ASSET PURCHASE AND SALE AGREEMENT
Exhibit 10.99
ASSET PURCHASE AND SALE AGREEMENT
THIS ASSET PURCHASE AND SALE AGREEMENT, dated as of March 15, 2006, is entered into by and between SENETEK PLC, a corporation duly organized and validly existing under the laws of England and having a place of business located at 000 Xxxxxx Xxxxx, Xxxx, Xxxxxxxxxx 00000 (“Senetek”), and RANBAXY PHARMACEUTICALS INC., a corporation duly organized and validly existing under the laws of the State of Florida, having a place of business located at 0000 Xxxxxxx Xxxxxx Xxxxxxxxx, Xxxxxxxxxxxx, Xxxxxxx 00000 (“Ranbaxy”).
PRELIMINARY STATEMENTS
WHEREAS, Senetek owns manufacturing equipment, supplies and other tangible property, and patents, trademarks, proprietary know-how, technology and other intangible property relating to the development, production and analytical methods of the Devices and the Products hereinafter described; and
WHEREAS, Ranbaxy wishes to purchase such Assets and undertake the regulatory approvals and commercial activities required to market the Products, all upon the terms and conditions recited hereinafter.
NOW, THEREFORE, in consideration of the foregoing Preliminary Statements and the mutual representations, warranties and covenants set forth herein, the Parties hereby agree as follows:
1. DEFINITIONS.
In addition to the terms defined elsewhere herein, as used in this Agreement the following terms shall have the meanings set forth in this Article 1 unless context dictates otherwise:
1.1 “AAA” shall have the meaning assigned to such term in Article 10.
1.2 “Affiliate” shall mean, with respect to any Party, any entity controlling, controlled by, or under common control with, such Party. For these purposes, “control” shall refer to (i) the possession, directly or indirectly, of the power to direct the management or policies of an entity, whether through the ownership of voting securities, by contract or otherwise, or (ii) the ownership, directly or indirectly, of at least fifty percent (50%) of the voting securities or other ownership interest of an entity.
1.3 “Agreement” shall mean this Lease and License Agreement together with all exhibits, schedules and attachments hereto.
1.4 “Ardana License” means the License Agreement, dated June 17, 2004, between Senetek and Ardana Bioscience Limited (“Ardana”) as set forth in Schedule 1.4, and any amendments or modifications thereto.
1.5 “Assets” shall mean all of Senetek’s existing (i) tangible assets related to the Devices and the Products, including, without limitation, all automated and semi-automated equipment necessary to assemble, fill and otherwise produce and manufacture the Reliaject Device more fully described in Paragraph A of Schedule 1.12 (currently configured for dental cartridges) (the “Reliaject Equipment”), and all existing components, raw materials, supplies, labels, packaging, parts, work-in-process, finished goods, goods on consignment and all other materials (“Inventory”) utilized in connection with the production, manufacture and packaging of the Devices and the Products, including that described in Schedule 1.36 (collectively, the “Tangible Assets”); (ii) intangible assets related to the Devices and the Products, including, without limitation, all Patents set forth on Schedule 1.31 hereto, which includes the patent numbers, patent titles and patent terms thereof, all statutory trademarks set forth on Schedule 1.43 hereto together with all common law trademarks related thereto, including, without limitation, “Reliaject” and “Adrenaject”, all Senetek Know-How, goodwill and all other intangibles, intellectual property and proprietary information related thereto, including, without limitation, all operating manuals, validation packages, unexpired warranties and indemnities of Third Parties, all rights and claims against Third Parties related to the Devices and the Products, and copies of all information relating to Senetek’s past ANDA submission for the Reliaject Device for the emergency treatment of anaphylaxis, including all correspondence between Senetek and the FDA regarding this ANDA submission; (iii) all accounting information related to the Devices and the Products and all media in which or on which any of the information, knowledge, data or records related to the Devices and the Products may be related or stored; and (iv) all computer software related to accounting and operations systems of Senetek in connection with the Devices and the Products (the Assets described in clauses (ii), (iii) and (iv), collectively, the “Intangible Assets”) .
1.6 “Calendar Quarter” shall mean a period of three (3) consecutive months in the Georgian calendar ending at midnight, Eastern Time on the last day of March, June, September, or December, respectively.
1.7 “CFR” shall mean the United States Code of Federal Regulations.
1.8 “cGMP” shall mean current Good Manufacturing Practice as defined in Parts 210 and 211 of Title 21 of the CFR, as may be amended from time to time, or any successor thereto.
1.9 “Commercially Reasonable Efforts” shall mean, with respect to a Party, those commercial efforts which a reasonable businessman having that Party’s business, legal, medical and scientific expertise and resources would use for a pharmaceutical product or device which is of similar market potential at a similar stage in development or its product or device life, taking into account the competitiveness of the marketplace, the proprietary position of the product or device and the profitability of the product or device, it being understood that a Party’s Commercially Reasonable Efforts will not in any event require that Party to take any action that
2
would be reasonably likely to result in a breach of any other provision of this Agreement, or any other agreement between the Parties, or any other agreement between a Party, Affiliate of such Party and/or Third Parties existing as of the Effective Date, or that the Party in good faith believes may violate any applicable law, regulation, rule, order, permit, direction or license of any court or governmental authority having appropriate jurisdiction over the Party and subject matter or would be reasonably likely to be disruptive of any material service conducted or product made at or from any of its facilities or impair its ability to provide services or products hereunder.
1.10 “Competing Product” means any product containing the same active ingredient as compared to a Ranbaxy product and providing for a patient-self-administered auto-injection delivery method (i.e., Epinephrine Product, Non-Epinephrine Product, etc.).
1.11 “Confidential Information” shall mean, with respect to either Party, all confidential or proprietary information and materials, patentable or otherwise, in any form (written, oral, photographic, electronic, magnetic, or otherwise) which is disclosed by or on behalf of such Party to the other Party pursuant to or in contemplation of this Agreement, including, without limitation, information relating to the Assets, the Devices, the Products or any other product of either Party or the pricing thereof; provided that such Confidential Information is identified as confidential either: (i) at the time of disclosure; or (ii) in writing, within thirty (30) days after disclosure, except as otherwise provided in Section 5.2.
1.12 “Devices” shall mean (i) the disposable automated injection device described in Paragraph A of Schedule 1.12 together with all modifications and enhancements thereof covered by any claim of the Patents or produced and assembled in whole or in part through use of the Reliaject Equipment (the “Reliaject Device”) and (ii) the reusable modified Xxxx Xxxxxxx automated injection device described in Paragraph B of Schedule 1.12 together with all modifications and enhancements thereof.
1.13 “Disclosing Party” shall have the meaning assigned to such term in Section 5.1
1.14 “DMF” shall mean the Drug Master File applicable to the Devices and/or the Products as from time to time in effect.
1.15 “Effective Date” shall mean the date of this Agreement as set forth in the Preamble.
1.16 “Encumbrance” means with respect to any Assets, any mortgage, lien, pledge, charge, security interest, conditional sale agreement, financing statement or encumbrance of any kind or any other type of preferential arrangement that has the practical effect of creating a security interest in respect of such Asset.
1.17 “Epinephrine Product” means the Devices unfilled or pre-filled with the active pharmaceutical ingredient epinephrine or its analogs or in kit form with ampoules or cartridges pre-filled with epinephrine.
3
1.18 “Excluded Liabilities” means any and all liabilities or obligations of Senetek or its Affiliates, of any kind or nature, whether or not relating to the Assets, and whether known or unknown, absolute, accrued, contingent or otherwise, or whether due or to become due, arising out of events, transactions, facts, acts or omissions occurring prior to, at or subsequent to the Effective Date, including, without limitation, all liabilities and obligations relating to the Ardana License and any costs, claims, damages, expenses and/or liabilities in connection with the action disclosed on Schedule 2.2.7.
1.19 “FDA” shall mean the United States Food and Drug Administration, or any successor agency having regulatory jurisdiction over the manufacture, distribution and sale of drugs in the United States and its territories and possessions.
1.20 “FDC Act” shall mean the Federal Food, Drug and Cosmetic Act, 21 U.S.C. §321 et seq., as amended, and the regulations promulgated thereunder from time to time.
1.21 “First Commercial Sale” shall mean the first sale of any of the Products for which payment has been received for use or consumption.
1.22 “GAAP” shall mean generally acceptable United States accounting principles consistently applied by Ranbaxy in preparing its audited consolidated financial statements.
1.23 “Gross Sales” shall mean, with respect to a specified quantity of the Products, such quantity of the Product multiplied by the gross price invoiced by Ranbaxy or its Affiliates therefor for sales to unaffiliated third parties, calculated in accordance with GAAP.
1.24 “Indemnitee” shall have the meaning assigned to such term in Section 8.3.
1.25 “Indemnitor” shall have the meaning assigned to such term in Section 8.3.
1.26 “Knowledge” means the actual knowledge of the executive officers and/or directors of the Company or any Subsidiary, assuming due inquiry.
1.27 “Net Sales” shall mean, with respect to a specified quantity of the Products, the Gross Sales with respect thereto, less the following deductions to the extent consistent with Ranbaxy’s standard trade terms for like products and with GAAP consistently applied: (i) trade, quantity or cash discounts applicable to gross amounts billed and allowed, paid or otherwise included in determining net sales, (ii) credits or allowances given or made on account of rejection, recall, withdrawal, return or destruction of Products previously delivered (if supported by appropriate documentation), (iii) charge-back payments and rebates (including, without limitation, Medicaid rebates and retroactive price reductions) granted to managed health care organizations or to national, federal, state or local governments, their agencies or reimbursers, or to trade customers, including wholesalers and chain and pharmacy buying groups, and (iv) tariffs, customs charges, duties, sales and excise taxes and any other like governmental charges imposed upon the importation or exportation of such Products. No marketing, promotional or advertising expenses of any kind will be deducted from Gross Sales to determine Net Sales.
4
1.28 “Non-Epinephrine Product” means the Devices unfilled or pre-filled with any active pharmaceutical ingredient other than epinephrine or its analogs, or in kit form with ampoules or cartridges pre-filled with any such pharmaceutical ingredient other than epinephrine or its analogs.
1.29 “North America Territory” means the United States of America and Canada.
1.30 “Party” shall mean Ranbaxy or Senetek and, when used in the plural, shall mean Ranbaxy and Senetek.
1.31 “Patents” shall mean the patents and patent applications set forth in Schedule 1.31 hereto.
1.32 “Person” means an individual, partnership, corporation, limited liability company, joint stock company, unincorporated organization or association, trust or joint venture, or a governmental agency or political subdivision thereof.
1.33 “Products” shall mean any of the Devices as offered for sale, whether in individual or multiple packs and whether sold unfilled, pre-filled with the active pharmaceutical ingredient epinephrine or another active pharmaceutical ingredient or in kit form with ampoules or cartridges pre-filled with any such pharmaceutical ingredient.
1.34 “Receiving Party” shall have the meaning assigned to such term in Section 5.1.
1.35 “Regulatory Authority” shall mean the FDA and any other regulatory authorities having jurisdiction over the manufacture, importation, distribution, mandating, use and sale of the Product in the Territory.
1.36 “Reliaject Equipment” shall mean the items listed on Schedule 1.36 hereto.
1.37 “Senetek Patent Rights” means the patent and patent applications listed in Schedule 1.31.
1.38 “Subsidiary” shall mean, with respect to a Party, an Affiliate of such Party all of the equity securities of which are owned, directly or indirectly, by such Party.
1.39 “Senetek Know-How” shall mean all information and data in Senetek’s possession or control which is not generally known, including formulae, procedures, protocols, techniques and results of experimentation and testing, which are necessary or useful to make, use, develop, sell or seek regulatory approval in the Territory to market the Product.
1.40 “Term” shall have the meaning assigned to such term in Section 6.1.
1.41 “Territory” shall mean the World.
1.42 “Third Party” shall mean any person who or which is neither a Party nor an Affiliate of a Party.
5
1.43 “Trademarks” shall mean the trademarks listed in Schedule 1.43 hereto.
1.44 “Valid Claim” means a claim of an issued and unexpired Patent included within the Senetek Patent Rights which has not been held permanently revoked, unenforceable or invalid by a decision of a court or other governmental agency of competent jurisdiction, unappealable or unappealed within the time allowed for appeal, and which has not been admitted to be invalid or unenforceable through reissue or disclaimer or otherwise.
2. REPRESENTATIONS AND WARRANTIES.
2.1 Representations by Each Party. Each Party hereby represents and warrants to the other Party, as of the Effective Date, as follows:
2.1.1 such Party (i) is a corporation duly organized, validly existing and in good standing under the laws of the jurisdiction in which it is incorporated; (ii) has the corporate power and authority and the legal right to own and operate its property and assets, to lease the property and assets it operates under lease, and to carry on its business as it is now being conducted; and (iii) is in compliance with all requirements of applicable law, except to the extent that any noncompliance would not have a material adverse effect on the properties, business, financial or other condition of such Party and would not materially adversely affect such Party’s ability to perform its obligations under this Agreement;
2.1.2 such Party (i) has the corporate power and authority and the legal right to enter into this Agreement and to perform its obligations hereunder; and (ii) has taken all necessary corporate action, including any required shareholder approval, on its part to authorize the execution and delivery of this Agreement and the performance of its obligations hereunder. This Agreement has been duly executed and delivered on behalf of such Party, and constitutes a legal, valid, binding obligation, enforceable against such Party in accordance with its terms, subject to enforcement of remedies pursuant to applicable bankruptcy, insolvency, reorganization, moratorium or similar laws affecting generally the enforcement of creditors’ rights and subject to a court’s discretionary authority with respect to the granting of a decree ordering specific performance or other equitable remedies;
2.1.3 such Party has obtained all necessary consents, qualifications, approvals, orders or authorizations, or filings with, any governmental authorities, including any court, and Third Parties required to be obtained by such Party in connection with the valid execution, delivery or performance of this Agreement or the consummation of any other transaction contemplated by this Agreement;
2.1.4 the execution and delivery of this Agreement and the performance of such Party’s obligations hereunder (i) do not, to the best of such Party’s knowledge, conflict with or violate any requirement of applicable laws or regulations; and (ii) do not conflict with, or constitute a default under, any contractual obligation of such Party; and
6
2.1.5 such Party has not entered into any agreement with any Third Party that is in conflict or inconsistent with the rights granted to the other Party pursuant to this Agreement.
2.2 Representations by Senetek. Senetek hereby represents and warrants to Ranbaxy, as of the Effective Date, as follows:
2.2.1 Title to Properties; Liens and Encumbrances. (a) Senetek has good and marketable title to and owns free and clear of any Encumbrances or restrictions on transfer, or, leases or has the right to use the Assets. Upon consummation of the transactions contemplated hereby, good and marketable title to each of the Assets shall be vested in Ranbaxy. The Assets constitute the entire right, title and interest owned by Senetek relating to the Devices and the Product. The Assets are, to the Knowledge of Senetek, structurally sound with no defects, have been maintained or stored in accordance with normal industry practices or Senetek’s normal storage practices and are, to the Knowledge of Senetek, in good and/or workable operating condition and repair (except for ordinary wear and tear).
(b) Schedule 1.36 is a true and complete list of all of the Reliaject Equipment, and Schedule 1.5 contains a true and correct list of all Inventory for the production, manufacture and packaging of the Devices, which are all of the Tangible Assets in Senetek’s possession or control necessary or useful to produce, manufacture and package the Devices.
2.2.2 Business. Senetek has no Knowledge that there exists, or there is pending or planned, any statute, rule, law, regulation, standard or code, including, without limitation, any regulations, notifications, correspondence, rules or interpretive position of the FDA, which would, or would reasonably be expected to have a material adverse effect on the Assets or the development or commercialization of the Devices or the Products.
2.2.3 Absence of Certain Changes. Since January 1, 2005, except as disclosed on Schedule 2.2.3, there has not been any change or any development which could reasonably be expected to result in a prospective material adverse effect on the Assets or the development or commercialization of the Products. During the past twelve months, Senetek has maintained the Assets in the ordinary and usual course consistent with past practices and has not:
(i) whether or not covered by insurance, incurred any material damage or loss to any Assets;
(ii) incurred, or become subject to, any obligation or liability (absolute or contingent, matured or unmatured, known or unknown) with respect to the Assets, or mortgaged, pledged or subjected to any Encumbrance any of its Assets which continues to exist as of the date hereof;
(iii) sold, exchanged, transferred or otherwise disposed of any of its Assets, or canceled any debts or claims with respect thereto;
(iv) written down the value of any Assets, except write-downs and write-offs in the ordinary course of business, none of which, individually or in the aggregate, are material to Senetek;
7
(v) entered into any transactions with respect to the Assets;
(vi) received any correspondence, subpoena or other information requesting data or material or indicating that the FDA or any other governmental entity had commenced, or was or is considering commencing, a review of any of the Assets; or
(vii) made an agreement to do any of the foregoing.
2.2.5 Leases, Contracts and Commitments. (a) Senetek has no outstanding contracts, agreements, loans, leases, license, guarantees, obligations or commitments associated with any of the Assets.
2.2.6 Suppliers. There have been no suppliers of any of the Assets during 2004 or 2005.
2.2.7 Litigation. Except as disclosed on Schedule 2.2.7, there are no actions, suits, proceedings, hearings or investigations before any governmental entity to which Senetek is a party in connection with the Assets. Senetek is not a party to or, to the Knowledge of Senetek, threatened to be made a party to any actions, suits, proceedings, hearings or investigations of, in or before any governmental entity that would have, individually or in the aggregate, a material adverse effect on the Assets or that questions or challenges the validity of this Agreement or any action taken or to be taken by Senetek pursuant to this Agreement. There is not outstanding against Senetek any decision, judgment, decree, injunction, rule or order of any governmental entity, that would have, individually or in the aggregate, a material adverse effect on the Assets. Without limiting the foregoing, Senetek is not involved in nor, to the Knowledge of Senetek, is it reasonably anticipated that Senetek would become involved in any dispute with any of its current or former employees, agents, brokers, customers or suppliers that would have, individually or in the aggregate, a material adverse effect on the Assets.
2.2.8 Insolvency. No insolvency proceedings of any character, including, without limitation, bankruptcy, receivership, reorganization, composition, or arrangement with creditors, voluntary or involuntary, affecting Senetek is pending or, to the Knowledge of Senetek, threatened; nor has Senetek made any assignment for the benefit of creditors, or taken any action in contemplation thereof, or taken any action which would constitute the basis for the institution of any such insolvency proceedings.
2.2.9 Licenses, Permits and Approvals. There are no licenses, permits, approvals, qualifications or the like, issued or to be issued to Senetek by any government or any governmental unit, agency, body or instrumentality, whether federal, state, municipal or local (within the U.S. or otherwise) in connection with the Assets. No registration with, approval by, consent or clearance from or pre-notification to any governmental agency is required in connection with the execution and performance of this Agreement by Senetek.
2.2.10 Environmental Matters. With respect to the Assets (a) Senetek is in compliance with all applicable environmental laws, and (b) Senetek has not received any written notice with respect to the Assets from any governmental entity or third party alleging that Senetek is not in material compliance with any environmental law.
8
2.2.11 ANDA Submissions. Schedule 2.2.11 is a true, correct and complete list of all of the information in Senetek’s possession or control relating to Senetek’s past ANDA submission for the Reliaject Device for the emergency treatment of anaphylaxis, including all correspondence between Senetek and the FDA regarding this ANDA submission.
2.2.12 Intellectual Property. (a) Schedules 1.31 and 1.43 are true, correct and complete lists of all of the Patents and statutory Trademarks, respectively, owned by Senetek and its Affiliates related to the Devices or the Products. Senetek has made all payments and filings required to maintain the same in full force and effect, and each is subsisting and in good standing as of the Effective Date except, in the case of the Trademarks, for the effect, if any, of lack of commercial use thereof. Except as described in Schedule 2.2.7, there are no oppositions, cancellations, invalidity proceedings, interferences or re-examination proceedings pending with respect to any Patents or Trademarks. To the Knowledge of Senetek, the use of the Patents, the Trademarks, the Senetek Know-How and the other Assets in the manner contemplated by this Agreement does not infringe the rights of any Third Party and no Third Party is infringing any of the Patents or the Trademarks.
(b) Senetek follows reasonable commercial practices common in the industry to protect its proprietary and confidential information, including requiring its employees, consultants and agents that have access to the know how and other proprietary information of Senetek, including, without limitation, any proprietary software developed by Senetek, to be bound in writing by obligations of confidentiality and non-disclosure, and requiring its employees, consultants and agents that have access to the know how and other proprietary information of Senetek to assign to it any and all inventions and discoveries discovered by such employees, consultants and/or agents within the scope of and during their employment and only disclosing proprietary and confidential information to third parties pursuant to written confidentiality and non-disclosure agreements.
2.2.13 Liabilities. Senetek has no liabilities or obligations (absolute, accrued, contingent or otherwise) relating to the Assets.
2.2.14 Ardana License. (a) The Ardana License as set forth in Schedule 1.4 is true and correct, and there are no amendments or modifications thereto except as set forth in such documents.
(b) Except for the Ardana License, Senetek has not granted any other licenses or other rights to use any of the Patents, the Trademarks, the Senetek Know-How or any other of the Assets to any Third Party.
2.2.15 Disclosure. No representation or warranty by Senetek in this Agreement contains an untrue statement of a material fact or omits any material fact necessary to make the statements of material fact contained herein, in light of the circumstances under which made, not misleading. To the Knowledge of Senetek, there is no fact or circumstance not disclosed to Ranbaxy which materially adversely affects the Assets
2.2.16 Brokers or Finders. No agent, broker, investment banker, financial advisor or other firm or Person is or will be entitled to any brokers’ or finder’s fee or other commission or similar fee in connection with the transactions contemplated by this Agreement.
9
2.2.17 Survival of Representations and Warranties of Senetek. The representations and warranties of Senetek made in this Agreement are correct, true and complete as of the Effective Date, and shall survive the Closing for a period of three (3) years except for the following:
(a) the representations and warranties contained in subsections 2.2.1 and 2.2.12 hereof shall survive for the maximum period permitted by law; and
(c) the representations and warranties of subsection 2.2.10 with respect to Environmental Matters shall survive for a period of four (4) years from the Effective Date.
Each of Senetek and Ranbaxy acknowledge and agree that as long as written notice is given on or prior to the three (3) year anniversary of the Effective Date relating to claims for indemnification for representations and warranties surviving for three (3) years, or the four (4) year anniversary of the Effective Date relating to claims for indemnification for representations and warranties surviving for four (4) years, any such claim for indemnification shall survive until such matter is resolved.
2.3 Representations by Ranbaxy. Ranbaxy hereby represents and warrants to Senetek, as of the Effective Date, as follows:
2.3.1 Litigation. Ranbaxy is not a party to or, to the Knowledge of Ranbaxy, threatened to be made a party to any actions, suits, proceedings, hearings or investigations of, in or before any governmental entity that would have, individually or in the aggregate, a material adverse effect on the transactions contemplated by this Agreement or that questions or challenges the validity of this Agreement or any action taken or to be taken by Ranbaxy pursuant to this Agreement.
2.3.2 Licenses, Permits and Approvals. No registration with, approval by, consent or clearance from or pre-notification to any governmental agency is required in connection with the execution and performance of this Agreement by Ranbaxy.
2.3.3 Disclosure. No representation or warranty by Ranbaxy in this Agreement contains an untrue statement of a material fact or omits any material fact necessary to make the statements of material fact contained herein, in light of the circumstances under which made, not misleading.
2.3.4 Brokers or Finders. No agent, broker, investment banker, financial advisor or other firm or Person is or will be entitled to any brokers’ or finder’s fee or other commission or similar fee in connection with the transactions contemplated by this Agreement.
3. PURCHASE AND SALE OF ASSETS.
3.1 Conveyance. Effective as of the Effective Date, Senetek, on its own behalf and on
10
behalf of its Affiliates, hereby unconditionally and irrevocably transfers and sells to Ranbaxy, and Ranbaxy hereby unconditionally and irrevocably purchases from Senetek, all of Senetek’s and its Affiliates’ right, title and interest in and to the Assets. Senetek hereby delivers to Ranbaxy a General Assignment and Xxxx of Sale, a Patent Assignment and a Trademark Assignment, in the forms annexed as Exhibits A through C hereto, duly executed by Senetek or its Affiliates, as appropriate, to evidence such transfer and assignment.
3.2 Purchase Price. (a) In consideration of the transfer and sale of the Assets, Ranbaxy hereby pays to Senetek, simultaneously with execution and delivery of this Agreement, by official bank or certified check to the order of Senetek, the non-refundable and non-creditable (subject to Section 6.2 and 6.3) sum of *** (the “Closing Payment”), and, subject to Sections 6.1, 6.2 and 6.3, covenants and agrees to pay to Senetek the following non-refundable and non-creditable sums as additional Purchase Price:
3.2.1 *** upon obtaining final ANDA or equivalent FDA approval for an Epinephrine Product for the emergency treatment of anaphylaxis;
3.2.2 *** upon achieving cumulative worldwide Net Sales of Products (including Products referred to in subsection 3.2.4 below) of *** ;
3.2.3 *** of the Net Sales of any Epinephrine Products in the North America Territory; and
3.2.4 *** of the Net Sales of all Non-Epinephrine Products identified on Schedule 3.2.4 in the North America Territory.
(b) Senetek and Ranbaxy acknowledge and agree that with respect to (i) all Non-Epinephrine Products not identified on Schedule 3.2.4, or (ii) any Product sold by Ranbaxy outside of the North America Territory, during the period of sixty (60) days after Ranbaxy gives Notice to Senetek of its desire to sell a Non-Epinephrine Product not identified in Schedule 3.2.4 or a Product in a country outside the North America Territory, Senetek and Ranbaxy shall negotiate in good faith with respect to the payments to Senetek applicable to the Net Sales of such Products, on a case-by-case basis, provided that if the Parties are unable to reach agreement within such period, as the same may be extended by mutual agreement, the payment applicable to the Net Sales of such Products shall be determined by adjusting the payment applicable pursuant to Section 3.2.3 or 3.2.4, as the case may be, to reflect the difference between the Gross Margin expected by Ranbaxy to be realized from such Net Sales of a Non-Epinephrine Product not identified in Schedule 3.2.4 or a Product in a country outside the North America Territory, as the case may be, during the first full calendar year after the commercial launch thereof, to the Gross Margin realized by Ranbaxy during the preceding full calendar year from Non-Epinephrine Products identified on Schedule 3.2.4 or from Products sold in the North America Territory, as the case may be, provided further that either Party may, by Notice after the first full calendar year after such commercial launch, require re-adjustment of such amount not more often than once per calendar year based upon the actual Gross Margins actually realized from
*** Confidential treatment has been requested
11
such sales and the Gross Margins realized from sales referred to in Section 3.2.3 and 3.2.4 during the last preceding full calendar year. For purposes hereof, “Gross Margin” shall mean the fraction of which the numerator shall be the Net Sales less the fully absorbed cost of goods of a Product and the denominator shall be the Net Sales of such Product, determined in accordance with GAAP.
3.3 Payments. Beginning with the Calendar Quarter in which the First Commercial Sale of any of the Products is made in the Territory and for each Calendar Quarter thereafter, within sixty (60) days after the end of each Calendar Quarter, Ranbaxy shall provide Senetek with a quarterly reconciliation report, in the form attached hereto as Exhibit D, that reflects the calculation of Gross Sales and Net Sales by Product and country, during such quarter, the reconciliation amount to be paid to Senetek pursuant to Section 3.2, and the cumulative Net Sales of all Products since the First Commercial Sale of any of the Products. Each such statement shall be accompanied by payment of the amount set forth in such reconciliation. Ranbaxy shall make all payments required under this Agreement by wire transfer to such bank account or other depositary as may be directed by Senetek from time to time, in U.S. Dollars.
3.4 Audit Rights. Ranbaxy shall keep, and shall require each contract manufacturer and distributor of any Products to keep, true, accurate and complete records with respect to the calculation of the Gross Sales, Net Sales and Gross Margin by Product and by country, and the reconciliation amount paid to Senetek for a period of two (2) years after the year in which the sale of the Product generating the same occurred, and in sufficient detail to allow Senetek to confirm the accuracy of all payments made hereunder. At the request of Senetek, Ranbaxy and its Affiliates shall, and shall require each such contract manufacturer and distributor to, permit an independent certified public accountant appointed by Senetek, at reasonable times and upon reasonable Notice (but in no event more than once per calendar year), to examine those records and all other documents relating to or relevant to Gross Sales, Net Sales and the reconciliation amount paid to Senetek in the possession or control of Ranbaxy or its Affiliates, for a period of two (2) years after same have accrued, as may be necessary: (i) to determine the correctness of any report or payment made under this Agreement; or (ii) to obtain information as to same with respect to any Calendar Quarter in the event of Ranbaxy’s failure to report or pay pursuant to this Agreement. The accounting firm shall disclose to Senetek only whether such payments are correct, or the amount of the discrepancy if not correct, but not the specific details concerning any discrepancies. The results of any such examination shall be made available to both Parties. Senetek shall bear the full cost of the performance of any such audit except as hereinafter set forth. If, as a result of any inspection of the books and records of Ranbaxy or its Affiliates, it is shown that Ranbaxy’s payments under this Agreement were less than the amount which should have been paid, then Ranbaxy shall make all payments required to be made to eliminate any discrepancy revealed by said inspection within forty-five (45) days after Senetek’s demand therefor, and if the payments made by Ranbaxy were less than *** of the amount of payments that should have been paid with respect to the period in question, Ranbaxy shall also reimburse Senetek for the cost of such examination, not to exceed *** If, as a result of any inspection of the books and records of Ranbaxy or its Affiliates, it is shown that Ranbaxy’s payments under this Agreement were more than the amount which should have been paid, then Senetek shall make all payments required to be made to eliminate any overpayment revealed by said inspection within forty-five (45) days after Ranbaxy’s demand therefor.
*** Confidential treatment has been requested
12
3.5 Taxes. In the event that Ranbaxy is required to withhold from any payment to Senetek any tax or other like impost payable by Senetek, such amount shall be deducted from the payment to be made by Ranbaxy, and Ranbaxy shall promptly notify Senetek of such withholding and, within a reasonable amount of time after making such deduction, furnish Senetek with copies of any tax certificate or other documentation evidencing such withholding. Each Party agrees to cooperate with the other Party in claiming exemptions from such deductions or withholdings under any agreement or treaty from time to time in effect.
3.6 Further Assurances. Senetek hereby agrees to execute or cause to be executed and to deliver from time to time such other and further assignments and other instruments as Ranbaxy may reasonably request to further perfect and evidence the transfer and sale provided for in Section 3.1.
3.7 Closing. The closing of any sale and purchase of the Assets pursuant to Section 3.1 (the “Closing”) shall take place at the offices of St. Xxxx & Xxxxx, L.L.C., Xxx Xxxx Xxxxx Xxxx, Xxxxxx, Xxx Xxxxxx 00000, unless another place, date and time is mutually agreed upon by Senetek and Ranbaxy. The Closing shall be deemed to be effective as of 6:00 PM on the Effective Date.
3.8 Liabilities. Ranbaxy shall not be the successor to Senetek and is not required to, does not and shall not assume, pay, perform, defend, discharge or guarantee or be deemed to have assumed, paid, performed, defended or discharged or guaranteed, or otherwise be responsible for any Excluded Liability. With respect to the action disclosed on Schedule 2.2.7, Senetek shall be responsible for the control and defense, as well as the satisfaction of all fees and expenses, of such action, subject to Senetek’s right to abandon the same and Ranbaxy’s right to assume the same as provided in Section 4.7. Senetek shall keep Ranbaxy informed about the status and progress of such action, including any difficulties encountered by Senetek during the course of such action, no less than on a monthly basis. RANBAXY SHALL ASSUME NO DEBTS, LIABILITIES, OBLIGATIONS OR COMMITMENTS OF SENETEK (WHETHER KNOWN OR UNKNOWN, ASSERTED OR UNASSERTED, FIXED, ABSOLUTE OR CONTINGENT, MATURED OR UNMATURED, ACCRUED OR UNACCRUED, LIQUIDATED OR UNLIQUIDATED, DUE OR TO BECOME DUE WHENEVER OR HOWEVER ARISING) WHATSOEVER.
3.9 Senetek Closing Deliveries. Senetek shall make the following deliveries at Closing, each in form and substance acceptable to the receiving party:
(a) deliver to Ranbaxy such bills of sale, endorsements, assignments and other good and sufficient instruments of sale, assignment, conveyance and transfer, including the Closing deliveries provided in Section 3.1, satisfactory in form and substance to Ranbaxy and its counsel, as shall be required to vest in Ranbaxy all of Senetek’s right, title and interest in and to the Assets. In addition, at the Closing, Senetek shall deliver to Ranbaxy such consents and assignments, in form and substance satisfactory to Ranbaxy and its counsel, to permit the assignment to Ranbaxy of all those leases, contracts, agreements, commitments or other undertakings comprising a part of the Assets;
13
(b) deliver to Ranbaxy certified copies of the resolutions of the Board of Directors of Senetek authorizing the execution and delivery of this Agreement and the transactions contemplated hereby;
(c) deliver copies of all UCC-3 Termination Statements which evidence the termination of any and all liens and encumbrances affecting the Assets as well as evidence of receipt of the consents and approvals described in Schedule 2.1.3 hereto;
(d) deliver an opinion of Senetek’s counsel to Ranbaxy, dated the Effective Date, in the form of Exhibit E attached hereto;
(e) deliver a certificate of good standing for Senetek dated within thirty (30) days of the Effective Date, issued by the Secretary of State of the state or jurisdiction of incorporation of Senetek and of each state in which Senetek is qualified to do business;
(f) deliver all of the certificates, documents, contracts, assignments, opinions, records, schedules and other exhibits required by this Agreement;
(g) deliver a certificate executed by the Secretary of Senetek certifying as of the Effective Date (i) a true and complete copy of the Articles of Association of Senetek; and
(h) deliver any other documents or instruments of conveyance and transfer as Ranbaxy may reasonably request not less than ten (10) days prior to the Closing Date for the purpose of assigning, transferring, granting, conveying and confirming the Assets to Ranbaxy.
Ranbaxy shall accept possession of the Assets, wherever located, as identified by Senetek by Notice as provided in Article 12, at or immediately prior to the Effective Date, and shall be responsible for removing all of the Tangible Assets and arranging with a third party storage facility for the storage by such third party of such Assets until such time as Ranbaxy transfers such Assets to its manufacturing facility.
3.10 Ranbaxy Closing Deliveries. At the Closing, in addition to any other documents specifically required to be delivered pursuant to this Agreement, Ranbaxy shall:
(a) pay the Closing Payment by wire transfer to such bank account or other depositary as directed at Closing by Senetek, in U.S. Dollars;
(b) deliver a certificate of good standing of Ranbaxy from the Florida Secretary of State dated within thirty (30) days of the Closing Date;
(c) deliver to Senetek the opinion of counsel for Ranbaxy, St. Xxxx & Xxxxx, L.L.C., in the form of Exhibit F, attached hereto, dated the Effective Date; and
(d) deliver to Senetek certified copies of the resolutions of the Board of Directors of Ranbaxy authorizing the execution and delivery of this Agreement and the transactions contemplated hereby.
14
4. PERFORMANCE AFTER THE EFFECTIVE DATE
4.1 Product Development and Regulatory Approvals. Commencing promptly after the Effective Date, Ranbaxy shall use Commercially Reasonable Efforts in the development and commercialization of the Products in the United States, including (i) the transportation and installation of the Reliaject Equipment and related materials and supplies to an appropriate contract manufacturing facility (the location and particulars of which shall be disclosed to Senetek prior to such installation) and commercial validation of the manufacturing processes for production of the Epinephrine Product in compliance with Section 4.2, (ii) submission of a full and complete ANDA filing, or equivalent, to the FDA for the Reliaject Device pre-filled with epinephrine for the emergency treatment of anaphylaxis, and (iii) establishment of a marketing and distribution program complying with Section 4.3 for commercialization of the Products in the United States. Notwithstanding the foregoing, however, should an Interfering Event exist or otherwise arise prior to Ranbaxy’s development of the Products (whether existing as of the Effective Date or arising prior to or during the development, promotion, sale or distribution of the Products), which materially restricts or impairs Ranbaxy’s ability to develop the Products’ ANDAs, Ranbaxy may delay or suspend the development, manufacture, sale, promotion and/or distribution of the Products until the Interfering Event no longer has such material effect. For purposes of this Section 4.1, an “Interfering Event” shall include (a) any decision, order, decree, ruling, etc., of any court, legislative, governmental or administrative body, or any regulatory changes affecting the development, sale, use, distribution and or marketing of the Products; (b) any pending claim that the products or any intellectual property used by Senetek in the development or manufacture of such Product infringes any intellectual property rights of any Third Party. Notwithstanding the foregoing, if Ranbaxy receives notice of a threatened claim by a Third Party of any Intellectual Property infringement relating to the Products, Ranbaxy shall notify Senetek to discuss its potential effect on the Assets and the Intellectual Property. Nothing in this Section 4.1 shall impair or otherwise restrict Ranbaxy’s right of termination as provided in Sections 6.2 and 6.3 hereof. In addition, Senetek acknowledges and agrees that Ranbaxy does not warrant or covenant that the Products will be submitted for applicable regulatory approval (including, without limitation, an ANDA with the FDA), receive applicable regulatory approval (including, without limitation, approval for an applicable Product ANDA from the FDA) or be successfully marketed or commercialized.
4.2 Manufacturing. Ranbaxy shall, and shall cause any contract manufacturer(s) to, manufacture the Products in accordance with cGMPs promulgated by the FDA and pursuant to the DMF and/or the procedures filed with the FDA (and any other Regulatory Authorities) via an ANDA or other marketing approval or exemption. Ranbaxy shall be responsible for the validation of all manufacturing processes and processing systems and shall establish programs for change control for the validated process, systems, and computer systems. Ranbaxy shall assure that the Products, at the time of shipment, shall not be adulterated or misbranded within the meaning of the FDC Act, as such FDC Act is constituted and effective at the time of shipment, and shall not be articles which may not under the provisions of Sections 404 and 505 of such FDC Act be introduced into interstate commerce. At any time during the Term of this
15
Agreement, Ranbaxy shall permit Senetek’s representatives to inspect, upon fifteen (15) days’ prior Notice and during normal business hours (but in no event more than once per calendar year), Ranbaxy’s and its contract manufacturer(s)’ facilities where the Product is manufactured or stored to review Ranbaxy’s compliance with this Section 4.2, including review of all batch records pertaining to production of Devices and Products and all other regulatory compliance documentation. In the event such representatives conclude that any non-conformity with this Agreement is continuing, the Parties, in addition to any other rights or remedies available to such Parties, shall use their respective diligent efforts to resolve the issue as promptly as possible. In addition, Ranbaxy shall promptly notify Senetek of any inspection by the FDA or any other Regulatory Authorities of any facility where the Product is manufactured or stored where such inspection could result in an adverse effect on the Product, and provide a summary of the results of that inspection and corrective actions taken or to be taken.
4.3 Sales and Marketing. During the term of this Agreement, Ranbaxy shall use Commercially Reasonable Efforts to promote, sell and distribute the Products within the Territory, with initial emphasis on the United States and subject to the receipt of approval from an applicable Regulatory Authority to manufacture, market, sell and/or distribute the applicable Product in such country of the Territory. Furthermore, Ranbaxy agrees that it shall use Commercially Reasonable Efforts to satisfy the demand for the Products throughout the Territory. Subject to the foregoing, Ranbaxy shall control and make all decisions regarding the strategy and tactics of marketing, selling and otherwise commercializing the Products hereunder, including, without limitation, the methods of sale and distribution, organization and management of sales and marketing, packaging and labeling, appointment of distributors hereof, and other terms and conditions of such sales and marketing, and shall exercise Commercially Reasonable Efforts in such regard. Ranbaxy shall have the right to take into consideration commercial and business factors when making any such determination, and in making such determinations, shall act in accordance with its reasonable business practices and judgment in such regard and in a manner consistent with which Ranbaxy evaluates for commercialization other Ranbaxy products of comparable market size in the Territory. Upon the First Commercial Sale of the Products in the Territory, Ranbaxy, either itself or through its Affiliates, distributors or sublicensees, shall market, distribute and sell the Products in the Territory under the Ranbaxy label to the extent reasonable in light of the size of the market and potential market for the Products and in a manner consistent in which it markets other Ranbaxy products of comparable market size in the Territory.
4.4 Adverse Experience Reporting. Ranbaxy shall report to Senetek any information of which it obtains knowledge concerning any adverse drug experience in connection with the use of the Products, including the incidence or severity thereof, whether the same is associated with non-clinical toxicity studies, clinical uses, studies, investigations or tests. Reports of routine adverse drug experiences of the type defined in Section 214.80 of Title 21 of the CFR shall be provided by Ranbaxy on a Calendar Quarter basis. Reports of serious adverse drug experiences of the type defined in Sections 312.32 and 314.80 of Title 21 of the CFR shall be provided by Ranbaxy within five (5) days after a Party becomes aware of such serious adverse drug experience. Notwithstanding the foregoing, Ranbaxy shall control the process of reporting of adverse drug experiences of the type specified above, including, without limitation, sole communication and/or correspondence with the applicable governmental agency.
16
4.5 Maintenance of Assets. Subject to Sections 6.2 and 6.3, Ranbaxy shall use Commercially Reasonable Efforts to maintain all of the Reliaject Equipment in good operating condition and repair. Promptly following the Effective Date, Ranbaxy will take all actions required to record and register the transfer and assignment into Ranbaxy’s name of the Patents and Trademarks, and shall maintain the Patents and Trademarks in good standing, including without limitation by timely payment of all renewal fees and the filing of affidavits of use or other documentation required therefore, and shall furnish to Senetek upon its request reasonable evidence of its compliance therewith.
4.6 Production of the Reliaject Device Pre-filled with Invicorp. Subject to any regulatory or other third party restrictions (for which Ranbaxy shall have no responsibility), Ranbaxy agrees to negotiate in good faith with the licensees or other acquirer(s) of Senetek’s rights to its erectile dysfunction treatment Invicorp® in Europe, the United States or elsewhere, including, without limitation, Ardana pursuant to the Ardana License, for Ranbaxy to contract manufacture the Reliaject Device pre-filled with Invicorp for such licensees and other acquirer(s). Unless otherwise agreed between Ranbaxy and Ardana, the Ardana License will be on a basis (i) reimbursing all of Ranbaxy’s fully absorbed manufacturing costs, including direct costs of manufacture (including labor, materials, tools, dies, molds, downtime for retooling and other overhead) and reasonable overhead allocation, plus a profit margin, which in no event shall be less than Ranbaxy’s average fully loaded net profit margin on sales of Epinephrine Products from time to time, and (ii) permitting Ranbaxy preferential treatment for its own production of Products in terms of scheduling available plant capacity. Notwithstanding the foregoing, Ranbaxy shall be under no obligation to expand plant capacity beyond its then current production.
4.7 Conduct of Reliaject Trademark Opposition. Senetek shall use Commercially Reasonable Efforts to defend against the Opposition proceeding involving the United States application for the Reliaject trademark referred to in Schedule 2.2.7 until such time, if any, as it shall be advised by its counsel of record that it is more likely than not that the Opposition will be successful. If Senetek shall be so advised, it shall give Notice to Ranbaxy within ten (10) days of receipt of such advice, and Ranbaxy shall then have thirty (30) days in which to give Notice to Senetek that it will assume control and defense thereof, as well as the satisfaction of all fees and expenses, if any, of such action. If Ranbaxy shall not give such Notice, Senetek shall be free to discontinue or settle such proceeding in its sole discretion. Subject to Senetek’s compliance with this Section 4.7, Ranbaxy waives any claim against Senetek based upon such proceeding or the outcome thereof, including any claim for damages or compensation for loss of the Reliaject trademark.
4.8 Suspension of Certain Performance Obligations. If at any time during the Term, an Interfering Event should arise or exists which restricts or impairs Ranbaxy’s ability to develop, market, distribute and/or commercialize the Products, Ranbaxy may delay or suspend the development or commercialization of the Products until the Interfering Event ceases to materially adversely effect such development, marketing, distribution and/or commercialization; provided that if the performance so suspended materially affects Ranbaxy’s covenants under Section 4.1 or 4.3 and the period of such suspension exceeds twenty-four (24) months, then unless the parties mutually agree in writing to the contrary, Senetek shall have the same rights as if it had terminated this Agreement pursuant to Section 6.2 and Ranbaxy shall have the same rights as if it had terminated this Agreement pursuant to Section 6.3.
17
5. CONFIDENTIALITY.
5.1 Confidential Treatment. Any Party receiving any Confidential Information (a “Receiving Party”) from the other Party (a “Disclosing Party”) in connection with the execution, delivery and performance of this Agreement agrees that all such Confidential Information (i) shall not be used by the Receiving Party except in connection with the activities contemplated by this Agreement or in order to further the purposes of this Agreement, (ii) shall be maintained in confidence by the Receiving Party and (iii) shall not be disclosed by the Receiving Party to any Third Party who is not a consultant of, or an advisor to, the Receiving Party or an Affiliate or sublicensee of the Receiving Party, without the prior written consent of the Disclosing Party.
5.2 Exceptions. The obligations of confidentiality and non-use set forth in Section 5.1 shall not apply to any such Confidential Information which:
5.2.1 either before or after the date of the disclosure to the Receiving Party becomes published or otherwise part of the public domain through no fault or omission on the part of the Receiving Party or its Affiliates;
5.2.2 either before or after the date of the disclosure to the Receiving Party is lawfully disclosed to the Receiving Party or its Affiliates by sources other than the Disclosing Party rightfully in possession of the Confidential Information and without restriction as to confidentiality or use;
5.2.3 was known or used by the Receiving Party or its Affiliates prior to its date of disclosure to the Receiving Party as demonstrated by legally admissible evidence available to the Receiving Party or its Affiliates;
5.2.4 is independently developed by or for the Receiving Party or its Affiliates without reference to or in reliance upon the Disclosing Party’s Confidential Information as demonstrated by competent written records; or
5.2.5 is required to be disclosed under applicable laws or regulations or by an order by a court or other regulatory body having competent jurisdiction; provided, however, that except where impracticable, the Receiving Party shall give the Disclosing Party reasonable advance Notice of such disclosure requirement (which shall include a copy of any applicable subpoena or order) and shall cooperate with the Disclosing Party to oppose, limit or secure confidential treatment for such required disclosure. In the event of any such required disclosure, the Receiving Party shall disclose only that portion of the Confidential Information of the Disclosing Party that the Receiving Party is legally required to disclose.
5.3 Term of Obligation. The obligations of the Parties under this Article 5 shall continue for a period of five (5) years after the expiration or earlier termination of the Term of this Agreement.
18
5.4 Return of Materials. Except as otherwise provided in Section 6.3, upon expiration or termination of this Agreement, the Receiving Party shall return to the Disclosing Party all Confidential Information of the Disclosing Party that the Receiving Party possesses in tangible form and shall return to the Disclosing Party, or destroy, at the Disclosing Party’s request, all Confidential Information of the Disclosing Party that the Receiving Party possesses in electronic form. Notwithstanding the foregoing, each Party may retain one copy of all Confidential Information provided by the other Party solely for archival and legal purposes.
6 TERM AND TERMINATION
6.1 The Term. The term of Ranbaxy’s obligation the pay the amounts provided for in Section 3.2 and to comply with Article 4 (the “Term”), shall expire fifteen (15) years from the date of its First Commercial Sale of the applicable Product unless a Competing Product shall be marketed or sold in the United States, in which event such fifteen (15) year period shall be reduced to ten (10) years from the date of its First Commercial Sale, and provided further that, without limiting the provisions of Sections 4.1, 4.3 and 4.7, Ranbaxy’s obligation to pay the amounts provided for in Section 3.2 shall be suspended during the existence of an Interfering Event or the effectiveness of any order of a court of competent jurisdiction binding upon Ranbaxy (which order has become final on appeal or the time to appeal has expired) enjoining Ranbaxy from manufacturing and marketing the Products (it being agreed that Ranbaxy shall give Senetek prompt Notice of any such threatened or pending injunctive proceedings).
6.2 Termination for Cause. Either Party (the “Non-breaching Party”) may, without prejudice to any other remedies available to it at law or in equity, terminate this Agreement in the event the other Party (the “Breaching Party”) shall have (i) materially breached any of its representations set forth in Article 2 or (ii) materially breached or defaulted in the performance of any of its material obligations under this Agreement and (if curable) such material breach or default in performance shall have continued for sixty (60) days after Notice thereof was provided to the Breaching Party by the Non-breaching Party (or, if such curable default cannot be cured within such sixty (60)-day period, if the Breaching Party does not commence and diligently continue actions to cure such default during such sixty (60)-day period and effect such cure within a reasonable period). Any such termination shall become effective at the end of such sixty (60)-day or longer reasonable period unless the Breaching Party has cured any such breach or default prior to the expiration of such period.
6.3 Termination by Ranbaxy. Ranbaxy may terminate this Agreement in the event that Ranbaxy, notwithstanding compliance with its obligations pursuant to Sections 4.1 and 4.2, fails to secure an ANDA or equivalent approval of the FDA for the Epinephrine Product for the emergency treatment of anaphylaxis within twenty four (24) months after its submission of a full and complete ANDA or equivalent application. Ranbaxy shall give Senetek sixty (60) days Notice of any determination to terminate pursuant to this Section 6.3 and will meet and confer with Senetek prior to effecting such termination.
6.4 Disposition of the Assets in Certain Events. In the event that Ranbaxy terminates this Agreement pursuant to Sections 6.3, or Senetek terminates this Agreement pursuant to
19
Section 6.2 based on Ranbaxy’s breach of its obligations contained in Article 4 herein, Ranbaxy and Senetek shall discuss and agree upon a mutually acceptable plan for the utilization and/or disposition of the Assets, provided, however, that in the case of a termination of this Agreement by Ranbaxy pursuant to Section 6.3, if the parties are unable to reach agreement with respect to a plan for utilization and/or disposition of the Assets, the Assets shall be disposed of to the highest bidder and the proceeds of disposition shall be divided evenly between Ranbaxy and Senetek, provided further that this Section 6.4 shall not apply if Ranbaxy fails to make a payment to Senetek pursuant to Section 3.3 in a timely manner.
7. ASSIGNMENT AND BINDING EFFECT. Neither this Agreement, nor any obligations or rights hereunder, shall be assignable by any Party hereto without the prior written consent of the other Party. Notwithstanding the above either Party may assign this Agreement, or any obligations or rights hereunder, in whole or in part, without the consent of the other Party to its Affiliates, if the assigning Party guarantees the full performance of its Affiliates’ obligations hereunder, or in connection with the sale or transfer of all or substantially all of its assets relating to this Agreement, whether by merger, sale of stock, operation of law or otherwise. Ranbaxy may subcontract any obligations under this Agreement, in whole or in part, without the consent of the other Party, if the subcontracting Party agrees to the full performance of its subcontractors, and such subcontractor agrees to be bound by the obligations of confidentiality and non-use set forth in Article 5 of this Agreement. Any purported assignment in contravention of this Article 7 shall, at the option of the non-assigning Party, be null and void and of no effect.
8. INDEMNIFICATION.
8.1 Indemnification by Ranbaxy. Ranbaxy shall indemnify and hold Senetek and its directors, officers, shareholders, employees, servants and agents harmless from and against any and all liabilities, claims, demands, actions, suits, losses, damages, costs and expenses (including reasonable attorney’s fees and disbursements, but excluding any anticipated or actual lost profits or revenues or other special, indirect, incidental or consequential damages), resulting (i) from Ranbaxy’s manufacturing, handling, storage, promotion, use, marketing, distribution or sale of the Products, or (ii) from Ranbaxy’s negligence, willful misconduct or breach of this Agreement (including, without limitation, of the representations and warranties hereunder); except to the extent caused by Senetek’s negligence or willful misconduct or its breach of this Agreement (including, without limitation, of the representations and warranties hereunder).
8.2 Indemnification by Senetek. Senetek shall indemnify and hold Ranbaxy and its directors, officers, shareholders, employees, servants and agents harmless from and against any and all liabilities, claims, demands, actions, suits, losses, damages, costs and expenses (including reasonable attorney’s fees and disbursements, but excluding any anticipated or actual lost profits or revenues or other special, indirect, incidental or consequential damages), resulting from (i) Senetek’s breach of this Agreement (including, without limitation, of the representations and warranties hereunder) or (ii) any Excluded Liability; except to the extent caused by Ranbaxy’s negligence or willful misconduct or its breach of this Agreement (including, without limitation, of the representations and warranties hereunder). Ranbaxy shall be entitled to set off against any amounts due and payable by it to Senetek hereunder any amounts which Senetek is contractually obligated to pay to Ranbaxy pursuant to this Section 8.2.
20
8.3 Procedure. In the event that any person entitled to indemnification under this Article 8 (an “Indemnitee”) is seeking such indemnification, such Indemnitee shall send Notice to the other Party (the “Indemnitor”) of the claim as soon as reasonably practicable after such Indemnitee receives notice of such claim, shall permit the Indemnitor to assume direction and control of the defense of the claim (including the sole right to settle it at the sole discretion of the Indemnitor, provided that such settlement provides for the unconditional release of the Indemnitee and does not impose any obligation on the Indemnitee or the other Party) and shall cooperate as requested (at the expense of the Indemnitor) in the defense of the claim.
8.4 Complete Indemnification. As the Parties intend complete indemnification, all costs and expenses incurred by an Indemnitee in connection with enforcement of this Article 8 shall also be reimbursed by the Indemnitor.
8.5 DISCLAIMER. NOTWITHSTANDING ANYTHING TO THE CONTRARY CONTAINED HEREIN, NEITHER PARTY SHALL BE LIABLE TO THE OTHER FOR ANY SPECIAL, INCIDENTAL, CONSEQUENTIAL OR CONTINGENT DAMAGES OR EXPENSES, INCLUDING BUT NOT LIMITED TO DAMAGES FOR LOST PROFITS, LOSS OF OPPORTUNITY OR USE OF ANY KIND SUFFERED BY THE OTHER PARTY, WHETHER IN CONTRACT, TORT OR OTHERWISE, OR ARISING OUT OF, CONNECTED WITH OR RESULTING FROM THE PERFORMANCE OF THEIR RESPECTIVE OBLIGATIONS UNDER THIS AGREEMENT OR OUT OF THE USE OR SALE OF THE ASSETS OR THE PRODUCTS.
9. INSURANCE.
Each Party shall use Commercially Reasonable Efforts to maintain insurance (including general and product liability coverage) and upon such terms (including coverages, deductible limits and self-insured retentions) as is customary for the activities to be conducted by it under this Agreement and is appropriate to cover its indemnification obligations hereunder. In satisfaction of its obligation under this Article 9, Senetek shall maintain for the entire Term of this Agreement liability insurance coverage of *** per occurrence/*** annual aggregate and Ranbaxy shall maintain for the entire Term of this Agreement product liability coverage for the Products of not less than *** per occurrence/*** annual aggregate and property casualty coverage on the Reliaject Equipment of not less than *** per occurrence. Each Party shall be named as an additional insured under the other Party’s policies with respect to claims arising out of the manufacture, use or sale of Product or otherwise under this Agreement. Each Party shall furnish to the other Party evidence of such insurance, upon request.
10. ARBITRATION.
Any dispute arising out of or relating to any provisions of this Agreement shall be finally settled by arbitration to be held in Newark, New Jersey, under the auspices and then current Commercial Arbitration Rules of the American Arbitration Association (the “AAA”). The
*** Confidential treatment has been requested
21
arbitration shall be conducted by one arbitrator who is knowledgeable in the subject matter which is at issue in the dispute and who is selected by mutual agreement of the Parties or, failing such agreement, shall be selected according to the AAA rules. The Parties shall have such discovery rights as the arbitrator may allow, but in no event broader than that discovery permitted under the Federal Rules of Civil Procedure. In conducting the arbitration, the arbitrator(s) shall apply the New Jersey Rules of Evidence and shall be able to decree any and all relief of an equitable nature, including but not limited to such relief as a temporary restraining order, a preliminary injunction, a permanent injunction, or replevin of property, as well as specific performance. The arbitrator(s) shall also be able to award direct damages but shall not award any other form of damages (e.g., consequential, punitive or exemplary damages). The reasonable fees and expenses of the arbitrator(s), along with the reasonable legal fees and expenses of the Parties (including all expert witness fees and expenses), the fees and expenses of a court reporter, and any expenses for a hearing room, shall be paid as follows: (i) if the arbitrator(s) rule in favor of one Party on all disputed issues in the arbitration, the losing Party shall pay *** of such fees and expenses; if the arbitrator(s) rule in favor of one Party on some issues and the other Party on other issues, the arbitrators shall issue with the rulings a written determination as to how such fees and expenses shall be allocated between the Parties. The decision of the arbitrators shall be final and may be entered, sued on or enforced by the Party in whose favor it runs in any court of competent jurisdiction at the option of such Party. Whether a claim, dispute or other matter in question would be barred by the applicable statute of limitations, which statute of limitations also shall apply to any claim or disputes subject to arbitration under this Article 10, shall be determined by binding arbitration pursuant to this Article 10. Notwithstanding the foregoing, nothing contained herein shall prohibit a Party from seeking injunctive relief from a court of competent jurisdiction in the event of a breach or prospective breach of this Agreement by the other Party which would cause irreparable harm to the non-breaching Party.
11. FORCE MAJEURE.
Any delay in the performance of any of the duties or obligations of either Party hereto (except the payment of money due hereunder) shall not be considered a breach of this Agreement, and the time required for performance shall be extended for a period equal to the period of such delay, if such delay has been caused by or is the result of acts of God; acts of public enemy; insurrections; riots; injunctions; embargoes; labor disputes, including strikes, lockouts, job actions, or boycotts; fires; explosions; floods; shortages of material or energy; governmental prohibition or restriction; the unavailability of active raw material that is being supplied by Third Parties; or other unforeseeable causes beyond the reasonable control and without the fault or negligence of the Party so affected. The Party so affected shall give prompt notice to the other Party of such cause, and shall take whatever reasonable steps are necessary to relieve the effect of such cause as rapidly as reasonably possible.
12. NOTICES.
Any notice or communication required or permitted to be given under or in connection with this Agreement (a “Notice”) shall be deemed to have been sufficiently given if in writing and personally delivered or sent by certified mail (return receipt requested), facsimile
22
transmission (receipt verified), or overnight express courier service (signature required), prepaid, to the Party for which such notice is intended as follows:
| (i) If to Ranbaxy, to: | Ranbaxy Pharmaceuticals Inc. | |||
| 0000 Xxxxxxx Xxxxxx Xxxxxxxxx | ||||
| Xxxxxxxxxxxx, Xxxxxxx 00000 | ||||
| Attention: | Vice President | |||
| Facsimile: | (000) 000-0000 | |||
| Telephone: | (000) 000-0000 | |||
| With a copy to: | Ranbaxy Inc. | |||
| 000 Xxxxxxx Xxxx Xxxx | ||||
| Xxxxxxxxx, Xxx Xxxxxx 00000 | ||||
| Attention: | President | |||
| Facsimile: | (000) 000-0000 | |||
| Telephone: | (000) 000-0000 | |||
| (ii) If to Senetek, to: | Senetek PLC | |||
| 000 Xxxxxx Xxxxx | ||||
| Xxxx, Xxxxxxxxxx 00000 | ||||
| Attention: Xxxxx X. Xxxxxxx | ||||
| Facsimile: 000-000-0000 | ||||
| Telephone: 000-000-0000 | ||||
or to such other address as the addressee shall have last furnished in writing to the addressor; such Notice shall be effective upon receipt by the addressee. If delivered personally or by facsimile transmission, the date of delivery shall be deemed to be the date on which such notice or request was given. If sent by overnight express courier service, the date of delivery shall be deemed to be the next business day after such notice or request was deposited with such service. If sent by certified mail, the date of delivery shall be deemed to be the third (3rd) business day after such notice or request was deposited with the U.S. Postal Service.
13. BOOKS AND RECORDS.
Any books and records to be maintained under this Agreement by a Party or its Affiliates shall be maintained in the continental United States, and any such financial books and records shall be prepared in accordance with GAAP, consistently applied.
14. GOVERNING LAW.
This Agreement shall be governed by the laws of the State of New York, in all respects of validity, construction and performance thereof, without regard to principles of conflicts of law.
23
15. MISCELLANEOUS.
15.1 Relationship of Parties. Nothing in this Agreement is intended or shall be deemed to constitute a partnership, agency, employer-employee or joint venture relationship between the Parties. No Party shall incur any debts or make any commitments for the other, except to the extent, if at all, specifically provided herein.
15.2 Further Actions. Each Party shall execute, acknowledge and deliver such further instruments, and do all such other acts, as may be necessary or appropriate in order to carry out the purposes and intent of this Agreement.
15.3 Use of Name; Publicity. Except as otherwise provided herein, (i) neither Party shall have any right, express or implied, to use in any manner the name or other designation of the other Party or any other trade name, trademark or logos (including the Trademark) of the other Party for any purpose in connection with the performance of this Agreement; and (ii) neither Party shall make any public announcement concerning this Agreement or the subject matter hereof without the prior written consent of the other Party; provided however, that nothing in this Section 15.3 shall prevent either Party from issuing statements that such Party determines to be necessary to comply with applicable law (including the disclosure requirements of the U.S. Securities and Exchange Commission, NASDAQ or any other stock exchange on which securities issued by such Party are traded), and provided further that the parties agree that on the Effective Date a press release in the form annexed as Schedule 15.3 shall be issued.
15.4 Waiver. A waiver by either Party of any of the terms and conditions of this Agreement in any instance shall not be deemed or construed to be a waiver of such term or condition for the future, or of any subsequent breach hereof. All rights, remedies, undertakings, obligations and agreements contained in this Agreement shall be cumulative and none of them shall be in limitation of any other remedy, right, undertaking, obligation or agreement of either Party.
15.5 Severability. When possible, each provision of this Agreement will be interpreted in such manner as to be effective and valid under applicable law, but if any provision of this Agreement is held to be prohibited by or invalid under applicable law, such provision will be ineffective only to the extent of such prohibition or invalidity, without invalidating the remainder of this Agreement.
15.6 Amendment. No amendment, modification or supplement of any provisions of this Agreement shall be valid or effective unless made in writing and signed by a duly authorized officer of each Party.
15.7 Entire Agreement. This Agreement together with the schedules and exhibits hereto, sets forth the entire agreement and understanding between the Parties as to the subject matter hereof and merges all prior discussions and negotiations between them, and neither of the Parties shall be bound by any conditions, definitions, warranties, understandings or representations with respect to such subject matter other than as expressly provided herein or as duly set forth on or subsequent to the date hereof in writing and signed by a proper and duly authorized officer or representative of the Party to be bound thereby.
24
15.8 Parties in Interest. All of the terms and provisions of this Agreement shall be binding upon, inure to the benefit of and be enforceable by the Parties hereto and their respective permitted successors and assigns.
15.9 Descriptive Headings. The descriptive headings of this Agreement are for convenience only, and shall be of no force or effect in construing or interpreting any of the provisions of this Agreement.
15.10 Counterparts. This Agreement may be executed simultaneously in any number of counterparts, any one of which need not contain the signature of more than one Party but all such counterparts taken together shall constitute one and the same agreement.
[The remainder of this page left intentionally blank]
25
IN WITNESS WHEREOF, the Parties hereto have caused this Agreement to be executed by their duly authorized representative as of the Effective Date.
| RANBAXY PHARMACEUTICALS INC. | SENETEK PLC | |||||||
| By: | /s/ Xxxxx Xxxxxx |
By: | /s/ Xxxxx X. Xxxxxxx | |||||
| Name: | Xxxxx Xxxxxx | Name: | Xxxxx X. Xxxxxxx | |||||
| Title: | Vice President – Sales, Marketing and Distribution | Title: | Chairman and Chief Executive Officer | |||||
26
Schedule 1.12
The Devices
A. The Reliaject Device
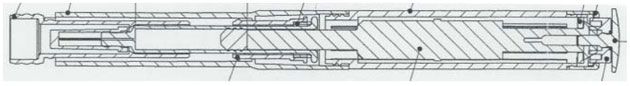
An automatic medicament injector employing a non-coring needle having side port geometry optimized to minimize or eliminate the coring of a rubber seal or septum when impaled by the internal needle tip of the cannula, fitted with a 27g needle and used for an epinephrine injection for allergic emergencies (anaphylaxis), 1 mg/mL (subcutaneous auto-injection), delivering 0.3 mg epinephrine.
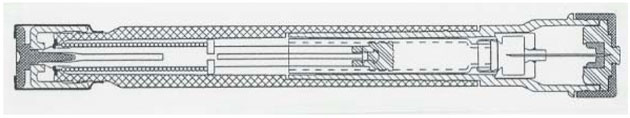
B. The OM Device—Autoject Mini for Epinephrine
An automatic medicament injector designed as a reusable device for use with a BD Hypak 1.0 xx xxxx glass syringe, with a fixed 27g x 1/2” needle and a soft needle shield, to be used for an epinephrine injection for allergic emergencies (anaphylaxis,. The device is designed to produce an extended needle length of 10mm +/- 1.5mm beyond the end of the syringe housing, and will deliver a dose of 0.3ml +/-10%.
27
SCHEDULE 1.31
THE PATENTS
| Country |
Patent No. | Filing Date | Issue Date | Title |
Expires | Status | Note | |||||||
| Argentina |
253898 | 10/4/1993 | 01/11/00 | Med Injector/Methods | 01/11/15 | Granted | ||||||||
| Australia |
671322 | 9/30/1993 | 12/10/96 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Canada |
2125179 | 9/30/1993 | Med Injector/Methods | 09/30/13 | Pending | |||||||||
| China |
93118246.8 | 10/5/1993 | Med Injector/Methods | 10/05/08 | Pending | |||||||||
| Denmark |
0616541 | 9/30/1993 | 11/25/98 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Egypt |
20095 | 10/4/1993 | 07/31/97 | Med Injector/Methods | 10/04/08 | Granted | ||||||||
| France |
0616541 | 9/30/1993 | 11/25/98 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Germany |
69322249.2 | 9/30/1993 | 11/25/98 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Ireland |
0616541 | 9/30/1993 | 11/25/98 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Israel |
107038 | 9/20/1993 | 07/16/97 | Med Injector/Methods | 09/20/13 | Granted | ||||||||
| Italy |
0616541 | 9/30/1993 | 11/25/98 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Japan |
509348/94 | 9/30/1993 | Med Injector/Methods | Pending | ||||||||||
| Kuwait |
77PA/93 | 9/29/1993 | Med Injector/Methods | Pending | ||||||||||
| Malaysia |
MY-109675 | 10/1/1993 | 04/30/97 | Med Injector/Methods | 04/30/11 | Granted | ||||||||
| Mexico |
187163 | 10/4/1993 | 11/28/97 | Med Injector/Methods | 10/04/13 | Granted | ||||||||
| New Zealand |
256977 | 9/30/1993 | 12/10/96 | Med Injector/Methods | 09/30/09 | Granted | ||||||||
| Phillipines |
31811 | 10/4/1993 | 02/02/99 | Med Injector/Methods | 10/04/13 | Granted | ||||||||
| Saudi Arabia |
94032104 | 12/29/1993 | Med Injector/Methods | Pending | ||||||||||
| South Korea |
317900 | 9/30/1993 | 12/05/01 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Spain |
0616541 | 9/30/1993 | 11/25/98 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Syria |
4512 | Med Injector/Methods | 10/03/08 | Pending | ||||||||||
| Taiwan |
069424 | 10/2/1993 | 12/21/94 | Med Injector/Methods | 10/02/13 | Granted | ||||||||
| Turkey |
27115 | 10/4/1993 | 11/08/94 | Med Injector/Methods | 10/04/08 | Granted | ||||||||
| UK |
0616541 | 9/30/1993 | 11/25/98 | Med Injector/Methods | 09/30/13 | Granted | ||||||||
| Uruguay |
23661 | 10/5/1993 | Med Injector/Methods | 10/05/13 | Pending | |||||||||
| Venezuela |
0-0000-00 | 10/4/1993 | Med Injector/Methods | 10/04/13 | Granted | |||||||||
| U.S. |
5,354,287 MUST BE IN ALL FOREIGN COUNTRIES |
33,254 | 10/11/94 | Program Auto Injector/Vial w Retract Needle |
10/11/13 | Granted | 54791 App 956,952 incorporated | |||||||
| U.S. |
5,709,668 MUST BE RETAINED IN US |
34,856 | 01/20/98 | Auto Med Injector Employ NonCoring Needle |
06/06/15 | Granted |
28
SCHEDULE 1.36
THE RELIAJECT EQUIPMENT
| Machine |
Specifications |
Model No | ||
| Automated Powerpak Assembly Machine Acro Automation Systems |
Overall dimen.: 25’ long x 13’ wide x 10’ high Power: 230V, 50Hz, 3 phase Air Supply: 85 PSI Production rate: 30 per minute |
98-2121 | ||
| Automated Final Assembly Machine EAM |
Overall dimen.: 72” long x 34” deep x 72” high Power: 110V, 60 Hz Production rate: 60 per minute |
60PPCNA | ||
| Automated Needle Case Assembly Machine Sortimat |
Overall dimen.: 24’ long x 14’ wide x 8’ high Power: 208V, 60 Hz, 3 phase Air supply: 85 PSI Production rate: 30 per minute |
M10-1593/1594 | ||
| Manual Powerpak Assembly Equipment EAM |
Overall dimen.: All pieces of equipment will fit within a 6’ x 12’ area Power: 110V, 60 Hz Air: 85 PSI Production rate 4,000/8 hr shift—8 operators |
|||
| Manual Powerpak/Cartridge Assembly Machine EAM |
Overall dimen.: 34” long x 36” deep x 53” high Power: 110V/220V, 60/50 Hz Production rate: Variable-set to operator speed |
30PPCA | ||
| Manual Final Assembly Machine EAM |
Overall dimen.: 34” long x 36” deep x 53” high Power: 110V, 60 Hz Production rate: Variable-set to operator speed |
30PPNA | ||
| Wrap Labeling System CCL Labeling Equipment |
Overall dimen.: 96” long x 46” deep x 84” high Power: 115V, 60 Hz, single phase, 20A Air: 80 PSI, 2 SCFM Production rate: 60 per minute |
330 | ||
| Custom Pouching Machine Eclipse Automation Corp. |
Overall dimen.: 64” long x 48” deep x 54” high Power: 110V, 60 Hz Compressed air-60 PSI Production rate: Variable-set to operator speed |
|||
| Automated Cartridge Filler EAM |
Overall dimen.: 73” long x 38” deep x 62” high Power: 220V, 60 Hz, 3 phase, 20A Production rate: 60 per minute |
C60 FSC | ||
| Cartridge Inspection Machine EAM |
Overall dimen.: 92” long x 28” deep x 62” high Power: 110V, 60 Hz, 20A Production rate: 60 per minute |
S1120 | ||
| Cartridge Inspection Machine Seidenader |
Overall dimen.: 96” long x 68” deep x 72” high Power: 230V, 50 Hz, single phase Compressed Air: 80 PSI, oil and water free Production rate: Variable up to 9,000/hr-dependent on operator speed |
V90-AVSB/60 | ||
| Lab Cartridge Capping Machine EAM |
Overall dimen.: 36” long x 36” deep x 60” high Power: 220V, 60 Hz, 3 phase, 20A Production rate: n/a |
C60C | ||
| Needle Processing Machine Sortimat |
Overall dimen.: 13’ long x 12’ deep x 8’ high Power: 000X, 00 Xx, 0 xxxxx Xxx: 80 PSI Production rate: 30/minute |
|||
29
| Company |
Machine/Component/Inventory Description |
Quantities | ||
| APR, Inc. |
Thermal Printmaster w/out auto tray | |||
| Labelview Software | ||||
| Pouchmaster Packaging System | ||||
| Acro | Washer Feeder Bowl | |||
| Safe Pin Feeder Bowl | ||||
| Half Collet Feeder Bowl | ||||
| Spring Feeder Bowl | ||||
| Cap Feeder Bowl | ||||
| Full Collet Feeder Bowl | ||||
| Housing Feeder Bowl | ||||
| Feeder Bowl/Inlines | ||||
| Vision Monitor w/ CE Marking | ||||
| 50h Generator Rental | ||||
| Auto Shrink Bander for Safe Pin | ||||
| CED | 4-up Firing Cap Assembly Press | |||
| 4-up Powerpak Assembly Press | ||||
| 4-up Pneumatic Powerpak Assembly Press | ||||
| 1/8 NPT x 5/32 Speed Control Valves | ||||
| CTP Plasro | Powerpak Housing 8 Impression Mold | |||
| EAM | Washer Insert Machine Mods | |||
| Repl Parts & Frght 220v coils & controller | ||||
| Repl/Spare Parts Inspection Machine | ||||
| Feeder for Needle Case Assembly | ||||
| X. Xxxxxx | Xxxxxxx Shrink Tunnel Conveyor Belt | |||
| SFM | End Caps 1008 | 500,000 | ||
| Needle guide 1004 | 510,000 | |||
| Gripper 1001 | 290,000 | |||
| Needle Housing 1005 | 510,000 | |||
| Stopper 1003 | 275,000 | |||
| 29g Messing Hubs | 440,000 | |||
| 27g Messing Hubs | 506,000 | |||
| 2 Cavity Injection Mold, Part #1005-70 | ||||
| Sortimat | Laser Head Mods | |||
| Hub Check Station | ||||
| Needle Length Check—Pull Station | ||||
| Needle Length Check—Guide Assembly | ||||
| Stopper Bowl Feeder Xxxxxx | ||||
| Add’l Auto Vision System @ Sta. 14 | ||||
| Spectrum | 8-Cavity Mold, 1001-10 | |||
| Insert Needle Guide, 0000-0X | ||||
| Xxxxxxx Xxxx, 0000-0X | ||||
| Xxxxxxx Case, 0000-0X | ||||
| Xxx & Xxx 0-Xxxxxx Xxxx, 0000-0X | ||||
| Des & Bld 8-Cavity Mold, 1102-1F | ||||
| D & B 8-Cavity Mold, 1106-1G | ||||
| D & B 8-Cavity Mold, 0000-0X | ||||
| X & X 0-XXxxxx Xxxx, 0000-0X | ||||
| Injector Xxxx/Xxxxxxxx Xxxxxxx, XX-000 | ||||
| Injector Case, 1106 | ||||
| 8-Cavity Mold for Needle Guide 1004-3A | ||||
| 1-Cavity Mold 1005-50 | ||||
| 1-Cavity Mold 1005-6A | ||||
| 8-Cavity Mold, Needle Guide 1004-40 | ||||
| Xxxxxxx | Inspection fix w/drill xxxxx | |||
| Adapter, Steel screw, Needle cup | ||||
| Plastic Discs | ||||
| Needle Fixture | ||||
30
SCHEDULE 1.43
THE TRADEMARKS
| Country |
Trademark No. | Issue Date | Title | Renewal Date | Status | Note | ||||||
| Canada |
896,992 | 10/18/98 | Adrenaject | Pending | Dec. of Comm. | |||||||
| China |
1,385,276 | 4/14/2000 | Adrenaject | 4/13/2010 | Registered | |||||||
| E.C. |
703,124 | 5/10/1999 | Adrenaject | 12/15/2007 | Registered | |||||||
| Japan |
4,300,605 | 7/30/1999 | Adrenaject | 7/30/2009 | Registered | |||||||
| United States |
76/258418 | Adrenaject | 6/19/2004 | Published | St. of Use | |||||||
| Canada |
896,991 | 11/18/98 | Reliaject | Published | Dec. of Comm. | |||||||
| China |
1,372,042 | 3/7/2000 | Reliaject | 3/6/2010 | Registered | |||||||
| E.C. |
703,256 | 5/10/1999 | Reliaject | 12/15/2007 | Registered | |||||||
| Japan |
4,300,606 | 7/30/1999 | Reliaject | 7/30/2009 | Registered | |||||||
| United States |
75/374002 | Reliaject | Published |
31
Schedule 2.2.1
Encumbrances
Senetek is a party to a Securities Purchase Agreement dated April 14, 1999, as amended by the First Amendment dated June 20, 2001, the Second Amendment dated September 4, 2003 and the Third Amendment dated September 30, 2004 (the “Securities Purchase Agreement”), among Silver Creek Investments, Ltd., Bomoseen Investments, Ltd., Elstree Holdings, Ltd. and Dandelion Investments, Ltd. (collectively, the “Holders”) and Senetek. Pursuant to the Securities Purchase Agreement Senetek sold and the Holders purchased senior secured notes of Senetek (the “Notes”) and granted to the Holders security interests in and to all of the Assets. The Notes mature on April 1, 2007 and are pre-payable at any time at the option of Senetek without premium or penalty. *** in principal amount of the Notes remain outstanding.
*** Confidential treatment has been requested
32
SCHEDULE 2.2.7
On January 6, 2005, Senetek’s trademark counsel received a letter from counsel for Ares Trading S.A. notifying Senetek of its contention that the trademark “Reliaject” for which Senetek had a pending trademark appliation with the U. S. Patent and Trademark Office, was confusingly similar to the trademark Rebiject issued to Ares Trading, S.A. Senetek’s counsel and Ares Trading’s Counsel corresponded, and on November 9, 2005 Ares Trading S.A. filed an Opposition to Senetek’s trademark application. On January 23, 2006, Senetek duly filed a response to the Opposition. True and correct copies of all Correspondence between Senetek and Ares or their respective counsel and of the parties filings with the USPTO are attached hereto.
33
Schedule 2.2.11
ANDA Information
| Content |
Author |
Date | ||
| Quality Manual |
Xxxx |
|||
| General Specifications |
Xxxx |
|||
| US Contract Filling |
Novocol |
1998 | ||
| File #2 Correspondence and Contact Logs |
1998-1999 | |||
| Fill II Correspondence and Contact Logs |
Novocol |
|||
| File III Correspoondence and Contact logs |
Xxxxxxx |
|||
| Xxxxx Xxxxxxxxx |
Xxxxxxx |
Xxxxx 0, 0000 | ||
| Xxxxxxxxxxxxxx (X. Xxxxx) |
Novocol |
Apr.-Sept. 95 | ||
| Correspondence (X. Xxxxxxxx) re Vasopotin |
Novocol |
Oct. 1995 | ||
| Correspondence (X. Xxxxxxxx) re Vasopotin |
Novocol |
Dec. 1995 | ||
| Shering Plough Due Diligence Visit to Novocol |
Dec. 4, 1997 | |||
| Certificate of Analysis, Vasopotin II |
Novocol |
Feb. 7, 1996 | ||
| Certificate of Analysis, Vasopotin II |
Novocol |
Mar. 15, 1996 | ||
| Certificate of Analysis, Vasopotin II Placebo |
Novocol |
Oct. 12, 1995 | ||
| Working Formula & Procedure-Xxxx Vasopotin 2 |
Novocol |
May 20, 1997 | ||
| Contact Reports |
Novocol |
April, 1998 | ||
| Contact Reports |
Novocol |
1995-1998 | ||
| Binder III |
Novocol |
8/98-10/17/98 | ||
| Binder II |
Novocol |
June-Aug. 1998 | ||
| Purchase Orders |
Novocol |
1995 | ||
| Batch Records (Blanks) |
Novocol |
|||
| Batch Records (.35, .30) old PMEA’s |
Novocol |
1996 | ||
| Corresp. to FDA; Amendment to Pending App. |
Novocol |
De. 1998 | ||
| Content |
Author |
Date | ||
| Informatoon/correspondence | Celltech/Medevale/Medeva |
1994-1995 | ||
| Epinephrine (X. Xxxxxx) | 1996-1997 | |||
| Senetek Epinephrine | 2000 | |||
| Aseptic Assembly Transfer to Novocol | 1995-1996 | |||
| Epinephrine Auto-injector FDA Protocol for In-Vitro Equiv. Test | Senetek |
Aug. 2, 1999 | ||
| STI (EPI) /FDA Information | Department of Health |
Aug. 3, 1995 | ||
| Epinephrine Auto-Inj, 0.3 mg ANDA Volume 1 of 3 | Xxxxxxx Cons. Serv’s (LCS) |
6/12/1998 | ||
| Epinephrine Auto-Inj, 0.3 mg ANDA Volume 2 of 3 | LCS |
June 12, 1998 | ||
| Epinephrine Auto-Inj, 0.3 mg ANDA Volume 3 of 3 | LCS |
June 12, 1998 | ||
| Epinephrine Injection USP, 0.3 mg per Delivery Amendment to Pending ANDA #75-397 | LCS |
Sept. 11, 1998 | ||
| ANDA Volume 1 0.3 mg Epinephrine Auto-Injector Auto-Injector for Subcutaneous Inj. of Epinephrine | Arent Fox |
Feb. 22, 1997 | ||
| ANDA Volume 2 0.3 mg Epinephrine Auto-Injector Auto-Injector for Subcutaneous Inj. of Epinephrine | Arent Fox |
Feb. 22, 1997 | ||
| Xxxxxxx Epinephrine | LCS |
Apr. 14, 1999 | ||
| Xxxxxxx Consultanat Services | LCS |
Apr. 21, 1997 | ||
| App. Part of the DMF Epinephrine (I-Adrenaline) | Boehringer Ingelheim |
Apr. 6, 1999 | ||
| App. Part of the DMF Epinephirne Bitartrate (I-Adrenaline Bitartrate) | Boehringer Ingelheim |
June 13, 1997 | ||
| Chemistry & Manufacturing Adrenoject Part 2 | ICN Canada LTD |
N/A | ||
| Stability Reports Auto-Injector | Novocol Pharm. Canada |
Sept. 1, 1998 | ||
| Epinephrine Auto-Injector, 0.3 mg ANDA Volume 1 of 3 Incomplete Copy | ||||
| Epinephrine Auto-Injector, 0.3 mg ANDA Volume 3 of 3 Incomplete Copy | ||||
| Epinephrine Inj. USP, 0.3 mg per Delivery Amend. to Pending ANDA #75-397 Incomplete Copy | LCS | Sept. 11, 1998 | ||
34
| Content |
Author |
Date | ||
| DIN Application Epinephrine Injection USP (0.3 and 0.15 mg) Adrenoject Volume 1 of 4 | ICN Canada Ltd | |||
| DIN Application Epinephrine Injection USP (0.3 and 0.15 mg) Adrenoject Volume 1 of 4 | LCS | Jan. 4, 1999 | ||
| EPI ANDA Second Document Submission | Novocol Pharma, Canada | Dec. 23, 1997 | ||
| Citizen Petition of the FDA for New Drug App. regarding Epinephrine Injection (In Quadruplicate) | Senetek DDT | Apr. 14, 1998 | ||
| Review of Senetek ANDA for Epinephrine Injection USP, Auto-Injector Original | LCS | May 5, 1997 | ||
| Review of Senetek ANDA for Epinephrine Injection USP, Auto-Injector Copy | LCS | May 5, 1997 | ||
| ANDA Volume1 0.3 mg Epinephrine Auto-Injector Auto-Injector for Subcutaneous Inj. fo Epine. Copy | Arent Fox | Feb. 22, 1997 | ||
| ANDA Volume2 0.3 mg Epinephrine Auto-Injector Auto-Injector for Subcutaneous Inj. fo Epine. Copy | Arent Fox | Feb. 22, 1998 | ||
| X. Xxxxxxx ANDA Package/Label | Xxxx Inc. | |||
| X. Xxxxxxx Epi 0.3mg ANDA Labeling Overflow | Xxxx Inc. | |||
| X. Xxxxxxx ANDA Graphics | LCS and Novocol | Nov. 14, 1997 | ||
| X. Xxxxxxx Epinephrine ANDA 0.3 mg Sterillization Assurance | Novocol | Feb. 7, 1997 | ||
| X. Xxxxxxx Epinephrine 0.3 mg Amendment | Novocol | Sept. 1, 1998 | ||
| X. Xxxxxxx Epinephrine 0.3mg ANDA Section XII Misc. Reports and Data | Novocol | June 9, 1997 | ||
| X. Xxxxxxx Epinephrine 0.3 mg ANDA Section XIV Reports and Summary | Novocol, The West Co, Xxxx Inc. | Dec. 11, 1997 | ||
| X. Xxxxxxx FDA-Eoubeogrube Approval | Novocol Dept. of Health | Apr. 12, 1996 | ||
| X. Xxxxxxx Adrenaject-ANDA History | LCS and Dept. of Health | Feb. 16, 1999 | ||
| Autoject Mini 510K Filing | Xxxx Xxxxxxx | |||
| Previously Distributed Material Content |
Author |
Date | ||
| Corresp. To X. Xxxxx, FDA: Request for Bioequivalence Testing Guidance for IM Rte of Admin.; .3mg per delivery | Xxxxxxx Consulting Services (LCS) | 3/12/99 | ||
| Corresp. To X. Xxxxxx, Senetek: 4 wk Rat Toxicology Study | FDA | 2/24/97 | ||
| Draft 1:1000 USP Epinephrine Submission Batch Validation Plan | Unk. | 6/24/97 | ||
| Corresp. To S. Lewinton, Senetek: Audit Schedule | LCS | 1/21/98 | ||
| Corresp. To S. Lewinton, Senetek: Audit Summary | LCS | 3/11/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Summary of Pending Issues | LCS | 6/2/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Reformatting MCA Submission | LCS | 7/3/98 | ||
| Corresp. To X. Xxxxxx, Senetek: FDA Acceptance of Filing, Novocol’s Inspection | LCS | 7/9/98 | ||
| Fax to Xxxxx Xxxxxx, CEKA: EpiPen Xxxx in UK | Moreton (X. Xxxxxx) | 7/13/98 | ||
| Corresp. To X. Xxxxxx, Senetek: EpiPen Xxxx Products in UK, Authorization to Market | LCS | 7/17/98 | ||
| Fax to Xxxxx Xxxxxx, CEKA: EpiPen Marking Exclusivity | Moreton (X. Xxxxxx) | 7/18/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Clarification of EpiPen Marking Exclusivity | LCS | 7/22/98 | ||
| Corresp. To E. Curely, Senetek: US Market Authorization for .15 mg | LCS | 8/20/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Novocol Facility Inspection | LCS | 10/9/98 | ||
| Corresp. To X. Xxxxx, ICN: Cover Ltr for Documents Forwarded | LCS | 11/24/98 | ||
| Corresp. To X. Xxxxxxx, Senetek: Anticipated Regulatory Activities & Est.’d Time Frames | LCS | 11/24/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Potential Substitutability with Epipen | LCS | 11/24/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Status of FDA Approval of the Adrenaject Projects | LCS | 12/1/98 | ||
| Fax To Xxxx Xxxxxxx et al: Draft Minutes of Conf Call 12/2/98 | S. Lewinton | 12/3/98 | ||
35
| Content |
Author |
Date | ||
| Fax To LCS/Xxxxxxx: Table of Information Required for Completion of Adrenaject NDS | ICN | 12/8/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Revised List of Documents for Addition of .15 mg to Application | LCS | 12/14/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Open Issues Pertaining to .3 mg ANDA | LCS | 12/15/98 | ||
| Corresp. To X. Xxxxx, Novocol: X. Xxxxxxxx Represents Novocol with FDA | LCS | 12/21/98 | ||
| Corresp. To X. Xxxxxx, Senetek: Meridian Petition to FDA to Deny .15 mg Product | LCS | 12/23/98 | ||
| Corresp. To X. Xxxxxx, Senetek: FDA Deficiency Letter for .3 mg ANDA | LCS | 1/4/99 | ||
| Response From FDA: Major Amendment re ANDA 75-397; Not Approved | FDA | 1/4/99 | ||
| Corresp. To X. Xxxxxx, Senetek: FDA Draft Guideline “ANDA’s: Impurites in Drug Products” | LCS | 1/8/99 | ||
| Corresp. To X. Xxxxx, Senetek: FDA response on Proposed 2 Year Rat Carcinogenicity Study | LCS | 1/5/99 | ||
| Corresp. To X. Xxxx, ICN: Cover Ltr for Documents Forwarded re .3mg ANDA | LCS | 1/11/99 | ||
| Memo to X. Xxxxxx, Senetek: Response to ANDA for .15 mg | FDA | 1/11/99 | ||
| Memo to X. Xxxxxx, Senetek: Response to ANDA for .3 mg | FDA | 1/11/99 | ||
| Corresp. To X. Xxxxxxx, Senetek: Issues to be Resolved re FDA 1/4/99 Deficiency Letter | LCS | 1/13/99 | ||
| Corresp. To X. Xxxxxxx, Senetek: Withdrawal of FDA Approval of ANDA Suitability Petition | LCS | 1/18/99 | ||
| Corresp. To X. Xxxxxxx, Senetek: FDA Warning Letter to Novocol | LCS | 1/18/99 | ||
| Corresp. To X. Xxxxxx, Senetek: Draft Response to FDA Not Approvable Letter | LCS | 2/8/99 | ||
| Corresp. To S. Lewinton, Senetek: Plan for ANDA Approval with Bio Waiver Request Option | LCS | 3/1/99 | ||
| Corresp. To S. L.: Device Comp. for Encl. with Bio Waiver | LCS | 3/9/99 |
36
| Content |
Author |
Date | ||
| Corresp. To X. Xxxxx, Senetek: Discussion of FDA Deficiency Letter | LCS | 3/8/99 | ||
| Corresp. To X. Xxxxxx, Senetek: Ltr to OGD Concerning Bioequivalance Testing Guidance | LCS | 3/12/99 | ||
| Corresp. To S. Lewinton, Senetek: Clarification of FDA’s Batch Size and Packaging Req’s | LCS | 3/23/99 | ||
| Corresp. To S. Lewinton, Senetek: Review of Protocol for Study ADJ001 | LCS | 3/29/99 | ||
| Corresp. To X. Xxxxx, ICN: Copy of Request for Bioequivalance Testing Guidance | LCS | 3/31/99 | ||
| Fax To X. Xxxxxx: Proposal for Packaging of ANDA Test Batches | LCS | 4//99 | ||
| Corresp. To X. Xxxxx: Review of Protocol for Novocol Validation Study | LCS | 6/25/99 | ||
| Corresp. To X. Xxx Xxxx, Senetek: Review of Proposal to Have ICN submit an ANDA for Xxx-Kit | LCS | 7/7/99 | ||
| Corresp. To X. Xxxxxxx, Senetek: Epinephrine Injection Project and ANDA | LCS | 7/9/99 | ||
| Corresp. To X. Xxxxx, Senetek: FDA requests re transgenic mouse study and Other Questions | LCS | 7/13/99 | ||
| Corresp. To X. Xxxxxxx, Xxxxxxx Cons. Services: Full Packaging Requirements | FDA | 7/13/99 | ||
| Corresp. To X. Xxxxxxx, Senetek: OGD Approval of Exhibit Batch Plan/Stability Proposal | LCS | 7/16/99 | ||
| Corresp. To X. Xxxxxxx, Senetek: Discussion of Contents of OGD Approval Letter | LCS | 7/23/99 | ||
| Content |
Author |
Date | ||
| Corresp. To X. Xxx Xxxx, Senetek: (Page 1 missing of 2 page letter) | LCS | 9/14/99 | ||
37
SCHEDULE 2.2.14
ARDANA’S LICENSE RIGHTS
Pursuant to Article 4 of the License Agreement dated June 17, 2004 between Senetek and Ardana Bioscience Limited (“Ardana”), Ardana was granted an exclusive license to market, sell and deliver the Reliaject Device pre-filled with Invicorp in its Territory, initially comprised of Europe. In addition, Article 7 of the License Agreement provides as follows:
“7. DELIVERY SYSTEM
7.1 Senetek will use its best endeavours to:
7.1.1 identify a suitable Third Party to manufacture the Delivery System on behalf of Senetek and its licensees;
7.1.2 enter into an agreement with such Third Party in terms of which Senetek grants to such Third Party a license under the Delivery System Patents to manufacture and supply the Delivery System on behalf of Senetek and its licensees;
7.1.3 cause such manufacturing licensee to enter into good faith negotiations with Ardana, solely at Ardana’s request, in relation to an exclusive manufacturing and supply contract, on reasonable and customary terms, for Ardana’s requirements of the Delivery System for marketing and sale in conjunction with the Product in the Territories for the period of this Agreement,
within twelve (12) months following the Commencement Date.”
38
Schedule 3.2.4
Identified Non-Epinephrine Products
Atropine
Morphine
Diazepam
Pralidoxine
Sumatriptan
Invicorp® (only if Ranbaxy or their Affiliates market and sell the Device pre-filled with Invicorp® to physicians and other retail or wholesale third party customers, but expressly excluding any sales to Plethora, Ardana or other third party licensees of Invicorp® for resale by such parties)
39
SCHEDULE 15.3
FORM OF PRESS RELEASE
Press Release
Source: Senetek PLC
SENETEK AND RANBAXY ANNOUNCE THE ACQUISITION BY
RANBAXY OF SENETEK’S RELIAJECT AUTOINJECTOR;
SENETEK TO RECEIVE MILESTONE AND ROYALTY PAYMENTS
NAPA, Calif., March , 2006 / (OTCBB: SNTKY - News), xxx.xxxxxxxxxx.xxx, a healthcare technologies company focused on developing and co-marketing products for the anti-aging markets worldwide, today announced the sale to Ranbaxy Pharmaceuticals Inc. of Jacksonville, Florida, of Senetek’s patents, trademarks and automated manufacturing equipment for its proprietary disposable autoinjector for self-administration of parenteral drugs, including epinephrine for emergency treatment of anaphylactic shock from peanut and other allergies.
The agreement provides for a non-refundable payment on signing and milestone payments based on regulatory approvals and cumulative sales milestones. Terms also include a percentage of Ranbaxy’s and/or its licensees’ quarterly net sales of the product(s). Initially, Ranbaxy will focus on pre-filling the autoinjector device with epinephrine. Ranbaxy will also evaluate the development of other parenteral drugs (including Senetek’s patented erectile dysfunction drug Invicorp®).
Ranbaxy will be making infrastructure investments, including building out the required clean room suites at its facility in New Brunswick, New Jersey to house the highly automated Reliaject production line. Under the agreement, Ranbaxy will be obtaining regulatory approvals and marketing the product. Ranbaxy has also agreed to negotiate with Ardana Bioscience Limited, Senetek’s exclusive licensee for Invicorp for Europe, and Plethora Solutions Holdings PLC, Senetek’s exclusive licensee for Invicorp in North America, for contract manufacturing agreements to produce the autoinjector pre-filled with Invicorp.
Xxxxx X. Xxxxxxx, Chairman and Chief Executive Officer of Senetek, commented, “Placing Senetek’s autoinjector technology and equipment with a strong commercial partner committed to bringing it to market has been one of the key goals of Senetek’s strategic plan. Ranbaxy is ideally suited to maximizing the commercial potential of this unique technology, and this transaction will further support our business plan to broaden and deepen Senetek’s position in the dermatologicals segment with Kinetin, Zeatin and the other new compounds under development or evaluation for in-licensing.”
40
Xxxxx Xxxxxxxxx, Chairman of Ranbaxy Inc., the U.S. parent company of Ranbaxy Pharmaceuticals Inc., said, “Anaphylactic shock due to allergic reaction to peanut-based food additives is a growing health risk that currently results in over 30,000 emergency room trips, and 150 to 200 preventable deaths, in the U.S. each year, on top of some 50 deaths from bee sting and other allergic reactions. Senetek’s patented, modular autoinjector is ideally suited for this acute self-medication market, which currently is growing at over 25% annually as health and educational organizations build popular awareness of these risks. This device also can be adapted for other acute applications, including in the military and homeland security sectors for administration of antidotes to chemical and biological agents. Ranbaxy Pharmaceuticals is well staffed and funded to shepherd this much needed technology through regulatory approval and bring it promptly to market.”
Ranbaxy Pharmaceuticals Inc. (RPI) based in Jacksonville, Florida, is a wholly owned subsidiary of Ranbaxy Laboratories Limited (RLL), India’s largest pharmaceutical company. RPI is engaged in the sale and distribution of generic and branded prescription products in the U.S. healthcare system.
Senetek is a life sciences-driven product development and licensing company focused on the high growth market for dermatological and skin care products primarily addressing photo-damage and age-related skin conditions. Senetek’s patented compound Kinetin is a naturally occurring cytokinin that has proven effective in improving the appearance of aging skin with virtually none of the side effects associated with acid-based active ingredients. Senetek has licensed Kinetin to leading global and regional dermatological and skin care marketers including Valeant Pharmaceuticals, The Body Shop and Revlon, and recently announced the grant to Valeant Pharmaceuticals of exclusive world-wide rights to Zeatin, a Kinetin analog shown to have significant differential advantages in clinical trials. Senetek’s researchers at the University of Aarhus, Denmark, also are collaborating with the Institute of Experimental Botany, Prague, and other leading governmental and private research institutions to identify and evaluate additional new biologically active compounds for this high growth field.
Senetek PLC Investor Relations Contact:
1-707-226-3900 ext. 102
E-mail: Xxxxxxxx@xxxxxxxxxxxxxxxxxxx.xxx
Safe Harbor Statement
This news release contains statements that may be considered “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act, including those that might imply Senetek’s expectation that the FDA will approve this autoinjector pre-filled with epinephrine for anaphylaxis and that Ranbaxy Pharmaceuticals will meet with commercial success with this product. Forward-looking statements by their nature involve substantial uncertainty, and actual results may differ materially from those that might be suggested by such statements. Important factors identified by the Company that it believes could result in such material differences are described in the Company’s Annual Report on Form 10-K for the year
41
2004. However, the Company necessarily can give no assurance that it has identified or will identify all of the factors that may result in any particular forward-looking statement materially differing from actual results, and the Company assumes no obligation to correct or update any forward-looking statements which may prove to be inaccurate, whether as a result of new information, future events or otherwise.
42
EXHIBIT A
GENERAL ASSIGNMENT AND XXXX OF SALE
This General Assignment and Xxxx of Sale (this “Assignment”) dated this day of March, 2006, is by and between Senetek PLC, a United Kingdom company with offices at 000 Xxxxxx Xxxxx, Xxxx, Xxxxxxxxxx 00000 (“Senetek”) and Ranbaxy Pharmaceuticals, Inc., a Florida corporation with offices at 0000 Xxxxxxx Xxxxxx Xxxxxxxxx, Xxxxxxxxxxxx, Xxxxxxx 00000 (“Ranbaxy”).
BACKGROUND
Senetek and Ranbaxy are parties to an Asset Purchase and Sale Agreement dated as of the date hereof (the “Purchase Agreement”) pursuant to which Senetek sold and Ranbaxy purchased certain tangible and intangible assets described in the Purchase Agreement (the “Assets”). Capitalized terms used herein and not otherwise defined are used with the meanings ascribed to them in the Purchase Agreement.
The parties hereto wish to enter into this Assignment to evidence the transfer and conveyance by Senetek and the purchase by Ranbaxy of all of the Assets other than the Patents and the Trademarks, the assignments of which are evidenced by other instruments dated the date hereof.
AGREEMENT
NOW, THEREFORE, for good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, Senetek and Ranbaxy agree as follows:
1. Assignment. Senetek hereby grants, assigns, conveys and delivers to Ranbaxy all of Senetek’s right, title and interest in and to the Assets other than the Patents and the Trademarks as the same exist on the date hereof (collectively the “Conveyed Assets”).
2. Acceptance. Ranbaxy hereby accepts all of Senetek’s right, title and interest in and to the Conveyed Assets
3. Further Assurances. Senetek agrees to do, execute, acknowledge and deliver to Ranbaxy or cause to be done, executed, acknowledged and delivered, upon its request, and without further consideration, all such further acts, conveyances, transfers, assignments, powers of attorney and assurances as may be required to more effectively convey, transfer and vest in Ranbaxy and put Ranbaxy in possession of the Conveyed Assets transferred and conveyed hereunder.
4. Choice of Law. This Assignment shall be governed by and interpreted in accordance with the laws of the State of New York (regardless of that or any other jurisdiction’s choice of law principles).
43
5. Counterparts. This Assignment may be executed in counterparts, each of which shall be deemed an original, but both of all of which together shall constitute or and the same instrument and shall become effective when one or more counterparts have been signed by each of the parties hereto and delivered to the other party.
6. Descriptive Headings. The descriptive headings herein are inserted for convenience only and are not intended to be part of or to affect the meaning or interpretation of this Assignment.
IN WITNESS WHEREOF, Senetek and Ranbaxy have executed and entered into this Assignment as of date first set forth above.
| SENETEK PLC | RANBAXY PHARMACEUTICALS, INC. | |||||||
| By: | /s/ Xxxxx X. Xxxxxxx |
By: |
| |||||
| Name: | Xxxxx X. Xxxxxxx | Name: |
| |||||
| Title: | Chairman and Chief Executive | Title: |
| |||||
44
EXHIBIT B
PATENT ASSIGNMENT
Senetek PLC, a United Kingdom company having its principal place of business at 000 Xxxxxx Xxxxx, Xxxx, Xxxxxxxxxx 00000 (“ASSIGNOR”) for good and valuable consideration, does hereby sell, assign, transfer and convey to Ranbaxy Pharmaceuticals, Inc., a Florida corporation having a principal place of business at 0000 Xxxxxxx Xxxxxx Xxxxxxxxx, Xxxxxxxxxxxx, Xxxxxxx 00000 (“ASSIGNEE”), its successors, assigns and legal representatives, the ASSIGNOR’s entire right, title and interest in and to the United States Letters Patents set forth in the schedule hereto and all United States rights to claim priority on the basis of such Letters Patent, to the subject matter disclosed therein, and to corresponding Letters Patent or Applications throughout the world.
ASSIGNOR further assigns to ASSIGNEE all causes of action for past or future infringements or violations by third parties of any of the above-assigned patents, patent applications and other rights, including the right to recover past and future damages for infringements or violations of such rights. ASSIGNOR will not take or omit any action which may result in the impairment or alteration of the Letters Patent or Applications or any of the rights therein. ASSIGNOR also agrees to sign all documents necessary to secure all any and all patents and other right based on or related to the above patent applications and patents, and to request issuance of all such patents to ASSIGNEE in accordance with this Assignment.
| March , 2006 | Senetek PLC | |||
| By: | /s/ Xxxxx X. Xxxxxxx | |||
| Xxxxx X. Xxxxxxx | ||||
| Chairman and Chief Executive Officer | ||||
00
| XXXXX XX XXX XXXX) | ||
| ) SS.: | ||
| COUNTY OF NEW YORK ) |
On the day of , in the year 2006, before me, the undersigned, personally appeared XXXXX XXXXXXX, personally known to me or proved to me on the basis of satisfactory evidence to be the individual whose name is subscribed to the within instrument and acknowledged to me that he executed the same in his capacity, and that by his signature on the instrument, the individual, or the person upon behalf of which the individual acted, executed the instrument.
|
Notary Public |
46
EXHIBIT C
TRADEMARK ASSIGNMENT
Senetek PLC, a United Kingdom company having its principal place of business at 000 Xxxxxx Xxxxx, Xxxx, Xxxxxxxxxx 00000 (“ASSIGNOR”) for good and valuable consideration, does hereby sell, assign, transfer and convey to Ranbaxy Pharmaceuticals, Inc., a Florida corporation having a principal place of business at 0000 Xxxxxxx Xxxxxx Xxxxxxxxx, Xxxxxxxxxxxx, XX 00000 (“ASSIGNEE”), its successors, assigns and legal representatives, the ASSIGNOR’s entire right, title and interest in and to the statutory trademarks set forth in the schedule hereto together with all common law trademarks related thereto, to the subject matter disclosed therein, and to corresponding rights throughout the world (the “Trademarks”).
ASSIGNOR further assigns, transfers and conveys to ASSIGNEE all goodwill symbolized by and associated with the Trademarks as used, all income, royalties and payments now or hereafter due or payable in respect thereto, and all causes of action for past, present or future infringements or violations of any of the above-assigned Trademarks and other rights, including the right to recover past and future damages for infringements or violations of such rights. ASSIGNOR also agrees to sign all documents necessary to secure any and all statutory trademarks and other right based on or related to the above assigned rights, and to request issuance of all such statutory trademarks and other rights to ASSIGNEE in accordance with this Assignment.
2. Further Assurances. ASSIGNOR agrees to execute and deliver to ASSIGNEE, upon its request, such other and further instruments of assignment and such other assistance as may be reasonably requested by ASSIGNEE in the establishment, registration, preservation and enforcement of Ranbaxy’s rights to the foregoing.
3. Choice of Law. This Assignment shall be governed by and interpreted in accordance with the laws of the State of New York (regardless of that or any other jurisdiction’s choice of law principles).
47
IN WITNESS WHEREOF, Senetek and Ranbaxy have executed and entered into this Assignment this day of March 2006.
| SENETEK PLC | ||
| By: | /s/ Xxxxx X. Xxxxxxx | |
| Name: | Xxxxx X. Xxxxxxx | |
| STATE OF NEW YORK) | ||
| ) SS.: | ||
| COUNTY OF NEW YORK ) |
On the day of , in the year 2006, before me, the undersigned, personally appeared XXXXX XXXXXXX, personally known to me or proved to me on the basis of satisfactory evidence to be the individual whose name is subscribed to the within instrument and acknowledged to me that he executed the same in his capacity, and that by his signature on the instrument, the individual, or the person upon behalf of which the individual acted, executed the instrument.
|
Notary Public |
48
EXHIBIT D
QUARTERLY PAYMENT RECONCILIATION
Ranbaxy Pharmaceuticals Co., Inc.
Quarterly Payment Reconciliation
For the Calendar Quarter Ended
| Product |
Gross Sales | Adjustments | Net Sales | Rate | Payment($) |
49

