.S.C. § 501(c)) and exempt from taxation under section 501(a) of the Internal Revenue Code (26 U.S.C. § 501(a)), or any nonprofit scientific or educational organization qualified under a State nonprofit organization statute.
“Practical application” means to manufacture, in the case of a composition of product; to practice, in the case of a process or method; or to operate, in the case of a machine or system; and, in each case, under such conditions as to establish that the invention is being utilized and that its benefits are, to the extent permitted by law or Government regulations, available to the public on reasonable terms.
“Subject invention” means any invention of Ark made in the performance of work under the Prime Contract.
(b) Ark’s rights
(1) Ownership. Ark may retain ownership of each subject invention throughout the world in accordance with the provisions of this clause.
(2) License
(i) Ark shall retain a nonexclusive royalty-free license throughout the world in each subject invention to which the Government obtains title, unless Ark fails to disclose the invention within the times specified in paragraph (c) of this clause. Ark’s license extends to any domestic subsidiaries and affiliates within the corporate structure of which Ark is a part, and includes the right to grant sublicenses to the extent Ark was legally obligated to do so at contract award. The license is transferable only with the written approval of the agency, except when transferred to the successor of that part of Ark’s business to which the invention pertains.
(ii) Ark’s license may be revoked or modified by the agency to the extent necessary to achieve expeditious practical application of the subject invention in a particular country in accordance with the procedures in FAR 27.302(i)(2) and 27.304-1(f).
(c) Contractor’s obligations
(1) Ark shall disclose in writing each subject invention to the Contracting Officer and Achaogen within two (2) months after the inventor discloses it in writing to Contractor personnel responsible for patent matters. The disclosure shall identify the inventor(s) and the Prime Contract under which the subject invention was made. It shall be sufficiently complete in technical detail to convey a clear understanding of the subject invention. The disclosure shall also identify any publication, on sale (i.e., sale or offer for sale), or public use of the subject invention, or whether a manuscript describing the subject invention has been submitted for publication and, if so, whether it
B-2
has been accepted for publication. In addition, after disclosure to the agency, Ark shall promptly notify the Contracting Officer of the acceptance of any manuscript describing the subject invention for publication and any on sale or public use.
(2) Ark shall elect in writing whether or not to retain ownership of any subject invention by notifying the Contracting Officer and Achaogen within two (2) years of disclosure to the agency. However, in any case where publication, on sale, or public use has initiated the one (1) year statutory period during which valid patent protection can be obtained in the United States, the period for election of title may be shortened by the agency to a date that is no more than sixty (60) days prior to the end of the statutory period.
(3) Ark shall file either a provisional or a nonprovisional patent application or a Plant Variety Protection Application on an elected subject invention within one (1) year after election. However, in any case where a publication, on sale, or public use has initiated the one (1) year statutory period during which valid patent protection can be obtained in the United States, Ark shall file the application prior to the end of that statutory period. If Ark files a provisional application, it shall file a nonprovisional application within ten (10) months of the filing of the provisional application. Ark shall file patent applications in additional countries or international patent offices within either ten (10) months of the first filed patent application (whether provisional or nonprovisional) or six (6) months from the date permission is granted by the Commissioner of Patents to file foreign patent applications where such filing has been prohibited by a Secrecy Order.
(4) Ark may request extensions of time for disclosure, election, or filing under paragraphs (c)(1), (c)(2), and (c)(3) of this clause.
(d) Government’s rights—
(1) Ownership. Ark shall assign to the agency, on written request, title to any subject invention—
(i) If Ark fails to disclose or elect ownership to the subject invention within the times specified in paragraph (c) of this clause, or elects not to retain ownership; provided, that the agency may request title only within sixty (60) days after learning of Ark’s failure to disclose or elect within the specified times.
(ii) In those countries in which Ark fails to file patent applications within the times specified in paragraph (c) of this clause; provided, however, that if Ark has filed a patent application in a country after the times specified in paragraph (c) of this clause, but prior to its receipt of the written request of the agency, Ark shall continue to retain ownership in that country.
B-3
(iii) In any country in which Ark decides not to continue the prosecution of any application for, to pay the maintenance fees on, or defend in reexamination or opposition proceeding on, a patent on a subject invention.
(2) License. If Ark retains ownership of any subject invention, the Government shall have a nonexclusive, nontransferable, irrevocable, paid-up license to practice, or have practiced for or on its behalf, the subject invention throughout the world.
(e) Contractor action to protect the Government’s interest
(1) Ark shall execute or have executed and promptly deliver to the agency all instruments necessary to—
(i) Establish or confirm the rights the Government has throughout the world in those subject inventions in which Ark elects to retain ownership; and
(ii) Assign title to the agency when requested under paragraph (d) of this clause and to enable the Government to obtain patent protection and plant variety protection for that subject invention in any country.
(2) Ark shall require, by written agreement, its employees, other than clerical and nontechnical employees, to disclose promptly in writing to personnel identified as responsible for the administration of patent matters and in Ark’s format, each subject invention in order that Ark can comply with the disclosure provisions of paragraph (c) of this clause, and to execute all papers necessary to file patent applications on subject inventions and to establish the Government’s rights in the subject inventions. The disclosure format should require, as a minimum, the information required by paragraph (c)(1) of this clause. Ark shall instruct such employees, through employee agreements or other suitable educational programs, as to the importance of reporting inventions in sufficient time to permit the filing of patent applications prior to U.S. or foreign statutory bars.
(3) Ark shall notify the Contracting Officer and Achaogen of any decisions not to file a nonprovisional patent application, continue the prosecution of a patent application, pay maintenance fees, or defend in a reexamination or opposition proceeding on a patent, in any country, not less than thirty (30) days before the expiration of the response or filing period required by the relevant patent office.
(4) Ark shall include, within the specification of any United States nonprovisional patent or plant variety protection application and any patent or plant variety protection certificate issuing thereon covering a subject invention, the following statement, “This invention was made with Government support under (identify the contract) awarded by (identify the agency). The Government has certain rights in the invention.”
B-4
(f) Reporting on utilization of subject inventions. Ark shall submit, on request, periodic reports no more frequently than annually on the utilization of a subject invention or on efforts at obtaining utilization of the subject invention that are being made by Ark or its licensees or assignees. The reports shall include information regarding the status of development, date of first commercial sale or use, gross royalties received by Ark, and other data and information as the agency may reasonably specify. Ark also shall provide additional reports as may be requested by the agency in connection with any march-in proceeding undertaken by the agency in accordance with paragraph (h) of this clause. Ark also shall ▇▇▇▇ any utilization report as confidential/proprietary to help prevent inadvertent release outside the Government. As required by 35 U.S.C. § 202(c)(5), the agency will not disclose that information to persons outside the Government without Ark’s permission.
(g) Preference for United States industry. Notwithstanding any other provision of this clause, neither Ark nor any assignee shall grant to any person the exclusive right to use or sell any subject invention in the United States unless the person agrees that any products embodying the subject invention or produced through the use of the subject invention will be manufactured substantially in the United States. However, in individual cases, the requirement for an agreement may be waived by the agency upon a showing by Ark or its assignee that reasonable but unsuccessful efforts have been made to grant licenses on similar terms to potential licensees that would be likely to manufacture substantially in the United States, or that under the circumstances domestic manufacture is not commercially feasible.
(h) March-in rights. Ark acknowledges that, with respect to any subject invention in which it has retained ownership, the agency has the right to require licensing pursuant to 35 U.S.C. §§ 203 and 210(c), and in accordance with the procedures in 37 CFR Part 401.6 and any supplemental regulations of the agency in effect on the date of contract award.
(i) Special provisions for contracts with nonprofit organizations. If Ark is a nonprofit organization, it shall—
(1) Not assign rights to a subject invention in the United States without the written approval of the agency, except where an assignment is made to an organization that has as one of its primary functions the management of inventions, provided, that the assignee shall be subject to the same provisions as Ark;
(2) Share royalties collected on a subject invention with the inventor, including Federal employee co-inventors (but through their agency if the agency deems it appropriate) when the subject invention is assigned in accordance with 35 U.S.C. § 202(e) and 37 CFR Part 401.10;
(3) Use the balance of any royalties or income earned by Ark with respect to subject inventions, after payment of expenses (including payments to
B-5
inventors) incidental to the administration of subject inventions for the support of scientific research or education; and
(4) Make efforts that are reasonable under the circumstances to attract licensees of subject inventions that are small business concerns, and give a preference to a small business concern when licensing a subject invention if Ark determines that the small business concern has a plan or proposal for marketing the invention which, if executed, is equally as likely to bring the invention to practical application as any plans or proposals from applicants that are not small business concerns; provided, that Ark is also satisfied that the small business concern has the capability and resources to carry out its plan or proposal. The decision whether to give a preference in any specific case will be at the discretion of Ark.
(5) Allow the Secretary of Commerce to review Ark’s licensing program and decisions regarding small business applicants, and negotiate changes to its licensing policies, procedures, or practices with the Secretary of Commerce when the Secretary’s review discloses that Ark could take reasonable steps to more effectively implement the requirements of paragraph (i)(4) of this clause.
(j) Communications.
For the sole purposes of fulfilling its obligations under this section, Ark shall submit communications to the Contracting Officer at the following address:
▇▇▇▇▇ ▇▇▇▇▇▇▇
Contracting Officer
Office of Acquisition Management, Contracts and Grants
Office of the Assistant Secretary for Preparedness and Response
United States Department of Health and Human Services
▇▇▇ ▇▇▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇, ▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Via e-mail: ▇▇▇▇▇.▇▇▇▇▇▇▇@▇▇▇.▇▇▇
ARK shall site the following contract number in all communications with the Contracting Officer: HHSO100201000046C
(k) Subcontracts
(1) Ark shall include the substance of this clause, including this paragraph (k), in all subcontracts for experimental, developmental, or research work to be performed by a small business concern or nonprofit organization.
B-6
(2) Ark shall include in all other subcontracts for experimental, developmental, or research work the substance of the patent rights clause required by FAR Subpart 27.3.
(3) At all tiers, the patent rights clause must be modified to identify the parties as follows: references to the Government are not changed, and the subcontractor has all rights and obligations of Ark in the clause. Ark shall not, as part of the consideration for awarding the subcontract, obtain rights in the subcontractor’s subject inventions.
(4) In subcontracts, at any tier, the agency, the subcontractor, and Ark agree that the mutual obligations of the parties created by this clause constitute a contract between the subcontractor and the agency with respect to the matters covered by the clause; provided, however, that nothing in this paragraph is intended to confer any jurisdiction under the Contract Disputes Act in connection with proceedings under paragraph (h) of this clause.
B-7
EXHIBIT C
APPLICABLE PRIME CONTRACT PROVISIONS
This Exhibit C to the Development Services Agreement between Achaogen Inc. and ARK Diagnostics, Inc. contains the language referenced in Section 7(a), in compliance with Achaogen’s obligations to BARDA under the Prime Contract.
The following Prime Contract provisions, as they may be amended by the United States Government over time, are incorporated by reference with the same force and effect as if set forth in full text. For the purposes of this Agreement, the term “contract” shall mean this Agreement; the term “Contractor” shall mean Ark; the term “prime contractor” shall mean Achaogen; and the terms “Government” and “Contracting Officer” may mean Achaogen or the United States Government depending on the context in which the term is used. The dollar amount listed parenthetically in the titles of some referenced clauses in this Exhibit is the applicable threshold for the clause. If the total cumulative amounts invoiced by Ark under the Prime Contract are expected to exceed this amount, the clause applies.
FEDERAL ACQUISITION REGULATION
| Clause |
Date |
Title | ||
| FAR 52.202-1 | Jul-04 | Definitions (Over $100,000) | ||
| FAR 52.203-3 | Apr-84 | Gratuities (Over $100,000) | ||
| FAR 52.203-5 | Apr-84 | Covenant Against Contingent Fees (Over $100,000) | ||
| FAR 52.203-6 | Sep-06 | Restrictions on Subcontractor Sales to the Government (Over $100,000) | ||
| FAR 52.203-7 | Jul-95 | Anti-Kickback Procedures (Over $100,000) | ||
| FAR 52.203-8 | Jan-97 | Cancellation, Rescission, and Recovery of Funds for Illegal or Improper Activity (Over $100,000) | ||
| FAR 52.203-10 | Jan-97 | Price or Fee Adjustment for Illegal or Improper Activity (Over $100,000) | ||
| FAR 52.203-12 | Sep-07 | Limitation on Payments to Influence Certain Federal Transactions (Over $100,000) | ||
| FAR 52.203-13 | Apr-10 | Contractor Code of Business Ethics and Conduct (applies if Agreement is over $5,000,000 and has a performance period greater than 120 days). Insert “and Achaogen” after “Government” throughout this clause. Disclosures made under this clause shall be made directly to the government entities listed in the clause. | ||
| FAR 52.209-6 | Sep-06 | Protecting the Government’s Interests When Subcontracting With Contractors Debarred, Suspended, or Proposed for Debarment (Over $30,000) | ||
| FAR 52.215-2 | Mar 09 | Audit and Records- Negotiation (Over $100,000) Insert “and Achaogen” after “Contracting Officer” and “Comptroller General of the United States”. | ||
| FAR 52.215-10 | Oct-97 | Price Reduction for Defective Cost or Pricing Data (Over $650,000) | ||
| FAR 52.215-12 | Oct-97 | Subcontractor Cost or Pricing Data (Over $650,000) | ||
| FAR 52.215-21 | Oct-97 | Requirements for Cost or Pricing Data or Information Other Than Cost or Pricing Data –Modifications. Substitute “Achaogen” for “Contracting Officer” throughout this clause. | ||
C-1
| Clause |
Date |
Title | ||
| FAR 52.219-8 | May-04 | Utilization of Small Business Concerns (Over $100,000) | ||
| FAR 52.222-21 | Feb-99 | Prohibition of Segregated Facilities | ||
| FAR 52.222-26 | Mar-07 | Equal Opportunity (Over $10,000) | ||
| FAR 52.222-35 | Sept-06 | Equal Opportunity for Special Disabled Veterans, Veterans of the Vietnam Era, and Other Eligible Veterans (Over $100,000) | ||
| FAR 52.222-36 | Jun-98 | Affirmative Action for Workers with Disabilities (Over $10,000) | ||
| FAR 52.222-37 | Sep-06 | Employment Reports on Special Disabled Veterans, Veterans of the Vietnam Era, and Other Eligible Veterans (Over $100,000) | ||
| FAR 52.222-50 | Feb-09 | Combating Trafficking in Persons Substitute “Achaogen” for “Contracting Officer” throughout this clause. In paragraph (e), insert “and Achaogen” after “Government”. | ||
| FAR 52.222-54 | Jan-09 | Employment Eligibility Verification. Applicable to services and construction subcontracts that: (1) exceed $3,000; and (2) include work performed in the United States. This clause does not apply to subcontracts for commercial services that are (a) part of the purchase of a Commercially Available Off the Shelf (COTS) item (or an item that would be a COTS item, but for minor modifications) (b) performed by the COTS provider, and (c) are normally provided for that COTS item. | ||
| FAR 52.224-1 | Apr-84 | Privacy Act Notification (If subcontract requires design, development, or operation of a system of records) | ||
| FAR 52.224-2 | Apr-84 | Privacy Act (If subcontract requires design, development, or operation of a system of records) | ||
| FAR 52.225-1 | Feb-09 | Buy American Act- Supplies | ||
| FAR 52.225-13 | Jun-08 | Restrictions on Certain Foreign Purchases | ||
| FAR 52.227-1 | Dec-07 | Authorization and Consent, Alternate I (Apr 1984) (Over $100,000) | ||
| FAR 52.227-2 | Dec-07 | Notice and Assistance Regarding Patent and Copyright Infringement (Over $100,000) | ||
| FAR 52.227-11 | Dec-07 | Patent Rights –Ownership by the Contractor (Note: In accordance with FAR 27.303(b)(2), paragraph (e) is modified to include the requirements in FAR 27.303(b)(2)(i) through (iv). The frequency of reporting in (i) is annual | ||
| FAR 52.227-16 | Jun-87 | Additional Data Requirements. Insert “and Achaogen” after “Government” throughout this clause. | ||
| FAR 52.242-15 | Aug-89 | Stop Work Order, with Alternate I (April 1984) Substitute “Achaogen” for “Contracting Officer” throughout this clause. In paragraph (e), insert “or Achaogen” after “Government”. | ||
| FAR 52.244-5 | Dec-96 | Competition in Subcontracting | ||
| FAR 52.244-6 | Jun-10 | Subcontracts for Commercial Items | ||
| FAR 52.245-1 | Aug-10 | Government Property Applicable where government property involved in performance of subcontract; “Contracting Officer” means “Achaogen” except in the definition of Property Administrator and in paragraph h(1)(iii) and where it is unchanged, and in paragraphs (c) and (h)(4) where it includes Achaogen. “Government” is unchanged in the phrases “Government property” and “Government furnished property” and where elsewhere used except in paragraph (d)(1) where it means Achaogen and except in paragraphs (d)(2) and (g) where the term includes Achaogen. | ||
C-2
THE DEPARTMENT OF HEALTH AND HUMAN SERVICES
SUPPLEMENTAL REGULATION PROVISIONS
| Clause |
Date |
Title | ||
| HHSAR 352.203-70 |
Jan-06 | Anti-lobbying | ||
| HHSAR 352.223-70 |
Jan-06 | Safety and Health | ||
| HHSAR 352.224-70 |
Jan-06 | Privacy Act (if subcontract requires design, development, or operation of a system of records) | ||
| HHSAR 352.234-2 |
Oct-08 | Full Earned Value Management System (applies if subcontractor is identified in the Prime Contract as one to whom EVMS will apply). | ||
| HHSAR 325.242-73 |
Jan-06 | Withholding of Contract Payments | ||
| HHSAR 352.270-4 |
Jan-06 | Protection of Human Subjects | ||
| HHSAR 352.270-5 |
Jan-06 | Care of Live Vertebrate Animals | ||
| HHSAR 352.270-6 |
Jan-06 | Publications and Publicity | ||
BARDA REQUIRED PROVISIONS
| Prime Contract Provision |
Clause | |
| H.5: Press Releases |
Company shall clearly state, when issuing statements, press releases, requests for proposals, bid solicitations and other documents describing projects or programs funded in whole or in part with Federal money: (1) the percentage of the total costs of the program or project which will be financed with Federal money; (2) the dollar amount of Federal funds for the project or program; and (3) the percentage and dollar amount of the total costs of the project or program that will be financed by nongovernmental sources. | |
| H.9: Publications and Publicity |
No information related to data obtained under this contract shall be released or publicized without the prior written consent of Achaogen and the Contracting Officer Technical Representative.
In addition to the requirements of HHSAR 352.227-70, Publications and Publicity incorporated by reference in section I of this contract shall acknowledge the support of the Biomedical Advanced Research and Development Authority whenever publicizing the work under this contract in any media by including an acknowledgment substantially as follows:
“This project has been funded in whole or in part with Federal funds from the Biomedical Advanced Research and Development Authority, office of the Assistant Secretary for Preparedness and response, Office of the Secretary, Department of Health and Human Services, Under Contract No. HHSO100201000046C.” | |
C-3
| Prime Contract Provision |
Clause | |
| H.10: Reporting Matters Involving Fraud, Waste and Abuse | Anyone who becomes aware of the existence or apparent existence of fraud, waste and abuse in BARDA funded programs is encouraged to report such matters to the HHS Inspector General’s Office in writing or on the Inspector General’s Hotline. The toll free number is ▇-▇▇▇-▇▇▇-▇▇▇▇ (▇-▇▇▇-▇▇▇-▇▇▇▇). All telephone calls will be handled confidentially. The e-mail address is ▇▇▇▇▇@▇▇.▇▇▇▇.▇▇▇ and the mailing address is:
Office of Inspector General Department of Health and Human Services TIPS HOTLINE ▇.▇. ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇, ▇.▇. ▇▇▇▇▇. | |
| H.15: Privacy Act Applicability | Notification is hereby given that Company and its employees are subject to criminal penalties for violation of the Privacy Act to the same extent as employees of the Government. | |
| H.16: Laboratory license requirement | Company shall comply with all applicable requirements of Section 353 of the Public Health Service Act (Clinical Laboratory Improvement Act as Amended). This requirement shall also be included in any subcontract for services under this contract. | |
| H.17: Dissemination of Information | No information related to data obtained under this contract shall be released or publicized without the prior written consent of the Contracting officer, to be obtained through Achaogen. | |
| H.18: Identification and Disposition of Data | Company will be required to provide certain data generated under this contract to the Department of Health and Human Services (DHHS). DHHS reserves the right to review any other data determined by DHHS to be directly related to and/or generated under this contract. Company shall keep copies of all data required by the Food and Drug Administration (FDA) relevant to this contract for the time period specified by the FDA. | |
C-4
| Prime Contract Provision |
Clause | |
| H.22: Registration with the Select Agent Program for Work involving the possession, use, and/or transfer of select biological agents or toxins | Company shall not conduct work involving select agents or toxins under this contract until it and any associated subcontractor(s) comply with the following:
For prime or subcontract awards to domestic institutions that possess, use, and/or transfer Select Agents under this contract, the institution must comply with the provisions of 42 C.F.R. part 73, 7 C.F.R. part 331, and/or 9 C.F.R. part 121 ( ▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇▇▇▇-▇▇-▇▇.▇▇▇ ) as required, before using NIH funds for work involving a Select Agent or Toxin. No government funds can be used for research involving a Select Agent or Toxin at a domestic institution without a valid registration certificate.
For prime or subcontract awards to foreign institutions that possess, use, and/or transfer a Select Agent or Toxin, before using NIH funds for any work directly involving a Select Agent or Toxin, the foreign institution must provide information satisfactory to the government that safety, security, and training standards equivalent to those described in 42 C.F.R. part 73, 7 C.F.R. part 331, and/or 9 C.F.R. part 121 are in place and will be administered on behalf of all Select Agent or Toxin work supported by these funds. The process for making this determination includes inspection of the foreign laboratory facility by a government representative. During this inspection, the foreign institution must provide the following information: concise summaries of safety, security, and training plans; names of individuals at the foreign institution who will have access to the Select Agents and procedures for ensuring that only approved and appropriate individuals, in accordance with institution procedures, will have access to the Select Agents under the contract; and copies of or links to any applicable laws, regulations, policies, and procedures applicable to that institution for the safe and secure possession, use, and/or transfer of select agents. No funds can be used for work involving a Select Agent or Toxin at a foreign institution without written approval from Achaogen.
Listings of HHS select agents and toxins, and overlap select agents or toxins as well as information about the registration process for domestic institutions, are available on the Select Agent Program Web site at http:// ▇▇▇.▇▇▇.▇▇▇/▇▇/▇▇▇/ and ▇▇▇▇://▇▇▇.▇▇▇.▇▇▇/▇▇/▇▇▇/▇▇▇▇/▇▇▇▇▇▇.▇▇▇.
Listings of USDA select agents and toxins as well as information about the registration process for domestic institutions are available on the APHIS/USDA website at: ▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/ index.html and: ▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇▇▇▇▇/▇▇_▇▇▇▇▇▇▇_▇▇▇▇▇.▇▇▇▇
For foreign institutions, see the NIAID Select Agent Award information: ▇▇▇▇://▇▇▇.▇▇▇▇▇.▇▇▇.▇▇▇/▇▇▇/▇▇▇▇▇▇▇▇/▇▇▇▇▇▇▇_▇▇▇▇▇▇▇▇▇▇.▇▇▇. | |
| H.23: EPA Energy Star Requirements | All microcomputers, including personal computers, monitors, and printers purchased with government funds in the performance of a contract shall be equipped with or meet the energy efficient low-power standby feature as defined by the EPA Energy Star program unless the equipment always satisfies Energy Star efficiency levels. | |
C-5
| Prime Contract Provision |
Clause | |
| H.24: Acknowledgement of Federal Funding | (a) Section 507 of P.L. 104-208 mandates that contractors funded with Federal dollars, in whole or in part, acknowledge Federal funding when issuing statements, press releases, requests for proposals, bid solicitations and other documents. Contractors are required to state (1) the percentage and dollar amounts of the total program or project costs financed with federal money, and (2) the percentage and dollar amount of the total costs financed by nongovernmental sources. This requirement is in addition to the continuing requirement to provide an acknowledgement of support and disclaimer on any publication reporting the results of a contract funded activity.
(b) Publication and Publicity. The contractor shall acknowledge the support of the Department of Health and Human Service, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority whenever publicizing the work under this contract in any media by including an acknowledgment substantially as follows: “This project has been funded in whole or in part with Federal funds from the Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under Contract no. HHSO100201000046C.
(c) Press Releases. Pursuant to Section 508 of Public Law 105-78, the contractor shall clearly state, when issuing statements, press releases, requests for proposals, bid solicitations and other documents describing projects or programs funded in whole or in part with federal money that: (1) the percentage of the total costs of the program or project which will be financed with federal money; (2) the dollar amount of Federal funds for the project or program; and (3) the percentage and dollar amount of the total costs of the project or program that will be financed by nongovernmental sources. | |
| H.25: Manufacturing Standards | The Current Good Manufacturing Practice Regulations (“cGMP”) (21 C.F.R. Parts 210-211) and regulations pertaining to biological products (21 C.F.R. Part 600) will be the standard to be applied for manufacturing, processing, packing, storage, and delivery of this product.
If at any time during the life of the contract, Company fails to comply with cGMP in the manufacturing, processing and packaging of this product and such failure results in a material adverse effect on the safety, purity or potency of this product (a material failure), the Contractor shall have thirty (30) calendar days from the time such material failure is identified to cure such material failure. If the Contractor fails to take such an action within the thirty (30) calendar day period, then the contract may be terminated for default. | |
| H.26: Export Control Notification | Company is responsible for ensuring compliance with all export control laws and regulations that may be applicable to the export of and foreign access to their proposed technologies. | |
C-6
| Prime Contract Provision |
Clause | |
| H.27: Institutional responsibility Regarding Conflicting Interests of Investigators | Company shall comply with the requirements of 45 CFR Part 94, Responsible Prospective Contractors, which promotes objectivity in research by establishing standards to ensure that investigators (defined as the principle investigator and any other person who is responsible for design, conduct, or reporting of research funded under BARDA contracts) will not be biased by any conflicting financial interest. For the purposes of this part relating to financial interest, “investigator” includes the investigator’s spouse and dependent children.
Company shall at a minimum:
(a) maintain a written, enforceable policy on conflict of interest and inform each investigator of the policy, the investigator’s reporting responsibilities, and the applicable regulations. The contractor must take reasonable steps to ensure that investigators working as collaborators or subcontractors comply with the regulations.
(b) Designate and official to review financial disclosure statements from each investigator participating in BARDA-funded research. Based on established guidelines consistent with the regulations, the designated official must determine whether a conflict of interest exists, and if so, determine what actions should be taken to manage, reduce, or eliminate such a conflict.
(c) Require updating of financial disclosure statements during the period of award.
(d) Maintain records taken under this provision for three years after final payment.
(e) Establish adequate enforcement mechanisms.
If a conflict of interest is identified, the Institution shall report to Achaogen the existence of the conflicting interest found. This report shall be made and the conflicting interest managed, reduced, or eliminated, at least on a temporary basis, within thirty (30) days of that identification. |
C-7
EXHIBIT D
ANIMAL USE PROVISIONS
This Exhibit D to the Development Services Agreement between Achaogen Inc. and ARK Diagnostics, Inc. contains the language referenced in Section 7(b), in compliance with Achaogen’s obligations to BARDA under the Prime Contract.
| 1.0 | ANIMAL USES |
| 1.1 | Purpose. Projects that involve the use of laboratory animals (“Animal Use”) by Ark or at Ark’s direction are subject to the terms of this section. |
| 1.2 | Applicable Rules and Regulations. Ark shall adhere to all applicable rules and regulations related to the conduct and oversight of Projects that involve Animal Use, including but not limited to the following, as they may be amended or updated over time: |
| 1.2.1 | Guide for the Care and Use of Laboratory Animals (NRC, 1996); |
| 1.2.2 | United States Public Health Service Policy on Humane Care and Use of Laboratory Animals; |
| 1.2.3 | Animal Welfare Act of 1966, as amended (7 U.S.C. 2131 et seq.) and associated regulations (9 C.F.R., subchapter A); |
| 1.2.4 | Procedures set forth in NIH Policy Manual 3044-2, entitled, “Protection of NIH Personnel Who Work with Nonhuman Primates;” |
| 1.2.5 | Defense Federal Acquisition Regulations Supplement (DFARS) and Health and Human Services Federal Acquisition Regulations Supplement (HHSAR) terms, including: |
| 1.2.5.1 | DFARS 252.235-7002 (DEC 91) Animal Welfare; and |
| 1.2.5.2 | HHSAR 352.270-9(b) (JAN 06) Care of Live Vertebrate Animals; and |
| 1.2.6 | All other applicable national, state and local laws associated with the care and use of laboratory animals. |
D-1
| 1.3 | Institutional Animal Care and Use Committee (IACUC) |
| 1.3.1 | Ark shall maintain an Institutional Animal Care and Use Committee or similar animal use review committee as required by the applicable rules and regulations in Section 1.2 (“IACUC”). |
| 1.3.2 | Animal Use shall undergo appropriate review and approval by the IACUC prior to initiation of work under the applicable Project. |
| 1.3.3 | Ark shall provide Achaogen the following within one (1) business day of its availability to Ark: |
| 1.3.3.1 | IACUC approvals and any regular renewal of Animal Use and associated protocols, including without limitation any amendments; and |
| 1.3.3.2 | IACUC-approved modifications, suspensions, and continuing reviews of Animal Use and associated protocols. |
| 1.3.4 | Within two (2) business days of receipt of an Achaogen request, Ark shall provide Achaogen with following documentation related to the IACUC and the Project at any time during the Project or within five (5) years following completion of the Project: |
| 1.3.4.1 | Any draft and final IACUC submissions or correspondence related to a Project; and |
| 1.3.4.2 | IACUC policies and procedures, identified by version number, date, and all required signatories. |
| 1.3.5 | Ark shall notify Achaogen within two (2) business days of any changes to the items in 20.3.4 related to a Project or, if general in nature, that have a material impact on a Project. |
| 1.3.6 | Third-Party Inspections and Certifications |
| 1.3.6.1 | Upon execution of this Agreement, Ark shall provide Achaogen with the following for each facility that may be involved in a Project under this Agreement: |
| (a) | Evidence of current accreditation by the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC); |
D-2
| (a) | Evidence of current certification by the Office of Animal Laboratory Welfare of the United States Public Health Service (OLAW); and |
| (b) | The most recent Facility Inspection Reports issued by the United States Department of Agriculture (USDA) or other applicable national regulatory agency. |
| 1.3.7 | Ark shall provide Achaogen within ten (10) business days of availability to Ark regular renewals or changes to the items in 20.4.1. |
| 1.3.8 | Ark shall notify Achaogen within two (2) business days of the occurrence of any of the following: |
| 1.3.8.1 | Reports to OLAW involving Animal Use regarding: |
| (a) | any serious or continuing noncompliance by Ark (regardless of Facility); |
| (b) | any serious deviation from the provisions of the Guide for the Care and Use of Laboratory Animals by Ark (regardless of Facility); or |
| (c) | any suspension of any Ark activity by the IACUC, or of any IACUC activity. |
| 1.3.8.2 | USDA or OLAW regulatory noncompliance evaluations; and |
| 1.3.8.3 | AAALAC, International status change (gain or loss of accreditation only). |
| 1.4 | Ark Animal Use Documentation, Protocols, and Procedures |
| 1.4.1 | Within two (2) business days of an Achaogen request, Ark shall provide Achaogen with following documentation related to a Project at any time during the Project or within five (5) years following completion of the Project: |
| 1.4.1.1 | Animal use procedures, including, but not limited to the administration of animal observations and health status assessment, interventions and euthanasia criteria, administration of |
D-3
| anesthesia/analgesia/tranquilization or non-pharmaceutical methods for relieving pain or distress or applying restraint; surgery; test article administration and biosampling; assessing and collecting safety data; and any other animal-use activities including in the Project and applicable protocol. |
| 1.4.1.2 | Documentation related to the training and qualifications of Ark’s staff responsible for the conduct of the research including, but not limited to: |
| (a) | Name and title of individual staff members; |
| (b) | Evidence and descriptions of the required training in the protection and handling of animals received by Ark staff members; |
| (c) | Evidence and descriptions of other qualifications or applicable experience of Ark staff members; |
| 1.4.1.3 | Applicable safety documentation and associated plans; |
| 1.4.1.4 | Pharmacy service records on the dosing material to be used and excipients; and |
| 1.4.1.5 | Any other item(s) reasonably related to Animal Use. |
| 1.4.2 | Ark shall notify Achaogen within two (2) business days of any changes to the items in 20.4.1 related to a Project or, if general in nature, that have a material impact on a Project. |
D-4
ATTACHMENT A
BACKGROUND IP
Achaogen Inc. (“Achaogen”) and Ark Diagnostics, Inc. (“Ark”) have entered into the Development Services Agreement (the “Agreement”) between them dated as of August 19, 2013. Capitalized terms used and not otherwise defined in this Attachment A shall have the meanings given to them in the Agreement. In accordance with the Agreement, each Party has identified that its Background IP includes but is not limited to the following.
Achaogen Background IP
• [***]
Ark Background IP
• [***]
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |
Attachment A
page 1
ACHAOGEN INC.
▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇ ▇▇▇
▇▇▇▇▇ ▇▇▇ ▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
August 19, 2013
ARK Diagnostics, Inc.
▇▇▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇
▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Dear ▇▇▇▇▇▇ ▇▇▇▇▇▇:
Reference is made to Section 8(d)(1) of the Development Services Agreement between Achaogen Inc. (“Achaogen”) and ARK Diagnostics, Inc. (“Ark”) dated August 19, 2013 (the “Agreement”), providing that Ark may not object to Third Party Suppliers (as defined therein) listed in a side letter between the parties provided that such Third Party Suppliers have agreed to the requirements of confidentiality and restrictions contained in the licenses provided in Section 8(d).
The parties agree that the following Third-Party Suppliers are to be those identified pursuant to Section 8(d)(1):
[***]
If the foregoing is acceptable to you, please countersign in the place indicated below.
| Sincerely, | ||
| ACHAOGEN INC. | ||
| By: | /s/ ▇▇▇▇▇▇ ▇▇▇ | |
| Name: | ▇▇▇▇▇▇ ▇▇▇ | |
| Title: | VP Finance & Corporate Development | |
| ACCEPTED AND AGREED TO: | ||
| ARK DIAGNOSTICS, INC. | ||
| By | /s/ ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |
| Name: | ▇▇▇▇▇▇ ▇▇▇▇▇▇ | |
| Title: | President & CEO | |
| [***] | Certain information in this document has been omitted and filed separately with the Securities and Exchange Commission. Confidential treatment has been requested with respect to the omitted portions. |

