MANUFACTURING SERVICES AGREEMENT FOR PBT 434 SUPPLY
Exhibit 4.36
MANUFACTURING SERVICES AGREEMENT FOR PBT 434 SUPPLY
| This MANUFACTURING SERVICES AGREEMENT (hereinafter called “Agreement”) is made on 28th day of March, 2014 by and between Prana Biotechnology Ltd ACN 080 699 065 (“Prana”), a company incorporated in Australia whose registered office and principal place of business is at ▇▇▇▇▇ ▇, ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇ and ▇▇. ▇▇▇▇▇’▇ Laboratories Limited (“▇▇. ▇▇▇▇▇’▇”), a company incorporated and existing under the laws of India, having its principal place of business at # ▇-▇-▇▇▇, ▇▇▇▇ ▇▇.▇, ▇▇▇▇▇▇▇ ▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇ 500 034, Andhra Pradesh, India. | |||
|
▇▇. ▇▇▇▇▇’▇ and Prana are individually referred to as a “Party” and jointly as the “Parties”.
|
|||
|
WHEREAS:
|
|||
|
A.
|
The parties executed a Confidentiality Agreement dated 19th August 2013 and Letter of Intent dated 24th January, 2014 in relation to Prana’s requirement for ▇▇. ▇▇▇▇▇’▇ to undertake process development, analytical method validations, cGMP manufacture and stability studies of Prana’s compound known as PBT 434. | ||
|
B.
|
The parties now enter this manufacturing services agreement to definitively record all the terms and conditions upon which ▇▇. ▇▇▇▇▇’▇ will perform the Project. | ||
|
NOW THEREFORE, IN CONSIDERATION TO THE MUTUAL COVENANTS AGREED HEREIN THE PARTIES HERETO HAVE AGREED TO BE LEGALLY BOUND BY THE FOLLOWING TERMS, WHICH SHALL HEREAFTER GOVERN THE TERMS OF THIS AGREEMENT
|
|||
|
1.
|
Definitions | ||
|
For the purposes of this Agreement, capitalized terms, whether used in the singular or plural, shall have the following meanings, unless the context clearly requires otherwise:
|
|||
|
(a)
|
“Affiliate” shall mean, with respect to a Party, any entity controlling, controlled by, or under common control with such Party. For these purposes, “control” shall refer to: (i) the possession, directly or indirectly, of the power to direct the management or policies of an entity, whether through the ownership of voting securities, by contract or otherwise; or (ii) the ownership, directly or indirectly, of more than fifty percent (50%) of the voting securities or other ownership interest of an entity.
|
||
|
(b)
|
“Agreement” means this agreement and includes any schedule or annexure to it.
|
||
|
DRL IRN – 100004118
|
|
 |
1
|
(c)
|
“API” means an active pharmaceutical ingredient of the Compound. | ||||
|
(d)
|
“Approved Purposes” for a given Party means the performance of the Project and its obligations under this Agreement, and for Prana also means the use of the Compound and Results for its business purposes which will necessarily include the satisfaction of any regulatory requirements in relation to the Compound. | ||||
|
(e)
|
“Business Day” means a day other than Saturday, Sunday or a public holiday or bank holiday in the place where an act is to be performed or a payment is to be made. | ||||
|
(f)
|
“Commencement Date” means 25th October, 2013. | ||||
|
(g)
|
“Compound” or “PBT434” means 5,7-dichloro-2-((ethylamino)methyl)-8-hydroxy-3- methylquinazolin-4(3H)-one hydrobromide. | ||||
|
(h)
|
“Confidential Information” means ▇▇. ▇▇▇▇▇’▇ Confidential Information or Prana Confidential Information as the context allows. | ||||
|
(i)
|
“Deliverables” means the quantities of Compound, reports, materials, licences or other deliverables specified in the Scope of Project Works for each Sub-Project. | ||||
|
(j)
|
“▇▇. ▇▇▇▇▇’▇ Background IP” means all Intellectual Property owned or controlled by or licensed to ▇▇. ▇▇▇▇▇’▇ or any Affiliate of it as at the Commencement Date. | ||||
|
(k)
|
“▇▇. ▇▇▇▇▇’▇ Confidential Information” means: | ||||
|
(i)
|
the Proposal (excluding any Prana Confidential Information contained in it);
|
||||
|
(ii)
|
▇▇. ▇▇▇▇▇’▇ Background IP;
|
||||
|
(iii)
|
all information concerning ▇▇. ▇▇▇▇▇’▇ comprising its research projects, plans and strategies, trade secrets, know-how, technology, business operations and financial dealings which is or has been disclosed to or obtained by Prana prior to or after the date of this Agreement (whether orally, electronically or in writing) other than Prana Confidential information and information that Prana can establish:
|
||||
|
(A)
|
was already in the public domain at the time of its provision to Prana; or
|
||||
|
(B)
|
became part of the public domain after its provision to Prana, otherwise than though a disclosure by Prana or any person to whom Prana has disclosed ▇▇. ▇▇▇▇▇’▇ Confidential Information;
|
||||
|
DRL IRN – 100004118
|
|
 |
2
|
(C)
|
is or came lawfully into the possession of Prana otherwise than as a result of disclosure in breach of an obligation of confidence; or
|
||||
|
(D)
|
was independently discovered by Prana without the aid, application or use of the ▇▇. ▇▇▇▇▇’▇ Confidential Information.
|
||||
|
(1)
|
“Force Majeure Event” has the meaning given to it in clause 13.
|
||||
|
(m)
|
“GMP” or “cGMP” means the current good manufacturing practices, standards and requirements specified in US 21 CFR parts 210 and 211 and ICH Q7.
|
||||
|
(n)
|
“Intellectual Property” (“IP”) means any and all Technology and all intellectual, industrial and commercial property rights throughout the world subsisting in or in relation thereto including rights and interests in respect of or in connection with Patents, trade secrets, rights in confidential information, copyright (including future copyright and rights in the nature of or analogous to copyright), trade marks, service marks, database rights, designs, whether or not registered or registrable and includes applications for any of the foregoing and the right to apply for any of the foregoing in any part of the world.
|
||||
|
(o)
|
“Letter of Intent” or “LOI” means the Letter of Intent between the parties in relation to the Project dated 24th January, 2014.
|
||||
|
(p)
|
“Material Form” in relation to information, includes any form (whether visible or not) of storage from which the information can be reproduced, and any form in which the information is embodied or encoded and in relation to Prana Materials, means the materials themselves.
|
||||
|
(q)
|
“Patents” mean all patent applications, patents, or letters patents, and any claims in any such patent applications or patents, in any part of the world, including, without limitation, all continuations, continuation-in-parts, reissues, extensions, substitutions, confirmations, registrations, re-validations, and additions, and any supplementary protection certificates in respect thereof.
|
||||
|
(r)
|
“Prana Arising IP” means all Intellectual Property generated, developed, conceived, created, invented, discovered, derived, modified, improved or adapted by ▇▇. ▇▇▇▇▇’▇, Prana or their respective Affiliates and Representatives in the course of performing the Project.
|
||||
|
(s)
|
“Prana Background IP” means all Intellectual Property owned or controlled by or licensed to Prana or any Affiliate of it as at the Commencement Date.
|
||||
|
(t)
|
“Prana Confidential Information” means:
|
||||
| (i) | the Prana Materials; | ||||
|
DRL IRN – 100004118
|
|
 |
3
|
(ii)
|
the Results and Deliverables;
|
||||
|
(iii)
|
the RFP;
|
||||
|
(iv)
|
Prana Background IP and Prana Arising IP;
|
||||
|
(v)
|
all information concerning Prana, the Compound and Prana’s research projects, plans and strategies, products, materials and compounds, trade secrets, know how, technology, business operations and financial dealings which is or has been disclosed to or obtained by ▇▇. ▇▇▇▇▇’▇ prior to or after the date of this Agreement (whether orally, electronically or in writing) other than information that ▇▇. ▇▇▇▇▇’▇ can establish:
|
||||
|
(A)
|
was already in the public domain at the time of its provision to ▇▇. ▇▇▇▇▇’▇; or
|
||||
|
(B)
|
became part of the public domain after its provision to ▇▇ ▇▇▇▇▇’▇, otherwise than though a disclosure by ▇▇. ▇▇▇▇▇’▇ or any person to whom ▇▇. ▇▇▇▇▇’▇ has disclosed Prana Confidential Information;
|
||||
|
(C)
|
is or came lawfully into the possession of ▇▇. ▇▇▇▇▇’▇ otherwise than as a result of disclosure in breach of an obligation of confidence; or
|
||||
|
(D)
|
was independently discovered by ▇▇. ▇▇▇▇▇’▇ outside the Project without the aid, application or use of the Prana Confidential Information.
|
||||
|
(u)
|
“Prana Materials” means: | ||||
|
(i)
|
samples of the Compound in the possession of ▇▇. ▇▇▇▇▇’▇ as at the Commencement Date and any other materials including reference standards provided by Prana to ▇▇. ▇▇▇▇▇’▇ for the purpose of the Project; and
|
||||
|
(ii)
|
any APIs of the Compound or other materials manufactured by ▇▇. ▇▇▇▇▇’▇ in the course of the Project.
|
||||
|
(v)
|
“Project” means a process development and manufacturing project to carry out (by way of Sub-Projects)process development, analytical method validations, cGMP manufacture and Stability Studies of the Compound. | ||||
|
(w)
|
“Project Price” means US$300,000 (subject to an increase of $20,000 if the bonus payment for Project item 6 specified in Appendix A becomes payable). | ||||
|
DRL IRN – 100004118
|
|
 |
4
|
(x)
|
“Project Works” means the works (comprising the Sub-Projects) described in Appendix A and specified in detail in Appendix B. | ||||
|
(y)
|
“Proposal” means ▇▇. ▇▇▇▇▇’▇ proposal for PBT434 manufacture provided in response to the RFP. The proposal is set out in ▇▇. ▇▇▇▇▇’▇ letter dated 3rd October, 2013. | ||||
|
(z)
|
“Quality Agreement” means the quality agreement put in place pursuant to the Manufacturing Service Agreement of PBT 2 HCI Supply dated 19th August, 2013 that will be modified and updated to support this Agreement (attached as Appendix C to this Agreement). | ||||
|
(aa)
|
“Representatives” in relation to a Party means a director, officer, employee, contractor, consultant, agent or adviser of that Party. | ||||
|
(bb)
|
“Results” means: | ||||
|
(i)
|
all results, data, information, processes, procedures, methodologies, techniques, concepts, ideas, compounds, materials, items or things conceived, created, developed, discovered, derived, modified, improved or adapted by ▇▇. ▇▇▇▇▇’▇ or Prana or their respective Affiliates and Representatives during, or as a consequence of, the Project; and
|
||||
|
(ii)
|
all papers, materials, records, laboratory notebooks and documents (in written or electronic form) which have been produced by ▇▇. ▇▇▇▇▇’▇ or Prana or their respective Affiliates and Representatives in relation to the Project and the Results.
|
||||
|
(cc)
|
“RFP” means Prana’s Request for Proposal: Process Development and cGMP API Manufacture of PBT434 dated 19th August 2013. | ||||
|
(dd)
|
“Scope of Project Works” means the specifications, requirements and Deliverables for the Project Works as set out in Appendix B. | ||||
|
(ee)
|
“Sub-Projects” means the sub-projects described in Appendix A and specified in detail in Appendix B for performance by ▇▇ ▇▇▇▇▇’▇, subject in each case to ▇▇ ▇▇▇▇▇’▇ receiving prior written approval from Prana. | ||||
|
(ff)
|
“Technology” means trade secrets, ideas, knowledge, information, discoveries, inventions, technology, data, results, reports, formulae, techniques, strategies, concepts, methodologies, processes, procedures for experiments and tests and manufacturing scale ups, compounds, materials, methods or schemes for synthesising compounds, uses of/or indications for chemical compounds, technical data, information or specifications, testing methods, assays, isolation and purification methods, designs, sketches, records, biological materials and analyses. | ||||
|
DRL IRN – 100004118
|
|
 |
5
|
(gg)
|
“Timetable” means the timetable contained in Appendix A for the commencement and completion of each Sub-Project.
|
|||
|
2.
|
Engagement and Obligations of ▇▇. ▇▇▇▇▇’▇
|
|||
|
(a)
|
Prana engages ▇▇. ▇▇▇▇▇’▇ to perform the Project (to the extent of those Sub-Projects authorised by Prana in accordance with clause 2 (b)), and ▇▇. ▇▇▇▇▇’▇ agrees to accept the engagement on the terms and conditions contained in this Agreement,
|
|||
|
(b)
|
▇▇. ▇▇▇▇▇’▇ must receive written authority from Prana’s Head of Discovery and Non-Clinical Development Manager (▇. ▇▇▇▇▇▇▇) or its Principal Scientist (▇. ▇▇▇▇▇▇▇) or its Head of Toxicology (▇. ▇▇▇▇▇) or its Chief Operating Officer (▇. ▇▇▇▇▇) before commencing any Sub-Project. Without such authority for a given Sub-Project, ▇▇. ▇▇▇▇▇’▇ must not undertake and may not charge Prana its fee for the Sub-Project or any other amount. The work undertaken and the costs of a Sub-Project cannot be altered without the express written permission of Prana. If in relation to a given Sub-Project, Prana provides written authority to undertake the Sub-Project after the relevant commencement date specified in the Timetable, then ▇▇. ▇▇▇▇▇’▇ shall commence the Sub-Project as soon as is reasonably practicable thereafter, and in any event, not later than 14 days after Prana provides written authority to undertake the Sub-Project.
|
|||
|
(c)
|
The parties acknowledge the grant of approval by Prana in the LOI for ▇▇. ▇▇▇▇▇’▇ to commence Sub-Projects 1, 2 and 3 in Appendix A. The parties also acknowledge and agree to the milestone payments totalling US$76,000 in relation to Sub-Project 3.
|
|||
|
(d)
|
▇▇. ▇▇▇▇▇’▇ will perform and carry out the Project Works with all due care and skill in accordance with this Agreement, and in particular the Scope of Project Works and the Timetable
|
|||
|
(e)
|
▇▇. ▇▇▇▇▇’▇ must provide the following updates and reports to Prana:
|
|||
|
(i)
|
weekly written updates (in a format acceptable to Prana, which will be communicated to ▇▇. ▇▇▇▇▇’▇ in a separate email) of the work undertaken and the Results obtained for the week, problems encountered by ▇▇. ▇▇▇▇▇’▇, the stage of the Timetable that ▇▇. ▇▇▇▇▇’▇ is up to and any other information that would be relevant to Prana in relation to the Project; and
|
|||
|
DRL IRN – 100004118
|
|
 |
6
|
(ii)
|
a written report (in a format acceptable to Prana) detailing all of the work carried out and all the Results obtained for each Sub-Project undertaken, including all practices, procedures, processes and data (including spectra) and information developed or generated in the conduct of the Sub-Project along with any future recommendations on completion of the work specified in the Scope of Project Works for that Sub-Project.
|
|||
|
(f)
|
▇▇. ▇▇▇▇▇’▇ will participate in weekly teleconferences with Prana to present its updates and reports and allow Prana to ask any questions that it may have concerning the Project and set the work priorities for the following week. A Representative of ▇▇. ▇▇▇▇▇’▇ must take the minutes of each telephone conference and prepare these for the consideration and approval of the Parties at the next telephone conference.
|
|||
|
(g)
|
All Results arising out of the Project must be recorded in a written format. These results captured in a written format must:
|
|||
|
(i)
|
be maintained and signed in accordance with best industry practice; and (ii) be made available for inspection by Prana upon request by Prana in writing.
|
|||
|
(h)
|
▇▇. ▇▇▇▇▇’▇ must comply with cGMP (in relation to the manufacturing work to be undertaken by it) and all applicable laws in the performance of its obligations under this Agreement.
|
|||
|
(i)
|
Prana and ▇▇. ▇▇▇▇▇’▇ have executed the Quality Agreement to the extent required for the purpose of the Project. The Quality Agreement shall be co terminus to this Agreement.
|
|||
|
(j)
|
On 15 days prior written notice and not more than twice in each twelve (12) month period, ▇▇. ▇▇▇▇▇’▇ must allow Prana or any Representative of it to attend any premises at which Project Works are being conducted for the purpose of auditing all Project Works, materials and information to ensure the compliance by ▇▇. ▇▇▇▇▇’▇ with its obligations under this Agreement. Prana’s Representative shall only have the right to access works, materials and information that relate exclusively to the Project, and only if such access would not compromise ▇▇. ▇▇▇▇▇’▇ confidentiality obligations to another party and/or its internal QA programs. Notwithstanding the foregoing, if Prana’s Representative is not an employee of Prana, (i) he/she must not be a competitor of ▇▇. ▇▇▇▇▇’▇ or any of its Affiliates, and (ii) will not be permitted to access or to examine any Project Works, materials and information, until he/she has entered into a non-disclosure agreement with ▇▇. ▇▇▇▇▇’▇.
|
|||
|
(k)
|
At any time during the conduct of the Project Works with prior notice by Prana, ▇▇. ▇▇▇▇▇’▇ must allow Prana or any Representative of it to attend any premises at which Project Works are being conducted for the purpose of assessing the progress of the Project Works. Notwithstanding the foregoing, if Prana’s Representative is not an employee of Prana, or ▇▇▇▇ ▇▇▇▇▇▇▇ or ▇▇▇▇ ▇▇▇▇▇▇ (in their capacity as contractors of Prana) (i) he/she must not be a competitor of ▇▇. ▇▇▇▇▇’▇ or any of its Affiliates, and (ii) will not be permitted to access or to examine any Project works, materials and information, until he/she has entered into a non-disclosure agreement with ▇▇. ▇▇▇▇▇’▇.
|
|||
|
DRL IRN – 100004118
|
|
 |
7
|
(1)
|
If at any time during or after the termination of this Agreement, Prana requires a third party to perform any work relating to PBT434 including its manufacture, then ▇▇. ▇▇▇▇▇’▇ must, at the request of Prana, co-operate with Prana and the third party and provide such assistance, advice, documentation and information (including the relevant Results) as is necessary to enable the third party to perform the work requested by Prana. Prana agrees to pay all out-of-pocket expenses reasonably incurred by ▇▇ ▇▇▇▇▇’▇, provided that any anticipated expenses in excess of USD$1,000 are approved by Prana in writing before they are incurred.
|
|||
|
(m)
|
It is agreed in relation to the Deliverables for each Sub-Project that Prana shall have fifteen (15) days (“Testing Period”) from the date they are received to perform acceptance testing on the Deliverables in order to confirm that they comply with the specifications set out in this Agreement (“Acceptance Testing”). If Prana is satisfied that the Deliverables meet specification, then it will confirm this in writing to ▇▇. ▇▇▇▇▇’▇ and pay the applicable milestone payment. If Prana finds that any Deliverables supplied by ▇▇. ▇▇▇▇▇’▇ do not meet specification, then Prana will immediately notify ▇▇. ▇▇▇▇▇’▇ in writing of any non-compliance. If the non-compliance for a Sub-Project relates to:
|
|||
|
(i)
|
Compound supplied to Prana, then Prana may by written notice:
|
|||
|
(A)
|
require that ▇▇. ▇▇▇▇▇’▇ manufacture and supply the applicable quantity of Compound based on a manufacture start within 28 days of Prana’s notice or 7 days of initiating plant modification required for the manufacturing, whichever is the earlier and a manufacturing period less than or equal to the period of time originally allocated for the activity in the corresponding Sub-Project in Appendix A; or
|
|||
|
(B)
|
terminate the Sub-Project in whole or in part, in which case Prana will not be liable to pay for, and ▇▇. ▇▇▇▇▇’▇ must immediately refund any moneys paid by Prana (if any) on account of, the Sub-Project or the terminated part of it;
|
|||
|
(ii)
|
services or documents or Results supplied, then:
|
|||
|
(A)
|
Prana may require that ▇▇. ▇▇▇▇▇’▇ immediately repeat the services or re-write the documents to rectify the non-compliance within the period specified by Prana; or
|
|||
|
(b)
|
terminate the Sub-Project in whole or in part, in which case Prana will not be liable to pay for, and ▇▇. ▇▇▇▇▇’▇ must immediately refund any moneys paid by Prana (if any) on account of, the Sub-Project or the terminated part of it.
|
|||
|
DRL IRN - 100004118
|
|
 |
8
|
3.
|
Payment
|
|||
|
(a)
|
The total cost of the Project (including all milestone payments) is three hundred and twenty thousand United States Dollars (USD 300,000/-) for Sub-Projects 1 to 6 inclusive, payable to ▇▇. ▇▇▇▇▇’▇. A further bonus payment of US$20,000 may be payable in respect of Sub-Project 6, subject to ▇▇. ▇▇▇▇▇’▇ complying with the conditions specified in Appendix A for the payment of that sum.
|
|||
|
(b)
|
The amount payable by Prana to ▇▇. ▇▇▇▇▇’▇ for each Sub-Project and the timelines and conditions attaching to each such payment are specified in Appendix A.
|
|||
|
(c)
|
All payments are to be made within 30 days of invoicing. Each of the Sub-Projects that attract and satisfy a milestone payment, other than Sub-Project 6(a) Stability Studies, shall be invoiced on completion of the Sub-Project. Each Sub-Project will be invoiced independent of the completion of other Sub-Projects. If any Sub-Project is not authorised by Prana (for whatever reason), then Prana will have no liability to pay ▇▇. ▇▇▇▇▇’▇ for the Sub-Project fees or any other amount. Sub-Project 6(a) will be invoiced within 30 days of completing the time points mentioned in Appendix A.
|
|||
|
(d)
|
All payments are subject to:-
|
|||
|
(i)
|
Prana having received the requisite Deliverables; and
|
|||
|
(ii)
|
Completion of the Sub-Project. Completion of a Sub-Project will be determined by the provision of all Deliverables for that Sub-Project that will include the required written documentation, records and Results and relevant materials in the quantities, form and purity as per the Scope of Project Works and satisfactory Acceptance Testing of the Deliverables.
|
|||
|
(e)
|
Property in the physical quantity of Compound or any API of it (or any other materials or samples) produced by ▇▇. ▇▇▇▇▇’▇ under any Sub-Project (Goods) will pass to Prana on delivery of the Compound to Prana’s carrier or payment for the Sub-Project in full, whichever is earlier. At Prana’s request (in case of delay in acceptance of Goods from freight forwarder), ▇▇. ▇▇▇▇▇’▇ will store the Goods at its cost and at the risk of Prana for up to 90 days at appropriate conditions. In the interim, ▇▇. ▇▇▇▇▇’▇ will liaise with Prana or its nominee in relation to the transport of the Goods to Prana and will obtain at its own risk and expense any export licence or other official authorisation and carry out where applicable all customs formalities necessary for the export of the Goods. On the agreed date, ▇▇. ▇▇▇▇▇’▇ will deliver the Goods to Prana’s carrier at ▇▇. ▇▇▇▇▇’▇ premises at which time risk in the Goods will pass to Prana. The payment amounts mentioned herein do not include freight, insurance and other shipping expenses for transportation of Goods from ▇▇. ▇▇▇▇▇’▇ premises to Australian port of entry or any other international port (Ex-works shipment) and Prana shall bear all such expenses, and shall reimburse ▇▇. ▇▇▇▇▇’▇ in full in case ▇▇. ▇▇▇▇▇’▇ is called upon to incur any such expenses. If requested by Prana, ▇▇. ▇▇▇▇▇’▇ will help Prana to identify an appropriate freight forwarder.
|
|||
|
DRL IRN – 100004118
|
|
 |
9
|
(f)
|
All payments by Prana to ▇▇. ▇▇▇▇▇’▇ pursuant to this Agreement shall be made without any withholding or deduction of any withholding tax or other tax or mandatory payment to governmental agencies. If Prana is legally required to make any such withholding or deduction from any payment to ▇▇. ▇▇▇▇▇’▇ under this Agreement, the sum payable by Prana upon which such withholding or deduction is based shall be increased to the extent necessary to ensure that, after such withholding or deduction, ▇▇. ▇▇▇▇▇’▇ receives and retains, free from liability for such withholding or deduction, a net amount equal to the amount ▇▇. ▇▇▇▇▇’▇ would have received and retained in the absence of such required withholding or deduction.
|
|||
|
4.
|
Intellectual Property
|
|||
|
(a)
|
▇▇. ▇▇▇▇▇’▇ acknowledges and agrees that the Prana Background IP will at all times remain the exclusive property of Prana or its relevant Affiliate. Similarly, Prana acknowledges and agrees that the ▇▇. ▇▇▇▇▇’▇ Background IP will at all times remain the exclusive property of ▇▇. ▇▇▇▇▇’▇ or its relevant Affiliate.
|
|||
|
(b)
|
The Parties acknowledge and agree that all Prana Arising IP is hereby assigned to and will vest in and be solely owned by Prana as and from the time of its creation.
|
|||
|
(c)
|
During the term of this Agreement Prana hereby grants ▇▇. ▇▇▇▇▇’▇ a royalty free, non exclusive, non-transferable, revocable licence for the term of this Agreement to use Prana Background IP and Prana Arising IP solely for the Approved Purposes. Similarly, during the term of this Agreement ▇▇. ▇▇▇▇▇’▇ hereby grants Prana a royalty free, non-exclusive, non-transferable licence to use ▇▇. ▇▇▇▇▇’▇ Background IP solely for the Approved Purposes.
|
|||
|
(d)
|
▇▇. ▇▇▇▇▇’▇ will provide all assistance and advice and execute all necessary documents as may be required by Prana from time to time, in relation to:
|
|||
|
(i)
|
any assignment that may be required to transfer Prana Arising IP to Prana;
|
|||
|
(ii)
|
any applications by Prana for Patents or other registrable intellectual property rights in respect of the Prana Arising IP; and
|
|||
|
(iii)
|
any applications, submissions or other documents that Prana seeks to file with a regulatory authority or other government department, agency or body to obtain an approval or consent in relation to the testing, manufacture or sale of the Compound or an API of it,
|
|||
| and Prana shall pay all reasonable costs and expenses incurred by ▇▇. ▇▇▇▇▇’▇ in providing such assistance. | ||||
|
5.
|
Term and Termination
|
|||
|
(a)
|
This Agreement shall commence on the Commencement Date and shall, subject always to earlier termination under this Clause 5, continue until ninety (90) days after delivery by ▇▇. ▇▇▇▇▇’▇ to Prana of the final written report for the last Sub-Project approved by Prana.
|
|||
|
DRL IRN – 100004118
|
|
 |
10
|
(b)
|
Notwithstanding any other provision of this Agreement, either Party shall have the right at any time by giving notice to the other to terminate this Agreement (or a Sub-Project in the case of the events described in paragraphs (i), (ii) or (iv)) forthwith in any of the following events:
|
|||
|
(i)
|
if the other Party commits a material breach of this Agreement and the breach is not capable of remedy;
|
|||
|
(ii)
|
if the other, Party commits a material breach of this Agreement and, where such breach is capable of remedy, that Party does not remedy such breach within 30 days from service of notice upon it that it is in breach and requiring it to remedy such breach; or
|
|||
|
(iii)
|
if the other Party enters into liquidation, whether compulsory or voluntary (other than for the purposes of solvent reconstruction or amalgamation where the resulting Party assumes all such Party’s obligations under this Agreement), or has a receiver, controller or administrator or similar official appointed over some or all it assets or compounds with its creditors or suffers any similar action in consequence of its indebtedness to creditors; or
|
|||
|
(iv)
|
if either Party is delayed or incapable of performing its obligations under this Agreement as a result of a matter described in Clause 13 (Force Majeure) for continuous period of 90 days or more.
|
|||
|
(c)
|
Notwithstanding any other provision of this Agreement, Prana may terminate this Agreement (in whole or in part) at any time by giving the ▇▇. ▇▇▇▇▇’▇ 30 days written notice.
|
|||
|
(d)
|
The obligations of the Parties under clauses 1, 2 (g) and (l) and (m), 3, 4, 5, 7, 8, 9 (b), 11,15, 16 and 17 will survive the expiry or termination of this Agreement. The obligations of the Parties under clause 6 will survive the expiry or termination of this Agreement for seven (7) years.
|
|||
|
(e)
|
On the expiry or termination of this Agreement or on the termination of a Sub-Project and subject to payment of consideration due under this Agreement:
|
|||
|
(i)
|
▇▇. ▇▇▇▇▇’▇ must provide Prana with all outstanding updates and reports as existing at the date of such expiry/termination under clause 2 (e) for any completed or partly completed Sub-Project;
|
|||
|
(ii)
|
▇▇. ▇▇▇▇▇’▇ must deliver to Prana all materials produced by it as part of any completed or partly completed Sub-Project in the quantities, form and purity that complies with Prana’s requirements as set forth in the Scope of Project Works (Appendix B);
|
|||
|
(iii)
|
Prana must pay all sums which have accrued or been invoiced by ▇▇. ▇▇▇▇▇’▇ up to the expiry or termination date and are payable in accordance with the terms of this Agreement. If a Sub-Project is only partly completed on the expiry or termination date, then provided this Agreement or the Sub-Project has not been terminated by Prana pursuant to clauses 2 (m) (i) or (ii) or 5 (b)), ▇▇. ▇▇▇▇▇’▇ will be entitled to a proportion of its fee for that Sub-Project and non-cancellable pass-through expenses necessary to wind down such Sub-Project. Proportion of the fee shall be calculated on the basis of the percentage of the Sub-Project completed by ▇▇. ▇▇▇▇▇’▇. If this amount is less than the total of the advance and other progress payments already paid by Prana for the Sub-Project, then ▇▇. ▇▇▇▇▇’▇ must refund the difference within 30 days of its fee being agreed with Prana.
|
|||
|
DRL IRN – 100004118
|
|
 |
11
|
(iv)
|
▇▇. ▇▇▇▇▇’▇ must return the Prana Materials and all Material Forms of the Prana Confidential Information to Prana. In the case of Prana Materials, the Parties acknowledge and agree that ▇▇. ▇▇▇▇▇’▇ may retain samples of the Prana Materials manufactured by it so that it may comply with its cGMP obligations;
|
|||
|
(v)
|
Prana must return all Material Forms of the ▇▇. ▇▇▇▇▇’▇ Confidential Information to ▇▇. ▇▇▇▇▇’▇.
|
|||
|
(f)
|
No expiry or termination of this Agreement shall affect any of the rights and obligations of the Parties accrued up to the date of expiry or termination.
|
|||
|
6.
|
Confidentiality
|
|||
|
(a)
|
Each Party acknowledges and agrees that the Confidential Information of the other Party will at all times remain the exclusive property of that other Party. Each Party also undertakes to keep the Confidential Information of the other secret and to protect and preserve the confidential nature and secrecy of that Confidential Information.
|
|||
|
(b)
|
Prana agrees and acknowledges in relation to ▇▇. ▇▇▇▇▇’▇ Confidential Information, and ▇▇. ▇▇▇▇▇’▇ agrees and acknowledges in relation to Prana Confidential Information, that it:
|
|||
|
(i)
|
may only use or reproduce the other Party’s Confidential Information for the Approved Purposes;
|
|||
|
(ii)
|
must not disclose the other Party’s Confidential Information to any person except as permitted by this Agreement;
|
|||
|
(iii)
|
must not permit unauthorised persons to have access to the other Party’s Confidential Information;
|
|||
|
(iv)
|
must not make, or assist or permit any person (including its Representatives) to make any unauthorised use, disclosure or reproduction of the other Party’s Confidential Information;
|
|||
|
DRL IRN – 100004118
|
|
 |
12
|
(v)
|
must ensure that any person who has access to the other Party’s Confidential Information does not make any unauthorised use, reproduction or disclosure of that information;
|
|||
|
(vi)
|
must enforce the confidentiality obligations imposed or required to be imposed by this Agreement, including diligently prosecuting at its cost any breach or threatened breach of those confidentiality obligations by a person to whom that Party has disclosed the other Party’s Confidential Information and, where appropriate, making applications for interim or interlocutory relief; and
|
|||
|
(vii)
|
must provide assistance reasonably requested by the other Party, in relation to any proceedings the other Party may take against any person for unauthorised use, copying or disclosure of the other Party’s Confidential Information.
|
|||
|
(c)
|
A Party may disclose the other Party’s Confidential Information to a Representative on a need to know basis but in each case, only to the extent necessary for the Approved Purposes, and provided the Representatives are placed under confidentiality obligations no less onerous than those set out in this Agreement.
|
|||
|
(d)
|
Each Party must procure that its Representatives (whether or not still employed or engaged in that capacity) do not do or omit to do anything which, if done or omitted to be done by that Party, would breach its obligations under this Agreement.
|
|||
|
(e)
|
The obligations of confidentiality and non-disclosure contained in this clause 6 do not apply if and to the extent that the Confidential Information is required to be supplied by virtue of any statute, law or regulation. Each Party must notify the other immediately if it becomes aware of any legal requirement to disclose part or all of the other Party’s Confidential Information.
|
|||
|
(f)
|
Each Party must:
|
|||
|
(i)
|
establish and maintain effective security measures to safeguard the other Party’s Confidential Information from access or use not authorised under this Agreement;
|
|||
|
(ii)
|
keep the other Party’s Confidential Information under its own control;
|
|||
|
(iii)
|
maintain complete, accurate and up-to-date records of use, copying and disclosure of the other Party’s Confidential Information and immediately produce these records to the other Party, on request; and
|
|||
|
DRL IRN – 100004118
|
|
 |
13
|
(iv)
|
immediately notify the other Party of any suspected or actual unauthorised use, copying or disclosure of the other Party’s Confidential Information.
|
|||
|
(g)
|
Either Party may at any time by notice in writing to the other Party request the return of all Material Forms of its Confidential Information in the possession, power or control of the other Party or any of its Representatives (whether or not those Material Forms were created by the other Party or its Representatives) and the other Party must immediately comply with such request. In the case of Prana Materials to be returned by ▇▇. ▇▇▇▇▇’▇, the parties acknowledge and agree that ▇▇. ▇▇▇▇▇’▇ may retain samples of the Prana Materials manufactured by it so that it may comply with its GMP obligations.
|
|||
|
(h)
|
Return of the Material Forms of Confidential Information under clause 6(g) does not release a Party from its obligations under this clause 6.
|
|||
|
7.
|
Liability
|
|||
|
(a)
|
Prana will defend, indemnify and hold harmless ▇▇. ▇▇▇▇▇’▇ and its Representatives and Affiliates from and against any and all liability losses, costs, damages or expenses (including court costs and reasonable attorneys fees) incurred from or arising in connection with any claim (including claims for infringing third party intellectual property rights) arising out of or are in any way relating to:
|
|||
|
(i)
|
Prana’s use of the Prana Arising IP, the Results, Compound, APIs or any materials produced during a Sub-Project and supplied to Prana, provided ▇▇. ▇▇▇▇▇’▇ has complied with this Agreement in relation to its performance of the Sub-Project and the Sub-Project Deliverables have been accepted by Prana in writing;
|
|||
|
(ii)
|
personal injuries or death to persons or property loss or damage which occur on ▇▇. ▇▇▇▇▇’▇ premises or the premises of ▇▇. ▇▇▇▇▇’▇ Affiliates as a result of the conduct of the Project to the extent that they are directly attributable to circumstances that could have been avoided by ▇▇. ▇▇▇▇▇’▇ if it had been aware of relevant information about the Compound that was knowingly or negligently withheld from ▇▇. ▇▇▇▇▇’▇ by Prana; or
|
|||
|
(iii)
|
the breach of clauses 4 or 6 by Prana or its Affiliates or Representatives.
|
|||
|
(b)
|
Prana will defend, indemnify and hold harmless ▇▇. ▇▇▇▇▇’▇ and its Representatives and Affiliates from and against any and all liability losses, costs, damages or expenses (including court costs and reasonable attorneys fees) incurred from or arising in connection with any claim for infringing third party intellectual property rights arising out of or are in any way relating to ▇▇. ▇▇▇▇▇’▇ use, for Approved Purposes, of the Prana Materials, Compound APIs, Prana Background IP or Prana Arising IP.
|
|||
|
DRL IRN – 100004118
|
|
 |
14
|
(c)
|
▇▇. ▇▇▇▇▇’▇ will indemnify and hold harmless Prana, its Representatives and Affiliates from and against all costs, expenses, liabilities, losses, damages, claims and proceedings suffered or incurred by them (including proceedings for infringing third party intellectual property rights) which have arisen out of or are in any way relating to:
|
|||
|
(i)
|
personal injuries or death to persons or property loss or damage which occur on ▇▇. ▇▇▇▇▇’▇’ premises or the premises of ▇▇. ▇▇▇▇▇’▇’ Affiliates as a result of or in connection with any act or omission, negligence or breach of this Agreement by ▇▇. ▇▇▇▇▇’▇ or its Affiliates or any of their respective Representatives;
|
|||
|
(ii)
|
any use (other than for the Approved Purposes) by ▇▇. ▇▇▇▇▇’▇ or its Affiliates (or by third parties under licence from or other arrangement with ▇▇. ▇▇▇▇▇’▇ or its Affiliates) of the Prana Materials, Prana Background IP, the Prana Arising IP, the Results or Compound APIs;
|
|||
|
(iii)
|
the use of ▇▇. ▇▇▇▇▇’▇ Background IP by Prana, its Representatives and Affiliates for the Approved Purposes; or
|
|||
|
(iv)
|
the breach of clauses 4 or 6 by ▇▇. ▇▇▇▇▇’▇ or its Affiliates or Representatives.
|
|||
|
(d)
|
Notwithstanding any other provision of this Agreement, neither Party will have any liability to the other Party (or any Affiliate of it) for any consequential or indirect loss or damage (including loss of profits) (“Consequential Loss”) suffered or incurred by the other Party (or any Affiliate of it), howsoever arising This paragraph (e) will not prevent a Party recovering from the other, Consequential Loss suffered or incurred by it (or an Affiliate of it) under paragraphs(a) and (b) and(c) above.
|
|||
|
(e)
|
In no event will the aggregate liability of either Party for any claims made by the other Party under or in connection with this Agreement exceed the Project Price (except in relation to their respective liabilities for any claims made under clauses 7 (a), (b) and (c) which will not be subject to this limitation).
|
|||
|
8.
|
Warranties
|
|||
|
(a)
|
Each Party warrants to the other that it is duly organised, validly existing and in good standing in accordance with the applicable laws and has all necessary power and authority to enter into this Agreement and to carry out its obligations under this Agreement and to consummate the transactions contemplated hereby and that it is duly licensed or qualified to do business in its principle place of business.
|
|||
|
(b)
|
Each Party warrants to the other that the execution and delivery by it of this Agreement, the performance by it of its obligations hereunder and the consummation of the transactions contemplated by this Agreement have been duly authorised by all requisite action on the part of the Party, and no other corporate proceedings by it or any of its Affiliates are required in connection therewith.
|
|||
|
(c)
|
Each Party represents that there is no litigation pending or threatened (judicial, regulatory or otherwise) or other operational issues within its business that would or might prevent or adversely interfere with the performance of its obligations under this Agreement.
|
|||
|
DRL IRN – 100004118
|
|
 |
15
|
(d)
|
During the term of this Agreement and before commencing the development and manufacture of the Compound, each Party warrants that it will (at its own cost) obtain, maintain and secure all permits, registrations and licences (including but not limited to those in respect of manufacturing and regulatory) required under applicable laws to allow it to perform its obligations under this Agreement. If requested by a Party, the other Party shall submit copies of any such documents for its inspection and records. Further, the Parties hereby agree that they will promptly notify the other of any notices and non- compliances issues that have been noticed, issued or reported by any regulatory or statutory authorities and ensure that they are complied with immediately without any delay or continuing default.
|
|||
|
(e)
|
Each Party warrants that it has the requisite skills, resources, technology and all the rights and licences necessary for rendering services and fulfilling its contractual obligations under this Agreement.
|
|||
|
(f)
|
Prana warrants that the Prana Background IP and other information provided by it for use by ▇▇. ▇▇▇▇▇’▇ in the manufacture and development of the Compound pursuant to this Agreement will not violate or infringe upon the intellectual property rights of any third person. ▇▇. ▇▇▇▇▇’▇ warrants that the use of ▇▇. ▇▇▇▇▇’▇ Background IP or other ▇▇. ▇▇▇▇▇’▇ Confidential Information by either Party for the Approved Purposes will not violate or infringe upon the intellectual property rights of any third person.
|
|||
|
(g)
|
EXCEPT AS AGREED UNDER THIS AGREEMENT ▇▇. ▇▇▇▇▇’▇ MAKES NO WARRANTIES, WHETHER EXPRESS, IMPLIED OR STATUTORY REGARDING OR RELATING TO THE PERFORMANCE OF THE OBLIGATIONS UNDER THIS AGREEMENT. TO THE EXTENT PERMITTED BY LAW, ▇▇. ▇▇▇▇▇’▇ SPECIFICALLY DISCLAIMS ALL IMPLIED WARRANTIES OR MERCHANTABILITY FOR A PARTICULAR PURPOSE OF RENDERING SERVICES FOR THE PROJECT UNDER THIS AGREEMENT.
|
|||
|
9.
|
Hazardous Information
|
|||
|
Prana will make all information (if any) which it has available to it concerning the health and other hazards of the Compound and its synthesisand any other materials including reference standards provided by Prana to ▇▇. ▇▇▇▇▇’▇ for the purpose of the Project. ▇▇. ▇▇▇▇▇’▇ must assess these hazards and take the necessary measures in relation to the Project to:
|
||||
|
(a)
|
ensure the safety of its Representatives; and
|
|||
|
(b)
|
avoid any loss or damage to its premises or property.
|
|||
|
DRL IRN – 100004118
|
|
 |
16
|
10.
|
Assignment and Subcontracting
|
|||
|
(a)
|
Neither Party shall assign this Agreement or any of its rights and obligations under it to any third party without first obtaining the prior written consent from the other.(b) ▇▇. ▇▇▇▇▇’▇ must not subcontract any of its obligations under this Agreement without the prior written consent of Prana.
|
|||
|
India
|
||||
|
▇▇▇▇ ▇▇. ▇▇▇, ▇.▇.▇▇-▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇, Jeedimetla, Hyderabad, Andhra Pradesh, India-500 055)
|
||||
|
(c)
|
If Prana, in its absolute discretion, consents to the subcontracting of the performance of any of the Project Works, then:
|
|||
|
(i)
|
▇▇. ▇▇▇▇▇’▇ shall remain fully responsible for the performance of the Project Works and must continue to comply with each and every one of its obligations under this Agreement;
|
|||
|
(ii)
|
without limitation, all acts or omissions of the subcontractor shall be deemed acts or omissions of ▇▇. ▇▇▇▇▇’▇; and
|
|||
|
(iii)
|
▇▇. ▇▇▇▇▇’▇ must ensure that any subcontractor so engaged complies with, and enters into a written agreement with ▇▇. ▇▇▇▇▇’▇ under the terms of which:
|
|||
|
a.
|
the subcontractor agrees to comply with all relevant provisions of this Agreement (including in respect of performance of the relevant subcontracted Project Works, compliance with cGMP and all applicable laws, record keeping, confidentiality and Intellectual Property) as if it were a party to this Agreement;
|
|||
|
b.
|
the subcontractor is prohibited itself from subcontracting any part of the performance of the subcontract.
|
|||
|
11.
|
Notices
|
|||
|
(a)
|
A notice, consent, approval or other communication (each a Notice) under this agreement must be signed by or on behalf of the Party giving it, addressed to the Party to whom it is to be given and:
|
|||
|
(i)
|
delivered to that Party’s address;
|
|||
|
(ii)
|
sent by pre-paid mail to that Party’s address; or
|
|||
|
(iii)
|
transmitted by facsimile to that Party’s address.
|
|||
|
DRL IRN – 100004118
|
|
 |
17
|
(b)
|
A Notice given to a Party in accordance with this clause 10 is treated as having been given and received:
|
||
|
(i)
|
if delivered to a Party’s address, on the day of delivery if a Business Day, otherwise on the next Business Day;
|
||
|
(ii)
|
if sent by pre-paid mail, on the tenth Business Day after posting; or
|
||
|
(iii)
|
if transmitted by facsimile to a Party’s address and a correct and complete transmission report is received, on the day of transmission if a Business Day, otherwise on the next Business Day.
|
||
|
(c)
|
For the purpose of this clause the address of a Party is the address set out below or another address of which that Party may from time to time give notice to the other Party:
|
||
|
If to Prana:
|
▇▇▇▇▇▇ ▇▇▇▇▇
|
|
|
Chief Operating Officer
|
||
|
▇▇▇▇▇ ▇, ▇▇▇ ▇▇▇▇▇ ▇▇▇▇▇▇, ▇▇▇▇▇▇▇▇▇ ▇▇▇▇▇▇▇▇ ▇▇▇▇
|
||
|
Facsimile: ▇▇▇ ▇ ▇▇▇▇ ▇▇▇▇
|
||
|
If to ▇▇. ▇▇▇▇▇’▇:
|
▇▇. ▇▇▇▇▇▇ ▇▇▇
|
|
|
Vice President – Global CPS head
|
||
|
Custom Pharmaceutical Services,
|
||
|
▇▇. ▇▇▇▇▇’▇ Laboratories Limited
|
||
|
▇▇▇▇▇▇▇▇ ▇▇▇▇, ▇▇▇▇▇▇▇,
|
||
|
▇▇▇▇▇▇▇▇▇, ▇▇▇▇▇▇ ▇▇▇▇▇▇▇, ▇▇▇▇▇- ▇▇▇▇▇▇
|
||
|
Facsimile: ▇▇▇ ▇▇ ▇▇▇▇ ▇▇▇▇
|
|
12.
|
Entire Agreement
|
|
The Confidentiality Agreement between the Parties dated 19th August 2013, the LOI, the Quality Agreement (as amended pursuant to clause 2 (i)) and this Agreement set forth the entire agreement between the Parties as to its subject matter. In the event of any incompatibility between the terms of this Agreement and the said Confidentiality Agreement LOI and Quality Agreement, the terms of this Agreement shall prevail and take priority. None of the terms of this Agreement shall be amended except in writing signed by both Parties.
|
DRL IRN – 100004118

18
|
13.
|
Force Majeure
|
|
A Party shall not be liable for a failure to perform any of its obligations under this Agreement (other than a payment obligation) due to any cause or circumstance which is beyond its reasonable control, including without limitation, acts of God, wars, riots, interference by military or para-military, strikes, lock-outs or other labour unrest, fires, explosions, shipwrecks, shortage in material if the supplier(s) of such material is unable to supply due to causes and circumstances beyond their control as exemplified above (“Force Majeure Event”) provided always that such failure or delay could not have been prevented by reasonable precautions. In the case of Force Majeure, the obligations of the Party affected shall be suspended and it shall not be liable for damages or for penalties for non-performance to the extent that such non-performance is caused by the Force Majeure event with the proviso that if the Force Majeure period should extend more than three (3) months then the other Party shall have the right to terminate this Agreement forthwith upon written notice at any time after expiration of said three (3) months period. In addition, non-performance shall only be excused during the continuation of the Force Majeure event.
|
|
14.
|
Independent Contractors
|
| The parties are independent contractors and this Agreement shall not be construed as creating or evidencing a partnership, agency, employment or joint venture relationship between them. |
|
15.
|
Dispute Resolution
|
||
|
(a)
|
If a dispute arises in connection with this Agreement or relating to this Agreement including its interpretation and any question regarding its existence, validity or termination, then a Party wishing to have the dispute resolved must give the other Party a notice specifying the dispute and requiring its resolution under this clause 15 (“Dispute Notice”).
|
||
|
(b)
|
Within 14 days of the date of service of the Dispute Notice, each Party must:
|
||
|
(i)
|
appoint a Representative with authority to negotiate and settle the dispute; and
|
||
|
(ii)
|
notify the other Party in writing of the appointed Representative’s name and contact details.
|
||
|
(c)
|
The authorised Representatives and the Parties that they represent must then use their reasonable endeavours to resolve the dispute within 42 days of the date of service of the Dispute Notice. If they fail to resolve the dispute within this period, then either Party may institute court proceedings.
|
||
|
(d)
|
A Party may not commence court or any other proceedings in relation to a dispute arising in connection with this Agreement until it has exhausted the procedures in this clause, unless the Party seeks injunctive or other interlocutory relief to preserve property or rights or to avoid losses that are not compensable in damages.
|
||
DRL IRN – 100004118

19
|
16.
|
Governing Law and Jurisdiction
|
||
|
(a)
|
This Agreement shall be construed, governed, interpreted, and applied in accordance with the laws of England and the courts located at London shall have the exclusive jurisdiction to entertain and resolve all the disputes between the Parties.
|
||
|
(b)
|
Each Party irrevocably and unconditionally:
|
||
|
(i)
|
submits to the jurisdiction of the courts of England; and
|
||
|
(ii)
|
waives any claim or objection based on absence of jurisdiction or inconvenient forum.
|
||
|
(c)
|
The rights and obligations of the parties under this Agreement shall not be governed by the United Nations Convention on Contracts for the International Sale of Goods (1980).
|
||
|
17.
|
Miscellaneous
|
||
|
(a)
|
No forbearance or tolerance on the part of the either Party of any breach of this Agreement by the other shall constitute waiver of the requirements of this Agreement. A right may only be waived in writing, signed by the Party giving the waiver.
|
||
|
(b)
|
The parties hereby agree that any provision/s of this agreement which are held to be invalid and unenforceable in law shall not by itself make this Agreement invalid nor effect the other provisions of this agreement and the other terms shall remain fully enforceable and valid in law.
|
||
|
(c)
|
Each Party agrees to do all things and execute all agreements, instruments, transfers or other documents as may be necessary or desirable to give effect to the provisions of this Agreement and the transactions contemplated by it.
|
||
|
(d)
|
This Agreement may be executed in any number of counterparts. All counterparts together will be taken to constitute one instrument.
|
||
DRL IRN – 100004118

20
Executed by the Parties by their duly authorised representatives
|
By
|
|||
|
(Name): ▇▇▇▇▇▇ ▇▇▇▇▇
|
|||
|
(Title): Chief Operating Officer
|
|||
|
(Date):
|
|||
▇▇. ▇▇▇▇▇’▇ Laboratories Limited
|
By
|
 |
||
|
(Name): ▇▇▇▇▇▇ ▇▇▇
|
|||
|
(Title): Vice President – Head of Global CPS business
|
|||
|
(Date):
|
28th March 2014
|
||
DRL IRN – 100004118

21
Appendix A
PROJECT WORKS AND TIMETABLE
|
Project item
|
Responsible
Party |
Milestone
Payment USD |
Timeline
|
||
|
1- Sourcing of Raw material (for process development of PBT 434).
|
▇▇. ▇▇▇▇▇’▇
|
Completion date 15th November 2013
|
|||
|
2- Submission of Manufacturing License
|
▇▇. ▇▇▇▇▇’▇**
|
||||
|
3- Process development of current and proposed route will take place in parallel.
|
▇▇. ▇▇▇▇▇’▇
|
Commencement date
- November 18th 2013 “four weeks after start date of procurement of raw materials”.
|
|||
|
(a) Process development work of current route.
|
USD 40,000*
|
Completion date December 13th 2013.
|
|||
|
(b) Process development work of proposed route
|
USD 36,000*
|
Completion date December 13th 2013.
|
DRL IRN – 100004118

22
|
4- Completion of both Process optimization of selected route and analytical method validation (selected route).
|
▇▇. ▇▇▇▇▇’▇
|
USD 24,000*
|
Commencement date - 16th December 2013.
Completion date - 19th January 2013
|
|||
|
5- Receipt of Manufacturing License
|
▇▇. ▇▇▇▇▇’▇
|
Within 4 weeks of commencing 4kg manufacturing campaign being no later than the 3rd February 2014.
|
||||
|
6- 4 Kg Campaign
a) Commencement of Manufacturing Campaign Commence 4kg PBT434 HBr Manufacture in plant under cGMP (as per agreed specifications)
b) Includes the contracting by ▇▇ ▇▇▇▇▇’▇ of AR Life Sciences to perform Step 4 (only) of a 7 step synthesis under cGMP conditions. Step 4 is a ▇▇▇▇▇ Nickel hydrogenation.
The optimised process for Step 4 has been developed by ▇▇. ▇▇▇▇▇’▇. AR Life Sciences will prepare Master Batch records for approval by ▇▇. ▇▇▇▇▇’▇ and Prana. Post mutual approval, ▇▇. ▇▇▇▇▇’▇ will oversee the batch execution at AR Life Sciences. The intermediate, CDCH4, will get tested and by AR Life Sciences in accordance with ▇▇. ▇▇▇▇▇’▇ method of analysis
|
▇▇. ▇▇▇▇▇’▇
|
USD 160,000*
Completion of 4 kg manufacturing of PBT 434 under agreed specifications and acceptance of COA.
Bonus payment of USD 20,000 if manufacturing of 4 kg PBT 434 is completed on or before the completion date.
|
Commencement date – 20th Jan 2014
Completion date – 31th March 2014
Timeline – Within 10 weeks from commence date.
Analysis and Dispatch – in the week of 1st April 2014
|
DRL IRN – 100004118

23
|
c) Completion of 4 kg Manufacturing Campaign of PBT 434 under cGMP (as per agreed specifications), acceptance of Certificate of Analysis and executed batch records by Prana. Analysis and Dispatch completed.
d) Manufacturing Report of PBT 434
|
USD 20,000
Submission of manufacturing report and executed batch records.
|
Milestone payment upon completion
|
||||
|
e) Stability Study (36 months as per ICH guidelines
|
Payment of USD $9000 towards initial pull point of 1st month. Remaining payments as incremental payments (USD 1000) per timepoint over 36 months
|
*Milestone payment on completion.
*Completion means completion of the laboratory work and finalization of the relevant Project Item in Development Report.
DRL IRN – 100004118

▇▇
▇▇▇▇▇▇▇▇ ▇
|
Sub Project 1: Sourcing of Raw Material (for process development of PBT434)
|
Raw materials for the project will be sourced and procured by ▇▇. ▇▇▇▇▇’▇
Deliverables:.
3-Chloro-2-nitrobenzoic acid with ▇▇. ▇▇▇▇▇’▇ to begin process development work.
|
Sub Project 2: Submission of Manufacturing License
|
Prana to send Purchase Orders to ▇▇. ▇▇▇▇▇’▇ for Manufacturing Licence for PBT434. ▇▇. ▇▇▇▇▇’▇ to apply for Manufacturing License
Deliverables:
Manufacturing Licence for PBT434
|
Sub Project 3: Process Development of current and proposed route
|
a) Process Development of current route
Deliverables
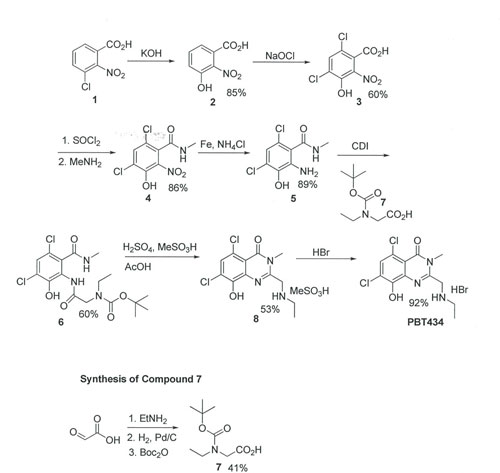
To repeat the synthesis of the current synthetic route from AMRI global as outlined in Scheme 1. Reaction of 3-chloro-2-nitrobenzoic acid with KOH to generate phenol 2 followed by reaction with sodium hypochlorite to provide 4,6-Dichloro-2-hydroxy-3-nitrobenzoic acid 3. Acid 3 is converted to the acid chloride followed by reaction with methylamine to generate amide 4. Reduction of amide 4 with Fe and ammonium chloride to afford Anthranilic acid derivative 5.
The coupling of intermediate 5 to N-Boc-N-Ethyl glycine 7 to provide the bis-amide 6. Cyclisation of bis-amide 6 to quinazolinone derivative 8 using methanesulfonic acid in acetic acid, in the presence of catalytic conc. H2SO4. Finally, conversion of quinazolinone derivative 8 to PBT434 with HBr. Yields will be comparable to those outlined in Scheme 1.
DRL IRN – 100004118

25
Scheme 1: Current Synthetic Route to PBT434
b) Process Development of Alternate Route
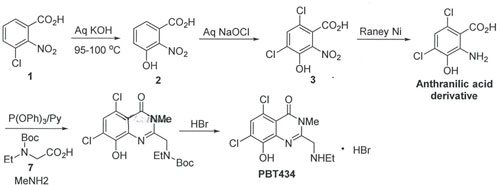
Alternate route to PBT434:
Alternatively, PBT434 synthesis can be evaluated based on a five step process, as enclosed below. This approach is based on known methods using a three component and one-pot reaction starting from anthranilic acid, aniline and
N-Boc glycine in the presence of coupling reagent, triphenyl phosphite. (see examples in Bioorg. Med. Chem. 2008, 16, 2570-2578 & Bioorg. Med. Chem. Lett. 2007, 17, 3339-3343).
DRL IRN – 100004118

26
Scheme 2: Alternative Route to PBT434
Based on this, to adopt this approach for PBT434 synthesis, anthranilic acid derivative could be synthesized starting from 3-chloro-2-nitrobenzoic acid 1 in a three step process. Further, anthranilic acid derivative on one-pot reaction with side chain 7 in the presence of triphenyl phosphite in pyridine can generate the intermediate benzoxazinone derivative, which on reaction with methyl amine (reaction requires anhydrous conditions, methyl amine in toluene may require for the synthesis and in-house preparation would need to be considered) can generate the required quinazolinone derivative. Finally, reaction with HBr can undergo Boc deprotection and generate PBT434 as a HBr salt.
This approach would be the novel route for synthesis of quinazolinone derivatives and require additional efforts in establishing the proposed one-pot chemistry and to familiarize the process.
Alternate route to N-Boc-N-ethylglycine (compound 7):
As per technical information, N-Boc-N-ethylglycine synthesis involved condensation of glyoxalic acid with ethyl amine and then reduction using Pd on carbon under hydrogenation conditions at 50 psi H2 pressure, and finally isolate product after Boc protection. Alternatively, compound 7 can also be synthesized from the methyl ester of glycine by reductive amination using acetaldehyde and sodium cyanoborohydride, followed by N-Boc protection and subsequent ester hydrolysis using LiOH (Bioorg. Med. Chem. Lett. 2009, 19, 2211-2214).
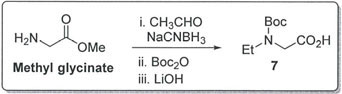
This approach is advantageous in avoiding the usage of palladium on carbon under hydrogenation conditions; however ester hydrolysis would be the additional stage in the process when compared with the current synthetic route
DRL IRN – 100004118

27
Another set of conditions to avoid hydrogenation is the use of chloroacetic acid and aqueous ethylamine solution to generate N-Ethylglycine in situ, followed by reaction with BOC anhydride to afford compound
7.
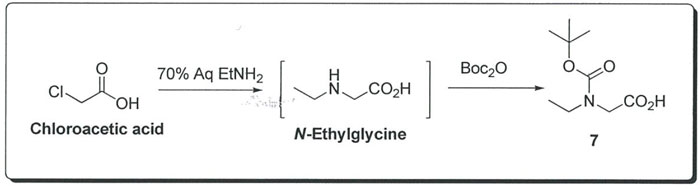
Deliverables for Sub-Project 3a) and 3b):
A comprehensive, detailed Familiarisation Report to be written up by ▇▇. ▇▇▇▇▇’▇ on the current and alternate routes to PBT434 and submitted to Prana for review and approval. Without limitation, the report will contain detailed synthetic methods and operations to manufacture PBT434 via the existing route. The report will contain reaction yields and HPLC purities for each step. The purity profiles will be tabulated with relative retention times and their percentages clearly shown. The report will also contain work to manufacture PBT434 via the alternative route detailed in Scheme 2, shown above.
|
Sub-Project 4: Completion of both Process optimisation of selected route and analytical method validation (selected route)
|
|
a)
|
Method development for specification tests:
|
|
|
i)
|
HPLC or GC method for starting material, intermediates and in-process controls will be in placed as per requirement. Retentions of reagents used; as well possible by-products will be recorded for the process development
|
|
|
ii)
|
HPLC method provided in the section 5.1 of document number HRC0000808 would be used to determine the purity in final compound. The method would be evaluated for the proposed scheme and would be modified/developed as per the requirements of process.
|
|
|
iii)
|
Method will be validated for parameters like LOD, LOQ, Linearity, repeatability, ruggedness, solution stability, mobile phase stability, accuracy and specificity with forced degradation (using LC-DAD) in compliance with current ICH guidelines and in compliance with EMA and FDA requirements for Phase I development.
|
|
|
iv)
|
Residual solvents in final compound will be monitored by GC and/or GCHS techniques. Method will be developed to analyse the samples for all process solvents in compliance with current ICH limits and in compliance with EMA and FDA requirements for Phase I development
|
|
|
v)
|
Method validation will be performed with minimum parameters like LOD, LOQ, Linearity, repeatability, ruggedness, recovery, solution stability, specificity of all solvents in intended methods (in compliance with current ICH limits and in compliance with EMA and FDA requirements for Phase I development)
|
DRL IRN – 100004118

28
|
vi)
|
Suitable method will be developed for quantification of Anion and Cation used in the process. Method would be verified for minimum parameters e.g. LOD, LOQ, Linearity, repeatability, ruggedness, recovery, solution stability (as applicable to the process).
|
|
|
vii)
|
An assay method for API will be developed and validated with minimum parameters including linearity, precision, and accuracy (in compliance with ICH, EMA and FDA requirements for Phase I development.
|
|
|
viii)
|
Reference markers for intermediates will be qualified for appropriate tests.
|
|
|
ix)
|
A reference standard of final API material will be qualified as per internal SOPs.
|
|
|
Specification testing
|
||
|
The limits/range can be mutually agreed between two parties based on process capabilities and phase requirements. The key raw material, isolated intermediates and API material will be analysed for several tests as below.
|
|
S. No.
|
Tests
|
Specification
|
MOA ref. No.
|
||||
|
1.
|
Description
|
White to light ▇▇▇▇▇ color powder
|
AR&D-GTP-001
|
||||
|
2.
|
Identification by
|
||||||
|
a) FT-IR
|
Shall matches to the standard
|
AR&D-GTP-003
|
|||||
|
|
|||||||
| The major peak in the sample | |||||||
|
b) HPLC
|
chromatogram should match
|
MF/CDCH7-002/00
|
|||||
|
with that of standard in assay
|
|||||||
|
preparation
|
|||||||
|
3.
|
Water content by KF (%w/w)
|
Not more than 2.0
|
AR&D-GTP-005
|
||||
|
4.
|
Residue on ignition (%w/w)
|
Not more than 0.30
|
AR&D-GTP-010
|
||||
|
5.
|
Heavy metals (%)
|
Not more than 0.002
|
AR&D-GTP-011
|
||||
|
6.
|
Assay by HPLC (on anhydrous and solvent free basis) (%w/w)
|
Between 97.0 and 103.0
|
MF/CDCH7-001/00
|
||||
|
7.
|
Related substances by HPLC (%area)
|
||||||
|
a) Any maximum impurity
|
|||||||
|
b) Total impurities
|
Not more than 0.25
|
MF/CDCH7-002/00
|
|||||
|
Not more than 1.5
|
|||||||
DRL IRN – 100004118

29
|
8.
|
Residual solvents by GC-HS (in ppm)
|
||||||
|
i) Ethyl acetate
|
|||||||
|
ii) Toluene
|
Not more than 5000
|
||||||
|
iii) Methanol
|
Not more than 890
|
MF/CDCH7-003/00
|
|||||
|
iv) Hexanes
|
Not more than 3000
|
||||||
|
v) Tetrahydrofuran
|
Not more than 290
|
||||||
|
|
Not more than 720
|
||||||
|
* Solvents are considered from stage 4 onwards
|
||
|
Deliverables for Sub-project 4 (a)
|
||
|
●
|
Develop and document analytical methods for key starting materials and intermediates for the manufacture of PBT434. Included are HPLC, Loss on Drying, GC and Infrared Spectroscopy.
|
|
|
●
|
Set specifications and document for each intermediate according to the developed analytical methods that have to be agreed and approved by Prana.
|
|
|
●
|
▇▇. ▇▇▇▇▇’▇ to write a comprehensive validation report for the assay methods and submit to Prana for review and approval.
|
|
|
●
|
▇▇. ▇▇▇▇▇’▇ to develop and document a Related Substance Method for PBT434
|
|
|
●
|
▇▇. ▇▇▇▇▇’▇ to develop and document a GC Methods to determine solvent levels for stage 4 intermediates onwards.
|
|
|
●
|
▇▇. ▇▇▇▇▇’▇ to develop and document analytical methods to determine the final purity of PBT434
|
|
|
●
|
Reference standard of PBT434 20 gm to be qualified according to ▇▇. ▇▇▇▇▇’▇ SOP’s and the standard is to be reviewed and approved by Prana in writing. Reference standard of PBT 434 will be taken from Lab assurance batches.
|
|
|
b)
|
Completion of both Process optimisation of selected route
|
Stage-1:
As per the given process shown in ▇▇▇▇▇▇ ▇, ▇▇▇▇▇-▇ involves 3-Chloro-2-nitrobenzoic acid 1 on treatment with aq KOH (14 eq) to generate the corresponding phenolic derivative 2 at reflux temperature. After completion of reaction, extract product at pH 2 with ethyl acetate and then product isolation by adding hexane.
Process optimization plan:
|
●
|
The requirement of excess usage of KOH (14 eq) and lot wise addition will be evaluated.
|
|
|
●
|
Product isolation at pH 2 directly from aqueous mixture will be studied, which can avoid ethyl acetate extraction and product isolation from ethyl acetate-hexane mixture.
|
|
|
●
|
Exploring the possibility of direct subjection of stage-1 wet cake to chlorination with aq NaOCl (in-situ process).
|
DRL IRN – 100004118

30
Stage-2:
Stage-2 involves aromatic chlorination using aqueous sodium hypochlorite solution at 80°C. After completion of reaction, extract product at pH 2 with ethyl acetate followed by concentration of organic layer and then finally product isolation by triturating with hexane.
Process optimization plan:
| ● | As per the technical information, stage-1 purity is more than 99%, which is the input for chlorination. After Chlorination, stage-2 product purity is ~90%. |
| ● | To improve the purity, purification of stage-2 will be explored. |
| ● | Product isolation at pH 2 directly from aqueous mixture will be evaluated, which can avoid ethyl acetate extraction, concentration and product isolation by triturating with hexane. |
Stage-3:
Stage-3 involves amide formation by converting acid to acid chloride using thionyl chloride followed by reaction with methyl amine. After completion of reaction, extract product at pH 2 with ethyl acetate followed by concentration of organic layer and then finally product isolation by triturating with hexane.
Process optimization plan:
| ● | Studying acid chloride formation in the presence of a solvent, i.e. in toluene media |
| ● | Complete removal of thionyl chloride from the reaction mixture after acid chloride formation is necessary for amide formation. |
| ● | Acid chloride synthesis in toluene will be advantageous in complete removal of SOCl2 from reaction mixture by co-distillation. |
| ● | As per scale up information from Prana, one of the batches is reported for low product purity (79% against the trend of ~94%) and yields varied from 86-99%. |
| ● | Since aq methyl amine is used in the process, re-generation of acid during the amide formation may be possible. Though, aromatic acid chlorides are stable to some extent, reaction conditions need to be established for robustness. |
Stage-4:
Process involves synthesis of key intermediate 9 by reduction of nitro functionality using iron powder in the presence of ammonium chloride.
Process optimization plan:
| ● | Based on our experience in reduction of aromatic nitro group, ▇▇▇▇▇ Ni would be the preferred reagent. |
| ● | Alternate reaction conditions will be explored using ▇▇▇▇▇ Ni under hydrogenation conditions and compare impurity profile and yield. |
Stage-5:
Process involves compound 9 on reaction with side chain 7 (generated by condensing glyoxalic acid and ethyl amine followed by reduction under hydrogenation conditions using Pd/C and finally Boc protection) in the presence of CDI and triethyl amine at room temperature to generate compound 10. After completion of reaction, extract product with ethyl acetate and concentrate. Finally, isolate product from ethyl acetate.
DRL IRN – 100004118

31
Process optimization plan:
| ● | Establish reaction conditions and product isolation process for compound 10 as per the given process. |
Stage-6:
Stage-6 is the quinazolinone ring formation from compound 10 using acetic acid in the presence of H2SO4 at 120°C and finally isolate compound 11 as methane sulfonic acid salt.
Process optimization plan:
| ● | We will try to replace Ethanol with isopropanol in the process. |
| ● | As per tech information, yields from the scale up batches varied from 36% to 52% with approximate product purity ~97%. |
| ● | Product purification trials will be explored to improve the purity. |
Stage-7:
PBT434 is synthesized by treating compound 11 with aq HBr at room temperature.
Process optimization plan:
| ● | We will try to replace Ethanol with isopropanol in the process. |
| ● | Specification for PBT434 is purity NLT 99% with single maximum impurity NMT 0.15%. |
| ● | One of batch from tech pack shows impurity at a level of 0.37% with product purity 99.49% |
| ● | Product purification trials will be explored to improve the purity. |
Synthesis of N-Boc-N-ethylglycine (compound 7):
Process involves condensation of glyoxalic acid with ethyl amine at 40 °C followed by reduction under hydrogenation conditions using Pd/C at 50 psi hydrogen pressure. After completion of reaction, filter the catalyst and then finally subject for Boc protection. After completion of reaction wash reaction mixture with MTBE and adjust aqueous layer pH to 2-3 with citric acid. Extract aqueous layer using ethyl acetate and concentrate under reduced pressure to generate compound 7.
Process optimization plan:
Establish reaction conditions for compound 7 as per the given process.
Deliverables for Sub-Project 4b
Dr. Reddys will write a comprehensive Process Development Report on the Selected Route. Without limitation, the report will contain reaction schemes to manufacture PBT434. There will be a detailed experimental write-up with HPLC, IR, proton and carbon NMR for each intermediate. Submit to Prana for review and written approval.
DRL IRN – 100004118

32
|
Sub-Project 5: Receipt of Manufacturing Licence
|
|
Deliverables for Sub-Project 5
|
||
|
▇▇. ▇▇▇▇▇’▇ to receive a manufacturing licence for PBT434 no later than February 3, 2014
|
|
Sub-Project 6: GMP Manufacture of 4kg PBT434 HBr
|
|
a)
|
Commence 4kg PBT434 HBr Manufacture in plant under cGMP by the 20th of January, 2014
|
|
|
(i) ▇▇. ▇▇▇▇▇’▇ will manufacture 4kg of PBT434 drug substance according to the applicable regulatory and statutory requirements of current Good Manufacturing Practices (GMP) (US 21 CFR parts 210 and 211 and ICH Q7) in the following scenario:
|
||
|
(ii) ▇▇ ▇▇▇▇▇’▇ will strictly adhere to all the decisions agreed and during Process Development (Sub-project 3) with regards to the type and material of construction of the equipment to be used in the manufacture, the quality and suppliers of reagents and solvents, the type of reactors to be used, the timing of use of the Cleanroom. Any changes in equipment, master batch records, specifications, methods or any other aspect of the manufacture from the previous 2010 manufacture that are not explicitly justified in the Familiarisation report (sub-project 3) will need written approval by Prana.
|
||
|
Deliverables for Sub-project 6a):
|
||
| ● |
Technology transfer for Stage 1 to be completed by ▇▇. ▇▇▇▇▇’▇ by the 20th of January, 2014
|
|
| ● |
Master batch records for Stage 1 to be approved and signed off in writing by Prana
|
|
| ● |
Reactors assigned to Stage 1 to be washed and ready for use in PBT2 manufacture.
|
|
| ● |
All personnel assigned to PBT434 manufacture to be identified in writing and available.
|
|
| ● |
3-Chloro-2-nitrobenzoic acid and all reagents and solvents to be used in Stage 1 to be received and released for use
|
|
| ● |
Stage 1 to be initiated in the plant by no later than January 20, 2014
|
|
|
b)
|
Completion of 4kg Manufacturing Campaign of PBT434 HBr under cGMP (as per agreed specifications), acceptance of Certificate of Analysis and executed batch records by Prana.
|
|
|
i) The manufacture will proceed as per the agreed master batch records. Prana will be informed of all deviations, however minor, and those will be dealt with as per the Quality Agreement
|
||
|
ii) All results will be communicated to Prana immediately. Any result that raises questions will be communicated to Prana ahead of any internal investigation so Prana can be take part in resolving issues.
|
||
|
iii) The existing analytical methods as well as the refined analytical methods as per Sub Project 3, will be used to release intermediates and final materials. All intermediates and final material will meet the specifications agreed in writing with Prana prior to manufacture
|
DRL IRN – 100004118

33
|
Deliverables for Sub-project 6c):
|
||
| ● |
Technology transfer for Stage 2 onwards to be completed by ▇▇. ▇▇▇▇▇’▇
|
|
| ● |
Master batch records for Stage 2 onwards to be approved and signed off in writing by Prana
|
|
| ● |
Release data for all intermediates and final material to be received and approved in writing by Prana
|
|
| ● |
Certificate of Analysis, Certificate of GMP compliance and other release documents to be received by Prana and approved in writing.
|
|
| ● |
Master Batch records for all stages to be provided to Prana, reviewed and any amendments and comments promptly attended to by ▇▇ ▇▇▇▇▇’▇.
|
|
| ● |
All information relevant to the manufacture and required for regulatory purposes(including FDA IND) to be provided to Prana, including but not limited to, the description of the manufacturing process, batch traceability, Certificates of Analysis of all reagents and solvents. In addition, ▇▇ ▇▇▇▇▇’▇ should record all suggestions for improvement in future campaigns or areas in need of further investigation. Such information should be provided in a Manufacturing Campaign Report.
|
|
|
c)
|
Strategic Business Partner- Step 4 ▇▇▇▇▇ Nickel Hydrogenation
|
|
|
Includes the use of AR Life Sciences to perform Stage 4 only of a 7 stage synthesis under cGMP conditions. Stage 4 is a ▇▇▇▇▇ Nickel hydrogenation.
|
||
|
The optimised process for Stage 4 has been developed by ▇▇. ▇▇▇▇▇’▇. AR Life Sciences will prepare Master Batch records for approval by ▇▇. ▇▇▇▇▇’▇ and Prana. Post mutual approval, ▇▇. ▇▇▇▇▇’▇ will oversee the batch execution at AR Life Sciences. Finally, the intermediate, CDCH4, will get tested and by AR Life Sciences in accordance with ▇▇. ▇▇▇▇▇’▇ method of analysis.
|
||
|
Deliverables
|
||
| ● |
AR Life Sciences to write batch records for Stage 4 that require approval from both ▇▇. ▇▇▇▇▇’▇ and Prana.
|
|
| ● |
AR Life Sciences to write methods of analyses and in-process controls for Step 4 that require approval from both ▇▇. ▇▇▇▇▇’▇ and Prana.
|
|
| ● |
Stage 4 Intermediate released by ▇▇. ▇▇▇▇▇’▇ according to methods of analyses from AR Life Sciences.
|
|
|
d)
|
Stability Study (36 Months as per ICH guideline)
|
|
|
A stability study, conducted according to the according to the ICH guideline “Q1A(R2) Stability Testing of New Drug Substances and Products”, will commence immediately after manufacture of 4 Kg GMP PBT434 API. The existing analytical methods as well as the refined analytical methods as per Sub Project 4a, will be used for the stability studies (6 month accelerated study and 36 months long term study). The accelerated study will have following data points - 0, 1, 2, 3, and 6 months. The long term study will have the following data points - 0, 1, 3, 6, 9, 12, 18, 24 and 36 months.
|
DRL IRN – 100004118

34
|
Deliverables for each timepoint:
|
||
| ● |
▇▇. ▇▇▇▇▇’▇ to write a QA-checked stability report to be received by Prana within 2 weeks of pulling the samples.
|
|
|
Raw data from each timepoint to be submitted to Prana.
|
DRL IRN – 100004118

35

