AMENDMENT NO. 1 to the RESEARCH FUNDING AGREEMENT by and between Affimed Therapeutics AG and The Leukemia & Lymphoma Society
Exhibit 10.7
EXECUTION COPY
CONFIDENTIAL TREATMENT REQUESTED UNDER RULE 406 UNDER THE SECURITIES ACT OF 1933, AS AMENDED.
[*****] INDICATES OMITTED MATERIAL THAT IS THE SUBJECT OF A CONFIDENTIAL TREATMENT REQUEST FILED SEPARATELY WITH THE COMMISSION. THE OMITTED MATERIAL HAS BEEN FILED SEPARATELY WITH THE COMMISSION.
AMENDMENT NO. 1
to the
by and between
Affimed Therapeutics AG
and
The Leukemia & Lymphoma Society
1

|

| |
| 0000 Xxxxxxxxxx Xxxxxx, Xxxxx 000 | Xx Xxxxxxxxxxx Xxxx 000 | |
| White Plains, NY 10605 USA | 69120 Heidelberg, Germany | |
| xxx.xxx.xxx | xxx.xxxxxxx.xxx | |
AMENDMENT NO. 1
to the
This Amendment (the “Amendment”) is made as of April 29th, 2014 (the “Amendment Effective Date”), by and between The Leukemia and Lymphoma Society, a New York nonprofit corporation with its principal place of business at 0000 Xxxxxxxxxx Xxxxxx, Xxxxx Xxxxxx, Xxx Xxxx 00000, Xxxxxx Xxxxxx xx Xxxxxxx (“LLS”) and Affimed Therapeutics AG, a German limited liability company with its principal place of business at Im Xxxxxxxxxxx Xxxx 000, 00000 Xxxxxxxxxx, Xxxxxxx (“Affimed”). LLS and Affimed are hereinafter referred to individually as the “Party” and together as the “Parties”.
WHEREAS, the Parties have concluded a Research Funding Agreement with effect as of August 26th, 2013 (“Agreement”), pursuant to which LLS has agreed to fund the AFM13 Development Program (as defined in the Agreement) according to a certain budget and certain defined milestones.
WHEREAS, the Parties have after the conclusion of the Agreement updated and modified the projected milestone timelines as well as the description of the study design and its rationale and now wish to document these updated and modified timelines and descriptions in a binding amendment to the Agreement and its Exhibits.
2
NOW, THEREFORE, in consideration of the mutual premises herein contained and for other good and valuable consideration the receipt and sufficiency of which is hereby acknowledged by the Parties, the Parties agree as follows.
| 1. | Amendment of Projected Milestone Dates. |
The Parties hereby agree on the modified projected timelines for milestone events as set forth in the Amended Exhibit C attached hereto which Amended Exhibit C shall replace and supersede the original Exhibit C to the Agreement.
| 2. | Amendment of Affimed Proposal. |
The Parties hereby agree on the amendment of the AFM13 Proposal (Exhibit D to the Agreement) as set forth in the Amendment to Exhibit D attached hereto. For the avoidance of doubt, the amendment solely relates to section VI) of the AFM13 Proposal All other sections of the AFM13 Proposal shall continue in full force and effect without modification and shall not be affected by this Amendment.
| 3. | General. |
Except as expressly set forth in this Amendment, the terms and conditions of the Agreement shall continue in full force and effect during the term of the Agreement.
IN WITNESS WHEREOF, the Parties have executed this Amendment as of the Amendment Effective Date.
| AFFIMED THERAPEUTICS AG | ||
| By: | /s/ Xxxx-Xxxxx Xxxxxxxxx | |
| Print Name: | Xxxx-Xxxxx Xxxxxxxxx | |
| Title: | CMO | |
3
| By: | /s/ Xxxxxxx Xxxxxxx | |
| Print Name: | Xxxxxxx Xxxxxxx | |
| Title: | CFO | |
| THE LEUKEMIA & LYMPHOMA SOCIETY | ||
| By: | /s/ Xxx Xxxxxxxxxxx | |
| Print Name: | Xxx Xxxxxxxxxxx | |
| Title: | CSO | |
4
AMENDED EXHIBIT C
Milestones and Payments
LLS and Affimed agree to the following provisions regarding Milestones and payments in performance of the AFM13 Development Program under the terms of the Agreement.
| Milestone | Milestone Payment | Milestone Event | Projected Date | |||||
| M1 |
***** | ***** |
***** | |||||
| M2 |
***** | ***** |
***** | |||||
| M3 |
***** | ***** |
***** | |||||
| M4 |
***** | ***** |
***** | |||||
| M5* |
***** | ***** |
***** | |||||
| M6* |
***** | ***** |
***** | |||||
| M7* |
***** | ***** |
***** | |||||
All milestone payments shall become due and payable by LLS within ***** after LLS’ receipt of a written notice from Affimed confirming that the respective milestone event has occurred.
| * | For clarification: Milestones M5 - M7 are each dependent on ***** as further described in the Affimed Proposal (Exhibit D). If no regimen *****, LLS reserves the right to make a No-go funding decision and cease all payments of Milestones M5 - M7. |
5
AMENDMENT TO EXHIBIT D
AFM13 Proposal
VI) AFM13 – Further clinical development – modified design
Because of further evaluation of clinical data of the phase 1 study AFM13-101 and further discussions with Key Opinion Leaders and statistical experts, the design of the originally proposed study was modified:
| • | A 2-stage Xxxxx’x design is used instead of a 2-stage Xxxxx design |
| • | The dose regimens investigated have been modified |
AFM13 will be investigated in a phase II study, using a two-stage Xxxxx’x design, in HL patients who failed or progressed on the therapy with Adcetris. This design allows for the rapid rejection of ineffective treatment at the end of the first stage and provides proof of concept at the end of the second stage. For this study a targeted response rate of at least 30% has been considered clinically meaningful. Compared to the original design 5 additional patients have to be recruited, otherwise there is no impact on the conduct of the study.
There will be two treatment groups in Stage 1. Each group will have 10 randomly allocated HL patients. One group will receive 1.5 mg/kg AFM13 dosed three times per week for 8 weeks and the other group will receive 1.5 mg/kg AFM13 dosed three times per week for 2 weeks followed by a weekly regimen of 7 mg/kg in weeks 3-8. The tumor responses will be assessed at the end of the cycle, in accordance with the Cheson criteria. Patients will receive a second cycle of therapy in case they show clinical benefit in terms of stable disease or response. The primary objective of this study will be objective response (OR) rate (PR and CR). The secondary objective will be, amongst others, progression free survival.
6

At the end of Stage 1 the number of objective responses (CR, PR) will be assessed. There must be at least two objective responses (OR) observed in one regimen in order for any dosing regimen to be considered for progress into Stage 2. If one or no OR is observed, the respective dosing regimen will be rejected and will not be investigated further. If both regimens qualify for Stage 2, the dosing regimen with the more favorable benefit-risk ratio will be selected. Therefore, the benefit-risk ratio will be evaluated exploratory by the investigators and the sponsor. If neither dosing schedule qualifies, LLS reserves the right to make a No-go funding decision and cease all payments of Milestones M5 - M7.
Additional 19 patients will be enrolled into Stage 2 and will be treated with the selected dosing regimen. Treatment and tumor assessment will be done as described for Stage 1. At the end of this study the XXX and other efficacy parameters of AFM13 will be estimated.
For the primary analysis an optimal Xxxxx’x two-stage design (Xxxxx, 1989) is used. The null hypothesis that the true overall response rate is 30% will be tested against a one-sided alternative. The null hypothesis will be rejected if 6 or more responses are observed in 29 patients with the selected dose regimen. This design yields a type I error rate of 0.0471 and power of 80% when the true response rate is 30%.
Affimed will conduct the Phase IIa study in collaboration with the German Hodgkin Study Group (GHSG). This group is a world leader in the field of HL and has a track record of highly scientific and high quality clinical trials. It is estimated that about 10 sites will participate in the study.
7
Rationale on the changed dose regimen in phase 2a
The doses and regimen for this phase 2a trial have been carefully selected considering preclinical experiments, PK modelling and results of the phase 1 study.
Phase 1 data revealed that activity was strongest with doses ³ 1.5 mg/kg. This could be demonstrated for pharmacodynamics in terms of NK cell activation and sCD30 depletion as well as for clinical response. The respective justification was given in the original proposal.
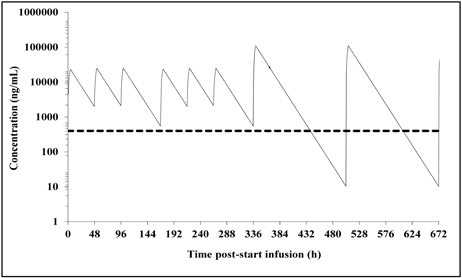
Based on the EC50 measured in preclinical experiments it was calculated that trough levels should be above 400 ng/ml. However, PK data of the phase 1 trial revealed that this was not the case with a weekly regimen for all doses tested. The figure below shows plasma concentrations for a patients who received 1.5 mg/kg as an example (the dotted line indicates the threshold of 400 ng/mL):

The same regimen administered 3 times per week, however, fulfills this requirement:

8
As it is not known how long the frequent regimen has to be given in order to maximize the efficacy and as it is a clear requirement by Key Opinion Leaders to investigate a more patient friendly regimen, an alternative regimen will be investigated in a second arm. Again starting with frequent dosing of 1.5 mg/kg over the first two weeks, a weekly regimen of a high dose, 7 mg/kg, will be administered in weeks 3 to 8. As shown by PK modelling below, this regimen still results in plasma levels above 400 ng/mL over the first 2.5 weeks and, in addition, in almost 5 of 7 days during the weekly regimen:
It was already explained and justified in the original study proposal, that a treatment of 8 weeks per cycle will be implemented. Further discussion with physicians and the FDA revealed that a second cycle has to be administered for ethical reasons in case patients benefit from the treatment, i.e. patients with stable disease, partial response or complete response after cycle 1.
9

