Exhibit 10.6
CERTAIN INFORMATION HAS BEEN REDACTED FROM THIS EXHIBIT IN ACCORDANCE WITH ITEM 601(B)(10)(IV) OF REGULATION S-K BECAUSE SUCH INFORMATION (1) IS NOT MATERIAL AND (2) IS THE TYPE OF INFORMATION THE REGISTRANT TREATS AS PRIVATE OR CONFIDENTIAL. INFORMATION THAT HAS BEEN SO REDACTED FROM THIS EXHIBIT IS MARKED AS FOLLOWS: “XXXXXXXXXX” TO INDICATE THE OMISSION.
SETTLEMENT AGREEMENT AND RELEASE
This Settlement Agreement and Release dated July 13, 2024 (“Settlement” or “Agreement”) is made and entered into by and among the following parties and by and through their respective counsel: (i) Altor BioScience, LLC (“Altor”), (ii) NantCell, Inc. (“NantCell” and, together with Xxxxx, “Claimants”), (iii) HCW Biologics, Inc. (“HCW”), and (iv) Xx. Xxxx X. Xxxx (“Xx. Xxxx” and, together with HCW, “Respondents,” and Respondents, together with Xxxxxxxxx, the “Settling Parties”). The parties to this Agreement are referred to herein collectively as the “Parties” and each a “Party.” The Settlement is intended by the Settling Parties to fully, finally, and forever resolve, discharge, and settle the Settled Claims subject to the terms and conditions hereof.
I.BRIEF OVERVIEW OF THE CLAIMS
A.On December 23, 2022, Xx. Xxxx filed a statement of claims against Xxxxxxxxx in JAMS Arbitration for declaratory relief, seeking a declaration that Xx. Xxxx was not liable to Claimants for breach of contract and other causes of action.[1]
B.Also on December 23, 2022, Claimants filed suit against HCW in the District Court for the Southern District of Florida for misappropriation of trade secrets and other causes of action.[2]
C. On January 9, 2023, Xxxxxxxxx filed a demand for arbitration against Xx. Xxxx in JAMS Arbitration for breach of contract and other causes of action.[3]
D.On May 1, 2023, Xxxxxxxxx filed a demand for arbitration against HCW for misappropriation of trade secrets and other causes of action.[4]
E.Ultimately, the federal action was dismissed in favor of the three arbitrations, which were consolidated into one action, referred to as JAMS Reference No. XXXXXXXXXX.
F.On April 1, 2024, Claimants filed an action against HCW in the Court of Chancery of the State of Delaware.[5]
II.CLAIMS OF CLAIMANTS AND BENEFITS OF SETTLEMENT; RESPONDENTS DENIAL OF WRONGDOING AND LIABILITY
The Settling Parties wish to settle the Actions solely as between the Claimants, on the one hand, and Respondents, on the other hand, by entering into this Settlement, solely to avoid the costs, disruption, and distraction of further litigation, and without admitting the validity of any allegations made in the Actions, or any liability with respect thereto, have concluded that it is desirable that the claims against them be settled and dismissed on the terms reflected in this Settlement. Further, entry into this Settlement by Claimants is not an admission as to the lack of merit of any of the claims asserted by any of them in the Actions, and entry into this Settlement by Respondents is not an admission as to the merit of any of the claims asserted against them in the Actions.
NOW, THEREFORE, in consideration of the mutual covenants and agreements contained herein and other valuable consideration, the receipt and sufficiency of which are hereby acknowledged and agreed, the Parties hereby agree as follows:
1.Definitions. As used in this Settlement, the following terms have the meanings specified below.
a.“Actions” refer to the civil litigation and arbitrations referenced in Section I above.
b.“Active Ingredient” means any clinically active material that provides pharmacological activity in a pharmaceutical product (excluding formulation components such as coatings, stabilizers, excipients or solvents, adjuvants or controlled release technologies).
c.“Additional Molecule” means any fusion protein developed by HCW utilizing the TOBI Platform as of the Effective Date other than the TGFb Molecules. Notwithstanding the foregoing, “Additional Molecule” specifically excludes HCW9302.
d.“Affiliate” means any Person that Controls, is Controlled by, or is under common Control with another Person.
e.XXXXXX XXX XX XXXX XXXXXXXXXX XXXXX XXXX XXXXXXX XXX XXXXXXXX XXXXXXXXXX XXXXX XXXXXX XXXXXXXXX XXXXX XX XX XXXX XXX XXXX XX XXX XXXXXXX XXXXXXXX XXX XXX.
f.“Cellular Therapy Products” means any pharmaceutical or biological product, process or therapy that contains or comprises cells (including without limitation, cytokine-induced memory-like Natural Killer Cells or T-Cells) that have been engineered, modified, or otherwise manipulated ex vivo, as an Active Ingredient, either alone or in combination with other Active Ingredients.
Notwithstanding the foregoing, for purposes of this Agreement, Cellular Therapy Products shall not include Treg Products.
g.“cGMP” means the then-current good manufacturing practices required by the FDA, as set forth in the FD&C Act, as amended, and the regulations promulgated thereunder, for the manufacture and testing of pharmaceutical materials, and comparable applicable law related to the manufacture and testing of pharmaceutical materials in jurisdictions outside the United States, including the quality guidelines promulgated by the ICH designated ICH Q7A, titled “Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical ingredients” and the regulations promulgated thereunder, in each case as they may be updated from time to time.
h.“CMC” means chemistry, manufacturing and controls processes with respect to any product or investigational agent, including the chemistry, manufacturing and controls section of any regulatory materials for such product or investigational agent.
i.“Commercialization” means any and all activities, other than manufacturing, directed to the preparation for sale of, or sale of the referenced products, including activities related to marketing, promoting, distributing, and importing the products, and interacting with Regulatory Agencies regarding any of the foregoing. When used as a verb, “to Commercialize” and “Commercializing” means to engage in Commercialization, and “Commercialized” has a corresponding meaning.
j.“Control” means the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of a Person, whether through the ability to exercise voting power, by contract or otherwise provided that with respect to any Intellectual Property Rights or information, “control” means that the applicable Person owns or has a license to such item or right and has the ability to grant to a party a license, sublicense, or rights of access and use under such item or right without (a) violating the terms or conditions of any agreement or other arrangement between such Person and any Third Party in existence as of the time such party would be required hereunder to grant such license, sublicense, or rights of access and use, and (b) paying any consideration to any Third Party. “Controlling” and “Controlled” have meanings correlative thereto.
k.“Development” means (i) with respect to ImmunityBio, all activities related to discovery, research, development, creation and prosecution of Intellectual Property Rights, pre-clinical and other non-clinical testing, test method development and stability testing, toxicology, formulation, process development, manufacturing scale-up, qualification and validation, quality assurance/quality control, clinical studies, including manufacturing in support thereof, statistical analysis and report writing, the preparation and submission of Drug Approval Applications, regulatory affairs with respect to the foregoing and all other activities necessary or reasonably useful or otherwise requested or required by a Regulatory Agency as a condition or in support of obtaining or maintaining a Regulatory Approval and (ii) with respect to HCW, all activities related to discovery, research, development, pre-clinical and other non-clinical testing, test method development and stability testing, toxicology, clinical studies, statistical analysis and report writing, the preparation and submission of Drug Approval Applications, regulatory affairs with respect to the foregoing and all other activities necessary or reasonably useful or otherwise requested or required by a Regulatory Agency as a condition or in support of obtaining or maintaining a Regulatory Approval and, solely with respect to Non-TGFb Products, subject to ImmunityBio’s rights under Paragraphs 2(d), 2(e), 2(f) or 5(e), all activities related to the creation and prosecution of Intellectual Property Rights generated after the Effective Date (other than with respect to a Non-TGFb Product in the Oncology Field that is Directed To a Licensed Target created by either Party utilizing the TOBI Platform). When used as a verb, “Develop” means to engage in Development.
l.“Directed To” means, with respect to any fusion protein, molecule and/or antibody and a biological target and/or its receptor or Licensed Target, that such fusion protein, molecule and/or antibody binds to, inhibits, modulates or otherwise interacts with such biological target and/or its receptor or Licensed Target.
m.“Drug Approval Application” means a New Drug Application submitted pursuant to Section 505 of the FD&C Act, a Biologics License Application, or any corresponding foreign application (in each case, including any amendment or supplement thereto) for any investigational agent.
n.“Effective Date” means the date of full execution of this Settlement by all of the Settling Parties.
o.“EirGenix” means EirGenix, Inc.
p.“Exclusive Licensed Field” means (i) with respect to TGFb Products covered by Group A Patents, Group B Patents or HCW Additional Assigned Patents, all Indications other than those in the Oncology Field and (ii) with respect to Non-TGFb Products covered by Group B Patents or HCW Additional Assigned Patents, all Indications other than neoadjuvant ovarian cancer Indications. For clarity, the “Exclusive Licensed Field” for Licensed Products that contain both TGFb Molecules and non-TGFb molecules shall be limited to Indications other than those in the Oncology Field.
q.“Exploit” or “Exploitation” means (i) with respect to ImmunityBio, the making, having made, using, having used, selling, having sold, offering for sale or otherwise disposing of, a product or investigational agent, including all discovery, research, Development (including the conduct of clinical trials), manufacturing, registration, modification, enhancement, improvement, labeling, storage, formulation, exportation, importation, optimization, transportation, distribution, promotion, marketing and Commercialization activities related thereto and (ii) with respect to HCW, using, having used, selling, having sold, offering for sale or otherwise disposing of, a product or investigational agent, including all discovery, research, Development (including the conduct of clinical trials), registration, labeling, storage, exportation, importation, transportation, distribution, promotion, marketing and Commercialization activities related thereto and, solely with respect to Non-TGFb Products (other than any Non-TGFb Product that is Directed To a Licensed Target created by either Party utilizing the TOBI Platform) and any product exclusively licensed by HCW to Wugen under the Wugen Agreement as of the Effective Date, the making or having made of a product or investigational agent and all manufacturing, modification, enhancement, improvement, formulation, and optimization activities related thereto.
r.“FDA” means the United States Food and Drug Administration or any successor federal agency thereto.
s.“Group A Patents” means the Group A Patent Rights as set forth in Schedule 1and all Patent Rights thereof and thereto.
t.“Group B Patents” means the Group B Patent Rights as set forth in Schedule 1and all Patent Rights thereof and thereto.
u.“Group C Patents” means the Group C Patent Rights as set forth in Schedule 1and all Patent Rights thereof and thereto.
v.“ImmunityBio” means ImmunityBio, Inc., a Delaware corporation.
w.“Improvements” means any improvements, enhancements or modifications to the products developed using the TOBI Platform and Directed To any Licensed Target, that are conceived, made, reduced to practice or developed by Respondents or any of their Affiliates after the Effective Date.
x.“IND” means (a) an Investigational New Drug Application as defined in the Federal Food, Drug, & Cosmetic Act (“FD&C Act”) and applicable regulations promulgated thereunder by the FDA, and (b) the equivalent application to the applicable Regulatory Agency in any other regulatory jurisdiction, the filing of which is necessary to initiate or conduct clinical testing of a pharmaceutical product in humans in such jurisdiction.
y.“Indication” means a class of human disease or condition for which a separate marketing authorization application (including any extensions or supplements) is required to be filed with a Regulatory Agency.
z.“Intellectual Property Rights” means any and all (A) patents, divisionals, applications, utility models, industrial rights and similar intellectual property rights registered or applied for in the United States and all other countries throughout the world (including all reissues, divisions, continuations, continuations-in-part, renewals, extensions and reexaminations thereof) (collectively, “Patent Rights”); (B) rights in trademarks, service marks, trade dress, logos, domain names, rights of publicity, trade names and corporate names (whether or not registered) in the United States and all other countries throughout the world, including all registrations and applications for registration of the foregoing
and all goodwill related thereto; (C) copyrights (whether or not registered) and rights in works of authorship, databases and mask works, and registrations and applications for registration thereof in the United States and all other countries throughout the world, including all renewals, extensions, reversions or restorations associated with such copyrights, now or hereafter provided by law, regardless of the medium of fixation or means of expression; (D) right in inventions, practices, methods, protocols, formulas, know-how, know-how related to manufacturing, testing, characterization and/or similar processes, specifications, formulae, software, algorithms, CMC information, formulations, expertise, test data, stability data, other study data and procedures, trade secrets, processes, assays, techniques and results of experimentation and testing, and other scientific, technical or regulatory information (including raw data) in the United States and all other countries throughout the world (collectively, “Know-How”); (E) other intellectual property or proprietary rights in the United States and all other countries throughout the world, including all neighboring rights and sui generis rights; (F) rights to apply for, file, register establish, maintain, extend or renew any of the foregoing; (G) rights to enforce and protect any of the foregoing, including the right to bring legal actions for past, present and future infringement, misappropriation or other violations of any of the foregoing; and (H) rights to transfer and grant licenses and other rights with respect to any of the foregoing.
aa.“Knowledge” means actual knowledge of XXX, XX. XXXX, XXXXX XXXXX, XXXX XXXX, XXX XXX, XXXXXXX XXX, XXX XXXX, XXXXXX XXX, XXXXX XXXXXXXX, XXXXXX XXXXXXXXXX, XXX/XX XXX XXX XXXXX XXXXXXXXXX XXXXX XXXXXXX, after performing due inquiry with respect to the applicable facts and information.
bb.“Licensed Field” means both the Exclusive Licensed Field and the Non-Exclusive Licensed Field.
cc.“Licensed Target” means the biological target and/or receptors of each of (a) PDL-1, (b) IL-7, (c) IL-12, (d) IL-18, (e) IL-21 and (f) such additional biological target selected pursuant to Paragraph 5(b).
dd.“Licensed Products” means (a) TGFb Products, and (b) Non-TGFb Products; provided that, the foregoing “(b)” expressly excludes any product that comprises or is Directed To, as applicable, subject matter that is subject to ImmunityBio’s exclusive rights pursuant to Paragraph 5.
ee.“Non-Exclusive Licensed Field” means neoadjuvant ovarian cancer Indications solely to the extent (i) used in combination with standard of care chemotherapy and (ii) limited to the treatment plan and the indication (if any) as submitted to the FDA by HCW as of the Effective Date.
ff.“Non-TGFb Product” means any pharmaceutical product in any form that does not (i) contain a TGFb Molecule or (ii) otherwise relate to human transforming growth factor receptors or TGFb traps.
gg.“Oncology Field” means all uses for oncology diseases, disorders or conditions in humans or animals, including prophylactic or therapeutic treatment, delay or prevention of any oncology diseases, disorders or conditions in humans and animals.
ii.“Person” means an individual, corporation, partnership, limited liability company, association, trust or other entity or organization, but not including a government or political subdivision or any agency or instrumentality of such government or political subdivision.
jj.“Regulatory Agency” means the FDA and any other governmental authority with responsibility for the approval of the marketing and sale of pharmaceuticals or biologics or other regulation of pharmaceuticals or biologics.
kk.“Regulatory Approval” means all approvals (including, without limitation, where applicable, Drug Approval Applications, pricing and reimbursement approval, labeling approval and schedule classifications), licenses, registrations, certificates, permits or authorizations of any Regulatory Agency necessary for the manufacture, use, storage, import, export, transport, offer for sale, or sale of any product or investigational agent, together with all amendments, supplements and updates thereto and all benefits arising therefrom, including any orphan drug exclusivities or other non-patent exclusivities.
ll.“ROFR Information Package” means (a) the following information Controlled by any Respondent at the time a ROFR Information Package is delivered that summarizes material data relating to the Additional Molecule including (i) structure, (ii) characterization, (iii) data and information supporting the promotion and activation of immune cells, (iv) clinical readouts, (v) pre-IND and IND enabling studies conducted or ongoing, (vi) the competitive advantages of using such fusion proteins for products, (vii) any other information provided to any prospective Third Party licensee, (b) physical samples of such fusion proteins of sufficient quality and quantity to permit ImmunityBio to conduct due diligence studies thereof, and (c) as applicable, the terms of any proposed Third Party license for any Additional Molecule (excluding the identity of the applicable Third Party).
mm.“SRS” means Shareholder Representative Services LLC.
nn.“T Cells” means a T-lymphocyte.
oo.“TGFb Assigned Patents” means the Group A Patents and Group B Patents.
pp.“TGFb Know-How” means all Know-How Controlled by the Respondents or any of their Affiliates as of the Effective Date or at any time thereafter that is necessary or reasonably useful for the Exploitation of the TGFb Molecules or any TGFb Products.
qq.“TGFb Molecules” means any molecules Controlled by any Respondent as of the Effective Date or thereafter that were generated through the use of the TOBI Platform related to the human transforming growth factor receptor and TGFb traps, including, without limitation, HCW9218, HCW9219, HCW9209 and any derivatives thereof or therefrom.
rr.“TGFb Product” means any pharmaceutical product in any form that contains a TGFb Molecule.
ss.“Third Party” means any Person other than the Claimants, Respondents, or its or their respective Affiliates.
tt.“TOBI Platform” means HCW’s TOBI™ immunotherapeutic drug design and discovery platform and any modifications, improvements and thereto.
uu.“Transferred Assets” means (i) the Transferred Intellectual Property Rights, (ii) Transferred Regulatory Materials, (iii) Transferred Invention Records and Prosecution Files, (iv) Transferred Inventory and (v) Transferred Contracts.
vv.“Transferred Contracts” means the contracts set forth on Schedule 3.
ww.“Transferred Intellectual Property Rights” means (i) the TGFb Assigned Patents, (ii) the TGFb Know-How, and (iii) other than the Group C Patents, all other Intellectual Property Rights (A) as of the Effective Date, necessary or reasonably useful for, and (B) any time thereafter, necessary for or otherwise specific to, the Exploitation of the TGFb Molecules or any TGFb Products Controlled by Respondents or any of their Affiliates (such Patent Rights in (iii), the “HCW Additional Assigned Patents”). For clarity, all rights, title, and interest in and to compositions of matter, all formulations, all methods of treatment, and all methods of manufacture necessary for or reasonably useful for ImmunityBio’s Exploitation of the TGFb Molecules or any TGFb Products that exist as of the Effective Date are included in the Transferred Intellectual Property Rights, and all formulations, all methods of treatment, and all methods of manufacture necessary for or otherwise specific to ImmunityBio’s Exploitation of the TGFb Molecules or any TGFb Products that exist after the Effective Date are included in the Transferred Intellectual Property Rights.
xx.“Transferred Invention Records and Prosecution Files” means (i) all records, books, documents and files pertaining to and/or demonstrating the inventorship of any Transferred Intellectual Property Rights and (ii) any other documents and materials relating to the prosecution, defense, maintenance, validity and enforceability of the Transferred Intellectual Property Rights.
yy.“Transferred Inventory” means the inventory of TGFb Molecules and any other biological materials necessary or reasonably useful to Develop the TGFb Molecules, to the extent provided for in Schedule 2(a)(iii).
zz.“Transferred Regulatory Materials” means all U.S. and foreign regulatory applications, submissions and approvals (including all INDs and Drug Approval Applications and foreign counterparts thereof), and all Regulatory Approvals for TGFb Molecules, and all correspondence with the FDA and other Regulatory Agencies relating to the TGFb Molecules or any of the foregoing regulatory applications, submission and approvals, that, in each case, are in the possession of or Controlled by, or held by Respondents or any of their Affiliates as of the Effective Date, whether generated, filed or held by or for Respondents or any of their Affiliates or licensees.
aaa.“Treg Products” means any pharmaceutical or biological product that contains or comprises Tregs that have been engineered, modified, or otherwise manipulated ex vivo, as an Active Ingredient, and the primary mechanism of action of such product is through the activities of such Tregs.
bbb.“Tregs” means regulatory T Cells that are a subpopulation of T Cells which negatively regulate the immune system, maintain tolerance to self-antigens, suppress immune system in cancers, abrogate autoimmune disease or alleviate inflammation.
ccc.“Wugen” means Wugen, Inc., a Delaware corporation.
“Wugen Agreement” means the Exclusive License Agreement entered into as of December 24, 2020 by and between Wugen and HCW, in the form as attached hereto as Exhibit A.
[1] Xx. Xxxx X. Xxxx x. Altor BioScience, LLC; NantCell, Inc. JAMS Arbitration Ref. No. XXXXXXXXXX.
[2] Altor BioScience, LLC., et al., v. HCW Biologics, Inc., Case No. 22-CV-62404-RAR (S.D. Fla. Dec. 23, 2022).
[3] Altor BioScience, LLC; NantCell, Inc. x. Xxxx X. Xxxx, JAMS Arbitration Ref. No. XXXXXXXXXX.
[4] Altor BioScience, LLC; NantCell, Inc. v. HCW Biologics, JAMS Arbitration Ref. No. XXXXXXXXXX.
[5] Altor BioScience, LLC and NantCell, Inc. v. HCW Biologics, Inc., C.A. No. 2024-0310-PAF (Del. Ch. Apr. 1, 2024).
2. All TGFb Molecules.
a.Assignment. Respondents hereby assign to ImmunityBio their entire right, title and interest in and to all Transferred Assets effective as of the Effective Date. In furtherance of the foregoing:
i.Assurances. Respondents agree to execute the Patent Assignment as set forth on Exhibit B hereto, as well as the Power of Attorney as set forth on Exhibit C hereto, each as of the Effective Date, and further agree going forward to cooperate with and assist ImmunityBio, and perform all acts deemed necessary or desirable by ImmunityBio, to apply for, obtain, establish, perfect, maintain, evidence, enforce or otherwise protect any of the full benefits, enjoyment, right, title and interest throughout the world in the TGFb Assigned Patents and TGFb Molecules. Such acts may include, but are not limited to, execution of assignments of title and other documents and assistance or cooperation in legal proceedings. Should ImmunityBio be unable to secure Respondents’ signature on any such document, in connection with and effective by the Power of Attorney attached hereto as Exhibit C, Respondents hereby irrevocably designate and appoint ImmunityBio and its duly authorized representatives as Respondents’ agents and attorneys-in-fact, with full power of substitution and delegation, to undertake such acts in Respondents’ name as if executed and delivered by Respondents (which appointment is coupled with an interest), and Respondents waive and quitclaim to ImmunityBio any and all claims of any nature whatsoever that they may have or may later have for infringement of any Transferred Intellectual Property Rights;
ii.Unassigned Assets. In the event that any Party becomes aware of any Transferred Assets (a) that come into existence after the Effective Date and/or (b) was otherwise not
assigned in accordance with Paragraph 2, including due to error or because such assignment would require an authorization, approval, consent or waiver from a Third Party, then such Party will notify the other Party and Respondents will take all actions necessary to effect the assignment of such unassigned Transferred Assets to ImmunityBio, including executing any additional legal instruments reflecting assignment as ImmunityBio deems necessary or useful and/or obtaining any required authorization, approval, consent or waiver from an applicable Third Party.
iii.Technology Transfer. Within XXXXXX XXX XXXX XXXXX XXX XXXXXXXXX XXXX, HCW shall (a) provide ImmunityBio with complete and accurate copies of the TGFb Know-How and all other Transferred Assets as provided for in Schedule 2(a)(iii) and (b) deliver to ImmunityBio the Transferred Inventory. HCW shall preserve the TGFb Know-How in the same state as immediately prior to the Effective Date until such TGFb Know-How is transferred in accordance with this Paragraph 2(a)(iii). ImmunityBio will be responsible for all reasonable, necessary and documented transportation costs for the shipment of any materials to ImmunityBio required under Schedule 2(a)(iii). Within XXXXXX XXX XXXX of any assignment of Transferred Assets under Paragraph 2(a)(ii), Respondents shall provide and transfer copies of (if applicable) such Transferred Assets to ImmunityBio, and Respondents or any of their Affiliates shall provide reasonable technical assistance with respect to any such additional Transferred Assets to the extent necessary to permit ImmunityBio’s Exploitation thereof. HCW grants to ImmunityBio a right of reference to any regulatory documentation, submissions or approvals with any Regulatory Agency, including in connection with the Transferred Regulatory Materials, submitted in HCW’s or its Affiliate’s name (or in the name of any of their respective designees). ImmunityBio grants to HCW a right of reference to the Transferred Regulatory Materials solely in connection with HCW’s Exploitation of HCW9218 in the Licensed Field.
iv.Respondents’ Non-Compete. On the Effective Date and thereafter, Respondents shall not, directly or indirectly, generate any derivatives of TGFb Molecules for use in the Oncology Field other than the Non-Exclusive Licensed Field.
b.Retained Liabilities; Indemnification. For the avoidance of doubt, the transfer provided in Paragraph 2(a) above is a transfer of assets only and the Settling Parties acknowledge and agree that ImmunityBio is not assuming any liabilities of any Respondents or any of their Affiliates arising, accruing or existing as of and prior to the Effective Date, which such liabilities are expressly retained by Respondent (“HCW Retained Liabilities”). For avoidance of doubt, HCW Retained Liabilities includes, without limitation, third party claims arising from infringing activity caused by Exploitation of the Transferred Intellectual Property Rights and for liabilities, including amounts accrued and/or owed, arising out of any Transferred Contract, in all cases prior to the Effective Date. Respondents shall defend, indemnify, and hold harmless ImmunityBio and its Affiliates and their respective officers, directors, employees, agents, successors and assigns (the “ImmunityBio Indemnitees”) from and against any and all losses, damages, liabilities, actually incurred expenses and costs, including reasonable legal expense and attorneys’ fees (“Losses”) to which any ImmunityBio Indemnitee may become subject as a result of any
claim, demand, action or other proceeding by any Third Party (“Third Party Claim”) arising out of, based on, or resulting from any HCW Retained Liabilities.
c.License to HCW. Subject to the terms and conditions of this Agreement, including the contingency referenced in this Paragraph 2(c) below, ImmunityBio hereby grants HCW (i) a worldwide, perpetual (subject to this Paragraph 2(c)), fully-paid up, royalty-free, exclusive license, with the right to sublicense, under the TGFb Assigned Patents, the HCW Additional Assigned Patents and the TGFb Know-How, to Exploit Licensed Products, as applicable, in the Exclusive Licensed Field and (ii) a worldwide, perpetual (subject to this Paragraph 2(c)), fully-paid up, royalty-free, non-exclusive license, with the right to sublicense, under the TGFb Assigned Patents, the HCW Additional Assigned Patents and the TGFb Know-How, to Exploit Licensed Products in the Non-Exclusive Licensed Field. HCW hereby grants ImmunityBio a right of first refusal to regain exclusive rights through termination of the license rights granted to HCW pursuant to this Paragraph 2(c) to Exploit Licensed Products for use in the Non-Exclusive Licensed Field, following the procedures as set forth in Paragraph 6 hereof, mutatis mutandis. HCW agrees to provide full access to all safety and clinical data generated in connection with Licensed Products, whether for the Exclusive or Non-Exclusive Licensed Field or otherwise, to ImmunityBio. Notwithstanding the foregoing, in the event that HCW does not initiate (i.e., dose a first patient) a clinical trial of a TGFb Molecule for use in the Non-Exclusive Licensed Field prior to December 31, 2024, then the applicable license granted to HCW as described in this Paragraph 2(c)(ii) shall automatically terminate effective as of such date.
d.Prosecution and Maintenance.
i.Rights. For the avoidance of doubt, ImmunityBio shall control and have the first right, but not the obligation, to prepare, file, prosecute, and maintain the TGFb Assigned Patents worldwide. Further, ImmunityBio shall have the first right, but not the obligation, to conduct any opposition, re-issuance, post-grant review, inter-partes review, reexamination request, nullity action, interference, or other similar post-grant proceedings and any appeals therefrom relating to the TGFb Assigned Patents worldwide. Notwithstanding the foregoing, and solely with respect to claims contained in a TGFb Assigned Patent that specifically cover the Exploitation of a Licensed Product in the Exclusive Licensed Field (an “Exclusive Licensed Field-Specific Claim”), ImmunityBio shall file, at HCW’s expense, any additional related applications HCW deems reasonably necessary to further protect a Licensed Product in the Exclusive Licensed Field, provided that, ImmunityBio may elect not to file such related application(s) to the extent ImmunityBio in good faith believes such application may have any adverse impact on any TGFb Assigned Patents, HCW Additional Assigned Patents or ImmunityBio TGFb Products. ImmunityBio shall provide HCW with a copy of any draft of a material and substantive filing directed to an Exclusive Licensed Field-Specific Claim reasonably in advance of ImmunityBio’s filing of such draft to permit HCW, using counsel of HCW’s choosing, an opportunity to review and provide reasonable comments and/or instructions thereto within XXXXXXX XXX XXXX of HCW’s receipt of the applicable draft (or a shorter period reasonably designated by ImmunityBio if XXXXXXX XXX XXXX is not practicable given the filing deadline). ImmunityBio will incorporate in good faith all reasonable comments and effect all reasonable instructions related directly to such Exclusive Licensed Field-Specific Claim thereto provided by HCW during the applicable comment period and in connection with the filing thereof; provided that, ImmunityBio may elect not to incorporate such reasonable comments to the extent ImmunityBio believes such reasonable comments may have any adverse impact on any TGFb Assigned Patents or ImmunityBio TGFb Products. Notwithstanding anything to the contrary herein, solely for purposes of this Paragraph 2(d), Paragraph 2(e) and Paragraph 2(f), the HCW Additional Assigned Patents shall be treated as TGFb Assigned Patents, mutatis mutandis.
ii.Costs. HCW and ImmunityBio shall XXXX XXXX XXXX XX XXX third party expenses (including reasonable attorneys’ fees) incurred in connection with the prosecution or maintenance activities that pertain to Patent Rights comprised of an Exclusive Licensed Field-Specific Claim and a claim that is not an Exclusive Licensed Field-Specific Claim. With respect to all third party expenses (including reasonable attorneys’ fees) incurred or to be incurred by ImmunityBio in connection with any prosecution or maintenance activities that pertain solely to an Exclusive Licensed Field-Specific Claim (a) HCW shall reimburse ImmunityBio for all such expenses, and (b) without limiting the foregoing, HCW shall, within HCW’s XXXXXXX XXX XXX (or shorter, as applicable) review and comment period, advance to ImmunityBio (or its designee) all such expenses. To the extent HCW does not advance such
Settlement Agreement and Release
Page 9
expenses, ImmunityBio shall have no obligation to take any action with respect to such Exclusive Licensed Field-Specific Claim.
iii.Step-In Right. ImmunityBio may cease prosecution and/or maintenance of any Exclusive Licensed Field-Specific Claim on a country-by-country basis by providing HCW written notice reasonably in advance (but at least XXXXX XXX XXXX before the applicable deadline with a relevant patent authority). If ImmunityBio elects to cease prosecution or maintenance of the relevant Exclusive Licensed Field-Specific Claim in a country, HCW, shall have the right, but not the obligation, at its sole discretion and cost, to continue prosecution or maintenance of such Exclusive Licensed Field-Specific Claim and in such country with counsel of its choosing.
i.Rights. If either Party becomes aware of any existing or threatened infringement of any TGFb Assigned Patent (“Infringement”), it shall promptly notify the other Party in writing to that effect. Respondents shall share with ImmunityBio all information available to it regarding such alleged Infringement, pursuant to a mutually agreeable “common interest agreement” executed by the Parties under which the Parties agree to their shared, mutual interest in the outcome of any suit or other action to enforce the TGFb Assigned Patent against such Infringement. ImmunityBio shall have (a) with respect to any Infringement of the TGFb Assigned Patents, the sole right; provided that, such Infringement is not subject to clause (b), and (b) with respect to any Infringement that is solely of an Exclusive Licensed Field-Specific Claim, the first right, and in each case of clause (a) and (b), but not the obligation, to bring an appropriate suit or other action against any Person engaged in the Infringement of any TGFb Assigned Patent or Exclusive Licensed Field-Specific Claim, as applicable, XX XXXXXXXXXXXXX XXXX XXX XXXXXXX. If the applicable Infringement is solely with respect to an Exclusive Licensed Field-Specific Claim and ImmunityBio notifies HCW in writing that it does not intend to commence a suit or other action to enforce the applicable Exclusive Licensed Field-Specific Claim against such Infringement or to take other action to secure the abatement of such Infringement, or fails to take any such action after a period of XXXXX XXXX XXXXXXXX XXXX following either Party’s receipt of the notice of Infringement pursuant to this Paragraph 2(e)(i) then, HCW shall have the right, but not the obligation, to commence such a suit or take such action, at XXXXX XXXX XXX XXXXXXX and using counsel of its choosing.
ii.Recoveries. Any amounts recovered in connection with an Infringement suit or action under Paragraph 2(e)(i) shall first be used to reimburse ImmunityBio and HCW for their costs and expenses incurred in connection with such Infringement suit or action and all remaining amounts shall be divided as follows: (a) with respect to an Infringement suit or action that relates to only claims that are not Exclusive Licensed Field-Specific Claims, XXXXXXXXXXX XXXXXXX XXX XXXXXXX XXXXXXX XXXXXX; (b) with respect to an Infringement suit or action (1) that relates to both claims that are not Exclusive Licensed Field-Specific Claims and Exclusive Licensed Field-Specific Claims or (2) that is controlled by ImmunityBio and relates to only Exclusive Licensed Field-Specific Claims, XXXXXXXXXXX XXX XXX XXXX XXXXXX XXXXX XXXXXXX XXXXX;and (c) with respect to an Infringement suit or action that is controlled by HCW and relates to only Exclusive Licensed Field-Specific Claims, XXXXXXXXXXX XXX XXX XXXX XXXXXXX XX XXXX XXXXX XX XXXXXXXXXX XXXXXXXXXX XX XXXXXXXXXX XX XXXXX XX XXXX XXXXXXX XXXXXXXXXX XXXXXXXXX XXXXXXXXX XXX XXXXXXXXXXXXX.
f.Cooperation. Each Party shall cooperate with the Party controlling the prosecution and/or enforcement of any TGFb Assigned Patent, at the controlling Party’s request and, subject to Paragraph 2(d)(ii) expense. To the extent that HCW exercises or intends to exercise its rights under Paragraphs 2(d) or 2(e), (i) XXX XXXX XXXX XXX XX XXX XXXXX XXX XXXXXXXX incurred in connection therewith and (ii) notwithstanding Paragraphs 2(d) and 2(e), if ImmunityBio reasonably believes that HCW’s initiation or continued prosecution, maintenance or enforcement, as applicable, of any such Exclusive Licensed Field-Specific Claim may have an adverse impact on any TGFb Assigned Patents or ImmunityBio TGFb Products, then HCW shall not have the right to continue the prosecution, maintenance or enforcement, as applicable, of such Exclusive Licensed Field-Specific Claim.
g.License to ImmunityBio. Respondents hereby grant ImmunityBio (i) a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, non-exclusive license, with the right to grant and authorize sublicenses through multiple tiers, under (A) the Group C Patents, and (B) any other
Settlement Agreement and Release
Page 10
Intellectual Property Rights Controlled by Respondents or any of their Affiliates after the Effective Date and reasonably useful for the Exploitation of TGFb Molecules or TGFb Products, in each of (A) and (B), for all uses in the Oncology Field; and (ii) a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, exclusive license (subject only to ImmunityBio’s license to HCW under Paragraph 2(c)(ii) to Exploit Licensed Products in the Non-Exclusive Licensed Field), with the right to grant and authorize sublicenses through multiple tiers, subject to and in accordance with Paragraph 2(a)(ii), any other Transferred Intellectual Property Rights that are unable to be assigned and until such time that such Transferred Intellectual Property Rights, as applicable, are assigned, for all uses in the Oncology Field.
h.Publicity. HCW agrees to update its corporate website pipeline to be consistent with this arrangement limiting HCW9218 to the Licensed Field.
3. NK Cell Memory Like Activation Technology.
a.Subject to the terms and conditions of this Agreement, Respondents hereby grant ImmunityBio a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, non-exclusive license, with the right to sublicense, under any Intellectual Property Rights Controlled by the Respondents or any of their Affiliates as of the Effective Date and thereafter, to Exploit HCW9201 (IL12/IL15/IL18) solely as a subcutaneous injection (in vivo). For clarity, the foregoing license grant excludes any rights exclusively granted to Wugen under the Wugen Agreement as of the Effective Date.
b.Within XXXXXX XXX XXXX XX XXX XXXXXXXXX XXXX (or such longer period as requested by ImmunityBio), HCW shall perform a manufacturing technology transfer to ImmunityBio or its designee sufficient to permit ImmunityBio or its designee to manufacture HCW9201, which shall comprise a transfer of complete and accurate copies of all Know-How Controlled by the Respondents or any of their Affiliates as of the Effective Date that is necessary or reasonably useful in exercising ImmunityBio’s rights under the license grant set forth in Paragraph 3(a) including, without limitation, transfer of the materials and documentation as set forth in Schedule 2(a)(iii), as well as any inventory and materials pertaining to HCW9201 (replacing HCW9218 and HCW 9101 with HCW9201, as appropriate) as set forth in Schedule 2(a)(iii).
c.Respondents shall defend, indemnify, and hold harmless ImmunityBio Indemnitees from and against any and all Losses to which any ImmunityBio Indemnitee may become subject as a result of any Third Party Claim arising out of, based on, or resulting from ImmunityBio’s exercise of the licenses granted under Paragraph 3(a), including the Exploitation of HCW9201, solely to the extent such Third Party Claim (i) alleges infringement or misappropriation of such Third Party’s Intellectual Property Rights as a direct result of ImmunityBio’s exercise of the licenses granted under Paragraph 3(a), (ii) arises in connection with the rights granted to Wugen under the Wugen Agreement or any amendments or modifications thereto, or (iii) arises in connection with the gross negligence or willful misconduct of Respondents or any of their Affiliates in connection with the performance of Respondents’ obligations under Paragraph 3(b).
4. All Affinity Purification and Column Technology.
a.Subject to the terms and conditions of this Agreement, Respondents hereby grant ImmunityBio a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, non-exclusive license, with the right to sublicense, under any Intellectual Property Rights Controlled by the Respondents or any of their Affiliates as of the Effective Date and thereafter, to Exploit (i) the HCW9101 master cell bank XXXXXXXXX XX XXX XX XXX XXX/XX XXX XXXXX XXXXX XXXXXXXX XXXXXXXXXXXXX XXXXXXXXXXXXXXX to manufacture and purify cGMP HCW9218 and (ii)
Settlement Agreement and Release
Page 11
master and research cell banks for the TGFb Molecules for manufacturing and purification of tissue factor based fusion proteins. Within XXXXXX XXX XXXX XXXXX XXX XXXXXXXXX XXXX, HCW shall provide ImmunityBio with complete and accurate copies of all Know-How Controlled by the Respondents or any of their Affiliates as of the Effective Date or thereafter and necessary or reasonably useful in exercising ImmunityBio’s rights under the license grant set forth in this Paragraph 4(a) including, without limitation, transfer the materials and documentation as set forth in Schedule 2(a)(iii), as well as any inventory and materials as set forth in Schedule 2(a)(iii). For clarity, such technology transfer shall include, without limitation, Know-How sufficient for XXXXXXXXXXX XX XXX XXXXXXXX XX XXX XXXXX XXX XXXXXXXXXXXXX XX XXX XXXXXX, XXXXX XXX XXXXXXXX XXXXXX and shall be subject to the same ongoing obligations and procedure as set forth in Paragraph 2(a)(ii), mutatis mutandis, including with respect to any applicable Improvements and ongoing support.
b.Respondents shall, as of the Effective Date, execute and deliver to each of ImmunityBio and EirGenix a signed letter in the form attached as Exhibit D. Further, Respondents hereby consent and agree to the assignment as set forth under the terms of this Agreement of the Transferred Contracts to ImmunityBio, subject only to the consent of EirGenix (or other third party thereto), as required. Further, nothing in this Agreement shall be interpreted as limiting or restricting ImmunityBio from entering into a direct contractual relationship with HCW’s Third Party contract manufacturing organization(s), including EirGenix, and Respondents represent that Respondents have not taken, and will not take, any action (or inaction) to interfere with or prevent ImmunityBio’s exercise of ImmunityBio’s rights with respect to EirGenix. Nothing in this Agreement shall be construed as an attem pt or agreement to assign or transfer any Transferred Contract to ImmunityBio which by its terms is not assignable or transferable without a consent or is cancelable by a Third Party in the event of an assignment or transfer (a “Non-Assignable Contract”), unless and until such consent shall have been obtained. Respondents and ImmunityBio shall obtain as expeditiously as possible any consent that may be required for the assignment or transfer of the Non-Assignable Contract to ImmunityBio, and Respondents shall take all such actions as may be necessary to effect the assignment and transfer of the Non-Assignable Contract. Unless and until any such consent that may be required is obtained, Respondents shall establish an arrangement reasonably satisfactory to ImmunityBio under which ImmunityBio would obtain the rights, claims and benefits under such Non-Assignable Contract (including by means of any subcontracting, sublicensing or subleasing arrangement) or under which Respondents would enforce for the benefit of ImmunityBio, any and all rights, claims and benefits of Respondents against a third party thereto.
c.For the avoidance of doubt, HCW shall control and have the first right, but not the obligation, to prepare, file, prosecute, and maintain the patents licensed to ImmunityBio pursuant to Paragraph 4(a) (other than the TGFb Assigned Patents which are subject to the assignment under Paragraph 2) (the “9101 Patents”) worldwide. Further, HCW shall have the first right, but not the obligation, to
Settlement Agreement and Release
Page 12
conduct any opposition, re-issuance, post-grant review, inter-partes review, reexamination request, nullity action, interference, or other similar post-grant proceedings and any appeals therefrom relating to the 9101 Patents. HCW shall provide ImmunityBio with a copy of the draft prepared for the filing of any claim contained in a 9101 Patent (a “9101 Specific Claim”) before the filing of such 9101 Specific Claim and will consider in good faith comments thereto provided by ImmunityBio in connection with the filing thereof. HCW shall provide ImmunityBio with regular updates on the prosecution of the 9101 Specific Claims. HCW may cease prosecution and/or maintenance of any 9101 Specific Claim on a country-by-country basis by providing ImmunityBio written notice reasonably in advance (but at least XXXXX XXX XXXX before the applicable deadline with a relevant patent authority). If HCW elects to cease prosecution or maintenance of the relevant 9101 Specific Claim in a country, ImmunityBio shall have the right, but not the obligation, at its sole discretion and cost, to continue prosecution or maintenance of such 9101 Specific Claim and in such country.
5. ImmunityBio’s Choice of Six (6) Licensed Targets and Associated TOBI Platform Products.
a.Respondents hereby grant to ImmunityBio, with respect to each Licensed Target, a worldwide, perpetual, irrevocable, fully-paid up, royalty-free, exclusive (even as to Respondents) license, with the right to sublicense through multiple tiers, under all Intellectual Property Rights Controlled by Respondents or any of their Affiliates as of the Effective Date and any Improvements thereto, to Exploit Licensed Targets and any products (other than the fusion proteins referred to as HCW9206 and HCW9302), including any fusion proteins, molecules and/or antibodies therein, created by Respondents prior to the Effective Date or by either Party thereafter utilizing the TOBI Platform Directed To a Licensed Target (“TOBI Platform Products”), solely in the Oncology Field; provided that, the foregoing license grant excludes the right to Exploit any Cellular Therapy Products ex vivo to the extent exclusively licensed to Wugen pursuant to the Wugen Agreement as of the Effective Date. For clarity, from and after the Effective Date, Respondents shall not, and shall cause its Affiliates, acquirors and/or sublicensees not to, institute or prosecute, any claim demand, action or other proceeding for damages, costs, expenses or compensation, or for an enjoinment, injunction or any other equitable remedy, against ImmunityBio or any of its Affiliates, acquirors and/or sublicensees alleging that the Exploitation of Licensed Targets or TOBI Platform Products in any way infringes any Intellectual Property Rights owned or Controlled by Respondents or any of its Affiliates, acquirors or sublicensees, including, without limitation, those covering HCW9206 or HCW9302.
b.ImmunityBio shall choose one (1) additional Licensed Target at its sole discretion within the next six (6) months following the Effective Date (“Target Evaluation Period”).
c.In connection with ImmunityBio’s right to choose one (1) additional Licensed Target at its sole discretion during the Target Evaluation Period, with respect to any biological target, Respondents shall provide ImmunityBio (i) all material data, results, presentations and other information related to such biological target and any fusion proteins, molecules and/or antibodies Directed To such target that is necessary or reasonably useful for ImmunityBio to evaluate whether to select such target to be a Licensed Target and (ii) at ImmunityBio’s reasonable request, direct access to personnel of Respondents for the purpose of evaluating and discussing the information described in clause (i). In the event that ImmunityBio selects a new biological target to be a Licensed Target pursuant to this Paragraph 5, it shall provide written notice of such selection to the Respondents. Upon expiration of the Target Evaluation Period, ImmunityBio shall have no further right under this Paragraph 5 to select biological targets not already selected to be a Licensed Target.
d.The Licensed Targets (i) shall be exclusive to ImmunityBio (even as to Respondents) as to any fusion protein, molecule and/or antibody created utilizing the TOBI Platform in the Oncology Field, such that Respondents cannot Develop, manufacture Commercialize, or Exploit, or license, authorize, appoint or otherwise enable any other Third Party to Develop, manufacture, Commercialize or Exploit, any fusion protein, molecule and/or antibody created utilizing the TOBI Platform Directed To such Licensed Target or that is a derivative of a pre-existing fusion protein, molecule and/or antibody created utilizing the TOBI Platform Directed To such Licensed Target, in either case, in the Oncology Field (but excluding the fusion proteins referred to as HCW9206 and HCW9302) and (ii) the
Settlement Agreement and Release
Page 13
sequence of such selected Licensed Target shall be the sole confidential information of ImmunityBio and subject to terms and conditions of Paragraph 19 of this Settlement.
ImmunityBio’s right to file, prosecute, maintain, and enforce any licensed Intellectual Property Rights granted under the license set forth in Paragraph 5(a) shall be as set forth in Paragraphs 2(d) through (f) (inclusive), mutatis mutandis.
6. Right of First Refusal for ImmunityBio.
a.Respondents hereby grant to ImmunityBio an exclusive option and right of first refusal to obtain an exclusive license to any Additional Molecule in the Oncology Field (the “ROFR”). If Respondents or any of their Affiliates desires to enter into any transaction with a Third Party for the license, transfer, or other disposition of any Additional Molecule for use in the Oncology Field (each, a “ROFR Transaction”), HCW will provide prompt written notice to ImmunityBio (“ROFR Notice”). Such ROFR Notice provided by HCW to ImmunityBio shall be accompanied by a ROFR Information Package and a Non-Disclosure Agreement to govern the treatment of the ROFR Information Package. ImmunityBio may exercise its ROFR by providing HCW with written notice thereof (“Exercise Notice”) by the date that is XXXXX XXX XXXXfollowing ImmunityBio’s receipt of a ROFR Notice by HCW. The Exercise Notice shall identify the Additional Molecule with respect to which ImmunityBio has an interest in exercising its ROFR. Following HCW’s receipt of such Exercise Notice, the Parties will enter into good faith negotiations for a license for a period not to exceed XXXXXX XXXX XXXX. The financial terms of any such license shall reasonably reflect the scope and content of the license grant, including its exclusivity, the Additional Molecule, the licensed Indication and the licensed territory. Notwithstanding the foregoing, and even if such aggregate XXX XXXXXXX XXX XXXXX XXXXX XXX period has expired, neither Respondents nor any of their Affiliates shall grant licenses or other rights to any Additional Molecule to any Third Party without first providing ImmunityBio with notice that a Third Party license is sought, including disclosure to ImmunityBio of all material terms of such Third Party’s offer, and giving ImmunityBio the opportunity to match such Third Party offer and exercise its ROFR with respect to such Additional Molecule. If ImmunityBio does not exercise its ROFR as provided in this Paragraph 6, or if the Parties do not complete negotiations for a license related to any Additional Molecule for which such Third Party license is sought within the time periods set forth in this Paragraph 6, then Respondents will be free to grant such Third Party license to the applicable Third Party for such Additional Molecule provided that such license shall be on terms no more favorable to such Third Party than the license terms last offered by ImmunityBio in writing; provided further that, if Respondents or any of their Affiliates desire to enter into a new ROFR Transaction, including, for clarity, for the same Additional Molecule, the procedure set forth in this Paragraph 6 shall apply to each such new ROFR Transaction. For the avoidance of doubt, Respondents agree that they will not, directly or indirectly, Develop, make, have made, use or Commercialize any Additional Molecule for use in the Oncology Field, without first complying with the procedures described in this Paragraph 6.
7. Other Provisions Regarding the License Grants.
a.Except as explicitly set forth in this Settlement, none of the Respondents nor Claimants shall be deemed by estoppel or implication to have granted any other party (including Claimants and Respondents, respectively) any license or other right to any intellectual property of such Respondent.
b.No later than XXX XXXXXXX XXXXXX XXXXX XXXX XXXXX XXX XXXXXXXXX XXXX, the Parties shall define and finalize the actions that the Parties shall employ with respect to Licensed Products in any Licensed Field to protect patients and promote their well-being in a written pharmacovigilance agreement (the “Pharmacovigilance Agreement”) for the Development and Commercialization of the Licensed Products in any Licensed Field globally. The Pharmacovigilance Agreement shall include mutually acceptable guidelines and procedures for the receipt, investigation, recording, communication, and exchange (as between the Parties) of adverse event reports, pregnancy reports, and any other information concerning the safety of the Licensed Products in any Licensed Field, and other routine pharmacovigilance reporting requirements. Such guidelines and procedures shall be in accordance with, and enable the Parties to fulfill, local and national regulatory reporting obligations under applicable laws. Furthermore, such agreed procedure shall be consistent with relevant ICH guidelines,
Settlement Agreement and Release
Page 14
except where said guidelines may conflict with existing local regulatory reporting requirements, in which case the local reporting requirements shall prevail. As between the Parties, ImmunityBio shall be responsible for preparing all adverse event reports and responses to safety issues and requests of Regulatory Agencies relating to Licensed Products in any Licensed Field. Each Party hereby agrees to comply with its respective obligations under such Pharmacovigilance Agreement and to cause its Affiliates and (sub)licensees to comply with such obligations.
8. Proceedings Against Claimants and ImmunityBio.
a.XXX. XX. XXXX XXXXXX XX XXXXXX XXXX XXX XXX XXXXXXXX XXXXX, XXX XXX XX XXXXXXXXXXX XX XXXXXX XX XXX XXX, XX XXXXX XXXXXXX XX XXX XXXXXX XXXXXXX XX XXXXX XXX XX XXXX XXXXXXXXX XXXXX XXXX XXXXXXXX XXXXXXXX XX XXX XX XXXXX XXXXXXX XXXXX, XXXXXXXXX. XX. XXXX XXXX XXXXXX XXX XXXXX XX XXXXXXXXXXX XXX XXXX XX XXXXXXXX - XX XXX - XXXXXXXX XX XXX XXX XXX XXXXXX XXXXX XXXXXXXXXXXX.
9. Forensic and Other Remediation of Certain Information and Data Repositories.
a.WithinXXXXXX XXXX XXXXXXXX XXXX XX XXX XXXXXXXXX XXXX, each Respondent (i.e., HCW and Xx. Xxxx) and each HCW employee who previously worked for Altor or NantCell and is still employed by or acting as a consultant for HCW shall affirm, by signing a declaration under oath in the form attached to this Agreement as Exhibit E, stating that: (1) they do not possess, are not using, and will not use any confidential and/or proprietary information of Claimants (“Claimants’ Confidential Materials”), including but not limited to (i) emails (and/or attachments) sent to or from their Altor or NantCell email addresses during their time at Altor or NantCell and (ii) documents stored on Altor’s private corporate servers and copies thereof; (2) they have conducted a reasonably diligent search of all Information Sources in their possession, have destroyed any Claimants’ Confidential Materials in their possession (other than HCW backups or archives that are not ordinarily accessible and which Xx. Xxxx agrees not to access and HCW agrees not to permit its employees to access), and have concluded that they no longer possess or have access to any Claimants’ Confidential Materials; (3) they understand they have an ongoing obligation to promptly destroy any Claimants’ Confidential Materials without using or disclosing such materials, if they later discover any such materials in their possession; and (4) they have not provided and will not provide any Claimants’ Confidential Materials to any third party. Each of Respondents and each HCW employee who previously worked for Altor or NantCell and is still employed by or acting as a consultant for HCW shall return such signed declaration to Claimants within thirty (30) calendar days after the Effective Date.
b.For the purposes of this Agreement, “Information Sources” shall include, but not be limited to, (i) HCW file servers, (ii) HCW email systems or email servers, (iii) HCW laptop or desktop computers, (iv) Altor laptops or desktop computers, (v) Altor lab notebooks or physical notebooks used for Altor work while at Altor, (vi) portable storage devices (including but not limited to USB devices) of Respondents or HCW employees who previously worked for Altor or NantCell, (vii) personal emails, files, or text messages of Xx. Xxxx and other HCW employees who previously worked for Altor or NantCell, and (viii) any backups and archives of the foregoing. A “reasonably diligent search” shall include, but not be limited to, diligent searches to identify all emails containing Altor or NantCell domain names, all documents bearing the logo of or referencing that it is an Altor or NantCell document, all documents describing or relating to Altor’s or NantCell’s research, and all documents previously downloaded from Altor or NantCell company servers.
c.For the purposes of this Agreement, “Claimants’ Confidential Materials” does not include information that (i) is or becomes generally available to the public other than as a result of disclosure by Respondents in violation of this Paragraph 9, so long as Respondents first obtained the information after they had become generally available to the public, or (ii) becomes available to Respondents on a non-confidential basis from a source that has the right to disclose such information. For the avoidance of doubt, “Claimants’ Confidential Materials” includes all materials, if any, in Respondents’
Settlement Agreement and Release
Page 15
or their representatives’ or Affiliates’ possession that were trade secret, confidential, proprietary, and/or internal to Claimants and were obtained as a result of, or in connection with, Xx. Xxxx’x or other HCW employees’ prior employment with Altor or NantCell.
d.Within XXXXXX XXXX XXXXXXXX XXXX XX XXX XXXXXXXXX XXXX, HCW’s counsel and XXXXXXXX XXXXXXXX XXXXXshall destroy or oversee the destruction of the following portable storage devices (including any copies, backups, or archives) which constitute Information Sources: (i) XXXXXXX XXXXXX XX XX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXXXXXXXXXXXXXX, (ii) XXXXXXX XXXXXX XXXXX XX XX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXXXXXXXXXXXXXX, (iii) XX XXXXXXXXXX XXX XX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXXXXXXXXXXXXXXXXXXXXXXX, and (iv) XXXXXXX XXX XXXXX XXXX XXXXXX XXXXXX XXXXXXXX XXXXXXXXX XXXXXX.
e.Within XXXXXX XXXX XXXXXXXX XXXX XX XXX XXXXXXXXX XXXX, HCW’s counsel shall oversee the forensic remediation process referenced in Paragraph 78-79 of the Rebuttal Expert Report XX XXXXXX XXXXX, dated April 23, 2024.
f.Claimants contend that a hard drive with serial number XXXXXXXXXXXXXXXXXXXXXXXX, a hard drive with serial number XXXXXXXXXXXXXX, and potential copies of the XXXXXXX XXXXX may also contain Claimants’ confidential information and trade secrets. Respondents agree that if any of these devices are located, they will either be (i) destroyed promptly, or (ii) provided to Respondents’ counsel who will promptly notify Claimants’ counsel within XXXXXXX XXXX XXXXXXXX XXXX of Respondents’ discovery of such devices.
g.Nothing in this Agreement shall limit or affect the Parties’ ongoing obligations under employment, confidentiality, and non-disclosure agreements previously executed in connection with Xx. Xxxx’x or other HCW employees’ prior employment with Altor or NantCell, to the extent any such ongoing obligations exist.
10. HCW Retained Rights.
a.Subject to the terms and conditions of this Agreement, HCW will retain freedom to develop the TOBI Platform for all indications and uses.
11. Legal Fees.
a.XXX XXXXXXXXX XX XXXXX, XXX XXXXXXX XXX XXXXXXXXXXX XXX XXXXX XXX XXXXX XXXX XXX XXXXXXXX XXXX XXX XX, XXXX XX, XXXXXXXXX XXX XXXXXX XXXXXXXX XXXX XXXX XXXX XXXXXXXXX XXX XXXXXXXXXXX XX XX. XXXX. XXX XXXXXX, XXXXXXXXXXX XXXX XXX XX XXXXXXX XX XXXXXXXXXX XX XXX XXXXXXXXXX XX XXX XXXXXXXX XX XX. XXXXXX XXXXXXX XXXX XXXX XXX XXXX XXXX XX XX XXX XX, XXXX, XXX XXXXXXXXXXX XXXX XXX XXXX XX XXXXXX XXXX XXXXXX XXXXXXXXXX XXX XXXXXXX XX XXX XXXX XX XX XXX XXX XXXX. All Parties will be responsible for their share of expenses, including expenses of the Arbitrator, invoiced by JAMS. ImmunityBio agrees to release its pending claim against HCW for contribution of funds it has advanced that was filed in the Delaware Court of Chancery, captioned Altor Bioscience, LLC, et al. v. HCW Biologics, Inc., C.A. No. 2024-0310-PAF, and to dismiss that action. Other than as set forth in this Paragraph 11, each Party shall bear its own expenses, costs, and fees in connection with the Action and this Agreement, including any expenses, costs, or fees incurred by its attorneys, experts, advisors, agents, or representatives.
12. Representations and Warranties of Respondents. Each Respondent, as to himself, herself, or itself, as applicable, hereby represents and warrants to, and covenants and agrees with, Xxxxxxxxx, as of the date hereof, as follows:
a.Respondents have the right to grant the rights, transfers and assignments granted herein, without the need for any assignments, releases, consents, approvals, immunities or other rights not yet obtained;
Settlement Agreement and Release
Page 16
b.The Transferred Assets are solely and exclusively owned by Respondents or its Affiliates and are free of and not subject to any restrictions or to any mortgages, liens, pledges, security interests, encumbrances or encroachments;
c.Each of the patents and trademarks included in the Transferred Intellectual Property Rights is valid, enforceable and subsisting and has not lapsed, expired, been cancelled or become abandoned and all applicable fees have been paid on or before the due date for payment;
d.Schedule 1 attached hereto is complete, true and accurate and contains all patents and patent applications Controlled by the Respondents, whether published or unpublished, that are related to the TGFb Molecules or necessary or reasonably useful for the research, Development, Commercialization, use, sale or Exploitation of the TGFb Molecules;
e.To the Knowledge of the Respondents, except for the Transferred Intellectual Property Rights, no other rights or licenses (including other Intellectual Property Rights) are necessary to use, Develop, manufacture, import Commercialize or Exploit the TGFb Molecules;
f.To the Knowledge of the Respondents, except for the Transferred Intellectual Property Rights, no other rights or licenses (including other Intellectual Property Rights) are necessary to use, Develop, manufacture, import, Commercialize or Exploit HCW9201 for use as a subcutaneous injection;
g.The materials, inventory, documentation and other technology transferred to ImmunityBio as set forth in Schedule 2(a)(iii), and pursuant to Paragraphs 2(a)(iii), 3 and 4, contain all that are needed to manufacture the TGFb Molecules and HCW9101 on a level consistent with Respondents and/or its Third Party contract manufacturers, including without limitation EirGenix, as of the Effective Date, and Respondents are not withholding anything necessary or reasonably useful for the manufacturing of the TGFb Molecules and HCW9101.
h.The Transferred Contracts set forth on Schedule 3 are all Third Party agreements to which HCW is a Party that are necessary for the use, Development, manufacture, importation, Commercialization of Exploitation of the Transferred Assets. HCW has made available to ImmunityBio prior to the Effective Date a true, complete and correct copy of each Transferred Contract as in effect on the date of this Agreement. HCW is not currently and, to the Knowledge of Respondents, no other party to a Transferred Contract is, in breach of or default of the terms of any Transferred Contract. Each Transferred Contract is a legal, valid and binding obligation of HCW or its Affiliates and is in full force and effect.
i.The Wugen Agreement attached as Exhibit Ais a true and complete copy as of the Effective Date.
As of the Effective Date, the Wugen Agreement has not been amended or modified and Wugen has not exercised any of Wugen’s option rights thereunder.
13. Release by Claimants.
a.Each of the Claimants and their respective present or former agents, affiliates, successors, assigns, predecessors, parents, subsidiaries, representatives, trustees, executors, heirs, spouses, marital communities, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively the “Claimant Releasing Parties”), without any other action being required to be taken, do hereby completely, finally, and fully forever release, remise, acquit, compromise, settle, extinguish, relinquish, and forever discharge without limitation, Respondents and their respective predecessors, successors-in-interest, direct and/or indirect parents, direct and/or indirect subsidiaries, affiliates, representatives, agents, trustees, executors, heirs, spouses, marital communities, assigns, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively, the “Respondent Released Parties”) (except for XXXXXXXX XXXXX XXXXXXXXXXXXX XX., XXX., XXXXXXXX XXXXXX XXXXXXX XXX., XXXX XXXXXX XXXXXXXXXX XXXXXXX, XXXX XXXXXXXXX, XXXXXXX XXXXXXX XXX., XXXXXXXX XXXXXXX, XXXXX XXX, XXX XXXX XX , which are expressly excluded from and shall not constitute Respondent Released Parties) from any and all causes of action, suits, charges, debts, dues, sums of money, accounts, reckonings, bonds, bills, specialties, covenants, contracts, appraisal rights, torts,
Settlement Agreement and Release
Page 17
controversies, agreements, promises, variances, trespasses, damages, judgments, executions, claims, and demands whatsoever, whether asserted or unasserted, contingent or remote, known or unknown, and whether arising in law, admiralty, or equity that the Claimant Releasing Parties or any of them had, now have, or that their successors or assigns hereinafter can, shall, or may have, for, upon, or by reason of any matter, cause, or thing whatsoever that have been or could have been asserted by the Claimants, in any forum, including class, derivative, individual, or other claims, whether state, federal, or foreign, common law, statutory, or regulatory, including, without limitation, claims under the federal securities laws (collectively, the “Claimants’ Settled Claims”); provided, however, that the Claimants’ Settled Claims shall not include (i) the right of the Claimants to enforce the terms of this Agreement, or (ii) any rights or claims that arise after the Effective Date.
14. Release by Respondents.
a.Respondents and their respective present or former agents, affiliates, successors, assigns, predecessors, parents, subsidiaries, representatives, trustees, executors, heirs, spouses, marital communities, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively the “Respondent Releasing Parties,” and together with the Claimant Releasing Parties, the “Releasing Parties”), without any other action being required to be taken, do hereby completely, finally, and fully forever release, remise, acquit, compromise, settle, extinguish, relinquish, and forever discharge without limitation, the Claimants and their respective predecessors, successors-in-interest, parents, subsidiaries, affiliates, representatives, agents, trustees, executors, heirs, spouses, marital communities, assigns, or transferees, immediate or remote, and any person or entity acting for or on behalf of any of them and each of them (collectively, the “Claimant Released Parties”) from any and all causes of action, suits, charges, debts, dues, sums of money, accounts, reckonings, bonds, bills, specialties, covenants, contracts, appraisal rights, torts, controversies, agreements, promises, variances, trespasses, damages, judgments, executions, claims, and demands whatsoever, whether asserted or unasserted, contingent or remote, known or unknown, and whether arising in law, admiralty, or equity that the Respondent Releasing Parties or any of them had, now have, or that their successors or assigns hereinafter can, shall, or may have, for, upon, or by reason of any matter, cause, or thing whatsoever that have been or could have been asserted by Respondents, in any forum, including class, derivative, individual, or other claims, whether state, federal, or foreign, common law, statutory, or regulatory (collectively, the “Respondents’ Settled Claims,” and together with the Claimants’ Settled Claims, the “Settled Claims”); provided, however, that the Respondents’ Settled Claims shall not include (i) the claims referenced in Paragraph 8 of this Settlement, subject to the terms therein, (ii) the right of Respondents to enforce the terms of this Settlement, or (iii) any rights or claims that arise after the Effective Date.
15. Release of Unknown Claims. The Parties understand and agree that the releases described herein shall extend to claims that the Releasing Parties do not know or suspect to exist at the time of the release, which if known, might have affected the Releasing Parties’ decisions to enter into the releases. The Releasing Parties shall be deemed to relinquish, to the extent applicable and to the full extent permitted by law, the provisions, rights, and benefits of Section 1542 of the California Civil Code, which states that:
A GENERAL RELEASE DOES NOT EXTEND TO CLAIMS THAT THE CREDITOR OR RELEASING PARTY DOES NOT KNOW OR SUSPECT TO EXIST IN HIS OR HER FAVOR
Settlement Agreement and Release
Page 18
AT THE TIME OF EXECUTING THE RELEASE AND THAT, IF KNOWN BY HIM OR HER, WOULD HAVE MATERIALLY AFFECTED HIS OR HER SETTLEMENT WITH THE DEBTOR OR RELEASED PARTY.
The Releasing Parties shall be deemed to waive any and all provisions, rights and benefits conferred by any law of any state or territory of the United States, or principle of common law, that is similar, comparable, or equivalent to Section 1542 of the California Civil Code. The Releasing Parties acknowledge that they may discover facts in addition to or different from those that they now know or believe to be true with respect to the subject matter of this Agreement, but that it is their intention to fully, finally, and forever settle and release any and all claims released in Paragraphs 13-14, whether known or unknown, suspected or unsuspected, which now exist or heretofore existed or may hereafter exist and without regard to the subsequent discovery or existence of such additional or different facts. Each Party acknowledges that the foregoing waiver was separately bargained for, is an integral element of this Agreement, and was relied upon by the other Parties in entering into this Agreement.
16. Dismissal of Arbitration and Delaware Action. Upon execution of this Agreement by all Parties, the Parties will inform the Arbitrator in the pending JAMS arbitration (Altor BioScience, LLC, et al. vs. Xxxx X. Xxxx, et al. - JAMS Ref No. XXXXXXXXXX (the “Arbitration”)) that the Arbitration has settled, and ask the Arbitrator to refrain from further work pending dismissal. Claimants and Respondents will cooperate to arrange for prompt dismissal of (i) the Arbitration; and (ii) the pending action in the Delaware Court of Chancery, Altor Bioscience, LLC v. HCW Biologics, Inc., C.A. No. 2024-0310-PAF, after Respondents’ compliance with Paragraphs 2 through 9 above. To that end, counsel for the Parties shall work together to submit stipulations of dismissal in the Arbitration and the pending action in the Delaware Court of Chancery no earlier than XXXXXX XXX XXXX XXXXX XXX XXXXXXXXX XXXX, but as soon as practicable thereafter.
17. No Assignment. The Parties represent that they have not assigned or transferred, or purported to assign or transfer, to any person or entity any claim released in this Agreement.
18. No Admission of Liability. This Agreement constitutes a compromise of disputed claims. This Agreement shall not be deemed a presumption, a concession, or an admission by any Party of any fault, liability, or wrongdoing as to any facts, claims, or defenses that have been or might have been alleged or asserted in the Action, and shall not be interpreted, construed, deemed, invoked, offered, received in evidence, or otherwise used by any person in any claim, action, proceeding, or settlement negotiation, except for any litigation or proceeding arising out of or relating to the terms of this Agreement, whether civil, criminal, or administrative, for any purpose other than as provided expressly herein. Notwithstanding the preceding sentence, this Agreement, proof of its execution, and payment of consideration under its terms shall be admissible to prove settlement and release of the claims set forth herein if such shall be necessary. In the event that this Agreement is rendered null and void for any reason,
Settlement Agreement and Release
Page 19
the existence of or the provisions contained in this Agreement shall not be deemed to prejudice in any way the respective positions of the Parties.
19. Disclosure and Confidentiality. This Settlement (its existence, and its terms) and all documents, communications, drafts and other materials of any kind relating to their negotiation, the circumstances leading thereto, or the implementation thereof shall be and remain confidential and shall not be disclosed to any other person without the Parties’ prior, express, written consent, except as required by applicable law, rule or regulation, or to comply with or enforce the terms of the Settlement itself. There will be no press releases, web site announcements or other public statements by any Party or any of their representatives regarding this Settlement or any of the matters referenced herein or therein other than as required by law. Notwithstanding the foregoing, each of the Parties may disclose the terms of this Settlement to his, her, or its respective attorneys, accountants, insurers, and/or regulators, and the Parties may disclose the terms as necessary in any required SEC or other regulatory filings or communications.
20. Entire Agreement; No Reliance. This Agreement constitutes the entire agreement of the Parties and replaces, cancels, and supersedes any and all prior agreements and understandings between them pertaining to the subject matter hereof. There is no separate agreement, representation, or other inducement between the Parties for the execution of this Agreement. In entering into this Agreement, no Party is relying upon any representation, commitment, warranty, or promise by any other Party unless expressly set forth in this Agreement. The Parties agree this provision is an “anti-reliance” provision under Delaware law precluding claims based on any representation, commitment, warranty, or promise whatsoever not set forth in this Agreement.
21. Counterparts. This Agreement may be executed in any number of counterparts, by original or electronically transmitted signature, all of which shall be considered one and the same agreement, and shall become effective when all such counterparts have been signed by each of the Parties and delivered to all of the other Parties.
22. Mutual Drafting. The Parties to this Agreement agree that they have thoroughly discussed all aspects of this Agreement with their attorneys, that they have read and fully understand all of the provisions of this Agreement, and that they are voluntarily entering into this Agreement. The Parties further agree that this Agreement has been jointly negotiated and prepared and that no Party shall be deemed to have prepared this Agreement for purposes of construing its terms.
23. Authorization. Each of the individuals executing this Agreement hereto on behalf of one or more of the Parties warrants and represents that he or she has been duly authorized and empowered to
Settlement Agreement and Release
Page 20
execute this Agreement on behalf of each such respective Party and to bind each such respective Party to the terms hereof.
24. Further Actions. The Parties and their attorneys agree to cooperate fully and to use their best efforts to effectuate expeditiously the terms and conditions of this Agreement, including the execution of all related documents, as soon as practicable. Counsel for the Parties are expressly authorized to enter into such changes, modifications, or amendments of this Agreement as to which they mutually agree as long as such changes are in writing.
25. No Waiver. Any failure by any Party to insist upon the strict performance by any other Party of any of the provisions of this Agreement shall not be deemed a waiver of any of the provisions hereof, and such Party, notwithstanding such failure, shall have the right thereafter to insist upon the strict performance of any and all of the provisions of this Agreement to be performed by such other Party.
26. Choice of Law and Forum. This Agreement and any and all disputes arising out of or relating in any way to this Agreement, whether in contract, tort, or otherwise, shall be governed by, and construed in accordance with, the laws of the state of Delaware, without regard to conflicts of law principles. Each of the Parties (a) irrevocably submits to the personal jurisdiction of any state or federal court sitting in Wilmington, Delaware, as well as to the jurisdiction of all courts to which an appeal may be taken from such courts, in any suit, action, or proceeding arising out of or relating to this Agreement and/or the Settlement, (b) agrees that all claims in respect of such suit, action, or proceeding shall be brought, heard, and determined exclusively in the Court (provided, however, that in the event that subject matter jurisdiction is unavailable in the Court, then all such claims shall be brought, heard, and determined exclusively in any other state or federal court sitting in Wilmington, Delaware), (c) agrees that it shall not attempt to deny or defeat such personal jurisdiction by motion or other request for leave from such court, (d) agrees not to bring any action or proceeding arising out of or relating to this Agreement in any other court, and (e) expressly waives and agrees not to plead or to make any claim that any such action or proceeding is subject (in whole or in part) to a jury trial. Each of the Parties waives any defense of inconvenient forum to the maintenance of any action or proceeding brought in accordance with this paragraph. Each of the Parties further agrees to waive any bond, surety, or other security that might be required of any other party with respect to any action or proceeding, including an appeal thereof. Each of the Parties further consents and agrees that process in any suit, action, or proceeding may be served on such Party by certified mail, return receipt requested, addressed to such Party or such Party’s registered agent in the state of its incorporation or organization, or in any other manner provided by law. Each of the Parties further consents and agrees that process in any suit, action, or proceeding may be served by mailing or emailing such written notice to:
If to Claimants:
XXXXX XXXXXXX XXXXXXXX X XXXXXXXX, XXX
XXXXX X. XXXXXX, XX.
XXXXXXXXXXXXXXXXXXXXXXXXXX
XXX X. XXXXXXXXX XX. XXXX XXXXX
XXX XXXXXXX, XXXXXXXXXX XXXXX-XXXX
If to Respondents:
XXXXXX, XXX
XXXXX XXXXXXXXX
XXXXXXXXXXXXXXXXXXXXX
XX XXXXXX XXXXX
Settlement Agreement and Release
Page 21
XXX XXXX, XXX XXXX XXXXX-XXXX
XXXXXX, XXXXXXXXXX X XXXXXXXXX XXX
XXXX XX
XXXXXXXXXXXXXX
XXX X. XXXXX XXX.
XXXXX XXX
27. Binding Effect. This Agreement shall be binding upon and inure to the benefit of the Parties and their respective heirs, executors, administrators, successors, and assigns, and upon any corporation or other entity with which any Party hereto may merge or consolidate. The releases in Paragraphs 13 through 15 of this Agreement shall inure to the benefit of, and be enforceable by, the respective released persons described therein.
28. Severability. If any provision in this Agreement is held by a court of competent jurisdiction to be invalid, void, or unenforceable, the remaining provisions shall nevertheless continue in full force and continue to be binding on the Parties without being impaired or invalidated in any way.
29. Notice/Cure/Settlement Conference. Before raising any potential breaches of this Settlement with any court, the Parties will meet and confer within XXXX XXX XXXXXXXX XXXX of notice of the breach to negotiate a resolution. Notice pursuant to this requirement shall be in writing and shall be deemed duly given: (i) upon actual receipt; (ii) XXXX XXX XXXXXXXX XXXX after mailing by first class, certified, or registered U.S. mail, postage prepared and addressed as indicated in Paragraph 26, return receipt requested; (iii) if given by email, once such notice or other communication is transmitted to the email address(es) specified in this Settlement, or (iv) if sent through a nationally-recognized overnight delivery service that guarantees next day delivery and addressed as indicated in this Settlement, the business day following its delivery to such service in time for next day delivery. The Party alleged to be in breach shall have XXXXX XXX XXXXXXXX XXXX in which to cure the breach. If no resolution can be negotiated or if the breach is not cured withinXXXXX XXX XXXXXXXX XXXX, or cannot be cured, the non-breaching Party may file an action seeking to enforce this Settlement.
30. Enforcement. Nothing herein shall be construed to limit or prejudice in any way any Party’s rights to seek enforcement of the terms of this Agreement against the breaching Party, including specifically, rights to sue for breach of contract and for specific performance and/or to seek appropriate legal and/or equitable relief to enforce this Agreement. The Parties agree that any Party found to have breached this Agreement shall reimburse the non-breaching Party for the actual and reasonable attorneys’ fees, costs, and expenses that the non-breaching Party incurs in connection with the enforcement of this Agreement or any claim, damages, or litigation relating to any breach of this Agreement.
IN WITNESS WHEREOF, the Parties have caused this Agreement to be executed and delivered as of the 13th day of July, 2024.
[SIGNATURE PAGES FOLLOW]
Settlement Agreement and Release
Page 22
ALTOR BIOSCIENCE, LLC
By:
Name: Xxxxxxx Xxxxxx
Title: President and Chief Executive Officer
NANTCELL, INC.
By:
Name: Xxxxxxx Xxxxxx
Title: President and Chief Executive Officer
IMMUNITYBIO, INC.
By:
Name: Xxxxxxx Xxxxxx
Title: President and Chief Executive Officer
HCW BIOLOGICS, INC.
By:
Name: Xx. Xxxx X. Xxxx
Title: Chief Executive Officer
XX. XXXX X. XXXX
By:
Name: Xx. Xxxx X. Xxxx
Settlement Agreement and Release
Page 23
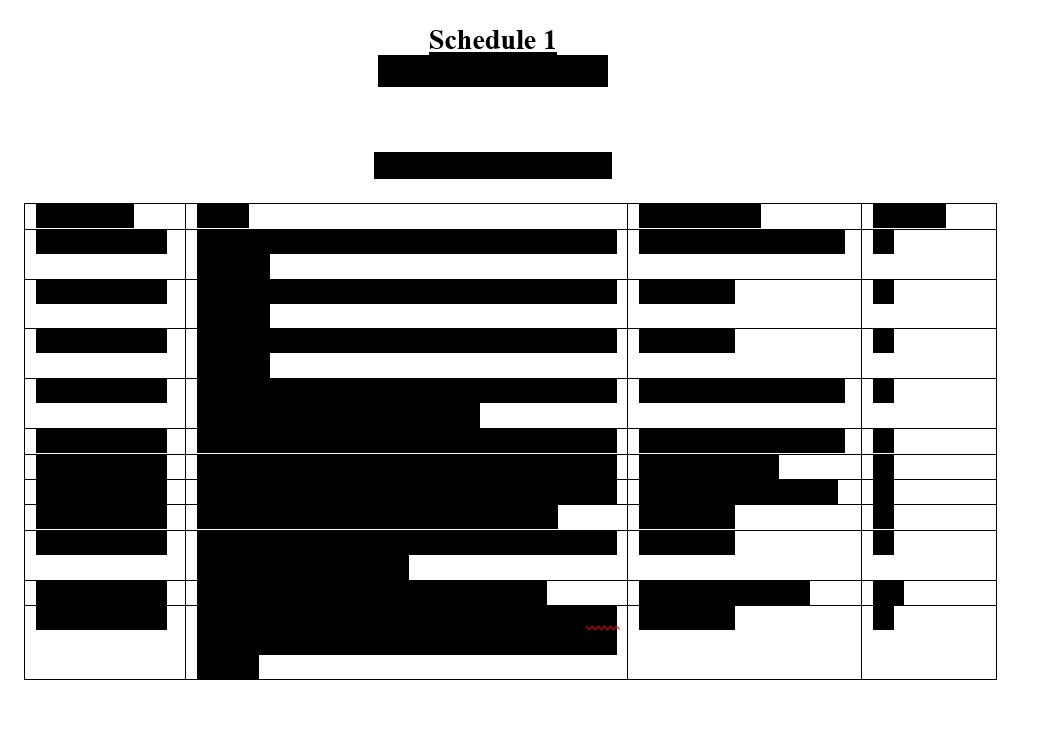
Settlement Agreement and Release
Page 24
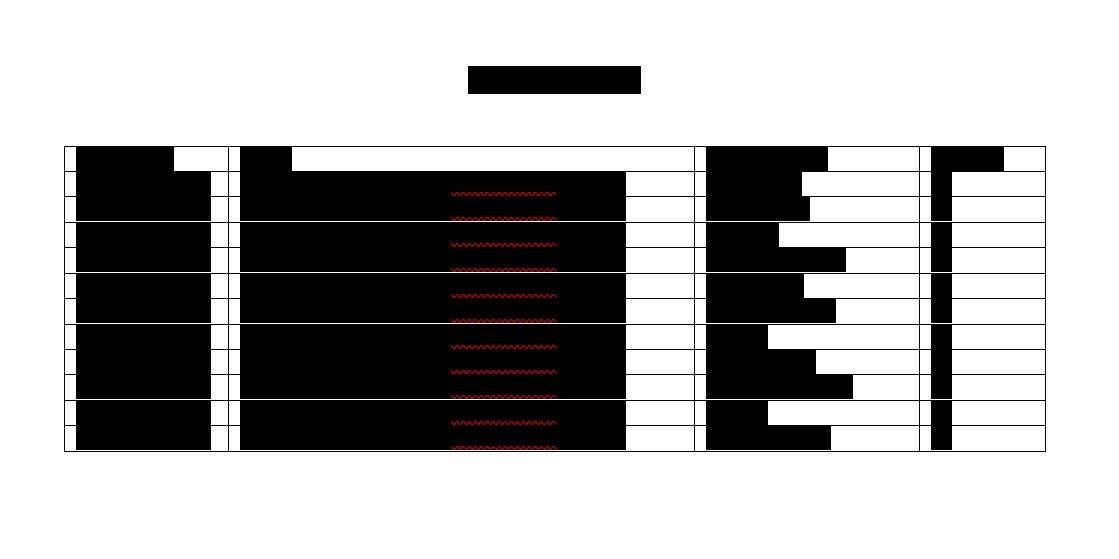
Settlement Agreement and Release
Page 25

Settlement Agreement and Release
Page 26
Settlement Agreement and Release
Page 27

Settlement Agreement and Release
Page 28

Settlement Agreement and Release
Page 29

Settlement Agreement and Release
Page 30

Settlement Agreement and Release
Page 31

Settlement Agreement and Release
Page 32











