Collaboration and License Agreement by and between KVK Tech, Inc. and KemPharm, Inc. Dated as of October 25, 2018
Exhibit 10.28
[*] = Certain confidential information contained in this document, marked by brackets, is filed with the Securities and Exchange Commission pursuant to Rule 24b-2 of the Securities Exchange Act of 1934, as amended.
Collaboration and License Agreement
by and between
KVK Tech, Inc.
and
Dated as of October 25, 2018
COLLABORATION AND LICENSE AGREEMENT
This Collaboration and License Agreement (the “Agreement”) is entered into as of October 25, 2018 (the “Effective Date”) by and between KemPharm, Inc., a Delaware corporation, with its principal place of business at ▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇ (“KemPharm”) and KVK Tech, Inc., a Delaware corporation having a place of business at ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇, ▇▇, ▇▇▇▇▇ (“KVK”). KemPharm and KVK are sometimes referred to herein individually as a “Party” and collectively as the “Parties.”
RECITALS
Whereas, KemPharm has developed the Product, as defined below, for the short-term management of acute pain, and has obtained regulatory approval of the Product in the U.S. for such Indication, as defined below; and
Whereas, KVK desires to obtain from KemPharm an exclusive license to conduct Regulatory Activities with respect to, manufacture and Commercialize, the Licensed Products in the U.S., and KemPharm is willing to grant such license to KVK, all under the terms and conditions hereof, with capitalized terms as defined below.
Now, Therefore, in consideration of the foregoing premises and the mutual promises, covenants and conditions contained in this Agreement, the Parties agree as follows:
Article 1
Definitions
1.1 “Act” shall mean, as applicable, the United States Federal Food, Drug and Cosmetic Act, 21 U.S.C. §§301 et seq., and/or the Public Health Service Act, 42 U.S.C. §§262 et seq., as such may be amended from time to time.
1.2 “Affiliate” means, with respect to a particular Party, a Person that controls, is controlled by or is under common control with such Party. For the purposes of this definition, the word “control” (including, with correlative meaning, the terms “controlled by” or “under common control with”) means the actual power, either directly or indirectly through one or more intermediaries, to direct or cause the direction of the management and policies of such entity, whether by the ownership of fifty percent (50%) or more of the voting stock of such entity, or by contract or otherwise. For clarity, once a Person ceases to be an Affiliate of a Party, then, without any further action, such Person shall cease to have any rights, including license and sublicense rights, under this Agreement by reason of being an Affiliate of such Party.
1.3 “Authorized Generic” means any version of Apadaz® specifically authorized by KVK for marketing and distribution as a generic in the Collaboration Territory.
1.4 “Business Day” means a day other than Saturday, Sunday or any day that banks in New York, New York are required or permitted to be closed.
1.5 “Change of Control” means with respect to either Party: (a) the sale of all or substantially all of such Party’s assets or business relating to this Agreement; (b) a merger, reorganization or consolidation involving such Party in which the voting securities of such Party outstanding immediately prior thereto cease to represent at least fifty percent (50%) of the combined voting power of the surviving entity immediately after such merger, reorganization or consolidation; or (c) a person or entity, or group of persons or entities, acting in concert acquire more than fifty percent (50%) of the voting equity securities or management control of such Party.
1.6 “Collaboration Territory” means the U.S., including its territories and possessions.
1.7 “CMC Information” means Information related to the chemistry, manufacturing and controls of the Licensed Products, as specified by the FDA and other applicable Regulatory Authorities.
1.8 “Commercialization,” with a correlative meaning for “Commercialize” and “Commercializing,” means all activities undertaken before and after obtaining Regulatory Approvals relating specifically to the pre-launch, launch, promotion, detailing, medical education and medical liaison activities, marketing, pricing, reimbursement, sale, and distribution of Licensed Products, including strategic marketing, sales force detailing, advertising, market Licensed Product support, all customer support, Licensed Product distribution and invoicing and sales activities.
1.9 “Commercialization/Regulatory Expenses” means the actual, out-of-pocket costs and expenses, in U.S. Dollars, as defined by KVK’s consistent application of U.S. GAAP, incurred by or on behalf of KVK in (a) the Commercialization of Licensed Products in the Field in the Collaboration Territory, including any payments made by KVK to Third Parties in connection with obtaining or maintaining intellectual property rights that are necessary or useful for the Commercialization of Licensed Products under this Agreement (“Third Party Commercial IP Costs”) (it being understood that, in the event that any other Third Party is receiving the benefit of such Third Party intellectual property from the applicable Third Party licensor, then the Parties, through the JSC, as defined in Section 3.2(a), shall mutually agree upon an appropriate allocation of such Third Party Commercial IP Costs as between, on the one hand, KVK and KemPharm, and on the other hand, such Third Party licensee, prior to KVK entering into an agreement with such Third Party) and (b) the maintenance of Regulatory Approvals for Licensed Products.
1.10 “Commercially Reasonable Efforts” means, with respect to either Party’s obligations under this Agreement, the carrying out of such obligations with a level of efforts and resources consistent with the commercially reasonable practices of a similarly situated company in the pharmaceutical industry for the active and diligent commercialization of a similarly situated branded pharmaceutical product as the Licensed Product at a similar stage of commercialization, taking into account efficacy, safety, patent and regulatory exclusivity, anticipated or approved labeling, present and future market potential, competitive market conditions, the profitability of the product in light of pricing and reimbursement issues, and all other relevant factors, but not taking into account any payment owed to KemPharm under this Agreement or any other pharmaceutical product that KVK is then researching, developing or commercializing, alone or with one or more collaborators.
1.11 [*].
1.12 “Confidential Information” of a Party means any and all Information, separately or in combination, of such Party that is disclosed to the other Party under this Agreement, whether in oral, written, graphic, or electronic form. In addition, all Information disclosed by a Party pursuant to the confidentiality agreement between the Parties dated November 28, 2017 (the “Confidentiality Agreement”) shall be deemed to be Confidential Information of such Party disclosed hereunder; provided, however, that any use or disclosure of any such Information that is authorized under Article 12 shall not be restricted by, or be deemed a violation of, the Confidentiality Agreement.
1.13 “Control” means, with respect to any material, Information, or intellectual property right, that a Party (a) owns or (b) has a license, other than a license granted to such Party under this Agreement, to such material, Information, or intellectual property right and, in each case, has the ability to grant to the other Party access, a license, or a sublicense, as applicable, to the foregoing on the terms and conditions set forth in this Agreement without violating the terms of any existing agreement or other arrangement with any Third Party or being obligated to pay any royalties or other consideration therefor.
1.14 “Data” means all data generated by or on behalf of a Party or its Affiliate or their respective sublicensees pursuant to activities conducted under this Agreement. For clarity, Data does not include (a) any patentable inventions or (b) any formulations of benzhydrocodone or (c) any patentable inventions or any formulations of benzhydrocodone with any other pharmaceutical ingredient(s) other than acetaminophen.
1.15 “Development,” with a correlative meaning for “Develop” and “Developing,” means all activities relating to preclinical and clinical trials, toxicology testing, statistical analysis and publication and presentation of study results with respect to Licensed Products (collectively, “Development Activities”) and (b) the reporting, preparation and submission of applications (including any CMC Information) for obtaining, registering and/or maintaining Regulatory Approval of Licensed Products (collectively, “Regulatory Activities”).
1.16 “FDA” means the U.S. Food and Drug Administration or any successor entity.
1.17 “Field” means the therapeutic treatment of any and all human diseases and conditions that are included in the KemPharm Indications for the Licensed Product(s) and as are specifically indicated and included as per the Label.
1.18 “First Commercial Sale” means the first sale of a Licensed Product in the Collaboration Territory to a Third Party after Regulatory Approval has been obtained in the Collaboration Territory.
1.19 “Fiscal Year” means KVK’s fiscal year that starts from January 1st and ends on December 31st.
1.20 “GCP” or “Good Clinical Practices” means the then-current standards, practices and procedures promulgated or endorsed by the FDA as set forth in the guidelines entitled “Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” including related regulatory requirements imposed by the FDA, as they may be updated from time to time.
1.21 “GLP” or “Good Laboratory Practices” means the then-current good laboratory practice standards promulgated or endorsed by the FDA as defined in 21 C.F.R. Part 58, as they may be updated from time to time.
1.22 “Governmental Authority” means any multi-national, national, federal, state, local, municipal, provincial or other governmental authority of any nature (including any governmental division, prefecture, subdivision, department, agency, bureau, branch, office, commission, council, court or other tribunal).
1.23 “Government Official” means (a) any official or employee of any Governmental Authority, or any department, agency, or instrumentality thereof (including without limitation commercial entities owned or controlled, directly or indirectly, by a Governmental Authority), (b) any political party or official thereof, or any candidate for political office, in the Collaboration Territory or any other country, or (c) any official or employee of any public international organization.
1.24 “IND” means an Investigational New Drug application filed with the FDA, or an equivalent filing outside of the U.S.
1.25 “Indication” means a separately defined, well-categorized class of human disease or condition for which a separate NDA (including any extensions or supplements) may be, or has been, filed with the FDA. For clarity, if an NDA is approved for a Licensed Product in a particular Indication and patient population, a Label expansion for such Licensed Product to include such Indication in a different patient population shall not be considered a separate Indication.
1.26 “Information” means any data, results, technology, business or financial information or information of any type whatsoever, in any tangible or intangible form, including know-how, copyrights, trade secrets, practices, techniques, methods, processes, inventions, developments, specifications, formulae, software, algorithms, marketing reports, expertise, technology, test data (including pharmacological, biological, chemical, biochemical, clinical test data and data resulting from non-clinical studies), CMC Information, stability data and other study data and procedures.
1.27 “Inventions” means any inventions and/or discoveries, including Information, processes, methods, assays, designs, protocols, and formulas, and improvements or modifications thereof, patentable or otherwise, that is generated, developed, conceived or reduced to practice by or on behalf of a Party or its Affiliate or their respective sublicensees pursuant to activities conducted under this Agreement and that are directly related to the Licensed Product (combination product of benzhydrocodone and acetaminophen, e.g. Apadaz® , in each case including all rights, title and interest in and to the intellectual property rights therein and thereto; provided, however, that Inventions shall exclude Data.
1.28 “KemPharm Indications” means (a) any separately defined, well-categorized class of human disease or condition for which KemPharm has filed either a separate IND to an appropriate Regulatory Authority or a separate application to an appropriate Regulatory Authority for approval to market Licensed Products in the Collaboration Territory, or for which KemPharm is otherwise developing or commercializing Licensed Products in the Collaboration Territory (whether before or after the Effective Date) (which, for clarity, includes the short-term management of acute pain) or (b) the Label
1.29 “KemPharm Product ▇▇▇▇” means KemPharm’s (or its Affiliates) trademark Apadaz®, or the alternative trademark selected by KemPharm for the Licensed Products in the Collaboration Territory pursuant to Section 9.6(a)(i), and related trade dress.
1.30 “KVK Inventions” means any Inventions generated by or on behalf of KVK, its Affiliates and their respective sublicensees, including their employees, agents and contractors.
1.31 “KVK Patents” means any Patents that claim KVK Inventions.
1.32 “Label” means any and all Indications as approved by an appropriate Regulatory Authority (e.g. FDA) for which the Licensed Product may be marketed, sold and used in the Collaboration Territory. For purposes of clarity, as of the Execution Date of this Agreement, the Label for the Licensed Product is attached to this Agreement as Exhibit C.
1.33 “Laws” means all laws, statutes, rules, regulations, ordinances and other pronouncements having the effect of law of any federal, national, multinational, state, provincial, county, city or other political subdivision, domestic or foreign.
1.34 “Licensed IP” means the Licensed Know-How and Licensed Patents.
1.35 “Licensed Know-How” means all Information (including Data and Regulatory Materials) that (a) (i) is Controlled by KemPharm or its Affiliates as of the Effective Date or (ii) becomes Controlled by KemPharm or its Affiliates during the Term, and (b) is necessary for the conduct of Regulatory Activities with respect to, Commercialization of, or the manufacture of, Licensed Products in the Field in the Collaboration Territory.
1.36 “Licensed Patents” means (a) with respect to Patents Controlled by KemPharm or any of its Affiliates as of the Effective Date, the Patents set forth in Exhibit A; and (b) with respect to any Patents that become Controlled by KemPharm or its Affiliates during the Term, such Patents that (i) are necessary for the conduct of Regulatory Activities with respect to, Commercialization of, or the manufacture of Licensed Products in the Field in the Collaboration Territory.
1.37 “Limited Licensed Priority Patents” means (a) with respect to Patents Controlled by KemPharm or any of its Affiliates as of the Effective Date, the Patents set forth in Exhibit A-1 which are those Patents from any of the Patents set forth in Exhibit A as noted in Section 1.35 herein. For sake of clarity, issued claims within said Limited Licensed Priority Patents directly related to, or encompassing, products, combinations, compounds etc. other than the combination product of benzhydrocodone with acetaminophen are not included in the scope of the Limited License.
1.38 “Licensed Product” shall mean (a) the Product (e.g. Apadaz®), including any Authorized Generic version thereof, or (b) any other salt or polymorphic form of the Product being developed or commercialized by KemPharm in the Collaboration Territory. For clarity, Licensed Products shall exclude any product that (i) contains benzhydrocodone, benzhydrocodone pro-drug or benzyhydrocodone product candidate as the sole active ingredient, or (ii) contains benzhydrocodone in combination with any pharmaceutical ingredient(s) other than acetaminophen
1.39 “Manufacturing Expenses” means the fully burdened manufacturing cost in U.S. Dollars, as defined by applicable Party’s consistent application of U.S. GAAP, of producing or obtaining supply of Licensed Products, which cost shall include, labor and material costs, quality assurance and control expenses, including required stability monitoring, and allocable facilities costs; provided, however, that if the applicable Party uses a contract manufacturer to perform any manufacturing activities with respect to the supply of Licensed Product under this Agreement, “Manufacturing Expenses” for such activities will be the sum of: (a) the price such Party pays such contract manufacturer for such activities, (b) such Party’s fully burdened cost of supporting the contract manufacturer’s performance of manufacturing activities with respect to Licensed Products and (c) any payments made by such Party to Third Parties in connection with obtaining or maintaining intellectual property rights that are necessary or useful for the manufacture of Licensed Products under this Agreement (irrespective of whether such agreements with Third Parties were entered into prior to the Effective Date or during the Term).
1.40 “NDA” means a New Drug Application filed with the FDA required for marketing approval for a Licensed Product in the U.S., but excluding pricing approvals.
1.41 “Net Profits” means, with respect to a given Rolling Year, the amount of Net Sales received by KVK, its Affiliates and their respective sublicensees in the Field in the Collaboration Territory for sales of Licensed Products, minus: (a) [*] incurred in such Rolling Year for Licensed Products and (b) [*] incurred in such Rolling Year for Licensed Products. If the amount received by KVK for Net Sales of Licensed Products in a given Rolling Year is less than the sum of the [*] and [*] in such Rolling Year, then the amount of such deficit shall be deemed a “Net Loss”.
1.42 “Net Sales” means the gross amounts billed or invoiced by KVK, its Affiliates and their respective sublicensees for sales of Licensed Products to unaffiliated Third Parties, less the following deductions to the extent reasonable and customary, provided to unaffiliated entities and actually allowed and taken with respect to such sales:
(a) [*]
(b) [*]
(c) [*]
(d) [*] and
(e) [*].
Notwithstanding the foregoing, amounts received, billed or invoiced by KVK, its Affiliates, or their respective sublicensees for the sale of Licensed Product among KVK, its Affiliates or their respective sublicensees shall not be included in the computation of Net Sales hereunder unless the purchasing entity is the end-user. For purposes of determining Net Sales, the Licensed Product shall be deemed to be sold when billed or invoiced. Net Sales shall be accounted for in accordance with standard KVK practices for operation by KVK, its Affiliates or their respective sublicensees, as practiced in the Collaboration Territory, but in any event in accordance with U.S. GAAP, consistently applied in the Collaboration Territory. For clarity, a particular item may only be deducted once in the calculation of Net Sales.
With respect to any transfer of any Licensed Product in the Collaboration Territory for any substantive consideration other than monetary consideration on arm’s length terms, for the purposes of calculating the Net Sales under this Agreement, such Licensed Product shall be deemed to be sold exclusively for money at the average Net Sales price charged to Third Parties for cash sales in the Collaboration Territory during the applicable reporting period, or if there were only de minimis cash sales in the Collaboration Territory, at the fair market value as determined by comparable markets.
KVK, its Affiliates, and their respective sublicensees shall sell the Licensed Product as a standalone product and will not sell the Licensed Product as a part of a bundle with other products or offer packaged arrangements to customers that include the Licensed Product, except with KemPharm’s prior written consent.
1.43 “Patents” means (a) pending patent applications, issued patents, utility models and designs; (b) all patents issuing from patent applications of any of the foregoing; (c) reissues, substitutions, confirmations, registrations, validations, re-examinations, additions, continuations, continued prosecution applications, continuations-in-part, or divisions of or to any of the foregoing; and (d) extensions, renewals or restorations of any of the foregoing by existing or future extension, renewal or restoration mechanisms, including supplementary protection certificate or the equivalent thereof.
1.44 “Patent Term Adjustment” means the calculation of an adjustment to any United States Patent term pursuant to the Rules of the United States Patent Office and U. S. patent law.
1.45 “Patent Term Extension” means extension of U.S. patent term under 35 U.S.C. § 156 and related statutes to compensate for product commercialization delays due to regulatory review of the Licensed Product.
1.46 “Person” means an individual, corporation, partnership, limited liability company, limited partnership, trust, business trust, association, joint stock company, joint venture, pool, syndicate, “group” as defined in Section 13(d)(3) of the Securities Exchange Act of 1934, as amended, sole proprietorship, unincorporated organization, Governmental Authority or any other form of entity not specifically listed herein.
1.47 “Product” means specifically KemPharm’s benzhydrocodone/acetaminophen combination product for the short-term management of acute pain, which is branded in the U.S. as Apadaz® and sold in tablet form.
1.48 “Proper Conduct Practices” means, KVK and each of its Representatives not, directly or indirectly, (a) making, offering, authorizing, providing or paying anything of value in any form, whether in money, property, services or otherwise to any Government Official, or other Person charged with similar public or quasi-public duties, or to any customer, supplier, or any other Person, or to any employee thereof, or failing to disclose fully any such payments in violation of the laws of any relevant jurisdiction to (i) obtain favorable treatment in obtaining or retaining business for it or any of its Affiliates, (ii) pay for favorable treatment for business secured, (iii) obtain special concessions or for special concessions already obtained, for or in respect of it or any of its Affiliates, in each case which would have been in violation of any applicable Law, (iv) influence an act or decision of the recipient (including a decision not to act) in connection with the Person’s or its Affiliate’s business, (v) induce the recipient to use his or her influence to affect any government act or decision in connection with the Person’s or its Affiliate’s business or (vi) induce the recipient to violate his or her duty of loyalty to his or her organization, or as a reward for having done so; (b) engaging in any transactions, establishing or maintaining any fund or assets in which it or any of its Affiliates shall have proprietary rights that have not been recorded in the books and records of it or any of its Affiliates; (c) making any unlawful payment to any agent, employee, officer or director of any Person with which it or any of its Affiliates does business for the purpose of influencing such agent, employee, officer or director to do business with it or any of its Affiliates; (d) making any payment in the nature of bribery, fraud, or any other unlawful payment under the applicable Laws of any jurisdiction where it or any of its Affiliates conducts business or is registered; or, (e) if such Person or any of its Representatives is a Government Official, improperly using his or her position as a Government Official to influence the award of business or regulatory approvals to or for the benefit of such Person, its Representatives or any of their business operations, or failing to recuse himself or herself from any participation as a Government Official in decisions relating to such Person, its Representatives or any of their business operations.
1.49 “Regulatory Approval” means all approvals necessary for the commercial sale of a Licensed Product in the Field in a given country or regulatory jurisdiction.
1.50 “Regulatory Authority” means, in a particular country or jurisdiction, any applicable Governmental Authority involved in granting Regulatory Approval in such country or jurisdiction.
1.51 “Regulatory Materials” means regulatory applications, submissions, notifications, communications, correspondence, registrations, Regulatory Approvals and/or other filings made to, received from or otherwise conducted with a Regulatory Authority in order to develop, manufacture, market, sell or otherwise commercialize Licensed Products in a particular country or jurisdiction.
1.52 “Representatives” means, as to any Person, such Person’s Affiliates and its and their successors, controlling Persons, directors, officers and employees.
1.53 “Retained Territory” means the world except for the Collaboration Territory.
1.54 “Rolling Year” means, with respect to a given quarter, the period of four (4) consecutive quarters immediately prior to such quarter.
1.55 “Territory” means worldwide.
1.56 “Third Party” means any entity other than KemPharm or KVK or an Affiliate of either of them.
1.57 “Third Party Supply Agreement” means, collectively (a) the Material Supply Agreement, dated November 2, 2009, by and between KemPharm, Inc. and ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Inc., as amended by the First Amendment to the Material Supply Agreement, dated December 9, 2016; (b) the Letter Agreement, dated February 19, 2018, by and between KemPharm, Inc. and ▇▇▇▇▇▇▇ Matthey Inc., in each case, as amended; and (c) the Quality Agreement dated May 2, 2018, by and between KemPharm, Inc. and ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Inc.
1.58 “Transaction Agreements” means, collectively, (a) this Agreement and (b) any other agreement executed between the Parties in connection with any of the foregoing.
1.59 “U.S. Dollar” means a U.S. dollar, and “US$” shall be interpreted accordingly.
1.60 “U.S.” means the United States of America, including all possessions and territories thereof.
1.61 “U.S. GAAP” means the generally accepted accounting principles as applied to the U.S., including other comprehensive basis of accounting (“OCBOA”) as consistently applied by KVK.
1.62 Additional Definitions: The following table identifies the location of definitions set forth in various Sections of the Agreement:
|
Defined Terms |
Section |
|
Agreement |
Preamble |
|
Alliance Manager |
3.1 |
|
Claims |
11.1 |
|
Commercialization Budget |
6.2(a) |
|
Commercialization Plan |
6.2(a) |
|
Confidentiality Agreement |
1.11 |
|
Development Activities |
1.14 |
|
Effective Date |
Preamble |
|
Enforcing Party |
9.3(c) |
|
Executive Officers |
14.1 |
|
ICC Rules |
14.2(a) |
|
Indemnified Party |
11.3 |
|
Indemnifying Party |
11.3 |
|
Infringement |
9.3(a) |
|
Infringement Actions |
9.4 |
|
Joint Steering Committee or JSC |
3.2(a) |
|
KemPharm |
Preamble |
|
KemPharm Indemnitees |
11.2 |
|
KemPharm Partner |
2.2 |
|
KVK |
Preamble |
|
KVK Housemarks |
9.6(b) |
|
KVK Indemnitees |
11.1 |
|
KVK Sublicense Agreement |
2.1(c) |
|
Losses |
11.1 |
|
NDA |
1.38 |
|
Net Loss |
1.39 |
|
Net Profit Report |
8.4(b) |
|
Net Profit Share |
8.4(a) |
|
Profit Sharing Percentage |
8.4(a) |
|
Party |
Preamble |
|
Pharmacovigilance Agreement |
5.9 |
|
POMV Fee |
8.1 |
|
Proof of Market Viability |
6.9 |
|
Proof of Market Viability Termination |
6.9 |
|
Regulatory Activities |
1.14 |
|
Remedial Action |
5.10 |
|
Subcommittee |
3.2(b) |
|
Tax Withholding |
8.9(c) |
|
Term |
13.1 |
|
Third Party Commercial IP Costs |
61.9 |
|
VAT |
8.9(d) |
Article 2
License
2.1 License to KVK.
(a) License Grant. Subject to the terms and conditions of this Agreement, KemPharm hereby grants KVK an exclusive, even as to KemPharm except as provided in Section 2.1(b) below, license, with the right to sublicense solely as provided in Section 2.1(c), under the Licensed IP, to conduct Regulatory Activities, distribute, market, promote, sell, have sold, offer for sale, import and otherwise Commercialize or manufacture, Licensed Products in the Field in the Collaboration Territory. For clarity, the foregoing license does not include a right for KVK to (i) Develop, except to conduct Regulatory Activities, or (ii) change the content of the Label or package of any Licensed Product, except to provide applicable labeling or packaging material for Licensed Products to KemPharm in accordance with Section 6.8. For further sake of clarity, the License Grant of this section does include the right for KVK, pursuant to the terms of the instant Agreement, to (1) Commercialize, including, but not limited to, as permitted or obligated to perform under Article 6 or the Commercialization Plan, (2) manufacture the Licensed Product, and (3) conduct other permitted activities, utilize any rights or perform any obligations under this Agreement or a Transaction Agreement.
(i) Furthermore, Subject to the terms and conditions of this Agreement, KemPharm grants KVK a limited non-exclusive license, with no right to sublicense without the prior express written consent of KemPharm, under the Limited License Priority Patents, for only Patent Enforcement and Third-Party Patent Infringement Actions as per Article 9 herein.
(b) KemPharm Retained Rights. Notwithstanding the exclusive rights granted to KVK in Section 2.1(a), KemPharm and its Affiliates retain the following: (i) the right to practice the Licensed IP within the scope of the license granted to KVK under Section 2.1(a) only in order to perform, or have performed by a Third Party contractor, KemPharm’s obligations under this Agreement or a Transaction Agreement; (ii) the right to use and conduct Development Activities with respect to Licensed Products anywhere in the world, including the Collaboration Territory not otherwise in violation of this Agreement or a Transaction Agreement; (iii) the right to manufacture or have manufactured Licensed Products in the Retained Territory for sale and use in the Retained Territory or for sale to KVK not otherwise in violation of this Agreement or a Transaction Agreement; and (iv) the right to practice and license the Licensed IP outside the scope of the license granted to KVK in Section 2.1(a). and (v) the right to manufacture, subject to the Third Party Supply Agreement, practice and license and otherwise commercialize the Limited License Priority Patents in full.
(c) Sublicense Rights. KVK shall have the right to grant sublicenses of the license granted in Section 2.1(a) only with KemPharm’s express prior written consent, not to be unreasonably denied or unreasonably conditioned. Following receipt of KemPharm’s consent, KVK shall, within [*] days after granting any sublicense under Section 2.1(a), notify KemPharm of the grant of such sublicense and provide KemPharm with a true and complete copy of the sublicense agreement (each, a “KVK Sublicense Agreement”). Unless agreed to in writing by KemPharm, each KVK Sublicense Agreement shall be consistent with the terms and conditions of this Agreement, and KVK shall be solely responsible for all of its sublicensees’ activities and any and all failures by its sublicensees to comply with the terms of this Agreement. Without limiting the foregoing, each KVK Sublicense Agreement shall include the following additional terms and conditions:
(i) the sublicensee shall be bound by non-use and non-disclosure obligations no less stringent than those set forth in this Agreement;
(ii) the sublicensee shall not have any right to grant further sublicenses to the Licensed IP or to engage subcontractors to perform its obligations to KVK, without the consent of KVK and KemPharm;
(iii) the sublicensee shall not have any right to prosecute or maintain or enforce any Licensed Patents;
(iv) the sublicensee shall assign to KVK all Data and Inventions generated by such sublicensee;
(v) the sublicensee shall not have the right to submit, own or control any Regulatory Materials for Licensed Products; and
(vi) if this Agreement terminates, KemPharm shall, at its sole option and discretion, have the right to either (a) assume KVK’s rights and obligations under the KVK Sublicense Agreement or (b) terminate the KVK Sublicense Agreement in its entirety without penalty or other obligation to the sublicensee.
2.2 KemPharm Partner. KemPharm has the right, in its sole discretion, to enter into one or more agreements with Third Parties and grant such Third Parties the right to exercise any of KemPharm’s retained rights under Section 2.1(b), including to conduct Development Activities or to manufacture Licensed Products in the Collaboration Territory, or to Develop, manufacture or Commercialize Licensed Products in the Retained Territory (each such Third Party, a “KemPharm Partner”), and KemPharm shall have the right, but not the obligation, to exercise any and/or all of its rights and/or fulfill any and/or all of its obligations under this Agreement through KemPharm Partner(s), including granting KemPharm Partner(s) the right to participate in any Committee meetings as one or more of KemPharm’s representatives. KemPharm Partner(s) shall be bound by the obligations of this Agreement to the extent applicable to any rights of KVK herein and KemPharm shall be held solely responsible for any breaches of this Agreement by KemPharm Partner(s). KVK shall cooperate fully with KemPharm Partner(s) to the extent that KVK has the obligation under this Agreement to cooperate with KemPharm. KemPharm shall have the right to disclose to KemPharm Partner(s) all information regarding Licensed Products, including all Regulatory Materials, disclosed by KVK to KemPharm under this Agreement, for use by KemPharm Partner(s) in their conduct of Development Activities or manufacturing of Licensed Products in the Collaboration Territory, or their Development, manufacture or Commercialization of Licensed Products in the Retained Territory, provided, however, that all such information disclosed to KemPharm Partner(s) by KemPharm shall be deemed the Confidential Information of KVK and KemPharm and KemPharm Partner(s) that receive any such information shall be obligated to abide by restrictions on disclosure and use substantially similar to the provisions set forth in Section 12.1.
2.3 Negative Covenant. KVK covenants that it will not, and further that it will not permit any of its Affiliates or sublicensees to, use or practice (i) any Licensed IP outside the scope of the license granted to it under Section 2.1(a) and (ii) any Limited Licensed Priority Patents outside the scope of the limited license granted to it under Section 2.1(a)(i). KemPharm covenants that it will not and will not permit any of its Affiliates or KemPharm Partner(s) to, use or practice any Licensed IP except as explicitly permitted under Section 2.1(b).
2.4 No Implied Licenses. Except as explicitly set forth in this Agreement, neither Party shall be deemed by estoppel or implication to have granted the other Party any license or other right to any intellectual property of such Party.
2.5 Exclusivity.
(a) During the Term of this Agreement with respect to any country in the Territory, [*] shall not, directly or indirectly, either by itself or through its Affiliates or any arrangement, or series of arrangements, with a Third Party, [*]; provided that, [*].
(b) During the Term of this Agreement. [*] shall not, directly or indirectly, either by itself or through its Affiliates or any arrangement, or series of arrangements, with a Third Party, [*].
Article 3
Governance
3.1 Alliance Managers. Within [*] days after the Effective Date, each Party shall appoint and notify the other Party of the identity of a representative having the appropriate qualifications, including a general understanding of pharmaceutical development and commercialization issues, to act as its alliance manager under this Agreement (the “Alliance Manager”). The Alliance Managers shall serve as the primary contact points between the Parties for the purpose of providing each Party with information on the progress and results of KVK’s conduct of Regulatory Activities with respect to, and Commercialization of, Licensed Products. The Alliance Managers shall also be primarily responsible for facilitating the flow of information and otherwise promoting communication, coordination and collaboration between the Parties with respect to Licensed Products. Each Party may replace its Alliance Manager at any time upon written notice to the other Party.
3.2 Joint Committees.
(a) JSC Formation and Role. Within [*] days after the Effective Date, the Parties shall establish a joint steering committee (the “Joint Steering Committee” or “JSC”) for the overall coordination and oversight of the Parties’ activities under this Agreement. The role of the JSC shall be:
(i) to review, discuss, coordinate, and approve the overall strategy for the conduct of Regulatory Activities with respect to, and Commercialization of, Licensed Products in the Collaboration Territory;
(ii) to review, discuss and coordinate the conduct of any Development Activities by KemPharm;
(iii) to discuss and coordinate manufacturing matters with respect to Licensed Products, including discussing and coordinating the commercial supply needs of KVK and sharing related supply forecasts, to ensure compliance with the requirements of the Third Party Supply Agreement;
(iv) to review, discuss and approve the Commercialization Plan and any proposed amendments or revisions to such plan, and review and discuss the Commercialization of Licensed Products in the Collaboration Territory;
(v) to coordinate the Commercialization of Licensed Products in the Collaboration Territory and Retained Territory to ensure consistent global marketing of Licensed Products;
(vi) to oversee the activities of the Subcommittees and attempt to resolve issues presented to it by and disputes within the Subcommittees; and
(vii) to perform such other functions as appropriate to further the purposes of this Agreement, as expressly set forth in this Agreement or as determined by the Parties in writing.
(b) Subcommittee(s). From time to time, the JSC may establish joint subcommittees to oversee particular projects or activities, such as Licensed Product supply and manufacturing, conduct of Regulatory Activities and Commercialization, as it deems necessary or advisable (each, a “Subcommittee”, and together with the JSC, the “Committees”).
(c) Members. Each Committee shall be comprised of an equal number of representatives from each Party. Each Party’s representatives shall be officer or employee of such Party or its Affiliate having sufficient seniority within the applicable Party to make decisions arising within the scope of the applicable Committee’s responsibilities. Each Party shall initially appoint [*] representatives to the JSC. Each Committee may change its size from time to time by mutual consent of its representatives, and each Party may replace its representatives at any time upon written notice to the other Party. Each Party shall appoint one (1) of its representatives on each Committee to act as the co-chairperson of such Committee. The role of the co-chairpersons shall be to convene and preside at the Committee meetings and to ensure the circulation of meeting agendas at least [*] days in advance of Committee meetings and the preparation of meeting minutes in accordance with Section 3.2(d), but the co-chairpersons shall have no additional powers or rights beyond those held by other Committee representatives.
(d) Meetings. The JSC shall meet at least twice per calendar year during the Term, unless the Parties mutually agree in writing to a different frequency for such meetings. Either Party may also call a special meeting of any Committee, by videoconference or teleconference, by at least [*] Business Days prior written notice to the other Party in the event such Party reasonably believes that a significant matter must be addressed prior to the next regularly scheduled meeting, and such Party shall provide the applicable Committee no later than [*] Business Days prior to the special meeting with materials reasonably adequate to enable an informed decision. Each Committee may meet in person, by videoconference or by teleconference, provided, however, that, unless the Parties otherwise mutually agree, at least one such JSC meeting per calendar year shall be in person, alternating between the headquarters of KemPharm and KVK. All Committee meetings shall be conducted in English and all communications under this Agreement shall be in English. The co-chairpersons shall be responsible for preparing reasonably detailed written minutes of the Committee meetings that reflect, without limitation, all material decisions made at such meetings. The co-chairpersons shall send draft meeting minutes to each representative of the applicable Committee for review and approval within [*] Business Days after the Committee meeting. Such minutes shall be deemed approved unless one or more Committee representatives object to the accuracy of such minutes within [*] Business Days of receipt.
3.3 Decision Making. Each Committee shall strive to seek consensus in its actions and decision-making process and all decisions by the Committees shall be made by consensus, with each Party having collectively one (1) vote in all decisions. If after reasonable discussion and good faith consideration of each Party’s view on a particular matter before any Subcommittee, the representatives of the Parties cannot reach an agreement as to such matter within [*] Business Days after such matter was brought to such Subcommittee for resolution, such disagreement shall be referred to the JSC for resolution. If after reasonable discussion and good faith consideration of each Party’s view on a particular matter before the JSC, the representatives of the Parties cannot reach an agreement as to such matter within [*] Business Days after such matter was brought to the JSC for resolution or after such matter has been referred to the JSC, such disagreement shall be referred to the Executive Officers for resolution. If the Executive Officers cannot resolve such matter within [*] days after such matter has been referred to them, then: (i) KemPharm’s Executive Officer shall have the final decision-making authority with respect to matters related [*]; and (ii) KVK’s Executive Officer shall have the final decision making authority with respect to [*], provided, however, that KemPharm’s Executive Officer shall have the right to [*], any such determination by KemPharm’s Executive Officer shall be in writing and provided to KVK:
(a) [*]; and
(b) [*].
For sake of clarity, KemPharm retains all authority and all decision-making authority with respect to matters related to [*], other than the [*] herein.
3.4 Limitation of Committee Authority. Each Committee shall only have the powers expressly assigned to it in this Article 3 and elsewhere in this Agreement and shall not have the authority to: (a) modify or amend the terms and conditions of this Agreement; (b) waive or determine either Party’s compliance with the terms and conditions of under this Agreement; or (c) decide any issue in a manner that would conflict with the express terms and conditions of this Agreement. In no event shall KemPharm’s Executive Officer pursuant to Section 3.3(i) or KVK’s Executive Officer pursuant to Section 3.3(ii) make any decision contrary to this Agreement without the written consent of the other Party.
3.5 Discontinuation of Committees. The activities to be performed by each Committee shall solely relate to governance under this Agreement and are not intended to be or involve the delivery of services. Each Committee shall continue to exist until the Parties mutually agreeing to disband such Committee. Once the Parties mutually agree, such Committee shall have no further obligations under this Agreement and, thereafter, each Party shall designate a contact person for the exchange of information relevant to such Committee under this Agreement and decisions of such Committee shall be decisions as between the Parties, subject to the other terms and conditions of this Agreement.
Article 4
DEVELOPMENT
4.1 Overview. Except with respect to the conduct of Regulatory Activities, as further described in Article 5, KemPharm will be solely responsible for the Development of Licensed Products in the Field in the Territory, at [*]. KVK shall not conduct any Development Activities, other than to conduct Regulatory Activities, with respect to Licensed Products at any time during the Term, including in the Field in the Collaboration Territory.
4.2 Transfer of Licensed Know-How. Promptly after the Effective Date, KemPharm shall provide KVK with complete and accurate copies of the Licensed Know-How that is reasonably necessary to maintain Regulatory Approval of, Commercialize of and manufacture of Licensed Products in the Field in the Collaboration Territory in existence as of the Effective Date; provided that, to the extent KVK requires additional Licensed Know-How during the Term in connection with the conduct of Regulatory Activities or Commercialization activities, KemPharm shall reasonably cooperate with KVK in providing KVK with copies of such Licensed Know-How promptly (but in no event later than [*] after receipt of KVK’s request. The Parties shall establish a process for ensuring that such Licensed Know-How, including CMC Information, is treated as the Confidential Information of KemPharm.
Article 5
Regulatory Matters
5.1 Regulatory Responsibilities. Subject to the terms and conditions of this Agreement, KVK will be responsible, at its sole cost and expense, for the conduct of all Regulatory Activities required to obtain and maintain Regulatory Approval of Licensed Products in the Field in the Collaboration Territory, including the preparation of all Regulatory Materials and all communications and interactions with Regulatory Authorities in the Collaboration Territory with respect to Licensed Products. Promptly after the Effective Date, in connection with the transfer of Licensed Know-How under Section 4.2, KemPharm shall transfer ownership of the NDA for the Product to KVK in accordance with 21 C.F.R. 314.72. For clarity, notwithstanding anything to the contrary in this Agreement, as of the Effective Date, KemPharm has obtained Regulatory Approval of the Product in the Collaboration Territory; as such, solely with respect to the Product, and for clarity, excluding other Licensed Products that are not the Product, the definition of “Regulatory Activities” shall be limited to the reporting, preparation and submission of applications for maintaining (i.e., not obtaining or registering) Regulatory Approval of Licensed Products in accordance with the applicable sections of 21 C.F.R. 314, as well as all post-marketing requirements and all post-approval activities, such as participation in required REMS programs, PREA deferred pediatric studies and annual PDUFA program fees; any other sections of this Agreement that reference the conduct of Regulatory Activities, even without using such defined term, shall be deemed amended accordingly.
5.2 Pricing. KVK shall be solely responsible for negotiating with applicable Governmental Authorities regarding the price and reimbursement status of Licensed Products in the Field in the Collaboration Territory. KVK shall keep KemPharm timely informed on the status of any applications for pricing approvals in the Collaboration Territory, including any discussion and correspondence with any Regulatory Authority with respect thereto. KVK shall, subject to KVK’s reasonable discretion, implement KemPharm’s reasonable comments with respect to such applications, discussions or correspondences. In the event of a dispute between KVK and KemPharm with respect to the inclusion of such a comment, KVK shall prevail. KVK shall promptly notify KemPharm of pricing approvals obtained for the Licensed Products in the Collaboration Territory.
5.3 Regulatory Information Sharing. KVK shall (i) provide KemPharm with the original documents, in the electronic format in which it has been prepared by KVK, of any supplemental NDA x related to the Regulatory Activities for the Licensed Products, for KemPharm’s review and comment, in connection with obtaining or maintaining any NDA approval for Licensed Products in the Field in the Collaboration Territory, prior to the submission of such documents to the Regulatory Authority in the Collaboration Territory; (ii) provide KemPharm with the original documents, in the electronic format in which it has been prepared by KVK, of any annual report or other regulatory submission within a reasonable time after each has been submitted to FDA; and (iii) shall keep KemPharm informed of any material verbal or written communication or question relating to Licensed Products received by KVK from the Regulatory Authority in the Collaboration Territory. Except as required by applicable Law, KVK, its Affiliates and sublicensees shall not submit any Regulatory Materials to, or communicate with, any Regulatory Authority in the Retained Territory regarding any Licensed Products. If such submission or communication is required by applicable Law, KVK shall promptly notify KemPharm in writing of such requirement and the content of such submission or communication.
5.4 Meetings with Regulatory Authorities. KVK shall lead all interactions with Regulatory Authorities in the Collaboration Territory with respect to Licensed Products. KVK shall keep KemPharm reasonably informed of regulatory developments related to Licensed Products in the Field in the Collaboration Territory. At each regularly scheduled JSC meeting, KVK shall provide KemPharm with a list and schedule of any in-person meeting or teleconference with the applicable Regulatory Authorities, or related advisory committees, in the Collaboration Territory planned for the next calendar quarter that relates to any Licensed Product in the Field. In addition, KVK shall notify KemPharm as soon as reasonably possible, but in no event later than [*], after KVK becomes aware of any additional such meetings or teleconferences that become scheduled for such calendar quarter. KemPharm shall provide all reasonable assistance requested by KVK to prepare for any such meeting or teleconference. To the extent permitted by applicable Laws, KemPharm shall have the right to participate, whether directly or through a representative, in all such meetings and teleconferences, at [*].
5.5 Regulatory Costs. Unless otherwise provided in this Agreement, KVK shall be responsible for the costs and expenses incurred in connection with the preparation and filing of any and all Regulatory Materials and the maintenance of any and all Regulatory Approvals, including supplemental NDA approvals, if any, participation in any required REMS programs, annual PDUFA program fees and any required PREA deferred pediatric studies for Licensed Products in the Field in the Collaboration Territory.
5.6 Right of Reference to Regulatory Materials. Each Party hereby grants to the other Party the right of reference to all Regulatory Materials pertaining to Licensed Products submitted by or on behalf of such Party. The receiving Party may use such right of reference solely for the purpose of seeking, obtaining and maintaining Regulatory Approval of Licensed Products in its respective territory.
5.7 No Harmful Actions. If KemPharm believes that KVK is taking or intends to take any action with respect to any Licensed Product that could reasonably be expected to have a material adverse impact upon the development, manufacture, commercialization or regulatory status of any Licensed Product in the Retained Territory, KemPharm may bring the matter to the attention of the JSC and the Parties shall discuss in good faith to promptly resolve such concern.
5.8 Notification of Threatened Action. Each Party shall immediately notify the other Party of any information it receives regarding any threatened or pending action, inspection or communication by or from any Third Party, including without limitation a Regulatory Authority, which may affect the development, manufacture, commercialization or regulatory status of any Licensed Product. Upon receipt of such information, the Parties shall consult with each other in an effort to arrive at a mutually acceptable procedure for taking appropriate action.
5.9 Adverse Event Reporting and Safety Data Exchange. No later than [*] days before the anticipated launch date of any Licensed Product in the Collaboration Territory, the Parties shall enter into a written pharmacovigilance agreement (the “Pharmacovigilance Agreement”) for the Commercialization of the Licensed Product. These responsibilities shall include mutually acceptable guidelines and procedures for the receipt, investigation, recordation, communication, and exchange, as between the Parties, of adverse event reports, pregnancy reports, and any other information concerning the safety of the Licensed Product. Such guidelines and procedures shall be in accordance with, and enable the Parties to fulfill, local and national regulatory reporting obligations under applicable Laws. Furthermore, such agreed procedure shall be consistent with relevant ICH guidelines, except where said guidelines may conflict with existing local regulatory reporting safety reporting requirement, in which case local reporting requirement shall prevail. The Pharmacovigilance Agreement shall provide for an adverse event database for the Licensed Products in the Collaboration Territory to be maintained by KVK at KVK’s expense, and also a global safety database for the Licensed Products to be maintained by KemPharm at KemPharm’s expense. As between the Parties, KVK shall be responsible for preparing and filing with Regulatory Authorities in the Collaboration Territory all adverse event reports and responses to safety issues and requests of Regulatory Authorities relating to Licensed Products in the Collaboration Territory. As between the Parties, KVK shall also be responsible for reporting quality complaints, adverse events and safety data related to Licensed Products to KemPharm for inclusion in the global safety database. Each Party hereby agrees to comply with its respective obligations under such Pharmacovigilance Agreement and to cause its Affiliates and permitted sublicensees to comply with such obligations.
5.10 Remedial Actions. Each Party will notify the other Party immediately, and promptly confirm such notice in writing, if it obtains information indicating that any Licensed Product may be subject to any recall, corrective action or other regulatory action taken by virtue of applicable Laws (a “Remedial Action”). The Parties will assist each other in gathering and evaluating such information as is necessary to determine the necessity of conducting a Remedial Action. KVK shall, and shall ensure that its Affiliates and sublicensees will, maintain adequate records to permit the Parties to trace the distribution, sale and use, to the extent possible, of the Licensed Product in the Collaboration Territory. KVK shall have sole discretion with respect to any matters relating to any Remedial Action in the Collaboration Territory, including the decision to commence such Remedial Action and the control over such Remedial Action in its territory, at its cost and expense; provided, however, that if KemPharm determines that any Remedial Action with respect to any Licensed Product in the Collaboration Territory should be commenced or is required by applicable Laws or Regulatory Authority, KemPharm shall discuss such Remedial Action with KVK and KVK shall carry out such Remedial Action upon KemPharm’s request. Each Party shall provide the other Party, at the other Party’s expense, with such assistance in connection with a Remedial Action as may be reasonably requested by such other Party.
Article 6
Commercialization
6.1 Overview. Subject to the terms and conditions of this Agreement, including the diligence obligations set forth below, KVK will be primarily responsible for and have primary operational control over all aspects of the Commercialization of Licensed Products in the Field in the Collaboration Territory, including: (a) negotiating with applicable Governmental Authorities regarding the price and reimbursement status of Licensed Products, subject to Section 5.2; (b) marketing, advertising and promotion; (c) booking sales and distribution and performance of related services; (d) handling all aspects of order processing, invoicing and collection, inventory and receivables; (e) providing customer support, including handling medical queries, and performing other related functions; and (f) conforming its practices and procedures to applicable Laws relating to the marketing, detailing and promotion of Licensed Products in the Field in the Collaboration Territory; provided that, the Parties shall jointly develop and execute a Commercialization Plan, as defined below, for the Licensed Products in the Field in the Collaboration Territory, pursuant to Section 6.2, including the Commercialization Budget (as defined below) described therein, and (y) KemPharm shall exercise operational control over certain aspects of Commercialization, as specified in the Commercialization Plan. KVK shall fund all of the costs and expenses incurred by or on behalf of KVK in connection with such Commercialization activities, and such costs and expenses shall constitute “Commercialization/Regulatory Expenses”.
6.2 Commercialization Plan.
(a) General. KVK shall Commercialize Licensed Products in the Field in the Collaboration Territory pursuant to a commercialization plan (the “Commercialization Plan”). The Commercialization Plan shall include (i) a detailed description of all key strategic decisions (including messaging, branding, marketing, advertising, sales force positioning, number of representatives and details, pricing strategy, etc.), implementation tactics and pre-launch and post-launch activities; (ii) a reasonably detailed description and timeline of KVK’s, its Affiliates’ and their respective sublicensees’ Commercialization activities for Licensed Products in the Collaboration Territory for the next Fiscal Year, including medical marketing activities, sales forecasts and projections, pricing, reimbursement, market research, sales training, distribution channels, customer service and sales force matters related to the launch and sale of Licensed Products in the Collaboration Territory, (iii) a mutually agreed-upon Commercialization budget (“Commercialization Budget”), it being understood that any proposed increase in the Commercialization Budget by either Party that is reasonably likely to result in the Commercialization Budget being [*] greater than the previously approved Commercialization Budget shall require mutual agreement of the Parties; and (iv) a strategic plan for Commercialization of Licensed Products in the Collaboration Territory for the following [*]. KemPharm shall provide ongoing support with respect to the Commercialization of Licensed Products in the Field in the Collaboration Territory, as specified in the Commercialization Plan.
(b) Initial Plan and Amendments. The initial Commercialization Plan is attached hereto as Exhibit B. Within a reasonable time, but no later than [*] months, prior to the anticipated Regulatory Approval of the technology transfer to KVK of each Licensed Product in the Collaboration Territory, the Parties shall jointly, through the JSC, prepare updates and amendments, as appropriate, to the initial Commercialization Plan, and shall present such plan to the JSC for its review and approval. Thereafter, from time to time during the Term, but at least on an annual basis, the Parties shall jointly, through the JSC, prepare further updates and amendments, as appropriate, to the then-current Commercialization Plan, and shall submit all updates and amendments to the Commercialization Plan to the JSC for review and approval. Once approved by the JSC, the Commercialization Plan shall become effective and supersede the previous Commercialization Plan as of the date of such approval. The Commercialization Plan shall specify the specific obligations of each Party with respect to the Commercialization of Licensed Products in the Field in the Collaboration Territory.
6.3 Pricing. Subject to any determination by applicable Regulatory Authorities, KVK shall have the sole right to determine the pricing of the Licensed Products in the Collaboration Territory, provided, however, that KVK shall price Licensed Products in a manner that is intended to maximize the profit of the Licensed Products and shall not price the Licensed Products in a manner intended to induce the sale of any other products. KemPharm shall not have any right to direct, control, or approve KVK’s pricing of the Licensed Products in the Collaboration Territory.
6.4 Commercial Diligence.
(a) KVK shall use Commercially Reasonable Efforts to Commercialize the Licensed Products in the Field in the Collaboration Territory.
6.5 Commercialization Reports. KVK shall keep KemPharm reasonably informed of KVK’s, its Affiliates’ and their respective sublicensees’ Commercialization activities with respect to the Licensed Products in the Field in the Collaboration Territory. Without limiting the foregoing, at each regularly scheduled JSC meeting, KVK shall provide KemPharm with a written report summarizing the significant Commercialization activities performed with respect to the Licensed Products since the last JSC meeting and comparing such activities with the Commercialization Plan for such time period. Such reports shall be at a level of detail reasonably requested by KemPharm and sufficient to enable KemPharm to determine KVK’s compliance with its diligence obligations under Section 6.4. At such JSC meeting, the Parties shall discuss the status, progress and results of KVK’s Commercialization activities. KVK shall promptly respond to KemPharm’s reasonable questions or requests for additional information relating to such Commercialization activities. In addition, within [*] days after the end of each Fiscal Year, KVK shall provide KemPharm with a detailed written annual report regarding the progress under the Commercialization Plan and results thereof.
6.6 Cross-Territorial Restrictions. KVK hereby covenants and agrees that it shall not, and shall ensure that its Affiliates and sublicensees will not, either directly or indirectly, promote, market, distribute, import, sell or have sold the Licensed Products, including via internet or mail order, into countries in the Retained Territory. As to such countries in the Retained Territory (which are exclusively reserved for KemPharm), KVK shall not, and shall ensure that its Affiliates and their respective sublicensees will not: (a) establish or maintain any branch, warehouse or distribution facility for Licensed Products in such countries, (b) engage in any advertising or promotional activities relating to Licensed Products that are directed primarily to customers or other purchaser or users of Licensed Products located in such countries, (c) solicit orders for Licensed Products from any prospective purchaser located in such countries, or (d) sell or distribute Licensed Products to any person in the Collaboration Territory who intends to sell or has in the past sold Licensed Products in such countries. If KVK receives any order for any Licensed Product from a prospective purchaser located in a country in the Retained Territory, KVK shall promptly refer that order to KemPharm and KVK shall not accept any such orders. KVK shall not deliver or tender, or cause to be delivered or tendered, Licensed Products into a country in the Retained Territory. KVK shall not, and shall ensure, that its Affiliates and their respective sublicensees will not, restrict or impede in any manner KemPharm’s exercise of its retained exclusive rights in the Retained Territory.
6.7 Label and Field Restrictions. KVK hereby covenants that it shall not, nor shall it permit any Affiliate or sublicensee to, directly or indirectly, market, promote, detail, sell or offer for sale Licensed Products in the Collaboration Territory for any use outside (1) the Label or (2) the Field. KemPharm acknowledges and understands that KVK cannot control the ultimate use of Licensed Products it sells and that the purpose of the foregoing covenant is to prevent KVK and its Affiliates and sublicensees from facilitating or encouraging uses outside (1) the Label and/or (2) the Field.
6.8 Labeling. KVK shall provide KemPharm with proposed content for labeling and packaging (including with respect to any product inserts) of Licensed Products in the Field in the Collaboration Territory, for KemPharm’s review and approval prior to use. Except with respect to KVK’s use of any KVK Housemark, as defined below, for the Licensed Product, KVK shall not materially deviate from KemPharm’s approved label for the Product in the design or content of the label for any Licensed Product unless required to by Law or approved by KemPharm in writing. KemPharm shall review KVK’s proposed labeling and packaging content and shall provide comments to KVK within [*] days of delivery of such materials to KemPharm, which comments KVK shall incorporate into the final labeling and packaging content provided to KemPharm.
6.9 Payors and PBMs. Subject to the direction and authorization of the JSC, and as set forth in the Commercialization Plan, KemPharm shall have the responsibility for outreach to pharmacy benefit managers and other payors to negotiate for utilization of the Licensed Products in place of traditional hydrocodone/acetaminophen combination products for their members where appropriate.
6.10 Proof of Market Viability Termination. In the event that the Parties’ joint efforts to Commercialize Licensed Products in the Field in the Collaboration Territory pursuant to this Article 6 does not result by the [*] of the effective date of this Agreement in KVK entering into agreements with Third Party payors and/or pharmacy benefit managers whereby, based on such Third Parties’ usage of hydrocodone and/or acetaminophen (for clarity, in all strengths, and irrespective of whether branded or generic) in the immediately preceding [*], KVK would be reasonably likely sell or have sold at least [*] units of tablets of Licensed Product within a given [*] period (the achievement of such result, the “Proof of Market Viability”), then either Party may, in its sole discretion, elect to terminate this Agreement in its entirety (the “Proof of Market Viability Termination”).
Article 7
API
7.1 Manufacture and Supply. As of the Effective Date, KemPharm has entered into a Third Party Supply Agreement, including a Third Party Supply Quality Agreement as per Section 1.56, pursuant to which ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ is manufacturing and supplying KemPharm API (benzhydrocodone hydrochloride) to KemPharm.
(a) KemPharm shall seek [*], pursuant to the terms of the Third Party Supply Agreement, from the Third Party Supplier (▇▇▇▇▇▇▇ Matthey) to [*] subject to the provisions of Section 7.1(c) below.
(b) KVK shall subsequently execute [*]. As part of this transfer assignment agreement, to be attached hereto as Exhibit D, KVK will [*] and shall [*] pursuant to Section 7.2 for purposes of manufacturing and commercializing the Licensed Product. KVK agrees that, [*], it shall [*], such that KemPharm’s supply obligations herein do not result in KemPharm’s breach of its Third Party Supply Agreement.
(c) Notwithstanding the above Section 7.1(a)-(b), KemPharm shall retain the rights to purchase API (benzhydrocodone hydrochloride), alone or in combination with other pharmaceutical ingredients, except for acetaminophen, for any and all products, other than the Licensed Product(s), from the Third Party Supplier pursuant to either the Third Party Supply Agreement or another agreement with ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ with substantially the same terms.
7.2 KVK shall exclusively purchase, subject to Section 7.1(c) above, the KemPharm API through KemPharm by way of the Third Party Supply Agreement, and KemPharm will use Commercially Reasonable Efforts to have manufactured and supplied directly to KVK through the Third Party Supply Agreement, all of KVK’s and its Affiliates’ and sublicensee’s requirements of KemPharm API and other raw materials for Commercialization use and manufacture in the Field in the Collaboration Territory under this Agreement. ▇▇▇▇▇▇▇ Matthey shall supply the KemPharm API and other raw materials, and KVK shall be responsible for the transportation, import and storage of the KemPharm API and/or raw materials at KVK’s own cost and expense. All KemPharm API shall be delivered by ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ to KVK in accordance with the terms of the Third Party Supply Agreement. The price for KemPharm API and other raw materials supplied by ▇▇▇▇▇▇▇ Matthey through KemPharm to KVK Pursuant to the Third Party Supply Agreement for Commercial use in the Field in the Collaboration Territory shall equal [*] (the “Supply Price”).
7.3 Notwithstanding the foregoing, nothing contained in this Section 7 shall require that [*]. Any requirement for KemPharm to make any extra payments of any kind to ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇, [*], shall be paid by [*]. Regarding the [*], [*] shall be responsible to pay an amount equal to ▇▇▇▇▇▇▇ Matthey’s cost, while [*] shall pay all other sums due to ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ under [*].
7.4 Distribution. KVK will be solely responsible for the distribution of Licensed Products in the Field in the Collaboration Territory.
7.5 Brand Security and Anti-Counterfeiting. The Parties will establish contacts for communication regarding brand security issues and will each reasonably cooperate with the other with respect thereto. Practices around these incidents will comply with KemPharm’s then-current standards, where they define product security features, warehouse/cargo protection requirements, and response and communication process for such incidents. KemPharm shall inform KVK of any changes to KemPharm’s standards with sufficient time for KVK to change any practices, if necessary.
Article 8
Compensation
8.1 POMV Fee. Within ten (10) Business Days after achievement of the Proof of Market Viability, KVK shall pay to KemPharm a one-time, upfront payment of two million U.S. Dollars (US$2,000,000), free of any Tax Withholding pursuant to Section 8.9, (the “POMV Fee”). The POMV Fee shall be fully creditable against the future amount of any Net Profit Share payments owed to KemPharm pursuant to Section 8.4.
8.2 Commercialization/Regulatory Expenses. Prior to achievement of Proof of Market Viability pursuant to Article 6, KemPharm shall fund [*] Commercialization/Regulatory Expenses incurred in connection with the Commercialization or maintenance of Regulatory Approvals of Licensed Products in the Field in the Collaboration Territory, in accordance with Articles 5 and 6. Subsequent to achievement of Proof of Market Viability pursuant to Article 6, KVK shall either directly fund, or reimburse KemPharm for, any expenses incurred in connection with the Commercialization/Regulatory Expenses of Licensed Products in the Field in the Collaboration Territory, such reimbursements to be paid within [*] days after receipt of the applicable invoice from KemPharm. For the avoidance of doubt, [*], as applicable.
8.3 Manufacturing Expenses. [*], as applicable.
8.4 Net Profit Share.
(a) Following the First Commercial Sale of any Licensed Product in the Field in the Collaboration Territory, KVK shall pay to KemPharm an initial percentage of the Net Profits (if any), in accordance with the table below (“Net Profit Sharing Percentage”; such profit share, the “Net Profit Share”).
|
Aggregate amount of Net Sales of Licensed Products sold in previous Rolling Year |
Net Profit Share |
|
Less than or equal to [*] |
70% to KVK / 30% to KemPharm |
|
Greater than [*] but less than or equal to [*] |
[*]% to KVK / [*]% to KemPharm |
|
Greater than [*] |
50% to KVK / 50% to KemPharm |
For clarity, once a certain Net Profit Sharing Percentage is reached with respect to a given Rolling Year, irrespective of the amount of future Net Sales in subsequent calendar quarters, the Net Profit Sharing Percentage shall not reset to a lower Net Profit Sharing Percentage for subsequent Rolling Years. An hypothetical example computation of Net Profit Share is attached hereto as Exhibit E.
(b) Net Profit Share Payments; Reports. Within [*] days after the end of each Calendar Quarter following the First Commercial Sale of a Licensed Product in the Field in the Collaboration Territory, KVK shall submit to KemPharm a report setting forth, with respect to the previous Rolling Year: (a) the amount of gross sales and Net Sales of Licensed Products in the Field in the Collaboration Territory in such Rolling Year, (b) an itemized calculation showing the deductions from gross sales (by major category as set forth in the definition of Net Sales) to determine Net Sales, (c) the amount of Manufacturing Expenses paid by KVK to KemPharm for Licensed Products purchased by KVK during such Rolling Year and (d) the amount of Commercialization/Regulatory Expenses incurred by KVK, which shall include amounts paid to KemPharm pursuant to Section 8.2, with respect to Licensed Products in the Field in the Collaboration Territory during such Rolling Year and (e) a calculation of the Net Profits or Net Losses, as applicable, for such Licensed Products (the “Net Profit Report”).
(c) Payment of Net Profit Sharing Percentage. KVK shall pay all Net Profit Share payments owed to KemPharm pursuant to this Section 8.4, if any, at the time of submission of the Net Profit Report. In any quarter in which there is a Net Loss, KVK shall be bear the full amount of such loss; provided that, KVK shall be entitled to [*]. Notwithstanding this Section 8.4, KVK shall not [*].
8.5 Sales Milestone Payments. KVK shall promptly notify KemPharm upon the achievement of the sales milestone events set forth below with respect to Net Sales of Licensed Products in the Field in the Collaboration Territory in a given Rolling Year. KVK shall pay to KemPharm the corresponding one-time, non-refundable and non-creditable sales milestone payments set forth below within [*] days after the achievement of such milestone event. For clarity, the sales milestone payments herein are free of any Tax Withholding pursuant to Section 8.9.
|
Sales Milestone Event |
Milestone Payment |
|
Net Sales in a Rolling Year equal or exceed [*] |
[*] |
|
Net Sales in a Rolling Year equal or exceed [*] |
[*] |
|
Net Sales in a Rolling Year equal or exceed [*] |
[*] |
|
Net Sales in a Rolling Year equal or exceed [*] |
[*] |
|
Net Sales in a Rolling Year equal or exceed [*] |
[*] |
8.6 Payment Method; Foreign Exchange. All payments owed by KVK under this Agreement shall be made by wire transfer in immediately available funds to a bank and account designated in writing by KemPharm. For clarity, all payments by KVK to KemPharm pursuant to Sections 8.1, 8.3, 8.4 and 8.5 shall be in U.S. Dollars. If applicable, the rate of exchange to be used in computing the amount of currency equivalent in U.S. Dollars of any amounts payable in U.S. Dollars by KVK to KemPharm under this Agreement shall be calculated in accordance with U.S. GAAP. KemPharm shall invoice KVK for all amounts payable under Sections 8.2 and 8.3 in U.S. Dollars.
8.7 Interest on Late Payments. If KemPharm does not receive payment of any sum due to it on or before the due date, [*] interest shall thereafter accrue on the sum due to KemPharm until the date of payment at the [*] rate of [*] over the then-current prime rate reported in The Wall Street Journal or the maximum rate allowable by applicable Laws, whichever is lower.
8.8 Records; Audits.
(a) KVK shall and shall ensure that its Affiliates and its and their respective sublicensees, maintain complete and accurate records in sufficient detail to permit KemPharm to confirm the accuracy of the calculation of Net Loss and/or Net Profit Share payments and the achievement of the sales milestone events, and KVK shall provide reconciliations of gross sales to net sales and cost of goods sold, detailing any adjustments made by KVK as a result of its application of OCBOA. All payments and other amounts under this Agreement shall be accounted for in accordance with U.S. GAAP. Upon reasonable prior notice, such records shall be available for examination during regular business hours for a period of [*] years from the end of the Fiscal Year to which they pertain, and not more often than once each Fiscal Year, by an independent certified public accountant selected by KemPharm and reasonably acceptable to KVK, for the sole purpose of verifying the accuracy of the financial reports furnished by KVK pursuant to this Agreement and any payments with respect thereto. Any such auditor shall not disclose KVK’s Confidential Information, except to the extent such disclosure is necessary to verify the accuracy of the financial reports furnished by KVK or the amount of payments due under this Agreement. Any amounts shown to be owed but unpaid shall be paid within [*] days from the accountant’s report, plus interest, as set forth in Section 8.7, from the original due date. [*] shall bear the full cost of such audit unless such audit discloses an [*] of more than [*] of the amount due for the audited period, in which case [*] shall bear the full cost of such audit.
(b) KemPharm shall, and shall ensure that its Affiliates and KemPharm Partners, maintain complete and accurate records in sufficient detail to permit KVK to confirm the accuracy of the calculation of the Commercialization/Regulatory Expenses and the Manufacturing Expenses. All such expenses shall be accounted for in accordance with U.S. GAAP. Upon reasonable prior notice, such records shall be available for examination during regular business hours for a period of [*] years from the end of the Fiscal Year to which they pertain, and not more often than once each Fiscal Year, by an independent certified public accountant selected by KVK and reasonably acceptable to KemPharm, for the sole purpose of verifying the accuracy of the amount of expenses furnished by KemPharm pursuant to this Agreement and any payments with respect thereto. Any such auditor shall not disclose KemPharm’s Confidential Information, except to the extent such disclosure is necessary to verify the accuracy of the amount of expenses furnished by KVK or the amount of payments due under this Agreement. Any overage assessed to KVK shall be refunded, if applicable, within [*] days from the accountant’s report, plus interest, in equal amounts as set forth in Section 8.7, from the original date such overage was applied. [*] shall bear the full cost of such audit unless such audit discloses an [*] of more than [*] of the for the audited period, in which case [*] shall bear the full cost of such audit
8.9 Taxes.
(a) Taxes on Income. Except as otherwise provided in this Section 8.9, each Party shall be solely responsible for the payment of all taxes imposed on its share of income arising directly or indirectly from the efforts of the Parties under this Agreement.
(b) Tax Cooperation. The Parties agree to cooperate with one another and use reasonable efforts to reduce or eliminate tax withholding or similar obligations in respect of the upfront fee, Net Profit Share payments, sales milestone payments or other payments made by KVK to KemPharm under this Agreement. To the extent KVK is required to deduct and withhold taxes from any payment to KemPharm, KVK shall pay the amounts of such taxes to the proper Governmental Authority in a timely manner and promptly transmit to KemPharm an official tax certificate or other evidence of such withholding sufficient to enable KemPharm to demonstrate such payment of taxes to any applicable Government Authority. KemPharm shall provide KVK any tax forms that may be reasonably necessary in order for KVK not to withhold tax or to withhold tax at a reduced rate under an applicable bilateral income tax treaty. Each Party shall provide the other with reasonable assistance to enable the recovery, as permitted by applicable Laws, of withholding taxes or similar obligations resulting from payments made under this Agreement, such recovery to be for the benefit of the Party bearing such withholding tax.
(c) Withholding Taxes. If KVK is required by applicable Laws to make any tax deduction, tax withholding or similar payment from any amount paid or payable by KVK to KemPharm (a “Tax Withholding”) under this Agreement, then KVK will pay KemPharm the actual stated amount set forth under this Agreement in full and shall also pay any such Tax Withholding, including any additional Tax Withholding required with respect to KVK’s additional payments under this Section 8.9, directly to the proper Governmental Authority. For clarity, KemPharm shall receive, without any deduction or offset with respect to taxes and free of any Tax Withholding, a net amount equal to, after payment of any Tax Withholding, the amount to which KemPharm was otherwise entitled under this Agreement and would have received had no Tax Withholding been required by Laws to be made.
(d) VAT. All payments due to KemPharm from KVK pursuant to this Agreement shall be paid exclusive of, and without reduction for, any value-added tax (“VAT”) (which, if applicable, shall be payable by KVK upon receipt of a valid VAT invoice). If KemPharm determines that it is required to report any such tax, KVK shall promptly provide KemPharm with applicable receipts and other documentation necessary or appropriate for such report. For clarity, this Section 8.9(d) is not intended to limit KVK’s right to deduct VAT in determining Net Sales.
Article 9
Intellectual Property Matters
9.1 Ownership of Data and Inventions.
(a) Data. KemPharm shall solely own all Data generated by KemPharm. The Data Controlled by KemPharm and related solely and specifically to the Licensed Products are included in the Licensed Know-How and licensed to KVK under Section 2.1(a). KVK shall solely own all Data generated by KVK in the conduct of Regulatory Activities that is solely and specifically related to Licensed Products in the Field in the Collaboration Territory. KVK hereby grants KemPharm an irrevocable, perpetual, royalty-free, fully paid-up, exclusive license with the right to grant sublicenses to use such Data generated and owned by KVK in the Retained Territory for all purposes, and a non-exclusive license in the Collaboration Territory upon expiration or termination of the Agreement, other than termination of the Agreement by KVK pursuant to Sections 13.4 or 13.5.
(b) Inventions. Inventorship of any Inventions will be determined in accordance with the standards of inventorship and conception under U.S. patent laws. For clarity, all Inventions directly related to the Licensed Product(s) (i.e. combination product of benzhydrocodone and acetaminophen) made by KemPharm are included in the Licensed IP and licensed to KVK under Section 2.1(a). KVK shall promptly disclose in writing to KemPharm all KVK Inventions. KVK hereby grants KemPharm an irrevocable, perpetual, royalty-free, fully paid-up, non-exclusive license with the right to grant sublicenses in such KVK Inventions directly related to the Licensed Products and invented during the Term in the Retained Territory, and in the Collaboration Territory upon expiration or termination of the Agreement. KVK shall have the sole right to file for patent protection for such KVK Inventions. However, KVK shall have no duty to file for patent protection for such KVK Inventions and shall prosecute such KVK Inventions in its sole discretion, subject to KemPharm having an opportunity to comment on any prosecution of KVK inventions which may affect the Licensed IP. If KVK elects not to file or continue prosecution of the KVK Inventions, and if KemPharm desires at its option to file or pursue prosecution of the KVK Inventions, KVK may, at its option, assign said KVK Inventions to KemPharm and KemPharm shall have the right to prosecute the assigned KVK Invention(s). KemPharm shall provide KVK with all reasonable assistance and cooperation in the patent prosecution efforts by KVK provided in this Section 9.1.
i. For sake of clarity, all inventions made by KemPharm, including but not limited to any benzhydrocodone, benzhydrocodone compounds, inventions, pro-drugs or combination of thereof with one or more additional pharmaceutical ingredients, other than Apadaz®, are not included in the Licensed IP and are not licensed to KVK.
(c) KVK’s Affiliates, Sublicensees and Subcontractors. KVK shall ensure that each of its Affiliates, sublicensees and subcontractors under this Agreement has a contractual obligation to disclose to KVK all Data and Inventions generated, invented, discovered, developed, made or otherwise created by them or their employees, agents or independent contractors, and to provide sufficient rights with respect thereto, so that KVK can comply with its obligations under Section 9.1(a).
9.2 Patent Prosecution.
(a) Prosecution by KemPharm. As between the Parties, KemPharm shall have the sole right to prepare, file, prosecute and maintain or abandon the Licensed Patents during the term of this Agreement. KemPharm will use Commercially Reasonable Efforts to prepare, file, prosecute, defend and maintain all Licensed Patents; provided, however, that KemPharm does not represent or warrant that any patent will issue or be granted based on patent applications contained in the Licensed Patents. KemPharm shall provide KVK reasonable opportunity to review and comment on such prosecution efforts regarding the Licensed Patents as follows: KemPharm shall promptly provide KVK with copies of all material communications from any patent authority and any translations in KemPharm possession in the Collaboration Territory regarding the Licensed Patents, and shall provide KVK, for its review and comment, with drafts of any material filings or responses to be made to such patent authorities in a reasonable amount of time in advance of submitting such filings or responses. KemPharm shall consider in good faith comments thereto provided by KVK in connection with the prosecution of the Licensed Patents. For the purpose of this Article 9, “prosecution” shall include any post-grant proceeding in the Collaboration Territory, including opposition proceedings.
(b) Retained Territory. For clarity, KVK does not have any rights pursuant to this Agreement with respect to any Licensed Patents in the Retained Territory and, as between the Parties, KemPharm shall have the sole right in its sole discretion to prepare, file, prosecute and maintain the Licensed Patents in the Retained Territory.
(c) Collaboration. KVK shall provide KemPharm with all reasonable assistance and cooperation in the patent prosecution efforts by KemPharm provided in this Section 9.2, including providing any necessary powers of attorney and executing any other required documents or instruments for such prosecution, including but not limited to the filing of Terminal Disclaimers.
9.3 Patent Enforcement.
(a) Notification. If either Party becomes aware of any existing or threatened infringement of any Licensed Patent (“Infringement”), it shall promptly notify the other Party in writing to that effect and the Parties will consult with each other regarding any actions to be taken with respect to such Infringement.
(b) Enforcement Rights. Each Party shall share with the other Party all Information available to it regarding such alleged Infringement, pursuant to a mutually agreeable “common interest agreement” executed by the Parties under which the Parties agree to their shared, mutual interest in the outcome of any suit to enforce the Licensed Patents against such Infringement. KemPharm shall have the first right, but not the obligation, to bring an appropriate suit or other action against any person or entity engaged in such Infringement, at [*]. If KemPharm elects to commence a suit to enforce the applicable Licensed Patents against such Infringement, then KVK shall have the right but not the obligation, to join such enforcement action upon notice to KemPharm, and in this case, the Parties shall [*] of the enforcement action. If KemPharm notifies KVK that it does not intend to commence a suit to enforce the applicable Licensed Patents against such Infringement or to take other action to secure the abatement of such Infringement, then, to the extent that such Infringement is resulting from a Third Party’s use or sale of a product that (i) competes with a Licensed Product in the Field and in the Collaboration Territory and (ii) consists of a combination of benzhydrocodone and acetaminophen, KVK shall have the right, subject to the foregoing, but not the obligation, to commence such a suit or take such action. In such case, [*] related to such actions. KVK shall not have the right to commence such an action without KemPharm’s consent if KemPharm believes in good faith that the commencement of any such suit or action by KVK would reasonably be likely to have a negative impact on any similar action that KemPharm is pursuing against such person or entity. In such case, KemPharm shall take reasonably appropriate actions in order to enable KVK to commence a suit or take the actions set forth in the preceding sentence. Neither Party shall settle any such suit or action in any manner that would negatively impact the Licensed Patents or that would limit or restrict the ability of KVK to sell the Licensed Products in the Collaboration Territory without the prior written consent of the other Party.
(c) Collaboration. Each Party shall provide to the Party bringing a claim, suit or action under Section 9.3(b) (the “Enforcing Party”) with reasonable assistance in such enforcement, at such Enforcing Party’s request and the Party’s expense, including joining such action as a party plaintiff if required by applicable Laws to pursue such action. If KVK is required to join such action as a party plaintiff, KemPharm shall reimburse KVK’s reasonable costs and attorney’s fees. The Enforcing Party shall keep the other Party regularly informed of the status and progress of such enforcement efforts and shall reasonably consider the other Party’s comments on any such efforts. The non-enforcing Party shall be entitled to separate representation in such matter by counsel of its own choice and at its own expense, except as otherwise provided herein, but such Party shall at all times cooperate fully with the Enforcing Party.
(d) Expenses and Recoveries. Subject to the terms of this Section 9.3(d), [*] shall be [*] it incurs as a result of such enforcement action, except that the Parties shall [*] of the enforcement action when [*] If the Enforcing Party recovers monetary damages in such claim, suit or action brought under Section 9.3(b), such recovery shall be allocated first to the reimbursement of any documented expenses incurred by the Parties in such enforcement action, and any remaining amounts shall be shared by the Parties as follows:
(i) [*];
(ii) [*];
(iii) [*];
(iv) [*];
(v) [*].
(e) Other Infringements. KemPharm shall have the sole right to enforce Licensed Patents in the Retained Territory, KemPharm shall be entitled to retain all recoveries resulting from all such enforcements, and KVK shall not be obligated to participate or assist in such enforcements.
9.4 Third Party Infringement Claims. If the manufacture, sale or use of the Licensed Products in the Field in the Collaboration Territory pursuant to this Agreement results in a claim, suit or proceeding alleging patent infringement against KemPharm or KVK, or their respective Affiliates, licensees or sublicensees (collectively, “Infringement Actions”), such Party shall promptly notify the other Party hereto in writing. KemPharm shall direct and control the defense of such Infringement Action, [*] with counsel of its choice; provided, however, that if KVK has not been named as a defendant, KVK may participate in the defense and/or settlement thereof, [*] with counsel of its choice. If KVK has been named as a defendant, KemPharm shall, whether through its own counsel or separate counsel in the event of a conflict, defend KVK [*], with regard solely to said Third Party Infringement Claims. In any event, KemPharm agrees to keep KVK reasonably informed of all material developments in connection with any such Infringement Action for which KemPharm exercises its right to direct and control the defense and shall provide any additional information about the Infringement Action that KVK reasonably requests. Each Party shall share with the other Party all Information available to it regarding such Infringement Actions, pursuant to a mutually agreeable “common defense agreement” executed by the Parties under which the Parties agree to their shared, mutual interest in the outcome of any such Infringement Actions. KemPharm agrees not to settle such Infringement Action, or make any admissions or assert any position in such Infringement Action, in a manner that would materially adversely affect the rights or interests of KVK, without the prior written consent of KVK, which shall not be unreasonably withheld or delayed. If a license from a Third Party is reasonably required in order for KVK to Commercialize the Licensed Products or otherwise perform its obligations under this agreement, the Parties shall mutually agree on such license and negotiate in good faith as to the allocation of such license. The JSC shall review the Third-Party licensed IP expenses at least annually. Notwithstanding the foregoing, under no circumstances shall KVK be responsible or pay for any licensing fees related to use or sale (i) prior to the Effective Date, (ii) after the Term of this Agreement, (iii) outside of the Field, or (iv) outside of the Collaboration Territory.
9.5 Patent Term Extensions. KemPharm will cooperate with KVK, [*] in seeking and obtaining patent term extensions, including any pediatric exclusivity extensions as may be available, patent term adjustments or supplemental protection certificates or their equivalents in the Collaboration Territory with respect to any Licensed Patents or Licensed Products. If elections with respect to obtaining such patent term extensions are to be made, KemPharm shall have the sole right to make such elections.
9.6 Trademarks.
(a) KemPharm Product ▇▇▇▇.
(i) KVK shall Commercialize Licensed Products in the Collaboration Territory under the applicable KemPharm Product ▇▇▇▇ selected by KemPharm. KVK shall, and shall ensure that its Affiliates and sublicensees will, (1) use the KemPharm Product ▇▇▇▇ solely in connection with the Commercialization and manufacture of Licensed Products in the Field in the Collaboration Territory, (2) comply with the requirements of KemPharm as to the form, manner, scale and context of the use of the KemPharm Product ▇▇▇▇, the use of the statements to accompany the KemPharm Product ▇▇▇▇, and the presentation or performance of the Licensed Products, and (3) adhere to any branding strategy policies, including, without limitation, logo design, product labeling, messaging points, and visual branding elements, implemented by KemPharm for the use of the KemPharm Product ▇▇▇▇. KVK shall display the proper form of trademark notice associated with the KemPharm Product ▇▇▇▇ in accordance with instructions received from KemPharm.
(ii) Upon KemPharm’s request, to the extent permitted by applicable Laws, KVK shall include KemPharm’s name and corporate logo on Licensed Product label, packaging, promotional/marketing materials to indicate that the Licensed Product is in-licensed from KemPharm, and shall display KemPharm’s name and corporate logo with equal prominence and comparable size, resolution, print quality, and location, as instructed by KemPharm from time to time, as KVK’s name and corporate logo is displayed.
(iii) KVK shall provide KemPharm with samples of proposed content for Licensed Product labeling and packaging in accordance with Section 6.8, together with any promotional/marketing materials that include the KemPharm Product Marks, for review and approval prior to use. If concerns arise based upon reviews of samples provided, KemPharm shall have a right to audit all uses of the KemPharm Product ▇▇▇▇.
(iv) KVK shall use the KemPharm Product ▇▇▇▇ upon or in relation to the Licensed Products only in such manner where the distinctiveness, reputation, and validity of the KemPharm Product ▇▇▇▇ shall not be impaired. Without prejudice to the generality of the foregoing, KVK shall ensure in particular that the KemPharm Product ▇▇▇▇ is correctly spelled, and that any text, graphics, or designs adjacent to the KemPharm Product ▇▇▇▇ do not put the KemPharm Product ▇▇▇▇ or KemPharm in a negative or derogatory light. KVK shall not, and shall ensure, that its Affiliates and sublicensees will not, engage in any action that will interfere with or diminish KemPharm’s rights or goodwill in the KemPharm Product ▇▇▇▇, including related names and logos.
(v) KemPharm shall register and maintain in the Collaboration Territory, at KemPharm’s cost and expense, the applicable KemPharm Product ▇▇▇▇ that KemPharm wishes to use in connection with the Commercialization of the Licensed Products in the Collaboration Territory.
(vi) KemPharm shall own all rights to the KemPharm Product ▇▇▇▇ throughout the world. KVK acknowledges that all use of the KemPharm Product ▇▇▇▇ and all rights and goodwill attached to or arising out of such use, shall accrue to the benefit of KemPharm. KVK shall at any time, whether during or after the Term, execute any documents that shall reasonably be required by KemPharm to confirm KemPharm’s ownership of the KemPharm Product ▇▇▇▇.
(b) KVK Housemarks. In addition to the KemPharm Product ▇▇▇▇, KVK shall have the right to brand the Licensed Products in the Field in the Collaboration Territory with those trademarks of KVK that are associated with KVK’s name or identity (“KVK Housemarks”); provided, however, that KVK shall not, and shall ensure that its Affiliates and sublicensees will not, make any use of trademarks that are confusingly similar to any trademarks or house marks of KemPharm or its Affiliates, including the corporate name of KemPharm or any of its Affiliates, without KemPharm’s prior written consent. KVK shall own all rights in the KVK Housemarks, and all goodwill in the KVK Housemarks shall accrue to KVK. KVK and its Affiliates and sublicensees shall not use any trademarks, other than the KemPharm Product Marks and the KVK Housemarks, in connection with the Commercialization of Licensed Products in the Field in the Collaboration Territory, without the prior written consent of KemPharm.
Article 10
Representations And Warranties; covenants
10.1 Mutual Representations and Warranties. Each Party hereby represents and warrants to the other Party as follows:
(a) Corporate Existence. As of the Effective Date, it is a company or corporation duly organized, validly existing, and in good standing under the Laws of the jurisdiction in which it is incorporated.
(b) Corporate Power, Authority and Binding Agreement. As of the Effective Date, (i) it has the corporate power and authority and the legal right to enter into this Agreement and perform its obligations hereunder; (ii) it has taken all necessary corporate action on its part required to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder; and (iii) this Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, and binding obligation of such Party that is enforceable against it in accordance with its terms, subject to applicable bankruptcy, insolvency, reorganization, moratorium and similar Laws affecting creditors' rights and remedies generally.
(c) No Debarment. Neither such Party nor any of its Affiliates is debarred or disqualified under the Act or comparable applicable Laws outside the U.S.
10.2 Additional Representations and Warranties of KemPharm. KemPharm represents and warrants to KVK as follows, as of the Effective Date:
(a) Title; Encumbrances. It has sufficient legal and/or beneficial title, ownership or license, free and clear from any mortgages, pledges, liens, security interests, conditional and installment sale agreement, encumbrances, charges or claim of any kind, of the Licensed IP to grant the licenses to KVK as purported to be granted pursuant to this Agreement;
(b) Notice of Infringement or Misappropriation. It has not received any notice, written or otherwise, from any Third Party asserting or alleging that the Commercialization of the Licensed Products in the Collaboration Territory would infringe or misappropriate the intellectual property rights of such Third Party;
(c) No Proceeding. There is no pending, and to KemPharm’s knowledge, no threatened, adverse action, suit or proceeding in the Collaboration Territory against KemPharm involving Licensed IP or the Licensed Products; and
(d) No Conflicts. KemPharm has not entered, and shall not enter, into any agreement with any Third Party that is in conflict with the rights granted to KVK under this Agreement with respect to the Licensed Product, and has not taken and shall not take any action that would in any way prevent it from granting the rights granted to KVK under this Agreement, or that would otherwise materially conflict with or adversely affect KVK’s rights under this Agreement.
(e) No Other Licenses. KemPharm has not granted and will not grant any licenses or other contingent or non-contingent right, title, or interest under or relating to Licensed IP that conflicts with the exclusivity granted to KVK herein, and is not nor will be under any obligation, that does or will conflict with or otherwise affect this Agreement with respect to such exclusivity.
(f) Prior Art; Litigation. No prior art or other information exists, as of the date of the execution of this Agreement, that would adversely affect the validity, enforceability, term, or scope of any Licensed IP (Exhibit A) and there is no settled, pending, or to its knowledge threatened litigation or reissue application, re-examination, post-grant, inter partes, or covered business method patent review, interference, derivation, opposition, claim of invalidity, or other claim or proceeding, including in the form of any offer to obtain a license, with respect to the Licensed IP.
(g) Non-Infringement. The Licensed Products do not infringe any Third Party’s intellectual property.
10.3 Additional Representations and Warranties of KVK. KVK represents and warrants to KemPharm as follows, as of the Effective Date:
(a) KVK has not entered, and shall not enter, into any agreement with any Third Party that is in conflict with the rights granted to KemPharm under this Agreement with respect to the Licensed Product, and has not taken and shall not take any action that would in any way prevent it from granting the rights granted to KemPharm under this Agreement, or that would otherwise materially conflict with or adversely affect KemPharm’s rights under this Agreement;
(b) KVK represents and warrants to KemPharm that, to KVK’s knowledge as of the Effective Date, any and all agreements, including, but not limited to confidentiality agreements, sale agreements, purchase agreements, license agreements, commercialization agreements, development agreements, or other agreements between KVK and any Third Party does not relate to, affect, involve, or impact any Intellectual Property rights of KVK for the manufacture and commercialization of the Licensed Product pursuant to this Agreement.
(c) KVK represents and warrants to KemPharm that, to KVK’s knowledge as of the Effective Date, any and all settlement agreements of past litigation, including, but not limited to actual or threatened litigation, do not adversely affect, in any material manner or regard, KVK’s ability to manufacture, market, sell or otherwise commercialize the Licensed Product(s) herein pursuant to this Agreement.
(d) KVK represents and warrants to KemPharm that, to KVK’s knowledge as of the Effective Date, that there is no action, suit, claim, investigation or proceeding pending or, to the reasonable knowledge of KVK, threatened against it which questions the validity of this Agreement or the transactions contemplated hereby, or any action taken or to be taken pursuant hereto or thereto. There are no outstanding orders, judgments, injunctions, awards or decrees of any court, arbitrator or Governmental Entity or agency against KVK that adversely affect, in any material manner or regard, KVK’s ability to manufacture, market, sell or otherwise commercialize the Licensed Product(s) herein pursuant to this Agreement.
(e) KVK represents and warrants to KemPharm that, to KVK’s knowledge as of the Effective Date, KVK does not Control any Patent that is necessary to make, use, import, offer for sale or sell Licensed Products in the Field.
(f) KVK represents and warrants to KemPharm that, to KVK’s knowledge as of the Effective Date, that its material representations of financial viability, stability, and general corporate financial health to KemPharm fairly represent the financial condition of the KVK at the dates of said materials representations and further represents and warrants that such material representations of financial viability, stability, and general corporate health are supported by information and documents prepared in accordance with U.S. GAAP consistently applied and consistent with the books and records of KVK.
(g) KVK represents and warrants to KemPharm that, to KVK’s knowledge as of the Effective Date, as evidenced as of the dates of the KVK's financial statements kept solely by the Company and not disclosed to KemPharm, KVK had no liabilities, either accrued or contingent, of a nature required to be reflected in the financial statements in accordance with U.S. GAAP, and whether due or to become due, which individually or in the aggregate are reasonably likely to have a material adverse effect on KVK or its ability to perform its duties and obligations under this Agreement.
(h) KVK represents and warrants to KemPharm that, to KVK's knowledge as of the Effective Date, there are no foreign party, foreign actor, domestic party, domestic actor, or any other third party known to KVK, or alternatively in contractual relationship with KVK, which will materially impact the operation and performance of this Agreement by KVK.
(i) KVK represents and warrants to KemPharm that, to KVK's knowledge as of the Effective Date, there is no existing contractual obligation of KVK or its Subsidiaries or Affiliates is with or for the direct benefit of (i) any party owning, or formerly owning, beneficially or of record, directly or indirectly, in excess of five percent (5%) of the outstanding capital stock of KVK and/or KemPharm, (ii) any director, officer or similar representative of KVK or KemPharm, other than employment agreements, (iii) any natural person related by blood, adoption or marriage to any party described in (i) or (ii), or (iv) any entity in which any of the foregoing parties has, directly or indirectly, at least a five percent (5%) beneficial interest (a "Related Party"). Without limiting the generality of the foregoing, no Related Party, directly or indirectly, owns or controls any material assets or material properties which are used in the KVK's business and to the knowledge of KVK, no Related Party, directly or indirectly, engages in or has any significant interest in or connection with any business which is, or has been within the last two years, a competitor, customer or supplier of KVK or has done business with the KVK or which currently sells or provides products or services which are similar or related to the products or services sold or provided in connection with the Business.
(j) KVK represents and warrants to KemPharm that there is no action, suit or proceeding pending or, to KVK's Knowledge, threatened against KVK or Affiliates which, if adversely determined, would, individually or together with all such other actions, reasonably be expected to have a Material Adverse Effect or otherwise affect KVK's performance of its obligations and responsibilities pursuant to this Agreement. As of the Effective Date, there is no action, suit or proceeding pending or, to KVK's Knowledge, threatened against KVK or any of their Subsidiaries or Affiliates which challenges or impairs the ability of KVK or any of their Subsidiaries or Affiliates to manufacture and sell the Licensed Product herein, or otherwise execute, deliver or perform its obligations under this Agreement.")
10.4 Compliance with Laws.
(a) Each Party shall, and shall ensure that its Affiliates and their respective sublicensees will, and KemPharm shall, and take all reasonable actions to ensure KemPharm Partner(s)’s shall, comply in all respects with Proper Conduct Practices and all applicable Laws in the conduct of Regulatory Activities with respect to, and Commercialization of, Licensed Products and performance of its obligations under this Agreement, including the GCP, GLP and any Regulatory Authority and Government Authority health care programs having jurisdiction in such Party’s respective territory, each as may be amended from time to time.
(b) Each Party shall immediately notify the other Party if it has any information or suspicion that there may be a violation of any applicable Laws in connection with its performance under this Agreement or the conduct of Regulatory Activities with respect to, or Commercialization of, any Licensed Product hereunder. In the event that either Party has violated or been suspected of violating any of its obligations, representations, warranties or covenants in Section 10.4(a), such Party will take reasonable actions to remedy such breach and to prevent further such breaches from occurring.
10.5 Additional KVK Covenants. In addition to any covenants made by KVK elsewhere in this Agreement, KVK hereby covenants to KemPharm as follows:
(a) neither KVK nor any of its Affiliates will employ or use the services of any Person who is debarred or disqualified under the Act, or comparable applicable Laws outside the U.S., in connection with activities relating to any Licensed Product; and in the event that KVK becomes aware of the debarment or disqualification or threatened debarment or disqualification of any Person providing services to KVK or any of its Affiliates with respect to any activities relating to any Licensed Product, KVK will promptly notify KemPharm in writing and KVK will cease, or cause its Affiliate to cease, as applicable, employing, contracting with, or retaining any such Person to perform any services relating to any Licensed Product; and
(b) neither it nor its Affiliates, or its or their sublicensees, shall exploit in any manner any Licensed Product outside of the scope of the licenses expressly granted to KVK under this Agreement.
10.6 No Other Representations or Warranties. EXCEPT AS EXPRESSLY STATED IN THIS AGREEMENT, NO REPRESENTATIONS OR WARRANTIES WHATSOEVER, WHETHER EXPRESS OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, NON-INFRINGEMENT OR NON-MISAPPROPRIATION OF THIRD PARTY INTELLECTUAL PROPERTY RIGHTS, ARE MADE OR GIVEN BY OR ON BEHALF OF A PARTY OR ITS AFFILIATE, AND ALL REPRESENTATIONS AND WARRANTIES, WHETHER ARISING BY OPERATION OF LAW OR OTHERWISE, ARE HEREBY EXPRESSLY EXCLUDED. For clarity and without limiting the foregoing, KemPharm makes no representation or warranty concerning the Licensed Products or Licensed IP except as expressly set forth in this Article 10.
Article 11
Indemnification
11.1 Indemnification by KemPharm. KemPharm shall defend, indemnify, and hold KVK and its Affiliates and their respective officers, directors, employees, and agents (the “KVK Indemnitees”) harmless from and against any and all losses, damages, liabilities, expenses and costs, including reasonable legal expense and attorneys’ fees (“Losses”) to which any KVK Indemnitee may become subject as a result of any claim, demand, action or other proceeding by any Third Party (collectively, “Claims”) arising out of, based on, or resulting from (a) the Development of Licensed Products in the Field in the Collaboration Territory by or on behalf of KemPharm, its Affiliates or KemPharm Partner(s) including, without limitation, the formulation of the Licensed Products or failure to warn regarding same, but excluding manufacture of said formulation or Licensed Products, (b) the breach of any of KemPharm’s obligations under this Agreement, including KemPharm’s representations and warranties set forth herein, (c) the willful misconduct or negligent acts of any KemPharm Indemnitee or KemPharm Partner(s) or such KemPharm Partner(s)’s respective officers, directors, employees, and agents, (d) any Infringement Actions. The foregoing indemnity obligation shall not apply to the extent that (i) the KVK Indemnitees fail to materially comply with the indemnification procedures set forth in Section 11.3 and KemPharm’s defense of the relevant Claim is prejudiced by such failure, or (ii) any Claim arises from, is based on, or results from any activity or occurrence for which KVK is obligated to indemnify the KemPharm Indemnitees under Section 11.2.
11.2 Indemnification by KVK. KVK shall defend, indemnify, and hold KemPharm and its Affiliates and their respective officers, directors, employees, and agents (the “KemPharm Indemnitees”) harmless from and against any and all Losses to which any KemPharm Indemnitee may become subject as a result of any Claims by any Third Party arising out of, based on, or resulting from (a) the conduct of Regulatory Activities with respect to, or Commercialization of, Licensed Products by or on behalf of KVK or its Affiliates or sublicensees (excluding (x) any Infringement Actions, (y) the conduct of Commercialization activities by or on behalf of KemPharm in accordance with the Commercialization Plan or otherwise performed at the request or under the direction of KVK, and (z) any action done at the request or demand of KemPharm or the JSC), (b) the breach of any of KVK’s obligations under this Agreement, including KVK’s representations and warranties set forth herein, (c) the manufacture of the Licensed Product and or formulations thereof or (d) the willful misconduct or negligent acts of any KVK Indemnitee. The foregoing indemnity obligation shall not apply to the extent that (i) the KemPharm Indemnitees fail to materially comply with the indemnification procedures set forth in Section 11.3 and KVK’s defense of the relevant Claim is prejudiced by such failure, or (ii) any Claim arises from, is based on, or results from any activity or occurrence for which KemPharm is obligated to indemnify the KVK Indemnitees under Section 11.1.
11.3 Indemnification Procedures. The Party claiming indemnity under this Article 11 (the “Indemnified Party”) shall give written notice to the Party from whom indemnity is being sought (the “Indemnifying Party”) promptly after learning of such Claim and shall offer control of the defense of such Claim to the Indemnifying Party. The Indemnified Party shall provide the Indemnifying Party with reasonable assistance, at the Indemnifying Party’s expense, in connection with the defense of the Claim for which indemnity is being sought. The Indemnified Party may participate in and monitor such defense with counsel of its own choosing at its sole expense; provided, however, the Indemnifying Party shall have the right to assume and conduct the defense of the Claim with counsel of its choice. The Indemnifying Party shall not settle any Claim without the prior written consent of the Indemnified Party, not to be unreasonably withheld, unless the settlement involves only the payment of money. So long as the Indemnifying Party is actively defending the Claim in good faith, the Indemnified Party shall not settle or compromise any such Claim without the prior written consent of the Indemnifying Party. If the Indemnifying Party does not assume and conduct the defense of the Claim as provided above, (a) the Indemnified Party may defend against, consent to the entry of any judgment, or enter into any settlement with respect to such Claim in any manner the Indemnified Party may deem reasonably appropriate, and the Indemnified Party need not consult with, or obtain any consent from, the Indemnifying Party in connection therewith, and (b) the Indemnifying Party shall remain responsible to indemnify the Indemnified Party as provided in this Article 11. Notwithstanding anything contained in the foregoing to the contrary, the provisions of Section 9.4 shall govern the defense of any Infringement Actions. Additionally, KVK shall not be obligated to indemnify KemPharm for any Claims related to such Infringement Action.
11.4 Limitation of Liability. NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR ANY SPECIAL, CONSEQUENTIAL, INCIDENTAL, PUNITIVE, OR INDIRECT DAMAGES ARISING FROM OR RELATING TO ANY BREACH OF THIS AGREEMENT, REGARDLESS OF ANY NOTICE OF THE POSSIBILITY OF SUCH DAMAGES. NOTWITHSTANDING THE FOREGOING, NOTHING IN THIS SECTION 11.4 IS INTENDED TO OR SHALL LIMIT OR RESTRICT THE INDEMNIFICATION RIGHTS OR OBLIGATIONS OF ANY PARTY UNDER SECTIONS 11.1, 11.2 or 11.3, OR DAMAGES AVAILABLE FOR A PARTY’S BREACH OF CONFIDENTIALITY OBLIGATIONS IN ARTICLE 12 OR FOR EITHER PARTY’S BREACH OF ITS OBLIGATIONS IN SECTION 2.5.
11.5 Insurance. Each Party shall procure and maintain insurance, including product liability insurance, adequate to cover obligations for which it is liable hereunder and consistent with normal business practices of prudent companies similarly situated. It is understood that such insurance shall not be construed to create a limit of either Party’s liability with respect to its indemnification obligations under this Article 11. Each Party shall provide the other Party with written evidence of such insurance upon request. Each Party shall provide the other Party with written notice at least [*] days prior to the cancellation, non-renewal or material change in such insurance.
Article 12
Confidentiality
12.1 Confidentiality. Each Party agrees that, during the Term and for a period of [*] years thereafter, it shall keep confidential and shall not publish or otherwise disclose and shall not use for any purpose other than as provided for in this Agreement or any other Transaction Agreement (which includes the exercise of any rights or the performance of any obligations hereunder or thereunder) any Confidential Information of the other Party, except to the extent expressly authorized by any Transaction Agreement or otherwise agreed in writing by the Parties. The foregoing confidentiality and non-use obligations shall not apply to any portion of the other Party’s Confidential Information that the receiving Party can demonstrate by competent written proof:
(a) was already known to the receiving Party or its Affiliate, other than under an obligation of confidentiality, at the time of disclosure by the other Party;
(b) was generally available to the public or otherwise part of the public domain at the time of its disclosure to the receiving Party;
(c) became generally available to the public or otherwise part of the public domain after its disclosure and other than through any act or omission of the receiving Party or its Affiliate in breach of this Agreement;
(d) was disclosed to the receiving Party or its Affiliate by a Third Party who has a legal right to make such disclosure and who did not obtain such information directly or indirectly from the other Party; or
(e) was independently discovered or developed by the receiving Party or its Affiliate without access to or aid, application, use of the other Party’s Confidential Information, as evidenced by a contemporaneous writing.
12.2 Authorized Disclosure. Notwithstanding the obligations set forth in Section 12.1, a Party may disclose the other Party’s Confidential Information and the terms of this Agreement to the extent:
(a) such disclosure is reasonably necessary (i) for the filing or prosecuting Patent rights as contemplated by any of the Transaction Agreements; (ii) to comply with the requirements of Regulatory Authorities with respect to obtaining and maintaining Regulatory Approval of Licensed Product; or (iii) for the prosecuting or defending litigation as contemplated by any of the Transaction Agreements;
(b) such disclosure is reasonably necessary to its or its Affiliate’s employees, agents, consultants, contractors, licensees or sublicensees on a need-to-know basis for the sole purpose of performing its obligations or exercising its rights under any of the Transaction Agreements; provided that in each case, the disclosees are bound by written obligations of confidentiality and non-use at least as restrictive as those contained in this Agreement;
(c) such disclosure is reasonably necessary to any bona fide potential or actual investor, acquiror, merger partner, or other financial or commercial partner for the sole purpose of evaluating or carrying out an actual or potential investment, acquisition or other business relationship; provided that in connection with such disclosure, such Party shall inform each disclosee of the confidential nature of such Confidential Information and require each disclosee to treat such Confidential Information as confidential; or
(d) such disclosure is reasonably necessary to comply with applicable Laws, including regulations promulgated by applicable security exchanges, court order, administrative subpoena or order.
Notwithstanding the foregoing, in the event a Party is required to make a disclosure of the other Party’s Confidential Information pursuant to Sections 12.2(a) or 12.2(d), such Party shall promptly notify the other Party of such required disclosure and shall use reasonable efforts to obtain, or to assist the other Party in obtaining, a protective order preventing or limiting the required disclosure.
12.3 Publicity; Terms of Agreement.
(a) The Parties agree that the terms of this Agreement are the Confidential Information of both Parties, subject to the special authorized disclosure provisions set forth in this Section 12.3.
(b) If either Party desires to make a public disclosure concerning the terms of this Agreement, such Party shall give reasonable prior advance notice of the proposed text of such disclosure to the other Party for its prior review and approval, except as otherwise provided herein, which approval shall not be unreasonably withheld or delayed. A Party commenting on such a proposed disclosure shall provide its comments, if any, within [*] Business Days after receiving the proposed disclosure for review, or such shorter period of time as necessitated by regulatory requirements. In addition, where required by applicable Law, including regulations promulgated by applicable security exchanges, either Party shall have the right to make a press release or other public disclosure regarding the achievement of each sales milestone under this Agreement as it is achieved, the achievements of Regulatory Approval in the Collaboration Territory as they occur, or the occurrence of other events that affect either Party’s rights or obligations under this Agreement, in each case subject only to the review procedure set forth in the preceding sentences. In relation to the other Party’s review of such an announcement, such other Party may make specific, reasonable comments on such proposed press release within the prescribed time for commentary. Neither Party shall be required to seek the permission of the other Party to repeat any information regarding the terms of this Agreement that has already been publicly disclosed by such Party, or by the other Party, in accordance with this Section 12.3.
(c) The Parties acknowledge that either or both Parties or their Affiliates may be obligated to file under applicable Laws a copy of this Agreement with Governmental Authorities. Each Party and its Affiliates shall be entitled to make such a required filing, provided that it requests confidential treatment of the commercial terms and sensitive technical terms hereof and thereof to the extent such confidential treatment is reasonably available. In the event of any such filing, each Party will provide the other Party with a copy of this Agreement marked to show provisions for which such Party or its Affiliate intends to seek confidential treatment and shall reasonably consider and incorporate the other Party’s timely comments thereon to the extent consistent with the legal requirements, with respect to the filing Party or Affiliate, governing disclosure of material agreements and material information that must be publicly filed.
12.4 Technical Publication. KVK may not publish peer reviewed manuscripts or provide other forms of public disclosure, including abstracts and presentations, pertaining to the Licensed Products or Licensed Know-How, without the prior written consent of KemPharm.
12.5 Equitable Relief. Each Party acknowledges that its breach of this Article 12 will cause irreparable harm to the other Party, which cannot be reasonably or adequately compensated in damages in an action at law. By reasons thereof, each Party agrees that the other Party shall be entitled, in addition to any other remedies it may have under this Agreement or otherwise, to preliminary and permanent injunctive and other equitable relief to prevent or curtail any actual or threatened breach of the obligations relating to Confidential Information set forth in this Article 12 by the other Party.
Article 13
Term And Termination
13.1 Term. The term of this Agreement (the “Term”) shall commence upon the Effective Date and, unless earlier terminated pursuant to this Article 13, shall expire on the later of (a) expiration of the last claim of a Licensed Patent in the Collaboration Territory and (b) KVK’s cessation of all Commercialization of Licensed Products in the Collaboration Territory.
13.2 Termination by KVK.
(a) KVK may terminate this Agreement in its entirety for convenience upon eighteen (18) months prior written notice given to KemPharm at any time after the third (3rd) anniversary of the Effective Date; provided, however, that the Parties may, upon mutual written consent, accelerate the effectiveness of such termination to the extent permitted by applicable Law in the Collaboration Territory.
(b) KVK may terminate this Agreement upon ninety (90) days prior written notice to KemPharm if a Regulatory Authority in the Collaboration Territory has ordered KVK to stop all sales of Licensed Products in the Collaboration Territory due to a safety concern; provided, however, that KVK has, for a period of ninety (90) days prior to the provision of such notice by KVK, used Commercially Reasonable Efforts to resolve such safety concern.
13.3 Termination by KemPharm.
(a) KemPharm may terminate this Agreement upon written notice to KVK, if KVK stops conducting Regulatory Activities with respect to, or Commercializing, Licensed Products in the Collaboration Territory for a period of six (6) months or more, unless the conduct of Regulatory Activities or Commercialization of Licensed Products was prevented throughout such period by a force majeure for which KVK provided notice pursuant to Section 15.2 prior to or at the start of such period and that persisted throughout such period despite KVK’s reasonable efforts to remove or mitigate it. Such termination shall go into effect on the date specified in the applicable termination notice.
(b) KemPharm may terminate this Agreement in its entirety upon written notice to KVK, if KVK or its Affiliates or their respective sublicensees, directly or indirectly, individually or in association with any other person or entity, challenges the validity, enforceability or scope of any Licensed Patent. Such termination shall go into effect on the date specified in the applicable termination notice.
13.4 Termination for Breach. Each Party shall have the right to terminate this Agreement in its entirety immediately upon written notice to the other Party if the other Party materially breaches its obligations under any Transaction Agreement and, after receiving written notice identifying such material breach in reasonable detail, fails to cure such material breach within thirty (30) days from the date of such notice.
13.5 Termination Due to Bankruptcy. Either Party may terminate this Agreement if, at any time, the other Party files in any court or agency pursuant to any statute or regulation of any state, country or jurisdiction, a petition in bankruptcy or insolvency or for reorganization or for an arrangement or for the appointment of a receiver or trustee of that Party or of its assets, or if the other Party proposes a written agreement of composition or extension of its debts, or if the other Party is served with an involuntary petition against it, filed in any insolvency proceeding, and such petition is not dismissed within sixty (60) days after the filing thereof, or if the other Party proposes or becomes a Party to any dissolution or liquidation, or if the other Party makes an assignment for the benefit of its creditors.
13.6 Proof of Market Viability Termination. Either Party may terminate this Agreement in accordance with Section 6.9. In the event that KemPharm terminates this Agreement pursuant to Section 6.9 and continues to Commercialize Licensed Products in the Field in the Collaboration Territory, in addition to the effects of termination described in Section 13.7, [*] prior to the effective date of termination.
13.7 Effect of Termination or Expiration. Upon the any termination or expiration of this Agreement, the following shall apply, in addition to any other rights and obligations under this Agreement with respect to such termination:
(a) Licenses. All licenses and other rights granted by KemPharm to KVK under this Agreement shall terminate, including all sublicenses granted by KVK unless such sublicenses are assumed by KemPharm as contemplated by Section 2.1(c)(vi), which shall survive expiration or termination. KemPharm shall have a reversion of all rights previously licensed to KVK hereunder for which the relevant licenses have terminated on a fully paid-up and royalty-free basis, itself or with or through an Affiliate or Third Party, to conduct Regulatory Activities with respect to, and Commercialize, the Licensed Products in the Field in the Collaboration Territory at KemPharm’s discretion.
(b) Regulatory Materials; Data. KVK shall transfer or assign to KemPharm or its designee all Regulatory Materials, including Regulatory Approvals, for the Licensed Products to the extent possible under applicable Law in the Collaboration Territory. KVK shall also promptly transfer or assign to KemPharm all Data, to the extent not already provided to KemPharm, including pharmacovigilance data, generated by or on behalf of KVK. In addition, each Party shall promptly return or destroy, at the other Party’s election, all Confidential Information of such Party. If this Agreement is terminated by KVK pursuant to Sections 13.4 or 13.5, KemPharm shall bear the cost arising out of this subsection. If this Agreement is terminated by KVK pursuant to Sections 13.2(a) or if this Agreement is terminated by KemPharm pursuant to Sections 13.3(a), 13.3(b), 13.4, or 13.5, [*]. If this Agreement is terminated by KVK pursuant to Section 13.2(b) or by either Party pursuant to Section 13.6, [*].
(c) Transition Assistance. Upon KemPharm’s request, KVK shall provide such assistance as may be reasonably necessary or useful for KemPharm to continue the Commercialization of Licensed Products in the Collaboration Territory, to the extent KVK or its Affiliate or sublicensee is then performing or having performed such activities, including upon request of KemPharm, assigning or amending as appropriate any agreements or arrangements with any Third Party for the distribution, sale or otherwise Commercialization of Licensed Products. If this Agreement is terminated by KVK pursuant to Sections 13.4 or 13.5, [*]. If this Agreement is terminated by KVK pursuant to Sections 13.2(a) or if this Agreement is terminated by KemPharm pursuant to Sections 13.3(a), 13.3(b), 13.4, or 13.5, [*]. If this Agreement is terminated by KVK pursuant to Sections 13.2(b) or by either Party pursuant to Section 13.6, [*]. Without limiting KVK’s obligations pursuant to Section 2.1(c)(vi), to the extent that any such contract between KVK or its Affiliate or sublicensee and a Third Party is not assignable to KemPharm, KVK shall reasonably cooperate with KemPharm, at KemPharm’s request [*], to arrange to continue to provide such services for a reasonable time after termination and to facilitate KemPharm’s entry into a replacement agreement with such Third Party for such services. Additionally, KVK shall provide KemPharm with copies of any promotional and marketing materials generated by or on behalf of KVK with respect to Licensed Products prior to the effective date of expiration or termination. Upon KemPharm’s request, KVK shall continue to Commercialize the Licensed Products in the Collaboration Territory for up to [*] after the effective date of expiration or termination of this Agreement, subject to [*].
(d) Inventory. In the event that this Agreement is terminated in its entirety, KemPharm shall have the right, but not the obligation to purchase any and all of the inventory of Licensed Products, including any samples and/or ingredients, held by KVK or its Affiliates or sublicensees as of the date of termination, at a price equal to (i) in the event that KVK is not the manufacturer of the Licensed Products, [*], or (ii) in the event that KVK is the manufacturer of the Licensed Products, [*]. Notwithstanding the above, if this Agreement is terminated by KVK pursuant to Sections 13.4 or 13.5, KVK shall have the right, at its sole discretion, to continue to be permitted to sell such inventory for up to at least [*] months after the effective date of termination of this Agreement.
13.8 Survival. Termination or expiration of this Agreement shall not affect rights or obligations of the Parties under this Agreement that have accrued prior to the date of termination or expiration. Notwithstanding anything to the contrary, the following provisions shall survive any expiration or termination of this Agreement: Articles 1 and 9 through 13, and Sections 2.1, 2.3, 2.4, 5.1, 5.3, 5.9, 5.10, 6.6, 6.7, 8.4 through 8.9, and 14.2.
13.9 Termination Not Sole Remedy. Termination is not the sole remedy under this Agreement and, whether or not termination is affected and notwithstanding anything contained in this Agreement to the contrary, all other remedies shall remain available except as agreed to otherwise herein.
Article 14
Dispute Resolution
14.1 Disputes; Internal Resolution. The Parties recognize that disputes as to certain matters may from time to time arise that relate to either Party’s rights and/or obligations hereunder. It is the objective of the Parties to establish procedures to facilitate the resolution of disputes arising under this Agreement in an expedient manner by mutual cooperation. To accomplish this objective, the Parties agree that, if a dispute arises under this Agreement with respect to which a Party does not have final decision-making authority pursuant to Section 3.3, and the Parties are unable to resolve such dispute within [*] days after such dispute is first identified by either Party in writing to the other, the Parties shall refer such dispute to the respective Chief Executive Officer of KemPharm, or one of its Affiliates, and KVK (the “Executive Officers”) for attempted resolution by good faith negotiations within [*] days after such notice is received, which shall include at least one (1) in person meeting of the Executive Officers within [*] days after such notice is received. If the dispute is not resolved within such [*] days, either Party may resolve such dispute in accordance with Section 14.2.
14.2 Governing Law. Resolution of all disputes and any remedies relating thereto, shall be governed by and construed under the laws of the State of Delaware, U.S., without giving effect to any choice of law principles that would require the application of the laws of a different state. Each of the Parties hereby irrevocably and unconditionally consents to submit to the exclusive jurisdiction of the courts of the State of Delaware for any matter arising out of or relating to this Agreement and the transactions contemplated hereby, and agrees not to commence any litigation relating thereto except in such courts. Each of the Parties hereby irrevocably and unconditionally waives any objection to the laying of venue of any matter arising out of this Agreement or the transactions contemplated hereby in the courts of the State of Delaware and hereby further irrevocably and unconditionally waives and agrees not to plead or claim in any such court that any such matter brought in any such court has been brought in an inconvenient forum.
Article 15
Miscellaneous
15.1 Entire Agreement; Amendment. This Agreement, including the Exhibits hereto, sets forth the complete, final and exclusive agreement and all the covenants, promises, agreements, warranties, representations, conditions and understandings between the Parties hereto with respect to the subject matter hereof and supersedes, as of the Effective Date, all prior and contemporaneous agreements and understandings between the Parties with respect to the subject matter hereof, including the Confidentiality Agreement. The foregoing shall not be interpreted as a waiver of any remedies available to either Party as a result of any breach, prior to the Effective Date, by the other Party of its obligations under the Confidentiality Agreement. There are no covenants, promises, agreements, warranties, representations, conditions or understandings, either oral or written, between the Parties other than as are set forth in this Agreement. No subsequent alteration, amendment, change or addition to this Agreement shall be binding upon the Parties unless reduced to writing and signed by an authorized officer of each Party.
15.2 Force Majeure. Both Parties shall be excused from the performance of their obligations under this Agreement to the extent that such performance is prevented by force majeure and the nonperforming Party promptly provides notice of the prevention to the other Party. Such excuse shall be continued so long as the condition constituting force majeure continues and the nonperforming Party takes reasonable efforts to remove the condition. For purposes of this Agreement, force majeure shall include conditions beyond the reasonable control of the applicable Party, which may include an act of God, war, civil commotion, terrorist act, labor strike or lock-out, epidemic, failure or default of public utilities or common carriers, destruction of production facilities or materials by fire, earthquake, storm or like catastrophe, and failure of plant or machinery. Notwithstanding the foregoing, a Party shall not be excused from making payments owed hereunder because of a force majeure affecting such Party. If a force majeure persists for more than ninety (90) days, then the Parties will discuss in good faith the modification of the Parties’ obligations under this Agreement in order to mitigate the delays caused by such force majeure.
15.3 Notices. Any notice required or permitted to be given under this Agreement shall be in writing, shall specifically refer to this Agreement, and shall be addressed to the appropriate Party at the address specified below or such other address as may be specified by such Party in writing in accordance with this Section 15.3, and shall be deemed to have been given for all purposes (a) when received, if hand-delivered or sent by a reputable courier service, or (b) [*] Business Days after mailing, if mailed by first class certified or registered airmail, postage prepaid, return receipt requested.
|
If to KemPharm: |
KemPharm Inc. |
▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attn: R. ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇, CFO, Secretary and Treasurer
with copies to, which shall not constitute notice:
▇▇▇▇▇▇ LLP
▇▇▇ ▇▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇-▇▇▇▇
Attn: ▇▇▇▇▇ ▇▇▇▇▇, Esq.
|
If to KVK: |
KVK Tech, Inc. |
▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇
▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attn: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇, CEO
with copies to, which shall not constitute notice:
▇▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇ LLP
▇▇▇▇ ▇▇▇▇▇▇ ▇▇▇▇▇▇
▇▇▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇
Attn: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇, ▇▇., Esq.
15.4 No Strict Construction; Headings. This Agreement has been prepared jointly by the Parties and shall not be strictly construed against either Party. Ambiguities, if any, in this Agreement shall not be construed against any Party, irrespective of which Party may be deemed to have authored the ambiguous provision. The headings of each Article and Section in this Agreement have been inserted for convenience of reference only and are not intended to limit or expand on the meaning of the language contained in the particular Article or Section. Except where the context otherwise requires, the use of any gender shall be applicable to all genders, and the word “or” is used in the inclusive sense (and/or). The term “including” as used herein means including, without limiting the generality of any description preceding such term.
15.5 Assignment; Change of Control.
(a) Neither Party may assign or transfer this Agreement or any rights or obligations hereunder without the prior written consent of the other Party, except that either Party may make such an assignment without the other Party’s express written consent to its Affiliates.
(b) Notwithstanding Section 15.5(a), either Party may without such consent but with prior written notice to the other Party, assign this Agreement and its rights and obligations hereunder in connection with a Change of Control, provided further that if the said assignee is engaged in a business that competes with the Licensed Product and/or the notified Party’s business, the notified Party shall have the right to terminate this Agreement without any obligation to the other Party, by providing written notice thereof within [*] after the receipt of such notice from the assigning Party. Any permitted assignee shall assume all obligations of its assignor under this Agreement. Any assignment or attempted assignment by either Party in violation of the terms of this Section 15.5 shall be null, void and of no legal effect.
15.6 Performance by Affiliates. Each Party may discharge any obligations and exercise any right hereunder through any of its Affiliates. Each Party hereby guarantees the performance by its Affiliates of such Party’s obligations under this Agreement and shall cause its Affiliates to comply with the provisions of this Agreement in connection with such performance. Any breach by a Party’s Affiliate of any of such Party’s obligations under this Agreement shall be deemed a breach by such Party, and the other Party may proceed directly against such Party without any obligation to first proceed against such Party’s Affiliate.
15.7 Further Actions. Each Party agrees to execute, acknowledge and deliver such further instruments, and to do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
15.8 Severability. If any one or more of the provisions of this Agreement is held to be invalid or unenforceable by any court of competent jurisdiction from which no appeal can be or is taken, the provision shall be considered severed from this Agreement and shall not serve to invalidate any remaining provisions hereof. The Parties shall make a good faith effort to replace any invalid or unenforceable provision with a valid and enforceable one such that the objectives contemplated by the Parties when entering this Agreement may be realized.
15.9 No Waiver. Any delay in enforcing a Party’s rights under this Agreement or any waiver as to a particular default or other matter shall not constitute a waiver of such Party’s rights to the future enforcement of its rights under this Agreement, except with respect to an express written and signed waiver relating to a particular matter for a particular period of time.
15.10 Independent Contractors. Each Party shall act solely as an independent contractor, and nothing in this Agreement shall be construed to give either Party the power or authority to act for, bind, or commit the other Party in any way. Nothing herein shall be construed to create the relationship of partners, principal and agent, or joint-venture partners between the Parties.
15.11 English Language. This Agreement was prepared in the English language, which language shall govern the interpretation of, and any dispute regarding, the terms of this Agreement.
15.12 Counterparts. This Agreement may be executed in one (1) or more counterparts, each of which shall be deemed an original, but all of which together shall constitute one and the same instrument.
{Signature Page Follows}
In Witness Whereof, the Parties have executed this License, Development and Commercialization Agreement in duplicate originals by their duly authorized officers as of the Effective Date.
|
By: /s/ R. ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Name: R. ▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇ Title: CFO, Secretary and Treasurer |
KVK Tech, Inc.
By: /s/ ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇ Name: ▇▇▇▇▇▇▇ ▇. ▇▇▇▇▇▇▇ Title: CEO and General Counsel |
Article 1 Definitions 1
Article 2 License 10
2.1 License to KVK 10
2.2 KemPharm Partner 11
2.3 Negative Covenant 12
2.4 No Implied Licenses 12
2.5 Exclusivity 12
Article 3 Governance 12
3.1 Alliance Managers 12
3.2 Joint Committees 13
3.3 Decision Making 14
3.4 Limitation of Committee Authority 15
3.5 Discontinuation of Committees 15
Article 4 DEVELOPMENT 15
4.1 Overview 15
4.2 Transfer of Licensed Know-How 15
Article 5 Regulatory Matters 16
5.1 Regulatory Responsibilities 16
5.2 Pricing 16
5.3 Regulatory Information Sharing 17
5.4 Meetings with Regulatory Authorities 17
5.5 Regulatory Costs 17
5.6 Right of Reference to Regulatory Materials 17
5.7 No Harmful Actions 18
5.8 Notification of Threatened Action 18
5.9 Adverse Event Reporting and Safety Data Exchange 18
5.10 Remedial Actions 18
Article 6 Commercialization 19
6.1 Overview 19
6.2 Commercialization Plan 19
6.3 Pricing 20
6.4 Commercial Diligence 20
6.5 Commercialization Reports 20
6.6 Cross-Territorial Restrictions 21
6.7 Field Restrictions 21
6.8 Labeling 21
6.9 Proof of Concept Termination 22
Article 7 MANUFACTURE AND SUPPLY 22
7.1 Manufacture and Supply 22
7.2 Supply Price 22
7.3 [Intentionally Deleted] 22
7.4 Quality Agreement 23
7.5 Distribution 23
7.6 Brand Security and Anti-Counterfeiting 23
Article 8 Compensation 23
8.1 POMV Fee 23
8.2 Commercialization/Regulatory Expenses 23
8.3 Manufacturing Expenses 23
8.4 Net Profit Share 23
8.5 Sales Milestone Payments 25
8.6 Payment Method; Foreign Exchange 25
8.7 Interest on Late Payments 25
8.8 Records; Audits 25
8.9 Taxes 26
Article 9 Intellectual Property Matters 27
9.1 Ownership of Data and Inventions 27
9.2 Patent Prosecution 27
9.3 Patent Enforcement 28
9.4 Third Party Infringement Claims 29
9.5 Patent Term Extensions 30
9.6 Trademarks 30
Article 10 Representations And Warranties; covenants 31
10.1 Mutual Representations and Warranties 32
10.2 Additional Representations and Warranties of KemPharm 32
10.3 Additional Representations and Warranties of KVK 32
10.4 Compliance with Laws 33
10.5 Additional KVK Covenants 33
10.6 No Other Representations or Warranties 33
Article 11 Indemnification 33
11.1 Indemnification by KemPharm 34
11.2 Indemnification by KVK 34
11.3 Indemnification Procedures 34
11.4 Limitation of Liability 35
11.5 Insurance 35
Article 12 Confidentiality 35
12.1 Confidentiality 35
12.2 Authorized Disclosure 36
12.3 Publicity; Terms of Agreement 37
12.4 Technical Publication 37
12.5 Equitable Relief 37
Article 13 Term And Termination 38
13.1 Term 38
13.2 Termination by KVK 38
13.3 Termination by KemPharm 38
13.4 Termination for Breach 38
13.5 Termination Due to Bankruptcy 39
13.6 Proof of Concept Termination 39
13.7 Effect of Termination or Expiration 39
13.8 Survival 40
13.9 Termination Not Sole Remedy 40
Article 14 Dispute Resolution 40
14.1 Disputes; Internal Resolution 41
14.2 Governing Law 41
Article 15 Miscellaneous 41
15.1 Entire Agreement; Amendment 41
15.2 Force Majeure 42
15.3 Notices 42
15.4 No Strict Construction; Headings 43
15.5 Assignment; Change of Control 43
15.6 Performance by Affiliates 43
15.7 Further Actions 43
15.8 Severability 43
15.9 No Waiver 44
15.10 Independent Contractors 44
15.11 English Language 44
15.12 Counterparts 44
List of Exhibits
Exhibit A: Licensed Patents
Exhibit A-1: Limited Licensed Priority Patents
Exhibit B: Initial Commercialization Plan
Exhibit C: Licensed Product Label
Exhibit D: [*]
Exhibit E: Net Profit Share Example
Exhibit A
Licensed Patents and Patent Applications
Title: [*]
Inventors: [*]
|
Application # |
Filing Date |
Patent # |
Grant Date |
|
|
1 |
[*] |
[*] |
[*] |
[*] |
|
2 |
[*] |
[*] |
[*] |
[*] |
|
3 |
[*] |
[*] |
Exhibit A-1
Limited Licensed Priority Patents and Patent Applications
Title: [*]
Inventors: [*]
|
Application # |
Filing Date |
Patent # |
Grant Date |
|
|
1 |
[*] |
[*] |
||
|
2 |
[*] |
[*] |
[*] |
[*] |
|
3 |
[*] |
[*] |
[*] |
[*] |
|
4 |
[*] |
[*] |
[*] |
[*] |
|
5 |
[*] |
[*] |
[*] |
[*] |
|
6 |
[*] |
[*] |
Exhibit B
Initial Commercialization Plan
[*]
Exhibit C
Licensed Product Label
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use APADAZ™ safely and effectively. See full prescribing information for APADAZ.
APADAZ (benzhydrocodone and acetaminophen) tablets, for oral use, CII
Initial U.S. Approval: 1982
|
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE- THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; NEONATAL OPIOID WITHDRAWAL SYNDROME; HEPATOTOXICITY, CYTOCHROME P450 3A4 INTERACTION, and RISKS FROM CONCOMITANT USE WITH BENZODIAZEPINES OR OTHER CNS DEPRESSANTS See full prescribing information for complete boxed warning. ●APADAZ exposes users to risks of addiction, abuse, and misuse, which can lead to overdose and death. Assess patient’s risk before prescribing and monitor regularly for these behaviors and conditions. (5.1) ●Serious, life-threatening, or fatal respiratory depression may occur. Monitor closely, especially upon initiation or following a dose increase. (5.2) ●Accidental ingestion of APADAZ, especially by children, can result in a fatal overdose of hydrocodone. (5.2) ●Prolonged use of APADAZ during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life- threatening if not recognized and treated. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available. (5.3) ●Concomitant use with CYP3A4 inhibitors (or discontinuation of CYP3A4 inducers) can result in a fatal overdose of hydrocodone from APADAZ. (5.4, 7, 12.3) ●APADAZ contains acetaminophen. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product. (5.5) ●Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing for use in patients for whom alternative treatment options are inadequate; limit dosages and durations to the minimum required; and follow patients for signs and symptoms of respiratory depression and sedation. (5.6, 7) |
INDICATIONS AND USAGE
APADAZ is a combination of benzhydrocodone, a prodrug of the opioid agonist hydrocodone, and acetaminophen, and is indicated for the short-term (no more than 14 days) management of acute pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. (1)
Limitations of Use (1)
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses, reserve APADAZ for use in patients for whom alternative treatment options [e.g., non-opioid analgesics]:
|
● |
Have not been tolerated, or are not expected to be tolerated, |
|
● |
Have not provided adequate analgesia, or are not expected to provide adequate analgesia. |
DOSAGE AND ADMINISTRATION
|
● |
Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals. (2.1) |
|
● |
Individualize dosing based on the severity of pain, patient response, prior analgesic experience, and risk factors for addiction, abuse, and misuse. (2.1) |
|
● |
Initiate treatment with APADAZ at 1 or 2 tablets every 4 to 6 hours as needed for pain. Dosage should not exceed 12 tablets in a 24- hour period. (2.2) |
|
● |
6.12 mg benzhydrocodone is equivalent to 4.54 mg hydrocodone or 7.5 mg hydrocodone bitartrate. If switching from immediate-release hydrocodone bitartrate/acetaminophen, substitute 6.12 mg/325 mg APADAZ for 7.5 mg/325 mg hydrocodone bitartrate/acetaminophen. Dosage of APADAZ should be adjusted according to the severity of the pain and the response of the patient. |
|
● |
Do not stop APADAZ abruptly in a physically-dependent patient. (2.5) |
DOSAGE FORMS AND STRENGTHS
Immediate-release tablets: 6.12 mg benzhydrocodone (equivalent to 6.67 mg benzhydrocodone hydrochloride) and 325 mg acetaminophen
(3)
CONTRAINDICATIONS
|
● |
Significant respiratory depression (4) |
|
● |
Acute or severe bronchial asthma in an unmonitored setting or in absence of resuscitative equipment (4) |
|
● |
Known or suspected gastrointestinal obstruction, including paralytic ileus (4) |
|
● |
Hypersensitivity to hydrocodone or acetaminophen (4) |
WARNINGS AND PRECAUTIONS
|
● |
Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration. (5.7) |
|
● |
Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. (5.8) |
|
● |
Severe Hypotension: Monitor during dosage initiation and titration. Avoid use of APADAZ in patients with circulatory shock. (5.9) |
|
● |
Serious Skin Reactions: Discontinue APADAZ immediately at the first appearance of skin rash and if symptoms associated with allergy or hypersensitivity occur. Do not use in patients with acetaminophen allergy. (5.10) |
|
● |
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness: Monitor for sedation and respiratory depression. Avoid use of APADAZ in patients with impaired consciousness or coma. (5.11) |
ADVERSE REACTIONS
Most common adverse reactions (>5%) are nausea, somnolence, vomiting, constipation, pruritus, dizziness, and headache. (6)
To report SUSPECTED ADVERSE REACTIONS, contact KemPharm, Inc. at 1-321-939-3416 or FDA at 1-800-FDA-1088 or ▇▇▇.▇▇▇.▇▇▇/▇▇▇▇▇▇▇▇
----------------------------- DRUG INTERACTIONS ----------------------------
|
● |
Serotonergic Drugs: Concomitant use may result in serotonin syndrome. Discontinue APADAZ if serotonin syndrome is suspected. (7) |
|
● |
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics: Avoid use with APADAZ because they may reduce analgesic effect of APADAZ or precipitate withdrawal symptoms. (7) |
|
● |
Monoamine Oxidase Inhibitors (MAOIs): Can potentiate the effects of hydrocodone. Avoid concomitant use in patients receiving MAOIs or within 14 days of stopping treatment with an MAOI. (7) |
USE IN SPECIFIC POPULATIONS
Pregnancy: May cause fetal harm (8.1).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 02/2018
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY DEPRESSION; ACCIDENTAL INGESTION; NEONATAL OPIOID WITHDRAWAL SYNDROME; AND HEPATOTOXICITY
|
1 |
INDICATIONS AND USAGE |
|
2 |
DOSAGE AND ADMINISTRATION |
|
2.1 |
Important Dosage and Administration Instructions |
|
2.2 |
Initial Dosage |
|
2.3 |
Conversion from Other Opioids to APADAZ |
|
2.4 |
Titration and Maintenance of Therapy |
|
2.5 |
Discontinuation of APADAZ |
|
3 |
DOSAGE FORMS AND STRENGTHS |
|
4 |
CONTRAINDICATIONS |
|
5 |
WARNINGS AND PRECAUTIONS |
|
5.1 |
Addiction, Abuse, and Misuse |
|
5.2 |
Life-Threatening Respiratory Depression |
|
5.3 |
Neonatal Opioid Withdrawal Syndrome |
|
5.4 |
Risks of Concomitant Use or Discontinuation of Cytochrome P450 CYP3A4 Inhibitors and Inducers |
|
5.5 |
Acetaminophen Hepatotoxicity |
|
5.6 |
Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants |
|
5.7 |
Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients |
|
5.8 |
Adrenal Insufficiency |
|
5.9 |
Severe Hypotension |
|
5.10 |
Serious Skin Reactions |
|
5.11 |
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness |
|
5.12 |
Hypersensitivity/Anaphylaxis |
|
5.13 |
Risk of Use in Patients with Gastrointestinal Conditions |
|
5.14 |
Increased Risk of Seizures in Patients with Seizure Disorders |
|
5.15 |
Withdrawal |
|
5.16 |
Risks of Driving and Operating Machinery |
|
6 |
ADVERSE REACTIONS |
|
6.1 |
Clinical Trials Experience |
|
6.2 |
Postmarketing Experience |
|
7 |
DRUG INTERACTIONS |
|
8 |
USE IN SPECIFIC POPULATIONS |
|
8.1 |
Pregnancy |
|
8.2 |
Lactation |
|
8.3 |
Females and Males of Reproductive Potential |
|
8.4 |
Pediatric Use |
|
8.5 |
Geriatric Use |
|
8.6 |
Hepatic Impairment |
|
8.7 |
Renal Impairment |
|
9 |
DRUG ABUSE AND DEPENDENCE |
|
9.1 |
Controlled Substance |
|
9.2 |
Abuse |
|
9.3 |
Dependence |
|
10 |
OVERDOSAGE |
|
11 |
DESCRIPTION |
|
12 |
CLINICAL PHARMACOLOGY |
|
12.1 |
Mechanism of Action |
|
12.2 |
Pharmacodynamics |
|
12.3 |
Pharmacokinetics |
|
13 |
NONCLINICAL TOXICOLOGY |
|
13.1 |
Carcinogenesis, Mutagenesis, Impairment of Fertility |
|
16 |
HOW SUPPLIED/STORAGE AND HANDLING |
|
17 |
PATIENT COUNSELING INFORMATION |
* Sections or subsections omitted from the full prescribing information are not listed
FULL PRESCRIBING INFORMATION
|
WARNING: ADDICTION, ABUSE, AND MISUSE; LIFE-THREATENING RESPIRATORY Addiction, Abuse, and Misuse APADAZ exposes patients and other users to the risks of opioid addiction, abuse, and misuse, which can lead to overdose and death. Assess each patient’s risk prior to prescribing APADAZ, and monitor all patients regularly for the development of these behaviors and conditions [see Warnings and Precautions (5.1)]. Life-Threatening Respiratory Depression Serious, life-threatening, or fatal respiratory depression may occur with use of APADAZ. Monitor for respiratory depression, especially during initiation of APADAZ or following a dose increase [see Warnings and Precautions (5.2)]. Accidental Ingestion Accidental ingestion of even one dose of APADAZ, especially by children, can result in a fatal overdose of hydrocodone [see Warnings and Precautions (5.2)]. Neonatal Opioid Withdrawal Syndrome Prolonged use of APADAZ during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. If prolonged opioid use is required in a pregnant woman, advise the patient of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Warnings and Precautions (5.3)]. Cytochrome P450 3A4 Interaction The concomitant use of APADAZ with all cytochrome P450 3A4 inhibitors may result in an increase in hydrocodone plasma concentrations, which could increase or prolong adverse reactions and may cause potentially fatal respiratory depression. In addition, discontinuation of a concomitantly used cytochrome P450 3A4 inducer may result in an increase in hydrocodone plasma concentration. Monitor patients receiving APADAZ and any CYP3A4 inhibitor or inducer [see Warnings and Precautions (5.4), Drug Interactions (7), Clinical Pharmacology (12.3)]. Hepatotoxicity APADAZ contains acetaminophen. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and often involve more than one acetaminophen-containing product [see Warnings and Precautions (5.5)]. Risks From Concomitant Use With Benzodiazepines Or Other CNS Depressants Concomitant use of opioids with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.6), Drug Interactions (7)]. •Reserve concomitant prescribing of APADAZ and benzodiazepines or other CNS depressants for use in patients for whom alternative treatment options are inadequate. •Limit dosages and durations to the minimum required. •Follow patients for signs and symptoms of respiratory depression and sedation. |
|
1 |
INDICATIONS AND USAGE |
APADAZ is indicated for the short-term (no more than 14 days) management of acute pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Limitations of Use
Because of the risks of addiction, abuse, and misuse with opioids, even at recommended doses [see Warnings and Precautions (5.1)], reserve APADAZ for use in patients for whom alternative treatment options [e.g., non-opioid analgesics]:
|
• |
Have not been tolerated, or are not expected to be tolerated, |
|
• |
Have not provided adequate analgesia, or are not expected to provide adequate analgesia. |
|
2 |
DOSAGE AND ADMINISTRATION |
|
2.1 |
Important Dosage and Administration Instructions |
|
• |
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [see Warnings and Precautions (5)]. The total dosage of APADAZ and any concomitant acetaminophen-containing products should not exceed 4000 mg of acetaminophen in a 24-hour period. |
|
• |
Initiate the dosing regimen for each patient individually, taking into account the patient’s severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [see Warnings and Precautions (5.1)]. |
|
• |
Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy and following dosage increases with APADAZ and adjust the dosage accordingly [see Warnings and Precautions (5.2)]. |
|
2.2 |
Initial Dosage |
Use of APADAZ as the First Opioid Analgesic
Initiate treatment with APADAZ at 1 to 2 tablets every 4 to 6 hours as needed for pain. Dosage should not exceed 12 tablets in a 24-hour period.
|
2.3 |
Conversion from Other Opioids to APADAZ |
There is inter-patient variability in the potency of opioid drugs and opioid formulations. Therefore, a conservative approach is advised when determining the total daily dosage of APADAZ. It is safer to underestimate a patient’s 24-hour APADAZ dosage than to overestimate the 24-hour APADAZ dosage and manage an adverse reaction due to overdose.
Conversion from Hydrocodone Bitartrate/Acetaminophen to APADAZ
Hydrocodone content in 6.12 mg benzhydrocodone is 4.54 mg hydrocodone or is equivalent to 7.5 mg hydrocodone bitartrate. If switching from immediate-release hydrocodone bitartrate/acetaminophen, substitute 6.12 mg/325 mg APADAZ for 7.5 mg/325 mg hydrocodone bitartrate/acetaminophen.
|
2.4 |
Titration and Maintenance of Therapy |
Individually titrate APADAZ to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving APADAZ to assess the maintenance of pain control and the relative incidence of adverse reactions, as well as monitoring for the development of addiction, abuse, or misuse [see Warnings and Precautions (5.1)]. Frequent communication is important among the prescriber, other members of the healthcare team, the patient, and the caregiver/family during periods of changing analgesic requirements, including initial titration.
If the level of pain increases after dosage stabilization, attempt to identify the source of increased pain before increasing the APADAZ dosage. If unacceptable opioid-related adverse reactions are observed, consider reducing the dosage. Adjust the dosage to obtain an appropriate balance between management of pain and opioid-related adverse reactions.
Total dosage of APADAZ and any concomitant acetaminophen-containing products should not exceed 4000 mg of acetaminophen in a 24-hour period.
|
2.5 |
Discontinuation of APADAZ |
When a patient who has been taking APADAZ regularly and may be physically dependent no longer requires therapy with APADAZ, taper the dose gradually, by 25% to 50% every 2 to 4 days, while monitoring carefully for signs and symptoms of withdrawal. If the patient develops these signs or symptoms, raise the dose to the previous level and taper more slowly, either by increasing the interval between decreases, decreasing the amount of change in dose, or both. Do not abruptly discontinue APADAZ in a physically dependent patient [see Warnings and Precautions (5.15), Drug Abuse and Dependence (9.3)].
|
3 |
DOSAGE FORMS AND STRENGTHS |
Immediate-release tablet. Each capsule-shaped white tablet debossed with “KP201” on one side contains 6.12 mg benzhydrocodone (equivalent to 6.67 mg benzhydrocodone hydrochloride) and 325 mg acetaminophen.
|
4 |
CONTRAINDICATIONS |
APADAZ is contraindicated in patients with:
|
• |
Significant respiratory depression [see Warnings and Precautions (5.2)] |
|
• |
Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see Warnings and Precautions (5.7)] |
|
• |
Known or suspected gastrointestinal obstruction, including paralytic ileus [see Warnings and Precautions (5.13)] |
|
• |
Hypersensitivity to hydrocodone or acetaminophen, or any other component of this product (e.g., anaphylaxis) [see Warnings and Precautions (5.12), Adverse Reactions (6)] |
|
5 |
WARNINGS AND PRECAUTIONS |
|
5.1 |
Addiction, Abuse, and Misuse |
APADAZ contains benzhydrocodone, a Schedule II controlled substance. As an opioid, APADAZ exposes users to the risks of addiction, abuse, and misuse [see Drug Abuse and Dependence (9)].
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed APADAZ. Addiction can occur at recommended dosages and if the drug is misused or abused.
Assess each patient’s risk for opioid addiction, abuse, or misuse prior to prescribing APADAZ, and monitor all patients receiving APADAZ for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as APADAZ, but use in such patients necessitates intensive counseling about the risks and proper use of APADAZ along with intensive monitoring for signs of addiction, abuse, and misuse.
Opioids are sought by drug abusers and people with addiction disorders and are subject to criminal diversion. Consider these risks when prescribing or dispensing APADAZ. Strategies to reduce these risks include prescribing the drug in the smallest appropriate quantity and advising the patient on the proper disposal of unused drug [see Patient Counseling Information (17)]. Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
|
5.2 |
Life-Threatening Respiratory Depression |
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status [see Overdosage (10)]. Carbon dioxide (CO2) retention from opioid- induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of APADAZ, the risk is greatest during the initiation of therapy or following a dosage increase.
Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy with and following dosage increases of APADAZ.
To reduce the risk of respiratory depression, proper dosing and titration of APADAZ are essential [see Dosage and Administration (2)]. Overestimating the APADAZ dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Accidental ingestion of even one dose of APADAZ, especially by children, can result in respiratory depression and death due to an overdose of hydrocodone.
|
5.3 |
Neonatal Opioid Withdrawal Syndrome |
Prolonged use of APADAZ during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for a prolonged period of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Use in Specific Populations (8.1), Patient Counseling Information(17)].
|
5.4 |
Risks of Concomitant Use or Discontinuation of Cytochrome P450 CYP3A4 Inhibitors and Inducers |
Concomitant use of APADAZ with a CYP3A4 inhibitor, such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), and protease inhibitors (e.g., ritonavir), may increase plasma concentrations of hydrocodone and prolong opioid adverse reactions, which may cause potentially fatal respiratory depression [see Warnings and Precautions (5.2)], particularly when an inhibitor is added after a stable dose of APADAZ is achieved. Similarly, discontinuation of a CYP3A4 inducer, such as rifampin, carbamazepine, and phenytoin, in APADAZ-treated patients may increase hydrocodone plasma concentrations and prolong opioid adverse reactions. When using APADAZ with CYP3A4 inhibitors or discontinuing CYP3A4 inducers in APADAZ-treated patients, monitor patients closely at frequent intervals and consider dosage reduction of APADAZ until stable drug effects are achieved [see Drug Interactions (7)].
Concomitant use of APADAZ with CYP3A4 inducers or discontinuation of an CYP3A4 inhibitor could decrease hydrocodone plasma concentrations, decrease opioid efficacy or, possibly, lead to a withdrawal syndrome in a patient who had developed physical dependence to hydrocodone. When using APADAZ with CYP3A4 inducers or discontinuing CYP3A4 inhibitors, monitor patients closely at frequent intervals and consider increasing the opioid dosage if needed to maintain adequate analgesia or if symptoms of opioid withdrawal occur [see Drug Interactions (7)].
|
5.5 |
Acetaminophen Hepatotoxicity |
APADAZ contains acetaminophen. Acetaminophen has been associated with cases of acute liver failure, at times resulting in liver transplant and death. Most of the cases of liver injury are associated with the use of acetaminophen at doses that exceed 4000 milligrams per day, and
often involve more than one acetaminophen-containing product [see Overdosage (10)]. The excessive intake of acetaminophen may be intentional to cause self-harm or unintentional as patients attempt to obtain more pain relief or unknowingly take other acetaminophen-containing products.
The risk of acute liver failure is higher in individuals with underlying liver disease and in individuals who ingest alcohol while taking acetaminophen.
Instruct patients to look for acetaminophen or APAP on package labels and not to use more than one product that contains acetaminophen. Instruct patients to seek medical attention immediately upon ingestion of more than 4000 milligrams of acetaminophen per day, even if they feel well.
|
5.6 |
Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants |
Profound sedation, respiratory depression, coma, and death may result from the concomitant use of APADAZ with benzodiazepines or other CNS depressants (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see Drug Interactions (7)].
If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Follow patients closely for signs and symptoms of respiratory depression and sedation.
Advise both patients and caregivers about the risks of respiratory depression and sedation when APADAZ is used with benzodiazepines or other CNS depressants (including alcohol and illicit drugs). Advise patients not to drive or operate heavy machinery until the effects of concomitant use of the benzodiazepine or other CNS depressant have been determined. Screen patients for risk of substance use disorders, including opioid abuse and misuse, and warn them of the risk for overdose and death associated with the use of additional CNS depressants including alcohol and illicit drugs [see Drug Interactions (7), Patient Counseling Information (17)].
|
5.7 |
Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients |
The use of APADAZ in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease: APADAZ-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive including apnea, even at recommended dosages of APADAZ [see Warnings and Precautions (5.2)].
Elderly, Cachetic, or Debilitated Patients: Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients because they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients [see Warnings and Precautions (5.2)].
Monitor such patients closely, particularly when initiating and titrating APADAZ and when APADAZ is given concomitantly with other drugs that depress respiration [see Warnings and Precautions (5.2)]. Alternatively, consider the use of non-opioid analgesics in these patients.
|
5.8 |
Adrenal Insufficiency |
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
|
5.9 |
Severe Hypotension |
APADAZ may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics) [see Drug Interactions (7)]. Monitor these patients for signs of hypotension after initiating or titrating the dosage of APADAZ. In patients with circulatory shock, APADAZ may cause vasodilation that can further reduce cardiac output and blood pressure. Avoid the use of APADAZ in patients with circulatory shock.
|
5.10 |
Serious Skin Reactions |
Rarely, acetaminophen may cause serious skin reactions such as acute generalized exanthematous pustulosis (AGEP), ▇▇▇▇▇▇▇-▇▇▇▇▇▇▇ Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. Inform patients about the signs of serious skin reactions and discontinue use at the first appearance of skin rash or any other sign of hypersensitivity.
|
5.11 |
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness |
In patients who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), APADAZ may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with APADAZ.
Opioids may also obscure the clinical course in a patient with a head injury. Avoid the use of APADAZ in patients with impaired consciousness or coma.
|
5.12 |
Hypersensitivity/Anaphylaxis |
There have been post-marketing reports of hypersensitivity and anaphylaxis associated with use of acetaminophen. Clinical signs included swelling of the face, mouth, and throat, respiratory distress, urticaria, rash, pruritus, and vomiting. There were infrequent reports of life-threatening anaphylaxis requiring emergency medical attention. Instruct patients to discontinue APADAZ tablets immediately and seek medical care if they experience these symptoms. Do not prescribe APADAZ tablets for patients with acetaminophen allergy.
|
5.13 |
Risks of Use in Patients with Gastrointestinal Conditions |
APADAZ is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The hydrocodone from APADAZ may cause spasm of the sphincter of Oddi. Opioids may cause increases in serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis for worsening symptoms.
|
5.14 |
Increased Risk of Seizures in Patients with Seizure Disorders |
The hydrocodone from APADAZ may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures occuring in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during APADAZ therapy.
|
5.15 |
Withdrawal |
Avoid the use of mixed agonist/antagonist (e.g, pentazocine, nalbuphine, and butorphanol) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving a full opioid agonist
analgesic, including APADAZ [see Drug Interactions (7)]. In these patients, mixed agonist/antagonist and partial agonist analgesics may reduce the analgesic effect and/or precipitate withdrawal symptoms.
When discontinuing APADAZ, gradually taper the dosage [see Dosage and Administration (2.5)]. Do not abruptly discontinue APADAZ [see Drug Abuse and Dependence (9.3)].
|
5.16 |
Risks of Driving and Operating Machinery |
APADAZ may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of APADAZ and know how they will react to the medication [see Patient Counseling Information (17)].
|
6 |
ADVERSE REACTIONS |
The following serious adverse reactions are described, or described in greater detail, in other sections:
|
• |
Addiction, Abuse, and Misuse [see Warnings and Precautions (5.1)] |
|
• |
Life-Threatening Respiratory Depression [see Warnings and Precautions (5.2)] |
|
• |
Neonatal Opioid Withdrawal Syndrome [see Warnings and Precautions (5.3)] |
|
• |
Hepatotoxicity [see Warnings and Precautions (5.5)] |
|
• |
Interactions with Benzodiazepines and other CNS Depressants [see Warnings and Precautions (5.6)] |
|
• |
Adrenal Insufficiency [see Warnings and Precautions (5.8)] |
|
• |
Severe Hypotension [see Warnings and Precautions (5.9)] |
|
• |
Serious Skin Reactions [see Warnings and Precautions (5.10)] |
|
• |
Anaphylaxis and Other Hypersensitivity Reactions [see Warnings and Precautions (5.12)] |
|
• |
Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.13)] |
|
• |
Seizures [see Warnings and Precautions (5.14)] |
|
• |
Withdrawal [see Warnings and Precautions (5.15)] |
|
6.1 |
Clinical Trials Experience |
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of APADAZ was evaluated in six Phase 1 studies in which a total of 200 healthy adult subjects receive at least one oral dose of APADAZ. The most common AEs (>5%) reported across these studies were: nausea (21.5%), somnolence (18.5%), vomiting (13.0%), constipation (12.0%), pruritus (11.5%), dizziness (7.5%), and headache (6.0%).
The following adverse reactions occurred with an incidence of 1% to 5% in single-dose or repeated-dose clinical trials of APADAZ.
Gastrointestinal disorder: abdominal distension, abdominal pain, flatulence
General disorders and administration site conditions: asthenia
Nervous system disorders: presyncope, tremor
Respiratory, thoracic and mediastinal disorders: dyspnea
Vascular disorders: hot flush, hypotension
Adverse reactions occurring at less than 1%: the following lists clinically relevant adverse reactions that occurred with an incidence of less than 1% in APADAZ clinical trials.
Eye disorders: eye pruritus
Gastrointestinal disorders: diarrhea, gastrooesophageal reflux disease, haematemesis
General disorders and administration site conditions: chest discomfort
Infections and infestations: rhinitis
Nervous system disorders: hypoesthesia, syncope
Psychiatric disorders: agitation, euphoric mood, nightmare
|
6.2 |
Postmarketing Experience |
The following adverse reactions have been identified during post-approval use of hydrocodone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in APADAZ.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology (12.2)].
|
7 |
DRUG INTERACTIONS |
Table 1 includes clinically significant drug interactions with APADAZ.
Table 1. Clinically Significant Drug Interactions with APADAZ
|
CYP3A4 and 2D6 Inhibitors |
|
|
Clinical Impact: |
The concomitant use of APADAZ and CYP3A4 inhibitors can increase the plasma concentration of hydrocodone, resulting in increased or prolonged opioid effects. These effects could be more pronounced with concomitant use of APADAZ and CYP2D6 and CYP3A4 inhibitors, particularly when an inhibitor is added after a stable dose of APADAZ is achieved [see Warnings and Precautions (5.4)]. After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the hydrocodone plasma concentration will decrease [see Clinical Pharmacology (12.3)], resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to hydrocodone. |
|
Intervention: |
If concomitant use is necessary, consider dosage reduction of APADAZ until stable drug effects are achieved. Monitor patients for respiratory depression and sedation at frequent intervals. If a CYP3A4 inhibitor is discontinued, consider increasing the APADAZ dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. |
|
Examples: |
Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g. ketoconazole), protease inhibitors (e.g., ritonavir) etc. |
|
CYP3A4 Inducers |
|
|
Clinical Impact: |
The concomitant use of APADAZ and CYP3A4 inducers can decrease the plasma concentration of hydrocodone [see Clinical Pharmacology (12.3)], resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence to hydrocodone [see Warnings and Precautions (5.15)]. After stopping a CYP3A4 inducer, as the effects of the inducer decline, the hydrocodone plasma concentration will increase [see Clinical Pharmacology (12.3)], which could increase or prolong both the therapeutic effects and adverse reactions, and may cause serious respiratory depression. |
|
Intervention: |
If concomitant use is necessary, consider increasing the APADAZ dosage until stable drug effects are achieved [see Dosage and Administration (2)]. Monitor for signs of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider APADAZ dosage reduction and monitor for signs of respiratory depression. |
|
Examples: |
Rifampin, carbamazepine, phenytoin etc. |
|
Benzodiazepines and Other Central Nervous System (CNS) Depressants |
|
|
Clinical Impact: |
Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, increases the risk of hypotension, respiratory depression, profound sedation, coma, and death. |
|
Intervention: |
Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation [see Dosage and Administration (2.5), Warnings and Precautions (5.6)]. |
|
Examples: |
Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol. |
|
Serotonergic Drugs |
|
|
Clinical Impact: |
The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. |
|
Intervention: |
If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue APADAZ if serotonin syndrome is suspected. |
|
Examples: |
Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). |
|
Monoamine Oxidase Inhibitors (MAOIs) |
|
|
Clinical Impact: |
MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precauitons (5.2)]. If urgent use of an opioid is necessary, use test doses and frequent titration of small doses to treat pain while closely monitoring blood pressure and signs and symptoms of CNS and respiratory depression. |
|
Intervention: |
The use of APADAZ is not recommended for patients taking MAOIs or within 14 days of stopping such treatment. |
|
Examples: |
phenelzine, tranylcypromine, linezolid |
|
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics |
|
|
Clinical Impact: |
May reduce the analgesic effect of APADAZ and/or precipitate withdrawal symptoms. |
|
Intervention: |
Avoid concomitant use. |
|
Examples: |
butorphanol, nalbuphine, pentazocine, buprenorphine |
|
Muscle Relaxants |
|
|
Clinical Impact: |
Hydrocodone may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. |
|
Intervention: |
Monitor patients for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of APADAZ and/or the muscle relaxant as necessary. |
|
Diuretics |
|
|
Clinical Impact: |
Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. |
|
Intervention: |
Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. |
|
Anticholinergic Drugs |
|
|
Clinical Impact: |
The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. |
|
Intervention: |
Monitor patients for signs of urinary retention or reduced gastric motility when APADAZ is used concomitantly with anticholinergic drugs. |
|
8 |
USE IN SPECIFIC POPULATIONS |
|
8.1 |
Pregnancy |
Risk Summary
Prolonged use of opioid analgesics during pregnancy may cause neonatal opioid withdrawal syndrome [see Warnings and Precautions (5.3)]. There are no available human data on hydrocodone or APADAZ use during pregnancy to inform any drug associated risks. However, neonatal opioid withdrawal and other adverse reactions during pregnancy and labor can occur with use of APADAZ [see Clinical Considerations].
Published studies with oral acetaminophen use during pregnancy have not reported an association with major congenital malformations. No reproductive or developmental toxicology studies in animals have been conducted with benzhydrocodone or the combination of benzhydrocodone and acetaminophen. Reproductive and developmental studies in rats and mice from the published literature identified adverse events at clinically relevant doses with acetaminophen. Treatment of pregnant rats with doses of acetaminophen approximately equal to the maximum human daily dose (MHDD) showed evidence of fetotoxicity and increases in bone variations in the fetuses. In another study, necrosis was observed in the liver and kidney of both pregnant rats and fetuses at doses approximately equal to the MHDD. In mice and rats treated with acetaminophen at doses within the clinical dosing range, cumulative adverse effects on reproductive capacity were reported. In mice, a reduction in number of litters of the parental mating pair was observed as well as retarded growth, abnormal sperm in their offspring, and reduced birth weight in the next generation. In rats, female fertility was decreased following in utero exposure to acetaminophen [see DATA].
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Prolonged use of opioid analgesics during pregnancy for medical or nonmedical purposes can result in physical dependence in the neonate and neonatal opioid withdrawal syndrome shortly after birth.
Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high pitched cry, tremor, vomiting, diarrhea and failure to gain weight. The onset, duration, and severity of neonatal opioid withdrawal syndrome vary based on the specific opioid used, duration of use, timing and amount of last maternal use, and rate of elimination of the drug by the newborn. Observe newborns for symptoms of neonatal opioid withdrawal syndrome and manage accordingly [see Warnings and Precautions (5.3)].
Labor or Delivery
Opioids cross the placenta and may produce respiratory depression and psycho-physiologic effects in neonates. An opioid antagonist, such as naloxone, must be available for reversal of opioid-induced respiratory depression in the neonate. APADAZ is not recommended for use in pregnant women during or immediately prior to labor, when other analgesic techniques are more appropriate. Opioid analgesics, including APADAZ, can prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However, this effect is not consistent and may be offset by an increased rate of cervical dilation, which tends to shorten labor. Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Data
Human Data
Acetaminophen:
Published data from a large population-based prospective cohort study and a population-based, case-control study do not clearly report an association with oral acetaminophen and major birth defects, miscarriage, or adverse maternal or fetal outcomes when acetaminophen is used during pregnancy. However, these studies cannot definitely establish the absence of any risk because of methodological limitations including recall bias.
Animal Data
No reproductive or developmental toxicology studies were conducted with benzhydrocodone or the combination of benzhydrocodone and acetaminophen. The following data are based on findings from studies performed with acetaminophen alone.
Studies in pregnant rats that received oral acetaminophen during organogenesis at doses up to 0.88 the maximum human daily dose (MHDD) of 3.9 grams/day based on a body surface area comparison showed evidence of fetotoxicity (reduced fetal weight and length) and a dose-related
increase in bone variations (reduced ossification and rudimentary rib changes). Offspring had no evidence of external, visceral, or skeletal malformations. When pregnant rats received oral acetaminophen throughout gestation at doses of 1.2 times the MHDD (based on a body surface area comparison), areas of necrosis occurred in both the liver and kidney of pregnant rats and fetuses. These effects did not occur in animals that received oral acetaminophen at doses 0.3 times the MHDD, based on a body surface area comparison. In a continuous breeding study, pregnant mice received 0.25, 0.5, or 1.0% acetaminophen via the diet (357, 715, or 1430 mg/kg/day). These doses are approximately 0.45, 0.89, and 1.78 times the MHDD, respectively, based on a body surface area comparison. A dose-related reduction in body weights of fourth and fifth litter offspring of the treated mating pair occurred during lactation and post-weaning at all doses. Animals in the high dose group had a reduced number of litters per mating pair, male offspring with an increased percentage of abnormal sperm, and reduced birth weights in the next generation pups.
|
8.2 |
Lactation |
Risk Summary
Hydrocodone is present in human milk. A published lactation study reports variable concentrations of hydrocodone and hydromorphone (an active metabolite) in breast milk with administration of hydrocodone to nursing mothers in the early post-partum period. This lactation study did not assess breastfed infants for potential adverse drug reactions. There is potential for sedation and respiratory depression resulting from infant exposure to hydrocodone and its metabolites in breast milk.
Acetaminophen is present in human milk in small quantities after oral administration. Based on data from more than 15 nursing mothers, the calculated infant daily dose of acetaminophen is approximately 1 to 2% of the maternal dose. There is one well-documented report of a rash in a breast-fed infant that resolved when the mother stopped acetaminophen use and recurred when she resumed acetaminophen use.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for APADAZ and any potential adverse effects on the breastfed child from APADAZ or from the underlying maternal condition.
Clinical Considerations
Infants exposed to APADAZ through breast milk should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
|
8.3 |
Females and Males of Reproductive Potential |
Infertility
Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Adverse Reactions (6.2),
Clinical Pharmacology (12.2)].
Published animal studies report that oral acetaminophen treatment of male animals at doses that are 1.2 times the MHDD and greater (based on a body surface area comparison) result in decreased testicular weights, reduced spermatogenesis, reduced fertility, and reduced implantation sites in females given the same doses. Additional published animal studies indicate that acetaminophen exposure in utero adversely impacts reproductive capacity of both male and female offspring at clinically relevant exposures [see Nonclinical Toxicology (13.1)].
|
8.4 |
Pediatric Use |
Safety and effectiveness in pediatric patients below the age of 18 years have not been established.
|
8.5 |
Geriatric Use |
Elderly patients (aged 65 years or older) may have increased sensitivity to hydrocodone. In general, use caution when selecting a dosage for an elderly patient, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
Respiratory depression is the chief risk for elderly patients treated with opioids, and has occurred after large initial doses were administered to patients who were not opioid-tolerant or when opioids were co-administered with other agents that depress respiration. Titrate the dosage of APADAZ slowly in geriatric patients and monitor closely for signs of respiratory depression [see Warnings and Precautions (5.2)].
Hydrocodone and acetaminophen are known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
|
8.6 |
Hepatic Impairment |
The effect of hepatic impairment on the pharmacokinetics of APADAZ has not been determined. Patients with hepatic impairment may have higher plasma concentrations than those with normal function. Use a low initial dose of APADAZ in patients with hepatic impairment or active liver disease and monitor closely for adverse events such as respiratory depression and hepatotoxicity [see Warnings and Precautions (5.2 and 5.5)].
|
8.7 |
Renal Impairment |
The effect of renal impairment on the pharmacokinetics of APADAZ has not been determined. Patients with renal impairment may have higher plasma concentrations than those with normal function. Use a low initial dose of APADAZ in patients with renal impairment and monitor closely for adverse events such as respiratory depression.
|
9 |
DRUG ABUSE AND DEPENDENCE |
|
9.1 |
Controlled Substance |
APADAZ contains benzhydrocodone, a Schedule II controlled substance.
|
9.2 |
Abuse |
APADAZ contains benzhydrocodone, a substance with a high potential for abuse similar to other opioids including fentanyl, hydromorphone, methadone, morphine, oxycodone, oxymorphone, and tapentadol. APADAZ can be abused and is subject to misuse, addiction, and criminal diversion [see Warnings and Precautions (5.1)].
All patients treated with opioids require careful monitoring for signs of abuse and addiction, because use of opioid analgesic products carries the risk of addiction even under appropriate medical use.
Prescription drug abuse is the intentional non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects.
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal.
“Drug-seeking” behavior is very common in persons with substance use disorders. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing, or referral, repeated “loss” of prescriptions, tampering with prescriptions, and reluctance to provide prior medical records or contact information for other treating healthcare provider(s). “Doctor shopping” (visiting multiple prescribers to obtain additional prescriptions) is common among drug abusers and people suffering from untreated addiction. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Health care providers should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction.
APADAZ, like other opioids, can be diverted for non-medical use into illicit channels of distribution. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests, as required by state and federal law, is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Risks Specific to Abuse of APADAZ
APADAZ is for oral use only. Abuse of APADAZ poses a risk of overdose and death. The risk is increased with concurrent use of APADAZ with alcohol and other central nervous system depressants.
With intravenous abuse, the inactive ingredients in APADAZ can result in local tissue necrosis, infection, pulmonary granulomas, embolism and death, and increased risk of endocarditis and valvular heart injury. Parenteral drug abuse is commonly associated with transmission of infectious diseases, such as hepatitis and HIV.
Abuse Deterrent Studies
In vitro and human abuse potential studies comparing APADAZ to an immediate-release hydrocodone/acetaminophen tablet control were conducted to assess the potential abuse deterrent properties of APADAZ.
In Vitro Testing
In vitro physical and chemical manipulation studies were performed to evaluate the ability of different methods to extract and convert benzyhydrocodone to hydrocodone for the purpose of preparing APADAZ for abuse by the intravenous route or by smoking. The efficiency of extracting benzhydrocodone from APADAZ was similar compared to the efficiency of extracting hydrocodone from the non-abuse-deterrent hydrocodone/acetaminophen control. Further conversion (hydrolysis) of benzhydrocodone to hydrocodone in vitro is a difficult process. Overall, these studies showed no advantage for APADAZ over the hydrocodone/acetaminophen control.
Oral Clinical Abuse Potential Study
In an oral, single-center, randomized, double-blind, active- and placebo-controlled, 7-period, crossover, human abuse potential study, 71 recreational opioid users were randomized into the Treatment Phase; 62 subjects completed the study. Treatment arms included APADAZ (4, 8, and 12 tablets, each containing 6.12 mg benzhydrocodone and 325 mg acetaminophen), hydrocodone/acetaminophen (4, 8 and 12 tablets, each containing 4.54 mg hydrocodone and 325 mg acetaminophen), and placebo. The respective dosage strengths for APADAZ and hydrocodone/acetaminophen contained equimolar amounts of hydrocodone. The rate (Cmax) and extent (AUClast, AUCinf) of hydrocodone exposure following APADAZ administration was comparable to that for hydrocodone/acetaminophen across all 3 dosage strengths. There were no statistically significant differences nor any clinically meaningful differences between APADAZ and the hydrocodone/acetaminophen control for the pre-specified primary endpoint of maximal score (Emax) for Drug Liking VAS or secondary endpoints of Emax for High VAS and Take Drug Again VAS. The results do not support a finding that APADAZ can be expected to deter abuse by the oral route of administration.
Intranasal Clinical Abuse Potential Study
In an intranasal single-center, randomized, double-blind, double-dummy, two-part human abuse potential study, 46 recreational opioid users were randomized into the Treatment Phase; 42
subjects completed the study. Five treatment arms included intranasal crushed and oral APADAZ (2 tablets, each containing 6.12 mg benzhydrocodone and 325 mg acetaminophen), intranasal crushed and oral hydrocodone/acetaminophen (2 tablets, each containing 4.54 mg hydrocodone and 325 mg acetaminophen), and intranasal placebo powder. The respective dosage strengths for APADAZ and hydrocodone/acetaminophen contained equimolar amounts of hydrocodone.
The pharmacokinetic data showed that overall (AUClast, AUCinf, and Cmax) hydrocodone exposure was comparable between intranasal crushed APADAZ and intranasal crushed hydrocodone/acetaminophen. These treatments were also comparable with cumulative hydrocodone exposure at the timepoints of 4, 8, and 24 hours (▇▇▇▇-▇, ▇▇▇▇-▇, ▇▇▇▇-▇▇). Over the first 2 hours post-dosing (AUC0-0.5, AUC0-1, and AUC0-2), the cumulative hydrocodone exposure was lower following intranasal APADAZ compared to intranasal hydrocodone/ acetaminophen.
There were numerically small but not statistically significant differences between APADAZ and the hydrocodone/acetaminophen control observed for the pre-specified primary endpoint, maximum effect on Drug Liking VAS (Emax), and the secondary endpoints of Emax for High VAS and Take Drug Again VAS.
|
Table 2: |
Summary Statistics of Maximum Scores (Emax) on Drug Liking, High and Take Drug Again, Following Intranasal Administration of Apadaz, Hydrocodone/APAP, and Placebo |
|
VAS Scale (100 point) intranasal (n=42) |
Apadaz |
Hydrocodone/APAP Crushed |
Placebo |
|
Drug Liking * |
|||
|
Mean (SE) |
75.9 (2.3) |
79.0 (2.7) |
53.0 (1.2) |
|
Median (Range) |
74.0 (50-100) |
80.0 (50-100) |
51.0 (50-85) |
|
High** |
|||
|
Mean (SE) |
61.8 (4.6) |
59.1 (5.1) |
8.8 (3.8) |
|
Median (Range) |
68.5 (0-100) |
67.5 (0-100) |
0.0 (0-100) |
|
Take Drug Again* |
|||
|
Mean (SE) |
69.5 (3.9) |
74.5 (3.9) |
48.2 (2.2) |
|
Median (Range) |
68.0 (0-100) |
81.5 (0-100) |
50.0 (0-100) |
*Bipolar scale (0=maximum negative response, 50=neutral response, 100=maximum positive response)
** Unipolar scale (0=maximum negative response, 100=maximum positive response)
Additional secondary analyses of Drug Liking based on area under the effect curve analyses (AUE) for the first half hour, hour, and 2 hours post-dosing, demonstrated numerically small differences between intranasal APADAZ and intranasal hydrocodone/acetaminophen. However, there were no differences between these two treatments with respect to the cumulative High experienced over the first 2 hours post-dosing using similar AUE analyses. There are no data to support that small differences in the early Drug Liking experience over the first 2 hours are clinically relevant findings consistent with possible abuse-deterrent effects, particularly in the
setting of the Emax analyses for Drug Liking, Take Drug Again, and High that do not support a deterrent effect. Based on the overall results, APADAZ cannot be expected to deter abuse by the intranasal route of administration.
Summary
The in vitro studies that evaluated physical manipulation and extraction for the purpose of preparing APADAZ for abuse by the intravenous route or by smoking did not find an advantage for APADAZ over the hydrocodone/acetaminophen control.
The results of the oral and intranasal human abuse potential studies do not support a finding that APADAZ can be expected to deter abuse by the oral or nasal routes of administration.
|
9.3 |
Dependence |
Both tolerance and physical dependence can develop during chronic opioid therapy. Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical dependence results in withdrawal symptoms after abrupt discontinuation or a significant dosage reduction of a drug. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity (e.g., naloxone, nalmefene), mixed agonist/antagonist analgesics (e.g., pentazocine, butorphanol, nalbuphine), or partial agonists (e.g., buprenorphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued opioid usage.
APADAZ should not be abruptly discontinued in a physically-dependent patient [see Dosage and Administration (2.5)]. If APADAZ is abruptly discontinued in a physically-dependent patient, a withdrawal syndrome may occur. Some or all of the following can characterize this syndrome: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other signs and symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal signs [see Use in Specific Populations (8.1)].
|
10 |
OVERDOSAGE |
Clinical Presentation
Following an acute overdosage, toxicity may result from hydrocodone or acetaminophen.
Hydrocodone
Acute overdose with APADAZ can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted
pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Clinical Pharmacology (12.2)].
Acetaminophen
In acute acetaminophen overdosage, dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect. Renal tubular necrosis, hypoglycemic coma, and thrombocytopenia also occur. Plasma acetaminophen levels > 300 mcg/mL at 4 hours after oral ingestion were associated with hepatic damage in 90% of patients; minimal hepatic damage is anticipated if plasma levels at 4 hours are < 150 mcg/mL or < 37.5 mcg/mL at 12 hours after ingestion. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis, and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment of Overdose
A single or multiple drug overdose with hydrocodone and acetaminophen is a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended. Immediate treatment includes support of cardiorespiratory function and measures to reduce drug absorption. Oxygen, intravenous fluids, vasopressors, assisted ventilation, and other supportive measures should be employed as indicated.
Hydrocodone
In case of overdose, priorities are the reestablishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support techniques.
The opioid antagonists, naloxone or nalmefene, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to hydrocodone overdose, administer an opioid antagonist. Opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression secondary to hydrocodone overdose.
Because the duration of opioid reversal is expected to be less than the duration of action of hydrocodone from APADAZ, carefully monitor the patient until spontaneous respiration is reliably re-established. If the response to an opioid antagonist is suboptimal or only brief in nature, administer additional antagonist as directed by the product’s prescribing information.
In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be begun with care and by titration with smaller than usual doses of the antagonist.
Acetaminophen
If an acetaminophen overdose is suspected, obtain a serum acetaminophen assay as soon as possible, but no sooner than 4 hours following oral ingestion. Obtain liver function studies initially and repeat at 24-hour intervals. Administer the antidote N-acetylcysteine (NAC) as early as possible. As a guide to treatment of acute ingestion, the acetaminophen level can be plotted against time since oral ingestion on a nomogram (Rumack-▇▇▇▇▇▇▇). The lower toxic line on the nomogram is equivalent to 150 mcg/mL at 4 hours and 37.5 mcg/mL at 12 hours. If serum level is above the lower line, administer the entire course of NAC treatment. Withhold NAC therapy if the acetaminophen level is below the lower line.
Gastric decontamination with activated charcoal should be administered just prior to N- acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatotoxicity; acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose-dependent and occurs early in the course of intoxication.
|
11 |
DESCRIPTION |
APADAZ (benzhydrocodone and acetaminophen) tablet is an immediate-release, fixed-dose combination of an opioid agonist and acetaminophen. APADAZ tablets are white to off-white, capsule shaped tablets with KP201 debossed on one side and contain 6.12 mg of benzhydrocodone (equivalent to 6.67 mg benzhydrocodone hydrochloride) and 325 mg of acetaminophen for oral administration.
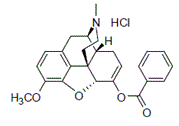
Benzhydrocodone hydrochloride is a prodrug of hydrocodone. It occurs as a fine white powder and is not affected by light. The chemical name is 6,7-didehydro-4,5á-epoxy-3-methoxy-17- methylmorphinan-6-yl benzoate hydrochloride. The molecular formula is C25H26ClNO4, which corresponds to a molecular weight of 439.93 g/mol. It has the following chemical structure:
Acetaminophen, 4’-hydroxyacetanilide, a slightly bitter, white, odorless, crystalline powder, is a non-opiate, non-salicylate analgesic and antipyretic. The molecular formula for acetaminophen is
C8H9NO2, which corresponds to a molecular weight of 151.16 g/mol. It has the following structural 
formula:
APADAZ tablets contain 6.12 mg of benzhydrocodone (equivalent to 6.67 mg benzhydrocodone hydrochloride) and 325 mg of acetaminophen and are white to off-white in color. In addition, each tablet contains the following inactive ingredients: crospovidone, microcrystalline cellulose, pregelatinized starch, Povidone K30, and stearic acid.
|
12 |
CLINICAL PHARMACOLOGY |
|
12.1 |
Mechanism of Action |
Benzhydrocodone
Benzhydrocodone is a prodrug of hydrocodone.
Hydrocodone
Hydrocodone is a full opioid agonist with relative selectivity for the mu-opioid receptor, although it can interact with other opioid receptors at higher doses. The principal therapeutic action of hydrocodone is analgesia. Like all full opioid agonists, there is no ceiling effect for analgesia with hydrocodone. Clinically, dosage is titrated to provide adequate analgesia and may be limited by adverse reactions, including respiratory and CNS depression.
The precise mechanism of the analgesic action is unknown. However, specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and are thought to play a role in the analgesic effects of this drug.
Acetaminophen
Acetaminophen is a non-opioid, non-salicylate analgesic. The site and mechanism for the analgesic effect of acetaminophen has not been determined but is thought to primarily involve central actions.
|
12.2 |
Pharmacodynamics |
Hydrocodone
Effects on the Central Nervous System
Hydrocodone produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.
Hydrocodone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Hydrocodone causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Hydrocodone produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.
Caution must be used in hypovolemic patients, such as those suffering acute myocardial infarction, because hydrocodone may cause or further aggravate their hypotension. Caution must also be used in patients with cor pulmonale who have received therapeutic doses of opioids.
Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans [see Adverse Reactions (6.2)]. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date. Patients presenting with symptoms of androgen deficiency should undergo laboratory evaluation [see Adverse Reactions (6.2)].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration–Efficacy Relationships
The minimum effective analgesic concentration will vary widely among patients, especially among patients who have been previously treated with potent agonist opioids. The minimum effective analgesic concentration of hydrocodone for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome, and/or the development of analgesic tolerance [see Dosage and Administration (2.1, 2.5)].
Concentration–Adverse Reaction Relationships
There is a relationship between increasing hydrocodone plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration (2.1, 2.2, 2.4)].
|
12.3 |
Pharmacokinetics |
APADAZ has met the bioequivalence criteria for hydrocodone AUC and Cmax to other immediate- release hydrocodone combination products. Benzhydrocodone was not detectable in plasma after oral administration in clinical studies, indicating that exposure to benzhydrocodone was minimal and transient. Steady state with APADAZ is attained within 24 to 36 hours of dosing. The systemic exposure to hydrocodone from APADAZ increases linearly after administration of single and multiple doses of 2 tablets of APADAZ.
Absorption
Single–Dose Studies
In 2 comparative bioavailability studies following oral administration of single dose to healthy subjects under fasted conditions, 6.67 mg/325 mg APADAZ tablet met the bioequivalence criteria for hydrocodone AUC and Cmax to immediate-release tablet of 7.5 mg hydrocodone/200 mg ibuprofen (N = 28); and the bioequivalence criteria for acetaminophen AUC and Cmax to immediate-release tablet of 37.5 mg tramadol/325 mg acetaminophen (N = 27)
In a comparative bioavailability study following oral administration of single dose under fasted conditions in 24 healthy subjects comparing 6.67 mg/325 mg APADAZ to immediate-release tablet of 7.5 mg hydrocodone/325 mg acetaminophen, APADAZ met the bioequivalence criteria for hydrocodone Cmax and AUC; and met the bioequivalence criteria for acetaminophen AUC, with comparable acetaminophen Cmax.
In a study to assess the effect of food on the bioavailability and pharmacokinetics of APADAZ in 38 healthy subjects, compared to fasted condition, coadministration of APADAZ with a high-fat, high-calorie meal showed a slight decrease in the rate but no change in the extent of hydrocodone absorption; and no difference in rate and extent of acetaminophen absorption. The effect of a high-fat, high-calorie meal on pharmacokinetics is similar between APADAZ and immediate- release tablet of 7.5 mg hydrocodone/325 mg acetaminophen. APADAZ can be administered without regard to food. The PK parameters for hydrocodone and acetaminophen after oral administration of APADAZ tablet, 6.67 mg/325 mg under fasted and fed conditions are shown in Table 3 below.
|
Table 3. |
PK parameters of hydrocodone and acetaminophen after oral administration of APADAZ tablet, 6.67 mg/325 mg under fasted and fed conditions. |
|
Parametera |
Fed |
Fasted |
|
Hydrocodone |
||
|
Cmax (ng/mL) |
16.04 ± 3.60 (40) |
19.18 ± 4.84 (38) |
|
Tmax (h) |
2.50 (40) [0.50–4.00] |
1.25 (38) [0.50–3.00] |
|
AUCinf (h●ng/mL) |
130.91 ± 29.45 (40) |
125.73 ± 36.78 (38) |
|
t½ (h) |
4.53 ± 0.70 (40) |
4.33 ± 0.67 (38) |
|
Acetaminophen |
||
|
Cmax (“g/mL) |
3.34 ± 1.01 (39) |
4.05 ± 1.30 (38) |
|
Tmax (h) |
1.50 (39) [0.50–4.00] |
1.00 (38) [0.50–3.00] |
|
AUCinf (h●“g/mL) |
15.0 ± 3.53 (36) |
14.7 ± 3.87 (36) |
|
t½ (h) |
5.64 ± 1.58 (36) |
4.78 ± 1.30 (36) |
a Arithmetic mean ± standard deviation (N) except Tmax for which the median (N) [Range] is reported
Multiple-Dose Study
A multiple-dose study in 24 healthy subjects showed no measurable exposure to benzhydrocodone, when 2 tablets of APADAZ, 6.67 mg/325 mg, was administered orally every 4 hours for a total of 13 doses. Steady state for hydrocodone and acetaminophen was achieved after 24 hours and between 24 and 36 hours, respectively. The accumulation ratios for hydrocodone Cmax and AUC values were 1.85-fold and 2.03-fold, respectively. The accumulation ratios for acetaminophen Cmax and AUC values were 1.38-fold and 1.80-fold, respectively.
Elimination
Hydrocodone is eliminated primarily from the kidneys. Elimination of acetaminophen is principally by liver metabolism and subsequent renal excretion of metabolites.
Metabolism
Benzhydrocodone is a prodrug of hydrocodone and is converted to active hydrocodone by enzymes in the intestinal tract.
Hydrocodone exhibits a complex pattern of metabolism, including O-demethylation, N- demethylation, and 6-keto reduction to the corresponding 6-á-and 6-®-hydroxy metabolites. Hydromorphone, a potent opioid, is formed from the O-demethylation of hydrocodone and contributes to the total analgesic effect of hydrocodone. The O- and N- demethylation processes are mediated by separate P-450 isoenzymes: CYP2D6 and CYP3A4, respectively. [see Drug Interactions (7)].
Acetaminophen is primarily metabolized in the liver by first-order kinetics and involves three principal separate pathways:
|
a) |
conjugation with glucuronide; |
|
b) |
conjugation with sulfate; and |
|
c) |
oxidation via the cytochrome, P450-dependent, mixed-function oxidase enzyme pathway |
In adults, the majority of acetaminophen is conjugated with glucuronic acid and, to a lesser extent, with sulfate. These glucuronide-, sulfate-, and glutathione-derived metabolites lack biologic activity. In premature infants, newborns, and young infants, the sulfate conjugate predominates.
Excretion
Hydrocodone and its metabolites are eliminated primarily in the kidneys, with a mean plasma half- life of 4.5 hours.
The half-life of acetaminophen is about 2 to 3 hours in adults. It is somewhat shorter in children and somewhat longer in neonates and in cirrhotic patients. Acetaminophen is eliminated from the body primarily by formation of glucuronide and sulfate conjugates in a dose-dependent manner. Less than 9% of acetaminophen is excreted unchanged in the urine.
Specific Populations
Age
For hydrocodone, no significant pharmacokinetic differences based on age have been demonstrated. For APAP, a population pharmacokinetic analysis of data obtained from a clinical trial in patients with chronic pain treated with immediate-release tablet of 7.5 mg hydrocodone/325 mg acetaminophen, which included 55 patients between 65 and 75 years of age and 19 patients over 75 years of age, showed no significant changes in the pharmacokinetics of acetaminophen in elderly patients with normal renal and hepatic function [see Use in Specific Populations (8.5)].
Sex
For hydrocodone, no significant pharmacokinetic differences based on gender have been demonstrated.
Renal Impairment
The effect of renal insufficiency on the pharmacokinetics of APADAZ has not been determined [see Use in Specific Populations (8.7)].
Hepatic Impairment
Because acetaminophen is extensively metabolized by the liver, the use of APADAZ in patients with severe hepatic impairment or severe active liver disease is contraindicated. The pharmacokinetics and tolerability of APADAZ in patients with impaired hepatic function have not been studied [see Contraindications (4), Use in Specific Populations (8.6)].
|
13 |
NONCLINICAL TOXICOLOGY |
|
13.1 |
Carcinogenesis, Mutagenesis, Impairment of Fertility |
Carcinogenesis
Long-term studies to evaluate the carcinogenic potential of benzhydrocodone or the combination of benzhydrocodone and acetaminophen have not been conducted.
Long-term studies in mice and rats have been completed by the National Toxicology Program to evaluate the carcinogenic potential of acetaminophen. In 2-year feeding studies, F344/N rats and B6C3F1 mice were fed a diet containing acetaminophen up to 6000 ppm. Female rats demonstrated equivocal evidence of carcinogenic activity based on increased incidences of mononuclear cell leukemia at 0.8 times the maximum human daily dose (MHDD) of 3.9 grams/day, based on a body surface area comparison. In contrast, there was no evidence of carcinogenic activity in male rats (0.7 times) or mice (1.3-1.5 times the MHDD, based on a body surface area comparison).
Mutagenesis
Benzhydrocodone was positive in an in vitro mammalian cell chromosome aberration assay in the presence of a metabolic activation (S9 mix) and negative in the absence of metabolic activation. Benzhydrocodone was negative in an in vitro bacterial mutation assay as well as in the in vivo rat micronucleus and comet assays.
Acetaminophen was not mutagenic in the bacterial reverse mutation assay (▇▇▇▇ test). In contrast, acetaminophen tested positive in the in vitro mouse lymphoma assay and the in vitro chromosomal aberration assay using human lymphocytes. In the published literature, acetaminophen has been reported to be clastogenic when administered at 1500 mg/kg/day to the rat model (3.7-times the MHDD, based on a body surface area comparison). In contrast, no clastogenicity was noted at a dose of 750 mg/kg/day (1.9-times the MHDD, based on a body surface area comparison), suggesting a threshold effect.
Impairment of Fertility
No nonclinical fertility studies have been conducted with benzhydrocodone or the combination of benzhydrocodone and acetaminophen.
In studies conducted by the National Toxicology Program, fertility assessments with acetaminophen have been completed in Swiss CD-1 mice via a continuous breeding study. There were no effects on fertility parameters in mice consuming up to 1.8 times the MHDD of acetaminophen, based on a body surface area comparison. Although there was no effect on sperm motility or sperm density in the epididymis, there was a significant increase in the percentage of abnormal sperm in mice consuming 1.8 times the MHDD (based on a body surface comparison) and there was a reduction in the number of mating pairs producing a fifth litter at this dose, suggesting the potential for cumulative toxicity with chronic administration of acetaminophen near the upper limit of daily dosing.
Published studies in rodents report that oral acetaminophen treatment of male animals at doses that are 1.2 times the MHDD and greater (based on a body surface comparison) result in decreased testicular weights, reduced spermatogenesis, reduced fertility, and reduced implantation sites in females given the same doses. These effects appear to increase with the duration of treatment.
In a published mouse study, oral administration of 50 mg/kg acetaminophen to pregnant mice from Gestation Day 7 to delivery (0.06 times the MHDD) reduced the number of primordial follicles in female offspring and reduced the percentage of full term pregnancies and number of pups born to these females exposed to acetaminophen in utero.
In a published study, pregnant rats oral administration of 350 mg/kg acetaminophen (0.9 times the MHDD) from Gestation Day 13 to 21 (dams), reduced the number of germ cells in the fetal ovary and decreased ovary weight and reduced number of pups per litter in F1 females as well as reduced ovary weights in F2 females.
|
16 |
HOW SUPPLIED/STORAGE AND HANDLING |
APADAZ (benzhydrocodone and acetaminophen) is a capsule-shaped white tablet debossed with “KP201” on one side. Each tablet contains 6.12 mg benzhydrocodone and 325 mg acetaminophen.
The tablets are supplied as:
NDC ▇▇▇▇▇-▇▇▇▇-▇: Bottles of 100 Tablets
NDC ▇▇▇▇▇-▇▇▇▇-▇: Blister Wallet Pack of 18 Tablets
Flush expired or unused APADAZ tablets that are no longer needed down the toilet or contact the Drug Enforcement Agency (DEA) to find the location of an authorized collector (▇-▇▇▇-▇▇▇-▇▇▇▇).
Storage
Store at 20°C to 25°C (68°F to 77°F). Excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature].
|
17 |
PATIENT COUNSELING INFORMATION |
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Important Administration Instructions
Instruct patients how to properly take APADAZ [see Dosage and Administration (2), Warnings and Precautions (5)].
|
• |
Do not take more than 4,000 milligrams of acetaminophen per day. Call your doctor if you took more than the recommended dose. |
|
• |
Use APADAZ exactly as prescribed to reduce the risk of life-threatening adverse reactions (e.g., respiratory depression) |
|
• |
Do not discontinue APADAZ without first discussing the need for a tapering regimen with your doctor. |
Addiction, Abuse, and Misuse
Inform patients that the use of APADAZ, even when taken as recommended, can result in addiction, abuse, and misuse, which can lead to overdose and death [see Warnings and Precautions (5.1)]. Instruct patients not to share APADAZ with others and to take steps to protect APADAZ from theft or misuse.
Life-Threatening Respiratory Depression
Inform patients of the risk of life-threatening respiratory depression, including information that the risk is greatest when starting APADAZ or when the dosage is increased, and that it can occur even at recommended dosages [see Warnings and Precautions (5.2)]. Advise patients how to recognize respiratory depression and to seek medical attention if breathing difficulties develop.
Accidental Ingestion
Inform patients that accidental ingestion, especially by children, may result in respiratory depression or death [see Warnings and Precautions (5.2)]. Instruct patients to take steps to store APADAZ securely and to dispose of unused APADAZ by flushing the tablets down the toilet.
Maximum Daily Acetaminophen Use
Advise patients not to take more than 4,000 milligrams of acetaminophen per day and call their doctor if they have taken more than the recommended dose. Advise patients not to take APADAZ in combination with other tramadol or acetaminophen-containing products, including over-the- counter preparations (see Warnings and Precautions (5.5)].
Interactions with Benzodiazepines and Other CNS Depressants
Inform patients that potentially fatal additive effects may occur if APADAZ is used with benzodiazepines or other CNS depressants, including alcohol, and not to use these unless supervised by a healthcare provider [see Warnings and Precautions (5.6), Drug Interactions (7)].
Serotonin Syndrome
Inform patients that opioids could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their healthcare providers if they are taking, or plan to take serotonergic medications. [see Drug Interactions (7)].
MAOI Interaction
Inform patients to avoid taking APADAZ while using any drugs that inhibit monoamine oxidase. Patients should not start MAOIs while taking APADAZ [see Drug Interactions (7)].
Adrenal Insufficiency
Inform patients that opioids could cause adrenal insufficiency, a potentially life-threatening condition. Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Advise patients to seek medical attention if they experience a constellation of these symptoms [see Warnings and Precautions (5.8)].
Hypotension
Inform patients that APADAZ may cause orthostatic hypotension and syncope. Instruct patients how to recognize symptoms of low blood pressure and how to reduce the risk of serious consequences should hypotension occur (e.g., sit or lie down, carefully rise from a sitting or lying position) [see Warnings and Precautions (5.9)].
Serious Skin Reactions
Advise patients to stop APADAZ immediately if they develop any type of rash and to contact their healthcare provider as soon as possible [see Warnings and Precautions (5.10)].
Anaphylaxis
Inform patients that anaphylaxis have been reported with ingredients contained in APADAZ. Advise patients how to recognize such a reaction and when to seek medical attention [see Contraindications (4), Warnings and Precautions (5.12), Adverse Reactions (6)].
Pregnancy
Neonatal Opioid Withdrawal Syndrome
Inform female patients of reproductive potential that prolonged use of APADAZ during pregnancy can result in neonatal opioid withdrawal syndrome, which may be life-threatening if not recognized and treated [see Warnings and Precautions (5.3), Use in Specific Populations (8.1)].
Embryo-Fetal Toxicity
Inform female patients of reproductive potential that APADAZ can cause fetal harm and to inform their healthcare provider of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise nursing mothers to monitor infants for increased sleepiness (more than usual), breathing difficulties, or limpness. Instruct nursing mothers to seek immediate medical care if they notice
these signs [see Use in Specific Populations (8.2)].
Infertility
Inform patients that chronic use of opioids may cause reduced fertility. It is not known whether these effects on fertility are reversible [see Use in Specific Populations (8.3)].
Driving or Operating Heavy Machinery
Inform patients that APADAZ may impair the ability to perform potentially hazardous activities such as driving a car or operating heavy machinery. Advise patients not to perform such tasks until they know how they will react to the medication [see Warnings and Precautions (5.16)].
Constipation
Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention [see Adverse Reactions (6)].
Disposal of Unused APADAZ
Advise patients to flush the unused tablets down the toilet when APADAZ is no longer needed or to contact the Drug Enforcement Agency (DEA) to find the location of an authorized collector (▇-▇▇▇-▇▇▇-▇▇▇▇).
Manufactured for:
▇▇▇▇ ▇▇▇▇▇▇▇▇▇ ▇▇▇▇
▇▇▇▇▇▇▇▇▇▇, ▇▇ ▇▇▇▇▇

PI-0167-01 R.02/18
Exhibit D
[*]
Exhibit E
Example Net Profit Share Computation
[*]

