API MANUFACTURING AND SUPPLY AGREEMENT
Exhibit 10.35
* Portions of this exhibit marked [*] are requested to be treated confidentially.
API MANUFACTURING AND SUPPLY AGREEMENT
This API Manufacturing and Supply Agreement (the “Agreement”) is entered into as of ________, 2015 (the “Effective Date”) by and between Cempra Pharmaceuticals, Inc., a company organized under the laws of Delaware, USA with a place of business at 0000 Xxxxxxxxxx Xxxxx, Xxxxx 000, Xxxxxx Xxxx, XX 00000, XXX (“Cempra”), and FUJIFILM Finechemicals Co., Ltd., a company organized under the laws of Japan with a place of business at 2-3, Higashiyawata 5-chome, Hiratsuka, Xxxxxxxx 000-0000, Xxxxx (“FFFC”). Cempra and FFFC may be referred to herein individually as a “Party”, and collectively as the “Parties”.
Recitals
A.Cempra and FFFC’s Affiliate, Toyama Chemical Co., Ltd. (“Toyama”), are parties to an Exclusive License and Development Agreement dated May 8, 2013 (the “Toyama License Agreement”) and a Supply Agreement dated May 8, 2013 (the “Toyama Supply Agreement”), under which Toyama obtained a license from Cempra to develop and commercialize certain products incorporating the API (as defined below) in Japan and Cempra agreed to supply Toyama with API for development and commercial purposes.
B.Cempra desires to engage FFFC to manufacture and supply to Cempra quantities of API for use in manufacturing solithromycin-based drug products.
C.FFFC possesses or plans to build the necessary facilities, equipment, manufacturing technology, professional expertise, personnel, and capacity to manufacture and supply such API, and desires to undertake such API manufacturing and supply for Cempra under the terms of this Agreement.
D.The Parties intend to conduct certain activities as part of preparing for the commercial manufacture and supply of API, which may include construction of a manufacturing plant, technology transfer, manufacture of registration lots, process development, process validation, stability studies and other pre-approval activities with respect to the manufacture of API under the terms of this Agreement.
Now, Therefore, the Parties hereby agree as follows:
|
1. |
Definitions |
The following capitalized words and phrases when used in this Agreement shall have the meaning provided in this Section 1.
1.1“Acceptance Tests” means the specific tests to be used to determine whether API manufactured by FFFC conforms to the API Specifications, which tests shall be established (and amended from time to time if required) in writing by Cempra in consultation with FFFC.
1
1.2 “Affiliate” means, with respect to a particular Party, any corporation, organization, or other business entity that, directly or indirectly, controls, is controlled by, or is under common control with such Party. The term “control” (including, with correlative meaning, the terms “controlled by” and “under common control with”), as used in this Section 1.2, means the possession of the power to direct, or cause the direction of, the management and business of the applicable corporation, organization, or other business entity, whether through the ownership or control of voting securities (or their voting power) or by contract, or court order, or otherwise.
1.3“API” means the active pharmaceutical ingredient known as solithromycin as further described in Exhibit A of this Agreement (“Solithromycin”).
1.4“API Improvements” means inventions, discoveries know-how or improvements specifically related to API or API Manufacturing Procedures (including analytical methods, manufacturing processes and packaging) that FFFC invents, develops, creates, discovers, conceives, or reduces to practice, in connection with or arising from its activities under this Agreement.
1.5“API Manufacturing Procedures” means the specific methods, techniques, processes and standard operating procedures (including Quality Control Procedures) that are to be used by FFFC (or any of its Affiliates or subcontractors) in manufacturing the API under this Agreement in accordance with the API Specifications.
1.6“API Specifications” mean the specifications, characteristics, qualities and labeling and packaging requirements established by Cempra in writing for API, with which API must conform (including release criteria and associated analytical methods), as such may be amended from time to time under the terms of this Agreement. The API Specifications shall be attached to this Agreement as Exhibit B.
1.7“Applicable Laws” means collectively all laws, regulations, ordinances, decrees, judicial and administrative orders, policies and other requirements of any applicable Regulatory Authority that cover or apply to the manufacture, supply, or distribution of API for use in human pharmaceuticals, including the FD&C Act and the regulations administered by the FDA (including 21 C.F.R. Parts 11, 210 and 211), any equivalent laws, rules, and regulations in the Territory, and the following to the extent not in conflict with any laws or regulations that are issued or enforced by the FDA, MHLW, and other Regulatory Authorities as in effect during the provision of and applicable to API Manufacturing and other services provided by FFFC hereunder: (a) USP/NF/EP and other applicable compendia standards; (b) guidance documents (including Guidelines, Points to Consider, Inspection Technical Guides, International Conference on Harmonization “Step 4 and 5” documents); and (c) cGMP.
2
1.8“Batch” means the total amount of Intermediate or API manufactured in one particular production run conducted by FFFC (or any of its Affiliates or subcontractors) for manufacturing API.
1.9“Batch Record” means, with respect to a particular production run conducted by FFFC for manufacturing one Batch of Intermediate or API, the completed manufacturing records, in the form of an executed Master Batch Record, for such production run containing all the relevant manufacturing details and information for the run, including quality control information and any deviations, and reviewed and approved by Quality Assurance.
1.10“Cempra Licensed Patents” means those Patents in the Territory listed on Exhibit C attached hereto and any Patents in the Territory claiming priority to such Patents that are Controlled by Cempra.
1.11“Certificate of Analysis” means a written document, for example in the form set forth in Exhibit D of this Agreement, which confirms that the quantity of the API manufactured and delivered by FFFC has been tested in accordance with the applicable Acceptance Tests and meets the API Specifications. The Certificate of Analysis will include the results of all Acceptance Tests performed by FFFC or, to the extent permitted by this Agreement, on behalf of FFFC by qualified Third Party subcontractors on the particular Batch of API.
1.12“Certificate of Compliance” means a document, in a form acceptable to Cempra, from FFFC that approves the release of API to Cempra and certifies that the manufacturing and supply of the API has been performed in compliance with all Applicable Laws, including cGMP requirements.
1.13“CMC” means the Chemistry, Manufacturing and Controls sections of any Regulatory Submission (including an IND, DMF, NDA, or equivalent of any of the foregoing in the Territory), as defined by Applicable Laws.
1.14‘“Confidential Information” means all information and know-how and any tangible or intangible embodiments thereof provided by or on behalf of one Party (the “Disclosing Party”) to the other Party (the “Receiving Party”) from time to time either in connection with the discussions and negotiations, whether in written or oral form, pertaining to this Agreement, or in the course of performing under or acting in relation to this Agreement, which may include data, knowledge, practices, processes, ideas, research plans, formulation or manufacturing processes and techniques, scientific, manufacturing, marketing and business plans, and financial and personnel matters relating to the Disclosing Party or to its present or future products, sales, suppliers, customers, employees, investors or business; provided, that, information or know-how of a Party will not be deemed Confidential Information of such Party for purposes of this Agreement if such information or know-how: (a) was already known to the Receiving Party, other than under an obligation of confidentiality or non-use, at the time of disclosure to such Receiving Party, as can be shown by written records; (b) was generally available or known to parties reasonably skilled in the field to which such information or know-how pertains, or was otherwise part of the public domain, at the time of its disclosure to such Receiving Party; (c) became generally available or known to parties reasonably skilled in the field to which such information or know-how pertains, or otherwise became part of the public domain, after its disclosure to such
3
Receiving Party through no fault of the Receiving Party; (d) was disclosed to such Receiving Party, other than under an obligation of confidentiality or non-use, by a Third Party who had no obligation to the Disclosing Party not to disclose such information or know-how to others, as can be shown by written records; or (e) was independently discovered or developed by such Receiving Party, as can be shown by its written records, without the use or benefit of, or reliance on, Confidential Information of the Disclosing Party. Notwithstanding anything to the contrary, (i) all information provided to Cempra, any Affiliate thereof, or any of Cempra’s or its Affiliates’ licensees by or on behalf of FFFC concerning API and (ii) the terms of this Agreement shall each be deemed the Confidential Information of both Parties.
1.15“Control” means, with respect to any intellectual property or right therein, the possession by Cempra of the ability to enable FFFC to practice under such rights in its manufacture of API hereunder as provided for herein without violating the terms of any arrangement or agreements between Cempra (or any Affiliate thereof) and any Third Party
1.16“Cover” means that the use, manufacture, sale, offer for sale, development, commercialization or importation of the subject matter in question by an unlicensed entity would infringe a Valid Claim of a Patent.
1.17“Current Good Manufacturing Practices” or “cGMP” means the then-current standards for the manufacture of pharmaceutical products, pursuant to (a) the FD&C Act; (b) relevant United States regulations in Title 21 of the United States Code of Federal Regulations (including Parts 11, 210, and 211); (c) EC Directive 2003/94 EC of October 8, 2003; (d) the EC Guide to Good Manufacturing Practice for Medicinal Intermediate Products; (e) International Conference on Harmonization (“ICH”) ICH Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients; (f) MHLW Ministerial Ordinance Xx. 000, 0000, XXX Xxxxxxxxxxx Xxxxxxxxx No. 2, 1961, and GMP Guideline for Drugs and Quasi-Drugs (Drug Products) 2005 and (g) all additional Regulatory Authority documents or regulations that replace, amend, modify, supplant or complement any of the foregoing.
1.18“DMF” means a Drug Master File, as provided for in Article 80-6 of Japan’s Pharmaceutical Products and Medical Equipment Law or similar submission to or file maintained with the MHLW or other Regulatory Authority that may be used to provide confidential detailed information about facilities, processes, or articles used in the manufacturing, processing, packaging, and storing of one or more human drugs or APIs.
1.19“Drug Product” means a finished dosage form of human pharmaceutical product containing API as an active pharmaceutical ingredient, alone or in combination with one or more other active pharmaceutical ingredients.
1.20“Facility” means the specific premises of FFFC (or its Affiliates or its subcontractors) where the API is Manufactured, as identified in Exhibit E of this Agreement.
1.21“FDA” means the United States Food and Drug Administration or any successor thereto.
1.22“FD&C Act” means the United States Food, Drug and Cosmetic Act (21 U.S.C. 321 et seq.), as amended from time to time.
4
1.23“FFFC Quality System” means the procedures and control documentation that FFFC has in place at its Facility during the Term that are necessary to evidence compliance with cGMP and all ICH guidelines, as well as any other requirements necessary to Manufacture the API in compliance with all Applicable Laws (including cGMP requirements) and the API Specifications.
1.24“IND” means an investigational new drug application filed with the MHLW, in order to commence human clinical testing of a drug.
1.25“Intermediates” means any of the compounds produced in the intermediate Manufacturing steps beginning with the initial modification of the relevant Raw Material and prior to the completion of final manufacturing steps to produce the API.
1.26“Losses” means any and all judgments, liabilities, losses, costs, damages and expenses (including, without limitation, reasonable attorneys’ fees and legal and court costs) together with any related interest, fines and penalties, resulting from any Claim (as defined below).
1.27“Lot” means one (1) discrete quantity of API as that term is defined under Title 21 of the United States Code of Federal Regulations §210(b)(10), specifically a batch, or a specific identified portion of a batch, having uniform character and quality within specified limits; or, in the case of a drug product produced by continuous process, it is a specific identified amount produced in a unit of time or quantity in a manner that assures its having uniform character and quality within specified limits.
1.28“Manufacture” or “Manufacturing” means the steps and activities conducted to produce the API from Raw Material and/or Intermediate in accordance with the API Manufacturing Procedures and the Master Batch Records, including obtaining all other needed raw materials and reagents, manufacturing steps and processing, packing, labeling, holding, testing, and quality control of the API and/or Intermediates, and actions taken to comply with Applicable Laws with respect to such manufacturing activities (e.g., equipment, methods and operations).
1.29“Marketing Approval” means an approval by MHLW to commence commercial marketing and distribution of the Drug Product for human therapeutic, prophylactic, or palliative use, or comparable approvals or registrations in countries or jurisdictions outside the Territory, including amendments and supplements to such approvals.
1.30“Master Batch Record” means a controlled document specifying the procedures to Manufacture the API or an Intermediate as established by the Parties under Section 2.2, including all applicable API Manufacturing Procedures, the in-process testing and release testing which are to be used in the Manufacture by FFFC hereunder of API.
1.31“MHLW” means Japanese Ministry of Health, Labor and Welfare, or any successor thereto, including the Pharmaceuticals and Medical Devices Agency.
1.32“NDA” means a New Drug Application for Marketing Approval filed in the United States.
5
1.33“Non-Conforming API” means API delivered by FFFC that does not comply with the API Specifications, that is otherwise defective, or that otherwise does not comply with the warranties set forth in Sections 7.4 and 7.5.
1.34“Out of Specification” or “OOS” means failure of API to meet the API Specifications.
1.35“Patents” means any granted patents and pending patent applications, together with all additions, divisionals, continuations, continuations-in-part, substitutions, reissues, re-examinations, extensions, registrations, patent term extensions, revalidations, supplementary protection certificates, and renewals of any of the foregoing, and all foreign applications and patents corresponding to or claiming priority from any of the foregoing.
1.36“Project Manager” means the individuals designated by FFFC and Cempra, respectively, to act as managers for the manufacturing project under this Agreement as provided in Section 3.1.
1.37“Quality Agreement” means the document mutually agreed upon by the Parties, a copy of which shall be attached hereto as Exhibit F, which contains the policies, procedures, and standards by which the Parties will coordinate and implement the operational and quality assurance activities needed to efficiently achieve regulatory compliance objectives, and as such agreement may be amended from time to time by the Parties in writing.
1.38“Product Failure” means (a) as indicated in writing by Cempra to FFFC, no Drug Product will be marketed or further developed by or on behalf of Cempra, any Affiliate thereof, or any of its or their licensees in the Territory or (b) Drug Product is taken off the market or no longer able to be marketed in the Territory by or on behalf of Cempra, any Affiliate thereof, or any of its or their licensees in the Territory for the following reasons: (i) any non-approvable or rejection letter or withdrawal of a Marketing Approval application in the Territory or any order from Regulatory Authority withdrawing Drug Product from the market or otherwise suspending use of Drug Product in the Territory, (ii) any serious safety problem with respect to the Drug Product, or (iii) any infringement of Patents or infringement or misappropriation of other intellectual property right arising from the manufacture, development, use, or commercialization of Drug Product or manufacture of API hereunder, which the terminating Party in reasonably determines in good faith cannot be reasonably and promptly resolved after consultation with the other Party regarding whether the infringement or misappropriation could be cured or remedied via a license or other settlement without material adverse affect on either Party, provided, that in the case of the circumstances described in clauses (i) and (ii), the terminating Party must reasonably determine in good faith that the Drug Product cannot be approved, commercially sold for human therapeutic use, re-launched, or marketed in the Territory, as applicable, within six (6) months of the occurrence of the circumstances originally constituting such Product Failure.
1.39“Raw Material” means the chemicals, compounds, water, solvents, reagents and other materials and supplies, including disposable manufacturing materials and labeling and packaging materials, used in Manufacturing.
6
1.40“Records” means all documents, reports, data, data listings, charts, process control/monitoring commands and data summaries, logs, notes, standard operating procedures, Master Batch Records, lot Batch Records, analyses, correspondences, notes, memoranda, (including, without limitation, production and quality assurance and quality control documentation) and other items containing information or data related to Manufacturing API from the Raw Material and/or Intermediate, whether in paper or electronic form, including originals and copies, and including any other items that would be considered manufacturing “records” under any Applicable Laws.
1.41“Regulatory Approval” means any and all approvals, licenses, registrations, clearances, or authorizations of any national, supra-national, regional, state or local regulatory agency, department, bureau, commission, council or other governmental entity, that are necessary for the commercial manufacture, distribution, use, and sale of a Licensed Product for human therapeutic, prophylactic, or palliative use in a particular jurisdiction, provided that Regulatory Approvals shall exclude pricing and reimbursement approvals
1.42“Regulatory Authority” means any multinational, federal, regional, state and/or local government authority (including public, quasi-public and private bodies contracted, certified or authorized by such governmental bodies) in a country or other jurisdiction with authority to regulate, approve, license, inspect, review or otherwise control or supervise the manufacture, sale, labeling, use, marketing, distribution, import, export, price or reimbursement for API or final Drug Product, including but not limited to the FDA, MHLW, and their counterparts in the European Union and other countries outside the Territory.
1.43“Regulatory Submission” means any document, correspondence, data, article, certifications, or physical samples that are, or that are required to be, delivered or made available for inspection or review by any Regulatory Authority in connection with the activities carried out by either Party relating to this Agreement, including applications, dossiers or reports supporting the manufacture, use, sale, or marketing of the API or Drug Product for investigational or commercial use, and including but not limited to any INDs, NDAs, applications for Marketing Authorizations, field reports, annual reports, adverse event and corrective action reports, and export approvals, change being effected reports, communication (e.g., meeting packages, teleconference, written correspondence) with any Regulatory Authorities and any amendments, supplements, corrections, and updates to any of the foregoing.
1.44“Territory” means Japan.
1.45“Third Party” means any party other than FFFC, Cempra, or an Affiliate of either of the foregoing.
1.46“Transfer Price” means the price charged by FFFC for the quantities of API delivered to Cempra to fill a Purchase Order submitted by Cempra, as provided in Section 4.1.
1.47“Valid Claim” means a claim of any pending patent application or any issued, unexpired or granted patent that has not been dedicated to the public, disclaimed, abandoned or held invalid or unenforceable by a court or other body of competent jurisdiction from which no further appeal can be taken, and that has not been explicitly disclaimed, or admitted in writing to
7
be invalid or unenforceable or of a scope not covering a particular product or service through reissue, disclaimer or otherwise.
1.48“Waste” means all hazardous waste, as defined by Applicable Laws, and all non-hazardous waste to the extent, in each case, arising out of Manufacturing and other activities performed by FFFC under this Agreement, including without limitation, rejected or unusable Raw Materials, Intermediates, or API, disposable manufacturing equipment, and materials (including solvents and other consumables).
1.49Miscellaneous Interpretation Aids.
(a)Each use in this Agreement of the term “including,” “comprising,” or “containing” (or a variant form thereof) shall be understood to have an open, non-limiting meaning. Thus, e.g., “including” shall be interpreted as meaning “including without limitation” or “including but not limited to,” regardless of whether the words “without limitation” or “but not limited to” actually follow the term “including.” Similarly, the terms “such as,” “for example,” and “e.g.” shall be understood as referring to non-limiting illustrations or examples.
(b) “Herein,” “hereby,” “hereunder,” “hereof,” and other equivalent words shall be understood as referring to this Agreement in its entirety, and not solely to the particular provision or portion of this Agreement in which any such word is used.
(c)Wherever used herein, any pronoun or pronouns shall be understood to cover all genders.
(d)All references to days, months, quarters, or years shall be understood to refer, respectively, to calendar days, calendar months, calendar quarters, or calendar years, unless otherwise indicated.
(e)Any reference to a supranational, national, federal, state, local, or foreign statute or law shall be understood to refer to the applicable version of the law or statute then in force (as it may have been amended or superseded) as well as all rules and regulations promulgated thereunder, unless the context requires otherwise.
(f)All references to “dollars,” “Dollars,” “US$,” or “$” shall mean United States dollars.
|
2. |
Manufacture and Supply of API |
2.1Obligation to Supply and Purchase. Pursuant to the terms of this Agreement, FFFC shall supply Cempra with, and Cempra shall purchase, the API, for use as the active pharmaceutical ingredient in human drug product to be used or sold in the Territory, in such quantities as Cempra may order pursuant to the provisions in of Section 2.6. FFFC shall deliver API in the quantity specified in each Purchase Order by Cempra as set forth in Section 5.4(b) and on the delivery date as specified by Cempra on such Purchase Order, or store such API as may be requested by Cempra for later delivery in accordance with Section 5.4(b)(ii). All manufacturing and storage of API under this Agreement shall be performed at the Facility, unless otherwise agreed to in writing by Cempra.
8
2.2Master Batch Records. The API Manufacturing Procedures contained in the Master Batch Records shall, except to the extent based on or reflecting methods, techniques, processes and standard operating procedures covered by Cempra Licensed Patents or Cempra’s know-how related thereto provided by Cempra to FFFC, be based upon applicable FFFC technology, and any applicable API Improvements. The Master Batch Records shall contain such items and requirements as typical and customary in the industry for manufacturing processes applicable to similar bulk pharmaceutical manufacturing, and shall be set forth in a written document. The API will be Manufactured to the then-current API Specifications at the time of manufacturing. If appropriate during the Term (such as, to include new API Improvements that are useful to Manufacturing the API), the Parties will agree on appropriate amendments or modifications to the API Specifications and/or the Master Batch Record. The details of the procedure for amending the API Specifications and/or the Master Batch Records shall be as specified in the Quality Agreement. FFFC will, at its cost, provide all documents required under the Quality Agreement or this Agreement to be provided to Cempra, in English. FFFC shall not have any obligations to disclose any information maintained in the DMF as confidential to Cempra or any Third Parties, provided that, if (i) either Party or any Affiliate is required by Applicable Law or to satisfy any obligation thereunder, (ii) either Party or any Affiliate thereof is requested by a Regulatory Authority, or (iii) it is reasonably necessary to satisfy any requests of any Regulatory Authority, in the case of (i), (ii) or (iii), to disclose any information maintained in the DMF as confidential, (X) Cempra and FFFC shall promptly use reasonable efforts to, as quickly as possible, determine the reasonable plan for satisfying such requests by mutual good-faith and reasonable consultation based on the careful study of confidentiality of such information maintained in the DMF and (Y) FFFC shall in any event be required to disclose such information if and as reasonably necessary to satisfy, or enable Cempra, any Affiliate thereof, or any licensee or sublicensee of either of the foregoing with respect to Drug Product in the Territory to satisfy, any such requests or requirements. For clarity, in this case, such information maintained in the DMF which is disclosed hereunder shall be used and disclosed only to the extent necessary for any such requests or requirements, and shall not be used or disclosed to any other party exceeding the scope necessary for any such requests or requirements.
2.3Registration Batches. Upon Cempra’s request, FFFC shall prepare registration Batches of API as needed for Cempra (or its Affiliate or its or its Affiliates’ licensee) to seek Regulatory Approval in the Territory, in accordance with a plan therefor (and related payment provisions) to be reasonably negotiated in good faith and agreed upon in writing by the Parties (which plan, upon such agreement in writing by the Parties, will be set forth in Exhibit G), and coordinate with Cempra on any request from any Regulatory Authority. Upon successful delivery of the registration Batches in accordance with such plan (i.e., such Batches meet the API Specifications and are manufactured in a way that they meet the criteria for registration Batches), Cempra shall pay FFFC for the delivery of such Batches in accordance with the payment provisions to be set forth in Exhibit G. FFFC shall work in good faith to establish, in consultation and cooperation with Cempra, and subject to Cempra’s written agreement, reasonably appropriate success criteria for the registration Batches for API. If a particular registration Batch supplied by FFFC pursuant to this Section fails to meet such criteria, appropriate representatives from each Party shall meet and discuss and seek to determine the causes of such Batch having failed to meet such criteria and shall cooperate diligently to try to find a solution to such causes, and FFFC shall use best efforts to rectify any such problems as soon as practicable. FFFC will replace any such failed Batches at its cost (including paying for needed Raw Materials and the internal costs of
9
conducting the manufacturing and supply). FFFC shall recommence manufacture and supply of the required registration Batches for API as soon as possible, and shall continue until such time as FFFC has successfully delivered to Cempra the number of consecutive Batches of API that meet the criteria that shall be set forth in Exhibit G, once agreed upon by the Parties as set forth above. Each such registration Batch supplied by FFFC shall meet the API Specifications and shall be suitable for use to support registration stability studies.
2.4Manufacturing Process Validation. Promptly after the Parties have completed the Master Batch Records, and at Cempra’s request, FFFC will commence and conduct certain validation studies (the “Validation Studies”) to validate the API Manufacturing Procedures pursuant to a mutually agreeable validation plan, in preparation for commercialization, to be reasonably negotiated in good faith and agreed upon in writing by the Parties (which plan, upon such agreement in writing by the Parties, will be set forth in Exhibit H). The actual detailed protocols for such Validation Studies shall be established by the FFFC, in consultation with, and subject to the written agreement of, Cempra, with FFFC preparing the initial proposed protocols for review and comment by Cempra and written approval by Cempra. FFFC shall disclose to Cempra in written reports all results of such Validation Studies and all other deliverables as required under the mutually-agreed upon plan for such Validation Studies. Notwithstanding the foregoing, unless otherwise agreed by FFFC, such protocols for the Validation Studies and such reports shall not contain any information of FFFC included as confidential in the DMF maintained by FFFC under this Agreement. Cempra shall pay FFFC as provided in the form of Exhibit H to be agreed upon for FFFC’s conduct of the Validation Studies. In the event that the Validation Studies are not successfully completed (i.e., they do not satisfy the predefined acceptance criteria in the validation protocol and related site SOPs), FFFC shall work cooperatively with Cempra using commercially diligent efforts to determine the cause of the failure, and shall work diligently and, as soon as possible, implement such changes in the Facility or as otherwise needed to assure that the Validation Studies are successfully completed. Each such validation Batch supplied by FFFC shall meet the API Specifications and shall be suitable for human clinical trial use and/or commercial use in humans, as applicable. FFFC shall use reasonable efforts to work in good faith with Cempra to obtain appropriate Marketing Approvals as needed.
2.5Stability Studies and Report. To the extent requested by Cempra in writing, FFFC shall conduct stability studies on the API manufactured by FFFC hereunder, in accordance with stability study protocols customary, reasonable, and typical for pharmaceutical manufacturing (e.g., ICH) to be negotiated in good faith and agreed upon by the Parties as soon as reasonably possible following the Effective Date, and which, upon mutual written agreement thereon by the Parties, shall be set forth on Exhibit I hereto. FFFC shall prepare and deliver to Cempra written reports setting forth the results of the studies, such reports to be in the form and at the time points described in such agreed protocols.
10
2.6Forecasts; Purchase Orders; Minimum Purchase Requirement.
(a)No later than the eighth (8th) day of each calendar month following the Effective Date, Cempra shall provide to FFFC a rolling forecast (each, a “Forecast”) of its anticipated orders for API to be placed during each of the [*] through (and including) the [*] calendar month (or, if earlier, the final calendar month of the Term) following the calendar month in which such forecast is provided. In each Forecast submitted by Cempra, the forecast for the first [*] months covered by the Forecast shall be binding on the Parties (pursuant to Purchase Orders placed under subsection (b) below), and the forecast for the last [*] months covered by each Forecast shall be non-binding on both Parties, not subject to any forecasting restrictions, provided that the quantity of API specified for any month in the nonbinding portion of any revised Forecast shall not (i) exceed [*] percent ([*]%), or be less than [*] percent ([*]%), of the quantity of API provided for such month in the initial Forecast including a forecast quantity of API for such month nor (ii) exceed [*] percent ([*]%), or be less than [*] percent ([*]%), of that quantity of API provided for such month in the most recent previous Forecast. FFFC shall notify Cempra in writing within three (3) business days of FFFC’s receipt of any Forecast if the quantities of API indicated in the non-binding portion thereof exceed FFFC’s production capacity therefor. Should Cempra wish to increase order quantities at any time in excess of the volumes permitted under this Section 2.6(a) or Section 2.6(b), Cempra may contact FFFC to request and FFFC shall use commercially reasonable efforts to supply any such increase in volumes.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
11
(b)Cempra shall have the right to place binding written purchase orders (each a “Purchase Order”) from time to time under and subject to the conditions regarding a Forecast set forth in Section 2.6(a), provided that, with respect to the first [*] Purchase Orders placed hereunder, Cempra may, at its option, place its Purchase Orders for API no less than [*] months in advance of the desired delivery date(s) (or, as contemplated by Section 5.4(b)(ii), storage date) therefor. Except as described above with respect to the first [*] Purchase Orders placed hereunder, Cempra shall issue each Purchase Order to FFFC no later than [*] calendar month preceding the date on which Cempra has requested FFFC to first deliver (or make available for storage pursuant to Section 5.4(b)(ii)) API pursuant to each such Purchase Order. Each Purchase Order shall specify the API to be supplied during the period from the [*] business day of the following calendar month to the end of the [*] calendar month of the period for which the Purchase Order is placed (corresponding to the binding portion of each Forecast). Such Purchase Order shall specify order quantity(ies), delivery (and/or storage) date(s), and other necessary matters. FFFC shall be obligated to supply to Cempra the amount of API as Cempra orders hereunder, which shall not be less than [*] ([*]%), nor more than [*] percent ([*]%), of the forecasted quantity for the applicable forecast period in the most recent Forecast, and will use commercially reasonable efforts to supply any additional quantities ordered by Cempra. Not later than five (5) days after receipt of a binding Purchase Order, FFFC will confirm in writing its receipt of the Purchase Order (“Order Acceptance”), and FFFC shall fulfill each Purchase Order. If there is any conflict between the Purchase Order or an Order Acceptance and the terms of this Agreement, this Agreement prevails and such conflicting terms are rejected and of no effect, unless the Parties mutually agree otherwise in writing. From time to time, due to significant unforeseen circumstances, Cempra may deliver to FFFC a Purchase Order for volumes of API in excess of those specified in the binding portion of any Forecast and, upon Cempra’s written request, FFFC shall use commercially reasonable efforts to provide Cempra with such excess API volumes; provided, however, that if FFFC is required to spend an additional unforeseen material expense, in excess of its typical expenses to supply API hereunder, in order to provide Cempra with such excess API volumes, (i) FFFC shall notify Cempra as soon as possible of the amount of such additional expense, (ii) Cempra shall have ten (10) business days following such notice within which to accept such additional expense in writing, and (iii) if Cempra does accept such additional expense in writing within such ten (10) business day period, Cempra shall be obligated to bear such expense upon and FFFC shall be obligated to supply such excess API volumes triggering such expense. If Cempra rejects such additional expense in writing, or otherwise does not accept such additional expense, within such ten (10) business day period, Cempra shall have no obligation to bear any such additional expense with respect to any excess API volume and FFFC shall not have any obligation to supply any such excess API volume.
(c)FFFC, on at least a quarterly basis, shall provide Cempra with a written schedule of all then-outstanding accepted Purchase Orders for API, including the status of manufacturing work in progress and expected delivery date(s).
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
12
(d)Cempra agrees that, for each of the first [*] Month Periods (as defined below) during the Term, Cempra shall be obligated to place Purchase Orders for the purchase of not less than [*] kilograms ([*] kg) of API in total per Month Period (i.e., such minimum purchase obligation shall apply during each of the first [*] Month Periods, and so Cempra’s minimum purchase obligation of this Agreement shall be its purchase of not less than [*] kilograms ([*] kg) of API in total during such first [*] Month Periods), provided that, notwithstanding the foregoing:
(i)such obligation shall not apply to the Month Period during which this Agreement terminates or expires unless such termination or expiration occurs on the last day of such Month Period; and
(ii)the requirement above shall not apply to a Month Period during which a Supply Failure occurs or the Month Period following such Supply Failure.
For purposes of clarity, the minimum purchase requirement set forth in this Section 2.6(d) shall, subject to clause (i) and (ii) above, only apply for up to the first (1st) [*] Month Periods as described above, and Cempra’s breach of such minimum purchase requirement shall be construed as a material breach enabling termination of this Agreement by FFFC as set forth in Section 10.2 (b). “Month Period” means (y) the twelve (12) consecutive complete calendar month period, following (a) successful completion of the Validation Studies pursuant to Section 2.4 and (b) written notice from FFFC that the Facility is completed and capable of Manufacturing [*] kg in the course of a twelve (12) consecutive month period, beginning with (and including) the first calendar month during which API is delivered, in accordance with a Purchase Order placed pursuant to Section 2.6(b), for use in the manufacture of Drug Product for commercial sale for human therapeutic use in Japan following Regulatory Approval of such Drug Product in Japan and (z) each subsequent twelve (12) consecutive calendar month period following the initial Month Period. Except to the extent otherwise agreed to in a separate written agreement between the Parties that shall not affect the terms of this Agreement, the Parties agree that FFFC shall not deliver any such API, pursuant to any Purchase Order placed by Cempra, prior to such initial delivery contemplated in clause (y) of the preceding sentence (i.e., prior to the first month of the first Month Period).
2.7Use of Affiliates or Subcontractors. FFFC shall have the right to fulfill its supply obligations hereunder through the engagement of any of its Affiliates or subcontractors, provided that the engagement of any such Affiliates or subcontractor shall be subject to the prior written approval of Cempra (such approval shall not be unreasonably withheld by Cempra). FFFC shall ensure that any Affiliate or subcontractor performing any obligations of FFFC hereunder agrees to be bound by the terms and conditions of this Agreement pertaining to the manufacture and supply of the API as if it is a party to this Agreement. FFFC shall remain fully responsible for its obligations under this Agreement, and the acts and omissions of its Affiliates and subcontractors with respect to this Agreement (as if such acts and omissions were those of FFFC hereunder), regardless of whether such obligations are performed by FFFC itself or through such Affiliate or subcontractor.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
13
2.8Raw Materials and Equipment. FFFC shall procure, at its own cost, all Raw Materials needed for Manufacturing the API ordered under this Agreement, provided that, if and as requested by Cempra, FFFC shall use reasonable efforts to consult in good faith with Cempra regarding the manner in which, and the Third Parties from which, any critical Raw Materials (e.g., clarithromycin) may be procured. FFFC shall conduct further audits of such Third Party vendors as needed or as reasonably requested by Cempra. FFFC shall be responsible for ensuring that the Raw Materials procured by FFFC in accordance with this Section 2.8 meet the quality requirements as set forth in the Quality Agreement. FFFC shall be responsible for procuring at its cost all equipment, personnel and other resources needed for Manufacturing and/or storing, as applicable, API ordered under this Agreement. FFFC shall be responsible for allocating appropriate space in the Facility, and for obtaining, installing and maintaining in such Facility all capital equipment, as needed to manufacture and/or store, as applicable, the amounts of API as ordered by Cempra in compliance with the terms of this Agreement. FFFC shall allocate sufficient time, effort, equipment and facilities to the program for manufacturing API, and shall dedicate and use personnel with sufficient skills and experience as are required to accomplish the manufacturing tasks, so as to manufacture and deliver API on a timely basis and in accordance with the terms of this Agreement. FFFC shall conduct its Manufacturing efforts and perform all of its other obligations under this Agreement in compliance with all Applicable Laws.
2.9Labeling and Packaging. FFFC shall label and package API to be supplied in accordance with the API Manufacturing Procedures, API Specifications (or other labeling and packaging specifications provided by Cempra), the Quality Agreement, and Applicable Laws, in each case that are applicable to active pharmaceutical ingredients for human use for shipment in bulk to Cempra or to one or more locations (e.g., manufacturing sites, distribution centers) designated by Cempra.
2.10Title to API. Title to all API shall remain with FFFC until it is delivered pursuant to Section 5.4 or stored on behalf of Cempra pursuant to Section 5.4(b)(ii). FFFC shall keep all Raw Material, Intermediates, and API stored in accordance with the API Specifications and Applicable Laws. FFFC shall bear the risk of loss, contamination or damage to the Raw Material, Intermediates, and API in its possession (including during such time as FFFC may be storing API on behalf of Cempra pursuant to Section 5.4(b)(ii)), until the finished API is actually delivered to Cempra or its designee pursuant to Section 5.4, and FFFC will pay the actual costs of replacing any Raw Material that is lost or damaged while in FFFC’s possession due storage or handling problems or losses or failures in Manufacturing or storage; provided, however, that in case that FFFC delivers API pursuant to Section 5.4 and enable Cempra to receive the same but Cempra fails to pickup, the risk of loss, contamination or damage to the Raw Material, Intermediates, and API shall pass to Cempra at the time of such delivery, except to the extent otherwise set forth in this Agreement, including but not limited to Section 5.4 hereof.
14
Limitation on Use; Supply for Outside the Territory. Cempra shall use reasonable efforts to ensure that API supplied hereunder is only used for the manufacture of Drug Product for use or sale in the Territory (or purposes related thereto). If Cempra desires FFFC to supply Cempra with API for use in manufacturing Drug Product for use or sale outside the Territory (within FFFC’s reasonable production capacity), the Parties shall, upon written notice from Cempra to FFFC, use reasonable efforts to negotiate in good faith an agreement, or amendment to this Agreement, providing for such supply on commercially reasonable terms.
|
3. |
Project Management |
3.1Project Managers. Each Party shall designate a representative (the “Project Manager” of such Party) with proper experience and authority as to technical matters to serve as the primary contact with the other Party regarding the Parties’ manufacturing and supply relationship for API under this Agreement. Each Project Manager shall be responsible for obtaining cooperation and input from other individuals within such Project Manager’s organization whose expertise and ability may be required from time to time to maximize the potential for successful relationship under this Agreement. The Project Managers shall develop procedures to optimize communication and collaboration between the Parties. The Project Managers will communicate regularly during the Term at mutually agreeable times, and, when necessary, hold meetings at mutually agreeable places, to review project management and status. The Project Managers shall use good faith, reasonable efforts to facilitate communication and collaboration between the Parties, but neither Project Manager shall have the ability or authority to modify the terms of this Agreement, to bind either Party, or to waive any rights or obligations of a Party.
3.2Monthly Progress & Budget Reports. Each calendar month (or on such other regular period as agreed by the Parties), FFFC shall provide Cempra with a status report on completion of outstanding obligations (e.g., production runs, process development, validation, stability data, Regulatory Submissions, and pending corrective actions). The status report shall indicate FFFC’s progress toward task or delivery milestones relative to planned completion schedules.
3.3Adverse Issues & Corrective Actions. FFFC shall inform Cempra promptly in writing of any events that might materially affect the ability of FFFC to timely and fully perform and/or deliver API ordered by Cempra under this Agreement, or otherwise affect the established schedule, including any unexpected adverse final or interim results or data from validation, stability or other studies. The status report also shall fully describe all Out of Specification (“OOS”) and out of trend events, failure investigations, process deviations, Batch failures and similar matters, as well as the corrective or other actions to be taken by FFFC. FFFC shall conduct periodic review of production records, on at least an annual basis, including trend analysis of Batch production records and other process data, and prepare a report for submission to Cempra summarizing FFFC’s findings, conclusions and recommendations. FFFC shall be responsible for ensuring that the adverse issues and corrective actions undertaken with respect thereto by FFFC in accordance with this Section 3.3 meet the quality requirements as set forth in the Quality Agreement.
15
4.1Transfer Prices. The Transfer Price for a particular shipment of API that is manufactured and supplied to Cempra by FFFC under this Agreement shall be equal to the total number of kilograms in such shipment, multiplied by the per-kilogram Transfer Price as set forth in the transfer price schedule and further otherwise determined as set forth on Exhibit J. Without any delay after the end of each Month Period, FFFC shall send Cempra a written report stating the Forecast-Based Price for such Month Period (or, if adjusted by mutual agreement of the Parties as contemplated by Exhibit J, the applicable Forecast-Based Prices for such Month Period and the volumes of API to which such Forecast-Based Prices applied) and the Final Price for such Month Period, and including a detailed calculation thereof. If the total amount that would have been owed or paid to FFFC for all API delivered during a particular Month Period meeting the API Specifications and accepted by Cempra in accordance with this Agreement (such Month Period’s “Accepted API”) had the Final Price been applicable thereto exceeds the total amount owed or paid to FFFC for all of such Month Period’s Accepted API based on the applicable Forecast-Based Price(s) therefor, then FFFC shall send Cempra an invoice for the amount of such excess and, within [*] days following Cempra’s receipt of such invoice, Cempra shall pay FFFC an amount equal to such excess. If the total amount owed or paid to FFFC for all of a particular Month Period’s Accepted API based on the applicable Forecast-Based Price(s) therefor exceeds the total amount that would have been owed or paid to FFFC for all of such Month Period’s Accepted API had the Final Price been applicable thereto, then FFFC shall pay Cempra an amount, or credit Cempra an amount against outstanding invoices or future amounts due under this Agreement, as elected in writing by Cempra in its sole discretion (with such payment to be made within [*] days, and such credit to become immediately effective, following such election by Cempra), equal to, in either case, such excess.
4.2Payments. Subject to any additional payments required of, or credits granted to Cempra under, Section 4.1, FFFC shall be paid for API meeting the API Specifications delivered and accepted in accordance with this Agreement within [*] days of receipt by Cempra of the applicable invoice setting forth the total Transfer Price applicable to such delivered API, as provided in Section 4.1. Subject to Section 5.5, payment of all undisputed invoices shall be delivered by wire transfer in US Dollars to the account provided in Exhibit J. Payment shall be considered received once funds become available to FFFC, or FFFC’s agent, at its bank account. In the case one invoice is in dispute, its payment shall not affect settlement of other outstanding and due invoices.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
16
4.3Access to Funds Received for Cempra’s Sale of API. Cempra and FFFC shall use reasonable efforts to work in good faith to establish, on commercially reasonable and customary terms of a separate agreement to be negotiated by the Parties and an internationally-recognized bank reasonably acceptable to both parties, a bank account in Cempra’s name and owned by Cempra into which Cempra will deposit payments received by Cempra from Toyama or other licensees or distributors of Cempra who commercialize Drug Products in the Territory, if any, for Toyama’s or such licensee’s or distributor’s purchases of API, acquired by Cempra from FFFC under this Agreement, sold to Toyama or such licensee or distributor by Cempra, and from which FFFC shall be entitled to withdraw the amount due FFFC for such API under this Agreement, and for which Toyama or such licensee or distributor has already paid Cempra, upon presentment to Cempra and the applicable bank of invoices therefor properly sent in accordance with this Agreement; provided, however, that Cempra shall use reasonable efforts to maintain and keep up the level of the amount of deposit in such bank account reasonably sufficient for the payments to FFFC of undisputed amounts due under Section 4.2 of this Agreement.
4.4No Liens. FFFC shall ensure that all API ordered by Cempra is delivered free of any liens, claims, or encumbrances, with good and marketable title.
|
5. |
Quality Control, Delivery And Acceptance |
5.1Quality Control. FFFC shall maintain and follow a quality control and quality assurance testing program consistent with the API Specifications, cGMP, the Quality Agreement, and all other requirements of Applicable Laws and consistent with industry standards (the “Quality Control Procedures”), which shall include performing the applicable Acceptance Tests on each Batch of API. FFFC shall ensure that all API supplied to Cempra hereunder shall be manufactured in accordance with the API Manufacturing Procedures, cGMP, the Quality Agreement, and all other Applicable Laws, and all other applicable requirements of Regulatory Authorities, (collectively, “Regulatory Standards”) and shall comply with the API Specifications. FFFC shall promptly notify Cempra of any deviation from API Manufacturing Procedures or the Regulatory Standards that occurs during any Batch process or Manufacturing or any Batch failure, including the details and causes thereof, to meet the quality requirements as set forth in the Quality Agreement.
5.2Certificates. FFFC shall provide to Cempra, accompanying each delivery of API: (a) the Batch number and Purchase Order number of the delivered API, (b) a completed and accurate Certificate of Analysis as to such Batch, and, upon Cempra’s request, copies of the analytical data used to generate such Certificate of Analysis, and (c) copies of all other documentation required for API release. Cempra or its Affiliate or designee may, but shall not be obligated to, test each amount of API delivered to confirm that it meets the API Specifications, that the assigned expiry/retest aligns with agreed upon period, and that the API otherwise complies with the terms of this Agreement.
5.3Quality Audits. FFFC shall maintain all quality control documentation and Acceptance Test results for each Batch of API for a period and in a manner consistent with Regulatory Standards, the Quality Agreement, and pharmaceutical industry standards. Cempra, its Affiliates, and any designees or licensees of Cempra or any Affiliate thereof may, from time-to-time, and at any time, periodically review, upon reasonable prior notice, such
17
documentation and results, and shall have the right, from time-to-time, and at any time, to audit, survey, verify the adherence of FFFC to the Quality Control Procedures and Regulatory Standards. In addition to the above and to Cempra representatives provided for in Section 8.13, and upon reasonable prior written notice to FFFC, Cempra, its Affiliates, and any designees or licensees of Cempra or any Affiliate thereof shall have the right, from time-to-time, and at any time, to have its representatives visit the Facility to audit or inspect the aspects of the Facility related to Manufacturing (including testing) of API and to discuss quality issues and any related issues with FFFC’s manufacturing and management personnel as relating to Manufacture of API. Except to the extent otherwise set forth in this Agreement, such audits or inspections shall not be limited in number or frequency, occur during regular business hours, and meet the quality requirements as set forth in the Quality Agreement. Audit report responses shall be provided as agreed upon by the Parties, and the Parties shall each use reasonable efforts in good faith to ensure completion of the action items. Follow up visits may, as reasonably determined by Cempra, be needed to confirm completion of action items and, in such cases, FFFC shall permit such visits and reasonable times upon reasonable notice. For critical observations, Cempra shall be permitted to assess impact to any product or filing documentation.
5.4Delivery of API.
(a)Release Testing. FFFC shall be responsible for analyzing each API lot for compliance with the API Specifications and for conducting all testing required prior to the release of any API for shipment as provided in this Section 5.4. FFFC shall send to Cempra a Certificate of Analysis and a Certificate of Compliance prior to or concurrent with each shipment of API. FFFC shall retain all Records necessary to fulfill the requirements established by cGMP and all other Applicable Laws. Prior to changing its testing methods, FFFC shall inform Cempra of such changes in writing and obtain Cempra’s written approval, including as set forth in the Quality Agreement.
(b)Shipment, Storage, and Delivery.
(i)Each amount of API to be delivered to Cempra shall be delivered by FFFC EXW (Incoterms 2010) at the Facility to Cempra’s designated carrier or shipper for shipping to Cempra’s, its Affiliate’s, or its or its Affiliates’ licensee’s designated manufacturing or storage facility, or to such other location as specified by Cempra. Cempra shall arrange for such shipping. FFFC shall be responsible for delivering the properly-packaged API to Cempra’s designated carrier or shipper. Deliveries of API under this Agreement (including the date on which API, initially being stored on behalf of Cempra pursuant to Section 5.4(b)(ii) following manufacture, shall be made available for shipment, as requested by Cempra) shall not vary by more than five (5) calendar days from the specified delivery date set forth in the applicable Purchase Order (or such later date as may be requested by Cempra for pick-up following storage of API on behalf of Cempra pursuant to subsection (ii) below) (i.e., may be between five (5) calendar days before the specified delivery date and five (5) calendar days after the specified delivery date). Such variance in actual date of delivery shall not constitute a breach of contract by FFFC. All risks of loss and all normal transport costs that occur after proper delivery by FFFC to the carrier or shipper shall be borne by Cempra. API shall be shipped in accordance with the shipping conditions and procedures established by this Agreement and written agreement of the
18
Parties. Each lot of API shall be accompanied by all required shipping documentation including the Certificates of Analysis and Certificate of Compliance.
(ii)Notwithstanding anything to the contrary, if Cempra notifies FFFC in writing, in conjunction with, as part of, or following the placement of, a particular Purchase Order that Cempra’s designated carrier will not be picking up all API ordered under such Purchase Order on the initial date on which the relevant API will be ready for delivery and requests storage thereof by FFFC, FFFC shall, with respect to such portion of any Purchase Order, store such API at the Facility in accordance Applicable Laws, the API Specifications, and cGMP, until such date as Cempra requests that such API be delivered EXW (Incoterms 2010) at the Facility to Cempra’s designated carrier or shipper for shipping pursuant to Section 5.4(b)(i). The reasonable, documented, direct cost incurred in connection with FFFC’s storing of such API under this Section 5.4(b)(ii) shall be borne by FFFC for the first month’s storage of each delivery of API following the initial delivery date thereof, and by Cempra for all periods thereafter; provided, however, that, notwithstanding the foregoing, such reasonable, documented, direct cost incurred in connection with FFFC’s storing of such API for all periods prior to the end of the second (2nd) Month Period shall in any event be borne by FFFC. In the event of such a notice and/or request by Cempra, Cempra shall be required to pay for such API as if it had been so delivered on the original intended delivery date therefor, and title to such API shall pass to Cempra upon such date, and risk of loss with respect to such API (and the obligation to insure against such loss) shall also pass to Cempra on such date, except to the extent that such loss of such API occurring during such period when FFFC stores such API at a Facility hereunder results from FFFC’s negligence, intentional misconduct, breach of this Agreement, or failure to comply with Applicable Laws, the applicable standards (which shall in any event include, but not be limited to, cGMP), or the applicable storage conditions for such API, which risks of loss shall be borne by FFFC. The Parties agree that any API that is stored in accordance with the foregoing pursuant to Cempra’s request or notice shall be included in determining the applicable Transfer Price.
5.5Acceptance/Rejection. Cempra (or its authorized representative or designee (which may include any of its Affiliates or its or its Affiliates’ licensees)) will inspect all deliveries of API (which, for API stored on Cempra’s behalf under Section 5.4(b)(ii), shall mean delivery following such storage, not at the time of storage) and Cempra will report to FFFC any Non-Conforming API that is reasonably discoverable by reasonable visible inspection within forty-five (45) days of receipt by Cempra, its Affiliate, its or its Affiliate’s licensee, or any of the foregoing’s designated manufacturer of Drug Product. If any API is found to be Non-Conforming API, then FFFC shall, at Cempra’s request and solely at its option (to be exercised by Cempra promptly), either: (a) replace said Non-Conforming API as soon as practically possible at no charge to Cempra and pay all round-trip shipping charges to and from the destination of the original shipment, (b) refund to Cempra the purchase price paid to FFFC for the Non-Conforming API, or (c) credit Cempra’s account in an amount equal to the purchase price paid for said Non-Conforming API. FFFC shall reimburse Cempra for the reasonable costs incurred by Cempra in properly disposing of any such Non-Conforming API. Any notice given hereunder shall specify the reason why such API was found to be Non-Conforming API. If Cempra does not report any defect or non-conformity of any API within forty-five (45) days of receipt by Cempra, then Cempra shall be deemed to have accepted such API, provided that, notwithstanding anything to the contrary, (a) Cempra shall have the right to rely on the data provided by FFFC in the Certificate of Analysis and the Certificate of Compliance for such inspection, and shall have the right to reject
19
such shipment at a later time for any inaccuracy in the Certificate of Analysis or Certificate of Compliance, and (b) if there is any latent defect that is not reasonably discoverable upon reasonable visual inspection in accordance with customary and reasonable procedures that causes such API to be Non-Confirming API, then Cempra may reject such Non-Conforming API within ten (10) business days of Cempra becoming aware of such latent defect.
5.6Dispute Regarding Rejection. If the Parties disagree as to whether a particular delivery of API contains Non-Conforming API, an independent and mutually acceptable independent, neutral Third Party arbitrator will be appointed to (a) review data that are in question and/or (b) to oversee the evaluation and testing of a sample of such purportedly Non-Conforming API at an independent, neutral referee laboratory. Such referee laboratory will conduct testing in accordance with the methods established for testing as set forth in the API Specifications. The costs of the referee testing will be charged to the Party whose position in the dispute was not supported by the referee’s findings. FFFC, if at fault, shall be solely responsible for the prompt replacement of all amounts of Non-Conforming API, or at Cempra’s election, FFFC shall refund the amounts paid or incurred by Cempra on account of the delivery of such Non-Conforming API (if previously paid for).
|
6. |
Supply Assurances |
6.1Production Site and Commercial Capacity Assurance. All Manufacturing of API (including all testing, filing and packaging activities) shall occur at the Facility, except as otherwise approved by Cempra in writing. No Manufacturing work shall be subcontracted to or performed by any Affiliate of FFFC or Third Party except with Cempra’s prior written approval (however, such approval shall not be unreasonably withheld by Cempra). If Cempra approves of any subcontracted Manufacturing or Manufacturing by an Affiliate of FFFC, FFFC shall be and remain fully responsible for the work of the subcontractor or Affiliate as if it was performed by FFFC directly.
6.2Change Control. Without Cempra’s prior written consent, FFFC shall make no change to any part of the API manufacturing process, including: (i) the API Manufacturing Procedures; (ii) any validated analytical methods used to test critical Raw Materials, Intermediates, or the API; (iii) any Regulatory Submission (including but not limited to any DMF) made by FFFC for the API product; (iv) the Master Batch Records; and (v) Batch records or other process documentation. In the event a change is requested and approved by Cempra in writing, FFFC will continue to Manufacture the API in accordance with the previously-applicable process changes pending the completion of process changes that require such changes. The implementation of changes shall be subject to Cempra’s prior written authorization. Where changes are implemented that reduce costs in the manufacturing process, the Parties will reduce the Transfer Price in an amount proportional to Cempra’s contributions to such changes. The API Specifications shall not be modified or revised except by the procedures are set forth below.
(a)Notice. A Party proposing a change to the API Specifications or the API Manufacturing Procedures shall provide reasonable advance written notice to the other Party, including as necessary to enable, in the case of Cempra in furtherance of its obligations under the any supply agreement between Cempra and any purchaser from Cempra (including Toyama) regarding Drug Products, Cempra to notify such purchaser thereof and, thereby, enable such
20
purchaser to notify and, if necessary, obtain approval of the relevant Regulatory Authority(ies) in the Territory. If the proposed change is required by a Regulatory Authority, then such notice shall include complete and full disclosure of the Regulatory Authority’s request and relevant correspondence, if any. Cempra, its Affiliates, its or their licensees, and any designees of any of the foregoing shall have the opportunity to directly participate in any dialogue FFFC has with the Regulatory Authority regarding the proposed change. If and as requested in writing by Cempra, FFFC will participate in any dialogue Cempra, any Affiliate thereof, or any licensee of Cempra or any Affiliate thereof has with the Regulatory Authority regarding the proposed change. If the change is proposed by Cempra or is required by a Regulatory Authority, then within thirty (30) days of such notice, FFFC shall notify Cempra in writing whether and the extent to which FFFC’s direct cost of Manufacturing and, therefore the Transfer Price, will increase or decrease if the proposed revision is implemented. Any proposed increase or decrease in FFFC’s Transfer Prices shall be supported by documentation, in a form and content satisfactory to, and subject to verification by, Cempra. If Cempra rejects any proposed price increase, the Parties agree to negotiate in good faith a mutually acceptable increase or decrease to the Transfer Prices based on the proposed change and its impact on Manufacturing Costs. If Cempra adopts the proposed API Specifications or API Manufacturing Procedures revision, the Transfer Prices for the API will be adjusted as per such agreement, upon the implementation of such revisions or as otherwise agreed by the Parties. Notwithstanding anything to the contrary, any changes API Specifications mandated by a Regulatory Authority shall be implemented (and Exhibit B correspondingly amended) by FFFC as soon as reasonably possible upon, and in accordance with, Cempra’s written request, subject only to Cempra’s written agreement to any price increases demanded by FFFC in amounts equal to the extent of any increase in FFFC’s Manufacturing Costs directly caused by such changes to API Specifications.
(b)Feasibility Determination. If Cempra, in consultation with FFFC, determines that FFFC cannot implement the proposed revision to the API Specifications or API Manufacturing Procedures in a cost-effective manner, it may withdraw the proposed revision. If the revision is required by a Regulatory Authority, however, then the Parties shall discuss in good faith to implement such revisions in a cost-effective manner upon mutual agreement of the Parties with respect thereto.
(c)Implementation Plan. Before implementing any agreed revision to the API Specifications or API Manufacturing Procedures, the Project Managers shall, if needed, develop and agree on a reasonable and appropriate implementation plan, which sets forth the specific procedures to be used in preparing for and implementing such change to the API Specifications and/or API Manufacturing Procedures.
(d)Regulatory Submissions. Cempra (or its Affiliate or its or its Affiliate’s licensee) will, except to the extent FFFC has filed and is maintaining a DMF in the Territory with respect to the API Manufactured by or on behalf of it hereunder, be responsible for any Regulatory Submission with the MHLW and other Regulatory Authorities in the Territory pertaining to the changes to the API Specifications and/or API Manufacturing Procedures. The Parties shall advise each other of the MHLW’s or other Regulatory Authorities’ approval and the effective date of any such changes to such API Specifications and/or API Manufacturing Procedures. FFFC’s responsibility shall be limited to the documents it prepares in connection with any Regulatory Submissions, and FFFC shall provide Cempra with all documentations to support such Regulatory
21
Submissions at the request of Cempra, including without limitation the right for Cempra, its Affiliates, or its or their licensees or other designees to reference FFFC’s DMF or the like pertaining to the API.
6.3Supply Failure and Right of Reference.
(a)Supply Failure. A “Supply Failure” shall be deemed to have taken place if (i) FFFC fails to supply (by making available to Cempra or storing at Cempra’s request, pursuant to Section 5.4(b)(ii)) at least[*] percent ([*]%) of the quantity of API ordered in any Purchase Order under this Agreement by the date(s) specified by such Purchase Order or (ii) FFFC fails to supply (by making available to Cempra or storing at Cempra’s request, pursuant to Section 5.4(b)(ii)), in the aggregate, [*] percent ([*]%) of the total quantity of API ordered by Cempra in any three (3) consecutive Purchase Orders. In the event of a Supply Failure, Cempra shall be entitled to, if and as elected by Cempra, (i) provide FFFC with a revised Forecast for the purchase of API which shall replace the then-existing Forecast (including any binding portion thereof) or (ii) terminate this Agreement under Section 10.2(d).
(b)Right of Reference. FFFC hereby grants Cempra and its Affiliates a sublicensable right of reference, transferable in accordance with Section 15.6, to the DMF owned or maintained by or on behalf of FFFC for the API and the information contained therein only for the purposes of Cempra’s, Cempra’s Affiliates’, and Cempra’s and its Affiliates’ licensees’ Regulatory Submissions or other development, manufacture or commercialization of Drug Product.
6.4Exclusivity. During the Term and until the later of (a) five (5) years after termination or expiration of this Agreement or (b) the date on which there are no remaining Valid Claims in the Patents set forth on Exhibit K or any other Patent claiming priority thereto in the Territory, FFFC will not manufacture, supply, sell or otherwise transfer API or any other form of Solithromycin to any Third Party or Affiliate of FFFC for any purpose or enable (by technology transfer, grant of rights, or otherwise) any Affiliate of FFFC or Third Party to manufacture (or assist in the manufacture by any of the foregoing of) API or any other form of Solithromycin, provided that these contractual limitations shall not apply following any termination of this Agreement by FFFC pursuant to Section 10.2(b) or 10.2(c) or by FFFC or Cempra pursuant to Section 10.2(e) as a result of a Product Failure directly and primarily resulting from Cempra’s gross negligence or intentional misconduct. Cempra shall have the right to at all times maintain and/or utilize one or more alternative or additional manufacturer(s) for the API or itself manufacture API.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
22
7.1Legal Authority; No Conflict. Each Party represents and warrants to the other Party that: (a) it has the legal power, authority and right to enter into this Agreement and to perform all of its respective obligations; (b) it is in good standing under the law of the jurisdiction in which it is incorporated or in which it is engaged in business activities; (c) it has no knowledge of any legal or other restriction, limitation, adverse financial or other conditions affecting its ability to fully perform under this Agreement; (d) that it shall not commit any act or fail to take any action that, in any significant way, would be in conflict with its material obligations under this Agreement; and (e) that it shall comply in all material respects with Applicable Laws, and in particular those related to API Manufacturing, and with all requirements under this Agreement. The execution and delivery of this Agreement and the performance of such Party’s obligations hereunder (i) do not conflict with or violate any requirement of applicable laws or regulations and (ii) do not conflict with, or constitute a default or require any consent under, any contractual obligation of such Party.
7.2Non-Infringement; Cempra Licensed Patents. FFFC represents and warrants to Cempra that, to FFFC’s best knowledge as of the Effective Date and thereafter during the Term, based on reasonable due diligence and investigation through the use of patent counsel, there are no Patents owned or controlled by Third Parties Covering the Manufacture of the API in accordance with the API Manufacturing Procedures or provision of any other services to be provided by FFFC under this Agreement, other than any Cempra Licensed Patents that may Cover the Manufacture of API or provision of other services under this Agreement. FFFC hereby agrees and covenants that neither it, any of its Affiliates, nor any of its or its Affiliates contractors will, in the course of Manufacturing API or performing any other activities under this Agreement, (i) practice any rights, or otherwise engage in any activity, perform or use any process, method, or procedure, or use any material that is, Covered by any Patents owned or controlled by any Third Party in Manufacturing API or otherwise performing its obligations under this Agreement, other than the Cempra Licensed Patents or Patents to which FFFC has directly obtained, independently of Cempra, sufficient rights to enable FFFC, its Affiliates, and its and its Affiliates’ contractors to Manufacture API and perform its obligations hereunder without infringing such Patents or causing Cempra, its Affiliates, or its or its Affiliates’ licensees to infringe such Patents or (ii) engage in any other misappropriation or violation of any Third Party’s intellectual property rights (including but not limited to trade secrets). FFFC acknowledges that Cempra and/or its Affiliates may have certain royalty, payment, and/or other obligations to Third Parties with respect to the Cempra Licensed Patents, and FFFC agrees that, for each amount of API supplied hereunder, it shall, prior to or simultaneously with its invoice for such API, confirm to Cempra in writing that the manufacturing methods, processes, and synthetic pathways followed or performed in the manufacture of such API were those specified by Cempra therefor or, solely to the extent not constituting or requiring the practice of, any Third Party’s intellectual property or rights thereto, those improved by FFFC and protected as confidential in the DMF, and FFFC further agrees and covenants that it will only utilize the methods, processes, and synthetic pathways specified by Cempra for the manufacture of API or, solely to the extent not constituting or requiring the practice of any Third Party’s intellectual property or rights thereto, those improved by FFFC and protected as confidential in the DMF in performing FFFC’s obligations hereunder.
23
7.3Ability and Capacity. FFFC represents and warrants that: (a) it has all permits, approvals, personnel, professional experience, equipment, facilities, funds, and capacity to fully perform it obligations under this Agreement; and (b) that it will not use in any manner, employ, engage or utilize the services of any person who has been or is threatened with debarment under the United States’ Generic Drug Enforcement Act of 1992 or any equivalent law, rule, or regulation outside of the United States, or subject to any other comparable administrative, institutional or other sanction for misconduct.
7.4Warranty of Title. FFFC represents and warrants that all API, when title therefor is to be transferred to Cempra pursuant to Section 5.4, shall be free and clear of any and all encumbrances, liens, or other claims, and FFFC can and does grant good and marketable title thereto.
7.5API Warranty. FFFC represents and warrants that all API, when delivered to Cempra under this Agreement, (a) will be manufactured, tested, packaged, handled, and stored in strict accordance with the API Manufacturing Procedures, the Quality Agreement, cGMP and all other Applicable Laws; (b) will meet the API Specifications; (c) will not be adulterated or misbranded within the meaning of the FD&C Act or any similar laws, regulations, or guidelines, or any applicable directives of applicable Regulatory Authorities; and (d) will not be articles that, under the provisions of the FD&C Act or any similar laws, regulations, or guidelines, or any applicable directives of applicable Regulatory Authorities, may not be introduced into interstate commerce. In the case of breach of the foregoing warranty, FFFC shall promptly replace the Non-Conforming API at no additional cost to Cempra, or refund the purchase price therefor, at Cempra’s election, and provided that the foregoing shall not limit FFFC’s recall obligations under Section 9 and indemnification obligation under Section 12.1.
7.6Compliance with Laws. Each Party covenants that it will comply with all Applicable Laws in its performance of this Agreement. Each Party certifies that it shall cooperate with the other Party as required to comply with Applicable Laws, including providing assistance with any disclosures required by Applicable Laws. Manufacturer represents and warrants that, as of the Effective Date, neither it, any Affiliate thereof, nor any facility of Manufacturer or any Affiliate thereof is the subject of any inquiries, notifications, inspection activity, suspensions, or holds by any Regulatory Authority with respect to the manufacture of any active pharmaceutical ingredients or finished pharmaceutical products (including but not limited to, any FDA Form 483 Establishment Inspection Reports, warning letters, or similar items).
7.7Disclaimer. EXCEPT AS EXPLICITLY SET FORTH IN THIS AGREEMENT, NEITHER PARTY MAKES ANY REPRESENTATIONS OR GRANTS ANY WARRANTIES, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE, AND, EXCEPT TO THE EXTENT SET FORTH IN THIS AGREEMENT, EACH PARTY SPECIFICALLY DISCLAIMS ANY AND ALL WARRANTIES, WHETHER WRITTEN OR ORAL, OR EXPRESS OR IMPLIED, INCLUDING ANY WARRANTY OF QUALITY, MERCHANTABILITY OR FITNESS FOR A PARTICULAR USE OR PURPOSE.
24
8.1Compliance with API Specifications and Other Requirements. FFFC shall Manufacture all API, and carry out all other obligations under this Agreement, in compliance with the API Specifications, the Quality Agreement, cGMP and all other Applicable Laws, and the other requirements under this Agreement.
8.2Licenses and Permits. FFFC shall obtain and maintain at its expense all permits, licenses and approvals (including facilities licenses) needed for FFFC to be able to manufacture and supply API in compliance with cGMP and Applicable Law (the “Facilities Licenses”), in a timely manner such that FFFC is able to meet its Manufacturing and supply obligations under this Agreement. FFFC shall keep Cempra regularly and fully informed about status of all such Facilities Licenses and shall provide Cempra copies thereof upon request. FFFC shall ensure that the Facility complies with cGMP and all other Applicable Laws with regards to its Manufacturing and supply of API. FFFC shall use best efforts to resolve as soon as possible any issues that arise in its seeking or maintaining Facilities Licenses, including completely addressing and rectifying any deviations or other issues raised in any regulatory compliance action or any similar warning or objection by any Regulatory Authority.
8.3Quality Agreement. The parties shall use reasonable efforts to work in good faith to negotiate and execute a customary form of quality agreement that is consistent with Applicable Law and industry standards (a “Quality Agreement”) as soon as reasonably possible following the Effective Date, but in any event no later than within six months of signing this Agreement; upon execution of the Quality Agreement, a copy thereof will be attached as Exhibit F of this Agreement. Such agreement may be amended by mutual agreement from time to time by the Parties. To the extent that the terms or conditions of the Quality Agreement, or any procedure, specification or requirement referenced by it, conflicts or is materially inconsistent with the terms of this Agreement, the terms of this Agreement shall prevail.
8.4Information for Regulatory Applications. FFFC shall prepare and maintain a DMF in the Territory for the API manufactured hereunder, and update such DMF as required by Applicable Law, with such DMF to contain reasonably appropriate information concerning Master Batch Records (and API Manufacturing Procedures) as necessary and appropriate for all Regulatory Submissions in the Territory and for the development, manufacture, commercialization, and use of Drug Product in the Territory. Except as may be agreed in writing by the Parties in any agreement for the supply of Drug Product for use or sale outside the Territory pursuant to any agreement negotiated in accordance with Section 2.11, FFFC shall not file any DMF for the API or any other form of Solithromycin outside of the Territory, nor enable or permit any Affiliate of FFFC or any Third Party to file a DMF concerning the API or any other form of Solithromycin or reference any such DMF filed or maintained by or on behalf of FFFC (provided that the foregoing shall not limit Cempra’s rights, including its rights to sublicense, under Section 6.3(b)), and FFFC shall ensure that no Affiliate of FFFC files any DMF for API or any other form of Solithromycin except to the extent otherwise agreed to in writing by Cempra. Upon Cempra’s written request, FFFC shall provide to Cempra, in English, the complete Master Batch Records, Batch Records, and any other API production records, and specific API Manufacturing Procedures and updates, and copies of the relevant documents containing any other FFFC technology used in manufacturing API, to the extent such technology and API Manufacturing Procedures are not
25
maintained as confidential in the DMF maintained by FFFC. Cempra has the right to review and copy the executed, completed Batch Records for each Batch, as needed for Cempra, its Affiliates, and its or its Affiliate’s licensees to prepare the CMC sections for any particular Regulatory Submissions that Cempra (or its Affiliate or its or its Affiliate’s licensee) intends to file or for any other appropriate regulatory purpose relating to API or any Drug Product to the extent such technology and API Manufacturing Procedures are not maintained as confidential in the DMF maintained by FFFC. FFFC shall prepare and maintain the Batch Records for each Batch of API manufactured hereunder, and shall provide Cempra, its Affiliates, and its and its Affiliates’ licensees access to such Batch Records for review and inspection, and shall provide copies thereof to Cempra upon request to the extent such technology and API Manufacturing Procedures are not maintained as confidential in the DMF maintained by FFFC.
8.5Regulatory Submissions. Cempra, its Affiliates, and its or its Affiliates’ licensees shall have the exclusive right to prepare and submit any and all Regulatory Submissions, other than the DMF to be filed by FFFC in the Territory as contemplated hereby, regarding API or Drug Products, and including filing any amendments or supplements thereto and pursuing such applications and filings to approval or registration. All Regulatory Submissions related to the API and/or the Drug Product, other than the DMF to be filed by FFFC in the Territory as contemplated hereby, shall be made, owned, and controlled by Cempra (or its Affiliates or its or its Affiliates’ licensees, as applicable) in its (or their) sole discretion. To the extent required or appropriate under Applicable Laws, any such Regulatory Submissions regarding API and/or Drug Product shall list FFFC as the manufacturer of any API supplied under this Agreement. FFFC shall have no rights in or to any such Regulatory Submissions. FFFC, in consultation with Cempra, shall prepare at its expense the description of the Manufacturing operations and related information (e.g., methods validation package, stability, representative data and Batch records) as required for inclusion in the Regulatory Submissions to the FDA, MHLW, and other Regulatory Authorities, which will contain all of the information relating to API Manufacturing Procedures as required in such Regulatory Submissions. FFFC will assist Cempra in the preparation of annual updates and other required or requested Regulatory Submissions, and in promptly responding to any questions from Regulatory Authorities. If and as requested by Cempra, FFFC shall provide qualified technical representatives to attend meetings and/or teleconferences with the FDA, MHLW, and other Regulatory Authorities as needed.
8.6Regulatory Assistance. Without limiting Section 8.5, with respect to any application or filings reasonably needed by Cempra (or its Affiliate or its or its Affiliate’s licensee) to obtain or maintain Regulatory Approvals for any Drug Product, and any record-keeping, audits, inspections and audits required by Regulatory Authorities relating to the manufacture and/or supply of all API by FFFC hereunder, FFFC shall reasonably cooperate with and assist Cempra in all such matters, including providing any additional information in FFFC’s control needed for such applications, filings or activities and any additional support relating to API as reasonably requested by Cempra, and Cempra shall reimburse FFFC for any actual out-of-pocket costs of providing such information and assistance, in amounts to be agreed prior to the services.
8.7FFFC Pre-Review of Regulatory Submission. FFFC accepts responsibility for the accuracy, integrity and completeness of all documentation prepared by or on behalf of FFFC that is filed with Regulatory Authorities, including but not limited to the DMF to be filed by FFFC as contemplated by this Agreement (collectively, the “FFFC Regulatory Documents”).
26
8.8OOS and Other Events. FFFC shall immediately inform Cempra in writing of all OOS and out-of-trend events (provided such trend constitutes a deviation) within two (2) business days, failure investigations, process deviations, Batch failures and similar matters (including any unexpected adverse final or interim results or data from stability or other studies) and provide Cempra with the applicable investigation report and corrective action plans prior to release of the in-process or finished Lots that are subject to the OOS event. All OOS and other investigations, and all corrective actions, shall be performed in accordance with Regulatory Standards and a written procedure acceptable to the Parties.
8.9Pre-Approval Inspections and Other Inspections. FFFC shall use its best efforts to successfully pass MHLW inspections, and all other regulatory inspections by the Regulatory Authorities and audits performed by or on behalf of Cempra, its Affiliates, or its or its Affiliates’ licensees, without material objection. Should FFFC fail MHLW inspection or review of Regulatory Submissions results in materially adverse actions by any Regulatory Authority (e.g., delay of Marketing Approval or requirement for corrective actions), in any event due to FFFC’s negligence, inadequate planning or implementation or failure to comply with Applicable Laws or other requirements under this Agreement, it shall immediately rectify such inadequacies and perform best efforts to prepare the Regulatory Submissions related to API Manufacturing and other services of FFFC provided hereunder. Except as specifically provided otherwise in this Agreement, FFFC shall bear the expense of establishing and maintaining its compliance with Applicable Laws and other requirements in their Agreement, including implementation of any corrective or other actions needed to bring about such compliance. FFFC shall allow Cempra’s quality assurance team (or that of its Affiliates or its or its Affiliates’ licensees) to conduct mock preapproval inspections upon their reasonable request.
8.10Records. FFFC shall prepare and maintain all Records relating to its activities under this Agreement, including all Batch Records. Records shall be prepared and maintained in compliance with cGMP and all other Applicable Laws and other requirements under this Agreement. All Records shall be complete, accurate, legible, valid, verifiable and contemporaneous with the events or activities described. All Records shall be available for Cempra’s, its Affiliates’, and its and its Affiliates’ licensees’ inspection and audit upon advance notice during business hours, and Cempra, its Affiliates, and its and its Affiliates’ licensees shall have the right to request and obtain copies thereof, which are accompanied by a written statement of an appropriate representative of FFFC certifying the authenticity and accuracy of such copies, during the Term and until the latest of (i) thirteen (13) years from the time of manufacture and release of the API to which the applicable Records relate, (ii) three (3) years from expiration of the Drug Product manufactured using such API, or (iii) such later date as may be required by Applicable Law, to the extent, in each case, such Records do not contain any information maintained as confidential in the DMF maintained by FFFC. Notwithstanding the foregoing, Cempra and/or its representative (including that of any Cempra Affiliate or licensee of Cempra or any Affiliate thereof) may at any time have access to the Records during business hours to the extent such the Records do not contain any information maintained as confidential in the DMF maintained by FFFC and the right to make copies thereof, in connection with investigation of any complaint or injury related to the API or the Drug Product or any dispute between the Parties. FFFC shall not destroy, alter (except for corrections as and in the manner permitted by Applicable Laws), remove or dispose of any Records without Cempra’s prior written consent and in which case Cempra may take possession and custody of such Records to the extent not containing
27
information not disclosed or required to be disclosed to Cempra (which may include information maintained as confidential from Cempra in the DMF).
8.11Retention Samples, Analytical Verification, and Qualification. FFFC shall collect and retain samples as required by the API Specifications and Applicable Laws. In addition, as directed by Cempra, FFFC also shall retain sufficient quantities of samples of API (including production samples taken during the Manufacturing process) to twice replicate the quality control and release testing applicable to the sample. These additional samples shall be maintained by FFFC for the longest of (i) eight (8) years from the date of manufacture and release of the corresponding API, (ii) three (3) years from expiration of the Drug Product manufactured using such API, or (iii) such longer period as may be required under Applicable law and, upon request, furnished to Cempra at any testing facility designated by Cempra. There shall be no charge for preparing these additional samples, other than any reasonable, documented costs incurred with special packing requirements or courier services. FFFC shall notify Cempra before disposing of any such samples, and upon Cempra’s request shall ship the requested samples to Cempra or any designee thereof at Cempra’s cost (which shall be reasonable and documented).
8.12Notice of Adverse Discovery. At any time following the Manufacture of a Lot of API, FFFC shall notify Cempra immediately in writing in the event FFFC discovers or has reason to believe that there may be defects or deviations of any kind whatsoever in such API, including any non-conformance with API Specifications, Applicable Law, or any requirements applicable to its Manufacture or any breach of the warranty in Section 7.5 as to such API.
8.13Inspection of Facility by Cempra. Cempra (or its Affiliate or any licensee of Cempra or any Affiliate thereof) shall have the right, and FFFC shall permit Cempra (or its Affiliate or any licensee of Cempra or any Affiliate thereof), from time-to-time, and at any time, to audit or inspect the portions of the Facility where API is Manufactured or stored and to review all Records and other documents relating to Manufacturing of API as is reasonably necessary for the purpose of assessing FFFC’s compliance with the Manufacturing SOPs, cGMP, the API Specifications, the Regulatory Standards, applicable chemical manufacturing controls, and this Agreement to the extent such Records do not contain any information maintained as confidential in the DMF maintained by FFFC. Such audits or inspections shall not be limited in number or frequency, but in principle once or twice a year, and any such audit or inspection and document review shall be conducted upon reasonable prior written notice by Cempra prior to the proposed audit or inspection (except in the event of a reasonable, urgent concern by Cempra regarding the quality of API, in which case Cempra may conduct the audit or inspection as soon as possible), at a time and date determined by Cempra, taking into account FFFC’s reasonable scheduling concerns. In addition, such audits or inspections shall be implemented during business hours of such Facility. Furthermore, Cempra shall have the right, from time-to-time, and at any time, to have an employee, agent, or representative of it, any Affiliate of Cempra, or any licensee of Cempra or any Affiliate thereof present at the Facility during the preparation for or conduct of any manufacturing or production run for Manufacture of a Batch of API, and such employee or agent shall be free to inspect and oversee all aspects of such preparation or production run and to comment to FFFC thereupon.
28
(a)Inspection by Regulatory Authorities. Upon the request of any Regulatory Authority having jurisdiction over the manufacture of API hereunder or Drug Product to be manufactured using such API, such Regulatory Authority shall have access to observe and inspect FFFC’s facilities and procedures used for all activities related to the manufacture and storage of the API including but not necessarily limited to the manufacture, testing and release, and/or warehousing of all API (and all Intermediates and Raw Material) and to audit such facilities for compliance with cGMP and/or other applicable Regulatory Standards. FFFC specifically agrees to cooperate with any inspection by a Regulatory Authority, whether prior to or after Regulatory Approval of a Drug Product, and to promptly provide Cempra a copy of any inspection or audit report resulting from any such inspection. If FFFC is purchasing Raw Materials from a Third Party manufacturer for use in manufacturing API, FFFC shall use commercially reasonable diligent efforts to ensure that such manufacturer’s facilities and procedures are subject to the provisions of this Section 8.14(a), or substantially similar contractual obligations, as to the manufacture of such Raw Materials, and to ensure that Cempra is provided copies of any inspection or audit report of such Third Party relating to such Raw Materials. For any Third Party manufacturers of Raw Materials selected by Cempra, FFFC will perform a reasonable and customary use test in accordance with relevant SOP or protocol with respect to any Raw Materials obtained from such Third Party.
(b)Notification of Inspections. FFFC agrees to notify Cempra in writing as soon as possible of any written or oral inquiries, notifications or inspection activity by any Regulatory Authority in regard to the API to be supplied to Cempra hereunder or to any Manufacturing activity related thereto. Cempra shall have the right to attend (or have any Affiliate thereof or licensee of Cempra or any Affiliate thereof attend) any such inspection that relates directly to Manufacturing (including testing) of API. FFFC shall provide a reasonable description of any such governmental or regulatory inquiries, notifications or inspections promptly, but in no event later than one (1) business day after such notification, inquiry or inspection. FFFC shall furnish to Cempra (i) as soon as possible and not to exceed within three (3) business days after receipt, any report or correspondence issued by any Regulatory Authority in connection with such notification, inquiry or inspection, including any List of Inspectional Observations, applicable portions of any Warning Letters, or any equivalent or similar form, letter, or notice in another country or jurisdiction which pertain to the API or any facility involved with the manufacture, handling, or storage thereof, and (ii) not later than ten (10) business days prior to the time it provides to any Regulatory Authority, copies of proposed responses or explanations relating to items set forth above (each, a “Proposed Response”), in each case redacted of trade secrets or other confidential or proprietary information of FFFC that are unrelated to the obligations under this Agreement or are unrelated to API or its manufacture. FFFC shall discuss with Cempra and consider in good faith any comments provided by Cempra on the Proposed Response. After the filing of a response with the Regulatory Authority, FFFC shall notify Cempra promptly in writing of any further contacts with such Regulatory Authority relating to the subject matter of the response until resolution and provide the final outcome (e.g. establishment inspection report (EIR)).
(c)Remedial Actions. FFFC shall notify Cempra immediately in writing in the event any action is taken or threatened by a Regulatory Authority relating to the manufacture,
29
handling, or storage of API by FFFC, or relating to the FFFC Facility in which such manufacture, handling, or storage occurs, or which may impair the ability of FFFC to manufacture or store API (including without limitation any impairment to FFFC’s ability to manufacture or store API conforming to the applicable API Specifications) in accordance with this Agreement. In any event, FFFC shall use best efforts to address and resolve as soon as possible any issues, concerns or warnings from any Regulatory Authority that might affect FFFC’s ability to manufacture, supply, and store API in accordance with this Agreement. To the extent FFFC must implement a plan of remediation or for other modifications or changes to its FFFC Facility or its manufacturing processes in order to address and resolve any such issues, concerns or warnings from any Regulatory Authority, FFFC shall prepare such plan as soon as possible, shall provide a draft of the plan to Cempra for review and comment, and shall implement all reasonable comments of Cempra as soon as possible, and shall implement and complete all aspects of the agreed plan as soon as possible.
(d)Damages for Regulatory Failures. If FFFC fails to deliver on a timely basis API ordered by Cempra under this Agreement due to either: (i) failure of FFFC to obtain or maintain all needed Facilities Licenses or (ii) a determination by the MHLW or any other Regulatory Authority that the API is “misbranded or adulterated” within the meaning of the FD&C Act (or equivalent determination under any Applicable Law in the Territory) due to any manufacturing problem or issue at the Facility, or any other similar disability or determination raised, imposed, or made by a Regulatory Authority, each arising from any reason, act, or omission attributable to FFFC, its Affiliates, or its subcontractors, then FFFC shall indemnify and hold Cempra harmless from any and all losses resulting from lost sales caused directly by such failure to deliver within the limitation set forth in Section 12.4.
8.15Other Conditions of Audits and Inspections. There shall be no charge for any inspections or audits as described in Sections 8.13 or 8.14 above, and FFFC shall cooperate with both, including providing of reasonable space for review and copying of Records and assistance of key personnel. Cempra representatives, when on FFFC’s premises, shall at all times comply with FFFC’s internal policies to the extent reasonable and provided in advance to Cempra. It is agreed that, except to the extent audits and observation are implemented by Cempra unreasonably and excessively frequently or unreasonably and excessively rigidly or for an unreasonably long period and such implementation by Cempra directly results in any prevention or inhibition of FFFC’s performance of its obligations under this Agreement, the audits and observation by Cempra representatives shall not in any way serve as a limitation on any of FFFC’s obligations or liabilities under this Agreement; although Cempra reserves the right to audit the Facility annually or more frequently if reasonable under the circumstances and will provide FFFC with the results of any quality audit performed by Cempra.
|
9. |
Recalls and Recall Costs |
9.1Responsibility for Recalls. If a Recall (as defined in Section 9.5 below) of any Drug Product distributed by Cempra or its Affiliate or any licensee of either of the foregoing is required or recommended by a Regulatory Authority or other governmental agency or authority of competent jurisdiction, or if a Recall is otherwise deemed advisable by Cempra (or its Affiliate or its or its Affiliate’s licensee), Cempra, its Affiliate, or its or its Affiliate’s licensee, as applicable shall, as between the Parties, be responsible for and determine, in their sole discretion, such Recall,
30
its planning, and its execution, provided that FFFC shall cooperate with Cempra, its Affiliates, and its or its Affiliates’ licensees, as applicable, with respect to any such Recall, as reasonably requested thereby, and further provided that, to the extent permitted and reasonably possible in light of any applicable terms of Cempra’s agreements with its licensees or distributors in the Territory (including but not limited to Toyama) without breach of such agreements, Cempra shall use reasonable efforts to consult with FFFC regarding any potential Recall prior to the initiation thereof. The costs of any Recall shall, as between the Parties, be borne by the Party or Parties whose actions or omissions caused the Recall to be necessary or deemed advisable, as provided in Section 9.4.
9.2Communication. Each Party shall keep the other fully and promptly informed of any notification, event or other information, whether received directly or indirectly, which might affect the marketability, safety or effectiveness of Drug Products sold or distributed by Cempra, its Affiliate, or its or its Affiliate’s licensee or might result in a Recall or Seizure (as defined in Section 9.5 below) of Drug Products by the FDA, MHLW, or other Regulatory Authority.
9.3Replacement; Refund. In the event of any Recall or Seizure of Drug Product arising out of or resulting from FFFC’s supplying defective API or other breach of this Agreement by FFFC, FFFC shall, if and as elected by Cempra, and in addition to any other obligations of FFFC under the terms of this Agreement available to Cempra for any breach of this Agreement by FFFC, either:
(a)promptly supply replacement API that meets the API Specifications and otherwise conforms to the warranty in Section 7.5, without charge to Cempra, in an amount sufficient to replace the amount of API needed to manufacture the Drug Product that is Recalled or Seized (including that amount of API incorporated into any Drug Products that are Recalled or Seized), or
(b)refund to Cempra, or give credit to Cempra against outstanding receivables due from Cempra against the price of API to be delivered to Cempra in the future, in amounts equal to the price paid by Cempra to FFFC for the API needed to manufacture the amounts of Drug Product so Recalled or Seized (including that API incorporated in the Drug Product so Recalled or Seized) plus the reasonable, documented transportation costs incurred by Cempra and not recovered by Cempra in respect of such Recalled or Seized Drug Product.
9.4Responsibility for Recall Costs. To the extent any Recall or Seizure of API or any Drug Product results from FFFC manufacturing defects in any API supplied by FFFC (for example, due to FFFC’s failure to manufacture a API included in such Drug Product in accordance with the API Specifications or cGMP), or otherwise arises out of, or is connected with any inaccuracy in, breach of, or non-fulfillment of, any representation, warranty, covenant or other obligation of FFFC under this Agreement, or any negligence, recklessness, willful misconduct, or failure to conform with the explicit quality standards or quality obligations imposed on FFFC in the Quality Agreement on the part of FFFC, its Affiliates, or its or its Affiliates’ directors, officers, employees, vendors or agents, then FFFC shall pay all the reasonable, documented, direct costs of such Recall or Seizure, including such costs incurred by Cempra, any Affiliate thereof, or any licensee of Cempra or any Affiliate with respect to the reasonable conduct of any such Recall or Seizure, including but not limited to shipping costs, repurchases, notification letters, direct
31
shipping expenses, and the costs of disposal and/or destruction of the Recalled items, and other direct costs and expenses directly related to such Recall or Seizure (such as costs of administering any Recall), provided that if such Recall or Seizure results from negligence, intentional misconduct, or failure of both Parties, Cempra and FFFC shall bear such costs and expenses pro rata in accordance with their share of fault, which shall be discussed in mutual good-faith and reasonable consultations between the Parties (for purpose of clarification, API, supplied by FFFC under this Agreement, that (x) was not manufactured, stored, or released by or on behalf of FFFC in accordance with the API Specifications or cGMP or (y) did not conform with the explicit quality standards or quality obligations imposed on FFFC in the Quality Agreement with respect to such API, shall, solely for purposes of this proviso, constitute such negligence, intentional misconduct, or failure on the part of FFFC). Notwithstanding the foregoing, FFFC shall have no obligation to pay costs of a Recall or Seizure of any Drug Product to the extent such Recall or Seizure is: (a) due to defects in the Drug Product other than those arising out of (x) manufacturing defects in the API as supplied by FFFC or (y) any inaccuracy in, breach of, or non-fulfillment of, any representation, warranty, covenant or other obligation of FFFC under this Agreement, or any negligence, recklessness, willful misconduct, or failure to conform with the explicit quality standards or quality obligations imposed on FFFC in the Quality Agreement on the part of FFFC, its Affiliates, or its or its Affiliates’ directors, officers, employees, vendors or agents, (b) due to defects in the Drug Product caused by improper actions (such as incorrect storage) occurring after the API is delivered by FFFC to Cempra’s carrier, (c) due to packaging or labeling defects for which Cempra or any Third Party has responsibility, or (d) due to and caused by any other breach by Cempra of its duties under this Agreement. For the avoidance of doubt and subject to the general limitations of liability under this Agreement, and without limitation of the foregoing, FFFC shall be solely responsible for the costs and expenses of a Recall or Seizure that is directly caused by a FFFC manufacturing defect in API supplied to Cempra.
9.5Definition of Recall and Seizure. For purposes of this Section 9, “Recall” shall mean any action by Cempra and/or its Affiliates or licensees to recover title or possession or halt distribution, prescription or consumption of Drug Products sold or shipped to Third Parties by Cempra or its Affiliate or licensee, including any market withdrawal. The term “Recall” also applies to Drug Product which would have been subject to recall or withdrawal if it had been sold or shipped. “Seizure” shall mean any action by the MHLW or other Regulatory Authority or governmental agency or authority of competent jurisdiction to detain or destroy API or Final Products or prevent the distribution, prescription, consumption or release of any API or Final Products.
|
10. |
Term And Termination |
10.1Term. Unless earlier terminated as provided for in Section 10.2 or Section 15.4(b), the initial term of this Agreement shall commence on the Effective Date and shall remain in full force and effect for a period of ten (10) years from the Effective Date (the “Initial Term”). The term shall be automatically renewed thereafter for one year periods (each, a “Renewal Term” and together with the Initial Term, the “Term”) unless either Party gives the other Party prior written notice of non-renewal at least [*] months prior to the end of the then-current Term.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
32
(a)Mutual Consent. The Parties may at any time terminate this Agreement by mutual written agreement.
(b)Material Breaches.
(i)A Party shall have the right to terminate this Agreement on written notice to the other Party if the other Party commits a material breach of its obligations under this Agreement and fails to remedy such breach within [*] days after notice of such breach. After the end of the applicable cure period, if the breach has not been cured, the Party having the right to termination may terminate in whole or in part immediately upon notifying the breaching Party in writing. Any termination of this Agreement shall not release the breaching Party from its obligations or otherwise affect or limit the Parties’ rights and remedies. For clarity, Cempra’s breach of its minimum purchase obligations under Section 2.6(d) of this Agreement shall be construed as a material breach of Cempra enabling termination as set forth in this Section 10.2 (b)(i).
(ii)Each Party shall notify the other Party in writing as soon as reasonably possible of any claim, threatened claim, or allegation made against it or any Affiliate thereof concerning any alleged, potential, or actual infringement, violation, or misappropriation of any Third Party’s intellectual property rights (including but not limited to Patents) in the Manufacture of API or performance of FFFC’s other obligations under this Agreement. Upon such notice, the Parties shall enter into good faith discussions concerning such claimed, alleged, possible, or actual infringement, violation, or misappropriation and potential appropriate or necessary measures that may enable FFFC to continue to perform this Agreement without infringing, violating, or misappropriating any Third Party’s intellectual property rights (including but not limited to Patents). If FFFC fails to propose a reasonable, feasible and practical solution embodying appropriate and necessary measures enabling such non-infringing, non-violating, and non-misappropriating continued performance by FFFC that (i) would not require any additional cost or expenditure by Cempra or otherwise adversely affect Cempra’s, its Affiliates’, or any of its or its Affiliates’ licensees’, sublicensees’, or distributors’ development, manufacture, or commercialization of API or Drug Product and (ii) is reasonably approved in writing by Cempra in its sole discretion within [*] days after one Party provides such notice to the other Party of the applicable claim, threatened claim, or allegation, then Cempra shall have the right to terminate this Agreement on written notice to FFFC.
(c)Bankruptcy. A Party shall have the right to terminate this Agreement effective upon written notice to the other Party in the event that: (a) such other Party files a petition in bankruptcy or makes a general assignment for the benefit of creditors, or is adjudged bankrupt, and such other Party (i) fails to assume this Agreement in any such bankruptcy proceeding within [*] days after filing or (ii) assumes and assigns this Agreement to a Third Party; (b) such other Party goes into or is placed in a process of complete liquidation; or (c) a trustee or receiver is appointed for any substantial portion of such Party’s business and such trustee or receiver is not discharged within [*] days after appointment.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
33
(d)Supply Failure. This Agreement may be terminated by Cempra by written notice to FFFC at any time following the occurrence of a Supply Failure.
(e)Product Failure. This Agreement may be terminated immediately by either Party on [*] days written notice to the other Party in the event of a Product Failure.
(f)Purchase Quantity Cause. This Agreement may be terminated by FFFC by written notice to Cempra at any time following the end of a complete Month Period during which the total quantity of API ordered by Cempra for delivery during such Month Period is less than a [*] kg.
(g)Validation or Process Issues. This Agreement may be terminated by Cempra on written notice to FFFC if there are manufacturing process issues that make it commercially impracticable to manufacture API at the Facility, such as, without limitation of any other circumstances enabling termination under this Section 10.2(g), that FFFC is unable to complete successfully the Validation Studies on a timely basis, or the registration or engineering Batches fail and cannot be completed to Cempra’s reasonable satisfaction.
10.3Effect of Termination.
(a)Remaining Portion of Forecast. Upon any termination of this Agreement, Cempra shall be obligated to purchase, and FFFC shall be obligated to deliver, API in accordance with any binding portion of the then current Forecast for which Purchase Orders have not been submitted, but Cempra shall not be obligated to submit any Purchase Orders or purchase any API in accordance with any non-binding portion of the then current Forecast for which Purchase Orders have not been submitted, except to the extent, with respect to all of the foregoing, explicitly provided for below.
(b)Elective Purchase of Inventories. In the event of this Agreement’s termination, Cempra shall be entitled, if and as elected by Cempra, to (i) purchase, at the lowest price indicated in Exhibit J, all remaining API in FFFC’s inventory that it would not otherwise be obligated to purchase under this Agreement and/or (ii) purchase, at FFFC’s reasonable, documented, direct purchase or production cost, as applicable, its Raw Materials or Intermediates (valued on a pro-rata basis to manufacturing cycle-time) reasonably purchased or produced for Manufacturing that, with the exercise of reasonable efforts by FFFC, are not reasonably able to be returned for credit or used for producing products for FFFC’s other customers, plus reasonable, documented shipping costs. In no event shall Cempra be charged an amount for Raw Materials or Intermediates that exceeds the purchase price set forth hereunder for the corresponding amounts of API specified by Cempra’s relevant Purchase Orders in effect at the date this Agreement is terminated (the “Termination Date”).
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
34
(c)Effects of Termination by Cempra for Product Failure or by FFFC for Cempra’s Breach or Bankruptcy. If this Agreement is terminated by Cempra under Section 10.2(e) or by FFFC under Section 10.2(b) or 10.2(c), then, except to the extent otherwise agreed to in writing by the Parties, (i) all amounts of completed API existing as of the date the termination notice is provided Manufactured under Purchase Orders previously accepted by FFFC shall be delivered and paid for by Cempra in accordance with the terms of this Agreement, (ii) all other Manufacturing work under this Agreement shall immediately cease and all other pending Purchase Orders shall be automatically cancelled, and (iii) Cempra shall, within thirty (30) days of an invoice from FFFC, reimburse FFFC for FFFC’s reasonable, documented direct cost of all unused Intermediates and all unused Raw Materials reasonably procured by FFFC prior to such notice of termination as necessary to manufacture API in satisfaction of the binding and non-binding portion of the most recent Forecast provided by Cempra, except to the extent (a) such Product Failure results from FFFC’s, its Affiliates’, or its or its Affiliates’ contractors’ negligence, intentional misconduct, breach of this Agreement, or failure to comply with Applicable Law or (b) such Intermediates or Raw Materials can, with the exercise of reasonable efforts, be used by FFFC in any other portions of its business. If and as requested by Cempra, any unused Intermediates or Raw Materials for which Cempra reimburses FFFC’s cost shall be promptly delivered (and all right, title, and interest therein assigned) to Cempra or its designee, at Cempra’s expense for such delivery.
35
(d)Additional Effect of Termination by FFFC for Cempra’s Breach. If this Agreement is terminated by FFFC under Section 10.2(b) and, prior to such termination, (i) FFFC has constructed a facility located at 0-00, Xxxxxxx, Xxxxxxxxxx, Xxxxxx-xxxxx, Xxxxxx-xxx, Xxxxxxxxx 000-0000, Xxxxx for the primary purpose of manufacturing API for Cempra hereunder and (ii) such facility is completed and fully operational and qualified for the manufacture of API for delivery hereunder, then, except to the extent otherwise agreed to in writing by the Parties, Cempra shall, within ninety (90) days of such termination and FFFC’s provision of a report accurately detailing and certifying to the facts, circumstances, and accounting supporting Cempra’s obligation hereunder (and the amount thereof), pay FFFC an amount equal to (a) the “Remaining Book Value” set forth on Exhibit L (which is denominated in millions of US dollars) less (b) the product of the number of kilograms of API ordered by Cempra under this Agreement prior to such termination times US$[*], provided that (x) if the amount described in clause (b) exceeds the amount described in clause (a), Cempra shall have no payment obligation under this Section 10.3(d), (y) if the total direct costs incurred by FFFC in the construction of the facility referenced above, net of any tax credits, tax refunds, government subsidies, or similar financial, monetary, or in-kind benefits provided by any governmental agency or authority (the “Construction Costs”), do not equal or exceed US$[*], then the “Remaining Book Value” shall be reduced by a pro rata amount, based on the ratios of the various amounts set forth in Exhibit L, based on the ratio of such lesser cost to $[*], and (z) no amount shall be payable hereunder if the Agreement terminates following December 31, 2025; provided, however, that if FFFC manufactures any product or performs any activities (other than the manufacture of API for Cempra under this Agreement) in, by, or using such facility prior to such termination and makes any profit thereby, the total amount of such profits shall be subtracted from the total payment amount due from Cempra to FFFC under this Section 10.3(d). As two examples of the payment requirements of this Section 10.3(d) (and in these examples, the total amount of such profits shall be assumed as zero (0)), (I) if the requirements for payment set forth in the first sentence of this Section 10.3(d) are satisfied, the Construction Costs equal US$[*], this Agreement is terminated in 2018, and Cempra has ordered [*] kg of API hereunder prior to such termination, Cempra shall owe FFFC US$[*] under this Section 10.3(d) and (II) if the requirements for payment set forth in the first sentence of this Section 10.3(d) are satisfied, the Construction Costs equal US$[*], this Agreement is terminated in 2018, and Cempra has ordered [*] kg of API hereunder prior to such termination, Cempra shall owe FFFC US$[*] under this Section 10.3(d).
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
36
(e)Effects of Termination by FFFC for Product Failure or by Cempra for FFFC-Caused Product Failure, FFFC’s Breach, Supply Failure, or Validation or Process Issues. If this Agreement is terminated (x) by Cempra under Section 10.2(b), 10.2(c), 10.2(d), 10.2(g), or 15.4(b), (y) by Cempra under Section 10.2(e) for a Product Failure resulting, in whole or in material part from FFFC’s, its Affiliates’, or its or its Affiliates’ contractors’ negligence, intentional misconduct, breach of this Agreement, or failure to comply with Applicable Law, or (z) by FFFC under Section 10.2(e), then, except to the extent otherwise agreed to in writing by the Parties or elected by Cempra under Section 10.3(b), Cempra shall not have any obligations to purchase any API in accordance with any binding or non-binding portion of the then current Forecast for which Purchase Orders have not been submitted or to purchase, or reimburse FFFC’s costs for, any API, Intermediates, or Raw Materials remaining in FFFC’s possession or control as of such termination, except, in the event of a termination by Cempra under Section 10.2(e), to the extent the applicable Product Failure did not result from FFFC’s, its Affiliates’, or its or its Affiliates’ contractors’ negligence, intentional misconduct, breach of this Agreement, or failure to comply with Applicable Law.
(f)Return of Materials. Upon termination of this Agreement, FFFC shall immediately return to Cempra copies of all documentation and information and materials relating to API Manufacturing (including copies of development reports and Master Batch Records to the extent not containing any information maintained as confidential in the DMF maintained by FFFC), the Product, and the Specifications. Any original documents provided by or on behalf of Cempra to FFFC during the Term shall be returned to Cempra, along with any copies thereof, provided that FFFC may keep one archival copy if required by a Regulatory Authority. Documents and materials shall be packaged and shipped in the manner reasonably requested by Cempra as needed to preserve their integrity and acceptability to Regulatory Authorities.
(g)Survival. Termination of this Agreement shall not operate to release any Party from any obligation or liability incurred under the terms of this Agreement before or upon termination hereof, nor shall it relieve the Parties of their obligations with respect to API supplied by FFFC under this Agreement. In addition Articles 1, 4, 5, 7, 9, 11, 12, 13, 14, and 15 and Sections 2.2, 2.7, 2.10, 3.3, 6.3(b) (for Drug Product manufactured with API supplied under this Agreement), 8.1, 8.4, 8.5, 8.6, 8.7, 8.8, 8.9, 8.10, 8.11, 8.12, 8.13, 8.14, 8.15, and 10.3 shall survive the expiration or termination of this Agreement on account of any cause.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
37
11.1Treatment of Confidential Information. The Parties acknowledge and agree that during the Term, either Party may disclose to the other Party its Confidential Information as needed for the conduct of this Agreement and that all “Confidential Information” (as defined in the Confidentiality Agreement) disclosed by either Party pursuant to the Non-Disclosure Agreement between Cempra and FFFC dated October 1, 2013, as amended (the “Confidentiality Agreement”) shall be deemed to be such Party’s Confidential Information hereunder. With respect to all such Confidential Information of a Disclosing Party, the Receiving Party agrees that (except as otherwise provided in Section 11.2 below) during the Term and for a period of [*] years after this Agreement expires or terminates, such Receiving Party shall (a) maintain in confidence such Confidential Information; (b) not disclose such Confidential Information to any Third Party without prior written consent of the Disclosing Party, except for, in the case of each Party, disclosures permitted of such Party under Section 11.2; and (c) not use such Confidential Information for any purpose other than the performance of or exercise of rights under this Agreement, or, in the case of Cempra as the Receiving Party, to the extent necessary or useful in developing, manufacturing, or commercializing Drug Products.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
38
11.2Authorized Disclosures. If a Receiving Party is required to disclose specific Confidential Information of the Disclosing Party to comply with Applicable Law, or order of a government authority or court of competent jurisdiction, such Receiving Party may disclose such Confidential Information only to the person(s) or entity(ies) required to receive such disclosure; provided, however, that the Receiving Party required to disclose such Confidential Information shall (a) to the extent reasonably practicable and permitted by such Applicable Law or order, first have given reasonable advance notice to such Disclosing Party to enable it to seek any available exemptions from or limitations on such disclosure requirement, and shall reasonably cooperate in such efforts by the Disclosing Party as reasonably requested thereby, (b) furnish only the portion of such Confidential Information which is legally required to be disclosed, (c) use reasonable efforts to secure confidential protection of such Confidential Information, and (d) continue to perform its obligations of confidentiality set out herein. Further, Cempra (or its Affiliate or its or its Affiliate’s licensee) shall be entitled to disclose Confidential Information of FFFC to the extent not containing any information maintained as confidential in the DMF maintained by FFFC (other than such information maintained as confidential in the DMF that is subject to disclosure pursuant to Section 2.2 (if any)) to: (i) Regulatory Authorities to the extent such disclosure is reasonably necessary or useful in Regulatory Submissions required for the development, manufacture, and/or commercialization of Drug Products; (ii) licensees, contractors, employees, and consultants who need to know such information for the development, manufacture and/or commercialization of Drug Products, (iii) potential or actual bankers, underwriters, lawyers, accountants, agents or other Third Parties in connection with due diligence or similar investigations, and (iv) potential or actual investors, licensees, acquirers, merger or acquisition targets, or other strategic partners; provided that any such Third Party is bound by obligations of confidentiality and non-use materially as protective as those set forth herein. Also, FFFC shall be entitled to disclose Confidential Information of Cempra under obligations of confidentiality and non-use materially as protective as those set forth herein to FFFC’s Affiliate or subcontractors set forth in Section 2.7 who have been approved by Cempra in writing and need to know such information for the performance of supply obligation in this Agreement; provided that any such Affiliate or Third Party is bound by obligations of confidentiality and non-use materially as protective as those set forth herein. In the case of each foregoing disclosure, the Party making such disclosure shall obtain reasonably appropriate confidential treatment of any such disclosure on its own responsibility, and shall not disclose Confidential Information of the other Party other than is reasonably necessary. Notwithstanding anything to the contrary, FFFC shall not disclose any of Cempra’s Confidential Information concerning any API Manufacturing Procedures to Toyama, any Affiliate thereof, or any other Affiliate of FFFC except to the extent approved in advance and in writing by Cempra (such approval not to be unreasonably withheld).
11.3Disclosure of Agreement. Except as otherwise provided below, neither Cempra nor FFFC shall release any information to any other person regarding the terms of this Agreement without the prior written consent of the other Party, which consent shall not be withheld unreasonably. The foregoing consent requirement includes, but is not limited to, press releases, educational and scientific conferences, promotional materials and discussions with the media. However, each Party shall be entitled to disclose the terms of this Agreement and specific information and terms relating to this Agreement to the extent such disclosure is required by applicable law or regulation or securities exchange rules or regulations, provided that, to the extent reasonably practicable, such Party shall notify the other Party of this requirement before releasing the information. The notice to the other Party shall include the text of the information proposed for
39
release, and the basis for the required disclosure. The other Party shall, to the extent reasonably practicable, be provided a reasonable opportunity to confer with the notifying Party regarding the necessity for the disclosure and the text of the information proposed for release. A Party shall further be entitled to disclose this Agreement in securities filings with the U.S. Securities and Exchange Commission (the “SEC”) or equivalent foreign agency to the extent required by Applicable Law. In such event, the Party seeking such disclosure shall prepare a proposed redacted version of this Agreement to request confidential treatment for this Agreement, and the other Party shall, to the extent reasonably practicable, be provided a reasonable opportunity (not in any event to be required to exceed two (2) business days after receipt of such proposed redactions) to promptly provide its comments. In addition, FFFC and Cempra shall each have the right to disclose the terms of this Agreement in confidence to persons engaged or proposing engagement in fiduciary relationships, such as banks extending credit with the Party, investors, and legal counsel, and potential investors, merger targets or acquirors and their legal counsel and professional advisors, if such persons are subject to reasonable confidentiality and non-use obligations. Further, FFFC shall have the right to disclose the terms of this Agreement in confidence to directors, officers, employees and agents of FUJIFILM Corporation and FUJIFILM Holdings Corporation, both parent companies of FFFC, who need to know such information for the conduct of this Agreement if they are subject to reasonable confidentiality and non-use obligations.
40
No Implied Licenses. Only licenses explicitly granted pursuant to the express terms of this Agreement or any separate agreement executed by the Parties shall be of any legal force and effect. No other license or any other proprietary rights shall be created by implication or estoppel, in the patents, know-how, trade-secrets, copyrights, trade and other marks, or other intellectual property rights, owned or licensed to the respective Parties. No other licenses are granted by Cempra to FFFC under this Agreement.
11.5Trade Names and Trademarks. Cempra hereby acknowledges that except as otherwise set forth in this Agreement, it does not have, and shall not acquire by virtue of this Agreement, any rights to or under any goodwill, trademark or trade name of FFFC, nor in any of FFFC’s trademark or trade names appearing on the label or packaging materials of API. FFFC hereby acknowledges that it does not have, and shall not acquire by virtue of this Agreement, any rights to or under any goodwill, trademark or trade name of Cempra, any Affiliate thereof, or any licensee of Cempra or any Affiliate thereof, nor in any of Cempra’s, its Affiliates’, or its or their licensees’ trademarks or trade names appearing on the label or packaging materials of API or Drug Product.
|
12. |
Indemnification; Limitation of Liability |
12.1FFFC’s Obligation to Indemnify. FFFC shall indemnify, defend and hold Cempra, its Affiliates, and its and their respective directors, officers, employees and agents (the “Cempra Indemnitees”) harmless against any and all Losses incurred by any of them as a result of any Third Party claim, demand, suit, action or proceeding (“Claims”) resulting from, arising out of, or connected with: (a) liability or personal injury claims arising directly from the manufacture of the API supplied hereunder or Drug Products incorporating the API supplied hereunder to the extent, in either case, caused only by or resulting only from the breach of any of FFFC’s obligations under this Agreement; (b) a breach of any of FFFC’s warranties or other obligations under this Agreement; (c) the clean-up, remediation and restoration arising out of or related to FFFC’s storage, handling, transportation, incineration or disposal of any Waste that may be generated by Manufacturing; (d) the alleged or actual infringement or misappropriation of a Third Party’s intellectual property rights (including but not limited to Patents) in the Manufacture of API or performance of FFFC’s other obligations under this Agreement; or (e) any negligence, intentional misconduct, or failure to comply with Applicable Law on the part of FFFC, its Affiliates, its or their contractors, or any employees, agents, or representatives of any of the foregoing with respect to this Agreement or the subject matter thereof. FFFC’s obligations set forth in this Section 12.1 shall not include Losses on any Claims to the extent that such Losses or Claims arise from the (x) alleged or actual infringement or misappropriation of a Third Party’s intellectual property rights (including but not limited to Patents) to the extent solely and directly (i) resulting from FFFC’s following any of Cempra’s clear technical instructions for the Manufacture of API hereunder or (ii) based on FFFC’s practice, in the Manufacture of API for Cempra hereunder, of the technology Covered by and described in the claims of the Cempra Licensed Patents (and not any technology not Covered or described in such claims); (y) breach by any Cempra Indemnitee of its obligations under this Agreement or (z) any negligence, intentional misconduct, or failure to comply with Applicable Law on the part of any Cempra Indemnitee.
41
12.2Cempra’s Obligation to Indemnify. Cempra shall indemnify, defend and hold harmless FFFC, its Affiliates, and its and their directors, officers, employees and agents (“FFFC Indemnitees”) against any and all Losses incurred by any of them as a result of any Third Party Claim resulting from, arising out of, or connected with: (a) product liability claims arising from Cempra’s, its Affiliates’, or its or their licensees’ testing, manufacturing, sale or use of Drug Product; (b) a breach of any of Cempra’s warranties or other obligations under this Agreement; (c) any negligence, intentional misconduct, or failure to comply with Applicable Law on the part of Cempra with respect to this Agreement or the subject matter thereof; or (d) alleged or actual infringement or misappropriation of a Third Party’s intellectual property rights (including but not limited to Patents) to the extent solely and directly (i) resulting from FFFC’s following any of Cempra’s clear technical instructions for the Manufacture of API hereunder or (ii) based on FFFC’s practice, in the Manufacture of API for Cempra hereunder, of the technology Covered by and described in the claims of Cempra Licensed Patents (and not any technology not Covered or described in such claims). Cempra obligations set forth in this Section 12.2 shall not include Losses on any Claims to the extent such Losses or Claims arise from the any of the circumstances described in clauses (a), (b), (c), (d), or (e) of the first sentence of Section 12.1.
12.3Indemnification Procedures. Each Party’s agreement to indemnify, defend, and hold harmless under Section 12.1 or 12.2, as applicable, is conditioned upon the indemnified party (a) providing written notice to the indemnifying Party of any claim, demand or action arising out of the allegedly or actually indemnified matter as soon as reasonably possible, and in any event no later than within [*] days after the indemnified Party has actual knowledge of such claim, demand or action, (b) permitting the indemnifying Party to assume control over the investigation of, preparation and defense against, and settlement or voluntary disposition of any such claim, demand or action, (c) assisting the indemnifying Party, as reasonably requested by the indemnifying Party and at the indemnifying Party’s reasonable expense, in the investigation, preparation, defense, and settlement or voluntary disposition of any such claim, demand or action, (d) not compromising, settling, or entering into any voluntary disposition of any such claim, demand or action without the indemnifying Party’s prior written consent, which consent shall not be unreasonably withheld, and (e) furnishing promptly to the indemnifying Party copies of all notices and documents (including court papers) received by any indemnified party in connection with the Claim for which indemnification is being sought; provided, however, that, if the party entitled to indemnification hereunder fails to comply with any of the foregoing conditions, the indemnifying Party will only be relieved of its indemnification obligation under this Agreement to the extent materially prejudiced by such failure. In no event may the indemnifying Party compromise, settle, or enter into any voluntary disposition of any claim, demand or action subject to indemnification under this Section 12 in any manner that admits material fault or wrongdoing on the part of the indemnified party or incurs non-indemnified liability on the part of the indemnified party without the prior written consent of the indemnified party, and in no event may the indemnifying Party settle, compromise, or agree to any voluntary disposition of any matter subject to indemnification hereunder in any manner which may materially and adversely affect Cempra’s (or its Affiliates’ or its or its Affiliates’ licensees) ability to develop, manufacture, or commercialize API or Drug Products without Cempra’s prior written consent.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
42
12.4Limitation of Liability. IN NO EVENT SHALL EITHER PARTY OR ANY AFFILIATE THEREOF BE LIABLE TO THE OTHER PARTY OR ANY AFFILIATE THEREOF FOR ANY CONSEQUENTIAL, INCIDENTAL, LIQUIDATED, SPECIAL OR INDIRECT DAMAGES OR LOSSES, HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY, REGARDLESS OF ANY FAILURE OF ESSENTIAL PURPOSE OF ANY REMEDY AVAILABLE UNDER THIS AGREEMENT; PROVIDED THAT, NOTWITHSTANDING ANYTHING TO THE CONTRARY, THE FOREGOING SHALL NOT BE CONSTRUED TO LIMIT THE INDEMNITY OBLIGATIONS SET FORTH IN SECTIONS 12.1 AND 12.2 ABOVE, EITHER PARTY’S LIABILITY FOR PATENT INFRINGEMENT OR BREACH OF ARTICLE 11, OR FFFC’S LIABILITY FOR ANY BREACH OF SECTION 6.4 OR 7.2.
Further, the total aggregate liability of a Party to the other Party with respect to all claims under this Agreement shall be limited to the greater of (i) an amount equal to [*] or (ii) US$[*]; provided that, notwithstanding anything to the contrary, the foregoing shall not (x) be applicable to any claim under this Agreement resulting from FFFC’s gross negligence or intentional misconduct or (y) be construed to limit FFFC’s indemnity obligations set forth in Sections 12.1 and 12.2 above, either Party’s liability for patent infringement or breach of Article 11, or FFFC’s liability for any breach of Section 6.4 or 7.2, except, with respect to clause (y), with respect to such obligations under clause (b) of Section 12.1 of this Agreement with respect to claims made by, or losses of, Third Parties with respect to any breach of contract or similar arrangement, any failure to perform under or comply with any contractual provision or similar obligation, or similar occurrence or on the basis of any similar theory or cause of action.
|
13. |
Insurance |
13.1Coverage. During the period starting from the date prior to validation campaign and ending at the date [*] years after the term of this Agreement, if issued on a claims made basis, FFFC shall maintain Commercial General Liability Insurance (including products liability insurance) providing not less than $[*] per occurrence and $[*] in the aggregate. All coverage shall be underwritten by reputable underwriters. Promptly after the Effective Date, FFFC shall add Cempra as an additional insured under FFFC’s policy. FFFC shall provide Cempra with a certificate of insurance upon request. FFFC shall provide Cempra with at least thirty (30) days prior written notice of any material change, cancellation or expiration of the above-required insurance.
13.2Review. On an annual basis, FFFC shall provide Cempra with a current certificate of coverage demonstrating that the coverage specified in Section 13.1 is in force and shall immediately notify Cempra of any actual or threatened reduction, termination, non-renewal or materially adverse change in terms of coverage. FFFC shall provide Cempra with thirty (30) days’ written notice of any cancellation or material change in the coverage specified in Section 13.1. FFFC represents and warrants that it has obtained and shall maintain all coverage, including its preparation of any applications and endorsements in compliance with its obligations under the terms of coverage of such polices and shall otherwise comply with all requirements under such policies. Failure to maintain the insurance coverage as set forth in this Section 13 shall be deemed a material breach of this Agreement.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
43
14.1Internal Mediation of Dispute. In the event of any controversy or claim arising out of or relating to any provision of this Agreement or otherwise between the Parties or their Affiliates, the Parties shall try to settle the differences amicably through the Chief Executive Officers of each Party (or, if none, highest ranking executive officer of a Party) for a period of [*] days. The designees shall be individuals who possess the authority to settle the dispute but who do not have direct responsibility for administration of this Agreement. Any disputes not resolved by the Parties’ executive officers as set forth above within [*] days shall, upon written notice from either party to the other Party as set forth below, be finally and exclusively resolved by confidential binding arbitration as provided in Section 14.2.
14.2Arbitration. If the Chief Executive Officers (or, if none, highest-ranking executive officers) are unable to resolve the dispute in accordance with Section 14.1, either Party will have the right to have the dispute resolved by binding arbitration, initiated by either Party on [*] Business Days notice to the other party following expiration of the [*] day period referenced above (such notice, the “Initiation Notice”), under the Rules of Arbitration of the International Chamber of Commerce (“ICC”) then pertaining, except where those rules conflict with this provision, in which case this provision controls, applying the laws of the State of New York, without regard to its conflicts of law provisions, before three (3) independent, neutral arbitrators experienced in the pharmaceutical industry and manufacturing relationships in such industry. Cempra and FFFC shall each be entitled to select one (1) such arbitrator, with the two (2) such arbitrators so selected selecting the third (3rd) such arbitrator. In the event either Party fails to select its arbitrator in accordance with the foregoing within [*] Business Days of the Initiation Notice, the arbitrator selected by the other Party within such [*] Business Day period shall be entitled to select such arbitrator, and, to the extent all three such arbitrators are not selected within [*] Calendar Days of the Initiation Notice, such arbitrators shall be appointed by the International Court of Arbitration of the ICC. Prior to the commencement of hearings, each of the arbitrators appointed must provide an oath of undertaking of impartiality. The decision of the arbitrators will be final and binding on the Patties, and judgment upon the award or determination rendered by the arbitrators may be entered and enforced in any court of competent jurisdiction. The arbitration shall be conducted in English, and the place of arbitration shall be New York, New York, USA. Each Party shall bear its own expenses and an equal share of the reasonable, documented expenses of the arbitration panel and any fees required by ICC to submit such matter to arbitration, unless the panel determines that any such fees or expenses are to be paid by the non-prevailing Party, and the Parties hereby agree that the panel shall be entitled and empowered to make such a determination.
14.3Injunctions. Notwithstanding anything to the contrary in this Agreement, either Party will have the right to seek injunctive or equitable relief in any court of competent jurisdiction as may be available to such Party under the laws and rules applicable in such jurisdiction with respect to any matters arising out of this Agreement. A Party seeking and/or obtaining injunctions shall not be required to prove the amount, irreparability, immediacy or likelihood of damages, nor shall it be required to post any bond (the posting of which is irrevocably waived).
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
44
14.4Choice of Law. This Agreement shall be construed and the rights of the Parties shall be determined in accordance with the laws of the State of New York, USA, without regard to its conflict of law provisions; provided, however, that patents and other intellectual property rights shall be construed and determined in accordance with the laws of the country under which such rights are granted. In no event shall the provisions of this Agreement be governed by the United Nations Convention on Contracts for the International Sale of Goods.
|
15. |
General Provisions |
15.1Integration & Severability. This Agreement, including its Exhibits, the Quality Agreement, and the Confidentiality Agreement, is the full and final negotiated agreement between the Parties regarding its subject matter. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original, but all of which together shall constitute a single agreement. In the event that any provision of this Agreement is judicially determined to be unenforceable, in part or in whole, the remaining provisions or portions of this Agreement shall be valid and binding to the fullest extent possible, and the Parties shall endeavor to negotiate modified or additional terms, as feasible, in a timely manner so as to fully effectuate the original intent of the Parties to the extent possible.
15.2Waivers & Amendment. Any failure by a Party to enforce any right which it may have hereunder in any instance shall not be deemed to waive any right which it or the other Party may have with respect to any provision of this Agreement, including the provision which such Party has failed to enforce. A waiver of a breach shall not act as a waiver or release of any other breach, regardless if prior, contemporaneous or subsequent, known or unknown or of the same or different nature, cause, effect or provision of this Agreement. No provision of this Agreement shall be waived, amended, supplemented or otherwise modified except in a writing signed by a duly authorized officer of each Party.
15.3Legal Relationship. The Parties acknowledge, agree, and declare that the relationship hereby established between them is solely that of provider and recipient of manufacturing services and that each Party hereto is an independent contractor with respect to the other, and not as a joint venturer, partner, distributor or any other type of relationship, and shall not be construed as an authorization of either Party to act as an agent of the other. Each may enter into similar or dissimilar arrangements with others and engage in activities for its own account, subject to their compliance with confidentiality and other provisions of this Agreement. The Parties agree that they have performed and shall at all times perform this Agreement in good faith.
15.4Force Majeure.
(a)Occurrences. Neither Party shall be responsible to the other Party for any failure, delay or interruption in the performance of any of its obligations under this Agreement if such failure, delay or interruption is caused by a matter reasonably outside of the control of the Party, which may include, but shall not be limited to, fire, flood, typhoon, earthquake, epidemic, riot, terrorist act, insurrection, war, failure or delay of normal sources of supply of materials, failure or delay of public utilities or carriers (“Force Majeure”), provided that the Party affected has used its best efforts to avoid the effects of such occurrence and to perform its obligations notwithstanding such occurrence, and such occurrence is not due to any fault or neglect of such
45
Party. If a Party believes that the performance of any of its obligations under this Agreement will be delayed or interrupted as a result of any Force Majeure event, then it shall promptly notify the other Party of the delay or interruption and the cause, shall use best efforts to perform its obligations notwithstanding the Force Majeure event, and shall provide the other Party with a good faith estimate of when performance of its obligations will resume. When the Party affected by a Force Majeure event is able to recommence the performance of obligations delayed or interrupted as a result of the Force Majeure event, it shall notify the other Party and, except as otherwise provided in this Agreement, it shall promptly resume performing its obligations.
(b)Production Assurance. For clarity, FFFC shall not be entitled to invoke the provisions of this Section 15.4 as an excuse for default or delay in performance of its obligations under this Agreement based upon its need to do work for others or on its own behalf resulting in constraints upon the availability of its manufacturing and packaging capacity, unless such constraints resulted from an event of Force Majeure as defined herein. In such an event, FFFC shall equitably allocate its available resources among its various customers, including Cempra. Additionally, in the event FFFC cannot provide Cempra with API for more than [*] days due to a Force Majeure event, FFFC will notify Cempra and Cempra shall be entitled, at its option, to terminate or suspend this Agreement in whole or in part upon written notice to FFFC. For clarification, during suspension of this Agreement as permitted in this Section 15.4(b), or if this Agreement is terminated pursuant to this Section 15.4(b), Cempra may utilize one or more other sources for all of Cempra’s API requirements and shall not be obligated to purchase API that was ordered for delivery from FFFC during such time. Furthermore, a suspension of this Agreement shall only be lifted, and the obligations of the parties resumed, after FFFC has demonstrated to the reasonable satisfaction of Cempra that FFFC has resolved the Force Majeure and can meet its obligations hereunder in full.
15.5Notice; Use of English. Any notice required or permitted to be given under this Agreement shall be in writing and shall be given in person, delivered by recognized overnight delivery service, sent by mail (certified or registered or air mail for addresses outside of the continental United States), or by telefax (or other similar means of electronic communication), the receipt of which is confirmed by confirming telefax, and addressed as indicated in Exhibit E, or such other person and/or address as may have been furnished in writing to the notifying Party of the change to such Exhibit. Except as otherwise provided herein, any notice shall be deemed delivered upon the earlier of: (a) actual receipt; (b) three (3) business days after delivery to such recognized overnight delivery service; (c) five (5) business days after deposit in the mail (ten (10) days for international mail); or (d) the date of receipt of the confirming telefax. All notices and other correspondence between the Parties shall be in English and English translations of all documents originally prepared, provided, or obtained in Japanese or any other non-English language shall be provided to Cempra simultaneously with the non-English originals thereof at FFFC’s expense.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
46
15.6Assignment. Neither Party may assign this Agreement, or any of its rights or obligations hereunder, without the other Party’s prior written consent, which consent shall not be unreasonably withheld or delayed; provided that, notwithstanding the foregoing, Cempra shall be entitled, without FFFC’s prior written consent, to assign or transfer this Agreement and Cempra’s rights and obligations hereunder: (i) in connection with the transfer or sale of all or substantially all of Cempra’s or any of its Affiliates’ assets or business (or that portion thereof related to the subject matter of this Agreement), (ii) in the event of Cempra’s or any of its Affiliates’ merger, consolidation, reorganization, change of control or similar transaction, or (iii) to an Affiliate of Cempra. Any purported assignment by a Party of this Agreement, or any of such Party’s rights or obligations hereunder, in violation of this Section 15.6 shall be null and void ab initio.
15.7Interpretation. All references to Sections shall refer to the Sections contained in this Agreement. All references to Exhibits shall, except as otherwise explicitly provided, refer to the Exhibits of this Agreement, which are appended to and made part of this Agreement. The captions of the Sections of this Agreement are for general information and reference only and shall not affect the interpretation of this Agreement. Where applicable in this Agreement, the singular includes the plural and vice versa. The term “including” shall be interpreted to mean “including without limitation”. English shall be the official language of this Agreement and all communications between the Parties hereto shall be conducted in that language. Both Parties acknowledge that they were represented by competent legal counsel and advisors, and fully negotiated the contract and each of its terms, and that ambiguities, if any, in this Agreement shall not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision.
«Signatures on Next Page»
47
In Witness Whereof, the Parties hereto have caused this Agreement to be executed as of the Effective Date.
|
Cempra Pharmaceuticals, Inc. By: /s/ Xxxxxxxxxxx Xxxxxxxxx Name: Xxxxxxxxxxx Xxxxxxxxx Title: President and CEO Date: December 16, 2015 |
FUJIFILM Finechemicals Co., Ltd. By: /s/ Xxxxxxxxx Xxxx Name: Xxxxxxxxx Xxxx Title: President and CEO Date: January 18, 2016 |
|
API Manufacturing and Supply Agreement Signature Page |
Confidential
Solithromycin
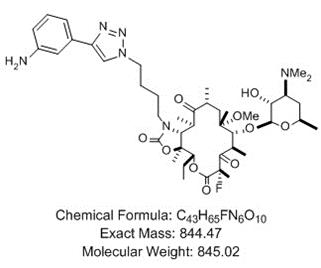
CAS# 760981-83-7
Confidential
[API Specifications*]
|
Test |
Method |
Oral Grade (Specification FP/IH/304995/4) |
Parenteral Grade (Specification FP/IH/304994/2) |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
|
|
|
|
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
|
|
|
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
|
|
[*] |
|
[*] |
[*] |
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential
|
Method |
Oral Grade (Specification FP/IH/304995/4) |
Parenteral Grade (Specification FP/IH/304994/2) |
||
|
[*] |
[*] |
|
|
|
|
|
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
||
|
|
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
||
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
|
|
|
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
[*] |
[*] |
|
|
|
[*] |
[*] |
[*] |
|
|
[*] |
[*] |
[*] |
[*] |
|
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential
Additional Non Release Tests for Drug Substance
|
Test |
Method Description |
Oral Grade Acceptance Criteria |
Parenteral Grade Acceptance Criteria |
|
|
|
|
|
|
[*] |
[*] |
[*] |
[*] |
|
[*] |
[*] |
[*] |
[*] |
|
[*] |
[*] |
[*] |
[*] |
|
[*] |
[*] |
[*] |
[*] |
|
[*] |
[*] |
[*] |
[*] |
|
[*] [*] [*] [*] [*] |
[*] |
[*] |
[*] |
|
[*] |
[*] |
[*] |
[*] |
N/A = Not applicable
* These are the current specifications as of Oct 2015. They are subject to revision. The current specifications in effect at the time of release should be used.
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential
Cempra Licensed Patents
|
Title |
Country |
Application Serial No. |
Filing Date |
Patent No. |
|
Copper-Catalysed Ligation of Azides and Acetylenes |
USA |
60/385,041 |
30 May 2002 |
n/a |
|
Copper-Catalysed Ligation of Azides and Acetylenes |
PCT |
PCT/US03/17311 |
30 May 2003 |
n/a |
|
Copper-Catalysed Ligation of Azides and Acetylenes |
Japan |
2004-509665 |
30 May 2003 |
4638225 |
|
Process for the Preparation of Macrolide Antibacterial Agents |
USA |
60/982,446 |
25 Oct 2007 |
n/a |
|
Process for the Preparation of Macrolide Antibacterial Agents |
PCT |
PCT/US2008/080936 |
23 Oct 2008 |
n/a |
|
Process for the Preparation of Macrolide Antibacterial Agents |
Japan |
2010-531238 |
23 Oct 2008 |
5698979 |
|
Process for the Preparation of Macrolide Antibacterial Agents |
Japan |
2014-227753 |
23 Oct 2008 |
|
|
Morphological Forms of CEM-101, and Uses Therefor |
USA |
61/316,063 |
22 Mar 2010 |
n/a |
|
Crystalline Forms of a Macrolide, and Uses Therefor |
PCT |
PCT/US2011/029424 |
22 Mar 2011 |
n/a |
|
Crystalline Forms of a Macrolide, and Uses Therefor |
Japan |
2013-501396 |
22 Mar 2011 |
5711352 |
|
Crystalline Forms of a Macrolide, and Uses Therefor |
Japan |
2014-231987 |
22 Mar 2011 |
|
|
Process For Preparing Triazole-Containing Ketolide Antibiotics |
USA |
61/346,664 |
20 May 2010 |
n/a |
|
Processes for Preparing Macrolides and Ketolides and Intermediates Therefor |
PCT |
PCT/US2011/037330 |
20 May 2011 |
n/a |
|
Processes for Preparing Macrolides and Ketolides and Intermediates Therefor |
Japan |
2013-511385 |
20 May 2011 |
|
|
Hydrogen Bond Forming Fluoro Ketolides for Treating Diseases |
USA |
61/381,794 |
10 Sep 2010 |
n/a |
|
Hydrogen Bond Forming Fluoro Ketolides for Treating Diseases |
PCT |
PCT/US2011/051064 |
9 Sep 2011 |
n/a |
Confidential
Confidential
Form of Certificate of Analysis
Confidential

Confidential
Exhibit E
FFFC Facilities, Notices, Project Managers and Other Key Personnel
Facility: [*]
Address: [* ]
TEL: [*]FAX: [*]
Notices:
|
To Cempra:
|
To FFFC:
|
Project Managers:
|
[*] Executive Vice President, Business Development E-mail [*] TEL: [*] FAX: [*]
|
For FFFC: [*] General Manager, Marketing Department E-mail [*] TEL: [*] FAX: [*] |
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential
|
For Cempra: [*] Senior Vice President, Chemistry E-mail [*] TEL: [*] FAX: [*]
[*] Vice President, Supply Chain E-mail [*] TEL: [*] FAX: [*]
[*] Director, Chemistry E-mail [*] TEL: [*] FAX: [*]
[*] Associate Director, Analytical Chemistry E-mail [*] TEL: [*] FAX: [*]
|
For FFFC: [*] General Manager, Production Engineering & Development Dept. E-mail [*] TEL: [*] FAX: [*]
[*] Manager, Organic Synthesis Research Laboratories E-mail [*] TEL: [*] FAX: [*]
[*] General Manager, HIRONO Factory Quality Assurance Department E-mail [*] TEL: [*] FAX: [*]
|
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential
Exhibit F
Copy of Quality Agreement
To be attached once agreed upon by the Parties.
Confidential
Exhibit G
Work Plan for Registration Batches Manufacturing
To be attached once agreed upon by the Parties.
Confidential
Exhibit H
Work Plan for Validation Campaign
To be attached once agreed upon by the Parties.
Confidential
Exhibit I
Stability Study Protocols
To be attached once agreed upon by the Parties.
Confidential
Transfer Price
|
Transfer Price per kg *1 |
Quantities of API Ordered for Delivery Per Month Period *2 |
|
No more than US$[*] |
Equal or more than [*] kg |
|
No more than US$[*] |
Equal or more than [*] kg and less than [*] kg |
|
No more than US$[*] |
Equal or more than [*] kg and less than [*] kg |
|
No more than US$[*] |
Equal or more than [*] kg and less than [*] kg |
|
No more than US$[*] |
Equal or more than [*] kg and less than [*] kg |
|
Subject to the reasonable quotation made by FFFC, and accepted in writing by Cempra, separately on an as-needed basis |
Less than [*] kg |
* 1 Each price does not include Japanese consumption tax imposed on the sale of API to Cempra under this Agreement.
|
* 2 |
If the Agreement terminates or expires on any day other than the last day of a Month Period, the Transfer Price for API ordered for delivery during that portion of the Term following the end of the last complete Month Period ending prior to such expiration or termination (such portion of the Term, the “Final Period”) shall, notwithstanding anything to the contrary, be calculated by dividing the quantity of API ordered for delivery during such Final Period by the number of the days of such Final Period and multiplying the number of such calculation’s result by 365, with the result of such calculation being used as the “Quantities of API Ordered for Delivery Per Month Period” in the table above to calculate the Transfer Price of API ordered for delivery during such Final Period. |
Provided, however, that:
|
|
· |
If the above Transfer Price becomes apparently unreasonable because of significant change of the economic environment applicable to the manufacture of API by FFFC, and either Party requests to the other Party the revision of such Transfer Price, the Parties shall use reasonable efforts to negotiate about a new Transfer Price applicable to the next Month Period or thereafter in good faith, provided that (i) the above-referenced obligation shall not require either party to agree to any new Transfer Price and (ii) the Transfer Price shall not be changed unless and until written agreement is reached by both Parties. |
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential
|
|
· |
If any change of API Manufacture Procedures, quality requirements, API Specifications, or other related matters of API requested pursuant to Cempra’s (or its Affiliate’s or its or its Affiliate’s licensee’s) instruction or direction following the Effective Date increases, by an amount greater than [*] percent ([*]%) of FFFC’s prior manufacturing cost (as calculated for the certain pricing tier set forth in the table above that will be applicable based on the quantities of API which are forecasted and/or scheduled to be ordered for delivery during the Month Period to which such increase applies and as compared to the previously-effective costs for such quantities of API during the prior Month Period), the manufacturing cost born by FFFC, FFFC shall notify Cempra promptly in writing of any such increase in FFFC’s direct cost of manufacturing API hereunder (including the amount of such increase) and, if Cempra elects in writing to proceed with such change following receipt of such notice, the Transfer Price shall be revised by mutual reasonable good-faith consultation between the Parties based on the new quotation issued by FFFC, provided that (i) any such increase in Transfer Price shall not exceed the increase in direct cost to FFFC of any such change and (ii) FFFC shall not proceed with any such change unless directed to do so by Cempra following Cempra’s receipt of notice of the relevant proposed change in Transfer Price. For clarity, in the absence of any agreement between the Parties regarding any increase in Transfer Price as a result of any requested changes of Cempra under this paragraph, FFFC shall not be required to manufacture any API pursuant to any such change in API Manufacture Procedures, quality requirements, API Specifications, or other related matters of API, and Cempra shall not be required to bear any increase in Transfer Price resulting therefrom. |
The Transfer Price in the table above to be applied pursuant to this Exhibit J for purposes of Sections 1.46, 4, 5.4, and 6.2 shall initially be (i) for the [*] [*] Month Periods, US$[*] per kilogram of API and, for all Month Periods other than the [*] [*] Month Periods, (ii) calculated based on the amounts of API forecasted for order and delivery during a particular Month Period based on the initial Forecast covering all twelve (12) months of such Month Period (such initial Forecast, the “Initial Forecast”, such initial price under the preceding clause (i) or clause (ii), the “Forecast-Based Price”) and then, following the end of such Month Period, recalculated based on the actual amounts of API actually ordered for delivery during such Month Period pursuant to Purchase Orders placed by Cempra (such recalculated price, the “Final Price”). If the amount of API forecasted for order and delivery during a particular Month Period, as reflected by any Forecasts, following the Initial Forecast, covering any portion of such Month Period, are materially inconsistent with the corresponding amount forecasted in the Initial Forecast (or any other previous Forecast following the Initial Forecast), the Parties shall, upon written notice from either Party to the other Party, use reasonable efforts to work together in good faith to mutually agree on a revised Forecast-Based Price for the remaining portion of such Month Period intended to minimize the difference between the total Forecast-Based Prices paid or due for API delivered in such Month Period and the total Final Price applicable to API delivered in such Month Period.
Confidential
FFFC Account Number [*]
|
|
Bank Name: |
[*] |
|
|
Branch Name: |
[*] |
|
|
|
|
|
|
Account Name: |
[*] |
|
|
Account #: |
[*] [*] |
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential
Cempra API Patents
|
Title |
Country |
Application Serial No. |
Filing Date |
Patent No. |
|
Process for the Preparation of Macrolide Antibacterial Agents |
Japan |
2010-531238 |
23 Oct 2008 |
5698979 |
|
Process for the Preparation of Macrolide Antibacterial Agents |
Japan |
2014-227753 |
23 Oct 2008 |
|
|
Crystalline Forms of a Macrolide, and Uses Therefor |
Japan |
2013-501396 |
22 Mar 2011 |
5711352 |
|
Crystalline Forms of a Macrolide, and Uses Therefor |
Japan |
2014-231987 |
22 Mar 2011 |
|
|
Processes for Preparing Macrolides and Ketolides and Intermediates Therefor |
Japan |
2013-511385 |
20 May 2011 |
|
|
Convergent Process for the Preparation of Macrolide Antibacterial Agents |
PCT |
PCT/US2014/29932 |
15-Mar-2014 |
n/a |
|
Process for Preparing Fluorinated Ketolide Antibiotics |
USA |
62/129,305 |
06-Mar-2015 |
n/a |
Confidential
[*]
[*] Confidential treatment requested; certain information omitted and filed separately with the SEC.
Confidential


