such Affiliate to Third Parties on the resale of such Product. A “sale” shall also include a transfer or other disposition for consideration other than cash, in which case such consideration shall be valued at the fair market value thereof. 1.4 Patent...
|
|
EXHIBIT 10.45 ROYALTY AGREEMENT This Agreement (this "Agreement") is made as of December 18, 2006, by and between Intersect Partners, LLC, a California limited liability (“Intersect"), and Abdominis, Inc., a Delaware corporation (“Abdominis”), (hereinafter referred to collectively as the “Parties” and individually as a "Party"). RECITALS A. Intersect, pursuant to that certain Intellectual Property Assignment Agreement, by and between Intersect and Abdominis dated September 16, 2005 (the “Assignment”), has previously assigned its interests in the Products and the Patent Rights to Abdominis. B. The Parties now wish to specify in certain detail the consideration that is to be paid to Intersect by Abdominis for the Assignment. NOW THEREFORE, in consideration of the mutual obligations set forth in this Agreement, Intersect and Abdominis hereby agree as follows: ARTICLE I DEFINITIONS 1.1 Affiliate. "Affiliate" shall mean any entity which controls, is controlled by or is under common control with another entity. 1.2 Net Proceeds. "Net Proceeds" shall mean the total proceeds received from the sale of either the Patent Rights, or the sale of Products by Abdominis, to a Third Party, less all reasonable costs incurred by Abdominis in completing such sale, including but not limited to, attorneys' fees, accountants’ fees, appraisers' fees and any commissions. 1.3 Net Sales. “Net Sales” shall mean the total amount invoiced to Third Parties by Abdominis or its Affiliates in connection with the sale, lease or use of a Product, Jess, to the extent actually incurred: (a) allowances and adjustments credited or payable, including credit for damaged, outdated and returned products; (b) trade, cost or quantity discounts earned or granted; (c) transportation charges (including insurance costs), sales taxes, excise taxes and duties, and other similar charges; (d) wholesaler chargebacks; and (e) taxes on sale, transportation or use paid by Abdominis. Net Sales shall be calculated in accordance with Abdominis’ standard internal policies and procedures. Net Sales shall not include sales by Abdominis to its Affiliates for resale, provided that if Abdominis sells Product to an Affiliate for resale, Net Sales shall include the amounts invoiced by |
|
|
such Affiliate to Third Parties on the resale of such Product. A “sale” shall also include a transfer or other disposition for consideration other than cash, in which case such consideration shall be valued at the fair market value thereof. 1.4 Patent Rights. "Patent Rights" shall mean any of the patent applications listed in Exhibit A attached to this Agreement; any continuing applications thereof including divisions; but excluding continuations-in-part except to the extent of claims entitled to the priority date of the parent case; any patents issuing on these applications including reissues and reexaminations; and any corresponding foreign patents or patent applications; all of which will be automatically incorporated in and added to Exhibit A and made a party of this Agreement 1.5 Product. "Product" shall mean any article, composition, apparatus, substance, or any other material whose manufacture, use or sale would constitute an infringement of any claim within Patent Rights, or any service, article, composition, apparatus, substance, or any other material made, used, or sold by or utilizing or practicing a method which would infringe a claim within the Patent Rights. 1.6 Third Party. ''Third Party” shall mean any individual, corporation, partnership, trust or other business organization or entity, and any other recognized organization other than the parties hereto and their Affiliates. ARTICLE II DISCLAIMER OF OWNERSHIP Disclaimer of Ownership. Intersect irrevocably disclaims any ownership or other interest in or to the Products and the Patent Rights and acknowledges that its sole rights with respect to the “Products and the Patent” Rights is the right to receive the payments set forth in Article III below. ARTICLE III CONSIDERATION TO BE RECEIVED BY INTERSECT 3.1 Royalties. On all sales of Products by Abdominis in territories where the Patent Rights exist, Abdominis shall pay Intersect a royalty of two and one-half percent (2 1/2%) of Net Sales. If any Product is manufactured and sold to a Third Party under license from Abdominis, Abdominis shall pay Intersect two and one-half percent (2 1/2%) of Net Sales of Products by such licensee, but not more than twenty-five percent (25%) of the amount actually received by Abdominis from such licensee. If any Product is sold in a series of transactions covered by more than one license or sublicense hereunder, only one royalty shall be payable, based upon the Net Sales for the first transaction in such series of transactions. 3.2 Reports and Payment. Abdominis shall, and shall cause its Affiliates and sublicensees, to keep complete and accurate records of Net Sales of all sales of Products with respect to which royalties are payable pursuant to this Agreement for a period of three (3) years following the year in which the sales were made. Forty-five (45) days following the close of each fiscal quarter, Abdominis shall submit to Intersect a written report setting forth its sales of Products, the Net Sales of such Products and the calculation of the amount of royalties due and payable to Intersect for the fiscal quarter just ended. Each report shall be accompanied by payment of the amount of royalty shown by the report to be due in accordance with the provisions of Article III hereof; provided, however, Abdominis shall accrue such payment of royalties until such time as one |
|
|
of the patent applications set forth on Exhibit A has had a patent issued by the U.S. Patent and Trademark Office containing a claim covering a Product at which time such accrued royalties shall be paid to Intersect. In the event Abdominis has not collected on an account after 180 days following delivery of an invoice therefor, Abdominis shall include in its next sales report to Intersect a statement of such failure to collect from such account and shall reduce the amount of royalties otherwise payable under such report by (i) the amount of royalties, if any, paid on account of such customer sates which have yet to be paid by such customer and (ii) on account of any sales to such customer since the most recent sales report. Abdominis shall pay all royalties due on such customer’s account once such customer has paid Abdominis. 3.3 Diligence. Abdominis shall have control over the development, manufacture and sale of the Product and Intersect shall have no claim against Abdominis for any failure of Abdominis to achieve any Net Sales or Net Proceeds. 3.4 Sale of the Rights to the Patents. If at any time during the term of this Agreement Abdominis sells its rights in one or more of the Patents to a Third Party, Abdominis, shall have the Third Party assume Abdominis's obligations hereunder, and such Third Party shall continue paying royalties to Intersect in accordance with Section 3.1 of this Agreement 3.5 Sale of Abdominis. If at any time during the term of this Agreement Abdominis shall be acquired by a Third Party (whether by purchase of stock, purchase of substantially all assets, merger or consolidation), Abdominis shall have the surviving entity assume Abdominis’s obligations hereunder, and such entity shall continue to pay royalties to Intersect in accordance with Section 3.1 of this Agreement. 3.6 Taxes. Any and all taxes levied by a proper taxing authority and paid by Abdominis, or its Affiliates or sublicensees, on account of royalties accruing to Intersect under this Agreement, remittable from a country in which provision is made in the law or by regulation for withholding of taxes, will be deducted from royalties paid· by Abdominis, provided that proof of payment is secured and promptly sent to Intersect as evidence of such payment. 3.7 Governmental Royalty Limitations. If the royalty rate specified herein should exceed the permissible rate established in any country which requires government approval for royalty remittance, the royalty rate for sales in such country shall be adjusted to the highest legally permissible or governmentally approved rate. 3.8 Payable in United States Funds. All royalty payments shall be made in United States funds. When Products are sold for currency other than United States dollars, the royalties will first be determined in the foreign currency of the country in which those Products were sold and converted into equivalent United States funds. Abdominis shall use the exchange rate set out in the Wall Street Journal or similar publication on the last day of the calendar quarter. |
|
|
ARTICLE IV RECORDS AND REPORTS 4.1 Records. Abdominis shall keep complete and accurate records with respect to which the royalties are determined pursuant to this Agreement for a period of three (3) years following the year in which such payments were made. 4.2 Accountants Review. Intersect shall have the right to nominate an independent accountant reasonably acceptable to Abdominis, to have access to the records of Abdominis during reasonable business hours, not more than one time per year for the purpose of verifying, at Intersect’s expense (unless Abdominis’ payment accountings are more than five percent (5%) at variance with Abdominis' records then Abdominis shall pay), the payments due to Intersect. Abdominis may request that such accountant hold all information received in confidence except as provided in the following sentence, including requiring the execution of a Confidentiality Agreement. Such accountant shall disclose to Intersect only information relating to the accuracy of the payment reports and payments made according to this Agreement. ARTICLE V DURATION AND TERMINATION 5.1 Duration of Agreement. Unless otherwise terminated by operation of law or by acts of the Parties in accordance with the terms of this Agreement, this Agreement is in force from the date first mentioned above and remains in effect for the life of the last-to-expire patent in the Patent Rights. ARTICLE VI CONFIDENTIALITY 6.1 Confidential Information. Confidential information shall consist of any information designated as confidential and relating to the Patent Rights and the Products, including the Reports and the payments made hereunder. Intersect shall not use any such confidential information other than to exercise its rights hereunder, or disclose confidential information to any third party without the prior written consent of Abdominis. 6.2 Exceptions. The restrictions set forth in Section 6.1 shall not apply to confidential information that (i) becomes generally known to the public subsequent to the date hereof through no wrongful act or omission of Intersect, (ii) is disclosed by Intersect pursuant to the order or requirement of a court, administrative agency or governmental body, provided, however, that Intersect shall provide prompt notice thereof to Abdominis to enable Abdominis to seek a protective order or otherwise prevent such disclosure, or (iii) has been approved for release in writing by Abdominis. 6.3 Remedies. Any breach of the restrictions contained in this Article VI is a breach of this Agreement which may cause irreparable harm to Abdominis entitling Abdominis to injunctive relief in addition to all legal remedies. |
|
|
ARTICLE VII MISCELLANEOUS 7.1 Entire Agreement. This Agreement sets forth the entire agreement and understanding of the Parties relating to the subject matter contained herein and merges all prior discussions between them. No claimed oral agreement in respect hereto shall be considered as any part thereof. No modification or claimed waiver of any of the provision hereof shall be valid unless in writing and signed by authorized representatives of the Party against whom such modification or waiver is sought to be enforced. 7.2 Severability. Should any part or provision of this Agreement be held unenforceable or in conflict with the law of any jurisdiction, the validity of the remaining parts or provisions shall not be affected by such holding. 7.3 Assignability. Intersect shall not assign this Agreement or its rights hereunder without the prior written consent of Abdominis. Abdominis shall have the rights to assign this Agreement subject to the terms of Section 3.4 and 3.5 herein. 7.4 Governing Law. This Agreement shall be interpreted and construed and the legal relations created herein shall be determined in accordance with the laws of the State of California, without reference to choice of laws principles, but the scope and validity of any patent or patent application will be governed by the applicable laws of the country of the patent or patent application. 7.5 General Assurances. The Parties agree to execute, acknowledge, and deliver all such further instruments, and do all such other acts, as may be necessary or appropriate from time to time in order to carry out the intent and purpose of this Agreement. 7.6 Disputes. If Intersect objects to Abdominis’ determination relating to payments, intersect shall cause to be delivered to Abdominis, within thirty (30) days of Abdominis' determination, written notice of such objection and its intent to submit the matter to an independent accountant as otherwise provided in Section 4.2 herein. Intersect shall select an independent accountant with at least 10 years experience within thirty (30) days of receipt of delivery of the quarterly statements provided in Section 3.2 herein by Abdominis. The accountant shall undertake its review of all such records within sixty (60) days of commencement of such accountant's review. The accountant shall deliver its decision to Intersect and Abdominis. The decision rendered by the accountant as to the determination in question shall be binding on the Parties. Intersect shall bear all costs associated with the accounting, unless the accountant determines that Abdominis underpaid the payment in question by more than five percent (5%), in which case Abdominis shall bear the expenses of the accountant. 7.7 Arbitration. Any controversy, dispute or claim arising out of or in connection with or relating to this Agreement (other than payment determinations covered in Section 7.6 above) will be submitted by the Parties to arbitration by the American Arbitration Association in Orange County, California in accordance with the commercial rules then in effect for that Association before a single arbitrator. The award rendered by the arbitrator shall include costs of arbitration, reasonable attorneys' fees and reasonable costs for expert and other witnesses, and judgment upon such award may be entered in any court having jurisdiction thereof. |
|
|
7.8 Notices and Statements. Any notice, statement, or report permitted or required to be given under the provision of this Agreement shall be in writing signed by the Party giving such notice, statement or report and shall be sent to the appropriate address given below: If to Intersect: With a copy to: If to Abdominis: Intersect Partners 00000 Xxxxxx Xxxxxxx Xxxxx, Xxxx 000 Xxxx Xxxxxx, Xxxxxxxxxx 00000 Attention: Manager Xxxxxxxxx Xxxxx Xxxxxxx & Xxxxx. 000 Xxxxxxx Xxxxxx Xxxxx Xxxxxxx Xxxxx, Xxxxxxxxxx 00000. Attention: Xxxxx Xxxxxxxx Fax: (000) 000-0000 Abdominis, Inc. 00000 Xxxxxx Xxxxxxx Xxxxx, Xxxx 000 Xxxx Xxxxxx, Xxxxxxxxxx 00000 Attention: Chief Executive Officer Any of the above addresses can be changed upon ten (10) days written notice to the other parties. Any such notice, statement or report that is dispatched by prepaid registered or certified mail shall be deemed to have been duly given upon mailing thereof. · 7.9 Waiver of Default. No waiver by a party of any provision of this Agreement shall be deemed a waiver of any other provision hereof or of any subsequent breach by a party of the same or any other provision. A Party’s consent to. or approval of, any act shall not be deemed to render unnecessary the obtaining of a Party’s consent to, or approval of, any subsequent act by the other Party. No remedy or election as provided for in this Agreement shall be deemed exclusive but shall, wherever possible, be cumulative with all other remedies at law or in equity. 7.10 Binding Effect. This Agreement shall be binding on and inure to the benefit of the Parties hereto and their respective heirs, successors and permitted the assignees. 7.11 Counterparts. This Agreement may be executed in several counterparts and such counterparts together shall constitute but one and the same instrument. 7.12 Headings. The headings of the several sections are inserted for convenience of reference only and are not intended to be a part of, or to affect the meaning or interpretation of, this Agreement. 7.13 No Agency. lntersect's and Abdominis’ activities hereunder shall be conducted as independent contractors and no agency relationship shall exist between the Parties. 7.14 Modification. This Agreement shall not be modified except by a writing signed on behalf of each of the parties hereto. [Signature Page Follows] 6 |
|
|
IN WITNESS WHEREOF, the parties have executed this Royalty Agreement as of the day and year first above written. INTERSECT PARTNERS, LLC By: Xxxxxx Xxxxxxx, Manager ABDOMINIS, INC. By: Xxxxx Xxxxx, Director |
|
|
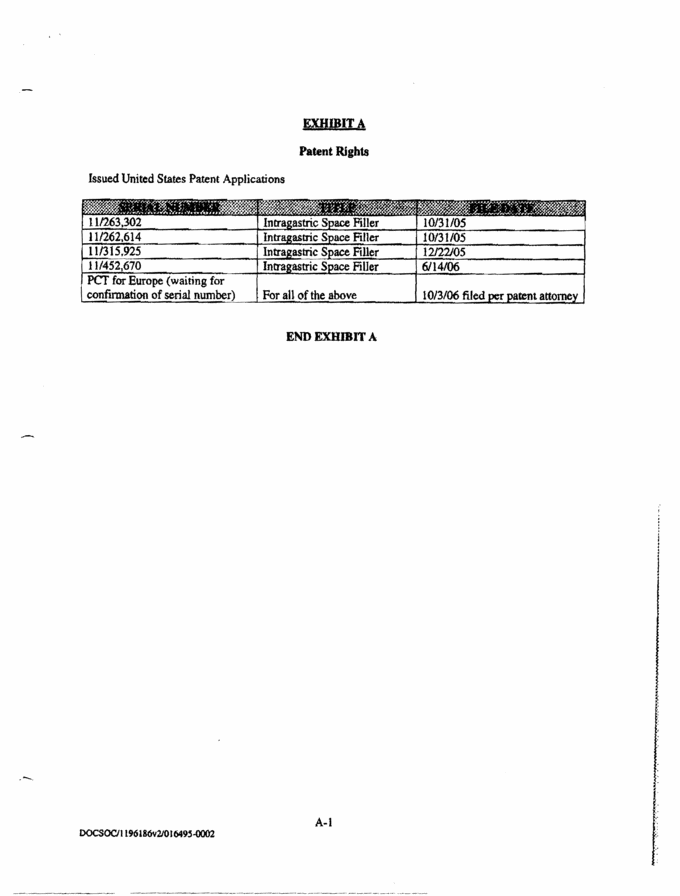
EXHIBIT A Patent Rights Issued United States Patent Applications SPRIAL NUMBER TITLE FILE DATE 11/263,302 Intragastric Space Filler 10/31/05 11/262,614 Intragastric Space Filler 10/31/05 11/315,925 Intragastric Space Filler 12/22/05 11/452,670 Intragastric Space Filler 6/14/06 PCT for Europe (waiting for confirmation of serial number) For all the above 10/3/06 filed per patent attorney END EXHIBIT A A-1 |
|
|
AMENDMENT NO. 1 to ROYALTY AGREEMENT This Amendment No. 1, dated as of April 16, 2013 (the “Effective Date”), is made and entered into by and between ReShape Medical, Inc. (formerly known as Abdominis, Inc.), a Delaware corporation (“ReShape”) and Intersect Partners, LLC, a California limited liability company (“Intersect”). RECITALS A. ReShape and Intersect (individually, a “Party”; collectively, the “Parties”) previously entered into a Royalty Agreement, effective as of December 18, 2006 (the “Original Royalty Agreement”). B. The Parties desire to amend the Original Royalty Agreement as set forth in this Amendment No. 1. Capitalized term(s) used but not defined in this Amendment No. 1 have the meaning assigned to such term(s) in the Original Royalty Agreement. AGREEMENT The Parties therefore agree as follows: 1. Patent Rights. Section 1.4 of the Original Royalty Agreement is hereby amended to read in its entirety as follows: "1.4 Patent Rights. “Patent Right” shall mean any United States and foreign patents and applications listed in Amended Exhibit A, including continuations, continuations-in-part, divisions, re-examinations, patents by addition, patent term extension, renewals, reissues, and extensions thereof.” 2. Reports and Payments. Section 3.2 of the Original Royalty Agreement is hereby amended to read in its entirety as follows: "3.2 Reports and Payments. Abdominis shall, and shall cause its Affiliates and sublicensees, to keep complete and accurate records of Net Sales of all sales of Products with respect to which royalties are payable pursuant to this Agreement for a period of three (3) years following the year in which the sales were made. Forty five (45) days following the close of each fiscal quarter, Abdominis shall submit to Intersect a written report setting forth its sales of Products, the Net Sales of such Products and the calculation of the amount of royalties due and payable to Intersect for the fiscal quarter just ended. Each report shall be accompanied by payment of the amount of royalty shown by the report to be due in accordance with the provisions of Article III hereof; provided, however Abdominis shall accrue such payment of royalties until such time as one of the U.S. or foreign patent applications set forth in Amended Exhibit A or Patents Rights as defined herein in Paragraph 1.4 is issued by the respective Patent Office and contains a claim covering a Product, at which time such accrued royalties shall be paid to Intersect.” |
|
|
3. Exhibit A. Exhibit A of the Original Royalty Agreement is hereby amended and replaced in its entirety with the Amended Exhibit A attached to this Amendment No. 1 4. Effective Dates. The amendments set forth in Paragraphs 1-3 of this Amendment No. 1 shall be effective as of December 18, 2006, the effective date of the Original Royalty Agreement. All other amendments set forth in this Amendment No. 1 will be effective as of the Effective Date set forth above. 5. Full Force and Effect. The Original Royalty Agreement, as amended by this Amendment No. 1, remains in full force and effect. This Amendment No. 1 sets forth the entire agreement, and supersedes any prior agreements, arrangements, or understanding relating to the subject matter hereof. In the event of any inconsistency between any provision of this Amendment No. 1 and any provision of the Original Royalty Agreement, the provision of this Amendment No. 1 shall govern. 6. Acknowledgement. For the avoidance of doubt, the Parties acknowledge that the only Parties to the Original Royalty Agreement are ReShape (formerly known as Abdominis, Inc.) and Intersect. 7. Counterparts. This Amendment No. 1 may be executed in counterparts, each of which shall be deemed to be an original, and all of which taken together shall constitute one and the same instrument. IN WITNESS WHEREOF, each of the Parties has caused this Amendment No. 1 to be made and executed by its duly authorized officer as of the Effective Date. ReShape Medical, Inc. Intersect Partners, LLC By: By: Xxxxxxx Xxxxxxxx Xxxxxx Xxxxxxx Chief Executive Officer Manager |
|
|
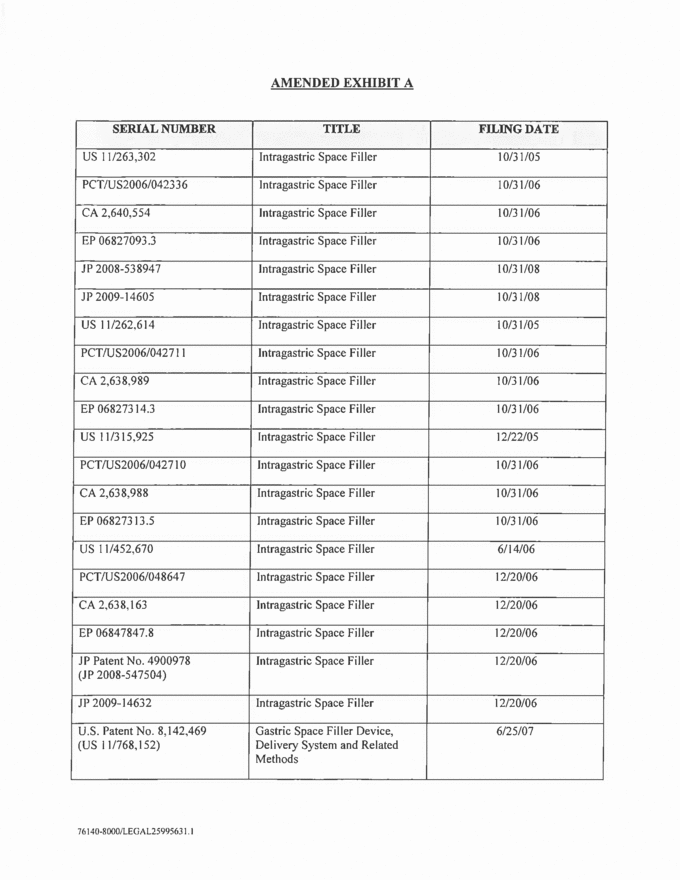
AMENDED EXHIBIT A SERIAL NUMBER TITLE FILING DATE US 11/263,302 Intragastric Space Filler 10/31/05 PCT/US2006/042336 Intragastric Space Filler 10/31/06 CA 2,640,554 Intragastric Space Filler 10/31/06 EP 06827093.3 Intragastric Space Filler 10/31/06 JP 2008-538947 Intragastric Space Filler 10/31/08 JP 2009-14605 Intragastric Space Filler 10/31/08 US 11/262,614 Intragastric Space Filler 10/31/05 PCT/US2006/042711 Intragastric Space Filler 10/31/06 CA 2,638,988 Intragastric Space Filler 10/31/06 EP 06827314.3 Intragastric Space Filler 10/31/06 US 11/315,925 Intragastric Space Filler 12/22/05 PCT/US2006/042710 Intragastric Space Filler 10/31/06 CA 2,638,989 Intragastric Space Filler 10/31/06 EP 06827314.3 Intragastric Space Filler 10/31/06 US 11/315,925 Intragastric Space Filler 6/14/06 PCT/US2006/042710 Intragastric Space Filler 12/20/06 CA 2,638,163 Intragastric Space Filler 12/20/06 EP 06847847.8 Intragastric Space Filler 12/20/06 JP Patent No. 4900978 (JP 2008-547504) Intragastric Space Filler 12/20/06 JP 2009-14632 Intragastric Space Filler 12/20/06 U.S. Patent No. 8,142,469 (US 11/768,152) Gastric Space Filler Device, Delivery System and Related Methods 6/25/07 |
|
|
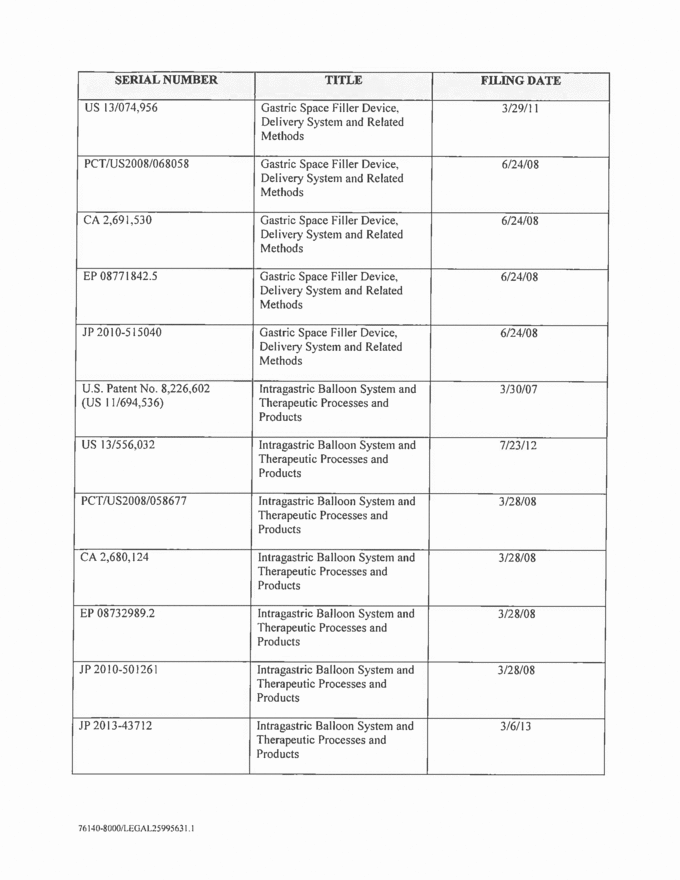
SERIAL NUMBER TITLE FILING DATE US 13/074,956 Gastric Space Filler Device, Delivery System and Related Methods 3/29/11 PCT/US2008/068058 Gastric Space Filler Device, Delivery System and Related Methods 6/24/08 CA 2,691,530 Gastric Space Filler Device, Delivery System and Related Methods 6/24/08 EP 08771842.5 Gastric Space Filler Device, Delivery System and Related Methods 6/24/08 JP 2010-515040 Gastric Space Filler Device, Delivery System and Related Methods 6/24/08 U.S. Patent No. 8,226,602 (US 11/694,536) Intragastric Balloon System and Therapeutic Processes and Products 3/30/07 US 13/556,032 Intragastric Balloon System and Therapeutic Processes and Products 7/23/12 PCT/US2008/058677 Intragastric Balloon System and Therapeutic Processes and Products 3/28/08 CA 2,680,124 Intragastric Balloon System and Therapeutic Processes and Products 3/28/08 EP 08732989.2 Intragastric Balloon System and Therapeutic Processes and Products 3/28/08 JP 2010-501261 Intragastric Balloon System and Therapeutic Processes and Products 3/28/08 JP 2013-43712 Intragastric Balloon System and Therapeutic Processes and Products 3/6/13 |