PROMOTION AGREEMENT by and between JANSSEN BIOTECH, INC. and IMMUNOMEDICS, INC. Dated as of: April 5, 2019
CERTAIN IDENTIFIED INFORMATION HAS BEEN EXCLUDED FROM THIS EXHIBIT BECAUSE IT IS NOT MATERIAL AND WOULD LIKELY CAUSE COMPETITIVE HARM TO THE REGISTRANT IF PUBLICLY DISCLOSED. [***] INDICATES THAT INFORMATION HAS BEEN REDACTED.
by and between
XXXXXXX BIOTECH, INC.
and
IMMUNOMEDICS, INC.
Dated as of: April 5, 2019
TABLE OF CONTENTS
ARTICLE 1 DEFINITIONS | 1 | |||
ARTICLE II BRAND PLAN | 10 | |||
2.1 | Brand Plan Generally | 10 | ||
2.2 | Contents of Brand Plan | 10 | ||
ARTICLE III PROMOTION | 12 | |||
3.1 | Scope | 12 | ||
3.2 | Detailing Requirements | 13 | ||
3.3 | Sales Representatives | 14 | ||
3.4 | Promotional Materials | 15 | ||
3.5 | Product Sales | 16 | ||
3.6 | Product Recall | 16 | ||
3.7 | Product Return | 17 | ||
ARTICLE IV GOVERNANCE | 17 | |||
4.1 | Authority | 17 | ||
4.2 | Joint Commercial Team | 17 | ||
4.3 | BALVERSA Sales Advisory Team | 18 | ||
ARTICLE V COMPENSATION | 18 | |||
5.1 | Definitions | 18 | ||
5.2 | Service Fees | 18 | ||
5.3 | Milestones | 19 | ||
5.4 | Costs | 20 | ||
5.5 | Reports and Payments | 20 | ||
ARTICLE VI REGULATORY MATTERS | 21 | |||
6.1 | Regulatory Approvals | 21 | ||
6.2 | Pharmacovigilance Procedures | 22 | ||
6.3 | Product Complaints | 22 | ||
6.4 | Post-Marketing Surveillance | 22 | ||
6.5 | Product Medical Inquiries | 22 | ||
6.6 | Companion Diagnostic Inquiries | 22 | ||
6.7 | Access, Affordability and Patient Support Inquiries | 23 | ||
ARTICLE VII BOOKS, RECORDS AND AUDIT RIGHTS | 23 | |||
7.1 | Books and Records | 23 | ||
7.2 | Books and Records Audits | 23 | ||
ARTICLE VIII TERM AND TERMINATION | 24 | |||
8.1 | Term; Termination | 24 | ||
8.2 | Effect of Termination or Expiration | 26 | ||
8.3 | Suspension of Product Promotion | 28 | ||
ARTICLE IX CONFIDENTIALITY; RESTRICTIVE COVENANTS | 28 | |||
9.1 | Confidentiality | 28 | ||
9.2 | Exclusivity | 31 | ||
9.3 | Restrictions on Promotions | 31 | ||
9.4 | Limitation on Soliciting Employees | 31 | ||
ARTICLE X INTELLECTUAL PROPERTY | 32 | |||
10.1 | Use of Trademarks | 32 | ||
10.2 | Ownership of Intellectual Property Rights | 32 | ||
10.3 | Prosecution and Maintenance | 33 | ||
10.4 | Enforcement against Infringement | 33 | ||
10.5 | Third Party Infringement Claims | 33 | ||
ARTICLE XI REPRESENTATIONS AND WARRANTIES; CERTAIN COVENANTS | 33 | |||
11.1 | Representations of Authority | 33 | ||
11.2 | Consents | 33 | ||
11.3 | No Conflict | 33 | ||
11.4 | Enforceability | 33 | ||
11.5 | Sales Representatives and Other Company Employees | 34 | ||
11.6 | Other Compliance Matters | 34 | ||
11.7 | Infringement of Third Party Intellectual Property; Clinical Trial Data | 36 | ||
11.8 | Disclaimer | 36 | ||
ARTICLE XII INDEMNIFICATION; LIMITS ON LIABILITY | 37 | |||
12.1 | Scope of Indemnification | 37 | ||
12.2 | Notice and Control of Actions | 38 | ||
12.3 | Limitations on Liability | 39 | ||
ARTICLE XIII DISPUTE RESOLUTION | 39 | |||
13.1 | Disputes | 39 | ||
13.2 | Negotiation | 39 | ||
13.3 | Mediation | 40 | ||
13.4 | Arbitration | 40 | ||
13.5 | Confidentiality | 42 | ||
ARTICLE XIV MISCELLANEOUS | 42 | |||
14.1 | Press Announcements | 42 | ||
14.2 | Force Majeure Event | 42 | ||
14.3 | Independent Contractors | 43 | ||
14.4 | Performance by Affiliates | 43 | ||
14.5 | Notices | 43 | ||
14.6 | Entire Agreement | 44 | ||
14.7 | Amendments; Assignment | 44 | ||
14.8 | Non-Waiver of Rights | ||
14.9 | Further Assurances and Cooperation | ||
14.1 | Severability | ||
14.11 | Binding Effect | ||
14.12 | Counterparts; Facsimile Signatures | ||
14.13 | Third Party Beneficiaries | ||
14.14 | Governing Law | ||
14.15 | Construction | ||
Schedule 1.16 | Xxxxxxx Universal Calendar | ||
Schedule 6.2 | Pharmacovigilance Provisions | ||
Exhibit A | Brand Plan | ||
Exhibit B | Detailing Requirements | ||
Exhibit C | Records and Information Management Requirements | ||
Exhibit D | Health Care Compliance Provisions | ||
This PROMOTION AGREEMENT (this “Agreement”) dated as of April 5, 2019 (the “Effective Date”), is entered into by and between Xxxxxxx Biotech, Inc., a corporation organized under the laws of Pennsylvania (“Xxxxxxx”) and Immunomedics, Inc., a corporation organized under the laws of Delaware (“Company”).
WHEREAS, before the Effective Date, Xxxxxxx submitted an application for approval to market and/or sell the Product (defined below) for the Initial Indication (defined below) in the Territory (defined below); and
WHEREAS, Xxxxxxx now wishes to engage Company to Promote (defined below) the Product for the Initial Indication in the Territory and Company wishes to be so engaged, subject to and upon the terms and conditions set forth in this Agreement;
NOW, THEREFORE, in consideration of the mutual representations, warranties, covenants and undertakings contained in this Agreement, and for other good and valuable consideration, the receipt and sufficiency of which are hereby acknowledged, the Parties, intending to be legally bound, agree as follows:
ARTICLE I
DEFINITIONS
Unless otherwise defined herein, capitalized terms used in this Agreement have the meanings set forth in this Article I.
1.1 “Acquirer” has the meaning set forth in Section 9.2.
1.2 “Acquisition” has the meaning set forth in Section 9.2.
1.3 “Action” means any claim, action, cause of action or suit (whether in contract or tort or otherwise), litigation (whether at law or in equity, whether civil or criminal), assessment, arbitration, investigation, hearing, charge, complaint, demand, notice or proceeding from, by or before any Governmental Authority.
1.4 “Affiliate” means with respect to a Party, any Person that is directly or indirectly controlling, controlled by or under common control with such Party at the time that the determination of affiliation is made. For the purposes of this definition, “control” of a Person means (a) beneficial ownership of at least fifty percent (50%) of the voting securities or other comparable equity interests of such Person (whether directly or pursuant to any option, warrant or other similar arrangement) or (b) the possession, directly or indirectly, of the power to direct the management and policies of such Person, whether through the ownership of voting securities, by contract, declaration of trust or otherwise, and the terms “controlling” and “controlled” shall have meanings correlative to the foregoing.
1.5 “Agreement” has the meaning set forth in the preamble to this Agreement.
1.6 “Approval Date” means the date upon which Marketing Approval is received for
the Product for the Initial Indication.
1.7 “Audit Report” has the meaning set forth in Section 7.2.2.
1.8 “Audited Party” has the meaning set forth in Section 7.2.1.
1.9 “Auditing Party” has the meaning set forth in Section 7.2.2.
1.10 “Balversa-only Target” has the meaning set forth in Exhibit B.
1.11 “Baseline” has the meaning set forth in Section 5.1.1.
1.12 “Books and Records” has the meaning set forth in Section 7.1.
1.13 “Brand Plan” has the meaning set forth in Section 2.1.
1.14 “BSAT” has the meaning set forth in Section 4.3.1.
1.15 “Business Day” means any day other than a Saturday or a Sunday or other day on which commercial banks are authorized or required to be closed in New York, New York.
1.16 “Calendar Quarter” means a calendar quarter based on that certain universal calendar system used by Xxxxxxx and each of its Affiliates for internal business purposes (a copy of which calendar for 2019 and 2020 is attached hereto as Schedule 1.16), such that each Calendar Quarter ends on the last date of the calendar quarter indicated on Schedule 1.16 (the “Quarter End Date”) and begins on the date following the Quarter End Date of the preceding Calendar Quarter.
1.17 “Calendar Year” means a calendar year based on that certain universal calendar system used by Xxxxxxx and each of its Affiliates for internal business purposes (a copy of which calendar for 2019 and 2020 is attached hereto as Schedule 1.16), such that each Calendar Year ends on the fourth Quarter End Date for such year and begins on the date following the fourth Quarter End Date of the preceding Calendar Year.
1.18 “Call” means an in-person visit by an adequately trained sales representative to the office of a health care professional in the Territory for the purpose of promoting or presenting one or more pharmaceutical products.
1.19 “Call Plan” has the meaning set forth in Section 2.2.3.
1.20 “CPR Mediation Procedure” has the meaning set forth in Section 13.3.1.
1.21 “CPR Rules” has the meaning set forth in Section 13.4.
1.22 “Companion Diagnostic” means the diagnostic test approved by FDA concurrently with the Product for use in conjunction with the Product.
1.23 “Company” has the meaning set forth in the preamble to this Agreement.
1.24 “Company Indemnified Parties” has the meaning set forth in Section 12.1.1.
1.25 “Company Internal Detail Reporting System” means the data and records collected by Company and its Affiliates, in accordance with its standard business practice, to monitor Details made by Sales Representatives, which, with respect to the Product, include the date a Detail was made, the name of the Target to whom the Detail was made, the indication(s) for which the Product was presented, and the identity of the Sales Representative who delivered the Detail.
1.26 “Company Product” means the drug that is being developed by Company on the Effective Date, known as “IMMU-132” or sacituzumab govitecan.
1.27 “Company Product Approval Date” means the date upon which Marketing Approval is first received in the Territory for the Company Product.
1.28 “Company Trademark” means any Trademark owned by Company or any of its Affiliates.
1.29 “Competing Product” means any pharmaceutical product that is (a) approved specifically for use, in the treatment of urothelial cancer in any patient population in the Territory or (b) a fibroblast growth factor receptor inhibitor. If the Parties agree to extend the Term beyond the Expiration Date, Competing Product shall not include Company Product after the Expiration Date.
1.30 “Confidential Information” of a Party means (a) all non-public or proprietary information and data (including clinical data, technology, trade secrets, design specifications, dossiers, manufacturing formulae, manufacturing procedures and instructions, methods and processes, formats, designs, applications and programs, raw material supply arrangements, projections, prescriber and target data, pharmacy data, sales data, analyses, rebate agreements, promotion plans, detailing information, financial statements, customer and target lists, marketing plans, budgets, Third Party contracts, market research data, pricing, reimbursement and costs relating to the Product) that is disclosed by or on behalf of such Party or any of its Affiliates to the other Party, any of its Affiliates or any of their respective employees, agents or contractors pursuant to or in connection with this Agreement and (b) any other non-public or proprietary information and data that is expressly deemed in this Agreement to be Confidential Information of such Party, whether or not disclosed by or on behalf of such Party to the other Party, any of its Affiliates or any of their respective employees, agents or contractors, in each case ((a) and (b)) without regard to whether any of the foregoing is marked “confidential” or “proprietary,” or in oral, written, graphic or electronic form.
1.31 “Cumulative Net Sales” has the meaning set forth in Section 5.1.1.
1.32 “Cure Period” has the meaning set forth in Section 8.1.4.
1.33 “Detail” means an in-person presentation of the Product and its uses for the Initial Indication made by an adequately trained sales representative during a Call to one or more Health Care Professionals in the Territory during which the sales representative describes the Product and such use(s) in a fair and balanced manner consistent with (a) the Product Label and Insert and any Promotional Materials approved in accordance with this Agreement, and (b) the other requirements of this Agreement, the Promotion Rules and applicable Laws, but shall not include reminder details or e-details, as such terms are generally understood in the pharmaceutical industry in the Territory, or any presentations made at conventions, consulting programs or similar gatherings, other than a pre-arranged or scheduled meeting at such gathering between the sales representative and a Health Care Professional. When used as a verb, “Detail” means to deliver the presentation described in this definition. “Detailing” shall have a corresponding meaning.
1.34 “Detailing Period” means each of the following periods: (a) the period beginning on the Start Date and ending on September 30, 2019; (b) October 1, 2019 through December 31, 2019; and (c) January 1, 2020 through March 31, 2020.
1.35 “Diligent Efforts” means, with respect to an activity to be undertaken by a Party pursuant to this Agreement, the level of efforts and resources normally used by such Party with respect to a pharmaceutical product owned or controlled by such Party, or to which such Party has similar rights, which product is of similar market potential and strategic value and is at a similar stage in its development or life as is the Product, taking into account all relevant factors, including issues of safety, efficacy, product profile, the competitiveness of the marketplace, the proprietary position of the Product, regulatory matters, profitability of the Product and other relevant commercial factors.
1.36 “Disclosing Party” has the meaning set forth in Section 9.1.3.
1.37 “Disputes” has the meaning set forth in Section 13.1.
1.38 “Dual Target” has the meaning set forth in Exhibit B.
1.39 “Effective Date” has the meaning set forth in the preamble to this Agreement.
1.40 “Expiration Date” means March 31, 2020.
1.41 “FDA” means the United States Food and Drug Administration or any successor agency thereto.
1.42 “Fee Notice” has the meaning set forth in Section 5.5.1.
1.43 “First Position Detail” means, with respect to any product, a detail or presentation that is dedicated solely to such product and constitutes at least 70% of the total presentation time for all products presented during a Call in which such product is the first product presented to the health care professional.
1.44 “Force Majeure” has the meaning set forth in Section 14.2.
1.45 “GAAP” means United States generally accepted accounting principles applied on a consistent basis. Unless otherwise defined or stated, financial terms shall be calculated by the accrual method under GAAP.
1.46 “Governmental Authority” means any government (including any national, federal, state or local government), or political subdivision thereof, or any multinational or other organization, authority, agency or commission entitled to exercise any administrative, executive, judicial, legislative, police, regulatory or taxing authority or power, any court or tribunal, or any governmental arbitrator or arbitral body (or any department, bureau or division of any of the foregoing).
1.47 “Health Care Professional” means a health care professional with prescribing authority who treats urothelial cancer.
1.48 “Indemnified Party” has the meaning set forth in Section 12.2.1.
1.49 “Indemnifying Party” has the meaning set forth in Section 12.2.1.
1.50 “Initial Indication” means the first indication for which the Product receives Marketing Approval in the Territory, which the Parties expect to be treatment of adult patients with locally advanced or metastatic urothelial carcinoma which has (a) susceptible FGFR 3 or 2 genetic alterations and (b) progressed during or following at least one line of prior platinum-containing chemotherapy including within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy, where patients are selected for therapy based on an FDA-approved companion diagnostic for the Product; provided, however, that, with respect to any such indication for which the Product receives Marketing Approval from the FDA, “Initial Indication” shall be defined by the exact wording used in the Product Label and Insert as so approved.
1.51 “Xxxxxxx” has the meaning set forth in the preamble to this Agreement.
1.52 “Xxxxxxx Brand Usage Guidelines” means Xxxxxxx’x group guidelines on the Xxxxxxx brand visual and verbal identity as they apply to the Trademarks of Xxxxxxx and its Affiliates and the use of other companies’ names and logos, as notified to Company by Xxxxxxx from time to time.
1.53 “Xxxxxxx Indemnified Parties” has the meaning set forth in Section 12.1.2.
1.54 “Joint Commercial Team” has the meaning set forth in Section 4.2.1.
1.55 “Launch Date” means the date of the commercial launch of the Product in the Territory selected by Xxxxxxx. As of the Effective Date, the Parties expect that the Launch Date shall be on or about May 1, 2019.
1.56 “Laws” means all laws, statutes, rules, regulations, ordinances and other pronouncements having the effect of law in any country, state, province, county, city or other political subdivision, and includes any rule or regulation of any Governmental Authority that may be in effect from time to time in the Territory.
1.57 “License Agreement” means that certain Collaboration and License Agreement between Xxxxxxx Pharmaceutica N.V. and Astex Therapeutics Limited executed in June 2008, as amended, pursuant to which Xxxxxxx was granted a license under one or more patents covering the Product.
1.58 “Losses” has the meaning set forth in Section 12.1.1.
1.59 “Marketing Approval” means, with respect to any product, approval by the FDA of an NDA for such product.
1.60 “Milestone Event” has the meaning set forth in Section 5.3.
1.61 “Milestone Payment” has the meaning set forth in Section 5.3.
1.62 “Minimum Number of Details Requirement” has the meaning set forth in Exhibit B.
1.63 “Minimum PDE Requirement” has the meaning set forth in Exhibit B.
1.64 “Minimum Reach Requirement” has the meaning set forth in Exhibit B.
1.65 “Minimum Top Target Requirement” has the meaning set forth in Exhibit B.
1.66 “NDA” means, with respect to any product, a New Drug Application for such product and all supplements to such New Drug Application filed pursuant to the requirements of the FDA.
1.67 “Net Sales” means [***].
1.68 “Other Company Employees” has the meaning set forth in Section 2.2.2(a).
1.69 “Party” means each of Xxxxxxx and Company, which together are referred to as the “Parties”.
1.70 “Passing Score” has the meaning set forth in Section 2.2.2(c).
1.71 “Payee Party” means, with regards to a payment pursuant to this Agreement, the Party that receives such payment from the other Party under this Agreement.
1.72 “Paying Party” means, with regards to a payment pursuant to this Agreement, the Party that makes such payment to the other Party under this Agreement.
1.73 “PDE” shall mean, with respect to any product, a primary detail equivalent, which consists of either a First Position Detail of such product or two Second Position Details of such product, such that a First Position Detail shall count as [***] PDE and a Second Position Detail shall count as [***] PDE.
1.74 “Performance Failure Notice” has the meaning set forth in Section 3.2.4(b).
1.75 “Person” means, as applicable, an individual, sole proprietorship, partnership, limited partnership, limited liability partnership, corporation, limited liability company, business trust, joint stock company, trust, incorporated association, joint venture or similar entity or organization, including a Governmental Authority.
1.76 “PMS” has the meaning set forth in Section 6.4.
1.77 “Product” means any or each of the tablets containing erdafitinib as its sole active ingredient in a dosage amount of 3 mg, 4 mg or 5 mg and that is currently expected to be approved by the FDA for the Initial Indication, as currently manufactured by or on behalf of Xxxxxxx or its Affiliate and planned to be marketed under the trademark BALVERSA™.
1.78 “Product Complaint” means an oral, written or electronic communication from any Person that implies dissatisfaction regarding the identity, purity, quality or stability of the Product.
1.79 “Product Label and Insert” means (a) all labels and other written, printed or graphic material affixed to any container, packaging or wrapper utilized with the Product; and (b) any written material physically accompanying the Product, including the Product package inserts.
1.80 “Product-Specific Training” means training with respect to (a) sales and scientific materials regarding the disease state information on urothelial cancer, (b) currently available clinical data supporting use of the Product for the treatment of urothelial cancer, and (c) clinical data for products that compete with the Product.
1.81 “Product Trademarks” means any Trademarks as may be selected by Xxxxxxx and its Affiliate, in their sole discretion, for use in connection with the Product in the Territory, including any Trademark owned or controlled by Xxxxxxx or its Affiliates that includes the name “BALVERSA”. For purposes of clarity, the term “Product Trademark” shall not include the corporate names and logos of either Party.
1.82 “Promotion” means the (a) Detailing of the Product in the Territory for the Initial Indication and (b) performance of the other promotional activities for the Product set forth in the Brand Plan. “Promote” and “Promoting,” when used as a verb, means to engage in such Promotion.
1.83 “Promotion Rules” means: (a) the PhRMA Code on Interactions with Health Care Professionals; and (b) upon reasonable notice by Xxxxxxx to Company, any other similar rules,
policies or procedures with respect to the promotion of pharmaceutical products in the Territory that Xxxxxxx deems necessary or advisable to follow (including Xxxxxxx’x compliance policies).
1.84 “Promotional Materials” has the meaning set forth in Section 3.4.2.
1.85 “Quarter End Date” has the meaning set forth in Section 1.16.
1.86 “Receiving Party” has the meaning set forth in Section 9.1.3.
1.87 “Regulatory Approval” means all technical, medical and scientific licenses, registrations, authorizations and approvals (including Marketing Approvals and labeling approvals) of all applicable Regulatory Authorities necessary for the commercial distribution, marketing, promotion, offer for sale, use, import and sale of a pharmaceutical product in a regulatory jurisdiction.
1.88 “Regulatory Authority” means any authority, agency, commission, official or other instrumentality inside or outside the Territory, including the FDA, having jurisdiction over the manufacture of Product for sale in the Territory, or over the commercial distribution, marketing, promotion, offer for sale, use, import or sale of the Product in the Territory.
1.89 “Remediation Plan” has the meaning set forth in Section 3.2.4(b).
1.90 “Sales Force” has the meaning set forth in Section 3.3.2(a).
1.91 “Sales Representative” means a sales representative used by Company to perform Details of the Product for the Initial Indication to Health Care Professionals in the Territory. Sales Representative shall not include any key account manager, medical science liaison or regional sales manager.
1.92 “Second Position Detail” means, with respect to any product, a detail or presentation that is dedicated solely to such product and constitutes at least 30% of the total presentation time for all products presented during a Call in which such product is the second product presented to the health care professional.
1.93 “Service Fee” has the meaning set forth in Section 5.1.
1.94 “Start Date” means the first date upon which Sales Representatives are able to Detail the Product in accordance with this Agreement and as approved by Xxxxxxx, which may be before, on or after the Launch Date.
1.95 “Supplementary Training” means supplemental training relating to a Product, including refresher training, training regarding any emerging Product safety information, or new Promotional Materials or Product messaging.
1.96 “Target” means a Health Care Professional who treats patients for locally advanced
or metastatic urothelial cancer and is included in the Target List in accordance with this Agreement.
1.97 “Target List” has the meaning set forth in Section 2.2.1.
1.98 “Tax” or “Taxes” means any present or future taxes, levies, imposts, duties, charges, assessments or fees of any nature (including interest, penalties and additions thereon or thereto) that are imposed upon a Party by a Governmental Authority or other taxing authority under any applicable Laws.
1.99 “Term” has the meaning set forth in Section 8.1.1.
1.100 “Territory” means the United States of America, including its territories and possessions.
1.101 “Third Party” means any Person other than a party to this Agreement or any of its Affiliates.
1.102 “Trademark” means any trademark, trade dress, trade name, brand name, logo, corporate name or service xxxx, used in connection with any product or service.
1.103 “Training Activities Plan” has the meaning set forth in Section 2.2.2(a).
ARTICLE II
BRAND PLAN
2.1 Brand Plan Generally. A written plan for the marketing and promotion of the Product for the Initial Indication in the Territory pursuant to this Agreement for Calendar Year 2019 is attached to this Agreement as Exhibit A (the “Brand Plan”). If the Launch Date is delayed beyond May 1, 2019, Xxxxxxx shall update the Brand Plan to adjust the Parties’ obligations appropriately to reflect such delay. Xxxxxxx shall have the sole authority and responsibility for updating the Brand Plan for Calendar Year 2020. Xxxxxxx shall use reasonable efforts to deliver the Brand Plan for Calendar Year 2020 to the Joint Commercial Team by no later than November 30, 2019.
2.2 Contents of Brand Plan. The Brand Plan shall include: (a) a description of the Target List; (b) the Training Activities Plan; (c) a description of the Call Plan; and (d) a description of the sales and promotional materials (including Health Care Provider and patient education sales materials and, where applicable, non-personal promotional materials) to be used during the relevant year in connection with the Product. The Brand Plan shall also include plans for other non-Detailing activities, if any, to be conducted in relation to the Product during the period covered by the Brand Plan, such as attendance at medical conferences and Xxxxxxx sales meetings, marketing plans for advisory boards and publication plans.
2.2.1 Target List. Prior to the Launch Date, Xxxxxxx shall provide to Company, in electronic form, a list that sets forth: (x) the name of each Target to which the Sales Representatives will perform Details; and (y) the priority classification of each such Target (high, medium or low). Such list, as amended from time to time by Xxxxxxx, shall be the “Target List”. Xxxxxxx shall furnish with or as part of the Target List the claims data upon which the Target priority classification was based if (a) Xxxxxxx is able to obtain an agreement with the relevant Third Party to provide such data to Company and (b) the Parties agree on which Party will bear the costs of providing such data to Company.
2.2.2 Training Activities Plan.
(a) Training Activities Plan. The Brand Plan includes a plan that sets forth all of the training that Xxxxxxx deems necessary or advisable for the Sales Representatives and any other employees of Company conducting activities under this Agreement (such other employees, the “Other Company Employees”) to complete prior to conducting activities under this Agreement (the “Training Activities Plan”). The Training Activities Plan shall indicate which Party is responsible for providing such training, when such training will be provided and how such training will be provided (e.g., in person or remotely, which may include live audio/video conference calls, or electronically such as via learning management systems). The initial Training Activities Plan includes a plan for conducting and completing the Product-Specific Training and (as applicable) state Law compliance training of the Sales Representatives before the Launch Date. The initial Training Activities Plan also includes a plan for conducting and completing before the Launch Date compliance training of the Sales Representatives and the Other Company Employees in a manner consistent with all applicable pharmaceutical industry standards. Xxxxxxx may update the Training Activities Plan from time to time to include any additional training that Xxxxxxx deems necessary or advisable to refresh or update the knowledge of the Sales Representatives and the Other Company Employees.
(b) Training Responsibilities. Xxxxxxx shall conduct all Product-Specific Training to the Sales Representatives and Other Company Employees. Company shall at all times ensure that each Sales Representative and Other Company Employee (including Sales Representatives and Other Company Employee that are engaged after Launch Date) has received the Product-Specific Training and any other training set forth in the Training Activities Plan.
(c) Examination. Xxxxxxx shall administer to each Sales Representative an examination of the Product-Specific Training topics and any other training topics that Xxxxxxx deems necessary or advisable. The first such examination of the Sales Representatives shall occur no later than the Launch Date. Xxxxxxx shall determine the minimum score that is considered a minimum passing score for each examination (the “Passing Score”). Company shall ensure that, before conducting any Detailing of the Product pursuant to this Agreement, each Sales Representative has completed the Product-Specific Training and other training described in the Training Activities Plan and has achieved a Passing Score on such examination. Upon Company’s request, Xxxxxxx shall provide, as soon as reasonably practicable, additional remedial training and re-testing of Sales Representatives who fail to achieve a Passing Score. Any Sales Representative
who does not obtain a Passing Score on such an examination shall not be permitted by Company to perform in-person presentations of the Product unless and until such Sales Representative is re-tested and achieves a Passing Score.
2.2.3 Call Plan. Xxxxxxx shall develop and provide to Company an annual plan that describes the amount, frequency and reach of Detailing to be performed by the Sales Representatives to the Targets on the Target List (the “Call Plan”).
ARTICLE III
PROMOTION
3.1 Scope.
3.1.1 Engagement; Obligations.
(a) Xxxxxxx hereby engages Company on a non-exclusive basis to Promote the Product for the Initial Indication in the Territory on the terms, and subject to the conditions, set forth in this Agreement, and Company hereby accepts such engagement. Company shall not Promote the Product for any indication other than the Initial Indication. Company shall not conduct any promotion or marketing activities with respect to the Product that are not set forth in the Brand Plan without the prior written consent of Xxxxxxx. Xxxxxxx and its Affiliates retain the right to Detail and otherwise promote the Product in the Territory.
(b) Each Party shall perform the obligations and activities assigned to it in, and comply with the applicable provisions of, the Brand Plan and this Agreement.
3.1.2 Retained Rights. Any rights of Xxxxxxx or any of its Affiliates related to the Product that are not expressly granted to Company hereunder shall be retained by Xxxxxxx or such Affiliate, including all decision-making and other authority relating to Product development, regulatory matters, medical affairs, distribution, manufacturing and supply, Product strategy, marketing, sales, pricing, discounting, reimbursement, life cycle management, positioning, marketing messages and other commercialization matters. Xxxxxxx shall book sales of the Product in the Territory and shall have the sole right and responsibility to manufacture the Product and to distribute the Product in the Territory. Company shall not distribute or sell the Product in the Territory, and nothing herein shall be construed to provide Company with any rights to develop, manufacture, supply, distribute or sell the Product in the Territory.
3.1.3 Compliance with Laws. Company shall ensure that all of its personnel involved in the activities set forth under this Agreement comply with all applicable Laws and the Promotion Rules. Company shall ensure that the Sales Representatives and Other Company Employees Promote the Product at all times in accordance with applicable Laws and the Promotional Materials provided and approved by Xxxxxxx, refrain from making any false or misleading statements about the Product and refrain from discussing any unapproved uses of the Product.
3.2 Detailing Requirements.
3.2.1 General. Subject to Xxxxxxx fulfilling its obligations under Section 2.2.2(b) to provide the initial training and under Section 3.4 to deliver the Promotional Materials, Company shall begin promoting and Detailing the Product for the Initial Indication to the Targets on the Start Date. Company shall perform Detailing during the Term in accordance with this Section 3.2, the Call Plan and the Detailing requirements set forth on Exhibit B. For reference, the term “Detail” is defined in Section 1.33.
3.2.2 Minimum Detailing Requirements. At a minimum, Company shall cause its Sales Force to satisfy the Minimum Number of Details Requirement, the Minimum Reach Requirement and, if applicable, the Minimum PDE Requirement and the Minimum Top Target Requirement set forth in Exhibit B in each Detailing Period. Company shall ensure that the Sales Force satisfies the Positioning Requirements set forth on Exhibit B. Details that do not satisfy the Positioning Requirements set forth on Exhibit B will not be counted for purposes of determining whether the Minimum Number of Details Requirement, the Minimum Reach Requirement, the Minimum PDE Requirement or the Minimum Top Target Requirement has been satisfied.
3.2.3 Effects of Failure to Meet Minimum Detailing Requirements. If Company fails to achieve the Minimum Number of Details Requirement, the Minimum Reach Requirement or, if applicable, the Minimum PDE Requirement or the Minimum Top Target Requirement in any Detailing Period, Xxxxxxx shall have the right to terminate this Agreement by giving thirty (30) days’ notice, unless:
(a) Company complied with and performed its Detailing activities in accordance with any Remediation Plans developed by Company and approved by Xxxxxxx during such Detailing Period; or
(b) if (i) neither Party provided a Performance Failure Notice under Section 3.2.4 during such Detailing Period and (ii) Company performs additional Details in the first month after such Detailing Period such that, if such Details had been performed during such Detailing Period, they would have been sufficient to cure the failure to achieve the Minimum Number of Details Requirement, the Minimum Reach Requirement, the Minimum PDE Requirement or the Minimum Top Target Requirement, as applicable. To avoid double-counting, such additional Details will not be taken into account when determining whether Company satisfies the Minimum Number of Details Requirement, Minimum Reach Requirement or, if applicable, the Minimum PDE Requirement or the Minimum Top Target Requirement in the then-current Detailing Period.
For clarity, (i) Company must achieve all of the applicable foregoing minimum requirements in order to avoid giving rise to Xxxxxxx’x rights and remedies under this Section 3.2.3, and (ii) such rights shall be in addition to any other rights and remedies that may be available to Xxxxxxx under applicable Laws in the event of any such failure on the part of Company.
3.2.4 Monthly Detailing Reports.
(a) No later than [***] ([***]) Business Days following the end of each month during the Term, Company shall report to Xxxxxxx the number of Details performed (and any other information necessary to determine whether the requirements set forth in Section 3.2.2 and Exhibit B have been satisfied) during such month by the Sales Representatives in accordance with this Agreement and the Call Plan, as reported by the Company Internal Detail Reporting System. The Joint Commercial Team shall review and discuss Company’s performance of its Detailing obligations on a monthly basis.
(b) In the event that either Party believes, based on such reports, review or discussion, that Company will fail to achieve the Minimum Number of Details Requirement, the Minimum Reach Requirement or, if applicable, the Minimum PDE Requirement or the Minimum Top Target Requirement under Section 3.2.2 for the then-current Detailing Period, such Party will promptly notify the other Party in writing (a “Performance Failure Notice”) and Company shall develop a plan to avoid such a failure within fifteen (15) Business Days after the end of the applicable month, which plan will be subject to Xxxxxxx’x approval, not to be unreasonably withheld or delayed (as so approved, a “Remediation Plan”).
(c) For clarity, the Joint Commercial Team shall have no authority to extend the time for performance or reduce the Minimum Number of Details Requirement, the Minimum Reach Requirement or, if applicable, the Minimum PDE Requirement or the Minimum Top Target Requirement without an amendment to this Agreement.
3.2.5 Ride-Alongs. Members of Xxxxxxx’x team shall have the right to conduct ride-alongs with the Sales Representatives for purposes of monitoring the Details delivered by the Sales Representatives upon Xxxxxxx’x request. Xxxxxxx will give reasonable notice to Company sales management of each request.
3.3 Sales Representatives.
3.3.1 Qualifications. Company shall ensure that each Sales Representative: (i) is a full-time employee of Company and a full-time member of its sales force; (ii) possesses skills, training and experience that are consistent with industry standards applicable to the promotion of an oncological pharmaceutical product; (iii) has completed the Product-Specific Training and other sales training described in this Agreement and the Brand Plan and achieved a Passing Score on an examination in accordance with Section 2.2.2(c); and (iv) has become adequately equipped and knowledgeable with respect to the Product, as determined in accordance with Company’s then-current standards for sales personnel selling pharmaceutical products in the Territory. No sales representative or other individual may be used by Company to perform in-person presentations of
the Product in the Territory unless and until such individual satisfies the conditions described in clauses (i) - (iv) above.
3.3.2 Size of Sales Force.
(a) At all times during the Term, Company shall use reasonable efforts to deploy and maintain a sales force (the “Sales Force”) of at least [***] ([***]) Sales Representatives who satisfy the conditions described in Section 3.3.1.
(b) Company shall notify Xxxxxxx (i) at least [***] ([***]) days in advance of any planned reduction by Company in the size of the Sales Force to less than [***] ([***]) Sales Representatives and (ii) promptly if the number of Sales Representatives on the Sales Force decreases to less than [***] ([***]). In either event, Company shall provide Xxxxxxx with a plan to continue meeting the Minimum Number of Details Requirements, Minimum Reach Requirements, Minimum PDE Requirements and, if applicable, Minimum Top Target Requirements under Section 3.2.2.
(c) If the average number of Sales Representatives on the Sales Force is less than twenty-five (25) over any forty-five (45)-day period, Xxxxxxx will have the right to terminate this Agreement by giving thirty (30) days’ notice.
3.3.3 Subcontracting. Company may not subcontract with or otherwise use any Affiliate or Third Party to perform any Detailing or any of its other obligations under this Agreement without the prior written consent of Xxxxxxx.
3.3.4 Compensation of Sales Force. In the event Company elects to provide incentives to Sales Representatives, such incentives will be appropriate, in accordance with the applicable Laws, and, in the aggregate, competitive in the marketplace with respect to the products promoted by the Sales Representatives. Xxxxxxx shall not have any responsibility for or authority over the hiring, supervision, termination or compensation of the Sales Representatives or any other Company employees or for any employee benefits of such employees.
3.3.5 Additional Obligations. Company shall ensure that the Sales Representatives do not identify or represent themselves as employees or agents of Xxxxxxx or any Affiliate of Xxxxxxx.
3.4 Promotional Materials.
3.4.1 Positioning and Messages. Xxxxxxx shall develop and, as deemed advisable or necessary by Xxxxxxx from time to time, update product positioning and core selling messages for the Promotion of the Product. Xxxxxxx agrees to consider in good faith Company’s feedback in the development of any such updates to such messaging.
3.4.2 Promotional Materials Development and Approval. Xxxxxxx shall be solely responsible for developing and providing to Company (at Xxxxxxx’x cost) all promotional materials
for use in connection with the Promotion of the Product (the “Promotional Materials”) and agrees to provide Company with sufficient quantities of the materials based on market demand and expected levels of Detailing efforts. Xxxxxxx agrees to consider in good faith Company’s feedback in the development of any new promotional materials during the Term. Such Promotional Materials shall comply with all applicable Laws and may include written sales and advertising materials, detail aids, brochures, hand-outs, reprints, booth panels and any other promotional support items. Company shall use only the Promotional Materials provided by Xxxxxxx and the Product Label and Insert in its Promotion of the Product in the Territory. Company shall not add any Company Trademark to the Promotional Materials or otherwise alter the Promotional Materials in any way. Company shall not develop or use any other promotional materials in its Promotion of the Product.
3.4.3 Xxxxxxx Right to Use Promotional Materials. Nothing in this Agreement shall restrict Xxxxxxx’x right to use any Promotional Materials for the purposes of promoting the Product in the Territory.
3.4.4 Revisions. Xxxxxxx may revise, update or develop additional Promotional Materials from time to time during the Term, as deemed necessary and appropriate by Xxxxxxx, including based on: (i) changes in the Product Label and Insert; (ii) requirements or mandates of the FDA or other Regulatory Authorities or any Laws; or (iii) changes in the Promotion Rules.
3.4.5 Revocation of Approval. If, at any time, Xxxxxxx notifies Company in writing that it no longer approves the use of specified Promotional Materials, Company shall immediately take action to remove the Promotional Materials from use by Sales Representatives and either (i) destroy such materials or (ii) return them to Xxxxxxx. The cost of such return shall be borne by Xxxxxxx.
3.5 Product Sales. Xxxxxxx shall have sole authority and responsibility for sale and distribution of the Product in the Territory. Company shall not, and shall not permit the Sales Representatives or Other Company Employees to, solicit or accept orders for the Product or otherwise engage in any distribution, sale or offer for sale of the Product, any Product samples or any other product containing erdafitinib, but rather shall promptly direct any orders that it receives for Product or Product samples, and cause the Sales Representatives and Other Company Employees to direct promptly any such orders they may receive, to Xxxxxxx or any Third Party designated by Xxxxxxx.
3.6 Product Recall. Xxxxxxx shall have sole authority and responsibility for any recall or withdrawal of the Product in the Territory. Following a decision by Xxxxxxx to conduct any such recall or withdrawal of the Product: (a) Xxxxxxx shall immediately notify Company of such decision, (b) Company shall immediately cease Detailing and all other promotion of the Product and (c) as soon as reasonably practicable, Xxxxxxx provide Company with a prepared statement for use in response to any inquiries regarding such recall or withdrawal. Company shall use such prepared statement to respond to any inquiries received with regard to the recall or withdrawal and shall not make any other statement regarding such recall or withdrawal except as required by
applicable Law. In the event of a recall or withdrawal, the obligations of the Parties under this Agreement (other than Xxxxxxx’x obligation to pay Service Fees or Milestone Payments to Company) will be suspended solely to the extent and for so long as necessary until the circumstances that were the reasons for the recall or withdrawal have been resolved.
3.7 Product Return. Xxxxxxx shall have the sole authority, right and responsibility to accept and handle, either directly or indirectly, any request to return Product in the Territory. Company shall not solicit the return of any Product and shall promptly direct any attempted returns and cause the Sales Representatives and Other Company Employees to direct promptly any attempted returns to Xxxxxxx or any Third Party designated by Xxxxxxx.
ARTICLE IV
GOVERNANCE
4.1 Authority. Xxxxxxx shall have sole decision-making authority with respect to all matters relating to the promotion and Detailing of the Product in the Territory under this Agreement (including making changes to the Brand Plan), but Xxxxxxx may not exercise such decision-making authority with respect to a change to the Brand Plan that would materially increase Company’s Detailing obligations or materially increase Company’s non-Detailing obligations.
4.2 Joint Commercial Team.
4.2.1 Formation; Purpose. Simultaneously with the execution of this Agreement the Parties shall establish a joint commercial team (the “Joint Commercial Team”) solely as a forum for the Parties’ representatives to discuss Company’s execution of the Brand Plan, potential changes to the Brand Plan and the promotion and Detailing of the Product to the Targets in the Territory. The Joint Commercial Team will have no decision-making authority. During the meetings of the Joint Commercial Team, the Parties may make recommendations to one another with respect to Company’s execution of the Brand Plan, potential changes to the Brand Plan and the promotion and Detailing of the Product to the Targets in the Territory.
4.2.2 Membership. The Joint Commercial Team shall consist of at least three (3) representatives of each Party, appointed by such Party from among its (or its Affiliates’) employees that have a level of experience customary for a committee of this type. Either Party may remove and replace any member that it appointed, with or without cause, at any time by prior notice to the other Party. The Joint Commercial Team shall at all times be chaired by a representative of Xxxxxxx. The chairperson shall be responsible for calling meetings, preparing and circulating an agenda in advance of each meeting, and preparing and issuing minutes of each meeting within thirty (30) days thereafter or within a timeframe agreed by the Parties.
4.2.3 Meetings. The Joint Commercial Team shall meet monthly. Meetings of the Joint Commercial Team may be held in person or by audio or video teleconference with the consent of each Party. Each Party shall bear its own costs associated with the attendance of its appointees at such meetings. Each Party shall ensure that at least two (2) of its appointed members
(or their alternates) attend each meeting. Other employee representatives of each Party may attend meetings of the Joint Commercial Team.
4.3 BALVERSA Sales Advisory Team.
4.3.1 Formation; Purpose. Upon request by Xxxxxxx, the Parties shall establish a BALVERSA Sales Advisory Team (the “BSAT”). The BSAT will serve solely as an advisory forum and will have no decision-making authority.
4.3.2 Membership. The BSAT shall consist of at least one Sales Representative per region, at least two (2) regional managers of Company and at least one representative of Xxxxxxx’x BALVERSA marketing team. Either Party may remove and replace any member that it appointed, with or without cause, at any time by prior notice to the other Party. The BSAT shall at all times be chaired by a representative of Xxxxxxx. The chairperson shall be responsible for calling meetings.
4.3.3 Meetings. The BSAT shall meet every other week during the first sixty (60) days after the Launch Date and monthly for the rest of the Term, with each meeting not to exceed ninety (90) minutes in duration. Meetings of the BSAT shall be held by audio or video teleconference and the parties agree to make reasonable efforts to ensure the meetings do not interfere with territory detailing time. Each Party shall bear its own costs associated with the attendance of its appointees at such meetings. Other employee representatives of each Party may attend meetings of the BSAT.
ARTICLE V
COMPENSATION
5.1 Definitions.
5.1.1 “Baseline” means (i) with respect to Calendar Year 2019, [***] Dollars ($[***]); and (ii) with respect to Calendar Year 2020, [***]Dollars ($[***]).
5.1.2 “Cumulative Net Sales” means, with respect to any Calendar Quarter, the aggregate amount of Net Sales that were made during such Calendar Quarter and any prior Calendar Quarter(s) during the same Calendar Year.
5.2 Service Fee. In partial consideration of Company’s Promotion of the Product in accordance with the terms of this Agreement, and subject to the terms and conditions of this Agreement, with respect to each Calendar Quarter during Calendar Year 2019 and Calendar Year 2020, Xxxxxxx shall pay Company a service fee (the “Service Fee”), as follows:
(a) with respect to each Calendar Quarter during Calendar Year 2019, an amount equal to (i) [***] percent ([***]%) of that portion of Cumulative Net Sales that is greater than the Baseline for Calendar Year 2019, less (ii) the total Service Fees that have been invoiced by Company to Xxxxxxx for all preceding Calendar Quarters of Calendar Year 2019; and
(b) with respect to each Calendar Quarter during Calendar Year 2020, an amount equal to (i) [***] percent ([***]%) of that portion of Cumulative Net Sales that is greater than the Baseline for Calendar Year 2020, less (ii) the total Service Fees that have been invoiced by Company to Xxxxxxx for all preceding Calendar Quarters of Calendar Year 2020.
Unless and until the Cumulative Net Sales exceed the Baseline for a particular Calendar Year, the Service Fee shall be zero. The foregoing calculation method is intended to ensure that each Service Fee for a Calendar Quarter includes a true-up amount of all Service Fees earned year-to-date in the same Calendar Year.
5.3 Milestones.
5.3.1 In partial consideration of Company’s Promotion of the Product in accordance with the terms of this Agreement, and subject to the terms and conditions of this Agreement, Xxxxxxx shall pay Company milestone payments in accordance with this Section 5.3. Xxxxxxx shall notify Company in the applicable Fee Notice the first time the Cumulative Net Sales in the applicable Calendar Year exceed the amounts set forth in the following table (each, a “Milestone Event”). Xxxxxxx shall pay to Company the applicable milestone payments set forth in the table below (each, a “Milestone Payment”) within [***] ([***]) days after receipt of an invoice from Company with respect to achievement of each Milestone Event. Each Milestone Payment shall be non-refundable and non-creditable.
Milestone Event | Milestone Payment |
Upon the first occasion that Cumulative Net Sales in Calendar Year 2019 exceed US$[***] | US$[***] |
Upon the first occasion that Cumulative Net Sales in Calendar Year 2019 exceed US$[***] | US$[***] |
Upon the first occasion that Cumulative Net Sales in Calendar Year 2019 exceed US$[***] | US$[***] |
Upon the first occasion that Cumulative Net Sales in Calendar Year 2020 exceed US$[***] | US$[***] |
5.3.2 Each Milestone Payment shall be payable only once upon the first occurrence of the relevant Milestone Event, even if the Milestone Event occurs multiple times.
In the event Regulatory Approval of the Product for the Initial Indication in the Territory is delayed beyond May 18, 2019, or in the event commercial availability of the Product is delayed beyond June 1, 2019, Xxxxxxx agrees to make proportional adjustments to the Milestone Events in 2019, Milestone Payments in 2019 and the Baseline for 2019
consistent with the period of delay in Regulatory Approval or commercial availability, whichever is greater, as shown in the following sample calculation.
Sample Calculation:
Example: Regulatory Approval date is June 18, 2019 – 30 days delayed approval
New Baseline for Calendar Year 2019:
$[***] – ($[***] X [***] days / [***] days) = $[***]
New Milestone Event and Payment for First 2019 Milestone:
Cumulative Net Sales:
$[***] – ($[***]X [***] days / [***] days) = $[***]
Payment:
$[***] – ($[***] X [***] days / [***] days) = $[***]
5.4 Costs. Unless otherwise expressly stated in this Agreement, each Party shall bear and be responsible for all internal and out-of-pocket costs and expenses incurred by such Party in the performance of this Agreement.
5.5 Reports and Payments.
5.5.1 After the end of each Calendar Quarter of Calendar Year 2019 and 2020, Xxxxxxx shall calculate in good faith, based on Xxxxxxx’x Books and Records and in accordance with Xxxxxxx’x customary and consistently-applied accounting practices, the Net Sales in such Calendar Quarter and for such Calendar Year in the aggregate, as well as the Cumulative Net Sales and the Service Fee for such Calendar Quarter. Xxxxxxx shall deliver to Company, within thirty (30) days following the last day of such Calendar Quarter, a report setting forth the Cumulative Net Sales, the Service Fee and, if applicable, the Milestone Payment for such Calendar Quarter (the “Fee Notice”).
5.5.2 Following receipt of a Fee Notice from Xxxxxxx pursuant to Section 5.5.1, Company shall invoice Xxxxxxx for the amount of the Service Fee payable with respect thereto, if any.
5.5.3 Subject to Xxxxxxx obtaining appropriate consents from its Third Party specialty pharmacy partner, and the Parties reaching mutual agreement on the allocation between them of the associated costs, if any, Xxxxxxx shall provide prescriber level unit sales data generated from the specialty pharmacy partner on a weekly basis for the Company to track business trends, direct resources, measure sales force effectiveness, detailing sensitivity, and to design an effective sales incentive program.
5.5.4 If a Party incurs any costs that are the responsibility of the other Party under this Agreement, such Party shall invoice the other Party for such costs promptly following the
Calendar Quarter during which such costs were incurred. Such invoice shall include reasonable documentation of the costs for which the invoicing Party is seeking reimbursement.
5.5.5 All invoices delivered in accordance with Section 5.5.2 or 5.5.4 shall be paid by the Paying Party within [***] ([***]) days after receipt of such invoice.
5.5.6 All payments hereunder will be paid in U.S. Dollars and made available by bank wire transfer, in immediately available funds, to the account designated in writing by the Payee Party from time to time. Any changes to such account designation shall be made at least thirty (30) Business Days before the due date of the applicable payment.
5.6 Tax Matters. The Paying Party shall make all payments to the Payee Party under this Agreement without deduction or withholding for any Taxes except to the extent that any such deduction or withholding is required by any Law in effect at the time of payment. Each Party shall otherwise be responsible for its own income taxes and corporate taxes and any other Taxes payable by such Party arising under or in connection with this Agreement and shall pay all such Taxes and file any applicable tax returns on a timely basis. Any Tax required to be withheld on amounts payable under this Agreement shall timely and promptly be paid by the Paying Party on behalf of the Payee Party to the appropriate Governmental Authority, and the Paying Party shall furnish the Payee Party with proof of payment of such Tax. Any such Tax required to be withheld shall be an expense of and borne by the Payee Party. If any such Tax is assessed against and paid by the Paying Party, then the amount of such Tax withheld shall be treated as paid by the Paying Party to the Payee Party and the Payee Party shall indemnify and hold harmless the Paying Party from and against such Tax. Both Parties will cooperate with respect to all documentation required by any taxing authority or reasonably requested by the Paying Party to secure a reduction in the rate of applicable withholding Taxes.
ARTICLE VI
REGULATORY MATTERS
6.1 Regulatory Approvals.
6.1.1 Obligations of Xxxxxxx. Xxxxxxx, either itself or through one of its Affiliates, shall use Diligent Efforts to obtain Regulatory Approval of the Product for the Initial Indication in the Territory and to maintain the validity of such Regulatory Approval throughout the Term, including the submission of any additional information requested by any Regulatory Authority in connection with such Regulatory Approval. Xxxxxxx shall have no obligation to file any application for Regulatory Approval for the Product in respect of any indication other than the Initial Indication.
6.1.2 Rights of Xxxxxxx. Xxxxxxx shall be the holder of any and all Regulatory Approvals for the Product in the Territory and shall retain sole authority over all regulatory matters relating to the Product in the Territory.
6.1.3 Communications with Regulatory Authorities. As between Xxxxxxx and Company, Xxxxxxx shall have the sole authority and responsibility for communicating with any Regulatory Authority regarding any Regulatory Approval of the Product in the Territory or any application or filing therefor, or regarding any other obligation to any Regulatory Authority in relation to the Product. Except as expressly set forth herein or as required by applicable Law or as approved in advance by Xxxxxxx in writing, Company shall not communicate directly with any Regulatory Authority regarding the Product or otherwise take any action concerning any application, registration, authorization or approval under which the Product is manufactured, imported, maintained, marketed, reimbursed or sold in the Territory.
6.2 Pharmacovigilance Procedures. The Parties shall comply, and Company shall cause the Sales Representatives and Other Company Employees to comply, with the provisions set forth on Schedule 6.2, which govern the reporting of adverse events/adverse drug reactions associated with the Product, Product quality complaints associated with adverse events and other information concerning the safety of the Product within the Territory.
6.3 Product Complaints. Xxxxxxx shall have the sole right and responsibility to accept and handle any Product Complaint associated with the use of the Product. Company shall, and shall cause each of its Sales Representatives and Other Company Employees to, notify Xxxxxxx as soon as possible, but no later than 24 hours after the time he or she becomes aware of any Product Complaint associated with the Product, which notice shall include the name of the person making such Product Complaint, the Target that prescribed the Product (if any), and the date the relevant Sales Representative or Other Company Employee received such Product Complaint. Details regarding the process for notifying Xxxxxxx of any such Product Complaints shall be as set forth in “product complaints standard operating procedures”, a copy of which Xxxxxxx will provide to Company promptly following the Effective Date.
6.4 Post-Marketing Surveillance. Xxxxxxx shall have the sole right to conduct any post marketing surveillance with respect to the Product (“PMS”), whether such PMS is elected by Xxxxxxx or required by applicable Law.
6.5 Product Medical Inquiries. Xxxxxxx shall handle all medical questions from members of the medical profession regarding the Product in the Territory. Company shall refer all medical inquiries regarding the Product to Xxxxxxx through the established process outlined by Xxxxxxx for reporting of medical information requests, a copy of which Xxxxxxx will provide to Company promptly following the Effective Date.
6.6 Companion Diagnostic Inquiries. Company shall direct all inquiries relating to the Companion Diagnostic in accordance with the procedures established by Xxxxxxx, a copy of which Xxxxxxx will provide to Company promptly following the Approval Date.
6.7 Access, Affordability and Patient Support Inquiries. Company shall direct all inquiries relating to access, affordability, or patient support for the Product to the dedicated specialty pharmacy responsible for addressing such questions, in accordance with the procedures
established by Xxxxxxx, a copy of which Xxxxxxx will provide to Company promptly following the Effective Date.
ARTICLE VII
BOOKS, RECORDS AND AUDIT RIGHTS
7.1 Books and Records. Xxxxxxx and Company shall each maintain true and complete books and records with respect to the performance of its obligations hereunder, including the Company Internal Detail Reporting System and items underlying all payment obligations and invoices related to this Agreement (the “Books and Records”). Company shall maintain and manage its Books and Records in accordance with the records and information management requirements set forth on Exhibit C.
7.2 Books and Records Audits.
7.2.1 Right to Audit. Upon [***] ([***]) days prior notice from a Party (the “Auditing Party”), the other Party (the “Audited Party”) will permit an independent certified public accounting firm of internationally recognized standing selected by the Auditing Party and reasonably acceptable to the Audited Party, to examine the relevant Books and Records of the Audited Party, as may be reasonably necessary to verify the accuracy of the reports provided by the Audited Party pursuant to Section 3.2.4 or Section 5.5.1, as applicable, and the payments made or invoiced under this Agreement. An examination by each Auditing Party under this Section shall occur not more than once with respect to the Term and will be limited to the pertinent Books and Records for Calendar Year 2019 and Calendar Year 2020.
7.2.2 Scope of Audit. The independent certified public accounting firm will be provided access to the Books and Records of the Audited Party, and such examination will be conducted during the Audited Party’s normal business hours. The Audited Party may require the accounting firm to sign a standard non-disclosure agreement before providing the accounting firm access to the Audited Party’s facilities or Books and Records. The draft report of the accounting firm will be provided to the Audited Party so that justifying remarks can be included in the final report to be shared with the Auditing Party. Upon completion of the audit, the accounting firm will provide both Parties a final copy of the written report disclosing any discrepancies in the reports submitted by the Audited Party or the payments made or owed by the Audited Party, if any, and shall not include any confidential information (or additional information that is ordinarily not included in the Fee Notice or Detailing reports, as applicable) disclosed to the auditor during the course of the audit (such report, an “Audit Report”).
7.2.3 Results of Audit. If an Audit Report shows that the Audited Party underpaid or failed to pay any amount due to the Auditing Party, then the Audited Party will pay to the Auditing Party the amount of such underpayment or non-payment. Such payment shall be made within [***] ([***]) days after the Audited Party’s receipt of the Audit Report. If an Audit Report shows that Company overstated or otherwise misreported any information relating to Calls and Details, then Xxxxxxx shall be entitled to exercise any rights and seek any remedies it would have
had if such information had been accurately reported. If the Audited Party disagrees with the findings of the Audit Report, the Parties will first seek to resolve the matter between themselves, and in the event they fail to reach agreement the dispute resolution provisions outlined in Article XIII shall be followed to resolve the dispute. Any unpaid Service Fees or Milestone Payments finally determined by such resolution to be payable shall be paid within [***] ([***]) days after such final resolution. If an Audit Report shows any overpayment by either Party, such Party will be entitled to receive, at its option, either a refund of such overpayment or a credit equal to such overpayment against the amounts otherwise payable by such Party to the other Party under this Agreement.
7.2.4 Costs of Audit. If an Audit Report shows unpaid Service Fees or Milestones that exceeds [***] percent ([***]%) of the total amount owed by the Audited Party for the period being audited, then the reasonable and documented fees and expenses of such independent public accountant performing the examination shall be paid by the Audited Party, subject to reasonable substantiation thereof. Otherwise, the costs of the examination shall be solely borne by the Auditing Party.
7.3 Compliance Audits. Xxxxxxx or an authorized representative of Xxxxxxx, and any governmental agency that regulates a Party, may, at reasonable times during the Term and upon reasonable notice to Company, inspect and audit the Books and Records of Company with respect to Company’s obligations under this Agreement for the sole purpose of evaluating Company’s compliance with Sections 3.1.3, 3.4.2, 11.5 and 11.6 of this Agreement, applicable Laws and the Promotion Rules. The costs of any such audit shall be borne by Xxxxxxx, unless such audit reveals noncompliance by Company due to a failure by Company that is not excused by Xxxxxxx under this Agreement, in which case Company shall reimburse Xxxxxxx for any out-of-pocket costs reasonably incurred in connection with the audit.
ARTICLE VIII
TERM AND TERMINATION
8.1 Term; Termination.
8.1.1 Term. This Agreement shall commence on the Effective Date and, unless earlier terminated, shall expire on the Expiration Date (the “Term”). For the avoidance of doubt, the Parties agree that Xxxxxxx’x obligations under Section 5.2 (subject to Section 8.2.5) and Section 5.3 (subject to Section 8.2.5) shall remain in effect beyond the Expiration Date.
8.1.2 Failure or Delay in Obtaining Regulatory Approval. This Agreement may be terminated by either Party, before the date that Marketing Approval has been obtained for the Product for the Initial Indication in the Territory, in the event that Xxxxxxx withdraws its application for Marketing Approval for the Product for the Initial Indication in the Territory or is notified by FDA that Xxxxxxx’x application for such Marketing Approval in the Territory has been or will be denied. Further, this Agreement may be terminated by either Party in the event that Marketing Approval for the Product in the Territory is not obtained before June 30, 2019. Any such
termination pursuant to this Section 8.1.2 shall be effective thirty (30) days following written notice of such termination being given to the non-terminating Party.
8.1.3 Termination Scenarios Following Regulatory Approval. Without limiting either Party’s rights under Section 8.3, this Agreement may be terminated by either Party with thirty (30) days’ prior written notice to the other, in the event that (a) a Governmental Authority requires Xxxxxxx to withdraw permanently the Product from the market in the Territory for the Initial Indication or Regulatory Approval for the Product for the Initial Indication is otherwise withdrawn, (b) Xxxxxxx permanently withdraws the Product from the market in the Territory for the Initial Indication for safety reasons or (c) promotion and sale of the Product in the Territory for the Initial Indication has been suspended for more than three (3) months or is permanently suspended, in either case, as a consequence of and pursuant to Section 8.3. To the extent practicable, each Party will consult with the other Party before terminating this Agreement pursuant to this Section and will consider the other Party’s input in good faith; provided, however, that the decision to withdraw the Product pursuant to clause (b) above will be made by Xxxxxxx in its sole discretion, acting in good faith.
8.1.4 Material Breach. This Agreement may be terminated by either Party in the event that the other Party commits a material breach of this Agreement and (a) such breach shall not have been cured within thirty (30) days after the giving of notice of such material breach, unless (i) the specific provision to which such breach relates expressly provides for a different period, or (ii) the Parties mutually agree in writing to an extension of such period (the “Cure Period”); or (b) such breach, by its nature, is not curable. Unless such breach in clause (a) is cured during the Cure Period, such termination will be effective immediately upon the expiration of the Cure Period without any further action or notice by the non-breaching Party. In the case of a breach in clause (b), such termination will be effective thirty (30) days following written notice of such breach being given to the breaching Party.
8.1.5 Performance Failure. This Agreement may be terminated by Xxxxxxx in accordance with Section 3.2.3 or 3.3.2(c).
8.1.6 Competing Products. In the event that Company commits a breach of Section 9.2 or Section 9.3, then Xxxxxxx shall have the right to terminate this Agreement in its entirety at any time immediately upon written notice to Company.
8.1.7 Insolvency Proceeding. This Agreement may be terminated by either Party, immediately and without notice, if the other Party at any time (a) commences a voluntary case or other proceeding seeking liquidation, reorganization or other relief with respect to itself or its debts under any bankruptcy, insolvency or other similar Law or seeking the appointment of a trustee, receiver, liquidator, custodian or similar official of it or of any substantial part of its property, or consents to any such relief or to the appointment of or taking possession by any such official in an involuntary case or other proceeding commenced against it, or makes a general assignment for the benefit of creditors, or takes any corporate action to authorize any of the foregoing, (b) has an involuntary case or other proceeding commenced against it seeking liquidation, reorganization or
other relief with respect to it or its debts under any bankruptcy, insolvency or other similar Law or seeking the appointment of a trustee, receiver, liquidator, custodian or other similar official of it or any substantial part of its property, and such involuntary case or other proceeding remains undismissed and unstayed for a period of ninety (90) days; or an order for relief is entered against such Party under applicable bankruptcy Laws, or (c) is insolvent or is generally unable to pay its debts as they become due.
8.1.8 Force Majeure. This Agreement may be terminated by either Party in accordance with Section 14.2.
8.1.9 Health Care Compliance. This Agreement may be terminated by Xxxxxxx in accordance with Section 2(d) of Exhibit D.
8.1.10 Third Party Agreement. This Agreement may be terminated by Xxxxxxx with effect on December 31, 2019 or January 31, 2020, in either case with fifteen (15) days’ advance written notice, and only in the event that Xxxxxxx has entered into an agreement with a Third Party that provides for (a) a license, sublicense, assignment, divestiture or other transfer or disposition of substantial rights or assets relating to the Product in the Territory, or (b) a collaboration involving the development and/or commercialization in the Territory of the Product or any other pharmaceutical product that contains erdafitinib. If Xxxxxxx terminates this Agreement pursuant to this Section 8.1.10, then Xxxxxxx shall, within [***] ([***]) days following the effective date of such termination, pay Company an amount equal to $[***] and, if the Milestone Event listed in the first line of the table in Section 5.3.1 has occurred, Xxxxxxx shall pay to Company an additional amount of $[***] (which shall be in addition to the Milestone Payment earned in respect of such Milestone Event).
8.1.11 Company Product Additional Indication. This Agreement may be terminated by Xxxxxxx with thirty (30) days’ prior written notice in the event that the Company Product is approved by the FDA for use in the treatment of urothelial cancer in any patient population in the Territory.
8.2 Effect of Termination or Expiration.
8.2.1 Materials. Upon the effective date of termination or expiration of this Agreement, Company shall immediately (a) cease, and cause the Sales Representatives and Other Company Employees to cease, all Promotion of the Product, (b) discontinue the use of any Promotional Materials, and (c) discontinue the use of any Xxxxxxx sales data, Target List and other documents and data related to the Product provided to Company by Xxxxxxx hereunder. As requested by Xxxxxxx, Company shall either maintain (subject to the provisions of Section 9.1 and Exhibit C) or promptly destroy (and certify to Xxxxxxx the destruction) or return to Xxxxxxx all Promotional Materials, all training materials and all other materials related to the Product provided by Xxxxxxx pursuant to this Agreement or the Brand Plan. With respect to any information, data, or reports provided by Xxxxxxx to Company under this Agreement, including Xxxxxxx sales data, that Xxxxxxx requests Company to destroy or return, Company shall upon the effective date of
termination or expiration of this Agreement remove such information from its internal systems and certify to Xxxxxxx to such removal; provided, however, that such information shall not be required to be removed from inactive back-up computer files created pursuant to standard, automated archiving procedures.
8.2.2 Confidential Information. Following the effective date of termination or expiration of this Agreement, without prejudice to Section 8.2.1, each Party shall use reasonable efforts to return, destroy or maintain (subject to the provisions of Section 9.1 and Exhibit C), at the Disclosing Party’s election, all Confidential Information of the other Party (provided that the Receiving Party may keep one copy of such Confidential Information subject to an ongoing obligation of confidentiality for archival purposes only).
8.2.3 Transition Plan. The Parties shall reasonably cooperate in good faith to effect the transition to Xxxxxxx of all Product promotional activities to minimize disruptions to customers and patients. In furtherance of the foregoing, and at the request of either Party, the Joint Commercial Team, reasonably in advance of the expected end of the Term shall develop and approve a transition plan that contains, among other things, a plan for notifying Targets and other customers or health care providers of such termination or expiration and transition, and, if applicable, provides for the completion of any events set forth in a Brand Plan which are already scheduled but will take place after the effective date of termination or expiration.
8.2.4 Non-Exclusive Remedies. The consequences set forth in this Section 8.2 are not intended to be the exclusive remedies of the Parties in connection with the breach of or termination of this Agreement.
8.2.5 Compensation in Certain Termination Events. If this Agreement is terminated pursuant to any of the following Sections, Xxxxxxx shall not be obligated to pay to Company (a) any Service Fees with respect to any period after the effective date of termination of this Agreement or (b) any Milestone Payments with respect to any Milestone Events that are achieved after the effective date of termination of this Agreement: Section 8.1.4 (if terminated by Xxxxxxx for breach by Company), 8.1.5, 8.1.6, 8.1.7 (if terminated by Xxxxxxx for the insolvency of Company), 8.1.8 (if terminated by Xxxxxxx for Force Majeure applicable to Company), 8.1.9, 8.1.10 (except that this Section 8.2.5 shall not affect Xxxxxxx’x obligation to pay the amounts set forth in Section 8.1.10 as being payable in accordance with and subject to the conditions set forth in such Section 8.1.10) or 8.1.11.
8.2.6 Survival. Termination or expiration of this Agreement shall not relieve a Party of any liability for any breach that occurred, or of any obligation to make payment that accrued, before or on the effective date of such termination or expiration, nor prejudice either Party’s right to obtain performance of any obligation provided for in this Agreement that survives termination or expiration. All provisions of this Agreement which, in accordance with their terms, are intended to have effect after the expiration or termination of this Agreement shall survive such termination or expiration, including: Sections 3.1.2, 3.2.4(a) (with respect to the last month of the Term), 5.2 (subject to Section 8.2.5), 5.3 (subject to Section 8.2.5), 5.5.1, 5.5.2, 5.5.5, 5.5.6, 5.6,
7.1, 7.2, 8.1.1 (last sentence only), 8.1.10 (only if the Agreement is terminated pursuant to Section 8.1.10), 8.2, 9.1, 10.2, 11.8, and Articles XII, XIII and XIV.
8.3 Suspension of Product Promotion
8.3.1 Right to Suspend. Xxxxxxx shall have the right to require that both Parties suspend the promotion of the Product in the Territory for the Initial Indication if Xxxxxxx decides, in its sole discretion, acting in good faith, that it is necessary to do so due to safety reasons, or to comply with applicable Law or a request or mandate of a Regulatory Authority, or because of any Third Party’s claim or potential claim of intellectual property infringement in relation to the Product. In any such event, Company shall cease promoting the Product in the Territory immediately upon Company’s receipt of notice from Xxxxxxx directing it to do so, and Xxxxxxx shall have the right to cease the sale and/or distribution of the Product for so long as promotion thereof is suspended. Xxxxxxx shall discuss its decision with Company as soon as it is practicable to do so and consider Company’s input in good faith; provided, however, that all decisions regarding such matters shall be made by Xxxxxxx in its sole discretion. If Xxxxxxx decides to end any such suspension of the promotion, sale or distribution of the Product in the Territory, Xxxxxxx shall immediately notify Company of its decision.
8.3.2 Adjustments Due to Suspension. In the event Xxxxxxx suspends the promotion and/or sale of the Product in the Territory for the Initial Indication pursuant to Section 8.3.1, and such suspension results in any restriction or prohibition on Detailing activities by Company for a period of one week or more, then the Parties will discuss and attempt to agree upon an appropriate adjustment to Company’s Detailing obligations under the Brand Plan and/or the Baselines.
ARTICLE IX
CONFIDENTIALITY; RESTRICTIVE COVENANTS
9.1 Confidentiality.
9.1.1 Non-Disclosure and Non-Use. Except to the extent expressly authorized by this Agreement or otherwise agreed in writing, each of Xxxxxxx and Company agrees that, during the Term and until the conclusion of the [***] ([***]) year period beginning upon the expiration or earlier termination of this Agreement, such Party shall: (a) maintain in confidence the Confidential Information of the other Party using not less than the efforts such Party uses to maintain in confidence its own confidential or proprietary information of similar kind and value (but not less than reasonable efforts); (b) not disclose the Confidential Information of the other Party to any Third Party; and (c) not use the Confidential Information of the other Party for any purpose other than as provided for in this Agreement.
9.1.2 Certain Information. The Brand Plan (including the Training Activities Plan and Call Plan), the Target List and all information and data contained within such documents is deemed to be the Confidential Information of Xxxxxxx. The reports of Details provided by Company pursuant to Section 3.2.4 and all data in the Company Internal Detailing System relating
to the Product are deemed to be (a) the Confidential Information of both Parties during the Term and (b) the Confidential Information of Xxxxxxx after the Term.
9.1.3 Exceptions. The obligations of Section 9.1.1 do not apply to any portion of the Confidential Information of a Party (the “Disclosing Party”) that the other Party (the “Receiving Party”) can show by competent written evidence:
(a) is already known to the Receiving Party before the time of disclosure by the Disclosing Party, as evidenced by the Receiving Party’s written records made or obtained before the date of disclosure; provided, however, that this clause (a) shall not apply to the reports and data described in the second sentence of Section 9.1.2;
(b) is disclosed to the Receiving Party on a non-confidential basis by a Third Party who, to the knowledge of the Receiving Party, is under no obligation to the Disclosing Party (or any of its Affiliates) with respect to confidentiality, secrecy or restriction on the use of such information or data;
(c) is now, or hereafter becomes, through no act or failure of the Receiving Party or any of its Affiliates in violation of this Agreement, generally known or available to the public;
(d) is independently discovered or developed by or on behalf of the Receiving Party or any of its Affiliates (i) not pursuant to or in connection with this Agreement and (ii) without the use of or reference to the Confidential Information of the Disclosing Party as evidenced by the Receiving Party’s written records; or
(e) is publicly disclosed by the Disclosing Party, either before or after it is disclosed to the Receiving Party under this Agreement.
9.1.4 Permitted Disclosure. The Receiving Party may disclose the Disclosing Party’s Confidential Information only to the extent such disclosure is reasonably necessary in the following instances, or to the extent permitted under the other applicable provisions of this Agreement:
(a) to those of the Receiving Party’s Affiliates and its and their respective officers, directors, employees, agents, advisors and consultants who (a) are bound in writing (or, with respect to counsel to the Receiving Party, by professional or ethical obligations) by obligations of confidentiality and non-use substantially similar to and consistent with those of this Section 9.1, (b) need to receive the Confidential Information in order for the Receiving Party to exercise its rights, conduct the activities required by or fulfill its other obligations under this Agreement and (c) are made aware of the confidential nature of the information, and then only to the extent required for the Receiving Party to exercise its rights under, conduct the activities required by or fulfill its other obligations under this Agreement; provided that the Receiving Party shall be responsible and liable for any breach of the provisions of this Section 9.1 by any Person who receives Confidential Information pursuant to this Section 9.1.4(a);
(b) with respect to Xxxxxxx as the Receiving Party, to the FDA or other applicable Regulatory Authority where such disclosure is required in connection with any filing, application, or request for any Regulatory Approval of the Product in the Territory;
(c) to the extent that such disclosure is necessary to prosecute litigation for the protection, preservation, or return of Confidential Information or to enforce its rights under this Agreement;
(d) to comply with applicable Law or the rules of any stock exchange on which such Party’s securities (or the securities of a Party’s Affiliate) are traded, subject to the terms of Section 9.1.5;
(e) with respect to Xxxxxxx as the Receiving Party, to counterparties under the License Agreement to the extent such disclosure is required under the License Agreement or is advisable for the purpose of carrying out more fully Xxxxxxx’x obligations under this Agreement or otherwise increasing Net Sales of the Product in the Territory; or
(f) to comply with court orders or administrative orders pursuant to Law.
In the case of disclosure pursuant to Section 9.1.4(c), 9.1.4(d) or 9.1.4(f), the Receiving Party (i) shall, to the extent reasonably practicable under the circumstances, give reasonable advance notice of the disclosure requirement to the Disclosing Party, so as to provide the Disclosing Party with the opportunity to secure, to the extent available, a protective order (or similar remedy) or other assurance of confidential treatment of the Confidential Information to be disclosed, and (ii) shall reasonably cooperate with the Disclosing Party, at its expense and request, in seeking such protective orders or other relief.
Any permitted use of the Disclosing Party’s Confidential Information by the Receiving Party for purposes of its performance hereunder will not be deemed a license or other right of the Receiving Party to use any such Confidential Information for any other purpose. The Receiving Party shall not acquire any right, title, or interest in or to any Confidential Information (including copies and summaries thereof and extracts therefrom, whether tangible or in electronic or other form) of the Disclosing Party by virtue of its disclosure hereunder.
9.1.5 Terms of this Agreement. The terms of this Agreement are deemed to be, and shall be treated by each Party as, Confidential Information of each Party. Either Party may disclose the terms of this Agreement and other information relating to this Agreement or the transactions contemplated by this Agreement to the extent required, in the reasonable opinion of such Party’s counsel, to comply with the rules and regulations promulgated by the United States Securities and Exchange Commission, New York Stock Exchange, Nasdaq Stock Market or similar security regulatory authorities or stock market in other countries. If a Party intends to disclose this Agreement or any of its terms or other Confidential Information of the other Party pursuant to this Section 9.1.5, such Party will, except where impracticable or not legally permitted, give reasonable advance notice to the other Party of such disclosure and seek confidential treatment
of portions of this Agreement or such terms or information, as may be reasonably requested by the other Party in a timely manner.
9.1.6 Prior Non-Disclosure Agreement. As of the Effective Date, the terms of this Section 9.1 supersede any prior non-disclosure, secrecy or confidentiality agreement between the Parties (or their Affiliates) relating to the subject matter of this Agreement, including the Mutual Confidentiality Agreement between the Parties dated February 1, 2019. Any information disclosed pursuant to any such prior agreement shall be deemed Confidential Information under this Agreement.
9.2 Exclusivity. During the Term, neither Company nor any of its Affiliates (including, for the avoidance of doubt, any Third Party that becomes an Affiliate of Company after the Effective Date) shall, alone or in collaboration with any Third Party, market, promote, sell, distribute or otherwise commercialize in the Territory any Competing Product without the prior written consent of Xxxxxxx. In the event that, after the Effective Date, a Third Party (an “Acquirer”) either (a) merges with Company, (b) acquires “control” (as defined in Section 1.4) of Company or (c) acquires substantially all the assets of the Company (each of (a), (b) and (c), an “Acquisition”), and such Acquirer or any of its Affiliates immediately prior to such Acquisition is commercializing a Competing Product in the Territory, then either Party shall have the right to terminate this Agreement on [***] ([***]) days written notice delivered within [***] ([***]) days of the closing of such Acquisition, and Company shall not be deemed to be marketing, promoting, selling, distributing or commercializing a Competing Product in breach of this Section for so long as it is conducting such activities solely through personnel who are not involved in any activities under this Agreement and do not have access to Xxxxxxx’x Confidential Information hereunder.
9.3 Restrictions on Promotion. During the Term, Company and its Affiliates (including, for the avoidance of doubt, any Third Party which becomes an Affiliate of Company after the Effective Date) (a) will not, whether alone or in collaboration with any Third Party or for itself or any Third Party, during the promotion of any product, compare such product (other than the Product) with the Product in any aspect nor disparage the Product in any manner, and (b) with respect to any such product that is a product of Company or its Affiliates and that Company or its Affiliates promotes, sells, distributes, or otherwise commercializes using or through a Third Party, will (i) cause any such Third Party, during the promotion of such product, not to compare such product with the Product in any aspect nor disparage the Product in any manner and (ii) not authorize any Third Party to make any such comparison or disparagement.
9.4 Limitation on Soliciting Employees. During the Term, Xxxxxxx shall not directly or indirectly solicit for employment any Sales Representative who is an employee of Company, and Company shall not directly or indirectly solicit for employment any employee of Xxxxxxx with whom Company has had contact in the course of the evaluation or negotiation of this Agreement or with whom Company interacts during the Term; provided, however, that the foregoing provision will not prohibit either Party from (a) conducting general solicitations of employment in publications (including but not limited to websites, newspapers and/or journals) available to the public, or solicitations through the use of search firms, and which, in any case, are not directed
specifically toward such employees of the other Party or (b) any contact with any such employee of the other Party (i) that was initiated by such employee without any solicitation prior thereto by the contacting Party (other than solicitation permitted by clause (a) of this sentence) or (ii) with whom the contacting Party is already in employment discussions as of the Effective Date, or (iii) by any person other than (A) one who was introduced to, or became aware of, the relevant employee of the other Party solely in connection with this Agreement, and (B) one who is acting at the direction or suggestion of a person described in (A).
ARTICLE X
INTELLECTUAL PROPERTY
10.1 Use of Trademarks. Xxxxxxx and its Affiliates shall retain all right, title and interest in and to its and their respective Trademarks. Company shall Promote pursuant to this Agreement only under the Product name and other Product Trademarks used by Xxxxxxx in the Territory. Xxxxxxx hereby grants to Company, during the Term, a non-exclusive, royalty free right to use such Product name and Product Trademarks, and Xxxxxxx corporate names and logos, solely to the extent they are included on the Promotional Materials and solely for the purpose of using the Promotional Materials to Promote in the Territory under this Agreement. Company shall not, without the express, prior written consent of Xxxxxxx, alter or modify in any manner any Product Trademark or any other Trademark of Xxxxxxx. Company agrees to comply with such Xxxxxxx standard guidelines regarding the use of the Product Trademarks and any other Trademarks of Xxxxxxx, and any amendments thereto, as Xxxxxxx provides to Company from time to time after the Effective Date (including the Xxxxxxx Brand Usage Guidelines).
10.2 Ownership of Intellectual Property Rights. Company acknowledges and agrees that Xxxxxxx or one of its Affiliates (a) is the sole and exclusive owner of all rights in and to the Product Trademarks and any other Trademarks of Xxxxxxx, including any form or embodiment thereof, and the goodwill now or hereafter associated therewith, (b) shall own the copyrights to all Promotional Materials and the Product Label and Insert, and (c) has the sole right to assert or control any action to enforce its rights in or to any of the Product Trademarks, any other Trademarks of Xxxxxxx or such copyrights and to receive the proceeds of any such action. Company further acknowledges and agrees that it does not, by virtue of this Agreement or its activities hereunder, obtain or acquire any right or interest in the Product Trademarks, any other Trademarks of Xxxxxxx, such copyrights, or any other intellectual property right of Xxxxxxx or its Affiliates. To the extent that Company, by operation of Law or otherwise, acquires any right (other than pursuant to this Agreement) to any of the Product Trademarks, any other Trademarks of Xxxxxxx, such copyrights or such other intellectual property rights, Company shall assign to Xxxxxxx all such rights at Xxxxxxx’x cost and will not claim ownership. Company agrees that it shall not seek to register or obtain ownership rights in any of Xxxxxxx’x corporate names, logos, or Product Trademarks (or any confusingly similar trademark).
10.3 Prosecution and Maintenance. Xxxxxxx will have the right (and not the obligation) to prepare, file, prosecute and maintain any intellectual property right of Xxxxxxx or its Affiliates claiming or covering the Product or its use in its sole discretion and at its own cost.
10.4 Enforcement against Infringement. Xxxxxxx and Company will each promptly notify the other in writing of any alleged or threatened infringement by a Third Party in the Territory of any intellectual property right of Xxxxxxx or its Affiliates claiming or covering the Product or its use in treating urothelial cancer, or any alleged or threatened assertion by a Third Party of invalidity of any of the intellectual property rights of Xxxxxxx or its Affiliates claiming or covering the Product or its use in treating urothelial cancer in the Territory, of which such Party becomes aware. Xxxxxxx and its Affiliates shall have the sole right (but not the obligation) to prosecute any such infringement in its sole discretion and at its sole cost.
10.5 Third Party Infringement Claims. In the event that Xxxxxxx or its Affiliate(s) decides to obtain a license to intellectual property from a Third Party in the Territory in order to commercialize the Product, whether or not due to a Third Party claim, notice, or suit or other inter partes proceeding against Xxxxxxx, Company and/or their Affiliates alleging that the commercialization of the Product in the Territory infringes or misappropriates any intellectual property rights of such Third Party, Xxxxxxx and its Affiliate(s) shall be solely responsible for the costs associated with such license and Company shall provide reasonable cooperation to Xxxxxxx or its applicable Affiliate(s) in procuring and complying with such license.
ARTICLE XI
REPRESENTATIONS AND WARRANTIES; CERTAIN COVENANTS
11.1 Representations of Authority. Xxxxxxx and Company each represents and warrants to the other Party that, as of the Effective Date, it has full right, power and authority to enter into this Agreement and to perform its respective obligations under this Agreement and that it has the right to grant to the other Party the rights granted pursuant to this Agreement as set forth herein.
11.2 Consents. Xxxxxxx and Company each represents and warrants to the other Party that all necessary consents, approvals, and authorizations of all Government Authorities and other Persons required to be obtained by it as of the Effective Date in connection with the execution, delivery, and performance of this Agreement have been obtained by the Effective Date.
11.3 No Conflict. Xxxxxxx and Company each represents and warrants to the other Party that the execution and delivery of this Agreement by it and the performance of its obligations hereunder (a) do not conflict with or violate any Laws existing as of the Effective Date as applicable to such Party and (b) do not conflict with, violate, breach, or constitute a default under any of its material contractual obligations existing as of the Effective Date.
11.4 Enforceability. Xxxxxxx and Company each represents and warrants to the other Party that, as of the Effective Date, this Agreement is a legal and valid obligation binding upon it and is enforceable against it in accordance with its terms, subject to the laws of bankruptcy, insolvency, and creditors’ rights.
11.5 Sales Representatives and Other Company Employees.
11.5.1 Company covenants to Xxxxxxx that:
(a) with respect to the Product, the Sales Representatives and Other Company Employees in the Territory shall make no statements, claims, or undertakings to any health care provider with whom they discuss or promote the Product that are not consistent with, nor provide nor use any labeling, literature, or other materials other than, the Product Label and Insert and those Promotional Materials provided and approved for use pursuant to this Agreement; and
(b) it shall ensure that all statements, comments and claims made by the Sales Representatives and Other Company Employees (i) about the Product, including as to efficacy and safety, are truthful and accurate and are consistent with and in strict compliance with the Product Label and Insert and all applicable Laws, and (ii) about Xxxxxxx in relation to the Product are truthful, accurate, and in strict compliance with all applicable Laws.
Any statement, claim or comment that is contained in the Promotional Materials or the Product Label and Insert, in each case, as in effect when such statement, claim or comment is made, shall be deemed not to violate this Section 11.5.1.
11.5.2 Company shall perform all Detailing and other promotional activities with respect to the Product in compliance with applicable Laws and the Promotion Rules.
11.6 Other Compliance Matters.
11.6.1 Company represents and warrants that it has established, and covenants that it will maintain during the Term, a compliance program consistent with the Compliance Program Guidance for Pharmaceutical Manufacturers published by the Office of Inspector General, U.S. Department of Health and Human Services.
11.6.2 Company represents and warrants that it has implemented, and covenants that it will maintain during the Term, adequate systems, policies, and procedures governing (1) interactions with health care professionals, (2) material that can be distributed or discussed with health care professionals, (3) the manner in which personnel should handle unsolicited requests for off-label information, and (4) the review and approval of all marketing, promotion, and sales materials, call plans, and incentive compensation structures. Company represents and warrants that such policies and procedures are and will be consistent with applicable Law and with this Agreement.
11.6.3 Company represents and warrants that neither Company, nor any of its employees, officers, directors, or agents, has been debarred by the FDA, is the subject of a conviction described in 21 U.S.C. 335a, or is subject to any similar sanction. Company represents and warrants that it has not, and covenants that it will not engage, in any capacity in connection with this Agreement, any person who has been debarred by FDA, is the subject of a conviction
described in 21 U.S.C. 335a, or is subject to any similar sanction. Company shall promptly inform Xxxxxxx in writing if it or any person performing services under this Agreement is debarred or is the subject of a conviction described in 21 U.S.C. 335a, or if any action, suit, claim, investigation, or legal or administrative proceeding is pending or threatened relating to the debarment or such conviction of Company or any such person performing services in connection with this Agreement. Upon written request from Xxxxxxx, Company shall, within ten (10) days, provide written confirmation that it has complied with the foregoing obligation.
11.6.4 Company represents and warrants that it is in compliance, and covenants that it will continue to comply during the Term, with all applicable Laws, rules and regulations, including the federal anti-kickback statute (42 U.S.C. § 1320a-7b), the related safe harbor regulations, and the Limitation on Certain Physician Referrals, also referred to as the “Xxxxx Law” (42 U.S.C. § 1395nn).
11.6.5 Company shall conduct activities in accordance with applicable state and federal Laws and any applicable regulations regarding Medicare, Medicaid, and other third party-payer programs, if any. Company represents and warrants that (1) it is not excluded from, and has not been convicted of any crime or engaged in any conduct that could result in exclusion from, participation in any state or federal healthcare program, as defined in 42 U.S.C. §1320a-7b(f), for the provision of items or services for which payment may be made by a federal healthcare program; (2) it has not contracted, and will not contract, with any employee, contractor, agent, or vendor to perform work under the Agreement who is excluded from participation in any state or federal healthcare program; and (3) it is not subject to a final adverse action, as defined in 42 U.S.C.§ 1320a-7a(e) and 42 U.S.C. § 1320a-7a(g), and has no adverse action pending or threatened against it. Company shall notify Xxxxxxx of any final adverse action, discovery of contract with an excluded entity or individual, or exclusion within thirty (30) days of such action.
11.6.6 Company will comply with Exhibit D.
11.6.7 Xxxxxxx represents and warrants that neither Xxxxxxx, nor any of its employees, officers, directors, or agents, has been debarred by the FDA, is the subject of a conviction described in 21 U.S.C. 335a, or is subject to any similar sanction. Xxxxxxx represents and warrants that it has not, and covenants that it will not engage, in any capacity in connection with this Agreement, any person who has been debarred by FDA, is the subject of a conviction described in 21 U.S.C. 335a or is subject to any similar sanction. Xxxxxxx shall promptly inform Company in writing if it or any person performing services under this Agreement is debarred or is the subject of a conviction described in 21 U.S.C. 335a, or if any action, suit, claim, investigation, or legal or administrative proceeding is pending or threatened relating to the debarment or such conviction of Xxxxxxx or any such person performing services in connection with this Agreement.
11.6.8 Xxxxxxx represents and warrants that it has established, and covenants that it will maintain during the Term, Promotional Materials which are truthful, accurate, and in strict compliance with all applicable Laws.
11.6.9 Xxxxxxx represents and warrants that it is in compliance, and covenants that it will continue to comply during the Term, with all applicable Laws, rules and regulations, including the federal anti-kickback statute (42 U.S.C. § 1320a-7b), the related safe harbor regulations, and the Limitation on Certain Physician Referrals, also referred to as the “Xxxxx Law” (42 U.S.C. § 1395nn).
11.6.10 Xxxxxxx shall conduct all activities hereunder in accordance with applicable state and federal Laws, including any applicable regulations regarding Medicare, Medicaid, and other third party-payer programs, if any. Xxxxxxx represents and warrants that (1) it is not excluded from, and has not been convicted of any crime or engaged in any conduct that could result in exclusion from, participation in any state or federal healthcare program, as defined in 42 U.S.C. §1320a-7b(f), for the provision of items or services for which payment may be made by a federal healthcare program; (2) it has not contracted, and will not contract, with any employee, contractor, agent, or vendor to perform work under the Agreement who is excluded from participation in any state or federal healthcare program; and (3) it is not subject to a final adverse action, as defined in 42 U.S.C.§ 1320a-7a(e) and 42 U.S.C. § 1320a-7a(g), and has no adverse action pending or threatened against it.
11.7 Infringement of Third Party Intellectual Property; Clinical Trial Data. Xxxxxxx represents and warrants to Company that, to its knowledge, as of the Effective Date, the manufacture, use, import, or sale of the Product in the Territory for the Initial Indication does not, and will not during the Term, infringe or misappropriate any intellectual property rights of any Third Party. Xxxxxxx represents and warrants to Company that, as of the Effective Date, [***].
11.8 Disclaimer. EXCEPT AS OTHERWISE EXPRESSLY SET FORTH IN THIS ARTICLE XI, NEITHER XXXXXXX NOR COMPANY, NOR ANY OF THEIR AFFILIATES, MAKES ANY REPRESENTATION OR EXTENDS ANY WARRANTY OF ANY KIND, EITHER EXPRESS OR IMPLIED, TO THE OTHER PARTY IN CONNECTION WITH THE PRODUCT, AND HEREBY DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE AND NON-INFRINGEMENT WITH RESPECT TO THE PRODUCT. EACH PARTY HEREBY DISCLAIMS ANY REPRESENTATION OR WARRANTY THAT THE EXPLOITATION OF THE PRODUCT PURSUANT TO THIS AGREEMENT WILL BE SUCCESSFUL OR THAT ANY PARTICULAR SALES LEVEL WITH RESPECT TO THE PRODUCT WILL BE ACHIEVED.
ARTICLE XII
INDEMNIFICATION; LIMITS ON LIABILITY
12.1 Scope of Indemnification.
12.1.1 Xxxxxxx shall indemnify and hold harmless Company, its Affiliates and its and their respective directors, officers, employees, and agents (collectively, the “Company Indemnified Parties”), from, against, and in respect of any and all liabilities, costs, fines, penalties, orders of any Governmental Authorities, Taxes, expenses, or amounts paid as damages or in settlement (in each case, including reasonable attorneys’ and experts fees and expenses), involving an Action asserted by a Third Party (collectively, “Losses”), incurred or suffered by the Company Indemnified Parties or any of them and arising out of or resulting from:
(a) any breach by Xxxxxxx or any of the other Xxxxxxx Indemnified Parties of any representation, warranty or covenant under this Agreement;
(b) the negligence or willful misconduct of Xxxxxxx or any of the other Xxxxxxx Indemnified Parties in connection with Xxxxxxx’x performance under this Agreement;
(c) any claim of death or bodily injury resulting from the use of the Product sold in the Territory; or
(d) any recall, withdrawal, product return or suspension of product promotion under Section 3.6, 3.7 or 8.3.
except, in each case ((a), (b) (c), and (d)), to the extent caused by the negligence or willful misconduct of Company or any of the other Company Indemnified Parties or the breach by Company of any of its representations, warranties or covenants set forth herein.
12.1.2 Company shall indemnify and hold harmless Xxxxxxx, its Affiliates, and its and their respective directors, officers, employees, and agents (collectively, the “Xxxxxxx Indemnified Parties”), from, against and in respect of any and all Losses incurred or suffered by the Xxxxxxx Indemnified Parties or any of them and arising out of or resulting from:
(a) any breach by Company or any of the other Company Indemnified Parties of any representation, warranty or covenant under this Agreement; or
(b) the negligence or willful misconduct of Company or any of the other Company Indemnified Parties in connection with Company’s performance under this Agreement;
except in each case ((a) and (b)), to the extent caused by the negligence or willful misconduct of Xxxxxxx or any of the other Xxxxxxx Indemnified Parties or the breach by Xxxxxxx of any of its representations, warranties or covenants set forth herein.
12.2 Notice and Control of Actions.
12.2.1 A Person entitled to indemnification under this Article XII (an “Indemnified Party”) shall give prompt written notification to the Person from whom indemnification is sought (the “Indemnifying Party”) of the assertion of any Action by a Third Party for which indemnification may be sought (it being understood and agreed, however, that the failure by an Indemnified Party to give such notice of a Third Party Action as provided in this Section 12.2.1 shall not relieve the Indemnifying Party of its indemnification obligation under this Agreement except and only to the extent that such Indemnifying Party is actually prejudiced as a result of such failure to give notice).
12.2.2 Within thirty (30) days after delivery of such notification, the Indemnifying Party may, upon written notice thereof to the Indemnified Party, assume control of the defense of such Action with counsel reasonably satisfactory to the Indemnified Party; provided, however, that the Indemnifying Party shall not have the right to control the defense of any Action against any Indemnified Party involving criminal charges or tax matters. If the Indemnifying Party does not assume control of the defense of an Action, the Indemnified Party shall control such defense.
12.2.3 The Party not controlling such defense shall reasonably cooperate with the other Party at such other Party’s request and expense, and may participate therein at its own expense; provided, however, that if the Indemnifying Party assumes control of such defense and the Indemnified Party reasonably concludes, based on advice from counsel, that the Indemnifying Party and the Indemnified Party have conflicting interests with respect to such Action, the Indemnifying Party shall be responsible for the reasonable fees and expenses of counsel to the Indemnified Party solely in connection with such Action; provided further, however, that in no event shall the Indemnifying Party be responsible for the fees and expenses of more than one counsel for all Indemnified Parties.
12.2.4 The Party controlling such defense shall keep the other Party advised of the status of such Action and the defense thereof and shall consider recommendations made by the other Party with respect thereto.
12.2.5 The Indemnified Party shall not agree to any settlement of such Action, consent to any judgment in respect thereof or admit any liability with respect thereto, without the prior written consent of the Indemnifying Party, which shall not be unreasonably withheld or delayed.
12.2.6 The Indemnifying Party shall not agree to any settlement of such Action or consent to any judgment in respect thereof without the prior written consent of the Indemnified Party, which shall not be unreasonably withheld or delayed; provided, however, that no such consent shall be required with respect to any such settlement, compromise or consent to judgment that (a) involves solely the payment of money damages as to which the Indemnifying Party has acknowledged its obligation to indemnify hereunder, (b) does not involve any claim for injunctive
or other equitable relief, and (c) effects a full and unconditional release of the Indemnified Party with respect to all claims related to the Action.
12.3 Limitations on Liability. SUBJECT TO AND WITHOUT LIMITING THE INDEMNIFICATION OBLIGATIONS OF EACH PARTY WITH RESPECT TO THIRD PARTY ACTIONS UNDER SECTIONS 12.1 AND 12.2, AND EXCEPT WITH RESPECT TO LIABILITY ARISING FROM BREACH OF SECTION 9.1 BY A PARTY, NO PARTY OR ANY OF ITS AFFILIATES WILL BE LIABLE TO THE OTHER PARTY OR ITS AFFILIATES UNDER ANY CONTRACT, WARRANTY, NEGLIGENCE, TORT, STRICT LIABILITY OR OTHER LEGAL OR EQUITABLE THEORY FOR ANY SPECIAL, INDIRECT, INCIDENTAL, PUNITIVE, MULTIPLIED OR CONSEQUENTIAL DAMAGES, OR OTHER DAMAGES FOR LOSS OF PROFIT, SALES OR FEES, ARISING OUT OF OR IN CONNECTION WITH THIS AGREEMENT OR ITS SUBJECT MATTER. FURTHER, SUBJECT TO AND WITHOUT LIMITING THE INDEMNIFICATION OBLIGATIONS OF EACH PARTY WITH RESPECT TO THIRD PARTY ACTIONS UNDER SECTIONS 12.1 AND 12.2, AND EXCEPT WITH RESPECT TO LIABILITY ARISING FROM BREACH OF SECTION 9.1 BY A PARTY OR ARISING FROM THE GROSS NEGLIGENCE OR WILLFUL MISCONDUCT OF A PARTY, EACH PARTY’S AGGREGATE LIABILITY TO THE OTHER PARTY FOR ALL CASES AND CONTROVERSIES ARISING OUT OF THE SUBJECT MATTER OF THIS AGREEMENT, REGARDLESS OF THE CAUSE OF ACTION AND WHETHER BROUGHT IN CONTRACT, TORT (INCLUDING NEGLIGENCE), STRICT LIABILITY OR OTHERWISE, WILL BE LIMITED TO $[***]. THE AMOUNT OF SERVICE FEES AND MILESTONE PAYMENTS PAID OR DUE TO COMPANY UNDER THIS AGREEMENT WILL NOT BE INCLUDED IN THE CALCULATION OF SUCH AGGREGATE LIABILITY AMOUNT.
12.4 Insurance. Company agrees to comply with Exhibit E attached hereto, which is incorporated herein by this reference.
ARTICLE XIII
DISPUTE RESOLUTION
13.1 Disputes. All disputes, claims or controversies (other than matters that are expressly stated herein to require the consent of either or both Parties) arising from or related to this Agreement, or to the interpretation, application, breach, termination or validity of this Agreement, whether based on contract, tort, statute, or other theory of liability (“Disputes”), shall be resolved in accordance with this Article XIII. It is the intent of the Parties that all Disputes relating in any way to this Agreement should be resolved in accordance with this Article, including Disputes that may involve the parent companies, subsidiaries, and other Affiliates of any Party.
13.2 Negotiation. Before any Dispute may be submitted to mediation or arbitration as provided below, the Dispute shall be referred to the President of Xxxxxxx and the Chief Executive Officer of Company for discussion and attempted resolution. No statements made by either Party during such discussions will be used by the other Party or admissible in arbitration or any other
subsequent proceeding for resolving the dispute. If such executives do not resolve the Dispute within thirty (30) days of such referral by either Party, then either Party may, upon written notice to the other Party, submit the Dispute to mediation pursuant to Section 13.3 and binding arbitration pursuant to Section 13.4.
13.3 Mediation.
13.3.1 The Parties shall first attempt in good faith to resolve any Dispute that is not resolved pursuant to Section 13.2 by confidential mediation in accordance with the then current Mediation Procedure of the International Institute for Conflict Prevention and Resolution (“CPR Mediation Procedure”) (xxxx://xxx.xxxxxx.xxx) before initiating arbitration. The CPR Mediation Procedure shall control, except where the CPR Mediation Procedure conflicts with these provisions, in which case these provisions control. The mediator shall be chosen pursuant to the CPR Mediation Procedure. The mediation shall be conducted in English in New York, New York. At the request of either Party (and at the shared expense of the Parties), the mediation shall have simultaneous translation from and into English.
13.3.2 Either Party may initiate mediation with respect to any Dispute that is not resolved pursuant to Section 13.2 by written notice to the other Party. The Parties agree to select the mediator within twenty (20) days of the notice and the mediation will begin promptly after the selection. The mediation will continue until the mediator or either Party, declares in writing, no sooner than after the conclusion of one full day of a substantive mediation conference attended on behalf of each Party by a senior business person with authority to resolve the Dispute, that the Dispute cannot be resolved by mediation. In no event, however, shall mediation continue more than sixty (60) days from the initial notice by a Party to initiate meditation unless the Parties agree in writing to extend that period.
13.3.3 Any period of limitations that would otherwise expire between the initiation of mediation and its conclusion shall be extended until twenty (20) days after the conclusion of the mediation.
13.4 Arbitration. If the Parties fail to resolve a Dispute by mediation under Section 13.3 and either Party desires to pursue resolution of the Dispute, the Dispute shall be submitted by either Party for resolution in arbitration pursuant to the then current CPR Rules for Non-Administered Arbitration of International Disputes (“CPR Rules”) (xxxx://xxx.xxxxxx.xxx), except where they conflict with these provisions, in which case these provisions control. CPR is designated as the Neutral Organization for all purposes.
13.4.2 Selection of Arbitrators.
(a) The arbitrators will be chosen from the CPR Panels of Distinguished Neutrals, unless a candidate not on the CPR Panels of Distinguished Neutrals is approved by both
Parties. Each arbitrator shall be a lawyer with at least fifteen (15) years’ experience with a law firm or corporate law department of over twenty-five (25) lawyers or who was a judge of a court of general jurisdiction. To the extent that the Dispute requires special expertise, the Parties will so inform CPR prior to the beginning of the selection process.
(b) The arbitration tribunal shall consist of three (3) arbitrators, chosen in accordance with Rules 5.3 and 6 of the CPR Rules. If, however, the aggregate award sought by the Parties is less than five million United States dollars (USD $5,000,000) and equitable relief is not sought, a single arbitrator shall be chosen in accordance with Rules 5.3 and 6 of the CPR Rules.
(c) Candidates for the arbitrator position(s) may be interviewed by representatives of the Parties in advance of their selection, provided that all Parties are represented.
(d) The Parties agree to select the arbitrator(s) within forty-five (45) days of initiation of the arbitration.
13.4.3 Conduct of Proceedings.
(a) The hearing will be concluded within nine (9) months after selection of the arbitrator(s) and the award will be rendered within 60 days of the conclusion of the hearing, or of any post hearing briefing, which briefing will be completed by both sides within 45 days after the conclusion of the hearing. In the event the Parties cannot agree upon a schedule, then the arbitrator(s) shall set the schedule following the time limits set forth above as closely as practical.
(b) The arbitrator(s) shall be guided, but not bound, by the IBA Rules on the Taking of Evidence in International Commercial Arbitration (xxx.xxxxxx.xxx).
(c) The hearing will be concluded in ten hearing days or less. Multiple hearing days will be scheduled consecutively to the greatest extent possible. A transcript of the testimony adduced at the hearing shall be made and shall be made available to either Party.
13.4.4 Applicable Law. The arbitrator(s) shall decide the merits of any Dispute in accordance with the law governing this Agreement, without application of any principle of conflict of laws that would result in reference to a different law. The arbitrator(s) may not apply principles such as “amiable compositeur” or “natural justice and equity.”
13.4.5 Award.
(a) The arbitrator(s) shall render a written opinion stating the reasons upon which the award is based. The arbitrator(s) may award the costs and expenses of the arbitration as provided in the CPR Rules, but each Party shall bear its own attorney fees.
(b) The award may be entered and enforced in any court of competent jurisdiction. If a court is called upon to enforce an award in a court proceeding, the Parties consent
to the court’s requiring the Party resisting enforcement to pay the reasonable attorneys’ fees and costs incurred in that proceeding by the Party seeking enforcement.
13.4.6 Provisional Relief. Any Party may seek emergency, interim, or provisional relief prior to the appointment of the arbitrator(s) from any court of competent jurisdiction, without waiver of the agreements to mediate and arbitrate. After appointment of the arbitrator(s), any request for emergency, interim, or provisional relief shall either be addressed to the arbitrator(s), which shall have the power to enter an interim award granting relief using the standards provided by applicable law, or to a court, but only with the permission of the arbitrator(s). Any interim award of the arbitrator(s) may be enforced in any court of competent jurisdiction.
13.4.7 WAIVER. EACH PARTY HERETO WAIVES: (A) ITS RIGHT TO TRIAL OF ANY ISSUE BY JURY, (B) WITH THE EXCEPTION OF RELIEF MANDATED BY STATUTE, ANY CLAIM FOR THE TYPES OF DAMAGES EXCLUDED BY SECTION 12.3 (SUBJECT TO THE EXCEPTIONS SPECIFIED IN SUCH SECTION), AND (C) ANY CLAIM FOR ATTORNEY FEES, COSTS AND PREJUDGMENT INTEREST.
13.5 Confidentiality. All proceedings and decisions of the mediator(s) and/or arbitrator(s) shall be deemed Confidential Information of each of the Parties and shall be subject to Section 9.1.
ARTICLE XIV
MISCELLANEOUS
14.1 Press Announcements. Neither Party, nor any of its Affiliates, shall issue any press release or make any other public statement relating to the terms and conditions of this Agreement or the relationship contemplated hereunder without the prior written consent of the other Party. Notwithstanding the foregoing, each Party (or its applicable Affiliate) may make any disclosure relating to the Product or the terms and conditions of this Agreement that such Party (or Affiliate), in the opinion of its counsel, is obligated to make pursuant to Laws applicable to publicly-traded companies, including, inter alia, regulations of the Securities and Exchange Commission, the New York Stock Exchange or the Nasdaq Stock Market. In such event, the announcement shall be brief and factual (to the extent consistent with applicable Laws), and the Party required to make such disclosure shall, to the extent practicable, notify the other Party of the method and content of such disclosure a reasonable period of time (at least five (5) Business Days if possible) in advance thereof, so as to allow such other Party to review it for the use of its name and disclosure of Confidential Information.
14.2 Force Majeure Event. All incidents of force majeure, being circumstances beyond the reasonable control of either Party and which have, or may have, a material effect on the ability of such Party to perform under this Agreement, including, failure of power or other utility or sanitary supplies; fire; flood; earthquake; other natural disaster; explosion; riot; strike or lock-out of such Party’s workforce; civil insurrection or unrest; terrorist activity; war (whether declared or not); and regulations of any Governmental Authority, in each case, to the extent beyond the
reasonable control of such Party (“Force Majeure”), shall, for the duration and to the extent of the effects caused thereby, release such Party from the performance of its contractual obligations hereunder. The Party who has suffered the Force Majeure shall notify the other Party without delay of any such incident(s) occurring, and the Parties shall discuss the effects and extent of such incident(s) on this Agreement and the measures to be taken. Each Party shall use Diligent Efforts to avoid or restrict Force Majeure and to mitigate any loss therefrom. In the event of an incident or incidents of Force Majeure, the Party whose performance has been affected thereby shall as soon as reasonably possible resume performance of its obligations hereunder. If any Force Majeure substantially prevents, hinders, or delays performance by a Party in a manner and to an extent that would, but for this Section, constitute a material breach or give rise to a right of termination hereunder, and the performance is not materially restored within one hundred eighty (180) days, the other Party may terminate this Agreement upon written notice to such Party.
14.3 Independent Contractors. Nothing in this Agreement shall create or imply an association, partnership, or joint venture between the Parties, it being agreed and understood that the Parties are independent contractors; and neither Party, with respect to a Third Party, shall have the power or authority to bind or obligate the other Party in any way. Neither Party shall have any responsibility for the hiring, termination or compensation of the other Party’s employees or for any employee benefits of such employee. No employee or representative of a Party shall have any authority to bind or obligate the other Party to this Agreement for any sum or in any manner whatsoever, or to create or impose any contractual or other liability on the other Party without said Party’s approval.
14.4 Performance by Affiliates. To the extent that this Agreement purports to impose obligations on the Affiliates of a Party, such Party agrees to cause its Affiliates to perform such obligations. Company shall not use an Affiliate to exercise any of its rights or perform any of its obligations or duties hereunder without Xxxxxxx’x prior written consent. Xxxxxxx may use an Affiliate to exercise its rights or perform its obligations and duties hereunder with prior written notice to Company. If either Xxxxxxx or Company uses an Affiliate to exercise any of its rights or perform any of its obligations or duties hereunder, as the case may be, such Party shall remain liable hereunder for the prompt payment and performance of all of its obligations hereunder.
14.5 Notices.
14.5.1 All notices, statements, requests or other documents that either Party shall be required or shall desire to give to the other hereunder shall be in writing and shall be given by the Parties only as follows: (a) by personal delivery; (b) by facsimile, receipt confirmed; (c) by addressing it as indicated below, and by depositing it certified mail, postage prepaid, in the mail, first class (airmail if the address is outside of the country in which such notice is deposited); or (d) by addressing it as indicated below, and by delivering it toll prepaid to a recognized courier service (e.g., Federal Express or DHL).
14.5.2 If so delivered, transmitted by facsimile, mailed, or couriered, each such notice, statement, request or other document shall, except as herein expressly provided, be
conclusively deemed to have been given when personally delivered or faxed during a Business Day, or on the fifth (5th) Business Day after the date of mailing, or on the second (2nd) Business Day after delivery to a courier service, as the case may be. The address of a Party shall be the address of which the other Party actually receives written notice pursuant to this Section 14.5 and until further notice such addresses are:
If to Xxxxxxx, to:
Xxxxxxx Biotech, Inc.
000 Xxxxxxxxx Xx.
Xxxxxxx, XX 00000
Attention: President, Oncology
Facsimile: [***]
With a copy (which shall not constitute notice) to:
Office of the General Counsel
Xxxxxxx & Xxxxxxx
Xxx Xxxxxxx & Xxxxxxx Xxxxx
Xxx Xxxxxxxxx, XX 00000
Attn: General Counsel, Pharmaceuticals
Fax No.: [***]
If to Company, to:
Immunomedics, Inc.
000 Xxx Xxxxxxxx Xxxx
Xxxxxx Xxxxxx, XX 00000
Attn: General Counsel
14.6 Entire Agreement. This Agreement, including the exhibits and schedules attached hereto (which are hereby incorporated by reference), sets forth the entire agreement and understanding between the Parties as to the subject matter hereof and supersedes all agreements or understandings, oral or written, made between the Parties before the Effective Date with respect to the subject matter hereof.
14.7 Amendments; Assignment. This Agreement may not be revised, amended, supplemented, or varied except by an instrument in writing signed by Xxxxxxx and Company. Neither this Agreement nor any rights or obligations of a Party may be assigned, delegated or otherwise transferred by such Party without the prior written consent of the other Party; provided, however, that Janssen may, without such consent but with prior written notice to Company, assign, delegate and transfer this Agreement or all or any of its rights and obligations under this Agreement to (a) any Third Party that acquires substantially all Xxxxxxx’x assets relating to the Product in the
Territory or (b) any Affiliate of Janssen. Any attempted assignment, transfer or delegation not in accordance with this Section shall be void.
14.8 Non-Waiver of Rights. Failure of a Party to enforce any of the provisions of or any rights with respect to this Agreement shall in no way be considered a waiver of such provisions or rights or in any way affect the validity of this Agreement. The failure of either Party to enforce any of such provisions or rights shall not preclude or prejudice such Party from later enforcing or exercising the same or any other provisions or rights which it may have under this Agreement. The waiver of any provision, right or obligation under this Agreement shall be effective only if in a written instrument signed by the Party to be bound thereby.
14.9 Further Assurances and Cooperation. Each Party agrees that after the Effective Date it will execute and deliver, or cause its Affiliates to execute and deliver, such further documents and instruments as may be reasonably necessary or proper to fully effectuate this Agreement and the transactions contemplated hereby.
14.10 Severability. This Agreement is intended to be valid and effective under any Laws and, to the extent permissible under Law, shall be construed in a manner to avoid violation of or invalidity under any Laws. Should any provisions of this Agreement be or become invalid, illegal, or unenforceable under any Laws, the other provisions of this Agreement shall not be affected and shall remain in full force and effect, and, to the extent permissible under the Laws, any such invalid, illegal, or unenforceable provision shall be deemed amended lawfully to conform with the intent of the Parties.
14.11 Binding Effect. This Agreement shall be binding upon and inure to the benefit of the Parties hereto and their respective successors and permitted assigns.
14.12 Counterparts; Facsimile Signatures. This Agreement may be executed in counterparts, each of which counterparts, when so executed and delivered, will be deemed to be an original, and all of which counterparts, taken together, will constitute one and the same instrument even if all parties have not executed the same counterpart. Signatures provided by any photocopy and transmitted by facsimile or other electronic means will be deemed to be original signatures.
14.13 Third Party Beneficiaries. The provisions of this Agreement are not intended legally to benefit or be enforceable by any Person who is not a party to this Agreement, and no such Person shall obtain any right under any such provisions or shall by reason of such provisions make any claim against a party to this Agreement.
14.14 Governing Law. The interpretation, construction and performance of this Agreement, and the rights granted and obligations arising hereunder, shall be governed in accordance with the substantive laws of the State of New York, without regard to its conflicts of law rules.
14.15 Construction. Except where expressly stated otherwise in this Agreement, the following rules of interpretation apply to this Agreement: (a) “include”, “includes”, and “including” are not limiting and mean include, includes, and including, without limitation; (b) definitions contained in this Agreement are applicable to the singular as well as the plural forms of such terms; (c) references to an agreement, statute, regulation, or instrument mean such agreement, statute, regulation, or instrument as from time to time amended, modified, or supplemented; (d) references to a Person are also to its successors and permitted assigns; (e) references to an “Article”, “Section”, “Exhibit”, or “Schedule” refer to an Article or Section of, or any Exhibit or Schedule to, this Agreement unless otherwise indicated; (f) the word “will” shall be construed to have the same meaning and effect as the word “shall”; (g) the use of any gender shall be applicable to all genders; and (i) the words “hereof” and “hereunder”, and words of similar import, shall be construed to refer to this Agreement as an entirety and not to any particular provision. The captions of this Agreement are for convenience of reference only and in no way define, describe, extend, or limit the scope or intent of this Agreement or the intent of any provision contained in this Agreement. Any reference in this Agreement to a matter or action being subject to the “mutual agreement” or “mutual consultation” of the Parties, or words of similar import, shall not be construed as an agreement that the Parties shall agree to such matter or action. The language of this Agreement shall be deemed to be the language mutually chosen by the Parties and no rule of strict construction shall be applied against either Party on the basis that such Party drafted this Agreement or any portion hereof.
[The remainder of this page is intentionally left blank.]
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the Effective Date.
XXXXXXX BIOTECH, INC. | |
By: | /s/Reshema Keups-Xxxxxxx |
Name: | Reshema Keups-Xxxxxxx |
Title: | VP, Sales and Marketing, Solid Tumor |
IMMUNOMEDICS, INC. | |
By: | /s/Xxxxx Xxxxxxxxx |
Name: | Xxxxx Xxxxxxxxx |
Title: | General Counsel |
Schedule 1.16
Janssen Universal Calendar

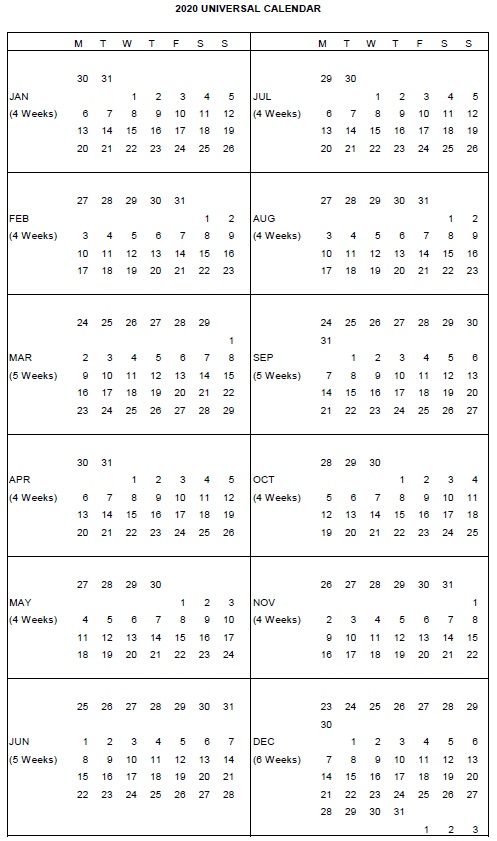
Schedule 6.2
Pharmacovigilance Provisions
1 Definitions
1.1 “Adverse Event” (AE) means any untoward medical occurrence in a patient or a clinical-trial subject administered a medicinal product and which does not necessarily have to have a causal relationship with this treatment. An adverse event can therefore be any unfavourable and unintended sign (for example, an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medicinal product, whether or not considered related to this medicinal product.
1.2 “Agreement” means the Promotion Agreement to which this Schedule is attached.
1.3 “Applicable Law” means the applicable laws, rules, regulations, including any guidelines or other requirements of any Regulatory Authority in the relevant country of the Territory, and industry guidelines or codes of conduct that may apply to the review and analysis of safety information, the reporting of safety information to Regulatory Authorities and the maintenance of records thereof.
1.4 “Company Employee” means any employee of Immunomedics, Inc. or any of its Affiliates conducting activities under the Agreement.
1.5 “Date of First Receipt” means the date of receipt or coming into possession or control of safety information, which contains at a minimum a suspect medicinal product and a suspect event i.e. an incomplete case. Unless otherwise indicated in the Applicable Law the Regulatory Clock Start Date or Day Zero for regulatory reporting, is the date the minimum criteria for reporting as defined by the Applicable Law becomes available (i.e., an identifiable subject/ patient, identifiable reporter, suspect product, and event).
1.6 “Incomplete Case” means a case that does not contain minimum criteria for reporting (as defined by the Applicable Law) to a Regulatory Authority (i.e., an identifiable subject/ patient, identifiable reporter, suspect medicinal product, and event), but at a minimum contains a suspect medicinal product and a suspect event. Such reports are entered on the safety database maintained by Janssen as potential cases of value for signal detection purposes.
1.7 “Personal Data” means any information relating to an identified or identifiable natural person.
1.8 “Product” has the meaning set forth in Section 1.77 of the Agreement.
1.9 “Product Quality Complaint” (PQC) Any written, electronic or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness or performance of a product after it is released for distribution.
1.10 “Regulatory Authority” means any applicable federal, national, regional, state, provincial or local regulatory agencies, departments, bureaus, commissions, councils or other government entities regulating or otherwise exercising authority with respect to the Product in the relevant Territory.
1.11 “Special Situation” Occurrences or reports that may not contain an adverse event, which must still be collected and reported in order to meet regulatory safety reporting requirements and Janssen policies:
• Overdose of Product,
• Pregnancy exposure (maternal and paternal),
• Exposure to the Product from breastfeeding,
• Suspected abuse/misuse of the Product,
• Inadvertent or accidental exposure to the Product (including occupational exposure),
• Any failure of expected pharmacological action (i.e. lack of effect) of the Product,
• Unexpected therapeutic or clinical benefit from use of the Product,
• | Medication error (includes potential, intercepted or actual) involving the Product with or without patient/consumer exposure to the Product, (e.g. name confusion) OR that caused an unintended effect or could cause an intended effect (e.g. adult medicine given to a young child), |
• Suspected transmission of an infectious agent via Product,
• Expired drug use and falsified medicine,
• | Off-label use - situations where the Product is intentionally used for a medical purpose not in accordance with the authorized product information. |
Off-label use without an associated AE, Special Situation, UE or AEPQC should be collected only when it is specifically and voluntarily brought to the attention of a Company Employee in an unsolicited manner by a reporter e.g., Health Care Professional or data obtained from databases where off-label use may be systematically collected (e.g., reimbursement database in US), and in accordance with local procedure in compliance with local laws and regulations. Follow-up of off-label use is not required.
1.12 “Territory” means the United States of America, including its territories and possessions.
1.13 "Undesirable Effect” (UE) shall mean an adverse reaction for human health attributable to the normal or reasonably foreseeable use of a cosmetic product.
Note: All capitalized terms used but not defined in this Schedule shall have the meanings ascribed to them (if any) in the Agreement.
2 Reporting Requirements
2.1 If any Company Employee receives or otherwise comes into possession or control of any information about the Product, regardless of source, relating to an Adverse Event (AE), Special Situation, AE associated with a Product Quality Complaint (AEPQC), Undesirable Effect (UE) or an Incomplete Case, such Company Employee shall provide such information immediately, but in no case later than twenty-four (24) hours from the Date of First Receipt by the Company Employee, to Janssen by using the Janssen Online Complaint Form available at Xxxxxxxxxxxxx.xxx. For the avoidance of doubt, all information regarding Incomplete Cases should also be provided immediately, but in no case later than twenty-four (24) hours from the date the Company Employee receives such information.
3 Training
4.1 Company shall ensure that all Company Employees are trained in the reporting of AEs, Special Situations, AEPQC or UEs, prior to the start of performing services under the Agreement and at least annually thereafter if such services remain in effect, to ensure
compliance with this Schedule and the Applicable Law. This includes, but is not limited to, monitoring applicable AE, Special Situation, AEPQC and UE training, and maintaining documentation of such training. Such training shall be conducted in the manner set forth in the Agreement using materials to be provided by Janssen. Janssen may require Company Employees to complete additional training provided by Janssen when there is a change in the governing contracts and/or processes or changes in Company’s personnel.
5 [Intentionally Omitted.]
5 Retention Policy
5.1 Company shall maintain and archive records of all source documentation generated by the activity (records, questionnaires, reports), personnel training records and other relevant information relating to its obligations under this Schedule for a period consistent with Section 7.1 of the Agreement (including Exhibit C thereto) and Applicable Law. Company must have appropriate storage capabilities (e.g., preventing accidental damage of physical records and appropriate back up of electronic storage systems) if storing original AE, Special Situations, AEPQC and UE documentation. Notwithstanding the above, before Company destroys any safety records it will notify Janssen of its intention to do so, affording Janssen the opportunity to retain such records if it so wishes.
6 Audit
6.1 Without prejudice to Section 7.5 of the Agreement, Janssen or its designee shall have the right to audit Company to verify Company’s compliance with this Schedule and the Applicable Law, provided that Janssen provides Company with at least [***] ([***]) calendar days prior written notice. The Parties shall agree upon the scope of the audit with a written audit plan to be submitted by Janssen [***] ([***]) calendar days prior to the audit. Company will allow such access to its facilities, systems, personnel and records, in whatever form and in any location (including locations owned or operated by a third party) as may reasonably be necessary to enable Janssen or its designee to evaluate and ensure compliance with this Schedule and the Applicable Law. Janssen shall communicate audit findings in a written audit report in a timely manner. The Parties undertake to cooperate with
each other to diligently investigate and resolve any such audit findings.
7 Data Privacy
7.1 In the performance of the above safety activities, both Parties will comply with all Applicable Laws in respect of data privacy in order to protect Personal Data.
7.2 Each Party shall collect, use and disclose any Personal Data obtained in the course of performing the safety activities under this Schedule solely for the purposes of complying with the regulatory obligations as described in this Schedule, or as otherwise required by Applicable Law or by a court order. Both Parties will use electronic, physical and any other safeguards appropriate to the nature of the information to prevent any use or disclosure of Personal Data other than as provided for above. Both Parties will also take reasonable precautions to protect the Personal Data from accidental, unauthorised, or unlawful alteration or destruction.
7.3 Each Party shall notify the other Party promptly of any accidental, unauthorised, unlawful destruction, loss, alteration, or disclosure of, or access to the Personal Data, and take immediate steps to rectify any such security breach.
8 Follow Up
8.1 Janssen will be responsible to diligently follow up on safety information.
9 Miscellaneous
9.1 Notwithstanding the above, in the event any Company Employee is informed of AE, Special Situations, AEPQC or UE related to the use of any other products of Janssen or its Affiliates that such Company Employee is aware of, such Company Employee shall report these to Janssen (in the same manner as any such report relating to the Products) within twenty-four (24) hours of the Date of First Receipt of such information by such Company Employee.
CONTACT DETAILS
For Janssen | Name: |
Company: | |
Telephone: | |
Fax: | |
Email: | |
For Company | Name: |
Company: | |
Telephone: | |
Fax: | |
Email: | |
EXHIBIT A
Brand Plan
[***]
EXHIBIT B
Detailing Requirements
[***]
EXHIBIT C
Records and Information Management (“RIM”) Requirements
1. Maintenance. Company shall maintain and manage all paper and electronic records, files, documents, work papers and other information in any form provided by Janssen or generated pursuant to this Agreement (the “Files and Work Papers”): (a) in accordance with Xxxxxxx’x records management policies (which may be changed by JBI from time to time and communicated to Company), including as set forth in “RIM Requirements” below, (b) separately from files generated, managed or maintained by Company under agreements with other customers, (c) as required by applicable statutes and regulations, and (d) as set out in any preservation request issued to Company by Janssen.
2. Preservation. Company shall comply promptly and fully with any request from Janssen, for any reason, to preserve Files and Work Papers or to promptly deliver such materials to Janssen. Steps to comply include, when requested by Janssen, periodic meetings to identify and implement documented procedures to preserve or deliver such data. Files and Work Papers created or modified by Company in electronic format must be delivered to Janssen in the same electronic format or as otherwise directed by Janssen.
3. Third Party Requests. Upon receipt from Third Parties of any request, demand, notice, subpoena, order, or other legal information-request for any Files and Work Papers, Company shall take all reasonable steps to protect Xxxxxxx’x legal rights in any response to such request and, to the extent that Company legally may do so, shall immediately notify Janssen, shall provide Janssen with a copy of such request, and shall meet and cooperate with Janssen in the implementation of procedures to comply with the request.
4. RIM Requirements. This section specifies RIM requirements applicable to Files and Work Papers that Company personnel create, maintain, manage or manipulate on behalf of Janssen. Company is responsible for understanding and complying with Xxxxxxx’x RIM requirements.
a. Records and Information Management requirements shall be applied consistently and regularly.
b. Company’s Files and Work Papers:
i) shall be created, stored and managed throughout their lifecycle using proper protection;
ii) | shall be protected and access controlled according to their value as described in the Xxxxxxx & Xxxxxxx Supplier Information Security Requirements; |
iii) shall be retained in accordance with the Xxxxxxx & Xxxxxxx
Enterprise Retention Schedule, which defines retention requirements for business, legal, regulatory and privacy purposes; and
iv) relevant to litigation or an investigation and subject to a legal hold shall be retained and preserved, regardless of the retention requirement set forth in the Xxxxxxx & Xxxxxxx Enterprise Retention Schedule.
c. | Company shall ensure that the Files and Work Papers are retained upon the departure of personnel employed by Company. |
x. | Xxxxxxx or the applicable Janssen Affiliate shall provide written approval prior to the disposition (disposal or deletion) of any Files and Work Papers. |
e. | Company personnel with access to Xxxxxxx’x network shall annually complete Records and Information Management training as specified by Janssen. |
EXHIBIT D
Health Care Compliance Provisions
1. “HCP” is defined as (i) any person who is licensed by a state to provide health care services directly or indirectly to patients, such as a physician, a nurse, a technician, a psychologist, or a lab specialist and/or (ii) any person or organization to whom a Party markets its products and services that is in a position to influence the selection of the products furnished or purchased, including but not limited to hospitals and health systems, administrators, procurement personnel, group purchasing organizations, pharmacy benefit managers, and business people.
2. Company shall, with respect to each HCP engaged under this Agreement:
a. Ensure that the HCP’s services are provided in compliance with all applicable laws and regulations, including but not limited to laws and regulations pertaining to the promotion of products regulated by the United States Food and Drug Administration (FDA); laws, regulations and guidance pertaining to federal and state anti-kickback and submission of false claims to governmental or private health care payors (collectively, “Health Care Compliance” or “HCC”); state and federal laws and regulations relating to the protection of individual and patient privacy; and any other laws and regulations applicable to such services.
b. Ensure that HCP’s services are provided in compliance with Xxxxxxx’x written policies and procedures of which Company is provided notice, including, but not limited to, policies and procedures related to FDA and Health Care Compliance and the protection of individual and patient privacy (collectively, “Janssen Policies”). The requirements of this Agreement and any additional policies attached to this Agreement shall constitute Janssen Policies of which Janssen provides notice to Company.
c. Comply with professional and/or employment rules (such as conflicts of interest or ethics policies) established by Company or a professional organization or institution with which HCP is affiliated when the provision of services by an HCP is subject to such rules, including, as applicable, obtaining any required approval(s) prior to providing services and making any required reports.
3. Company shall provide notice to each HCP of the following:
The Physician Payments Transparency Requirements of the Patient Protection and Affordable Care Act of 2010 (codified at 42 U.S.C. 1320a-7h) and implementing regulations, require certain pharmaceutical, medical device, and other companies to annually report to the Centers for Medicare and Medicaid Services (CMS) certain information about payments and transfers of value provided directly or indirectly to U.S. physicians and teaching hospitals, which CMS will make publicly available. This includes any payments or transfers of value that Janssen provides indirectly through Company to U.S. physicians and teaching hospitals. As required by law, Janssen will report to CMS information about payments and transfers of value that Company
provides to U.S. physicians and teaching hospitals pursuant to this Agreement. This includes any portion of any payment or transfer of value that Janssen furnishes to Company which Company then provides directly or indirectly to U.S. physicians or teaching hospitals, including its employees, agents, or contractors. Information that Janssen must report includes the identity and business address of each relevant U.S. physician or teaching hospital, the value and purpose of any payments or transfers of value that are furnished, and any other information as may be required by law. To enable Janssen to comply with its legal obligations, Company shall track, maintain, and provide Janssen information and data related to any payments or transfers of value that Company provides to U.S. physicians and teaching hospitals under this Agreement. Company shall provide such information and data in the form and manner that Janssen requests in a timely manner. Janssen may also report information about compensation, payments or transfers of value that Company provides to U.S. physicians and teaching hospitals as otherwise required by law and Janssen reserves the right to post on a website accessible to the public such information, whether or not required by law.
4. In accordance with Xxxxxxx’x request, Company shall, within thirty (30) days thereafter, provide or upload to Xxxxxxx’x health care compliance data system (the “Totality Third Party Company Portal”) or any similar system, all compliance documents and data templates related to services. Data requirements regarding Totality Third Party Company Portal can be found at xxxxx://xxxxxxxxxxxxxxx.xxx.xxx. Compliance documents and data templates include the following:
a. Copies of written agreements including compensation terms, with each HCP providing services.
b. Documentation indicating that each HCP providing services is not excluded or debarred and, for any health care practitioner, duly licensed under state law, as set forth above. Company shall obtain such documentation prior to engaging such HCP to provide services.
c. Documentation of services provided by such HCP (e.g., a written report, comments collected at a meeting, presentation materials, etc.).
d. HCP data templates capturing details on HCP value exchange. Value exchanges shall include, without limitation, any gifts, meals, compensation, travel reimbursement and patient-related materials provided to HCPs in connection with this Agreement.
e. Documentation that shows that Company provided notice to each HCP that information provided pursuant to this Agreement may be made publicly available at any time at the sole discretion of Janssen.
f. Electronic report of overall expenses paid to or on behalf of each HCP and electronic copies of all original receipts documenting such expenses; and
g. Written evidence of any required ethics or other authorizations allowing HCPs employed by federal, state or local government agencies, including but not limited to pharmacy and therapeutics committees, to provide services under this Agreement.
5. In the event that Janssen is charged any fee or penalty because Company failed to comply with the requirements set forth in this Exhibit, Company agrees to reimburse Janssen for such fees or penalties. Janssen reserves the right to reduce or not pay any invoice in the event that Company fails to comply with the requirements set forth in this Exhibit.
6. Company shall produce and send to Janssen electronic reports each month in which payments were made or gifts or meals were provided to HCPs by Company on behalf of Janssen, listing the following:
a. value of any gifts, meals, compensation paid, and/or entertainment provided to HCPs, whether their services were obtained through a written agreement or not;
b. nature, purpose and date of payments or other items of value provided; and
c. names, addresses, and federal Tax I.D. number of HCPs who were paid remuneration for services relating to Janssen.
7. Company shall report any violations of the compliance obligations set forth in this Agreement to Janssen at the name and address listed in Section 14.5 (Notices) or through the Vendor & Distributor Hotline at 0-000-000-0000.
8. Company, at its expense, shall ensure that all personnel and subcontractors involved in providing services attend and participate in training and educational programs reasonably scheduled by Janssen. Company, at its expense, agrees to train and periodically provide refresher training to all its new and current personnel and subcontracted personnel providing services regarding the compliance obligations set forth in this Agreement, including any Janssen Policies applicable to services. Company shall, upon request, provide Janssen with a record of the training provided and the dates training was attended by any Company personnel and subcontractors.
EXHIBIT E
Insurance Requirements
[***]

