PRC IP PURCHASE AGREEMENT
Execution Copy
This PRC IP Purchase Agreement (the “Agreement”) is entered into as of December 4, 2017 (the “Sale Date”), by and between OptAsia Healthcare Limited, a Hong Kong company (“OAHL”) and Capnia, Inc., a Delaware corporation (“Capnia” or the “Company”).
The Company is a wholly-owned subsidiary of Soleno Therapeutics, Inc. (“Soleno”), a company that has itself and through its affiliates engaged in the development and commercialization of the "CoSense"technology and related commercial products that utilize a portfolio of Intellectual Property assets referred to as the "Sensalyze Technology Platform", which business has historically involved the design, manufacture and sale of medical monitors that measure end-tidal carbon monoxide in breath to assist in the detection of excessive hemolysis in neonates, and which the parties contemplate may in the future also involve the commercialization of other applications utilizing the Sensalyze Technology Platform including extensions and improvements thereon (collectively, the “Business”). Soleno has prior to the date hereof transferred to the Company all of Soleno’s right, title and interest in all of Soleno’s worldwide Intellectual Property assets used by Soleno and its Affiliates in relation to the Business, including but not limited to all such assets worldwide consisting of Intellectual Property in the United States, the European Union, the PRC, Japan and Korea, and quality assurance and regulatory affairs assets, including but not limited to all Food and Drug Administration 510(k) submissions and clearances, quality management systems and related certificates under the ISO 13485 standard of the International Organization for Standardization, and any other related regulatory documents, submissions and/or approvals (the “CoSense Worldwide IP Assets”). OAHL desires to acquire from the Company, and the Company desires to sell to OAHL, all right, title and interest of CoSense in the CoSense Worldwide IP Assets in the People’s Republic of China (“PRC”)(the “CoSense PRC IP Assets”) on the terms and subject to the conditions set forth in this Agreement.
In consideration of the mutual agreements, representations, warranties and covenants set forth below, OAHL and the Company agree as follows:
1.1 Transfer of IP Assets. Under the terms and conditions of this Agreement, the Company hereby absolutely and irrevocably (except as set forth in Section 1.4 (Company Repurchase Option) sells, assigns, grants, transfers, and delivers to OAHL and OAHL hereby purchases and accepts from the Company as of the Sale Date the exclusive right, title and interest in the People’s Republic of China (the “PRC”) in and to all of the CoSense PRC IP Assets that are owned by the Company (including but not limited to the CoSense PRC IP Assets listed on Exhibit A attached hereto and the Future IP Assets (as defined below)) free and clear of all charge, claim, community property interest, condition, easement, covenant, warrant, demand, encumbrance, equitable interest, lien, mortgage, option, purchase right, pledge, security interest, right of first
-1-
refusal, license, assignment, or other right of third parties or restriction of any kind, including any restriction on use, voting, transfer, receipt of income or exercise of any other attribute of ownership (collectively, “Encumbrances”), other than (if and to the extent applicable) the International Distribution Agreement effective February 1, 2016 (the “PRC Distribution Agreement”), by and between Capnia UK Limited, a corporation registered under the laws of England and Wales and a wholly-owned subsidiary of the Company and Shanghai CiiC Science and Technology Development Company (“PRC Distributor”).
For any and all Future IP Assets (as defined below), the Company agrees to assign and hereby assigns to OAHL (i) in the case of Future IP Assets created in the PRC, the exclusive right to apply for registration worldwide with respect to such Future IP Assets; (ii) in the case of Future IP Assets other than those described in the foregoing subclause (i), the exclusive right to apply for registration in respect of such Future IP Assets in the PRC; and (iii) the worldwide right, title and interest in and to all other Intellectual Property rights in and to such Future IP Assets relating to the Business in the PRC, in each case including without limitation trade secrets, source codes protected as trade secrets, and unregistered copyrights and in each case free and clear of any Encumbrances other than (if and to the extent applicable) the PRC Distribution Agreement.
The sale, assignment, grant, transfer and deliverance to OAHL contemplated hereunder shall not be terminated for any failure of OAHL to exercise such transferred rights in whole or in part. All right, title and interest in and to the CoSense PRC IP Assets and the Future IP Assets shall not be waived if not exercised by OAHL within one (1) year of the Sale Date.
“Future IP Assets” means any Intellectual Property which relates to the Business created, made or acquired in any manner by or on behalf of the Company or any third party as appointed by the Company on or after the Sale Date worldwide, regardless of whether patentable or not (including without limitation copyrights (registered or unregistered), trademarks and applications for registrations thereof, trade secrets and other confidential information, patents granted or patent application(s) filed in the future, including future patent application(s) and those currently pending, and improvements, derivations, or modifications to the CoSense Worldwide IP Assets).
“Intellectual Property” has the meaning set forth in the JV Agreement.
1.2 Escrow.
(a) Establishment of Escrow. Within thirty (30) days after the Sale Date, the Company will deposit with Iron Mountain, Inc. (the “Escrow Agent”) a copy of any source code included in the CoSense Worldwide IP Assets and any and all related documentation (the “Escrow Materials”) pursuant to the terms of an escrow agreement to be entered into among the Company, OAHL and the Escrow Agent. If the Company creates any Future IP Asset prior to the occurrence of the Release Event (as described below in Section 1.2(b) (Release Event and Procedure)), the Company shall deposit such Future IP Asset with the Escrow Agent within thirty (30) days following the creation of such Future IP Asset if such Future IP Asset would have been deposited as part of the Escrow Materials had it been in existence at the time of the Company’s deposit of the initial
-2-
Escrow Materials. Upon deposit, any and all deposited Future IP Asset shall be considered part of the Escrow Materials for the purposes of this Agreement. OAHL shall pay all costs associated with establishing and maintaining such escrow account.
(b) Release Event and Procedure. The “Release Event” shall be OAHL’s investment of its full capital commitment under to the Joint Venture Agreement. Upon the occurrence of the Release Event, OAHL and the Company will jointly notify the Escrow Agent and the Escrow Agent shall release the Escrow Materials to OAHL.
(c) Termination of the Escrow Agreement. In the event the Company exercises the Repurchase Option (as defined in Section 1.5 (Company Repurchase Option)), upon payment by the Company to OAHL of the Purchase Price, the Company shall be entitled to terminate the escrow agreement with the Escrow Agent and the Escrow Agent shall return any and all deposited Escrow Materials to the Company.
1.3 Mandatory Laws. If and to the extent that, as a matter of law, ownership, title, or any rights or interest in or to any of the CoSense PRC IP Assets cannot be assigned as provided in Section 1.1 (Transfer of IP Assets): (a) the Company irrevocably agrees to assign and transfer, and hereby assigns and transfers to OAHL all rights (including, without limitation, all economic and commercialization rights) that can be assigned pursuant to Section 1.1 (Transfer of IP Assets) to the fullest extent permissible; and (b) the Company irrevocably agrees to grant, and hereby grants, OAHL an unlimited, exclusive, irrevocable, assignable, transferable, sublicenseable, worldwide, perpetual, royalty-free license to use, exploit and commercialize in any manner now known or in the future discovered and for whatever purpose, any rights to CoSense PRC IP Assets that cannot be assigned as contemplated by Section 1.1 (Transfer of IP Assets).
1.4 Purchase Price. On the Sale Date, OAHL is paying to the Company, as the purchase price for the CoSense PRC IP Assets, US$40,000.00 in immediately available funds (the “Purchase Price”). The Company acknowledges receipt of the Purchase Price. Any and all recording fees or related asset transfer fees shall be paid by OAHL.
1.5 Company Repurchase Option. In connection with the Company’s sale of the CoSense PRC IP Assets (including without limitation the Future IP Assets) hereunder, OAHL has agreed to make certain quarterly investments in the Company pursuant to a Joint Venture Agreement dated on or about December 4, 2017 among OAHL, Soleno and the Company (the “Joint Venture Agreement”). Upon such time as OAHL delivers, or is deemed to deliver, an Investment Termination Notice (as defined in the Joint Venture Agreement), the Company shall have the right to repurchase the CoSense PRC IP Assets from OAHL at the Purchase Price (the “Company Repurchase Option”). The Company Repurchase Option shall become exercisable by the Company beginning on the date the Investment Termination Notice is delivered or deemed to be delivered (the “Company Repurchase Option Commencement Date”). The Company may exercise the Company Repurchase Option on or after the Company Repurchase Option Commencement Date by providing written notice of such exercise to OAHL in accordance with Section 5.7 (Notices). If the Company exercises the Company Repurchase Option on or following the Company Repurchase Option Commencement Date, then effective as of such date of such exercise OAHL hereby automatically assigns the CoSense PR
-3-
C IP Assets (including any Future IP Assets) to the Company upon exercise of the Company Repurchase Option, the license from the Company to OAHL set forth in Section 1.3 shall automatically terminate, and, if OAHL is unable to assign the CoSense PRC IP Assets or Future IP Assets to the Company, then effective as of such date OAHL hereby grants a license to the Company under the same terms as set forth in Section 1.3, in each case subject to payment by the Company to OAHL of an amount in cash in immediately available funds equal to the Purchase Price (the “Repurchase Price”).
2. License to Related IP. To the extent the Company owns (presently or in the future) any Intellectual Property and/or any Intellectual Property rights that are necessary for OAHL to exercise its rights in and to the CoSense PRC IP Assets and Future IP Assets (“Related IP”), the Company hereby grants to OAHL, without further consideration, a perpetual, transferable, irrevocable, fully-paid license, throughout the world, with the right to sublicense through multiple levels of sub-licensees to any and all Related IP to: (a) reproduce, create derivative works of, distribute, publicly perform, publicly display, transmit, and otherwise use the Related IP in any medium or format, whether now known or hereafter discovered; (b) use, make, have made, sell, have sold, offer to sell, market, promote, import, and otherwise exploit any Related IP; and (c) exercise any and all similar present or future rights in the Related IP; in each case, solely as necessary for OAHL to exercise its rights in and to the CoSense PRC IP Assets and Future IP Assets; provided, however, OAHL may not commercialize the Related IP as a standalone product.
3. Representations and Warranties of the Company. The Company represents and warrants to OAHL that:
(a) the execution, delivery and performance of this Agreement by the Company has been duly authorized by all necessary corporate and partnership action of the Company, and this Agreement constitutes the valid and binding obligation of the Company, enforceable in accordance with its terms, except to the extent enforceability may be limited by bankruptcy, insolvency, reorganization, moratorium or other similar laws affecting the enforcement of creditors' rights in general and subject to general principles of equity (regardless of whether such enforceability is considered in a proceeding in equity or at law);
(b) the execution, delivery and performance of this Agreement by the Company will not (i) violate any law, regulation, order, writ, judgment, injunction or decree; or (ii) conflict with, or result in the breach of the provisions of, or constitute a default under, any agreement, license, transfer, assignment, pledge, permit or other instrument to which the Company is a party or is bound or by which any of the IP Assets are bound;
(c) Exhibit A sets forth a full and complete list of all patent registrations and applications for patent registration in respect of the CoSense Worldwide IP Assets in the PRC;
(d) the Company owns (beneficially and of record) all right, title and interest in and to all Worldwide CoSense IP Assets, free and clear of all Encumbrances, other than (if and to the extent applicable) the PRC Distribution Agreement and has the power and the right to grant exclusively to OAHL its full right, title and interest in and to each of the CoSense PRC IP Assets. All of the
-4-
patents and trademark applications within the CoSense PRC IP Assets have been duly filed in the jurisdiction named in each such application, are being actively prosecuted and have not been abandoned or allowed to lapse;
(e) the Company has not received any communication from any third party asserting any ownership interest in the Worldwide CoSense IP Assets (including without limitation the CoSense PRC IP Assets), and there is no private or governmental action, suit, proceeding, litigation, arbitration or investigation (“Action”) pending or, to the knowledge of the Company, threatened that challenges the rights of the Company in respect of any of the Worldwide CoSense IP Assets (including without limitation the CoSense PRC IP Assets) or the validity, enforceability or effectiveness thereof;
(f) the Company has not received any written communication or is aware of any allegations alleging that the Business has infringed the intellectual property rights or proprietary rights of any third party and there are no Actions that are pending or, to the knowledge of the Company, threatened against the Company with respect thereto;
(g) OAHL’s exploitation of the CoSense PRC IP Assets (including without limitation the Future IP Assets) will not constitute a violation, infringement or misappropriation of any third party intellectual property right;
(h) to the knowledge of the Company, there is no unauthorized use, infringement or misappropriation of the Worldwide CoSense IP Assets (including without limitation the CoSense PRC IP Assets) by any third party and there is no Action that is pending or threatened by the Company with respect thereto; and
(i) effective as of the Sale Date, OAHL will acquire good and marketable title to the CoSense PRC IP Assets free and clear of any and all Encumbrances, other than (if and to the extent applicable) the PRC Distribution Agreement.
(a) At the time of execution and delivery hereby, the Company shall deliver to OAHL all tangible materials related to the CoSense PRC IP Assets in the Company’s possession including, without limitation, all copies of trade secrets and other confidential information; provided, however, that this Section 4(a) shall not apply to the Escrow Materials.
(b) The Company shall, from time to time, execute and deliver, upon the request and expense of OAHL, all such other and further materials and documents and instruments of conveyance, transfer or assignment as may reasonably be requested by OAHL to effect, record or verify the transfer to, and vesting in OAHL, of the Company’s right, title and interest in and to the CoSense PRC IP Assets (including without limitation the Future IP Assets), free and clear of all Encumbrances, other than (if and to the extent applicable) the PRC Distribution Agreement, in accordance with the terms of this Agreement.
-5-
(c) From time to time after the Sale Date, at the request of OAHL and at its expense, and without further consideration, the Company shall assist OAHL (and any transferee of OAHL) as OAHL may reasonably require in connection with the defense or prosecution of any claim by or against any third party with respect to the ownership, validity, enforceability, infringement or other violation of or by the CoSense PRC IP Assets; provided that from and after the Sale Date (until any exercise by the Company of the Company Repurchase Option) OAHL shall be responsible for the appropriate prosecution and maintenance of the CoSense PRC IP Assets.
(d) Each party shall indemnify, defend and hold harmless the other party, its officers, directors, Affiliates, partners, members, managers, shareholders, employees, agents and other representatives from and against any damages, claims, losses, liabilities, costs and expenses (including, without limitation, reasonable attorneys’ fees) incurred by any of the foregoing arising out of (i) with respect to the Company, any inaccuracy or breach of any representation or warranty of the Company contained in this Agreement, and/or (ii) with respect to either party, any breach of any covenant or agreement of the other party contained in this Agreement.
5. Taxes. OAHL shall be responsible for paying any sales or use, transfer, excise, stamp, or other similar taxes arising from, imposed on or attributable to this Agreement.
6. Miscellaneous.
6.1 Counterparts. This Agreement may be executed in two or more counterparts, each of which shall be deemed an original and all of which together shall constitute one instrument.
6.2 Section Headings. The section headings used in this Agreement are for convenience only and are not to be considered in construing or interpreting this Agreement.
6.3 Amendments and Waivers. This Agreement may be amended only by a written agreement executed by each of the parties hereto. No amendment of or waiver of, or modification of any obligation under this Agreement will be enforceable unless set forth in a writing signed by the party against which enforcement is sought. Any amendment effected in accordance with this section will be binding upon all parties hereto and each of their respective successors and assigns. No delay or failure to require performance of any provision of this Agreement shall constitute a waiver of that provision as to that or any other instance.
6.4 Successors and Assigns. The terms and conditions of this Agreement shall inure to the benefit of and be binding upon the respective successors and assigns of the parties. Nothing in this Agreement, express or implied, is intended to confer upon any party other than the parties hereto or their respective successors and assigns any rights, remedies, obligations, or liabilities under or by reason of this Agreement, except as expressly provided in this Agreement.
6.5 Governing Law; Jurisdiction. This Agreement and all acts and transactions pursuant hereto and the rights and obligations of the parties hereto shall be governed, construed and interpreted in accordance with the laws of the State of California without giving effect to principles of conflicts of law. Each of the parties to this Agreement consents to the exclusive
-6-
jurisdiction and venue of the courts of the state and federal courts of San Francisco County, California.
6.6 Severability. If one or more provisions of this Agreement are held to be unenforceable under applicable law, the parties agree to renegotiate such provision in good faith, in order to maintain the economic position enjoyed by each party as close as possible to that under the provision rendered unenforceable. In the event that the parties cannot reach a mutually agreeable and enforceable replacement for such provision, then: (i) such provision shall be excluded from this Agreement; (ii) the balance of the Agreement shall be interpreted as if such provision were so excluded; and (iii) the balance of the Agreement shall be enforceable in accordance with its terms.
6.7 Notices. Any notice required or permitted by this Agreement shall be in writing and shall be deemed sufficient when delivered personally or sent by fax or forty-eight (48) hours after being deposited in the U.S. mail, as certified or registered mail, with postage prepaid, and addressed to the party to be notified at such party’s address or fax number as set forth above or as subsequently modified by written notice.
6.8 Advice of Legal Counsel. Each party acknowledges and represents that, in executing this Agreement, it has had the opportunity to seek advice as to its legal rights from legal counsel and that the person signing on its behalf has read and understood all of the terms and provisions of this Agreement. This Agreement shall not be construed against any party by reason of the drafting or preparation thereof.
6.9 Entire Agreement. This Agreement is the product of both of the parties hereto, and constitutes the entire agreement between such parties pertaining to the subject matter hereof, and merges all prior negotiations and drafts of the parties with regard to the transactions contemplated herein. Any and all other written or oral agreements existing between the parties hereto regarding such transactions are expressly canceled.
[Signature pages follow]
-7-
This Agreement has been duly executed and delivered by the duly authorized officers of the Company and OAHL as of the date first above written.
CAPNIA, INC.
By: /s/ Xxxxx Xxxxxxxxx
Name: Xxxxx Xxxxxxxxx
Title: CEO
OPTASIA HEALTHCARE LIMITED
By: /s/ Xxxxxxx Xx
Name: Xxxxxxx Xx
Title: Director
-8-
Exhibit A
CoSense PRC IP Assets
Table 1: Patent Portfolio Overview
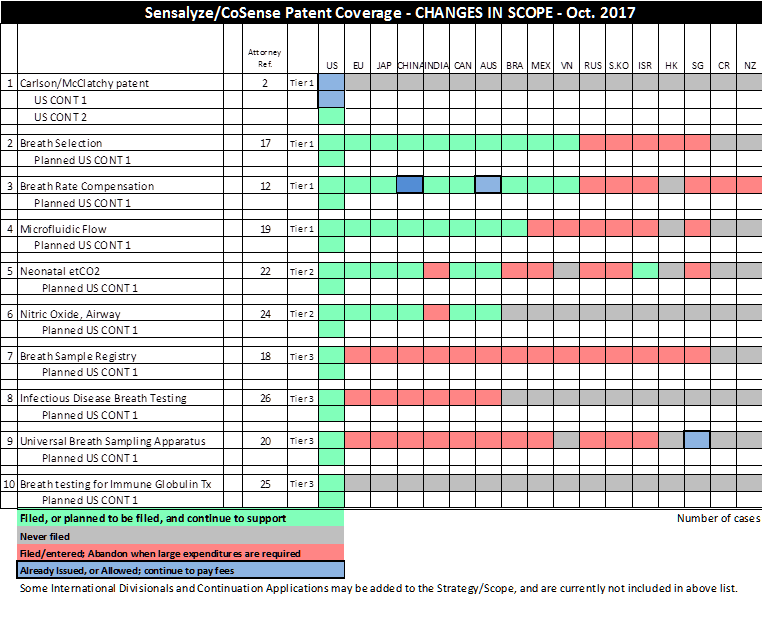
Table 2: Case List
Xxxxxx Docket No. | Title/Xxxx | Application No. | Registration No. | Country |
CAPN-002/00US | BREATH END-TIDAL GAS MONITOR | 10/561,561 | 8,021,308 | United States of America |
CAPN-002/01US | BREATH END-TIDAL GAS MONITOR | 13/153,169 | 9,095,276 | United States of America |
CAPN-002/02US | BREATH END-TIDAL GAS MONITOR | 14/812,822 | United States of America | |
CAPN-012/00US | COLLECTION AND ANALYSIS OF A VOLUME OF EXHALED GAS USING BREATH RATE COMPENSATION | 61/578,811 | United States of America | |
CAPN-012/01IN | “ | 6091DELNP2014 | India | |
CAPN-012/01KR | “ | 00-0000-0000000 | Republic of Korea | |
CAPN-012/01MX | “ | MXA2014007542 | Mexico | |
CAPN-012/01RU | “ | 2014129561 | Russian Federation | |
CAPN-012/01WO | “ | PCT/US2012/071085 | Patent Cooperation Treaty | |
CAPN-012/01CN | DATABASE FOR CORRECTION OF COLLECTION AND ANALYSIS OF EXHALED GAS WITH BREATHING PARAMETER FREQUENCY COMPENSATION | 2012800700205 | China | |
CAPN-012/01BR | “ | 1120140151458 | Brazil | |
CAPN-012/01AU | COLLECTION AND ANALYSIS OF A VOLUME OF EXHALED GAS WITH COMPENSATION FOR THE FREQUENCY OF A BREATHING PARAMETER | 2012358370 | Australia | |
CAPN-012/01CA | “ | 2860247 | Canada | |
CAPN-012/01EP | “ | 12860711.6 | European Patent Office | |
CAPN-012/01IL | “ | 000000 | Xxxxxx | |
CAPN-012/01JP | “ | 2014548919 | Japan | |
CAPN-012/01US | “ | 13/722,950 | United States of America | |
CAPN-012/01VN | “ | 0000000000 | Vietnam | |
CAPN-012/02AU | “ | Xxxxxxxxx | ||
XXXX-000/00XX | “ | United States of America | ||
CAPN-017/00US | SELECTION AND ANALYSIS OF PHYSIOLOGICALLY VALID BREATHS FOR BREATH GAS ANALYSIS | 61/750,305 | United States of America | |
CAPN-017/01AU | BREATH SELECTION FOR ANALYSIS | 0000000000 | Australia | |
CAPN-017/01BR | “ | 1120150164340 | Brazil | |
CAPN-017/01CA | “ | 2897533 | Canada | |
CAPN-017/01CN | “ | 201480009292.3 | China | |
CAPN-017/01EP | “ | 14737690.9 | European Patent Office | |
CAPN-017/01HK | “ | 16105282.8 | Hong Kong | |
CAPN-017/01IL | “ | 239822 | Israel | |
CAPN-017/01IN | “ | 0000/XXXXX/0000 | Xxxxx | |
CAPN-017/01JP | “ | 2015-551865 | Japan | |
CAPN-017/01KR | “ | 1020157021302 | Republic of Korea | |
CAPN-017/01MX | “ | MX/a2015/008838 | Mexico | |
CAPN-017/01RU | “ | 2015133209 | Russian Federation | |
CAPN-017/01US | “ | 14/150,625 | United States of America | |
CAPN-017/01VN | “ | 1201502810 | Vietnam | |
CAPN-017/01WO | “ | PCT/US2014/010746 | Patent Cooperation Treaty | |
CAPN-019/00US | BREATH ANALYSIS SYSTEM WITH ANTI-MIXING FEATURES | 61/872,270 | United States of America | |
CAPN-019/01AU | COLUMNAR FLOW GAS SAMPLING AND MEASUREMENT SYSTEM | 2014312040 | Australia | |
CAPN-019/01BR | “ | 1120160040651 | Brazil | |
CAPN-019/01CA | “ | 2922347 | Canada | |
CAPN-019/01CN | “ | 201480054258.8 | China | |
CAPN-019/01EP | “ | 14840760.4 | European Patent Office | |
CAPN-019/01IL | “ | 000000 | Xxxxxx | |
CAPN-019/01IN | “ | 201617009208 | India | |
CAPN-019/01JP | “ | 0000-000000 | Xxxxx | |
CAPN-019/01KR | “ | 1020167008189 | Republic of Korea | |
CAPN-019/01MX | “ | MX/a/2016/002629 | Mexico | |
CAPN-019/01RU | “ | 2016111649 | Russian Federation | |
CAPN-019/01SG | “ | 11201601439Q | Singapore | |
CAPN-019/01US | “ | 14/474,019 | United States of America | |
CAPN-019/01WO | “ | PCT/US2014/053567 | Patent Cooperation Treaty | |
CAPN-020/00US | UNIVERSAL BREATH ANALYSIS SAMPLING DEVICE | 61/872,514 | United States of America | |
CAPN-020/01AU | “ | 2014312042 | Australia | |
CAPN-020/01BR | “ | BR1120160040961 | Brazil | |
CAPN-020/01CA | “ | 2922349 | Canada | |
CAPN-020/01CN | “ | 201480054249.9 | China | |
CAPN-020/01EP | “ | 14838958.8 | European Patent Office | |
CAPN-020/01IL | “ | 000000 | Xxxxxx | |
CAPN-020/01IN | “ | 201617009369 | India | |
CAPN-020/01JP | “ | 0000-000000 | Xxxxx | |
CAPN-020/01KR | “ | 1020167008190 | Republic of Korea | |
CAPN-020/01MX | “ | MX/a/2016/002628 | Mexico | |
CAPN-020/01RU | “ | 2016111651 | Russian Federation | |
CAPN-020/01SG | “ | 11201601440Q | Singapore | |
CAPN-020/01US | “ | 14/473,878 | United States of America | |
CAPN-020/01WO | “ | PCT/US2014/053569 | Patent Cooperation Treaty | |
CAPN-020/02SG | “ | 10201703241U | Singapore | |
CAPN-022/00US | NEONATAL CARBON DIOXIDE MEASUREMENT SYSTEM | 61/872,415 | United States of America | |
CAPN-022/01AU | “ | 2014312044 | Australia | |
CAPN-022/01BR | “ | BR1120160040945 | Brazil | |
CAPN-022/01CA | “ | 2922356 | Canada | |
CAPN-022/01CN | “ | 2014800548866 | China | |
CAPN-022/01EP | “ | 14839697.1 | European Patent Office | |
CAPN-022/01IL | “ | 000000 | Xxxxxx | |
CAPN-022/01IN | “ | 201617009209 | India | |
CAPN-022/01JP | “ | 0000-000000 | Xxxxx | |
CAPN-022/01KR | “ | 0000000000000 | Republic of Korea | |
CAPN-022/01MX | “ | MX/a/2016/002627 | Mexico | |
CAPN-022/01RU | “ | 2016111654 | Russian Federation | |
CAPN-022/01SG | “ | 11201601442U | Singapore | |
CAPN-022/01US | “ | 14/473,888 | United States of America | |
CAPN-022/01WO | “ | PCT/US2014/053572 | Patent Cooperation Treaty | |
CAPN-024/00US | SELECTION, SEGMENTATION, AND ANALYSIS OF EXHALED BREATH FOR AIRWAY DISORDERS ASSESSMENT | 61/968,290 | United States of America | |
CAPN-024/01AU | “ | 2015231003 | Australia | |
CAPN-024/01CA | “ | 2943243 | Canada | |
CAPN-024/01CN | “ | 201580023183.1 | China | |
CAPN-024/01EP | “ | 15764503.7 | European Patent Office | |
CAPN-024/01IN | “ | 201617034345 | India | |
CAPN-024/01JP | “ | 0000-000000 | Xxxxx | |
CAPN-024/01US | “ | 14/664,728 | United States of America | |
CAPN-024/01WO | “ | PCT/US2015/021852 | Patent Cooperation Treaty | |
CAPN-025/00US | IVIG TREATMENT: METHODS FOR SELECTION OF PATIENTS, MONITORING FOR HEMOLYSIS AND DOSE DETERMINATION | 62/042,762 | United States of America | |
CAPN-025/01US | METHODS FOR IMMUNE GLOBULIN ADMINISTRATION | 14/838,241 | United States of America | |
CAPN-025/01WO | “ | PCT/US2015/047296 | Patent Cooperation Treaty | |
CAPN-026/00US | BREATH ANALYSIS SYSTEMS AND METHODS FOR SCREENING FOR INFECTIOUS DISEASE | 62/066,094 | United States of America | |
CAPN-026/01AU | “ | 2015336025 | Australia | |
CAPN-026/01CA | “ | 2965142 | Canada | |
CAPN-026/01CN | “ | 201580062714.8 | China | |
CAPN-026/01EP | “ | 15852238.3 | European Patent Office | |
CAPN-026/01IN | “ | 201717016477 | India | |
CAPN-026/01JP | “ | 0000-000000 | Xxxxx | |
CAPN-026/01US | “ | 14/918,484 | United States of America | |
CAPN-026/01WO | “ | PCT/US2015/056527 | Patent Cooperation Treaty | |
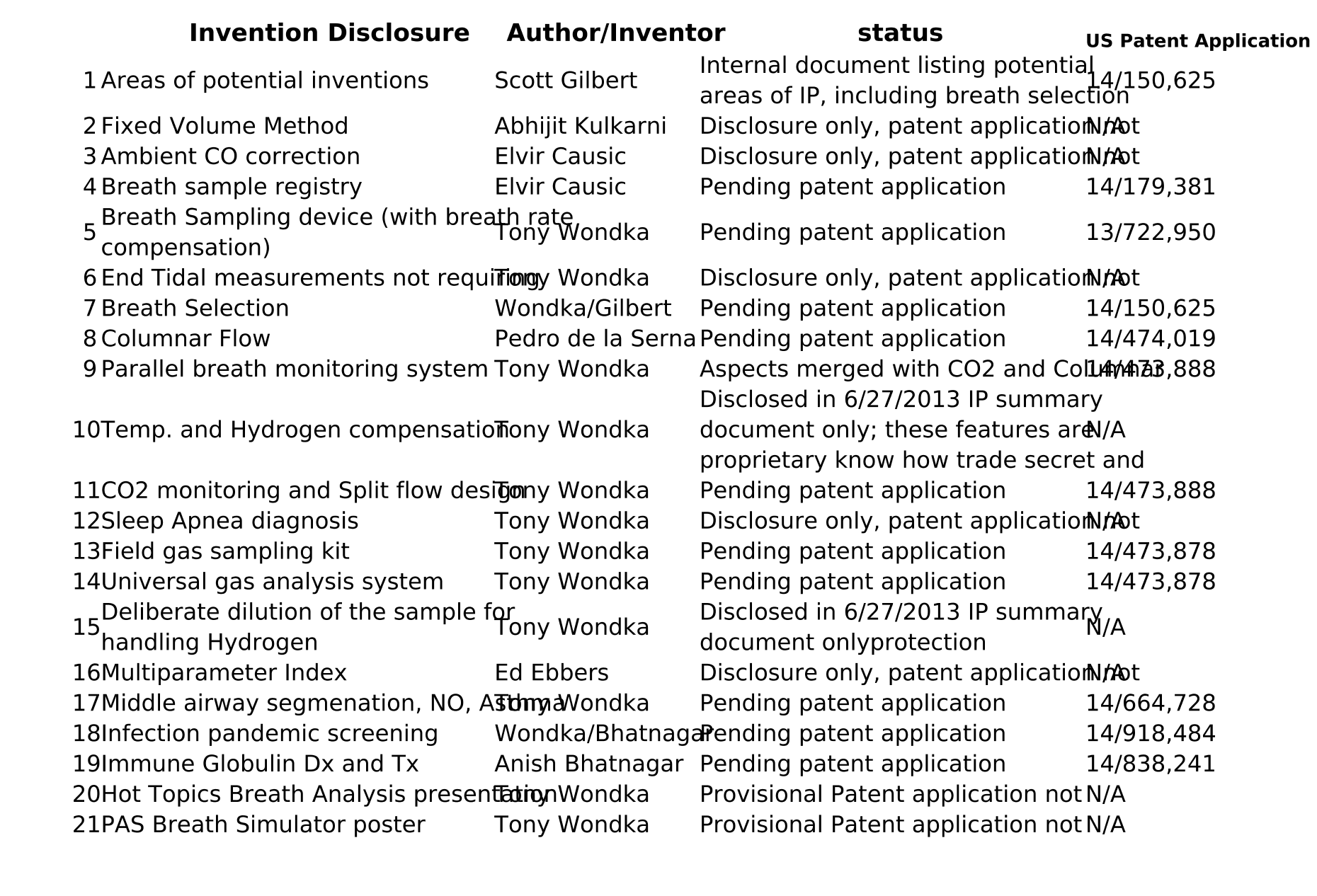
Table 3: Invention Disclosure List
(d) Licenses used in the product or in the manufacturing of the product
1. | uboot (Embest CD) : this is mainly taken from Embest CD together with the Embest SBC6000X board. The original work is from DENX Software Engineering Open-Source Community. The source codes are released under the terms of the GNU General Public License. |
2. | At91bootstrap (manages hardware initialization such as clock speed, PIO settings, RAM, Flash). This is taken from Embest CD. The original work is provided by Atmel and GNU Open-source community to support Atmel micro-controller chips. |
3. | Linux Kernel: taken from Embest CD with Capnia's modifications to adapt to Capnia's purpose. The original work for Linux Kernel is from Linux Kernel Organization and the source-codes released under the terms of GNU General Public License. |
4. | Linux File-system: taken from Embest CD with Capnia's modifications. The original work is from Busybox open-source project and released under the term of GNU General Public License. |
5. |
6. | Protothreads Library (mini version of multi-threading) taken from Xxxx Xxxxxxx, Swedish Institute of Computer Science, without any modification from Capnia. The library is released under open-source license without any restrictions. The only requirement of the license is that the credit should be given the source-codes and in the documentation. Further information is available at xxxx://xxxxxxx.xxx/xxxx/xx/xxxxxxx.xxxx |
7. | UART library - QextSerialPort (provides interface to serial ports used in Qt-based projects) The library is released under open-source license under the terms of the MIT license. Capnia uses the library without any modification to the original source codes and library. |
8. | Qt Libraries: the libraries are released under the terms of General Lesser Public License (LGPL-2+). Capnia used the libraries files without any modification on the original source-codes or libraries. |
9. | Microchip Library: the free library is provided by Microchip company to customize and configure to adapt to Microchip micro-controllers used on both BASS and XXXX boards. |
10. | Solidworks 3D solid modeling software |
11. | MP Lab Compiler |
12. | Hyperterminal |
13. | Microsoft Office |
14. | Adobe |
15. | Microsoft Windows 7 |
-9-


